Abstract
The Golgi apparatus is tightly integrated into the cellular system where it plays essential roles required for a variety of cellular processes. Its vital functions include not only processing and sorting of proteins and lipids, but also serving as a signaling hub and a microtubule-organizing center. Golgi stacks in mammalian cells are interconnected into a compact ribbon in the perinuclear region. However, the ribbon can undergo distinct disassembly processes that reflect the cellular state or environmental demands and stress. For instance, its most dramatic change takes place in mitosis when the ribbon is efficiently disassembled into vesicles through a combination of ribbon unlinking, cisternal unstacking and vesiculation. Furthermore, the ribbon can also be detached and positioned at specific cellular locations to gain additional functionalities during differentiation, or fragmented to different degrees along disease progression or upon cell death. Here, we describe the major morphological alterations of Golgi ribbon disassembly under physiological and pathological conditions and discuss the underlying mechanisms that drive these changes.
Structure and function of the Golgi apparatus
The Golgi apparatus is a key membrane-bound organelle in the secretory pathway that is essential for all eukaryotic cells. The morphology of the Golgi is highly conserved and is featured by the densely packed cisternae that are layered on top of each other to form stacks [1]. Despite similar appearance, each individual cisterna houses a specific set of enzymes and represents a functionally distinct reaction chamber for processing incoming substrates [2]. Upon export from the endoplasmic reticulum, the newly synthesized proteins arrive at the cis-Golgi network (CGN) and take on their journey through individual cisternae of the stacks [3]. On their way from cis to medial to trans cisternae, the cargo proteins undergo various types of post-translational modifications including glycosylation, phosphorylation, sulfation, acetylation, methylation and proteolytic cleavage [4]. The molecules then exit the stacked cisternae at the trans-Golgi network (TGN), where they are sorted into specific vesicles and delivered to their final destinations such as the endosomal-lysosomal compartments, the cell surface, or the extracellular space. In this sense, the Golgi serves as a processing and sorting station for proteins and lipids in the biosynthetic pathway. This role is shared among all eukaryotes and is carried out in most cases by stacked cisternae [5].
Although a single stack or even unstacked cisternae are sufficient to sustain secretion in simple protozoa and budding yeast, most organisms contain multiple stacks that are distributed throughout the cell [6,7]. Uniquely to mammals, the stacks are further connected by tubular membranes into an elongated, twisted, but continuous structure named the Golgi ribbon. The interconnection of stacks is not strictly required for the secretory function of the Golgi, since disrupting the ribbon into discrete mini-stacks by microtubule depolymerization does not prevent cargo trafficking to the cell surface. However, transport kinetics is reduced in the initial phase but resumed to normal level at later stages [8], indicating that mini-stacks need to be fully dispersed and matured to become transport-competent [9].
Though dispensable for secretion, the ribbon organization greatly expands the functional repertoire of the Golgi in mammalian cells. The homotypic connections between adjacent cisternae of the stacks enlarge the membrane compartment such that large cargos as collagen can be readily accommodated [10,11*]. Similarly, a continuous ribbon allows proper packing of the von Willebrand factor into larger Weibel-Palade bodies, which impacts platelet aggregation [12**,13]. Furthermore, by laterally linking stacks, the Golgi apparatus is consolidated into one single entity that in most cases is asymmetrically positioned in the juxtanuclear region and in close proximity to centrosomes, the primary microtubule-organizing center (MTOC) in proliferating cells [6]. This confined pericentriolar localization of the ribbon plays an important role in establishing and maintaining cell polarity. The orientation of the ribbon guides membrane traffic towards a particular area of the plasma membrane, which lays the cornerstones for many polarization events in mammalian cells, such as neurite outgrowth [14], epithelial polarization [15] and directional cell migration [16–18]. In addition to these secretory and polarity functions, the Golgi has emerged as a versatile platform that supports a broad range of cellular processes. The Golgi can actively modulate the microtubule network [19,20], forms a hub for a variety of signaling pathways [21–24] and participates in the regulation of calcium and pH homeostasis [25], stress response [26,27], apoptosis [28] and autophagy [29,30**].
Golgi ribbon and the microtubule network
In proliferating cells, the structural integrity and the perinuclear positioning of the Golgi ribbon are tightly coordinated with the microtubule cytoskeleton and depend on the minus end-directed motor dynein. Disruption of the microtubule network with nocodazole or inhibition of dynein function at the Golgi disperses the ribbon into mini-stacks that are scattered throughout the cytoplasm [31–33]. Upon removal of nocodazole, microtubules regrow from Golgi membranes in addition to the centrosomes, exemplifying the function of the Golgi as an MTOC [34–36]. Two distinct microtubule networks originating from the centrosomes and the Golgi contribute to build a pericentriolar ribbon in cycling interphase cells [37]. In support of microtubule growth from the Golgi, the core component and modulators of the microtubule-nucleating y-tubulin ring complexes (γ-TuRCs), including γ-tubulin, AKAP450, Cdk5Rap2 and myomegalin, have been found localized to Golgi membranes where they collaborate to initiate microtubule nucleation [38–40,20]. Moreover, microtubule-associated proteins (MAPs), such as the minus-end binding protein CAMSAP2 and the plus-end tracking proteins CLASPs that recruits the microtubule-crosslinking protein MTCL1 to the Golgi, also help anchor and stabilize Golgi-derived microtubules and thus contribute to Golgi organization and function [36,41,42*].
The unique architecture and organization of the Golgi ribbon best exemplifies the hierarchical assembly of the cellular organelles [5]. Interestingly, this also implies that distinct mechanisms must be in place to disintegrate a larger structure such as the Golgi ribbon into its simpler units (stacks, cisternae, vesicles). These steps can occur simultaneously or independently, thus giving rise to different degrees of disassembly or fragmentation phenotypes. In many cases, disintegration of the Golgi ribbon also accompanies the rearrangement of the microtubule network. Below we describe the major morphological transformations during physiological processes including ribbon disassembly in mitosis and structural variations upon differentiation, as well as fragmentation under pathological conditions.
Mitotic disassembly of the Golgi ribbon
Best characterized among the fragmentation processes are the mechanisms that drive the disassembly of the Golgi during mitosis when it is most extensive and complete [43–45]. Interestingly, mitotic Golgi disassembly is not common to all organisms [6]. In plants and yeast, the stacked cisternae stay intact and are fully functional in secretion throughout the entire cell cycle [46,47]. The reason why the Golgi is disassembled in animal mitosis has long been an open question [48]. The fact that Golgi stacks are interconnected into a continuous ribbon poses a challenge for mammalian cells to equally partition the organelle. Disassembly of the ribbon into vesicles thus helps to segregate the Golgi membranes into the daughter cells. Furthermore, the disassembly of the ribbon also controls mitotic progression and spindle dynamics [49,50*,51*]. This mutual regulation of Golgi inheritance and cell division ensures the propagation of a functional Golgi ribbon through successive generations [52].
Once committed to mitotic entry, mammalian cells rapidly remodel their cellular structures to prepare for division [53]. To this end, the continuous Golgi ribbon swiftly disassembles into a collection of tubular-vesicular membranes, which are then partitioned with the help of the spindle into the daughter cells where they reassemble into a Golgi ribbon [54–56] (Figure 1a). The morphological changes of the Golgi in early mitosis are referred to as mitotic Golgi disassembly or mitotic Golgi fragmentation and both terms are often used interchangeably. Here we use the term mitotic Golgi disassembly because Golgi fragmentation is also used for irreversible processes such as apoptosis or necrosis [57,26]. Mitotic Golgi disassembly constitutes a series of highly orchestrated actions that are coordinated with the reorganization of other cellular contents (Figure 1a). More specifically, it is a multi-step process achieved through a combination of ribbon unlinking, cisternal unstacking, as well as tubulation and vesiculation of the Golgi membranes (Figure 1b) [43]. All these distinct mechanisms contribute to the rapid and drastic remodeling of mitotic Golgi membranes, though the extent of their interdependency and redundancy are not completely defined yet.
Figure 1.
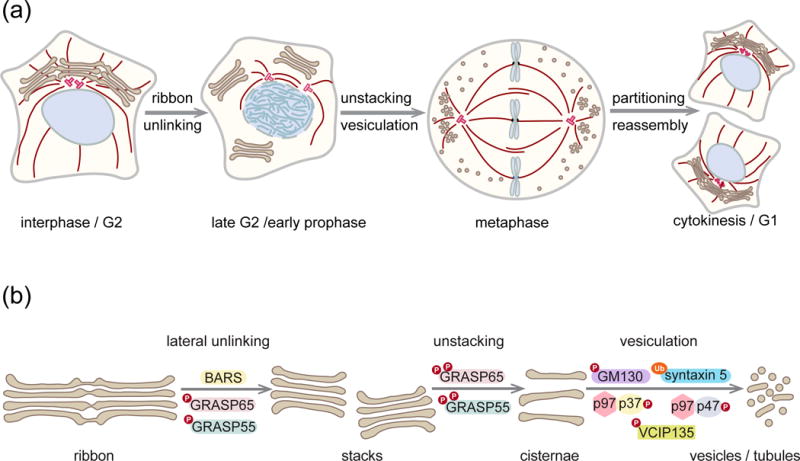
Golgi ribbon disassembly in mitosis. (a) During interphase, the Golgi stacks are interconnected into a ribbon that is localized close to the centrosomes. In late G2, the lateral connections between stacks are severed, which unlinks the ribbon and allows progression into mitosis. The cisternae then further unstack and vesiculate. Upon partitioning with the aid of the spindle, the mitotic Golgi membranes reassemble a ribbon in both daughter cells. (b) Stages and main players in mitotic Golgi disassembly. Ribbon unlinking in late G2 requires the membrane fission protein BARS and the phosphorylation of GRASP65 and GRASP55. Once the cells entered mitosis, further phosphorylation of GRASP 65 and GRASP55 induces cisternal unstacking. Simultaneously the cisternae vesiculate due to inhibition of both vesicle tethering (mediated by phosphorylation of GM130) and heterotypic fusion (mediated by ubiquitination of the t-SNARE syntaxin 5). Meanwhile, homotypic fusion of Golgi membranes is also blocked by phosphorylation of the p97 adaptors p37 and p47 and the co-factor VCIP135.
Lateral unlinking: from the ribbon to stacks
Ribbon unlinking initiates Golgi disassembly in late G2 phase before cells commit to mitosis. At this step, the lateral connections between cisternae are severed and individual stacks are released [58]. Scission of the interconnecting tubules requires the membrane fission protein CtBP/BARS. Blocking its activity by microinjection of inhibitory antibodies or dominant negative mutant proteins interferes with G2/M transition, indicating that ribbon unlinking is an important first step in mitotic Golgi disassembly [59,60]. In parallel, mitotic entry is delayed when blocking the Golgi proteins GRASP65 and GRASP55 [61–64], which function as lateral tethers of adjacent stacks during ribbon formation [65]. Ribbon unlinking further depends on JNK2 to phosphorylate GRASP65 at Ser277 [66], the same site that is also phosphorylated by ERK in interphase and by Cdk1 during mitosis [62,64,16]. These results have led to a proposed Golgi-based G2/M checkpoint [61,60], although the precise surveillance cascade that halts mitotic entry remains to be determined. Recent studies have begun to shed light on the key players in this pathway. Failure in ribbon unlinking in G2 prevents activation of the Src kinase at the TGN. Consequently, the Aurora-A kinase fails to be recruited to the centrosomes and remains inactive. As active Aurora A is a prerequisite of centrosome maturation and spindle formation, mitotic entry is thus forfeited [67,68*].
Surprisingly, severing of the stacks in late G2 is also monitored in Drosophila S2 cells where the Golgi stacks are present in pairs but not interconnected into a centralized ribbon. Analogous to ribbon unlinking in mammalian cells, pairs of fly Golgi become separated in late G2, which is also required for the transition into mitosis [69]. Despite their resemblance, the two processes are driven by very distinct mechanisms. While stack separation in flies is caused by actin depolymerization, ribbon unlinking in mammals is mediated through GRASP65/55 and/or BARS [70].
In late G2 phase, the unlinked stacks remain concentrated in the perinuclear region of the mammalian cells [60]. Upon mitotic entry, when the interphase microtubules are rapidly dismantled and remodeled to form a bipolar spindle, the stacks begin to scatter. Proper dispersal of the Golgi requires the dissociation of the microtubule motor cytoplasmic dynein from its Golgi receptor golgin160 [33]. Concomitant with the dispersal, the cisternae further unstack and vesiculate, leading to the complete disassembly of the Golgi apparatus.
Unstacking: from stacks to cisternae
Unstacking of cisternae in early mitosis is mediated through phosphorylation of GRASP65 and GRASP55, which were first identified as stacking factors that align cisternae into stacks [71,72]. Both GRASPs are homodimers that are attached via lipid modifications to the cytoplasmic face of the Golgi cisternae. During interphase, GRASP proteins assemble into antiparallel homo-tetramers in trans, which link apposing cisternae into stacks as well as laterally tether stacks within the ribbon [73,74]. Upon phosphorylation in early mitosis, trans-oligomerization of GRASP proteins is reversed, causing cisternal unstacking [74,75]. GRASP65 is phosphorylated by Cdk1/cyclin B and Plk1 at multiple sites [76], while GRASP55 is a mitotic target of the MAP kinase ERK2 [74,77].
Vesiculation: from cisternae to vesicles and tubules
Coinciding with unstacking, the membranes further disassemble into vesicles. Unstacking not only physically releases the cisternae, but also significantly speeds up vesiculation. The unstacked cisternae expose a larger surface area that becomes more accessible to recruit the components required for vesicle budding [78]. During interphase, budding and fusion of COPI transport vesicles at the Golgi are delicately balanced to maintain its function and morphology [79]. Upon entry into mitosis, phosphorylation of GM130 by Cdk1 prevents the vesicle tethering factor p115 from binding and thus blocks vesicle docking [80].
Furthermore, membrane fusion is also suppressed in mitosis [81]. Heterotypic fusion of vesicles with Golgi cisternae is inhibited via the ubiquitin E3 ligase HACE1 that monoubiquitinates the SNARE protein syntaxin 5 and thus prevents SNARE complex formation [82**]. In addition, homotypic fusion of Golgi membranes mediated by the AAA-ATPase p97 is also blocked upon mitotic phosphorylation of its adaptor proteins p47/VCIP135 and p37 [83–85]. In sum, vesicles continue to bud but fail to dock and fuse with cisternae, which quickly drives the equilibrium towards vesiculation [86].
Together, Golgi disassembly upon mitotic entry is facilitated through several processes that are driven by mechanistically distinct pathways to disassemble different parts of the Golgi [5]. Employing multiple mechanisms to drive disassembly makes this process extremely robust and efficient. In support of this notion, cells can still progress through mitosis when each individual process is blocked. These have been demonstrated by manipulating GRASP65 [62,74], p47 [85], BARS [60], syntaxin 5 ubiquitination [82**] or COPI vesicle budding [87].
The consequence of challenging such robustness in mitotic disassembly has been recently revealed. By filling the Golgi lumen with a unbreakable polymer that physically prevents its remodeling before progression into mitosis, cells could progress into M-phase with an intact Golgi, but strikingly centrosome separation is blocked [51*]. Accordingly, the cells fail to set up a bipolar spindle and become arrested with monoasters by an active spindle assembly checkpoint (SAC). This demonstrates that mitotic Golgi disassembly, just like spindle formation, is closely monitored by a signaling pathway that cross-talks with the SAC. Furthermore, upon disassembly the vesiculated Golgi membranes further participate in spindle formation. This is achieved by GM130 that binds and recruits importin α to the Golgi membranes [50*]. Sequestration of importin α relieves its inhibition on the spindle assembly factor TPX2, which in turn triggers microtubule nucleation in the vicinity of Golgi membranes. GM130 then captures the nascent microtubules and thus couples the Golgi membranes to the forming spindle. Collectively, mitotic Golgi disassembly not only passively serves a means to divide the organelle per se but also proactively mediates mitotic progression and spindle assembly.
Morphological variations of the Golgi ribbon during differentiation
In contrast to extensive disassembly during cell division, the ribbon possesses relatively minor morphological variations upon differentiation. These structural alterations enable the Golgi to fulfill specialized functions in post-mitotic differentiated cells (Figure 2). In neurons, for example, Golgi stacks can be detached from the somatic ribbon and are frequently found in dendrites [88]. These dendritic Golgi outposts function as local secretion units for synaptic receptors as well as sites for microtubule nucleation, thus regulating dendritic outgrowth and branching (Figure 2b) [14,89,90]. Golgi outposts are not locally established by de novo formation within major dendrites. Instead, they are generated through deployment and fission of tubules that originate from the somatic Golgi ribbon. This process is regulated by a RhoA-ROCK signaling pathway that activates two Golgi-localized kinases, protein kinase D1 (PKD1) and LIM domain kinase 1 (LIMK1), to promote tubule fission [91*]. In addition to outposts, dendrites further contain Golgi satellites, which represent simplified secretory micro-compartments that, in contrast to Golgi outposts, are seemingly deprived of essential Golgi proteins functioning in sorting and structural organization [92]. Whether these secretory units are indeed Golgi elements derived from the somatic ribbon, their exact ultrastructure and how they relate to Golgi outposts await further clarification.
Figure 2.
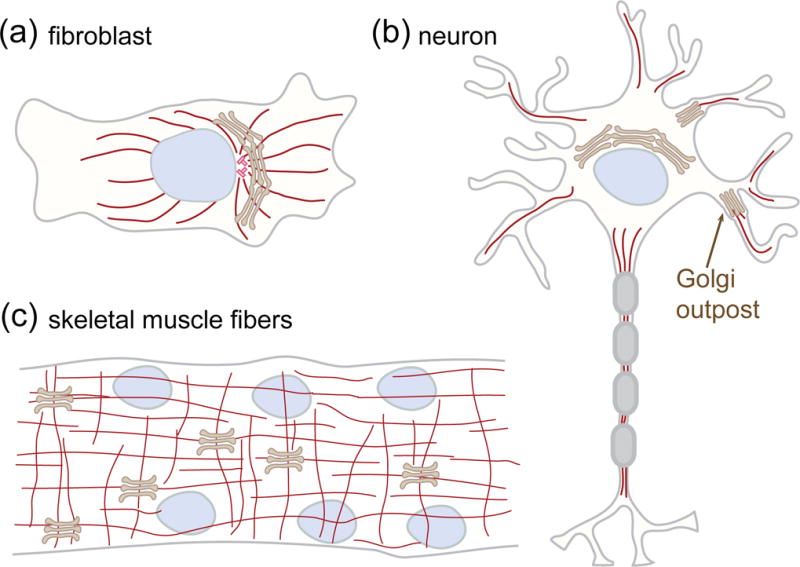
Golgi stacks in proliferating and differentiated mammalian cells. (a) Golgi ribbon in fibroblasts. The stacks are laterally linked together into a continuous ribbon that localizes in the perinuclear and pericentriolar region of the cell. (b) Golgi outposts in neurons. During neuronal differentiation, some stacks detach from the somatic ribbon and relocated to dendrites. These Golgi outpost function as sites for local secretion and microtubule nucleation to regulate dendrite outgrowth. (c) Golgi stacks in muscle fibers. In skeletal muscle fibers, the ribbon is broken up into stacks. Microtubules originating from the nuclear membrane and from Golgi stacks form a grid-like network.
Golgi ribbon fragmentation under pathological conditions
A fragmented Golgi ribbon is commonly associated with many stress and pathological conditions, including apoptosis [26,93], pathogen infection [94], amyotrophic lateral sclerosis (ALS) [95,96], Alzheimer’s disease [97,98], Parkinson’s disease [99,100] and various forms of cancer [101–103]. Despite similar phenotypic characteristics among these diseases, the mechanisms that cause Golgi fragmentation and dysfunction can range from imbalanced membrane flux, altered microtubule dynamics, to post-translational modifications or irreversible proteolytic cleavage of Golgi structural proteins. It is not clear whether the mechanisms that drive Golgi ribbon disassembly in mitosis or differentiation are also underpinning Golgi fragmentation during disease progression. In fact, the correlation between the observed morphological alterations and dysfunction of the Golgi is often unclear, as the fragmentation may directly cause, partially contribute to, or merely be the outcome of pathology.
In an effort to determine the contributions of Golgi fragmentation to neurodegenerative diseases, a recent study showed that gene deletion of the golgin GM130 in mice causes severe trafficking defects, concomitant with disruption and aberrant positioning of the Golgi, resulting in the death of Purkinje neurons and ataxia [104**]. Likewise, down-regulation of GM130 in zebrafish leads to microcephaly and muscle defects, which were also observed in a human patient with GM130 mutations who developed microcephaly and neuromuscular disorders [105]. Furthermore, GM130 knock out mice showed reduced body size and male infertility due to abnormal spermatogenesis caused by defects in sorting Golgi-derived vesicles [106]. These findings suggest that Golgi disruption and secretion dysfunction might be sufficient to cause severe phenotypes associated with neurodegenerative and other diseases.
On the other hand, the microtubule network also plays an important part in Golgi fragmentation during pathogenesis that share some morphological similarities with those during cell division and differentiation [107]. During differentiation of many cell types, including keratinocytes, hippocampal neurons, skeletal muscle and pancreatic cells [108–111], the microtubule nucleation activity of centrosomes is attenuated, leaving the Golgi as the key organelle to nucleate microtubules [112,88]. In pancreatic beta cells, for instance, microtubules predominantly grow from Golgi membranes instead of centrosomes [113*]. Furthermore, Golgi outposts in neurons can promote dendrite branching by generating non-centrosomal microtubules [90,114], although additional nucleation sites persist after experimentally removing Golgi outposts from dendrites [115]. Intriguingly, suppression of microtubule dynamics at Golgi outposts reduces terminal but not initial branching of dendrites in flies. This was observed upon deletion of the fly homologue of AKAP450, a γ-TuRC binding protein that is targeted to the Golgi by GM130 [116] and is indispensable for microtubule nucleation [42*]. A comparable phenotype is seen in Purkinje neurons of GM130 knockout mice where only terminal branching and arborization but not initial formation of dendrites is affected. Deletion of GM130 coincides with the loss of AKAP450 from the Golgi [104**], suggesting that defective dendrite branching may be partially attributable to the impaired microtubule nucleation from the Golgi. However, since the extensive dendrite branching of Purkinje cells depends on a functional secretory pathway, it is likely that the severe secretory perturbation further aggravates the phenotype [104**].
Reduced microtubule nucleation in combination with defective Golgi transport has also been linked to ALS, where loss of the Golgi-localized tubulin-binding cofactor E (TBCE) disrupts Golgi-derived microtubules and fragments the ribbon in motor neurons [95,117]. Similarly, a mouse model with impaired dynein/dynactin function exhibited a fragmented Golgi and developed ALS-like phenotypes [118,119], corroborating that Golgi ribbon disruption induced by cytoskeletal alterations are associated with various neurodegenerative disorders [120].
During differentiation and fusion of myoblasts (Figure 2c), the Golgi ribbon becomes dispersed and the microtubule network is reorganized while centrosomes lose their microtubule nucleation activity [121,111]. In skeletal muscle, microtubules are nucleated from the Golgi and nuclear membranes [112]. Dysregulation of these membrane-associated microtubules may lead to defective organization and thus alter the muscle function as found in Duchenne muscular dystrophy [111].
Concluding remarks
The Golgi ribbon represents a higher level of structural organization that correlates with more complex and advanced functions. Building on this new module, mammalian cells can expand its functionality by refining or disabling it at specific time and place. Recent progresses in studying differentiated cell types have suggested that ribbon disassembly could benefit the organism as a whole by meeting the needs for local secretion in specialized cells. On the other hand, accumulating evidence revealed that ribbon disassembly could also be the backstage driving force and/or the outcome of several notorious diseases. Stemming from our current understanding of Golgi disassembly in normal conditions, we have just begun to decipher the complexity of these processes, which may or may not share unifying mechanisms that underlie these morphological changes. More importantly, it is especially challenging but definitely important to dissect whether the structure-function relationship is actually causal or merely correlative, as this could completely change the strategy for diagnosis, drug development and treatment of the related diseases.
Highlights.
Golgi disassembles in mitosis via ribbon unlinking, unstacking and vesiculation
Golgi ribbon disassembly is required for mitotic progression and spindle assembly
Golgi ribbon disassembly often accompanies with microtubule remodeling
During differentiation the Golgi ribbon disassembles to gain additional functions
Fragmentation and dysfunction of the Golgi ribbon are linked to several diseases
Acknowledgments
This work is supported by the National Institute of Health (GM096070) and the Welch Foundation (I-1910) to J.S. and by the UCSF Program for Breakthrough Biomedical Research (PBBR), funded in part by the Sandler Foundation, to J.H.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lowe M. Structural organization of the Golgi apparatus. Curr Opin Cell Biol. 2011;23:85–93. doi: 10.1016/j.ceb.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Papanikou E, Glick BS. Golgi compartmentation and identity. Curr Opin Cell Biol. 2014;29:74–81. doi: 10.1016/j.ceb.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandizzi F, Barlowe C. Organization of the ER-Golgi interface for membrane traffic control. Nat Rev Mol Cell Biol. 2013;14:382–392. doi: 10.1038/nrm3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Potelle S, Klein A, Foulquier F. Golgi post-translational modifications and associated diseases. J Inherit Metab Dis. 2015;38:741–751. doi: 10.1007/s10545-015-9851-7. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura N, Wei J-H, Seemann J. Modular organization of the mammalian Golgi apparatus. Curr Opin Cell Biol. 2012;24:467–474. doi: 10.1016/j.ceb.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei J-H, Seemann J. Unraveling the Golgi ribbon. Traffic. 2010;11:1391–1400. doi: 10.1111/j.1600-0854.2010.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Seemann J. Golgi biogenesis. Cold Spring Harb Perspect Biol. 2011;3:a005330. doi: 10.1101/cshperspect.a005330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole NB, Sciaky N, Marotta A, Song J, Lippincott-Schwartz J. Golgi dispersal during microtubule disruption: regeneration of Golgi stacks at peripheral endoplasmic reticulum exit sites. Mol Biol Cell. 1996;7:631–650. doi: 10.1091/mbc.7.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fourriere L, Divoux S, Roceri M, Perez F, Boncompain G. Microtubule-independent secretion requires functional maturation of Golgi elements. J Cell Sci. 2016;129:3238–3250. doi: 10.1242/jcs.188870. [DOI] [PubMed] [Google Scholar]
- 10.Puthenveedu MA, Bachert C, Puri S, Lanni F, Linstedt AD. GM130 and GRASP65-dependent lateral cisternal fusion allows uniform Golgi-enzyme distribution. Nat Cell Biol. 2006;8:238–248. doi: 10.1038/ncb1366. [DOI] [PubMed] [Google Scholar]
- 11*.Lavieu G, Dunlop MH, Lerich A, Zheng H, Bottanelli F, Rothman JE. The Golgi ribbon structure facilitates anterograde transport of large cargoes. Mol Biol Cell. 2014;25:3028–3036. doi: 10.1091/mbc.E14-04-0931. This paper shows that large secretory cargos such as collagen are more efficiently transported through stacks that are interconnected into a ribbon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Ferraro F, Kriston-Vizi J, Metcalf DJ, Martin-Martin B, Freeman J, Burden JJ, Westmoreland D, Dyer CE, Knight AE, Ketteler R, et al. A Two-Tier Golgi-Based Control of Organelle Size Underpins the Functional Plasticity of Endothelial Cells. Dev Cell. 2014;29:292–304. doi: 10.1016/j.devcel.2014.03.021. The authors showed that the continuous lumen of the Golgi generated by connecting cisternae into a ribbon facilitates the packing of large Weibel-Palade bodies that are important upon secretion to recruit platelets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferraro F, Mafalda Lopes da S, Grimes W, Lee HK, Ketteler R, Kriston-Vizi J, Cutler DF. Weibel-Palade body size modulates the adhesive activity of its von Willebrand Factor cargo in cultured endothelial cells. Sci Rep. 2016;6:32473. doi: 10.1038/srep32473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horton AC, Rácz B, Monson EE, Lin AL, Weinberg RJ, Ehlers MD. Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron. 2005;48:757–771. doi: 10.1016/j.neuron.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 16.Bisel B, Wang Y, Wei J-H, Xiang Y, Tang D, Miron-Mendoza M, Yoshimura S, Nakamura N, Seemann J. ERK regulates Golgi and centrosome orientation towards the leading edge through GRASP65. J Cell Biol. 2008;182:837–843. doi: 10.1083/jcb.200805045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yadav S, Puri S, Linstedt AD. A primary role for Golgi positioning in directed secretion, cell polarity, and wound healing. Mol Biol Cell. 2009;20:1728–1736. doi: 10.1091/mbc.E08-10-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preisinger C, Short B, De Corte V, Bruyneel E, Haas A, Kopajtich R, Gettemans J, Barr FA. YSK1 is activated by the Golgi matrix protein GM130 and plays a role in cell migration through its substrate 14-3-3zeta. J Cell Biol. 2004;164:1009–1020. doi: 10.1083/jcb.200310061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu X, Kaverina I. Golgi as an MTOC: making microtubules for its own good. Histochem Cell Biol. 2013;140:361–367. doi: 10.1007/s00418-013-1119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanders AAWM, Kaverina I. Nucleation and Dynamics of Golgi-derived Microtubules. Front Neurosci. 2015;9:431. doi: 10.3389/fnins.2015.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farhan H, Rabouille C. Signalling to and from the secretory pathway. J Cell Sci. 2011;124:171–180. doi: 10.1242/jcs.076455. [DOI] [PubMed] [Google Scholar]
- 22.Mayinger P. Signaling at the Golgi. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chia J, Goh G, Racine V, Ng S, Kumar P, Bard F. RNAi screening reveals a large signaling network controlling the Golgi apparatus in human cells. Mol Syst Biol. 2012;8:629. doi: 10.1038/msb.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luini A, Parashuraman S. Signaling at the Golgi: sensing and controlling the membrane fluxes. Curr Opin Cell Biol. 2016;39:37–42. doi: 10.1016/j.ceb.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Demaegd D, Foulquier F, Colinet A-S, Gremillon L, Legrand D, Mariot P, Peiter E, Van Schaftingen E, Matthijs G, Morsomme P. Newly characterized Golgi-localized family of proteins is involved in calcium and pH homeostasis in yeast and human cells. Proc Natl Acad Sci U S A. 2013;110:6859–6864. doi: 10.1073/pnas.1219871110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machamer CE. The Golgi complex in stress and death. Front Neurosci. 2015;9:421. doi: 10.3389/fnins.2015.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taniguchi M, Yoshida H. TFE3, HSP47, and CREB3 pathways of the mammalian Golgi stress response. Cell Struct Funct. 2017 doi: 10.1247/csf.16023. [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee S, Chiu R, Leung S-M, Shields D. Fragmentation of the Golgi apparatus: an early apoptotic event independent of the cytoskeleton. Traffic. 2007;8:369–378. doi: 10.1111/j.1600-0854.2007.00542.x. [DOI] [PubMed] [Google Scholar]
- 29.Geng J, Nair U, Yasumura-Yorimitsu K, Klionsky DJ. Post-Golgi Sec proteins are required for autophagy in Saccharomyces cerevisiae. Mol Biol Cell. 2010;21:2257–2269. doi: 10.1091/mbc.E09-11-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30**.Joachim J, Jefferies HBJ, Razi M, Frith D, Snijders AP, Chakravarty P, Judith D, Tooze SA. Activation of ULK Kinase and Autophagy by GABARAP Trafficking from the Centrosome Is Regulated by WAC and GM130. Mol Cell. 2015;60:899–913. doi: 10.1016/j.molcel.2015.11.018. This study shows a direct signaling role for the Golgi in regulating autophagy. GM130 directly recruits the autophagy regulators WAC and the Atg8 homologue GABARAP to the Golgi, thereby acting as a negative regulator of autophagy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burkhardt JK, Echeverri CJ, Nilsson T, Vallee RB. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roghi C, Allan VJ. Dynamic association of cytoplasmic dynein heavy chain 1a with the Golgi apparatus and intermediate compartment. J Cell Sci. 1999;112(Pt 24):4673–4685. doi: 10.1242/jcs.112.24.4673. [DOI] [PubMed] [Google Scholar]
- 33.Yadav S, Puthenveedu MA, Linstedt AD. Golgin160 recruits the Dynein motor to position the Golgi apparatus. Dev Cell. 2012;23:153–165. doi: 10.1016/j.devcel.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chabin-Brion K, Marceiller J, Perez F, Settegrana C, Drechou A, Durand G, Poüs C. The Golgi complex is a microtubule-organizing organelle. Mol Biol Cell. 2001;12:2047–2060. doi: 10.1091/mbc.12.7.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Efimov A, Kharitonov A, Efimova N, Loncarek J, Miller PM, Andreyeva N, Gleeson P, Galjart N, Maia ARR, McLeod IX, et al. Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev Cell. 2007;12:917–930. doi: 10.1016/j.devcel.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller PM, Folkmann AW, Maia ARR, Efimova N, Efimov A, Kaverina I. Golgi-derived CLASP-dependent microtubules control Golgi organization and polarized trafficking in motile cells. Nat Cell Biol. 2009;11:1069–1080. doi: 10.1038/ncb1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vinogradova T, Paul R, Grimaldi AD, Loncarek J, Miller PM, Yampolsky D, Magidson V, Khodjakov A, Mogilner A, Kaverina I. Concerted effort of centrosomal and Golgi-derived microtubules is required for proper Golgi complex assembly but not for maintenance. Mol Biol Cell. 2012;23:820–833. doi: 10.1091/mbc.E11-06-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rivero S, Cardenas J, Bornens M, Rios RM. Microtubule nucleation at the cis-side of the Golgi apparatus requires AKAP450 and GM130. EMBO J. 2009;28:1016–1028. doi: 10.1038/emboj.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z, Wu T, Shi L, Zhang L, Zheng W, Qu JY, Niu R, Qi RZ. Conserved motif of CDK5RAP2 mediates its localization to centrosomes and the Golgi complex. J Biol Chem. 2010;285:22658–22665. doi: 10.1074/jbc.M110.105965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Zhang C, Qi RZ. A newly identified myomegalin isoform functions in Golgi microtubule organization and ER–Golgi transport. J Cell Sci. 2014;127:4904–4917. doi: 10.1242/jcs.155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato Y, Hayashi K, Amano Y, Takahashi M, Yonemura S, Hayashi I, Hirose H, Ohno S, Suzuki A. MTCL1 crosslinks and stabilizes non-centrosomal microtubules on the Golgi membrane. Nat Commun. 2014;5:5266. doi: 10.1038/ncomms6266. [DOI] [PubMed] [Google Scholar]
- 42*.Wu J, de Heus C, Liu Q, Bouchet BP, Noordstra I, Jiang K, Hua S, Martin M, Yang C, Grigoriev I, et al. Molecular Pathway of Microtubule Organization at the Golgi Apparatus. Dev Cell. 2016;39:44–60. doi: 10.1016/j.devcel.2016.08.009. This paper shows that AKAP450 is essential for microtubule nucleation from the Golgi. Proper tethering of microtubules to the Golgi is mediated through the microtubule binding protein CAMSAP2 that is recruited by the Golgi-localized AKAP450/myomegalin complex. [DOI] [PubMed] [Google Scholar]
- 43.Shorter J, Warren G. Golgi architecture and inheritance. Annu Rev Cell Dev Biol. 2002;18:379–420. doi: 10.1146/annurev.cellbio.18.030602.133733. [DOI] [PubMed] [Google Scholar]
- 44.Lowe M, Barr FA. Inheritance and biogenesis of organelles in the secretory pathway. Nat Rev Mol Cell Biol. 2007;8:429–439. doi: 10.1038/nrm2179. [DOI] [PubMed] [Google Scholar]
- 45.Valente C, Colanzi A. Mechanisms and Regulation of the Mitotic Inheritance of the Golgi Complex. Front Cell Dev Biol. 2015;3:79. doi: 10.3389/fcell.2015.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Preuss D, Mulholland J, Franzusoff A, Segev N, Botstein D. Characterization of the Saccharomyces Golgi complex through the cell cycle by immunoelectron microscopy. Mol Biol Cell. 1992;3:789–803. doi: 10.1091/mbc.3.7.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seguí-Simarro JM, Staehelin LA. Cell cycle-dependent changes in Golgi stacks, vacuoles, clathrin-coated vesicles and multivesicular bodies in meristematic cells of Arabidopsis thaliana: a quantitative and spatial analysis. Planta. 2006;223:223–236. doi: 10.1007/s00425-005-0082-2. [DOI] [PubMed] [Google Scholar]
- 48.Lucocq JM, Pryde JG, Berger EG, Warren G. A mitotic form of the Golgi apparatus in HeLa cells. J Cell Biol. 1987;104:865–874. doi: 10.1083/jcb.104.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei J-H, Seemann J. The mitotic spindle mediates inheritance of the Golgi ribbon structure. J Cell Biol. 2009;184:391–397. doi: 10.1083/jcb.200809090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Wei J-H, Zhang ZC, Wynn RM, Seemann J. GM130 Regulates Golgi-Derived Spindle Assembly by Activating TPX2 and Capturing Microtubules. Cell. 2015;162:287–299. doi: 10.1016/j.cell.2015.06.014. Upon mitotic entry, GM130 on the Golgi actives the spindle assembly factor TPX2 to locally nucleate microtubules. GM130 further captures the filaments thereby linking mitotic Golgi membranes to the nascent spindle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51*.Guizzunti G, Seemann J. Mitotic Golgi disassembly is required for bipolar spindle formation and mitotic progression. Proc Natl Acad Sci U S A. 2016;113:E6590–E6599. doi: 10.1073/pnas.1610844113. Preventing Golgi vesiculation arrests cells in early mitosis with an active spindle assembly checkpoint, showing that Golgi disassembly is a required step for mitosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colanzi A, Corda D. Mitosis controls the Golgi and the Golgi controls mitosis. Curr Opin Cell Biol. 2007;19:386–393. doi: 10.1016/j.ceb.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 53.Champion L, Linder MI, Kutay U. Cellular Reorganization during Mitotic Entry. Trends Cell Biol. 2016 doi: 10.1016/j.tcb.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Hiller G, Weber K. Golgi detection in mitotic and interphase cells by antibodies to secreted galactosyltransferase. Exp Cell Res. 1982;142:85–94. doi: 10.1016/0014-4827(82)90412-8. [DOI] [PubMed] [Google Scholar]
- 55.Shima DT, Haldar K, Pepperkok R, Watson R, Warren G. Partitioning of the Golgi apparatus during mitosis in living HeLa cells. J Cell Biol. 1997;137:1211–1228. doi: 10.1083/jcb.137.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seemann J, Pypaert M, Taguchi T, Malsam J, Warren G. Partitioning of the matrix fraction of the Golgi apparatus during mitosis in animal cells. Science. 2002;295:848–851. doi: 10.1126/science.1068064. [DOI] [PubMed] [Google Scholar]
- 57.Nozawa K, Casiano CA, Hamel JC, Molinaro C, Fritzler MJ, Chan EKL. Fragmentation of Golgi complex and Golgi autoantigens during apoptosis and necrosis. Arthritis Res. 2002;4:R3. doi: 10.1186/ar422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rabouille C, Kondylis V. Golgi ribbon unlinking: an organelle-based G2/M checkpoint. Cell Cycle. 2007;6:2723–2729. doi: 10.4161/cc.6.22.4896. [DOI] [PubMed] [Google Scholar]
- 59.Hidalgo Carcedo C, Bonazzi M, Spanò S, Turacchio G, Colanzi A, Luini A, Corda D. Mitotic Golgi partitioning is driven by the membrane-fissioning protein CtBP3/BARS. Science. 2004;305:93–96. doi: 10.1126/science.1097775. [DOI] [PubMed] [Google Scholar]
- 60.Colanzi A, Hidalgo Carcedo C, Persico A, Cericola C, Turacchio G, Bonazzi M, Luini A, Corda D. The Golgi mitotic checkpoint is controlled by BARS-dependent fission of the Golgi ribbon into separate stacks in G2. EMBO J. 2007;26:2465–2476. doi: 10.1038/sj.emboj.7601686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sütterlin C, Hsu P, Mallabiabarrena A, Malhotra V. Fragmentation and dispersal of the pericentriolar Golgi complex is required for entry into mitosis in mammalian cells. Cell. 2002;109:359–369. doi: 10.1016/s0092-8674(02)00720-1. [DOI] [PubMed] [Google Scholar]
- 62.Preisinger C, Körner R, Wind M, Lehmann WD, Kopajtich R, Barr FA. Plk1 docking to GRASP65 phosphorylated by Cdk1 suggests a mechanism for Golgi checkpoint signalling. EMBO J. 2005;24:753–765. doi: 10.1038/sj.emboj.7600569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duran JM, Kinseth M, Bossard C, Rose DW, Polishchuk R, Wu CC, Yates J, Zimmerman T, Malhotra V. The role of GRASP55 in Golgi fragmentation and entry of cells into mitosis. Mol Biol Cell. 2008;19:2579–2587. doi: 10.1091/mbc.E07-10-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoshimura S, Yoshioka K, Barr FA, Lowe M, Nakayama K, Ohkuma S, Nakamura N. Convergence of cell cycle regulation and growth factor signals on GRASP65. J Biol Chem. 2005;280:23048–23056. doi: 10.1074/jbc.M502442200. [DOI] [PubMed] [Google Scholar]
- 65.Rabouille C, Linstedt AD. GRASP: A Multitasking Tether. Front Cell Dev Biol. 2016;4:1. doi: 10.3389/fcell.2016.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cervigni RI, Bonavita R, Barretta ML, Spano D, Ayala I, Nakamura N, Corda D, Colanzi A. JNK2 controls fragmentation of the Golgi complex and the G2/M transition through phosphorylation of GRASP65. J Cell Sci. 2015;128:2249–2260. doi: 10.1242/jcs.164871. [DOI] [PubMed] [Google Scholar]
- 67.Persico A, Cervigni RI, Barretta ML, Corda D, Colanzi A. Golgi partitioning controls mitotic entry through Aurora-A kinase. Mol Biol Cell. 2010;21:3708–3721. doi: 10.1091/mbc.E10-03-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68*.Barretta ML, Spano D, D’Ambrosio C, Cervigni RI, Scaloni A, Corda D, Colanzi A. Aurora-A recruitment and centrosomal maturation are regulated by a Golgi-activated pool of Src during G2. Nat Commun. 2016;7:11727. doi: 10.1038/ncomms11727. In this paper, the authors uncovered a signaling pathway that activates Src kinase upon ribbon unlinking in late G2. Src in turn phosphorylates Aurora A kinase which gets recruited to centrosomes and enables mitotic progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kondylis V, van Nispen tot Pannerden HE, Herpers B, Friggi-Grelin F, Rabouille C. The golgi comprises a paired stack that is separated at G2 by modulation of the actin cytoskeleton through Abi and Scar/WAVE. Dev Cell. 2007;12:901–915. doi: 10.1016/j.devcel.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 70.Kondylis V, Rabouille C. The Golgi apparatus: lessons from Drosophila. FEBS Lett. 2009;583:3827–3838. doi: 10.1016/j.febslet.2009.09.048. [DOI] [PubMed] [Google Scholar]
- 71.Barr FA, Puype M, Vandekerckhove J, Warren G. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 1997;91:253–262. doi: 10.1016/s0092-8674(00)80407-9. [DOI] [PubMed] [Google Scholar]
- 72.Shorter J, Watson R, Giannakou ME, Clarke M, Warren G, Barr FA. GRASP55, a second mammalian GRASP protein involved in the stacking of Golgi cisternae in a cell-free system. EMBO J. 1999;18:4949–4960. doi: 10.1093/emboj/18.18.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feinstein TN, Linstedt AD. GRASP55 regulates Golgi ribbon formation. Mol Biol Cell. 2008;19:2696–2707. doi: 10.1091/mbc.E07-11-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiang Y, Wang Y. GRASP55 and GRASP65 play complementary and essential roles in Golgi cisternal stacking. J Cell Biol. 2010;188:237–251. doi: 10.1083/jcb.200907132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y, Seemann J, Pypaert M, Shorter J, Warren G. A direct role for GRASP65 as a mitotically regulated Golgi stacking factor. EMBO J. 2003;22:3279–3290. doi: 10.1093/emboj/cdg317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tang D, Yuan H, Vielemeyer O, Perez F, Wang Y. Sequential phosphorylation of GRASP65 during mitotic Golgi disassembly. Biol Open. 2012;1:1204–1214. doi: 10.1242/bio.20122659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jesch SA, Lewis TS, Ahn NG, Linstedt AD. Mitotic phosphorylation of Golgi reassembly stacking protein 55 by mitogen-activated protein kinase ERK2. Mol Biol Cell. 2001;12:1811–1817. doi: 10.1091/mbc.12.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y, Wei J-H, Bisel B, Tang D, Seemann J. Golgi cisternal unstacking stimulates COPI vesicle budding and protein transport. PloS One. 2008;3:e1647. doi: 10.1371/journal.pone.0001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Glick BS, Malhotra V. The curious status of the Golgi apparatus. Cell. 1998;95:883–889. doi: 10.1016/s0092-8674(00)81713-4. [DOI] [PubMed] [Google Scholar]
- 80.Lowe M, Rabouille C, Nakamura N, Watson R, Jackman M, Jämsä E, Rahman D, Pappin DJ, Warren G. Cdc2 kinase directly phosphorylates the cis-Golgi matrix protein GM130 and is required for Golgi fragmentation in mitosis. Cell. 1998;94:783–793. doi: 10.1016/s0092-8674(00)81737-7. [DOI] [PubMed] [Google Scholar]
- 81.Lowe M, Nakamura N, Warren G. Golgi division and membrane traffic. Trends Cell Biol. 1998;8:40–44. doi: 10.1016/s0962-8924(97)01189-6. [DOI] [PubMed] [Google Scholar]
- 82**.Huang S, Tang D, Wang Y. Monoubiquitination of Syntaxin 5 Regulates Golgi Membrane Dynamics during the Cell Cycle. Dev Cell. 2016;38:73–85. doi: 10.1016/j.devcel.2016.06.001. This paper shows that in early mitosis the Golgi t-SNARE protein sytnaxin 5 is monoubiquitinated by the ubiquitin ligase HACE1. Monoubiquitinated syntaxin 5 fails to bind to its cognate v-SNARE Bet1, which prevents vesicle fusion and thus contributes to Golgi vesiculation in mitosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaneko Y, Tamura K, Totsukawa G, Kondo H. Phosphorylation of p37 is important for Golgi disassembly at mitosis. Biochem Biophys Res Commun. 2010;402:37–41. doi: 10.1016/j.bbrc.2010.09.097. [DOI] [PubMed] [Google Scholar]
- 84.Totsukawa G, Matsuo A, Kubota A, Taguchi Y, Kondo H. Mitotic phosphorylation of VCIP135 blocks p97ATPase-mediated Golgi membrane fusion. Biochem Biophys Res Commun. 2013;433:237–242. doi: 10.1016/j.bbrc.2013.02.090. [DOI] [PubMed] [Google Scholar]
- 85.Uchiyama K, Jokitalo E, Lindman M, Jackman M, Kano F, Murata M, Zhang X, Kondo H. The localization and phosphorylation of p47 are important for Golgi disassembly-assembly during the cell cycle. J Cell Biol. 2003;161:1067–1079. doi: 10.1083/jcb.200303048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei J-H, Seemann J. Mitotic division of the mammalian Golgi apparatus. Semin Cell Dev Biol. 2009;20:810–816. doi: 10.1016/j.semcdb.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 87.Altan-Bonnet N, Phair RD, Polishchuk RS, Weigert R, Lippincott-Schwartz J. A role for Arf1 in mitotic Golgi disassembly, chromosome segregation, and cytokinesis. Proc Natl Acad Sci. 2003;100:13314–13319. doi: 10.1073/pnas.2234055100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hanus C, Ehlers MD. Specialization of biosynthetic membrane trafficking for neuronal form and function. Curr Opin Neurobiol. 2016;39:8–16. doi: 10.1016/j.conb.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 89.Ye B, Zhang Y, Song W, Younger SH, Jan LY, Jan YN. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell. 2007;130:717–729. doi: 10.1016/j.cell.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ori-McKenney KM, Jan LY, Jan Y-N. Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron. 2012;76:921–930. doi: 10.1016/j.neuron.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91*.Quassollo G, Wojnacki J, Salas DA, Gastaldi L, Marzolo MP, Conde C, Bisbal M, Couve A, Cáceres A. A RhoA Signaling Pathway Regulates Dendritic Golgi Outpost Formation. Curr Biol. 2015;25:971–982. doi: 10.1016/j.cub.2015.01.075. Golgi outposts are generated from the somatic ribbon through a RhoA-controlled mechanism of tubulation and fission. [DOI] [PubMed] [Google Scholar]
- 92.Mikhaylova M, Bera S, Kobler O, Frischknecht R, Kreutz MR. A Dendritic Golgi Satellite between ERGIC and Retromer. Cell Rep. 2016;14:189–199. doi: 10.1016/j.celrep.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 93.Lane JD, Lucocq J, Pryde J, Barr FA, Woodman PG, Allan VJ, Lowe M. Caspase-mediated cleavage of the stacking protein GRASP65 is required for Golgi fragmentation during apoptosis. J Cell Biol. 2002;156:495–509. doi: 10.1083/jcb.200110007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jimenez A, Chen D, Alto NM. How Bacteria Subvert Animal Cell Structure and Function. Annu Rev Cell Dev Biol. 2016;32:373–397. doi: 10.1146/annurev-cellbio-100814-125227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haase G, Rabouille C. Golgi Fragmentation in ALS Motor Neurons. New Mechanisms Targeting Microtubules, Tethers, and Transport Vesicles Front Neurosci. 2015;9:448. doi: 10.3389/fnins.2015.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sundaramoorthy V, Sultana JM, Atkin JD. Golgi fragmentation in amyotrophic lateral sclerosis, an overview of possible triggers and consequences. Front Neurosci. 2015;9:400. doi: 10.3389/fnins.2015.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baloyannis SJ. Golgi apparatus and protein trafficking in Alzheimer’s disease. J Alzheimers Dis. 2014;42(Suppl 3):S153–162. doi: 10.3233/JAD-132660. [DOI] [PubMed] [Google Scholar]
- 98.Joshi G, Bekier ME, Wang Y. Golgi fragmentation in Alzheimer’s disease. Front Neurosci. 2015;9:340. doi: 10.3389/fnins.2015.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cho HJ, Yu J, Xie C, Rudrabhatla P, Chen X, Wu J, Parisiadou L, Liu G, Sun L, Ma B, et al. Leucine-rich repeat kinase 2 regulates Sec16A at ER exit sites to allow ER-Golgi export. EMBO J. 2014;33:2314–2331. doi: 10.15252/embj.201487807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang T, Hay JC. Alpha-synuclein Toxicity in the Early Secretory Pathway: How It Drives Neurodegeneration in Parkinsons Disease. Front Neurosci. 2015;9:433. doi: 10.3389/fnins.2015.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baschieri F, Uetz-von Allmen E, Legler DF, Farhan H. Loss of GM130 in breast cancer cells and its effects on cell migration, invasion and polarity. Cell Cycle. 2015;14:1139–1147. doi: 10.1080/15384101.2015.1007771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Petrosyan A. Onco-Golgi: Is Fragmentation a Gate to Cancer Progression? Biochem Mol Biol J. 2015;1 doi: 10.21767/2471-8084.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rizzo R, Parashuraman S, D’Angelo G, Luini A. GOLPH3 and oncogenesis: What is the molecular link? Tissue Cell. 2016 doi: 10.1016/j.tice.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 104**.Liu C, Mei M, Li Q, Roboti P, Pang Q, Ying Z, Gao F, Lowe M, Bao S. Loss of the golgin GM130 causes Golgi disruption, Purkinje neuron loss, and ataxia in mice. Proc Natl Acad Sci. 2016 doi: 10.1073/pnas.1608576114. GM130 knockout in mice leads to altered morphology and aberrant positioning of the Golgi in Purkinje cells. Protein trafficking is also severely impaired, causing degeneration of Purkinje neurons and ataxia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shamseldin HE, Bennett AH, Alfadhel M, Gupta V, Alkuraya FS. GOLGA2, encoding a master regulator of golgi apparatus, is mutated in a patient with a neuromuscular disorder. Hum Genet. 2016;135:245–251. doi: 10.1007/s00439-015-1632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Han F, Liu C, Zhang L, Chen M, Zhou Y, Qin Y, Wang Y, Chen M, Duo S, Cui X, et al. Globozoospermia and lack of acrosome formation in GM130-deficient mice. Cell Death Dis. 2017;8:e2532. doi: 10.1038/cddis.2016.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Joshi G, Wang Y. Golgi defects enhance APP amyloidogenic processing in Alzheimer’s disease. BioEssays. 2015;37:240–247. doi: 10.1002/bies.201400116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stiess M, Maghelli N, Kapitein LC, Gomis-Rüth S, Wilsch-Bräuninger M, Hoogenraad CC, Tolić-Nørrelykke IM, Bradke F. Axon extension occurs independently of centrosomal microtubule nucleation. Science. 2010;327:704–707. doi: 10.1126/science.1182179. [DOI] [PubMed] [Google Scholar]
- 109.Sanchez AD, Feldman JL. Microtubule-organizing centers: from the centrosome to non-centrosomal sites. Curr Opin Cell Biol. 2016 doi: 10.1016/j.ceb.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Muroyama A, Seldin L, Lechler T. Divergent regulation of functionally distinct γ-tubulin complexes during differentiation. J Cell Biol. 2016;213:679–692. doi: 10.1083/jcb.201601099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Folker ES, Baylies MK. Nuclear positioning in muscle development and disease. Front Physiol. 2013;4:363. doi: 10.3389/fphys.2013.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Oddoux S, Zaal KJ, Tate V, Kenea A, Nandkeolyar SA, Reid E, Liu W, Ralston E. Microtubules that form the stationary lattice of muscle fibers are dynamic and nucleated at Golgi elements. J Cell Biol. 2013;203:205–213. doi: 10.1083/jcb.201304063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113*.Zhu X, Hu R, Brissova M, Stein RW, Powers AC, Gu G, Kaverina I. Microtubules Negatively Regulate Insulin Secretion in Pancreatic β Cells. Dev Cell. 2015;34:656–668. doi: 10.1016/j.devcel.2015.08.020. In insulin-secreting cells, Golgi-derived microtubules form a dense meshwork, which interferes with the secretion of insulin granules. Glucose destabilizes these microtubules and thus enhances insulin secretion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lewis TL, Polleux F. Neuronal morphogenesis: Golgi outposts, acentrosomal microtubule nucleation, and dendritic branching. Neuron. 2012;76:862–864. doi: 10.1016/j.neuron.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 115.Nguyen MM, McCracken CJ, Milner ES, Goetschius DJ, Weiner AT, Long MK, Michael NL, Munro S, Rolls MM. Γ-tubulin controls neuronal microtubule polarity independently of Golgi outposts. Mol Biol Cell. 2014;25:2039–2050. doi: 10.1091/mbc.E13-09-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hurtado L, Caballero C, Gavilan MP, Cardenas J, Bornens M, Rios RM. Disconnecting the Golgi ribbon from the centrosome prevents directional cell migration and ciliogenesis. J Cell Biol. 2011;193:917–933. doi: 10.1083/jcb.201011014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bellouze S, Schäfer MK, Buttigieg D, Baillat G, Rabouille C, Haase G. Golgi fragmentation in pmn mice is due to a defective ARF1/TBCE cross-talk that coordinates COPI vesicle formation and tubulin polymerization. Hum Mol Genet. 2014;23:5961–5975. doi: 10.1093/hmg/ddu320. [DOI] [PubMed] [Google Scholar]
- 118.Teuling E, van Dis V, Wulf PS, Haasdijk ED, Akhmanova A, Hoogenraad CC, Jaarsma D. A novel mouse model with impaired dynein/dynactin function develops amyotrophic lateral sclerosis (ALS)-like features in motor neurons and improves lifespan in SOD1-ALS mice. Hum Mol Genet. 2008;17:2849–2862. doi: 10.1093/hmg/ddn182. [DOI] [PubMed] [Google Scholar]
- 119.van Dis V, Kuijpers M, Haasdijk ED, Teuling E, Oakes SA, Hoogenraad CC, Jaarsma D. Golgi fragmentation precedes neuromuscular denervation and is associated with endosome abnormalities in SOD1-ALS mouse motor neurons. Acta Neuropathol Commun. 2014;2:38. doi: 10.1186/2051-5960-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jaarsma D, Hoogenraad CC. Cytoplasmic dynein and its regulatory proteins in Golgi pathology in nervous system disorders. Front Neurosci. 2015;9:397. doi: 10.3389/fnins.2015.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lu Z, Joseph D, Bugnard E, Zaal KJ, Ralston E. Golgi complex reorganization during muscle differentiation: visualization in living cells and mechanism. Mol Biol Cell. 2001;12:795–808. doi: 10.1091/mbc.12.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]


