Abstract
Serotonin (5-HT) is an important neurotransmitter in the central nervous system where it modulates circuits involved in mood, cognition, movement, arousal, and autonomic function. The 5-HT transporter (SERT; SLC6A4) is a key regulator of 5-HT signaling, and genetic variations in SERT are associated with various disorders including depression, anxiety, and autism. This review focuses on the role of SERT in the sympathetic nervous system. Autonomic / sympathetic dysfunction is evident in patients with depression, anxiety, and other diseases linked to serotonergic signaling. Experimentally, loss of SERT function (SERT knockout mice or chronic pharmacological block) has been reported to augment the sympathetic stress response. Alterations to serotonergic signaling in the CNS and thus central drive to the peripheral sympathetic nervous system are presumed to underlie this augmentation. Although less widely recognized, SERT is robustly expressed in chromaffin cells of the adrenal medulla, the neuroendocrine arm of the sympathetic nervous system. Adrenal chromaffin cells do not synthesize 5-HT but accumulate small amounts by SERT-mediated uptake. Recent evidence demonstrated that 5-HT1A receptors inhibit catecholamine secretion from adrenal chromaffin cells via an atypical mechanism that does not involve modulation of cellular excitability or voltage-gated Ca2+ channels. This raises the possibility that the adrenal medulla is a previously unrecognized peripheral hub for serotonergic control of the sympathetic stress response. As a framework for future investigation, a model is proposed in which stress-evoked adrenal catecholamine secretion is fine-tuned by SERT-modulated autocrine 5-HT signaling.
Keywords: serotonin transporter, adrenal chromaffin cell, sympathetic nervous system, calcium channel, exocytosis, catecholamine, 5-HT receptor
Graphical abstract
Introduction
Serotonin (5-HT) is an important neurotransmitter and neuromodulator in the central nervous system and controls a variety of physiological processes including mood, cognition, movement, arousal, and autonomic functions. The 5-HT transporter (SERT; SLC6A4) is a key regulator of 5-HT signaling; SERT-mediated reuptake by serotonergic neurons is one of the main mechanisms for clearing extracellular 5-HT after release, thereby terminating signaling and recycling 5-HT for subsequent rounds of release. Genetic variations that alter the expression or activity of SERT have been linked with depression, anxiety disorders, obsessive compulsive disorder, and autism 1-3. Moreover, SERT is a target for psychostimulant drugs (e.g. cocaine and MDMA / ecstasy) and widely prescribed antidepressants (e.g. selective serotonin reuptake inhibitors - SSRIs) 4.
In this article we focus on the role of SERT / 5-HT signaling in the peripheral sympathetic nervous system. Autonomic dysfunction, including an aberrant sympathoadrenal stress response, is evident in depression, anxiety, and other diseases linked to serotonergic signaling5-12. Experimental evidence has also implicated SERT in the sympathetic stress response; SSRIs enhance the counterregulatory release of epinephrine in response to hypoglycemic stress in rodents 13 and humans 14, 15. In other studies, restraint stress evoked an exaggerated increase in plasma epinephrine in mice with constitutive (global) knockout of SERT (SERT-/- mice) 16-18. The underlying mechanism is presumed to involve altered SERT / 5-HT signaling in the CNS which increases central drive to the peripheral sympathetic nervous system. Although not widely recognized, SERT is robustly expressed in adrenal chromaffin cells, the neuroendocrine arm of the sympathetic nervous system 19 and in a subset (∼30%) of neurons in the sympathetic ganglia 20. Here we outline the pathways by which SERT / 5-HT signaling in the CNS might modulate central drive to the sympathetic nervous system. We discuss the potential role(s) of SERT in the sympathoadrenal system, and the possibility that the adrenal medulla is a peripheral hub for serotonergic control of the sympathetic stress response. A working model is also outlined and intended to provide a framework for future experiments to test this central hypothesis.
Overview of the sympathoadrenal system and potential sites of serotonergic regulation in the CNS
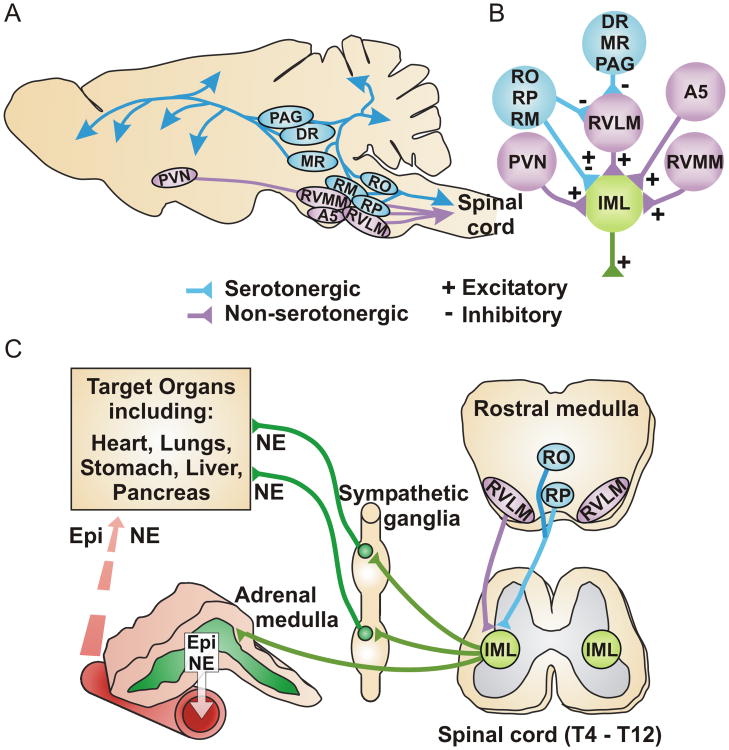
Serotonergic neurons in the CNS are clustered in the raphe nuclei of the brainstem and, although relatively few in number, project extensively throughout the brain and spinal cord 21-25 (Figure 1A). The various raphe nuclei are interconnected and their activity is modulated by input from multiple other brain regions. In general, the rostral midbrain raphe nuclei send ascending projections to brain regions including cerebral cortex, hippocampus, hypothalamus, thalamus, basal ganglia and amygdala whereas the caudal raphe nuclei (raphe pallidus, raphe magnus and raphe obscurus) send afferents to the cerebellum and descending projections to the spinal cord. Both the rostral and caudal raphe nuclei innervate brainstem neurons that regulate the sympathetic stress response, including those in the rostral ventrolateral medulla (RVLM). The RVLM contains a heterogeneous population of neurons (predominantly glutamatergic and catecholaminergic) that provide the primary excitatory drive to preganglionic sympathetic neurons in the intermediolateral columns of the thoracic spinal cord. The preganglionic neurons also receive direct input from the rostral ventromedial medulla, A5 noradrenergic cell group, paraventricular nucleus of the hypothalamus, and the caudal raphe nuclei 25-30 (Figure 1B). Spinal preganglionic neurons project to peripheral sympathetic ganglia where they release acetylcholine (ACh) to excite postganglionic sympathetic neurons. In turn, the postganglionic neurons innervate specific target organs, releasing norepinephrine (NE) and other co-transmitters to exert local control of these effectors (e.g. heart, vascular smooth muscle, immune system, etc.) (Figure 1C). Preganglionic sympathetic neurons also project via the splanchnic nerve to chromaffin cells in the adrenal medulla. Derived from the neural crest, adrenal chromaffin cells behave much like postganglionic sympathetic neurons and comprise the neuroendocrine arm of the sympathetic nervous system. Upon stimulation they release catecholamines and neuropeptides into the bloodstream to exert widespread effects on the cardiovascular, endocrine, immune, and nervous systems 31-36 (Figure 1C). Unlike sympathetic neurons, most (∼80%) adrenal chromaffin cells express phenylethanolamine-N-methyltransferase (PNMT), the enzyme that converts norepinephrine into epinephrine (Epi). These “adrenergic” chromaffin cells release primarily epinephrine while the “noradrenergic” chromaffin cells lacking PNMT release norepinephrine. The vast majority of plasma epinephrine (>95%) is due to secretion from adrenal chromaffin cells whereas plasma norepinephrine is partly due to secretion from chromaffin cells (10-30%) but mostly due to overflow from postganglionic nerve terminals 37, 38. Together, the sympathoneural and sympathoadrenal arms of the sympathetic nervous system coordinate the physiological and/or pathophysiological responses to environmental, metabolic, and emotional/psychological stressors. Distinct subsets of RVLM neurons can be activated in a stressor specific manner resulting in preferential recruitment of the sympathoneural (e.g. cold stress) or sympathoadrenal responses (hypoglycemia) 27, 39-42. Additionally, evidence suggests distinct preganglionic innervation and stressor specific activation of adrenergic and noradrenergic chromaffin cells 43-46.
Figure 1. Relationship between central serotonergic neurons and the sympathetic nervous system.
A) Simplified schematic of an adult rodent brain showing serotonergic nuclei (blue) and other brainstem regions (purple) important for central drive to the peripheral sympathetic nervous system. Abbreviations: PAG periaqueductal grey; DR dorsal raphe; MR median raphe; RO raphe obscurus; RM raphe magnus; RP raphe pallidus; PVN paraventricular nucleus of the hypothalamus; RVMM rostral ventromedial medulla; RVLM rostral ventrolateral medulla; A5 A5 noradrenergic cell group. B) Simplified schematic showing that preganglionic sympathetic neurons in the intermediolateral column (IML) of the thoracic spinal cord receive central drive from the RVLM and other brainstem / forebrain regions. The RVLM integrates input from multiple regions but only serotonergic input is shown; this is predominantly inhibitory, although some excitation might also occur. Serotonergic nuclei also send projections directly to the spinal cord to modulate central drive. C) Simplified schematic showing descending projections from the rostral medulla to the preganglionic sympathetic neurons in the intermediolateral column of the thoracic spinal cord (IML). Preganglionic neurons in the IML project to the peripheral sympathetic ganglia and the adrenal medulla. Postganglionic sympathetic neurons project from the ganglia to specific target organs and release norepinephrine (NE) to exert local control. Adrenal chromaffin cells comprise the neuroendocrine arm of the sympathetic nervous system and exert widespread effects by secreting epinephrine (Epi), norepinephrine (NE), and various neuropeptides into the bloodstream.
As noted above, SERT is expressed on the axons and synaptic terminals of serotonergic neurons that project widely throughout the CNS. Consequently, genetic loss or pharmacological block of SERT at multiple sites within the CNS could ultimately alter the excitability (increase or decrease) of sympathetic preganglionic neurons. For example, 5-HT can act directly within the RVLM to inhibit the sympathetic premotor neurons, likely via 5-HT1A receptors, although excitation via 5-HT2 receptors has been reported 47-51. Altered SERT function could also have less direct effects on the excitability of RVLM neurons by modulating input from other brain regions. In the spinal cord, immunocytochemical and retrograde labeling studies show that SERT is expressed on presynaptic boutons innervating the dendrites and soma of preganglionic sympathetic neurons, including those that innervate the adrenal gland 52-55. These serotonergic projections are thought to arise directly from the caudal raphe nuclei, although there is a small population of spinal serotonergic neurons 56, 57. The density of serotonergic innervation is not uniform along the rostral-caudal axis of the thoracolumbar spinal cord, which may result in differential modulation of specific sympathetic responses by SERT/5-HT 25, 55, 58. Although inhibition has been reported 59, 60, 5-HT is generally thought to excite preganglionic sympathetic neurons in the spinal cord by 5-HT2 receptor dependent mechanisms or through 5-HT1A receptors that produce a disinhibition of spinal interneuron input to the sympathetic neurons 59-64.
To summarize, SERT / 5-HT signaling in multiple regions of the brain and spinal cord can potentially increase or decrease central drive to the peripheral sympathetic nervous system. The overall effect will vary depending on the neuronal pathways recruited by the stressor and the serotonergic innervation and receptor expression in those respective pathways. For example, loss of SERT function enhanced the increase in plasma epinephrine, but not norepinephrine, evoked by restraint stress or hypoglycemia 13-16. This could be explained if loss of central SERT selectively altered the activity of neurons innervating the adrenergic population of chromaffin cells. Alternatively, as discussed below, SERT could act locally within the adrenal gland to modulate epinephrine secretion.
Multiple roles for SERT in sympathoadrenal chromaffin cells
In addition to mechanisms that modulate drive from the CNS, the sympathetic nervous system can also be regulated at peripheral sites including plasticity in ganglionic synaptic transmission and autocrine regulation of norepinephrine secretion in target tissues by presynaptic G protein coupled receptors (GPCRs) 65-70. Similarly, catecholamine secretion can be regulated locally within the adrenal medulla by plasticity at the splanchnic-chromaffin cell synapse 71 and by a variety of GPCRs on chromaffin cells that respond to autocrine, paracrine, endocrine and neuronal transmitters 72-74. Autocrine / paracrine control of adrenal catecholamine secretion is physiologically significant in vivo. For example, elevated sympathetic tone and plasma catecholamines are hallmarks of heart failure 75. In a rodent model of heart failure, this elevation involved upregulation of GRK2 in the adrenal gland and consequent loss of GPCR-mediated autocrine inhibition 76, 77. Correcting this imbalance using in vivo viral transduction to inhibit GRK2 selectively in adrenal chromaffin cells reduced plasma catecholamines and improved heart failure symptoms.
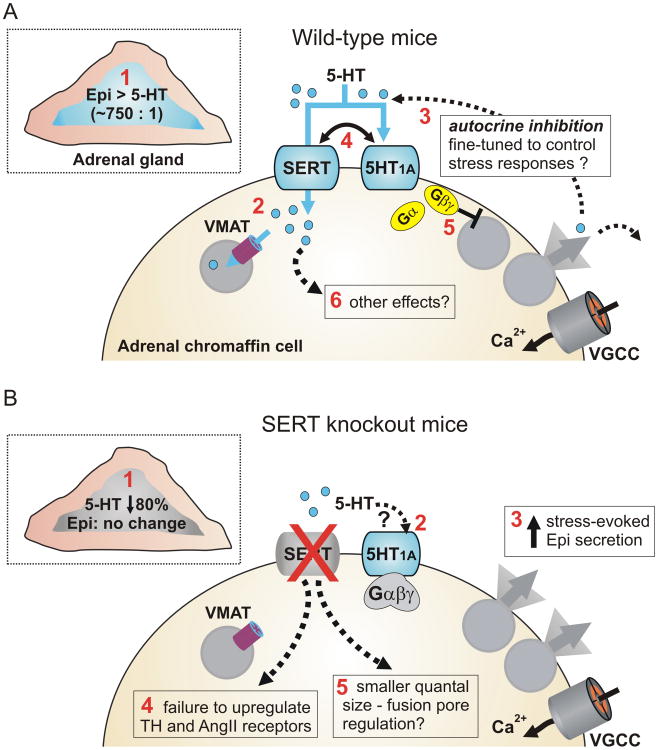
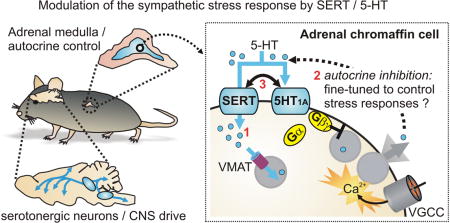
Although not widely recognized, SERT is highly expressed and co-localizes with PNMT in chromaffin cells of the adrenal medulla 19. This raises the possibility that SERT / 5-HT signaling might act locally within the adrenal gland to control the sympathetic stress response. Consistent with that, it is becoming clear that SERT has several distinct effects on chromaffin cell function and might coordinate serotonergic regulation of catecholamine exocytosis via 5-HT1A receptors (Figure 2). One role for SERT is to accumulate 5-HT into the adrenergic chromaffin cells, which lack the rate limiting enzyme for 5-HT synthesis, tryptophan hydroxylase 78-82. The amount of 5-HT in chromaffin cells is ≈ 750 fold lower than epinephrine, but this is still an appreciable amount considering that vesicular catecholamine concentrations approach 0.5-1 molar 83, 84. Moreover, when 5-HT was infused into rodents, the adrenal medulla displayed prominent SERT-mediated accumulation of exogenous 5-HT 82. Conversely, the 5-HT content of adrenal glands isolated from SERT-/- mice or rats was dramatically reduced (≈ 80%) compared to wild-type controls, with no change in the catecholamine content 16, 82, 85. A likely source of 5-HT is the circulation; the adrenal gland is highly vascularized and blood contains large amounts of 5-HT, although most of this is sequestered into platelets by SERT-mediated uptake 86, 87. Other possible sources include mast cells in the adrenal cortex which are capable of synthesizing 5-HT 88, or neuronal input. Preganglionic splanchnic nerve terminals do not contain 5-HT, but sensory and vagal neuron terminals are also present in the adrenal medulla 89, and some of these might contain 5-HT 90. Following SERT-mediated uptake, some of the 5-HT is likely transported into secretory vesicles by vesicular monoamine transporters and the balance subject to metabolism by monoamine oxidase in the cytoplasm. Thus 5-HT would then be available for exocytosis along with catecholamines and neuropeptides. Given the small amounts of 5-HT present in the adrenal gland and the efficient uptake of 5-HT by circulating platelets, we speculate that sufficient concentrations of 5-HT for receptor action are only achieved locally within the adrenal gland where the indoleamine would act as an autocrine / paracrine agent.
Figure 2. SERT and serotonergic signaling in adrenal chromaffin cells.
A) Schematic showing the mouse adrenal gland (inset) and an adrenal chromaffin cell. SERT can exert multiple effects indicated by the red numbering. 1) SERT is expressed in the adrenal medulla (blue) but not the adrenal cortex. 5-HT is found in the adrenal gland largely due to SERT-mediated uptake. Levels of 5-HT are approximately 1/750th of epinephrine levels. 2) Following SERT-mediated uptake into chromaffin cells 5-HT is packaged into secretory vesicles by the vesicular monoamine transporter (VMAT). 3) Chromaffin cells also express 5-HT1A receptors providing for potential autocrine regulation by 5-HT. 4) SERT constrains the ability of 5-HT to recruit the 5-HT1A receptor signaling pathway. 5) The 5-HT1A receptors reduce catecholamine secretion but this does not involve the typical mechanisms used by inhibitory GPCRs that target chromaffin cell voltage-gated Ca2+ channels (VGCC), K+ channels, or Ca2+ signaling. 6) Other effects of SERT and/or intracellular 5-HT are also possible. B) Known and potential effects of SERT knockout on adrenal chromaffin cells. 1) The adrenal catecholamine content is unaltered in glands from SERT-/- mice, but the 5-HT content is reduced by ≈80%. 2) 5-HT1A receptors are still present and functional, but the loss of cellular 5-HT content presumably prevents autocrine inhibition via these receptors. Conversely, other sources of 5-HT might be expected to more efficiently recruit the 5HT1A receptors as the opposing action of SERT is no longer present. 3) The sympathoadrenal response to acute restraint stress (increase in plasma epinephrine) is exaggerated in SERT knockout mice. 4) There is a failure to upregulate expression of tyrosine hydroxylase (TH) or angiotensin II (Ang II) receptors in response to acute stress in SERT knockouts. 5) The quantal size of unitary vesicular fusion events detected using carbon fiber amperometry is reduced in cells isolated from SERT-/- mice.
SERT modulates 5-HT1A receptor-mediated inhibition of catecholamine secretion
Consistent with a local signaling role for 5-HT in adrenal chromaffin cells, our recent work revealed that catecholamine secretion can be inhibited by 5-HT1A receptors 85. In these studies, we monitored catecholamine release from mouse chromaffin cells ex vivo using carbon fiber amperometry, which enables the detection of quantal vesicular release events. We found that exocytosis was inhibited by 5-HT1A receptors and that SERT-mediated uptake opposed the ability of 5-HT to recruit this pathway. The inhibition by 5-HT1A receptors resulted in fewer vesicles undergoing exocytosis, whereas the amount (quantal size) and kinetics of transmitter release from individual vesicular fusion events were not changed. Voltage-gated Ca2+ channels play a pivotal role in stimulus-secretion coupling and are an important target for regulation by GPCRs at presynaptic terminals and adrenal chromaffin cells 65, 66, 91-93. In the CNS, 5-HT1A receptors inhibit voltage-gated Ca2+channel currents (ICa) and somatodendritic 5-HT1A receptors can also modulate cellular excitability through K+ channel activation 94-99. Surprisingly, although the inhibition of catecholamine secretion by 5-HT1A receptors in adrenal chromaffin cells involved Gi/o-type G proteins and Gβγ signaling, it did not involve modulation of Ca2+ channels, K+ channels, or changes in intracellular [Ca2+ ] 85. Previous reports have shown that Gβγ can inhibit secretion independently from Ca2+ channel regulation in chromaffin cells 100, 101 and neurons 102-104. The “downstream” molecular target(s) of Gβγ in chromaffin cells remain to be defined, but one possible mechanism involves binding of Gβγ directly to SNARE proteins which comprise the core exocytotic fusion machinery 105. Gβγ can compete with Ca2+-bound synaptotagmin-1 for binding to the C-terminus of SNAP25, which might disrupt the triggering of exocytosis 106, 107. Another proposed target of Gβγ is dynamin 101 which can modulate both exocytosis and endocytosis in chromaffin cells 108-110.
The 5-HT mediated autocrine inhibition outlined above for adrenal chromaffin cells has some parallels with serotonergic signaling in the CNS. In the CNS, 5-HT1A receptors are predominantly expressed in the somatodendritic compartment of neurons whereas 5-HT1B receptors are predominantly expressed at presynaptic sites 111-113. Locally released 5-HT can control serotonergic neuron excitability through somatodendritic 5-HT1A receptors which activate potassium channels 97-99, whilst 5-HT1B receptors are thought to mediate direct presynaptic autocrine inhibition of 5-HT release and heterosynaptic inhibition of glutamatergic transmission. The mechanisms underlying 5-HT1B receptor-mediated inhibition of neurotransmitter release include the classical modulation of presynaptic voltage-gated Ca2+channels 114, and Ca2+channel independent effects perhaps involving Gβγ-mediated modulation of the SNARE machinery 104. Also of note, 5-HT1B receptors can modulate SERT function in the CNS 115, 116.
SERT / 5-HT might modulate the late steps of exocytosis
SERT might have additional effects in adrenal chromaffin cells beyond those discussed above. For example, the amount of transmitter released by unitary vesicular fusion events (i.e. quantal size) was decreased by ≈35 % in SERT-/- cells compared to wild-type cells, even in the absence of extracellular 5-HT and regardless of 5-HT receptor activation 85. Further experiments will be required to confirm this finding and identify the underlying mechanism. It is possible that the amount of monoamine transmitters packaged per individual vesicle is reduced in SERT-/- mice. However, the 80% reduction in 5-HT accounts for only a very small fraction (< 0.25%) of the total monoamines and the catecholamine content was unchanged in SERT-/- mice 85. Alternatively, loss of SERT might favor partial rather than full release of the vesicular content. The properties and stability of the initial fusion pore that forms between the vesicle and extracellular space to allow efflux of transmitters can be regulated 117, 118. This can result in transient fusion events (sometimes referred to as “kiss-and-run” exocytosis) that release only some of the vesicle cargo. In chromaffin cells the prevalence of these transient fusion events can be modulated by cellular firing patterns, G proteins, kinases, and remodeling of the actin cytoskeleton 100, 101, 117, 119-121. Notably, different peptide cargo in the vesicles can also alter fusion kinetics and quantal size 122. Perhaps the presence of intracellular and/or intravesicular 5-HT in wild-type cells somehow favors more complete emptying of vesicle contents. 5-HT has been reported to act as an intracellular messenger following SERT-mediated uptake in smooth muscle, platelets, and pancreatic β-cells 123-127. This appears to involve transglutaminase-mediated covalent modification of proteins such as actin and monomeric G proteins (termed “serotonylation”) 128-130. In both platelets and β-cells one effect of serotonylation is to augment exocytosis, but whether this novel signaling paradigm contributes to serotonergic control of catecholamine secretion or other chromaffin cell functions remains an open question.
Stress-evoked transcriptional regulation of tyrosine hydroxylase is altered in SERT knockout mice
Tyrosine hydroxylase is the rate limiting enzyme for catecholamine synthesis. Acute stress leads to increased expression / function of tyrosine hydroxylase in the adrenal medulla, and this is postulated to maintain catecholamine stores in the face of increased demand 40, 131-135. Stress-evoked catecholamine secretion and upregulation of tyrosine hydroxylase both depend on the neuropeptide PACAP which is released from preganglionic splanchnic nerve terminals and acts on PAC1 receptors expressed on adrenal chromaffin cells 133, 136, 137. Baseline (resting) plasma catecholamine levels were similar in wild type and SERT-/- mice 16. Notably, catecholamine secretion evoked by restraint stress was enhanced in SERT-/- mice compared to wild-type controls, but SERT-/- mice failed to upregulate expression of tyrosine hydroxylase or angiotensin II receptors 17, 138. Moreover, adrenal catecholamine content was reduced in the SERT-/- mice but not in wild type controls following restraint stress 16, 17. Thus, two parallel effects of acute stress on chromaffin cells that are mediated by PACAP were differentially altered in the SERT-/- mice. The relationship between SERT and upregulation of tyrosine hydroxylase is intriguing and its nature remains to be elucidated. Stress also increases expression of several neuropeptides including galanin, NPY, and enkephalin; it will be interesting to determine if these changes are also disrupted by loss of SERT, and if local serotonergic signaling in the adrenal chromaffin cells is involved in this phenomenon. Clinically relevant doses of selective 5-HT reuptake inhibitors result in ≈80 - 90 % occupancy of SERT in the CNS 139, 140. Assuming a similar occupancy in the adrenal gland, this could have a substantial impact on serotonergic regulation of adrenal chromaffin cells. Thus, determining if stress evoked transcriptional regulation is altered by SSRIs is also pertinent.
Is stress-evoked catecholamine secretion fine-tuned by serotonergic signaling/autocrine regulation?
There are several autocrine signals that can control adrenal chromaffin cells, but we postulate that the properties of 5-HT mediated inhibition are tuned to control stress-evoked secretion. One autocrine mediator is catestatin, a neuropeptide produced by proteolytic cleavage of chromogranins in large dense core secretory vesicles of chromaffin cells 141. Catestatin is a noncompetitive antagonist of nicotinic ACh receptors, and inhibits secretion evoked by ACh, but has no effect on secretion evoked by other stimuli such as direct membrane depolarization or PACAP 141, 142. Adrenal chromaffin cells also express GPCRs for a variety of autocrine, paracrine, endocrine, and neuronal transmitters. These include inhibitory autocrine receptors for catecholamines (α2 adrenergic), ATP (P2Y purinergic) and opioids (μ-opioid), and receptors for paracrine signals including inflammatory mediators (e.g. prostaglandin EP3 receptors) 143-147. These receptors are all thought to act predominantly by inhibition of ICa 72, 93. In contrast, 5-HT1A receptors do not modulate Ca2+ channels, K+ channels, or intracellular Ca2+ signaling in chromaffin cells 85. We postulate that the distinct mechanism of 5-HT1A receptors, coupled with the modulatory influence of SERT, might fine-tune serotonergic signaling to control catecholamine secretion during periods of intense stimulation (i.e. stress) (Figure 3). With basal or modest stimulation the secretory vesicles release substantial amounts of ATP along with catecholamines, neuropeptides, and 5-HT. SERT-mediated clearance of the 5-HT would prevent activation of the 5-HT1A receptors whereas P2Y purinergic receptors, activated by released ATP, robustly inhibit Ca2+ channels and thus exocytosis 143, 146, 148, 149 (Figure 3A). Endogenous opioids and catecholamines might also mediate autocrine inhibition of Ca2+ channels and exocytosis through activation of μ-opioid and α2 adrenergic receptors respectively. During more intense / sustained stimulation (i.e. stress) several factors change that favor a dominant role for serotonergic modulation. GPCRs can recruit several pathways to inhibit Ca2+channels, but the most widespread mechanism involves direct binding of Gβγ to the pore forming α1 subunit of the channels 150, 151. One notable feature of this “voltage-dependent inhibition” is that strong depolarization / repetitive firing can reverse Gβγ binding and thus channel inhibition 152-157 (for review see 91, 158). Channel inactivation can also be slowed by Gβγ 159, 160. Together, the altered inactivation, reversal of Gβγ binding, and build-up of residual Ca2+ within the cells are predicted to diminish the effectiveness of autocrine inhibition by P2Y receptors and other GPCRs that target Ca2+channels during intense / sustained stimulation (Figure 3B). On the other hand, increased stimulation would lead to greater amounts of 5-HT being released that could overcome the opposing influence of SERT and activate the 5-HT1A receptors. Since the 5-HT1A receptors act through a distinct mechanism that does not involve Ca2+ channels or membrane excitability, the serotonergic inhibition might persist and become the dominant mechanism for feedback control during stress-like stimuli (Figure 3B). If plasma membrane SERT levels or transport activity are changed rapidly, or as a consequence of prolonged chromaffin cell activation, additional opportunities to modulate 5-HT1A signaling could emerge.
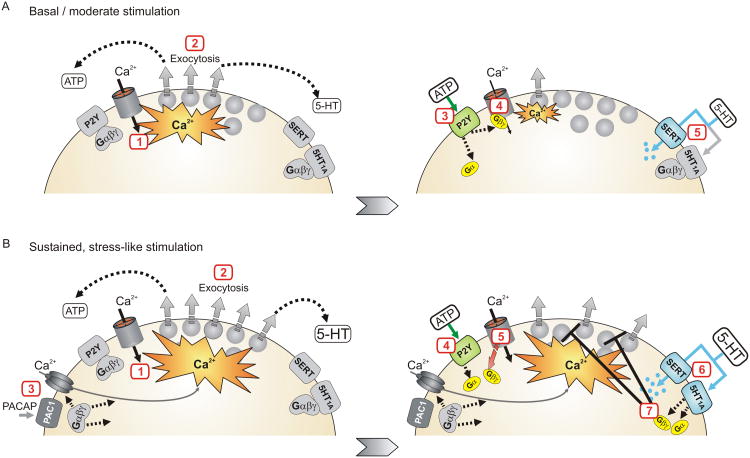
Figure 3. Proposed model in which serotonergic inhibition is fine-tuned to control stress-evoked catecholamine secretion.
A) During periods of basal or brief stimulation purinergic (and other) receptors that inhibit voltage-gated Ca2+channels mediate autocrine regulation of exocytosis. Left panel: 1) Acetylcholine released from the splanchnic nerve depolarizes chromaffin cells opening voltage-gated Ca2+ channels. 2) The Ca2+ entry triggers exocytosis of catecholamines and other transmitters including ATP, opioids, and 5-HT. Right panel: 3) ATP activates P2Y autoreceptors. 4) Gβγ inhibits the Ca2+ channels, reducing Ca2+ entry and thereby exocytosis. 5) SERT mediated uptake of 5-HT opposes activation of the 5-HT1A receptors so this signaling pathway is not recruited. B) With sustained, stress-like stimulation 5-HT signaling becomes the dominant pathway for autocrine regulation. Left panel: 1-2) As in panel A, acetylcholine released from the splanchnic nerve depolarizes chromaffin cells opening voltage-gated Ca2+ channels and triggering exocytosis. 3) With sustained stimulation PACAP might also be released from the splanchnic nerve and become the main stress mediator activating the chromaffin cells. PACAP acts via PAC1 receptors which recruit additional Ca2+ entry pathways perhaps including CaV3 (T-type) channels and TRPC channels. Right panel: 4) ATP activates P2Y autoreceptors. 5) Inhibition of Ca2+ channels becomes ineffective with strong, sustained depolarization in part because Gβγ dissociates from the channels. 6) The stronger stimulation leads to greater increase in local 5-HT and activation of 5-HT1A receptors. 7) The 5-HT1A receptors inhibit exocytosis by an atypical mechanism independent from cellular excitability / Ca2+ entry that can persist during the sustained stimulation. In this manner, 5-HT signaling becomes the dominant pathway for autocrine regulation.
Another consideration is that the neuropeptide PACAP might supplant acetylcholine (ACh) as the dominant excitatory transmitter at the splanchnic nerve – chromaffin cell synapse during stress-like stimuli 133, 136, 137. Thus, any autocrine inhibition of chromaffin cells by catestatin will be lost because the peptide blocks nicotinic ACh receptors but has no effect on PACAP-evoked secretion 141, 142. Furthermore, the stimulus-secretion coupling pathway recruited by PACAP is distinct from that of cholinergic stimulation and might not be effectively regulated by the autocrine GPCRs. Acetylcholine acts via nicotinic ACh receptors to depolarize the adrenal chromaffin cells, fire action potentials, and open the high voltage-activated Ca2+ channels that are modulated by GPCRs. Muscarinic receptors will also be activated and might contribute to stimulus-secretion coupling 161. In contrast, PACAP acts on the PAC1 GPCR which has complex downstream signaling through both Gs and Gq-type G protein pathways 162. In chromaffin cells PACAP is a potent secretagogue; it causes modest membrane depolarization and sustained Ca2+entry through poorly defined channels that might include low-voltage-activated CaV3.2 (T-type) channels, TRP channels, and perhaps other pathways 163-166. Thus, the Ca2+entry pathways recruited by PACAP are distinct from those activated by cholinergic stimulation and might not be effectively regulated by the autocrine GPCRs that target CaV2 voltage-gated Ca2+ channels. In contrast, the 5-HT1A receptors might still mediate autocrine inhibition of PACAP-evoked secretion (Figure 3B).
Further experiments are needed to directly test the above model for autocrine regulation of adrenal chromaffin cells by 5-HT, to dissect the molecular mechanism(s) utilized by 5-HT1A receptors, and to determine if serotonergic signaling inhibits PACAP-evoked catecholamine secretion. However, it is clear that the components for autocrine regulation of adrenal chromaffin cells by 5-HT are in place and that SERT plays an important role in this pathway. First, SERT is responsible for accumulating 5-HT into the chromaffin cells; second, the transporter constrains the ability of 5-HT to recruit the 5-HT1A receptor-mediated inhibition 85. Consequently, the loss of SERT function might have complex effects. Acute block of SERT is predicted to enhance serotonergic inhibition by removing the constraint for receptor activation. The effects of chronic loss or block of SERT might be even more complex, and derive in part from a loss of the local source of 5-HT that drives autocrine feedback. As demonstrated in the SERT-/- mice, chronic loss of SERT depletes 5-HT from chromaffin cells 16, 82, 85 , which we suggest will diminish or preclude autocrine serotonergic inhibition. Indeed, SERT-/- mice display an exaggerated increase in plasma epinephrine evoked by acute restraint stress 16, 17, and chronic block of SERT with SSRIs enhances the increase in plasma epinephrine produced by hypoglycemia in rats and humans 13-15. These phenotypes are consistent with loss of autocrine inhibition within the adrenal gland, although the global nature of the knockout / block of SERT means that other sites could also contribute; loss of SERT in the CNS could alter central drive to the peripheral sympathetic nervous system, and 5-HT will also be depleted from other tissues including platelets.
Future direction exploiting novel transgenic mouse models
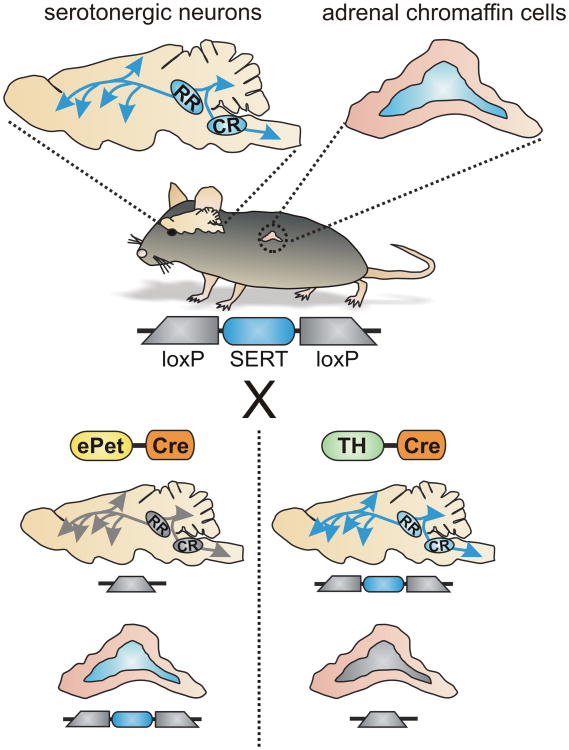
The SERT-/- mouse is a valuable model for investigating serotonergic signaling and the mechanisms of antidepressants and other drugs that target SERT 3, 18, 167, 168. However, the global nature of the SERT-/- knockout makes it difficult to determine the site(s) where SERT might be acting to control the sympathetic stress response. As discussed in this article, SERT could act at multiple sites within the brain and spinal cord to control central drive to the peripheral sympathetic nervous system. SERT is also expressed in the adrenal chromaffin cells, and a subset (∼30%) of sympathetic neurons in the rat superior cervical ganglion is reported to express SERT 20. It will be important to establish if the expression and function of SERT is conserved in human adrenal chromaffin cells. Mechanistically, it remains unclear which site(s) of SERT expression are most pertinent for controlling the sympathetic stress response. Recent advances that enable conditional, tissue-selective excision of SERT using Cre/LoxP technology promise to provide new insight into this question 169. In the initial report using this approach, SERT expression was efficiently reduced in CNS serotonergic neurons by crossing ePet-Cre mice with the “floxed” SERT mice 169. Notably, Cre is not expressed in the adrenal gland of ePet-Cre mice 170, so SERT will remain intact in the peripheral sympathoadrenal system in this model. We have performed preliminary experiments with the goal of generating the complementary model, where SERT is selectively excised in the peripheral sympathetic nervous system, but not the CNS. As SERT is not expressed in catecholaminergic neurons in the CNS, we initially crossed floxed SERT mice with TH-Cre mice (mice expressing Cre driven by the tyrosine hydroxylase promoter) 171. As expected, Cre is robustly expressed in adrenal chromaffin cells and catecholaminergic cells in the peripheral and central nervous systems. It is also expressed in some other CNS neurons, presumably due to transient expression of TH during development 171. Nonetheless, our preliminary data show that the SERT gene is excised from adrenal chromaffin cells but remains intact in the CNS (unpublished observation) (Figure 4). One could also imagine the use of temporally-restricted transgenic or viral approaches to limit SERT excision in the peripheral nervous system to adult animals, lessening concerns of developmental effects. Together, these approaches have the capacity to reveal pertinent and functionally distinct sites of SERT action and directly test the hypothesis that adrenal chromaffin cells form a peripheral hub for serotonergic regulation of the sympathoadrenal stress response.
Figure 4. Schematic depicting use of transgenic mice to selectively excise SERT from the peripheral sympathoadrenal system or central serotonergic neurons.
In the “floxed” SERT mouse, SERT is expressed in both central serotonergic neurons and adrenal chromaffin cells (depicted by blue shading). Crossing these mice with Cre driver lines provides a strategy to selectively excise SERT in a tissue specific manner. For example, ePet-Cre drives expression of Cre and excision of SERT in serotonergic neurons of the CNS but not in adrenal chromaffin cells. Conversely, using TH-Cre will result in excision of SERT in the adrenal chromaffin cells but not in CNS raphe neurons.
It is well-documented that SERT function and its expression at the plasma membrane can be regulated by genetic polymorphisms, promoter variants, and cell signaling pathways 1, 2, 18, 172, 173. Little is known about what impact this might have on sympathoadrenal chromaffin cells, although the response to acute restraint stress was similar in heterozygous SERT+/- and wild type mice whereas this response was enhanced in SERT-/- mice 16. As noted above, interpretation of this observation is complicated because of the global nature of the genetic deletion in this model and conditional heterozygous mice might provide additional insight. Other transgenic mice have been engineered to model human variants with altered SERT function 1. One such model bears a single, autism-associated missense mutation in SERT (Gly56Ala) that confers elevated transport function. These mice display hyperserotonemia, deficits in social interactions, repetitive behavior, and altered gastrointestinal function 174, 175. Determining how these and other (patho)physiological alterations of SERT impact the sympathoadrenal stress response both in vivo and at a cellular / mechanistic level could provide important insights into the autonomic dysfunction associated with depression, anxiety, and other disorders in which serotonergic signaling is disrupted.
Acknowledgments
Work on this project was supported by the National Institutes of Health [Grant R21 NS081429] and [Grant P50 MH096972]. The sponsors had no role in design, interpretation, or writing of the manuscript.
Abbreviations
- 5-HT
5-hydroxytryptamine
- ACh
acetylcholine
- Epi
epinephrine
- NE
norepinephrine
- GPCR
G protein coupled receptor
- SERT
serotonin transporter
- SERT-/-
SERT knockout mice
- SSRI
selective serotonin reuptake inhibitor
Footnotes
All authors contributed to the conception, research, writing and editing of the manuscript.
The authors declare no conflict of interest.
Literature Cited
- 1.Ye R, Blakely RD. Natural and engineered coding variation in antidepressant-sensitive serotonin transporters. Neuroscience. 2011;197:28–36. doi: 10.1016/j.neuroscience.2011.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daws LC, Gould GG. Ontogeny and regulation of the serotonin transporter: providing insights into human disorders. Pharmacol Ther. 2011;131:61–79. doi: 10.1016/j.pharmthera.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy DL, Fox MA, Timpano KR, Moya PR, Ren-Patterson R, Andrews AM, Holmes A, Lesch KP, Wendland JR. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55:932–960. doi: 10.1016/j.neuropharm.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- 5.Paine NJ, Watkins LL, Blumenthal JA, Kuhn CM, Sherwood A. Association of depressive and anxiety symptoms with 24-hour urinary catecholamines in individuals with untreated high blood pressure. Psychosomatic medicine. 2015;77:136–144. doi: 10.1097/PSY.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 7.Gold PW, Wong ML, Goldstein DS, Gold HK, Ronsaville DS, Esler M, Alesci S, Masood A, Licinio J, Geracioti TD, Jr, Perini G, DeBellis MD, Holmes C, Vgontzas AN, Charney DS, Chrousos GP, McCann SM, Kling MA. Cardiac implications of increased arterial entry and reversible 24-h central and peripheral norepinephrine levels in melancholia. Proc Natl Acad Sci U S A. 2005;102:8303–8308. doi: 10.1073/pnas.0503069102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinstein AA, Deuster PA, Francis JL, Bonsall RW, Tracy RP, Kop WJ. Neurohormonal and inflammatory hyper-responsiveness to acute mental stress in depression. Biol Psychol. 2010;84:228–234. doi: 10.1016/j.biopsycho.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coughlin SS. Post-traumatic Stress Disorder and Cardiovascular Disease. Open Cardiovasc Med J. 2011;5:164–170. doi: 10.2174/1874192401105010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pervanidou P, Chrousos GP. Neuroendocrinology of post-traumatic stress disorder. Prog Brain Res. 2010;182:149–160. doi: 10.1016/S0079-6123(10)82005-9. [DOI] [PubMed] [Google Scholar]
- 11.Bedi US, Arora R. Cardiovascular manifestations of posttraumatic stress disorder. J Natl Med Assoc. 2007;99:642–649. [PMC free article] [PubMed] [Google Scholar]
- 12.Southwick SM, Paige S, Morgan CA, 3rd, Bremner JD, Krystal JH, Charney DS. Neurotransmitter alterations in PTSD: catecholamines and serotonin. Semin Clin Neuropsychiatry. 1999;4:242–248. doi: 10.153/SCNP00400242. [DOI] [PubMed] [Google Scholar]
- 13.Sanders NM, Wilkinson CW, Taborsky GJ, Jr, Al-Noori S, Daumen W, Zavosh A, Figlewicz DP. The selective serotonin reuptake inhibitor sertraline enhances counterregulatory responses to hypoglycemia. American journal of physiology Endocrinology and metabolism. 2008;294:E853–860. doi: 10.1152/ajpendo.00772.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briscoe VJ, Ertl AC, Tate DB, Dawling S, Davis SN. Effects of a selective serotonin reuptake inhibitor, fluoxetine, on counterregulatory responses to hypoglycemia in healthy individuals. Diabetes. 2008;57:2453–2460. doi: 10.2337/db08-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Briscoe VJ, Ertl AC, Tate DB, Davis SN. Effects of the selective serotonin reuptake inhibitor fluoxetine on counterregulatory responses to hypoglycemia in individuals with type 1 diabetes. Diabetes. 2008;57:3315–3322. doi: 10.2337/db08-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tjurmina OA, Armando I, Saavedra JM, Goldstein DS, Murphy DL. Exaggerated adrenomedullary response to immobilization in mice with targeted disruption of the serotonin transporter gene. Endocrinology. 2002;143:4520–4526. doi: 10.1210/en.2002-220416. [DOI] [PubMed] [Google Scholar]
- 17.Tjurmina OA, Armando I, Saavedra JM, Li Q, Murphy DL. Life-long serotonin reuptake deficiency results in complex alterations in adrenomedullary responses to stress. Ann N Y Acad Sci. 2004;1018:99–104. doi: 10.1196/annals.1296.011. [DOI] [PubMed] [Google Scholar]
- 18.Murphy DL, Lesch KP. Targeting the murine serotonin transporter: insights into human neurobiology. Nat Rev Neurosci. 2008;9:85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- 19.Schroeter S, Levey AI, Blakely RD. Polarized expression of the antidepressant-sensitive serotonin transporter in epinephrine-synthesizing chromaffin cells of the rat adrenal gland. Mol Cell Neurosci. 1997;9:170–184. doi: 10.1006/mcne.1997.0619. [DOI] [PubMed] [Google Scholar]
- 20.Nishimura M, Sato K, Shimada S, Tohyama M. Expression of norepinephrine and serotonin transporter mRNAs in the rat superior cervical ganglion. Brain Res Mol Brain Res. 1999;67:82–86. doi: 10.1016/s0169-328x(99)00041-8. [DOI] [PubMed] [Google Scholar]
- 21.Hale MW, Shekhar A, Lowry CA. Stress-related serotonergic systems: implications for symptomatology of anxiety and affective disorders. Cell Mol Neurobiol. 2012;32:695–708. doi: 10.1007/s10571-012-9827-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore RY, Halaris AE, Jones BE. Serotonin neurons of the midbrain raphe: ascending projections. J Comp Neurol. 1978;180:417–438. doi: 10.1002/cne.901800302. [DOI] [PubMed] [Google Scholar]
- 23.Bang SJ, Jensen P, Dymecki SM, Commons KG. Projections and interconnections of genetically defined serotonin neurons in mice. Eur J Neurosci. 2012;35:85–96. doi: 10.1111/j.1460-9568.2011.07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. The brainstem and serotonin in the sudden infant death syndrome. Annual review of pathology. 2009;4:517–550. doi: 10.1146/annurev.pathol.4.110807.092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerman IA, Shabrang C, Taylor L, Akil H, Watson SJ. Relationship of presympathetic-premotor neurons to the serotonergic transmitter system in the rat brainstem. J Comp Neurol. 2006;499:882–896. doi: 10.1002/cne.21129. [DOI] [PubMed] [Google Scholar]
- 26.Jansen AS, Nguyen XV, Karpitskiy V, Mettenleiter TC, Loewy AD. Central command neurons of the sympathetic nervous system: basis of the fight-or-flight response. Science. 1995;270:644–646. doi: 10.1126/science.270.5236.644. [DOI] [PubMed] [Google Scholar]
- 27.Strack AM, Sawyer WB, Platt KB, Loewy AD. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res. 1989;491:274–296. doi: 10.1016/0006-8993(89)90063-2. [DOI] [PubMed] [Google Scholar]
- 28.Bacon SJ, Zagon A, Smith AD. Electron microscopic evidence of a monosynaptic pathway between cells in the caudal raphe nuclei and sympathetic preganglionic neurons in the rat spinal cord. Experimental brain research. 1990;79:589–602. doi: 10.1007/BF00229327. [DOI] [PubMed] [Google Scholar]
- 29.Loewy AD. Raphe pallidus and raphe obscurus projections to the intermediolateral cell column in the rat. Brain Res. 1981;222:129–133. doi: 10.1016/0006-8993(81)90946-x. [DOI] [PubMed] [Google Scholar]
- 30.Verberne AJ, Sabetghadam A, Korim WS. Neural pathways that control the glucose counterregulatory response. Frontiers in neuroscience. 2014;8:38. doi: 10.3389/fnins.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livett BG, Dean DM, Whelan LG, Udenfriend S, Rossier J. Co-release of enkephalin and catecholamines from cultured adrenal chromaffin cells. Nature. 1981;289:317–319. doi: 10.1038/289317a0. [DOI] [PubMed] [Google Scholar]
- 32.Winkler H, Fischer-Colbrie R, Schober M, Rieker S, Weiler R. Catecholamine storing cells secrete a blended mixture of catecholamines, neuropeptides and chromogranins. In: Thorn NA, Treiman M, Peterson OH, editors. Molecular Mechanisms in Secretion: Alfred Benzon Symposium. Vol. 25. Munksgaard; Copenhagen: 1988. pp. 347–359. [Google Scholar]
- 33.Wegrzyn J, Lee J, Neveu JM, Lane WS, Hook V. Proteomics of neuroendocrine secretory vesicles reveal distinct functional systems for biosynthesis and exocytosis of peptide hormones and neurotransmitters. J Proteome Res. 2007;6:1652–1665. doi: 10.1021/pr060503p. [DOI] [PubMed] [Google Scholar]
- 34.Tank AW, Lee Wong D. Peripheral and central effects of circulating catecholamines. Comprehensive Physiology. 2015;5:1–15. doi: 10.1002/cphy.c140007. [DOI] [PubMed] [Google Scholar]
- 35.Parmer RJ, Zinder O. Catecholaminergic pathways, chromaffin cells, and human disease. Ann N Y Acad Sci. 2002;971:497–505. doi: 10.1111/j.1749-6632.2002.tb04514.x. [DOI] [PubMed] [Google Scholar]
- 36.Aunis D, Langley K. Physiological aspects of exocytosis in chromaffin cells of the adrenal medulla. Acta Physiol Scand. 1999;167:89–97. doi: 10.1046/j.1365-201x.1999.00580.x. [DOI] [PubMed] [Google Scholar]
- 37.McCarty R. Learning about stress: neural, endocrine and behavioral adaptations. Stress. 2016;19:449–475. doi: 10.1080/10253890.2016.1192120. [DOI] [PubMed] [Google Scholar]
- 38.Goldstein DS, McCarty R, Polinsky RJ, Kopin IJ. Relationship between plasma norepinephrine and sympathetic neural activity. Hypertension. 1983;5:552–559. doi: 10.1161/01.hyp.5.4.552. [DOI] [PubMed] [Google Scholar]
- 39.Pacak K, Palkovits M, Yadid G, Kvetnansky R, Kopin IJ, Goldstein DS. Heterogeneous neurochemical responses to different stressors: a test of Selye's doctrine of nonspecificity. Am J Physiol. 1998;275:R1247–1255. doi: 10.1152/ajpregu.1998.275.4.R1247. [DOI] [PubMed] [Google Scholar]
- 40.Sabban EL, Nankova BB, Serova LI, Hiremagalur B, Rusnak M, Saez E, Spiegelman B, Kvetňanský R. Regulation of Gene Expression of Catecholamine Biosynthetic Enzymes by Stress. In: David S, Goldstein GE, Richard M, editors. Advances in Pharmacology. Academic Press; 1997. pp. 564–567. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein DS. Differential responses of components of the autonomic nervous system. Handbook of clinical neurology. 2013;117:13–22. doi: 10.1016/B978-0-444-53491-0.00002-X. [DOI] [PubMed] [Google Scholar]
- 42.Morrison SF, Reis DJ. Responses of sympathetic preganglionic neurons to rostral ventrolateral medullary stimulation. Am J Physiol. 1991;261:R1247–1256. doi: 10.1152/ajpregu.1991.261.5.R1247. [DOI] [PubMed] [Google Scholar]
- 43.Cao WH, Morrison SF. Differential chemoreceptor reflex responses of adrenal preganglionic neurons. American journal of physiology Regulatory, integrative and comparative physiology. 2001;281:R1825–1832. doi: 10.1152/ajpregu.2001.281.6.R1825. [DOI] [PubMed] [Google Scholar]
- 44.Morrison SF, Cao WH. Different adrenal sympathetic preganglionic neurons regulate epinephrine and norepinephrine secretion. American journal of physiology Regulatory, integrative and comparative physiology. 2000;279:R1763–1775. doi: 10.1152/ajpregu.2000.279.5.R1763. [DOI] [PubMed] [Google Scholar]
- 45.Wolf K, Zarkua G, Chan SA, Sridhar A, Smith C. Spatial and activity-dependent catecholamine release in rat adrenal medulla under native neuronal stimulation. Physiological reports. 2016;4 doi: 10.14814/phy2.12898. published online Sept 7, 2016. doi:2010.14814/phy14812.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verberne AJ, Korim WS, Sabetghadam A, Llewellyn-Smith IJ. Adrenaline: insights into its metabolic roles in hypoglycaemia and diabetes. Br J Pharmacol. 2016;173:1425–1437. doi: 10.1111/bph.13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Helke CJ, Capuano S, Tran N, Zhuo H. Immunocytochemical studies of the 5-HT(1A) receptor in ventral medullaryneurons that project to the intermediolateral cell column and contain serotonin or tyrosine hydroxylase immunoreactivity. J Comp Neurol. 1997;379:261–270. [PubMed] [Google Scholar]
- 48.Bago M, Sprtel BM, Dean C. Modulation of sympathetic nerve activity by microinjection of the 5-HT1A receptor agonist 8-OH-DPAT into the rostroventrolateral medulla. J Auton Nerv Syst. 1999;76:127–134. doi: 10.1016/s0165-1838(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 49.Miyawaki T, Goodchild AK, Pilowsky PM. Rostral ventral medulla 5-HT1A receptors selectively inhibit the somatosympathetic reflex. American journal of physiology Regulatory, integrative and comparative physiology. 2001;280:R1261–1268. doi: 10.1152/ajpregu.2001.280.5.R1261. [DOI] [PubMed] [Google Scholar]
- 50.Ramage AG, Villalon CM. 5-hydroxytryptamine and cardiovascular regulation. Trends Pharmacol Sci. 2008;29:472–481. doi: 10.1016/j.tips.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 51.Horiuchi J, McDowall LM, Dampney RA. Role of 5-HT(1A) receptors in the lower brainstem on the cardiovascular response to dorsomedial hypothalamus activation. Auton Neurosci. 2008;142:71–76. doi: 10.1016/j.autneu.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 52.Bacon SJ, Smith AD. Preganglionic sympathetic neurones innervating the rat adrenal medulla: immunocytochemical evidence of synaptic input from nerve terminals containing substance P, GABA or 5-hydroxytryptamine. J Auton Nerv Syst. 1988;24:97–122. doi: 10.1016/0165-1838(88)90140-3. [DOI] [PubMed] [Google Scholar]
- 53.Holets V, Elde R. The differential distribution and relationship of serotoninergic and peptidergic fibers to sympathoadrenal neurons in the intermediolateral cell column of the rat: a combined retrograde axonal transport and immunofluorescence study. Neuroscience. 1982;7:1155–1174. doi: 10.1016/0306-4522(82)91123-x. [DOI] [PubMed] [Google Scholar]
- 54.Appel NM, Wessendorf MW, Elde RP. Coexistence of serotonin- and substance P-like immunoreactivity in nerve fibers apposing identified sympathoadrenal preganglionic neurons in rat intermediolateral cell column. Neurosci Lett. 1986;65:241–246. doi: 10.1016/0304-3940(86)90268-5. [DOI] [PubMed] [Google Scholar]
- 55.Sur C, Betz H, Schloss P. Localization of the serotonin transporter in rat spinal cord. Eur J Neurosci. 1996;8:2753–2757. doi: 10.1111/j.1460-9568.1996.tb01570.x. [DOI] [PubMed] [Google Scholar]
- 56.Llewellyn-Smith IJ, Weaver LC, Keast JR. Effects of spinal cord injury on synaptic inputs to sympathetic preganglionic neurons. Prog Brain Res. 2006;152:11–26. doi: 10.1016/S0079-6123(05)52001-6. [DOI] [PubMed] [Google Scholar]
- 57.Newton BW, Hamill RW. Immunohistochemical distribution of serotonin in spinal autonomic nuclei: I. Fiber patterns in the adult rat. J Comp Neurol. 1989;279:68–81. doi: 10.1002/cne.902790107. [DOI] [PubMed] [Google Scholar]
- 58.Jensen I, Llewellyn-Smith IJ, Pilowsky P, Minson JB, Chalmers J. Serotonin inputs to rabbit sympathetic preganglionic neurons projecting to the superior cervical ganglion or adrenal medulla. J Comp Neurol. 1995;353:427–438. doi: 10.1002/cne.903530310. [DOI] [PubMed] [Google Scholar]
- 59.Lewis DI, Coote JH. The influence of 5-hydroxytryptamine agonists and antagonists on identified sympathetic preganglionic neurones in the rat, in vivo. Br J Pharmacol. 1990;99:667–672. doi: 10.1111/j.1476-5381.1990.tb12987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pickering AE, Spanswick D, Logan SD. 5-Hydoxytryptamine evokes depolarizations and membrane potential oscillations in rat sympathetic preganglionic neurones. J Physiol. 1994;480(Pt 1):109–121. doi: 10.1113/jphysiol.1994.sp020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lewis DI, Sermasi E, Coote JH. Excitatory and indirect inhibitory actions of 5-hydroxytryptamine on sympathetic preganglionic neurones in the neonate rat spinal cord in vitro. Brain Res. 1993;610:267–275. doi: 10.1016/0006-8993(93)91410-t. [DOI] [PubMed] [Google Scholar]
- 62.Madden CJ, Morrison SF. Serotonin potentiates sympathetic responses evoked by spinal NMDA. J Physiol. 2006;577:525–537. doi: 10.1113/jphysiol.2006.116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Madden CJ, Morrison SF. Brown adipose tissue sympathetic nerve activity is potentiated by activation of 5-hydroxytryptamine (5-HT)1A/5-HT7 receptors in the rat spinal cord. Neuropharmacology. 2008;54:487–496. doi: 10.1016/j.neuropharm.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zimmerman AL, Sawchuk M, Hochman S. Monoaminergic modulation of spinal viscero-sympathetic function in the neonatal mouse thoracic spinal cord. PLoS One. 2012;7:e47213. doi: 10.1371/journal.pone.0047213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schlicker E, Feuerstein T. Human presynaptic receptors. Pharmacol Ther. 2016 doi: 10.1016/j.pharmthera.2016.11.005. published online Dec 3, 2016. [DOI] [PubMed] [Google Scholar]
- 66.Kubista H, Boehm S. Molecular mechanisms underlying the modulation of exocytotic noradrenaline release via presynaptic receptors. Pharmacol Ther. 2006;112:213–242. doi: 10.1016/j.pharmthera.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 67.Alkadhi KA, Alzoubi KH, Aleisa AM. Plasticity of synaptic transmission in autonomic ganglia. Prog Neurobiol. 2005;75:83–108. doi: 10.1016/j.pneurobio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 68.Brown TH, McAfee DA. Long-term synaptic potentiation in the superior cervical ganglion. Science. 1982;215:1411–1413. doi: 10.1126/science.6278593. [DOI] [PubMed] [Google Scholar]
- 69.Cifuentes F, Arias ER, Morales MA. Long-term potentiation in mammalian autonomic ganglia: an inclusive proposal of a calcium-dependent, trans-synaptic process. Brain Res Bull. 2013;97:32–38. doi: 10.1016/j.brainresbull.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 70.Starke K, Gothert M, Kilbinger H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev. 1989;69:864–989. doi: 10.1152/physrev.1989.69.3.864. [DOI] [PubMed] [Google Scholar]
- 71.Wang M, Wang Q, Whim MD. Fasting induces a form of autonomic synaptic plasticity that prevents hypoglycemia. Proc Natl Acad Sci U S A. 2016;113:E3029–3038. doi: 10.1073/pnas.1517275113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Currie KP. Inhibition of Ca2+ channels and adrenal catecholamine release by G protein coupled receptors. Cell Mol Neurobiol. 2010;30:1201–1208. doi: 10.1007/s10571-010-9596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garcia AG, Garcia-De-Diego AM, Gandia L, Borges R, Garcia-Sancho J. Calcium signaling and exocytosis in adrenal chromaffin cells. Physiol Rev. 2006;86:1093–1131. doi: 10.1152/physrev.00039.2005. [DOI] [PubMed] [Google Scholar]
- 74.Lymperopoulos A, Brill A, McCrink KA. GPCRs of adrenal chromaffin cells & catecholamines: The plot thickens. Int J Biochem Cell Biol. 2016;77:213–219. doi: 10.1016/j.biocel.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 75.Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res. 2013;113:739–753. doi: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lymperopoulos A, Rengo G, Funakoshi H, Eckhart AD, Koch WJ. Adrenal GRK2 upregulation mediates sympathetic overdrive in heart failure. Nature medicine. 2007;13:315–323. doi: 10.1038/nm1553. [DOI] [PubMed] [Google Scholar]
- 77.Lymperopoulos A, Rengo G, Gao E, Ebert SN, Dorn GW, 2nd, Koch WJ. Reduction of sympathetic activity via adrenal-targeted GRK2 gene deletion attenuates heart failure progression and improves cardiac function after myocardial infarction. J Biol Chem. 2010;285:16378–16386. doi: 10.1074/jbc.M109.077859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Holzwarth MA, Sawetawan C, Brownfield MS. Serotonin-immunoreactivity in the adrenal medulla: distribution and response to pharmacological manipulation. Brain Res Bull. 1984;13:299–308. doi: 10.1016/0361-9230(84)90131-x. [DOI] [PubMed] [Google Scholar]
- 79.Verhofstad AA, Jonsson G. Immunohistochemical and neurochemical evidence for the presence of serotonin in the adrenal medulla of the rat. Neuroscience. 1983;10:1443–1453. doi: 10.1016/0306-4522(83)90125-2. [DOI] [PubMed] [Google Scholar]
- 80.Meyer T, Brinck U. Differential distribution of serotonin and tryptophan hydroxylase in the human gastrointestinal tract. Digestion. 1999;60:63–68. doi: 10.1159/000007590. [DOI] [PubMed] [Google Scholar]
- 81.Kent C, Coupland RE. On the uptake and storage of 5-hydroxytryptamine, 5-hydroxytryptophan and catecholamines by adrenal chromaffin cells and nerve endings. Cell Tissue Res. 1984;236:189–195. doi: 10.1007/BF00216530. [DOI] [PubMed] [Google Scholar]
- 82.Linder AE, Beggs KM, Burnett RJ, Watts SW. Body distribution of infused serotonin in rats. Clinical and experimental pharmacology & physiology. 2009;36:599–601. doi: 10.1111/j.1440-1681.2009.05147.x. [DOI] [PubMed] [Google Scholar]
- 83.Winkler H, Westhead E. The molecular organization of adrenal chromaffin granules. Neuroscience. 1980;5:1803–1823. doi: 10.1016/0306-4522(80)90031-7. [DOI] [PubMed] [Google Scholar]
- 84.Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–512. doi: 10.1038/39081. [DOI] [PubMed] [Google Scholar]
- 85.Brindley RL, Bauer MB, Blakely RD, Currie KP. An interplay between the serotonin transporter (SERT) and 5-HT receptors controls stimulus-secretion coupling in sympathoadrenal chromaffin cells. Neuropharmacology. 2016;110:438–448. doi: 10.1016/j.neuropharm.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mercado CP, Kilic F. Molecular mechanisms of SERT in platelets: regulation of plasma serotonin levels. Molecular interventions. 2010;10:231–241. doi: 10.1124/mi.10.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Watts SW, Morrison SF, Davis RP, Barman SM. Serotonin and blood pressure regulation. Pharmacol Rev. 2012;64:359–388. doi: 10.1124/pr.111.004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lefebvre H, Compagnon P, Contesse V, Delarue C, Thuillez C, Vaudry H, Kuhn JM. Production and metabolism of serotonin (5-HT) by the human adrenal cortex: paracrine stimulation of aldosterone secretion by 5-HT. The Journal of clinical endocrinology and metabolism. 2001;86:5001–5007. doi: 10.1210/jcem.86.10.7917. [DOI] [PubMed] [Google Scholar]
- 89.Parker TL, Kesse WK, Mohamed AA, Afework M. The innervation of the mammalian adrenal gland. J Anat. 1993;183(Pt 2):265–276. [PMC free article] [PubMed] [Google Scholar]
- 90.Fernandez-Vivero J, Rodriguez-Sanchez F, Verastegui C, Cordoba Moriano F, Romero A, de Castro JM. Immunocytochemical distribution of serotonin and neuropeptide Y (NPY) in mouse adrenal gland. Histol Histopathol. 1993;8:509–520. [PubMed] [Google Scholar]
- 91.Zamponi GW, Currie KP. Regulation of Ca(V)2 calcium channels by G protein coupled receptors. Biochim Biophys Acta. 2013;1828:1629–1643. doi: 10.1016/j.bbamem.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stephens GJ. G-protein-coupled-receptor-mediated presynaptic inhibition in the cerebellum. Trends Pharmacol Sci. 2009;30:421–430. doi: 10.1016/j.tips.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 93.Jewell ML, Currie KPM. Control of CaV2 calcium channels and neurosecretion by heterotrimeric G protein coupled receptors. In: Stephens GJ, Mochida S, editors. Modulation of presynaptic calcium channels. Springer Publishing; 2013. pp. 101–130. [Google Scholar]
- 94.Penington NJ, Kelly JS. Serotonin receptor activation reduces calcium current in an acutely dissociated adult central neuron. Neuron. 1990;4:751–758. doi: 10.1016/0896-6273(90)90201-p. [DOI] [PubMed] [Google Scholar]
- 95.Foehring RC. Serotonin modulates N- and P-type calcium currents in neocortical pyramidal neurons via a membrane-delimited pathway. J Neurophysiol. 1996;75:648–659. doi: 10.1152/jn.1996.75.2.648. [DOI] [PubMed] [Google Scholar]
- 96.Bayliss DA, Li YW, Talley EM. Effects of serotonin on caudal raphe neurons: inhibition of N- and P/Q-type calcium channels and the afterhyperpolarization. J Neurophysiol. 1997;77:1362–1374. doi: 10.1152/jn.1997.77.3.1362. [DOI] [PubMed] [Google Scholar]
- 97.Courtney NA, Ford CP. Mechanisms of 5HT1A receptor-mediated transmission in dorsal raphe serotonin neurons. J Physiol. 2016;594:953–965. doi: 10.1113/JP271716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Penington NJ, Kelly JS, Fox AP. Whole-cell recordings of inwardly rectifying K+ currents activated by 5HT1A receptors on dorsal raphe neurones of the adult rat. J Physiol. 1993;469:387–405. doi: 10.1113/jphysiol.1993.sp019819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- 100.Yoon EJ, Hamm HE, Currie KP. G protein betagamma subunits modulate the number and nature of exocytotic fusion events in adrenal chromaffin cells independent of calcium entry. J Neurophysiol. 2008;100:2929–2939. doi: 10.1152/jn.90839.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen XK, Wang LC, Zhou Y, Cai Q, Prakriya M, Duan KL, Sheng ZH, Lingle C, Zhou Z. Activation of GPCRs modulates quantal size in chromaffin cells through G(betagamma) and PKC. Nat Neurosci. 2005;8:1160–1168. doi: 10.1038/nn1529. [DOI] [PubMed] [Google Scholar]
- 102.Blackmer T, Larsen EC, Takahashi M, Martin TF, Alford S, Hamm HE. G protein betagamma subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca2+ entry. Science. 2001;292:293–297. doi: 10.1126/science.1058803. [DOI] [PubMed] [Google Scholar]
- 103.Gerachshenko T, Blackmer T, Yoon EJ, Bartleson C, Hamm HE, Alford S. Gbetagamma acts at the C terminus of SNAP-25 to mediate presynaptic inhibition. Nat Neurosci. 2005;8:597–605. doi: 10.1038/nn1439. [DOI] [PubMed] [Google Scholar]
- 104.Hamid E, Church E, Wells CA, Zurawski Z, Hamm HE, Alford S. Modulation of neurotransmission by GPCRs is dependent upon the microarchitecture of the primed vesicle complex. J Neurosci. 2014;34:260–274. doi: 10.1523/JNEUROSCI.3633-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Betke KM, Wells CA, Hamm HE. GPCR mediated regulation of synaptic transmission. Prog Neurobiol. 2012;96:304–321. doi: 10.1016/j.pneurobio.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yoon EJ, Gerachshenko T, Spiegelberg BD, Alford S, Hamm HE. Gbetagamma interferes with Ca2+-dependent binding of synaptotagmin to the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex. Mol Pharmacol. 2007;72:1210–1219. doi: 10.1124/mol.107.039446. [DOI] [PubMed] [Google Scholar]
- 107.Zurawski Z, Rodriguez S, Hyde K, Alford S, Hamm HE. Gbetagamma Binds to the Extreme C Terminus of SNAP25 to Mediate the Action of Gi/o-Coupled G Protein-Coupled Receptors. Mol Pharmacol. 2016;89:75–83. doi: 10.1124/mol.115.101600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jackson J, Papadopulos A, Meunier FA, McCluskey A, Robinson PJ, Keating DJ. Small molecules demonstrate the role of dynamin as a bi-directional regulator of the exocytosis fusion pore and vesicle release. Molecular psychiatry. 2015;20:810–819. doi: 10.1038/mp.2015.56. [DOI] [PubMed] [Google Scholar]
- 109.Anantharam A, Bittner MA, Aikman RL, Stuenkel EL, Schmid SL, Axelrod D, Holz RW. A new role for the dynamin GTPase in the regulation of fusion pore expansion. Mol Biol Cell. 2011;22:1907–1918. doi: 10.1091/mbc.E11-02-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chan SA, Doreian B, Smith C. Dynamin and myosin regulate differential exocytosis from mouse adrenal chromaffin cells. Cell Mol Neurobiol. 2010;30:1351–1357. doi: 10.1007/s10571-010-9591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of5HT1Aand preterminal axonal localization of 5HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- 112.Boschert U, Amara DA, Segu L, Hen R. The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience. 1994;58:167–182. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 113.Hen R. Of mice and flies: commonalities among 5-HT receptors. Trends Pharmacol Sci. 1992;13:160–165. doi: 10.1016/0165-6147(92)90054-a. [DOI] [PubMed] [Google Scholar]
- 114.Mizutani H, Hori T, Takahashi T. 5-HT1B receptor-mediated presynaptic inhibition at the calyx of Held of immature rats. Eur J Neurosci. 2006;24:1946–1954. doi: 10.1111/j.1460-9568.2006.05063.x. [DOI] [PubMed] [Google Scholar]
- 115.Daws LC, Gould GG, Teicher SD, Gerhardt GA, Frazer A. 5-HT(1B) receptor-mediated regulation of serotonin clearance in rat hippocampus in vivo. J Neurochem. 2000;75:2113–2122. doi: 10.1046/j.1471-4159.2000.0752113.x. [DOI] [PubMed] [Google Scholar]
- 116.Hagan CE, McDevitt RA, Liu Y, Furay AR, Neumaier JF. 5-HT(1B) autoreceptor regulation of serotonin transporter activity in synaptosomes. Synapse (New York, NY) 2012;66:1024–1034. doi: 10.1002/syn.21608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cardenas AM, Marengo FD. How the stimulus defines the dynamics of vesicle pool recruitment, fusion mode, and vesicle recycling in neuroendocrine cells. J Neurochem. 2016;137:867–879. doi: 10.1111/jnc.13565. [DOI] [PubMed] [Google Scholar]
- 118.Chang CW, Chiang CW, Jackson MB. Fusion pores and their control of neurotransmitter and hormone release. J Gen Physiol. 2017 doi: 10.1085/jgp.201611724. published online Feb 6, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Elhamdani A, Palfrey HC, Artalejo CR. Quantal size is dependent on stimulation frequency and calcium entry in calf chromaffin cells. Neuron. 2001;31:819–830. doi: 10.1016/s0896-6273(01)00418-4. [DOI] [PubMed] [Google Scholar]
- 120.Fulop T, Radabaugh S, Smith C. Activity-dependent differential transmitter release in mouse adrenal chromaffin cells. J Neurosci. 2005;25:7324–7332. doi: 10.1523/JNEUROSCI.2042-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fulop T, Smith C. Physiological stimulation regulates the exocytic mode through calcium activation of protein kinase C in mouse chromaffin cells. Biochem J. 2006;399:111–119. doi: 10.1042/BJ20060654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Weiss AN, Anantharam A, Bittner MA, Axelrod D, Holz RW. Lumenal protein within secretory granules affects fusion pore expansion. Biophys J. 2014;107:26–33. doi: 10.1016/j.bpj.2014.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Walther DJ, Peter JU, Winter S, Holtje M, Paulmann N, Grohmann M, Vowinckel J, Alamo-Bethencourt V, Wilhelm CS, Ahnert-Hilger G, Bader M. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell. 2003;115:851–862. doi: 10.1016/s0092-8674(03)01014-6. [DOI] [PubMed] [Google Scholar]
- 124.Paulmann N, Grohmann M, Voigt JP, Bert B, Vowinckel J, Bader M, Skelin M, Jevsek M, Fink H, Rupnik M, Walther DJ. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol. 2009;7:e1000229. doi: 10.1371/journal.pbio.1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Watts SW, Priestley JR, Thompson JM. Serotonylation of vascular proteins important to contraction. PLoS One. 2009;4:e5682. doi: 10.1371/journal.pone.0005682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ahmed BA, Jeffus BC, Bukhari SI, Harney JT, Unal R, Lupashin VV, van der Sluijs P, Kilic F. Serotonin transamidates Rab4 and facilitates its binding to the C terminus of serotonin transporter. J Biol Chem. 2008;283:9388–9398. doi: 10.1074/jbc.M706367200. [DOI] [PubMed] [Google Scholar]
- 127.Penumatsa K, Abualkhair S, Wei L, Warburton R, Preston I, Hill NS, Watts SW, Fanburg BL, Toksoz D. Tissue transglutaminase promotes serotonin-induced AKT signaling and mitogenesis in pulmonary vascular smooth muscle cells. Cell Signal. 2014;26:2818–2825. doi: 10.1016/j.cellsig.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hummerich R, Costina V, Findeisen P, Schloss P. Monoaminylation of Fibrinogen and Glia-Derived Proteins: Indication for Similar Mechanisms in Posttranslational Protein Modification in Blood and Brain. ACS Chem Neurosci. 2015;6:1130–1136. doi: 10.1021/cn5003286. [DOI] [PubMed] [Google Scholar]
- 129.Muma NA, Mi Z. Serotonylation and Transamidation of Other Monoamines. ACS Chem Neurosci. 2015;6:961–969. doi: 10.1021/cn500329r. [DOI] [PubMed] [Google Scholar]
- 130.Walther DJ, Stahlberg S, Vowinckel J. Novel roles for biogenic monoamines: from monoamines in transglutaminase-mediated post-translational protein modification to monoaminylation deregulation diseases. FEBS J. 2011;278:4740–4755. doi: 10.1111/j.1742-4658.2011.08347.x. [DOI] [PubMed] [Google Scholar]
- 131.Wang Q, Wang M, Whim MD. Neuropeptide y gates a stress-induced, long-lasting plasticity in the sympathetic nervous system. J Neurosci. 2013;33:12705–12717. doi: 10.1523/JNEUROSCI.3132-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu L, Chen X, Sun B, Sterling C, Tank AW. Evidence for regulation of tyrosine hydroxylase mRNA translation by stress in rat adrenal medulla. Brain Res. 2007;1158:1–10. doi: 10.1016/j.brainres.2007.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stroth N, Kuri BA, Mustafa T, Chan SA, Smith CB, Eiden LE. PACAP controls adrenomedullary catecholamine secretion and expression of catecholamine biosynthetic enzymes at high splanchnic nerve firing rates characteristic of stress transduction in male mice. Endocrinology. 2013;154:330–339. doi: 10.1210/en.2012-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nankova BB, Tank AW, Sabban EL. Transient or sustained transcriptional activation of the genes encoding rat adrenomedullary catecholamine biosynthetic enzymes by different durations of immobilization stress. Neuroscience. 1999;94:803–808. doi: 10.1016/s0306-4522(99)00290-0. [DOI] [PubMed] [Google Scholar]
- 135.Kvetnansky R, Lu X, Ziegler MG. Stress-triggered changes in peripheral catecholaminergic systems. Adv Pharmacol. 2013;68:359–397. doi: 10.1016/B978-0-12-411512-5.00017-8. [DOI] [PubMed] [Google Scholar]
- 136.Smith CB, Eiden LE. Is PACAP the major neurotransmitter for stress transduction at the adrenomedullary synapse? J Mol Neurosci. 2012;48:403–412. doi: 10.1007/s12031-012-9749-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mustafa T. Pituitary adenylate cyclase-activating polypeptide (PACAP): a master regulator in central and peripheral stress responses. Adv Pharmacol. 2013;68:445–457. doi: 10.1016/B978-0-12-411512-5.00021-X. [DOI] [PubMed] [Google Scholar]
- 138.Armando I, Tjurmina OA, Li Q, Murphy DL, Saavedra JM. The serotonin transporter is required for stress-evoked increases in adrenal catecholamine synthesis and angiotensin II AT(2) receptor expression. Neuroendocrinology. 2003;78:217–225. doi: 10.1159/000073705. [DOI] [PubMed] [Google Scholar]
- 139.Meyer JH, Wilson AA, Sagrati S, Hussey D, Carella A, Potter WZ, Ginovart N, Spencer EP, Cheok A, Houle S. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. The American journal of psychiatry. 2004;161:826–835. doi: 10.1176/appi.ajp.161.5.826. [DOI] [PubMed] [Google Scholar]
- 140.Owens MJ, Krulewicz S, Simon JS, Sheehan DV, Thase ME, Carpenter DJ, Plott SJ, Nemeroff CB. Estimates of serotonin and norepinephrine transporter inhibition in depressed patients treated with paroxetine or venlafaxine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:3201–3212. doi: 10.1038/npp.2008.47. [DOI] [PubMed] [Google Scholar]
- 141.Mahata SK, O'Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, Gill BM, Parmer RJ. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Invest. 1997;100:1623–1633. doi: 10.1172/JCI119686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mahapatra NR, Mahata M, Mahata SK, O'Connor DT. The chromogranin A fragment catestatin: specificity, potency and mechanism to inhibit exocytotic secretion of multiple catecholamine storage vesicle co-transmitters. J Hypertens. 2006;24:895–904. doi: 10.1097/01.hjh.0000222760.99852.e0. [DOI] [PubMed] [Google Scholar]
- 143.Currie KPM, Fox AP. ATP serves as a negative feedback inhibitor of voltage-gated Ca2+ channel currents in cultured bovine adrenal chromaffin cells. Neuron. 1996;16:1027–1036. doi: 10.1016/s0896-6273(00)80126-9. [DOI] [PubMed] [Google Scholar]
- 144.Albillos A, Gandia L, Michelena P, Gilabert JA, del Valle M, Carbone E, Garcia AG. The mechanism of calcium channel facilitation in bovine chromaffin cells. J Physiol. 1996;494(Pt 3):687–695. doi: 10.1113/jphysiol.1996.sp021524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jewell ML, Breyer RM, Currie KP. Regulation of calcium channels and exocytosis in mouse adrenal chromaffin cells by prostaglandin EP3 receptors. Mol Pharmacol. 2011;79:987–996. doi: 10.1124/mol.110.068569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Carabelli V, Carra I, Carbone E. Localized secretion of ATP and opioids revealed through single Ca2+ channel modulation in bovine chromaffin cells. Neuron. 1998;20:1255–1268. doi: 10.1016/s0896-6273(00)80505-x. [DOI] [PubMed] [Google Scholar]
- 147.Brede M, Nagy G, Philipp M, Sorensen JB, Lohse MJ, Hein L. Differential control of adrenal and sympathetic catecholamine release by alpha 2-adrenoceptor subtypes. Molecular endocrinology (Baltimore, Md. 2003;17:1640–1646. doi: 10.1210/me.2003-0035. [DOI] [PubMed] [Google Scholar]
- 148.Powell AD, Teschemacher AG, Seward EP. P2Y purinoceptors inhibit exocytosis in adrenal chromaffin cells via modulation of voltage-operated calcium channels. J Neurosci. 2000;20:606–616. doi: 10.1523/JNEUROSCI.20-02-00606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Harkins AB, Fox AP. Activation of purinergic receptors by ATP inhibits secretion in bovine adrenal chromaffin cells. Brain Res. 2000;885:231–239. doi: 10.1016/s0006-8993(00)02952-8. [DOI] [PubMed] [Google Scholar]
- 150.Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 151.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 152.Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- 153.Williams S, Serafin M, Muhlethaler M, Bernheim L. Facilitation of N-type calcium current is dependent on the frequency of action potential-like depolarizations in dissociated cholinergic basal forebrain neurons of the guinea pig. J Neurosci. 1997;17:1625–1632. doi: 10.1523/JNEUROSCI.17-05-01625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Park D, Dunlap K. Dynamic regulation of calcium influx by G-proteins, action potential waveform, and neuronal firing frequency. J Neurosci. 1998;18:6757–6766. doi: 10.1523/JNEUROSCI.18-17-06757.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tosetti P, Taglietti V, Toselli M. Action-potential-like depolarizations relieve opioid inhibition of N-type Ca2+ channels in NG108-15 cells. Pflugers Arch. 1999;437:441–448. doi: 10.1007/s004240050799. [DOI] [PubMed] [Google Scholar]
- 156.Brody DL, Patil PG, Mulle JG, Snutch TP, Yue DT. Bursts of action potential waveforms relieve G-protein inhibition of recombinant P/Q-type Ca2+ channels in HEK 293 cells. J Physiol. 1997;499(Pt 3):637–644. doi: 10.1113/jphysiol.1997.sp021956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Currie KPM, Fox AP. Differential facilitation of N- and P/Q-type calcium channels during trains of action potential-like waveforms. J Physiol. 2002;539:419–431. doi: 10.1113/jphysiol.2001.013206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Proft J, Weiss N. G protein regulation of neuronal calcium channels: back to the future. Mol Pharmacol. 2015;87:890–906. doi: 10.1124/mol.114.096008. [DOI] [PubMed] [Google Scholar]
- 159.McDavid S, Currie KP. G-proteins modulate cumulative inactivation of N-type (Cav2.2) calcium channels. J Neurosci. 2006;26:13373–13383. doi: 10.1523/JNEUROSCI.3332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Weiss N, Tadmouri A, Mikati M, Ronjat M, De Waard M. Importance of voltage-dependent inactivation in N-type calcium channel regulation by G-proteins. Pflugers Arch. 2007;454:115–129. doi: 10.1007/s00424-006-0184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Olivos L, Artalejo AR. Muscarinic excitation-secretion coupling in chromaffin cells. Acta physiologica (Oxford, England) 2008;192:213–220. doi: 10.1111/j.1748-1716.2007.01816.x. [DOI] [PubMed] [Google Scholar]
- 162.Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- 163.Hill J, Chan SA, Kuri B, Smith C. Pituitary adenylate cyclase-activating peptide (PACAP) recruits low voltage-activated T-type calcium influx under acute sympathetic stimulation in mouse adrenal chromaffin cells. J Biol Chem. 2011;286:42459–42469. doi: 10.1074/jbc.M111.289389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kuri BA, Chan SA, Smith CB. PACAP regulates immediate catecholamine release from adrenal chromaffin cells in an activity-dependent manner through a protein kinase C-dependent pathway. J Neurochem. 2009;110:1214–1225. doi: 10.1111/j.1471-4159.2009.06206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tanaka K, Shibuya I, Nagamoto T, Yamashita H, Kanno T. Pituitary adenylate cyclase-activating polypeptide causes rapid Ca2+ release from intracellular stores and long lasting Ca2+ influx mediated by Na+ influx-dependent membrane depolarization in bovine adrenal chromaffin cells. Endocrinology. 1996;137:956–966. doi: 10.1210/endo.137.3.8603609. [DOI] [PubMed] [Google Scholar]
- 166.Morita K, Sakakibara A, Kitayama S, Kumagai K, Tanne K, Dohi T. Pituitary adenylate cyclase-activating polypeptide induces a sustained increase in intracellular free Ca(2+) concentration and catechol amine release by activating Ca(2+) influx via receptor-stimulated Ca(2+) entry, independent of store-operated Ca(2+) channels, and voltage-dependent Ca(2+) channels in bovine adrenal medullary chromaffin cells. J Pharmacol Exp Ther. 2002;302:972–982. doi: 10.1124/jpet.102.033456. [DOI] [PubMed] [Google Scholar]
- 167.Fox MA, Andrews AM, Wendland JR, Lesch KP, Holmes A, Murphy DL. A pharmacological analysis of mice with a targeted disruption of the serotonin transporter. Psychopharmacology (Berl) 2007;195:147–166. doi: 10.1007/s00213-007-0910-0. [DOI] [PubMed] [Google Scholar]
- 168.Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biological psychiatry. 2003;54:953–959. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 169.Chen X, Ye R, Gargus JJ, Blakely RD, Dobrenis K, Sze JY. Disruption of Transient Serotonin Accumulation by Non-Serotonin-Producing Neurons Impairs Cortical Map Development. Cell reports. 2015;10:346–358. doi: 10.1016/j.celrep.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, Jiang W, Conlon RA, Strowbridge BW, Deneris ES. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci U S A. 2005;102:16472–16477. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lindeberg J, Usoskin D, Bengtsson H, Gustafsson A, Kylberg A, Soderstrom S, Ebendal T. Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis (New York, NY: 2000) 2004;40:67–73. doi: 10.1002/gene.20065. [DOI] [PubMed] [Google Scholar]
- 172.Baganz L, Blakely RD. A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chem Neurosci. 2013;4:48–63. doi: 10.1021/cn300186b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bermingham DP, Blakely RD. Kinase-dependent Regulation of Monoamine Neurotransmitter Transporters. Pharmacol Rev. 2016;68:888–953. doi: 10.1124/pr.115.012260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Cohen J, Mannangatti P, Jessen T, Thompson BJ, Ye R, Kerr TM, Carneiro AM, Crawley JN, Sanders-Bush E, McMahon DG, Ramamoorthy S, Daws LC, Sutcliffe JS, Blakely RD. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci U S A. 2012;109:5469–5474. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Margolis KG, Li Z, Stevanovic K, Saurman V, Israelyan N, Anderson GM, Snyder I, Veenstra-VanderWeele J, Blakely RD, Gershon MD. Serotonin transporter variant drives preventable gastrointestinal abnormalities in development and function. J Clin Invest. 2016;126:2221–2235. doi: 10.1172/JCI84877. [DOI] [PMC free article] [PubMed] [Google Scholar]