Abstract
Circulating plasma cells at diagnosis, prior to auto-transplant and at relapse have a negative impact on survival in multiple myeloma. However, the impact of kinetics of circulating plasma cells along the course of illness has not been defined. We have analyzed 247 newly diagnosed multiple myeloma patients undergoing early auto-transplant who had paired evaluation of circulating plasma cells at diagnosis and pre-transplant by 6-color flow cytometry. A total of 117 patients had no detectable circulating plasma cells at both time points (CPC−/−), 82 had circulating plasma cells at diagnosis followed by complete eradication after induction (CPC+/−) and 48 had circulating plasma cells at transplant, including persistence of cells (CPC+/+; n=45) or emergence of new cells (CPC−/+; n=3) after induction. The rate of post-transplant stringent complete response was 32% in the CPC−/−, 30% in CPC+/− and 12% in CPC+/+ or −/+ groups (P=0.018). At a median follow up of 58 months from transplantation, the median progression-free survival in the 3 respective groups were 30, 24 and 14 months, and the 5-year overall survival rates were 83%, 70% and 43% (P<0.001 for both comparisons). On a multivariate analysis for overall survival, the risk of mortality was higher in CPC +/− (hazard ratio 2.7, 95%CI: 1.3–5.8; P=0.009) and CPC+/+ or −/+ (hazard ratio 5.7, 95%CI: 2.5–13.1; P<0.001) groups compared to the CPC−/− group. Monitoring for circulating plasma cells before induction therapy and before transplant by 6-color flow cytometry is predictive of survival in newly diagnosed myeloma and should be incorporated into clinical trials.
Introduction
The presence of clonal circulating plasma cells (CPCs) has a negative impact on survival in newly diagnosed and relapsed/refractory multiple myeloma (MM)1–4 despite the use of proteasome inhibitor (PI) and immunomodulator (IMiD)-based therapy. Furthermore, the presence of CPCs prior to autologous stem cell transplantation (ASCT) is a predictor of inferior progression-free survival (PFS) and overall survival (OS), independent of the post-induction depth of response.5–7 However, the impact of changes in CPCs along the course of the disease has not been reported. Since MM is a multi-clonal disease with dynamic evolution along the course of illness, serial assessment of bone marrow (BM) and extramedullary disease burden is important in the treatment decision-making process. Using highly sensitive next-generation flow cytometry, clonal CPCs have been shown to be present in up to 96–100% of patients with newly diagnosed MM (NDMM).8,9 Hence, serial assessment of peripheral blood for detection of CPCs or cell-free DNA could be a feasible approach for detection of minimal residual disease (MRD) outside of the bone marrow.
We hypothesized that the reduction of the CPC burden after PI and/or IMiD-based induction therapy would lead to an improved PFS and OS in NDMM patients undergoing upfront ASCT. To test our hypothesis, we retrospectively analyzed NDMM patients undergoing upfront ASCT with available data on paired measurement of CPCs before initiation of induction therapy and before ASCT.
Methods
Patients
This study was approved by the Mayo Clinic Institutional Review Board and was conducted in accordance with federal regulations and the principles of the Declaration of Helsinki. Informed consent was obtained from all patients for review of their electronic medical records. We have included all consecutive patients who had a serial evaluation for the presence of clonal CPCs at diagnosis (prior to initiating therapy) and prior to stem cell mobilization for ASCT by 6-color multiparameter flow cytometry (MFC). All patients underwent upfront ASCT (<12 months from diagnosis) in Mayo Clinic between January 2007 and May 2015 (the era of PI and IMiD-based induction therapy).
Mononuclear cells from peripheral blood samples were isolated by Ficoll gradient and stained with antibodies to CD19, CD38, CD45, CD138 and cytoplasmic kappa and lambda immunoglobulin light chains.10 The CPCs were detected by analysis of CD19, CD45, CD38 and CD138. Clonality was assessed by light chain restriction [Kappa: lambda expression ratio of >4:1 (Kappa restricted) or <1:2 (Lambda restricted)]. The target for collection was more than 150,000 cellular events. The clonal CPCs were reported as the number of clonal PCs/150,000 total mononuclear cells. Patients were considered to be negative for clonal CPCs at a sensitivity of 10−4 clonal plasma cells in all tested events. Patients were classified as having high-risk cytogenetics by fluorescent in situ hybridization (FISH) if they had deletion (17p), t(4;14), t(14;20), t(14;16) or +1q at diagnosis or at first presentation to the Mayo Clinic prior to ASCT.
The primary end point of this study was best post-transplant response, PFS and OS. Response was determined according to the current International Myeloma Working Group (IMWG) response criteria.11 Best post-transplant response was defined as the best response at any time after ASCT. PFS was defined as time from ASCT to disease progression or death due to any cause. OS was defined as time from ASCT to death due to any cause. Patients who were alive and free of disease were censored at the last follow-up visit. Stem cell mobilization, conditioning and transplant management in the Mayo Clinic have been previously described.12,13
Statistical analysis
Two-sided Fisher’s exact tests were used to test for differences between categorical variables and two-sided Wilcoxon rank sum tests were used to compare continuous variables. Survival analysis was carried out using the method described by Kaplan and Meier.14 Differences in survival between groups were tested for statistical significance using the two-sided log-rank test. Univariate analysis using the Cox proportional hazards model was performed with the following variables: age ≥65 years at transplant, high-risk cytogenetics by FISH, International Staging System (ISS) stage 3 at diagnosis, CPC kinetics, very good partial response (VGPR) or better at transplant, lactate dehydrogenase (LDH) more than upper normal limit (UNL) at diagnosis, plasma cell labeling index (PCLI) greater than 1 at diagnosis, PI-based induction therapy, IMiD-based induction therapy and PI and IMiD-based induction therapy. Prognostic factors for PFS and OS with P<0.1 in the univariate analysis were studied in a multivariate analysis. The JMP 10.0.0 (SAS Institute Inc., Cary, NC, USA) statistical package was used for all statistical analysis.
Results
Baseline characteristics
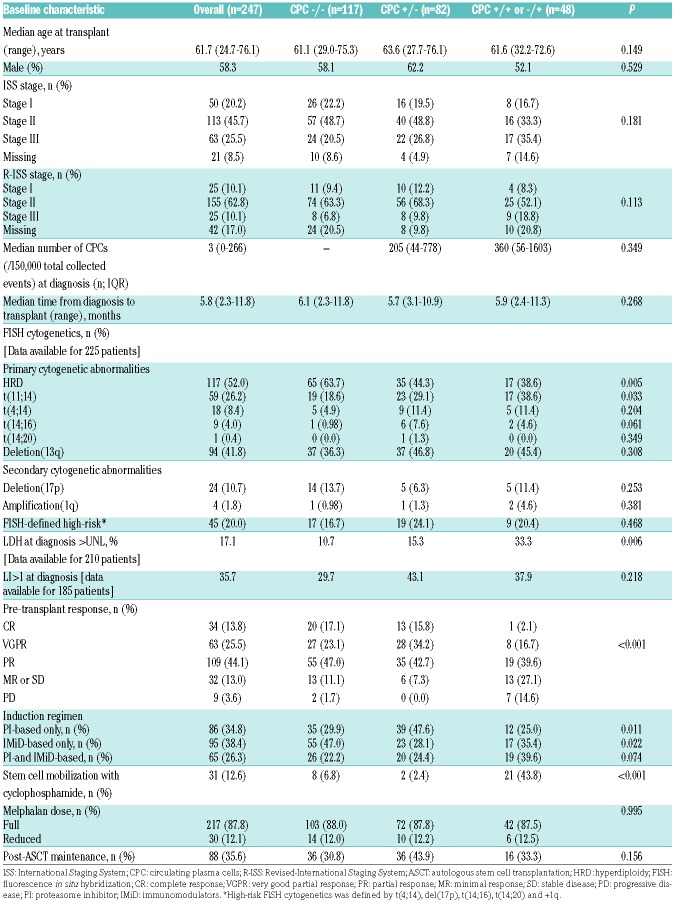
A total of 247 patients had available data on paired evaluation of clonal CPCs at diagnosis and prior to ASCT in our database in the designated time period. The median age at transplant was 62 years (range, 25–76). Clonal CPCs were detected in 127 (51.4%) patients at diagnosis. The median number of clonal CPCs at diagnosis was 247/150,000 total collected events (range 1–88,383). A total of 117 (47.4%) patients did not have detectable CPCs at diagnosis and transplant (CPC−/−). Eighty-two (33.2%) patients had detectable CPCs at diagnosis followed by complete eradication after induction therapy (CPC +/−). Among patients with detectable CPCs at transplant (n=48; 19.4%), persistence of CPCs was seen in 45 patients (CPC +/+) and emergence of new CPCs (CPC−/+) was seen in 3 patients. The median percentage change in the number of CPCs at transplant from that at diagnosis in patients with incomplete resolution after induction therapy was −82% (range, −99% to +122%). There was no significant difference in the median number of CPCs at diagnosis in patients with complete (CPC+/−) or incomplete (CPC+/+) resolution of CPCs after induction therapy (median, 206 vs. 415, respectively; P=0.11). The median number of post-induction CPCs in patients with incomplete resolution of CPCs after induction therapy was 75/150,000 total collected events (range 1–9645). The 3 patients with emergence of new CPCs after induction had a total of 30, 10 and 168 CPCs/150,000 total collected events.
For analysis, we divided the patients into 3 groups based on the detection of CPCs before and after induction therapy: CPC−/− (n=117), CPC+/− (n=82) and CPC+/+ or −/+ (n=48). The baseline clinical characteristics in the 3 groups are shown in Table I. There was no significant difference in the ISS stage or revised-ISS stage at presentation in the 3 groups (P=0.181 and 0.113, respectively). Among primary cytogenetic abnormalities, the incidence of hyperdiploidy was significantly higher in patients with CPC−/−, compared to those with CPC+/− and CPC+/+ or −/+ (64%, 44% and 39%, respectively; P=0.005). On the other hand, the incidence of t(11;14) in patients with CPC−/− was 19%, compared to 29% in those with CPC+/−and 39% in patients with CPC+/+ or −/+ (P=0.033). There was no significant difference in the incidence of FISH-defined high-risk cytogenetic signatures (including deletion [17p], t[4;14], t[14;16], t[14;20] and/or +1q) in any of the groups (P=0.468). The proportion of patients with an elevated LDH level at diagnosis was 11% in CPC−/−, 15% in CPC+/− and 33% in CPC+/+ or −/+ groups (P=0.006); in other words, patients with elevated LDH had a higher incidence of having CPCs at diagnosis compared to those with normal LDH (42% vs. 17%). There was no statistically significant correlation between the kinetics of CPCs and LDH level at transplant. The proportion of patients with pre-transplant PCLI of bone marrow plasma cells (BMPCs) greater than 1 was higher in patients in the CPC+/+ or −/+ group (39%), compared to those in CPC+/− (26%) and CPC−/− groups (16%) (P=0.018), suggesting that proliferating plasma cell clones in the bone marrow were associated with the presence of CPCs resistant to clearance after induction therapy.
Table 1.
Baseline clinical characteristics.
The median time from diagnosis to ASCT was six months, and was similar in the 3 groups. Patients with CPC+/+ or −/+ had a lower incidence of receiving only a PI-based induction regimen (P=0.011) and a trend towards a higher incidence of receiving both PI and IMiD-based induction regimens (P=0.074) prior to ASCT (Table 1). There was no significant difference in the proportion of patients receiving triplet induction regimens in the CPC+/−and CPC+/+ or −/+ groups (68% vs. 56%; P=0.170). Very good partial response (VGPR) or better response prior to transplant was achieved in 40% of patients with CPC−/−, 50% in CPC+/− and 19% of patients with CPC+/+ or −/+ groups (P=0.001). The frequency of administration of post-transplant maintenance therapy was 36%, with no significant difference in the 3 groups (P=0.156).
Post-transplant response and survival
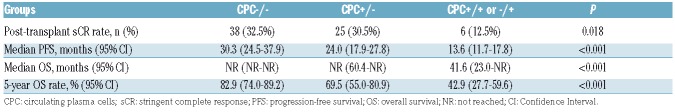
The median follow up was 58 months (95%CI: 50–64) from ASCT. Patients in CPC−/− and +/− groups had a higher incidence of achieving post-transplant stringent complete response (sCR), compared to those with CPC+/+ or −/+ (32%, 30% and 12%, respectively; P=0.018 for comparison of 3rd with 1st and 2nd groups) (Table 2). The Kaplan-Meier curves for PFS and OS are shown in Figure 1A and B. The median PFS from transplant in the 3 respective groups was 30, 24 and 14 months, respectively, and the 5-year OS rates were 83%, 70% and 43%, respectively (P<0.001 for both comparisons).
Table 2.
Post-transplant response and survival.
Figure 1.
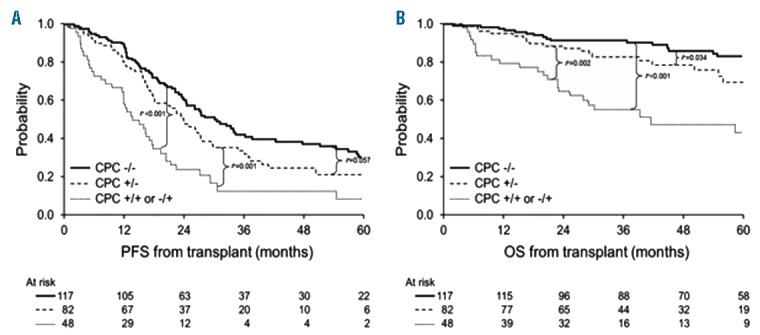
Kaplan-Meier curves depicting progression-free survival (PFS) (A) and overall survival (OS) (B) in groups stratified by kinetics of circulating plasma cells (CPCs) before and after induction therapy: CPC−/−, CPC +/− and CPC +/+ or −/+.
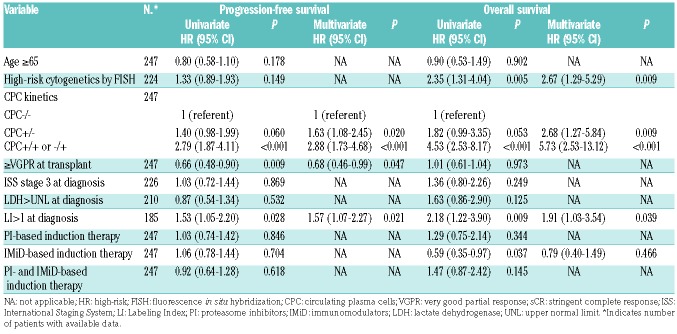
On a multivariate analysis (MVA) (Table 3), using CPC−/− group as the comparator, PFS and OS was significantly inferior in CPC+/− (P=0.020 and 0.009 for PFS and OS, respectively) and CPC +/+ or −/+ groups (P<0.001 for both PFS and OS). Presence of high-risk cytogenetics by FISH and PCLI greater than 1 at diagnosis also retained their independent prognostic impact for OS on MVA.
Table 3.
Univariate and multivariate analysis for progression-free and overall survival by Cox proportional hazards model.
Data on clonal CPCs at day 100 post transplant was available in 213 out of 247 patients. A total of 10 (4.7%) patients had detectable CPCs on day 100, with the median number of CPCs being 770/150,000 total collected events (range 32–118,285). This included 6 patients with incomplete resolution of pre-transplant CPCs and 4 patients with emergence of new CPCs after transplant. The median PFS in these 10 patients with clonal CPCs on day 100 was 5.3 months (95%CI: 1.1–17.5) and the median OS was 6.6 months (95%CI: 4.9–24.2) from ASCT. The median PFS and OS in patients with complete resolution of pre-transplant CPCs by day 100 (n=35) was 16.4 months (95%CI: 12.0–20.5) and 58.4 months (95%CI: 29.0–NR), respectively, the 5-year OS rate being 48% (95%CI: 29–67). The Kaplan-Meier curve for PFS and OS stratified by kinetics of CPCs before and after ASCT is shown in the Online Supplementary Appendix.
Impact of maintenance therapy
A total of 88 (36%) patients had received post-transplant maintenance therapy. PI-based maintenance was received by 13%, IMiD-based 20% and both PI and IMiD-based therapy was received by 2% of patients after ASCT. The Kaplan-Meier curves for PFS in the 3 groups (CPC−/−, CPC+/− and CPC+/+ or −/+) stratified by receipt of maintenance therapy are shown in Figure 2. Improvement in PFS with administration of post-transplant maintenance therapy was demonstrated only in the group with complete resolution of CPCs (CPC+/−) after induction therapy (median PFS, 27 vs. 22 months with maintenance vs. no maintenance, respectively; P=0.029). There was no statistically significant difference in OS noted between groups.
Figure 2.
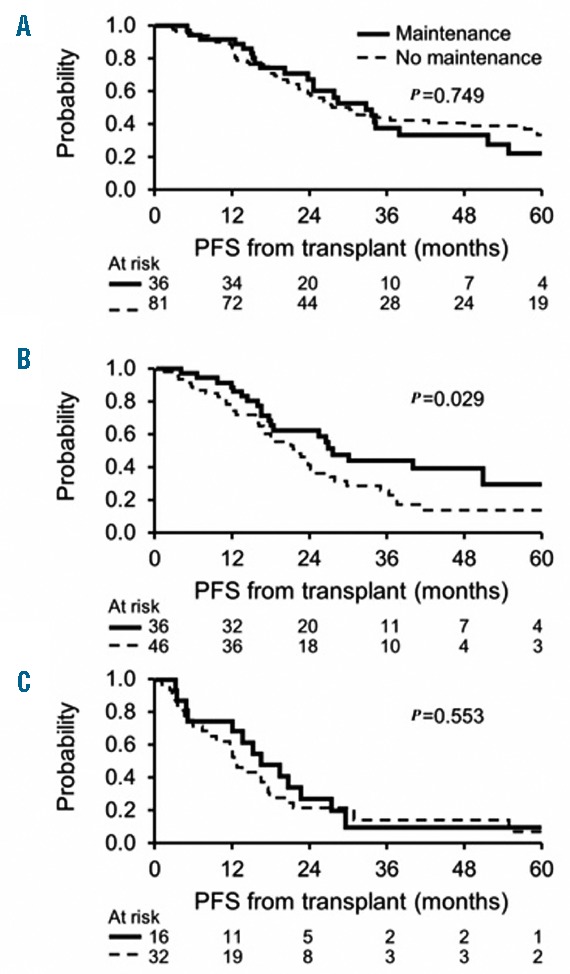
Kaplan-Meier curves depicting progression-free survival (PFS) in different groups stratified by the receipt of post-transplant maintenance therapy. (A) CPC−/−; (B) CPC+/−; (C) CPC+/+ or −/+.
Discussion
Our study shows that in patients with clonal CPCs at diagnosis, complete eradication of CPCs after induction therapy is associated with a marked improvement in PFS and OS from transplant. However, their survival is still inferior compared to patients with no detectable CPCs at diagnosis and prior to ASCT. Furthermore, in patients with incomplete resolution of CPCs after induction therapy with PIs and/or IMiDs, 5-year OS rate from ASCT is only 43%. The prognostic impact of kinetics of CPCs before induction and before ASCT was independent of other known prognostic variables, including ISS stage, high-risk FISH cytogenetics, LDH at diagnosis and pre-transplant response by IMWG criteria. Interestingly, among patients with CPCs at diagnosis, administration of post-transplant maintenance therapy led to PFS improvement only in the group with complete eradication of CPCs after induction therapy which signifies that resistance of clonal CPCs to induction therapy cannot be overcome by maintenance.
Evaluation of CPCs for assessing disease burden in MM is emerging as an attractive modality for non-invasive risk stratification and response assessment. However, it has not been evaluated in prospective studies. The presence of CPCs in newly diagnosed MM has been shown to predict an inferior PFS and OS in various retrospective studies, independent of the cytogenetic risk status at diagnosis.1,3,7,15 However, a uniform cut off for CPCs cannot be ascertained, primarily due to the heterogeneity of flow cytometry techniques used across studies. Using a highly sensitive next-generation flow cytometry method9 with the median limit of detection being 3×10−6, clonal CPCs have been detected in 60% of patients with monoclonal gammopathy of unknown significance, 75% with smoldering MM, 96–100% of patients with newly diagnosed MM, and 85% with relapsed/refractory MM.8,16 The authors in the study by Burgos et al.8 also reported a 100% concordance in FISH-defined cytogenetic abnormalities in 10 paired BMPC and CPC analysis and 75% concordance in gene expression profiling signatures, based on paired data on BMPCs and CPCs in 12 patients. Another study by Lohr et al. on genomic characterization of CPCs in 24 MM patients showed the co-existence of several targeted mutations in BMPCs and CPCs. However, in 3 of 24 patients, the proportion of clonal CPCs harboring TP53R273C, BRAFG469A and NRASG13D was significantly higher compared to that of clonal BMPCs harboring similar mutations.17 Interestingly, in another study in relapsed MM, there was a lack of concordance in the mutational profile in all 6 patients among BMPCs, CPCs and clonal PCs from extramedullary (EM) plasmacytomas. The somatic mutational burden in BMPCs, CPCs and EM PCs was 75%, 77% and 85%, respectively (P=0.07), likely indicating independence from the bone marrow microenvironment in myeloma cells with increasing mutational burden.18 It would be interesting to explore the mutational profile of CPCs at several time points in the course of illness to identify the dynamics of clonal evolution.
There was no significant difference in the frequency of FISH-defined high-risk cytogenetic abnormalities between groups in our study. However, patients with CPCs at diagnosis had a higher frequency of harboring t(11;14) compared to those with no CPCs (34 vs. 19%; P=0.033). t(11;14) is known to be more prevalent in patients with primary plasma cell leukemia (PCL) (25–65%) compared to those with newly diagnosed MM (15%).19 Although the frequency of t(11;14) was higher in patients with incomplete resolution of CPCs compared to those with complete eradication after induction therapy (40% vs. 29%, respectively), this difference was not statistically significant (P=0.209). Conversely, the incidence of hyperdiploidy (HRD) was lower in patients with CPCs at diagnosis compared to those without (33% vs. 52%, respectively; P=0.005). HRD as a primary cytogenetic abnormality is extremely rare in primary PCL (0–9%), compared to a prevalence of 50% or more in newly diagnosed MM.19
In our study, 82 out of 127 patients with CPCs at diagnosis had complete resolution after induction therapy with PI and/or IMiDs and this subgroup also achieved PFS benefit from PI/IMiD-based post-transplant maintenance therapy, indicating greater chemosensitivity of the CPC clone in this subgroup. This also highlights the heterogeneity in sensitivity to induction therapy among clonal CPCs present at diagnosis. Patients with incomplete resolution of CPCs after induction therapy, who subsequently achieved complete clearance by day 100 of ASCT still had an inferior OS (5-year OS rate, 48%) compared to those with complete resolution of CPCs after induction (5-year OS rate, 70%). Only 3 of 247 patients in our study had no CPCs at diagnosis followed by emergence of new CPCs after induction therapy. With serial CPC monitoring by higher sensitivity flow cytometry methods, this number might increase and reflect subclonal evolution of PCs developing anchorage independence and growth potential outside of the bone marrow microenvironment under therapeutic and immunological pressure.
This study has limitations. The flow cytometry technique employed was not highly sensitive, as demonstrated by the detection of CPCs in only 51% of newly diagnosed patients, compared to 96% with next-generation flow technology.8 With the use of homogeneous high-sensitivity flow cytometry techniques, the impact of quantitative cut offs for decrease in CPCs after induction therapy on survival should be explored, ideally in the setting of clinical trials. Some recent studies have also shown down-regulation of CD138 expression on CPCs,20,21 hence CD138-independent strategies should be employed to identify CPCs in MM. Furthermore, there were significant differences in induction regimens received across different groups and the groups were not well balanced in relation to LDH at diagnosis. However, CPC kinetics retained its independent prognostic impact on multivariate analysis.
To the best of our knowledge, this is the first study to demonstrate the independent prognostic value of serial assessment of CPC burden on multivariate analysis in newly diagnosed MM patients undergoing early ASCT. Furthermore, it also shows that post-transplant maintenance did not offer any PFS benefit to patients who did not achieve complete eradication of clonal CPCs at the end of induction therapy. Flow cytometry of peripheral blood for CPCs is a non-invasive and cost-effective technique which can be easily employed in resource-limited settings to assess disease burden outside of the bone marrow. Future prospective studies using highly sensitive methods for detection of CPCs should incorporate several time points to explore impact on survival and establish a predictive marker for maintenance therapy. However, the findings presented here provide additional evidence the already established role of CPCs as a prognostic marker in myeloma.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/8/1439
References
- 1.Gonsalves WI, Rajkumar SV, Gupta V, et al. Quantification of clonal circulating plasma cells in newly diagnosed multiple myeloma: implications for redefining high-risk myeloma. Leukemia. 2014;28(10):2060–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonsalves WI, Morice WG, Rajkumar V, et al. Quantification of clonal circulating plasma cells in relapsed multiple myeloma. Br J Haematol. 2014;167(4):500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An G, Qin X, Acharya C, et al. Multiple myeloma patients with low proportion of circulating plasma cells had similar survival with primary plasma cell leukemia patients. Ann Hematol. 2015;94(2):257–264. [DOI] [PubMed] [Google Scholar]
- 4.Peceliunas V, Janiulioniene A, Matuzeviciene R, Zvirblis T, Griskevicius L. Circulating plasma cells predict the outcome of relapsed or refractory multiple myeloma. Leuk Lymphoma. 2012; 53(4):641–647. [DOI] [PubMed] [Google Scholar]
- 5.Chakraborty R, Muchtar E, Kumar SK, et al. Risk stratification in myeloma by detection of circulating plasma cells prior to autologous stem cell transplantation in the novel agent era. Blood Cancer J. 2016;6(12):e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dingli D, Nowakowski GS, Dispenzieri A, et al. Flow cytometric detection of circulating myeloma cells before transplantation in patients with multiple myeloma: a simple risk stratification system. Blood. 2006; 107(8):3384–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nowakowski GS, Witzig TE, Dingli D, et al. Circulating plasma cells detected by flow cytometry as a predictor of survival in 302 patients with newly diagnosed multiple myeloma. Blood. 2005;106(7):2276–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgos L, Alignani D, Garces J-J, et al. Non-Invasive Genetic Profiling Is Highly Applicable in Multiple Myeloma (MM) through Characterization of Circulating Tumor Cells (CTCs). Blood. 2016;128(22):801–801 [Meeting Abstract]. [Google Scholar]
- 9.Flores-Montero J, Sanoja-Flores L, Paiva B, et al. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia. 2017. March 10 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar S, Kimlinger T, Morice W. Immunophenotyping in multiple myeloma and related plasma cell disorders. Best Pract Res Clin Haematol. 2010;23(3):433–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–46. [DOI] [PubMed] [Google Scholar]
- 12.Gertz MA, Dingli D. How we manage autologous stem cell transplantation for patients with multiple myeloma. Blood. 2014;124(6):882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gertz MA, Ansell SM, Dingli D, et al. Autologous stem cell transplant in 716 patients with multiple myeloma: low treatment-related mortality, feasibility of outpatient transplant, and effect of a multidisciplinary quality initiative. Mayo Clin Proc. 2008;83(10):1131–1138. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 15.Vagnoni D, Travaglini F, Pezzoni V, et al. Circulating plasma cells in newly diagnosed symptomatic multiple myeloma as a possible prognostic marker for patients with standard-risk cytogenetics. Br J Haematol. 2015;170(4):523–531. [DOI] [PubMed] [Google Scholar]
- 16.Sanoja-Flores L, Paiva B, Flores-Montero JA, et al. Next Generation Flow (NGF): A High Sensitive Technique to Detect Circulating Peripheral Blood (PB) Clonal Plasma Cells (cPC) in Patients with Newly Diagnosed of Plasma Cell Neoplasms (PCN). Blood. 2015;126(23):4180–4180 [Meeting Abstract]. [Google Scholar]
- 17.Lohr JG, Kim S, Gould J, et al. Comprehensive Genetic Interrogation of Circulating Multiple Myeloma Cells at Single Cell Resolution. Blood. 2016; 128(22):800–800 [Meeting Abstract]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bretones G, Paiva B, Valdes-Mas R, et al. Genomic Profiles of Bone Marrow (BM) Clonal Plasma Cells (PCs) Vs Circulating Tumor Cells (CTCs) and Extramedullary (EM) Plasmacytomas in Multiple Myeloma (MM). Blood. 2016;128(22):4442–4442 [Meeting Abstract]. [Google Scholar]
- 19.van de Donk NW, Lokhorst HM, Anderson KC, Richardson PG. How I treat plasma cell leukemia. Blood. 2012;120(12):2376–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paiva B, Paino T, Sayagues JM, et al. Detailed characterization of multiple myeloma circulating tumor cells shows unique phenotypic, cytogenetic, functional, and circadian distribution profile. Blood. 2013;122(22):3591–3598. [DOI] [PubMed] [Google Scholar]
- 21.Muz B, de la Puente P, Azab F, et al. A CD138-independent strategy to detect minimal residual disease and circulating tumour cells in multiple myeloma. Br J Haematol. 2016;173(1):70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.