Abstract
Odorant receptors (ORs) constitute the molecular basis for the detection of volatile odorous molecules and the perception of smell. Our understanding of chemical senses has been greatly expanded by the discovery of the OR gene families in vertebrates and in the nematode Caenorhabditis elegans. Recently, candidate Drosophila OR genes have been identified. The putative ORs do not possess any primary sequence identity with known vertebrate or C. elegans receptors, but belong to the family of G protein-coupled receptors according to their predicted seven transmembrane topology. To prove olfactory function of these proteins, we expressed a member of the putative Drosophila OR gene family, Or43a, in Xenopus laevis oocytes. Using two-electrode voltage-clamp recording we identified four odors (cyclohexanone, cyclohexanol, benzaldehyde, and benzyl alcohol) that activated the receptor at low micromolar concentration and structurally related substances that did not. This report shows the function and specificity of a member of the recently identified family of Drosophila ORs expressed in a heterologous system.
The olfactory system performs the complex task of discriminating the quality and assessing the concentration of thousands of different odorants. The molecular units constituting the basis for the detection of volatile molecules and the perception of smell are the odorant receptors (ORs), which are expressed in olfactory receptor neurons (ORNs). Recently, candidate OR genes have been identified in Drosophila (1, 2). Each of the 59 genes identified so far in the Drosophila genome encodes a putative seven-transmembrane domain protein of about 380 aa and do not show any primary sequence identity with known vertebrate or Caenorhabditis elegans receptors (3–5). The members of the gene family in the fly are extremely divergent, with an average amino acid identity of ≈20%. Consistent with a role in odor recognition some ORs are expressed in small subsets of ORNs of the olfactory sensory organs of adult Drosophila, the antenna, or the maxillary palp (1, 2). Functional evidence that expressed candidate OR genes in fact encode ORs in other animals could be obtained by using various experimental approaches. In C. elegans, genetic loss-of-function analysis led to the identification of the diacetyl receptor ODR10 (6). Functional expression of putative OR cDNAs in vertebrate ORNs (7, 8) as well as in heterologous systems (9–14) proved candidate OR genes to encode functional proteins and succeeded in identification of odors that were capable to activate the expressed receptors. Such sets of data are not available for the recently identified Drosophila genes so far.
We performed two-electrode voltage-clamp recordings of Xenopus laevis oocytes heterologously expressing a member of the candidate Drosophila OR gene family, Or43a (Drosophila Receptor Nomenclature Committee 2000), to (i) show the function of the candidate receptor protein acting as OR, and (ii) screen the odor profile activating the Or43a to investigate the tuning of this receptor.
Methods
Construction and in Vitro Transcription of pRc/CMV-Or43a and pSGEM-Gα15.
The plasmid pRc/CMV-Or43a was constructed by cloning the 1-kB SacI/NotI fragment of the bluescript vector containing the complete reading frame of Or43a (cDNA kindly provided by L. Vosshall, The Rockefeller University, New York) blunt end into the HindIII/XbaI sites of pRc/CMV (Invitrogen). As a template for in vitro transcription, a PCR product consisting of the coding region of pRc/CMV-Or43a, a T7-promoter at the 5′ end as well as a poly(A) stretch of 30 nt at the 3′ end was obtained by PCR. The PCR mix contained Pfu buffer, 1.5 mM MgCl2, 0.2 mM of each dNTP, 10 ng pOr43a, 0.5 mM primer P1 (CGGGATCCAGATCTCGAAATTAATACGACTCACTATAGG), P2 (T30AGGAAAGGACAGTGGGAGTGGCACC), and 2.5 units Pfu polymerase (Stratagene). PCR amplification was performed according to the following schedule: 94°C for 1 min, 60°C for 1 min, 72°C for 2 min, for 25 cycles. The PCR product was purified by agarose gel electrophoresis. For the transcription of the human Gα15 RNA, pSGEM-Gα15, that contains the 1.4-kB ClaI/XbaI fragment of pCISGα15 (15) cloned blunt end into the SmaI/EcoRV sites of pSGEM (16), was linearized with PacI. RNA was synthesized in the presence of capping analogue m7G(5′)ppp(5′)G (Amersham Pharmacia) by using RNA polymerase. The RNA was treated with DNaseI, extracted with phenol/chloroform (1:1), ethanol-precipitated, and redissolved in water to give a final concentration of 1 μg/μl. RNA was analyzed on an agarose gel to ensure that no degradation had occurred.
Expression of Odorant Receptor cDNA in X. laevis Oocytes.
Ovarian lobes were obtained from mature female X. laevis anesthetized by immersion in 0.15% 3-aminobenzoic acid ethyl ester (methansulfonate salt; Sigma). Ovarian tissue was removed and placed in Barth's solution [88 mM NaCl/1 mM KCl/0.82 mM MgSO4/0.33 mM Ca(NO3)2/0.41 mM CaCl2/2.4 mM NaHCO3/5 mM Tris⋅HCl, pH 7.4/100 units/ml penicillin/50 μg/ml streptomycin] sterilized by filtration. After treatment of the ovarian tissue with collagenase (type II, Sigma C-6885, 2 mg/ml in Ca2+-free Barth's solution) for 2 h at room temperature, the oocytes were incubated overnight at 18.5°C in fresh Barth's solution. After 24 h, mature healthy oocytes (stages V–VI) were selected for cytoplasmic injection of cRNA (about 50 ng per oocyte) with a sharp pipette using a pressure injector (NPI Instruments PDES 04T, Tamm, Germany). Afterward, injected oocytes were placed again in fresh Barth's solution and incubated at 18.5°C. Oocytes were tested for functional expression of Or43a protein after 5–7 days.
Electrophysiological Recording.
We used two-electrode voltage-clamp recording of injected X. laevis oocytes to obtain current responses to Drosophila odorants in various concentrations. Odorants were purchased in the highest purity available from Riedel-de-Haen, Seelze, Germany (benzyl alcohol), J.T. Baker (benzaldehyde, butanol, cyclohexanol, cyclohexanone, propionaldehyde), Fluka (1,3-cyclohexanedione, 1,4-cyclohexanedione, 3-phenylpropionaldehyde, hexanone), or Sigma (phenylacetaldehyde, toluene, isopropylacetate, hexanol, octanal). Odors were diluted to the indicated concentrations with Xenopus-Ringer (115 mM NaCl/2.5 mM KCl/1.8 mM CaCl2/10 mM Hepes, pH 7.2) and applied by means of a multibarrel single-tip superfusion device. Membrane potential was controlled, and membrane current was recorded by using a TURBO TEC-03 amplifier (NPI Instruments) and pclamp software (Axon Instruments, Foster City, CA). Odorant-induced currents were recorded at a holding potential of −80 mV.
Results and Discussion
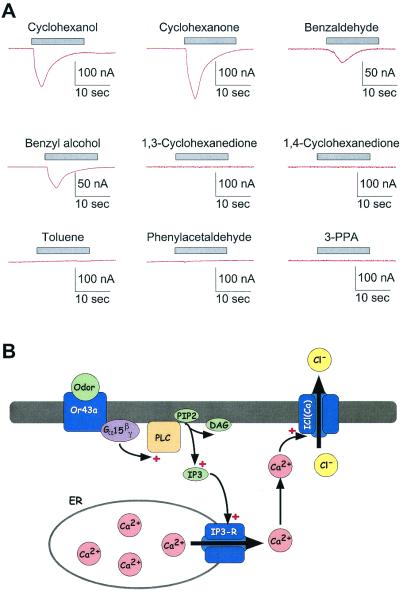
To study the function and properties of a candidate Drosophila OR we investigated X. laevis oocytes injected with cRNA coding for a member of the candidate Drosophila OR gene family, Or43a, by means of two-electrode voltage-clamp recording. Or43a was coexpressed with a Gα15 subunit (17), because this subunit was shown to couple activated ORs to the inositol 1,4,5-trisphosphate pathway and induce Ca2+ release from internal stores (12), leading to the activation of endogenous Ca2+-induced Cl− currents (Fig. 1B). Five to 7 days after injection of the cRNA, the oocytes were voltage-clamped (Vhold = −80 mV) and challenged with a panel of odors (Fig. 2), known to be perceived by Drosophila and used in other studies as olfactory stimuli (18–20) (Fig. 2). Oocytes injected with Or43a and Gα15 cRNAs responded to application of only four individual odors out of this panel, cyclohexanol, cyclohexanone, benzaldehyde, or benzyl alcohol (Fig. 1A). Cyclohexanol or cyclohexanone induced inward currents, which varied in amplitude from cell to cell, presumably reflecting different levels of receptor expression. The currents developed in 2–5 s and peaked at 40–290 nA in n = 15 oocytes (average: 156 ± 47 nA for cyclohexanol and 186 ± 28 nA for cyclohexanone; Figs. 1A and 2A). The current declined despite the continued presence of odor (application time 15 s), indicating desensitization or adaptation of at least one component in the signal transduction pathway. Benzaldehyde or benzyl alcohol induced smaller, but measurable inward currents in n = 6 oocytes (average: 60 ± 20 nA for benzaldehyde and 50 ± 25 nA for benzyl alcohol), indicating that these two odors were weaker agonists (Figs. 1A and 2A).
Figure 1.
(A) Original two-electrode voltage-clamp recordings of X. laevis oocytes injected with cRNA coding for the Drosophila Or43a and Gα15 subunit. The membrane potential (Vhold) was set to −80 mV, and the oocytes were challenged with various odorants (1 mM each) as indicated by the bar. The odors were delivered by a multibarrel single-tip superfusion device for 15 s. Using this system, the complete exchange of the solutions (odor-free or containing the particular compounds) in the recording chamber could be obtained within less than 1 s. Stimulation of the oocytes with cyclohexanol, cyclohexanone, benzaldehyde, or benzyl alcohol resulted in prominent inward currents that developed in 2–5 s, reached a maximum, and decreased in continued presence of odor. Other odors [phenylacetaldehyde, toluene, 1,3-cyclohexanediol, 1,4-cyclohexanediol, 3-phenylpropionaldehyde (3-PPA)] were not active as agonists. (B) Schematic drawing of the suggested signal transduction pathway in oocytes injected with cRNA coding for the Drosophila Or43a and Gα15 subunit. The odor interacts with the OR protein inserted in the plasma membrane and activates the Gα15 subunit that couples the activation to an endogenous phospholipase C (PLC). The synthesized inositol 1,4,5-trisphosphate (IP3) leads to the liberation of free Ca2+ from internal stores (endoplasmatic reticulum, ER) and activation of endogenous Ca2+-dependent Cl− currents.
Figure 2.
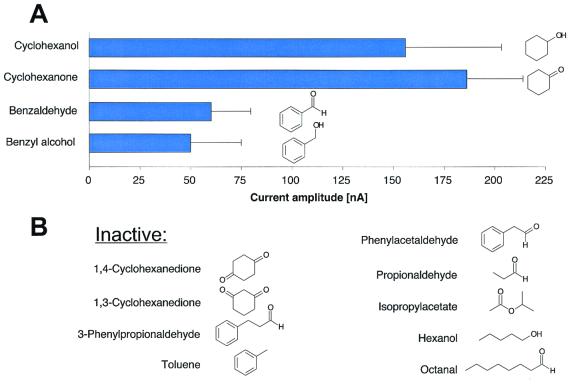
(A) Diagram showing the structure and the activity of the compounds tested on oocytes injected with Drosophila Or43a together with Gα15 cRNA. The stimulatory action of agonists is presented as the peak amplitude of the induced currents (mean ± SE). The odor concentration was 1 mM. Only cyclohexanol, cyclohexanone, benzyl alcohol, and benzaldehyde were active as agonists at the Or43a. (B) Structures of compounds that were inactive at millimolar concentration at the Or43a.
Six structurally related substances (1,3-cyclohexanedione, 1,4-cyclohexanedione, toluene, 3-phenylpropionaldehyde, phenylacetaldehyde, hexanol) and two structurally unrelated Drosophila odorants (propionaldehyde or isopropylacetate) (in millimolar concentrations) failed to activate Or43a (Figs. 1A and 2). All active ligands share the cyclic 6-carbon structure with a polar functional group attached to the ring. Adding a second polar group to the active ligand cyclohexanone, as it is the case for 1,3- or 1,4-cyclohexanedione, resulted in a complete loss of activity.
As we only have tested a limited set of odorants, we cannot exclude a possible activity of odorants that are different in structure and may interact with yet unknown binding sites at the receptor protein.
To exclude possible unspecific effects of odorants on the signal transduction pathway or on ion channels expressed in the oocytes, we injected cRNA for the rat OR I7 (7, 12, 14) together with Gα15 and tested the oocytes with the I7-specific agonist octanal and the odorants used to activate the Or43a and found that only octanal activated these oocytes. Oocytes not injected with the Or43a RNA failed to respond to application of cyclohexanone, cyclohexanol, benzyl alcohol, or benzaldehyde even at millimolar concentrations, indicating that the stimulatory effects of these odorants were specific to the Or43a (data not shown).
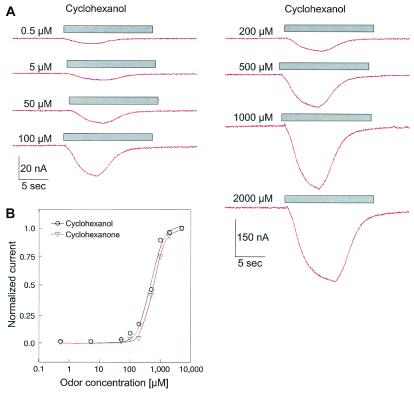
Cyclohexanol and cyclohexanone induced measurable currents at concentrations down to 500 nM (Fig. 3). The amplitude of the induced inward current increased in a dose-dependent manner with the concentration of the odorants, saturating at about 2 mM. Fitting the averaged data with the Hill equation, resulted in an EC50 of 492 ± 43 μM and a Hill coefficient of nH = 2 (n = 3) for cyclohexanol and an EC50 of 601 ± 20 μM and a Hill coefficient of nH = 1.9 ± 0.2 (n = 4) for cyclohexanone (Fig. 3). The time to peak of the cyclohexanol-induced current was not decreased by increasing odor concentrations as would be expected for currents induced by direct ligand-activated ion channels, suggesting that the kinetics of the responses reflect several amplifying stages in the signal transduction pathway.
Figure 3.
(A) Original two-electrode voltage-clamp recordings of a X. laevis oocyte injected with cRNA coding for the Drosophila Or43a and Gα15 subunit, showing the dose dependency of cyclohexanol-induced currents. The membrane potential (Vhold) was set to −80 mV, and the oocyte was challenged with cyclohexanol at various concentrations for 15 s as indicated by the bar. (B) Diagram depicting the corresponding dose-response curves for the experiment shown in A (cyclohexanol) and for cyclohexanone. The normalized peak current amplitudes (I/Imax) are plotted vs. the odor concentration.
Comparing the response characteristics of the Or43a expressed in our heterologous system with the specificity of the olfactory system of the transformed flies that are misexpressing the Or43a (27) revealed no qualitative differences in specificity, even though it has been proposed that the perception of odorants in Drosophila is influenced by perireceptor events such as odorant binding proteins (OBPs) (21) or a possible contribution from Or83b, a candidate receptor that seems to be expressed ubiquitously in all Drosophila ORNs (5). The similarity of the in vivo and in vitro results argues against perireceptor events (i) influencing the rank order of potency of odorants to which the OR can respond, (ii) broadening the tuning of the ORs, or (iii) forming a ligand-OBP complex that is recognized by the receptor. However, our results do not exclude that OBPs facilitate the interaction of odorants with ORs.
The signal transduction pathway of Drosophila ORNs in vivo is not known. Although components of the cyclic nucleotide and phosphatidylinositol signaling pathway have been identified in Drosophila (22–25), an essential role for either pathway has not emerged. The fact that the Gα15 subunit couples the activity of vertebrate seven-transmembrane receptors to the phospholipase C/inositol 1,4,5-trisphosphate pathway (12, 15), the functionality of this pathway in the heterologous system does not necessarily imply that this pathway functions in Drosophila ORNs in situ. ORs, like other seven-transmembrane receptors, presumably have the capacity to use diverse signal transduction pathways, depending on the signaling compounds present in the particular cell. Mammalian ORs that in situ couple to the cAMP pathway via Golf, activate (i) the inositol 1,4,5-trisphosphate pathway upon stimulation in HEK293 cells (12, 14), (ii) the cAMP pathway in X. laevis oocytes (14), and (iii) G protein-gated potassium channels (GirK; refs. 13 and 26) in X. laevis oocytes (unpublished observations).
In summary, we have functionally characterized Drosophila OR Or43a heterologously expressed in X. laevis oocytes and identified cyclohexanol, cyclohexanone, benzaldehyd, and benzyl alcohol as agonists in nanomolar concentrations. The long-awaited identification and functional expression of an OR in insects opens up this system to more complete functional characterization by using a variety of techniques, including receptor mutagenesis, pharmacology, and computational modeling. The relative simplicity of olfactory system in Drosophila melanogaster compared with mammalian systems should favor investigation of the logic of odor perception and olfactory discrimination.
Acknowledgments
We thank M. Bathen, H. Bartel, and A. Niehaus for technical assistance, Dr. L. Vosshall for providing the Or43a cDNA, and Dr. B. Ache for critical reading of the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft (grants to H.H., C.H.W., K.F.S., and B.H.).
Abbreviations
- OR
odorant receptor
- ORN
olfactory receptor neuron
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 8936.
References
- 1.Clyne P J, Warr C G, Freeman M R, Lessing D, Kim J. Neuron. 1999;22:327–338. doi: 10.1016/s0896-6273(00)81093-4. [DOI] [PubMed] [Google Scholar]
- 2.Vosshall L B, Amrein H, Morozov P S, Rzhetsky A, Axel R. Cell. 1999;96:725–736. doi: 10.1016/s0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- 3.Buck L, Axel R. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 4.Troemel E R, Chou J H, Dwyer N D, Colbert H A, Bargmann C I. Cell. 1995;83:207–218. doi: 10.1016/0092-8674(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 5.Vosshall L B, Wong A M, Axel R. Cell. 2000;102:147–159. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- 6.Sengupta P, Chou J H, Bargmann C I. Cell. 1996;84:899–909. doi: 10.1016/s0092-8674(00)81068-5. [DOI] [PubMed] [Google Scholar]
- 7.Zhao H, Ivic L, Otaki J M, Hashimoto M, Mikoshiba K, Firestein S. Science. 1998;279:237–242. doi: 10.1126/science.279.5348.237. [DOI] [PubMed] [Google Scholar]
- 8.Touhara K, Sengoku S, Inaki K, Tsuboi A, Hirono J, Sato T, Sakano H, Haga T. Proc Natl Acad Sci USA. 1999;96:4040–4045. doi: 10.1073/pnas.96.7.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raming K, Krieger J, Strotmann J, Boekhoff I, Kubick S, Baumstark C, Breer H. Nature (London) 1993;361:353–356. doi: 10.1038/361353a0. [DOI] [PubMed] [Google Scholar]
- 10.Wellerdieck C, Oles M, Pott L, Korsching S, Gisselmann G, Hatt H. Chem Sens. 1997;22:467–476. doi: 10.1093/chemse/22.4.467. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Chou J H, Bradley J, Bargmann C I, Zinn K. Proc Natl Acad Sci USA. 1997;94:12162–12167. doi: 10.1073/pnas.94.22.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krautwurst D, Yau K W, Reed R R. Cell. 1998;95:917–926. doi: 10.1016/s0092-8674(00)81716-x. [DOI] [PubMed] [Google Scholar]
- 13.Speca D J, Lin D M, Sorensen P W, Isacoff E Y, Ngai J. Neuron. 1999;23:487–498. doi: 10.1016/s0896-6273(00)80802-8. [DOI] [PubMed] [Google Scholar]
- 14.Wetzel C H, Oles M, Wellerdieck C, Kuczkowiak M, Gisselmann G, Hatt H. J Neurosci. 1999;19:7426–7433. doi: 10.1523/JNEUROSCI.19-17-07426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkie T M, Scherle P A, Strathmann M P, Slepak V Z, Simon M I. Proc Natl Acad Sci USA. 1991;88:10049–10053. doi: 10.1073/pnas.88.22.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villmann C, Bull L, Hollmann M. J Neurosci. 1997;17:7634–7643. doi: 10.1523/JNEUROSCI.17-20-07634.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Offermanns S, Simon M I. J Biol Chem. 1995;270:15175–15180. doi: 10.1074/jbc.270.25.15175. [DOI] [PubMed] [Google Scholar]
- 18.Ayer R K, Jr, Carlson J. Proc Natl Acad Sci USA. 1991;88:5467–5471. doi: 10.1073/pnas.88.12.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayer R K, Jr, Carlson J. J Neurobiol. 1992;23:965–982. doi: 10.1002/neu.480230804. [DOI] [PubMed] [Google Scholar]
- 20.Siddiqi O. In: Olfactory Neurogenetics of Drosophila. Chopra V L, Joshi B C, Sharma R P, Bansal H C, editors. New Delhi: Oxford; 1983. pp. 243–261. [Google Scholar]
- 21.Pikienly C W, Hasan G, Rouyer F, Rosbash M. Neuron. 1994;12:35–49. doi: 10.1016/0896-6273(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 22.Baumann A, Frings S, Godde M, Seifert R, Kaupp U B. EMBO J. 1994;13:5040–5050. doi: 10.1002/j.1460-2075.1994.tb06833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubin A E, Harris G L. J Neurobiol. 1997;32:123–137. doi: 10.1002/(sici)1097-4695(199701)32:1<123::aid-neu11>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 24.Marx T, Gisselmann G, Störtkuhl K F, Hovemann B T, Hatt H. Inverteb Neurosci. 1999;4:55–63. doi: 10.1007/pl00022368. [DOI] [PubMed] [Google Scholar]
- 25.Riesgo-Escovar J, Raha D, Carlson J R. Proc Natl Acad Sci USA. 1995;92:2864–2868. doi: 10.1073/pnas.92.7.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spauschus A, Lentes K U, Wischmeyer E, Dissmann E, Karschin A. J Neurosci. 1996;16:930–938. doi: 10.1523/JNEUROSCI.16-03-00930.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Störtkuhl K F, Kettler K. Proc Natl Acad Sci USA. 2001;98:9381–9385. doi: 10.1073/pnas.151105698. [DOI] [PMC free article] [PubMed] [Google Scholar]