Abstract
Cyclic AMP receptor protein (Crp) is a major transcriptional regulator in bacteria. This study demonstrated that Crp affects numerous virulence-related phenotypes, including colonization of mice, motility, fimbria-mediated adhesion, and glucose stress tolerance in uropathogenic Proteus mirabilis. Diabetic mice were more susceptible to kidney colonization by wild-type strain than nondiabetic mice, in which the crp mutant exhibited increased kidney colonization. Loss of crp or addition of 10% glucose increased the P. mirabilis adhesion to kidney cells. Direct negative regulation of pmpA (which encodes the major subunit of P-like fimbriae) expression by Crp was demonstrated using a reporter assay and DNase I footprinting. Moreover, the pmpA/crp double mutant exhibited reduced kidney adhesion comparable to that of the pmpA mutant, and mouse kidney colonization by the pmpA mutant was significantly attenuated. Hence, the upregulation of P-like fimbriae in the crp mutant substantially enhanced kidney colonization. Moreover, increased survival in macrophages, increased stress tolerance, RpoS upregulation, and flagellum deficiency leading to immune evasion may promote kidney colonization by the crp mutant. This is the first study to elucidate the role of Crp in the virulence of uropathogenic P. mirabilis, underlying mechanisms, and related therapeutic potential.
Introduction
Cyclic AMP receptor protein (Crp) is a transcriptional regulator that modulates bacterial metabolism and coordinates the expression of virulence factors through a process known as carbon catabolite repression (CCR), which is triggered in response to the availability of digestible sugars1–5. CCR is a common process used by bacteria to optimize their metabolism and enhance their fitness in their natural environments. Therefore, CCR and virulence factor production are often linked in pathogenic bacteria4, such as type I fimbriae in Escherichia coli and Pla (the plasminogen activator) in Yersinia pestis, which causes the pneumonic plague6, 7. The mechanisms by which Crp regulates gene expression in response to variable cytoplasmic levels of cAMP have been extensively investigated1–5, 8. Crp-regulated genes can be identified by the presence of a Crp box sequence within their promoter regions, and Crp and its cofactor molecular cAMP bind to this sequence to activate or repress gene transcription1–3. Crp from several other bacterial species has been characterized and a disparity in Crp regulation was demonstrated among different bacteria; for example, E. coli Crp represses its own gene expression9 in response to glucose10, whereas crp itself is not autoregulated in Y. pestis. Instead, Y. pestis crp is regulated by the PhoPQ two-component system (TCS)11.
Proteus mirabilis is a common opportunistic urinary tract pathogen, particularly in patients with indwelling catheters12. Several virulence factors of P. mirabilis have been demonstrated to contribute to infections, including fimbriae, flagella, hemolysin, urease, and ZapA metalloprotease12–14. Mannose-resistant Proteus-like (MR/P) fimbriae were synthesized in vivo and implicated in adherence and biofilm formation15. P pili (or P fimbriae) were shown to be highly associated with acute pyelonephritis caused by uropathogenic E. coli (UPEC)16. The pyelonephritis-associated pili (pap) gene cluster encodes the proteins required for the biogenesis of P fimbriae. Notably, the P. m irabilis P (pmp) fimbria-like gene cluster was found in almost all uropathogenic P. mirabilis species17. In addition, P. mirabilis exhibits a form of multicellular behavior termed swarming13. The regulation of swarming motility depends on several factors, including the flagellar master regulator FlhDC12 and specific nutrients and environmental cues, such as amino acids (L-arginine and L-glutamine)18 and some carbohydrates19. In this regard, Crp mediates glucose repression of the swarming associated sdh operon19, 20. Because Crp-cAMP binds to the flhDC promoter region and interacts with RNA polymerase to activate transcription, the cAMP-dependent catabolite repression systems of E. coli and Serratia marcescens are required for the production of flagella and flagellum-based motility21, 22.
Urinary tract infections (UTIs) are common in patients with diabetes, with associated complications such as emphysematous cystitis and pyelonephritis if not properly managed23–25. Poor glycemic control, various immune system impairments, and bladder dysfunction unique to diabetes may increase the risk of UTIs in these patients23, 24, 26. In addition, higher glucose concentrations in urine may promote the growth of pathogenic bacteria. Notably, Proteus species are among the most common pathogens isolated from the urine of diabetic patients with UTIs24, 27, 28. However, the reasons for this high incidence have not been thoroughly investigated.
In this study, we investigated the roles of Crp in uropathogenic P. mirabilis by creating a crp mutant and using different glucose conditions to mimic alterations in Crp activity. We observed that crp expression and Crp activity affected numerous virulence-related phenotypes, including urinary tract colonization of mice, motility, fimbria-mediated adhesion, and stress tolerance. In addition, the relationship of Crp with the glucose PTS transporter (PtsG)10, 29, adenylate cyclase (CyaA)30, or RNA chaperone (Hfq)31 was investigated. Hyperglycemia is a hallmark of patients with diabetes, and excessive glucose in the urine and/or blood is generally considered to reduce the ability of immune cells to break down bacteria26. However, few studies have investigated the role of the virulence factors of pathogens in UTIs in hosts with diabetes. Hence, we also created a hyperglycemic mouse model to investigate the influence of Crp on urinary tract colonization. Diabetic mice exhibited significantly increased kidney colonization (p < 0.01) by the wild-type P. mirabilis rather than the crp mutant compared with nondiabetic mice, which implies that Crp plays a role in kidney colonization in diabetic mice. Crp-mediated upregulation of P-like fimbriae, increased survival in macrophages, increased stress tolerance, upregulation of RpoS, and flagellum deficiency (leading to immune evasion) caused by crp loss or reduced Crp activity may promote mouse kidney colonization. This is the first study to elucidate the Crp-regulated virulence factors of uropathogenic P. mirabilis, underlying mechanisms, and related therapeutic potential. Moreover, this study provides new insights into the role of P. mirabilis Crp in urinary tract colonization of a host with diabetes.
Results
Identification and characterization of P. mirabilis crp
As shown in Supplementary Fig. S1a, crp was identified from nucleotides 3093805–3094437 in the genome of P. mirabilis strain HI4320. It is located between PMI2819 and argD. P. mirabilis Crp comprises 210 amino acids and shares 98–99% sequence identity with E. coli MG1655, Salmonellla enterica Typhimurium LT2, Y. pestis CO92, and S. marcescens Db11. Using primers annealing to conserved sequences, we cloned and sequenced the DNA fragment containing crp and its upstream region of P. mirabilis N2. The Crp amino acid sequence of P. mirabilis N2 was 100% identical to that of P. mirabilis HI4320. Because E. coli Crp is activated in response to low glucose (LB broth), a condition that activates CyaA for the production of cAMP10, we monitored the expression of the plac-gfpuv reporter, which is regulated by Crp-cAMP at 3, 5, 7, 9, 11 and 24 h in wild-type P. mirabilis after exposure to 0%, 2%, and 10% glucose. Crp activity increased over time in the absence of glucose, but was inhibited in the presence of 2% or 10% glucose (Fig. 1a). Crp has been described as a master regulator, activating or repressing the expression of many genes in E. coli 3. In contrast to ptsG activation29, E. coli Crp-cAMP negatively regulates the expression of cyaA (adenylate cyclase gene)32 and hfq 31. Notably, Y. pestis Hfq exhibits specific transcriptional and posttranscriptional regulation of Crp7. To investigate the role of Crp, we generated an isogenic mutant lacking the entire crp coding sequence. We monitored the growth of the wild-type strain, crp mutant, and crp-complemented strain (crpc) in LB broth. A growth defect was detected in the crp mutant compared with the wild-type and crpc strains (Supplementary Fig. S1d). Overnight cultures of the crp mutant routinely reached a density at 600 nm of 0.6–0.7 rather than 1 for the wild-type strain.
Figure 1.
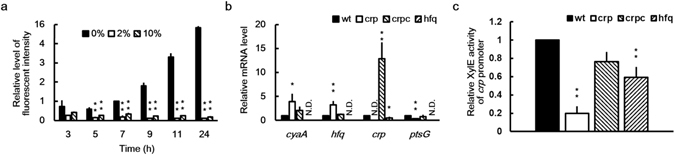
Crp activity was inhibited by high glucose levels; cyaA, hfq, ptsG, and crp itself were regulated by Crp, and crp was regulated by Hfq in P. mirabilis. (a) Crp activity increased in the absence of glucose and decreased by 2% and 10% (2000 and 10,000 mg/dL) glucose. The plac-gfpuv reporter plasmid-transformed wild-type P. mirabilis was grown in LB broth with or without glucose, and the fluorescent intensity of GFPuv was monitored after incubation for 3, 5, 7, 9, 11, and 24 h. The fluorescent intensity in the absence of glucose at 7 h was set at 1, and all other data are expressed relative to this value after being normalized against the OD600 value. The data are the averages and standard deviations of three independent experiments. The significant difference between the presence and absence of glucose at each time point was determined using the Student’s t test (**P < 0.01). (b) Crp regulated the expression of cyaA, hfq, and ptsG mRNA, and crp expression was regulated by Hfq in P. mirabilis. Overnight bacterial cultures were diluted in LB broth and incubated for 7 h before total RNA was prepared for measuring mRNA levels of cyaA, hfq, crp, and ptsG in the wild-type strain, crp mutant, crp-complemented strain, or hfq mutant by using real-time RT-PCR. The mRNA level for the wild-type strain was set at 1, and all other data are expressed relative to this value. N.D., not determined. (c) crp promoter activities of wild-type P. mirabilis, crp mutant, crp-complemented strain, and hfq mutant. The activities of XylE in the crp-xylE reporter plasmid-transformed P. mirabilis strains were determined using the reporter assay at 7 h after incubation. The value obtained for the wild-type strain was set at 1. In (b) and (c), the data are the averages and standard deviations of three independent experiments. Significant difference from the wild-type strain is indicated with an asterisk (*P < 0.05; **P < 0.01 by using Student’s t test). wt, wild-type; crp, crp mutant; crpc, crp-complemented strain; hfq, hfq mutant.
To investigate the effect of crp loss on the expression of cyaA, hfq, and ptsG, we assessed the mRNA levels of cyaA, hfq, and ptsG by using real-time reverse transcription polymerase chain reactions (RT-PCRs) in wild-type P. mirabilis, crp mutant, and crpc strains. We observed that Crp positively regulated the expression of ptsG mRNA and negatively regulated that of hfq and cyaA (Fig. 1b). The XylE reporter assay revealed a positive autoregulation in crp itself (Fig. 1c). In addition, we observed that the loss of hfq reduced both the mRNA level (Fig. 1b) and promoter activity (Fig. 1c) of crp. Collectively, these findings imply that the expression of P. mirabilis ptsG, cyaA, and hfq can be modulated by Crp-cAMP under normal LB culture conditions. Moreover, Crp is positively autoregulated and also upregulated by Hfq.
The loss of crp has been demonstrated to have metabolic defects in some bacterial species20, 33, 34. Accordingly, a metabolic analysis with the VITEK GN ID card (bioMérieux) revealed that the P. mirabilis crp mutant had defects in citrate utilization, trehalose fermentation, succinate alkalinization, and activities of ornithine decarboxylase and γ-glutamyl transferase (data not shown). Furthermore, we used a transcriptome to compare the gene expression of wild-type P. mirabilis in the presence of 10% glucose (wt-10% glc) and the crp mutant. Total RNA was extracted and subjected to differential RNA-sequencing (RNA-seq) analysis35. Supplementary Fig. S1e presents a scattergram of the gene expression of the 10% glucose-treated wild-type strain (x-axis) and crp mutant (y-axis) relative to the wild-type strain (log2 fold-change values). Among 3217 genes detected in wild-type P. mirabilis by using RNA-seq, 1253 genes were upregulated (quadrant I) and 628 genes were downregulated (quadrant III) in both the 10% glucose-treated wild-type strain and crp mutant. A correlation (up to 60%) was observed between the gene expression profiles of the 10% glucose-treated wild-type strain and crp mutant. In other words, an up to 60% correlation of gene expression profiles between the 10% glucose-treated wild-type strain and crp mutant was observed, and more than 40% of the genes were expressed in an opposite manner between them. When more than twofold selection was applied to the expression change, 202 genes were upregulated (quadrant I) and 211 genes were downregulated (quadrant III) in both the 10% glucose-treated wild-type strain and crp mutant. Moreover, 64 genes exhibited an opposite expression trend between the 10% glucose-treated wild-type strain and the crp mutant both in quadrants II and IV (Supplementary Excel file).
The crp mutant exhibited an increased ability to colonize the kidneys of nondiabetic mice, and Crp might play a role in kidney colonization in diabetic mice
Proteus spp. are among the most common pathogens in diabetic patients with UTIs24, 27, 28. Because Crp is often linked to virulence factor production in pathogenic bacteria4–7, 36 and because its activity is associated with glucose levels, we hypothesized that P. mirabilis Crp-cAMP (correlated with glucose levels) controls the expression of virulence factors for establishing UTIs in the diabetic host. Therefore, we studied streptozotocin-induced diabetic ICR mice, whose blood and urine glucose levels were approximately 440.17 and more than 1000 mg/dL, respectively (approximately 152 and less than 50 mg/dL for nondiabetic mice, respectively). First, we tested whether high-glucose conditions alter P. mirabilis Crp activity in diabetic mice. The mice were injected transurethrally with wild-type P. mirabilis harboring the plac-gfpuv reporter plasmid, and the gfpuv mRNA levels of P. mirabilis in the bladders and kidneys were measured. The results indicated that the Crp activity (shown as the gfpuv mRNA level) of P. mirabilis in diabetic mice significantly decreased relative to that in nondiabetic mice (Fig. 2a). Thus, P. mirabilis Crp activity was modulated by high glucose in vivo and could be involved in UTIs. Subsequently, we injected diabetic and nondiabetic ICR mice with wild-type P. mirabilis and the crp mutant, respectively, to examine the effect of crp loss on the urinary tract colonization of mice. On day 3 after inoculation, the mice were sacrificed to collect kidney and bladder samples, and the samples were homogenized to determine the viable bacterial count. Diabetic mice were more susceptible to kidney and bladder colonization by wild-type P. mirabilis than nondiabetic mice (Fig. 2b and c). The loss of crp abolished this phenomenon in kidneys but not in bladders. Notably, crp mutant colonization of nondiabetic mice significantly increased in the kidneys (p < 0.05) (although the difference was small) and nonsignificantly decreased in the bladder, compared to the wild-type strain. No difference in the ability to colonize the bladders and kidneys of diabetic mice was observed between the wild-type strain and the crp mutant, possibly due to the downregulation of Crp activity under high-glucose conditions in diabetic mice. In summary, P. mirabilis crp expression and Crp activity were involved in kidney colonization in diabetic mice, and the loss of crp increased kidney colonization in nondiabetic mice. We then investigated the underlying mechanisms.
Figure 2.
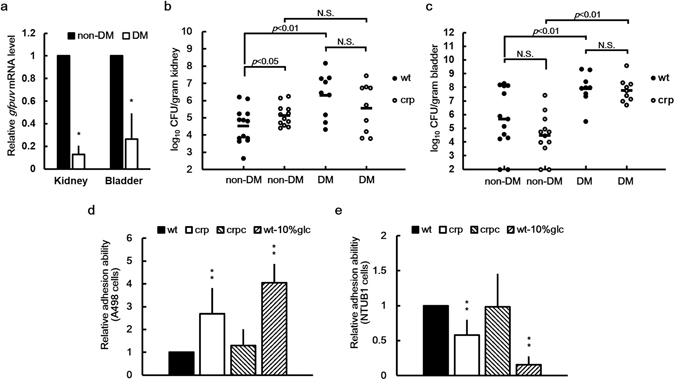
Crp activity, urinary tract colonization, and urothelial cell adhesion of P. mirabilis were affected by the diabetic environment, loss of crp or the high glucose level. (a) Crp activity of P. mirabilis was decreased in the kidneys and bladders of diabetic mice. Six-week-old female mice were injected transurethrally with overnight cultures of the plac-gfpuv reporter plasmid-transformed wild-type P. mirabilis strains (107 CFU per mouse). On day 3 after injection, the mice were sacrificed, and bladder and kidney samples were collected to quantify the mRNA content of gfpuv using real-time RT-PCR. The mRNA level for each organ of nondiabetic mice was set at 1. The data are the averages and standard deviations of three independent experiments. The significant difference between nondiabetic mice (non-DM) and diabetic mice (DM) was observed using Student’s t test (*P < 0.05). Kidney (b) and bladder (c) colonization in DM and non-DM by wild-type P. mirabilis (wt) or crp mutant (crp). Diabetic or nondiabetic ICR mice (at least nine mice per group) were inoculated transurethrally with overnight cultures of bacteria at a dose of 107 CFU per mouse. Bacterial loads (CFU) in the kidneys and bladders were determined on day 3 after inoculation. Horizontal bars indicate the average for each group, and the limit of detection was 100 CFU/g organ. Significant differences were determined using the Mann–Whitney test, except for the difference between colonization by the wild-type strain and crp mutant in the kidneys of nondiabetic mice as determined using the Student’s t test. N.S., no significant difference. Adhesion of wild-type in the absence (wt) or presence of 10% glucose (wt-10%glc), the crp mutant (crp), and the crp-complemented strain (crpc) to kidney (d) and bladder (e) epithelial cells. Adhesion abilities to A498 cells and NTUB1 cells were determined as described in the Materials and Methods section. The adhesion ability of the wild-type strain was set at 1 and other data are relative to this value. The data represent the averages and standard deviations of three independent experiments. The significant difference from the wild-type strain is indicated with an asterisk (**P < 0.01, using Student’s t test).
Loss of crp or high glucose reduced swarming, production of flagellin/flagella, and flhDC expression in P. mirabilis
In general, the flagella of bacterial pathogens assist in colonization and dissemination within the host13. In addition, antigenic variation through flagellin gene rearrangement enables P. mirabilis to evade host immune response during catheter-associated UTIs13. Because Crp-cAMP regulates the expression of flagella in E. coli and S. marcescens 21, 22, swimming and swarming assays were performed. The crp mutant exhibited almost no swimming (Supplementary Fig. S4) and swarming (Fig. 3a) motility compared with the wild-type and crpc strains. In addition, 10% glucose had an obvious inhibitory effect on swarming and swimming motility (Figs 3a and S4). We then examined the expression of flhDC by determining the promoter activity and mRNA level. We observed that flhDC expression was significantly reduced in the crp mutant compared with the wild-type and crpc strains (Fig. 3b,c). An electrophoretic mobility shift assay (EMSA) with purified His-tagged Crp and the infrared (IR) dye-labeled flhDC promoter DNA fragment was conducted to determine whether Crp-cAMP can directly bind the putative flhDC promoter in P. mirabilis. Polyacrylamide gel electrophoresis (PAGE) revealed a band shift after the labeled flhDC promoter DNA was incubated with adequate His-tagged Crp, and the unlabeled flhDC promoter DNA acted as a competitor in reducing the binding of the IRDye-labeled flhDC promoter DNA with His-Crp (Fig. 3d), in the presence of a predicted Crp binding site in the flhDC promoter region (Fig. 3e). No band shift was observed in a sample from the IRDye-labeled negative control DNA incubated with His-Crp (Supplementary Fig. S7). Accordingly, an analysis of flagellin expression through sodium dodecyl sulfate-PAGE revealed that the crp mutant produced almost no flagellin compared with the wild-type and crpc strains (Fig. 3f). Further transmission electron microscopy validated that the crp mutant lacked flagella (Fig. 3g). In contrast to the fewer flagella seen in the 10% glucose-treated wild-type strain (Fig. 3g), the wild-type and crpc strains had many flagella. Notably, the crp mutant cells failed to differentiate into elongated swarmer cells at 4 h after inoculation as opposed to the wild-type strain (Fig. 3g). Moreover, transcriptome analysis indicated that the loss of crp reduced the flhDC mRNA level (Supplementary Excel file). These findings suggest a contribution by the downregulation of flagella in the crp mutant to immune evasion and consequent colonization during infection.
Figure 3.
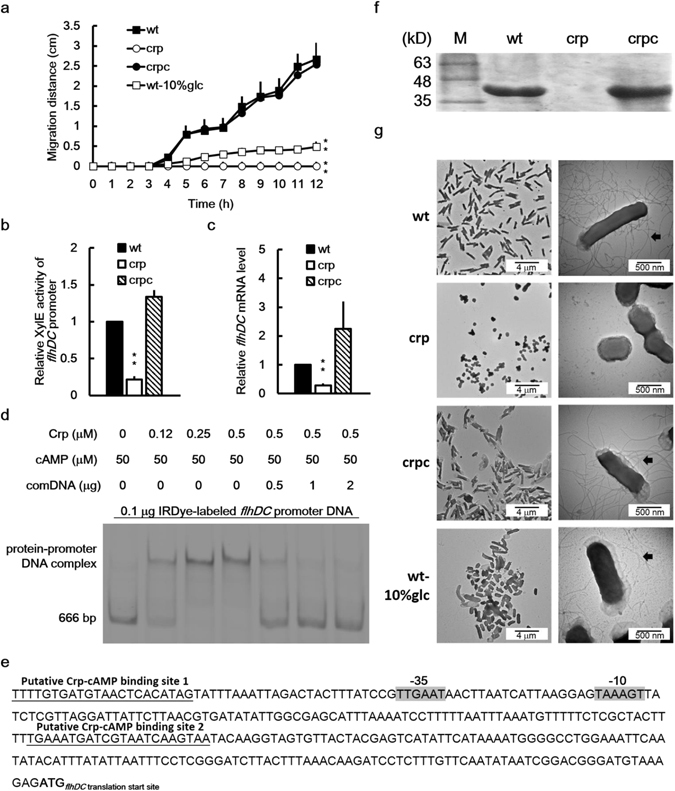
Loss of crp or high glucose reduced swarming, production of flagellin/flagella and flhDC expression in P. mirabilis. (a) Swarming migration of the wild-type in the absence or presence of 10% glucose, crp mutant and the crp-complemented strain. An aliquot (5 μl) of overnight cultures was inoculated centrally onto the swarming plate. The migration distance was measured hourly after inoculation. The significant difference from wild-type is indicated with an asterisk at 12 h. (b) Promoter activities of flhDC in wild-type, crp mutant, and crp-complemented strain. The activities of XylE in the flhDC-xylE reporter plasmid-transformed bacterial strains were determined using the reporter assay at 7 h after incubation. The value obtained for the wild-type was set at 1. (c) The flhDC mRNA levels in the wild-type, crp mutant, and crp-complemented strain. The flhDC mRNA amounts were quantified using real-time RT-PCR at 7 h after incubation. The value obtained for the wild-type was set at 1. In (a),(b) or (c), the data represent the averages and standard deviations of three independent experiments. Significant difference from the wild-type is indicated with an asterisk (**P < 0.01 by Student’s t test). (d) The binding of P. mirabilis Crp-cAMP to the flhDC promoter region revealed using an EMSA. IRDye-labeled DNA fragments (0.1 μg) of the flhDC promoter region (666 bp) obtained by PCR were incubated with the purified His-tagged Crp (0–0.5 μM) in the presence of cAMP. The protein-DNA complex was resolved on a 5% non-denaturing polyacrylamide gel and the gel image was obtained by the quantitative infrared fluorescent imaging system. The unlabeled flhDC promoter DNA acted as a competitor to verify the binding specificity. comDNA, competitive DNA fragments. (e) A diagram showing the Crp-cAMP binding sites upstream of flhDC gene. The putative Crp-cAMP binding sequences are underlined and the putative −10 and −35 promoter sequences of sigma 70 are shadowed. (f) The flagellin levels of wild-type, crp mutant and crp-complemented strain. Flagellin levels were examined after seeding on the swarming plates for 4 h by the SDS-PAGE. The band of flagellin is 40 kD. M, molecular weight marker. (g) TEM pictures of wild-type in the absence or presence of 10% glucose, crp mutant and the crp-complemented strain. Bacterial cultures were applied onto a carbon-coated grid, cells were stained with 1% PTA and TEM pictures were taken. Flagella are indicated by arrows. In (a–c,f and g), wt, wild-type; wt-10%glc, wild-type with 10% glucose; crp, crp mutant; crpc, crp-complemented strain. Full-length gels for (d) and (f) are shown in Supplementary Fig. S8.
Loss of P. mirabilis crp increased adhesion to kidney epithelial cells and mRNA expression of Pmp fimbriae
Because P. mirabilis Crp activity was involved in kidney colonization in diabetic mice and the loss of crp affected kidney colonization in nondiabetic mice, we determined the Crp-associated virulence factors participating in mouse colonization. Adherence is a key initiating step in urinary tract colonization. Accordingly, critical components of P. mirabilis pathogenesis center on fimbriae, which mediate adherence to epithelial cells of the urinary tract as well as catheters15, 17, and loss of Mrp fimbriae correlates with decreased bladder colonization of mice37. Therefore, we first performed a cell adhesion assay by using kidney epithelial cells (A498) and bladder urothelial cells (NTUB1). We observed that the crp mutant exhibited an increased adhesion ability to A498 cells but a decreased adhesion ability to NTUB1 cells compared with the wild-type and crpc strains; similarly, 10% glucose increased the adhesion of the wild-type strain to A498 cells but reduced the adhesion to NTUB1 cells (Fig. 2d and e). The data suggest that Crp-regulated adhesion contributes to kidney colonization. Although a synergistic relationship might exist between type 1 and P fimbriae for colonization and subsequent UTIs, UPEC has the potential to predominantly express P fimbriae during the infection of the kidney epithelium16, 38. Consequently, we searched for the pmp locus in the P. mirabilis HI4320 genome. An analysis of the deduced amino acid sequences revealed that the locus may encode nine proteins, with 60%, 59%, 51%, 73%, 78%, 50%, 56%, 55%, and 41% similarity to UPECJ96 (accession no. ALIN02000070) papI, papA, papH, papC, papD, papJ, papK, papF, and papG, respectively (Fig. 4a). In view of activating papA expression by Crp in E. coli 39, we examined the effect of crp loss on pmpA expression, encoding the major subunit protein of P. mirabilis P-like fimbriae, in the wild-type strain, crp mutant, and crpc strain by using real-time RT-PCR and a pmpA-xylE reporter assay. The results indicate that Crp negatively regulated pmpA expression (Fig. 4b,c). An EMSA with a negative control (Supplementary Fig. S7) revealed a visible band shift in the sample of the labeled pmpA promoter DNA incubated with His-Crp and that the unlabeled pmpA promoter DNA can act as a competitor to reduce the binding of the IRDye-labeled pmpA promoter DNA with His-Crp (Fig. 4d). We further probed the binding site of Crp in the upstream region of pmpA using the DNase I footprinting assay. The assay revealed two Crp binding sites located between −95 and −142 upstream of pmpA start codon (Fig. 4e). The Crp binding sites of the pmpA promoter overlapped the −10 and −35 regions of the putative pmpA promoter (Fig. 4f), as opposed to the binding site upstream of the −10 and −35 regions of the E. coli papA promoter39.
Figure 4.
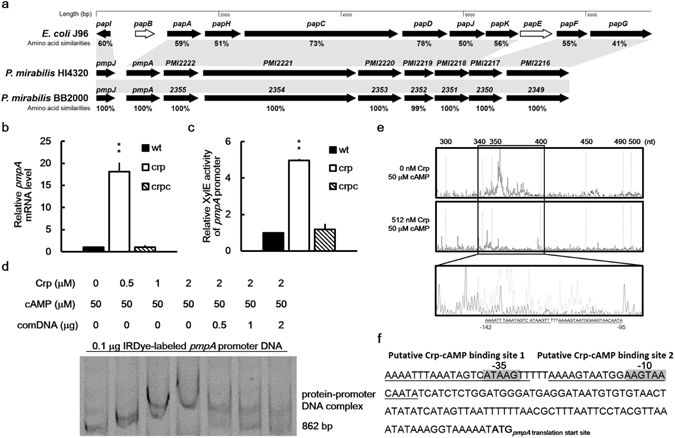
Regulation of pmpA expression by P. mirabilis Crp-cAMP. (a) The P-like fimbrial gene locus (pmp) similar to the well characterized pap locus in UPEC J96 in P. mirabilis genome. An amino acid sequence analysis of the pmp locus in P. mirabilis HI4320 (accession no. AM942759) and its counterparts in E. coli J96 (accession no. ALIN02000070) and P. mirabilis BB2000 (accession no. NC_022000) was performed using position-specific iterative BLAST. The nine proteins of the pmp locus in P. mirabilis HI4320 or BB2000 are similar to the PapI, PapA, PapH, PapC, PapD, PapJ, PapK, PapF and PapG in E. coli J96 with corresponding genes in shadows. The percent amino acid similarities between P. mirabilis HI4320 and E. coli J96 or P. mirabilis BB2000 were shown below each gene. The white arrows represent genes that are not found in either P. mirabilis HI4320 or P. mirabilis BB2000. (b) Loss of crp increased the pmpA mRNA level. The pmpA mRNA levels of the wild-type, crp mutant, and crp-complemented strain were quantified using real-time RT-PCR at 24 h after incubation. The value obtained for the wild-type was set at 1. (c) Loss of crp increased the promoter activity of pmpA. The activities of XylE in the pmpA-xylE reporter plasmid-transformed wild-type, crp mutant, and crp-complemented strain were determined using the reporter assay at 7 h after incubation. The value obtained for the wild-type was set at 1. In (b and c), the data represent the averages and standard deviations of three independent experiments. The significant difference from the wild-type was determined by Student’s t test (**P < 0.01). wt, wild-type; crp, crp mutant; crpc, crp-complemented strain. (d) The binding of P. mirabilis Crp-cAMP to pmpA promoter region revealed using an EMSA. The EMSA was performed as described in Fig. 3d, except that IRDye-labeled pmpA promoter region DNA fragments (862 bp) were incubated with 0–2 μM of the purified His-tagged Crp. (e) Identification of the Crp-cAMP binding site in the pmpA promoter region by a DNase I footprinting assay. FAM was used to label the pmpA promoter DNA fragment and the DNA fragment was incubated with cAMP (50 μM) with or without the recombinant Crp (512 nM) followed by DNase I treatment. The mixture was subject to electrophoresis. The fluorescence intensity of the FAM-labeled DNA fragment (ordinate) was plotted against the sequence length of the fragment. Two Crp-cAMP binding sites (underlined) located between −95 and −142 upstream of pmpA start codon were shown in an expanded view. (f) A diagram showing the Crp-cAMP binding sites upstream of pmpA gene. The putative Crp-cAMP binding sequences are underlined and the putative −10 and −35 promoter sequences of sigma 70 are shadowed. The full-length gel for (d) is shown in Supplementary Fig. S8.
To verify the significance of Crp-regulated PmpA in adhesion to A498 cells, we performed a cell adhesion assay involving an isogenic pmpA mutant (Supplementary Fig. S3) and pmpA/crp double mutant. A nearly 50% reduction in adhesion was observed for the pmpA mutant compared with the wild-type strain (Fig. 5a), and the adhesion ability of the pmpA/crp double mutant was comparable to that of the pmpA mutant (Fig. 5a). Subsequently, we assessed whether Pmp fimbriae (PmpA) played an important role in kidney colonization in mice. Kidney colonization by the pmpA mutant was significantly attenuated compared with that by the wild-type strain (Fig. 5b). In conclusion, a large part of the increased adhesion to kidney cells of the crp mutant could be attributed to the upregulation of Pmp fimbriae. Moreover, the transcriptome analysis through RNA-seq indicates that pmp genes were upregulated in both the crp mutant and 10% glucose-treated wild-type strain (Supplementary Excel file).
Figure 5.
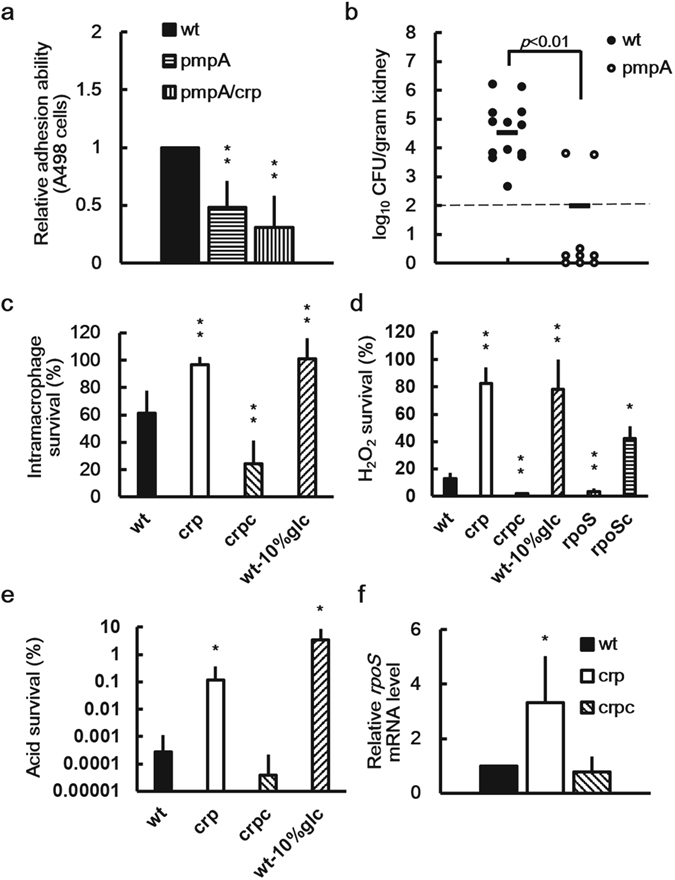
Loss of pmpA abolished the increased kidney adhesion of crp mutant and decreased kidney colonization, and crp mutant displayed enhanced stress tolerance and upregulation of RpoS. (a) Adhesion of wild-type P. mirabilis, pmpA mutant or pmpA/crp double mutant to A498 cells. Adhesion abilities were determined as in Fig. 2. (b) Mouse kidney colonization by the wild-type or pmpA mutant. Colonization was determined as in Fig. 2b. (c) Intramacrophage survival of the wild-type in the absence or presence of 10% glucose, crp mutant and crp-complemented strain. THP-1 cells were infected with bacteria for 30 min at an MOI of 10. Intramacrophage survival was determined as the percentage of viable bacteria that survived in the macrophages for 4 h versus those for 1 h after outside bacteria were killed with streptomycin. (d) Survival in H2O2 of wild-type in the absence or presence of 10% glucose, crp mutant, crp-complemented strain, rpoS mutant and rpoS-complemented strain. Overnight cultures were regrown and adjusted to a density of 108 cells/ml before cells were exposed to 30 mM H2O2 for 20 min at 37 °C. The percent cell survival was determined by colony counting relative to the untreated control. (e) Survival in acid of wild-type in the absence or presence of 10% glucose, crp mutant and crp-complemented strain. Overnight bacterial cultures were regrown before cells were challenged with acid (pH 3.0) for 2 h. The percent cell survival was obtained as in (d). (f) Loss of crp increased the rpoS mRNA level. The mRNA amounts in the wild-type, crp mutant and crp-complemented strain were quantified sing real-time RT-PCR at 3 h after incubation. The value obtained for the wild-type was set at 1. In (a,c–f), the data represent the averages and standard deviations of three independent experiments. The significant difference from the wild-type was determined by Student’s t test (*P < 0.05; **P < 0.01). wt, wild-type; pmpA, pmpA mutant; crp, crp mutant; pmpA/crp, pmpA/crp double mutant; crpc, crp-complemented strain; wt-10%glc, wild-type with 10% glucose; rpoS, rpoS mutant; rpoSc, rpoS-complemented strain.
Loss of crp increased survival of P. mirabilis in macrophages
Successful colonization of the urinary tract requires P. mirabilis to overcome a barrage of innate host defenses, including killing by macrophages and the generation of reactive oxygen species (ROS)13. To determine whether Crp is involved in counteracting the innate ability of macrophages to eliminate P. mirabilis, we challenged macrophages with the wild-type strain or its derivatives, killed bacteria outside the macrophages with streptomycin, and assessed the survival of internalized bacteria. No significant difference was observed between the colony-forming units (CFU) of the wild-type strain and crp mutant within macrophages immediately after outside bacteria were killed with streptomycin. The relative survival of the wild-type strain was reduced to approximately 60% at 4 h versus 1 h after outside bacteria were killed with streptomycin (Fig. 5c). However, the survival of the crp mutant or 10% glucose-treated wild-type strain remained 100% (Fig. 5c). The crpc strain exhibited a significant reduction in relative survival compared with the wild-type strain, possibly because of the overexpression of Crp in the crpc strain (Fig. 1b).
Loss of crp increased the resistance of P. mirabilis to H2O2 and acid
Because crp expression and Crp activity affected the intramacrophage survival of P. mirabilis, we investigated whether Crp was involved in coping with challenges within the urinary tract or macrophages such as oxidative and acid stresses during infection. The survival of the wild-type strain, crp mutant, and crpc strain was assessed after exposure to H2O2 or acid. The loss of crp significantly increased survival after exposure to 30 mM H2O2 or HCl at pH 3 (Fig. 5d,e). Furthermore, the 10% glucose-treated wild-type strain was more resistant to H2O2 and acid than the wild-type strain (Fig. 5d,e). The crpc strain was nearly as susceptible to stresses as the wild-type strain or more susceptible than the wild-type strain (Fig. 5d,e).
Loss of crp increased the expression of sigma factor RpoS of P. mirabilis
Crp has been reported to regulate the expression of the general stress response regulator RpoS of E. coli 40. We demonstrated that loss of rpoS rendered P. mirabilis more sensitive to H2O2 than the wild-type strain (Fig. 5d). Previously, we found that P. mirabilis Hfq positively regulated the expression of rpoS mRNA41. In the present study, we demonstrated that Crp negatively regulated the expression of hfq mRNA (Fig. 1b) and Crp participated in the tolerance to H2O2 and acid (Fig. 5d,e). Hence, we investigated the effect of crp loss on the expression of rpoS. We observed that rpoS mRNA level was increased in the crp mutant compared with the wild-type and crpc strains (Fig. 5f). Accordingly, transcriptome data also revealed that the loss of crp increased the expression of rpoS (Supplementary Excel file). These results suggest that the loss of crp or high-glucose conditions (low Crp activity) protect P. mirabilis from stressful conditions (acid or H2O2) through the RpoS-dependent pathway.
Discussion
The Crp regulatory system is a paradigm of bacterial gene regulation and was extensively investigated in E. coli and Salmonella in a prior study42. However, despite decades of intensive studies, new information has continued to emerge. Crp-cAMP was previously coopted to control different regulons in bacteria that occupy distinct niches4. In this study, we characterized P. mirabilis Crp in terms of the response to glucose, gene regulation, and virulence traits including host colonization, fimbria-mediated adhesion, stress tolerance, and motility. We provided several lines of evidence to demonstrate the Crp counterpart in uropathogenic P. mirabilis. First, P. mirabilis Crp shares a high sequence identity with its homologue in other bacteria. Second, P. mirabilis Crp activity was associated with the glucose level, and the loss of crp caused defects in growth and metabolism. Third, P. mirabilis Crp-cAMP regulated the expression of ptsG, cyaA, and hfq similarly to how it did in E. coli. In addition, P. mirabilis Hfq positively regulated the expression of crp, as in Yersinia 7. In the normal LB culture condition, the CyaA of P. mirabilis can exist in an active form, catalyzing the conversion of ATP to cAMP. Once the Crp-cAMP complex was formed, CyaA positively regulated the expression of ptsG, but negatively regulated the expression of hfq and cyaA, possibly by binding to the respective promoter region. In E. coli, extracellular glucose is taken up by the PtsG transporter. High glucose levels inhibit CyaA activity, resulting in decreased cAMP levels and the repression of PtsG, thus forming the feedback regulation of glucose uptake10. Our data suggest that the feedback regulation of PtsG by Crp-cAMP in glucose uptake exists in P. mirabilis. Many studies have demonstrated that crp expression must be appropriately controlled to ensure optimal cell physiology. Environmental alterations during infection could serve as signals to activate the TCS or trigger the expression of regulatory small RNAs (sRNAs) that influence Crp expression in P. mirabilis. For example, Y. pestis crp is regulated by the PhoPQ TCS11. Our data suggest that Hfq-dependent sRNAs are likely to posttranscriptionally stimulate Crp synthesis, thereby affecting the expression of the Crp regulons such as pmp during infection. Additional studies are required to further delineate the sRNAs that contribute to the regulation of Crp and the host signals detected by P. mirabilis to influence the Crp regulon.
Crp is a regulator of not only genes involved in the catabolism of sugars1, 3 but also a multitude of genes associated with stress tolerance and virulence2, 3, 43, 44. The involvement of Crp, a bacterial factor, in kidney rather than bladder colonization in diabetic mice, as well as increased colonization in nondiabetic mice in the kidney but not in the bladder by the crp mutant, as revealed in this study (Fig. 2b,c), indicates the complexity of gene regulation by Crp in different niches. UPEC Crp-cAMP was reported to be responsible for effective bladder colonization in a mouse infection model, probably due to the regulatory effects of Crp-cAMP on factors required for resistance to stresses generated in the niche36. In this study, bladder colonization in nondiabetic mice by the P. mirabilis crp mutant was not significantly attenuated (Fig. 2c); however, Mrp fimbriae (a factor for bladder colonization by P. mirabilis) were downregulated in the crp mutant (Supplementary Fig. S2). Other factors, such as resistance to H2O2 and killing by macrophages, may counteract the effect of Mrp fimbriae on bladder colonization by the crp mutant in nondiabetic mice. Notably, the P. mirabilis crp mutant was more successful in colonizing the kidneys of nondiabetic mice than the wild-type strain (Fig. 2b). Yet the effect of the loss of crp on kidney colonization remains to be addressed. Our data suggest that Crp-regulated P-like fimbriae (Pmp), survival in macrophages, stress tolerance, and immune evasion due to flagellum deficiency are involved in this process. Our study was the first to demonstrate the inhibition of P. mirabilis Crp activity in the presence of high glucose levels (Fig. 1a) and its significantly lower level in diabetic mice than in nondiabetic mice (Fig. 2a). Because the urine glucose concentration of normal adults ranges from 0.002% to 0.02% (2–20 mg/dL) and that in people with diabetes is higher than 3.14% (3140 mg/dL) (http://www.urinemetabolome.ca/), Crp inactivation might be biologically significant, leading to increased kidney colonization and subsequent infection in patients with diabetes.
P fimbriae, as a prominent feature of UPEC, mediate its adherence and persistence in mammalian kidneys16, 45. P fimbria adhesin (PapG) is known to bind to the globoseries of glycolipids abundantly expressed on the surface of kidney cells46. The similarity of P. mirabilis putative adhesin encoded by PMI2216 (one gene of the pmp operon) to PapG indicates that P. mirabilis P-like fimbriae (Pmp) might mediate adhesion to kidney cells, as in UPEC. Hence, we conducted the first investigation of the influence of P. mirabilis P-like fimbriae on kidney adhesion and colonization and the regulation of P-like fimbriae by Crp. Several lines of evidence in this study support that the upregulation of P-like fimbriae in the crp mutant accounts for a large part of the enhanced kidney colonization. First, the crp mutant exhibited increased adhesion to kidney cells (Fig. 2d), and the pmpA mutant exhibited a reduced kidney adhesion ability comparable to that of the pmpA/crp double mutant (Fig. 5a). Second, the loss of crp upregulated pmpA expression (Fig. 4b,c). Furthermore, EMSA (Fig. 4d) and DNase I footprinting (Fig. 4e) validated that Crp can bind to the Crp box located in the promoter region of pmpA (Fig. 4f). Third, colonization of the kidney by the pmpA mutant was significantly attenuated (Fig. 5b). The upregulation of pmpA (P-like fimbriae) expression in the crp mutant also implies that the increased kidney colonization in diabetic mice by wild-type P. mirabilis compared with nondiabetic mice might have resulted from the increased expression of P-like fimbriae in the diabetic condition (high glucose) with reduced Crp activity.
The UPEC Crp-cAMP complex plays a positive regulatory role in pap transcription47. Unlike UPEC, the expression of P. mirabilis pmpA was under the negative control of Crp-cAMP. Because Crp-cAMP can act as an activator or a repressor1–3, the mode of transcriptional regulation by Crp-cAMP could correlate with its DNA binding site relative to the promoter of the target gene48. The discrepancy of P fimbria regulation by Crp between P. mirabilis and E. coli may be because of the overlapping of the Crp-cAMP binding site with the −35 and −10 regions of the pmpA promoter in P. mirabilis but not in UPEC49. The E. coli pap promoter is highly regulated by numerous factors, including PapI, Lrp, Cpx, and Crp47, 49, 50. Recently, RcsB, a two-component response regulator, was reported to increase pmpA expression in P. mirabilis (www.ncbi.nlm.nih.gov/geo/; accession number: GSE76341). Investigation of the molecular mechanisms of pmp expression in P. mirabilis is therefore warranted.
Bacterial flagellin is recognized by Toll-like receptor 5, resulting in inflammatory cytokine production, immune cell recruitment to the infectious site, and elimination of the invading bacteria51. In the urinary tract, the immune mechanisms of the host favor the removal of bacteria expressing immunogenic surface structures such as flagella13. In this regard, we observed that the P. mirabilis crp mutant produced almost no flagella (Fig. 3g). This finding implies that Crp participates in the regulation of expression of the surface flagellin protein to alter the immunogenicity of the bacterium for preventing clearance by the host. Similarly, Crp-cAMP regulates flagellin expression in E. coli and S. marcescens 21, 22. Although flagella are believed to contribute to the virulence of uropathogenic P. mirabilis by allowing movement from the catheter to the bladder and the kidney in ascending UTIs, P. mirabilis mutants lacking flagella were demonstrated to cause ascending UTIs in mice52. Additional studies are required to determine the movement of the P. mirabilis crp mutant lacking flagella to the kidneys.
Although P. mirabilis is not an intracellular pathogen, intramacrophage survival during early infection was proposed to be an important fitness index53. The increased number of surviving crp mutant cells inside macrophages and the increased ability of the crp mutant to withstand H2O2 and acid (Fig. 5c,d and e) indicate that the loss of crp can improve the fitness of P. mirabilis during infection. RpoS, a bacterial stationary phase sigma factor, regulates the expression of various genes to enable bacteria to overcome multiple environmental stresses, including ROSs such as H2O2 54, 55. In addition, rpoS transcription was demonstrated to be regulated by Crp-cAMP56, and Crp could inhibit RpoS expression through Spf, an Hfq-dependent sRNA that facilitates acid resistance by activating RpoS expression in E. coli 40. Our unpublished data also revealed that P. mirabilis Crp negatively regulated Spf expression, and the loss of hfq reduced the Spf RNA level. The previous finding that P. mirabilis Hfq positively regulated RpoS expression41, along with the present study’s findings of Crp-mediated negative regulation of hfq mRNA expression, increased tolerance of the crp mutant to H2O2 and upregulation of rpoS mRNA in the crp mutant (Fig. 5f), and decreased H2O2 resistance in the rpoS mutant (Fig. 5d), suggest that the upregulation of the RpoS pathway through Hfq due to the loss of crp strengthens the resistance of P. mirabilis to stressful environments.
This is the first study to report that P. mirabilis Crp can regulate multiple virulence traits, including colonization of mice, production of flagella, fimbria-mediated adhesion, survival in macrophages, and stress tolerance in response to glucose. We demonstrated that the upregulation of P-like fimbriae, enhanced stress tolerance, and host immune evasion due to flagellum deficiency in the crp mutant facilitated kidney colonization. Hence, the reduced Crp activity in a high-glucose environment in diabetic mice may contribute to the increased kidney colonization by P. mirabilis. Moreover, this study shows that P. mirabilis is capable of optimizing metabolic and virulence gene expression through the Crp regulon to link catabolite repression with multiple regulatory pathways involving Hfq and sRNAs. Understanding the regulatory network that governs the expression of virulence or colonization factors might facilitate the development of novel strategies for treating bacterial infections. Therefore, we suggest that P-like fimbriae can serve as a target for the design of antibacterial strategies. For this purpose, a recombinant strain E. coli 83972::lgtCE, a nonpathogenic E. coli strain exhibiting a P fimbria receptor mimic on its surface46, may act as a prophylactic agent for preventing UTIs caused by P. mirabilis.
Methods
Bacterial strains, plasmids, reagents and growth conditions
The bacterial strains and plasmids used in this study are listed in Supplementary Table S1. Construction of P. mirabilis mutants and crp-complemented strain were described in the Supplemental Material. All chemicals were obtained from the Sigma-Aldrich unless otherwise indicated, and primer sequences are listed in Supplementary Table S2. Bacteria were routinely cultured in Luria-Bertani (LB) broth at 37 °C. The LSW− agar was used to prevent the phenotypic expression of swarming motility for selecting mutant clones and determining colony forming units (CFU). Ampicillin (100 μg/ml), chloramphenicol (20 μg/ml), gentamicin (50 μg/ml), kanamycin (100 μg/ml), tetracycline (20 μg/ml), or glucose (2 or 10% (2000 or 10000 mg/dl)) was added to the medium as needed.
Real-time reverse transcription PCR (RT-PCR)
To study the effect of crp loss on the expression of ptsG, hfq, cyaA, crp, pmpA, flhDC, rpoS and mrpA mRNAs, overnight LB cultures of the wild-type, crp mutant and the crpc strain were diluted 100-fold in the same medium and incubated for 3 h (rpoS), 7 h (ptsG, hfq, cyaA, crp, flhDC), or 24 h (pmpA, mrpA) at 37 °C. Total RNA was extracted, and real-time RT-PCR was carried out as described previously57 to monitor the mRNA expression normalized against the gyrB mRNA.
GFPuv and XylE reporter assays
Because the lac promoter is positively regulated by Crp-cAMP, a plac-gfpuv reporter plasmid was constructed to assess the effect of glucose on the activity of Crp-cAMP in P. mirabilis by measuring GFP fluorescence intensity. gfpuv gene fragment was obtained by PCR from pGFPuv (Clontech) and cloned into the TA cloning vector, pGEM-T Easy, to produce pGgfpuv, a plac-gfpuv reporter plasmid; gfpuv gene is thus driven by the lac promoter in the plasmid. The wild-type P. mirabilis transformed with pGgfpuv was grown overnight in LB broth containing ampicillin. The cultures were diluted 100-fold in the same medium with 0%, 2%, or 10% glucose and incubated for 3, 5, 7, 9, 11 and 24 h at 37 °C. One milliliter of the cell suspension at each time point was centrifuged and the pellet was re-suspended in sterile phosphate-buffered saline (PBS) (1 ml) for fluorescence intensity determination (excitation at 395 nm and emission at 507 nm) normalized against the OD600 value.
For the XylE reporter assay, the promoter region of the crp, pmpA or flhDC was amplified using SphI and PstI-included primer pairs (Supplementary Table S2) and cloned into the pGEM-T Easy. These promoter-containing plasmids were cut by SphI and PstI, and the promoter-containing fragment was cloned, respectively to the xylE-containing pACYC184 to construct the different reporter plasmids. The wild-type, crp mutant, and crpc strain were transformed with respective reporter plasmids and grown overnight in LB broth containing chloramphenicol. The cultures were diluted 100-fold in the same medium and incubated for 7 h at 37 °C. The XylE activity was measured as described previously58.
Animal experiments
Induction of diabetes in ICR mice was performed as described previously59 with modifications. Six-week-old female mice were treated with two consecutive daily intravenous (i.v. 125 mg/kg body weight) injections of streptozotocin (STZ, Sigma-Aldrich) freshly dissolved in sodium citrate buffer (50 mM sodium citrate, pH 4.5) to induce β-cell death of the pancreatic islet. Control mice were treated with the same amount (50 μl) of citrate buffer without STZ. On day one, all food except water was removed from cages at 4 h prior to STZ treatment. After injection, all mice were fed with a normal diet. Blood and urine glucose levels were measured daily after three days of the second STZ injection. Mice with two consecutive blood glucose levels ≥250 mg/dl were considered diabetic. All STZ-injected mice became diabetic within 1 week after the second STZ injection.
The mouse model of UTIs was used as described previously60. Six-week-old female ICR mice were injected transurethrally with overnight cultures of bacteria at a dose of 107 CFU per mouse. On day 3 after injection, mice were sacrificed and bladder and kidney samples were collected to determine the viable bacterial count. All animal experiments were performed in strict accordance to the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Laboratory Animal Center (Taiwan), and the protocol was approved by the Institutional Animal Care and Use Committee of National Taiwan University College of Medicine.
Cell adhesion assay
Human A498 kidney epithelial cells and human NTUB1 bladder urothelial cells57 were grown on 12-well cell culture plates (Corning) in 10% fetal bovine serum-containing advanced Minimum Essential Medium (MEM, Gibco) and RPMI-1640 medium (Gibco), respectively, to confluent monolayers before being washed with PBS. Overnight bacterial cultures were centrifuged and the pellet was re-suspended in MEM or RPMI-1640 before applying to each well at an MOI of 10 (107 CFU/well). Bacteria were brought into contact with epithelial cells by centrifugation, followed by incubation for 1 h at 37 °C. Monolayers of cells were lysed with 1% Triton X-100 to determine bacterial CFU after being washed with PBS. The cell adhesion ability was the percentage of viable bacteria that adhered to the A498 cells or NTUB1 cells versus the total inoculum, and the relative adhesion ability to the wild-type was expressed.
Swarming assay
The swarming assay was performed as described previously on LB agar (1.5%, w/v) plates41 with or without 10% glucose. The swarming migration distance was monitored by following swarm fronts of the bacterial cells and recording progress at 60-min intervals.
Measurement of the flagellin level
Flagellin levels were determined as described previously41. The bacterial suspension washed from the LB agar plate with PBS (10 ml, OD600nm = 0.6) was used to prepare flagellin by vigorous vortexing and subsequent centrifugation. Flagellin in the supernatant was precipitated with trichloroacetic acid (5%, w/v), separated on 12% SDS-PAGE and stained with Coomassie Brilliant Blue R (Sigma-Aldrich B7920).
Transmission electron microscopy (TEM)
TEM was performed as described previously by using 1% phosphotungstic acid (PTA)-stained bacteria on a carbon-coated grid and TEM pictures were obtained with a Hitachi H-7100 electron microscope41.
Expression and purification of the His-tagged recombinant Crp
crp was cloned into the plasmid pET32a(+) (Novagen) to generate the pET32a-crp plasmid. Overexpression and purification of His-tagged Crp were performed as described previously57. An overnight culture of E. coli BL21 harboring pET32a-crp was diluted and grown at 37 °C with vigorous shaking to an optical density at 600 nm of 0.5 to 0.6 followed by addition of isopropyl-β-D- thiogalactopyranoside (IPTG) (0.1 mM) to induce Crp expression. The His-tagged Crp was purified by using a Ni2+-nitrilotriacetic acid column (Invitrogen) and its purity was confirmed by SDS-PAGE (Supplementary Fig. S5).
Electrophoretic mobility shift assay (EMSA)
An EMSA using purified His-tagged Crp and the IRDye-labeled flhDC or pmpA promoter DNA in the presence of cAMP was performed. The unlabeled promoter DNA fragment acted as a competitor to verify the binding specificity. The flhDC and pmpA promoter sequence were PCR- amplified by using the primer pairs flhDC-reF/flhDC-reR and pmp-fpF/pmp-koR, respectively (Supplementary Table S2). The PCR product was cloned into pGEM-T Easy (Promega) to generate pGflhDCp or pGpmpAp. The IRDye-labeled flhDC and pmpA promoter fragments were amplified from the pGflhDCp and pGpmpAp, respectively, using the IRDye® 700-labeled M13F/M13R primers (Supplementary Table S2, Integrated DNA Technologies). The amplified product (0.1 μg) was incubated with His-Crp (0–2 or 0–0.5 μM) and cAMP (50 μM) in a 10-μl binding buffer (150 mM KCl, 10 mM MgCl2, 30 mM Tris-HCl, 50 μM cAMP, pH 8.0). After incubation for 30 min at room temperature, the reaction mixtures were loaded onto a 5% non-denaturing polyacrylamide gel buffered with Tris-acetate-EDTA containing 50 μM cAMP, and then the gel was electrophoresed at 100 V for 1.5 to 2 h. The gel image was obtained by the quantitative infrared fluorescent imaging system (Odyssey, LI-COR). The unlabeled flhDC and pmpA promoter DNA fragments (0.5, 1, 2 μg) amplified from pGflhDCp and pGpmpAp, respectively, using unlabeled M13 primers, acted as a competitor. The DNA fragment amplified from the pGEM®-T Easy without the promoter sequence by the same primer pair was used as a negative control (Supplementary Fig. S7).
DNase I footprinting
A DNase I footprinting assay was modified from the protocol described by Yindeeyoungyeon and Schell61. The 597-bp fragment from nucleotide position −415 to 182 of pmpA start codon was PCR-amplified by using the primer pair pmp-fpF/pmp-koR and cloned into pGEM-T Easy to generate the plasmid pGpmp-fp. The 6-carboxyfluorescein (FAM)-labeled DNA fragment was generated by using PCR with the 5′ FAM-labeled T7 primer (Genomics, Taiwan) and the unlabeled SP6 primer. The FAM-labeled DNA fragment (0.2 μg) was incubated with Crp (512 nM) in a 150-μl solution containing 10 mM Tris-HCl (pH 7.5), 0.1 mM EDTA, 5.5 mM MgCl2, 20 μg/ml of poly(dI-dC), 50 μM cAMP and 0.2 mg/ml of BSA for 30 min at room temperature. After addition of freshly prepared DNase I solution (150 μl) containing 10−4 U/μl DNase I (Epicentre, USA), 20 mM Tris-HCl (pH 7.5), 5 mM MgCl2, and 1 mM CaCl2, the mixture was further incubated at 26 °C for 5 min, followed by incubation at 37 °C for 30 min in a stop solution (300 μl) containing 0.2 M NaCl, 40 mM EDTA, 1% SDS, and 125 μg/ml of tRNA to stop DNase I digestion. The DNA sample was extracted with a phenol:chloroform:isoamyl alcohol solution (25:24:1), precipitated with absolute ethanol, washed with 70% ethanol, and dissolved in 10 μl deionized water. A portion of the DNA sample (0.5 μl) combined with the LIZ-500 molecular size standard (Applied Biosystems) (0.5 μl) and deionized formamide (9 μl) was denatured at 95 °C for 5 min and quickly chilled on ice. Electrophoresis by using a 3730xl capillary DNA analyzer (Applied Biosystems) and an analysis by using Peak Scanner Software, version 1.0 (Applied Biosystems) were performed according to the instructions supplied by the manufacturer.
Macrophage infection assay
The assay was performed as described previously60 with some modifications. Briefly, overnight bacterial cultures were applied to a 12-well plate containing differentiated THP-1 cells at an MOI of 10 (106 CFU/well). After infection for 30 min, bacteria outside THP-1 cells were killed with streptomycin (250 μg/ml). The 12-well plate was incubated further in the medium containing streptomycin (32 μg/ml o) for 1 and 4 h. At each time point, cells in wells were lysed by using 1% Triton X-100 to determine CFU of intracellular bacterial cells. Intramacrophage survival was the percentage of viable bacteria that survived in the macrophages for 4 h versus those for 1 h.
Stress tolerance assays
Acid resistance assay was performed as described by Wu et al. with some modifications40. Overnight cultures were diluted 500-fold in fresh LB broth and incubated at 37 °C with shaking at 250 rpm for 4 h. After adjusting the LB broth with HCl to pH 3.0, bacterial cells were treated statically with acid for 2 h. Cells were washed with PBS (pH 7.2) and plated on LSW− plates to determine the CFU. H2O2 survival test was performed as described previously41, except that 30 mM H2O2 was used. The percent cell survival of acid or H2O2 was calculated as 100× (CFU of treated cells/CFU of untreated cells).
Electronic supplementary material
Acknowledgements
This work was supported by grant MOST102-2320-B002-022 from the Ministry of Science and Technology of Taiwan. We thank Yeong-Shiau Pu, Che-Ming Teng, Betty A. Wu-Hsieh, Chishih Chu, Tsuey-Ching Yang and Hao-Chieh Chiu for providing NTUB1 cell line, A498 cell line, THP-1 cells, pGFPuv plasmid, pX1918GT plasmid and pKD4 plasmid, respectively. We thank Jin-Ying Lu for information about diabetes. Also Graduate Institute of Anatomy and Cell Biology, College of Medicine, National Taiwan University and GenoInfo Core Facility funded by NCFPB of MOST Taiwan (MOST105-2319-B-010-001) provided assistance in TEM examination and Illumina sequencing for transcriptome, respectively.
Author Contributions
Y.L.T., K.T.H. and W.Y.L. performed experiments and data analysis. Y.L.T., H.F.C. and S.J.L. designed experiments. Y.L.T. and S.J.L. prepared the manuscript. Y.L.T. and S.J.L. wrote the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-07304-7
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shimada T, Fujita N, Yamamoto K, Ishihama A. Novel roles of cAMP receptor protein (CRP) in regulation of transport and metabolism of carbon sources. PLoS One. 2011;6:e20081. doi: 10.1371/journal.pone.0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gosset G, Zhang Z, Nayyar S, Cuevas WA, Saier MH. Transcriptome analysis of Crp-dependent catabolite control of gene expression in Escherichia coli. J. Bacteriol. 2004;186:3516–3524. doi: 10.1128/JB.186.11.3516-3524.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng D, Constantinidou C, Hobman JL, Minchin SD. Identification of the CRP regulon using in vitro and in vivo transcriptional profiling. Nucleic Acids Res. 2004;32:5874–5893. doi: 10.1093/nar/gkh908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green J, et al. Cyclic-AMP and bacterial cyclic-AMP receptor proteins revisited: adaptation for different ecological niches. Curr. Opin. Microbiol. 2014;18:1–7. doi: 10.1016/j.mib.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heroven AK, Dersch P. Coregulation of host-adapted metabolism and virulence by pathogenic yersiniae. Front. Cell Infect. Microbiol. 2014;4:146. doi: 10.3389/fcimb.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller CM, et al. Type 1 fimbriae, a colonization factor of uropathogenic Escherichia coli, are controlled by the metabolic sensor CRP-cAMP. PLoS Pathog. 2009;5:e1000303. doi: 10.1371/journal.ppat.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lathem WW, et al. Posttranscriptional regulation of the Yersinia pestis cyclic AMP receptor protein Crp and impact on virulence. MBio. 2014;5:e01038–13. doi: 10.1128/mBio.01038-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolb A, Busby S, Buc H, Garges S, Adhya S. Transcriptional regulation by cAMP and its receptor protein. Annu. Rev. Biochem. 1993;62:749–795. doi: 10.1146/annurev.bi.62.070193.003533. [DOI] [PubMed] [Google Scholar]
- 9.Hanamura A, Aiba H. Molecular mechanism of negative autoregulation of Escherichia coli crp gene. Nucleic Acids Res. 1991;19:4413–4419. doi: 10.1093/nar/19.16.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu K. Metabolic regulation of a bacterial cell system with emphasis on Escherichia coli metabolism. ISRN Biochem. 2013;2013:645983. doi: 10.1155/2013/645983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, et al. Autoregulation of PhoP/PhoQ and positive regulation of the cyclic AMP receptor protein-cyclic AMP complex by PhoP in Yersinia pestis. J. Bacteriol. 2013;195:1022–1030. doi: 10.1128/JB.01530-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armbruster CE, Mobley HL. Merging mythology and morphology: the multifaceted lifestyle of Proteus mirabilis. Nat. Rev. Microbiol. 2012;10:743–754. doi: 10.1038/nrmicro2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobsen SM, Stickler DJ, Mobley HL, Shirtliff ME. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin. Microbiol. Rev. 2008;21:26–59. doi: 10.1128/CMR.00019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielubowicz GR, Mobley HL. Host-pathogen interactions in urinary tract infection. Nat. Rev. Urol. 2010;7:430–441. doi: 10.1038/nrurol.2010.101. [DOI] [PubMed] [Google Scholar]
- 15.Jansen AM, Lockatell V, Johnson DE, Mobley HL. Mannose-resistant Proteus-like fimbriae are produced by most Proteus mirabilis strains infecting the urinary tract, dictate the in vivo localization of bacteria, and contribute to biofilm formation. Infect. Immun. 2004;72:7294–7305. doi: 10.1128/IAI.72.12.7294-7305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lane MC, Mobley HL. Role of P-fimbrial-mediated adherence in pyelonephritis and persistence of uropathogenic Escherichia coli (UPEC) in the mammalian kidney. Kidney Int. 2007;72:19–25. doi: 10.1038/sj.ki.5002230. [DOI] [PubMed] [Google Scholar]
- 17.Kuan L, Schaffer JN, Zouzias CD, Pearson MM. Characterization of 17 chaperone-usher fimbriae encoded by Proteus mirabilis reveals strong conservation. J. Med. Microbiol. 2014;63:911–922. doi: 10.1099/jmm.0.069971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armbruster CE, Hodges SA, Mobley HL. Initiation of swarming motility by Proteus mirabilis occurs in response to specific cues present in urine and requires excess L-glutamine. J. Bacteriol. 2013;195:1305–1319. doi: 10.1128/JB.02136-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alteri CJ, Himpsl SD, Engstrom MD, Mobley HL. Anaerobic respiration using a complete oxidative TCA cycle drives multicellular swarming in Proteus mirabilis. MBio. 2012;3:e00365–12. doi: 10.1128/mBio.00365-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nam TW, Park YH, Jeong HJ, Ryu S, Seok YJ. Glucose repression of the Escherichia coli sdhCDAB operon, revisited: regulation by the CRP* cAMP complex. Nucleic Acids Res. 2005;33:6712–6722. doi: 10.1093/nar/gki978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stella NA, Kalivoda EJ, O’Dee DM, Nau GJ, Shanks RM. Catabolite repression control of flagellum production by Serratia marcescens. Res. Microbiol. 2008;159:562–568. doi: 10.1016/j.resmic.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soutourina O, et al. Multiple control of flagellum biosynthesis in Escherichia coli: role of H-NS protein and the cyclic AMP-catabolite activator protein complex in transcription of the flhDC master operon. J. Bacteriol. 1999;181:7500–7508. doi: 10.1128/jb.181.24.7500-7508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stapleton A. Urinary tract infections in patients with diabetes. Am. J. Med. 2002;113(1A):80S–84S. doi: 10.1016/S0002-9343(02)01062-8. [DOI] [PubMed] [Google Scholar]
- 24.Nitzan O, Elias M, Chazan B, Saliba W. Urinary tract infections in patients with type 2 diabetes mellitus: review of prevalence, diagnosis, and management. Diabetes Metab. Syndr. Obes. 2015;8:129–136. doi: 10.2147/DMSO.S51792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nkume FA, Chongsi ME, Mbuntum JF, Tanjeko AT, Kwenti TE. Glycemic control and urinary tract infection in diabetes mellitus: a cross sectional study. Res. Rev.: J. Med. Health Sci. 2014;3:83–88. [Google Scholar]
- 26.Burekovic A, Dizdarevic-Bostandzic A, Godinjak A. Poorly regulated blood glucose in diabetic patients-predictor of acute infections. Med. Arch. 2014;68:163–166. doi: 10.5455/medarh.2014.68.163-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shkurti S. Prevalence of urinary tract infection among patients with diabetes melitus in Tirana district. Mediterr. J. Med. Sci. 2015;2:13–18. [Google Scholar]
- 28.Chiţă T, et al. Prevalence of urinary tract infections in diabetic patients. Rom. J. Diabetes. Nutr. Metab. Dis. 2013;20:99–105. [Google Scholar]
- 29.Kimata K, Takahashi H, Inada T, Postma P, Aiba H. cAMP receptor protein-cAMP plays a crucial role in glucose-lactose diauxie by activating the major glucose transporter gene in Escherichia coli. Proc. Natl. Acad. Sci. USA. 1997;94:12914–12919. doi: 10.1073/pnas.94.24.12914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qu S, et al. Cyclic AMP receptor protein is a repressor of adenylyl cyclase gene cyaA in Yersinia pestis. Can. J. Microbiol. 2013;59:304–310. doi: 10.1139/cjm-2012-0705. [DOI] [PubMed] [Google Scholar]
- 31.Lin HH, et al. Negative effect of glucose on ompA mRNA stability: a potential role of cyclic AMP in the repression of hfq in Escherichia coli. J. Bacteriol. 2011;193:5833–5840. doi: 10.1128/JB.05359-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aiba H. Transcription of the Escherichia coli adenylate cyclase gene is negatively regulated by cAMP-cAMP receptor protein. J. Biol. Chem. 1985;260:3063–3070. [PubMed] [Google Scholar]
- 33.Bott M. Anaerobic citrate metabolism and its regulation in enterobacteria. Arch. Microbiol. 1997;167:78–88. doi: 10.1007/s002030050419. [DOI] [PubMed] [Google Scholar]
- 34.Wright JM, Satishchandran C, Boyle SM. Transcription of the speC (ornithine decarboxylase) gene of Escherichia coli is repressed by cyclic AMP and its receptor protein. Gene. 1986;44:37–45. doi: 10.1016/0378-1119(86)90040-5. [DOI] [PubMed] [Google Scholar]
- 35.Wu CJ, Huang YW, Lin YT, Ning HC, Yang TC. Inactivation of SmeSyRy two-component regulatory system inversely regulates the expression of SmeYZ and SmeDEF efflux pumps in Stenotrophomonas maltophilia. PLoS One. 2016;11:e0160943. doi: 10.1371/journal.pone.0160943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donovan GT, Norton JP, Bower JM, Mulvey MA. Adenylate cyclase and the cyclic AMP receptor protein modulate stress resistance and virulence capacity of uropathogenic Escherichia coli. Infect. Immun. 2013;81:249–258. doi: 10.1128/IAI.00796-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bahrani FK, et al. Construction of an MR/P fimbrial mutant of Proteus mirabilis: role in virulence in a mouse model of ascending urinary tract infection. Infect. Immun. 1994;62:3363–3371. doi: 10.1128/iai.62.8.3363-3371.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melican K, et al. Uropathogenic Escherichia coli P and type 1 fimbriae act in synergy in a living host to facilitate renal colonization leading to nephron obstruction. PLoS Pathog. 2011;7:e1001298. doi: 10.1371/journal.ppat.1001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goransson M, Forsman P, Nilsson P, Uhlin BE. Upstream activating sequences that are shared by two divergently transcribed operons mediate cAMP-CRP regulation of pilus-adhesin in Escherichia coli. Mol. Microbiol. 1989;3:1557–1565. doi: 10.1111/j.1365-2958.1989.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 40.Wu J, Li Y, Cai Z, Jin Y. Pyruvate-associated acid resistance in bacteria. Appl. Environ. Microbiol. 2014;80:4108–4113. doi: 10.1128/AEM.01001-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang MC, Chien HF, Tsai YL, Liu MC, Liaw SJ. The RNA chaperone Hfq is involved in stress tolerance and virulence in uropathogenic Proteus mirabilis. PLoS One. 2014;9:e85626. doi: 10.1371/journal.pone.0085626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saier, M. H., Ramseier, T. M. & Reizer, J. Regulation of carbon utilization. in Escherichia coli and Salmonella Typhimurium: cellular and molecular biology (eds Neidhardt, F. C., et al.) American Society for Microbiology, Washington, DC. 1, 1325–1343 (1996).
- 43.Chen ZW, et al. Mutations in the Salmonella enterica serovar Choleraesuis cAMP-receptor protein gene lead to functional defects in the SPI-1 type III secretion system. Vet. Res. 2010;41:5. doi: 10.1051/vetres/2009053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalivoda EJ, Stella NA, O’Dee DM, Nau GJ, Shanks RM. The cyclic AMP-dependent catabolite repression system of Serratia marcescens mediates biofilm formation through regulation of type 1 fimbriae. Appl. Environ. Microbiol. 2008;74:3461–3470. doi: 10.1128/AEM.02733-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khandige S, Kronborg T, Uhlin BE, Moller-Jensen J. sRNA-mediated regulation of P-fimbriae phase variation in uropathogenic Escherichia coli. PLoS Pathog. 2015;11:e1005109. doi: 10.1371/journal.ppat.1005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watts RE, et al. Escherichia coli 83972 expressing a P fimbriae oligosaccharide receptor mimic impairs adhesion of uropathogenic E. coli. J. Infect. Dis. 2012;206:1242–1249. doi: 10.1093/infdis/jis493. [DOI] [PubMed] [Google Scholar]
- 47.Forsman K, Sonden B, Goransson M, Uhlin BE. Antirepression function in Escherichia coli for the cAMP-cAMP receptor protein transcriptional activator. Proc. Natl. Acad. Sci. USA. 1992;89:9880–9884. doi: 10.1073/pnas.89.20.9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishihama A. Protein-protein communication within the transcription apparatus. J. Bacteriol. 1993;175:2483–2489. doi: 10.1128/jb.175.9.2483-2489.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hung DL, Raivio TL, Jones CH, Silhavy TJ, Hultgren SJ. Cpx signaling pathway monitors biogenesis and affects assembly and expression of P pili. EMBO J. 2001;20:1508–1518. doi: 10.1093/emboj/20.7.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spurbeck RR, Alteri CJ, Himpsl SD, Mobley HL. The multifunctional protein YdiV represses P fimbria-mediated adherence in uropathogenic. Escherichia coli. J. Bacteriol. 2013;195:3156–3164. doi: 10.1128/JB.02254-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steiner TS. How flagellin and toll-like receptor 5 contribute to enteric infection. Infect. Immun. 2007;75:545–552. doi: 10.1128/IAI.01506-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Legnani-Fajardo C, Zunino P, Piccini C, Allen A, Maskell D. Defined mutants of Proteus mirabilis lacking flagella cause ascending urinary tract infection in mice. Microb. Pathog. 1996;21:395–405. doi: 10.1006/mpat.1996.0070. [DOI] [PubMed] [Google Scholar]
- 53.Hamrick TS, Havell EA, Horton JR, Orndorff PE. Host and bacterial factors involved in the innate ability of mouse macrophages to eliminate internalized unopsonized Escherichia coli. Infect. Immun. 2000;68:125–132. doi: 10.1128/IAI.68.1.125-132.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dong T, Schellhorn HE. Role of RpoS in virulence of pathogens. Infect. Immun. 2010;78:887–897. doi: 10.1128/IAI.00882-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Battesti A, Majdalani N, Gottesman S. The RpoS-mediated general stress response in Escherichia coli. Annu. Rev. Microbiol. 2011;65:189–213. doi: 10.1146/annurev-micro-090110-102946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lange R, Hengge-Aronis R. The cellular concentration of the sigma S subunit of RNA polymerase in Escherichia coli is controlled at the levels of transcription, translation, and protein stability. Genes Dev. 1994;8:1600–1612. doi: 10.1101/gad.8.13.1600. [DOI] [PubMed] [Google Scholar]
- 57.Jiang SS, et al. Proteus mirabilis pmrI, an RppA-regulated gene necessary for polymyxin B resistance, biofilm formation, and urothelial cell invasion. Antimicrob. Agents Chemother. 2010;54:1564–1571. doi: 10.1128/AAC.01219-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang SS, et al. Characterization of UDP-glucose dehydrogenase and UDP-glucose pyrophosphorylase mutants of Proteus mirabilis: defectiveness in polymyxin B resistance, swarming, and virulence. Antimicrob. Agents Chemother. 2010;54:2000–2009. doi: 10.1128/AAC.01384-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu JH, Tsai CG. Infectivity of hepatic strain Klebsiella pneumoniae in diabetic mice. Exp. Biol. Med. (Maywood) 2005;230:757–761. doi: 10.1177/153537020523001009. [DOI] [PubMed] [Google Scholar]
- 60.Liu MC, Kuo KT, Chien HF, Tsai YL, Liaw SJ. New aspects of RpoE in uropathogenic Proteus mirabilis. Infect. Immun. 2015;83:966–977. doi: 10.1128/IAI.02232-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yindeeyoungyeon W, Schell MA. Footprinting with an automated capillary DNA sequencer. Biotechniques. 2000;29:1034–1041. doi: 10.2144/00295st05. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


