Abstract
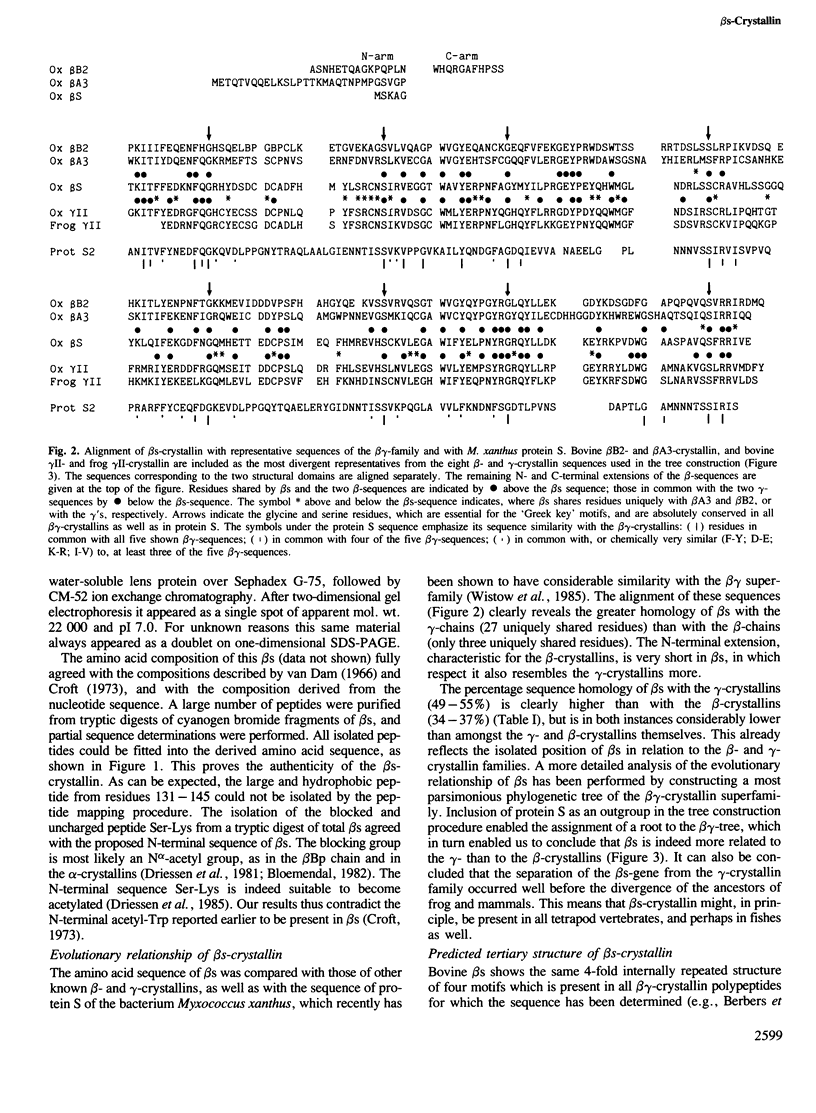
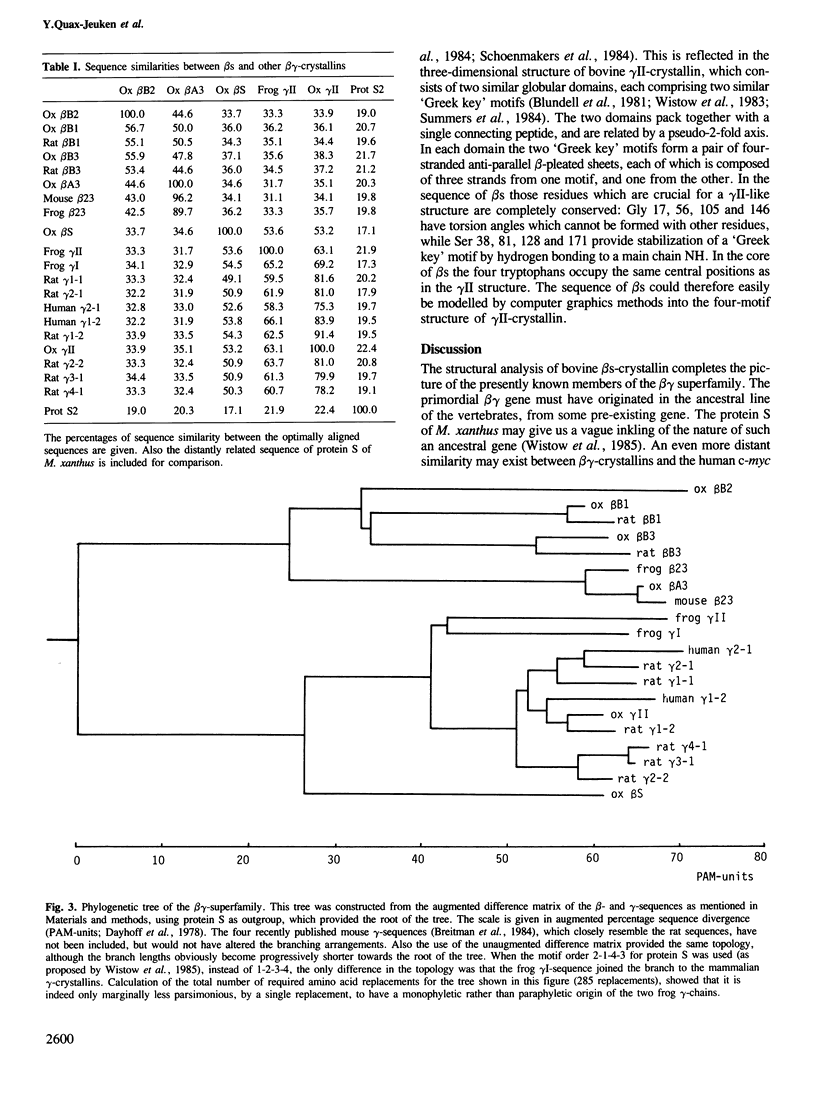
The nucleotide sequence of the cDNA of bovine lens beta s-crystallin has been determined, and the derived amino acid sequence has been confirmed by amino acid compositions and partial sequences of the tryptic peptides of this monomeric protein. beta s-Crystallin has a length of 177 residues, corresponding to a mol. wt. of 20 773, and a blocked N-terminal serine. Comparison of beta s with the known sequences of other beta- and gamma-crystallins, and computer construction of a phylogenetic tree of these sequences, shows beta s to be more closely related to the monomeric gamma-crystallins than to the oligomeric beta-crystallins. Also the tertiary structure of beta s modelled by interactive computer graphics on the coordinates of gamma II-crystallin, revealed similarities with the gamma-crystallins which might explain its monomeric behavior: the presence of a very short N-terminal 'arm' as compared with the beta-crystallins; a distribution of charged residues on the surface as in the gamma-crystallins; and finally the nature of certain residues of its inter-domain contacts. beta s-Crystallin seems to be an old and isolated offshoot of the gamma-family, and, considering its ancient origin, might well be present in other, non-mammalian, vertebrate classes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berbers G. A., Brans A. M., Hoekman W. A., Slingsby C., Bloemendal H., De Jong W. W. Aggregation behavior of the bovine beta-crystallin Bp chain studied by limited proteolysis. Biochim Biophys Acta. 1983 Oct 28;748(2):213–219. doi: 10.1016/0167-4838(83)90297-2. [DOI] [PubMed] [Google Scholar]
- Berbers G. A., Hoekman W. A., Bloemendal H., de Jong W. W., Kleinschmidt T., Braunitzer G. Homology between the primary structures of the major bovine beta-crystallin chains. Eur J Biochem. 1984 Mar 15;139(3):467–479. doi: 10.1111/j.1432-1033.1984.tb08029.x. [DOI] [PubMed] [Google Scholar]
- Berbers G. A., Hoekman W. A., Bloemendal H., de Jong W. W., Kleinschmidt T., Braunitzer G. Proline- and alanine-rich N-terminal extension of the basic bovine beta-crystallin B1 chains. FEBS Lett. 1983 Sep 19;161(2):225–229. doi: 10.1016/0014-5793(83)81013-8. [DOI] [PubMed] [Google Scholar]
- Bhat S. P., Spector A. Complete nucleotide sequence of a cDNA derived from calf lens gamma-crystallin mRNA: presence of Alu I-like DNA sequences. DNA. 1984 Aug;3(4):287–295. doi: 10.1089/dna.1.1984.3.287. [DOI] [PubMed] [Google Scholar]
- Bindels J. G., Koppers A., Hoenders H. J. Structural aspects of bovine beta-crystallins: physical characterization including dissociation-association behavior. Exp Eye Res. 1981 Sep;33(3):333–343. doi: 10.1016/s0014-4835(81)80056-5. [DOI] [PubMed] [Google Scholar]
- Bindels J. G., de Man B. M., Hoenders H. J. High-performance gel permeation chromatography of bovine eye lens proteins in combination with low-angle laser light scattering. Superior resolution of the oligomeric beta-crystallins. J Chromatogr. 1982 Dec 3;252:255–267. doi: 10.1016/s0021-9673(01)88416-8. [DOI] [PubMed] [Google Scholar]
- Bloemendal H. Lens proteins. CRC Crit Rev Biochem. 1982;12(1):1–38. doi: 10.3109/10409238209105849. [DOI] [PubMed] [Google Scholar]
- Blundell T., Lindley P., Miller L., Moss D., Slingsby C., Tickle I., Turnell B., Wistow G. The molecular structure and stability of the eye lens: x-ray analysis of gamma-crystallin II. Nature. 1981 Feb 26;289(5800):771–777. doi: 10.1038/289771a0. [DOI] [PubMed] [Google Scholar]
- Breitman M. L., Lok S., Wistow G., Piatigorsky J., Tréton J. A., Gold R. J., Tsui L. C. Gamma-crystallin family of the mouse lens: structural and evolutionary relationships. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7762–7766. doi: 10.1073/pnas.81.24.7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe M. J. Partial sequence homology of human myc oncogene protein to beta and gamma crystallins. FEBS Lett. 1985 Feb 11;181(1):157–159. doi: 10.1016/0014-5793(85)81133-9. [DOI] [PubMed] [Google Scholar]
- Driessen H. P., Herbrink P., Bloemendal H., de Jong W. W. Primary structure of the bovine beta-crystallin Bp chain. Internal duplication and homology with gamma-crystallin. Eur J Biochem. 1981 Dec;121(1):83–91. doi: 10.1111/j.1432-1033.1981.tb06433.x. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- Gorin M. B., Horwitz J. Cloning and characterization of a cow beta crystallin cDNA. Curr Eye Res. 1984 Jul;3(7):939–948. doi: 10.3109/02713688409167211. [DOI] [PubMed] [Google Scholar]
- Inana G., Piatigorsky J., Norman B., Slingsby C., Blundell T. Gene and protein structure of a beta-crystallin polypeptide in murine lens: relationship of exons and structural motifs. Nature. 1983 Mar 24;302(5906):310–315. doi: 10.1038/302310a0. [DOI] [PubMed] [Google Scholar]
- Inouye S., Franceschini T., Inouye M. Structural similarities between the development-specific protein S from a gram-negative bacterium, Myxococcus xanthus, and calmodulin. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6829–6833. doi: 10.1073/pnas.80.22.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabasawa I., Tsunematsu Y., Barber G. W., Kinoshita J. H. Low molecular weight proteins of the bovine lenses. Exp Eye Res. 1977 May;24(5):437–448. doi: 10.1016/0014-4835(77)90265-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McDevitt D. S., Croft L. R. On the existence of gamma-crystallin in the bird lens. Exp Eye Res. 1977 Nov;25(5):473–481. doi: 10.1016/0014-4835(77)90176-2. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Piatigorsky J., Nickerson J. M., King C. R., Inana G., Hejtmancik J. F., Hawkins J. W., Borras T., Shinohara T., Wistow G., Norman B. Crystallin genes: templates for lens transparency. Ciba Found Symp. 1984;106:191–207. doi: 10.1002/9780470720875.ch11. [DOI] [PubMed] [Google Scholar]
- Prager E. M., Wilson A. C. Construction of phylogenetic trees for proteins and nucleic acids: empirical evaluation of alternative matrix methods. J Mol Evol. 1978 Jun 20;11(2):129–142. doi: 10.1007/BF01733889. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Quax-Jeuken Y., Janssen C., Quax W., van den Heuvel R., Bloemendal H. Bovine beta-crystallin complementary DNA clones. Alternating proline/alanine sequence of beta B1 subunit originates from a repetitive DNA sequence. J Mol Biol. 1984 Dec 15;180(3):457–472. doi: 10.1016/0022-2836(84)90022-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schoenmakers J. G., den Dunnen J. T., Moormann R. J., Jongbloed R., van Leen R. W., Lubsen N. H. The crystallin gene families. Ciba Found Symp. 1984;106:208–218. doi: 10.1002/9780470720875.ch12. [DOI] [PubMed] [Google Scholar]
- Slingsby C., Croft L. R. Developmental changes in the low molecular weight proteins of the bovine lens. Exp Eye Res. 1973 Nov 25;17(4):369–376. doi: 10.1016/0014-4835(73)90246-7. [DOI] [PubMed] [Google Scholar]
- Tomarev S. I., Krayev A. S., Skryabin K. G., Bayev A. A., Gause G. G., Jr The nucleotide sequence of a cloned cDNA corresponding to one of the gamma-crystallins from the eye lens of the frog Rana temporaria. FEBS Lett. 1982 Sep 20;146(2):314–318. doi: 10.1016/0014-5793(82)80942-3. [DOI] [PubMed] [Google Scholar]
- Tomarev S. I., Zinovieva R. D., Chalovka P., Krayev A. S., Skryabin K. G., Gause G. G., Jr Multiple genes coding for the frog eye lens gamma-crystallins. Gene. 1984 Mar;27(3):301–308. doi: 10.1016/0378-1119(84)90074-x. [DOI] [PubMed] [Google Scholar]
- Tréton J. A., Jones R. E., King C. R., Piatigorsky J. Evidence against gamma-crystallin DNA or RNA sequences in the chicken. Exp Eye Res. 1984 Oct;39(4):513–522. doi: 10.1016/0014-4835(84)90051-4. [DOI] [PubMed] [Google Scholar]
- Wistow G., Slingsby C., Blundell T., Driessen H., De Jong W., Bloemendal H. Eye-lens proteins: the three-dimensional structure of beta-crystallin predicted from monomeric gamma-crystallin. FEBS Lett. 1981 Oct 12;133(1):9–16. doi: 10.1016/0014-5793(81)80460-7. [DOI] [PubMed] [Google Scholar]
- Wistow G., Summers L., Blundell T. Myxococcus xanthus spore coat protein S may have a similar structure to vertebrate lens beta gamma-crystallins. 1985 Jun 27-Jul 3Nature. 315(6022):771–773. doi: 10.1038/315771a0. [DOI] [PubMed] [Google Scholar]
- Wistow G., Turnell B., Summers L., Slingsby C., Moss D., Miller L., Lindley P., Blundell T. X-ray analysis of the eye lens protein gamma-II crystallin at 1.9 A resolution. J Mol Biol. 1983 Oct 15;170(1):175–202. doi: 10.1016/s0022-2836(83)80232-0. [DOI] [PubMed] [Google Scholar]
- Zigler J. S., Jr, Horwitz J., Kinoshita J. H. Studies on the low molecular weight proteins of human lens. Exp Eye Res. 1981 Jan;32(1):21–30. doi: 10.1016/s0014-4835(81)80035-8. [DOI] [PubMed] [Google Scholar]
- den Dunnen J. T., Moormann R. J., Schoenmakers J. G. Rat lens beta-crystallins are internally duplicated and homologous to gamma-crystallins. Biochim Biophys Acta. 1985 Apr 19;824(4):295–303. doi: 10.1016/0167-4781(85)90035-1. [DOI] [PubMed] [Google Scholar]
- van Dam A. F. Purification and composition of beta-s-crystallin. Exp Eye Res. 1966 Oct;5(4):255–266. doi: 10.1016/s0014-4835(66)80035-0. [DOI] [PubMed] [Google Scholar]


