Abstract
Incidence of IBD is rising in parallel with overweight and obesity. Contrary to conventional belief, about 15–40% of patients with IBD are obese, which might contribute to the development of IBD. Findings from cross-sectional and retrospective cohort studies are conflicting on the effect of obesity on natural history and course of IBD. Most studies are limited by small sample size, low event rates, non-validated assessment of disease activity and lack robust longitudinal follow-up and have incomplete adjustment for confounding factors. The effect of obesity on the efficacy of IBD-related therapy remains to be studied, though data from other autoimmune diseases suggests that obesity results in suboptimal response to therapy, potentially by promoting rapid clearance of biologic agents leading to low trough concentrations. These data provide a rationale for using weight loss interventions as adjunctive therapy in patients with IBD who are obese. Obesity also makes colorectal surgery technically challenging and might increase the risk of perioperative complications. In this Review, we highlight the existing literature on the epidemiology of obesity in IBD, discuss its plausible role in disease pathogenesis and effect on disease course and treatment response, and identify high-priority areas of future research.
With an estimated 2.1 billion adults worldwide overweight (BMI ≥25 kg/m2), of which 600 million are obese (BMI ≥30kg/m2), the prevalence of overweight and obesity has reached epidemic proportions1. From 1980 to 2013, the proportion of overweight adults has increased 28% in developed countries and nearly 60% in developing countries, with no country reporting a decrease in prevalence during the same period1. In the USA, more than one-third (35.7%) of adults are obese, with the estimated annual medical costs of a patient with obesity being US$1,429 higher than individuals of a normal weight2.
In parallel with the obesity epidemic, the incidence and prevalence of IBD is rising globally. In a systematic review of 260 population-based studies, the estimated annual incidence of IBD ranged from 10–30 cases per 100,000 persons in the Western world; an estimated 0.5% of adults in the West suffer from IBD3. A time-trend analysis suggests that incidence of IBD has increased over time, particularly in newly industrialized countries4,5. Various environmental exposures have been implicated in these epidemiological trends in IBD, including smoking, improving hygienic standards, infections and antibiotics and dietary factors such as high-fat or low-fibre diets. A potentially understudied factor might be obesity, which has been associated with an increased risk of several autoimmune diseases such as rheumatoid arthritis, psoriasis and psoriatic arthritis6,7. Beyond the potential epidemiological association, the rising prevalence of obesity implies that a substantial proportion of patients with IBD would be obese. Obesity negatively affects disease course and treatment response in other autoimmune diseases, but there is limited synthesis of data on the effect of obesity in IBD.
In this Review, we discuss the epidemiology and pathophysiology of obesity in IBD, the potential effect of obesity on disease course and complications, treatment response and surgical outcomes in patients with IBD, and whether treatment of obesity could modify disease course in patients with IBD.
Epidemiology
Prevalence of obesity in IBD
Cross-sectional studies in patients with IBD show that about 15–40% of adults with IBD are obese, and an additional 20–40% are overweight8–14. In a population-based study of 489 patients with IBD conducted in Scotland, 18% of patients were obese (compared with 23% of the general population), and 38% of patients were overweight; this proportion was comparable between patients with Crohn’s disease (18% obese) or ulcerative colitis (17.5% obese)15. By contrast, only 3% of patients with Crohn’s disease and 0.5% of patients with ulcerative colitis were underweight (BMI <18.5 kg/m2). Large US-based, single-centre adult cohort studies have observed similar rates of obesity (TABLE 1).
Table 1.
Key studies on obesity epidemiology and its effect on disease course and complications in IBD
| Study | Study characteristics | Patient characteristics | Prevalence of obese and overweight | Key findings | Refs |
|---|---|---|---|---|---|
| Flores et al. (2015) |
|
Crohn’s disease:
|
Crohn’s disease:
|
Crohn’s disease:
|
8 |
| Seminerio et al. (2015) |
|
|
|
|
12 |
| Pringle et al. (2015) |
|
|
|
|
10 |
| Hass et al. (2006) |
|
|
32% overweight or obese (at Crohn’s disease diagnosis) |
|
77 |
CRP, C-reactive protein; OR, odds ratio.
A similar trend has also been observed in paediatric patients with IBD. In two North American multicentre inception cohorts of paediatric patients with IBD formed between 2000 and 2002, about 9–10% of children with Crohn’s disease and 20–34% of children with ulcerative colitis had a sex-specific BMI-for-age above the 85th percentile at diagnosis16. In another multiple-site registry of 1,598 children with IBD, Long and colleagues9 observed 23.6% of children were above the 85th sex-specific BMI-for-age percentile (20.0% patients with Crohn’s disease, 30.1% patients with ulcerative colitis), including 9.5% children who were above the 95th percentile; by contrast, only 2.9% of children were below the 5th percentile at diagnosis. A greater proportion of Hispanic children with IBD were obese or overweight than non-Hispanic children with IBD (35.2% versus 23.1%, P = 0.02). These rates of obesity in patients with IBD are not different from rates observed in the general population.
This rise in the prevalence of obesity in patients with IBD seems to parallel the global obesity epidemic. In an early single-centre study conducted in France including 2,065 patients with Crohn’s disease seen between 1974 and 2000, the prevalence of obesity was 3%, gradually rising from 1.7% of patients diagnosed with Crohn’s disease before 1981 to 4% of patients diagnosed with Crohn’s disease after 1990 (P for trend <0.01)17. By contrast, contemporary cohorts estimate the prevalence of obesity in IBD at 15–40%, as discussed earlier. On analysing 40 trials of 10,282 patients with Crohn’s disease conducted between 1991 and 2008, an increase in trial participant weight was observed over time18. The minimum mean weight of participants increased from 57.1 kg in 1997 to a maximum mean weight of 89.1 kg in 2008.
Premorbid obesity and IBD risk
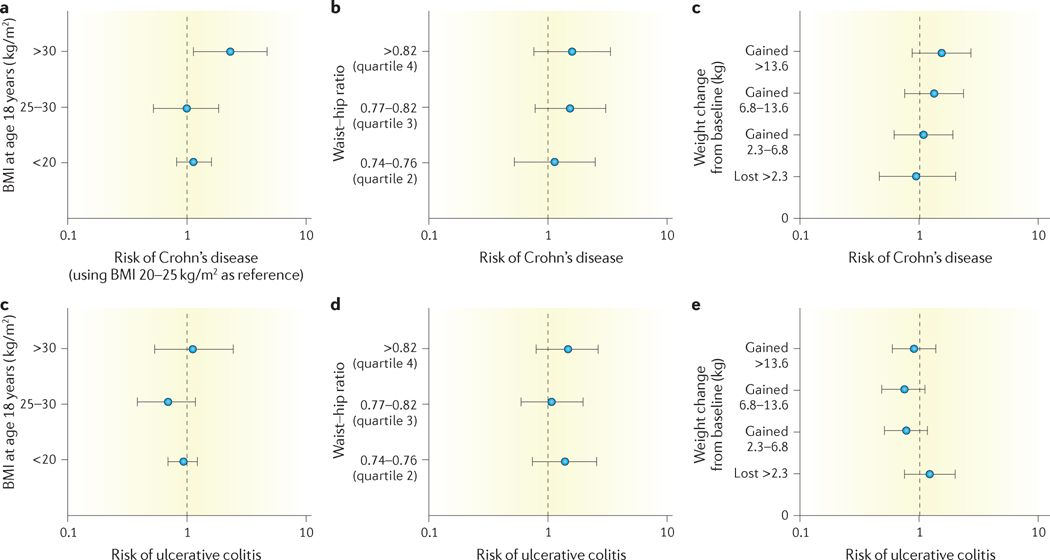
Besides a high prevalence of obesity in patients with IBD, premorbid obesity has also been associated with a risk of developing Crohn’s disease, but not ulcerative colitis, though this relationship has not been consistently observed19–21 (TABLE 2). In the Danish National Birth Cohort of over 75,000 women, prepregnancy obesity was associated with a 1.9-fold increase in risk of developing Crohn’s disease (hazard ratio (HR) 1.88, 95% CI 1.02–3.47), but not ulcerative colitis (HR 0.77, 95% CI 0.48–1.25)20. Similar effect estimates were observed in the Nurses’ Health Study, with BMI at age 18 years being predictive of risk of Crohn’s disease (BMI >30 versus normal BMI: HR 2.33, 95% CI 1.15–4.69) but not ulcerative colitis (HR 1.17, 95% CI 0.54–2.52)19 (FIG. 1). Interestingly, in the Danish National Birth cohort, the association between premorbid BMI and risk of Crohn’s disease was U-shaped, as women with a BMI <18.5 were also at increased risk of developing Crohn’s disease compared with women of a normal BMI (HR 2.57, 95% CI 1.30–5.06)20. The effect of obesity on risk of developing IBD might be age-dependent, with obesity in teenage or young adulthood associated with higher risk of developing Crohn’s disease than obesity at later ages. Using the population-based Swedish Hospital Discharge Register, Hemminki et al.22 observed that among patients hospitalized with a primary diagnosis of obesity, the relative incidence of Crohn’s disease is highest when obesity is diagnosed before 30 years of age (standardized incidence rate (SIR) 1.92, 95% CI 1.17–2.96), and decreases with increasing age at obesity diagnosis (SIR for Crohn’s disease when obesity diagnosed between 40–49 years of age 1.04, 95% CI 0.37–2.28). Additionally, magnitude of weight gain after age 18 years has also been associated with increased risk of Crohn’s disease, but not ulcerative colitis. In the Nurses’ Health Study, women who gained >13.61 kg (30 lbs) between age 18 years and enrolment in the cohort had a 1.5-fold higher risk of developing Crohn’s disease, as compared with women who remained within 2.27 kg (5 lbs) of their weight at age 18, in a dose-dependent manner19 (FIG. 1).
Table 2.
Key studies on the association between pre-morbid obesity and risk of developing incident IBD
| Study | Study characteristics | Patient characteristics | Key findings | Refs |
|---|---|---|---|---|
| Khalili et al. (2014) | US population-based cohort of 111,498 women (Nurses’ Health Study II) followed over 18 years | Incident Crohn’s disease and ulcerative colitis in 153 and 229 participants, respectively |
|
18 |
| Harpsoe et al. (2015) | Danish National Birth Cohort study of 75,008 women (median 30 years of age at cohort entry), followed over median 11 years | Incident Crohn’s disease and ulcerative colitis in 138 and 394 participants, respectively |
|
19 |
| Chan et al. (2013) | Nested case–control study within the European Prospective Cohort Study of 300,724 participants, from 23 centres in 10 countries | Incident Crohn’s disease and ulcerative colitis in 75 and 177 participants, respectively | No association between obesity and risk of developing Crohn’s disease (obese vs normal BMI: OR 0.85, 95% CI 0.29–2.45) or ulcerative colitis (obese vs normal BMI: OR 1.15, 95% CI 0.62–2.12) | 20 |
Figure 1. Association between premorbid obesity, waist–hip ratio and weight gain since age 18 years and IBD risk.
Data from the Nurses’ Health Cohort18 showing the relationship between premorbid obesity at 18 years of age, waist–hip ratio and weight gain since age 18 years on the risk of developing Crohn’s disease and ulcerative colitis.
Of the several compartments of body fat, visceral adiposity is now well recognized to be the metabolically active fraction23, and might be more predictive of the risk of developing IBD than overall obesity as determined by BMI. Patients with IBD have higher visceral fat volumes than those without IBD24. In a prospective cohort study, Khalili and colleagues19 observed a trend towards increased risk of Crohn’s disease, but not ulcerative colitis, in patients with a high waist–hip ratio (WHR). Among patients with Crohn’s disease, participants with a premorbid WHR ≥0.83 had a 1.6-fold higher risk of developing Crohn’s disease than those with a WHR ≤0.73 (HR, 1.58, 95% CI 0.78–3.21, P = 0.14) (FIG. 1). Beyond obesity, obesity-related lifestyle factors such as physical activity might also modify risk of IBD. In the Nurses’ Health Study, high level of physical activity (recreational or occupational) was associated with decreased risk of developing IBD25,26.
Can IBD contribute to obesity?
The high prevalence of obesity in patients with IBD might also suggest an independent effect of IBD on the risk of obesity development. Although current epidemiological data is unable to accurately assess the directionality of this association, preclinical data suggests that dysbiosis and altered metabolic gut signalling induced by IBD and acting through hormones (including incretins such as gastric inhibitory polypeptide and glucagon-like peptide), satiety-related peptides (such as ghrelin and peptide YY) and bile acids might contribute to development of obesity and dysmetabolism in patients with IBD27,28. In addition, smoking cessation and use of corticosteroids could contribute to weight gain in patients with IBD; in the general population, the average magnitude of weight gain with smoking cessation and corticosteroid use is 3–5 kg; ~11% of patients who quit smoking and ~24% of patients on corticosteroids for >1 year gain >10 kg body weight29–31.
Limitations of current literature
Most of the available epidemiological data on prevalence of obesity in IBD is cross-sectional, which limits the ability to infer causality or even directionality of the association (that is, whether obesity contributes to IBD pathogenesis, or vice versa). A limited number of prospective cohort studies (Nurses’ Health Study, Danish National Birth Cohort and European Prospective Investigation into Cancer and Nutrition study) have measured BMI in adulthood (generally in the 3rd to 5th decades of life) and subsequent development of IBD in an older population. These studies might not be representative of the general IBD population. Moreover, the Nurses’ Health Study and the Danish National Birth Cohort have only studied women, and it is not possible to study whether obesity would have a similar effect on risk of developing IBD in men. Finally, given the increasing understanding of the metabolically active nature of visceral fat, overall obesity measured using BMI probably does not adequately capture the association with IBD; current studies have included limited assessment of visceral fat and its effect of development of IBD, usually through a surrogate measure like WHR.
The ideal study to evaluate the association between obesity and risk of developing IBD would involve creating a prospective cohort of paediatric or young adult patients with assessment of overall obesity and visceral adiposity at multiple time points, through both anthropometry as well as more sophisticated methods (such as the ratio of visceral adipose tissue (VAT) to subcutaneous adipose tissue on cross-sectional imaging), and subsequent close follow-up for the development and accurate phenotyping of IBD. However, as IBD would be expected to be observed in only 1 in 200 participants, such a study would involve following a large number of participants over many years, and would be cost prohibitive. As an intermediary step, prospective identification of patients with IBD in diverse historical cohorts established decades ago, similar to efforts from the Nurses’ Health Study and the European Prospective Investigation into Cancer and Nutrition study, might help understand the association between obesity and risk of developing IBD.
Obesity in the pathogenesis of IBD
Increasingly, obesity is recognized as a perpetual state of chronic low-grade inflammation32. Whether via the systemic and paracrine increase in levels of cytokines, chemokines and adipokines, the expression of innate immune receptors on preadipocytes and adipocytes or the conversion of preadipocytes to macrophages, adipose tissue is integrally involved in the regulation of inflammation.
Role of adipose tissue
Subcutaneous and visceral adipose tissue compartments display distinct metabolic and immunological profiles. Mesenteric VAT has a predominance of pro-inflammatory M1 macrophages that secrete several inflammatory cytokines, including TNF and IL-1. In addition, adipocytes also produce other pro-inflammatory cytokines such as IL-6, chemokines such as C-C motif chemokine 2 (also known as chemoattractant protein-1) and adipokines such as leptin and resistin. These adipokines affect satiety and lipid metabolism, and regulate the effects of insulin and glucose metabolism through a network of interactions with cytokines33. In obesity, this altered network of adipokine and cytokine interactions contributes to impaired adipocyte metabolism; animal models and humans with obesity have increased levels of circulating cytokines, which can induce innate immune responses and influence the expression of several inflammatory mediators34. As low-grade inflammation is involved in the pathophysiology of obesity, circulating cytokines have been named as potential therapeutic targets for the treatment of obesity.
Patients with IBD show a unique locally restrictive form of VAT — creeping fat — whereby mesenteric fat hyperplasia is limited to areas of inflamed bowel35,36. Predominately seen in Crohn’s disease, creeping fat is thought to be more immunologically active than other VAT, and the extent of creeping fat correlates closely with the extent of histological inflammation and degree of lymphocyte or macrophage infiltration23. In a case–control study, Zulian and colleagues36 observed that the gene expression profile of omental VAT, which is distant from unhealthy mesenteric depots, from patients with Crohn’s disease was similar to that of VAT adipocytes from IBD-free individuals with obesity, with high expression of pro-inflammatory genes, and was distinct from VAT adipocytes in healthy, normal-weight individuals. Furthermore, expression of leptin and adipo nectin has been demonstrated to be increased in the hypertrophied mesenteric fat of patients with Crohn’s disease, and specific risk alleles (such as ATG16L1) associated with alterations in adiponectins have also been associated with an increased risk of developing Crohn’s disease. Levels of other adipokines, such as resistin, also correlate with Crohn’s disease severity and disease activity, further supporting the interaction of adipokines and cytokines to promote mucosal inflammation37.
Role of dysbiosis
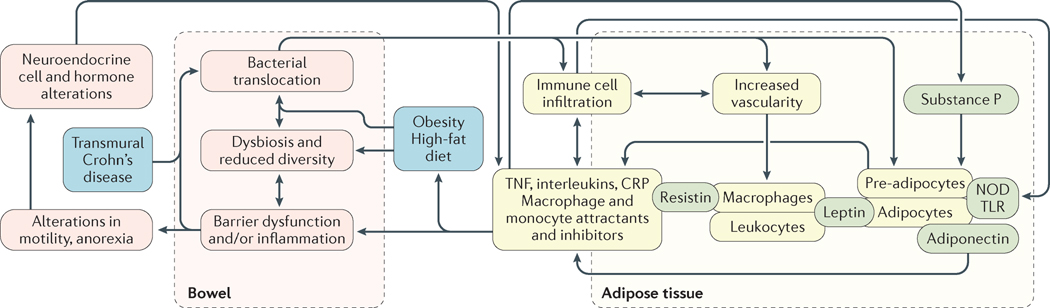
Central to the pathogenesis of IBD is mucosal barrier dysfunction, bacterial translocation and loss of intestinal immune homeostasis38. Translocation of bacteria has been linked to adipocyte and preadipocyte activation, with ensuing alterations in pro-inflammatory cytokine expression and immune homeostasis28,35,38–60 (FIG. 2). Increased gut bacterial translocation is pertinent given its independent links to both obesity and Crohn’s disease; both conditions have a reduction in bacterial diversity with accompanying dysbiosis61. A colonic-mucosa–bacteria–adipose-tissue feedback loop is then slowly established and reinforced by alterations in motility and immunoendocrine axes62,63. High-fat diet (both directly and by contributing to obesity) has been associated with an increased risk of Crohn’s disease in preclinical models, with effects mediated through dysbiosis and altered gut permeability64–66. Epidemiological studies have also reported an association between a diet rich in omega fatty acids and saturated, monounsaturated and polyunsaturated fats and risk of developing Crohn’s disease and/or ulcerative colitis67.
Figure 2. Postulated pathogenesis and feedback loop between visceral adipose tissue and intestinal inflammation in IBD.
In patients with IBD, the T-cell compartment shifts from a homeostatic regulatory environment consisting of M2 macrophages, natural killer cells and CD4+ Treg cells toward a pro-inflammatory environment characterized by M1 macrophages, CD4+ TH1 and CD8+ cytotoxic subtypes. Leptin, a member of the IL-6 protein family, is produced by adipocytes in proportion to fat mass. Leptin increases synthesis of pro-inflammatory cytokines in monocytes and macrophages, and is involved in T-cell-mediated immune responses. Adiponectin, produced by adipocytes in an inverse proportion to fat mass, functions as an insulin sensitizer and anti-inflammatory adipokine in patients who do not have IBD. However, in IBD, and in particular in Crohn’s disease, evidence supports its role as a pro-inflammatory adipokine that results in increased cytokine secretion and epithelial cell proliferation. Macrophages and monocytes are the main source of resistin, the expression of which is induced by pro-inflammatory cytokines (IL-1, IL-6 and TNF), and circulating levels correlate with acute-phase reactants (C-reactive protein, CRP). Resistin can promote expression of TNF and IL-6. Evidence from the past decade has demonstrated the existence of an adipose-tissue–colonic-mucosa feedback loop, in which preadipocytes release IL-17A in response to substance P; the colonic mucosa from these individuals shows an increase in IL-17A receptors. Obesity and a high-fat diet, as well as transmural inflammation in Crohn’s disease, results in impaired mucosal barrier function through alterations in tight-junction proteins, thereby promoting bacterial translocation. TLR, Toll-like receptor.
Studies in Crohn’s disease have identified genetic polymorphisms in enteroendocrine transcription factors (PHOX2B) as well as autoantibodies to specific cell components68,69. This finding, coupled with alterations in the levels of enteroendocrine hormones such as PYY, GLP-1 and GLP-2 (REF. 70), suggests a possible role for enteroendocrine cells in the propagation of inflammation in IBD. Treatment with GLP-2, an epithelial growth factor with anti-inflammatory properties, reduces the severity of injury in animal colitis models71, and its use as a treatment option for Crohn’s disease is currently under investigation72. Circulating PYY levels correlate with Crohn’s-disease-related nausea and anorexia73, and evidence has demonstrated that enteroendocrine cells might be key producers and mediators of pro-inflammatory cytokines, such as IL-17 (REF. 74). With evidence demonstrating an adipose-tissue–colon feedback loop between substance P, adipocytes and IL-17, alterations in neuroendocrine cells and hormones could play into and further strengthen this feedback mechanism to further promote inflammation. Hence, through a combination of paracrine, enteroendocrine and microbial factors, obesity — in particular visceral adiposity — could contribute to development and perpetuation of inflammation in IBD, particularly Crohn’s disease.
Effect of obesity on IBD course
Obesity has been associated with high disease activity and worse clinical outcomes in several autoimmune diseases, including rheumatoid arthritis, psoriasis and psoriatic arthritis75,76. Studies in patients with Crohn’s disease suggest that overall obesity (as measured by BMI) is not consistently associated with an increased prevalence of IBD-related complications. In a cross-sectional study of 846 patients with Crohn’s disease, Pringle and colleagues10 observed a lower prevalence of penetrating disease complications in patients who were obese (odds ratio (OR) 0.56, 95% CI 0.31–0.99), but comparable risk of stricturing and perianal complications, compared with adults of a normal BMI. In another cross-sectional study of 297 patients with Crohn’s disease (30.3% obese), there was no difference in the prevalence of penetrating (20% versus 22%) or stricturing complications (17% versus 22%) between individuals who were obese or those of a normal weight8. Obesity, in particular class II (BMI ≥35 kg/m2) or class III obesity (BMI ≥40 kg/m2) might be associated with increased prevalence of colonic involvement with Crohn’s disease, but this finding has not been consistently observed8,10,11,13,14. Similarly, in patients with ulcerative colitis, obesity has not been associated with disease severity. In a cohort of 202 patients with ulcerative colitis (13.4% of whom were obese), Stabroth-Akil and colleagues14 observed a lower prevalence of pancolitis in patients who were obese than in patients of a normal BMI (33% versus 61%, respectively).
Longitudinal studies show variable effects of obesity on IBD disease course and the development of complications (TABLE 1). Inferior IBD-related quality of life and a higher frequency of elevated levels of serum C-reactive protein (an inflammatory marker) have been observed in patients with obesity (particularly class II or III obesity) compared with patients of normal weight; however, there was no statistically significant difference in the risk of IBD-related surgery, hospitalization or emergency department use between patients who were obese, overweight or a normal BMI12. A shorter time to first surgery in those who were overweight or obese versus undernourished individuals (2 years versus 21 years, respectively) has also been found, although there were no differences in the number of surgeries related to Crohn’s disease or escalation of medical therapy in patients with BMI ≥25 kg/m2 versus BMI <25 kg/m2 (REF. 77). By contrast, Flores and colleagues8 observed a lower risk of IBD-related surgery (41% versus 52% versus 61% for patients who were obese, overweight, or normal or underweight, respectively), hospitalization (42% versus 44% versus 66%) and initiation of anti-TNF therapy (25% versus 26% versus 43%), both in patients with Crohn’s disease and ulcerative colitis. Outcomes during an index hospitalization are generally worse among patients with IBD who are obese compared with non-obese patients with IBD. In a cross-sectional study using the Nationwide Inpatient Sample, we observed that among 6,742 hospitalized patients with ulcerative colitis, adults who are obese have higher rates of surgery (23.1% versus 14.2%), severe hospitalization (need for surgery or hospital stay >7days: 35.3% versus 26.2%) and longer hospital stay (mean 6.0 versus 5.4 days) than patients who are not obese78.
Contribution of visceral adiposity
Although overall obesity has not been consistently associated with more severe disease activity and progression to disease complications, visceral adiposity has been independently associated with increased risk of IBD-related complications. In a cross-sectional study, Erhayiem and colleagues79 observed that patients with complications of Crohn’s disease had a higher CT-measured visceral fat area and a higher visceral to subcutaneous fat area ratio (mesenteric fat index) than patients with inflammatory Crohn’s disease; on multivariate analysis, mesenteric fat index was independently associated with Crohn’s disease complications. In a retrospective cohort study of 114 paediatric patients with IBD (101 with Crohn’s disease), high VAT volume was associated with increased risk of penetrating or stricturing Crohn’s disease complications (OR 1.7, 95% CI 1.1–2.9), Crohn’s-disease-related hospitalization (OR, 1.9, 95% CI 1.2–3.4), shorter time interval to surgery (HR 1.4, 95% CI 1.0–2.0) and moderate–severe disease activity scores (OR 1.8, 95% CI 1.1–3.1), after adjusting for age and BMI24. High visceral fat area has also been associated with increased risk of recurrence of Crohn’s disease after surgical resection (endoscopic recurrence: HR 8.6, 95% CI 1.6–47.1; clinical recurrence: HR 2.6, 95% CI 1.0–6.7)80. Likewise, the metabolic consequences of obesity, in particular of visceral adiposity, have also been associated with IBD-related disease severity. In a single-centre retrospective cohort study of 868 patients with Crohn’s disease (19.0% of whom were obese), an increased incidence of IBD-related hospitalization was observed in the 4% of the cohort with metabolic syndrome (defined as >3 of the following features: obesity, diabetes mellitus, hypertension, high levels of circulating triglycerides and low levels of circulating HDL cholesterol), compared with those without metabolic syndrome (incidence rate ratio 1.79, 95% CI 1.06–3.00). However, on evaluating different components of metabolic syndrome, presence of diabetes mellitus, high circulating triglyceride levels and low HDL cholesterol levels, but not obesity, were associated with increased incidence of IBD-related hospitalization81. In another retrospective cohort study of 240 patients with Crohn’s disease (12.5% of whom were obese), although obesity was identified as a risk factor for surgery on univariate analysis (OR 2.2, 95% CI 1.0–4.8), it was not an independent predictor of surgery after adjustment for confounding variables. On the other hand, diabetes mellitus (which affected 6.7% of cohort) was a strong independent predictor of surgery, even after adjustment for corticosteroid use (OR 5.4, 95% CI 1.7–17.6)82. Patients with diabetes also had lower quality of life and higher clinical disease activity than individuals without diabetes.
Limitations of current literature
Current studies on the association between obesity and natural history of IBD have several limitations, and need to be interpreted carefully. Cross-sectional and cohort studies are retrospective in nature, which limits the ability to make causal inference given the dynamic nature of weight as well as IBD activity. This approach is limited by confounding by disease severity, wherein patients with severe disease activity (and at higher risk of disease-related complications) are likely to lose weight, resulting in potential misclassification of their BMI. On the other hand, patients with mild disease activity are more likely to maintain (or might gain) weight, as is the trend in the general population83; in these patients, obesity or overweight might be a manifestation rather than the aetiology of a mild disease course. Additionally, most studies failed to adjust for other potential confounding factors such as smoking and corticosteroid use. Smoking cessation might improve Crohn’s disease course, and could also result in weight gain causing a misclassification of obesity category. Similarly, use of corticosteroids, often prescribed to patients with more severe disease, is frequently associated with weight gain, potentially resulting in misclassification of BMI categories. Moreover, single tertiary-centre retrospective cohort studies have low event rates with limited follow-up, hence the independent effect of overweight and obesity on IBD course and development of complications has not been comprehensively studied. Design considerations for a more robust observational study of the relationship between IBD and obesity are described in BOX 1.
Box 1. Observational studies of obesity and IBD: clarifying the relationship.
A better designed observational study to evaluate the association between obesity and disease course and outcomes in IBD would possess the following features:
Inclusion of incident or prevalent cases with IBD
Measurement of BMI at cohort inception (preferably at a time when weight has been stable, when patients are in remission, off corticosteroids, in patients without recent change in smoking status)
Prospective evaluation of disease-related complication risk, stratified by baseline BMI
Adjustment for confounding factors (such as disease severity and current or prior disease complications, prior surgery and IBD-related therapy, among other factors)
Given the increasing recognition of metabolic differences in visceral (and mesenteric) and subcutaneous adiposity, overall BMI might not adequately capture the association between obesity and IBD. As observed in several studies, measurement of visceral fat (through VAT area or volume, or at least measurement of central obesity using surrogate measures such as WHR) could help us better understand the true effect of obesity on IBD.
Obesity and IBD-related therapy
Observational comparative effectiveness studies in Crohn’s disease suggest that infliximab is associated with lower rates of IBD-related hospitalization and abdominal surgery than adalimumab and certolizumab pegol84,85; these observations are also supported by indirect treatment comparison network meta-analyses86. These differences could be due to differences in systemic drug exposure related to body weight; infliximab dose is weight-based and intravenously administered, whereas other agents are administered subcutaneously in a fixed dose. For example, in a single-centre retrospective cohort study, Bhalme et al.87 observed — in adalimumab-treated but not infliximab-treated patients — a higher likelihood of dose escalation in patients who were obese than in patients who were not obese (BMI >35 kg/m2 versus BMI <25 kg/m2, 40% versus 20%, respectively). In an exploratory analysis of trials of adalimumab in patients with psoriasis, the response rate decreased progressively with increasing quartile of weight, from 74–79% in the lowest quartile to 62–71% in the highest quartile88. By contrast, in a pooled analysis of three randomized controlled trials of infliximab in psoriasis, the response rates were comparable in patients who were normal weight, overweight and obese (78% versus 78% versus 74%, no statistically significant difference between groups)88. Importantly, patients who are obese, particularly those who are morbidly obese, are less likely to receive optimal weight-appropriate therapy. Seminerio and colleagues12 observed that the average dose of infliximab in patients with class III obesity was ~4 mg/kg body weight, compared with 7.9 mg/kg body weight in those with normal BMI and 6.4 mg/kg body weight in patients who were overweight. Similarly, the usual per kilogram body weight dose of immunomodulators was lower in patients with class III obesity than in patients with normal BMI (azathioprine dose 1.1 mg/kg body weight per day versus 1.7 mg/kg body weight per day, respectively, and 6-mercaptopurine dose 0.7 mg/kg body weight per day versus 1.2 mg/kg body weight per day, respectively).
However, obesity might affect treatment response to biologics independent of drug exposure. Population pharmacokinetic studies of all biologic agents approved for use in IBD, including anti-TNF agents (infliximab89, adalimumab90, certolizumab pegol91, golimumab92,) and anti-integrin agents (vedolizumab)93, have identified high body weight as a risk factor associated with increased drug clearance, resulting in short half-life and low trough drug concentrations. This effect might be related to rapid proteolysis94 and to a ‘TNF sink’ phenomenon in patients with obesity, whereby increased levels of adipose-secreted TNF sequester anti-TNF agents. In a retrospective cohort study of 124 infliximab-treated patients with IBD, patients with obesity were 3–9 times more likely to have an IBD flare and require biologic dose escalation than normal weight patients95. Each unit increase in BMI was associated with a 6% higher likelihood of Crohn’s disease flare (HR 1.06, 95% CI 1.02–1.11) and 30% higher likelihood of ulcerative colitis flare (HR 1.30, 95% CI 1.07–1.58). In another single-centre prospective cohort of 199 patients with Crohn’s disease treated with adalimumab and followed over a median of 1 year, over one-third of patients were dose-escalated to weekly adalimumab within a median 5 months of initiating therapy, and BMI was the only independent predictor of dose escalation96. However, these findings have not been consistent. In a prospective, single-centre experience, no statistically significant association was observed between BMI and infliximab or adalimumab trough levels97.
Although data on obesity and treatment response in IBD is limited, similar observations have been made in biologic-agent-treated patients with other autoimmune diseases. In a prospective cohort of 89 patients with rheumatoid arthritis treated with infliximab, patients who were obese had lower rates of clinical response (measured using Disease Activity Score in 28 joints) than patients with a normal BMI, even after adjustment for baseline disease activity and anti-citrullinated protein antibody status (BMI >30 kg/m2 versus BMI 20–30 kg/m2 versus BMI <20 kg/m2, 50% versus 75% versus 84%, respectively)98. Similarly, in a prospective cohort of 641 patients with rheumatoid arthritis (10.3% of whom were obese), the cumulative rate of clinical remission at 12 months after treatment initiation was lowest in patients with obesity, compared with individuals who were overweight or normal BMI (15.2% versus 30.4% versus 32.9%, respectively)99. Likewise, in 557 patients with psoriatic arthritis (35.4% of whom were obese), likelihood of achieving sustained minimal disease activity was lowest in patients who were obese (OR 0.52, 95% CI 0.40–0.67) and overweight (OR 0.65, 95% CI 0.50–0.85), compared with patients with normal BMI100.
Besides biologic therapy, obesity might also modify response to immunomodulator therapy in IBD. Among 132 patients with IBD treated with thiopurines dosed according to body weight, patients who were obese were less likely to achieve therapeutic 6-thioguanine nucleotide concentrations, with thiopurines instead preferentially metabolized via the 6-methyl mercaptopurine nucleotide pathway101.
Effect of obesity on IBD-related surgery
Intra-abdominal surgeries in patients with obesity are both technically challenging and are usually associated with higher rates of postoperative complications than surgeries in patients with a normal BMI102. In a systematic review of 33 studies on the effect of obesity on perioperative outcomes with laparoscopic colorectal resection for any aetiology, Makino and colleagues103 observed longer operative times and an increased likelihood of conversion to open procedure in patients who were obese. Compared with patients who were not obese, patients with obesity had more comorbidities, a higher risk of postoperative complications (in particular wound infection) and a longer length of hospital stay. In a cohort of patients undergoing colorectal cancer surgery, increased perioperative mortality, higher rates of surgical complications, increased hospital costs and more frequent need for discharge to short-term rehabilitation facilities were observed in those with obesity compared with those who were not obese.
Key studies on the effect of obesity on surgical outcomes in patients with IBD are summarized in TABLE 3. In a nationwide US study of 382,637 inpatient hospitalizations for surgery in patients with IBD (using the Nationwide Inpatient Sample), Jain and colleagues105 observed that patients with obesity had increased rates of postoperative wound complications (OR 1.35, P = 0.01), infections (OR 1.16, P = 0.02), pulmonary complications (OR 1.21, P = 0.02), and shock (OR 1.30, P = 0.02). No difference in the risk of cardiovascular complications (OR 1.09, P = 0.52), perforations (OR 1.04, P = 0.71), venous thromboembolism (OR 1.18, P = 0.40) or death (OR 0.73, P = 0.07) was observed between obese and non-obese IBD surgeries. Two aspects of surgery that might be particularly challenging in patients with IBD merit special mention. First, obesity makes creating a stoma challenging, as it tends to retract, and is associated with higher rates of stoma-related complications such as parastomal hernia, mucocutaneous separation and stoma prolapse106,107. Second, the mesentery of patients with obesity tends to be foreshortened by the mesenteric fat, making it more challenging to create a J-pouch in patients with ulcerative colitis. Obesity increases risk of short-term postoperative complications in patients undergoing ileal pouch–anal anastomosis, although long-term outcomes might be comparable to those in patients without obesity in experienced centres108,109.
Table 3.
Key studies on the effect of obesity on surgical outcomes in patients with IBD.
| Study | Study characteristics | Patient characteristics | Obesity rate | Key findings | Refs |
|---|---|---|---|---|---|
| Krane et al. (2013) |
|
626 patients (all with BMI >18.5 kg/m2) undergoing laparoscopic colorectal resection |
|
|
124 |
| Causey et al. (2011) | Multi-centre, retrospective cohort study (National Surgical Quality Improvement Program), USA, 2005–2008 | 2,319 patients undergoing abdominal surgery for Crohn’s disease (25% performed laparoscopically) | 16% obese (2% morbidly obese) |
|
125 |
| Klos et al. (2014) |
|
178 patients with ulcerative colitis undergoing IPAA | 42% obese |
|
108 |
IPAA, ileal pouch–anal anastomosis.
Beyond overall obesity, visceral adiposity might be an independent predictor of surgical outcomes and complications in patients with IBD. Using analytic morphomics (the application of machine learning to biomedical images to derive patient-specific biomarkers), Stidham and colleagues observed that a high visceral to subcutaneous fat ratio, and not high BMI, is associated with increased risk of infectious complications after surgical resection for Crohn’s disease110. In another retrospective cohort of 164 patients undergoing primary Crohn’s disease-related surgery, a high visceral fat area (≥130 cm2) was independently associated with a 2.7-fold increased risk of overall postoperative complications111.
Besides surgery, an area that merits discussion in patients with IBD who are obese is medical imaging. Two types of abdominal imaging, CT and MRI, are frequently used in patients with IBD, and can impose special challenges in patients who are morbidly obese112. Equipment-specific issues include table weight limit, gantry width and tube capacity; imaging via MRI brings greater equipment-specific issues, as the standard MRI table weight capacity is 350 lbs (158.8 kg) and the standard machine bore diameter is 60 cm, limiting the ability to place patients in the scanner. Image-quality issues are also present, as a result of photon starvation, reduced contrast-to-noise ratio and truncation artefacts112. Owing to difficult intravenous access and risk of extra-vasation, and the need for weight-based contrast administration, there can be issues with intravenous contrast in patients who are obese. Additionally, claustrophobia and patient discomfort are substantial concerns when using MRI. Hence, despite inherent limitations, CT might be the best and easiest way to image larger patients. Specific solutions during imaging and post-image processing might be implemented to optimize image quality in patients who are obese.
Obesity treatment and IBD outcomes
Although there is considerable evidence that obesity adversely affects response to IBD-related therapy, there is a paucity of data on whether treating obesity can favourably affect outcomes in patients with IBD. No interventional studies of interventional weight loss in IBD exist; however, trials of diet and/or lifestyle-induced weight loss in other autoimmune diseases suggest improvement in outcomes with this form of adjunctive therapy. In a meta-analysis of five studies in patients with psoriasis who were obese or overweight, those randomized to weight loss intervention were 2.9 times more likely to achieve 75% reduction in Psoriasis Area and Severity Index score, compared with those not receiving the weight loss intervention (OR 2.92, 95% CI 1.39–6.13)113. Achieving as little as 5% weight loss is associated with favourable outcomes. In a 6-month randomized trial comparing a low-calorie or a free-managed diet (comprising dietary advice but no calorific restriction) in 126 patients with psoriatic arthritis starting anti-TNF therapy, Di Munno and colleagues114 observed a greater reduction in levels of pain and inflammatory markers, and higher likelihood of achieving minimal disease activity, in patients receiving a low-calorie diet (42.9% versus 34.9% of patients achieved minimal disease activity on low-calorie diet versus free-managed diet, respectively). Importantly, regardless of the intervention, magnitude of intentional weight loss was associated with improvement in outcomes: the proportion of patients attaining minimal disease activity in those achieving >10% weight loss, 5–10% weight loss and <5% weight loss was 59.5%, 44.8% and 23.1%, respectively. Patients who achieved at least 5% weight loss were 4.2 times more likely to achieve minimal disease activity compared with those who experienced <5% weight loss (OR 4.20, 95% CI 1.82–9.66).
Whether similar benefits would be observed among patients with IBD who are obese is speculative. Although bariatric surgery is technically feasible in patients with IBD, long-term consequences of major restrictive and malabsorptive procedures in these individuals are poorly understood. Case series have suggested that these procedures are safe in highly selected patients who are morbidly obese and have IBD, and have also anecdotally observed improvement in disease activity after weight loss115,116. However, short of bariatric surgery, other weight loss interventions would also probably be beneficial. Although dietary interventions carry low risk of adverse events, adherence to specific diets is limited, and despite intensive lifestyle interventions remaining the cornerstone of initial obesity management, their long-term effectiveness in a real-world setting is limited by small average weight loss with commercial weight-loss programmes and frequent regain of lost weight over time117. Weight loss achieved through pharmacological or endoscopic bariatric interventions might achieve the same effect on outcomes in IBD as in other autoimmune diseases, but has not been studied. With five weight-loss medications now approved by the FDA for long-term management of obesity, with average excess weight loss (over placebo) ranging from 2.6 kg (with orlistat) to 8.8 kg (phentermine–topiramate), and an estimated 44–75% of patients achieving the threshold of at least 5% weight loss, it is very appealing to consider pilot trials of the efficacy and safety of these medications as adjunct therapies in patients with IBD who are obese118. Two medications in particular merit attention: phentermine–topiramate and naltrexone–bupropion. Both of these medications result in statistically and clinically significant weight loss (mean 8.8 kg and 5.0 kg excess weight loss over placebo, respectively, and 75% and 50% patients achieving at least 5% weight loss, respectively), with low rates of serious adverse events. Preclinical data supports an anti-inflammatory role of both topiramate and phentermine. Using a novel computational approach for drug repositioning, Dudley and colleagues119 systematically compared the gene expression profiles of a compendium of 164 drug compounds in human cell lines to the gene expression signature of IBD derived from public microarray data of patient biopsy samples. On the basis of this analysis, they predicted that topiramate is very likely to be effective against both Crohn’s disease and ulcerative colitis. Subsequently, in vivo testing of topiramate was performed in a 2,4,6-trinitrobenzenesulfonic acid (TNBS) rat model of IBD, with three independent validations. Administration of topiramate for 7 days after TNBS-induced colitis resulted in markedly reduced diarrhoea and reduced pathological inflammation. Similarly, several lines of evidence have shown that noradrenaline might regulate intestinal mucosal immune responses mediated by intraepithelial lymphocytes via β1-adrenoreceptors120. The individual components of naltrexone–bupropion have also shown favourable effects in IBD. One 12-week trial of low-dose naltrexone (4.5 mg per day) in 40 patients with Crohn’s disease found higher rates of clinical and endoscopic remission in the intervention group than the placebo group; at this low dose, this observed benefit might be mediated through naltrexone’s anti-inflammatory effect rather than through weight loss121. Preclinical studies have suggested that bupropion might have an anti-TNF effect, with small case series reporting the effectiveness of this medication in inducing clinical remission122,123.
Conclusions
The prevalence of obesity in patients with IBD is sizeable and parallels rates in the general population, contrary to conventional beliefs that patients with IBD are malnourished. Although from a pathophysiological perspective, obesity, and in particular visceral adiposity, seems to promote intestinal inflammation, epidemiological studies implicating obesity in the development of IBD are limited. Understanding of the effects of obesity on cross-sectional disease severity and development of disease complications is incomplete, with studies conducted to date (with flawed designs and inherent limitations) showing conflicting results. On the basis of pharmacokinetic data, obesity seems to promote rapid clearance of biologic agents, regardless of drug dose. The effect of obesity on response to therapy remains to be studied, though data from other autoimmune diseases suggests that obesity is an independent predictor of poor response to medical therapy. This association provides an opportunity for using treatment directed towards obesity, either through lifestyle, pharmacological or endoscopic interventions, as adjunctive therapy in patients with IBD who are obese.
Key points.
~20–40% of patients with IBD in Western countries are obese
Premorbid obesity, in particular visceral adiposity, might increase the risk of developing Crohn’s disease
Obesity might contribute to the pathogenesis of IBD through dysbiosis, mucosal barrier dysfunction with bacterial translocation and activation of adipocytes
Risk of complications, such as surgery, hospitalization and infection, might be increased in patients with IBD who are obese
Obesity is associated with rapid clearance of biologic agents, resulting in low trough concentrations, and could result in suboptimal response to biologics
Treating obesity could be a potential adjunct therapeutic target in patients with IBD who are obese
Acknowledgments
S.S. is supported by the NIH/National Library of Medicine training grant T15LM011271, the American College of Gastroenterology Junior Faculty Development Award and the Crohn’s and Colitis Foundation of American Career Development Award. P.S.D. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases training grant 5T32DK007202. A.Z. has received support from NIH K08 DK102902, the American Association for the Study of Liver Diseases Liver Scholar Award, and the American Gastroenterological Association Microbiome Junior Investigator Research Award. The Authors thank L. J. Prokop, Senior Medical Librarian at Mayo Clinic, Rochester, USA, who assisted with a systematic literature review on this topic.
Footnotes
Author contributions
S.S. and P.S.D. researched data for the article. S.S., P.S.D., A.Z., S.R. and W.J.S. substantially contributed to the discussion of content for the article. S.S. and P.S.D. wrote the article, and all authors contributed equally to reviewing and editing of the manuscript before submission.
Competing interests statement
W.J.S. has received consulting fees from Abbvie, Janssen Biotech, Prometheus Laboratories and UCB Pharma, research grants from Abbvie, Janssen Pharmaceutical Research & Development and UCB Pharma, and payments for lectures or speakers bureau from Abbvie, Janssen Pharmaceutical Research & Development. S.S., P.S.D., A.Z. and S.R. declare no competing interests.
References
- 1.Ng M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff. 2009;28:w822–w831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 3.Molodecky NA, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Ng SC, et al. Early course of inflammatory bowel disease in a population-based inception cohort study from 8 countries in Asia and Australia. Gastroenterology. 2016;150:86–95.e3. doi: 10.1053/j.gastro.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- 6.Qin B, et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res Ther. 2015;17:86. doi: 10.1186/s13075-015-0601-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterry W, Strober BE, Menter A, International Psoriasis Council Obesity in psoriasis: the metabolic, clinical and therapeutic implications Report of an interdisciplinary conference and review. Br J Dermatol. 2007;157:649–655. doi: 10.1111/j.1365-2133.2007.08068.x. [DOI] [PubMed] [Google Scholar]
- 8.Flores A, Burstein E, Cipher DJ, Feagins LA. Obesity in inflammatory bowel disease: a marker of less severe disease. Dig Dis Sci. 2015;60:2436–2445. doi: 10.1007/s10620-015-3629-5. [DOI] [PubMed] [Google Scholar]
- 9.Long MD, et al. Prevalence and epidemiology of overweight and obesity in children with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:2162–2168. doi: 10.1002/ibd.21585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pringle PL, et al. Body mass index, genetic susceptibility, and risk of complications among individuals with Crohn’s disease. Inflamm Bowel Dis. 2015;21:2304–2310. doi: 10.1097/MIB.0000000000000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendall MA, Gunasekera AV, John BJ, Kumar D. Is obesity a risk factor for Crohn’s disease? Dig Dis Sci. 2011;56:837–844. doi: 10.1007/s10620-010-1541-6. [DOI] [PubMed] [Google Scholar]
- 12.Seminerio JL, et al. Impact of obesity on the management and clinical course of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:2857–2863. doi: 10.1097/MIB.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 13.Nic Suibhne T, et al. High prevalence of overweight and obesity in adults with Crohn’s disease: associations with disease and lifestyle factors. J Crohns Colitis. 2013;7:e241–e248. doi: 10.1016/j.crohns.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Stabroth-Akil D, Leifeld L, Pfutzer R, Morgenstern J, Kruis W. The effect of body weight on the severity and clinical course of ulcerative colitis. Int J Colorectal Dis. 2015;30:237–242. doi: 10.1007/s00384-014-2051-3. [DOI] [PubMed] [Google Scholar]
- 15.Steed H, Walsh S, Reynolds N. A brief report of the epidemiology of obesity in the inflammatory bowel disease population of Tayside, Scotland. Obes Facts. 2009;2:370–372. doi: 10.1159/000262276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kugathasan S, et al. Body mass index in children with newly diagnosed inflammatory bowel disease: observations from two multicenter North American inception cohorts. J Pediatr. 2007;151:523–527. doi: 10.1016/j.jpeds.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Blain A, et al. Crohn’s disease clinical course and severity in obese patients. Clin Nutr. 2002;21:51–57. doi: 10.1054/clnu.2001.0503. [DOI] [PubMed] [Google Scholar]
- 18.Moran GW, Dubeau MF, Kaplan GG, Panaccione R, Ghosh S. The increasing weight of Crohn’s disease subjects in clinical trials: a hypothesis-generatings time-trend analysis. Inflamm Bowel Dis. 2013;19:2949–2956. doi: 10.1097/MIB.0b013e31829936a4. [DOI] [PubMed] [Google Scholar]
- 19.Khalili H, et al. Measures of obesity and risk of Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2015;21:361–368. doi: 10.1097/MIB.0000000000000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harpsoe MC, et al. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol. 2014;43:843–855. doi: 10.1093/ije/dyu045. [DOI] [PubMed] [Google Scholar]
- 21.Chan SSM, et al. Body mass index and the risk for Crohn’s disease and ulcerative colitis: data from a European Prospective Cohort Study (The IBD in EPIC Study) Am J Gastroenterol. 2013;108:575–582. doi: 10.1038/ajg.2012.453. [DOI] [PubMed] [Google Scholar]
- 22.Hemminki K, Li X, Sundquist J, Sundquist K. Risk of asthma and autoimmune diseases and related conditions in patients hospitalized for obesity. Ann Med. 2012;44:289–295. doi: 10.3109/07853890.2010.547515. [DOI] [PubMed] [Google Scholar]
- 23.Kredel LI, Siegmund B. Adipose-tissue and intestinal inflammation — visceral obesity and creeping fat. Front Immunol. 2014;5:462. doi: 10.3389/fimmu.2014.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uko V, et al. Impact of abdominal visceral adipose tissue on disease outcome in pediatric Crohn’s disease. Inflamm Bowel Dis. 2014;20:2286–2291. doi: 10.1097/MIB.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 25.Khalili H, et al. Physical activity and risk of inflammatory bowel disease: prospective study from the Nurses’ Health Study cohorts. BMJ. 2013;347:f6633. doi: 10.1136/bmj.f6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bilski J, Brzozowski B, Mazur-Bialy A, Sliwowski Z, Brzozowski T. The role of physical exercise in inflammatory bowel disease. Biomed Res Int. 2014;2014:429031. doi: 10.1155/2014/429031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zietek T, Rath E. Inflammation meets metabolic disease: gut feeling mediated by GLP-1. Front Immunol. 2016;7:154. doi: 10.3389/fimmu.2016.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karmiris K, et al. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:100–105. doi: 10.1097/01.MIB.0000200345.38837.46. [DOI] [PubMed] [Google Scholar]
- 29.Tian J, Venn A, Otahal P, Gall S. The association between quitting smoking and weight gain: a systemic review and meta-analysis of prospective cohort studies. Obes Rev. 2015;16:883–901. doi: 10.1111/obr.12304. [DOI] [PubMed] [Google Scholar]
- 30.Berthon BS, MacDonald-Wicks LK, Wood LG. A systematic review of the effect of oral glucocorticoids on energy intake, appetite, and body weight in humans. Nutr Res. 2014;34:179–190. doi: 10.1016/j.nutres.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Wung PK, et al. Effects of glucocorticoids on weight change during the treatment of Wegener’s granulomatosis. Arthritis Rheum. 2008;59:746–753. doi: 10.1002/art.23561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winer DA, Luck H, Tsai S, Winer S. The intestinal immune system in obesity and insulin resistance. Cell Metab. 2016;23:413–426. doi: 10.1016/j.cmet.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Balistreri CR, Caruso C, Candore G. The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediators Inflamm. 2010;2010:802078. doi: 10.1155/2010/802078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 35.Kredel L, Batra A, Siegmund B. Role of fat and adipokines in intestinal inflammation. Curr Opin Gastroenterol. 2014;30:559–565. doi: 10.1097/MOG.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 36.Zulian A, et al. Visceral adipocytes: old actors in obesity and new protagonists in Crohn’s disease? Gut. 2012;61:86–94. doi: 10.1136/gutjnl-2011-300391. [DOI] [PubMed] [Google Scholar]
- 37.Batra A, Zeitz M, Siegmund B. Adipokine signaling in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1897–1905. doi: 10.1002/ibd.20937. [DOI] [PubMed] [Google Scholar]
- 38.Dulai PS, Levesque BG, Feagan BG, D’Haens G, Sandborn WJ. Assessment of mucosal healing in inflammatory bowel disease: review. Gastrointest Endosc. 2015;82:246–255. doi: 10.1016/j.gie.2015.03.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 41.Schaffler A, Scholmerich J, Salzberger B. Adipose tissue as an immunological organ: Toll-like receptors, C1q/TNFs and CTRPs. Trends Immunol. 2007;28:393–399. doi: 10.1016/j.it.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Stroh T, et al. Nucleotide oligomerization domains 1 and 2: regulation of expression and function in preadipocytes. J Immunol. 2008;181:3620–3627. doi: 10.4049/jimmunol.181.5.3620. [DOI] [PubMed] [Google Scholar]
- 43.Charriere G, et al. Preadipocyte conversion to macrophage: evidence of plasticity. J Biol Chem. 2003;278:9850–9855. doi: 10.1074/jbc.M210811200. [DOI] [PubMed] [Google Scholar]
- 44.Desreumaux P, et al. Inflammatory alterations in mesenteric adipose tissue in Crohn’s disease. Gastroenterology. 1999;117:73–81. doi: 10.1016/s0016-5085(99)70552-4. [DOI] [PubMed] [Google Scholar]
- 45.Paul G, et al. Profiling adipocytokine secretion from creeping fat in Crohn’s disease. Inflamm Bowel Dis. 2006;12:471–477. doi: 10.1097/00054725-200606000-00005. [DOI] [PubMed] [Google Scholar]
- 46.La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- 47.Batra A, et al. Leptin: a critical regulator of CD4+ T-cell polarization in vitro and in vivo. Endocrinology. 2010;151:56–62. doi: 10.1210/en.2009-0565. [DOI] [PubMed] [Google Scholar]
- 48.Siegmund B, Lehr HA, Fantuzzi G. Leptin: a pivotal mediator of intestinal inflammation in mice. Gastroenterology. 2002;122:2011–2025. doi: 10.1053/gast.2002.33631. [DOI] [PubMed] [Google Scholar]
- 49.Nishihara T, et al. Effect of adiponectin on murine colitis induced by dextran sulfate sodium. Gastroenterology. 2006;131:853–861. doi: 10.1053/j.gastro.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 50.Fayad R, et al. Adiponectin deficiency protects mice from chemically induced colonic inflammation. Gastroenterology. 2007;132:601–614. doi: 10.1053/j.gastro.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 51.Ohashi K, et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285:6153–6160. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogunwobi OO, Beales IL. Adiponectin stimulates proliferation and cytokine secretion in colonic epithelial cells. Regul Pept. 2006;134:105–113. doi: 10.1016/j.regpep.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 53.Schaffler A, Scholmerich J. The role of adiponectin in inflammatory gastrointestinal diseases. Gut. 2009;58:317–322. doi: 10.1136/gut.2008.159210. [DOI] [PubMed] [Google Scholar]
- 54.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 55.Konrad A, et al. Resistin is an inflammatory marker of inflammatory bowel disease in humans. Eur J Gastroenterol Hepatol. 2007;19:1070–1074. doi: 10.1097/MEG.0b013e3282f16251. [DOI] [PubMed] [Google Scholar]
- 56.Sideri A, et al. Effects of obesity on severity of colitis and cytokine expression in mouse mesenteric fat. Potential role of adiponectin receptor 1. Am J Physiol Gastrointest Liver Physiol. 2015;308:G591–G604. doi: 10.1152/ajpgi.00269.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zulian A, et al. Differences in visceral fat and fat bacterial colonization between ulcerative colitis and Crohn’s disease. An in vivo and in vitro study. PLoS ONE. 2013;8:e78495. doi: 10.1371/journal.pone.0078495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peyrin-Biroulet L, et al. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn’s disease. Gut. 2012;61:78–85. doi: 10.1136/gutjnl-2011-300370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamamoto K, et al. Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn’s disease. Gut. 2005;54:789–796. doi: 10.1136/gut.2004.046516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheehan AL, Warren BF, Gear MW, Shepherd NA. Fat-wrapping in Crohn’s disease: pathological basis and relevance to surgical practice. Br J Surg. 1992;79:955–958. doi: 10.1002/bjs.1800790934. [DOI] [PubMed] [Google Scholar]
- 61.Kim A. Dysbiosis: a review highlighting obesity and inflammatory bowel disease. J Clin Gastroenterol. 2015;49(Suppl. 1):S20–S24. doi: 10.1097/MCG.0000000000000356. [DOI] [PubMed] [Google Scholar]
- 62.Worthington JJ. The intestinal immunoendocrine axis: novel cross-talk between enteroendocrine cells and the immune system during infection and inflammatory disease. Biochem Soc Trans. 2015;43:727–733. doi: 10.1042/BST20150090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cani PD, Everard A, Duparc T. Gut microbiota, enteroendocrine functions and metabolism. Curr Opin Pharmacol. 2013;13:935–940. doi: 10.1016/j.coph.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 64.Leone VA, Cham CM, Chang EB. Diet, gut microbes, and genetics in immune function: can we leverage our current knowledge to achieve better outcomes in inflammatory bowel diseases? Curr Opin Immunol. 2014;31:16–23. doi: 10.1016/j.coi.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee D, et al. Diet in the pathogenesis and treatment of inflammatory bowel diseases. Gastroenterology. 2015;148:1087–1106. doi: 10.1053/j.gastro.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gruber L, et al. High fat diet accelerates pathogenesis of murine Crohn’s disease-like ileitis independently of obesity. PLoS ONE. 2013;8:e71661. doi: 10.1371/journal.pone.0071661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106:563–573. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 68.Rioux JD, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sakiyama T, Fujita H, Tsubouchi H. Autoantibodies against ubiquitination factor E4A (UBE4A) are associated with severity of Crohn’s disease. Inflamm Bowel Dis. 2008;14:310–317. doi: 10.1002/ibd.20328. [DOI] [PubMed] [Google Scholar]
- 70.Harrison E, Lal S, McLaughlin JT. Enteroendocrine cells in gastrointestinal pathophysiology. Curr Opin Pharmacol. 2013;13:941–945. doi: 10.1016/j.coph.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 71.Qi KK, Wu J, Wan J, Men XM, Xu ZW. Purified PEGylated porcine glucagon-like peptide-2 reduces the severity of colonic injury in a murine model of experimental colitis. Peptides. 2014;52:11–18. doi: 10.1016/j.peptides.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 72.Blonski W, Buchner AM, Aberra F, Lichtenstein G. Teduglutide in Crohn’s disease. Expert Opin Biol Ther. 2013;13:1207–1214. doi: 10.1517/14712598.2013.815721. [DOI] [PubMed] [Google Scholar]
- 73.Moran GW, Leslie FC, McLaughlin JT. Crohn’s disease affecting the small bowel is associated with reduced appetite and elevated levels of circulating gut peptides. Clin Nutr. 2013;32:404–411. doi: 10.1016/j.clnu.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 74.Friedrich M, Diegelmann J, Schauber J, Auernhammer CJ, Brand S. Intestinal neuroendocrine cells and goblet cells are mediators of IL-17A-amplified epithelial IL-17C production in human inflammatory bowel disease. Mucosal Immunol. 2015;8:943–958. doi: 10.1038/mi.2014.124. [DOI] [PubMed] [Google Scholar]
- 75.Iannone F, et al. Impact of obesity on the clinical outcome of rheumatologic patients in biotherapy. Autoimmun Rev. 2016;15:447–450. doi: 10.1016/j.autrev.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 76.Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev. 2014;13:981–1000. doi: 10.1016/j.autrev.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 77.Hass DJ, Brensinger CM, Lewis JD, Lichtenstein GR. The impact of increased body mass index on the clinical course of Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:482–488. doi: 10.1016/j.cgh.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 78.Singh S, Khera R, Sandborn WJ. Obesity is associated with worse outcomes in hospitalized patients with inflammatory bowel diseases: a nationwide study. Am J Gastroenterol. 2016;111:S271. [Google Scholar]
- 79.Erhayiem B, Dhingsa R, Hawkey CJ, Subramanian V. Ratio of visceral to subcutaneous fat area is a biomarker of complicated Crohn’s disease. Clin Gastroenterol Hepatol. 2011;9:684–687.e1. doi: 10.1016/j.cgh.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 80.Li Y, et al. Visceral fat area is associated with a high risk for early postoperative recurrence in Crohn’s disease. Colorectal Dis. 2015;17:225–234. doi: 10.1111/codi.12798. [DOI] [PubMed] [Google Scholar]
- 81.Fitzmorris PS, et al. Impact of metabolic syndrome on the hospitalization rate of Crohn’s disease patients seen at a tertiary care center: a retrospective cohort study. Digestion. 2015;91:257–262. doi: 10.1159/000380763. [DOI] [PubMed] [Google Scholar]
- 82.Harper JW, Welch MP, Sinanan MN, Wahbeh GT, Lee SD. Co-morbid diabetes in patients with Crohn’s disease predicts a greater need for surgical intervention. Aliment Pharmacol Ther. 2012;35:126–132. doi: 10.1111/j.1365-2036.2011.04915.x. [DOI] [PubMed] [Google Scholar]
- 83.Dutton GR, et al. 25-year weight gain in a racially balanced sample of U.S. adults: The CARDIA study. Obesity. 2016;24:19620–1968. doi: 10.1002/oby.21573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh S, et al. Comparative effectiveness and safety of anti-tumor necrosis factor agents in biologic-naive patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2016;14:1120–1129.e6. doi: 10.1016/j.cgh.2016.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Osterman MT, et al. Comparative effectiveness of infliximab and adalimumab for Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:811–817.e3. doi: 10.1016/j.cgh.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh S, et al. Comparative efficacy of biologic therapy in biologic-naive patients with Crohn disease: a systematic review and network meta-analysis. Mayo Clin Proc. 2014;89:1621–1635. doi: 10.1016/j.mayocp.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 87.Bhalme M, Sharma A, Keld R, Willert R, Campbell S. Does weight-adjusted anti-tumour necrosis factor treatment favour obese patients with Crohn’s disease? Eur J Gastroenterol Hepatol. 2013;25:543–549. doi: 10.1097/MEG.0b013e32835d1f15. [DOI] [PubMed] [Google Scholar]
- 88.Puig L. Obesity and psoriasis: body weight and body mass index influence the response to biological treatment. J Eur Acad Dermatol Venereol. 2011;25:1007–1011. doi: 10.1111/j.1468-3083.2011.04065.x. [DOI] [PubMed] [Google Scholar]
- 89.Dotan I, et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis. 2014;20:2247–2259. doi: 10.1097/MIB.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 90.Sharma S, et al. Pharmacokinetics and exposure-efficacy relationship of adalimumab in pediatric patients with moderate to severe Crohn’s disease: results from a randomized, multicenter, phase-3 study. Inflamm Bowel Dis. 2015;21:783–792. doi: 10.1097/MIB.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 91.Colombel JF, et al. Association between plasma concentrations of certolizumab pegol and endoscopic outcomes of patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:423–431.e1. doi: 10.1016/j.cgh.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 92.Xu ZH, et al. Population pharmacokinetics of golimumab in patients with ankylosing spondylitis: impact of body weight and immunogenicity. Int J Clin Pharmacol Ther. 2010;48:596–607. doi: 10.5414/cpp48596. [DOI] [PubMed] [Google Scholar]
- 93.Rosario M, et al. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment Pharmacol Ther. 2015;42:188–202. doi: 10.1111/apt.13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brill MJ, et al. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51:277–304. doi: 10.2165/11599410-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 95.Harper JW, Sinanan MN, Zisman TL. Increased body mass index is associated with earlier time to loss of response to infliximab in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2118–2124. doi: 10.1097/MIB.0b013e31829cf401. [DOI] [PubMed] [Google Scholar]
- 96.Bultman E, et al. Predictors of dose escalation of adalimumab in a prospective cohort of Crohn’s disease patients. Aliment Pharmacol Ther. 2012;35:335–341. doi: 10.1111/j.1365-2036.2011.04946.x. [DOI] [PubMed] [Google Scholar]
- 97.Bond A, et al. Comparative analysis of the influence of clinical factors including BMI on adalimumab and infliximab trough levels. Eur J Gastroenterol Hepatol. 2016;28:271–276. doi: 10.1097/MEG.0000000000000544. [DOI] [PubMed] [Google Scholar]
- 98.Klaasen R, Wijbrandts CA, Gerlag DM, Tak PP. Body mass index and clinical response to infliximab in rheumatoid arthritis. Arthritis Rheum. 2011;63:359–364. doi: 10.1002/art.30136. [DOI] [PubMed] [Google Scholar]
- 99.Gremese E, et al. Obesity and reduction of the response rate to anti-tumor necrosis factor α in rheumatoid arthritis: an approach to a personalized medicine. Arthritis Care Res. 2013;65:94–100. doi: 10.1002/acr.21768. [DOI] [PubMed] [Google Scholar]
- 100.Eder L, Thavaneswaran A, Chandran V, Cook RJ, Gladman DD. Obesity is associated with a lower probability of achieving sustained minimal disease activity state among patients with psoriatic arthritis. Ann Rheum Dis. 2015;74:813–817. doi: 10.1136/annrheumdis-2013-204448. [DOI] [PubMed] [Google Scholar]
- 101.Poon SS, et al. Body mass index and smoking affect thioguanine nucleotide levels in inflammatory bowel disease. J Crohns Colitis. 2015;9:640–646. doi: 10.1093/ecco-jcc/jjv084. [DOI] [PubMed] [Google Scholar]
- 102.Boutros M, Maron D. Inflammatory bowel disease in the obese patient. Clin Colon Rectal Surg. 2011;24:244–252. doi: 10.1055/s-0031-1295687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Makino T, Shukla PJ, Rubino F, Milsom JW. The impact of obesity on perioperative outcomes after laparoscopic colorectal resection. Ann Surg. 2012;255:228–236. doi: 10.1097/SLA.0b013e31823dcbf7. [DOI] [PubMed] [Google Scholar]
- 104.Hussan H, et al. Morbid obesity is associated with increased mortality, surgical complications, and incremental health care utilization in the peri-operative period of colorectal cancer surgery. World J Surg. 2016;40:987–994. doi: 10.1007/s00268-015-3358-0. [DOI] [PubMed] [Google Scholar]
- 105.Jain A, Limketkai BN, Hutfless S. The effect of obesity on post-surgical complications during hospitalizations for inflammatory bowel disease: a nationwide analysis. Gastroenterology. 2014;146:S595–S596. [Google Scholar]
- 106.Duchesne JC, Wang YZ, Weintraub SL, Boyle M, Hunt JP. Stoma complications: a multivariate analysis. Am Surg. 2002;68:961–966. [PubMed] [Google Scholar]
- 107.Beck SJ. Stoma issues in the obese patient. Clin Colon Rectal Surg. 2011;24:259–262. doi: 10.1055/s-0031-1295689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Klos CL, et al. Obesity increases risk for pouch-related complications following restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) J Gastrointest Surg. 2014;18:573–579. doi: 10.1007/s11605-013-2353-8. [DOI] [PubMed] [Google Scholar]
- 109.Kiran RP, et al. Complications and functional results after ileoanal pouch formation in obese patients. J Gastrointest Surg. 2008;12:668–674. doi: 10.1007/s11605-008-0465-3. [DOI] [PubMed] [Google Scholar]
- 110.Stidham RW, et al. Body fat composition assessment using analytic morphomics predicts infectious complications after bowel resection in Crohn’s disease. Inflamm Bowel Dis. 2015;21:1306–1313. doi: 10.1097/MIB.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ding Z, et al. Association between high visceral fat area and postoperative complications in patients with Crohn’s disease following primary surgery. Colorectal Dis. 2016;18:163–172. doi: 10.1111/codi.13128. [DOI] [PubMed] [Google Scholar]
- 112.Carucci LR. Imaging obese patients: problems and solutions. Abdom Imaging. 2013;38:630–646. doi: 10.1007/s00261-012-9959-2. [DOI] [PubMed] [Google Scholar]
- 113.Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes. 2015;39:1197–1202. doi: 10.1038/ijo.2015.64. [DOI] [PubMed] [Google Scholar]
- 114.Di Minno MN, et al. Weight loss and achievement of minimal disease activity in patients with psoriatic arthritis starting treatment with tumour necrosis factor α blockers. Ann Rheum Dis. 2014;73:1157–1162. doi: 10.1136/annrheumdis-2012-202812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Colombo F, et al. Bariatric surgery in patients with inflammatory bowel disease: an accessible path? Report of a case series and review of the literature. J Crohns Colitis. 2015;9:185–190. doi: 10.1093/ecco-jcc/jju011. [DOI] [PubMed] [Google Scholar]
- 116.Aminian A, et al. Outcomes of bariatric surgery in patients with inflammatory bowel disease. Obes Surg. 2016;26:1186–1190. doi: 10.1007/s11695-015-1909-y. [DOI] [PubMed] [Google Scholar]
- 117.Johnston BC, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA. 2014;312:923–933. doi: 10.1001/jama.2014.10397. [DOI] [PubMed] [Google Scholar]
- 118.Khera R, et al. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA. 2016;315:2424–2434. doi: 10.1001/jama.2016.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dudley JT et al. Computational repositioning of the anticonvulsant topiramate for inflammatory bowel disease. Sci Transl Med. 2011;3:96ra76. doi: 10.1126/scitranslmed.3002648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Takayanagi Y, et al. Norepinephrine suppresses IFN-gamma and TNF-alpha production by murine intestinal intraepithelial lymphocytes via the beta(1) adrenoceptor. J Neuroimmunol. 2012;245:66–74. doi: 10.1016/j.jneuroim.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 121.Smith JP, et al. Therapy with the opioid antagonist naltrexone promotes mucosal healing in active Crohn’s disease: a randomized placebo-controlled trial. Dig Dis Sci. 2011;56:2088–2097. doi: 10.1007/s10620-011-1653-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brustolim D, Ribeiro-dos-Santos R, Kast RE, Altschuler EL, Soares MB. A new chapter opens in anti-inflammatory treatments: the antidepressant bupropion lowers production of tumor necrosis factor-alpha and interferon-gamma in mice. Int Immunopharmacol. 2006;6:903–907. doi: 10.1016/j.intimp.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 123.Kane S, Altschuler EL, Kast RE. Crohn’s disease remission on bupropion. Gastroenterology. 2003;125:1290. doi: 10.1016/j.gastro.2003.02.004. [DOI] [PubMed] [Google Scholar]
- 124.Krane MK, et al. Does morbid obesity change outcomes after laparoscopic surgery for inflammatory bowel disease? Review of 626 consecutive cases. J Am Coll Surg. 2013;216:986–996. doi: 10.1016/j.jamcollsurg.2013.01.053. [DOI] [PubMed] [Google Scholar]
- 125.Causey MW, et al. The impact of obesity on outcomes following major surgery for Crohn’s disease: an American College of Surgeons National Surgical Quality Improvement Program assessment. Dis Colon Rectum. 2011;54:1488–1495. doi: 10.1097/DCR.0b013e3182342ccb. [DOI] [PubMed] [Google Scholar]