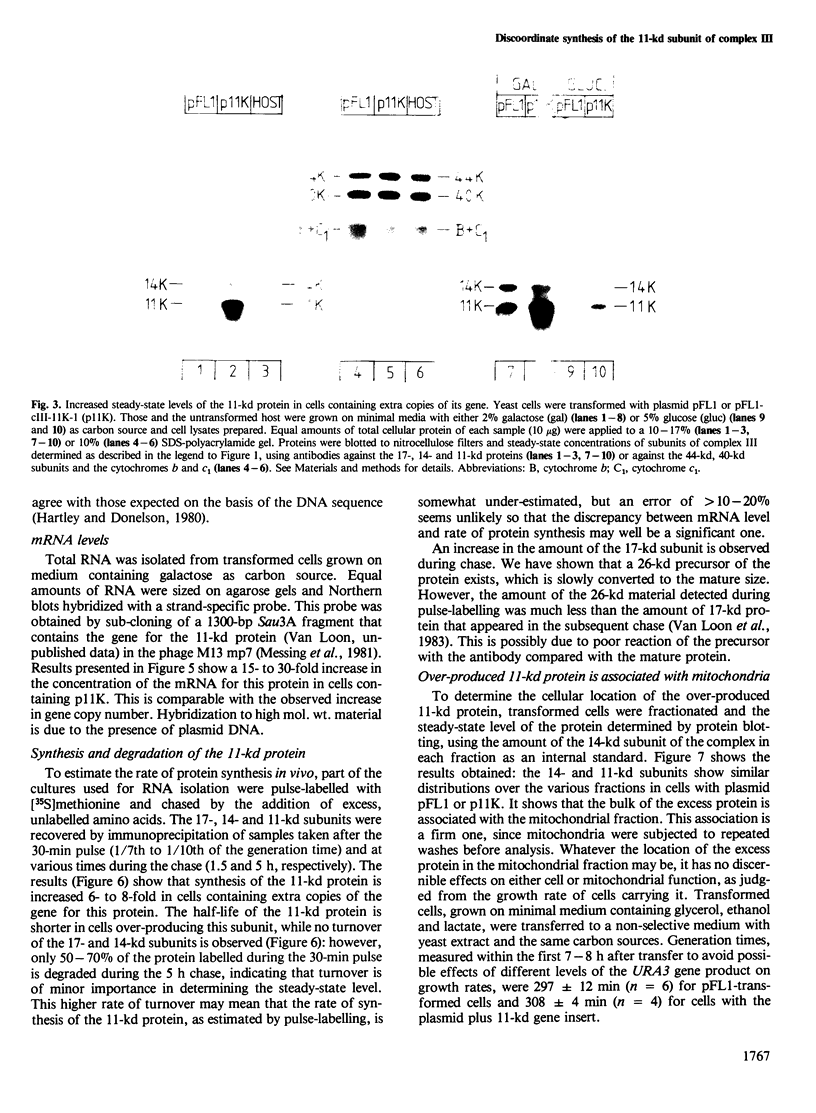
Abstract
In wild-type yeast cells, steady-state concentrations of subunits of the ubiquinol-cytochrome c reductase complex (complex III) and the levels of their translatable mRNAs change coordinately in response to the need for mitochondrial function. Despite this, re-introduction of the cloned gene for one of the subunits (11 kd) into cells by transformation with a free-replicating plasmid results in the discoordinate synthesis of this subunit only, without effects on either the synthesis or degradation of the other subunits. The overproduced subunit is associated with the mitochondrial fraction, yet does not interfere with mitochondrial function, as judged by the growth of transformed cells on nonfermentable media. Quantitative analysis of both mRNA and protein levels suggests that both translational controls and elevated turnover of excess protein contribute to a partial compensation for the effects of increased gene dosage in transformed cells. These contain approximately 30 copies of the cloned gene and 15-30 times the normal level of its mRNA. Nevertheless, synthesis of the 11-kd protein is only 6- to 8-fold higher than normal, and steady-state levels are increased only 5- to 10-fold. These findings imply that synthesis of the various subunits of complex III is not tightly coupled and that for the 11-kd subunit at least, the level of mRNA is likely to be the most important means of regulating protein level. Fine-tuning may be additionally achieved by control of translation and degradation of excess protein which is not assembled in the complex.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M. L., Lacroute F., Botstein D. Evidence for transcriptional regulation of orotidine-5'-phosphate decarboxylase in yeast by hybridization of mRNA to the yeast structural gene cloned in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):386–390. doi: 10.1073/pnas.76.1.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. Purification and properties of poliovirus double-stranded ribonucleic acid. J Mol Biol. 1966 Jul;18(3):421–428. doi: 10.1016/s0022-2836(66)80034-7. [DOI] [PubMed] [Google Scholar]
- Beggs J. D. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978 Sep 14;275(5676):104–109. doi: 10.1038/275104a0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chevallier M. R., Bloch J. C., Lacroute F. Transcriptional and translational expression of a chimeric bacterial-yeast plasmid in yeasts. Gene. 1980 Oct;11(1-2):11–19. doi: 10.1016/0378-1119(80)90082-7. [DOI] [PubMed] [Google Scholar]
- Côté C., Solioz M., Schatz G. Biogenesis of the cytochrome bc1 complex of yeast mitochondria. A precursor form of the cytoplasmically made subunit V. J Biol Chem. 1979 Mar 10;254(5):1437–1439. [PubMed] [Google Scholar]
- Gasser S. M., Ohashi A., Daum G., Böhni P. C., Gibson J., Reid G. A., Yonetani T., Schatz G. Imported mitochondrial proteins cytochrome b2 and cytochrome c1 are processed in two steps. Proc Natl Acad Sci U S A. 1982 Jan;79(2):267–271. doi: 10.1073/pnas.79.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. L., Donelson J. E. Nucleotide sequence of the yeast plasmid. Nature. 1980 Aug 28;286(5776):860–865. doi: 10.1038/286860a0. [DOI] [PubMed] [Google Scholar]
- Katan M. B., Harten-Loosbroek N. V., Groot G. S. The cytochrome bc) complex of yeast mitochondria. Site of translation of the polypeptides in vivo. Eur J Biochem. 1976 Nov 15;70(2):409–417. doi: 10.1111/j.1432-1033.1976.tb11031.x. [DOI] [PubMed] [Google Scholar]
- Katan M. B., Pool L., Groot G. S. The cytochrome bc1 complex of yeast mitochondria. Isolation and partial characterization of the cytochrome bc1 complex and cytochrome b. Eur J Biochem. 1976 May 17;65(1):95–105. doi: 10.1111/j.1432-1033.1976.tb10393.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacroute F. Regulation of pyrimidine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1968 Mar;95(3):824–832. doi: 10.1128/jb.95.3.824-832.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marcu K., Dudock B. Characterization of a highly efficient protein synthesizing system derived from commercial wheat germ. Nucleic Acids Res. 1974 Nov;1(11):1385–1397. doi: 10.1093/nar/1.11.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi A., Gibson J., Gregor I., Schatz G. Import of proteins into mitochondria. The precursor of cytochrome c1 is processed in two steps, one of them heme-dependent. J Biol Chem. 1982 Nov 10;257(21):13042–13047. [PubMed] [Google Scholar]
- Ohnishi T., Kawaguchi K., Hagihara B. Preparation and some properties of yeast mitochondria. J Biol Chem. 1966 Apr 25;241(8):1797–1806. [PubMed] [Google Scholar]
- Osley M. A., Hereford L. M. Yeast histone genes show dosage compensation. Cell. 1981 May;24(2):377–384. doi: 10.1016/0092-8674(81)90327-5. [DOI] [PubMed] [Google Scholar]
- Papa S. Proton translocation reactions in the respiratory chains. Biochim Biophys Acta. 1976 Apr 30;456(1):39–84. doi: 10.1016/0304-4173(76)90008-2. [DOI] [PubMed] [Google Scholar]
- Pearson N. J., Fried H. M., Warner J. R. Yeast use translational control to compensate for extra copies of a ribosomal protein gene. Cell. 1982 Jun;29(2):347–355. doi: 10.1016/0092-8674(82)90151-9. [DOI] [PubMed] [Google Scholar]
- Perlman P. S., Mahler H. R. Derepression of mitochondria and their enzymes in yeast: regulatory aspects. Arch Biochem Biophys. 1974 May;162(1):248–271. doi: 10.1016/0003-9861(74)90125-8. [DOI] [PubMed] [Google Scholar]
- Rieske J. S. Composition, structure, and function of complex III of the respiratory chain. Biochim Biophys Acta. 1976 Sep 27;456(2):195–247. doi: 10.1016/0304-4173(76)90012-4. [DOI] [PubMed] [Google Scholar]
- Ross E., Schatz G. Cytochrome c1 of bakers' yeast. II. Synthesis on cytoplasmic robosomes and influence of oxygen and heme on accumulation of the apoprotein. J Biol Chem. 1976 Apr 10;251(7):1997–2004. [PubMed] [Google Scholar]
- Siedow J. N., Power S., de la Rosa F. F., Palmer G. The preparation and characterization of highly purified, enzymically active complex III from baker's yeast. J Biol Chem. 1978 Apr 10;253(7):2392–2399. [PubMed] [Google Scholar]
- Slater E. C. Mechanism of oxidative phosphorylation. Annu Rev Biochem. 1977;46:1015–1026. doi: 10.1146/annurev.bi.46.070177.005055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suissa M., Schatz G. Import of proteins into mitochondria. Translatable mRNAs for imported mitochondrial proteins are present in free as well as mitochondria-bound cytoplasmic polysomes. J Biol Chem. 1982 Nov 10;257(21):13048–13055. [PubMed] [Google Scholar]
- Vaessen R. T., Kreike J., Groot G. S. Protein transfer to nitrocellulose filters. A simple method for quantitation of single proteins in complex mixtures. FEBS Lett. 1981 Feb 23;124(2):193–196. doi: 10.1016/0014-5793(81)80134-2. [DOI] [PubMed] [Google Scholar]
- Van Ommen G. J., Groot G. S., Grivell L. A. Transcription maps of mtDNAs of two strains of saccharomyces: transcription of strain-specific insertions; Complex RNA maturation and splicing. Cell. 1979 Oct;18(2):511–523. doi: 10.1016/0092-8674(79)90068-0. [DOI] [PubMed] [Google Scholar]
- van Loon A. P., de Groot R. J., van Eyk E., van der Horst G. T., Grivell L. A. Isolation and characterization of nuclear genes coding for subunits of the yeast ubiquinol-cytochrome c reductase complex. Gene. 1982 Dec;20(3):323–337. doi: 10.1016/0378-1119(82)90201-3. [DOI] [PubMed] [Google Scholar]