ABSTRACT
Microbial N2 fixation (diazotrophy) represents an important nitrogen source to oligotrophic peatland ecosystems, which are important sinks for atmospheric CO2 and are susceptible to the changing climate. The objectives of this study were (i) to determine the active microbial group and type of nitrogenase mediating diazotrophy in an ombrotrophic Sphagnum-dominated peat bog (the S1 peat bog, Marcell Experimental Forest, Minnesota, USA); and (ii) to determine the effect of environmental parameters (light, O2, CO2, and CH4) on potential rates of diazotrophy measured by acetylene (C2H2) reduction and 15N2 incorporation. A molecular analysis of metabolically active microbial communities suggested that diazotrophy in surface peat was primarily mediated by Alphaproteobacteria (Bradyrhizobiaceae and Beijerinckiaceae). Despite higher concentrations of dissolved vanadium ([V] 11 nM) than molybdenum ([Mo] 3 nM) in surface peat, a combination of metagenomic, amplicon sequencing, and activity measurements indicated that Mo-containing nitrogenases dominate over the V-containing form. Acetylene reduction was only detected in surface peat exposed to light, with the highest rates observed in peat collected from hollows with the highest water contents. Incorporation of 15N2 was suppressed 90% by O2 and 55% by C2H2 and was unaffected by CH4 and CO2 amendments. These results suggest that peatland diazotrophy is mediated by a combination of C2H2-sensitive and C2H2-insensitive microbes that are more active at low concentrations of O2 and show similar activity at high and low concentrations of CH4.
IMPORTANCE Previous studies indicate that diazotrophy provides an important nitrogen source and is linked to methanotrophy in Sphagnum-dominated peatlands. However, the environmental controls and enzymatic pathways of peatland diazotrophy, as well as the metabolically active microbial populations that catalyze this process, remain in question. Our findings indicate that oxygen levels and photosynthetic activity override low nutrient availability in limiting diazotrophy and that members of the Alphaproteobacteria (Rhizobiales) catalyze this process at the bog surface using the molybdenum-based form of the nitrogenase enzyme.
KEYWORDS: Alphaproteobacteria, Sphagnum, acetylene, diazotrophy, methanotrophs, molybdenum, nitrogen cycle enzymes, nitrogen fixation, peatland, vanadium
INTRODUCTION
High-latitude peatlands store approximately one-third of global soil carbon and may pose a climatic threat if rising global temperatures accelerate the release of this stored carbon in gaseous forms, as either carbon dioxide or methane (1–3). Mineral-poor (ombrotrophic) peatlands receive most of their nutrient inputs from atmospheric deposition and contain Sphagnum moss as their primary plant cover (2, 4). The peat moss Sphagnum is a keystone genus in these ecosystems and is responsible for much of the primary production and recalcitrant dead organic matter (5, 6). Sphagnum mosses also host complex microbiomes (7–10), including N2 fixers (diazotrophs) that are significant nitrogen sources for peatland ecosystems (11).
Despite decades of research, there is still much debate on the identity of the dominant diazotrophs in ombrotrophic peatlands. Early work implicated Cyanobacteria (12–14) or heterotrophic bacteria (15) based primarily on microscopic studies, while more recent molecular analyses argue for the importance of methanotrophic Beijerinckiaceae (16) as major diazotrophs in Sphagnum peat bogs (17–20). Possible contributions from other potential diazotrophs, such as strictly anaerobic methanogenic Euryarchaeota, remain unknown. However, it is quite possible that diverse diazotrophs exist within defined niches of peatland environments (21).
Diazotrophy is catalyzed by the nitrogenase metalloenzyme, a complex of thee subunits (H, D, and K) that contains abundant iron as Fe-S clusters. This enzyme is extremely O2 sensitive (22) and must be protected from exposure to O2 for diazotrophy to occur (23). The most common form of nitrogenase, encoded by nif genes, contains molybdenum (Mo) as its cofactor. When Mo is scarce, some species of Bacteria and Archaea express nitrogenases containing vanadium ([V] vnf genes) or iron ([Fe] anf genes) in place of Mo, but these “alternative” nitrogenases are less efficient than the Mo form (24, 25). The most conserved nitrogenase gene, nifH (26), has become the marker gene of choice for environmental diazotrophy (27–29). Phylogenetic studies show five nifH clusters: aerobic bacteria (cluster I), alternative nitrogenases (cluster II), anaerobic bacteria and archaea (cluster III), uncharacterized sequences (cluster IV), and paralogs related to chlorophyll biosynthesis (cluster V) (30). Because vnfH and anfH genes in cluster II cannot be differentiated by sequence alone, the D subunit (nifD-vnfD-anfD) has become the preferred marker gene for studies of alternative nitrogenases (31). Consistent with higher concentrations of V than Mo in most rocks (32), microbes from diverse soils have been shown to contain vnfD genes (31, 33–37). Given that oligotrophic conditions dominate in peatlands, trace metals may limit diazotrophy. However, little is known about trace metal availability and the role of alternative nitrogenase pathways in ombrotrophic peatlands.
Similarly, methane monooxygenase ([MMO] the enzyme that catalyzes the first step of methane oxidation) occurs in particulate copper (Cu)-containing (pMMO) and soluble Fe-containing (sMMO) forms. While pMMO has more specific substrate requirements, pathways that employ sMMO can use a wider range of compounds (38). Both forms of MMO are inhibited by acetylene (C2H2) (39, 40). In organisms with both sets of genes, pMMO is expressed when Cu is abundant, whereas Cu limitation induces sMMO expression (41). The dominant peatland methanotrophs in Alphaproteobacteria and Gammaproteobacteria tend to possess both MMOs (42–46), although Methylocella and Methyloferula species containing solely sMMO have been isolated from peat bogs (47–49). While most studies have primarily targeted the pmoA gene (43, 45), mmoX genes and transcripts have also been reported from peatlands (46, 50, 51), raising questions about the relative importance of each form for peatland methane oxidation.
The acetylene reduction assay (ARA) is commonly used as a proxy for diazotroph activity (52, 53). This assay is effective for capturing the potential activity of diazotrophs that are not inhibited by C2H2, such as Cyanobacteria and nonmethanotrophic Proteobacteria (e.g., Bradyrhizobiaceae) (54). However, a number of functional guilds of microorganisms, including methanotrophs, methanogens, sulfate reducers, and nitrifiers, are inhibited by C2H2 (55–60). If these or other C2H2-sensitive microbes perform diazotrophy and/or provide substrates to other diazotrophs (see Fulweiler et al. [61]), ARA may underestimate diazotrophy in that system. Thus, recent studies have shifted to tracking diazotrophy by incorporation of the stable isotope tracer, 15N2 (20, 21, 62, 63).
In this study of the S1 peat bog at the Marcell Experimental Forest in northern Minnesota, USA, dissolved macronutrients (NH4+, NO3−, and PO43−) and micronutrients (Fe, Cu, V, and Mo) were profiled along with the community composition and abundance of diazotrophic microorganisms. We also performed separate laboratory incubation experiments to measure potential rates of ARA and 15N2 incorporation to (i) assess environmental controls (light, O2, and CH4) on diazotrophy; (ii) quantify the effect of C2H2 on rates of diazotrophy and methanotrophy; and (iii) search for diagnostic markers for alternative nitrogenase activity, such as a low conversion factor of ARA to 15N2 incorporation (31) and C2H2 reduction to ethane (64). Finally, we make recommendations on universal nifH primers for amplicon sequencing and quantitative PCR based on our findings.
RESULTS
Macro- and micronutrient profiles.
In S1 bog hollows, NH4+ was at a concentration of ∼2 μM from the surface to the 25-cm depth and increased at greater depths (Fig. 1; see also Fig. S1a in the supplemental material). Nitrate was at a concentration of <1 μM in surface peat and decreased with depth (Fig. S1b). Phosphate was at a concentration of <0.1 μM from the surface to the 25-cm depth and then increased with depth (Fig. S1c). For the metal pairs of greatest interest to this study, V (at 5 to 21 nM) was consistently more abundant than Mo (at 1 to 7 nM) (Fig. 2), and the concentration of Fe (at 7 to 35 μM) was three orders of magnitude higher than that of Cu (7 to 38 nM) (see Fig. S3) at all three depth intervals (0 to 30, 30 to 50, and 100 to 150 cm) and at three sampling dates (September 2014, June 2015, and September 2015) (data for June 2015 are shown in Fig. 2 and also Fig. S2). Other trace nutrients were in the nano- to micromolar range: Co, 5 to 20 nM; Ni, 10 to 80 nM; Zn, 50 to 250 nM; and Mn, 60 to 2,220 nM (see Table S1). Essentially identical macro- and micronutrient profiles were obtained from the Zim bog, another ombrotrophic bog ∼80 km southeast of the S1 bog, sampled in September 2014, with the exception of a lower Fe concentration at the surface (data not shown).
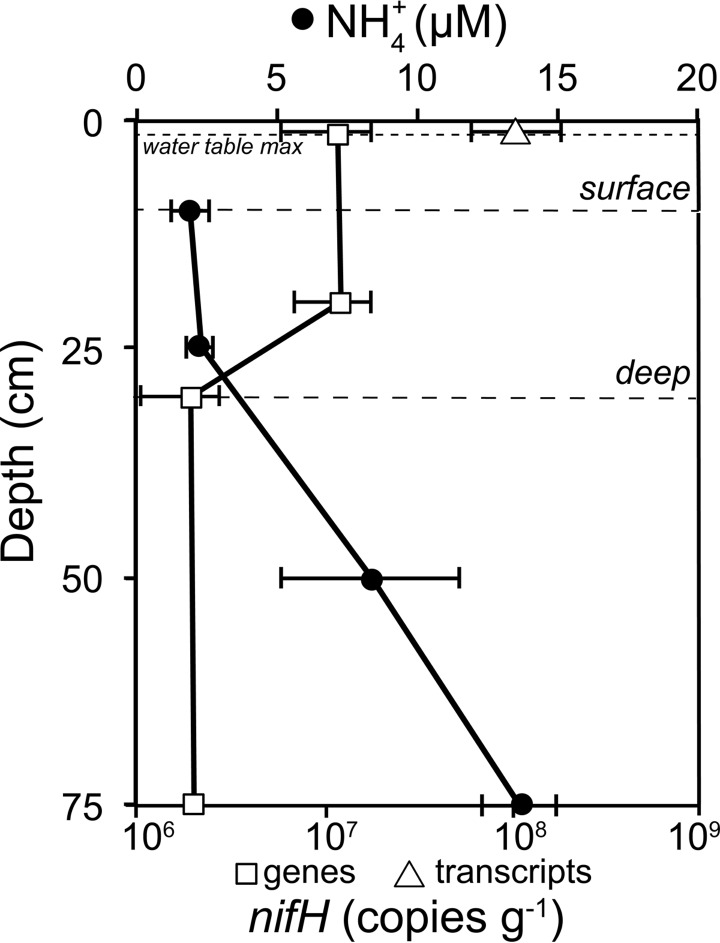
FIG 1.
Depth profiles of NH4+ concentrations (black circles) and nifH gene copies (white squares) and transcripts (white triangle) in units of copies per gram (dry weight) for the S1 bog T3 middle site. Ammonium concentrations are means from measurements from May, June, and September 2014. nifH copy numbers are from July 2013. Error bars are standard errors. nifH transcripts were not detected at 20-, 30-, and 75-cm depths. Surface (0 to 10 cm) and deep (10 to 30 cm) peat depth intervals used for rate measurements are designated by dashed lines. The maximum water table depth at the S1 site in July 2013 was 2 cm below the hollow surface (dotted line) (97).
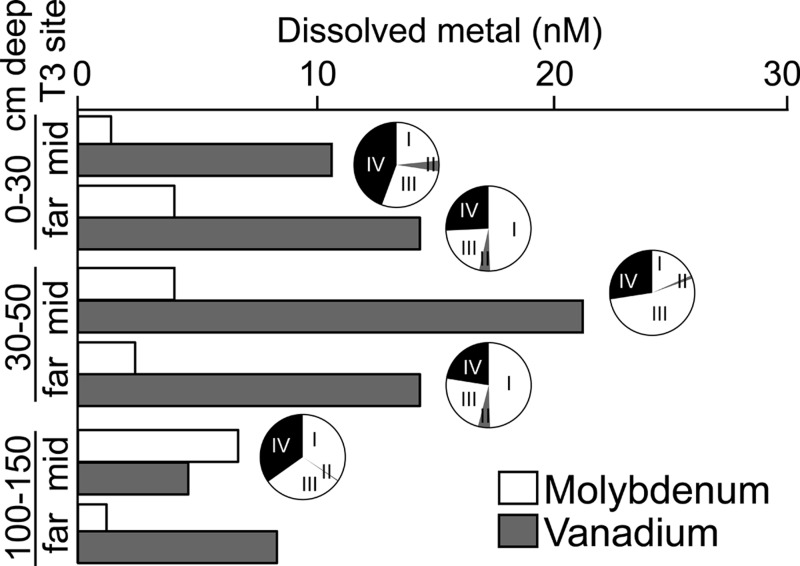
FIG 2.
Dissolved molybdenum (white) and vanadium (gray) concentrations in porewater from three depths in S1 peat hollows (middle and far sites along T3 transect) from June 2015. Pie charts show the relative abundances of genes encoding the five nitrogenase H subunit clusters from metagenomes for each depth; clusters I and III encode Mo-Fe nitrogenases (nifH); cluster II encodes alternative (vnfH, anfH) nitrogenases; cluster IV encodes nitrogenase paralogs. Deepest metagenomes were from 75 cm; insufficient numbers of nitrogenase H subunit sequences were recovered from the far site for cluster analysis.
Nitrogenase expression and phylogeny.
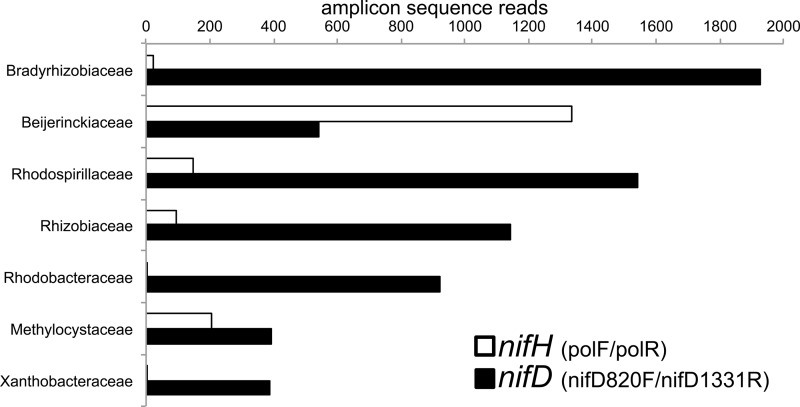
With the polF/polR primer pair, we measured 1.2 × 107 copies of nifH · g−1 at 1 and 20 cm and 0.2 × 107 copies · g−1 at 30 and 75 cm; nifH transcripts (12.2 × 107 copies · g−1) were only detected at 1 cm (10:1 transcript-to-gene ratio) and not at deeper depths (Fig. 1; see also Fig. S3a). Sequencing of cDNA from surface peat amplified with polF/polR (for nifH) and nifD820F/nifD1331R (for nifD) primers showed that the majority of nitrogenase transcripts belonged to cluster I (Alphaproteobacteria), with Beijerinckiaceae dominating for the largest number of nifH sequences and Bradyrhizobiaceae dominating for nifD sequences (Fig. 3). Additional alphaproteobacterial nif transcripts matched to Rhodospirillaceae, Rhizobiaceae, Rhodobacteraceae, Methylocystaceae, and Xanthobacteraceae (Fig. 3). Gammaproteobacteria, Cyanobacteria (Oscillatoriophycideae), and Nitrospira were also observed at lower abundances in cDNA amplicon libraries (data not shown).
FIG 3.
Numbers of cDNA amplicon sequence reads for nifH and nifD alphaproteobacterial transcripts. Primer sets were polF/polR and nifD820F/nifD1331R for nifH and nifD, respectively.
In metagenomes, nifH genes were roughly equally distributed between Mo-dependent clusters I and III and cluster IV/V paralogs (Fig. 3; see also Table S2). Sequences from cluster II (alternative nitrogenases, vnfH and anfH) were scarce at all depths (<5% overall); two vnfD genes from metagenomes showed phylogenetic similarity to those from soil Proteobacteria (see Fig. S4). Attempts to amplify vnfD-anfD from cDNA yielded few reads; those recovered were most similar to Alphaproteobacteria anfD from Rhodopseudomonas species (Fig. S4).
Methane-related gene expression and phylogeny.
Like nifH, particulate methane monooxygenase (pmoA) and methyl coenzyme M reductase (mcrA) transcripts showed the highest abundances in surface peat (Fig. S3b and c). Surface pmoA transcripts mapped to Methylocystaceae (75%) and Methylococcaceae (25%). Surface mcrA transcripts mapped to Methanosarcina (58%), Methanocella (28%), and Methanoregula (11%). Attempts to amplify mmoX from cDNA were unsuccessful (data not shown). In metagenomes, genes for pmoA were dominant in surface peat, whereas the relative abundance of mmoX transcripts increased with depth (Fig. S2).
Rates of diazotrophy and methanotrophy.
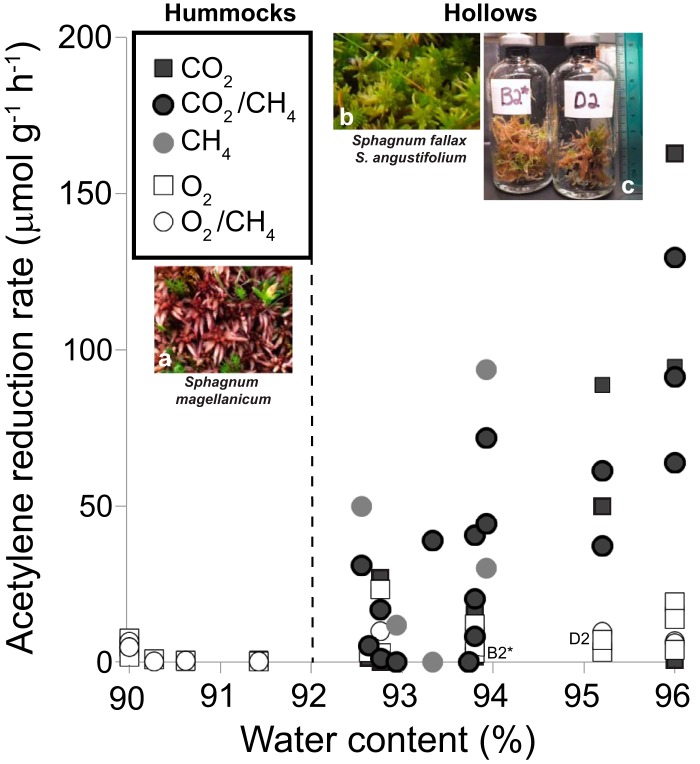
Potential rates of acetylene reduction were measured for peat collected from S1 bog hollows and hummocks in April, June, August and September of 2013 to 2015 and incubated for 1 week at 25°C. Acetylene reduction to ethylene was only detected in surface (0 to 10 cm) peat samples incubated in the light and not in deep (10 to 30 cm) peat or in surface peat incubated in the dark. Acetylene reduction to ethane was not detected in any incubation (data not shown). Sphagnum in peat incubations exposed to light became visibly greener over the course of the incubation (Fig. 4).
FIG 4.
Acetylene reduction rates for hummocks (90 to 91% water content) and hollows (93 to 96% water content) at the S1 bog, T3 transect (0- to 10-cm depth incubated in the light at 25°C for 7 days). ARA units are μmol ethylene produced per gram (dry weight) per hour. Photo insets show dominant Sphagnum species in hummocks (S. magellanicum) (a) and hollows (S. fallax and S. angustifolium) (b). Photo inset (c) shows Sphagnum greening after incubation of hollow samples in the light for 7 days at 25°C; bottle B2* (April 2014) received air headspace with 1% CH4 and D2 (Sept 2013) received air headspace without CH4. The vertical dotted line divides the hummock samples (dominated by S. magellanicum) from the hollow samples (dominated by S. fallax/angustifolium) in terms of water content (92%).
In hollows, where surface peat was dominantly covered by a mix of Sphagnum fallax and S. angustifolium, ARA rates were higher and more variable in degassed versus oxic incubations (0 to 163 versus 2 to 23 μmol C2H4 · g−1 · h−1, respectively) and were unaffected by the presence or absence of 20% CO2. The ARA rates in hollows incubated with degassed headspace were positively correlated (P < 0.0001) with peat water content (93 to 96%). In both hollows and hummocks, ARA rates were not affected by the addition of 1% CH4. In hummocks with lower water contents (90 to 91%), surface peat was dominantly covered by S. magellanicum, and oxic and degassed treatments had similarly low ARA rates (0 to 8 μmol C2H4 · g−1 · h−1). nifH transcripts in surface peat from hollows incubated under degassed headspace with 1% C2H2, with or without 1% CH4, ranged from 104 to 107 copies · g−1 (July 2014) (data not shown), which were 1 to 4 orders of magnitude lower than those from field samples from the previous summer (108 copies · g−1) (Fig. 1) and higher and more variable in hollows than nifH transcripts from hummock incubations (see Fig. S5).
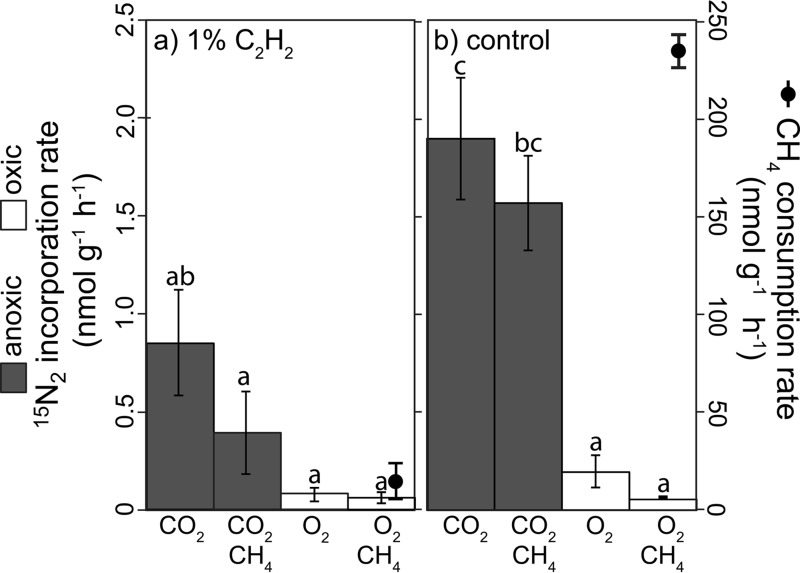
15N2 incorporation showed overall trends similar to those of ARA rates (e.g., 90% higher rates in degassed versus oxic conditions and no significant CH4 effect) (Fig. 5). In degassed treatments, 1% C2H2 inhibited 15N2 incorporation by 55% but had no effect on oxic treatments. In oxic treatments, CH4 consumption rates were 100 times higher than 15N2 incorporation rates, and 1% C2H2 addition suppressed CH4 oxidation rates by 95% (Fig. 5). Using the four sites measured with both methods, a conversion factor of 3.9 for 15N2 to ARA was calculated (see Fig. S6). In sum, laboratory incubations of native peats revealed that diazotrophy was stimulated by light, suppressed by O2, and minimally affected by CH4 and CO2.
FIG 5.
Effects of 1% C2H2 on15N2 incorporation and CH4 consumption for S1 bog surface peat. Rates were measured for samples collected from the northwest (NW) S1 bog transect in September 2014. 15N2 incorporation treatment conditions were 80% N2 plus 20% CO2 with and without 1% CH4 (shaded bars) or 80% N2 plus 20% O2 with and without 1% CH4 (white bars) with (a) and without (b) 1% C2H2; units are nmol 15N2 incorporated per gram (dry weight) per hour. CH4 consumption treatments were 80% N2 plus 20% CO2 plus 1% CH4 (black circles) with (a) and without (b) 1% C2H2; units are nmol CH4 consumed per gram (dry weight) per hour. Error bars are standard errors. Lowercase letters indicate statistically different elemental contents (P < 0.05 based on Tukey-Kramer HSD test).
DISCUSSION
Diazotrophs are active in surface peat.
By definition, the only source of nutrients to ombrotrophic peat bogs is the atmosphere (65). Scarce (low μM concentrations) dissolved nitrogen and phosphorus in S1 peat porewater suggests oligotrophic conditions (65–67), consistent with low rates of atmospheric deposition in Minnesota peat bogs (68). Low nutrient concentrations add further evidence to previous suggestions that these nutrients may limit Sphagnum productivity (21) and/or complex mechanisms may exist for nutrient scavenging at ultralow concentrations. Diazotroph activity (ARA and nifH transcription) was solely detected in surface peat samples incubated in the light. The apparent absence of diazotrophy at greater depths is consistent with previous reports (69) and may be due to light limitation and/or remineralization of organic nitrogen to ammonium, which is preferentially used as a nitrogen source by microbes.
Nitrogenase sequences from Alphaproteobacteria (Rhizobiales) dominate in peatland.
Diazotrophic methanotrophs have the potential to serve as a methane biofilter and a nitrogen source for peatland ecosystems. Previous work showed that Alphaproteobacteria (Rhizobiales), including type II methanotrophs (20, 50), were the dominant diazotrophs in Sphagnum-dominated peatlands and may provide the unaccounted nitrogen input resulting from an imbalance in atmospheric nitrogen deposition and an accumulation in Sphagnum mosses (19–21, 70, 71). However, the carbon metabolism of peatland diazotrophs remains unclear, because the two dominant Rhizobiales families grow on both complex organics (Bradyrhizobiaceae [72]) and simple alkanes and C1 compounds (Beijerinckiaceae [16]). Consistent with results from previous studies, Rhizobiales showed the highest relative abundance in transcript libraries in this study. nifH and nifD amplicons from Beijerinckiaceae and Bradyrhizobiaceae, respectively, dominated. However, the complex taxonomic classification of Rhizobiales nifH and nifD genes (18) prevented distinguishing methanotrophic Beijerinckiaceae from heterotrophic Beijerinckia indica and Bradyrhizobiaceae solely on the basis of nifH and nifD phylogenies.
Diazotrophy is catalyzed by the molybdenum form of nitrogenase.
Peatland conditions such as low pH, nitrogen, and temperature would be expected to favor diazotrophy by alternative nitrogenases. Molybdenum sorption to peat is enhanced at low pH (73), biological requirements for Mo are higher when bacteria are fixing N2 than when growing on other nitrogen compounds (74), and alternative nitrogenases have higher activity and expression at lower temperatures (24, 25). Below 10 nM Mo, diazotrophy is limited in laboratory cultures (75–79), and alternative nitrogenases, if present, are expressed (80). Transcription of alternative nitrogenase genes has been reported for Peltigera cyanolichens (35) but, to our knowledge, has not previously been investigated for Sphagnum peatlands.
Molybdenum concentrations in S1 bog porewaters were 1 to 7 nM, which are within the same range as Mo in other oligotrophic freshwaters (74) and lower than those of V (5 to 21 nM) and Fe (7 to 35 μM). Similar metal concentrations among (i) sampling dates in September 2014, June 2015, and September 2015, (ii) those in another ombrotrophic Minnesota peatland, Zim bog, ∼80 km from the S1 bog, and (iii) those in acidic peatlands in Northern Europe (81) suggest that the values we measured are spatially and temporally representative for diverse northern peatlands.
Intriguingly, despite the presence of conditions that would be expected to favor alternative nitrogenase expression (e.g., low pH, long winters, and [Fe] > [V] > [Mo]), our collective evidence suggests that diazotrophy at the S1 bog was catalyzed by the Mo-containing form of the nitrogenase enzyme. The majority of nifH genes retrieved from metagenomes belonged to Mo-containing clusters I and III. A significant number of sequences came from the uncharacterized cluster IV, recently shown to contain a functional nitrogenase (82) that likely binds a Mo-Fe cofactor (83). The metagenomes contained very few cluster II vnfD-anfD genes, and minimal numbers of vnfD-anfD transcripts were amplified from peat cDNA. Ethane, a biomarker of alternative nitrogenase, was undetectable in ARA incubations. Finally, the 15N2-to-ARA conversion factor (3.9) was within the same range (31, 80) as other peat bogs (20, 52, 84) and matched that of Mo-nitrogenase in pure culture experiments as opposed to the lower values measured for alternative nitrogenases (31). The question of how peatland diazotrophs access scarce Mo remains uncertain; it is possible that Mo bound to peat organic matter can be scavenged by diazotrophs as is the case in forest soils (85, 86).
Methanotrophs are active in incubations and inhibited by acetylene.
We performed bottle experiments to test whether methanotrophs were active. Complete CH4 consumption in air amended with 1% CH4 showed that methanotrophs were active in our incubations and that the black bromobutyl stoppers we used were not toxic to peatland methanotrophs, whereas nonhalogenated stoppers are toxic to aquatic aerobic methanotrophs (87). Acetylene fully inhibited CH4 consumption, demonstrating that the methanotrophs in our incubations were C2H2 sensitive, similar to laboratory strains tested in previous studies (55, 60). Like diazotrophy, methanotrophy was apparently also mediated by the enzyme requiring the scarcer metal; concentrations of dissolved Fe were consistently orders of magnitude higher than those of Cu in peat porewater, yet pmoA sequences were more abundant than mmoX sequences in surface peat where the highest CH4 consumption was observed (43, 46). This finding is also consistent with a higher transcription of pmoA versus mmoX in other acidic peatlands (50, 81, 88). In laboratory studies, the “copper switch” from sMMO- to pMMO-supported growth occurs when the concentration of Cu is >1 μM (41), which is several orders of magnitude higher than Cu concentrations measured at the S1 bog (7 to 38 nM). It is possible that the copper switch occurs at a lower threshold in peat bog or that there are other factors controlling the type of MMO expressed, such as CH4 or O2 availability. Additionally, the inherent nature of the peat matrix, with characteristically high levels of particulate and dissolved organic matter, likely also affects metal bioavailability in complex ways (89) not addressed in our study.
Surface peatland diazotrophy is sensitive to oxygen and acetylene.
If methanotrophs were the dominant diazotrophs in peatlands, as previously proposed (20, 21), CH4 addition should have stimulated 15N2 incorporation in our bottle experiments, and C2H2 should have inhibited it. Instead, this and previous (63) works found that 15N2 incorporation was not affected by CH4 addition. Acetylene partially inhibited 15N2 incorporation under degassed conditions (as reported by Kox et al. [90]) and had a minimal effect on oxic 15N2 incorporation, suggesting that C2H2- and O2-sensitive microbial clades contributed approximately half of the diazotrophic activity in our incubations. This finding highlights the importance of quantifying peatland diazotrophy by 15N2 incorporation instead of, or in addition to, ARAs.
Based on nif, pmoA, and mcrA phylogenies from native peat, the O2- and C2H2-sensitive diazotrophs were likely methanotrophic Beijerinckiaceae that can only fix N2 under microoxic conditions and/or strict anaerobes in cluster III, such as methanogenic Euryarchaeota. These two families are the most active in surface peat based on the numbers of transcripts and amplicon sequences. Since the numbers of nifH transcripts were 1 to 4 orders of magnitude lower in our incubations than in native peat, it is likely that diazotrophs were stressed, possibly due to O2 exposure during the sampling process. Indeed, we observed the highest ARA rates in hollows where the water table was typically at the bog surface, limiting O2 penetration into the peat. The other half of the O2-sensitive diazotrophic activity was likely performed by C2H2-insensitive heterotrophic Bradyrhizobiaceae and/or Beijerinckiaceae (91, 92) or C2H2-insensitive methanotrophic Methylocystaceae, which can adapt to a wide range of CH4 concentrations (93). Less-O2-sensitive microbes, such as heterotrophic Beijerinckiaceae (92) and photosynthetic Oscillatoriophycideae (23), likely contributed to the minor amount of ARA activity in the presence of O2.
Molecular markers for diazotrophy.
We end with a word of caution with regard to the molecular detection of diazotrophs. The majority of studies in peatlands have employed PCR amplification and sequencing of the nifH marker gene for studying the dynamics of diazotrophs in peatlands. A wide range of nifH primer sets exist, with varied universality (28). Peat bog sequencing efforts have used polF/polR (this study and reference 50), F1/R6 (20), FGPH19-polF/polR-AQER (63), and 19F/nifH3 plus nifH1(1)/nifH2 (12) with nested PCR (17, 90). In silico evaluation predicts that the polF/polR primer set will not amplify the majority of Proteobacteria and/or Cyanobacteria and group III nifH sequences (28); however, this primer set yields the highest efficiency for qPCR (94). Of the nifH primer sets used previously, F1/R6, 19F/nifH3, and nifH1(1)/nifH2 are predicted to have the highest coverage for soils (>80% predicted primer binding for sequences from soil ecosystems). However, it is important to be aware that the F1/R6 primer set contains a number of mismatches with sequences from cluster III in peatlands, including of methanogenic Euryarchaeota (see Fig. S7). To maximize sequence coverage, we suggest using primer sets that can amplify nifH from cluster III, such as IGK(3)/DVV (28), for future studies.
Conclusions.
This study revealed that peatland diazotrophs preferentially transcribed the Mo-based, rather than the V-based, form of the nitrogenase enzyme, despite the dominance of V over Mo in the environment. It also highlighted the sensitivity of diazotrophic peatland communities to O2 exposure during sample collection and quantified the inhibitory effect of C2H2 addition on peatland diazotrophy. Under our experimental conditions in lab incubations, we did not observe CH4-stimulated diazotrophy. However, the quantification of the relative contributions of methanotrophic and heterotrophic diazotrophy in situ awaits further investigation.
MATERIALS AND METHODS
Site description and sample collection.
Samples were collected from the S1 (black spruce Sphagnum spp.) peat bog at Marcell Experimental Forest (MEF; 47°30.476′N, 93°27.162′W), the site of the DOE SPRUCE (Spruce and Peatland Responses Under Climatic and Environmental Change) experiment in northern Minnesota, USA (95). The S1 bog is ombrotrophic and acidic (average pH, 3.5 to 4 [66, 96]). Over the summer months, the water table is ±5 cm from the hollow surface (66, 97). Dissolved O2 levels decrease to below detection (∼20 ppb) within the top 5 cm of the bog. Three locations were sampled along S1 bog transect 3 (T3) at near, middle, and far sites (see Lin et al. [98] for further details). Surface (0- to 10-cm depth) peat was collected from hollows dominated by a mixture of Sphagnum fallax and S. angustifolium and from hummocks dominated by S. magellanicum. Peat depth cores (0 to 200 cm) were sampled from hollows where the water level reached the surface of the Sphagnum layer.
Macronutrients.
Peat porewater was collected using piezometers from depths of 0, 10, 25, 50, 75, 150, and 200 cm. Piezometers were recharged the same day as collection, and porewater was pumped to the surface, filtered through sterile 0.2-μm polyethersulfone membrane filters, and stored frozen until analysis. Nitrate (NO3−) and nitrite (NO2−) were analyzed using the spectrophotometric assay described by García-Robledo et al. (99). Ammonium (NH4+) concentrations were determined with the indophenol blue assay (100). Phosphate concentrations were measured with the molybdate-antimony ascorbic acid colorimetric assay (101).
Micronutrients.
Peat porewater was collected from two locations in the S1 bog from cores at depths of 0 to 30 cm, 30 to 50 cm, and 100 to 150 cm by filtration through 0.15-μm Rhizon soil samplers (Rhizosphere Research Products). All plastics were washed with HCl prior to sampling; Rhizon soil samplers were cleaned by pumping 10 ml of 1 N HCl through them, followed by rinsing with ultrapure water until the pH returned to neutral (∼100 ml/filter). After collection, samples were acidified with 0.32 M HNO3 (Fisher Optima) and analyzed using a Thermo Element 2 high-resolution inductively coupled plasma mass spectrometer (HR-ICP-MS; National High Magnetic Field Laboratory, Florida State University). Initial analyses resulted in the frequent clogging of the nebulizer, likely due to the abundance of dissolved organic carbon. Therefore, samples were diluted 1:10 to minimize interruptions from nebulizer clogs. Concentrations were quantified with a 7-point external calibration using standards prepared in 0.32 N HNO3 from a multi-element standard mix (High-Purity Standards).
To generate an organic-free sample matrix suitable for ICP-MS analysis without contaminating or diluting the sample, subsequent samples were digested as follows: 1-ml aliquots of the porewater samples were heated in 15-ml Teflon beakers (Savillex) with 1 ml of 16 N HNO3 (Ultrex II, JT Baker) and 100 μl of 30% H2O2 (Ultrex II, JT Baker) for 36 h at 230°C in a trace-metal-clean polypropylene exhaust hood. The HNO3-H2O2 mixture oxidizes any dissolved organic matter (DOM) to CO2, but the resulting matrix is too acidic for direct ICP-MS introduction. Therefore, samples were evaporated to near dryness, resuspended in a 0.32 N HNO3 matrix suitable for ICP-MS analysis, and analyzed using the Element2 ICP-MS along with parallel blank solutions.
Quantification and sequencing of gene and transcript amplicons.
Peat was frozen on dry ice at the field site in July 2013 or in liquid N2 after 7-day incubations at 25°C in the light under a degassed (80% N2 plus 20% CO2) headspace with 1% C2H2, with or without 1% CH4, for June 2014 incubations (see “Acetylene reduction and methane consumption rates” below). DNA and RNA were extracted with Mo Bio PowerSoil DNA and total RNA extraction kits, respectively, as described by Lin et al. (46). RNA was cleaned with a Turbo DNA-free kit (Ambion). Nucleic acid purity was analyzed for the 260/280 absorbance ratio (of 1.8 to 2.0) on a NanoDrop spectrophotometer. cDNA was synthesized using the GoScript reverse transcription system (Promega) according to the manufacturer's protocol.
Plasmid standards for qPCR were constructed according to the method of Lin et al. (102). Primer pairs are given in Table 1. The gene fragments of nifH, pmoA, and mcrA for constructing plasmid standards for qPCR were amplified from genomic DNA of Rhodobacter sphaeroides, Methylococcus capsulatus strain Bath, and S1 peat bog peat soil, respectively. To prepare cDNA standards, plasmid DNA with a positive gene insert was linearized with NcoI restriction enzyme following the manufacturer's protocol (Promega) and purified by using a MinElute PCR purification kit (Qiagen). RNA was synthesized from the linearized plasmid DNA by using the Riboprobe in vitro transcription system (Promega) followed by cDNA synthesis using the GoScript reverse transcription system (Promega) according to the manufacturer's protocols.
TABLE 1.
qPCR and sequencing primers used in this studya
The abundance of functional gene transcripts was quantified in samples run in duplicates on a StepOnePlus real-time PCR system (ABI) using Power SYBR green PCR master mix. Reaction mixtures of 20 μl comprised 2 μl of template cDNA (10 to 100 ng/μl) added to 10 μl of SYBR green master mix, 0.5 to 1.6 μl of each forward and reverse primer (0.3 to 0.8 μM final concentration; Table 1), and 4.8 to 6.5 μl of PCR-grade water. Samples were run against a cDNA standard curve (101 to 107 copies of plasmid gene fragment) on a StepOnePlus qPCR instrument with 96 wells with an initial denaturation step of 2 to 5 min at 95°C and 40 cycles of denaturation at 95°C for 15 to 30 s, annealing at 55 to 64°C for 30 to 45 s, extension at 72°C for 30 to 45 s, and data acquisition at 83 to 86.5°C for 16 to 30 s. To minimize the effects of inhibitors in assays, peat DNA was diluted to 1/40 of the original concentrations, and duplicate 20-μl reaction mixtures, each containing 2 μl of diluted DNA, were run for each sample. Functional gene and transcript copy numbers were normalized to the dry weight of peat or 16S rRNA transcript copies for incubation samples. Amplicons were sent to the University of Illinois at Chicago for DNA sequencing using a 454 platform. Raw sequences were demultiplexed, trimmed, and quality filtered in CLC bio software. The phylogenies of vnfD-anfD sequences were inferred using the maximum likelihood method based on the Kimura 2-parameter model in MEGA5 (103).
Acetylene reduction and methane consumption rates.
Samples of bulk peat (Sphagnum spp. and surrounding soil) were collected from 0- to 10- and 10- to 30-cm depths in September 2013, April 2014, June 2014, September 2014, and August 2015 and stored at 4°C until the start of laboratory incubations. Samples from the 0- to 10-cm depth were gently homogenized so as not to rupture Sphagnum sp. tissues, while peat samples from the 10- to 30-cm depth were fully homogenized. For each sample, 5 g of bulk peat was placed in 70-ml glass serum bottles, stoppered with black bromobutyl stoppers (Geo-Microbial Technologies) (pretreated by boiling 3 times in 0.1 M NaOH), and sealed with an aluminum crimp seal. Headspaces were oxic (room air, 80% N2 plus 20% O2) or degassed (100% N2 or 80% N2 plus 20% CO2) with or without 1% C2H2 or 1% CH4. Treatments were incubated for 1 week at 25°C in the light or dark. A gas chromatograph with a flame ionization detector (SRI Instruments) equipped with a HayeSep N column was used to quantify CH4, C2H2, and C2H4. Samples were measured for C2H4 production daily until C2H4 production was linear (∼7 days). Controls not amended with C2H2 did not produce ethylene (C2H4). Incubations of hollow peat from June 2014 incubated under an oxic headspace with and without 1% C2H2 were also monitored for the consumption of 1% CH4. Statistical analysis was performed with JMP Pro (v. 12.1.0) using the Tukey-Kramer honestly significant difference (HSD) comparison of all means.
15N2 incorporation rates.
In September 2014, samples were quantified for N2 fixation rates by 15N2 incorporation in parallel with ARA measurements. Incubations were set up as described above and supplemented with 7 ml of 98% 15N2 (Cambridge Isotope Laboratories, Tewksbury, MA, USA). After 7 days, samples were dried at 80°C, homogenized into a fine powder, and analyzed for N content and δ15N by isotope ratio mass spectrometry (IRMS) with a MICRO cube elemental analyzer and IsoPrime100 IRMS (Elementar) at the University of California, Berkeley, corrected relative to National Institute of Standards and Technology (Gaithersburg, MD, USA) standards.
Metagenomic analyses.
Metagenomes were generated in a previous study (104). Diazotrophic and methanotrophic pathways were investigated using the following bioinformatics approaches. Briefly, Illumina reads were filtered by quality (Phred33 score threshold of Q25) using Trim Galore (Babraham Bioinformatics) and a minimum sequence length cutoff of 100 bp. The sequences were then queried using RAPSearch2 (105) against the NCBI nr database of nonredundant protein sequences as of November 2013. Sequences with bit scores of 50 and higher were retained to determine the total number of functional genes for normalization across the different samples. The taxonomic composition of protein-coding sequences was determined based on the taxonomic annotation of each gene according to the NCBI nr taxonomy in MEGAN5 (106; minimum score, 50; maximum expected, 0.01; top percent, 10; minimum complexity, 0.3).
To classify sequences by nitrogenase cluster type, genes were analyzed using BLASTX (E value, 0.1; bit score, 50) versus a custom nifH database that includes a phylogenetic tree to distinguish the principal clusters (I, V, and III) in the nifH phylogeny, as well as paralogous cluster IV nifH-like sequences (27). Abundances of nifH genes from the four clusters were normalized to those of total protein-coding genes from RAPSearch2 output sequences. The relative abundance of particulate (pmoA) versus soluble (mmoX) methane monooxygenase was based on previous analyses reported by Lin et al. (46).
Accession number(s).
Metagenomes were reported in a previous study (104) and deposited in BioProject PRJNA382698 (SAMN06712535-06712540). pmoA cDNA amplicons were reported in a previous study (43) and deposited in BioProject PRJNA311735. nifH, mcrA, nifD, and vnfD-anfD cDNA amplicons were deposited in BioProjects PRJNA382268, PRJNA382282, PRJNA382288, and PRJNA382295, respectively.
Supplementary Material
ACKNOWLEDGMENTS
This research was funded by DOE support to J.E.K. under grant numbers DE-SC0007144 and DE-SC0012088, DOE support to D.J.W. and C.W.S. under grant number DE-AC05-00OR22725, and NASA Exobiology grant NNX14AJ87G and a Center for Dark Energy Biosphere Investigations small research grant (NSF-CDEBI OCE-0939564) to J.B.G. Work at Lawrence Livermore National Laboratory (LLNL) was conducted under the auspices of DOE contract DE-AC52-07NA27344, with funding provided by LDRD 14-ER-038.
We thank Loren Dean Williams for inspiration with visuals, Heather Dang (UC Berkeley) for IRMS analysis, and Will Overholt and Damian Horton for technical assistance.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01174-17.
REFERENCES
- 1.Gorham E, Lehman C, Dyke A, Clymo D, Janssens J. 2012. Long-term carbon sequestration in North American peatlands. Quat Sci Rev 58:1–14. doi: 10.1016/j.quascirev.2012.09.018. [DOI] [Google Scholar]
- 2.Limpens J, Berendse F, Blodau C, Canadell J, Freeman C, Holden J, Roulet N, Rydin H, Schaepman-Strub G. 2008. Peatlands and the carbon cycle: from local processes to global implications—a synthesis. Biogeosciences 5:1475–1491. doi: 10.5194/bg-5-1475-2008. [DOI] [Google Scholar]
- 3.Yu Z. 2012. Northern peatland carbon stocks and dynamics: a review. Biogeosciences 9:4071–4085. doi: 10.5194/bg-9-4071-2012. [DOI] [Google Scholar]
- 4.Clymo R. 1984. The limits to peat bog growth. Philos Trans R Soc Lond B Biol Sci 303:605–654. doi: 10.1098/rstb.1984.0002. [DOI] [Google Scholar]
- 5.Clymo R, Hayward P. 1982. The ecology of Sphagnum, p 229–289. In Smith AJE. (ed), Bryophyte ecology. Chapman and Hall, London, United Kingdom. [Google Scholar]
- 6.Weston DJ, Timm CM, Walker AP, Gu L, Muchero W, Schmutz J, Shaw AJ, Tuskan GA, Warren JM, Wullschleger SD. 2015. Sphagnum physiology in the context of changing climate: emergent influences of genomics, modeling and host-microbiome interactions on understanding ecosystem function. Plant Cell Environ 38:1737–1751. doi: 10.1111/pce.12458. [DOI] [PubMed] [Google Scholar]
- 7.Bragina A, Oberauner-Wappis L, Zachow C, Halwachs B, Thallinger GG, Müller H, Berg G. 2014. The Sphagnum microbiome supports bog ecosystem functioning under extreme conditions. Mol Ecol 23:4498–4510. doi: 10.1111/mec.12885. [DOI] [PubMed] [Google Scholar]
- 8.Kostka JE, Weston DJ, Glass JB, Lilleskov EA, Shaw AJ, Turetsky MR. 2016. The Sphagnum microbiome: new insights from an ancient plant lineage. New Phytol 211:57–64. doi: 10.1111/nph.13993. [DOI] [PubMed] [Google Scholar]
- 9.Opelt K, Berg C, Schonmann S, Eberl L, Berg G. 2007. High specificity but contrasting biodiversity of Sphagnum-associated bacterial and plant communities in bog ecosystems independent of the geographical region. ISME J 1:502–516. doi: 10.1038/ismej.2007.58. [DOI] [PubMed] [Google Scholar]
- 10.Opelt K, Chobot V, Hadacek F, Schonmann S, Eberl L, Berg G. 2007. Investigations of the structure and function of bacterial communities associated with Sphagnum mosses. Environ Microbiol 9:2795–2809. doi: 10.1111/j.1462-2920.2007.01391.x. [DOI] [PubMed] [Google Scholar]
- 11.Berg A, Danielsson Å, Svensson BH. 2013. Transfer of fixed-N from N2-fixing cyanobacteria associated with the moss Sphagnum riparium results in enhanced growth of the moss. Plant Soil 362:271–278. doi: 10.1007/s11104-012-1278-4. [DOI] [Google Scholar]
- 12.Basilier K, Granhall U, Stenström T-A. 1978. Nitrogen fixation in wet minerotrophic moss communities of a subarctic mire. Oikos 31:236–246. doi: 10.2307/3543568. [DOI] [Google Scholar]
- 13.Granhall U, Hofsten AV. 1976. Nitrogenase activity in relation to intracellular organisms in Sphagnum mosses. Physiol Plant 36:88–94. doi: 10.1111/j.1399-3054.1976.tb05033.x. [DOI] [Google Scholar]
- 14.Granhall U, Selander H. 1973. Nitrogen fixation in a subarctic mire. Oikos 24:8–15. doi: 10.2307/3543247. [DOI] [Google Scholar]
- 15.Schwintzer CR. 1983. Nonsymbiotic and symbiotic nitrogen fixation in a weakly minerotrophic peatland. Am J Bot 70:1071–1078. doi: 10.2307/2442817. [DOI] [Google Scholar]
- 16.Dedysh SN, Dunfield PF. 2016. Beijerinckiaceae, p 1–4. In Whitman WB. (ed), Bergey's manual of systematics of Archaea and Bacteria. John Wiley & Sons, Inc., Hoboken, NJ. [Google Scholar]
- 17.Bragina A, Maier S, Berg C, Müller H, Chobot V, Hadacek F, Berg G. 2011. Similar diversity of alphaproteobacteria and nitrogenase gene amplicons on two related Sphagnum mosses. Front Microbiol 2:275. doi: 10.3389/fmicb.2011.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dedysh SN, Ricke P, Liesack W. 2004. NifH and NifD phylogenies: an evolutionary basis for understanding nitrogen fixation capabilities of methanotrophic bacteria. Microbiology 150:1301–1313. doi: 10.1099/mic.0.26585-0. [DOI] [PubMed] [Google Scholar]
- 19.Ho A, Bodelier PL. 2015. Diazotrophic methanotrophs in peatlands: the missing link? Plant Soil 389:419–423. doi: 10.1007/s11104-015-2393-9. [DOI] [Google Scholar]
- 20.Vile MA, Wieder RK, Živković Scott KD, Vitt DH, Hartsock JA, Iosue CL, Quinn JC, Petix M, Fillingim HM. 2014. N2-fixation by methanotrophs sustains carbon and nitrogen accumulation in pristine peatlands. Biogeochemistry 121:317–328. doi: 10.1007/s10533-014-0019-6. [DOI] [Google Scholar]
- 21.Larmola T, Leppänen SM, Tuittila E-S, Aarva M, Merilä P, Fritze H, Tiirola M. 2014. Methanotrophy induces nitrogen fixation during peatland development. Proc Natl Acad Sci U S A 111:734–739. doi: 10.1073/pnas.1314284111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong PP, Burris R. 1972. Nature of oxygen inhibition of nitrogenase from Azotobacter vinelandii. Proc Natl Acad Sci U S A 69:672–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fay P. 1992. Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol Rev 56:340–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller R, Eady R. 1988. Molybdenum and vanadium nitrogenases of Azotobacter chroococcum. Low temperature favours N2 reduction by vanadium nitrogenase. Biochem J 256:429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walmsley J, Kennedy C. 1991. Temperature-dependent regulation by molybdenum and vanadium of expression of the structural genes encoding 3 nitrogenases in Azotobacter vinelandii. Appl Environ Microbiol 57:622–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Normand P, Simonet P, Bardin R. 1988. Conservation of nif sequences in Frankia. Mol Gen Genet 213:238–246. doi: 10.1007/BF00339587. [DOI] [PubMed] [Google Scholar]
- 27.Gaby JC, Buckley DH. 2014. A comprehensive aligned nifH gene database: a multipurpose tool for studies of nitrogen-fixing bacteria. Database (Oxford) 2014:bau001. doi: 10.1093/database/bau001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaby JC, Buckley DH. 2012. A comprehensive evaluation of PCR primers to amplify the nifH gene of nitrogenase. PLoS One 7:e42149. doi: 10.1371/journal.pone.0042149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zehr JP, Jenkins BD, Short SM, Steward GF. 2003. Nitrogenase gene diversity and microbial community structure: a cross-system comparison. Environ Microbiol 5:539–554. doi: 10.1046/j.1462-2920.2003.00451.x. [DOI] [PubMed] [Google Scholar]
- 30.Raymond J, Siefert JL, Staples CR, Blankenship RE. 2004. The natural history of nitrogen fixation. Mol Biol Evol 21:541–554. doi: 10.1093/molbev/msh047. [DOI] [PubMed] [Google Scholar]
- 31.Bellenger JP, Xu Y, Zhang X, Morel FMM, Kraepiel AML. 2014. Possible contribution of alternative nitrogenases to nitrogen fixation by asymbiotic N2-fixing bacteria in soils. Soil Biol Biochem 69:413–420. doi: 10.1016/j.soilbio.2013.11.015. [DOI] [Google Scholar]
- 32.Wedepohl KH. 1995. The composition of the continental crust. Geochem Cosmochim Acta 59:1217–1232. doi: 10.1016/0016-7037(95)00038-2. [DOI] [Google Scholar]
- 33.Betancourt DA, Loveless TM, Brown JW, Bishop PE. 2008. Characterization of diazotrophs containing Mo-independent nitrogenases, isolated from diverse natural environments. Appl Environ Microbiol 74:3471–3480. doi: 10.1128/AEM.02694-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darnajoux R, Constantin J, Miadlikowska J, Lutzoni F, Bellenger JP. 2014. Is vanadium a biometal for boreal cyanolichens? New Phytol 202:765–771. doi: 10.1111/nph.12777. [DOI] [PubMed] [Google Scholar]
- 35.Hodkinson B, Allen J, Forrest L, Goffinet B, Seurasiaux E, Andresson O, Miao V, Bellenger JP, Lutzoni F. 2014. Lichen-symbiotic cyanobacteria associated with Peltigera have an alternative vanadium-dependent nitrogen fixation system. Eur J Phycol 49:11–19. doi: 10.1080/09670262.2013.873143. [DOI] [Google Scholar]
- 36.Loveless TM, Saah JR, Bishop PE. 1999. Isolation of nitrogen-fixing bacteria containing molybdenum-independent nitrogenases from natural environments. Appl Environ Microbiol 65:4223–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McRose DL, Zhang X, Kraepiel AM, Morel FM. 2017. Diversity and activity of alternative nitrogenases in sequenced genomes and coastal environments. Front Microbiol 8:267. doi: 10.3389/fmicb.2017.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crombie AT, Murrell JC. 2014. Trace-gas metabolic versatility of the facultative methanotroph Methylocella silvestris. Nature 510:148–151. doi: 10.1038/nature13192. [DOI] [PubMed] [Google Scholar]
- 39.Colby J, Dalton H. 1976. Some properties of a soluble methane mono-oxygenase from Methylococcus capsulatus strain Bath. Biochem J 157:495–497. doi: 10.1042/bj1570495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prior SD, Dalton H. 1985. Acetylene as a suicide substrate and active site probe for methane monooxygenase from Methylococcus capsulatus (Bath). FEMS Microbiol Lett 29:105–109. doi: 10.1111/j.1574-6968.1985.tb00843.x. [DOI] [Google Scholar]
- 41.Semrau JD, DiSpirito AA, Yoon S. 2010. Methanotrophs and copper. FEMS Microbiol Rev 34:496–531. doi: 10.1111/j.1574-6976.2010.00212.x. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Dumont MG, Neufeld JD, Bodrossy L, Stralis-Pavese N, McNamara NP, Ostle N, Briones MJ, Murrell JC. 2008. Revealing the uncultivated majority: combining DNA stable isotope probing, multiple displacement amplification and metagenomic analyses of uncultivated Methylocystis in acidic peatlands. Environ Microbiol 10:2609–2622. doi: 10.1111/j.1462-2920.2008.01683.x. [DOI] [PubMed] [Google Scholar]
- 43.Esson KC, Lin X, Kumaresan D, Chanton JP, Murrell JC, Kostka JE. 2016. Alpha-and gammaproteobacterial methanotrophs codominate the active methane-oxidizing communities in an acidic boreal peat bog. Appl Environ Microbiol 82:2363–2371. doi: 10.1128/AEM.03640-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta V, Smemo KA, Yavitt JB, Basiliko N. 2012. Active methanotrophs in two contrasting North American peatland ecosystems revealed using DNA-SIP. Microb Ecol 63:438–445. doi: 10.1007/s00248-011-9902-z. [DOI] [PubMed] [Google Scholar]
- 45.Kip N, Dutilh BE, Pan Y, Bodrossy L, Neveling K, Kwint MP, Jetten MS, Op den Camp HJ. 2011. Ultra-deep pyrosequencing of pmoA amplicons confirms the prevalence of Methylomonas and Methylocystis in Sphagnum mosses from a Dutch peat bog. Environ Microbiol Rep 3:667–673. doi: 10.1111/j.1758-2229.2011.00260.x. [DOI] [PubMed] [Google Scholar]
- 46.Lin X, Tfaily MM, Green S, Steinweg JM, Chanton P, Imvittaya A, Chanton JP, Cooper W, Schadt CW, Kostka JE. 2014. Microbial metabolic potential for carbon degradation and nutrient (nitrogen and phosphorus) acquisition in an ombrotrophic peatland. Appl Environ Microbiol 80:3531–3540. doi: 10.1128/AEM.00206-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dedysh SN, Liesack W, Khmelenina VN, Suzina NE, Trotsenko YA, Semrau JD, Bares AM, Panikov NS, Tiedje JM. 2000. Methylocella palustris gen. nov., sp. nov., a new methane-oxidizing acidophilic bacterium from peat bogs, representing a novel subtype of serine-pathway methanotrophs. Int J Syst Evol Microbiol 50:955–969. doi: 10.1099/00207713-50-3-955. [DOI] [PubMed] [Google Scholar]
- 48.Dedysh SN, Panikov NS, Liesack W, Großkopf R, Zhou J, Tiedje JM. 1998. Isolation of acidophilic methane-oxidizing bacteria from northern peat wetlands. Science 282:281–284. doi: 10.1126/science.282.5387.281. [DOI] [PubMed] [Google Scholar]
- 49.Vorobev AV, Baani M, Doronina NV, Brady AL, Liesack W, Dunfield PF, Dedysh SN. 2011. Methyloferula stellata gen. nov., sp. nov., an acidophilic, obligately methanotrophic bacterium that possesses only a soluble methane monooxygenase. Int J Syst Evol Microbiol 61:2456–2463. doi: 10.1099/ijs.0.028118-0. [DOI] [PubMed] [Google Scholar]
- 50.Liebner S, Svenning MM. 2013. Environmental transcription of mmoX by methane-oxidizing Proteobacteria in a subarctic palsa peatland. Appl Environ Microbiol 79:701–706. doi: 10.1128/AEM.02292-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rahman MT, Crombie A, Chen Y, Stralis-Pavese N, Bodrossy L, Meir P, McNamara NP, Murrell JC. 2011. Environmental distribution and abundance of the facultative methanotroph Methylocella. ISME J 5:1061–1066. doi: 10.1038/ismej.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hardy R, Burns R, Holsten R. 1973. Applications of the acetylene-ethylene assay for measurement of nitrogen fixation. Soil Biol Biochem 5:47–81. doi: 10.1016/0038-0717(73)90093-X. [DOI] [Google Scholar]
- 53.Hardy RWF, Holsten RD, Jackson EK, Burns RC. 1968. The acetylene-ethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiol 43:1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keister D. 1975. Acetylene reduction by pure cultures of Rhizobia. J Bacteriol 123:1265–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dalton H, Whittenbury R. 1976. The acetylene reduction technique as an assay for nitrogenase activity in the methane oxidizing bacterium Methylococcus capsulatus strain Bath. Arch Microbiol 109:147–151. doi: 10.1007/BF00425127. [DOI] [Google Scholar]
- 56.Hynes R, Knowles R. 1982. Effect of acetylene on autotrophic and heterotrophic nitrification. Can J Microbiol 28:334–340. doi: 10.1139/m82-049. [DOI] [Google Scholar]
- 57.Oremland RS, Taylor BF. 1975. Inhibition of methanogenesis in marine sediments by acetylene and ethylene: validity of the acetylene reduction assay for anaerobic microcosms. Appl Microbiol 30:707–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Payne W, Grant M. 1982. Influence of acetylene on growth of sulfate-respiring bacteria. Appl Environ Microbiol 43:727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sprott G, Jarrell K, Shaw K, Knowles R. 1982. Acetylene as an inhibitor of methanogenic bacteria. J Gen Microbiol 128:2453–2462. [Google Scholar]
- 60.Stirling DI, Dalton H. 1977. Effect of metal-binding agents and other compounds on methane oxidation by two strains of Methylococcus capsulatus. Arch Microbiol 114:71–76. doi: 10.1007/BF00429633. [DOI] [PubMed] [Google Scholar]
- 61.Fulweiler RW, Heiss EM, Rogener MK, Newell SE, LeCleir GR, Kortebein SM, Wilhelm SW. 2015. Examining the impact of acetylene on N-fixation and the active sediment microbial community. Front Microbiol 6:418. doi: 10.3389/fmicb.2015.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knorr KH, Horn MA, Borken W. 2015. Significant nonsymbiotic nitrogen fixation in Patagonian ombrotrophic bogs. Glob Chang Biol 21:2357–2365. doi: 10.1111/gcb.12849. [DOI] [PubMed] [Google Scholar]
- 63.Leppänen SM, Rissanen AJ, Tiirola M. 2015. Nitrogen fixation in Sphagnum mosses is affected by moss species and water table level. Plant Soil 389:185–196. doi: 10.1007/s11104-014-2356-6. [DOI] [Google Scholar]
- 64.Dilworth MJ, Eady RR, Robson RL, Miller RW. 1987. Ethane formation from acetylene as a potential test for vanadium nitrogenase in vivo. Nature 327:167–168. doi: 10.1038/327167a0. [DOI] [Google Scholar]
- 65.Limpens J, Heijmans MM, Berendse F. 2006. The nitrogen cycle in boreal peatlands, p 195–230. In Wieder RK, Vitt DH (ed), Boreal peatland ecosystems. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 66.Sebestyen SD, Dorrance C, Olson DM, Verry ES, Kolka RK, Elling AE, Kyllander R. 2011. Long-term monitoring sites and trends at the Marcell Experimental Forest, p 15–71. In Kolka R, Sebestyen S, Verry ES, Brooks KN (ed), Peatland biogeochemistry and watershed hydrology at the Marcell Experimental Forest. CRC Press LLC, Boca Raton, FL. [Google Scholar]
- 67.Urban N, Eisenreich S. 1988. Nitrogen cycling in a forested Minnesota bog. Can J Bot 66:435–449. doi: 10.1139/b88-069. [DOI] [Google Scholar]
- 68.Hill BH, Jicha TM, Lehto LL, Elonen CM, Sebestyen SD, Kolka RK. 2016. Comparisons of soil nitrogen mass balances for an ombrotrophic bog and a minerotrophic fen in northern Minnesota. Sci Total Environ 550:880–892. doi: 10.1016/j.scitotenv.2016.01.178. [DOI] [PubMed] [Google Scholar]
- 69.Rosswall T, Granhall U. 1980. Nitrogen cycling in a subarctic ombrotrophic mire. Ecol Bull 30:209–234. [Google Scholar]
- 70.Kip N, van Winden JF, Pan Y, Bodrossy L, Reichart G-J, Smolders AJ, Jetten MS, Damsté JSS, Op den Camp HJM. 2010. Global prevalence of methane oxidation by symbiotic bacteria in peat-moss ecosystems. Nat Geosci 3:617–621. doi: 10.1038/ngeo939. [DOI] [Google Scholar]
- 71.Raghoebarsing AA, Smolders AJ, Schmid MC, Rijpstra WIC, Wolters-Arts M, Derksen J, Jetten MS, Schouten S, Damsté JSS, Lamers LP, Roelofs JGM, Op den Camp HJM, Strous M. 2005. Methanotrophic symbionts provide carbon for photosynthesis in peat bogs. Nature 436:1153–1156. doi: 10.1038/nature03802. [DOI] [PubMed] [Google Scholar]
- 72.Garrity G, Bell J, Lilburn T. 2015. Bradyrhizobiaceae fam. nov., p 1 In Whitman WB. (ed), Bergey's manual of systematics of Archaea and Bacteria. John Wiley & Sons, Inc., Hoboken, NJ. [Google Scholar]
- 73.Bertine K. 1972. The deposition of molybdenum in anoxic waters. Mar Chem 1:43–53. doi: 10.1016/0304-4203(72)90005-9. [DOI] [Google Scholar]
- 74.Glass JB, Axler RP, Chandra S, Goldman CR. 2012. Molybdenum limitation of microbial nitrogen assimilation in aquatic ecosystems and pure cultures. Front Microbiol 3:331. doi: 10.3389/fmicb.2012.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Attridge EM, Rowell P. 1997. Growth, heterocyst differentiation and nitrogenase activity in the cyanobacteria Anabaena variabilis and Anabaena cylindrica in response to molybdenum and vanadium. New Phytol 135:517–526. doi: 10.1046/j.1469-8137.1997.00666.x. [DOI] [Google Scholar]
- 76.Fay P, Vasconcelos L. 1974. Nitrogen metabolism and ultrastructure in Anabaena cylindrica. II. The effect of molybdenum and vanadium. Arch Microbiol 99:221–230. doi: 10.1007/BF00696236. [DOI] [PubMed] [Google Scholar]
- 77.Glass JB, Wolfe-Simon FL, Elser JJ, Anbar AD. 2010. Molybdenum—nitrogen co-limitation in freshwater and coastal heterocystous cyanobacteria. Limnol Oceanogr 55:667–676. doi: 10.4319/lo.2010.55.2.0667. [DOI] [Google Scholar]
- 78.Scherer P. 1988. Vanadium and molybdenum requirement for the fixation of molecular nitrogen by two Methanosarcina strains. Arch Microbiol 151:44–48. doi: 10.1007/BF00444667. [DOI] [Google Scholar]
- 79.Zerkle AL, House CH, Cox RP, Canfield DE. 2006. Metal limitation of cyanobacterial N2 fixation and implications for the Precambrian nitrogen cycle. Geobiology 4:285–297. doi: 10.1111/j.1472-4669.2006.00082.x. [DOI] [Google Scholar]
- 80.Bellenger JP, Wichard T, Xu Y, Kraepiel AML. 2011. Essential metals for nitrogen fixation in a free-living N2-fixing bacterium: chelation, homeostasis and high use efficiency. Environ Microbiol 13:1395–1411. doi: 10.1111/j.1462-2920.2011.02440.x. [DOI] [PubMed] [Google Scholar]
- 81.Kip N, Ouyang W, van Winden J, Raghoebarsing A, van Niftrik L, Pol A, Pan Y, Bodrossy L, van Donselaar EG, Reichart G-J. 2011. Detection, isolation, and characterization of acidophilic methanotrophs from Sphagnum mosses. Appl Environ Microbiol 77:5643–5654. doi: 10.1128/AEM.05017-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng H, Dietrich C, Radek R, Brune A. 2016. Endomicrobium proavitum, the first isolate of Endomicrobia class. nov. (phylum Elusimicrobia)—an ultramicrobacterium with an unusual cell cycle that fixes nitrogen with a group IV nitrogenase. Environ Microbiol 18:191–204. doi: 10.1111/1462-2920.12960. [DOI] [PubMed] [Google Scholar]
- 83.McGlynn SE, Boyd ES, Peters JW, Orphan VJ. 2013. Classifying the metal dependence of uncharacterized nitrogenases. Front Microbiol 3:419. doi: 10.3389/fmicb.2012.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leppänen SM, Salemaa M, Smolander A, Mäkipää R, Tiirola M. 2013. Nitrogen fixation and methanotrophy in forest mosses along a N deposition gradient. Environ Exp Bot 90:62–69. doi: 10.1016/j.envexpbot.2012.12.006. [DOI] [Google Scholar]
- 85.Marks J, Perakis S, King E, Pett-Ridge J. 2015. Soil organic matter regulates molybdenum storage and mobility in forests. Biogeochemistry 125:167–183. doi: 10.1007/s10533-015-0121-4. [DOI] [Google Scholar]
- 86.Wichard T, Mishra B, Myneni SCB, Bellenger JP, Kraepiel AML. 2009. Storage and bioavailability of molybdenum in soils increased by organic matter complexation. Nat Geosci 2:625–629. doi: 10.1038/ngeo589. [DOI] [Google Scholar]
- 87.Niemann H, Steinle L, Blees J, Bussmann I, Treude T, Krause S, Elvert M, Lehmann MF. 2015. Toxic effects of lab-grade butyl rubber stoppers on aerobic methane oxidation. Limnol Oceangr Methods 13:40–52. doi: 10.1002/lom3.10005. [DOI] [Google Scholar]
- 88.Chen Y, Dumont MG, McNamara NP, Chamberlain PM, Bodrossy L, Stralis-Pavese N, Murrell JC. 2008. Diversity of the active methanotrophic community in acidic peatlands as assessed by mRNA and SIP-PLFA analyses. Environ Microbiol 10:446–459. doi: 10.1111/j.1462-2920.2007.01466.x. [DOI] [PubMed] [Google Scholar]
- 89.Vaughan D, Lumsdon D, Linehan D. 1993. Influence of dissolved organic matter on the bio-availability and toxicity of metals in soils and aquatic systems. Chem Ecol 8:185–201. [Google Scholar]
- 90.Kox MA, Lüke C, Fritz C, van den Elzen E, van Alen T, Op den Camp HJM, Lamers LP, Jetten MS, Ettwig KF. 2016. Effects of nitrogen fertilization on diazotrophic activity of microorganisms associated with Sphagnum magellanicum. Plant Soil 406:83–100. doi: 10.1007/s11104-016-2851-z. [DOI] [Google Scholar]
- 91.Keister DL, Evans WR. 1976. Oxygen requirement for acetylene reduction by pure cultures of rhizobia. J Bacteriol 127:149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Spiff E, Odu C. 1973. Acetylene reduction by Beijerinckia under various partial pressures of oxygen and acetylene. J Gen Microbiol 78:207–209. [Google Scholar]
- 93.Dam B, Dam S, Blom J, Liesack W. 2013. Genome analysis coupled with physiological studies reveals a diverse nitrogen metabolism in Methylocystis sp. strain SC2. PLoS One 8:e74767. doi: 10.1371/journal.pone.0074767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gaby JC, Buckley DH. 7 April 2017. The use of degenerate primers in qPCR analysis of functional genes can cause dramatic quantification bias as revealed by investigation of nifH primer performance. Microb Ecol doi: 10.1007/s00248-017-0968-0. [DOI] [PubMed] [Google Scholar]
- 95.Hanson PJ, Riggs JS, Nettles WR, Phillips JR, Krassovski MB, Hook LA, Gu L, Richardson AD, Aubrecht DM, Ricciuto DM, Warren JM, Barbier C. 2017. Attaining whole-ecosystem warming using air and deep-soil heating methods with an elevated CO2 atmosphere. Biogeosciences 14:861–883. doi: 10.5194/bg-14-861-2017. [DOI] [Google Scholar]
- 96.Kolka R, Sebestyen S, Verry ES, Brooks K (ed). 2011. Peatland biogeochemistry and watershed hydrology at the Marcell Experimental Forest. CRC Press LLC, Boca Raton, FL. [Google Scholar]
- 97.Griffiths NA, Sebestyen SD. 2016. Dynamic vertical profiles of peat porewater chemistry in a northern peatland. Wetlands 36:1119–1130. doi: 10.1007/s13157-016-0829-5. [DOI] [Google Scholar]
- 98.Lin X, Tfaily MM, Steinweg JM, Chanton P, Esson K, Yang ZK, Chanton JP, Cooper W, Schadt CW, Kostka JE. 2014. Microbial community stratification linked to utilization of carbohydrates and phosphorus limitation in a boreal peatland at Marcell Experimental Forest, Minnesota, USA. Appl Environ Microbiol 80:3518–3530. doi: 10.1128/AEM.00205-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.García-Robledo E, Corzo A, Papaspyrou S. 2014. A fast and direct spectrophotometric method for the sequential determination of nitrate and nitrite at low concentrations in small volumes. Mar Chem 162:30–36. doi: 10.1016/j.marchem.2014.03.002. [DOI] [Google Scholar]
- 100.Strickland JDH, Parsons TR. 1972. A practical handbook of seawater analysis, vol 167, 2nd ed Fisheries Research Board of Canada, Ottawa, Canada. [Google Scholar]
- 101.Murphy J, Riley J. 1962. A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. doi: 10.1016/S0003-2670(00)88444-5. [DOI] [Google Scholar]
- 102.Lin X, Green S, Tfaily M, Prakash O, Konstantinidis K, Corbett J, Chanton J, Cooper W, Kostka J. 2012. Microbial community structure and activity linked to contrasting biogeochemical gradients in bog and fen environments of the Glacial Lake Agassiz Peatland. Appl Environ Microbiol 78:7023–7031. doi: 10.1128/AEM.01750-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin X, Handley KM, Gilbert JA, Kostka JE. 2015. Metabolic potential of fatty acid oxidation and anaerobic respiration by abundant members of Thaumarchaeota and Thermoplasmata in deep anoxic peat. ISME J 9:2740–2744. doi: 10.1038/ismej.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao Y, Tang H, Ye Y. 2012. RAPSearch2: a fast and memory-efficient protein similarity search tool for next-generation sequencing data. Bioinformatics 28:125–126. doi: 10.1093/bioinformatics/btr595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huson DH, Mitra S, Ruscheweyh H-J, Weber N, Schuster SC. 2011. Integrative analysis of environmental sequences using MEGAN4. Genome Res 21:1552–1560. doi: 10.1101/gr.120618.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hutchens E, Radajewski S, Dumont MG, McDonald IR, Murrell JC. 2004. Analysis of methanotrophic bacteria in Movile Cave by stable isotope probing. Environ Microbiol 6:111–120. doi: 10.1046/j.1462-2920.2003.00543.x. [DOI] [PubMed] [Google Scholar]
- 108.Kolb S, Knief C, Stubner S, Conrad R. 2003. Quantitative detection of methanotrophs in soil by novel pmoA-targeted real-time PCR assays. Appl Environ Microbiol 69:2423–2429. doi: 10.1128/AEM.69.5.2423-2429.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Steinberg LM, Regan JM. 2008. Phylogenetic comparison of the methanogenic communities from an acidic, oligotrophic fen and an anaerobic digester treating municipal wastewater sludge. Appl Environ Microbiol 74:6663–6671. doi: 10.1128/AEM.00553-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Poly F, Monrozier LJ, Bally R. 2001. Improvement in the RFLP procedure for studying the diversity of nifH genes in communities of nitrogen fixers in soil. Res Microbiol 152:95–103. doi: 10.1016/S0923-2508(00)01172-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.