Abstract
The emergence of pandemic influenza strains, particularly the reemergence of the swine-derived influenza A (H1N1) in 2009, is reaffirmation that influenza viruses are very adaptable and influenza remains as a significant global public health treat. As recommended by the World Health Organization (WHO), the use of adjuvants is an attractive approach to improve vaccine efficacy and allow dose-sparing during an influenza emergency. In this study, we utilized CaPtivate Pharmaceutical’s proprietary calcium phosphate nanoparticles (CaPNP) vaccine adjuvant and delivery platform to formulate an inactivated whole virus influenza A/CA/04/2009 (H1N1pdm) vaccine as a potential dose-sparing strategy. We evaluated the relative immunogenicity and the efficacy of the formulation in BALB/c mice following single intramuscularly administration of three different doses (0.3, 1, or 3 μg based on HA content) of the vaccine in comparison to non-adjuvanted or alum-adjuvant vaccines. We showed that, addition of CaPNP in vaccine elicited significantly higher hemagglutination inhibition (HAI), virus neutralization (VN), and IgG antibody titers, at all dose levels, relative to the non-adjuvanted vaccine. In addition, the vaccine containing CaPNP provided equal protection with 1/3rd of the antigen dose as compared to the non-adjuvanted or alum-adjuvanted vaccines. Our data provided support to earlier studies indicating that CaPNP is an attractive vaccine adjuvant and delivery system and should play an important role in the development of safe and efficacious dose-sparing vaccines. Our findings also warrant further investigation to validate CaPNP’s capacity as an alternative adjuvant to the ones currently licensed for influenza/pandemic influenza vaccination.
Keywords: Calcium phosphate nanoparticle, adjuvant, dose-sparing, influenza, vaccine
1. Introduction
Global influenza epidemics occur annually and cause nearly 500,000 deaths and 3–5 millions of hospitalizations every year from severe respiratory complications related to influenza [WHO estimates]. Apart from seasonal outbreaks, influenza A strains caused three devastating pandemics in human in the 20th century: H1N1 in 1918, H2N2 in 1957, and H3N2 in 1968 [1–4]. A novel strain of influenza A H1N1 virus (A/H1N1/pdm/09) emerged in 2009. Although far less deadly than its 1918 ancestor, it was highly contagious nevertheless and was very efficient in human-to-human transmission compared to previous swine influenza strains [5,6]. The estimates from the CDC indicate that between 43 million and 90 million people have been infected in the US with 2009 H1N1 during the 2009–2010 seasons [7]. On the global scale, during the first year that the virus circulated among human, between 151,700 and 575,400 people lost their lives from 2009 H1N1 infection and related complications [8]. The emergence of highly pathogenic avian influenza strains A/H5N1 in 1997 [9] and 2003–2004, and A/H7N9 in 2013 [10], and the reemergence of a pandemic H1N1 in 2009 remind us that influenza is a very powerful and adaptable virus which must not be ignored.
Vaccination provides the most feasible and efficient way to prevent influenza infections and to reduce the morbidity and mortality from infection-related complications. As confirmed in CDC reports, the previous seasonal vaccinations of children or adults did not elicit cross-reactive antibody responses to the pandemic H1N1 strain of 2009 [11]. Based on the WHO pandemic preparedness estimates, the global production capacity for pandemic vaccines is approximately 3 billion doses per year [12], although predictions for future capacity are more optimistic [13]. In any case, when a pandemic caused by a more pathogenic strain than 2009 H1N1 emerges, there is a risk that most of the world’s population would be left unprotected. Thus, WHO recommends the use of adjuvants in pandemic influenza vaccines for dose-sparing [14].
Adjuvanted vaccines are considered to enhance rapidity or intensity of immune response, to induce longer-lasting and broader/cross-protective immune response (breadth), provide antigen-dose sparing to allow immunization of more people using smaller amount of antigen, reduce the need for booster dosing, and/or to improve vaccine efficacy in individuals with weaker immune system [15–17]. Thus, availability of effective adjuvants safe for human use is critical for pandemic preparedness. There are currently four inactivated pandemic influenza A(H1N1)2009 monovalent vaccines approved by the FDA in the US [18] but none are adjuvanted. A single 15 μg antigen dose in adults and a second dose given 21 days after the first are recommended in young children [19–21]. Among the adjuvants considered for inclusion in H1N1(pdm) vaccines, alum showed no significant benefit in clinical studies compared to the vaccine without the adjuvant [20]. Human volunteers at different age groups were administered an influenza A(H1N1)2009 monovalent split-virus vaccine at doses of 7.5 μg, 15 μg, or 30 μg HA antigen with or without alum adjuvant. Immunogenicity outcomes were evaluated with respect to HAI titers where HAI titer greater than 1:40 was considered as protective immune response. It was reported that a single dose of 15 μg or 30 μg vaccine without alum induced ≥1:40 HAI titers in 97% of the subjects (ages 12 to 60). Alum-adjuvanted vaccine induced ≥1:40 HAI titers in less than 90% of the subjects. Thus it was concluded that the alum did not enhance immune response to A(H1N1)2009 vaccine [20]. On the other hand, in clinical studies, MF59-adjuvanted Focetria™ [22–24] and AS03-adjuvanted Pandermix™ [25–27] at 3.75 μg A/H1N1 antigen dose were reported as being highly immunogenic and both vaccines were approved by the European Medicines Agency (EMEA). Recently, MF59 also gained FDA-approval but for limited use in seasonal influenza vaccine (Fluad™) [28].
Dose-sparing through use of adjuvants is an attractive strategy for ensuring sufficient vaccine will be available during a pandemic. On the other hand, the benefits of enhancing immunogenicity and boosting the vaccine capacity must be weighed against the risk of adjuvant itself inducing local or systemic adverse reactions. The safety of vaccine additives in general and the use of adjuvants (e.g. alum, squalene) in particular has been a long going public concern, particularly in the US [29]. Thus, development of adjuvants that can reduce such anxieties and gain public acceptance is also critical from the compliance standpoint.
Previously, a proprietary method of synthetically manufactured calcium phosphate nanoparticle (CaPNP) technology was disclosed [30,31] and its use to deliver therapeutic drugs [32,33] and vaccine antigens [34,35] by systemic or mucosal routes were reported. In preclinical safety and toxicity studies, CaPNP was shown safe for intramuscular, subcutaneous, intradermal, oral, and inhalation routes. In a Phase I study conducted in the US, subcutaneously administered CaPNP caused no toxicity, pathology, or adverse reactions in healthy volunteers [32,34]. In various preclinical studies conducted by the inventors of the CaPNP technology or other researchers using the technology, CaPNP-adjuvanted vaccines were shown to induce significant antigen-specific cell mediated and humoral immune responses and provided protection against live pathogens in challenge experiments [34–39]. In in vitro and in vivo studies, a multi-peptide dengue vaccine formulated with CaPNP demonstrated ability of CaPNP to efficiently deliver antigens to antigen presenting cells (e.g. DCs), to directly activate DCs and upregulate surface expression of MHC class I/II, CD107a, and co-stimulatory molecule CD86 to generate a robust CD8+ CTL response [40,41]. These studies provide scientific support suggesting that vaccines formulated with CaPNP have the capacity to induce broad spectrum of protective immune responses.
We report here an inactivated whole virus influenza A 2009 (H1N1pdm) vaccine (IIV) formulation containing CaPNP as an adjuvant and carrier. We evaluated the immune response generated by three different doses (0.3, 1, or 3 μg) of IIV+CaPNP vaccine in BALB/c mice after single intramuscular (i.m.) injections. We tested the protective efficacy of the vaccine in immunized mice following challenge with a lethal dose of live 2009 (H1N1pdm) virus adapted to replicate in the lungs of BALB/c mice. Non-adjuvanted IIV vaccine was used as the control and alum-adjuvanted IIV vaccine was used for comparison. Our data indicate that CaPNP could allow dose-sparing of antigens in vaccines and also support the previous suggestions that it must be involved in multiple mechanisms of immune responses to induce protection against infection. Our findings also warrant further investigation to improve the immunogenicity and the efficacy seen from the current formulation.
2. Materials and methods
2.1. Ethics regulation of laboratory animals
This study was conducted in accordance with the approval of the Institutional Animal Care and Use Committee of Utah State University (USU) dated 20 September 2013 (expiration date 19 September 2016). The work was performed in the AAALAC-accredited Laboratory Animal Research Center of Utah State University. The U. S. Government (NIH) approval was renewed on 1 April 2010 (PHS Assurance No. A3801-01) in accordance with the NIH guide for the Care and Use of Laboratory Animals.
2.2. Animals
Pathogen free female BALB/c mice, 18–20 g, were obtained from Charles River Laboratories (Wilmington, MA) and maintained in accordance with USU Institutional Animal Care and Use Committee (IACUC). Animals were quarantined for 72 hours and maintained on Tekland Rodent Diet (Harlan Tekland) and tap water at the Laboratory Animal Research Center of USU. The mouse efficacy studies were conducted in accordance with the approval of the IACUC of USU dated 20 September 2013. Eleven mice per group were used in most experiments.
2.3. Virus
Influenza A/CA/04/2009 (H1N1pdm) was used for lethal challenge. Influenza A/CA/04/2009 (H1N1) strain designation 175190 was received from Dr. Elena Govorkova, Department of Infectious Diseases, St. Jude Children’s Research Hospital, Memphis TN. The virus was adapted to replication in the lungs of BALB/c mice by 9 sequential passages in mice [42]. Virus was plaque purified in Madin-Darby Canine Kidney (MDCK) cells and a virus stock was prepared by growth in embryonated chicken eggs and then MDCK cells as described earlier [43].
2.4. Vaccine and adjuvants
The inactivated whole virus vaccine produced at USU consisted of the influenza A/CA/04/2009 (H1N1pdm) virus grown in MDCK cells. After virus was harvested from MDCK cells, it was inactivated by addition of binary ethyleneimine (BEI) and then clarified/concentrated using tangential flow filtration using a Pellicon® XL 50 cassette (100kd, Millipore) membrane. USU has the facility, technical, and scientific resources and expertise of producing an inactivated whole virus vaccine with similar quality as those made in industry, except at a much smaller scale. At USU, Dr. Bart Tarbet (co-author) was responsible for the production and the quality control of the “inactivated whole virus vaccine” used in the study. Dr. Tarbet has developed and licensed two inactivated influenza virus vaccines during his previous employments in industry. That “production” experience was utilized in a 5-year grant from BARDA to work with the WHO to train personnel from developing countries in the manufacturer of influenza vaccines [44].
The vaccine stock used in formulations contained 2048 H1N1 HA Unit/ml. CaPNPs (2% suspension) in the 450–500 nm size range were manufactured by CaPtivate Pharma using a process modified from previously described [34,Supplement]. The final concentration of CaPNP in vaccines was at 0.3% (weight/volume) which was based on a different study (H5N1+CaPNP Vaccine), thus it was partially arbitrary [45,Supplement]. Alhydrogel (Aluminium hydroxide (alum), 2% gel) was purchased from InvivoGen (San Diego, CA) and used at final concentration of 0.2% in vaccines. Three different doses of inactivated influenza A/CA/04/2009 (H1N1pdm) vaccines (IIV) were formulated based on the HA amount. The vaccines contained 0.3 μg, 1 μg, or 3 μg H1N1 HA either without an adjuvant or adsorbed on pre-formulated CaPNP or commercial alum adjuvant. In preliminary investigation, an IIV vaccine at 3 μg H1N1 HA provided about 90% protection in mice against lethal challenge with mouse-adapted A/CA/04/2009 (H1N1pdm) virus (B. Tarbet, unpublished). Thus, in this study we used sub-optimal doses of IIV (≤ 3 μg) in order to observe the effect of adjuvants. Placebo vaccines contained physiological sterile saline (PSS) or 0.3% CaPNP.
2.5. Experimental design for animal studies
2.5.1. Immunogenicity studies
Mice were assigned to groups (11 mice per group) by random number generator. Five untreated mice were observed for normal weight gain. Mice were vaccinated with a single intramuscular (i.m.) dose of “IIV alone”, “IIV + 0.3% CaPNP”, or “IIV + 0.2% alum” vaccine containing 0.3, 1, or 3 μg H1N1 HA in 50 μl. Placebo vaccines were administered by the same route and volume. Blood was collected via cheek bleed on day 0 and day 21. Serum samples were prepared by centrifugation and stored at −70°C until used in assays.
2.5.2. Vaccine efficacy studies
On day 21 post immunization, mice were anesthetized by intraperitoneal (i.p.) injection of ketamine/xylazine (50 mg/kg//5 mg/kg). Anesthetized mice were infected by the intranasal (i.n.) route with approximately 1 × 104 cell culture infectious doses (CCID50) of A/CA/04/2009 (H1N1pdm) per mouse in a 90 μl inoculum. Post challenge, animals were monitored for morbidity and mortality and change in body weights for 21 days.
2.6. Virus-specific IgG ELISA
Virus-specific immunoglobulin levels, IgG1 and IgG2a, in mice sear were determined by using a mouse IgG enzyme immunoassay (EIA) kit (Bethyl Laboratories, Montgomery, TX) according to the manufacturer’s instructions with some modifications. Instead of coating plates with capture antibody, purified influenza A/CA/04/2009 (H1N1pdm) virus (0.84 μg/well) was bound to microtiter plates (Nunc MaxiSorp C; Fisher Scientific, Pittsburg, PA) by incubation in carbonate-bicarbonate coating buffer overnight at room temperature. Influenza virus bound to plates enable the detection of virus-specific immunoglobulins using the IgG immunoassay kits. Serum samples were diluted in PBS and the manufacturer’s directions were followed for detection of bound antibody with a minor modification. The concentration of goat anti-mouse IgG-subtype specific antibody conjugated to horseradish peroxidase was increased 3× to increase the sensitivity of the assay. Antibody concentrations were read off from a standard curve generated by using the mouse reference serum.
2.7. Humoral Immunity
Humoral immunity was measured in serum samples collected 21 days post-vaccination by hemagglutination inhibition (HAI) and virus-specific neutralization (VN) assays.
2.7.1. Hemagglutination inhibition (HAI) Assay
HAI assay was performed as previously described [43]. Prior to the HAI assay, immune sera were pretreated with receptor-destroying enzyme (RDE) II (Vibrio cholerae neuraminidase, Accurate Chemical and Scientific, Westbury NY) to remove non-specific inhibitors by diluting one part serum with three parts enzyme and incubating at 37°C for 18 hrs (WHO manual-2002). RDE was subsequently inactivated by heating in a 56°C water bath for 45 min. Serum samples were diluted in PBS in 96-well round-bottom microtiter plates (Fisher Scientific, Pittsburg, PA). Subsequently, 8 HA units/well of influenza A/CA/04/2009 (H1N1pdm) virus plus turkey red blood cells (Lampire Biological Laboratories, Pipersville, PA) were added (50 μl of each per well), mixed briefly, and incubated for 60 min at room temperature. The serum HAI titers are reported as the reciprocal of the highest serum dilution at which hemagglutination was completely inhibited and shown as Log2 geometric mean titers (GMT) in graphs.
2.7.2. Anti-influenza virus neutralizing (VN) antibody assay
MDCK cells were seeded in 96-well plates at 1 × 104 cells per well in MEM containing 5% FBS (Hyclone, Logan, UT) 24 hours prior to use. On the next day, 2-fold serial dilutions of serum samples from five mice were prepared in serum-free media, containing 10 units/ml trypsin and 1 μg/ml EDTA, starting at 1:32 dilution and ending at 1:4096. Each serum dilution was mixed 1:1 (0.1 ml) with serum-free media (containing trypsin and EDTA) containing approximately 100 CCID50/well of influenza A/CA/04/2009 (H1N1pdm) virus. After incubation at room temperature for 1 h, the serum-influenza virus mixture (0.2 ml) was transferred to a well containing MDCK cells and incubated for 3 days. Anti-influenza VN antibodies were measured as cytopathic effect (CPE) inhibition. CPE was scored from duplicate samples by examining the MDCK cell monolayers under a light microscope on day 3 post-infection.
2.8. Statistical analysis
Kaplan-Meier survival curves were generated and compared by the Log-rank (Mantel-Cox) test followed by pairwise comparison using the Gehan-Breslow-Wilcoxon test in Prism 6.0f (GraphPad Software Inc., La Jolla, CA). The mean body weights were analyzed by analysis of variance (ANOVA) followed by Tukey’s multiple comparison test using Prism 6.0f. In addition, the results from HAI and VN assays were analyzed by ANOVA followed by Tukey’s multiple comparison tests using Prism 6.0f.
3. Results
This study determined the immunogenicity and the efficacy provided by single i.m. administration of three different doses (0.3, 1.0 and 3.0 μg) of an inactivated influenza A/CA/04/2009 (H1N1pdm) virus vaccine (IIV) alone or in combination with 0.3% CaPNP or 0.2% alum against challenge with influenza A/CA/04/2009 (H1N1pdm) virus. Along with corresponding figures, all data is summarized in Table 1 for easy comparison.
Table 1.
Summary of immunogenicity and efficacy IIV vaccine formulations in mice
| Vaccines | Dose (μg HA) |
HAI Titers | VN Titers | IgG2a/IgG1 Ratios | Survival | |||
|---|---|---|---|---|---|---|---|---|
| GMT | % SP ≥40 | GMT | % SP ≥64 | Number | (%) | |||
| VII | 0.3 | 22b | 0 | 64c | 80 | 2.00 | 3/11 | 27 |
| VII + CaPNP | 0.3 | 64b | 70 | 96c | 100 | 1.20 | 6/11 | 55* |
| VII + alum | 0.3 | 48b | 50 | 80b | 90 | 0.47 | 7/11 | 64** |
|
| ||||||||
| VII | 1 | 64b | 70 | 110a | 80 | 2.79 | 7/11 | 64* |
| VII + CaPNP | 1 | 77b | 80 | 150a | 90 | 0.89 | 9/11 | 82*** |
| VII + alum | 1 | 95b | 90 | 160b | 100 | 0.37 | 5/11 | 45** |
|
| ||||||||
| VII | 3 | 90c | 90 | 512b | 100 | 0.70 | 9/11 | 82** |
| VII + CaPNP | 3 | 125c | 90 | 512b | 100 | 0.51 | 9/11 | 82*** |
| VII + alum | 3 | 128c | 90 | 700b | 100 | 0.30 | 9/11 | 82*** |
|
| ||||||||
| Placebo 1 (PSS) | 0 | n.d. | n.d. | n.d. | n.d. | n.d. | 1/11 | 9 |
| Placebo 2 (CaP) | 0 | n.d. | n.d. | n.d. | n.d. | n.d | 0/11 | 0 |
Mice were immunized by the i.m. route on day 0 with the IIV vaccines listed above and challenged on day 21 with 1 × 104 CCID50 mouse-adapted A/CA/04/2009 (H1N1pdm). Post-challenged, mice (n=11) were monitored for 21 days for mortality.
GMT: Geometric mean titers from Figure 2. % SP: Percentage of mice with seroprotective HAI titers ≥ 40 or VN Titers ≥ 64
n.d. None detected.
P<0.05 (*),
P<0.01 (**),
P<0.001 (***)
3.1. Virus-specific antibody response
Influenza virus-specific immunoglobulin levels in serum samples were evaluated by ELISA. Figure 1A and 1B shows virus-specific IgG1 and IgG2a levels in mice following single dose i.m. vaccination with non-adjuvanted IIV or IIV formulated with CaPNP or alum at the 0.3, 1, or 3 μg antigen doses. As shown in Figure 1A, including adjuvants (CaPNP or alum) provided significant and dose-dependent increases in virus-specific IgG1 titers at all three dose levels compared to non-adjuvanted IIV. There was no significant difference in the IgG1 titers produced by the vaccine containing CaPNP or alum. With respect to IgG2a levels (Figure 1B), alum induced lower IgG2a levels than both non-adjuvanted and CaP-adjuvanted vaccines at all dose levels. However, the differences were not statistically significant at lower vaccine doses of 0.3 μg or 1 μg. Although there was no statistically significant difference in the IgG2a produced by the vaccine with or without CaPNP, CaPNP-adjuvanted vaccine at 3 μg level induced slightly higher IgG2a. At 3 μg dose level, CaPNP-adjuvanted vaccine also induced significantly higher IgG2a than the vaccine with alum at the same dose. The IgG2a/IgG1 ratios for each vaccine are shown in Table 1 and are discussed in relation to vaccine efficacy in Discussion section.
Figure 1. Virus-specific serum antibody response as measured by ELISA.
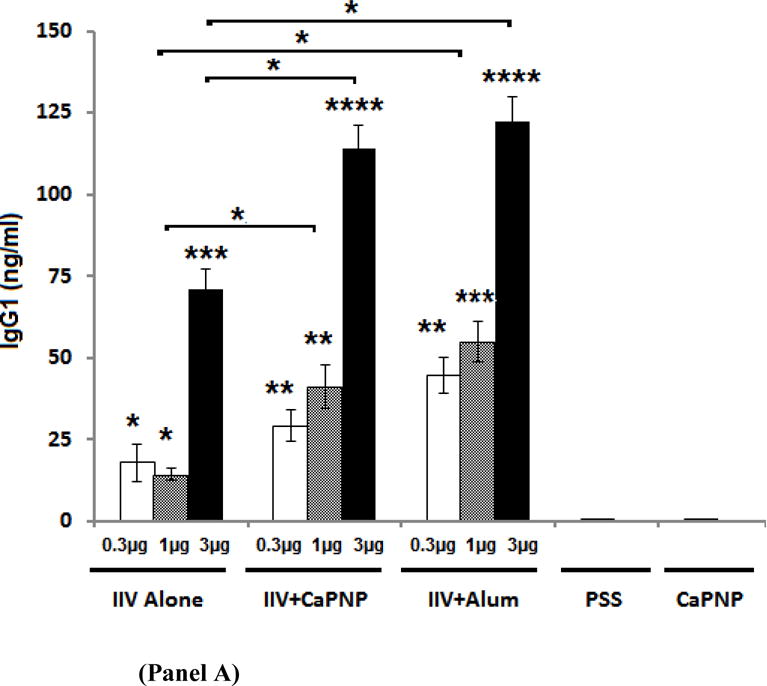
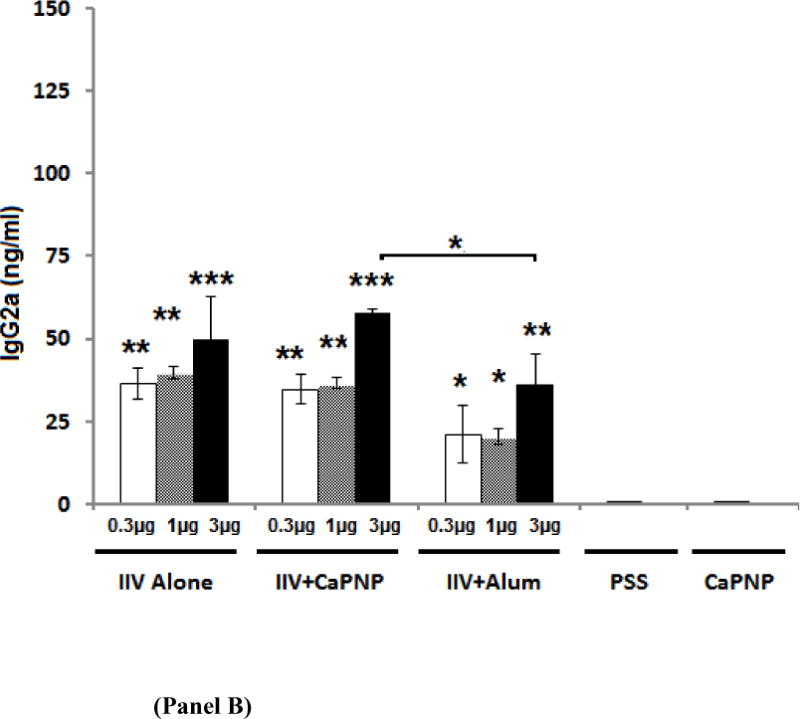
IgG1 (Panel A) and IgG2a levels (Panel B) are shown in serum from mice on day 21 following i.m. vaccination with an inactivated influenza virus (IIV) vaccine containing CaPNP or Alhydrogel (alum) as adjuvant. Data reported for IgG1 include 10 mice/group, IgG2a includes 5 mice/group. On top of the bars, “*” represent a significant increase (*P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001) compared to saline (PSS) and CaPNP only controls (placebos) in 1-way ANOVA/Turkey test. Significant differences (*P<0.05) between groups are linked by brackets.
3.2. Hemagglutination inhibition (HAI) titers
Figure 2 shows HAI titers following a single i.m. vaccination with varying doses of IIV vaccine with or without adjuvants. Non-adjuvanted IIV provided significant dose-dependent HAI titers compared to placebo. All vaccines with or without adjuvants at all dose levels, except 0.3 μg non-adjuvanted IIV, achieved HAI titers ≥ 40 (i.e. ≥1:40) which is generally defined as the threshold for a seroprotective response [20,46]. At the lowest dose level of 0.3 μg, HAI titers for the vaccines containing CaPNP or alum adjuvants were significantly higher compared to non-adjuvanted IIV. At that low dose level, the HAI titers produced by CaPNP adjuvant were the highest (GMT= 64) compared with that of IIV plus alum (GMT= 48) or non-adjuvanted IIV (GMT= 22) (ranked as IIVCaPNP>IIValum>IIV). In addition, higher % of mice immunized with 0.3 μg IIV+CaPNP (70% of mice) showed ≥40 HAI titers than the vaccine with alum (50% mice) (Fig. 2, Table 1). No mice in the non-adjuvanted IIV groups showed ≥ 40 HAI titers. At 1 μg vaccine dose, HAI titers with either CaPNP or alum were slightly higher than the vaccine without the adjuvant but the differences were not significant. At the highest vaccine dose of 3 μg, the IIV+CaPNP and IIV+alum vaccines induced comparable HAI titers (GMT=125–128) which were slightly higher than that of produced by the non-adjuvanted IIV vaccine (GMT=90). At the 3 μg vaccine dose, 90% of animals in all vaccine groups indicated ≥ 40 HAI titers. Thus, with respect to HAI titers, our preclinical data also suggested that presence of alum (or CaPNP) does not substantially enhance the HAI titer which is in line with the reports from human studies [20].
Figure 2.
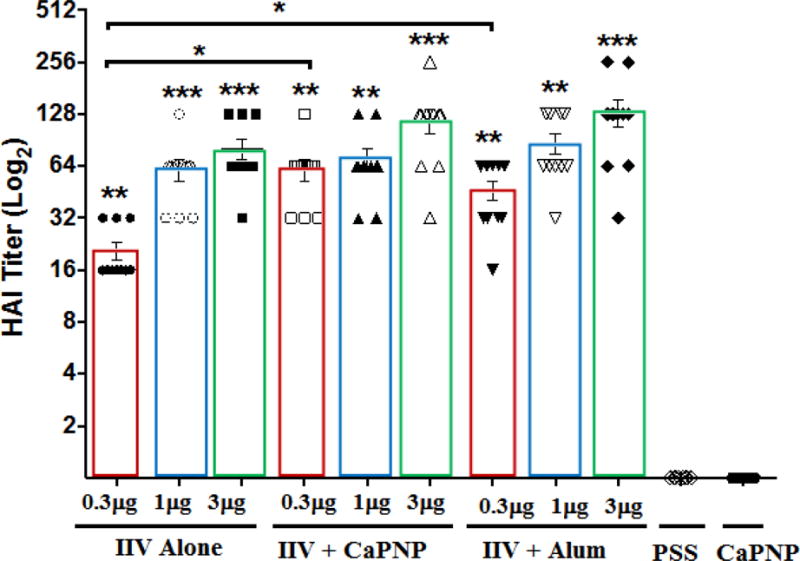
Hemagglutination inhibition titers on day 21 following i.m. vaccination with an inactivated influenza virus (IIV) vaccine without adjuvant or containing CaPNP or Alhydrogel (alum) as adjuvant. Transparent bars framing the data sets are manually overlaid on the plotted data to make comparisons between groups easier to the reader. Geometric means and 95% confidence intervals are shown. On top of the bars, “*” represent a significant increase (*P<0.05, **P<0.01, and ***P<0.001) compared to saline (PSS) and CaPNP only controls. Significant differences (*P<0.05) between groups are linked by brackets.
3.3. Virus neutralizing antibody (VN) titers
Figure 3 show virus neutralizing (VN) antibody titers on days 21 following i.m. vaccination with non-adjuvanted or CaPNP- or alum-adjuvanted IIV measured against influenza A H1N1pdm virus. Vaccination produced significant VN antibody titers in a dose-dependent manner in all vaccine groups. Inclusion of CaPNP or alum produced significantly higher VN titers than non-adjuvanted vaccine in most cases. The highest titers (GMT in the 512–700 range) were observed at the 3 μg dose level where mean titers peaked at 1024. Mice vaccinated with PSS or CaPNP placebo had no detectable serum VN titers against the virus. Although there is a lack of consensus, most studies suggest a good correlation between VN and HAI titers. However, our data did not show a clear correlation between VN and HAI for any of the vaccines (Table 1).
Figure 3.
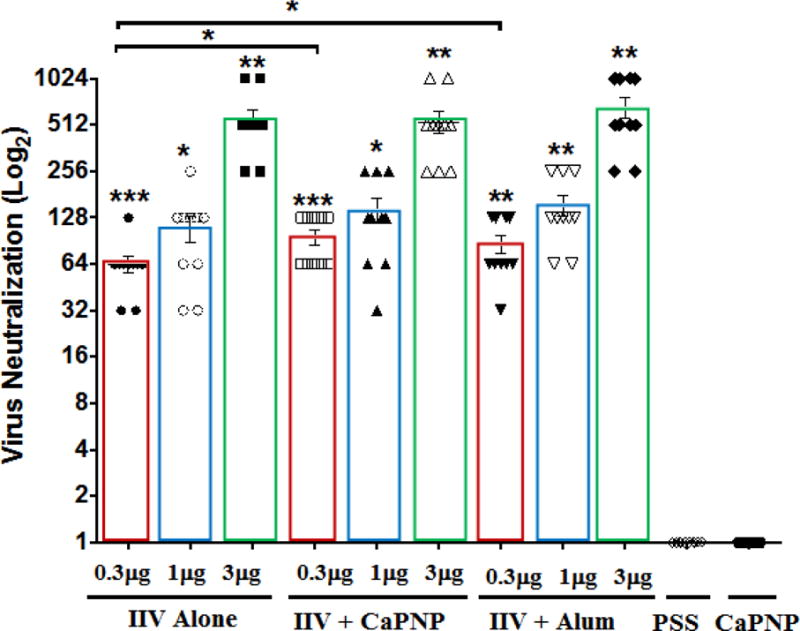
Virus neutralization (VN) titers on day 21 following i.m. vaccination with an inactivated influenza virus (IIV) vaccine containing CaPNP or Alhydrogel (alum) as adjuvant. Transparent bars framing the data sets are manually overlaid on the plotted data to make the comparisons easier. Geometric means and 95% confidence intervals are shown. On top of the bars, asterisks represent a significant increase (*P<0.05, **P<0.01, and ***P<0.001) compared to saline (PSS) and CaPNP only controls. Significant differences (*P<0.05) between groups are linked by brackets.
3.4. Vaccine efficacy
Figure 4 shows Kaplan-Meier survival curves for immunized mice after a lethal viral challenge with mouse-adapted A/California/04/2009 (H1N1pdm) virus. Animal survival on day 21 post-infection is also tabulated in Table 1 for direct comparison. As mentioned earlier, we used sub-optimal doses of IIV for immunization in order to observe the relative effect of CaPNP adjuvant on vaccine immunogenicity and efficacy. Thus, it was not surprising to see that none of the vaccine formulations provided complete protection as follows: (i) non-adjuvanted IIV provided 27%, 64%, and 82% protection, (ii) IIV plus CaPNP provided 55%, 82%, and 82% protection and, (iii) alum-adjuvanted vaccine provided 64%, 45%, and 82% protection at the 0.3, 1, and 3.0 μg doses levels, respectively. In other words, the IIV+CaPNP provided significantly better protection than the non-adjuvanted IIV with 0.3 or 1 μg doses (30% and 20% more, respectively). In addition, at 1 μg vaccine dose, IIV+CaPNP also provided significantly better protection (~40% more) than the IIV+alum vaccine. Furthermore, IIV+CaPNP at 1 μg dose provided same level of protection (82%) to mice as the vaccines (non-adjuvnated or adjuvanted) given at 3 μg dose level, indicating a 3-fold dose sparing.
Figure 4.
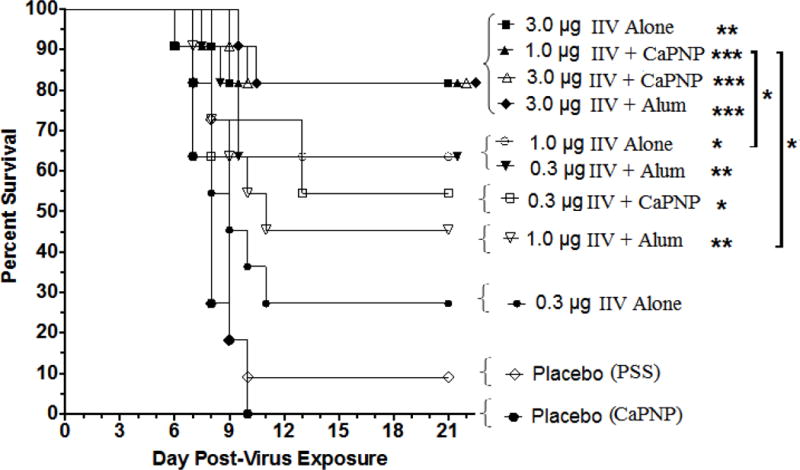
Survival following intramuscular vaccination with an inactivated influenza virus (IIV) vaccine containing CaPNP or Alhydrogel (alum) as adjuvant, and challenge with 1 × 104 CCID50 (or 3 × LD50) influenza A/CA/04/2009 (pandemic H1N1). Infected mice (n=11) were monitored for 21 days post challenge. Significant increase in survival compared to PSS and CaPNP placebo treatments are denoted as *P<0.05, **P<0.01, ***P<0.001. Significant differences (*P<0.05) between any two groups are linked by brackets.
The greatest weight loss in animals in all vaccine groups occurred within the 7–9 days of post-viral infection. Except the mice in placebo and in 0.3 μg or 1 μg non-adjuvanted IIV groups, animals started recovering from weight loss the next day after the maximum weight loss was observed. The mice in 3.0 μg IIV+alum group lost the least weight (19%) while mice in 0.3 μg or 1 μg non-adjuvanted IIV groups lost the most weight (≥30% (cut-off)). All the other groups lost maximum 23–26% weight and started gaining weight by the next time point. With the mouse-adapted H1N1(pdm) challenge, we did not observe the typical signs of disease seen in human (sneezing, fever etc.) which was expected.
4. Discussion
In this study we investigated the potential of CaPtivate’s CaP nanoparticle technology platform to provide dose-sparing for inactivated whole virus pandemic influenza A/CA/04/2009 (H1N1pdm) vaccine. CaPNP is a synthetic formulation of calcium phosphate containing all GRAS components and has been shown safe for human vaccination in a Phase I study conducted in the US [32]. As a chemical entity, calcium phosphate is a natural/biocompatible constituent of the body. It is significantly better tolerated than alum and has been safely used in human vaccines in Europe for decades [47–50]. CaPNP technology represents a unique formulation of calcium phosphate (not to be confused with its commercial counterpart) as spherical/round nanoparticles with physicochemical properties, morphology, and adjuvant action superior to those of aluminum salts or commercial calcium phosphate [40]. By itself, it is inert to body. When combined in vaccines, it indicates capacity to induce a broad range of immune responses (e.g. humoral, cellular, mucosal) [30,32–37,39,43]. In various vaccine studies, CaPNP demonstrated ability to induce robust antigen- or virus-specific CTL responses [37,40]. CaPNP also demonstrated ability to boost antigen uptake by APCs and to efficiently stimulate maturation and activation of DCs [40]. It was reported that, people who developed mild illness during the 2009 H1N1 pandemic epidemic also had abundance of CD8+T cells (CTLs). CTLs recognize parts of flu viruses that are most stable among all strains [51]. In such situations, CTLs may provide extra layer of protection against the virus strains to which the recipient or the general human population are immunologically naïve. Thus, using a vaccine adjuvant/carrier that could induce humoral and also cell-mediated immune response should further enhance vaccine’s effectiveness and stop people dying during a pandemic.
This study presents preclinical data documenting the immunogenicity and efficacy after single i.m. administration of an inactivated A/CA/04/2009 (H1N1pdm) virus vaccine containing CaPNP in mice. As summarized in Table 1, immunogenicity testing clearly showed that IIV+CaPNP formulation could induce significantly higher serum HAI, VN, and anti-virus IgG1 titers than the non-adjuvanted IIV (Fig. 1A). In most cases, IIV+CaPNP demonstrated comparable HAI, VN, and IgG1 levels to that induced by IIV+alum but the vaccine containing CaPNP, at all dose levels, produced approximately 1.7× higher virus-specific IgG2a antibodies than alum (Table 1). The IgG2a levels for non-adjuvanted and CaPNP-adjuvanted vaccines were comparable at all three dose levels (Fig. 1B). It has been previously demonstrated in mice that the Th2-type immune response, which is associated with stimulation of IgG1 antibodies, is the typical response to influenza vaccination by inactivated or subunit influenza vaccines [52,53 (Cross referenced in 54)]. However, stimulation of IgG2a, which is associated with Th1-type immune response, has been associated with survival from infection and increased vaccine efficacy [53,55]. The studies by Huber et al. [54] suggested that IgG1 antibodies, not the IgG2a antibodies, played as specific role in the neutralization of the virus. In their study, the specific induction of IgG2a was mostly correlated with viral clearance and increased protection against lethal influenza challenge. The increased induction of both IgG1 and IgG2a, but not IgG2a alone, has been indicated a better correlate for vaccine efficacy than neutralization alone [54]. Our data also suggest a similar association. Although the IgG2a/IgG1 ratios for non-adjuvanted IIV were higher than the IIV+CaPNP at all dose levels, IIV+CaPNP vaccine demonstrated significantly better protection at lower vaccine doses, particularly at 1 μg, than IIV without CaPNP. This may be because IIV+CaPNP vaccine also induced higher IgG1, HAI, and VN titers than non-adjuvanted IIV while it was inducing comparable levels of IgG2a. In comparison to alum, the vaccine containing CaPNP adjuvant indicated a balanced Th1/Th2-type antibody response while alum induced primarily a Th2-type response (see IgG2a/IgG1 ratios in Table 1).
With the exception of non-adjuvanted IIV at 3 μg vaccine dose, all three vaccines at all dose levels produced >40 HAI and > 64 VN titers in a dose-dependent manner. Based on previous studies concerning inactivated influenza virus vaccines, it was suggested that an HAI antibody titer of 1:40 against HA should be regarded as a relative correlate of protection (e.g. 50%–70%) [Reviewed in 52]. Our HAI data and its relation to protection against challenge infection were generally in agreement with this approximation. Although there is a lack of consensus, most studies also suggest a good correlation between VN and HAI titers (i.e. 1:40 dilution both) but there are others suggesting 1:64 VN titers as the correlate of protection [53 (Cross-referenced in 52)]. In our study, there was no close correlation between the HAI and VN titers which may relate to differences in the antibody binding characteristics inherent in these functional assays [46]. There was a dose-dependent increase in VN-titers for all vaccines but the increases in magnitude of VN titers did not directly correlate with increased protection.
We observed the most notable difference in vaccine efficacy with the 1 μg dose level. The IIV+ CaPNP vaccine at the 1 μg dose level (1/3rd of the high dose) was sufficient to provided equal level of protection against the live A/CA/04/2009 (H1N1pdm) challenge as seen with the “high dose” (3 μg) IIV with or without adjuvants. Interestingly, this was in spite of significantly lower HAI, VT, and IgG titers were produced with 1 μg IIV+CaPNP vaccine compared to those at 3 μg dose levels. Although not evaluated in this study, we suggest that CaPNP may be stimulating a broader immune response than IIV alone or IIV containing alum to enable equal protection using only 1/3rd of the highest dose associated with the highest protection. In previous preclinical investigations conducted by various industrial partners of CaPtivate, vaccines containing CaPNP were shown to stimulate production of both Th1- (e.g. IL-12, IFN-ɣ) and Th2-type cytokines (e.g. IL-5) [41] and also Th17-promoting cytokines (e.g. IL-22) (unpublished). With acknowledging further investigation is needed, we speculate that activation of multiple cytokine profiles may also be contributing to the good efficacy seen with IIV+CaPNP vaccine at lower doses. In a previous study that used the methods of Q. He and T. Morcol et al. in Ref. 34, a foot and mouth disease virus (FMDV) vaccine containing CaPNP induced significant humoral and T-cell mediated responses [37]. FMDV+CaPNP also provided 90% protection against virus challenge with ½ dose of the antigen as compared to vaccine without the adjuvant and in spite of inducing lower serum VN antibody titers. In another study, a CaPNP-adjuvanted fusion protein vaccine formulation was superior to free antigen in eliciting long-lasting CD8+ T cell and enhanced recall responses, despite producing lower IgG1 response compared to vaccine without the adjuvant [54]. Reports from these investigators echo our findings in this study.
In conclusion, we suggest that including CaPNPs in influenza vaccines may be an important contribution to antigen-sparing strategies. Current data also warrant further investigation in order to improve vaccine efficacy and dose-sparing potential. We have future studies designed to (i) optimize the vaccine formulation with respect to CaPNP particle size vs vaccine immunogenicity (ii) demonstrate the IIV+CaPNP vaccine’s capacity to induce Th1 response and trigger T-cell activation, (iii) in challenge studies (with homologous and heterologous virus strains), the IIV+CaPNP vaccine eliciting the broader immune response relative to IIV will be evaluated for protective efficacy and its potential to induce longer-lasting immunity, (iv) virus titters in lung lavage fluids will be determined, (v) the cytokine/chemokine profiles will also be evaluated in lung lavage fluids by mouse cytokine screen (16-plex) multiplex ELISA.
Supplementary Material
Acknowledgments
B.L. Hurst and E.B. Tarbet were involved in the execution of the experimental work. All authors were jointly involved in the study design, processing and interpretation of data, decision to publish, and preparation of the manuscript. The authors would like to thank members of the laboratory animal research center at Utah State University for performing or assisting with animal experiments. We also thank New Technologies New Vaccines (NTNV) organizing committee for allowing on-line link to conference presentation in Ref. 40.
Funding
The research represented in this paper was funded under the NIAID, NIH Animal Models of Infectious Diseases Preclinical Services Program, Contract HHSN272201000039I from the Respiratory Diseases Branch, DMID.
Appendix A
Supplementary Information: “CaPNP dose-response effect in inactivated whole virus influenza A(H1N1)2009 vaccine”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: T. Morcol is the founder of CaPtivate Pharmaceuticals (spin-off from BioSante Pharmaceuticals) and a co-inventor of the CaPNP technology used in the study. B.L. Hurst, E.B. Bart, or Utah State University has no competing interests in CaPtivate and vice versa.
References
- 1.Palese P, Shaw ML. Orthomyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. 5th. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 1647–1689. [Google Scholar]
- 2.Cox NJ, Neumann G, Donis RO, Kawaoka Y. Orthomyxoviruses: influenza. In: Mahy BWJ, ter Meulen V, editors. Topley and Wilson’s microbiology and microbial infections. London: Hodder Arnold Press; 2005. pp. 634–698. 2005. [Google Scholar]
- 3.Rambaut A, Pybus OG, Nelson MI, Viboud C, Taubenberger JK, Holmes EC. The genomic and epidemiological dynamics of human influenza A virus. Nature. 2008;45:615–619. doi: 10.1038/nature06945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taubenberger JK, Reid AH, Lourens RM, Wang R, Jin G, Fanning TG. Characterization of the 1918 influenza virus polymerase genes. Nature. 2005;437:889–893. doi: 10.1038/nature04230. [DOI] [PubMed] [Google Scholar]
- 5.Neumann G, Noda T, Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 2009;459:931–939. doi: 10.1038/nature08157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naffakh N, van der Werf S. April 2009: an outbreak of swine-origin influenza A (H1N1) virus with evidence for human-to-human transmission. Microbes Infect. 2009;11:725–728. doi: 10.1016/j.micinf.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Shrestha SS, Swerdlow DL, Borse RH, Prabhu VS, Finelli L, Atkins CY, et al. Estimating the burden of 2009 pandemic influenza A (H1N1) in the United States (April 2009–April 2010) Clin Infect Dis. 2011;52(Suppl 1):S75–S82. doi: 10.1093/cid/ciq012. [DOI] [PubMed] [Google Scholar]
- 8.Dawood FS, Iuliano AD, Reed C, Meltzer MI, Shay DK, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. The Lancet-Infectious Diseases. 2012;12:687–695. doi: 10.1016/S1473-3099(12)70121-4. [DOI] [PubMed] [Google Scholar]
- 9.de Jong JC, Claas ECJ, Osterhaus ADME, Webster RG, Lim WL. A pandemic warning? Nature. 1997;389:554. doi: 10.1038/39218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao R, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 11.CDC. Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR Morb Mortal Wkly Report. 2009;58:521–524. [PubMed] [Google Scholar]
- 12.World Health Organization (WHO) Influenza pandemic preparedness and response. Available from: http://www.who.int/csr/disease/swineflu/notes/pandemic_influenza_vaccines_20090924/en/
- 13.McLean KA, Goldin S, Nannei C, Sparrow E, Torelli G. The 2015 global production capacity of seasonal and pandemic influenza vaccine. Vaccine. 2016;34:5410–5413. doi: 10.1016/j.vaccine.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Technical Advisory Group on Vaccine-Preventable Diseases. Final Recommendations of Pandemic Influenza. Pan American Health Organization, Regional Office of the World Health Organization; 2009. Available at: http://www.paho.org/english/ad/fch/im/PandemicFlu_TAGReco_Aug2009_e.pdf. [Google Scholar]
- 15.FDA Workshop on adjuvants and adjuvanted preventive and therapeutic vaccines. 2008 Dec 2; http://www.fda.gov/downloads/biologicsbloodvaccines/newsevents/workshopsmeetingsconferences/ucm095708.pdf.
- 16.Walker WT, Faust SN. Monovalent inactivated split-virion, AS03-adjuvanted pandemic influenza A (H1N1) vaccine. Expert Rev Vaccines. 2010;9:1385–98. doi: 10.1586/erv.10.141. [DOI] [PubMed] [Google Scholar]
- 17.Nichol KL, Treanor JJ. Vaccines for seasonal and pandemic influenza. J Infect Dis. 2006;194(Suppl 2):S111–S118. doi: 10.1086/507544. [DOI] [PubMed] [Google Scholar]
- 18.FDA Press Release. 2009 Sep 15; http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm182399.htm.
- 19.Plennevaux E, Sheldon E, Blatter M, Reeves-Hoche MK, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomized controlled phase 2 trials. Lancet. 2010;375:41–48. doi: 10.1016/S0140-6736(09)62026-2. [DOI] [PubMed] [Google Scholar]
- 20.Zhu FC, Wang H, Fang HH, Yang JG, Lin XJ, Liang XF, et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med. 2009;361:2414–2423. doi: 10.1056/NEJMoa0908535. [DOI] [PubMed] [Google Scholar]
- 21.Nolan T, McVernon J, Skeljo M, Richmond P, Wadia U, Lambert S, et al. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: a randomized trial. JAMA. 2010;303:37–46. doi: 10.1001/jama.2009.1911. [DOI] [PubMed] [Google Scholar]
- 22.Block SL, Ruiz-Palacios GM, Guerrero ML, Beygo J, Sales V, Holmes SJ. Dose-range study of MF59-adjuvanted versus nonadjuvanted monovalent A/H1N1 pandemic influenza vaccine in six- to less than thirty-six-month-old children. Pediatr Infect Dis J. 2012;31:e92–e98. doi: 10.1097/INF.0b013e318257644f. [DOI] [PubMed] [Google Scholar]
- 23.Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, Stephenson I. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361:2424–2435. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 24.Esposito S, Pugni L, Daleno C, Ronchi A, Valzano A, Serra D, Mosca F, Principi N. Influenza A/H1N1 MF59-Adjuvanted Vaccine in Preterm and Term Children Aged 6 to 23 Months. Pediatrics. 2011;127:e1161–e1168. doi: 10.1542/peds.2010-1920. [DOI] [PubMed] [Google Scholar]
- 25.Roman F, Vaman T, Kafeja F, Hanon E, Van Damme P. AS03(A)-Adjuvanted influenza A (H1N1) 2009 vaccine for adults up to 85 years of age. Clin Infect Dis. 2010;51:668–677. doi: 10.1086/655830. [DOI] [PubMed] [Google Scholar]
- 26.Carmona A, Omenaca F, Tejedor JC, Merino JM, Vaman T, Dieussaert I, Gillard P, Aristegui J. Immunogenicity and safety of AS03-adjuvanted 2009 influenza A H1N1 vaccine in children 6–35 months. Vaccine. 2010;28:5837–5844. doi: 10.1016/j.vaccine.2010.06.065. [DOI] [PubMed] [Google Scholar]
- 27.Wichmann O, et al. Pandemic influenza A(H1N1) 2009 breakthrough infections and estimates of vaccine effectiveness in Germany 2009–2010. Eurosurveillance. 2010;15 [PubMed] [Google Scholar]
- 28.FDA News release: FDA Approves first seasonal influenza vaccine containing an adjuvant. 2015 Nov 24; http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm474295.htm.
- 29.Chatterjee Archana, O’Keefe Catherine. Current controversies in the USA regarding vaccine safety. Expert Rev Vaccines. 2010;9(5):497–502. doi: 10.1586/erv.10.36. [DOI] [PubMed] [Google Scholar]
- 30.Bell SJD, Morcol T, He Q. Therapeutic calcium phosphate particles and methods of manufacture and use. US Patent 6,355,271. 2002 [Google Scholar]
- 31.Bell SJD, Morcol T, He Q. Therapeutic calcium phosphate particles and methods of manufacture and use. US Patent 8,431,221 [Google Scholar]
- 32.Morcol T, Nagappan P, Nerenbaum L, Mitchel AR, Bell SJD. Particulate drug delivery systems for protein drugs. In: Kumar R, editor. Particlute Drug Delivery. Vol. 2. American Scientific Publishers; Stevenson Ranch, CA: 2008. pp. 223–241. Chapter 11. [Google Scholar]
- 33.Garcia-Contreras L, Morçöl T, Bell SJD, Hickey AJ. Evaluation of Novel particles as pulmonary delivery system for insulin in rats. AAPS PharmSci. 2003;5:10–20. doi: 10.1208/ps050209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He Q, Mitchell A, Johnson SL, Wagner-Bartak C, Morcol T, Bell SJD. Calcium phosphate nanoparticle adjuvant. Clin Diagn Lab Immunol. 2000;6:899–903. doi: 10.1128/cdli.7.6.899-903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He Q, Mitchell A, Morcol T, Bell SJD. Calcium Phosphate Nanoparticles Induce Mucosal Immunity and Protection against Herpes Simplex Virus Type 2. Clin Diagnos Lab Immunol. 2002;9:1021–1024. doi: 10.1128/CDLI.9.5.1021-1024.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karam HM, Shaaban EA, Mohamed AF, Zaki HF, Kenawy SA. New Approach for Improving Production for Naja haje Snake Venom. Int J Sci Res Pub. 2015;5(3) [Google Scholar]
- 37.Joyappa DH, Kumar CA, Banumathi N, Reddy GR, Suryanarayana VVS. Calcium phosphate nanoparticle prepared with foot and mouth disease virus P1-3CD gene construct protects mice and guinea pigs against the challenge virus. Vet Microbiol. 2009;139:58–66. doi: 10.1016/j.vetmic.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Volkova MA, Irza AV, Chvala IA, Frolov SF, Drygin VV, Kapczynski DR. Adjuvant Effects of Chitosan and Calcium Phosphate Particles in an Inactivated Newcastle Disease Vaccine. Avian Diseases. 2014;58:46–52. doi: 10.1637/10510-020413-Reg.1. [DOI] [PubMed] [Google Scholar]
- 39.Olmedo H, Herrera M, Rojas L, Villalta M, Vargas M, Leiguez E, et al. Comparison of the adjuvant activity of aluminum hydroxide and calcium phosphate on the antibody response towards Bothrops asper snake venom. J Immunotox. 2014;11:44–49. doi: 10.3109/1547691X.2013.772267. [DOI] [PubMed] [Google Scholar]
- 40.Morcol T, Philip R, Karabudak A, Huang X. Novel Calcium Phosphate Nanoparticle (CaPNP) Vaccine Adjuvant and Delivery System. Oral presentation at New Technologies New Vaccines 2015; Wilmington DE. 2015; March 22–25; http://www.ntnv.org/wp-content/uploads/2016/12/1600-Morcol.pdf. [Google Scholar]
- 41.Huang X, Karabudak A, Comber J, Morcol T, Philip R. Novel vaccination approach for dengue infection based on conserved T cell epitopes formulated in calcium phosphate nanoparticles. Human Vaccines Immotherapeutics. 2016 Nov; doi: 10.1080/21645515.2017.1369639. Submitted to. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ilyushina NA, Khalenkov AM, Seiler JP, Forrest HL, Bovin NV, et al. Adaptation of Pandemic H1N1 Influenza Viruses in Mice. J Virology. 2010;84:8607–16. doi: 10.1128/JVI.00159-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu G, Tarbet B, Song L, Reiserova L, Weaver B, Chen Y, et al. Immunogenicity and Efficacy of Flagellin-Fused Vaccine Candidates Targeting 2009 Pandemic H1N1 Influenza in Mice. PLoS One. 2011;6:e220928. doi: 10.1371/journal.pone.0020928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tarbet B, Dorward J, Day C, Rashid KA. Vaccine Production Training to Develop the Workforce of Foreign Institutions Supported by the BARDA Influenza Vaccine Capacity Building Program. Vaccine. 2013;31:1646–1649. doi: 10.1016/j.vaccine.2012.06.041. [DOI] [PubMed] [Google Scholar]
- 45.Morcol T, Dell A, Lieb L. CaP Adjuvant Enhances Th1 and Th2 Response to a Single/Low Dose Inactivated 2009 Pandemic H1N1 Virus Vaccine. Poster session presented at: Adjuvants & Delivery Systems. Immunotherapeutics and Vaccine Summit (ImVacS); 2010 August 17–19; Cambridge MA. Selected data in “Supplementary Material”. [Google Scholar]
- 46.Papenburg J, Baz M, Hamelin ME, Rheaume C, Carbonneau J, Ouakki M, Rouleau I, De Serres G, Boivin G. Evaluation of serological diagnostic methods for the 2009 pandemic influenza A (H1N1) virus. Clin Vaccine Immunol. 2011;18:520–522. doi: 10.1128/CVI.00449-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ickovic MR, Relyveld EH, Henocq E. Calcium phosphate adjuvanted allergens: Total and specific IgE levels before and after immunotherapy with house dust and mite extracts. Ann Immunol. 1983;134D:385–398. doi: 10.1016/s0769-2625(83)80029-4. [DOI] [PubMed] [Google Scholar]
- 48.Kato H, Shibamo M. Relationship between hemolytic activity and absorption capacity of aluminum and calcium phosphate as immunological adjuvants for biologicals. Microbiol Immunol. 1994;38:543–548. doi: 10.1111/j.1348-0421.1994.tb01820.x. [DOI] [PubMed] [Google Scholar]
- 49.Relyveld EH. Preparation and use of calcium phosphate adsorbed vaccines. Dev Biol Stand. 1986;65:131–6. [PubMed] [Google Scholar]
- 50.Relyveld EH. A history of toxoids. In: Plotkin SA, Fantini B, editors. Vaccinia, vaccination, vaccinology. New York: Elsevier; 1996. p. 95. [Google Scholar]
- 51.Sridhar S, Shaima Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nature Med. 2013;19:1305–1312. doi: 10.1038/nm.3350. [DOI] [PubMed] [Google Scholar]
- 52.Benne CA, Harmsen M, van der Graaff W, Verheul AF, Snippe H, Kraaijeveld CA. Influenza virus neutralizing antibodies and IgG isotype profiles after immunization of mice with influenza A subunit vaccine using various adjuvants. Vaccine. 1997;15:1039–1044. doi: 10.1016/s0264-410x(96)00287-3. [DOI] [PubMed] [Google Scholar]
- 53.Moran TM, Park H, Fernandez-Sesma A, Schulman JL. Th2 responses to inactivated influenza virus can be converted to Th1 responses and facilitate recovery from heterosubtypic virus infection. J Infect Dis. 1999;180:579–585. doi: 10.1086/314952. [DOI] [PubMed] [Google Scholar]
- 54.Huber VC, McKeon RM, Brackin MN, Laura A, Miller LA, et al. Distinct Contributions of Vaccine-Induced Immunoglobulin G1 (IgG1) and IgG2a Antibodies to Protective Immunity against Influenza. Clin Vaccine Immunol. 2006;13:981–990. doi: 10.1128/CVI.00156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hovden AO, Cox RJ, Haaheim LR. Whole influenza virus vaccine is more immunogenic than split influenza virus vaccine and induces primarily an IgG2a response in BALB/c mice. Scand J Immunol. 2005;62:36–44. doi: 10.1111/j.1365-3083.2005.01633.x. [DOI] [PubMed] [Google Scholar]
- 56.Trombetta CM, Perini D, Mather S, Temperton N, Montomoli E. Overview of Serological Techniques for Influenza Vaccine Evaluation: Past, Present and Future. Vaccines (Basel) 2014;2:707–734. doi: 10.3390/vaccines2040707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allwinn R, Geiler J, Berger A, Cinat J, Doerr HW. Determination of serum antibodies against swine origin influenza A virus H1N1/09 by immunofluorescence, haemagglutination inhibition, and by neutralization tests: How is the prevalence rate of protecting antibodies in humans? Med Microbiol Immunol. 2010;199:117–121. doi: 10.1007/s00430-010-0143-4. [DOI] [PubMed] [Google Scholar]
- 58.Zhou W, Mogucheb AO, Chiua D, Murali-Krishna K, Baneyx F. Just-in-time vaccines: Biomineralized calcium phosphate core-immunogen shell nanoparticles induce long-lasting CD8+ T cell responses in mice. Nanomedicine: Nanotech Biol Med. 2014;10:571–578. doi: 10.1016/j.nano.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


