Abstract
A novel peripheral membrane protein (2c18) that interacts directly with the gamma ‘ear' domain of the adaptor protein complex 1 (AP-1) in vitro and in vivo is described. Ultrastructural analysis demonstrates a colocalization of 2c18 and γ1-adaptin at the trans-Golgi network (TGN) and on vesicular profiles. Overexpression of 2c18 increases the fraction of membrane-bound γ1-adaptin and inhibits its release from membranes in response to brefeldin A. Knockdown of 2c18 reduces the steady-state levels of γ1-adaptin on membranes. Overexpression or downregulation of 2c18 leads to an increased secretion of the lysosomal hydrolase cathepsin D, which is sorted by the mannose-6-phosphate receptor at the TGN, which itself involves AP-1 function for trafficking between the TGN and endosomes. This suggests that the direct interaction of 2c18 and γ1-adaptin is crucial for membrane association and thus the function of the AP-1 complex in living cells. We propose to name this protein γ-BAR.
Keywords: AP-1, gamma-adaptin, mannose-6-phosphate receptor, post-Golgi transport, TGN
Introduction
The different membrane organelles within cells are formed and maintained through the continuous addition and retrieval of proteins and lipids, which travel back and forth between compartments. Such transport occurs in specific tubular and vesicular carriers that are formed in the donor compartment utilizing a protein coat congregating the selected cargo (Aridor et al, 1998). The best-characterized coats are those containing clathrin, a protein complex capable of forming a ‘cage' around the membrane protein coat. Clathrin assembles with multimeric adaptor protein (AP) complexes AP-1 and AP-2, although the requirement of the homologous complexes AP-3 and AP-4 for clathrin is still unclear (reviewed in Boehm and Bonifacino, 2001; Robinson and Bonifacino, 2001; Robinson, 2004). The subunits of these different coats are thought to select membrane protein cargo by interacting directly or indirectly with specific amino-acid motifs present in their cytoplasmic domain, thereby facilitating protein sorting (Kirchhausen et al, 1997; Bonifacino and Dell'Angelica, 1999). In addition, it is known that the association of at least the AP-1, AP-3 and AP-4 adaptor proteins with membranes is dependent on the small GTPase ARF (ARF: ADP ribosylation factor), although ARF itself is not a component of the final clathrin-coated vesicle (Robinson and Kreis, 1992; Stamnes and Rothman, 1993; Zhu et al, 1999).
The AP-1 adaptor complex localizes to the trans-Golgi network (TGN) (Robinson, 1990) and endosomes (Le Borgne et al, 1996; Futter et al, 1998; Deneka et al, 2003) and is implicated in the traffic between these compartments (Meyer et al, 2000; and reviewed in Hinners and Tooze, 2003). It comprises two large chains of adaptor proteins, or ‘adaptins', γ1 and β1, in addition to a medium (μ1) and small (σ1) chain. Of these, the μ1 chain is believed to be responsible for the interaction with the cytoplasmic domains of cargo proteins containing a YXXΦ motif (where Φ is a hydrophobic residue) (Ohno et al, 1995), while the β1 chain has been shown to interact with dileucine-based signals of cargo molecules (Rapoport et al, 1998) in addition to clathrin (Gallusser and Kirchhausen, 1993). Recently, binding of a γ1–σ1 hemicomplex to dileucine-based signals has been reported (Janvier et al, 2003). A recent report has also indicated the γ1-adaptin subunit has a domain capable of binding clathrin (Doray and Kornfeld, 2001), and additionally is believed to function as a platform for binding other regulatory proteins (Kent et al, 2002; Nogi et al, 2002).
In addition to AP-1, another related family of proteins, the Golgi-localized, gamma ear-containing, ARF-binding proteins (GGAs), has also been identified as a factor that controls post-TGN trafficking (Boman et al, 2000; Dell'Angelica et al, 2000; Hirst et al, 2000). GGAs localize to the TGN, have similarity to the γ1-adaptin subunit of AP-1, bind ARF-GTP and possess a clathrin-binding site. Bonifacino and co-workers (Puertollano et al, 2001) have shown that the cytoplasmic domain sequence used by mannose-6-phosphate receptors (MPRs) for sorting to endosomes is specifically recognized by GGA proteins, and that the GGA and the receptor leave the Golgi together. Recently, it has been shown that GGAs and AP-1 actually bind each other, and might cooperate in packaging MPRs at the TGN (Doray et al, 2002).
AP-mediated transport in the late secretory pathway must therefore be tightly controlled and involves many regulatory proteins. Indeed in the case of AP-2/clathrin-mediated endocytosis from the cell surface, a large number of accessory factors that bind the α-adaptin subunit have been identified (reviewed in Slepnev and De Camilli, 2000). However, the scenario is somewhat different for the γ1-adaptin subunit of AP-1. To date, γ-synergin (Page et al, 1999), Rabaptin-5 (Shiba et al, 2002), p56 (Lui et al, 2003), the ENTH domain-containing protein EpsinR (Kalthoff et al, 2002; Wasiak et al, 2002; Hirst et al, 2003; Mills et al, 2003), NECAP and aftiphilin (Mattera et al, 2004) are among the few proteins that can bind γ1-adaptin; however, the specific role of most of these in AP-1 sorting is unclear. Recently, the identification of a GGA and γ1-adaptin ear-binding motif (GAE), namely ΨG(P/D/E)(Ψ/L/M) (where Ψ represents an aromatic residue), in all of these accessory proteins provides a possible strategy to identify further interactors (described in Mattera et al, 2004).
Here, we describe a peripheral membrane protein that localizes to membranes of the late secretory pathway, and interacts with the γ1-adaptin subunit of the AP-1 sorting machinery. This direct interaction of 2c18 and γ1-adaptin is crucial for the membrane association and thus function of the AP-1 complex in living cells.
Results
A novel peripheral membrane protein that interacts with the ‘ear' domain of γ1-adaptin
Using a visual screen based on green fluorescent protein (GFP) tagging of novel full-length cDNAs (Wiemann et al, 2001), we recently identified new proteins that localize to structures of the secretory pathway and thus potentially regulate membrane traffic (Simpson et al, 2000). One of these proteins (DKFZp564C182; henceforth abbreviated 2c18), when expressed in Vero cells as a C-terminally tagged GFP fusion protein, localized to the juxta-nuclear Golgi region and to smaller discrete punctate or tubular structures throughout the cytoplasm, which were highly mobile and cycling between the juxta-nuclear area and the cell periphery (Figure 1A and Supplementary video). The appearance and kinetics of these structures strongly resembled previously described post-TGN membrane carriers (see, for example, Waguri et al, 2003).
Figure 1.
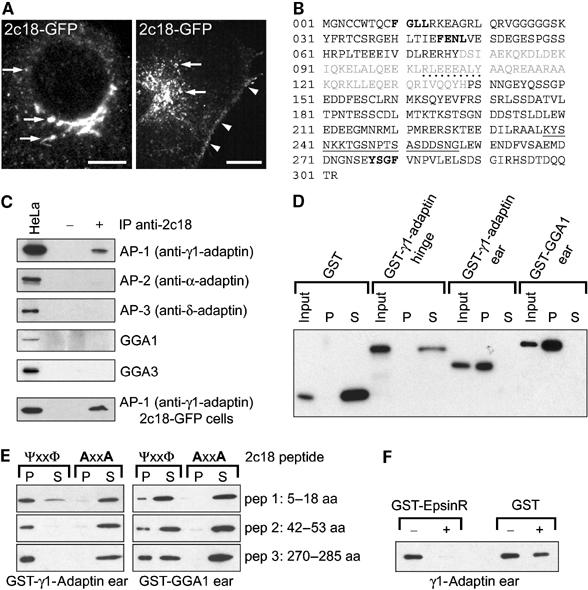
Characterization of 2c18 and its interaction with γ1-adaptin. (A) Fixed (left panel) and living (right panel) Vero cells expressing GFP-tagged 2c18 showing the protein localizing to the juxta-nuclear Golgi region, punctate and tubular structures (arrows) and the plasma membrane (arrowheads). Bars represent 10 μm. A time-lapse video showing the dynamics of 2c18-GFP is available as Supplementary data (video 1). (B) Amino-acid sequence of the 2c18 ORF. Residues in gray represent the predicted coiled-coil domain, dotted underlined residues indicate a predicted tyrosine kinase phosphorylation site and solid underlined residues designate the peptide sequence used to generate the anti-2c18 antibodies. Bold residues show potential GAE-binding motifs. (C) Protein extracts prepared from HeLa cells were immunoprecipitated with anti-2c18 antibodies followed by Western blotting for different components of various adaptor complexes as indicated. Only co-immunoprecipitation of γ1-adaptin by 2c18 was detectable, and to a higher degree from 2c18-GFP-transfected cells. No co-immunoprecipitation of α-adaptin, δ-adaptin, GGA1 or GGA3 was detected under these conditions. Lane 1 contains 20 μg of total HeLa extract from nontransfected cells. (D) Various purified GST fusion proteins were incubated with immobilized His-tagged 2c18 and subsequently separated by centrifugation into pellet (P) and soluble (S) fractions to determine binding to 2c18. Following SDS–PAGE and Western blotting, the samples were probed with anti-GST antibodies. Only the γ1-adaptin and GGA1 ‘ear' domains interact with recombinant 2c18. (E) 2c18 peptides containing putative GAE-binding motifs, FGLL (aa 5–18), FENL (aa 35–55), YSGF (aa 270–287), and their counterparts containing alanine substitutions were incubated with GST-γ1-adaptin and GST-GGA1 ‘ears'. All the wild-type peptides bound strongly to the γ1-adaptin ‘ear', but less so to the GGA1 ‘ear'. None of the peptides containing the alanine substitutions bound to either of the GST-tagged proteins. (F) GST-γ1-adaptin ‘ear' was bound to immobilized 2c18 and then competitively eluted with either purified GST-EpsinR or GST alone. The remaining 2c18-bound γ1-adaptin ‘ear' was determined by SDS–PAGE and Western blotting. EpsinR protein was able to competitively remove the γ1-adaptin ‘ear' from 2c18.
Sequence analysis of 2c18 predicted a protein of 302 aa (Figure 1B). The Coils program (Lupas et al, 1991) indicated the presence of a coiled-coil region from residues 78 to 138, but other motif prediction software did not predict the presence of either a signal peptide or transmembrane domains. Analysis of protein solubility by sodium carbonate (pH 11) buffer extraction demonstrated that 2c18 is a peripheral membrane protein (not shown), as also predicted by sequence analysis using the PSORT program (Nakai and Horton, 1999). Amino-acid alignments and comparisons did not reveal significant homologies to any known proteins. Database analyses however indicated that the equivalent protein exists in mouse (89% identical at the protein level) and antibodies raised against the human protein crossreacted with a protein of similar size in extracts from a variety of rat tissues (not shown), suggesting that a closely related protein also exists in rat.
Yeast two-hybrid screening using an N-terminal 2c18 fragment (residues 1–151) as ‘bait' revealed several potential 2c18-interacting proteins (not shown). The strongest interaction was observed with a fragment that could be mapped to aa 253–822 of human γ1-adaptin (Supplementary Figure 1). Other potential 2c18 interactors identified in this screen included the Rab5 GTPase activating protein (GAP) tuberin (Xiao et al, 1997), the Rab5 effector Rabaptin-5 (Lippe et al, 2001) and the ARF-related protein ARD1 (Vitale et al, 1998). All these proteins are known to be associated with post-Golgi traffic. However, none of the potential interactors identified by the yeast two-hybrid screen mapped to subunits of other adaptor complexes such as AP-2, AP-3, AP-4 or GGAs or to components of the COPI and COPII vesicular coat protein complexes.
Immunoprecipitation of endogenous 2c18 from HeLa cell extracts specifically co-precipitated γ1-adaptin (Figure 1C). In contrast, co-precipitation of the α-adaptin subunit of AP-2, the δ-adaptin subunit of AP-3, GGA1 or the GGA3 protein from both nontransfected (Figure 1C) and 2c18-GFP-transfected cells (not shown) was not detected. In 2c18-GFP-overexpressing cells, increased amounts of γ1-adaptin could be co-precipitated (Figure 1C).
To further establish the significance of this potential interaction, we performed direct binding assays with purified glutathione-S-transferase (GST)-tagged γ1-adaptin domains and recombinant histidine-tagged 2c18 protein (Figure 1D). Both GST and GST-γ1-adaptin ‘hinge' (aa 595–683) showed no binding affinity for 2c18, as both of these proteins were found in the supernatant fractions of the binding assays. In contrast, the GST-γ1-adaptin ‘ear' domain (aa 703–822) showed a very strong affinity for 2c18, indicating that the binding of γ1-adaptin to 2c18 is direct and occurs via the ‘ear' domain. Due to the high homology between the ‘ear' domains of γ1-adaptin and the GGA proteins (GAE domains), we also looked for a 2c18–GGA interaction in the direct binding assay. These experiments revealed a significant in vitro interaction between recombinant 2c18 and GGA1 ‘ear' despite our inability to co-precipitate the GGA proteins with the 2c18 antibodies from cells.
It has recently been proposed that GAE domains may bind to a series of accessory proteins via a common motif. In particular, the consensus sequence ΨG(P/D/E)(Ψ/L/M), preceded by negatively charged residues, appears to be critical in this respect (Collins et al, 2003; Miller et al, 2003; Mattera et al, 2004). Examination of the 2c18 sequence revealed three similar motifs in the molecule, namely the sequences FGLL, FENL and YSGF (Figure 1B, marked in bold), suggesting that 2c18 and γ1-adaptin interaction could occur via one or all of these motifs. To test this hypothesis, we synthesized peptides containing both the ‘wild-type' motifs and mutated versions ‘AGLA', ‘AENA' and ‘ASGA', respectively, and tested their ability to bind to recombinant GST-γ1-adaptin and GST-GGA1 ‘ears'. While each of the peptides containing the wild-type sequence showed an affinity for the γ1-adaptin ‘ear', the presence of the two point mutations totally abolished this interaction (Figure 1E). Equivalent experiments revealed a lower affinity between the wild-type peptides and the GGA1 ‘ear', with more protein detectable in the supernatants (Figure 1E).
Since such GAE-binding motifs are found in all the AP-1 accessory proteins described so far, we wanted to determine if these proteins were able to compete with 2c18 for the same binding site on the γ1-adaptin ‘ear'. One example of such an accessory protein is EpsinR (Kalthoff et al, 2002; Wasiak et al, 2002; Hirst et al, 2003; Mills et al, 2003). As shown in Figure 1F, EpsinR was seen to competitively interact with the γ1-adaptin.
Cellular localization of 2c18
Immunofluorescence analysis revealed that the localization of endogenous 2c18 was similar to that seen with the GFP-tagged protein, predominantly displaying a juxta-nuclear pattern with a few small dot-like structures throughout the cell (Figure 3A). Costainings with the cis-Golgi marker GM130 showed no apparent overlap in the localization of these two proteins (Supplementary Figure 2). In contrast, costainings with the late Golgi marker TGN46 showed many overlapping structures in the juxta-nuclear region (Supplementary Figure 2). Consistent with the interaction data above, 2c18 colocalization with γ1-adaptin showed significant overlap in the juxta-nuclear area but only little in dot-like structures throughout the cell (Figure 3A).
Figure 3.
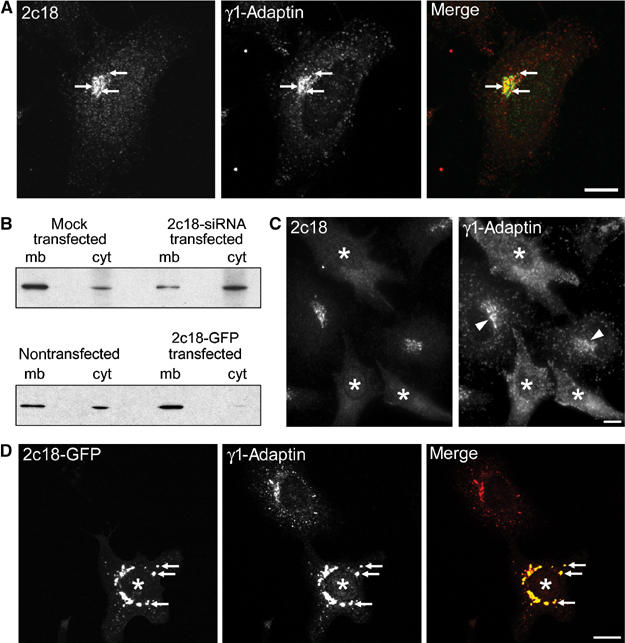
2c18 expression levels influence γ1-adaptin membrane association. (A) HeLa cells were immunostained for endogenous 2c18 and γ1-adaptin. Colocalizing structures are marked by arrows. (B) Membrane (mb) and cytosol (cyt) fractions were prepared from HeLa cell extracts and then subjected to SDS–PAGE followed by Western blotting for γ1-adaptin. (C) HeLa cells were treated with siRNA oligonucleotides against 2c18 and then fixed and double stained for 2c18 and γ1-adaptin. Cells showing a decreased 2c18 staining are marked with asterisks. Arrowheads point to γ1-adaptin's juxta-nuclear localization. (D) Vero cells were transfected with a plasmid encoding 2c18-GFP for 18 h and then fixed and stained for γ1-adaptin. Overexpression of 2c18-GFP causes a more compact staining of γ1-adaptin, which now almost totally overlaps with the 2c18-GFP (arrows). Transfected cells are denoted with asterisks. Bars represent 10 μm.
We next examined the localization of 2c18 at the ultrastructural level by immunogold electron microscopy (EM) in HeLa SA:48 cells, a cell line stably transfected with the TGN marker sialyltransferase (Rabouille et al, 1995). 2c18 (Figure 2A, C and D, arrows) was observed on membrane structures in close proximity to the TGN, as identified by the presence of the sialyltransferase (Figure 2A, C and D, arrowheads). In addition, 2c18 was also observed on membranes resembling endocytic structures (marked E in Figure 2A). To further confirm this endosomal localization, we incubated HeLa SA:48 cells with gold-BSA for 10 min followed by a 20 min chase period in order to label late endosomes. Colocalization of 2c18 and internalized gold-BSA could be observed on large, highly invaginated membrane structures, typical of late endosomes (Griffiths et al, 1988; reviewed in Gruenberg, 2001) (Figure 2B). It was also possible to visualize 2c18 at the plasma membrane (Figure 2E), consistent to that seen by light microscopy in cells expressing GFP-tagged 2c18 (Figure 1A). Quantification revealed that the majority of all of the 2c18-gold particles (48.2%) were found on late endocytic structures, with a small number of the total on the TGN (3.6%) and early endosomes (4.3%) (Table I). In colocalization studies with 2c18 and γ1-adaptin in rat pancreatic tissue, an organ known for its highly active secretory pathway, a number of colocalizing structures on both the TGN and vesicular profiles in close proximity to the TGN were observed (Figure 2F and G). Quantification of these double-labeling experiments revealed that half of all the 2c18-positive structures at or close to the TGN also contained γ1-adaptin (Table II).
Figure 2.
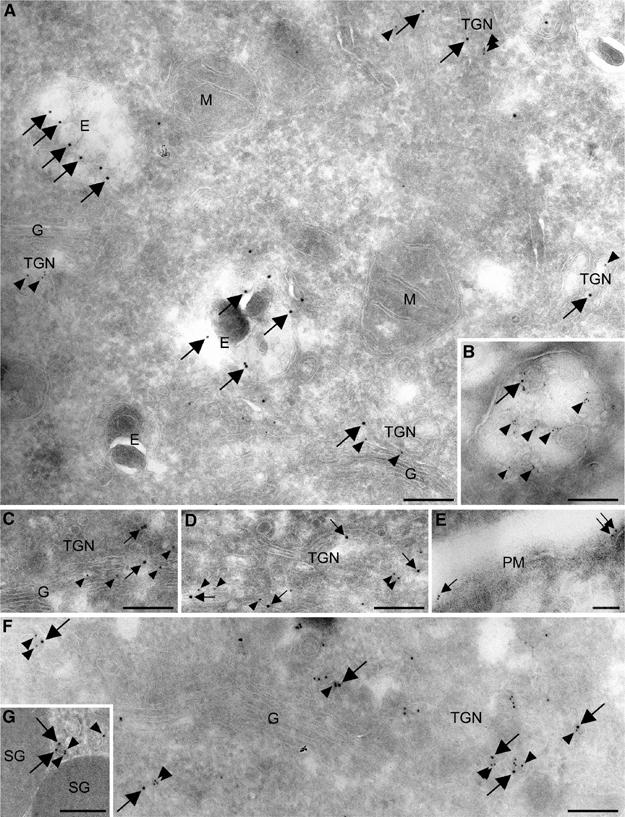
Ultrastructural localization of 2c18. (A, C, D) Double labeling of HeLa SA:48 cells for the TGN marker sialyltransferase (12 nm gold, arrowheads) and endogenous 2c18 (15 nm gold, arrows). 2c18 localizes in close proximity to the Golgi complex (G) and the TGN (TGN), in addition to endocytic structures (E). No labeling is detectable on mitochondria (M). (B) The 5 nm gold-BSA (arrowheads) was internalized for 10 min and chased for 20 min to label late endosomes. 2c18 (10 nm gold, arrows) is present on the inner membranes of this multivesicular structure. (E) 2c18 (10 nm gold, arrows) is also seen to localize to the plasma membrane (PM). (F, G) Double labeling of rat pancreatic tissue for γ1-adaptin (10 nm gold, arrowheads) and endogenous 2c18 (15 nm gold, arrows). Both markers are seen to colocalize on TGN buds and vesicular profiles. ‘SG' denotes secretory granules. Bars represent 200 nm.
Table 1.
Relative distribution of 2c18 labeling in SA:48 cells
| Compartment | % distribution | % s.d. |
|---|---|---|
| TGN | 3.6 | ±1.6 |
| Golgi stacks | 0.0 | ±0.0 |
| Late endocytic structures | 48.2 | ±3.5 |
| Early endosomes | 4.3 | ±0.4 |
| Plasma membrane | 8.2 | ±3.6 |
| Vesicular profiles | 3.9 | ±2.3 |
| Unknown membranes | 18.4 | ±2.8 |
| Others | 13.0 | NA |
| Cells were immunogold labeled for endogenous 2c18. A total of 2000 randomly selected gold particles were counted from 10 grids at a magnification of 13 500 and each of these was assigned to a compartment. Membrane buds within 400 nm of the Golgi were considered to be the TGN. ‘Others' include cytoplasm, endoplasmic reticulum and mitochondria. Standard deviation was determined between grids. | ||
| NA=not applicable. | ||
Table 2.
Colocalization of 2c18 and γ1-adaptin labeling at the TGN of rat exocrine pancreas
| % single labeled for 2c18 | % double labeled for 2c18 and γ1-adaptin | % single labeled for γ1-adaptin | |
|---|---|---|---|
| Labeled TGN structures | 15±1 | 15±2 | 69±2 |
| Cryosections of rat pancreatic tissue were labeled for γ1-adaptin and 2c18. Labeled structures were assigned as either being single or double labeled for these markers. Three grids were counted at a magnification of 16 000. Membrane buds within 400 nm of the Golgi were considered to be the TGN. Standard deviation was determined between grids. | |||
Relationship between 2c18 and γ1-adaptin in living cells
In order to investigate the relationship between 2c18 and AP-1, we first transfected HeLa cells with small interfering RNAs (siRNAs) targeted against the 2c18 mRNA. Western blot analysis of such cells indicated little remaining 2c18 under these conditions (Supplementary Figure 3A). No apparent effect on GGA3, COPI, AP-2 or early endosome antigen I (EEA1) distribution was observed in cells treated with siRNAs targeting 2c18 (Supplementary Figure 3). Analysis of cytosol and membrane fractions from these cells showed that significantly less γ1-adaptin was detectable in the membrane fraction (mb/cyt=1.5/1.0; Figure 3B, upper panel) than in the mock-transfected cells where γ1-adaptin was present in both fractions (mb/cyt=5.3/1.0). These results could be confirmed in immunofluorescence experiments, where in those cells clearly lacking 2c18 staining, the γ1-adaptin was seen to have lost its juxta-nuclear localization, now being distributed throughout the cytoplasm (Figure 3C).
Since lowered levels of 2c18 resulted in reduced membrane-bound γ1-adaptin, we next asked what the consequence of excess 2c18 might be. In extracts prepared from 2c18-GFP-overexpressing cells, the γ1-adaptin was now substantially enriched in the membrane fraction (mb/cyt=9.3/1.0) compared to nontreated control cells (mb/cyt=4.0/1.0) (Figure 3B, lower panel). These biochemical observations were further confirmed by immunofluorescence analysis. A drastic redistribution of endogenous γ1-adaptin could be observed upon overexpression of 2c18-GFP (Figure 3D). The levels of γ1-adaptin were significantly reduced in the fine vesicle-like peripheral structures and appeared now to be concentrated in larger structures strongly colabeled for overexpressed 2c18. A similar effect was also observed in cells overexpressing a nontagged 2c18 construct (not shown). No such effect on the distributions of AP-2, AP-3 or GGA3 was observed at 2c18-GFP expression levels similar to that shown in Figure 3D (not shown). Together, these data suggest that γ1-adaptin and therefore AP-1 membrane localization is dependent on 2c18 function.
To investigate the 2c18 distribution in cells lacking a functional AP-1 complex, we made use of a recently characterized mouse fibroblast cell line in which the μ1A-adaptin subunit of AP-1 has been genetically deleted (Meyer et al, 2000), and HeLa cells where the μ1A protein had been downregulated more than 90% by treatment with siRNAs (Hirst et al, 2003). Both of these situations result in an inability of the remainder of the AP-1 complex to bind membranes, and in both cases the 2c18 was seen to have lost its juxta-nuclear staining and was now spread predominantly throughout the cytoplasm (Supplementary Figure 4). When 2c18 and γ1-adaptin were monitored over time in cells treated with siRNAs targeting the μ1A protein, γ1-adaptin was released from membranes earlier than 2c18 (compare Supplementary Figure 4C with D). This suggests that 2c18 can bind membranes independent of γ1-adaptin but fails to do so in the continuous absence of γ1-adaptin from membranes, possibly as a result of indirect effects.
To further characterize the effects of 2c18 on the membrane association of AP-1, we transfected Vero cells with 2c18-GFP DNA, and treated them with brefeldin A (BFA) for increasing periods of time followed by immunostaining for γ1-adaptin and various other coat protein subunits (Figure 4). In nontransfected cells, both γ1-adaptin and the COPI coat complex rapidly dissociated from membranes after just 2 min of BFA treatment, as previously described (Robinson and Kreis, 1992). In contrast, in 2c18-overexpressing cells (asterisks), γ1-adaptin, but not COPI, largely retained a juxta-nuclear localization that persisted for at least 30 min of treatment (Figure 4A and B). Moreover, in cells overexpressing 2c18, treatment with BFA still resulted in the cytoplasmic redistribution of both δ-adaptin (AP-3; Figure 4C) and GGA3 (Figure 4D).
Figure 4.
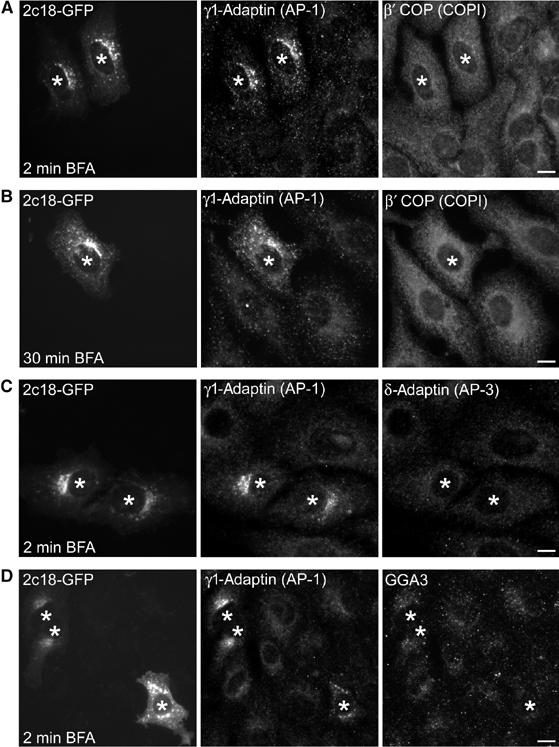
2c18 overexpression stabilizes AP-1 on membranes in the presence of BFA. Vero cells expressing 2c18-GFP were treated with 5 μg/ml BFA for increasing lengths of time as indicated and then fixed and immunostained for γ1-adaptin and other coat complex components. (A) AP-1 but not COPI retains its membrane localization in cells overexpressing 2c18-GFP after 2 min of BFA treatment. (B) After 30 min BFA treatment, AP-1 is still present on membranes of 2c18-GFP cells. (C) AP-3 and (D) GGA3 are not stabilized on membranes by 2c18-GFP in the presence of BFA. Transfected cells are denoted with asterisks. Bars represent 10 μm.
Our data suggest that 2c18 is involved in the control of membrane association/dissociation of the AP-1 adaptor complex by its direct interaction with γ1-adaptin. If this is indeed the case, one prediction would be that overexpression of a non-γ1-adaptin binding variant of 2c18 should not result in the observed changes to AP-1 distribution. To test this, we generated a series of point mutants of 2c18, initially concentrating on the predicted GAE-binding motifs. We transfected HeLa cells with these and first determined if they were still able to co-precipitate γ1-adaptin from cells. Surprisingly, even a 2c18 protein with mutations in all three GAE-binding motifs (2c18ΔBM-CFP; CFP: cyan fluorescent protein) was still able to co-precipitate γ1-adaptin (Figure 5A), and likewise was able to redistribute γ1-adaptin to the membrane fraction when overexpressed (Figure 5B). Another possible mechanism of interaction between adaptors and their accessory proteins is via coiled-coil structures, as has been reported for Rabaptin-5 (Mattera et al, 2003). To test directly if the coiled-coil region of 2c18 might be able to interact with γ1-adaptin, we performed in vitro binding assays. Small amounts of γ1-adaptin ‘ear' could be bound to the 2c18 coiled-coil domain, but binding of the GGA1 ‘ear' to this domain was not detectable under these conditions (Figure 5D). Database analysis revealed that a naturally occurring 2c18 splice variant lacking the coiled-coil domain of the full-length protein existed. Due to the skipping of a single exon, the protein encoded is 33 residues shorter than the full-length 2c18, crucially removing the majority of the coiled-coil domain (Figure 1B). Overexpression of this 2c18 variant was unable to co-precipitate γ1-adaptin or redistribute it to the membrane fraction (Figure 5A and B). Furthermore, this variant was unable to confer BFA resistance to AP-1, a key feature of the full-length 2c18 (Figure 5C). Yeast two-hybrid analysis with this splice variant also failed to identify γ1-adaptin as a potential interactor (not shown). This splice variant does however still contain all three GAE motifs present in the full-length 2c18, and might therefore still have the ability to bind to GAE domains. Indeed, in vitro binding experiments confirmed that, similar to the full-length 2c18, this variant could bind both γ1-adaptin and GGA1 ‘ear' domains (Figure 5D). Together, these data imply that the observed effects on AP-1, as described above, are a consequence of the 2c18–γ1-adaptin interaction in cells, most likely mediated through the 2c18 coiled-coil region.
Figure 5.
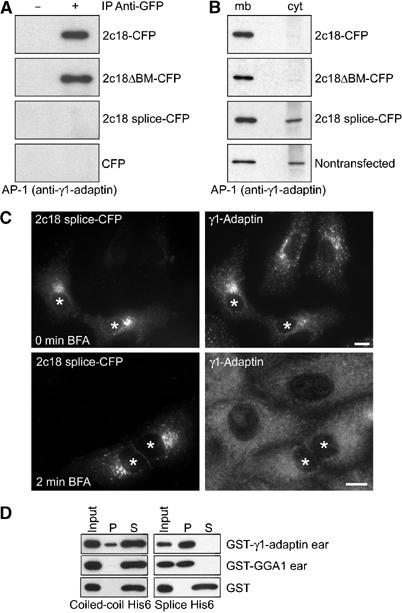
Analysis of 2c18 variants. (A) Protein extracts prepared from HeLa cells, transfected with either 2c18-CFP, 2c18ΔBM-CFP, 2c18 splice-CFP or CFP alone, were immunoprecipitated with anti-GFP/CFP antibodies and blotted for γ1-adaptin. The construct 2c18ΔBM is comprised of full-length 2c18 with the three putative GAE-binding motifs mutated as described in Figure 1. (B) Membrane (mb) and cytosol (cyt) fractions were prepared from HeLa cell extracts expressing various 2c18 constructs as described and then subjected to SDS–PAGE followed by Western blotting for γ1-adaptin. (C) Vero cells expressing 2c18 splice-CFP were fixed and immunostained for γ1-adaptin (upper panel) or treated with 5 μg/ml BFA for 2 min prior to immunostaining (lower panel). Bar represents 10 μm. (D) Purified GST fusion proteins were incubated with immobilized His-tagged 2c18 splice variant or 2c18 coiled-coil domain (aa 78–138) and subsequently separated by centrifugation into pellet (P) and soluble (S) fractions to determine binding to the 2c18 variants. Following SDS–PAGE and Western blotting, the samples were probed with anti-GST antibodies.
2c18 is required for AP-1 function
If the 2c18–γ1-adaptin interaction is important for AP-1-mediated transport in cells, an obvious prediction would be that traffic from the TGN and/or endosomes of proteins such as the MPRs, which have been shown to be present in AP-1-coated carriers (Le Borgne and Hoflack, 1997; Waguri et al, 2003), would be affected by changes in the intracellular levels of 2c18. In 2c18-overexpressing cells, the MPRs were redistributed compared to nontransfected control cells (Figure 6A). In nontransfected control cells, the MPR staining was characterized by an increased juxta-nuclear staining in 87% of the cells, which could be observed in only 33% of the 2c18-overexpressing cells (Figure 6A). In the transfected cells, the MPRs were more diffusely distributed throughout the cytoplasm in structures containing 2c18-GFP (Figure 6A; 63±17% colocalization). The majority of these 2c18-GFP- and MPR-positive structures also contained γ1-adaptin (Figure 6B, arrows). We next investigated whether this 2c18-induced MPR redistribution had any effect on cargo molecule trafficking. Newly synthesized lysosomal enzymes such as cathepsin D are transported from the TGN to lysosomes, after binding to MPRs, by being packaged into clathrin-coated carriers in a process involving AP-1 (Doray et al, 2002). Pro-cathepsin D is intracellularly sorted and is first found in early endosomes, where it dissociates from the MPR due to the low pH. Processing to the intermediate form starts during its transit to late endosomes, and its maturation is completed in lysosomes. Therefore, any interference with AP-1 function and thus MPR trafficking should either lead to secretion of cathepsin D precursors or their intracellular accumulation. Indeed, in cells either lacking a functional AP-1 complex or overexpressing AP-1-interacting proteins, a fraction of pro-cathepsin D is missorted and secreted (Meyer et al, 2000; Mills et al, 2003). In the 2c18-overexpressing cells, a three-fold increase in the secretion of cathepsin D compared to GFP-transfected cells, and a 2.5-fold increase compared to nontransfected cells was observed (Figure 6C). Furthermore, if we also factor in the observation that, at most, 80% of these cells were in fact transfected with 2c18-GFP (as determined by immunofluorescence, not shown), the degree of missorting and thus secretion of cathepsin D becomes even more pronounced. Similar to the overexpression experiments, in cells with downregulated 2c18 (approximately 80% of the initial levels; see Supplementary Figure 3A), about four times more cathepsin D was secreted as compared to nontransfected cells (Figure 6D). A similar degree of cathepsin D missorting was observed when μ1A-adaptin was downregulated (Figure 6D). This suggests that 2c18 is necessary for AP-1-dependent transport between the TGN and endosomes.
Figure 6.
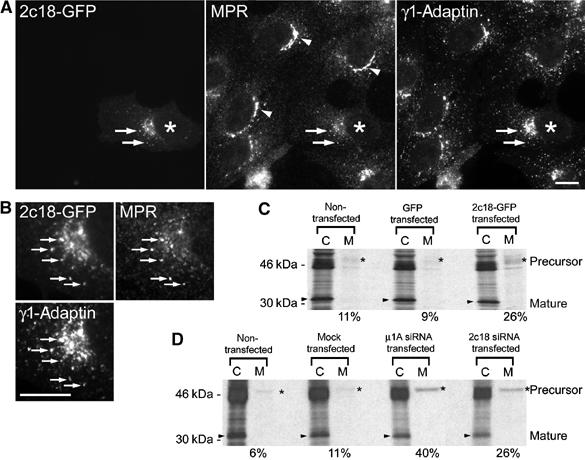
MPR trafficking in 2c18-knockdown or 2c18-overexpressing cells. (A) Vero cells expressing 2c18-GFP were fixed and stained for MPRs and γ1-adaptin. Arrows indicate colocalizing structures and arrowheads show juxta-nuclear staining of the MPRs. (B) Enlarged view of the juxta-nuclear region of the transfected cell, showing all three markers colocalizing to the same structures (arrows). Bars represent 10 μm. (C) HeLa cells were transfected with a plasmid encoding 2c18-GFP. After 24 h, the maturation and secretion of cathepsin D was determined as described in Materials and methods. ‘C' indicates the cathepsin D recovered from total cell extracts and ‘M' that recovered from the cell culture medium. Arrowheads mark mature cathepsin D and asterisks precursor cathepsin D. Numbers at the bottom of (C, D) indicate the amount of cathepsin D from the ‘M lane' (secreted cathepsin D) divided by the sum of cathepsin D secreted and processed inside cells to the mature form (indicated by an arrowhead in C lane). (D) HeLa cells were transfected with siRNAs as described in Materials and methods. The maturation and secretion of cathepsin D was analyzed as described above.
In the experiments described above, 2c18, MPRs and γ1-adaptin colocalized in 2c18-overexpressing cells (Figure 6B), suggesting that all three proteins are retained on the same structures. Due to the lack of resolution of light microscopy, it was unclear from these experiments whether these structures resembled TGN/TGN-derived membranes or endosomes. To address this, cells were treated for 2 h with nocodazole. This has the effect of separating Golgi and endosomal markers into distinct structures, whereas Golgi stack and TGN localizing proteins are found in Golgi ministacks opposing each other (Shima et al, 1997). Under these conditions, in 2c18-overexpressing cells, the MPRs colocalized with 2c18-CFP in structures lying adjacent to GM130-positive structures (Figure 7A) and colocalizing with the TGN marker p230 (Figure 7B). Comparison of nontransfected with transfected cells showed an increase in colocalization of MPRs and p230 from 12±2 to 55±6%. Colocalization of MPRs with the early endosomal marker EEA1 was similar for nontransfected and transfected cells (Figure 7C; 36±3 and 41±8%, respectively). Only 5±1% of Golgi structures colocalized with the endosomal marker EEA1 in the presence of nocodazole, independent of 2c18 overexpression.
Figure 7.
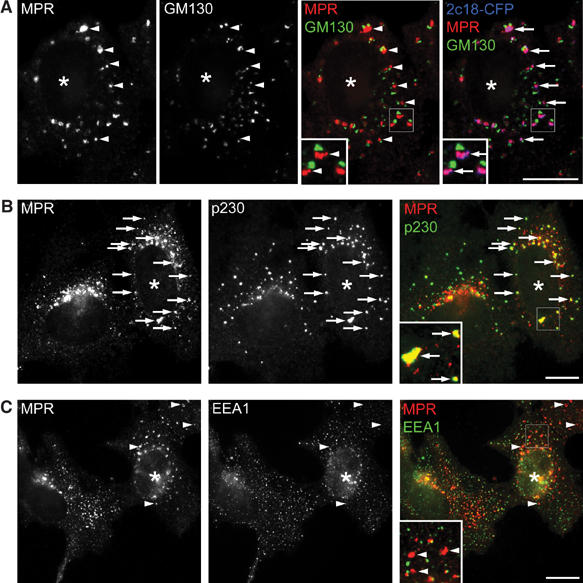
Retention of the MPR at the TGN in 2c18-overexpressing cells. (A–C) HeLa cells were transfected with 2c18-CFP, treated with nocodazole (20 μg/ml) for 2 h and then fixed and immunostained for MPR (red) and either GM130 (A) as a cis Golgi marker, p230 (B) as a trans Golgi marker or EEA1 (C) as an early endosome marker (all green). The insets show an enlarged area of the transfected cells in each case. Arrows mark colocalizing structures and arrowheads those that do not colocalize. Transfected cells are denoted with asterisks. Bars represent 10 μm.
Discussion
In this work, we introduce and describe a newly identified peripheral membrane protein (2c18) of the late secretory pathway. We demonstrate that 2c18 can directly interact with the ‘ear' domain of γ1-adaptin, and that the cellular levels of this protein strongly influence the amount of γ1-adaptin associated with membranes. When cellular levels of 2c18 are increased, γ1-adaptin becomes enriched in the membrane fraction, and concentrated on 2c18-positive membrane structures. Further, the γ1-adaptin dislocation into the cytoplasm in the presence of BFA is inhibited, suggesting that 2c18 interaction with γ1-adaptin attenuates the release of membrane-bound γ1-adaptin. Consistent with this, depletion of 2c18 results in an increased amount of γ1-adaptin recovered from the cytosolic fraction of cells. By visual inspection, the γ1-adaptin pool appears to be more diffuse, no longer concentrated at the juxta-nuclear area. These observed changes in γ1-adaptin membrane association also result in defects in AP-1-mediated transport, demonstrated by an increased secretion of the lysosomal enzyme cathepsin D. The striking γ1-adaptin brefeldin A resistance observed in the presence of excess 2c18 leads us to propose that 2c18 should be named γ-BAR.
Similar to other γ1-adaptin-interacting proteins, we determined that γ-BAR binds specifically to the ‘ear' domain of this AP-1 subunit. Bonifacino and co-workers (Mattera et al, 2004) have recently used in vitro binding and two-hybrid analysis in a systematic way to determine the consensus sequence ΨG(P/D/E)(Ψ/L/M), preceded by negatively charged residues, for GAE-binding proteins. γ-BAR contains three sequence motifs that largely fit this consensus. Although these motifs present in γ-BAR show some small variance from the proposed consensus, peptide binding experiments demonstrated that they are nevertheless still able to bind in vitro to GAE domains, most likely because they possess the critical hydrophobic residues at the 0 and +3 positions (Collins et al, 2003; Miller et al, 2003). The N-terminal binding motif however is not preceded by negatively charged residues, possibly explaining the lower affinity of this peptide for γ1-adaptin compared with the other two motifs, which are actually preceded by such residues. We were also able to detect an in vitro preference of the γ-BAR peptides for the GAE of γ1-adaptin over GGA1. Such preferences have also been shown for peptides of γ-synergin, NECAP1 and EpsinR (Mills et al, 2003; Mattera et al, 2004), but the explanation for this remains unclear, and indicates that the residues around this core motif might play a role. In vivo preferences for particular GAEs have also been noted, for example the case of γ-synergin binding to γ1-adaptin (Lui et al, 2003), and are consistent with our finding that γ-BAR interacts with γ1-adaptin but not with GGA1 or GGA3 in vivo. This is somehow surprising as both adaptor proteins share significant homologies in their ‘ear' domains, which can be interchanged without loss of function (Hirst et al, 2001), and even share the same fold as determined by X-ray crystallography (Lui et al, 2003).
The fact that all the GAE-binding accessory proteins identified so far appear to contain such motifs indicates that they are likely to bind to the same site on the ‘ear'. Indeed for the γ1-adaptin ‘ear', this has been shown for EpsinR and Eps15 (Wasiak et al, 2003), and more recently for interactions of GGA1 with Rabaptin-5 or p56 (Bai et al, 2004). In this study, we also observed such competition between γ-BAR and EpsinR, further suggesting that only one accessory molecule at a time can bind the ‘ear'. In an attempt to correlate our in vitro data with in vivo experiments, we generated a mutant of γ-BAR containing mutations in all three GAE-binding motifs. Surprisingly, such a mutant was still able to co-precipitate γ1-adaptin and cause its redistribution to a membrane fraction biochemically isolated from cells. In contrast, a γ-BAR variant lacking the central coiled-coil domain did not behave in this way, suggesting that the in vivo γ-BAR–γ1-adaptin interaction involves more than just the GAE-binding motifs. The importance of a coiled-coil domain for Rabaptin-5 interaction with the GGAs has previously been noted (Mattera et al, 2003), and here we suggest another protein that may use such a feature for binding. Since the coiled-coil region of γ-BAR interacts in vitro with γ1-adaptin, but not with GGA1, this might be another way of determining the specificity of accessory proteins binding to GAE domains. We cannot formally exclude the possibility that another unidentified protein assists the γ-BAR–γ1-adaptin interaction in vivo; however, we consider it more likely that γ-BAR–γ1-adaptin binding may be a consequence of both the coiled-coil domain and the GAE-binding motifs. Further in vivo work will be necessary to clarify this.
However, since the γ-BAR variant defective in the in vivo binding to γ1-adaptin does not increase γ1-adaptin levels on membranes and does not inhibit the release of γ1-adaptin from membranes in BFA-treated cells, our data provide strong evidence that it is the interaction between wild-type γ-BAR and γ1-adaptin that plays a critical role in the regulation of membrane association of γ1-adaptin.
Of particular note is that we observed increased cathepsin D secretion in both our γ-BAR-overexpressing and γ-BAR-knockdown cells. One explanation for these seemingly contradictory results could be that in the presence of elevated γ-BAR levels, when the γ1-adaptin is retained more tightly on membranes, the MPRs are also held at these sites, and indeed in support of this we observed many structures colocalizing for all three markers. The MPRs may therefore be unavailable for sorting newly synthesized cathepsin D cargo, and thereby in the absence of an active sorting system, it is secreted. Such a phenotype has been previously described in MPR-deficient fibroblasts (Dittmer et al, 1999). In cells with reduced γ-BAR levels however, the MPRs now find that functional or membrane-associated AP-1 is limiting, again resulting in their aberrant transport to the cell surface. This second result is particularly compelling, since although overexpression experiments can perturb membrane traffic via the titration of other interacting factors, downregulation studies can only produce a phenotype if the protein itself, in this case γ-BAR, is rate limiting.
Although we observe cathepsin D missorting in cells with altered levels of γ-BAR, the respective experiments do not resolve whether this effect is occurring at the level of the TGN, endosomes or both. The precise role of AP-1 in MPR trafficking is still unclear, and it has been proposed that AP-1 is involved in anterograde, retrograde or both directions of MPR trafficking between the TGN and endosomes (reviewed in Hinners and Tooze, 2003). However, our EM analyses showing a colocalization of γ-BAR and γ1-adaptin at the TGN and that upon γ-BAR overexpression MPRs show an increased colocalization on structures colabeled with TGN markers but not with early endosomal markers suggest that MPRs are predominantly retained at the TGN due to the overexpression of γ-BAR. Although we consider it less likely, we cannot formally rule out that missorting of cathepsin D occurs also in part due to an interaction of γ-BAR with γ1-adaptin on endosomes.
Since we did not observe a total colocalization of γ-BAR and γ1-adaptin, this suggests that this interaction is transient, and that the remaining proportion of γ-BAR is available for binding other molecules in other locations. This is consistent with the high number of potential interactors identified in the two-hybrid screen, including those such as tuberin and Rabaptin-5 known to be involved in endosomal pathways. Other proteins present on the plasma membrane could be responsible for the observed cell surface localization of γ-BAR. The role of these putative interactions is presently unclear. Similarly, the lack of total colocalization between γ1-adaptin and γ-BAR is also consistent with the fact that γ1-adaptin binds other accessory proteins in a competitive manner, with γ-BAR being only one component of the AP-1 regulatory network.
Despite the numerous proteins that have been identified as interacting with the AP-2 adaptor complex in order to regulate clathrin-mediated endocytosis, interacting partners for the AP-1 complex have until recently largely remained elusive. In this work, we add a new protein that can act as a regulator of AP-1 traffic. The direct interaction between γ1-adaptin and γ-BAR, attenuating the release of the AP-1 complex from membranes, might constitute the basis of the molecular mechanism of this regulation step.
Materials and methods
DNA constructs
The 2c18 cDNA (clone DKFZp564C182) (GenBank accession number AL136628) and a shorter splice variant (clone IMAGE:3623656) (GenBank accession number BC009485) were recently identified in large-scale cDNA sequencing projects carried out by the German cDNA Consortium (Wiemann et al, 2001) and the Mammalian Gene Collection (Strausberg et al, 2002), respectively.
Antibodies
Two antibodies recognizing 2c18 were raised in rabbits. The first one (anti-KYSN) was prepared by coupling a peptide (aa 238–257 of the 2c18 open reading frame (ORF)) to KLH followed by injection into rabbits (Genaxis). The second antibody (anti-2c18) was raised against the full-length His-tagged recombinant 2c18. Both antibodies were affinity purified against the respective antigens and stored at −20°C.
Western blotting and immunoprecipitation
For immunoprecipitation, HeLa cells cultured in 15 cm dishes were lysed with IP buffer (50 mM Tris pH 8, 150 mM NaCl, 1% Nonidet P-40 and protease inhibitors). Lysates (8 mg per immunoprecipitation, except GGA3 (1 mg)) were precleared with protein A Sepharose (Amersham Biosciences), centrifuged at 15 000 g and then the supernatant fraction was incubated overnight with 10 μg of anti-2c18 or anti-GFP antibodies. Complexes were recovered with protein A Sepharose, washed five times with PBS and 0.1% Nonidet P-40, and then treated for SDS–PAGE and Western blotting analysis. Horseradish peroxidase-conjugated secondary antibodies and ECL (Amersham Biosciences) were used for detection. Total protein extracts (20 μg) were included on the gels as controls.
siRNA experiments
For siRNA treatments, double-stranded RNA oligonucleotides were designed against the 2c18 mRNA sequence (adenine 370 to adenine 390) AACAUGCUCAAGAGGUGAGCA and μ1A-adaptin as described previously (Hirst et al, 2003). Cells were incubated with 200 nM of these siRNA oligonucleotides (Dharmacon) and Oligofectamine (Invitrogen) according to the manufacturer's instructions for 72 or 144 h.
Fluorescence microscopy
Unless otherwise stated, images were acquired with a ‘Cell Observer' Zeiss Axiovert 200 microscope. With this system, bleed through of signals into different color channels was below the detection levels as tested in experiments with single-labeled samples of intensities comparable to those obtained in multilabeling experiments. For Figure 3A, 20 confocal slices at a z-resolution of 300 nm were acquired on a Leica AOBS laser scanning microscope (Leica Microsystems, Mannheim, Germany) and assembled as a maximum projection.
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Materials and Methods
Supplementary Video Legend
Supplementary Video
Acknowledgments
We thank Brigitte Joggerst-Thomalla for purification and testing of antibodies, Arie Geerlof and Ario de Marco for assistance with protein purification, Gareth Griffiths and Anja Habermann for assistance with EM and Juan Bonifacino for antibodies. In particular, we also thank Margaret Robinson for antibodies and DNA constructs. We also thank Gareth Griffiths for critical comments on the manuscript. We thank the ALMF team for their help with light microscopy aspects throughout the project and Eppendorf, Perkin Elmer, Leica Microsystems and Carl Zeiss for instrument support to the ALMF. The Dotti lab is partially supported by an EU grant (DIADEM). The Wiemann and Pepperkok labs are supported by grants from the BMBF nos. 01KW9987 (German cDNA Consortium), 01KW0012 (to SW), 01KW0013 (to RP) and 01GR0101 (to SW and RP). VEN is supported by a Schering PhD Fellowship. JCS was in part supported by an EMBO Long Term Fellowship.
References
- Aridor M, Weissman J, Bannykh S, Nuoffer C, Balch WE (1998) Cargo selection by the COPII budding machinery during export from the ER. J Cell Biol 141: 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H, Doray B, Kornfeld S (2004) GGA1 interacts with the adaptor protein AP-1 through a WNSF sequence in its hinge region. J Biol Chem 279: 17411–17417 [DOI] [PubMed] [Google Scholar]
- Boehm M, Bonifacino JS (2001) Adaptins: the final recount. Mol Biol Cell 12: 2907–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman AL, Zhang C, Zhu X, Kahn RA (2000) A family of ADP-ribosylation factor effectors that can alter membrane transport through the trans-Golgi. Mol Biol Cell 11: 1241–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Dell'Angelica EC (1999) Molecular bases for the recognition of tyrosine-based sorting signals. J Cell Biol 145: 923–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins BM, Praefcke GJ, Robinson MS, Owen DJ (2003) Structural basis for binding of accessory proteins by the appendage domain of GGAs. Nat Struct Biol 10: 607–613 [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC, Puertollano R, Mullins C, Aguilar RC, Vargas JD, Hartnell LM, Bonifacino JS (2000) GGAs: a family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex. J Cell Biol 149: 81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneka M, Neeft M, Popa I, van Oort M, Sprong H, Oorschot V, Klumperman J, Schu P, van der Sluijs P (2003) Rabaptin-5alpha/rabaptin-4 serves as a linker between rab4 and gamma(1)-adaptin in membrane recycling from endosomes. EMBO J 2: 2645–2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer F, Ulbrich EJ, Hafner A, Schmahl W, Meister T, Pohlmann R, von Figura K (1999) Alternative mechanisms for trafficking of lysosomal enzymes in mannose 6-phosphate receptor-deficient mice are cell type-specific. J Cell Sci 112: 1591–1597 [DOI] [PubMed] [Google Scholar]
- Doray B, Ghosh P, Griffith J, Geuze HJ, Kornfeld S (2002) Cooperation of GGAs and AP-1 in packaging MPRs at the trans-Golgi network. Science 297: 1700–1703 [DOI] [PubMed] [Google Scholar]
- Doray B, Kornfeld S (2001) Gamma subunit of the AP-1 adaptor complex binds clathrin: implications for cooperative binding in coated vesicle assembly. Mol Biol Cell 12: 1925–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter CE, Gibson A, Allchin EH, Maxwell S, Ruddock LJ, Odorizzi G, Domingo D, Trowbridge IS, Hopkins CR 1998. In polarized MDCK cells basolateral vesicles arise from clathrin-gamma-adaptin-coated domains on endosomal tubules. J Cell Biol 141: 611–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallusser A, Kirchhausen T (1993) The beta 1 and beta 2 subunits of the AP complexes are the clathrin coat assembly components. EMBO J 12: 5237–5244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Hoflack B, Simons K, Mellman I, Kornfeld S (1988) The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell 52: 329–341 [DOI] [PubMed] [Google Scholar]
- Gruenberg J (2001) The endocytic pathway: a mosaic of domains. Nat Rev Mol Cell Biol 2: 721–730 [DOI] [PubMed] [Google Scholar]
- Hinners I, Tooze SA (2003) Changing directions: clathrin-mediated transport between the Golgi and endosomes. J Cell Sci 116: 763–771 [DOI] [PubMed] [Google Scholar]
- Hirst J, Lindsay MR, Robinson MS (2001) GGAs: roles of the different domains and comparison with AP-1 and clathrin. Mol Biol Cell 12: 3573–3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Lui WW, Bright NA, Totty N, Seaman MN, Robinson MS (2000) A family of proteins with gamma-adaptin and VHS domains that facilitate trafficking between the trans-Golgi network and the vacuole/lysosome. J Cell Biol 149: 67–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Motley A, Harasaki K, Peak Chew SY, Robinson MS (2003) EpsinR: an ENTH domain-containing protein that interacts with AP-1. Mol Biol Cell 14: 625–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvier K, Kato Y, Boehm M, Rose JR, Martina JA, Kim BY, Venkatesan S, Bonifacino JS (2003) Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 gamma-sigma1 and AP-3 delta-sigma3 hemicomplexes. J Cell Biol 163: 1281–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalthoff C, Groos S, Kohl R, Mahrold S, Ungewickell EJ (2002) Clint: a novel clathrin-binding ENTH-domain protein at the Golgi. Mol Biol Cell 13: 4060–4073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent HM, McMahon HT, Evans PR, Benmerah A, Owen DJ (2002) Gamma-adaptin appendage domain: structure and binding site for Eps15 and gamma-synergin. Structure 10: 1139–1148 [DOI] [PubMed] [Google Scholar]
- Kirchhausen T, Bonifacino JS, Riezman H (1997) Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr Opin Cell Biol 9: 488–495 [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Griffiths G, Hoflack B (1996) Mannose 6-phosphate receptors and ADP-ribosylation factors cooperate for high affinity interaction of the AP-1 Golgi assembly proteins with membranes. J Biol Chem 271: 2162–2170 [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Hoflack B (1997) Mannose 6-phosphate receptors regulate the formation of clathrin-coated vesicles in the TGN. J Cell Biol 137: 335–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippe R, Miaczynska M, Rybin V, Runge A, Zerial M (2001) Functional synergy between Rab5 effector Rabaptin-5 and exchange factor Rabex-5 when physically associated in a complex. Mol Biol Cell 12: 2219–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui WWY, Collins BM, Hirst J, Motley A, Millar C, Schu P, Owen DJ, Robinson MS (2003) Binding partners for the COOH-terminal appendage domains of the GGAs and gamma-adaptin. Mol Biol Cell 14: 2385–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A, Van Dyke M, Stock J (1991) Predicting coiled coils from protein sequences. Science 252: 1162–1164 [DOI] [PubMed] [Google Scholar]
- Mattera R, Arighi CN, Lodge R, Zerial M, Bonifacino JS (2003) Divalent interaction of the GGAs with the Rabaptin-5–Rabex-5 complex. EMBO J 22: 78–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattera R, Ritter B, Sidhu SS, McPherson PS, Bonifacino JS (2004) Definition of the consensus motif recognized by gamma-adaptin ear domains. J Biol Chem 279: 8018–8028 [DOI] [PubMed] [Google Scholar]
- Meyer C, Zizioli D, Lausmann S, Eskelinen EL, Hamann J, Saftig P, von Figura K, Schu P (2000) Mu1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J 19: 2193–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GJ, Mattera R, Bonifacino JS, Hurley JH (2003) Recognition of accessory protein motifs by the gamma-adaptin ear domain of GGA3. Nat Struct Biol 10: 599–606 [DOI] [PubMed] [Google Scholar]
- Mills IG, Praefcke GJK, Vallis Y, Peter BJ, Olesen LE, Gallop JL, Butler PJG, Evans PR, McMahon HT (2003) EpsinR: an AP1/clathrin interacting protein involved in vesicle trafficking. J Cell Biol 160: 213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai K, Horton P 1999. PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem Sci 1: 34–36 [DOI] [PubMed] [Google Scholar]
- Nogi T, Shiba Y, Kawasaki M, Shiba T, Matsugaki N, Igarashi N, Suzuki M, Kato R, Takatsu H, Nakayama K, Wakatsuki S (2002) Structural basis for the accessory protein recruitment by the gamma-adaptin ear domain. Nat Struct Biol 9: 527–531 [DOI] [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS (1995) Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science 269: 1872–1875 [DOI] [PubMed] [Google Scholar]
- Page LJ, Sowerby PJ, Lui WWY, Robinson MS (1999) Gamma-synergin: an EH domain-containing protein that interacts with gamma-adaptin. J Cell Biol 146: 993–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puertollano R, Randazzo PA, Presley JF, Hartnell LM, Bonifacino JS (2001) The GGAs promote ARF-dependent recruitment of clathrin to the TGN. Cell 105: 93–102 [DOI] [PubMed] [Google Scholar]
- Rabouille C, Hui N, Hunte F, Kieckbusch R, Berger EG, Warren G, Nilsson T (1995) Mapping the distribution of Golgi enzymes involved in the construction of complex oligosaccharides. J Cell Sci 108: 1617–1627 [DOI] [PubMed] [Google Scholar]
- Rapoport I, Chen YC, Cupers P, Shoelson SE, Kirchhausen T (1998) Dileucine-based sorting signals bind to the beta chain of AP-1 at a site distinct and regulated differently from the tyrosine-based motif-binding site. EMBO J 17: 2148–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS (1990) Cloning and expression of gamma-adaptin, a component of clathrin-coated vesicles associated with the Golgi apparatus. J Cell Biol 111: 2319–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS (2004) Adaptable adaptors for coated vesicles. Trends Cell Biol 14: 167–174 [DOI] [PubMed] [Google Scholar]
- Robinson MS, Bonifacino JS (2001) Adaptor-related proteins. Curr Opin Cell Biol 13: 444–453 [DOI] [PubMed] [Google Scholar]
- Robinson MS, Kreis TE (1992) Recruitment of coat proteins onto Golgi membranes in intact and permeabilized cells: effects of brefeldin A and G protein activators. Cell 69: 129–138 [DOI] [PubMed] [Google Scholar]
- Shiba Y, Takatsu H, Shin HW, Nakayama K (2002) Gamma-adaptin interacts directly with Rabaptin-5 through its ear domain. J Biochem 131: 327–336 [DOI] [PubMed] [Google Scholar]
- Shima DT, Haldar K, Pepperkok R, Watson R, Warren G (1997) Partitioning of the Golgi apparatus during mitosis in living HeLa cells. J Cell Biol 137: 1211–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JC, Wellenreuther R, Poustka A, Pepperkok R, Wiemann S (2000) Systematic subcellular localization of novel proteins identified by large-scale cDNA sequencing. EMBO Rep 1: 287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepnev VI, De Camilli P (2000) Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat Rev Neurosci 1: 161–172 [DOI] [PubMed] [Google Scholar]
- Stamnes MA, Rothman JE (1993) The binding of AP-1 clathrin adaptor particles to Golgi membranes requires ADP-ribosylation factor, a small GTP-binding protein. Cell 73: 999–1005 [DOI] [PubMed] [Google Scholar]
- Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler GD, Altschul SF, Zeeberg B, Buetow KH, Schaefer CF, Bhat NK, Hopkins RF, Jordan H, Moore T, Max SI, Wang J, Hsieh F, Diatchenko L, Marusina K, Farmer AA, Rubin GM, Hong L, Stapleton M, Soares MB, Bonaldo MF, Casavant TL, Scheetz TE, Brownstein MJ, Usdin TB, Toshiyuki S, Carninci P, Prange C, Raha SS, Loquellano NA, Peters GJ, Abramson RD, Mullahy SJ, Bosak SA, McEwan PJ, McKernan KJ, Malek JA, Gunaratne PH, Richards S, Worley KC, Hale S, Garcia AM, Gay LJ, Hulyk SW, Villalon DK, Muzny DM, Sodergren EJ, Lu X, Gibbs RA, Fahey J, Helton E, Ketteman M, Madan A, Rodrigues S, Sanchez A, Whiting M, Madan A, Young AC, Shevchenko Y, Bouffard GG, Blakesley RW, Touchman JW, Green ED, Dickson MC, Rodriguez AC, Grimwood J, Schmutz J, Myers RM, Butterfield YS, Krzywinski MI, Skalska U, Smailus DE, Schnerch A, Schein JE, Jones SJ, Marra MA, Mammalian Gene Collection Program Team (2002) Generation and initial analysis of more than 15 000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci USA 99: 16899–16903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale N, Horiba K, Ferrans VJ, Moss J, Vaugha M (1998) Localization of ADP-ribosylation factor domain protein 1 (ARD1) in lysosomes and Golgi apparatus. Proc Natl Acad Sci USA 95: 8613–8618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waguri S, Dewitte F, Le Borgne R, Rouille Y, Uchiyama Y, Dubremetz JF, Hoflack B (2003) Visualization of TGN to endosome trafficking through fluorescently labeled MPR and AP-1 in living cells. Mol Biol Cell 14: 142–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasiak S, Denisov AY, Han Z, Leventis PA, de Heuvel E, Boulianne GL, Kay BK, Gehring K, McPherson PS (2003) Characterization of a gamma-adaptin ear-binding motif in enthoprotin. FEBS Lett 555: 437–442 [DOI] [PubMed] [Google Scholar]
- Wasiak S, Legendre-Guillemin V, Puertollano R, Blondeau F, Girard M, de Heuvel E, Boismenu D, Bell AW, Bonifacino JS, McPherson PS (2002) Enthoprotin: a novel clathrin-associated protein identified through subcellular proteomics. J Cell Biol 158: 855–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemann S, Weil B, Wellenreuther R, Gassenhuber J, Glassl S, Ansorge W, Bocher M, Blocker H, Bauersachs S, Blum H, Lauber J, Dusterhoft A, Beyer A, Kohrer K, Strack N, Mewes HW, Ottenwalder B, Obermaier B, Tampe J, Heubner D, Wambutt R, Korn B, Klein M, Poustka A (2001) Toward a catalog of human genes and proteins: sequencing and analysis of 500 novel complete protein coding human cDNAs. Genome Res 11: 422–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao GH, Shoarinejad F, Jin F, Golemis EA, Yeung RS (1997) The tuberous sclerosis 2 gene product, tuberin, functions as a Rab5 GTPase activating protein (GAP) in modulating endocytosis. J Biol Chem 2: 6097–6100 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Drake MT, Kornfeld S (1999) ADP-ribosylation factor 1 dependent clathrin-coat assembly on synthetic liposomes. Proc Natl Acad Sci USA 96: 5013–5018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Materials and Methods
Supplementary Video Legend
Supplementary Video


