Abstract
Objective
Aerobic capacity is the most powerful predictor of all-cause mortality in humans; however, its role in the development of obesity and susceptibility for high fat diet (HFD)-induced weight gain is not completely understood.
Methods
Herein, we utilized a rodent model system of divergent intrinsic aerobic capacity [high capacity running (HCR) and low capacity running (LCR)], to evaluate the role of aerobic fitness on 1-week HFD-induced (45% & 60% kcal) weight gain. Food/energy intake, body composition analysis, and brown adipose tissue gene expression were assessed as important potential factors involved in modulating HFD-induced weight gain.
Results
HCR rats had reduced 1-week weight gain on both HFDs compared to LCR. Reduced HFD-induced weight gain was associated with greater adaptability to decrease food intake following initiation of the HFDs. Further, the HCR rats were observed to have reduced feeding efficiency, greater brown adipose mass, and expression of genes involved in thermogenesis.
Conclusion
Rats with high intrinsic aerobic capacity have reduced susceptibility to 1-week HFD-induced weight gain, which is associated with greater food intake adaptability to control intake of energy dense HFDs, reduced weight gain per kcal consumed, and greater brown adipose tissue mass and thermogenic gene expression.
Keywords: food intake, energy intake, physical fitness, weight gain, obesity phenotypes
Introduction
Obesity is a global epidemic, impacting over 35% of the world population (1, 2) and is fundamental to the development and progression of metabolic disease states. Therefore, understanding the factors influencing weight gain and obesity are critically important. A striking characteristic is the presence of individual phenotypes that are resistant to obesity despite exposure to the same obesogenic environment (3). This is undoubtedly driven by a complex interplay between polygenetic factors. The importance of these polygenetic characteristics is emphasized by the observation that up to 2/3 of variability in body mass index is due to heritable factors (4). These putative genetic factors predispose individuals to susceptibility or protection against weight gain through metabolic, hedonic, hormonal, and satiety driven mechanisms (5, 6, 7, 8).
Weight gain occurs following a shift to a positive energy balance through some combination of increased energy intake and/or reduced energy expenditure (9). However, the multi-system physiology and polygenetic factors that interact to ultimately control energy balance make it difficult to tease out individual components of weight gain that may be used as predictive tools or therapeutic targets. Observed changes in energy intake and body weight are not constant, with daily and weekly weight fluctuations observed. As such, long-term weight gain results as a series of small positive weight fluctuations due to changes in energy intake and expenditure in time scales such as weekends (10), holidays (11, 12), and seasonally (13). Thus, any factors that could act to reduce the propensity to acutely gain weight in the face of the current energy dense food landscape could be important tools in mitigating the increasing number of overweight and obesity.
Aerobic capacity has been described as the most powerful predictor of all-cause mortality in adults(14), and 40 – 70% of an individual’s aerobic capacity is driven by complex genetic components (15). However, the mechanisms by which intrinsic aerobic capacity impacts diet-induced weight gain are not completely known. To assess how intrinsic aerobic capacities impact metabolic health, a rat model was produced based on selective breeding for high capacity running (HCR) or low capacity running (LCR) rats, resulting in divergent intrinsic aerobic capacities (16). Several studies have found that the HCR rats are resistant to high-fat diet (HFD)-induced metabolic diseases and increased adiposity (17, 18), while in contrast, the LCR are extremely susceptible to dietary-induced obesity, insulin resistance, and fatty liver (19, 20). Therefore, the HCR/LCR model presents a unique opportunity to study the role of aerobic capacity upon susceptibility or protection against weight gain. Here we examine the potential role of intrinsic fitness to impact short-term, dose-dependent, HFD-induced weight gain through modulation of food/energy intake and excess energy storage. Our findings suggest that the high aerobic capacity HCR rat is protected from obesity due in part to a better acute regulation of energy intake and reduced feeding efficiency compared to LCR rats.
Methods
An overview of the methods utilized is provided below, detailed methods are provided in supplemental information.
Animals
HCR/LCR rats development and characterization are previously described (16, 17, 18, 19, 20, 21, 22). Rats 25–30 weeks of age were singly housed (~75–77°F, 12-hour light cycle), and acclimatized to the low-fat, control diet (LFD) (D12110704 (10% kcal fat 3.5% kcal sucrose, 3.85 kcal/gm), Research Diets, Inc., New Brunswick, NJ, USA) for two weeks prior to the initiation of the 1 week HFD, (D12451 (45% kcal fat, 17% kcal sucrose, 4.73 kcal/gm) or D12492 (60% kcal fat, 6.8% kcal sucrose, 5.24 kcal/gm)). Food intake was monitored for 1-week prior to HFD (week 0), and during the 1-week HFD (week 1). Change in food intake and change in energy intake was calculated as the difference between the week 0 and week 1 values. Feeding efficiency was calculated as the weight gained per kcals consumed during 1-week. The animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Missouri and the Subcommittee for Animal Safety at the Harry S Truman Memorial VA Hospital.
Body Composition Analysis
Fat mass was measured by magnetic resonance using the EchoMRI-900 analyzer (EchoMRI, Houston, Texas, USA) as previously described (18).
Adipose Staining & Morphometrics
Retroperitoneal and brown adipose tissue was collected at sacrifice and formalin fixed. Hematoxylin/eosin staining was performed and retroperitoneal fat pad images from each animal were analyzed for cell volume and number as previously described (19).
mRNA Expression
RNA and cDNA were prepared as previously described (23).
Western Blot
Brown fat samples were prepared, separated by SDS-PAGE, and analyzed as previously described (23).
Citrate Synthase Activity
Citrate synthase activity was determined as previously described (23).
Statistical Analysis
All data is presented as means ± standard error mean. The main effects of phenotype and diet were tested by using two-way ANOVA utilizing SPSS (SPSS Inc., Armonk, NY). Two-way ANCOVA with body weight as the co-variate was performed for several outcomes to statistically control for the consistent difference in body weight between the strains. Where significant main effects were observed, post hoc analysis was performed using least significant difference to test for any specific pairwise differences. Linear regression analysis was performed utilizing GraphPad Prism (GraphPad Software, Inc., La Jolla, CA). Data are represented as the regression lines within strains across diets, with dietary groups separately annotated. Goodness-of-fit as r2 and the equation of the regression line for comparison of the associations are presented. For all analysis, statistical significance was set at p<0.05.
Results
Animal Characteristics
Body weight was ~15% less in the HCR compared to LCR before and after the HFD, due to both lower initial fat-free and fat mass (data not shown). The 60% HFD increased body weight in both strains (5%, p<0.05). One week of both the 45% and 60% HFD resulted in increased fat mass in both HCR and LCR rats (Table 1, p<0.05). Final fat-free mass was lower in the HCR than LCR (p<0.05), with no observed diet effect.
Table 1.
Anthropometrics, Food, and Energy Intake Data. Body weight (n=16–24), fat-free mass (FFM, n=8–16), fat mass (FM, n=8–16), and percent body fat after the one week HFD are expressed as means ± SEM. Food and energy intake (n=16–24) during the one week HFD are expressed as means ± SEM. Food and energy intake following ANCOVA for body weight are expressed as the estimated mean ± SEM.
| HCR | LCR | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| LFD | 45% HFD | 60% HFD | LFD | 45% HFD | 60% HFD | Interaction | |
| Anthropometrics | |||||||
|
|
|||||||
| Body Weight (g) | 361.1 ± 7.8 | 363.7 ± 11.8 | 382.0 ± 10.0† | 441.6 ± 8.4* | 446.8 ± 10.6* | 465.6 ± 11.6*,† | |
| Fat-Free Mass (g) | 320.7 ± 8.9 | 322.8 ± 13.6 | 325.2.7 ± 7.9 | 370.3 ± 9.5* | 374.8 ± 11.4* | 369.2 ± 8.8* | |
| Fat Mass (g) | 50.0 ± 4.1 | 64.6 ± 7.8† | 56.6 ± 2.9† | 72.4 ± 4.3* | 89.2 ± 6.1*,† | 89.1 ± 4.8*,† | |
| % Body Fat | 14.0 ± 1.0 | 16.4 ± 1.4† | 15.26 ± 0.8† | 16.20 ± 0.8* | 19.1 ± 0.9*,† | 19.33 ± 0.9*,† | |
|
| |||||||
| Intake | |||||||
|
|
|||||||
| Food (g) | 103.5 ± 3.6 | 98.8 ± 4.2 | 93.4 ± 3.3 | 111.6 ± 4.0* | 122.7 ± 5.6* | 122.5 ± 3.8* | p<0.05 |
| Energy (kcal) | 398.0 ± 13.9 | 467.3 ± 19.7† | 489.2 ± 17.1† | 429.5 ± 15.3* | 580.4 ± 26.7*,† | 641.8 ± 20.1*,†,&& | p<0.05 |
|
| |||||||
| Adjusted Intake | |||||||
|
|
|||||||
| Food (g, covariate Final BW) | 116.3 ± 3.5 | 110.3 ± 3.8 | 101.3 ± 3.8†† | 105.2 ± 3.2** | 113.2 ± 3.7 | 107.0 ± 4.2 | p<0.05 |
| Co-variate Effect Size | Co-variate | p-value | Partial Eta2 | ||||
|
|
|||||||
| BW | p<0.05 | 0.361 | |||||
|
| |||||||
| Energy (kcal, covariate Final BW | 455.1 ± 15.4 | 519.1 ±17.0† | 525.1 ± 16.8† | 399.9 ± 14.1** | 537.6 ± 16.6† | 572.3 ± 18.6† | p<0.05 |
| Co-variate Effect Size | Co-variate | p-value | Partial Eta2 | ||||
|
|
|||||||
| BW | p<0.05 | 0.366 | |||||
Estimated effect size of the covariate for each ANCOVA analysis are presented as partial eta squared.
p<0.05 main effect HCR vs. LCR,
p<0.05 main effect LFD vs. HFD,
p<0.05 HCR vs. LCR within diet,
p<0.05 LFD vs. HFD within strain,
p<0.05 45% HFD vs 60% HFD within strain.
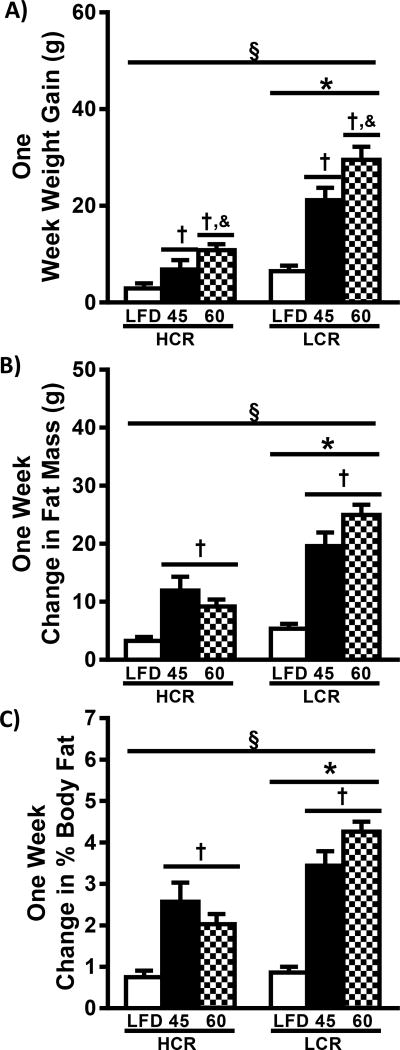
The 45% HFD resulted in increased 1-week weight gain in both HCR and LCR rats (2.3× and 3.3×, respectively, p<0.05) compared to LFD (Figure1A). 60% HFD produced greater weight gain in HCR and LCR rats than both the LFD (3.7× and 4.6×, respectively, p<0.05) and the 45% HFD (58% and 40%, respectively, p<0.05). Importantly, HCR rats demonstrated less weight gain (~65%) compared to LCR regardless of diet (p<0.05). Together these findings resulted in a significant interaction of strain and diet on 1-week weight gain (p<0.05). ANCOVA for body weight was utilized to determine if the difference in body weight influenced HFD-induced weight gain. Using final body weight as a covariate did not change the outcomes (Figure 1B, p<0.05). Further, the partial Eta2 for the analysis was relatively low (0.117) suggesting that only a small percentage of the observed variance was due to body weight differences. Importantly, differences in weight gain between the HCR and LCR strains was driven by increases in fat mass (Figure 1C). HCR rats gained less fat mass than LCR within all diets (p<0.05), while both the 45% and 60% HFD resulted in greater fat mass gain in both strains compared to LFD (p<0.05). The observed differences in fat mass resulted in similar alterations in change in percent body fat (Figure 1D). 1-week change in fat-free mass was minimal, highly variable, and not different between strains or diets (data not shown). ANCOVA analysis of the change in fat and fat-free mass was not possible as both data sets failed the homogeneity of regression test. Together these data add to our previous data (18) suggesting higher intrinsic aerobic capacity is associated with reduced acute HFD-induced weight gain that is primarily driven by increased adiposity in the LCR rats.
Figure 1.
High intrinsic aerobic capacity reduces HFD-induced weight gain. Body weight and composition was determined prior to and following one week of HFD. One week weight gain (A), one week change in fat mass (B, n=8–16), and one week change in percent body fat (C, n=8–16) are presented as means ± SEM. § p<0.05 interaction, * p<0.05 main effect HCR vs. LCR, † p<0.05 main effect LFD vs. HFD, & p<0.05 main effect 45% HFD vs 60% HFD.
Food and Energy Intake
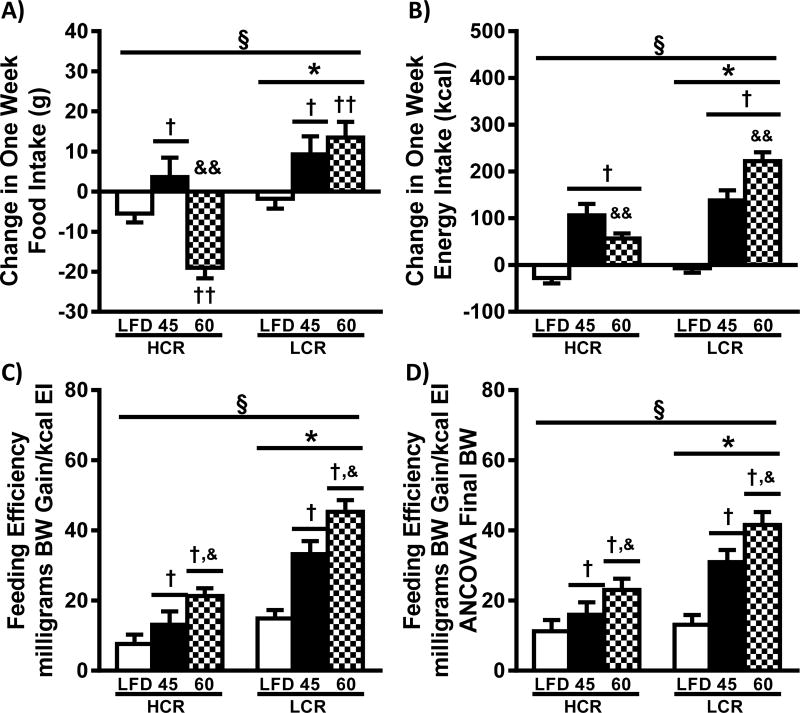
Data for food and energy intake (FI and EI, respectively) are presented in Table 1 and Figure 2. FI during the 1-week 45% and 60% HFD’s was lower in HCR compared to LCR (Table 1, p<0.05). Compared to the LFD, HFD’s trended to increase FI in the LCR (p=0.060). Following adjustment of FI by ANCOVA, the main effect of strain disappears (Table 1). The partial Eta2 (0.361) highlights that the body weight difference between the strains is a moderate determinant of FI. We also examined how FI was altered within each animal during the transition from the LFD to the HFD (Figure 2A). FI after 1 week of HFD was subtracted by the LFD FI that was measured 1-week prior. Introduction of the 45% HFD increased FI in both strains (p<0.05). Interestingly, introduction of the 60% HFD resulted in a similar increase in FI in the LCR, while the HCR displayed a dramatic reduction in FI following transition to the 60% HFD (p<0.05).
Figure 2.
High intrinsic aerobic capacity is associated with food intake adaptability and reduced feeding efficiency following HFD. Change in food and energy intake were calculated as the difference between the LFD lead-in week and one week of HFD, and feeding efficiency is the weight gain in milligrams for the week divided by the total weekly energy intake: A) change in food intake, B) change in energy intake, C) feeding efficiency, and D) estimated feeding efficiency adjusted for final body weight (ANCOVA) (n=16–24) are presented as means ± SEM. § p<0.05 interaction,* p<0.05 main effect HCR vs. LCR, † p<0.05 main effect LFD vs. HFD, & p<0.05 main effect 45% HFD vs 60% HFD, †† p<0.05 LFD vs. HFD within strain, && p<0.05 45% HFD vs 60% HFD within strain.
Because HFD’s have different energy density than LFD it is important to additionally examine EI. Both HFDs resulted in increased EI compared to LFD condition within both strains (Table1, p<0.05). While no difference was observed between HFD diets in HCR, the LCR rats on 60% HFD had a 10% higher EI compared to 45% HFD (p<0.05). HCR rats had lower EI compared to LCR, effects primarily driven by the 24% and 31% lower EI on the HCR on 45% and 60% HFD, respectively. There was a significant interaction for EI as a result of the LCR significantly increasing EI to a greater degree on both of the HFD’s compared to the HCR (p<0.05). ANCOVA analysis of EI once again diminished differences between the strains (Table 1, Eta2 ~ 0.366). However, the weight adjusted mean EI of LFD for the HCR rats was higher compared to LCR (p<0.05). Both, HFDs resulted in greater weight adjusted EI compared to LFD in HCR and LCR rats (p<0.05). Change in EI (Figure 2B) was lower in HCR rats compared to LCR (p<0.05), with both HFD diets being higher than LFD across both strains (p<0.05). The divergent change in FI observed on transition to the 60% HFD resulted in a smaller increase in EI in the HCR compared to 45% HFD, while the LCR demonstrated a significantly larger increase in EI compared to 45% HFD. Together these data suggest that the HCR rats have greater adaptability than the LCR for lowering FI when provided with an energy dense HFD, a trait that significantly influences HFD-induced weight gain.
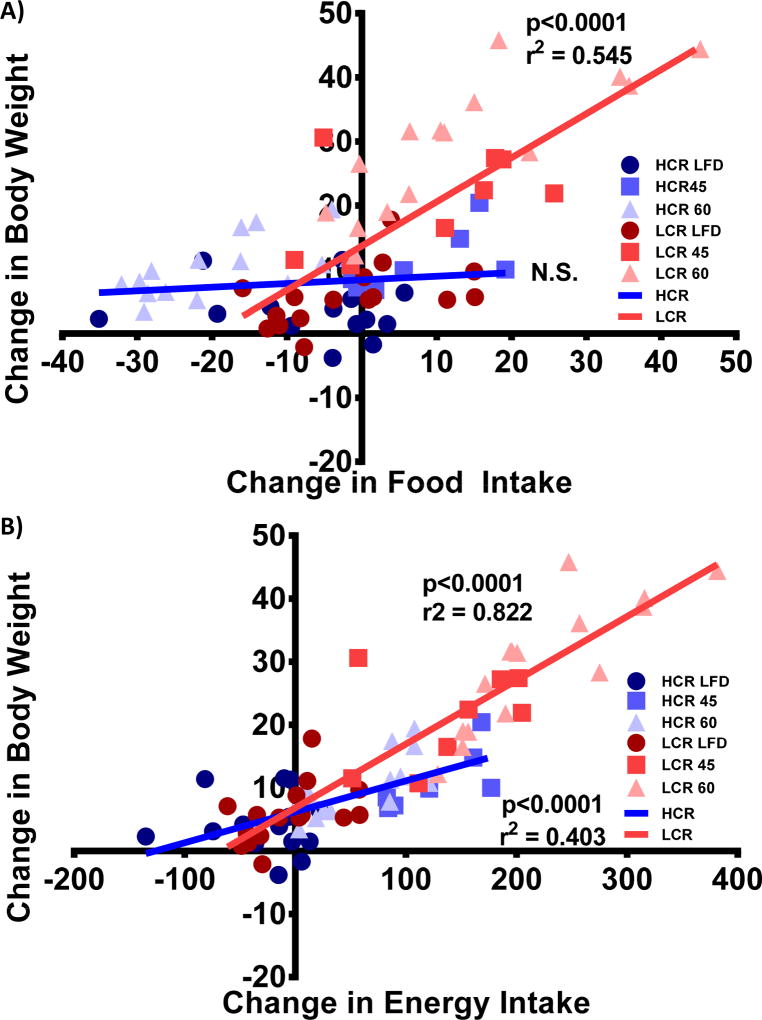
Relationship between FI/EI and Weight Gain
Regression analysis of the body weight gain versus change in FI for the HCR and LCR rats is displayed in Figure 3A. The dynamic differences in FI and lower weight gain changes result in no observed correlation within the HCR, while the LCR rats demonstrate a significant association (p<0.0001, r2=0.545). As previously observed (24), change in EI was highly associated with change in body weight across strains and diets (p<0.0001, r2=0.751, y=0.094× + 6.134). Figure 3B represents the within strain association of body weight gain versus change in EI across all three diets, with both HCR and LCR rats having significant associations (p<0.0001, r2=0.403, y=0.049× + 6.203; p<0.0001, r2=0.822, y=0.101× + 6.822, respectively). Together these data suggest a stronger association between FI/EI and weight gain in the LCR than in the HCR rats.
Figure 3.
Change in food/energy intake versus weight gain. Linear regression of on week body weight gain versus B) change in food intake and D) change in energy intake (blue = HCR, red = LCR, LFD = ●, 45% = ■, 60% = ▲) are presented with p-value and r2 when appropriate.
Feeding Efficiency
Feeding efficiency (FE) is displayed in Figure 2C and represents a comparison of weight gained per kcal consumed during the one-week HFD’s. HCR rats displayed ~50% lower FE than LCR across all diets (p<0.05). 45% HFD increased FE in both HCR and LCR rats (70% and 2.2×, respectively, p<0.05) compared to LFD. The 60% HFD produced greater FE in HCR and LCR rats than LFD (2.8× and 3.0, respectively, p<0.05) and 45% HFD conditions (62% and 36%, respectively, p<0.05). While the adjustment of FE for final body weight by ANCOVA was significant (Figure 2D, p<0.05), the relationships are similar to the absolute data and the very small partial Eta2 (~0.043) suggests that body weight differences were not responsible for the observed differences in FE. The large differences in FE between HCR and LCR rats across diets suggests that additional factors such as previously described differences in weight-adjusted resting energy expenditure (18) and metabolic efficiency (21), impact the observed association between intrinsic aerobic capacity and HFD-induced weight gain.
Adipose Mass, Morphometrics and Gene Expression
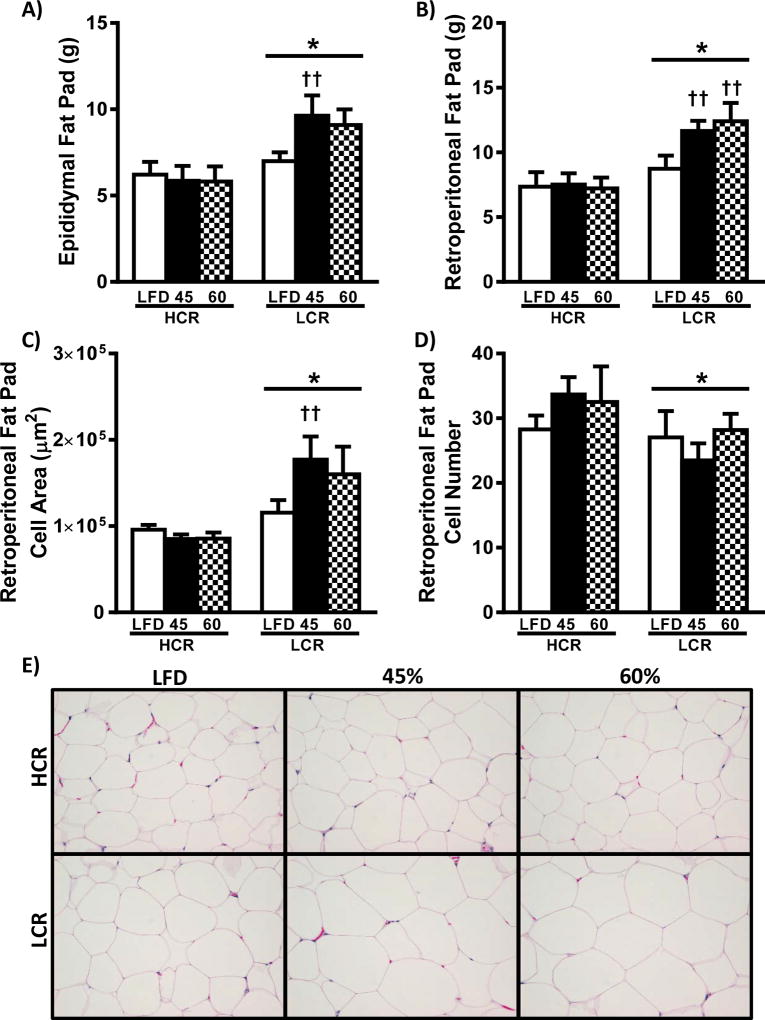
Adipose depot weights and retroperitoneal adipose tissue morphometric analysis are shown in Figure 4. As expected, epididymal and retroperitoneal adipose mass was less in HCR rats compared to LCR (Figure 4A & B, p<0.05). The LCR rats increased epididymal and retroperitoneal adipose mass following the HFD, while HCR on HFDs was not different from LFD. Adipocyte cell size in the retroperitoneal fat pad was smaller in HCR compared to LCR. (Figure 4C & 4E, p<0.05). Only the LCR demonstrated an increase following the HFD, with only the 45% HFD reaching significance. Further, HCR had more adipocytes per frame compared to LCR (Figure 4D & 4E, p<0.05), but this was not altered by the HFD in either strain.
Figure 4.
White adipose tissue. Epididymal (A) and retroperitoneal (B) fat pad masses at the time of sacrifice. Morphometric analysis of the retroperitoneal fat pad are presented as (C) cell area and (D) cell number are presented as means ± SEM (n=8). (E) Representative H&E images of retroperitoneal fat pad. * p<0.05 main effect HCR vs. LCR, †† p<0.05 LFD vs. HFD within strain.
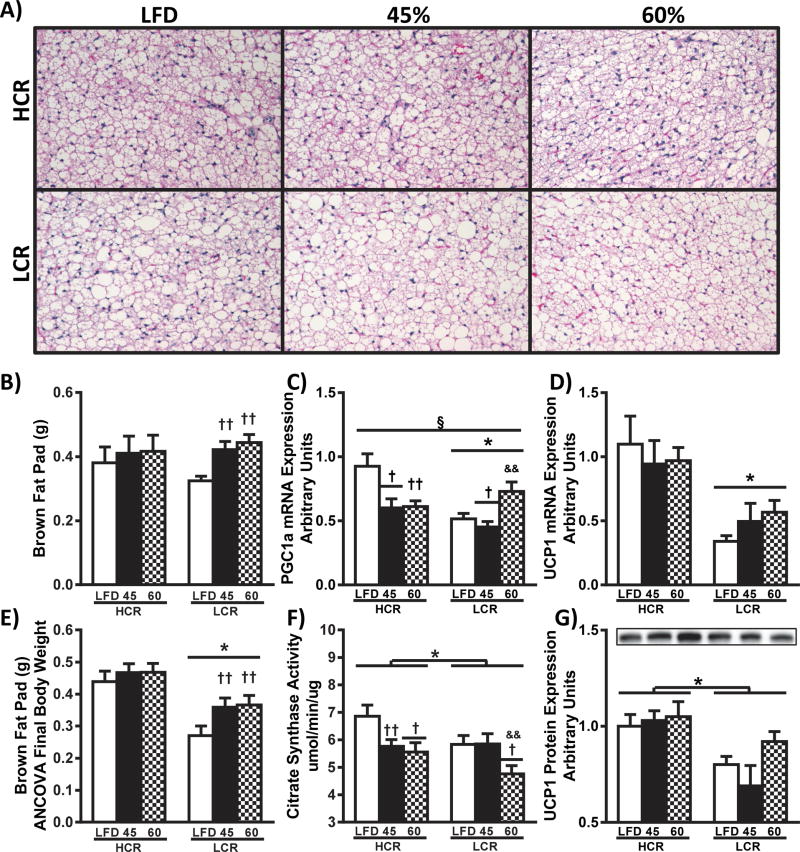
The previously described weight adjusted differences in resting energy expenditure (18) and the observed differences in FE and weight gain could be due in part to differences in brown adipose tissue (BAT) mass-dependent thermogenesis. We observed no differences in BAT mass between strains, and only the LCR increased BAT mass in response to both HFD’s (Figure 5B, p<0.05). However, body weight-adjusted BAT mass was greater in HCR compared to LCR regardless of diet (Figure 5E, p<0.05). Previously, our group has observed greater BAT mass as a percentage of body weight and reduced weight gain in female HCR rats compared to LCR following ovariectomy (20).
Figure 5.
Brown adipose tissue. Representative H&E images of brown adipose tissue are presented in panel A. (B) Fat pad mass was determined at sacrifice and estimated mass of brown fat pad adjusted for final body weight (ANCOVA) is shown in panel E. Gene expression in brown adipose was determined by RT-PCR and western blot analysis: relative mRNA expression of (C) UCP1 and (D) PGC1a were normalized to CycA, and western blot analysis of brown adipose homogenate for (F) UCP1. Brown adipose mitochondrial content was assessed by (G) citrate synthase activity. All data are presented as means ± SEM (n=8). § p<0.05 interaction,* p<0.05 main effect HCR vs. LCR, † p<0.05 main effect LFD vs. HFD, & p<0.05 main effect 45% HFD vs 60% HFD, †† p<0.05 LFD vs. HFD within strain, && p<0.05 45% HFD vs 60% HFD within strain.
To evaluate whether these weight-adjusted differences in BAT were associated with potential functional changes, we assessed tissue H&E and the expression of genes relevant to non-shivering thermogenesis and mitochondrial content. In Figure 5A, representative H&E images suggest that BAT of HCR rats have increased locularity and mitochondrial content compared to LCR. The mRNA expression of mitochondrial biogenesis transcriptional activator, Peroxisome proliferator-activated receptor γ co-activator-1α (PGC-1a), was higher in the HCR compared to LCR rats (Figure 5C, p<0.05), with significant changes occurring due to both HFD’s compared to LFD (p<0.05). Citrate synthase activity, a marker of mitochondrial content, was increased in BAT homogenate from HCR rats compared to LCR (Figure 5F, p<0.05), with reductions due to HFD in both strains. Uncoupling protein-1 (UCP1) mRNA expression was greater in HCR rats compared to LCR (p<0.05), with no diet effects (Figure 5B). Importantly, the reduced UCP1 mRNA expression was associated with increased UCP1 protein expression in HCR BAT compared to LCR rats (Figure 5G, p<0.05). Interestingly, the observed differences in PGC-1a mRNA did not produce similar differences in protein expression (Supplemental figure 1). Additionally, expression of certain genes involved in FAO, BAT activation, and lipid storage were differentially expressed across the strains (Supplemental figure 1). Collectively these data suggest that the observed increase in weight-adjusted resting energy expenditure in the HCR may due to greater non-shivering thermogenesis (18), which may help protect against the more dramatic acute HFD-induced weight gain observed in the LCR.
Discussion
Aerobic capacity is arguably the most powerful predictor of all-cause mortality, (14) but its impact on susceptibility for weight gain and obesity is relatively unknown. Herein we report that HCR rats bred for high intrinsic aerobic capacity display reduced susceptibility for HFD-induced acute weight gain compared to LCR rats bred for low intrinsic aerobic capacity. The protective phenotype in the HCR rats is marked by reduced FI/EI and FE. Moreover, the reduced FE in the HCR rats may be due to a greater weight-adjusted brown adipose mass that also display higher mitochondrial content and higher expression of thermogenic genes and proteins (18).
In sedentary mature ad lib fed rodents, acute HFD-induced weight gain is driven by increased FI/EI. Increased voluntary FI/EI of HFDs in both rodents and humans (25, 26) is influenced by a complex interplay of metabolic and hedonic factors (5, 6, 7, 8). In agreement with previous studies using obese prone/resistant rats (24, 27), the HCR/LCR HFD-induced change in EI strongly associated with subsequent weight gain. EI is a function of both the energy density and quantity of the food consumed, with greater energy density of HFDs resulting in increased weight gain in both humans (28) and rodents (29). In this study, the HCR maintains or reduces FI following transition to the HFDs, while the LCR consumed more food despite the higher energy density of the HFD’s. All told, the smaller weight gain in the HCR rat following transition to the HFDs is partially due to avoiding over-consumption.
To date, we are aware of no detailed studies that have solely focused on hedonic pathways driving FI/EI in the HCR/LCR. However, a recent study examining the interaction between hunger and environmental novelty on FI found that the LCR consumed food (normal chow) more quickly after a 24 hour fast in a novel environment than the HCR (30). This suggests that the drive for the LCR to re-acquire dietary energy is more powerful than the need to explore or be vigilant to potential threats in a new environment. These results in the LCR were associated with differential expression of CART in the nucleus accumbens and higher circulating levels of leptin and ghrelin. In the present study, we explored a different paradigm, changes in FI/EI after the transition from a low fat to a more palatable and energy dense HFD. The differences in FI/EI following a transition to the HFD in the HCR/LCR model could be due to hepatic energy sensing and vagal afferent pathways mediating control in the CNS (18), a hypothesis originally tested separately by Friedman (31) and Langhans (32). These groups showed that reductions in hepatic fat oxidation lead to greater food intake. We have shown repeatedly that the LCR have reduced hepatic mitochondrial content and fat oxidation compared to the HCR. Additional potential metabolic predictors of weight gain in human subjects have included low REE, low whole body fatty acid oxidation capacity (FAO), and metabolic inflexibility (3, 5), all characteristics that we show to occur in the LCR rats compared to HCR (18). The numerous combinations of potential metabolic modulators of differences in EI and weight gain between the HCR and LCR rats underscores the difficulty of dissecting out any individual causal factors; however, it emphasizes that the polygenetic nature of selection for divergent aerobic capacity has resulted in multi-factorial components that underlie their susceptibility for HFD-induced weight gain. Importantly, other factors that control EI, such as hedonic control, as well as, hormonal and satiety feedback signaling cannot be ignored (5, 7), but are beyond the scope of this manuscript.
As previously stated, susceptibility for HFD-induced weight gain has been ascribed to low metabolic rate (8), decreased fat oxidation (33), reduced spontaneous activity (8), low sympathetic nervous system activity, and increased storage of dietary fat (34). In this study, we observed the HCR rats had lower FE compared to LCR within all diets. The decreased FE of the HCR rats could be due to greater metabolic flexibility as suggested by the previously reported lower RQ on HFD compared to LCR (18, 22). Importantly, in the same study HCR rats have higher whole-body FAO on the LFD compared to LCR (18). The higher whole body FAO in the HCR over the LCR is likely due to the greater skeletal muscle and hepatic mitochondrial content and FAO capacity witnessed in the HCR (17, 19, 35). In humans, subjects classified as high fat oxidizers had lower weight gain compared to low fat oxidizers, which was independent of 24 hr metabolic rate (36). Further, maintenance of weight loss in previously obese subjects is associated with higher FAO rates compared to those who regained weight (37). These data suggest that the observed reduction in FE of HCR rats on HFD is due in part to increased adaptability to utilize fat for energy production. However, other factors that could impact FE such as increased tone of sympathetic nervous system (21), higher resting energy expenditure, and cage activity (18, 19, 20, 22) have also been observed in HCR compared to LCR rats.
REE represents ~75% of total energy expenditure in sedentary, caged rodents, and thus significantly impacts energy balance following a transition to a HFD. The HCR display higher weight-adjusted REE on LFD and following 3-days of HFD (18, 20). Elevated brown adipose tissue (BAT) thermogenesis produces an increase in REE, and results in improvements in numerous metabolic disease states (38). Recent studies suggest that increased BAT thermogenesis attenuates HFD-induced weight gain (39, 40). Following body weight adjustment, the HCR display greater BAT regardless of diet. This data is in agreement with our previous finding that female HCR rats display greater BAT (per body weight) which correlated with higher REE, and protection from weight gain following ovariectomy (20). In this study, we observed HCR BAT was more multi-locular, had greater mitochondrial content, and altered expression of several genes and proteins putatively involved in BAT thermogenesis between HCR and LCR rats. Classic markers of increased BAT thermogenesis, PGC-1α and UCP-1, were higher in HCR rats compared to LCR. These data suggest that high intrinsic aerobic capacity results in a phenotype of greater weight-adjusted BAT mass and possibly enhanced BAT thermogenesis, as evidenced by greater mitochondrial content and UCP-1 protein, which could potentially explain the observed higher REE reported in our previous studies (18, 20). More studies are needed to validate the role of BAT in the HCR phenotype.
In summary, we demonstrate that selection for high intrinsic aerobic capacity results in a phenotype of reduced 1-week weight gain following initiation of both a 45% and a 60% HFD. The protection against weight gain in the HCR is linked to a greater adaptability to control FI upon transition to the HFDs, reduced FE, and possibly increased BAT thermogenesis. In contrast, the LCR display greater acute HFD-induced weight gain, putatively due to an inability to adapt FI/EI after transition to HFD’s, augmented FE, and reduced BAT size and thermogenic gene expression. These data provide insight into potential mechanisms by which intrinsic aerobic capacity impacts weight gain, factors which may have particular importance in our obesogenic environment in which energy is available in over-abundance.
Supplementary Material
What is already known about this subject?
Aerobic fitness is the most powerful predictor of mortality and metabolic disease development in humans.
High-fat diets result in increased weight gain in humans and animal models proportional to the quantity of fat in the diet.
In humans and animals models, a phenotype exists with reduced susceptibility to high-fat diet-induced weight gain and obesity.
What does this study add?
High aerobic capacity is associated with greater capacity to adjust food intake following transition to high-fat diet to reduce excess energy intake and subsequent acute weight gain.
Selection for increased aerobic capacity has resulted in a phenotype that reduces high-fat diet-induced acute weight gain in part through decreased weight gain per kcal of energy consumed.
High aerobic capacity is associated with greater brown adipose tissue mass adjusted for body weight and increased gene expression of brown adipose genes involved in thermogenesis in male rats.
Acknowledgments
GRANTS and ACKNOLDGEMENTS
This work was partially supported by NIH grants DK088940 (JPT), 5T32AR48523-8 and AHA 14POST20110034 (EMM), P50 HD073063 (PSM). The LCR-HCR rat model system was funded by the Office of Research Infrastructure Programs/OD grant P40OD021331 and by grant R01DK099034 (LGK and SLB) from the NIH. Contact LGK lgkoch@umich.edu or SLB brittons@umich.edu for information on the LCR and HCR rats: these rat models are maintained as an international resource with support from the Department of Anesthesiology at the University of Michigan, Ann Arbor, Michigan. This work was supported with resources and the use of facilities at the Harry S Truman Memorial VA Hospital in Columbia, MO.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose for this research.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blundell JE, Stubbs RJ, Golding C, Croden F, Alam R, Whybrow S, et al. Resistance and susceptibility to weight gain: individual variability in response to a high-fat diet. Physiol Behav. 2005;86:614–622. doi: 10.1016/j.physbeh.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 4.Bouchard C, Tremblay A, Despres JP, Nadeau A, Lupien PJ, Theriault G, et al. The response to long-term overfeeding in identical twins. N Engl J Med. 1990;322:1477–1482. doi: 10.1056/NEJM199005243222101. [DOI] [PubMed] [Google Scholar]
- 5.Hopkins M, Jeukendrup A, King NA, Blundell JE. The relationship between substrate metabolism, exercise and appetite control: does glycogen availability influence the motivation to eat, energy intake or food choice? Sports medicine. 2011;41:507–521. doi: 10.2165/11588780-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev. 2010;23:270–299. doi: 10.1017/S0954422410000168. [DOI] [PubMed] [Google Scholar]
- 7.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 8.Galgani J, Ravussin E. Energy metabolism, fuel selection and body weight regulation. International journal of obesity. 2008;32(Suppl 7):S109–119. doi: 10.1038/ijo.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall KD, Heymsfield SB, Kemnitz JW, Klein S, Schoeller DA, Speakman JR. Energy balance and its components: implications for body weight regulation. The American journal of clinical nutrition. 2012;95:989–994. doi: 10.3945/ajcn.112.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Racette SB, Weiss EP, Schechtman KB, Steger-May K, Villareal DT, Obert KA, et al. Influence of weekend lifestyle patterns on body weight. Obesity (Silver Spring) 2008;16:1826–1830. doi: 10.1038/oby.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hull HR, Radley D, Dinger MK, Fields DA. The effect of the Thanksgiving holiday on weight gain. Nutr J. 2006;5:29. doi: 10.1186/1475-2891-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanovski JA, Yanovski SZ, Sovik KN, Nguyen TT, O'Neil PM, Sebring NG. A prospective study of holiday weight gain. N Engl J Med. 2000;342:861–867. doi: 10.1056/NEJM200003233421206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Y, Olendzki BC, Li W, Hafner AR, Chiriboga D, Hebert JR, et al. Seasonal variation in food intake, physical activity, and body weight in a predominantly overweight population. European journal of clinical nutrition. 2006;60:519–528. doi: 10.1038/sj.ejcn.1602346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 15.Costa AM, Breitenfeld L, Silva AJ, Pereira A, Izquierdo M, Marques MC. Genetic inheritance effects on endurance and muscle strength: an update. Sports medicine. 2012;42:449–458. doi: 10.2165/11650560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics. 2001;5:45–52. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- 17.Noland RC, Thyfault JP, Henes ST, Whitfield BR, Woodlief TL, Evans JR, et al. Artificial selection for high-capacity endurance running is protective against high-fat diet-induced insulin resistance. Am J Physiol Endocrinol Metab. 2007;293:E31–41. doi: 10.1152/ajpendo.00500.2006. [DOI] [PubMed] [Google Scholar]
- 18.Morris EM, Jackman MR, Johnson GC, Liu TW, Lopez JL, Kearney ML, et al. Intrinsic aerobic capacity impacts susceptibility to acute high-fat diet-induced hepatic steatosis. Am J Physiol Endocrinol Metab. 2014;307:E355–364. doi: 10.1152/ajpendo.00093.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thyfault JP, Rector RS, Uptergrove GM, Borengasser SJ, Morris EM, Wei Y, et al. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol. 2009;587:1805–1816. doi: 10.1113/jphysiol.2009.169060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vieira-Potter VJ, Padilla J, Park YM, Welly RJ, Scroggins RJ, Britton SL, et al. Female rats selectively bred for high intrinsic aerobic fitness are protected from ovariectomy-associated metabolic dysfunction. Am J Physiol Regul Integr Comp Physiol. 2015;308:R530–542. doi: 10.1152/ajpregu.00401.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gavini CK, Mukherjee S, Shukla C, Britton SL, Koch LG, Shi H, et al. Leanness and Heightened Non-Resting Energy Expenditure: Role of Skeletal Muscle Activity Thermogenesis. Am J Physiol Endocrinol Metab. 2014 doi: 10.1152/ajpendo.00555.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novak CM, Escande C, Burghardt PR, Zhang M, Barbosa MT, Chini EN, et al. Spontaneous activity, economy of activity, and resistance to diet-induced obesity in rats bred for high intrinsic aerobic capacity. Horm Behav. 2010;58:355–367. doi: 10.1016/j.yhbeh.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris EM, Jackman MR, Meers GM, Johnson GC, Lopez JL, Maclean PS, et al. Reduced hepatic mitochondrial respiration following acute high-fat diet is prevented by PGC-1alpha overexpression. Am J Physiol Gastrointest Liver Physiol. 2013;305:G868–880. doi: 10.1152/ajpgi.00179.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gayles EC, Pagliassotti MJ, Prach PA, Koppenhafer TA, Hill JO. Contribution of energy intake and tissue enzymatic profile to body weight gain in high-fat-fed rats. Am J Physiol. 1997;272:R188–194. doi: 10.1152/ajpregu.1997.272.1.R188. [DOI] [PubMed] [Google Scholar]
- 25.Pagliassotti MJ, Knobel SM, Shahrokhi KA, Manzo AM, Hill JO. Time course of adaptation to a high-fat diet in obesity-resistant and obesity-prone rats. Am J Physiol. 1994;267:R659–664. doi: 10.1152/ajpregu.1994.267.3.R659. [DOI] [PubMed] [Google Scholar]
- 26.Donahoo W, Wyatt HR, Kriehn J, Stuht J, Dong F, Hosokawa P, et al. Dietary fat increases energy intake across the range of typical consumption in the United States. Obesity (Silver Spring) 2008;16:64–69. doi: 10.1038/oby.2007.31. [DOI] [PubMed] [Google Scholar]
- 27.Jackman MR, MacLean PS, Bessesen DH. Energy expenditure in obesity-prone and obesity-resistant rats before and after the introduction of a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1097–1105. doi: 10.1152/ajpregu.00549.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill JO, Melanson EL, Wyatt HT. Dietary fat intake and regulation of energy balance: implications for obesity. The Journal of nutrition. 2000;130:284S–288S. [PubMed] [Google Scholar]
- 29.Ghibaudi L, Cook J, Farley C, van Heek M, Hwa JJ. Fat intake affects adiposity, comorbidity factors, and energy metabolism of sprague-dawley rats. Obes Res. 2002;10:956–963. doi: 10.1038/oby.2002.130. [DOI] [PubMed] [Google Scholar]
- 30.Burghardt PR, Krolewski DM, Dykhuis KE, Ching J, Pinawin AM, Britton SL, et al. Nucleus accumbens cocaine-amphetamine regulated transcript mediates food intake during novelty conflict. Physiol Behav. 2016;158:76–84. doi: 10.1016/j.physbeh.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedman MI. Control of energy intake by energy metabolism. The American journal of clinical nutrition. 1995;62:1096S–1100S. doi: 10.1093/ajcn/62.5.1096S. [DOI] [PubMed] [Google Scholar]
- 32.Langhans W. Fatty acid oxidation in the energostatic control of eating--a new idea. Appetite. 2008;51:446–451. doi: 10.1016/j.appet.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Houmard JA. Intramuscular lipid oxidation and obesity. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1111–1116. doi: 10.1152/ajpregu.00396.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horton TJ, Drougas H, Brachey A, Reed GW, Peters JC, Hill JO. Fat and carbohydrate overfeeding in humans: different effects on energy storage. The American journal of clinical nutrition. 1995;62:19–29. doi: 10.1093/ajcn/62.1.19. [DOI] [PubMed] [Google Scholar]
- 35.Naples SP, Borengasser SJ, Rector RS, Uptergrove GM, Morris EM, Mikus CR, et al. Skeletal muscle mitochondrial and metabolic responses to a high-fat diet in female rats bred for high and low aerobic capacity. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2010;35:151–162. doi: 10.1139/h09-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol. 1990;259:E650–657. doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]
- 37.Froidevaux F, Schutz Y, Christin L, Jequier E. Energy expenditure in obese women before and during weight loss, after refeeding, and in the weight-relapse period. The American journal of clinical nutrition. 1993;57:35–42. doi: 10.1093/ajcn/57.1.35. [DOI] [PubMed] [Google Scholar]
- 38.Poher AL, Altirriba J, Veyrat-Durebex C, Rohner-Jeanrenaud F. Brown adipose tissue activity as a target for the treatment of obesity/insulin resistance. Front Physiol. 2015;6:4. doi: 10.3389/fphys.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crane JD, Palanivel R, Mottillo EP, Bujak AL, Wang H, Ford RJ, et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat Med. 2015;21:166–172. doi: 10.1038/nm.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jun HJ, Joshi Y, Patil Y, Noland RC, Chang JS. NT-PGC-1alpha activation attenuates high-fat diet-induced obesity by enhancing brown fat thermogenesis and adipose tissue oxidative metabolism. Diabetes. 2014;63:3615–3625. doi: 10.2337/db13-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.