Abstract
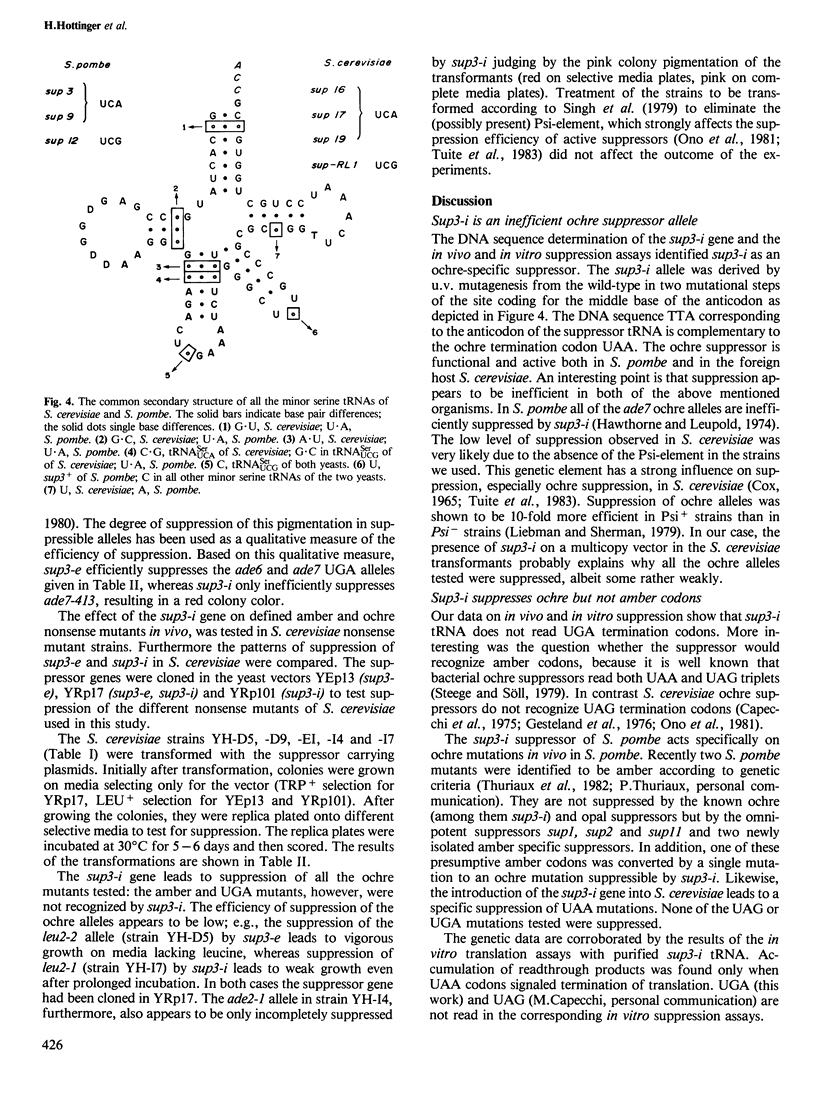
The inefficient suppressor sup3-i of the fission yeast Schizosaccharomyces pombe is an ochre suppressor. Sup3-i was derived from the efficient serine inserting UGA suppressor sup3-e. The cloning and sequencing of the sup3-i gene indicate that the suppressor is different from the parent sup3-e by a C----T substitution in the sequence coding for the middle position of the anticodon. In vitro translation assays supplemented with purified sup3-i tRNA and programmed with Xenopus globin mRNAs lead to the accumulation of a readthrough product in response to UAA termination signals, but not in response to UGA termination codons. Transformation of Saccharomyces cerevisiae nonsense mutant strains with plasmid DNA carrying the S. pombe sup3-i gene, led to ochre, but not amber or UGA suppression in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R., Hughes S. H., Wahl G. M. Yeast super-suppressors are altered tRNAs capable of translating a nonsense codon in vitro. Cell. 1975 Nov;6(3):269–277. doi: 10.1016/0092-8674(75)90178-6. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer D. R., Eccleshall R., Marmur J. Isolation of yeast DNA. Methods Cell Biol. 1975;12:39–44. doi: 10.1016/s0091-679x(08)60950-4. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Vournakis J. N., Donis-Keller H., Chaconas G., Dougall D. K., Kafatos F. C. End labeling of enzymatically decapped mRNA. Nucleic Acids Res. 1977 Dec;4(12):4165–4174. doi: 10.1093/nar/4.12.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egel R., Kohli J., Thuriaux P., Wolf K. Genetics of the fission yeast Schizosaccharomyces pombe. Annu Rev Genet. 1980;14:77–108. doi: 10.1146/annurev.ge.14.120180.000453. [DOI] [PubMed] [Google Scholar]
- Gauss D. H., Sprinzl M. Compilation of sequences of tRNA genes. Nucleic Acids Res. 1983 Jan 11;11(1):r55–103. [PMC free article] [PubMed] [Google Scholar]
- Gauss D. H., Sprinzl M. Compilation of tRNA sequences. Nucleic Acids Res. 1983 Jan 11;11(1):r1–53. [PMC free article] [PubMed] [Google Scholar]
- Gesteland R. F., Wolfner M., Grisafi P., Fink G., Botstein D., Roth J. R. Yeast suppressors of UAA and UAG nonsense codons work efficiently in vitro via tRNA. Cell. 1976 Mar;7(3):381–390. doi: 10.1016/0092-8674(76)90167-7. [DOI] [PubMed] [Google Scholar]
- Hawthorne D. C., Leupold U. Suppressors in yeast. Curr Top Microbiol Immunol. 1974;64(0):1–47. doi: 10.1007/978-3-642-65848-8_1. [DOI] [PubMed] [Google Scholar]
- Hirsh D. Tryptophan transfer RNA as the UGA suppressor. J Mol Biol. 1971 Jun 14;58(2):439–458. doi: 10.1016/0022-2836(71)90362-7. [DOI] [PubMed] [Google Scholar]
- Hottinger H., Pearson D., Yamao F., Gamulin V., Cooley L., Cooper T., Söll D. Nonsense suppression in Schizosaccharomyces pombe: the S. pombe Sup3-e tRNASerUGA gene is active in S. cerevisiae. Mol Gen Genet. 1982;188(2):219–224. doi: 10.1007/BF00332678. [DOI] [PubMed] [Google Scholar]
- Kay R. M., Harris R., Patient R. K., Williams J. G. Molecular cloning of cDNA sequences coding for the major alpha- and beta-globin polypeptides of adult Xenopus laevis. Nucleic Acids Res. 1980 Jun 25;8(12):2691–2707. doi: 10.1093/nar/8.12.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knöchel W., Meyerhof W., Hummel S., Grundmann U. Molecular cloning and sequencing of mRNAs coding for minor adult globin polypeptides of Xenopus laevis. Nucleic Acids Res. 1983 Mar 11;11(5):1543–1553. doi: 10.1093/nar/11.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli J., Hottinger H., Munz P., Strauss A., Thuriaux P. Genetic Mapping in SCHIZOSACCHAROMYCES POMBE by Mitotic and Meiotic Analysis and Induced Haploidization. Genetics. 1977 Nov;87(3):471–489. doi: 10.1093/genetics/87.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
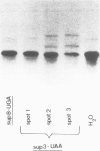
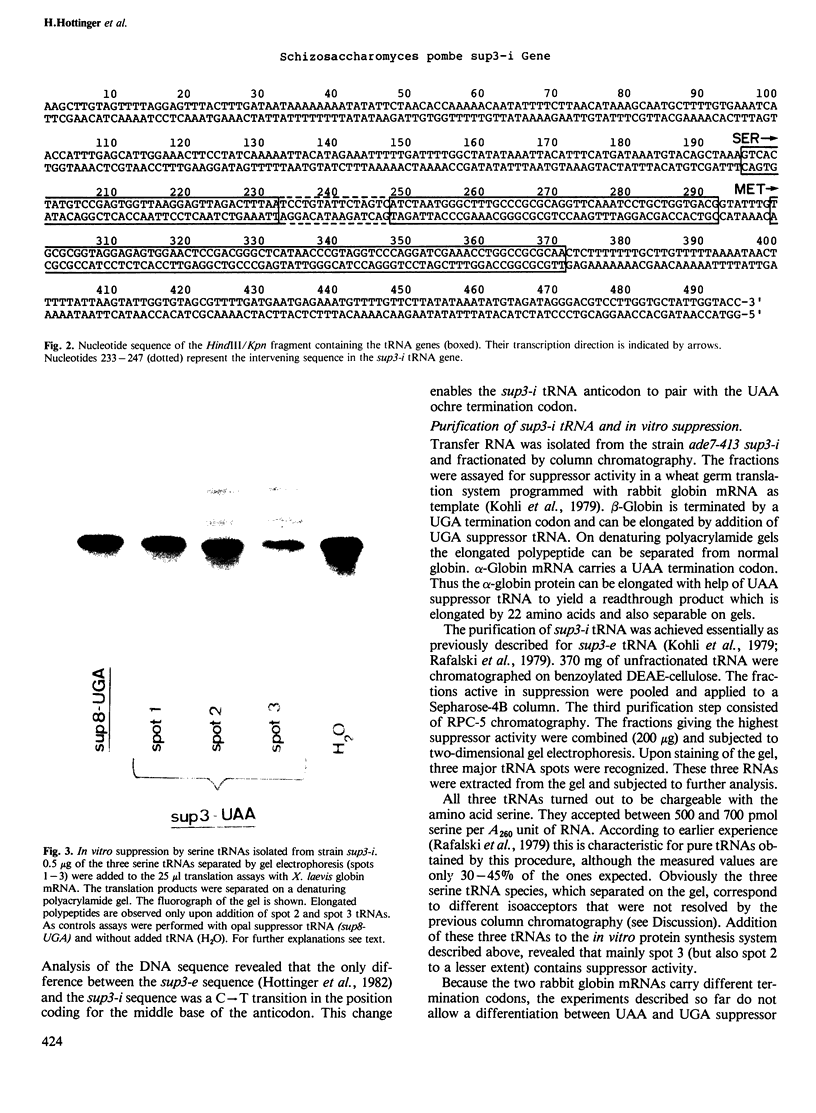
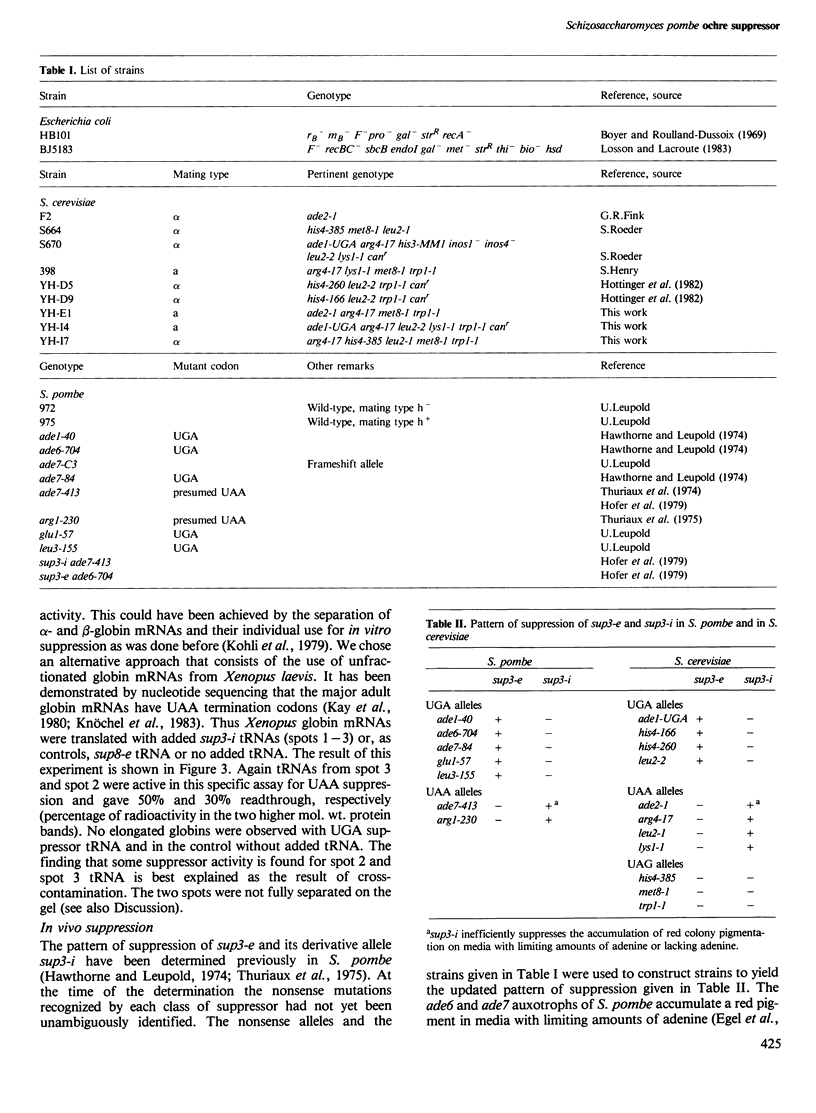
- Kohli J., Kwong T., Altruda F., Söll D., Wahl G. Characterization of a UGA-suppressing serine tRNA from Schizosaccharomyces pombe with the help of a new in vitro assay system for eukaryotic suppressor tRNAs. J Biol Chem. 1979 Mar 10;254(5):1546–1551. [PubMed] [Google Scholar]
- Liebman S. W., Sherman F. Extrachromosomal psi+ determinant suppresses nonsense mutations in yeast. J Bacteriol. 1979 Sep;139(3):1068–1071. doi: 10.1128/jb.139.3.1068-1071.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losson R., Lacroute F. Plasmids carrying the yeast OMP decarboxylase structural and regulatory genes: transcription regulation in a foreign environment. Cell. 1983 Feb;32(2):371–377. doi: 10.1016/0092-8674(83)90456-7. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H., Kutter E., Nakanishi M. A restriction map of the bacteriophage T4 genome. Mol Gen Genet. 1980;179(2):421–435. doi: 10.1007/BF00425473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono B. I., Wills N., Stewart J. W., Gesteland R. F., Sherman F. Serine-inserting UAA suppression mediated by yeast tRNASer. J Mol Biol. 1981 Aug 15;150(3):361–373. doi: 10.1016/0022-2836(81)90552-0. [DOI] [PubMed] [Google Scholar]
- Rafalski A., Kohli J., Agris P., Söll D. The nucleotide sequence of a UGA suppressor serine tRNA from Schizosaccharomyces pombe. Nucleic Acids Res. 1979 Jun 25;6(8):2683–2695. doi: 10.1093/nar/6.8.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T. M., Swanberg S. L., Poteete A., Riedel G., Backman K. A plasmid cloning vehicle allowing a positive selection for inserted fragments. Gene. 1980 Dec;12(1-2):123–127. doi: 10.1016/0378-1119(80)90022-0. [DOI] [PubMed] [Google Scholar]
- Singh A., Helms C., Sherman F. Mutation of the non-Mendelian suppressor, Psi, in yeast by hypertonic media. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1952–1956. doi: 10.1073/pnas.76.4.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuriaux P., Minet M., Hofer F., Leupold U. Genetic analysis of antisuppressor mutants in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1976 Dec 31;142(4):251–261. doi: 10.1007/BF00271250. [DOI] [PubMed] [Google Scholar]
- Tuite M. F., Cox B. S., McLaughlin C. S. In vitro nonsense suppression in [psi+] and [psi-] cell-free lysates of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 May;80(10):2824–2828. doi: 10.1073/pnas.80.10.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walseth T. F., Johnson R. A. The enzymatic preparation of [alpha-(32)P]nucleoside triphosphates, cyclic [32P] AMP, and cyclic [32P] GMP. Biochim Biophys Acta. 1979 Mar 28;562(1):11–31. doi: 10.1016/0005-2787(79)90122-9. [DOI] [PubMed] [Google Scholar]
- Widmer H. J., Andres A. C., Niessing J., Hosbach H. A., Weber R. Comparative analysis of cloned larval and adult globin cDNA sequences of Xenopus laevis. Dev Biol. 1981 Dec;88(2):325–332. doi: 10.1016/0012-1606(81)90176-7. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Takeishi K., Ukita T. Structural studies on a yeast glutamic acid tRNA specific to GAA codon. Biochim Biophys Acta. 1971 Jan 1;228(1):153–166. doi: 10.1016/0005-2787(71)90555-7. [DOI] [PubMed] [Google Scholar]