Abstract
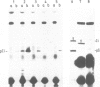
The invariant (Ii) chain is a membrane-spanning glycoprotein found intracellularly associated with class II major histocompatibility complex (MHC) molecules. Using hybrid-selected translation and the Ii-specific monoclonal antibody In-1, we have isolated a cDNA clone (pIi-5) coding for most of the Ii chain. Sequence analysis of this clone reveals an open reading frame encoding 169 amino acid residues. The protein is rich in methionine and contains two potential N-glycosylation sites. No stretch of uncharged amino acid residues, characteristic for a membrane-spanning segment, is found close to the COOH-terminal end. There is one, however, close to the NH2-terminal end. As it is know that approximately 20 amino acid residues of Ii chain are exposed on the cytoplasmic side, we conclude that the Ii chain spans the membrane exposing the NH2 terminus on the cytoplasmic side and the COOH terminus on the luminal side.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastia D. Determination of restriction sites and the nucleotide sequence surrounding the relaxation site of ColE1. J Mol Biol. 1978 Oct 5;124(4):601–639. doi: 10.1016/0022-2836(78)90174-2. [DOI] [PubMed] [Google Scholar]
- Benacerraf B. Role of MHC gene products in immune regulation. Science. 1981 Jun 12;212(4500):1229–1238. doi: 10.1126/science.6165083. [DOI] [PubMed] [Google Scholar]
- Benoist C. O., Mathis D. J., Kanter M. R., Williams V. E., 2nd, McDevitt H. O. Regions of allelic hypervariability in the murine A alpha immune response gene. Cell. 1983 Aug;34(1):169–177. doi: 10.1016/0092-8674(83)90147-2. [DOI] [PubMed] [Google Scholar]
- Charron D. J., Aellen-Schulz M. F., St Geme J., 3rd, Erlich H. A., McDevitt H. O. Biochemical characterization of an invariant polypeptide associated with Ia antigens in human and mouse. Mol Immunol. 1983 Jan;20(1):21–32. doi: 10.1016/0161-5890(83)90101-3. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Choi E., McIntyre K., Germain R. N., Seidman J. G. Murine I-A beta chain polymorphism: nucleotide sequences of three allelic I-A beta genes. Science. 1983 Jul 15;221(4607):283–286. doi: 10.1126/science.6407114. [DOI] [PubMed] [Google Scholar]
- Drickamer K. Complete amino acid sequence of a membrane receptor for glycoproteins. Sequence of the chicken hepatic lectin. J Biol Chem. 1981 Jun 10;256(11):5827–5839. [PubMed] [Google Scholar]
- Drickamer L. K. Orientation of the band 3 polypeptide from human erythrocyte membranes. Identification of NH2-terminal sequence and site of carbohydrate attachment. J Biol Chem. 1978 Oct 25;253(20):7242–7248. [PubMed] [Google Scholar]
- Finn O. J., Stackhouse C. J., Metzgar R. S. Human Ia beta chains and the invariant chain share a common antigenic determinant. J Exp Med. 1983 Oct 1;158(4):1344–1349. doi: 10.1084/jem.158.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Garoff H., Lehrach H. A subcloning strategy for DNA sequence analysis. Nucleic Acids Res. 1980 Dec 11;8(23):5541–5549. doi: 10.1093/nar/8.23.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Jones P. P., Murphy D. B., Hewgill D., McDevitt H. O. Detection of a common polypeptide chain in I--A and I--E sub-region immunoprecipitates. Mol Immunol. 1979 Jan;16(1):51–60. doi: 10.1016/0161-5890(79)90027-0. [DOI] [PubMed] [Google Scholar]
- Klein J. The major histocompatibility complex of the mouse. Science. 1979 Feb 9;203(4380):516–521. doi: 10.1126/science.104386. [DOI] [PubMed] [Google Scholar]
- Koch N., Hämmerling G. J. Structure of Ia antigens: identification of dimeric complexes formed by the invariant chain. J Immunol. 1982 Mar;128(3):1155–1158. [PubMed] [Google Scholar]
- Koch N., Koch S., Hämmerling G. J. Ia invariant chain detected on lymphocyte surfaces by monoclonal antibody. Nature. 1982 Oct 14;299(5884):644–645. doi: 10.1038/299644a0. [DOI] [PubMed] [Google Scholar]
- Kvist S., Bregegere F., Rask L., Cami B., Garoff H., Daniel F., Wiman K., Larhammar D., Abastado J. P., Gachelin G. cDNA clone coding for part of a mouse H-2d major histocompatibility antigen. Proc Natl Acad Sci U S A. 1981 May;78(5):2772–2776. doi: 10.1073/pnas.78.5.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvist S., Wiman K., Claesson L., Peterson P. A., Dobberstein B. Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell. 1982 May;29(1):61–69. doi: 10.1016/0092-8674(82)90090-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larhammar D., Hammerling U., Denaro M., Lund T., Flavell R. A., Rask L., Peterson P. A. Structure of the murine immune response I-A beta locus: sequence of the I-A beta gene and an adjacent beta-chain second domain exon. Cell. 1983 Aug;34(1):179–188. doi: 10.1016/0092-8674(83)90148-4. [DOI] [PubMed] [Google Scholar]
- Larhammar D., Hammerling U., Denaro M., Lund T., Flavell R. A., Rask L., Peterson P. A. Structure of the murine immune response I-A beta locus: sequence of the I-A beta gene and an adjacent beta-chain second domain exon. Cell. 1983 Aug;34(1):179–188. doi: 10.1016/0092-8674(83)90148-4. [DOI] [PubMed] [Google Scholar]
- Lemke H., Hämmerling G. J., Hämmerling U. Fine specificity analysis with monoclonal antibodies of antigens controlled by the major histocompatibility complex and by the Qa/TL region in mice. Immunol Rev. 1979;47:175–206. doi: 10.1111/j.1600-065x.1979.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMillan M., Frelinger J. A., Jones P. P., Murphy D. B., McDevitt H. O., Hood L. Structure of murine Ia antigens. Two dimensional electrophoretic analyses and high pressure liquid chromatography tryptic peptide maps of products of the I-A and I-E subregions and of an associated invariant polypeptide. J Exp Med. 1981 Apr 1;153(4):936–950. doi: 10.1084/jem.153.4.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosic J. P., Nilson A., Hämmerling G. J., McKean D. J. Biochemical characterization of Ia antigens. I. Characterization of the 31K polypeptide associated with I-A subregion Ia antigens. J Immunol. 1980 Oct;125(4):1463–1469. [PubMed] [Google Scholar]
- Nagy Z. A., Baxevanis C. N., Ishii N., Klein J. Ia antigens as restriction molecules in Ir-gene controlled T-cell proliferation. Immunol Rev. 1981;60:59–83. doi: 10.1111/j.1600-065x.1981.tb00362.x. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Norgard M. V., Emigholz K., Monahan J. J. Increased amplification of pBR322 plasmid deoxyribonucleic acid in Escherichia coli K-12 strains RR1 and chi1776 grown in the presence of high concentrations of nucleoside. J Bacteriol. 1979 Apr;138(1):270–272. doi: 10.1128/jb.138.1.270-272.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmsdorf H. J., Koch N., Mallick U., Herrlich P. Regulation of MHC class II invariant chain expression: induction of synthesis in human and murine plasmocytoma cells by arresting replication. EMBO J. 1983;2(6):811–816. doi: 10.1002/j.1460-2075.1983.tb01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowekamp W., Firtel R. A. Isolation of developmentally regulated genes from Dictyostelium. Dev Biol. 1980 Oct;79(2):409–418. doi: 10.1016/0012-1606(80)90126-8. [DOI] [PubMed] [Google Scholar]
- Sung E., Jones P. P. The invariant chain of murine Ia antigens: its glycosylation, abundance and subcellular localization. Mol Immunol. 1981 Oct;18(10):899–913. doi: 10.1016/0161-5890(81)90013-4. [DOI] [PubMed] [Google Scholar]
- Swiedler S. J., Hart G. W., Freed J. H. Characterization of the oligosaccharides from the invariant chain associated with murine Ia antigens. J Immunol. 1983 Jul;131(1):352–358. [PubMed] [Google Scholar]
- Wahli W., Ryffel G. U., Wyler T., Jaggi F. B., Weber R., Dawid I. B. Cloning and characterization of synthetic sequences from the Xenopus iaevis vitellogenin structural gene. Dev Biol. 1978 Dec;67(2):371–383. doi: 10.1016/0012-1606(78)90207-5. [DOI] [PubMed] [Google Scholar]