Abstract
Familial hypercholesterolaemia is an autosomal dominant inherited disorder characterised by elevated low-density lipoprotein cholesterol levels and consequently an increased risk of atherosclerotic cardiovascular disease (ASCVD). Familial hypercholesterolaemia is relatively common, but is often underdiagnosed and undertreated. Cardiologists are likely to encounter many individuals with familial hypercholesterolaemia; however, patients presenting with premature ASCVD are rarely screened for familial hypercholesterolaemia and fasting lipid levels are infrequently documented. Given that individuals with familial hypercholesterolaemia and ASCVD are at a particularly high risk of subsequent cardiac events, this is a missed opportunity for preventive therapy. Furthermore, because there is a 50% chance that first-degree relatives of individuals with familial hypercholesterolaemia will also be affected by the disorder, the underdiagnosis of familial hypercholesterolaemia among patients with ASCVD is a barrier to cascade screening and the prevention of ASCVD in affected relatives. Targeted screening of patients with ASCVD is an effective strategy to identify new familial hypercholesterolaemia index cases. Statins are the standard treatment for individuals with familial hypercholesterolaemia; however, low-density lipoprotein cholesterol targets are not achieved in a large proportion of patients despite treatment. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have been shown to reduce low-density lipoprotein cholesterol levels considerably in individuals with familial hypercholesterolaemia who are concurrently receiving the maximal tolerated statin dose. The clinical benefit of PCSK9 inhibitors must, however, also be considered in terms of their cost-effectiveness. Increased awareness of familial hypercholesterolaemia is required among healthcare professionals, particularly cardiologists and primary care physicians, in order to start early preventive measures and to reduce the mortality and morbidity associated with familial hypercholesterolaemia and ASCVD.
Keywords: Cardiovascular disease, familial hypercholesterolaemia, PCSK9, proprotein convertase subtilisin/kexin type 9
Introduction
Familial hypercholesterolaemia (FH) is an autosomal dominant inherited lipid disorder that, in most cases, is caused by mutations occurring in one (or more) of three genes: the low-density lipoprotein (LDL) receptor gene (LDLR), the apolipoprotein B gene (APOB) and the proprotein convertase subtilisin/kexin type 9 gene (PCSK9).1 Mutations in these genes lead to impaired LDL metabolism and elevation of plasma LDL-cholesterol. FH can also be caused by mutations in other genes, including signal transducing adaptor family member 1 (STAP1);2 however, mutations in such genes are rare. Regardless of the underlying cause, patients with FH have elevated LDL-cholesterol levels and are therefore at increased risk of premature atherosclerotic cardiovascular disease (ASCVD). ASCVD most commonly manifests as coronary heart disease (CHD),3 but patients may also present with stroke.4
Recent studies have shown that the prevalence of heterozygous FH (HeFH) is one in 200–250 people5,6 and that of homozygous FH (HoFH) is one in 160,000–300,000 people.7 Although HeFH is not uncommon, it is often underdiagnosed and undertreated. This leads to substantial mortality and morbidity;6 approximately 50% of men and 30% of women with FH will develop CHD before the age of 50 years if the disorder is left untreated.3 ASCVD in patients with FH is therefore an important public health challenge. Patients with confirmed FH should receive high-intensity lipid-lowering therapy (LLT), which has been shown to improve their life expectancy8 and quality of life markedly.9
As a result of the high prevalence of ASCVD in patients with FH, it is important that individuals presenting with ASCVD are assessed for FH.10 However, screening for FH among this patient population remains low,5 which leads to suboptimal management of patients with FH and ASCVD. Furthermore, given the autosomal dominant inheritance of FH, family members of individuals diagnosed with FH should be screened in a cascade approach to identify affected relatives. As the prevalence of FH is much greater in those presenting with ASCVD than in the general population,5,11 patients with ASCVD represent a key target population for FH screening.5 It is, therefore, vital that cardiologists and other medical professionals are aware of current guidelines and consensus statements on the diagnosis and treatment of patients with FH.6,7,9 Understanding of the molecular pathology and genetic basis of FH is vital to support screening so that LLT is initiated in individuals with FH in order to prevent ASCVD events.12
Cardiovascular disease risk in patients with FH
Patients with FH are up to 16 times more likely to develop ASCVD than the overall population.13–16 In a population-based cohort study comprising 69,016 individuals from Denmark, it was estimated that 33% of patients with FH had CHD.13 Patients with FH typically develop premature ASCVD, with ASCVD events often occurring in patients with HeFH before 55 years of age in men and before 60 years of age in women.6 A recent cohort study of CHD risk in patients with FH, which included 65,565 people and a follow-up of 78,985–308,378 person-years, found that CHD risk was accelerated in patients with FH by 10–20 years in men and 20–30 years in women.17 ASCVD is a leading cause of death in those with FH;18 in a 21-year cohort study of 5518 patients with FH, ASCVD was the most common cause of death, accounting for 42% of the 189 deaths occurring during the study period.19
Given the relative rarity of HoFH (estimated prevalence is one in 160,000–300,000 people),7 little is known about the exact ASCVD risk and associated mortality in these patients.20 Sjouke et al. found that 29% of 49 patients with HoFH had ASCVD.21 Patients with HoFH develop ASCVD much younger than those with HeFH, often before 20 years of age. In a study of 149 patients with HoFH, Raal et al. found that, in those who were untreated, the age (mean ± SD) at first non-fatal major adverse cardiac event (MACE) was 12.8 ± 5.9 years and the age of ASCVD-related death was 17.7 ± 10.1 years.22
Cardiovascular disease risk stratification
Not all patients with FH develop atherosclerosis and ASCVD to the same extent.23 ASCVD risk depends mainly on plasma LDL-cholesterol levels.24 The underlying genetic mutation, patient comorbidities and lifestyle factors influence LDL-cholesterol levels and ASCVD risk.23 Stratification of patients by their individual ASCVD risk factors may help to identify those who would benefit from high-intensity LLT.25
Mutation of a known FH-causing gene can be found in approximately 80% of patients with definite FH.23 Among patients with an identifiable genetic mutation, the most common cause of FH is mutations in LDLR, affecting over 90% of these patients.6 These fall into six classes: class 1 mutations are null mutations that result in no detectable LDLR protein; class 2 mutations disrupt the transport of LDLR from the endoplasmic reticulum to the Golgi apparatus; class 3 mutations lead to the expression of non-functional LDLR; class 4 mutations result in LDLR–LDL complexes that cannot cluster in coated pits; class 5 mutations lead to inefficient recycling of LDLR; and class 6 mutations disrupt the targeting of the receptor to the basolateral membrane.9
Null mutations in LDLR are consistently found to be associated with the most severe forms of FH (in terms of both LDL-cholesterol levels and ASCVD risk).21,26,27 In a study of 1088 patients with premature myocardial infarction (MI), it was found that, compared with the general population, those with a class 1 mutation in LDLR had a 13-fold increased risk of MI, while those with other classes of LDLR mutations had a 2.4-fold increased risk.14 Furthermore, LDLR mutations overall confer a more severe phenotype than APOB mutations. In a study of CHD risk in patients with FH and their unaffected relatives, individuals with any class of LDLR mutation had a 8.5-fold increased risk of CHD, whereas those with an APOB mutation had a 2.7-fold increased risk compared with unaffected relatives.28
Mutations in APOB occur in 5–10% of patients with FH.1 However, the frequency of mutation in this gene varies by country and has not been found to occur in Finland, Spain, Russia and Japan.29 The most frequent FH-causing mutation in this gene is the R3500Q (Arg3500Gln) mutation.1 Patients carrying this mutation have been shown to have significantly increased LDL-cholesterol levels and a seven times increased risk of ischaemic heart disease compared with the general population.30 Not all mutations in APOB are associated with FH; for example, patients with the R3531C (Arg3531Cys) mutation have been shown not to have an increased risk of ischaemic heart disease compared with the general population.30
Mutations in PCSK9 are relatively rare, occurring in fewer than 1% of patients with HeFH,31 which makes it difficult to obtain sufficient data to assess the magnitude of the ASCVD risk specifically associated with mutations in this gene.32 More than 20 different mutations have been identified in PCSK9 and all of these have different effects on lipid levels and ASCVD risk.6,33 In a study of 130 patients with FH without mutations in LDLR or APOB, it was found that different mutations in PCSK9 cause variable phenotypes, and that the type and severity of hyperlipidaemia and level of ASCVD risk could vary among individuals from the same family.33 Furthermore, one particular mutation in PCSK9 has been shown to be associated with a very severe phenotype; in a retrospective analysis of 49 patients with FH, over a 30-year follow-up period, individuals carrying the D374Y (Asp374Tyr) PCSK9 mutation were affected by premature CHD more than 10 years earlier than those with severe mutations in LDLR.34
The degree of elevation of LDL-cholesterol is the main factor driving ASCVD risk in patients with FH, and those with LDL-cholesterol levels greater than 10 mmol/L are at particularly high risk.7,25 In addition to LDL-cholesterol, lipoprotein a (Lp[a]) has recently been identified as a possible independent risk factor for ASCVD, both in the general population and in patients with FH. In a cross-sectional analysis of 1960 patients with FH and 957 relatives without FH, patients with FH had higher plasma levels of Lp(a) than their unaffected relatives. In individuals with FH, ASCVD-free survival was significantly lower in patients who had Lp(a) levels above 50 mg/dL than in those with Lp(a) levels below 50 mg/dL.26 Furthermore, in a recent prospective cohort study of 46,200 individuals, patients with FH and high Lp(a) levels had the highest risk of MI; compared with individuals without FH and lipoprotein(a) concentrations of 50 mg/dL or less, hazard ratios (HRs) for MI were 1.4 for those without FH and Lp(a) levels above 50 mg/dL, 3.2 for those with FH and Lp(a) levels of 50 mg/dL or less and 5.3 in those with FH and Lp(a) levels above 50 mg/dL.35
Other ASCVD risk factors that apply to the general population also play a role in ASCVD risk in patients with FH, but their predictive value differs from that of the general population.23 These factors include diabetes mellitus, obesity, hypertension, smoking, renal insufficiency, low high-density lipoprotein (HDL)-cholesterol levels, and a family history of premature ASCVD.26,36–39 A family history of premature ASCVD in a patient with FH probably reflects the autosomal dominant inheritance of the disorder. Thus, it is important to take a family history of premature ASCVD events to gain a full picture of ASCVD risk. Similar to the general population, male sex increases the risk of premature ASCVD in the FH population: men with FH have been shown to develop ASCVD approximately 7 years earlier than women with FH.36,40 This difference in ASCVD risk is probably driven by the cardioprotective effects of oestrogen.41 In addition, high levels of testosterone may be linked to premature ASCVD,42 but the relative contribution of this factor has not been established in patients with FH. Clinical characteristics specific to patients with FH, such as the presence of tendon xanthomas, do not appear to be independently associated with ASCVD risk in individuals with FH.39
Subclinical atherosclerosis in the coronary arteries is an independent risk factor for ASCVD in the general population.43 This is also the case in patients with FH; a recent prospective study of 101 patients with HeFH, in whom 21 MACEs occurred during a median follow-up of 941 days, found that an increased coronary atherosclerotic plaque score was independently associated with coronary events.44 Non-invasive imaging techniques, such as ultrasonography and computed tomography, can be used to determine the extent of subclinical atherosclerosis.45 Use of such tests could help to identify patients with FH and advanced atherosclerosis who may be at high risk of ASCVD.25
Risk calculators, such as the US Framingham risk score and the European SCORE (systematic coronary risk evaluation), are not suitable for those with FH because these patients are at considerably higher risk of ASCVD due to lifelong exposure to elevated plasma LDL-cholesterol levels.6 Evidence of the suitability of existing criteria for assessing the ASCVD risk in patients with FH is limited. Therefore, the International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel has provided a consensus statement, based on expert opinion, that suggests the following criteria to identify patients with severe FH who are at high risk of ASCVD: patients with LDL-cholesterol levels above 10 mmol/L (>400 mg/dL) at diagnosis, or greater than 8 mmol/L (>310 mg/dL) or 5 mmol/L (>190 mg/dL) at diagnosis if another one or two risk factors, respectively, are present (i.e. age >40 years, smoking, male sex, high Lp(a) (>75 nmol/L), low HDL-cholesterol (<1 mmol/L), hypertension, diabetes mellitus, impaired renal function, body mass index (BMI) >30 kg/m2, family history of premature ASCVD). Patients with advanced subclinical atherosclerosis or those who have previously experienced a cardiovascular event should also be considered as having severe FH and being at high risk of ASCVD.25
Underdiagnosis and undertreatment
FH is common among patients presenting with ASCVD.10The European Action on Secondary and Primary Prevention by Intervention to Reduce Events (EUROASPIRE) IV cohort study of 7998 individuals with CHD across 24 European countries found that, among 7044 evaluable patients, 8.3% had potential FH (defined as a score of ≥6 using a modified version of the make early diagnosis to prevent early deaths (MEDPED)/World Health Organization (WHO) criteria and the Dutch Lipid Clinic Network (DLCN) diagnostic criteria).5 Rates of potential FH in patients with CHD varied considerably across European regions, ranging from as low as 3.4% in the Finnish centres to 20.8% in Bosnia and Herzegovina. These large regional differences in FH prevalence may relate to genetic founder effects.46–48 In addition, the types of centres participating in the study in each region may have impacted on the reported FH prevalence.5 Lifestyle factors, such as variations in lipid intake across regions,49 may also lead to misdiagnoses of FH in some countries.
The EUROASPIRE IV study found that FH prevalence in individuals with CHD was inversely related to age; the prevalence of potential FH was eight times greater in patients younger than 50 years than in those older than 70 years.5 This association with age may partly be explained by the fact that patients with FH die earlier resulting in a decline of the prevalence of potential FH by age.5 Furthermore, CHD occurred prematurely in 78% and 73% of men and women with potential FH, respectively, compared with 33% in men and 37% in women without FH.5 Similarly, in a smaller cohort study of 4778 patients with acute coronary syndromes (ACSs), FH prevalence (defined by the Simon Broome Register and DLCN diagnostic criteria) inversely correlated with age of ACS onset.10 These recent studies reflect the results of seminal work from Genest et al. in 1992, who found that more than half of patients with premature CHD had a familial lipoprotein disorder.11
Prevention of ASCVD in individuals with FH is failing, partly because of underdiagnosis in this patient population. Yudi et al. conducted a retrospective analysis of 210 patients admitted to hospital in Australia for premature coronary artery disease (events occurring in male patients aged ≤55 years and female patients aged ≤60 years) in a 12-month period, which found that only 96 patients (46%) had their fasting lipid levels recorded following a hospital admission for premature coronary artery disease.50 Among individuals for whom lipids were measured, three (1%) were found to have probable FH and 50 (24%) had possible FH, as assessed using the DLCN criteria.50 In a Norwegian registry study of 5538 patients with genotype-verified FH, 1411 patients were hospitalised over a 15-year period; ischaemic heart disease was reported in the hospitalisation of 90% of these patients. However, the diagnosis of FH was registered in only 46% of the patients at discharge.51
The underdiagnosis of FH among individuals with ASCVD has led to inadequate administration of therapy to prevent further ASCVD events. In the Australian study by Yudi et al., 23% of individuals with retrospectively diagnosed possible or probable FH were discharged from hospital without LLT.50 Furthermore, data from the EUROASPIRE IV study showed that only 55% of patients with CHD and potential FH received high-intensity statin therapy,5 and data from the Danish study by Benn et al. showed that only 48% of patients with clinically defined FH received LLT.13 Even in patients who are receiving LLT, the therapy may not be sufficient to reduce ASCVD risk adequately; a recent observational study, conducted in Europe, China, Canada, Russia, Africa and the Middle East, which included 54,811 patients receiving statin therapy found that 60.1% of patients with probable FH had CHD, compared with 38.8% of those in the total study population.52 Taken together, the results of these studies suggest that patients with ASCVD are receiving suboptimal treatment. Given that appropriate treatment can reduce the risk of ASCVD, the issues of underdiagnosis and undertreatment of FH and ASCVD require attention.53 Until recently, however, there were few available agents with the potency and tolerability needed to treat FH adequately.
In many patients with FH, the disease only becomes evident after the first major cardiovascular event and cardiologists are, therefore, frequently the first to diagnose a patient with FH.54 A lack of awareness among cardiologists of the relatively high prevalence of FH in patients with ASCVD may account for the low level of FH screening in these individuals. In a survey conducted among American College of Cardiology CardioSurve members in 2011, the majority of whom had over 10 years of experience in cardiovascular clinical practice, most (∼80%) were unaware of the true prevalence of FH. Although more than 95% of cardiologists surveyed agreed that patients with FH are at a moderate/high risk of future ASCVD events, only 10% reported feeling very or extremely confident about their understanding of FH.55 Furthermore, fewer than 30% of cardiologists recognised FH when they were shown a case brought by the National Lipid Association.55 The survey also revealed a lack of understanding of the genetic causes of FH; 60% of cardiologists were unaware of the fact that, given the autosomal dominant mode of inheritance, there is a 50% chance that first-degree relatives of a patient with FH will also have the disorder.55 Increased awareness of FH among cardiologists is required to improve the diagnosis of this condition in patients with ASCVD and to facilitate initiation of appropriate treatment earlier in the disease course. Moreover, increasing the understanding of the genetic basis of FH may support cardiologist-led initiation of screening and ASCVD prevention by referral of a patient’s relatives to primary care physicians or lipid specialists.
Screening
As a result of the prevalence of FH, a systematic approach to screening is warranted. Cascade screening, whereby first, second and third-degree relatives of an established index case are assessed for FH via genetic testing and LDL-cholesterol measurement, has been shown to be a cost-effective approach.56 Targeted screening in selected groups that have a high prevalence of FH, such as patients presenting with premature ASCVD, is an efficient method of identifying new FH index cases.57 Universal screening, in which a population is systematically screened, could be applied to FH via cholesterol measurement or genotyping of children. Although this approach has not been used in FH, universal screening has been successful in detecting other disorders such as phenylketonuria and cystic fibrosis.58 Universal genotyping may not identify patients whose FH is caused by novel mutations or polygenic mutations and may be most effective in populations in which genetic founder effects restrict the number of prevalent mutations.57 Data suggest, however, that it may be prudent to initiate cholesterol screening in children, in whom elevated levels of LDL-cholesterol alone are strongly diagnostic of FH.59 In addition, screening of children has been shown to be an effective method for the diagnosis of affected parents and siblings through a cascade approach.60 Furthermore, identifying FH in childhood enables treatment to be initiated early, which could result in improved long-term outcomes for patients, although research is needed to ascertain the exact age to begin treatment and the long-term safety of LLTs.61
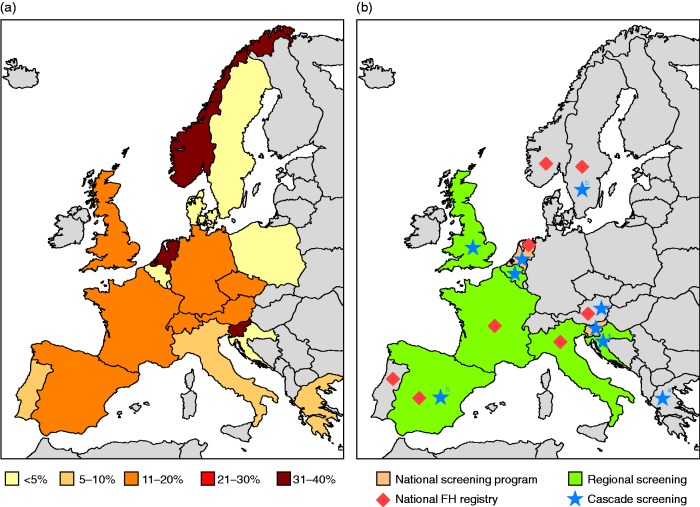
Unfortunately, screening programmes are not conducted on a large scale in most countries. Slovenia initiated universal genetic screening for FH among 5-year old children in 2009.62 A national cascade screening programme has been conducted in the Netherlands,63 and several countries, including Spain, the UK and Norway, have regional screening programmes.64,65 In addition, a pilot screening programme has recently started in Croatia (I Pećin, personal communication). It is important to note that the effectiveness of screening programmes varies and there is disparity in detection levels between countries, ranging from 20% of patients with confirmed FH in Spain to 36% in the Netherlands and 39% in Slovenia.6,62,64 To improve detection levels in Spain, a national cascade screening approach has recently been advocated.66 Further improvements, in both the number of countries with screening programmes and the effectiveness of established screening programmes, are required to tackle the ASCVD burden associated with FH (Figure 1).
Figure 1.
(a) FH diagnosis rates and (b) map of FH current screening programmes, ongoing or planned, across Europe. FH: familial hypercholesterolaemia.
aCascade screening planned.
bLimited cascade screening.
Diagnosis rates were based on the following estimated prevalences: 1/500 Austria, Finland, France, Germany, Italy, Portugal, Spain, UK, Switzerland; 1/300 Belgium, Czech Republic, Netherlands, Norway; 1/250 Greece, Poland, Sweden; 1/200 Denmark and Slovenia.62,64 Please note, because of a lack of data availability on FH prevalence in Austria and Germany a 1/500 rate has been applied for these countries.
Lack of awareness of FH prevalence may contribute to the absence of nationwide screening programmes in many countries. It is also likely that the initial cost of implementing such programmes acts as a barrier to their establishment and use; however, screening for FH has been shown to be cost-effective. In an Australian study it was estimated that genetic cascade screening for FH would reduce the 10-year incidence of CHD from 50% to 25% among people with FH, leading to a gain of 29 quality-adjusted life-years for every 100 individuals screened.67 Understanding the long-term benefits in terms of quality of life and healthcare resource utilisation associated with early diagnosis of FH and prevention of ASCVD may act as an incentive for screening programmes to be initiated. Further support for genetic cascade screening in FH recently came from a study analysing FH severity in patients diagnosed with FH as part of the Netherlands cascade screening programme. This study showed that the deleterious effect of FH, both in terms of LDL-cholesterol levels and ASCVD risk is the same in people distantly related to the index patient compared with those who are more closely related, suggesting that FH severity is mainly determined by the underlying mutation.68
Electronic screening of patient medical records may be a further cost-effective approach to increase the rate of diagnosis of FH in primary care.69,70 For example, Troeung et al. retrospectively screened the primary care medical records of 3708 patients using the TARB-Ex electronic screening tool, which extracts routine clinical information from electronic medical records to derive a DLCN criteria score and identify patients at risk of FH, who may therefore require clinical investigation.69 The records of patients with potential FH (DLCN score ≥5) identified by TARB-Ex were then reviewed by a primary care physician and a lipid specialist; patients subsequently considered to be at high risk of FH were recalled for clinical assessment. The TARB-Ex identified 32 patients at risk of FH compared with 22 identified by a physician-led manual review of medical records, which was considered the ‘gold standard’ for FH screening in this study. Sensitivity was 95.5%, specificity was 96.7%, negative predictive accuracy was 99.7% and positive predictive accuracy was 65.6%. Ten patients were recalled for clinical examination, seven of whom attended. Six of these patients were diagnosed with phenotypic FH according to clinical criteria and one patient was referred for FH genetic testing. Electronic screening with TARB-Ex was completed in 10 minutes, compared with 60 hours for manual record review.
International and national patient registries collect data on individuals with FH in a systematic and standardised manner. This information can be useful for understanding the epidemiology of the disorder and risk factors associated with the development of ASCVD, as well as for recruiting for clinical trials, improving healthcare services, facilitating patient education and identifying gaps in knowledge.71 Through cascade screening, such registries also support the cost-effective identification of additional patients with FH.71,72 Examples of FH registries include international registries such as the European Atherosclerosis Society (EAS)-FH Studies Collaboration, the 10 Countries Project and the ScreenPro FH programme, the HoFH International Clinical Collaborators (HICC) registry, and national registries such as the DLCN, Spanish FH Foundation, Lipid TransPort Disorders Italian Genetic Network (LIPIGEN, Italy), CASCADE FH Registry (USA), SWEDEHEART (Sweden), the Czech MEDPED database and the Portuguese FH Study.64,73–75 Several countries, including Austria, Greece and Poland, have recently established new national FH registries, with the aim of increasing awareness of FH and stimulating the initiation of nationwide screening programmes.64 Furthermore, the international EAS-FH Studies Collaboration (FHSC), which aims to disseminate information on the detection and management of FH, is a first step towards creating a global consensus on best practice in FH diagnosis and treatment.64
Diagnosis
For screening programmes to be effective, physicians need to be aware of the diagnostic criteria for FH. Several clinical criteria algorithms are used to diagnose FH (Table 1). The DLCN criteria are widely accepted and can be used to estimate the likelihood of FH.76 The Simon Broome Register diagnostic criteria18 and the MEDPED/WHO criteria77,78 are also used. In routine clinical practice, however, some data needed for the diagnostic algorithms may be inaccurate or incomplete (e.g. detailed family history of ASCVD, xanthomas).79 It is important to note that diagnostic criteria are likely to differ by geographical region because certain clinical presentations of FH vary across different patient populations; for example, xanthelasmas have been shown to occur in 32% of patients with FH in Finland compared with 8% of patients in Norway.80 In addition, with the increased use of LLTs among the general population, some characteristics of FH may be masked preventing diagnosis of FH in the assessed individual as well as in affected family members. In recognition of this issue, Haralambos et al. recently developed modified FH diagnostic criteria based on the DLCN criteria that additionally provide a LDL-cholesterol correction factor to estimate pretreatment LDL-cholesterol levels in patients receiving LLT.80 It goes without saying that secondary causes of hypercholesterolaemia, such as nephrotic syndrome, hypothyroidism, diabetes mellitus, or medication, must be excluded prior to applying any of the above algorithms.1,81
Table 1.
FH diagnostic criteria.
| (a) Dutch Lipid Clinic Network diagnostic criteria | |||||
|---|---|---|---|---|---|
| Criteria |
Points | ||||
| Family history | |||||
| First-degree relative with prematurea ASCVD OR | 1 | ||||
| First-degree relative with LDL-C ≥ 95th percentile for age and sex | 1 | ||||
| First-degree relative with tendon xanthomas and/or arcus cornealis OR | 2 | ||||
| Children ≤18 years old with LDL-C ≥ 95th percentile for age and sex | 2 | ||||
| Clinical history | |||||
| Patient with premature ASCVD | 2 | ||||
| Patient with prematurea cerebral or peripheral vascular disease | 1 | ||||
| Physical examination | |||||
| Tendinous xanthomas | 6 | ||||
| Arcus cornealis in patients ≤45 years old | 4 | ||||
| LDL-C level, mmol/L (mg/dL) | |||||
| ≥8.5 (330) | 8 | ||||
| 6.5–8.4 (250–329) | 5 | ||||
| 5.0–6.4 (190–249) | 3 | ||||
| 4.0–4.9 (155–189) | 1 | ||||
| DNA analysis | |||||
| Functional mutation in LDLR, APOB or PCSK9 gene | 8 | ||||
| Diagnosis (point total): definite FH, >8 points; probable FH, 6–8 points; possible FH, 3–5 points, unlikely FH, <3 points (b) Simon Broome Register diagnostic criteria | |||||
| Diagnosis of definite FH Functional mutation in LDLR, APOB or PCSK9 gene OR Adult: cholesterol >7.5 mmol/dL or LDL-C >4.9 mmol/dL Child:b cholesterol >6.7 mmol/dL or LDL-C >4.0 mmol/dL PLUS Tendon xanthomas in patient of first- or second-degree relative | |||||
| Diagnosis of probable FH Adult: cholesterol >7.5 mmol/dL or LDL-C >4.9 mmol/dL Child:b cholesterol >6.7 mmol/dL or LDL-C >4.0 mmol/dL PLUS Family history of ASCVD: <60 years of age in a first-degree relative or <50 years of age in a second-degree relative OR Family history of raised total cholesterol level: >7.5 mmol/dL in an adult first- or second-degree relative or >6.7 mmol/dL in a childb or sibling | |||||
| (c) MEDPED diagnostic criteria | |||||
| Total cholesterol cut-off points, mmol/dL |
|||||
| Age (years) |
First-degree relative with FH |
Second-degree relative with FH |
Third-degree relative with FH |
General population | |
| <20 | 5.7 | 5.9 | 6.2 | 7.0 | |
| 20–29 | 6.2 | 6.5 | 6.7 | 7.5 | |
| 30–39 | 7.0 | 7.2 | 7.5 | 8.8 | |
| ≥40 | 7.5 | 7.8 | 8.0 | 9.3 | |
| Diagnosis is made if total cholesterol levels exceed cut-off points | |||||
APOB: apolipoprotein B gene; ASCVD: atherosclerotic cardiovascular disease; FH: familial hypercholesterolaemia; LDL-C: low-density lipoprotein cholesterol; LDLR: low-density lipoprotein receptor gene; PCSK9: proprotein convertase subtilisin/kexin type 9 gene.
Premature ASCVD, cerebral or peripheral vascular disease defined as occurring in males aged <55 years and in women aged <60 years.
<16 years of age.
Simplified diagnostic criteria would facilitate the identification of patients with FH. The recent publication of the familial hypercholesterolaemia ascertainment tool (FAMCAT), which uses data collected from primary care records, including total cholesterol levels and family history of FH, may help clinicians to identify patients with a high probability of having FH. To facilitate routine identification of patients with FH, work is underway to integrate the FAMCAT algorithm into the UK primary care computer systems, supported by a user-friendly interface.82
In addition, using clinical and genetic data from 64,106 patients who were screened for FH in the Dutch FH screening programme, Besseling et al. developed a model to predict the presence of a FH-causing mutation based on factors routinely collected in clinical practice, including: age; sex; levels of LDL-cholesterol, HDL-cholesterol and triglycerides; history of CVD and age at onset; use of statins; smoking; alcohol; and presence of hypertension. Validation of the model in a separate patient cohort confirmed that the model showed good discrimination of patients at risk of FH. The model will be available as an online calculator to aid physicians in deciding whether or not to refer patients for genetic testing.70
Genomic tests to identify pathogenic mutations in LDLR, APOB and PCSK9 are also available and may be considered the ‘gold standard’ for FH diagnosis; however, their use varies widely between countries, probably owing to issues of cost and availability.6 Therefore, in clinical practice, FH is most commonly diagnosed by clinical examination and laboratory tests, because a high LDL-cholesterol level is the main clinical factor contributing to an increased ASCVD risk, and should be treated regardless of the results of mutational analysis. Genetic testing may also have an impact on issues related to life insurance reimbursement and access to treatment.83 In the Netherlands, issues of genetic discrimination have been circumvented by the implementation of guidelines to protect patients with FH under the Medical Examination Act (1998).84,85 When setting up screening programmes, countries may also need to consider introducing relevant guidelines or laws to mitigate the potential for such genetic discrimination.
Lipid targets for patients with FH
The aim of FH treatment is to reduce LDL-cholesterol levels to prevent ASCVD. The 2016 joint European Society of Cardiology and EAS guidelines recommend target LDL-cholesterol levels of less than 3.5 mmol/L (<135 mg/dL) in children with FH over 10 years of age, a 50% reduction of LDL-cholesterol at younger ages, less than 2.6 mmol/L (<100 mg/dL) in adults with FH, or less than 1.8 mmol/L (<70 mg/dL) in adults with FH in the presence of ASCVD.86 LDL-cholesterol targets apply for both HeFH and HoFH; however, with current treatment options, these are very difficult to achieve in children and adults with HoFH.6
Treatment options
Diet and lifestyle modifications
Diet and lifestyle have an effect on LDL-cholesterol levels and ASCVD risk.87,88 Patients with FH should be counselled regarding lifestyle modifications to reduce fat and cholesterol intake, to avoid tobacco products, and to balance physical activity with caloric intake to maintain a healthy BMI.1 It is important to note that, although FH cannot be managed by diet and lifestyle changes alone, healthy lifestyle modifications should be used in conjunction with optimised LLT in order achieve LDL-cholesterol targets and minimise ASCVD risk.89
Statins and other LLTs
Statins are the cornerstone of treatment for patients with FH; however, target LDL-cholesterol levels are not reached in a large proportion of patients, despite the use of statins and additional LLT.90 Statins lower LDL-cholesterol by inhibiting 3-hydroxy-3-methylglutaryl-coenzyme A reductase, which results in upregulation of the LDLR.91 The 2013 EAS consensus statement recommends that patients with FH receive high-dose statin treatment (atorvastatin 80 mg/day, rosuvastatin 40 mg/day or pitavastatin 4 mg/day) at diagnosis.6 The efficacy of statins in reducing morbidity and improving survival rates in patients with FH has been demonstrated in retrospective and cohort studies. In a retrospective study, statins were shown to reduce the risk of CHD by 76% in patients with FH who received statins before the onset of CHD.53 In addition, the results of a cohort study of 3382 patients with FH indicated that treatment with statins resulted in a 37% reduction in CHD mortality.92 There is, however, a lack of data from randomised placebo controlled trials on the clinical benefit of statins in patients with FH. Given the high risk of ASCVD in these patients, such trials are not ethically justifiable. However, the randomised placebo controlled Lipid Research Clinics Coronary Primary Prevention Trial, which studied a patient population that was likely to be enriched for individuals with FH, may provide some insights into the clinical benefit of statins. The study showed that patients receiving the statin cholestryramine had a 12% reduction in LDL-cholesterol and a 19% reduction in CHD risk compared with placebo-treated patients.93 In addition, a real-world retrospective analysis of 2447 patients with HeFH found that moderate to high-intensity statin therapy lowered the risk of ASCVD and death by 44% compared with the risk in patients who had never received statins.12
Despite the administration of high-dose potent statins, nearly 80% of patients with HeFH do not achieve target LDL-cholesterol levels.90 These individuals require additional agents to enable them to reach these targets. Statin intolerance may contribute to an insufficient LDL-cholesterol response to therapy. There is, however, a lack of data on the incidence of statin intolerance and it is likely that non-adherence to statin treatment is the main cause of suboptimal outcomes in patients prescribed statins.94,95
Ezetimibe decreases cholesterol absorption at the brush border of the small intestine by inhibiting Niemann–Pick C1-like protein (NPC1L1)96 and can be used in combination with statin therapy when LDL-cholesterol targets have not been met with statin monotherapy.6,86 Ezetimibe is useful in the management of patients with HoFH because the mechanism of action does not rely on LDLR expression.96 The clinical efficacy of ezetimibe was questioned following results from the Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial, which showed that the addition of ezetimibe to simvastatin led to a reduction in LDL-cholesterol, but not to a reduction in carotid intima–media thickness.97 Recent data from the IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT), however, suggest that the further reduction in cholesterol levels in patients receiving ezetimibe provides clinical benefit, in terms of reducing both LDL-cholesterol levels and cardiovascular events.98 Ezetimibe can also be prescribed as monotherapy to individuals who are intolerant to statins.99,100
Bile-acid-binding resins (cholestyramine, colestipol or colesevelam) decrease the absorption of bile acid, resulting in increased conversion of cholesterol to bile acids and enhanced production of LDLR.96 For patients with FH and established ASCVD, combination therapy with a statin, ezetimibe and a bile-acid-binding resin is recommended.8,86
Statins are less effective in patients with HoFH than in those with HeFH due to the severely decreased LDLR function in HoFH patients.101 The therapy of choice for these patients is LDL apheresis in combination with high-intensity statin treatment, with or without ezetimibe.6,7 LDL apheresis should also be considered in patients with HeFH who are intolerant to statins or in whom LDL-cholesterol levels and ASCVD risk remain high following maximally tolerated LLT.102 Although apheresis lowers LDL-cholesterol levels by 55–70%, the effect is transient and levels rebound to pre-apheresis concentrations within a few days.103 In addition, apheresis is expensive and time-consuming to administer, and therefore is not widely available.103
Lomitapide is a new LLT that inhibits the microsomal triglyceride transfer protein, which functions in the production of LDL. Phase 3 data from a single-arm study in 29 patients with HoFH showed that, at week 26, the addition of lomitapide to current LLT reduced LDL-cholesterol levels by 50% from baseline.104
Mipomersen is an antisense oligonucleotide that inhibits APOB protein synthesis. In phase 3 trials, compared with placebo, the addition of mipomersen to maximally tolerated statin therapy (with or without other LLTs) has been shown to reduce LDL-cholesterol levels significantly in patients with HeFH and CHD,105 and in patients with HoFH.106 Mipomersen is approved in the USA in combination with LLTs for the treatment of patients with HoFH.107 Approval has not been granted in Europe as a result of hepatic and cardiac safety concerns.108
Fibrates are agonists of peroxisome proliferator-activated receptor-α, which via regulation of transcription factors regulate various steps in lipid and lipoprotein metabolism.86 In combination with the maximum tolerated dose of statins, fibrates can be considered in patients with FH who have elevated triglycerides and low HDL-cholesterol, or in those with serum triglyceride levels greater than 5.7 mmol/L (>500 mg/dL).6 However, it must be noted that the lipid-lowering effect of fibrates is minimal, and with more potent therapies now available, they are unlikely to be a standard treatment choice for patients with FH.
Overall, current data suggest that the treatment of patients with FH is often not optimal. In a population-based cohort study, individuals with FH who were not treated with LLT had a 13-fold increased risk of CHD compared with those without FH. This risk was reduced, to 10-fold, when LLTs were used.13 This highlights the need to monitor response to therapy and to ensure that patients with FH are receiving treatment of sufficient intensity. A 2-year prospective study of 325 patients with FH showed that high-intensity statin therapy (high-dose atorvastatin) was more effective at reducing LDL-cholesterol levels and decreasing carotid intima–media thickness than lower-intensity statin therapy (simvastatin).109 Furthermore, pharmacokinetic alterations have been reported when statins are co-administered with drugs metabolised through the cytochrome P450, 3A4 or 2C9 pathways (through which most statins are metabolised). These changes could lead to reduced efficacy and an increase in adverse events (AEs), making statins unsuitable for patients who require certain concomitant medications.110,111 Novel treatments are required to prevent cardiovascular events effectively in patients with FH, particularly in individuals with established ASCVD or homozygous mutations, and in those who are intolerant to statin therapy.
PCSK9 inhibitors
Monoclonal antibodies that inhibit PCSK9 are a promising treatment modality for patients with FH.112 PCSK9 is a serine protease secreted by hepatocytes that binds to the LDLR and promotes its degradation.113 Monoclonal antibodies to PCSK9 prevent its interaction with the LDLR and thereby restore LDLR recycling and LDL-cholesterol uptake.114 A hint towards a beneficial effect of PCSK9 inhibition was derived from studies in patients carrying non-sense mutations in PCSK9 that were found to be associated with low LDL-cholesterol levels and a reduced risk of CHD.115 Two antibodies to PCSK9, evolocumab and alirocumab, were approved in 2015 in the USA, Canada and Europe for the treatment of patients with FH in whom target LDL-cholesterol levels are not achieved with available therapies.116–120 Recently, the global clinical development programme for a third PCSK9 antibody, bococizumab, was discontinued because of an unanticipated attenuation of efficacy over time associated with higher immunogenicity and a higher rate of injection-site reactions than seen with other agents in this class.121 RNA interference inhibition of proprotein convertase subtilisin/kexin type 9 (PCSK9) is also being investigated.122 ALN-PCSsc is a first-in-class RNA interference that acts by switching off PCSK9 synthesis in the liver.122 Promising efficacy and safety results have been reported in a phase 1 study in healthy volunteers and in a recent phase 2 study in patients at high risk of ASCVD who had elevated LDL-cholesterol levels;123,124 however, phase 3 data in patients with FH are required to evaluate the role of this therapy in FH.
Evolocumab and alirocumab have shown efficacy in randomised controlled trials in reducing LDL-cholesterol levels in patients with FH at high risk of developing ASCVD. Evolocumab has been evaluated in a broad patient population, including individuals whose LDL-cholesterol levels were not controlled by statin therapy, patients with HoFH and those with severe atherosclerosis. In the Reduction of LDL-cholesterol with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) phase 3 trial, 331 patients with HeFH whose LDL-cholesterol levels were not adequately controlled with LLT received evolocumab (140 mg every 2 weeks or 420 mg monthly) in addition to current therapy. Following 12 weeks of treatment, LDL-cholesterol levels were reduced by 60% in patients receiving evolocumab. These patients also experienced a 30% reduction in Lp(a) levels compared with those receiving placebo.125 This might have a large clinical impact because elevated Lp(a) is an independent risk factor for ASCVD in patients with FH.26,35 Evolocumab has also shown efficacy in patients with HoFH. In the phase 3 Trial Evaluating PCSK9 Antibody in Subjects with LDL Receptor Abnormalities (TESLA) study involving 50 patients who received LLT but did not undergo apheresis, 12 weeks of treatment with evolocumab 420 mg monthly led to a significant mean LDL-cholesterol reduction of 31% compared with placebo.126
Alirocumab has shown efficacy in reducing LDL-cholesterol and Lp(a) levels in patients with HeFH. In the multicentre, placebo controlled randomised phase 3 trials ODYSSEY FH I and II, 735 patients with HeFH, with or without a history of ASCVD, whose LDL-cholesterol levels were not controlled by the maximum tolerated dose of statins, were randomly allocated to receive alirocumab 75 mg every 2 weeks, increasing to 150 mg every 2 weeks if LDL-cholesterol levels remained above 70 mg/dL, or placebo. After 24 weeks, alirocumab treatment led to a 49% reduction in LDL-cholesterol levels, with 40% of patients requiring the 150 mg dose.127
PCSK9 inhibitors have also shown promising results in treating patients with FH whose LDL-cholesterol levels are difficult to control and for whom regular LDL-cholesterol apheresis is required. In a small study of three patients with FH and CHD, switching from LDL-cholesterol apheresis to evolocumab maintained LDL-cholesterol lowering.128 After apheresis, HDL-cholesterol levels increased and remained constant on evolocumab treatment. Evolocumab was also associated with a non-significant trend towards improved patient quality of life.128 The ODYSSEY ESCAPE study assessed the efficacy of alirocumab in 62 patients with severe HeFH who had been receiving regular LDL-cholesterol apheresis for a mean of 7 years; 46% of patient receiving alirocumab and 62% of those receiving placebo were also receiving a statin therapy. Treatment with alirocumab 150 mg every 2 weeks significantly reduced the frequency of required apheresis treatments by 75% (P < 0.0001) from week 7 to week 18. Apheresis was no longer required in 63% of patients receiving alirocumab compared with 0% of patients receiving placebo.129
As well as a strong efficacy profile, PCSK9 inhibitors have been shown to be well tolerated. In the phase 3 RUTHERFORD trial, the rates of AEs with evolocumab were similar to those seen with placebo. The most common AEs in patients receiving evolocumab were nasopharyngitis and muscle-related AEs, occurring in 9% and 5% of patients, respectively, compared with 5% and 1% of those receiving placebo, respectively.125 Injection-site reactions occurred at similar frequencies in patients receiving evolocumab (6%) and in those receiving placebo (4%). No patients discontinued treatment because of an AE.125 As with evolocumab, similar AEs were associated with alirocumab. In the phase 3 ODYSSEY FH I and II trials, the most common AEs were injection-site reactions and nasopharyngitis. Injection-site reactions occurred in 12% and 11% of patients receiving alirocumab in ODYSSEY FH I and FH II, respectively, compared with 11% and 7% of those receiving placebo, respectively. Nasopharyngitis occurred in 11% and 13% of those receiving alirocumab in FH I and FH II, respectively, compared with 7% and 22% of those receiving placebo. Few patients discontinued treatment because of AEs.127 A recent meta-analysis on the long-term safety of PCSK9 inhibitors suggested that PCSK9 inhibitors are not associated with an increased risk of cumulative severe AEs, musculoskeletal effects or stroke compared with standard of care.130 A subgroup analysis of larger outcome studies found a two-fold increase in the incidence of neurocognitive events with PCSK9 inhibitors compared with standard of care.130 However, the results of the non-inferiority EBBINGHAUS cognitive function study, conducted in 1900 patients enrolled in the Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) study, found that evolocumab did not increase the risk of impairment of cognitive function compared with placebo.131
PCSK9 inhibitors have been shown to reduce LDL-cholesterol levels, but their impact on long-term disease progression and clinical outcomes is less well established. The recent Global Assessment of Plaque Regression with a PCSK9 Antibody as Measured by Intravascular Ultrasound (GLAGOV) study of 968 patients with angiographic coronary disease assessed the impact of PCSK9 inhibition on the progression of coronary atherosclerosis. After 78 weeks, compared with patients receiving statins and placebo, those receiving evolocumab and statins had a significantly greater percentage reduction in atheroma volume (−0.95% vs. 0.05%; P < 0.0001) and absolute atheroma volume (−5.8 mm3 vs. −0.9 mm3; P < 0.001).132 Although this study was not conducted in patients with FH, these data suggest that the addition of evolocumab to statin therapy could lead to significant regression of atherosclerotic plaques. This could be beneficial for individuals with FH and ASCVD, if the results are replicated in this patient population.
Preliminary long-term efficacy data on PCSK9 inhibitors preventing MACEs are also encouraging. In two open-label randomised trials of evolocumab (OSLER-1 and OSLER-2), 4465 patients, of whom 10% had FH, received standard therapy or evolocumab (140 mg every 2 weeks or 420 mg monthly) plus standard therapy. At 1 year, patients receiving evolocumab had a significant reduction in the rate of MACEs compared with individuals receiving standard therapy alone (0.95% vs. 2.18%; P = 0.003).133 Similarly, in the phase 3 ODYSSEY Long Term trial of alirocumab, 2341 patients at high risk of ASCVD, of whom 18% had HeFH, received alirocumab 150 mg every 2 weeks for 78 weeks.134 In a post hoc analysis, the rate of MACEs was lower for patients receiving alirocumab than for those receiving placebo (1.7% vs. 3.3%; P = 0.02).
It should be noted, however, that in OSLER-1 and OSLER-2 and ODYSSEY Long Term, the MACE event numbers were very low in both the treatment and control groups, and a longer follow-up period is required to confirm the long-term impact of PCSK9 inhibitors on the rate of MACEs. The results of such prospective interim analyses give support to, but are not proof of, the efficacy of PCSK9 inhibitors in the prevention of MACEs. Recently, the FOURIER trial reported that at a median follow-up of 2.2 years additional LDL-cholesterol lowering with evolocumab (in combination with optimised statin therapy) significantly reduced the risk of cardiovascular events in patients with ASCVD and high LDL-cholesterol levels (≥1.8 mmol/L) by 15% compared with placebo (HR 0.85; P < 0.001).135 The ODYSSEY OUTCOMES trial assessing the extent to which alirocumab reduces the risk of MACEs is ongoing.136,137 In the phase 3 SPIRE-2 trial (median follow-up, 12 months) the now discontinued PCSK9 inhibitor bococizumab significantly reduced MACEs in patients with a high risk of MACEs (LDL-cholesterol ≥2.6 mmol/L) compared with placebo (HR 0.79; P = 0.02). However, in the SPIRE-1 trial (median follow-up, 7 months) bococizumab provided no benefit compared with placebo in patients at low risk of MACEs (LDL-cholesterol level ≥1.8 mmol/L; HR 0.99; P = 0.94).138
As with all new treatments, the benefit of PCSK9 inhibitors in patients with FH must be considered in relation to their cost. A recent cost-effectiveness analysis conducted in the USA determined that at their 2015 prices, PCSK9 inhibitors did not meet incremental cost-effectiveness thresholds.139 However, it must be noted that the pricing structure and the cost-effectiveness model used in that analysis apply specifically to the USA and are not applicable to other regions. Indeed, in Europe the National Institute for Health and Care Excellence has determined that both evolocumab and alirocumab have favourable incremental cost-effectiveness ratios in patients with HeFH.140,141
Treatment: when to use PCSK9 inhibitors
Patients with FH and ASCVD, or another major risk factor for ASCVD such as diabetes mellitus with target organ damage or hypertension, or those with severe HeFH should receive statins (preferably atorvastatin or rosuvastatin) at the maximally tolerated dose plus ezetimibe.142 Patients who have a less than anticipated response on maximally tolerated statin therapy (<50% reduction in LDL-cholesterol) should be assessed for adherence by evaluating the number of missed statin doses per month and any barriers to adherence. Patients who are unable to tolerate even a moderate-intensity statin should be evaluated for statin intolerance and considered for referral to a lipid specialist.143 Physicians should consider adding a PCSK9 inhibitor to the regimen for patients with ASCVD, or a major risk factor for ASCVD, if LDL-cholesterol levels are more than 3.6 mmol/L (>140 mg/dL) or more than 2.6 mmol/L (>100 mg/dL) with evidence of rapid progression of ASCVD. Patients with severe HeFH without ASCVD should be considered for PCSK9 inhibition therapy if LDL-cholesterol levels are more than 5.0 mmol/L (>200 mg/dL) or more than 4.5 mmol/L (>175 mg/dL) in the presence of one or more risk factors for ASCVD including diabetes mellitus, elevated lipoprotein levels (>50 mg/L), hypertension and premature familial ASCVD. Most patients with HoFH should receive maximal LLT including LDL apheresis plus a PCSK9 inhibitor. However, it should be noted that patients with a homozygous null mutation in LDLR should not receive a PCSK9 inhibitor.142 Treatment options for patients with HeFH and HoFH are presented in Table 2.
Table 2.
Summary of treatment options for patients with FH.
| Treatment | Mechanism of action | HeFH | HoFH |
|---|---|---|---|
| Statins (atorvastatin, fluvastatin, pravastatin, rosuvastatin, simvastatin) | Upregulate LDLR through inhibition of HMG-CoA reductase | A first-line treatment option for patients with HeFH. Patients should receive up to the maximum approved/tolerated dose in order to lower LDL-C levels6 | A first-line treatment option for patients with HoFH. Patients should receive up to the maximum approved/tolerated dose in order to lower LDL-C levels6 |
| Ezetimibe | Inhibits cholesterol absorption in the small intestine | Can be administered in combination with statins for patients not reaching LDL-C levels6 or as a single agent for those who are intolerant to statins99 | Can be administered in combination with statins for patients not reaching LDL-C levels6 or as a single agent for patients who are intolerant to statins99 |
| Bile acid sequestrants (colesevelam, colestipol, colestyramine) | Bind bile components in the gastrointestinal tract leading to increased production of bile, which requires LDL | Can be administered in combination with statins and ezetimibe for patients with a very high risk of CHD/established CHD/type 2 diabetes mellitus/LDL-C levels >1.8 mmol/dL or ∼70 mg/dL6 | Can be administered in combination with statins and ezetimibe for patients with a very high risk of CHD/established CHD/type 2 diabetes mellitus/LDL-C levels >1.8 mmol/dL or ∼70 mg/dL6 |
| Fibrates (bezafibrate, ciprofibrate, fenofibrate, gemfibrozil) | Increase lipid catabolism through activation of peroxisome proliferator activated receptors | Can be administered in combination with other LLTs after first-line therapy has failed; however, combination with statins increases risk of myopathy. Fibrates are not recommended in patients without elevated triglyceride levels144 | Can be administered in combination with other LLTs after first-line therapy has failed; however, combination with statins increases risk of myopathy. Fibrates are not recommended in patients without elevated triglyceride levels144 |
| Apheresis | Physical removal of LDL from the blood | Can be administered in treatment-resistant patients with CHD6 | A first-line treatment option for patients with HoFH. Apheresis should be initiated as early as possible following diagnosis. Treat every 1–2 weeks7 |
| Lomitapide | Inhibits VLDL assembly | X | Recommended in combination with other LLTs, with or without apheresis7 |
| Mipomersen | Inhibits synthesis of apolipoprotein B-100 in the liver | X | Recommended in combination with other LLTs in the USA.107 Not currently approved in Europe108 |
| PCSK9 inhibitors (evolocumab, alirocumab) | Block LDLR degradation | Indicated in adults in combination with a statin or statin with other LLTs in patients unable to reach target LDL-C levels with the maximum tolerated statin dose, or alone or in combination with other LLTs in patients who are statin intolerant, or for whom a statin is contraindicated116,117 | Evolocumab is indicated in adults and adolescents aged 12 years and over in combination with other LLTs.116 Alirocumab is not indicated in patients with HoFH117 |
CHD: coronary heart disease; FH: familial hypercholesterolaemia; HeFH: heterozygous familial hypercholesterolaemia; HMG-CoA: 3-hydroxy-3-methylglutaryl-coenzyme A; HoFH: homozygous familial hypercholesterolaemia; LDL: low-density lipoprotein; LDL-C: low-density lipoprotein cholesterol; LDLR: low-density lipoprotein receptor; LLTs, lipid-lowering therapies; PCSK9: proprotein convertase subtilisin/kexin type 9; VLDL: very low-density lipoprotein cholesterol.
Conclusions
FH is common in patients presenting with cardiovascular events, particularly when the events occur at an early age. Screening for FH in individuals with ASCVD is currently inadequate, leading to a missed opportunity to initiate preventive therapies and to reduce the morbidity and mortality associated with FH. Primary care physicians are at the front line of FH screening and thus need to be informed about the prevalence of FH and how to diagnose the disorder so that interventional treatments can be administered before the onset of ASCVD. Cardiologists are likely to encounter a large proportion of patients with FH and with increased awareness and appropriate support, they can make a substantial positive impact on outcomes in these individuals. Ultimately, the care of people with FH requires a multi-disciplinary approach involving primary care physicians, lipid specialists, cardiologists, nutritionists, nurses, pharmacists and patient support groups. Statin therapy reduces LDL-cholesterol levels and ASCVD risk, and treatment with high-dose statins should be initiated in patients with FH. For individuals at highest risk of developing ASCVD, additional therapies are required to control their disease adequately. Furthermore, data suggest that patients with potential FH should also be treated because the associated raised LDL-cholesterol levels substantially increase the risk of ASCVD.5 New PCSK9 inhibitors offer an effective and well tolerated treatment option for patients with FH.
Acknowledgements
The authors would like to thank Kelly Soady of Oxford PharmaGenesis, Oxford, UK for medical writing support. Editorial support was provided by Carine Thual of Amgen (Europe) GmbH.
Author contribution
Ivan Pećin, Gerard Kees Hovingh, Ricardo Dent and Željko Reiner contributed to the conception and design of the manuscript. All authors contributed to the acquisition and interpretation of data, critically reviewed the manuscript, provided their final approval and agree to be accountable for all aspects of the work.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Ivan Pećin received speaker’s fees from Genzyme, Servier and Sanofi. Gerard Kees Hovingh or his institution received honoraria for consultancy, advisory boards, and/or conduct of clinical trials from Amgen, Aegerion, Pfizer, Astra Zeneca, Sanofi, Regeneron, Kowa, Ionis pharmaceuticals and Cerenis. He has received research support from Aegerion, Amgen, Sanofi, Astra Zeneca and Synageva. Ricardo Dent was an employee of Amgen at time of this work and owns stocks in Amgen and Esperion Therapeutics. Željko Reiner and Merel L Hartgers report no relationships that could be construed as a conflict of interest.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for medical writing support for this manuscript was provided by Amgen (Europe) GmbH.
References
- 1.Goldberg AC, Hopkins PN, Toth PP, et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol 2011; 5: S1–S8. [DOI] [PubMed] [Google Scholar]
- 2.Fouchier SW, Dallinga-Thie GM, Meijers JC, et al. Mutations in STAP1 are associated with autosomal dominant hypercholesterolemia. Circ Res 2014; 115: 552–555. [DOI] [PubMed] [Google Scholar]
- 3.Slack J. Risks of ischaemic heart-disease in familial hyperlipoproteinaemic states. Lancet 1969; 2: 1380–1382. [DOI] [PubMed] [Google Scholar]
- 4.Barkas F, Elisaf M, Milionis H. Statins decrease the risk of stroke in individuals with heterozygous familial hypercholesterolemia: a systematic review and meta-analysis. Atherosclerosis 2015; 243: 60–64. [DOI] [PubMed] [Google Scholar]
- 5.De Backer G, Besseling J, Chapman J, et al. Prevalence and management of familial hypercholesterolaemia in coronary patients: an analysis of EUROASPIRE IV, a study of the European Society of Cardiology. Atherosclerosis 2015; 241: 169–175. [DOI] [PubMed] [Google Scholar]
- 6.Nordestgaard BG, Chapman MJ, Humphries SE, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J 2013; 34: 3478–3490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuchel M, Bruckert E, Ginsberg HN, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J 2014; 35: 2146–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiner Z. A comparison of European and US guidelines for familial hypercholesterolaemia. Curr Opin Lipidol 2015; 26: 215–220. [DOI] [PubMed] [Google Scholar]
- 9.Gidding SS, Ann Champagne M, de Ferranti SD, et al. The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation 2015; 132: 2167–2192. [DOI] [PubMed] [Google Scholar]
- 10.Nanchen D, Gencer B, Auer R, et al. Prevalence and management of familial hypercholesterolaemia in patients with acute coronary syndromes. Eur Heart J 2015; 36: 2438–2445. [DOI] [PubMed] [Google Scholar]
- 11.Genest JJ, Jr., Martin-Munley SS, McNamara JR, et al. Familial lipoprotein disorders in patients with premature coronary artery disease. Circulation 1992; 85: 2025–2033. [DOI] [PubMed] [Google Scholar]
- 12.Besseling J, Hovingh GK, Huijgen R, et al. Statins in familial hypercholesterolemia: consequences for coronary artery disease and all-cause mortality. J Am Coll Cardiol 2016; 68: 252–260. [DOI] [PubMed] [Google Scholar]
- 13.Benn M, Watts GF, Tybjaerg-Hansen A, et al. Familial hypercholesterolemia in the Danish general population: prevalence, coronary artery disease, and cholesterol-lowering medication. J Clin Endocrinol Metab 2012; 97: 3956–3964. [DOI] [PubMed] [Google Scholar]
- 14.Do R, Stitziel NO, Won HH, et al. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature 2015; 518: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huijgen R, Kindt I, Defesche JC, et al. Cardiovascular risk in relation to functionality of sequence variants in the gene coding for the low-density lipoprotein receptor: a study among 29,365 individuals tested for 64 specific low-density lipoprotein-receptor sequence variants. Eur Heart J 2012; 33: 2325–2330. [DOI] [PubMed] [Google Scholar]
- 16.Wong B, Kruse G, Kutikova L, et al. Cardiovascular disease risk associated with familial hypercholesterolemia: a systematic review of the literature. Clin Ther 2016; 38: 1696–1709. [DOI] [PubMed] [Google Scholar]
- 17.Perak AM, Ning H, de Ferranti SD, et al. Long-term risk of atherosclerotic cardiovascular disease in US adults with the familial hypercholesterolemia phenotype. Circulation 2016; 134: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scientific Steering Committee on behalf of the Simon Broome Register Group. Risk of fatal coronary heart disease in familial hypercholesterolaemia. BMJ 1991; 303: 893–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mundal L, Igland J, Ose L, et al. Cardiovascular disease mortality in patients with genetically verified familial hypercholesterolemia in Norway during 1992–2013. Eur J Prev Cardiol 2017; 24: 137–144. [DOI] [PubMed] [Google Scholar]
- 20.Reiner Z. Management of patients with familial hypercholesterolaemia. Nat Rev Cardiol 2015; 12: 565–575. [DOI] [PubMed] [Google Scholar]
- 21.Sjouke B, Kusters DM, Kindt I, et al. Homozygous autosomal dominant hypercholesterolaemia in the Netherlands: prevalence, genotype-phenotype relationship, and clinical outcome. Eur Heart J 2015; 36: 560–565. [DOI] [PubMed] [Google Scholar]
- 22.Raal FJ, Pilcher GJ, Panz VR, et al. Reduction in mortality in subjects with homozygous familial hypercholesterolemia associated with advances in lipid-lowering therapy. Circulation 2011; 124: 2202–2207. [DOI] [PubMed] [Google Scholar]
- 23.Sharifi M, Rakhit RD, Humphries SE, et al. Cardiovascular risk stratification in familial hypercholesterolaemia. Heart 2016; 102: 1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huijgen R, Vissers MN, Kindt I, et al. Assessment of carotid atherosclerosis in normocholesterolemic individuals with proven mutations in the low-density lipoprotein receptor or apolipoprotein B genes. Circ Cardiovasc Genet 2011; 4: 413–417. [DOI] [PubMed] [Google Scholar]
- 25.Santos RD, Gidding SS, Hegele RA, et al. Defining severe familial hypercholesterolaemia and the implications for clinical management: a consensus statement from the International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel. Lancet Diabetes Endocrinol 2016; 4: 850–861. [DOI] [PubMed] [Google Scholar]
- 26.Alonso R, Andres E, Mata N, et al. Lipoprotein(a) levels in familial hypercholesterolemia: an important predictor of cardiovascular disease independent of the type of LDL receptor mutation. J Am Coll Cardiol 2014; 63: 1982–1989. [DOI] [PubMed] [Google Scholar]
- 27.Descamps OS, Gilbeau JP, Leysen X, et al. Impact of genetic defects on atherosclerosis in patients suspected of familial hypercholesterolaemia. Eur J Clin Invest 2001; 31: 958–965. [DOI] [PubMed] [Google Scholar]
- 28.Fouchier SW, Defesche JC, Kastelein JJ, et al. Familial defective apolipoprotein B versus familial hypercholesterolemia: an assessment of risk. Semin Vasc Med 2004; 4: 259–264. [DOI] [PubMed] [Google Scholar]
- 29.Bednarska-Makaruk M, Bisko M, Pulawska MF, et al. Familial defective apolipoprotein B-100 in a group of hypercholesterolaemic patients in Poland. Identification of a new mutation Thr3492Ile in the apolipoprotein B gene. Eur J Hum Genet 2001; 9: 836–842. [DOI] [PubMed] [Google Scholar]
- 30.Tybjaerg-Hansen A, Steffensen R, Meinertz H, et al. Association of mutations in the apolipoprotein B gene with hypercholesterolemia and the risk of ischemic heart disease. N Engl J Med 1998; 338: 1577–1584. [DOI] [PubMed] [Google Scholar]
- 31.Marduel M, Carrie A, Sassolas A, et al. Molecular spectrum of autosomal dominant hypercholesterolemia in France. Hum Mutat 2010; 31: E1811–E1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Castro-Orós I, Pocoví M, Civeira F. The genetic basis of familial hypercholesterolemia: inheritance, linkage, and mutations. Appl Clin Genet 2010; 3: 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allard D, Amsellem S, Abifadel M, et al. Novel mutations of the PCSK9 gene cause variable phenotype of autosomal dominant hypercholesterolemia. Hum Mutat 2005; 26: 497–497. [DOI] [PubMed] [Google Scholar]
- 34.Naoumova RP, Tosi I, Patel D, et al. Severe hypercholesterolemia in four British families with the D374Y mutation in the PCSK9 gene: long-term follow-up and treatment response. Arterioscler Thromb Vasc Biol 2005; 25: 2654–2660. [DOI] [PubMed] [Google Scholar]
- 35.Langsted A, Kamstrup PR, Benn M, et al. High lipoprotein(a) as a possible cause of clinical familial hypercholesterolaemia: a prospective cohort study. Lancet Diabetes Endocrinol 2016; 4: 577–587. [DOI] [PubMed] [Google Scholar]
- 36.Allard MD, Saeedi R, Yousefi M, et al. Risk stratification of patients with familial hypercholesterolemia in a multi-ethnic cohort. Lipids Health Dis 2014; 13: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Besseling J, Kindt I, Hof M, et al. Severe heterozygous familial hypercholesterolemia and risk for cardiovascular disease: a study of a cohort of 14,000 mutation carriers. Atherosclerosis 2014; 233: 219–223. [DOI] [PubMed] [Google Scholar]
- 38.Chan DC, Pang J, Hooper AJ, et al. Elevated lipoprotein(a), hypertension and renal insufficiency as predictors of coronary artery disease in patients with genetically confirmed heterozygous familial hypercholesterolemia. Int J Cardiol 2015; 201: 633–638. [DOI] [PubMed] [Google Scholar]
- 39.Jansen AC, van Aalst-Cohen ES, Tanck MW, et al. The contribution of classical risk factors to cardiovascular disease in familial hypercholesterolaemia: data in 2400 patients. J Intern Med 2004; 256: 482–490. [DOI] [PubMed] [Google Scholar]
- 40.Maas A, Appelman YEA. Gender differences in coronary heart disease. Neth Heart J 2010; 18: 598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 1999; 340: 1801–1811. [DOI] [PubMed] [Google Scholar]
- 42.Thirumalai A, Rubinow KB, Page ST. An update on testosterone, HDL and cardiovascular risk in men. Clin Lipidol 2015; 10: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA 2012; 308: 788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tada H, Kawashiri MA, Okada H, et al. Assessment of coronary atherosclerosis in patients with familial hypercholesterolemia by coronary computed tomography angiography. Am J Cardiol 2015; 115: 724–729. [DOI] [PubMed] [Google Scholar]
- 45.Lester SJ, Eleid MF, Khandheria BK, et al. Carotid intima-media thickness and coronary artery calcium score as indications of subclinical atherosclerosis. Mayo Clin Proc 2009; 84: 229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamalainen T, Palotie A, Aalto-Setala K, et al. Absence of familial defective apolipoprotein B-100 in Finnish patients with elevated serum cholesterol. Atherosclerosis 1990; 82: 177–183. [DOI] [PubMed] [Google Scholar]
- 47.Miserez AR, Muller PY. Familial defective apolipoprotein B-100: a mutation emerged in the mesolithic ancestors of Celtic peoples? Atherosclerosis 2000; 148: 433–436. [DOI] [PubMed] [Google Scholar]
- 48.Pecin I, Whittall R, Futema M, et al. Mutation detection in Croatian patients with familial hypercholesterolemia. Ann Hum Genet 2013; 77: 22–30. [DOI] [PubMed] [Google Scholar]
- 49.Linseisen J, Welch AA, Ocke M, et al. Dietary fat intake in the European Prospective Investigation into Cancer and Nutrition: results from the 24-h dietary recalls. Eur J Clin Nutr 2009; 63: S61–S80. [DOI] [PubMed] [Google Scholar]
- 50.Yudi M, Omera L, McCubbery N, et al. Suboptimal consideration and management of potential familial hypercholesterolaemia in patients with suspected premature coronary artery disease. Singapore Med J 2012; 53: 174–178. [PubMed] [Google Scholar]
- 51.Mundal L, Veierod MB, Halvorsen T, et al. Cardiovascular disease in patients with genotyped familial hypercholesterolemia in Norway during 1994–2009, a registry study. Eur J Prev Cardiol 2016; 23: 1962–1969. [DOI] [PubMed] [Google Scholar]
- 52.Catapano AL, Lautsch D, Tokgozoglu L, et al. Prevalence of potential familial hypercholesteremia (FH) in 54,811 statin-treated patients in clinical practice. Atherosclerosis 2016; 252: 1–8. [DOI] [PubMed] [Google Scholar]
- 53.Versmissen J, Oosterveer DM, Yazdanpanah M, et al. Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. BMJ 2008; 337: a2423–a2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hopkins PN, Toth PP, Ballantyne CM, et al. Familial hypercholesterolemias: prevalence, genetics, diagnosis and screening recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol 2011; 5: S9–S17. [DOI] [PubMed] [Google Scholar]
- 55.American College of Cardiologists. Familial hypercholesterolaemia: cardiologist and patient needs assessment. www.acc.org/membership/member-benefits-and-resources/acc-member-publications/cardiosurve/newsletter/archive/2012/07/familial%20hypercholesterolemia%20cardiologist%20and%20patient%20perspectives (2012, accessed 12 November 2015).
- 56.National Institute for Health and Care Excellence. Familial hypercholesterolaemia: costing report. www.nice.org.uk/guidance/cg71/resources/familial-hypercholesterolaemia-costing-report-241873741 (2009, accessed 25 January 2017).
- 57.Henderson R, O’Kane M, McGilligan V, et al. The genetics and screening of familial hypercholesterolaemia. J Biomed Sci 2016; 23: 39–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Green NS, Dolan SM, Murray TH. Newborn screening: complexities in universal genetic testing. Am J Public Health 2006; 96: 1955–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reiner Z. Impact of early evidence of atherosclerotic changes on early treatment in children with familial hypercholesterolemia. Circ Res 2014; 114: 233–235. [DOI] [PubMed] [Google Scholar]
- 60.Wald DS, Bestwick JP, Morris JK, et al. Child–parent familial hypercholesterolemia screening in primary care. N Engl J Med 2016; 375: 1628–1637. [DOI] [PubMed] [Google Scholar]
- 61.Wiegman A, Gidding SS, Watts GF, et al. Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur Heart J 2015; 36: 2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klančar G, Grošelj U, Kovač J, et al. Universal screening for familial hypercholesterolemia in children. J Am Coll Cardiol 2015; 66: 1250–1257. [DOI] [PubMed] [Google Scholar]
- 63.Umans-Eckenhausen MA, Defesche JC, Sijbrands EJ, et al. Review of first 5 years of screening for familial hypercholesterolaemia in the Netherlands. Lancet 2001; 357: 165–168. [DOI] [PubMed] [Google Scholar]
- 64.Vallejo-Vaz AJ, Kondapally Seshasai SR, Cole D, et al. Familial hypercholesterolaemia: a global call to arms. Atherosclerosis 2015; 243: 257–259. [DOI] [PubMed] [Google Scholar]
- 65.Defesche JC. Defining the challenges of FH screening for familial hypercholesterolemia. J Clin Lipidol 2010; 4: 338–341. [DOI] [PubMed] [Google Scholar]
- 66.Mata P, Alonso R, Perez-Jimenez F. Screening for familial hypercholesterolemia: a model for preventive medicine. Rev Esp Cardiol (Engl Ed) 2014; 67: 685–688. [DOI] [PubMed] [Google Scholar]
- 67.Bell DA, Pang J, Burrows S, et al. Effectiveness of genetic cascade screening for familial hypercholesterolaemia using a centrally co-ordinated clinical service: an Australian experience. Atherosclerosis 2015; 239: 93–100. [DOI] [PubMed] [Google Scholar]
- 68.Besseling J, Huijgen R, Martin SS, et al. Clinical phenotype in relation to the distance-to-index-patient in familial hypercholesterolemia. Atherosclerosis 2016; 246: 1–6. [DOI] [PubMed] [Google Scholar]
- 69.Troeung L, Arnold-Reed D, Chan She Ping-Delfos W, et al. A new electronic screening tool for identifying risk of familial hypercholesterolaemia in general practice. Heart 2016; 102: 855–861. [DOI] [PubMed] [Google Scholar]
- 70.Besseling J, Reitsma JB, Gaudet D, et al. Selection of individuals for genetic testing for familial hypercholesterolaemia: development and external validation of a prediction model for the presence of a mutation causing familial hypercholesterolaemia. Eur Heart J 2017; 38: 565–573. [DOI] [PubMed]
- 71.Ellis KL, Pang J, Watts GF. Registries, codifications and cardiovascular outcomes in familial hypercholesterolaemia. Eur J Prev Cardiol 2017; 24: 133–136. [DOI] [PubMed] [Google Scholar]
- 72.Ademi Z, Watts GF, Pang J, et al. Cascade screening based on genetic testing is cost-effective: evidence for the implementation of models of care for familial hypercholesterolemia. J Clin Lipidol 2014; 8: 390–400. [DOI] [PubMed] [Google Scholar]
- 73.European Atherosclerosis Society. HoFH international clinical collaboration – HICC. www.eas-society.org/page/hicc (2016, accessed 17 November 2016).
- 74.Ceska R, Freiberger T, Susekov AV, et al. ScreenPro FH – screening project for familial hypercholesterolemia in central, southern and eastern Europe: basic epidemiology. Vnitr Lek 2017; 63: 25–30. [PubMed] [Google Scholar]
- 75.Ceska R, Paragh G, Reiner Z, et al. ScreenPro FH – screening project for familial hypercholesterolemia in central, southern and eastern Europe: rationale and design. Vnitr Lek 2017; 63: 43–48. [PubMed] [Google Scholar]
- 76.Defesche JC, Lansberg PJ, Umans-Eckenhausen MA, et al. Advanced method for the identification of patients with inherited hypercholesterolemia. Semin Vasc Med 2004; 4: 59–65. [DOI] [PubMed] [Google Scholar]
- 77.Williams RR, Hunt SC, Schumacher MC, et al. Diagnosing heterozygous familial hypercholesterolemia using new practical criteria validated by molecular genetics. Am J Cardiol 1993; 72: 171–176. [DOI] [PubMed] [Google Scholar]
- 78.World Health Organization. Familial hypercholesterolaemia (FH). Report of a second WHO consultation. http://apps.who.int/iris/bitstream/10665/66346/1/WHO_HGN_FH_CONS_99.2.pdf (1998, accessed 11 December 2015).
- 79.Haase A, Goldberg AC. Identification of people with heterozygous familial hypercholesterolemia. Curr Opin Lipidol 2012; 23: 282–289. [DOI] [PubMed] [Google Scholar]
- 80.Haralambos K, Whatley SD, Edwards R, et al. Clinical experience of scoring criteria for familial hypercholesterolaemia (FH) genetic testing in Wales. Atherosclerosis 2015; 240: 190–196. [DOI] [PubMed] [Google Scholar]
- 81.Qureshi N, Humphries SE, Seed M, et al. Identification and management of familial hypercholesterolaemia: what does it mean to primary care? Br J Gen Pract 2009; 59: 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weng SF, Kai J, Andrew Neil H, et al. Improving identification of familial hypercholesterolaemia in primary care: derivation and validation of the familial hypercholesterolaemia case ascertainment tool (FAMCAT). Atherosclerosis 2015; 238: 336–343. [DOI] [PubMed] [Google Scholar]
- 83.National Human Genome Research Institute. Genetic discrimination. http://www.genome.gov/10002077 (2015, accessed 23 December 2015).
- 84.Homsma SJ, Huijgen R, Middeldorp S, et al. Molecular screening for familial hypercholesterolaemia: consequences for life and disability insurance. Eur J Hum Genet 2008; 16: 14–17. [DOI] [PubMed] [Google Scholar]
- 85.Huijgen R, Homsma SJ, Hutten BA, et al. Improved access to life insurance after genetic diagnosis of familial hypercholesterolaemia: cross-sectional postal questionnaire study. Eur J Hum Genet 2012; 20: 722–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS Guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) developed with the special contribution of the European Assocciation for Cardiovascular Prevention and Rehabilitation (EACPR). Atherosclerosis 2016; 253: 281–344. [DOI] [PubMed] [Google Scholar]
- 87.Chiuve SE, Cook NR, Shay CM, et al. Lifestyle-based prediction model for the prevention of CVD: the Healthy Heart Score. J Am Heart Assoc 2014; 3: e000954–e000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chow CK, Jolly S, Rao-Melacini P, et al. Association of diet, exercise, and smoking modification with risk of early cardiovascular events after acute coronary syndromes. Circulation 2010; 121: 750–758. [DOI] [PubMed] [Google Scholar]
- 89.Arsenault BJ, Perrot N, Couture P. Does lifestyle contribute to disease severity in patients with inherited lipid disorders? Curr Opin Lipidol 2017; 28: 177–185. [DOI] [PubMed] [Google Scholar]
- 90.Pijlman AH, Huijgen R, Verhagen SN, et al. Evaluation of cholesterol lowering treatment of patients with familial hypercholesterolemia: a large cross-sectional study in the Netherlands. Atherosclerosis 2010; 209: 189–194. [DOI] [PubMed] [Google Scholar]
- 91.Ooi EM, Barrett PH, Watts GF. The extended abnormalities in lipoprotein metabolism in familial hypercholesterolemia: developing a new framework for future therapies. Int J Cardiol 2013; 168: 1811–1818. [DOI] [PubMed] [Google Scholar]
- 92.Neil A, Cooper J, Betteridge J, et al. Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: a prospective registry study. Eur Heart J 2008; 29: 2625–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.The Lipid Research Clinics Coronary Primary Prevention Trial collaborators. The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA 1984; 251: 351–364. [DOI] [PubMed] [Google Scholar]
- 94.Galema-Boers JM, Lenzen MJ, van Domburg RT, et al. Predicting non-adherence in patients with familial hypercholesterolemia. Eur J Clin Pharmacol 2014; 70: 391–397. [DOI] [PubMed] [Google Scholar]
- 95.Reiner Z. Resistance and intolerance to statins. Nutr Metab Cardiovasc Dis 2014; 24: 1057–1066. [DOI] [PubMed] [Google Scholar]
- 96.Varghese MJ. Familial hypercholesterolemia: a review. Ann Pediatr Cardiol 2014; 7: 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kastelein JJ, Akdim F, Stroes ES, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med 2008; 358: 1431–1443. [DOI] [PubMed] [Google Scholar]
- 98.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015; 372: 2387–2397. [DOI] [PubMed] [Google Scholar]
- 99.Wierzbicki AS, Humphries SE, Minhas R. Familial hypercholesterolaemia: summary of NICE guidance. BMJ 2008; 337: a1095–a1095. [DOI] [PubMed] [Google Scholar]
- 100.Roeters van Lennep HW, Liem AH, Dunselman PH, et al. The efficacy of statin monotherapy uptitration versus switching to ezetimibe/simvastatin: results of the EASEGO study. Curr Med Res Opin 2008; 24: 685–694. [DOI] [PubMed] [Google Scholar]
- 101.Goldberg AC. Emerging low-density lipoprotein therapies: microsomal triglyceride transfer protein inhibitors. J Clin Lipidol 2013; 7: S16–S20. [DOI] [PubMed] [Google Scholar]
- 102.Vishwanath R, Hemphill LC. Familial hypercholesterolemia and estimation of US patients eligible for low-density lipoprotein apheresis after maximally tolerated lipid-lowering therapy. J Clin Lipidol 2014; 8: 18–28. [DOI] [PubMed] [Google Scholar]
- 103.Reiner Z. Management of patients with familial hypercholesterolaemia. Nat Rev Cardiol 2015; 12: 565–575. [DOI] [PubMed] [Google Scholar]
- 104.Cuchel M, Meagher EA, du Toit Theron H, et al. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet 2013; 381: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stein EA, Dufour R, Gagne C, et al. Apolipoprotein B synthesis inhibition with mipomersen in heterozygous familial hypercholesterolemia: results of a randomized, double-blind, placebo-controlled trial to assess efficacy and safety as add-on therapy in patients with coronary artery disease. Circulation 2012; 126: 2283–2292. [DOI] [PubMed] [Google Scholar]
- 106.Raal FJ, Santos RD, Blom DJ, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 375: 998–1006. [DOI] [PubMed] [Google Scholar]
- 107.Genzyme. Kynamro® (mipomersen): highlights of prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2013/203568s000lbl.pdf (2015, accessed 3 August 2016).
- 108.European Medicines agency. Kynamro® (mipomersen): assessment report. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002429/WC500144511.pdf (2013, accessed 3 August 2016).
- 109.Smilde TJ, van Wissen S, Wollersheim H, et al. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet 2001; 357: 577–581. [DOI] [PubMed] [Google Scholar]
- 110.Shitara Y, Sugiyama Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug–drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther 2006; 112: 71–105. [DOI] [PubMed] [Google Scholar]
- 111.Corsini A, Bellosta S, Baetta R, et al. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther 1999; 84: 413–428. [DOI] [PubMed] [Google Scholar]
- 112.Reiner Ž. PCSK9 inhibitors – past, present and future. Expert Opin Drug Metab Toxicol 2015; 11: 1517–1521. [DOI] [PubMed] [Google Scholar]
- 113.Abifadel M, Varret M, Rabes JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003; 34: 154–156. [DOI] [PubMed] [Google Scholar]
- 114.Dias CS, Shaywitz AJ, Wasserman SM, et al. Effects of AMG 145 on low-density lipoprotein cholesterol levels: results from 2 randomized, double-blind, placebo-controlled, ascending-dose phase 1 studies in healthy volunteers and hypercholesterolemic subjects on statins. J Am Coll Cardiol 2012; 60: 1888–1898. [DOI] [PubMed] [Google Scholar]
- 115.Cohen JC, Boerwinkle E, Mosley TH, Jr., et al. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006; 354: 1264–1272. [DOI] [PubMed] [Google Scholar]
- 116.European Medicines Agency. Repatha® (evolocumab) summary of product characteristics. Amgen. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003766/WC500191398.pdf (2015, accessed 7 December 2015).
- 117.European Medicines Agency. Praluent® (alirocumab) summary of product characterisitics. Sanofi-Regeneron. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003882/WC500194521.pdf (2016, accessed 2 February 2016).
- 118.Amgen Inc. Repatha® (evolocumab) prescribing information. http://pi.amgen.com/united_states/repatha/repatha_pi_hcp_english.pdf (2015, accessed 3 February 2016).
- 119.Regeneron Pharmaceuticals Inc/Sanofi-Aventis US. Praluent® (alirocumab) prescribing information. http://products.sanofi.us/praluent/praluent.pdf (2015, accessed 3 February 2016).
- 120.Health Canada. Repatha® (evolocumab) product monograph. Amgen. www.amgen.ca/Repatha_PM.pdf (2015, accessed 3 February 2016).
- 121.Pfizer. Pfizer discontinues global development of bococizumab, its investigational PCSK9 inhibitor. www.pfizer.com/news/press-release/press-release-detail/pfizer_discontinues_global_development_of_bococizumab_its_investigational_pcsk9_inhibitor (2016, accessed 17 November 2016).
- 122.Strat AL, Ghiciuc CM, Lupusoru CE, et al. New class of drugs: therapeutic RNAi inhibition of PCSK9 as a specific LDL-C lowering therapy. Rev Med Chir Soc Med Nat Iasi 2016; 120: 228–232. [PubMed] [Google Scholar]
- 123.Fitzgerald K, White S, Borodovsky A, et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med 2017; 376: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med 2017; 376: 1430–1440. [DOI] [PubMed] [Google Scholar]
- 125.Raal FJ, Stein EA, Dufour R, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet 2015; 385: 331–340. [DOI] [PubMed] [Google Scholar]
- 126.Raal FJ, Honarpour N, Blom DJ, et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet 2015; 385: 341–350. [DOI] [PubMed] [Google Scholar]
- 127.Kastelein JJ, Ginsberg HN, Langslet G, et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J 2015; 36: 2996–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lappegard KT, Enebakk T, Thunhaug H, et al. Transition from LDL apheresis to evolocumab in heterozygous FH is equally effective in lowering LDL, without lowering HDL cholesterol. Atherosclerosis 2016; 251: 119–123. [DOI] [PubMed] [Google Scholar]
- 129.Moriarty PM, Parhofer KG, Babirak SP, et al. Alirocumab in patients with heterozygous familial hypercholesterolaemia undergoing lipoprotein apheresis: the ODYSSEY ESCAPE trial. Eur Heart J 2016; 37: 3588–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Khan AR, Bavishi C, Riaz H, et al. Increased risk of adverse neurocognitive outcomes with proprotein convertase subtilisin-kexin type 9 inhibitors. Circ Cardiovasc Qual Outcomes 2017; 10: pii: e003153–pii: e003153. DOI: 10.1161/CIRCOUTCOMES.116.003153. [DOI] [PubMed] [Google Scholar]
- 131.PRNewswire. Amgen announces Repatha® (evolocumab) significantly reduced the risk of cardiovascular events in FOURIER outcomes study. www.prnewswire.com/news-releases/amgen-announces-repatha-evolocumab-significantly-reduced-the-risk-of-cardiovascular-events-in-fourier-outcomes-study-300401508.html (2017, accessed 20 February 2017).
- 132.Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA 2016; 316: 2373–2384. [DOI] [PubMed] [Google Scholar]
- 133.Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372: 1500–1509. [DOI] [PubMed] [Google Scholar]
- 134.Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372: 1489–1499. [DOI] [PubMed] [Google Scholar]
- 135.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376: 1713–1722. [DOI] [PubMed]
- 136.Schwartz GG, Bessac L, Berdan LG, et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J 2014; 168: 682–689. [DOI] [PubMed] [Google Scholar]
- 137.Pecin I, Reiner Z. Alirocumab: targeting PCSK9 to treat hypercholesterolemia. Drugs of Today (Barcelona, Spain: 1998) 2015; 51: 681–687. [DOI] [PubMed] [Google Scholar]
- 138.Ridker PM, Revkin J, Amarenco P, et al. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med 2017; 376: 1527–1539. [DOI] [PubMed] [Google Scholar]
- 139.Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA 2016; 316: 743–753. [DOI] [PubMed] [Google Scholar]
- 140.National Institute for Health and Care Excellence. Evolocumab for treating primary hypercholesterolaemia and mixed dyslipidaemia. www.nice.org.uk/guidance/ta394/chapter/1-Recommendations (2016, accessed 26 January 2017).
- 141.National Institute for Health and Care Excellence. Alirocumab for treating primary hypercholesterolaemia and mixed dyslipidaemia. www.nice.org.uk/guidance/TA393 (2016, accessed 26 January 2017).
- 142.Landmesser U, John Chapman M, Farnier M, et al. European Society of Cardiology/European Atherosclerosis Society Task Force consensus statement on proprotein convertase subtilisin/kexin type 9 inhibitors: practical guidance for use in patients at very high cardiovascular risk. Eur Heart J 2016; pii: ehw480. [DOI] [PubMed]
- 143.Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2016 ACC Expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2016; 68: 92–125. [DOI] [PubMed] [Google Scholar]
- 144.Sjouke B, Kusters DM, Kastelein JJ, et al. Familial hypercholesterolemia: present and future management. Curr Cardiol Rep 2011; 13: 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]