Abstract
Background and Aims
Sex differences in clinical pain severity and response to experimental pain are commonly reported, with women generally showing greater vulnerability. Affect, including state (a single rating) and stable (average daily ratings over two weeks) positive affect and negative affect has also been found to impact pain sensitivity and severity, and research suggests that affect may modulate pain differentially as a function of sex. The current study aimed to examine sex as a moderator of the relationships between affect and pain-related outcomes among participants with knee osteoarthritis (KOA).
Methods
One hundred and seventy-nine participants (59 men) with KOA completed electronic diaries assessing clinical pain, positive affect, and negative affect. A subset of participants (n = 120) underwent quantitative sensory testing, from which a single index of central sensitization to pain was derived. We used multiple regression models to test for the interactive effects of sex and affect (positive versus negative and stable versus state) on pain-related outcomes. We used mixed effects models to test for the moderating effects of sex on the relationships between state affect and pain over time.
Results
Sex differences in affect and pain were identified, with men reporting significantly higher stable positive affect and lower central sensitization to pain indexed by quantitative sensory testing, as well as marginally lower KOA-specific clinical pain compared to women. Moreover, there was an interaction between stable positive affect and sex on KOA-specific clinical pain and average daily non-specific pain ratings. Post-hoc analyses revealed that men showed trends toward an inverse relationship between stable positive affect and pain outcomes, while women showed no relationship between positive affect and pain. There was also a significant interaction between sex and stable negative affect and sex on KOA-specific pain such that men showed a significantly stronger positive relationship between stable negative affect and KOA-specific pain than women. Sex did not interact with state affect on pain outcomes.
Conclusions
Findings suggest that men may be particularly sensitive to the effects of stable positive affect and negative affect on clinical pain. Future work with larger samples is needed in order to identify potential mechanisms driving the sex-specific effects of affect on pain.
Implications
The current study provides novel data that suggesting that the association of positive affect, negative affect, and pain are different in men versus women with KOA. Further understanding of the difference in affective expression between men and women may lead to the development of novel therapeutic interventions and help to identify additional modifiable factors in the prevention and management of pain.
Keywords: knee osteoarthritis, sex, pain, quantitative sensory testing
1. INTRODUCTION
Research suggests that women are more vulnerable to pain than men [1, 2]. Women with chronic pain report more severe pain [3–5] and disability [3, 5–8], and greater sensitivity to laboratory pain [9–12]. Women have an increased risk of chronic pain disorders, including osteoarthritis [3, 13–16]. However, other studies have evidenced increased clinical pain severity among men [17], or shown no sex differences in laboratory pain sensitivity [18] nor clinical pain severity [19, 20]. These inconsistencies suggest other factors, including psychological factors, may influence pain differentially across sexes.
One promising and modifiable factor is affective experience, including positive and negative affect. Research indicates that positive and negative affect are psychometrically distinct [6, 21–23] and independently impact pain outcomes. Negative affect is associated with enhanced pain and disability [22, 24–27]. Positive affect improves resilience in managing chronic pain [28, 29]. Furthermore, positive affect may mitigate the detrimental effects of negative affect on pain [21, 30–32]. The temporal stability of affect (stable vs. state) may also influence the experience of pain [18]. Stable (i.e., trait-like) measures of affect reflect mood over time versus state measures, which reflect transient changes. Both stable and state positive affect are associated with reduced pain [21]. Our lab has demonstrated that stable negative affect is a better predictor of clinical compared with stable positive affect; however, state positive affect is a better predictor of knee-specific and experimental pain than state negative affect [33]. Thus elucidating distinguishable roles of both affective valence and stability is warranted in pain research [34].
Further evidence suggests that sex differences in affective expression [35] may contribute to sex discrepancies in the pain experience. Women have an increased risk of depressive and anxiety disorders [36–38], which augment negative affect in pain [3, 25]. Women may be more emotionally expressive [39], however, men react more to positive stimuli [40]. Rhudy & Williams [25] suggest that women may be more vulnerable to the pain-enhancing effects of negative affect [20, 41, 42]; while men may be protected by pain-reducing effects of positive affect [43]. However, this model [25] was based largely on healthy participants. In osteoarthritis, positive affect may serve as a resilience factor in women [31]; however, men were not included as a comparison group. While studies of individuals with osteoarthritis show sex differences in the experience of pain [44, 45], how affect may modulate clinical pain as a function of sex in individuals with osteoarthritis remains unclear.
This study is a secondary analysis on a subset of individuals with knee osteoarthritis (KOA) and extends previous findings from our group [33]. The primary aim of this study was to investigate the relationships between positive and negative affect and pain-related outcomes (i.e., daily clinical pain, KOA-specific pain, pain catastrophizing, and central sensitization) as a function of sex in adults with KOA. We evaluated the effects of positive and negative affect in two separate ways: state (i.e., affect ratings collected at a single time point) vs. stable (i.e., average affect ratings over a two week period). We hypothesize that men with KOA will show stronger inverse relationships between both state and stable positive affect and pain, while women will show stronger positive relationship between both state and stable negative affect and pain.
2. MATERIALS AND METHODS
2.1. Participants
One hundred and seventy-nine participants (59 men) were recruited as a part of a larger study designed to evaluate the role of sleep on pain modulation in older adults with and without KOA [46]. In order to qualify for inclusion in the study, participants had to meet the American College of Rheumatology criteria for KOA as diagnosed by a board-certified rheumatologist, needed radiographic evidence of KOA with a Kellgren-Lawrence grade > 1 for at least one knee, and report typical pain ratings of > 2 out of 10 at least 4 days per week for 6 months or more before entering the study. Participants were excluded if they had serious comorbid medical conditions that would affect sleep or pain, or were diagnosed with severe or unstable psychiatric illness, cognitive impairments/dementia, or current substance use disorders (or a positive urine toxicology screen). Participants agreed to discontinue any analgesic and sedative medications for 24 hours prior to laboratory pain testing. Due to the larger sleep-related aims of the project, we over-sampled for patients reporting insomnia and conducted at-home polysomnographic (PSG) studies on all participants to test for sleep apnea. The majority of the sample (n = 143; 79.9%) met diagnostic criteria for insomnia disorder. Results from the at-home PSG studies also revealed a broad range of sleep apnea severity (Apnea-Hypopnea Index (AHI) range = 0 – 46.75; Mean AHI = 8.82; SD = 9.53; 17.9% of participants had AHI > 15). Please see past publications stemming from this data set [33, 46] for more details on sample selection. The study was approved by the Johns Hopkins School of Medicine Institutional Review Board.
2.2. Procedures
After obtaining informed consent, participants completed questionnaires, the Structured Interview for Sleep Disorders (SIS-D [47]), and bilateral knee x-rays to confirm study eligibility. They were trained to complete daily measures of pain and affect using a personal digital assistant (Palm Pilot). Participants were instructed to complete diaries in the evening before bed every day for two weeks. Diaries were completed for 1992 (85.8%) of 2322 possible days. We compared those who completed <15% of diary entries with those who completed >15% and did not see differences in terms of baseline pain measures. Participants returned after two weeks for a second baseline visit, during which they returned the Palm Pilot and completed quantitative sensory testing (QST) measures of pain. A subset of participants provided baseline laboratory ratings of positive affect and negative affect immediately prior to QST (n = 120). These baseline laboratory studies were added later as a part of an ancillary study of inflammatory response to pain, and will be presented in the current study as measures of state affect (referred to as “lab affect” hereafter in order to differentiate these ratings from the individual daily ratings of state affect collected from daily Palm Pilot diaries) in analyses that include QST assessments as the outcome variable. In summary, the analyses examining state (“lab”) positive affect and negative affect assessed immediately prior to QST included only this subsample of 120 people, while all other analyses included the larger sample of 179. Participants in this subsample did not differ from the full participant sample in terms of age, race, sex, or clinical pain.
2.3. Measures
2.3.1. Insomnia symptom measure
2.3.1.1. Insomnia Severity Index (ISI; [48])
The ISI is a 7-item self-report questionnaire used to measure insomnia symptom severity based on DSM-IV criteria. ISI scores were examined as a potential covariate in the current study.
2.3.2. Positive affect and negative affect
2.3.2.1. Daily diary measures of positive and negative affect
Before going to bed each night, patients completed several affect ratings reflecting how they felt on average over the course of the day using a Palm Pilot. Specifically, patients rated three positive emotions (happy, calm, and agreeable) and three negative emotions (unhappy, anxious, annoyed) on separate sliding visual analog scales (VAS) ranging from 0 (“not at all”) to 100 (“extremely”). Only six (total) emotions were collected nightly to minimize the burden of daily documentation. As described elsewhere [33], the three positive emotion items were averaged to provide an index of daily positive affect, and two of the negative emotion items (anxious and annoyed) were averaged to provide an index of daily negative affect. “Unhappy” was not included in the computation of negative affect due to concerns that it would artificially increase the correlation of positive affect and negative affect due to its similarity to the “Happy” positive emotion item.
2.3.2.2. Baseline laboratory assessment of state positive and negative affect
A subset of patients (n = 120) completed baseline state (“lab”) positive affect and negative affect ratings immediately prior to commencing QST procedures, using individual items from the PANAS-X and POMS-Bipolar. Each participant was asked to rate the extent to which they felt three positive emotions (happy, calm, and pleasant) and six negative emotions (anxious, nervous, tense, frustrated, irritable, and annoyed) at that moment, on a 0 to 100 scale, where 0 = “not at all” and 100 = “extremely”. As laboratory assessment may induce a greater range of negative emotions, six negative emotions were collected in this setting. The positive and negative items were each averaged to create indices of baseline laboratory positive affect and negative affect, respectively. These variables were specifically used as the measures of state affect in our analyses examining the interaction between state affect and sex on laboratory pain sensitivity (quantified as central sensitization index scores) under QST.
2.3.3. Clinical pain and pain-related catastrophizing
2.3.3.1. Diary Pain Intensity Index (PII-D)
Before going to bed each night, patients completed ratings of “usual pain “ experienced during the entire day on a Palm Pilot using a VAS ranging from 0 (“no pain”) to 100 (“worst pain imaginable”)[49]. For the current study, these daily ratings were used as our index of nonspecific clinical pain because participant ratings included any pain related both to the knee or otherwise.
2.3.3.2. Western Ontario McMaster Universities Osteoarthritis Scale (WOMAC, 3.1) [50]
The WOMAC is a widely used and well validated KOA-specific outcomes questionnaire, which yields three scales: 1) pain severity, 2) disability and 3) joint stiffness, as well as a total score. Ratings are made on a 10 cm VAS. For the current study, we used the pain severity subscale as our measure of KOA-specific clinical pain.
2.3.3.3. Pain Catastrophizing Scale (PCS; [51])
The PCS is a 13-item self-report questionnaire which asks patients to rate the extent to which they experience certain thoughts and feelings when they are in pain on a scale of 0 (“not at all”) to 4 (“all the time”). Responses to items on the PCS can be used to calculate a PCS total score, as well as three sub-scales designed to capture Rumination, Magnification, and Helplessness. For the current study, we used the PCS total score as our index of pain-related catastrophizing.
2.3.4. Quantitative sensory testing (QST)
A battery of QST measures were administered over the course of a single daytime session lasting approximately 1.5 hours. Following methods previously employed by our group [52], we created a summary measure of central sensitization (CS) by z-scoring individual tests and averaging across the z-scored values. CS occurs when application of noxious nociceptive stimuli triggers increased excitability and synaptic efficacy of neurons in central nociceptive pathways, resulting in a prolonged, but reversible, hypersensitivity to pain [53]. CS has been implicated in both animal and human studies as a mechanism underlying clinical pain augmentation, specifically in OA [54]. The CS index score for the current study was comprised of z-scored summary measures of thermal temporal summation, mechanical temporal summation, and cold pressor after-sensations (pain ratings on 0–100 scale provided by patients at 30 seconds, 1-minute, and 2-minutes post-removal from cold water bath), (all described thoroughly in [53]). We then computed the mean of all thermal and mechanical temporal summation z-scores and cold pressor after sensation z-scores to create one measure of CS, which was the primary QST-derived variable of interest. Higher scores on the CS index are indicative of greater pain sensitivity.
2.4. Data analytic method
Primary analyses were conducted in SPSS version 22.0 (IBM corporation); for all significant interactions, post-hoc analyses of simple slopes [55] were conducted and interaction graphs were generated using Interaction! version 1.7.22.11. We computed stable (i.e. trait-like) positive affect and negative affect ratings as the mean daily diary ratings over the course of two weeks (see [33] for similar uses of these measures). We examined how state positive affect and negative affect interact with sex to affect daily pain ratings either on the same day, or on the following day; and additionally, we used the baseline ratings of positive affect and negative affect (i.e., “lab” affect ratings) that were collected immediately prior to QST procedures to test the effects of state affect on CS as a function of sex. Age, race, insomnia severity (ISI scores), and AHI were covaried in all models as age, race, and sleep disturbances are risk factors for pain sensitivity [56–58]. Additionally, in our study population, differences between the two sexes were noted for age, race, ISI scores and AHI further necessitating adjustment for covariates. Similar to previous findings [59] Kellgren-Lawrence score did not correlate with pain ratings, and was not significantly different between sexes, and was not included as a covariate.
2.4.1. Descriptives and Correlations
T-tests were used to compare demographic and sleep variables between sexes (see Table 1) and Pearson’s product-moment correlations were calculated to examine the relationship between affect and pain-related outcomes (see Table 2).
Table 1.
Sample Demographic and Sleep Characteristics
| Males (n = 59) |
Females (n = 120) |
Total (n = 179) |
|
|---|---|---|---|
| Age (Mean years + SD) | 62.78+10.11□ | 59.88+9.58 | 60.83+9.82 |
| Insomnia Diagnosis (%) | 71.2* | 84.2 | 79.9 |
| ISI Score (Mean + SD) | 13.27+8.39 | 14.87+6.94 | 14.34+7.47 |
| AHI (Mean + SD) | 11.49+11.89** | 7.51+7.86 | 8.82+9.53 |
| K-L scale (Mean + SD) | 2.32+1.22 | 2.46+1.25 | 2.00+1.14 |
| Race | |||
| % African American | 25.4 | 44.2 | 38.0 |
| % White | 67.8□ | 54.2 | 58.7 |
| % Asian | 5.1 | 1.7 | 2.8 |
| % More than one race | 1.7 | 0.0 | 0.6 |
| Marital status | |||
| % Married | 50.8 | 39.5 | 43.3 |
| Education | |||
| % College graduate | 59.0 | 47.0 | 51.7 |
| Employment | |||
| % Employed | 37.3 | 42.9 | 41.1 |
| Household Income | |||
| % > $50,000 | 49.1 | 42.4 | 44.6 |
p < .10;
p < .05;
p < .01; SD = standard deviation
Table 2.
Means and correlations of primary measures
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Male M(SD) |
Female M(SD) |
Total M(SD) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Avg daily PA | 1 | −.62** | .43** | −.14 | −.16* | −.16* | −.26** | −.11 | 68.31(15.14)* | 63.45(13.49) | 65.04(14.19) |
| 2. Avg daily NA | 1 | −.33** | .33** | .46** | .48** | .44** | .11 | 25.90(18.77) | 25.85(15.45) | 25.86(16.56) | |
| 3. Lab PA | 1 | −.42** | .05 | .05 | −.16 | −.13 | 75.50(21.00) | 74.33(18.81) | 74.67(19.39) | ||
| 4. Lab NA | 1 | .21* | .18 | .40** | .29** | 8.83(13.64) | 11.07(16.18) | 10.42(15.46) | |||
| 5. Avg daily pain | 1 | .81** | .41** | .27** | 42.09(18.80) | 43.93(18.71) | 43.33(18.70) | ||||
| 6. WOMAC | 1 | .40** | .26** | 3.96(2.44)□ | 4.63(2.26) | 4.40(2.34) | |||||
| 7. PCS-Tot | 1 | .13 | 12.85(11.88) | 13.32(10.27) | 13.16(10.81) | ||||||
| 8. QST CS index | 1 | −0.152(.588)** | 0.196(.597) | 0.084(0.614) |
PA: positive affect; NA: negative affect; PCS: pain catastrophizing score; QST: quantitative sensory testing; CS: central sensitization
p < .10;
p < .05;
p < .01; M = mean; SD = standard deviation
2.4.2. Stable positive affect and negative affect analyses
We used multiple regression models to test for the interactive effects of sex and stable positive affect/negative affect on pain-related outcomes (e.g., average daily diary-based pain ratings, KOA-specific pain ratings based on the WOMAC, pain-related catastrophizing scores on the PCS, and QST CS index scores). In separate models, we entered either mean positive affect or mean negative affect as the independent variable, along with sex and the sex × stable positive affect/negative affect interaction term. For significant sex × stable affect interactions, post-hoc analyses were conducted using a simple slopes approach to determine the nature of the relationships between stable affect and the pain-related variables independently for men and women. As we had more than one factor in our models, partial eta squared is reported as the variance explained by a given variable of the variance remaining after excluding variance explained by other factors [60, 61].
2.4.3. State positive affect and negative affect analyses
To test for the moderating effects of sex on the relationships between state positive affect/negative affect and daily diary pain, we used mixed effects models using methods described previously [33]. Specifically, state positive affect and negative affect variables from the daily diary affect ratings were computed by centering the daily diary affect scores around each individual’s mean across days, resulting in daily within-person deviations. Following the methods described in Singer and Willet [62], we then tested a Level 1 (i.e., within person) model in which daily pain was the dependent variable and state positive affect (or negative affect) was the independent variable, against a Level 2 (between person) model that included sex and the interaction between sex and state positive affect (or negative affect) as additional independent variables. We ran each Level 2 model twice: once with same day diary pain as the dependent variable, and once with next-day diary pain as the dependent variable in order to see if variation in today’s state negative affect or positive affect impacts tomorrow’s pain levels. The intercepts were allowed to randomly vary, and autoregression was controlled.
Laboratory measures of state (“lab”) positive affect and negative affect were operationalized as the baseline positive affect and negative affect reports given by participants immediately before engaging in the series of QST tests. These variables were entered as predictors in separate multiple regression models, along with sex, the sex × affect interaction, and identified covariates (i.e., age, race, ISI scores, and AHI), with the QST CS index score entered as the dependent variable.
3. RESULTS
3.1. Sample characteristics and descriptives
One hundred and seventy-nine eligible participants (59 men) were recruited to participate. Demographic data is displayed in Table 1. Men were marginally older (p = .06), were significantly less likely to carry an insomnia diagnosis (p = .042), had higher AHI scores (p = .008), and marginally more likely to be White (p = .08) relative to women.
Pearson correlations between affect and pain-related measures, as well as the means and standard deviations for males, females, and the total sample, are presented in Table 2. Men evidenced significantly higher average (stable) positive affect from daily diaries (p = .03) and significantly lower central sensitization index scores (p < .001). A marginally significant sex difference was observed on WOMAC pain scores, with men reporting lower KOA-specific clinical pain (p = .07). No other measures were significantly different between sexes.
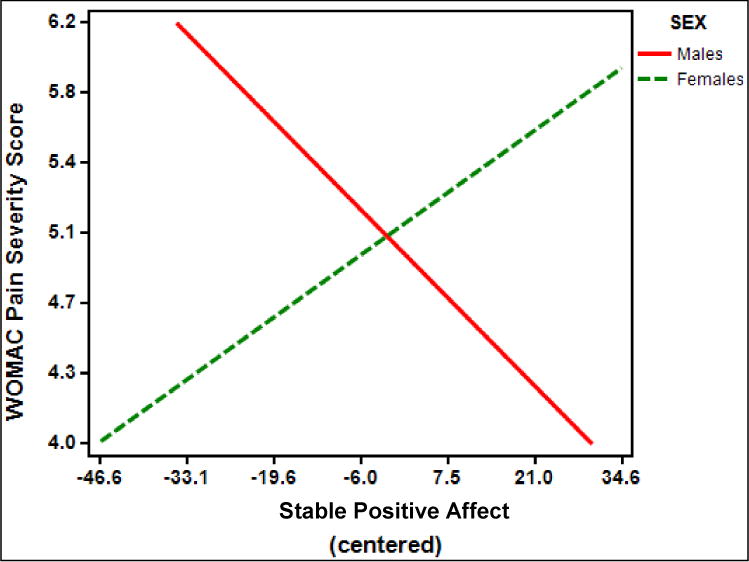
Multiple linear regression analyses revealed a significant interaction between stable positive affect and sex on KOA-specific clinical pain as measured by WOMAC pain severity scores (B = −.06, standard error [SE] = .02; t (169) = −2.45, p = .02, partial η2 = .04) (Figure 1). Post-hoc examination revealed that for males, there was a trend toward an inverse relationship between stable positive affect and WOMAC pain severity scores (t(162) = −1.71; simple slope = −.034; SE of simple slope = .02; p = .089). For females, there was no relationship between stable positive affect and WOMAC pain severity scores (t(162) = 1.53; simple slope = .02; SE of simple slope = .016; p = .13).
Figure 1.
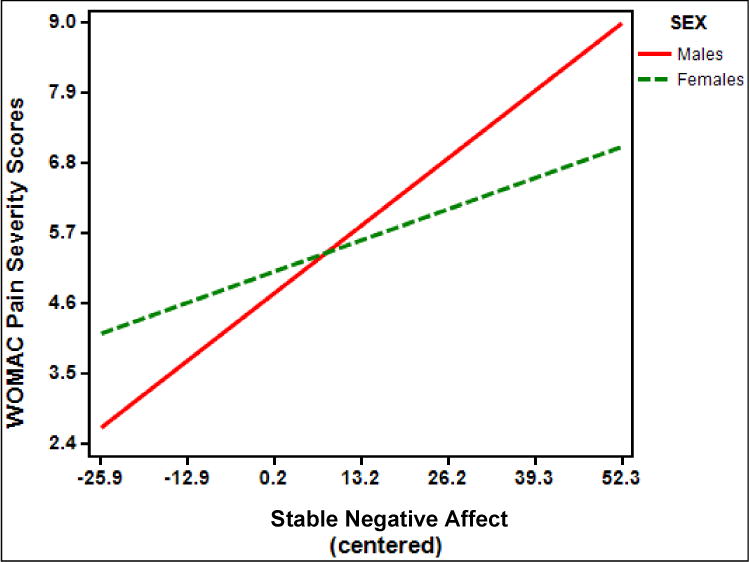
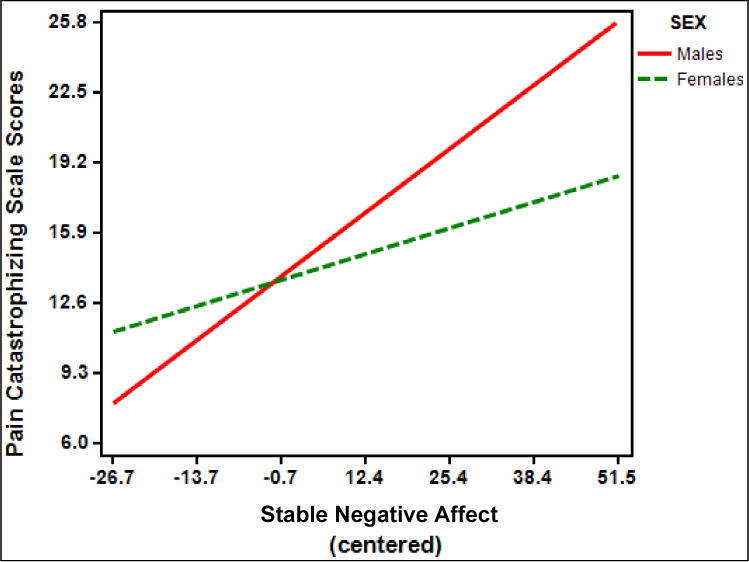
There was also a significant interaction between stable negative affect and sex (B = .04, standard error [SE] = .02; t (169) = 2.37, p = .02, partial η2 = .03) on KOA-specific clinical pain, indicating that women had a significant positive relationship between stable negative affect and WOMAC pain severity scores (t(162) = 2.94; simple slope = .037; SE of simple slope = .013; p = .004); however, the positive relationship between stable negative affect and WOMAC pain severity was significantly stronger for men (t(162) = 5.25; simple slope = .081; SE of simple slope = .015; p < .001) (Figure 2).
Figure 2.
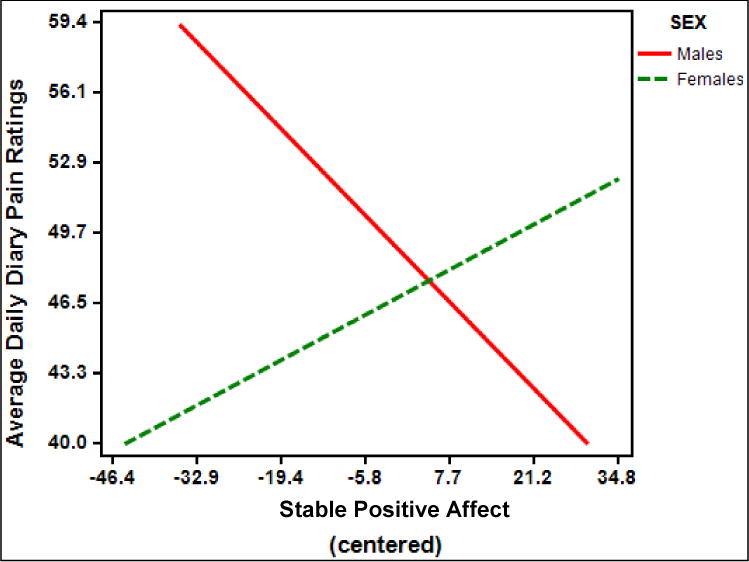
Regarding non-specific clinical pain, there was an interaction between stable positive affect and sex on average daily pain ratings (B = −.45, standard error [SE] = .20; t (172) = −2.290, p = .02, partial η2 = .03) (Figure 3). Post-hoc examination of simple slopes revealed that for males, there was a trend toward an inverse relationship between stable positive affect and average daily diary pain ratings (t(165) = −1.78; simple slope = −.29; SE of simple slope = .16; p = .077). For females, there was no relationship between stable positive affect and average daily diary pain ratings (p = .22).
Figure 3.
The interaction between stable negative affect and sex on average daily pain ratings across diary days was not significant (p = .12, partial η2 = .02).
In separate models, we tested sex × stable affect interactions on pain catastrophizing. Neither the sex × stable positive affect interaction (p = .07; partial η2 = .02; Figure 4) nor the sex × stable negative affect interaction (p = .054, partial η2 = .02; Figure 5) was significant. However, the direction of each effect was similar to those observed in WOMAC and average daily diary pain analyses, and the effect size estimates were comparable.
Figure 4.
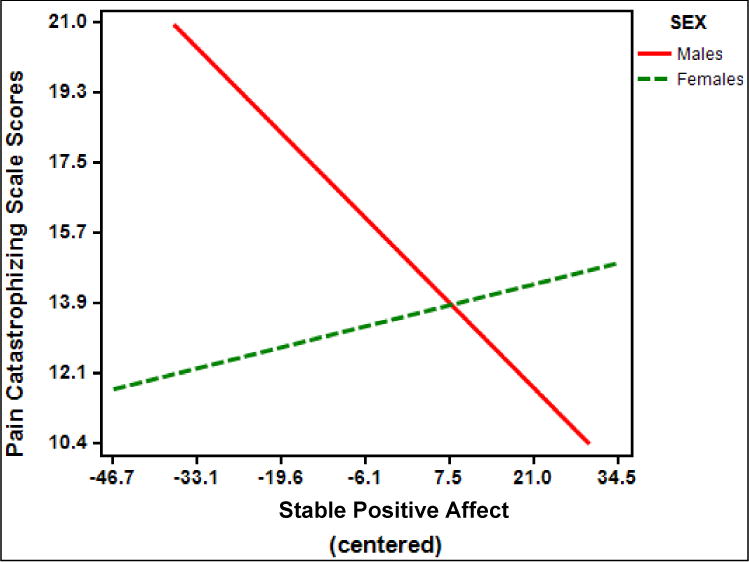
Figure 5.
Three-way interactions between sex, stable positive affect, and stable negative affect failed to reach significance in all models (p’s > .26).
3.3. Interactions between stable affect and sex on laboratory pain
Multiple linear regression analyses failed to show a significant interaction between stable positive affect and sex (p = .11) or a significant interaction between stable negative affect and sex (p = .43) on CS index scores derived from QST procedures. There also was no significant three-way interaction between sex, stable positive affect, and stable negative affect on CS index scores (p = .72).
3.4. Interactions between state affect and sex on daily clinical pain
Separate mixed effects models were conducted to test for significant interactions between state positive affect/negative affect and sex on both same-day pain and next-day pain from the daily diary measures. Regarding same-day pain, there were no significant interactions between sex and state positive affect (p = .65) or state negative affect (p = .51). Similarly, when next-day pain was included as the dependent variable, there were no significant interactions between sex and state positive affect (p = .15) or state negative affect (p = .55). There also was no significant three-way interaction between sex, state positive affect, and state negative affect on same-day pain (p = .16) or next-day pain (p = .78).
3.5. Interactions between state (“lab”) affect and sex on laboratory pain
Multiple linear regression analyses revealed no significant sex × state affect (i.e., baseline positive affect and negative affect ratings collected immediately prior to QST procedures) interactions on CS index scores (p’s > .42).
4. DISCUSSION
The current study aimed to examine whether sex moderates the relationships between affect and pain outcomes in a sample of adults with KOA. Two dimensions of affect were examined: valence (positive affect and negative affect) and temporal stability (stable and state). Based on previous empirical and theoretical work on sex, affect, and pain [10, 25, 43], we hypothesized that women would evidence stronger positive associations between negative affect and pain, and men would show stronger inverse associations between positive affect and pain.
Consistent with previous research [28, 30], we found that stable positive affect was inversely associated with KOA-specific, non-specific clinical pain, and pain catastrophizing; while, stable negative affect was positively associated with all pain outcomes [25, 33, 63]. Neither stable positive affect nor stable negative affect was associated with CS to pain measured by our QST battery. In contrast, state (“lab”) positive affect assessed immediately prior to QST was not associated with any of the pain outcome variables; however, state (“lab”) negative affect was positively associated with non-specific clinical pain, pain catastrophizing, and CS to pain under QST. CS index scores were positively associated with KOA-specific and non-specific clinical pain, providing further support for the use of CS index scores as a meaningful way of characterizing individual differences in pain sensitivity, and perhaps as a biomarker of clinical pain severity and outcomes [52, 54, 64].
Notable findings of sex differences included that men reported higher stable positive affect than women. Men also evidenced significantly lower CS and marginally lower KOA-specific clinical pain ratings than women. This finding extends work by Bartley et al [65] which demonstrated that women with KOA exhibited greater experimental pain sensitivity compared to men. Overall, these findings support the extant literature that women, including those with osteoarthritis, experience greater pain severity than men[16, 65, 66] and experience greater sensitivity to certain types of laboratory pain [9–12]. Taken together, further investigation of sex-specific biological mechanisms of central sensitization are indicated.
Examining the relationships between affect and pain as a function of sex revealed significant interactions between sex and stable positive affect on both KOA-specific and nonspecific clinical pain, and a marginally significant sex × stable positive affect interaction on pain catastrophizing. Only men showed trends toward inverse relationships between stable positive affect and pain, suggesting that men may be more sensitive to potentially pain-related benefits of positive affect.
Indeed, numerous studies of healthy volunteers have demonstrated that positive emotions inhibit pain [67, 68]. Moreover, previous studies suggest that men have a greater tendency than women to experience positive emotions [25, 40, 67, 69], and this sex difference in positive affect was supported in our sample. Our findings, although not significant, were consistent with the hypothesis that men would show inverse relationships between positive affect and pain. Notably, our sample included fewer men (n = 59) than women (n = 120), thus reducing our power to detect significant pain-affect associations among men. However, given that men in our sample evidenced significantly higher stable positive affect, lower CS index scores, and marginally lower KOA-specific pain severity than women, our findings indicate a small but potentially meaningful impact of stable positive affect as a resilience factor against pain that is specific to male KOA patients. As such, interventions that aim to increase positive affect may be particularly fruitful for reducing pain in men.
Sex also interacted with stable negative affect on KOA-specific pain severity. A similar interaction between sex and stable negative affect on pain catastrophizing trended toward significance. Contrary to our hypotheses, however, post-hoc analyses revealed that the positive relationship between stable negative affect and pain outcomes was significantly stronger for men than women, suggesting that men in our sample may also be more sensitive to the pain-enhancing effects of negative affect than women.
There have been some reports of men showing a greater sensitivity to the effects of negative affect on pain. For example, Jones and colleagues [70] found that men, but not women, with high trait anxiety reported higher pain intensity and unpleasantness, and lower pain tolerance during a cold pain task than men with low trait anxiety. Other studies have found no moderating effect of gender on the relationship between negative affect and pain [71, 72]. Clearly, more research is needed in order to better understand the nature of sex-specific effects of negative affect on pain, but our findings suggest an association between stable negative affect and KOA-specific clinical pain, and perhaps an association with pain-related catastrophizing and stable negative affect, in men. Our findings have important therapeutic implications as clinicians may help patients understand their inherent affective vulnerabilities or strengths in managing chronic pain [73]. Notably, the relationships between state positive affect/negative affect and pain outcomes did not differ as a function of sex. Additionally, there were no interactions between sex and either stable or state affect on CS to pain under QST. Therefore, additional research is also needed to elucidate potential mechanisms driving the sex difference in CS that was identified.
Previous studies within women-only arthritis samples have reported that positive affect can serve as a resilience factor to protect against pain [31], yet we did not find this effect among women in the current sample. While it is unclear why females in the current study failed to show a significant relationship between positive affect and pain, various hypotheses could be considered. First, women may experience social reward for endorsing heightened clinical pain and distress. Studies show that women are more likely than men to seek social support to cope with pain in particular [74]. While engagement in social interactions may promote positive affect [75], seeking emotional support from others has been linked to increased pain [75]. It is possible that women with increased pain may be more likely to seek social support, which may promote enhanced positive affect without necessarily improving pain, and may even reinforce their tendencies to report higher clinical pain and distress. Future studies are warranted to investigate how specific interpersonal events relate to affect and pain in osteoarthritis patients.
Gender differences in the affect-pain association may also be related to hormonal effects. Our finding of increased CS among women provides some indication that biological mechanisms may be at work in differentiating vulnerabilities to pain between men and women; however, no study to our knowledge has directly examined the relationship between affect, sex hormones, and pain. As such, further investigations of the effects of sex hormones on negative affect, positive affect, and pain among KOA patients are warranted.
Several limitations should be considered. First, this study was not specifically designed to examine sex differences in psychological factors impacting pain. Indeed, the larger aims of the study were focused on the role of sleep disturbances in KOA; therefore, the majority of the sample met diagnostic criteria for insomnia, thus potentially introducing selection bias of our results [76] and possibly limiting generalizability of the current findings to non-sleep disordered populations of KOA patients. It is worth noting, however, that sleep complaints are extremely common among individuals with KOA, with as many as 81% of patients reporting difficulty with sleep maintenance on one or more nights per week [77].
Additionally, the gender ratio of our sample was unbalanced in that it included more women than men, and we did not match men and women on key factors, such as insomnia status, that may have impacted pain levels. However, we controlled for ISI scores in all analyses, in order to mitigate the effects of insomnia on the differential effects of affect on pain as a function of sex. Future studies should aim to recruit larger, matched samples of men and women to better understand the association of affect and pain as a function of sex. The current study also did not include any objective measures of stress or affective functioning, such as psychophysiological data or cortisol levels, nor did we measure sex hormones that may have influenced findings. Also, the analyses utilized in the current study preclude us from drawing conclusions about the directionality of the identified relationships between affect and pain. Finally, our understanding of the role of central sensitization in clinical research remains elusive. As discussed in the extant literature, a clear set of diagnostic criteria for the phenomenon of central sensitization remains undefined. Fortunately, previous work from our group [52] and others [65] have shown that CS index scores associate with KOA-specific and non-specific clinical pain. However, findings of pain-related hypersensitivity remain limited in the implications and translatability of centrally-mediated pain mechanisms [78, 79]. Despite these limitations, the current study provides novel data that, to our knowledge, have not been previously reported, suggesting that the association of positive affect, negative affect, and pain are different in men versus women with KOA. Despite decades of osteoarthritis research, a paucity of literature exists on sex differences. More work is clearly needed to elucidate biologic mechanisms underlying potential centrally mediated differences in pain between men and women. Further understanding of the difference in affective expression between men and women may lead to the development of novel therapeutic interventions and help to identify additional modifiable factors that may uniquely serve as more effective resilience factors in the prevention and management of pain.
Highlights.
In adults with knee osteoarthritis, sex moderates relationship of pain and affect.
Men reported higher stable positive affect and lower central sensitization to pain.
Women showed no relationship between stable positive affect and pain.
Men showed stronger relationship between stable negative affect and pain.
Men may be more sensitive to sex-specific effects of affect on pain.
Acknowledgments
This work was supported by NIH grants R01 AR054871 (MTS), R01 AR059410 (MTS), K23 DA035915 (PHF), P30 NR014131 (PHF), and T32 NS070201-12 (TJS, JMR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We have no conflicts of interest to disclose.
Conflict of interest
Authors Speed and Richards have no disclosures or financial or personal conflicts of interest to report. Finan and Smith both serve as consultants for PainCare, LLC. The authors report no other financial or personal conflicts.
Ethical issues
All participants provided written consent. The study was approved by the Johns Hopkins School of Medicine Institutional Review Board. The study is registered on clinicaltrials.gov as NCT00592449.
Reference List
- 1.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. Sex, gender, and pain: a review of recent clinical and experimental findings. The journal of pain : official journal of the American Pain Society. 2009;10(5):447–85. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keogh EM. Sex differences in pain across the life course. In: Moore R, editor. Handbook of Pain and Palliative Care. Springer; 2013. [Google Scholar]
- 3.Bingefors K, Isacson D. Epidemiology, co-morbidity, and impact on health-related quality of life of self-reported headache and musculoskeletal pain–a gender perspective. European journal of pain (London, England) 2004;8(5):435–50. doi: 10.1016/j.ejpain.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Kindler LL, Valencia C, Fillingim RB, George SZ. Sex differences in experimental and clinical pain sensitivity for patients with shoulder pain. European journal of pain (London, England) 2011;15(2):118–23. doi: 10.1016/j.ejpain.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65(2–3):123–67. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 6.Keogh MJ, Atkinson S, Maniscalco JM. Body condition and endocrine profiles of Steller sea lion (Eumetopias jubatus) pups during the early postnatal period. General and comparative endocrinology. 2013;184:42–50. doi: 10.1016/j.ygcen.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Blyth FM, March LM, Brnabic AJ, Jorm LR, Williamson M, Cousins MJ. Chronic pain in Australia: a prevalence study. Pain. 2001;89(2–3):127–34. doi: 10.1016/s0304-3959(00)00355-9. [DOI] [PubMed] [Google Scholar]
- 8.Keefe FJ, Lefebvre JC, Egert JR, Affleck G, Sullivan MJ, Caldwell DS. The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: the role of catastrophizing. Pain. 2000;87(3):325–34. doi: 10.1016/S0304-3959(00)00296-7. [DOI] [PubMed] [Google Scholar]
- 9.Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. British journal of anaesthesia. 2013;111(1):52–8. doi: 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. Sex, gender, and pain: a review of recent clinical and experimental findings. The journal of pain : official journal of the American Pain Society. 2009;10(5):447–85. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riley JL, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74(2–3):181–7. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 12.Riley JL, Robinson ME, Wise EA, Price DD. A meta-analytic review of pain perception across the menstrual cycle. Pain. 1999;81(3):225–35. doi: 10.1016/S0304-3959(98)00258-9. [DOI] [PubMed] [Google Scholar]
- 13.Berkley KJ. Sex differences in pain. The Behavioral and brain sciences. 1997;20(3):371–80. doi: 10.1017/s0140525x97221485. discussion 435. [DOI] [PubMed] [Google Scholar]
- 14.Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. The journal of pain : official journal of the American Pain Society. 2010;11(11):1230–9. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Shinal RM, Fillingim RB. Overview of orofacial pain: epidemiology and gender differences in orofacial pain. Dental clinics of North America. 2007;51(1):1–18. v. doi: 10.1016/j.cden.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2005;13(9):769–81. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 17.George SZ, Fritz JM, Childs JD, Brennan GP. Sex differences in predictors of outcome in selected physical therapy interventions for acute low back pain. The Journal of orthopaedic and sports physical therapy. 2006;36(6):354–63. doi: 10.2519/jospt.2006.2270. [DOI] [PubMed] [Google Scholar]
- 18.Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G. Choini+¿re M. A systematic literature review of 10 years of research on sexgender and experimental pain perceptionpart are there really differences between women and men Pain 153602. 2012;1 doi: 10.1016/j.pain.2011.11.025. SRC - GoogleScholar. [DOI] [PubMed] [Google Scholar]
- 19.Bush FM, Harkins SW, Harrington WG, Price DD. Analysis of gender effects on pain perception and symptom presentation in temporomandibular pain. Pain. 1993;53(1):73–80. doi: 10.1016/0304-3959(93)90058-W. [DOI] [PubMed] [Google Scholar]
- 20.Robinson ME, Dannecker EA, George SZ, Otis J, Atchison JW, Fillingim RB. Sex differences in the associations among psychological factors and pain report: a novel psychophysical study of patients with chronic low back pain. The journal of pain : official journal of the American Pain Society. 2005;6(7):463–70. doi: 10.1016/j.jpain.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Pressman SD, Cohen S. Does positive affect influence health? Psychological bulletin. 2005;131(6):925–71. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- 22.Reich JW, Zautra AJ, Davis M. Dimensions of affect relationships: Models and their integrative implications. Review of General Psychology. 2003;7(1):66–83. SRC - GoogleScholar. [Google Scholar]
- 23.Gable SL, Reis HT, Elliot AJ. Behavioral activation and inhibition in everyday life. J Pers Soc Psychol. 2000;78(6):1135–49. doi: 10.1037//0022-3514.78.6.1135. [DOI] [PubMed] [Google Scholar]
- 24.Edwards RR, Cahalan C, Calahan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nature reviews Rheumatology. 2011;7(4):216–24. doi: 10.1038/nrrheum.2011.2. [DOI] [PubMed] [Google Scholar]
- 25.Rhudy JL, Williams AE. Gender differences in pain: do emotions play a role? Gender medicine. 2005;2(4):208–26. doi: 10.1016/s1550-8579(05)80051-8. [DOI] [PubMed] [Google Scholar]
- 26.Keefe FJ, Smith SJ, Buffington AL, Gibson J, Studts JL, Caldwell DS. Recent advances and future directions in the biopsychosocial assessment and treatment of arthritis. J Consult Clin Psychol. 2002;70(3):640–55. doi: 10.1037//0022-006x.70.3.640. [DOI] [PubMed] [Google Scholar]
- 27.Zautra AJ, Smith BW. Depression and reactivity to stress in older women with rheumatoid arthritis and osteoarthritis. Psychosom Med. 2001;63(4):687–96. doi: 10.1097/00006842-200107000-00022. [DOI] [PubMed] [Google Scholar]
- 28.Strand EB, Kerns RD, Christie A, Haavik-Nilsen K, Klokkerud M, Finset A. Higher levels of pain readiness to change and more positive affect reduce pain reports–a weekly assessment study on arthritis patients. Pain. 2007;127(3):204–13. doi: 10.1016/j.pain.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Fisher MN, Snih SA, Ostir GV, Goodwin JS. Positive affect and disability among older Mexican Americans with arthritis. Arthritis Rheum. 2004;51(1):34–9. doi: 10.1002/art.20079. [DOI] [PubMed] [Google Scholar]
- 30.Finan PH, Garland EL. The role of positive affect in pain and its treatment. Clin J Pain. 2015;31(2):177–87. doi: 10.1097/AJP.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zautra AJ, Johnson LM, Davis MC. Positive affect as a source of resilience for women in chronic pain. J Consult Clin Psychol. 2005;73(2):212–20. doi: 10.1037/0022-006X.73.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porter LS, Gil KM, Carson JW, Anthony KK, Ready J. The role of stress and mood in sickle cell disease pain: an analysis of daily diary data. J Health Psychol. 2000;5(1):53–63. doi: 10.1177/135910530000500109. [DOI] [PubMed] [Google Scholar]
- 33.Finan PH, Quartana PJ, Smith MT. Positive and negative affect dimensions in chronic knee osteoarthritis: effects on clinical and laboratory pain. Psychosomatic medicine. 2013;75(5):463–70. doi: 10.1097/PSY.0b013e31828ef1d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zautra AJ, Sturgeon JA. Examining the complexities of affective experience will enhance our understanding of pain and inform new interventions designed to bolster resilience. Pain. 2016;157(8):1586–7. doi: 10.1097/j.pain.0000000000000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vigil JM. A socio-relational framework of sex differences in the expression of emotion. The Behavioral and brain sciences. 2009;32(5):375–90. doi: 10.1017/S0140525X09991075. discussion 391. [DOI] [PubMed] [Google Scholar]
- 36.Bekker MHJ, van Mens-Verhulst J. Anxiety disorders: sex differences in prevalence, degree, and background, but gender-neutral treatment. Gender medicine. 2007;4(Suppl B):S178–93. doi: 10.1016/s1550-8579(07)80057-x. [DOI] [PubMed] [Google Scholar]
- 37.Marcus SM, Kerber KB, Rush AJ, Wisniewski SR, Nierenberg A, Balasubramani GK, Ritz L, Kornstein S, Young EA, Trivedi MH. Sex differences in depression symptoms in treatment-seeking adults: confirmatory analyses from the Sequenced Treatment Alternatives to Relieve Depression study. Comprehensive psychiatry. 2008;49(3):238–46. doi: 10.1016/j.comppsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLean CP, Anderson ER. Brave men and timid women? A review of the gender differences in fear and anxiety. Clinical psychology review. 2009;29(6):496–505. doi: 10.1016/j.cpr.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 39.LaFrance M, Hecht MA, Paluck EL. The contingent smile: a meta-analysis of sex differences in smiling. Psychological bulletin. 2003;129(2):305–34. doi: 10.1037/0033-2909.129.2.305. [DOI] [PubMed] [Google Scholar]
- 40.Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: sex differences in picture processing. Emotion (Washington, D.C) 2001;1(3):300–19. [PubMed] [Google Scholar]
- 41.Logan H, Lutgendorf S, Rainville P, Sheffield D, Iverson K, Lubaroff D. Effects of stress and relaxation on capsaicin-induced pain. The journal of pain : official journal of the American Pain Society. 2001;2(3):160–70. doi: 10.1054/jpai.2001.21597. [DOI] [PubMed] [Google Scholar]
- 42.Logan HL, Gedney JJ. Sex differences in the long-term stability of forehead cold pressor pain. The journal of pain : official journal of the American Pain Society. 2004;5(7):406–12. doi: 10.1016/j.jpain.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Fillingim RB, Hastie BA, Ness TJ, Glover TL, Campbell CM, Staud R. Sex-related psychological predictors of baseline pain perception and analgesic responses to pentazocine. Biological psychology. 2005;69(1):97–112. doi: 10.1016/j.biopsycho.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Keefe FJ, Affleck G, France CR, Emery CF, Waters S, Caldwell DS, Stainbrook D, Hackshaw KV, Fox LC, Wilson K. Gender differences in pain, coping, and mood in individuals having osteoarthritic knee pain: a within-day analysis. Pain. 2004;110(3):571–7. doi: 10.1016/j.pain.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 45.Affleck G, Tennen H, Keefe FJ, Lefebvre JC, Kashikar-Zuck S, Wright K, Starr K, Caldwell DS. Everyday life with osteoarthritis or rheumatoid arthritis: independent effects of disease and gender on daily pain, mood, and coping. Pain. 1999;83(3):601–9. doi: 10.1016/S0304-3959(99)00167-0. [DOI] [PubMed] [Google Scholar]
- 46.Quartana PJ, Finan PH, Page GG, Smith MT. Effects of insomnia disorder and knee osteoarthritis on resting and pain-evoked inflammatory markers. Brain behavior and immunity. 2014 doi: 10.1016/j.bbi.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schramm E, Hohagen F, Grasshoff U, Riemann D, Hajak G, Weess HG, Berger M. Test-retest reliability and validity of the Structured Interview for Sleep Disorders According to DSM-III-R. The American journal of psychiatry. 1993;150(6):867–72. doi: 10.1176/ajp.150.6.867. [DOI] [PubMed] [Google Scholar]
- 48.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep medicine. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 49.Jensen MP, McFarland CA. Increasing the reliability and validity of pain intensity measurement in chronic pain patients. Pain. 1993;55(2):195–203. doi: 10.1016/0304-3959(93)90148-I. [DOI] [PubMed] [Google Scholar]
- 50.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. The Journal of rheumatology. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 51.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychological assessment. 1995;5247 SRC - GoogleScholar. [Google Scholar]
- 52.Campbell CM, Buenaver LF, Finan P, Bounds SC, Redding M, McCauley L, Robinson M, Edwards RR, Smith MT. Sleep, Pain Catastrophizing, and Central Sensitization in Knee Osteoarthritis Patients With and Without Insomnia. Arthritis care & research. 2015;67(10):1387–96. doi: 10.1002/acr.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Finan PH, Buenaver LF, Bounds SC, Hussain S, Park RJ, Haque UJ, Campbell CM, Haythornthwaite JA, Edwards RR, Smith MT. Discordance between pain and radiographic severity in knee osteoarthritis: findings from quantitative sensory testing of central sensitization. Arthritis and rheumatism. 2013;65(2):363–72. doi: 10.1002/art.34646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aiken LS, West SG, Reno RR. Multiple regression: Testing and interpreting interactions. Singer; 1991. [Google Scholar]
- 56.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Medicine Reviews. 2004;8(2):119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 57.Campbell CM, Edwards RR. Ethnic differences in pain and pain management. Pain Manag. 2012;2(3):219–230. doi: 10.2217/pmt.12.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Riley JL, 3rd, Cruz-Almeida Y, Glover TL, King CD, Goodin BR, Sibille KT, Bartley EJ, Herbert MS, Sotolongo A, Fessler BJ, Redden DT, Staud R, Bradley LA, Fillingim RB. Age and race effects on pain sensitivity and modulation among middle-aged and older adults. J Pain. 2014;15(3):272–82. doi: 10.1016/j.jpain.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Frontiers in Psychology. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: Academic Press; 1977. [Google Scholar]
- 62.Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence Oxford university press. 2003 [Google Scholar]
- 63.Edwards RR, Cahalan C, Calahan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nature reviews Rheumatology. 2011;7(4):216–24. doi: 10.1038/nrrheum.2011.2. [DOI] [PubMed] [Google Scholar]
- 64.Malfait AM, Schnitzer TJ. Towards a mechanism-based approach to pain management in osteoarthritis. Nature reviews Rheumatology. 2013;9(11):654–64. doi: 10.1038/nrrheum.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bartley EJ, King CD, Sibille KT, Cruz-Almeida Y, Riley JL, 3rd, Glover TL, Goodin BR, Sotolongo AS, Herbert MS, Bulls HW, Staud R, Fessler BJ, Redden DT, Bradley LA, Fillingim RB. Enhanced Pain Sensitivity Among Individuals With Symptomatic Knee Osteoarthritis: Potential Sex Differences in Central Sensitization. Arthritis Care Res (Hoboken) 2016;68(4):472–80. doi: 10.1002/acr.22712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman BN, Aliabadi P, Levy D. The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1995;38(10):1500–5. doi: 10.1002/art.1780381017. [DOI] [PubMed] [Google Scholar]
- 67.Meagher MW, Arnau RC, Rhudy JL. Pain and emotion: effects of affective picture modulation. Psychosomatic medicine. 2001;63(1):79–90. doi: 10.1097/00006842-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 68.Weaver J, Zillmann D. Effect of humor and tragedy on discomfort tolerance. Perceptual and motor skills. 1994;78(2):632–4. doi: 10.2466/pms.1994.78.2.632. [DOI] [PubMed] [Google Scholar]
- 69.Murnen SK, Smolak L. Femininity, masculinity, and disordered eating: a meta-analytic review. The International journal of eating disorders. 1997;22(3):231–42. doi: 10.1002/(sici)1098-108x(199711)22:3<231::aid-eat2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 70.Jones A, Zachariae R, Arendt-Nielsen L. Dispositional anxiety and the experience of pain: gender-specific effects. European journal of pain (London, England) 2003;7(5):387–95. doi: 10.1016/S1090-3801(02)00139-8. [DOI] [PubMed] [Google Scholar]
- 71.Rhudy JL, Meagher MW. Fear and anxiety: divergent effects on human pain thresholds. Pain. 2000;84(1):65–75. doi: 10.1016/S0304-3959(99)00183-9. [DOI] [PubMed] [Google Scholar]
- 72.Willer JC, Albe-Fessard D. Electrophysiological evidence for a release of endogenous opiates in stress-induced’analgesia’ in man. Brain research. 1980;198(2):419–26. doi: 10.1016/0006-8993(80)90755-6. [DOI] [PubMed] [Google Scholar]
- 73.Linton SJF, AE A hybrid emotion-focused exposure treatment for chronic pain: a feasibility study. Scandinavian Journal of pain. 2014;5:151–158. doi: 10.1016/j.sjpain.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 74.Weir A, Lipman M, Congleton J. Co-trimoxazole in Wegener’s granulomatosis. The New England journal of medicine. 1996;335(23):1769–70. [PubMed] [Google Scholar]
- 75.Finan PH, Okun MA, Kruszewski D, Davis MC, Zautra AJ, Tennen H. Interplay of concurrent positive and negative interpersonal events in the prediction of daily negative affect and fatigue for rheumatoid arthritis patients. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2010;29(4):429–37. doi: 10.1037/a0020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–25. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 77.Wilcox S, Brenes GA, Levine D, Sevick MA, Shumaker SA, Craven T. Factors related to sleep disturbance in older adults experiencing knee pain or knee pain with radiographic evidence of knee osteoarthritis. Journal of the American Geriatrics Society. 2000;48(10):1241–51. doi: 10.1111/j.1532-5415.2000.tb02597.x. [DOI] [PubMed] [Google Scholar]
- 78.Hansson P. Translational aspects of central sensitization induced by primary afferent activity: what it is and what it is not. Pain. 2014;155(10):1932–4. doi: 10.1016/j.pain.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 79.Woolf CJ. What to call the amplification of nociceptive signals in the central nervous system that contribute to widespread pain? Pain. 2014;155(10):1911–2. doi: 10.1016/j.pain.2014.07.021. [DOI] [PubMed] [Google Scholar]


