Abstract
Aconitum species (belonging to the Ranunculaceae) are well known herbaceous medicinal ingredients and have great economic value in Asian countries. However, there are still limited genomic resources available for Aconitum species. In this study, we sequenced the chloroplast (cp) genomes of two Aconitum species, A. coreanum and A. carmichaelii, using the MiSeq platform. The two Aconitum chloroplast genomes were 155,880 and 157,040 bp in length, respectively, and exhibited LSC and SSC regions separated by a pair of inverted repeat regions. Both cp genomes had 38% GC content and contained 131 unique functional genes including 86 protein-coding genes, eight ribosomal RNA genes, and 37 transfer RNA genes. The gene order, content, and orientation of the two Aconitum cp genomes exhibited the general structure of angiosperms, and were similar to those of other Aconitum species. Comparison of the cp genome structure and gene order with that of other Aconitum species revealed general contraction and expansion of the inverted repeat regions and single copy boundary regions. Divergent regions were also identified. In phylogenetic analysis, Aconitum species positon among the Ranunculaceae was determined with other family cp genomes in the Ranunculales. We obtained a barcoding target sequence in a divergent region, ndhC–trnV, and successfully developed a SCAR (sequence characterized amplified region) marker for discrimination of A. coreanum. Our results provide useful genetic information and a specific barcode for discrimination of Aconitum species.
Introduction
The genus Aconitum belongs to the Ranunculaceae and consists of approximately 400 species [1]. These grow in temperate regions and are well known herbaceous medicinal ingredients with great economic value in Asian countries. The dried root tubers of A. carmichaelii and A. coreanum have been used as different herbal medicines in Korea and China, namely, Aconiti Lateralis Radix (Bu-ja in Korean and Fu-zi in Chinese) and Aconiti Koreani Tuber (Baek-bu ja in Korean and Guan-bai-fu in Chinese), respectively. In addition, the root tubers of A. kusnezoffii, A. ciliare (a synonym of A. volubile var. pubescens), and A. triphyllum (a synonym of A. jaluense var. triphyllum) have also been used as Aconiti Kusnezoffii Tuber in both countries (Cho-oh in Korean and Cao-wu in Chinese) [2]. The root tubers of A. carmichaelii have pharmacological effects such as cardiotonic action, impact on blood vessels and blood pressure, anti-arrhythmic effects, anti-inflammation, and analgesic action [3], whereas those of A. coreanum have anti-arrhythmic, analgesic, and anti-inflammatory activities [4]. The A. kusnezoffii root tuber (Aconiti Kusnezoffii Tuber) has analgesic, cardiotonic, antioxidant, and immunological activities [5, 6]. Thus, root tubers of Aconitum species have been used as a variety of herbal medicines. Aconitum plants also contain highly toxic components such as diester-diterpene alkaloids including aconitine, mesaconitine, and hypaconitine [3]. Although they are a widely distributed and important source of medicinal herbs, improvements in the accurate identification of Aconitum species are required because it is difficult to determine the species based on the morphological features of plants and medicinal materials.
Chloroplasts (cp) play an important role in photosynthesis and carbon fixation as well as in biosynthesis [7]. The cp genome ranges from 120 to 180 kb in size in higher plants and has a conserved quadripartite structure with a large single copy (LSC) region, a small single copy (SSC) region, and two copies of inverted repeats (IRs) [8]. The cp genome has undergone diverse changes such as changes in size, gene intron gains and losses, expansion/contraction of IRs, and structure rearrangements [9]. However, gene content and orientation are considerably conserved between species [10]. The cp genome consists of 110 to 130 genes with up to 80 unique protein-coding genes, four ribosomal RNAs (rRNAs), and approximately 30 transfer RNAs (tRNAs) [11, 12]. The cp genome has been widely used to understand phylogenetic relationships and to identify useful molecular markers, which are used for DNA barcoding, namely super DNA barcoding and to authenticate and identify herbal medicines [13]. The universal plant DNA barcodes ITS, matk, and rbcL have been used for the evaluation of phylogenetic relationships [14, 15]. In a previous report, the psbA–trnH intergenic region showed genetic variation in 134 individuals belonging to 19 taxa of Aconitum [16], indicating indicating interspecies variation of more than 85% in the psbA–trnH intergenic spacer. Nineteen taxa of Aconitum were classified into ten subgroups. However, in Aconitum species, more genomic information is required for accurate identification. Although Aconitum species are important in herbal medicine, there is little genomic and sequence information available.
The sequence characterized amplified region (SCAR) markers are valuable genetic tools for the authentication of medicinal plants and herbal medicines because they are simple, reliable, and reproducible [13, 17]. The SACR marker was developed based on sequence information obtained using DNA fingerprinting with the random amplified polymorphic DNA (RAPD) method, and has been used to identify closely related species by PCR amplification of specific target regions [18, 19]. Species identification based on SCAR markers does not need DNA subcloning or sequencing and is more applicable to large scale analyses using multiple samples than the DNA barcoding method. Furthermore, the SCAR assay method can easily discriminate contaminants in a single PCR amplification reaction. Because the SCAR assay is easy to perform, yields highly reproducible results, and allows discrimination between genera and/or species, it is a simple and rapid tool for authenticating herbal medicines and identifying species [17]. In a previous study, important medicinal herbs in Korea, Aralia continentalis (Araliae Continentalis Radix) and Angelica biserrata (Angelicae Pubescentis Radix), were clearly distinguished and tested for adulteration using a SCAR marker. Also, commercial herbal medicines were tested and were confirmed to be derived from refined herbal medicines and to be free of adulteration [13]. Therefore, a SCAR marker is a powerful molecular tool for determining the botanical origin of herbs used in herbal medicine and for detecting adulterants [20–22].
In this study, we completed de novo assembly of cp genomes from A. coreanum and A. carmichaelii and compared them with those from other Aconitum species to discover highly divergent regions. We also developed a SCAR marker, based on a sequence from a variable gene region in cp DNA, that can distinguish A. coreanum from other Aconitum species. The results will be useful for the standardization and quality control of the herbal medicine Aconiti Koreani Tuber, and could help provide important and valuable information for the verification of the evolutional traits and genetic diversity of A. coreanum and species belonging to the genus Aconitum L., as well as for the genetic engineering of these species.
Materials and methods
Plant material, DNA extraction and Illumina sequencing
Plant materials were collected from native habitats and a private farming field in Korea. A sample collection permit of the Korean Institute of Oriental Medicine was obtained from Korean National Parks Service for native habitats at June, 2011 (permit number, Korean National Parks Service-1112). The leaves of A. carmichaelii and A. coreanum were used for next-generation sequencing (NGS) analysis, and 27 Aconitum germplasms were used for validation of the SCAR marker. DNA was extracted using a DNeasy Plant Maxi kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Total libraries from A. carmichaelii and A. coreanum were generated using the MiSeq platform (Illumina, San Diego, CA, USA) by LabGenomics, Korea.
Chloroplast genome assembly and annotation
Cp genomes were obtained by de novo assembly from the low coverage whole genome sequence derived from the Phyzen pipeline (http://phyzen.com). Paired-end reads (Phred scores of 20 or more) were assembled using CLC genome assembler (ver. 4.06 beta, CLC Inc, Aarhus, Denmark) with an autonomously controlled overlap size parameter of 200 to 600 bp. The principal contigs in the cp genome were retrieved from total contigs using Nucmer [23] with the cp genome sequence of Aconitum barbatum var. puberulum (KC844054) as the reference sequence. The contigs were ordered based on the results of BLASTZ analysis [24]. Filtered contigs were linked into a single draft sequence by joining the overlapping terminal sequences of contigs. Gene annotation was performed using the DOGMA program [25] and manual curation was performed using BLAST searches. To verify the exact gene region, reference genomes were compared. Circular maps of A. carmichaelii and A. coreanum were obtained using OGDRAW [26]. Codon usage and base composition were determined using MEGA6 [27].
Genome comparison and repeat analysis
mVISTA was used to analyze similarities between eight Aconitum species [28]: A. carmichaelii (KY407560), A. coreanum (KU318669), A. volubile (KU556690), A. austrokoreense (KY407559), A. chiisanense (NC029829), A. pseudolaeve (KY407562), A. longecassidatum (KY407561), and A. barbatum (KC844054). The boundaries between IR and SC regions were also compared with those of eight Aconitum species: A. carmichaelii, A. coreanum, A. volubile, A. austrokoreense, A. chiisanense, A. pseudolaeve, A. longecassidatum, A. barbatum, Nicotiana tabacum (NC001879) and Thalictrum coreanum (KM206568). Tandem repeats were detected using Tandem Repeat Finder [29] using a length of over 20 bp as the default parameter and the identity of repeats was set to over 90%. Simple sequence repeats (SSRs) were detected using MISA (http://pgrc.ipk-gatersleben.de/misa/misa) with a minimum number of repeats of 10, 5, 4, 3, 3 and 3 for mono-, di- tri- tetra- and hexa nucleotides, respectively, as the default parameter. IRs were detected using the Inverted Repeats Finder [30] with default parameters. The IRs were 20 bp or more in length and showed 90% similarity.
Phylogenetic analysis
A molecular phylogenetic tree was constructed using 70 protein-coding genes from 38 species, and 36 complete cp genomes were downloaded from GenBank (S1 Table). To identify the phylogenetic position of Aconitum species in the Ranunculaes, 20 cp genomes of 13 Aconitum species, seven from other species of Ranunclaceae, and 8 species of five other families were used with Nicotian tabacum (NC001879) and Arabidopsis thaliana (AP000423) as the outgroup. Seventy protein-coding genes were aligned using MAFFT. (http://mafft.cbrc.jp/alignment/server/). Maximum likelihood analysis was performed using MEGA6 with 1,000 bootstrap replicates [27].
Development of a SCAR marker for A. coreanum
Highly divergent regions between eight Aconitum species were confirmed based on mVISTA similarities, and primers were designed using Primer-BLAST (NCBI). To amplify divergent regions, about 20 ng genomic DNA was added to 20 ul PCR mixture (Solg™ 2× Taq PCR smart mix 1, Solgent, Daejeon, Korea) containing 10 pmol primers (Bioneer, Daejeon, Korea). Amplification was performed on a Pro Flex PCR system (Applied Biosystems, Waltham, MA, USA) according to the following step-cycle program: initial denaturing step at 95°C for 2 min; 35 cycles at 95°C for 40 s, 55°C for 30 s, and 72°C for 90 s; and a final extension at 72°C for 5 min. The PCR products were separated on a 2% agarose gel for 40 min at 150 V. Amplified DNA fragments were extracted from agarose gels using a Gel Extraction Kit (Qiagen, Valencia, CA, USA) and subcloned into the pGEM-T Easy vector (Promega, Madison, WI, USA). Inserted DNA was sequenced using a DNA sequence analyzer (ABI 3730, Applied Biosystems Inc., Foster City, CA, USA). NGS data and Sanger sequencing data were aligned using CLUSTALW by Bioedit [31]. A. coreanum-specific primers were designed for amplification of the SCAR marker. PCR reactions were performed with A. coreanum-specific primers. Amplification conditions were as follows: 95°C for 2 min; 35 cycles at 95°C for 30 s, 63°C for 30 s, and 72°C for 30 s; and a final extension at 72°C for 5 min. Amplified fragments were verified using 1.5% agarose gel electrophoresis.
Results and discussion
Complete chloroplast genome sequence of two Aconitum species
Illumina sequencing of paired-end libraries revealed approximately 2.84 Gb of trimmed reads from A. carmichaelii and A. coreanum (S2 Table). From aligned contigs against the reference cp genome, we obtained five and ten contigs covering the whole chloroplast genome sequences of A. carmichaelii and A. coreanum, respectively (S1 Fig). Single circular sequence was completed after gap filling and manual editing. The complete circular chloroplast genomes of A. carmichaelii and A. coreanum were 155,880 bp and 157,040 bp, respectively (S3 Table). Complete cp genomes were compared with the A. barbatum reference genome [32] using BLASTZ analysis (S1A Fig). We also confirmed by read mapping that the average read mapping depth was 206.52× in A. carmichaelii and 268.92× in A. coreanum (S1B Fig). Furthermore, several divergent regions were confirmed by Sanger sequencing. To perform efficient cp genome sequencing, we used low coverage whole genome sequencing with a reference genome sequence [33]. This approach requires less time and has a lower cost than the previously used method [33, 34].
Both A. carmichaelii and A. coreanum chloroplast genomes had the quadripartite structure found in most land plants consisting of a pair of IRs (52,586 and 52,488 bp, respectively) separated by LSC (86,348 and 87,628 bp) and SSC (16,946 and 19,924 bp) regions (Fig 1 and Table 1). The genomes are similar to those of A. barbatum (156,749 bp) and A. chiisanense (155,934 bp). The total GC content was 38.13% and 37.99% for A. carmichaelii and A. coreanum, respectively, which is similar to published Aconitum species [32, 35]. The GC content of the IRs (42.98%, 43.02%) was higher than that of LSC (36.25%, 36.02%) and SSC (32.69%, 32.66%) regions in both A. carmichaelii and A. coreanum chloroplast genomes. The IR region showed a higher GC content due to the presence of four rRNA (rrn4.5, rrn5, rrn16, and rrn23) sequences. Previous studies have reported that high GC content in IR regions is due to the presence of rRNA [36, 37]. The Aconitum genomes were AT-rich (61.87% in A. carmichaelii, 62.01% in A. coreanum). The chloroplast genomes of A. carmichaelii and A. coreanum encoded a total of 112 unique genes, of which 18 were duplicated in IR regions. Among the 112 genes, there were 78 protein-coding genes, four rRNA genes, and 30 tRNA genes (Table 1 and Table 2).
Fig 1. Circular gene map of the two Aconitum species.
Genes drawn inside the circle are transcribed clockwise, and those outside the circle are transcribed counterclockwise. The darker gray in the inner circle corresponds to GC content.
Table 1. Size comparison of two Aconitum chloroplast genomic regions.
| Species | A. camichaelii | A. coreanum |
|---|---|---|
| Total cp genome size (bp) | 155,880 | 157,040 |
| Large single copy (LSC) region (bp) | 86,348 | 87,628 |
| Inverted repeat (IR) region (bp) | 52,586 | 52,488 |
| Small single copy (SSC) region (bp) | 16,946 | 16,924 |
| GC content (%) | 38.13 | 37.99 |
| LSC (%) | 36.25 | 36.02 |
| IR (%) | 42.98 | 43.02 |
| SSC (%) | 32.69 | 32.66 |
| Total number of genes | 131 | 131 |
| Protein-coding gene | 86 | 86 |
| rRNA | 8 | 8 |
| tRNA | 37 | 37 |
Table 2. Genes present in the two Aconitum chloroplast genomes.
| Gene products of Aconitum species | |
|---|---|
| Photosystem I | psaA, B, C, I, J |
| Photosystem II | psbA, B, C, D, E, F, H, I, J, K, L, M, N, T, Z |
| Cytochrome b6_f | petA, B1), D1), G, L, N |
| ATP synthase | atpA, B, E, F1), H, I |
| Rubisco | rbcL |
| NADH oxidoreductase | ndhA1), B1) 3), C, D, E, F, G, H, I, J, K |
| Large subunit ribosomal proteins | rpl21) 3), 14, 161), 20, 22, 233), 32, 33, 36 |
| Small subunit ribosomal proteins | rps2, 3, 4, 73), 8, 11, 12 2) 3) 4), 14, 15, 18, 19 |
| RNA polymerase | rpoA, B, C11), C2 |
| Unknown function protein-coding gene | ycf13), 23), 32), 4 |
| Other genes | accD, ccsA, cemA, clpP2), infA, matK |
| Ribosomal RNAs | rrn163), rrn233), rrn4.53), rrn53) |
| Transfer RNAs | trnA-UGC1) 3), trnC-GCA, trnD-GUC, trnE-UUC, trnF-GAA, trnG-UCC1), trnG-GCC, trnH-GUG, trnI-CAU3), trnI-GAU1) 3) trnK-UUU1), trnL-UAA1), trnL-UAG, trnL-CAA3), trnM-CAU, trnfM-CAU, trnN-GUU3), trnP-UGG, trnQ-UUG, trnR-ACG3), trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-UAC1), trnV-GAC3), trnW-CCA, trnY-GUA |
1) Gene containing a single intron
2) gene containing two introns
3) two gene copies in IRs
4) Trans-spliced gene.
The two Aconitum chloroplast genomes had 17 intron-containing genes, among which 14 (eight protein-coding and six tRNA genes) had a single intron and two (ycf3, clpP) had two introns (S4 Table). Twelve genes were located in the LSC region (nine protein-coding and three tRNA genes), a protein-coding gene in the SSC region, and four genes in the IR region (two protein-coding and two tRNA genes). The rps12 gene was trans-spliced because its 5’ end was located in the LSC region and its duplicated 3’ end in the IR region [38]. We also detected the non-canonical start codon ACG in two genes, rpl2 and ndhD. The Aconitum cp genomes consisted of 51.05% and 50.59% protein-coding genes (79,590 bp in A. carmichaelii and 79,461 bp in A. coreanum), 1.8% and 1.79% tRNA (2,813 bp in both species), and 5.8% and 5.76% rRNA (9,050 bp in both species). The remaining regions were non-coding sequences with intergenic spacers, introns, and pseudogenes. Two pseudogenes were identified: rps16 had one exon deletion in both A. carmichaelii and A. coreanum. The ycf1 pseudogene was located in the boundary region between IRa and SSC. The codon usage and recognition pattern of the cp genomes are summarized in S5 Table. The 30 unique tRNA genes encode all 20 amino acids essential for protein biosynthesis. A total of 26,530 codons and 26,487 codons were found in A. carmichaelii and A. coreanum, respectively (S5 Table). Among these codons, those coding for leucine and isoleucine were the most common in both Aconitum cp genomes. In the CDS regions, the percentage AT content at each codon position was 54.6%, 61.4% and 69.6% in A. carmichaelii and 54.6% 61.4% and 69.7% in A. coreanum. There was a bias towards a higher AT content at the third position of the CDS, as observed for other Aconitum genomes (S6 Table) [39].
Repeat analysis
Repeat sequences were abundant and diverse in the two genomes, where they play an important role in gene duplication, gene expansion, and DNA rearrangement [40]. Repeat sequences in the Aconitum chloroplast genomes were analyzed using MISA and tandem repeat finder. We detected 18 tandem repeats of over 20 bp in length in both Aconitum cp genomes (S7 Table). The tandem repeats were 26–54 bp in length. Of these, 44.4% were in intergenic spaces, 38.8% in exons, and 16.6% in introns. Most of the tandem repeats (66.6%) were located in the LSC region. The longest repeat (54 bp) was located in ycf2 in both Aconitum cp genomes. Among the coding regions, the most tandem repeats were present in the ycf2 gene, which included three tandem repeats. The ycf1 and ycf2 genes are associated with a divergent region with many repeat events [41, 42]. We also detected palindromic repeats in both cp genomes. Seven and six palindromic repeats were identified in A. carmichaelii and A. coreanum, respectively (Table 3). Among these, six repeats were shared between the two Aconitum cp genomes, and were located in the LSC (four repeats) and IR (two repeats) regions. The length of repeats ranged from 21 to 36 bp.
Table 3. Palindromic repeats in the two Aconitum cp genomes.
| Spbcies | Positiona | Repeat unit length (bp) | Repeat unit sequences | Regionb |
|---|---|---|---|---|
| A. carmichaelii | IGS (trnE-UUC, trnT-GGU) | 23 | TTATTTCTATATATTCTAATGAT | LSC |
| IGS (petA, psbJ) | 36 |
GTAAGAATAAGAACTCAATGGAC CTTGCCCCTCGAA |
LSC | |
| IGS (psbT, psbN) | 26 | TTGAAGTAAAGTAATGAGCCTCCCAT | LSC | |
| IGS (petD, rpoA) | 24 | ATGTATCTAGGGACTAGTCGCTTC | LSC | |
| Exon (ycf2) | 24 | AGATCCATTAGATAATGAACTATT | IR | |
| Exon (ycf15) | 21 | TGGTTGTTCGCCGTTCAAGAA | IR | |
| Exon (ycf1) | 25 | CTTGATTTAGCGAATCTAAATCAAG | SSC | |
| A. coreanum | IGS (trnE-UUC, trnT-GGU) | 23 | TTATTTCTATATATTCTAATGAT | LSC |
| IGS (petA, psbJ) | 36 |
GTAAGAATAAGAACTCAATGGACCTTG CCCCTCAAA |
LSC | |
| IGS (psbT, psbN) | 28 | TTGAAGTAAAGTAATGAGCCTCC-ATAT | LSC | |
| IGS (petD, rpoA) | 24 | ATGTATCTAGGGACTAGTCGCTTC | LSC | |
| Exon (ycf2) | 24 | AGATCCATTAGATAATGAACTATT | IR | |
| Exon (ycf15) | 21 | TGGTTGTTCGCCGTTCAAGAA | IR |
a IGS, Intergenic sequence
b LSC, Large single copy; IR, Inverted repeat region; SSC, Small single copy.
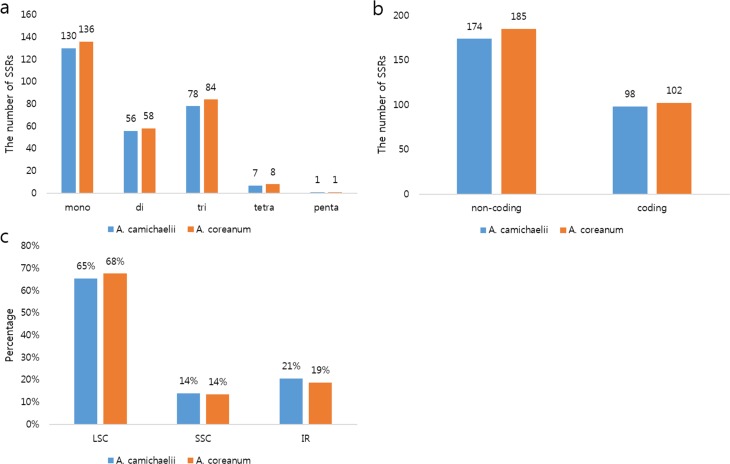
SSRs, or microsatellites, are repeating sequences of 1–6 nucleotides and are widely distributed in genomes [36]. They are easily identified in whole genome sequences and are used as molecular markers due to high interspecies polymorphism, locus-specific co-dominance, and high transferability [43]. We identified 272 and 287 SSRs in the cp genomes of A. carmichaelii and A. coreanum, respectively (Fig 2). No hexa-nucleotide repeats were found. Mononucleotide motifs were the most abundant and trinucleotide motifs the second most abundant type of repeat in both Aconitum genomes (Fig 2A). A and T repeat units occupied the highest portion due to short polyamine (poly A) or polythymine (poly T) repeats [39]. Furthermore, these SSRs contributed to the AT richness of the Aconitum cp genomes. The SSRs were more abundant in non-coding than in coding regions: 64% of all SSRs in both genomes resided in non-coding regions (Fig 2B). Most SSRs were located in the LSC region (65% in A. carmichaelii and 68% in A. coreanum). The numbers of SSRs in SSC and IR regions were similar (Fig 3C). These SSRs could be widely used as molecular markers for discrimination of species as well as for genetic diversity and evolution studies.
Fig 2. Distribution of SSRs in the two Aconitum cp genomes.
(A) SSR type distribution in the two cp genomes. (B) The proportion of SSRs in different regions of the Aconitum cp genomes. (C) SSR distribution between coding and non-coding regions.
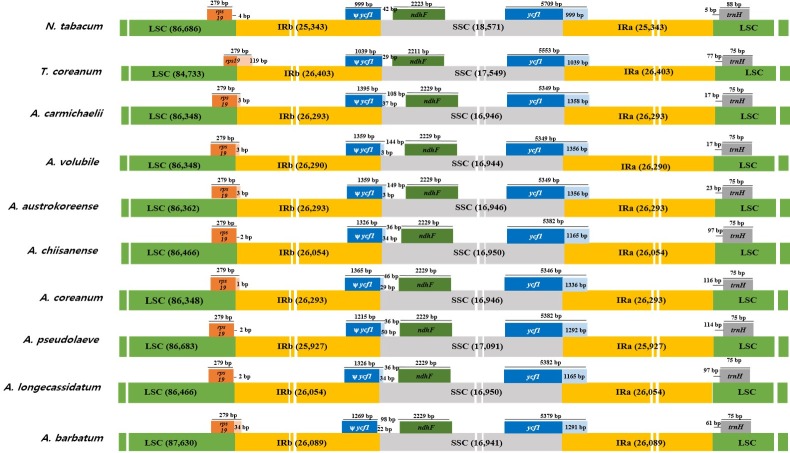
Fig 3. Schematic representations of LSC, SSC, and IR border regions in the eight Aconitum species as well as in N. tabacum and T. coreanum.
Comparison of chloroplast genomes with those of other Aconitum species
The two Aconitum cp genomes had 98.1% sequence identity and their genome structures, gene contents, and gene orders were similar. The cp genomes of Aconitum species are greatly conserved with regard to genome structure and gene orientation and length. Rearrangement such as translocation and inversion was not detected in the two genomes. However, the LSC region of A. coreanum was 1,280 bp longer than that of A. carmichaelii, which is similar to that of other plant genomes [32, 35].
Although the IR regions are highly conserved, the expansion and contraction of IR regions are a general feature of chloroplast genomes, where they are mainly responsible for variations in cp genome size and rearrangement [8, 44]. We analyzed the border structures of eight Aconitum cp genomes and compared them with those of T. coreanum [45] and N. tabacum [46] cp genomes (Fig 3). The lengths of the IR regions of Aconitum species were similar and ranged from 25,927 to 26,293 bp, but the expansion and contraction of IR regions differed. The rps19 genes of three species (A. chiisanense, A. pseudolaeve, and A. longecassidatum) were located exclusively in LSC regions; those of other Aconitum species extended into the IR regions. The ycf1 genes were located at the junctions of IRb/SSC and SSC/IRa border. IRb/SSC extended into ycf1 genes in all Aconitum species except for A. barbatum. The length of ѱycf1 ranged from 1,215 to 1,395 bp in Aconitum species. The ycf1 gene was embedded at the IRa/SSC border. The trnH genes were all located in LSC regions, 17–114 bp away from the IRa/LSC boundary (Fig 3). The cp genomes are probably preserved in closely related species; species belonging to other families might exhibit considerable variation in their cp genomes, for example, in gene orientation and size, causing expansion and contraction of IR regions.
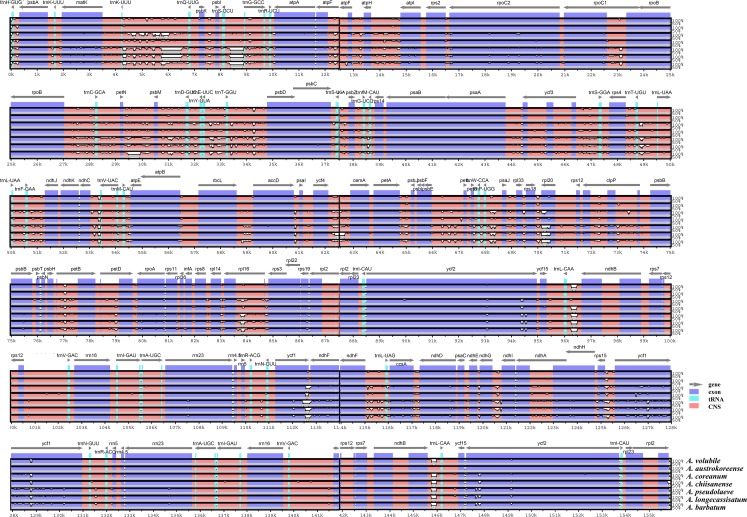
The overall sequences of eight Aconitum chloroplast genomes were represented using mVISTA with A. carmichaelii as reference (Fig 4). The results show that the Aconitum chloroplast genomes share highly conserved identity. IR regions were more conserved than other regions due to copy correction by gene conversion following mutation in the IR region [47]. These results are generally consistent with the phylogenetic tree of Aconitum species. As expected, the IR regions were more conserved than the LSC and SSC regions. Furthermore, non-coding regions were more divergent than coding regions. In our alignment, the most divergent regions were found in trnK-UUU–trnQ-UUG, trnS-GCU–trnG-GCC, petN–psbM, ndhC–trnV-UAC, ycf4–cemA, rpl18–rpl20, and trnT–psbD regions. These intergenic regions have been applied in phylogenetic studies [12, 40]. For the coding regions, the most divergent regions were ycf1, ycf2, rpl20, and rpoc2. These are called hotspot regions as they contain clustered variation such as single-nucleotide polymorphisms and indels [48, 49]. The cp genomes of Aconitum species also contain general hotspot regions for variation similar to those of other plants. These divergent regions of Aconitum species could be developed as genomic information for development of molecular markers for use in DNA barcoding and phylogenic analysis in Aconitum species.
Fig 4. Comparison of eight Aconitum chloroplast genomes using mVISTA.
Complete cp genomes of eight Aconitum species were used for comparison. Genic regions were identified using the DOGMA program, and a comparative map was prepared using mVISTA. Blue block, conserved gene; sky-blue block, tRNA and rRNA; red block, intergenic region. White peaks indicate the regions with sequence variation among Aconitum species.
Phylogenic relationship of Aconitum species within Ranunculaceae
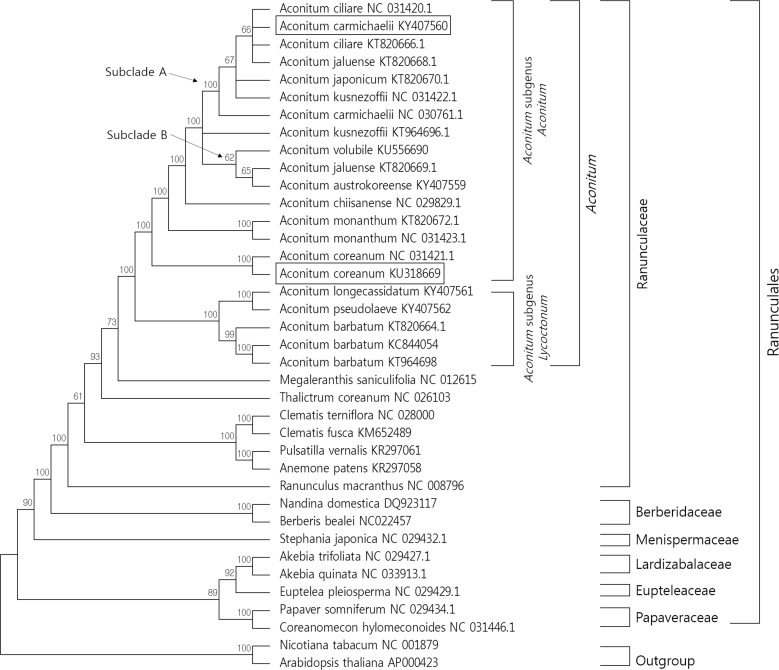
To identify the phylogenetic positon of Aconitum species within Ranunculaceae and their relationship to other families within Ranunculales, 70 protein-coding sequences shared by the 38 cp genomes were aligned (Fig 5). Two species, Nicotiana tabacum (NC001879) and Arabidopsis thaliana (AP000423), were set as outgroups. Maximum likelihood (ML) analysis was performed with 1,000 bootstrap replicates. The reconstructed phylogeny showed that 21 nodes had bootstrap values >95%, and 19 of these had bootstrap values of 100%. The clusters consisting of 38 species, including 21 Aconitum species, were well supported in six families; Ranunculaceae, Berberidaceae, Menispermaceae, Lardizabalacea, Euptrleaceae, and Papaveraceae in Ranunculales. Ranunculaceae and Berberidaceae are the closest families according to the APG III system of flowering plant classification [50] (Fig 5). Aconitum species were strongly clustered, and M. saniculifilia and T. coreanum were closer than other species in Ranunculaceae. Twenty-one Aconitum species formed a clade as two subgenera, Aconitum subgen. Aconitum and Aconitum subgen. Lycoctonum. A. barbatum clustered with A. longecassidatum and A. pseudolaveve in the subgenus Lycoctonum [51]. In Aconitum subgen. Aconitum, A. ciliare (NC031420), A. carmichaelii (KY407560), A. ciliare (KT820666), A. jaluense (KT820668), A. japonicum (KT820670), A. kusnezoffii (NC031422), and A. carmichaelii (NC030761) clustered monophyletically (subclade A) with a bootstrap value of 100%. Another monophyletic subclade (subclade B) also formed a cluster and included A. volubile (KU556690), A. jaluense (KT820669), A. austrokoreense (KY407559), with a weak bootstrap value of 62%. A. kusnezoffii (KT964696) clustered paraphyletically with subclade A and B. In addition, A. jaluense was included in both subclades (subclade A and B) (Fig 5). To verify the reasons for the polyphyletic division of the two species, A. kusnezoffii and A. jaluense, we critically analyzed the coding sequences by performing sequence alignments. Interestingly, we identified diverse intra-species specific sequence variabilities for A. kusnezoffii, and A. jaluense, but not for other Aconitum species such as A. ciliare, A. monanthum, and A. coreanum. Thus, we estimate that the two subclades separated by weak bootstrap values branched via intra-specific variation of A. kusnezoffii and A. jaluense cp genomes in each species. To identify the exact position of A. kusnezoffii and A. jaluense, we additionally confirmed phylogenic relationships based on one accession of each A. kusnezoffii and A. jaluense, A. kusnezoffii (NC031422) and A. jaluense (KT820668) (S2A Fig) and A. kusnezoffii (KT964696) and A. jaluense (KT820669) (S2B Fig), respectively.
Fig 5. Molecular phylogenetic tree of 38 plants including 21 Aconitum species based on 70 protein-coding genes in the cp genome.
The tree was constructed by maximum likelihood analysis using MEGA6 with a bootstrap test of 1,000 replications.
The results showed that the positions of A. kusnezoffii and A. jaluense were unchanged in the Aconitum subgen. Aconitum (S2 Fig). It has been reported that Aconitum subgen. Aconitum exist as diploid, tetraploid, and hexaploid varieties in East Asia, of which the tetraploid variety is the most common. [52]. Interestingly, A. kusnezoffii and A. jaluense, A. carmichaelii, A. japonicum, and A. ciliare were reported to be tetraploids in a previous report [52, 53]. Polyploidization is an evolutionally common event in natural populations [54, 55]. Two different accessions of A. ciliare (NC 031420 and KT820666) clustered monophyletically (Fig 5) and seemed to have the same ploidy and maternal inheritance pattern. However, four Aconitum species (A. kusnezoffii and A. jaluense, A. carmichaelii, and A. japonicum) having different accessions from the same species did not clearly cluster monophyletically (Fig 5). We inferred that the Aconitum accessions of the four species had different ploidy even if they correspond to the same species reported previously [52]. As shown Fig 5, polyphyletic relationship of different individuals from the same species including A. kusnezoffii and A. jaluense seem to be depending on ploidy within the species.
Taken together, the results suggest that the species identification of Aconitum subgen. Aconitum is limited depending on cp genome sequence in several taxa because of the low inter-species variability and high intra-species variability.
In previous studies, several phylogenetic trees were constructed to verify the relationships between species of Aconitum subgen. Aconitum based on ITS, trnL- F sequences [53]. ITS sequence-based phylogenetic analysis of 52 species of Aconitum subgen. Aconitum classified them into two seed groups. In addition, A. austrokoreense and A. monanthum formed a paraphyly with A. variegatum, A. napellus, and unidentified Aconitum samples using ITS sequences [53]. Likewise, in cp phylogenetic analysis, A. monanthum and A. austrokoreense were consistent with previous study. A. carmichaelii (KY407560) and A. coreanum (KU318669) showed a polyphyletic relationship in Aconitum subgen. Aconitum (Fig 5). These results indicate that phylogenetic analysis of cp genome sequences could provide useful information for analysing the phylogenetic relationships between Aconitum subgen. Aconitum species. However, the results suggest that additional largescale genomic analyses using numerous and accurately identified Aconitum species will be required to clarify the taxonomy and phylogenetic relationships of Aconitum subgen. Aconitum at low taxonomic levels, including species identification.
Authentication of A. coreanum using a SCAR marker
In comparative analysis of the complete cp genome sequences of Aconitum species, we obtained a highly divergent and specific sequence with an indel mutation only in the ndhC–trnV region of A. coreanum. Firstly, Sanger sequencing of the ndhC–trnV region and sequence alignment were performed using multiple accessions of germplasm to determine whether or not A. coreanum-specific sequence variation is detected only in A. coreanum. The results showed that A. coreanum had a 6 bp insertion that was absent in the other Aconitum species (S3 Fig). Based on these specific sequences, specific primers for amplifying SCAR were designed and those specifies were confirmed (Fig 6). To confirm the specificity of SCAR primers, we employed a set of nine species and 1 variety in the genus Aconitum consisting of a total of 27 samples (Fig 6 and Table 4). PCR amplification resulted in a DNA fragment with the expected SCAR amplicon size from A. coreanum (131 bp), whereas no PCR product was observed for other Aconitum species (Fig 6). Thus, we were successful in obtaining a species-specific SCAR marker for A. coreanum.
Fig 6. Schematic diagram of the indel region and development of A. coreanum-specific SCAR marker for the identification of Aconiti Koreani Tuber.
1–2. A. coreanum; 3–6. A. carmichaelii; 7–8 A. voluvile var. pubescens; 9–13. A. jaluense var. triphyllum; 14–16. A. kusnezoffii; 17–18. A. jaluense; 19–22. A. austrokoreense; 23–24. A. kirinense; 25–26. A. barbatum; 27. A. chiisanense; M. 100bp DNA ladder. * 6 taxa of Aconitum species were used in this study.
Table 4. Primers for SCAR marker development.
| Primer name | Primer sequence (5' > 3') | Product size | Position |
|---|---|---|---|
| AC-F | GCC AAA ATA GGA ATA ACA CTT GAT ATT | 131 bp | ndhC–trnV (IGS) |
| AC-R | GAA TCG ACT AAT ACG AAT ACG GAT |
In a previous study, 19 Aconitum species were classified into ten subgroups based on sequence variabilities in the psbA-trnH region. Of these, A. camichaelii and A. kusnezoffii had the same nucleotide sequences, whereas A. coreanum showed sequence variability with respect to those of other Aconitum species [16]. However, our study analyzing the same sequences of nine Aconitum species found that A. coreanum could not be differentiated from either A. volubile or A. austrokoreense. Thus, sequence variability in the psbA-trnH region could not differentiate A. coreanum from A. volubile or A. austrokoreense at the species level because they had the same sequences in our preliminarily analysis. Furthermore, to develop a genetic authentication tool for Aconitum-based herbal medicines, we analyzed DNA barcode regions (ITS, matK, and psbA-trnH) in Aconitum germplasms. We observed several nucleotide substitutions that could be used to identify A. coreanum and A. kiriensis from other Aconitum species using comparative analysis of entire sequences of the nrDNA-ITS regions. However, the number of nucleotide substitutions in the nrDNA-ITS regions was not enough to develop a SCAR marker, and matK gene sequences did not show any sequences differences similar to those found in the psbA-trnH region. Therefore, we conducted further comparative analysis of complete cp genome sequences to overcome the limitations of the universal DNA barcode used previously to distinguish Aconitum species. Since Aconitum cp genomes have high similarity (97.36–99.9%), it was difficult to develop species-specific markers for each Aconitum species. Although plants of Aconitum species show morphological differences in their areal parts, identification of the botanical origin of the dried tubers used in herbal medicines is very difficult. In this study, we developed an A. coreanum-specific SCAR marker using ten Aconitum taxa. However, further study will be required to distinguish between different Aconiti herbal medicines employing Aconitum species and to identify the botanical origins at the species level for quality control purposes. The SCAR marker developed in this study will help authenticate rapidly and accurately herbal medicines, and determine whether they have been adulterated or not.
Conclusions
In this paper, we obtained the complete chloroplast sequences of A. coreanum and A. carmichaelii belonging to the Ranunculaceae family. The two Aconitum species had slightly different genome sizes, a feature shared by other Aconitum species. Furthermore, the distributions and locations of SSRs were determined. The cp genome sequences were compared with those of six other Aconitum species. The most divergent regions were found in the trnK-UUU–trnQ-UUG, trnS-GCU–trnG-GCC, petN–psbM, ndhC–trnV-UAC, ycf4–cemA, rpl18–rpl20, and trnT–psbD non-coding regions, and in the ycf1-, ycf2-, rpl20-, and rpoc2-coding regions. A phylogenetic analysis was performed on the 20 cp genomes of 13 Aconitum species with the cp genomes of five other families within the Ranunculales. We also developed a SCAR marker for A. coreanum using a divergent region of the Aconitum cp genome. These results provide useful information for phylogenetic studies on the Ranunculaceae and the biological systematics of Aconitum L., as well as for the conservation biology of A. coreanum. Furthermore, the SCAR maker could be useful in distinguishing A. coreanum from other Aconitum species, particularly with respect to the authentication of herbal medicines.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank the “Classification and Identification Committee of the KIOM” for the identification of plant materials and the Herbarium of Korea Standard Herbal Resources (herbarium code KIOM) for the provision of plant materials.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a grant from Development of Foundational Techniques for the Domestic Production of Authentic Herbal Medicines based on the Establishment of Molecular Authentication System (K16403) from the Korea Institute of Oriental Medicine (KIOM). The grant was funded by the Ministry of Science, ICT, and Future Planning (MSIP) of Republic of Korea. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chen S, Gilbert M. Flora of China. Science, Beijing and Missouri Botanical Garden Press, St Louis: 2006. [Google Scholar]
- 2.KIOM. Defining Dictionary for Medicinal Herbs. http://boncho.kiom.re.kr/codex/: Korea Institute of Oriental Medicine; 2016.
- 3.Zhou G, Tang L, Zhou X, Wang T, Kou Z, Wang Z. A review on phytochemistry and pharmacological activities of the processed lateral root of Aconitum carmichaelii Debeaux. J Ethnopharmacol 2015;160:173–93. doi: 10.1016/j.jep.2014.11.043 . [DOI] [PubMed] [Google Scholar]
- 4.Yao S, Liu RM, Huang XF, Kong LY. Preparative isolation and purification of chemical constituents from the root of Adenophora tetraphlla by high-speed counter-current chromatography with evaporative light scattering detection. J Chromatogr A 2007;1139(2):254–62. doi: 10.1016/j.chroma.2006.11.056 [DOI] [PubMed] [Google Scholar]
- 5.Gao TT, Ma S, Song JY, Bi HT, Tao YD. Antioxidant and immunological activities of water-soluble polysaccharides from Aconitum kusnezoffii Reichb. Int J Biol Macromol 2011;49(4):580–6. doi: 10.1016/j.ijbiomac.2011.06.017 [DOI] [PubMed] [Google Scholar]
- 6.Xu N, Zhao DF, Liang XM, Zhang H, Xiao YS. Identification of Diterpenoid Alkaloids from the Roots of Aconitum kusnezoffii Reihcb. Molecules 2011;16(4):3345–50. doi: 10.3390/molecules16043345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniell H, Lin CS, Yu M, Chang WJ. Chloroplast genomes: diversity, evolution, and applications in genetic engineering. Genome Biol 2016;17(1):134 doi: 10.1186/s13059-016-1004-2 ; PubMed Central PMCID: PMC4918201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang M, Zhang X, Liu G, Yin Y, Chen K, Yun Q, et al. The complete chloroplast genome sequence of date palm (Phoenix dactylifera L.). PLoS One 2010;5(9):e12762 doi: 10.1371/journal.pone.0012762 ; PubMed Central PMCID: PMC2939885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wicke S, Schneeweiss GM, dePamphilis CW, Muller KF, Quandt D. The evolution of the plastid chromosome in land plants: gene content, gene order, gene function. Plant Mol Biol 2011;76(3–5):273–97. doi: 10.1007/s11103-011-9762-4 ; PubMed Central PMCID: PMC3104136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi KS, Park S. The complete chloroplast genome sequence of Aster spathulifolius (Asteraceae); genomic features and relationship with Asteraceae. Gene 2015;572(2):214–21. doi: 10.1016/j.gene.2015.07.020 . [DOI] [PubMed] [Google Scholar]
- 11.Yang JB, Tang M, Li HT, Zhang ZR, Li DZ. Complete chloroplast genome of the genus Cymbidium: lights into the species identification, phylogenetic implications and population genetic analyses. BMC Evol Bio 2013;13:84 doi: 10.1186/1471-2148-13-84 ; PubMed Central PMCID: PMC3644226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw J, Lickey EB, Schilling EE, Small RL. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: the tortoise and the hare III. Am J Bot 2007;94(3):275–88. doi: 10.3732/ajb.94.3.275 . [DOI] [PubMed] [Google Scholar]
- 13.Kim WJ, Moon BC, Yang S, Han KS, Choi G, Lee AY. Rapid Authentication of the Herbal Medicine Plant Species Aralia continentalis Kitag. and Angelica biserrata C.Q. Yuan and R.H. Shan Using ITS2 Sequences and Multiplex-SCAR Markers. Molecules 2016;21(3):270 doi: 10.3390/molecules21030270 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollingsworth PM, Graham SW, Little DP. Choosing and using a plant DNA barcode. PLoS One. 2011;6(5):e19254 doi: 10.1371/journal.pone.0019254 ; PubMed Central PMCID: PMC3102656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Las Rivas J, Lozano JJ, Ortiz AR. Comparative analysis of chloroplast genomes: functional annotation, genome-based phylogeny, and deduced evolutionary patterns. Genome Res 2002;12(4):567–83. doi: 10.1101/gr.209402 ; PubMed Central PMCID: PMC187516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He J, Wong KL, Shaw PC, Wang H, Li DZ. Identification of the medicinal plants in Aconitum L. by DNA barcoding technique. Planta Med 2010;76(14):1622–8. doi: 10.1055/s-0029-1240967 . [DOI] [PubMed] [Google Scholar]
- 17.Lam KY, Chan GK, Xin GZ, Xu H, Ku CF, Chen JP, et al. Authentication of Cordyceps sinensis by DNA Analyses: Comparison of ITS Sequence Analysis and RAPD-Derived Molecular Markers. Molecules 2015;20(12):22454–62. doi: 10.3390/molecules201219861 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YM, Ji Y, Kang YM, Kim WJ, Choi G, Moon BC. Molecular authentication of Pinelliae Tuber and its common adulterants using RAPD-derived multiplex sequence characterized amplified region (multiplex-SCAR) markers. Int J Clin Exp Med 2016;9(1):40–50. [Google Scholar]
- 19.Bhagyawant SS. RAPD-SCAR Markers: An Interface Tool for Authentication of Traits. J Biosci Med (Irvine) 2016;04(01):1–9. 0.4236/jbm.2016.41001. [Google Scholar]
- 20.Lee MY, Doh EJ, Park CH, Kim YH, Kim ES, Ko BS, et al. Development of SCAR marker for discrimination of Artemisia princeps and A. argyi from other Artemisia herbs. Bio Pharm Bull 2006;29(4):629–33. [DOI] [PubMed] [Google Scholar]
- 21.Dnyaneshwar W, Preeti C, Kalpana J, Bhushan P. Development and Application of RAPD-SCAR Marker for Identification of Phyllanthus emblica LINN. Bio Pharm Bull 2006;29(11):2313–6. [DOI] [PubMed] [Google Scholar]
- 22.Adinolfi B, Chicca A, Martinotti E, Breschi MC, Nieri P. Sequence characterized amplified region (SCAR) analysis on DNA from the three medicinal Echinacea species. Fitoterapia 2007;78(1):43–5. doi: 10.1016/j.fitote.2006.09.012 [DOI] [PubMed] [Google Scholar]
- 23.Delcher AL, Salzberg SL, Phillippy AM. Using MUMmer to identify similar regions in large sequence sets. Curr Protoc Bioinformatics 2003;Chapter 10:Unit 10 3. doi: 10.1002/0471250953.bi1003s00 . [DOI] [PubMed] [Google Scholar]
- 24.Schwartz S, Kent WJ, Smit A, Zhang Z, Baertsch R, Hardison RC, et al. Human-mouse alignments with BLASTZ. Genome Res 2003;13(1):103–7. doi: 10.1101/gr.809403 ; PubMed Central PMCID: PMC430961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wyman SK, Jansen RK, Boore JL. Automatic annotation of organellar genomes with DOGMA. Bioinformatics 2004;20(17):3252–5. doi: 10.1093/bioinformatics/bth352 . [DOI] [PubMed] [Google Scholar]
- 26.Lohse M, Drechsel O, Bock R. OrganellarGenomeDRAW (OGDRAW): a tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr Genet 2007;52(5–6):267–74. doi: 10.1007/s00294-007-0161-y . [DOI] [PubMed] [Google Scholar]
- 27.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Bio Evol 2013;30(12):2725–9. doi: 10.1093/molbev/mst197 ; PubMed Central PMCID: PMCPMC3840312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: computational tools for comparative genomics. Nucleic Acids Res 2004;32(Web Server issue):W273–9. doi: 10.1093/nar/gkh458 ; PubMed Central PMCID: PMCPMC441596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res 1999;27(2):573–80. ; PubMed Central PMCID: PMC148217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warburton PE, Giordano J, Cheung F, Gelfand Y, Benson G. Inverted repeat structure of the human genome: the X-chromosome contains a preponderance of large, highly homologous inverted repeats that contain testes genes. Genome Res 2004;14(10A):1861–9. doi: 10.1101/gr.2542904 ; PubMed Central PMCID: PMC524409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.TA H. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acid Symp Ser (Oxf) 1999;41(Oxford University Press).
- 32.Chen X, Li Q, Li Y, Qian J, Han J. Chloroplast genome of Aconitum barbatum var. puberulum (Ranunculaceae) derived from CCS reads using the PacBio RS platform. Front Plant Sci 2015;6:42 doi: 10.3389/fpls.2015.00042 ; PubMed Central PMCID: PMC4319492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bayes de Luna A, Rovai D, Pons Llado G, Gorgels A, Carreras F, Goldwasser D, et al. The end of an electrocardiographic dogma: a prominent R wave in V1 is caused by a lateral not posterior myocardial infarction-new evidence based on contrast-enhanced cardiac magnetic resonance-electrocardiogram correlations. Eur Heart J 2015;36(16):959–64. doi: 10.1093/eurheartj/ehv035 . [DOI] [PubMed] [Google Scholar]
- 34.Cho KS, Yun BK, Yoon YH, Hong SY, Mekapogu M, Kim KH, et al. Complete Chloroplast Genome Sequence of Tartary Buckwheat (Fagopyrum tataricum) and Comparative Analysis with Common Buckwheat (F. esculentum). PLoS One. 2015;10(5):e0125332 doi: 10.1371/journal.pone.0125332 ; PubMed Central PMCID: PMC4428892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim CE, Kim GB, Baek S, Han SM, Yu HJ, Mun JH. The complete chloroplast genome of Aconitum chiisanense Nakai (Ranunculaceae). Mitochondrial DNA 2015:1–2. doi: 10.3109/19401736.2015.1110805 . [DOI] [PubMed] [Google Scholar]
- 36.Curci PL, De Paola D, Danzi D, Vendramin GG, Sonnante G. Complete chloroplast genome of the multifunctional crop globe artichoke and comparison with other Asteraceae. PLoS One 2015;10(3):e0120589 doi: 10.1371/journal.pone.0120589 ; PubMed Central PMCID: PMCPMC4361619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang JB, Yang SX, Li HT, Yang J, Li DZ. Comparative chloroplast genomes of Camellia species. PLoS One 2013;8(8):e73053 doi: 10.1371/journal.pone.0073053 ; PubMed Central PMCID: PMC3751842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hao Z, Cheng T, Zheng R, Xu H, Zhou Y, Li M, et al. The Complete Chloroplast Genome Sequence of a Relict Conifer Glyptostrobus pensilis: Comparative Analysis and Insights into Dynamics of Chloroplast Genome Rearrangement in Cupressophytes and Pinaceae. PLoS One 2016;11(8):e0161809 doi: 10.1371/journal.pone.0161809 ; PubMed Central PMCID: PMC4999192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qian J, Song J, Gao H, Zhu Y, Xu J, Pang X, et al. The complete chloroplast genome sequence of the medicinal plant Salvia miltiorrhiza. PLoS One 2013;8(2):e57607 doi: 10.1371/journal.pone.0057607 ; PubMed Central PMCID: PMC3584094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nie X, Lv S, Zhang Y, Du X, Wang L, Biradar SS, et al. Complete chloroplast genome sequence of a major invasive species, crofton weed (Ageratina adenophora). PLoS One 2012;7(5):e36869 doi: 10.1371/journal.pone.0036869 ; PubMed Central PMCID: PMC3350484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bausher MG, Singh ND, Lee SB, Jansen RK, Daniell H. The complete chloroplast genome sequence of Citrus sinensis (L.) Osbeck var 'Ridge Pineapple': organization and phylogenetic relationships to other angiosperms. BMC Plant Biol 2006;6:21 doi: 10.1186/1471-2229-6-21 ; PubMed Central PMCID: PMCPMC1599732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li R, Ma PF, Wen J, Yi TS. Complete sequencing of five araliaceae chloroplast genomes and the phylogenetic implications. PLoS One 2013;8(10):e78568 doi: 10.1371/journal.pone.0078568 ; PubMed Central PMCID: PMC3799623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park I, Kim J, Lee J, Kim S, Cho O, Yang K, et al. Development of SSR markers by next-generation sequencing of Korean landraces of chamoe (Cucumis melo var. makuwa). Mol Biol Rep 2013;40(12):6855–62. doi: 10.1007/s11033-013-2803-0 . [DOI] [PubMed] [Google Scholar]
- 44.Raubeson LA, Peery R, Chumley TW, Dziubek C, Fourcade HM, Boore JL, et al. Comparative chloroplast genomics: analyses including new sequences from the angiosperms Nuphar advena and Ranunculus macranthus. BMC Genomics 2007;8:174 doi: 10.1186/1471-2164-8-174 ; PubMed Central PMCID: PMC1925096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park S, Jansen RK, Park S. Complete plastome sequence of Thalictrum coreanum (Ranunculaceae) and transfer of the rpl32 gene to the nucleus in the ancestor of the subfamily Thalictroideae. BMC Plant Biol 2015;15:40 doi: 10.1186/s12870-015-0432-6 ; PubMed Central PMCID: PMC4329224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shinozaki K, Ohme M, Tanaka M, Wakasugi T, Hayashida N, Matsubayashi T, et al. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J 1986;5(9):2043–9. ; PubMed Central PMCID: PMC1167080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khakhlova O, Bock R. Elimination of deleterious mutations in plastid genomes by gene conversion. Plant J 2006;46(1):85–94. doi: 10.1111/j.1365-313X.2006.02673.x . [DOI] [PubMed] [Google Scholar]
- 48.Redwan RM, Saidin A, Kumar SV. Complete chloroplast genome sequence of MD-2 pineapple and its comparative analysis among nine other plants from the subclass Commelinidae. BMC Plant Biol 2015;15:196 doi: 10.1186/s12870-015-0587-1 ; PubMed Central PMCID: PMCPMC4534033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song Y, Dong W, Liu B, Xu C, Yao X, Gao J, et al. Comparative analysis of complete chloroplast genome sequences of two tropical trees Machilus yunnanensis and Machilus balansae in the family Lauraceae. Front Plant Sci 2015;6:662 doi: 10.3389/fpls.2015.00662 ; PubMed Central PMCID: PMCPMC4548089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bremer B, Bremer K, Chase M, Fay M, Reveal J, Soltis D, et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc 2009. [Google Scholar]
- 51.Hong Y, Luo Y, Gao Q, Ren C, Yuan Q, Yang QE. Phylogeny and reclassification of Aconitum subgenus Lycoctonum (Ranunculaceae). PLos One 2017;12(1):e0171038 doi: 10.1371/journal.pone.0171038 ; PubMed Central PMCID: PMCPMC5334035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kita Y, Ito M. Nuclear ribosomal ITS sequences and phylogeny in East Asian Aconitum subgenus Aconitum (Ranunculaceae), with special reference to extensive polymorphism in individual plants. Plant Syst Evol 2000;225(1–4):1–13. [Google Scholar]
- 53.Luo Y, Zhang F-m, Yang Q-E. Phylogeny of Aconitum subgenus Aconitum (Ranunculaceae) inferred from ITS sequences. Plant Syst Evol 2005;252(1):11–25. [Google Scholar]
- 54.Russell A, Samuel R, Klejna V, Barfuss MH, Rupp B, Chase MW. Reticulate evolution in diploid and tetraploid species of Polystachya (Orchidaceae) as shown by plastid DNA sequences and low-copy nuclear genes. Ann Bot 2010;106(1):37–56. doi: 10.1093/aob/mcq092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Govindarajulu R, Parks M, Tennessen JA, Liston A, Ashman T-L. Comparison of nuclear, plastid, and mitochondrial phylogenies and the origin of wild octoploid strawberry species. Am J Bot 2015;102(4):544–54. doi: 10.3732/ajb.1500026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.