Abstract
Introduction
Neglected tropical diseases (NTDs) are important public health problems in Ethiopia. In 2013, the Federal Ministry of Health (FMOH) has launched a national NTD master plan to eliminate major NTDs of public health importance by 2020. Benchmarking the current status of NTDs in the country is important to monitor and evaluate the progress in the implementation of interventions and their impacts. Therefore, this study aims to assess the trends of mortality and Disability-adjusted Life-Years (DALY) for the priority NTDs over the last 25 years.
Methods
We used the Global Burden of Disease (GBD) 2015 estimates for this study. The GBD 2015 data source for cause of death and DALY estimation included verbal autopsy (VA), Demographic and Health Surveys (DHS), and other disease specific surveys, Ministry of Health reports submitted to United Nations (UN) agencies and published scientific articles. Cause of Death Ensemble modeling (CODEm) and/or natural history models were used to estimate NTDs mortality rates. DALY were estimated as the sum of Years of Life Lost (YLL) due to premature mortality and Years Lived with Disability (YLD).
Results
All NTDs caused an estimated of 6,293 deaths (95% uncertainty interval (UI): 3699-10,080) in 1990 and 3,593 deaths (95% UI: 2051 – 6178) in 2015, a 43% reduction over the 25 years. Age-standardized mortality rates due to schistosomiasis, STH and leshmaniasis have declined by 91.3%, 73.5% and 21.6% respectively between 1990 to 2015. The number of DALYs due to all NTDs has declined from 814.4 thousand (95% UI: 548 thousand–1.2million) in 1990 to 579.5 thousand (95%UI: 309.4 thousand–1.3 million) in 2015. Age-standardized DALY rates due to all NTDs declined by 30.7%, from 17.6 per 1000(95%UI: 12.5-26.5) in 1990 to 12.2 per 1000(95%UI: 6.5 – 27.4) in 2015. Age-standardized DALY rate for trachoma declined from 92.7 per 100,000(95% UI: 63.2 – 128.4) in 1990 to 41.2 per 100,000(95%UI: 27.4–59.2) in 2015, a 55.6% reduction between 1990 and 2015. Age-standardized DALY rates for onchocerciasis, schistosomiasis and lymphiaticfilariasis decreased by 66.2%, 29.4% and 12.5% respectively between 1990 and 2015. DALY rate for ascariasis fell by 56.8% over the past 25 years.
Conclusions
Ethiopia has made a remarkable progress in reducing the DALY rates for most of the NTDs over the last 25 years. The rapid scale of interventions and broader system strengthening may have a lasting impact on achieving the 2020 goal of elimination of most of NTDs. Ethiopia should strengthen the coverage of integrated interventions of NTD through proper coordination with other health programs and sectors and community participation to eliminate NTDs by 2020.
Keywords: NTDs, DALY, Ethiopia
Introduction
Ethiopia has demonstrated significant progress in the health sector and has been described as one of the success stories to deliver health services at low cost (1). The country achieved most of the health related Millennium Development Goals (MDSs) by 2013 (2). Despite this significant progress, communicable diseases including neglected tropical diseases (NTDs) remain the major public health problems of the country during the Sustainable Development Goals (SDG) era. To address these important public health problems, Ethiopia launched its first NTD master plan in 2013(3,4). Eight NTDs have been prioritized in the master plan: lymphiatic filariasis (LF), onchocerciasis, schistosomiasis, soil transmitted helminths (STH), leishmaniasis, trachoma, podoconiosis and dracunculiasis (3–5).
Neglected tropical diseases (NTDs) are a cluster of tropical diseases that affect more than one billion people worldwide, they occur mainly among poor populations who have low access to the health care system. NTDs can cause disability, disfigurement, undernutrition and cognitive impairments, yet most of them can easily be controlled and prevented (6,7). The global effort to control NTDs was shaped by the launching of the WHO NTD Roadmap in 2012(8) and through the London declaration, where partners, donors and pharmaceutical companies pledged to eliminate 10 NTDs by 2020 (9).
Ethiopia has completed the mapping of most of the NTDs. The results suggest that in 2013 there were an estimated 80 million Ethiopians living in areas that are endemic to one or more of NTDs (10). Considering the high burden and public health impacts of NTDs, the Federal Ministry of Health (FMOH) and development partners have collaborated to implement high impact interventions such as mass drug administration (MDA), integrated vector control measures, intensified case management and environmental sanitation and safe water supply over the last decade(10,11).
The national NTD master plan envisaged to control and eliminate the eight priority NTDs. The master plan focuses on four pillar strategies to achieve its goal: government ownership and partnership; enhance planning for financial sustainability; scale up of high impact interventions through community participation; and enhance monitoring and evaluation, surveillance and research (5). As stipulated in the master plan monitoring and evaluation of the program impact are progress are important. The prerequisite for the implementation of effective program monitoring and evaluation is benchmarking. Nonetheless, despite their importance the mortality and Disability Adjusted life-years (DALY) due to NTDs have not been comprehensively evaluated in Ethiopia. In this paper, we used the GBD 2015 estimates (11,12) to assess mortality and DALY trends for common NTDs such as LF, onchocerciasis, schistosomiasis, soil transmitted helminthiasis (STH), leishmaniasis, and trachoma over the last 25 years. These estimates provide important insights into the performance of Ethiopia during the MGD era, and serve as benchmark to track future progress during the SDG period.
Methods
Settings
Ethiopia has an estimated population of 94 million, the second largest in Africa, and 86% the population live in rural areas (13). The country has nine regional states and two city administrations which are further divided into Woredas and Kebeles (lowest administrative units). Ethiopia has a decentralized health care delivery system and the national health policy focuses on both health promotion and disease prevention, and curative services. The country has launched an innovative Health Extension Programme (HEP) in 2003 to deliver cost-effective basic health services to all Ethiopians at the grass root level, mainly women and children through the health extension workers (14,15).
Data sources
GBD 2015 data sources for Ethiopia have been detailed elsewhere (2). In brief, the 2015 GBD data sources for estimation of causes of death and DALYs included verbal autopsy (VA), Demographic and Health Surveys (DHS), other disease specific surveys, FMOH reports submitted to United Nations (UN) agencies and published scientific articles (2,16–18).
Causes of death modeling
The data analysis for this paper is part of the GBD global data analysis framework that included seven supper regions, 21 regions and 195 countries (11,12). The detailed data analysis steps and techniques to estimate cause-specific mortality rates (2,11,19) and DALYs (17,18) are published elsewhere. Briefly, the GBD cause list relies on assigning a single cause for each death in accordance with the principle of International Classification of Diseases (ICD). The cause of death (i.e. underlying cause of death) may initiate a series of events leading to death (11,17,19). In the first step, the causes of death (CoD) database was developed from the main data sources as described earlier. During the database development, multiple data formats were standardized to a single GBD standard and each ICD or verbal autopsy variant was mapped to the GBD cause list. Deaths assigned to causes that cannot be underlying causes of death (i.e., garbage coded) were re-assigned to their likely underlying cause of death (11,20). Cause of Death Ensemble modeling (CODEm) and/or natural history models were used to estimate NTDs and malaria related mortality rates. Detail descriptions of CODEm published elsewhere (16,20–22). In short, CODEm tests a wide range of sub-models that vary based on model type (mixed effects linear models versus spatial-temporal Gaussian process regression (ST-GPR) models), outcome (mortality rate versus cause fraction) and covariate selection. Each sub-model is tested for out-of-sample predictive performance using crossvalidation, and the final estimates are based on an ensemble of the best performing sub-models (16). A natural history model was applied to estimate leishmaniasis mortality rate. This is because leishmaniasis mortality is not captured in VA due to either geographic location of death or potential systematic bias in VA methodology (11). Similarly, for malaria in sub-Saharan Africa including Ethiopia, a natural history model was used based on the incidence estimated by the Malaria Atlas Project and age–sex-specific case-fatality rates estimated from available data (11).
Estimation of DALY
Detailed DALY estimation methods were described in previous GBD publications (12,23). In summary, DALY combines years of life lost (YLL) due to premature mortality and years lived with disability (YLD), a measure of non-fatal health loss, in a single metric. One DALY can be thought of as one lost year of healthy life. YLL were estimated using standard GBD methods whereby each death is multiplied by the normative standard life expectancy at each age. The normative standard life expectancy at birth is 86·59 years, based on the lowest observed death rates for each 5-year age group in populations larger than 5 million. YLD were estimated using sequelae prevalence and disability weights derived from population-based surveys of the general public to assign disability weights to each sequela and combination of sequelae (12,23).
Ethical considerations
Permission to conduct this study was sought from the Institute of Health Metrics and Evaluation in Washington University in Seattle. The study follows the World Health Organization (WHO) Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) (11).
Results
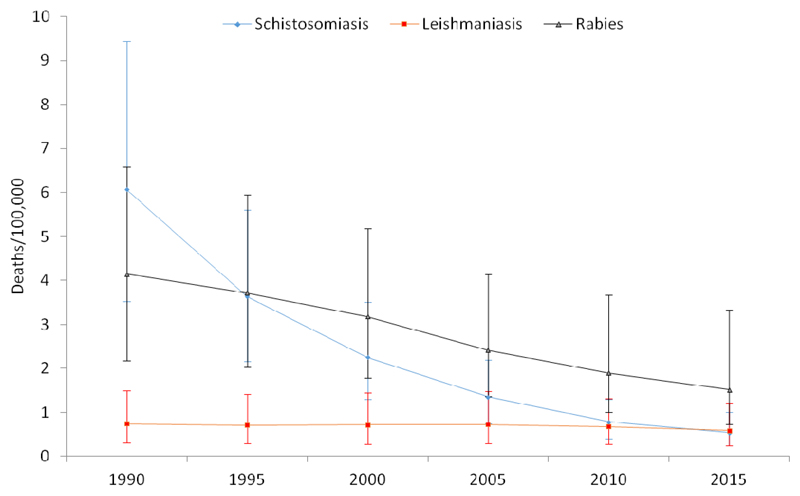
All NTDs caused an estimated of 6,293 deaths (95% uncertainty interval, UI: 3699 – 10,080) in 1990 and 3,593 deaths (95% UI: 2051–6178) in 2015, a 43% reduction over the 25 years. Age-standardized mortality rates due to schistosomiasis was 6.1/100,000 (95% UI: 3.5-9.4) in 1990 and 0.53/100,000 (95% UI: 0.23–1.0) in 2015, a 91.3% reduction over the 25 years. Age-standardized mortality rates due to all NTDs declined by 70% between 1990 and 2015 (Table 1). Age-standardized mortality rates for schistosomiasis, STH and rabies have declined by 91.3%, 73.5% and 63.1% respectively, between 1990 and 2015. The annualized mortality rate of change (ARC) for all NTD was 5% (95% UI: 3.4-7.6%). The least progress in terms of median % of mortality change (21.6%) was observed for leishmaniasis during 1990-2015 (Table 1 and Figure1).
Table 1.
Number of deaths and mortality rate of common neglected tropical diseases in Ethiopia, both sex, 1990 - 2015
| Diseases | 1990 | 2015 | Median % change mortality rate, 1990-2015 | ||
|---|---|---|---|---|---|
| Number of deaths (95% UI) | Deaths/100,000 (95%UI) | Number of deaths (95%UI) | Deaths/100,000 95%UI) | ||
| All NTDs | 6293(3699, 10080) | 15.5(10.6,23.2) | 3593(2051, 6178) | 3.9(2.2,7.0) | -70.0 |
| Schistosomiasis | 993.4(599.2, 1470.7) | 6.1(3.5,9.4) | 249.4(118.1,453.4) | 0.53(0.23,1.0) | -91.3 |
| Leishmaniasis | 373.9(139.0, 800.8) | 0.74(0.3,1.49) | 615.0(248.5,1240.3) | 0.58(0.23,1.22) | -21.6 |
| Intestinal nematode infections | 300.2(89.7,481.4) | 0.34(0.12,0.54) | 122.6(69.1,184.9) | 0.09(0.05,0.13) | -73.5 |
| Rabies | 1786.9(854.9,3159.1) | 4.1(2.2,6.6) | 1242.5(632.6,2659.3) | 1.5(0.72,3.33) | -63.1 |
Figure 1.
Trends of age-standardized mortality rates for all NTDs, Schistosomiasis, Leishmaniasis and rabies, 1990-2015
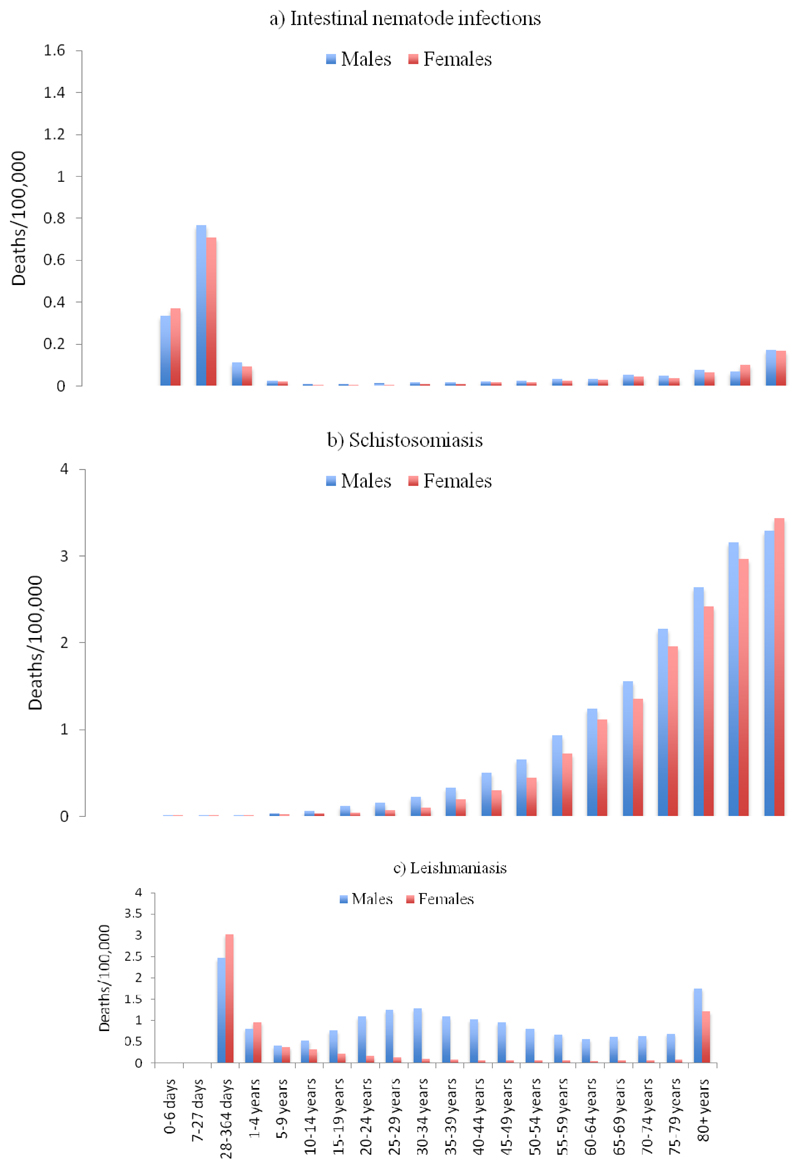
STH mortality rates peaked in the age group of 1-4 years for males (0.76 per 100,000; 95%UI: 0.32-1.36) and females (0.71 per 100,000; 95%UI: 0.29-1.27)b (Figure 2a). Schistosomiasis mortality rates rose substantially as age increased (Figure 2b). Leishmaniasis mortality rates were higher among males were more than 5 times higher than mortality rate among females for individuals older than 10 years (Figure 2c).
Figure 2a, 2b & 2c.
Mortality rate due to Schistosomiasis, Leishmaniasis and Intestinal nematode by age groups and sex in 2015
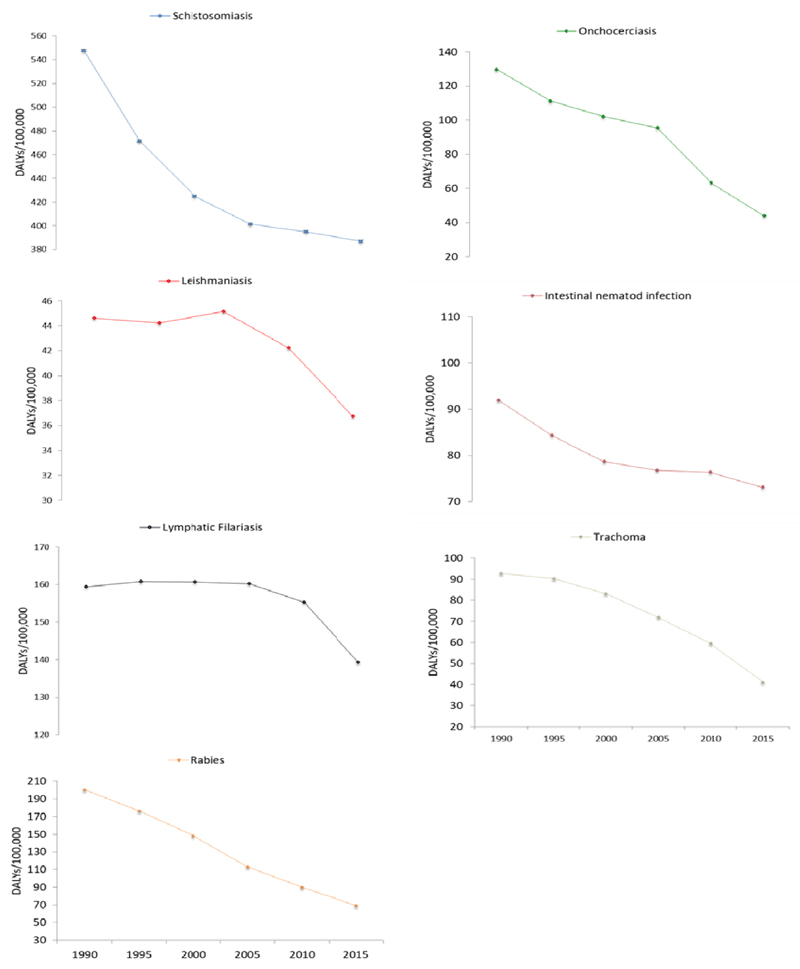
The number of DALY due to all NTDs has declined from 814.4 thousand (95% UI: 548 thousand–1.2 million) in 1990 to 579.5 thousand (95% UI: 309.4 thousand–1.3 million) in 2015. Age-standardized DALY rates due to all NTDs declined by 30.4%, from 17.6 per 1000 (95%UI: 12.5-26.5) in 1990 to 12.2 per 1000 (95%UI:6.5–27.4) in 2015. Age-standardized DALY rate for trachoma declined from 92.7 per 100,000 (95% UI: 63.2–128.4) in 1990 to 41.2 per 100,000 (95%UI: 27.4–59.2) in 2015, a 55.6% reduction between 1990 and 2015. Age-standardized DALY rates for onchocerciasis, schistosomiasis and LF decreased by 66.2%, 29.4% and 12.5% respectively between 1990 and 2015. DALY rate for ascariasis fell by 56.8% over the past 25 years (Table 2).
Table 2.
Number of DALY and DALY rates for common neglected tropical Diseases in Ethiopia, both sexes between 1990 and 2015
| Diseases | 1990 | 2015 | Median % change DALY rate, 1990-2015 | ||
|---|---|---|---|---|---|
| Number of DALYs (95%UI) | DALYs/100,000 | Number of DALYs (95% UI) | DALYs/100,000 | ||
| All NTDs | 814410 (548520, 1223541) | 1762 (1249.9, 2650.9) | 579574 (309380,1309453) | 1225.6 (653.2,2741.3) | -30.4 |
| Schistosomiasis | 208298.3 (122544.6) | 547.8(338.2, 924.1) | 367556.8 (187441.7,695405.3) | 386.8 (192.1,747.2) | -29.4 |
| Leishmaniasis | 26442.9 (9993.4,56347.4) | 44.6(18.9,90.9) | 43850.3 (18879.3,84765.2) | 36.8 (16.3,70.8) | -17.5 |
| Lymphatic Filariasis | 54734.6 (29059.1(93370.2) | 159.4(83.2,271.8) | 105045.2 (54565.3,176643.9) | 139.4 (70.5,238.4) | -12.5 |
| Onchocerciasis | 44137.2 (21847.5,77146.4) | 129.7(66.4,228.9) | 31214.4 (13580.3,57597.1) | 43.9 (20.0,81.7) | -66.2 |
| Intestinal nematode infections | 57277.9 (36196.2,81770.6) | 91.9(61.3,131.9) | 82707.6 (53644.3,123177.1) | 73.0 (45.7,111.1) | -20.6 |
| Ascariasis | 29763.8 (11887.3,45399.6) | 35.9(16.9,53.5) | 19752.9 (,12837.7, 28278.9) | 15.5 (10.2,22.7) | -56.8 |
| Trichuriasis | 4566.0 (2527.6,7843.0) | 8.8(4.7,15.2) | 9299.7 (4998.7,15531.7) | 8.6(4.6,14.4) | -2.3 |
| Hookwork | 22948.1 (14309.7,35072.0) | 47.1(29.0,73.5) | 53654.9 (33334.6,81706.4) | 48.9 (29.5,75.8) | 3.8 |
| Trachoma | 18211.7 (12257.1,25644.5) | 92.7(63.2,128.4) | 17293.6 (11505.2,25198.7) | 41.2 (27.4,59.2) | -55.6 |
| Rabies | 112196.8 (47222.9,214928.3) | 200.4 (99.8,339.6) | 74287.4 (37885.7,158420.8) | 69.3 (34.4,148.4) | -65.4 |
The age-standardized DALY rates for rabies, leishmaniasis and STH declined by 65.4%, 17.5%, 20.6% respectively during 1990 and 2015 (Figure 3).
Figure 3.
Trends of age-standardized DALY rates for common NTD, both sexes, 1990-2015
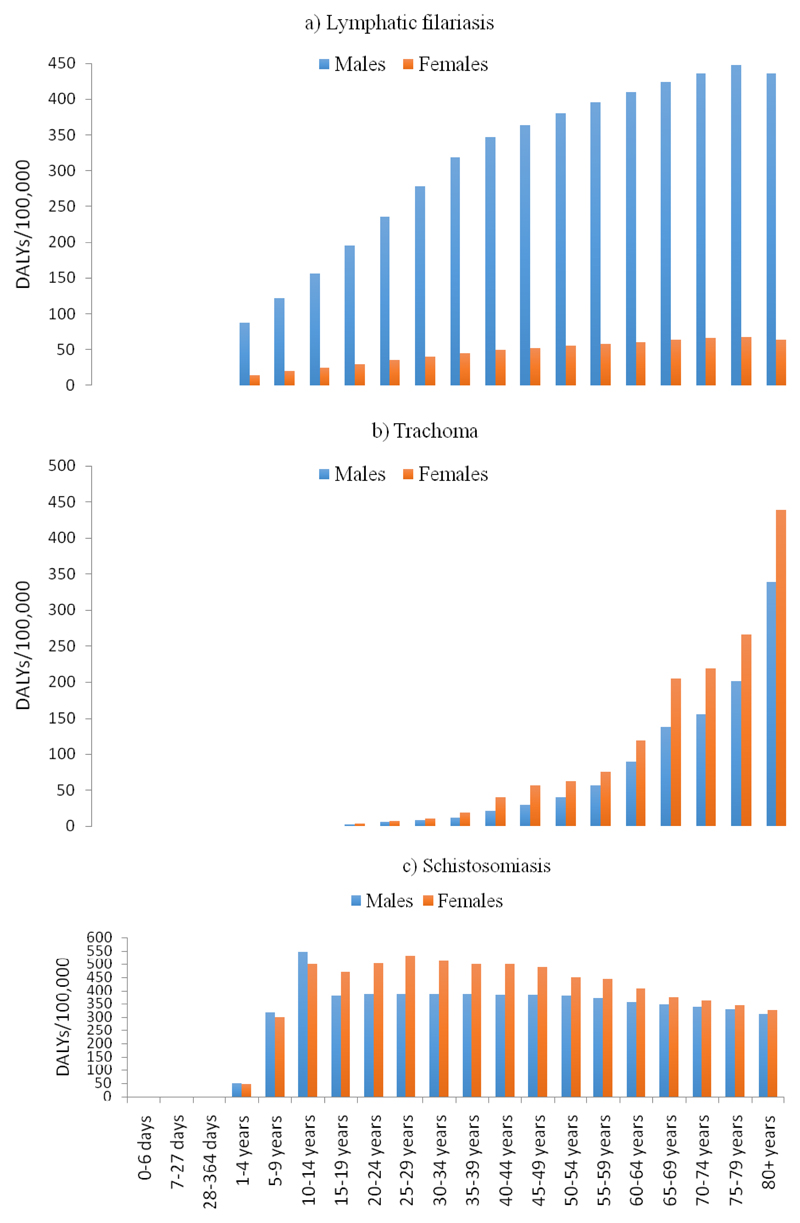
DALY rates for LF progressively increased with age and were more than five times higher among males than among females in all age groups (Figure 4a). Trachoma DALY rates increased significantly with age, with trachoma DALY rates being more than 200 per 100,000 among females 65 years and older, compared to 0.9 per 100,000 among females ages 15-19 years (Figure 4b).
Figure 4.
DALY rates for common NTDs by age group and sex in 2015
Discussion
In recent years unprecedented support for the NTDs has been given globally. The launching of the World Health Organization NTD roadmap and the London declaration contributed to this global momentum (8,9). In line with this global increased commitment Ethiopia launched the first ever NTD master plan in 2013 and renewed the second master plan in 2016 (3,4). To monitor and evaluate the impact of interventions and to track the progress in the NTD control and elimination it is critical to have robust benchmark. This study advances that goal and provides important account to the progress and current status of NTDs burden in Ethiopia.
This study presents a comprehensive evaluation of mortality and DALYs for common NTDs in Ethiopia between 1990 and 2015. Ethiopia has made remarkable progress in reducing the burden of NTDs during the MDG era. Age-standardized mortality rates for schistosomiasis have declined by more than 91.3% over the last 25 years. Age-standardized DALY rates for trachoma and onchocerciasis have decreased by more than 50% during the same period. However, less progress was observed for LF, leishmaniasis, trichuriasis, for which DALY rates declined by 12%, 17.5%, and 2.3%, respectively. On the other hand, DALY rates have increased by nearly 4% for hookworm during the MDG era.
NTDs are diseases of poverty and they affect the most disadvantaged and vulnerable population, particularly in Sub-Saharan Africa and Ethiopia. Poor housing conditions, illiteracy, lack of safe water supply and poor sanitations are among the strongest risk factors for NTDs, and these risks are common in sub-Saharan Africa (24). Several NTDs such as LF, onchocerciasis and Trachoma are non-fatal; and their public health impact is, therefore, best understood in terms of DALYs (24). Ethiopia has made significant progress in reducing health loss from most NTDs. First, the rapid economic growth in the country over the last two decades could have positive impact in reducing the burden of poverty related NTDs (2). Second, the FMOH in collaboration with implementing partners and donors have launched and improved the coverage of integrated and effective interventions such as mass drug administration (MDA), integrated vector control activities, SAFE for trachoma (surgery, antibiotics, face washing and environmental sanitation) and intensified case management. These interventions likely underlie the observed reductions in the burden of NTDs (5,10,25). As of 2016, the SAFE intervention has reached 510 districts in Ethiopia, and 871,646 peoples received TT surgery for trichiasis from 2003 to end of 2014. In addition more than 186 million people have been treated with annual Zithromax since the program start the programme in 2003(5). More than 20 million school aged children have been treated for schistosomiasis and STH yearly since 2007(5). MDA for onchocerciasis in west Ethiopia has reduced the prevalence of microfilaridermia by 46% between 2006 and 2012 (26) . Similarly, annual MDA for LF has been implemented since 2009, and by 2015 nearly two million individuals were treated (5).
Despite the remarkable progress in fighting NTDs, several bottlenecks must be addressed to eliminate NTDs in Ethiopia during the SDG era. First, the coexistence of HIV with common NTDs such as leishmaniasis pose a diagnostic and treatment challenge. Diro et al have reported that the use of antimonials for the treatment of leishmaniasis in HIV patients could result in treatment failure (27). The slow reductions in leishmaniasis mortality and DALY rates reported here could be the result of treatment failures that demand new drugs be made available to reduce mortality. Second, individuals with NTDs such as LF, trachoma and onchocerciasis are prone to social stigma and discrimination, and requires strong community engagement and mobilization activities to address that stigma and improve intervention coverage (28). Third, some NTDs such as rabies are zoonotic diseases that require a one health approach and collaboration with veterinary medicine. The one health approach emphasizes the linkage and collaboration between human and animal health and the environment (2,29). Lastly, Ethiopia’s future economy will be based on mega projects such as hydroelectric dams and irrigation systems that might favor the breeding of mosquitoes and transmission of vector borne diseases such as LF that calls for tailored interventions in such high-risk areas (30–32).
GBD is the first study to provide comprehensive estimates of mortality and DALYs for NTDs during the MDG era. However, the study has some limitations. First, quantity of data for estimation of DALYs and mortality rates are sparse in Ethiopia that makes the uncertainty intervals very wide (Table 2 and Figure 2). Second, GBD 2015did not produce sub-national estimates for Ethiopia and we are, therefore, unable to describe heterogeneity in the burden of NTDs within Ethiopia.
In conclusion, Ethiopia has made remarkable progress in reducing DALY rates for trachoma, onchocerciasis and some STH such as ascariasis. Slow progress has been observed for LF and leishmaniasis. Ethiopia should continue to strengthen integrated intervention coverage for NTDs through proper coordination with other health programs and sectors, and community participation to eliminate NTDs by 2020. The health management information system for NTDs should be strengthened by districts and regions to track progresses during the SDG period.
Acknowledgments
We are grateful to the GBD team at the Institute of Health Metrics and Evaluation (IHME) to support us to establish the Ethiopian national disease burden team. KD is funded by Wellcome Trust Intermediate Fellowship in Public Health and Tropical Medicine (grant number 201900).
References
- 1.Balabanova D, Mills A, Conteh L, et al. Good Health at Low Cost 25 years on: lessons for the future of health systems strengthening. Lancet. 2013;381(9883):2118–33. doi: 10.1016/S0140-6736(12)62000-5. [DOI] [PubMed] [Google Scholar]
- 2.Nenoff P, Simon JC, Muylowa GK, Davey G. Podoconiosis - non-filarial geochemical elephantiasis - a neglected tropical disease? J Dtsch Dermatol Ges. 2009;8(1):7–14. doi: 10.1111/j.1610-0387.2009.07099_supp.x. [DOI] [PubMed] [Google Scholar]
- 3.Federal Democratic Republic of Ethiopia Ministry of Health. Ethiopia National Master Plan For Neglected Tropical Diseases. Addis Ababa, Ethiopia: 2013. [Accessed on 03 April 2014]. Available at http://ntdenvision.org/resource/ethiopia_national_master_plan_for_neglected_tropical_diseases. [Google Scholar]
- 4.Federal Democratic Republic of Ethiopia Ministry of Health. Second Edition of Ethiopia National Master Plan For Neglected Tropical Diseases. Addis Ababa, Ethiopia: 2016. [Google Scholar]
- 5.Deribe K, Tomczyk S, Tekola-Ayele F. Ten years of podoconiosis research in Ethiopia. PLoS Negl Trop Dis. 2013;7(10):e2301. doi: 10.1371/journal.pntd.0002301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deribe K, Meribo K, Gebre T, et al. The burden of Neglected Tropical Diseases in Ethiopia, and opportunities for integrated control and elimination. Parasit Vectors. 2012;5(1):240. doi: 10.1186/1756-3305-5-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotez PJ, Fenwick A, Savioli L, Molyneux DH. Rescuing the “bottom billion” through neglected tropical disease control. Lancet. 2009;373:1570–6. doi: 10.1016/S0140-6736(09)60233-6. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases a Roadmap for Implementation. World Health Organization; 20 Avenue Appia, 1211 Geneva 27, Switzerland: 2012. [Google Scholar]
- 9.Uniting to Combat Neglected Tropical Diseases. Ending the Neglect & Reaching 2020 Goals. [Accessed 02 May 2015];London Declaration on Neglected Tropical Diseases. 2012 Available at http://unitingtocombatntds.org/sites/default/files/resource_file/london_declaration_on_ntds.pdf. [Google Scholar]
- 10.Mengitsu B, Shafi O, Kebede B, et al. Ethiopia and its steps to mobilize resources to achieve 2020 elimination and control goals for neglected tropical diseases webs joined can tie a lion. Int Health. 2016;8(Suppl 1):i34–52. doi: 10.1093/inthealth/ihw007. [DOI] [PubMed] [Google Scholar]
- 11.Mortality GBD, Causes of Death C. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DALYs GBD, Collaborators, H. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1603–58. doi: 10.1016/S0140-6736(16)31460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Addisu S, El-Metwally TH, Davey G, Worku Y, Titheradge MA. The role of transforming growth factor-beta1 and oxidative stress in podoconiosis pathogenesis. Br J Dermatol. 2010;162(5):998–1003. doi: 10.1111/j.1365-2133.2010.09652.x. [DOI] [PubMed] [Google Scholar]
- 14.Wakabi W. Extension workers drive Ethiopia's primary health care. Lancet. 2008;372(9642):880. doi: 10.1016/s0140-6736(08)61381-1. [DOI] [PubMed] [Google Scholar]
- 15.Karim AM, Admassu K, Schellenberg J, et al. Effect of Ethiopia's Health Extension Program on Maternal and Newborn Health care practices in 101 rural districts: a dose-response study. PLoS One. 2013;8(6):e65160. doi: 10.1371/journal.pone.0065160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray CJ, Ortblad KF, Guinovart C, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9947):1005–70. doi: 10.1016/S0140-6736(14)60844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Global Burden of Disease Study C. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Liddell CA, Coates MM, et al. Global, regional, and national levels of neonatal, infant, and under-5 mortality during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9947):957–79. doi: 10.1016/S0140-6736(14)60497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foreman KJ, Lozano R, Lopez AD, Murray CJ. Modeling causes of death: an integrated approach using CODEm. Popul Health Metr. 2012;10:1. doi: 10.1186/1478-7954-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray CJ, Ezzati M, Flaxman AD, et al. GBD 2010: design, definitions, and metrics. Lancet. 2012;380(9859):2063–6. doi: 10.1016/S0140-6736(12)61899-6. [DOI] [PubMed] [Google Scholar]
- 23.DALYs GBD, Collaborators H. Murray CJ, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990-2013: quantifying the epidemiological transition. Lancet. 2015;386(10009):2145–91. doi: 10.1016/S0140-6736(15)61340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davey G, Tekola F, Newport MJ. Podoconiosis: non-infectious geochemical elephantiasis. Trans R Soc Trop Med Hyg. 2007;101(12):1175–80. doi: 10.1016/j.trstmh.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Price E. Podoconiosis:Non-filarial Elephantiasis. Oxford Medical Publications; Oxford, UK: 1990. [Google Scholar]
- 26.Samuel A, Belay T, Yehalaw D, Taha M, Zemene E, Zeynudin A. Impact of Six Years Community Directed Treatment with Ivermectin in the Control of Onchocerciasis, Western Ethiopia. PLoS One. 2016;11(3):e0141029. doi: 10.1371/journal.pone.0141029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diro E, Lynen L, Mohammed R, Boelaert M, Hailu A, van Griensven J. High parasitological failure rate of visceral leishmaniasis to sodium stibogluconate among HIV co-infected adults in Ethiopia. PLoS Negl Trop Dis. 2014;8(5):e2875. doi: 10.1371/journal.pntd.0002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofstraat K, van Brakel WH. Social stigma towards neglected tropical diseases: a systematic review. Int Health. 2016;8(Suppl 1):i53–70. doi: 10.1093/inthealth/ihv071. [DOI] [PubMed] [Google Scholar]
- 29.Gebreyes WA, Dupouy-Camet J, Newport MJ, et al. The global one health paradigm: challenges and opportunities for tackling infectious diseases at the human, animal, and environment interface in low-resource settings. PLoS Negl Trop Dis. 2014;8(11):e3257. doi: 10.1371/journal.pntd.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yewhalaw D, Legesse W, Van Bortel W, et al. Malaria and Water Resource Development: the case of Gilgel-Gibe hydroelectric dam in Ethiopia. Malar J. 2009;8:21. doi: 10.1186/1475-2875-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kibret S, Wilson GG, Tekie H, Petros B. Increased Malaria Transmission around irrigation schemes in Ethiopia and the potential of canal water management for malaria vector control. Malar J. 2014;13:360. doi: 10.1186/1475-2875-13-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deribew A, Birhanu Z, Sena L, et al. The effect of household heads training about the use of treated bed nets on the burden of malaria and anaemia in under-five children: a cluster randomized trial in Ethiopia. Malar J. 2012;11:8. doi: 10.1186/1475-2875-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]