Abstract
Background
Miltefosine, an anti-cancer drug that has been successfully repositioned for treatment of Leishmania infections, has recently also shown promising effects against Schistosoma spp targeting all life cycle stages of the parasite. The current study examined the effect of treating Schistosoma mansoni adult worms with miltefosine on exposure of worm surface antigens in vitro.
Methodology/Principal findings
In an indirect immunofluorescence assay, rabbit anti-S.mansoni adult worm homogenate and anti-S. mansoni infection antisera gave strong immunofluorescence of the S. mansoni adult worm surface after treatment with miltefosine, the latter antiserum having previously been shown to synergistically enhance the schistosomicidal activity of praziquantel. Rabbit antibodies that recognised surface antigens exposed on miltefosine-treated worms were recovered by elution off the worm surface in low pH buffer and were used in a western immunoblotting assay to identify antigenic targets in a homogenate extract of adult worms (SmWH). Four proteins reacting with the antibodies in immunoblots were purified and proteomic analysis (MS/MS) combined with specific immunoblotting indicated they were the S. mansoni proteins: fructose-1,6 bisphosphate aldolase (SmFBPA), Sm22.6, alkaline phosphatase and malate dehydrogenase. These antibodies were also found to bind to the surface of 3-hour schistosomula and induce immune agglutination of the parasites, suggesting they may have a role in immune protection.
Conclusion/Significance
This study reveals a novel mode of action of miltefosine as an anti-schistosome agent. The immune-dependent hypothesis we investigated has previously been lent credence with praziquantel (PZQ), whereby treatment unmasks parasite surface antigens not normally exposed to the host during infection. Antigens involved in this molecular mechanism could have potential as intervention targets and antibodies against these antigens may act to increase the drug’s anti-parasite efficacy and be involved in the development of resistance to re-infection.
Author summary
Schistosomiasis (Bilharzia) is a serious public health problem caused by a parasite of genus Schistosoma. There is an increasing concern about development of parasite resistance to the only drug available for treatment, praziquantel (PZQ). Miltefosine, a repurposed anti-cancer drug for treatment of Leishmania infection, was shown to have activity against Schistosoma in animal models at all the parasite’s life cycle stages. In this work, we examined the potential that miltefosine could act to expose parasite surface antigens that are normally hidden during natural infection as a way to avoid lethal effects of host immunity. We used two immunobinding techniques, immunofluorescence and western immunoblotting, and a protein identification technique, namely mass spectrometry, to identify proteins exposed on the worm surface following incubation with miltefosine. Four S. mansoni proteins were shown to be exposed by miltefosine treatment: fructose-bisphosphate aldolase (SmFBPA), Sm22.6, alkaline phosphatase and malate dehydrogenase. Antibodies specific for these antigens recognised and bound to the surface of early-stage schistosome larvae and antibodies specific for SmFBPA induced clumping of the larvae, suggesting a potential role in early parasite killing and protection against infection. These antibodies may be utilised to increase miltefosine’s anti-parasite efficacy and may be involved in resistance to re-infection.
Introduction
Despite extensive control efforts, schistosomiasis is still prevalent in many countries [1]. Concerns are growing over development of resistance to the only drug available for treatment, namely praziquantel (PZQ), because of its extensive use in control programs for almost three decades [2,3]. The suboptimal efficacy of PZQ against immature stages of the infection is another concern [4]. Thus, the search for an alternative therapeutic control or a combination strategy that is effective against multiple stages of the schistosome life cycle has become necessary [5,6]. One approach that may accelerate discovery of new drugs is to identify and develop alternative uses for existing, approved drugs that have had their pharmacokinetics, toxicity and pre-clinical data evaluated − an approach known as ‘Drug-Repositioning’ [7,8]. One promising example is miltefosine, a hexa-decyl-phosphocholine discovered in the late 1980s and approved as Miltex in 1992 for topical treatment of cutaneous metastasis of breast cancer [9,10]. The drug later demonstrated effectiveness against antimony-resistant Leishmania [11,12] and (under the trade name Impavido) was approved for oral treatment of visceral leishmaniasis in some countries [13–15].
Recent studies by Eissa et al. [16–18], Bertao et al. [19,20] and El-Moslemany et al. [21] demonstrated significant effectiveness of miltefosine against Schistosoma mansoni (S. mansoni) and Schistosoma haematobium in vitro and against different developmental stages of S. mansoni in vivo. The drug exhibited an advantage over PZQ by clearing the infection at the invasive, immature and adult parasite stages [4,17]. The mechanism by which miltefosine exerts its effect is unclear, although it is believed to act mainly on cell membranes producing biophysical perturbation of lipid rafts and interfering with phospholipid metabolism and cell growth signalling pathways, subsequently promoting cell apoptosis [19,22–24]. One of the drug targets is sphingomyelin, a schistosome membrane phospholipid (ceramide phosphorylcholine) [25]. Biosynthesis of this molecule was shown to be impeded by miltefosine [25]. Sphingomyelin acts to conceal surface membrane proteins from the host immune system through formation of a tight barrier of hydrogen bonds with water molecules [26]. Evidence of damage to the schistosome tegument following treatment with miltefosine has been referred to in several reports [16–20], but no studies have yet been conducted to clearly establish if the drug induces exposure of antigens on the parasite surface.
In the present work, we evaluated the in vitro effect of miltefosine, relative to PZQ, on a Puerto Rican strain of S. mansoni and investigated whether the drug treatment would ‘unmask’ hidden antigens on the adult worm tegument using immunofluorescence (IF) and western immunoblotting assays linked to a proteomic approach i.e. tandem mass spectrometry (MS/MS). The likelihood of expression of the proteins exposed by the drug on the surface of adults and 3-hour schistosomula has also been investigated. Exposure of cryptic adult membrane proteins has previously been reported after treatment with PZQ and other anti-schistosomal drugs [27–34], an event which was thought to be an important factor in host immune responses directed at intact worms and to be potentially involved in development of resistance to re-infection by targeting migratory schistosomula [35–37].
Methods
Ethics statement
All work with laboratory animals, including maintenance of life cycle of Schistosoma mansoni by repeated passage through random-bred CD1 strain mice and laboratory-bred Biomphalaria glabrata snails, was conducted according to the UK Animal (Scientific Procedures) Act 1986 with personal and project license authorities held by MJD (numbers PIL 70/3255 and PPL 40/3024 respectively). Work performed under these authorities was approved by Nottingham University’s Animal Welfare and Ethical Review Body and was carried out in strict accordance with UK government regulations for animal welfare and amelioration of suffering in force at the time. The biological materials obtained from the animals under the above authorities was utilised for immunobinding experiments in accordance with the Egyptian National Animal Welfare Standards, approved by the Alexandria University’s Faculty of Medicine Ethics Committee (protocol approval number 0303186).
Chemicals and drug
All chemicals were purchased from Sigma-Aldrich (UK) unless otherwise indicated. Miltefosine (hexa-decyl-phosphocholine) was purchased from Calbiochem (Cambridge, UK) and praziquantel (PZQ) from Merck Ltd (UK). For in vitro treatment, drugs were dissolved in a minimal volume of 0.5% dimethyl sulfoxide (DMSO) diluted in Roswell Park Memorial Institute (RPMI) 1640 medium (Invitrogen, UK) to generate a stock solution of 5 mg/ml [16,38,39].
Parasites and animals
A Puerto Rican strain of S. mansoni was maintained by repeated passage through random-bred female CD1 strain mice and laboratory-bred Biomphalaria glabrata snails using previously described methods [40]. The life cycle of the parasite has been maintained in the laboratory for more than 40 years. The S. mansoni strain used in the present study was originally isolated from eggs obtained from a Puerto Rican patient in 1960s by Dr. Smithers SR, NIMR, London. This strain was analyzed anonymously and is currently kept at the University of Nottingham. Cercariae were used for animal infection or mechanically transformed into schistosomula as previously described [41]. Mice were percutaneously infected with 200 cercariae using methods previously described [29,42]. 42 days after infection, animals were sacrificed using an overdose of pentobarbitone anaesthetic and worms were recovered from their portal system as described elsewhere [43].
Preparation of aqueous parasite extract
A crude protein extract from S. mansoni adult worms (whole worm homogenate), SmWH, was prepared according to methods previously described by Doenhoff et al. [44]. S. mansoni adult worms washed in phosphate-buffered saline (PBS) were mechanically homogenized using a ground-glass homogenizer, and the homogenate centrifuged at 2000 x g for 10 minutes at room temperature. The supernatant was collected, freeze-dried and kept at −80°C. To prepare S. mansoni detergent-soluble adult worm extract, freshly perfused worms were incubated in PBS, pH 7.4, the gravity-sedimented worm pellet being resuspended in an equal volume of buffer containing 2% sodium deoxycholate [44]. The suspension was gently agitated on a rocker for 3 hours at room temperature, and then centrifuged at 5,000 x g for 30 minutes at room temperature. The supernatant was collected, freeze-dried and kept at −80°C. When required for use, samples were reconstituted with distilled water and the protein concentration was measured by the BioRad DC Protein Assay (BioRad Laboratories, Inc., Hercules, California USA) using bovine serum albumin as the reference standard.
Rabbit anti-S. mansoni antisera
A polyspecific rabbit anti-S. mansoni infection antiserum, anti-SmI, was developed as described by Doenhoff et al. [44] by exposing New Zealand white rabbits (B&K Universal, UK) to 5 rounds of percutaneous S. mansoni infections, each of approximately 15 x 103 cercariae, via the ear at two week intervals. Two weeks after the last cercarial exposure, 3 samples of 20 ml blood were removed from an ear vein at two-week intervals after which the rabbits were exsanguinated by cardiac puncture. Sera from the serial and final bleeds were pooled and stored at −20°C until required. A rabbit antiserum polyspecifically reactive against S. mansoni adult worm homogenate (anti-SmWH) was prepared as described before [44,45]. New Zealand white rabbits were immunized subcutaneously at two week intervals with 1 ml of the homogenate containing approximately 5 mg protein emulsified with an equal volume of Freund’s complete (CFA) and incomplete adjuvants for the first and subsequent booster injections, respectively. The rabbits were serially bled via an ear vein every two weeks and when sera showed what was considered sufficient immunoreactivity in an immunoelectrophoretic analysis, animals were serially bled, followed by terminal anaesthesia and exsanguination by cardiac puncture. Sera from serial and final bleeds were pooled and stored at −20°C until needed. Rabbit antiserum monospecifically reactive against S. mansoni alkaline phosphatase (SmAP) was prepared by immunization of rabbits subcutaneously with replicates of immunoprecipitin arcs containing the enzyme excised from immunoelectrophoresis gels, homogenized and emulsified with an equal volume of Freund’s adjuvants [45,46]. The immunization protocol was as described above for anti-SmWH. Sera from rabbits injected with Freund’s adjuvants alone, developed as above, and normal rabbit sera from naive uninfected animals were used as controls for the IF experiments. The rabbit antibodies were prepared using Freund's Complete Adjuvant during the period from 1990- to 2000, before a ban was placed on the use of CFA in Europe [47].
In vitro miltefosine and praziquantel treatment and immunofluorescence (IF) of schistosome adult worms
Intact freshly-recovered S. mansoni adult worms (males/couples) obtained by perfusion from infected mice were rinsed in RPMI-1640 medium (supplemented with 5% fetal calf serum (FCS), 2 mM L-glutamine, 100 μg/ml streptomycin and 100 IU/ml penicillin), placed in a 24-well culture plate containing 1 ml/well of the supplemented RPMI-1640 medium and incubated at 37°C in 5% CO2 incubator for 2 hours to recover, the method being adapted from Eissa et al. [16], Bertao et al. [19,20] and Mossallam et al. [48]. Worms were divided into three groups, each being tested in triplicate, as follows: Group I, untreated worms in a medium containing 0.5% DMSO (vehicle) were used as negative controls (n = 10); Group II, worms treated with PZQ; Group III, worms treated with miltefosine. Groups II and III were further subdivided into 5 and 4 subgroups, respectively (n = 10 for each) to test different concentrations of the PZQ: 2, 4, 6, 8 and 10 μg/ml [38] and the miltefosine: 5, 10, 20 and 40 μg/ml [16]. All groups were incubated at 37°C in 5% CO2 incubator for 96 hours. Worms were examined under an inverted microscope (Olympus Inverted Microscope Model IX70, Olympus, Tokyo, Japan) every 24 hours and observed for changes in their motility, pairing, tegument and for mortality, and the data was analysed with respect to both the drug concentration and the incubation time. Mortality was indicated by absence of any body movement detected during 2 minutes of observation [49,50]. Lethal concentrations of miltefosine that killed 50% of worms (LC50) at the examination times of 24, 48, 72 and 96 hours were calculated using Excel Microsoft software and a curve for LC50s at different time points was generated using GraphPad Prism 4 software (Inc., La Jolla—CA, USA).
After 96 hours or in the case of death, worms from all groups were rinsed three times in RPMI medium, fixed in 4% paraformaldehyde (PFA) in PBS, pH 7.4 overnight at 4°C, washed thoroughly three times in PBS, each for 5 minutes. Fixed worms were blocked by incubation with 1% bovine serum albumin (BSA) in PBS-Tween (PBST) (0.5% v/v Tween20 in PBS) for 1 hour at room temperature and washed as above in PBST. Worms from untreated Group I and all subgroups in groups II and III were further subdivided into two subgroups/(sub)group, each was tested in triplicate wells (n = 5/well), incubated with the following heat-inactivated (56°C for 30 minutes) rabbit sera: anti-S. mansoni infection antiserum (anti-SmI); anti-S. mansoni worm homogenate antiserum (anti-SmWH). All rabbit sera were diluted 1:20 in PBST and incubated with the worms overnight at 4°C. After washing in PBST, fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG antibodies (diluted 1:40) were added for 30 minutes in the dark at room temperature as previously described [51]. Labelled worms were examined under ultraviolet light microscopy (GMXL3201LED) and worms displaying representative staining patterns were photographed using a GXCAM-FLUOMA-X camera (GT Vision Ltd) and GXCapture7 software. All images were obtained under consistent microscope settings.
Elution of rabbit antibodies bound to the schistosome adult worm surface
120 worms freshly perfused from S. mansoni-infected mice at day 42 after infection were divided into three groups (n = 40/group): Group I, untreated; Groups II and III, treated with the drug dose and for the duration that produced the greatest changes in worm tegument in vitro and the most intense immunofluorescence (6 μg/ml PZQ for 24 hours and 40 μg/ml miltefosine for 48 hours). Treatment of worms, washings, fixation in 4% PFA and blocking were carried out as described above. Worms in each group were then divided into four subgroups (n = 10 for each), (A): incubated with the rabbit anti-SmI serum; (B): incubated with the rabbit anti-SmWH serum; (C): incubated with a control serum from rabbits that received adjuvant alone; (D): incubated with a control serum from naive rabbits. Before use, rabbit sera were heat inactivated for 30 minutes at 56°C, diluted 1:20 in PBST and incubated with the worms overnight at 4°C. Four worms from each subgroup were incubated with 1:40 FITC-conjugated goat anti-rabbit IgG and examined under the fluorescence microscope to check for antibody binding. The rest of the worms were used to recover rabbit antibodies bound to their surfaces from the groups that showed immunofluorescence staining using a method adapted from Doenhoff et al. [52] for elution of antibodies from antigens fixed to a nitrocellulose film. After incubation with the primary rabbit sera worms were washed thoroughly three times in PBST, pH 7.4, each for 5 minutes. The worms were then incubated in a low-pH buffer, 0.1 M glycine, pH 2.8 for 10 minutes to elute off surface-bound antibodies, after which the eluates were collected and neutralized using 0.1 ml of 1M Tris-HCl, pH 8.0. The process of incubation with primary antibody, washing and antibody-elution using low pH buffer was repeated up to 4 times. The eluates from each subgroup were pooled, concentrated to 5% of the starting volume using Amicon ultra centrifugal filters, 3000 molecular weight cut-off (Millipore, Corrigtwohill, Co. Cork, Ireland), aliquoted and kept frozen at -20°C until needed.
Western immunoblotting for detection of protein targets in S. mansoni worm extract
Protein samples (10 μg/lane) from S. mansoni adult worm crude extract were analyzed in reducing 10% or 8% SDS-PAGE gels prepared as described elsewhere [53,54]. SDS-PAGE gels were run using a Bio-Rad Mini Protean II electrophoresis system (Bio-Rad Laboratories, California, USA) and were stained with SimplyBlue SafeStain (Invitrogen, UK) according to the manufacturer’s instructions. Proteins resolved with SDS-PAGE were transferred to nitrocellulose paper (NCP) strips at 50 V for two hours as previously described [45,55]. After visualizing the protein marker, the membranes were blocked with 5% skim milk in TBST (TBS-0.5% v/v Tween20) overnight at 4°C. Blot strips were washed x3 in TBST and probed with the primary rabbit antibodies (diluted 1:100 in TBST) for two hours at room temperature. After washing, the membranes were incubated with the secondary antibody: horse radish peroxidise (HRP)-conjugated goat anti-rabbit IgG antibody (Sigma, UK) diluted 1:1000 for 2 hours at room temperature. The immunoblots were then developed using 4-chloro-1-naphthol substrate (Sigma, UK) as described by the manufacturer.
Immunofluorescence for detection of reactivity of antibodies recovered from drug-treated adult worms on the surface of schistosomula
Schistosomula that had been mechanically transformed from freshly-harvested cercariae [41] were incubated in RPMI-1640 medium supplemented with 5% FCS, 2 mM L-glutamine, 100 μg/ml streptomycin and 100 IU/ml penicillin in a 24-well culture plate containing about 500 parasites in 1ml/well medium at 37°C, 5% CO2 for 3 hours, then fixed in 4% PFA in PBS for 1 hour at room temperature. Thereafter, the parasites were transferred to 5 ml glass test tubes, blocked by incubation in 1% BSA in PBST for 1 hour at room temperature. Fixed, blocked schistosomula were incubated with rabbit anti-SmI or anti-SmWH antibodies eluted from PZQ or miltefosine-treated worms. Control groups included schistosomula incubated with heat-inactivated sera from naive rabbits and from rabbits that had received adjuvant alone. Parasites were incubated with the eluted rabbit antibodies or control sera diluted 1:20 overnight at 4°C, washed three times in PBST and incubated with FITC-conjugated goat anti-rabbit IgG antibodies diluted 1:40 in PBST for 30 minutes in the dark at room temperature. IF staining was evaluated by fluorescence microscopy as described above. At least 40 parasites were observed for each group and those displaying representative staining patterns were photographed.
Antigen purification
About 150–200 μg of SmWH was placed in broad wells (6.2 cm long) and fractionated in reducing 12% SDS-PAGE gels and the gels were stained with the SimplyBlue SafeStain. Target proteins were identified by matching western blots bearing the immunoreactivities of interest to protein bands in SimplyBlue-stained SDS-PAGE gels. The proteins were purified by excision of the respective bands and elution of the proteins from the gel by incubation in a buffer containing 10% SDS as previously described [56,57]. The eluates were centrifuged at 14,000 x g, 37°C for 30 minutes and kept frozen at −20°C until use. Solutions of eluted purified proteins were concentrated using Amicon ultra centrifugal filters, 3000 molecular weight cut-off (Millipore, Corrigtwohill, Co. Cork, Ireland), their protein concentrations measured using the BioRad DC Protein Assay (BioRad Laboratories, USA) and their molecular weights and antigenic reactivity were confirmed by SDS-PAGE and western blotting. Purified, concentrated proteins were stored at −20°C.
Tandem mass spectrometry (MS/MS) analysis of purified proteins
SDS-PAGE gel slices bearing the target proteins were manually and individually excised, de-stained, reduced (DTT), carbamidomethylated (iodoacetamide) and digested with trypsin (20 μg/ml overnight at 37°C). The resulting peptides were submitted to tandem mass spectrometry (MS/MS) for protein identification [58]. The MS/MS data were used to search the public database NCBInr (version 20140927) using the Mascot software, version 2.3.01 (Matrixscience, UK, http://www.matrixscience.com) for peptide matching [59].
Results
Miltefosine pre-treatment of Puerto Rican S. mansoni adult worms induces tegumental changes and exposure of surface proteins
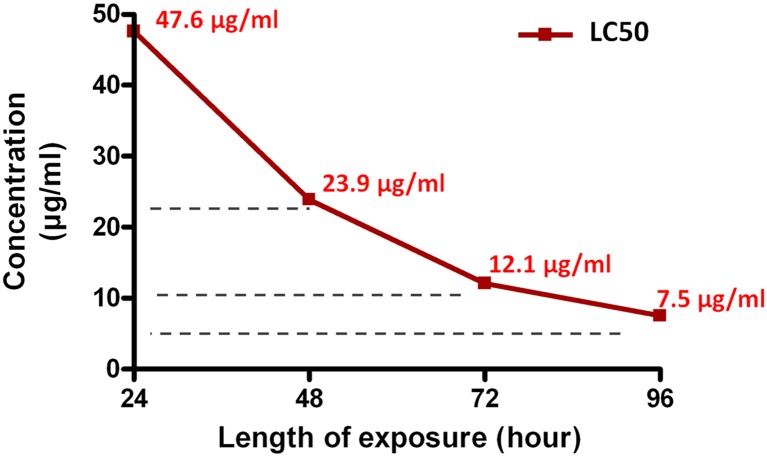
The effect of in vitro treatment of S. mansoni with miltefosine was evaluated every 24 hours over a time-course of 96 hours at different drug concentrations (5–40 μg/ml) relative to the control untreated and PZQ-treated worms. All control worms (incubated in supplemented RPMI medium containing 0.5% DMSO) survived and exhibited normal appearance with no tegumental changes throughout the examination period (Table 1) consistent with previously described results [50,60]. Parasites in the control group showed natural peristaltic motility of the body and were occasionally seen attached to the bottom of the well by their ventral suckers, male and female couples remaining paired. PZQ treatment at tested doses of 4–10 μg/ml resulted in instant muscular contraction with complete loss of motor activity in all worms and 90%-100% parasite mortality within 24 hours (Table 1). Light microscopic examination demonstrated tegumental disruption (S1 Fig) most obviously seen in the subgroup treated with 6 μg/ml PZQ. Extensive tegumental damage was seen with miltefosine doses of 10–40 μg/ml in 70–80% of the worms within 48–96 hours under an inverted microscope. The drug eventually resulted in worm death in 70–100% of worms (Table 1). These changes, however, developed at a slower rate than those observed with PZQ; the intensity of the effects by miltefosine was both dose- and time-dependent. The LC50 (drug concentration needed to cause 50% worm killing) in 24 hours was 47.6 μg/ml, while 23.9 μg/ml, 12.1 μg/ml and 7.5 μg/ml miltefosine were needed to kill 50% of worms in 48, 72 and 96 hours, respectively (Fig 1). Worm separation into individual males and females was observed within 24 hours in 70% of worms with miltefosine doses as low as 10 μg/ml. In contrast, only 10% of paired worms in the PZQ-treated group showed separation of couples (Table 1).
Table 1. In vitro activity of miltefosine against S. mansoni adult worms.
| Group | Dose μg/ml (μM) | Hours of incubation | Number | UncoupledWorms | Dead | Reduced activity a | Tegumental changes b | ||
|---|---|---|---|---|---|---|---|---|---|
| + | ++ | + | ++ | ||||||
| Control | 96 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Praziquantel | 2 (06.4) | 24 | 10 | 0 | 05 | 0 | 5 | 0 | 0 |
| 4 (12.8) | 24 | 10 | 0 | 09 | 0 | 1 | 8 | 0 | |
| 6 (19.2) | 24 | 10 | 1 | 10 | 0 | 0 | 3 | 7 | |
| 8 (25.6) | 24 | 10 | 1 | 10 | 0 | 0 | 5 | 5 | |
| 10 (32.0) | 24 | 10 | 1 | 10 | 0 | 0 | 6 | 4 | |
| Miltefosine | 5 (12.5) | 24 | 10 | 3 | 0 | 0 | 0 | 0 | 0 |
| 48 | 10 | 7 | 0 | 0 | 0 | 0 | 0 | ||
| 72 | 10 | 10 | 0 | 3 | 2 | 2 | 1 | ||
| 96 | 10 | 10 | 0 | 5 | 5 | 5 | 2 | ||
| 10 (25.0) | 24 | 10 | 7 | 0 | 1 | 0 | 0 | 0 | |
| 48 | 10 | 8 | 2 | 1 | 1 | 2 | 2 | ||
| 72 | 10 | 10 | 4 | 3 | 1 | 2 | 5 | ||
| 96 | 10 | 10 | 7 | 3 | 0 | 3 | 7 | ||
| 20 (50.0) | 24 | 10 | 10 | 2 | 1 | 2 | 1 | 2 | |
| 48 | 10 | 10 | 4 | 2 | 6 | 3 | 5 | ||
| 72 | 10 | 10 | 10 | 0 | 0 | 3 | 7 | ||
| 40 (100.0) | 24 | 10 | 10 | 4 | 3 | 2 | 6 | 4 | |
| 48 | 10 | 10 | 10 | 0 | 0 | 2 | 8 | ||
a Dead worms were excluded. +, Slight. ++, Significant.
b S1 Fig.
Fig 1. In vitro lethal effect of miltefosine on S. mansoni adult worms.
LC50s of the drug (concentrations that kill 50% of worms) 24, 48, 72 and 96 hours after in vitro exposure to miltefosine were calculated from concentration-response curves at the respective time points and LC50 values were plotted against length of incubation.
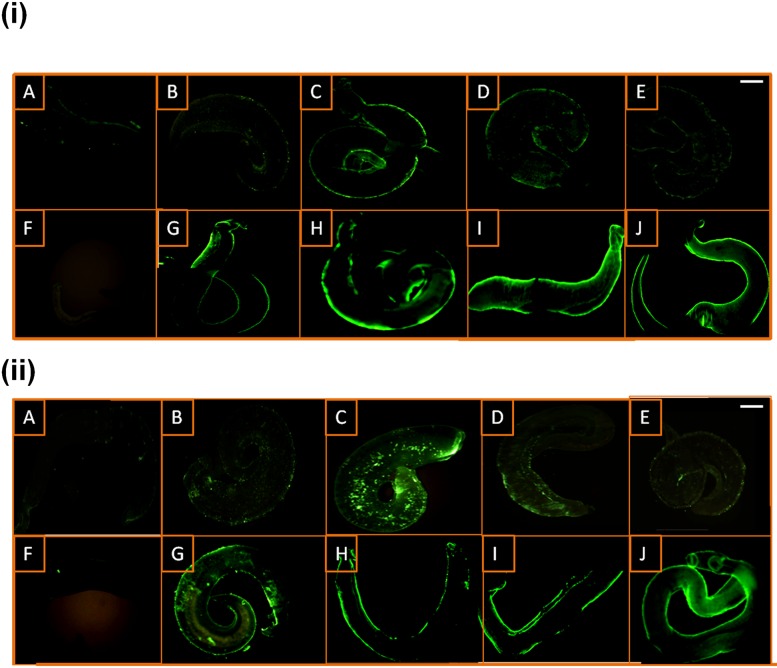
The surface of drug-treated worms was recognised by IgG antibodies from the anti-infection rabbit serum, anti-SmI (Fig 2iA–2iE & 2iG–2iI), while no staining could be seen on the untreated worms using the same antibodies (Fig 2iF). With PZQ, the fluorescence was minimal at both extremes of the dose-spectrum at 2, 4 and 10 μg/ml (Fig 2iA, 2iB and 2iE) and most intensive at 6 μg/ml (Fig 2iC). Fig 2 illustrates that, in general, the staining resulting from miltefosine treatment at all the tested doses was of a higher intensity compared to that resulting from PZQ treatment. The fluorescence was observed to be dose-dependent, most evident at 20 and 40 μg/ml miltefosine (Fig 2iI and 2iJ). Comparable results were obtained using a rabbit anti-S. mansoni worm homogenate although at relatively lower staining intensities, particularly with the PZQ treated-subgroup (Fig 2iiA–2iiE), while no staining could be detected on untreated worms (Fig 2iiF). In a second experiment, no staining was detected on PZQ-treated (6 μg/ml for 24 hours) or miltefosine-treated (40 μg/ml for 48 hours) worms by sera from a naive rabbit and a rabbit injected with Freund’s adjuvants alone (Figs 3iC and 3iD and 4iC and 4iD). The miltefosine and PZQ doses of 40 μg/ml and 6 μg/ml were, respectively found to have the most intense effects in 24 and 48 hours (Table 1 and Fig 2); these groups were therefore selected for subsequent antibody-elution experiments.
Fig 2. Immunofluorescent staining of miltefosine- and PZQ-treated S. mansoni adult worms.
The surface of worms treated with praziquantel (PZQ) (A-E), untreated worms (F) and worms treated with miltefosine (G-J) and exposed to: rabbit anti-S. mansoni infection (anti-SmI) IgG (i), rabbit anti-S. mansoni adult worm homogenate (anti-SmWH) antisera (ii). A, 2 μg/ml PZQ; B, 4 μg/ml PZQ; C, 6 μg/ml PZQ; D, 8 μg/ml PZQ; E, 10 μg/ml PZQ; G, 5 μg/ml miltefosine; H, 10 μg/ml miltefosine; I, 20 μg/ml miltefosine; J, 40 μg/ml miltefosine. Scale bar = 100 μm.
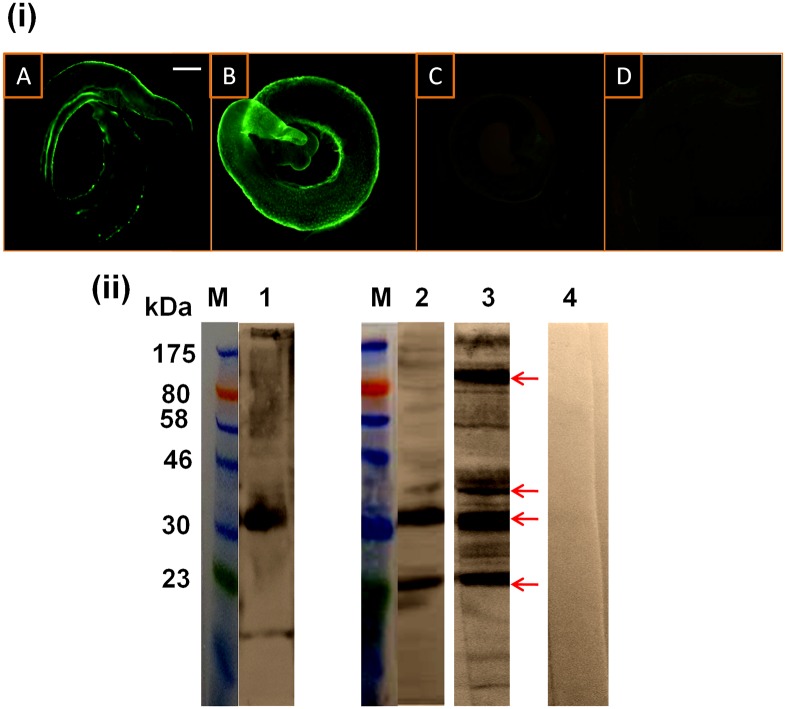
Fig 3. Characterization of worm surface antigens recognized by rabbit anti-SmI antibodies after miltefosine- and PZQ-treatment.
(i) Immunofluorescent staining of PZQ- (6 μg/ml for 24 hours) (A and C) and miltefosine- (40 μg/ml for 48 hours) (B and D) treated S. mansoni adult worms detected with rabbit anti-SmI IgG antibodies (A and B) and IgG antibodies from a normal rabbit serum (C and D). Scale bar = 100 μm. (ii) Western immunoblots of a S. mansoni crude worm homogenate preparation (SmWH) were probed with rabbit anti-SmI antiserum (lane 1), rabbit anti-SmI antibodies eluted from PZQ- (6 μg/ml for 24 hours) (lane 2) and miltefosine- (40 μg/ml for 48 hours) (lane 3) treated worms. A blot of SmWH probed with a normal rabbit serum (lane 4) was used as a control. Lane M, protein molecular weight markers. Antigenic extract was loaded onto the gel with 10 μg protein/lane. Blots were detected using HRP-conjugated anti-rabbit IgG secondary antibodies. Detected antigens with the most intense reactivities are arrowed.
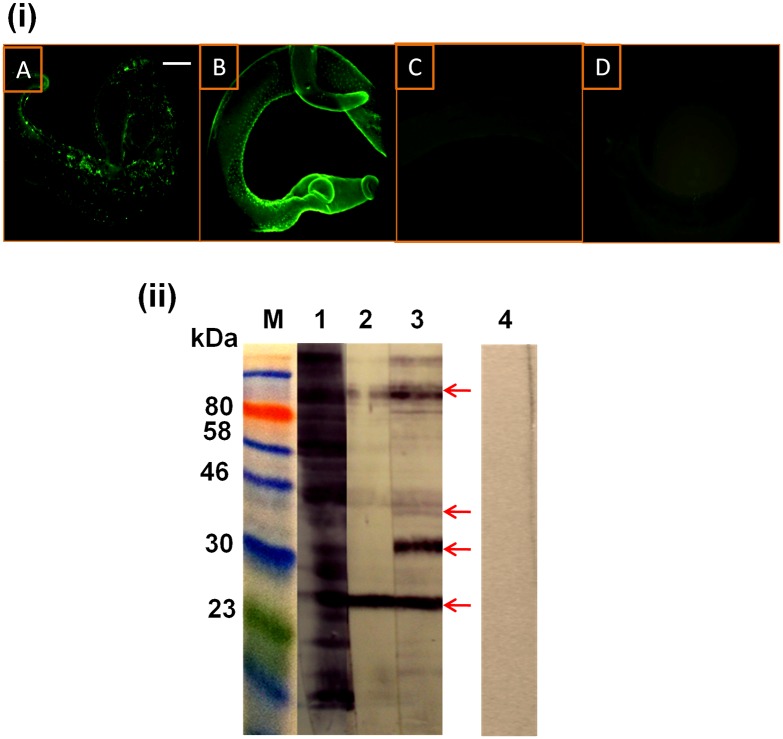
Fig 4. Detection by rabbit anti-SmWH antibodies of worm surface antigens exposed by miltefosine or PZQ.
(i) Immunofluorescent staining of PZQ- (6 μg/ml for 24 hours) (A and C) and miltefosine- (40 μg/ml for 48 hours) (B and D) treated S. mansoni adult worms detected with rabbit anti-SmWH IgG antibodies (A and B) and IgG antibodies from a rabbit that received adjuvant alone (C and D). Scale bar = 100 μm. (ii) Western immunoblots of S. mansoni crude worm homogenate preparation (SmWH) were probed with rabbit anti-SmWH antiserum (lane 1), rabbit anti-SmWH antibodies eluted from PZQ- (6 μg/ml for 24 hours) (lane 2) and miltefosine- (40 μg/ml for 48 hours) (lane 3) treated worms. A blot of SmWH probed with a serum from a rabbit injected with Freund’s adjuvant alone (lane 4) was used as a control. Lane M, protein molecular weight markers. Antigenic extract was loaded onto the gel with 10 μg protein/lane. Blots were detected using HRP-conjugated anti-rabbit IgG secondary antibodies. Detected antigens with the most intense reactivities are arrowed.
Proteins exposed by miltefosine and praziquantel on S. mansoni worm surface were characterized by western immunoblotting
Rabbit antibodies in the anti-SmI and the anti-SmWH sera were found to bind to the surface of worms treated earlier with 6 μg/ml PZQ for 24 hours or 40 μg/ml miltefosine for 48 hours (Figs 3iA and 3iB and 4iA and 4iB, respectively), thus confirming that treatment induced antigen exposure. No staining was detected on drug-treated worms incubated with control sera from naive and adjuvant-immunized rabbits (Figs 3iC and 3iD and 4iC and 4iD).
Surface-bound antibodies were recovered from the worm surface and used to probe western immunoblots of a crude worm extract (Figs 3 and 4, S2 and S3 Figs). The reactivity of the precursor anti-SmI antiserum with SmWH was predominantly against a ~ 33 kDa protein band (Fig 3, lane 1). Following PZQ treatment, two of these proteins (at ~23, 33 kDa) were also strongly reacted against by the anti-SmI antibodies, while antibody reactivities against the bands at ~37 and ~120–130 were of much lower intensities (Fig 3, lane 2). Our results showed that miltefosine treatment resulted in exposure of four main proteins having molecular sizes of approximately 23, 33, 37 and 120–130 kDa (the last was determined in a western immunblot transferred from an 8% gel [S4 Fig]). These protein bands were detected in blots of S. mansoni worm homogenate extract reacted against, respectively, by both the anti-SmI and the anti-SmWH antibodies that had been acid-eluted off the surface of treated worm (Figs 3 and 4, lane 3). As regards the anti-SmWH antibodies eluted after treated worms, the above four protein bands were detected following miltefosine treatment (Fig 4, lane 3), whereas in the case of PZQ, the ~33 kDa reactive band was not identified (Fig 4, lane 2). No antigenic reactivities were detected in the blots of the worm extract probed with sera from control normal/adjuvant-alone injected rabbits (Figs 3 and 4, lane 4).
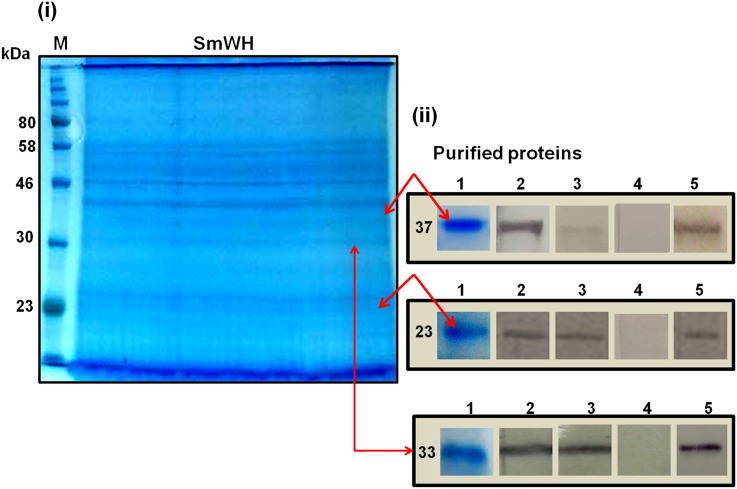
The protein bands at ~23 (pr23) and ~37 (pr37) kDa in the SmWH extract were purified by excision and elution from the gel films used for SDS-PAGE (Fig 5). The immunodominant band detected following treatment with miltefosine at ~33 kDa (pr37), and similarly recognised by anti-SmI on PZQ-treated parasites, was also purified from SDS-PAGE bearing the fractionated crude worm proteins. After these purification steps the three protein bands pr23, pr33 and pr37 were antigenically reactive in western blots with solutions of respective anti-SmI and anti-SmWH antibodies eluted from treated worms (Fig 5ii and S5–S7 Figs).
Fig 5. Purification and characterisation of the SmWH 23 kDa, 33 kDa and 37 kDa protein bands.
(i) Coomassie blue-stained reducing 12% SDS-PAGE gel of SmWH. Protein bands at ~23, 33 and 37 kDa (arrowed) were purified from by excision and elution from the gel. Lane M, protein molecular weight marker. (ii) Purified 37, 33 and 23 kDa protein bands in Coomassie blue-stained SDS-PAGE gel (1) and western immunoblots (2–5) probed with anti-SmI eluted antibodies from miltefosine- (2) and PZQ- (3) treated worms, a normal rabbit serum (4) and a rabbit anti-SmI antiserum (5). Samples were loaded onto the gel with 10 μg protein/lane.
MS/MS analysis of the detected protein bands pr33, pr23 and pr37
Purified protein bands at ~33 kDa, ~23 and 37 kDa from SmWH were subjected to MS/MS mass spectrometry. Mascot identification output of fragmented peptides in the reactive bands was searched against NCBInr database with the tandem MS data and found to best match, respectively S. mansoni fructose-bisphosphate aldolase (SmFBPA), S. mansoni tegument protein Sm22.6 and S. mansoni malate dehydrogenase (SmMLD) having significant Mascot scores of 1085 (S1 Table), 1262 (S2 Table) and 469 (S3 Table), respectively. The SmFBPA has a calculated mass of 39963 da (Uniprot Smp_042160, gi: 1703248. The Sm22.6 (pr23) has a calculated mass of 22563 da (UniProtKB P14202, gi: 135578,) and SmMLD (pr37) has a mass of 36305 da (UniProtKB G4V7Z7, gi: 353230847), thus matching the molecular masses of the reactivities detected in western immunoblots (Figs 3 and 4).
Identification of the protein band at ~120–130 kDa as S. mansoni alkaline phosphatase
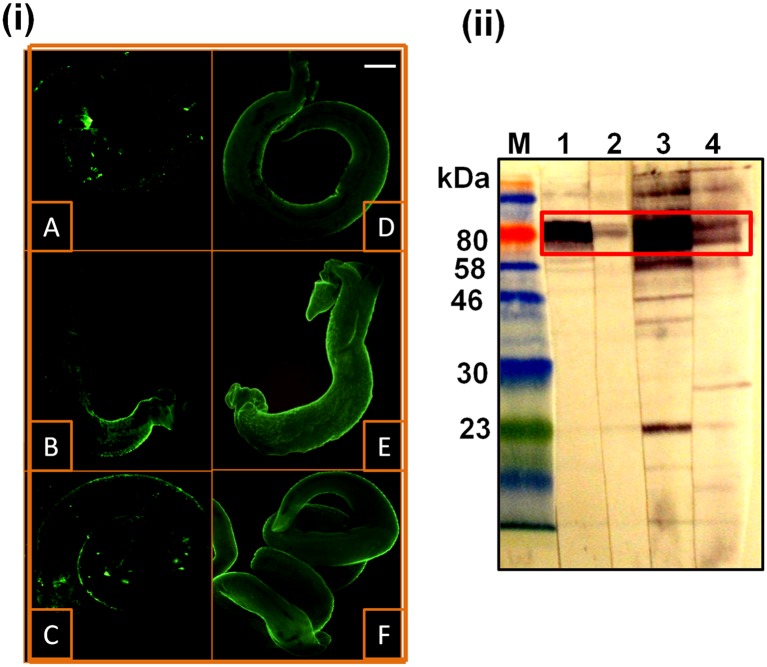
The possibility that the rabbit anti-S. mansoni antibodies eluted from treated worms could be recognising the S. mansoni alkaline phosphatase (SmAP) protein sequences in the ~120–130 kDa doublet band (a molecular size determined using 8% SDS-PAGE (S4 Fig), having a molecular mass similar to that described in the literature for a SmAP dimer of ~130 kDa [61]), was investigated. Worms treated with 40 μg/ml miltefosine for 48 hours or 6 μg/ml PZQ for 24 hours were incubated with a rabbit antiserum specific to SmAP followed by FITC-conjugated anti-IgG antibodies and examined under a fluorescence microscope. Both PZQ and miltefosine-treated worms showed IF staining of their surface, while only minimal fluorescence was detected on untreated parasites (Fig 6i). The intensity of staining was higher after miltefosine treatment than after PZQ. Anti-SmAP antibodies eluted from the surface of miltefosine-treated worms (after they were incubated with the rabbit anti-SmAP antiserum) were used to probe immunoblots of S. mansoni worm protein extracts. Reactivities at approximately 120–130 kDa were comparable, in terms of molecular mass and pattern, to those observed using the precursor antiserum (Fig 6ii) and with our earlier blots probed with anti-SmI and anti-SmWH elutions from treated worms (Figs 3 and 4). These results indicate that the protein exposed by miltefosine treatment at ~120–130 kDa is the SmAP.
Fig 6. Detection of S. mansoni alkaline phosphatase (SmAP) after miltefosine treatment by immunofluorescence (i) and western blotting (ii).
(i) Immunofluorescence staining of drug-treated S. mansoni adult worms that had been incubated with a rabbit anti-S. mansoni alkaline phosphatase (anti-SmAP) antiserum. A, untreated worms; B, 6 μg/ml PZQ; C, 8 μg/ml PZQ; D, 10 μg/ml miltefosine; E, 20 μg/ml miltefosine; F, 40 μg/ml miltefosine. Scale bar = 100 μm. (ii) Western immunoblots of a detergent-soluble extract of S. mansoni adult worms (lanes 1 and 2) and SmWH (lanes 3 and 4) probed with the rabbit anti-SmAP-antiserum (lanes 1 and 3) and anti-SmAP antibody eluted from miltefosine-treated worms (lanes 2 and 4); M, protein molecular weight markers. Antigenic extract was loaded onto the gel with 10 μg protein/lane. Detected SmAP is boxed.
Antibodies recovered from miltefosine-treated worms induced agglutination of schistosomula and detected the parasite surface by immunofluorescence
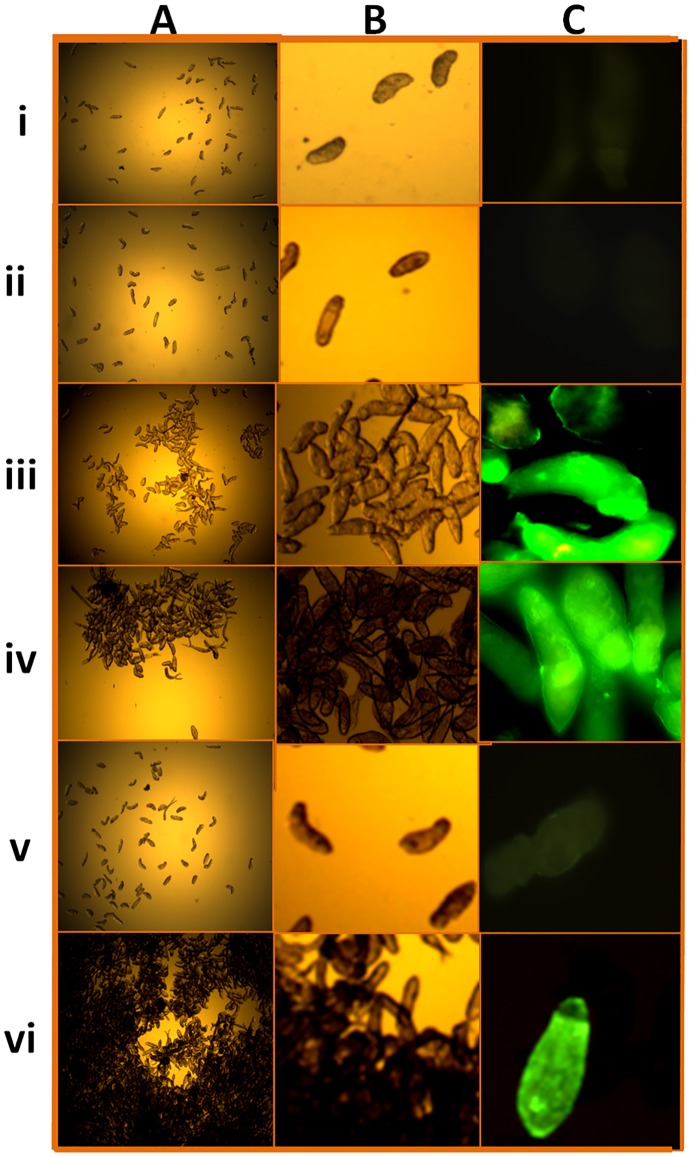
An indirect immunofluorescence assay was used to examine the antigenicity of the surfaces of intact 3-hour schistosomula. Parasites were incubated with rabbit anti-SmI and anti-SmWH antibodies recovered from miltefosine- and PZQ-treated adult worms, followed by FITC-conjugated anti-IgG Fc-specific secondary antibodies. The rabbit anti-SmI and anti-SmWH antibodies eluted from miltefosine-treated worms were observed to agglutinate schistosomula (without secondary antibody), as did anti-SmI antibodies recovered from PZQ-treated worms (Fig 7). This was most obviously seen with anti-SmWH antibodies from the miltefosine-treated worms (Fig 7vi). Anti-SmWH antibodies eluted from PZQ-treated worms, and sera from naive or adjuvant-alone immunized rabbits did not have this property (Fig 7i, 7ii and 7v). Schistosomula that had agglutinated were also positive in indirect immunofluorescence labelling of their surfaces (Fig 7C).
Fig 7. Agglutination and indirect immunofluorescence reactivity of 3-hour schistosomula with rabbit anti-S. mansoni antibodies specific for drug-exposed antigens.
Representative microscopic images after incubation with the primary antibody under white light (A and B) and after processing for indirect immunofluorescence following incubation with anti-rabbit IgG conjugated to FITC (secondary antibody) (C). Schistosomula probed with: serum from naive rabbits (i); serum from rabbits immunized with adjuvant-alone (ii); anti-SmI antibodies eluted from PZQ-treated worms (iii); anti-SmI antibodies eluted from miltefosine-treated worms (iv); anti-SmWH antibodies eluted from PZQ-treated worms (v); anti-SmWH antibodies eluted from miltefosine-treated worms (vi). Scale bars = 100 μm.
Discussion
Miltefosine, a hexa-phospho-choline, was previously shown to have anti-schistosomal properties and to induce extensive changes in the tegument of adult worms of Egyptian and Brazilian strains of S. mansoni in vitro and in vivo [16,17,19,20]. In the current study, we assessed the in vitro action of miltefosine on a Puerto Rican isolate of S. mansoni relative to PZQ. We particularly investigated the effect of the drug on adult worm teguments in terms of surface antigen exposure.
The present data showed that miltefosine is effective in vitro against adult worms of a Puerto Rican isolate of S. mansoni at concentrations of 10–40 μg/ml within 24–96 hours. These results are consistent with previous reports showing in vitro efficacies of miltefosine against other isolates of S. mansoni [16,19,20]. Our calculated LC50 value were 12.1 and 7.5 μg/ml miltefosine after, respectively 72 and 96 hours of incubation with drug, showing greater efficacy than those in previous studies by Eissa et al. [16] and Bertao et al. [19], who respectively reported LC50s of 5.8 μg/ml and 16 ± 0.2 μg/ml after 120 hours incubation of the Egyptian Sambon and the Brazilian BH strains. Miltefosine was conversely found to have lower effects on the S. mansoni strain ‘LE’ in a study by Yepes et al. [62] at the same doses we tested, though interestingly, the authors demonstrated a higher susceptibility to PZQ than that reported here. Given the reported difference in sensitivities of different strains of Leishmania and Schistosoma to miltefosine and PZQ, respectively [38,63,64], our results on the Puerto Rican strain of S. mansoni showing relatively different susceptibility to miltefosine than the Sambon, the BH and the LE strains are as expected. The possible differences in the parasite membrane properties, phospholipid composition or the drug(s) sensitive sites between different strains could be a plausible explanation [65,66]. Being effective against three schistosome strains (the Puerto Rican, the Egyptian Sambon and the Brazilian BH strains), this work further validates possible use of miltefosine as an anti-schistosomal against different S. mansoni strains. The knowledge of variation in miltefosine susceptibility of S. mansoni strains may help define drug schedules (dose/duration regimens) in future clinical trials with the drug and suggests that a laboratory pre-evaluation step is needed. Although the highest effective LC50 of the three studies that investigated miltefosine efficacy against different strains (16 μg/ml) may be the concentration to be aimed for, re-assessment of miltefosine efficacy in multiple clinical trials in various geographical areas of endemicity is needed. According to the results of the present study, it is expected that lower doses/shorter durations of treatment with miltefosine than previously reported [16,19] may be needed to treat patients with S. mansoni infection. We demonstrated that the schistosomicidal effects of miltefosine progressed slowly over 96 hours of incubation of the worms and were intensified using higher concentrations of the drug, consistent with earlier findings [16,19]. The efficacy of alkyl-phospholipids was reported earlier to depend on the drug’s contact time with the cell membrane, which could last for days [67]. PZQ, on the other hand, induced immediate muscular contraction and extensive tegumental disruption and 90%-100% parasite mortality at 6 μg/ml within 24 hours. The observed difference in time progression of drug-induced events can be accounted for by the disparate difference in the modes of action between the two drugs. On one hand, PZQ exerts its effect by inducing a rapid influx of calcium, an increase in membrane fluidity and interfering with uptake of glucose and ATP [68] resulting in worm death as soon as 3 hours [38], whereas miltefosine acts more slowly by weakly modifying lipid ordering in lipid rafts, interfering with membrane complex renewal and cell growth and survival pathways [19,22–24,69]. At doses of PZQ lower than 6 μg/ml (i.e., 2 and 4 μg/ml), no or minimal changes in worm teguments could be observed under the inverted microscope and only a little staining by IF; and at higher PZQ doses (8 and 10 μg/ml), the effects on the tegument were also seen to decrease. In accordance, Mendonca et al. [38] examined a range of PZQ doses in vitro and showed that the most extensive changes on the worm tegument occurred at 6.5 μg/ml PZQ. These findings can possibly be attributed to too low drug concentrations at 2 and 4 μg/ml (that were not enough to induce tegumental disruption) or a short time of contact with the worm tegument due to early induction of worm death at the 8–10 μg/ml levels. This early killing without significantly damaging the tegument could have resulted from the effect of PZQ on energy metabolic pathways essential to parasite survival [70] through interference with uptake of glucose and ATP or due to accumulation of lactate as a result of sustained muscle contraction [68].
In the current study, we used a rabbit anti-S. mansoni infection antiserum (anti-SmI) to detect protein targets exposed by miltefosine on the surface of treated worms. This serum was developed in a rabbit, a relatively non-permissive host to S. mansoni infection compared to the more permissive human or murine hosts [71,72]. Antibodies in the rabbit immune antiserum were previously reported to enhance PZQ activity in mice [44] and to partially protect the animals against a challenge S. mansoni infection when injected into them during the first week of infection [73]. Hence, the anti-SmI rabbit serum may be useful for detecting potential antigenic targets of antibodies that enhance worm mortality caused by the drug treatment or that protect against a new infection. These antigens may be potential candidates for development of a vaccine against S. mansoni infection. A host treated with miltefosine and that has developed specific antibodies to exposed adult worm tegumental proteins may be resistant to subsequent infection through targeting antigenic epitopes on young migrating schistosomula, leading to destruction or disablement of larval migration in the skin or lungs. This concept has been proposed for praziquantel following observations from field studies indicating that protective immunity against re-infection with schistosomiasis can be developed by cumulative exposure to antigens released from or that become exposed at the surface of worms following treatment with PZQ (post-treatment development of resistance) or of worms naturally-dying in untreated populations (age-dependent immunity) [35,74,75]. On the other hand, surface antigens would not be detectable on intact adult schistosome worms of a new infection in the absence of the drug; hence such mechanism of protection by rabbit antibodies is unlikely to occur at the adult worm stage.
In accordance with our inverted microscopy observations, strong immunofluorescence of the worm surface following treatment with miltefosine was recognised using the anti-SmI antiserum, while no fluorescent staining was detected on untreated worms using the same antiserum or on drug-treated worms incubated with a serum from naive rabbits. The staining was found to increase with higher concentrations of miltefosine and the fluorescence intensity was more than that observed on the PZQ-treated worms; for the latter, the brightest staining was induced by 6 μg/ml. Although schistosome adult worm proteins in the subtegument and deeper tissue might become exposed as a result of drug treatment, the epitopes that appear to be recognized utilising rabbit anti-schistosome antibodies are likely those on or very near the parasite surface as demonstrated by the surface labeling observed in the fluorescent microscopy studies. Miltefosine doses/durations of treatment found to produce adequate unmasking, as indicated by fluorescence staining intensity, were 40 ug/ml for 48 hours and 20ug/ml for 72 hours. These concentrations could be potentially achieved in vivo based on previous data on clinical pharmacokinetics of miltefosine against Leishmania infections [76], yet smaller doses for longer periods not tested in this work might achieve similar effects.
Rabbit antibodies eluted from miltefosine-treated worms recognised two dominant proteins at ~33 and 23, as did the antibodies recovered from PZQ-treated parasites, yet the reactivities of the latter were of relatively lower intensities. Two additional bands at ~37 and ~120–130 kDa were detectable by the anti-SmI antibodies detached from miltefosine-treated worms, but hardly seen with the antibodies from PZQ-treated worms. Miltefosine thus seems to expose more antigens/epitopes when compared to PZQ.
The immunodominant reactivity at ~33 kDa exposed by treatment with miltefosine was analysed by MS/MS and was found to have peptide sequences that gave 77% sequence coverage of S. mansoni fructose bisphosphate aldolase. SmFBPA belongs to the class I FBPA family with a predicted molecular size of 39.6 kDa (Uniprot Smp_042160). The enzyme has 65–66% similar amino-acid sequence to human class I C and A isoenzymes [77]. Class I aldolases are characterised by having a (β/α) 8-barrel domain [78], a structure that was reported to be difficult to be completely disrupted by detergents and even high temperature [79]. These properties have been claimed to result in anomalously fast protein migration in SDS-PAGE gels [80]. De Montigny and colleagues [81] detected an aldolase subunit from the eubacteria Thermus aquaticus with a mass of 34 kDa on reducing SDS-PAGE, a size lower than the theoretical 41 kDa (homotetramer mass estimated to be 165 kDa). The other possibility is that the protein detected here at ~33 kDa might correspond to a lower molecular-sized SmFBPA isoform. Class I aldolases in eukaryotes have been reported to differ in molecular mass [82], with aldolase I B subunits (~70% homologous to both aldolases I A and C in amino-acid sequence) having a smaller molecular size of 36 kDa [83], a size near to the SmFBPA-band detected in the present work. Two different isoforms of Schistosoma haematobium adult worm FBPA were recently characterised and reported to have disparate immunogenic properties [84]. The situation in S. haematobium may be similar in S. mansoni; i.e., there may be two different SmFBPA isoforms possessing different immunogenic properties. Further investigations to characterise the relationship between SmFBPA p33 and the previously described SmFBPA p42 [77] are needed. SmFBPA is a tegument protein expressed in different schistosome life cycle stages and plays a central role in glycolysis and generation of the energy needed by the parasite for its survival [77,85]. The enzyme was shown to be recognised by antibodies specific to the lung stage schistosomulum antigens released in culture medium and antibodies from rabbits vaccinated with irradiated cercariae [86,87]. Recombinant- and DNA-vaccines based on SmFBPA induced 41.7–57% protection against S. mansoni challenge infections and significant reductions in the number of hepatic granulomas in mice [84,87].
IgG antibodies in the anti-SmWH antiserum eluted from PZQ-treated worms showed a different antigenic pattern when compared to all other elutions, in so far as the SmFBPA-containing band at ~33 kDa was not evidenced in the blots, although treatment with PZQ might be predicted to expose the same antigenic epitopes as those recognized by eluted anti-SmI antibodies. These findings may imply that miltefosine acts to unmask epitopes on SmFBPA protein that are different from those exposed by PZQ. The epitopes unmasked by the first were bound by specific IgG antibodies in the anti-SmWH serum following miltefosine treatment. The anti-SmWH antiserum, unlike the anti-SmI, failed previously to enhance the efficiency of PZQ in mice [44]. Since the anti-SmWH antibodies eluted off PZQ-treated worms did not recognise the SmFBPA pr33, our present findings suggest this antigen may have an important role in mediating the PZQ/antibody synergistic effect.
Our MS/MS analysis of the 23 kDa band showed the presence of peptide sequences for the S. mansoni tegument protein Sm22.6, a cryptic allergen like surface membrane protein (TAL1) with unknown function, although it was thought to inhibit human thrombin [88]. Sm22.6 is a major target of specific IgE antibody in infected and PZQ-treated individuals [89,90]. This antibody response was implicated in resistance to re-infection [89,91,92]. The protein may have an important role in entry into the host vasculature since it becomes critically up-regulated in schistosomula [93]. Recombinant Sm22.6 was shown to induce specific anti-Sm22.6 IgG antibodies in mice and to partly protect the animals against infection [94].
The high molecular weight protein band detected at ~120–130 kDa had a molecular mass similar to that of the dimeric form of S. mansoni alkaline phosphatase (SmAP) of ~130 kDa [61]. To investigate whether SmAP was exposed by miltefosine treatment, we used a rabbit anti-SmAP-specific antiserum to probe the surface of S. mansoni worms that had been treated with the drug. Since anti-SmAP antibody was available, providing an opportunity to specifically detect SmAP without the need for mass spectrometry, the antibody was used to identify SmAP. Immunofluorescence analysis confirmed the protein exposure by inducing intense, bright staining of the surface of miltefosine-treated worms compared to the untreated controls. The differences in labeling between untreated control worms treated with the anti-SmAP and the polyspecific anti-infection or anti-worm homogenate sera could be related to different anti-SmAP antibody concentrations and/or epitope specificities between the last two sera and the anti-SmAP antiserum.
Anti-SmAP antibodies eluted from these worms reacted with a protein band of molecular mass similar to that detected by the anti-SmI and anti-SmWH antibody elutions from miltefosine-treated worms. SmAP is a marker for the schistosome adult worm tegument, where its exposure was found to be enhanced by PZQ [95–99], but also present in internal tissues of the parasite [98]. The enzyme was shown to be involved in host invasion, nutrient uptake and immune evasion of host immune responses by generating anti-inflammatory and immunosuppressant molecules such as adenosine [98,100].
MS/MS analysis of the ~37 kDa protein, the fourth band detected following miltefosine treatment, identified the protein as S. mansoni malate dehydrogenase, SmMDH. SmMDH is an important enzyme involved in anaerobic carbohydrate metabolism through catalysing reduction of oxaloacetate to malate, the main pathway of energy production for the Schistosoma parasite [101]. The enzyme is expressed in schistosome adult worms, cercariae and eggs stages [102].
Another three proteins: a tubercle glycoprotein Sm200 [27], an unidentified 27 kDa protein with an esterase activity [44] and a 43 kDa actin [103] were previously reported to be exposed on S. mansoni adult worms by PZQ. In the present work, reactive protein bands at similar molecular sizes to the above, which were not selected for MS/MS analysis due to their low intensity relative to the 4 studied bands, were also detected in our western blots by antibodies eluted from miltefosine-treated worms. These reactivities were not detected by rabbit antibodies recovered from PZQ-treated parasites, possibly due to different epitopes being exposed by the two drugs.
The presence/accessibility of the drug-exposed epitopes on the surface of 3-hour schistosomula was assessed by an indirect IF assay. Schistosomula were heavily agglutinated following incubation with the rabbit anti-SmI antibodies recovered from worms exposed to PZQ and miltefosine. Schistosomulum-agglutination was most extensive with anti-SmWH antibodies eluted from miltefosine-treated parasites. These results indicate that the epitopes exposed by the drugs on adult worms are also expressed on early schistosomula. This agglutination is supposed to induce parasite immobilization preventing its migration and leading to parasite killing in vivo by antibody-dependent cell-mediated cytotoxicity (ADCC) [104–107]. The key feature of radiation-attenuated cercarial vaccines (RA-vaccine) may be the parasite’s slow migration and its subsequent attack by the host immune system in the lung [108]. No agglutination or fluorescence was observed with sera from naive rabbits or animals immunized with adjuvant alone or from the anti-SmWH antibodies recovered from PZQ-treated worms. The latter non-agglutinating antibodies showed reactivities against Sm22.6 and SmAP but not against the SmFBPA pr33 in western blots. The low levels of expression of the first two proteins on 3-hour schistosomula could be an explanation [102,109].
In conclusion, our results suggest that the S. mansoni proteins FBPA, Sm22.6, alkaline phosphatase, malate dehydrogenase are exposed by miltefosine and PZQ treatment on the surface of adult worms, the degree of exposure of the last two being greater with miltefosine. Future experiments may seek to investigate the combined effect of both drugs on exposure of schistosome worm surface proteins. These antigens could be possibly implicated in a proposed miltefosine-mediated immune-dependent action in vivo as specific antibody response against miltefosine-exposed antigens could enhance the drug activity. Results of experiments with sera from S. mansoni infected humans to investigate whether anti-schistosome antibodies would react similarly to the rabbit antibodies would be instructive. Miltefosine doses and durations of treatment found to produce adequate unmasking of tegumental proteins, as indicated by relative intensity of fluorescence staining in this study, were 40 ug/ml for 48 hours and 20ug/ml for 72 hours. These concentrations could potentially be achieved in vivo based on previous data on clinical pharmacokinetics of miltefosine against Leishmania infections [76], though exposure to smaller doses for longer periods (not tested in the present work) may achieve in vivo the effects observed in our in vitro study. Our data predict that SmFBPA pr33 may have a dominant role in drug-mediated immune-dependent effect and could be a principal antigen on the surface of 3-hour shistosomula and thus a target for immunological intervention. Further experiments are ongoing to evaluate the effect of antibody-miltefosine interaction in vivo and the individual roles of the recognised antigens in miltefosine-mediated worm destruction.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(PPTX)
(PPTX)
(PPTX)
(PPTX)
(PPTX)
(PPTX)
(PPTX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Murray CJL, Vos T, Lozano R. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012; 384: 582–582. [DOI] [PubMed] [Google Scholar]
- 2.Doenhoff MJ, Hagan P, Cioli D, Southgate V, Pica-Mattoccia L, et al. Praziquantel: its use in control of schistosomiasis in sub-Saharan Africa and current research needs. Parasitology. 2009; 136: 1825–1835. doi: 10.1017/S0031182009000493 [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Wang L, Liang Y.S. Susceptibility or resistance of praziquantel in human schistosomiasis: a review. Parasitology Research. 2012; 111: 1871–1877. doi: 10.1007/s00436-012-3151-z [DOI] [PubMed] [Google Scholar]
- 4.Sabah AA, Fletcher C, Webbe G, Doenhoff M.J. Schistosoma mansoni: The effect of chemotherapy on infections of different ages. Experimental Parasitolology. 1986; 61: 294–303. [DOI] [PubMed] [Google Scholar]
- 5.Bergquist R, Utzinger J, McManus DP. Trick or Treat: The Role of Vaccines in Integrated Schistosomiasis Control. PLOS Neglected Tropical Diseases. 2008; 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagan P, Appleton CC, Coles GC, Kusel JR, Tchuem-Tchuente LA. Schistosomiasis control: keep taking the tablets. Trends in Parasitology. 2004; 20: 92–97. [DOI] [PubMed] [Google Scholar]
- 7.Panic G, Duthaler U, Speich B, Keiser J Repurposing drugs for the treatment and control of helminth infections. International Journal for Parasitology-Drugs and Drug Resistance. 2014; 4: 185–200. doi: 10.1016/j.ijpddr.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Persaud-Sharma V, Zhou S.F. Drug Repositioning: A Faster Path to Drug Discovery. Advances in Pharmacoepidemiology & Drug Safety. 2012; 1: e117. [Google Scholar]
- 9.Hilgard P, Klenner T, Stekar J, Unger C. Alkylphosphocholines—a new class of membrane-active anticancer agents. Cancer Chemotherapy and Pharmacology. 1993; 32: 90–95. [DOI] [PubMed] [Google Scholar]
- 10.Unger C, Damenz W, Fleer EAM, Kim DJ, Breiser A, et al. Hexadecylphosphocholine, a new ether lipid analog—studies on the antineoplastic activity in vitro and in vivo. Acta Oncologica. 1989; 28: 213–217. [DOI] [PubMed] [Google Scholar]
- 11.Escobar P, Matu S, Marques C, Croft SL. Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH3 (edelfosine) and amphotericin B. Acta Tropica. 2002; 81: 151–157. [DOI] [PubMed] [Google Scholar]
- 12.Ganguly NK. Oral miltefosine may revolutionize treatment of visceral leishmaniasis. a. WHO TDR news. 2002. www.who.int/entity/tdr/publications/documents/tdrnews-issue-68.pdf?ua=1. Cited 14 March 2017.
- 13.Dorlo TPC, Balasegaram M, Beijnen JH, de Vries PJ Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. Journal of Antimicrobial Chemotherapy. 2012; 67: 2576–2597. doi: 10.1093/jac/dks275 [DOI] [PubMed] [Google Scholar]
- 14.Sundar S, Jha TK, Thakur CP, Bhattacharya SK, Rai M. Oral miltefosine for the treatment of Indian visceral leishmaniasis. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2006; 100: S26–S33. doi: 10.1016/j.trstmh.2006.02.011 [DOI] [PubMed] [Google Scholar]
- 15.U.S. FDA. FDA approves Impavido to treat tropical disease leishmaniasis. FDA News Release. 2014. https://newdrugapprovals.org/2014/03/20/the-u-s-fda-approved-impavido-miltefosine-to-treat-a-tropical-disease-called-leishmaniasis/. Cited 14 March 2017.
- 16.Eissa MM, El Bardicy S, Tadros M. Bioactivity of miltefosine against aquatic stages of Schistosoma mansoni, Schistosoma haematobium and their snail hosts, supported by scanning electron microscopy. Parasites & Vectors. 2011; 4: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eissa MM, El-Azzouni MZ, Amer EI, Baddour NM. Miltefosine, a promising novel agent for schistosomiasis mansoni. International Journal for Parasitology. 2011; 41: 235–242. doi: 10.1016/j.ijpara.2010.09.010 [DOI] [PubMed] [Google Scholar]
- 18.Eissa MM, El-Moslemany RM, Ramadan AA, Amer EI, El-Azzouni MZ, et al. Miltefosine lipid nanocapsules for single dose oral treatment of schistosomiasis mansoni: A Preclinical Study. PLOS One. 2015; 10: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertao HG, da Silva R.A.R., Radis-Baptista G., Albuquerque M.C.P.D. Miltefosine, an alkylphosohocholine originally developed as an antitumoral, is an effective compound against Schistosoma mansoni. International Journal of Pharma Medicine and Biological Sciences. 2012; 1: 2278–5221. [Google Scholar]
- 20.Bertao HG, da Silva RAR, Padilha RJR, Albuquerque M, Radis-Baptista G. Ultrastructural analysis of miltefosine-induced surface membrane damage in adult Schistosoma mansoni BH strain worms. Parasitology Research. 2012; 110: 2465–2473. doi: 10.1007/s00436-011-2786-5 [DOI] [PubMed] [Google Scholar]
- 21.El-Moslemany RM, Eissa MM, Ramadan AA, El-Khordagui LK, El-Azzouni MZ. Miltefosine lipid nanocapsules: Intersection of drug repurposing and nanotechnology for single dose oral treatment of pre-patent schistosomiasis mansoni. Acta Tropica. 2016; 159: 142–148. doi: 10.1016/j.actatropica.2016.03.038 [DOI] [PubMed] [Google Scholar]
- 22.Pachioni JD, Magalhaes JG, Lima EJC, Bueno LD, Barbosa JF, et al. Alkylphospholipids—A Promising Class of Chemotherapeutic Agents with a Broad Pharmacological Spectrum. Journal of Pharmacy & Pharmaceutical Sciences. 2013; 16: 742–759. [DOI] [PubMed] [Google Scholar]
- 23.van Blitterswijk WJ, Verheij M. Anticancer alkylphospholipids: Mechanisms of action, cellular sensitivity and resistance, and clinical prospects. Current Pharmaceutical Design. 2008; 14: 2061–2074. [DOI] [PubMed] [Google Scholar]
- 24.van der Luit AH, Buddle M., Ruurs P., Verheij M., van Blitterswijk W.J. Alkyl-lysophospholipid accumumlates in lipid rafts and induces apoptosis via raft-dependent endocytosis and inhibition of phosphatidylcholine synthesis. The Journal of Biological Chemistry. 2002; 277: 39541–39547. doi: 10.1074/jbc.M203176200 [DOI] [PubMed] [Google Scholar]
- 25.Wieder T, Reutter W, Orfanos CE, Geilen CC. Mechanisms of action of phospholipid analogs as anticancer compounds. Progress in Lipid Research. 1999; 38: 249–259. [DOI] [PubMed] [Google Scholar]
- 26.El Ridi R, Tallima H. Equilibrium in lung schistosomula sphingomyelin breakdown and biosynthesis allows very small molecules, but not antibody, to access proteins at the host-parasite interface. Journal of Parasitology. 2006; 92: 730–737. doi: 10.1645/GE-745R1.1 [DOI] [PubMed] [Google Scholar]
- 27.Brindley PJ, Strand M, Norden AP, Sher A. Role of host antibody in the chemotherapeutic action of praziquantel against Schistosoma mansoni—identification of target antigens. Molecular and Biochemical Parasitology. 1989; 34: 99–108. [DOI] [PubMed] [Google Scholar]
- 28.Doenhoff MJ. The immune-dependence of chemotherapy in experimental schistosomiasis. Memorias Do Instituto Oswaldo Cruz. 1989; 84: 31–37. [DOI] [PubMed] [Google Scholar]
- 29.Doenhoff MJ, Bain J. Immune-dependence of schistosomicidal chemotherapy—relative lack of efficacy of an antimonial in Schistosoma-mansoni-infected mice deprived of their T-cells and demonstration of drug-antiserum synergy. Clinical and Experimental Immunology. 1978; 33: 232–238. [PMC free article] [PubMed] [Google Scholar]
- 30.Doenhoff MJ, Modha J, Lambertucci JR, McLaren DJ. The immune dependence of chemotherapy. Parasitology Today. 1991; 7: 16–18. [DOI] [PubMed] [Google Scholar]
- 31.Doenhoff MJ, Sabah AAA, Fletcher C, Webbe G, Bain J. Evidence for an immune-dependent action of praziquantel on Schistosoma-mansoni in mice. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1987; 81: 947–951. [DOI] [PubMed] [Google Scholar]
- 32.Reimers N, Homann A, Hoschler B, Langhans K, Wilson RA, et al. Drug-Induced Exposure of Schistosoma mansoni Antigens SmCD59a and SmKK7. PLOS Neglected Tropical Diseases. 2015; 9: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabah AA, Fletcher C, Webbe G, Doenhoff MJ. Schistosoma-mansoni—reduced efficacy of chemotherapy in infected T-cell-deprived MICE. Experimental Parasitology. 1985; 60: 348–354. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro F, Mello R.T., Tavares C.A., Kusel J.R., Coelho P.M. Synergistic action of praziquantel and host specific immune response against Schistosoma mansoni at different phases of infection. The Revista do Instituto de Medicina Tropical de Sao Paulo. 2004; 46: 231–233. [DOI] [PubMed] [Google Scholar]
- 35.Black CL, Muok EMO, Mwinzi PNM, Carter JM, Karanja DMS, et al. Increases in levels of schistosome-specific immunoglobulin E and CD23(+) B cells in a cohort of Kenyan children undergoing repeated treatment and reinfection with Schistosoma mansoni. Journal of Infectious Diseases. 2010; 202: 399–405. doi: 10.1086/653828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fitzsimmons CM, Joseph S, Jones FM, Reimert CM, Hoffmann KF, et al. Chemotherapy for schistosomiasis in Ugandan fishermen: Treatment can cause a rapid increase in interleukin-5 levels in plasma but decreased levels of eosinophilia and worm-specific immunoglobulin E. Infection and Immunity. 2004; 72: 4023–4030. doi: 10.1128/IAI.72.7.4023-4030.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joseph S, Jones FM, Walter K, Fulford AJ, Kimani G, et al. Increases in human T helper 2 cytokine responses to Schistosoma mansoni worm and worm-tegument antigens are induced by treatment with praziquantel. Journal of Infectious Diseases. 2004; 190: 835–842. doi: 10.1086/422604 [DOI] [PubMed] [Google Scholar]
- 38.Mendonca AMB, Feitosa APS, Veras DL, Matos-Rocha TJ, Cavalcanti MGD, et al. The susceptibility of recent isolates of Schistosoma mansoni to praziquantel. Revista Do Instituto De Medicina Tropical De Sao Paulo. 2016; 58: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DE LA Torre Paloma, T. S., Torrado S. Preparation, Dissolution and Characterization of Praziquantel Solid Dispersions. Chemical and Pharmaceutical Bulletin. 1999; 47: 1629–1633. [Google Scholar]
- 40.Taylor MG, Amin M.B.A., Nelson G.S. 'Parthenogenesis' in Schistosoma mattheei. Journal of Helminthol. 1969; 43: 197–206. [DOI] [PubMed] [Google Scholar]
- 41.Colley DG, Wikel SK. Simplified method for the production of schistosomules. Experimental Parasitololgy. 1974; 35:44–51. [DOI] [PubMed] [Google Scholar]
- 42.Smithers SR, Terry R.J. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology Research. 1965; 55: 695–700. [DOI] [PubMed] [Google Scholar]
- 43.Doenhoff M, Long E. Factors affecting the acquisition of resistance against Schistosoma-mansoni in the mouse .4. Inability of T-cell-deprived mice to resist re-infection, and other in vivo studies on the mechanisms of resistance. Parasitology. 1979; 78: 171–183. [DOI] [PubMed] [Google Scholar]
- 44.Doenhoff MJ, Modha J, Lambertucci JR. Anti-schistosome chemotherapy enhanced by antibodies specific for a parasite esterase. Immunology. 1988; 65: 507–510. [PMC free article] [PubMed] [Google Scholar]
- 45.Dunne DW, Agnew AM, Modha J, Doenhoff MJ. Schistosoma-mansoni egg antigens—preparation of rabbit antisera with monospecific immunoprecipitating activity, and their use in antigen characterization. Parasite Immunology. 1986; 8: 575–586. [DOI] [PubMed] [Google Scholar]
- 46.Goudie RB, Horne CHW, Wilkinson PC. A simple method for producing antibody specific to a single selected diffusible antigen. Lancet. 1966; 2: 1224 [DOI] [PubMed] [Google Scholar]
- 47.Hendriksen CFM. Laboratory animals and immunization procedures: challenges and opportunities. Institute for Laboratory Animal Research Journal. 2005; 46: 227–229. [DOI] [PubMed] [Google Scholar]
- 48.Mossallam SF, Amer EI, El-Faham MH. Efficacy of Synriam (TM), a new antimalarial combination of OZ277 and piperaquine, against different developmental stages of Schistosoma mansoni. Acta Tropica. 2014; 143: 36–46. doi: 10.1016/j.actatropica.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 49.Ramirez B, Bickle Q, Yousif F, Fakorede F, Mouries MA. et al. Schistosomes: challenges in compound screening. Expert Opinion on Drug Discovery. 2007; 2: S53–S61. doi: 10.1517/17460441.2.S1.S53 [DOI] [PubMed] [Google Scholar]
- 50.de Moraes J, Carvalho AAL, Nakano E, de Almeida AAC, Marques THD, et al. Anthelmintic activity of carvacryl acetate against Schistosoma mansoni. Parasitology Research. 2013; 112: 603–610. doi: 10.1007/s00436-012-3172-7 [DOI] [PubMed] [Google Scholar]
- 51.Melo TT, Sena I.C., Araujo N, Fonseca CT. Antibodies are involved in the protective immunity induced in mice by Schistosoma mansoni schistosomula tegument (Smteg) immunization. Parasite Immunology. 2014; 36: 107–111. [DOI] [PubMed] [Google Scholar]
- 52.Doenhoff MJ, El-Faham M, Liddell S, Fuller HR, Stanley RG, et al. Cross-Reactivity between Schistosoma mansoni Antigens and the Latex Allergen Hev b 7: Putative Implication of Cross-Reactive Carbohydrate Determinants (CCDs). PLOS One. 2016; 11: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970; 277: 680–685. [DOI] [PubMed] [Google Scholar]
- 54.Studier FW. Analysis of bacteriophage t7 early RNAs and proteins on slab gels. Journal of Molecular Biology. 1973; 79: 237 [DOI] [PubMed] [Google Scholar]
- 55.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets—procedure and some applications. Proceedings of the National Academy of Sciences of the United States of America. 1979; 76: 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beyer NH, Schou C, Houen G, Heegaard NHH. Extraction and identification of electroimmunoprecipitated proteins from agarose gels. Journal of Immunological Methods. 2008; 330: 24–33. doi: 10.1016/j.jim.2007.10.016 [DOI] [PubMed] [Google Scholar]
- 57.Igetei JE, Liddell S, El-Faham M, Doenhoff MJ. Purification of a chymotrypsin-like enzyme present on adult Schistosoma mansoni worms from infected mice and its characterization as a host carboxylesterase. Parasitology. 2016; 143: 646–657. doi: 10.1017/S0031182016000184 [DOI] [PubMed] [Google Scholar]
- 58.Steen H, Mann M. The ABC's (and XYZ's) of peptide sequencing. Nature Reviews Molecular Cell Biology. 2004; 5: 699–711. doi: 10.1038/nrm1468 [DOI] [PubMed] [Google Scholar]
- 59.Perkins DN, Pappin DJC, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999; 20: 3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2 [DOI] [PubMed] [Google Scholar]
- 60.de Moraes J, Nascimento C, Lopes P, Nakano E, Yamaguchi LF, et al. Schistosoma mansoni: In vitro schistosomicidal activity of piplartine. Experimental Parasitology. 2011; 127: 357–364. doi: 10.1016/j.exppara.2010.08.021 [DOI] [PubMed] [Google Scholar]
- 61.Pujol FH, Cesari IM. Antigenicity of adult Schistosoma-mansoni alkaline-phosphatase. Parasite Immunology. 1990; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 62.Yepes E, Varela-M RE, Lopez-Aban J, Dakir ELH, Mollinedo F, et al. In vitro and in vivo Anti-schistosomal activity of the Alkylphospholipid analog edelfosine. PLOS One. 2014; 9: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coelho AC. Miltefosine susceptibility and resistance in Leishmania: from the laboratory to the field. Journal of Tropical Diseases & Public Health. 2016; 4: 203. [Google Scholar]
- 64.Fernandez OL, Diaz-Toro Y, Ovalle C, Valderrama L, Muvdi S, et al. Miltefosine and antimonial drug susceptibility of Leishmania viannia species and populations in regions of high transmission in Colombia. PLOS Neglected Tropical Diseases. 2014; 8: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Redman CA, Robertson A, Fallon PG, Modha J, Kusel JR, et al. Praziquantel: An urgent and exciting challenge. Parasitology Today. 1996; 12: 14–20. [DOI] [PubMed] [Google Scholar]
- 66.Rumjanek FD. Biochemistry and Physiology in the biology of schistosomes, from Genes to latrines. Rollinson DASA, editor: Academic Press; 1987. [Google Scholar]
- 67.Engelmann J, Henke J, Willker W, Kutscher B, Nossner G, et al. Early stage monitoring of miltefosine induced apoptosis in KB cells by multinuclear NMR spectroscopy. Anticancer Research. 1996; 16: 1429–1439. [PubMed] [Google Scholar]
- 68.Andrews P. Praziquantel: mechanisms of anti-schistosomal activity. Pharmacology & Theraputics. 1985; 29: 129–156. [DOI] [PubMed] [Google Scholar]
- 69.Vial HJ, Torpier G, Ancelin ML, Capron A. Renewal of the membrane complex of Schistosoma-mansoni is closely associated with lipid-metabolism. Molecular and Biochemical Parasitology. 1985; 17: 203–218. [DOI] [PubMed] [Google Scholar]
- 70.Shapiro TA, Talalay P. Schistosoma mansoni: mechanisms in regulation of glycolysis. Experimental Parasitology. 1982; 54: 379–390. [DOI] [PubMed] [Google Scholar]
- 71.Cheever AW, Lenzi JA, Lenzi HL, Andrade ZA. Experimental models of Schistosoma mansoni infection. Memorias Do Instituto Oswaldo Cruz. 2002; 97: 917–940. [DOI] [PubMed] [Google Scholar]
- 72.Cioli D, Knopf PM, Senft AW. Study of Schistosoma-mansoni transferred into permissive and nonpermissive hosts. International Journal for Parasitology. 1977; 7: 293–297. [DOI] [PubMed] [Google Scholar]
- 73.Fallon PG, Fookes RE, Doenhoff MJ. Protection of mice against Schistosoma mansoni infection by passive transfer of sera from infected rabbits. Parasite Immunology. 2003; 18: 7–14. [DOI] [PubMed] [Google Scholar]
- 74.Gazzinelli G, Viana IR, Bahia-Oliveira LM, Silveira AM, Queiroz CC, et al. Immunological profiles of patients from endemic areas infected with Schistosoma mansoni. Memorias Do Instituto Oswaldo Cruz. 1992; 87: 139–142. [DOI] [PubMed] [Google Scholar]
- 75.Correa-Oliveira R, Caldas IR, Gazzinelli G. Natural versus drug-induced resistance in Schistosoma mansoni infection. Parasitology Today. 2000; 16: 397–399. [DOI] [PubMed] [Google Scholar]
- 76.Dorlo TPC; van Thiel PPAM; Huitema ADR, et al. Pharmacokinetics of miltefosine in Old World cutaneous leishmaniasis patients. Antimicrobial Agents Chemotherapy. 2008; 52: 2855–1860. doi: 10.1128/AAC.00014-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.El-Dabaa E, Mel H, El-Sayed A, Karim AM, Eldesoky HM, et al. Cloning and characterization of Schistosoma mansoni fructose-1,6-bisphosphate aldolase isoenzyme. Journal of Parasitology. 1998; 84: 954–960. [PubMed] [Google Scholar]
- 78.Goldman AD, Beatty JT, Landweber LF. The TIM barrel architecture facilitated the early evolution of protein-mediated metabolism. Journal of Molecular Evolution. 2016; 82: 17–26. doi: 10.1007/s00239-015-9722-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Babu MM. TIM Barrel Analysis. Center for Biotechnology, Anna University. http://www.mrc-lmb.cam.ac.uk/genomes/madanm/articles/timanal.htm. Cited 14 March 2017.
- 80.Rath A, Glibowicka M, Nadeau VG, Chen G, Deber CM. Detergent binding explains anomalous SDS-PAGE migration of membrane proteins. Proceedings of the National Academy of Sciences of the United States of America. 2009; 106: 1760–1765. doi: 10.1073/pnas.0813167106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DeMontigny C, Sygusch J. Functional characterization of an extreme thermophilic class II fructose-1,6-bisphosphate aldolase. European Journal of Biochemistry, 1996; 241: 243–248. [DOI] [PubMed] [Google Scholar]
- 82.Rutter WJ. Evolution of aldolase. Federation Proceedings. 1964; 23: 1248 [PubMed] [Google Scholar]
- 83.Rottmann WH, Tolan DR, Penhoet EE. Complete amino-acid-sequence for human aldolase-B derived from cDNA and genomic clones. Proceedings of the National Academy of Sciences of the United States of America. 1984; 81: 2738–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mutapi F, Bourke C, Harcus Y, Midzi N, Mduluza T, et al. Differential recognition patterns of Schistosoma haematobium adult worm antigens by the human antibodies IgA, IgE, IgG1 and IgG4. Parasite Immunology. 2011; 33: 181–192. doi: 10.1111/j.1365-3024.2010.01270.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rumjanek F.D., Biochemistry and Physiology in the biology of schistosomes, from Genes to latrines. Rollinson D, Simpson AJ eds. London: Academic Press; 1987. pp 163–183 [Google Scholar]
- 86.Harrop R, Coulson PS, Wilson RA. Characterization, cloning and immunogenicity of antigens released by lung-stage larvae of Schistosoma mansoni. Parasitology. 1999; 118: 583–594. [DOI] [PubMed] [Google Scholar]
- 87.Marques HH, Zouain CS, Torres CBB, Oliveira JS, Alves JB, et al. Protective effect and granuloma down-modulation promoted by RP44 antigen a fructose 1,6 bisphosphate aldolase of Schistosoma mansoni. Immunobiology. 2008; 213: 437–446. doi: 10.1016/j.imbio.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 88.Lin YL, He S. Sm22.6 antigen is an inhibitor of human thrombin. Molecular Biochemical Parasitololgy. 2006; 147: 95–100. [DOI] [PubMed] [Google Scholar]
- 89.Dunne DW, Butterworth AE, Fulford AJC, Kariuki HC, Langley JG, et al. Immunity after treatment of human schistosomiasis—association between IgE antibodies to adult worm antigens and resistance to reinfection. European Journal of Immunology. 1992; 22: 1483–1494. doi: 10.1002/eji.1830220622 [DOI] [PubMed] [Google Scholar]
- 90.Fitzsimmons CM, Stewart TJ, Hoffmann KF, Grogan JL, Yazdanbakhsh M, et al. Human IgE response to the Schistosoma haematobium 22.6 kDa antigen. Parasite Immunology. 2004; 26: 371–376. doi: 10.1111/j.0141-9838.2004.00721.x [DOI] [PubMed] [Google Scholar]
- 91.Dunne DW, Webster M, Smith P, Langley JG, Richardson BA, et al. The isolation of a 22 kDa band after SDS-PAGE of Schistosoma mansoni adult worms and its use to demonstrate that IgE responses against the antigen(s) it contains are associated with human resistance to reinfection. Parasite Immunology. 1997; 19: 79–89. [DOI] [PubMed] [Google Scholar]
- 92.Pinot de Moire A., Fulford A.J., Kabatereine N.B., Ouma J.H., Booth M., et al. Analysis of complex patterns of human exposure and immunity to schistosomiasis mansoni: the influence of age, sex, ethnicity and IgE. PLOS Neglected Tropical Diseases. 2010; 14, e820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gobert GN, Tran MH, Moertel L, Mulvenna J, Jones MK, et al. Transcriptional Changes in Schistosoma mansoni during early schistosomula development and in the presence of erythrocytes. PLOS Neglected Tropical Diseases. 2010; 4: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pacifico LGG, Fonseca CT, Chiari L, Oliveira SC. Immunization with Schistosoma mansoni 22.6 kDa antigen induces partial protection against experimental infection in a recombinant protein form but not as DNA vaccine. Immunobiology. 2006; 211: 97–104. doi: 10.1016/j.imbio.2005.06.004 [DOI] [PubMed] [Google Scholar]
- 95.Roberts SM, Macgregor AN, Vojvodic M, Wells E, Crabtree JE, et al. Tegument surface-membranes of adult Schistosoma-mansoni—development of a method for their isolation. Molecular and Biochemical Parasitology. 1983; 9: 105–127. [DOI] [PubMed] [Google Scholar]
- 96.Fallon PG, McNeice C, Probert AJ, Doenhoff MJ. Quantification of praziquantel-induced damage on the surface of adult Schistosoma-mansoni worms—estimation of esterase and alkaline-phosphatase activity. Parasitology Research. 1994; 80: 623–625. [DOI] [PubMed] [Google Scholar]
- 97.Cesari IM, Bouty I, Bout D, Denoya BA, Hoebeke J. Parasite enzymes as a tool to investigate immune-responses. Memorias Do Instituto Oswaldo Cruz. 1992; 87: 55–65. [DOI] [PubMed] [Google Scholar]
- 98.Fallon PG, Smith P, Nicholls T, Modha J, Doenhoff MJ. Praziquantel-induced exposure of Schistosoma-mansoni alkaline-phosphatase—drug-antibody synergy which acts preferentially against female worms. Parasite Immunology. 1994; 16: 529–535. [DOI] [PubMed] [Google Scholar]
- 99.Araujo-Montoya BO, Rofatto HK, Tararam CA, Farias LP, Oliveira KC, et al. Schistosoma mansoni: Molecular characterization of alkaline phosphatase and expression patterns across life cycle stages. Experimental Parasitology. 2011; 129: 284–291. doi: 10.1016/j.exppara.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 100.Bhardwaj R, Skelly PJ Characterization of schistosome tegumental alkaline phosphatase (SmAP). PLOS Neglected Tropical Diseases. 2001; 5: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tielens AGM, Hellemond van JJ. Unusual aspects of metabolism in flatworm parasites Parasitic flatworms: molecular biology, biochemistry, immunology and physiology. Ed: Maule AG, Marks NJ. Oxfordshire: CABI Publishing; 2006. pp. 390–401. 387–401. [Google Scholar]
- 102.Akhiani AA, Nilsson LA, Ouchterlony O. Comparative antigen-analysis of different life stages of Schistosoma-mansoni by crossed immunoelectrophoresis. International Archives of Allergy and Applied Immunology. 1991; 95: 266–272. [DOI] [PubMed] [Google Scholar]
- 103.Linder E, Thors C. Schistosoma mansoni: praziquantel-induced tegumental lesion exposes actin of surface spines and allows binding of actin depolymerizing factor, gelsolin. Parasitology. 1992; 105: 71–79. [DOI] [PubMed] [Google Scholar]
- 104.Newsome J. Immune opsonins in Schistosoma infestations. Nature. 1962; 195: 1175–1179. [DOI] [PubMed] [Google Scholar]
- 105.Capron A, Dessaint JP, Capron M. Specific IgE antibodies in immune adherence of normal macrophages to Schistosoma mansoni schistosomules. Nature. 1974; 253: 474–475. [DOI] [PubMed] [Google Scholar]
- 106.Dean DA, Wistar R, Murrell KD. Combined in vitro effects of rat antibody and neutrophilic leukocytes on schistosomula of Schistosoma mansoni. American Journal of Tropical Medicine and Hygiene. 1974; 23: 420–428. [DOI] [PubMed] [Google Scholar]
- 107.Dean DA, Wistar R, Chen P. Immune response of guinea pigs to Schistosoma mansoni. I. In vitro effects of antibody and neutrophils, eosinophils and macrophages on schistosomula. American Journal of Tropical Medicine and Hygiene. 1975; 24: 74–82. [DOI] [PubMed] [Google Scholar]
- 108.Wilson RA, Coulson PS, Dixon B. Migration of the schistosomula of Schistosoma mansoni in mice vaccinated with radiation-attenuated cercariae, and normal mice: an attempt to identify the timing and site of parasite death. Parasitology. 1986; 92: 101–116. [DOI] [PubMed] [Google Scholar]
- 109.Gobert GN, McInnes R, Moertel L, Nelson C, Jones MK, et al. Transcriptomics tool for the human Schistosoma blood flukes using microarray gene expression profiling. Experimental Parasitology. 2006; 114: 160–172. doi: 10.1016/j.exppara.2006.03.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(PPTX)
(PPTX)
(PPTX)
(PPTX)
(PPTX)
(PPTX)
(PPTX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.