Abstract
Background
Smith-Magenis syndrome (SMS) is a complex neurobehavioral disorder associated with recurrent otitis. Most SMS cases result from heterozygous interstitial chromosome 17p11.2 deletions that encompass not only the intellectual disability gene RAI1 but also other genes associated with immunodeficiency, autoimmunity and/or malignancy.
Objectives
The goals of this study were to describe the immunological consequence of 17p11.2 deletions by determining the prevalence of immunological diseases in SMS subjects and by assessing their immune systems via laboratory methods.
Methods
We assessed clinical histories of 76 SMS subjects with heterozygous 17p11.2 deletions and performed in-depth immunological testing on 25 representative cohort members. Laboratory testing included determination of serum antibody concentrations, vaccine titers and lymphocyte subset frequencies. Detailed reactivity profiles of SMS serum antibodies were performed using custom-made antigen microarrays.
Results
74 of 76 SMS subjects reported recurrent infections including otitis (88%), pneumonia (47%), sinusitis (42%), and gastroenteritis (34%). Infections were associated with worsening SMS-related neurobehavioral symptoms. The prevalence of autoimmune and atopic diseases was not increased. Malignancy was not reported. Laboratory evaluation revealed most SMS subjects to be deficient of isotype-switched memory B cells and many to lack protective antipneumococcal antibodies. SMS antibodies were not more reactive than control antibodies to self-antigens.
Conclusions
SMS patients with heterozygous 17p.11.2 deletions display an increased susceptibility to sinopulmonary infections, but not to autoimmune, allergic or malignant diseases. SMS sera display an antibody reactivity profile favoring neither recognition of pathogen-associated or self-antigens. Prophylactic strategies to prevent infections may also provide neurobehavioral benefits to selected SMS patients.
Keywords: Smith-Magenis syndrome, chromosome 17p11.2 deletion, immune deficiency, autoantibody, TNFRSF13B, FLCN and TOM1L2, B-cell tolerance
INTRODUCTION
Smith–Magenis syndrome (SMS; OMIM #182290, *607642) is a complex genetic disorder, estimated prevalence 1: 15,000–25,000. SMS is characterized by intellectual disability, sleep disturbances, self-injurious behaviors and skeletal abnormalities.1–3 Although ear infections are commonly described in SMS patients,4 it is unclear if the predisposition is due to an anatomic or immunologic abnormality. Diminished anti-pneumococcal antibodies have been described in SMS sera5 but neither a comprehensive clinical evaluation of the SMS immune system nor a detailed account of the full spectrum of infections experienced by SMS patients have been reported Similarly, it is unknown is if the SMS immune system is prone to the development of autoimmune, malignant, and/or atopic diseases as is the case in many primary immunodeficiencies.6–9
Approximately 90% of SMS cases are caused by the heterozygous 3.7 Mb interstitial deletion of 17p11.2, a region encompassing the retinoic acid-induced 1 (RAI1) gene locus.3 In rare cases, SMS may be caused by deleterious RAI1 point mutations, without deletion of 17p11.2, suggesting that RAI1 is the gene primarily responsible for the neuro-developmental features of SMS.10 RAI1 serves no known immunologic function,11 but proximate genes also lost to 17p11.2 deletion, including TNFRSF13B, FLCN and TOM1L2, do. TNFRSF13B encodes TACI, which controls T-independent humoral responses and B cell tolerance.12–15 Heterozygous missense TNFRSF13B mutations are associated with Common Variable Immune Deficiency (CVID),16,17 an antibody deficiency disorder often complicated by autoantibody production and hematologic malignancy.18 Autoimmune disease occurs in 41% of CVID patients with heterozygous TNFRSF13B missense mutations.19 FLCN is a tumor suppressor gene mutated in Birt-Hogg-Dubé syndrome (BHDS).20 BHDS patients accumulate both benign and malignant tumors.20 TOM1L2 is not implicated in a human disease, but Tom1l2-deficient mice are susceptible to infections and tumors.21
Since many SMS patients are hemizygous for multiple genes associated with immunodeficiency, autoimmunity and/or malignancy, we hypothesized they may also be susceptible to these diseases. To test this hypothesis, we surveyed medical histories, spanning 970 person-years, from a large cohort of 76 SMS subjects ages 6 months to 37 years (mean 13.8 years) with 17p11.2 deletions. We obtained peripheral blood samples on 25 representative subjects from our cohort, all with deletions encompassing RAI1, TNFRSF13B, FLCN and TOM1L2, to create in-depth immunologic profiles via laboratory assessments that included serum immunoglobulin quantification, vaccine titers, lymphocyte flow-cytometry and custom-made antigen microarrays. Our results indicate that SMS patients are antibody deficient and frequently experience sinopulmonary infections, including severe bacterial illnesses like pneumonia. Unlike many primary immunodeficiency patients, SMS subjects were not more susceptible to autoimmune, allergic or malignant diseases, nor did they frequently display increased serum autoantibodies or elevated IgE.
METHODS
Study subjects and clinical history evaluations
We enrolled 76 SMS subjects with heterozygous chromosome 17p11.2 deletions. Subjects ranged from 6 months to 37 years in age (mean 13.8 years); 52% were female (Table 1). Informed consent was obtained for all individuals before study enrollment. TThe study protocol was approved by the Human Subjects Committee of Yale University, the Institutional Review Board of the Children’s Hospital of Philadelphia and the professional advisory board of Parents and Researchers Interested in Smith-Magenis Syndrome (PRISMS). An immunological diseases questionnaire was distributed to families of SMS subjects at the international PRISMS family meeting, on the PRISMS web-site or in the course of the authors’ clinical practice. When subjects were identifiable (n=12), survey responses were secondarily confirmed for accuracy using electronic medical records (EMR). Overall good concordance between survey and EMR data was observed. For survey responses, a recurrent infection was defined as at least 4 infections per year. Peripheral blood samples, paired to survey data, were obtained either at the 2014 International PRISMS Conference and family meeting (n=18), at Yale New Haven Hospital (n=5), or the Children’s Hospital of Philadelphia (n=2). Blood based screening evaluations were performed on 25 SMS subjects; all possessed a chromosome 17p11.2 deletion spanning TNFRSF13B, RAI1, FLCN and TOM2L1 as determined by florescence in situ hybridization or chromosomal microarray (see Table E1 in the Online Repository); all had completed a primary vaccination series; none were receiving antibody replacement or immunosuppressive therapies. Healthy control adult serum samples were obtained from 3 first-degree relatives of SMS subjects and 6 unrelated adult donors after obtaining informed consent. Serum samples from 8 healthy unrelated children were purchased as comparators (Biodesign International Inc., Saco, Maine).
Table 1.
Clinical serum antibody testing and infectious histories of 25 SMS subjects not receiving antibody replacement therapy
| Agea | Sex | IgM (mg/dL) | IgA (mg/dL) | IgG (mg/dL) | IgG2 (mg/dL) | Tetanus Ab (IU/ml) | Hib Ab (μg/ml) | Pneumococca Ab (μg/ml), 14 serotypes | IgE (ng/mL) | Infections | |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Reference | __b | __b | __b | __b | ≥0.15 | ≥0.15 | ≥0.35 | __b | |||
| SMS1 | 4 | M | 41 (L) | 76 | 863 | 114 | >7 | 0.4 | 6/14 (L) | 158 (H) | O, P, S, G, U |
| SMS2 | 6 | M | 82 | 106 | 1040 | 122 | >7 | 1.5 | 11/14 | <4 | P, U |
| SMSY2 | 6 | F | 113 | 124 | 891 | 117 | <0.1 (L) | 6.25 | 11/14 | <2 | O |
| SMS4d | 7 | M | 44 (L) | 38 (L) | 508 (L) | 95 | 0.7 | 0.8 | 3/14c (L) | 3 | O |
| SMS5 | 7 | F | 51 | 12 (L) | 650 | 42 (L) | 3.2 | 1.1 | 3/14c (L) | <2 | W, O |
| SMSY6 | 9 | M | 134 | 303 | 490 (L) | 194 | 0.37 | 0.5 | 9/14 | 43 | O |
| SMS7 | 10 | M | 113 | 91 | 928 | 93 | 0.23 | 0.6 | 7/14 | 3 | O, S, P, G, U |
| SMS8 | 10 | F | 83 | 67 (L) | 675 | 119 | 0.26 | 0.4 | 11/14 | <2 | P, U |
| SMS9 | 11 | M | 72 | 73 | 879 | 190 | 0.75 | 0.5 | 11/14 | 14 | O |
| SMSY3 | 12 | F | 51 | 12 (L) | 680 | 121 | >7 | 1.1 | 6/14 (L) | ND | O, W, U |
| SMS10 | 13 | F | 118 | 54 (L) | 642 (L) | 114 | 1.76 | 1.4 | 12/14 | 32 | O, S, G, W, |
| SMS11d | 15 | F | 53 (L) | 97 | 585 (L) | 176 | 2.02 | 0.7 | 4/14c (L) | 38 | O, S, P |
| SMS12 | 16 | F | 99 | 32 (L) | 718 | 112 | 1.06 | 4.3 | 6/14 (L) | 5 | O, S, P, G |
| SMS13 | 16 | M | 77 | 126 | 1470 | 304 | 1 | 3.6 | 14/14 | 15 | O |
| SMS14 | 20 | F | 62 | 217 | 1210 | 404 | 0.1 (L) | 0.7 | 8/14 | 5 | C, O, S, G, W |
| SMSY5 | 20 | F | 72 | 114 | 1400 | 377 | 0.53 | 0.9 | 14/14 | <2 | O, G |
| SMSY7 | 20 | M | 18 (L) | <7 (L) | 1050 | ND | 0.23 | 19.5 | 13/14 | 45 | O, S, P, B |
| SMS17 | 20 | F | 59 (L) | 83 | 720 | 257 | 0.4 | 1.1 | 13/14 | 5 | O, S, P |
| SMS18 | 21 | F | 26 (L) | 195 | 925 | 178 | 2.55 | 1.4 | 11/14 | 5 | O, S, P |
| SMS19 | 22 | M | 48 | 181 | 1150 | 214 | 3.7 | 1.5 | 5/14 (L) | 7 | O, OE |
| SMS20 | 22 | F | 71 | 90 | 1280 | 351 | 2.05 | 4.1 | 11/14 | 42 | O, U, G |
| SMS21 | 23 | M | 119 | 71 | 1250 | 148 | 1.64 | 4.4 | 14/14 | 22 | O, P, O, U |
| SMS22 | 23 | F | 174 | 140 | 1500 | 190 | 1.24 | 2.1 | 11/14 | 5 | O, S, |
| SMSY4 | 26 | F | 83 | 278 | 1360 | 67 (L) | 5.36 | 4.3 | 4/14c (L) | 5 | O, C, W |
| SMS23 | 27 | M | 68 | 82 | 904 | 170 | 1 | 4.8 | 13/14 | 8 | O, U |
Ab, antibody; B, bacteremia; C, cellulitis; F, female; G, gastroenteritis; H, higher than normal range; Hib, Haemophilus influenza type B; L, below normal/protective range; M, male; ND, not determined; O, otitis; OE, osteomyelitis; P, pneumonia; S, sinusitis; U, upper respiratory tract infection;
Age (years) at time of testing
Reference range varies with age20
Patient failed a challenge with the 23-valent pneumococcal vaccine. The number of protective serotypes displayed reflect pre-challenge values.
Patient meets diagnostic criteria for Common Variable Immune Deficiency including vaccine challenge failure.
Quantitative and qualitative antibody testing
Measurement of IgM, IgA, IgE, IgG, IgG subclass 1–4 concentrations and antibody responses to tetanus toxoid, Haemophilus influenza type B and 14 serotypes of Streptococcus pneumonia (1, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 12F, 14, 18C, 19F, 23F) were performed on 20 serum samples by the Yale New Haven Hospital clinical laboratory. Results from 5 additional SMS patients, performed by other clinical reference laboratories were also included. Age specific normal value ranges were used to assess if a subject’s laboratory assessments were abnormal.22 Anti-Haemophilus influenza type B and anti-tetanus toxoid antibody concentrations were considered protective at concentrations of ≥0.15 μg/ml and ≥0.15 IU/ml respecitively.23 Anti-pneumococcal antibodies were considered protective at concentrations >0.35 μg/ml.24 For the subset of 6 patients challenged with the 23-valent pneumococcal vaccine, an adequate vaccine response was defined as anti-pneumococcal antibody concentrations of 1.3 μg/ml to >50% serotypes assessed.25
Flow cytometry
Flow cytometry sample acquisition was performed on a LSR Fortessa (BD Biosciences, Mountain View, Calif). The following antibodies were used for flow cytometric stainings: anti-TACI PE (clone 1A1), anti-CD19 APC-Cy7, anti-CD27 AF700, anti-CD4 APC-Cy7, anti-CD8 BV711, anti-CD25 Pe/Dazzle 594, anti-CD127 PerCPCy5.5 (all from BioLegend, San Diego, Calif), anti-IgM PerCPCy5.5 and anti-CD3 eFluor 605NC (BD). Intracellular staining for FOXP3 Alexa Fluor 488 (clone 150D; Biolegend) was performed using the FOXP3/Transcription Factor Staining Buffer Set (eBioscience, San Diego, Calif) in accordance with the manufacturer’s instructions. Subset analysis was performed with FlowJo software (Tree Star, Ashland, Ore).
Antigen microarrays
Antigen microarrays were generated using a VersArray ChipWriter™ Pro microarrayer (Bio-Rad, Hercules, Calif) and using customized printheads from Silicon Microarray Spotting Pins (Parallel Synthesis Technologies, Santa Clara, Calif) as previously described.26 Briefly, 337 purified biomolecules including autoantigens, cytokines, and chemokines were purchased from multiple vendors and printed in triplicate at dilutions of 200μg/ml onto Nexterion E epoxysilane-coated glass slides (Schott, Duryea, Pa). A complete list of molecules printed can be found in Figure E1. Arrays were blocked, then washed in 7% fetal bovine serum in PBS plus 0.1% Tween (PBST). Arrays were probed for one hour with sera, diluted 1:150 in 30% FBS 1% PBS, from 18 SMS subjects with heterozygous 17p11.2 deletions, 3 of their healthy first-degree relatives and 14 healthy unrelated pediatric controls. After washing in PBST, serum reactivity was detected using an Alexafluor 647-conjugated goat anti-human IgG (Fc-specific) secondary antibody (Jackson, West Grove, Pa). After washing, arrays were dried under negative pressure and scanned using an Agilent microarray scanner. Data were bioinformatically processed using GenePix 6 software. Mean fluorescence intensity (MFI) values for each antigen were calculated by taking the mean of median fluorescence intensity for each feature. From this value was subtracted the value of MFI reactivity by probing with secondary antibody alone.
Statistical Methods
Linear regression modeling was conducted using PRISM software (GraphPad, San Diego, CA). Significance Analysis of Microarrays (SAM), a permutation-based algorithm for determining statistically significant differences in large datasets, was used to determine differences in antibody reactivities between SMS and control serum samples.27 For SAM analyses, a false discovery rate (FDR) of <0.001 was accepted and an adjusted P value of <0.05 was considered statistically significant. Antigen microarray analyses were powered (>0.8) to detect at least 1.5-fold reactivity differences between subject and control sera.
RESULTS
SMS subjects are susceptible to sinopulmonary infections
A history of recurrent and/or severe infections was reported in 72 of 76 (95%) SMS subjects (Table 2). Sinopulmonary infections were most commonly described and included recurrent otitis media (88% of subjects), recurrent upper respiratory tract infections (61%), pneumonia (47%) and recurrent sinusitis (42%). Recurrent gastroenteritis was described by 34% of respondents. Skin infections were also reported, including bacterial cellulitis (17%) and warts (16%) primarily affecting the hands and feet (Figure E2). A history of bacteremia was reported in 2 subjects; hematogenously seeded osteomyelitis in another. No cases of abscesses, deep tissue infections or joint infections were identified. Excluding warts, other opportunistic infections like mucocutaneous Candidiasis, Pneumocystis pneumonia, Cryptococcosis, Cryptosporidiosis, molluscum and cytomegalovirus infections were not identified.
Table 2.
Infections reported in 76 SMS subjects (ages 6 months-37 years)
| Percentage (n) | |
|---|---|
| Recurrent ear infections | 88.2 (67) |
| Recurrent viral respiratory | 60.5 (46) |
| Pneumonia | 47.4 (36) |
| Recurrent sinus infections | 42.1 (32) |
| Recurrent gastroenteritis | 34.2 (26) |
| Bacterial cellulitis | 17.1 (13) |
| Warts | 15.8 (12) |
| Bacteremia | 2.6 (2) |
| Osteomyelitis | 1.3 (1) |
25 (33%) SMS subjects surveyed had previously received an immunological evaluation. Of those evaluations, 68% were performed by allergists/clinical immunologists, 21% by clinical geneticists and 8% by infectious disease physicians. 67 (87%) SMS subjects had received a complete primary vaccine series. 6 (9%) SMS subjects were currently receiving, or had at one time received, antibody replacement therapy. Altogether, these results show that SMS patients display an increased susceptibility to sinopulmonary infections.
Infections negatively impact SMS-associated sleep disturbances, maladaptive behaviors and seizures
SMS-associated sleep disturbances and maladaptive behaviors, which included self-injury by onychotillomania or polyembolokoilamania, temper tantrums and attention deficit/hyperactivity, were described by all respondents. During acute infections, 72% of SMS caregivers perceived a worsening of sleep disturbances and 79% perceived a worsening of behavioral issues (Figure 1). Of those reporting a negative impact, the majority described the effect of infections to be “significant.” Seizures were reported in 10 SMS subjects; infections increased seizure frequency and severity in 8 of these (Figure 1).
Figure 1.
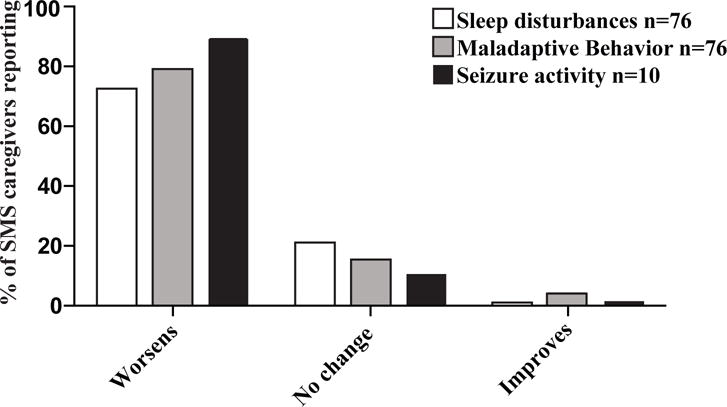
Most SMS caregivers perceive acute infections to worsen SMS-associated sleep disturbances, maladaptive behaviors and seizures.
Autoimmune, atopic and malignant diseases do not occur frequently in SMS subjects
Autoimmune diseases, which included autoimmune thyroiditis (n=2), autoimmune neutropenia (n=1) and pernicious anemia (n=1), were reported in 5% of SMS subjects (Table E2). This frequency was identical to that reported in subjects’ siblings suggesting autoimmunity was not increased in our young SMS cohort. Atopy was reported in 27% of subjects with a prevalence and variety (Table E3) similar to large national health surveys.28,29 A modestly elevated serum IgE concentration was identified in 1 subject (Table 1). Malignant diseases were not reported in our cohort.
Antibody responses are impaired in SMS
We assessed SMS serum for immunoglobulin isotypes and IgG subclass concentrations and identified at least 1 abnormal result in the majority of subjects (60%) (Table 1). IgM, IgA, and IgG concentrations were beneath age-adjusted institutional normal ranges in 22%, 16%, and 28% of serum samples respectively. 2 subjects were selectively IgG2 deficient. All subjects possessed protective anti-Haemophilus influenza type B (HiB) antibody concentrations and most possessed protective concentrations to tetanus toxoid (92%). In contrast, 32% lacked protective antibody concentrations (>0.35 μg/ml) to the majority of the 14 Streptococcus pneumonia serotypes tested. Vaccine challenges with the 23-valent pneumococcal vaccine were performed on 6 SMS subjects, 4 of these were unable to generate appropriate anti-pneumococcal antibody responses. 2 vaccine non-responders met CVID diagnostic criteria (Table 1). Although there was a trend of improving pneumococcal vaccine titers with advancing age, we conclude that many SMS subjects suffer from decreased antibody production and impaired pneumococcal responses.
Many primary antibody deficiency diseases are associated with diminished class-switched memory B cells.30,31 In our SMS cohort, total B-cell and total memory B-cell frequencies were not diminished compared to age-matched institutional normal ranges whereas isotype-switched memory B cells were diminished in 17 of 19 SMS subjects. This is consistent with our previous study analyzing fewer subjects.32 Enumeration of T cell subsets including CD4 T cells, CD8 T cells and T regulatory cells in our cohort revealed no consistent abnormal trends (Table E4). Significant T cell lymphopenia was identified in only one SMS subject (SMS2), a boy with a history of partial thymectomy secondary to surgical correction of a congenital heart defect. He did not experience opportunistic infections. Natural killer cell deficiency was not identified in our SMS cohort.
SMS antibody reactivities to pathogens and to self-antigens are limited
To create an unbiased and in-depth reactivity profile of SMS protective IgG antibodies and autoantibodies, we designed and fabricated antigen microarrays and probed them with sera from 18 SMS subjects and 17 healthy controls. Antigen microarrays contained a total of 337 antigens including 19 pathogen-specific antigens and autoantigens, including cytokines, chemokines, and growth factors.33 Serum reactivities measured by microarray and by conventional laboratory testing linearly correlated (R2=0.54, p<0.0005) and were generally concordant (Figure E3). For instance, 10 of 10 of the most reactive SMS serum samples to veterinary tetanus vaccine also demonstrated protective tetanus specific IgG levels (≥0.15μg/ml) by conventional clinical laboratory testing.
To measure differences in the levels of antibodies between SMS patients and age-matched, related and unrelated controls, we performed Significance Analysis of Microarrays (SAM), a permutation-based algorithm for determining statistically significant differences in large datasets.27 SAM analysis of the 19 pathogen-associated antigens on the array demonstrated that antibodies against 4 pathogens were decreased in SMS patient sera compared to healthy controls. These antigens were the HiB-conjugate vaccine, Hepatitis B surface antigen, bacterial flagellin, and horse tetanus vaccine (Figure 2). No anti-pathogen antibodies tested were significantly elevated in SMS sera compared to controls.
Figure 2.
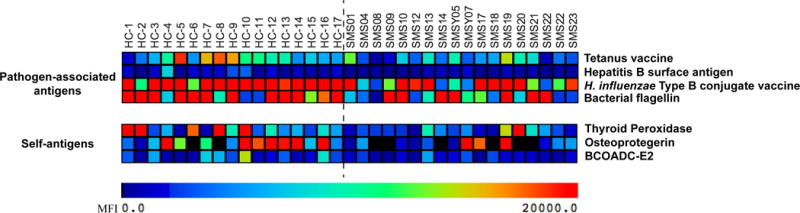
SMS sera are less reactive than control sera to pathogen-associated and self-antigens. The heat map displays sera reactivities to 4 pathogen-associated antigens (upper panel) and 3 self-antigens (lower panel) lower in 18 SMS serum samples than 17 healthy-related and unrelated control samples. Colorimetric differences corresponding to array MFIs are indicated.
Antigen microarrays have previously identified diverse and numerous autoantibodies in the sera from patients with autoimmune diseases including systemic lupus erythematosus, juvenile dermatomyositis and RAG deficiency compared to healthy controls.34–36 We therefore assessed autoantibody profiles between SMS and healthy control sera by performing SAM a second time using array values for autoantibody reactivity against classical self-antigens. Of the 94 self-antigens tested, no autoantibody was significantly increased in the SMS population. There were 3 autoantibodies found at lower levels in SMS sera than healthy sera, among these was anti-thyroid peroxidase (Figure 2). Given the newly recognized importance of anti-cytokine autoantibodies in primary immunodeficiencies,33,36,37 we also performed SAM for sera reactivities to cytokines, chemokines and growth factors. No statistically significant differences were identified. Taken together, these data demonstrate a deficiency of pathogen protective antibodies in SMS subjects without an increased presence of autoantibodies.
DISCUSSION
Herein, we report that SMS patients display an increased susceptibility to sinopulmonary infections including otitis but also invasive bacterial infections. During our study, a 35-year-old female subject succumbed to pneumonia, an infection affecting nearly half our cohort, underscoring the potential infectious acuity of SMS. Our results are consistent with the limited number of previously published reports on the immunologic phenotype of SMS patients that were either more limited in scope38 or were mechanistic and not clinical.5,32 Here, we provide a unique and detailed analysis of SMS antibody reactivities to pathogen-associated antigens and self-antigens by traditional clinical laboratory testing and by in-depth antigen microarray We have demonstrated decreased pathogen-specific antibodies, impaired pneumococcal responses and fewer isotype-switched memory B cells to be general features of SMS. Such immune defects mirror those of CVID patients and reinforce the essential role TACI in mediating T-independent humoral responses and regulating late-stage B cell differentiation.15,32 Yet, despite such dysfunction, SMS B cells do not preferentially produce serum autoantibodies as CVID B cells do.14 Why are SMS patients and TNFRSF13B mutated CVID patients similarly susceptible to infections but not to autoimmune diseases? One possible explanation is that the single unmutated TNFRSF13B allele possessed by most SMS patients is sufficient to establish B-cell tolerance whereas a CVID-associated TNFRSF13B mutant allele, likely encoding a dominant-negative product, interferes with it.14,32 Unlike tolerance formation, optimal antibody production requires B cells with two functional TNFRSF13B alleles. Hence, SMS and CVID patients with TNFRSF13B mutations are both susceptible to antibody deficiency-associated sinopulmonary infections.
Several SMS subjects reported warts, an uncharacteristic finding for pure antibody deficiency diseases, but one that may be explained by “skin picking,” a compulsive SMS behavior. Warts may also be related to fibrofolliculomas, benign hair follicle tumors pathognomonic for BHDS. However, since 30% of BHDS patients also develop renal cancer, and cancer was not reported in our cohort, it appears heterozygous BHDS-associated FLCN mutations and SMS-associated FLCN hemizygosity are not equivalent.20 Longitudinal study of our relatively young cohort may provide further confirmation of this hopeful finding.
Despite a significant infectious burden, family members of SMS subjects consistently rate behavioral issues and sleep disturbances to be the most challenging aspects of the disease.39 Such prioritization is understandable and may partially explain why only 35% of our cohort received a prior immunological evaluation. Yet, we report here that SMS caregivers also perceive infections to significantly aggravate SMS-associated neurobehavioral problems. As many laboratory abnormalities we identified in SMS subjects, including hypogammaglobulinemia, IgG subclass deficiency and specific antibody deficiency, are indications for prophylactic antibody replacement, a trial of this therapy in selected SMS patients may improve both infectious and non-infectious outcomes.
Supplementary Material
Figure E1: A detailed account of the reactivities of SMS sera versus control sera to 337 antigens. A heat map displays reactivities of sera from 18 SMS subjects 14 healthy unrelated and 3 related controls to the 337 listed antigens. Colorimetric differences on the heat map correspond.
Figure E2: Persistent verrucous disease of the hand (A) and foot (B) of SMSY4.
Figure E3: Bivariate analysis of serum reactivity to tetanus toxoid by conventional laboratory testing versus antigen microarray reveals fair concordance. Linearity (R2) and statistical significance (P) calculated via linear regression analysis. MFI, mean florescence intensity.
Table E1- Location, size and relevant genes contained within the deleted regions of 25 SMS blood donors.
Table E2: History of autoimmune diseases in 76 SMS subjects (ages 6 months-37 years).
Table E3: Atopic history in 76 SMS subjects (ages 6 months-37 years).
Table E4: Relative frequencies of lymphocyte subsets in the peripheral blood of 19 SMS subjects.
Highlights box.
What’s is already known on this topic?
Smith-Magenis syndrome (SMS) is a complex neurobehavioral disorder associated with otitis. Most SMS cases result from chromosome 17p11.2 deletions that encompass the intellectual disability gene RAI1 and also genes associated with immunodeficiency, autoimmunity and/or malignancy.
What does this article add to our knowledge?
Description of the immunopathologies and laboratory immunological features of a large cohort of 76 SMS patients reveals a consistent susceptibly to sinopulmonary infections, including pneumonia, but not to autoimmune, allergic or malignant diseases.
How does this study impact current management guidelines
As with other genetic syndromes associated with antibody deficiency listed in the AAAI practice parameters for diagnosis and management of primary immunodeficiency, all SMS patients should receive an immunologic evaluation. Infectious prophylaxis should be considered in selected SMS patients.
Acknowledgments
This work was supported by grant number K23AI115001 from National Institutes of Health-National Institute of Allergy and Infectious Diseases (NIH-NIAID), K12HD0141401-10 from UL1, TR000142 from NIH-National Institutes of Health-National Center for Advancing Translational Sciences, and the Jeffrey Modell Foundation (all to NR), the Donald E. and Delia B. Baxter Foundation Career Development Award, a gift from The Floren Family Trust, U19-AI1110491, R01 AI125197 both from the NIH-NIAID, and Alliance for Lupus Research Grant No. 296550 (all to PJU), AI061093 from NIH-NIAID (to EM).
Abbreviations
- BHDS
Birt-Hogg-Dubé syndrome
- CVID
Common variable immune deficiency
- FCLN
Folliculin
- MFI
Mean fluorescence intensity
- RAI1
Retinoic acid-induced 1
- SAM
Significance Analysis of Microarrays
- SMS
Smith-Magenis syndrome
- TNFRSF13B
Tumor necrosis factor receptor superfamily member 13b
- TOM1L2
Target of myb1 like 2 membrane trafficking protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith AC, McGavran L, Robinson J, Waldstein G, Macfarlane J, Zonona J, et al. Interstitial deletion of (17)(p11.2p11.2) in nine patients. Am J Med Genet. 1986 Jul;24(3):393–414. doi: 10.1002/ajmg.1320240303. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg F, Lewis RA, Potocki L, Glaze D, Parke J, Killian J, et al. Multidisciplinary clinical study of Smith-Magenis syndrome (deletion 17p11.2) Am J Med Genet. 1996 Mar 29;62(3):247–54. doi: 10.1002/(SICI)1096-8628(19960329)62:3<247::AID-AJMG9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 3.Elsea SH, Girirajan S. Smith–Magenis syndrome. Eur J Hum Genet. 2008 Jan 30;16(4):412–21. doi: 10.1038/sj.ejhg.5202009. [DOI] [PubMed] [Google Scholar]
- 4.Di Cicco M, Padoan R, Felisati G, Dilani D, Moretti E, Guerneri S, et al. Otorhinolaringologic manifestation of Smith-Magenis syndrome. Int J Pediatr Otorhinolaryngol. 2001 Jun 7;59(2):147–50. doi: 10.1016/s0165-5876(01)00475-x. [DOI] [PubMed] [Google Scholar]
- 5.Chinen J, Martinez-Gallo M, Gu W, Cols M, Cerutti A, Radigan L, et al. Transmembrane activator and CAML interactor (TACI) haploinsufficiency results in B-cell dysfunction in patients with Smith-Magenis syndrome. J Allergy Clin Immunol. 2011 Jun;127(6):1579–86. doi: 10.1016/j.jaci.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham-Rundles C. Autoimmunity in primary immune deficiency: taking lessons from our patients. Clin Exp Immunol. 2011 Jun;164(Suppl 2):6–11. doi: 10.1111/j.1365-2249.2011.04388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yong PFK, Freeman AF, Engelhardt KR, Holland S, Puck JM, Grimbacher B. An update on the hyper-IgE syndromes. Arthritis Res Ther. 2012;14(6):228. doi: 10.1186/ar4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Notarangelo LD. PIDs and cancer: an evolving story. Blood. 2010 Aug 26;116(8):1189–90. doi: 10.1182/blood-2010-06-286179. [DOI] [PubMed] [Google Scholar]
- 9.Meffre E. The establishment of early B cell tolerance in humans: lessons from primary immunodeficiency diseases. Ann N Y Acad Sci. 2011 Dec;1246:1–10. doi: 10.1111/j.1749-6632.2011.06347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slager RE, Newton TL, Vlangos CN, Finucane B, Elsea SH. Mutations in RAI1 associated with Smith-Magenis syndrome. Nat Genet. 2003 Apr;33(4):466–8. doi: 10.1038/ng1126. [DOI] [PubMed] [Google Scholar]
- 11.Vilboux T, Ciccone C, Blancato JK, Cox GF, Deshpande C, Introne WJ, et al. Molecular Analysis of the Retinoic Acid Induced 1 Gene (RAI1) in Patients with Suspected Smith-Magenis Syndrome without the 17p11.2 Deletion. PLoS ONE. 2011 Aug 8;6(8):e22861. doi: 10.1371/journal.pone.0022861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castigli E, Wilson SA, Elkhal A, Ozcan E, Garibyan L, Geha RS. Transmembrane activator and calcium modulator and cyclophilin ligand interactor enhances CD40-driven plasma cell differentiation. J Allergy Clin Immunol. 2007 Oct;120(4):885–91. doi: 10.1016/j.jaci.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He B, Santamaria R, Xu W, Cols M, Chen K, Puga I, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol. 2010 Sep;11(9):836–45. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romberg N, Chamberlain N, Saadoun D, Gentile M, Kinnunen T, Ng YS, et al. CVID-associated TACI mutations affect autoreactive B cell selection and activation. J Clin Invest. 2013 Oct 1;123(10):4283–93. doi: 10.1172/JCI69854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Bülow GU, van Deursen JM, Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity. 2001 May;14(5):573–82. doi: 10.1016/s1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 16.Salzer U, Chapel HM, Webster ADB, Pan-Hammarström Q, Schmitt-Graeff A, Schlesier M, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet. 2005 Aug;37(8):820–8. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 17.Castigli E, Wilson SA, Garibyan L, Rachid R, Bonilla F, Schneider L, et al. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet. 2005 Aug;37(8):829–34. doi: 10.1038/ng1601. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham-Rundles C. How I treat common variable immune deficiency. Blood. 2010 Jul 8;116(1):7–15. doi: 10.1182/blood-2010-01-254417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salzer U, Bacchelli C, Buckridge S, Pan-Hammarström Q, Jennings S, Lougaris V, et al. Relevance of biallelic versus monoallelic TNFRSF13B mutations in distinguishing disease-causing from risk-increasing TNFRSF13B variants in antibody deficiency syndromes. Blood. 2009 Feb 26;113(9):1967–76. doi: 10.1182/blood-2008-02-141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nickerson ML, Warren MB, Toro JR, Matrosova V, Glenn G, Turner ML, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dubé syndrome. Cancer Cell. 2002 Aug;2(2):157–64. doi: 10.1016/s1535-6108(02)00104-6. [DOI] [PubMed] [Google Scholar]
- 21.Girirajan S, Hauck PM, Williams S, Vlangos CN, Szomju BB, Solaymani-Kohal S, et al. Tom1l2 hypomorphic mice exhibit increased incidence of infections and tumors and abnormal immunologic response. Mamm Genome Off J Int Mamm Genome Soc. 2008 Apr;19(4):246–62. doi: 10.1007/s00335-008-9100-6. [DOI] [PubMed] [Google Scholar]
- 22.Jolliff CR, Cost KM, Stivrins PC, Grossman PP, Nolte CR, Franco SM, et al. Reference intervals for serum IgG, IgA, IgM, C3, and C4 as determined by rate nephelometry. Clin Chem. 1982 Jan 1;28(1):126–8. [PubMed] [Google Scholar]
- 23.Anderson P. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1984 Jun;149(6):1034–5. doi: 10.1093/infdis/149.6.1034. [DOI] [PubMed] [Google Scholar]
- 24.Expert Committee on Biological Standardization. Recommendations for the production and control of pneumococcal conjugate vaccines. WHO Press; 2009. Oct, [Google Scholar]
- 25.Orange JS, Ballow M, Stiehm ER, Ballas ZK, Chinen J, De La Morena M, et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2012 Sep;130(3 Suppl):S1–24. doi: 10.1016/j.jaci.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Robinson WH, DiGennaro C, Hueber W, Haab BB, Kamachi M, Dean EJ, et al. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat Med. 2002 Mar;8(3):295–301. doi: 10.1038/nm0302-295. [DOI] [PubMed] [Google Scholar]
- 27.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001 Apr 24;98(9):5116–21. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bloom B, Cohen RA, Freeman G. Summary health statistics for us Children: national health interview survey, 2011. Vital Health Stat 10. 2012;(254):1–88. [PubMed] [Google Scholar]
- 29.Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997–2011. NCHS Data Brief. 2013;121:1–8. [PubMed] [Google Scholar]
- 30.Warnatz K, Denz A, Drager R, Braun M, Groth C, Wolff-Vorbeck G, et al. Severe deficiency of switched memory B cells (CD27+IgM-IgD-) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002 Mar 1;99(5):1544–51. doi: 10.1182/blood.v99.5.1544. [DOI] [PubMed] [Google Scholar]
- 31.Agematsu K, Nagumo H, Shinozaki K, Hokibara S, Yasui K, Terada K, et al. Absence of IgD-CD27(+) memory B cell population in X-linked hyper-IgM syndrome. J Clin Invest. 1998 Aug 15;102(4):853–60. doi: 10.1172/JCI3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romberg N, Virdee M, Chamberlain N, Oe T, Schickel J-N, Perkins T, et al. TNF receptor superfamily member 13b (TNFRSF13B) hemizygosity reveals transmembrane activator and CAML interactor haploinsufficiency at later stages of B-cell development. J Allergy Clin Immunol. 2015 Jun 19; doi: 10.1016/j.jaci.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg JM, Price JV, Barcenas-Morales G, Ceron-Gutierrez L, Davies S, Kumararatne DS, et al. Protein microarrays identify disease-specific anti-cytokine autoantibody profiles in the landscape of immunodeficiency. J Allergy Clin Immunol. 2016 Jan;137(1):204–13.e3. doi: 10.1016/j.jaci.2015.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haddon DJ, Diep VK, Price JV, Limb C, Utz PJ, Balboni I. Autoantigen microarrays reveal autoantibodies associated with proliferative nephritis and active disease in pediatric systemic lupus erythematosus. Arthritis Res Ther. 2015;17:162. doi: 10.1186/s13075-015-0682-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balboni I, Niewold TB, Morgan G, Limb C, Eloranta M-L, Rönnblom L, et al. Interferon-α induction and detection of anti-ro, anti-la, anti-sm, and anti-rnp autoantibodies by autoantigen microarray analysis in juvenile dermatomyositis. Arthritis Rheum. 2013 Sep;65(9):2424–9. doi: 10.1002/art.38038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walter JE, Rosen LB, Csomos K, Rosenberg JM, Mathew D, Keszei M, et al. Broad-spectrum antibodies against self-antigens and cytokines in RAG deficiency. J Clin Invest. 2015 Nov 2;125(11):4135–48. doi: 10.1172/JCI80477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Browne SK. Anticytokine autoantibody-associated immunodeficiency. Annu Rev Immunol. 2014;32:635–57. doi: 10.1146/annurev-immunol-032713-120222. [DOI] [PubMed] [Google Scholar]
- 38.Edelman EA, Girirajan S, Finucane B, Patel PI, Lupski JR, Smith ACM, et al. Gender, genotype, and phenotype differences in Smith-Magenis syndrome: a meta-analysis of 105 cases. Clin Genet. 2007 Jun;71(6):540–50. doi: 10.1111/j.1399-0004.2007.00815.x. [DOI] [PubMed] [Google Scholar]
- 39.Hodapp RM, Fidler DJ, Smith ACM. Stress and coping in families of children with Smith-Magenis syndrome. J Intellect Disabil Res. 2002 Jan 5;42(5):331–40. doi: 10.1046/j.1365-2788.1998.00148.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure E1: A detailed account of the reactivities of SMS sera versus control sera to 337 antigens. A heat map displays reactivities of sera from 18 SMS subjects 14 healthy unrelated and 3 related controls to the 337 listed antigens. Colorimetric differences on the heat map correspond.
Figure E2: Persistent verrucous disease of the hand (A) and foot (B) of SMSY4.
Figure E3: Bivariate analysis of serum reactivity to tetanus toxoid by conventional laboratory testing versus antigen microarray reveals fair concordance. Linearity (R2) and statistical significance (P) calculated via linear regression analysis. MFI, mean florescence intensity.
Table E1- Location, size and relevant genes contained within the deleted regions of 25 SMS blood donors.
Table E2: History of autoimmune diseases in 76 SMS subjects (ages 6 months-37 years).
Table E3: Atopic history in 76 SMS subjects (ages 6 months-37 years).
Table E4: Relative frequencies of lymphocyte subsets in the peripheral blood of 19 SMS subjects.


