ABSTRACT
Upregulation of programmed death ligand 1 (PD-L1) is a mechanism of immune escape utilized by a variety of tumors. PD-L1 expression in tumor cells or in the surrounding infiltrate correlates with clinical responsiveness to novel therapies targeting the PD-1/PD-L1 immune checkpoint. In the context of HIV-1 infection, Kaposi's sarcoma (KS) is largely responsive to restoration of immunity following combination antiretroviral therapy (cART), but there is a subset that is not. We hypothesized that this subset of cART-refractory KS may utilize the PD-L1 pathway of immune escape. We found that PD-L1 expressing KS had a denser CD8+ T cell (p = 0.03) and PD-L1 positive macrophage peritumoral infiltrate (p = 0.04) to suggest the involvement of PD-L1 in shaping an immune-tolerogenic microenvironment in cART-refractory KS. The presence of PD-L1 expression in association with immune-infiltrating cells provides rationale for the clinical development PD-1/PD-L1-targeted checkpoint inhibitors in cART-refractory KS.
KEYWORDS: cART, HIV, KS, microenvironment, PD-L1
Programmed death ligand 1 (PD-L1, also called B7H1) is an immunosuppressive co-stimulatory molecule that interacts with the T-cell co-receptor, programmed death-1 (PD-1), inhibiting T-cell proliferation and promoting T-cell apoptosis in peripheral tissues.1,2 Physiologically, this interaction limits T-cell-mediated inflammatory responses to infection and restricts autoimmunity.3,4 PD-L1 is upregulated in many different tumor types, where it inhibits local antitumor T-cell responses, thus maintaining the immunosuppressive tumor microenvironment.5,6 Furthermore, PD-1 is expressed on the majority of tumor-infiltrating lymphocytes.7 In recent years, agents targeting the PD-L1/PD-1 pathway have been of major interest as a novel immunotherapeutic approach to cancer therapy.8 The remarkable success of these therapies in early trials in otherwise untreatable cancers, most notably melanoma, renal-cell carcinoma and non-small-cell lung carcinoma,9-11 led to them being named “Breakthrough of the Year” by Science journal in 2013.12
Kaposi's sarcoma (KS) is a mesenchymal tumor caused by an infection by human herpesvirus 8 (HHV8), usually in the context of immunosuppression. The outbreak of the HIV-1 epidemic led to an exponential rise in the number of KS cases, a trend that was partially reversed by the introduction of combination antiretroviral therapy (cART).13 Although the majority of KS cases presenting in cART-naive HIV-1-positive patients can be successfully treated by cART-mediated restoration of immunity, a small number present with new lesions despite plasma HIV-1 RNA load suppression and respectable CD4+ T-cell counts.14-19 In this study, we sought to determine whether, in these cases, PD-L1 overexpression by KS tumor cells or the surrounding microenvironment may influence antitumor immune-escape in these tumors that are otherwise uniquely responsive to immune restoration.
From our prospectively maintained database of 688 patients with HIV-associated KS diagnosed in the post cART era (1996–2016) at the National Centre for HIV Malignancy, Chelsea and Westminster Hospital, UK, a total of 115 patients were established on cART for >3 mo at the time of KS diagnosis, of whom 56 had undetectable plasma HIV viral load. From this patient cohort, we prospectively recruited 10 patients who had progressive or relapsing KS despite being on established, successful cART following written, informed consent. Ethical approval was obtained from Riverside Research Ethics Committee.
The cases selected for this study are distinct from KS in the context of immune reconstitution inflammatory syndrome, where there is a paradoxical worsening of pre-existing KS or unmasking of subclinical KS following cART-mediated immune restoration.19 All patients were male (age range: 33–59 y), the median time since HIV-1 diagnosis was 9 y (range: 2–26), all had undetectable plasma HIV-1 viral loads, and all but two had CD4+ counts above 450 cells/mm3 (Table 1). None of the patients had any other AIDS-defining illness.
Table 1.
Expression of programmed death ligand 1 (PD-L1) in Kaposi's sarcoma (KS).
| Patient | Age | Years on cART | Years HIV positive | Years since first KS diagnosed | Peripheral blood CD4+ count (cells/mm3) | Peripheral blood CD8+ count (cells/mm3) | KS case or normal skin control | Amount of tumor | PD-L1 tumor-cell expression | PD-L1-expressing macrophages | Tissue CD8+ count (cells/mm2) | KS Staging (ACTG criteria) | KS grade | Plasma HHV8 viral load copies/mL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S926 | 53 | 2.8 | 26.3 | 1.3 | 499 | 830 | Case | Small | 0 | Absent | 76 | T1I0S0 | Patch | 42400 |
| Control | Absent | 0 | Absent | 24 | ||||||||||
| M907 | 59 | 16.2 | 16.3 | 13.7 | 111 | 1101 | Case | Small | 0 | Rare | 106 | T1I0S0 | Plaque | 0 |
| Control | Absent | 0 | Absent | 32 | ||||||||||
| M515 | 54 | 10.5 | 13.2 | 13.2 | 913 | 482 | Case | Abundant | 1+ | Numerous | 292 | T1I0S0 | Nodular | 0 |
| Control | Absent | 0 | Absent | 15 | ||||||||||
| M610 | 49 | 9.0 | 9.9 | 3.0 | 574 | 820 | Case | Moderate | 1+ | Rare | 44 | T1I0S0 | Nodular | 0 |
| Control | Absent | 0 | Absent | 5 | ||||||||||
| L292 | 37 | 1.2 | 1.2 | 1.2 | 298 | 1497 | Case | Abundant | 1+ | Moderate | 225 | T1I1S1 | Nodular | 0 |
| Control* | Focal | 1+ (focal) | Rare | 111 | ||||||||||
| S371 | 58 | 8.3 | 8.3 | 8.3 | 677 | 961 | Case | Focal | 0 | Absent | 36 | T1I0S1 | Lymphangiectatic | 0 |
| Control | Absent | 0 | Absent | 15 | ||||||||||
| L432 | 45 | 0.8 | 4.4 | 0.8 | 459 | 709 | Case | Small | 1+ (focal) | Rare | 190 | T0I0S0 | Plaque | 1650 |
| Control | Absent | 0 | Absent | 13 | ||||||||||
| R366 | 39 | 11.0 | 12.0 | 11.1 | 1200 | 961 | Case | Small | 0 | Absent | 72 | T0I0S0 | Patch | 0 |
| Control | Absent | 0 | Absent | 7 | ||||||||||
| G099 | 43 | 10.5 | 13.4 | 0.1 | 1219 | 732 | Case | Abundant | 1 | Rare | 156 | T0I0S0 | Plaque | 0 |
| Control | Absent | 0 | Absent | 34 | ||||||||||
| G963 | 33 | 1.0 | 2.0 | 0.9 | 661 | 738 | Case | Absent | 0 | Absent | 22 | T0I0S0 | Patch | 541 |
| Control | Absent | 0 | Absent | 23 |
This table summarizes the results of histological examination of Kaposi's sarcoma (KS) biopsies (cases) and normal skin controls from 10 patients with KS despite established, successful antiretroviral therapy, undetectable plasma HIV-1 RNA load and good CD4+ T-cell counts. The amount of KS tumor in each was semi-quantitatively assessed. Expression of programmed death ligand 1 (PD-L1) by tumor-cells was judged according to a scoring system from 0 to 3+. No tumors showed expression greater than 1+ (any staining above background). The number of tumor-infiltrating PD-L1-expressing macrophages was semi-quantitatively assessed. The number of tumor-infiltrating CD8+ T cells was calculated as cells per mm2.
indicates this control biopsy contained a focal area of tumor not clinically apparent.
Biopsies were taken from areas of visible cutaneous tumor (cases) and adjacent normal skin (controls), fresh frozen in liquid nitrogen, and then fixed in buffered formalin. Following review of cases and controls on freshly cut hematoxylin and eosin (H&E) sections, immunostaining for PD-L1, CD68 (to identify tumor-infiltrating macrophages) and CD8+ (to identify tumor-infiltrating cytotoxic T lymphocytes) was performed by MEDTOX Scientific Inc., Minneapolis, USA. Full protocols are available in the Supplementary methods. Human tonsil was used as a positive control for each reaction. Semi-quantitative grading of PD-L1 expression was performed by a single pathologist based on a scoring scale from 0 to 3+ previously validated for reproducibility in other PD-L1-expressing tumors. The presence of PD-L1-expressing, CD68-positive macrophages was recorded on a semi-quantitative scale (absent/rare/moderate/numerous). Manual microscope counts of CD8+ T lymphocytes within the entire dermis of biopsies were recorded and converted to a CD8+ count per mm2. The amount of KS present in each biopsy was characterized semi-quantitatively (absent/focal/moderate/abundant) according to H&E staining as shown in Table 1. One of the control biopsies (L292) contained a focal area of tumor that had not been apparent to the naked eye.
We observed evidence of PD-L1 expression in KS cells in a total of 5 out of 10 cases, where the pattern of immunopositivity was cytoplasmic in all cases and judged level 1+ (Fig. 1). One case (L432) showed focal level 1+ tumor-cell PD-L1 staining and the focal tumor observed in the control biopsy from patient L292 also showed focal level 1+ PD-L1 staining. All other cases and controls were judged negative. PD-L1-expressing macrophages were observed in six cases and the one control that contained focal tumor (control L292). In four cases and the L292 control, they were rare in number, in one case (L292) they were moderate and in one case (M515) they were numerous.
Figure 1.
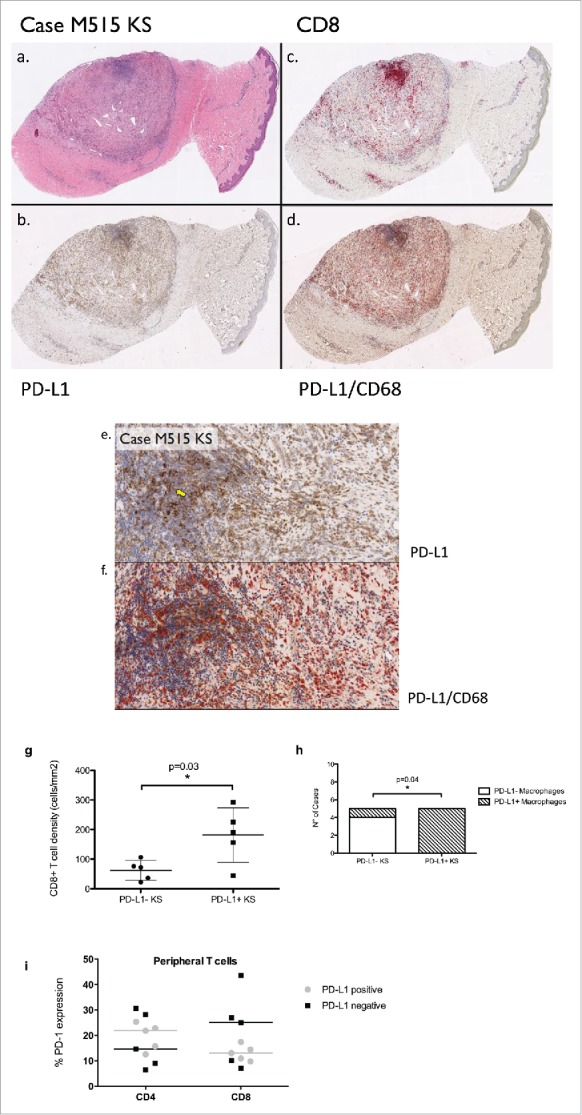
A) Hemotoxylin and eosin, showing the presence of abundant Kaposi's sarcoma tumor spindle cells. (B) Programmed death ligand 1 (PD-L1) expression, showing tumor-cell expression of PD-L1 that was judged to be 1+ in our scoring system, representing weak cytoplasmic expression above baseline. (C) CD8+ staining shows the infiltration of cytotoxic T lymphocytes. (D) Dual staining with PD-L1 and CD68 shows the presence of numerous tumor-infiltrating PD-L1-expressing macrophages. (E, F) Representative sections of PD-L1 positive KS (E) and CD68/PD-L1 co-immunoexpression (F) at high magnification (200×). A yellow arrow identifies areas of immunopositive KS. (G) The positive relationship between PD-L1 positivity in KS cells and CD8+ peritumoral immune infiltrate. (H) The positive relationship between PD-L1 positivity in KS and in the surrounding PD-L1 expressing macrophage infiltrate. (I) The relationship between PD-L1 expression in KS and PD-1 expression in peripheral CD4+ and CD8+ T cells. Bars are representative of median values of PD-1 expression in PD-L1 positive (gray) and PD-L1 negative (black) KS samples.
Tumor-infiltrating CD8+ lymphocytes in histologically identifiable tumors ranged from 44 to 292 cells/mm2 (mean = 133.0), and in controls ranged from 5 to 34 cells/mm2 (mean = 18.7). Cases that showed cytoplasmic tumor-cell PD-L1 expression (n = 5) had significantly higher levels of CD8+ T-cell infiltrate (mean = 181.4, standard error = 41.0) compared with PD-L1 negative counterparts (n = 5, mean 62.4, standard error = 15.0, p = 0.03). Similarly, we found a positive association tumor-cell PD-L1 expression and the presence of a PD-L1-positive macrophage infiltrate (p = 0.04). We found no association between PD-L1 expression and peripheral CD4+ count, HIV viral load or years on cART. Because peripheral immune responses are being explored as a surrogate biomarker of antitumor activity in prospective clinical trials of anti-PD-1/PD-L1 checkpoint inhibitors, we performed phenotypical analysis of PD-1 expressing peripheral T cells isolated at the moment of the biopsy to detect differences in PD-1 expression in patients with PD-L1 positive (n = 5) and negative KS (n = 5). Median PD-1 expression in peripheral lymphocytes expressed as percentage of immunopositive cells was 18.7 in CD4+ (range 8.9–30.6) and 13.7 in CD8+ cells (range 7–43.5). We found no association between PD-1 expression in CD4+ (p = 0.84) or CD8+ cells (p = 0.54) and KS PD-L1 status (Fig. 1G and H).
PD-L1 is upregulated by many cell types (epithelial, hematopoietic, and endothelial) in response to pro-inflammatory cytokines.20 PD-L1 expression in either tumor cells or surrounding infiltrate has emerged as a predictive correlate of response to PD-1/PD-L1-targeted checkpoint inhibitors in a growing variety of solid tumors.9 Hence, there is strong reason to seek to identify other tumors that may be using the same immune escape mechanism and therefore benefit from these remarkable novel therapies. A recent study investigated PD-L1 expression in a wide range of lymphomas and virus-associated malignancies.21 They reported robust upregulation of PD-L1 in most lymphoma sub-types, including several Epstein Barr virus-related malignancies and HHV8-associated primary effusion lymphoma. They did not detect expression of PD-L1 in nine KS cases. In contrast to these findings, a second case-series of sarcomas including five KS samples has shown high prevalence of PD-L1 expression in 80% of KS patients.22 However, in both studies, cases were collected retrospectively and were not selected according to any specific patient or disease characteristics, which is likely to account for the high inter-study heterogeneity in PD-L1 expression.
As such, the true prevalence of PD-L1 expression in the cART-refractory KS population, in whom systemic anticancer treatment is indicated to alter the natural progression of the disease, is not known. This is a significant limitation to an efficient planning of clinical studies of immune checkpoint inhibitors, where PD-L1 expression has been utilized as a predictive correlate of response. In addition, the relative rarity of the disease makes it particularly difficult to qualify PD-L1 as a novel therapeutic target in the specific setting of cART refractoriness, where previous studies investigating PD-L1 expression have been elusive.21,22
Our study, although preliminary in nature and limited by sample size, is uniquely different because of the strict selection criteria applied to define our population and the prospective nature of patient accrual, where cART refractoriness was confirmed using uniform and pre-defined follow-up schedules. In attempting to investigate specifically whether PD-L1 upregulation could explain cART refractoriness, we confirmed a high prevalence of PD-L1 upregulation, where evidence of weak cytoplasmic PD-L1 expression was seen in 50% of patients with cART refractory KS. While different from the intense membranous expression pattern expression seen in epithelial malignancies that are clinically responsive to PD-L1/PD-1 blockade,23 PD-L1 positive KS was associated with higher T-cell infiltrate and macrophage recruitment, hallmarks of an exhausted antitumor-immune response. Importantly, the lack of association found between PD-L1 positivity and HIV infection-specific parameters including peripheral CD4+ counts, HIV RNA load and duration of infection further strengthens the pathophysiologic relevance of the local tolerogenic environment as a mechanism of KS progression independent from the underlying HIV infection control. Interestingly, PD-1 expression in peripheral blood lymphocytes did not correlate with PD-L1 positivity in KS cells, suggesting a divergence between peripheral and peri-tumoral immune responses, an important finding in the qualification of surrogate biomarkers of the antitumor immune response. A limitation of our work that should be addressed in future studies is the lack of a pairwise, prospective comparison with cART-sensitive KS, where regulation of immune-tolerogenic signals in the tumor and the surrounding microenviroment might be different and could explain the discrepancy in PD-L1 expression reported in previous studies.21 Similarly, our pilot study supports but does not definitely prove an involvement of PD-L1 in shaping the antitumor immune response, whose pathophysiology is complex and determined by a wide spectrum of molecular mediators including cytokines and chemokines that can upregulate PD-L1 expression as a mechanism of immune-adaptation.
While we lack comprehensive phenotypic characterization of tumor-infiltrating lymphocytes, where other immune-modulatory pathways or cell populations including regulatory T cells and NK cells might play a role,24 our results are provocative in suggesting PD-L1 as a potential immune-tolerogenic mechanism underlying the pathogenesis and progression of cART-refractory KS, suggesting a potential role as a therapeutic target in this disease. Our preliminary findings require confirmation in prospective, multi-center clinical studies.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by an educational grant from Chelsea and Westminster Joint Research Committee and a non-interventional clinical research grant from ViiV Healthcare Limited. DJP is supported by the National Institute for Health Research (NIHR) as well as grant funding from the Academy of Medical Sciences and the Imperial NIHR Biomedical Research Centre (BRC).
References
- 1.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999; 5:1365-9; PMID:10581077; http://dx.doi.org/ 10.1038/70932 [DOI] [PubMed] [Google Scholar]
- 2.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC et al.. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000; 192:1027-34; PMID:11015443; http://dx.doi.org/ 10.1084/jem.192.7.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006; 439:682-7; PMID:16382236; http://dx.doi.org/ 10.1038/nature04444 [DOI] [PubMed] [Google Scholar]
- 4.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26:677-704; PMID:18173375; http://dx.doi.org/ 10.1146/annurev.immunol.26.21607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K et al.. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8:793-800; PMID:12091876; http://dx.doi.org/ 10.1038/nm0902-1039c [DOI] [PubMed] [Google Scholar]
- 6.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 2008; 8:467-77; PMID:18500231; http://dx.doi.org/ 10.1038/nri2326 [DOI] [PubMed] [Google Scholar]
- 7.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009; 114:1537-44; PMID:19423728; http://dx.doi.org/ 10.1182/blood-2008-12-195792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012; 24:207-12; PMID:22236695; http://dx.doi.org/ 10.1016/j.coi.2011.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/ 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K et al.. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122-33; PMID:23724867; http://dx.doi.org/ 10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K et al.. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455-65; PMID:22658128; http://dx.doi.org/ 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013; 342:1432-3; PMID:24357284; http://dx.doi.org/ 10.1126/science.342.6165.1432 [DOI] [PubMed] [Google Scholar]
- 13.Rubinstein PG, Aboulafia DM, Zloza A. Malignancies in HIV/AIDS: from epidemiology to therapeutic challenges. AIDS 2014; 28:453-65; PMID:24401642; http://dx.doi.org/ 10.1097/QAD.0000000000000071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bower M, Weir J, Francis N, Newsom-Davis T, Powles S, Crook T, Boffito M, Gazzard B, Nelson M. The effect of HAART in 254 consecutive patients with AIDS-related Kaposi's sarcoma. AIDS 2009; 23:1701-6; PMID:19550283; http://dx.doi.org/ 10.1097/QAD.0b013e32832d080d [DOI] [PubMed] [Google Scholar]
- 15.Maurer T, Ponte M, Leslie K. HIV-associated Kaposi's sarcoma with a high CD4 count and a low viral load. N Engl J Med 2007; 357:1352-3; PMID:17898112; http://dx.doi.org/ 10.1056/NEJMc070508 [DOI] [PubMed] [Google Scholar]
- 16.Daly ML, Fogo A, McDonald C, Morris-Jones R. Kaposi sarcoma: no longer an AIDS-defining illness? A retrospective study of Kaposi sarcoma cases with CD4 counts above 300/mm(3) at presentation. Clin Exp Dermatol 2014; 39:7-12; PMID:23691969; http://dx.doi.org/ 10.1111/ced.12163 [DOI] [PubMed] [Google Scholar]
- 17.Mocroft A, Furrer HJ, Miro JM, Reiss P, Mussini C, Kirk O, Abgrall S, Ayayi S, Bartmeyer B, Braun D et al.. The incidence of AIDS-defining illnesses at a current CD4 count ≥ 200 cells/μL in the post-combination antiretroviral therapy era. Clin Infect Dis 2013; 57:1038-47; PMID:23921881; http://dx.doi.org/ 10.1093/cid/cit423 [DOI] [PubMed] [Google Scholar]
- 18.Unemori P, Leslie KS, Hunt PW, Sinclair E, Epling L, Mitsuyasu R, Effros RB, Dock J, Dollard SG, Deeks SG et al.. Immunosenescence is associated with presence of Kaposi's sarcoma in antiretroviral treated HIV infection. AIDS 2013; 27:1735-42; PMID:23435301; http://dx.doi.org/ 10.1097/QAD.0b013e3283601144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robey RC, Bower M. Facing up to the ongoing challenge of Kaposi's sarcoma. Curr Opin Infect Dis 2015; 28:31-40; PMID:25490104; http://dx.doi.org/ 10.1097/QCO.0000000000000122 [DOI] [PubMed] [Google Scholar]
- 20.Wilke CM, Wei S, Wang L, Kryczek I, Kao J, Zou W. Dual biological effects of the cytokines interleukin-10 and interferon-gamma. Cancer Immunol Immunother 2011; 60:1529-41; PMID:21918895; http://dx.doi.org/ 10.1007/s00262-011-1104-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, Yu H, Fletcher CD, Freeman GJ, Shipp MA et al.. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res 2013; 19:3462-73; PMID:23674495; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-0855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paydas S, Bagir EK, Deveci MA, Gonlusen G. Clinical and prognostic significance of PD-1 and PD-L1 expression in sarcomas. Med Oncol 2016; 33:93; PMID:27421997; http://dx.doi.org/ 10.1007/s12032-016-0807-z [DOI] [PubMed] [Google Scholar]
- 23.Pinato DJ, Shiner RJ, White SD, Black JR, Trivedi P, Stebbing J, Sharma R, Mauri FA. Intra-tumoral heterogeneity in the expression of programmed-death (PD) ligands in isogeneic primary and metastatic lung cancer: Implications for immunotherapy. Oncoimmunology 2016; 5:e1213934; PMID:27757309; http://dx.doi.org/ 10.1080/2162402X.2016.1213934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beldi-Ferchiou A, Lambert M, Dogniaux S, Vely F, Vivier E, Olive D et al.. PD-1 mediates functional exhaustion of activated NK cells in patients with Kaposi sarcoma. Oncotarget 2016; 7:72961-77. PMID: 27662664; http://dx.doi.org/ 10.18632/oncotarget.12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


