Abstract
The mid-day nap, sometimes called a siesta, is a ubiquitous occurrence across the lifespan. It is well established that in addition to reducing sleepiness, mid-day naps offer a variety of benefits: memory consolidation, preparation for subsequent learning, executive functioning enhancement, and a boost in emotional stability. These benefits are present even if a sufficient amount of sleep is obtained during the night prior. However, we present a paradox: in spite of these reported benefits of naps, frequent napping has also been associated with numerous negative outcomes (eg, cognitive decline, hypertension, diabetes), particularly in older populations. This association exists even when statistically controlling for relevant health- and sleep-affecting determinants. An emerging hypothesis suggests inflammation is a mediator between mid-day naps and poor health outcomes, yet further research is necessary. Given this, it may be premature to ‘prescribe’ naps as a health enhancer. Herein, we aggregate findings from several branches of sleep research (eg, developmental neuroscience, cognitive neuroscience, sleep medicine) to critically examine the paradoxical role of naps in cognitive and somatic health. This review uncovers gaps in the literature to guide research opportunities in the field.
Keywords: Nap, cognition, learning, memory, emotion, inflammation
1. Introduction
The mid-day nap, sometimes called a siesta, is ubiquitous. Naps are most frequent in infancy and into toddlerhood [1]. Young adult naps are less frequent, depending on cultural expectations, geographic location [2], and employment status [3]. In late life, especially after retirement, napping again becomes more prevalent [4], either because of age-related changes in sleep and circadian rhythmicity or because of psychosocial or psychological changes (eg, more free time, higher incidence of depression) [5].
The cognitive benefits of a mid-day nap have become more apparent in recent years. Naps facilitate executive functioning [6,23,24], memory formation [10–18] subsequent learning [19,20] and emotional processing [21–25]. Yet, paradoxically, there are also a multitude of studies linking frequent napping with negative outcomes, especially in older populations [26–29].
Here we review recent research that has unveiled the unique properties of naps and their functional contribution to cognitive and emotional processing. We first characterize the physiological architecture of naps. Next, we turn to behavioral studies that provide evidence of the beneficial functions naps serve. We then review evidence that naps may be detrimental to health, including evidence that inflammation may be related to naps and health outcomes. Finally, we discuss the implications of napping and examine whether napping should be prescribed to enhance health.
1.1 Physiology of naps
1.1.1 Nap architecture
Sleep is not homogenous; rather, it is composed of multiple physiologically unique stages. Non-rapid eye movement (NREM) stages, which are further divided into stage 1 (N1), stage 2 (N2), and stage 3 (N3 or slow wave sleep (SWS)), is associated with low energy expenditure and high neuronal synchronization [30]. Conversely, rapid eye movement (REM) sleep is associated with high brain activity and energy expenditure comparable to wake.
Only recently has the physiology of naps in healthy individuals been considered. In infants, naps are indistinguishable from nocturnal sleep, as both are REM-rich [1] (Figure 1). Later during early childhood, naps are predominantly composed of NREM sleep with very little REM [31]. Young adult naps, if of substantial length, will contain both NREM and REM bouts [32]. Naps of older adults are dominated by lighter NREM stages, a short bout of SWS, and less often, REM sleep [10].
Figure 1.
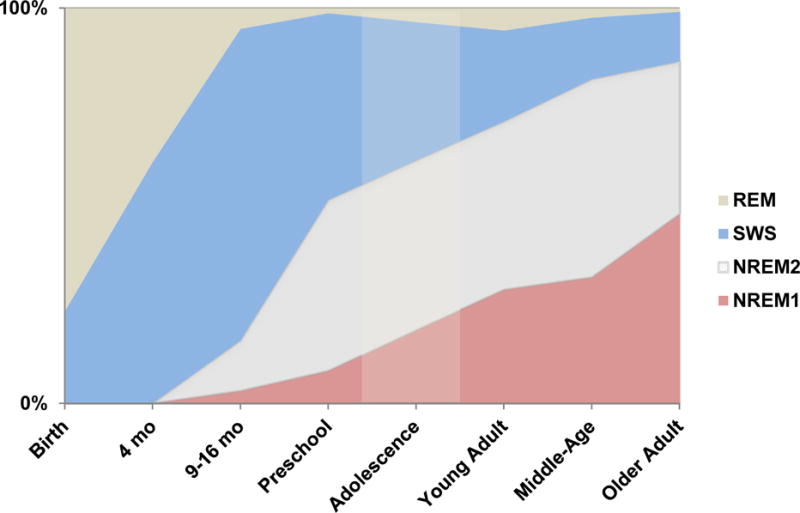
Nap architecture from infancy to older adulthood. Dimmed region represents extrapolated data. [1,10,31,124–126]
1.1.2 Homeostatic pressure and circadian rhythmicity govern daytime sleep characteristics
Sleep is hypothesized to be regulated by two processes: Process S, which reflects homeostatic ‘sleep pressure,’ and Process C, which constitutes circadian (ie, endogenous) rhythmicity [33]. On a simplified level, process S has been hypothesized to be the result of extracellular adenosine accumulation [34], which intensifies with the amount of time spent awake. Process C, on the other hand, has been hypothesized to be the result of genetically-driven changes in alertness via the suprachiasmatic nucleus of the hypothalamus [33], in addition to other factors (eg, the REM-on and REM-off switch posited to be in the tegmentum of the brainstem [35,36]). Process C cycles non-linearly, and troughs of alertness typically occur during the night and postprandially (ie, after lunch). Sleep pressure accumulated during a normal day, via Process S, is believed to initiate the onset of NREM sleep upon sleep onset [17]. Process C modulates REM sleep. Circadian influences, which affect core temperature and hormonal fluctuations, modulate REM onset, both during the night and the day.
The effects of Processes S and C on sleep are important to understanding nap sleep architecture (ie, sleep staging). Given the influence of sleep pressure on NREM, naps taken following sleep deprivation or those taken later in the day comprise mostly NREM sleep [37]. On the other hand, circadian rhythmicity resulting from Process C induces REM-rich naps early in the day. The post-prandial nap, which occurs during a circadian alertness dip but also after many hours spent awake, tends to contain both NREM and REM, although this may vary with age[10].
2. Naps benefit cognitive functions
2.1 Sleepiness and cognition
Following sleep deprivation, sleep restriction, or even a normal night of sleep, sleepiness increases with time spent awake, while cognitive abilities, such as working memory, decrease. However, a mid-day nap has been shown to effectively assist with ‘recovery’ of these faculties by minimizing homeostatic sleep pressure.
2.2.1 Homeostatic sleep pressure
The search for which “sleep factor” contributes to the rise and dissipation of homeostatic sleep pressure has been lengthy. Much evidence points to adenosine, a byproduct of cellular energy and metabolism (ie, hydrolysis of adenosine tri-phosphate [ATP]), and a neuromodulator that orchestrates the release of post-synaptic neurotransmitters [38], as being a critical sleep factor [34]. In theory, when cerebral energy (ie, glycogen) is required, glycogenolysis takes place, leaving an adenosine byproduct. During subsequent NREM sleep, the activity of neurotransmitters that heavily utilize glycogen during wake is reduced, and synthesis of new glycogen stores can begin. Accumulated adenosine dissipates to provide energy for glycogen replenishment. Thus, after a sufficient amount of NREM, homeostatic sleep pressure is reduced, and the process may begin anew (Figure 2).
Figure 2.
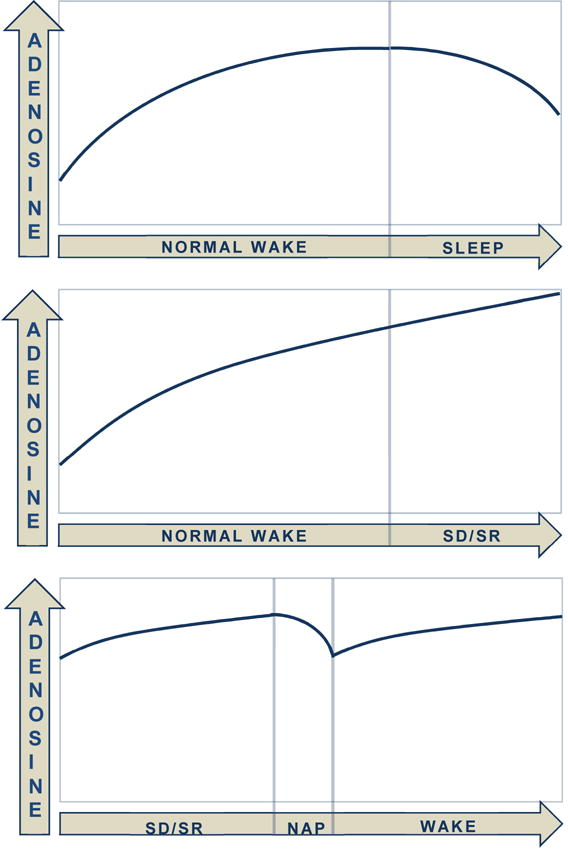
Adenosine, one of the so called “sleep factors,” is thought to accumulate and dissipate depending on the state [34]. SD = sleep deprivation; SR = sleep restriction.
However, adenosine does not affect the brain uniformly, as there are several adenosine receptor types (ie, A1, A2a, A2b and A3) with differing downstream effects [39]. Sleep-impacting effects of adenosine seem to mainly involve A1 and A2a receptors. For example, blocking A1 receptors decreases sleep [40], whereas infusing adenosine to A1 receptors promotes sleep [41]. Further, SWS is induced when A2a receptors in the subarachnoid space below the basal forebrain are promoted [42]. Additional evidence for both receptor types playing a role in sleep regulation comes from studies showing caffeine promotes wakefulness by blocking adenosine’s activation of both A1 and A2a receptors [39]. A2b and A3 receptors have a relatively low affinity for adenosine and their sleep-promoting effects, if any, are poorly understood.
Although adenosine has been the focus of many recent studies, it is not the only identified sleep factor. Several other chemicals, including interleukin-1, tumor necrosis factor-alpha, GH-releasing factor, and prostaglandin D2, have also been identified as potential sleep factors and likely contribute to these processes [43,44]. However, work on the relative contribution of each factor (and potential interactions between factors) remains limited, and thus is a promising future direction.
2.2.2 Naps reduce homeostatic sleep drive
Homeostatic drive can be quantified through EEG delta activity (1–4 Hz, also known as slow wave activity (SWA)). Naps following sleep deprivation/restriction are architecturally distinct from naps that follow normal nocturnal sleep such that SWA is elevated. However, SWA and sleep pressure are reduced by a recovery nap [45]. At times, naps reduce sleep pressure so thoroughly that subsequent nocturnal sleep can be disturbed, even if occurring hours later. Thus, even a brief daytime nap may restore the Process S imbalance that has been altered by extended wakefulness.
If a recovery nap is successful, the negative effects of wakefulness, including sleepiness and poor cognitive functioning, should be reduced. Indeed, this is the case, as naps reduce both subjective and objective sleepiness, gauged via questionnaire or psychomotor vigilance test [46], respectively, after sleep restriction. However, recovery depends on the architecture of the preceding nap. ‘Ultrashort’ naps minimize homeostatic sleep drive and sleepiness, yet naps containing SWS increase sleepiness immediately after waking [7]. This phenomenon is called sleep inertia, and many investigations have attempted to ‘maximize’ sleepiness reduction by increasing alertness while avoiding sleep inertia. Overall, it seems lighter stages of sleep benefit alertness immediately after waking, while deeper stages benefit long-term alertness (Figure 3).
Figure 3.
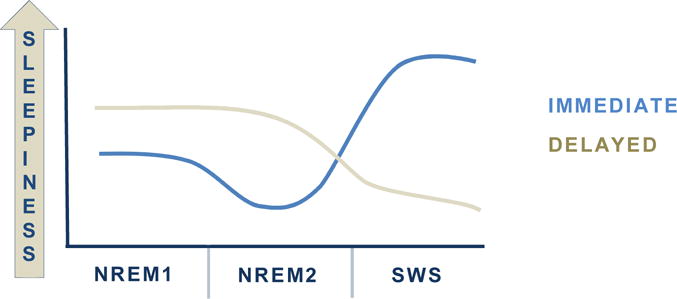
Nap architecture impacts immediate and long-term sleepiness following a nap. A brief nap with ligher sleep stages (NREM1 and NREM2) reduces sleepiness immediately after the nap, while a nap containing SWS may bring on sleep inertia [7,8]. However, there is a delayed benefit of naps that contain SWS. After sleep inertia has dissipated, sleepiness is lower for longer.
For example, a study investigating the effects of differing nap lengths (5, 10, 20 and 30 minutes) found a 10-minute nap increased immediate alertness to a greater extent than the other nap durations [7]. Both the 5 and 10 minute naps predominantly contained N2, but naps longer than 10 minutes were more likely to contain SWS. The authors concluded that a sustained period of N2 (> 4 minutes) or a 10-minute period of any combination of stages are sufficient to reduce immediate sleepiness, whereas SWS does the opposite. This notion has been corroborated by investigations showing decreased sleepiness immediately upon wakening when naps only contain N1 and N2 [6].
Although SWS may induce inertia immediately after waking, it may provide longer-lasting benefits than N1 and N2. For example, following the dissipation of sleep inertia, sleepiness is reduced to the extent that it would have been if a shorter nap without SWS had occurred [8], and this reduction can be maintained for an extended period of time [6] (Figure 3).
2.2.3 Naps improve executive functioning
In addition to minimizing sleepiness, napping also promotes executive functioning (eg, working memory). Both reduced sleepiness and improved cognition rely on adenosine A1 and A2A receptors (discussed below), and therefore it is difficult to separate these mechanisms. That is, one possible avenue through which cognition is improved following a nap is through reduced sleepiness. Alertness (ie, reduced sleepiness) plays a large role in cognitive performance [47], and thus this relationship seems viable. As such, in almost every investigation discussed, both faculties improve simultaneously [7,8,46,48]. However, the impact of adenosine on executive functioning may be separate from that governing sleepiness, as A1 receptor agonist injection into the hippocampus, a critical region for encoding, promotes working memory in rodents [49]. There is also evidence of an interaction of adenosine and dopamine in brain regions involved in executive functioning (ie, cortico-striatal regions) reviewed in [50]. Thus, naps may improve cognition and minimize sleepiness through separate pathways.
2.3 Memory and emotional regulation
During wake, sensory information, facts, and procedural skills are continually acquired. After acquisition, certain information should be permanently retained while other information is discarded to enhance subsequent information capacity. We propose napping facilitates this process in two ways: (1) by solidifying previously-learned information in long-term storage (ie, consolidation), and (2) by enhancing subsequent learning capacity.
2.3.1 Naps promote memory consolidation
Sleep is thought to facilitate memory consolidation [51]. Consolidation is evidenced by enhanced retention of memory traces or skills compared to an equivalent period spent awake. Many investigations have examined the effects of sleep-dependent consolidation over a period of nocturnal sleep. Yet a brief nap can also enhance consolidation beyond waking levels, and specific sleep stages within a nap provide information of underlying brain processes that facilitate this activity.
2.3.2 SWS and declarative memory consolidation
The encoding of declarative memory traces relies on the hippocampus [52], and consolidation of such information is also hippocampal-dependent. There are multiple hypotheses as to how consolidation occurs. According to the systems level hypothesis, encoding occurs in both the hippocampus and the neocortex, and during consolidation, memory traces are solidified in the neocortex while the hippocampal role is reduced [53]. Conversely, the multiple trace theory posits that the hippocampus is still ‘linked’ with the consolidated memory traces, and thus the hippocampus and neocortex continue to play a role post-consolidation [54,55]. Nevertheless, in both of these models, SWS has been implicated in facilitating memory consolidation [56]. Notably, the opportunistic hypothesis states consolidation occurs whenever the hippocampus is ‘quiet’ ie, free from interference), and therefore SWS is the opportune time at which consolidation occurs [57].
Memory consolidation is promoted when neurons activated in the hippocampus during encoding are reactivated during subsequent SWS [58], and this reactivation is believed to facilitate the solidification of information in the neocortex. Additionally, neurotransmitter fluctuation during SWS is optimal for consolidation [59]. During wake, levels of circulating neurotransmitters, including acetylcholine, are high, yet acetylcholine drops during SWS. Incidentally, low acetylcholine is necessary for consolidation to occur. The administration of physostigmine, which blocks cholinergic inhibition, disrupts sleep-dependent consolidation[60], but only for declarative memory traces, suggesting neurotransmitter tone during SWS uniquely supports declarative consolidation. Furthermore, norepinephrine has been shown to impact declarative memory consolidation [61]. Specifically, norepinephrine suppression via clonidine administration during early-night SWS disrupted memory consolidation for the sequential order of learned events in a story, relative to a placebo group. Of note, suppressing norepinephrine did not block memory consolidation for story content, and therefore, the authors suggested brain regions (eg, hippocampus and parahippocampal cortex for temporal order and perirhinal cortex for content) were differentially impacted by norepinephrine blunting. Taken together, these investigations indicate hormonal fluctuations during SWS uniquely and discreetly impact sleep-dependent memory consolidation.
There is also clear behavioral evidence of the importance of SWS in nap-dependent memory consolidation. Declarative memory is greatest following a nap containing SWS when compared to an equivalent period spent awake or a short nap with no SWS. For instance, in a nap paradigm in which participants learned bi-model paired associates (eg, a picture paired with a sound clip), participants with naps containing SWS exhibited both greater immediate and long-term (ie, after one week) memory consolidation effects [11]. Furthermore, these memories were less prone to subsequent interference, indicating SWS influenced the stability of these memories.
Additionally, we have shown greater SWA during a nap predicts better subsequent declarative memory recall and less hippocampal engagement during retrieval, signifying consolidation had occurred [10]. Notably, in older adults who have reductions in SWA, we found nap-dependent consolidation of declarative traces is reduced and hippocampal disengagement from the frontal lobe is not present. Thus, following a nap with less SWA, declarative memory traces might be at an “earlier” stage of consolidation in older adults, rendering retrieval poorer. In this population, therefore, SWS within a nap might not be sufficient for complete declarative consolidation.
Although the amount of SWS during a nap has been found to correlate with consolidation (e, sleep-dependent increases in memory performance) [12], several investigations have found that SWS alone is not sufficient for consolidation. For instance, Alger and colleagues [13] found the amount of time in SWS and the amount of time spent awake between the learning session and sleep interact to predict consolidation. A longer time spent awake following the learning session, combined with more SWS, unexpectedly maximized memory performance for learned neutral images. Further, a separate group found naps containing predominantly NREM sleep enhanced declarative memory consolidation of three distinct types of declarative memories (eg, via a paired-associates task, visuo-spatial maze task, and a complex figure-tracing task), but preferentially enhanced memories that had been encoded more strongly during the learning session [14]. The authors suggest that individuals who learn more efficiently during encoding also consolidate more efficiently, thus indicating a trait-like quality (eg, learning capacity), in addition to a state-like quality (eg, amount of SWS) influenced consolidation.
2.3.3 N2 and procedural memory consolidation
Motor skill learning has been the predominant probe of procedural memory consolidation in the field. The consolidation of procedural memory traces is anatomically distinct from declarative traces, although there may be overlap [62]. As opposed to declarative learning, which relies on the hippocampus, procedural learning relies on the bilateral motor cortex, sensorimotor cortex, and cerebellum [63]. Depending on the type of task (eg, motor sequence learning vs. motor adaptation), memories are posited to either be encoded through a cortico-striatal loop or through a cortico-cerebellar loop, respectively [64]. During subsequent sleep, reactivation is present in the same regions that were active during encoding [65]. Reactivation enhances procedural consolidation by reorganizing motor representations into more efficient procedural memories [63]. Although the hippocampal-neocortical system appears somewhat distinct from these motor networks, sleep benefits the consolidation of both types of memories equivalently.
A mid-day nap, particularly a nap rich in N2, has been shown to benefit the consolidation of skills in the procedural domain. For instance, following encoding of a finger-to-opposition task, a short nap improved performance while minimizing susceptibility to experimental interference that occurred after the nap [15], and time spent in N2 correlated with sleep-dependent gains in motor skill. Similarly, performance on a mirror-tracing task improved across a nap period, which was predominantly N2 [16]. However, the latter finding regarding N2 and motor consolidation is not always consistent. Specifically, when participants were trained on both a declarative task and a mirror-tracing task prior to a nap, only the declarative memories were consolidated, even though the nap contained a high amount of N2 (~41% of total nap time) [66]. Similarly, a separate study found a daytime nap promotes the consolidation of the perceptual, but not the motor element of a motor sequencing task [67].
Notably, in populations who are traditionally thought to exhibit sleep-dependent reduced motor consolidation (ie, children and older adults, for exceptions, see [68,69]) a mid-day nap has a “delayed” effect on motor consolidation. Specifically, in children, we have shown performance gains 24 hr after skill acquisition are present only when a nap occurs after initial skill learning [70]. This finding suggests a mid-day nap might stabilize memory traces and exhibit a delayed benefit after a nocturnal sleep period. We postulate that in the developing brain, multiple sleep bouts might be necessary for measurable consolidation to occur. An alternative or perhaps additional explanation is that in this population, both N2 and REM are necessary for motor consolidation. Given that mid-day naps in preschoolers contain little REM (Figure 1), consolidation may not emerge until after nocturnal sleep, which is rich in REM.
Likewise, older adults, who also do not exhibit sleep-dependent consolidation of motor skills, similarly show delayed benefits of a nap [71], suggesting naps play a subtle yet important role in learning in this population. Unfortunately, sleep staging was not available in either of the aforementioned studies, limiting interpretations on sleep-stage dependency.
2.3.4 N2 and Other Memory Domains
N2 has been linked with other memory domains and examining the ‘microstructure’ of nap N2 has yielded further insight into memory consolidation. Sleep spindles, which reflect firing between the thalamus and the cortex [72], are critical to N2 memory consolidation. Although originally implicated in procedural learning, sleep spindles have been linked with the consolidation of a host of memory types, including emotional and declarative memory. For example, higher spindle activity in a mid-day nap was linked to enhanced consolidation of an associative task [17] and was also shown to moderate emotional memory consolidation [73]. In preschool-aged children, we demonstrated that higher spindle density in habitual nappers was correlated with enhanced consolidation of a visuo-spatial task [31]. Lastly, a causal examination of spindle characteristics via pharmacological manipulation of sleep (Ambien, a GABAA agonist), increased sleep spindles compared to a placebo, and, in turn, yielded greater consolidation of declarative memory traces [74].
Given the diverse range of tasks linked with sleep spindles, their presence in naps seems to promote the general consolidation of knowledge (or general plasticity) rather than being specific to one type of learning. Further, it is likely that consolidation benefits from N2 characteristics in accordance with other sleep-dependent mechanisms (eg, those underlying slow oscillations and slow activity) [75].
2.3.5 REM-dependent processes
The role of REM in naps is largely unexplored, as naps contain little to no REM (Figure 1). However, recent work has demonstrated that REM plays a unique role in promoting relational memory [18]. When participants were presented with directly related images (eg, A – B and B – C) and a pair of indirectly rated images (A – C), REM sleep during a nap was positively correlated with memory for the related images, but correlated negatively with memory for the direct pairs. The authors concluded that while NREM sleep promotes veridical learning (ie, consolidation of directly associated images), REM plays a role in reorganizing and integrating information into existing schemas.
2.4 Naps enhance subsequent learning
Sleep also promotes subsequent encoding (ie, learning). Without sleep (eg, after nocturnal sleep deprivation), encoding is reduced relative to encoding after a normal night of sleep [76]. Hippocampal activation during encoding following sleep deprivation is also altered, and it has been suggested that this hippocampal dysfunction mediates the relationship between sleep loss and encoding deficits. This notion is in support of the synaptic homeostasis hypothesis [77], which suggests that sleep, namely SWS, is a state of global synaptic depotentiation. The synaptic load, potentiated during the day when learning occurs, is reduced during sleep. In this way, the stronger neuronal connections (eg, those that were heavily activated during wake) are preserved following sleep, while weaker connections are removed. The removal of weak traces provides more space and more opportunity for new encoding. Consequently, without sleep, the brain is not as well prepared for new learning to occur.
Several investigations utilizing mid-day naps have corroborated this finding (outlined in Figure 4). In the first, encoding was found to be superior following a nap relative to an equivalent interval awake [20]. Next, others demonstrated enhanced encoding following a SWA-boosted nap (via transcranial direct-current stimulation) when compared to a natural nap [19]. Notably, both investigations showed enhancements for declarative but not procedural encoding, demonstrating the essential role of SWS in preparing for subsequent declarative encoding.
Figure 4.
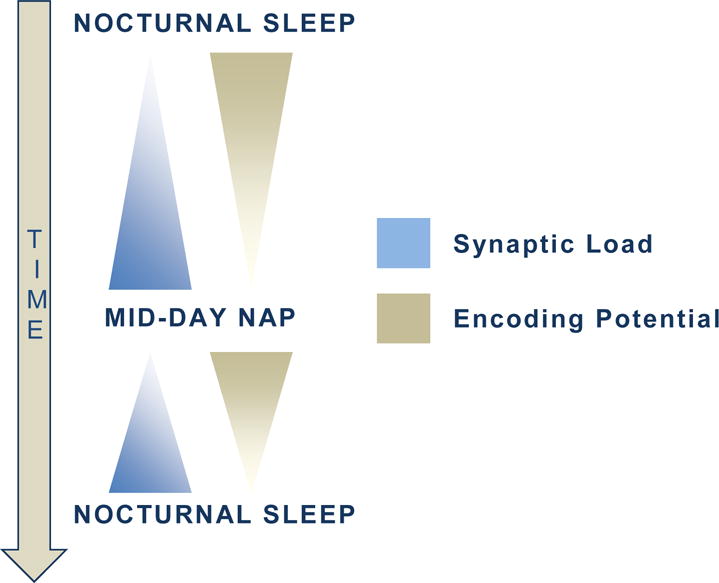
The potentiated synaptic burden accumulates with wakefulness, while encoding potential decreases. The mid-day nap reduces this burden and enhances future learning. In this way, a mid-day nap facilitates learning in 2 ways: through global downscaling (as in the synaptic homeostasis hypothesis [77]), and by boosting subsequent learning potential [19].
2.5 Naps promote emotion processing
There is a bi-directional relationship between sleep and emotion. Poor sleep quality is both an antecedent to and a consequence of poor mental health [78]. Emotional functioning, however, is complex and multi-faceted. Therefore, many investigations have probed discreet aspects of emotion (eg, reactivity), rather than complex processes (eg, regulation) [79]. Of these studies, few have probed emotion using a nap paradigm, as REM sleep, which has been implicated in emotional processing [25,80], is scarce during mid-day naps.
Recently, there have been several investigations assessing whether mid-day naps impact emotion processing in children. This population is a primary focus given emotional competency occurs at this age [81] and given that, anecdotally, children who miss a daily nap are more emotionally dysregulated. We have demonstrated that a nap reduces the emotional attention bias in preschool aged children relative to wake [21]. Using a “dot probe” task, which measures emotional reactivity by quantifying the attentional response bias towards emotional compared to neutral stimuli, we found the bias toward emotional stimuli is only present when children do not nap. Further, greater SWA in the nap predicted a greater reduction in emotional bias, suggesting a SWS-specific mechanism underlying the nap-dependent change in emotional reactivity.
Additionally, studies in early childhood have demonstrated that naps improve emotion responsivity. For example, when presented with an unsolvable puzzle, children (~3 yrs) who napped used more mature and efficient self-regulation skills than those who were nap deprived [22]. Similarly, children in the same age range who were denied a nap responded emotionally-inappropriately to a range of stimuli with differing emotional valences (ie, displayed less positivity to positive stimuli and more negativity to neutral and negative stimuli) [23].
A mid-day nap can also impact emotional perception. Over wake, the repeated presentation of the same emotional stimuli induces emotional habituation. That is, ratings of emotional stimuli become more neutral (ie, less negative) with each subsequent presentation. However, we have demonstrated that a nap between presentations preserves emotional valence of negative stimuli (stimuli originally perceived as negative will remain negative) [24]. Thus, it is plausible that either sleep disrupts wake-dependent emotional habituation, or alternatively, that sleep actively promotes the preservation of the emotional valence [80].
On the other hand, others have reported diverging findings of those discussed immediately above. Specifically, in a group of young adults, those who remained awake between two face viewing sessions rated negative faces as more negative during the second viewing session (rather than less negative, as above). Individuals who napped, however, and especially those who had a nap containing REM, rated the faces as less negative [25]. Differences in findings between these two investigations may have occurred because of dissimilarities in study design. Specifically, in the prior study, participants viewed images with varied content (eg, bloody limbs, needles), while in the latter study, participants viewed and rated only faces. Therefore, perhaps a mid-day nap has stimulus-specific effects depending on the emotional content.
Given that naps enhance consolidation while also impacting emotional processing, a reasonable hypothesis is that these two processes are linked. In other words, might the synaptic burden that is purportedly decreased during sleep(as discussed in context of the synaptic homeostasis hypothesis[77]) also decrease emotional burden? If this were so, depotentiation might enable better emotional regulation during future encounters. However, to our knowledge, no work has yet linked these two mechanisms, and our studies that have investigated both memory and emotional processing have found no correlation between these factors [80,82].
3. Frequent napping is associated with negative outcomes
3.1 Frequent napping predicts negative health outcomes
In contrast to the benefits of napping described above, frequent habitual napping (“essential napping”) has been linked with a number of subsequent negative outcomes, including increased risk for hypertension [83], microvascular disease [26], depression [28], diabetes [84], osteoporosis [85], functional limitations [86], general medical morbidity [27], increased mortality [87], and cognitive decline [5]. These associations have predominantly been identified in older adults but also exist in middle age [26,83] and young adults [89].
Given the unexpected nature of these associations, examining directionality between napping and poor health is critical. Here, we will specifically focus on the link between napping and both mortality and cognitive decline.
3.1.1 Essential napping and mortality
Prospective investigation of causality (ie, random experimental group assignment) between frequent napping and mortality is not feasible, yet there are several studies that have attempted to disentangle this relationship. It has been posited that this association may not be due to excess sleep per se, but instead due to excess bouts of wake. The morning waking period corresponds with an increase in blood pressure, heart rate, and platelet aggregability [90], potentially due to reactivation of the sympathetic nervous system that occurs upon awakening [91]. In response to this rise, both vascular sheer stress and myocardial oxygen demand increase [66], and incidence of cardiovascular events rises. It is proposed, then, that the afternoon nap provides a second waking opportunity and thus a second opportunity for cardiovascular events to occur [87].
Supporting this hypothesis, individuals who frequently nap but also have a history of myocardial infarction (heart attack) do not have an increased risk of mortality [87]. It has been suggested that these individuals, who are likely regularly taking beta-blockers or aspirin (which both provide favorable cardiac benefits), may be unknowingly decreasing their risk of nap-induced cardiac issues [93]. Additionally, as blood pressure and heart rate decrease linearly with longer sleep periods [94], it is plausible that longer periods of sleep trigger larger increases in blood pressure/heart rate upon awakening. This hypothesis would explain why longer naps seem to be particularly dangerous [5,27,85].
Frequent napping and long nocturnal sleep duration have also been linked with a number of other outcomes that increase the risk for cardiovascular events. Specifically, long-napping middle-aged adults had an increased risk for metabolic syndrome [95]. Similarly, the incidence of diabetes, another cardiac event risk factor, is higher in habitual nappers [84]. Therefore, if naps were theoretically causing poor outcomes, they may be affecting the cardiovascular system indirectly through metabolic syndrome and diabetes. Although directionality is still unknown between napping and cardiac events, the presence of these other negative outcomes presents further research opportunities.
3.1.2 Essential napping and cognitive decline
The link between cognitive decline and essential napping has been viewed predominantly with the opposite causal directionality as the link between mortality and napping. That is, rather than naps being a detrimental factor, they are predicted to be a byproduct of related factors. For instance, it has been posited that age-related changes in brain integrity lead to sleepiness, which then induces napping. Brain damage and neurofibrillary tangle deposition brought about by early cognitive decline, particularly in the brainstem [96], alter cholinergic activity that is necessary for proper sleep/wake maintenance [97]. Similarly, age-related loss and/or functional degradation of wake-active cells, including orexinergic, dopaminergic, cholinergic, histaminergic, noradrenergic, and serotonergic types, is caused by age-related changes in brain integrity [98]. Therefore, reduction in brain integrity and functioning may increase sleep propensity and subsequently increase daytime napping.
If a loss in brain integrity contributes to napping, more severe brain degradation should lead to greater sleepiness. Conversely, individuals who are sleepier should be the most cognitively impaired. Indeed, healthy older individuals with higher self-reported daytime sleepiness perform worse on range of cognitive tasks, including tasks that are not alertness-dependent (eg, spatial orientation tasks rather than reaction time tasks) [99]. Moreover, older adults reporting daytime sleepiness are twice as likely to be diagnosed with dementia three years later[100], and demented individuals (those with mild-moderate Alzheimer’s disease) with higher sleep propensity perform worse on cognitive tests [101]. These results suggest that sleepiness and napping may be the result of cognitive degradation in older adults, yet directionality between these factors remains unknown.
3.1.3 The role of nocturnal sleep
Frequent napping may merely be a reflection of poor nocturnal sleep. If this were the case, poor nocturnal sleep, rather than consequential frequent napping, could create or exacerbate negative consequences. Certainly, short nocturnal sleep has been linked to a number of unfavorable health outcomes, including cognitive decline [102] and mortality [103], and poor sleep quality leads to more subsequent daytime napping [104]. However, counter to this hypothesis, many studies have statistically controlled for nocturnal sleep length or have removed individuals with poor sleep from analyses [26,84,89], and others have found that napping and non-napping older adults do not differ in terms of nocturnal sleep efficiency [28]. Nevertheless, these studies only take into account total sleep time and/or sleep efficiency and do not account for sleep architecture (ie, sleep staging). The quality of a full night of shallow sleep differs markedly from a night with deep sleep, and thus the possibility remains that poor nocturnal sleep could be a mediating factor between napping and illness.
3.1.4 Other factors to consider
Other health factors, including daily physical activity and medication use, may also contribute to napping behavior. Regular physical activity is crucial for maintaining physical health, and it could be surmised that those who nap more often are less physically active than those who do not nap. However, multiple studies suggest that this is not the case: older adults who nap are more likely to engage in exercise than those who do not nap [29,105]. It has also been proposed that medications increase daytime sleepiness, but several investigations examining health-related associations of a mid-day nap negate this hypothesis by removing or controlling for individuals on medications [28,29].
Sleep disorders must also be considered. Sleep apnea, a disorder in which breathing ceases periodically during the night [106], has been suggested to mediate essential napping and negative outcomes [26,83,85]. Sleep apnea prevalence increases dramatically in aged individuals, and it has been shown to exacerbate a number of disorders [107] and cause or contribute to cognitive decline [81]. Those with sleep apnea also take naps more frequently [110], and therefore the link between napping and disease may be driven by underlying disordered sleep. Given the high rate of undiagnosed sleep apnea in older adults [111], it is difficult to control for this factor without prospective polysomnography, and thus this factor warrants further exploration.
3.2 An emerging hypothesis: Naps and inflammatory mediation
Inflammation, the immune response to cell injury or foreign pathogen, might mediate the relationship between frequent mid-day napping and poor subsequent health outcomes. Sleep is a known regulator of immune processes [112], and, notably, siestas are proposed to be an evolutionary response to disease [113]. Therefore, it is possible that mid-day naps occur to mediate inflammation when disease or cell injury is present.
3.2.1 Sleep and inflammation: a bi-directional relationship
Sleep impacts the immune system [114], potentially via the glymphatic system [115]. Consequently, individuals with disturbed sleep, such as those with insomnia and untreated sleep apnea, tend to have immune alterations and chronic inflammation, a non-specific immune response [116]. On the other hand, the immune system modulates sleep. Through two separate immune cascades, inflammation causes daytime sleepiness [112]. Within one pathway, IL-1α elevates body temperature to prepare for immune recovery [117]; elevating temperature is metabolically costly. Given that energy expenditure is low during NREM sleep relative to wake and REM [118], having a large portion of NREM is advantageous for immune-induced recovery. Therefore, sleep occurs in response to this metabolism/temperature increase as a means of energy preservation [112]. Notably, daytime naps–especially in older adults–are NREM rich [10] (Figure 1), and thus they may be optimal for responding to such increased energy demands.
The link between daytime napping and inflammation is ambiguous. On one hand, a daytime nap is beneficial for immune (IL-6) recovery following experimental immune dysregulation via sleep restriction [119]. Naps also facilitate immune recovery by working in concert with nocturnal sleep. Specifically, following sleep deprivation, subsequent nocturnal sleep is sometimes not sufficient for recovery, and both a nap and nocturnal sleep are required for immune cytokines to be returned to baseline levels [120]. Therefore, a mid-day nap is an efficient method for enhancing immune recovery beyond what is provided by nocturnal sleep [121].
On the other hand, frequent daytime napping has been linked to negative immune outcomes (eg, increased inflammation) in both young [89] and older populations [122]. However, despite these seemingly contradictory findings, they may not be mutually exclusive. Acute inflammation, occurring following sleep deprivation, may be regulated with a short nap bout or with multiple subsequent sleep bouts, while chronic inflammation, potentially caused by disease or illness, may be too severe to be regulated by sleep. Nevertheless, the body may still induce daytime sleep in an attempt to re-normalize. Thus, daytime naps, in a naturalistic setting, may occur in response to increased inflammation.
3.2.2 Napping, inflammation, and negative outcomes
We posit a three-way relationship between essential napping, inflammatory burden, and poor health outcomes. In older populations, age-related immune alterations or comorbid disease (eg, hypertension) elevate chronic inflammation levels. As a consequence of the two pathways described above, individuals with higher inflammation nap more frequently [89,122]. In some instances, multiple sleep bouts are required to return inflammatory markers to normal levels [120]. Thus, daytime sleepiness could be recurrent if chronic inflammation is present, and napping could become more frequent as a consequence.
In parallel, an elevated inflammatory burden is a risk factor for a number of negative outcomes, including cognitive decline and mortality. For instance, interleukin-6 and several other inflammatory markers predict cognitive functioning six years later [91]. Similarly, inflammatory burden predicts increased risk for heart attack and stroke [92]. Therefore, elevated inflammation is a risk factor for poor health outcomes and it also induces daytime sleepiness and napping. Given this, it is possible that inflammation is the mediating factor that links frequent napping with poor subsequent health.
An emerging body of evidence supports this hypothesis. First, in a study focusing on the link between nocturnal sleep duration and poor health, the relationship between both short and long sleep duration and mortality was attenuated when controlling for inflammatory burden and several other lifestyle factors [14]. Additionally, using a longitudinal cohort, we found frequent napping predicts both cognitive decline and mortality eight years later only in individuals with inflammatory disease [unpublished results]. This suggests that napping itself is not detrimental and that inflammation may precede both napping and poor health outcomes; however, prospective investigation is required to confirm this hypothesis.
3.3 The nap paradox
Given what has been discussed above, a paradox exists within the literature: napping seems to be simultaneously both beneficial and detrimental for cognitive and physical health. We propose several explanations that may elaborate on these seemingly contradictory findings.
First, it is possible that well-controlled, empirical studies investigating the acute effects of naps may not be detecting or searching for concurrent negative effects of napping. Thus, both beneficial and negative effects could be present in these studies, but they may remain undetected. Further, these investigations may not have the statistical power to detect effects that become apparent in large cohort studies. To address this possibility, future work studies examine multiple dependent variables.
Second, the discrepancy in findings may exist because chronic napping (ie, frequent napping over the course of many months or years) could be distinct from acute napping (ie, a single nap in a well-controlled setting). This situation is comparable to that of sleep deprivation and sleep restriction; a full night of sleep deprivation in a laboratory setting is not comparable to years of chronic, acute sleep deprivation. Thus, those who nap chronically may experience cumulative negative effects that are not detectable or present in the short-term. To address this hypothesis, nap frequency could be tracked periodically using objective measures (eg, actigraphy) in conjunction with measures of health and cognition.
Third, characteristics unrelated to sleep could impact how a nap influences each individual. For instance, the negative effects of a nap on health may increase with age. This could explain why findings linking naps to negative outcomes have been predominantly identified in older populations. However, this age-related hypothesis is speculative and not supported by current literature, as positive effects provided by naps do not change with age [10].
Next, the majority of the work discussed above did not take into account nap ‘types’ that were originally proposed by Broughton & Dinges [2] (Table 1). It is possible that, for instance, recovery naps are more beneficial to health than appetitive naps, yet rarely are these differentiations made. Similarly, in prospective investigations, nap habituality (ie, an individual’s typical nap routine) is seldom considered, although recent evidence suggests that nap habituality moderates the relationship between naps and cognition [31,123]. Future work taking into account both nap type and nap habituality may yield greater insight into the relationship between napping and subsequent outcomes.
Table 1.
Types of naps [2].
| Nap Type | Definition |
|---|---|
| Recovery | Due to sleep loss |
| Prophylactic | In preparation for sleep loss |
| Appetitive | For enjoyment |
| Fulfillment | Due to increased sleep need (during development) |
| Essential | Due to sickness or inflammatory burden |
Finally, as has been discussed, it is possible that naps themselves are not detrimental but instead are a byproduct or side-effect of pre-existing health issues or typical age-related brain and body degradation. Based on the literature discussed, we believe this to be the most plausible explanation. However, prospective investigation is necessary in order to confirm this notion.
4. General conclusion: Should naps be prescribed?
In healthy, young individuals, a mid-day nap is observably beneficial. A bout of mid-day sleep minimizes sleepiness while enhancing executive functioning [7]. Naps also facilitate memory consolidation [10], subsequent learning [19], and emotional processing [21], while providing additional somatic benefits [119]. In young, healthy populations who are in need of emotional or cognitive intervention, napping could be prescribed.
On the other hand, in older populations, as opposed to the obvious benefits of a mid-day nap outlined above, excessive napping has been linked with negative outcomes. Yet there is no direct evidence suggesting that mid-day napping is detrimental. Although it is unlikely that essential naps are causal in inducing comorbidities, it is perhaps premature to make this statement definitively. Therefore, it is also premature to prescribe napping in this population. In the future, studies focusing on the link between napping and negative outcomes, as well aspotential interactions with inflammatory markers, would be useful in disentangling directionality.
Highlights.
Napping can facilitate cognitive and emotional health
Frequent napping is associated with negative health outcomes in older adults
Inflammation may mediate the link between frequent napping and poor health outcomes
Contradictions in the literature and future research recommendations are discussed
Acknowledgments
This work was supported in part by National Institutes of Health grants NIH R01 HL111695 and NIH R01 AG040133.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest, financial support, or off-label/investigational uses to disclose.
References
- 1.Bhat RY, Hannam S, Pressler R, Rafferty GF, Peacock JL, Greenough A. Effect of prone and supine position on sleep, apneas, and arousal in preterm infants. Pediatrics. 2006;18:101–7. doi: 10.1542/peds.2005-1873. [DOI] [PubMed] [Google Scholar]
- 2.Broughton RJ, Dinges DF. Sleep and Alertness: Chronobiological, Behavioural, and Medical Aspects of Napping. New York, NY: 1989. [Google Scholar]
- 3.Bursztyn M, Stessman J. The Siesta and Mortality: Twelve Years of Prospective Observations in 70-Year-Olds. Sleep. 2005;28:345–7. [PubMed] [Google Scholar]
- 4.Buysse DJ, Browman KE, Monk TH, Reynolds CF, Fasiczka AL, Kupfer DJ. Napping and 24‐hour sleep/wake patterns in healthy elderly and young adults. J Am Geriatr Soc. 1992;40:779–86. doi: 10.1111/j.1532-5415.1992.tb01849.x. [DOI] [PubMed] [Google Scholar]
- 5.Cross N, Terpening Z, Rogers NL, Duffy SL, Hickie IB, Lewis SJ, et al. Napping in older people “at risk” of dementia: relationships with depression, cognition, medical burden and sleep quality. J Sleep Res. 2015;24:494–502. doi: 10.1111/jsr.12313. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi M, Motoyoshi N, Hori T. Recuperative power of a short daytime nap with or without stage 2 sleep. Sleep. 2005;28:829–36. [PubMed] [Google Scholar]
- 7.Brooks A, Lack L. A brief afternoon nap following nocturnal sleep restriction: which nap duration is most recuperative? Sleep. 2006;29:831–40. doi: 10.1093/sleep/29.6.831. [DOI] [PubMed] [Google Scholar]
- 8.Tietzel AJ, Lack LC. The short-term benefits of brief and long naps following nocturnal sleep restriction. Sleep. 2001;24:293–300. doi: 10.1093/sleep/24.3.293. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi M, Watanabe M, Hori T. The effects of a 20 min nap in the mid-afternoon on mood, performance and EEG activity. Clin Neurophysiol. 1999;110:272–9. doi: 10.1016/s1388-2457(98)00003-0. [DOI] [PubMed] [Google Scholar]
- 10.Baran B, Mantua J, Spencer RM. Age-related Changes in the Sleep-dependent Reorganization of Declarative Memories. J Cogn Neurosci. 2016 doi: 10.1162/jocn_a_00938. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alger SE, Lau H, Fishbein W. Slow wave sleep during a daytime nap is necessary for protection from subsequent interference and long-term retention. Neurobiol Learn Mem. 2012;98:188–96. doi: 10.1016/j.nlm.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Tucker MA, Hirota Y, Wamsley EJ, Lau H, Chaklader A, Fishbein W. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem. 2006;86:241–7. doi: 10.1016/j.nlm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Alger SE, Lau H, Fishbein W. Delayed Onset of a Daytime Nap Facilitates Retention of Declarative Memory. PLoS One. 2010;5:e12131. doi: 10.1371/journal.pone.0012131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tucker MA, Fishbein W. Enhancement of declarative memory performance following a daytime nap is contingent on strength of initial task acquisition. Sleep. 2008;31:197–203. doi: 10.1093/sleep/31.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korman M, Doyon J, Doljansky J, Carrier J, Dagan Y, Karni A. Daytime sleep condenses the time course of motor memory consolidation. Nat Neurosci. 2007;10:1206–13. doi: 10.1038/nn1959. [DOI] [PubMed] [Google Scholar]
- 16.Backhaus J, Junghanns K. Daytime naps improve procedural motor memory. Sleep Med. 2006;7:508–12. doi: 10.1016/j.sleep.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Ruch S, Markes O, Duss SB, Oppliger D, Reber TP, Koenig T, et al. Sleep stage II contributes to the consolidation of declarative memories. Neuropsychologia. 2012;50:2389–96. doi: 10.1016/j.neuropsychologia.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Alger SE, Payne JD. The differential effects of emotional salience on direct associative and relational memory during a nap. Cogn Affect Behav Neurosci. 2016;16:1150–63. doi: 10.3758/s13415-016-0460-1. [DOI] [PubMed] [Google Scholar]
- 19.Antonenko D, Diekelmann S, Olsen C, Born J, Mölle M, Molle M. Napping to renew learning capacity: enhanced encoding after stimulation of sleep slow oscillations. Eur J Neurosci. 2013;37:1142–51. doi: 10.1111/ejn.12118. [DOI] [PubMed] [Google Scholar]
- 20.Mander BA, Santhanam S, Saletin JM, Walker MP. Wake deterioration and sleep restoration of human learning. Curr Biol. 2011;21 doi: 10.1016/j.cub.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cremone A, Kurdziel LBF, Fraticelli-Torres A, McDermott JM, Spencer RMC. Napping Reduces Emotional Attention Bias During Early Childhood. Dev Sci. 2016 doi: 10.1111/desc.12411. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller AL, Seifer R, Crossin R, Lebourgeois MK. Toddler’s self-regulation strategies in a challenge context are nap-dependent. J Sleep Res. 2015;24:279–87. doi: 10.1111/jsr.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berger RH, Miller AL, Seifer R, Cares SR, LeBourgeois MK. Acute sleep restriction effects on emotion responses in 30- to 36-month-old children. J Sleep Res. 2012;21:235–46. doi: 10.1111/j.1365-2869.2011.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pace-Schott EF, Shepherd E, Spencer RMC, Marcello M, Tucker M, Propper RE, et al. Napping promotes inter-session habituation to emotional stimuli. Neurobiol Learn Mem. 2011;95:24–36. doi: 10.1016/j.nlm.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gujar N, McDonald SA, Nishida M, Walker MP. A Role for REM Sleep in Recalibrating the Sensitivity of the Human Brain to Specific Emotions. Cereb Cortex. 2010;21:115–23. doi: 10.1093/cercor/bhq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen G, Tang K, Chen F, Wei S, Lin F, Yao J, et al. Afternoon nap and nighttime sleep with risk of micro- and macrovascular disease in middle-aged and elderly population. Int J Cardiol. 2015;187:553–5. doi: 10.1016/j.ijcard.2015.03.404. [DOI] [PubMed] [Google Scholar]
- 27.Dautovich ND, Kay DB, Perlis ML, Dzierzewski JM, Rowe MA, McCrae CS. Day-to-day variability in nap duration predicts medical morbidity in older adults. Health Psychol. 2012;31:671–6. doi: 10.1037/a0027374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cross N, Terpening Z, Rogers NLNL, Duffy SL, Hickie IB, Lewis SJG, et al. Napping in older people “at risk” of dementia: relationships with depression, cognition, medical burden and sleep quality. J Sleep Res. 2015;24:494–502. doi: 10.1111/jsr.12313. [DOI] [PubMed] [Google Scholar]
- 29.Cao Z, Shen L, Wu J, Yang H, Fang W, Chen W, et al. The effects of midday nap duration on the risk of hypertension in a middle-aged and older Chinese population: a preliminary evidence from the Tongji-Dongfeng Cohort Study, China. J Hypertens. 2014;32:1993–8. doi: 10.1097/HJH.0000000000000291. [DOI] [PubMed] [Google Scholar]
- 30.Fontvieille AM, Rising R, Spraul M, Larson DE, Ravussin E. Relationship between sleep stages and metabolic rate in humans. Am J Physiol. 1994;267:E732–7. doi: 10.1152/ajpendo.1994.267.5.E732. [DOI] [PubMed] [Google Scholar]
- 31.Kurdziel L, Duclos K, Spencer RMC. Sleep spindles in midday naps enhance learning in preschool children. Proc Natl Acad Sci U S A. 2013;110:17267–72. doi: 10.1073/pnas.1306418110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDevitt EA, Alaynick WA, Mednick SC. The effect of nap frequency on daytime sleep architecture. Physiol Behav. 2012;107:40–4. doi: 10.1016/j.physbeh.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 34.Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–60. doi: 10.1016/0301-0082(94)00057-O. [DOI] [PubMed] [Google Scholar]
- 35.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–94. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 36.Van Dort CJ, Zachs DP, Kenny JD, Zheng S, Goldblum RR, Gelwan NA, et al. Optogenetic activation of cholinergic neurons in the PPT or LDT induces REM sleep. Proc Natl Acad Sci. 2015;112:584–9. doi: 10.1073/pnas.1423136112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karacan I, Williams RL, Finley WW, Hursch CJ. The effects of naps on nocturnal sleep: influence on the need for stage-1 REM and stage 4 sleep. Biol Psychiatry. 1970;2:391–9. [PubMed] [Google Scholar]
- 38.Cunha RA. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int. 2001;38:107–25. doi: 10.1016/s0197-0186(00)00034-6. [DOI] [PubMed] [Google Scholar]
- 39.Holst SC, Landolt HP. Sleep Homeostasis, Metabolism, and Adenosine. Curr Sleep Med Reports. 2015;1:27–37. [Google Scholar]
- 40.Strecker RE, Morairty S, Thakkar MM, Porkka-Heiskanen T, Basheer R, Dauphin LJ, et al. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behav Brain Res. 2000;115:183–204. doi: 10.1016/s0166-4328(00)00258-8. [DOI] [PubMed] [Google Scholar]
- 41.Portas CM, Thakkar M, Rainnie DG, Greene RW, McCarley RW. Role of adenosine in behavioral state modulation: A microdialysis study in the freely moving cat. Neuroscience. 1997;79:225–35. doi: 10.1016/S0306-4522(96)00640-9. [DOI] [PubMed] [Google Scholar]
- 42.Urade Y, Hayaishi O. Prostaglandin D2 and sleep regulation. Biochim Biophys Acta. 1999;1436:606–15. doi: 10.1016/S0005-2760(98)00163-5. [DOI] [PubMed] [Google Scholar]
- 43.Porkka-Heiskanen T, Kalinchuk AV. Adenosine, energy metabolism and sleep homeostasis. Sleep Med Rev. 2011;15:123–35. doi: 10.1016/j.smrv.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Szymusiak R, McGinty D. Hypothalamic regulation of sleep and arousal. Ann N Y Acad Sci. 2008;1129:275–86. doi: 10.1196/annals.1417.027. [DOI] [PubMed] [Google Scholar]
- 45.Werth E, Dijk DJ, Achermann P, Borbely AA, Borbély AA. Dynamics of the sleep EEG after an early evening nap: experimental data and simulations. Am J Physiol. 1996;271:R501–10. doi: 10.1152/ajpregu.1996.271.3.R501. [DOI] [PubMed] [Google Scholar]
- 46.Gillberg M, Kecklund G, Axelsson J, Akerstedt T. The effects of a short daytime nap after restricted night sleep. Sleep. 1996;19:570–5. doi: 10.1093/sleep/19.7.570. [DOI] [PubMed] [Google Scholar]
- 47.Oken BS, Salinsky MC, Elsas SM. Vigilance, alertness, or sustained attention: physiological basis and measurement. Clin Neurophysiol. 2006;117:1885–901. doi: 10.1016/j.clinph.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayashi M, Ito S, Hori T. The effects of a 20-min nap at noon on sleepiness, performance and EEG activity. Int J Psychophysiol. 1999;32:173–80. doi: 10.1016/s0167-8760(99)00009-4. [DOI] [PubMed] [Google Scholar]
- 49.De Mendonça A, Costenla AR, Ribeiro JA. Persistence of the neuromodulatory effects of adenosine on synaptic transmission after long-term potentiation and long-term depression. Brain Res. 2002;932:56–60. doi: 10.1016/S0006-8993(02)02281-3. [DOI] [PubMed] [Google Scholar]
- 50.Reichert CF, Maire M, Schmidt C, Cajochen C. Sleep-Wake Regulation and Its Impact on Working Memory Performance: The Role of Adenosine. Biology (Basel) 2016;5:11. doi: 10.3390/biology5010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–8. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 52.Rawlins JNP. Associations across time: The hippocampus as a temporary memory store. Behav Brain Sci. 1985;8:479. doi: 10.1017/S0140525X00001291. [DOI] [Google Scholar]
- 53.Diekelmann S, Born J. The Memory Function of Sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 54.Nadel L, Samsonovich A, Ryan L, Moscovitch M. Multiple trace theory of human memory: Computational, neuroimaging, and neuropsychological results. Hippocampus. 2000;10:352–68. doi: 10.1002/1098-1063(2000)10:4<352::AID-HIPO2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 55.Moscovitch M, Cabeza R, Winocur G, Nadel L. Episodic memory and beyond: The hippocampus and neocortex in transformation. Annu Rev Psychol. 2016;67:105–34. doi: 10.1146/annurev-psych-113011-143733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marshall L, Helgadóttir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–3. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 57.Mednick SC, Cai DJ, Shuman T, Anagnostaras S, Wixted JT. An opportunistic theory of cellular and systems consolidation. Trends Neurosci. 2011;34:504–14. doi: 10.1016/j.tins.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–9. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 59.Hasselmo ME. Neuromodulation: Acetylcholine and memory consolidation. Trends Cogn Sci. 1999;3:351–9. doi: 10.1016/S1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- 60.Gais S, Born J. Low acetylcholine during slow-wave sleep is critical for declarative memory consolidation. Proc Natl Acad Sci U S A. 2004;101:2140–4. doi: 10.1073/pnas.0305404101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Groch S, Wilhelm I, Diekelmann S, Sayk F, Gais S, Born J. Contribution of norepinephrine to emotional memory consolidation during sleep. Psychoneuroendocrinology. 2011;36:1342–50. doi: 10.1016/j.psyneuen.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 62.Schönauer M, Geisler T, Gais S. Strengthening procedural memories by reactivation in sleep. J Cogn Neurosci. 2014;26:143–53. doi: 10.1162/jocn_a_00471. [DOI] [PubMed] [Google Scholar]
- 63.Debas K, Carrier J, Orban P, Barakat M, Lungu O, Vandewalle G, et al. Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proc Natl Acad Sci U S A. 2010;107:17839–44. doi: 10.1073/pnas.1013176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41:252–62. doi: 10.1016/S0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- 65.Maquet P, Laureys S, Peigneux P, Fuchs S, Petiau C, Phillips C, et al. Experience-dependent changes in cerebral activation during human REM sleep. Nat Neurosci. 2000;3:831–6. doi: 10.1038/77744. [DOI] [PubMed] [Google Scholar]
- 66.Tucker Ma, Hirota Y, Wamsley EJ, Lau H, Chaklader A, Fishbein W. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem. 2006;86:241–7. doi: 10.1016/j.nlm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 67.Albouy G, Fogel S, Pottiez H, Nguyen VA, Ray L, Lungu O, et al. Daytime sleep enhances consolidation of the spatial but not motoric representation of motor sequence memory. PLoS One. 2013;8:e52805. doi: 10.1371/journal.pone.0052805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tucker M, McKinley S, Stickgold R. Sleep optimizes motor skill in older adults. J Am Geriatr Soc. 2011;59:603–9. doi: 10.1111/j.1532-5415.2011.03324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilhelm I, Metzkow‐Mészàros M, Knapp S, Born J. Sleep‐dependent consolidation of procedural motor memories in children and adults: The pre‐sleep level of performance matters. Dev Sci. 2012;15:506–15. doi: 10.1111/j.1467-7687.2012.01146.x. [DOI] [PubMed] [Google Scholar]
- 70.Desrochers PC, Kurdziel LBF, Spencer RMC. Delayed benefit of naps on motor learning in preschool children. Exp Brain Res. 2015;234:763–72. doi: 10.1007/s00221-015-4506-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Korman M, Dagan Y, Karni A. Nap it or leave it in the elderly: A nap after practice relaxes age-related limitations in procedural memory consolidation. Neurosci Lett. 2015;606:173–6. doi: 10.1016/j.neulet.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 72.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–85. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 73.Cairney SA, Durrant SJ, Jackson R, Lewis PA. Sleep spindles provide indirect support to the consolidation of emotional encoding contexts. Neuropsychologia. 2014;63:285–92. doi: 10.1016/j.neuropsychologia.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 74.Mednick SC, McDevitt EA, Walsh JK, Wamsley E, Paulus M, Kanady JC, et al. The critical role of sleep spindles in hippocampal-dependent memory: a pharmacology study. J Neurosci. 2013;33:4494–504. doi: 10.1523/JNEUROSCI.3127-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buzsáki G. The hippocampo-neocortical dialogue. Cereb Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- 76.Yoo S-S, Hu PT, Gujar N, Jolesz Fa, Walker MP. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10:385–92. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- 77.Tononi G, Cirelli C. Sleep and synaptic homeostasis: A hypothesis. Brain Res Bull. 2003;62:143–50. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 78.Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-analysis Arch Gen Psychiatry. 1992;49:651–68-70. doi: 10.1001/archpsyc.1992.01820080077011. [DOI] [PubMed] [Google Scholar]
- 79.Palmer CA, Alfano CA. Sleep and Emotion Regulation: An Organizing, Integrative Review. 2016 doi: 10.1016/j.smrv.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 80.Baran B, Pace-Schott EF, Ericson C, Spencer RMC. Processing of emotional reactivity and emotional memory over sleep. J Neurosci. 2012;32:1035–42. doi: 10.1523/JNEUROSCI.2532-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Denham SA. Dealing with feelings: How children negotiate the worlds of emotions and social relationships. Cogniţie Creier Comport. 2007;XI:1–48. [Google Scholar]
- 82.Jones BJ, Schultz KS, Adams S, Baran B, Spencer RMC. Emotional bias of sleep-dependent processing shifts from negative to positive with aging. Neurobiol Aging. 2016 doi: 10.1016/j.neurobiolaging.2016.05.019. [Accepted] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cao Z, Shen L, Wu J, Yang H, Fang W, Chen W, et al. The effects of midday nap duration on the risk of hypertension in a middle-aged and older Chinese population: a preliminary evidence from the Tongji-Dongfeng Cohort Study, China. J Hypertens. 2014;32:1993–8. doi: 10.1097/HJH.0000000000000291. [DOI] [PubMed] [Google Scholar]
- 84.Fang W, Li Z, Wu LL, Cao Z, Liang Y, Yang H, et al. Longer habitual afternoon napping is associated with a higher risk for impaired fasting plasma glucose and diabetes mellitus in older adults: results from the Dongfeng–Tongji cohort of retired workers. Sleep Med. 2013;14:950–4. doi: 10.1016/j.sleep.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 85.Chen G, Chen L, Wen J, Yao J, Li L, Lin L, et al. Associations between sleep duration, daytime nap duration, and osteoporosis vary by sex, menopause, and sleep quality. J Clin Endocrinol Metab. 2014;99:2869–77. doi: 10.1210/jc.2013-3629. [DOI] [PubMed] [Google Scholar]
- 86.Goldman SE, Stone KL, Ancoli-Israel S, Blackwell T, Ewing SK, Boudreau R, et al. Poor sleep is associated with poorer physical performance and greater functional limitations in older women. Sleep. 2007;30:1317–24. doi: 10.1093/sleep/30.10.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bursztyn M, Ginsberg G, Hammerman-Rozenberg R, Stessman J. The siesta in the elderly: risk factor for mortality? Arch Intern Med. 1999;159:1582–6. doi: 10.1001/archinte.159.14.1582. [DOI] [PubMed] [Google Scholar]
- 88.Asada T, Motonaga T, Yamagata Z, Uno M, Takahashi K. Associations between retrospectively recalled napping behavior and later development of Alzheimer’s disease: association with APOE genotypes. Sleep. 2000;23:629–34. [PubMed] [Google Scholar]
- 89.Mantua J, Spencer RMC. The interactive effects of nocturnal sleep and daytime naps in relation to serum C-reactive protein. Sleep Med. 2015;16:1213–6. doi: 10.1016/j.sleep.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tofler GH, Brezinski D, Schafer AI, Czeisler CA, Rutherford JD, Willich SN, et al. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1987;316:1514–8. doi: 10.1097/00006534-198808000-00105. [DOI] [PubMed] [Google Scholar]
- 91.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–7. doi: 10.1097/00132586-199308000-00067. [DOI] [PubMed] [Google Scholar]
- 92.Bursztyn M, Ginsberg G, Stessman J. The siesta and mortality in the elderly: effect of rest without sleep and daytime sleep duration. Sleep. 2002;25:187–91. doi: 10.1093/sleep/25.2.187. [DOI] [PubMed] [Google Scholar]
- 93.Cheng TO. Afternoon nap is good for the elderly. Arch Intern Med. 2000;160:711. doi: 10.1001/archinte.160.5.711. author reply 711-2. [DOI] [PubMed] [Google Scholar]
- 94.Bursztyn M, Mekler J, Ben-Ishay D. The siesta and ambulatory blood pressure in hypertensive diabetics: attenuated decline during day and night time sleep. J Hypertens. 1998;32:377–8. [Google Scholar]
- 95.Wu J, Xu G, Shen L, Zhang Y, Song L, Yang S, et al. Daily sleep duration and risk of metabolic syndrome among middle-aged and older Chinese adults: cross-sectional evidence from the Dongfeng-Tongji cohort study. BMC Public Health. 2015;15:178. doi: 10.1186/s12889-015-1521-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ishii T. Distribution of Alzheimer’s neurofibrillary changes in the brain stem and hypothalamus of senile dementia. Acta Neuropathol. 1992;6:181–7. doi: 10.1007/BF00686763. [DOI] [PubMed] [Google Scholar]
- 97.Merlino G, Piani A, Gigli GL, Cancelli I, Rinaldi A, Baroselli A, et al. Daytime sleepiness is associated with dementia and cognitive decline in older Italian adults: A population-based study. Sleep Med. 2010;11:372–7. doi: 10.1016/j.sleep.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 98.Stern AL, Naidoo N. Wake-active neurons across aging and neurodegeneration: a potential role for sleep disturbances in promoting disease. Springerplus. 2015;4 doi: 10.1186/s40064-014-0777-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ohayon MM, Vecchierini M-F. Daytime sleepiness and cognitive impairment in the elderly population. Arch Intern Med. 2002;162:201–8. doi: 10.1001/archinte.162.2.201. [DOI] [PubMed] [Google Scholar]
- 100.Foley D, Monjan A, Masaki K, Ross W, Havlik R, White L, et al. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. J Am Geriatr Soc. 2001;49:1628–32. doi: 10.1046/j.1532-5415.2001.t01-1-49271.x. [DOI] [PubMed] [Google Scholar]
- 101.Bonanni E, Maestri M, Tognoni G, Fabbrini M, Nucciarone B, Manca ML, et al. Daytime sleepiness in mild and moderate Alzheimer’s disease and its relationship with cognitive impairment. J Sleep Res. 2005;14:311–7. doi: 10.1111/j.1365-2869.2005.00462.x. [DOI] [PubMed] [Google Scholar]
- 102.Xu L, Jiang CQ, Lam TH, Liu B, Jin YL, Zhu T, et al. Short or long sleep duration is associated with memory impairment in older Chinese: the Guangzhou Biobank Cohort Study. Sleep. 2011;34:575–80. doi: 10.1093/sleep/34.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goldman SE, Hall M, Boudreau R, Matthews KA, Cauley JA, Ancoli-Israel S, et al. Association between nighttime sleep and napping in older adults. Sleep. 2008;31:733–40. doi: 10.1093/sleep/31.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fang W, Li Z, Wu L, Cao Z, Liang Y, Yang H, et al. Longer habitual afternoon napping is associated with a higher risk for impaired fasting plasma glucose and diabetes mellitus in older adults: results from the Dongfeng–Tongji cohort of retired workers. Sleep Med. 2013;14:950–4. doi: 10.1016/j.sleep.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 106.Norman D, Loredo JS. Obstructive Sleep Apnea in Older Adults. Clin Geriatr Med. 2008;24:151–65. doi: 10.1016/j.cger.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 107.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: A population-based study. Am J Respir Crit Care Med. 2005;172:1590–5. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Osorio RS, Gumb T, Pirraglia E, Varga AW, Lu SE, Lim J. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84:1964–71. doi: 10.1212/WNL.0000000000001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Osorio RS, Ayappa I, Mantua J, Gumb T, Varga A, Mooney AM, et al. The interaction between sleep-disordered breathing and apolipoprotein E genotype on cerebrospinal fluid biomarkers for Alzheimer’s disease in cognitively normal elderly individuals. Neurobiol Aging. 2014;35:1318–24. doi: 10.1016/j.neurobiolaging.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Masa JF, Rubio M, Pérez P, Mota M, de Cos JS, Montserrat JM. Association between habitual naps and sleep apnea. Sleep. 2006;29:1463–8. doi: 10.1016/S8756-3452(08)70714-X. [DOI] [PubMed] [Google Scholar]
- 111.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–95. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barone TL. Is the siesta an adaptation to disease? Hum Nat. 2000;11:233–58. doi: 10.1007/s12110-000-1012-4. [DOI] [PubMed] [Google Scholar]
- 114.Redwine L, Dang J, Irwin M. Cellular adhesion molecule expression, nocturnal sleep, and partial night sleep deprivation. Brain Behav Immun. 2004;18:333–40. doi: 10.1016/j.bbi.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 115.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–7. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Simpson N, Dinges DF. Sleep and Inflammation. Nutr Rev. 2007;65 doi: 10.1111/j.1753-4887.2007.tb00371.x. [DOI] [PubMed] [Google Scholar]
- 117.Olivadoti MD, Opp MR. Effects of i.c.v administration of interleukin-1 on sleep and body temperature of interleukin-6-deficient mice. Neuroscience. 2008;153:338–48. doi: 10.1016/j.neuroscience.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011;589:235–44. doi: 10.1113/jphysiol.2010.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Faraut B, Nakib S, Drogou C, Elbaz M, Sauvet F, De Bandt JP, et al. Napping reverses the salivary interleukin-6 and urinary norepinephrine changes induced by sleep restriction. J Clin Endocrinol Metab. 2015;100:E416–26. doi: 10.1210/jc.2014-2566. [DOI] [PubMed] [Google Scholar]
- 120.Faraut B, Boudjeltia KZ, Dyzma M, Rousseau A, David E, Stenuit P, et al. Benefits of napping and an extended duration of recovery sleep on alertness and immune cells after acute sleep restriction. Brain Behav Immun. 2011;25:16–24. doi: 10.1016/j.bbi.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 121.Faraut B, Andrillon T, Vecchierini MF, Leger D. Napping: A public health issue. From epidemiological to laboratory studies. Sleep Med Rev. 2016 doi: 10.1016/j.smrv.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 122.Leng Y, Ahmadi-Abhari S, Wainwright NWJ, Cappuccio FP, Surtees PG, Luben R, et al. Daytime napping, sleep duration and serum C reactive protein: a population-based cohort study. BMJ Open. 2014;4:e006071–e006071. doi: 10.1136/bmjopen-2014-006071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Milner CE, Fogel SM, Cote KA. Habitual napping moderates motor performance improvements following a short daytime nap. Biol Psychol. 2006;73:141–56. doi: 10.1016/j.biopsycho.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 124.Milner CE, Cote KA. A dose-response investigation of the benefits of napping in healthy young, middle-aged and older adults. Sleep Biol Rhythms. 2008;6:2–15. doi: 10.1111/j.1479-8425.2007.00328.x. [DOI] [Google Scholar]
- 125.Butte NF, Jensen CL, Moon JK, Glaze DG, Frost JD., Jr Sleep organization and energy expenditure of breast-fed and formula-fed infants. Pediatr Res. 1992;32:514–9. doi: 10.1203/00006450-199211000-00003. [DOI] [PubMed] [Google Scholar]
- 126.Friedrich M, Wilhelm I, Born J, Friederici AD. Generalization of word meanings during infant sleep. Nature. 2015;6 doi: 10.1038/ncomms7004. [DOI] [PMC free article] [PubMed] [Google Scholar]


