Abstract
Objective: Pulmonary rehabilitation improves exercise tolerance in patients with chronic obstructive pulmonary disease (COPD). However, many patients do not have access to pulmonary rehabilitation programs. We hypothesized that an alternative to pulmonary rehabilitation to improve exercise tolerance is the practice of pranayama, or yoga breathing, which could be done independently at home. We also sought to determine whether yoga nonprofessionals could adequately teach pranayama to patients.
Design: Proof-of-concept, randomized, double-blind, controlled pilot trial.
Settings/Location: Two academic pulmonary practices.
Subjects: Forty-three patients with symptomatic, moderate-to-severe COPD.
Interventions: Twelve weeks of pranayama plus education versus education alone. Two yoga professionals trained the research coordinators to conduct all pranayama teaching and monitored the quality of the teaching and the practice of pranayama by study participants.
Outcome measures: The primary outcome was a change in the 6-min walk distance (6MWD). Secondary outcomes included changes in lung function, markers of oxidative stress and systemic inflammation, and measures of dyspnea and quality of life.
Results: The 6MWD increased in the pranayama group (least square mean [95% confidence interval] = 28 m [−5 to 61]) and decreased in the control group (−15 m [−47 to 16]), with a nearly significant treatment effect (p = 0.06) in favor of pranayama. Pranayama also resulted in small improvements in inspiratory capacity and air trapping. Both groups had significant improvements in various measures of symptoms, but no overall differences in respiratory system impedance or markers of oxidative stress or systemic inflammation.
Conclusion: This pilot study successfully demonstrated that pranayama was associated with improved exercise tolerance in patients with COPD. Lay personnel were able to adequately teach patients to practice pranayama. These results suggest that pranayama may have significant clinical benefits for symptomatic patients with COPD, a concept that needs to be confirmed in future, larger clinical trials.
Keywords: : pranayama, yoga, breathing exercises, pulmonary rehabilitation, COPD
Introduction
Patients with chronic obstructive pulmonary disease (COPD) primarily suffer from poor exercise tolerance and dyspnea.1–5 Inhaled bronchodilator therapy is currently the mainstay of therapy in COPD.6 However, bronchodilators are expensive and may have significant side-effects, and many patients continue to have symptoms despite therapy.
A validated, nonpharmacologic approach to combating the dyspnea and exercise intolerance of COPD is pulmonary rehabilitation.7,8 Two common breathing techniques are taught during pulmonary rehabilitation: diaphragmatic breathing and pursed-lip breathing.7 Both are beneficial because they result in a slowing of the breathing rate, which leads to longer exhalation time, better lung emptying, and reduced dynamic hyperinflation.9–11 Unfortunately, the beneficial effects of pulmonary rehabilitation diminish with time,12 and pulmonary rehabilitation programs are not widely available, especially to lower-income, minority, and rural patients.7 These programs are also poorly reimbursed, making them expensive to run and difficult for patients to afford.7 Multiple factors contribute to poor attendance at pulmonary rehabilitation programs, including smoking status, severity of dyspnea, anxiety and depression, length of program, frequency of hospitalizations, and long journey time.13,14
Pursed-lip and diaphragmatic breathing focus on the mechanics of breathing, whereas the breathing techniques of pranayama (yoga breathing) encourage participants to also focus on relaxation, which is important in reducing anxiety in patients with COPD.11 Early studies indicate that yoga and pranayama are feasible and well tolerated in COPD patients, and they may result in decreased dyspnea, increased exercise capacity, improved oxygenation, or better quality-of-life scores.15–19 However, these prior studies were limited because they involved a single yoga breathing session,17 had no control group,20 or might be difficult to achieve in practice because they required participation in an extensive yoga program15,18–20 or involved multifaceted pranayama.16
We hypothesized that focusing only on a simple pranayama would be feasible to teach in the clinic, easy to practice at home, and result in increased exercise tolerance in patients with symptomatic COPD. As a pilot study, we also measured multiple aspects of pulmonary function related to dynamic hyperinflation,2,4,5,21 as well as biomarkers of inflammation22 and oxidative stress,23,24 which are associated with COPD.
Materials and Methods
Patient population
We enrolled patients who were 18 years of age and older who had a physician diagnosis of COPD with symptoms of shortness of breath as indicated by a modified medical research council (mMRC) Dyspnea Scale25 score >2, and airway obstruction defined by forced expiratory volume in 1 sec (FEV1)/forced vital capacity (FVC) ratio <0.7 and FEV1 < 80% predicted. They had to be current nonsmokers with stable disease over the previous 4 weeks, and not enrolled in pulmonary rehabilitation or practicing yoga.
Study design
This study design was a 12-week, randomized, double-blind, controlled trial of pranayama in patients with COPD. The study was conducted at the Vermont Lung Center of the University of Vermont, in Burlington, Vermont, and at Baylor College of Medicine in Houston, Texas, over the period from January 2013 to October 2015.
Based on a computer-generated randomization scheme administered by the statistician, participants were randomly assigned to receive pranayama teaching, in addition to education (pranayama group) or education alone (control group). Participants in both groups were not fully informed of the study's purpose to test the efficacy of pranayama; rather, they were told that this was a study of education in COPD, with different groups receiving different emphasis on breathing techniques. In addition, one set of research coordinators who knew of group assignment conducted all pranayama teaching and educational sessions, whereas a different set of research coordinators who were blinded to group assignment conducted all measurements and assessments.
For those randomized to the pranayama group, research coordinators conducted pranayama teaching after first undergoing direct personal instruction in pranayama by a local, certified Kripalu yoga (a type of Hatha yoga) instructor. We selected the pranayama method known as the Dirgha (meaning “long”) Three-Part breath because of its simplicity and ease of practice. Specifically, the Dirgha breath calms the mind and body, reducing stress and anxiety. This pranayama also promotes relaxed, slow, complete inhalations and exhalations, which are important to enhance lung emptying, a particular problem in patients with COPD.4
The yoga instructors trained the research coordinators to teach the Dirgha breath in the following manner: While sitting in a chair, participants were instructed to breathe in slowly through their nose to fill first the bottom of their lungs, then the middle of their lungs, and finally the top of their lungs. They could then empty in the same order from bottom to top, or from top to bottom if that felt more comfortable, from nose or mouth. There were no restrictions on the speed or frequency of the breaths. Although this is similar to diaphragmatic breathing, the focus on visualization of lung filling and emptying and on relaxation make the Dirgha breath more ideally suited to patients with COPD.
The yoga instructors monitored the pranayama teaching by the research coordinators for consistent quality throughout the study. First, they initially certified that the research coordinators were proficient in teaching and practicing the Dirgha breath. Then, the yoga instructors reviewed the teaching interaction between the research coordinator and the participant after the first three participants were enrolled. Finally, the yoga instructors also reviewed videotapes of the interaction between the research coordinator and each participant when that participant returned for his or her 6-week visit. Any deficiencies that were identified at any of these points of time were resolved before the research coordinator could continue with the study.
Both groups received usual care as prescribed by their personal physician, in addition to focused, standardized sessions of education provided by the research coordinators. We used standardized, instructional materials published by the American College of Chest Physicians Patient Education Guide (“Living Well with COPD,” 2004, www.chestnet.org) and the Canadian Thoracic Society (“Living Well with COPD,” www.livingwellwithCOPD.com). Participants spent 1 h at each visit, with those in the pranayama group spending 30 min on the educational materials and 30 min learning and practicing pranayama, and those in the control group spending 60 min on the educational materials. Of particular importance, during the session that covered breathing exercises, the pursed-lip and diaphragmatic breathing techniques were described briefly to both groups, but they were not emphasized. The research coordinators were careful to balance these sessions so that both groups received the same overall content, although a slightly different time was spent on these educational materials. Both groups received the same total time of attention during all visits.
A different set of research coordinators, blinded to group assignment, conducted all the measurements and assessments in this study. At baseline, we recorded age, sex, height, weight, and body–mass index (BMI), and we then made multiple measurements of symptoms (Borg dyspnea scale,26 mMRC dyspnea scale,25 Baseline Dyspnea Index/Transitional Dyspnea Indices [BDI/TDI],27 COPD Assessment Test [CAT]28).
We also measured multiple aspects of lung function: (FEV1, FVC, FEV1/FVC, inspiratory capacity [IC; MGC Diagnostics, Inc.], inspiratory time to total breathing cycle time [Ti/Ttot], 6-min walk distance [6MWD],29 and body-mass index, airflow obstruction, dyspnea, exercise capacity [BODE] index).30 In addition, we measured lung volumes31 (total lung capacity [TLC], functional residual capacity [FRC], residual volume [RV]) and specific airway conductance (sGaw) by body plethysmography (MGC Diagnostics, Inc.), and the diffusing capacity of the lung for carbon monoxide (DLCO)32 (MGC Diagnostics, Inc.).
We measured respiratory system impedance (resistance at 5 Hz [R5], resistance at 20 Hz [R20], reactance at 5 Hz [X5], resonant frequency [Fres], and area under the reactance curve [AX]), measured by the forced oscillation technique (FOT)33 (IOS, CareFusion Corp.), and quality of life (St. George Respiratory Questionnaire–COPD [SGRQ-C]).34
We collected exhaled breath condensate (EBC) for measurement of oxidative stress (hydrogen peroxide [H2O2]35 and 8-isoprostane23) (by enzyme-linked immunosorbent assay [ELISA]; R&D Systems, Inc.), and blood for measurement of inflammation (C-reactive protein [CRP]22 [by nephelometry, Beckman Immage 800; Beckman Coulter, Inc.], coefficient of variation of red cell distribution width [RDW-CV]22 [Beckman Coulter DxH 800 Cell Counter, Beckman Coulter, Inc.], and interleukin-6 [IL-6]36) (by ELISA assay; R&D Systems, Inc.). To specifically address dynamic hyperinflation and its consequences, spirometry, FOT, and EBC were performed and analyzed as described earlier, both before and after the 6-min walk tests.
All participants then returned for two 1-h visits per week, for 2 weeks. During these visits, participants received instruction in pranayama plus education about COPD (pranayama group), or education alone (control group). We asked participants in the pranayama group to practice pranayama every day, building up to 30 min per day, and provided them with a brief, instructional DVD created by our yoga instructors to use at home. Both groups kept a daily diary of symptoms and time spent on exercise and breathing activities. At 6 weeks, all participants returned for another hour-long session of pranayama practice plus education (pranayama group), or education alone (control group). At 12 weeks, all participants returned for repeat measurements and assessments as were made at the baseline visit. All participants received weekly telephone reminders to practice breathing exercises and complete their diaries.
The study was approved by each institution's Institutional Review Board (M12-131 at Vermont, H-30071 at Baylor), each participant provided informed consent, and the trial was listed on ClinicalTrials.gov (NCT01633697). An independent data safety monitoring committee consisting of three pulmonologists with expertise in pulmonary rehabilitation reviewed study progress and safety at baseline and at quarterly intervals throughout the study.
Statistical analysis
Baseline demographics, symptoms, and lung function were compared between groups by using t tests for continuous measures and chi-square tests for dichotomous measures. Mean values per week were calculated for the measures collected in the daily journals. Due to the skewed nature of these measures, group comparisons were done by using Wilcoxon rank sum tests. Spearman correlations were used to examine the association between 6MWD and time spent on physical activity and on breathing exercises.
The primary outcome was the change in 6MWD over the 12 weeks compared between groups. Based on this outcome, we estimated that we needed 18 subjects per group to detect a difference of 50 m in distance with 80% power and α = 0.05. To account for dropout and lost data, we recruited a total of 42 subjects.
We used SAS Proc Mixed to perform repeated-measures analysis of variance [ANOVA] on the primary and secondary outcome data by using the intent-to-treat population. For the primary outcome, the difference in means and 95% confidence intervals were evaluated by using the least square means estimates generated from the repeated-measures ANOVA. In the event of non-normality, the analysis was done on the log- or square-root-transformed data and the back-transformed means and interquartile ranges (IQR) were presented. A suitable transformation could not be found for the respiratory system impedance measures, so the Wilcoxon signed-rank test was used to compare baseline with 12 weeks within each group and the medians and IQR were presented. Correlations between baseline 6MWD and baseline lung function measures and between the change in 6MWD and change in lung function from baseline to 12 weeks were examined with Pearson correlation coefficients. All analyses were performed by using SAS Version 9 statistical software (SAS Institute, Cary, NC). Statistical significance was determined based on α = 0.05.
Results
Participant characteristics
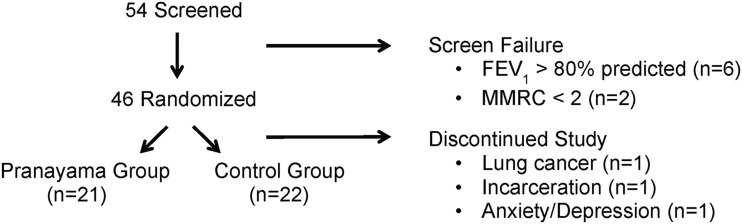
The study flow (CONSORT) diagram is shown in Figure 1. All participants attended all visits throughout the study. Participants were well matched for age, BMI, symptoms, and lung function, with a slightly higher predominance of women than men in the pranayama group (Table 1).
FIG. 1.
Study flowchart. Patients were screened for eligibility and then eligible subjects were randomized on enrollment in the study. Eight patients failed screening: six because of high lung function and two because of low dyspnea. After randomization, three participants failed to complete the study: one due to newly diagnosed lung cancer, one due to incarceration, and one due to severe anxiety and depression.
Table 1.
Baseline Demographics, Symptoms, and Lung Function
| Characteristic | Pranayama (n = 21) | Control (n = 22) |
|---|---|---|
| Age (years) | 68 ± 7 | 68 ± 9 |
| Sex (% female) | 67 | 55 |
| BMI (kg/m2) | 29 ± 7 | 30 ± 4 |
| GOLD (%I/II/III/IV) | 0/29/52/19 | 0/36/41/23 |
| mMRC score (0–4, higher worse) | 3.1 ± 0.3 | 3.0 ± 0.3 |
| BDI | 5.71 ± 2.22 | 5.54 ± 1.87 |
| CAT (0–40, higher worse) | 19.1 ± 6.5 | 21.0 ± 7.0 |
| BODE index (0–10, higher worse) | 5.24 ± 1.81 | 5.73 ± 1.75 |
| SGRQ-C (0–100, higher worse) | 48.6 ± 12.7 | 52.1 ± 20.2 |
| SGRQ-C symptom score | 62.6 ± 18.9 | 60.3 ± 17.7 |
| SGRQ-C activity score | 70.2 ± 14.2 | 73.2 ± 23.1 |
| SGRQ-C impact score | 30.98 ± 16.7 | 36.7 ± 21.4 |
| FEV1 (% predicted, prebronchodilator) | 43 ± 16 | 42 ± 13 |
| IC (L) | 1.95 ± 0.73 | 2.04 ± 0.69 |
| RV/TLC (% predicted) | 150 ± 26 | 151 ± 20 |
| DLCO (% predicted) | 37.4 ± 13.9 | 45.2 ± 19.9 |
Values represent means ± SD unless otherwise specified. p-Values are from t tests for continuous characteristics and from chi-square tests for categorical characteristics.
BDI, baseline dyspnea index; BMI, body–mass index; BODE, body–mass index, airflow obstruction, dyspnea and exercise capacity index; CAT, COPD assessment test; COPD, chronic obstructive pulmonary disease; DLCO, diffusing capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 sec; GOLD, Global Initiative on Obstructive Lung Disease; IC, inspiratory capacity; mMRC, modified medical research council; RV/TLC, residual volume to total lung capacity ratio; SGRQ-C, St. George Respiratory Questionnaire–COPD.
Primary outcome
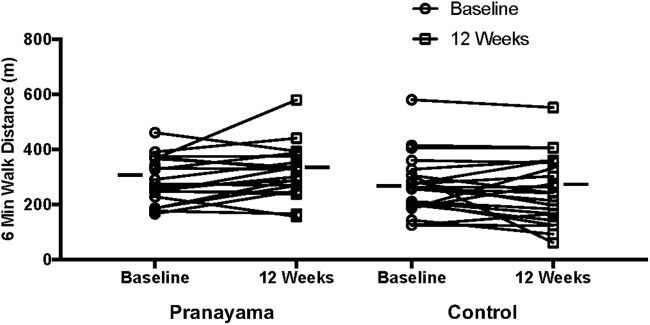
Within groups, the 6MWD increased from baseline to 12 weeks in the pranayama group (28 m [−5 to 61]) and decreased in the control group (−15 m [−47 to 16]), with a borderline-significant treatment effect (p = 0.06), suggesting that the change over time indicated an improvement in 6MWD in the pranayama group only (Table 2 and Fig. 2). Given the borderline statistical significance of the treatment effect, we examined the simple effects of time and group. There was no significant difference in 6MWD between the pranayama group and the control group at baseline (22 m [−41 to 85], p = 0.48). However, at 12 weeks, the pranayama group had a significantly greater 6MWD than the control group (65 m [2–129], p = 0.04).
Table 2.
Measures of Pulmonary Function and Exercise Capacity at Baseline and 12 Weeks
| Pranayama (n = 21) | Control (n = 22) | p | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | Baseline | 12 Weeks | Baseline | 12 Weeks | Group | Time | Group × time |
| 6MWD (m) | 290 ± 81 | 316 ± 95 | 268 ± 106 | 252 ± 122 | 0.14 | 0.57 | 0.06 |
| FEV1 (% predicted) | 43 ± 16 | 45 ± 14 | 42 ± 13 | 43 ± 13 | 0.75 | 0.32 | 0.75 |
| IC (L) | 1.95 ± 0.73 | 2.07 ± 0.77 | 2.04 ± 0.69 | 2.04 ± 0.65 | 0.75 | 0.65 | 0.71 |
| RV/TLC (% predicted) | 150 ± 26 | 142 ± 27 | 151 ± 20 | 153 ± 20 | 0.50 | 0.48 | 0.13 |
| sGaw mean (IQR)a | 0.08 (0.05, 0.21) | 0.07 (0.04, 0.19) | 0.12 (0.05, 0.18) | 0.10 (0.05, 0.14) | 0.80 | 0.52 | 0.67 |
| DLCO (% predicted) (ml/min/mmHg) | 37.4 ± 13.9 | 42.0 ± 19.5 | 45.2 ± 19.9 | 43.1 ± 17.8 | 0.74 | 0.92 | 0.11 |
| VA/TLC | 60.1 ± 15.9 | 60.8 ± 13.7 | 60.7 ± 11.9 | 60.7 ± 11.5 | 0.75 | 0.77 | 0.80 |
| Ti/Ttot | 0.40 ± 0.08 | 0.36 ± 0.05 | 0.40 ± 0.09 | 0.38 ± 0.11 | 0.64 | 0.16 | 0.75 |
Values represent the mean and standard deviations unless otherwise noted. p-Values for group, time, and group × time interaction are from the repeated-measures model.
Values represent back-transformed means with IQR.
6MWD, 6-min walk distance; IQR, interquartile range; sGaw, specific airway conductance; Ti/Ttot, inspiratory time to total breathing cycle time ratio; VA/TLC, alveolar volume to total lung capacity ratio.
FIG. 2.
Individual changes in 6MWD from baseline to week 12 in the pranayama and control groups. Horizontal bars indicate mean values. The change in 6MWD over time nearly met statistical significance with a greater change in the pranayama group, based on a p-value of 0.06 for the interaction of group × time. 6MWD, 6-min walk distance.
Secondary outcomes
Within groups, there were improvements in IC and RV/TLC in the pranayama group, but not in the control group, although these changes were small and not statistically significant. The Ti/Ttot ratios fell in both groups (Table 2).
There were significant changes over time within groups in multiple measures of symptoms and disease severity (Table 3). Though none of the interactions were significant, the simple effects of time within groups were examined, revealing favorable changes in the mMRC and BODE index in both groups, the CAT score in the control group, and the SGRQ-C and SGRQ-C impact score in the pranayama group.
Table 3.
Measures of Symptoms and Quality of Life at Baseline and 12 Weeks
| Pranayama (n = 21) | Control (n = 22) | p | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | Baseline | 12 Weeks | Baseline | 12 Weeks | Group | Time | Group × time |
| mMRC score (0–4, MID = 1)a | 3.1 ± 0.3 | 2.1 ± 1.0b | 3.0 ± 0.3 | 2.4 ± 0.9b | 0.68 | <0.001 | 0.21 |
| BDI (baseline), TDI (12 weeks) | 5.71 ± 2.22 | 0.89 ± 1.88 | 5.55 ± 1.87 | −0.05 ± 2.19 | 0.78, 0.16 | ||
| CAT (0–40, MID = 2)a | 19.1 ± 6.5 | 17.7 ± 6.1 | 21.0 ± 7.0 | 17.5 ± 7.8b | 0.59 | 0.002 | 0.31 |
| BODE index (0–10, MID = 1)a | 5.24 ± 1.81 | 4.06 ± 1.86b | 5.73 ± 1.75 | 5.00 ± 2.37b | 0.24 | <0.001 | 0.42 |
| SGRQ-C (0–100, MID = 4)a | 48.6 ± 12.7 | 42.2 ± 11.6b | 52.1 ± 20.2 | 49.8 ± 21.6 | 0.35 | 0.02 | 0.39 |
| SGRQ-C symptom score | 62.6 ± 18.9 | 61.2 ± 20.0 | 60.4 ± 17.72 | 56.0 ± 21.3 | 0.43 | 0.33 | 0.33 |
| SGRQ-C activity score | 70.2 ± 14.2 | 63.4 ± 18.7 | 73.2 ± 23.12 | 71.3 ± 26.6 | 0.45 | 0.12 | 0.46 |
| SGRQ-C impact score | 31.0 ± 16.7 | 23.1 ± 9.9b | 36.7 ± 21.4 | 34.8 ± 21.5 | 0.14 | 0.02 | 0.16 |
Values represent means ± SD unless otherwise specified. p-Values for group, time, or group × time interaction are from the repeated-measures model, except for BDI and TDI, where p-values are from the t test comparing groups within time points.
Range of scores (lower is better), with MID indicated.
p < 0.05 baseline versus 12 weeks within treatment group based on simple effects.
MID, minimal important difference; TDI, transitional dyspnea index.
Among respiratory system impedance parameters, there was a significant decrease in R5 in the pranayama group after 12 weeks, but otherwise there were no other significant changes in respiratory system impedance between baseline and 12 weeks in either group (Table 4). We demonstrated statistically significant increases in markers of oxidative stress (exhaled breath H2O2 and 8-isoprostane) in the pranayama group, but otherwise there were no changes in any systemic markers of inflammation (serum CRP, RDW-CV, IL-6) in either group (Table 5). When examined before and after the 6-min walk test at baseline, and again at 12 weeks, there were no significant differences in Borg levels of dyspnea, IC, respiratory system impedance, or exhaled breath H2O2, but there was a statistically significant drop in exhaled breath 8-isoprostane after the 6-min walk at 12 weeks versus at baseline in the pranayama group (Table 6).
Table 4.
Respiratory System Impedance at Baseline and 12 Weeks
| Pranayama | Control | |||||
|---|---|---|---|---|---|---|
| Characteristic | Baseline | 12 Weeks | p | Baseline | 12 Weeks | p |
| R5 (cm H2O.s/L) | 1.28 (0.63, 6.03) (n = 19) | 0.73 (0.57, 4.13) (n = 18) | 0.04 | 2.46 (0.64, 7.26) (n = 22) | 2.92 (0.62, 7.31) (n = 20) | 0.55 |
| R20 (cm H2O.s/L) | 0.78 (0.37, 3.75) (n = 19) | 0.39 (0.32, 3.20) (n = 17) | 0.07 | 1.47 (0.40, 3.88) (n = 22) | 1.80 (0.38, 4.03) (n = 20) | 0.74 |
| X5 (cm H2O.s/L) | −0.38 (−2.52, −0.33) (n = 19) | −0.39 (−1.35, −0.29) (n = 18) | 0.33 | −0.48 (−3.47, −0.33) (n = 21) | −1.66 (−4.15, −0.19) (n = 20) | 0.54 |
| Fres (Hz) | 25.8 (20.5, 29.4) (n = 18) | 23.5 (19.2, 29.5) (n = 18) | 0.71 | 24.3 (20.8, 27.9) (n = 21) | 25.5 (21.4, 28.8) (n = 20) | 0.10 |
| AX (cm H2O/L) | 4.77 (3.13, 19.93) (n = 18) | 4.51 (2.29, 8.75) (n = 18) | 0.17 | 4.51 (2.48, 35.56) (n = 21) | 16.82 (2.23, 33.64) (n = 20) | 0.83 |
Values represent the median and IQR. p-values are from Wilcoxon signed-rank tests comparing baseline with 12 weeks within groups.
AX, area under the reactance curve; Fres, resonant frequency; R20, resistance at 20 Hz; R5, resistance at 5 Hz; X5, reactance at 5 Hz.
Table 5.
Markers of Inflammation and Oxidative Stress at Baseline and 12 Weeks
| Pranayama | Control | p | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | Baseline | 12 Weeks | Baseline | 12 Weeks | Group | Time | Group × time |
| CRP (mg/L)a | 0.96 (0.70, 1.80) (n = 19) | 0.83 (0.70, 1.20) (n = 17) | 1.56 (0.40, 2.55) (n = 20) | 0.93 (0, 1.80) (n = 19) | 0.41 | 0.22 | 0.43 |
| RDW-CV (%)a | 14.9 (13.7, 15.4) (n = 21) | 14.4 (13.5, 15.1) (n = 18) | 14.7 (13.7, 15.2) (n = 21) | 14.8 (13.6, 15.3) (n = 20) | 0.90 | 0.29 | 0.11 |
| IL-6 (pg/mL)a | 0.47 (0, 1.47) (n = 20) | 1.14 (0, 2.06) (n = 18) | 0.32 (0, 1.47) (n = 20) | 0.28 (0, 1.18) (n = 19) | 0.20 | 0.36 | 0.26 |
| H2O2 (μmol/L)b | 0.010 (0.009, 0.011) (n = 12) | 0.015c (0.014, 0.019) (n = 13) | 0.013 (0.010, 0.016) (n = 12) | 0.014 (0.010, 0.025) (n = 14) | 0.65 | 0.01 | 0.10 |
| 8-isoprostane (pg/mL)d | 0.019 ± 0.11 (n = 9) | 0.26 ± 0.06c (n = 11) | 0.23 ± 0.04 (n = 12) | 0.26 ± 0.05 (n = 14) | 0.36 | 0.01 | 0.16 |
Data were square root transformed and expressed as back-calculated means and IQR. p-Values for group, time, or group × time interaction are from the repeated-measures model.
Data were log transformed and expressed as back-calculated means and IQR. p-Values for group, time, or group × time interaction are from the repeated-measures model.
p < 0.05 baseline versus 12 weeks within treatment group based on simple effects.
Mean ± SD.
CRP, C-reactive protein; H2O2, hydrogen peroxide; IL-6, interleukin-6; RDW-CV, coefficient of variation of red cell distribution width.
Table 6.
Changes (Δ) in Symptoms, Lung Function, and Exhaled Breath Oxidative Stress Pre- Versus Post-6-Min Walk Test, at Baseline and 12 Weeks
| Pranayama (n = 21) | Control (n = 22) | p | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | Baseline | 12 Weeks | Baseline | 12 Weeks | Group | Time | Group × time |
| Δ Borg | 3.05 ± 2.01 (n = 21) | 2.18 ± 1.67 (n = 17) | 2.64 ± 1.84 (n = 22) | 2.50 ± 2.02 (n = 22) | 0.91 | 0.18 | 0.32 |
| ΔIC (L) | −0.14 ± 0.25 (n = 20) | −0.12 ± 0.35 (n = 17) | −0.03 ± 0.26 (n = 22) | −0.10 ± 0.34 (n = 21) | 0.51 | 0.60 | 0.42 |
| ΔR5 (cm H2O.s/L) | 0.13 ± 0.81 (n = 19) | 0.06 ± 1.21 (n = 17) | 0 ± 1.33 (n = 22) | 0.25 ± 2.36 (n = 20) | 0.93 | 0.79 | 0.59 |
| ΔR20 (cm H2O.s/L) | 0.02 ± 0.75 (n = 19) | 0.14 ± 0.48 (n = 17) | 0.09 ± 0.51 (n = 22) | 0.01 ± 0.84 (n = 20) | 0.88 | 0.93 | 0.41 |
| ΔX5 (cm H2O.s/L) | 0.20 ± 1.81 (n = 19) | −0.33 ± 0.84 (n = 17) | −0.50 ± 0.88 (n = 22) | −0.47 ± 1.66 (n = 20) | 0.22 | 0.39 | 0.33 |
| ΔFres (Hz) | 2.22 ± 3.95 (n = 18) | 1.46 ± 2.75 (n = 17) | 1.75 ± 3.16 (n = 21) | 1.30 ± 3.03 (n = 20) | 0.68 | 0.41 | 0.85 |
| ΔAX (cm H2O/L) | 2.78 ± 5.89 (n = 18) | 1.89 ± 7.77 (n = 17) | 2.24 ± 8.36 (n = 20) | −1.54 ± 10.61 (n = 20) | 0.25 | 0.28 | 0.53 |
| Δ8-isoprostane (pg/mL) | 0.05 ± 0.07 (n = 9) | −0.01 ± 0.04a (n = 11) | 0 ± 0.06 (n = 12) | 0.01 ± 0.03 (n = 14) | 0.29 | 0.14 | 0.05 |
| ΔH2O2 (μmol/L) | −0.005 ± 0.006 (n = 12) | −0.006 ± 0.007 (n = 13) | −0.007 ± 0.008 (n = 12) | −0.003 ± 0.013 (n = 14) | 0.77 | 0.21 | 0.29 |
Mean ± SD. p-Values for group, time, or group × time interaction are from the repeated-measures model.
p < 0.05 baseline versus 12 weeks within treatment group based on simple effects.
There were significant correlations between baseline lung function and 6MWD (FEV1 %predicted, r = 0.54, p < 0.001; IC, r = 0.36, p = 0.02; RV/TLC, r = −0.29, p = 0.06; and X5, r = 0.38, p = 0.02). However, there were no significant correlations between changes in these baseline variables and change in 6MWD at baseline versus at 12 weeks. In a forward stepwise regression procedure predicting baseline 6MWD with the baseline lung function measures, only IC entered the model (R2 = 0.34, p < 0.0001).
Participant performance
Participant diaries revealed that participants in the pranayama group (n = 17 with full diary data) had lower COPD symptom scores (0–3, 0 = no symptoms: median [IQR], 1.18 [1.00, 1.66] versus 2.00 [1.51, 2.08], p = 0.02), rated less shortness of breath (0–10, 0 = no shortness of breath: 1.46 [1.00, 2.08] versus 2.28 [1.74, 3.13], p = 0.01), spent more time on physical activities (107 [89, 187] versus 79 [20, 110] min/week, p = 0.05), and spent more time on breathing exercises (82.5 [53.3, 108.7] versus 0 [0, 28] min/week, p < 0.001), compared with those in the control group (n = 22 with full diary data). On average, participants in the pranayama group spent 12 min per day on pranayama. There were no differences between groups in days of rescue medication use, antibiotic use, steroid use, or hospitalizations. There were no correlations between 6MWD and either time spent on physical activity or time spent on breathing exercises. There were no adverse events related to the interventions, and all participants rated their experience in the study as highly positive. The local yoga professionals validated the adequate teaching of pranayama to study participants by the research coordinators.
Discussion
This is the first randomized, controlled study to demonstrate that simple pranayama alone is associated with improved exercise tolerance in patients with symptomatic COPD. Further, we have demonstrated the feasibility of teaching the technique to patients in the clinical setting by using lay-trained research coordinators as teachers.15–20 This general approach makes our findings applicable in the primary care clinic, in pulmonary rehabilitation programs, and at home, where the approach would be particularly useful for patients who do not have access to a formal program of yoga or pulmonary rehabilitation.
Our rationale for using pranayama as an intervention is that it involves slow, relaxed breathing, which is particularly true of the Three-Part Dirgha breath. Slow, relaxed breathing results in a longer time for expiration and relieves gas trapping.37 The Ti/Ttot ratios fell in both groups, suggesting that patients were altering their breathing patterns to allow a longer time for exhalation. However, only the pranayama group had evidence of reduced resting gas trapping, as seen by the lower RV/TLC ratio at 12 weeks. Our data also demonstrate less resting hyperinflation at 12 weeks, as seen by the increase in IC in the pranayama group compared with the control group. The importance of resting hyperinflation in determining exercise capacity38 is also illustrated by the finding that only the baseline IC was associated with 6MWD in a multifactor logistic regression model. However, immediately after exercise, the change in IC was not different between groups, so we were unable to prove that dynamic hyperinflation was improved during this clinical trial.
We measured other aspects of lung function, including respiratory system impedance, but we were unable to demonstrate a statistically significant effect of pranayama on these parameters over 12 weeks. We expected improvements in parameters that reflect lung compliance, such as X5 or AX,39 but we suspect, in part, that the wide variability in these measurements and relatively small sample size precluded the ability to see such effects. However, it was interesting to note that X5 correlated with 6MWD, suggesting that increased respiratory system compliance (greater [less negative] X5) is associated with increased 6MWD, which would be expected with less hyperinflation.
Slow, relaxed breathing should also enhance well-being and reduce anxiety.11 Although we did not measure anxiety specifically, there were significant improvements in the SGRQ-C impact score in the pranayama group, which associates strongly with other measures of anxiety and depression.40 Finally, there is evidence that a complete yoga program, not just pranayama, can reduce oxidative stress.24 We attempted to assess this via EBC levels of 8-isoprostane and H2O2. Although we found statistically significant increases in exhaled breath H2O2 and 8-isoprostane in the pranayama group at 12 weeks, and an acute decrease in 8-isoprostane after the 6-min walk in the pranayama group at 12 weeks, the clinical significance of these findings is unclear, and we suspect that they may be spurious due to the high variability and small sample sizes associated with these data. We also attempted to track the effect of pranayama on systemic inflammation by measuring serum levels of CRP, RDW-CV, and IL-6. Our data show no difference in these measures within or between groups.
There are some limitations to this study. First, it involved only four initial sessions of pranayama teaching, was only of a 12-week total duration, and involved a small group of patients. We chose a limited number of teaching sessions to mimic real-world feasibility, and the total 12-week length as demonstrated by other studies that have shown benefits of yoga.15,16 Second, we did not specifically test participant comprehension of pranayama practice, but we did monitor that actual practice by a review of videotapes at the halfway point of the trial. Third, we tried to enhance retention and adherence by having the subjects keep diaries and by reminding them to complete their daily practice through weekly telephone calls. Nonetheless, our data show that on average, participants in the pranayama group practiced only 12 min per day at home, which could have reduced the impact of the intervention. Fourth, we tried to blind participants and coordinators involved in assessing outcomes to the true nature of the intervention, although there was certainly the possibility that group assignment may have been revealed, which could have contaminated the results. Fifth, the research coordinators, not professional yoga instructors, provided training in pranayama for the participants, with the potential for teaching improper technique. However, this study design was intentional, as we wanted to determine whether lay instructors could reliably learn to teach pranayama. Sixth, the diary data revealed that participants in the pranayama group spent more time not only on breathing exercises, as intended, but also on physical activity. It is possible that the practice of pranayama made participants feel better and, therefore, more likely to engage in physical activity, which may have contributed to their improved exercise tolerance by enhancing their physical conditioning. Finally, the control group also demonstrated many benefits in this study, which might have diminished our ability to detect the effect of pranayama. This improvement might have been due to the slightly greater amount of time spent in the control group compared with the pranayama group on the educational components of the intervention, which was necessary to balance the total time and attention received by both groups. However, the improvement in the control group may have also been due to the general beneficial effect of participating in a clinical trial (“Hawthorne Effect”).
Conclusion
Our findings demonstrate that practice of a simple method of pranayama by patients with COPD, who were taught by yoga nonprofessionals, is feasible, well tolerated, and associated with improved exercise capacity. This method should be easy to implement in the primary care setting, and it may be helpful for patients who cannot participate in a formal program of yoga or pulmonary rehabilitation but can practice pranayama at home. Future, larger studies will need to confirm this finding and assess the effect of pranayama on other patient-centered outcomes such as symptoms and quality of life.
Acknowledgments
The authors acknowledge Albert van der Vliet, PhD, for performing the biochemical analyses for H2O2, 8-isoprostane, and IL-6; Kathleen Dwinell for administrative support; Ed Dixon and Alexis Walker for help with data compilation; and Jaideep Sood, MD, for early discussions and pilot work related to pranayama and COPD. This study was funded by the National Institutes of Health, NHLBI R34 HL113290.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Cooper C. Airflow obstruction and exercise. Respir Med 2009;103:325–334 [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Rio F, Lores V, Mediano O, et al. Daily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflation. Am J Respir Crit Care Med 2009;180:506–512 [DOI] [PubMed] [Google Scholar]
- 3.Hannink J, van Helvoort H, Dekhuijzen P, Heijdra Y. Dynamic hyperinflation during daily activities. Does COPD Global Initiative for Chronic Lung Disease stage matter? Chest 2010;137:1116–1121 [DOI] [PubMed] [Google Scholar]
- 4.O'Donnell D. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006;3:180–184 [DOI] [PubMed] [Google Scholar]
- 5.O'Donnell D, Lam M, Webb K. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;158:1557–1565 [DOI] [PubMed] [Google Scholar]
- 6.O'Donnell D, Sciurba F, Celli B, et al. Effect of fluticasone propionate/salmeterol on lung hyperinflation and exercise endurance in COPD. Chest 2006;130:647–656 [DOI] [PubMed] [Google Scholar]
- 7.Casaburi R, ZuWallack R. Pulmonary rehabilitation for management of chronic obstructive pulmonary disease. N Engl J Med 2009;360:1329–1335 [DOI] [PubMed] [Google Scholar]
- 8.McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015;CD003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casaburi R, Porszasz J, Burns M, et al. Physiologic benefits of exercise training in rehabilitation of patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997;155:1541–1551 [DOI] [PubMed] [Google Scholar]
- 10.Martarelli D, Cocchioni M, Scuri S, Pompei P. Diaphragmatic breathing reduces exercise-induced oxidative stress. Evid Based Complement Alternat Med 2011;2011:932430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valenza C, Valenza-Pena G, Torres-Sanchez I, et al. Effectiveness of controlled breathing techniques on anxiety and depression in hospitalized patients with COPD: A randomized clinical trial. Respir Care 2014;59:209–215 [DOI] [PubMed] [Google Scholar]
- 12.Ries A, Kaplan R, Myers R, Prewitt L. Maintenance after pulmonary rehabilitation in chronic lung disease: A randomized trial. Am J Respir Crit Care Med 2003;167:880–888 [DOI] [PubMed] [Google Scholar]
- 13.Fan V, Giardino N, Bough D, et al. Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD 2008;5:105–116 [DOI] [PubMed] [Google Scholar]
- 14.Sabit R, Griffiths T, Watkins A, et al. Predictors of poor attendance at an outpatient pulmonary rehabilitation programme. Respir Med 2008;102:819–824 [DOI] [PubMed] [Google Scholar]
- 15.Donesky-Cuenco D, Nguyen H, Paul S, Carrieri-Kohlman V. Yoga therapy decreases dyspnea-related distress and improves functional performance in people with chronic obstructive pulmonary diseae: A pilot study. J Altern Complement Med 2009;15:225–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katiyar S, Bihari S. Role of pranayama in rehabilitation of COPD patients—A randomized controlled study. Indian J Allergy Asthma Immunol 2006;20:98–104 [Google Scholar]
- 17.Pomidori L, Campigotto F, Amatya T, et al. Efficacy and tolerability of yoga breathing in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev 2009;29:133–137 [DOI] [PubMed] [Google Scholar]
- 18.Tandon M. Adjunct treatment with yoga in chronic severe airways obstruction. Thorax 1978;33:514–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta A, Gupta R, Sood S, Arkham M. Pranayama for treatment of chronic obstructive pulmonary disease: Results from a randomized, controlled trial. Integrative Med 2014;13:26–31 [PMC free article] [PubMed] [Google Scholar]
- 20.Fulambarker A, Farooki B, Kheir F, et al. Effect of yoga in chronic obstructive pulmonary disease. Am J Ther 2012;19:96–100 [DOI] [PubMed] [Google Scholar]
- 21.Loring S, Garcia-Jacques M, Malhotra A. Pulmonary characteristics and mechanisms of increased work of breathing. J Appl Physiol 2009;107:309–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Rio F, Miravitlles M, Soriano J, et al. Systemic inflammation in chronic obstructive pulmonary disease: A population-based study. Respir Res 2010;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinho R, Chiesa D, Messomo K, et al. Oxidative stress in chronic obstructive pulmonary disease patients submitted to a rehabilitation program. Respir Med 2007;101:1830–1835 [DOI] [PubMed] [Google Scholar]
- 24.Sinha S, Singh S, Monga Y, Ray R. Improvement of glutathione and total antioxidant status with yoga. J Altern Complement Med 2007;13:1085–1090 [DOI] [PubMed] [Google Scholar]
- 25.Fletcher C. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J 1959;2:257–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borg G. Psychophysical basis of perceived exertion. Med Sci Sports Exerc 1982;14:377–381 [PubMed] [Google Scholar]
- 27.Mahler D, Weinberg D, Wells C, Feinstein A. The measurement of dyspnea: Contents, interobserver agreement, and physiologic correlates of two new clinical indeces. Chest 1984;85:751–758 [DOI] [PubMed] [Google Scholar]
- 28.Jones P, Harding G, Berry P, et al. Development and first validation of the COPD assessment test. Eur Respir J 2009;34:648–665 [DOI] [PubMed] [Google Scholar]
- 29.American Thoracic Society Statement. Guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–117 [DOI] [PubMed] [Google Scholar]
- 30.Celli B, Cote C, Marin J, et al. The body-mass index, airflow obstruction, dyspnea and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005–1012 [DOI] [PubMed] [Google Scholar]
- 31.Wanger J, Clausen J, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005;26:511–522 [DOI] [PubMed] [Google Scholar]
- 32.MacIntyre N, Crapo R, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005;26:720–735 [DOI] [PubMed] [Google Scholar]
- 33.Oostveen E, MacLeod D, Lorino H, et al. The forced oscillation technique in clinical practice: Methodology, recommendations and future developments. Eur Respir J 2003;22:1026–1041 [DOI] [PubMed] [Google Scholar]
- 34.Meguro M, Barley E, Spencer S, Jones P. Development and validation of an improved, COPD-specific version of the St. George Respiratory Questionnaire. Chest 2007;132:456–463 [DOI] [PubMed] [Google Scholar]
- 35.Sham D, Wesley U, Hristova M, van der Vliet A. ATP-mediated transactivation of the epidermal growth factor receptor in airway epithelial cells involves DUOX1-dependent oxidation of Src and ADAM17. PLoS One 2013;8:e54391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahaman I, Kilty I. Antioxidant therapeutic targets in COPD. Current Drug Targets 2006;7:707–720 [DOI] [PubMed] [Google Scholar]
- 37.Dechman G, Wilson C. Evidence underlying breathing retraining in people with chronic obstructive pulmonary disease. Phys Ther 2004;84:1189–1197 [PubMed] [Google Scholar]
- 38.Marin J, Carrizo S, Gascon M, et al. Inspiratory capacity, dynamic hyperinflation, breathlessness, and exercise performance during the 6 minute walk test in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:1395–1399 [DOI] [PubMed] [Google Scholar]
- 39.Kolsum U, Borrill Z, Roy K, et al. Impulse oscillometry in COPD: Identification of measurements related to airway obstrution, airway conductance, and lung volumes. Respir Med 2009;103:136–143 [DOI] [PubMed] [Google Scholar]
- 40.Jones P, Quirk F, Baveystock C, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992;145:1321–1327 [DOI] [PubMed] [Google Scholar]