Abstract
Introduction
This study aims to identify novel quantitative EEG measures associated with mindfulness meditation. As there is some evidence that meditation is associated with higher integration of brain networks, we focused on EEG measures of network integration.
Methods
Sixteen novice meditators and sixteen experienced meditators participated in the study. Novice meditators performed a basic meditation practice that supported effortless awareness, which is an important quality of experience related to mindfulness practices, while their EEG was recorded. Experienced meditators performed a self-selected meditation practice that supported effortless awareness. Network integration was analyzed with maximum betweenness centrality and leaf fraction (which both correlate positively with network integration) as well as with diameter and average eccentricity (which both correlate negatively with network integration), based on a phase-lag index (PLI) and minimum spanning tree (MST) approach. Differences between groups were assessed using repeated-measures ANOVA for the theta (4–8 Hz), alpha (8–13 Hz) and lower beta (13–20 Hz) frequency bands.
Results
Maximum betweenness centrality was significantly higher in experienced meditators than in novices (P=0.012) in the alpha band. In the same frequency band, leaf fraction showed a trend toward being significantly higher in experienced meditators than in novices (P=0.056), while diameter and average eccentricity were significantly lower in experienced meditators than in novices (P=0.016 and P=0.028 respectively). No significant differences between groups were observed for the theta and beta frequency bands.
Conclusion
These results show that alpha band functional network topology is better integrated in experienced meditators than in novice meditators during meditation. This novel finding provides the rationale to investigate the temporal relation between measures of functional connectivity network integration and meditation quality, for example using neurophenomenology experiments.
Mindfulness meditation programs have increasingly shown beneficial effects on a variety of medical disorders. For example, a recent meta-analysis showed that these programs may have similar effects on anxiety and depression as medication, perhaps without the associated side effects (Goyal et al., 2014). Currently, the most consistent EEG findings associated with meditation are increased theta and alpha power (for a recent systematic review see Lomas et al. (2015)). However, as Brandmeyer and Delorme (2013) point out, “Recent research suggests that complex brain activity during meditation may not be adequately described by basic EEG analyses”. More specifically, a limitation of power analysis is that it provides no information about the organization of interactions between brain regions. As such, more advanced EEG measures, such as network integration, i.e. how efficiently information is exchanged across the whole network, may provide potential targets for neurofeedback. Indeed, there is some neuroimaging evidence that meditation may be associated with increased network integration. In a recent EEG study, assessing the relationship between meditation and network integration in the theta band, it was found that a meditation intervention increased global and local network efficiency (which can be considered measures of network integration) during resting-state (Xue et al., 2014). However, in this study, synchronization likelihood was used to calculate functional connectivity, which is sensitive to volume conduction and EEG reference effects. Volume conduction relates to nearby electrodes being highly likely to pick up activity from the same, i.e. common, sources, which then gives rise to spurious correlations between time series at these electrodes (Stam et al., 2007). Fortunately, new methodologies have been developed to address these limitations. A solution to this issue would be to use a measure that assesses phase synchrony (and focuses on non-zero phase differences), such as the phase lag index (PLI), which minimizes the effect of volume conduction (Stam et al., 2007). Functional connectivity matrices based on a phase synchrony measure can then be calculated for the network of all electrodes. Sophisticated signal processing analysis methods, such as recent innovations in graph analysis (Tewarie et al., 2015), can subsequently be used to analyze this matrix, providing the opportunity to investigate advanced potential markers of meditation quality.
Recently, the minimal spanning tree (MST) started to being applied in neuroscience (Tewarie et al., 2015; Utianski et al., 2016; van Dellen et al., 2015). This approach yields an unweighted backbone graph that is considered to reflect the functional core of the network (Stam et al., 2014). The MST approach is thought to avoid several methodological biases in comparing graphs between populations, such as the use of an arbitrary threshold for unweighted graph analysis and differences in average connectivity strength between groups for weighted graph analysis (Tewarie et al., 2015; van Wijk et al., 2010). The MST approach provides the opportunity to assess several aspects of network organization. Specifically, maximum betweenness centrality, leaf fraction, diameter and average eccentricity all represent overall integration of the MST network. Characterization of network integration using this approach has been used for several neurological disorders, including dementia with Lewy bodies, Alzheimer’s disease and Parkinson’s disease (Utianski et al., 2016; van Dellen et al., 2015), but is yet to be applied to meditation training.
Based on the current literature, we hypothesize that during meditation, experienced meditators will show increased integration of their brain networks relative to novice meditators, as assessed using maximum betweenness centrality, leaf fraction, diameter and average eccentricity in an approach that utilizes the PLI and the MST.
Methods
Utilizing a dataset from a previous study (Van Lutterveld et al., 2016), sixteen novice meditators (defined as having no meditation practice in the previous year and <20 entire lifetime hours) and 16 experienced meditators (defined as meditating ≥30 minutes per day for at least 5 days per week over the past 5 years) were matched for age, gender and handedness. Exclusion criteria for both novice meditators and experienced meditators were: (i) any neurological condition, including head injury or head trauma, (ii) any serious psychiatric, cognitive or medical disorder which could interfere with completion of the study (anxiety and depressive disorders in remission were not considered exclusion criteria), (iii) not being on a stable dose for the last 6 months if using anxiolytic or antidepressant medication, (iv) alcohol abuse, specified as drinking more than 14 alcoholic drinks per week at any one time or more than 4 drinks at any one time for a male, and drinking more than 7 alcoholic drinks per week at any one time or more than 3 drinks at any one time for a female, (v) illegal or recreational drug use in the past 6 weeks. Additional exclusion criteria for the novice meditators were (vi) practicing any meditation practice or yoga, Tai Chi or Qigong in the last year or over 20 hours ever in life, attendance of a meditation or yoga retreat, and participation in any meditation course. Demographics for both groups are shown in Table 1. Participants were paid 30 US dollars for their participation in the study. The study was approved by the University of Massachusetts Medical School Institutional Review Board and all participants were provided a fact sheet before participation in the study.
Table 1.
Demographics. Differences in sex, work status, marital status and race were tested using Fisher’s exact tests. Highest completed level of education was tested using the Mann-Whitney test. Differences in age were tested using an independent samples t-test after testing for normality. N/A: not applicable.
| Novice (N=16) | Experienced (N=16) | P | |
|---|---|---|---|
| Gender (male / female) | 11/5 | 13/3 | 0.685 |
| Age (mean with standard deviation in parentheses) | 51 (14) | 50 (14) | 0.970 |
| Handedness (right / non-right) | 13/3 | 14/2 | 1.000 |
| Highest level of completed education (college or university/graduate school ) | 6/10 | 4/12 | 0.453 |
| Work status (full-time / part-time / homemaker / retired / unemployed / not in labor force (student) | 9/3/1/2/1/0 | 12/2/0/1/0/1 | 0.648 |
| Marital status (never married/married/living in permanent relationship/divorced) | 1/11/2/2 | 5/6/3/2 | 0.231 |
| Race (White / African American / Asian) | 13/1/2 | 16/0/0 | 0.226 |
| Meditation practice (vajrayana / theravada / mindfulness / zen / vedanta and mindfulness / zen and mindfulness / theravada and zen and mindfulness / theravada and zen and vajrayana / zen and contemplative) | 5/3/2/1/1/1/1/1/1 | ||
| Lifetime meditation practice hours (median, range) | N/A | 9688 (2046–50978) |
Effortless awareness
All participants were first taught the concept of effortless awareness. The subjective experience of effortless awareness is a major component of meditation practice and consists of the factors “concentration”, “observing sensory experience”, “not ‘efforting’” and “contentment” (Garrison et al., 2013a). Novice meditators were taught “noting practice” meditation, which is theoretically thought to support and train effortless awareness. During noting practice, novices were instructed to silently label the sensory experience that was most predominant from moment-to-moment (i.e. seeing, hearing, feeling or thinking)(Fronsdal, 2008). Before the experiment, novices performed a short noting practice session (~30 s) in which they verbalized the noting practice out loud to confirm that the participants understood the instructions. Next, they completed a short silent practice session (~30 s). Exact instructions for the noting practice and the practice session are provided in Supplementary Text S1. Experienced meditators performed the meditation practice in which it was easiest for them to foster effortless awareness. Exact instructions for the experienced meditators are also provided in Supplementary Text S1. Meditation was performed with eyes open, with the gaze fixated on a fixation cross.
EEG
Technical setup
The participants sat in a quiet room and watched a flat-panel monitor with a viewing distance of 70 cm. Electroencephalography data were recorded with a high-density EEG system using a cap with 128 active electrodes (BioSemi, Amsterdam, The Netherlands). For off-line horizontal electrooculography (EOG) assessment, two electrodes were placed at the outer canthus of the left and right eye, respectively. For off-line vertical EOG assessment two electrodes were placed infra- and supraorbitally at the right eye, respectively. Signals were digitized on-line by a computer at a rate of 2048 Hz.
Task design and experimental procedure
Each run started with a 30-second baseline task during which participants viewed trait-adjectives and assessed if the words described themselves (adapted from (Kelley et al., 2002)). After completing the baseline task, participants performed effortless awareness meditation for 3.5 minutes. This procedure was part of a larger test battery, which is described in detail in (Van Lutterveld et al., 2016). The baseline task was included to familiarize participants with it as it was an essential component of the other tasks in the test battery. To familiarize themselves with the task and the research setting, participants performed an initial run of the paradigm. After this, the main run was performed.
EEG analysis
Preprocessing
For keeping in line with the previous MST literature (that reports on 19 to 21 electrodes), we will focus on a subset of nineteen scalp electrodes in similar locations (Engels et al., 2015; Utianski et al., 2016; van Dellen et al., 2015). As the 128-electrode Biosemi system does not exactly follow the 10/20 system, for four out of nineteen electrodes the electrodes closest to the 10/20 configuration were included. A detailed visual representation is provided in supplementary figure S2. Data preprocessing was performed using the BrainVision Analyzer software suite (BrainProducts, Munich, Germany) and in-house developed Matlab scripts (Natick, MA, USA). First, data of the meditation part of the run was visually inspected for bad channels. Subsequently, data from bad channels was recreated from the surrounding leads in the 128-channel configuration (no more than 2 channels for any subject). Data were filtered between 0.5 and 100 Hz using a Butterworth Infinite Impulse Response (IIR) filter (48 dB/octave). Eye blinks were detected by creating a bipolar vertical EOG channel by subtracting activity in the infraorbitally placed electrode from the superorbitally placed electrode. Horizontal eye movements were detected by creating a bipolar horizontal EOG channel by subtracting activity in the electrode placed at the outer canthus of the left eye from the electrode placed at the outer canthus of the right eye. Ocular correction was performed using Gratton and Coles algorithm (Gratton et al., 1983). After this, data of the meditation part of the run were segmented in 2-second epochs. Artifact rejection was performed in two stages. First, automated artifact rejection took place using the following parameters: 1) maximal allowed voltage step: 50 μV/ms, 2) maximal allowed difference of values in 200 ms intervals: 200 μV, 3) minimal allowed amplitude: −100 μV and maximal allowed amplitude: 100 μV, 4) lowest allowed activity in 100 ms intervals: 0.5 μV. Second, all segments surviving automated artifact rejection were visually inspected for undetected artifacts by two EEG researchers (RvL and HY). Hereafter, twenty artifact-free segments per EEG were randomly selected for further analysis. To avoid carry-over effects of the baseline task into the meditation task, no segments were selected from the first 10 seconds of the meditation part of each run.
Stability analysis of number of selected segments
To verify that the number of segments was sufficient to reach a stable measure of functional connectivity, we plotted the average PLI value of the functional connectivity matrix for the range of 2 – 20 segments for each frequency band (see Supplementary figure S3).
Graph analysis
Brain Wave (version 0.9.152.2.17) software was used for graph analysis (http://home.kpn.nl/stam7883/). Data were filtered in the following frequency bands: theta (4–8 Hz); alpha (8–13 Hz); and beta (13–20 Hz). Oscillations under 4 Hz and above 20 Hz were not analyzed because of expected muscle artifact contamination (Hagemann and Naumann, 2001; Whitham et al., 2007; Yuval-Greenberg et al., 2008). An average reference was applied.
Functional connectivity
Functional connectivity between EEG time-series of all 19 electrode pairs was calculated using the Phase Lag Index (PLI). Briefly, the PLI is a measure for phase synchronization between time series. It is based on the consistency of the nonzero phase lag between those time series and can be calculated from a time series of phase differences Δφ(tk), k = 1 … N in the following way:
The advantage of the PLI is that it is less likely to be contaminated by volume conduction (Porz et al., 2014; Stam et al., 2007). The PLI ranges between 0 (no phase synchronization) and 1 (complete phase synchronization). The PLI has been used extensively to characterize functional connectivity using EEG (e.g. Engels et al., 2015; Utianski et al., 2016; van Dellen et al., 2015). An extensive description of the PLI and its mathematical theory is provided by Stam et al (2007). Average functional connectivity was calculated by calculating the arithmetic mean between all pairwise PLI measurements.
Minimum spanning tree (MST) and graph measures
Network topology of the PLI functional connectivity matrix was characterized using graph theory. In this approach, every electrode constitutes a node in the network and each connection between two electrodes constitutes an edge representing functional connectivity strength between those two electrodes. The network was further characterized using the minimum spanning tree (MST)(Stam et al., 2014). The MST is a subgraph of the weighted graph, which connects all nodes such that the strongest connections in the weighted graph are included, while avoiding loops. This results in an unweighted backbone graph (i.e. binarized graph containing edge weights of 0 and 1) that is considered to reflect the functional core of the network (Stam et al., 2014). Importantly, the MST is thought to avoid several methodological biases in comparing graphs between populations, such as the utilization of an arbitrary threshold for unweighted graph analysis, and differences in average connectivity strength between groups for weighted graph analysis (Tewarie et al., 2015; van Wijk et al., 2010). Figure 1 shows a graphical representation of MST calculation. The MST was used to assess network topology using several key graph measures indicating network integration. Table 2 shows definitions and interpretations of these measures (Utianski et al., 2016), while figure 2 shows more information on MSTs and its association to network integration and graph measures. Summarizing, the MST is a structure that is considered to reflect the structural backbone of the functional connectivity network. Importantly, the structure of the MST is based on the spatial distribution of the underlying functional connectivity network. This means that two networks that have the same average functional connectivity (i.e., the arithmetic mean of all pairwise PLI values), but do not have the same pairwise PLI values at each pair of electrodes, the two networks can have different MSTs, leading to different values of graph measures. Supplementary figure S4 provides an example.
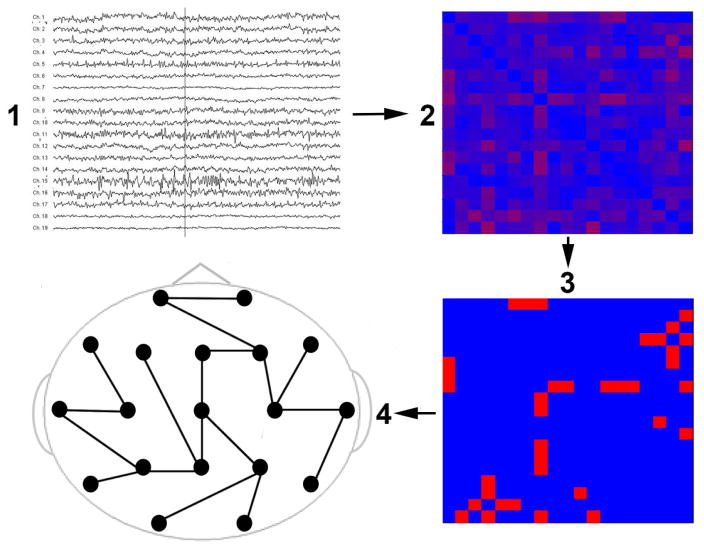
Figure 1.
MST calculation pipeline. Step 1 shows the preprocessed EEG. In step 2, a functional connectivity matrix was calculated using the phase-lag index (PLI) for each pair of electrodes. In step 3, the minimum spanning tree (MST) was constructed from the functional connectivity matrix by including the strongest connections while avoiding loops. All the connections in the MST are set to 1 while all other connections are set to 0. As such, the MST is a structure that is considered to reflect the backbone of the functional connectivity network. Step 4 shows an example of a MST.
Table 2.
definitions and interpretations of minimum spanning tree-based graph measures.
| Minimum Spanning Tree graph measure | Definition | Correlations |
|---|---|---|
| Maximum betweenness centrality | Betweenness centrality indicates the number of shortest paths passing through a node. Maximum betweenness centrality indicates the highest value of betweenness centrality in the network. | Correlates positively with network integration |
| Leaf Fraction | The ratio of the number of nodes with only one edge (i.e. ‘end-points’ in the graph) and the maximum possible number of nodes with only one edge (i.e. the number of nodes minus 1, this indicates a star-shaped graph). | Correlates positively with network integration |
| Diameter | The length of the longest path in the network. | Correlates negatively with network integration |
| Average eccentricity | Eccentricity of a node is defined as the longest distance (measured in number of edges) between that node and any other node. Average eccentricity is the artithmetic mean of all nodes. | Correlates negatively with network integration |
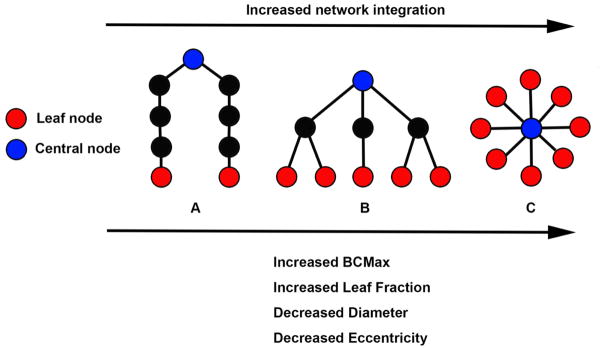
Figure 2.
Schematic representation of three minimum spanning trees (MSTs) consisting of nine nodes. MST structures can range between a path-like tree (i.e., less integrated network) to a star-like tree (i.e. more integrated network). The figure shows examples of a path-like (A), hierarchical (B) and star-like tree (C). Red nodes represent leaf nodes (i.e. end-nodes in the graph). Blue nodes represent central nodes. In A, the blue node characteristics are: betweenness centrality = 16 (indicating there are 16 “shortest-paths” connecting any two nodes through that node) and eccentricity = 4 (indicating the longest distance between that node and any other node). Red node characteristics are: betweenness centrality = 0 and eccentricity = 8. Tree characteristics are BCMax = 16, leaf fraction = 0.25 (indicating 2 leaf nodes divided by the potential maximum of 8 leaf nodes as can be observed in figure C), Diameter (i.e. the longest path in the network) = 8, average eccentricity = 6.2. In B, blue node characteristics are: betweenness centrality = 21 and eccentricity = 2. Red node characteristics are betweenness centrality = 0 and eccentricity = 4. Tree characteristics are BCMax = 21, leaf fraction = 0.625, diameter = 4 and average eccentricity = 3.4. In C, blue node characteristics are betweenness centrality = 28 and eccentricity = 1. Red node characteristics are betweenness centrality = 0 and eccentricity = 2. Tree characteristics are BCMax = 28, leaf fraction = 1, diameter = 2, average eccentricity = 1.9. Path-like trees have the disadvantage of being inefficient in the transfer of information, while the star-like tree at the other side of the spectrum has the advantage that information can spread easily across the network, but the central node might suffer from overloading of information. A hierarchical tree is a hypothesized optimal topology. Please note that the values described above indicate the raw graph values. In the present study, these values are normalized for network size, yielding graph measure values ranging from 0 to 1. BCMax = maximum betweenness centrality. Figure is adjusted from (Numan et al., 2017; van Dellen et al., 2014).
Control analyses
Eye blinks
As participants meditated with their eyes open, and regressing out eye-blinks can theoretically influence the underlying connectivity matrix, we investigated whether excluding segments with eye-blinks would affect results. In this analysis, per participant the first ten segments without eye-blinks were selected from the original twenty segments to keep the number of segments consistent across participants. Participants were excluded in this analysis if there were less than 10 segments that contained no eye-blinks.
Interpolation of bad leads 1
As recreation of bad channels influences the underlying connectivity matrix, we investigated whether excluding the bad channels in MST calculation and subsequent graph measures for the datasets that had bad channels would affect group results. Six out of 16 datasets for novice meditators and for 4 out of 16 datasets for experienced meditators contained either 1 or 2 bad leads. Importantly, as calculation of MST graph measures in the BrainWave software suite includes application of a normalization factor to account for graph size, graph measures can be statistically tested across different graph sizes. Bad leads were excluded in MST and subsequent graph measures calculation. Thus, for these datasets the MST and subsequent graph measures were calculated based on an 18x18 or a 17x17 matrix. For the datasets without bad leads, MST and subsequent graph measures calculation were performed on all 19 channels. Differences across groups were then statistically analyzed identical to the main analysis.
Interpolation of bad leads 2
To further explore the effect of lead interpolation, we investigated whether interpolating leads in in a dataset that did not contain bad leads affected results. For this, one dataset was randomly picked. In the datasets containing bad leads, they were observed in 1–2 positions in a total of 6 different combinations. For each combination, leads of the randomly picked dataset were interpolated and preprocessing and calculation of subsequent graph measures was performed for the same 20 time-windows of 2 seconds each as described above. These outcomes were then statistically tested versus the outcomes of the chosen dataset without lead interpolation.
Statistical analysis
Graph analysis
All statistical analyses were performed with SPSS (version 22.0). For each of the three frequency bands, differences between groups in average functional connectivity of the network were assessed using Mann-Whitney U tests. Correction for multiple comparisons for the three frequency bands was performed using the Bonferroni procedure. Differences between groups for the four graph measures were assessed using a repeated measures ANOVA with between factor ‘group’ (novice or experienced) and within factor ‘graph measure’ (maximum betweenness centrality, leaf fraction, diameter and average eccentricity). Correction for multiple comparisons for the three ANOVAs was performed using the Bonferroni procedure. Levene's test was used to confirm the equality of error variances and Box's M statistic was used to confirm the assumption of homogeneity of covariance matrices. The Greenhouse–Geisser correction was used to adjust the degrees of freedom when the assumption of sphericity was violated as assessed by Mauchly’s test. Post-hoc testing for significant main effects of ‘group’ or significant interaction effects of ‘group’ and ‘graph measure’ was performed using Mann-Whitney U-tests with a correction for multiple comparisons using the Bonferroni procedure. Statistical significance was set at P<0.05.
Control analysis
Eye blinks
Nine novice meditators and twelve experienced meditators met selection criteria of at least 10 segments without eye-blinks in the twenty segments per EEG. Because of this relatively small, uneven number, non-parametric Mann-Whitney U tests were performed without correction for multiple comparisons.
Interpolation of bad leads 1
Statistical analysis was performed identical to the main analysis.
Interpolation of bad leads 2
Differences between the non-interpolated dataset and any of the 6 combinations of interpolation were assessed using separate repeated measures ANOVAs for each frequency band (theta, alpha and beta) and for each outcome measure (PLI, maximum betweenness centrality, leaf fraction, diameter and average eccentricity). The Greenhouse–Geisser correction was used to adjust the degrees of freedom when the assumption of sphericity was violated as assessed by Mauchly’s test. Post-hoc testing was performed by comparing each measure of the interpolated datasets to the non-interpolated dataset using Wilcoxon signed-rank tests. Importantly, to increase sensitivity of the statistical tests in this control analysis, no correction for multiple comparisons was performed for the 15 ANOVAs and the post-hoc tests.
Results
Theta band
No significant difference in average functional connectivity were observed [median experienced: 0.232, median novice: 0.241, Mann-Whitney U=95.000; P=0.214; Pcorrected=0.642]. For the graph measures, no main effect of ‘group’ was observed [F(1,30)=0.964, P=0.334, Pcorrected=1.000] and no significant interaction effects of ‘group’ and ‘graph measure’ were observed [F(1.106,33.174)=0.052, P=0.845, Pcorrected=1.000]. These results show that novice and experienced meditators were not significantly different across the four graph measures of network integration in the theta band. Figure 3 shows a graphical representation of the network integration EEG measures for each frequency band.
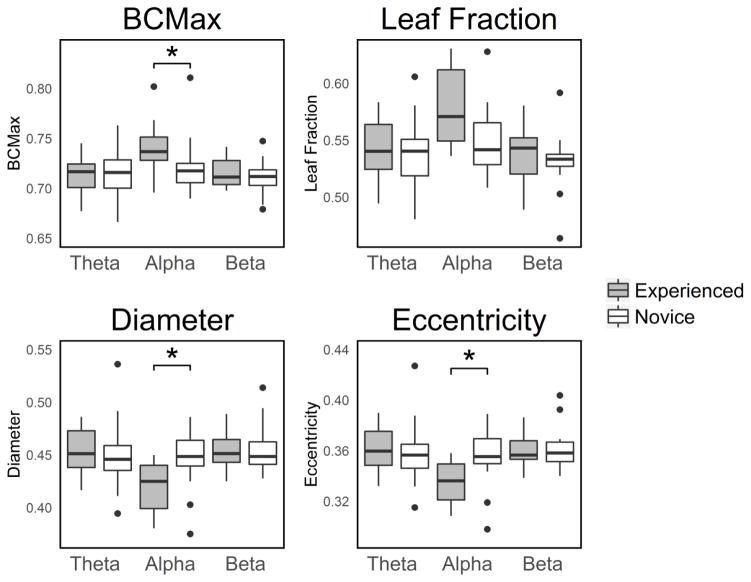
Figure 3.
Boxplots of the network integration graph measures. *: statistically significant at Pcorrected < 0.05. BCMax: maximum betweenness centrality. Eccentricity: Average eccentricity.
Alpha band
No significant difference in average functional connectivity were observed [median experienced: 0.271, median novice: 0.230; Mann-Whitney U=72.000; P=0.035; Pcorrected=0.105]. For the graph measures, no main effect of ‘group’ was observed [F(1,30)=0.911, P=0.347, Pcorrected=1.000]. A significant interaction effect of ‘group’ and ‘graph measure’ was observed [F(1.203,36.078)=6.711, P=0.010, Pcorrected=0.030]. Post-hoc testing showed that maximum betweenness centrality was significantly higher in the experienced group than in the novice group [median experienced: 0.737, median novice: 0.718; P=0.003; Pcorrected=0.012]. Leaf Fraction showed a trend toward being significantly higher in the experienced group than in the novice group [median experienced: 0.571, median novice: 0.542; P=0.014; Pcorrected=0.056]. Diameter was significantly lower in the experienced group than in the novice group [median experienced: 0.425, median novice: 0.449; P=0.004; Pcorrected=0.016]. Average eccentricity was also significantly lower in the experienced group [median experienced: 0.336, median novice: 0.355; P=0.007; Pcorrected=0.028]. These results show that the brain network in the alpha band is more integrated in experienced than in novice meditators during meditation. Figure 3 shows a graphical representation of the network integration EEG measures for each frequency band.
Beta band
No significant difference in average functional connectivity were observed in the beta band [median experienced: 0.181, median novice: 0.184, Mann-Whitney U=96.000; P=0.228, Pcorrected=0.684]. For the graph measures, no main effect of ‘group’ was observed [F(1,30)=1.293, P=0.265, Pcorrected=0.768] and no significant interaction effects of ‘group’ and ‘graph measure’ were observed [F(1.239,37.156)=0.305, P=0.632, Pcorrected=1.000]. These results show that novice and experienced meditators were not significantly different across the four graph measures of network integration in the beta band. Figure 3 shows a graphical representation of the network integration EEG measures for each frequency band.
Stability analysis
Supplementary data S3 shows the stability analysis for the various frequency bands, indicating relative stability of average functional connectivity across frequency bands for the 20 segments used in this study.
Control analysis
Eye-blinks
In the analysis in which segments with eye-blinks were excluded, no significant differences in average functional connectivity were observed in any of the three frequency bands, identical to the main analysis. For the theta band, no significant differences were observed for any of the four graph measures, identical to the main analysis. For the alpha band, maximum betweenness centrality was significantly higher in the experienced group than in the novice group, identical to the main analysis [median experienced: 0.745, median novice: 0.707; P=0.007]. Leaf Fraction was significantly higher in the experienced group than in the novice group, which was a trend in the same direction in the main analysis [median experienced: 0.583, median novice: 0.539; P=0.023]. Diameter was significantly lower in the experienced group than in the novice group, identical to the main analysis [median experienced: 0.422, median novice: 0.444; P=0.012]. Average eccentricity was also significantly lower in the experienced group than in the novice group, identical to the main analysis [median experienced: 0.335, median novice: 0.358; P=0.009]. For the beta band, Leaf Fraction was significantly higher in the experienced group than in the novice group, which was non-significant in the main analysis [median experienced: 0.556, median novice: 0.528; P=0.034]. For the other measures in the beta band, no significant differences between groups were observed, which is identical to the main analysis. These results show that the results in the alpha-band are robust regarding the occurrence of eye-blinks. An overview of median values and associated P-values is provided in supplementary table S5.
Interpolation of bad leads 1
For the theta band, no significant differences in average functional connectivity were observed, identical to the main analysis. In addition, no main effect of ‘group’ was observed and no significant interaction effects of ‘group’ and ‘graph measure’ was observed, identical to the main analysis. For the alpha band, no significant differences in functional connectivity were observed, identical to the main analysis. A significant interaction effect of ‘group’ and ‘graph measure’ was observed, identical to the main analysis [F(1.208,36.241)=6.870, P=0.009, Pcorrected=0.027]. Post-hoc testing showed that maximum betweenness centrality was significantly higher in the experienced group than in the novice group, identical to the main analysis [median experienced: 0.742, median novice: 0.716; P=0.004; Pcorrected=0.016]. No significant differences in Leaf Fraction were observed across groups, which showed a trend in the main analysis [median experienced: 0.571, median novice: 0.552; P=0.035; Pcorrected=0.105]. Diameter was significantly lower in the experienced group than in the novice group, identical to the main analysis [median experienced: 0.426, median novice: 0.450; P=0.003; Pcorrected=0.012]. Average eccentricity was also significantly lower in the experienced group, identical to the main analysis [median experienced: 0.343, median novice: 0.361; P=0.004; Pcorrected=0.016]. For the beta band, no significant differences in average functional connectivity were observed, identical to the main analysis. In addition, no main effect of ‘group’ was observed and no significant interaction effects of ‘group’, identical to the main analysis. These results suggest that the results in the main analysis are relatively robust to the interpolation of bad leads performed. An overview of median values and associated P-values is provided in supplementary table S6.
Interpolation of bad leads 2
No significant results were observed for any combination of frequency band and functional connectivity and graph measure. These results suggest that the outcome measures are relatively robust to the interpolations performed in this study. An overview of median values and associated P-values is provided in supplementary table S7.
Discussion
This is the first study assessing EEG measures of network integration associated with meditation using a minimal spanning tree approach. We partly confirmed our hypothesis that experienced meditators showed increased EEG measures of network integration during meditation relative to novice meditators. In the alpha band, three out of four measures indicated increased network integration in experienced meditators relative to novice meditators in the alpha band, while the fourth measure showed the same trend nearing significance.
These results suggest that the alpha band functional connectivity network is more integrated in experienced meditators than in novice meditators, which could facilitate information exchange between different brain areas. Speculating, increased integration of brain networks may underlie some of the beneficial effects of meditation, such as improved cognition (for a recent meta-analysis see Sedlmeier et al. (2012)). For example, a recent study observed an association between cognition and the same four MST EEG network integration measures as in the present study in Parkinson’s patients with varying degrees of cognitive functioning (Utianski et al., 2016). Specifically, all sixteen correlations indicated positive (though small) relationships between network integration in alpha sub-bands and measures of cognition, from which four reached statistical significance. A longitudinal MEG study in Parkinson’s patients also observed a link between declining cognitive performance and decreasing MST network integration in an alpha sub-band (Olde Dubbelink et al., 2014). In addition, another study found correlations between MST EEG measures of network integration and measures of cognition in patients with Dementia with Lewy Bodies (van Dellen et al., 2015). Together, these findings provide the groundwork for future studies that directly link these measures of network integration with the salutary effects that are being found with various meditation practices.
To the best of our knowledge, to date one previous EEG study has investigated the relationship between meditation and network integration. This group observed that a meditation intervention increased network efficiency (which can be considered a measure of network integration) in the theta band as assessed by the harmonic mean of the shortest path length between each pair of electrodes (Xue et al., 2014). Differences between this finding and the observation of no association between meditation experience and network integration in the theta-band in the present study may be explained by the present study’s cross-sectional design, the present study’s focus on effortless awareness during meditation, and the difference in network integration measures used. Also, the Xue et al study used synchronization likelihood to calculate functional connectivity. A main issue with EEG functional connectivity calculation, such as synchronization likelihood, is that it is sensitive to volume conduction, potentially interfering with accurate functional connectivity assessment (Stam et al., 2007).
Control analyses
An important issue in the present study regards the interpolation of bad leads, as this can affect calculation of the functional connectivity matrix and hence subsequent MST and graph measure calculation. Ideally, one would want clean data without bad leads. However, as this study is a secondary data-analysis of an existing dataset, this was unfortunately not feasible. To verify the validity of the results, a control analysis was conducted in which bad leads were excluded instead of interpolated, finding similar results as in the interpolation analysis. In line with this finding was a second control analysis, showing no significant effects of interpolation on outcome measures. As such, the present findings seem to be relatively robust to the interpolations performed in this study. Another important issue that could affect calculation of the functional connectivity matrix is the occurrence of eye-blinks. However, when only segments without eye-blinks were analyzed, similar results as in the main analysis were observed, which is suggestive of a relative robustness of the outcome measures to the occurrence of eye-blinks.
Importantly, the results of this cross-sectional study provide the rationale to investigate whether there is an association between the network integration measures in the alpha band and the subjective experience of meditation quality in real-time, similar to our previously reported link between 40–57 Hz gamma activity in the posterior cingulate cortex and the subjective experience of effortless awareness (Van Lutterveld et al., 2016). If in future neurophenomenology experiments a temporal relation can be observed between these new measures and the subjective experience of meditation quality, this would provide the rationale to investigate the efficacy of this measure in a neurofeedback randomized controlled trial. Further, these measures could be studied by themselves, or in combination with previously established markers to optimize specificity and sensitivity as the field homes in on markers not just of meditation itself, but specific aspects and qualities therein. Advances such as these are important for moving the field forward as our knowledge of different domains of meditative experience are further defined and elucidated (Lutz et al., 2015).
Limitations
We recruited our meditation sample from a variety of traditions as this approach was intended to increase generalizability across meditation traditions and techniques. Importantly, both novice and experienced meditators performed a meditation that supported effortless awareness. This approach was chosen as effortless awareness has been found to be a major component of meditation practice across a variety of meditation traditions, including Theravada, Zen, Catholic Contemplative and Gelugpa of Tibetan Buddhism (Garrison et al., 2013b). Still, meditation practices may vary in how much emphasis is placed on factors that underlie effortless awareness. In addition, it should be noted that novice meditators performed a meditation practice (noting practice) that was different from the experienced meditators, who performed an individually chosen meditation practice that they considered most effortless. As such, group instructions were different. However, in pilot studies we found that for novices, noting practice was the least effortful form of meditation. Moreover, novices are easily trained to proficiency in this meditation practice (van Lutterveld et al, 2016). In addition, in pilot experiments we observed that experienced meditators who were proficient in a different meditation practice than noting practice, actually found noting practice more effortful than their own meditation practice. For this reason, we chose to implement instructions for both groups that focused on the method that was most effortless for that group. An important consideration in the present study is that, although there is evidence that meditation induces effortless awareness in experienced meditators (Garrison et al, 2013) and in novice meditators (van Lutterveld et al, 2016) , it should be noted that it is unclear to exactly what extent the noting practice task induced the subjective experience of effortless awareness. Also, meditation practices typically also involve contextual components, such as for example intentions for practice, ethical consideration, related conceptual beliefs, and community support. As such, the interpretation of current results remains limited to that of effortless awareness meditation in a decontextualized research setting (Garrison et al., 2013a). It is important to note that we focused on a limited number of EEG electrodes. The prime rationale behind this was future scalability. The end-goal of this research is to develop new neurofeedback measures that can easily be used to inform meditators on their meditation quality, i.e. outside of research lab settings. As such, a limited number of electrodes is paramount as this boosts future ease-of-use. In addition, this kept in line with the previous MST literature (that reports on 19 to 21 electrodes) (Engels et al., 2015; Utianski et al., 2016; van Dellen et al., 2015) and facilitated segment selection. Another limitation of the current study is the relatively small sample size. However, all of our measures in the alpha band showed convergence in pointing toward increased network integration, providing confidence in the present results. An alternative design of the present study to identify a possible association between meditation and graph measures of network integration would be a within-subjects design with experienced meditators, contrasting the meditation state with a resting state. However, during piloting, several of the experienced meditators reported that their resting-state was actually a meditation-state, i.e. they had come to a point in their meditation practice where they were in a continuous meditative state. To be in a non-meditative state would actually be effortful for them, as the meditative state has become their ‘default state’ (Brewer et al., 2011). To be able to include these very advanced practitioners, who constitute the end-goal of a potential neurofeedback paradigm, we felt a cross-section design would be more appropriate. As well, self-report measures on the “effortlessness” of the meditations during the runs were not acquired, which could have shed additional light on the present results. Finally, we conducted this study in a hypothesis-driven way. Machine learning approaches could be employed to identify other markers of meditation quality.
In sum, experienced meditators showed increased measures of network integration in the alpha band. These findings provide the rationale to investigate the relation between these measures and depth of meditation in neurophenomenology experiments.
Supplementary Material
Acknowledgments
This research was supported by NIH/NCCIH grant R01 AT007922-01 and UMASS Medical School institutional funds. Previous versions of this work were presented as a poster at the Neuroscience 2016 conference.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Remko van Lutterveld, Center for Mindfulness, University of Massachusetts Medical School, Worcester, MA, USA.
Edwin van Dellen, Department of Psychiatry, Brain Center Rudolf Magnus, University Medical Center Utrecht, Utrecht, The Netherlands.
Prasanta Pal, Center for Mindfulness, University of Massachusetts Medical School, Worcester, MA, USA.
Hua Yang, Center for Mindfulness, University of Massachusetts Medical School, Worcester, MA, USA.
Cornelis Jan Stam, Department of Clinical Neurophysiology and MEG Centre, VU University Medical Center, Amsterdam, The Netherlands.
Judson Brewer, Center for Mindfulness, University of Massachusetts Medical School, Worcester, MA, USA.
References
- Brandmeyer T, Delorme A. Meditation and neurofeedback. Front Psychol. 2013;4:688. doi: 10.3389/fpsyg.2013.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR, Tang YY, Weber J, Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:20254–20259. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels MM, Stam CJ, van der Flier WM, Scheltens P, de Waal H, van Straaten EC. Declining functional connectivity and changing hub locations in Alzheimer's disease: an EEG study. BMC Neurol. 2015;15:145. doi: 10.1186/s12883-015-0400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fronsdal G. [accessed August 3, 2015];Mental Noting. 2008 http://www.insightmeditationcenter.org/books-articles/articles/mental-noting.
- Garrison KA, Santoyo JF, Davis JH, Thornhill TAt, Kerr CE, Brewer JA. Effortless awareness: using real time neurofeedback to investigate correlates of posterior cingulate cortex activity in meditators' self-report. Front Hum Neurosci. 2013a;7:440. doi: 10.3389/fnhum.2013.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison KA, Scheinost D, Worhunsky PD, Elwafi HM, Thornhill TAt, Thompson E, Saron C, Desbordes G, Kober H, Hampson M, Gray JR, Constable RT, Papademetris X, Brewer JA. Real-time fMRI links subjective experience with brain activity during focused attention. Neuroimage. 2013b;81:110–118. doi: 10.1016/j.neuroimage.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal M, Singh S, Sibinga EM, Gould NF, Rowland-Seymour A, Sharma R, Berger Z, Sleicher D, Maron DD, Shihab HM, Ranasinghe PD, Linn S, Saha S, Bass EB, Haythornthwaite JA. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med. 2014;174:357–368. doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hagemann D, Naumann E. The effects of ocular artifacts on (lateralized) broadband power in the EEG. Clinical Neurophysiology. 2001;112:215–231. doi: 10.1016/s1388-2457(00)00541-1. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Lomas T, Ivtzan I, Fu CH. A systematic review of the neurophysiology of mindfulness on EEG oscillations. Neuroscience and Biobehavioral Reviews. 2015;57:401–410. doi: 10.1016/j.neubiorev.2015.09.018. [DOI] [PubMed] [Google Scholar]
- Lutz A, Jha AP, Dunne JD, Saron CD. Investigating the phenomenological matrix of mindfulness-related practices from a neurocognitive perspective. American Psychologist. 2015;70:632–658. doi: 10.1037/a0039585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan T, Slooter AJC, van der Kooi AW, Hoekman AML, Suyker WJL, Stam CJ, van Dellen E. Functional connectivity and network analysis during hypoactive delirium and recovery from anesthesia. Clinical Neurophysiology. 2017;128:914–924. doi: 10.1016/j.clinph.2017.02.022. [DOI] [PubMed] [Google Scholar]
- Olde Dubbelink KT, Hillebrand A, Stoffers D, Deijen JB, Twisk JW, Stam CJ, Berendse HW. Disrupted brain network topology in Parkinson's disease: a longitudinal magnetoencephalography study. Brain. 2014;137:197–207. doi: 10.1093/brain/awt316. [DOI] [PubMed] [Google Scholar]
- Porz S, Kiel M, Lehnertz K. Can spurious indications for phase synchronization due to superimposed signals be avoided? Chaos. 2014;24:033112. doi: 10.1063/1.4890568. [DOI] [PubMed] [Google Scholar]
- Sedlmeier P, Eberth J, Schwarz M, Zimmermann D, Haarig F, Jaeger S, Kunze S. The psychological effects of meditation: a meta-analysis. Psychological Bulletin. 2012;138:1139–1171. doi: 10.1037/a0028168. [DOI] [PubMed] [Google Scholar]
- Stam CJ, Nolte G, Daffertshofer A. Phase lag index: assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Human Brain Mapping. 2007;28:1178–1193. doi: 10.1002/hbm.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ, Tewarie P, Van Dellen E, van Straaten EC, Hillebrand A, Van Mieghem P. The trees and the forest: Characterization of complex brain networks with minimum spanning trees. International Journal of Psychophysiology. 2014;92:129–138. doi: 10.1016/j.ijpsycho.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Tewarie P, van Dellen E, Hillebrand A, Stam CJ. The minimum spanning tree: an unbiased method for brain network analysis. Neuroimage. 2015;104:177–188. doi: 10.1016/j.neuroimage.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Utianski RL, Caviness JN, van Straaten EC, Beach TG, Dugger BN, Shill HA, Driver-Dunckley ED, Sabbagh MN, Mehta S, Adler CH, Hentz JG. Graph theory network function in Parkinson's disease assessed with electroencephalography. Clinical Neurophysiology. 2016;127:2228–2236. doi: 10.1016/j.clinph.2016.02.017. [DOI] [PubMed] [Google Scholar]
- van Dellen E, de Waal H, van der Flier WM, Lemstra AW, Slooter AJ, Smits LL, van Straaten EC, Stam CJ, Scheltens P. Loss of EEG Network Efficiency Is Related to Cognitive Impairment in Dementia With Lewy Bodies. Movement Disorders. 2015;30:1785–1793. doi: 10.1002/mds.26309. [DOI] [PubMed] [Google Scholar]
- van Dellen E, Douw L, Hillebrand A, de Witt Hamer PC, Baayen JC, Heimans JJ, Reijneveld JC, Stam CJ. Epilepsy surgery outcome and functional network alterations in longitudinal MEG: a minimum spanning tree analysis. Neuroimage. 2014;86:354–363. doi: 10.1016/j.neuroimage.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Van Lutterveld R, Houlihan SD, Pal P, Sacchet MD, McFarlane-Blake C, Patel PR, Sullivan JS, Ossadtchi A, Druker S, Bauer C, Brewer JA. Source-space EEG neurofeedback links subjective experience with brain activity during effortless awareness meditation. Neuroimage. 2016 doi: 10.1016/j.neuroimage.2016.02.047. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk BC, Stam CJ, Daffertshofer A. Comparing brain networks of different size and connectivity density using graph theory. PLoS ONE. 2010;5:e13701. doi: 10.1371/journal.pone.0013701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham EM, Pope KJ, Fitzgibbon SP, Lewis T, Clark CR, Loveless S, Broberg M, Wallace A, DeLosAngeles D, Lillie P, Hardy A, Fronsko R, Pulbrook A, Willoughby JO. Scalp electrical recording during paralysis: quantitative evidence that EEG frequencies above 20 Hz are contaminated by EMG. Clinical Neurophysiology. 2007;118:1877–1888. doi: 10.1016/j.clinph.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Xue SW, Tang YY, Tang R, Posner MI. Short-term meditation induces changes in brain resting EEG theta networks. Brain and Cognition. 2014;87:1–6. doi: 10.1016/j.bandc.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58:429–441. doi: 10.1016/j.neuron.2008.03.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.