Abstract
Vitamin C plays a role in neuronal differentiation, maturation, myelin formation and modulation of the cholinergic, catecholinergic, and glutaminergic systems. This review evaluates the link between vitamin C status and cognitive performance, in both cognitively intact and impaired individuals. We searched the PUBMED, SCOPUS, SciSearch and the Cochrane Library from 1980 to January 2017, finding 50 studies, with randomised controlled trials (RCTs, n = 5), prospective (n = 24), cross-sectional (n = 17) and case-control (n = 4) studies. Of these, 36 studies were conducted in healthy participants and 14 on cognitively impaired individuals (including Alzheimer’s and dementia). Vitamin C status was measured using food frequency questionnaires or plasma vitamin C. Cognition was assessed using a variety of tests, mostly the Mini-Mental-State-Examination (MMSE). In summary, studies demonstrated higher mean vitamin C concentrations in the cognitively intact groups of participants compared to cognitively impaired groups. No correlation between vitamin C concentrations and MMSE cognitive function was apparent in the cognitively impaired individuals. The MMSE was not suitable to detect a variance in cognition in the healthy group. Analysis of the studies that used a variety of cognitive assessments in the cognitively intact was beyond the scope of this review; however, qualitative assessment revealed a potential association between plasma vitamin C concentrations and cognition. Due to a number of limitations in these studies, further research is needed, utilizing plasma vitamin C concentrations and sensitive cognitive assessments that are suitable for cognitively intact adults.
Keywords: vitamin C, ascorbic acid, central nervous system, cognition, Alzheimer’s, dementia, MMSE
1. Introduction
The biological benefits of the water soluble molecule vitamin C (l-ascorbic acid or ascorbate) have been well documented [1,2,3,4,5]. Based on its unique chemistry, the biological role of ascorbate is to act as a reducing agent, donating electrons in various enzymatic and non-enzymatic reactions [6]. It is a cofactor for at least eight enzymatic reactions involved in key bodily processes including the production of collagen, preventing harmful genetic mutations, protecting white blood cells [7] and the production of carnitine, vital for energy [8]. Ascorbate is reversibly oxidized with the loss of two electrons to form dehydroascorbic acid (DHAA).
Despite the extensive research into its enzymatic roles and antioxidant properties, the biological roles of vitamin C on the brain have only recently been described in detail. Animal studies have explored this biological link. In particular, research has focused on guinea pigs, due to their inability to biosynthesize vitamin C from glucose, similar to humans [9]. As a result of this biological limitation, the human brain relies on dietary sources of vitamin C. Animal studies have shown that vitamin C plays a vital role in neurodevelopment by influencing neuronal differentiation and the general development of neurons and myelin formation [9]. Additional, specific neurotransmitter functions include modulation of the cholinergic, catecholinergic, and glutaminergic systems of the brain. Ascorbic acid affects synaptic neurotransmission by preventing neurotransmitter binding to receptors [10], by modulating their release and reuptake [11], and also acting as a cofactor in neurotransmitter synthesis [12]. Another neuromodulatory role of Vitamin C appears to be its involvement in presynaptic re-uptake of glutamate [13], exhibiting a direct effect in the prevention of neuronal over-stimulation by glutamate [14].
Less research has been conducted on ascorbate in collagen synthesis in brain than in other organs, but minimal amounts are essential for blood vessel formation (angiogenesis). Vitamin C is essential for the formation of procollagen which then acts as an intracellular “glue” that gives support, shape and bulk to blood vessels [15]. Studies indicate that vitamin C deficiency in the brain is associated with a reduction in angiogenesis and vascular dysfunction [16,17] and the production of nitric oxide, responsible for vasodilation.
Neurons are especially sensitive to ascorbate deficiency, possibly due to 10-fold higher rates of oxidative metabolism than supporting glia [18]. Ascorbate at the concentrations present in CSF and neurons in vivo has been shown to effectively scavenge superoxide [19]. Once a superoxide radical is formed in the mitochondria of neurons, ascorbate catalyses its conversion to H2O2 and is oxidised in the process to an ascorbate free radical and DHAA. Ascorbate also supports the regeneration of other antioxidants, such as vitamin E and glutathione [19].
Indicative of its vital role in the brain is its recycling, homeostatic mechanism [20] which maintains vitamin C concentrations in the brain and neuronal tissues relative to other bodily organs and tissues. In the healthy brain, the content of vitamin C in cerebrospinal fluid (CSF) is highly concentrated compared to plasma (2–4 times more, 150–400 µmol/L) [21]. In whole brain, 1 to 2 mM of ascorbic acid has been detected, while intracellular neuronal concentrations are much higher, reaching up to 10 mM [22]. These high concentrations are the result of DHAA being recycled into ascorbate within astrocytes, which consist of glutathione [23]. The most saturated vitamin C brain regions include the cerebral cortex, hippocampus and amygdala [24,25].
Although higher plasma ascorbic acid concentrations generally result in higher CSF concentrations, these concentrations start to reach a steady state. As plasma concentrations decline, relatively more ascorbate is pumped into the CSF in order to maintain homeostasis [26]. Studies have demonstrated a higher CSF: plasma ratio in those with lower plasma vitamin C [26,27]. This could be a reflection of the increased “consumption” of ascorbate by the oxidative stressed brain, leading to lower plasma concentrations [26].
Thus, not only is it difficult to deplete brain ascorbate, it is also difficult to increase levels above those set by uptake and recycling mechanisms. In neuronal cells, the apparent Michaelis–Menten transport kinetics (Km) for ascorbate appears to be somewhat high (113 µmol/L); this affinity corresponds well to plasma ascorbate concentrations of 30–60 μmol/L [28]. Thus, plasma vitamin C can only relate to brain vitamin C status in a narrow window, likely levels below 30 μmol/L.
Duration of deficiency has shown to influence brain ascorbate concentrations to a higher degree than the amount of depletion. This is exemplified by observations in acute scurvy where brain concentrations of ascorbate are relatively maintained through depletion of peripheral tissues [29], whereas marginal deficiency for longer periods of time resulted in greater brain ascorbate depletions [30].
Given the various biological roles on the central nervous system, a number of studies have been conducted with the intention of exploring whether vitamin C status is associated with cognitive performance in cognitively intact participants as well as those diagnosed with a neurodegenerative condition. This systematic review is the first to explore the effects of blood vitamin C status and cognitive performance in both cognitively impaired and intact groups of participants. This systematic review summarises current knowledge and provides recommendations for future studies.
2. Methods
2.1. Search Strategy
We searched the PUBMED, SCOPUS, SciSearch and the Cochrane Library for publications from 1980 to January 2017. Keywords used were vitamin C, ascorbic acid, antioxidant, cognition, memory, Alzheimer’s and dementia. Additional published reports were obtained by checking references of screened articles. Studies only examining cognitive function and vitamin C status were included.
2.2. Selection of Trials
Study designs included randomised controlled trials, prospective cohort, cross-sectional, and case-control, restricted to those in the English language. This selection included adult participants who were either cognitively intact or diagnosed with a neurodegenerative condition such as Alzheimer’s or dementia. Studies that administered some form of vitamin C measure and quantitative cognitive assessment were accepted.
2.3. Quality Assessment
Quality of studies was independently assessed by two investigators (NT and KR). Appraisal was determined using established guidelines for randomised, controlled trials (RCT), and observational studies (prospective and cohort) established from the Cochrane collaboration [31]. Quality was assessed on selection bias, allocation bias, attrition bias, methods to control confounding factors, and conflict of interest. Compliance was further assessed in RCTs. Higher-quality trials (score ≥4 of 8 points for RCT, ≥3 of 4 points for prospective and ≥2 of 3 for cross-sectional and case control) were compared with lower-quality studies.
2.4. Analysis of Trials Using Comparable Methods
An initial survey of the literature revealed that many studies used comparable cognitive and vitamin C measures—The Mini Mental State Examination (MMSE) and blood plasma vitamin C concentrations. Given this consistency in measurement we decided to further explore these trends across studies. A brief summary of these inclusions and methods is presented below. We contacted authors for mean values and standard deviations of studies which did not report numerical mean vitamin C concentrations or MMSE scores (0–30) but instead placed the means into categories (e.g., MMSE score of over/under 27, vitamin C concentrations into deficient/adequate ranges).
2.5. Blood Plasma Vitamin C
Given the practicality and accuracy of measuring absorbed vitamin C status through blood plasma, plasma vitamin C has been considered the ideal measure of vitamin C status [32]. A number of investigated studies have used this measure to determine vitamin C status. vitamin C blood concentrations, based on population studies, indicate that a plasma concentration of <11 μmol/L is considered to be deficient, 11–28 μmol/L is depleted or marginally deficient, 28–40 μmol/L is adequate, and >40 μmol/L is optimal [33]. Other studies measured CSF vitamin C concentrations or incorporated a variety of FFQs and supplementation questionnaires, measuring daily intake in milligrams. A recommended daily intake of 200 mg/day has been suggested, as this corresponds with optimal vitamin C blood concentrations [34].
2.6. Measure of Cognition
The MMSE is a simple validated and reliable paper and pen questionnaire designed to estimate the severity and progression of cognitive impairment and used to follow the course of cognitive changes in an individual over time [35]. Any score greater than or equal to 24 points (out of 30) indicates normal cognition. Below this, scores can indicate severe (≤9 points), moderate (10–18 points) or mild (19–23 points) cognitive impairment [36]. The cognitive domains measured include attention and calculation, recall, language, ability to follow simple commands and orientation. Descriptive analyses were conducted for all included studies, which assessed vitamin C concentrations (means and standard deviations in µmol/L for blood tests and mg/day for FFQs), and mean MMSE scores.
2.7. Z Statistical Analysis-Correlation Between Blood Vitamin C and MMSE Score
Using IBM SPSS (version 23, Chicago, IL, USA) t-tests were conducted, comparing the baseline blood vitamin C concentrations and baseline MMSE scores between cognitively intact and impaired participants. Due to the ordinal nature of MMSE scores and ratio scales for blood test concentrations, a Spearman’s correlation coefficient analysis (r values) was conducted. R-squared values, assessing goodness of fit and test of normality were conducted to establish the correlation between mean vitamin C concentrations and MMSE scores.
Only studies which measured blood vitamin C concentrations and cognition through the MMSE were compared. Comparable mean vitamin C blood concentrations and MMSE scores were extracted as separate data points from each of the studies and plotted graphically. A number of studies assessing cognitively impaired individuals also used healthy controls. The mean MMSE and vitamin C concentrations from these controls was added to the mean scores of other cognitively intact samples for comparison.
FFQ-based vitamin C levels were also converted to predicted blood concentrations, where every 1.97 mg of consumed vitamin C equates to 1 µmol/L of ascorbate plasma. A constant plateau in ascorbic acid concentration (60–80 µmol/L) is reached at 150 mg of consumed vitamin C [34]. Given the non-linear link between vitamin C consumption and absorption, the converted FFQ blood concentrations were added to the scatterplot for comparison, but were not included in the analysis. Additionally, ascorbate CSF concentrations were not included in the analysis due to a non-linear relationship with plasma vitamin C.
Additionally, qualitative analyses were conducted on the studies that utilized a range of other cognitive assessments and direct plasma vitamin C measures. These studies were reported qualitatively due to a large diversity in cognitive assessments and statistical reporting of results (odds ratios, confidence intervals, etc.). The overall trend of results and quality of these trials was taken into account for the qualitative analysis.
3. Results
The search captured exactly 500 articles, of which 50 studies were included in the systematic review (Figure 1). Of these, 14 studies involved cognitively impaired participants, e.g., dementia including Alzheimer’s disease and 36 studies were conducted on cognitively intact participants. The cognitively impaired subgroup included 3 RCTS [37,38,39], 4 prospective [26,40,41], 4 cross-sectional [42,43,44,45] and 4 case-control [46,47,48,49] studies (Table 1). The cognitively intact subgroup included 2 RCTS [50,51], 21 prospective [52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72], 13 cross-sectional [73,74,75,76,77,78,79,80,81,82,83,84,85], and no case-control studies (Table 2). Table 3 summarises the trials that were excluded from the review, and the reason for their exclusion.
Figure 1.
Flow chart of steps in systematic review.
Table 1.
Characteristics and outcomes of studies using cognitively impaired samples.
| Paper | Study Design | N | Age (years) | Condition | Quality Rating | Cognitive Measure | Vitamin C Measure | Outcome |
|---|---|---|---|---|---|---|---|---|
| Arlt, 2012 [37] | RCT | 23 | 60–80 | AD | 6 | MMSE, Word fluency, Immediate/delayed verbal recall, Trail-making task | CSF | 1000 mg/day of vit C and E (400 mg/day) increased CSF concentrations after 1 year, but decreased MMSE score and no effect on other measures |
| Galasko, 2012 [47] | RCT | 78 | 50–85 | AD | 4.5 | MMSE | CSF | Decline in MMSE score occurred in E/C/ALA group. (500 mg/day vit C, vit E, alpha lipoic acid) did not influence CSF biomarkers related to amyloid |
| Burns, 1989 [39] | RCT | 81 | ≥65 | Senile Dementia, Community dementia | 4.5 | MMSE | Blood tests | 200 mg Vit C, vits B1, B2, B3 No correlation between vit C intake and cognitive impairment |
| Bowman, 2009 [26] | Pros | 32 | 71 | AD | 5 | MMSE | CSF, plasma ascorbate | Neither Plasma nor CSF AA predictive of AD across 1 year |
| Zandi, 2004 [40] | Pros | 4740 (4540 healthy) | ≥65 | AD | 3.5 | 3MS, Dementia Questionnaire (DQ) | Supplement, Interview | vit E (>400 IU) and C (500 mg) supplements reduced the AD prevalence and incidence. Supplements alone had no protective affect across 2 years |
| Deijen, 2003 [41] | Pros | 90 | >65 | Psychiatry nursing home | 4.5 | Dutch geriatric nursing scale, Zorg Index geriatrie (ZIG) | Food record | Higher vitamin intakes were associated with a worse daily functioning across 6 months |
| Rinaldi, 2003 [42] | Cross | 141 | >70 | MCI, AD | 3 | Clinical dementia rating scale, MMSE, clock drawing test, Babcock story recall, auditory verbal learning test, Corsi block tapping test, Token test, category naming test, Oral word association test, visual search test, digit forward and backward test, Raven’s progressive colored matrices | Plasma ascorbate | Lower vit C concentrations in patients with AD and MCI. MCI sig lower then controls |
| Polidori, 2004 [43] | Cross | 141 | ≥65 | AD, VaD | 2 | MMSE | Plasma ascorbate | Plasma AA lower in AD and VD |
| Richardson, 2002 [44] | Cross | 37 | 65–97 | In-patient ward | 2 | MMSE | Plasma ascorbate | 75% with dementia had low concentrations of vitamin C |
| Lu, 2016 [45] | Cross | 2892 (768 MCI) | 58 | MCI | 2.5 | Montreal cognitive assessment | FFQ | Carotenoids, vit C, and vitamin B6 exhibited the highest protective factor loadings |
| Charlton, 2004 [46] | CC | 93 | ≥65 | Dementia | 4 | MMSE | Plasma Ascorbate/FFQ | Plasma AA lower in dementia, not explained by diet |
| Glaso, 2004 [47] | CC | 38 | 75–85 | AD | 4 | MMSE | Serum ascorbate/CSF | Both plasma vitamin C and CSF lower in AD. CSF: plasma AA ratio higher in AD |
| Riviere,1999 [48] | CC | 69 | >75 | Severe AD, Moderate AD, Hospitalised AD | 3.5 | MMSE | Plasma ascorbate, FFQ | Nutritional intake lower in Severe AD, plasma vit C lower in more severe AD, not explained by vit C intake |
| Masaki, 2000 [49] | CC | 3735 men | 71–93 | Dementia | 3 | Hasegawa scale, MMSE | Self-report supplementation | After controlling for factors such as age, education, stroke, there was an association with cognitive performance |
Key: MCI = Mild cognitive impairment, AD = Alzheimer’s, VaD = vascular dementia RCT = Randomized control trial, Pros = prospective, Cross = cross-sectional, CC = case-control, Vit = vitamin, FFQ = food frequency questionnaire, CSF = cerebrospinal fluid, MMSE = Mini mental state examination, 3MS = Modified Mini Mental State Examination, ALA = alpha lipoic acid.
Table 2.
Characteristics and outcomes of studies using cognitively intact samples.
| Paper | Study Design | N | Age (years) | Quality Assessment | Cognitive Measure | Vitamin C Measure | Outcome |
|---|---|---|---|---|---|---|---|
| Chandra, 2001 [50] | RCT | 86 | ≥65 | 5.5 | Wechsler memory test, Halstead-Reitan categories test, Buschke consistent long-term retrieval, digit span forward, salthouse listening span test, long-term memory recall, MMSE | Plasma spectrophotometry | 80 mg of vitamin C in a multivitamin improved cognitive performance, not Long-term memory across 1 year |
| Dror, 1996 [51] | RCT | 21 | >80 | 3.5 | MMSE | Plasma Assay | No changes in MMSE scores following 42-day supplementation with 45mg/day of vitamin C with other vitamins (Vit D, E B12, B6) |
| Gale, 1996 [52] | Pros | 921 | ≥65 | 2.5 | Hodkinson mental test (Dementia assessment) | Dietary intake/Ascorbate plasma | Cognitive function was poorest in those with the lowest vitamin C over 1 year |
| La Rue, 1997 [53] | Pros | 137 | 66–90 | 5 | Abstract performance, visuospatial performance, memory assessment | Plasma Ascorbate, Nutritional status | Visuospatial performance was higher with higher ascorbate concentrations after 6 years |
| Paleologos, 1998 [54] | Pros | 117 | 69–91 | 4 | MMSE, Reid brief neuropsychological Screen, the animals test of category fluency, the F, A, S test of verbal fluency | Semi-quantitative food frequency | After adjusting for age, sex, smoking, education, energy, vit C supplement linked to less severe cognitive decline, not verbal/category fluency across 4 years |
| Devore, 2002 [55] | Pros | 16,010 | >70 Women | 5 | MMSE, Telephone interview for cognitive status (TICS). East Boston memory test (immediate/delayed) category fluency, Delayed TICS, Digit span backwards | Semi-quantitative food frequency | Dietary vitamin C intake not associated with cognitive decline. Supplemental vit C associated with worse decline over 6 years |
| Engelhart, 2002 [56] | Pros | 5395 | >55 | 3.5 | DSM-III-R criteria, MMSE | Semi-quantitative food frequency (SFFQ) | Higher dietary vit C intake associated with less AD after a mean of 6.5 years, controlling for supplements |
| Kalmijn, 1997 [57] | Pros | 342 Men | 69–89 | 3 | MMSE | Dietary history FFQ | Higher vit C intake not correlated with cognitive decline or impairment after 3 years |
| Laurin, 2003 [58] | Pros | 2549 Men | 45–68 | 4 | Hasegawa dementia screening instrument, MMSE, 3MS | 24-h dietary recall | Vit C was not associated with the risk of dementia or its subtypes across an 8-year period |
| Basambombo, 2016 [59] | Pros | 5269 | ≥65 | 2.5 | Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R) | Self-reported supplementation | The use of vitamin C supplements associated with a reduced risk of cognitive decline during 3, 5 year intervals |
| Nooyens, 2015 [60] | Pros | 2613 | 43–70 | 5 | 15 Words Learning Test, the Stroop Test, Word Fluency test, Letter Digit Substitution Test | 178-item semi-quantitative FFQ | No associations between intakes of vit C and cognitive decline across 5 years |
| Peneau, 2011 [61] | Pros | 2533 | 45–60 | 4.5 | RI-48 cued recall, semantic, and phonemic fluency tests, trail-making and forward and backward digit span tests | 24-h dietary record | vit C–rich FVs (P-trend = 0.03), vitamin C (P-trend = 0.005) positively associated with verbal memory across 13 years |
| Fotuhi, 2008 [62] | Pros | 3376 | ≥65 | 2.5 | 3MS | Self-report | Combined vit C, E, and anti-inflammatory resulted in a lower decline on the 3MS across 8 years. Vit C alone had no affect |
| Gray, 2008 [63] | Pros | 2969 | ≥65 | 3.5 | Cognitive abilities screening instrument | Self-report | No association between vitamin C and AD incidence, or vit C and E together after 2.8–8.7 years |
| Wengreen, 2007 [64] | Pros | 3831 | ≥65 | 3.5 | 3MS | Food frequency | Higher quartiles of vit C intake had a greater 3MS score and lower vit C intake had a greater rate of decline during 7 years |
| Fillenbaum, 2005 [65] | Pros | 616 | 65–105 | 3.5 | Short portable mental status questionnaire | In home interview | Vitamin C did not reduce AD or dementia incidence over either 3 or 14-year interval |
| Maxwell, 2005 [66] | Pros | 894 | ≥65 | 3.5 | 3MS | Self-report | Subjects reporting supplementation of vit C were less likely to have cognitive decline or to be diagnosed with VCI after 5 years |
| Grodstein, 2003 [67] | Pros | 14,968 | 70–79 women | 4.5 | Telephone Interview of Cognitive Status, Delayed recall of 10 word lists, Immediate and delayed recall of paragraph, Verbal fluency, Digit span backwards | Supplementation questionnaire | Vit C and E had higher mean global scores than non-supplemented. Vit C alone did not affect global score after 5 years |
| Luchsinger, 2003 [68] | Pros | 980 | ≥65 | 4.5 | Neuropsychological test battery | Semi quantitative food frequency | Neither dietary, supplemental nor total intake of vit C across 4 years was linked to AD Incidence |
| Morris, 2002 [69] | Pros | 815 | >65 | 3 | Consortium Established for Research on AD | FFQ | Intake of vitamin C was not significantly associated with risk of AD across 3.9 years |
| Peacock, 2000 [70] | Pros | 12,187 | 48–67 | 4.5 | Delayed word recall test, Wechsler adult intelligence scale, Revised digit symbol subtest, word fluency test | Food frequency questionnaire | No consistent association between dietary and supplemental vit C and cognition across 8 years |
| Morris, 1998 [71] | Pros | 633 | ≥65 | 3.5 | Criteria for clinical diagnosis | Supplementation questionnaire | None of the vitamin C users were diagnosed after a mean of 4.3 years |
| Mendelsohm, 1996 [72] | Pros | 1059 | ≥65 | 2.5 | Neuropsychological battery (15 items) | 297 vitamin C self-report supplementation | After adjustment for age, race, income, education, vit C supplementation did not relate to cognitive scores during 2 years |
| Berti, 2015 [73] | Cross | 52 Women | 54–66 | 1.5 | Clinical dementia rating, Global deterioration score, MMSE | Harvard/Willet FFQ | Antioxidant consumption positively associated with METglc (p < 0.001) |
| Beydoun, 2015 [74] | Cross | 1274 | 30–60 | 2 | MMSE, CLVT-list A, CVLT-DFR, digit span forward/backwards, Benten visual retention test, Animal fluency test, Brief test of attention, trail making test, Clock drawing test, card rotations, identical pictures | Two 24-h recalls | Vitamin C not associated with cognition on either cognitive task, MMSE error count (p = 0.17) |
| Chaudhari, 2015 [75] | Cross | 582 | 40–96 | 2 | Repeatable battery for the assessment of neurological status, The executive interview | Ascorbate supplementation (self-report) | Vit C led to better immediate memory (p = 0.04), visuospatial skills (p = 0.002), language (p = 0.01), global cognition (p = 0.006) |
| Goodwin, 1983 [76] | Cross | 260 | >60 | 2 | Halstead-Reitan Categories, (Non-verbal abstract thinking), Wechsler Memory Test | Dietary intake/Ascorbate plasma | Performance worse on both tasks in those with low vit C (5–10% lowest levels) |
| Jama, 1996 [77] | Cross | 5182 | 55–95 | 2.5 | MMSE | Semi-quantitative food frequency questionnaire | No association between cognitive function and intake of vitamin C intake (<70mg/day (odd ratio) = 1.14, 130–160 mg/day (od) = 1.21 |
| Lindemann, 2000 [78] | Cross | 195 | ≥65 | 3 | MMSE, WAIS-R Digits Forward, Fuld Object Memory Evaluation, Clock drawing, Two Color Trail Making Tests | Serum ascorbate | Lower vit C not associated with cognition. There was a trend. Low vit C linked with a history of depression |
| Perrig, 1997 [79] | Cross | 442 | ≥65 | 3 | Computerised cognitive test (assessed working, implicit and explicit memory), WAIS-R vocabulary test | Plasma Ascorbate | Free recall, recognition, and vocabulary (not priming or working memory) correlated with ascorbic acid concentrations (semantic memory p = 0.034, vocabulary test p ≤ 0.021) |
| Schmidt, 1998 [80] | Cross | 1769 | 50–75 | 2 | Mattis Dementia Rating Scale | Plasma (chromatograph) | No association between cognitive scores and plasma concentrations (odds ratio = 1, p = 0.87) |
| Sato, 2006 [81] | Cross | 544 | ≥65 | 2.5 | Digit symbol substitution task (DSST), MMSE | Ascorbate plasma, Block’s FFQ | Highest fifth of plasma ascorbate associated with better DSST, marginally with MMSE |
| Whalley, 2003 [82] | Cross | 176 | 77 | 2.5 | MMSE, Raven’s Progressive Matrices | Ascorbate plasma, FFQ (MONICA) | No difference between those taking vitamin C supplements and controls, after controlling for childhood IQ, education, socioeconomic status and cardiovascular health |
| Perkins, 1998 [83] | Cross | 4809 | >60 | 2 | Delayed word recall, Delayed story recall | Serum ascorbate | After adjusting for socioeconomic factors and other trace elements, vitamin C concentrations were not associated with poor memory performance |
| Ortega, 1997 [84] | Cross | 260 | 65–90 | 1.5 | MMSE, Pfeiffer’s mental status questionnaire | Food frequency for 7 days | Higher cognition correlated with great vitamin C intake across 7 days |
| Requejo, 2003 [85] | Cross | 168 | 65–90 | 0.5 | MMSE | Food record | Those with a greater intake of vitamin C were more likely to display adequate cognitive ability |
Key: MCI = Mild cognitive impairment, AD = Alzheimer’s, VaD = vascular dementia RCT = Randomized control trial, Pros = prospective, Cross = cross-sectional, CC = case-control, Vit = vitamin, FFQ = food frequency questionnaire, CSF = cerebrospinal fluid, MMSE = Mini mental state examination, 3MS = Modified Mini Mental State Examination.
Table 3.
List of studies with reasons for exclusion.
| Study | Study Design | Reason for Exclusion |
|---|---|---|
| Kennedy (2011) [86] | RCT | Mood/fatigue primary measures, vitamin C status not assessed |
| Smith (1999) [87] | RCT | Self-reported cognitive failures (subjective cognitive assessment) |
| Kumar (2008) [88] | RCT | Vitamin C status not assessed |
| Yaffe (2004) [89] | RCT | Cognition not assessed at baseline, vitamin C status not assessed |
| Kang (2009) [90] | RCT | Cognition not assessed at baseline, only 3.5 years after intervention |
| Chui (2008) [91] | RCT | Vitamin C status not assessed, no placebo/blinding |
| Day (1988) [92] | RCT | Vitamin C status not assessed, assessed only confusion |
| Paraskevas (1997) [93]/Quinn (2004) [27]/Woo (1989) [94]/Polidori (2002) [95]/Foy (1998) [96] | CS | No cognitive tests administered |
| Talley [97] | Pre-test post-test | Simple orientation/consciousness assessment |
Legend: RCT = Randomised control trial, CS = case-control.
In the cognitively impaired samples, eight out of 14 studies used blood tests to measure vitamin C [26,39,42,43,44,46,47,48], two used CSF [37,38] and four used FFQs alone [40,41,45,49]. A series of cognitive tests were conducted in these studies. Eleven studies [26,37,38,39,42,43,44,47,48,49] used the MMSE and six [37,40,41,42,45,49] used alternate forms of cognitive assessment. In the cognitively intact samples, 11 out of 36 used blood tests to measure vitamin C status [50,51,52,53,76,78,79,80,81,82,83], and 25 studies conducted FFQs [54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,77,84,85]. A series of cognitive tests were conducted in these studies. Fifteen studies [50,51,54,55,56,57,58,73,74,77,78,81,82,84,85] used the MMSE and 31 studies [50,52,53,54,55,56,58,59,60,61,62,63,64,65,66,67,69,70,71,72,73,74,75,76,78,79,80,81,82,83,84,98] used other forms of cognitive assessment.
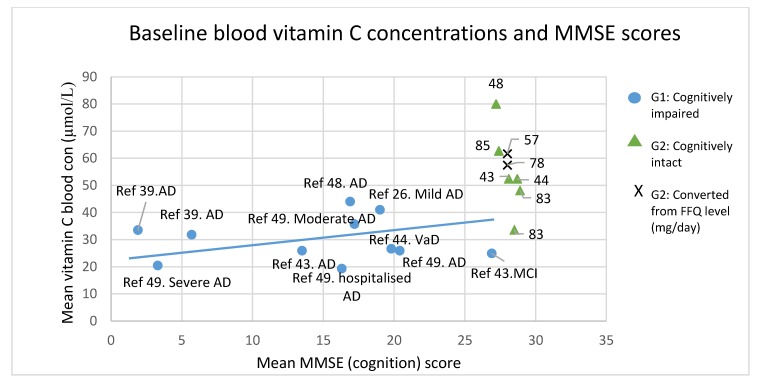
Mean MMSE scores and measured or derived blood vitamin C concentrations are plotted in Figure 2 and presented in Table 4 and Table 5. In the cognitively impaired group, these means were extracted from seven studies (sample sizes ranged from 12–88 participants, with a total of 391 participants). Independent samples t-tests revealed that mean vitamin C concentrations in the cognitively intact subgroup were significantly higher than in the cognitively impaired (t (15) = 4.5, p < 0.01) and mean MMSE scores were also significantly higher in this subgroup (t (10.3) = 5.7, p < 0.01).
Figure 2.
Scatterplot of baseline mean MMSE scores against blood vitamin C concentrations. Blue circles represent cognitively impaired groups of participants, and green triangles and crosses represent cognitively intact groups (triangles: direct plasma vit C measure, cross: converted from FFQ). No correlation analyses were conducted on the cognitively intact data points. The blue line represents the correlation slope amongst the studies of cognitively impaired groups of participants (rs (11) = 0.009, p = 0.98). Key: Ref = study reference, * Not included in the analysis, AD = Alzheimer’s disease, CSF = Cerebral Spinal Fluid, FFQ = Food Frequency Questionnaire; MCI = mild cognitive impairment, mg/day = milligram per day, VaD = Vascular dementia, Con = concentration, MMSE = Mini Mental State Examination.
Table 4.
Cognitively impaired participants (Mean blood vitamin C/MMSE scores).
| Paper | Study Design | N | Mean Vitamin C Level in μmol/L (SD) | Mean MMSE Score (SD) |
|---|---|---|---|---|
| Burns (1989) [39] | RCT | 81 | Intervention baseline-33.5 (28) Placebo baseline-31.8 (31) Placebo final-25 (28) # |
1.9 (3.3) 5.7 (9.1) 5.7 (10.6) # |
| Bowman (2009) [26] | Pros | 32 | 41 (30) | 19 (5) |
| Rinaldi (2003) [42] | CS | 25 63 |
MCI-24.9 (2.4) AD-25.9 (8.9) |
26.9 (2) 13.5 (6.5) |
| Polidori (2004) [43] | CS | 63 23 |
AD-25.9 (8.9) Vascular AD-26.6 (11.3) |
20.4 (3) 19.8 (3) |
| Glaso (2004) [47] | CC | 20 | AD-44 (25) | 16.9 |
| Rivierie (1999) [48] | CC | 24 9 20 |
Moderate AD-35.7 Hospitalized AD-19.3 Severe AD-20.4 |
17.2 (4.9) 16.3 (6.1) 3.3 (3.1) |
Legend: SD, standard deviation; RCT = randomised controlled trial, Pros = prospective, CS = cross-sectional, CC = case-control, # not a baseline value therefore not included in analysis, blue circles representing cognitively impaired blood values.
Table 5.
Cognitively intact participants (Mean blood vitamin C/MMSE scores).
| Paper | Study Design | N | Vitamin C Level in μmol/L (SD) | MMSE Score (SD) |
|---|---|---|---|---|
| Engelhart (2002) [56] * | Pros | 5395 | 61.7 (27) | 28 |
| Jama (1996) [77] * | CS | 5182 | 57.5 | 28 |
| Ortega (1997) [84] | CS | 260 | 62.7 (33.5) | 27.4 (4.8) |
| Whalley (2003) [82] | CS | 79 31 |
Non-supplement user-33.7 (26.2) Supplement user-48.2 (25.7) |
28.5 (1.4) 28.9 (1.4) |
| Glaso (2004) [47] | CC | 18 | Control group-80 (28) | 27.2 |
| Polidori (2004) [43] | CS | 55 | Control group-52.4 (16.4) | 28.7 (1) |
| Rinaldi (2003) [42] | CS | 53 | Control group-52.4 (16.5) | 28.1 (1.4) |
| Chandra (2001) [50] # | RCT | 86 | Adequate Deficient |
28 (6.3) 17 (4) |
| Lindemann (2003) [78] # | CC | 195 | >57 <57 |
27.2 (2.4) 26.4 (2.9) |
| Sato (2006) [81] # | CC | 544 | Median = 74.9 (interquartile range = 57.8–90.7) Median = 78.9 (interquartile range = 64.1–99.2) |
<27 >27 |
| Richardson (2002) [44] # | CC | 37 | <11 11–40 40–100 |
23 (12.3) 25 (6.0) 27 (5.1) |
Legend: RCT = randomised controlled trial, Pros = prospective, CS = cross-sectional, CC = case-control, * converted FFQ to blood vitamin C (μmol/L) represented by crosses on Figure 2 (not included in analysis), green circles representing cognitively intact blood values (Figure 2), # Not included in analysis.
In the cognitively impaired subgroup, there was a wide distribution of both MMSE scores (mean score range = 1.9–26.9) and vitamin C concentrations (19–44 µmol/L) (Figure 2). Mean vitamin C concentration (Mean score ± standard deviation (SD) = 29.91 ± 8 µmol/L) corresponded with a borderline vitamin C depletion (<28 µmol/L) [33]. Mean MMSE scores (Mean score = 14.63 ± 7.8) corresponded to a severe cognitive impairment (scores >17) [99].
In the cognitively intact subgroup, mean vitamin C and MMSE scores were extracted from 5 studies (sample sizes ranged 18–260 participants, with a total of 496 participants). In this group, mean vitamin C concentrations (Mean score ± SD = 54.9 ± 16) µmol/L) were widely spread (33.7–80 µmol/L) but mean MMSE scores (Mean score = 28.1 ± 0.7) were not (27.2–28.9). The lack of variance in MMSE scores precluded correlational analysis in this subgroup.
In the cognitively impaired subgroup the scatterplot (Figure 2/Table 4) and a Pearson r2 value of 0.0016 revealed low variance and a spread in means around the fitted regression line. The Spearman’s correlation also revealed no significant correlation between MMSE scores and vitamin C concentrations (rs (11) = 0.009, p = 0.98).
A number of studies [44,50,78,81] (Table 5) did not report numerical mean vitamin C concentrations or MMSE scores (0–30) but instead placed the means into categories (e.g., MMSE score of over/under 27, Vitamin C concentrations into deficient/adequate ranges). The results from these studies followed our observed trend where participants whose vitamin C concentrations were categorized into adequate ranges produced higher mean MMSE scores and those who were categorized into scoring under 27 on the MMSE had lower mean vitamin C concentrations.
Additional studies using cognitively intact groups of participants (Table 2) assessed cognition using a number of different cognitive measures and plasma vitamin C. Examples of these cognitive measures included the digit span backwards/forwards, the East Boston memory test, Wechsler memory test, clock drawing, delayed word recall, etc. (Table 2). A majority of these studies [50,52,78,79,81] revealed an association between vitamin C blood concentrations and cognitive performance on various cognitive tasks. Some of the cognitive domains included short-term memory, information processing, abstract thinking and working memory. A number of studies [80,82,83] did fail to demonstrate a link between vitamin C and cognition. However, the quality assessment revealed lower ratings for these studies than for those demonstrating a link. Additionally, one study [42] using cognitively impaired groups of participants (Table 1) assessed cognition with alternative assessments to the MMSE and demonstrated superior performance in those with higher vitamin C concentrations.
The predicted blood vitamin C concentrations generated from FFQs in the cognitively intact participants when plotted (Figure 2), were relatively similar to the blood concentrations generated by studies primarily using blood tests. These converted values were not used in correlation analyses.
4. Discussion
This review evaluated 50 studies exploring the link between vitamin C and cognitive function. Extrapolated mean vitamin C concentrations and MMSE scores from a number of these studies indicated that the cognitively intact groups of participants had higher mean vitamin C concentrations and MMSE scores than the cognitively impaired groups. However, there was no significant correlation between mean vitamin C concentrations and mean MMSE scores in the cognitively impaired studies (n = 7, n = 391 participants). In contrast, correlation analysis between blood vitamin C concentrations and MMSE scores in the cognitively intact studies was not feasible due to the low variance in MMSE scores, demonstrating the unsuitability of the MMSE in the cognitively healthy participants. Quantitative assessment of those studies in the cognitively intact groups revealed a potential association between plasma vitamin C concentrations and cognition. Our findings are consistent with a number of studies [42,48,95] that showed a significantly lower vitamin C blood concentrations between cognitively impaired compared to healthy individuals.
This may be explained by a reduction in dietary intake amongst the elderly in general [100], and those living alone or in aged care/hospital facilities [101] who are often unable to prepare their own meals, may have chewing problems, and may make poor food choices such as not including fruits and vegetables in their diet.
Subjects with AD may be nutrient deficient, particularly in the later phase of the disease. However, case-control studies have also demonstrated lower plasma vitamin C concentrations in the early AD stages in well-nourished subjects [48].
A more recent, second hypothesis for the depleted blood vitamin C concentrations in the cognitively impaired is the increased oxidation of vitamin C in response to elevated free radical production in the brain. Vitamin C has been reported to be the first barrier to free radicals produced in biological fluids [102]. In the cognitively impaired, studies have demonstrated an increased sensitivity to free radicals in the cerebral cortex [103]. The mechanisms of free radical production hypothesized for AD include: activated microglia surrounding senile plaques [104], neuronal mitochondrial dysfunction [105], intraneuronal amyloid accumulation [106] and presence of redox active metals [107]. Thirdly, disturbances in iron metabolism found in the vicinity of the senile plaques [108], could catalyse the production of free radicals. Noradrenergic and serotoninergic deficiencies have also been reported in AD [109], requiring the utilisation of vitamin C to restore these deficiencies.
The lack of linearity in vitamin C concentrations and MMSE scores in the cognitively impaired group could be explained by the non-linear relationship between plasma vitamin C and ascorbate CSF absorption. Due to a homeostatic mechanism [26], the amount of ascorbate CSF and vitamin C reaching the brain could show little variability at varying plasma concentrations, even with deficient plasma concentrations (<28 µmol/L). This could result in similar cognitive scores at varying plasma vitamin C concentrations.
4.1. Limitations
The results from the current review do need to be interpreted cautiously due to a number of limitations:
While blood samples are a more reliable measure of vitamin C status than FFQ-based Vitamin C determination, a number of further methodical issues may exist. Many factors can contribute to the instability of ascorbic acid in biological samples due to the oxidation of vitamin C in plasma is accelerated by heat, light, and elevated pH (acidity). These issues arise as a result of a lack of full appreciation of the redox chemistry and biology of ascorbic acid [110]. A number of handling techniques should be incorporated in order to ensure quality measures.
A majority of studies included in this review failed to thoroughly explain blood sample handling and biochemical analysis. Ideal handling conditions of samples intended for ascorbate analysis include immediate coverage from light, immediate plasma isolation, rapid acidification, and freezing below −20 °C to avoid misinterpretations compounded by the use of poorly preserved samples [110]. In order for plasma to be transported, it needs to be covered from light and transported on dry ice (−70 °C) before thawing and analysis.
Underestimation of vitamin C concentrations could occur if samples were not handled properly. Frequent freeze-thaw cycles or exposure to any metals (such as iron in the haemolysis of red blood cells) could both lead to rapid degradation of vitamin C in the sample [111]. It has been shown that there is a significant loss of ascorbate plasma in EDTA tubes [112], with lithium heparin tubes being ideal.
Several limitations can arise from the use of FFQs in determining nutrient level [32]. Plasma vitamin C concentrations are dependent on recent dietary intake, due to the vitamin’s water soluble properties and excretion, therefore blood plasma measures would be most reflective of foods consumed recently (1–2 weeks). Incorporating food questionnaires relating to most recent food consumption, would be most indicative of blood concentrations. Given the overreliance on FFQs in the reviewed studies, especially in those incorporating prospective designs, instead of blood samples interpretation of findings is limited. A direct comparison between FFQ and blood samples could validate the effective of the questionnaire. A recent meta-analysis demonstrated that FFQ and food diaries have a moderate relationship with plasma vitamin C, with multiple factors affecting this relationship [32].
While converted FFQ-based vitamin C levels were of a similar range to blood concentrations, this conversion needs to be interpreted with caution. The conversion ratio of 1.95 mg to 1 µmol/L in plasma was based on a study that used 8 healthy participants [34]. However, this ratio may not be applicable for all individuals as individual factors could affect vitamin C absorption and distribution (i.e., oxidative stress, infection, etc.).
Plasma vitamin C differs according to polymorphisms of sodium dependent active transporters (SVCT2 and SVCT1) despite equivalent vitamin C intake indicating that SVCT1 and 2 genotype may determine the strength of the association between vitamin C intake and circulating vitamin C concentrations [113]. Some people may require greater than the recommended daily allowance to maintain optimal vitamin C concentrations. These differences could render food diary information even less accurate as perceived intake may not be equivalent to absorption [111].
In addition, dietary assessment has reliability and validity issues in relation to even mild cognitive deficits, which are frequent in older populations [114]. These include recall errors but even when food types and amounts are recalled correctly, differences in storage and cooking can decrease the vitamin C level in the food [115]. It is close to impossible to determine the concentrations retained in foods following manipulations such as cooking [116]. Furthermore, high levels of vitamin C gained from dietary sources will often be accompanied by higher levels of a number of other beneficial compounds (vitamins, phytochemicals) also found from the same sources [111].
Moreover, the reviewed randomised controlled studies have failed to assess the effects of a vitamin C intervention on its own, by using multivitamins. A large portion of the included studies have made efforts to statistically control for potential confounders. Although our review did demonstrate lower plasma vitamin C concentrations in the cognitively impaired, other studies using impaired samples have shown depletions in a number of other vitamin and minerals including: vitamin B12 [117], vitamin E [118], vitamin D [119], vitamin K [120], folate [117], and elevated homocysteine [117]. Additionally, it is important to note that when antioxidant function is involved, vitamins can work synergistically with other vitamins, e.g., vitamin C recycles α-tocopherol radical (vitamin E) [111]. The consumption and supplementation of these vitamins should be considered as potential confounders and should be monitored, especially in cognitive impaired participants.
Moreover, it can be speculated that a consistently high Vitamin C status acts in a preventive manner, while vitamin C supplementation per se is not a treatment for clinical AD [48]. Thus, infrequent supplement users may not achieve the same benefits as individuals with consistent intake of adequate vitamin C. Controlling for vitamin C supplementation use, or taking it into account, is crucial.
Intake at the time of measurement may not reflect lifetime dietary habits and given data that suggest that amyloid plaque burden begins to form well before middle age [121], intakes during younger adulthood may be equally as important as supplements taken by older adults, perhaps contributing to a biological buffer against disease pathogenesis. Measuring and controlling for a history of consumption and supplementation is crucial, especially in longer prospective studies where the development of neurodegeneration is being investigated.
In addition to the limitations on vitamin C levels, there were limitations regarding the type of cognitive measures. A number of long term prospective studies incorporated cognitive tests suitable for screening and assessing the incidence of Alzheimer’s, such as the MMSE. Given the simplicity of such tests, and the scales used to measure performance, it becomes difficult to establish cognitive changes unless the cognitive decline is extremely severe. These MMSE scales have been effective in measuring cognition in those clinically diagnosed with a neurodegenerative condition [96,48], and were useful in the cognitively impaired subgroup in this review.
The sensitivity of the MMSE to detect differences in cognitively intact samples has been questioned [122,123]. This can lead to a lack of variance in MMSE scores. In our review, the mean MMSE score ranged 27.2–28.9 in this group (<24 = mild cognitive impairment). In this review, a number of studies conducted on the cognitively intact group did use a range of other, more suitable cognitive tests, including the digit span forwards/backwards, delayed word recall, letter digit substitution test, etc., with mixed results. A number of these studies [55,67,70,74,83] failed to demonstrate a link between vitamin C status and cognition whereas a number of studies [50,61,76,79,81] demonstrated the effects of vitamin C on a number of cognitive domains such as free recall, short-term memory, abstract thinking, visuospatial performance and recognition. However, comparison of different cognitive tests was beyond the scope of this review.
A further limitation to be considered is the often self-selection of healthier, more cognitively-able population in population studies. As a consequence of high baseline performance in cognitively intact participants, ceiling effects with narrow ranges in results can occur [124]. This effectively minimizes several confounding factors, but narrows the chance of detecting cognitive effects.
In cognitively intact samples, cognitive tests sensitive to age-associated cognitive decline should be employed to maximize the observation of any potential effects. Programs such as The Cambridge Neuropsychological Test Automated Battery [125] and The National Institute of Health (NIH) Toolbox [126] are available that tap into a wide range of cognitive domains sensitive to change from mid adulthood such as fluid intelligence would be ideal for establishing its association with nutrition or intervention [127]. In the present review, one study [79] using cognitively intact participants incorporated a computerized test battery assessing a number of cognitive domains. This study demonstrated a significant link between vitamin C status and free recall, recognition and vocabulary.
4.2. Future Directions
Future studies should incorporate a number of recommendations. Firstly, the most reliable and practical measure of vitamin C is the measurement of biological blood samples. Moreover, the incorporation of FFQs would allow a measure of possible confounding variables (vitamin B12, vitamin E, etc.). Age-sensitive cognitive tests assessing response time and accuracy should be administered [127], particularly in the case of cognitively intact individuals. A number of potential confounding factors such as supplementation, and the long term intake of other vitamins and minerals associated with cognition need to be take into account.
5. Conclusions
In summary, studies included in this systematic review demonstrated higher mean vitamin C concentrations in the cognitively intact groups of participants compared to the impaired groups. No correlation was found between vitamin C concentrations and MMSE scores in the cognitively impaired groups of participants. Analysis of the studies that used a variety of cognitive assessments was beyond the scope of this review, however, qualitative assessment in the cognitively intact groups revealed a potential association between plasma vitamin C concentrations and cognition. Due to a number of limitations, further research, assessing plasma vitamin C concentrations, taking confounding factors such as vitamin B12 and vitamin E into account, and the use of more sensitive cognitive assessment methodology for cognitively intact participants are needed to provide more insights into the relationship between vitamin C and cognition.
Author Contributions
A.S. and N.T. conceptualised the study in discussion with K.R. and A.P. I.H. provided statistical knowledge advice. N.T. undertook data analysis and interpreted findings in discussion with K.R. and I.H. N.T. and K.R. prepared the manuscript with contributions from co-authors A.P., A.Sa., and A.Sc. All authors approved the final version.
Conflicts of Interest
A.Sc. and A.P. have received research funding, consultancy, travel support and speaking fees from the nutrition and supplement industry. N.T., K.R., A.S. and I.H. declare no conflict of interest.
References
- 1.Trout D.L. Vitamin c and cardiovascular risk factors. Am. J. Clin. Nutr. 1991;53:322S–325S. doi: 10.1093/ajcn/53.1.322S. [DOI] [PubMed] [Google Scholar]
- 2.Vojdani A., Ghoneum M. In vivo effect of ascorbic acid on enhancement of human natural killer cell activity. Nutr. Res. 1993;13:753–764. doi: 10.1016/S0271-5317(05)80799-7. [DOI] [Google Scholar]
- 3.Jacques P.F., Chylack L.T. Epidemiologic evidence of a role for the antioxidant vitamins and carotenoids in cataract prevention. Am. J. Clin. Nutr. 1991;53:352S–355S. doi: 10.1093/ajcn/53.1.352S. [DOI] [PubMed] [Google Scholar]
- 4.Hatch G.E. Asthma, inhaled oxidants, and dietary antioxidants. Am. J. Clin. Nutr. 1995;61:625S–630S. doi: 10.1093/ajcn/61.3.625S. [DOI] [PubMed] [Google Scholar]
- 5.Hemilä H. Does vitamin c alleviate the symptoms of the common cold?—A review of current evidence. Scand. J. Infect. Dis. 1994;26:1–6. doi: 10.3109/00365549409008582. [DOI] [PubMed] [Google Scholar]
- 6.Gund P. Progress in Molecular and Subcellular Biology. Springer; Berlin/Heidelberg, Germany: 1977. Three-dimensional pharmacophoric pattern searching; pp. 117–143. [Google Scholar]
- 7.Gaby S.K., Bendich A., Singh V., Machlin L.J. Vitamin Intake and Health: A Scientific Review. CRC Press; Boca Raton, FL, USA: 1991. pp. 71–103. [Google Scholar]
- 8.Levine M., Asher A., Pollard H., Zinder O. Ascorbic acid and catecholamine secretion from cultured chromaffin cells. J. Biol. Chem. 1983;258:13111–13115. [PubMed] [Google Scholar]
- 9.Hansen S.N., Tveden-Nyborg P., Lykkesfeldt J. Does vitamin c deficiency affect cognitive development and function? Nutrients. 2014;6:3818–3846. doi: 10.3390/nu6093818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majewska M.D., Bell J.A. Ascorbic acid protects neurons from injury induced by glutamate and nmda. Neuroreport. 1990;1:194–196. doi: 10.1097/00001756-199011000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Levine M., Morita K., Heldman E., Pollard H.B. Ascorbic acid regulation of norepinephrine biosynthesis in isolated chromaffin granules from bovine adrenal medulla. J. Biol. Chem. 1985;260:15598–15603. [PubMed] [Google Scholar]
- 12.Levine M., Morita K., Pollard H. Enhancement of norepinephrine biosynthesis by ascorbic acid in cultured bovine chromaffin cells. J. Biol. Chem. 1985;260:12942–12947. [PubMed] [Google Scholar]
- 13.Sandstrom M.I., Rebec G.V. Extracellular ascorbate modulates glutamate dynamics: Role of behavioral activation. BMC Neurosci. 2007;8:1. doi: 10.1186/1471-2202-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majewska M.D., Bell J.A., London E.D. Regulation of the nmda receptor by redox phenomena: Inhibitory role of ascorbate. Brain Res. 1990;537:328–332. doi: 10.1016/0006-8993(90)90379-P. [DOI] [PubMed] [Google Scholar]
- 15.Liu X., Wu H., Byrne M., Krane S., Jaenisch R. Type iii collagen is crucial for collagen i fibrillogenesis and for normal cardiovascular development. Proc. Natl. Acad. Sci. USA. 1997;94:1852–1856. doi: 10.1073/pnas.94.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang J., Agus D.B., Winfree C.J., Kiss S., Mack W.J., McTaggart R.A., Choudhri T.F., Kim L.J., Mocco J., Pinsky D.J. Dehydroascorbic acid, a blood-brain barrier transportable form of vitamin c, mediates potent cerebroprotection in experimental stroke. Proc. Natl. Acad. Sci. USA. 2001;98:11720–11724. doi: 10.1073/pnas.171325998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iyer N.V., Kotch L.E., Agani F., Leung S.W., Laughner E., Wenger R.H., Gassmann M., Gearhart J.D., Lawler A.M., Aimee Y.Y. Cellular and developmental control of o2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hediger M.A. New view at c. Nat. Med. 2002;8:445–446. doi: 10.1038/nm0502-445. [DOI] [PubMed] [Google Scholar]
- 19.Jackson T.S., Xu A., Vita J.A., Keaney J.F. Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ. Res. 1998;83:916–922. doi: 10.1161/01.RES.83.9.916. [DOI] [PubMed] [Google Scholar]
- 20.Spector R., Johanson C.E. Sustained choroid plexus function in human elderly and alzheimer’s disease patients. Fluids Barriers CNS. 2013;10:1. doi: 10.1186/2045-8118-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison F., Allard J., Bixler R., Usoh C., Li L., May J., McDonald M. Antioxidants and cognitive training interact to affect oxidative stress and memory in app/psen1 mice. Nutr. Neurosci. 2009;12:203–218. doi: 10.1179/147683009X423364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison F.E., Green R.J., Dawes S.M., May J.M. Vitamin c distribution and retention in the mouse brain. Brain Res. 2010;1348:181–186. doi: 10.1016/j.brainres.2010.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.May J.M. Water Soluble Vitamins. Springer; Dordrecht, The Netherlands: 2012. Vitamin c transport and its role in the central nervous system; pp. 85–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mefford I.N., Oke A.F., Adams R.N. Regional distribution of ascorbate in human brain. Brain Res. 1981;212:223–226. doi: 10.1016/0006-8993(81)90056-1. [DOI] [PubMed] [Google Scholar]
- 25.Oke A.F., May L., Adams R.N. Ascorbic acid distribution patterns in human brain. Ann. N. Y. Acad. Sci. 1987;498:1–12. doi: 10.1111/j.1749-6632.1987.tb23747.x. [DOI] [PubMed] [Google Scholar]
- 26.Bowman G.L., Dodge H., Frei B., Calabrese C., Oken B.S., Kaye J.A., Quinn J.F. Ascorbic acid and rates of cognitive decline in alzheimer’s disease. J. Alzheimers Dis. 2009;16:93–98. doi: 10.3233/JAD-2009-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinn J., Suh J., Moore M.M., Kaye J., Frei B. Antioxidants in alzheimer’s disease-vitamin c delivery to a demanding brain. J. Alzheimers Dis. 2003;5:309–313. doi: 10.3233/JAD-2003-5406. [DOI] [PubMed] [Google Scholar]
- 28.May J.M., Li L., Hayslett K., Qu Z.-C. Ascorbate transport and recycling by sh-sy5y neuroblastoma cells: Response to glutamate toxicity. Neurochem. Res. 2006;31:785–794. doi: 10.1007/s11064-006-9077-z. [DOI] [PubMed] [Google Scholar]
- 29.Spector R. Vitamin homeostasis in the central nervous system. N. Engl. J. Med. 1977;296:1393–1398. doi: 10.1056/NEJM197706162962409. [DOI] [PubMed] [Google Scholar]
- 30.Hornig D. Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann. N. Y. Acad. Sci. 1975;258:103–118. doi: 10.1111/j.1749-6632.1975.tb29271.x. [DOI] [PubMed] [Google Scholar]
- 31.Higgins J.P., Green S. Cochrane Handbook for Systematic Reviews of Interventions. Volume 4 John Wiley & Sons; Hoboken, NJ, USA: 2011. [Google Scholar]
- 32.Dehghan M., Akhtar-Danesh N., McMillan C.R., Thabane L. Is plasma vitamin c an appropriate biomarker of vitamin c intake? A systematic review and meta-analysis. Nutr. J. 2007;6:41. doi: 10.1186/1475-2891-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hampl J.S., Taylor C.A., Johnston C.S. Vitamin c deficiency and depletion in the united states: The third national health and nutrition examination survey, 1988 to 1994. Am. J. Public Health. 2004;94:870–875. doi: 10.2105/AJPH.94.5.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine M., Conry-Cantilena C., Wang Y., Welch R.W., Washko P.W., Dhariwal K.R., Park J.B., Lazarev A., Graumlich J.F., King J. Vitamin c pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA. 1996;93:3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tombaugh T.N., McIntyre N.J. The mini-mental state examination: A comprehensive review. J. Am. Geriatr. Soc. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 36.Mungas D. Iii-office mental status testing: A practical guide. Geriatrics. 1991;46:54–67. [PubMed] [Google Scholar]
- 37.Arlt S., Müller-Thomsen T., Beisiegel U., Kontush A. Effect of one-year vitamin c-and e-supplementation on cerebrospinal fluid oxidation parameters and clinical course in alzheimer’s disease. Neurochem. Res. 2012;37:2706–2714. doi: 10.1007/s11064-012-0860-8. [DOI] [PubMed] [Google Scholar]
- 38.Galasko D.R., Peskind E., Clark C.M., Quinn J.F., Ringman J.M., Jicha G.A., Cotman C., Cottrell B., Montine T.J., Thomas R.G. Antioxidants for alzheimer disease: A randomized clinical trial with cerebrospinal fluid biomarker measures. Arch. Neurol. 2012;69:836–841. doi: 10.1001/archneurol.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burns A., Marsh A., Bender D.A. A trial of vitamin supplementation in senile dementia. Int. J. Geriatr. Psychiatry. 1989;4:333–338. doi: 10.1002/gps.930040606. [DOI] [Google Scholar]
- 40.Zandi P.P., Anthony J.C., Khachaturian A.S., Stone S.V., Gustafson D., Tschanz J.T., Norton M.C., Welsh-Bohmer K.A., Breitner J.C. Reduced risk of alzheimer disease in users of antioxidant vitamin supplements: The cache county study. Arch. Neurol. 2004;61:82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 41.Deijen J., Slump E., Wouters-Wesseling W., De Groot C., Galle E., Pas H. Nutritional intake and daily functioning of psychogeriatric nursing home residents. J. Nutr. Health Aging. 2002;7:242–246. [PubMed] [Google Scholar]
- 42.Rinaldi P., Polidori M.C., Metastasio A., Mariani E., Mattioli P., Cherubini A., Catani M., Cecchetti R., Senin U., Mecocci P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in alzheimer’s disease. Neurobiol. Aging. 2003;24:915–919. doi: 10.1016/S0197-4580(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 43.Polidori M.C., Mattioli P., Aldred S., Cecchetti R., Stahl W., Griffiths H., Senin U., Sies H., Mecocci P. Plasma antioxidant status, immunoglobulin g oxidation and lipid peroxidation in demented patients: Relevance to alzheimer disease and vascular dementia. Dement. Geriatr. Cogn. Disord. 2004;18:265–270. doi: 10.1159/000080027. [DOI] [PubMed] [Google Scholar]
- 44.Richardson T., Ball L., Rosenfeld T. Will an orange a day keep the doctor away? Postgrad. Med. J. 2002;78:292–294. doi: 10.1136/pmj.78.919.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu Y., An Y., Guo J., Zhang X., Wang H., Rong H., Xiao R. Dietary intake of nutrients and lifestyle affect the risk of mild cognitive impairment in the chinese elderly population: A cross-sectional study. Front. Behav. Neurosci. 2016;10:229. doi: 10.3389/fnbeh.2016.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charlton K.E., Rabinowitz T.L., Geffen L., Dhansay M. Lowered plasma vitamin c, but not vitamin e, concentrations in dementia patients. J. Nutr. Health Aging. 2004;8:99–108. [PubMed] [Google Scholar]
- 47.Glasø M., Nordbø G., Diep L., Bøhmer T. Reduced concentrations of several vitamins in normal weight patients with late-onset dementia of the alzheimer type without vascular disease. J. Nutr. Health Aging. 2003;8:407–413. [PubMed] [Google Scholar]
- 48.Rivière S., Birlouez-Aragon I., Nourhashémi F., Vellas B. Low plasma vitamin c in alzheimer patients despite an adequate diet. Int. J. Geriatr. Psychiatry. 1998;13:749–754. doi: 10.1002/(SICI)1099-1166(1998110)13:11<749::AID-GPS860>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 49.Masaki K., Losonczy K., Izmirlian G., Foley D., Ross G., Petrovitch H., Havlik R., White L. Association of vitamin e and c supplement use with cognitive function and dementia in elderly men. Neurology. 2000;54:1265–1272. doi: 10.1212/WNL.54.6.1265. [DOI] [PubMed] [Google Scholar]
- 50.Chandra R.K. Retracted: Effect of vitamin and trace-element supplementation on cognitive function in elderly subjects. Nutrition. 2001;17:709–712. doi: 10.1016/S0899-9007(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 51.Dror Y., Stern F., Nemesh L., Hart J., Grinblat J. Estimation of vitamin needs—Riboflavin, vitamin b6 and ascorbic acid-according to blood parameters and functional-cognitive and emotional indices in a selected well-established group of elderly in a home for the aged in israel. J. Am. Coll. Nutr. 1996;15:481–488. doi: 10.1080/07315724.1996.10718628. [DOI] [PubMed] [Google Scholar]
- 52.Gale C.R., Martyn C.N., Cooper C. Cognitive impairment and mortality in a cohort of elderly people. BMJ. 1996;312:608–611. doi: 10.1136/bmj.312.7031.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.La Rue A., Koehler K.M., Wayne S.J., Chiulli S.J., Haaland K.Y., Garry P.J. Nutritional status and cognitive functioning in a normally aging sample: A 6-y reassessment. Am. J. Clin. Nutr. 1997;65:20–29. doi: 10.1093/ajcn/65.1.20. [DOI] [PubMed] [Google Scholar]
- 54.Paleologos M., Cumming R.G., Lazarus R. Cohort study of vitamin c intake and cognitive impairment. Am. J. Epidemiol. 1998;148:45–50. doi: 10.1093/oxfordjournals.aje.a009559. [DOI] [PubMed] [Google Scholar]
- 55.Devore E.E., Kang J.H., Stampfer M.J., Grodstein F. The association of antioxidants and cognition in the nurses’ health study. Am. J. Epidemiol. 2013;177:33–41. doi: 10.1093/aje/kws202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Engelhart M.J., Geerlings M.I., Ruitenberg A., van Swieten J.C., Hofman A., Witteman J.C., Breteler M.M. Dietary intake of antioxidants and risk of alzheimer disease. JAMA. 2002;287:3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 57.Kalmijn S., Feskens E., Launer L.J., Kromhout D. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am. J. Epidemiol. 1997;145:33–41. doi: 10.1093/oxfordjournals.aje.a009029. [DOI] [PubMed] [Google Scholar]
- 58.Laurin D., Masaki K.H., Foley D.J., White L.R., Launer L.J. Midlife dietary intake of antioxidants and risk of late-life incident dementia the honolulu-asia aging study. Am. J. Epidemiol. 2004;159:959–967. doi: 10.1093/aje/kwh124. [DOI] [PubMed] [Google Scholar]
- 59.Basambombo L.L., Carmichael P.-H., Côté S., Laurin D. Use of vitamin e and c supplements for the prevention of cognitive decline. Ann. Pharmacother. 2016;51:118–124. doi: 10.1177/1060028016673072. [DOI] [PubMed] [Google Scholar]
- 60.Nooyens A.C., Milder I.E., Van Gelder B.M., Bueno-de-Mesquita H.B., Van Boxtel M.P., Verschuren W.M. Diet and cognitive decline at middle age: The role of antioxidants. Br. J. Nutr. 2015;113:1410–1417. doi: 10.1017/S0007114515000720. [DOI] [PubMed] [Google Scholar]
- 61.Péneau S., Galan P., Jeandel C., Ferry M., Andreeva V., Hercberg S., Kesse-Guyot E., Group S.V.M.R. Fruit and vegetable intake and cognitive function in the su. Vi. Max 2 prospective study. Am. J. Clin. Nutr. 2011;94:1295–1303. doi: 10.3945/ajcn.111.014712. [DOI] [PubMed] [Google Scholar]
- 62.Fotuhi M., Zandi P.P., Hayden K.M., Khachaturian A.S., Szekely C.A., Wengreen H., Munger R.G., Norton M.C., Tschanz J.T., Lyketsos C.G. Better cognitive performance in elderly taking antioxidant vitamins e and c supplements in combination with nonsteroidal anti-inflammatory drugs: The cache county study. Alzheimers Dement. 2008;4:223–227. doi: 10.1016/j.jalz.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gray S.L., Anderson M.L., Crane P.K., Breitner J., McCormick W., Bowen J.D., Teri L., Larson E. Antioxidant vitamin supplement use and risk of dementia or alzheimer’s disease in older adults. J. Am. Geriatr. Soc. 2008;56:291–295. doi: 10.1111/j.1532-5415.2007.01531.x. [DOI] [PubMed] [Google Scholar]
- 64.Wengreen H., Munger R., Corcoran C., Zandi P. Antioxidant intake and cognitive function of elderly men and women: The cache county study. J. Nutr. Health Aging. 2007;11:230. [PubMed] [Google Scholar]
- 65.Fillenbaum G.G., Kuchibhatla M.N., Hanlon J.T., Artz M.B., Pieper C.F., Schmader K.E., Dysken M.W., Gray S.L. Dementia and alzheimer’s disease in community-dwelling elders taking vitamin c and/or vitamin e. Ann. Pharmacother. 2005;39:2009–2014. doi: 10.1345/aph.1G280. [DOI] [PubMed] [Google Scholar]
- 66.Maxwell C.J., Hicks M.S., Hogan D.B., Basran J., Ebly E.M. Supplemental use of antioxidant vitamins and subsequent risk of cognitive decline and dementia. Dement. Geriatr. Cogn. Disord. 2005;20:45–51. doi: 10.1159/000085074. [DOI] [PubMed] [Google Scholar]
- 67.Grodstein F., Chen J., Willett W.C. High-dose antioxidant supplements and cognitive function in community-dwelling elderly women. Am. J. Clin. Nutr. 2003;77:975–984. doi: 10.1093/ajcn/77.4.975. [DOI] [PubMed] [Google Scholar]
- 68.Luchsinger J.A., Tang M.-X., Shea S., Mayeux R. Antioxidant vitamin intake and risk of alzheimer disease. Arch. Neurol. 2003;60:203–208. doi: 10.1001/archneur.60.2.203. [DOI] [PubMed] [Google Scholar]
- 69.Morris M.C., Evans D.A., Bienias J.L., Tangney C.C., Bennett D.A., Aggarwal N., Wilson R.S., Scherr P.A. Dietary intake of antioxidant nutrients and the risk of incident alzheimer disease in a biracial community study. JAMA. 2002;287:3230–3237. doi: 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- 70.Peacock J.M., Folsom A.R., Knopman D.S., Mosley T.H., Goff D.C., Szklo M. Dietary antioxidant intake and cognitive performance in middle-aged adults. Public Health Nutr. 2000;3:337–343. doi: 10.1017/S1368980000000380. [DOI] [PubMed] [Google Scholar]
- 71.Morris M.C., Beckett L.A., Scherr P.A., Hebert L.E., Bennett D.A., Field T.S., Evans D.A. Vitamin e and vitamin c supplement use and risk of incident alzheimer disease. Alzheimer Dis. Assoc. Disord. 1998;12:121–126. doi: 10.1097/00002093-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 72.Mendelsohn A.B., Belle S.H., Stoehr G.P., Ganguli M. Use of antioxidant supplements and its association with cognitive function in a rural elderly cohort the movies project. Am. J. Epidemiol. 1998;148:38–44. doi: 10.1093/oxfordjournals.aje.a009556. [DOI] [PubMed] [Google Scholar]
- 73.Berti V., Murray J., Davies M., Spector N., Tsui W., Li Y., Williams S., Pirraglia E., Vallabhajosula S., McHugh P. Nutrient patterns and brain biomarkers of alzheimer’s disease in cognitively normal individuals. J. Nutr. Health Aging. 2015;19:413–423. doi: 10.1007/s12603-014-0534-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beydoun M.A., Kuczmarski M.F., Kitner-Triolo M.H., Beydoun H.A., Kaufman J.S., Mason M.A., Evans M.K., Zonderman A.B. Dietary antioxidant intake and its association with cognitive function in an ethnically diverse sample of us adults. Psychosom. Med. 2015;77:68. doi: 10.1097/PSY.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chaudhari K., Sumien N., Johnson L., D’Agostino D., Edwards M., Paxton R., Hall J., O’Bryant S.E. Vitamin c supplementation, apoe4 genotype and cognitive functioning in a rural-dwelling cohort. J. Nutr. Health Aging. 2016;20:841–844. doi: 10.1007/s12603-016-0705-2. [DOI] [PubMed] [Google Scholar]
- 76.Goodwin J.S., Goodwin J.M., Garry P.J. Association between nutritional status and cognitive functioning in a healthy elderly population. JAMA. 1983;249:2917–2921. doi: 10.1001/jama.1983.03330450047024. [DOI] [PubMed] [Google Scholar]
- 77.Jama J.W., Launer L.J., Witteman J., Den Breeijen J., Breteler M., Grobbee D., Hofman A. Dietary antioxidants and cognitive function in a population-based sample of older persons the rotterdam study. Am. J. Epidemiol. 1996;144:275–280. doi: 10.1093/oxfordjournals.aje.a008922. [DOI] [PubMed] [Google Scholar]
- 78.Lindeman R.D., Romero L.J., Koehler K.M., Liang H.C., LaRue A., Baumgartner R.N., Garry P.J. Serum vitamin b12, c and folate concentrations in the new mexico elder health survey: Correlations with cognitive and affective functions. J. Am. Coll. Nutr. 2000;19:68–76. doi: 10.1080/07315724.2000.10718916. [DOI] [PubMed] [Google Scholar]
- 79.Perrig W.J., Perrig P., Stähelin H. The relation between antioxidants and memory performance in the old and very old. J. Am. Geriatr. Soc. 1997;45:718–724. doi: 10.1111/j.1532-5415.1997.tb01476.x. [DOI] [PubMed] [Google Scholar]
- 80.Schmidt R., Hayn M., Reinhart B., Roob G., Schmidt H., Schumacher M., Watzinger N., Launer L. Plasma antioxidants and cognitive performance in middle-aged and older adults: Results of the austrian stroke prevention study. J. Am. Geriatr. Soc. 1998;46:1407–1410. doi: 10.1111/j.1532-5415.1998.tb06008.x. [DOI] [PubMed] [Google Scholar]
- 81.Sato R., Helzlsouer K., Comstock G., Hoffman S. A cross-sectional study of vitamin c and cognitive function in older adults: The differential effects of gender. J. Nutr. Health Aging. 2006;10:37. [PubMed] [Google Scholar]
- 82.Whalley L., Fox H., Lemmon H., Duthie S., Collins A., Peace H., Starr J., Deary I. Dietary supplement use in old age: Associations with childhood iq, current cognition and health. Int. J. Geriatr. Psychiatry. 2003;18:769–776. doi: 10.1002/gps.915. [DOI] [PubMed] [Google Scholar]
- 83.Perkins A.J., Hendrie H.C., Callahan C.M., Gao S., Unverzagt F.W., Xu Y., Hall K.S., Hui S.L. Association of antioxidants with memory in a multiethnic elderly sample using the third national health and nutrition examination survey. Am. J. Epidemiol. 1999;150:37–44. doi: 10.1093/oxfordjournals.aje.a009915. [DOI] [PubMed] [Google Scholar]
- 84.Ortega R.M., Requejo A.M., Andrés P., López-Sobaler A.M., Quintas M.E., Redondo M.R., Navia B., Rivas T. Dietary intake and cognitive function in a group of elderly people. Am. J. Clin. Nutr. 1997;66:803–809. doi: 10.1093/ajcn/66.4.803. [DOI] [PubMed] [Google Scholar]
- 85.Requejo A., Ortega R., Robles F., Navia B., Faci M., Aparicio A. Influence of nutrition on cognitive function in a group of elderly, independently living people. Eur. J. Clin. Nutr. 2003;57:S54–S57. doi: 10.1038/sj.ejcn.1601816. [DOI] [PubMed] [Google Scholar]
- 86.Kennedy D.O., Veasey R.C., Watson A.W., Dodd F.L., Jones E.K., Tiplady B., Haskell C.F. Vitamins and psychological functioning: A mobile phone assessment of the effects of a b vitamin complex, vitamin c and minerals on cognitive performance and subjective mood and energy. Hum. Psychopharmacol. 2011;26:338–347. doi: 10.1002/hup.1216. [DOI] [PubMed] [Google Scholar]
- 87.Smith A.P., Clark R., Nutt D., Haller J., Hayward S., Perry K. Vitamin c, mood and cognitive functioning in the elderly. Nutr. Neurosci. 1999;2:249–256. doi: 10.1080/1028415X.1999.11747281. [DOI] [PubMed] [Google Scholar]
- 88.Kumar M.V., Rajagopalan S. Trial using multiple micronutrient food supplement and its effect on cognition. Indian J. Pediatr. 2008;75:671–678. doi: 10.1007/s12098-008-0127-1. [DOI] [PubMed] [Google Scholar]
- 89.Yaffe K., Clemons T., McBee W., Lindblad A. Impact of antioxidants, zinc, and copper on cognition in the elderly: A randomized, controlled trial. Neurology. 2004;63:1705–1707. doi: 10.1212/01.wnl.0000142969.19465.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kang J.H., Cook N.R., Manson J.E., Buring J.E., Albert C.M., Grodstein F. Vitamin e, vitamin c, beta carotene, and cognitive function among women with or at risk of cardiovascular disease. Circulation. 2009;119:2772–2780. doi: 10.1161/CIRCULATIONAHA.108.816900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chui M.H., Greenwood C.E. Antioxidant vitamins reduce acute meal-induced memory deficits in adults with type 2 diabetes. Nutr. Res. 2008;28:423–429. doi: 10.1016/j.nutres.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 92.Day J., Bayer A., McMahon M., Pathy M., Spragg B., Rowlands D. Thiamine status, vitamin supplements and postoperative confusion. Age Ageing. 1988;17:29–34. doi: 10.1093/ageing/17.1.29. [DOI] [PubMed] [Google Scholar]
- 93.Paraskevas G., Kapaki E., Libitaki G., Zournas C., Segditsa I., Papageorgiou C. Ascorbate in healthy subjects, amyotrophic lateral sclerosis and alzheimer’s disease. Acta Neurol. Scand. 1997;96:88–90. doi: 10.1111/j.1600-0404.1997.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 94.Woo J., Ho S., Mak Y., MacDonald D., Swaminathan R. Vitamin nutritional status in elderly chinese subjects living in chronic care institutions. Nutr. Res. 1989;9:1071–1080. doi: 10.1016/S0271-5317(89)80042-9. [DOI] [Google Scholar]
- 95.Polidori M.C., Mecocci P. Plasma susceptibility to free radical-induced antioxidant consumption and lipid peroxidation is increased in very old subjects with alzheimer disease. J. Alzheimers Dis. 2002;4:517–522. doi: 10.3233/JAD-2002-4608. [DOI] [PubMed] [Google Scholar]
- 96.Foy C., Passmore A., Vahidassr M., Young I., Lawson J. Plasma chain-breaking antioxidants in alzheimer’s disease, vascular dementia and parkinson’s disease. QJM. 1999;92:39–45. doi: 10.1093/qjmed/92.1.39. [DOI] [PubMed] [Google Scholar]
- 97.Talley V H.C., Wicks M.N., Carter M., Roper B. Ascorbic acid does not influence consciousness recovery after anesthesia. Biol. Res. Nurs. 2009;10:292–298. doi: 10.1177/1099800408323222. [DOI] [PubMed] [Google Scholar]
- 98.Luchsinger J.A., Mayeux R. Dietary factors and alzheimer’s disease. Lancet Neurol. 2004;3:579–587. doi: 10.1016/S1474-4422(04)00878-6. [DOI] [PubMed] [Google Scholar]
- 99.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 100.Mowe M., Bøhmer T., Kindt E. Reduced nutritional status in an elderly population (>70 years) is probable before disease and possibly contributes to the development of disease. Am. J. Clin. Nutr. 1994;59:317–324. doi: 10.1093/ajcn/59.2.317. [DOI] [PubMed] [Google Scholar]
- 101.Monget A., Galan P., Preziosi P., Keller H., Bourgeois C., Arnaud J., Favier A., Hercberg S. Micronutrient status in elderly people. Geriatrie/min. Vit. Aux network. Int. J. Vitam. Nutr. Res. 1996;66:71–76. [PubMed] [Google Scholar]
- 102.Frei B., Stocker R., Ames B.N. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc. Natl. Acad. Sci. USA. 1988;85:9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Richardson J.S. Free radicals in the genesis of alzheimer’s disease. Ann. N. Y. Acad. Sci. 1993;695:73–76. doi: 10.1111/j.1749-6632.1993.tb23031.x. [DOI] [PubMed] [Google Scholar]
- 104.Markesbery W.R., Carney J.M. Oxidative alterations in alzheimer’s disease. Brain Pathol. 1999;9:133–146. doi: 10.1111/j.1750-3639.1999.tb00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Beal M.F. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann. Neurol. 1995;38:357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- 106.Gouras G.K., Tsai J., Naslund J., Vincent B., Edgar M., Checler F., Greenfield J.P., Haroutunian V., Buxbaum J.D., Xu H. Intraneuronal aβ42 accumulation in human brain. Am. J. Pathol. 2000;156:15–20. doi: 10.1016/S0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sayre L., Perry G., Atwood C., Smith M. The role of metals in neurodegenerative diseases. Cell. Mol. Biol. 2000;46:731–741. [PubMed] [Google Scholar]
- 108.Connor J., Menzies S., St Martin S., Mufson E. A histochemical study of iron, transferrin, and ferritin in alzheimer’s diseased brains. J. Neurosci. Res. 1992;31:75–83. doi: 10.1002/jnr.490310111. [DOI] [PubMed] [Google Scholar]
- 109.Thomas T., Thomas G., McLendon C., Sutton T., Mullan M. Beta-amyloid-mediated vasoactivity and vascular endothelial damage. Nature. 1996;380:168. doi: 10.1038/380168a0. [DOI] [PubMed] [Google Scholar]
- 110.Michels A.J., Frei B. Myths, artifacts, and fatal flaws: Identifying limitations and opportunities in vitamin c research. Nutrients. 2013;5:5161–5192. doi: 10.3390/nu5125161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Harrison F.E. A critical review of vitamin c for the prevention of age-related cognitive decline and alzheimer’s disease. J. Alzheimers Dis. 2012;29:711–726. doi: 10.3233/JAD-2012-111853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Benzie I., Strain J. Simultaneous automated measurement of total’antioxidant’(reducing) capacity and ascorbic acid concentration. Redox Rep. 1997;3:233–238. doi: 10.1080/13510002.1997.11747115. [DOI] [PubMed] [Google Scholar]
- 113.Cahill L.E., El-Sohemy A. Vitamin c transporter gene polymorphisms, dietary vitamin c and serum ascorbic acid. J. Nutrigenet. Nutrigenomics. 2010;2:292–301. doi: 10.1159/000314597. [DOI] [PubMed] [Google Scholar]
- 114.Bowman G.L., Shannon J., Ho E., Traber M.G., Frei B., Oken B.S., Kaye J.A., Quinn J.F. Reliability and validity of food frequency questionnaire and nutrient biomarkers in elders with and without mild cognitive impairment. Alzheimer Dis. Assoc. Disord. 2011;25:49. doi: 10.1097/WAD.0b013e3181f333d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weinstein M., Babyn P., Zlotkin S. An orange a day keeps the doctor away: Scurvy in the year 2000. Pediatrics. 2001;108:e55. doi: 10.1542/peds.108.3.e55. [DOI] [PubMed] [Google Scholar]
- 116.Vizuete A.A., Robles F., Rodríguez-Rodríguez E., López-Sobaler A.M., Ortega R.M. Association between food and nutrient intakes and cognitive capacity in a group of institutionalized elderly people. Eur. J. Nutr. 2010;49:293–300. doi: 10.1007/s00394-009-0086-y. [DOI] [PubMed] [Google Scholar]
- 117.Clarke R., Smith A.D., Jobst K.A., Refsum H., Sutton L., Ueland P.M. Folate, vitamin b12, and serum total homocysteine levels in confirmed alzheimer disease. Arch. Neurol. 1998;55:1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 118.Grundman M. Vitamin e and alzheimer disease: The basis for additional clinical trials. Am. J. Clin. Nutr. 2000;71:630S–636S. doi: 10.1093/ajcn/71.2.630s. [DOI] [PubMed] [Google Scholar]
- 119.Evatt M.L., DeLong M.R., Khazai N., Rosen A., Triche S., Tangpricha V. Prevalence of vitamin d insufficiency in patients with parkinson disease and alzheimer disease. Arch. Neurol. 2008;65:1348–1352. doi: 10.1001/archneur.65.10.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Presse N., Shatenstein B., Kergoat M.-J., Ferland G. Low vitamin k intakes in community-dwelling elders at an early stage of alzheimer’s disease. J. Am. Diet. Assoc. 2008;108:2095–2099. doi: 10.1016/j.jada.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 121.Rodrigue K., Kennedy K., Devous M., Rieck J., Hebrank A., Diaz-Arrastia R., Mathews D., Park D. B-amyloid burden in healthy aging regional distribution and cognitive consequences. Neurology. 2012;78:387–395. doi: 10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.WIND A.W., Schellevis F.G., Van Staveren G., Scholten R.J., Jonker C., Van Eijk J.T.M. Limitations of the mini-mental state examination in diagnosing dementia in general practice. Int. J. Geriatr. Psychiatry. 1997;12:101–108. doi: 10.1002/(SICI)1099-1166(199701)12:1<101::AID-GPS469>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 123.Crum R.M., Anthony J.C., Bassett S.S., Folstein M.F. Population-based norms for the mini-mental state examination by age and educational level. JAMA. 1993;269:2386–2391. doi: 10.1001/jama.1993.03500180078038. [DOI] [PubMed] [Google Scholar]
- 124.Polidori M.C., Praticó D., Mangialasche F., Mariani E., Aust O., Anlasik T., Mang N., Pientka L., Stahl W., Sies H. High fruit and vegetable intake is positively correlated with antioxidant status and cognitive performance in healthy subjects. J. Alzheimers Dis. 2009;17:921–927. doi: 10.3233/JAD-2009-1114. [DOI] [PubMed] [Google Scholar]
- 125.Sahakian B.J., Morris R.G., Evenden J.L., Heald A., Levy R., Philpot M., Robbins T.W. A comparative study of visuospatial memory and learning in alzheimer-type dementia and parkinson’s disease. Brain. 1988;111:695–718. doi: 10.1093/brain/111.3.695. [DOI] [PubMed] [Google Scholar]
- 126.Weintraub S., Dikmen S.S., Heaton R.K., Tulsky D.S., Zelazo P.D., Bauer P.J., Carlozzi N.E., Slotkin J., Blitz D., Wallner-Allen K. Cognition assessment using the nih toolbox. Neurology. 2013;80:S54–S64. doi: 10.1212/WNL.0b013e3182872ded. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pipingas A., Harris E., Tournier E., King R., Kras M., Stough C.K. Assessing the efficacy of nutraceutical interventions on cognitive functioning in the elderly. Curr. Top. Nutraceutical Res. 2010;8:79. [Google Scholar]