ABSTRACT
Accurate and rapid identification of methicillin-resistant Staphylococcus aureus (MRSA) is needed to screen MRSA carriers and improve treatment. The current widely used duplex PCR methods are not able to differentiate MRSA from coexisting methicillin-susceptible S. aureus (MSSA) or other methicillin-resistant staphylococci. In this study, we aimed to develop a direct method for accurate and rapid detection of MRSA in clinical samples from open environments, such as nasal swabs. The new molecular assay is based on detecting the cooccurrence of nuc and mecA markers in a single bacterial cell by utilizing droplet digital PCR (ddPCR) with the chimeric lysin ClyH for cell lysis. The method consists of (i) dispersion of an intact single bacterium into nanoliter droplets, (ii) temperature-controlled release of genomic DNA (gDNA) by ClyH at 37°C, and (iii) amplification and detection of the markers (nuc and mecA) using standard TaqMan chemistries with ddPCR. Results were analyzed based on MRSA index ratios used for indicating the presence of the duplex-positive markers in droplets. The method was able to achieve an absolute limit of detection (LOD) of 2,900 CFU/ml for MRSA in nasal swabs spiked with excess amounts of Escherichia coli, MSSA, and other mecA-positive bacteria within 4 h. Initial testing of 104 nasal swabs showed that the method had 100% agreement with the standard culture method, while the normal duplex qPCR method had only about 87.5% agreement. The single-bacterium duplex ddPCR assay is rapid and powerful for more accurate detection of MRSA directly from clinical specimens.
KEYWORDS: methicillin-resistant Staphylococcus aureus, single-bacterium duplex droplet digital PCR, temperature-controlled lysis, chimeric lysin ClyH, MRSA index ratios
INTRODUCTION
Staphylococcus aureus is a Gram-positive pathogen associated with a variety of problems ranging from skin infections to bloodstream infections (1, 2). Infections due to methicillin-resistant S. aureus (MRSA) lead to significantly higher mortality rates and in-ward costs (3–5). Rapid screening of MRSA carriers by PCR methods is currently recommended as an effective way to prevent nosocomial MRSA infections (6–8). A nasal or skin swab is normally collected from a patient and subjected to different PCR tests. Because different Staphylococcus species might coexist in the swab and have different pathogenicity mechanisms, pathogenesis, or peculiar transmission aspects, it is important to develop PCR methods which can detect MRSA correctly in the sample.
The most common PCR tests are to detect a specific gene of S. aureus, such as nuc, and the methicillin-resistance marker mecA simultaneously using duplex real-time quantitative PCR (RT-qPCR) (9–11). If both genes are detected in a sample, it is considered positive for MRSA. This way is straightforward. But false-positive results could happen if methicillin-susceptible S. aureus (MSSA) (nuc positive) and other methicillin-resistant coagulase-negative staphylococci (CoNS), such as methicillin-resistant Staphylococcus haemolyticus (mecA positive), are present in the same swab (12, 13).
Another PCR method for MRSA detection is to find the staphylococcal cassette chromosome mec element (SCCmec) right extremity junction (MREJ) region between the mecA cassette and the orfX gene (14). Because only MRSA could have such a junction, the PCR method is quite specific. The major problem for this method is that multiple primers must be used, since there are multiple MREJ genotypes in MRSA (15–18). The primers need adjusting when new types of MRSA need to be accommodated (18). False-positive results would also appear during testing of mecA dropout strains (19).
In recent years, droplet digital PCR (ddPCR) has been developed and used for many clinical applications due to its absolute quantitation without a calibration curve, unparalleled sensitivity, and precision (20–22). One of the key features of ddPCR is its ability to disperse the target nucleic acid molecules into nanoliter liquid droplets so that there can be one molecule in each droplet to facilitate the absolute quantitation. Based on a similar principle, if bacteria could be dispersed into nanoliter droplets so that a single bacterium exists in an individual droplet, accurate detection of MRSA in mixtures may be realized using duplex ddPCR to detect if there is cooccurrence of an S. aureus-specific gene and mecA in the droplets. In this study, this novel method was developed and evaluated using samples from nasal swabs.
RESULTS
Optimization of the annealing temperature for the ddPCR assay.
Because the duplex ddPCR assay needed to amplify both nuc and mecA simultaneously, the annealing temperature for the ddPCR assay was optimized by using temperature gradients. As shown in Fig. S1 in the supplemental material, when the annealing temperature was set at 57°C, the fluorescence differences between the negative and the positive droplets were largest for both nuc and mecA. Therefore, an optimized annealing temperature of 57°C was chosen for the subsequent ddPCR.
Optimizing conditions for bacteria lysis by ClyH.
It was critical to disperse intact bacteria into the droplets and then lyse the bacteria before PCR so that the target genes could be amplified for the successful application of single-bacterium duplex ddPCR. We showed previously that ClyH is an effective enzyme for releasing genomic DNA from MRSA at room temperature (23). Since the droplet generator has no cooling function, we tried to precool the solutions with a low concentration of ClyH on ice in order to slow the lysis speed of ClyH. As shown in Fig. S2, after cooling of the ddPCR mixture containing MRSA strain N315 and ClyH on ice for 10 min, minimum bacteria lysis could be detected in terms of optical density at 600 nm (OD600) after the mixture was placed at room temperature for 10 min. With a further increase of the temperature to 37°C, the activity of ClyH could be reactivated to lyse the bacteria completely within 30 min. In contrast, without the precooling on ice, the decreased OD600 indicated that some bacteria were lysed by ClyH during 10 min of incubation at room temperature. Since the time required is about 5 to 6 min for the droplet generator to complete droplet generation, the precooling could reduce the activity of ClyH and ensure the integrity of the bacterial cells before dispersing them into nanoliter droplets.
Evaluation of the duplex ddPCR performance for detection of MRSA.
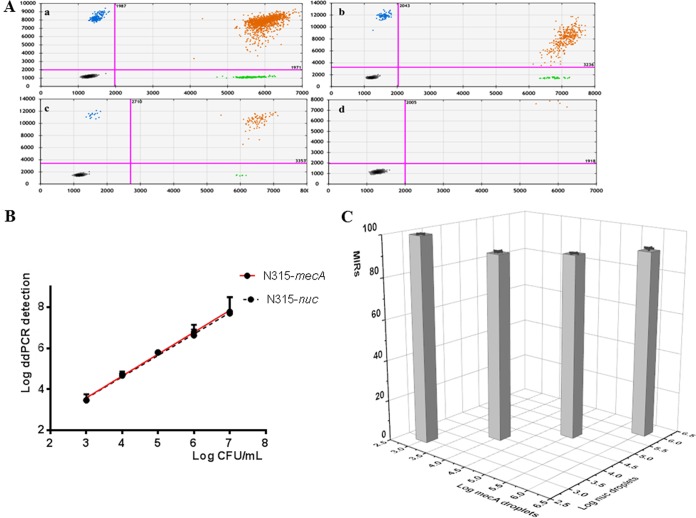
MRSA strain N315 solutions with different concentrations were tested first using the duplex ddPCR assay. From the two-dimensional (2D) plots of the assay (Fig. 1A), one can see that under different N315 concentrations, the duplex-positive droplets were dominant among the positive droplets. The MRSA index ratio was above 80% under all the concentrations tested (Fig. 1C and Table S2).
FIG 1.
Two-dimensional (2D) plots (A), calibration curves (B), and the average MRSA index ratio (MIR) of MRSA (C) of duplex ddPCR for detection of MRSA N315 under different concentrations (a, 1 × 106 CFU/ml; b, 1 × 105 CFU/ml; c, 1 × 104CFU/ml; and d. 1 × 103 CFU/ml). In the 2D plots, the x axis shows the fluorescence amplitude corresponding to the HEX fluorophore (nuc), and the y axis represents the fluorescence amplitude corresponding to the FAM fluorophore (mecA). Each point represents a droplet with a given fluorescence level, and the droplet colors indicate which target was amplified (blue, mecA positive; green, nuc positive; black, negative; orange, both nuc and mecA positive). In panel C, the x axis shows log of the number of mecA-positive droplets per ml, and the y axis shows log of the number of nuc-positive droplets per ml. The z axis shows the MRSA index ratio. Standard deviations are shown as error bars in the calibration curve.
The duplex ddPCR assay was also quite sensitive with a limit of detection about 2,900 CFU/ml and good linearity (slope = 1.033 ± 0.045, coefficient of determination [R2] = 0.9943 for detecting nuc; slope = 1.020 ± 0.045, R2 = 0.9941 for detecting mecA) ranging from 2.90 × 103 CFU/ml to 1 × 107 CFU/ml (Fig. 1B). Furthermore, under bacteria concentrations lower than 1 × 106 CFU/ml, the bacteria concentrations (or copies/ml) given by the software based on the numbers of positive nuc or mecA droplets were quite close to the actual bacteria numbers counted by CFU. Because almost all the droplets were positive when the concentration was 1 × 107 CFU/ml (by calculation, 2 μl of the bacteria solution contained about 20,000 bacteria theoretically in one PCR, where the maximum droplets in one ddPCR reaction are about 20,000 according to Bio-Rad's instruction), the maximum concentrations of bacteria were limited to 1 × 106 CFU/ml in the following experiments.
Evaluation of duplex ddPCR performance for detection of MSSA, MR-CoNS, and their mixtures.
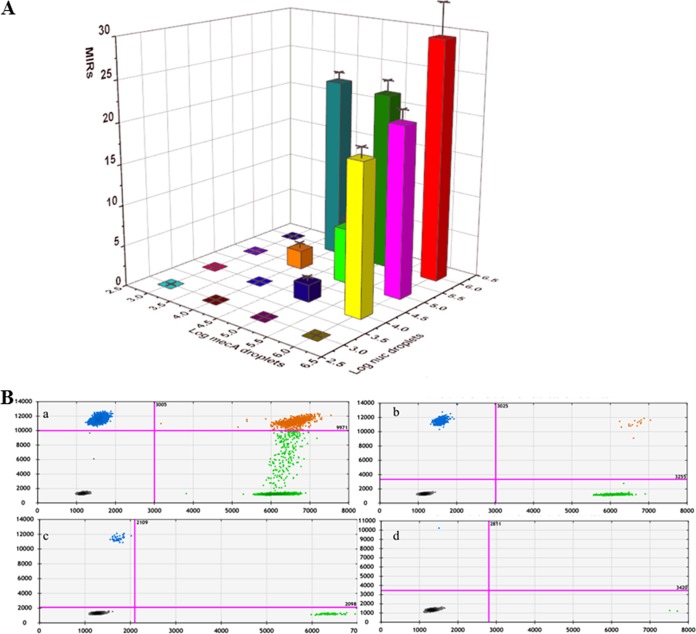
Further analyzing MSSA strain 91118 and methicillin-resistant Staphylococcus haemolyticus (MR-CoNS) strain WH01 alone by the duplex ddPCR assay showed that only nuc-positive or mecA-positive droplets could be detected (data not shown). Therefore, the MRSA index ratios (MIRs) were 0 for pure MSSA 91118 and pure MR-CoNS strain WH01. However, when MSSA 91118 and MR-CoNS WH01 were mixed under different concentrations, duplex-positive droplets could be detected, especially under high concentrations (Fig. 2B, Fig. S3 to S5, and Table S3). The MIRs increased from 0 with the increasing concentrations of either MSSA 91118 or MR-CoNS in the mixtures (Fig. 2A). When the concentrations in the mixture of both MSSA 91118 and MR-CoNS were 1 × 106 CFU/ml, the MIR was highest, about 29.48 ± 4.07 (Table S3).
FIG 2.
The average MRSA index ratios (MIRs) (A) and two-dimensional (2D) plots (B) of duplex ddPCR for detection of the mixture of MSSA 91118 and MR-CoNS WH01 under different concentrations (a, 1 × 106 CFU/ml each; b, 1 × 105 CFU/ml each; c, 1 × 104CFU/ml each; and d, 1 × 103 CFU/ml each). In panel A, the x axis shows log of the number of mecA-positive droplets per ml, the y axis shows log of the number of nuc-positive droplets per ml, and the z axis (colored bars) shows the MRSA index ratio. Standard deviations are shown as error bars in the calibration curve. In panel B, the x axis shows the fluorescence amplitude corresponding to the HEX fluorophore (nuc), and the y axis represents the fluorescence amplitude corresponding to the FAM fluorophore (mecA). Each point represents a droplet with a given fluorescence level, and the droplet colors indicate which target was amplified (blue, mecA positive; green, nuc positive; black, negative; orange, both nuc and mecA positive).
Performance of duplex ddPCR for analyzing nasal swab samples spiked with different bacteria.
In order to test if the duplex ddPCR assay could be used for accurate detection of MRSA in mixtures, 18 spiked nasal swab samples were prepared as shown in Table 1. Based on the results, MIRs were calculated and used to determine if MRSA was present or not in the sample by comparison with the MIRs of the MSSA and MR-CoNS mixtures with similar nuc-positive and mecA-positive droplets. If the MIR of a sample was larger than that of the MSSA 91118 and MR-CoNS WH01 mixture plus 2 standard deviations (SDs) (MIRcutoff), it suggested that MRSA was present in the sample. Using this method, we found that the duplex ddPCR could detect MRSA correctly in all cases. Notably, 0.1% MRSA (about 5 × 103 CFU/ml, close to the limit of detection [LOD] of the ddPCR assay) in the spiked nasal swab was detected correctly, even in the presence of the high background of nuc-positive MSSA 91118 (1 × 106 CFU/ml) and mecA-positive MR-CoNS WH01 (1 × 105 CFU/ml).
TABLE 1.
Detection results of 18 simulative nasal swab samples based on the MRSA index ratios of duplex ddPCR
| Sample no.a | Sample contents | Average Mb | Average N | Average MN | MIR | MIRcutoff | Detection result |
|---|---|---|---|---|---|---|---|
| 1 | 1 × 105 CFU/ml S. aureus 91118 | 0 | 295 | 0 | 0 | 0 | MSSA |
| 2 | 1 × 106 CFU/ml E. coli | 0 | 0 | 0 | 0 | 0 | No MRSA |
| 3 | 2.7 × 105 CFU/ml MR-CoNS | 512 | 0 | 0 | 0 | 0 | MR-CoNS |
| 4 | 3 × 103 CFU/ml MRSA ZX3 | 4 | 4 | 4 | 100 | 0 | MRSA |
| 5 | 4 × 105 CFU/ml MRSA ZX108 | 826 | 768 | 690 | 89.84 | 8.65 | MRSA |
| 6 | 4 × 104 CFU/ml MRSA FY16 | 91 | 84 | 77 | 91.67 | 0 | MRSA |
| 7 | 7 × 103 CFU/ml MRSA WH70 | 12 | 13 | 11 | 91.67 | 0 | MRSA |
| 8 | 3 × 103 CFU/ml MRSA N315 | 4 | 4 | 4 | 100 | 0 | MRSA |
| 9 | 4.5 × 105 CFU/ml MRSA YN22 | 833 | 805 | 691 | 85.84 | 8.65 | MRSA |
| 10 | 3 × 104 CFU/ml MRSA KQ6 | 63 | 52 | 43 | 82.69 | 0 | MRSA |
| 11 | 2.5 × 104 CFU/ml MRSA FY17 | 48 | 49 | 39 | 81.25 | 0 | MRSA |
| 12 | 2 × 105 CFU/ml MRSA ZX54 | 433 | 422 | 386 | 91.47 | 8.65 | MRSA |
| 13 | 5 × 103 CFU/ml MRSA N315 + 1 × 105 CFU/ml MR-CoNS + 1.7 × 106 CFU/ml MSSA | 207 | 3,427 | 51 | 24.63 | 23.96 | MRSA with mixture of MSSA (more) and MR-CoNS (less) |
| 14 | 1 × 105 CFU/ml MR-CoNS + 1.7 × 106 CFU/ml MSSA | 193 | 3,419 | 37 | 19.17 | 23.96 | Mixture of MSSA and MR-CoNS |
| 15 | 2 × 106 CFU/ml MRSA N315 + 1 × 106 CFU/ml MR-CoNS + 1.5 × 106 CFU/ml MSSA | 6,037 | 7,204 | 4,768 | 78.79 | 37.62 | MRSA with nearly equal mixture of MSSA and MR-CoNS |
| 16 | 4 × 103 CFU/ml MR-CoNS + 4 × 103 CFU/ml MSSA | 8 | 8 | 0 | 0 | 0 | Mixture of MSSA and MR-CoNS |
| 17 | 1.5 × 105 CFU/ml MRSA N315 + 1 × 106 CFU/ml MR-CoNS + 3 × 106 CFU/ml MSSA | 2,305 | 6,261 | 951 | 41.25 | 37.62 | MRSA with nearly equal mixture of MSSA and MR-CoNS |
| 18 | 1 × 106 CFU/ml MR-CoNS + 3 × 106 CFU/ml MSSA | 2,051 | 5,996 | 681 | 33.2 | 37.62 | Mixture of MSSA and MR-CoNS |
All spiked nasal swab samples contained E. coli (1 × 106 CFU/ml) as background. Each sample was tested in triplicate.
M, the total number of mecA gene-positive droplets; N, the total number of nuc gene-positive droplets; MN, the number of duplex-positive droplets which were both nuc and mecA positive; MIR, MRSA index ratio; MIRcutoff, MRSA index ratio of the MSSA and MR-CoNS mixture with similar nuc-positive and mecA-positive droplets plus 2 × SD.
Comparative evaluation of the performance of duplex ddPCR and duplex qPCR for analyzing nasal swab specimens.
In order to compare the performance of duplex ddPCR with that of duplex qPCR, 104 nasal swabs were taken and subjected to MRSA detection. As shown in Table 2 and Table S4, the results showed that the sensitivity and the specificity of the ddPCR (both 100%) were much better than those of the duplex qPCR, which were 38.89% and 97.67%, respectively, in comparison with the standard culture method. It was also found that the ddPCR assay was less prone to the possible inhibitors in the swabs, enabling the detection of a lower number of bacteria in the samples, while the duplex qPCR assay could not detect MRSA from 11 specimens (Table S4). Among the samples, specimens 13 and 25 were detected incorrectly as MRSA by the duplex qPCR. Notably, sample 68 was found to contain an excess amount of mecA (about 2 × 106 copies/ml by the ddPCR assay, cycle threshold (CT) 24.61 by the qPCR test), which is about 1,000 times more than the amount of nuc (Table S4) under the direct test. Since the amount of mecA exceeded the detection range recommended for the ddPCR assay, further testing the 10 × dilution of the specimen showed that it was MRSA negative.
TABLE 2.
Sensitivity and specificity of the qPCR and ddPCR methods for detection of MRSA in 104 nasal specimens
| Method and result | Culture result |
Sensitivity (% [95% CI]) | Specificity (% [95% CI]) | Agreement (% [95% CI]) | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| qPCR | 38.89 (18.26–63.86) | 97.67 (91.06–99.6) | 87.5 (79.22–92.91) | ||
| Positive | 7 | 2 | |||
| Negative | 11 | 84 | |||
| ddPCR | 100 (78.12–100) | 100 (94.67–100) | 100 (95.56–100) | ||
| Positive | 18 | 0 | |||
| Negative | 0 | 86 | |||
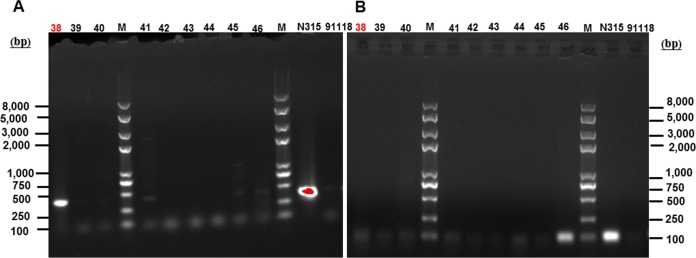
Another thing worth noting is that one mecA dropout strain (MREJ region positive and mecA negative) was isolated from specimen 38 as shown in Fig. 3. The MRSA-negative result given by the duplex ddPCR method for specimen 38 (Table S4) showed that the mecA dropout did not affect the detection of the single-bacterium duplex ddPCR method.
FIG 3.
PCR amplicons of the MREJ region (A) using the primer pair rjmec and ORFX1r and mecA (B) for colonies isolated from different specimens. MRSA strain N315 and MSSA strain 91118 were used as the controls. Lanes 38, 39, and 40 were isolated from specimen 38; lanes 41, 42, and 43 were isolated from specimen 39; lanes 44, 45, and 46 were isolated from specimen 40.
DISCUSSION
In this study, a new approach for the accurate detection of MRSA in mixtures was developed utilizing single-bacterium duplex ddPCR. The idea behind this approach is to disperse bacteria into nanoliter droplets so that a single bacterium can exist in an individual droplet. Duplex ddPCR is then performed to detect if there is cooccurrence of S. aureus-specific nuc and methicillin-resistance marker mecA in one droplet. Therefore, it is very important to keep the bacteria intact before inclusion into the droplets and then break down the bacteria inside the droplets to release their genomic DNA for PCR. How to meet these two paradoxical requirements is critical for the success of the approach.
Because S. aureus is Gram positive, it is difficult to break its cell walls by boiling, which means that it is difficult to release genomic DNA using the denaturation temperature of the PCR. Chemicals normally used for lysing bacteria, such as surfactants, could not be used since they would inactivate the enzymes for PCR. In our previous research, lysin ClyH was found to be effective in lysing S. aureus at room temperature and compatible with PCRs. By using a low temperature (cooling on ice) to slow the activity of ClyH, the integrity of the bacteria was successfully maintained before dispersing them into droplets (see Fig. S2 in the supplemental material). This temperature-controlled enzyme lysis was a key factor for the single-bacterium duplex ddPCR.
Through analyzing the ratios of the duplex-positive droplets over the positive droplets obtained by the duplex ddPCR assay in cases of MRSA, MSSA, and MR-CoNS, a simple MRSA index ratio (MIR) was created and used to identify MRSA in the samples. The theoretical MIRs for MRSA should be 100%. However, the MIRs for pure MRSA strain N315 and other clinical MRSA isolates spiked in the nasal swabs was found to be less than 100% but more than 80% in our study (Fig. 1C and Table S2). The reason may be that few bacteria were still lysed by ClyH during the droplet generation performed at room temperature, although there was a precooling on ice, since ClyH is a very effective enzyme (23). The MIRs for pure MRSA might increase further if a droplet generator with cooling function is used.
The MIRs for pure MSSA and pure MR-CoNS were 0%, identical to the theoretical values. However, in the mixtures of MSSA and MR-CoNS bacteria, droplets with cooccurrence of nuc and mecA were detected (Fig. 2B). The reason might be that two or more bacterial cells were dispersed into one droplet, since the number of the bacteria in one droplet is distributed with Poisson distribution. When the concentrations of bacteria are high, there is a greater chance for two or more bacteria to be distributed into one droplet. Therefore, besides the MIR, one must also check the total number of droplets positive for both nuc and mecA (which are correlated with the concentration of the target bacteria in the sample) when analyzing real samples. Ideally, MIRs under different combinations of MSSA and MR-CoNS could be determined first to obtain the different cutoff MIRs (MIRcutoff) under different numbers of droplets positive for nuc and mecA. Then, the MIR of a real sample is compared with the MIRcutoff under similar numbers of droplets positive for nuc and mecA.
Using the current approach, the results demonstrated that as low as 0.1% MRSA could be correctly identified even in the samples with high backgrounds of MSSA strain 91118 (1 × 106 CFU/ml), MR-CoNS WH01 (1 × 105 CFU/ml), and E. coli (1 × 106 CFU/ml). This result shows that the single-bacterium duplex ddPCR assay is very powerful and can overcome the false-positive problems in current PCR methods when detecting MSSA and MR-CoNS mixtures. The testing results (Table 2 and Table S4) of 104 nasal specimens not only further confirmed this conclusion but also showed that ddPCR has a higher sensitivity for detecting MRSA directly in nasal swabs than normal duplex qPCR. In Table S4, the low sensitivity of the qPCR is mainly because nuc was not detected in 8 samples (numbers 3, 19, 24, 33, 45, 49, 72, and 96), while the ddPCR could detect it, although with low copies. Since it is well known that ddPCR has higher tolerance to inhibitors, a DNA purification process after ClyH lysis would help improve the sensitivity of qPCR. Furthermore, the MRSA-negative result given by the duplex ddPCR for mecA-dropout-containing specimen 38 implies that mecA dropouts do not affect the specificity of the duplex ddPCR method. This feature would be an added advantage compared with other qPCR methods targeting SCCmec elements and the neighboring chromosome-borne orfX (MREJ region), which could give false-positive results when detecting mecA dropouts.
Last but not least, due to the upper quantification limit (UQL) of ddPCR (the limit numbers of droplets in one reaction), samples with high concentrations of MRSA, MSSA, and/or MR-CoNS need dilution before testing. Normally, if after the ddPCR assay all the droplets are found positive for a sample, the sample needs dilution until some percentage of the droplets is negative. Although the dilution might decrease the chance of detecting a minimum amount of MRSA in the sample, the reduced MIRcutoff after the dilution might help to identify MRSA more easily. From the results of the 104 nasal specimens, it seems that bacteria concentrations in the swabs are normally low. Only one specimen (number 68) needed dilution for the ddPCR.
In conclusion, a novel approach for accurate detection of MRSA in mixtures has been developed by using single-bacterium duplex ddPCR coupled with temperature-controlled lysis of S. aureus by ClyH. Since the whole assay is easy to perform and powerful for identifying MRSA in samples with high background of MSSA, MR-CoNS, and other bacteria, duplex ddPCR will be useful for more accurate detection of MRSA in clinical specimens, especially specimens from open environments such as nasal swabs.
MATERIALS AND METHODS
Bacterial strains.
MRSA reference strain N315, methicillin-resistant Staphylococcus haemolyticus strain WH01 (MR-CoNS), methicillin-susceptible S. aureus (MSSA) strain CCTCC AB91118 (called 91118 in this study), and E. coli strain ATCC 25922 were used as the control bacteria for optimizing and characterizing the method. Eight MRSA clinical isolates were obtained from local hospitals in China (see Table S1 in the supplemental material).
Preparation of bacteria.
Bacteria were maintained by growth on Luria-Bertani (LB) agar plates. To prepare bacteria suspensions, one single colony on each plate was picked and separately cultured overnight in 5 ml LB liquid media at 37°C with continuous shaking at 160 rpm. Then the pellets were harvested by centrifugation (6000 × g for 5 min) and suspended in sterilized phosphate-buffered saline (PBS) buffer (consisting of 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4; pH 7.4). The turbidity of the bacteria solutions was adjusted to OD600 0.45 (corresponding to about 6 × 108 CFU/ml in bacteria concentration) before use.
Primers, probes, and thermal gradient optimization of ddPCR assay.
S. aureus-specific nuc and methicillin-resistance marker mecA were selected as the detection targets. The primers and probes are the same as described previously by Wang et al. (10) and synthesized by Sangon Biotech Co., Ltd. (Shanghai, China): for nuc, forward primer 5′-AAAGCGATTGATGGTGATACGGTT-3′, reverse primer 5′-TGCTTTGTTTCAGGTGTATCAACCA-3′. and probe 5′-HEX-ATGTACAAAGGTCAACCAATGACATTYAGA-BHQ1-3′; for mecA, forward primer 5′-GCTCAAATTTCAAACAAAAATTTAGATAATG3′, reverse primer 5′ TGAAAGGATCTGTACTGGGTTAATCAGT-3′, and probe 5′-FAM-AGCTGATTCAGGTTACGGACAAGGTGA-BHQ1-3′. The concentrations of the primers and the probes used in ddPCR were 900 nM and 250 nM, respectively. To assess the optimal annealing temperature of the ddPCR assay, the annealing temperature gradient between 54°C and 60°C was performed in a Bio-Rad T100 Thermal Cycler using genomic DNA extracted form MRSA strain N315 as the template.
Optimizing conditions for bacteria lysis by ClyH.
The lyophilized recombinant lysin ClyH was provided by Wuhan Scithera Microbial Technologies, Inc. (Wuhan, China) and reconstituted to 1 mg/ml as the stock solution by adding water. In order to ensure the target bacteria were intact before dispersing them into nanoliter liquid droplets, a microplate reader (Synergy H1, BioTek) was used to monitor the OD600 changes of MRSA strain N315 solutions mixed with different concentrations of ClyH under different temperatures. Briefly, all of the solutions, including the bacteria solution/samples, the ClyH stock solution, and the duplex ddPCR mixture, were precooled on ice for 10 min. Then mixtures were made on ice by pipetting different amounts of the solutions into wells of a 96-well microplate. After that, the microplate was placed at room temperature (25°C) for 10 min. Finally, the microplate was placed into the microplate reader at 37°C to monitor the OD600 of the mixtures for 30 min.
Procedure of the duplex droplet digital PCR assay.
Duplex ddPCR assays were conducted on a QX200 ddPCR system (Bio-Rad) according to the manufacturer's recommendations. Briefly, the ddPCR reaction mixtures (22 μl each) were prepared on ice by mixing 11 μl of 2 × Supermix (Bio-Rad), 0.25 μM each of the probes, and 0.9 μM each of the primers for detection of mecA and nuc, 10 μg/ml chimeric lysin ClyH, and 2 μl of the bacteria suspension or the samples. Then droplets were generated at room temperature using an automated droplet generator (Bio-Rad), where a vacuum was applied to the outlet wells to simultaneously partition the PCR mixtures into nanoliter-sized droplets. The PCR plate obtained was subsequently heat-sealed with pierceable foil using a PX1 PCR plate sealer (Bio-Rad) and then amplified in a conventional thermal cycler (C100 Touch, Bio-Rad). The thermocycling was set as bacteria lysis at 37°C for 30 min, denaturation at 95°C for 10 min, then 40 cycles of denaturation at 94°C for 30 s, annealing at 57°C for 1 min (temperature ramp 2°C/s), and finally, incubation at 98°C for 10 min. The first incubation at 37°C for 30 min was to ensure that the bacteria inside droplets were lysed completely by ClyH. After the cycling, the 96-well plate was fixed into a plate holder and placed into a Q200 droplet reader (Bio-Rad) to measure the fluorescence of the droplets. The numbers of positive, negative, and duplex-positive droplets obtained were analyzed by the software package provided (QuantaSoft, Bio-Rad). Total droplet counts below 10,000 were unacceptable and discarded.
Evaluation of the duplex ddPCR performance for detection of MRSA, MSSA, MR-CoNS, and their mixtures.
MRSA strain N315, MSSA strain 91118, and MR-CoNS strain WH01 were tested alone or in mixtures by the duplex ddPCR to find the right parameters which could be used to identify MRSA correctly in mixtures. Briefly, bacteria suspensions with different concentrations of strains N315, 91118, and MR-CoNS were prepared and analyzed by the duplex PCR as described above. After the analysis, three data, i.e., the total number of nuc-positive droplets (N), the total number of mecA-positive droplets (M), and the number of duplex-positive droplets that were both nuc and mecA positive (MN), were counted. Every sample was performed in triplicate, and the average numbers of the positive droplets were used to calculate the MRSA index ratio (MIR) by the following formula:
MIR = 100 × MN/min{M,N}, where “min{M,N}” means selecting the smaller number of M or N.
Since both nuc and mecA exist in a MRSA cell, the MIR for a sample containing pure MRSA will be 100% in theory because all the droplets will be duplex-positive droplets. For MSSA or MR-CoNS, since there is only nuc or mecA in a bacterial cell, MIR for a sample containing pure MSSA, pure MR-CoNA, or their mixtures will be 0% in theory because all the droplets will be single-gene-positive droplets, supposing that there is only one bacterium in one droplet.
Evaluation of duplex ddPCR performance by spiking bacteria into nasal swabs.
Based on the MRSA index ratios for pure MRSA and the mixtures of MSSA and MR-CoNS, the performance of duplex ddPCR for identification of MRSA in nasal swabs was tested by spiking different bacteria into a negative nasal swab (no S. aureus or mecA was detected by both culture and single-bacterium duplex ddPCR assay) collected from one healthy volunteer. Briefly, a swab was carefully inserted a short distance into the nostril and gently rotated for 10 s. Then the swab was taken out and inserted into 1 ml PBS buffer immediately. After vigorous shaking for 30 s, the swab was discarded. The PBS solution was centrifuged at 6000 × g for 5 min, and the supernatant was collected for spiking clinical MRSA isolates, MRSA strain N315, S. aureus 91118, MRCoNS WH01, and/or E. coli at different concentrations. Finally, all the spiked samples were tested by the duplex ddPCR assay described above.
Comparative evaluation of the performance of duplex ddPCR and qPCR for analyzing nasal swab specimens.
A total of 104 clinical nasal swab specimens were collected from different healthy individuals. Briefly, a sterile cotton swab was carefully inserted a short distance into the nostril and gently rotated for 10 s. Then the swab was taken out and inserted into 1 ml PBS buffer immediately. After vigorous shaking for 30 s, the swab was discarded, and the PBS buffer was used for detection by ddPCR, standard culture, and qPCR directly. The ddPCR tests were performed using the same procedures described above. The same method we reported previously (23) was used for MRSA detection by qPCR. The qPCR mixtures (20 μl each) were prepared by mixing 10 μl of Premix Ex Taq (Probe qPCR, Takara Bio) containing 50 μg/ml chimeric lysin ClyH, 0.4 μM of each probe and each primer for detection of mecA and nuc genes, and 5 μl of the bacterial sample. The qPCR running program was as follows: bacteria lysis at 37°C for 10 min, denaturation at 95°C for 30 s, 40 cycles of denaturation at 95°C for 5 s, and annealing at 60°C for 1 min. The culture method (24–27) was performed by plating 10 μl of each of the above swab-PBS suspensions on selective Baird-Parker agar plates (Guangdong Huankai Microbial Sci. & Tech. Co., Ltd.) and then incubating them at 35°C for 24 to 48 h. The suspected colonies with gray-black shiny color were picked and cultured again on Mueller-Hinton agar containing 4% NaCl and 6 μg of oxacillin per ml (Qingdao Binder Bio Technologies Co., Ltd.) in accordance with NCCLS recommendations (28) to detect oxacillin-resistant strains. Finally, the MRSA colonies were further confirmed by qPCR to detect nuc and mecA.
Isolation of mecA dropout strains from nasal specimens.
mecA dropout strains were isolated from the 104 nasal specimens according to the method described by Mendes et al. (19), except using a single primer pair specific for SCCmec elements and the neighboring chromosome-borne orfX (MREJ region) as described by Cuny and Witte (14). The MSSA strains which are MREJ region positive and mecA negative are considered mecA dropout strains. Briefly, the 104 nasal specimens were inoculated on selective Baird-Parker agar plates, respectively. After incubation, the suspected colonies with gray-black shiny color were picked and cultured again on Mueller-Hinton agar containing 4% NaCl and 6 μg of oxacillin per ml to detect oxacillin-sensitive strains. Finally, the MSSA colonies were tested by qPCR to detect nuc, mecA, and the MREJ region.
The MREJ region was amplified using the primer pair rjmec, 5′-TATGATATGCTTCTCC-3′ and ORFX1r, 5′-AACGTTTAGGCCCATACACCA-3′. The MREJ PCR mixtures (20 μl each) were prepared by mixing 0.8 μl of recombinant Taq (rTaq) DNA polymerase, 0.8 μl of each primer (10 μM), 2 μl of PCR buffer, 1.6 μl of dNTP (250 mM), 13 μl of water, 1 μl of DNA template. The PCR running program was as follows: denaturation at 94°C for 3 min, 30 cycles of denaturation at 94°C for 30 s, and annealing at 55°C for 30s and 72°C for 30 s, with a final extension at 72°C for 4 min.
Supplementary Material
ACKNOWLEDGMENTS
This work is supported financially by the Natural Science Foundation of China (grant 31570175).
We have no conflicts of interest to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00716-17.
REFERENCES
- 1.Miller LG, Eells SJ, Taylor AR, David MZ, Ortiz N, Zychowski D, Kumar N, Cruz D, Boyle-Vavra S, Daum RS. 2012. Staphylococcus aureus colonization among household contacts of patients with skin infections: risk factors, strain discordance, and complex ecology. Clin Infect Dis 54:1523–1535. doi: 10.1093/cid/cis213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomer L, Schneewind O, Missiakas D. 2016. Pathogenesis of Staphylococcus aureus bloodstream infections. Annu Rev Pathol 11:343–364. doi: 10.1146/annurev-pathol-012615-044351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graffunder EM, Venezia RA. 2002. Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials. J Antimicrob Chemother 49:999–1005. doi: 10.1093/jac/dkf009. [DOI] [PubMed] [Google Scholar]
- 4.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. 2003. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 36:53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 5.Reich PJ, Boyle MG, Hogan PG, Johnson AJ, Wallace MA, Elward AM, Warner BB, Burnham CA, Fritz SA. 2016. Emergence of community-associated methicillin-resistant Staphylococcus aureus strains in the neonatal intensive care unit: an infection prevention and patient safety challenge. Clin Microbiol Infect 22:645.e1–645.e8. doi: 10.1016/j.cmi.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grisold AJ, Leitner E, Mühlbauer G, Marth E, Kessler HH. 2002. Detection of methicillin-resistant Staphylococcus aureus and simultaneous confirmation by automated nucleic acid extraction and real-time PCR. J Clin Microbiol 40:2392–2397. doi: 10.1128/JCM.40.7.2392-2397.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huletsky A, Giroux R, Rossbach V, Gagnon M, Vaillancourt M, Bernier M, Gagnon F, Truchon K, Bastien M, Picard FJ, van Belkum A, Ouellette M, Roy PH, Bergeron MG. 2004. New real-time PCR assay for rapid detection of methicillin-resistant Staphylococcus aureus directly from specimens containing a mixture of staphylococci. J Clin Microbiol 42:1875–1884. doi: 10.1128/JCM.42.5.1875-1884.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Awad S, Alshami I, Alharbi AE. 2013. Evaluation of a duplex real-time PCR assay to detect MRSA from broth culture, human sera seeded with MRSA and from patient's serum. Bioinformation 9:896–900. doi: 10.6026/97320630009896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa AM, Kay I, Palladino S. 2005. Rapid detection of mecA and nuc genes in staphylococci by real-time multiplex polymerase chain reaction. Diagn Microbiol Infect Dis 51:13–17. doi: 10.1016/j.diagmicrobio.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Wang HY, Kim S, Kim J, Park SD, Uh Y, Lee H. 2014. Multiplex real-time PCR assay for rapid detection of methicillin-resistant staphylococci directly from positive blood cultures. J Clin Microbiol 52:1911–1920. doi: 10.1128/JCM.00389-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JU, Cha CH, An HK, Lee LJ, Kim MN. 2013. Multiplex real-time PCR assay for detection of methicillin-resistant Staphylococcus aureus (MRSA) strains suitable in regions of high MRSA endemicity. J Clin Microbiol 51:1008–1013. doi: 10.1128/JCM.02495-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francois P, Pittet D, Bento M, Pepey B, Vaudaux P, Lew D, Schrenzel J. 2003. Rapid detection of methicillin-resistant Staphylococcus aureus directly from sterile or nonsterile clinical samples by a new molecular assay. J Clin Microbiol 41:254–260. doi: 10.1128/JCM.41.1.254-260.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu B, Liu L, Liu L, Li X, Li X, Wang X. 2012. A multiplex PCR assay for the rapid and sensitive detection of methicillin-resistant Staphylococcus aureus and simultaneous discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J Food Sci 77:638–642. doi: 10.1111/j.1750-3841.2012.02959.x. [DOI] [PubMed] [Google Scholar]
- 14.Cuny C, Witte W. 2005. PCR for the identification of methicillin-resistant Staphylococcus aureus (MRSA) strains using a single primer pair specific for SCCmec elements and the neighbouring chromosome-borne orfX. Clin Microbiol Infect 11:834–837. doi: 10.1111/j.1469-0691.2005.01236.x. [DOI] [PubMed] [Google Scholar]
- 15.Kondo Y, Ito T, Ma XX, Watanabe S, Kreiswirth BN, Etienne J, Hiramatsu K. 2007. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob Agents Chemother 51:264–274. doi: 10.1128/AAC.00165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bosch T, Pluister GN, van Luit M, Landman F, van Santen-Verheuvel M, Schot C, Witteveen S, van der Zwaluw K, Heck ME, Schouls LM. 2015. Multiple-locus variable number tandem repeat analysis is superior to spa typing and sufficient to characterize MRSA for surveillance purposes. Future Microbiol 10:1155–1162. doi: 10.2217/fmb.15.35. [DOI] [PubMed] [Google Scholar]
- 17.Petersdorf S, Herma M, Rosenblatt M, Layer F, Henrich B. 2015. A novel staphylococcal cassette chromosome mec type XI primer for detection of mecC-harboring methicillin-resistant Staphylococcus aureus directly from screening specimens. J Clin Microbiol 53:3938–3941. doi: 10.1128/JCM.02328-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Zee A, Roorda L, Hendriks WD, Ossewaarde JM, Buitenwerf J. 2011. Detection of novel chromosome-SCCmec variants in methicillin resistant Staphylococcus aureus and their inclusion in PCR based screening. BMC Res Notes 4:150. doi: 10.1186/1756-0500-4-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendes RE, Watters AA, Rhomberg PR, Farrell DJ, Jones RN. 2016. Performance of BD Max StaphSR for screening of methicillin-resistant Staphylococcus aureus isolates among a contemporary and diverse collection from 146 institutions located in nine U.S. Census regions: prevalence of mecA dropout mutants. J Clin Microbiol 54:204–207. doi: 10.1128/JCM.02047-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huggett JF, Cowen S, Foy CA. 2015. Considerations for digital PCR as an accurate molecular diagnostic tool. Clin Chem 61:79–88. doi: 10.1373/clinchem.2014.221366. [DOI] [PubMed] [Google Scholar]
- 21.Hayden RT, Gu Z, Ingersoll J, Abdul-Ali D, Shi L, Pounds S, Caliendo AM. 2013. Comparison of droplet digital PCR to real-time PCR for quantitative detection of cytomegalovirus. J Clin Microbiol 51:540–546. doi: 10.1128/JCM.02620-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hindson BJ, Ness KD, Masquelier DA, Belgrader P, Heredia NJ, Makarewicz AJ, Bright IJ, Lucero MY, Hiddessen AL, Legler TC, Kitano TK, Hodel MR, Petersen JF, Wyatt PW, Steenblock ER, Shah PH, Bousse LJ, Troup CB, Mellen JC, Wittmann DK, Erndt NG, Cauley TH, Koehler RT, So AP, Dube S, Rose KA, Montesclaros L, Wang S, Stumbo DP, Hodges SP, Romine S, Milanovich FP, White HE, Regan JF, Karlin-Neumann GA, Hindson CM, Saxonov S, Colston BW. 2011. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem 83:8604–8610. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Y, Yang H, Wang J, Zhang Y, Yu J, Wei H. 2016. Comparison between a chimeric lysin ClyH and other enzymes for extracting DNA to detect methicillin resistant Staphylococcus aureus by quantitative PCR. World J Microbiol Biotechnol 32:1. doi: 10.1007/s11274-015-1971-6. [DOI] [PubMed] [Google Scholar]
- 24.Hombach M, Pfyffer GE, Roos M, Lucke K. 2010. Detection of methicillin-resistant Staphylococcus aureus (MRSA) in specimens from various body sites: performance characteristics of the BD GeneOhm MRSA assay, the Xpert MRSA assay, and broth-enriched culture in an area with a low prevalence of MRSA infections. J Clin Microbiol 48:3882–3887. doi: 10.1128/JCM.00670-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Safdar N, Narans L, Gordon B, Maki DG. 2003. Comparison of culture screening methods for detection of nasal carriage of methicillin-resistant Staphylococcus aureus: a prospective study comparing 32 methods. J Clin Microbiol 41:3163–3166. doi: 10.1128/JCM.41.7.3163-3166.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Micheel V, Hogan B, Köller T, Warnke P, Crusius S, Hinz R, Hagen RM, Schwarz NG, Frickmann H. 2015. Screening agars for MRSA: evaluation of a stepwise diagnostic approach with two different selective agars for the screening for methicillin-resistant Staphylococcus aureus (MRSA). Mil Med Res 2:18. doi: 10.1186/s40779-015-0046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee HJ, Jeong JM, Park YH, Choi SS, Kim YH, Chae JS, Moon JS, Park H, Kim S, Eo SK. 2004. Evaluation of the methicillin-resistant Staphylococcus aureus (MRSA)-Screen latex agglutination test for detection of MRSA of animal origin. J Clin Microbiol 42:2780–2782. doi: 10.1128/JCM.42.6.2780-2782.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard—6th ed. NCCLS document M07-A6. National Committee for Clinical Laboratory Standards, Wayne, PA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.