Abstract
Nanoscale engineering is revolutionizing the development of vaccines and immunotherapies. Viruses have played a key role in this field because they can function as prefabricated nanoscaffolds with unique properties that are easy to modify. Viruses are immunogenic through multiple pathways, and antigens displayed naturally or by engineering on the surface can be used to create vaccines against the cognate virus, other pathogens, specific molecules or cellular targets such as tumors. This review focuses on the development of virus-based nanoparticle systems as vaccines indicated for the prevention or treatment of infectious diseases, chronic diseases, cancer, and addiction.
Introduction
Vaccines are designed to elicit a strong immune response and to provide long-lasting protective immunity by generating neutralizing antibodies, activating cellular immunity and inducing immune memory 1, 2. The earliest reports of vaccination were subjects protected against smallpox by exposure to powders from infected scabs. However, Edward Jenner presented the first formal description of a vaccine in 1798, when he observed that milkmaids previously infected with the less virulent Cowpox virus were no longer susceptible to smallpox. In 1967, the World Health Organization (WHO) oversaw a worldwide smallpox eradication program, which was completed by 1980 3. Since then, eradication programs have been established for other diseases such as polio, measles, mumps, rubella, and malaria 4, 5. Vaccines have also been developed against other prevalent infectious diseases, such as hepatitis B, rabies, anthrax, and cholera 6–9. The development of vaccines has achieved an immense socioeconomic impact by reducing the burden of erstwhile pandemic diseases responsible for widespread morbidity and mortality. Even so, several major pathogens cannot yet be controlled by vaccines including Human immunodeficiency virus (HIV) and hemorrhagic fever viruses, such as those responsible for the recent Ebola outbreak affecting West African countries. More recently vaccines have also been against non-infectious diseases including cancer and chronic disease.
Virus-based nanoparticles as platform technologies
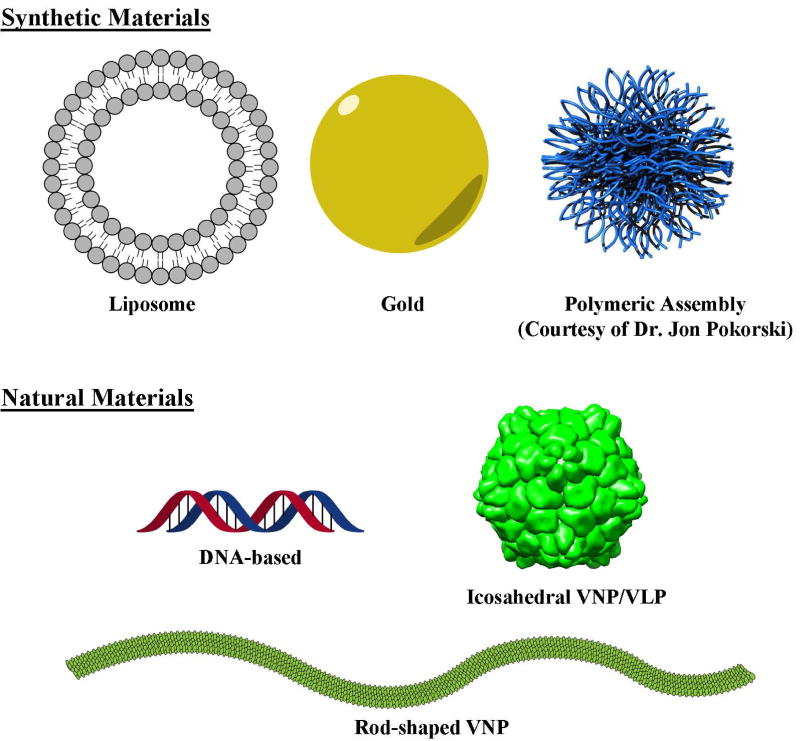
Nanoparticle-based vaccines have been developed using a diverse range of materials (Figure 1), including synthetic particles (e.g. gold, polymers or lipid micelles) and biological particles (e.g. nucleic acids and proteins, including viruses) 10, 11. We consider two broad types of particles in the latter category: virus-based nanoparticles (VNPs) that feature a modified capsid encapsulating the virus genome, and virus-like nanoparticles (VLPs) that comprise protein components alone.
Figure 1.
Virus-based nanoparticles (VNPs) as platform technologies for vaccine development.
Virus-based materials have many beneficial properties. Their proteinaceous, highly-ordered, multivalent structures, when combined with an appropriate immune adjuvant, often elicit robust cellular and humoral immune responses 12. They display antigens in a repetitive array (which promotes B cell crosslinking and subsequent activation) and pathogen-associated molecular patterns (PAMPs) that induce stronger and longer-lasting antigen-specific immune responses than soluble antigens 13–15. The single-stranded viral RNA (ssRNA) found in VNPs is also a PAMP, and this is a natural ligand for Toll-like receptors 7 and 8 that induce cytokine expression 16,17–19. The size range of virus particles (20–500 nm) means they are efficiently taken up by antigen presenting cells (APCs), including dendritic cells (DCs) and other phagocytes, thus stimulating T cells 20, 21.
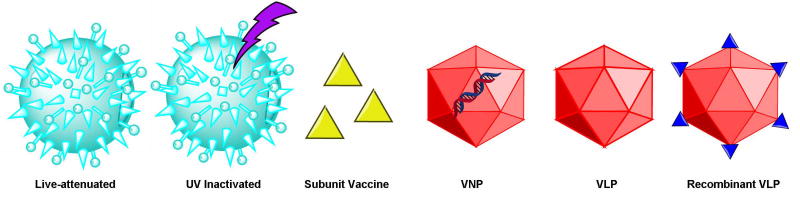
Viral vaccines can be divided into four categories (Figure 2): live-attenuated, inactivated, subunit vaccines, and native or recombinant VNP/VLP structures. The latter are considered safer because there is no risk of virulence, yet stronger than inactivated viruses or subunit vaccines because they induce a robust immune response without multiple doses 22, 23. Native VLPs lack the viral genome but are otherwise identical to the infectious virus, making them highly immunogenic but unable to replicate. These are particularly suitable when the native virus replicates and causes disease in humans. VNPs retain the genome and are therefore easier to produce by relying on natural virus replication. This format is particularly suitable when the native virus does not replicate in humans, i.e. bacteriophage and plant viruses. Recombinant VLP/VNP formats add an important further layer of advantages because they can be engineered to present antigenic epitopes of a counterpart virus or any other disease-associated antigen. VLPs and VNPs can be manufactured in heterologous production systems, including plants, mammalian cells, yeast and bacteria 24.
Figure 2.
Categories of viral vaccines; UV = ultraviolet; VNP = viral nanoparticle; VLP = virus-like particle
Chemical and genetic engineering of virus-based scaffolds
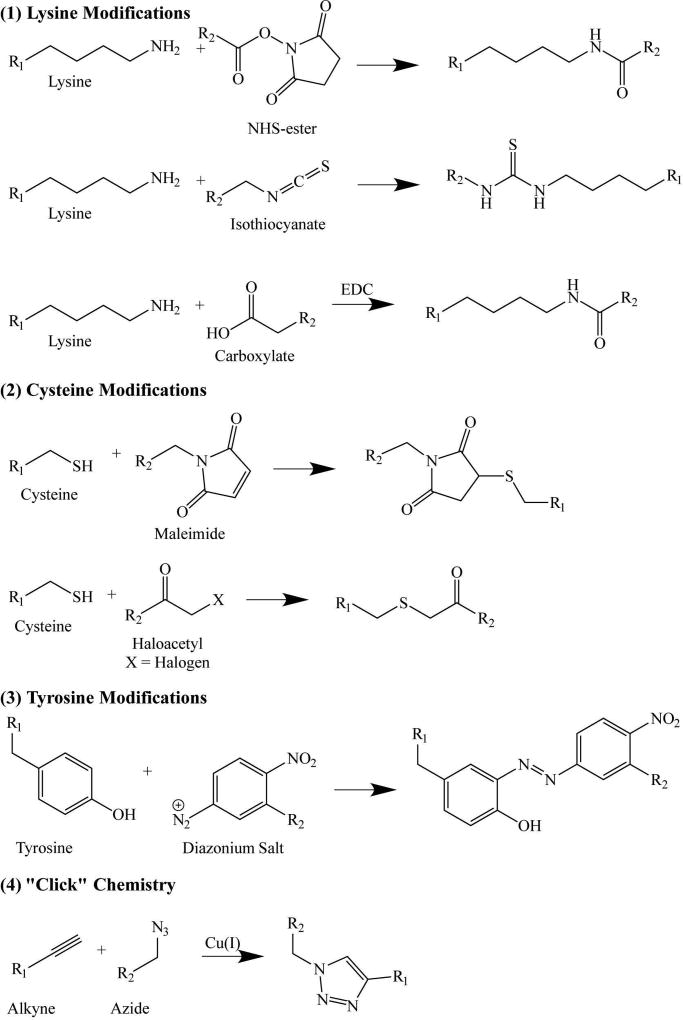
Viruses comprise many identical copies of one or more coat proteins arranged in helical or icosahedral symmetry to form a capsid that encapsulates the genome. The structure of many virus capsids has been solved at atomic resolution, allowing site-specific modification and the multivalent display of antigenic epitopes on particular surface loops or the N/C-terminal region of the coat protein. Epitopes and/or other immunostimulatory molecules can be introduced by chemical engineering (bioconjugation) of particular residues (Figure 3) or genetic engineering of the coat protein sequence (Figure 4).
Figure 3.
Chemical conjugation strategies (bioconjugation); NHS = N-hydroxysuccinimde
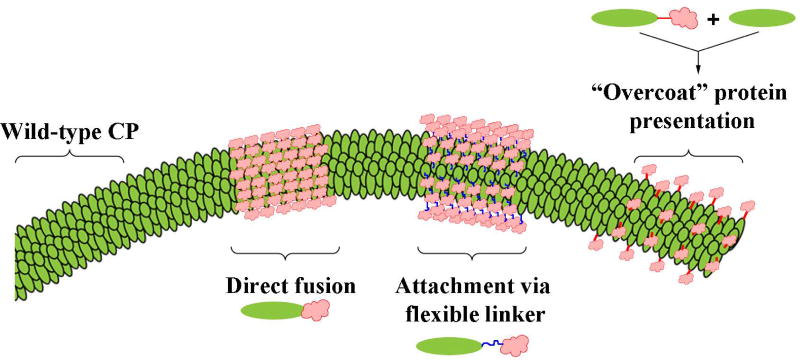
Figure 4.
Genetic engineering strategies for the display of epitopes on viral coat proteins.
Chemical conjugation strategies
Antigenic peptide sequences can be added to a virus coat protein by chemical modification strategies that target five of the 20 naturally occurring amino acids: lysine (amine functional group), glutamic and aspartic acid (carboxylate functional group), cysteine (thiol functional group), and tyrosine (hydroxyl functional group). Lysine residues contain a highly nucleophilic amine that reacts with isothiocyanate or N-hydroxysuccinimide (NHS) esters. Amines can also be covalently attached to carboxylate groups through formation of an amide (peptide) bond, facilitated by a carboxylate-selective coupling agent such as 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC). Glutamic and aspartic acid residues contain carboxylate groups that can be modified using EDC to react with amine-functionalized peptides resulting in the formation of a stable amide bond, in the mirror image of the reaction described above. Cysteine residues contain thiol groups that can be reacted with haloacetyls or maleimides. Finally, tyrosine residues contain a phenolic hydroxyl group, which can be modified using diazonium coupling strategies, although this is more complex than the other reactions listed above.
As well as these direct bioconjugation strategies, bifunctional linkers can be used to introduce additional functionalities that are not naturally found in virus coat proteins. Bio-orthogonal reactions, including ‘click chemistries’ such as Cu(I)-catalyzed azide-alkyne cycloaddition are particularly useful because the kinetics of the reaction are much more efficient than standard coupling. Ligation handles can be introduced via the bioconjugation of an azide or alkyne-NHS ester to a lysine side chain, or through the incorporation of non-natural amino acids in vitro. The diverse chemistries used to engineer viruses have been reviewed in detail 25.
Genetic engineering strategies
Unlike synthetic nanoparticles, VNPs can be modified not only chemically but also genetically, i.e. the nucleic acid sequence encoding the coat protein can be changed to exchange particular amino acids or introduce additional contiguous amino acids to form linear epitopes. Three major approaches are used to insert additional peptides into the virus coat protein, resulting in a coat protein fusion or chimera: direct fusion, linker fusion, and the “protein overcoat” strategy. In the direct fusion approach, the foreign peptide is linked directly to the N-terminus 26–28 or C-terminus 28–31 of the coat protein or inserted into flexible surface loops presented on the capsid surface 29, 32. Although the external surface is usually chosen for presentation of native antigens recognized by B cells, the internal surface may be more suitable in some applications that involve the presentation of processed peptides 33, 34. In contrast, linker fusion involves the inclusion of a short sequence of amino acids (e.g. multiple glycine residues) between the foreign peptide and the end of the coat protein to allow flexibility. Finally, the “protein overcoat” strategy places the Foot and mouth disease virus (FMDV) 2A sequence between the foreign peptide and coat protein sequences, causing an inconsistent ribosomal skip during translation. The outcome is a mixture of native coat proteins and fusion proteins, which is useful if the inserted sequence is so large that its presence on every copy of the coat protein would prevent virus assembly 35.
VNP and VLP vaccines and immunotherapies
The first vaccines were developed against infectious diseases and likewise the first VLP and VNP vaccines were developed as strategies to contain the disease caused by the corresponding native form of the virus. However, as chemical and genetic engineering strategies have become more sophisticated, VLPs and VNPs have been adapted into platform technologies for the presentation of more diverse antigens, including abnormal self-proteins that can be used to treat chronic diseases and cancer. Several key vaccines based on VLPs or chimeric VNPs are summarized in Table 1.
Table 1.
Key VLP/VNP-based vaccines approved or under development.
| Vaccine Target | Platform | Composition | Stage of Development |
References |
|---|---|---|---|---|
| HIV | AP205 | gp41 epitope | Animal studies | 36 |
| HIV | PVX | gp41 epitope | Animal studies | 27 |
| HIV | BPV-1 | CCR5 peptide | Animal studies | 37 |
| HIV | Qβ | CCR5 peptides | NHP studies | 38 |
| HIV | Canarypox | Env, gag, pol genes + AIDSVAX (gp120) | Testing in humans | 39–43 |
| HIV/SIV | RABV | SIV envelope | Animal studies | 44 |
| HIV/SIV | Virosomes | gp41 epitopes | NHP studies | 45 |
| Ebola | Ebola | VP40 and GP | Animal studies | 46, 47 |
| Ebola | Ebola | GP, NP, and VP40 | NHP studies | 48 |
| Ebola | Ebola | EBOVΔVP30 | NHP studies | 49 |
| Ebola | rVSV | GP | Testing in humans | 50 |
| Ebola | RABV | GP | NHP Studies | 51, 52 |
| Sudan virus and Marburg Virus | RABV | GP | Animal studies | 52 |
| Influenza (pandemic) | Influenza | HA, NA, and M1 from H1N1 | Animal studies | 53 |
| Influenza (pandemic) | Influenza | HA and NA from H7N9; M1 from H5N1 | Animal studies | 54 |
| Influenza (pandemic) | Influenza | HA and NA from H1N1; M1 from H5N1 | Testing in humans | 55 |
| Influenza (pandemic) | Influenza | H5 and H1 | Testing in humans | 56 |
| Influenza (universal vaccine) | Influenza | HA, NA, and M1 from H5N1 | Animal studies | 57 |
| Influenza (universal vaccine) | HBc | HA | Animal studies | 58 |
| Influenza (universal vaccine) | Dd | M1 | Cell studies | 59 |
| Influenza (universal vaccine) | PapMV | M2e | Animal studies | 60 |
| Influenza (universal vaccine) | IBDV | HA and M2 from H1N1 | Animal studies | 61 |
| Influenza (universal vaccine) | PapMV | NP | Animal studies | 62 |
| Influenza (universal vaccine) | PVX | NP | Animal studies | 63 |
| Influenza (universal vaccine) | P22 | NP | Animal studies | 34 |
| Influenza (universal vaccine) | sHSP | n/a | Animal studies | 64, 65 |
| Leukemia | TMV | TACA | Animal studies | 66 |
| Leukemia | Qβ | TACA | Animal studies | 67 |
| Melanoma | TMV | p15e and Trp2 | Animal studies | 68 |
| Lymphoma | PVX | Id | Animal studies | 69 |
| Ductal adenocarcinoma | BPV | MUC-1 | Animal studies | 70 |
| HPV (prophylactic) | HPV-16, −18 | L1 | Clinically available | Cervarix |
| HPV (prophylactic) | HPV-6, −11, −16, −18 | L1 | Clinically available | Gardasil® |
| HPV (prophylactic) | MS2 | L2 from HPV-16 and −31 | Animal studies | 71, 72 |
| HPV (prophylactic) | PP7 | L2 from HPV-1, −16, and −18 | Animal studies | 72 |
| HPV (prophylactic) | HPV-18 L1 VLP | L2 from HPV-18, −45, and −59 | Animal studies | 73 |
| HPV (therapeutic) | HPV-16 | E7 | Testing in humans | 74 |
| HPV (therapeutic) | HPV-16 L1 VLP | E6 and E7 | Animal studies | 75 |
| HBV (prophylactic) | HBV | HBsAg | Clinically available | Recombivax HB |
| HBV (therapeutic) | HBc | HBx-derived cytotoxic T lymphocyte epitopes and PADRE | Animal studies | 76 |
| HER-2+ breast cancer | IRIV | P4, P6, and P7 | Testing in humans | 77 |
| HER-2+ breast cancer | Influenza | GPI-HER-2 | Animal studies | 78 |
| HER-2+ breast cancer | MPyV | HER-21–683 | Animal studies | 79 |
| HER-2+ breast cancer | T7 | p66 | Animal studies | 80 |
| HER-2+ breast cancer | PVX | P4 | Animal studies | 81 |
| Nicotine addiction | Qβ | Nicotine | Testing in humans | 82 |
| Nicotine addiction | dAd5 | AM1 | Animal studies | 83 |
| Cocaine addiction | dAd5 | GNC | Animal studies | 84 |
| Cocaine addiction | dAd5 | GNE | Animal studies | 85 |
| Hypertension | Qβ | Angiotensin II peptide (8 aa) | Testing in humans | 28 |
| Alzheimer’s disease | HPV-16 | Aβ peptides | Animal studies | 86 |
| Alzheimer’s disease | Qβ | Aβ1–9 | Animal studies | 86 |
| Alzheimer’s disease | Qβ | Aβ1–6 | Testing in humans | 87, 88 |
| Alzheimer’s disease | HBcΔ | Aβ1–15 | Animal studies | 89 |
| Alzheimer’s disease | BPV1 | Aβ1–9 | Animal studies | 90 |
Infectious diseases
HIV
HIV (Figure 5) is unusual in that it primarily attacks the immune system and therefore destroys the very cells whose function is to neutralize it. By disabling the immune system, HIV not only achieves a successful infection but it also renders the body susceptible to other adventitious pathogens, i.e. acquired immunodeficiency syndrome (AIDS). There is no cure for HIV/AIDS. More than 35 million people are currently infected with HIV, two thirds of the infected population living in sub-Saharan Africa 92. The current best treatment option is highly active antiretroviral therapy, a cocktail of drugs consisting of a non-nucleoside reverse transcriptase inhibitor and two nucleoside analog reverse transcriptase inhibitors 93. Early HIV vaccine candidates based on inactivated or attenuated viruses were ineffective or unsafe 94, 95. More recent vaccine development strategies have focused on eliciting both humoral and cell-mediated responses targeting HIV envelope proteins.
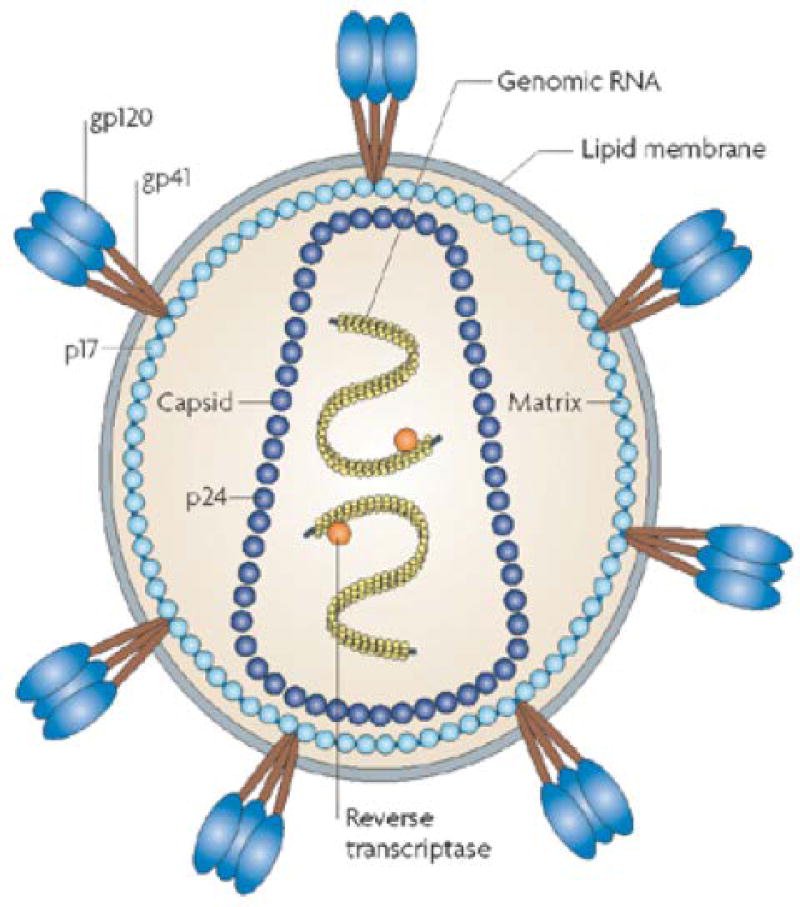
Figure 5.
In HIV-1, trimeric gp120-gp40 complexes are embedded in the membrane. The transmembrane glycoprotein gp41 and the external envelope glycoprotein gp120 are depicted in non-covalent association. The cytoplasmic tail of gp41 interacts with the HIV-1 matrix protein p17. The capsid protein, p24, makes up the cone-shaped core, which contains two positive-strand RNA copies of the HIV-1 genome that are surrounded by the nucleocapsid protein (yellow). Reverse transcriptase protein is also packaged into the particle. (Adapted by permission from Macmillan Publishers Ltd: Nature Reviews Microbiology; Ref 91 Copyright 2008)
Recombinant VLPs and VNPs displaying full-length HIV envelope proteins, individual glycoproteins, glycoprotein precursors or fragments thereof, on the surface of carriers such as Flock House virus (FHV) 96, Hepatitis B virus (HBV) 97, papillomaviruses 37, bacteriophages Qβ, AP205 36 and MS2 98, and plant viruses such as Tobacco mosaic virus (TMV) 99, 100 and Potato virus X (PVX) 27. The membrane-proximal external region (MPER) of gp41 can be recognized and neutralized by monoclonal antibodies thus providing a good target for a vaccine candidate 36. Accordingly, a set of gp41 peptides chemically conjugated to VLPs derived from phage AP205 elicited high-titer, peptide-specific antibodies in mice. Depending on the peptide, sera were able to neutralize a highly-sensitive laboratory strain of HIV-1 and a less-sensitive primary isolate, but not a clade C primary isolate. Some sera exhibited antibody-dependent cell-mediated cytotoxicity (ADCC) in infected cells, indicating that ADCC epitopes are most likely located in the distal region of gp41 36. The highly conserved epitope of gp41 (ELDKWA) has been genetically fused to the N-terminus of PVX, among other platforms 27, 101, 102. Sera from mice immunized with these chimeric virus particles contained high IgG titers specific for HIV-1 MN gp160-derived synthetic peptide (H66), and were able to neutralize HIV-1. Additionally, human DCs pulsed with the vaccine triggered the proliferation of peripheral blood lymphocytes in vivo 27. Multiple gp41 epitopes such as a trimeric recombinant gp41 (rgp41), which contains several conserved gp41epitopes, were conjugated to influenza virosomes. Vaccinated rhesus monkeys were challenged intravaginally 13 times with a heterologous simian HIV (SHIV). All subjects vaccinated through intramuscular or intranasal route were protected from challenge, whereas only 50% of the intramuscular-only group was protected 45.
Other HIV vaccine development approaches include the targeting of host cell receptors such as the C-C chemokine receptor CCR5 co-receptor used by macrophage strains of HIV-1 103–107. Bovine papillomavirus type 1 (BPV-1) VLPs were engineered to express CCR5 peptides. Vaccinated mice produced high antibody titers against CCR5, and functional studies demonstrated that sera displaced the native CCR5 ligand in a competition assay. Most importantly, sera from immunized mice neutralized HIV-1 in cells transfected with a human-mouse chimeric receptor of CCR5 37. CCR5 peptides have also been conjugated to bacteriophage Qβ. Two peptides, representing the N-terminus (EC1) or second extracellular loop (ECL2) of macaque CCR5 (mCCR5), were conjugated to Qβ and administered to rhesus macaques. Animals immunized with Qβ-EC1 and Qβ-ECL2 produced high titers of anti-CCR5 antibodies. When vaccinated animals were challenged with SIV, the viral load was lower than in non-vaccinated controls 38.
Another promising strategy is the combination of ALVAC-HIV, a vaccine based on Canarypox virus (vCP1521), and AIDSVAX (VaxGen), which consists of gp120 from two different HIV strains 39, 43. Unlike the vaccines discussed above, the ALVAC Canarypox virus vector contains the HIV env, gag, and pol genes 40. The two treatments were tested together in a clinical trial in Thailand (RV 144). Vaccinated subjects showed a 31% lower rate of HIV infection compared to the placebo group 43. ALVAC-HIV was also tested alone in a pediatric HIV trial in Africa, involving infants born to HIV-positive mothers. Low levels of binding antibodies were detected in one subject as expected because the subjects did not receive a gp120 boost 40. The addition of recombinant glycoprotein subunit vaccine (rgp120) to ALVAC-HIV resulted in higher levels of specific-binding serum antibodies in infants that were distinguishable from maternal antibodies. Additionally, 50% of subjects who received both ALVAC-HIV and rgp120 generated neutralizing antibodies against homologous strains of HIV 41.
Ebola virus disease and related diseases
Ebola virus disease is caused by four different filoviruses of the genus Ebolavirus: Bundibugyo ebolavirus, Sudan ebolavirus, Tai Forest ebolavirus, and the eponymous Ebola virus (formerly Zaire ebolavirus) which is the most dangerous and prolific (Figure 6). The ease of infection 108, 109 and lack of clinically approved treatment produces mortality rates of up to 90% 110.
Figure 6.
Structure of Ebola virus (Courtesy of David S. Goodsell and RCSB PDB)
VLP/VNP vaccines against Ebola virus are currently in the development pipeline, using either complete Ebola VLPs or specific components such as the viral matrix protein (VP40), nucleoprotein (NP) and glycoprotein (GP) displayed on other viruses. Recombinant VLPs containing Ebola virus VP40 and GP were constructed using a baculovirus system. The system yielded high levels of GP-specific antibodies in mice, particularly the IgG2a subtype which is needed to achieve protective immunity. Additionally, VLPs induced the secretion of IL-6, IL-10, IL-12, and TNFα from DCs, confirming their adjuvant and immunostimulatory properties. Serum from vaccinated mice was also able to block the infection of JC53 cells by a pseudotyped virus 47. The immunization of rodents with VLPs comprising the viral envelope (including GP, NP, and VP40) has also conferred protection against Ebola challenge 48. Furthermore, immunized cynomolgus macaques were completely protected when challenged with Ebola virus 48. VLPs based on recombinant Vesicular stomatitis virus (rVSV) expressing Ebola virus glycoproteins were shown to protect mice and non-human primates against a lethal challenge with homologous Ebola virus after a single injection 111, 112. Post-exposure protection was also conferred 113. The rVSV-EBOV vaccine showed a good safety profile in non-human primates and pigs 114, 115 and was able to prevent the disease when administered during an outbreak in a ring vaccination strategy 50.
Other examples include the application of an inactivated Rabies virus fused to Ebola virus GP (INAC-RV-GP) resulting in a strong, multivalent humoral response against both viruses in mice and non-human primates, protecting the animals against both diseases 51, 116. The titer of neutralizing antibodies was increased further by the addition of an adjuvant and resulted in 100% protection from a lethal challenge 52. The inactivated Rabies virus platform has also been expanded to express the GP from other filoviruses, including Sudan ebolavirus and Marburg virus 52.
Influenzavirus
Seasonal influenza epidemics cause up to 500,000 deaths every year 117–119 as well as regular pandemics, which can result in millions of deaths in a relatively short time span 118, 120. Seasonal influenza epidemics are typically caused by human influenzaviruses (Figure 7) that have acquired mutations, whereas pandemics occur when the influenzaviruses cross species barriers 121, 122. Seasonal vaccines are typically based on whole-inactivated viruses or live-attenuated viruses, both of which achieve good protection and significantly improve public health 123. However, these vaccines are based on hemagglutinin (HA) and neuraminidase (NA), the major targets of the immune system 123. Epitopes on both proteins are highly prone to genetic drift, leading to the rapid emergence of influenza strains that are not genetically similar to the strains covered by the vaccine 53, 124–126. Seasonal vaccines must therefore be updated every year, and vaccines against pandemic strains must be developed in response to the pathogen, a retroactive strategy that places millions of people at risk. Preclinical and clinical research has focused on the development of a proactive universal influenza vaccine based on more conserved epitopes such as the matrix proteins (M1 and M2) and nucleoprotein (NP), which undergo drift more slowly 127, 128. Other strategies include the development of multivalent VLPs with HA and/or NA epitopes from diverse strains, combined with immunostimulatory molecules.
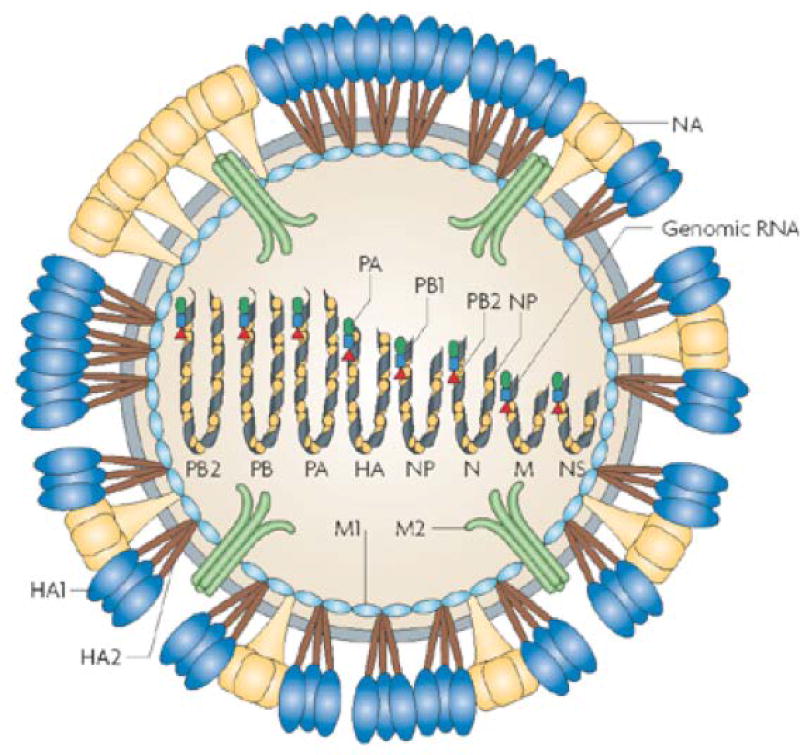
Figure 7.
In influenza A virus, 3 viral proteins are exposed on the outside of virus particles: haemagglutinin (HA, which forms trimers), neuraminidase (NA) (which forms tetramers) and M2 (which forms tetramers that make up ion-channels). Upon proteolytic cleavage, HA0 (not shown) is processed to HA1 and HA2. The influenza virus matrix protein M1 associates inside the viral membrane, and the viral genome consists of eight negative-strand RNA segments and is packaged into the particle as a ribonucleoprotein in complex with nucleocapsid protein (NP) and the viral polymerases PA, PB1 and PB2. (Adapted by permission from Macmillan Publishers Ltd: Nature Reviews Microbiology; Ref 91 Copyright 2008)
Recombinant influenzavirus VLPs based on HA, NA, and M1 have recently been used to develop heterotypic vaccines 53–55, 57. Studies in mice and ferrets indicate that H1N1 VLP vaccination protects against challenges with the homologous subtype (H1N1) as well as a heterologous subtype (H5N1), and intranasal administration elicited higher IgG and IgA titers than intramuscular vaccination 53. These VLP vaccines were well-tolerated in a phase II study 55. Other VLPs have been developed based on the highly pathogenic avian influenza (HPAI) H5N1 and avian-origin influenza A (H7N9) 54, 57. The H5N1 VLP was composed of HA, NA, and M1 from H5N1, whereas the H7N9 vaccine was composed of HA and NA from H7N9 and M1 from H5N1 54, 57. Mice vaccinated with H5N1 VLPs were challenged with homologous or heterologous (H5N8) strains and all survived. Ferrets immunized with H7N9 VLPs plus adjuvant produced high titers of H7N9-specific neutralizing antibodies, and viral loads in the lungs and viral shedding were both reduced compared to controls 54.
Influenzavirus epitopes have also been expressed on the surface of VLPs based on HPV core protein (HBc) 58, Infectious bursal disease virus (IBDV) 61, PVX 63, Papaya mosaic virus (PapMV) 60, 62, Adenovirus59, and Simian virus 40 (SV40) 129. Several of these platforms were used to display HA, NA, and matrix proteins, similar to the influenzavirus VLPs.
Influenza vaccines can also be developed to promote the formation of inducible bronchus-associated lymphoid tissue (iBALT), which plays a role in adaptive immunity in the lungs, similar to the role of the spleen in primary adaptive immunity 130. Small heat shock protein (sHSP) cages are structurally similar to VLPs, and when administered to the lung they promote the formation of iBALT, which includes B cells, follicular DCs, and CD4+ and CD8+ T cells. Mice treated with sHSP cages were protected from primary influenzavirus infection, as well as from a secondary infection from a different strain of the virus 65. The sHSP cages also induced antibody class-switching when mice were challenged with influenzavirus, increasing the amount of IgA and IgG present in the lung 64.
Seasonal influenza vaccines are usually produced in eggs, or in avian or mammalian cell cultures. However, pandemic strains that develop when viruses cross the species barrier (particularly the avian-to-human barrier) are more difficult to produce in avian cells and alternative production platforms are required. One particularly attractive option is to produce the influenzavirus VLPs in plants. The H5 and H1 proteins have each been successfully expressed in Nicotiana benthamiana plants and can self-assemble into VLPs. The molecular farming of VLPs has several advantages: in planta production greatly reduces the rissts associated with human viruses since plants do not support the replication of human viruses; and the process is highly scalable. For example, Figure 8 shows the Medicago production facility. Mice immunized with plant-derived H5-VLPs were protected from homologous and heterologous viral challenge 131. Furthermore, when the plant-derived VLPs were tested in a phase I clinical trial, none of the subjects developed allergy or hypersensitivity symptoms, and the IgG and IgE responses to plant-derived epitopes returned to baseline after 6 months. Additionally, no IgE response was observed in response to the glycan motif, MMXF, which is associated with allergenicity 56, demonstrating a good safety profile of the vaccine.
Figure 8.
Production facility at Medicago
Cancer
Cancer is one of the leading causes of death worldwide, with 14 million new cases diagnosed every year and over 8 million cancer-related deaths. Although cancer includes a diverse spectrum of diseases with different causes, sites of origin, and clinical outcomes, they are all defined by six hallmarks: sustained proliferative signaling, evasion of growth suppressors, promotion of invasion and metastasis, limitless replicative potential, induction of angiogenesis, and resistance to programmed cell death132. Some cancers are caused by viral infections and can thus be prevented with vaccines. The first VLP vaccine offering protection against a cancer-causing virus (Hepatitis B virus, HBV) was approved in 1981 for infants. Two vaccines against Human papillomavirus (HPV) have been approved more recently for the prevention of cervical cancer, or oropharyngeal cancers. In addition to these FDA-approved vaccines, numerous VLP vaccine candidates are being investigated for the prevention or treatment of lymphoma, leukemia, melanoma, and breast cancer.
VLP-based cancer vaccines can also be developed to enhance the tumor-antigen specific T-cell response and elicit antibodies against tumor-specific surface antigens. In addition to papillomaviruses 70, such vaccines have been developed using bacteriophages 67 and plant viruses 66, 68, 69 as delivery platforms because the native viruses do not infect or replicate in human cells so the virus genome can be left intact. The coat proteins of TMV and bacteriophage Qβ have been modified to present the tumor-associated carbohydrate antigen (TACA), which normally has low immunogenicity. TMV-TACA generated much higher titers of antigen-specific antibodies than the soluble form of the antigen, whereas Qβ-TACA elicited a stronger humoral response to the TACA than the soluble form or TACA attached to other nanoparticles 67. The resulting IgG antibodies also reacted strongly in vitro against cells expressing the antigen 67.
Tolerance against self-peptides with low immunogenicity can be broken using VLP/VNP-based immunotherapy platforms. For example, the immunogenicity of melanoma T-cell epitopes p15e and tyrosinase-related protein-2 (Trp2) can be increased by presenting them together on a single bivalent TMV particle, improving cellular immunity and conferring protection against tumor challenge 68. PVX has been modified with the idiotypic (Id) immunoglobulin from B-cell lymphomas, a weak tumor antigen. When administered to mice, Id-PVX induced high titers of anti-Id antibodies, which prolonged survival after lymphoma challenge 69.
The FDA-approved vaccines for HPV are based on papillomavirus VLPs. However, these VLPs can also be modified to express other tumor antigens, such as human mucin-1 (MUC-1), which is a marker of ductal adenocarcinoma. BPV-1 particles modified with a MUC-1 epitope were administered to mice, which were later challenged with a MUC-1+ lymphoma cell line. T cells were strongly induced in the vaccinated mice and their tumors grew more slowly, resulting in a smaller tumor mass at the end of the study 70. We will consider HPV vaccines and hepatocellular carcinoma vaccines in more detail in the following sections because commercial vaccines are already available. We will also discuss HER-2+ breast cancer vaccines.
Prophylactic vaccines to protect against cervical cancer caused by HPV
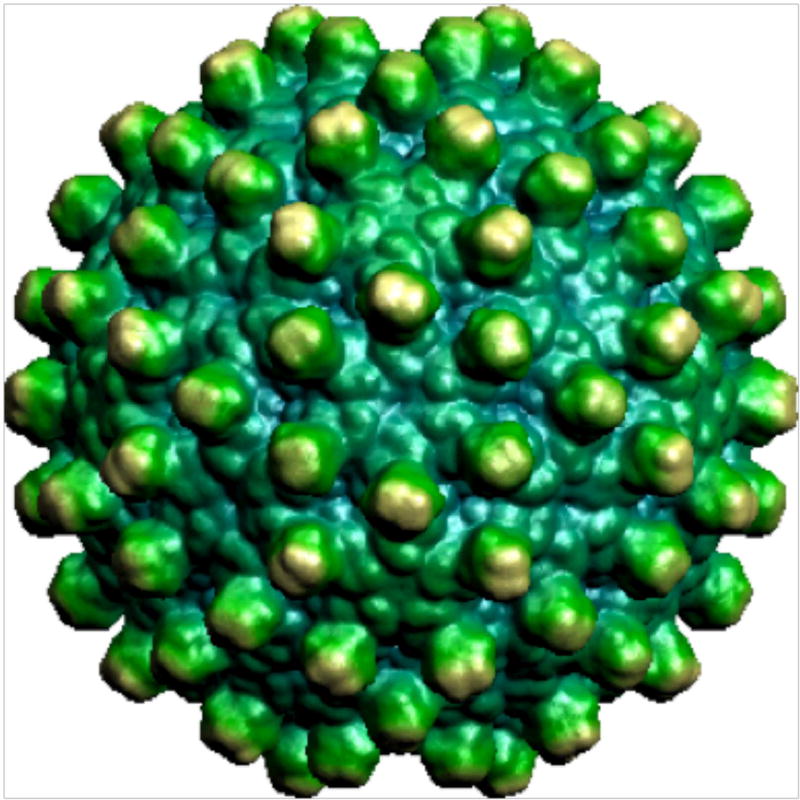
Cervical cancer is the fourth most common cancer in women and more than 500,000 new cases are diagnosed each year 133, 134. HPV, usually sexually transmitted, is implicated in 90% of cervical cancers 135, 136. Among more than 150 known strains of HPV, up to 20 are designated high risk because they cause almost all cervical cancers 137. The two highest-risk strains are HPV-16 and HPV-18, which are responsible for 70% of all cases 138, 139. There are currently two FDA-approved prophylactic vaccines for HPV: a bivalent vaccine (Cervarix) that protects against strains 16 and 18, and a quadrivalent vaccine (Gardasil) that protects against strains 6, 11, 16, and 18. Both vaccines are based on VLPs composed of the HPV L1 coat protein (Figure 9) 71 combined with adjuvants to further boost the immune system. Both vaccines have proven efficacious after a three-dose schedule. However, the L1 protein is not conserved across all serotypes, so these vaccines only offer protection against the specific serotypes within each formulation 140–142.
Figure 9.
Human papillomavirus 16 L1 capsid (viperdb.scripps.edu)
The development of VLPs based on the more conserved L2 coat protein would offer increased cross-protection against multiple serotypes, and the next generation of HPV vaccines is likely to be based on this principle 72. L2 is naturally shielded from the immune system 143 but vaccination with L2 can nevertheless protect against a range of HPV serotypes 144–146. The first vaccines based on L2 were limited in efficacy by low antibody titers and the need to sufficiently protect against all high-risk serotypes 147, 148 so VLPs have been considered as a strategy to overcome these limitations.
Bacteriophage MS2 has been used to express L2 epitopes from HPV strains 16 and 31, individually or as a bivalent formulation. MS2-16L2 was previously shown to confer protection against 11 HPV serotypes but not HPV31 71. Mice immunized with the individual constructs (MS2-16L2 or MS2-31L2) were protected against some strains, whereas the bivalent formulation (MS2-16/31L2) elicited high antibody titers across a panel of HPV serotypes and strongly neutralized all HPV pseudoviruses 72. In a complementary approach, bacteriophage PP7 was modified to express L2 from HPV strains 16 and 18 (which are closely related) and 1 (which is more distant) individually and in pairwise combinations (PP7-18L2, PP7-18/1L2 and PP7-16/18L2). Mice immunized with PP7-18/1L2 only produced antibodies against HPV1 peptides, whereas PPV-18/16L2 elicited antibodies that bound strongly to HPV16 and HPV18, as well as HPV1, HPV5, and HPV6. Only mice vaccinated with PPV-18/16L2 were able to neutralize an HPV-6 pseudovirus, a heterologous serotype 72.
The high-risk HPV strains 18, 45, and 59 have been targeted by inserting a cross-neutralizing epitope from HPV45 L2 into a surface loop of HPV18 L1 and creating VLPs from the chimeric construct (18L1-45RG1). L2-specific antibodies from vaccinated rabbits reacted against HPV strains 39, 45, 68, and 70 (which are members of the same clade as HPV45 and HPV18). Additionally, when mice were passively immunized with immune sera from rabbits, they were protected against a challenge with HPV strains 18, 39, 45, and 6873.
Vaccines to treat cancers caused by HPV
Although prophylactic vaccines have been successful, they are unable to treat established tumors. The development of HPV therapeutic vaccines has focused on the E6 and E7 oncoproteins, which are necessary for tumor development and are expressed in all cervical cancer cells 149. For example, HPV16L1 was genetically modified to express the HPV16 E7 protein. In mice, these recombinant VLPs induced L1-specific antibodies, as well as cytotoxic T cells that recognized L1 and E7 150–153. In a phase I trial, patients with proven ectocervical CIN 2/3 lesions who were also HPV16 mono-infected, were treated with HPV16L1E7 VLPs. Approximately 50% of vaccinated patients exhibited a 50% reduction in lesion size following the final vaccination 74. Further improvements of this therapeutic strategy include the incorporation of T-cell epitopes from HPV16 E6 and E7. Preclinical studies in mice showed an 85% reduction in tumor size when immunized with such recombinant VLPs 75.
Vaccines targeting hepatocellular carcinoma caused by HBV
Liver cancer causes 600,000 deaths per year and there are 700,000 new cases, approximately 500,000 of whom are male, making it the fourth most common form of cancer in men 133. Among these cases, 95% are classified as hepatocellular carcinoma 154, which is associated with risk factors such as alcoholism, hepatitis B, hepatitis C, and liver cirrhosis 155, 156. HBV (Figure 10) is responsible for about 50% of all primary hepatocellular carcinomas. A vaccine against HBV has been available since 1981, and is on the World Health Organization’s List of Essential Medicines. This vaccine is a VLP comprising the HBV surface antigen (HBsAg), which is administered as two or three injections within one year. The vaccine provides lasting immunity against HBV by producing anti-HBV antibodies 157, 158.
Figure 10.
Human Hepatitis B virus (viperdb.scripps.edu)
The prophylactic HBV vaccine has greatly reduced the incidence of hepatocellular carcinoma but a therapeutic vaccine is needed to treat established disease. One of the key targets is the HBV X protein (HBx), a regulatory protein that promotes carcinogenesis and is expressed at high levels in hepatocellular carcinoma 76, 159. The HBc has therefore been genetically modified to express dominant HBx-derived cytotoxic T-cell epitopes, as well as the universal Th-cell epitope, pan-HLA DR-binding epitope (PADRE). These chimeric proteins self-assemble into VLPs, and mice vaccinated with this formulation inhibited tumor growth up to 30 days after tumor challenge, while also eliciting a strong T-cell response 76.
Vaccines targeting HER-2+ breast cancer
Breast cancer is the most common form of cancer in women, as well as a minor form of cancer in men, with over one million cases diagnosed each year 133. There are five molecular subtypes of breast cancer: normal-like, luminal A, luminal B, HER-2+, and triple negative 160. Each is defined by the expression of different receptors and the prognosis varies accordingly. HER-2+ tumors overexpress the HER-2/neu/ERBb2 receptor. They do not express hormone receptors and they are associated with aggressive tumors, high rates of metastasis, and an overall poor prognosis 161. FDA-approved treatments include the HER2-specific antibodies trastuzumab and pertuzumab, which are used for passive immunotherapy and require repetitive administration 162, 163. Passive immunization requires prolonged therapeutic delivery, cannot be developed as a prophylactic, and does not induce cellular immune responses 77, 78. To overcome these challenges, HER-2+ breast cancer research is now focused on active immunotherapy that elicits long-lasting cellular and humoral responses.
VLPs that display full-length HER-2 or specific immunogenic epitopes have been tested in clinical trials 77–81. Peptides derived from the extracellular domain of HER-2 were incorporated into reconstituted influenzavirus virosomes (IRIVs) 164–166. Three immunogenic peptides that are known to elicit B cell responses were tested: P4 (378–394), P6 (545–560), and P7 (610–623) 167. The clinical trial revealed that 80% of vaccinated patients produced peptide-specific antibodies, and HER-2-specific IgG was elicited in 70% of the patients after immunization. A cellular immune response was also observed following vaccination, involving the increased secretion of IL-2, TNFα, and IFNγ 77. In a different approach, enveloped influenzavirus VLPs were modified to incorporate glycosylphosphatidylinositol (GPI)-anchored HER-2 (GPI-HER-2-VLP). Preclinical studies showed strong anti-D2F2/E2 (HER-2+) serum IgG responses in mice, with comparable levels of IgG1, IgG2a, and IgG2b in the serum of vaccinated animals, indicating a balanced Th1 and Th2 response. In contrast, the vaccination of mice with soluble GPI-HER-2 predominantly elicited a Th2 response, whereas Th1-biased responses are needed to induce a potent anti-tumor reaction. When mice were challenged with HER-2+ cells, those vaccinated with GPI-HER-2 VLPs showed a slower tumor growth rate compared to those administered GPI-HER-2 alone. 67% of the mice vaccinated with GPI-HER-2 VLPs remained tumor free 78.
VLPs have also been genetically modified to express HER-2 peptides. The internal face of the murine polyomavirus (MPyV) major capsid protein (VP1) can bind to the minor capsid protein (VP2) 168. VP2 was genetically modified to express the N-terminal domain of HER-2, which contains the extracellular and transmembrane domains (VP2Her21–683). VP1 and VP2Her21–683 were produced in a baculovirus vector to obtain Her21–683PyVLPs. Immunized mice were challenged with HER-2+ D2F2/E2 cells, and 87% of the vaccinated mice did not develop tumors. Similar results were obtained using transgenic BALB-neuT mice, which overexpress the rat HER-2 oncogene. The mice did not produce HER-2-specific antibodies, but did induce HER-2-specific T cells 79.
A T7 bacteriophage was genetically modified to express a H-2kd-restricted cytotoxic T lymphocyte (CTL) epitope (p66) derived from rat HER-2 to investigate whether a CTL response is required for cancer immunotherapy. Preclinical studies showed that splenocytes from mice immunized with T7-p66 produced a higher IFNγ response than controls. Interestingly, splenocytes from mice vaccinated with a mixture of unconjugated T7 and p66 did not yield a strong IFNγ response when challenged with p66, indicating that the CTL peptide must be attached to T7 in order to elicit the CTL response. Splenocytes from mice vaccinated with T7-p66 were also able to lyse target cells pulsed with p66-peptide in vitro. Healthy mice vaccinated with T7-p66 rejected HER-2+ TUBO cells, with five of the six mice remaining tumor-free 42 days after challenge. Furthermore, therapeutic vaccination with T7-p66 slowed the growth of pre-implanted tumors, eventually resulting in the full regression of HER-2+ tumors 80.
We have recently worked on the development of a HER-2+ breast cancer vaccine using the plant virus PVX. The chemical conjugation of PVX with the P4 B-cell epitope, which contains amino acids 387–394 from the extracellular domain of HER-2, elicited higher titers of HER-2-specific antibodies in mice than soluble P4 alone. These antibodies selectively recognized HER-2+ breast cancer cells 81.
Addiction (cocaine and nicotine)
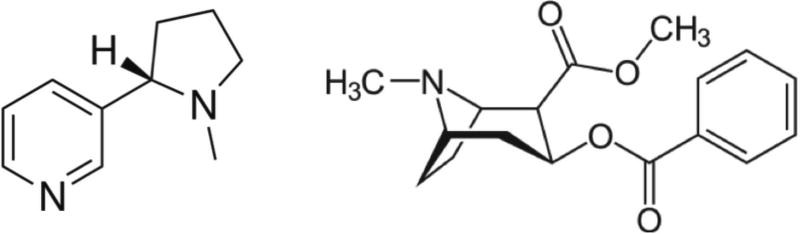
Addictive substances, such as nicotine and cocaine (Figure 11), affect the body by interacting with the nervous system. Nicotine and cocaine both modulate dopamine levels in the brain, thereby affecting the reward pathway. Vaccines against these addictive substances can help to reduce the severity of withdrawal symptoms and prevent relapse. However, small molecules tend to have low immunogenicity, so VLP platform technologies are required to elicit potent and long-lasting immune responses against the drugs 169–171. VLPs displaying nicotine or cocaine as multivalent arrays have been shown to elicit potent humoral responses, yielding drug-specific antibodies that prevent the substances from crossing the blood-brain barrier to exert their effects 172–174. Nicotine is the addictive component of tobacco, and tobacco use is the leading preventable cause of disease, disability, and death in the industrialized world 172. Several vaccines against nicotine are currently undergoing clinical trials, including NicVax™, NIC002, SEL-068, Ta-NIC, and IP18-KLH, but similar approaches are also under development for cocaine addition.
Figure 11.
Chemical structures of nicotine (left) and cocaine (right).
NIC002 is a VLP vaccine in which bacteriophage Qβ is chemically modified to display nicotine (NicQb) 82, 172, 174. Preclinical studied in mice demonstrated development of nicotine-specific antibodies 172. Importantly, when challenged with nicotine, vaccinated mice showed a higher concentration of nicotine remaining in the blood and a corresponding reduction of nicotine levels in the brain, compared to non-vaccinated mice 172. In phase I trials, NicQb was immunogenic, well tolerated and efficacious in patients with high antibody titers, but the subsequent phase II trials did not support the primary endpoint of the study and the product was discontinued 82. In an alternative approach, a nicotine analog was chemically linked to disrupted serotype-5 adenovirus (dAd5). The dAd5 VLP lacks the E1 and E3 proteins, allowing the particle to circumvent any pre-existing Ad5 immunity, which is prevalent in the population 83. Sera from vaccinated mice contained high titers of anti-nicotine antibodies for a prolonged duration, resulting in lower concentrations of nicotine in the brain than naïve mice, inversely related to the levels of nicotine in the serum 83.
The dAd5 VLP has also been conjugated with the cocaine analog. Vaccinated mice challenged with cocaine were found to have 41% less cocaine in the brain than naïve mice. Locomotor activity in vaccinated mice challenged with cocaine was the same as in non-challenged mice treated with PBS, confirming that the vaccine reduced the cognitive impact of cocaine 84. Similar strategies are being developed using alternative cocaine haptens 85.
Chronic diseases
Chronic diseases are persistent or even life-long conditions that require regular therapeutic intervention as a form of disease management. The prevalence of chronic diseases is increasing globally due to the ageing population and various dietary and lifestyle factors, which means that such diseases represent a significant and increasing public health burden in almost every country. Where chronic diseases are caused by malfunctioning self-proteins, vaccination can be used to induce the generation of autoantibodies. This approach has been tested in numerous disease models, including rheumatoid arthritis 175,176–180, osteoporosis 177, 181, experimental autoimmune encephalitis 179, myocarditis 182, and obesity 183. Many of these diseases are currently treated by passive immunotherapy, i.e. the regular administration of therapeutic antibodies targeting pathogenic self-proteins, which is expensive and restricts the patient. We will discuss hypertension and Alzheimer’s disease as case studies for the alternative active immunization approach. The reader is recommended to consult further review articles for information about the development of vaccines against other chronic diseases184, 185, 186.
Hypertension
Hypertension (high blood pressure) is an underlying risk factor that promotes the development of cardiovascular disease, which can lead to life threatening events such as heart attack and stroke. Although hypertension can be regulated with drugs, many hypertensive individuals never receive a diagnosis and therapeutic compliance tends to be poor even in diagnosed hypertensive patients. Another risk factor is the so-called morning pressure surge, a steep increase in blood pressure prior to waking, before medication can be taken. Active immunotherapy could overcome many of these challenges by inducing long-lasting immune responses targeting key regulators of blood pressure.
Angiotensin I and II could be the first targets of hypertension immunotherapy. These are small, soluble regulatory peptides (10 and 8 amino acids in length, respectively) which do not elicit a strong immune response in their native state. As described above, the immunogenicity of small molecules can be increased by exploiting VLP technology. A vaccine candidate has therefore been developed in which angiotensin II is chemically coupled to bacteriophage Qβ VLPs (AngQb). Preclinical studies in a rat model of hypertension showed that vaccination produced high titers of angiotensin II-specific IgGs and resulted in the normalization of blood pressure 187. In clinical trials, the AngQb vaccine was well tolerated and no serious adverse effects were observed. The immunization of patients with mild to moderate hypertension reduced blood pressure during the daytime and especially in the early morning 188.
Alzheimer’s disease
Alzheimer’s disease is a neurodegenerative disorder characterized by a decline in cognitive ability accompanied by neuropathological features such as the loss of neurons in the hippocampus and neocortex and the accumulation of intracellular and extracellular protein deposits 189. Extracellular protein deposits (amyloid plaques) contain the amyloid-β (Aβ) peptide, which is 42 amino acids in length 190, 191. Previous reports have shown that immunization with the Aβ peptide reduced the deposition of amyloid plaques in transgenic mouse models 192. Furthermore, passive immunization with Aβ antibodies had a similar effect 193. However, a clinical trial (AN1792) using synthetic Aβ peptides for immunization showed minimal efficacy and was stopped after meningoencephalitis was reported in 6% of the subjects 194. This unanticipated effect was attributed to a T-cell-mediated autoimmune response caused by the adjuvant QS21 194–196. The safety of Aβ-derived immunotherapies could therefore be improved by triggering a predominantly Th2-based immune response by delivering only B-cell epitopes, which are found on the N-terminus of the Aβ peptide 197, 198. VLPs based on HPV, bacteriophage Qβ, HBc, and BPV-1 have already been used to display Aβ peptides 86, 89, 90.
HPV-16 displaying Aβ peptides such as full-length Aβ (Aβ1–40), N-terminal Aβ (Aβ1–9 and Aβ1–16), mid-domain Aβ (Aβ12–28), and C-terminal Aβ (Aβ17–40) have been tested as vaccines. HPV-Aβ1–40 elicited IgG responses without the use of Freund’s adjuvant in mice, whereas free Aβ1–40 required Freund’s adjuvant to obtain comparable titers. HPV conjugated to peptides from the N-terminal domain of Aβ elicited higher antibody titers than HPV conjugated to peptides from either the mid or C-terminal domains, indicating that N-terminal Aβ peptides are the most immunogenic when presented on HPV particles. Importantly, these antibodies were predominantly of subtype IgG1, indicating a Th2-biased immune response 86.
Aβ peptides have been directly conjugated to bacteriophage Qβ. The N-terminal modified peptide (Aβ1–9) with a C-terminal –GGC linker was conjugated to the bacteriophage using a bifunctional linker with both amine and sulfhydryl reactive arms (SMPH). Mice immunized with Qβ-Aβ1–9 VLPs without adjuvant produced higher titers of antibodies than those immunized with HPV-Aβ1–9 and similar titers to those immunized with HPV-Aβ40. The inclusion of incomplete Freund’s adjuvant increased the IgG titers even further 86.
Bacteriophage Qβ has also been conjugated with Aβ1–6, which is shorter than the typical T-cell epitope. Mice were immunized three times with Qβ-Aβ1–6 and did not activate Aβ-specific T cells. Additionally, mice immunized with Qβ-Aβ1–6 produced high antibody titers against the Aβ peptide and formed fewer plaques than control mice 199. Qβ-Aβ1–6 was tested in a phase I trial (CAD106) and was deemed safe and tolerable in a double-blind, placebo-controlled, 52-week study. Importantly, no subjects recorded clinical or subclinical cases of meningoencephalitis, which halted the previous Alzheimer’s disease immunotherapy clinical trial 88. In a phase II trial, CAD106 was administered to 47 patients with Alzheimer’s disease (n = 11 for placebo). The patients received three subcutaneous or intramuscular doses of CAD106, followed by four additional subcutaneous or intramuscular injections. Long-term treatment induced prolonged high titers of Aβ-specific antibodies suggesting that CAD106 could be developed into an effective immunotherapy for Alzheimer’s disease 87.
A C-terminally truncated version of the HBc protein (HBcΔ) was genetically modified to include two copies of Aβ1–15 in the MIR (Aβ-HBc) 89. The MIR was chosen because epitopes inserted there tend to be highly antigenic and immunogenic compared to other insertion sites 200. Preclinical studies in mice showed that anti-Aβ antibodies, predominantly IgG1 and IgG2b subtypes (indicating a Th2-biased immune response) were developed. Free Aβ peptide with adjuvant also elicited high antibody titers, but was dominated by the IgG2a subtype. Sera from immunized mice also prevented formation of Aβ fibrils and reduced the toxicity of the Aβ peptide towards PC12 cells 89.
In the final example based on BPV-1, the Aβ1–9 peptide was fused with the L1 protein and the chimeric Aβ-VLPs self-assembled to form a structure resembling the native virus particle. Preclinical vaccination studies in rabbits indicated that sera from the treated rabbits recognized Aβ1–9 and full length Aβ, and that Aβ fibril formation was inhibited in vitro. Transgenic APP/PS1 mice, which spontaneously form Aβ plaques, were also immunized with Aβ-VLP without adjuvant eliciting high titers of Aβ-specific antibodies. Higher levels of circulating Aβ peptide were detected in these mice compared to naïve controls, corresponding to lower levels of Aβ peptide in the brain 90.
Conclusion
Vaccines based on viruses could be developed for the prevention and/or treatment of diverse diseases, including infectious diseases, cancer, addiction, and chronic disorders. One of the key advantages of viruses as a vaccine development platform is that they are naturally immunogenic, and are therefore ideal for the induction of immune responses even in the absence of an adjuvant. The success of prophylactic vaccines against HPV and HBV highlights the potential of this platform for the treatment of many other diseases. Many virus-based vaccines have shown promising results in non-human primates but a number of challenges remain to be overcome before such vaccines can be deployed in the clinic. The first barrier is safety: the natural immunogenicity of virus-based particles makes them ideal for the display of antigenic epitopes but increases the risk of toxicity. Bacteriophage Qβ vaccines for Alzheimer’s disease and nicotine addiction have recently completed phase I safety tests, but other platforms remain to be evaluated in clinical trials. Nevertheless, the phase I trials indicate that virus-based vaccines offer an alternative to other vaccine materials, and the promising results in primates indicate that this platform could be used to develop novel vaccines against a wide range of diseases.
Acknowledgments
This work was supported in part by grants from the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (T32EB007509) to K.L.L., the National Science Foundation (CMMI NM 1333651 for VNP nanomanufacturing, and CMMI NM 1509232 for Ebola virus diagnostics), a Susan G. Komen grant (CCR14298962 for cancer vaccines); and Mt. Sinai Foundation and Case Western Reserve University startup funds to N.F.S.
Contributor Information
Karin L. Lee, Department of Biomedical Engineering, Case Western Reserve University Schools of Engineering and Medicine, Cleveland, OH 44106
Richard M. Twyman, TRM Ltd, PO Box 463, York YO11 PFJ, United Kingdom
Steven Fiering, Department of Microbiology and Immunology and Norris Cotton Cancer Center, The Geisel School of Medicine at Dartmouth, Lebanon, NH 03756.
Nicole Steinmetz, Departments of Biomedical Engineering, Radiology, Materials Science and Engineering, and Macromolecular Science and Engineering, Case Western Reserve University and Medicine, Cleveland, OH 44106; nicole.steinmez@case.edu.
References
- 1.Clem AS. Fundamentals of vaccine immunology. J Glob Infect Dis. 2011;3:73–78. doi: 10.4103/0974-777X.77299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12:509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riedel S. Edward Jenner and the history of smallpox and vaccination. Proc (Bayl Univ Med Cent) 2005;18:21–25. doi: 10.1080/08998280.2005.11928028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller M, Barrett S, Henderson DA. Control and Eradication. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, editors. Disease Control Priorities in Developing Countries. 2. Washington (DC): 2006. [PubMed] [Google Scholar]
- 5.Knobler S, Lederberg J, Pray LA, editors. Considerations for Viral Disease Eradication: Lessons Learned and Future Strategies: Workshop Summary. Washington (DC): 2002. [PubMed] [Google Scholar]
- 6.Hicks DJ, Fooks AR, Johnson N. Developments in rabies vaccines. Clin Exp Immunol. 2012;169:199–204. doi: 10.1111/j.1365-2249.2012.04592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geier MR, Geier DA, Zahalsky AC. A review of hepatitis B vaccination. Expert Opin Drug Saf. 2003;2:113–122. doi: 10.1517/14740338.2.2.113. [DOI] [PubMed] [Google Scholar]
- 8.Lopez AL, Gonzales ML, Aldaba JG, Nair GB. Killed oral cholera vaccines: history, development and implementation challenges. Ther Adv Vaccines. 2014;2:123–136. doi: 10.1177/2051013614537819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scorpio A, Blank TE, Day WA, Chabot DJ. Anthrax vaccines: Pasteur to the present. Cell Mol Life Sci. 2006;63:2237–2248. doi: 10.1007/s00018-006-6312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao L, Seth A, Wibowo N, Zhao CX, Mitter N, Yu C, Middelberg AP. Nanoparticle vaccines. Vaccine. 2014;32:327–337. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 11.Gregory AE, Titball R, Williamson D. Vaccine delivery using nanoparticles. Front Cell Infect Microbiol. 2013;3:13. doi: 10.3389/fcimb.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lua LH, Connors NK, Sainsbury F, Chuan YP, Wibowo N, Middelberg AP. Bioengineering virus-like particles as vaccines. Biotechnol Bioeng. 2014;111:425–440. doi: 10.1002/bit.25159. [DOI] [PubMed] [Google Scholar]
- 13.Jennings GT, Bachmann MF. The coming of age of virus-like particle vaccines. Biol Chem. 2008;389:521–536. doi: 10.1515/bc.2008.064. [DOI] [PubMed] [Google Scholar]
- 14.Peacey M, Wilson S, Baird MA, Ward VK. Versatile RHDV virus-like particles: incorporation of antigens by genetic modification and chemical conjugation. Biotechnol Bioeng. 2007;98:968–977. doi: 10.1002/bit.21518. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Wang L, Liu Y, Chen X, Liu Q, Jia J, Yang T, Qiu S, Ma G. Immune responses to vaccines involving a combined antigen-nanoparticle mixture and nanoparticle-encapsulated antigen formulation. Biomaterials. 2014;35:6086–6097. doi: 10.1016/j.biomaterials.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 17.Jegerlehner A, Maurer P, Bessa J, Hinton HJ, Kopf M, Bachmann MF. TLR9 signaling in B cells determines class switch recombination to IgG2a. J Immunol. 2007;178:2415–2420. doi: 10.4049/jimmunol.178.4.2415. [DOI] [PubMed] [Google Scholar]
- 18.Bessa J, Jegerlehner A, Hinton HJ, Pumpens P, Saudan P, Schneider P, Bachmann MF. Alveolar macrophages and lung dendritic cells sense RNA and drive mucosal IgA responses. J Immunol. 2009;183:3788–3799. doi: 10.4049/jimmunol.0804004. [DOI] [PubMed] [Google Scholar]
- 19.Hou B, Saudan P, Ott G, Wheeler ML, Ji M, Kuzmich L, Lee LM, Coffman RL, Bachmann MF, DeFranco AL. Selective utilization of Toll-like receptor and MyD88 signaling in B cells for enhancement of the antiviral germinal center response. Immunity. 2011;34:375–384. doi: 10.1016/j.immuni.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cubas R, Zhang S, Kwon S, Sevick-Muraca EM, Li M, Chen C, Yao Q. Virus-like particle (VLP) lymphatic trafficking and immune response generation after immunization by different routes. J Immunother. 2009;32:118–128. doi: 10.1097/CJI.0b013e31818f13c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 22.Vogel FR. Improving vaccine performance with adjuvants. Clin Infect Dis. 2000;30(Suppl 3):S266–270. doi: 10.1086/313883. [DOI] [PubMed] [Google Scholar]
- 23.Goldman MaL P-H. Immunological safety of vaccines: facts, hypotheses, and allegation. In: Kaufmann SHE, editor. Novel Vaccination Strategies. Germany: Wiley-VCH; 2004. pp. 595–611. [Google Scholar]
- 24.Schneemann A, Young MJ. Viral assembly using heterologous expression systems and cell extracts. Adv Protein Chem. 2003;64:1–36. doi: 10.1016/s0065-3233(03)01001-5. [DOI] [PubMed] [Google Scholar]
- 25.Pokorski JK, Steinmetz NF. The art of engineering viral nanoparticles. Mol Pharm. 2011;8:29–43. doi: 10.1021/mp100225y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shukla S, Dickmeis C, Nagarajan AS, Fischer R, Commandeur U, Steinmetz NF. Molecular farming of fluorescent virus-based nanoparticles for optical imaging in plants, human cells and mouse models. Biomaterials Science. 2014;2:784–797. doi: 10.1039/c3bm60277j. [DOI] [PubMed] [Google Scholar]
- 27.Marusic C, Rizza P, Lattanzi L, Mancini C, Spada M, Belardelli F, Benvenuto E, Capone I. Chimeric plant virus particles as immunogens for inducing murine and human immune responses against human immunodeficiency virus type 1. J Virol. 2001;75:8434–8439. doi: 10.1128/JVI.75.18.8434-8439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tissot AC, Renhofa R, Schmitz N, Cielens I, Meijerink E, Ose V, Jennings GT, Saudan P, Pumpens P, Bachmann MF. Versatile virus-like particle carrier for epitope based vaccines. PLoS One. 2010;5:e9809. doi: 10.1371/journal.pone.0009809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turpen TH, Reinl SJ, Charoenvit Y, Hoffman SL, Fallarme V, Grill LK. Malarial epitopes expressed on the surface of recombinant tobacco mosaic virus. Biotechnology (N Y) 1995;13:53–57. doi: 10.1038/nbt0195-53. [DOI] [PubMed] [Google Scholar]
- 30.Denis J, Majeau N, Acosta-Ramirez E, Savard C, Bedard MC, Simard S, Lecours K, Bolduc M, Pare C, Willems B, et al. Immunogenicity of papaya mosaic virus-like particles fused to a hepatitis C virus epitope: evidence for the critical function of multimerization. Virology. 2007;363:59–68. doi: 10.1016/j.virol.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Tremblay MH, Majeau N, Gagne ME, Lecours K, Morin H, Duvignaud JB, Bolduc M, Chouinard N, Pare C, Gagne S, et al. Effect of mutations K97A and E128A on RNA binding and self assembly of papaya mosaic potexvirus coat protein. FEBS J. 2006;273:14–25. doi: 10.1111/j.1742-4658.2005.05033.x. [DOI] [PubMed] [Google Scholar]
- 32.Phelps JP, Dang N, Rasochova L. Inactivation and purification of cowpea mosaic virus-like particles displaying peptide antigens from Bacillus anthracis. J Virol Methods. 2007;141:146–153. doi: 10.1016/j.jviromet.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Neil A, Prevelige PE, Basu G, Douglas T. Coconfinement of fluorescent proteins: spatially enforced communication of GFP and mCherry encapsulated within the P22 capsid. Biomacromolecules. 2012;13:3902–3907. doi: 10.1021/bm301347x. [DOI] [PubMed] [Google Scholar]
- 34.Patterson DP, Rynda-Apple A, Harmsen AL, Harmsen AG, Douglas T. Biomimetic antigenic nanoparticles elicit controlled protective immune response to influenza. ACS Nano. 2013;7:3036–3044. doi: 10.1021/nn4006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donnelly ML, Luke G, Mehrotra A, Li X, Hughes LE, Gani D, Ryan MD. Analysis of the aphthovirus 2A/2B polyprotein ‘cleavage’ mechanism indicates not a proteolytic reaction, but a novel translational effect: a putative ribosomal ‘skip’. J Gen Virol. 2001;82:1013–1025. doi: 10.1099/0022-1317-82-5-1013. [DOI] [PubMed] [Google Scholar]
- 36.Pastori C, Tudor D, Diomede L, Drillet AS, Jegerlehner A, Rohn TA, Bomsel M, Lopalco L. Virus like particle based strategy to elicit HIV-protective antibodies to the alpha-helic regions of gp41. Virology. 2012;431:1–11. doi: 10.1016/j.virol.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Chackerian B, Lowy DR, Schiller JT. Induction of autoantibodies to mouse CCR5 with recombinant papillomavirus particles. Proc Natl Acad Sci U S A. 1999;96:2373–2378. doi: 10.1073/pnas.96.5.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Rompay KK, Hunter Z, Jayashankar K, Peabody J, Montefiori D, LaBranche CC, Keele BF, Jensen K, Abel K, Chackerian B. A vaccine against CCR5 protects a subset of macaques upon intravaginal challenge with simian immunodeficiency virus SIVmac251. J Virol. 2014;88:2011–2024. doi: 10.1128/JVI.02447-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adis International L. HIV gp120 vaccine - VaxGen: AIDSVAX, AIDSVAX B/B, AIDSVAX B/E, HIV gp120 vaccine - Genentech, HIV gp120 vaccine AIDSVAX - VaxGen, HIV vaccine AIDSVAX - VaxGen. Drugs R D. 2003;4:249–253. doi: 10.2165/00126839-200304040-00007. [DOI] [PubMed] [Google Scholar]
- 40.Kaleebu P, Njai HF, Wang L, Jones N, Ssewanyana I, Richardson P, Kintu K, Emel L, Musoke P, Fowler MG, et al. Immunogenicity of ALVAC-HIV vCP1521 in infants of HIV-1-infected women in Uganda (HPTN 027): the first pediatric HIV vaccine trial in Africa. J Acquir Immune Defic Syndr. 2014;65:268–277. doi: 10.1097/01.qai.0000435600.65845.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McFarland EJ, Johnson DC, Muresan P, Fenton T, Tomaras GD, McNamara J, Read JS, Douglas SD, Deville J, Gurwith M, et al. HIV-1 vaccine induced immune responses in newborns of HIV-1 infected mothers. AIDS. 2006;20:1481–1489. doi: 10.1097/01.aids.0000237363.33994.45. [DOI] [PubMed] [Google Scholar]
- 42.Pitisuttithum P, Rerks-Ngarm S, Bussaratid V, Dhitavat J, Maekanantawat W, Pungpak S, Suntharasamai P, Vanijanonta S, Nitayapan S, Kaewkungwal J, et al. Safety and reactogenicity of canarypox ALVAC-HIV (vCP1521) and HIV-1 gp120 AIDSVAX B/E vaccination in an efficacy trial in Thailand. PLoS One. 2011;6:e27837. doi: 10.1371/journal.pone.0027837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 44.Dunkel A, Shen S, LaBranche CC, Montefiori D, McGettigan JP. A Bivalent, Chimeric Rabies Virus Expressing Simian Immunodeficiency Virus Envelope Induces Multifunctional Antibody Responses. AIDS Res Hum Retroviruses. 2015 doi: 10.1089/aid.2014.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bomsel M, Tudor D, Drillet AS, Alfsen A, Ganor Y, Roger MG, Mouz N, Amacker M, Chalifour A, Diomede L, et al. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011;34:269–280. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 46.Martins KA, Steffens JT, van Tongeren SA, Wells JB, Bergeron AA, Dickson SP, Dye JM, Salazar AM, Bavari S. Toll-like receptor agonist augments virus-like particle-mediated protection from Ebola virus with transient immune activation. PLoS One. 2014;9:e89735. doi: 10.1371/journal.pone.0089735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye L, Lin J, Sun Y, Bennouna S, Lo M, Wu Q, Bu Z, Pulendran B, Compans RW, Yang C. Ebola virus-like particles produced in insect cells exhibit dendritic cell stimulating activity and induce neutralizing antibodies. Virology. 2006;351:260–270. doi: 10.1016/j.virol.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 48.Warfield KL, Swenson DL, Olinger GG, Kalina WV, Aman MJ, Bavari S. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J Infect Dis. 2007;196(Suppl 2):S430–437. doi: 10.1086/520583. [DOI] [PubMed] [Google Scholar]
- 49.Marzi A, Halfmann P, Hill-Batorski L, Feldmann F, Shupert WL, Neumann G, Feldmann H, Kawaoka Y. Vaccines. An Ebola whole-virus vaccine is protective in nonhuman primates. Science. 2015;348:439–442. doi: 10.1126/science.aaa4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henao-Restrepo AM, Longini IM, Egger M, Dean NE, Edmunds WJ, Camacho A, Carroll MW, Doumbia M, Draguez B, Duraffour S, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)61117-5. [DOI] [PubMed] [Google Scholar]
- 51.Blaney JE, Wirblich C, Papaneri AB, Johnson RF, Myers CJ, Juelich TL, Holbrook MR, Freiberg AN, Bernbaum JG, Jahrling PB, et al. Inactivated or live-attenuated bivalent vaccines that confer protection against rabies and Ebola viruses. J Virol. 2011;85:10605–10616. doi: 10.1128/JVI.00558-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Willet M, Kurup D, Papaneri A, Wirblich C, Hooper JW, Kwilas SA, Keshwara R, Hudacek A, Beilfuss S, Rudolph G, et al. Preclinical Development of Inactivated Rabies Virus-Based Polyvalent Vaccine Against Rabies and Filoviruses. J Infect Dis. 2015 doi: 10.1093/infdis/jiv251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perrone LA, Ahmad A, Veguilla V, Lu X, Smith G, Katz JM, Pushko P, Tumpey TM. Intranasal vaccination with 1918 influenza virus-like particles protects mice and ferrets from lethal 1918 and H5N1 influenza virus challenge. J Virol. 2009;83:5726–5734. doi: 10.1128/JVI.00207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu YV, Massare MJ, Pearce MB, Sun X, Belser JA, Maines TR, Creager HM, Glenn GM, Pushko P, Smith GE, et al. Recombinant virus-like particles elicit protective immunity against avian influenza A(H7N9) virus infection in ferrets. Vaccine. 2015;33:2152–2158. doi: 10.1016/j.vaccine.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lopez-Macias C, Ferat-Osorio E, Tenorio-Calvo A, Isibasi A, Talavera J, Arteaga-Ruiz O, Arriaga-Pizano L, Hickman SP, Allende M, Lenhard K, et al. Safety and immunogenicity of a virus-like particle pandemic influenza A (H1N1) 2009 vaccine in a blinded, randomized, placebo-controlled trial of adults in Mexico. Vaccine. 2011;29:7826–7834. doi: 10.1016/j.vaccine.2011.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ward BJ, Landry N, Trepanier S, Mercier G, Dargis M, Couture M, D’Aoust MA, Vezina LP. Human antibody response to N-glycans present on plant-made influenza virus-like particle (VLP) vaccines. Vaccine. 2014;32:6098–6106. doi: 10.1016/j.vaccine.2014.08.079. [DOI] [PubMed] [Google Scholar]
- 57.Ren Z, Ji X, Meng L, Wei Y, Wang T, Feng N, Zheng X, Wang H, Li N, Gao X, et al. H5N1 influenza virus-like particle vaccine protects mice from heterologous virus challenge better than whole inactivated virus. Virus Res. 2015;200:9–18. doi: 10.1016/j.virusres.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 58.Chen S, Zheng D, Li C, Zhang W, Xu W, Liu X, Fang F, Chen Z. Protection against multiple subtypes of influenza viruses by virus-like particle vaccines based on a hemagglutinin conserved epitope. Biomed Res Int. 2015;2015:901817. doi: 10.1155/2015/901817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Naskalska A, Szolajska E, Chaperot L, Angel J, Plumas J, Chroboczek J. Influenza recombinant vaccine: matrix protein M1 on the platform of the adenovirus dodecahedron. Vaccine. 2009;27:7385–7393. doi: 10.1016/j.vaccine.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 60.Denis J, Acosta-Ramirez E, Zhao Y, Hamelin ME, Koukavica I, Baz M, Abed Y, Savard C, Pare C, Lopez Macias C, et al. Development of a universal influenza A vaccine based on the M2e peptide fused to the papaya mosaic virus (PapMV) vaccine platform. Vaccine. 2008;26:3395–3403. doi: 10.1016/j.vaccine.2008.04.052. [DOI] [PubMed] [Google Scholar]
- 61.Pascual E, Mata CP, Gomez-Blanco J, Moreno N, Barcena J, Blanco E, Rodriguez-Frandsen A, Nieto A, Carrascosa JL, Caston JR. Structural basis for the development of avian virus capsids that display influenza virus proteins and induce protective immunity. J Virol. 2015;89:2563–2574. doi: 10.1128/JVI.03025-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Babin C, Majeau N, Leclerc D. Engineering of papaya mosaic virus (PapMV) nanoparticles with a CTL epitope derived from influenza NP. J Nanobiotechnology. 2013;11:10. doi: 10.1186/1477-3155-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lico C, Mancini C, Italiani P, Betti C, Boraschi D, Benvenuto E, Baschieri S. Plant-produced potato virus X chimeric particles displaying an influenza virus-derived peptide activate specific CD8+ T cells in mice. Vaccine. 2009;27:5069–5076. doi: 10.1016/j.vaccine.2009.06.045. [DOI] [PubMed] [Google Scholar]
- 64.Richert LE, Servid AE, Harmsen AL, Rynda-Apple A, Han S, Wiley JA, Douglas T, Harmsen AG. A virus-like particle vaccine platform elicits heightened and hastened local lung mucosal antibody production after a single dose. Vaccine. 2012;30:3653–3665. doi: 10.1016/j.vaccine.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wiley JA, Richert LE, Swain SD, Harmsen A, Barnard DL, Randall TD, Jutila M, Douglas T, Broomell C, Young M, et al. Inducible bronchus-associated lymphoid tissue elicited by a protein cage nanoparticle enhances protection in mice against diverse respiratory viruses. PLoS One. 2009;4:e7142. doi: 10.1371/journal.pone.0007142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yin Z, Nguyen HG, Chowdhury S, Bentley P, Bruckman MA, Miermont A, Gildersleeve JC, Wang Q, Huang X. Tobacco mosaic virus as a new carrier for tumor associated carbohydrate antigens. Bioconjug Chem. 2012;23:1694–1703. doi: 10.1021/bc300244a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yin Z, Comellas-Aragones M, Chowdhury S, Bentley P, Kaczanowska K, Benmohamed L, Gildersleeve JC, Finn MG, Huang X. Boosting immunity to small tumor-associated carbohydrates with bacteriophage qbeta capsids. ACS Chem Biol. 2013;8:1253–1262. doi: 10.1021/cb400060x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCormick AA, Corbo TA, Wykoff-Clary S, Palmer KE, Pogue GP. Chemical conjugate TMV-peptide bivalent fusion vaccines improve cellular immunity and tumor protection. Bioconjug Chem. 2006;17:1330–1338. doi: 10.1021/bc060124m. [DOI] [PubMed] [Google Scholar]
- 69.Jobsri J, Allen A, Rajagopal D, Shipton M, Kanyuka K, Lomonossoff GP, Ottensmeier C, Diebold SS, Stevenson FK, Savelyeva N. Plant virus particles carrying tumour antigen activate TLR7 and Induce high levels of protective antibody. PLoS One. 2015;10:e0118096. doi: 10.1371/journal.pone.0118096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pejawar-Gaddy S, Rajawat Y, Hilioti Z, Xue J, Gaddy DF, Finn OJ, Viscidi RP, Bossis I. Generation of a tumor vaccine candidate based on conjugation of a MUC1 peptide to polyionic papillomavirus virus-like particles. Cancer Immunol Immunother. 2010;59:1685–1696. doi: 10.1007/s00262-010-0895-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tumban E, Peabody J, Tyler M, Peabody DS, Chackerian B. VLPs displaying a single L2 epitope induce broadly cross-neutralizing antibodies against human papillomavirus. PLoS One. 2012;7:e49751. doi: 10.1371/journal.pone.0049751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tyler M, Tumban E, Peabody DS, Chackerian B. The use of hybrid virus-like particles to enhance the immunogenicity of a broadly protective HPV vaccine. Biotechnol Bioeng. 2014;111:2398–2406. doi: 10.1002/bit.25311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huber B, Schellenbacher C, Jindra C, Fink D, Shafti-Keramat S, Kirnbauer R. A chimeric 18L1-45RG1 virus-like particle vaccine cross-protects against oncogenic alpha-7 human papillomavirus types. PLoS One. 2015;10:e0120152. doi: 10.1371/journal.pone.0120152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaufmann AM, Nieland JD, Jochmus I, Baur S, Friese K, Gabelsberger J, Gieseking F, Gissmann L, Glasschroder B, Grubert T, et al. Vaccination trial with HPV16 L1E7 chimeric virus-like particles in women suffering from high grade cervical intraepithelial neoplasia (CIN 2/3) Int J Cancer. 2007;121:2794–2800. doi: 10.1002/ijc.23022. [DOI] [PubMed] [Google Scholar]
- 75.Monroy-Garcia A, Gomez-Lim MA, Weiss-Steider B, Hernandez-Montes J, Huerta-Yepez S, Rangel-Santiago JF, Santiago-Osorio E, Mora Garcia Mde L. Immunization with an HPV-16 L1-based chimeric virus-like particle containing HPV-16 E6 and E7 epitopes elicits long-lasting prophylactic and therapeutic efficacy in an HPV-16 tumor mice model. Arch Virol. 2014;159:291–305. doi: 10.1007/s00705-013-1819-z. [DOI] [PubMed] [Google Scholar]
- 76.Ding FX, Wang F, Lu YM, Li K, Wang KH, He XW, Sun SH. Multiepitope peptide-loaded virus-like particles as a vaccine against hepatitis B virus-related hepatocellular carcinoma. Hepatology. 2009;49:1492–1502. doi: 10.1002/hep.22816. [DOI] [PubMed] [Google Scholar]
- 77.Wiedermann U, Wiltschke C, Jasinska J, Kundi M, Zurbriggen R, Garner-Spitzer E, Bartsch R, Steger G, Pehamberger H, Scheiner O, et al. A virosomal formulated Her-2/neu multi-peptide vaccine induces Her-2/neu-specific immune responses in patients with metastatic breast cancer: a phase I study. Breast Cancer Res Treat. 2010;119:673–683. doi: 10.1007/s10549-009-0666-9. [DOI] [PubMed] [Google Scholar]
- 78.Patel JM, Kim MC, Vartabedian VF, Lee YN, He S, Song JM, Choi HJ, Yamanaka S, Amaram N, Lukacher A, et al. Protein transfer-mediated surface engineering to adjuvantate virus-like nanoparticles for enhanced anti-viral immune responses. Nanomedicine. 2015;11:1097–1107. doi: 10.1016/j.nano.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tegerstedt K, Lindencrona JA, Curcio C, Andreasson K, Tullus C, Forni G, Dalianis T, Kiessling R, Ramqvist T. A single vaccination with polyomavirus VP1/VP2Her2 virus-like particles prevents outgrowth of HER-2/neu-expressing tumors. Cancer Res. 2005;65:5953–5957. doi: 10.1158/0008-5472.CAN-05-0335. [DOI] [PubMed] [Google Scholar]
- 80.Pouyanfard S, Bamdad T, Hashemi H, Bandehpour M, Kazemi B. Induction of protective anti-CTL epitope responses against HER-2-positive breast cancer based on multivalent T7 phage nanoparticles. PLoS One. 2012;7:e49539. doi: 10.1371/journal.pone.0049539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shukla S, Wen AM, Commandeur U, Steinmetz NF. Presentation of HER2 epitopes using a filamentous plant virus-based vaccination platform. Journal of Materials Chemistry B. 2014;2:6249–6258. doi: 10.1039/c4tb00749b. [DOI] [PubMed] [Google Scholar]
- 82.Montoya ID. Biologics (Vaccines, Antibodies, Enzymes) to Treat Drug Addictions. In: Nady el-Guebaly GC, Marc Galanter, editors. Textbook of Addiction Treatment: International Perspectives. Springer; 2015. pp. 683–692. [Google Scholar]
- 83.De BP, Pagovich OE, Hicks MJ, Rosenberg JB, Moreno AY, Janda KD, Koob GF, Worgall S, Kaminsky SM, Sondhi D, et al. Disrupted adenovirus-based vaccines against small addictive molecules circumvent anti-adenovirus immunity. Hum Gene Ther. 2013;24:58–66. doi: 10.1089/hum.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hicks MJ, De BP, Rosenberg JB, Davidson JT, Moreno AY, Janda KD, Wee S, Koob GF, Hackett NR, Kaminsky SM, et al. Cocaine analog coupled to disrupted adenovirus: a vaccine strategy to evoke high-titer immunity against addictive drugs. Mol Ther. 2011;19:612–619. doi: 10.1038/mt.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wee S, Hicks MJ, De BP, Rosenberg JB, Moreno AY, Kaminsky SM, Janda KD, Crystal RG, Koob GF. Novel cocaine vaccine linked to a disrupted adenovirus gene transfer vector blocks cocaine psychostimulant and reinforcing effects. Neuropsychopharmacology. 2012;37:1083–1091. doi: 10.1038/npp.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chackerian B, Rangel M, Hunter Z, Peabody DS. Virus and virus-like particle-based immunogens for Alzheimer’s disease induce antibody responses against amyloid-beta without concomitant T cell responses. Vaccine. 2006;24:6321–6331. doi: 10.1016/j.vaccine.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 87.Farlow MR, Andreasen N, Riviere ME, Vostiar I, Vitaliti A, Sovago J, Caputo A, Winblad B, Graf A. Long-term treatment with active Abeta immunotherapy with CAD106 in mild Alzheimer’s disease. Alzheimers Res Ther. 2015;7:23. doi: 10.1186/s13195-015-0108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Winblad B, Andreasen N, Minthon L, Floesser A, Imbert G, Dumortier T, Maguire RP, Blennow K, Lundmark J, Staufenbiel M, et al. Safety, tolerability, and antibody response of active Abeta immunotherapy with CAD106 in patients with Alzheimer’s disease: randomised, double-blind, placebo-controlled, first-in-human study. Lancet Neurol. 2012;11:597–604. doi: 10.1016/S1474-4422(12)70140-0. [DOI] [PubMed] [Google Scholar]
- 89.Feng G, Wang W, Qian Y, Jin H. Anti-Abeta antibodies induced by Abeta-HBc virus-like particles prevent Abeta aggregation and protect PC12 cells against toxicity of Abeta1–40. J Neurosci Methods. 2013;218:48–54. doi: 10.1016/j.jneumeth.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 90.Zamora E, Handisurya A, Shafti-Keramat S, Borchelt D, Rudow G, Conant K, Cox C, Troncoso JC, Kirnbauer R. Papillomavirus-like particles are an effective platform for amyloid-beta immunization in rabbits and transgenic mice. J Immunol. 2006;177:2662–2670. doi: 10.4049/jimmunol.177.4.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Karlsson Hedestam GB, Fouchier RA, Phogat S, Burton DR, Sodroski J, Wyatt RT. The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nat Rev Microbiol. 2008;6:143–155. doi: 10.1038/nrmicro1819. [DOI] [PubMed] [Google Scholar]
- 92.Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet. 2014;384:258–271. doi: 10.1016/S0140-6736(14)60164-1. [DOI] [PubMed] [Google Scholar]
- 93.Brechtl JR, Breitbart W, Galietta M, Krivo S, Rosenfeld B. The use of highly active antiretroviral therapy (HAART) in patients with advanced HIV infection: impact on medical, palliative care, and quality of life outcomes. J Pain Symptom Manage. 2001;21:41–51. doi: 10.1016/s0885-3924(00)00245-1. [DOI] [PubMed] [Google Scholar]
- 94.Esparza J. A brief history of the global effort to develop a preventive HIV vaccine. Vaccine. 2013;31:3502–3518. doi: 10.1016/j.vaccine.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 95.Dowdle W. The search for an AIDS vaccine. Public Health Rep. 1986;101:232–233. [PMC free article] [PubMed] [Google Scholar]
- 96.Buratti E, Tisminetzky SG, Scodeller ES, Baralle FE. Conformational display of two neutralizing epitopes of HIV-1 gp41 on the Flock House virus capsid protein. J Immunol Methods. 1996;197:7–18. doi: 10.1016/0022-1759(96)00097-x. [DOI] [PubMed] [Google Scholar]
- 97.Berkower I, Raymond M, Muller J, Spadaccini A, Aberdeen A. Assembly, structure, and antigenic properties of virus-like particles rich in HIV-1 envelope gp120. Virology. 2004;321:75–86. doi: 10.1016/j.virol.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 98.Sun S, Li W, Sun Y, Pan Y, Li J. A new RNA vaccine platform based on MS2 virus-like particles produced in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 2011;407:124–128. doi: 10.1016/j.bbrc.2011.02.122. [DOI] [PubMed] [Google Scholar]
- 99.Yusibov V, Modelska A, Steplewski K, Agadjanyan M, Weiner D, Hooper DC, Koprowski H. Antigens produced in plants by infection with chimeric plant viruses immunize against rabies virus and HIV-1. Proc Natl Acad Sci U S A. 1997;94:5784–5788. doi: 10.1073/pnas.94.11.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sugiyama Y, Hamamoto H, Takemoto S, Watanabe Y, Okada Y. Systemic production of foreign peptides on the particle surface of tobacco mosaic virus. FEBS Lett. 1995;359:247–250. doi: 10.1016/0014-5793(95)00054-d. [DOI] [PubMed] [Google Scholar]
- 101.Lu Y, Xiao Y, Ding J, Dierich M, Chen YH. Immunogenicity of neutralizing epitopes on multiple-epitope vaccines against HIV-1. Int Arch Allergy Immunol. 2000;121:80–84. doi: 10.1159/000024300. [DOI] [PubMed] [Google Scholar]
- 102.Arnold GF, Velasco PK, Holmes AK, Wrin T, Geisler SC, Phung P, Tian Y, Resnick DA, Ma X, Mariano TM, et al. Broad neutralization of human immunodeficiency virus type 1 (HIV-1) elicited from human rhinoviruses that display the HIV-1 gp41 ELDKWA epitope. J Virol. 2009;83:5087–5100. doi: 10.1128/JVI.00184-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 104.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, Wu L, Mackay CR, LaRosa G, Newman W, et al. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 105.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 106.Doranz BJ, Rucker J, Yi Y, Smyth RJ, Samson M, Peiper SC, Parmentier M, Collman RG, Doms RW. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 107.Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 108.Park DJ, Dudas G, Wohl S, Goba A, Whitmer SL, Andersen KG, Sealfon RS, Ladner JT, Kugelman JR, Matranga CB, et al. Ebola Virus Epidemiology, Transmission, and Evolution during Seven Months in Sierra Leone. Cell. 2015;161:1516–1526. doi: 10.1016/j.cell.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Goeijenbier M, van Kampen JJ, Reusken CB, Koopmans MP, van Gorp EC. Ebola virus disease: a review on epidemiology, symptoms, treatment and pathogenesis. Neth J Med. 2014;72:442–448. [PubMed] [Google Scholar]
- 110.Baize S, Pannetier D, Oestereich L, Rieger T, Koivogui L, Magassouba N, Soropogui B, Sow MS, Keita S, De Clerck H, et al. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med. 2014;371:1418–1425. doi: 10.1056/NEJMoa1404505. [DOI] [PubMed] [Google Scholar]
- 111.Matassov D, Marzi A, Latham T, Xu R, Ota-Setlik A, Feldmann F, Geisbert JB, Mire CE, Hamm S, Nowak B, et al. Vaccination With a Highly Attenuated Recombinant Vesicular Stomatitis Virus Vector Protects Against Challenge With a Lethal Dose of Ebola Virus. J Infect Dis. 2015 doi: 10.1093/infdis/jiv316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jones SM, Stroher U, Fernando L, Qiu X, Alimonti J, Melito P, Bray M, Klenk HD, Feldmann H. Assessment of a vesicular stomatitis virus-based vaccine by use of the mouse model of Ebola virus hemorrhagic fever. J Infect Dis. 2007;196(Suppl 2):S404–412. doi: 10.1086/520591. [DOI] [PubMed] [Google Scholar]
- 113.Feldmann H, Jones SM, Daddario-DiCaprio KM, Geisbert JB, Stroher U, Grolla A, Bray M, Fritz EA, Fernando L, Feldmann F, et al. Effective post-exposure treatment of Ebola infection. PLoS Pathog. 2007;3:e2. doi: 10.1371/journal.ppat.0030002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Geisbert TW, Feldmann H. Recombinant vesicular stomatitis virus-based vaccines against Ebola and Marburg virus infections. J Infect Dis. 2011;204(Suppl 3):S1075–1081. doi: 10.1093/infdis/jir349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.de Wit E, Marzi A, Bushmaker T, Brining D, Scott D, Richt JA, Geisbert TW, Feldmann H. Safety of recombinant VSV-Ebola virus vaccine vector in pigs. Emerg Infect Dis. 2015;21:702–704. doi: 10.3201/eid2104.142012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Papaneri AB, Wirblich C, Cooper K, Jahrling PB, Schnell MJ, Blaney JE. Further characterization of the immune response in mice to inactivated and live rabies vaccines expressing Ebola virus glycoprotein. Vaccine. 2012;30:6136–6141. doi: 10.1016/j.vaccine.2012.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.World Health Organization. Influenza (Seasonal) Fact Sheet. Available at: http://www.who.int/mediacentre/factsheets/fs211/en/
- 118.Lambert LC, Fauci AS. Influenza vaccines for the future. N Engl J Med. 2010;363:2036–2044. doi: 10.1056/NEJMra1002842. [DOI] [PubMed] [Google Scholar]
- 119.Krammer F. Emerging influenza viruses and the prospect of a universal influenza virus vaccine. Biotechnol J. 2015;10:690–701. doi: 10.1002/biot.201400393. [DOI] [PubMed] [Google Scholar]
- 120.Taubenberger JK, Morens DM. Influenza: the once and future pandemic. Public Health Rep. 2010;125(Suppl 3):16–26. [PMC free article] [PubMed] [Google Scholar]
- 121.World Health Organization Global Influenza Program Surveillance N. Evolution of H5N1 avian influenza viruses in Asia. Emerg Infect Dis. 2005;11:1515–1521. doi: 10.3201/eid1110.050644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Riedel S. Crossing the species barrier: the threat of an avian influenza pandemic. Proc (Bayl Univ Med Cent) 2006;19:16–20. doi: 10.1080/08998280.2006.11928118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fiore AE, Bridges CB, Cox NJ. Seasonal influenza vaccines. Curr Top Microbiol Immunol. 2009;333:43–82. doi: 10.1007/978-3-540-92165-3_3. [DOI] [PubMed] [Google Scholar]
- 124.Krammer F, Palese P. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Opin Virol. 2013;3:521–530. doi: 10.1016/j.coviro.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.de Jong JC, Beyer WE, Palache AM, Rimmelzwaan GF, Osterhaus AD. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J Med Virol. 2000;61:94–99. [PubMed] [Google Scholar]
- 126.Tricco AC, Chit A, Soobiah C, Hallett D, Meier G, Chen MH, Tashkandi M, Bauch CT, Loeb M. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med. 2013;11:153. doi: 10.1186/1741-7015-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ito T, Gorman OT, Kawaoka Y, Bean WJ, Webster RG. Evolutionary analysis of the influenza A virus M gene with comparison of the M1 and M2 proteins. J Virol. 1991;65:5491–5498. doi: 10.1128/jvi.65.10.5491-5498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Epstein SL, Kong WP, Misplon JA, Lo CY, Tumpey TM, Xu L, Nabel GJ. Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein. Vaccine. 2005;23:5404–5410. doi: 10.1016/j.vaccine.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 129.Kawano M, Morikawa K, Suda T, Ohno N, Matsushita S, Akatsuka T, Handa H, Matsui M. Chimeric SV40 virus-like particles induce specific cytotoxicity and protective immunity against influenza A virus without the need of adjuvants. Virology. 2014;448:159–167. doi: 10.1016/j.virol.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 130.Moyron-Quiroz JE, Rangel-Moreno J, Hartson L, Kusser K, Tighe MP, Klonowski KD, Lefrancois L, Cauley LS, Harmsen AG, Lund FE, et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 2006;25:643–654. doi: 10.1016/j.immuni.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 131.D’Aoust MA, Lavoie PO, Couture MM, Trepanier S, Guay JM, Dargis M, Mongrand S, Landry N, Ward BJ, Vezina LP. Influenza virus-like particles produced by transient expression in Nicotiana benthamiana induce a protective immune response against a lethal viral challenge in mice. Plant Biotechnol J. 2008;6:930–940. doi: 10.1111/j.1467-7652.2008.00384.x. [DOI] [PubMed] [Google Scholar]
- 132.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 133.American Cancer Society. Global Cancer Facts & Figures, American. 3. 2015. [Google Scholar]
- 134.Castellsague X, Bosch FX. [Advances in cervical cancer prevention: HPV vaccines] Farm Hosp. 2007;31:261–263. doi: 10.1016/s1130-6343(07)75388-6. [DOI] [PubMed] [Google Scholar]
- 135.Bosch FX, de Sanjose S. The epidemiology of human papillomavirus infection and cervical cancer. Dis Markers. 2007;23:213–227. doi: 10.1155/2007/914823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Clifford GM, Gallus S, Herrero R, Munoz N, Snijders PJ, Vaccarella S, Anh PT, Ferreccio C, Hieu NT, Matos E, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet. 2005;366:991–998. doi: 10.1016/S0140-6736(05)67069-9. [DOI] [PubMed] [Google Scholar]
- 137.Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol. 2010;117:S5–10. doi: 10.1016/j.ygyno.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 138.Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsague X, Skinner SR, Apter D, Naud P, Salmeron J, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:89–99. doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 139.Munoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Brown DR, Koutsky LA, Tay EH, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010;102:325–339. doi: 10.1093/jnci/djp534. [DOI] [PubMed] [Google Scholar]
- 140.Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, Tay EH, Garcia P, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis. 2009;199:926–935. doi: 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- 141.Wheeler CM, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Perez G, Brown DR, Koutsky LA, Tay EH, Garcia P, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16–26 years. J Infect Dis. 2009;199:936–944. doi: 10.1086/597309. [DOI] [PubMed] [Google Scholar]
- 142.Smith JF, Brownlow M, Brown M, Kowalski R, Esser MT, Ruiz W, Barr E, Brown DR, Bryan JT. Antibodies from women immunized with Gardasil cross-neutralize HPV 45 pseudovirions. Hum Vaccin. 2007;3:109–115. doi: 10.4161/hv.3.4.4058. [DOI] [PubMed] [Google Scholar]
- 143.Roden RB, Yutzy WHt, Fallon R, Inglis S, Lowy DR, Schiller JT. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology. 2000;270:254–257. doi: 10.1006/viro.2000.0272. [DOI] [PubMed] [Google Scholar]
- 144.Gambhira R, Karanam B, Jagu S, Roberts JN, Buck CB, Bossis I, Alphs H, Culp T, Christensen ND, Roden RB. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J Virol. 2007;81:13927–13931. doi: 10.1128/JVI.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pastrana DV, Gambhira R, Buck CB, Pang YY, Thompson CD, Culp TD, Christensen ND, Lowy DR, Schiller JT, Roden RB. Cross-neutralization of cutaneous and mucosal Papillomavirus types with anti-sera to the amino terminus of L2. Virology. 2005;337:365–372. doi: 10.1016/j.virol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 146.Schellenbacher C, Kwak K, Fink D, Shafti-Keramat S, Huber B, Jindra C, Faust H, Dillner J, Roden RB, Kirnbauer R. Efficacy of RG1-VLP vaccination against infections with genital and cutaneous human papillomaviruses. J Invest Dermatol. 2013;133:2706–2713. doi: 10.1038/jid.2013.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Karanam B, Jagu S, Huh WK, Roden RB. Developing vaccines against minor capsid antigen L2 to prevent papillomavirus infection. Immunol Cell Biol. 2009;87:287–299. doi: 10.1038/icb.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tumban E, Peabody J, Peabody DS, Chackerian B. A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2. PLoS One. 2011;6:e23310. doi: 10.1371/journal.pone.0023310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst. 2000;92:690–698. doi: 10.1093/jnci/92.9.690. [DOI] [PubMed] [Google Scholar]
- 150.Muller M, Zhou J, Reed TD, Rittmuller C, Burger A, Gabelsberger J, Braspenning J, Gissmann L. Chimeric papillomavirus-like particles. Virology. 1997;234:93–111. doi: 10.1006/viro.1997.8591. [DOI] [PubMed] [Google Scholar]
- 151.Schafer K, Muller M, Faath S, Henn A, Osen W, Zentgraf H, Benner A, Gissmann L, Jochmus I. Immune response to human papillomavirus 16 L1E7 chimeric virus-like particles: induction of cytotoxic T cells and specific tumor protection. Int J Cancer. 1999;81:881–888. doi: 10.1002/(sici)1097-0215(19990611)81:6<881::aid-ijc8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 152.Kaufmann AM, Nieland J, Schinz M, Nonn M, Gabelsberger J, Meissner H, Muller RT, Jochmus I, Gissmann L, Schneider A, et al. HPV16 L1E7 chimeric virus-like particles induce specific HLA-restricted T cells in humans after in vitro vaccination. Int J Cancer. 2001;92:285–293. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1181>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 153.Ohlschlager P, Osen W, Dell K, Faath S, Garcea RL, Jochmus I, Muller M, Pawlita M, Schafer K, Sehr P, et al. Human papillomavirus type 16 L1 capsomeres induce L1-specific cytotoxic T lymphocytes and tumor regression in C57BL/6 mice. J Virol. 2003;77:4635–4645. doi: 10.1128/JVI.77.8.4635-4645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.London WTMK. Liver Cancer. In: Schottenfeld DFJJ, editor. Cancer Epidemiology and Prevention. 3. New York: Oxford University Press; 2006. pp. 763–786. [Google Scholar]
- 155.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47(Suppl):S2–6. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 157.McAleer WJ, Buynak EB, Maigetter RZ, Wampler DE, Miller WJ, Hilleman MR. Human hepatitis B vaccine from recombinant yeast. Nature. 1984;307:178–180. doi: 10.1038/307178a0. [DOI] [PubMed] [Google Scholar]
- 158.Andre FE, Safary A. Summary of clinical findings on Engerix-B, a genetically engineered yeast derived hepatitis B vaccine. Postgrad Med J. 1987;63(Suppl 2):169–177. [PubMed] [Google Scholar]
- 159.Su F, Schneider RJ. Hepatitis B virus HBx protein sensitizes cells to apoptotic killing by tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1997;94:8744–8749. doi: 10.1073/pnas.94.16.8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 162.Jhaveri K, Esteva FJ. Pertuzumab in the treatment of HER2+ breast cancer. J Natl Compr Canc Netw. 2014;12:591–598. doi: 10.6004/jnccn.2014.0059. [DOI] [PubMed] [Google Scholar]
- 163.Hortobagyi GN. Trastuzumab in the treatment of breast cancer. N Engl J Med. 2005;353:1734–1736. doi: 10.1056/NEJMe058196. [DOI] [PubMed] [Google Scholar]
- 164.Zurbriggen R. Immunostimulating reconstituted influenza virosomes. Vaccine. 2003;21:921–924. doi: 10.1016/s0264-410x(02)00541-8. [DOI] [PubMed] [Google Scholar]
- 165.Bovier PA. Epaxal: a virosomal vaccine to prevent hepatitis A infection. Expert Rev Vaccines. 2008;7:1141–1150. doi: 10.1586/14760584.7.8.1141. [DOI] [PubMed] [Google Scholar]
- 166.Thompson FM, Porter DW, Okitsu SL, Westerfeld N, Vogel D, Todryk S, Poulton I, Correa S, Hutchings C, Berthoud T, et al. Evidence of blood stage efficacy with a virosomal malaria vaccine in a phase IIa clinical trial. PLoS One. 2008;3:e1493. doi: 10.1371/journal.pone.0001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wagner S, Jasinska J, Breiteneder H, Kundi M, Pehamberger H, Scheiner O, Zielinski CC, Wiedermann U. Delayed tumor onset and reduced tumor growth progression after immunization with a Her-2/neu multi-peptide vaccine and IL-12 in c-neu transgenic mice. Breast Cancer Res Treat. 2007;106:29–38. doi: 10.1007/s10549-006-9469-4. [DOI] [PubMed] [Google Scholar]
- 168.Chen XS, Stehle T, Harrison SC. Interaction of polyomavirus internal protein VP2 with the major capsid protein VP1 and implications for participation of VP2 in viral entry. EMBO J. 1998;17:3233–3240. doi: 10.1093/emboj/17.12.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Carrera MR, Ashley JA, Parsons LH, Wirsching P, Koob GF, Janda KD. Suppression of psychoactive effects of cocaine by active immunization. Nature. 1995;378:727–730. doi: 10.1038/378727a0. [DOI] [PubMed] [Google Scholar]
- 170.Fox BS, Kantak KM, Edwards MA, Black KM, Bollinger BK, Botka AJ, French TL, Thompson TL, Schad VC, Greenstein JL, et al. Efficacy of a therapeutic cocaine vaccine in rodent models. Nat Med. 1996;2:1129–1132. doi: 10.1038/nm1096-1129. [DOI] [PubMed] [Google Scholar]
- 171.Kinsey BM, Jackson DC, Orson FM. Anti-drug vaccines to treat substance abuse. Immunol Cell Biol. 2009;87:309–314. doi: 10.1038/icb.2009.17. [DOI] [PubMed] [Google Scholar]
- 172.Maurer P, Jennings GT, Willers J, Rohner F, Lindman Y, Roubicek K, Renner WA, Muller P, Bachmann MF. A therapeutic vaccine for nicotine dependence: preclinical efficacy, and Phase I safety and immunogenicity. Eur J Immunol. 2005;35:2031–2040. doi: 10.1002/eji.200526285. [DOI] [PubMed] [Google Scholar]
- 173.Maoz A, Hicks MJ, Vallabhjosula S, Synan M, Kothari PJ, Dyke JP, Ballon DJ, Kaminsky SM, De BP, Rosenberg JB, et al. Adenovirus capsid-based anti-cocaine vaccine prevents cocaine from binding to the nonhuman primate CNS dopamine transporter. Neuropsychopharmacology. 2013;38:2170–2178. doi: 10.1038/npp.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bachmann MF, Jennings GT. Therapeutic vaccines for chronic diseases: successes and technical challenges. Philos Trans R Soc Lond B Biol Sci. 2011;366:2815–2822. doi: 10.1098/rstb.2011.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chackerian B, Lowy DR, Schiller JT. Conjugation of a self-antigen to papillomavirus-like particles allows for efficient induction of protective autoantibodies. J Clin Invest. 2001;108:415–423. doi: 10.1172/JCI11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Spohn G, Keller I, Beck M, Grest P, Jennings GT, Bachmann MF. Active immunization with IL-1 displayed on virus-like particles protects from autoimmune arthritis. Eur J Immunol. 2008;38:877–887. doi: 10.1002/eji.200737989. [DOI] [PubMed] [Google Scholar]
- 177.Spohn G, Bachmann MF. Targeting osteoporosis and rheumatoid arthritis by active vaccination against RANKL. Adv Exp Med Biol. 2007;602:135–142. doi: 10.1007/978-0-387-72009-8_17. [DOI] [PubMed] [Google Scholar]
- 178.Spohn G, Guler R, Johansen P, Keller I, Jacobs M, Beck M, Rohner F, Bauer M, Dietmeier K, Kundig TM, et al. A virus-like particle-based vaccine selectively targeting soluble TNF-alpha protects from arthritis without inducing reactivation of latent tuberculosis. J Immunol. 2007;178:7450–7457. doi: 10.4049/jimmunol.178.11.7450. [DOI] [PubMed] [Google Scholar]
- 179.Rohn TA, Jennings GT, Hernandez M, Grest P, Beck M, Zou Y, Kopf M, Bachmann MF. Vaccination against IL-17 suppresses autoimmune arthritis and encephalomyelitis. Eur J Immunol. 2006;36:2857–2867. doi: 10.1002/eji.200636658. [DOI] [PubMed] [Google Scholar]
- 180.Nakajima A, Seroogy CM, Sandora MR, Tarner IH, Costa GL, Taylor-Edwards C, Bachmann MH, Contag CH, Fathman CG. Antigen-specific T cell-mediated gene therapy in collagen-induced arthritis. J Clin Invest. 2001;107:1293–1301. doi: 10.1172/JCI12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Spohn G, Schwarz K, Maurer P, Illges H, Rajasekaran N, Choi Y, Jennings GT, Bachmann MF. Protection against osteoporosis by active immunization with TRANCE/RANKL displayed on virus-like particles. J Immunol. 2005;175:6211–6218. doi: 10.4049/jimmunol.175.9.6211. [DOI] [PubMed] [Google Scholar]
- 182.Sonderegger I, Rohn TA, Kurrer MO, Iezzi G, Zou Y, Kastelein RA, Bachmann MF, Kopf M. Neutralization of IL-17 by active vaccination inhibits IL-23-dependent autoimmune myocarditis. Eur J Immunol. 2006;36:2849–2856. doi: 10.1002/eji.200636484. [DOI] [PubMed] [Google Scholar]
- 183.Fulurija A, Lutz TA, Sladko K, Osto M, Wielinga PY, Bachmann MF, Saudan P. Vaccination against GIP for the treatment of obesity. PLoS One. 2008;3:e3163. doi: 10.1371/journal.pone.0003163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chackerian B. Virus-like particles: flexible platforms for vaccine development. Expert Rev Vaccines. 2007;6:381–390. doi: 10.1586/14760584.6.3.381. [DOI] [PubMed] [Google Scholar]
- 185.Bachmann MF, Whitehead P. Active immunotherapy for chronic diseases. Vaccine. 2013;31:1777–1784. doi: 10.1016/j.vaccine.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 186.Jennings GT, Bachmann MF. Immunodrugs: therapeutic VLP-based vaccines for chronic diseases. Annu Rev Pharmacol Toxicol. 2009;49:303–326. doi: 10.1146/annurev-pharmtox-061008-103129. [DOI] [PubMed] [Google Scholar]
- 187.Ambuhl PM, Tissot AC, Fulurija A, Maurer P, Nussberger J, Sabat R, Nief V, Schellekens C, Sladko K, Roubicek K, et al. A vaccine for hypertension based on virus-like particles: preclinical efficacy and phase I safety and immunogenicity. J Hypertens. 2007;25:63–72. doi: 10.1097/HJH.0b013e32800ff5d6. [DOI] [PubMed] [Google Scholar]
- 188.Tissot AC, Maurer P, Nussberger J, Sabat R, Pfister T, Ignatenko S, Volk HD, Stocker H, Muller P, Jennings GT, et al. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: a double-blind, randomised, placebo-controlled phase IIa study. Lancet. 2008;371:821–827. doi: 10.1016/S0140-6736(08)60381-5. [DOI] [PubMed] [Google Scholar]
- 189.Liebscher S, Meyer-Luehmann M. A Peephole into the Brain: Neuropathological Features of Alzheimer’s Disease Revealed by in vivo Two-Photon Imaging. Front Psychiatry. 2012;3:26. doi: 10.3389/fpsyt.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 191.Haass C, Selkoe DJ. Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell. 1993;75:1039–1042. doi: 10.1016/0092-8674(93)90312-e. [DOI] [PubMed] [Google Scholar]
- 192.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 193.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 194.Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 195.Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 196.Ferrer I, Boada Rovira M, Sanchez Guerra ML, Rey MJ, Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bard F, Barbour R, Cannon C, Carretto R, Fox M, Games D, Guido T, Hoenow K, Hu K, Johnson-Wood K, et al. Epitope and isotype specificities of antibodies to beta -amyloid peptide for protection against Alzheimer’s disease-like neuropathology. Proc Natl Acad Sci U S A. 2003;100:2023–2028. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Seabrook TJ, Bloom JK, Iglesias M, Spooner ET, Walsh DM, Lemere CA. Species-specific immune response to immunization with human versus rodent A beta peptide. Neurobiol Aging. 2004;25:1141–1151. doi: 10.1016/j.neurobiolaging.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 199.Wiessner C, Wiederhold KH, Tissot AC, Frey P, Danner S, Jacobson LH, Jennings GT, Luond R, Ortmann R, Reichwald J, et al. The second-generation active Abeta immunotherapy CAD106 reduces amyloid accumulation in APP transgenic mice while minimizing potential side effects. J Neurosci. 2011;31:9323–9331. doi: 10.1523/JNEUROSCI.0293-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Karpenko LI, Ivanisenko VA, Pika IA, Chikaev NA, Eroshkin AM, Veremeiko TA, Ilyichev AA. Insertion of foreign epitopes in HBcAg: how to make the chimeric particle assemble. Amino Acids. 2000;18:329–337. doi: 10.1007/s007260070072. [DOI] [PubMed] [Google Scholar]