Summary
Confidence and actions are normally tightly interwoven—if I am sure that it is going to rain, I will take an umbrella—therefore, it is difficult to understand their interplay. Stimulated by the ego-dystonic nature of obsessive-compulsive disorder (OCD), where compulsive actions are recognized as disproportionate, we hypothesized that action and confidence might be independently updated during learning. Participants completed a predictive-inference task designed to identify how action and confidence evolve in response to surprising changes in the environment. While OCD patients (like controls) correctly updated their confidence according to changes in the environment, their actions (unlike those of controls) mostly disregarded this knowledge. Therefore, OCD patients develop an accurate, internal model of the environment but fail to use it to guide behavior. Results demonstrated a novel dissociation between confidence and action, suggesting a cognitive architecture whereby confidence estimates can accurately track the statistic of the environment independently from performance.
Keywords: beliefs, action, confidence, metacognition, compulsivity, learning, uncertainty, computational psychiatry, obsessive-compulsive disorder
Highlights
-
•
Action can be functionally dissociated from confidence
-
•
Patients with obsessive-compulsive disorder (OCD) show exaggerated action updating
-
•
Confidence in OCD is intact and reflects information that is not used to control action
-
•
Degree of action-confidence dissociation correlates with symptom severity in OCD
Vaghi, Luyckx et al. provide evidences of a novel dissociation between confidence and action in OCD patients with confidence accessing information that is not used to guide action.
Introduction
Intelligent agents have to act on incomplete and fragmented information. Typically, incoming information is processed to reduce uncertainty so as to make more accurate inferences about the causal structure of the environment (Knill and Pouget, 2004, O’Reilly et al., 2012). Subjects are generally able to learn from experience, and often actions are dictated by subjects’ beliefs acquired flexibly through this inference process. The strength of belief (or “confidence”) is generally tightly coupled to behavior—I will study more for an exam if I am in doubt about my level of knowledge. Bayesian accounts of learning suggest that current levels of uncertainty in the estimate of the action’s value influence behavior (Behrens et al., 2007, Nassar et al., 2010). In particular, the impact of information on behavior depends on the level of epistemic uncertainty (i.e., confidence) held by the agent. In other words, information is mostly influential when the agent is more uncertain about the environment. In contrast, when an agent has little uncertainty, it is less influenced by upcoming new evidence (Behrens et al., 2007, Nassar et al., 2010, Yu and Dayan, 2005). However, because of a tight link between behavior and confidence, it has been difficult to study how confidence and action evolve and interact during learning. In turn, obsessive-compulsive disorder (OCD) provides a paradigmatic example whereby the link between strength of beliefs and action can be disrupted. Compulsive rituals, such as hand washing, are called ego-dystonic, since patients recognize them as disproportionate and excessive but nevertheless cannot stop performing them (Kozak and Foa, 1994). Therefore, we capitalized on this distinctive feature of the disorder to test opposing views about how subjective beliefs are accessed for metacognitive reports of confidence and action control: specifically, are they accessed in parallel or is confidence necessarily informed by action monitoring?
At the same time, we sought to provide a novel computational insight in the etiology of this debilitating psychiatric condition.
Results
Experimental Design
We studied 24 OCD patients and 25 matched controls (Table S1) on a modified predictive-inference task (McGuire et al., 2014, Nassar et al., 2010) (Figures 1A and 1B; STAR Methods). In each trial, participants were required to position a bucket on a circular ring to catch particles flying from the middle of the ring. After positioning the bucket (and before seeing where the particle would land), participants reported their degree of confidence in their prediction. During the task, subjects were required to gauge the value of a new piece of information and differentiate periods of time in which unexpected outcomes should be ignored as noise and those in which abrupt changes were likely to require updating of action and beliefs. In such unstable, dynamic environments, recent events are only informative in the presence of abrupt changes; meanwhile, during a stable period, most recent events are less informative, and decisions should be driven by averaging over the outcomes of many previous actions, i.e., historical information should be taken into account.
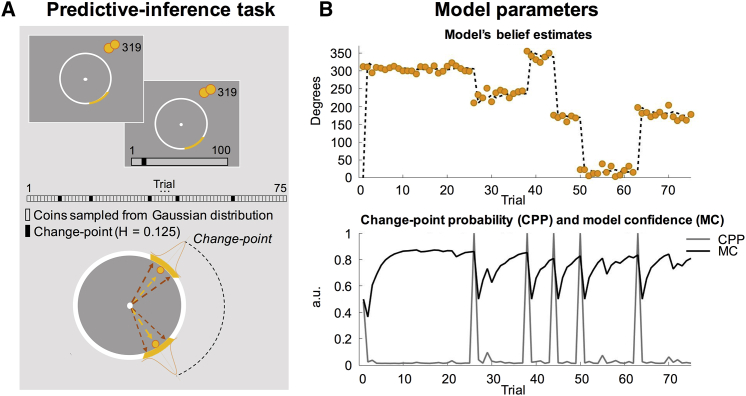
Figure 1.
Diagram of the Predictive-Inference Task and Model Parameters
(A) On each trial, participants chose a position for the bucket (orange segment) and scored their confidence for their prediction on a bar appearing thereafter. One particle was then fired from the center of the big circle. Throughout each experimental block, particles were drawn from a Gaussian distribution. Mean of the distribution could change on any trial with a probability of 0.125 (H, hazard rate) determining a change in action-outcome contingencies in the environment (change-point).
(B) Top: example of a sequence of trials. Points mark the position at which particles landed on the big circle (0°–360°). The dotted line identifies the predictions of the quasi-optimal Bayesian model. Bottom: two theoretical factors, change-point probability (CPP) and model confidence (MC), jointly influence learning rate. When unexpected observations occur, CPP is high and MC attenuated.
Behavioral Analyses
To investigate how new evidence collected in a noisy environment influences behavior (action) and confidence reports, we computed for each trial in each participant a spatial learning rate expressed as the magnitude of change in the prediction (chosen bucket position from one trial to the next) as a fraction of the error made on the previous prediction (difference between chosen bucket position and position of new evidence). We then considered the same sequence of observations experienced by each participant and computed the behavior of a quasi-optimal Bayesian learning model (STAR Methods for the model specification; Nassar et al., 2010, Nassar et al., 2012, McGuire et al., 2014). This model has no free parameters since it is not fitted to the participant behavior and provides a benchmark Bayesian model against which to compare participants’ behavior. Following a procedure used previously (McGuire et al., 2014) and commonly used in model-based fMRI (O’Doherty et al., 2007), we then constructed regression models to test how participants’ behavioral measures relate to different parameters of the benchmark Bayesian model with the aim to identify and quantify statistical differences between the two groups.
Learning Rate
In our task, participants’ LRs ( Equation 1) were estimated on each trial by taking the ratio of bucket displacement and the spatial prediction error (McGuire et al., 2014, Nassar et al., 2010), measuring the extent to which each new outcome influenced subsequent prediction.
| Equation 1 |
| Equation 2 |
In Equations 1 and 2, bt and bt+1 are the chosen bucket position (i.e., where participant positioned the bucket) for one trial and the next one. is the spatial prediction error, which is the difference between the location of particle at trial t (Xt) and chosen bucket position at trial t (bt).
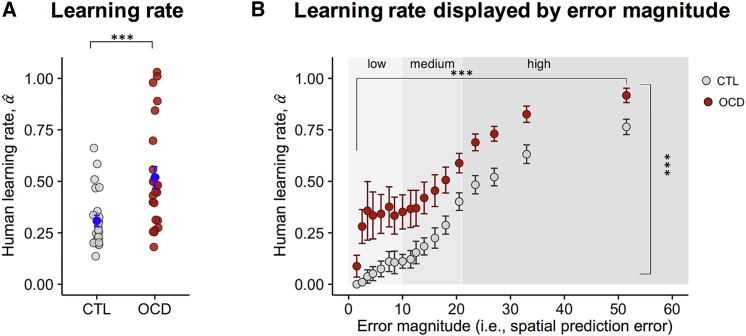
OCD patients were strongly influenced by recent outcomes as shown by their LR being significantly higher than the controls’ (OCD, 0.52 ± 0.05; CTL, 0.31 ± 0.03; Welch two-tailed t test t34 = −3.587, p = 0.001) (Figure 2A). To investigate the effect of error magnitude (Figure 2B) on LR , we divided the range of the spatial prediction error into quantiles and extracted data for controls and patients separately (Table S2; STAR Methods). Results suggested that LRs were highest after subjects made larger errors (effect of error magnitude, F2,94 = 190.604, p < 0.001), showing that both groups correctly monitored their actions, thus adapting their learning (Figure 2B). However, independently of error magnitude, LRs were systematically higher in patients than controls (effect of group, F1,47 = 13.388, p < 0.001) (Figure 2B), reflecting a more marked influence of a new outcome on subsequent prediction in the OCD group, regardless of the magnitude of error (groups pairwise comparisons low, t26.627 = −3.267, p = 0.003; medium, t34.811 = −2.874, p = 0.007; high, t47 = −3.758, p = 0.0005, Bonferroni corrected). Increased LRs in OCD patients did not correlate with medication dosage tested via Spearman correlation (OCD, n = 24, ρ = −0.173, p = 0.418). In addition, we tested the presence of an effect of medication by treating dosage as a categorical variable and testing the association with LRs divided into evenly spaced quantiles. Absence of an association was confirmed for multiple discretization of the LRs (all p values > 0.116). In the patient group, more marked updating of the bucket’s position indexed by increased LRs was not associated with impulsivity as measured with the Barratt Impulsiveness Scale (Patton et al., 1995) (OCD, n = 24, Pearson’s correlation, r = 0.162, p = 0.449). Therefore, increased LR is likely driven by processes other than impulsiveness in OCD patients.
Figure 2.
Learning Rates in OCD and Controls
(A) Learning rate (LR) for participants (Equation 1). Patients showed significantly higher LRs compared with controls. Dots represent individual subjects. Mean ± SEM are displayed in blue.
(B) LR for participants (Equation 1) plotted as a function of the error magnitude (Equation 2). The distribution of the values of the spatial prediction error was divided in 20 quantiles (Table S2 for mean and SEM for each quantile). For visualization purposes, data from 18 quantiles are shown. Mean ± SEM are shown. Subjects tended to use variable LRs spanning the entire allowed range, with higher LRs for higher spatial prediction error. However, LR was higher in OCD patients, regardless of error magnitude. ∗∗∗p < 0.001.
Dynamics of Learning and Confidence Update
To determine how participants’ beliefs evolved over time, we compared the performance of our human participants to that of a quasi-optimal Bayesian learner carrying out the same task (Equation 3). The quasi-optimal Bayesian learner is a computationally parsimonious algorithmic implementation of an optimal Bayesian learner (Nassar et al., 2010 and McGuire et al., 2014 for a full comparison). In such simple tasks, it can achieve performance that is comparable to that of the optimal Bayesian learner at a fraction of the cost through a delta-rule type of learning in belief space.
| Equation 3 |
| Equation 4 |
| Equation 5 |
In Equation 3, Bt and Bt+1 are the model’s belief estimate about the mean of the distribution at trial t and t+1. The LR of the model (α) (Equation 4) is jointly influenced by the change-point probability (CPP, Ω) (Equation 6) and the model confidence (MC, ) (Equation 8) (Figure 1B). δ (Equation 5) is the prediction error (PE), which is the discrepancy between the model belief estimate (Bt) and the location of the new sample (Xt). Note that unlike a full Bayesian model, in this Bayesian delta-rule model, beliefs are not represented by probability density but are point-like estimates that are iteratively updated like values in classic reinforcement learning models (McGuire et al., 2014, Nassar et al., 2010). Another feature of this algorithmic implementation is that there is a direct mapping between beliefs and action, which are not linked through a softmax function that converts values (or belief distributions) into action probabilities.
In response to change-points in the environment, when the information about the previous average position becomes irrelevant, the quasi-optimal Bayesian learner reacted by increasing its LR (α); subsequent to a change-point, as more evidence is accumulated from the same generative distribution, LR (α) steadily decreases, relying on integration of previous observations (Figure 3A). Our human participants followed a similar temporal dynamic in adjusting their LRs (Figure 3B and Figure S1 for a close overlap at the trial-by-trial level between subject’s bucket position computed in Equation 1 and model term Bt computed with Equation 3). However, in OCD patients, accumulation of more knowledge about the environment was unable to reduce LRs sufficiently. In the extreme cases (patients with mean LR = 1) behavior was exclusively driven by the last observed sample, ignoring all previously observed evidence. Therefore, LRs were driven predominantly by the most recent outcomes disregarding previously experienced ones. Strikingly, and unlike action, the way in which confidence changed over time in OCD patients was indistinguishable from that of controls (Figure 3E) and closely resembled the quasi-optimal Bayesian learner’s confidence (MC), which is bound to increase as more evidence is accumulated after a change-point (Figure 3D). This dissociation between action and confidence suggests that, in the OCD group, reports of confidence accurately reflected the increase in strength of beliefs following the accumulation of more evidence even when this evidence was underutilized for behavioral control.
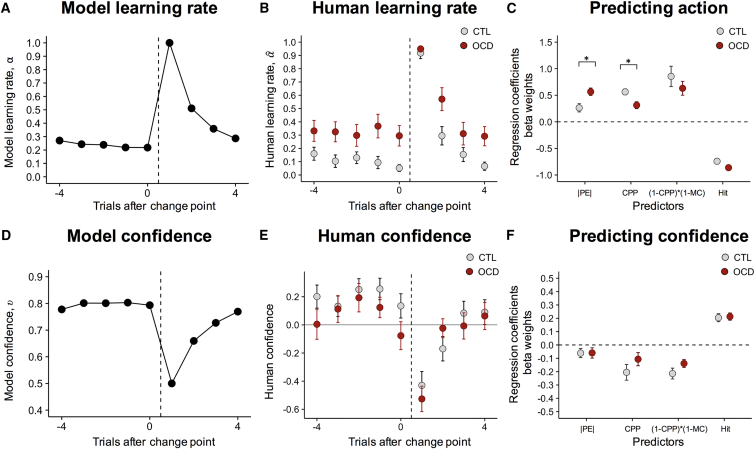
Figure 3.
Regression-Based Analysis for Learning Rates and Confidence
(A and B) (A) Model learning rate (LR) (α) and (B) human LR aligned to change-points (vertical dashed line). LRs were highest after change-point trials and decayed thereafter. OCD patients showed increased LRs on trials before and after change-points.
(C) Regression analysis of behavioral data for human action, constructed similar to the update formula of the quasi-optimal Bayesian model, multiplying the LR by the absolute spatial prediction error .
(D and E) (D) Model confidence (υ) and (E) human confidence (Z-scored) aligned to change-points. The effects of confidence on LRs are greatest on the trials immediately after a change-point when confidence drops. Confidence recovers over several trials thereafter, with no between-group differences.
(F) Regression analysis of behavioral data for human confidence (Z-scored). Error bars represent SEM. Plotted predictors for action and confidence regressions correspond to absolute prediction error (|δ|), change-point probability , model confidence (υ), and hit/miss categorical predictor. (A) (model learning rate) and (D) (model confidence) represent value of the first change-point. For (B) (human learning rate) and (E) (human confidence), all epochs were identified per subject where a change-point was preceded by five data points and followed by four. Group-level mean and SEM were calculated separately for controls and OCD patients. See also Table S3. ∗p < 0.05.
To quantify and compare between-group differences in trial-wise adjustment in LRs , we performed linear regression analysis using the different parameters of the quasi-optimal Bayesian learner as predictors. Compared with controls, patients showed a stronger influence of PEs, indexing the tendency to update the bucket toward the most recent particle, in driving trial-wise adjustment in LRs and reduced responses to abrupt changes in the environment indexed by CPP (PE: OCD, 0.57 ± 0.07; CTL, 0.26 ± 0.08; Wilcoxon rank-sum test, p = 0.018; CPP: OCD, 0.31 ± 0.07; CTL, 0.57 ± 0.06; Wilcoxon rank-sum test, p = 0.011) (Figure 3C; Table S3). There were no between-group differences for MC and for a categorical predictor indexing whether the previous trial resulted in a hit or miss (this was included in the regression since it has been proposed that OCD patients might exhibit differential sensitivity to reward and punishment; Endrass et al., 2011) (Table S3).
Using the same approach described above for action, we performed regression analysis to predict participants’ confidence (Figure 3F). In contrast to the regression predicting participants’ action (Figure 3C), there were no between-group differences for confidence in any predictor (Figure 3F; Table S3). Therefore, OCD patients made full use of accumulated knowledge about the position of the particle to infer the underlying statistics of the environment and built accurate confidence estimates. This belief was nevertheless underutilized to control action, resulting in excessive reactivity to the most recent evidence and therefore elevated LRs .
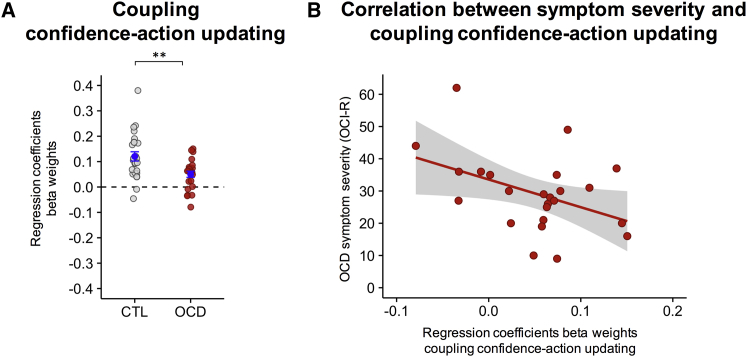
Such mismatching was formally tested by a new regression model in which action updating was predicted by confidence updating. In OCD patients, there was a weakened relationship between action control and metacognitive reports of confidence (OCD, 0.05 ± 0.01; CTL, 0.12 ± 0.02; Wilcoxon rank-sum test, z = 2.690, p = 0.007) (Figure 4A). Reduced coupling between action and belief was most prominent in more severely ill patients (OCD, n = 24, Pearson’s correlation, r = −0.426, p = 0.038) (Figure 4B), thus relating inter-individual patient variability to symptom severity and suggesting that this computational deficit is a core feature of the multifaceted OCD psychiatric manifestation (Robbins et al., 2012, Stephan and Mathys, 2014).
Figure 4.
Uncoupling between Confidence and Action in OCD and Relationship with Symptom Severity
(A) Regression model whereby action updating (i.e., the absolute difference between where participants positioned bucket on trial t and t-1) is predicted by confidence updating (i.e., the absolute difference between Z-scored confidence reports on trial t and t-1). Dots represent individual subjects. Mean ± SEM are displayed in blue.
(B) Association between self-reported symptom severity and coupling confidence-action updating in patients. OCI-R, obsessive-compulsive inventory revised. Shaded gray area represents 95% confidence interval. ∗∗p < 0.01.
Discussion
In this study, we investigated how confidence interplays with action. We showed that both controls and OCD patients integrated historical information to update their confidence flexibly in keeping with the non-stationary features of the environment. More specifically, when the environment abruptly changed (i.e., change-point probability or CPP) confidence first decreased and then gradually increased again on subsequent trials when more evidence was accumulated as shown previously in healthy controls (Nassar et al., 2010, Nassar et al., 2012). The dynamic by which confidence evolved over time closely matched that of a quasi-optimal Bayesian learner (McGuire et al., 2014, Nassar et al., 2010). Critically, we showed that while the estimation of confidence was intact in OCD patients, their actions largely disregarded this accumulated information. In other words, OCD patients constantly updated the position of the bucket in favor of the most recent outcomes rather than the average of the preceding trials. In extreme cases (participants with a LR close to one), this behavior thus reflected abandonment of historical information acquired on previous trials, and as a consequence, the behavior was driven only by the last observation.
Hyperactive error signals have been linked to OCD and have been associated to the “not-just-right” experience, which forces these patients to monitor their behavior more carefully (Pitman, 1987). Here, however, higher LRs in OCD were found even in the absence of an error and independently of its magnitude (Figure 2B), suggesting that elevated action update was not simply driven by response feedback. Instead, our computational analyses showed that, when updating their actions, OCD patients were more driven by prediction errors compared with controls.
More prominent action updating based on most recent outcomes in OCD is possibly due to an inability to take into account the broader context (i.e., history of outcomes during the experimental task) in order to build an internal map of the external world. These functions are generally attributed to model-based strategies exploited to generate goal-directed choices and held to produce cognitive predictions of future values based on representations of the environment, expectations, and prospective calculations (Daw et al., 2005). A weakness of this system in OCD might leave room for producing automatic stimulus-response habits. Development of action-outcome predictions and PE is heavily dependent on dopamine in the ventral striatum (VS) (O’Doherty et al., 2004). Blunted VS reactivity in response to reward has been shown several times in OCD (Figee et al., 2013, Marsh et al., 2015).
In addition, the anterior cingulate cortex (ACC) might underpin the behavioral adjustments required to perform our task. ACC has been shown to be critically involved in updating learning rates when circumstance in the environment are changing (Behrens et al., 2007, Kennerley et al., 2006, McGuire et al., 2014). Therefore, an intriguing possibility is that the exaggerated behavioral updating in OCD patients shown here might implicate a dysfunction in a frontostriatal loop involving the VS and the ACC (Haber and Behrens, 2014) and might possibly explain how deep brain stimulation successfully targets this frontostriatal circuit (Denys et al., 2010, Figee et al., 2013) in the treatment of OCD.
Furthermore, our work shows that the degree of uncoupling between confidence and action is correlated with the severity of the OCD symptomatology, showing that this uncoupling (that we report here, we believe, for the first time) might be at the core of the computational deficit that characterizes OCD. Previous work has proposed that OCD patients might suffer from a metacognitive deficit (Hauser et al., 2017, Hermans et al., 2008). Our results are consistent with a metacognitive deficit in OCD (i.e., patients’ confidence reports were not tracking their actions) but suggest that OCD metacognitive deficits might not be triggered by an abnormal confidence response but by the uncoupling between action and subjective confidence ratings that we have isolated.
These findings might also help to distinguish between competing accounts of human cognition by clarifying the link between confidence and behavioral control. A prevailing view is that confidence is built from internal cues related to object-level processes and through monitoring one’s own actions (Metcalfe and Shimamura, 1994) to support error detection (Yeung and Summerfield, 2012). In the context of signal-detection theory, it has been proposed that confidence is built from a subjective internal state (decision variable) that is influenced by sensory evidence. Confidence is then construed as the absolute distance between a decision variable and a criterion (Kepecs et al., 2008, Vickers, 1979). A similar logic underpins sequential sampling models with optional stopping (e.g., race models, drift diffusion models) in which confidence is computed from the state of the decision variable when the choice is emitted (De Martino et al., 2013, Fetsch et al., 2014, Kiani and Shadlen, 2009, Vickers, 1979). An exciting recent development of this approach suggests that confidence is also informed by parameters relating to the subject’s own actions such as response latency (Fetsch et al., 2014, Kiani et al., 2014). Our findings nuance this view by showing that OCD patients can build accurate confidence estimates based on information that correctly reflects the statistics of their environment, even when their actions are not guided by this information. This finding has broader implications that go beyond OCD. Notably, the simple fact that this population is able to build accurate confidence estimates (integrating information over time) even if actions are not driven by this information (but only by the most recent samples) speaks against a general view in which actions must be monitored in order to build accurate confidence reports. Based on these results, we therefore suggest a parallel coding scheme in which “beliefs” can independently control actions and give rise to a metacognitive sense of confidence.
The notion that confidence and performance can be dissociated is not entirely new. Recent studies have proposed that confidence, in order to become available for self-report, needs to be “read out” by an anatomically distinct network in the prefrontal cortex; during this process, information can be corrupted by further noise that does not affect action (De Martino et al., 2013, Fleming et al., 2012, Insabato et al., 2010, Maniscalco and Lau, 2012). Moreover, confidence estimates might be modified by further information processed in the time elapsing between when an action is performed and when a confidence rating is reported (Fleming, 2016, Moran et al., 2015, Navajas et al., 2016, Resulaj et al., 2009) (for a comprehensive review, see Fleming and Daw, 2017). However, all these accounts suggest that such a dissociation is driven by the fact that actions correctly reflect the information available when the choice is elicited, possibly incorporating extra information that is inaccessible to metacognitive reports of confidence (Meyniel et al., 2015). In this contribution, inspired by the distinctive ego-dystonic nature of OCD, we were able to demonstrate a novel dissociation in which actions can underutilize (or fail to access) information about the environment that is fully available to the decision maker and accessible to reports of confidence. We further suggest that a correct coupling between actions and confidence might be critical for fully functional behavior. In conclusion, we hope that this novel dissociation might help to constrain models of how beliefs are used to control behavior in the healthy brain while at the same time informing our understanding of the mechanisms underpinning OCD.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited Data | ||
| Behavioral data | This paper | https://github.com/BDMLab/Vaghi_Luyckx_et_al_2017 and https://doi.org/10.17863/CAM.13236 for raw, unprocessed behavioral data |
| Software and Algorithms | ||
| MATLAB | MathWorks | Matlab_R2015b |
| R | R Development Core Team (2008) | http://www.R-project.org. |
| Custom code (experiment, model, analyses) | This paper | https://github.com/BDMLab/Vaghi_Luyckx_et_al_2017 |
Contact for Reagent and Resource Sharing
All resources, including data and codes used for the analyses on this paper, are publicly available (see Data Software Availability and Key Resource Table). Further information and requests for resource sharing should be directed to and will be fulfilled by the Lead Contact, Matilde M. Vaghi (matilde.vaghi@gmail.com).
Experimental Model and Subject Details
Human Subjects
The study included 49 participants, consisting of 24 patients with Obsessive-Compulsive Disorder (OCD) and 25 healthy volunteers matched for gender, age, and estimated verbal IQ using the National Adult Reading Test (data from one participant were not included being Spanish mother tongue) (Table S1). Control subjects were recruited from the community; none of them were on psychiatric medication and they never suffered from a psychiatric disorder. Patients were recruited through clinical referral from local psychiatric and psychological services or local advertisement. We ensured that patients met criteria for OCD diagnosis and did not suffer from any current comorbidity. When recruitment was conducted through advertisement, a consultant psychiatrist (N.A.F. or A.S.) made DSM-5 diagnoses using an extended clinical interview, supplemented by the Mini International Neuropsychiatric Interview (Sheehan et al., 1998). Exclusion criteria for all participants were current substance dependence, head injury and current depression, indexed by Montgomery-Åsberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979) exceeding 16 during screening. OCD patients were not enrolled in the study if they scored less than 12 on the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) (Goodman et al., 1989) and if they reported hoarding symptomatology. Self-reported measures of anxiety were collected using the State-Trait Anxiety Inventory (STAI) (Spielberger, 1983); and, in addition to Y-BOCS scores, self-reported measures of OCD symptomatology were collected using the Obsessive-Compulsive Inventory-Revised (OCI-R) (Foa et al., 2002). OCD patients reported higher levels of depressive symptoms and anxiety, though well below clinical threshold (Table S1). Sixteen of the 24 patients were taking a stable dose of serotonin reuptake inhibitors (SSRIs) medication for a minimum of 8 weeks prior to taking part in the study. Eight unmedicated patients were included in the study, being either drug-naive or off medication for at least 8 weeks prior taking part of the study. Due to insufficient power, we were limited in the possibility of making direct comparison between medicated and unmedicated patients for the relevant behavioral measures. However, to test for a potential role of medication on behavioral measures of interest, and to overcome this limitation, we divided patients into 4 categories according to medication dosages considered to represent clinical equivalents. The study was approved by the NHS East of England, Cambridge Central Research Ethics Committee. Participants were reimbursed for their time and informed consent was obtained prior to participation. Participants completed two other behavioral tasks, unrelated to the present study. No statistical methods were used to pre-determine sample size but our sample sizes are similar to those generally employed in the field.
Method Details
Behavioral Task
The task consisted of a particle released from the center of a large circle, which participants were asked to catch with a bucket (orange segment in Figure 1A) placed at the edge of the circle. After they positioned the bucket, participants gave a score between 1 and 100 on how confident they were the particle would land in their bucket. The particle’s location was determined on each trial by sampling a Gaussian distribution; thus particles usually landed in the same location with small variations only determined by noise. This procedure introduced uncertainty into the estimation. The mean of this distribution usually remained stable over a block of trials but changed at random intervals (change-points) when it was resampled from a uniform distribution, thus requiring the participant to form a new belief about the mean of the new generative Gaussian distribution (Figures 1A and 1B).
A trial started with participants choosing a location for the bucket. The bucket could be moved around with the rotary controller. When a location was chosen, they confirmed by pressing the spacebar. After 150 ms a confidence bar would appear below the big circle, where participants could indicate how confident they were the particle would land in the bucket. The confidence pointer would always start on a random score between 25 and 75, so participants were forced to move the pointer even when their bucket position had not changed. The confidence pointer could be moved around with the rotary controller and a decision was confirmed by pressing the spacebar. A particle was released 150 ms after a reporting of confidence. If the particle landed within the boundaries of the bucket, the bucket would turn white for 1 frame, creating a short flash. Subsequently the center dot would turn green for 800 ms and a consonant tone was played simultaneously for 400 ms. Alternatively, when the particle missed the bucket, the center dot turned red and a dissonant tone was played for 400 ms. Catching the particle resulted in a gain of 10 points, while missing a particle resulted in a loss of 10 points, thus payment was fixed throughout the task and we did not incentivize confidence. Each block started with 0 points and their total score was the sum of points gathered by the end of each block. Payment was performance contingent: the more points participants gathered, the more money they earned at the end up, to a maximum of £ 5.
Before the start of the experiment, participants were shown the layout of the experiment while being instructed on the purpose of the experiment. Participants then completed 20 practice trials that were excluded from any analysis and did not count for their final score. The actual experiment lasted for 4 blocks of 75 particles, thus each block consisted of 75 trials (Figures 1A and 1B). There was no time limit during a trial, but participants were instructed to act as quickly and accurately as possible. In total, the full experiment lasted around 18 min.
Procedure
The experiment was programmed in MATLAB (MathWorks) using Psychtoolbox 3. Input was given through the Griffin PowerMate USB rotary controller - to comply with our circular design - and a spacebar was pressed for confirmation responses.
For each participant, particles were drawn from a normal Gaussian distribution with a low fixed standard deviation of 12. The mean of the distribution could change on any trial with a probability of 0.125 (hazard rate, change-point, Figure 1A), drawn from a uniform distribution U(1,360).
A white circle (ø = 500 px) with a dot (ø = 16 px) in the center was displayed permanently in the middle of the screen on a gray background. The so-called ‘bucket’ was designed as a portion of the circle displaced outwardly, moving along the outside border of the big circle. The bucket spanned 3 times the SD of the generative distribution, covering an area of 36 possible locations. Particles were represented by a yellow dot with a diameter of 16 pixels that flew from the center of the big circle to the edge at a speed of 30 frames per second (approx. 500 ms). A white confidence bar (w = 500px, h = 20px) appeared below the big circle, with a black pointer (w = 5 px) starting at a random location between a score of 25 and 75. The numbers 1 and 100 below the left and right corner respectively of the confidence bar indicated the range of possible scores. The points accumulated during the running block was presented in the top right corner, so participants could keep track of their performance. After each block, the total score was also displayed. Data collection and analysis were not performed blind to the conditions of the experiment. See quantification and statistical analysis for inclusion and exclusion criteria of any data.
Computational Model
Our model was an implementation of the reduced quasi-optimal Bayesian observer from McGuire et al. (2014), with slight adjustments to work with our circular data. The model, originally proposed by Nassar et al. (2010), attempts to approximate the behavior of a full Bayesian model, without the computational complexity of having to search through the full state space. It employs a simple delta rule to estimate a new belief estimate about the environment on every trial.
In our case, the model’s belief estimate Bt about the environment is equal to a point estimation of the mean of the current normal Gaussian distribution from which samples are drawn (Equation 3). The learning rate (α) (Equation 4) determines how much the new sample will influence the model’s belief estimate. If αt = 0, the model will not alter its current belief estimate at all, but when αt = 1, the most recent outcome will determine the updated belief estimate entirely.
In contrast to common reinforcement learning models where learning rate is fixed, the model employs a dynamic learning rate that can change on any trial (Equation 4). This allows the model to take into account large changes in the environment - indicating a change of location - and disregard outliers when beliefs estimates about the mean are well established. Learning rate (α) thus consists of two components that are also updated on each trial. The first component is the change-point probability (CPP) Ωt (Equation 6), which indicates the model’s suspicion that a change in location has occurred, and the second is the model confidence (MC) υt (Equation 8), which takes into account the uncertainty arising from the imprecise estimation of the mean. In other words, learning rate (α) will be high when the model assumes a change in location has occurred or when the model is uncertain about the mean.
CPP in Equation 6 is constructed as the relative likelihood that a new sample is drawn from the same Gaussian distribution (N), centered around the current belief estimate Bt of the model, or alternatively from a uniform distribution (U) over all 360 possible locations.
| Equation 6 |
H is the hazard rate, the probability that the mean of the distribution has changed. When comparing behavior to the quasi-optimal model, we set H equal to the hazard rate of the experiment (H = 0.125, but see Figures S3 and S4). CPP will be close to 1 when the probability of the sample coming from the uniform distribution is greater than the probability of it coming from the normal distribution (i.e., a surprising outcome). σ2t is the estimated variance of the predictive distribution (not to be confused with σ2N, the variance of the generative Gaussian distribution).
| Equation 7 |
This formula (Equation 7) has been validated in a previous paper (Nassar et al., 2012). It consists of two terms: the variance of the generative Gaussian distribution σ2N and the same variance but modulated by model confidence υt. Because σ2t is modulated by υt, the variance on trials immediately after a change-point will be larger than the generative variance, but will slowly decay toward the generative variance again. As a result, new samples drawn shortly after a change-point will be interpreted more conservatively, i.e., the model will be less inclined to assume a new change-point under the conditional distribution.
In contrast to the other model variables, MC in Equation 8 is computed for the subsequent trial. It takes into account the uncertainty arising from the imprecise estimation of the mean, opposed to uncertainty arising from noise (i.e., σ2N).
| Equation 8 |
Analytically model confidence is (1-RU), the additive inverse of relative uncertainty (RU), from McGuire et al. (2014). For comparison purposes with humans’ reported confidence we chose to represent this parameter as a confidence measure. The two models are mathematically identical. The first term of the nominator computes the variance when a change-point is assumed to have occurred (υt = 0.5), while the second term is conditional on no change-point (slowly decaying uncertainty). The third term of the nominator reflects a rise in uncertainty when the model is not sure whether a change-point has in fact occurred. The same three terms are repeated in the denominator, with an added variance term reflecting the uncertainty arising from noise.
Quantification and Statistical Analysis
Learning Rate Computation: Humans and Model
For each participant, on each trial, LR was computed according to the formula in Equation 1. Namely, LR corresponded to the ratio of difference between chosen bucket position (i.e., where participant positioned the bucket) from one trial to the next and the spatial prediction error (i.e., the difference between the location of particle at trial t and chosen bucket position at trial t). Therefore, LR was empirically derived for each individual subject on each trial based on the experimental data. For the quasi-optimal Bayesian model, learning rate on each trial (αt) was computed according to the formula in Equation 4. Computation of CPP was construed as the relative likelihood that a new sample is drawn from the same Gaussian distribution as shown in Equations 6 and 7. Therefore, the position of the new sample at a given trial corresponded to the empirical data (i.e., where the particle landed). The belief estimate of the model for the first trial (Bt, t = 1) was initialized as 0. MC was implemented according to Equation 8 and initialized for the first trial (υt, t = 1) as 0.5 (i.e., the same value assigned for when a change-point was assumed to have occurred). Values of the hazard rate (H = 0.125) and variance of the generative Gaussian distribution (σ2N = 12) were fixed as described in previous section.
Behavioral Analysis
All analyses were conducted in MATLAB (MathWorks) with used in-house scripts and functions and R version 3.3.1 via RStudio version 0.99.878 (https://www.r-project.org/). All statistical tests were two-sided, and parametric or nonparametric tests applied as needed according to assumptions of the specific statistical test chosen.
We excluded trials from the analysis where the estimated LR exceeded the 99th percentile (calculated separately for control and patient group) or where the spatial prediction error (i.e., = Xt-bt, Equation 2) was equal to 0. Those were respectively thought to be due to processes other than error-driven learning or provided no information about error-driven learning (Nassar et al., 2016). Consequently, we omitted 3.1% of all trials. All participants (OCD, n = 24; CTL, n = 25) were included for the statistical analyses if not reported otherwise.
All confidence measures were z-scored within subjects to make comparison between groups possible. Additional analyses were performed in order to ensure that there were no differences between the groups that may have arisen by chance. First, we computed the number of change-points occurring for each subject and compared between groups. There were no differences in the total number of change-points occurring (OCD, 42.70 ± 1.16; Controls, 41.56 ± 1.98; t47 = 0.760, p = 0.451). Second, because change-points might have occurred at any point, we identified periods of stability (periods in between change-points). There were no groups differences in number of trials occurring between consecutive change-points (OCD, 6.95 ± 0.20; Controls, 7.07 ± 0.16; t47 = 0.491, p = 0.625). Third, because position of change-points was re-drawn from a Gaussian distribution, particles at change-points might have landed on any location of the circumference. The distance between the position of the particle preceding a change-point and the position of the particle at a change-point was not different between groups (OCD, 83.39 ± 1.20; Controls, 80.41 ± 2.08; t47 = 1.228, p = 0.226). Fourth, because of the jittering during periods of stability (periods in between change-points) particles might have landed in the proximities of the mean. As expected, given the fixed standard deviation of the Gaussian distribution, there were no significant differences in the position of the particles during periods of stability (OCD, 13.64 ± 0.13; Controls, 13.74 ± 0.18; t47 = 0.406, p = 0.686).
LR was compared across groups (Figure 2A) using Welch’s two-tailed t test adjusting degrees of freedom to account for unequal variances.
For data in Figure 2B we computed, for each participant, for each trial, the error magnitude (i.e., = Xt-bt, Equation 2 corresponding to the difference between the location of particle at trial t and chosen bucket position at trial t). The distribution of the values of the spatial prediction error was divided into 20 quantiles. The MATLAB function quantile was used dividing the frequency distribution of the spatial prediction error into 20 groups (see Table S2 for mean and SEM for each quantile). As a result, the quantiles were not equally spaced but contained the same fraction of the total data distribution. For each quantile, we computed the mean LR separately in controls and patients. Only for visualization purposes, data from the first 18 quantiles are shown in Figure 2B (but see Table S2 for mean and SEM of each quantile). For statistical analysis, the distribution of the values of the spatial prediction error was divided in 3 quantiles (i.e., low, medium, and high error magnitude), data extracted for controls and OCD for each of the three quantiles, and statistical analysis performed. A mixed two factor within subject design was used to analyze the data having group as the between subject factor and magnitude of error as the within subject. “Ez” R package yielding ANOVA results and assumptions check (Mauchly’s test for sphericity, sphericity corrections and Levene’s test for homogeneity of variance) was employed. Sphericity violations were corrected using the Greenhouse-Geisser procedure. Pairwise between-groups comparisons were performed and Bonferroni correction applied.
Regression Model
For all regression models, we additionally excluded the last trial of each block, as no learning rate could be estimated for these trials. All regression models were run at the participant level and reported statistics were calculated on group level averages.
In order to estimate how much participants updated their action according to the benchmark Bayesian model, we ran a linear regression model with four regressors: (a) absolute prediction error (|δ|), (b) change-point probability , (c) model confidence (υ) and (d) hit/missed as categorical predictor. Model confidence was inserted as (1-CPP)∗(1-MC) to reflect the second component of the learning rate in Equation 4. The second and third regressors should be positive (and close to 1) if participant behavior approximates the benchmark Bayesian model. Since both components were linearly predictive of learning rate in the benchmark Bayesian model, all regressors – except PE – were implemented as interaction terms with PE. The last regressor coded whether the particle was caught or could alternatively be seen as positive feedback. This information was unavailable to the benchmark Bayesian model and thus the model would not predict any influence of this term. The dependent variable ‘action’ was constructed similar to the update formula of the quasi-optimal model, by multiplying the LR by the absolute spatial prediction error .
For reported confidence we ran a linear regression model with similar predictors, now without the interaction term with PE. All predictors were z-scored at the subject level. The dependent variable ‘confidence’ was the z-scored reported confidence provided by the participants. As confidence increases when uncertainty decreases, we expect negative beta weights for the parameters of CPP and model confidence.
Regression fits were as follows: action controls, median r2 = 0.814; action patients, median r2 = 0.846; confidence controls, median r2 = 0.152; confidence patients, median r2 = 0.110. The posterior model probability was used for Bayesian model selection (Stephan et al., 2009) among a finite set of models (Table S4; Figure S2). Wilcoxon rank-sum test was used for between-group comparisons on values of the predictors of the selected regression models (Figures 3C, 3F, and 4A).
Finally, to bring together the results of the previous two regression models, we wanted to investigate whether the link between confidence- and action-updating was in fact weakened in the patient group (Figure 4A). We therefore constructed a new regression model with the absolute confidence-update (i.e., the absolute difference between z-scored confidence reports on trial t and t-1) as the independent variable and absolute action-update (i.e., the absolute difference between where participants positioned bucket on trial t and t-1) as the dependent variable. We reasoned that if confidence and action were linked, then on trials where the participant had to adjust the position of the bucket more, confidence reports would also have to be adjusted more, irrespective of directionality (hence the absolute). Pearson’s correlation was also used to measure the association between symptom severity and strength of coupling between action control and metacognitive report of confidence (Figure 4B).
Data Software Availability
Data and Code Availability
The data and the analysis scripts are available on https://github.com/BDMLab and https://doi.org/10.17863/CAM.13236 for raw, unprocessed behavioral data.
Author Contributions
Conceptualization, M.M.V., F.L., T.W.R., and B.D.M.; Methodology, M.M.V., F.L., and B.D.M.; Model implementation, F.L.; Data acquisition, M.M.V.; Data analysis, M.M.V. and F.L.; Resources, N.A.F. and A.S.; Writing – Review and Editing, M.M.V., F.L., N.A.F., A.S., T.W.R., and B.D.M.; Supervision, B.D.M.; Funding acquisition, T.W.R.
Acknowledgments
B.D.M. was supported by Wellcome Trust and Royal Society (Sir Henry Dale Fellowship 102612/A/13/Z). T.W.R. was supported by Wellcome Trust (Senior Investigator Award 104631/Z/14/Z). M.M.V. is supported by a Pinsent Darwin Scholarship in Mental Pathology and an Angharad Dodds John Bursary in Mental Health and Neuropsychiatry. We also thank Steve Fleming for his comments on a previous draft of this article.
Published: September 28, 2017
Footnotes
Supplemental Information includes four figures and four tables and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2017.09.006.
Contributor Information
Matilde M. Vaghi, Email: matilde.vaghi@gmail.com.
Benedetto De Martino, Email: benedettodemartino@gmail.com.
Supplemental Information
References
- Behrens T.E.J., Woolrich M.W., Walton M.E., Rushworth M.F.S. Learning the value of information in an uncertain world. Nat. Neurosci. 2007;10:1214–1221. doi: 10.1038/nn1954. [DOI] [PubMed] [Google Scholar]
- Daw N.D., Niv Y., Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat. Neurosci. 2005;8:1704–1711. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- De Martino B., Fleming S.M., Garrett N., Dolan R.J. Confidence in value-based choice. Nat. Neurosci. 2013;16:105–110. doi: 10.1038/nn.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denys D., Mantione M., Figee M., van den Munckhof P., Koerselman F., Westenberg H., Bosch A., Schuurman R. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch. Gen. Psychiatry. 2010;67:1061–1068. doi: 10.1001/archgenpsychiatry.2010.122. [DOI] [PubMed] [Google Scholar]
- Endrass T., Kloft L., Kaufmann C., Kathmann N. Approach and avoidance learning in obsessive-compulsive disorder. Depress. Anxiety. 2011;28:166–172. doi: 10.1002/da.20772. [DOI] [PubMed] [Google Scholar]
- Fetsch C.R., Kiani R., Shadlen M.N. Predicting the accuracy of a decision: a neural mechanism of confidence. Cold Spring Harb. Symp. Quant. Biol. 2014;79:185–197. doi: 10.1101/sqb.2014.79.024893. [DOI] [PubMed] [Google Scholar]
- Figee M., Luigjes J., Smolders R., Valencia-Alfonso C.-E., van Wingen G., de Kwaasteniet B., Mantione M., Ooms P., de Koning P., Vulink N. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat. Neurosci. 2013;16:386–387. doi: 10.1038/nn.3344. [DOI] [PubMed] [Google Scholar]
- Fleming S.M. Changing our minds about changes of mind. eLife. 2016;5:e14790. doi: 10.7554/eLife.14790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming S.M., Daw N.D. Self-evaluation of decision-making: a general Bayesian framework for metacognitive computation. Psychol. Rev. 2017;124:91–114. doi: 10.1037/rev0000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming S.M., Huijgen J., Dolan R.J. Prefrontal contributions to metacognition in perceptual decision making. J. Neurosci. 2012;32:6117–6125. doi: 10.1523/JNEUROSCI.6489-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa E.B., Huppert J.D., Leiberg S., Langner R., Kichic R., Hajcak G., Salkovskis P.M. The Obsessive-Compulsive Inventory: development and validation of a short version. Psychol. Assess. 2002;14:485–496. [PubMed] [Google Scholar]
- Goodman W.K., Price L.H., Rasmussen S.A., Mazure C., Fleischmann R.L., Hill C.L., Heninger G.R., Charney D.S. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch. Gen. Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Haber S.N., Behrens T.E.J. The neural network underlying incentive-based learning: implications for interpreting circuit disruptions in psychiatric disorders. Neuron. 2014;83:1019–1039. doi: 10.1016/j.neuron.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser T.U., Allen M., Rees G., Dolan R.J., NSPN Consortium Metacognitive impairments extend perceptual decision making weaknesses in compulsivity. Sci. Rep. 2017;7:6614. doi: 10.1038/s41598-017-06116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D., Engelen U., Grouwels L., Joos E., Lemmens J., Pieters G. Cognitive confidence in obsessive-compulsive disorder: distrusting perception, attention and memory. Behav. Res. Ther. 2008;46:98–113. doi: 10.1016/j.brat.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Insabato A., Pannunzi M., Rolls E.T., Deco G. Confidence-related decision making. J. Neurophysiol. 2010;104:539–547. doi: 10.1152/jn.01068.2009. [DOI] [PubMed] [Google Scholar]
- Kennerley S.W., Walton M.E., Behrens T.E.J., Buckley M.J., Rushworth M.F.S. Optimal decision making and the anterior cingulate cortex. Nat. Neurosci. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- Kepecs A., Uchida N., Zariwala H.A., Mainen Z.F. Neural correlates, computation and behavioural impact of decision confidence. Nature. 2008;455:227–231. doi: 10.1038/nature07200. [DOI] [PubMed] [Google Scholar]
- Kiani R., Shadlen M.N. Representation of confidence associated with a decision by neurons in the parietal cortex. Science. 2009;324:759–764. doi: 10.1126/science.1169405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani R., Corthell L., Shadlen M.N. Choice certainty is informed by both evidence and decision time. Neuron. 2014;84:1329–1342. doi: 10.1016/j.neuron.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knill D.C., Pouget A. The Bayesian brain: the role of uncertainty in neural coding and computation. Trends Neurosci. 2004;27:712–719. doi: 10.1016/j.tins.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Kozak M.J., Foa E.B. Obsessions, overvalued ideas, and delusions in obsessive-compulsive disorder. Behav. Res. Ther. 1994;32:343–353. doi: 10.1016/0005-7967(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Maniscalco B., Lau H. A signal detection theoretic approach for estimating metacognitive sensitivity from confidence ratings. Conscious. Cogn. 2012;21:422–430. doi: 10.1016/j.concog.2011.09.021. [DOI] [PubMed] [Google Scholar]
- Marsh R., Tau G.Z., Wang Z., Huo Y., Liu G., Hao X., Packard M.G., Peterson B.S., Simpson H.B. Reward-based spatial learning in unmedicated adults with obsessive-compulsive disorder. Am. J. Psychiatry. 2015;172:383–392. doi: 10.1176/appi.ajp.2014.13121700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire J.T., Nassar M.R., Gold J.I., Kable J.W. Functionally dissociable influences on learning rate in a dynamic environment. Neuron. 2014;84:870–881. doi: 10.1016/j.neuron.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J., Shimamura A.P. The MIT Press; 1994. Knowing about Knowing. [Google Scholar]
- Meyniel F., Sigman M., Mainen Z.F. Confidence as bayesian probability: from neural origins to behavior. Neuron. 2015;88:78–92. doi: 10.1016/j.neuron.2015.09.039. [DOI] [PubMed] [Google Scholar]
- Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Moran R., Teodorescu A.R., Usher M. Post choice information integration as a causal determinant of confidence: novel data and a computational account. Cognit. Psychol. 2015;78:99–147. doi: 10.1016/j.cogpsych.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Nassar M.R., Wilson R.C., Heasly B., Gold J.I. An approximately Bayesian delta-rule model explains the dynamics of belief updating in a changing environment. J. Neurosci. 2010;30:12366–12378. doi: 10.1523/JNEUROSCI.0822-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar M.R., Rumsey K.M., Wilson R.C., Parikh K., Heasly B., Gold J.I. Rational regulation of learning dynamics by pupil-linked arousal systems. Nat. Neurosci. 2012;15:1040–1046. doi: 10.1038/nn.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar M.R., Bruckner R., Gold J.I., Li S.-C., Heekeren H.R., Eppinger B. Age differences in learning emerge from an insufficient representation of uncertainty in older adults. Nat. Commun. 2016;7:11609. doi: 10.1038/ncomms11609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navajas J., Bahrami B., Latham P.E. Post-decisional accounts of biases in confidence. Curr. Opin. Behav. Sci. 2016;11:55–60. [Google Scholar]
- O’Doherty J., Dayan P., Schultz J., Deichmann R., Friston K., Dolan R.J. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- O’Doherty J.P., Hampton A., Kim H. Model-based fMRI and its application to reward learning and decision making. Ann. N Y Acad. Sci. 2007;1104:35–53. doi: 10.1196/annals.1390.022. [DOI] [PubMed] [Google Scholar]
- O’Reilly J.X., Jbabdi S., Behrens T.E.J. How can a Bayesian approach inform neuroscience? Eur. J. Neurosci. 2012;35:1169–1179. doi: 10.1111/j.1460-9568.2012.08010.x. [DOI] [PubMed] [Google Scholar]
- Patton J.H., Stanford M.S., Barratt E.S. Factor structure of the barratt impulsiveness scale. J. Clin. Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pitman R.K. A cybernetic model of obsessive-compulsive psychopathology. Compr. Psychiatry. 1987;28:334–343. doi: 10.1016/0010-440x(87)90070-8. [DOI] [PubMed] [Google Scholar]
- Resulaj A., Kiani R., Wolpert D.M., Shadlen M.N. Changes of mind in decision-making. Nature. 2009;461:263–266. doi: 10.1038/nature08275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins T.W., Gillan C.M., Smith D.G., de Wit S., Ersche K.D. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn. Sci. 2012;16:81–91. doi: 10.1016/j.tics.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Spielberger C.D. Consulting Psychologists Press; 1983. Manual for the State-Trait Anxiety Inventory (STAI) [Google Scholar]
- Stephan K.E., Mathys C. Computational approaches to psychiatry. Curr. Opin. Neurobiol. 2014;25:85–92. doi: 10.1016/j.conb.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Stephan K.E., Penny W.D., Daunizeau J., Moran R.J., Friston K.J. Bayesian model selection for group studies. Neuroimage. 2009;46:1004–1017. doi: 10.1016/j.neuroimage.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers D. Academic Press; 1979. Decision Processes in Visual Perception. [Google Scholar]
- Yeung N., Summerfield C. Metacognition in human decision-making: confidence and error monitoring. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367:1310–1321. doi: 10.1098/rstb.2011.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A.J., Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46:681–692. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and the analysis scripts are available on https://github.com/BDMLab and https://doi.org/10.17863/CAM.13236 for raw, unprocessed behavioral data.