Abstract
The blood-brain barrier (BBB) is a major obstacle to drug delivery into the central nervous system (CNS), in particular for macromolecules such as peptides and proteins. However, certain macromolecules can reach the CNS via a receptor-mediated transcytosis (RMT) pathway, and low-density lipoprotein receptor-related protein 1 (LRP1) is one of the promising receptors for RMT. An LRP1 ligand peptide, Angiopep-2, was reported to pass through the BBB and deliver covalently conjugated drugs into the CNS. While conjugation of LRP1 ligands with drugs would be an effective approach for drug delivery to the CNS, no other reliable LRP1 ligands have been reported to date. In this study, we aimed to identify novel LRP1 ligands to further investigate LRP1-mediated RMT. Using phage display technology, we obtained a novel peptide, L57 (TWPKHFDKHTFYSILKLGKH-OH), with an EC50 value of 45 nM for binding to cluster 4 (Ser3332–Asp3779) of LRP1. L57 was stable in mouse plasma for up to 20 min. In situ brain perfusion assay in mice revealed the significantly high BBB permeability of L57. In conclusion, we discovered L57, the first artificial LRP1-binding peptide with BBB permeability. Our findings will contribute to the development of RMT-based drugs for the treatment of CNS diseases.
Abbreviations: BBB, blood brain barrier; CNS, central nervous system; LRP1, low-density lipoprotein receptor-related protein 1; RMT, receptor-mediated transcytosis
Keywords: Blood brain barrier, Low-density lipoprotein receptor-related protein 1, Peptide, Phage display, Receptor-mediated transcytosis
Highlights
-
•
The first artificial LRP1-binding peptide L57 was discovered by phage display.
-
•
L57 binds the extracellular domain of LRP1 with an EC50 binding value of 24 nM.
-
•
L57 exhibits brain uptake in in situ brain perfusion and i.v. injection in mice.
1. Introduction
Many substances are prevented from entering the central nervous system (CNS) by the blood-brain barrier (BBB), formed by brain capillary endothelial cells and pericytes, to maintain brain homeostasis. Although the BBB is a major obstacle to drug delivery into the CNS, there are several mechanisms to cross the BBB [1]. Passive diffusion is a major mechanism that facilitates the delivery of lipophilic small molecules to the CNS; however, macromolecules, such as peptides, proteins, and nucleic acids, cannot cross the BBB via this mechanism because of their hydrophilicity. Certain macromolecules, including endogenous proteins, are known to pass the BBB via adsorptive-mediated transcytosis (AMT) or receptor-mediated transcytosis (RMT), where vesicles are formed and translocated across brain endothelial cells through an endocytosis-like mechanism [2]. Several polycationic molecules, such as protamine, avidin, and albumin, have been reported to accumulate on the cellular surface to induce AMT [3]. However, non-specific adsorption of such cationic molecules may cause adverse effects.
Several receptors have been identified as receptors for RMT, including the insulin, transferrin, and leptin receptors, and low-density lipoprotein receptor-related protein 1 (LRP1). LRP1 is a large single-pass transmembrane receptor (600 kDa) that is highly expressed in the CNS, including brain endothelial cells [4]. A variety of LRP1 ligands, such as aprotinin, apolipoprotein E, lipoprotein lipase, and factor XIa, have been identified. In 2008, Demeule et al. identified Angiopep-2, a 19-amino-acid peptide (TFFYGGSRGKRNNFKTEEY-OH), by sequence alignment of aprotinin and other LRP1-binding proteins harboring a Kunitz-type domain [2], [5], [6], [7], [8], [9]. Angiopep-2 was shown to cross the BBB, and its conjugate with paclitaxel (ANG1005) is now in Phase II study for the treatment of recurrent brain metastases of pretreated breast cancer patients [7], [9]. Thus, conjugation of LRP1 ligands with drugs such as ANG1005 is an effective approach for drug delivery to the CNS. However, to date, no reliable LRP1 ligands apart from Angiopep-2 have been reported. The identification of other LRP1 ligands would be valuable for verifying the potential of LRP1-mediated RMT and a variety of ligands with different affinity for LRP-1 are expected to provide further clarification of RMT. Therefore, we are investigating LRP1-mediated RMT using other ligands to further improve drug delivery to the brain.
As targeting molecules, peptides are very attractive as they bind target proteins potently and selectively, and display technologies are available for discovering binding peptides [10]. Moreover, a variety of derivatization methods exist to ensure the generation of peptide analogs with adequate pharmacokinetic profiles; peptide analogs are prepared homogeneously by chemical synthesis.
In this study, we used the phage display technology to discover LRP1-binding peptides. This technology offers a powerful approach to finding novel artificial peptides binding to target proteins from highly diversified peptide libraries displayed on phage particles [11], [12], [13], [14]. As targeting domains, cluster 2 (Arg786–Leu1165) and cluster 4 (Ser3332–Asp3779) of LRP1 were selected because these two clusters interact with various endogenous ligands [4]. Random peptide libraries displayed on T7 phage were screened against Fc-fused LRP1(Arg786–Leu1165) (LRP1(CL2)-Fc) and Fc-fused LRP1(Ser3332–Asp3779) (LRP1(CL4)-Fc). Subsequently, in situ brain perfusion and intravenous (i.v.) administration were carried out to evaluate the BBB permeability of the novel peptides in mice.
2. Material and methods
2.1. Peptide screening by phage display
Human LRP1(CL2)-Fc (2368-L2) and human LRP1(CL4)-Fc (5395-L4) were purchased from R&D Systems (Minneapolis, MN, USA). To generate T7 phage libraries displaying random peptides, X12, X16, X20 (X is a mixture of twenty natural amino acids) mixed-oligonucleotides as template DNA were internally constructed, purified with a QIAquick PCR Purification kit (QIAGEN, Hilden, Germany), and ligated into the T7Select 10-3 vector (Merck Millipore, Darmstadt, Germany), according to the manufacturer's manual. The total library diversity was estimated to be 3.1 × 109 plaque-forming units (pfu). For the screening of T7 phage libraries, 20 μg of LRP1(CL2)-Fc or LRP1(CL4)-Fc was immobilized on Dynabeads Protein A (200 μL; Invitrogen, Carlsbad, CA, USA) in PBS (045–29795; Wako, Osaka, Japan) containing 0.5% BSA. After washing of the beads with PBS containing 0.1% Tween-20 (PBST), they were incubated with 1.0 × 1012 pfu of phage libraries (mixture of X12, X16, and X20) for 1 h at room temperature, and then washed with PBST (three times for the first round, five times for the second round, and 10 times for the third to sixth rounds). Bound phages were eluted with 200 μL of 0.5% SDS and then transfected into 80 mL of E. coli BLT5615 cells (Merck Millipore) in the log phase of growth for amplification. After bacteriolysis, the phages were recovered from the culture supernatant by centrifugation and PEG precipitation, according to the T7Select manual. The recovered phages were dissolved in 1 mL of PBS and 0.5 mL of the phages was used for the next round of biopanning.
2.2. Evaluation of phage- or synthetic peptide-binding to recombinant proteins by plate ELISA
The wells of a Nunc Maxisorp microplate (460−518) were coated with an anti-human Fc goat polyclonal antibody (10 μg/mL) (Jackson ImmunoResearch, West Grove, PA, USA) at 4 °C overnight and then blocked with 0.5% BSA in PBS at room temperature for 2 h. Fc-fused proteins (1 μg/mL) were captured by the antibody, and phage solution (1.0 × 1011 pfu/mL) or biotinylated peptide solution was added to the wells and incubated at room temperature for 1 h. After three times washing with PBST, bound phages or peptides were detected using horseradish peroxidase (HRP)-conjugated anti-T7 antibody (5000-fold dilution in PBS with 0.5% BSA) (Merck Millipore) and HRP-conjugated streptavidin (1000-fold dilution in PBS with 0.5% BSA) (Vector Laboratories Inc., Burlingame, CA, USA), respectively. The amount of HRP in each well was measured colorimetrically with the chromogenic reagent tetramethylbenzidine (Wako, Osaka, Japan). EC50 binding values were calculated with Prism 5 (GraphPad Software, La Jolla, CA, USA).
2.3. Chemical synthesis of peptides
L57 (TWPKHFDKHTFYSILKLGKH-OH), Angiopep-2, and Angiopep-7 (TFFYGGSRGRRNNFRTEEY-OH) were synthesized by fluorenylmethoxycarbonyl-based solid-phase peptide synthesis [15]. Crude peptides were purified with preparative reversed-phase (RP) high-performance liquid chromatography (HPLC) using a Kinetex® 5-µm XB-C18 column (250 × 21.10 mm I.D.) (Phenomenex Inc., Torrance, CA, USA) at a flow rate of 8 mL/min with a linear gradient of acetonitrile (MeCN)/H2O (10% increases) containing 0.1% TFA over 1 h. Peptide purity was ascertained by analytical HPLC, and structures were assigned by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry on a Bruker autoflex speed system (Bruker, Billerica, MA, USA).
2.4. Biotinylation of peptides
After Cys-L57, an L57 derivative with additional Cys at the N-terminus, was prepared and reacted with N-[2-[2-[2-[(3-maleimidopropionyl)amino]ethoxy]ethoxy]ethyl]biotinamide in N,N,-dimethylformamide, purification by preparative RP-HPLC yielded biotinylated L57. Angiopep-7 was N-terminally modified with a polyethylene glycol-based linker and biotin by solid-phase peptide synthesis. Cleavage from the resins and purification by preparative RP-HPLC yielded biotinylated Angiopep-7.
2.5. Evaluation of L57 stability in mouse plasma
An aliquot of each peptide (1 mM, 3 μL) was incubated with mouse plasma (10 μL) prepared internally from C57BL/6 J mice. After 0, 5, or 20 min of incubation at 37 °C, acetone (100 μL) was added. The mixture was stored at 4 °C for 10 min and centrifuged at 13,000 rpm at 4 °C for 10 min. The supernatant was recovered, evaporated under a nitrogen flow, and dissolved in DMSO (50 μL). The remaining amount of unmodified peptide in the sample was determined by RP-HPLC.
2.6. Preparation of radiolabeled peptides
Peptides were labeled with 125I using the lactoperoxidase-mediated iodination method. In brief, 10 μL of 1 mM peptides and 10 μL of 0.01 mg/mL lactoperoxidase (L2005; Sigma-Aldrich, St. Louis, MO, USA) were added to Na125I (74 MBq, 0.02 mL, NEZ033A; PerkinElmer, Hopkinton, MA, USA). After pipetting, 10 μL of 0.0001% H2O2 (Wako) was added and incubated for 20 min at room temperature. Labeled peptides were purified over a TSKgel® Super-ODS Column (TOSO, Tokyo, Japan) on an HPLC system (Shimadzu, Kyoto, Japan).
2.7. In situ brain perfusion in mice
In situ brain perfusion was performed as described previously [16]. Eight-week-old male C57BL/6J mice purchased from Charles River Laboratories Japan (Kanagawa, Japan) had ad libitum access to diet and tap water under controlled temperature (20–26 °C), humidity (40–70%), and a 12-h:12-h light/dark cycle (lights on 8:00–20:00). Krebs buffer containing 128 mM NaCl, 4.2 mM KCl, 0.9 mM MgCl2, 2.4 mM KH2PO4, 24 mM NaHCO3, 1.5 mM CaCl2, and 20 mM HEPES was prepared. Before an experiment, 10 mM D-glucose, 0.05% BSA, and protease inhibitors (1.5 mM E-64, 2 mM leupeptin and 1 mM pepstatin A) were added, and the perfusate was oxygenated for 10 min with 95% O2–5% CO2. Male mice anesthetized with pentobarbital at a dose of 40 mg/kg were placed on a heating pad to maintain the body temperature at 37 °C during surgery and perfusion. The right common carotid artery was exposed, and the common carotid artery and external common artery were ligated. A catheter (AMI-4; Eicom, Kyoto, Japan) was inserted into the common carotid artery and connected to an infusion pump. After severing the heart ventricle, 125I-labeled peptides dissolved in Krebs buffer with 0.1% BSA (Sigma) and 10 mM D-glucose (1.15 mL/min, 5 min) were perfused, followed by perfusion of Krebs buffer for washing (1.15 mL/min, 1 min). The right hemisphere was isolated and radioactivity was measured with an AccuFLEX (HITACHI, Tokyo, Japan). All animal experiments were conducted in accordance with the protocol reviewed by the Institutional Animal Care and Use Committee (IACUC) of Takeda Pharmaceutical Company Limited.
2.8. In vivo i.v. injection of peptides to mice
Seven- to eight-week-old male C57BL/6J mice purchased from Charles River Laboratories Japan were used. 125I-labeled peptides were dissolved in 200 μL of saline and administered intravenously. One hour after administration, the mice were anesthetized with tribromoethanol (T48402; Sigma-Aldrich) at a dose of 250 mg/kg in saline and laparotomized. Heart blood was withdrawn with a 25-G needle, and was subsequently removed by introducing saline from the left ventricle to the left atrial appendage of the heart using an infusion pump (2 mL/min × 8 min). The right hemisphere was isolated and radioactivity was measured.
2.9. Statistical analysis
Each experiment was repeated three times. Data are presented as the mean ± SD. Means were compared using Student's t-test. A p-value < 0.05 was considered significant.
3. Results
3.1. Identification of L57 by phage display screening
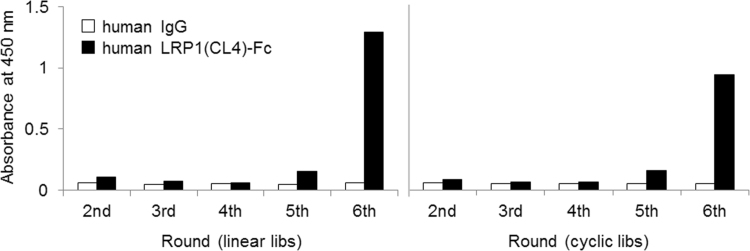
To discover artificial LRP1-binding peptides, we panned random peptide libraries displayed on T7 phage including random linear peptides or disulfide-constrained cyclic peptides against human LRP1(CL2)-Fc or human LRP1(CL4)-Fc. After six rounds of biopanning, phages binding to LRP1(CL4)-Fc were successfully concentrated in both types of libraries (Fig. 1). In contrast, phages binding to LRP1(CL2)-Fc were not enriched (data not shown). There are two possible explanations for these results: 1) no binding peptide motifs against LRP1(CL2)-Fc were included in the phage libraries, 2) no acceptable binding sites for such peptides are present on LRP1(CL2)-Fc. After binding screening of the monoclonal phages and DNA sequencing, 11 independent sequences were identified. Among them, L57 showed a three times higher concentration than the other sequences. Therefore, we selected L57 as a candidate sequence for further investigation.
Fig. 1.
Binding activity of polyclonal phages to LRP1(CL4)-Fc. Binding activity of polyclonal phages to LRP1(CL4)-Fc was evaluated by plate ELISA. Phage binding was detected with an HRP-labeled anti-T7 phage antibody and was measured by determining the absorbance at 450 nm. (A) Binding activity of polyclonal phages displaying linear peptide, and (B) binding activity of polyclonal phages displaying disulfide-constrained cyclic peptide. IgG is a counter protein.
3.2. Binding activity of biotinylated L57 to LRP1(CL4)-Fc
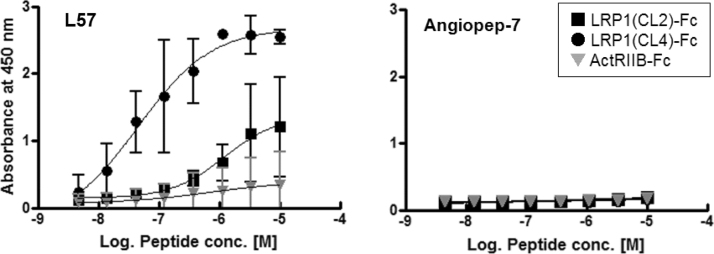
Biotinylated L57 was chemically synthesized and its binding activity to LRP1(CL4)-Fc was evaluated by plate ELISA. Biotinylated L57 was added to target protein-immobilized wells, and specific binding was detected with HRP-conjugated streptavidin. Biotinylated L57 bound to LRP1(CL4)-Fc in a concentration-dependent manner, but not to LRP1(CL2)-Fc and ActRIIB-Fc (negative control protein). The EC50 value of L57 for binding to LRP1(CL4)-Fc was calculated to be 45 nM (Fig. 2). Conversely, biotinylated Angiopep-7, an analog with Arg substitutions at positions 10 and 15 of Angiopep-2, did not bind to LRP1(CL4)-Fc. Although Angiopep-2 showed more potent binding activity to LRP1(CL2)-Fc than to LRP1(CL4)-Fc, the binding activity was not saturated even at 10 μM (data not shown). These data indicated that L57 selectively interacts with cluster 4 (Ser3332–Asp3779) of LRP1.
Fig. 2.
Binding activity of biotinylated synthetic peptides. Binding activity and selectivity of biotinylated L57 and Angiopep-7 to LRP1(CL4)-Fc were evaluated by plate ELISA. Binding activity was detected with HRP-conjugated streptavidin and was measured by determining the absorbance at 450 nm. LRP1(CL2)-Fc and ActRIIB-Fc are counter proteins. The absorbance at 450 nm of biotinylated L57 was expressed as the mean ± SD (n = 2).
3.3. Evaluation of L57 peptide stability in mouse plasma
The stability of L57 was evaluated in mouse plasma. L57 was incubated with mouse plasma at 37 °C for different periods and the remaining amount of the parent peptide was determined by RP-HPLC. By comparison with the peak area of peptide without plasma incubation, the remaining amount of the parent peptide after plasma incubation was roughly estimated. As shown in Fig. S1, L57 is stable for at least up to 20 min under the condition inducing marked degradation of a control linear octapeptide NMU8 (YFLFRPRN-NH2), which is considered to be decomposed at a rate comparable to or faster than that of the linear peptide NMU25 (complete degradation after 30-min incubation in mouse plasma) [17].
3.4. CNS delivery of 125I-radiolabeled peptides in mice
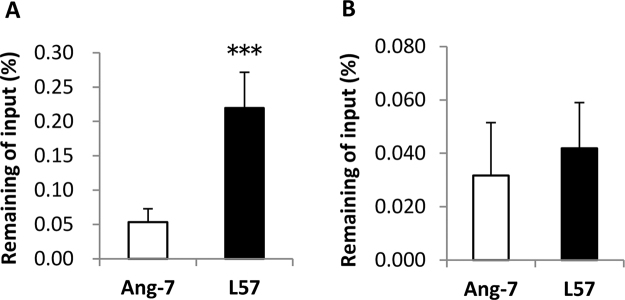
The CNS delivery of L57 was evaluated in an in situ brain perfusion assay in mice. First, CNS entry of 125I-Angiopep-2 and 125I-Angiopep-7, which have been reported to be less permeable than Angiopep-2 [8], [9], was examined. Radioisotope (RI) counts in the right brain hemisphere were measured after a 5-min cerebral perfusion of the peptides. The “% input” value represents the enrichment of radiolabeled peptides in the brain region, and was calculated as follows: % input = brain count/input count. 125I-Angiopep-2 and 125I-Angiopep-7 showed similarly low permeability across the BBB (Fig. S2 and Table S1); therefore, we used 125I-Angiopep-7 as a reference compound in subsequent tests. 125I-L57 showed higher count per minute values in the right brain hemisphere than 125I-Angiopep-7 (Fig. 3A, Table 1). The % input values also indicated the improved CNS delivery of 125I-L57 (0.220 ± 0.052%) as compared to 125I-Angiopep-7 (0.053 ± 0.019%). Next, the delivery of 125I-L57 and 125I-Angiopep-7 to the brain was examined by i.v. injection in mice. The brain uptake of 125I-L57 was equal to or slightly more efficient than that of 125I-Angiopep-7 (Fig. 3B, Table 1). Together, these results indicated that L57 has the potential to function as a targeting peptide to the brain in mice.
Fig. 3.
Brain uptake of Angiopep-7 and L57 in mice. (A) The brain uptake of 125I-labeled peptides [Angiopep-7 (Ang-7) and L57] was evaluated by in situ brain perfusion in mice. Radioisotope counts in the right brain hemisphere were measured after 5 min perfusion. (B) The brain uptake of 125I-labeled peptides (Ang-7 and L57) was evaluated after i.v. injection in mice. Radioisotope counts in the whole brain were measured 60 min post injection. Data are means + SDs (n = 6, ***p < 0.001, t-test).
Table 1.
Brain uptake of 125I-labeled peptides in mice.
| Name | Sequence | In situ brain perfusion (% input) | i.v. injection (% input) |
|---|---|---|---|
| L57 | TWPKHFDKHTFYSILKLGKH-OH | 0.220 ± 0.052*** | 0.042 ± 0.017 |
| Angiopep-7 | TFFYGGSRGRRNNFRTEEY-OH | 0.053 ± 0.019 | 0.032 ± 0.020 |
Data are means ± SDs (n = 6, t-test).
p < 0.001.
4. Discussion
Drug delivery to the brain for the treatment of CNS diseases remains challenging, although various approaches have been investigated to date. RMT through LRP1 is one of the promising mechanisms to realize CNS delivery. It is conceivable that LRP1 ligands can deliver covalently conjugated drugs into the CNS similar to ANG1005, which is the most advanced example based on RMT. In this study, we aimed to discover new LRP1 ligands and to further develop the LRP1-based drug delivery technology to the brain.
Using phage display technology, we found L57 as a novel artificial peptide binding to LRP1. L57 selectively bound to cluster 4 of LRP1 and did not show sequence homology with Angiopep-2. Recently, several studies reported that cell-penetrating peptides, such as TAT (GRKKRRQRRRPPQ) and octaarginine (RRRRRRRR), can enter the brain across the BBB [18], [19], [20]. These highly basic peptides are likely to transit via AMT, and there remains a concern about non-specific binding to negatively charged surfaces in the body. In contrast, L57 is less basic than TAT and octaarginine, indicating a low risk of non-specific binding. In addition, L57 was stable in mouse plasma up to 20 min at 37 °C, suggesting the potential of L57 for use in vivo.
The BBB permeability of L57 was significantly higher than that of Angiopep-7, which showed comparable or slightly better BBB permeability than Angiopep-2 and thus was used as a reference peptide in the in situ brain perfusion assay in mice. The difference between Lys10,15 residues in Angiopep-2 and Arg10,15 residues in Angiopep-7 was not considered to affect the BBB permeability of Angiopep peptides. Indeed, ANG1005, in which Lys10,15 residues are conjugated with paclitaxel, has been used to treat recurrent brain metastases of pretreated breast cancer patients in a Phase II study. The improved permeability of L57 indicates that L57 is a more effective carrier for CNS delivery than Angiopep-7, because in situ brain perfusion can minimize the effects of distribution to and metabolism in other organs. Biotinylated L57 bound to LRP1(CL4)-Fc more efficiently than Angiopep-7, which might explain the improved BBB permeability of L57. It has been a matter of debate whether higher binding affinity of ligands contributes to the efficiency of transcytosis [21], [22], [23]. Intracellular access of Angiopep-2 peptides has been shown to be independent of the interaction with LRP-1 in human neuroblastoma cells [24], suggesting that the BBB permeability of Angiopep-2 peptides is largely owing to receptor-independent pathways. Further investigation of the relationship between the binding affinity of L57 analogs and their BBB permeability would elucidate the potential of LRP1(CL4)-mediated transcytosis.
In the case of i.v. administration in mice, the brain delivery of L57 was slightly better than or equal to that of Angiopep-7; however, the difference was smaller than that in the in situ brain perfusion assay. This suggests that the pharmacokinetic property of L57 may be limiting BBB permeability in i.v. administration. Thus, more effective LRP1 ligands for drug delivery to the brain would be obtained by optimizing L57 (e.g., replacement with natural/unnatural amino acids, modification with polyethylene glycols or alkyl chains, and cyclization) to improve the pharmacokinetic parameters.
In conclusion, we discovered L57, the first artificial LRP1-binding peptide, and significant BBB permeability of L57 was demonstrated in mice. To further clarify the function of L57, conjugation of L57 with centrally acting agents and evaluation of physiological effects will contribute to the development of RMT-based drugs for the treatment of CNS diseases.
Author contributions
K. Sakamoto supervised phage display screening and wrote most of this paper. T. Shinohara conducted phage display screening and in situ/in vivo evaluations. Y. Adachi conducted chemical synthesis of peptides. T. Asami and T. Ohtaki supervised and supported this work.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We would like to thank Editage (www.editage.jp) for English language editing.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.07.003.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.07.003.
Appendix A. Transparency document
Supplementary material
Appendix A. Supplementary material
Supplementary material
References
- 1.Stockwell J., Abdi N., Lu S., Maheshwari O., Taghibiglou C. Novel central nervous system drug delivery systems. Chem. Biol. Drug Des. 2014;83:507–520. doi: 10.1111/cbdd.12268. [DOI] [PubMed] [Google Scholar]
- 2.Oller-Salvia B., Sánchez-Navarro M., Giralt E., Teixidó M. Blood-brain barrier shuttle peptides: an emerging paradigm for brain delivery. Chem. Soc. Rev. 2016;45:4690–4707. doi: 10.1039/c6cs00076b. [DOI] [PubMed] [Google Scholar]
- 3.Brasnjevic B., Steinbusch H.W.M., Schmitz C., Martinez-Martinez P. Delivery of peptide and protein drugs over the blood–brain barrier. Prog. Neurobiol. 2009;87:212–251. doi: 10.1016/j.pneurobio.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Spuch C., Ortolanoand S., Navarro C. LRP-1 and LRP-2 receptors function in the membrane neuron. Trafficking mechanisms and proteolytic processing in Alzheimer's disease. Front. Physiol. 2012;3:269. doi: 10.3389/fphys.2012.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demeule M., Régina A., Ché C., Poirier J., Nguyen T., Gabathuler R. Identification and design of peptides as a new drug delivery system for the brain. J. Pharmacol. Exp. Ther. 2008;324:1064–1072. doi: 10.1124/jpet.107.131318. [DOI] [PubMed] [Google Scholar]
- 6.Demeule M., Currie J.C., Bertrand Y., Ché C., Nguyen T., Régina A. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. J. Neurochem. 2008;106:1534–1544. doi: 10.1111/j.1471-4159.2008.05492.x. [DOI] [PubMed] [Google Scholar]
- 7.Régina A., Demeule M., Ché C., Lavallée I., Poirier J., Gabathuler R. Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2. Br. J. Pharmacol. 2008;155:185–197. doi: 10.1038/bjp.2008.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertrand Y., Currie J.C., Demeule M., Régina A., Ché C., Abulrob A. Transport characteristics of a novel peptide platform for CNS therapeutics. J. Cell Mol. Med. 2010;14:2827–2839. doi: 10.1111/j.1582-4934.2009.00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertrand Y., Currie J.C., Poirier J., Demeule M., Abulrob A., Fatehi D. Influence of glioma tumour microenvironment on the transport of ANG1005 via low-density lipoprotein receptor-related protein 1. Br. J. Cancer. 2011;105:1697–1707. doi: 10.1038/bjc.2011.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilad Y., Firer M., Gellerman G. Recent innovations in peptide based targeted drug delivery to cancer cells. Biomedicines. 2016;4:11. doi: 10.3390/biomedicines4020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakamoto K., Sogabe S., Kamada Y., Sakai N., Asano K., Yoshimatsu M. Discovery of high-affinity BCL6-binding peptide and its structure-activity relationship. Biochem. Biophys. Res. Commun. 2017;482:310–316. doi: 10.1016/j.bbrc.2016.11.060. [DOI] [PubMed] [Google Scholar]
- 12.Sakamoto K., Adachi Y., Komoike Y., Kamada Y., Koyama R., Fukuda Y. Novel DOCK2-selective inhibitory peptide that suppresses B-cell line migration. Biochem. Biophys. Res. Commun. 2017;483:183–190. doi: 10.1016/j.bbrc.2016.12.170. [DOI] [PubMed] [Google Scholar]
- 13.Sakamoto K., Kawata Y., Masuda Y., Umemoto T., Ito T., Asami T. Discovery of an artificial peptide agonist to the fibroblast growth factor receptor 1c/βKlotho complex from random peptide T7 phage display. Biochem. Biophys. Res. Commun. 2016;480:55–60. doi: 10.1016/j.bbrc.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Ohnishi T., Sakamoto K., Asami-Odaka A., Nakamura K., Shimizu A., Ito T. Generation of a novel artificial TrkB agonist, BM17d99, using T7 phage-displayed random peptide libraries. Biochem. Biophys. Res. Commun. 2017;483:101–106. doi: 10.1016/j.bbrc.2016.12.186. [DOI] [PubMed] [Google Scholar]
- 15.Adachi Y., Sakamoto K., Umemoto T., Fukuda Y., Tani A., Asami T. Investigation on cellular uptake and pharmacodynamics of DOCK2-inhibitory peptides conjugated with cell-penetrating peptides. Bioorg. Med. Chem. 2017;25:2148–2155. doi: 10.1016/j.bmc.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Smith Q.R., Allen D.D. In situ brain perfusion technique. Methods Mol. Med. 2003;89:209–218. doi: 10.1385/1-59259-419-0:209. [DOI] [PubMed] [Google Scholar]
- 17.Dalbøge L.S., Pedersen S.L., van Witteloostuijn S.B., Rasmussen J.E., Rigbolt K.T., Jensen K.J. Synthesis and evaluation of novel lipidated neuromedin U analogs with increased stability and effects on food intake. J. Pept. Sci. 2015;21:85–94. doi: 10.1002/psc.2727. [DOI] [PubMed] [Google Scholar]
- 18.Stalmans S., Bracke N., Wynendaele E., Gevaert B., Peremans K., Burvenich C. Cell-penetrating peptides selectively cross the blood-brain barrier in vivo. PLoS One. 2015;10:e0139652. doi: 10.1371/journal.pone.0139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou L.L., Ma J.L., Wang T., Yang T.B., Liu C.B. Cell-penetrating peptide-mediated therapeutic molecule delivery into the central nervous system. Curr. Neuropharmacol. 2013;11:197–208. doi: 10.2174/1570159X11311020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim S., Kim W.J., Kim Y.H., Lee S., Koo J.H., Lee J.A. dNP2 is a blood-brain barrier-permeable peptide enabling ctCTLA-4 protein delivery to ameliorate experimental autoimmune encephalomyelitis. Nat. Commun. 2015;6:8244. doi: 10.1038/ncomms9244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Y.J., Zhang Y., Kenrick M., Hoyte K., Luk W., Lu Y., Atwal J., Elliott J.M., Prabhu S., Watts R.J., Denni M.S. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl. Med. 2011;3:84ra44. doi: 10.1126/scitranslmed.3002230. [DOI] [PubMed] [Google Scholar]
- 22.Bien-Ly N., Yu Y.J., Bumbaca D., Elstrott J., Boswell C.A., Zhang Y., Luk W., Lu Y., Dennis M.S., Weimer R.M., Chung I., Watts R.J. Transferrin receptor (TfR) trafficking determines brain uptake of TfR antibody affinity variants. J. Exp. Med. 2014;211:233–244. doi: 10.1084/jem.20131660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanimirovic D., Kemmerich K., Haqqani A.S., Farrington G.K. Engineering and pharmacology of blood-brain barrier-permeable bispecific antibodies. Adv. Pharmacol. 2014;71:301–335. doi: 10.1016/bs.apha.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Kim J.A., Casalini T., Brambilla D., Leroux J.C. Presumed LRP1-targeting transport peptide delivers β-secretase inhibitor to neurons in vitro with limited efficiency. Sci. Rep. 2016;6:34297. doi: 10.1038/srep34297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material