Abstract
Body odours reportedly portray information about an individual's genotype at the major histocompatibility complex (MHC, called human leucocyte antigen, HLA, in humans). While there is strong experimental support for MHC-associated mating behaviour in animals, the situation in humans is more complex. A lot of effort has been spent on testing HLA-associated odour preferences of women. To date, only very few studies have looked at HLA-linked olfactory preferences in men and these studies have revealed inconsistent results. Here, we investigate men's HLA-associated preferences for women's body odours. Importantly, and in contrast to previous studies, these odours were gathered at peak fertility (i.e. just before ovulation) when any HLA-associated odour preferences should be strongest. We scrutinized whether men's preference for women's body odours is influenced by (i) the number of shared HLA alleles between men and women, (ii) HLA heterozygosity, and (iii) the frequency of rare HLA alleles. We found that men could readily differentiate between odours they found attractive and odours they found less attractive, but that these preferences were not associated with HLA. Specifically, men did not prefer odours from women who are HLA dissimilar, HLA heterozygous, or who have rare HLA alleles. Together, these findings suggest that HLA has no effect on men's odour preferences.
Keywords: major histocompatibility complex, human leucocyte antigen, mating preference, heterozygosity, olfaction
1. Introduction
Body odours reportedly portray information about an individual's genotype at the major histocompatibility complex (MHC, or human leucocyte antigen system, HLA, in humans, see [1] for a review). The MHC is a large chromosomal region containing highly polymorphic genes which play a central role in the process of adaptive immunity (cf. [2,3]). MHC-associated mating preferences can be adaptive because they may help individuals to choose mates possessing MHC genotypes that differ from their own [4], mates possessing rare alleles [5], and/or mates that are MHC heterozygous [6]. Choosing mates with dissimilar MHC alleles leads to heterozygous offspring resulting in increased resistance to infectious diseases (e.g. [7]) and helps to avoid inbreeding effects [8]. Mates with rare MHC alleles may have a selective advantage as no pathogen strain is adapted to them [9]. Choosing MHC heterozygous mates is beneficial because they might be more resistant to parasites than homozygous individuals and thus might be healthier (e.g. [6]). Here, we investigate whether men exhibit HLA-associated preferences for body odours of women. Importantly, and in contrast to previous studies, all odours were gathered during the late follicular phase of the women's menstrual cycle phase. Controlling for menstrual cycle phase is essential, because women's body odours vary significantly across the cycle [10–13].
Evidence from a range of vertebrates supports preference for MHC-dissimilar mating partners (for a recent review, see [14]) but work on human mating preferences has yielded disparate and controversial results (for reviews, see [1,15,16]).
In the current research, we investigated whether men show HLA-mediated preferences for women's body odours. To date, a lot more effort has been spent on testing MHC-linked odour preferences of women (for reviews, see [1,15,16]) and more studies that look at male preferences are needed to make generalizations for men. The few existing studies that have included men as odour raters have yielded inconclusive findings [17–20]. While some studies suggest that men show a preference for body odours of HLA-dissimilar women [19,20], others report no such preference [17,18]. Regarding preferences for body odours of individuals with rare HLA alleles, there seems to be no evidence for men preferring odours of potential mates with rare alleles [17,18]. Moreover, Thornhill et al. [19] found that men showed no preferences for the scent of HLA heterozygous women. Similarly, Kromer et al. [17] found that men with heterozygous partners did not rate their partner's body odour as being more attractive than men who have homozygous partners did. Note that Kromer et al. [17] asked couples in a romantic relationship to rate the attractiveness of their partners' body odour while the other studies reviewed here presented participants with body odour samples of strangers and asked them to rate how attractive the odour smelt.
A common shortcoming of previous studies is that none of them controlled for the menstrual cycle phase during which the body odours were collected. Controlling for menstrual cycle phase during which odour collection takes place is important because body odour varies across the cycle. An increasing number of studies report that women's body odour is rated as more attractive if gathered near ovulation compared with odour that was collected in other cycle phases [10–13]. In fact, a recent study suggests that approximately 25% of the total variance in women's odour pleasantness and intensity is accounted for by the ovulatory cycle [21].
Further methodological issues to consider in HLA studies include the number of odour donors, the number of odour raters, and the number of HLA loci that are typed. Most previous studies have included a small number of female odour donors and a small sample of male raters. Regarding the number of HLA loci, most previous studies have typed their participants at only three from a total of the nine classical HLA loci (HLA-A, HLA-B, and HLA-DR [19,20,22–24]) or at only two loci (HLA-A, HLA-B [18]).
The current research aimed to address the methodological limitations of previous work in order to offer a more conclusive view on men's preferences of women's body odours. A crucial improvement of the present study is that we not only controlled for cycle phase but specifically targeted odour collection to happen during the late follicular cycle phase (i.e. at peak fertility). Specifically, we collected odours of 49 women during the late follicular phase of their cycle (i.e. just before ovulation). Menstrual cycle monitoring was completed by using OvaCUE© devices to determine the fertile window and ovulation was confirmed with tests of luteinizing hormone (LH). Odour donors and raters were typed at the six loci that show the greatest variability [25], three at Class I (HLA-A, HLA-B, and HLA-C) and three at Class II (HLA-DRB1, HLA-DQA1, and HLA-DQB1). This enabled us to test whether putative HLA effects are locus specific [26]. By improving the methodology used in previous studies, we attempt to determine more conclusively the effect of HLA on men's preferences for women's body odour. Specifically, we investigated whether (i) the number of shared HLA alleles between men and women, (ii) the frequency of rare HLA alleles, and (iii) the HLA heterozygosity of women influenced men's preference for women's odours.
2. Methods
(a). Participants
Initially, 49 women (odour donors, mean age = 23.27, s.d. = 3.80) and 96 men (odour raters, mean age = 23.41, s.d. = 3.71) agreed to participate in this study. The final sample size consisted of 94 male raters and 42 female odour donors (see below and flow chart in the electronic supplementary material 1D). All participants were Caucasian and of European descent (at least back to their grandparents). All participants gave written informed consent prior to participating.
(i). Odour donors
Forty-nine women participated as odour donors. All donors were selected on the basis of the following inclusion criteria: (i) aged between 17 and 40 years, (ii) medication-free (including hormonal contraception for at least three previous months), (iii) regular menstrual cycle (average length of between 25 and 35 days), (iv) not pregnant or breastfeeding, and (v) non-smoker. Women received 140 Swiss Francs (CHF) for their participation.
(ii). Odour raters
Of the 96 men initially interested in taking part in this study, 94 participated as odour raters. All raters were non-smokers. Sample size was determined using G-Power [27]. To detect a conservatively medium effect size of d = 0.3 [19] with an alpha-error probability of 0.05 and power of 0.8, the required sample size is N = 90. Men received 60 CHF for their participation.
(b). Blood sampling procedure
Eligible participants (49 women and 96 men) were invited to the laboratory for venous blood sampling. Before blood sampling, participants read the study information and gave written informed consent. The participants' blood samples (10 ml) were genotyped for HLA-class I (HLA-A, HLA-B, and HLA-C) and class II (HLA-DRB1, HLA-DQA1, and HLA-DQB1) using LinkSēq™ test kits (Linkage Biosystems™). These test kits are based on real-time polymerase chain reaction (PCR) using allele-specific exponential amplification (sequence-specific primers). The resulting amplimers were subjected at endpoint to a melting curve analysis to identify specific DNA based on melting temperature using SYBR® Green. Attribution of HLA genotypes was performed using SureTyper™ software. Ambiguities were resolved using alternative typing methods via routine HLA-typing.
(c). Odour collection procedure
All odour donors (all female) were initially screened in a telephone interview for the required inclusion and exclusion criteria. In the same telephone interview, we also collected demographic information and information about their menstrual cycle (regularity, length, and onset of last menstrual bleeding).
(i). Scheduling female odour collection procedures
We used OvaCUE© fertility monitors to predict high fertility days for odour collection (cf. electronic supplementary material 1B). One day before the date of predicted ovulation, the participants collected body odour using cotton axillary pads (see §2c(ii)).
We verified that odour collection took place near ovulation by having participants complete a series of urine tests measuring the LH using one-step urine ovulation tests with a reported LH sensitivity of 10 mlU ml−1 (David One Step Ovulation Tests, Runbio Biotech, China, http://www.runbio-bio.com). Women were instructed to complete the urine test twice a day (morning and evening) starting 1 day before the date of predicted ovulation. After a positive test result, participants continued performing the tests until the results became negative for two subsequent days. Participants photographed each test using their smart phones and sent the picture to the study manager, who verified whether the test was positive or not.
(ii). Odour collection
The odour collection procedure is described in detail in electronic supplementary material 1A. In brief, odour donors (all female) followed a strict schedule of dietary and behavioural restrictions while collecting their body odour. Odours were collected from both armpits using axillary pads (100% cotton). Women collected body odour on three consecutive nights using new pads every night. Cotton pads were returned to the laboratory in sealable plastic bags. On delivery, pads were frozen at −30°C until use. Previous studies have shown that freezing has no significant effect on attractiveness ratings [23,28].
(iii). Compliance interview and dropouts
When returning their body odour samples to the laboratory, donors were asked a series of questions in a structured face-to-face interview, assessing how long they had worn their axillary pads, whether they had complied with the dietary and behavioural restrictions (see electronic supplementary material 1C). We followed and slightly adapted the structured interview used by Gildersleeve et al. [21].
Of a total of 49 women initially participating in the study, two women withdrew without giving a reason, two women took medication during the odour collection, two women did not show evidence of an LH-surge, and one woman violated behavioural restrictions (i.e. slept in the same bed as her partner during odour collection). These seven women were excluded from all analyses. Additionally, we excluded four pads because odour collection took place more than 2 days after the LH-surge and two pads because women violated behaviour restriction (i.e. did not take a shower before wearing the pads). The final sample of odour donors consisted of 42 women and a total of 120 odour pads (see flow chart in the electronic supplementary material, 1D for an overview of the donors who dropped out and the reasons for non-participation at each stage).
(d). Odour rating procedure
We closely followed the procedures reported in [23]. Axillary pads from each female donor were thawed 3 h before the test and were placed in a 500 ml opaque glass jar. Three research assistants smelled the pads and confirmed that none was contaminated with extraneous odours (e.g. perfume and smoke). Only left-arm odour samples were used in the present study, in order to control for potential effects of body side [29].
To prepare for the rating session, odour raters (all male) were asked to refrain from eating and drinking caffeinated or alcoholic beverages for 1 h prior to testing, as these activities are known to affect smelling ability. To assess the odour preferences, we closely followed the procedures reported in [24,30]. Odour raters rated the body odour of eight pads. For each rater, we pre-selected four HLA similar and four HLA dissimilar body odour pads (see §2e). The order of pads was randomized for each rater. Odour raters were asked to rate the female body odour samples on a visual analogue scale (0–100) for (i) attractiveness, (ii) pleasantness, and (iii) intensity. If a rater found any of the samples too weak to asses, he was asked to select ‘I cannot smell the sample’ instead of using the rating scales; these samples were not included in further analysis. Sniffing time was not restricted. After assessing the odour of a pad, raters were asked to sniff at a neutral pad to neutralize the nose.
(e). Human leucocyte antigen-genetics
To test the first hypothesis that HLA dissimilarity has an effect on odour attractiveness or pleasantness, we pre-selected four HLA similar and four HLA dissimilar body odour pads. To do so, we first calculated an HLA similarity-index for each rater-donor pair. This index was primarily based on the three most investigated loci in human studies (HLA-A, HLA-B, and HLA-DR [19,20,22–24]) and secondarily on all loci samples (HLA-A, HLA-B, HLA-C, HLA-DRB1, HLA-DQA1, HLA-DQB1). For details, see the electronic supplementary material 2. In a second step, for each rater we pre-selected four HLA-dissimilar female donors and four HLA-similar female donors. For each male rater, we chose as HLA-dissimilar donors those with the lowest value of HLA similarity and as HLA-similar donors those with the highest value of HLA similarity. On average, male raters shared 0.88 alleles with HLA-dissimilar female donors (s.d. = 0.77) and 6.25 alleles with the HLA-similar female donors (s.d. = 1.53) over all the six HLA loci. These numbers compare nicely with the shared alleles in other studies (e.g. [23,24]).
To test the second hypothesis that rare alleles are positively correlated with odour attractiveness and pleasantness, we calculated the mean allele frequency of HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1 based on a Berne population (N = 3,545 retrieved from http://www.allelefrequencies.net/, see the electronic supplementary material 3 for details on the filters used, frequencies of all alleles within the sample, and how these compare with the European, German, and Berne population).
To test the third hypothesis that HLA heterozygosity positively influences odour pleasantness and attractiveness, we calculated the measure of heterozygosity. For this, we calculated a continuous measure of homozygosity by adding up for each donor the number of alleles that were homozygous.
3. Results
(a). Descriptive statistics
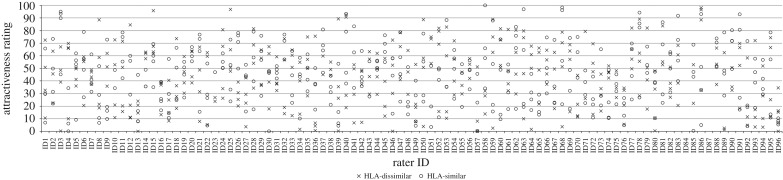
A total of 752 (94 × 8) ratings were completed. Of these, 37 (4.96%) were rated as not perceivable and six (0.8%) had to be discarded because data were not recorded due to technical problems. Furthermore, we excluded 21 ratings of pads which ex-post were found to be sampled more than 2 days after peak of the LH. This was done to ensure that all odour pads that were rated in this study were collected at high fertility. The mean attractiveness rating was 43.67 (s.d. = 23.47), mean pleasantness ratings were 42.92 (s.d. = 24.45), and mean intensity ratings were 49.65 (s.d. = 25.12). Odour attractiveness was strongly correlated with ratings of pleasantness (r = 0.89, n = 688, p < 0.001). Both attractiveness and pleasantness were equally and negatively associated with perceived odour intensity (r's > −0.28, n = 688, p's < 0.001). The results of the attractiveness ratings are shown in figure 1 (for results of the pleasantness ratings, see electronic supplementary material 4A, figure S2). As can be seen in figure 1, every rater showed a great variance in his attractiveness ratings (mean range = 56.4) suggesting that men could readily differentiate between more and less attractive odours.
Figure 1.
Attractiveness ratings for HLA-similar (circles) and HLA-dissimilar (cross symbols) female body odours.
(b). Preferences for human leucocyte antigen dissimilarity
We analysed the mean scores given to the four HLA-similar and -dissimilar women using paired t-tests (cf. [23,24]). We found no evidence that men preferred women's odours with dissimilar HLA genotypes (attractiveness: M = 43.35, s.d. = 13.36; pleasantness: M = 42.45, s.d. = 12.25) compared to women's odour with more similar HLA genotypes (attractiveness: M = 43.78, s.d. = 15, t93 = −0.23, p = 0.813; pleasantness: M = 43.24, s.d. = 15.68, t93 = −0.40, p = 0.694). Furthermore, there were no differences in intensity between women's odour with dissimilar HLA genotypes compared to women's odour with more similar MHC genotypes (t93 = −0.56, p = 0578).
To test whether odour preferences might be moderated by the rater's relationship status, we calculated a repeated measure analysis of variance with HLA similarity (dissimilar versus similar) as within-subject factor and relationship status (single versus in a relationship) as between-subjects factor, revealing no significant main effect of MHC similarity (F1,89 = 0.01, p = 0.905, 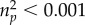 ) or relationship status (F1,89 = 0.26, p = 0.611,
) or relationship status (F1,89 = 0.26, p = 0.611, 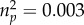 ). Furthermore, there was no significant interaction between HLA similarity and relationship status (F1,89 = 1.21, p = 0.275,
). Furthermore, there was no significant interaction between HLA similarity and relationship status (F1,89 = 1.21, p = 0.275, 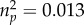 ).
).
To test for potential locus-specific effects [26], we correlated individuals' ratings of attractiveness with the number of HLA alleles shared at each HLA locus, separately for each odour pad. Adopting the procedure used by Wedekind & Furi [20] and Thornhill et al. [19], we then tested whether these correlations differed significantly from zero in the predicted negative direction. Again, we found no evidence that men prefer the odour of HLA-dissimilar women at the loci HLA-A, HLA-B, HLA-C, HLA-DRB1, HLA-DQA1, or HLA-DQB1 (mean −0.04 > r < 0.07; t's < 1.54, p > 0.126).
(c). Preferences for rare human leucocyte antigen alleles
We examined preferences for rare HLA alleles by correlating the mean allele frequency at each HLA-locus (i.e. HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1) and odour ratings. Because individual men varied considerably in their use of the rating scale and each man smelled only a subsample of the total sample of women, we used z-transformed scores rather than raw scores. We found no association between the rareness of the women's alleles and men's ratings of women's odour attractiveness, pleasantness, or intensity (attractiveness: r = 0.057, p = 0.718; pleasantness: r = 0.058, p = 0.713). To enable a comparison with other studies [19,20], we calculated the mean frequency of HLA-A, HLA-B, and HLA-DRB1 alleles and again found no relationship between ratings and the rareness of HLA alleles (attractiveness: r = 0.091, p = 0.566; pleasantness: r = 0.096, p = 0.545). The results are displayed in the electronic supplementary material 4B.
(d). Preferences for human leucocyte antigen heterozygosity
Again, we used z-transformed scores rather than raw scores. We found no significant correlation between any of the rating measures and the number of women's heterozygous alleles (attractiveness ratings: r = 0.02, p = 0.902; pleasentness ratings: r = 0.07, p = 0.674; intensity ratings: r = −0.11, p = 0.480). We also found no significant correlation when we analysed the preferences for HLA heterozygosity at the three most studied HLA loci (HLA-A, HLA-B, and HLA-DRB1; attractiveness ratings: r = 0.07, p = 0.667; pleasentness ratings: r = 0.13, p = 0.430; intensity ratings: r = −0.11, p = 0.478).
4. Discussion
Motivated by conflicting evidence in the literature, we reinvestigated the effects of HLA on men's preferences for women's body odours. A crucial improvement of the present study in comparison with earlier work is that we collected women's body odours during the late follicular phase of their menstrual cycle. Controlling women's fertility status is essential, because previous studies have demonstrated that men rate the scent of women as being most attractive during the fertile window. Hence, if not controlled, cycle phase might override any HLA-mediated odour preferences. In this study, we not only controlled for menstrual cycle phase, but in fact also targeted odour collection to take place during the late follicular phase. In a mating context, the late follicular phase is the most relevant phase of the menstrual cycle, since only then women can conceive. Any putative HLA-mediated mating preference should be most pronounced when the chances of reproduction are highest, because only then any selective benefits of preferring a mate with advantageous HLAs can be effective. A further improvement is that the present study was based on ratings of a large number of raters and on a large sample of odours. Finally, we typed our participants at six HLA loci (compared to only three or two loci). We found no evidence that men prefer odours of HLA-dissimilar women, odours of HLA-heterozygous women, or women with rare HLA alleles. Overall, these results can be taken as strong evidence that HLA plays an insignificant role in men's preferences for women's body odours.
Preferring individuals with dissimilar HLA alleles might be adaptive because it would produce offspring with higher MHC variability, which in turn would increase resistance to infectious diseases. Hence, our first hypothesis was that men prefer odours of HLA-dissimilar women. Our results suggest that this is not the case: HLA dissimilarity is not taken into account when judging the preference for women's body odours. This finding is in contrast to studies reporting a positive effect of HLA dissimilarity on men's preferences for female odour [19,20]. Instead, our findings add to the literature suggesting that HLA dissimilarity is not related to men's preferences for female body odours [18] or to men's rating of partner odour pleasantness [17]. Interestingly, men were indeed capable of discriminating between the odours of different women: some odours were clearly rated as being more attractive than others. The men used most of the range provided by the scale to rate the attractiveness of the odours. However, these preferences were not mediated by HLA similarity.
Our second hypothesis alludes to the notion that odours of heterozygous individuals should be preferred because individuals with heterozygous HLA might be more resistant to parasites than homozygous individuals and thus might be healthier (e.g. [6]). We hence expected that odours of HLA-heterozygous individuals should be preferred over odours of HLA-homozygous individuals. Again, this hypothesis was not supported by the data: we found no indication for a preference for odours of HLA-heterozygous women. This finding is consistent with the results of other studies suggesting no effect of HLA heterozygosity on men's odour preferences [19].
Finally, we hypothesized a general preference for odours of women with rare HLA alleles, because mates with rare MHC alleles may have a selective advantage as no pathogen strain is adapted to them [9] and should hence increase resistance to infectious diseases in offspring. Consistent with previous findings [17,19,20], we found no evidence that men prefer odours of women with rare HLA alleles.
Studies from across the animal kingdom suggest that females rely more strongly on MHC-mediated mating preferences than males ([31–34], for a review, see [15]). Because males have a higher potential reproductive rate [35] and females usually bear greater reproductive costs [36], males might seek females with high perceived fertility to increase the probability for reproduction, while women seek males who are most likely to maximize offspring survival. In the present study, we included only men as odour raters and only women as odour donors. It is plausible that HLA might have a more pronounced effect on women's preferences for men's body odour than the other way round. It will have to be the aim of future studies using similarly comprehensive methodology to assert whether this is the case.
In sum, while previous studies investigating the effects of HLA on body odour preferences in humans have yielded contradictory findings, the present results suggest that HLA does not affect men's preferences for women's body odours. Because of the rigorous methodology used, this study is perhaps the most conclusive to suggest that HLA plays an insignificant role in men's preferences for women's body odours.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Peggy Kübler for her organizational support at every stage of this work, Susanne Kanzler, Rahel Gross, Amina Bachmann, and Sarah Calmonte for their assistance in recruiting and testing participants. We are grateful to Jitka Fialova for helpful input on the study procedure. We thank Sandra Augsburger, Andrea Odermatt, and Annalea Klainguti for HLA-typing and Kelly Gildersleeve for sharing an unpublished study protocol.
Ethics
The study was approved by the Ethics Committee of the Canton of Bern (KEK-no.: 242/15) and was conducted according to the principles expressed in the Declaration of Helsinki.
Data accessibility
The dataset used for this manuscript is available from the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.270h8) [37].
Authors' contributions
F.P., U.F., J.S.L., and D.K. participated in the study design; U.W. performed HLA typing; F.P. collected behavioural data; F.P. and U.F. analysed the data; F.P., J.S.L., and D.K. wrote the manuscript; U.F. und U.W. provided helpful input on manuscript drafts.
Competing interests
All authors gave final approval for publication and have no competing interests.
Funding
This work was supported by a grant from the Typhaine Foundation awarded to D.K. F.P. was supported by a grant from the Mens Sana Foundation awarded to D.K. and a grant from the Swiss National Science Foundation awarded to J.S.L. (grant no. PP00P1_163758/1).
References
- 1.Havlicek J, Roberts SC. 2009. MHC-correlated mate choice in humans: a review. Psychoneuroendocrinology 34, 497–512. ( 10.1016/j.psyneuen.2008.10.007) [DOI] [PubMed] [Google Scholar]
- 2.Klein J. 1986. Natural history of the histocompatibility complex. New York, NY: Wiley. [Google Scholar]
- 3.Reche PA, Reinherz EL. 2003. Sequence variability analysis of human class I and class II MHC molecules: functional and structural correlates of amino acid polymorphisms. J. Mol. Biol. 331, 623–641. ( 10.1016/S0022-2836(03)00750-2) [DOI] [PubMed] [Google Scholar]
- 4.Neff BD, Pitcher TE. 2005. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 14, 19–38. ( 10.1111/j.1365-294X.2004.02395.x) [DOI] [PubMed] [Google Scholar]
- 5.Penn DJ, Potts WK. 1999. The evolution of mating preferences and major histocompatibility complex genes. Am. Nat. 153, 145–164. ( 10.1086/303166) [DOI] [PubMed] [Google Scholar]
- 6.Penn DJ, Damjanovich K, Potts WK. 2002. MHC heterozygosity confers a selective advantage against multiple-strain infections. Proc. Natl Acad. Sci. USA 99, 11 260–11 264. ( 10.1073/pnas.162006499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milinski M. 2006. The major histocompatibility complex, sexual selection, and mate choice. Annu. Rev. Ecol. Evol. Syst. 37, 159–186. ( 10.1146/annurev.ecolsys.37.091305.110242) [DOI] [Google Scholar]
- 8.Potts WK, Wakeland EK. 1993. Evolution of MHC genetic diversity: a tale of incest, pestilence and sexual preference. Trends Genet. 9, 408–412. ( 10.1016/0168-9525(93)90103-O) [DOI] [PubMed] [Google Scholar]
- 9.Slade RW, McCallum HI. 1992. Overdominant vs. frequency-dependent selection at MHC loci. Genetics 132, 861–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerda-Molina AL, Hernandez-Lopez L, de la OCE, Chavira-Ramirez R, Mondragon-Ceballos R. 2013. Changes in men's salivary testosterone and cortisol levels, and in sexual desire after smelling female axillary and vulvar scents. Front. Endocrinol. 4, 159 ( 10.3389/fendo.2013.00159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gildersleeve KA, Haselton MG, Larson CM, Pillsworth EG. 2012. Body odor attractiveness as a cue of impending ovulation in women: evidence from a study using hormone-confirmed ovulation. Horm. Behav. 61, 157–166. ( 10.1016/j.yhbeh.2011.11.005) [DOI] [PubMed] [Google Scholar]
- 12.Havlicek J, Dvorakova R, Bartos L, Flegr J. 2006. Non-advertized does not mean concealed: body odour changes across the human menstrual cycle. Ethology 112, 81–90. ( 10.1111/j.1439-0310.2006.01125.x) [DOI] [Google Scholar]
- 13.Singh D, Bronstad PM. 2001. Female body odour is a potential cue to ovulation. Proc. R. Soc. Lond. B 268, 797–801. ( 10.1098/rspb.2001.1589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamiya T, O'Dwyer K, Westerdahl H, Senior A, Nakagawa S. 2014. A quantitative review of MHC-based mating preference: the role of diversity and dissimilarity. Mol. Ecol. 23, 5151–5163. ( 10.1111/mec.12934) [DOI] [PubMed] [Google Scholar]
- 15.Winternitz J, Abbate JL, Huchard E, Havlicek J, Garamszegi LZ. 2017. Patterns of MHC-dependent mate selection in humans and nonhuman primates: a meta-analysis. Mol. Ecol. 26, 668–688. ( 10.1111/mec.13920) [DOI] [PubMed] [Google Scholar]
- 16.Winternitz J, Abbate JL. 2015. Examining the evidence for major histocompatibility complex-dependent mate selection in humans and nonhuman primates. Res. Rep. Biol. 6, 73–88. [Google Scholar]
- 17.Kromer J, Hummel T, Pietrowski D, Giani AS, Sauter J, Ehninger G, Schmidt AH, Croy I. 2016. Influence of HLA on human partnership and sexual satisfaction. Sci. Rep. 6, 32550 ( 10.1038/srep32550) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos PSC, Schinemann JA, Gabardo J, Bicalho MD. 2005. New evidence that the MHC influences odor perception in humans: a study with 58 Southern Brazilian students. Horm. Behav. 47, 384–388. ( 10.1016/j.yhbeh.2004.11.005) [DOI] [PubMed] [Google Scholar]
- 19.Thornhill R, Gangestad SW, Miller R, Scheyd G, McCollough JK, Franklin M. 2003. Major histocompatibility complex genes, symmetry, and body scent attractiveness in men and women. Behav. Ecol. 14, 668–678. ( 10.1093/beheco/arg043) [DOI] [Google Scholar]
- 20.Wedekind C, Furi S. 1997. Body odour preferences in men and women: do they aim for specific MHC combinations or simply heterozygosity? Proc. R. Soc. Lond. B 264, 1471–1479. ( 10.1098/rspb.1997.0204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gildersleeve KA, Fales MR, Haselton MG. 2017. Women's evaluations of other women's natural body odor depend on target's fertility status. Evol. Hum. Behav. 38, 155–163. ( 10.1016/j.evolhumbehav.2016.08.003) [DOI] [Google Scholar]
- 22.Jacob S, McClintock MK, Zelano B, Ober C. 2002. Paternally inherited HLA alleles are associated with women's choice of male odor. Nature Genet 30, 175–179. ( 10.1038/ng830) [DOI] [PubMed] [Google Scholar]
- 23.Roberts SC, Gosling LM, Carter V, Petrie M. 2008. MHC-correlated odour preferences in humans and the use of oral contraceptives. Proc. R. Soc. B 275, 2715–2722. ( 10.1098/rspb.2008.0825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wedekind C, Seebeck T, Bettens F, Paepke AJ. 1995. MHC-dependent mate preferences in humans. Proc. R. Soc. Lond. B 260, 245–249. ( 10.1098/rspb.1995.0087) [DOI] [PubMed] [Google Scholar]
- 25.Robinson J, Halliwell JA, Hayhurst JH, Flicek P, Parham P, Marsh SGE. 2015. The IPD and IPD-IMGT/HLA Database: allele variant databases. Nucleic Acids Res. 43, D423–D431. ( 10.1093/nar/gku1161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hedrick PW, Black FL. 1997. HLA and mate selection: No evidence in South Amerindians. Am. J. Hum. Genet. 61, 505–511. ( 10.1086/515519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faul F, Erdfelder E, Lang AG, Buchner A. 2007. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191. ( 10.3758/BF03193146) [DOI] [PubMed] [Google Scholar]
- 28.Lenochova P, Roberts SC, Havlicek J. 2009. Methods of human body odor sampling: the effect of freezing. Chem. Senses 34, 127–138. ( 10.1093/chemse/bjn067) [DOI] [PubMed] [Google Scholar]
- 29.Ferdenzi C, Schaal B, Roberts SC. 2009. Human axillary odor: are there side-related perceptual differences? Chem. Senses 34, 565–571. ( 10.1093/chemse/bjp037) [DOI] [PubMed] [Google Scholar]
- 30.Roberts SC, Little AC, Gosling LM, Perrett DI, Carter V, Jones BC Penton-Voak I, Petrie M. 2005. MHC-heterozygosity and human facial attractiveness. Evol. Hum. Behav. 26, 213–226. ( 10.1016/j.evolhumbehav.2004.09.002) [DOI] [Google Scholar]
- 31.Neff BD, Garner SR, Heath JW, Heath DD. 2008. The MHC and non-random mating in a captive population of Chinook salmon. Heredity (Edinb) 101, 175–185. ( 10.1038/hdy.2008.43) [DOI] [PubMed] [Google Scholar]
- 32.Egid K, Brown JL. 1989. The major histocompatibility complex and female mating preferences in mice. Anim. Behav. 38, 548–550. ( 10.1016/S0003-3472(89)80051-X) [DOI] [Google Scholar]
- 33.Eklund A. 1997. The major histocompatibility complex and mating preferences in wild house mice (Mus domesticus). Behav. Ecol. 8, 630–634. ( 10.1093/beheco/8.6.630) [DOI] [Google Scholar]
- 34.Huchard E, Knapp LA, Wang J, Raymond M, Cowlishaw G. 2010. MHC, mate choice and heterozygote advantage in a wild social primate. Mol. Ecol. 19, 2545–2561. ( 10.1111/j.1365-294X.2010.04644.x) [DOI] [PubMed] [Google Scholar]
- 35.Clutton-Brock TH, Parker GA. 1992. Potential reproductive rates and the operation of sexual selection. Q. Rev. Biol. 67, 437–456. ( 10.1086/417793) [DOI] [Google Scholar]
- 36.Trivers R. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man (ed. Campbell B.), pp. 136–179. Chicago, IL: Aldine-Atherton. [Google Scholar]
- 37.Probst F, Fischbacher U, Lobmaier JS, Wirthmüller U, Knoch D. 2017. Data from: Men's preferences for women's body odours are not associated with human leucocyte antigen Dryad Digital Repository. ( 10.5061/dryad.270h8) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Probst F, Fischbacher U, Lobmaier JS, Wirthmüller U, Knoch D. 2017. Data from: Men's preferences for women's body odours are not associated with human leucocyte antigen Dryad Digital Repository. ( 10.5061/dryad.270h8) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The dataset used for this manuscript is available from the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.270h8) [37].