ABSTRACT
We previously reported a novel phenotype of vancomycin-intermediate Staphylococcus aureus (VISA), i.e., “slow VISA,” whose colonies appear only after 72 h of incubation. Slow-VISA strains can be difficult to detect because prolonged incubation is required and the phenotype is unstable. To develop a method for detection of slow-VISA isolates, we studied 23 slow-VISA isolates derived from the heterogeneous VISA (hVISA) clinical strain Mu3. We identified single nucleotide polymorphisms (SNPs) in genes involved in various pathways which have been implicated in the stringent response, such as purine/pyrimidine synthesis, cell metabolism, and cell wall peptidoglycan synthesis. We found that mupirocin, which also induces the stringent response, caused stable expression of vancomycin resistance. On the basis of these results, we developed a method for detection of slow-VISA strains by use of 0.032 μg/ml mupirocin (Yuki Katayama, 7 March 2017, patent application PCT/JP2017/008975). Using this method, we detected 53 (15.6%) slow-VISA isolates among clinical methicillin-resistant S. aureus (MRSA) isolates. In contrast, the VISA phenotype was detected in fewer than 1% of isolates. Deep-sequencing analysis showed that slow-VISA clones are present in small numbers among hVISA isolates and proliferate in the presence of vancomycin. This slow-VISA subpopulation may account in part for the recurrence and persistence of MRSA infection.
KEYWORDS: HA-MRSA, MRSA, hVISA, mupirocin, ppGpp, recurrent infection, slow VISA, stringent response, tolerance, vancomycin resistance
INTRODUCTION
Two major issues with vancomycin as a treatment for severe methicillin-resistant Staphylococcus aureus (MRSA) infections, including bacteremia, are treatment failure (1–3) and recurrent infection. The role of bacterial factors in poor outcomes has not been well defined. To address these issues, we have been focusing on the possible involvement of a novel phenotype, namely, slow-growing vancomycin-intermediate S. aureus (“slow VISA”). Slow-VISA strains have the following three major growth properties: (i) a low growth rate, (ii) resistance to concentrations of vancomycin higher than those resisted by other extant clinical strains of VISA (MIC ≥ 8 μg/ml), and (iii) unstable resistance and colony morphology. rpoB or rpoC mutations can produce the slow-VISA phenotype (4, 5).
Detection of slow-VISA and heterogeneous VISA (hVISA) strains (6) is problematic. The vancomycin MICs of hVISA are, by definition, less than 4 μg/ml (the susceptibility breakpoint for VISA), and thus hVISA is classified as vancomycin-susceptible S. aureus (VSSA) by MIC testing. Nonetheless, hVISA is distinguishable from VSSA by the presence of cells resistant to 4 μg/ml vancomycin within the cell population, at a frequency of 1 × 10−6 or greater. hVISA is thought to be an intermediate step in the development of resistance from VSSA to VISA. hVISA and slow-VISA cells may also lose their resistance in the process of repeated isolation and incubation or may not be detected at all under the standard incubation period of 48 h recommended by the Clinical and Laboratory Standards Institute (CLSI) guidelines.
In this study, we found numerous mutations in hVISA strain Mu3-derived slow-VISA strains, in genes involved in various metabolic pathways, including purine/pyrimidine synthesis and 5P-d-ribosyl-1-pyrophosphate (PRPP) synthesis, as well as in rpoB and rpoC. These genes are known to be involved in the stringent response, a phenomenon originally identified in cells subjected to the stress of amino acid starvation, during which cells undergo massive physiological changes to save energy and conserve cellular resources (7). Mupirocin also induces the stringent stress response and amino acid starvation by blocking the charging of isoleucyl-tRNA with isoleucine through competition with Ile-AMP for isoleucyl-tRNA synthetases. Accumulation of unloaded isoleucyl-tRNA results in upregulation of the isoleucyl-tRNA synthetase (ileS) gene (8–10). Mupirocin-mediated amino acid starvation in S. aureus results in downregulation of genes involved in nucleotide biosynthesis, DNA metabolism, energy metabolism, and translation (11). The stringent response is mediated by (p)ppGpp, which regulates transcription by RNA polymerase (12). The stringent stress response induces high-level, homogeneous resistance to beta-lactams and depends on the transglycosylase activity of PBP2 (13, 14).
On the basis of these observations, we hypothesized that the mupirocin-induced stringent stress response could enhance expression of the slow-VISA phenotype. We developed a method for detecting slow-VISA strains by use of mupirocin and then investigated the prevalence of slow-VISA strains among clinical isolates by using the newly developed method.
RESULTS
Mutations present in slow-VISA mutants of Mu3.
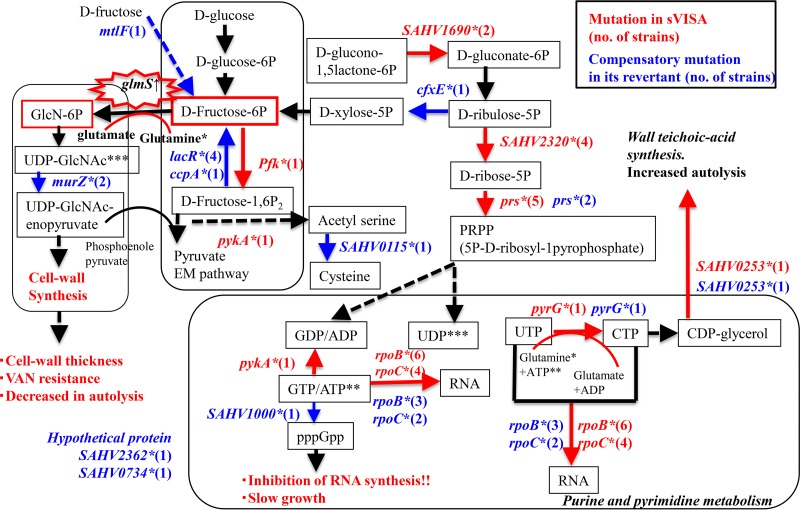
We identified genetic mutations other than those in the rpoB and rpoC genes in 23 slow-VISA isolates, derived from the hVISA strain Mu3 in the presence of 6 μg/ml vancomycin for 72 to 144 h (4), and in their revertants. Mutations were detected in several genes involved in various metabolic pathways, such as cell envelope synthesis (capP), purine/pyrimidine synthesis (pyrG [CTP synthase], pykA [pyruvate kinase], and SAHV1000 [GTP pyrophosphokinase]), the stringent response (PRPP synthesis, prs [ribose-phosphate pyrophosphokinase], SAHV2320 [ribose 5-phosphate isomerase A], cfxE [ribulose phosphate 3-epimerase], and SAHV1690 [similar to bifunctional oligoribonuclease] genes), and the Embden-Meyerhof (EM) pathway (pfk) (see Table S3 in the supplemental material). The pykA and GTP pyrophosphokinase genes have been associated with the VISA phenotype (5, 15). We mapped the main genetic mutations identified in the 23 slow-VISA isolates (Fig. 1, highlighted in red) and their 23 revertants (hVISA) (Fig. 1, highlighted in blue) to the corresponding metabolic pathways.
FIG 1.
Proposed model for the mechanism underlying vancomycin resistance in slow-VISA (sVISA) strains. We compared genotypes between the 23 slow-VISA clinical isolates and 23 revertant hVISA strains shown in Table S3 in the supplemental material. Mutations in slow-VISA isolates and revertant hVISA strains are highlighted in red and blue, respectively.
The vancomycin MIC was decreased compared to that of the mutant for 21 revertants obtained from the slow-VISA isolates (Table S3). Some mutations in the large-colony (LC) revertants occurred in the same genes but at different locations from those in the slow-VISA isolates (rpoB, rpoC, prs, pyrG, and SAHV0253). Mutations in other genes, for example, the murZ (encoding UDP-N-acetylglucosamine 1-carboxyvinyltransferase, the first enzyme in the biosynthetic pathway for production of peptidoglycan monomer units from UDP-GlcNAc), lacR, ccpA (16), SAHV1000, and rpoC genes, were detected in 10 LC revertants (Fig. 1).
In vivo metabolomics analysis.
To determine the effect on metabolomics induced by mupirocin in the slow-VISA strain Mu3-6R, carrying the RpoB(R512P) mutation, we performed a comparative analysis of strains expressing three different vancomycin-nonsusceptible phenotypes. There were clear phenotypic differences between hVISA strain Mu3 and slow-VISA strain Mu3-6R, with each expressing different amounts of products related to the mutations shown in Fig. 1 (Table 1; Table S4). The concentrations of fructose 1,6-P2, fructose 6-P, ribose-5P, ribulose-5P, and PRPP were significantly decreased in slow-VISA strain Mu3-6R compared to those in hVISA strain Mu3 and VISA strain Mu3-5-5d in the absence and presence of mupirocin, as expected based on the mutations shown in Fig. 1.
TABLE 1.
In vivo concentrations of major substances and ppGpp
| Strain | Phenotype | Treatment | Concn (pmol/ml)a |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | Fructose 1,6-P2 | Fructose 6-P | Ribose 5-P | Ribulose 5-P | PRPP | GTP | GDP | ATP | UTP | UDP | CTP | ppGppc | |||
| Mu3 | hVISA | Drug-free | 31,657 | 2,081 | 44 | 54 | 293 | 1,024 | 595 | 229 | 1,371 | 441 | 157 | 194 | ND |
| Mupirocinb | 32,824 | 560 | 23 | 13 | 328 | 739 | 414 | 154 | 3,205 | 1,498 | 292 | 1,454 | 41 ± 6 | ||
| Mu3-3-5dd | VISA | Drug-free | 36,738 | 539 | 25 | 11 | 88 | 615 | 40 | 118 | 315 | 124 | 44 | 159 | ND |
| Mupirocinb | 31,140 | 463 | 22 | 10 | 91 | 729 | 44 | 102 | 1,004 | 817 | 373 | 309 | ND | ||
| Mu3-6Re | Slow VISA | Drug-free | 31,384 | 472 | 12 | 13 | 149 | 504 | 245 | 31 | 1,415 | 1,717 | 498 | 534 | ND |
| Mupirocinb | 30,759 | 231 | 11 | 7 | 87 | 283 | 175 | 56 | 2,566 | 1,364 | 235 | 1,362 | 12 ± 6 | ||
Data calculated from two biological replicates. Each value indicates the average. Glucose concentration was used as a control value.
The strain was induced with mupirocin (0.032 μg/ml) for 30 min. We detected more than 1 nmol/mg of CDW.
Data are means ± standard deviations. ND, not detected. ppGpp was measured in triplicate.
Mu3-3-5d showing the VISA phenotype carried the TagH(D26E) mutation.
Mu3-6R showing the slow-VISA phenotype carried the RpoB(R512P) mutation.
Nucleotides, especially CTP, UTP, and UDP, used in peptidoglycan synthesis, were concentrated >4- to 10-fold in slow-VISA strain Mu3-6R compared to those in hVISA strain Mu3 and VISA strain Mu3-5-5d (Table 1). Low concentrations of GTP were apparent in slow-VISA strain Mu3-6R and VISA strain Mu3-5-5d in the absence and presence of mupirocin. However, the decrease in GTP concentration in Mu3-5-5d carrying TagH(D26E) was not accompanied by induction of (p)ppGpp (undetectable concentration) (Table 1). Mupirocin induces a decrease in GTP concentration (11, 17) and elicits a response in S. aureus that is linked to the stringent response induced by (p)ppGpp (8, 18).
Mupirocin-induced stable resistance to and tolerance of vancomycin in slow-VISA strain Mu3-6R.
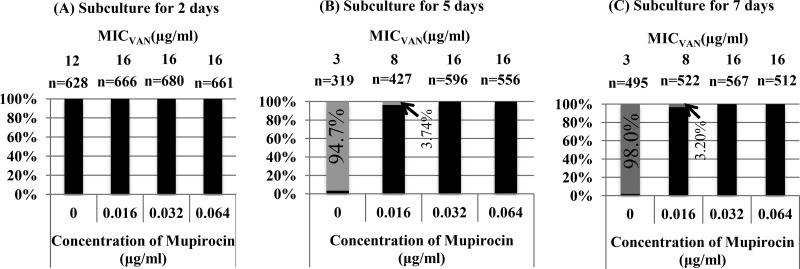
To evaluate the effect of mupirocin on phenotypic expression of slow VISA, Mu3-6R was subcultured in an antibiotic-free medium for 2, 5, or 7 days. When the strain was subcultured for 5 days, more than 90% of the small colonies of slow-VISA strain Mu3-6R converted to the hVISA phenotype (vancomycin MIC = 3 μg/ml) (Fig. 2). The expression of the slow-VISA phenotype in Mu3-6R was maintained by cultivation in brain heart infusion (BHI) broth containing mupirocin at 0.032 or 0.064 μg/ml after subculture for 5 days (Fig. 2). The growth curve for Mu3-6R was shifted to the right (inhibition of growth) at a mupirocin concentration of 0.064 μg/ml (Fig. S1), and therefore a mupirocin concentration of 0.032 μg/ml was used for further experiments.
FIG 2.
Stability of slow-VISA strain Mu3-6R showing high resistance to vancomycin induced by mupirocin. Black and gray bars indicate slow-VISA and hVISA strains, respectively; n is the number of tested colonies in a population. The y axis shows the proportion of hVISA cells among slow-VISA cells. The vancomycin MIC (MICVAN) was determined after incubation for 48 h. Mu3-6R was subcultured without or with mupirocin at 0.016, 0.032, or 0.064 μg/ml for 2 days (A), 5 days (B), and 7 days (C).
Mupirocin accelerated the growth rate of Mu3-6R in the presence of vancomycin.
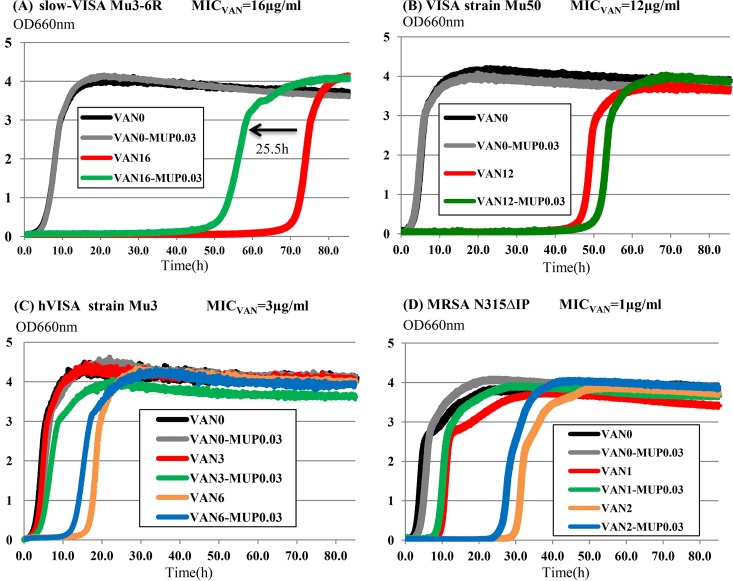
When slow-VISA strain Mu3-6R was cultured in BHI broth containing 16 μg/ml of vancomycin (1× MIC) plus 0.032 μg/ml of mupirocin, the growth curve shifted to the left (i.e., growth was accelerated by 25.5 h) compared to that for growth in BHI broth without mupirocin (Fig. 3A). The growth curves for strains without the slow-VISA phenotype were only slightly affected by mupirocin (Fig. 3B to D).
FIG 3.
Increased growth rate of slow-VISA cells under the influence of mupirocin (0.032 μg/ml). Cultures were incubated in BHI broth. Black, drug-free conditions; gray, mupirocin; red, vancomycin at 1× MIC; green, vancomycin at 1× MIC plus mupirocin; orange, vancomycin at 2× MIC; blue, vancomycin at 2× MIC plus mupirocin. The growth curve was accelerated by 25.5 h compared to that for growth in BHI containing vancomycin at 16 μg/ml (1× MIC) without mupirocin. MICVAN indicates the vancomycin MIC for the strain.
Reduced susceptibility of Mu3-6R to the bactericidal activity of vancomycin is induced by mupirocin.
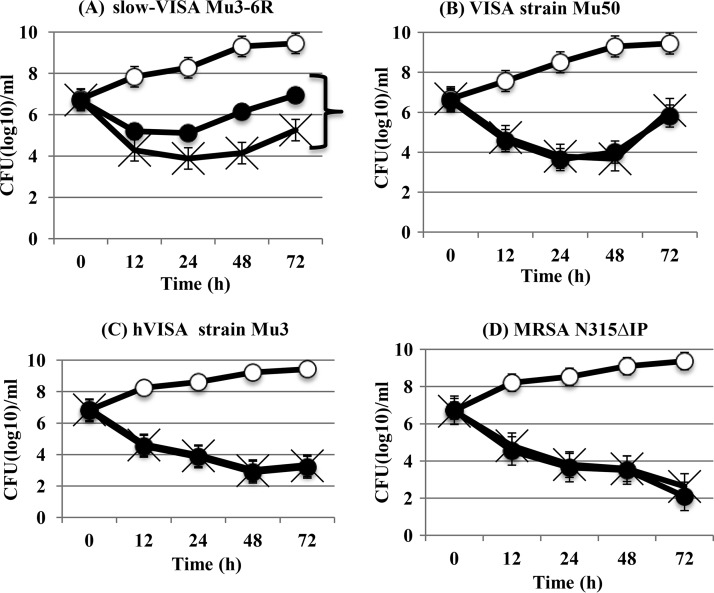
Time-kill assays were performed to compare the in vitro activities of vancomycin at 20 μg/ml plus mupirocin at 0.032 μg/ml against slow-VISA, VISA, hVISA, and VSSA (MRSA) strains. Mupirocin induced tolerance (i.e., loss of bactericidal activity) of vancomycin in slow-VISA strains but not in the other strains (Fig. 4). The same effect on bactericidal activity was observed for vancomycin at 1× or 2× MIC (data not shown).
FIG 4.
Mupirocin induced tolerance of vancomycin only in slow-VISA strain Mu3-6R. A time-kill assay was performed in BHI broth without a drug (○), with vancomycin at 20 μg/ml (×), and with vancomycin at 20 μg/ml plus mupirocin at 0.032 μg/ml (●) against slow-VISA strain Mu3-6R, VISA strain Mu50, hVISA strain Mu3, and VSSA (MRSA) strain N315ΔIP. Each symbol represents the average CFU (log10)/ml. The data are means ± standard deviations for triplicate wells.
Prevalence of clinical slow-VISA isolates among MRSA isolates from blood.
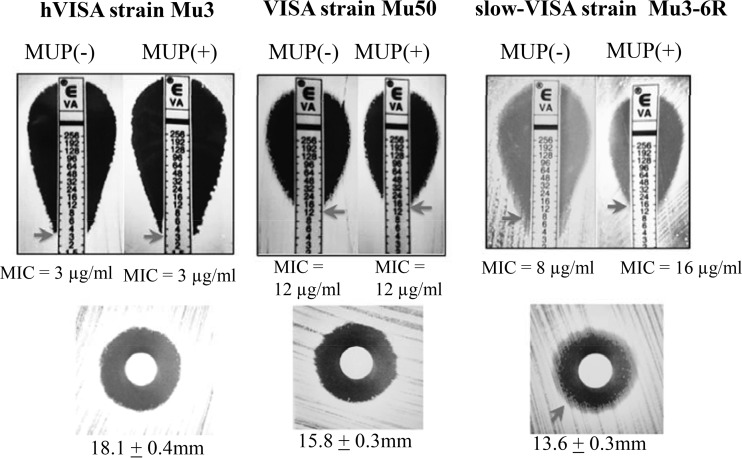
We tested 340 Japanese clinical MRSA isolates (156 blood culture isolates from a single hospital [Table S1] and 184 randomly selected blood culture isolates collected from 9 hospitals [Table S2]) (19, 20) to determine the prevalence of the slow-VISA phenotype detected by use of mupirocin induction. Determination of the MIC on BHI agar containing mupirocin at 0.032 μg/ml detected the slow-VISA phenotype after 48 h of incubation instead of a 72-h or longer incubation in the absence of mupirocin (Table 2 and Fig. 5). In total, we identified 53 (15.6%) slow-VISA isolates (vancomycin MICs of >4 mg/liter). Less than 1% of the isolates were VISA strains (Table 2; Tables S1 and S2). The slow-VISA phenotype showed vancomycin MICs of >4 μg/ml after 48 h of incubation, with increasing resistance to vancomycin after 72 h of incubation. The hVISA strain Mu3 and the VISA strain Mu50 had stable vancomycin MICs of 3 μg/ml and 12 μg/ml over time, respectively, in the presence and absence of mupirocin (Table 2 and Fig. 5), and they showed no growth defects.
TABLE 2.
Detection of slow-VISA cells among clinical MRSA isolates by use of mupirocin
| Strain | Phenotypeb |
Vancomycin MIC (μg/ml) by Etest after various periods of incubation |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLSI classification (48 h) | New classification | Without antibiotics |
With mupirocin at 0.032 μg/ml |
|||||||||
| 48 h | 72 h | 96 h | 120 h | 144 h | 48 h | 72 h | 96 h | 120 h | 144 h | |||
| 209P | VSSA (MSSA) | VSSA (MSSA) | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| N315ΔIP | VSSA (MRSA) | VSSA (MRSA) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Mu3 | hVISA | hVISA | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Mu50 | VISA | VISA | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 |
| MI (HIP5827) | VISA | VISA | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 |
| Mu3-6R | VISA | Slow VISA | 8 | 8 | 12 | 12 | 16 | 16 | 16 | 16 | 16 | 16 |
| Clinical blood culture isolates from 9 hospitals | ||||||||||||
| MRSA212 | VSSA | VSSA | 1.5 | 2 | 2 | 2 | 2 | 3 | 3 | 3 | 3 | 3 |
| MRSA84 | VSSA | hVISA | 2 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| MRSA220 | VSSA | hVISA | 1.5 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| MRSA192 | VSSA | hVISA | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| MRSA190 | VISA | Slow VISA | 4 | 8 | 8 | 8 | 8 | 12 | 12 | 12 | 12 | 12 |
| MRSA194 | VISA | Slow VISA | 4 | 6 | 8 | 12 | 12 | 8 | 12 | 12 | 12 | 12 |
| T series clinical blood culture isolatesa | ||||||||||||
| T12 | VISA | Slow VISA | 4 | 4 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| T15 | VISA | Slow VISA | 4 | 6 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| T61 | VISA | Slow VISA | 4 | 6 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| T89 | VISA | Slow VISA | 4 | 8 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| T122 | VISA | Slow VISA | 4 | 8 | 8 | 8 | 8 | 6 | 6 | 8 | 8 | 8 |
| T123 | VISA | Slow VISA | 4 | 6 | 8 | 8 | 8 | 6 | 8 | 8 | 8 | 8 |
| T12-L | hVISA | hVISA | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| T15-L | hVISA | hVISA | 3 | 3 | 3 | 3 | 4 | 4 | 4 | 4 | 4 | 4 |
| T61-L | hVISA | hVISA | 3 | 3 | 3 | 3 | 4 | 6 | 6 | 6 | 6 | 6 |
| T89-L | hVISA | hVISA | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| T122-L | VSSA | VSSA | 2 | 2 | 2 | 2 | 2 | 3 | 3 | 3 | 3 | 3 |
| T123-L | VSSA | VSSA | 2 | 2 | 2 | 2 | 2 | 3 | 3 | 3 | 3 | 3 |
T-L strains are revertants obtained from the original clinical strains by subculture for 5 days in the absence of antibiotics.
Slow-VISA strains showed increasing resistance to vancomycin after 72 h of incubation (vancomycin MICs of >4 μg/ml), although they had vancomycin MICs of >4 μg/ml after 48 h of incubation. The Mu3 and Mu50 isolates with the hVISA and VISA phenotypes had stable vancomycin MICs of 3 and 12 μg/ml, respectively, over time.
FIG 5.
(Top) Etest in BHI agar plates containing mupirocin (MUP) at 0.032 μg/ml or no drug. After 48 h of incubation, the slow-VISA strain showed a stable MIC value. (Bottom) Vancomycin (30 μg/ml) disk diffusion test in BHI agar plates. Slow-VISA strains formed a hazy zone around the inhibition zone. The values given under each panel are the means and standard deviations for the inhibition zone diameter for 3 experiments.
We confirmed the unstable expression of the slow-VISA phenotype in the absence of antibiotics. The levels of resistance to vancomycin in six slow-VISA strains (T series strains in Table 2) were reduced after a 5-day subculture in the absence of antibiotic. All six revertants (L strains derived from slow-VISA T strains) reverted to the VSSA or hVISA phenotype.
The zone of inhibition around a 30-μg/ml vancomycin disk in the disk diffusion test was hazy for slow-VISA Mu3-6R on a BHI agar plate (Fig. 5). The 30-μg/ml vancomycin disk diffusion test may be a useful method for detection of slow-VISA strains.
Sequence analysis of emergence of the slow-VISA phenotype from VSSA via hVISA.
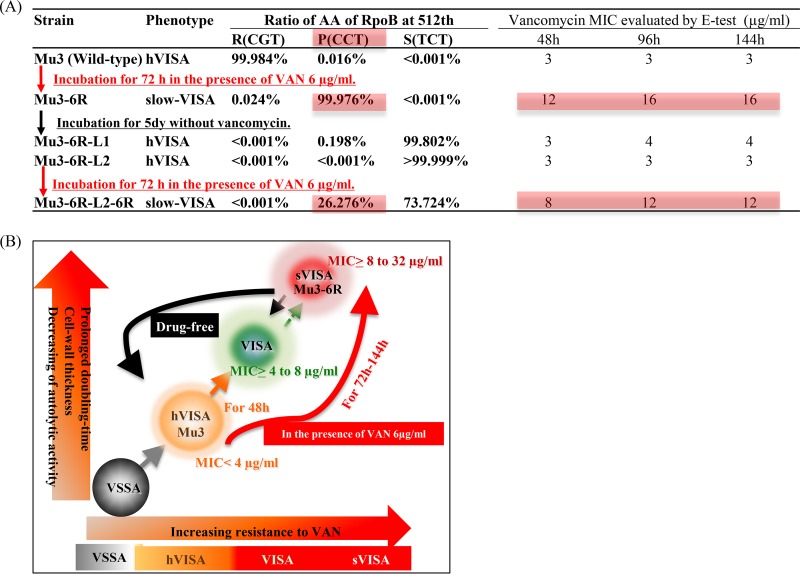
We previously reported that a single mutation of RpoB (R512P substitution) is responsible for nonsusceptibility to vancomycin (4). The Mu3-6R-L1 (hVISA) and Mu3-6R-L2 (hVISA) revertant mutants showed the large-colony phenotype and were isolated from slow-VISA strain Mu3-6R after antibiotic-free subculture for 5 days, as described previously (4). Mu3-6R-L2-6R (slow VISA) was obtained from a pinpoint colony of Mu3-6R-L2 growing on a BHI agar plate containing vancomycin at 6 μg/ml. We previously reported that when RpoB(R512P) in slow-VISA strain Mu3-6R was changed to an R512S, R512L, or R512H mutant, it resulted in the hVISA phenotype (4). We used deep sequencing to study the relationship between phenotype and the proportions of these various amino acid substitutions in the rpoB gene.
At baseline, Mu3 or Mu3-6R grown in the presence of 6 μg/ml of vancomycin carried >99.9% RpoB(R512) or RpoB(R512P), respectively (Fig. 6A). The Mu3-6R-L1 (hVISA) revertant mutant cells had 0.20% RpoB(R512P) and 99.8% RpoB(R512S). The Mu3-6R-L2 (hVISA) revertant mutant had >99.9% RpoB(R512S), whereas Mu3-6R-L2-6R, expressing the slow-VISA phenotype, contained 26.3% RpoB(R512P) and 73.7% RpoB(R512S). Thus, various RpoB R512 substitution mutants were associated with the slow-VISA Mu3-6R phenotype (Fig. 6A). The slow-VISA phenotype emerged in the presence of vancomycin, and the phenotype was lost in the absence of vancomycin, reverting to the hVISA phenotype (Fig. 6B).
FIG 6.
Emergence of the slow-VISA phenotype from VSSA via hVISA or VISA. (A) Proportions of mutations at the 512th residue of the RpoB gene, which confers high resistance to vancomycin, according to analysis of the rpoB amplicon by deep sequencing of Mu3-6R derivative clones. (B) The slow-VISA (sVISA) strain converted to VISA and hVISA in the absence of vancomycin.
DISCUSSION
We found that several mutations associated with the stringent response are found in slow-VISA mutant strains. A mupirocin-induced stringent response likely accounts for detection of the slow-VISA phenotype. The mechanism of resistance to vancomycin of slow-VISA strains differs from that of VISA strains because mupirocin induces the phenotype of the former but not the latter, and the associated mutations are different. For example, slow-VISA strain Mu3-6R has the RpoB(R512P) mutation, whereas VISA strain Mu3-5-5d has TagH(D26E).
Among the 23 slow-VISA isolates and their revertants, mutations in the rpoB and rpoC genes occurred more frequently in the slow-VISA strains than in the revertants, accounting for 32% of the total. Although rpoB is not a regulatory gene, its mutation drastically changes the transcription profile of the cell—much more than any mutations in regulators do. An rpoB mutation may act as a regulatory mutation (4, 5, 21, 22).
Besides RpoB mutation, we identified several other mutations in genes involved in various metabolic pathways, such as purine/pyrimidine synthesis in the pentose phosphate pathway. The increase in pools of CTP, UTP, and UMP shown in Table 1 is associated with the thickness of the cell wall and the VISA phenotype (5, 23–25). Pyrimidine upregulation has been reported to be normal with respect to the synthesis of ribosomal components but abnormal in terms of the ppGpp pool (26). Therefore, we speculate that the vancomycin resistance of slow VISA is due to an increase in pools of CTP, UTP, and UDP.
Mutations of pfk and pykA altered the amounts of the metabolites d-fructose-6P, d-fructose-1,6P2, and pyruvate in slow-VISA isolates. It is likely that d-fructose-6P pooled in the slow-VISA strain and contributed to cell wall synthesis.
The capP gene encodes an enzyme that converts UDP-GlcNac to UDP-GlcManNAc (27). Mutations in this gene may stimulate the pathway of cell wall synthesis at the expense of purine/pyrimidine synthesis by way of d-fructose-6P. Upregulated glmS increases production of glucosamine 6-P, which is required for the synthesis of peptidoglycan and wall teichoic acids, major components of the S. aureus cell wall (34). These mutations altering the metabolic pathways of slow VISA may result in activation of cell wall synthesis, causing thickening of the cell walls and consequent acquisition of resistance to vancomycin.
We compared the mutations in the 23 revertants (VSSA or hVISA strains) obtained from slow-VISA cells in the absence of antibiotics. These mutations localized in genes involved in the stringent response, likely affecting the flow of metabolites in the cell and promoting cell wall peptidoglycan synthesis by concentrating energy and metabolites within that pathway.
Colistin has also been reported to induce vancomycin tolerance that is associated with quorum sensing. Nevertheless, the mechanisms are different from mupirocin's mode of action (28). Further research into the mechanisms by which mupirocin induces the slow-VISA phenotype is needed.
We identified the following three characteristics of slow-VISA induction by mupirocin: (i) stable resistance to vancomycin, (ii) an accelerated growth rate, and (iii) tolerance of bactericidal activity. These were utilized to develop a method for detection of slow VISA in this study. Using this method, we found that the slow-VISA phenotype was prevalent in Japanese hospitals, being detected at a frequency of about 15.6% in the tested MRSA clinical isolates from blood cultures. We also isolated cells with the slow-VISA phenotype (vancomycin MIC of ≥16 μg/ml) from three VISA strains, namely, HIP07920 (isolated in 1999, in Rhode Island, from the blood of a patient with bacteremia), HIP12864 (isolated in 2003, in Oklahoma, from the blood of a patient with bacteremia) (29), and P1V44 (isolated in 1999, in Belgium, from sputum of a patient with cystic fibrosis) (30), in the absence of antibiotics (T. Azechi, unpublished data). Slow VISA may be prevalent in hospitals worldwide.
We also found that slow VISA emerged in the presence of vancomycin, lost resistance in the absence of vancomycin, and reverted to the hVISA or VSSA phenotype (Fig. 6). Slow-VISA cells were present in small numbers among the hVISA revertants, and their numbers increased again when they were incubated in the presence of vancomycin. Slow-VISA strains may account in part for recurrent and persistent MRSA infections (Fig. 6). Rapid detection of slow VISA may be an important tool for predicting and preventing vancomycin treatment failure.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Bacterial strains used in this study were routinely grown in BHI medium (Eiken Chemical Co., Ltd., Tochigi, Japan) at 37°C. Two clinical strain collections were tested for the presence of slow VISA. We selected 156 MRSA blood isolates (described as the T series) collected from 1987 to 2007, before the use of daptomycin and linezolid in Japan (see Table S1 in the supplemental material) (20), and we randomly selected 184 MRSA blood isolates from 803 clinical strains of MRSA collected from January 2008 through May 2011 at nine university hospitals in Japan (Table S2) (19). The study protocol was approved by the Institutional Review Board.
DNA methods.
The standard methods for DNA manipulations were described previously (31). Genomic DNAs were prepared with a Miniamp kit (Qiagen Inc., Valencia, CA). Routine PCR amplification was conducted by means of the Expand High-Fidelity system (Roche, Mannheim, Germany).
Antibiotic susceptibility tests.
MIC values were determined by Etest (AB Biodisk, Solna, Sweden). BHI agar was used instead of Mueller-Hinton agar because the former is more supportive of the expression of the VISA phenotype than are other agar types (32). Plates were then incubated at 37°C and analyzed after 48 h to 144 h. The Etest to determine the MIC of vancomycin (AB Biodisk, Solna, Sweden), the 30-μg/ml vancomycin agar disk diffusion susceptibility test (Eiken), and population analysis were carried out on BHI agar following the CLSI guidelines. Mupirocin (Roche) was used for the selection of slow VISA.
VSSA was defined as S. aureus with a vancomycin MIC of <4 μg/ml (CLSI breakpoint) that was unaffected by prolonged incubation. hVISA was defined as S. aureus with a vancomycin MIC of <4 μg/ml (CLSI breakpoint) that was unaffected by prolonged incubation or incubation with mupirocin but had a subpopulation of cells resistant to 4 μg/ml of vancomycin, at a frequency of 1 × 10−7 or greater. Slow VISA was defined as S. aureus with increasing resistance to vancomycin with prolonged incubation of >48 h, to an MIC of >8 μg/ml. Other properties of slow VISA are a low growth rate, unstable resistance and growth phenotypes, tolerance to killing by vancomycin, and a small or pinpoint colony morphology. VISA was defined as S. aureus with a stable vancomycin MIC of >4 μg/ml with prolonged incubation. Other properties are a stable phenotypic expression, colony morphology, and growth rate.
Emergence of slow-VISA isolates from hVISA strain Mu3.
Twenty-three revertants were obtained from the 23 slow-VISA isolates, derived from Mu3 by selection with 6 μg/ml of vancomycin (4), by growth in the absence of antibiotics after subculture for 5 days. Doubling times and vancomycin MICs were determined (Table S3).
Genome analysis.
We carried out single nucleotide polymorphism (SNP) detection according to a previously described protocol (4) and by deep sequencing of the amplified rpoB gene according to the manufacturer's recommendations (TaKaRa). In brief, 150-bp DNA fragments of the rpoB gene were amplified by PCR using the primers described previously (4). The amplicon library was constructed by means of a Nextera XT DNA sample preparation kit, and 150-bp paired-end read sequencing was performed on a Miseq sequencing platform (Illumina, Inc., San Diego, CA). Reads of 30 Mb were obtained from each amplicon DNA library. Each read was mapped to the reference rpoB sequence of strain N315.
Metabolome analysis.
hVISA strain Mu3, VISA strain Mu3-3-5d (showing a stable vancomycin MIC of 8 μg/ml over time), and slow-VISA strain Mu3-6R carrying the RpoB(R512P) mutation each was grown as described above in defined synthetic medium. VISA strain Mu3-3-5d was isolated from hVISA Mu3 by selection on vancomycin at 6 μg/ml and was found to carry the TagH(D26E) mutation affecting the teichoic acid export ATP-binding protein (4). The cells were treated with 0.032 μg/ml mupirocin, and samples were harvested immediately after the addition of mupirocin (0 min) and after 30 min. The cells were washed and quenched, and metabolites were extracted according to the manufacturer's recommendations (Human Metabolome Technology [HMT], Tokyo, Japan). Briefly, the metabolite extraction procedure was conducted as follows. Cells were grown in 40 ml of medium in 100-ml Erlenmeyer flasks with shaking at 180 rpm and 37°C. For each strain, three cultures were incubated in parallel. Cells were harvested by vacuum filtration through a filter with a 0.45-μm pore size. The cells on the filter were immersed in 2 ml of methanol containing 10 μM internal standard solution 1 (HMT). Analytical biochemistry detection of nucleotides was performed by capillary electrophoresis with mass spectrometry (CE-MS; HMT). Identification and calibration of calibration curves for all the metabolites were carried out via comparison and measurement of a pure standard compound, (p)ppGpp (Trilink Biotechnology, San Diego CA). The results for intracellular metabolites were normalized to the cell dry weight (CDW). Experiments were performed in duplicate.
Development of a method for detecting slow VISA by use of mupirocin.
Measurement of the appearance rate of hVISA (large-colony [LC] phenotype) from slow VISA (small colonies) and construction of growth curves were performed as described previously (4). Time-kill analyses were conducted to assess killing of bacteria (33). In brief, an inoculum of 106 CFU/ml of fresh overnight culture was used. Four-milliliter aliquots of BHI broth were used for all the time-kill analyses. Bacterial colony counts were determined in duplicate at baseline and 12, 24, 48, and 72 h after incubation at 37°C. Mupirocin concentrations ranged from 0.016 to 0.064 μg/ml (Fig. S1). The vancomycin MIC was determined by Etest (AB Biodisk, Solna, Sweden), using BHI agar containing mupirocin at 0.032 μg/ml and drug-free BHI agar (Yuki Katayama, 7 March 2017, patent application PCT/JP2017/008975).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant-in-aid for St. Luke's International University Support Unit for Conducting Clinically Essential Studies, Takeda Science Foundation, and grants-in-aid (grants 24791029, 15K09581, and S1201013) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) for the Foundation.
We thank Tomomi Hishinuma and Yoshifumi Aiba for their invaluable help in sample preparation for sequencing. We thank Keiichi Hiramatsu and Hajime Arai for their total support of this report.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00452-17.
REFERENCES
- 1.Howden BP, Ward PB, Charles PG, Korman TM, Fuller A, du Cros P, Grabsch EA, Roberts SA, Robson J, Read K, Bak N, Hurley J, Johnson PD, Morris AJ, Mayall BC, Grayson ML. 2004. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin Infect Dis 38:521–528. doi: 10.1086/381202. [DOI] [PubMed] [Google Scholar]
- 2.Entenza JM, Veloso TR, Vouillamoz J, Giddey M, Moreillon P. 2011. Failure of vancomycin continuous infusion against experimental endocarditis due to vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother 55:385–387. doi: 10.1128/AAC.00811-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore MR, Perdreau-Remington F, Chambers HF. 2003. Vancomycin treatment failure associated with heterogeneous vancomycin-intermediate Staphylococcus aureus in a patient with endocarditis and in the rabbit model of endocarditis. Antimicrob Agents Chemother 47:1262–1266. doi: 10.1128/AAC.47.4.1262-1266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saito M, Katayama Y, Hishinuma T, Iwamoto A, Aiba Y, Kuwahara-Arai K, Cui L, Matsuo M, Aritaka N, Hiramatsu K. 2014. “Slow VISA,” a novel phenotype of vancomycin resistance, found in vitro in heterogeneous vancomycin-intermediate Staphylococcus aureus strain Mu3. Antimicrob Agents Chemother 58:5024–5035. doi: 10.1128/AAC.02470-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuo M, Hishinuma T, Katayama Y, Hiramatsu K. 2015. A mutation of RNA polymerase β′ subunit (RpoC) converts heterogeneously vancomycin-intermediate Staphylococcus aureus (hVISA) into “slow VISA”. Antimicrob Agents Chemother 59:4215–4225. doi: 10.1128/AAC.00135-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, Fukuchi Y, Kobayashi I. 1997. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 7.Potrykus K, Cashel M. 2008. (p)ppGpp: still magical? Annu Rev Microbiol 62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- 8.Reiss S, Pane-Farre J, Fuchs S, Francois P, Liebeke M, Schrenzel J, Lindequist U, Lalk M, Wolz C, Hecker M, Engelmann S. 2012. Global analysis of the Staphylococcus aureus response to mupirocin. Antimicrob Agents Chemother 56:787–804. doi: 10.1128/AAC.05363-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grundy FJ, Haldeman MT, Hornblow GM, Ward JM, Chalker AF, Henkin TM. 1997. The Staphylococcus aureus ileS gene, encoding isoleucyl-tRNA synthetase, is a member of the T-box family. J Bacteriol 179:3767–3772. doi: 10.1128/jb.179.11.3767-3772.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee AS, Gizard Y, Empel J, Bonetti EJ, Harbarth S, Francois P. 2014. Mupirocin-induced mutations in ileS in various genetic backgrounds of methicillin-resistant Staphylococcus aureus. J Clin Microbiol 52:3749–3754. doi: 10.1128/JCM.01010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corrigan RM, Bellows LE, Wood A, Grundling A. 2016. ppGpp negatively impacts ribosome assembly affecting growth and antimicrobial tolerance in Gram-positive bacteria. Proc Natl Acad Sci U S A 113:E1710–E1719. doi: 10.1073/pnas.1522179113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivatsan A, Wang JD. 2008. Control of bacterial transcription, translation and replication by (p)ppGpp. Curr Opin Microbiol 11:100–105. doi: 10.1016/j.mib.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Mwangi MM, Kim C, Chung M, Tsai J, Vijayadamodar G, Benitez M, Jarvie TP, Du L, Tomasz A. 2013. Whole-genome sequencing reveals a link between beta-lactam resistance and synthetases of the alarmone (p)ppGpp in Staphylococcus aureus. Microb Drug Resist 19:153–159. doi: 10.1089/mdr.2013.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aedo S, Tomasz A. 2016. Role of the stringent stress response in the antibiotic resistance phenotype of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 60:2311–2317. doi: 10.1128/AAC.02697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuo M, Cui L, Kim J, Hiramatsu K. 2013. Comprehensive identification of mutations responsible for heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA)-to-VISA conversion in laboratory-generated VISA strains derived from hVISA clinical strain Mu3. Antimicrob Agents Chemother 57:5843–5853. doi: 10.1128/AAC.00425-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barriere C, Veiga-da-Cunha M, Pons N, Guedon E, van Hijum SA, Kok J, Kuipers OP, Ehrlich DS, Renault P. 2005. Fructose utilization in Lactococcus lactis as a model for low-GC gram-positive bacteria: its regulator, signal, and DNA-binding site. J Bacteriol 187:3752–3761. doi: 10.1128/JB.187.11.3752-3761.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bittner AN, Kriel A, Wang JD. 2014. Lowering GTP level increases survival of amino acid starvation but slows growth rate for Bacillus subtilis cells lacking (p)ppGpp. J Bacteriol 196:2067–2076. doi: 10.1128/JB.01471-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonenshein AL. 2005. CodY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr Opin Microbiol 8:203–207. doi: 10.1016/j.mib.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Hanaki H, Cui L, Ikeda-Dantsuji Y, Nakae T, Honda J, Yanagihara K, Takesue Y, Matsumoto T, Sunakawa K, Kaku M, Tomono K, Fukuchi K, Kusachi S, Mikamo H, Takata T, Otsuka Y, Nagura O, Fujitani S, Aoki Y, Yamaguchi Y, Tateda K, Kadota J, Kohno S, Niki Y. 2014. Antibiotic susceptibility survey of blood-borne MRSA isolates in Japan from 2008 through 2011. J Infect Chemother 20:527–534. doi: 10.1016/j.jiac.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki M, Takata T, Yoshimura H, Matsunaga A, Ohta D, Ishikura H, Futo M, Hara S, Kamimura H, Tamura K, Ngo D, Tsuji BT. 2011. Vancomycin bactericidal activity as a predictor of 30-day mortality in patients with methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 55:1819–1820. doi: 10.1128/AAC.01536-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao W, Cameron DR, Davies JK, Kostoulias X, Stepnell J, Tuck KL, Yeaman MR, Peleg AY, Stinear TP, Howden BP. 2013. The RpoB H(4)(8)(1)Y rifampicin resistance mutation and an active stringent response reduce virulence and increase resistance to innate immune responses in Staphylococcus aureus. J Infect Dis 207:929–939. doi: 10.1093/infdis/jis772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiramatsu K, Katayama Y, Matsuo M, Sasaki T, Morimoto Y, Sekiguchi A, Baba T. 2014. Multi-drug-resistant Staphylococcus aureus and future chemotherapy. J Infect Chemother 20:593–601. doi: 10.1016/j.jiac.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Yu R, Dale SE, Yamamura D, Stankus V, Lee C. 2012. Daptomycin-nonsusceptible, vancomycin-intermediate, methicillin-resistant Staphylococcus aureus endocarditis. Can J Infect Dis Med Microbiol 23:e48–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jorgensen CM, Hammer K, Jensen PR, Martinussen J. 2004. Expression of the pyrG gene determines the pool sizes of CTP and dCTP in Lactococcus lactis. Eur J Biochem 271:2438–2445. doi: 10.1111/j.1432-1033.2004.04168.x. [DOI] [PubMed] [Google Scholar]
- 25.Hanaki H, Kuwahara-Arai K, Boyle-Vavra S, Daum RS, Labischinski H, Hiramatsu K. 1998. Activated cell-wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J Antimicrob Chemother 42:199–209. doi: 10.1093/jac/42.2.199. [DOI] [PubMed] [Google Scholar]
- 26.Vogel U, Pedersen S, Jensen KF. 1991. An unusual correlation between ppGpp pool size and rate of ribosome synthesis during partial pyrimidine starvation of Escherichia coli. J Bacteriol 173:1168–1174. doi: 10.1128/jb.173.3.1168-1174.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiser KB, Lee JC. 1998. Staphylococcus aureus cap5O and cap5P genes functionally complement mutations affecting enterobacterial common-antigen biosynthesis in Escherichia coli. J Bacteriol 180:403–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haaber J, Friberg C, McCreary M, Lin R, Cohen SN, Ingmer H. 2015. Reversible antibiotic tolerance induced in Staphylococcus aureus by concurrent drug exposure. mBio 6:e02268-14. doi: 10.1128/mBio.02268-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe Y, Neoh HM, Cui L, Hiramatsu K. 2008. Improved antimicrobial activity of linezolid against vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother 52:4207–4208. doi: 10.1128/AAC.00676-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denis O, Nonhoff C, Byl B, Knoop C, Bobin-Dubreux S, Struelens MJ. 2002. Emergence of vancomycin-intermediate Staphylococcus aureus in a Belgian hospital: microbiological and clinical features. J Antimicrob Chemother 50:383–391. doi: 10.1093/jac/dkf142. [DOI] [PubMed] [Google Scholar]
- 31.Katayama Y, Sekine M, Hishinuma T, Aiba Y, Hiramatsu K. 2016. Complete reconstitution of the vancomycin-intermediate Staphylococcus aureus phenotype of strain Mu50 in vancomycin-susceptible S. aureus. Antimicrob Agents Chemother 60:3730–3742. doi: 10.1128/AAC.00420-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother 40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 33.Aiba Y, Katayama Y, Hishinuma T, Murakami-Kuroda H, Cui L, Hiramatsu K. 2013. Mutation of RNA polymerase beta-subunit gene promotes heterogeneous-to-homogeneous conversion of beta-lactam resistance in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:4861–4871. doi: 10.1128/AAC.00720-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lunse CE, Schmidt MS, Wittmann V, Mayer G. 2011. Carba-sugars activate the glmS-riboswitch of Staphylococcus aureus. ACS Chem Biol 6:675–678. doi: 10.1021/cb200016d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.