Summary
Understanding the molecular basis of zinc (Zn) uptake and transport in staple cereal crops is critical for improving both Zn content and tolerance to low‐Zn soils. This study demonstrates the importance of group F bZIP transcription factors and ZIP transporters in responses to Zn deficiency in wheat (Triticum aestivum). Seven group F TabZIP genes and 14 ZIPs with homeologs were identified in hexaploid wheat. Promoter analysis revealed the presence of Zn‐deficiency‐response elements (ZDREs) in a number of the ZIPs. Functional complementation of the zrt1/zrt2 yeast mutant by TaZIP3, ‐6, ‐7, ‐9 and ‐13 supported an ability to transport Zn. Group F TabZIPs contain the group‐defining cysteine–histidine‐rich motifs, which are the predicted binding site of Zn2+ in the Zn‐deficiency response. Conservation of these motifs varied between the TabZIPs suggesting that individual TabZIPs may have specific roles in the wheat Zn‐homeostatic network. Increased expression in response to low Zn levels was observed for several of the wheat ZIPs and bZIPs; this varied temporally and spatially suggesting specific functions in the response mechanism. The ability of the group F TabZIPs to bind to specific ZDREs in the promoters of TaZIPs indicates a conserved mechanism in monocots and dicots in responding to Zn deficiency. In support of this, TabZIPF1‐7DL and TabZIPF4‐7AL afforded a strong level of rescue to the Arabidopsis hypersensitive bzip19 bzip23 double mutant under Zn deficiency. These results provide a greater understanding of Zn‐homeostatic mechanisms in wheat, demonstrating an expanded repertoire of group F bZIP transcription factors, adding to the complexity of Zn homeostasis.
Keywords: zinc, micronutrient, biofortification, ZIP transporter, membrane transport, bZIP, wheat (Triticum aestivum), transcription factor
Significance Statement
Understanding the molecular basis of Zn uptake, transport throughout the plant and homeostasis is an important step toward the improvement of both Zn content and tolerance to low Zn soils of the staple cereal, wheat. Here we identify and characterise Zn‐responsive members of the wheat ZIP membrane transporter family and also wheat F group bZIP transcription factors and determine their role in the response to Zn deficiency.
Introduction
Micronutrient deficiency in humans is an issue of global concern. Enhancing the micronutrient content of staple crops is therefore an important objective for modern agriculture. Cereals such as wheat (Triticum aestivum) are relatively low in essential micronutrients such as zinc (Zn) and iron in their edible tissues. This presents a major problem when cereals form the main part of the diet and is a particular issue in developing countries (Kumssa et al., 2015). Crop yield is also detrimentally affected when plants are deficient in micronutrients as they are required throughout plant development (Brown et al., 1993). Zn has essential roles in plant growth, phytohormone activity, enzyme activation and modification of gene expression (Broadley et al., 2007). The yield reductions from reduced Zn availability are ultimately associated with damage to cell proteins, lipids and DNA (Cakmak, 2000). The development of crops that maintain growth and yield under low Zn availability would have clear benefits, and for that an understanding of the homeostatic network that determines Zn efficiency is required. Wheat cultivation occupies the largest area of any crop, therefore this study addresses mechanisms contributing to adaptation to low Zn in this monocot cereal.
Plants have developed sophisticated sensing and response mechanisms allowing them to adapt to variations in micronutrient availability. The complex nature of these homeostatic mechanisms is starting to be resolved in the model plant Arabidopsis. In this dicot, two bZIP (basic‐leucine zipper domain) transcription factors, bZIP19 and ‐23, are involved in adapting to Zn deficiency by inducing the expression of particular family members of membrane transporters, the ZIPs (ZRT, IRT‐related proteins) (Assunção et al., 2010). These have emerged as a key membrane transporter family in the journey of Zn from soil to seed (Palmgren et al., 2008). In Arabidopsis, eight members of the ZIP family have ability to transport Zn (AtIRT1, AtZIP1, ‐2, ‐3, ‐7, ‐10, ‐11 and ‐12) (Grotz et al., 1998; Milner et al., 2013), and several have been shown to be induced in response to Zn deficiency. ZIPs have also been characterised in cereals such as rice (Oryza sativa) and barley (Hordeum vulgare). In rice, OsZIP3, ‐4, ‐5 and ‐8 are functional Zn transporters (Ishimaru et al., 2005; Yang et al., 2009b; Lee et al., 2010a) while OsIRT1 and ‐2 as well as OsZIP6 and ‐7 can transport Fe but not Zn (Bughio et al., 2002; Ishimaru et al., 2006; Kavitha et al., 2015). The expression of OsZIP4, ‐5 and ‐8 is induced by Zn deficiency in both the root and shoot (Suzuki et al., 2012). In barley HvIRT1 and HvZIP3, ‐5 and ‐8 have all been shown to rescue the zrt1/zrt2 Zn mutant yeast strain to a varying degree, indicating the ability to transport Zn (Pedas et al., 2008, 2009). Some of the rice and barley ZIPs are also induced by Zn deficiency, including OsZIP1, ‐3, ‐4, ‐5 and ‐8 (Ramesh et al., 2003; Ishimaru et al., 2005; Lee et al., 2010b) and HvZIP3, ‐5, ‐7, ‐8, ‐10 and ‐13 (Pedas et al., 2009; Tiong et al., 2013, 2015). In wheat little is known about this family except for TdZIP1 from wild emmer wheat (Triticum turgidum ssp. dicoccoides), a Zn transporter with higher expression under Zn deficiency (Durmaz et al., 2011). In this study we have identified Zn‐responsive ZIPs in wheat (T. aestivum) and confirmed their Zn transport capability. A particular step forward was to investigate the mechanism that leads to changes in the expression of these transporters in response to Zn deficiency.
In Arabidopsis, increases observed in expression of particular ZIPs are proposed to occur via binding of AtbZIP19 and AtbZIP23 to Zn‐deficiency‐response elements (ZDREs) in ZIP promoters (Assunção et al., 2010). This operates as an adaptive mechanism to increase uptake and distribution of Zn in response to Zn deficiency. Evidence for this was provided by the extreme hypersensitivity of a bzip19 bzip23 double mutant to low‐Zn conditions and the lack of Zn‐induced expression of key Zn transporters, including AtZIP1, ‐3, ‐4, ‐5, ‐9 and ‐10 (Assunção et al., 2010). These genes contain one or more copies of the ZDRE in their promoter and direct binding by AtbZIP19 to this motif in AtZIP4 was demonstrated previously (Assunção et al., 2010).
Further analysis of the bzip19 and bzip23 single mutants indicates that while they may overlap in their targets, AtbZIP19 and AtbZIP23 may also operate to alter the expression of a specific subset of genes (Inaba et al., 2015). The mechanism whereby low Zn is sensed is still to be elucidated, but it is suggested that under normal cellular Zn concentrations a Zn2+ ion binds to the cysteine–histidine‐rich motifs present in the group F bZIPs, making them inactive. Upon a reduction in cellular Zn concentration, Zn2+ dissociates, thereby activating the bZIP dimer which in turn binds to the ZDRE motif and brings about an increase in transcription of Zn‐responsive genes (Assunção et al., 2013). We show that wheat contains an additional level of complexity in adapting to Zn deficiency with an expanded number of group F bZIP transcription factors, allowing further modulation of the response to variations in Zn growth conditions.
Results
A multi‐gene family of ZIPs is present in wheat
A comprehensive analysis of the genomic information recently available for hexaploid wheat was performed. From this analysis, 14 TaZIP genes were identified (Figure 1). Modern bread wheat (T. aestivum) is hexaploid, containing three genomes – A, B and D; homeologous genes are present on all three genomes. Full homeolog complements were found for all of the TaZIPs in each of the A, B and D genomes (Figure S1 in the Supporting Information). A previous phylogenetic analysis of TaZIPs identified 11 homologs (Tiong et al., 2015); TaZIP2, ‐8 and ‐9 have not formerly been identified. The TaZIP homeologs group closely to one another (Figure S1) and for 12 of the TaZIPs the most closely related homolog was that from barley, followed by Brachypodium and rice. TaZIP9 and TaZIP13 showed high relatedness: TaZIP9‐2DS [TaZIP9 present on the short arm (S) of chromosome 2D] and TaZIP13‐2DL [TaZIP13 present on the long arm (L) of chromosome 2D] had 83% nucleotide sequence similarity (Table S1 and Figure S2), and both are located on chromosome 2, suggesting a wheat‐specific gene duplication. This is further supported by there being only one barley gene in this group.
Figure 1.
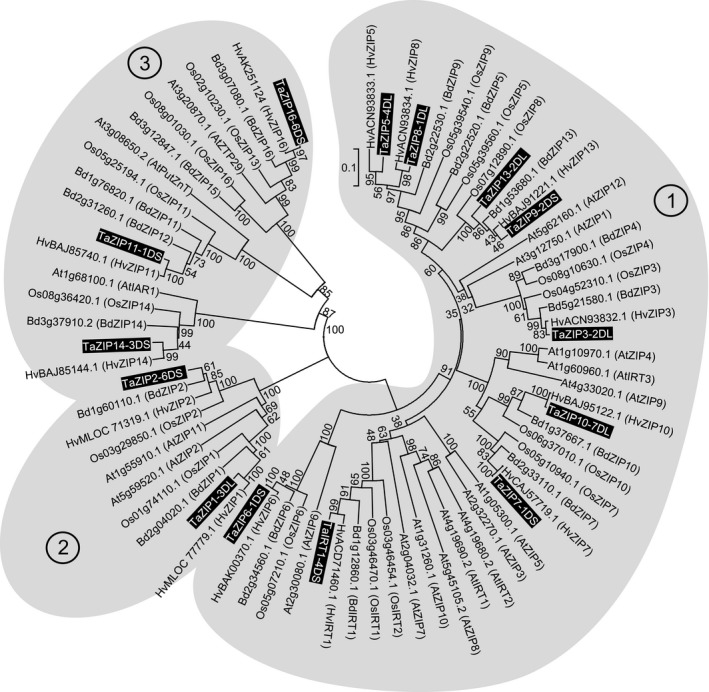
Phylogenetic tree of ZIP proteins from cereals and Arabidopsis.
A neighbour‐joining tree was generated for Arabidopsis (At), rice, Oryza sativa (Os), Brachypodium distachyon (Bd), barley, Hordeum vulgare (Hv), and wheat, Triticum aestivum (Ta) ZIP coding sequence translations. The Muscle algorithm (Edgar, 2004) was used for the alignment of sequences and the phylogenetic tree was created using mega (v.5.2) software. Evolutionary distances were computed using the p‐distance method and are in the units of the number of amino acid differences per site. A thousand bootstrap replicates were used and bootstrap values are shown as percentages. Gene names of Arabidopsis, rice, Brachypodium and barley are shown in brackets, these gene names are in accordance with Tiong et al. (2015). D genome homeologs for T. aestivum are given in this figure. Full accession information for the wheat genes is provided in Table S2.
The phylogeny of ZIPs displays three distinct clades (Figure 1). All clades contain both monocot and dicot ZIP homologs, suggesting that ZIPs were present before the divergence of monocots and dicots. AtPutZnT, AtIAR1 and AtZTP29 and their cereal homologs (TaZIP11, TaZIP14 and TaZIP16) are situated in a subclade of clade 3 and are more distantly related to the other ZIPs included in the phylogenetic analysis. Each sub‐clade also contains monocot and dicot members, but there is also evidence suggesting species‐specific ZIP expansion with possible functional divergence.
Wheat ZIPs functionally complement zrt1/zrt2, a Zn‐sensitive yeast mutant
A functional role in Zn homeostasis for a selection of the wheat ZIPs (TaZIP3, ‐6, ‐7, ‐9 and ‐13) was tested using yeast complementation. These TaZIPs were selected due to their location in the main clade of the ZIP phylogeny which contains previously confirmed Zn‐transporting ZIPs from Arabidopsis, rice and barley (Figure 1). These TaZIPs were tested using the zrt1/zrt2 yeast mutant strain. This strain is defective in both the ZRT1 high‐affinity and the ZRT2 low‐affinity uptake transporters, and is susceptible to low‐Zn conditions (Zhao and Eide, 1996a,b). As seen in Figure 2, the vector‐transformed zrt1/zrt2 yeast was markedly inhibited in growth compared with the wild type (DY1457) in the absence of Zn (achieved by addition of the chelator EGTA). Heterologous expression of TaZIP3, ‐6, ‐7, ‐9 and ‐13 partially rescued the Zn‐deficiency phenotype. Growth levels in Zn‐deficient media (5 mm EGTA and 7.5 mm EGTA) were higher in the zrt1/zrt2 strain expressing the TaZIPs compared with the empty pYES2 vector control, consistent with them functioning as Zn transporters. None of the TaZIPs heterologously expressed in the fet3/fet4 Fe‐uptake mutant strain were able to rescue the Fe‐deficient phenotype (Figure 2e–g), suggesting that none of the TaZIPs investigated are functional Fe transporters.
Figure 2.
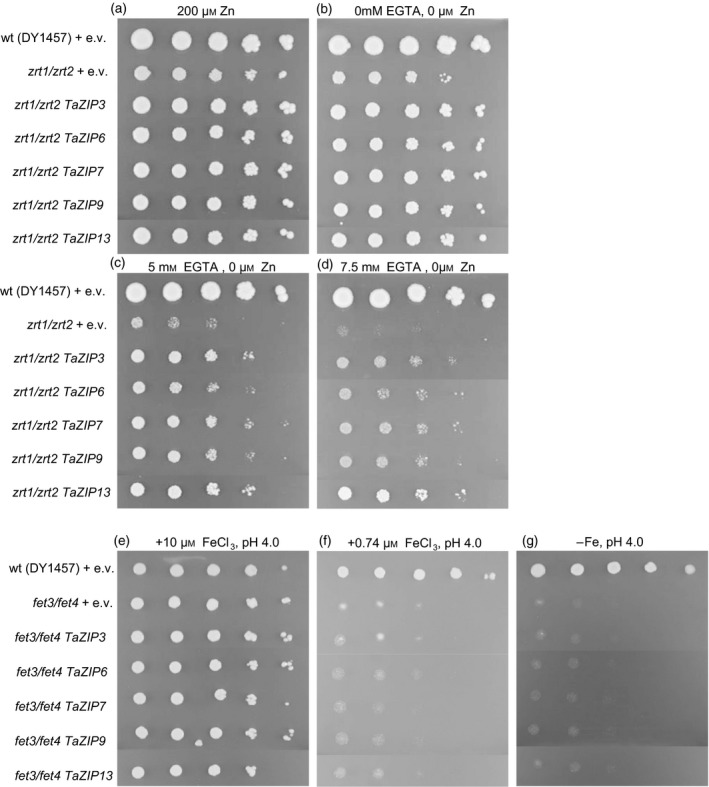
Complementation of Zn and Fe yeast uptake mutants with TaZIP genes.
The yeast Zn uptake mutant zrt1/zrt2 (a–d) and the Fe uptake mutant fet3/fet4 (e–g) were transformed with five wheat TaZIP genes, shown here with empty vector controls (e.v.) in both the mutant and wild‐type yeast strains. Each spot is a dilution of the culture starting on the left of each plate (undiluted, 1:2, 1:10, 1:100, 1:1000) with the contents of selective media described above each plate.
TaZIP expression is induced by Zn deficiency
Relative gene expression levels of TaZIP3, ‐5, ‐6, ‐7 and ‐13 were determined in root and shoot tissue obtained during a 1‐week hydroponic Zn‐starvation period. Under these conditions there were no observable differences in wheat plants after 1 week and they showed similar root and shoot fresh weight at the end of this period (Figure S3). However, Zn levels were clearly reduced in both roots and shoots (Figure S3). Other elements were measured, but they showed little change over this period (Figure S4). There was a small but significant increase in root Mn levels in −Zn grown plants but no corresponding change in shoots (Figure S4). Thus the major effect was on the Zn content in both roots and shoots. Having established a system for testing the effects of Zn deficiency, we determined the transcriptional changes of a selected number of ZIPs. In roots, TaZIP3, ‐5, ‐7 and ‐13 showed increased expression under the −Zn treatment, with only TaZIP6 expression remaining fairly stable (Figure 3). TaZIP3, ‐5 and ‐13 showed significantly higher expression levels from the first time point (day 1); TaZIP7 expression was significantly increased from day 3. In the shoot material, expression of all five TaZIPs increased under −Zn conditions but the timing varied between individual genes. TaZIP6 was the slowest to respond, remaining constant until day 5. As in the roots, TaZIP3 showed significantly higher expression from day 1; however, TaZIP5 and ‐13 were slower to respond in the shoot compared with the root. The magnitude of gene‐expression changes varied between the TaZIPs as well as between roots and shoots. TaZIP5 expression increased to a greater extent in the shoots compared with roots, whereas for TaZIP13 the opposite was observed. TaZIP induction under Zn‐deficient conditions was also observed over a longer starvation period (Figure S5). In this case, plants were only grown for 7 days rather than 14 days prior to the Zn starvation. Slight differences were observed, indicating that the developmental stage at which Zn starvation was implemented could have an effect, but overall the results were similar. In this case an induction was seen in TaZIP6 expression in the root but again it was not as high as the induction observed for the other genes at day seven.
Figure 3.
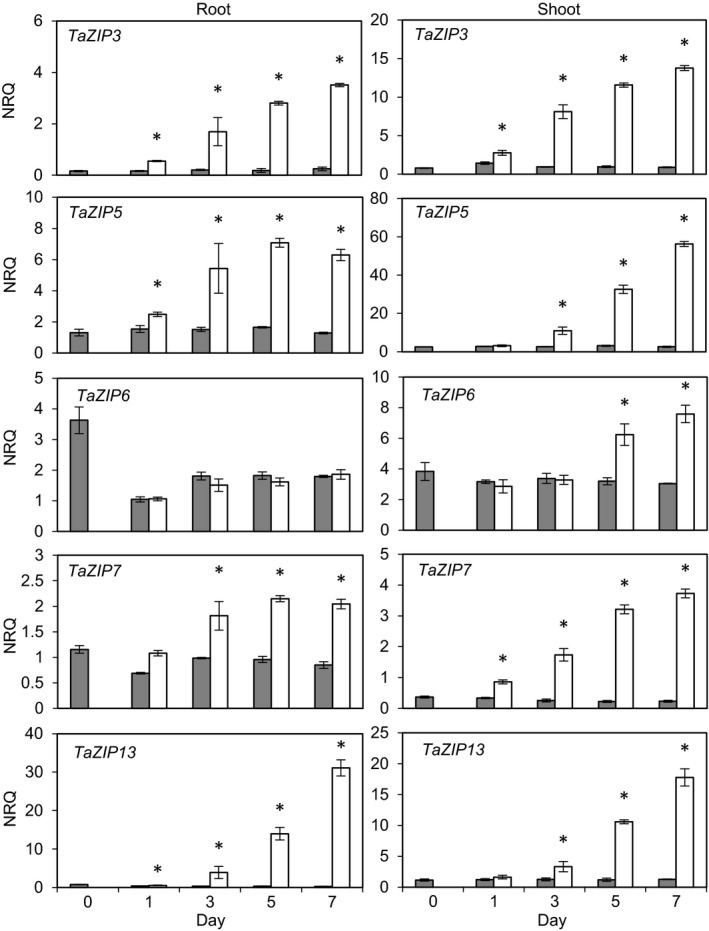
TaZIP gene expression analysis in wheat material throughout a 1‐week Zn starvation period.
Relative expression levels (normalised relative quantification, NRQ) of five wheat ZIP transporter gene transcripts in root and shoot material throughout 1 week of Zn starvation. NRQ values are normalised to TaActin3 and TaSuccDH expression and means of three biological replicates are given (±SEM). Bars within individual graphs displaying an asterisk show a significant difference between treatment means at a given time point. Significance (P < 0.05) was tested post hoc, using Fisher's least significant difference test on log2(1/NRQ)‐transformed data. +Zn = 8 μm Zn (grey bars), −Zn = 0 μm Zn (white bars).
Wheat contains seven group F TabZIPs with a full set of homeologs identified in the wheat genome
Searching the wheat genome revealed seven group F TabZIPs with homeologs identified on the A, B and D genomes. The previous genome‐wide identification of TabZIPs in the wheat genome (Li et al., 2015) reported 11 group F TabZIPs. However, this was inaccurate as closer examination showed that a considerable proportion of these are homeologs of the same gene (Table S2). Our analysis identified seven TabZIPFs, each with three homeologs. The phylogenetic analysis presented in Figure 4 shows that these have a related barley homolog. TabZIPF3a and TabZIPF3b are closely related to TabZIPF3a‐7AL and TabZIPF3b‐7AL, sharing 88% sequence similarity. Their sequence similarity, the location of each on chromosome seven and the fact that each has a barley homolog suggests they have evolved through gene duplication, which is likely to have occurred before the speciation of wheat and barley. TabZIPF1, ‐2 and ‐4 also form a clade more closely related to TabZIPF3a and ‐3b than TabZIPF5 and ‐6. TabZIPF1, ‐2, ‐3a, ‐3b and ‐4 cluster more closely to the two group F Arabidopsis bZIPs shown to be involved in the Zn‐deficiency response (AtbZIP19 and AtbZIP23) rather than to AtbZIP24, which has a reported function in salt tolerance (Yang et al., 2009a).
Figure 4.
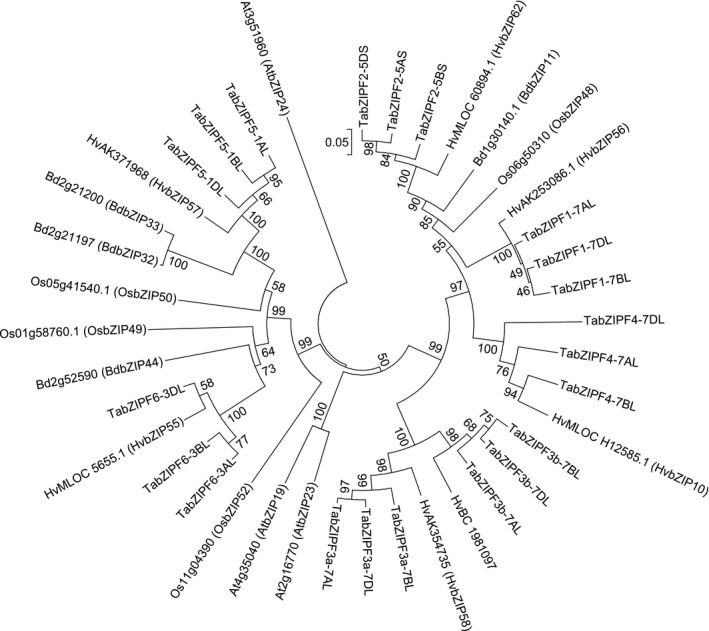
Phylogenetic tree of group F bZIP proteins from cereals and Arabidopsis.
A neighbour‐joining tree was generated for Arabidopsis (At), rice, Oryza sativa (Os), Brachypodium distachyon (Bd), barley, Hordeum vulgare (Hv), and wheat, Triticum aestivum (Ta) group F bZIP coding sequence translations. The Muscle algorithm (Edgar, 2004) was used for the alignment of sequences and the phylogenetic tree was created using mega (v.5.2) software. Evolutionary distances were computed using the p‐distance method and are in units of the number of amino acid differences per site. A thousand bootstrap replicates were used and bootstrap values are shown as percentages. Gene nomenclature for Arabidopsis is from Jakoby et al. (2002), Brachypodium is from Liu and Chu (2015), rice is from Corrêa et al. (2008) and barley is from Pourabed et al. (2015). Full accession information for the wheat genes is provided in Table S2.
Group F bZIP expression is induced by low‐Zn conditions
The relative gene expression levels of TabZIPF1, ‐3a, ‐3b and ‐4 were determined in root and shoot material from a 1‐week period of Zn starvation. The four TabZIPFs were all induced under Zn‐deficient conditions in both roots and shoots but to rather different extents (Figure 5). TabZIPF3a, ‐3b and ‐4 showed the greatest induction in both roots and shoots. In contrast, TabZIPF1 was slower to respond to the −Zn treatment, taking until day 5 in the root and day 3 in the shoot to be expressed to a significantly higher level. The magnitude of induction of TabZIPF1 was less than that of the other TabZIPFs examined.
Figure 5.
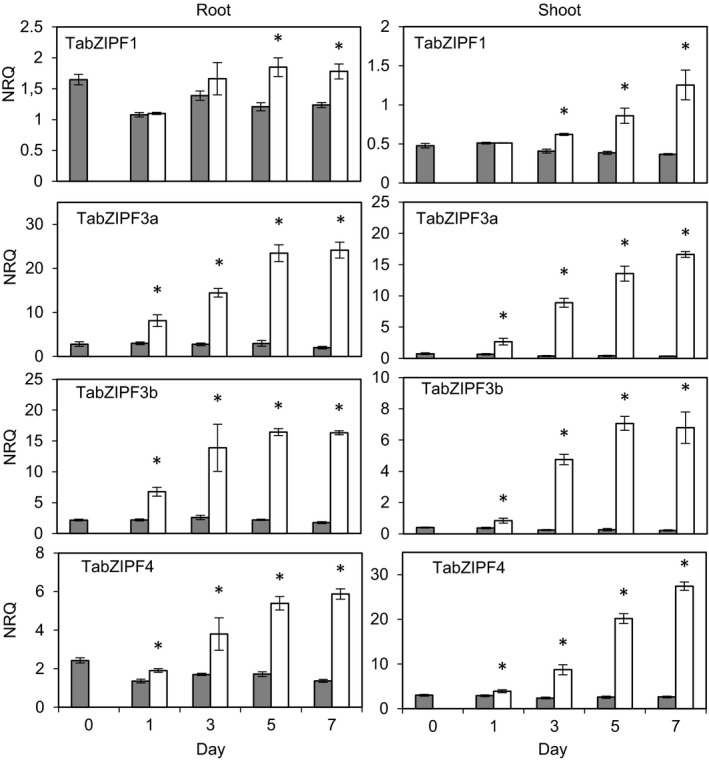
TabZIP gene expression analysis in wheat material throughout a 1‐week Zn starvation period.
Relative expression levels (normalised relative quantification, NRQ) of five wheat bZIP transcription factor gene transcripts in root and shoot material throughout 1 week of Zn starvation. NRQ values are normalised to TaActin3 and TaSuccDH expression and means of three biological replicates are given (±SEM). Bars within individual graphs displaying an asterisk show a significant difference between treatment means at a given time point. Significance (P < 0.05) was tested post hoc, using Fisher's least significant difference test on log2(1/NRQ)‐transformed data. +Zn = 8 μm Zn (grey bars), −Zn = 0 μm Zn (white bars).
Group F bZIPs rescue the Zn‐deficiency phenotype of an Arabidopsis bzip19‐4 bzip23‐2 mutant indicating conserved homeostatic mechanisms
TabZIPF1‐7DL, TabZIPF3b‐7BL, TabZIPF4‐7AL and TabZIPF4‐7DL were investigated further due to variations in the conservation of their group F motif 1. They were cloned into the Arabidopsis expression vector pMDC32 (Curtis and Grossniklaus, 2003), which contains a dual 35S CaMV constitutive promoter. The sequences of the four cloned TabZIPFs are shown in Figure S6. All four cloned TabZIPs show high similarity to AtbZIP19 and AtbZIP23 in both the bZIP domain and group F motif 2; however, the group F motif 1 is less conserved. TabZIPF3b‐7BL and TabZIPF4‐7DL both contain a truncated group F motif 1 compared with the other group F bZIPs as well as their homeologs. TabZIPF1‐7DL and TabZIPF4‐7AL have a group F motif 1 with higher similarity to AtbZIP19 and AtbZIP23. To test their importance in the Zn‐deficiency response mechanism, the ability of these four TabZIPs to rescue the Zn hypersensitivity of the Arabidopsis bzip19 bzip23 double mutant was tested. An alternative double mutant Arabidopsis line, bzip19‐4 bzip23‐2, was createdby Nazri et al. (2017) that demonstrates the same Zn hypersensitivity as the Arabidopsis bzip19‐1 bzip23‐1 double mutant previously characterised (Assunção et al., 2010). The bzip19‐4 bzip23‐2 double mutant was transformed with the four TabZIPFs and two independent transgenic lines were characterised in detail. These were compared with the wild type and the non‐transformed double mutants on half MS media with (15 μm) and without (0 μm) Zn. Expression of both TabZIPF1‐7DL and TabZIPF4‐7AL significantly improved the performance of the bzip19‐4 bzip23‐2 double mutant under low‐Zn conditions and plants displayed a significantly higher fresh weight than the mutant (Figures 6 and S7). TabZIPF3b‐7BL expression slightly increased the growth of the double mutant in one line only under low Zn, though to a lesser extent than both TabZIPF1‐7DL and TabZIPF4‐7AL. TabZIPF4‐7DL provided no rescue to the double mutant (Figure 6).
Figure 6.
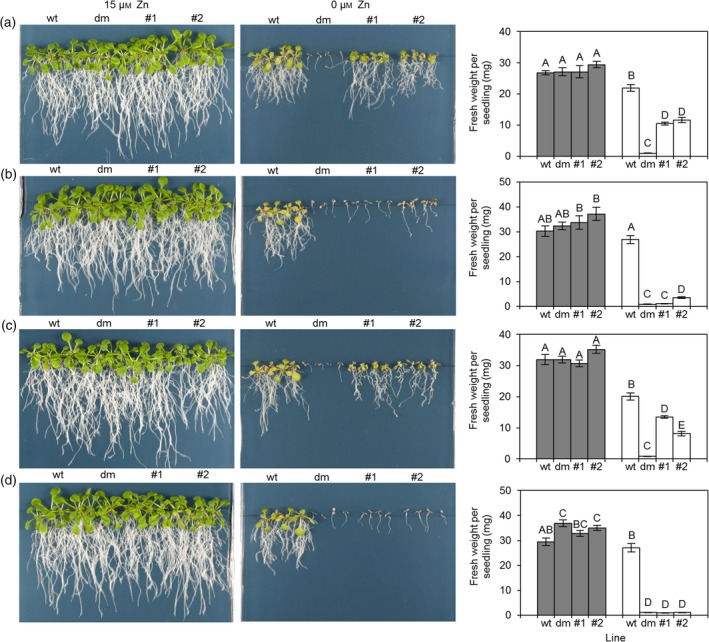
Functional complementation of the Arabidopsis bzip19‐4 bzip23‐2 mutant with group F TabZIPs.
Complementation analysis with (a) TabZIPF1‐7DL, (b) TabZIPF3b‐7BL, (c) TabZIPF4‐7AL and (d) TabZIPF4‐7DL. Fresh weight analysis results shown are mean average fresh weights per seedling ± SEM from six plates with four seedlings per plate, per line (n = 24). Bars with different letters indicate a significant difference (P < 0.05) tested on log‐transformed data using Fisher's least significant difference test. +Zn = 15 μm Zn (grey bars) and –Zn = 0 μm Zn (white bars). Representative plates at both the +Zn (15 μm Zn) and −Zn (0 μm Zn) media concentrations are shown. wt indicates the wild‐type line and dm indicates the untransformed bzip19‐4 bzip23‐2 line. #1 and #2 are two corresponding, independent TabZIPF transformed bzip19‐4 bzip23‐2 lines. All plates illustrated are 18 days post‐germination.
Ability of TabZIP to bind to ZDRE motifs identified in TaZIP promoters
The rescue of the bzip19 bzip23 double mutant by the wheat bZIPs suggested a conserved mechanism of action for these group F bZIP transcription factors. In Arabidopsis bZIP19 functions in binding to ZDRE motifs in Zn‐responsive genes (Assunção et al., 2010). Therefore, the presence of ZDREs in the promoters of TaZIP genes was determined. Regions upstream of the start codon were analysed at up to 2000 bp where possible. Motifs were regarded as potential ZDREs if they had no more than one mismatch to the ZDRE consensus (RTGTCGACAY) reported by Assunção et al. (2010). ZDREs were present in the promoter regions of a number of the ZIP genes; these are shown in Table S3 with their location relative to the predicted start codon. TaZIPs with a completely conserved ZDRE domain include TaZIP5, ‐7, ‐8 and ‐10; additionally TaZIP1, ‐3, ‐6, ‐9 and ‐13 contain at least one ZDRE motif with one mismatch to the consensus in their promoter sequence.
To directly test the regulatory link between the TabZIPs and TaZIPs, the binding ability of in vitro synthesised bZIPs to putative ZDRE motifs discovered in the promoter regions of TaZIPs was determined (Figure 7). AtbZIP19, used as a control, created a band shift (indicative of binding) when two or three copies of the AtZIP4 ZDRE were present (probe names Ass2Z and Ass3Z), but not with a mutated three‐copy AtZIP4 ZDRE probe (Ass3Zmut). AtbZIP19 also produced band shifts with the previously untested TaZIP ZDRE probes 3, 5, 13 and 7. No shift was observed with the TaZIP6 ZDRE probe, suggesting AtbZIP19 does not interact with the putative ZDRE identified in the promoter of TaZIP6 (Figure 7a).
Figure 7.
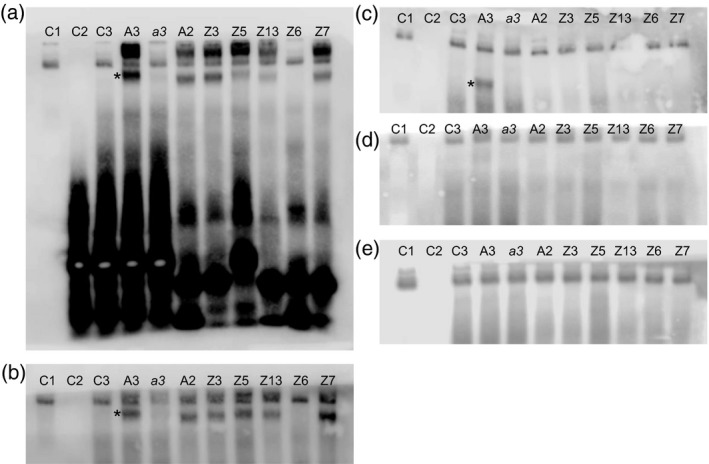
Electrophoretic mobility shift assays determining the binding ability of AtbZIP19 and TabZIPs to Zn‐deficiency‐response element (ZDRE)‐containing probes.
Electrophoretic mobility shift assays were used to investigate the binding ability of AtbZIP19 (a) and five TabZIPs [TabZIPF1‐7DL (b), TabZIPF3b‐7BL (c), TabZIPF4‐7AL (d) and TabZIPF4‐7DL (e)] to Arabidopsis ZDRE‐containing probes and wheat ZDRE‐containing probes. Lanes C1–C3 are control lanes: C1 is TabZIPF protein only, no probe. C2 is probe (Ass3Z), no protein. C3 is probe (Ass3Z) with TNT wheat germ mix (no template). All other lanes contain in vitro synthesised TabZIPs (as labelled) with the following probes: lane A3 is Ass3Z, a3 is Ass3Zmut (mutated Ass3Z) and A2 is Ass2Z which all contain Arabidopsis ZDREs. Z3–Z13 are TaZIP3ZDRE–TaZIP13ZDRE, which all contain wheat ZDREs. Asterisks indicate a band shift. Probe sequences are provided in Table S7.
TabZIPF1‐7DL created a band shift with Ass3z and Ass2Z, which are based on AtZIP4 ZDREs, but not the mutated version, Ass3Zmut. TaZIP ZDRE probes 3, 5, 13 and 7 all produced a band shift indicating an interaction of TabZIPF1‐7DL with these ZDREs. As with AtbZIP19, however, TaZIP6 ZDRE did not produce a band shift (Figure 7b). TabZIPF3b‐7BL was only able to bind to the probe containing three AtZDREs (Ass3Z) and none of the TaZIP ZDRE probes (Figure 7c). Ass3Z is a modification of the ZDREs present in the promoter of AtZIP4. It has three ZDREs adjacent to each other whereas the promoter has two ZDREs spaced 128‐bp apart (Figure 7). Neither TabZIPF4‐7AL nor TabZIPF4‐7DL bound to any of the ZIP ZDRE probes tested (Figure 7d, e).
Discussion
TaZIPs have an important role in the transport of Zn
Analysis of the wheat genome revealed 14 TaZIPs, each with a homeolog on the A, B and D genomes. This in an increase in the number in the previous phylogenetic analysis of TaZIPs (Tiong et al., 2015) where 12 TaZIPs were identified and homeologs were not reported. The lengths ranged from 277 to 578 amino acids with much of the variation coming from the more divergent homologs TaZIP11, ‐14 and ‐16 (homologs of AtPutZnT, AtIAR and AtZTP29, respectively). TaZIP11, ‐14 and ‐16 were more distantly related to the other TaZIPs identified, suggesting a different role or cellular localisation in the plant. This is supported in knockout studies of AtIAR in Arabidopsis and heterologous expression of AtZTP29 in yeast (Lasswell et al., 2000; Wang et al., 2010).
Functional characterisation using the yeast zrt1/zrt2 mutant shows that TaZIP3, ‐6, ‐7, ‐9 and ‐13 rescue the Zn hypersensitivity of this mutant. When considered alongside the Zn‐induced gene expression patterns of the TaZIPs determined in this study, this is strong evidence that ZIP members from this clade are important Zn transporters in wheat. Although not tested in yeast, TaZIP5 was induced under Zn‐deficient conditions, suggesting a role in the Zn homeostatic framework. Barley homologs of three TaZIPs investigated in this study have shown Zn‐responsive expression (TaZIP3/HvZIP3, TaZIP5/HvZIP5 and TaZIP7/HvZIP7) (Pedas et al., 2009; Tiong et al., 2015). Previously, from yeast studies, Pedas et al. (2009) indicated that HvIRT1 and HvZIP3, ‐5 and ‐8 could transport Zn. Additionally, work in yeast suggests that the wild emmer wheat (T. turgidum ssp. dicoccoides) TdZIP1 (TaZIP3 homolog) may also function as a Zn transporter (Durmaz et al., 2011). The five TaZIPs characterised in this study were able to complement the yeast zrt1/zrt2 mutant but were unable to rescue the Fe‐uptake mutant fet3/fet4, suggesting there is substrate selectivity for these particular ZIPs.
The barley TaZIP6 homolog HvZIP6 is unresponsive to Zn status in the shoot but expression has been shown to increase in the root (Tiong et al., 2015). Additionally, OsZIP6 expression is induced in both the roots and the shoots of Zn‐deficient rice plants (Kavitha et al., 2015). In this study we show that TaZIP6 expression was not induced in the root during 1 week of Zn‐deficient growth but was significantly induced in the shoot after 5 days. The specific spatiotemporal expression patterns indicate that individual TaZIPs may have precise roles within the plant. Feasibly the Zn requirement of different regions in the root and shoot varies and may explain the differential pattern of increased gene expression initiation observed and its magnitude.
Specific group F bZIPs induce the transcription of key Zn transporters, indicating a conserved homeostatic mechanism
The analysis presented here discovered seven distinct sets of group F bZIPs present in the wheat genome. Amongst the TabZIPFs identified (and between homeologs), conservation of the cysteine–histidine‐rich group F motif 1 varied. The group F motif 2 was widely conserved, as was the general bZIP domain. Previously, the two cysteine–histidine‐rich motifs present in group F bZIPs were hypothesised to be the binding site for Zn2+ ions (Assunção et al., 2010, 2013). As a small and efficient electron acceptor, Zn2+ is able to form tetrahedral complexes with sulphur and nitrogen ligands found in the side chains of cysteine and histidine (Tauris et al., 2009; Pace and Weerapana, 2014).
The role of four TabZIPFs in the Zn homeostatic mechanism was investigated by expression in the Arabidopsis bzip19‐4 bzip23‐2 double mutant line. TabZIPF1‐7DL and TabZIPF4‐7AL provided partial complementation of this mutant, resulting in improved growth under the –Zn treatment compared with the untransformed bzip19‐4 bzip23‐2 line. This indicates that the role of these genes in wheat is likely to be in the Zn‐deficiency response, and furthermore that they play a similar role in wheat and Arabidopsis. They did not completely rescue the bzip19‐4 bzip23‐2 mutant. This may be due to slight differences in Zn2+ affinity of the TabZIPs, or a reduced binding ability to ZDREs in the promoter regions of Zn‐responsive Arabidopsis genes. TabZIPF1‐7DL and TabZIPF4‐7AL show divergence from AtbZIP19 and AtbZIP23 in the 3′ end of group F motif 1 (Figure S6), this may increase the Zn2+ affinity of these TabZIPs, although this requires further study.
Binding of AtbZIP19 and TabZIPF1‐7DL to ZDREs found in the promoter of AtZIP4 as well as TaZIP3, ‐5, ‐7 and ‐13 demonstrated that an analogous Zn‐homeostatic mechanism exists in the two species. TabZIPF4‐7AL partially rescued the bzip19‐4 bzip23‐2 line, but did not bind to the ZDRE‐containing probes in vitro. This suggests this bZIP functions in the Zn‐deficiency response of wheat but the lack of binding indicates that either it acts through a different binding mechanism or that, potentially, binding is not observed here due to a requirement for native ZIP promoter flanking sequence or for a narrow and specific Zn concentration range not simulated in the electrophoretic mobility shift assay (EMSA).
TabZIPF3b‐7BL and TabZIPF4‐7DL were unable to restore the Zn‐deficiency response of the bzip19‐4 bzip23‐2 mutant. Neither of these TabZIPFs contains a well‐conserved group F motif 1, which could be a causal factor for their lack of rescue ability. Despite being homeologs with high sequence similarity (84%), TabZIPF4‐7AL and TabZIPF4‐7DL showed different rescue abilities. This is most likely due to their different levels of group F motif 1 conservation. Neither TabZIPF3b‐7BL nor TabZIPF4‐7DL were able to bind to TaZDRE‐containing probes in vitro. Their presence in the wheat genome and expression in planta could be the result of genetic redundancy due to the hexaploidy of wheat, although an alternative hypothesis is a role in the fine tuning of the Zn homeostatic mechanism in wheat. The truncated motif in these TabZIPFs may facilitate the increased expression of specific Zn homeostatic genes at precise plant tissue locations. This may permit the plant to alter expression of particular genes in response to certain conditions or developmental stages without changing the expression of an entire suite of genes throughout the whole plant at a single given point in the Zn concentration continuum.
The expression profiles of TabZIPF1, ‐3a, ‐3b and ‐4 show they were induced, although to different degrees, under Zn‐deficient conditions. The current Zn‐homeostatic model for adapting to Zn deficiency suggests that dissociation of Zn2+ from group F bZIPs results in their binding to ZDRE motifs in the promoters of Zn‐responsive genes to increase their expression, seen particularly in the ZIPs (Assunção et al., 2010). Transcriptional regulation of the bZIPs themselves was not included as part of the model. Modifications may include a further level of intricacy, revealed here in wheat, whereby induction of bZIPs would allow for a sustained adaptive response under prolonged deficiency. Due to the differential effects observed across the TabZIPs in response to Zn deficiency, it is possible that certain bZIPs, notably the members with lower induction due to Zn deficiency, are ‘master regulators’ of other bZIPs. TabZIPF1 may exert a global induction of ZIPs at critical levels of Zn deficiency while also influencing other bZIPs that then contribute to further adaptive responses. As such, TabZIPF1 may be a promising target for future breeding or transgenic strategies.
In summary, our findings demonstrate that wheat contains a complex system for regulating Zn homeostasis. It has an expanded number of group F bZIP transcription factors that serve in altering the expression of ZIPs by binding to ZDREs in their promoters. These genes show differences in spatial and temporal expression patterns, indicating that there is a homeostatic network allowing adaptation to low Zn availability by contributing to Zn uptake and distribution under changing nutrient availability. The variation in conservation of cysteine–histidine‐rich motifs throughout the wheat group F bZIPs may provide additional refinement to the homeostatic mechanism.
Experimental procedures
Bioinformatics analysis
Gene and coding sequences of wheat ZIPs and bZIPs were identified using a Blast analysis of the IWGSC wheat survey sequence database and the TGACv1 assembly (http://www.plants.ensemble.org) with rice, barley and Brachypodium homologs. Phylogenetic analysis was performed by multiple protein sequence alignment using geneious v.8.1.3 (http://www.geneious.com; Kearse et al., 2012) with the Muscle algorithm (Edgar, 2004). mega5 (Tamura et al., 2011) was used for calculation of phylogenetic trees using the neighbour‐joining method with evolutionary distances computed using the p‐distance method and 1000 bootstrap replicates conducted for each phylogeny.
Plant growth (T. aestivum and Arabidopsis thaliana)
Hydroponic culture 1‐week starvation experiment
Triticum aestivum cv. Paragon seedlings were germinated on sterile water‐soaked soft paper tissue for 7 days before transfer to single‐plant hydroponic aerated culture. Plants were cultivated for the first 3 days on half‐strength before changing to full‐strength modified Letcombe liquid nutrient solution [1.5 mm Ca(NO3)2, 5 mm KNO3, 2 mm NaNO3, 1 mm MgSO4, 0.5 mm KH2PO4, 25 μm FeEDTA, 0.2 μm CuCl2·2H2O, 1 μm H3BO3, 0.6 μm MnCl2.4H2O, 0.1 μm Na2MoO4·2H2O, 5 μm KCl, 8 μm ZnCl2, 2.56 mm 2‐(N‐morpholine)‐ethanesulphonic acid (MES) buffer, pH = 5.8; Drew and Saker, 1984] including the chelator 75.5 μm HEDTA. The solution was changed three times a week. Two weeks after germination, plants (apart from the +Zn control plants) were Zn‐starved by omitting the ZnCl2 from the culture solution (−Zn). Plants were grown in a growth chamber with 16‐h day conditions of 20°C/70% humidity and 500 μmol m−2 sec−1 light and night conditions of 16°C/80% humidity. Samples were taken at day 0, 1, 3, 5 and 7 of Zn starvation. Whole roots (washed with deionised water and dried briefly on soft paper towels) and whole shoots were sampled, weighed and immediately frozen in liquid nitrogen and stored at −80°C.
Hydroponic culture 3‐week starvation experiment
Conditions as above except for a reduction to 7 days rather than 14 days of hydroponic culture in +Zn solution prior to Zn‐starving the plants.
Arabidopsis thaliana plants were grown in growth chambers with 16‐h day conditions, 23°C and 120 μmol m−2 sec−1 light, night conditions 18°C. Soil used was autoclaved and comprised equal proportions of vermiculite, Levingtons M2 and John Innes No. 2 compost in 8‐cm pots supplemented with 0.28 g L−1 Intercept insecticide (Bayer, https://www.bayer.com/).
Plant total RNA isolation
All wheat material was homogenised using a SPEX freezer mill (SPEX CertiPrep Ltd, http://www.spexcsp.com/) in liquid nitrogen, aliquotted into 2‐ml micro‐tubes and stored at −80°C. Total RNA was isolated by a modified method based on Verwoerd et al. (1989) with additional phenol–chloroform–isoamyl alcohol extractions. Possible genomic DNA contamination was removed by RNase‐free DNase treatment. The final air‐dried pellet was dissolved in an appropriate volume of RNase‐free water.
Cloning full‐length TabZIPs and TaZIPs
First‐strand cDNA synthesis was performed from 2 μg total RNA and a dT‐adapter primer (Invitrogen Superscript III, http://www.invitrogen.com/; standard protocol, 2 h synthesis time). Full‐length TabZIPs were amplified using the oligonucleotide primers specified in Table S4 with Pfu DNA polymerase (Promega, http://www.promega.com/). TabZIP amplicons were subsequently topoisomerase‐cloned into pENTR/D‐TOPO (Invitrogen) and transformed into Escherichia coli. TabZIP plant expression vectors were created by Gateway (Invitrogen) recombination of pENTR:TabZIP vectors into pMDC32 (Curtis and Grossniklaus, 2003).
Full‐length TaZIPs were amplified using Q5 High‐fidelity DNA polymerase (New England Biolabs, https://www.neb.com/). TaZIP amplicons were cloned into pGEM‐T Easy vector (Promega) and subcloned into a pYES2 yeast expression vector using EcoRI digestion and subsequent ligation. All vectors were sequenced before Arabidopsis or yeast transformation.
Functional complementation in yeast
The following strains of the yeast Saccharomyces cerevisiae were used in this study: wild‐type DY1457 (MATa, ade1/+ can1, his3, leu2, trp1, ura3), zrt1/zrt2 (DY1457 + zrt1::LEU2, zrt2::HIS3) and fet3/fet4 (DY1457 + fet3‐2::HIS3, fet3‐1::LEU2). Yeast strains were transformed with pYES2TaZIP vectors using the S.c. EasyComp Transformation Kit (Invitrogen) according to the manufacturer's instructions. Following transformation, PCR‐confirmed pYES2TaZIP containing zrt1/zrt2 colonies was inoculated in 10 ml of SC‐glucose minus uracil (pH 5.3) overnight (30°C with shaking at 200 r.p.m.). Inoculums were pelleted (3 min, 1300 g), suspended in SC‐galactose minus uracil (pH 5.3) and incubated for 4 h (30°C with shaking at 200 r.p.m.) to allow gene induction. Inoculums were then pelleted and washed with SC‐galactose minus uracil minus Zn (pH 5.3) to remove excess Zn from the pellet. Inoculums were diluted to OD600 = 0.4 using SC‐glucose minus uracil minus Zn (pH 5.3) and serial dilutions made (1:2, 1:10, 1:100 and 1:1000). Seven microlitres of the dilutions was plated onto SC‐galactose minus uracil plates (pH 5.3) containing either 200 μm ZnSO4 (+Zn), 0 Zn + 0 mm, 2 mm, 5 mm and 7.5 mm EGTA, incubated at 30°C and photographed after 8 days.
Following fet3/fet4 transformation, positive colonies were confirmed using PCR and inoculated in 10 ml SC‐glucose minus uracil + 10 μm FeCl3 (pH 4.0) overnight (30°C with shaking at 200 r.p.m.). Inoculums were pelleted (3 min, 1300 g), suspended in SC‐galactose minus uracil + 10 μm FeCl3 (pH 4.0) and incubated for 4 h (30°C with shaking at 200 r.p.m.) to allow gene induction. Inoculums were diluted to OD600 = 0.4 using SC‐glucose minus uracil + 10 μm FeCl3 (pH 4.0) and serial dilutions made (1:2, 1:10, 1:100 and 1:1000). Seven microlitres of the dilutions was plated onto SC‐galactose minus uracil minus Fe (pH 4.0) plates supplemented with 0 μm, 0.74 μm or 10 μm FeCl3, incubated at 30°C and photographed after 3 days.
Expression analysis of TaZIPs and TabZIPs
Real‐time PCR was performed using the Applied Biosystems 7500 Real Time PCR System and the SYBR® Green Jumpstart™ Taq ReadyMix™ (Sigma‐Aldrich, http://www.sigmaaldrich.com). The 25‐μl reactions contained 1 μl of cDNA and 250 nm of each primer. The primers used are given in Table S5 and were designed to cover gene expression of the homeologous genes from all three wheat genomes. Mean primer efficiencies were estimated using the linear phase of all individual reaction amplification curves (Ramakers et al., 2003) calculated using the LinRegPCR package (Ruijter et al., 2009). The stable, constitutive genes Actin 3 and Succinate Dehydrogenase were used to determine the normalised quantification of expression. The normalised relative quantity (NRQ) of expression was calculated in relation to the Ct values and the primer efficiency (E) of both the target gene (X) and the mean of the two normalisation reference genes (N) as normalised relative expression (NRE) based on Rieu and Powers (2009):
Statistically significant changes in relation to Zn treatment were calculated using a two‐way analysis of variance (anova) on log2(1/NRQ)‐transformed data followed by a post hoc Fisher's least significant difference test (LSD) at the 5% significance level. All statistical analyses were done in Genstat 17th edition (VSN International, https://www.vsni.co.uk/).
Complementation of the Arabidopsis bzip19‐4 bzip23‐2 mutant line with group F TabZIPs
pMDC32TabZIPF plasmids were transformed into Agrobacterium tumefaciens GV3850 by electroporation. Arabidopsis thaliana (Col‐8) bzip19‐4 bzip23‐2 double mutants (Nazri et al., 2017) were transformed using the floral dip method (Clough and Bent, 1998) with an additional 3‐h pre‐induction of vir genes by the addition of 100 μm acetosyringone to the culture prior to floral dipping. Homozygous T3 plants were used in subsequent phenotype assays.
Growth assays were conducted using two independent T3 homozygous lines from each pMDC32‐TabZIPF transformation. Four sterilised seeds of each T3 line, the Col‐8 wild type and the untransformed bzip19‐4 bzip23‐2 were plated onto six square plates of 0.5 × MS medium (Murashige and Skoog, 1962) containing 1% (w/v) sucrose and 0.8% (w/v) agarose containing 15 μm ZnSO4 (+Zn) and 0 μm ZnSO4 (−Zn). Following 18 days of growth, plates were photographed and root and shoot fresh weights were measured. Individual fresh weights were obtained by calculating the average from the combined weight of four seedlings prior to statistical analysis. A two‐way anova was performed on log‐transformed data for each complementation experiment (the four TabZIPFs investigated) followed by a post hoc Fisher's LSD at the 5% significance level.
Electrophoretic mobility shift assay
Full‐length coding sequences of bZIPs were PCR‐amplified from sequenced vectors using primer pairs containing an SP6 promoter and Kozak region (forward) and a poly‐A tail (reverse) (Table S6). bZIP proteins were in vitro translated using the TNT SP6 High‐Yield Wheat Germ Protein Expression Kit (Promega) following the manufacturer's instructions with 690 ng of purified PCR product added to 18 μl of wheat germ master mix. ZDREs containing biotinylated oligonucleotides and nonbiotin‐labelled complementary oligonucleotides were synthesised (Table S7) (Eurofins, https://www.eurofins.com/) and annealed at a concentration of 1 pmol μl−1, 10 mm 2‐amino‐2‐(hydroxymethyl)‐1,3‐propanediol (TRIS), 1 mm EDTA, 50 mm NaCl for 5 min at 95°C and slowly cooling to room temperature (20°C) overnight. In vitro translated proteins (3 μl of the 30‐μl reaction) were incubated with 4 μl of the annealed oligonucleotide solution in a 20‐μl binding reaction containing 20 mm TRIS‐HCl (pH 7.5), 10 mm KCl, 1 mm EDTA, 0.25 μg μl−1 BSA, 1 mm DTT and 0.25 μg μl−1 salmon sperm DNA at 28°C for 30 min. The DNA–protein complex was analysed on a 6% native PAGE DNA retardation gel (Thermo Fisher Scientific, https://www.thermofisher.com/) using 0.5 × TRIS borate–EDTA at 100 V for 75 min. After electrophoresis, the gel was blotted to Amersham Hybond‐N+ membrane (GE Healthcare, http://www3.gehealthcare.com/en/global_gateway) using the XCell II™ Blot module (Thermo Fisher Scientific). The membrane was crosslinked using a Stratalinker® UV crosslinker and the signal detected using Chemiluminescent Nucleic Acid Detection Module (Thermo Fisher Scientific) according to manufacturer's instructions using the Odyssey® FC imaging system (Li‐Cor, https://www.licor.com/), with a 2‐min exposure.
Determination of the Zn content in plant material
Cryogenically milled roots and shoots of hydroponically grown T. aestivum cv. Paragon were freeze dried for 2 days, then digested in 5 ml of nitric acid:perchloric acid (85:15, v/v; 70% concentration, trace analysis grade; Fisher Scientific) for a minimum of 2 h at room temperature followed by a 5‐h programmed thermoblock cycle. Five millilitres of 25% (v/v) nitric acid was added to the solution and the tubes were reheated for 1 h at 80°C. Ultra‐pure water (>18 MΩ) was added to to makel up to 9 ml, mixed well and re‐warmed for a further 30 min at 80°C. After cooling, the solutions were made up to final volumes of 10 ml with deionised H2O. Inductively coupled plasma optical emission spectrometry analysis was carried out using an Optima inductively coupled plasma–optical emission spectrometer (Perkin Elmer Life and Analytical Sciences, http://www.perkinelmer.com/).
Conflicts of interest
The authors confirm that they have no conflicts of interest to declare.
Supporting information
Figure S1. Phylogenetic tree of Triticum aestivum and barley ZIP proteins showing homeolog grouping.
Figure S2. Multiple sequence alignment of TaZIPs.
Figure S3. Phenotypic effect of Zn starvation throughout a 3‐week growth period.
Figure S4. Mineral concentration analysis of wheat root and shoot samples during a 1‐week Zn‐ starvation period.
Figure S5. Gene expression analysis of TaZIPs in wheat root and shoot material throughout an extended Zn starvation period of 3 weeks.
Figure S6. Multiple sequence alignment of cloned group F TabZIPs, AtbZIP19 and AtbZIP23.
Figure S7. Confirmatory PCR of 35S::TabZIP‐transformed Arabidopsis lines.
Table S1. TaZIP identity matrix.
Table S2. Wheat TaZIP and TabZIP gene identification details.
Table S3. Overview of ZDREs present in promoters of TaZIPs.
Table S4. Oligonucleotide primer sequences used for cloning of full length TaZIPs and TabZIPs.
Table S5. Oligonucleotide primer sequences used for SYBR Green real time RT‐PCR expression analysis.
Table S6. Oligonucleotide primer sequences used for PCR‐amplification of TabZIPs. With SP6 promoters and Poly‐A tails prior to in vitro transcription translation.
Table S7. Complementary oligonucleotides used in EMSAs.
Acknowledgements
This study was supported by a University of Southampton and Rothamsted Research BBSRC DTP studentship (BB/J014451/1) and the 20:20 Wheat® programme (BBS/E/C/00005202) at Rothamsted Research. Rothamsted Research receives strategic funding from the BBSRC.
Contributor Information
Lorraine E. Williams, Email: l.e.williams@soton.ac.uk
Malcolm J. Hawkesford, Email: malcolm.hawkesford@rothamsted.ac.uk
References
- Assunção, A.G.L. , Herrero, E. , Lin, Y. , Huettel, B. , Talukdar, S. and Smaczniak, C. (2010) Arabidopsis thaliana transcription factors bZIP19 and bZIP23 regulate the adaptation to zinc deficiency. Proc. Natl. Acad. Sci. U. S. A. 107, 10296–10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assunção, A.G.L. , Persson, D.P. , Husted, S. , Schjørring, J.K. , Alexander, R.D. and Aarts, M.G.M. (2013) Model of how plants sense zinc deficiency. Metallomics, 5, 1110–1116. [DOI] [PubMed] [Google Scholar]
- Broadley, M.R. , White, P.J. , Hammond, J.P. , Zelko, I. and Lux, A. (2007) Zinc in plants. New Phytol. 173, 677–702. [DOI] [PubMed] [Google Scholar]
- Brown, P. , Cakmak, I. and Zhang, Q. (1993) Form and function of zinc in plants In Zinc in Soils and Plants (Robson A.D. ed). Dordecht, the Netherlands: K. A. Publishers , pp. 93–106. [Google Scholar]
- Bughio, N. , Yamaguchi, H. , Nishizawa, N.K. , Nakanishi, H. and Mori, S. (2002) Cloning an iron‐regulated metal transporter from rice. J. Exp. Bot. 53, 1677–1682. [DOI] [PubMed] [Google Scholar]
- Cakmak, I. (2000) Tansley Review No.111: possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 146, 185–205. [DOI] [PubMed] [Google Scholar]
- Clough, S.J. and Bent, A.F. (1998) Floral dip: a simplified method for Agrobacterium‐mediated transformation of Arabidopsis thaliana . Plant J. 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Corrêa, L.G.G. , Riaño‐Pachón, D.M. , Schrago, C.G. , dos Santos, R.V. , Mueller‐Roeber, B. and Vincentz, M. (2008) The role of bZIP transcription factors in green plant evolution: adaptive features emerging from four founder genes. PLoS ONE, 3, e2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M.D. and Grossniklaus, U. (2003) A Gateway cloning vector set for high‐throughput functional analysis of genes in planta. Plant Physiol. 133, 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew, M.C. and Saker, L.R. (1984) Uptake and long‐distance transport of phosphate, potassium and chloride in relation to internal ion concentrations in barley; evidence of non‐allosteric regulation. Planta, 160, 500–507. [DOI] [PubMed] [Google Scholar]
- Durmaz, E. , Coruh, C. , Dinler, G. et al. (2011) Expression and cellular localization of ZIP1 transporter under zinc deficiency in wild emmer wheat. Plant Mol. Biol. 29, 582–596. [Google Scholar]
- Edgar, R.C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotz, N. , Fox, T. , Connolly, E. , Park, W. , Guerinot, M.L. and Eide, D. (1998) Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc. Natl. Acad. Sci. U. S. A. 95, 7220–7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba, S. , Kurata, R. , Kobayashi, M. , Yamagishi, Y. , Mori, I. , Ogata, Y. and Fukao, Y. (2015) Identification of putative target genes of bZIP19, a transcription factor essential for Arabidopsis adaptation to Zn deficiency in roots. Plant J. 84, 323–334. [DOI] [PubMed] [Google Scholar]
- Ishimaru, Y. , Suzuki, M. , Kobayashi, T. , Takahashi, M. , Nakanishi, H. , Mori, S. and Nishizawa, N.K. (2005) OsZIP4, a novel zinc‐regulated zinc transporter in rice. J. Exp. Bot. 56, 3207–3214. [DOI] [PubMed] [Google Scholar]
- Ishimaru, Y. , Suzuki, M. , Tsukamoto, T. et al. (2006) Rice plants take up iron as an Fe3 + ‐phytosiderophore and as Fe2 + . Plant J. 45, 335–346. [DOI] [PubMed] [Google Scholar]
- Jakoby, M. , Weisshaar, B. , Dröge‐Laser, W. , Vicente‐Carbajosa, J. , Tiedemann, J. , Kroj, T. and Parcy, F. (2002) bZIP transcription factors in Arabidopsis . Trends Plant Sci. 7, 106–111. [DOI] [PubMed] [Google Scholar]
- Kavitha, P. , Kuruvilla, S. and Mathew, M. (2015) Functional characterization of a transition metal ion transporter, OsZIP6 from rice (Oryza sativa L.). Plant Physiol. Biochem. 97, 165–174. [DOI] [PubMed] [Google Scholar]
- Kearse, M. , Moir, R. , Wilson, A. et al. (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28, 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumssa, D.B. , Joy, E.J.M. , Ander, E.L. , Watts, M.J. , Young, S.D. , Walker, S. and Broadley, M.R. (2015) Dietary calcium and zinc deficiency risks are decreasing but remain prevalent. Sci. Rep. 5, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasswell, J. , Rogg, L.E. , Nelson, D.C. , Rongey, C. and Bartel, B. (2000) Cloning and characterization of IAR1, a gene required for auxin conjugate sensitivity in Arabidopsis . Plant Cell, 12, 2395–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. , Jeong, H.J. , Kim, S.A. , Lee, J. , Guerinot, M.L. and An, G. (2010a) OsZIP5 is a plasma membrane zinc transporter in rice. Plant Mol. Biol. 73, 507–517. [DOI] [PubMed] [Google Scholar]
- Lee, S. , Kim, S.A. , Lee, J. , Guerinot, M.L. and An, G. (2010b) Zinc deficiency‐inducible OsZIP8 encodes a plasma membrane‐localized zinc transporter in rice. Mol. Cells, 29, 551–558. [DOI] [PubMed] [Google Scholar]
- Li, X. , Gao, S. , Tang, Y. , Li, L. , Zhang, F. , Feng, B. , Fang, Z. , Ma, L. and Zhao, C. (2015) Genome‐wide identification and evolutionary analyses of bZIP transcription factors in wheat and its relatives and expression profiles of anther development related TabZIP genes. BMC Genom. 16, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. and Chu, Z. (2015) Genome‐wide evolutionary characterization and analysis of bZIP transcription factors and their expression profiles in response to multiple abiotic stresses in Brachypodium distachyon . BMC Genom. 16, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner, M. , Seamon, J. , Craft, E. and Kochain, L. (2013) Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J. Exp. Bot. 64, 369–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T. and Skoog, F. (1962) A revised medium for rapid growth and bio assays with Tobacco tissue cultures. Physiol. Plant. 15, 473–497. [Google Scholar]
- Nazri, A.Z. , Griffin, J.H.C. , Peaston, K.A. , Alexander‐Webber, D.G.A. and Williams, L.E. (2017) F‐group bZIPs in barley ‐ a role in Zn deficiencies. Plant Cell Environ. https://doi.org/10.1111/pce.13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace, N. and Weerapana, E. (2014) Zinc‐binding cysteines: diverse functions and structural motifs. Biomolecules, 4, 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren, M.G. , Clemens, S. , Williams, L.E. , Krämer, U. , Borg, S. , Schjørring, J.K. and Sanders, D. (2008) Zinc biofortification of cereals: problems and solutions. Trends Plant Sci. 13, 464–473. [DOI] [PubMed] [Google Scholar]
- Pedas, P. , Ytting, C.K. , Fuglsang, A.T. , Jahn, T.P. , Schjoerring, J.K. and Husted, S. (2008) Manganese efficiency in barley: identification and characterization of the metal ion transporter HvIRT1. Plant Physiol. 148, 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedas, P. , Schjoerring, J.K. and Husted, S. (2009) Identification and characterization of zinc‐starvation‐induced ZIP transporters from barley roots. Plant Physiol. Biochem. 47, 377–383. [DOI] [PubMed] [Google Scholar]
- Pourabed, E. , Ghane Golmohamadi, F. , Soleymani Monfared, P. , Razavi, S.M. and Shobbar, Z.S. (2015) Basic leucine zipper family in barley: genome‐wide characterization of members and expression analysis. Mol. Biotechnol. 57, 12–26. [DOI] [PubMed] [Google Scholar]
- Ramakers, C. , Ruijter, J.M. , Lekanne Deprez, R.H. and Moorman, A.F.M. (2003) Assumption‐free analysis of quantitative real‐time polymerase chain reaction (PCR) data. Neurosci. Lett. 339, 62–66. [DOI] [PubMed] [Google Scholar]
- Ramesh, S.A. , Shin, R. , Eide, D.J. and Schachtman, D.P. (2003) Differential metal selectivity and gene expression of two zinc transporters from rice. Plant Physiol. 133, 126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieu, I. and Powers, S.J. (2009) Real‐time quantitative RT‐PCR: design, calculations, and statistics. Plant Cell, 21, 1031–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruijter, J.M. , Ramakers, C. , Hoogaars, W.M.H. , Karlen, Y. , Bakker, O. , Hoff, M.J.B. , Van Den Hoff, M.J. and Moorman, A.F.M. (2009) Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 37, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, M. , Bashir, K. , Inoue, H. , Takahashi, M. , Nakanishi, H. and Nishizawa, N.K. (2012) Accumulation of starch in Zn‐deficient rice. Rice, 5, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, K. , Peterson, D. , Peterson, N. , Stecher, G. , Nei, M. and Kumar, S. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauris, B. , Borg, S. , Gregersen, P.L. and Holm, P.B. (2009) A roadmap for zinc trafficking in the developing barley grain based on laser capture microdissection and gene expression profiling. J. Exp. Bot. 60, 1333–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiong, J. , McDonald, G.K. , Genc, Y. , Pedas, P. , Hayes, J.E. , Toubia, J. , Langridge, P. and Huang, C.Y. (2013) HvZIP7 mediates zinc accumulation in barley (Hordeum vulgare) at moderately high zinc supply. New Phytol. 201, 131–143. [DOI] [PubMed] [Google Scholar]
- Tiong, J. , Mcdonald, G. , Genc, Y. , Shirley, N. , Langridge, P. and Huang, C.Y. (2015) Increased expression of six ZIP family genes by zinc (Zn) deficiency is associated with enhanced uptake and root‐to‐shoot translocation of Zn in barley (Hordeum vulgare). New Phytol. 207, 1097–1109. [DOI] [PubMed] [Google Scholar]
- Verwoerd, T. , Dekker, B. and Hoekema, A. (1989) A small‐scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 17, 2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , Xu, Q. , Yu, J. and Yuan, M. (2010) The putative Arabidopsis zinc transporter ZTP29 is involved in the response to salt stress. Plant Mol. Biol. 73, 467–479. [DOI] [PubMed] [Google Scholar]
- Yang, O. , Popova, O.V. , Süthoff, U. , Lüking, I. , Dietz, K.‐J. and Golldack, D. (2009a) The Arabidopsis basic leucine zipper transcription factor AtbZIP24 regulates complex transcriptional networks involved in abiotic stress resistance. Gene, 436, 45–55. [DOI] [PubMed] [Google Scholar]
- Yang, X. , Huang, J. , Jiang, Y. and Zhang, H.S. (2009b) Cloning and functional identification of two members of the ZIP (Zrt, Irt‐like protein) gene family in rice (Oryza sativa L.). Mol. Biol. Rep. 36, 281–287. [DOI] [PubMed] [Google Scholar]
- Zhao, H. and Eide, D. (1996a) The yeast ZRT1 gene encodes the zinc transporter protein of a high‐affinity uptake system induced by zinc limitation. Proc. Natl. Acad. Sci. U. S. A. 93, 2454–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, H. and Eide, D. (1996b) The ZRT2 gene encodes the low affinity zinc transporter in Saccharomyces cerevisiae. J. Biol. Chem. 271, 23203–23210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Phylogenetic tree of Triticum aestivum and barley ZIP proteins showing homeolog grouping.
Figure S2. Multiple sequence alignment of TaZIPs.
Figure S3. Phenotypic effect of Zn starvation throughout a 3‐week growth period.
Figure S4. Mineral concentration analysis of wheat root and shoot samples during a 1‐week Zn‐ starvation period.
Figure S5. Gene expression analysis of TaZIPs in wheat root and shoot material throughout an extended Zn starvation period of 3 weeks.
Figure S6. Multiple sequence alignment of cloned group F TabZIPs, AtbZIP19 and AtbZIP23.
Figure S7. Confirmatory PCR of 35S::TabZIP‐transformed Arabidopsis lines.
Table S1. TaZIP identity matrix.
Table S2. Wheat TaZIP and TabZIP gene identification details.
Table S3. Overview of ZDREs present in promoters of TaZIPs.
Table S4. Oligonucleotide primer sequences used for cloning of full length TaZIPs and TabZIPs.
Table S5. Oligonucleotide primer sequences used for SYBR Green real time RT‐PCR expression analysis.
Table S6. Oligonucleotide primer sequences used for PCR‐amplification of TabZIPs. With SP6 promoters and Poly‐A tails prior to in vitro transcription translation.
Table S7. Complementary oligonucleotides used in EMSAs.


