Abstract
Collagen type 4 alpha 1 (COL4A1) and collagen type 13 alpha 1 (COL13A1) produced by urothelial cancer cells support the vital oncogenic property of tumor invasion. We investigated the diagnostic and prognostic capability of COL4A1 and COL13A1 in voided urine and compared the observed values with those of fragments of cytokeratin‐19 (CYFRA21‐1), nuclear matrix protein 22 (NMP‐22), and voided urine cytology in bladder cancer (BCa). We collected voided urine samples from 154 patients newly diagnosed with BCa, before surgery and from 61 control subjects. Protein levels of COL4A1, COL13A1, CYFRA21‐1, and NMP‐22 in urine supernatants were measured using enzyme‐linked immunosorbent assays. Diagnostic performance and optimal cut‐off values were determined by receiver operating characteristic analysis. Urine levels of COL4A1, COL13A1, the combined values of COL4A1 and COL13A1 (COL4A1 + COL13A1), and CYFRA21‐1 were significantly elevated in urine from patients with BCa compared to the controls. Among these biomarkers, the optimal cut‐off value of COL4A1 + COL13A1 at 1.33 ng/mL resulted in 57.4%, 83.7%, 56.1%, 80.7%, and 91.7% sensitivity for low‐grade tumors, high‐grade tumors, Ta, T1, and muscle invasive disease, respectively. We evaluated the prognostic value of preoperative urine levels in 130 non‐muscle invasive BCa samples after the initial transurethral surgery. A high urinary COL4A1 + COL13A1 was found to be an independent risk factor for intravesical recurrence. Although these data need to be externally validated, urinary COL4A1 and COL13A1 could be a potential diagnostic and prognostic biomarker for BCa. This easy‐to‐use urinary signature identifies a subgroup of patients with a high probability of recurrence and progression in non‐muscle invasive and muscle invasive BCa.
Keywords: Collagen, enzyme‐linked immunosorbent assay, recurrence, urinary bladder neoplasm, urine
In the clinical management of BCa, VUC is the most widely used non‐invasive urine test, with reported sensitivities ranging from 13% to 75% and specificities ranging from 85% to 100%.1, 2 None of the urine‐based biomarkers is recommended for routine examination by bladder cancer guideline panels owing to insufficient clinical benefit. Highly sensitive and specific urine tests are therefore strongly desirable in screening and post‐surgical surveillance for BCa. Among the numerous classes of urine‐based biomolecules, proteins represent the most intensively studied class in this setting.3
Our recent report demonstrated that COL4A1 and COL13A1 support the vital oncogenic property of tumor invasion and are strongly associated with poor clinical outcome in human BCa.4 COL4A1 and COL13A1 localize predominantly in the stroma around the tumor cells and cytoplasm, respectively. Collagen type 4 is the most abundant collagen and is essential for the formation of basement membranes, which play critical roles including regulation of tumor cell behavior.5 A couple of previous reports demonstrated that COL4A1 acts as a promoter of angiogenesis and tumor progression.4, 5 Collagen type 13 is a transmembrane protein expressed at cell–matrix junctions.6, 7 Although this type of collagen has not been well studied, in the oncological field, overexpression of COL13A1 as well as COL4A1 is associated with high risk of disease progression through the aggressive invasion pattern called tumor budding in BCa as reported previously.4
Our previous findings raised the hypothesis that these collagens have potential as detection markers as well as prognostic markers for BCa. The protein levels of these two types of collagen secreted into the urine are measurable by ELISA. In the present study, the diagnostic and prognostic performance of urine COL4A1 and COL13A1 was evaluated in comparison with other existing urine tests including CYFRA21‐1, NMP‐22, and VUC.
Materials and Methods
Patient selection and data collection
The Ethics Committee of Nara Medical University approved this study (study reference number: 287 and 1256) and it conforms to the provisions of the Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013). All patients provided informed consent and patient anonymity was preserved.
Figure 1 shows the schema, including the summary of our previous work,4 and the design of the present study. The study included 154 patients with pathologically diagnosed primary BCa who were treated by TURBT and/or radical cystectomy at Nara Medical University. Patient demographics are shown in TableS1. Clinical information and follow‐up data were collected by retrospective chart review. Follow up was carried out according to our institutional protocol.8, 9 The control cohort consisted of 61 healthy volunteers (Table 1). All controls underwent routine medical check‐up once a year and revealed no evidence of malignant disease including urogenital cancer and chronic kidney disease, which could cause proteinuria.
Figure 1.
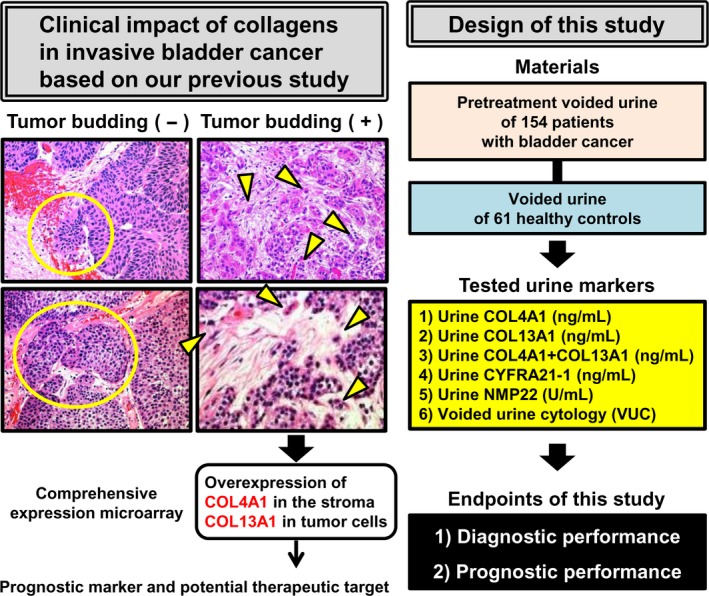
Flow chart of the present study. Tumor budding is characterized by loss of cell‐to‐cell junctions, and cell‐to‐basal membrane and is strongly associated with poor clinical outcome. Representative images of H&E‐stained specimens (200 × magnification) from two patients with T1 BCa each with or without tumor budding are shown. Yellow circles indicate the indolent invasion pattern. Yellow arrowheads indicate isolated tumor cells or small clusters of tumor cells (buds). Our previous report demonstrated that two types of collagen, namely collagen type 4 alpha 1 (COL4A1) and collagen type 13 alpha 1 (COL13A1), produced by cancer cells play a critical role in tumor invasion through induction of tumor budding. Based on robust molecular evidence, we hypothesized that these collagens in voided urine samples had potential not only as detection markers but also as prognostic markers for bladder cancer. The study was designed to evaluate the diagnostic and prognostic capability of preoperative urine COL4A1, COL13A1, and their sum (COL4A1 + COL13A1) in comparison with three existing urine tests including fragments of cytokeratin‐19 (CYFRA21‐1), nuclear matrix protein 22 (NMP‐22), and voided urine cytology (VUC).
Table 1.
Median values of COL4A1, COL13A1, CYFRA 21‐1, and NMP22 measurements in preoperative voided urine
| Variables | Category | N | Concentration in voided urine obtained before TURBT | ||||
|---|---|---|---|---|---|---|---|
| COL4A1 (ng/mL) | COL13A1 (ng/mL) | COL4A1 + COL13A1 (ng/mL) | CYFRA21‐1 (ng/mL) | NMP‐22 (U/mL) | |||
| Control | 61 | 0.39 (0.00–1.47) | 0.28 (0.16–0.48) | 0.82 (0.40–2.24) | 0.43 (0.17–1.17) | NA | |
| Bladder cancer | Total | 154 | 2.89 (0.24–11.71) | 0.63 (0.13–1.76) | 3.64 (1.14–13.34) | 4.11 (1.09–22.77) | 6.32 (3.19–15.75) |
| P‐value* | <0.0001 | 0.002 | <0.0001 | <0.0001 | NA | ||
| T stage | Ta | 66 | 1.19 (0.00–3.85) | 0.21 (0.02–0.89) | 1.72 (0.40–4.36) | 1.46 (0.64–4.77) | 4.70 (2.96–7.95) |
| T1 | 57 | 7.28 (1.30–34.81) | 0.68 (0.30–1.76) | 9.32 (1.91–36.32) | 8.82 (2.95–41.67) | 11.04 (4.15–24.91) | |
| Isolated Tis | 7 | 2.92 (1.74–10.96) | 0.50 (0.22–1.41) | 3.96 (1.96–12.72) | 8.99 (1.28–20.29) | 20.30 (1.42–36.10) | |
| T2‐4 | 24 | 4.48 (0.00–34.30) | 3.21 (1.37–4.50) | 8.94 (2.55–36.17) | 12.98 (5.52–46.73) | 28.49 (11.10–43.57) | |
| P‐value** | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Tumor grade | Low grade | 68 | 1.30 (0.00–3.88) | 0.25 (0.03–0.90) | 1.78 (0.51–4.66) | 1.80 (0.67–7.16) | 5.10 (3.10–8.40) |
| High grade | 86 | 5.78 (1.07–34.10) | 1.15 (0.39–2.29) | 8.94 (1.96–36.12) | 8.71 (2.40–39.28) | 11.88 (4.00–36.10) | |
| P‐value*** | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Tumor budding | No | 133 | 2.73 (0.15–9.80) | 0.50 (0.12–1.58) | 3.38 (0.93–10.98) | 3.79 (1.19–19.82) | 6.32 (3.40–13.5) |
| Yes | 21 | 6.37 (1.06–42.07) | 1.93 (0.78–4.15) | 10.95 (2.71–44.53) | 12.68 (3.06–15.47) | 12.53 (5.00–21.10) | |
| P‐value*** | 0.04 | 0.002 | 0.01 | 0.10 | 0.06 | ||
*Compared between Control and Bladder cancer; **Kruskal–Wallis test; ***Mann–Whitney U‐test. Interquartile range in parentheses. COL13A1, collagen type 13 alpha 1; COL4A1, collagen type 4 alpha 1; CYFRA21‐1, fragments of cytokeratin‐19; NA, not analyzed; NMP‐22, nuclear matrix protein 22; TURBT, transurethral resection of bladder tumor.
Processing of voided urine samples
In total, 50‐mL fresh voided urine samples were obtained from patients before therapeutic intervention and from control subjects. Of these, 20 mL was sent to the pathological department for VUC examination by the Papanicolaou procedure,10 and the remainder was centrifuged at 400 g for 5 min at 20°C. The supernatant was decanted, and 2‐mL aliquots were prepared with urine stabilizer containing 2 mM Tris‐HCL (pH 7.6), 0.1% BSA, 0.09% sodium azide, protease inhibitor cocktail (Cat# P8340; Sigma‐Aldrich, St Louis, MO, USA), and the pellet was snap frozen. Both the supernatant and the pellet were stored at –80°C prior to analysis.
Measurement of urine protein markers
Information on the ELISA kits used is available in Table S2. Absorbance was measured immediately following color development using a microplate spectrophotometer (Infinite 200M PRO; Tecan, Männedorf, Switzerland) equipped with i‐control version 1.8 software. Calibration curves were prepared using purified standards for each protein. Curve fitting was accomplished by either linear or four‐parameter logistic regression. Measured COL4A1, COL13A1, and CYFRA21‐1 concentrations in the urine were normalized to corresponding creatinine values and expressed as ng protein/mg creatinine. CYFRA 21‐1 is a cytokeratin‐19 fragment that is soluble in serum and reported to be a useful urine tumor marker. Urine NMP22 in the controls was not measured because the optimal cut‐off value was already established based on previous studies.
Histopathological assessment including tumor budding
Histopathological review was conducted by two experienced uropathologists (K.S. and N.K.) to determine the T category (2010 AJCC TNM Staging system) and tumor grade (2004 WHO classification). For the cohort of stage ≥T1 BCa (n = 81), tumor budding was determined histologically according to Ueno's definition (10 buds in a 25 × field) as previously described.4, 11 Non‐invasive tumors without stromal invasion (Ta/Tis, n = 73) were considered negative for tumor budding. Representative H&E‐stained samples of BCa with or without tumor budding are shown in Figure 1.
Statistical analysis
Two‐sided Mann–Whitney U‐test or Kruskal–Wallis test was applied to evaluate differences in the concentration of urine biomarkers between BCa and controls, with respect to tumor stage, grade, and presence of tumor budding. Data of urinary concentrations are visualized as scatterplots and tabulated with the median and IQR. Diagnostic performance of urinary COL4A1, COL13A1, and CYFRA21‐1 was determined using ROC curve analyses and AUROC values, followed by determining the optimal cut‐off value by calculating the Youden index.12 PPV, NPV, and diagnostic accuracy were determined according to standard methods.13 Sensitivity of the biomarkers and VUC were compared using the McNemar test. Wilcoxon signed‐rank test was applied to compare urine levels in paired samples obtained before TURBT and 3 months after complete tumor resection. Based on published literature, a cut‐off value of 10 U/mL was used to define a positive NMP‐22 test.2, 14
Cut‐offs for the prognostic performance of urinary COL4A1, COL13A1, CYFRA21‐1, and NMP22 were determined by median urine concentration. In the cohort of NMIBC, n = 130, intravesical RFS and PFS from the day of TURBT were obtained using the Kaplan–Meier method and compared by the log–rank test. Multivariate analysis was used to identify independent prognostic variables using a stepwise Cox proportional hazards regression model. Possible confounding factors detected in univariate analysis were included in the multivariate analysis.
PRISM software version 7.00 (GraphPad Software Inc., La Jolla, CA, USA) and IBM SPSS Version 21 (SPSS Inc., Chicago, IL, USA) were used for statistical analyses and plotting the data, respectively. For all analyses, P < 0.05 was considered statistically significant.
Results
Urinary levels of COL4A1, COL13A1, CYFRA21‐1, and NMP22
The cohort of 215 subjects consisted of 154 subjects with active BCa and 61 healthy control subjects. Median values and interquartile range of urine levels are listed in Table 1. Urine levels of COL4A1, COL13A1, the combined value of COL4A1 and COL13A1 (hereafter referred to as COL4A1 + COL13A1), and CYFRA21‐1 were significantly higher in the BCa compared to those in the controls (Fig. 2a–d). There was no significant difference in the analysis of urine total soluble collagen (Fig. 2e). Thus, we excluded total soluble collagen from further analysis. Next, we investigated the associations of urine levels of COL4A1, COL13A1, CYFRA21‐1, and NMP22 with tumor stage, grade, and tumor budding. For all biomarkers, higher values were associated with higher stage and higher grade (Table 1). Patients with muscle‐invasive ≥T2 tumors were clearly distinguishable from those with NMIBC tumors. The urine levels of the five markers studied were higher in high‐grade BCa compared to those in low‐grade BCa (P < 0.0001). An additional comparison according to tumor budding revealed that high levels of urine COL4A1, COL13A1, and COL4A1 + COL13A1 were associated with tumor budding, whereas CYFRA21‐1 and NMP22 were not (Table 1). Preoperative serum levels of COL4A1, COL13A1, COL4A1 + COL13A1, and total soluble collagen did not differ significantly according to tumor stage, grade, and tumor budding in patients with BCa (data not shown). Serum samples of controls were not available for this study.
Figure 2.
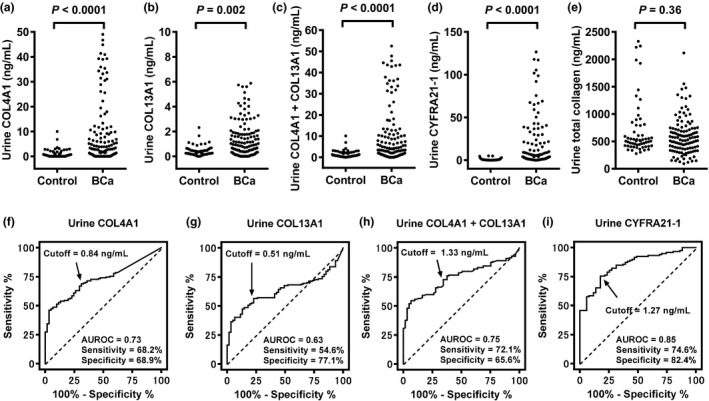
Diagnostic performance of the tested urinary biomarkers. Urinary concentrations of (a) collagen type 4 alpha 1 (COL4A1), (b) collagen type 13 alpha 1 (COL13A1), (c) COL4A1 + COL13A1, (d) cytokeratin‐19 (CYFRA21‐1), and (e) total soluble collagen are shown as scatterplots. Mann–Whitney U‐test was used to compare concentrations between bladder cancer (BCa) and healthy control groups. Receiver operator characteristic (ROC) curves were calculated from analysis of urine samples obtained from a cohort of 215 subjects (154 patients with confirmed BCa and 61 healthy controls) for (f) COL4A1, (g) COL13A1, (h) COL4A1 + COL13A1, and (i) CYFRA21‐1. Optimal cut‐off values were determined by calculating the Youden index12 and are indicated with arrows. AUROC, area under the ROC curve.
Diagnostic performance of COL4A1, COL13A1, CYFRA21‐1, and VUC
We carried out ROC analyses to evaluate the diagnostic performance of urine markers for distinction between patients with BCa and the controls, and to define the optimal cut‐off value for each marker. ROC images of non‐normalized makers and creatinine‐normalized markers are shown in Figure 2(f–i) and Figure S1, respectively. ROC curve analysis of the model allowed the definition of cut‐off values. Table 2 summarizes the AUROC, optimal cut‐off value, and diagnostic accuracy of both non‐normalized values and creatinine‐normalized values. We found that normalization of urine COL4A1 to creatinine instead of urine COL4A1 alone did not improve accuracy significantly (McNemar test, P = 0.62). In addition, creatinine normalization for COL13A1, COL4A1 + COL13A1, and CYFRA21‐1 did not help significantly (Table 2: P = 0.61, 0.18, and 0.99, respectively). We therefore decided to use non‐normalized urine levels for further analyses. Overall sensitivity for COL4A1, COL13A1, COL4A1 + COL13A1, CYFRA21‐1, and VUC was 68.2%, 54.6%, 72.1%, 74.6%, and 40.0%, respectively. Using the McNemar test, the studied biomarkers were more sensitive than VUC as the current reference standard for BCa detection. Of all the studied markers, CYFRA21‐1 had the greatest diagnostic accuracy, which was significantly different from that of the other biomarkers as well as VUC (P < 0.001).
Table 2.
Performance characteristics of urine COL4A1, COL13A1, and CYFRA 21‐1 in preoperative voided urine
| Performance characteristics | COL4A1 | COL13A1 | COL4A1 + COL13A1 | CYFRA21‐1 | NMP‐22 | VUC | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Alone (ng/mL) | Creatinine normalized (ng/mg Cre) | Alone (ng/mL) | Creatinine normalized (ng/mg Cre) | Alone (ng/mL) | Creatinine normalized (ng/mg Cre) | Alone (ng/mL) | Creatinine normalized (ng/mg Cre) | |||
| Area under ROC | 0.73 | 0.75 | 0.63 | 0.61 | 0.75 | 0.74 | 0.85 | 0.84 | NA | NA |
| Optimal cut‐off value | 0.84 | 9.62 | 0.51 | 3.85 | 1.33 | 15.92 | 1.27 | 15.92 | NA | NA |
| Total (n = 154) | ||||||||||
| Sensitivity (%) | 68.2 | 69.5 | 54.6 | 51.7 | 72.1 | 65.3 | 74.6 | 81.4 | 38.1 | 40.0 |
| Specificity (%) | 68.9 | 72.6 | 77.1 | 76.5 | 65.6 | 78.4 | 82.4 | 74.5 | NA | 100 |
| Positive predictive value (%) | 84.7 | 88.2 | 85.7 | 83.6 | 84.1 | 87.5 | 90.7 | 91.4 | NA | 100 |
| Negative predictive value (%) | 46.2 | 52.6 | 40.2 | 40.6 | 48.2 | 49.4 | 58.3 | 65.6 | NA | 52.1 |
| Accuracy (%) | 68.3 | 72.1 | 60.9 | 59.2 | 70.2 | 69.2 | 76.9 | 81.6 | NA | 74.7 |
| McNemar test, P‐value Alone vs creatinine normalized | 0.62 | 0.61 | 0.18 | 0.99 | NA | NA | ||||
| McNemar test, P‐value for comparison with VUC | <0.001 | <0.001 | 0.003 | 0.029 | <0.001 | 0.002 | <0.001 | <0.001 | NA | NA |
| Sensitivity (%) for grade/stage | ||||||||||
| Low‐grade (n = 68) | 57.4 | 55.6 | 35.3 | 33.9 | 57.4 | 48.2 | 62.5 | 69.6 | 22.2 | 17.6 |
| High‐grade (n = 86) | 76.7 | 79.0 | 69.8 | 67.7 | 83.7 | 80.6 | 85.5 | 91.9 | 54.0 | 58.1 |
| Ta (n = 66) | 56.1 | 55.6 | 33.3 | 31.5 | 56.1 | 37.9 | 47.0 | 56.1 | 18.2 | 16.7 |
| T1 (n = 57) | 79.0 | 80.7 | 63.2 | 70.2 | 80.7 | 80.7 | 89.5 | 94.7 | 52.6 | 56.1 |
| Isolated Tis (n = 7) | 85.7 | 85.7 | 57.1 | 57.1 | 85.7 | 85.7 | 85.7 | 85.7 | 71.4 | 57.1 |
| T2‐4 (n = 24) | 70.8 | 83.3 | 95.8 | 95.8 | 91.7 | 95.8 | 87.5 | 87.5 | 50.0 | 62.5 |
COL13A1, collagen type 13 alpha 1; COL4A1, collagen type 4 alpha 1; Cre, creatinine; CYFRA21‐1, fragments of cytokeratin‐19; NA, not analyzed; NMP‐22, nuclear matrix protein 22; ROC, receiver operating characteristic; VUC, voided urine cytology.
Sensitivity of urine biomarkers and VUC according to tumor grade and stage of the primary tumors is listed in Table 2. The optimal cut‐off value of COL4A1 + COL13A1 at 1.33 ng/mL resulted in 57.4%, 83.7%, 56.1%, 80.7%, and 91.7% sensitivity for low‐grade tumors, high‐grade tumors, Ta, T1, and muscle invasive disease, respectively. The COL4A1 + COL13A1 urine assay detected more than 80% of patients with high‐grade, invasive disease (≥T1). Both NMP22 and VUC provided only about 20% sensitivity for the detection of low‐grade, Ta tumors, whereas the COL4A1 + COL13A1 and CYFRA 21‐1 urine assays detected 50%–60% of indolent tumors.
Postoperative restoration of biomarker urine levels
To examine the crucial link between the presence of bladder tumor and elevated urine levels of biomarkers, urine levels were compared in preoperative and postoperative urine samples obtained from seven NMIBC patients 3 months after complete TURBT. Wilcoxon signed‐rank test revealed postoperative restoration of COL4A1, COL13A1, COL4A1 + COL13A1, and CYFRA21‐1 urine levels, whereas there were no significant differences in urine NMP22 (Fig. 3). Urine COL4A1 in one of the seven patients did not decrease to less than the cut‐off value. In addition, urine CYFRA21‐1 in two patients did not decrease to less than the cut‐off value. Urine COL13A1 and COL4A1 + COL13A1 decreased to less than the cut‐off in all seven patients.
Figure 3.
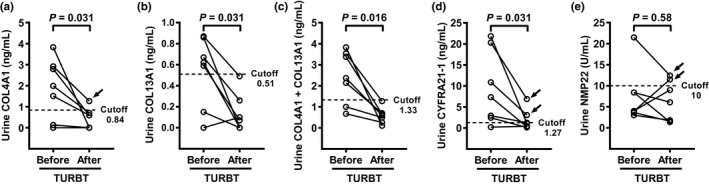
Postoperative restoration of biomarker urine levels. Urine levels of (a) collagen type 4 alpha 1 (COL4A1), (b) collagen type 13 alpha 1 (COL13A1), (c) COL4A1 + COL13A1, (d) cytokeratin‐19 (CYFRA21‐1), and (e) nuclear matrix protein 22 (NMP‐22) were compared in preoperative and postoperative urine samples obtained from seven non‐muscle invasive bladder cancer (NMIBC) patients 3 months after complete transurethral resection of bladder tumor (TURBT). Wilcoxon signed‐rank test was used to evaluate postoperative restoration in urine levels. Dashed lines indicate cut‐off values determined by receiver operator characteristic (ROC) analysis. Black arrows indicate the urine biomarkers that did not decrease to less than the cut‐off values.
Prognostic capability of biomarkers for intravesical recurrence and progression in NMIBC
In the cohort of 130 patients with NMIBC, median follow up was 32 months (IQR: 16–43). In total, 47 (36%) patients received weekly instillation of BCG, whereas 10 (8%) patients received a single immediate postoperative prophylactic intravesical instillation of anthracyclines. Univariate Kaplan–Meier analysis for RFS and PFS was carried out to evaluate potential prognostic capability of the biomarkers. Cut‐offs of the biomarkers for prognostic performance were determined by the medians. Figures S2 and S3 depict survival curves generated using the same cut‐off values as the analysis of diagnostic performance. Patients with high preoperative urinary levels of COL4A1, COL4A1 + COL13A1, and CYFRA21‐1 had higher recurrence rates than those with low urinary levels (Table 3) that were included in the multivariate analysis. We carried out an additional analysis of the combination of COL4A1 and COL13A1 as categorical data. However, the prognostic capability of the number of high collagen (COL) (0, 1, or 2) in the univariate analysis was not superior to the sum of COL4A1 and COL13A1. Multivariate analysis including tumor grade, urinary COL4A1 + COL13A1, and urinary CYFRA21‐1 identified high urinary COL4A1 + COL13A1 as an independent risk factor for intravesical recurrence. In contrast, patients with high urinary levels of COL4A1 and COL4A1 + COL13A1 had significantly higher progression rates than those with low urinary COL4A1 levels, whereas high progression rate was marginally associated with high urinary levels of COL13A1, CYFRA21‐1, and NMP‐22. In the univariate analysis, clinicopathological features including older age, female gender, T1 stage, high‐grade tumor, tumor size ≥3 cm, and carcinoma in situ were detected as possible poor prognostic factors for progression (Table 3). Consecutive multivariate analysis showed that only the T1 stage tumor was found as an independent predictor of progression, whereas urinary COL4A1 + COL13A1 was not. Figure 4 shows the urine level of COL4A1 + COL13A1 from all 130 NMIBC patients in order of magnitude. Notably, the higher level is likely to recur and progress after TURBT. Median levels (IQR) of the non‐recurrence group, recurrence groups without progression, and progression groups were 2.0 (0.55–8.95), 4.8 (2.0–18.2), and 23.0 (4.1–104), respectively. Addition of COL4A1 and COL13A1 into voided urine before TURBT can be an indicator of the outcome after TURBT.
Table 3.
Prognostic factors for recurrence and progression in 130 patients with NMIBC
| Variables | Intravesical recurrence‐free survival | Progression‐free survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariatea | Univariate | Multivariatea | |||||||||
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Age (years) | ||||||||||||
| ≥70/<70 | 1.39 | 0.73–2.66 | 0.34 | NA | 6.70 | 2.09–21.5 | 0.035 | 3.35 | 0.74–15.1 | 0.12 | ||
| Sex | ||||||||||||
| Female/Male | 1.27 | 0.45–3.54 | 0.62 | NA | 3.83 | 0.65–22.5 | 0.018 | 3.57 | 0.95–13.3 | 0.06 | ||
| T stage | ||||||||||||
| T1 or isolated Tis/Ta | 1.64 | 0.85–3.36 | 0.12 | NA | 9.44 | 2.87–31.1 | 0.0002 | 5.58 | 1.51–20.6 | 0.01 | ||
| Tumor grade | ||||||||||||
| High/Low | 1.73 | 0.91–3.30 | 0.083 | 0.99 | 0.47–2.07 | 0.97 | 9.64 | 3.08–30.2 | 0.0002 | 0.89 | 0.62–12.5 | 0.93 |
| Multiplicity | ||||||||||||
| Multiple/Solitary | 1.63 | 0.86–3.08 | 0.14 | NA | 2.49 | 0.76–8.12 | 0.16 | NA | ||||
| Carcinoma in situ | ||||||||||||
| Yes/No | 1.60 | 0.79–3.23 | 0.14 | NA | 6.67 | 1.96–22.7 | 0.001 | 1.92 | 0.50–7.28 | 0.35 | ||
| COL4A1 (ng/mL) | ||||||||||||
| ≥2.76/<2.76 | 3.26 | 1.71–6.23 | 0.002 | NA | 5.00 | 1.52–16.2 | 0.023 | NA | ||||
| COL13A1 (ng/mL) | ||||||||||||
| ≥0.45/<0.45 | 2.64 | 1.38–5.03 | 0.005 | NA | 2.79 | 0.85–9.10 | 0.11 | NA | ||||
| No. high COL | ||||||||||||
| 1/0 | 2.39 | 0.84–6.75 | 0.11 | NA | 3.48 | 1.08–11.3 | 0.047 | NA | ||||
| 2/0 | 2.91 | 1.44–8.28 | 0.008 | NA | 4.32 | 1.27–15.2 | 0.04 | NA | ||||
| COL4A1 + COL13A1 (ng/mL) | ||||||||||||
| ≥3.40/<3.40 | 3.10 | 1.61–5.87 | 0.001 | 2.13 | 1.11–7.11 | 0.029 | 5.06 | 1.55–16.5 | 0.021 | 1.97 | 0.48–8.10 | 0.24 |
| CYFRA21‐1 (ng/mL) | ||||||||||||
| ≥4.06/<4.06 | 3.42 | 1.74–6.72 | 0.0007 | 2.12 | 0.91–4.97 | 0.083 | 2.78 | 0.85–9.07 | 0.11 | NA | ||
| NMP‐22 (U/mL) | ||||||||||||
| ≥6.36/<6.36 | 1.58 | 0.83–3.01 | 0.16 | NA | 2.88 | 0.88–9.41 | 0.10 | NA | ||||
Treatment‐adjusted multivariate Cox regression analysis; CI, confidence interval; COL, collagen; COL13A1, collagen type 13 alpha 1; COL4A1, collagen type 4 alpha 1; CYFRA21‐1, fragments of cytokeratin‐19; HR, hazard ratio; NA, not analyzed; NMIBC, non‐muscle invasive BCa; NMP‐22, nuclear matrix protein 22.
Figure 4.
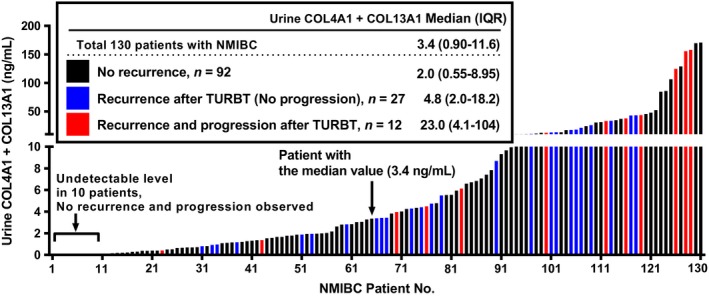
Preoperative urine levels of collagen type 4 alpha 1 (COL4A1) + collagen type 13 alpha 1 (COL13A1) in non‐muscle invasive bladder cancer (NMIBC) patients. Preoperative COL4A1 + COL13A1 levels of 130 NMIBC patients are aligned in order of magnitude. Blue and red colored bars indicate patients who experienced intravesical recurrence only and both recurrence and progression, respectively. Median levels with interquartile range (IQR) of three groups are shown. TURBT, transurethral resection of bladder tumor.
Discussion
In the clinical management of BCa, non‐invasive urine tests for early detection and postoperative surveillance are highly desirable for both the patient and the health‐care system. Despite many advances in the medical field, the clinical need for accurate diagnostic urine markers for BCa remains urgent. None of the current guidelines recommends the routine use of urinary biomarkers in the management of BCa because of their insufficient accuracy.3 A vast number of studies have identified proteomic markers,3, 15, 16 genomic markers including mutations in FGFR3 and p53,15, 17, 18 and epigenetic markers,19, 20 and have been evaluated in a bid to improve the accuracy of urine‐based diagnosis of BCa. Genomic markers and epigenetic markers generally require time‐consuming and complex processes, such as extracting DNA or RNA following reverse transcription and/or PCR. In this sense, proteomic markers soluble in the urine are measurable in the urine supernatant. Most proteins can be quantified rapidly and easily, thus enabling point‐of‐care testing or laboratory‐based enzyme immunoassays including ELISA. We investigated the diagnostic and prognostic performance of urine COL4A1 and COL13A1 by comparison with other existing urine tests including CYFRA21‐1, NMP‐22, and VUC. COL4A1 produced by BCa cells is a secretory collagen, whereas COL13A1 localizes in the cytoplasm and the cellular membrane.4 To our knowledge, this study is the first to focus on the urine levels of two types of collagens, COL4A1 and COL13A1, as potential biomarkers of BCa.
Based on evidence that the diagnostic and prognostic value of circulating COL6A, another type of collagen, in serum has been addressed,21, 22 collagens can be good targets for both detection tests and prognostic indicators of malignancies. Therefore, we expected that COL4A1 and COL13A1 in urine and serum samples might be clinically useful in human BCa. Our ELISA results indicated that urine COL4A1 (AUROC = 0.73) and COL13A1 (0.63) showed less diagnostic performance for BCa compared to urine CYFRA21‐1 (0.85). In this study, we evaluated the addition of COL4A1 and COL13A1 (COL4A1 + COL13A1) in an attempt to improve diagnostic performance. AUROC and diagnostic accuracy of COL4A1 + COL13A1 was 0.75 and 70.2%, respectively, offering a slight improvement compared to COL4A1 alone and COL13A1 alone (Table 2). In addition, we compared the performance between values with and without urine creatinine‐normalization based on reports showing the advantage of normalization.23 However, in accordance with other studies,24, 25 we found that using urine COL4A1 and COL13A1 concentration normalized to urine creatinine (ng/mg Cre) instead of urinary concentration alone (ng/mL) did not improve the diagnostic accuracy. Our results are similar to those of other urinary protein‐based tests, aiming for measurement of soluble proteins related to BCa; for instance, analysis of growth factors, matrix metalloproteases, or other cytokines in urine.3, 26 The greater the progression of the BCa, the higher the urine collagen concentration and detection sensitivity (Tables 1, 2). As Kumari et al. reported, a panel of serum cytokines had a predictive role in the invasion and recurrence of BCa;26 serum can be a useful source of non‐invasive diagnostic and prognostic biomarkers for BCa. Kang et al. demonstrated the potential clinical utility of serum COL6A3 in pancreatic ductal adenocarcinoma.22 Our findings did not prove the clinical significance of COL4A1 and COL13A1 serum levels. This may be because the majority of this cohort (84%, 130/154) included patients with non‐advanced BCa (i.e. NMIBC). Low‐stage diseases do not seem to affect the serum level of tumor‐related biomarkers as much as advanced high‐stage diseases. The present cohort reflected the epidemiological reports for BCa, in which more than 80% of patients with newly diagnosed and recurrent disease presented with NMIBC.27 Further studies are required to evaluate the true diagnostic and prognostic value of serum COL4A1 and COL13A1 levels in an independent cohort consisting of advanced BCa, such as muscle invasive BCa.
Our previous study found that COL4A1 and COL13A1 produced by BCa cells promote tumor budding and poor prognosis.4 Thus, we hypothesized that urine levels of COL4A1 and COL13A1 would be associated with poor clinical outcome through tumor budding. Notably, urinary COL4A1 and COL13A1 levels were significantly higher in tumors with tumor budding, whereas there was no significant association of CYFRA21‐1 and NMP22 urine levels with tumor budding. Although in this study and in previous reports, urine CYFRA21‐1 was a useful diagnostic test for BCa,24, 25 there was only moderate prognostic impact of urine CYFRA21‐1 on the intravesical recurrence of NMIBC (Table 3). In contrast, the high level of urine COL4A1 + COL13A1 proved to be an independent predictor for intravesical recurrence by multivariate analysis (HR = 2.13, 95% CI 1.11–7.11) and was strongly associated with disease progression by univariate analysis (HR = 5.06, 95% CI 1.55–16.5), but not by multivariate analysis (HR = 1.97, 95% CI 0.48–8.10). Here, urine COL4A1 + COL13A1 provides additional risk stratification to clinicopathological prognosticators.
Patients with NMIBC need to undergo rigorous surveillance by cystoscopy after TURBT with or without neoadjuvant intravesical treatment. The challenge is to establish supplementary biomarkers that assist with increasing the low sensitivity of VUC and maintain its high specificity. We previously reported a highly sensitive detection method for the FGFR‐3 gene using peptide nucleic acid‐mediated PCR in urine exfoliated cells, which could potentially be available as a surveillance marker after TURBT.17, 18 In the present study, we examined the link between the presence of bladder tumor and elevated urine levels of tested biomarkers. Although the sample numbers were low (n = 7), urine levels of COL4A1, COL13A1, and COL4A1 + COL13A1 showed postoperative restoration, which is considered essential for surveillance markers. Considering that urine COL13A1 and COL4A1 + COL13A1 decreased under the cut‐off in all patients, these markers might earn broad acceptance as surveillance markers. Monitoring the levels of two collagens may be used at intervals between cystoscopies with a negative result warranting avoidance of cystoscopy. Patients with low‐grade NMIBC represent a population that may require less intensive surveillance and may benefit from intermittent confirmatory urine testing during surveillance. We expect combinations of different molecular biomarkers including COL4A1 and COL13A1 and histopathological features to be introduced into the development of surveillance protocols for NMIBC.28
One of the major advantages of this study is that it includes a cohort of patients treated homogeneously in a single center, with prospective sample collection. However, this study has some limitations. First, there is a significant difference in sex and age between BCa patients and healthy controls (TableS1). Second, the number of events in the disease progression is limited. Interpretation of this part of the results must therefore be considered with care until a validation study in a larger cohort is conducted.
Although the data need to be externally validated, urinary COL4A1 and COL13A1 would be novel potential diagnostic and prognostic biomarkers for BCa. This easy‐to‐use urinary signature identifies a subgroup of patients with high probability of recurrence and progression in NMIBC. An accurate prediction of post‐TURBT risk could help develop appropriate adjuvant therapy such as BCG, re‐staging, immediate radical cystectomy, or more intensive surveillance. The diagnostic and prognostic performance of the urine COL4A1 and COL13A1 signature will be more reliable if validation is conducted in an independent external cohort.
Disclosure Statement
Authors declare no conflicts of interest for this article.
Abbreviations
- AUROC
area under the curve of ROC
- BCa
bladder cancer
- BCG
bacille Calmette Guérin
- COL
collagen
- COL13A1
collagen type 13 alpha 1
- COL4A1
collagen type 4 alpha 1
- CYFRA 21‐1
fragments of cytokeratin‐19
- FGFR3
fibroblast growth factor receptor 3
- HR
hazard ratio
- IQR
interquartile range
- NMIBC
non‐muscle invasive BCa
- NMP‐22
nuclear matrix protein 22
- NPV
negative predictive value
- PFS
progression‐free survival
- PPV
positive predictive value
- RFS
recurrence‐free survival
- ROC
receiver operating characteristic
- TURBT
transurethral resection of bladder tumor
- VUC
voided urinary cytology
Supporting information
Table S1. Clinicopathological characteristics of the bladder cancer cohort and healthy controls.
Table S2. ELISA kits used for immunohistochemical staining analysis and Western blotting analysis.
Fig. S1. Diagnostic performance of tested urinary biomarkers normalized to urine creatinine levels. Receiver operator characteristic (ROC) curves were calculated from analysis of urine samples obtained from a cohort of 215 subjects (154 with confirmed bladder cancer) for creatinine‐normalized (a) COL4A1, (b) COL13A1, (c) COL4A1 + COL13A1, and (d) CYFRA21‐1. Values are expressed as ng protein/mg creatinine. Optimal cut‐off values were determined by calculating the Youden index(12) and are indicated with arrows. AUROC, area under the ROC curve.
Fig. S2. Kaplan–Meier curves for survival after transurethral resection of bladder tumor (TURBT) in the cohort of 130 patients with non‐muscle invasive bladder cancer (NMIBC). Kaplan–Meier curves for intravesical recurrence‐free survival and progression‐free survival were compared for (a) COL4A1, (b) COL13A1, and (c) COL4A1 + COL13A1. Log–rank test was used for comparison.
Fig. S3. Kaplan–Meier curves for survival after transurethral resection of bladder tumor (TURBT) in the cohort of 130 patients with non‐muscle invasive bladder cancer (NMIBC). Kaplan–Meier curves for intravesical recurrence‐free survival and progression‐free survival were compared for (a) CYFRA21‐1 and (b) NMP‐22. Log–rank test was used for comparison.
Acknowledgments
This study was supported by JSPS KAKENHI grant numbers 16K20159 (M.M.) and 15K10605 (K.F.) and ‘Fiscal Years 2015–2016’ Nara Medical University Grant‐in‐Aid for Collaborative Research Projects (M.M. and K.F.).
Cancer Sci 108 (2017) 2221–2228
Funding Information
Nara Medical University Grant‐in‐Aid for Collaborative Research Projects, (Grant/Award Number: ‘Fiscal Years 2015–2016’) JSPS KAKENHI, (Grant/Award Number: ‘15K10605’,‘16K20159’).
References
- 1. Miyake M, Fujimoto K, Hirao Y. Active surveillance for nonmuscle invasive bladder cancer. Investig Clin Urol 2016; 57: S4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miyake M, Goodison S, Giacoia EG et al Influencing factors on the NMP‐22 urine assay: an experimental model. BMC Urol 2012; 12: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. D'Costa JJ, Goldsmith JC, Wilson JS et al A systematic review of the diagnostic and prognostic value of urinary protein biomarkers in urothelial bladder cancer. Bladder Cancer 2016; 2: 301–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miyake M, Hori S, Morizawa Y et al Collagen type IV alpha 1 (COL4A1) and collagen type XIII alpha 1 (COL13A1) produced in cancer cells promote tumor budding at the invasion front in human urothelial carcinoma of the bladder. Oncotarget 2017; 8: 36099–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer 2003; 3: 422–33. [DOI] [PubMed] [Google Scholar]
- 6. Hägg P, Rehn M, Huhtala P et al Type XIII collagen is identified as a plasma membrane protein. J Biol Chem 1998; 273: 15590–7. [DOI] [PubMed] [Google Scholar]
- 7. Hägg P, Väisänen T, Tuomisto A et al Type XIII collagen: a novel cell adhesion component present in a range of cell‐matrix adhesions and in the intercalated discs between cardiac muscle cells. Matrix Biol 2001; 19: 727–42. [DOI] [PubMed] [Google Scholar]
- 8. Miyake M, Gotoh D, Shimada K et al Exploration of risk factors predicting outcomes for primary T1 high‐grade bladder cancer and validation of the Spanish Urological Club for Oncological Treatment scoring model: long‐term follow‐up experience at a single institute. Int J Urol 2015; 22: 541–7. [DOI] [PubMed] [Google Scholar]
- 9. Morizawa Y, Miyake M, Shimada K et al Neutrophil‐to‐lymphocyte ratio as a detection marker of tumor recurrence in patients with muscle‐invasive bladder cancer after radical cystectomy. Urol Oncol 2016; 34: 257. e11‐17. [DOI] [PubMed] [Google Scholar]
- 10. Papanicolaou GN, Marshall VF. Urine sediment smears as a diagnostic procedure in cancers of the urinary tract. Science 1945; 101: 519–20. [DOI] [PubMed] [Google Scholar]
- 11. Ueno H, Mochizuki H, Hashiguchi Y et al Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology 2004; 127: 385–94. [DOI] [PubMed] [Google Scholar]
- 12. Miyake M, Lawton A, Dai Y et al Clinical implications in the shift of syndecan‐1 expression from the cell membrane to the cytoplasm in bladder cancer. BMC Cancer 2014; 14: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lalkhen AG, McCluskey A. Clinical tests: sensitivity and specificity. Contin Educ Anaesth Crit Care Pain 2008; 8: 221–3. [Google Scholar]
- 14. Sözen S, Biri H, Sinik Z et al Comparison of the nuclear matrix protein 22 with voided urine cytology and BTA stat test in the diagnosis of transitional cell carcinoma of the bladder. Eur Urol 1999; 36: 225–9. [DOI] [PubMed] [Google Scholar]
- 15. Shirodkar SP, Lokeshwar VB. Potential new urinary markers in the early detection of bladder cancer. Curr Opin Urol 2009; 19: 488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hori S, Miyake M, Onishi S et al Clinical significance of α‐ and β‐Klotho in urothelial carcinoma of the bladder. Oncol Rep 2016; 36: 2117–25. [DOI] [PubMed] [Google Scholar]
- 17. Miyake M, Sugano K, Kawashima K et al Sensitive detection of FGFR3 mutations in bladder cancer and urine sediments by peptide nucleic acid‐mediated real‐time PCR clamping. Biochem Biophys Res Commun 2007; 362: 865–71. [DOI] [PubMed] [Google Scholar]
- 18. Miyake M, Sugano K, Sugino H et al Fibroblast growth factor receptor 3 mutation in voided urine is a useful diagnostic marker and significant indicator of tumor recurrence in non‐muscle invasive bladder cancer. Cancer Sci 2010; 101: 250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim YK, Kim WJ. Epigenetic markers as promising prognosticators for bladder cancer. Int J Urol 2009; 16: 17–22. [DOI] [PubMed] [Google Scholar]
- 20. Yu J, Zhu T, Wang Z et al A novel set of DNA methylation markers in urine sediments for sensitive/specific detection of bladder cancer. Clin Cancer Res 2007; 13: 7296–304. [DOI] [PubMed] [Google Scholar]
- 21. Qiao J, Fang CY, Chen SX et al Stroma derived COL6A3 is a potential prognosis marker of colorectal carcinoma revealed by quantitative proteomics. Oncotarget 2015; 6: 29929–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kang CY, Wang J, Axell‐House D et al Clinical significance of serum COL6A3 in pancreatic ductal adenocarcinoma. J Gastrointest Surg 2014; 18: 7–15. [DOI] [PubMed] [Google Scholar]
- 23. Goodison S, Ogawa O, Matsui Y et al A multiplex urinary immunoassay for bladder cancer detection: analysis of a Japanese cohort. J Transl Med 2016; 14: 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nisman B, Barak V, Shapiro A et al Evaluation of urine CYFRA 21‐1 for the detection of primary and recurrent bladder carcinoma. Cancer 2002; 94: 2914–22. [DOI] [PubMed] [Google Scholar]
- 25. Pariente JL, Bordenave L, Jacob F et al Analytical and prospective evaluation of urinary cytokeratin 19 fragment in bladder cancer. J Urol 2000; 163: 1116–9. [PubMed] [Google Scholar]
- 26. Kumari N, Agrawal U, Mishra AK et al Predictive role of serum and urinary cytokines in invasion and recurrence of bladder cancer. Tumour Biol 2017; 39: 1–14. [DOI] [PubMed] [Google Scholar]
- 27. Babjuk M, Burger M, Zigeuner R et al European Association of Urology: EAU guidelines on non‐muscle‐invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 2013; 64: 639–53. [DOI] [PubMed] [Google Scholar]
- 28. Zuiverloon TC, Beukers W, van der Keur KA et al Combinations of urinary biomarkers for surveillance of patients with incident nonmuscle invasive bladder cancer: the European FP7 UROMOL project. J Urol 2013; 189: 1945–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinicopathological characteristics of the bladder cancer cohort and healthy controls.
Table S2. ELISA kits used for immunohistochemical staining analysis and Western blotting analysis.
Fig. S1. Diagnostic performance of tested urinary biomarkers normalized to urine creatinine levels. Receiver operator characteristic (ROC) curves were calculated from analysis of urine samples obtained from a cohort of 215 subjects (154 with confirmed bladder cancer) for creatinine‐normalized (a) COL4A1, (b) COL13A1, (c) COL4A1 + COL13A1, and (d) CYFRA21‐1. Values are expressed as ng protein/mg creatinine. Optimal cut‐off values were determined by calculating the Youden index(12) and are indicated with arrows. AUROC, area under the ROC curve.
Fig. S2. Kaplan–Meier curves for survival after transurethral resection of bladder tumor (TURBT) in the cohort of 130 patients with non‐muscle invasive bladder cancer (NMIBC). Kaplan–Meier curves for intravesical recurrence‐free survival and progression‐free survival were compared for (a) COL4A1, (b) COL13A1, and (c) COL4A1 + COL13A1. Log–rank test was used for comparison.
Fig. S3. Kaplan–Meier curves for survival after transurethral resection of bladder tumor (TURBT) in the cohort of 130 patients with non‐muscle invasive bladder cancer (NMIBC). Kaplan–Meier curves for intravesical recurrence‐free survival and progression‐free survival were compared for (a) CYFRA21‐1 and (b) NMP‐22. Log–rank test was used for comparison.


