Aberrant signaling by the TGF-β and BMP pathways is associated with numerous human diseases—from common connective tissue disorders (e.g., osteoarthritis and osteoporosis) to rare genetic syndromes.
Abstract
The transforming growth factor β (TGF-β) family of signaling molecules, which includes TGF-βs, activins, inhibins, and numerous bone morphogenetic proteins (BMPs) and growth and differentiation factors (GDFs), has important functions in all cells and tissues, including soft connective tissues and the skeleton. Specific TGF-β family members play different roles in these tissues, and their activities are often balanced with those of other TGF-β family members and by interactions with other signaling pathways. Perturbations in TGF-β family pathways are associated with numerous human diseases with prominent involvement of the skeletal and cardiovascular systems. This review focuses on the role of this family of signaling molecules in the pathologies of connective tissues that manifest in rare genetic syndromes (e.g., syndromic presentations of thoracic aortic aneurysm), as well as in more common disorders (e.g., osteoarthritis and osteoporosis). Many of these diseases are caused by or result in pathological alterations of the complex relationship between the TGF-β family of signaling mediators and the extracellular matrix in connective tissues.
The transforming growth factor β (TGF-β) family of cytokines comprises the three TGF-β proteins (TGF-β1, TGF-β2, and TGF-β3) and related growth and differentiation factors such as activins, inhibins, bone morphogenic proteins (BMPs), and growth and differentiation factors (GDFs). These molecules play critical roles both in normal development and in several pathological conditions, including inflammation, fibrosis, and cancer (reviewed in Li and Flavell 2006; Gordon and Blobe 2008; Ikushima and Miyazono 2010). This review focuses on the role of these proteins in development and homeostasis of the skeleton and other connective tissues (summarized in Fig. 1) and on the diseases that ensue when these pathways are altered. Aberrant signaling in these pathways has been associated with common connective tissue disorders such as osteoarthritis and osteoporosis. In addition, mutations in several members of the TGF-β family, their receptors, or signaling mediators have been shown to cause hereditable connective tissue syndromes that are characterized by defects in the development and homeostasis of soft and hard connective tissues (Table 1).
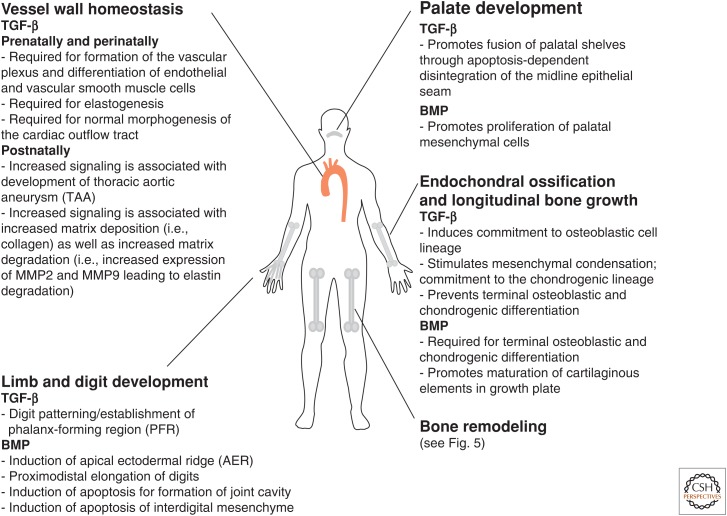
Figure 1.
Summary of the roles of transforming growth factor β (TGF-β) and bone morphogenetic protein (BMP) signaling in the development and homeostasis of connective tissue and the skeletal system.
Table 1.
Connective tissue and skeletal disorders caused by known mutations in the transforming growth factor β (TGF-β) family signaling pathways
| Disorder (official symbol) [MIM #] | Gene mutation | Change in signal activity | Clinical manifestation | |||
|---|---|---|---|---|---|---|
| Cardiovascular | Ocular | Skeletal | Other | |||
| Acromesomelic dysplasia (AMD) | GDF5 | ↓ | All AMD: acromesomelic limbs and brachydactyly | |||
| Hunter–Thompson type (AMDH) [201250] | Joint dislocation in elbow and ankles, also frequently found in hip and knee | |||||
| Grebe type (AMDG) [200700] | Ball-shaped reduction of phalanges | |||||
| Du Pan syndrome [228900] | Hypo-/aplasia of fibula, short limbs, ball-shaped reduction of phalanges, partial syndactyly | |||||
| Brachydactyly (BD) | All BD: shortened digits | |||||
| BD type A2 (BDA2) [112600] |
BMPR1B GDF5 BMP2 |
↓ | Shortening of middle phalanx in second and fifth digit | |||
| BD type B2 (BDB2) [611377] | NOG | ↑ | Shortening of distal phalanges symphalangism and partial syndactyly, fusion of carpal/tarsal bones | |||
| BD type C (BDC) [113100] | GDF5 | ↓ | Shortening of middle phalanx in second, third and fifth digit, shortening of first metacarpal, hypersegmentation of second and/or third digit | |||
| Camurati–Engelmann disease (CED) [131300] | TGFB1 | ↑ | Sclerosing bone dysplasia, thickening of the diaphyses of the long bones | |||
| Fibrodysplasia ossificans progressiva (FOP) [135100] | ACVR1 | ↑ | Extra-articular joint ankylosis, tibial osteochondroma, cervical spine and femoral neck malformation, big toe malformation | Postnatal soft tissue ossification | ||
| Hereditary hemorrhagic telangiectasia (HHT) HHT1 [187300] HHT2 [600376] |
ENG ACVRL1 |
↓ | Telangiectases and arteriovenous malformations of skin, mucosa, and viscera | |||
| Loeys–Dietz syndrome (LDS) LDS1 [609192] LDS2 [610168] LDS3 [613795] LDS4 [614816] LDS5 |
TGFBR1 TGFBR2 SMAD3 TGFB2 TGFB3 |
↓/↑ | Aortic root aneurysm, arterial tortuosity, aneurysm of other vessels | Hyper-telorism | Arachnodactyly, cleft palate/bifid uvula, craniosynostosis, pectus deformity, scoliosis, joint laxity, malar hypoplasia | Increased susceptibility to asthma, allergy, eczema, gastrointestinal inflammation |
| Marfan syndrome (MFS) [154700] | FBN1 | ↑ | Aortic root aneurysm | Ectopia lentis | Arachnodactyly, dolichostenomelia, pectus deformity, scoliosis, joint laxity, malar hypoplasia | |
| Multiple synostosis syndrome (SYNS) | ||||||
| SYNS1 [186500] | NOG | ↑ | Progressive symphalangism, carpal, tarsal, and vertebral fusions | Progressive hearing loss | ||
| SYNS2 [610017] | GDF5 | |||||
| Orofacial cleft 11 (OFC11) [600625] | BMP4 | a | Nonsyndromic cleft lip with or without cleft palate | |||
| Proximal symphalangism (SYM1) | ||||||
| SYM1A [185800] | NOG | ↑ | Multiple joint fusions restricted to proximal interphalangeal joints in hand and feet, fusion of carpal/tarsal bones | Common: conductive hearing loss | ||
| SYM1B [615298] | GFD5 | |||||
| Sclerosteosis (SOST1) [269500] | SOST | - | Sclerosing bone dysplasia, tall stature, syndactyly | |||
| Shprintzen–Goldberg syndrome (SGS) [182212] | SKI | ↑ | Aortic root aneurysm | Hypertelorism | Arachnodactyly, dolichostenomelia, craniosynostosis, pectus deformity, malar hypoplasia, joint laxity | |
| Stiff skin syndrome (SSS) [184900] | FBN1 | ↑ | Syndesmodysplasic, dwarfism, cutaneous nodules in distal interphalangeal joints | Skin fibrosis | ||
| Van Buchem disease (VBD) [239100] | SOST | - | Sclerosing bone dysplasia, increased inner and outer diameter of metacarpals | |||
SNPs in components of BMP/TGF-β pathway are further implicated in osteoarthritis and osteoporosis but have not been functionally determined.
aMutant protein activity has not been functionally determined.
OVERVIEW OF TGF-β SIGNALING
All members of the TGF-β family of cytokines share conserved structural motifs and signal through similar mechanisms, although the exact signaling pathways activated by any given ligand differ. Signaling is initiated by binding of the ligand to tetrameric complexes formed by two type I and two type II receptors, all possessing serine, threonine, and tyrosine kinase activity. Ligand binding induces autophosphorylation of type II receptors, which in turn phosphorylate and activate type I receptors. Activated type I receptors phosphorylate, and thus activate receptor-regulated Smad proteins (R-Smads). Phosphorylated R-Smads bind the common Smad (co-Smad, known as Smad4 or DPC4), translocate into the nucleus and modulate gene expression by interacting with other transcription factors to regulate target gene expression (reviewed in Schmierer and Hill 2007; Massagué 2012; Hata and Chen 2016). In this review, we have broadly grouped the TGF-β family into two general pathways, TGF-β and its main signaling molecules Smad2 and Smad3 (Fig. 2) and BMP and its main signaling molecules Smad1, Smad5, and Smad8 (Fig. 3).
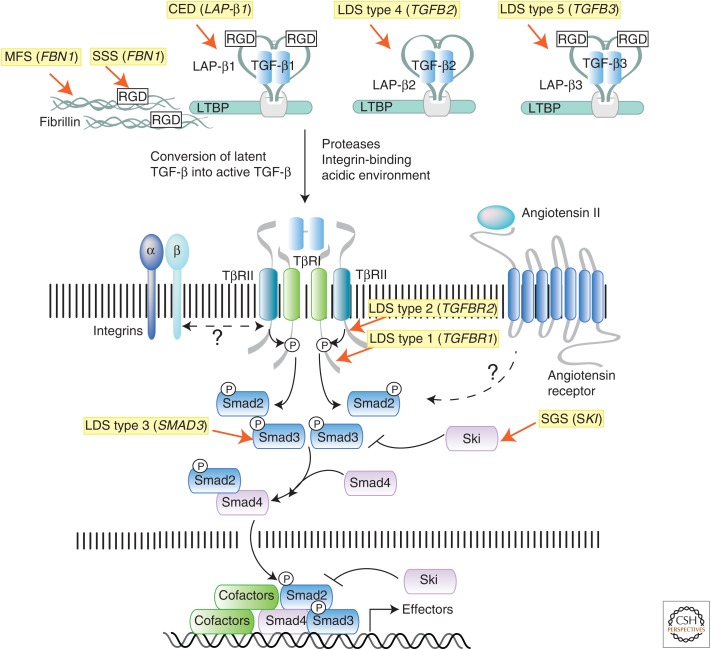
Figure 2.
Summary of the transforming growth factor β (TGF-β) signaling pathway and mutations causing connective tissue disorders. TGF-β molecules (TGF-β1, -β2, and -β3) are secreted in latent forms that are unable to interact with the receptors. In the latent form, dimeric TGF-β molecules are noncovalently associated with dimeric latency-associated peptides (LAPs), which are made from the same gene as the corresponding TGF-β molecule. This complex, which is referred to as the small latent complex (SLC), is then covalently linked by disulfide bonds to a member of the latent TGF-β binding protein (LTBP) family, to form the larger complex called large latent complex (LLC). The LLC complex interacts noncovalently with various components of the extrcellular matrix (ECM), including fibrillin microfibrils and integrins, the major adhesion molecule linking cells to the ECM. Both fibrillin and TGF-β (TGF-β1 and -β3, but not TGF-β2) contain an arginine-glycine-aspartic acid (RGD) domain that can bind integrin molecules. Cross talk between LTBP, fibrillin, and integrins is thought to be critical for proper localization, sequestration, and conversion of latent TGF-β to active TGF-β. Once activated, dimeric TGF-β binds to a tetrameric receptor complex formed by two type I (TβRI) and type II (TβRII) receptor subunits, leading to direct phosphorylation of R-Smads (Smad2 or Smad3), complex formation with co-Smad (Smad4), and induction or repression of target gene expression. Heterozygous inactivating mutations in both “positive” and “negative” regulators of this pathway have been identified as the cause of genetic disorders that are characterized by pathological alterations in the connective tissue due to misregulated expression of TGF-β gene targets (i.e., tissue metalloproteinases, collagen, integrins, and several other ECM components). The syndromes associated with these mutations, and the human gene mutated in each condition, are highlighted in yellow.
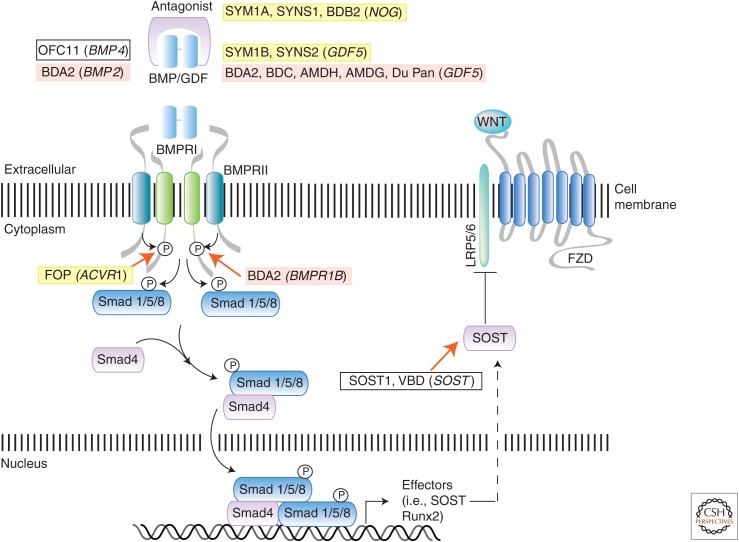
Figure 3.
Summary of the bone morphogenetic protein (BMP) and growth and differentiation factor (GDF) signaling pathway and mutations causing connective tissue and skeletal disorders. The BMP signaling pathway is activated by extracellular ligands, such as BMPs and GDFs, through binding to type I (BMPRI) and type II (BMPRII) receptor complexes. Extracellular antagonists, such as noggin, can block ligand–receptor binding and pathway activation. Smad (shown) and non-Smad (not shown) mechanisms mediate BMP signal transduction to regulate transcriptional target genes, including sclerostin (SOST), a Wnt pathway inhibitor. Key components of the BMP pathway are shown to illustrate positions at which gene mutations act to cause specific human connective tissue and skeletal syndromes. Cross talk with the Wnt pathway is also shown. Specific disorders and their gene mutations (in parentheses), as discussed in text and Table 1, are shaded in yellow if the mutation enhances pathway signaling or in red if the pathway has diminished activation. White boxes indicate disorders for which the BMP pathway is either not directly affected or the functional consequence of the mutation is unknown. BDA2, Brachydactyly type A2; BDC, brachydactyly type C; AMDH, acromesomelic dysplasia Hunter–Thompson type; AMDG, acromesomelic dysplasia Grebe type; FOP, fibrodysplasia ossificans progressiva; VBD, Van Buchem disease.
Seven type I receptors and five type II receptors have been identified in humans (Heldin and Moustakas 2016). Type I receptors are also known as activin receptor-like kinases (ALK-1 to -7). The BMP type I receptors (BMPRIs), ALK-1 (also known as ACVRL1, activin A receptor-like 1), ALK-2 (ActRI, ACVR1, activin A receptor type I), BMPRIA (bone morphogenetic protein receptor type IA, also known as ALK-3), and BMPRIB (bone morphogenetic protein receptor type IB, also known as ALK-6), primarily signal by phosphorylation of Smad1, Smad5, and Smad8. ALK-4 (ActRIB, ACVR1B, activin A receptor type IB), TβRI (transforming growth factor-β receptor, type I, also known as ALK-5), and ALK-7 (ACVR1C, activin A receptor type IC), primarily signal by phosphorylation of Smad2 and Smad3. The type II receptors are ActRII (also known as ACVR2, activin A receptor type IIA), ActRIIB (ACVR2B, activin A receptor type IIB), TβRII (TGFBR2, transforming growth factor-β receptor type II), BMPRII (bone morphogenetic protein receptor type II), and AMHRII (anti-Müllerian hormone receptor type II). The type III receptors, betaglycan (TGFBR3, transforming growth factor-β receptor type III) and endoglin (ENG, also known as CD105), and decoy receptors, such as BAMBI (BMP and activin membrane-bound inhibitor), do not signal independently but influence the signaling properties of receptor tetramers formed by type I and type II subunits.
The preferential pairing of each type II receptor with a specific type I receptor, the affinity of each cytokine for different receptor complexes, temporal and spatial coexpression of receptors, coreceptors, or decoy receptors, all vary and influence the probability that a certain complex will form and activate a specific signaling cascade. TGF-β1, -β2, and -β3 ligands are thought to primarily bind and activate tetramers composed of TβRI and TβRII subunits, resulting in phosphorylation of Smad2 and Smad3. Activins and some GDFs (including myostatin/GDF-8 and GDF-11) also signal through Smad2 and Smad3. Other GDFs and BMP ligands are thought to primarily function through receptor complexes that cause activation of Smad1, Smad5, and Smad8 through tetramers formed by BMPRIs and BMPRII, ActRII, or ActRIIB. Although specific combinations of tetramers are more likely to form in response to a given ligand, data suggest that a certain degree of promiscuous pairing occurs. For example, TGF-β-bound TβRII interacts and activates not only TβRI but also ALK-1 (Goumans et al. 2003), thus leading to activation of both Smad2 and Smad3 and Smad1, Smad5, and Smad8 in cells that express both types of type I receptors. In addition to activation of Smad-dependent pathways, TGF-β family ligands can also activate additional “noncanonical” pathways including the TRAF4–TRAF6 (TNF-receptor-associated factor 4 and/or 6)-TAK1 (TGF-β-activated kinase 1)-p38 MAPK pathway, the PI3K (phosphoinositide 3-kinase)-Akt pathway, the Rho-ROCK (Rho-associated protein kinase) pathway, and the JNK (c-Jun amino-terminal kinase), Erk (extracellular signal-regulated kinase) MAPK, and NF-κB (nuclear factor-κB) pathways (reviewed in Derynck and Zhang 2003). Activation of these noncanonical pathways is highly cell-specific and context-dependent.
EXTRACELLULAR MATRIX–DEPENDENT REGULATION OF TGF-β BIOAVAILABILITY
Connective tissue disorders are a family of diseases characterized by alterations in the composition, structure, or turnover of the extracellular matrix (ECM), a complex mixture of carbohydrates and proteins that includes collagens, proteoglycans, and glycoproteins (Mouw et al. 2014) that is secreted by cells in multicellular organisms. The specific properties of each type of connective tissue (i.e., cartilage, bone, ligaments, skin, muscle, and vessel walls) are dependent on the tissue-specific molecular composition of the ECM. In addition to providing physical support to cells, tissues, and organs, the ECM participates in regulating complex cellular behaviors including proliferation, migration, apoptosis, and differentiation. Dynamic remodeling of the ECM through regulated deposition, assembly, and degradation is critical for all ECM functions. ECM exerts its influence on surrounding cells through its interaction with matrix-sensing receptors on the cell surface, such as integrins (Campbell and Humphries 2011), and by regulation of the bioavailability of signaling molecules through regulated sequestration, release, and activation (Hynes 2009). The importance of the ECM to normal development and homeostasis is highlighted by the number of disorders that are either caused or exacerbated by abnormal ECM function (reviewed in Bateman et al. 2009; Bonnans et al. 2014).
TGF-β ligands (TGF-β1, TGF-β2, and TGF-β3) are secreted as biologically inactive latent complexes that are converted into an active form through a tightly regulated process that depends on interactions with components of the ECM (Munger and Sheppard 2011; Robertson and Rifkin 2016). TGF-β latent complexes (referred to as the small latent complex, or SLC) are comprised of TGF-β dimers and latency-associated peptide (LAP) dimers. LAPs are translated as prodomains from the same genes and mRNAs encoding the corresponding TGF-βs and are referred to as β1-LAP for TGF-β1, β2-LAP for TGF-β2, and β3-LAP for TGF-β3. LAP prodomains are cleaved during posttranslational processing and remain associated with the corresponding TGF-β molecule through noncovalent interactions (Munger and Sheppard 2011). Disruption of noncovalent interactions between TGF-β and LAP molecules, through LAP degradation (Lyons et al. 1988; Yu and Stamenkovic 2000), chemical modification, such as that caused by reactive oxygen species or low pH (Lyons et al. 1988; Barcellos-Hoff and Dix 1996), and/or through binding-induced conformational change (Munger et al. 1997; Shi et al. 2011), results in conversion of latent TGF-β into active TGF-β. TGF-β–LAP dimers (SLC) covalently bind to one of the latent TGF-β binding proteins (LTBP-1, -3, and -4) through disulfide bonds formed between LAP and LTBPs (Todorovic and Rifkin 2012), resulting in the large latent complex, or LLC. Most cell-types do not secrete TGF-β as a SLC but rather already in complex with LTBP proteins. Interactions between LTBPs and ECM components, in particular with fibrillin microfibrils, are thought to be important for proper localization, concentration, and activation of latent TGF-β (Ramirez et al. 2007). Importantly, integrins (and in particular αvβ6, αvβ8, and αvβ1) have been shown to be necessary for activation of TGF-β1 and TGF-β3 through their interaction with arginine-glycine-aspartic acid (RGD) amino acid domains present in β1-LAP and β3-LAP (but not β2-LAP) (Munger et al. 1998; Mu et al. 2002; Dong et al. 2014; also reviewed in Munger and Sheppard 2011). Although prodomains similar to LAPs are present in other members of the TGF-β family and associate noncovalently with their corresponding ligands, they are not known, with the exception of myostatin, to be associated with latency. These prodomains might, however, facilitate binding to the ECM through interactions with fibrillin microfibrils. For example, fibrillin has been shown by surface plasmon resonance to bind BMP-2, BMP-4, BMP-7, BMP-10, and GDF-5 (Sengle et al. 2008). In particular, the interaction between fibrillin-1 and BMP-4 has been shown to be important for regulating BMP-4 bioavailability (Nistala et al. 2010). Although latent TGF-β can also be found in association with glycoprotein-A repetitions predominant protein (GARP) (Stockis et al. 2009; Wang et al. 2012), expression of GARP appears to be limited to platelets and regulatory T lymphocytes (Macaulay et al. 2007; Tran et al. 2009), suggesting that this regulatory molecule is not a major regulator of TGF-β bioavailability in the ECM (Stockis et al. 2009; Tran et al. 2009; Wang et al. 2012).
ROLES OF TGF-β AND BMP SIGNALING IN BONE FORMATION, SKELETAL DEVELOPMENT, AND PATTERNING
Bone is described as a hard connective tissue because the terminally differentiated osteoblasts that form much of the bone tissue produce an ECM that mineralizes, and provides bone with its rigid structure. Bone tissue forms through two distinct mechanisms that either depend exclusively on osteoblasts or additionally on chondrocytes (Yang 2013). Most flat bones, such as craniofacial bones and part of the clavicle, are intramembranous, developing by direct differentiation of mesenchymal progenitors into osteoblasts (Soltanoff et al. 2009). The entire appendicular and most of the axial skeleton have an endochondral origin, forming through a cartilage template that is subsequently replaced by osteoblasts and bone (Soltanoff et al. 2009). TGF-β and BMP signaling are crucial for the development of both bone types (Chen et al. 2012) and for regulating chondrogenesis and osteogenesis (Kulkarni et al. 1993; Mishina et al. 1995; Winnier et al. 1995; Zhang and Bradley 1996; Sanford et al. 1997; Sirard et al. 1998; Gu et al. 1999; Beppu et al. 2000; Dunker and Krieglstein 2002; Wang et al. 2014; Salazar et al. 2016; Wu et al. 2016).
Although BMP signaling promotes differentiation and maturation of osteoblasts, TGF-β exerts a dual role during osteogenesis by inducing commitment to the osteoblast lineage while at the same time preventing terminal differentiation (Moses and Serra 1996; Serra et al. 1999; Alliston et al. 2001; Derynck and Akhurst 2007). R-Smads physically interact with and regulate the transcriptional activity of runt-related transcription factor 2 (Runx2, also known as Cbfa1), a master regulator of mesenchymal differentiation toward the osteoblastic cell lineage (Komori et al. 1997; Otto et al. 1997; Leboy et al. 2001; Miyazono et al. 2004; Hecht et al. 2007; Javed et al. 2008, 2009; reviewed in Lian et al. 2004; Schroeder et al. 2005). Runx2 function is repressed by TGF-β and Smad2 and Smad3 signaling, and enhanced by BMPs and Smad1, Smad5, and Smad8 signaling (Cohen 2009; van der Kraan et al. 2009).
Activin A, another member of the TGF-β family of ligands, is also expressed in bone and cartilage, and activates the Smad2- and Smad3-dependent pathway with potential overlap with TGF-β functions (Ogawa et al. 1992; Massagué and Gomis 2006; Eijken et al. 2007; Tsuchida et al. 2009; Pauklin and Vallier 2015; Salazar et al. 2016).
Regulation of Chondrogenesis and Longitudinal Bone Growth by TGF-β and BMP Signaling
In addition to its dual role in osteoblastogenesis, TGF-β signaling exerts a similar dual role in chondrogenesis by inducing the condensation of undifferentiated mesenchymal cells, stimulating their commitment to the chondrogenic lineage, and promoting proliferation of chondrocytes and deposition of cartilage-specific ECM, but also inhibiting terminal differentiation of chondrocytes (Leonard et al. 1991; Ballock et al. 1993; Mackay et al. 1998; Worster et al. 2000; Yang et al. 2001; Tuli et al. 2003; Zhang et al. 2004; Song et al. 2007; Mueller and Tuan 2008; Furumatsu et al. 2009). BMP signaling, in contrast, is dispensable for the initial step of mesenchymal condensation but required for commitment to the chondrogenic lineage, proliferation, differentiation, and maturation of chondrocytes (Li et al. 2003; Horiki et al. 2004; Yoon et al. 2005; Retting et al. 2009; Culbert et al. 2014). BMP-mediated chondrogenic induction is achieved through the direct transcriptional activation of the transcription factor Sex determining region Y-box 9 (Sox9), which is the master regulator of chondrogenesis (Zehentner et al. 1999; Akiyama 2008; Pan et al. 2008). Genetic disruption of Sox9 results in perturbed skeletogenesis throughout the body (Akiyama et al. 2002; Mori-Akiyama et al. 2003). Differential regulation of Runx2 by TGF-β and BMP signaling, as discussed above, also participates in regulation of chondrocyte maturation. TGF-β and BMP signaling regulate chondrogenesis not only during embryonic skeletogenesis but also during postnatal bone growth. Longitudinal growth of long bones is mediated by proliferation and differentiation of chondrocytes within the growth plate, where morphologically distinct zones are recognized, each containing chondrocytes at different stages of differentiation and producing stage-specific ECM. Growth plates, located between the epiphysis and diaphysis at the ends of long bones, are normally spared from ossification until the end of longitudinal growth (Kronenberg 2003). TGF-β and BMP signaling are both required for growth plate progression and maintenance (Brunet et al. 1998; Yang et al. 2001; Zhang et al. 2003, 2005; Yoon et al. 2005; Pogue and Lyons 2006; Seo and Serra 2007; Keller et al. 2011; Jing et al. 2013).
BMP signaling occurs throughout the various zones of the growth plate indicating multiple roles in growth plate development and maintenance (Solloway et al. 1998; reviewed in Pogue and Lyons 2006). Mice deficient for the BMP-specific antagonist, noggin, show overgrowth of skeletal elements (Brunet et al. 1998) supporting that BMP signaling promotes cell proliferation, differentiation, and maturation within the growth plate. Conversely, chondrocyte-specific overexpression of noggin results in gross reduction of cartilaginous elements (Tsumaki et al. 2002). A balance between positive and negative regulators of the BMP pathway is essential for proper bone growth, as shown by dysregulated bone development when the BMP pathway is over- or underactivated in genetically modified mouse models. Both genetically enhanced and genetically ablated BMP signaling result in impaired bone growth, which is caused by either premature onset of chondrocyte maturation toward hypertrophy or impaired proliferation within the growth plate (Francis-West et al. 1999; Mikic 2004; Kobayashi et al. 2005; Yoon et al. 2005; Pogue and Lyons 2006; Jing et al. 2013).
TGF-β signaling in the growth plate occurs in proliferating and hypertrophic chondrocytes and in the periosteum, suggesting a role in maintenance of these tissues (Ellingsworth et al. 1986; Sandberg et al. 1988; Gatherer et al. 1990; Pelton et al. 1990; Millan et al. 1991; Morales 1991; Serra et al. 2002; Ivkovic et al. 2003; Serra and Chang 2003; Minina et al. 2005). TGF-β signaling controls longitudinal bone growth through inhibition of chondrocyte terminal differentiation and maintenance of the proliferating chondrocyte pool (Serra et al. 1997; Alvarez et al. 2001; Yang et al. 2001; Alvarez and Serra 2004). Consequently, conditional inactivation of Tgfbr2 or Tgfbr1, which leads to ablation of TGF-β signaling, results in severe shortening of the limbs among other skeletal manifestations (Baffi et al. 2004; Seo and Serra 2007; Matsunobu et al. 2009; Hiramatsu et al. 2011).
Growth Plate Defects Caused by Altered TGF-β and BMP Signaling
Acromesomelic dysplasias (AMDs) are characterized by brachydactyly (BD) and short stature (Table 1). Various forms of AMD are caused by mutations in the gene coding for GDF-5, also known as cartilage-derived morphogenetic protein 1 (CDMP-1), and impaired BMP signaling. Growth retardation and the decreased size of appendicular skeletal elements are a result of premature growth plate closure (Thomas et al. 1996). Distal skeletal elements are more severely affected by size reduction than proximal elements. Clinical manifestations in acromesomelic dysplasia Hunter–Thompson type (AMDH), Grebe type (AMDG), and Du Pan syndrome are overlapping with the most severe growth retardation in AMDG and with specific fibula malformation in Du Pan syndrome (Langer and Garrett 1980; Langer et al. 1989; Costa et al. 1998; Faiyaz-Ul-Haque et al. 2002; Szczaluba et al. 2005).
AMD-associated GDF5 mutations result in loss of GDF-5 protein function. The AMDG-specific GDF5 mutation, C400Y, results in an inactive protein that is not secreted, and acts in a dominant-negative manner by preventing the secretion of other BMP molecules, consistent with the severe phenotype in patients carrying this mutation (Thomas et al. 1996, 1997). Although homozygous carriers of AMDG mutations display severe autopod (digit) malformation with reduction of fingers and toes to ball-shaped structures, heterozygous carriers are only mildly affected, indicating a dose-dependent effect of GDF-5 on growth plates. The phenotypic finding of AMD is recapitulated in mice deficient for Gdf5, including severe size reduction of long bones and abnormal joint development (Storm et al. 1994).
Roles of TGF-β and BMP Signaling in Limb Formation and Digit Patterning
During embryogenesis, the development of the appendicular skeleton first becomes evident by the outgrowth of limb buds. Limb bud outgrowth and patterning is a multistep process that is orchestrated by “signaling centers” within the limb field that provide positional and instructive information to the surrounding cells through gradients of growth factors, including BMPs, fibroblast growth factors (FGFs), Sonic hedgehog (SHH), and Wnts (Johnson and Tabin 1997; Martin 1998). BMPs are widely expressed in a dynamic pattern during limb development and have a range of diverse functions (Bandyopadhyay et al. 2006; Robert 2007). During limb bud initiation, BMP signaling is crucial for the induction of the apical ectodermal ridge (AER), a specialized epithelial structure located at the distal tip of the developing limb that controls formation of the proximal–distal axis from body center toward tips of appendages (Johnson and Tabin 1997; Martin 1998; Ahn et al. 2001). Early ablation of the AER or conditional inactivation of BMP signaling in the ectoderm leads to limb truncation (Saunders 1948; Summerbell et al. 1973; Rowe et al. 1982; Ahn et al. 2001). The AER functions to initiate and maintain normal gene expression patterns in the progress zone (PZ), an area of undifferentiated cells in the mesoderm directly underlying the AER, and is furthermore required for the maintenance of SHH expression within the zone of polarizing activity (ZPA), a crucial signaling center located at the posterior margin of the limb that controls anterior-posterior pattering. Together, the AER and ZPA coordinate gradients of growth factors to regulate formation, size, shape, and location of future bone and joints in the appendicular skeleton (reviewed in Johnson and Tabin 1997; Galloway and Tabin 2008). Interlinked feedback loops between AER, ZPA, and mesenchymal BMP signaling regulate initiation, propagation, and termination of the limb signaling system (reviewed in Benazet et al. 2009; Zeller et al. 2009). Disruption of these feedback loops result in impaired progression of limb bud development and specification of digit identity (Kawakami et al. 1996; Ahn et al. 2001; Khokha et al. 2003; Ovchinnikov et al. 2006; Montero et al. 2008; Suzuki et al. 2008; Benazet et al. 2009).
BMP signaling controls patterning of digits via a crescent-like structure located at the distal tip of each digit ray, called the phalanx-forming region (PFR). The PFR is marked by high levels of BMP activity and is formed and maintained by continuous recruitment of cells from the PZ. Cells recruited from the PZ aggregate distal to metatarsal and metacarpal condensations, commit to chondrogenic fate, and are eventually integrated into the condensing underlying digital ray. PFR-ablation or inhibition of BMP signaling via noggin results in truncation of the digit (Montero et al. 2008; Suzuki et al. 2008). TGF-β and activin signaling are also needed to establish the PFR and to induce Sox9 expression (Chimal-Monroy et al. 2011).
To form joints, the initially continuous digital rays become segmented. Many studies showed the importance of a balanced interplay among multiple factors for both joint formation and homeostasis (Storm and Kingsley 1996; Brunet et al. 1998; Rountree et al. 2004; Koyama et al. 2007). Location of a future synovial joint is initially indicated by cell condensations within an area of the digit ray called the interzone (reviewed in Archer et al. 2003; Decker et al. 2014). Cells in this region do not undergo chondrogenic differentiation but adopt a flattened morphology. The joint interzone later divides into three discrete layers. Cells within the medial layer express BMP-2 and subsequently undergo cell death, thereby forming the joint cavity, whereas the remaining outer layers will give rise to the articular cartilage (reviewed in Pacifici et al. 2005). During joint formation, noggin is expressed within the intermedial layer of the interzone (Brunet et al. 1998; Capdevila and Johnson 1998; Merino et al. 1998; Goumans and Mummery 2000; Pizette and Niswander 2000; Seemann et al. 2005; Lorda-Diez et al. 2013), where it counteracts the effect of GDF-5, the key molecule required for defining the future joint interzone within the unpatterned digital ray. Loss of GDF-5 function leads to abnormal joint development with complete or partial fusion of consecutive skeletal elements and structural changes in autopods, along with other skeletal defects, whereas loss of noggin activity results in failure to initiate joint formation and thus symphalangism (Storm and Kingsley 1996, 1999; Brunet et al. 1998).
Digit Malformations Caused by Disruption of TGF-β and BMP Signaling
Depending on the specific perturbation, disruption in the BMP signaling pathway can cause various types of BD, all characterized by reduction or absence of phalanges in one or more digits and resulting in shortened toes and/or fingers (Bell 1951; Stricker and Mundlos 2011). Although BD can occur as an isolated malformation, more commonly it is one of several features of syndromes caused by mutations that impair BMP ligand or receptor function, for example, in AMDs (see previous section; Table 1).
Depending on the affected digit, BD is classified into groups A to E (Table 2) (Fitch 1979). Inactivating mutations in genes coding for the BMPRIB receptor or its ligand GDF-5 (Nishitoh et al. 1996; Lehmann et al. 2003, 2006) cause BDA2, which is characterized by short middle phalanges in the second and fifth digits. A microduplication in a downstream regulatory element of BMP-2, which is thought to affect limb-specific expression, was also found in some patients with BDA2 (Dathe et al. 2009). GDF5 mutations also can cause BDC, characterized by short or absent middle phalanges in second, third, and fifth fingers, short first metacarpal and hypersegmentation of the second and/or third finger (Polinkovsky et al. 1997; Robin et al. 1997; Galjaard et al. 2001; Everman et al. 2002; Savarirayan et al. 2003; Holder-Espinasse et al. 2004; Schwabe et al. 2004). These BD phenotypes are recapitulated in vivo by ablation of Bmpr1b or Gdf5 in mice, which results in impaired proliferation of mesenchymal progenitors and increased apoptosis in phalanges, with preservation of interdigital mesenchyme (Baur et al. 2000; Yi et al. 2000). That impaired signaling through BMPRIB or GDF-5 in the developing autopod results in very specific malformations and does not affect all phalanges or digit identities suggests that other BMP ligands or pathways compensate for this specific deficiency during limb development.
Table 2.
Summary of brachydactyly types and associated clinical manifestations
| Brachydactyly type (OMIM #) | Gene mutation | Clinical manifestation in the skeletal system |
|---|---|---|
| A1 (112500) | IHH | Hypoplasia/aplasia of all middle phalanges |
| A2 (112600) | GDF5, BMP2 | Hypoplasia/aplasia middle phalanges of digit 2 and 5 |
| B1 (113000) | ROR2 | Hypoplasia/aplasia of distal phalanges of digits 2 to 5, fusion of distal interphalangeal joints; duplication of distal phalange in digit 1 |
| B2 (611377) | NOG | Hypoplasia/aplasia of distal and middle phalanges, syndactyly (variable) |
| C (113100) | GDF5 | Shortening of metacarpal 1, shortening or absence of middle phalanges of digit 2, 3, and 5, hypersegmentation of digit 2 and/or 3 |
| E1 (113300) | HOXD13 | Shortening metacarpal 4; sometimes shortening of metatarsal 4 |
| E2 (613382) | PTHLH | Shortening of metacarpals (variable in affected digits but most common in digit 4 and 5) |
Although reduced GDF-5 and BMPRIB signaling causes BDA2, excessive BMP signaling activity through GDF-5 and BMPRIA leads to proximal symphalangism (SYM1), multiple synostosis syndromes (SYNS1 and SYNS2), and BDB2. SYM1, SYNS1, and SYNS2 syndromes are characterized by multiple joint fusions that are restricted to proximal interphalangeal joints of the hands and feet and carpal and tarsal bones; SYNS1 and SYNS2 patients additionally display multiple joint fusions in vertebrae and the hip (da-Silva et al. 1984; Gaal et al. 1987; Gong et al. 1999; Takahashi et al. 2001; Mangino et al. 2002). SYM1 is caused by gain-of-function mutations in GDF5 that strongly increase ligand affinity for the BMPRIA receptor and result in loss of binding specificity for BMPRIB (Nickel et al. 2005; Seemann et al. 2005). Increased BMP signaling activity also follows from GDF5 mutations that render GDF-5 resistant to noggin and cause SYNS2 (Schwaerzer et al. 2012; Degenkolbe et al. 2013). SYNS1 and SYM1 can also result from heterozygous missense mutations in the gene coding for the BMP inhibitor noggin. Although secretion of noggin is only reduced in SYM1, it is completely abolished in SYNS1, explaining the more severe SYNS1 phenotype and pointing toward a dosage-dependent effect of this inhibitor during skeletogenesis (Marcelino et al. 2001).
The importance of balanced levels of BMP signaling during digit development is further emphasized by other congenital syndromes, in which mutations, such as those found in BDB2, induce a shift in this tightly regulated process. Short distal phalanges in combination with symphalangism, fusion of carpal and tarsal bones and partial cutaneous syndactyly are characteristic skeletal malformations in BDB2 patients. Point mutations within the NOGGIN (NOG) gene cause reduced binding capacity to BMP ligands and result in reduced sequestration of BMP ligand by the mutant protein, which shifts the state of the BMP signaling pathway balance toward activation (Lehmann et al. 2007) and results in disruption of digit patterning.
Roles of TGF-β and BMP Signaling in Craniofacial Development
TGF-β and BMP signaling are required for proper formation of craniofacial structures, and in particular for proper development of cranial bones and palate (Rigueur et al. 2015). During craniofacial development, plates of calvarial bone interconnected via fibrous sutures envelop the brain. The fibrous sutures serve as the sites of bone expansion during postnatal growth and provide plasticity to the skull in response to the growing brain (reviewed in Opperman 2000; Warren et al. 2001). Alterations in both TGF-β and BMP signaling have been associated with premature closure of sutures and craniosynostosis. Increased Smad-dependent BMP signaling induced in cranial neural crest cells by a constitutively active BMPRIA receptor causes premature suture closure in mice, which can be rescued by genetically or chemically inhibiting this pathway (Komatsu et al. 2013). During calvarial development, a major role of FGF is to suppress expression of the BMP inhibitor noggin via Erk1 and Erk2 MAPK signaling and thus induce suture closure by increasing BMP signaling (Warren et al. 2003). TGF-β ligands are also expressed in the developing skull and most likely involved in regulating suture closure. TGF-β1 and TGF-β2 are expressed in sutures undergoing fusion, whereas TGF-β3 expression is restricted to patent sutures, possibly suggesting that TGF-β1 and TGF-β2 promote, whereas TGF-β3 prevents, closure (Opperman et al. 1997; Most et al. 1998; Chong et al. 2003). In accordance with these observations, TGF-β2 has been shown to induce suture closure in an Erk1 and Erk2 MAPK-dependent manner in an ex vivo murine calvaria model (Opperman et al. 2006).
In addition to their role in calvaria fusion, BMP and TGF-β signaling are also essential for development and fusion of the palatal shelves (Bush and Jiang 2012). Fusion between the shelves requires first formation and then disintegration of the midline epithelial seam (MES). This leads to mesenchymal fusion of the palate, and subsequent bone formation, which occurs through intramembranous ossification (Baek et al. 2011). The main function of TGF-β in palate development is to promote fusion of the shelves. Before palatal shelf elevation, epithelial expression of TGF-β1 and TGF-β3 is induced within the future adhesion site, and then diminishes during disintegration of the MES (Cui et al. 1998; Cui and Shuler 2000; Yang and Kaartinen 2007; Bush and Jiang 2012). Global ablation of TGF-β2 or TGF-β3 ligands or cranial neural crest specific knockout of Tgfbr2 or Tgfbr1 causes cleft palate, highlighting the importance of this signaling pathway in palatogenesis (Kaartinen et al. 1995; Proetzel et al. 1995; Sanford et al. 1997; Ito et al. 2003; Dudas et al. 2006; Xu et al. 2006). In Tgfb3-null mutants, palatal shelf adhesion occurs, but impaired apoptosis causes persistence of the MES resulting in non-union of the palatal mesenchyme. Treatment of these mice with recombinant TGF-β3 or overexpression of Smad2 rescues palate fusion (Kaartinen et al. 1997; Taya et al. 1999; Cui et al. 2005; Dudas et al. 2006; Xu et al. 2006). BMP signaling regulates specification and migration of cranial neural crest cells to the facial primordia (reviewed in Nie et al. 2006), and also controls mesenchymal cell proliferation. Tissue-specific inhibition of this signaling pathway by conditional inactivating mutations in BMP receptors or overexpression of the antagonist noggin causes a reduced proliferation rate within the palatal mesenchyme and results in palate clefting (Dudas et al. 2004; Liu et al. 2005; He et al. 2010; Baek et al. 2011).
Craniofacial Defects Caused by Altered TGF-β or BMP Signaling
Cleft palate with or without involvement of the lip is a common congenital defect in humans (Parada and Chai 2012) that has been associated with loss-of-function mutations in BMP4 (orofacial cleft 11; OFC11) and BMP7 (Suzuki et al. 2009; Wyatt et al. 2010). Although the functional consequences of these mutations are not fully understood, some observations support that they alter posttranslational processing to reduce protein activity in a tissue-dependent manner (Akiyama et al. 2012). In addition, both cleft palate and craniosynostosis are associated with Loeys–Dietz syndrome, which is caused by mutations in critical mediators and regulators of TGF-β signaling, and is discussed below in greater detail.
TGF-β SIGNALING AND VESSEL WALL HOMEOSTASIS
Structure of the Arterial Vessel Wall
The vessel wall of major arteries is composed of three connective tissue layers: an inner layer (tunica intima), a middle layer (tunica media), and an outer layer (tunica adventitia) (Wagenseil and Mecham 2009), each composed of different types of cells and ECM components (Fig. 4). Endothelial cells line the lumen of the vessel and are anchored to a basement membrane that separates these cells from the tunica intima. The internal elastic lamina separates the tunica intima from the tunica media, which is populated by vascular smooth muscle cells (VSMCs) and contains elastic fibers, complex structures that contain both elastin and fibrillin-containing microfibrils, collagen fibers, and proteoglycans (Wagenseil and Mecham 2009). Although the majority of VSMCs reside in the tunica media, some VSMCs or VSMC-like cells are also present in the tunica intima and/or can be recruited to the intima in response to vascular injury (Shanahan and Weissberg 1998; Owens et al. 2004; Fukuda and Aikawa 2010; Iwata et al. 2010). The tunica intima, which is generally much thicker in human arteries compared with other mammals, does not contain elastic fibers. The outer layer or tunica adventitia is populated by adventitial fibroblasts, is rich in collagen, does not contain elastic fibers, and is separated by the tunica media by the external elastic lamina (reviewed in Wagenseil and Mecham 2009). Some studies have suggested that the adventitial layer contains progenitors that trans-differentiate into VSMCs (Sartore et al. 2001; Hoofnagle et al. 2004; Hu et al. 2004; Passman et al. 2008). The composition of the ECM secreted by vascular cell types, and by VSMCs in particular, varies depending on the type of blood vessel and in response to developmental, physiological, and pathological stimuli (Kelleher et al. 2004; Xu and Shi 2014). For example, a study of the type of ECM components secreted by VSMCs at various stages of mouse development identified a critical period from embryonic day E14 to ∼2 weeks after birth during which expression of matrix proteins, such as elastin and fibrillar collagens, is very pronounced, and which is followed by a period of low-expression that continues into adulthood (Kelleher et al. 2004; Wagenseil and Mecham 2009). Under physiological circumstances, elastin is thought to only be secreted and productively deposited by VSMCs during the fetal and neonatal periods (Davis 1993; Kelleher et al. 2004).
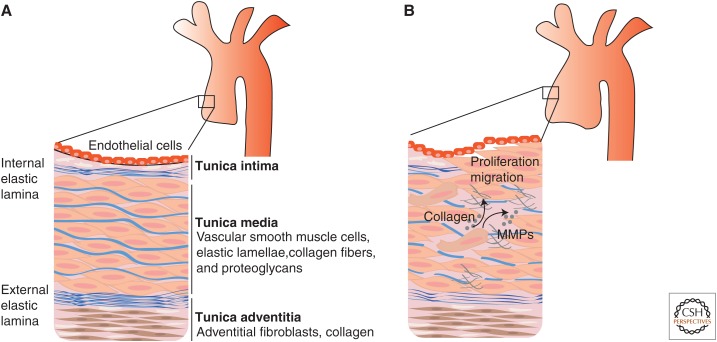
Figure 4.
Schematic representation of the vessel wall of major arteries. (A) The vessel wall of arteries is organized in layers, which are referred to as tunica intima, media, and adventitia. Endothelial cells line the lumen and are attached to a basement membrane. The connective tissue immediately below the endothelial cell layer is referred to as tunica intima. The internal elastic lamina separates the tunica intima from the tunica media, which contains vascular smooth muscle cells (VSMCs) and elastic fibers, organized in fenestrated sheets (also called lamellae). The external elastic lamina separates the tunica media from the tunica adventitia, which contains mostly fibroblasts and collagen, but no elastic fibers. (B) Arterial wall remodeling associated with aneurysm development include increased VSMC migration and proliferation (and associated loss of contractile function), increased secretion of matrix-degrading enzymes (such as matrix metalloproteinases [MMPs]), and increased deposition of collagen and fragmentation of elastic fibers. The ultimate effect of these processes is the replacement of the ordered and layered architecture of elastic lamellae and vascular smooth muscle cells with disorganized and weak connective tissue.
Both mechanical stimuli and biochemical signals regulate the interaction between the ECM and vascular cells (Owens et al. 2004; Mack 2011; Humphrey et al. 2015). Changes in the ECM are sensed by vascular cells through matrix-binding receptors, such as integrins, and influence cell migration, proliferation, and survival (Moiseeva 2001); in turn, vascular cells can modify the composition and structure of the ECM by increasing or decreasing secretion of matrix proteins and matrix-remodeling enzymes (Leung et al. 1976; O’Callaghan and Williams 2000).
TGF-β Signaling in Vascular Homeostasis
The importance of TGF-β signaling regulation in the complex relationship between ECM and vascular cells is highlighted by the number of vascular disorders that result from failed ECM-dependent regulation of TGF-β signaling or from mutations in critical mediators of TGF-β signal transduction (reviewed in Goumans and Mummery 2000; Goumans et al. 2009). Of particular importance to the focus of this review are the effects of altered TGF-β signaling on the development of aneurysm, a pathological dilation of a blood vessel that can result in fatal tear and rupture.
The effects of TGF-β and BMP signaling on the biology of the vessel wall vary depending on developmental stage, cellular context, and presence of other ligands or stimuli. Prenatally, TGF-β and BMP signaling are key regulators of both development and morphogenesis of the vascular system (Goumans and Mummery 2000; Goumans et al. 2009). Early during embryonic development, TGF-β signaling is required for formation of the vascular plexus and differentiation of both endothelial cells and VSMCs (Dickson et al. 1995; Oshima et al. 1996; Goumans and Mummery 2000; Larsson et al. 2001; Carvalho et al. 2007; Goumans et al. 2009). TGF-β signaling is also required for morphogenesis of the cardiac outflow tract and VSMC-dependent deposition of elastic fibers (Wurdak et al. 2005; Choudhary et al. 2006).
Prenatal or perinatal ablation of TGF-β signaling in mouse models by deletion of Tgfbr2 in VSMCs with a VSMC-specific Cre (Langlois et al. 2010), neural crest- and mesoderm-specific Cre (Choudhary et al. 2009), or an inducible VSMC-specific Cre (Li et al. 2014) results in defective elastogenesis, vessel wall dilation, aneurysm, and dissection. However, deletion of Tgfbr2 in VSMCs after ∼8 weeks of age has no apparent consequence on VSMC function and vessel wall homeostasis (Li et al. 2014), suggesting that complete loss of TGF-β signaling in adult VSMCs does not cause major pathology in the adult vessel wall. The early developmental sensitivity to TGF-β signaling ablation in VSMCs might relate to the timing of physiological elastin deposition, which is complete once animals reach adulthood, at ∼4–8 weeks after birth (Davis 1993; Kelleher et al. 2004; Wagenseil and Mecham 2009). The expression of other determinants of vessel wall homeostasis might also require TGF-β signaling during this critical time window, but then become irrelevant, redundant, or independent of TGF-β signaling later in life.
In the adult aorta, excessive TGF-β signaling, rather than impaired TGF-β signaling, has been linked to maladaptive remodeling of the vascular ECM, VSMC dysfunction, and development of thoracic aortic aneurysm (TAA), a common form of aneurysm often associated with genetic syndromes (Chen et al. 2013). TGF-β-dependent changes during aneurysm development include increased expression and/or activities of matrix-degrading enzymes such as metalloproteinases, degradation of elastic fibers, enhanced proliferation and migration of VSMCs, and excessive collagen secretion and deposition (reviewed in Jones et al. 2009). Overall, the effects of these alterations, which target both matrix degradation and matrix deposition, weaken the aortic wall rendering it more prone to dilatation, dissection, and rupture.
Marfan Syndrome
The importance of TGF-β signaling in the context of ECM homeostasis is well illustrated by the case of Marfan syndrome (MFS). MFS is an autosomal dominant disorder caused by heterozygous mutations in the gene that encodes fibrillin-1 (FBN1), the main component of extracellular microfibrils (Dietz et al. 1991). It is characterized by cardiovascular, ocular, and musculoskeletal defects (Table 1), including a high risk for aortic root aneurysm and dissection (Judge and Dietz 2005). MFS pathology was initially attributed to a structural deficit caused by defective fibrillin-1 assembly into microfibrils. However, simple structural changes could not explain some MFS manifestations, such as craniofacial dysmorphism, bone overgrowth, myxomatous degeneration of the mitral valve, and low-muscle mass and fat stores, all of which pointed to a more complex relationship between the ECM and developmental signals. This observation led to the hypothesis that dysregulation of TGF-β signaling contributed to MFS pathology. It is now recognized that most manifestations of MFS can be understood as a failure in the ECM-dependent control of TGF-β signaling (Neptune et al. 2003). Under physiological circumstances, extracellular microfibrils serve complex and somewhat divergent functions in regulating TGF-β signaling. They serve both as “positive” regulators of TGF-β signaling, by concentrating latent TGF-β at sites of intended function, and as “negative” regulators of TGF-β signaling, by sequestering latent TGF-β and preventing its conversion to the active form (Ramirez et al. 2004; Robertson et al. 2015).
Despite these two contrasting roles, several studies examining a diverse set of MFS phenotypes, both in patients and mouse models, have shown that a signature consistent with increased activation of the TGF-β signaling pathway is associated with MFS pathology, and that pharmacological antagonism of TGF-β with neutralizing antibodies attenuates or prevents disease progression (Neptune et al. 2003; Ng et al. 2004; Habashi et al. 2006; Cohn et al. 2007). Studies conducted in MFS mouse models have suggested that effects of TGF-β signaling attenuation with neutralizing antibodies are dependent on the developmental stage of the vessel wall, with early TGF-β inhibition, between three and six weeks of age, being deleterious, and late TGF-β inhibition, after 6 weeks of age, being beneficial (Cook et al. 2015). These observations are consistent with the studies discussed above suggesting that TGF-β signaling has a protective role prenatally and perinatally, when it is required to complete vessel wall development and elastogenesis, and a pathogenic role after such events are completed.
Importantly for clinical management of MFS patients, the Food and Drug Administration (FDA)-approved angiotensin II type 1 receptor (AT1) blocker losartan, an antihypertensive drug, has been shown to reduce TGF-β signaling and ameliorate many manifestations of MFS in mouse models (Neptune et al. 2003; Ng et al. 2004; Habashi et al. 2006; Cohn et al. 2007; Lacro et al. 2007). Additionally, in a randomized clinical trial of pediatric MFS patients, losartan, at the FDA-recommended dose for hypertension, was shown to be equally effective as atenolol used at a dose far above the FDA-recommended daily dose, with both drugs leading to a significant decline in Z-score over time (body size-indexed aortic root dimension over time) (Lacro et al. 2014; Mallat and Daugherty 2015). A trial in adults with MFS showed that use of losartan was associated with a significant reduction in aortic root growth rate and also attenuated dilatation of the more distal ascending aorta in patients that had undergone aortic root replacement (Groenink et al. 2013).
Although several studies have shown that antagonism of angiotensin II signaling results in decreased TGF-β signaling in a variety of tissues, including kidney, lung, skeletal muscle, heart, and aorta (Shihab et al. 1997; Sun et al. 1998; Lavoie et al. 2005; Habashi et al. 2006; Yao et al. 2006; Cohn et al. 2007; Podowski et al. 2012), the exact mechanism by which this occurs is not fully understood (Gibbons et al. 1992; Stouffer and Owens 1992; Wolf et al. 1993, 1999; Kagami et al. 1994; Lee et al. 1995; Campbell and Katwa 1997; Fukuda et al. 2000; Boffa et al. 2003; Naito et al. 2004; Rodriguez-Vita et al. 2005; Zhou et al. 2006; Chen et al. 2013). Suppression of excessive Erk1 and Erk2 MAPK activation with losartan or an inhibitor of MAPK kinase (MAPKK, also known as MEK) has been shown to normalize aortic architecture and aneurysm pathology in MFS mouse models (Habashi et al. 2011), indicating that Erk MAPK activation is critical to aneurysm progression. Although not fully elucidated, the pathogenic effects of Erk1 and Erk2 MAPK signaling might relate to induced expression of metalloproteinases (Ghosh et al. 2012) or TGF-β1 ligand (Hamaguchi et al. 1999).
Perturbed sequestration of TGF-β ligands in bone and cartilage might also explain the skeletal features of MFS. MFS patients show increased length of appendicular skeletal elements, especially in phalanges. Although the TGF-β bioavailability in skeletal tissue of MFS patients has not been evaluated, TGF-β ligands are abundantly expressed in growth plates and bone (Pedrozo et al. 1998; Minina et al. 2005). Osteoblasts derived from fibrillin (Fbn1 or Fbn2)-deficient mice show abnormal activation of TGF-β signaling (Nistala et al. 2010). The stimulatory effect of TGF-β on cell proliferation and chondrogenesis in the growth plate and its inhibition of chondrocyte maturation suggest that bone overgrowth in MFS may result from growth plate enlargement. In addition to perturbed TGF-β bioavailability, MFS mutations might also alter binding of fibrillin-1 to BMPs (Arteaga-Solis et al. 2001; Sengle et al. 2008; Nistala et al. 2010), suggesting that skeletal abnormalities in MFS might result from altered, and perhaps misbalanced, BMP and TGF-β signaling.
Loeys–Dietz Syndrome
Loeys–Dietz syndrome (LDS) is an autosomal dominant Mendelian disorder with widespread phenotypic overlap with MFS, including bone overgrowth, skeletal deformity, and a strong predisposition for aortic root aneurysm and dissection (Loeys et al. 2005). When compared with MFS, LDS also shows many unique features (Table 1) in the craniofacial, skeletal, cutaneous, and cardiovascular systems (MacCarrick et al. 2014). Patients with LDS can also show evidence of immunologic dysregulation including asthma, eczema, and gastrointestinal inflammation that associates with increased numbers of T-regulatory cells that inappropriately secrete proinflammatory T-helper 2 cytokines (Frischmeyer-Guerrerio et al. 2013). Inactivating mutations in the genes encoding TGF-β receptors (TGFBR1 or TGFBR2) (Loeys et al. 2005), intracellular mediators of TGF-β signaling (SMAD3 and SMAD2) (van de Laar et al. 2011; Micha et al. 2015), or TGF-β ligands (TGFB2 and TGFB3) (Boileau et al. 2012; Lindsay et al. 2012; Bertoli-Avella et al. 2015), have all been shown to cause LDS or LDS-like syndromes. In contrast, mutations in genes coding for TGF-β family receptors primarily expressed in endothelial cells, such as the ENG (McAllister et al. 1994) and ACVRL1 (Lesca et al. 2004) genes, cause hereditary hemorrhagic telangiectasia type 1 and type 2, syndromes characterized by dilated small blood vessels (telangiectasias) and improper connections between arteries and veins (arteriovenous malformation, or AVMs), but typically without aortic aneurysm or widespread connective tissue involvement (and as such not the focus of this review). The phenotypic similarities between MFS and LDS suggest that these syndromes share a common pathogenic mechanism. However, although the role of enhanced TGF-β signaling is well established in MFS, the mechanisms by which LDS mutations cause disease and a tissue signature for increased TGF-β signaling during aneurysm progression remain unclear. In vitro experiments consistently show that mutant receptors are expressed, traffic to the cell surface, and bind ligand but fail to phosphorylate Smad2 in response to exogenous TGF-β (Mizuguchi et al. 2004; Horbelt et al. 2010; Gallo et al. 2014). However, histological and biochemical assessment of aortic tissue derived from patients affected by LDS, or mouse models, show a paradoxical signature of high TGF-β signaling (van de Laar et al. 2011; Lindsay et al. 2012; Gallo et al. 2014). These contrasting observations have generated controversy in the field regarding the specific role of TGF-β in the initiation and progression of aneurysm (Lavoie et al. 2005; Dietz 2010; Mallat and Daugherty 2015). Various mechanisms have been proposed to explain how LDS mutations can paradoxically result in increased TGF-β signaling in vivo, including the possibility that angiotensin II receptor signaling, rather than TGF-β signaling, might be responsible for the observed increase in Smad2 and Smad3 phosphorylation and up-regulation of TGF-β-driven gene products (Davis et al. 2014), or that different intrinsic susceptibility to LDS-causing mutations in cells of different type or embryonic origin might result in paracrine signaling overdrive caused by excessive secretion of TGF-β1 ligand (Lindsay and Dietz 2011). However, none of these mechanisms has been validated to date. A possible paracrine mechanism is suggested by the observation that expression of TGF-β1 is increased in tissues derived from LDS-3 and LDS-4 patients and in LDS mouse models (van de Laar et al. 2011; Lindsay et al. 2012; Gallo et al. 2014), and that treatment of LDS mouse models with losartan, which normalizes aortic root growth and aortic wall architecture in these animals, is associated with reduction of excessive TGF-β1 expression and Smad2 and Erk MAPK activation in LDS mice (Gallo et al. 2014).
Perturbations in TGF-β signaling in LDS are also associated with cleft palate (Table 1). In LDS patients, defective TGF-β signaling during palate development might result in failed mesenchymal fusion of palatal shelves (Yang and Kaartinen 2007) and thus in cleft palate. An alternative, yet controversial, explanation (Iwata et al. 2012) is that in Tgfbr2-null mice, cleft palate might result from an imbalance between Smad and non-Smad TGF-β signaling caused by “noncanonical” pairing of TβRI and TβRIII/betaglycan, and overactivation of the TRAF6–TAK1–p38 signaling pathway, which leads to a proliferation defect responsible for nonunion of the palatal shelves (Iwata et al. 2012). This idea was supported by the observation that concomitant deletion of the Tgfbr1 gene rescued the cleft palate phenotype in cranial neural crest-specific Tgfbr2−/− mice. TβRI or TβRII receptors with LDS mutations, which traffic to the cell surface and can bind ligand, might in a similar fashion increase signaling by favoring “noncanonical” pairing of the remaining functional receptors in the developing palate of LDS mouse models and patients.
Shprintzen–Goldberg Syndrome
Shprintzen–Goldberg syndrome (SGS) is characterized by virtually all the craniofacial, skeletal, cutaneous and cardiovascular manifestations found in LDS and MFS, with the addition of highly penetrant intellectual disability. It is caused by heterozygous loss-of-function mutations in the Sloan Kettering Institute proto-oncogene (SKI) (Carmignac et al. 2012; Doyle et al. 2012), a potent inhibitor of TGF-β-induced Smad signaling. Ski-dependent inhibition of TGF-β signaling occurs through suppression of Smad2 and Smad3 phosphorylation and nuclear translocation, by displacement of positive regulators of TGF-β-induced transcription, such as p300 and CREB-binding protein (CBP), and recruitment of negative regulators such as histone deacetylases (Akiyoshi et al. 1999; Nomura et al. 1999; Prunier et al. 2003). All mutations causing SGS occur in amino-terminal domains of Ski that participate in Smad2 and Smad3 binding, and cultured fibroblasts from patients with SGS show increased Smad- and non-Smad-mediated TGF-β signaling and expression of TGF-β-driven genes (Doyle et al. 2012). The fact that mutations in SKI that cause SGS increase TGF-β signaling and fully phenocopy LDS supports the notion that enhanced TGF-β signaling contributes to the pathogenesis of both conditions.
INTEGRIN–FIBRILLIN INTERACTIONS AND OVERACTIVATION OF TGF-β SIGNALING
As discussed above, fibrillin-1 regulates the bioavailability of TGF-β by both concentrating and sequestering latent TGF-β in the ECM, and mutations that interfere with this functions cause MFS. Interestingly, a specific subset of fibrillin-1 mutations causes stiff skin syndrome (SSS), a condition characterized by perinatal onset of dense dermal fibrosis and joint contracture, in the absence of any features of MFS. Skin derived from SSS patients shows large macroaggregates of disorganized microfibrils that concentrate latent TGF-β in the dermis and increased levels of Smad2 phosphorylation, indicative of overactivation of TGF-β signaling (Loeys et al. 2010).
SSS-causing mutations all cluster in the only fibrillin-1 domain that harbors an RGD domain used to mediate cell–matrix interactions via binding to integrin receptors, suggesting that disruption of integrin–fibrillin-1 interactions contributes to the pathogenesis of this disorder. Fibrillin-1 fragments with SSS mutations fail to support attachment and spreading of cells that express integrins αvβ3 and/or α5β1, which are the integrins that were reported to bind the RGD domain in fibrillin-1 (Loeys et al. 2010). In addition, mice heterozygous for an RGD to RGE encoding mutation in Fbn1, predicted to cause an obligate loss of integrin binding, recapitulate the dermal sclerosis found in patients and mice heterozygous for SSS mutations (Gerber et al. 2013). Taken together, these data indicate that failed integrin binding to fibrillin-1 initiates and sustains a sclerotic phenotype in the skin.
A unique population of cells that express high levels of integrins αvβ3 and/or α5β1 is present in the dermis of SSS mouse models (Gerber et al. 2013), suggesting that disruption of the interaction between fibrillin-1 and integrins results, directly or indirectly, in increased expression of these integrins and/or recruitment of cells that naturally express high levels of αvβ3 and/or α5β1. Treatments that mimic engagement of β1 integrin, such as administration of a β1 integrin-activating antibody, ameliorate skin stiffness and normalize expression of both αvβ3 and/or α5β1 in the dermis (Gerber et al. 2013). Similar results are observed by inactivating the gene encoding β3 integrin, treatment with TGF-β neutralizing antibody, or inhibition of the Erk MAPK pathway, suggesting cross-talk between integrins and TGF-β signaling pathways in SSS (Gerber et al. 2013). Increased TGF-β signaling in SSS skin might be caused by excessive integrin-mediated TGF-β activation and/or increased TGF-β bioavailability and activation of Erk1 and Erk2 MAPK in a β3 integrin-dependent manner, with the bulk of current evidence favoring the latter (Scaffidi et al. 2004; Munger and Sheppard 2011; Gerber et al. 2013).
SSS mice recapitulate multiple aspects of systemic sclerosis (SSc), including tightening and hardening of the skin, a tissue signature for excessive TGF-β signaling, and a propensity to develop autoantibodies and other markers of autoinflammation. In this light, it is notable that SSS mice show excessive concentration and activation of plasmacytoid dendritic cells (pDCs) in the dermis. pDCs normally perform a surveillance function for viral pathogens (Swiecki and Colonna 2010). On activation, pDCs express high levels of interferon-α, interleukin (IL)-6, and TGF-β; this can lead to autoantibody production and T-helper cell skewing toward proinflammatory subsets (as seen in both SSc and mouse models of SSS). Wild-type pre-pDCs showed increased adherence and activation when plated on ECM produced by SSS fibroblasts, when compared with wild-type cell-derived ECM, suggesting that the altered matrix environment is sufficient to initiate this process. The current view is that the altered matrix in SSS increases integrin expression by pDCs and the local concentration and activity of TGF-β, known to induce pDCs to produce even more TGF-β. The resulting increased concentrations of profibrotic and proinflammatory cytokines in the skin provide a plausible mechanism by which point mutations in a connective tissue protein can phenocopy autoimmune scleroderma (SSc), a condition that also shows microfibrillar macroaggregates and increased dermal concentration of TGF-β, albeit through an unknown initiating event (Loeys et al. 2010).
ROLE OF TGF-β AND BMP SIGNALING IN BONE HOMEOSTASIS
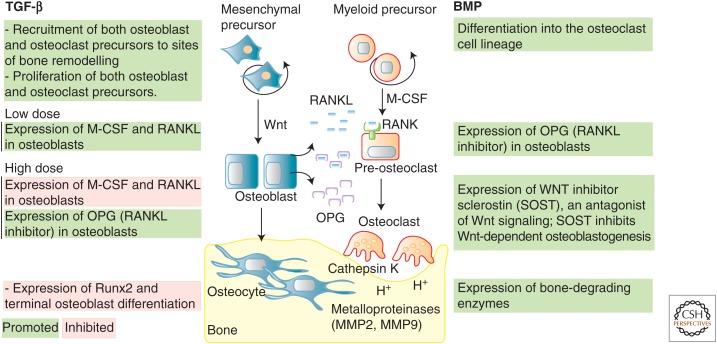
In addition to their crucial functions in skeletal development, it has become clear over the last few years that balanced BMP and TGF-β signaling is necessary for postnatal bone homeostasis (Fig. 5). Bone remodeling maintains bone quality and stability, and occurs through continuous cycles of bone resorption by osteoclasts and new bone formation by osteoblasts and osteocytes (Teitelbaum and Ross 2003; Capulli et al. 2014). Osteoblasts exert key regulatory roles in bone homeostasis by controlling the differentiation of osteoclasts from the hematopoietic cell lineage and by regulating osteoclast activity and survival. Osteoblasts secrete RANKL (receptor activator of NF-κB ligand, also known as OPGL, TRANCE, ODF) and osteoprotegerin (OPG, also known as OCIF, TNFSF11B), the antagonist of RANKL. Binding of RANKL to its cognate surface receptor RANK on preosteoclasts induces osteoclast differentiation and bone resorption. OPG protects the skeleton from excessive bone loss by sequestering and thus neutralizing RANKL by inhibiting binding to its receptor. Balanced levels of RANK, RANKL, and OPG are required to maintain bone homeostasis, and imbalance results in excess resorption, as found in osteoporosis, or excess bone formation, as found in sclerosing bone dysplasias and osteopetrosis. Osteoblasts, furthermore, promote osteoclast proliferation, function and survival by secretion of macrophage colony-stimulating factor (M-CSF, also known as CSF1) that binds to its cognate surface receptor CSFR1 (aliases C-FMS, CD115) on the membrane of osteoclasts (Sherr et al. 1988; Suda et al. 1999). Targeted inactivation of the gene encoding M-CSF in mouse models leads to an osteopetrotic phenotype because of complete absence of osteoclasts (Wiktor-Jedrzejczak et al. 1990; Marks et al. 1992). OPG expression in osteoblasts is controlled by multiple bone metabolic regulators, including vitamin D, prostaglandin E2, tumor necrosis factors (TNFs), and IL-1, and also by TGF-βs and BMPs (Hofbauer et al. 1998; Murakami et al. 1998; Takai et al. 1998; Thirunavukkarasu et al. 2001; Wan et al. 2001; Sato et al. 2009). TGF-β signaling has both positive and negative effects on osteoclastogenesis, which initially led to controversial reports on the role of TGF-β in bone homeostasis. It is now understood that TGF-β acts as a chemoattractant and recruits both preosteoblast mesenchymal stromal cells and preosteoclasts to areas of bone remodeling (Pfeilschifter et al. 1990; Pilkington et al. 2001; Tang et al. 2009). TGF-β can promote bone resorption by directly regulating proliferation, differentiation, and survival of osteoclasts (Tashjian et al. 1985; Dieudonne et al. 1991; Kaneda et al. 2000) or indirectly by regulating the expression and secretion of pro-osteoclastic proteins in osteoblasts (Quinn et al. 2001; Mohammad et al. 2009; Tang and Alliston 2013). The TGF-β effects on osteoblasts are dose-dependent, with low doses increasing secretion of RANKL and suppressing expression of OPG to promote osteoclastogenesis, and high doses suppressing RANKL and increasing OPG to inhibit osteoclastogenesis (Karst et al. 2004). This mechanism functions to limit bone resorption when vast amounts of latent TGF-β are released from the bone matrix during high osteoclast activity. Excessive bone resorption is also prevented by TGF-β’s action as chemoattractant for preosteoblast mesenchymal stromal cells, thus coupling bone resorption with new bone formation.
Figure 5.
Roles of transforming growth factor β (TGF-β) and bone morphogenetic protein (BMP) signaling in bone homeostasis. Bone homeostasis is maintained through continuous cycles of bone resorption by osteoclasts and new bone formation by osteoblasts and osteocytes. Both TGF-β and BMP signaling participate in regulating this process. TGF-β can act as a chemoattractant and recruit both preosteoblast and preosteoclasts to areas of bone remodeling and also promotes proliferation, differentiation, and survival of osteoclasts. At low doses, TGF-β increases secretion of RANKL (receptor activator of NF-κB ligand) and suppresses expression of the inhibitor OPG (osteoprotegerin), thus promoting osteoclastogenesis by activating RANK signaling in preosteoclasts. At high doses, it suppresses RANKL and increases OPG expression and thus inhibits osteoclastogenesis. This biphasic effect limits excessive bone degradation in the presence of high levels of active TGF-β derived from conversion of latent TGF-β by proteases and acid secreted by osteoclasts during bone degradation. BMP signaling suppresses osteoclast differentiation by increasing expression of OPG in osteoblasts. It can, however, also promote bone resorption by increasing the expression of sclerostin (SOST), an inhibitor of Wnt signaling which is a pathway that normally promotes osteoblastogenesis and bone formation. M-CSF, Macrophage-colony stimulating factor.
BMP signaling is well characterized for its bone-protective functions both in vitro and in vivo. This effect is in part mediated by the BMP-dependent increase of OPG expression in osteoblasts, and consequent suppression of osteoclast differentiation (Kaneko et al. 2000; Wan et al. 2001; Jensen et al. 2010; Kamiya and Mishina 2011). However, BMP signaling also regulates bone resorption, as is apparent by the high bone mass phenotype when Bmpr1a expression is specifically inactivated in osteoblasts, which results in failed osteoclastogenesis (Kamiya et al. 2008). This effect appears to be mediated by BMP-dependent effects on the Wnt signaling pathway. Wnt signaling controls commitment of mesenchymal progenitors to the osteoblastic lineage and stimulates the expression of the osteoclast inhibitor OPG (Glass et al. 2005; Hill et al. 2005; Kennell and MacDougald 2005; Rodda and McMahon 2006; Kamiya and Mishina 2011; Baron and Kneissel 2013). Wnt activating gene mutations cause high bone mass, whereas inactivating mutations cause osteopenia characterized by severely reduced bone mineral density (Gong et al. 2001; Boyden et al. 2002; Little et al. 2002; Patel and Karsenty 2002; Babij et al. 2003; Winkler et al. 2003). The high-bone-mass phenotype in mice with osteoblast-specific Bmpr1a silencing was found to be triggered by impaired expression of sclerostin (SOST) (Kamiya et al. 2008), an antagonist of Wnt signaling that acts by sequestering the co-receptor low-density lipoprotein receptor-related protein 5 (LRP5) into inactive complexes (van Bezooijen et al. 2007; Kamiya et al. 2010; Krause et al. 2010), as also discussed below in the context of sclerosteosis and Van Buchem disease. In addition, treatment of osteoclastic precursors with BMP induces differentiation along the osteoclast cell lineage, and further stimulates expression of key enzymes for bone degradation (Kaneko et al. 2000; Jensen et al. 2010). The observation that BMP can regulate both bone formation and bone resorption is consistent with the notion that, with the exception of fibrodysplasia ossificans progressiva (FOP), mutations in BMP signaling components do not generally cause anabolic bone diseases.
Heterotopic Ossification Caused by Dysregulation of BMP Signaling
Postnatal bone formation is normally restricted to the skeleton during fracture healing; however, heterotopic ossification (HO) can occur at extraskeletal sites, frequently in response to severe tissue trauma (Kaplan et al. 2004; McCarthy and Sundaram 2005; Evans et al. 2014). In a rare genetic form of HO, FOP, ectopic endochondral ossification forms episodically and progressively at extraskeletal sites within soft connective tissue, including skeletal muscle, fascia, tendon, and ligaments, resulting in severe disability (Kaplan et al. 2008a; Shore and Kaplan 2008, 2010). At birth, only minor skeletal abnormalities occur in FOP patients, with congenital big toe malformation as the most characteristic feature. Most cases of FOP are caused by a c.617G>A point mutation in the ACVR1 gene, which encodes the ACVR1/ALK-2 type I receptor and confers a moderate gain-of-function in this BMP type I receptor (Shafritz et al. 1996; de la Pena et al. 2005; Fiori et al. 2006; Shore et al. 2006; Billings et al. 2008; Fukuda et al. 2009; Kaplan et al. 2009; Shen et al. 2009; Chaikuad et al. 2012; Culbert et al. 2014). Signaling through the ALK-2 receptor occurs in chondrocytes and osteoblasts, and is required for early stages of chondrogenesis (Culbert et al. 2014). Because surgical removal of ectopic bone frequently leads to recurrent or even exacerbation of HO shortly after surgery in FOP patients, treatment has been limited to pain management that is associated with the bone formation process by counteracting the inflammatory reaction using high doses of glucocorticoids (Kaplan et al. 2008b; Pignolo et al. 2013). The discovery of the genetic cause of FOP is leading to the development of alternative therapeutic strategies to directly prevent HO formation (Kaplan et al. 2013). Palovarotene, a retinoic acid receptor γ (RARγ) agonist, has emerged as a potent candidate for treatment of HO. Pretreatment with palovarotene renders cells unresponsive to BMP-2 and decreases BMP pathway-specific R-Smad phosphorylation in vitro; it also successfully blocks HO in transgenic mice expressing a constitutively active form of the ALK-2 receptor not found in individuals with FOP (Shimono et al. 2011) and in a knockin mouse with the FOP ALK2 c.617G>A (R206H) mutation (Chakkalakal et al. 2016). The efficacy of palovarotene is currently tested in a phase II clinical trial (ClinicalTrials.gov, NCT02190747). Moreover, a monoclonal antibody against activin A was reported to impair HO in a mouse model and in iPS cells with the FOP mutation (Hatsell et al. 2015; Hino et al. 2015); although the cellular mechanism for this mode of action has not been determined, the data suggest that activin A may be a therapeutic target for FOP.
In progressive osseous heteroplasia (POH), a second genetic form of HO, ectopic bone forms by intramembraneous ossification and is caused by inactivating mutations in the guanine nucleotide binding protein alpha stimulating (GNAS) gene (Kaplan and Shore 2000; Shore et al. 2002; Shore and Kaplan 2010). Although a detailed mechanism for HO formation in POH remains to be elucidated, data suggest that the mutations lead to overactivation of the hedgehog and BMP pathways and failure to inhibit osteogenic differentiation (Bowler et al. 1998; Wang and Wong 2009; Zhang et al. 2012; Regard et al. 2013). Currently, there is no effective treatment for POH other than surgical intervention (Pignolo et al. 2015).
Gain of Bone Mass in Camurati–Engelmann Disease
Sclerosing bone dysplasias are human syndromes characterized by abnormal accumulation of bone within the skeleton. There is a wide range of clinical manifestations, severity of bone accumulation, and genetic causes. In Camurati–Engelmann disease (CED), one form of sclerosing bone dysplasia, progressive diaphyseal cortical thickening occurs in long bones (Janssens et al. 2006), with first manifestations usually occurring in femur and tibia, and then expanding progressively toward distal areas of the limb, accompanied by chronic pain (Ribbing 1949; Janssens et al. 2006). Calvarial and pelvic bone thickening are also common (Janssens et al. 2006). CED is caused by TGFB1 missense mutations in the LAP region or signal peptide that destabilize the disulfide binding needed for homodimerization of latent TGF-β1, resulting in increased TGF-β signaling through either premature activation or intracellular retention of the protein and intracrine signal transmission (Saito et al. 2001; Janssens et al. 2003, 2006). Clinical manifestations do not include fibrosis or aneurysm, and no evidence for increased TGF-β signaling has been described in the aortic wall or other tissues, suggesting that these mutations have tissue-specific effects on TGF-β activity. It appears that patients with CED have increased rates of bone resorption as well as bone formation, with the overall balance shifted toward bone formation, possibly because of increased proliferation of osteoblasts (Hernandez et al. 1997; Saito et al. 2001; McGowan et al. 2003). CED can be successfully treated with glucocorticosteroids, which decrease proliferation, maturation and ECM synthesis in osteoblasts and osteocytes, and also increase the rate of apoptosis of these cells, thus resulting in a net decrease in bone mass (Low et al. 1985; Naveh et al. 1985).
High-Bone-Mass Disorders, Sclerosteosis and Van Buchem Disease, Caused by Mutations in Sclerostin (SOST)
Autosomal recessive loss-of-function mutations in the SOST gene, a direct target of BMP signaling, cause sclerosteosis (SOST1) (Fig. 2; Table 1) (Beighton 1988; Balemans et al. 2001; Brunkow et al. 2001). SOST1 is characterized by progressive skeletal overgrowth and increased bone mass and strength (Brunkow et al. 2001). Syndactyly and gigantism are also frequently found in patients with SOST1, and the appearance of a square-shaped jaw is a common facial feature (Beighton 1988). Heterozygous carriers are only mildly affected and display age-related thickening of the calvaria, mandible, ribs, clavicles, and long bones with increased bone mineral density in otherwise clinically inconspicuous individuals (Beighton et al. 1977; Stein et al. 1983; Gardner et al. 2005).
Deletion of a long-range regulatory element distal to the SOST gene results in decreased expression of sclerostin, the protein product of the SOST gene, and causes a form of sclerosing bone dysplasia known as Van Buchem disease (VBD) (Wergedal et al. 2003; Loots et al. 2005; van Lierop et al. 2013). Patients with VBD develop progressive increased thickness in calvaria, ribs, diaphysis of long bones, and tubular bones of hand and feet accompanied by hearing impairment and facial palsy (Van Buchem et al. 1962; Fryns and Van den Berghe 1988; Van Hul et al. 1998; Brunkow et al. 2001; Balemans et al. 2002; Staehling-Hampton et al. 2002; Wergedal et al. 2003; van Lierop et al. 2013).
The overall VBD phenotype is similar to SOST1, but less severe and without the appearance of hand malformation or gigantism. Deletion of an evolutionarily conserved region required for sclerostin expression in osteoblast-like cells and skeletal anlagen is able to recapitulate the VBD phenotype in mouse models (Loots et al. 2005). Sclerostin-deficient mice or mice expressing a dominant missense mutation in the LRP5 gene (encoding a Wnt receptor component) that leads to reduced sclerostin binding show a high-bone-mass phenotype with increased bone strength, thereby recapitulating the key features of SOST1 and VBD (Semenov and He 2006; Li et al. 2008). Conversely, sclerostin overexpression in mice shows a reciprocal phenotype—that is, low bone mass and decreased bone strength—emphasizing the negative regulatory function of sclerostin on bone formation (Winkler et al. 2003).
Analysis of serum from patients with SOST1 and VBD revealed an inverse relationship between levels of the bone formation marker procollagen I amino-terminal propeptide (PINP) and sclerostin. PINP is the larger precursor of collagen type I that, when cleaved to its mature form, constitutes ∼90% of all collagens in bone (Parfitt et al. 1987; Prockop and Kivirikko 1995; Young 2003). Serum from VBD patients contains a low level of sclerostin coupled with a significant increase in PINP, whereas heterozygous carriers display sclerostin and PINP levels intermediate between those in unaffected controls and homozygous patients (van Lierop et al. 2014). Sclerostin protein levels are undetectable in SOST1 patients, explaining the more severe phenotype in this disease in comparison to VBD (van Lierop et al. 2011).
Knowledge gained from both genetic diseases, SOST1 and VBD, has led to the development of novel treatment strategies against osteoporosis by using humanized antibodies against sclerostin to counteract the osteocatabolic effect of sclerostin (Kim et al. 2013; Clarke 2014). Romosozumab is currently in phase II clinical trial, whereas a phase I trial of blosozumab is completed. Current treatment of SOST1 and VBD, however, is limited to either surgical removal of excess bone, which harbors a high risk (du Plessis 1993; Tholpady et al. 2014) or treatment with the glucocorticoid prednisone, which can successfully counteract bone accumulation as well as bone resorption in a VBD patient (van Lierop et al. 2010).
Dysregulation of TGF-β or BMP Signaling in Catabolic Bone Diseases
Signaling through TGF-β and BMP pathways has been implicated in catabolic bone diseases, such as osteoarthritis and osteoporosis, that are common in the population but with limited therapeutic options. These conditions, unlike the genetic disorders already described in this review, appear to have a complex genetic basis with contributions from multiple genes and interacting pathways.
Arthritis is a chronic, destructive disease affecting articular cartilage and subchondral bone leading to loss of joint function and pain. Osteoarthritis (OA) is the most common form with increased prevalence in the aged population and characterized by progressive degeneration of articular cartilage, aberrant chondrocyte maturation, and osteophyte formation in subchondral bone, resulting in remodeling and functional loss of the joint (Zhang and Jordan 2010). Proinflammatory cytokines (TNF-α, IL-1) and matrix metalloproteinases (MMPs) play central roles in OA (Lories and Luyten 2005; Wojdasiewicz et al. 2014). Elevated BMP-2 expression was found in articular chondrocytes of OA mouse models (Blaney Davidson et al. 2006). In cartilage, BMP-2 has both anabolic and catabolic effects by stimulating expression of ECM proteins, such as collagen type II, and matrix degrading enzymes, such as MMPs (Blaney Davidson et al. 2007; van der Kraan et al. 2010). Single-nucleotide polymorphisms (SNPs) in BMP2 were found in OA patients but their functional relevance is unknown (Valdes et al. 2004, 2006). The expression levels of BMP7 decline with increasing age and with progression of OA (Chubinskaya et al. 2002), and the expression domains of GDF5 and GDF6 expand in adult human OA articular cartilage (Erlacher et al. 1998). BMP-7 possesses cartilage-protective properties by enhancing the expression of cartilage ECM and counteracting catabolic enzyme synthesis (Huch et al. 1997; Im et al. 2003). Clinical application of BMP-7 as a treatment for OA is promising with greater symptomatic improvement in comparison to placebo treated group (Hunter et al. 2010; Matthews and Hunter 2011).
The role of TGF-β signaling in human OA pathology is not well understood, but increasing evidence supports its function in maintaining articular cartilage. Age-related loss of TβRI expression, which may shift signal transduction from TβRI and Smad2 and Smad3 to ALK-1 and Smad1, Smad5 and Smad8, was found in two independent OA mouse models (van der Kraan et al. 2012). This resulted in the formation of transcriptionally active Runx2–Smad1 or Runx2–Smad5 complexes, which in turn activated terminal chondrocyte maturation and MMP13 expression (Blaney Davidson et al. 2009). MMP13 is the main collagenase that cleaves type II collagen in articular cartilage leading to OA (Reboul et al. 1996; Billinghurst et al. 1997). Expression of a truncated form of TβRII (Serra et al. 1997) or silencing Smad3 expression (Yang et al. 1999) also results in an OA phenotype in mice, further showing the importance of TGF-β signaling in the maintenance of joint homeostasis. OA is observed in all characterized etiologies of LDS (mutations in TGFBR1, TGFBR2, SMAD3, and TGFB2), but appears to be particularly frequent in patients with SMAD3 mutations (van de Laar et al. 2011). Although all of these alterations lead to impaired TGF-β signaling in vitro, all also associate with paradoxically increased signaling in vivo, precluding a definitive conclusion regarding the precise role of TGF-β in this process.
The TGF-β family member GDF-8, also known as myostatin, was reported to be highly expressed in human rheumatoid arthritis (RA) synovial tissues (Dankbar et al. 2015). Myostatin was found to increase RANKL-mediated osteoclast differentiation through Smad2 activation and regulation of nuclear factor of activated T cells (NFAT) c1. The absence of myostatin in genetically null (Mstn−/−) mice or depletion of myostatin by myostatin-specific neutralizing antibodies decreases osteoclast formation and bone destruction at joints in a RA mouse model (Dankbar et al. 2015), suggesting a potential new therapeutic target for RA.
Osteoporosis occurs when bone remodeling is unbalanced and bone loss exceeds bone formation, either by insufficient new bone formation or by excessive resorption, and results in increased fracture risk (Raisz 2005). The prevalence of this disease increases with age. Many candidate genes are associated with osteoporosis, including multiple TGFB1 polymorphisms that may reduce the secretion of TGF-β1 (Yamada et al. 1998; Langdahl et al. 2003). SNPs in TGFBR1 and TGFBR2, SMAD2, SMAD3, and SMAD4/DPC4 genes, as well as SMAD7 were correlated with osteoporosis, but their relevance needs to be confirmed (Watanabe et al. 2002). Polymorphisms in BMP2 and BMP4 are also linked to osteoporosis, low bone mineral density, and fragility fracture risk (Styrkarsdottir et al. 2003; Ramesh Babu et al. 2005), although a correlation between a SNP in BMP2 and osteoporosis remains to be confirmed (Medici et al. 2006). Osteoporosis, which presents with a high risk of pathologic fractures and delayed bone healing, is also a feature of LDS, and appears to be particularly frequent and most severe in patients with heterozygous missense mutations in TGFBR1 or TGFBR2 (Kirmani et al. 2010; Tan et al. 2013). Given the dual roles of TGF-β signaling in bone formation and resorption, and the paradoxical effects of LDS mutations on TGF-β signaling in vivo, the mechanisms leading to osteoporosis in LDS remain unclear. That the osteoporotic skeletal phenotype in LDS patients is recapitulated in LDS mouse models with heterozygous missense mutations in TGFBR2 (Dewan et al. 2015) suggests that this mouse model will provide informative mechanistic insight into the bone pathology of LDS and osteoporosis.
Osteogenesis imperfecta (OI) is an inherited disorder characterized by brittle bones and fractures due to aberrant connective tissue matrix that is caused by mutations in collagen type I (dominant OI) or in posttranslational modifiers of collagen I (recessive OI) (Van Dijk and Sillence 2014). Increased TGF-β signaling was shown in mouse models for each form of OI, and the OI phenotype could be rescued by TGF-β inhibition, consistent with aberrant TGF-β activity causing the underlying OI bone pathology (Grafe et al. 2014). These results emphasize the sensitivity of the TGF-β-mediated balance between bone resorption and deposition; however, further investigations are needed to fully understand the roles of BMP and TGF-β in bone homeostasis and quality.
CONCLUDING REMARKS
Numerous human syndromes are caused by mutations in the TGF-β and BMP pathways, reflecting the fundamental functions of these signaling molecules and their pathways in development, patterning, and homeostasis in many tissues. Both pathways have synergistic and overlapping effects but also possess discrete functions that are revealed in pathway-specific human genetic diseases of connective tissues. Mutations affecting the functions of the BMP pathway are predominantly associated with skeletal tissue diseases, whereas those affecting TGF-β-specific components are more diverse in their manifestation, including cardiovascular pathologies, along with a range of connective tissue effects.
ACKNOWLEDGMENTS
This work is supported in part through the Howard Hughes Medical Institute, the Leducq Foundation, the Center for Research in FOP and Related Disorders at the University of Pennsylvania, the International Fibrodysplasia Ossificans Progressiva Association (IFOPA), the Progressive Osseous Heteroplasia Association (POHA), the Ian Cali Endowment, the Weldon Family Endowment, the Cali/Weldon Professorship (to E.M.S.), and by Grants from the National Institutes of Health (R01-AR41916, R01-AR046831, R01-AR41135-21, K99-HL121287). We thank the members of our laboratories for helpful discussions.
REFERENCES
*Reference is also in this collection.
- Ahn K, Mishina Y, Hanks MC, Behringer RR, Crenshaw EB III. 2001. BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal–ventral patterning of the limb. Development 128: 4449–4461. [DOI] [PubMed] [Google Scholar]
- Akiyama H. 2008. Control of chondrogenesis by the transcription factor Sox9. Mod Rheumatol 18: 213–219. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. 2002. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev 16: 2813–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Marques G, Wharton KA. 2012. A large bioactive BMP ligand with distinct signaling properties is produced by alternative proconvertase processing. Sci Signal 5: ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyoshi S, Inoue H, Hanai J, Kusanagi K, Nemoto N, Miyazono K, Kawabata M. 1999. c-Ski acts as a transcriptional co-repressor in transforming growth factor-β signaling through interaction with Smads. J Biol Chem 274: 35269–35277. [DOI] [PubMed] [Google Scholar]
- Alliston T, Choy L, Ducy P, Karsenty G, Derynck R. 2001. TGF-β-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J 20: 2254–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J, Serra R. 2004. Unique and redundant roles of Smad3 in TGF-β-mediated regulation of long bone development in organ culture. Dev Dyn 230: 685–699. [DOI] [PubMed] [Google Scholar]
- Alvarez J, Horton J, Sohn P, Serra R. 2001. The perichondrium plays an important role in mediating the effects of TGF-β1 on endochondral bone formation. Dev Dyn 221: 311–321. [DOI] [PubMed] [Google Scholar]
- Archer CW, Dowthwaite GP, Francis-West P. 2003. Development of synovial joints. Birth Defects Res C Embryo Today 69: 144–155. [DOI] [PubMed] [Google Scholar]
- Arteaga-Solis E, Gayraud B, Lee SY, Shum L, Sakai L, Ramirez F. 2001. Regulation of limb patterning by extracellular microfibrils. J Cell Biol 154: 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ, Bouxsein ML, Reddy PS, Bodine PV, Robinson JA, Bhat B, et al. 2003. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res 18: 960–974. [DOI] [PubMed] [Google Scholar]
- Baek JA, Lan Y, Liu H, Maltby KM, Mishina Y, Jiang R. 2011. Bmpr1a signaling plays critical roles in palatal shelf growth and palatal bone formation. Dev Biol 350: 520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baffi MO, Slattery E, Sohn P, Moses HL, Chytil A, Serra R. 2004. Conditional deletion of the TGF-β type II receptor in Col2a expressing cells results in defects in the axial skeleton without alterations in chondrocyte differentiation or embryonic development of long bones. Dev Biol 276: 124–142. [DOI] [PubMed] [Google Scholar]
- Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, et al. 2001. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 10: 537–543. [DOI] [PubMed] [Google Scholar]
- Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, et al. 2002. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet 39: 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballock RT, Heydemann A, Wakefield LM, Flanders KC, Roberts AB, Sporn MB. 1993. TGF-β1 prevents hypertrophy of epiphyseal chondrocytes: Regulation of gene expression for cartilage matrix proteins and metalloproteases. Dev Biol 158: 414–429. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ. 2006. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet 2: e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Dix TA. 1996. Redox-mediated activation of latent transforming growth factor-β1. Mol Endocrinol 10: 1077–1083. [DOI] [PubMed] [Google Scholar]
- Baron R, Kneissel M. 2013. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat Med 19: 179–192. [DOI] [PubMed] [Google Scholar]
- Bateman JF, Boot-Handford RP, Lamande SR. 2009. Genetic diseases of connective tissues: Cellular and extracellular effects of ECM mutations. Nat Rev Genet 10: 173–183. [DOI] [PubMed] [Google Scholar]
- Baur ST, Mai JJ, Dymecki SM. 2000. Combinatorial signaling through BMP receptor IB and GDF5: Shaping of the distal mouse limb and the genetics of distal limb diversity. Development 127: 605–619. [DOI] [PubMed] [Google Scholar]
- Beighton P. 1988. Sclerosteosis. J Med Genet 25: 200–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beighton P, Davidson J, Durr L, Hamersma H. 1977. Sclerosteosis—An autosomal recessive disorder. Clin Genet 11: 1–7. [DOI] [PubMed] [Google Scholar]
- Bell J. 1951. On brachydactyly and symphalangism. In Treasury of human inheritance (ed. Penrose LS), Vol. 5, pp. 1–31. Cambridge University Press, London. [Google Scholar]
- Benazet JD, Bischofberger M, Tiecke E, Goncalves A, Martin JF, Zuniga A, Naef F, Zeller R. 2009. A self-regulatory system of interlinked signaling feedback loops controls mouse limb patterning. Science 323: 1050–1053. [DOI] [PubMed] [Google Scholar]
- Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, Miyazono K. 2000. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol 221: 249–258. [DOI] [PubMed] [Google Scholar]
- Bertoli-Avella AM, Gillis E, Morisaki H, Verhagen JM, de Graaf BM, van de Beek G, Gallo E, Kruithof BP, Venselaar H, Myers LA, et al. 2015. Mutations in a TGF-β ligand, TGFB3, cause syndromic aortic aneurysms and dissections. J Am Coll Cardiol 65: 1324–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, et al. 1997. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest 99: 1534–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings PC, Fiori JL, Bentwood JL, O’Connell MP, Jiao X, Nussbaum B, Caron RJ, Shore EM, Kaplan FS. 2008. Dysregulated BMP signaling and enhanced osteogenic differentiation of connective tissue progenitor cells from patients with fibrodysplasia ossificans progressiva (FOP). J Bone Miner Res 23: 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaney Davidson EN, Vitters EL, van der Kraan PM, van den Berg WB. 2006. Expression of transforming growth factor-β (TGF-β) and the TGF-β signalling molecule SMAD-2P in spontaneous and instability-induced osteoarthritis: Role in cartilage degradation, chondrogenesis and osteophyte formation. Ann Rheum Dis 65: 1414–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaney Davidson EN, Vitters EL, van Lent PL, van de Loo FA, van den Berg WB, van der Kraan PM. 2007. Elevated extracellular matrix production and degradation upon bone morphogenetic protein-2 (BMP-2) stimulation point toward a role for BMP-2 in cartilage repair and remodeling. Arthritis Res Ther 9: R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaney Davidson EN, Remst DF, Vitters EL, van Beuningen HM, Blom AB, Goumans MJ, van den Berg WB, van der Kraan PM. 2009. Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J Immunol 182: 7937–7945. [DOI] [PubMed] [Google Scholar]
- Boffa JJ, Lu Y, Placier S, Stefanski A, Dussaule JC, Chatziantoniou C. 2003. Regression of renal vascular and glomerular fibrosis: Role of angiotensin II receptor antagonism and matrix metalloproteinases. J Am Soc Nephrol 14: 1132–1144. [DOI] [PubMed] [Google Scholar]
- Boileau C, Guo DC, Hanna N, Regalado ES, Detaint D, Gong L, Varret M, Prakash SK, Li AH, d’Indy H, et al. 2012. TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat Genet 44: 916–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnans C, Chou J, Werb Z. 2014. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15: 786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler WB, Gallagher JA, Bilbe G. 1998. G-protein coupled receptors in bone. Front Biosci 3: d769–780. [DOI] [PubMed] [Google Scholar]
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. 2002. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med 346: 1513–1521. [DOI] [PubMed] [Google Scholar]
- Brunet LJ, McMahon JA, McMahon AP, Harland RM. 1998. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science 280: 1455–1457. [DOI] [PubMed] [Google Scholar]
- Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, et al. 2001. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet 68: 577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush JO, Jiang R. 2012. Palatogenesis: Morphogenetic and molecular mechanisms of secondary palate development. Development 139: 231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell ID, Humphries MJ. 2011. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol 3: a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SE, Katwa LC. 1997. Angiotensin II stimulated expression of transforming growth factor-β1 in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol 29: 1947–1958. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Johnson RL. 1998. Endogenous and ectopic expression of noggin suggests a conserved mechanism for regulation of BMP function during limb and somite patterning. Dev Biol 197: 205–217. [DOI] [PubMed] [Google Scholar]
- Capulli M, Paone R, Rucci N. 2014. Osteoblast and osteocyte: Games without frontiers. Arch Biochem Biophys 561: 3–12. [DOI] [PubMed] [Google Scholar]
- Carmignac V, Thevenon J, Ades L, Callewaert B, Julia S, Thauvin-Robinet C, Gueneau L, Courcet JB, Lopez E, Holman K, et al. 2012. In-frame mutations in exon 1 of SKI cause dominant Shprintzen–Goldberg syndrome. Am J Hum Genet 91: 950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho RL, Itoh F, Goumans MJ, Lebrin F, Kato M, Takahashi S, Ema M, Itoh S, van Rooijen M, Bertolino P, et al. 2007. Compensatory signalling induced in the yolk sac vasculature by deletion of TGF-β receptors in mice. J Cell Sci 120: 4269–4277. [DOI] [PubMed] [Google Scholar]
- Chaikuad A, Alfano I, Kerr G, Sanvitale CE, Boergermann JH, Triffitt JT, von Delft F, Knapp S, Knaus P, Bullock AN. 2012. Structure of the bone morphogenetic protein receptor ALK2 and implications for fibrodysplasia ossificans progressiva. J Biol Chem 287: 36990–36998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakkalakal SA, Uchibe K, Convente MR, Zhang D, Economides AN, Kaplan FS, Pacifici M, Iwamoto M, Shore EM. 2016. Palovarotene inhibits heterotopic ossification and maintains limb mobility and growth in mice with the human ACVR1 R206H fibrodysplasia ossificans progressiva (FOP) mutation. J Bone Miner Res 31: 1666–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Deng C, Li YP. 2012. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci 8: 272–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Lu H, Rateri DL, Cassis LA, Daugherty A. 2013. Conundrum of angiotensin II and TGF-β interactions in aortic aneurysms. Curr Opin Pharmacol 13: 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimal-Monroy J, Abarca-Buis RF, Cuervo R, Diaz-Hernandez M, Bustamante M, Rios-Flores JA, Romero-Suarez S, Farrera-Hernandez A. 2011. Molecular control of cell differentiation and programmed cell death during digit development. IUBMB Life 63: 922–929. [DOI] [PubMed] [Google Scholar]
- Chong SL, Mitchell R, Moursi AM, Winnard P, Losken HW, Bradley J, Ozerdem OR, Azari K, Acarturk O, Opperman LA, et al. 2003. Rescue of coronal suture fusion using transforming growth factor-β3 (Tgf-β3) in rabbits with delayed-onset craniosynostosis. Anat Rec A Discov Mol Cell Evol Biol 274: 962–971. [DOI] [PubMed] [Google Scholar]
- Choudhary B, Ito Y, Makita T, Sasaki T, Chai Y, Sucov HM. 2006. Cardiovascular malformations with normal smooth muscle differentiation in neural crest-specific type II TGFβ receptor (Tgfbr2) mutant mice. Dev Biol 289: 420–429. [DOI] [PubMed] [Google Scholar]
- Choudhary B, Zhou J, Li P, Thomas S, Kaartinen V, Sucov HM. 2009. Absence of TGF-β signaling in embryonic vascular smooth muscle leads to reduced lysyl oxidase expression, impaired elastogenesis, and aneurysm. Genesis 47: 115–121. [DOI] [PubMed] [Google Scholar]
- Chubinskaya S, Kumar B, Merrihew C, Heretis K, Rueger DC, Kuettner KE. 2002. Age-related changes in cartilage endogenous osteogenic protein-1 (OP-1). Biochim Biophys Acta 1588: 126–134. [DOI] [PubMed] [Google Scholar]
- Clarke BL. 2014. Anti-sclerostin antibodies: Utility in treatment of osteoporosis. Maturitas 78: 199–204. [DOI] [PubMed] [Google Scholar]
- Cohen MM Jr. 2009. Perspectives on RUNX genes: An update. Am J Med Genet A 149A: 2629–2646. [DOI] [PubMed] [Google Scholar]
- Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, ap Rhys CM, Holm TM, Loeys BL, et al. 2007. Angiotensin II type 1 receptor blockade attenuates TGF-β-induced failure of muscle regeneration in multiple myopathic states. Nat Med 13: 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JR, Clayton NP, Carta L, Galatioto J, Chiu E, Smaldone S, Nelson CA, Cheng SH, Wentworth BM, Ramirez F. 2015. Dimorphic effects of transforming growth factor-β signaling during aortic aneurysm progression in mice suggest a combinatorial therapy for Marfan syndrome. Arterioscler Thromb Vasc Biol 35: 911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa T, Ramsby G, Cassia F, Peters KR, Soares J, Correa J, Quelce-Salgado A, Tsipouras P. 1998. Grebe syndrome: Clinical and radiographic findings in affected individuals and heterozygous carriers. Am J Med Genet 75: 523–529. [DOI] [PubMed] [Google Scholar]
- Cui XM, Shuler CF. 2000. The TGF-β type III receptor is localized to the medial edge epithelium during palatal fusion. Int J Dev Biol 44: 397–402. [PubMed] [Google Scholar]
- Cui XM, Warburton D, Zhao J, Crowe DL, Shuler CF. 1998. Immunohistochemical localization of TGF-β type II receptor and TGF-β3 during palatogenesis in vivo and in vitro. Int J Dev Biol 42: 817–820. [PubMed] [Google Scholar]
- Cui XM, Shiomi N, Chen J, Saito T, Yamamoto T, Ito Y, Bringas P, Chai Y, Shuler CF. 2005. Overexpression of Smad2 in Tgf-β3-null mutant mice rescues cleft palate. Dev Biol 278: 193–202. [DOI] [PubMed] [Google Scholar]
- Culbert AL, Chakkalakal SA, Theosmy EG, Brennan TA, Kaplan FS, Shore EM. 2014. Alk2 regulates early chondrogenic fate in fibrodysplasia ossificans progressiva heterotopic endochondral ossification. Stem Cells 32: 1289–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankbar B, Fennen M, Brunert D, Hayer S, Frank S, Wehmeyer C, Beckmann D, Paruzel P, Bertrand J, Redlich K, et al. 2015. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat Med 21: 1085–1090. [DOI] [PubMed] [Google Scholar]
- da-Silva EO, Filho SM, de Albuquerque SC. 1984. Multiple synostosis syndrome: Study of a large Brazilian kindred. Am J Med Genet 18: 237–247. [DOI] [PubMed] [Google Scholar]
- Dathe K, Kjaer KW, Brehm A, Meinecke P, Nurnberg P, Neto JC, Brunoni D, Tommerup N, Ott CE, Klopocki E, et al. 2009. Duplications involving a conserved regulatory element downstream of BMP2 are associated with brachydactyly type A2. Am J Hum Genet 84: 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EC. 1993. Stability of elastin in the developing mouse aorta: A quantitative radioautographic study. Histochemistry 100: 17–26. [DOI] [PubMed] [Google Scholar]
- Davis F, Rateri DL, Daugherty A. 2014. Aortic aneurysms in Loeys–Dietz syndrome—A tale of two pathways? J Clin Invest 124: 79–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker RS, Koyama E, Pacifici M. 2014. Genesis and morphogenesis of limb synovial joints and articular cartilage. Matrix Biol 39: 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenkolbe E, Konig J, Zimmer J, Walther M, Reissner C, Nickel J, Ploger F, Raspopovic J, Sharpe J, Dathe K, et al. 2013. A GDF5 point mutation strikes twice—Causing BDA1 and SYNS2. PLoS Genet 9: e1003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Pena LS, Billings PC, Fiori JL, Ahn J, Kaplan FS, Shore EM. 2005. Fibrodysplasia ossificans progressiva (FOP), a disorder of ectopic osteogenesis, misregulates cell surface expression and trafficking of BMPRIA. J Bone Miner Res 20: 1168–1176. [DOI] [PubMed] [Google Scholar]
- Derynck R, Akhurst RJ. 2007. Differentiation plasticity regulated by TGF-β family proteins in development and disease. Nat Cell Biol 9: 1000–1004. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. 2003. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425: 577–584. [DOI] [PubMed] [Google Scholar]
- Dewan AK, Tomlinson RE, Mitchell S, Goh BC, Yung RM, Kumar S, Tan EW, Faugere MC, Dietz HC III, Clemens TL, et al. 2015. Dysregulated TGF-β signaling alters bone microstructure in a mouse model of Loeys–Dietz syndrome. J Orthop Res 33: 1447–1454. [DOI] [PubMed] [Google Scholar]
- Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. 1995. Defective haematopoiesis and vasculogenesis in transforming growth factor-β1 knock out mice. Development 121: 1845–1854. [DOI] [PubMed] [Google Scholar]
- Dietz HC. 2010. TGF-β in the pathogenesis and prevention of disease: A matter of aneurysmic proportions. J Clin Invest 120: 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM, et al. 1991. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 352: 337–339. [DOI] [PubMed] [Google Scholar]
- Dieudonne SC, Foo P, van Zoelen EJ, Burger EH. 1991. Inhibiting and stimulating effects of TGF-β1 on osteoclastic bone resorption in fetal mouse bone organ cultures. J Bone Miner Res 6: 479–487. [DOI] [PubMed] [Google Scholar]
- Dong X, Hudson NE, Lu C, Springer TA. 2014. Structural determinants of integrin β-subunit specificity for latent TGF-β. Nat Struct Mol Biol 21: 1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle AJ, Doyle JJ, Bessling SL, Maragh S, Lindsay ME, Schepers D, Gillis E, Mortier G, Homfray T, Sauls K, et al. 2012. Mutations in the TGF-β repressor SKI cause Shprintzen-Goldberg syndrome with aortic aneurysm. Nat Genet 44: 1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudas M, Sridurongrit S, Nagy A, Okazaki K, Kaartinen V. 2004. Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mech Dev 121: 173–182. [DOI] [PubMed] [Google Scholar]
- Dudas M, Kim J, Li WY, Nagy A, Larsson J, Karlsson S, Chai Y, Kaartinen V. 2006. Epithelial and ectomesenchymal role of the type I TGF-β receptor ALK5 during facial morphogenesis and palatal fusion. Dev Biol 296: 298–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker N, Krieglstein K. 2002. Tgfb2−/− Tgfb3−/− double knockout mice display severe midline fusion defects and early embryonic lethality. Anat Embryol (Berl) 206: 73–83. [DOI] [PubMed] [Google Scholar]
- du Plessis JJ. 1993. Sclerosteosis: Neurosurgical experience with 14 cases. J Neurosurg 78: 388–392. [DOI] [PubMed] [Google Scholar]
- Eijken M, Swagemakers S, Koedam M, Steenbergen C, Derkx P, Uitterlinden AG, van der Spek PJ, Visser JA, de Jong FH, Pols HA, et al. 2007. The activin A–follistatin system: Potent regulator of human extracellular matrix mineralization. FASEB J 21: 2949–2960. [DOI] [PubMed] [Google Scholar]
- Ellingsworth LR, Brennan JE, Fok K, Rosen DM, Bentz H, Piez KA, Seyedin SM. 1986. Antibodies to the N-terminal portion of cartilage-inducing factor A and transforming growth factor β. Immunohistochemical localization and association with differentiating cells. J Biol Chem 261: 12362–12367. [PubMed] [Google Scholar]
- Erlacher L, Ng CK, Ullrich R, Krieger S, Luyten FP. 1998. Presence of cartilage-derived morphogenetic proteins in articular cartilage and enhancement of matrix replacement in vitro. Arthritis Rheum 41: 263–273. [DOI] [PubMed] [Google Scholar]
- Evans KN, Potter BK, Brown TS, Davis TA, Elster EA, Forsberg JA. 2014. Osteogenic gene expression correlates with development of heterotopic ossification in war wounds. Clin Orthop Relat Res 472: 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everman DB, Bartels CF, Yang Y, Yanamandra N, Goodman FR, Mendoza-Londono JR, Savarirayan R, White SM, Graham JM Jr, Gale RP, et al. 2002. The mutational spectrum of brachydactyly type C. Am J Med Genet 112: 291–296. [DOI] [PubMed] [Google Scholar]
- Faiyaz-Ul-Haque M, Ahmad W, Zaidi SH, Haque S, Teebi AS, Ahmad M, Cohn DH, Tsui LC. 2002. Mutation in the cartilage-derived morphogenetic protein-1 (CDMP1) gene in a kindred affected with fibular hypoplasia and complex brachydactyly (DuPan syndrome). Clin Genet 61: 454–458. [DOI] [PubMed] [Google Scholar]
- Fiori JL, Billings PC, de la Pena LS, Kaplan FS, Shore EM. 2006. Dysregulation of the BMP-p38 MAPK signaling pathway in cells from patients with fibrodysplasia ossificans progressiva (FOP). J Bone Miner Res 21: 902–909. [DOI] [PubMed] [Google Scholar]
- Fitch N. 1979. Classification and identification of inherited brachydactylies. J Med Genet 16: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis-West PH, Abdelfattah A, Chen P, Allen C, Parish J, Ladher R, Allen S, MacPherson S, Luyten FP, Archer CW. 1999. Mechanisms of GDF-5 action during skeletal development. Development 126: 1305–1315. [DOI] [PubMed] [Google Scholar]
- Frischmeyer-Guerrerio PA, Guerrerio AL, Oswald G, Chichester K, Myers L, Halushka MK, Oliva-Hemker M, Wood RA, Dietz HC. 2013. TGF-β receptor mutations impose a strong predisposition for human allergic disease. Sci Transl Med 5: 195ra194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryns JP, Van den Berghe H. 1988. Facial paralysis at the age of 2 months as a first clinical sign of van Buchem disease (endosteal hyperostosis). Eur J Pediatr 147: 99–100. [DOI] [PubMed] [Google Scholar]
- Fukuda D, Aikawa M. 2010. Intimal smooth muscle cells: The context-dependent origin. Circulation 122: 2005–2008. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Hu WY, Kubo A, Kishioka H, Satoh C, Soma M, Izumi Y, Kanmatsuse K. 2000. Angiotensin II upregulates transforming growth factor-β type I receptor on rat vascular smooth muscle cells. Am J Hypertens 13: 191–198. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Kohda M, Kanomata K, Nojima J, Nakamura A, Kamizono J, Noguchi Y, Iwakiri K, Kondo T, Kurose J, et al. 2009. Constitutively activated ALK2 and increased SMAD1/5 cooperatively induce bone morphogenetic protein signaling in fibrodysplasia ossificans progressiva. J Biol Chem 284: 7149–7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furumatsu T, Ozaki T, Asahara H. 2009. Smad3 activates the Sox9-dependent transcription on chromatin. Int J Biochem Cell Biol 41: 1198–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaal SA, Doyle JR, Larsen IJ. 1987. Symphalangism and its introduction into Hawaii: A pedigree. Hawaii Med J 46: 305–307. [PubMed] [Google Scholar]
- Galjaard RJ, van der Ham LI, Posch NA, Dijkstra PF, Oostra BA, Hovius SE, Timmenga EJ, Sonneveld GJ, Hoogeboom AJ, Heutink P. 2001. Differences in complexity of isolated brachydactyly type C cannot be attributed to locus heterogeneity alone. Am J Med Genet 98: 256–262. [DOI] [PubMed] [Google Scholar]
- Gallo EM, Loch DC, Habashi JP, Calderon JF, Chen Y, Bedja D, van Erp C, Gerber EE, Parker SJ, Sauls K, et al. 2014. Angiotensin II-dependent TGF-β signaling contributes to Loeys–Dietz syndrome vascular pathogenesis. J Clin Invest 124: 448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway JL, Tabin CJ. 2008. Classic limb patterning models and the work of Dennis Summerbell. Development 135: 2683–2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JC, van Bezooijen RL, Mervis B, Hamdy NA, Lowik CW, Hamersma H, Beighton P, Papapoulos SE. 2005. Bone mineral density in sclerosteosis; affected individuals and gene carriers. J Clin Endocrinol Metab 90: 6392–6395. [DOI] [PubMed] [Google Scholar]
- Gatherer D, Ten Dijke P, Baird DT, Akhurst RJ. 1990. Expression of TGF-β isoforms during first trimester human embryogenesis. Development 110: 445–460. [DOI] [PubMed] [Google Scholar]
- Gerber EE, Gallo EM, Fontana SC, Davis EC, Wigley FM, Huso DL, Dietz HC. 2013. Integrin-modulating therapy prevents fibrosis and autoimmunity in mouse models of scleroderma. Nature 503: 126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, DiMusto PD, Ehrlichman LK, Sadiq O, McEvoy B, Futchko JS, Henke PK, Eliason JL, Upchurch GR Jr. 2012. The role of extracellular signal-related kinase during abdominal aortic aneurysm formation. J Am Coll Surg 215: 668–680.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons GH, Pratt RE, Dzau VJ. 1992. Vascular smooth muscle cell hypertrophy vs. hyperplasia. Autocrine transforming growth factor-β1 expression determines growth response to angiotensin II. J Clin Invest 90: 456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass DA II, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, et al. 2005. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8: 751–764. [DOI] [PubMed] [Google Scholar]
- Gong Y, Krakow D, Marcelino J, Wilkin D, Chitayat D, Babul-Hirji R, Hudgins L, Cremers CW, Cremers FP, Brunner HG, et al. 1999. Heterozygous mutations in the gene encoding noggin affect human joint morphogenesis. Nat Genet 21: 302–304. [DOI] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, et al. 2001. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107: 513–523. [DOI] [PubMed] [Google Scholar]
- Gordon KJ, Blobe GC. 2008. Role of transforming growth factor-β superfamily signaling pathways in human disease. Biochim Biophys Acta 1782: 197–228. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Mummery C. 2000. Functional analysis of the TGF-β receptor/Smad pathway through gene ablation in mice. Int J Dev Biol 44: 253–265. [PubMed] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, Karlsson S, ten Dijke P. 2003. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGF-β/ALK5 signaling. Mol Cell 12: 817–828. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Liu Z, ten Dijke P. 2009. TGF-β signaling in vascular biology and dysfunction. Cell Res 19: 116–127. [DOI] [PubMed] [Google Scholar]
- Grafe I, Yang T, Alexander S, Homan EP, Lietman C, Jiang MM, Bertin T, Munivez E, Chen Y, Dawson B, et al. 2014. Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfecta. Nat Med 20: 670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenink M, den Hartog AW, Franken R, Radonic T, de Waard V, Timmermans J, Scholte AJ, van den Berg MP, Spijkerboer AM, Marquering HA, et al. 2013. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: A randomized controlled trial. Eur Heart J 34: 3491–3500. [DOI] [PubMed] [Google Scholar]
- Gu Z, Reynolds EM, Song J, Lei H, Feijen A, Yu L, He W, MacLaughlin DT, van den Eijnden-van Raaij J, Donahoe PK, et al. 1999. The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo. Development 126: 2551–2561. [DOI] [PubMed] [Google Scholar]
- Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, et al. 2006. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 312: 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habashi JP, Doyle JJ, Holm TM, Aziz H, Schoenhoff F, Bedja D, Chen Y, Modiri AN, Judge DP, Dietz HC. 2011. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through Erk antagonism. Science 332: 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi A, Kim S, Izumi Y, Zhan Y, Yamanaka S, Iwao H. 1999. Contribution of extracellular signal-regulated kinase to angiotensin II–induced transforming growth factor β1 expression in vascular smooth muscle cells. Hypertension 34: 126–131. [DOI] [PubMed] [Google Scholar]
- *.Hata A, Chen YG. 2016. TGF-β signaling from receptors to Smads. Cold Spring Harb Perspect Biol 8: a022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsell SJ, Idone V, Wolken DM, Huang L, Kim HJ, Wang L, Wen X, Nannuru KC, Jimenez J, Xie L, et al. 2015. ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci Transl Med 7: 303ra137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Xiong W, Wang Y, Matsui M, Yu X, Chai Y, Klingensmith J, Chen Y. 2010. Modulation of BMP signaling by Noggin is required for the maintenance of palatal epithelial integrity during palatogenesis. Dev Biol 347: 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht J, Seitz V, Urban M, Wagner F, Robinson PN, Stiege A, Dieterich C, Kornak U, Wilkening U, Brieske N, et al. 2007. Detection of novel skeletogenesis target genes by comprehensive analysis of a Runx2−/− mouse model. Gene Expr Patterns 7: 102–112. [DOI] [PubMed] [Google Scholar]
- *.Heldin C-H, Moustakas A. 2016. Signaling receptors for TGF-β family members. Cold Spring Harb Perspect Biol 8: a022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez MV, Peris P, Guanabens N, Alvarez L, Monegal A, Pons F, Ponce A, Munoz-Gomez J. 1997. Biochemical markers of bone turnover in Camurati-Engelmann disease: A report on four cases in one family. Calcif Tissue Int 61: 48–51. [DOI] [PubMed] [Google Scholar]
- Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. 2005. Canonical Wnt/β-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell 8: 727–738. [DOI] [PubMed] [Google Scholar]
- Hino K, Ikeya M, Horigome K, Matsumoto Y, Ebise H, Nishio M, Sekiguchi K, Shibata M, Nagata S, Matsuda S, et al. 2015. Neofunction of ACVR1 in fibrodysplasia ossificans progressiva. Proc Natl Acad Sci 112: 15438–15443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu K, Iwai T, Yoshikawa H, Tsumaki N. 2011. Expression of dominant negative TGF-β receptors inhibits cartilage formation in conditional transgenic mice. J Bone Miner Metab 29: 493–500. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. 1998. Osteoprotegerin production by human osteoblast lineage cells is stimulated by vitamin D, bone morphogenetic protein-2, and cytokines. Biochem Biophys Res Commun 250: 776–781. [DOI] [PubMed] [Google Scholar]
- Holder-Espinasse M, Escande F, Mayrargue E, Dieux-Coeslier A, Fron D, Doual-Bisser A, Boute-Benejean O, Robert Y, Porchet N, Manouvrier-Hanu S. 2004. Angel shaped phalangeal dysplasia, hip dysplasia, and positional teeth abnormalities are part of the brachydactyly C spectrum associated with CDMP-1 mutations. J Med Genet 41: e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoofnagle MH, Wamhoff BR, Owens GK. 2004. Lost in transdifferentiation. J Clin Invest 113: 1249–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horbelt D, Guo G, Robinson PN, Knaus P. 2010. Quantitative analysis of TGFBR2 mutations in Marfan-syndrome-related disorders suggests a correlation between phenotypic severity and Smad signaling activity. J Cell Sci 123: 4340–4350. [DOI] [PubMed] [Google Scholar]
- Horiki M, Imamura T, Okamoto M, Hayashi M, Murai J, Myoui A, Ochi T, Miyazono K, Yoshikawa H, Tsumaki N. 2004. Smad6/Smurf1 overexpression in cartilage delays chondrocyte hypertrophy and causes dwarfism with osteopenia. J Cell Biol 165: 433–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. 2004. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest 113: 1258–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch K, Wilbrink B, Flechtenmacher J, Koepp HE, Aydelotte MB, Sampath TK, Kuettner KE, Mollenhauer J, Thonar EJ. 1997. Effects of recombinant human osteogenic protein 1 on the production of proteoglycan, prostaglandin E2, and interleukin-1 receptor antagonist by human articular chondrocytes cultured in the presence of interleukin-1β. Arthritis Rheum 40: 2157–2161. [DOI] [PubMed] [Google Scholar]
- Humphrey JD, Schwartz MA, Tellides G, Milewicz DM. 2015. Role of mechanotransduction in vascular biology: Focus on thoracic aortic aneurysms and dissections. Circ Res 116: 1448–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DJ, Pike MC, Jonas BL, Kissin E, Krop J, McAlindon T. 2010. Phase 1 safety and tolerability study of BMP-7 in symptomatic knee osteoarthritis. BMC Musculoskelet Disord 11: 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. 2009. The extracellular matrix: Not just pretty fibrils. Science 326: 1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikushima H, Miyazono K. 2010. Cellular context-dependent “colors” of transforming growth factor-β signaling. Cancer Sci 101: 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im HJ, Pacione C, Chubinskaya S, Van Wijnen AJ, Sun Y, Loeser RF. 2003. Inhibitory effects of insulin-like growth factor-1 and osteogenic protein-1 on fibronectin fragment- and interleukin-1β-stimulated matrix metalloproteinase-13 expression in human chondrocytes. J Biol Chem 278: 25386–25394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Yeo JY, Chytil A, Han J, Bringas P Jr, Nakajima A, Shuler CF, Moses HL, Chai Y. 2003. Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects. Development 130: 5269–5280. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM. 2003. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development 130: 2779–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata H, Manabe I, Fujiu K, Yamamoto T, Takeda N, Eguchi K, Furuya A, Kuro-o M, Sata M, Nagai R. 2010. Bone marrow–derived cells contribute to vascular inflammation but do not differentiate into smooth muscle cell lineages. Circulation 122: 2048–2057. [DOI] [PubMed] [Google Scholar]
- Iwata J, Hacia JG, Suzuki A, Sanchez-Lara PA, Urata M, Chai Y. 2012. Modulation of noncanonical TGF-β signaling prevents cleft palate in Tgfbr2 mutant mice. J Clin Invest 122: 873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens K, ten Dijke P, Ralston SH, Bergmann C, Van Hul W. 2003. Transforming growth factor-β1 mutations in Camurati–Engelmann disease lead to increased signaling by altering either activation or secretion of the mutant protein. J Biol Chem 278: 7718–7724. [DOI] [PubMed] [Google Scholar]
- Janssens K, Vanhoenacker F, Bonduelle M, Verbruggen L, Van Maldergem L, Ralston S, Guanabens N, Migone N, Wientroub S, Divizia MT, et al. 2006. Camurati–Engelmann disease: Review of the clinical, radiological, and molecular data of 24 families and implications for diagnosis and treatment. J Med Genet 43: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed A, Bae JS, Afzal F, Gutierrez S, Pratap J, Zaidi SK, Lou Y, van Wijnen AJ, Stein JL, Stein GS, et al. 2008. Structural coupling of Smad and Runx2 for execution of the BMP2 osteogenic signal. J Biol Chem 283: 8412–8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed A, Afzal F, Bae JS, Gutierrez S, Zaidi K, Pratap J, van Wijnen AJ, Stein JL, Stein GS, Lian JB. 2009. Specific residues of RUNX2 are obligatory for formation of BMP2-induced RUNX2–SMAD complex to promote osteoblast differentiation. Cells Tissues Organs 189: 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen ED, Pham L, Billington CJ Jr, Espe K, Carlson AE, Westendorf JJ, Petryk A, Gopalakrishnan R, Mansky K. 2010. Bone morphogenic protein 2 directly enhances differentiation of murine osteoclast precursors. J Cell Biochem 109: 672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Ren Y, Zong Z, Liu C, Kamiya N, Mishina Y, Liu Y, Zhou X, Feng JQ. 2013. BMP receptor 1A determines the cell fate of the postnatal growth plate. Int J Biol Sci 9: 895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RL, Tabin CJ. 1997. Molecular models for vertebrate limb development. Cell 90: 979–990. [DOI] [PubMed] [Google Scholar]
- Jones A, Deb R, Torsney E, Howe F, Dunkley M, Gnaneswaran Y, Gaze D, Nasr H, Loftus IM, Thompson MM, et al. 2009. Rosiglitazone reduces the development and rupture of experimental aortic aneurysms. Circulation 119: 3125–3132. [DOI] [PubMed] [Google Scholar]
- Judge DP, Dietz HC. 2005. Marfan’s syndrome. Lancet 366: 1965–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. 1995. Abnormal lung development and cleft palate in mice lacking TGF-β3 indicates defects of epithelial–mesenchymal interaction. Nat Genet 11: 415–421. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Cui XM, Heisterkamp N, Groffen J, Shuler CF. 1997. Transforming growth factor-β3 regulates transdifferentiation of medial edge epithelium during palatal fusion and associated degradation of the basement membrane. Dev Dyn 209: 255–260. [DOI] [PubMed] [Google Scholar]
- Kagami S, Border WA, Miller DE, Noble NA. 1994. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-β expression in rat glomerular mesangial cells. J Clin Invest 93: 2431–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya N, Mishina Y. 2011. New insights on the roles of BMP signaling in bone—A review of recent mouse genetic studies. Biofactors 37: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya N, Ye L, Kobayashi T, Lucas DJ, Mochida Y, Yamauchi M, Kronenberg HM, Feng JQ, Mishina Y. 2008. Disruption of BMP signaling in osteoblasts through type IA receptor (BMPRIA) increases bone mass. J Bone Miner Res 23: 2007–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya N, Kobayashi T, Mochida Y, Yu PB, Yamauchi M, Kronenberg HM, Mishina Y. 2010. Wnt inhibitors Dkk1 and Sost are downstream targets of BMP signaling through the type IA receptor (BMPRIA) in osteoblasts. J Bone Miner Res 25: 200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda T, Nojima T, Nakagawa M, Ogasawara A, Kaneko H, Sato T, Mano H, Kumegawa M, Hakeda Y. 2000. Endogenous production of TGF-β is essential for osteoclastogenesis induced by a combination of receptor activator of NF-κB ligand and macrophage-colony-stimulating factor. J Immunol 165: 4254–4263. [DOI] [PubMed] [Google Scholar]
- Kaneko H, Arakawa T, Mano H, Kaneda T, Ogasawara A, Nakagawa M, Toyama Y, Yabe Y, Kumegawa M, Hakeda Y. 2000. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone 27: 479–486. [DOI] [PubMed] [Google Scholar]
- Kaplan FS, Shore EM. 2000. Progressive osseous heteroplasia. J Bone Miner Res 15: 2084–2094. [DOI] [PubMed] [Google Scholar]
- Kaplan FS, Glaser DL, Hebela N, Shore EM. 2004. Heterotopic ossification. J Am Acad Orthop Surg 12: 116–125. [DOI] [PubMed] [Google Scholar]
- Kaplan FS, Le Merrer M, Glaser DL, Pignolo RJ, Goldsby RE, Kitterman JA, Groppe J, Shore EM. 2008a. Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol 22: 191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan FS, Xu M, Glaser DL, Collins F, Connor M, Kitterman J, Sillence D, Zackai E, Ravitsky V, Zasloff M, et al. 2008b. Early diagnosis of fibrodysplasia ossificans progressiva. Pediatrics 121: e1295–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan FS, Xu M, Seemann P, Connor JM, Glaser DL, Carroll L, Delai P, Fastnacht-Urban E, Forman SJ, Gillessen-Kaesbach G, et al. 2009. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat 30: 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan FS, Pignolo RJ, Shore EM. 2013. From mysteries to medicines: Drug development for fibrodysplasia ossificans progressive. Expert Opin Orphan Drugs 1: 637–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst M, Gorny G, Galvin RJ, Oursler MJ. 2004. Roles of stromal cell RANKL, OPG, and M-CSF expression in biphasic TGF-β regulation of osteoclast differentiation. J Cell Physiol 200: 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y, Ishikawa T, Shimabara M, Tanda N, Enomoto-Iwamoto M, Iwamoto M, Kuwana T, Ueki A, Noji S, Nohno T. 1996. BMP signaling during bone pattern determination in the developing limb. Development 122: 3557–3566. [DOI] [PubMed] [Google Scholar]
- Kelleher CM, McLean SE, Mecham RP. 2004. Vascular extracellular matrix and aortic development. Curr Top Dev Biol 62: 153–188. [DOI] [PubMed] [Google Scholar]
- Keller B, Yang T, Chen Y, Munivez E, Bertin T, Zabel B, Lee B. 2011. Interaction of TGF-β and BMP signaling pathways during chondrogenesis. PLoS ONE 6: e16421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell JA, MacDougald OA. 2005. Wnt signaling inhibits adipogenesis through β-catenin-dependent and -independent mechanisms. J Biol Chem 280: 24004–24010. [DOI] [PubMed] [Google Scholar]
- Khokha MK, Hsu D, Brunet LJ, Dionne MS, Harland RM. 2003. Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nat Genet 34: 303–307. [DOI] [PubMed] [Google Scholar]
- Kim JH, Liu X, Wang J, Chen X, Zhang H, Kim SH, Cui J, Li R, Zhang W, Kong Y, et al. 2013. Wnt signaling in bone formation and its therapeutic potential for bone diseases. Ther Adv Musculoskelet Dis 5: 13–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirmani S, Tebben PJ, Lteif AN, Gordon D, Clarke BL, Hefferan TE, Yaszemski MJ, McGrann PS, Lindor NM, Ellison JW. 2010. Germline TGF-β receptor mutations and skeletal fragility: A report on two patients with Loeys–Dietz syndrome. Am J Med Genet A 152A: 1016–1019. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Lyons KM, McMahon AP, Kronenberg HM. 2005. BMP signaling stimulates cellular differentiation at multiple steps during cartilage development. Proc Natl Acad Sci 102: 18023–18027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y, Yu PB, Kamiya N, Pan H, Fukuda T, Scott GJ, Ray MK, Yamamura K, Mishina Y. 2013. Augmentation of Smad-dependent BMP signaling in neural crest cells causes craniosynostosis in mice. J Bone Miner Res 28: 1422–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, et al. 1997. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89: 755–764. [DOI] [PubMed] [Google Scholar]
- Koyama E, Ochiai T, Rountree RB, Kingsley DM, Enomoto-Iwamoto M, Iwamoto M, Pacifici M. 2007. Synovial joint formation during mouse limb skeletogenesis: Roles of Indian hedgehog signaling. Ann NY Acad Sci 1116: 100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause C, Korchynskyi O, de Rooij K, Weidauer SE, de Gorter DJ, van Bezooijen RL, Hatsell S, Economides AN, Mueller TD, Lowik CW, et al. 2010. Distinct modes of inhibition by sclerostin on bone morphogenetic protein and Wnt signaling pathways. J Biol Chem 285: 41614–41626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg HM. 2003. Developmental regulation of the growth plate. Nature 423: 332–336. [DOI] [PubMed] [Google Scholar]
- Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. 1993. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci 90: 770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacro RV, Dietz HC, Wruck LM, Bradley TJ, Colan SD, Devereux RB, Klein GL, Li JS, Minich LL, Paridon SM, et al. 2007. Rationale and design of a randomized clinical trial of β-blocker therapy (atenolol) versus angiotensin II receptor blocker therapy (losartan) in individuals with Marfan syndrome. Am Heart J 154: 624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacro RV, Dietz HC, Sleeper LA, Yetman AT, Bradley TJ, Colan SD, Pearson GD, Selamet Tierney ES, Levine JC, Atz AM, et al. 2014. Atenolol versus losartan in children and young adults with Marfan’s syndrome. N Engl J Med 371: 2061–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdahl BL, Carstens M, Stenkjaer L, Eriksen EF. 2003. Polymorphisms in the transforming growth factor β1 gene and osteoporosis. Bone 32: 297–310. [DOI] [PubMed] [Google Scholar]
- Langer LO, Garrett RT. 1980. Acromesomelic dysplasia. Radiology 137: 349–355. [DOI] [PubMed] [Google Scholar]
- Langer LO Jr, Cervenka J, Camargo M. 1989. A severe autosomal recessive acromesomelic dysplasia, the Hunter-Thompson type, and comparison with the Grebe type. Hum Genet 81: 323–328. [DOI] [PubMed] [Google Scholar]
- Langlois D, Hneino M, Bouazza L, Parlakian A, Sasaki T, Bricca G, Li JY. 2010. Conditional inactivation of TGF-β type II receptor in smooth muscle cells and epicardium causes lethal aortic and cardiac defects. Transgenic Res 19: 1069–1082. [DOI] [PubMed] [Google Scholar]
- Larsson J, Goumans MJ, Sjostrand LJ, van Rooijen MA, Ward D, Leveen P, Xu X, ten Dijke P, Mummery CL, Karlsson S. 2001. Abnormal angiogenesis but intact hematopoietic potential in TGF-β type I receptor-deficient mice. EMBO J 20: 1663–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie P, Robitaille G, Agharazii M, Ledbetter S, Lebel M, Lariviere R. 2005. Neutralization of transforming growth factor-β attenuates hypertension and prevents renal injury in uremic rats. J Hypertens 23: 1895–1903. [DOI] [PubMed] [Google Scholar]
- Leboy P, Grasso-Knight G, D’Angelo M, Volk SW, Lian JV, Drissi H, Stein GS, Adams SL. 2001. Smad–Runx interactions during chondrocyte maturation. J Bone Joint Surg Am 83-A: S15–S22. [PubMed] [Google Scholar]
- Lee AA, Dillmann WH, McCulloch AD, Villarreal FJ. 1995. Angiotensin II stimulates the autocrine production of transforming growth factor-β1 in adult rat cardiac fibroblasts. J Mol Cell Cardiol 27: 2347–2357. [DOI] [PubMed] [Google Scholar]
- Lehmann K, Seemann P, Stricker S, Sammar M, Meyer B, Suring K, Majewski F, Tinschert S, Grzeschik KH, Muller D, et al. 2003. Mutations in bone morphogenetic protein receptor 1B cause brachydactyly type A2. Proc Natl Acad Sci 100: 12277–12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann K, Seemann P, Boergermann J, Morin G, Reif S, Knaus P, Mundlos S. 2006. A novel R486Q mutation in BMPR1B resulting in either a brachydactyly type C/symphalangism-like phenotype or brachydactyly type A2. Eur J Hum Genet 14: 1248–1254. [DOI] [PubMed] [Google Scholar]
- Lehmann K, Seemann P, Silan F, Goecke TO, Irgang S, Kjaer KW, Kjaergaard S, Mahoney MJ, Morlot S, Reissner C, et al. 2007. A new subtype of brachydactyly type B caused by point mutations in the bone morphogenetic protein antagonist NOGGIN. Am J Hum Genet 81: 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CM, Fuld HM, Frenz DA, Downie SA, Massagué J, Newman SA. 1991. Role of transforming growth factor-β in chondrogenic pattern formation in the embryonic limb: Stimulation of mesenchymal condensation and fibronectin gene expression by exogenenous TGF-β and evidence for endogenous TGF-β-like activity. Dev Biol 145: 99–109. [DOI] [PubMed] [Google Scholar]
- Lesca G, Plauchu H, Coulet F, Lefebvre S, Plessis G, Odent S, Riviere S, Leheup B, Goizet C, Carette MF, et al. 2004. Molecular screening of ALK1/ACVRL1 and ENG genes in hereditary hemorrhagic telangiectasia in France. Hum Mutat 23: 289–299. [DOI] [PubMed] [Google Scholar]
- Leung DY, Glagov S, Mathews MB. 1976. Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science 191: 475–477. [DOI] [PubMed] [Google Scholar]
- Li MO, Flavell RA. 2006. TGF-β, T-cell tolerance and immunotherapy of autoimmune diseases and cancer. Expert Rev Clin Immunol 2: 257–265. [DOI] [PubMed] [Google Scholar]
- Li X, Ionescu AM, Schwarz EM, Zhang X, Drissi H, Puzas JE, Rosier RN, Zuscik MJ, O’Keefe RJ. 2003. Smad6 is induced by BMP-2 and modulates chondrocyte differentiation. J Orthop Res 21: 908–913. [DOI] [PubMed] [Google Scholar]
- Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D, Kurahara C, Gao Y, Cao J, Gong J, et al. 2008. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23: 860–869. [DOI] [PubMed] [Google Scholar]
- Li W, Li Q, Jiao Y, Qin L, Ali R, Zhou J, Ferruzzi J, Kim RW, Geirsson A, Dietz HC, et al. 2014. Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis. J Clin Invest 124: 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian JB, Javed A, Zaidi SK, Lengner C, Montecino M, van Wijnen AJ, Stein JL, Stein GS. 2004. Regulatory controls for osteoblast growth and differentiation: Role of Runx/Cbfa/AML factors. Crit Rev Eukaryot Gene Expr 14: 1–41. [PubMed] [Google Scholar]
- Lindsay ME, Dietz HC. 2011. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature 473: 308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay ME, Schepers D, Bolar NA, Doyle JJ, Gallo E, Fert-Bober J, Kempers MJ, Fishman EK, Chen Y, Myers L, et al. 2012. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat Genet 44: 922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, et al. 2002. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet 70: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Sun X, Braut A, Mishina Y, Behringer RR, Mina M, Martin JF. 2005. Distinct functions for Bmp signaling in lip and palate fusion in mice. Development 132: 1453–1461. [DOI] [PubMed] [Google Scholar]
- Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, et al. 2005. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 37: 275–281. [DOI] [PubMed] [Google Scholar]
- Loeys BL, Gerber EE, Riegert-Johnson D, Iqbal S, Whiteman P, McConnell V, Chillakuri CR, Macaya D, Coucke PJ, De Paepe A, et al. 2010. Mutations in fibrillin-1 cause congenital scleroderma: Stiff skin syndrome. Sci Transl Med 2: 23ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loots GG, Kneissel M, Keller H, Baptist M, Chang J, Collette NM, Ovcharenko D, Plajzer-Frick I, Rubin EM. 2005. Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res 15: 928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorda-Diez CI, Montero JA, Rodriguez-Leon J, Garcia-Porrero JA, Hurle JM. 2013. Expression and functional study of extracellular BMP antagonists during the morphogenesis of the digits and their associated connective tissues. PLoS ONE 8: e60423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lories RJ, Luyten FP. 2005. Bone morphogenetic protein signaling in joint homeostasis and disease. Cytokine Growth Factor Rev 16: 287–298. [DOI] [PubMed] [Google Scholar]
- Low LC, Stephenson JB, Stuart-Smith DA. 1985. Progressive diaphyseal dysplasia mimicking childhood myopathy: Clinical and biochemical response to prednisolone. Aust Paediatr J 21: 193–196. [DOI] [PubMed] [Google Scholar]
- Lyons RM, Keski-Oja J, Moses HL. 1988. Proteolytic activation of latent transforming growth factor-β from fibroblast-conditioned medium. J Cell Biol 106: 1659–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaulay IC, Tijssen MR, Thijssen-Timmer DC, Gusnanto A, Steward M, Burns P, Langford CF, Ellis PD, Dudbridge F, Zwaginga JJ, et al. 2007. Comparative gene expression profiling of in vitro differentiated megakaryocytes and erythroblasts identifies novel activatory and inhibitory platelet membrane proteins. Blood 109: 3260–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCarrick G, Black JH III, Bowdin S, El-Hamamsy I, Frischmeyer-Guerrerio PA, Guerrerio AL, Sponseller PD, Loeys B, Dietz HC III. 2014. Loeys–Dietz syndrome: A primer for diagnosis and management. Genet Med 16: 576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack CP. 2011. Signaling mechanisms that regulate smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol 31: 1495–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. 1998. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng 4: 415–428. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Daugherty A. 2015. AT1 receptor antagonism to reduce aortic expansion in Marfan syndrome: Lost in translation or in need of different interpretation? Arterioscler Thromb Vasc Biol 35: e10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangino M, Flex E, Digilio MC, Giannotti A, Dallapiccola B. 2002. Identification of a novel NOG gene mutation (P35S) in an Italian family with symphalangism. Hum Mutat 19: 308. [DOI] [PubMed] [Google Scholar]
- Marcelino J, Sciortino CM, Romero MF, Ulatowski LM, Ballock RT, Economides AN, Eimon PM, Harland RM, Warman ML. 2001. Human disease-causing NOG missense mutations: Effects on noggin secretion, dimer formation, and bone morphogenetic protein binding. Proc Natl Acad Sci 98: 11353–11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks SC Jr, Wojtowicz A, Szperl M, Urbanowska E, MacKay CA, Wiktor-Jedrzejczak W, Stanley ER, Aukerman SL. 1992. Administration of colony stimulating factor-1 corrects some macrophage, dental, and skeletal defects in an osteopetrotic mutation (toothless, tl) in the rat. Bone 13: 89–93. [DOI] [PubMed] [Google Scholar]
- Martin GR. 1998. The roles of FGFs in the early development of vertebrate limbs. Genes Dev 12: 1571–1586. [DOI] [PubMed] [Google Scholar]
- Massagué J. 2012. TGF-β signalling in context. Nat Rev Mol Cell Biol 13: 616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J, Gomis RR. 2006. The logic of TGF-β signaling. FEBS Lett 580: 2811–2820. [DOI] [PubMed] [Google Scholar]
- Matsunobu T, Torigoe K, Ishikawa M, de Vega S, Kulkarni AB, Iwamoto Y, Yamada Y. 2009. Critical roles of the TGF-β type I receptor ALK5 in perichondrial formation and function, cartilage integrity, and osteoblast differentiation during growth plate development. Dev Biol 332: 325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews GL, Hunter DJ. 2011. Emerging drugs for osteoarthritis. Expert Opin Emerg Drugs 16: 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J, et al. 1994. Endoglin, a TGF-β binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet 8: 345–351. [DOI] [PubMed] [Google Scholar]
- McCarthy EF, Sundaram M. 2005. Heterotopic ossification: A review. Skeletal Radiol 34: 609–619. [DOI] [PubMed] [Google Scholar]
- McGowan NW, MacPherson H, Janssens K, Van Hul W, Frith JC, Fraser WD, Ralston SH, Helfrich MH. 2003. A mutation affecting the latency-associated peptide of TGF-β1 in Camurati–Engelmann disease enhances osteoclast formation in vitro. J Clin Endocrinol Metab 88: 3321–3326. [DOI] [PubMed] [Google Scholar]
- Medici M, van Meurs JB, Rivadeneira F, Zhao H, Arp PP, Hofman A, Pols HA, Uitterlinden AG. 2006. BMP-2 gene polymorphisms and osteoporosis: The Rotterdam study. J Bone Miner Res 21: 845–854. [DOI] [PubMed] [Google Scholar]
- Merino R, Ganan Y, Macias D, Economides AN, Sampath KT, Hurle JM. 1998. Morphogenesis of digits in the avian limb is controlled by FGFs, TGF-βs, and noggin through BMP signaling. Dev Biol 200: 35–45. [DOI] [PubMed] [Google Scholar]
- Micha D, Guo DC, Hilhorst-Hofstee Y, van Kooten F, Atmaja D, Overwater E, Cayami FK, Regalado ES, van Uffelen R, Venselaar H, et al. 2015. SMAD2 mutations are associated with arterial aneurysms and dissections. Hum Mutat 36: 1145–1149. [DOI] [PubMed] [Google Scholar]
- Mikic B. 2004. Multiple effects of GDF-5 deficiency on skeletal tissues: Implications for therapeutic bioengineering. Ann Biomed Eng 32: 466–476. [DOI] [PubMed] [Google Scholar]
- Millan FA, Denhez F, Kondaiah P, Akhurst RJ. 1991. Embryonic gene expression patterns of TGF β1, β2 and β3 suggest different developmental functions in vivo. Development 111: 131–143. [DOI] [PubMed] [Google Scholar]
- Minina E, Schneider S, Rosowski M, Lauster R, Vortkamp A. 2005. Expression of FGF and TGF-β signaling related genes during embryonic endochondral ossification. Gene Expr Patterns 6: 102–109. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Suzuki A, Ueno N, Behringer RR. 1995. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev 9: 3027–3037. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Maeda S, Imamura T. 2004. Coordinate regulation of cell growth and differentiation by TGF-β superfamily and Runx proteins. Oncogene 23: 4232–4237. [DOI] [PubMed] [Google Scholar]
- Mizuguchi T, Collod-Beroud G, Akiyama T, Abifadel M, Harada N, Morisaki T, Allard D, Varret M, Claustres M, Morisaki H, et al. 2004. Heterozygous TGFBR2 mutations in Marfan syndrome. Nat Genet 36: 855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad KS, Chen CG, Balooch G, Stebbins E, McKenna CR, Davis H, Niewolna M, Peng XH, Nguyen DH, Ionova-Martin SS, et al. 2009. Pharmacologic inhibition of the TGF-β type I receptor kinase has anabolic and anti-catabolic effects on bone. PLoS ONE 4: e5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseeva EP. 2001. Adhesion receptors of vascular smooth muscle cells and their functions. Cardiovasc Res 52: 372–386. [DOI] [PubMed] [Google Scholar]
- Montero JA, Lorda-Diez CI, Ganan Y, Macias D, Hurle JM. 2008. Activin/TGF-β and BMP crosstalk determines digit chondrogenesis. Dev Biol 321: 343–356. [DOI] [PubMed] [Google Scholar]
- Morales TI. 1991. Transforming growth factor-β1 stimulates synthesis of proteoglycan aggregates in calf articular cartilage organ cultures. Arch Biochem Biophys 286: 99–106. [DOI] [PubMed] [Google Scholar]
- Mori-Akiyama Y, Akiyama H, Rowitch DH, de Crombrugghe B. 2003. Sox9 is required for determination of the chondrogenic cell lineage in the cranial neural crest. Proc Natl Acad Sci 100: 9360–9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses HL, Serra R. 1996. Regulation of differentiation by TGF-β. Curr Opin Genet Dev 6: 581–586. [DOI] [PubMed] [Google Scholar]
- Most D, Levine JP, Chang J, Sung J, McCarthy JG, Schendel SA, Longaker MT. 1998. Studies in cranial suture biology: up-regulation of transforming growth factor-β1 and basic fibroblast growth factor mRNA correlates with posterior frontal cranial suture fusion in the rat. Plast Reconstr Surg 101: 1431–1440. [DOI] [PubMed] [Google Scholar]
- Mouw JK, Ou G, Weaver VM. 2014. Extracellular matrix assembly: A multiscale deconstruction. Nat Rev Mol Cell Biol 15: 771–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. 2002. The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J Cell Biol 157: 493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller MB, Tuan RS. 2008. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum 58: 1377–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Sheppard D. 2011. Cross talk among TGF-β signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb Perspect Biol 3: a005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Harpel JG, Gleizes PE, Mazzieri R, Nunes I, Rifkin DB. 1997. Latent transforming growth factor-β: Structural features and mechanisms of activation. Kidney Int 51: 1376–1382. [DOI] [PubMed] [Google Scholar]
- Munger JS, Harpel JG, Giancotti FG, Rifkin DB. 1998. Interactions between growth factors and integrins: Latent forms of transforming growth factor-β are ligands for the integrin αvβ1. Mol Biol Cell 9: 2627–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Yamamoto M, Ono K, Nishikawa M, Nagata N, Motoyoshi K, Akatsu T. 1998. Transforming growth factor-β1 increases mRNA levels of osteoclastogenesis inhibitory factor in osteoblastic/stromal cells and inhibits the survival of murine osteoclast-like cells. Biochem Biophys Res Commun 252: 747–752. [DOI] [PubMed] [Google Scholar]
- Naito T, Masaki T, Nikolic-Paterson DJ, Tanji C, Yorioka N, Kohno N. 2004. Angiotensin II induces thrombospondin-1 production in human mesangial cells via 38 MAPK and JNK: A mechanism for activation of latent TGF-β1. Am J Physiol Renal Physiol 286: F278–287. [DOI] [PubMed] [Google Scholar]
- Naveh Y, Alon U, Kaftori JK, Berant M. 1985. Progressive diaphyseal dysplasia: Evaluation of corticosteroid therapy. Pediatrics 75: 321–323. [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. 2003. Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat Genet 33: 407–411. [DOI] [PubMed] [Google Scholar]
- Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, Gabrielson KL, Hausladen JM, Mecham RP, Judge DP, et al. 2004. TGF-β-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest 114: 1586–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel J, Kotzsch A, Sebald W, Mueller TD. 2005. A single residue of GDF-5 defines binding specificity to BMP receptor IB. J Mol Biol 349: 933–947. [DOI] [PubMed] [Google Scholar]
- Nie X, Luukko K, Kettunen P. 2006. BMP signalling in craniofacial development. Int J Dev Biol 50: 511–521. [DOI] [PubMed] [Google Scholar]
- Nishitoh H, Ichijo H, Kimura M, Matsumoto T, Makishima F, Yamaguchi A, Yamashita H, Enomoto S, Miyazono K. 1996. Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5. J Biol Chem 271: 21345–21352. [DOI] [PubMed] [Google Scholar]
- Nistala H, Lee-Arteaga S, Smaldone S, Siciliano G, Ramirez F. 2010. Extracellular microfibrils control osteoblast-supported osteoclastogenesis by restricting TGF-β stimulation of RANKL production. J Biol Chem 285: 34126–34133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Khan MM, Kaul SC, Dong HD, Wadhwa R, Colmenares C, Kohno I, Ishii S. 1999. Ski is a component of the histone deacetylase complex required for transcriptional repression by Mad and thyroid hormone receptor. Genes Dev 13: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan CJ, Williams B. 2000. Mechanical strain-induced extracellular matrix production by human vascular smooth muscle cells: Role of TGF-β1. Hypertension 36: 319–324. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Schmidt DK, Nathan RM, Armstrong RM, Miller KL, Sawamura SJ, Ziman JM, Erickson KL, de Leon ER, Rosen DM, et al. 1992. Bovine bone activin enhances bone morphogenetic protein-induced ectopic bone formation. J Biol Chem 267: 14233–14237. [PubMed] [Google Scholar]
- Opperman LA. 2000. Cranial sutures as intramembranous bone growth sites. Dev Dyn 219: 472–485. [DOI] [PubMed] [Google Scholar]
- Opperman LA, Nolen AA, Ogle RC. 1997. TGF-β1, TGF-β2, and TGF-β3 exhibit distinct patterns of expression during cranial suture formation and obliteration in vivo and in vitro. J Bone Miner Res 12: 301–310. [DOI] [PubMed] [Google Scholar]
- Opperman LA, Fernandez CR, So S, Rawlins JT. 2006. Erk1/2 signaling is required for Tgf-β2-induced suture closure. Dev Dyn 235: 1292–1299. [DOI] [PubMed] [Google Scholar]
- Oshima M, Oshima H, Taketo MM. 1996. TGF-β receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol 179: 297–302. [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, et al. 1997. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89: 765–771. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov DA, Selever J, Wang Y, Chen YT, Mishina Y, Martin JF, Behringer RR. 2006. BMP receptor type IA in limb bud mesenchyme regulates distal outgrowth and patterning. Dev Biol 295: 103–115. [DOI] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. 2004. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801. [DOI] [PubMed] [Google Scholar]
- Pacifici M, Koyama E, Iwamoto M. 2005. Mechanisms of synovial joint and articular cartilage formation: Recent advances, but many lingering mysteries. Birth Defects Res C Embryo Today 75: 237–248. [DOI] [PubMed] [Google Scholar]
- Pan Q, Yu Y, Chen Q, Li C, Wu H, Wan Y, Ma J, Sun F. 2008. Sox9, a key transcription factor of bone morphogenetic protein-2-induced chondrogenesis, is activated through BMP pathway and a CCAAT box in the proximal promoter. J Cell Physiol 217: 228–241. [DOI] [PubMed] [Google Scholar]
- Parada C, Chai Y. 2012. Roles of BMP signaling pathway in lip and palate development. Front Oral Biol 16: 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt AM, Simon LS, Villanueva AR, Krane SM. 1987. Procollagen type I carboxy-terminal extension peptide in serum as a marker of collagen biosynthesis in bone. Correlation with iliac bone formation rates and comparison with total alkaline phosphatase. J Bone Miner Res 2: 427–436. [DOI] [PubMed] [Google Scholar]
- Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, Bautch VL, Majesky MW. 2008. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci 105: 9349–9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MS, Karsenty G. 2002. Regulation of bone formation and vision by LRP5. N Engl J Med 346: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Pauklin S, Vallier L. 2015. Activin/Nodal signalling in stem cells. Development 142: 607–619. [DOI] [PubMed] [Google Scholar]
- Pedrozo HA, Schwartz Z, Gomez R, Ornoy A, Xin-Sheng W, Dallas SL, Bonewald LF, Dean DD, Boyan BD. 1998. Growth plate chondrocytes store latent transforming growth factor (TGF)-β1 in their matrix through latent TGF-β1 binding protein-1. J Cell Physiol 177: 343–354. [DOI] [PubMed] [Google Scholar]
- Pelton RW, Dickinson ME, Moses HL, Hogan BL. 1990. In situ hybridization analysis of TGFβ3 RNA expression during mouse development: Comparative studies with TGFβ1 and β2. Development 110: 609–620. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J, Wolf O, Naumann A, Minne HW, Mundy GR, Ziegler R. 1990. Chemotactic response of osteoblastlike cells to transforming growth factor β. J Bone Miner Res 5: 825–830. [DOI] [PubMed] [Google Scholar]
- Pignolo RJ, Shore EM, Kaplan FS. 2013. Fibrodysplasia ossificans progressiva: Diagnosis, management, and therapeutic horizons. Pediatr Endocrinol Rev 10: 437–448. [PMC free article] [PubMed] [Google Scholar]
- Pignolo RJ, Ramaswamy G, Fong JT, Shore EM, Kaplan FS. 2015. Progressive osseous heteroplasia: Diagnosis, treatment, and prognosis. Appl Clin Genet 8: 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkington MF, Sims SM, Dixon SJ. 2001. Transforming growth factor-β induces osteoclast ruffling and chemotaxis: Potential role in osteoclast recruitment. J Bone Miner Res 16: 1237–1247. [DOI] [PubMed] [Google Scholar]
- Pizette S, Niswander L. 2000. BMPs are required at two steps of limb chondrogenesis: Formation of prechondrogenic condensations and their differentiation into chondrocytes. Dev Biol 219: 237–249. [DOI] [PubMed] [Google Scholar]
- Podowski M, Calvi C, Metzger S, Misono K, Poonyagariyagorn H, Lopez-Mercado A, Ku T, Lauer T, McGrath-Morrow S, Berger A, et al. 2012. Angiotensin receptor blockade attenuates cigarette smoke-induced lung injury and rescues lung architecture in mice. J Clin Invest 122: 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue R, Lyons K. 2006. BMP signaling in the cartilage growth plate. Curr Top Dev Biol 76: 1–48. [DOI] [PubMed] [Google Scholar]
- Polinkovsky A, Robin NH, Thomas JT, Irons M, Lynn A, Goodman FR, Reardon W, Kant SG, Brunner HG, van der Burgt I, et al. 1997. Mutations in CDMP1 cause autosomal dominant brachydactyly type C. Nat Genet 17: 18–19. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Kivirikko KI. 1995. Collagens: Molecular biology, diseases, and potentials for therapy. Annu Rev Biochem 64: 403–434. [DOI] [PubMed] [Google Scholar]
- Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, Ding J, Ferguson MW, Doetschman T. 1995. Transforming growth factor-β3 is required for secondary palate fusion. Nat Genet 11: 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunier C, Pessah M, Ferrand N, Seo SR, Howe P, Atfi A. 2003. The oncoprotein Ski acts as an antagonist of transforming growth factor-β signaling by suppressing Smad2 phosphorylation. J Biol Chem 278: 26249–26257. [DOI] [PubMed] [Google Scholar]
- Quinn JM, Itoh K, Udagawa N, Hausler K, Yasuda H, Shima N, Mizuno A, Higashio K, Takahashi N, Suda T, et al. 2001. Transforming growth factor β affects osteoclast differentiation via direct and indirect actions. J Bone Miner Res 16: 1787–1794. [DOI] [PubMed] [Google Scholar]
- Raisz LG. 2005. Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. J Clin Invest 115: 3318–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh Babu L, Wilson SG, Dick IM, Islam FM, Devine A, Prince RL. 2005. Bone mass effects of a BMP4 gene polymorphism in postmenopausal women. Bone 36: 555–561. [DOI] [PubMed] [Google Scholar]
- Ramirez F, Sakai LY, Dietz HC, Rifkin DB. 2004. Fibrillin microfibrils: Multipurpose extracellular networks in organismal physiology. Physiol Genomics 19: 151–154. [DOI] [PubMed] [Google Scholar]
- Ramirez F, Sakai LY, Rifkin DB, Dietz HC. 2007. Extracellular microfibrils in development and disease. Cell Mol Life Sci 64: 2437–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboul P, Pelletier JP, Tardif G, Cloutier JM, Martel-Pelletier J. 1996. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes. A role in osteoarthritis. J Clin Invest 97: 2011–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regard JB, Malhotra D, Gvozdenovic-Jeremic J, Josey M, Chen M, Weinstein LS, Lu J, Shore EM, Kaplan FS, Yang Y. 2013. Activation of Hedgehog signaling by loss of GNAS causes heterotopic ossification. Nat Med 19: 1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retting KN, Song B, Yoon BS, Lyons KM. 2009. BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development 136: 1093–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbing S. 1949. Hereditary, multiple, diaphyseal sclerosis. Acta radiol 31: 522–536. [DOI] [PubMed] [Google Scholar]
- Rigueur D, Brugger S, Anbarchian T, Kim JK, Lee Y, Lyons KM. 2015. The type I BMP receptor ACVR1/ALK2 is required for chondrogenesis during development. J Bone Miner Res 30: 733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert B. 2007. Bone morphogenetic protein signaling in limb outgrowth and patterning. Dev Growth Differ 49: 455–468. [DOI] [PubMed] [Google Scholar]
- *.Robertson IB, Rifkin DB. 2016. Regulation of the bioavailability of TGF-β and TGF-β-related proteins. Cold Spring Harb Perspect Biol 8: a021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson IB, Horiguchi M, Zilberberg L, Dabovic B, Hadjiolova K, Rifkin DB. 2015. Latent TGF-β-binding proteins. Matrix Biol 47: 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin NH, Gunay-Aygun M, Polinkovsky A, Warman ML, Morrison S. 1997. Clinical and locus heterogeneity in brachydactyly type C. Am J Med Genet 68: 369–377. [DOI] [PubMed] [Google Scholar]
- Rodda SJ, McMahon AP. 2006. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 133: 3231–3244. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Vita J, Sanchez-Lopez E, Esteban V, Ruperez M, Egido J, Ruiz-Ortega M. 2005. Angiotensin II activates the Smad pathway in vascular smooth muscle cells by a transforming growth factor-β-independent mechanism. Circulation 111: 2509–2517. [DOI] [PubMed] [Google Scholar]
- Rountree RB, Schoor M, Chen H, Marks ME, Harley V, Mishina Y, Kingsley DM. 2004. BMP receptor signaling is required for postnatal maintenance of articular cartilage. PLoS Biol 2: e355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe DA, Cairns JM, Fallon JF. 1982. Spatial and temporal patterns of cell death in limb bud mesoderm after apical ectodermal ridge removal. Dev Biol 93: 83–91. [DOI] [PubMed] [Google Scholar]
- Saito T, Kinoshita A, Yoshiura K, Makita Y, Wakui K, Honke K, Niikawa N, Taniguchi N. 2001. Domain-specific mutations of a transforming growth factor (TGF)-β1 latency-associated peptide cause Camurati–Engelmann disease because of the formation of a constitutively active form of TGF-β1. J Biol Chem 276: 11469–11472. [DOI] [PubMed] [Google Scholar]
- Salazar VS, Gamer LW, Rosen V. 2016. BMP signalling in skeletal development, disease and repair. Nat Rev Endocrinol 12: 203–221. [DOI] [PubMed] [Google Scholar]
- Sandberg M, Vuorio T, Hirvonen H, Alitalo K, Vuorio E. 1988. Enhanced expression of TGF-β and c-fos mRNAs in the growth plates of developing human long bones. Development 102: 461–470. [DOI] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. 1997. TGF-β2 knockout mice have multiple developmental defects that are non-overlapping with other TGF-β knockout phenotypes. Development 124: 2659–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. 2001. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: From innocent bystander to active participant. Circ Res 89: 1111–1121. [DOI] [PubMed] [Google Scholar]
- Sato MM, Nakashima A, Nashimoto M, Yawaka Y, Tamura M. 2009. Bone morphogenetic protein-2 enhances Wnt/β-catenin signaling-induced osteoprotegerin expression. Genes Cells 14: 141–153. [DOI] [PubMed] [Google Scholar]
- Saunders JW Jr. 1948. The proximo-distal sequence of origin of the parts of the chick wing and the role of the ectoderm. J Exp Zool 108: 363–403. [DOI] [PubMed] [Google Scholar]
- Savarirayan R, White SM, Goodman FR, Graham JM Jr, Delatycki MB, Lachman RS, Rimoin DL, Everman DB, Warman ML. 2003. Broad phenotypic spectrum caused by an identical heterozygous CDMP-1 mutation in three unrelated families. Am J Med Genet A 117A: 136–142. [DOI] [PubMed] [Google Scholar]
- Scaffidi AK, Petrovic N, Moodley YP, Fogel-Petrovic M, Kroeger KM, Seeber RM, Eidne KA, Thompson PJ, Knight DA. 2004. αvβ3 Integrin interacts with the transforming growth factor β (TGF-β) type II receptor to potentiate the proliferative effects of TGF-β 1 in living human lung fibroblasts. J Biol Chem 279: 37726–37733. [DOI] [PubMed] [Google Scholar]
- Schmierer B, Hill CS. 2007. TGF-β–SMAD signal transduction: Molecular specificity and functional flexibility. Nat Rev Mol Cell Biol 8: 970–982. [DOI] [PubMed] [Google Scholar]
- Schroeder TM, Jensen ED, Westendorf JJ. 2005. Runx2: A master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res C Embryo Today 75: 213–225. [DOI] [PubMed] [Google Scholar]
- Schwabe GC, Turkmen S, Leschik G, Palanduz S, Stover B, Goecke TO, Mundlos S. 2004. Brachydactyly type C caused by a homozygous missense mutation in the prodomain of CDMP1. Am J Med Genet A 124A: 356–363. [DOI] [PubMed] [Google Scholar]
- Schwaerzer GK, Hiepen C, Schrewe H, Nickel J, Ploeger F, Sebald W, Mueller T, Knaus P. 2012. New insights into the molecular mechanism of multiple synostoses syndrome (SYNS): Mutation within the GDF5 knuckle epitope causes noggin-resistance. J Bone Miner Res 27: 429–442. [DOI] [PubMed] [Google Scholar]
- Seemann P, Schwappacher R, Kjaer KW, Krakow D, Lehmann K, Dawson K, Stricker S, Pohl J, Ploger F, Staub E, et al. 2005. Activating and deactivating mutations in the receptor interaction site of GDF5 cause symphalangism or brachydactyly type A2. J Clin Invest 115: 2373–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov MV, He X. 2006. LRP5 mutations linked to high bone mass diseases cause reduced LRP5 binding and inhibition by SOST. J Biol Chem 281: 38276–38284. [DOI] [PubMed] [Google Scholar]
- Sengle G, Charbonneau NL, Ono RN, Sasaki T, Alvarez J, Keene DR, Bachinger HP, Sakai LY. 2008. Targeting of bone morphogenetic protein growth factor complexes to fibrillin. J Biol Chem 283: 13874–13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HS, Serra R. 2007. Deletion of Tgfbr2 in Prx1-cre expressing mesenchyme results in defects in development of the long bones and joints. Dev Biol 310: 304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra R, Chang C. 2003. TGF-β signaling in human skeletal and patterning disorders. Birth Defects Res C Embryo Today 69: 333–351. [DOI] [PubMed] [Google Scholar]
- Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, Moses HL. 1997. Expression of a truncated, kinase-defective TGF-β type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol 139: 541–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra R, Karaplis A, Sohn P. 1999. Parathyroid hormone-related peptide (PTHrP)-dependent and -independent effects of transforming growth factor β (TGF-β) on endochondral bone formation. J Cell Biol 145: 783–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M, Pastor J, Domenzain C, Bassols A. 2002. Effect of transforming growth factor-β1, insulin-like growth factor-I, and hepatocyte growth factor on proteoglycan production and regulation in canine melanoma cell lines. Am J Vet Res 63: 1151–1158. [DOI] [PubMed] [Google Scholar]
- Shafritz AB, Shore EM, Gannon FH, Zasloff MA, Taub R, Muenke M, Kaplan FS. 1996. Overexpression of an osteogenic morphogen in fibrodysplasia ossificans progressiva. N Engl J Med 335: 555–561. [DOI] [PubMed] [Google Scholar]
- Shanahan CM, Weissberg PL. 1998. Smooth muscle cell heterogeneity: Patterns of gene expression in vascular smooth muscle cells in vitro and in vivo. Arterioscler Thromb Vasc Biol 18: 333–338. [DOI] [PubMed] [Google Scholar]
- Shen Q, Little SC, Xu M, Haupt J, Ast C, Katagiri T, Mundlos S, Seemann P, Kaplan FS, Mullins MC, et al. 2009. The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J Clin Invest 119: 3462–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ, Roussel MF, Rettenmier CW. 1988. Colony-stimulating factor-1 receptor (c-fms). J Cell Biochem 38: 179–187. [DOI] [PubMed] [Google Scholar]
- Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. 2011. Latent TGF-β structure and activation. Nature 474: 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihab FS, Bennett WM, Tanner AM, Andoh TF. 1997. Angiotensin II blockade decreases TGF-β1 and matrix proteins in cyclosporine nephropathy. Kidney Int 52: 660–673. [DOI] [PubMed] [Google Scholar]
- Shimono K, Tung WE, Macolino C, Chi AH, Didizian JH, Mundy C, Chandraratna RA, Mishina Y, Enomoto-Iwamoto M, Pacifici M, et al. 2011. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-γ agonists. Nat Med 17: 454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore EM, Kaplan FS. 2008. Insights from a rare genetic disorder of extra-skeletal bone formation, fibrodysplasia ossificans progressiva (FOP). Bone 43: 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore EM, Kaplan FS. 2010. Inherited human diseases of heterotopic bone formation. Nat Rev Rheumatol 6: 518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore EM, Ahn J, Jan de Beur S, Li M, Xu M, Gardner RJ, Zasloff MA, Whyte MP, Levine MA, Kaplan FS. 2002. Paternally inherited inactivating mutations of the GNAS1 gene in progressive osseous heteroplasia. N Engl J Med 346: 99–106. [DOI] [PubMed] [Google Scholar]
- Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, et al. 2006. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet 38: 525–527. [DOI] [PubMed] [Google Scholar]
- Sirard C, de la Pompa JL, Elia A, Itie A, Mirtsos C, Cheung A, Hahn S, Wakeham A, Schwartz L, Kern SE, et al. 1998. The tumor suppressor gene Smad4/Dpc4 is required for gastrulation and later for anterior development of the mouse embryo. Genes Dev 12: 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solloway MJ, Dudley AT, Bikoff EK, Lyons KM, Hogan BL, Robertson EJ. 1998. Mice lacking Bmp6 function. Dev Genet 22: 321–339. [DOI] [PubMed] [Google Scholar]
- Soltanoff CS, Yang S, Chen W, Li YP. 2009. Signaling networks that control the lineage commitment and differentiation of bone cells. Crit Rev Eukaryot Gene Expr 19: 1–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JJ, Aswad R, Kanaan RA, Rico MC, Owen TA, Barbe MF, Safadi FF, Popoff SN. 2007. Connective tissue growth factor (CTGF) acts as a downstream mediator of TGF-β1 to induce mesenchymal cell condensation. J Cell Physiol 210: 398–410. [DOI] [PubMed] [Google Scholar]
- Staehling-Hampton K, Proll S, Paeper BW, Zhao L, Charmley P, Brown A, Gardner JC, Galas D, Schatzman RC, Beighton P, et al. 2002. A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. Am J Med Genet 110: 144–152. [DOI] [PubMed] [Google Scholar]
- Stein SA, Witkop C, Hill S, Fallon MD, Viernstein L, Gucer G, McKeever P, Long D, Altman J, Miller NR, et al. 1983. Sclerosteosis: Neurogenetic and pathophysiologic analysis of an American kinship. Neurology 33: 267–277. [DOI] [PubMed] [Google Scholar]
- Stockis J, Colau D, Coulie PG, Lucas S. 2009. Membrane protein GARP is a receptor for latent TGF-β on the surface of activated human Treg. Eur J Immunol 39: 3315–3322. [DOI] [PubMed] [Google Scholar]
- Storm EE, Kingsley DM. 1996. Joint patterning defects caused by single and double mutations in members of the bone morphogenetic protein (BMP) family. Development 122: 3969–3979. [DOI] [PubMed] [Google Scholar]
- Storm EE, Kingsley DM. 1999. GDF5 coordinates bone and joint formation during digit development. Dev Biol 209: 11–27. [DOI] [PubMed] [Google Scholar]
- Storm EE, Huynh TV, Copeland NG, Jenkins NA, Kingsley DM, Lee SJ. 1994. Limb alterations in brachypodism mice due to mutations in a new member of the TGFβ-superfamily. Nature 368: 639–643. [DOI] [PubMed] [Google Scholar]
- Stouffer GA, Owens GK. 1992. Angiotensin II-induced mitogenesis of spontaneously hypertensive rat-derived cultured smooth muscle cells is dependent on autocrine production of transforming growth factor-β. Circ Res 70: 820–828. [DOI] [PubMed] [Google Scholar]
- Stricker S, Mundlos S. 2011. Mechanisms of digit formation: Human malformation syndromes tell the story. Dev Dyn 240: 990–1004. [DOI] [PubMed] [Google Scholar]
- Styrkarsdottir U, Cazier JB, Kong A, Rolfsson O, Larsen H, Bjarnadottir E, Johannsdottir VD, Sigurdardottir MS, Bagger Y, Christiansen C, et al. 2003. Linkage of osteoporosis to chromosome 20p12 and association to BMP2. PLoS Biol 1: E69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. 1999. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 20: 345–357. [DOI] [PubMed] [Google Scholar]
- Summerbell D, Lewis JH, Wolpert L. 1973. Positional information in chick limb morphogenesis. Nature 244: 492–496. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhang JQ, Zhang J, Ramires FJ. 1998. Angiotensin II, transforming growth factor-β1 and repair in the infarcted heart. J Mol Cell Cardiol 30: 1559–1569. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hasso SM, Fallon JF. 2008. Unique SMAD1/5/8 activity at the phalanx-forming region determines digit identity. Proc Natl Acad Sci 105: 4185–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Marazita ML, Cooper ME, Miwa N, Hing A, Jugessur A, Natsume N, Shimozato K, Ohbayashi N, Suzuki Y, et al. 2009. Mutations in BMP4 are associated with subepithelial, microform, and overt cleft lip. Am J Hum Genet 84: 406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiecki M, Colonna M. 2010. Accumulation of plasmacytoid DC: Roles in disease pathogenesis and targets for immunotherapy. Eur J Immunol 40: 2094–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczaluba K, Hilbert K, Obersztyn E, Zabel B, Mazurczak T, Kozlowski K. 2005. Du Pan syndrome phenotype caused by heterozygous pathogenic mutations in CDMP1 gene. Am J Med Genet A 138: 379–383. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Takahashi I, Komatsu M, Sawaishi Y, Higashi K, Nishimura G, Saito H, Takada G. 2001. Mutations of the NOG gene in individuals with proximal symphalangism and multiple synostosis syndrome. Clin Genet 60: 447–451. [DOI] [PubMed] [Google Scholar]
- Takai H, Kanematsu M, Yano K, Tsuda E, Higashio K, Ikeda K, Watanabe K, Yamada Y. 1998. Transforming growth factor-β stimulates the production of osteoprotegerin/osteoclastogenesis inhibitory factor by bone marrow stromal cells. J Biol Chem 273: 27091–27096. [DOI] [PubMed] [Google Scholar]
- Tan EW, Offoha RU, Oswald GL, Skolasky RL, Dewan AK, Zhen G, Shapiro JR, Dietz HC, Cao X, Sponseller PD. 2013. Increased fracture risk and low bone mineral density in patients with Loeys–Dietz syndrome. Am J Med Genet A 161A: 1910–1914. [DOI] [PubMed] [Google Scholar]
- Tang SY, Alliston T. 2013. Regulation of postnatal bone homeostasis by TGF-β. Bonekey Rep 2: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, Hu J, et al. 2009. TGF-β1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med 15: 757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian AH Jr, Voelkel EF, Lazzaro M, Singer FR, Roberts AB, Derynck R, Winkler ME, Levine L. 1985. α and β human transforming growth factors stimulate prostaglandin production and bone resorption in cultured mouse calvaria. Proc Natl Acad Sci 82: 4535–4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taya Y, O’Kane S, Ferguson MW. 1999. Pathogenesis of cleft palate in TGF-β3 knockout mice. Development 126: 3869–3879. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL, Ross FP. 2003. Genetic regulation of osteoclast development and function. Nat Rev Genet 4: 638–649. [DOI] [PubMed] [Google Scholar]
- Thirunavukkarasu K, Miles RR, Halladay DL, Yang X, Galvin RJ, Chandrasekhar S, Martin TJ, Onyia JE. 2001. Stimulation of osteoprotegerin (OPG) gene expression by transforming growth factor-β (TGF-β): Mapping of the OPG promoter region that mediates TGF-β effects. J Biol Chem 276: 36241–36250. [DOI] [PubMed] [Google Scholar]
- Tholpady S, Dodd ZH, Havlik RJ, Fulkerson DH. 2014. Cranial reconstruction for treatment of intracranial hypertension from sclerosteosis: Case-based update. World Neurosurg 81: 442.e1–442.e5. [DOI] [PubMed] [Google Scholar]
- Thomas JT, Lin K, Nandedkar M, Camargo M, Cervenka J, Luyten FP. 1996. A human chondrodysplasia due to a mutation in a TGF-β superfamily member. Nat Genet 12: 315–317. [DOI] [PubMed] [Google Scholar]
- Thomas JT, Kilpatrick MW, Lin K, Erlacher L, Lembessis P, Costa T, Tsipouras P, Luyten FP. 1997. Disruption of human limb morphogenesis by a dominant negative mutation in CDMP1. Nat Genet 17: 58–64. [DOI] [PubMed] [Google Scholar]
- Todorovic V, Rifkin DB. 2012. LTBPs, more than just an escort service. J Cell Biochem 113: 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran DQ, Andersson J, Wang R, Ramsey H, Unutmaz D, Shevach EM. 2009. GARP (LRRC32) is essential for the surface expression of latent TGF-β on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci 106: 13445–13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida K, Nakatani M, Hitachi K, Uezumi A, Sunada Y, Ageta H, Inokuchi K. 2009. Activin signaling as an emerging target for therapeutic interventions. Cell Commun Signal 7: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumaki N, Nakase T, Miyaji T, Kakiuchi M, Kimura T, Ochi T, Yoshikawa H. 2002. Bone morphogenetic protein signals are required for cartilage formation and differently regulate joint development during skeletogenesis. J Bone Miner Res 17: 898–906. [DOI] [PubMed] [Google Scholar]
- Tuli R, Tuli S, Nandi S, Huang X, Manner PA, Hozack WJ, Danielson KG, Hall DJ, Tuan RS. 2003. Transforming growth factor-β-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem 278: 41227–41236. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Hart DJ, Jones KA, Surdulescu G, Swarbrick P, Doyle DV, Schafer AJ, Spector TD. 2004. Association study of candidate genes for the prevalence and progression of knee osteoarthritis. Arthritis Rheum 50: 2497–2507. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Van Oene M, Hart DJ, Surdulescu GL, Loughlin J, Doherty M, Spector TD. 2006. Reproducible genetic associations between candidate genes and clinical knee osteoarthritis in men and women. Arthritis Rheum 54: 533–539. [DOI] [PubMed] [Google Scholar]
- van Bezooijen RL, Svensson JP, Eefting D, Visser A, van der Horst G, Karperien M, Quax PH, Vrieling H, Papapoulos SE, ten Dijke P, et al. 2007. Wnt but not BMP signaling is involved in the inhibitory action of sclerostin on BMP-stimulated bone formation. J Bone Miner Res 22: 19–28. [DOI] [PubMed] [Google Scholar]
- Van Buchem FS, Hadders HN, Hansen JF, Woldring MG. 1962. Hyperostosis corticalis generalisata. Report of seven cases. Am J Med 33: 387–397. [DOI] [PubMed] [Google Scholar]
- van de Laar IM, Oldenburg RA, Pals G, Roos-Hesselink JW, de Graaf BM, Verhagen JM, Hoedemaekers YM, Willemsen R, Severijnen LA, Venselaar H, et al. 2011. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet 43: 121–126. [DOI] [PubMed] [Google Scholar]
- van der Kraan PM, Blaney Davidson EN, Blom A, van den Berg WB. 2009. TGF-β signaling in chondrocyte terminal differentiation and osteoarthritis: Modulation and integration of signaling pathways through receptor-Smads. Osteoarthritis Cartilage 17: 1539–1545. [DOI] [PubMed] [Google Scholar]
- van der Kraan PM, Blaney Davidson EN, van den Berg WB. 2010. Bone morphogenetic proteins and articular cartilage: To serve and protect or a wolf in sheep’s clothing? Osteoarthritis Cartilage 18: 735–741. [DOI] [PubMed] [Google Scholar]
- van der Kraan PM, Goumans MJ, Blaney Davidson E, ten Dijke P. 2012. Age-dependent alteration of TGF-β signalling in osteoarthritis. Cell Tissue Res 347: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk FS, Sillence DO. 2014. Osteogenesis imperfecta: Clinical diagnosis, nomenclature and severity assessment. Am J Med Genet A 164A: 1470–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hul W, Balemans W, Van Hul E, Dikkers FG, Obee H, Stokroos RJ, Hildering P, Vanhoenacker F, Van Camp G, Willems PJ. 1998. Van Buchem disease (hyperostosis corticalis generalisata) maps to chromosome 17q12-q21. Am J Hum Genet 62: 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lierop AH, Hamdy NA, Papapoulos SE. 2010. Glucocorticoids are not always deleterious for bone. J Bone Miner Res 25: 2796–2800. [DOI] [PubMed] [Google Scholar]
- van Lierop AH, Hamdy NA, Hamersma H, van Bezooijen RL, Power J, Loveridge N, Papapoulos SE. 2011. Patients with sclerosteosis and disease carriers: Human models of the effect of sclerostin on bone turnover. J Bone Miner Res 26: 2804–2811. [DOI] [PubMed] [Google Scholar]
- van Lierop AH, Hamdy NA, van Egmond ME, Bakker E, Dikkers FG, Papapoulos SE. 2013. Van Buchem disease: Clinical, biochemical, and densitometric features of patients and disease carriers. J Bone Miner Res 28: 848–854. [DOI] [PubMed] [Google Scholar]
- van Lierop AH, Moester MJ, Hamdy NA, Papapoulos SE. 2014. Serum Dickkopf 1 levels in sclerostin deficiency. J Clin Endocrinol Metab 99: E252–256. [DOI] [PubMed] [Google Scholar]
- Wagenseil JE, Mecham RP. 2009. Vascular extracellular matrix and arterial mechanics. Physiol Rev 89: 957–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan M, Shi X, Feng X, Cao X. 2001. Transcriptional mechanisms of bone morphogenetic protein-induced osteoprotegrin gene expression. J Biol Chem 276: 10119–10125. [DOI] [PubMed] [Google Scholar]
- Wang K, Wong YH. 2009. G protein signaling controls the differentiation of multiple cell lineages. Biofactors 35: 232–238. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhu J, Dong X, Shi M, Lu C, Springer TA. 2012. GARP regulates the bioavailability and activation of TGF-β. Mol Biol Cell 23: 1129–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Rigueur D, Lyons KM. 2014. TGF-β signaling in cartilage development and maintenance. Birth Defects Res C Embryo Today 102: 37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SM, Greenwald JA, Spector JA, Bouletreau P, Mehrara BJ, Longaker MT. 2001. New developments in cranial suture research. Plast Reconstr Surg 107: 523–540. [DOI] [PubMed] [Google Scholar]
- Warren SM, Brunet LJ, Harland RM, Economides AN, Longaker MT. 2003. The BMP antagonist noggin regulates cranial suture fusion. Nature 422: 625–629. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Kinoshita A, Yamada T, Ohta T, Kishino T, Matsumoto N, Ishikawa M, Niikawa N, Yoshiura K. 2002. A catalog of 106 single-nucleotide polymorphisms (SNPs) and 11 other types of variations in genes for transforming growth factor-β1 (TGF-β1) and its signaling pathway. J Hum Genet 47: 478–483. [DOI] [PubMed] [Google Scholar]
- Wergedal JE, Veskovic K, Hellan M, Nyght C, Balemans W, Libanati C, Vanhoenacker FM, Tan J, Baylink DJ, Van Hul W. 2003. Patients with Van Buchem disease, an osteosclerotic genetic disease, have elevated bone formation markers, higher bone density, and greater derived polar moment of inertia than normal. J Clin Endocrinol Metab 88: 5778–5783. [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW Jr, Ahmed-Ansari A, Sell KW, Pollard JW, Stanley ER. 1990. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci 87: 4828–4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, et al. 2003. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22: 6267–6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. 1995. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev 9: 2105–2116. [DOI] [PubMed] [Google Scholar]
- Wojdasiewicz P, Poniatowski LA, Szukiewicz D. 2014. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm 2014: 561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G, Mueller E, Stahl RA, Ziyadeh FN. 1993. Angiotensin II–induced hypertrophy of cultured murine proximal tubular cells is mediated by endogenous transforming growth factor-β. J Clin Invest 92: 1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G, Ziyadeh FN, Stahl RA. 1999. Angiotensin II stimulates expression of transforming growth factor β receptor type II in cultured mouse proximal tubular cells. J Mol Med (Berl) 77: 556–564. [DOI] [PubMed] [Google Scholar]
- Worster AA, Nixon AJ, Brower-Toland BD, Williams J. 2000. Effect of transforming growth factor β1 on chondrogenic differentiation of cultured equine mesenchymal stem cells. Am J Vet Res 61: 1003–1010. [DOI] [PubMed] [Google Scholar]
- Wu M, Chen G, Li YP. 2016. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res 4: 16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurdak H, Ittner LM, Lang KS, Leveen P, Suter U, Fischer JA, Karlsson S, Born W, Sommer L. 2005. Inactivation of TGF-β signaling in neural crest stem cells leads to multiple defects reminiscent of DiGeorge syndrome. Genes Dev 19: 530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt AW, Osborne RJ, Stewart H, Ragge NK. 2010. Bone morphogenetic protein 7 (BMP7) mutations are associated with variable ocular, brain, ear, palate, and skeletal anomalies. Hum Mutat 31: 781–787. [DOI] [PubMed] [Google Scholar]
- Xu J, Shi GP. 2014. Vascular wall extracellular matrix proteins and vascular diseases. Biochim Biophys Acta 1842: 2106–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Han J, Ito Y, Bringas P Jr, Urata MM, Chai Y. 2006. Cell autonomous requirement for Tgfbr2 in the disappearance of medial edge epithelium during palatal fusion. Dev Biol 297: 238–248. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Miyauchi A, Goto J, Takagi Y, Okuizumi H, Kanematsu M, Hase M, Takai H, Harada A, Ikeda K. 1998. Association of a polymorphism of the transforming growth factor-β1 gene with genetic susceptibility to osteoporosis in postmenopausal Japanese women. J Bone Miner Res 13: 1569–1576. [DOI] [PubMed] [Google Scholar]
- Yang Y. 2013. Skeletal morphogenesis and embryonic development. In Primer on the metabolic bone diseases and disorders of mineral metabolism, 8th ed. (ed. Rosen C), pp. 3–10. ASBMR, Washington, DC. [Google Scholar]
- Yang LT, Kaartinen V. 2007. Tgfb1 expressed in the Tgfb3 locus partially rescues the cleft palate phenotype of Tgfb3 null mutants. Dev Biol 312: 384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Letterio JJ, Lechleider RJ, Chen L, Hayman R, Gu H, Roberts AB, Deng C. 1999. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-β. EMBO J 18: 1280–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Chen L, Xu X, Li C, Huang C, Deng CX. 2001. TGF-β/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol 153: 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HW, Zhu JP, Zhao MH, Lu Y. 2006. Losartan attenuates bleomycin-induced pulmonary fibrosis in rats. Respiration 73: 236–242. [DOI] [PubMed] [Google Scholar]
- Yi SE, Daluiski A, Pederson R, Rosen V, Lyons KM. 2000. The type I BMP receptor BMPRIB is required for chondrogenesis in the mouse limb. Development 127: 621–630. [DOI] [PubMed] [Google Scholar]
- Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM. 2005. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc Natl Acad Sci 102: 5062–5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MF. 2003. Bone matrix proteins: Their function, regulation, and relationship to osteoporosis. Osteoporos Int 14: S35–S42. [DOI] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. 2000. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev 14: 163–176. [PMC free article] [PubMed] [Google Scholar]
- Zehentner BK, Dony C, Burtscher H. 1999. The transcription factor Sox9 is involved in BMP-2 signaling. J Bone Miner Res 14: 1734–1741. [DOI] [PubMed] [Google Scholar]
- Zeller R, Lopez-Rios J, Zuniga A. 2009. Vertebrate limb bud development: Moving towards integrative analysis of organogenesis. Nat Rev Genet 10: 845–858. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bradley A. 1996. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 122: 2977–2986. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jordan JM. 2010. Epidemiology of osteoarthritis. Clin Geriatr Med 26: 355–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Schwarz EM, Rosier RN, Zuscik MJ, Puzas JE, O’Keefe RJ. 2003. ALK2 functions as a BMP type I receptor and induces Indian Hedgehog in chondrocytes during skeletal development. J Bone Miner Res 18: 1593–1604. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ziran N, Goater JJ, Schwarz EM, Puzas JE, Rosier RN, Zuscik M, Drissi H, O’Keefe RJ. 2004. Primary murine limb bud mesenchymal cells in long-term culture complete chondrocyte differentiation: TGF-β delays hypertrophy and PGE2 inhibits terminal differentiation. Bone 34: 809–817. [DOI] [PubMed] [Google Scholar]
- Zhang J, Tan X, Li W, Wang Y, Wang J, Cheng X, Yang X. 2005. Smad4 is required for the normal organization of the cartilage growth plate. Dev Biol 284: 311–322. [DOI] [PubMed] [Google Scholar]
- Zhang S, Kaplan FS, Shore EM. 2012. Different roles of GNAS and cAMP signaling during early and late stages of osteogenic differentiation. Horm Metab Res 44: 724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Poczatek MH, Berecek KH, Murphy-Ullrich JE. 2006. Thrombospondin 1 mediates angiotensin II induction of TGF-β activation by cardiac and renal cells under both high and low glucose conditions. Biochem Biophys Res Commun 339: 633–641. [DOI] [PubMed] [Google Scholar]