Abstract
The regulatory networks governing morphogenesis of a pleomorphic fungus, Candida albicans are extremely complex and remain to be completely elucidated. This study investigated the function of C. albicans yeast casein kinase 2 (CaYck2p). The yck2Δ/yck2Δ strain displayed constitutive pseudohyphae in both yeast and hyphal growth conditions, and formed enhanced biofilm under non-biofilm inducing condition. This finding was further supported by gene expression analysis of the yck2Δ/yck2Δ strain which showed significant upregulation of UME6, a key transcriptional regulator of hyphal transition and biofilm formation, and cell wall protein genes ALS3, HWP1, and SUN41, all of which are associated with morphogenesis and biofilm architecture. The yck2Δ/yck2Δ strain was hypersensitive to cell wall damaging agents and had increased compensatory chitin deposition in the cell wall accompanied by an upregulation of the expression of the chitin synthase genes, CHS2, CHS3, and CHS8. Absence of CaYck2p also affected fungal-host interaction; the yck2Δ/yck2Δ strain had significantly reduced ability to damage host cells. However, the yck2Δ/yck2Δ strain had wild-type susceptibility to cyclosporine and FK506, suggesting that CaYck2p functions independently from the Ca+/calcineurin pathway. Thus, in C. albicans, Yck2p is a multifunctional kinase that governs morphogenesis, biofilm formation, cell wall integrity, and host cell interactions.
Introduction
Candida albicans is a part of the normal microbiota of human hosts. As an opportunistic pathogen, it can cause life-threatening infections under immunocompromised conditions [1, 2]. Its ability to switch morphologies in various host environments is a key virulence trait [3–8]. Morphologic transition is regulated by complex pathways [9–12], and regulatory cross talk among the signaling pathways plays a significant role in governing morphogenesis in response to various environmental cues [13, 14]. Morphogenesis is closely associated with biofilm formation. The C. albicans biofilm architecture contains a mixture of yeast and hyphal cells in structured layers [15–18], and is largely related to its dimorphic nature in response to environmental factors such as temperature, pH, and media composition [19–21]. Previous studies suggest that many genes involved in hyphal formation are also tied to genes governing biofilm formation [16, 22–24]. It is also known that cell wall biogenesis directly contributes to the morphogenesis and pathogenicity of C. albicans, as the cell wall structure dynamically changes during progression of the cell cycle [25]. Thus, it is necessary to understand the interconnections amongst the various regulatory pathways that govern cell wall biogenesis and morphogenesis for therapeutic and prophylactic interventions.
CaYck2p is one of the fungal homologs of the casein kinase 1 (CK1) family of highly conserved serine/threonine kinases that are present in most eukaryotic organisms [26]. In higher eukaryotes, the CK1 family is part of the Wnt signaling pathway [27] and regulates cellular differentiation [28], membrane trafficking [29], DNA damage response [30], and circadian rhythms [28]. The Candida genome project has revealed three CK1 paralogs (YCK2, YCK3, and HRR25) that share amino acid similarity to other fungal CK1s, such as ScYck1p and ScYck2p in Saccharomyces cerevisiae (S1 Fig). In S. cerevisiae, CK1 members are functionally related, governing the cell cycle, morphology, and cell wall integrity [26, 31, 32]. Furthermore, in S. cerevisiae, ScYck1p and ScYck2p are known to regulate endocytosis, cell morphogenesis, mRNA localization, and nutrient sensing [26, 31, 33, 34]. Based on its sequence similarity to ScYck1p and ScYck2p, C. albicans Yck2p (CaYck2p) is predicted to play a role in nutrient sensing [34, 35]. A recent large-scale screening of a C. albicans protein kinase insertion library revealed that CaYck2p and CaYck3p are critical for cell wall regulation [36]. Yet, further analysis of individual CK1s in C. albicans has not been performed.
Our previous study discovered that transcription of the YCK2 gene was significantly upregulated when C. albicans interacted with host endothelial cells, and analysis of a yck2 insertion mutant suggested that CaYck2p may govern host cell interactions [37]. However, a limitation of this work was that it analyzed a yck2 mutant in which the insertion cassette was integrated at bp 1130 in the YCK2 open reading frame. As a result, the truncated 376 amino acid-protein likely retained partial function (S1 Fig). To better elucidate the function of CaYck2p in C. albicans and to determine the functional conservation among members of the fungal CK1 family, we generated and investigated a homozygous yck2Δ/yck2Δ strain in which the entire protein coding region of the YCK2 was deleted. We identified a new function of CaYck2p in governing cell growth and hyphal transition, in vitro biofilm formation, and cell wall biogenesis. We also uncovered several potential downstream molecules affected by CaYck2p that govern these cellular processes of C. albicans.
Materials and methods
Strains and media
All C. albicans strains constructed and used in this study are listed in S1 Table. Strains were maintained on YPD agar (1% yeast extract (Difco), 2% peptone (Difco) and 2% glucose) at 30°C. Synthetic complete medium [SC, 0.67% yeast nitrogen base without amino acids (Difco), 0.065% synthetic complete supplement mixture without histidine, arginine, and uridine (Qbiogene), 2% glucose, and 2% agar, supplemented with 100 μg/ml arginine, 50 μg/ml histidine, and 20 μg/ml uridine as needed] was used for strain construction and screening. YPD, RPMI 1640 (Hyclone), Spider (1% nutrient broth, 1% mannitol, 0.2% K2PO4) and YPGly (YP, 2% glycerol) broth were used for testing hyphal induction and biofilm formation.
Strains construction
The yck2Δ/yck2Δ strain was derived from C. albicans BWP17 by successive transformation with ARG4 and HIS1 deletion cassettes generated by PCR using the primers YCK2 KO-5 and YCK2 KO-3 (S2 Table) [38]. Transformants with ARG4 and HIS1 markers were selected on SC medium supplemented with 50 μg/ml histidine and 20 μg/ml uridine amino acids, respectively. The resulting yck2Δ/yck2Δ strain was subsequently transformed with Not I (Promega) and Pst I (Promega) digested 3.8 Kb pBSK-URA3 to re-integrate URA3 at its native locus as previously described [39]. To construct the YCK2 complemented strain (yck2Δ/yck2Δ::YCK2), a 2.85 Kb fragment containing YCK2 was amplified from SC5314 genomic DNA with the primers YCK2 Comp-5 and YCK2 Comp-3 by high fidelity PCR (Takara). This PCR product was then cloned into Not I digested pBSK-URA3 [39]. The resulting construct was linearized with Nru I for direct integration at the URA3 locus of the yck2Δ/yck2Δ strain. Targeted deletion of YCK2 was confirmed by whole cell PCR with the primers, YCK2 Confirm-5 and YCK2 Confirm-3.
Morphology analysis
To induce hyphal growth of C. albicans, the cells were grown in 3 ml YPD medium for overnight. The blastopores were then harvested by centrifugation for 10 min at 1000 × g and washed twice with 1X phosphate buffered saline (PBS). The final concentration of 3 × 106 cells/ml blastospores were added into RPMI 1640 medium (Hyclone) and incubated at 37°C for 3 h. Then 10 μl aliquots of the final resuspension was added to glass slides and mixed with 10 μl Vectashield Antifade Mounting Media (Vector Laboratories) for microscopic evaluation under a Zeiss Apotome microscope (Zeiss).
Biofilm assay
The extent of biofilm formation by the various C. albicans strains was measured by a 96-well microtiter plate biofilm assay [40]. Briefly, the overnight grown cells were washed with PBS and then diluted to 107 cells/ml, in various media, and 100 μl of each suspension was transferred in quadruplicate into 96-well polystyrene, round-bottom microtiter plates (Thermo Fisher Scientific). Medium without C. albicans cells was used as a baseline control. The extent of biofilm was quantified after 24 h by crystal violet staining. First, the wells were washed twice with 200 μl of PBS and then dried for 45 minutes at room temperature. Then, 110 μl of 1% crystal violet solution (Thermo Fisher Scientific) was added and incubated for 45 minutes at room temperature. The wells were then washed four times with 350 μl sterile water to remove the unbound crystal violet. Finally, the biofilm-associated crystal violet was solubilized with 110 μl of 95% ethanol (Sigma-Aldrich) at room temperature for 45 min. Subsequently, 100 μl was transferred into a new 96 well plate for reading at 570 nm (VictorX, Perkin Elmer, Waltham, MA).
Susceptibility testing
A spot dilution assay was used to test the susceptibility of various C. albicans strains to the cell membrane and cell wall stressors, as previously described [41]. Briefly, the cells were grown for overnight in YPD at 30°C and counted using a hemocytometer. Serial 10-fold dilutions of the strains in 5 μl PBS (range 105 to 101 colony forming units (CFU) per spot) were then plated onto YPD agar containing 300 μg/ml congo red, 10 μg/ml calcofluor white, 2 mg/ml protamine sulfate, 0.1% sodium dodecyl sulfate, 10 μg/ml cyclosporine A, 5 μg/ml FK506 and 5 μg/ml pyrvinium pamoate, respectively, and incubated at 30°C for 48 h. The plates were photographed in UVP Gel Doc-It system (UVP, CA).
The minimum Inhibitory Concentrations (MIC) of various C. albican strains to fluconazole and caspofungin were tested using the broth microdilution method of the CLSI according to document M27-A3 [42]. MIC values were determined after 24 h and 48 h of incubation.
Calcofluor white staining and fluorescent microscopy
The C. albicans strains were grown to log phase in YPD medium at 30°C and then further incubated with 0.05% calcofluor white solution (Sigma) for 5 min, after which they were washed once and resuspended in PBS. To mount the cells, 10 μl of final resuspension was added to the glass slide and mixed with 10 μl of Vectashield Antifade Mounting Media (Vector Laboratories). To compare the degree of the calcofluor white fluorescent signal, the UV laser intensity and the exposure time were fixed at the same level for all observation. The images were photographed with 63X magnification under a Zeiss Apotome microscope.
Host cell damage assay
Human umbilical vein endothelial cells (HUVECs) were harvested as previously described [43]. The cells were maintained in M-199 medium (Gibco) supplemented with 10% fetal bovine serum, 10% bovine calf serum, 2 mM L-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin. The immortalized oral mucosal keratinocytes (OKF6/TERT) were kindly provided from Dr. Rheinwald [44] and the immortalized vaginal epithelial cells (VK2/E6E7) were purchased from American Type Culture Collection (ATCC) [45]. The immortalized cells were maintained in keratinocyte serum-free medium (Gibco) supplemented with 50 μg/ml bovine pituitary extract, 0.1 ng/ml epidermal growth factor, 100 U/ml penicillin, and 100 μg/ml streptomycin [44, 45]. All cells were grown at 37°C in a humidified environment containing 5% CO2. The extent of damage caused by various C. albicans strains was measured using a 51Cr release assay as described previously [46]. The host cells were grown in a 96-well tissue culture plate with detachable wells and incubated overnight with 6 μCi Na251CrO4 (MP Biomedicals) per well. The following day, the unincorporated tracer was removed by extensive rinsing. When HUVECs were used, they were infected with 4 × 104 C. albicans blastospores per well in RPMI 1640. As the epithelial cells are less susceptible to C. albicans-induced damage, they were infected with 105 C. albicans blastospores per well in the same medium. To measure the spontaneous release of 51Cr, uninfected host cells were exposed to medium alone. The infected host cells were incubated for 3 h and then, the amount of 51Cr released into the medium and retained by the cells was determined by liquid scintillation counter 6500 (Beckman Coulter).
Quantitative real time RT-PCR analysis
C. albicans cells were grown overnight in 3ml of YPD and adjusted to a 3 × 106 cells/ml subculture. The cells were then incubated for two h in YPD at 30°C for yeast phase growth and in RPMI at 37°C for hyphal growth. Total RNAs were extracted with RiboPure RNA extraction kit following the manufacturer’s protocol (Ambion). cDNAs were synthesized from 1 μg of total RNAs with Retroscript Reverse Transcription Kit (Ambion) following the manufacturer’s protocol. Quantitative real-time PCR was carried out using 5-Prime SYBR green PCR kit (Thermo Fisher Scientific) and Eppendorf Realplex System (Eppendorf) following the manufacturers’ protocol. The primers used in this study are listed in S2 Table. Relative gene expression was calculated by the 2ΔΔCT method [47] using the transcript level of CaACT1 as the endogenous control [37].
Statistical analysis
Unless stated otherwise, each experiment was performed in triplicate on at least three separate occasions. Raw data were analyzed with Microsoft Excel software and statistical significance of any differences between experimental groups was determined by ANOVA with Dunnett’s test posthoc analysis. *, **, and *** denotes a p value of < 0.05, < 0.001, and < 0.0001, respectively.
Results
Yck2p governs morphologic transition of C. albicans
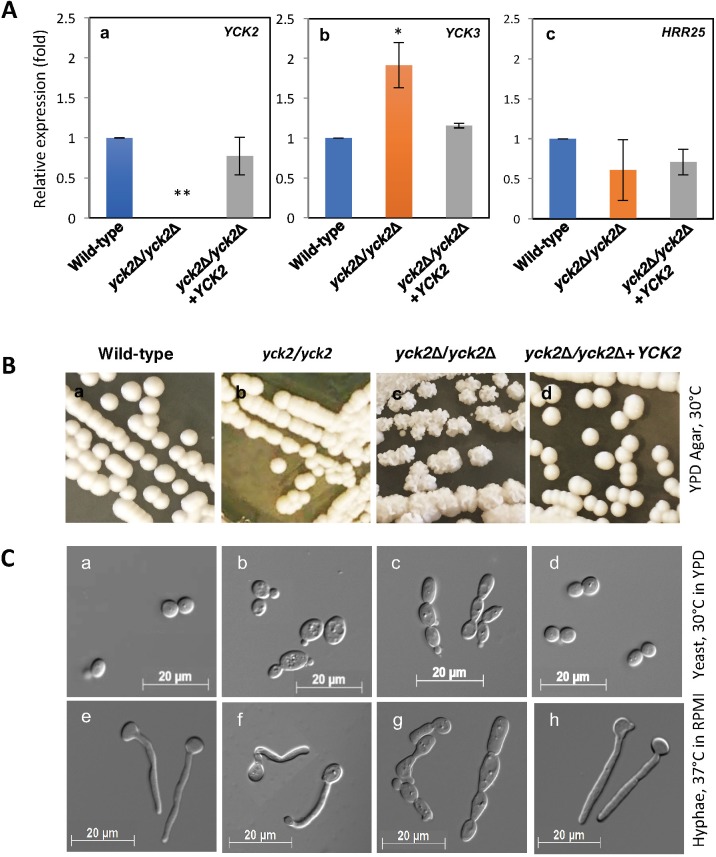
C. albicans switches morphologies between yeast and hyphae forms in response to various environmental cues. We sought to define the role of CaYck2p during C. albicans morphogenesis. The yck2Δ/yck2Δ strain was constructed as summarized in S1 Fig and YCK2 transcription was found to be below detection (p<0.001, Fig 1A). As there are two additional paralogs of CK1 family, YCK3 and HRR25, their transcription was measured to determine any compensatory upregulation in the absence of YCK2. As shown in Fig 1A, YCK3 transcript levels increased (p<0.05), but HRR25 transcript levels were not changed in the yck2Δ/yck2Δ strain compared to the wild-type strain and the yck2Δ/yck2Δ+YCK2 complemented strain.
Fig 1. Morphology of the yck2Δ/yck2Δ strain.
A, relative mRNA expression of YCK2 (a), YCK3 (b), and HRR25 (c) for the designated strains. Shown are mean ± S.D. of three biological replicates (* p<0.05, ** p<0.001). B, colony morphologies of the wild-type (a), the yck2/yck2 insertion mutant (b), the yck2Δ/yck2Δ (c), and the yck2Δ/yck2Δ+YCK2 complemented (d) strains grown on YPD plate at 30°C for 2 days. C, microscopic morphologies of the wild-type (a and e), the yck2/yck2 insertion mutant (b and f), the yck2Δ/yck2Δ (c and g), and the complemented (d and h) strains grown in YPD medium for two h at 30°C and in RPMI 1640 medium at 37°C, respectively.
The colony morphology of the yck2Δ/yck2Δ strain was significantly different from the wild-type strain when grown on YPD agar at 30°C. As shown on Fig 1B, the wild-type strain formed smooth convex colonies. The phenotype of the yck2 insertion mutant strain (yck2-Tn7::UAU1/yck2-Tn7::URA3, called yck2 insertion strain hereafter) [37] generated in our previous study was similar to that of the wild-type strain. By contrast, the yck2Δ/yck2Δ strain formed wrinkled colonies with a rough texture, which were different from both the wild-type and the yck2 insertion mutant strains. Complementing the yck2Δ/yck2Δ strain with an intact copy of YCK2 (yck2Δ/yck2Δ+YCK2, complemented strain hereafter) restored the wild-type colony morphology, indicating that the wrinkled colonies of the yck2Δ/yck2Δ strain were solely due to the complete loss of functional YCK2 alleles. In contrast, all strains formed wrinkled colonies on YPD with 10% serum agar at 37°C as shown in S2 Fig. It was noted that the wild-type and the complemented strain formed more flat and fuzzy colonies than the yck2Δ/yck2Δ strain.
Next, the cells were viewed by microscopy to observe the morphology of the yck2Δ/yck2Δ strain as shown on Fig 1C. The wild-type strain grew as yeast in YPD medium at 30°C, and grew as hyphae in RPMI 1640 medium at 37°C. Similarly, the yck2 insertion strain grew as yeast form in YPD medium at 30°C and formed short hyphae in RPMI medium at 37°C, similarly to our previous study [37]. However, the yck2Δ/yck2Δ strain grew as chains of elongated yeast cells in both YPD medium at 30°C and RPMI medium at 37°C. The chains of elongated cells were reminiscent of pseudohyphae. The complemented strain had wild-type morphology, indicating that the observed pseudohyphal-like morphology of the yck2Δ/yck2Δ strain was solely due to the loss of functional YCK2 alleles.
Yck2p is involved in biofilm formation
While assessing the morphology of the yck2Δ/yck2Δ strain, we noticed that the cells were aggregated and adhered to the plastic culture tubes in YPD broth at 30°C, which was not usually observed with the wild-type strain. Therefore, we suspected that the yck2Δ/yck2Δ strain may have increased biofilm capability. First, we assessed the extent of biofilm by the wild-type strain in various conditions, to determine the effect of media and temperature on biofilm formation (S2 Fig). Growth in RPMI 1640 medium induced the noticeable biofilm formation by the wild-type strain at 30°C, whereas neither YPD nor YPGly media induced biofilm formation at 30°C. The Spider medium induced less biofilm formation by the wild-type strain at 30°C. Thus, we considered the spider medium at 30°C as a moderate biofilm inducing condition. The extent of biofilm formed at 37°C suggest that all media except YPGly at 37°C are biofilm-inducing conditions for the wild-type strain.
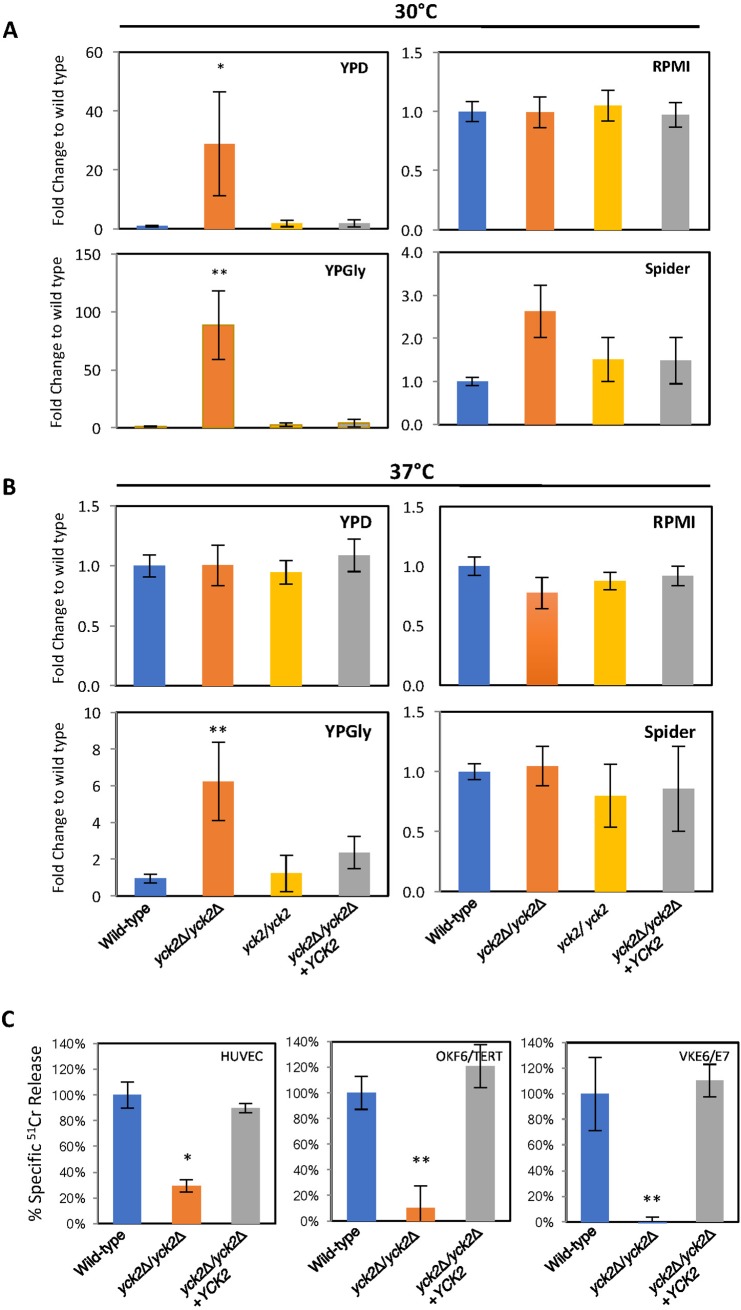
Next, we measured the extent of biofilm formed by the yck2Δ/yck2Δ strain in both non-biofilm inducing and biofilm-inducing conditions. Indeed, the yck2Δ/yck2Δ strain formed significantly increased biofilm in all non-biofilm inducing conditions as compared to the wild-type strain. As shown in Fig 2A, the wild-type strain did not form a biofilm in either YPD or YPGly media at 30°C. In contrast, the yck2Δ/yck2Δ strain developed 28-fold (p<0.05) and 80-fold (p<0.001) more biofilm in YPD and YPGly, compared to the wild-type strain. The insertion mutant and the complemented strains did not form biofilms in those conditions, similar to the wild-type strain. The extent of biofilm by all strains grown in RPMI 1640 medium at 30°C was comparable, whereas the yck2Δ/yck2Δ strain formed 2 fold increased biofilm as compared to the wild type strain under spider medium at 30°C. Fig 2B demonstrates that the yck2Δ/yck2Δ strain formed 6-fold (p<0.001) increased biofilm relative to the wild-type strain in YPGly at 37°C, a non-biofilm inducing condition for the wild-type strain. This result suggests that the yck2Δ/yck2Δ strain has an enhanced capacity to form biofilms when grown under conditions that induce minimal biofilm formation by the wild-type strain. The yck2Δ/yck2Δ strain also formed a similar level of biofilm as the wild type did in biofilm inducing condition, suggesting that the yck2Δ/yck2Δ strain likely formed a constitutive biofilm in all conditions tested for this study.
Fig 2. Functional analysis of YCK2 deletion.
A and B, The extent of biofilm formation by the indicated strains after 48 h in YPD, RPMI1640, YPGly, and Spider media at 30°C (A) and at 37°C (B). (* p<0.001). C, The extent of cell damage of endothelial cells (HUVECs), oral epithelial cells (OKF6/TERT), and vaginal epithelial cells (VK2E6/E7) after 180 min co-incubation with the indicated strains. (*, p<0.001 compared to both the wild-type strain and complemented strain).
Yck2p is required for normal host cell damage by C. albicans
The ability of C. albicans to switch morphology greatly affects its ability to damage host cells [3, 10]. We analyzed whether the yck2Δ/yck2Δ strain with constitutive pseudohyphae was capable of damaging host cells in vitro. As shown in Fig 2C, the yck2Δ/yck2Δ strain caused significantly less damage to all three types of host cells, human umbilical vein endothelial cells (HUVECs), immortalized oral mucosal keratinocytes (OKF6/TERT), and immortalized vaginal epithelial cells (VK2/E6E7), as compared to the wild-type strain. Complementing the yck2Δ/yck2Δ strain with a wild-type copy of YCK2 restored its capacity to damage host cells. This result suggests that CaYck2p is required to damage both endothelial and epithelial cells in vitro.
YCK2 is involved in cell wall integrity of C. albicans
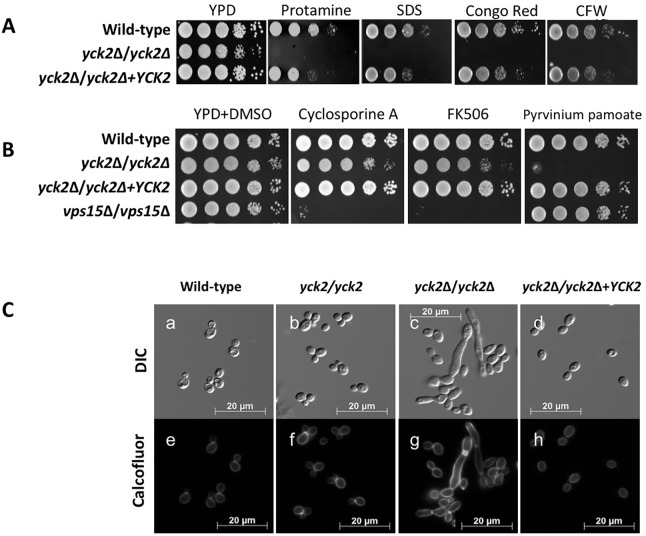
The yck2Δ/yck2Δ strain displaying altered morphology and biofilm likely has cell wall architecture change, which often leads to altered cell wall integrity that in turn may impact cell membrane function. Thus, we examined the susceptibility of the yck2Δ/yck2Δ strain to various cell wall and cell membrane stressors. As shown in Fig 3A, the yck2Δ/yck2Δ strain had markedly increased susceptibility to protamine, SDS, congo red, and calcofluor white. In contrast, the yck2Δ/yck2Δ strain grew normally in the presence of NaCl or H2O2, indicating that CaYck2p is not required for its resistance to either osmotic or oxidative stress. This result suggests that CaYck2p is involved in governing cell wall integrity but is not related to either osmotic or oxidative stress response.
Fig 3. Effect of YCK2 deletion on cell wall integrity.
A, susceptibility of the indicated strains to various cell wall stressors, 2 mg/ml protamine sulfate, 0.1% SDS, 300 μg/ml congo red, and 10 μg/ml calcofluor white (CFW) after 48 h at 30°C. B, susceptibility of the designated strains to calcineurin inhibitors, 10 μg/ml cyclosporine A and 5 μg/ml FK506 and 5 μg/ml pyrvinium pamoate. Shown are representative images for three independent experiments with the same outcome. C, microscopic analysis of chitin deposition in the cell wall of the wild-type (a, e), the insertion mutant (b, f), the yck2Δ/yck2Δ (c, g), and the complemented (d, h) strains grown in YPD at 30°C and stained with 0.05% calcofluor white.
To further investigate the degree of hypersensitivity of the yck2Δ/yck2Δ strain to the cell wall stressors, the minimum inhibitory concentration (MIC) values of fluconazole and caspofungin were measured. As shown on Table 1, the 24 h and 48 h fluconazole MICs for the yck2Δ/yck2Δ strain were 0.062 μg/ml and 0.125 μg/ml, respectively, which were hypersusceptible compared with those for wild type (0.5 μg/ml at 24 h and 1 μg/ml at 48 h) and complemented strains (0.25 μg/ml at 24 h and 0.5 μg/ml at 48 h). The MIC values of caspofungin demonstrated similar results; the MICs for yck2Δ/yck2Δ strain were significantly lower (0.031 μg/ml at 24 h and 0.125 μg/ml at 48 h) than those for the wild-type strain and the complemented strains (0.5 μg/ml at 24 h and 1 μg/ml at 48 h).
Table 1. Minimum inhibitory concentrations of fluconazole and caspofungin for the yck2Δ/yck2Δ strain.
| Minimum Inhibitory Concentration (μg/mL) | ||||
|---|---|---|---|---|
| C. albcans strains | Fluconazole | Caspofungin | ||
| 24 h | 48 h | 24 h | 48 h | |
| Wild-type (DIC185) | 0.5 | 1 | 0.5 | 1 |
| yck2Δ/yck2Δ (1041U) | 0.062 | 0.125 | 0.031 | 0.125 |
| yck2Δ/yck2Δ + YCK2 (10410) | 0.5 | 0.5 | 0.5 | 1 |
We sought to identify potential regulatory mechanisms by which CaYck2p maintains cell wall integrity. To test whether CaYck2p is associated with the Ca2+/calcineurin pathway in governing cell wall integrity, the susceptibility of the yck2Δ/yck2Δ strain to cyclosporine A and FK506 was tested [48]. As shown on Fig 3B, the wild-type strain was resistant to 10 μg/ml cyclosporine A and 5 μg/ml FK506. The C. albicans vps15Δ/vps15Δ strain is known to be hypersusceptible to both inhibitors [37], and, thus, was used as a positive control for susceptibility to these drugs. The yck2Δ/yck2Δ strain grew similarly to the wild-type strain, suggesting that Yck2p does not influence cell wall integrity via the calcineurin pathway. To test the conserved function of the CK1 family among eukaryotic system, the yck2Δ/yck2Δ strain was also tested for its susceptibility to pyrvinium pamoate, a drug that modulates the CK1 and Wnt signaling pathways [49, 50]. The yck2Δ/yck2Δ strain displayed significantly increased sensitivity to pyrvinium pamoate as compared to the wild-type strain (Fig 3B), which provides evidence that CaYck2p is functions in the CK1 and Wnt signaling pathways.
Compensatory chitin synthesis was induced by the loss of Yck2p function
The finding that the yck2Δ/yck2Δ strain was sensitive to cell wall targeting drugs suggested that the yck2Δ/yck2Δ strain may have a compensatory increase in chitin deposition. When the yck2Δ/yck2Δ strain was stained with calcofluor white to assess chitin levels and distribution in the cell wall, there was notably increased fluorescence in the cell wall and the septum of the yck2Δ/yck2Δ strain as compared to both the wild-type and the complemented strains (Fig 3C). This result suggests that there is an increased chitin synthesis in the absence of functional CaYck2p.
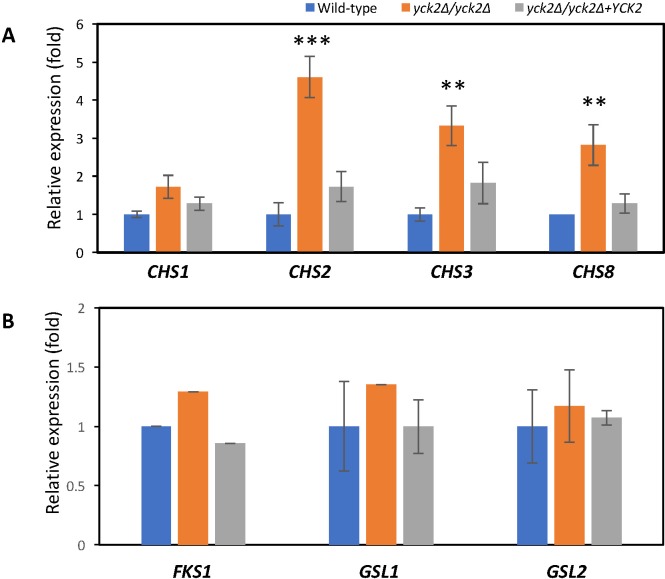
Transcriptional analysis of the chitin synthase genes in the yck2Δ/yck2Δ strain demonstrated that the expression of CHS2, CHS3, and CHS8 genes was induced by 5-fold (p<0.0001), 3.3-fold (p<0.01), and 3-fold (p<0.01), respectively, compared to the wild-type strain and the complemented strains (Fig 4A). CHS1 expression was slightly, but not significantly increased in the yck2Δ/yck2Δ strain. These data suggest that the loss of functional CaYck2p results in impaired cell wall integrity, which then leads to a compensatory increase in chitin synthase mRNA expression, leading to enhance cell wall chitin deposition. However, the transcription level of glucan synthase genes (FKS1, GSL1, GSL2) were similar between the yck2Δ/yck2Δ and the wild-type strains (Fig 4B).
Fig 4. Quantitative gene expression analysis of cell wall synthase genes.
A, the relative mRNA expression of chitin synthase genes compared to wild type in the indicated strains grown in YPD at 30°C to mid log phase (** p<0.001 and *** p<0.0001). B, the relative mRNA expression of glucan synthase genes compared to wild type in the indicated strains grown in YPD at 30°C to mid log phase.
Yck2p is involved in hyphal specific gene regulation
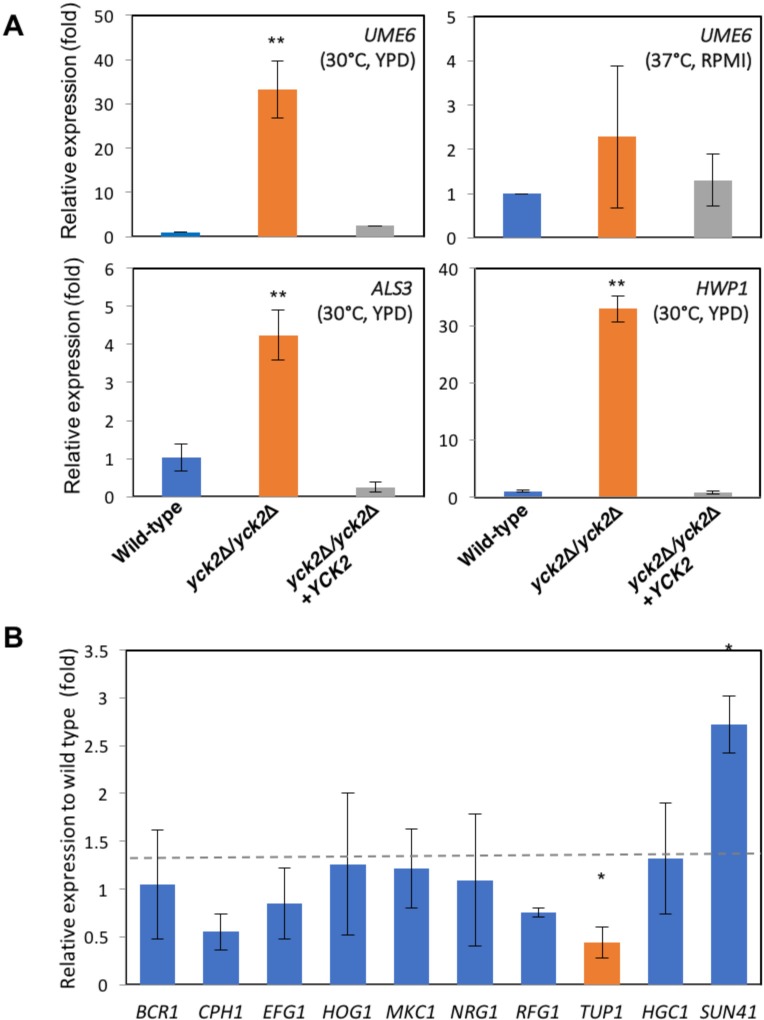
To determine how CaYck2p regulates C. albicans morphology, biofilm formation, and host cell damage, we assessed the transcription levels of the genes associated with these processes. First, we tested the mRNA expression of a transcription factor, UME6, for which has increased expression during the yeast-to-hyphal transition [51]. UME6 mRNA levels in the yck2Δ/yck2Δ strain grown yeast-inducing condition (YPD at 30°C) were 35-fold higher than in the wild-type strain (p<0.001) (Fig 5A, top left). The complemented strain expressed wild-type levels of UME6. When the strains were grown under hyphal inducing conditions, all strains expressed similar levels of UME6 (Fig 5A, top right). The increased UME6 expression in the yck2Δ/yck2Δ strain is likely the cause of its pseudohyphal growth and increased biofilm formation under yeast-inducing condition. We also measured the transcription of ALS3 and HWP1, both of which are highly expressed by hyphae, and involved in biofilm formation [52]. Fig 5A (bottom) demonstrated that there was a 4-fold increased expression of ALS3 (p<0.001) and a 32-fold increased level of HWP1 (p<0.001) relative to the wild-type strain, even when the organisms were grown under yeast-inducing conditions.
Fig 5. Quantitative gene expression analysis.
A, relative mRNA expression of UME6, ALS3, and HWP1 in the indicated strains grown in YPD at 30°C and in RPMI at 37°C, respectively. Shown are means ± S.D. of three independent experiments (** p<0.001). B, relative mRNA expression of genes involved in morphology transition and biofilm formation in the yck2Δ/yck2Δ strain compared to the wild type strain grown in YPD at 30°C. Shown are means ± SD for three biological replicates (* p<0.05).
To further elucidate the molecular mechanisms of the altered phenotype of the yck2Δ/yck2Δ strain, we measured the mRNA expression levels of BCR1, CPH1, and EFG1, which encode transcription factors known to be responsible for hyphal transition and/or biofilm formation. CPH1 expression was slightly decreased but the change was not statistically significant. Either BCR1 or EFG1 was not differentially expressed in the yck2Δ/yck2Δ strain relative to the wild-type, as shown on Fig 5B, suggesting that CaYck2p is not involved in the transcriptional regulation of these genes. The transcription of HOG1 and MKC1, which specify MAP kinases involved in stress response, was not altered in the yck2Δ/yck2Δ strain as compared to the wild-type strain. The expression of transcription genes encoding the repressor proteins, NRG1, RFG1, and TUP1, which represses UME6 transcription and hyphal transition was also tested. Only TUP1 expression was decreased in the yck2Δ/yck2Δ strain as compared to the wild type. The expression of SUN41 was increased in the yck2Δ/yck2Δ strain as compared to the wild-type strain, which is consistent with previous finding that increased UME6 expression enhances SUN41 transcription [23]. Collectively, these results suggest that CaYck2p is associated with de-repression of UME6 and its downstream hyphal specific genes, ALS3, HWP1, and SUN41 when C. albicans is grown under yeast-inducing conditions.
Discussion
This study identified a new function of the C. albicans yeast casein kinase 2 (CaYck2p) in controlling morphogenesis, biofilm formation, host cell damage, and cell wall integrity. Our results suggest that CaYck2p is the dominant CK1 homolog of C. albicans, by which governs similar biological processes as ScYck1p/ScYck2p [26, 31, 53, 54] of S. cerevisiae and other fungal CK1 homologs [32, 55, 56]. We noticed that the morphology of the yck2Δ/yck2Δ strain differed significantly from that of the insertion mutant strain reported in our previous study [37]. The insertion mutant strain, expressing a truncated CaYck2p with the conserved catalytic domain, had delayed yeast-to-hyphal transition [37]. By contrast, the yck2Δ/yck2Δ strain was not able to switch between yeast and hyphae and formed constitutive pseudohyphae-like cells. Thus, the N-terminus catalytic domain of CaYck2p seems to be crucial for the yeast-to-hyphal transition. The transcription of YCK3 was increased in the yck2Δ/yck2Δ strain, suggesting that there was compensatory upregulation of the other CK1 homolog in the absence of CaYck2p. Still, CaYck2p and CaYck3p do not seem to play interchangeable function as the lack of functional CaYck2p alone resulted similar morphological changes, similar to the double ScYck1p/ScYck2p mutations [26, 31, 53, 54].
The yck2Δ/yck2Δ strain grew as a constitutive pseudohyphae-like form in both yeast and hyphal growth conditions. Yet, the microscopic observation alone did not provide adequate evidence of whether the mutant filaments were bona fide pseudohyphae or defected filamentation under hyphal inducing condition. The capacity of C. albicans to switch between yeast, pseudohyphae, and hyphae forms contributes significantly to its ability to form biofilm [23, 52, 57]. The yck2Δ/yck2Δ strain formed a biofilm under various non-biofilm inducing conditions, and formed similar wild-type biofilm under strong biofilm inducing conditions. Thus, it is likely that the constitutive pseudohyphae of the yck2Δ/yck2Δ strain enabled the organism to form a biofilm even under non-inducing condition. Studies suggest that the pseudohyphae are intermediate to yeast and hyphae and the genes associated with pseudohyphal form are a subset of those with hyphal form [7, 58]. Of those genes, UME6 is transcriptionally regulated, and its mRNA expression levels escalate as the cell transition from yeast to pseudohyphae to true hyphae. Previous studies also demonstrated that overexpression of UME6 in a non-biofilm forming mutant restored biofilm formation [20, 59] and significantly increased levels of ALS3, which encodes an agglutinin-like protein [60–62] and invasin [63, 64], and HWP1, which specifies a hyphal specific protein involved in adherence and biofilm formation [52, 65–67]. We found that the yck2Δ/yck2Δ strain overexpresses UME6, as well as ALS3, and HWP1. Thus, we speculate that in the yck2Δ/yck2Δ strain, de-repression of UME6 led to the increased ALS3 and HWP1 to the enhanced biofilm formation.
As the filamentous growth program is initiated by relief of transcriptional repression [68], CaYck2p is likely associated with transcriptional repression of UME6 and its downstream genes. It was reported that the transcription of UME6 is upregulated by Efg1p and negatively regulated by Nrg1p-Tup1p and Rfg1p-Tup1p repression complexes [51, 69, 70]. Thus, it is possible that CaYck2p interacts with the transcription factors that regulate UME6 transcription. Our study found the expression of TUP1 was slightly decreased in the yck2Δ/yck2Δ strain. Thus, reduced TUP1 expression in the yck2Δ/yck2Δ strain likely decreases the repressive activity the Tup1 complexes, leading to de-repression of UME6 transcription. Nevertheless, the yck2Δ/yck2Δ strain is locked in pseudohyphae form even with the elevated UME6 expression, in contrast to the previous result that constitutive expression of UME6 induces the formation of true hyphae under non-hyphae inducing condition [7, 23, 51, 71]. As tup1Δ/tup1Δ mutant is known to grow constitutively as pseudohyphae [4], reduced TUP1 expression may be one reason that the yck2Δ/yck2Δ strain has this morphology. Therefore, we conclude that the elevated UME6 expression is insufficient to induce the formation of true hyphae in the absence of functional CaYck2p. This study also suggests that true hyphal formation is not required for in vitro biofilm formation; the increased ALS3 and HWP1 expression in pseudohyphae of the yck2Δ/yck2Δ strain was sufficient to promote constitutive biofilm formation. Still, the biofilm formed by the yck2Δ/yck2Δ strain might have different ultrastructure as compared to the wild-type strain. In the future, a closer investigation of the role of Yck2p in governing biofilm architecture and the process of cell dispersal from mature biofilms will provide additional insight into the role of Yck2p in biofilm formation and dispersal.
Many studies suggest that the C. albicans strains with impaired morphogenesis also have reduced virulence [72, 73]. Our result demonstrated that the yck2Δ/yck2Δ strain, even with increased expression of UME6 and its downstream cell surface adhesins, ALS3 and HWP1, had significantly decreased ability to damage both endothelial and epithelial cells tested in this study. These results strongly suggest that the yck2Δ/yck2Δ strain would have reduced virulence in mouse models of mucosal and disseminated infection.
Cell wall dynamics are controlled by a broad signaling network [74, 75] and are tightly linked to C. albicans growth, morphogenesis, and pathogenicity [76]. The yck2Δ/yck2Δ strain was hypersensitive to cell wall stressors and demonstrated increased chitin deposition in the cell wall. Transcriptional analysis confirmed increased expression of three chitin synthase genes, CHS2, CHS3, and CHS8, which is consistent with previous studies showing that fungal cells increase chitin synthesis to overcome compromised cell wall integrity [77–79]. The transcription of SUN41, a cell wall glycosidase involved in cell wall biogenesis and biofilm formation [80], was also induced in the yck2Δ/yck2Δ strain as compared to the wild-type. Taken together, these results indicate that CaYck2p is important to cell wall integrity. These data are also consistent with those of a previous study that found that CaYck2p is a part of a carbohydrate utilization protein kinase cluster involved in cell wall biogenesis [36].
One possible mechanism by which CaYck2p is involved in regulation of cell wall integrity is by interacting with one of the cell wall integrity regulatory pathways [81–84]. Our results indicated that the yck2Δ/yck2Δ strain displayed wild-type sensitivity to cyclosporine A and FK506, suggesting that the absence of CaYck2p does not activate the calcineurin pathway. The PKC-Mkc1p pathway regulates cell wall integrity by directly increasing chitin synthase gene expression when the cell wall is damaged by cell wall perturbing drugs [36, 85, 86]. As the yck2Δ/yck2Δ strain had increased chitin deposition on the cell wall and the upregulation of chitin synthase genes, it is likely that aberrant cell wall formation in the yck2Δ/yck2Δ strain mimics stress conditions and leads to constitutive upregulation of compensatory chitin synthesis. A previous study suggested that Cck1p, the CK1 homolog in C. neoformans, regulates the expression and phosphorylation of Mpk1p and Hog1p under oxidative and osmotic stress conditions [32]. By contrast, we found that the yck2Δ/yck2Δ strain displayed wild-type resistance to osmotic and oxidative stress. Thus, it is likely that there is functional divergence of CK1 family among fungal species. Finally, we found that pyrvinium pamoate, an anthelminthic agent known to modulate CK1 and Wnt signaling pathway in higher eukaryotic systems, has specific inhibitory effect on the yck2Δ/yck2Δ strain. This result indicates that Yck2p functions in the CK1/Wnt signaling pathway and suggests that the combination of pyrvinium pamoate and a CK1 inhibitor could be developed into a new antifungal agent.
In summary, the fungal CK1 homologs have conserved their functions in maintaining cellular processes, but species specific functional divergence has occurred. In C. albicans, Yck2p regulates a range of cellular functions including morphogenesis, biofilm formation, cell wall integrity, and host cell interaction. In particular, CaYck2p likely plays a significant role in governing genetic repression of hyphal specific genes, and in regulating compensatory cell wall biogenesis.
Supporting information
(A) Protein similarity analysis of C. albicans Yck2p, Yck3p, S. cerevisiae Yck1p, and Yck2p. (B) The protein conserved domain information of the CaYck2p (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). (C) Illustration of two-step gene disruption with HIS1 and ARG4 markers. (D) PCR amplified YCK2 alleles with YCK2 Confirm-5 and YCK2 Confirm-3 primers. Indicated strains’ genomic DNAs were amplified with YCK2 Confirm-5 and YCK2 Confirm-3 primers, run on 0.8% agarose gel with 1 Kb ladder (New England Biolabs), and imaged with UVP Gel Doc-It system (UVP, CA).
(TIFF)
Colony morphologies of the wild-type (a), the yck2Δ/yck2Δ (b), and the yck2Δ/yck2Δ+YCK2 complemented (c) strains grown on YPD with 10% serum plate at 37°C for 2 days.
(TIFF)
The extent of biofilm formation in RPMI1640, spider, YPD and YPglycerol media at 30°C and 37°C was tested by colormetric absorbance analysis at 595nm. (** p<0.01 in OneWay ANOVA with Dunnett’s test posthoc analysis).
(TIFF)
(PDF)
(PDF)
Acknowledgments
We thank Jesses Garcia, and Peng-Yi Zhu and Lythou Yeo and Norma Solis for technical assistance in fluorescent microscopy, and biofilm assay, and host cell damage assay, respectively.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the National Institutes of Health, URL: https://www.nih.gov, grant numbers: GM61331, SC3 GM103699, SC1 GM096916, R01DE022600; and National Research Foundation of Korea, URL: https://www.nrf.re.kr/index, grant number: NRF-2013R1A1A3010554. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36(1):1–53. doi: 10.3109/10408410903241444 . [DOI] [PubMed] [Google Scholar]
- 2.Arendrup MC. Epidemiology of invasive candidiasis. Curr Opin Crit Care. 2010;16(5):445–52. doi: 10.1097/MCC.0b013e32833e84d2 . [DOI] [PubMed] [Google Scholar]
- 3.Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90(5):939–49. . [DOI] [PubMed] [Google Scholar]
- 4.Braun BR, Johnson AD. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science. 1997;277(5322):105–9. . [DOI] [PubMed] [Google Scholar]
- 5.Braun BR, Johnson AD. TUP1, CPH1 and EFG1 make independent contributions to filamentation in Candida albicans. Genetics. 2000;155(1):57–67. ; PubMed Central PMCID: PMCPMC1461068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumamoto CA, Vinces MD. Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cell Microbiol. 2005;7(11):1546–54. doi: 10.1111/j.1462-5822.2005.00616.x . [DOI] [PubMed] [Google Scholar]
- 7.Carlisle PL, Banerjee M, Lazzell A, Monteagudo C, Lopez-Ribot JL, Kadosh D. Expression levels of a filament-specific transcriptional regulator are sufficient to determine Candida albicans morphology and virulence. Proc Natl Acad Sci U S A. 2009;106(2):599–604. doi: 10.1073/pnas.0804061106 ; PubMed Central PMCID: PMCPMC2626749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gow NA, Brown AJ, Odds FC. Fungal morphogenesis and host invasion. Curr Opin Microbiol. 2002;5(4):366–71. . [DOI] [PubMed] [Google Scholar]
- 9.Brown AJ, Gow NA. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 1999;7(8):333–8. . [DOI] [PubMed] [Google Scholar]
- 10.Park H, Myers CL, Sheppard DC, Phan QT, Sanchez AA, E Edwards J, et al. Role of the fungal Ras‐protein kinase A pathway in governing epithelial cell interactions during oropharyngeal candidiasis. Cellular microbiology. 2005;7(4):499–510. doi: 10.1111/j.1462-5822.2004.00476.x [DOI] [PubMed] [Google Scholar]
- 11.Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol. 2012;10(2):112–22. doi: 10.1038/nrmicro2711 ; PubMed Central PMCID: PMCPMC3624162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inglis DO, Sherlock G. Ras signaling gets fine-tuned: regulation of multiple pathogenic traits of Candida albicans. Eukaryot Cell. 2013;12(10):1316–25. doi: 10.1128/EC.00094-13 ; PubMed Central PMCID: PMCPMC3811338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saputo S, Kumar A, Krysan DJ. Efg1 directly regulates ACE2 expression to mediate cross talk between the cAMP/PKA and RAM pathways during Candida albicans morphogenesis. Eukaryot Cell. 2014;13(9):1169–80. doi: 10.1128/EC.00148-14 ; PubMed Central PMCID: PMCPMC4187626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol. 2011;9(10):737–48. doi: 10.1038/nrmicro2636 . [DOI] [PubMed] [Google Scholar]
- 15.Nobile CJ, Mitchell AP. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr Biol. 2005;15(12):1150–5. doi: 10.1016/j.cub.2005.05.047 . [DOI] [PubMed] [Google Scholar]
- 16.Ramage G, Saville SP, Thomas DP, Lopez-Ribot JL. Candida biofilms: an update. Eukaryot Cell. 2005;4(4):633–8. doi: 10.1128/EC.4.4.633-638.2005 ; PubMed Central PMCID: PMCPMC1087806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richard ML, Nobile CJ, Bruno VM, Mitchell AP. Candida albicans biofilm-defective mutants. Eukaryot Cell. 2005;4(8):1493–502. doi: 10.1128/EC.4.8.1493-1502.2005 ; PubMed Central PMCID: PMCPMC1214533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fanning S, Xu W, Beaurepaire C, Suhan JP, Nantel A, Mitchell AP. Functional control of the Candida albicans cell wall by catalytic protein kinase A subunit Tpk1. Mol Microbiol. 2012;86(2):284–302. doi: 10.1111/j.1365-2958.2012.08193.x ; PubMed Central PMCID: PMCPMC3947901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183(18):5385–94. doi: 10.1128/JB.183.18.5385-5394.2001 ; PubMed Central PMCID: PMCPMC95423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai JV, Bruno VM, Ganguly S, Stamper RJ, Mitchell KF, Solis N, et al. Regulatory role of glycerol in Candida albicans biofilm formation. MBio. 2013;4(2):e00637–12. doi: 10.1128/mBio.00637-12 ; PubMed Central PMCID: PMCPMC3622937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukherjee PK, Mohamed S, Chandra J, Kuhn D, Liu S, Antar OS, et al. Alcohol dehydrogenase restricts the ability of the pathogen Candida albicans to form a biofilm on catheter surfaces through an ethanol-based mechanism. Infect Immun. 2006;74(7):3804–16. doi: 10.1128/IAI.00161-06 ; PubMed Central PMCID: PMCPMC1489753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finkel JS, Mitchell AP. Genetic control of Candida albicans biofilm development. Nat Rev Microbiol. 2011;9(2):109–18. doi: 10.1038/nrmicro2475 ; PubMed Central PMCID: PMCPMC3891587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banerjee M, Uppuluri P, Zhao XR, Carlisle PL, Vipulanandan G, Villar CC, et al. Expression of UME6, a key regulator of Candida albicans hyphal development, enhances biofilm formation via Hgc1- and Sun41-dependent mechanisms. Eukaryot Cell. 2013;12(2):224–32. doi: 10.1128/EC.00163-12 ; PubMed Central PMCID: PMCPMC3571304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blankenship JR, Mitchell AP. How to build a biofilm: a fungal perspective. Curr Opin Microbiol. 2006;9(6):588–94. doi: 10.1016/j.mib.2006.10.003 . [DOI] [PubMed] [Google Scholar]
- 25.Gow NA, Hube B. Importance of the Candida albicans cell wall during commensalism and infection. Curr Opin Microbiol. 2012;15(4):406–12. doi: 10.1016/j.mib.2012.04.005 . [DOI] [PubMed] [Google Scholar]
- 26.Robinson LC, Hubbard EJ, Graves PR, DePaoli-Roach AA, Roach PJ, Kung C, et al. Yeast casein kinase I homologues: an essential gene pair. Proc Natl Acad Sci U S A. 1992;89(1):28–32. ; PubMed Central PMCID: PMCPMC48168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davidson G. The cell cycle and Wnt. Cell Cycle. 2010;9(9):1667–8. doi: 10.4161/cc.9.9.11595 . [DOI] [PubMed] [Google Scholar]
- 28.Cheong JK, Virshup DM. Casein kinase 1: Complexity in the family. Int J Biochem Cell Biol. 2011;43(4):465–9. doi: 10.1016/j.biocel.2010.12.004 . [DOI] [PubMed] [Google Scholar]
- 29.Panek HR, Stepp JD, Engle HM, Marks KM, Tan PK, Lemmon SK, et al. Suppressors of YCK-encoded yeast casein kinase 1 deficiency define the four subunits of a novel clathrin AP-like complex. EMBO J. 1997;16(14):4194–204. ; PubMed Central PMCID: PMCPMC1170045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kearney PH, Ebert M, Kuret J. Molecular cloning and sequence analysis of two novel fission yeast casein kinase-1 isoforms. Biochem Biophys Res Commun. 1994;203(1):231–6. doi: 10.1006/bbrc.1994.2172 . [DOI] [PubMed] [Google Scholar]
- 31.Robinson LC, Menold MM, Garrett S, Culbertson MR. Casein kinase I-like protein kinases encoded by YCK1 and YCK2 are required for yeast morphogenesis. Mol Cell Biol. 1993;13(5):2870–81. ; PubMed Central PMCID: PMCPMC359678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Liu TB, Patel S, Jiang L, Xue C. The casein kinase I protein Cck1 regulates multiple signaling pathways and is essential for cell integrity and fungal virulence in Cryptococcus neoformans. Eukaryot Cell. 2011;10(11):1455–64. doi: 10.1128/EC.05207-11 ; PubMed Central PMCID: PMCPMC3209051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson LC, Bradley C, Bryan JD, Jerome A, Kweon Y, Panek HR. The Yck2 yeast casein kinase 1 isoform shows cell cycle-specific localization to sites of polarized growth and is required for proper septin organization. Mol Biol Cell. 1999;10(4):1077–92. ; PubMed Central PMCID: PMCPMC25234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moriya H, Johnston M. Glucose sensing and signaling in Saccharomyces cerevisiae through the Rgt2 glucose sensor and casein kinase I. Proc Natl Acad Sci U S A. 2004;101(6):1572–7. doi: 10.1073/pnas.0305901101 ; PubMed Central PMCID: PMCPMC341776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown V, Sabina J, Johnston M. Specialized sugar sensing in diverse fungi. Curr Biol. 2009;19(5):436–41. doi: 10.1016/j.cub.2009.01.056 ; PubMed Central PMCID: PMCPMC2762733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blankenship JR, Fanning S, Hamaker JJ, Mitchell AP. An extensive circuitry for cell wall regulation in Candida albicans. PLoS Pathog. 2010;6(2):e1000752 doi: 10.1371/journal.ppat.1000752 ; PubMed Central PMCID: PMCPMC2816693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park H, Liu Y, Solis N, Spotkov J, Hamaker J, Blankenship JR, et al. Transcriptional responses of Candida albicans to epithelial and endothelial cells. Eukaryotic cell. 2009;8(10):1498–510. doi: 10.1128/EC.00165-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson RB, Davis D, Mitchell AP. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J Bacteriol. 1999;181(6):1868–74. ; PubMed Central PMCID: PMCPMC93587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phan QT, Eng DK, Mostowy S, Park H, Cossart P, Filler SG. Role of endothelial cell septin 7 in the endocytosis of Candida albicans. MBio. 2013;4(6):e00542–13. doi: 10.1128/mBio.00542-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin Y, Yip HK, Samaranayake YH, Yau JY, Samaranayake LP. Biofilm-forming ability of Candida albicans is unlikely to contribute to high levels of oral yeast carriage in cases of human immunodeficiency virus infection. J Clin Microbiol. 2003;41(7):2961–7. doi: 10.1128/JCM.41.7.2961-2967.2003 ; PubMed Central PMCID: PMCPMC165379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park H, Liu Y, Solis N, Spotkov J, Hamaker J, Blankenship JR, et al. Transcriptional responses of Candida albicans to epithelial and endothelial cells. Eukaryot Cell. 2009;8(10):1498–510. doi: 10.1128/EC.00165-09 ; PubMed Central PMCID: PMCPMC2756863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Espinel-Ingroff A, Canton E, Peman J, Rinaldi MG, Fothergill AW. Comparison of 24-hour and 48-hour voriconazole MICs as determined by the Clinical and Laboratory Standards Institute broth microdilution method (M27-A3 document) in three laboratories: results obtained with 2,162 clinical isolates of Candida spp. and other yeasts. J Clin Microbiol. 2009;47(9):2766–71. doi: 10.1128/JCM.00654-09 ; PubMed Central PMCID: PMCPMC2738097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52(11):2745–56. doi: 10.1172/JCI107470 ; PubMed Central PMCID: PMCPMC302542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, et al. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000;20(4):1436–47. ; PubMed Central PMCID: PMCPMC85304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fichorova RN, Rheinwald JG, Anderson DJ. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol Reprod. 1997;57(4):847–55. . [DOI] [PubMed] [Google Scholar]
- 46.Filler SG, Swerdloff JN, Hobbs C, Luckett PM. Penetration and damage of endothelial cells by Candida albicans. Infect Immun. 1995;63(3):976–83. ; PubMed Central PMCID: PMCPMC173098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262 . [DOI] [PubMed] [Google Scholar]
- 48.Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentino D, et al. The mechanism of action of cyclosporin A and FK506. Clin Immunol Immunopathol. 1996;80(3 Pt 2):S40–5. . [DOI] [PubMed] [Google Scholar]
- 49.Saraswati S, Alfaro MP, Thorne CA, Atkinson J, Lee E, Young PP. Pyrvinium, a potent small molecule Wnt inhibitor, promotes wound repair and post-MI cardiac remodeling. PLoS One. 2010;5(11):e15521 doi: 10.1371/journal.pone.0015521 ; PubMed Central PMCID: PMCPMC2993965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu L, Zhang L, Hu C, Liang S, Fei X, Yan N, et al. WNT pathway inhibitor pyrvinium pamoate inhibits the self-renewal and metastasis of breast cancer stem cells. Int J Oncol. 2016;48(3):1175–86. doi: 10.3892/ijo.2016.3337 . [DOI] [PubMed] [Google Scholar]
- 51.Banerjee M, Thompson DS, Lazzell A, Carlisle PL, Pierce C, Monteagudo C, et al. UME6, a novel filament-specific regulator of Candida albicans hyphal extension and virulence. Mol Biol Cell. 2008;19(4):1354–65. doi: 10.1091/mbc.E07-11-1110 ; PubMed Central PMCID: PMCPMC2291399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nobile CJ, Nett JE, Andes DR, Mitchell AP. Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot Cell. 2006;5(10):1604–10. doi: 10.1128/EC.00194-06 ; PubMed Central PMCID: PMCPMC1595337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang PC, Vancura A, Mitcheson TG, Kuret J. Two genes in Saccharomyces cerevisiae encode a membrane-bound form of casein kinase-1. Mol Biol Cell. 1992;3(3):275–86. ; PubMed Central PMCID: PMCPMC275529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Babu P, Bryan JD, Panek HR, Jordan SL, Forbrich BM, Kelley SC, et al. Plasma membrane localization of the Yck2p yeast casein kinase 1 isoform requires the C-terminal extension and secretory pathway function. J Cell Sci. 2002;115(Pt 24):4957–68. . [DOI] [PubMed] [Google Scholar]
- 55.Shimada M, Yamamoto A, Murakami-Tonami Y, Nakanishi M, Yoshida T, Aiba H, et al. Casein kinase II is required for the spindle assembly checkpoint by regulating Mad2p in fission yeast. Biochem Biophys Res Commun. 2009;388(3):529–32. doi: 10.1016/j.bbrc.2009.08.030 . [DOI] [PubMed] [Google Scholar]
- 56.Koyano T, Barnouin K, Snijders AP, Kume K, Hirata D, Toda T. Casein kinase 1gamma acts as a molecular switch for cell polarization through phosphorylation of the polarity factor Tea1 in fission yeast. Genes Cells. 2015;20(12):1046–58. doi: 10.1111/gtc.12309 ; PubMed Central PMCID: PMCPMC4737401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Desai JV, Mitchell AP. Candida albicans Biofilm Development and Its Genetic Control. Microbiol Spectr. 2015;3(3). doi: 10.1128/microbiolspec.MB-0005-2014 ; PubMed Central PMCID: PMCPMC4507287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carlisle PL, Kadosh D. A genome-wide transcriptional analysis of morphology determination in Candida albicans. Mol Biol Cell. 2013;24(3):246–60. doi: 10.1091/mbc.E12-01-0065 ; PubMed Central PMCID: PMCPMC3564527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Desai JV, Cheng S, Ying T, Nguyen MH, Clancy CJ, Lanni F, et al. Coordination of Candida albicans Invasion and Infection Functions by Phosphoglycerol Phosphatase Rhr2. Pathogens. 2015;4(3):573–89. doi: 10.3390/pathogens4030573 ; PubMed Central PMCID: PMCPMC4584273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao X, Oh SH, Cheng G, Green CB, Nuessen JA, Yeater K, et al. ALS3 and ALS8 represent a single locus that encodes a Candida albicans adhesin; functional comparisons between Als3p and Als1p. Microbiology. 2004;150(Pt 7):2415–28. doi: 10.1099/mic.0.26943-0 . [DOI] [PubMed] [Google Scholar]
- 61.Argimon S, Wishart JA, Leng R, Macaskill S, Mavor A, Alexandris T, et al. Developmental regulation of an adhesin gene during cellular morphogenesis in the fungal pathogen Candida albicans. Eukaryot Cell. 2007;6(4):682–92. doi: 10.1128/EC.00340-06 ; PubMed Central PMCID: PMCPMC1865654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y, Filler SG. Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryot Cell. 2011;10(2):168–73. doi: 10.1128/EC.00279-10 ; PubMed Central PMCID: PMCPMC3067396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, Welch WH, et al. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007;5(3):e64 doi: 10.1371/journal.pbio.0050064 ; PubMed Central PMCID: PMCPMC1802757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu Y, Phan QT, Luo G, Solis NV, Liu Y, Cormack BP, et al. Investigation of the function of Candida albicans Als3 by heterologous expression in Candida glabrata. Infect Immun. 2013;81(7):2528–35. doi: 10.1128/IAI.00013-13 ; PubMed Central PMCID: PMCPMC3697595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Staab JF, Bradway SD, Fidel PL, Sundstrom P. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science. 1999;283(5407):1535–8. . [DOI] [PubMed] [Google Scholar]
- 66.Dwivedi P, Thompson A, Xie Z, Kashleva H, Ganguly S, Mitchell AP, et al. Role of Bcr1-activated genes Hwp1 and Hyr1 in Candida albicans oral mucosal biofilms and neutrophil evasion. PLoS One. 2011;6(1):e16218 doi: 10.1371/journal.pone.0016218 ; PubMed Central PMCID: PMCPMC3026825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fan Y, He H, Dong Y, Pan H. Hyphae-specific genes HGC1, ALS3, HWP1, and ECE1 and relevant signaling pathways in Candida albicans. Mycopathologia. 2013;176(5–6):329–35. doi: 10.1007/s11046-013-9684-6 . [DOI] [PubMed] [Google Scholar]
- 68.Kadosh D, Johnson AD. Induction of the Candida albicans filamentous growth program by relief of transcriptional repression: a genome-wide analysis. Mol Biol Cell. 2005;16(6):2903–12. doi: 10.1091/mbc.E05-01-0073 ; PubMed Central PMCID: PMCPMC1142434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Childers DS, Kadosh D. Filament condition-specific response elements control the expression of NRG1 and UME6, key transcriptional regulators of morphology and virulence in Candida albicans. PLoS One. 2015;10(3):e0122775 doi: 10.1371/journal.pone.0122775 ; PubMed Central PMCID: PMCPMC4374957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeidler U, Lettner T, Lassnig C, Muller M, Lajko R, Hintner H, et al. UME6 is a crucial downstream target of other transcriptional regulators of true hyphal development in Candida albicans. FEMS Yeast Res. 2009;9(1):126–42. doi: 10.1111/j.1567-1364.2008.00459.x . [DOI] [PubMed] [Google Scholar]
- 71.Carlisle PL, Kadosh D. Candida albicans Ume6, a filament-specific transcriptional regulator, directs hyphal growth via a pathway involving Hgc1 cyclin-related protein. Eukaryot Cell. 2010;9(9):1320–8. doi: 10.1128/EC.00046-10 ; PubMed Central PMCID: PMCPMC2937344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sanchez AA, Johnston DA, Myers C, Edwards JE Jr., Mitchell AP, Filler SG. Relationship between Candida albicans virulence during experimental hematogenously disseminated infection and endothelial cell damage in vitro. Infect Immun. 2004;72(1):598–601. doi: 10.1128/IAI.72.1.598-601.2004 ; PubMed Central PMCID: PMCPMC344013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Phan QT, Belanger PH, Filler SG. Role of hyphal formation in interactions of Candida albicans with endothelial cells. Infect Immun. 2000;68(6):3485–90. ; PubMed Central PMCID: PMCPMC97632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bruno VM, Kalachikov S, Subaran R, Nobile CJ, Kyratsous C, Mitchell AP. Control of the C. albicans cell wall damage response by transcriptional regulator Cas5. PLoS Pathog. 2006;2(3):e21 doi: 10.1371/journal.ppat.0020021 ; PubMed Central PMCID: PMCPMC1401495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rauceo JM, Blankenship JR, Fanning S, Hamaker JJ, Deneault JS, Smith FJ, et al. Regulation of the Candida albicans cell wall damage response by transcription factor Sko1 and PAS kinase Psk1. Mol Biol Cell. 2008;19(7):2741–51. doi: 10.1091/mbc.E08-02-0191 ; PubMed Central PMCID: PMCPMC2441657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chaffin WL, Lopez-Ribot JL, Casanova M, Gozalbo D, Martinez JP. Cell wall and secreted proteins of Candida albicans: identification, function, and expression. Microbiol Mol Biol Rev. 1998;62(1):130–80. ; PubMed Central PMCID: PMCPMC98909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Walker LA, Munro CA, de Bruijn I, Lenardon MD, McKinnon A, Gow NA. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 2008;4(4):e1000040 doi: 10.1371/journal.ppat.1000040 ; PubMed Central PMCID: PMCPMC2271054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walker LA, Lenardon MD, Preechasuth K, Munro CA, Gow NA. Cell wall stress induces alternative fungal cytokinesis and septation strategies. J Cell Sci. 2013;126(Pt 12):2668–77. doi: 10.1242/jcs.118885 ; PubMed Central PMCID: PMCPMC3687699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stevens DA, Ichinomiya M, Koshi Y, Horiuchi H. Escape of Candida from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin; evidence for beta-1,6-glucan synthesis inhibition by caspofungin. Antimicrob Agents Chemother. 2006;50(9):3160–1. doi: 10.1128/AAC.00563-06 ; PubMed Central PMCID: PMCPMC1563524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hiller E, Heine S, Brunner H, Rupp S. Candida albicans Sun41p, a putative glycosidase, is involved in morphogenesis, cell wall biogenesis, and biofilm formation. Eukaryot Cell. 2007;6(11):2056–65. doi: 10.1128/EC.00285-07 ; PubMed Central PMCID: PMCPMC2168408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lagorce A, Hauser NC, Labourdette D, Rodriguez C, Martin-Yken H, Arroyo J, et al. Genome-wide analysis of the response to cell wall mutations in the yeast Saccharomyces cerevisiae. J Biol Chem. 2003;278(22):20345–57. doi: 10.1074/jbc.M211604200 . [DOI] [PubMed] [Google Scholar]
- 82.Boorsma A, de Nobel H, ter Riet B, Bargmann B, Brul S, Hellingwerf KJ, et al. Characterization of the transcriptional response to cell wall stress in Saccharomyces cerevisiae. Yeast. 2004;21(5):413–27. doi: 10.1002/yea.1109 . [DOI] [PubMed] [Google Scholar]
- 83.Eisman B, Alonso-Monge R, Roman E, Arana D, Nombela C, Pla J. The Cek1 and Hog1 mitogen-activated protein kinases play complementary roles in cell wall biogenesis and chlamydospore formation in the fungal pathogen Candida albicans. Eukaryot Cell. 2006;5(2):347–58. doi: 10.1128/EC.5.2.347-358.2006 ; PubMed Central PMCID: PMCPMC1405885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Munro CA, Selvaggini S, de Bruijn I, Walker L, Lenardon MD, Gerssen B, et al. The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans. Mol Microbiol. 2007;63(5):1399–413. doi: 10.1111/j.1365-2958.2007.05588.x ; PubMed Central PMCID: PMCPMC2649417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Navarro-Garcia F, Sanchez M, Pla J, Nombela C. Functional characterization of the MKC1 gene of Candida albicans, which encodes a mitogen-activated protein kinase homolog related to cell integrity. Mol Cell Biol. 1995;15(4):2197–206. ; PubMed Central PMCID: PMCPMC230448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Valdivia RH, Schekman R. The yeasts Rho1p and Pkc1p regulate the transport of chitin synthase III (Chs3p) from internal stores to the plasma membrane. Proc Natl Acad Sci U S A. 2003;100(18):10287–92. doi: 10.1073/pnas.1834246100 ; PubMed Central PMCID: PMCPMC193553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Protein similarity analysis of C. albicans Yck2p, Yck3p, S. cerevisiae Yck1p, and Yck2p. (B) The protein conserved domain information of the CaYck2p (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). (C) Illustration of two-step gene disruption with HIS1 and ARG4 markers. (D) PCR amplified YCK2 alleles with YCK2 Confirm-5 and YCK2 Confirm-3 primers. Indicated strains’ genomic DNAs were amplified with YCK2 Confirm-5 and YCK2 Confirm-3 primers, run on 0.8% agarose gel with 1 Kb ladder (New England Biolabs), and imaged with UVP Gel Doc-It system (UVP, CA).
(TIFF)
Colony morphologies of the wild-type (a), the yck2Δ/yck2Δ (b), and the yck2Δ/yck2Δ+YCK2 complemented (c) strains grown on YPD with 10% serum plate at 37°C for 2 days.
(TIFF)
The extent of biofilm formation in RPMI1640, spider, YPD and YPglycerol media at 30°C and 37°C was tested by colormetric absorbance analysis at 595nm. (** p<0.01 in OneWay ANOVA with Dunnett’s test posthoc analysis).
(TIFF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.