Abstract
Bacteria require iron for growth, with only a few reported exceptions. In many environments, iron is a limiting nutrient for growth and high affinity uptake systems play a central role in iron homeostasis. However, iron can also be detrimental to cells when it is present in excess, particularly under aerobic conditions where its participation in Fenton chemistry generates highly reactive hydroxyl radicals. Recent results have revealed a critical role for iron efflux transporters in protecting bacteria from iron intoxication. Systems that efflux iron are widely distributed amongst bacteria and fall into several categories: P1B-type ATPases, cation diffusion facilitator (CDF) proteins, major facilitator superfamily (MFS) proteins, and membrane bound ferritin-like proteins. Here, we review the emerging role of iron export in both iron homeostasis and as part of the adaptive response to oxidative stress.
Graphical abstract
Introduction
Iron is critical for cell growth and survival. However, when present in excess, it is also detrimental to cells. Under aerobic conditions, iron toxicity is closely related to oxidative stress through Fenton chemistry1. Hydrogen peroxide (H2O2) reacts with ferrous iron (Fe2+) to generate highly reactive hydroxyl radicals that damage macromolecules such as DNA, proteins and fatty acids, resulting in disruption of cell metabolism and ultimately cell death2. Therefore, the toxicity of reactive oxygen species (ROS) is generally thought to be exacerbated by conditions that elevate the intracellular iron pool. Conversely, high levels of intracellular iron may also be toxic independent of ROS, presumably due to the ability of iron to compete with other transition metals, such as manganese, for binding to metal-dependent enzymes or regulators, resulting in mismetallation and inactivation of these proteins3, 4. ROS such as H2O2 and superoxide radical can disrupt iron-sulfur clusters and mononuclear iron centers of iron-enzymes, thereby leading to iron release5, 6. Therefore, iron intoxication may also be exacerbated by an elevation in ROS. Clearly, the toxicity of iron and ROS are closely intertwined, with each potentially increasing the toxicity of the other.
Bacteria adapt to environmental stresses by activation of specific transcriptional programs. In the case of iron homeostasis, bacteria monitor intracellular iron levels using metal-sensing (metalloregulatory) proteins7, 8. The ferric uptake regulator (Fur) protein is the most widespread bacterial iron sensor9, but it can be replaced by functionally analogous proteins such as IdeR (in actinomycetes)10, 11 and Irr (in alpha-proteobacteria)12–14. Fur helps to maintain iron homeostasis by regulating genes implicated in iron uptake, storage, and efflux15. Typically, Fur is considered to function as an Fe2+-activated transcriptional repressor for most of its targets, but there are increasing examples where Fur functions as a transcriptional activator or where it binds DNA in the absence of bound iron16–18.
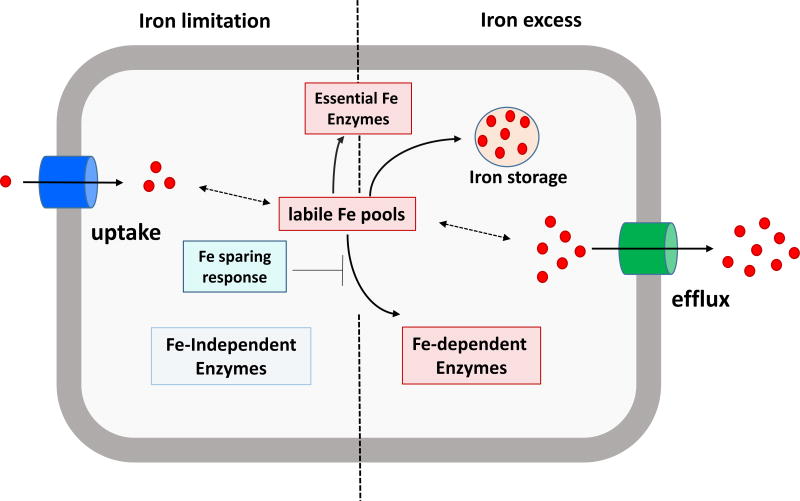
Iron-sensing regulators such as Fur play a central role in the control of iron homeostasis19. The Escherichia coli Fur regulon illustrates the diverse roles that Fur may play. E. coli Fur (FurEC) binds to DNA when associated with Fe2+ and serves to repress the expression of target operons20. This repression is relieved under iron-limited conditions, and this results in the derepression of iron uptake systems, including the synthesis of the high-affinity iron-chelating compound siderophore known as enterobactin and its cognate import system21. Fur also helps bacteria to remodel their proteomes to prioritize the utilization of iron, in a process known as "iron-sparing" (Fig. 1)22–24. In E. coli, the loss of FurEC DNA-binding activity (under low iron conditions) results in expression of the RyhB small RNA (sRNA) that represses translation of non-essential iron-enzymes22–24. Fur also participates in the regulation of gene expression under conditions of iron excess. For example, FurEC positively regulates expression of the iron storage protein ferritin by occluding the binding of the H-NS transcriptional repressor25. In general, adaptation to iron excess often involves expression of iron storage functions (including heme-containing bacterioferritins, ferritins, and Dps-family mini-ferritins) but may additionally require iron efflux systems (Fig. 1). In light of the central role of Fur in coordinating iron homeostasis, it is not surprising that some iron efflux systems are induced by Fur in response to iron excess26, 27.
fig. 1. Iron homeostasis in bacteria.
Under iron deficient conditions (left), high affinity iron uptake systems are induced to scavenge iron from the surroundings to maintain the cell's labile iron pool. when iron is limiting, it is selectively partitioned to the most essential functions and incorporation into lower priority iron enzymes is translationally inhibited as part of an iron sparing response. in many cases, iron-independent enzymes may be derepressed to replace functions that would otherwise depend on iron. under iron excess conditions, the cell will have a full complement of iron-requiring enzymes, and iron in excess of immediate needs will be either stored for future use or exported by fe2+ efflux transporters to prevent iron overload.
Bacteria also adapt to oxidative stress by the induction of specific defensive genes. For example, H2O2 induces a specific peroxide-stress response that is regulated by the OxyR repressor in E. coli28 and by the PerR repressor in Bacillus subtilis29. In both model organisms, a rise in intracellular H2O2 triggers the induction of defensive enzymes such as catalase and alkyl hydroperoxide reductase, which can directly detoxify H2O2. In addition, cells scavenge excess iron from the cytosol by sequestration into mini-ferritin proteins, including Dps in E. coli30 and the Dps ortholog MrgA in B. subtilis31. The co-regulation of H2O2 degradation enzymes and iron-sequestering proteins further highlights the central role of iron in peroxide intoxication. In addition to scavenging iron, peroxide stress also frequently modulates metal uptake and efflux systems32. In E. coli, H2O2 induces an OxyR-activated Mn2+ uptake system (MntH)33, 34, and in B. subtilis H2O2 induces a PerR-regulated iron efflux system, PfeT35, 36. PfeT is a member of the P1B4-type ATPases, and recent results indicate that several close homologs also function as Fe2+ efflux pumps27, 37–39. Fe2+ efflux pumps have now been documented in a wide variety of bacteria, and include P1B-type ATPases, cation diffusion facilitator (CDF) proteins, major facilitator superfamily (MFS) proteins, and membrane bound ferritin-like proteins (Table 1 & Fig. 2). Here, we summarize the emerging role of these ferrous iron efflux pumps in helping ameliorate the deleterious effects of excess iron and peroxide.
Table 1.
Fe2+ efflux transporters in bacteria.
| Protein | Organism | Function | Category | Substrate specificity* |
Transcription regulation | References |
|---|---|---|---|---|---|---|
| PfeT | Bacillus subtilis | Fe2+ efflux | P1B4-type ATPase | Fe2+ a, b, Co2+ a | PerR & Fur | 36 |
| FrvA | Listeria monocytogenes | Fe2+ efflux | P1B4-type ATPase | Fe2+ a, b, Co2+ a, Zn2+ a | PerR & Fur | 27 |
| CtpD | Mycobacterium tuberculosis | Fe2+ efflux | P1B4-type ATPase | Fe2+ a, b, Co2+ a | Unknown | 37 |
| PmtA | group A Streptococcus | Fe2+ efflux | P1B4-type ATPase | Fe2+ b | PerR | 38, 39 |
| Nia | Sinorhizobium meliloti | Fe2+ or Ni2+ efflux | P1B5-type ATPase | Fe2+ a, b, Ni2+ a, b | Unknown | 73 |
| FieF (YiiP) | Escherichia coli | Fe2+ or Zn2+ efflux | CDF family | Fe2+ b, Zn2+ a, Cd2+ a | Unknown | 92–94 |
| AitP | Pseudomonas aeruginosa | Fe2+ or Co2+ efflux | CDF family | Fe2+ b | Unknown | 97 |
| FeoE | Shewanella oneidensis | Fe2+ efflux | CDF family | Fe2+ b | Unknown | 101 |
| IceT | Salmonella typhimurium | Fe2+ citrate or citrate efflux | MFS family | Fe2+ b | BaeSR | 120 |
| MbfA | Agrobacterium tumefaciens | Fe2+ efflux | Membrane bound ferritin | Fe2+ b | Irr | 125 |
| MbfA | Bradyrhizobium japonicum | Fe2+ efflux | Membrane bound ferritin | Fe2+ b | Irr | 127 |
Note: the substrate specificity of the transporters is either based on biochemical measurements (a), inferred from physiology studies (b), or both (a, b).
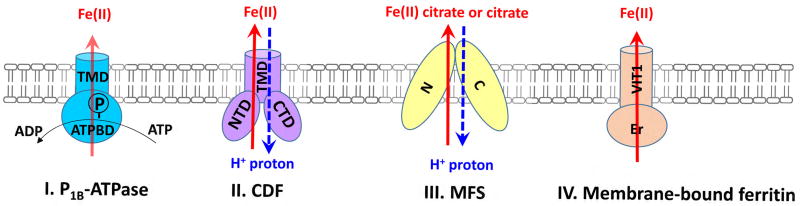
fig. 2. Ferrous iron efflux systems in bacteria.
Four different groups of transporters can function as fe2+ efflux pumps. i. p1b-atpase; ii. cation diffusion facilitator (cdf); iii. major facilitator superfamily (mfs); iv. membrane-bound ferritin. a typical p1b-atpase consists of a transmembrane domain (tmd) that has 6–8 helices, a soluble actuator domain (not shown), and an atp-binding domain (atp-bd)52. a cdf transporter contains a n-terminal domain (ntd), a transmembrane domain (tmd) that has 6 helices, a histine-rich interconnecting loop (il) between tm4 and tm5 (not shown), and a c-terminal cytoplasmic domain (ctd)75. the common structural fold (mfs fold) of a mfs transporter is composed of two distinct domains, n domain and c domain. each domain has six consecutive transmembrane helices103. a membrane-bound ferritin transporter has two major domains, n-terminal ferritin-like or er domain (er) and c-terminal membrane-embedded vacuolar iron transporter domain (vit1).
P-type ATPases
The P-type ATPases are a large group of transmembrane proteins that transport ions and lipids across cellular membranes, energetically driven by ATP hydrolysis40. Five subgroups of P-type ATPases have been defined based on sequence homology and substrate specificity41. These are the P1-type (K+ and transition metal transporters), P2-type (Ca2+, Na+/K+, and H+/K+ pumps), P3-type (H+ pumps), P4-type (phospholipid transporters), and P5-type ATPases (unknown substrate). The P2-type ATPases have been well studied and are more prevalent in eukaryotes than in prokaryotes. The majority of P3-type ATPases are H+ pumps found in plants and fungi. Some of the P4-type ATPases have been revealed to be phospholipid transporters42, 43. No specific substrate has yet been identified for the P5-type ATPases that are only found in eukaryotes.
The P1-type ATPases exist predominately in prokaryotes but are omnipresent across all domains of life44: P1A-ATPases are involved in K+ transport whereas P1B-ATPases are important for maintaining transition metal homeostasis. P1B-ATPases are known to transport Cu+ 45, 46, Ag+ 47, Zn2+ 48, Cd2+ 49, Cu2+ 50, Co2+ 51 and Fe2+ 27, 36, 37. The structure of a typical P1B-ATPase includes a transmembrane domain with 6–8 helices, a soluble actuator domain, and an ATP-binding domain52 (Fig. 2). The P1B-ATPases can be further divided into seven subclasses based on sequence similarity and metal substrate specificity52. The P1B4-type ATPases were originally assigned a role in Co2+ export, based on the properties of some of the first characterized members53. However, P1B4-type ATPases have recently been found to function instead, or in addition, as Fe2+ efflux transporters including Bacillus subtilis PfeT36, Listeria monocytogenes FrvA27, Mycobacterium tuberculosis CtpD37, and group A Streptococcus PmtA38, 39.
PfeT in Bacillus subtilis
B. subtilis is a Gram-positive soil microorganism and encodes two transcriptional regulators critical for iron homeostasis, FurBs and PerR. FurBs is a global transcriptional regulator of iron homeostasis analogous to FurEC54 and PerR mediates the adaptive response to peroxide stress by regulating genes involved in iron storage and peroxide detoxification29. The regulons for both FurBs and PerR have been well defined55, 56. FurBs senses intracellular iron sufficiency and represses genes that are involved in siderophore synthesis and uptake54, 57. FurBs also regulates an iron sparing response mediated by the small non-coding RNA FsrA (Fig. 1) and its coregulators FbpA, FbpB and FbpC58–60. This system, analogous to RyhB in E. coli, blocks the translation of non-essential iron-containing enzymes such as aconitase and succinate dehydrogenase58–60. PerR regulates peroxide detoxification enzymes (catalase, alkyl hydroperoxide reductase), iron sequestration (MrgA) and the P1B4-type ATPase (PfeT). Although the Fur and PerR regulons are largely non-overlapping, pfeT is the exception and is regulated by both proteins26. The result is that pfeT is induced by either peroxide stress or by iron excess (unpublished data, Pinochet-Barros A & Helmann JD).
PfeT is one of three P1B ATPases encoded by B. subtilis. CopA is a P1B1-ATPase that functions as a Cu+ efflux transporter and, appropriate to its function, is regulated by the CsoR Cu+ sensor61. CadA is a P1B2-ATPase that confers resistance to Cd2+, Zn2+, and Co2+ and is regulated by the divalent cation sensor CzrA62. PfeT (formerly named as ZosA) is a P1B4-type ATPase and was discovered as a transporter induced by H2O2 that plays a role in protecting cells against oxidative stress35. Initial results indicated that deletion of pfeT enhanced Zn2+ tolerance, as monitored in cells lacking the CadA efflux system35. This led to the proposal that PfeT might function as a Zn2+ importer under oxidative stress conditions, consistent with the idea that Zn2+ has a role in protecting cells against oxidative damage35. As a result, PfeT was originally named for this proposed role as ZosA (Zn2+ uptake under oxidative stress)35.
Contrary to this model, most P1B-type ATPases function in metal export rather than import, which motivated a reinvestigation of the role of PfeT. Further study revealed that a pfeT null mutant is sensitive to Fe2+ and Fe3+, particularly under acidic media conditions, but not to Zn2+ or Co2+. Moreover, a pfeT null mutant accumulates elevated levels of intracellular Fe2+, as judged by sensitivity to the Fe2+-activated antibiotic streptonigrin and by direct chemical measurement36. Biochemical studies confirmed that the ATPase activity of PfeT is induced the most by Fe2+, with modest induction by Co2+ but not with other metals, including Zn2+. In addition to H2O2, pfeT is strongly and specifically induced by iron, but not by other metals. Together, these findings indicate that PfeT function as a peroxide- and iron induced ferrous efflux transporter36. The ability of PfeT to protect against H2O2 is secondary to that of the detoxification enzymes catalase and alkyl hydroperoxide reductase. However, PfeT plays a dominant role in protecting cells from iron overload with the MrgA miniferritin playing a secondary role36. The revelation that PfeT functions in Fe2+ efflux, in turn, prompted a re-evaluation of the roles of several other P1B4-type ATPases in bacterial iron homeostasis.
FrvA in Listeria monocytogenes
L. monocytogenes is the causative agent of the foodborne disease listeriosis, which is associated with central nervous system infections and bacteraemia. FrvA (Lmo0641) is a P1B4-ATPase originally described as a Fur-regulated virulence factor63. FrvA was proposed to function as a heme exporter that was suggested to be induced by iron deficiency and to be under negative regulation of both Fur and PerR63, 64. However, a different transcriptome study showed a downregulation of frvA in a fur null mutant65, indicating a positive regulatory role of Fur in frvA expression.
To resolve these contradictory reports of iron regulation, and to test if FrvA might function in Fe2+ efflux, the mutant phenotype was reinvestigated and the FrvA protein was purified for biochemical studies27. As predicted based on studies of pfeT, a frvA null mutant was sensitive to iron intoxication, but not to other metals or heme. Like B. subtilis pfeT, frvA is positively regulated by Fur in response to high Fe2+ levels and is repressed by PerR27, 64. Biochemical studies indicate the FrvA ATPase activity is stimulated most strongly by Fe2+ with weaker stimulation in the presence of Co2+ or Zn2+. Based on the Fe2+ concentration dependence of ATPase activity, FrvA seems to have a higher affinity for Fe2+ than B. subtilis PfeT. Consistent with this, not only does FrvA complement the iron-sensitive phenotype of a B. subtilis pfeT null mutant, its expression depletes the cytosol of iron (even under iron-rich conditions) thereby leading to derepression of the Fur regulon27. These results support the hypothesis that FrvA functions as a Fe2+ efflux transporter that protects cells from Fe2+ intoxication27.
FrvA is required for virulence in murine and insect (Galleria mellonella) infection models63. The frvA null mutant strain shows strong attenuation in virulence, but is still able to invade and propagate inside antigen-presenting cells66, suggesting an important link between iron homeostasis and virulence, but it is not clear at which stage(s) of the L. monocytogenes life cycle FrvA is important. The phagocytic vacuole is generally considered to be an iron-limited environment. One possibility is that the expression of high affinity iron uptake systems by iron limitation during infection or in the phagocytic vacuole can contribute to iron overload upon escape of cells into the relatively iron-rich cytosol. Alternatively, the imposition of oxidative damage from host immune cells may trigger iron release from listerial iron enzymes and this may lead to iron overload. The points in the infection cycle where FrvA plays a critical role are not yet clearly defined and provide an interesting avenue for future research.
CtpD in Mycobacterium tuberculosis
M. tuberculosis is an obligate pathogen and the causative agent of human tuberculosis. Nearly one-third of the world's population is infected with M. tuberculosis, which can persist in a latent state for decades and then later emerge (in ~10% of cases) as an active lung infection. M. tuberculosis encodes a total of 11 P-type ATPases, which have been suggested to be possible targets for therapeutic intervention67. Of these, two encode P1B4-ATPases: CtpD (Rv1469) and CtpJ (Rv3743)37. CtpD, but not CtpJ, was found to be important for survival in macrophages and the mouse lung37. Biochemical studies had previously highlighted the activity of these two P1B4-ATPases with Co2+, but it was not clear why M. tuberculosis would encode two such proteins, nor was it understood why Co2+ efflux would be important for survival in the host.
In light of the finding that PfeT functions as an Fe2+ efflux transporter, the roles of CtpD and CtpJ were reinvestigated. Biochemical studies indicated that the ATPase activity of CtpD is most strongly activated by Fe2+. Although Co2+ also activates ATPase activity, the maximal activity (Vmax) is 10-fold lower than with ferrous iron37. CtpD also binds Fe2+ with 3-fold higher affinity than Co2+. In contrast, the CtpJ ATPase activity is activated by both Fe2+ and Co2+, and has a slightly higher affinity for Co2+ than Fe2+. To better understand their roles in vivo, metal accumulation and sensitivity was monitored for strains lacking either ctpD or ctpJ. The ctpD mutant strain did not accumulate Co2+ and was impaired in growth in iron-amended medium, consistent with a primary role in resistance to iron intoxication37. Mutation of ctpJ led to a significant increase in Co2+ accumulation and expression was induced by Co2+, consistent with a primary role in Co2+ resistance37. However, the ctpJ mutant was also growth impaired in the presence of excess iron. Thus, these two paralogous transporters seem to have overlapping metal selectivity, but largely distinct physiological roles. Further studies are needed to understand the molecular mechanism of substrate specificity, but based on X-ray absorption spectroscopy (XAS) analysis, it is likely that distinct metal coordination geometry plays an important role37.
During infection, M. tuberculosis propagates in the host macrophages, which are considered iron-poor environments. Just as noted for L. monocytogenes, it is not yet clear where in the infection process cells experience iron intoxication. Further studies are needed to better understand the conditions that lead to induction of ctpD. In prior work ctpD was not induced by metals such as Co2+, Zn2+, and Ni2+, but its cognate substrate Fe2+ was not tested68. It might be induced by Fe2+ and, by analogy with its orthologs, this might involve an iron-sensing transcription factor. IdeR, a member of DtxR family, is the major iron-dependent transcriptional regulator in M. tuberculosis10, 11. IdeR represses transcription of genes involved in iron uptake and siderophore biosynthesis and activates expression of genes encoding iron-storage proteins such as bacterioferritin and a ferritin-like protein10, 11. Since M. tuberculosis is primarily a pathogen of the mammalian respiratory system it might frequently encounter oxidative stress. Thus, it is also possible that ctpD might be induced in response to H2O2 stress. Future work to monitor the expression of ctpD in vitro in response to specific stresses and in vivo during the course of infection will be needed to elucidate the physiological role of CtpD during the infection process.
PmtA in group A Streptococcus
Group A Streptococcus (GAS), a human pathogen, is the causative agent of a wide range of diseases, from mild skin infection to life-threatening diseases such as necrotizing fasciitis69. GAS encodes a P1B4-type ATPase under regulation of PerR, and was therefore named a PerR-regulated metal transporter (PmtA). In a perR null mutant, high level expression of pmtA is associated with derepression of genes normally responsive to cellular zinc status due to repression by AdcR70, a Zn2+-dependent repressor. This simplest interpretation of this result is that PmtA may function as a Zn2+ efflux transporter. Consistent with this notion, a perR null mutant has an increased resistance to Zn2+, and this depends on PmtA70. However, it is unclear why cells would efflux Zn2+ in response to H2O2 stress, nor is there any evidence that PmtA is important for Zn2+ resistance in wild-type cells, which presumably relies on the Zn2+-inducible CzcD efflux pump to ameliorate Zn2+-toxicity.
By analogy with PfeT and its orthologs, an alternative interpretation is that the primary role of PmtA is as a H2O2-inducible Fe2+-efflux pump and that activity with Zn2+ may only be revealed when it is constitutively overexpressed in a perR null mutant. Two recent studies have confirmed the primary role of PmtA as an Fe2+-efflux pump38, 39. PmtA is important for resistance to iron intoxication, and a pmtA null mutant accumulates elevated levels of intracellular iron. As expected, expression of pmtA is strongly induced by Fe2+. Although a pmtA null mutant shows similar sensitivity to peroxide stress as a wild type strain in the absence of excess Fe2+, it exhibits significantly increased susceptibility to peroxide stress when treated with Fe2+. Since GAS is catalase negative, PmtA might be a frontline defense against peroxide stress. PmtA is also a critical virulence factor and is required for survival during infection in both intramuscular and subcutaneous mouse models38, which again links iron efflux and peroxide resistance to pathogen virulence.
Nia in Sinorhizobium meliloti
In addition to the P1B4-ATPases featured above, it is possible that P1B-ATPases of other groups may also have physiologically relevant activity with iron. One example is Nia, a P1B5-ATPase with a C-terminal hemerythrin domain. Since hemerythrin domains bind O2 via a diiron active site, this suggests a possible role in O2-sensing71, 72. Nia is encoded by the symbiotic plasmid A of Sinorhizobium meliloti, a nitrogen fixing microbe in the Rhizobiales lineage that has a symbiotic relationship with legumes in which it establishes nodules associated with roots.
Consistent with a possible role in Fe2+ efflux, a nia null mutant accumulates Fe2+ under excess metal conditions73. However, Nia also functions with Ni2+ and a nia null mutant accumulates Ni2+ when in excess. The precise physiological role of Nia is not yet resolved. Biochemical assays suggest that Nia interacts with both Fe2+ and Ni2+ (but not Co2+). However, a nia null mutant showed moderate sensitivity to Ni2+, but not to Fe2+, under the conditions tested73. Expression of nia was moderately induced by Fe2+ (3-fold), Ni2+ (3-fold), and Co2+ (2-fold), but not by other metals. Interestingly, nia was most strongly induced (20-fold) in root nodules, thought to be a microaerobic, iron-rich environment74. These results lead to a model in which Nia is expressed in nitrogen-fixing root nodules, in response to either iron excess or microaerobic conditions. The C-terminal hemerythrin domain may also participate in or regulate transport activity, perhaps in response to O273. More work needs to be done to characterize the details of nia gene regulation and to more clearly define the physiological role of Nia during the S. meliloti -plant symbiosis.
Cation diffusion facilitator (CDF) proteins
Cation diffusion facilitators (CDFs) are a family of membrane-bound proteins that export and thereby confer tolerance to heavy metal ions75, 76. CDF proteins are ubiquitous in bacteria, archaea, and eukaryotes77. Collectively, bacterial CDF proteins have been implicated in transport of a wide range of metal ions (Zn2+, Cd2+, Co2+, Ni2+, Fe2+ and Mn2+) with some transporters able to transport multiple metals78–82. Phylogenetic analysis of the CDF transporters defines three major groups corresponding to substrate specificity: 1) manganese efflux, 2) iron/zinc efflux, 3) zinc and other metals (but not manganese or iron) efflux83.
A typical bacterial CDF contains an N-terminal domain (NTD), 6 transmembrane helices (TM), a histidine-rich interconnecting loop (IL) between TM4 and TM5, and a C-terminal cytoplasmic domain (CTD)75 (Fig 2). However, the detailed mechanisms of metal selectivity are unknown. Some studies suggest the cytoplasmic domain or the IL loop is important for metal specificity84–86, but other studies highlight the role of residues in the TM3 helix on metal selectivity87. For the E. coli FieF transporter, evidence supports a role for a tetrahedral metal-binding site formed between TM2 and TM5 in metal selectivity88. So far, there is no unifying model that can account for metal selectivity of CDF proteins.
FieF in E. coli: Zn2+ vs. Fe2+ efflux
There are two CDF transporters in E. coli: ZitB and FieF (also named as YiiP). ZitB is the secondary zinc efflux system that is critical for maintaining zinc homeostasis only when the zinc efflux ATPase ZntA is absent89. FieF has been studied for more than a decade, but its physiological function has been controversial. In 2004, the first two reports of its structural analysis were built on the assumption that FieF acts as a zinc efflux protein90, 91. In fact, prior studies had demonstrated that fieF is induced by either zinc or iron89. However, ectopic expression of FieF does not restore zinc tolerance in a zinc-sensitive strain, suggesting it might not play a role in zinc homeostasis89.
Physiological studies suggest that the major physiological role of FieF may be in iron tolerance. Indeed, FieF is important for full resistance to iron intoxication in a fur null mutant, where iron homeostasis is disrupted and iron uptake systems are constitutively expressed92. Ectopic expression of FieF leads to reduced accumulation of iron in a fieF null mutant. Moreover, reconstitution of FieF in proteoliposomes showed that it mediates iron transport in vitro92. These results all support the assignment of FieF (ferrous iron efflux) as an iron efflux transporter. However, this notion has been challenged by others. For example, FieF was shown to selectively bind zinc and cadmium with high affinity, but not iron or other metals tested93. Based on the site-directed fluorescence resonance energy transfer (FRET) measurements, Lu et al. proposed an autoregulation model of transport activity in response to intracellular zinc levels94. Currently, FieF (YiiP) is referred to as a Zn2+ transporter in most published papers.
Ever since its structure was solved in 200779, FieF has been considered as a prototype for bacterial CDF proteins, which makes it more frustrating that its physiological role has remained controversial. The regulation of fieF expression has not been well defined, but it does not appear to be regulated by Fur92. The physiological studies of FieF are certainly supportive of a role in Fe(II) efflux. This inference is further supported by the observation that the FieF homologs MamM and MamB form a heterodimeric CDF protein required for Fe(II) import into vesicles in support of magnetosome formation in the magnetotatic bacterium Magnetospirillum gryphiswaldense95, 96.
AitP in Pseudomonas aeruginosa
Pseudomonas aeruginosa is Gram-negative, opportunistic pathogen that is highly antibiotic resistant. P. aeruginosa encodes three paralogous CDF efflux systems: CzcD (PA0397), AitP (PA1297), and YiiP (PA3963). Of these, the alternative iron transport protein (AitP) most likely functions physiologically in Fe2+ efflux. Deletion of aitP leads to an increased sensitivity to both Fe2+ and Co2+, increased intracellular accumulation of both ions, and decreased survival in presence of H2O297. The observed sensitivity to H2O2 is most consistent with a role in Fe2+ efflux, as noted above for P-type ATPases. In contrast with AitP, the CzcD and YiiP proteins were inferred to function physiologically in Zn2+ resistance, although this role is largely masked in wild-type cells by the activity of the Zn2+ efflux P-type ATPase, ZntA98. All the three transporters are critical for virulence in a plant infection model97. However, it remains unclear why this organism requires multiple classes of Zn2+ efflux proteins or under what conditions the three proteins are physiologically important during the infection process.
FeoE in Shewanella oneidensis MR-1
Shewanella oneidensis MR-1 is a facultative anaerobe in the γ-proteobacterium family that is capable of respiration using metals (e.g. manganese, lead, uranium and ferric iron) as electron acceptors99. S. oneidensis cells are usually pink or red, reflective of a high iron content in hemoproteins and cytochromes100. When Fe3+ is used as a terminal electron acceptor, cells generate a large amount of soluble Fe2+ which could potentially lead to iron intoxication. FeoE, a CDF protein, is required for cell growth during anaerobic iron respiration, and deletion of feoE increased susceptibility to Fe2+ intoxication, consistent with a physiological role in Fe2+ efflux101. Further work is required to understand how feoE expression is regulated. It is unclear, for example, whether feoE is induced in response to excess iron. Fur is the primary regulator that modulates iron acquisition in S. oneidenis102, and is a candidate for an iron-responsive transcription factor that could be involved.
Major facilitator superfamily (MFS)
The major facilitator superfamily (MFS) of membrane transporters function with a wide scope of small molecules such as ions, nucleosides, amino acids, small peptides, and lipids103. They can be categorized into three groups: uniporters that transport a single substrate, symporters that transport a substrate coupled with another ion (generally a proton), and antiporters that transport two substrates in opposite directions104, 105. All the MFS transporters share a canonical structural fold composed of two distinct domains [Fig. 2], each consisting of six transmembrane helices. The substrate binding site is located at the interface between these two domains103.
The mechanism of transport by MFS proteins is not clear, but several related models have been proposed. The first, an alternate-access model, was proposed more than five decades ago106. This model speculates that the transporters undergo a conformational change that alternates between a form where substrate can bind from one side of the membrane to one where it can only bind from the other side. This has been validated by many structural studies such as the xylose/H+ symporter XylE and for LacY107–109. The second, a rocker-switch model, postulates that conformational changes are accomplished through rocker-switch-type rotation between the N and C domain. This model is supported by some open-conformation structures110 but not by the structures in occluded states111–114. A third, clamp-and-switch model, provides a two-step transport mechanism: a clamping step that mediates occlusion of the binding site and a switching step that mediates the exposure of the binding site. This model postulates four conformational states: inward open, outward open, inward-facing occlusion, and outward-facing occlusion105. This model is in a good agreement with studies of some MFS transporters115, 116, but more structural analyses combined with biochemical and computational analyses are needed to further understand the transport mechanism of MFS transporters.
IceT (iron and citrate efflux transporter) in Salmonella Typhimurium
Salmonella Typhimurium is a Gram-negative pathogen commonly found in the gastrointestinal tract. IceT (MdtD) is a member of the MFS superfamily in S. Typhimurium. The mdtABCD baeSR operon encodes IceT and two other systems: a RND (resistance-nodulation-division) drug efflux system MdtABC and a two-component regulatory system BaeSR that regulates antibiotic resistance and efflux117–119. IceT is proposed to be an iron-citrate efflux transporter and it can export either iron citrate or citrate alone120. The iceT null mutant shows increased susceptibility to the antibiotic streptonigrin (SN), the activity of which is modulated by the level of intracellular free iron121. This result suggests that the mutation of iceT leads to an increase in intracellular labile iron pools. Consistent with this result, induction of IceT expression leads to reduced levels of intracellular iron120.
Although the mdtABCD baeSR operon is not induced directly by high Fe2+ 122, it is induced by disruption of iron homeostasis in a fur null mutant where iron uptake systems are constitutively expressed, supportive of a physiological role for IceT in iron efflux. Although IceT confers resistance to peroxide stress in a fur null mutant, the mdtABCD baeSR operon is not induced by H2O2 or superoxide-generating reagents such as paraquat120. However, it is induced by nitric oxide, which is also known to interact with the labile iron pool120. The significance of the regulation of IceT, together with its co-transcribed ABC transporter, by the BaeSR two-component system is not understood, nor is it yet clear whether or not IceT is important for pathogenesis.
Membrane bound ferritin A (MbfA) in Agrobacterium tumefaciens and Bradyrhizobium japonicum
Agrobacterium tumefaciens belongs to the Rhizobiales lineage and is the causative agent of the economically important plant disease, crown gall. MbfA was originally described as membrane-bound ferritin A, and is a member of the erythrin-vacuolar iron transport (Er-VIT1) ferritin-like superfamily. MbfA has two major domains: an N-terminal ferritin-like or Er domain (Er) and a C-terminal membrane-embedded vacuolar iron transporter domain (VIT1) (Fig. 2). The Er domain has a di-iron binding site and the VIT1 domain shows sequence homology to Arabidopsis VIT1, which is responsible for transferring iron into vacuoles123. Ferritin is a cytosolic iron storage protein ubiquitous in prokaryotes and eukaryotes124, however, MbfA is not a bona fide ferritin and its physiological function was not immediately apparent.
Plant hosts often produce reactive oxygen species as a defense mechanism in response to microbial infection. Initial studies revealed that MbfA confers resistance to H2O2 stress, suggesting that it may play an important role in plant-pathogen interaction125. Moreover, mbfA expression was induced in response to high iron conditions as sensed by the iron response regulator protein, Irr125. However, these results could not distinguish between a role for MbfA in sequestration of iron (through its ferritin domain) or iron efflux. A follow up study revealed that MbfA is important for resistance to iron intoxication under acidic conditions (pH 5.5), which enhances iron solubility thereby promoting toxicity126. Compared to wild-type, an mbfA null mutant had a modest increase in intracellular total iron as well as labile iron125. Since its expression is induced by high iron under acidic conditions125, and leads to reduced intracellular iron levels, MbfA was postulated to function as an iron efflux transporter125.
Bradyrhizobium japonicum also encodes an MbfA protein implicated in iron efflux127. B. japonicum is a nitrogen-fixing endosymbiotic microbe that, like A. tumefaciens, belongs to the Rhizobiales lineage. As in A. tumefaciens, iron homeostasis in B. japonicum is also under control of Irr128, which regulates iron uptake, storage, and utilization129. MbfA in B. japonicum is specifically induced by high iron and confers resistance to iron intoxication and H2O2 stress. Moreover, an mbfA null mutant accumulates significantly high levels of iron.
Collectively, these data support the idea that MbfA functions physiologically as an iron efflux transporter127. Interestingly, the N-terminal ferritin-like domain located on the cytoplasmic side of inner membrane is required for iron transport activity and stress resistance. The purified ferritin domain forms a dimer in solution, which suggests that MbfA may dimerize to form a functional channel127. By mediating the efflux of Fe2+, MbfA functions cooperatively with bacterioferritin (Bfr), which functions in iron sequestration, to prevent iron intoxication130. Mutation of either mbfA or bfr increases Fe2+ sensitivity, but a double mbfA bfr mutant is extremely sensitive to iron130.
Conclusions
Efflux systems play a central role in the resistance of bacteria to heavy metals, but their role in iron homeostasis has been relatively slow to emerge. This is perhaps a reflection of the fact that iron limitation is a far more prevalent challenge for bacteria than iron intoxication131, due in part to the very low solubility of iron under aerobic conditions of near neutral pH. Recent results, however, have greatly expanded our appreciation of the central importance of iron efflux systems and their contribution to virulence in human pathogens27, 37, 38. This implies that iron intoxication imposes a selective pressure during infection, although how this arises is not yet clear. For example, iron intoxication may arise from an uncontrolled influx of iron into the cell from the outside. Indeed, it is thought that macrophages impose Zn2+ and Cu+ toxicity on engulfed bacteria by import of metals into the phagolysosome132. However, iron is not known to be imported into the phagocytic vacuole. Iron overload may also result when bacteria exposed to an iron limited environment, and therefore expressing high affinity uptake systems, transition to an iron-rich environment. The sudden influx of iron may then be best accommodated by storage or efflux. Alternatively, or in addition, iron intoxication may arise from within the cell. For example, oxidative stress may lead to the release of iron from abundant iron-sulfur and mononuclear iron enzymes, thereby leading to an increase in cytosolic iron levels.
Iron intoxication may also be present in specific environments. For example, acidophilic bacteria grow in low pH environments where iron concentrations may be 1018 times higher than that found in pH neutral environments133. In the case of iron-respiring bacteria, high local concentrations of Fe2+ may be produced by reduction of Fe3+-containing minerals101. Further work is needed to better define the prevalence of iron intoxication in natural environment settings and the role of iron efflux in these environments.
With the identification of the several families of iron efflux systems noted here, the stage is now set for further structural, biochemical and genetic studies to address their mechanisms of metal selectivity. It is presently unclear how these efflux transporters discriminate Fe2+ from competing substrates and how, at a structural level, efflux is coupled to substrate binding and energy consumption. It is also unclear why some cells rely on ATP-dependent P-type transporters and others utilize CDF proteins, which are coupled to the proton motive force. It is notable that in several cases efflux pumps were initially assigned a role for substrates others than Fe2+ (PfeT, FrvA, CtpD), and in other cases (FieF, Nia) the most relevant physiological substrate is still unclear. This highlights the fact that metal selectivity cannot be easily predicted from protein sequence alone, and biochemical assays need to be interpreted in context of the physiology of the organisms. In several of the cases described, the most compelling evidence to assign function has emerged from a careful analysis of mutant phenotypes combined with detailed analysis of regulation to infer those conditions that specifically induce expression.
Supplementary Material
Acknowledgments
We thank Pete Chandrangsu for helpful comments. This work was supported by a grant from the NIH (GM059323) to JDH.
References
- 1.Imlay JA. Pathways of oxidative damage. Annual review of Microbiology. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 2.Park S, You X, Imlay JA. Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in Hpx-mutants of Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9317–9322. doi: 10.1073/pnas.0502051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imlay JA. The mismetallation of enzymes during oxidative stress. The Journal of biological chemistry. 2014;289:28121–28128. doi: 10.1074/jbc.R114.588814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barwinska-Sendra A, Waldron KJ. Advances in Microbial Physiology. Academic Press; DOI: http://doi.org/10.1016/bs.ampbs.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Anjem A, Imlay JA. Mononuclear iron enzymes are primary targets of hydrogen peroxide stress. The Journal of biological chemistry. 2012;287:15544–15556. doi: 10.1074/jbc.M111.330365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang S, Imlay JA. Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. The Journal of biological chemistry. 2007;282:929–937. doi: 10.1074/jbc.M607646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waldron KJ, Rutherford JC, Ford D, Robinson NJ. Metalloproteins and metal sensing. Nature. 2009;460:823–830. doi: 10.1038/nature08300. [DOI] [PubMed] [Google Scholar]
- 8.Chandrangsu P, Rensing C, Helmann JD. Metal homeostasis and resistance in bacteria, Nature reviews. Microbiology. 2017 doi: 10.1038/nrmicro.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleischhacker AS, Kiley PJ. Iron-containing transcription factors and their roles as sensors. Current opinion in chemical biology. 2011;15:335–341. doi: 10.1016/j.cbpa.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez GM, Voskuil MI, Gold B, Schoolnik GK, Smith I. ideR, An essential gene in Mycobacterium tuberculosis: role of IdeR in iron-dependent gene expression, iron metabolism, and oxidative stress response. Infection and immunity. 2002;70:3371–3381. doi: 10.1128/IAI.70.7.3371-3381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sritharan M. Iron Homeostasis in Mycobacterium tuberculosis: Mechanistic Insights into Siderophore-Mediated Iron Uptake. Journal of bacteriology. 2016;198:2399–2409. doi: 10.1128/JB.00359-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodionov DA, Gelfand MS, Todd JD, Curson AR, Johnston AW. Computational reconstruction of iron- and manganese-responsive transcriptional networks in alpha-proteobacteria. PLoS computational biology. 2006;2:e163. doi: 10.1371/journal.pcbi.0020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Todd JD, Sawers G, Rodionov DA, Johnston AW. The Rhizobium leguminosarum regulator IrrA affects the transcription of a wide range of genes in response to Fe availability. Molecular genetics and genomics: MGG. 2006;275:564–577. doi: 10.1007/s00438-006-0115-y. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Sangwan I, Lindemann A, Hauser F, Hennecke H, Fischer HM, O'Brian MR. Bradyrhizobium japonicum senses iron through the status of haem to regulate iron homeostasis and metabolism. Molecular Microbiology. 2006;60:427–437. doi: 10.1111/j.1365-2958.2006.05101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helmann JD. Specificity of metal sensing: iron and manganese homeostasis in Bacillus subtilis. The Journal of biological chemistry. 2014;289:28112–28120. doi: 10.1074/jbc.R114.587071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo SW, Kim D, Latif H, O'Brien EJ, Szubin R, Palsson BO. Deciphering Fur transcriptional regulatory network highlights its complex role beyond iron metabolism in Escherichia coli. Nature communications. 2014;5:4910. doi: 10.1038/ncomms5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delany I, Rappuoli R, Scarlato V. Fur functions as an activator and as a repressor of putative virulence genes in Neisseria meningitidis. Molecular Microbiology. 2004;52:1081–1090. doi: 10.1111/j.1365-2958.2004.04030.x. [DOI] [PubMed] [Google Scholar]
- 18.Yu C, Genco CA. Fur-mediated activation of gene transcription in the human pathogen Neisseria gonorrhoeae. Journal of bacteriology. 2012;194:1730–1742. doi: 10.1128/JB.06176-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrews SC, Robinson AK, Rodriguez-Quinones F. Bacterial iron homeostasis. FEMS Microbiology reviews. 2003;27:215–237. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 20.McHugh JP, Rodriguez-Quinones F, Abdul-Tehrani H, Svistunenko DA, Poole RK, Cooper CE, Andrews SC. Global iron-dependent gene regulation in Escherichia coli. A new mechanism for iron homeostasis. The Journal of biological chemistry. 2003;278:29478–29486. doi: 10.1074/jbc.M303381200. [DOI] [PubMed] [Google Scholar]
- 21.Hunt MD, Pettis GS, McIntosh MA. Promoter and operator determinants for fur-mediated iron regulation in the bidirectional fepA-fes control region of the Escherichia coli enterobactin gene system. Journal of bacteriology. 1994;176:3944–3955. doi: 10.1128/jb.176.13.3944-3955.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masse E, Gottesman S. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:4620–4625. doi: 10.1073/pnas.032066599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masse E, Salvail H, Desnoyers G, Arguin M. Small RNAs controlling iron metabolism. Current opinion in Microbiology. 2007;10:140–145. doi: 10.1016/j.mib.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Masse E, Vanderpool CK, Gottesman S. Effect of RyhB small RNA on global iron use in Escherichia coli. Journal of bacteriology. 2005;187:6962–6971. doi: 10.1128/JB.187.20.6962-6971.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nandal A, Huggins CC, Woodhall MR, McHugh J, Rodriguez-Quinones F, Quail MA, Guest JR, Andrews SC. Induction of the ferritin gene (ftnA) of Escherichia coli by Fe2+-Fur is mediated by reversal of H-NS silencing and is RyhB independent. Molecular Microbiology. 2010;75:637–657. doi: 10.1111/j.1365-2958.2009.06977.x. [DOI] [PubMed] [Google Scholar]
- 26.Faulkner MJ, Ma Z, Fuangthong M, Helmann JD. Derepression of the Bacillus subtilis PerR peroxide stress response leads to iron deficiency. Journal of bacteriology. 2012;194:1226–1235. doi: 10.1128/JB.06566-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pi H, Patel SJ, Arguello JM, Helmann JD. The Listeria monocytogenes Fur-regulated virulence protein FrvA is an Fe(II) efflux P1B4 -type ATPase. Molecular Microbiology. 2016;100:1066–1079. doi: 10.1111/mmi.13368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Storz G, Altuvia S. OxyR regulon. Methods in enzymology. 1994;234:217–223. doi: 10.1016/0076-6879(94)34088-9. [DOI] [PubMed] [Google Scholar]
- 29.Herbig AF, Helmann JD. Roles of metal ions and hydrogen peroxide in modulating the interaction of the Bacillus subtilis PerR peroxide regulon repressor with operator DNA. Molecular Microbiology. 2001;41:849–859. doi: 10.1046/j.1365-2958.2001.02543.x. [DOI] [PubMed] [Google Scholar]
- 30.Calhoun LN, Kwon YM. Structure, function and regulation of the DNA-binding protein Dps and its role in acid and oxidative stress resistance in Escherichia coli: a review. Journal of applied Microbiology. 2011;110:375–386. doi: 10.1111/j.1365-2672.2010.04890.x. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Helmann JD. Bacillus subtilis MrgA is a Dps(PexB) homologue: evidence for metalloregulation of an oxidative-stress gene. Molecular Microbiology. 1995;18:295–300. doi: 10.1111/j.1365-2958.1995.mmi_18020295.x. [DOI] [PubMed] [Google Scholar]
- 32.Faulkner MJ, Helmann JD. Peroxide stress elicits adaptive changes in bacterial metal ion homeostasis. Antioxidants & redox signaling. 2011;15:175–189. doi: 10.1089/ars.2010.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anjem A, Varghese S, Imlay JA. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Molecular Microbiology. 2009;72:844–858. doi: 10.1111/j.1365-2958.2009.06699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kehres DG, Zaharik ML, Finlay BB, Maguire ME. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Molecular Microbiology. 2000;36:1085–1100. doi: 10.1046/j.1365-2958.2000.01922.x. [DOI] [PubMed] [Google Scholar]
- 35.Gaballa A, Helmann JD. A peroxide-induced zinc uptake system plays an important role in protection against oxidative stress in Bacillus subtilis. Molecular Microbiology. 2002;45:997–1005. doi: 10.1046/j.1365-2958.2002.03068.x. [DOI] [PubMed] [Google Scholar]
- 36.Guan G, Pinochet-Barros A, Gaballa A, Patel SJ, Arguello JM, Helmann JD. PfeT, a P1B4 -type ATPase, effluxes ferrous iron and protects Bacillus subtilis against iron intoxication. Molecular Microbiology. 2015;98:787–803. doi: 10.1111/mmi.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel SJ, Lewis BE, Long JE, Nambi S, Sassetti CM, Stemmler TL, Arguello JM. Fine-tuning of Substrate Affinity Leads to Alternative Roles of Mycobacterium tuberculosis Fe2+-ATPases. The Journal of biological chemistry. 2016;291:11529–11539. doi: 10.1074/jbc.M116.718239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.VanderWal AR, Makthal N, Pinochet-Barros A, Helmann JD, Olsen RJ, Kumaraswami M. Iron Efflux by PmtA Is Critical for Oxidative Stress Resistance and Contributes Significantly to Group A Streptococcus Virulence. Infection and immunity. 2017;85 doi: 10.1128/IAI.00091-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner AG, Ong CY, Djoko KY, West NP, Davies MR, McEwan AG, Walker MJ. The PerR-Regulated P1B-4-Type ATPase (PmtA) Acts as a Ferrous Iron Efflux Pump in Streptococcus pyogenes. Infection and immunity. 2017;85 doi: 10.1128/IAI.00140-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuhlbrandt W. Biology, structure and mechanism of P-type ATPases, Nature reviews. Molecular cell biology. 2004;5:282–295. doi: 10.1038/nrm1354. [DOI] [PubMed] [Google Scholar]
- 41.Chan H, Babayan V, Blyumin E, Gandhi C, Hak K, Harake D, Kumar K, Lee P, Li TT, Liu HY, Lo TC, Meyer CJ, Stanford S, Zamora KS, Saier MH., Jr The P-type ATPase superfamily. J Mol Microbiol Biotechnol. 2010;19:5–104. doi: 10.1159/000319588. [DOI] [PubMed] [Google Scholar]
- 42.Lenoir G, Williamson P, Holthuis JC. On the origin of lipid asymmetry: the flip side of ion transport. Current opinion in chemical biology. 2007;11:654–661. doi: 10.1016/j.cbpa.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Lopez-Marques RL, Poulsen LR, Hanisch S, Meffert K, Buch-Pedersen MJ, Jakobsen MK, Pomorski TG, Palmgren MG. Intracellular targeting signals and lipid specificity determinants of the ALA/ALIS P4-ATPase complex reside in the catalytic ALA alpha-subunit. Molecular biology of the cell. 2010;21:791–801. doi: 10.1091/mbc.E09-08-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thever MD, Saier MH., Jr Bioinformatic characterization of P-type ATPases encoded within the fully sequenced genomes of 26 eukaryotes. The Journal of membrane biology. 2009;229:115–130. doi: 10.1007/s00232-009-9176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez-Guerrero M, Eren E, Rawat S, Stemmler TL, Arguello JM. Structure of the two transmembrane Cu+ transport sites of the Cu+-ATPases. The Journal of biological chemistry. 2008;283:29753–29759. doi: 10.1074/jbc.M803248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan B, Rosen BP. Biochemical characterization of CopA, the Escherichia coli Cu(I)-translocating P-type ATPase. The Journal of biological chemistry. 2002;277:46987–46992. doi: 10.1074/jbc.M208490200. [DOI] [PubMed] [Google Scholar]
- 47.Mandal AK, Cheung WD, Arguello JM. Characterization of a thermophilic P-type Ag+/Cu+-ATPase from the extremophile Archaeoglobus fulgidus. The Journal of biological chemistry. 2002;277:7201–7208. doi: 10.1074/jbc.M109964200. [DOI] [PubMed] [Google Scholar]
- 48.Liu J, Dutta SJ, Stemmler AJ, Mitra B. Metal-binding affinity of the transmembrane site in ZntA: implications for metal selectivity. Biochemistry. 2006;45:763–772. doi: 10.1021/bi051836n. [DOI] [PubMed] [Google Scholar]
- 49.Nucifora G, Chu L, Misra TK, Silver S. Cadmium resistance from Staphylococcus aureus plasmid pI258 cadA gene results from a cadmium-efflux ATPase. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:3544–3548. doi: 10.1073/pnas.86.10.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mana-Capelli S, Mandal AK, Arguello JM. Archaeoglobus fulgidus CopB is a thermophilic Cu2+-ATPase: functional role of its histidine-rich-N-terminal metal binding domain. The Journal of biological chemistry. 2003;278:40534–40541. doi: 10.1074/jbc.M306907200. [DOI] [PubMed] [Google Scholar]
- 51.Zielazinski EL, Cutsail GE, 3rd, Hoffman BM, Stemmler TL, Rosenzweig AC. Characterization of a cobalt-specific P(1B)-ATPase. Biochemistry. 2012;51:7891–7900. doi: 10.1021/bi3006708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith AT, Smith KP, Rosenzweig AC. Diversity of the metal-transporting P1B-type ATPases. Journal of biological inorganic chemistry: JBIC. 2014;19:947–960. doi: 10.1007/s00775-014-1129-2. a publication of the Society of Biological Inorganic Chemistry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arguello JM. Identification of ion-selectivity determinants in heavy-metal transport P1B-type ATPases. The Journal of membrane biology. 2003;195:93–108. doi: 10.1007/s00232-003-2048-2. [DOI] [PubMed] [Google Scholar]
- 54.Ollinger J, Song KB, Antelmann H, Hecker M, Helmann JD. Role of the Fur regulon in iron transport in Bacillus subtilis. Journal of bacteriology. 2006;188:3664–3673. doi: 10.1128/JB.188.10.3664-3673.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baichoo N, Wang T, Ye R, Helmann JD. Global analysis of the Bacillus subtilis Fur regulon and the iron starvation stimulon. Molecular Microbiology. 2002;45:1613–1629. doi: 10.1046/j.1365-2958.2002.03113.x. [DOI] [PubMed] [Google Scholar]
- 56.Fuangthong M, Herbig AF, Bsat N, Helmann JD. Regulation of the Bacillus subtilis fur and perR genes by PerR: not all members of the PerR regulon are peroxide inducible. Journal of bacteriology. 2002;184:3276–3286. doi: 10.1128/JB.184.12.3276-3286.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee JW, Helmann JD. Functional specialization within the Fur family of metalloregulators, Biometals: an international journal on the role of metal ions in biology. Biochemistry, and medicine. 2007;20:485–499. doi: 10.1007/s10534-006-9070-7. [DOI] [PubMed] [Google Scholar]
- 58.Gaballa A, Antelmann H, Aguilar C, Khakh SK, Song KB, Smaldone GT, Helmann JD. The Bacillus subtilis iron-sparing response is mediated by a Fur-regulated small RNA and three small, basic proteins. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11927–11932. doi: 10.1073/pnas.0711752105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smaldone GT, Antelmann H, Gaballa A, Helmann JD. The FsrA sRNA and FbpB protein mediate the iron-dependent induction of the Bacillus subtilis lutABC iron-sulfur-containing oxidases. Journal of bacteriology. 2012;194:2586–2593. doi: 10.1128/JB.05567-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smaldone GT, Revelles O, Gaballa A, Sauer U, Antelmann H, Helmann JD. A global investigation of the Bacillus subtilis iron-sparing response identifies major changes in metabolism. Journal of bacteriology. 2012;194:2594–2605. doi: 10.1128/JB.05990-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smaldone GT, Helmann JD. CsoR regulates the copper efflux operon copZA in Bacillus subtilis. Microbiology (Reading, England) 2007;153:4123–4128. doi: 10.1099/mic.0.2007/011742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore CM, Gaballa A, Hui M, Ye RW, Helmann JD. Genetic and physiological responses of Bacillus subtilis to metal ion stress. Molecular Microbiology. 2005;57:27–40. doi: 10.1111/j.1365-2958.2005.04642.x. [DOI] [PubMed] [Google Scholar]
- 63.McLaughlin HP, Xiao Q, Rea RB, Pi H, Casey PG, Darby T, Charbit A, Sleator RD, Joyce SA, Cowart RE, Hill C, Klebba PE, Gahan CG. A putative P-type ATPase required for virulence and resistance to haem toxicity in Listeria monocytogenes. PloS one. 2012;7:e30928. doi: 10.1371/journal.pone.0030928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rea R, Hill C, Gahan CG. Listeria monocytogenes PerR mutants display a small-colony phenotype, increased sensitivity to hydrogen peroxide, and significantly reduced murine virulence. Applied and environmental Microbiology. 2005;71:8314–8322. doi: 10.1128/AEM.71.12.8314-8322.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ledala N, Sengupta M, Muthaiyan A, Wilkinson BJ, Jayaswal RK. Transcriptomic response of Listeria monocytogenes to iron limitation and Fur mutation. Applied and environmental Microbiology. 2010;76:406–416. doi: 10.1128/AEM.01389-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McLaughlin HP, Bahey-El-Din M, Casey PG, Hill C, Gahan CG. A mutant in the Listeria monocytogenes Fur-regulated virulence locus (frvA) induces cellular immunity and confers protection against listeriosis in mice. Journal of medical Microbiology. 2013;62:185–190. doi: 10.1099/jmm.0.049114-0. [DOI] [PubMed] [Google Scholar]
- 67.Novoa-Aponte L, Soto Ospina CY. Mycobacterium tuberculosis P-type ATPases: possible targets for drug or vaccine development. BioMed research international. 2014:296986. doi: 10.1155/2014/296986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raimunda D, Long JE, Padilla-Benavides T, Sassetti CM, Arguello JM. Differential roles for the Co2+ /Ni2+ transporting ATPases, CtpD and CtpJ, in Mycobacterium tuberculosis virulence. Molecular Microbiology. 2014;91:185–197. doi: 10.1111/mmi.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olsen RJ, Shelburne SA, Musser JM. Molecular mechanisms underlying group A streptococcal pathogenesis. Cellular Microbiology. 2009;11:1–12. doi: 10.1111/j.1462-5822.2008.01225.x. [DOI] [PubMed] [Google Scholar]
- 70.Brenot A, Weston BF, Caparon MG. A PerR-regulated metal transporter (PmtA) is an interface between oxidative stress and metal homeostasis in Streptococcus pyogenes. Molecular Microbiology. 2007;63:1185–1196. doi: 10.1111/j.1365-2958.2006.05577.x. [DOI] [PubMed] [Google Scholar]
- 71.Xiong J, Kurtz DM, Jr, Ai J, Sanders-Loehr J. A hemerythrin-like domain in a bacterial chemotaxis protein. Biochemistry. 2000;39:5117–5125. doi: 10.1021/bi992796o. [DOI] [PubMed] [Google Scholar]
- 72.Karlsen OA, Ramsevik L, Bruseth LJ, Larsen O, Brenner A, Berven FS, Jensen HB, Lillehaug JR. Characterization of a prokaryotic haemerythrin from the methanotrophic bacterium Methylococcus capsulatus (Bath) The FEBS journal. 2005;272:2428–2440. doi: 10.1111/j.1742-4658.2005.04663.x. [DOI] [PubMed] [Google Scholar]
- 73.Zielazinski EL, Gonzalez-Guerrero M, Subramanian P, Stemmler TL, Arguello JM, Rosenzweig AC. Sinorhizobium meliloti Nia is a P(1B-5)-ATPase expressed in the nodule during plant symbiosis and is involved in Ni and Fe transport. Metallomics: integrated biometal science. 2013;5:1614–1623. doi: 10.1039/c3mt00195d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Becker A, Berges H, Krol E, Bruand C, Ruberg S, Capela D, Lauber E, Meilhoc E, Ampe F, de Bruijn FJ, Fourment J, Francez-Charlot A, Kahn D, Kuster H, Liebe C, Puhler A, Weidner S, Batut J. Global changes in gene expression in Sinorhizobium meliloti 1021 under microoxic and symbiotic conditions. Molecular plant-microbe interactions: MPMI. 2004;17:292–303. doi: 10.1094/MPMI.2004.17.3.292. [DOI] [PubMed] [Google Scholar]
- 75.Haney CJ, Grass G, Franke S, Rensing C. New developments in the understanding of the cation diffusion facilitator family. Journal of industrial Microbiology & biotechnology. 2005;32:215–226. doi: 10.1007/s10295-005-0224-3. [DOI] [PubMed] [Google Scholar]
- 76.Kolaj-Robin O, Russell D, Hayes KA, Pembroke JT, Soulimane T. Cation Diffusion Facilitator family: Structure and function. FEBS letters. 2015;589:1283–1295. doi: 10.1016/j.febslet.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 77.Nies DH. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiology reviews. 2003;27:313–339. doi: 10.1016/S0168-6445(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 78.Munkelt D, Grass G, Nies DH. The chromosomally encoded cation diffusion facilitator proteins DmeF and FieF from Wautersia metallidurans CH34 are transporters of broad metal specificity. Journal of bacteriology. 2004;186:8036–8043. doi: 10.1128/JB.186.23.8036-8043.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu M, Fu D. Structure of the zinc transporter YiiP. Science (New York, N.Y.) 2007;317:1746–1748. doi: 10.1126/science.1143748. [DOI] [PubMed] [Google Scholar]
- 80.Cubillas C, Vinuesa P, Tabche ML, Davalos A, Vazquez A, Hernandez-Lucas I, Romero D, Garcia-de los Santos A. The cation diffusion facilitator protein EmfA of Rhizobium etli belongs to a novel subfamily of Mn(2+)/Fe(2+) transporters conserved in alpha-proteobacteria. Metallomics : integrated biometal science. 2014;6:1808–1815. doi: 10.1039/c4mt00135d. [DOI] [PubMed] [Google Scholar]
- 81.Raimunda D, Elso-Berberian G. Functional characterization of the CDF transporter SMc02724 (SmYiiP) in Sinorhizobium meliloti: Roles in manganese homeostasis and nodulation. Biochimica et biophysica acta. 2014;1838:3203–3211. doi: 10.1016/j.bbamem.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 82.Rosch JW, Gao G, Ridout G, Wang YD, Tuomanen EI. Role of the manganese efflux system mntE for signalling and pathogenesis in Streptococcus pneumoniae. Molecular Microbiology. 2009;72:12–25. doi: 10.1111/j.1365-2958.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Montanini B, Blaudez D, Jeandroz S, Sanders D, Chalot M. Phylogenetic and functional analysis of the Cation Diffusion Facilitator (CDF) family: improved signature and prediction of substrate specificity. BMC genomics. 2007;8:107. doi: 10.1186/1471-2164-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blindauer CA, Schmid R. Cytosolic metal handling in plants: determinants for zinc specificity in metal transporters and metallothioneins. Metallomics: integrated biometal science. 2010;2:510–529. doi: 10.1039/c004880a. [DOI] [PubMed] [Google Scholar]
- 85.Podar D, Scherer J, Noordally Z, Herzyk P, Nies D, Sanders D. Metal selectivity determinants in a family of transition metal transporters. The Journal of biological chemistry. 2012;287:3185–3196. doi: 10.1074/jbc.M111.305649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kawachi M, Kobae Y, Mimura T, Maeshima M. Deletion of a histidine-rich loop of AtMTP1, a vacuolar Zn(2+)/H(+) antiporter of Arabidopsis thaliana, stimulates the transport activity. The Journal of biological chemistry. 2008;283:8374–8383. doi: 10.1074/jbc.M707646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin H, Burton D, Li L, Warner DE, Phillips JD, Ward DM, Kaplan J. Gain-of-function mutations identify amino acids within transmembrane domains of the yeast vacuolar transporter Zrc1 that determine metal specificity. The Biochemical journal. 2009;422:273–283. doi: 10.1042/BJ20090853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hoch E, Lin W, Chai J, Hershfinkel M, Fu D, Sekler I. Histidine pairing at the metal transport site of mammalian ZnT transporters controls Zn2+ over Cd2+ selectivity. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7202–7207. doi: 10.1073/pnas.1200362109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grass G, Fan B, Rosen BP, Franke S, Nies DH, Rensing C. ZitB (YbgR), a member of the cation diffusion facilitator family, is an additional zinc transporter in Escherichia coli. Journal of bacteriology. 2001;183:4664–4667. doi: 10.1128/JB.183.15.4664-4667.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chao Y, Fu D. Thermodynamic studies of the mechanism of metal binding to the Escherichia coli zinc transporter YiiP. The Journal of biological chemistry. 2004;279:17173–17180. doi: 10.1074/jbc.M400208200. [DOI] [PubMed] [Google Scholar]
- 91.Wei Y, Li H, Fu D. Oligomeric state of the Escherichia coli metal transporter YiiP. The Journal of biological chemistry. 2004;279:39251–39259. doi: 10.1074/jbc.M407044200. [DOI] [PubMed] [Google Scholar]
- 92.Grass G, Otto M, Fricke B, Haney CJ, Rensing C, Nies DH, Munkelt D. FieF (YiiP) from Escherichia coli mediates decreased cellular accumulation of iron and relieves iron stress. Archives of Microbiology. 2005;183:9–18. doi: 10.1007/s00203-004-0739-4. [DOI] [PubMed] [Google Scholar]
- 93.Wei Y, Fu D. Selective metal binding to a membrane-embedded aspartate in the Escherichia coli metal transporter YiiP (FieF) The Journal of biological chemistry. 2005;280:33716–33724. doi: 10.1074/jbc.M506107200. [DOI] [PubMed] [Google Scholar]
- 94.Lu M, Chai J, Fu D. Structural basis for autoregulation of the zinc transporter YiiP. Nature structural & molecular biology. 2009;16:1063–1067. doi: 10.1038/nsmb.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nies DH. How iron is transported into magnetosomes. Molecular Microbiology. 2011;82:792–796. doi: 10.1111/j.1365-2958.2011.07864.x. [DOI] [PubMed] [Google Scholar]
- 96.Uebe R, Junge K, Henn V, Poxleitner G, Katzmann E, Plitzko JM, Zarivach R, Kasama T, Wanner G, Posfai M, Bottger L, Matzanke B, Schuler D. The cation diffusion facilitator proteins MamB and MamM of Magnetospirillum gryphiswaldense have distinct and complex functions, and are involved in magnetite biomineralization and magnetosome membrane assembly. Molecular Microbiology. 2011;82:818–835. doi: 10.1111/j.1365-2958.2011.07863.x. [DOI] [PubMed] [Google Scholar]
- 97.Salusso A, Raimunda D. Defining the Roles of the Cation Diffusion Facilitators in Fe2+/Zn2+ Homeostasis and Establishment of Their Participation in Virulence in Pseudomonas aeruginosa. Frontiers in cellular and infection Microbiology. 2017;7:84. doi: 10.3389/fcimb.2017.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pederick VG, Eijkelkamp BA, Begg SL, Ween MP, McAllister LJ, Paton JC, McDevitt CA. ZnuA and zinc homeostasis in Pseudomonas aeruginosa. Scientific reports. 2015;5:13139. doi: 10.1038/srep13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hau HH, Gralnick JA. Ecology and biotechnology of the genus Shewanella. Annual review of Microbiology. 2007;61:237–258. doi: 10.1146/annurev.micro.61.080706.093257. [DOI] [PubMed] [Google Scholar]
- 100.Meyer TE, Tsapin AI, Vandenberghe I, de Smet L, Frishman D, Nealson KH, Cusanovich MA, van Beeumen JJ. Identification of 42 possible cytochrome C genes in the Shewanella oneidensis genome and characterization of six soluble cytochromes. Omics: a journal of integrative biology. 2004;8:57–77. doi: 10.1089/153623104773547499. [DOI] [PubMed] [Google Scholar]
- 101.Bennett BD, Brutinel ED, Gralnick JA. A Ferrous Iron Exporter Mediates Iron Resistance in Shewanella oneidensis MR-1. Applied and environmental Microbiology. 2015;81:7938–7944. doi: 10.1128/AEM.02835-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang Y, Harris DP, Luo F, Wu L, Parsons AB, Palumbo AV, Zhou J. Characterization of the Shewanella oneidensis Fur gene: roles in iron and acid tolerance response. BMC genomics. 2008;9(Suppl 1):S11. doi: 10.1186/1471-2164-9-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yan N. Structural Biology of the Major Facilitator Superfamily Transporters. Annual review of biophysics. 2015;44:257–283. doi: 10.1146/annurev-biophys-060414-033901. [DOI] [PubMed] [Google Scholar]
- 104.Forrest LR, Kramer R, Ziegler C. The structural basis of secondary active transport mechanisms. Biochimica et biophysica acta. 2011;1807:167–188. doi: 10.1016/j.bbabio.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 105.Quistgaard EM, Low C, Guettou F, Nordlund P. Understanding transport by the major facilitator superfamily (MFS): structures pave the way, Nature reviews. Molecular cell biology. 2016;17:123–132. doi: 10.1038/nrm.2015.25. [DOI] [PubMed] [Google Scholar]
- 106.Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;211:969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- 107.Quistgaard EM, Low C, Moberg P, Tresaugues L, Nordlund P. Structural basis for substrate transport in the GLUT-homology family of monosaccharide transporters. Nature structural & molecular biology. 2013;20:766–768. doi: 10.1038/nsmb.2569. [DOI] [PubMed] [Google Scholar]
- 108.Kumar H, Finer-Moore JS, Kaback HR, Stroud RM. Structure of LacY with an alpha-substituted galactoside: Connecting the binding site to the protonation site. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:9004–9009. doi: 10.1073/pnas.1509854112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kumar H, Kasho V, Smirnova I, Finer-Moore JS, Kaback HR, Stroud RM. Structure of sugar-bound LacY. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:1784–1788. doi: 10.1073/pnas.1324141111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dang S, Sun L, Huang Y, Lu F, Liu Y, Gong H, Wang J, Yan N. Structure of a fucose transporter in an outward-open conformation. Nature. 2010;467:734–738. doi: 10.1038/nature09406. [DOI] [PubMed] [Google Scholar]
- 111.Yin Y, He X, Szewczyk P, Nguyen T, Chang G. Structure of the multidrug transporter EmrD from Escherichia coli. Science (New York, N.Y.) 2006;312:741–744. doi: 10.1126/science.1125629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Newstead S, Drew D, Cameron AD, Postis VL, Xia X, Fowler PW, Ingram JC, Carpenter EP, Sansom MS, McPherson MJ, Baldwin SA, Iwata S. Crystal structure of a prokaryotic homologue of the mammalian oligopeptide-proton symporters, PepT1 and PepT2. The EMBO journal. 2011;30:417–426. doi: 10.1038/emboj.2010.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yan H, Huang W, Yan C, Gong X, Jiang S, Zhao Y, Wang J, Shi Y. Structure and mechanism of a nitrate transporter. Cell reports. 2013;3:716–723. doi: 10.1016/j.celrep.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 114.Fukuda M, Takeda H, Kato HE, Doki S, Ito K, Maturana AD, Ishitani R, Nureki O. Structural basis for dynamic mechanism of nitrate/nitrite antiport by NarK. Nature communications. 2015;6:7097. doi: 10.1038/ncomms8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Deng D, Sun P, Yan C, Ke M, Jiang X, Xiong L, Ren W, Hirata K, Yamamoto M, Fan S, Yan N. Molecular basis of ligand recognition and transport by glucose transporters. Nature. 2015;526:391–396. doi: 10.1038/nature14655. [DOI] [PubMed] [Google Scholar]
- 116.Nomura N, Verdon G, Kang HJ, Shimamura T, Nomura Y, Sonoda Y, Hussien SA, Qureshi AA, Coincon M, Sato Y, Abe H, Nakada-Nakura Y, Hino T, Arakawa T, Kusano-Arai O, Iwanari H, Murata T, Kobayashi T, Hamakubo T, Kasahara M, Iwata S, Drew D. Structure and mechanism of the mammalian fructose transporter GLUT5. Nature. 2015;526:397–401. doi: 10.1038/nature14909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Baranova N, Nikaido H. The baeSR two-component regulatory system activates transcription of the yegMNOB (mdtABCD) transporter gene cluster in Escherichia coli and increases its resistance to novobiocin and deoxycholate. Journal of bacteriology. 2002;184:4168–4176. doi: 10.1128/JB.184.15.4168-4176.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leblanc SK, Oates CW, Raivio TL. Characterization of the induction and cellular role of the BaeSR two-component envelope stress response of Escherichia coli. Journal of bacteriology. 2011;193:3367–3375. doi: 10.1128/JB.01534-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nagakubo S, Nishino K, Hirata T, Yamaguchi A. The putative response regulator BaeR stimulates multidrug resistance of Escherichia coli via a novel multidrug exporter system, MdtABC. Journal of bacteriology. 2002;184:4161–4167. doi: 10.1128/JB.184.15.4161-4167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Frawley ER, Crouch ML, Bingham-Ramos LK, Robbins HF, Wang W, Wright GD, Fang FC. Iron and citrate export by a major facilitator superfamily pump regulates metabolism and stress resistance in Salmonella Typhimurium. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:12054–12059. doi: 10.1073/pnas.1218274110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yeowell HN, White JR. Iron requirement in the bactericidal mechanism of streptonigrin. Antimicrobial agents and chemotherapy. 1982;22:961–968. doi: 10.1128/aac.22.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bjarnason J, Southward CM, Surette MG. Genomic profiling of iron-responsive genes in Salmonella enterica serovar typhimurium by high-throughput screening of a random promoter library. Journal of bacteriology. 2003;185:4973–4982. doi: 10.1128/JB.185.16.4973-4982.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim SA, Punshon T, Lanzirotti A, Li L, Alonso JM, Ecker JR, Kaplan J, Guerinot ML. Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science (New York, N.Y.) 2006;314:1295–1298. doi: 10.1126/science.1132563. [DOI] [PubMed] [Google Scholar]
- 124.Arosio P, Elia L, Poli M. Ferritin, cellular iron storage and regulation. IUBMB life. 2017 doi: 10.1002/iub.1621. [DOI] [PubMed] [Google Scholar]
- 125.Ruangkiattikul N, Bhubhanil S, Chamsing J, Niamyim P, Sukchawalit R, Mongkolsuk S. Agrobacterium tumefaciens membrane-bound ferritin plays a role in protection against hydrogen peroxide toxicity and is negatively regulated by the iron response regulator. FEMS Microbiology letters. 2012;329:87–92. doi: 10.1111/j.1574-6968.2012.02509.x. [DOI] [PubMed] [Google Scholar]
- 126.Johnson DB, Kanao T, Hedrich S. Redox Transformations of Iron at Extremely Low pH: Fundamental and Applied Aspects. Frontiers in Microbiology. 2012;3:96. doi: 10.3389/fmicb.2012.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sankari S, O'Brian MR. A bacterial iron exporter for maintenance of iron homeostasis. The Journal of biological chemistry. 2014;289:16498–16507. doi: 10.1074/jbc.M114.571562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hamza I, Qi Z, King ND, O'Brian MR. Fur-independent regulation of iron metabolism by Irr in Bradyrhizobium japonicum. Microbiology (Reading, England) 2000;146(Pt 3):669–676. doi: 10.1099/00221287-146-3-669. [DOI] [PubMed] [Google Scholar]
- 129.Rudolph G, Semini G, Hauser F, Lindemann A, Friberg M, Hennecke H, Fischer HM. The Iron control element, acting in positive and negative control of iron-regulated Bradyrhizobium japonicum genes, is a target for the Irr protein. Journal of bacteriology. 2006;188:733–744. doi: 10.1128/JB.188.2.733-744.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sankari S, O'Brian MR. Synthetic Lethality of the bfr and mbfA Genes Reveals a Functional Relationship between Iron Storage and Iron Export in Managing Stress Responses in Bradyrhizobium japonicum. PloS one. 2016;11:e0157250. doi: 10.1371/journal.pone.0157250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen–host interface. Nat Rev Micro. 2012;10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Flannagan RS, Heit B, Heinrichs DE. Antimicrobial Mechanisms of Macrophages and the Immune Evasion Strategies of Staphylococcus aureus. Pathogens (Basel, Switzerland) 2015;4:826–868. doi: 10.3390/pathogens4040826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Osorio H, Martinez V, Nieto PA, Holmes DS, Quatrini R. Microbial iron management mechanisms in extremely acidic environments: comparative genomics evidence for diversity and versatility. BMC Microbiology. 2008;8:203. doi: 10.1186/1471-2180-8-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.