Abstract
The key to preventing brain aging, mild cognitive impairment (MCI), and Alzheimer disease (AD) via vitamin intake is first to understand molecular mechanisms, then to deduce relevant biomarkers, and subsequently to test the level of evidence for the impact of vitamins in the relevant pathways and their modulation of dementia risk. This narrative review infers information on mechanisms from gene and metabolic defects associated with MCI and AD, and assesses the role of vitamins using recent results from animal and human studies. Current evidence suggests that all known vitamins and some “quasi-vitamins” are involved as cofactors or influence ≥1 of the 6 key sets of pathways or pathologies associated with MCI or AD, relating to 1) 1-carbon metabolism, 2) DNA damage and repair, 3) mitochondrial function and glucose metabolism, 4) lipid and phospholipid metabolism and myelination, 5) neurotransmitter synthesis and synaptogenesis, and 6) amyloidosis and Tau protein phosphorylation. The contemporary level of evidence for each of the vitamins varies considerably, but it is notable that B vitamins are involved as cofactors in all of the core pathways or pathologies and, together with vitamins C and E, are consistently associated with a protective role against dementia. Outcomes from recent studies indicate that the efficacy and safety of supplementation with vitamins to prevent MCI and the early stages of AD will most likely depend on 1) which pathways are defective, 2) which vitamins are deficient and could correct the relevant metabolic defects, and 3) the modulating impact of nutrient-nutrient and nutrient-genotype interaction. More focus on a precision nutrition approach is required to realize the full potential of vitamin therapy in preventing dementia and to avoid causing harm.
Keywords: vitamins, brain, aging, Alzheimer, biomarkers, epidemiology, interventions, mechanisms, knowledge gaps
Introduction
Aging of populations worldwide is increasing the numbers of people at high risk of degenerative diseases, including Alzheimer disease (AD), for which no cure exists (1–6). Mild cognitive impairment (MCI) is the prodromal stage of AD, and the risk for both increases with malnutrition (2–6). An understanding of which nutritional factors are associated with the risk of MCI and AD is essential in order to design appropriate preventive strategies based on dietary intervention. It is well recognized that prevention of AD requires intervention before or very early during the onset of MCI (5, 6). For this reason, biomarkers associated prospectively with eventual risk of MCI and AD are an important tool to determine the potential preventive effects of vitamins ingested either via nutrient-rich whole foods or as supplements. This review focuses on current knowledge regarding vitamins that have been associated with MCI and AD in animal models and in human epidemiological and interventional studies. It identifies important knowledge gaps and suggests new research directions, based on precision nutrition, to achieve more successful outcomes with vitamin therapy for preventing dementia.
Current Status of Knowledge
Biomarkers associated with MCI and AD
Although specific tissues and organs may age in unique ways, fundamental hallmarks of aging that occur at the cellular and genome levels are common to all tissues; these include genomic instability at the chromosomal and DNA sequence levels; telomere attrition and dysfunction; shifts in epigenetic marks at the DNA and histone levels, which affect gene expression; and loss of mitochondrial function due in part to mitochondrial DNA mutations. These fundamental genomic deficits may then lead to increased cellular senescence, reduced capacity for stem cell regeneration, altered nutrient sensing, loss of proteostasis, and deleterious changes in cellular phenotypes and in intercellular communication (7, 8). Assuming that MCI and AD are manifestations of accelerated aging occurring systemically within the body, one could reasonably hypothesize that those at higher risk may already be exhibiting a heightened level of the above-mentioned hallmarks of aging in easily accessible peripheral tissues (e.g., blood, buccal cells, and urine) before the symptoms of MCI start to become evident.
Age-related changes that are biochemically more directly associated with MCI and AD have been identified and are progressively being better characterized as knowledge accumulates (1, 9, 10). The best-characterized temporal sequence of pathology indicative of increased risk of MCI and AD is reduced amyloid β-42 (Aβ42) in cerebrospinal fluid (CSF); amyloid accumulation in the brain, which is detected by positron emission tomography using Pittsburgh compound B (PIB-PET); followed by accumulation of tau protein in CSF, after which brain atrophy and reduced uptake of glucose become evident in the brain, as detected by MRI and fluorodeoxyglucose positron emission tomography, respectively (1, 10). The accumulation of these biomarkers precedes the onset of cognitive impairment that is measured using a defined set of cognitive function tests specific for memory, attention, and executive function (1, 6).
Total tau, phosphorylated tau, neurofilament light protein, and Aβ42 in CSF have the strongest diagnostic value (1, 9, 10), but CSF collection is a more invasive procedure than blood collection, requiring highly skilled personnel to achieve success and minimize adverse effects. Total tau and chitinase-3-like protein 1 (an inflammatory protein) in blood are also associated with MCI and AD risk and may be useful in a research setting. Tau, neurofilament light protein, and chitinase-3-like protein 1 are biomarkers of axonal damage. Aβ42 is a toxic form of amyloid generated through the inappropriate processing of amyloid precursor protein (APP) and can accumulate intracellularly or extracellularly to a concentration that induces neuronal cell death and brain atrophy (11, 12).
Untargeted approaches have also yielded several metabolomic and proteomic biomarkers in blood that indicate significant risk for AD. These include biomarkers associated with cell growth (insulin-like growth factor binding protein), pancreatic function (pancreatic polypeptide), stress (cortisol), 1-carbon metabolism (homocysteine), antioxidant function (superoxide dismutase), and kidney function (β-2 microglobulin) (13–15). Other studies showed strong associations of single FAs or combinations of specific SFAs, MUFAs, PUFAs, and ω-3 FAs with MCI (16–19). Such biomarkers, when combined with other risk factors such as age, sex, and APOE genotype may provide a stronger predictive potential for identifying those with an increased probability of MCI and AD.
We recently reviewed the various biomarkers in peripheral tissue that are associated with risk for MCI and AD (20). One of the increasingly investigated tissues are buccal cells because they are easily obtained in a minimally invasive manner. It has been hypothesized that buccal cell tissue could be a good candidate to reflect AD-related pathology if the disease was systemic, given that both brain and buccal cells are of ectodermal origin (21). In a pioneering study, we showed that buccal cells of patients with AD and patients with Down syndrome have increased levels of DNA damage, measured as micronuclei, reduced frequencies of dying cells, and a much reduced frequency of basal cells, which represents the regenerative capacity of the tissue. The combination of the low frequency of karyorrhexis cells (biomarker 1) and basal cells (biomarker 2) was strongly associated with AD [positive predictive value: 98%; negative predictive value: 77%; sensitivity: 82%; specificity: 97%; likelihood ratio: 25, OR: 140 (95% CI: 17, 1165); P = 0.0001 for biomarker 1 + 2 <41/1000 cells] (22, 23). The area under the receiver operating characteristic curve (AUROC) for the association of basal cell frequency with AD was 0.96 (P < 0.0001), for karyorrhexis cells it was 0.88 (P < 0.0001), and for both basal and karyorrhexis cells the AUROC was 0.91 (P < 0.0001). We observed that the lower frequency of basal cells and karyorrhexis cells in patients with AD may be explained by lower vitamin B-12 and higher homocysteine concentrations in plasma, respectively (22, 23).
The association with low basal cell frequency was further verified using the basal cell–specific cytokeratin 14 (CK14) marker. CK14 was significantly reduced in buccal cells of patients with MCI and AD, and its diagnostic risk value was better than that of plasma homocysteine (24). The diagnostic value for detecting a case of MCI based on the AUROC was 0.57, 0.90, and 0.93 for homocysteine, CK14, and the CK14-to-homocysteine ratio, respectively. The diagnostic value for detecting a case of AD based on the AUROC was 0.67, 0.77, and 0.79 for homocysteine, CK14, and the CK14-to-homocysteine ratio, respectively. Furthermore, it was also noted that CK14 was positively correlated with vitamin B-12 in the MCI and AD groups (r = 0.51 and 0.63, respectively). In addition, Aβ1–42 was detectable in buccal cells and increased significantly in MCI and even further in AD relative to controls (21, 24, 25). Telomere shortening in buccal cells, like lymphocytes, was also associated with increased risk for dementia, but the association tended to be stronger with blood lymphocytes in our studies (26, 27). It is interesting to note that some of the dietary and metabolic factors associated with protection against (e.g., Mediterranean diet and higher ω-3 FA intake) or aggravation of (e.g., high homocysteine and obesity) telomere shortening are also often linked in the same directions with AD risk (28–33).
Which vitamin combinations might provide an effective metabolic tune-up to prevent brain aging and the early stages of MCI and AD? Does it depend on genotype?
It is essential to understand the biology of brain aging and the metabolic pathways involved, before attempting to work out which vitamin combination (nutriome) might be required to optimize (tune up) metabolic function in a manner that decelerates brain aging and the pathologies that lead to MCI and AD (34). Which metabolic pathways matter most can be thoroughly understood by examining 1) genetic risk factors, 2) changes in gene expression in the brain with age and with MCI or AD, 3) using bioinformatics, to identify which metabolic pathways are affected by such genetic changes, and 4) metabolic imbalances that indicate where metabolic blocks may be occurring. Once the metabolic pathways are identified, it becomes possible to establish which vitamins might be required as cofactors and substrates in these pathways and therefore reasonably have a putative role in preventing MCI and AD. These could then be considered as legitimate candidates for a metabolic tune-up to prevent brain aging and dementia. The aim would be to intervene with the appropriate vitamin intake combination well before the symptoms of MCI emerge; current evidence indicates AD-related brain pathology and atrophy commence several years before the onset of MCI (6, 35, 36).
Highly penetrant mutations have been identified that increase risk of dementia, and recent studies have shed some light on the protective effects of vitamins in these genetic backgrounds. The most important of these mutations occur in the APP, presenilin (PSEN) 1, and PSEN2 genes, which cause AD through their role in the metabolism of the APP by shifting APP processing toward the generation of Aβ1–42 and fibrillogenesis, leading to amyloid plaque formation (37). Such mutations have strong causative effects, but they are relatively rare (<1%) and are associated with early onset of AD. Dietary factors that influence APP processing have not yet been systematically defined, but several studies of mouse models with mutations in the App and Psen genes indicate the potential of folate, thiamine, nicotinamide, and vitamins C, D, and E to exert a substantial beneficial modifying effect on either APP processing, amyloidosis, or amyloid plaque formation (38–48).
Other gene mutations increase risk of AD through alternative mechanisms. One of the more common of these mutations associated with AD risk is the APOEε4 mutation, which occurs at a frequency of 2–5% in the homozygous state and 10–20% in the heterozygous state, depending on ethnicity. Relative to non–ε4 carriers, APOEε4 carriers have a higher cholesterol concentration in their blood and show higher Aβ deposition in the form of senile plaques in the brain (49). Emerging evidence of the interactive effects of the APOEε4 genotype with specific vitamin status (e.g., vitamin B-12 deficiency) in modifying risk for AD suggests the possibility that APOEε4 carriers may respond differently to micronutrient intervention relative to noncarriers. For example, a significant positive correlation between serum concentrations of vitamin B-12 and volume of brain regional gray matter was observed in APOEε4 carriers with AD but not in noncarriers (50). Furthermore, in cross-sectional studies in the elderly in Norway and Singapore, researchers observed that cognitive decline was increased with low vitamin B-12 status, but only in those carrying the APOE ε4 allele (51, 52).
Other gene mutations that confer lower risk for AD but are common (15–50% frequency) include those related to cholesterol metabolism [ATP binding cassette subfamily A member 7 (ABCA7) and solute carrier family 24 member 4 (SLC24A4)], endocytosis [Ras and Rab interactor 3 (RIN3), phosphatidylinositol binding clathrin assembly protein (PICALM), and bridging integrator 1 (BIN1)], immune response [complement C3b/C4b receptor 1 (CR1), CD33 molecule (CD33), and inositol polyphosphate-5- phosphatase D (INPP5D)], and cytoskeletal function or axonal transport [CUGPB Elav-like family member 1 (CELF1) and NME/NM23 family member 8 (NME8)] (37). Mechanisms for some of these mutations are known; for example, ABCA7 deficiency alters the brain lipid profile and accelerates the processing of APP to amyloid β by increasing β-site APP cleaving enzyme 1 (BACE1) (53). However, whether vitamin status might substantially alter the impact of the genetic susceptibilities conferred by these mutations remains untested.
Age-related changes in brain gene expression due to oxidative damage and DNA methylation of gene promoter methylation, and the role of vitamins in mitigating these effects
Apart from inherited gene mutations, it is also possible that normal genes associated with MCI or AD risk could be silenced by DNA damage in the promoter sequence or as a result of methylation of cytosine-guanine dinucleotide islands sites within it. Limited evidence shows differential DNA methylation of promoters between normal and AD brains for coding genes involved in myelination and microRNA genes that express microRNAs which control expression of genes required for the myelination process (54, 55). Furthermore, methylation of promoters of nucleolar ribosomal RNA (rRNA) genes are decreased in MCI and AD relative to healthy control brains (56, 57). This observation is consistent with other evidence that nuclear ribosomal RNA expression and nucleolar size is diminished in the cells of AD brains (58). The synthesis of rRNA is carefully tuned to match nutritional conditions such that rRNA gene expression is depressed when intracellular energy status is diminished (59). Furthermore, rodent studies suggest that maternal protein calorie malnutrition downregulates ribosomal DNA transcription in fetal tissues and that such effects might be carried over throughout the life span with the consequence that inhibition of rRNA gene expression could lead to neurodegeneration (60). The effects of vitamin deficiencies on nucleolar structure and rRNA gene expression is barely explored, and the limited evidence available suggests that vitamin E deficiency can adversely affect nucleolar structure and function and reduce RNA transcription rate, similar to aging (61).
Oxidation of DNA in gene promoters is another important mechanism affecting gene expression in the aging brain. Lu et al. (62), using transcriptomic profiling, showed progressively reduced expression of key genes involved in synaptic plasticity, vesicular transport, calcium homeostasis, and mitochondrial function in frontal cortex brain tissue from human subjects aged ≥40 y. Furthermore, the promoters of these genes were selectively susceptible to oxidative damage as a result of reduced base-excision repair of oxidized DNA bases. The results of this study suggest that oxidative stress may reduce the expression of selectively vulnerable genes involved in learning, memory, and neuronal survival by damaging their promoters. In further studies, the same group showed that repressor element 1–silencing transcription factor (REST) provides protection against oxidative damage and amyloid β toxicity by repressing genes that promote cell death, thereby improving survival of neurons, and that expression of REST is lost in MCI and AD (63). These observations indicate the potential importance of dietary factors that protect against oxidative damage by supporting antioxidant defenses (e.g., vitamin C and vitamin E) and by improving efficiency of base-excision repair (zinc, magnesium, niacin, and folate) to remove oxidized guanine in DNA (64–69). However, the impact of dietary factors on the expression of REST remains unknown.
In this context, it is important to note the strong dependence of the human brain on vitamin C and folate; concentrations of these vitamins are much higher in the brain than in plasma (70, 71). Arguably, this could be mainly to prevent oxidative damage to DNA and optimize its repair. However, alternative explanations have emerged relating to regulation of promoter methylation since the 2009 discovery of the sixth deoxyribonucleoside 5-hydroxymethyldeoxycytidine in DNA, which is most abundant in neurons (72), and the concurrent discovery of the three 10–11 translocation (TET) enzymes, which not only synthesize but also oxidize 5-hydroxymethyldeoxycytidine in DNA, before removing glycosylase and repairing base excisions (73, 74). TET enzymes require vitamin C as a cofactor to demethylate DNA, whereas reduced folates are needed to maintain DNA methylation, to synthesize mitochondrial and nuclear DNA, and to perform DNA repair synthesis (75, 76). A study in knockout Tet1 rodents suggest that neuronal Tet1 is critical for extinction of memory and regulation of the expression of key neuronal activity–regulated genes and neuronal plasticity (73).
It is also interesting to note that another important role of reduced folates is to metabolize formaldehyde and its oxidized form formate, which has been shown to be elevated in MCI and AD and to inhibit spatial learning and spatial memory, resulting in topographic amnesia, which is commonly observed in AD (77–79). Formate is incorporated into 1-carbon metabolism via the activity of 10-formyltetrahydrofolate synthetase, which catalyses the reaction of formate with tetrahydrofolate to form 10-formyltetrahydrofolate, which is a precursor of the methyl donors 5,10-methylenetetrahydrofolate and 5-methyltetrahydrofolate required for nucleotide and methione synthesis, respectively (80). When folate and vitamin B-12 are deficient, the concentration of tetrahydrofolate is diminished; as a consequence, formate increases, as do the pathological consequences of its excess (80, 81).
Another approach that could inform which nutriome provides the best metabolic tune-up to prevent brain aging and the early stages of AD is to integrate transcriptomic data from patients with AD through a genome-wide computational human metabolic model in order to characterize the altered metabolism in AD, then deduce with metabolic modeling methods which nutrients can help to overcome bottlenecks. Research using this approach showed that the AD brain exhibits highly significant decreases in 1) mitochondrial nutrient transport (P = 4.8 × 10−11), 2) the activity of the carnitine shuttle (P = 3.5 × 10−18), and 3) folate metabolism (P = 3.8 × 10−13), indicating the possibility that specific micronutrients relevant to mitochondrial and 1-carbon metabolism might provide some metabolic stress relief and possibly delay the onset of the later stages of AD (82).
The plausibility of mitochondrial and 1-carbon metabolism involvement is supported by evidence that folate deficiency increases the frequency of large deletions in mitochondrial DNA in rodent models (83, 84) and that mitochondrial DNA deletions increase with age in the temporal and frontal cortices and the putamen of human brain (85). An initial pilot study showed that mitochondrial DNA deletions were increased 15-fold in the frontal cortex of younger patients with AD (<75 y old) relative to age-matched healthy controls, but this trend was not evident in older cases and controls (86). A recent review of all studies published in the past 2 decades was inconclusive regarding the role of mitochondrial DNA deletions in the brain as a cause of AD because of inconsistencies between studies (87).
Decreases in mitochondrial transport may be partly explained by mutations in translocase of the mitochondrial outer membrane (TOM) 40 or blockage of translocase of the TOM by Aβ1–42; TOM40 is the channel-forming subunit of the TOM complex that is essential for importing of protein precursors into mitochondria (88, 89). There is a lack of knowledge regarding the direct and indirect effects of vitamin deficiencies on the transport of proteins into mitochondria.
As is evident from basic biochemistry, several vitamins are required as cofactors for the biochemical reactions within the mitochondria—which include the citric acid cycle (thiamine, riboflavin, niacin, pantothenic acid, and biotin), the electron transport chain (riboflavin, niacin, and pantothenic acid), 1-carbon metabolism (riboflavin and vitamin B-6)—and for methylmalonyl-CoA mutase (vitamin B-12) (90). The latter is required to obtain energy in the form of succinyl-CoA from methylmalonyl-CoA, which is primarily derived from propionyl-CoA, a substance formed from the catabolism and digestion of isoleucine, valine, threonine, methionine, thymine, cholesterol, or odd-chain FAs. This suggests that correcting thiamine, riboflavin, niacin, pantothenic acid, vitamin B-6, biotin, vitamin B-12, and folate deficiencies has the potential to boost mitochondrial metabolism in the aging brain, potentially optimizing ATP production efficiency.
Associations of vitamins with MCI and AD deduced from epidemiological studies, interventions, and brain imaging
A more direct approach to investigate the association between micronutrients and MCI and AD is to study the correlation with brain atrophy, metabolic function (e.g., glucose metabolism), and accumulation of amyloid, which can be measured with brain MRI, fluorodeoxyglucose standardized uptake value ratio, and PIB-PET, respectively (1, 37).
Using MRI, Vogiatzoglou et al. (91) and Smith et al. (92) reported that the rate of brain atrophy in patients with MCI was significantly positively correlated with homocysteine concentration; homocysteine is a biomarker of either folate deficiency or vitamin B-12 deficiency, or both. In addition, brain atrophy in amnestic or nonamnestic patients with MCI was prevented by supplementation with folic acid (0.8 mg/d), vitamin B-12 (0.5 mg/d), and vitamin B-6 (20 mg/d) for 2 y (92). These beneficial effects were attributable to improved vitamin B-12 status and mainly found in those with homocysteine >11 μmol/L or plasma ω-3 FAs >390 μmol/L, and were reflected in improved cognition scores (93–95). The same investigators also observed a significant interactive effect of vitamin B-12 status with APOE genotype, such that those carrying the ε4 allele benefitted most from cognitive improvements associated with higher plasma concentrations of vitamin B-12 and lower concentrations of methylmalonic acid, the metabolic biomarker of vitamin B-12 deficiency (51). Furthermore, they observed that ω-3 FA status has to be optimal to achieve the full benefit of vitamin B supplementation. A clinical trial of B vitamins combined with ω-3 FAs is needed to determine whether it is possible to further improve the prevention of conversion from MCI to AD (36, 95). It is evident from these observations that interventions with B vitamins are unlikely to be successful in preventing cognitive decline and brain atrophy unless they are tailored to meet the specific needs of subgroups and individuals according to their vitamin B and ω-3 FA status, their metabolic profile, and their genetic susceptibility. This could explain the apparent lack of substantive evidence from meta-analyses of vitamin B homocysteine-lowering trials designed to prevent cognitive decline with aging or in MCI and AD (96–98). This conundrum needs to be resolved with better-designed controlled interventions performed in communities with cohorts showing the strongest associations between combined high homocysteine and low vitamin B-12 with AD risk, such as that recently reported in China [adjusted OR: 30.5 (95% CI: 9.7, 95.9); P < 0.0001] (99).
Another reason for past failures with vitamin interventions could be unexpected or previously unknown nutrient-nutrient and nutrient-gene interactions. The fact that nutrient intake combinations may have specific effects on health outcomes is not surprising given the evidence that nutrient-nutrient interactions affect fundamental pathologies such as DNA damage—sometimes in unexpected ways. For example, our studies of dietary nutrient intake and DNA damage in healthy Australians showed that micronucleus frequency in lymphocytes (a biomarker of chromosome breakage or loss), associated with increased risk for cancer, cardiovascular disease, diabetes, dementia, cognitive dysfunction, and microcephaly (100–104), is significantly affected by 1) interactions of folate with riboflavin and calcium, such that micronuclei are increased when riboflavin is increased in a low folate background and when both folate and calcium intakes are low (105, 106), and 2) interactions between polymorphisms in folate metabolism genes such as methionine synthase (MTR; A2756G), methylene tetrahydrofolate reductase (MTHFR; C677T), and reduced folate carrier (A80G) (107, 108). It was evident from genotype combination analyses that the frequency of micronuclei in lymphocytes was highest in those with the combined MTR (2756) AA and reduced folate carrier (79) GA or AA genotype and in those with the TT genotype for the C677T MTHFR polymorphism (107, 108).
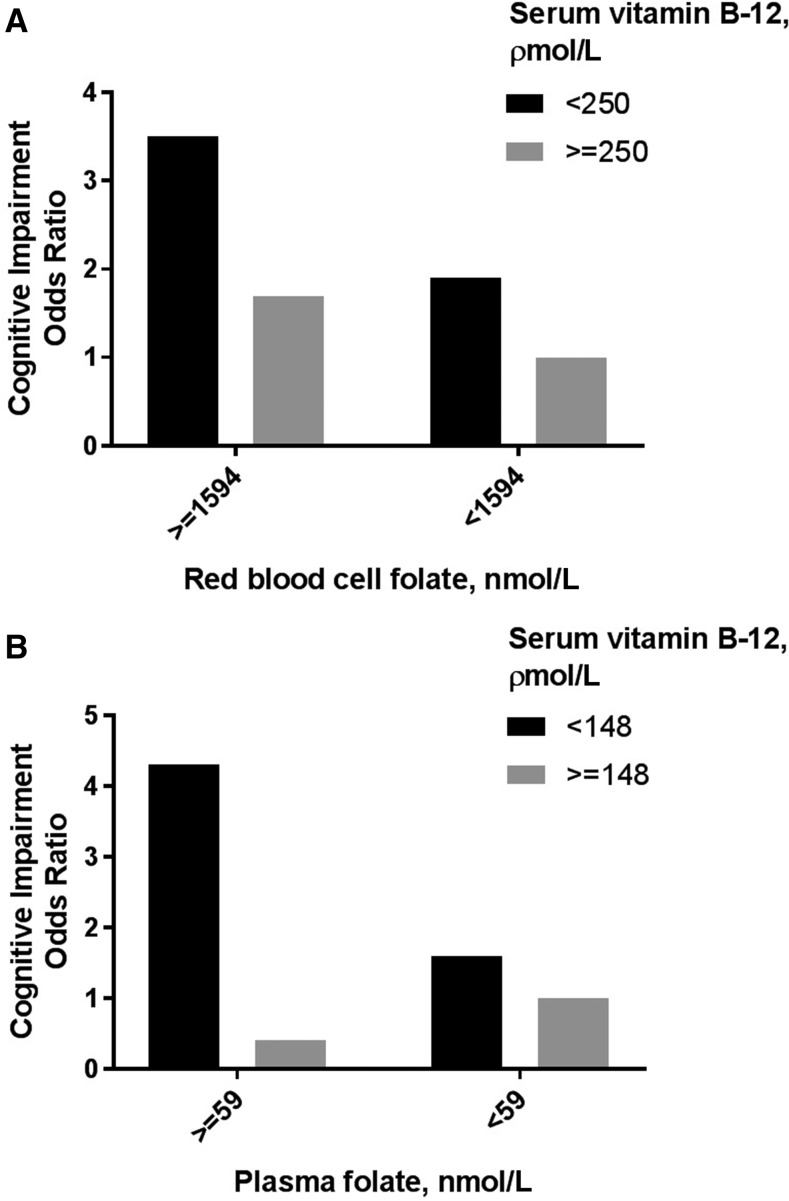
In this regard there is an increasing concern about the cognitive effects of mandatory folic acid fortification in a low vitamin B-12 background, because of the observation in the United States and Australia that older people with low plasma concentrations of vitamin B-12 and high concentrations of folate in the blood have a substantially higher risk of cognitive impairment compared to those with low blood folate and low plasma vitamin B-12 (36, 109–111) (Figure 1). This also raises concerns as to whether the shift to plant-based diets may be jeopardized by folic acid fortification, because vitamin B-12 status in those choosing to shift to vegetarianism may decline substantially unless staple foods are more prevalently supplemented with vitamin B-12. These concerns also raise the urgent need to consider inclusion of vitamin B-12 together with folic acid in fortification programs to minimize risk of vitamin B-12 deficiency and mitigate cognitive deficits induced by high folic acid intake in the elderly (112). The plausibility that excessive intake of folic acid may contribute to cognitive decline is supported by 2 other observations: 1) cognitive decline in the presence of a high plasma folate concentration is observed mainly in those homozygous for the 19-bp deletion in the dihydrofolate reductase gene, which codes for the enzyme that reduces folic acid to the active THF form required for participation in 1-carbon metabolism (113) and 2) excess folic acid in animal models inhibits Mthfr and Mtr expression and promotes thymidylate synthase expression, favoring DNA nucleotide synthesis over homocysteine methylation and the generation of methionine and the methyl donor s-adenosyl methionine required for neurotransmitter synthesis (114, 115). Whether this observation in animal models applies to humans remains undetermined. Nevertheless, these observations indicate the risks inherent in the use of single-vitamin supplementation without considering interactions with other vitamins and genotype that affect the same metabolic pathways. The much higher complexity of nutrient-nutrient interactions and nutrient-genotype interactions across multiple interconnected pathways may vary greatly between individuals when using multinutrient supplementation. This could partly explain the relative inefficacy of Souvenaid [a complex of ω-3 FAs, the nucleotide uridine monophosphate, phospholipids, B complex vitamins (pyridoxine, cyanocobalamin, and folate), choline, vitamin E, and the micronutrient selenium] in preventing cognitive decline in the early stages of AD (116).
FIGURE 1.
High blood folate concentration in combination with low serum vitamin B-12 concentration is associated with a higher risk for cognitive impairment. The graphs show results from a study performed in Australia (109) (A) and a study performed in the United States (110) (B). Data were adapted from references 109 and 110 with permission.
The homocysteine and B vitamins in cognitive impairment intervention (92) illustrated the importance of combining brain neuroimaging with nutritional studies, an approach that is also yielding valuable insights on the impact of a wider spectrum of vitamins and other nutrients in cross-sectional studies. For example, a cross-sectional neuroimaging pilot study investigated dietary nutrient intake and brain biomarkers of AD in at-risk but otherwise cognitively normal individuals (117). In that investigation, fluorodeoxyglucose positron emission tomography and PIB-PET were used to measure glucose uptake and metabolism, and amyloid deposition in the brain, respectively. Glucose uptake and metabolism in the brain were correlated positively with higher intake of folate and β-carotene and were reduced with more saturated fat; on the other hand, amyloid deposition in the brain seemed to be substantially reduced in those with higher intake of vitamin D, vitamin B-12, and the ω-3 PUFA EPA. Using a similar approach, but also including gray matter volume measurements and focusing on nutrient intake patterns, the same group of researchers showed that intake of a nutrient combination that was protective against AD was associated with higher consumption of fresh fruit and vegetables, whole grains, fish, and low-fat dairy products, and lower intake of sweets, fried potatoes, high-fat dairy products, processed meat, and butter in cognitively normal individuals (118).
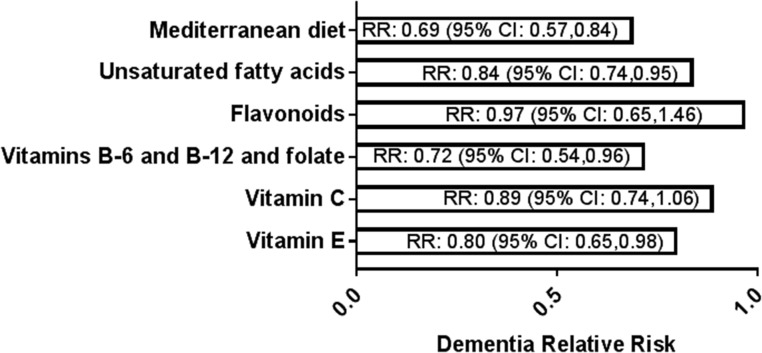
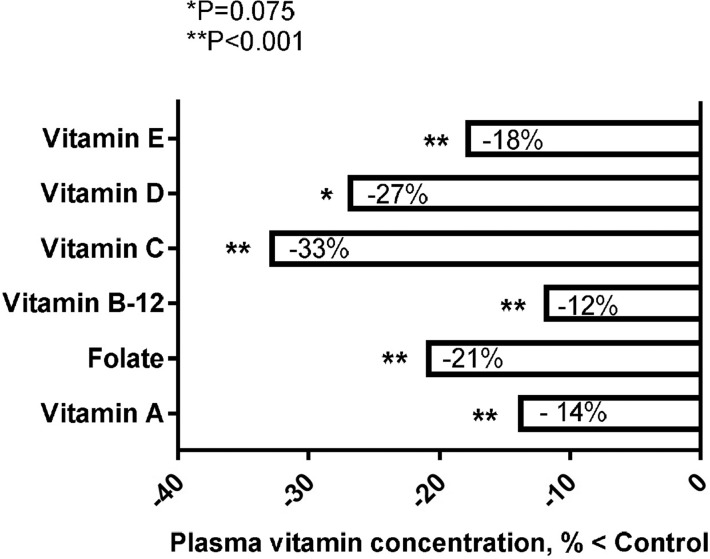
The above associations of folate, β-carotene, vitamin D, vitamin B-12, and ω-3 FA with a healthier brain imaging profile are supported by 1) results from a meta-analysis of 43 prospective cohort studies, showing that the relative risk for dementia was decreased with dietary patterns that reflected a Mediterranean diet [RR: 0.69 (95% CI: 0.57, 0.84); P < 0.0001], higher intake of vitamin B-6, folate, and vitamin B-12 [RR: 0.72 (95% CI: 0.54, 0.96); P = 0.026], vitamin E [RR: 0.80 (95% CI: 0.65, 0.98); P = 0.034], unsaturated FAs [RR: 0.84 (95% CI: 0.74, 0.95); P = 0.006], vitamin C [RR: 0.89 (95% CI: 0.74, 1.06); P = 0.192], and flavonoids [RR: 0.97 (95% CI: 0.65, 1.46); P = 0.896] (119) (Figure 2) and 2) the outcome from a meta-analysis of 106 investigations of plasma vitamin concentration in patients with AD, showing significantly lower values of vitamin B-12 (−12%), vitamin A (−14%), vitamin E (−18%), folate (−21%), vitamin D (−27%), and vitamin C (−33%) than those in healthy controls (120) (Figure 3). However, it is important to note that studies may not always be consistent across populations and cohorts, because dementia may still emerge in cohorts that are not deficient in the above-mentioned vitamins or do not have additional risk factors such as obesity, hyperhomocysteinemia, or inflammation, as was, for example, reported in a recent small cross-sectional study in Norway (121).
FIGURE 2.
Results of a meta-analysis of 43 prospective cohort studies showing dietary factors associated with a reduced RR for dementia (119). The numbers in brackets in the figure are the 95% CIs. Data were adapted from reference 119 with permission.
FIGURE 3.
Results of a meta-analysis of 106 investigations showing statistically significant reductions in the concentration of 6 key vitamins in plasma of patients with AD relative to healthy controls (120). The meta-analysis only included studies that used established criteria for identifying AD cases and cognitively intact controls. Data were adapted from reference 120 with permission. AD, Alzheimer disease.
The effects of less investigated vitamins and “quasi-vitamins”
Less is known about the effects of other vitamins on MCI and AD risk, but results from initial studies suggest the possibility that vitamin B-1 (thiamin), vitamin B-3 (niacin), vitamin K, and other essential nutrients such as inositol, choline, and carnitine may also contribute to the prevention of dementia.
Thiamine-dependent enzymes (transketolase, pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, and branched-chain α-ketoacid dehydrogenase) play a critical role in glycolysis and the Krebs cycle, and deficiencies in the activity of these enzymes may contribute to reduced glucose metabolism, as evident in the brain of patients with dementia (122, 123). Preclinical models of thiamine deficiency and human thiamine deficiency (Korsakoff syndrome) exhibit memory deficits, neuritic plaques, and hyperphosphorylation of tau (123). In addition, dietary supplementation with benfotiamine, a more bioavailable analog of thiamine, enhanced the spatial memory of APP/PSEN1 mutant mice and reduced amyloid plaque and phosphorylated tau in their brains (124). Furthermore, a recent case-control study of humans showed that blood thiamine diphosphate concentration was significantly reduced in patients with AD relative to controls and had an AUROC of 77.4%, sensitivity of 78.1%, and specificity of 77.2% (125). A subsequent study reported that thiamine phophatases are increased in the blood of patients with AD, which may explain the low thiamine diphosphate concentration in AD (126).
Niacin plays an important role in carbohydrate and energy metabolism through its critical involvement as a cofactor in the Krebs cycle for conversion of acetyl-CoA generated from proteins, fats, and carbohydrates to ATP. Furthermore, niacin is important as a cofactor in myelination and DNA repair (68, 127, 128). Niaspan (a prolonged-release formulation of niacin) promoted synaptic plasticity and axon growth in a rodent model of stroke (129). These potential neuroprotective effects of niacin are also supported by a prospective cohort study in 3718 elderly aged ≥65 y which showed that the RR (95% CI) of AD in those in the second, third, fourth, and fifth quintiles of niacin intake was 0.3 (0.1–0.6), 0.3 (0.1–0.7), 0.6 (0.3–1.3), and 0.3 (0.1–0.7), respectively, when compared with those in the first quintile of intake during a 6-y follow-up, and that cognitive decline was diminished with higher niacin intake (130).
An increasing body of evidence suggests a role for vitamin K in brain physiology via its participation in sphingolipid metabolism and biological activation of the vitamin K–dependent growth arrest-specific 6 protein (Gas6), which may protect against neuronal apoptosis induced by amyloid β or oxidative stress (131–133). Four human studies reported an association of low vitamin K intake or low blood concentrations of vitamin K with cognitive impairment or AD (134–137). Studies of dietary intake among older adults indicated that 1) those with serious subjective memory complaints had a lower mean dietary vitamin K intake than those with normal memory (298.0 compared with 393.8 μg/d; P = 0.005) (134), 2) mean vitamin K intake in patients at an early stage of AD, on a person-day basis, was 63 μg/d, compared with 139 μg/d in control subjects (135), and 3) dietary phylloquinone intake was positively associated with better cognitive function (136). A single study of measurements of vitamin K-1 (phylloquinone) and vitamin K-2 (menaquinone) in plasma showed significantly reduced plasma vitamin K-1 concentrations in patients with mild and severe AD compared with healthy controls, but no difference in plasma vitamin K-2 between cases and controls (137). So far, to my knowledge, no intervention studies have been reported to determine whether depletion or supplementation with vitamin K affected cognitive function or prevented brain atrophy.
Evidence of a role for “quasi-vitamins” in providing protection against dementia is available: 1) myo-inositol and scyllo-inositol were observed to have a capacity to inhibit β-secretase-1, which is required to convert APP to amyloid-β or amyloid-β oligomerization (138, 139), 2) choline has a role as precursor of the neurotransmitter acetylcholine, as a methyl donor in 1-carbon metabolism, and as a critical component of membrane phospholipids, and evidence shows that specific choline-containing phospholipids such as CDP-choline and choline alphoscerate improve the cognitive abilities of patients affected by neurodegenerative diseases (140, 141), and 3) carnitine functions to transport acetyl groups from FAs into mitochondria for ATP generation, has a role in acetylcholine synthesis, and has reported restorative effects on mitochondrial function, physical activity, and memory in older rats (142, 143). Furthermore, a double-blind placebo-controlled trial showed that 1-y supplementation with acetyl-carnitine protected against decline in 13 of 14 measures of cognitive and functional performance in MCI and AD (144)
Summary and Conclusions
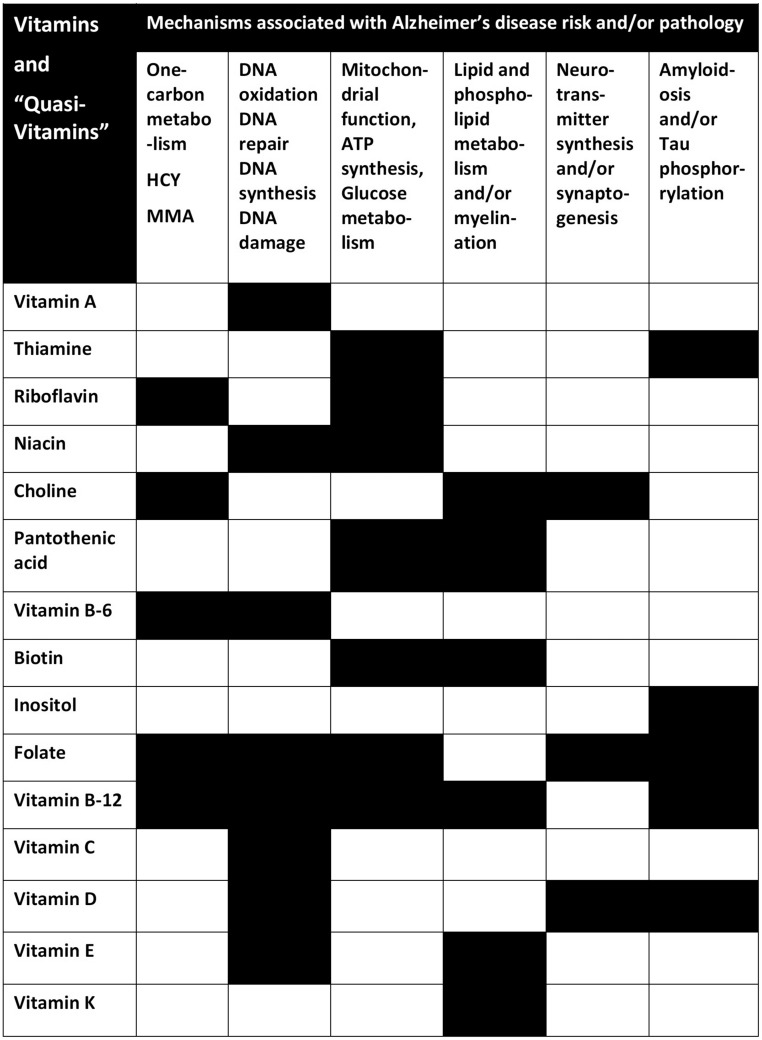
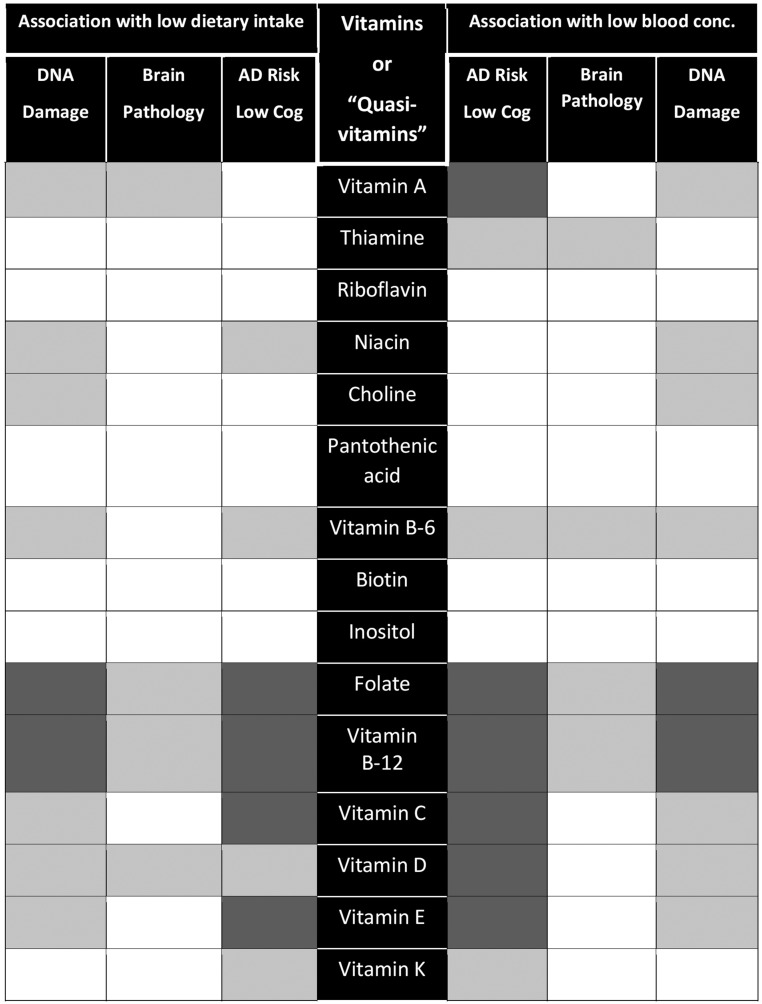
Thirteen vitamins and 3 quasi-vitamins play a substantial role in ≥1 of 6 relevant core pathways or pathologies associated with AD (Figure 4). The level of evidence for each of these vitamins varies considerably (Figure 5), and in most cases it may be insufficient to make specific recommendations. Although it is notable that some vitamins, such as folate and vitamin B-12, are involved as cofactors in ≥5 core pathways or pathologies, it remains challenging to identify and test a combination of vitamins that would best contribute to the prevention of dementia in a precise and predictable manner at the individual level. Furthermore, several outstanding questions remain:
FIGURE 4.
The 6 metabolic pathways or pathologies associated with Alzheimer disease risk and the various vitamins that are required as cofactors in these pathways or that influence the severity of brain pathology (black cells). HCY, homocysteine; MMA, methylmalonic acid.
FIGURE 5.
An overview of the current level of evidence of the association of low dietary intake or low blood concentration of various vitamins with risk for AD or cognitive dysfunction, brain pathology (e.g., brain atrophy, low glucose metabolism, and amyloid plaques), and DNA damage. Dark gray shading indicates substantial evidence, light gray shading indicates limited evidence, and no shading indicates insufficient or no evidence. AD, Alzheimer disease; Cog, cognitive function; conc., concentration.
Which vitamins and which pathways should be prioritized for further research?
Should more effort be invested in replication studies, especially for some of the vitamins for which mechanistic plausibility exists but only minimal preliminary data are available from humans?
The homocysteine and B vitamins in cognitive impairment intervention suggest that supplementation with just 3 B vitamins targeted to a relevant pathway (i.e., 1-carbon metabolism) may contribute to slowing cognitive decline in the early stages of dementia. Could effects be improved further by including other vitamins targeting other pathways, or would this be counterproductive?
Efficacy is likely to be modest unless dosages are adequate and combinations are personalized based on need, metabolic phenotype, and genotype. Which combinations and doses should be used and for which subgroups?
When and for how long should we intervene? The timing of intervention should be as early as possible before symptoms of MCI become evident, but how soon before remains unclear. Is 2 y the minimum intervention period to see a reliable effect on reversal of brain pathology and cognitive decline? Will it be enough to predict that if a benefit occurs in an individual or subgroup it will be reliably sustained in the long-term?
Detrimental interaction effects are also possible, suggesting the importance of including biomarkers of safety in intervention trials. Which biomarkers should be used to ensure that no harm is done? Would biomarkers of DNA damage be sufficient for this purpose, or would a more comprehensive pathology assessment be required?
In conclusion, future research should focus much more on a precision nutrition approach. Multiple omics technologies and bioinformatics, in combination with brain imaging and cognitive function tests, will be required to both design and test multivitamin combinations (nutriomes) that are tailored appropriately to match the metabolic phenotype and genotype of individuals and subgroups.
Acknowledgments
The sole author was responsible for all parts of the manuscript.
Footnotes
Abbreviations used: AD, Alzheimer disease; APP, amyloid precursor protein; AUROC, area under the receiver operating characteristic curve; Aβ42, amyloid beta 42; CK14, cytokeratin 14; CSF, cerebrospinal fluid; MCI, mild cognitive impairment; MTHFR, methylene tetrahydrofolate reductase; MTR, methionine synthase; PIB-PET, Pittsburgh compound B-positron emission tomography; PSEN, presenilin; REST, repressor element 1–silencing transcription factor; rRNA, ribosomal RNA; TET, 10–11 translocation; TOM, translocase of the mitochondrial outer membrane.
References
- 1.Jack CR Jr., Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, et al. . Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013;12:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rege SD, Geetha T, Broderick TL, Babu JR. Can diet and physical activity limit Alzheimer’s disease risk? Curr Alzheimer Res 2017;14:76–93. [DOI] [PubMed] [Google Scholar]
- 3.Monti JM, Moulton CJ, Cohen NJ. The role of nutrition on cognition and brain health in ageing: a targeted approach. Nutr Res Rev 2015;28:167–80. [DOI] [PubMed] [Google Scholar]
- 4.Gustafson DR, Clare Morris M, Scarmeas N, Shah RC, Sijben J, Yaffe K, Zhu X. New perspectives on Alzheimer’s disease and nutrition. J Alzheimers Dis 2015;46:1111–27. [DOI] [PubMed] [Google Scholar]
- 5.Gillette-Guyonnet S, Secher M, Vellas B.. Nutrition and neurodegeneration: epidemiological evidence and challenges for future research. Br J Clin Pharmacol 2013;75:738–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferry M, Coley N, Andrieu S, Bonhomme C, Caubère JP, Cesari M, Gautry J, Garcia Sanchez I, Hugonot L, Mansuy L, et al. . How to design nutritional intervention trials to slow cognitive decline in apparently healthy populations and apply for efficacy claims: a statement from the International Academy on Nutrition and Aging Task Force. J Nutr Health Aging 2013;17:619–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013;153:1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López-Otín C, Galluzzi L, Freije JM, Madeo F, Kroemer G. Metabolic control of longevity. Cell 2016;166:802–21. [DOI] [PubMed] [Google Scholar]
- 9.Olsson B, Lautner R, Andreasson U, Öhrfelt A, Portelius E, Bjerke M, Hölttä M, Rosén C, Olsson C, Strobel G, et al. . CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol 2016;15:673–84. [DOI] [PubMed] [Google Scholar]
- 10.Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, Hampel H, Jagust WJ, Johnson KA, Knopman DS, et al. . A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016;87:539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg RN, Lambracht-Washington D, Yu G, Xia W. Genomics of Alzheimer disease: a review. JAMA Neurol 2016;73:867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfe MS. Processive proteolysis by γ-secretase and the mechanism of Alzheimer’s disease. Biol Chem 2012;393:899–905. [DOI] [PubMed] [Google Scholar]
- 13.Olazarán J, Gil-de-Gómez L, Rodríguez-Martín A, Valentí-Soler M, Frades-Payo B, Marín-Muñoz J, Antúnez C, Frank-García A, Acedo-Jiménez C, Morlán-Gracia L, et al. . A blood-based, 7-metabolite signature for the early diagnosis of Alzheimer’s disease. J Alzheimers Dis 2015;45:1157–73. [DOI] [PubMed] [Google Scholar]
- 14.Muenchhoff J, Poljak A, Song F, Raftery M, Brodaty H, Duncan M, McEvoy M, Attia J, Schofield PW, Sachdev PS. Plasma protein profiling of mild cognitive impairment and Alzheimer’s disease across two independent cohorts. J Alzheimers Dis 2015;43:1355–73. [DOI] [PubMed] [Google Scholar]
- 15.Doecke JD, Laws SM, Faux NG, Wilson W, Burnham SC, Lam CP, Mondal A, Bedo J, Bush AI, Brown B, et al. ; Alzheimer’s Disease Neuroimaging Initiative; Australian Imaging Biomarker and Lifestyle Research Group. Blood-based protein biomarkers for diagnosis of Alzheimer disease. Arch Neurol 2012;69:1318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zarrouk A, Riedinger JM, Ahmed SH, Hammami S, Chaabane W, Debbabi M, Ben Ammou S, Rouaud O, Frih M, Lizard G, et al. . Fatty acid profiles in demented patients: identification of hexacosanoic acid (C26:0) as a blood lipid biomarker of dementia. J Alzheimers Dis 2015;44:1349–59. [DOI] [PubMed] [Google Scholar]
- 17.Olde Rikkert MG, Verhey FR, Sijben JW, Bouwman FH, Dautzenberg PL, Lansink M, Sipers WM, van Asselt DZ, van Hees AM, Stevens M, et al. . Differences in nutritional status between very mild Alzheimer’s disease patients and healthy controls. J Alzheimers Dis 2014;41:261–71. [DOI] [PubMed] [Google Scholar]
- 18.Milte CM, Sinn N, Street SJ, Buckley JD, Coates AM, Howe PR. Erythrocyte polyunsaturated fatty acid status, memory, cognition and mood in older adults with mild cognitive impairment and healthy controls. Prostaglandins Leukot Essent Fatty Acids 2011;84:153–61. [DOI] [PubMed] [Google Scholar]
- 19.Yuan L, Zhen J, Ma W, Cai C, Huang X, Xiao R. The erythrocyte fatty acid profile and cognitive function in old Chinese adults. Nutrients 2016;8:E385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.François M, Leifert W, Martins R, Thomas P, Fenech M.. Biomarkers of Alzheimer’s disease risk in peripheral tissues; focus on buccal cells. Curr Alzheimer Res 2014;11:519–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.François M, Fenech MF, Thomas P, Hor M, Rembach A, Martins RN, Rainey-Smith SR, Masters CL, Ames D, Rowe CC, et al. . High content, multi-parameter analyses in buccal cells to identify Alzheimer’s disease. Curr Alzheimer Res 2016;13:787–99. [DOI] [PubMed] [Google Scholar]
- 22.Thomas P, Fenech M. Buccal cytome biomarkers and their association with plasma folate, vitamin B12 and homocysteine in Alzheimer’s disease. J Nutrigenet Nutrigenomics 2015;8:57–69. [DOI] [PubMed] [Google Scholar]
- 23.Thomas P, Hecker J, Faunt J, Fenech M. Buccal micronucleus cytome biomarkers may be associated with Alzheimer’s disease. Mutagenesis 2007;22:371–9. [DOI] [PubMed] [Google Scholar]
- 24.Leifert WR, Tuli JF, Francois M, Nguyen T, Rembach A, Rumble RL, Rainey-Smith S, Martins R, Fenech MF. Buccal cell cytokeratin 14 identifies mild cognitive impairment and Alzheimer’ s disease in the AIBL study of aging. Curr Alzheimer Res 2015;12:233–41. [DOI] [PubMed] [Google Scholar]
- 25.Leifert WR, Nguyen T, Rembach A, Martins R, Rainey-Smith S, Masters CL, Ames D, Rowe CC, Macaulay SL, François M, et al. . Buccal cell cytokeratin 14 correlates with multiple blood biomarkers of Alzheimer’s disease risk. J Alzheimers Dis 2015;48:443–52. [DOI] [PubMed] [Google Scholar]
- 26.Forero DA, González-Giraldo Y, López-Quintero C, Castro-Vega LJ, Barreto GE, Perry G. Meta-analysis of telomere length in Alzheimer’s disease. J Gerontol A Biol Sci Med Sci 2016;71:1069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas P, O’ Callaghan NJ, Fenech M. Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer’s disease. Mech Ageing Dev 2008;129:183–90. [DOI] [PubMed] [Google Scholar]
- 28.Dhillon V, Bull C, Fenech M. Telomere, ageing and nutrition Malavolta E, Mocchegiani M, editors. Molecular basis of nutrition and ageing. London: Elsevier; 2016. p. 129–40. [Google Scholar]
- 29.Rafie N, Golpour Hamedani S, Barak F, Safavi SM, Miraghajani M. Dietary patterns, food groups and telomere length: a systematic review of current studies. Eur J Clin Nutr 2017;71:151–8. [DOI] [PubMed] [Google Scholar]
- 30.van de Rest O, Berendsen AA, Haveman-Nies A, de Groot LC. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr 2015;6:154–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feart C, Samieri C, Barberger-Gateau P. Mediterranean diet and cognitive health: an update of available knowledge. Curr Opin Clin Nutr Metab Care 2015;18:51–62. [DOI] [PubMed] [Google Scholar]
- 32.Swaminathan A, Jicha GA. Nutrition and prevention of Alzheimer’s dementia. Front Aging Neurosci 2014;6:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pedditizi E, Peters R, Beckett N. The risk of overweight/obesity in mid-life and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies. Age Ageing 2016;45:14–21. [DOI] [PubMed] [Google Scholar]
- 34.Ames BN. The metabolic tune-up: metabolic harmony and disease prevention. J Nutr 2003;133(5 Suppl 1):1544S–8S. [DOI] [PubMed] [Google Scholar]
- 35.Guyonnet S, Secher M, Vellas B. Nutrition, frailty, cognitive frailty and prevention of disabilities with aging. Nestle Nutr Inst Workshop Ser 2015;82:143–52. [DOI] [PubMed] [Google Scholar]
- 36.Smith AD, Refsum H. Homocysteine, B vitamins, and cognitive impairment. Annu Rev Nutr 2016;36:211–39. [DOI] [PubMed] [Google Scholar]
- 37.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM. Alzheimer’s disease. Lancet 2016;388:505–17. [DOI] [PubMed] [Google Scholar]
- 38.Liu H, Tian T, Qin S, Li W, Zhang X, Wang X, Gao Y, Huang G. Folic acid deficiency enhances abeta accumulation in APP/PS1 mice brain and decreases amyloid-associated miRNAs expression. J Nutr Biochem 2015;26:1502–8. [DOI] [PubMed] [Google Scholar]
- 39.Li W, Liu H, Yu M, Zhang X, Zhang M, Wilson JX, Huang G. Folic acid administration inhibits amyloid β-peptide accumulation in APP/PS1 transgenic mice. J Nutr Biochem 2015;26:883–91. [DOI] [PubMed] [Google Scholar]
- 40.Dixit S, Bernardo A, Walker JM, Kennard JA, Kim GY, Kessler ES, Harrison FE. Vitamin C deficiency in the brain impairs cognition, increases amyloid accumulation and deposition, and oxidative stress in APP/PSEN1 and normally aging mice. ACS Chem Neurosci 2015;6:570–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harrison FE, Hosseini AH, McDonald MP, May JM. Vitamin C reduces spatial learning deficits in middle-aged and very old APP/PSEN1 transgenic and wild-type mice. Pharmacol Biochem Behav 2009;93:443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Q, Yang G, Li W, Fan Z, Sun A, Luo J, Ke ZJ. Thiamine deficiency increases β-secretase activity and accumulation of β-amyloid peptides. Neurobiol Aging 2011;32:42–53. [DOI] [PubMed] [Google Scholar]
- 43.Bennett L, Kersaitis C, Macaulay SL, Münch G, Niedermayer G, Nigro J, Payne M, Sheean P, Vallotton P, Zabaras D, et al. . Vitamin D2-enriched button mushroom (Agaricus bisporus) improves memory in both wild type and APPswe/PS1dE9 transgenic mice. PLoS One 2013;8:e76362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gong B, Pan Y, Vempati P, Zhao W, Knable L, Ho L, Wang J, Sastre M, Ono K, Sauve AA, et al. . Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol Aging 2013;34:1581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu J, Gattoni-Celli M, Zhu H, Bhat NR, Sambamurti K, Gattoni-Celli S, Kindy MS. Vitamin D3-enriched diet correlates with a decrease of amyloid plaques in the brain of AβPP transgenic mice. J Alzheimers Dis 2011;25:295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishida Y, Ito S, Ohtsuki S, Yamamoto N, Takahashi T, Iwata N, Jishage K, Yamada H, Sasaguri H, Yokota S, et al. . Depletion of vitamin E increases amyloid beta accumulation by decreasing its clearances from brain and blood in a mouse model of Alzheimer disease. J Biol Chem 2009;284:33400–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fenili D, Brown M, Rappaport R, McLaurin J. Properties of scyllo-inositol as a therapeutic treatment of AD-like pathology. J Mol Med (Berl) 2007;85:603–11. [DOI] [PubMed] [Google Scholar]
- 48.Sung S, Yao Y, Uryu K, Yang H, Lee VM, Trojanowski JQ, Praticò D. Early vitamin E supplementation in young but not aged mice reduces Abeta levels and amyloid deposition in a transgenic model of Alzheimer’s disease. FASEB J 2004;18:323–5. [DOI] [PubMed] [Google Scholar]
- 49.Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 2013;9:106–18. Erratum in: Nat Rev Neurol 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee YM, Ha JK, Park JM, Lee BD, Moon E, Chung YI, Kim JH, Kim HJ, Mun CW, Kim TH, et al. . Apolipoprotein E genotype modulates effects of vitamin B12 and homocysteine on grey matter volume in Alzheimer’s disease. Psychogeriatrics 2016;16:3–11. [DOI] [PubMed] [Google Scholar]
- 51.Vogiatzoglou A, Smith AD, Nurk E, Drevon CA, Ueland PM, Vollset SE, Nygaard HA, Engedal K, Tell GS, Refsum H. Cognitive function in an elderly population: interaction between vitamin B12 status, depression, and apolipoprotein E ε4: the Hordaland Homocysteine Study. Psychosom Med 2013;75:20–9. [DOI] [PubMed] [Google Scholar]
- 52.Feng L, Li J, Yap KB, Kua EH, Ng TP. Vitamin B-12, apolipoprotein E genotype, and cognitive performance in community-living older adults: evidence of a gene-micronutrient interaction. Am J Clin Nutr 2009;89:1263–8. [DOI] [PubMed] [Google Scholar]
- 53.Sakae N, Liu CC, Shinohara M, Frisch-Daiello J, Ma L, Yamazaki Y, Tachibana M, Younkin L, Kurti A, Carrasquillo MM, et al. . ABCA7 deficiency accelerates amyloid-β generation and Alzheimer’s neuronal pathology. J Neurosci 2016;36:3848–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Humphries CE, Kohli MA, Nathanson L, Whitehead P, Beecham G, Martin E, Mash DC, Pericak-Vance MA, Gilbert J. Integrated whole transcriptome and DNA methylation analysis identifies gene networks specific to late-onset Alzheimer’s disease. J Alzheimers Dis 2015;44:977–87. [DOI] [PubMed] [Google Scholar]
- 55.Villela D, Ramalho RF, Silva AR, Brentani H, Suemoto CK, Pasqualucci CA, Grinberg LT, Krepischi AC, Rosenberg C. Differential DNA methylation of microRNA genes in temporal cortex from Alzheimer’s disease individuals. Neural Plast 2016;2016:2584940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pietrzak M, Rempala G, Nelson PT, Zheng JJ, Hetman M. Epigenetic silencing of nucleolar rRNA genes in Alzheimer’s disease. PLoS One 2011;6:e22585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hernández-Ortega K, Garcia-Esparcia P, Gil L, Lucas JJ, Ferrer I. Altered machinery of protein synthesis in Alzheimer’s: from the nucleolus to the ribosome. Brain Pathol 2016;26:593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mann DM, Yates PO, Marcyniuk B. Some morphometric observations on the cerebral cortex and hippocampus in presenile Alzheimer’s disease, senile dementia of Alzheimer type and Down’s syndrome in middle age. J Neurol Sci 1985;69:139–59. [DOI] [PubMed] [Google Scholar]
- 59.Grummt I, Ladurner AG. A metabolic throttle regulates the epigenetic state of rDNA. Cell 2008;133:577–80. [DOI] [PubMed] [Google Scholar]
- 60.Denisenko O, Lucas ES, Sun C, Watkins AJ, Mar D, Bomsztyk K, Fleming TP. Regulation of ribosomal RNA expression across the lifespan is fine-tuned by maternal diet before implantation. Biochim Biophys Acta 2016;1859:906–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malatesta M, Bertoni-Freddari C, Fattoretti P, Caporaloni C, Fakan S, Gazzanelli G. Altered RNA structural constituents in aging and vitamin Edeficiency. Mech Ageing Dev 2003;124:175–81. [DOI] [PubMed] [Google Scholar]
- 62.Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature 2004;429:883–91. [DOI] [PubMed] [Google Scholar]
- 63.Lu T, Aron L, Zullo J, Pan Y, Kim H, Chen Y, Yang TH, Kim HM, Drake D, Liu XS, et al. . REST and stress resistance in ageing and Alzheimer’s disease. Nature 2014;507:448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sram RJ, Binkova B, Rossner P Jr. Vitamin C for DNA damage prevention. Mutat Res 2012;733:39–49. [DOI] [PubMed] [Google Scholar]
- 65.Traber MG. Vitamin E inadequacy in humans: causes and consequences. Adv Nutr 2014;5:503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ames BN, Atamna H, Killilea DW. Mineral and vitamin deficiencies can accelerate the mitochondrial decay of aging. Mol Aspects Med 2005;26:363–78. [DOI] [PubMed] [Google Scholar]
- 67.Fenech M. Folate (vitamin B9) and vitamin B12 and their function in the maintenance of nuclear and mitochondrial genome integrity. Mutat Res 2012;733:21–33. [DOI] [PubMed] [Google Scholar]
- 68.Kirkland JB. Niacin requirements for genomic stability. Mutat Res 2012;733:14–20. [DOI] [PubMed] [Google Scholar]
- 69.Sharif R, Thomas P, Zalewski P, Fenech M. The role of zinc in genomic stability. Mutat Res 2012;733:111–21. [DOI] [PubMed] [Google Scholar]
- 70.May JM. Vitamin C transport and its role in the central nervous system. Subcell Biochem 2012;56:85–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hyland K, Shoffner J, Heales SJ. Cerebral folate deficiency. J Inherit Metab Dis 2010;33:563–70. [DOI] [PubMed] [Google Scholar]
- 72.Cheng Y, Bernstein A, Chen D, Jin P.. 5-Hydroxymethylcytosine: a new player in brain disorders? Exp Neurol 2015;268:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rudenko A, Dawlaty MM, Seo J, Cheng AW, Meng J, Le T, Faull KF, Jaenisch R, Tsai LH. Tet1 is critical for neuronal activity-regulated gene expression and memory extinction. Neuron 2013;79:1109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaas GA, Zhong C, Eason DE, Ross DL, Vachhani RV, Ming GL, King JR, Song H, Sweatt JD. TET1 controls CNS 5-methylcytosine hydroxylation, active DNA demethylation, gene transcription, and memory formation. Neuron 2013;79:1086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yin R, Mao SQ, Zhao B, Chong Z, Yang Y, Zhao C, Zhang D, Huang H, Gao J, Li Z, et al. . Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. J Am Chem Soc 2013;135:10396–403. [DOI] [PubMed] [Google Scholar]
- 76.Minor EA, Court BL, Young JI, Wang G. Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J Biol Chem 2013;288:13669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tong Z, Wang W, Luo W, Lv J, Li H, Luo H, Jia J, He R. Urine formaldehyde predicts cognitive impairment in post-stroke dementia and Alzheimer’s disease. J Alzheimers Dis 2017;55:1031–8. [DOI] [PubMed] [Google Scholar]
- 78.Tong Z, Zhang J, Luo W, Wang W, Li F, Li H, Luo H, Lu J, Zhou J, Wan Y, et al. . Urine formaldehyde level is inversely correlated to mini mental state examination scores in senile dementia. Neurobiol Aging 2011;32:31–41. [DOI] [PubMed] [Google Scholar]
- 79.Tong Z, Han C, Qiang M, Wang W, Lv J, Zhang S, Luo W, Li H, Luo H, Zhou J, et al. . Age-related formaldehyde interferes with DNA methyltransferase function, causing memory loss in Alzheimer’s disease. Neurobiol Aging 2015;36:100–10. [DOI] [PubMed] [Google Scholar]
- 80.Brosnan ME, Brosnan JT. Formate: the neglected member of one-carbon metabolism. Annu Rev Nutr 2016;36:369–88. [DOI] [PubMed] [Google Scholar]
- 81.Lamarre SG, Morrow G, Macmillan L, Brosnan ME, Brosnan JT. Formate: an essential metabolite, a biomarker, or more? Clin Chem Lab Med 2013;51:571–8. [DOI] [PubMed] [Google Scholar]
- 82.Stempler S, Yizhak K, Ruppin E. Integrating transcriptomics with metabolic modelling predicts biomarkers and drug targets for Alzheimer’s disease. PLoS One 2014;9:e105383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chou YF, Huang RF. Mitochondrial DNA deletions of blood lymphocytes as genetic markers of low folate-related mitochondrial genotoxicity in peripheral tissues. Eur J Nutr 2009;48:429–36. [DOI] [PubMed] [Google Scholar]
- 84.Chou YF, Yu CC, Huang RF. Changes in mitochondrial DNA deletion, content, and biogenesis in folate-deficient tissues of young rats depend on mitochondrial folate and oxidative DNA injuries. J Nutr 2007;137:2036–42. [DOI] [PubMed] [Google Scholar]
- 85.Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, Beal MF, Wallace DC. Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat Genet 1992;2:324–9. [DOI] [PubMed] [Google Scholar]
- 86.Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, McKee AC, Beal MF, Graham BH, Wallace DC. Marked changes in mitochondrial DNA deletion levels in Alzheimer brains. Genomics 1994;23:471–6. [DOI] [PubMed] [Google Scholar]
- 87.Phillips NR, Simpkins JW, Roby RK. Mitochondrial DNA deletions in Alzheimer’s brains: a review. Alzheimers Dement 2014;10:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dhillon VS, Fenech M. Mutations that affect mitochondrial functions and their association with neurodegenerative diseases. Mutat Res Rev Mutat Res 2014;759:1–13. [DOI] [PubMed] [Google Scholar]
- 89.Gottschalk WK, Lutz MW, He YT, Saunders AM, Burns DK, Roses AD, Chiba-Falek O. The broad impact of TOM40 on neurodegenerative diseases in aging. J Parkinsons Dis Alzheimers Dis 2014;1 pii.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kennedy DO. B vitamins and the brain: mechanisms, dose and efficacy–a review. Nutrients 2016;8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vogiatzoglou A, Refsum H, Johnston C, Smith SM, Bradley KM, de Jager C, Budge MM, Smith AD. Vitamin B12 status and rate of brain volume loss in community-dwelling elderly. Neurology 2008;71:826–32. [DOI] [PubMed] [Google Scholar]
- 92.Smith AD, Smith SM, de Jager CA, Whitbread P, Johnston C, Agacinski G, Oulhaj A, Bradley KM, Jacoby R, Refsum H. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS One 2010;5:e12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oulhaj A, Jernerén F, Refsum H, Smith AD, de Jager CA. Omega-3 fatty acid status enhances the prevention of cognitive decline by B vitamins in mild cognitive impairment. J Alzheimers Dis 2016;50:547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Jager CA, Oulhaj A, Jacoby R, Refsum H, Smith AD. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: a randomized controlled trial. Int J Geriatr Psychiatry 2012;27:592–600. [DOI] [PubMed] [Google Scholar]
- 95.Douaud G, Refsum H, de Jager CA, Jacoby R, Nichols TE, Smith SM, Smith AD. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc Natl Acad Sci U S A 2013;110:9523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith AD, de Jager CA, Refsum H, Rosenberg IH. Homocysteine lowering, B vitamins, and cognitive aging. Am J Clin Nutr 2015;101:415–6. [DOI] [PubMed] [Google Scholar]
- 97.Clarke R, Bennett D, Parish S, Lewington S, Skeaff M, Eussen SJ, Lewerin C, Stott DJ, Armitage J, Hankey GJ, et al. . Effects of homocysteine lowering with B vitamins on cognitive aging: meta-analysis of 11 trials with cognitive data on 22,000 individuals. Am J Clin Nutr 2014;100:657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li MM, Yu JT, Wang HF, Jiang T, Wang J, Meng XF, Tan CC, Wang C, Tan L. Efficacy of vitamins B supplementation on mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. Curr Alzheimer Res 2014;11:844–52. [PubMed] [Google Scholar]
- 99.Chen H, Liu S, Ji L, Wu T, Ma F, Ji Y, Zhou Y, Zheng M, Zhang M, Huang G. Associations between Alzheimer’s disease and blood homocysteine, vitamin B12, and folate: a case-control study. Curr Alzheimer Res 2015;12:88–94. [DOI] [PubMed] [Google Scholar]
- 100.Bonassi S, El-Zein R, Bolognesi C, Fenech M. Micronuclei frequency in peripheral blood lymphocytes and cancer risk: evidence from human studies. Mutagenesis 2011;26:93–100. [DOI] [PubMed] [Google Scholar]
- 101.Andreassi MG, Barale R, Iozzo P, Picano E. The association of micronucleus frequency with obesity, diabetes and cardiovascular disease. Mutagenesis 2011;26:77–83. [DOI] [PubMed] [Google Scholar]
- 102.Migliore L, Coppedè F, Fenech M, Thomas P. Association of micronucleus frequency with neurodegenerative diseases. Mutagenesis 2011;26:85–92. [DOI] [PubMed] [Google Scholar]
- 103.Lee SL, Thomas P, Hecker J, Faunt J, Fenech M. Chromosomal DNA damage measured using the cytokinesis-block micronucleus cytome assay is significantly associated with cognitive impairment in South Australians. Environ Mol Mutagen 2015;56:32–40. [DOI] [PubMed] [Google Scholar]
- 104.Martin CA, Murray JE, Carroll P, Leitch A, Mackenzie KJ, Halachev M, Fetit AE, Keith C, Bicknell LS, Fluteau A, et al. . Mutations in genes encoding condensin complex proteins cause microcephaly through decatenation failure at mitosis. Genes Dev 2016;30:2158–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fenech M, Baghurst P, Luderer W, Turner J, Record S, Ceppi M, Bonassi S. Low intake of calcium, folate, nicotinic acid, vitamin E, retinol, beta-carotene and high intake of pantothenic acid, biotin and riboflavin are significantly associated with increased genome instability–results from a dietary intake and micronucleus index survey in South Australia. Carcinogenesis 2005;26:991–9. [DOI] [PubMed] [Google Scholar]
- 106.Kimura M, Umegaki K, Higuchi M, Thomas P, Fenech M. Methylenetetrahydrofolate reductase C677T polymorphism, folic acid and riboflavin are important determinants of genome stability in cultured human lymphocytes. J Nutr 2004;134:48–56. [DOI] [PubMed] [Google Scholar]
- 107.Dhillon V, Thomas P, Fenech M. Effect of common polymorphisms in folate uptake and metabolism genes on frequency of micronucleated lymphocytes in a South Australian cohort. Mutat Res 2009;665:1–6. [DOI] [PubMed] [Google Scholar]
- 108.Dhillon VS, Thomas P, Iarmarcovai G, Kirsch-Volders M, Bonassi S, Fenech M. Genetic polymorphisms of genes involved in DNA repair and metabolism influence micronucleus frequencies in human peripheral blood lymphocytes. Mutagenesis 2011;26:33–42. [DOI] [PubMed] [Google Scholar]
- 109.Moore EM, Ames D, Mander AG, Carne RP, Brodaty H, Woodward MC, Boundy K, Ellis KA, Bush AI, Faux NG, et al. . Among vitamin B12 deficient older people, high folate levels are associated with worse cognitive function: combined data from three cohorts. J Alzheimers Dis 2014;39:661–8. [DOI] [PubMed] [Google Scholar]
- 110.Selhub J, Morris MS, Jacques PF, Rosenberg IH. Folate-vitamin B-12 interaction in relation to cognitive impairment, anemia, and biochemical indicators of vitamin B-12 deficiency. Am J Clin Nutr 2009;89:702S–6S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Selhub J, Rosenberg IH. Excessive folic acid intake and relation to adverse health outcome. Biochimie 2016;126:71–8. [DOI] [PubMed] [Google Scholar]
- 112.Allen LH, Rosenberg IH, Oakley GP, Omenn GS. Considering the case for vitamin B12 fortification of flour. Food Nutr Bull 2010;31(1 Suppl):S36–46. [DOI] [PubMed] [Google Scholar]
- 113.Philip D, Buch A, Moorthy D, Scott TM, Parnell LD, Lai CQ, Ordovás JM, Selhub J, Rosenberg IH, Tucker KL, et al. . Dihydrofolate reductase 19-bp deletion polymorphism modifies the association of folate status with memory in a cross-sectional multi-ethnic study of adults. Am J Clin Nutr 2015;102:1279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Christensen KE, Mikael LG, Leung KY, Lévesque N, Deng L, Wu Q, Malysheva OV, Best A, Caudill MA, Greene ND, et al. . High folic acid consumption leads to pseudo-MTHFR deficiency, altered lipid metabolism, and liver injury in mice. Am J Clin Nutr 2015;101:646–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ortbauer M, Ripper D, Fuhrmann T, Lassi M, Auernigg-Haselmaier S, Stiegler C, König J. Folate deficiency and over-supplementation causes impaired folate metabolism: regulation and adaptation mechanisms in Caenorhabditis elegans. Mol Nutr Food Res 2016;60:949–56. [DOI] [PubMed] [Google Scholar]
- 116.Onakpoya IJ, Heneghan CJ. The efficacy of supplementation with the novel medical food, Souvenaid, in patients with Alzheimer’s disease: a systematic review and meta-analysis of randomized clinical trials. Nutr Neurosci 2017;20:219–227. [DOI] [PubMed] [Google Scholar]
- 117.Mosconi L, Murray J, Davies M, Williams S, Pirraglia E, Spector N, Tsui WH, Li Y, Butler T, Osorio RS, et al. . Nutrient intake and brain biomarkers of Alzheimer’s disease in at-risk cognitively normal individuals: a cross-sectional neuroimaging pilot study. BMJ Open 2014;4:e004850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Berti V, Murray J, Davies M, Spector N, Tsui WH, Li Y, Williams S, Pirraglia E, Vallabhajosula S, McHugh P, et al. . Nutrient patterns and brain biomarkers of Alzheimer’s disease in cognitively normal individuals. J Nutr Health Aging 2015;19:413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cao L, Tan L, Wang HF, Jiang T, Zhu XC, Lu H, Tan MS, Yu JT. Dietary patterns and risk of dementia: a systematic review and meta-analysis of cohort studies. Mol Neurobiol 2016;53:6144–54. [DOI] [PubMed] [Google Scholar]
- 120.Lopes da Silva S, Vellas B, Elemans S, Luchsinger J, Kamphuis P, Yaffe K, Sijben J, Groenendijk M, Stijnen T. Plasma nutrient status of patients with Alzheimer’s disease: systematic review and meta-analysis. Alzheimers Dement 2014;10:485–502. [DOI] [PubMed] [Google Scholar]
- 121.Ulstein I, Bøhmer T. Normal vitamin levels and nutritional indices in Alzheimer’s disease patients with mild cognitive impairment or dementia with normal body mass indexes. J Alzheimers Dis 2017;55:717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen Z, Zhong C.. Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Prog Neurobiol 2013;108:21–43. [DOI] [PubMed] [Google Scholar]
- 123.Gibson GE, Hirsch JA, Fonzetti P, Jordan BD, Cirio RT, Elder J. Vitamin B1 (thiamine) and dementia. Ann N Y Acad Sci 2016;1367:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pan X, Gong N, Zhao J, Yu Z, Gu F, Chen J, Sun X, Zhao L, Yu M, Xu Z, et al. . Powerful beneficial effects of benfotiamine on cognitive impairment and beta-amyloid deposition in amyloid precursor protein/presenilin-1 transgenic mice. Brain 2010;133:1342–51. [DOI] [PubMed] [Google Scholar]
- 125.Pan X, Fei G, Lu J, Jin L, Pan S, Chen Z, Wang C, Sang S, Liu H, Hu W, et al. . Measurement of blood thiamine metabolites for Alzheimer’s disease diagnosis. EBioMedicine 2015;3:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pan X, Sang S, Fei G, Jin L, Liu H, Wang Z, Wang H, Zhong C. Enhanced activities of blood thiamine diphosphatase and monophosphatase in Alzheimer’s disease. PLoS One 2017;12:e0167273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xu P, Sauve AA. Vitamin B3, the nicotinamide adenine dinucleotides and aging. Mech Ageing Dev 2010;131:287–98. [DOI] [PubMed] [Google Scholar]
- 128.Nakashima Y, Suzue R. Effect of nicotinic acid on myelin lipids in brain of developing rat. J Nutr Sci Vitaminol (Tokyo) 1982;28:491–500. [DOI] [PubMed] [Google Scholar]
- 129.Cui X, Chopp M, Zacharek A, Roberts C, Buller B, Ion M, Chen J. Niacin treatment of stroke increases synaptic plasticity and axon growth in rats. Stroke 2010;41:2044–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Morris MC, Evans DA, Bienias JL, Scherr PA, Tangney CC, Hebert LE, Bennett DA, Wilson RS, Aggarwal N. Dietary niacin and the risk of incident Alzheimer’s disease and of cognitive decline. J Neurol Neurosurg Psychiatry 2004;75:1093–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ferland G. Vitamin K and the nervous system: an overview of its actions. Adv Nutr 2012;3:204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ferland G. Vitamin K and brain function. Semin Thromb Hemost 2013;39:849–55. [DOI] [PubMed] [Google Scholar]
- 133.Denisova NA, Booth SL. Vitamin K and sphingolipid metabolism: evidence to date. Nutr Rev 2005;63:111–21. [DOI] [PubMed] [Google Scholar]
- 134.Soutif-Veillon A, Ferland G, Rolland Y, Presse N, Boucher K, Féart C, Annweiler C. Increased dietary vitamin K intake is associated with less severe subjective memory complaint among older adults. Maturitas 2016;93:131–6. [DOI] [PubMed] [Google Scholar]
- 135.Presse N, Shatenstein B, Kergoat MJ, Ferland G. Low vitamin K intakes in community-dwelling elders at an early stage of Alzheimer’s disease. J Am Diet Assoc 2008;108:2095–9. [DOI] [PubMed] [Google Scholar]
- 136.Chouet J, Ferland G, Féart C, Rolland Y, Presse N, Boucher K, Barberger-Gateau P, Beauchet O, Annweiler C. Dietary vitamin K intake is associated with cognition and behaviour among geriatric patients: the CLIP study. Nutrients 2015;7:6739–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sato Y, Honda Y, Hayashida N, Iwamoto J, Kanoko T, Satoh K. Vitamin K deficiency and osteopenia in elderly women with Alzheimer’s disease. Arch Phys Med Rehabil 2005;86:576–81. [DOI] [PubMed] [Google Scholar]
- 138.Abe TK, Taniguchi M. Identification of myo-inositol hexakisphosphate (IP6) as a β-secretase 1 (BACE1) inhibitory molecule in rice grain extract and digest. FEBS Open Bio 2014;4:162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jin M, Selkoe DJ. Systematic analysis of time-dependent neural effects of soluble amyloid β oligomers in culture and in vivo: prevention by scyllo-inositol. Neurobiol Dis 2015;82:152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tayebati SK, Amenta F. Choline-containing phospholipids: relevance to brain functional pathways. Clin Chem Lab Med 2013;51:513–21. [DOI] [PubMed] [Google Scholar]
- 141.Traini E, Bramanti V, Amenta F. Choline alphoscerate (alpha-glyceryl-phosphoryl-choline) an old choline-containing phospholipid with a still interesting profile as cognition enhancing agent. Curr Alzheimer Res 2013;10:1070–9. [DOI] [PubMed] [Google Scholar]
- 142.Hagen TM, Ingersoll RT, Wehr CM, Lykkesfeldt J, Vinarsky V, Bartholomew JC, Song MH, Ames BN. Acetyl-L-carnitine fed to old rats partially restores mitochondrial function and ambulatory activity. Proc Natl Acad Sci U S A 1998;95:9562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, Cotman CW, Ames BN. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid. Proc Natl Acad Sci U S A 2002;99:2356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Spagnoli A, Lucca U, Menasce G, Bandera L, Cizza G, Forloni G, Tettamanti M, Frattura L, Tiraboschi P, Comelli M, et al. . Long-term acetyl-L-carnitine treatment in Alzheimer’s disease. Neurology 1991;41:1726–32. [DOI] [PubMed] [Google Scholar]