Abstract
Purpose
The transcription factor brachyury has been shown in preclinical studies to be a driver of the epithelial-to-mesenchymal transition (EMT) and resistance to therapy of human tumor cells. This study describes the characterization of a Modified Vaccinia Ankara (MVA) vector-based vaccine expressing the transgenes for brachyury and three human costimulatory molecules (B7.1, ICAM-1, and LFA-3, designated TRICOM) and a phase I study with this vaccine.
Experimental Design
Human dendritic cells (DCs) were infected with MVA-brachyury-TRICOM to define their ability to activate brachyury-specific T cells. A dose escalation phase I study (NCT02179515) was conducted in advanced cancer patients (n = 38) to define safety and to identify brachyury-specific T-cell responses.
Results
MVA-brachyury-TRICOM-infected human DCs activated CD8+ and CD4+ T cells specific against the self-antigen brachyury in vitro. No dose-limiting toxicities were observed due to vaccine in cancer patients at any of the three dose levels. One transient grade 3 adverse event (AE) possibly related to vaccine (diarrhea) resolved without intervention and did not recur with subsequent vaccine. All other AEs related to vaccine were transient and ≤ grade 2. Brachyury-specific T-cell responses were observed at all dose levels and in most patients.
Conclusions
The MVA-brachyury-TRICOM vaccine directed against a transcription factor known to mediate EMT can be administered safely in patients with advanced cancer and can activate brachyury-specific T cells in vitro and in patients. Further studies of this vaccine in combination therapies are warranted and planned.
Keywords: cancer vaccines, brachyury, EMT, TRICOM, immunotherapy
Introduction
Brachyury was first identified as an embryonic transcription factor of the T-box family that regulates the formation of the posterior mesoderm in the developing murine embryo, a process that involves the conversion of epithelial layers into mesenchymal cells (1). Subsequent studies have found that brachyury is absent in most normal adult human tissues, with the exception of low levels found in normal testis, thyroid, and a subset of B cells (2,3). High levels of brachyury have been found, however, in the primary and/or metastatic sites of non-small cell lung cancer (NSCLC) and small cell lung cancer (4,5), colon (6), hepatocellular (7), prostate (8), and breast carcinomas (9), including triple-negative breast cancer (TNBC) (10). High levels of brachyury are also characteristic of chordoma (11,12), a rare tumor type thought to originate from remnants of the notochord where brachyury is normally found in the human embryo.
We and others have now characterized the role of brachyury in the biology of epithelial tumors, and demonstrated its ability to induce the process of carcinoma mesenchymalization (13), i.e., a phenotypic conversion of tumor cells from an epithelial to a mesenchymal-like phenotype (also designated as an epithelial-mesenchymal transition, or EMT) (14,15). Tumor cells undergoing this phenotypic transition exhibit enhanced motility and invasiveness in vitro, a propensity to metastasize in vivo, and features of tumor stemness (16), including resistance to a range of therapeutics such as chemotherapy, radiation, small molecule therapies, and, potentially, immunotherapy (17–20). In agreement with a role for brachyury in the progression of carcinomas, multiple studies have now shown that the level of brachyury in the primary tumor correlates with poor patient prognosis in carcinomas of the lung (21), colon (6), breast (9), triple-negative breast (10), and gastrointestinal stromal tumor (GIST) (22). Brachyury expression has also been shown to be correlated with advanced-stage prostate cancer (8).
In addition to having a tumor-restricted pattern of expression and a relevant role in several aspects of tumor progression, brachyury has been shown to be an immunogenic target. Utilizing 9-mer peptides of the brachyury protein, for example, brachyury-specific CD8+ T cells have been expanded in vitro from the blood of cancer patients. These brachyury-specific CD8+ T cells were utilized in cytotoxic assays for effective lysis of human tumor cells that endogenously express brachyury (23,24). These combined properties, i.e., tumor-restricted expression, relevant function in tumor progression, and immunogenicity, make the self-antigen brachyury a potential target for immunotherapy-mediated approaches against cancer.
Here we describe the preclinical work that led to the development of a recombinant brachyury-specific poxviral vaccine, and the phase I clinical trial of this agent. This poxviral platform, which encodes the transgenes for the target antigen(s) as well as a triad of human T-cell costimulatory molecules (TRICOM: B7.1, LFA-3, and ICAM-1) (25), has been previously evaluated across multiple clinical trials (26–32). The vaccine in this brachyury-TRICOM clinical trial (NCT02179515) differs from the previous generation of TRICOM-based vaccines in that the priming vector is not vaccinia virus, but a replication-incompetent form of vaccinia designated Modified Vaccinia Ankara (MVA). MVA has an improved safety profile compared with vaccinia, and its inability to replicate allows MVA to be administered more than once without a significant host-neutralizing immune response (33–38). This dose-escalation phase I study was conducted to demonstrate the safety of MVA-brachyury-TRICOM and to determine its ability to generate brachyury-specific CD4+ or CD8+ T cells in patients with advanced cancer. Future phase II studies of this vaccine will be based on the preclinical rationale of vaccine studies in combination with checkpoint inhibitor monoclonal antibodies (MAbs), immune modulators, inhibitors of immune entities, and combinations of these (6,39–43).
Materials and Methods
Preclinical
Vaccine construct
MVA-brachyury-TRICOM vaccine comprises MVA, a strain of vaccinia that lacks replication capacity despite maintaining the ability to infect human cells and express transgenes, and the transgenes for brachyury and the triad of human costimulatory molecules (B7.1, LFA-3, and ICAM-1) (44). The vaccine was developed as part of a Cooperative Research and Development Agreement (CRADA) between the National Cancer Institute (NCI) and Bavarian Nordic.
Culture and infection of human DCs
Peripheral blood mononuclear cells (PBMCs) were separated from heparinized blood of normal donors obtained from the NIH Blood Bank by centrifugation on a Ficoll density gradient (Lymphocyte Separation Medium, LSM, MP Biomedicals). For preparation of dendritic cells (DCs), PBMCs were resuspended in AIM-V medium (ThermoFisher) and allowed to adhere to the surface of T-150 flasks (Corning) for 2 hours at 37° C. The non-adherent cell fraction was removed with a gentle rinse. Adherent cells were cultured for 6 days in AIM-V medium containing 100 ng/mL of recombinant human GM-CSF and 20 ng/mL of recombinant human IL-4 (Peprotech). The culture medium was replenished every 3 days. On day 6, DCs (1 × 106) were incubated with MVA-brachyury-TRICOM or a control MVA empty vector (MVA-wild type (WT)) or MVA-TRICOM (devoid of the brachyury transgene), for 1 hour at 37°C in 1 mL of Opti-MEM medium (ThermoFisher) at a multiplicity of infection (MOI) of 10:1. Infected DCs were subsequently suspended in 10 mL of fresh, warmed RPMI-1640 medium (ThermoFisher) containing 10% FBS and cultured for 24 hours prior to analysis.
Flow cytometric analysis
Infected DCs were washed with cold PBS 1X and stained for 40 minutes at 4°C using phycoerythrin (PE)-labeled antibodies against human B7.1 (CD80), ICAM-1 (CD54), and LFA-3 (CD58), or a control isotype IgG (BD Biosciences). Following staining, cells were washed and resuspended in PBS 1X and analyzed using a FACSCalibur cell analyzer (BD Biosciences) and FlowJo 9.9 software (FlowJo, LLC). Results are shown in percent positive cells for each marker and mean fluorescence intensity (MFI).
Western blot
Protein lysates from uninfected DCs and DCs infected with 10 MOI of the MVA-WT or MVA-brachyury-TRICOM vectors were prepared with RIPA lysis buffer (Santa Cruz Biotechnology) supplemented with 1mM phenylmethanesulfonyl fluoride (PMSF, Sigma-Aldrich). Protein lysates (20 μg) were resolved on SDS-PAGE and transferred onto nitrocellulose membranes using a standard western blot protocol. Membranes were probed overnight at 4°C with a primary rabbit MAb against brachyury (MAb 54-1) (3), followed by 1-hour incubation with a secondary anti-rabbit antibody conjugated with IRDye. Membranes were imaged using the Odyssey Infrared Imaging System (LI-COR Biosciences).
Immunofluorescence
DCs were grown on glass cover slips and infected by direct addition of 10 MOI of the MVA-WT or MVA-brachyury-TRICOM vectors in 1mL Opti-MEM medium. After a 1-hour infection, warmed RPMI-1640 medium containing 10% FBS was added for an additional 24-hour culture. Cells were fixed with 3% paraformaldehyde, permeabilized with 0.05% Triton X-100 and blocked with PBS containing 10% goat serum and 1% BSA. Coverslips were incubated overnight with anti-brachyury MAb 54-1, followed by incubation with an AlexaFluor-488-conjugated secondary antibody (ThermoFisher) and staining with DAPI (ThermoFisher). Coverslips were mounted using Fluorogel (Electron Microscopy Sciences) and imaged utilizing a fluorescence microscope (Leica Microsystems). Percentage of DCs expressing brachyury was calculated as the average of brachyury positive cells relative to the total number of DAPI positive nuclei counted from two 20X fields.
Activation of brachyury-specific T cells
For activation of brachyury-specific CD8+ T cells, DCs were prepared from PBMCs of a normal donor and infected on day 6 with 10 MOI of MVA-brachyury-TRICOM, MVA-WT, or MVA-TRICOM (encoding for the three costimulatory molecules but not brachyury), for 1 hour at 37°C. Cells were then cultured in serum containing medium for an additional 24-hour period. Allogeneic, brachyury-specific CD8+ T cells (1 × 105 T cells, generated against a brachyury-specific 9-mer peptide, designated as Tp2) (23) were stimulated with irradiated DCs at a T cells:DCs ratio of 10:1. After 24 hours, supernatants were collected and evaluated for IFN-γ production by ELISA; background observed with T cells only was subtracted from the values observed with MVA-WT–, MVA-TRICOM– and MVA-brachyury-TRICOM–infected DCs.
For activation of autologous brachyury-specific T cells, DCs were prepared from PBMCs of four normal donors and infected with MVA-brachyury-TRICOM, as indicated above. Autologous PBMCs were stimulated with irradiated MVA-brachyury-TRICOM–infected DCs at a PBMC:DC ratio of 10:1. After 3 days, IL-2 (20 U/mL) was added to the culture; each stimulation cycle lasted 7 days. At the end of two stimulation cycles (IVS2), CD4+ T cells were isolated by negative selection with magnetic beads (Miltenyi) and assayed for proliferation in response to autologous PBMCs pulsed with control HSA protein or a recombinant, purified His-brachyury protein. Proliferation of CD4+ T cells was measured on day five by [3H]-thymidine incorporation. Production of IFN-γ was evaluated with total T cells stimulated for two IVS as indicated above, and subsequently activated in the presence of autologous DCs pulsed with control HSA or His-brachyury protein. Supernatants were collected at 72–96 hours and assayed for IFN-γ production by ELISA.
Detection of brachyury-specific T cells
PBMCs from patients enrolled in the clinical trial were separated by Ficoll-Hypaque density gradient separation, washed three times, and cryopreserved in 90% heat-inactivated human AB serum and 10% DMSO in liquid nitrogen at a concentration of 1 × 107 cells/mL until assayed. Analysis of antigen-specific responses following therapy was assessed by intracellular cytokine staining following a period of in vitro stimulation (IVS) with overlapping 15-mer peptide pools encoding the tumor-associated antigen (TAA) brachyury as previously described (45). The TAA peptide pool was designed to contain a brachyury agonist epitope that had been previously identified (24). Peptide pools encoding for HLA and CEFT (a mixture of peptides of cytomegalovirus, EBV, influenza, and tetanus toxin) served as negative and positive controls, respectively. Peptide mixes were purchased from JPT, reconstituted in DMSO, and utilized immediately.
Cryopreserved PBMCs from patients before therapy and on days 28 (post I cycle of vaccine), 56 (post II cycles of vaccine), 84 (post III cycles of vaccine), and at a late time point (day 168–190, where available) were assayed as previously described (45). Using a BD LSR-II flow cytometer equipped with a UV, violet, blue, and red laser, 3 × 105 events in the live gate were acquired. FCS files were analyzed with FlowJo V.9.7 for Macintosh (TreeStar). Nonviable cells were excluded and fluorescence minus one controls were used for gating. The absolute number of CD4+ or CD8+ T lymphocytes producing cytokine or positive for CD107a was calculated per 1 × 106 cells plated at the start of the IVS. The background signal (obtained with the HLA peptide pool) and any value obtained prior to vaccination, was subtracted from those obtained after vaccination ([post-brachyury − post-HLA] − [pre-brachyury − pre-HLA]). An antigen-specific immune response to brachyury was scored as positive if a patient had more than 250 CD4+ or CD8+ T cells that produced IFN-γ, TNF, IL-2, or were positive for CD107a at the end of the stimulation assay per 1×106 cells that were plated at the start of the assay.
Immunoassay for brachyury-specific antibodies
Serum samples were collected from patients enrolled in the clinical trial in serum separator tubes, spun down, and stored at −80°C. Immulon 4HBX 96-well plates (Thermo Scientific) were coated with 1 μg/mL of a purified, recombinant brachyury protein or 5% BSA in PBS for 4 hours at room temperature. Assay plates were washed twice with PBS and blocked for three hours at room temperature with 5% BSA in PBS. Serum collected from patients before vaccination and on days 28, 56, and 84 post-vaccination was added at a 1:100 dilution in PBS with 1% BSA. Following overnight incubation at 4°C, plates were washed four times with PBST buffer (PBS + 0.05 % Tween-20). A secondary HRP-labeled anti-human IgG antibody (BD Biosciences) was added at 1:6000 dilution in PBS with 1% BSA. After a 1-hour incubation at room temperature, plates were washed four times with PBST; SureBlue TMB Microwell Peroxidase Substrate (KPL) was added, and color was allowed to develop for 30 minutes in the dark at room temperature. The reaction was stopped utilizing TBM stop solution (KPL) and absorbance was read at 405 nm. Titer was defined as the maximum dilution where the absorbance at 450 nm was twice that obtained from the same sample on the control BSA plate at a 1:50 dilution of sera.
Statistical analysis
Data were analyzed using GraphPad Prism (GraphPad Software, La Jolla, CA). Mean comparisons among groups was conducted by one-way ANOVA; multiple comparison analysis used the Tukey test. Graphs depict the mean±SD from replicate measures for each group.
Clinical trial
Patients
Eligibility for the trial required patients to be >18 years of age with evaluable (not necessarily measurable) metastatic or unresectable locally advanced solid tumors, including chordoma, and good ECOG performance status (0–1). Patients must have completed at least one line of standard therapy (if one existed) at least 4 weeks prior to enrollment, with resolution of any grade ≥2 adverse events (AEs) from prior therapy and could not be eligible for curative therapy. Patients with resected metastatic disease at high risk of recurrence were eligible. Essentially normal organ function and barrier contraception and/or abstinence was required. During dose escalation, no other cancer treatment was allowed. Exclusion criteria included chronic infection, including hepatitis B or C and HIV, altered mental status, autoimmune disorders of clinical significance, concurrent systemic corticosteroid use, untreated central nervous system metastases, history of allergic reaction to components of vaccine, serious uncontrolled medical issues, and pregnancy.
Trial design and oversight
The trial was a phase I dose-escalation study to demonstrate the safety and immunogenicity of MVA-brachyury-TRICOM in patients with advanced solid malignancies. Three vaccine dose levels (DLs) were evaluated. Doses were administered subcutaneously. Each injection site received 5 × 108 plaque-forming units (PFU). At increasing DLs, one, two, and four injection sites were given per dose to make total PFU delivery 5 × 108, 1 × 109, and 2 × 109 at DLs 1, 2, and 3, respectively. After safety was established, expansion of the two highest doses for further safety and immune response analysis was conducted. In these cohorts, eligibility was modified to allow patients receiving specific standard therapies. Patients with EGFR-mutated lung cancer who were being treated with erlotinib for at least 3 months with ongoing response or stable disease were allowed to continue erlotinib and enter the study. Patients with ER+ breast cancer could continue hormonal therapy. Patients with Her2+ breast cancer could continue on maintenance Her2-directed therapy. Finally, patients with colorectal cancer who had completed front-line combination chemotherapy and were on maintenance capecitabine and/or bevacizumab were eligible
The trial was run in accordance with the Declaration of Helsinki after approval by the Scientific Review Committee and Institutional Review Board (IRB) of the Intramural National Cancer Institute (NCI) and the Center for Cancer Research, NCI. NCI sponsored this study. Ongoing safety monitoring was conducted by the IRB and the Intramural Data Safety Monitoring Board. All serious AEs were reported to the U.S. Food and Drug Administration for review per guidelines. Informed consent was obtained from each participant, including consent for treatment, primary and secondary endpoints, and correlative studies.
Results
In vitro studies
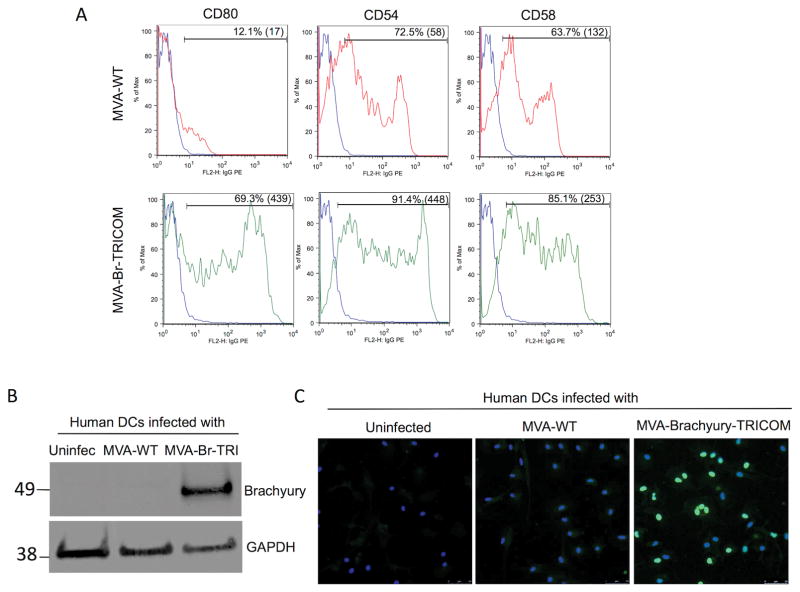
The ability of the MVA-brachyury-TRICOM vector to infect human DCs in vitro was first evaluated. As shown in Fig. 1A, whereas human DCs endogenously express costimulatory molecules, expression of the encoded human costimulatory molecule transgenes B7.1, ICAM-1, and LFA-3 was markedly enhanced in MVA-brachyury-TRICOM–infected cells vs. control MVA-WT–infected cells. Expression of the encoded brachyury protein was also observed in MVA-brachyury-TRICOM–infected human DCs, and compared with uninfected and control MVA-WT–infected cells (Fig. 1B). Immunofluorescent analysis demonstrated expression of brachyury protein in 63 ± 11% of DCs infected with MVA-brachyury-TRICOM (Fig. 1C).
Figure 1.
(A) Flow cytometric analysis of CD80 (B7.1), CD54 (ICAM-1), and CD58 (LFA-3) expression in human DCs infected with indicated vectors (10 MOI, 24-hour). Shown is the percent positive cells for each marker and the mean fluorescence intensity (MFI). (B) Western blot analysis of brachyury expression in protein lysates of indicated DC cultures. GAPDH is used as a loading control protein for each sample. (C) Immunofluorescence analysis of brachyury expression in indicated DC cultures; green corresponds to brachyury and blue corresponds to DAPI-stained nuclei. Magnification 20X.
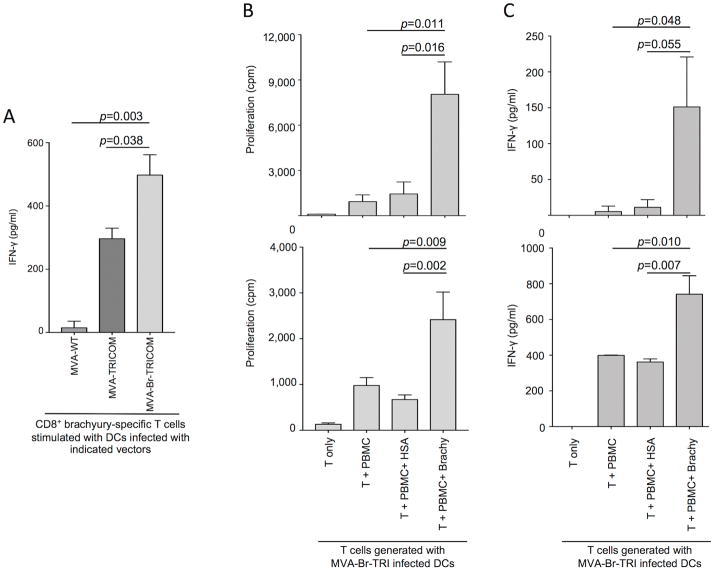
MVA-brachyury-TRICOM–infected human DCs were subsequently used in vitro as antigen-presenting cells (APCs) to stimulate HLA-A2–restricted, brachyury-specific human CD8+ T cells. Brachyury-specific T cells were generated against a 9-mer epitope of brachyury (Tp2, WLLPGTSTL), as previously described (23). DCs generated from PBMCs of an HLA-A2+ healthy donor were infected with MVA-WT, MVA-TRICOM, or MVA-brachyury-TRICOM, subsequently irradiated, and used as APCs for stimulation of brachyury-specific CD8+ T cells. As control, a recombinant vector encoding for the three costimulatory molecules (MVA-TRICOM) and not the target brachyury was used. As shown in Fig. 2A, MVA-brachyury-TRICOM–infected DCs efficiently stimulated brachyury-specific CD8+ T cells to secrete high levels of IFN-γ, compared to the levels observed with control-infected DCs.
Figure 2.
(A) DCs infected with 10 MOI of MVA-WT, MVA-TRICOM, or MVA-brachyury-TRICOM vectors were used for stimulation of allogeneic, brachyury-specific CD8+ T cells generated against a brachyury-specific 9-mer peptide. After 24 hours, supernatants were collected and evaluated for IFN-γ production by ELISA assay. Shown are the IFN-γ levels (pg/mL) after subtraction of background in response to T cells only. One-way ANOVA p=0.0036. Shown p-values for comparison between indicated groups were calculated by the Tukey’s multiple comparisons test. (B) DCs infected with 10 MOI of MVA-brachyury-TRICOM vector were used for stimulation of autologous PBMCs, as described in detail in the Materials and Methods section. Following two cycles of stimulation, CD4+ T cells were isolated and stimulated in the presence of autologous PBMCs pulsed with control HSA protein or a recombinant, purified His-brachyury protein. Shown is the proliferation of CD4+ T cells measured as [3H] thymidine incorporation (cpm = counts per minute) for two representative donors. One-way ANOVA p= 0.0046 (top panel) and p=0.004 (bottom panel). (C) Supernatants from autologous T cells were collected at 72–96 hours and assayed for IFN-γ production by ELISA. One-way ANOVA p= 0.0329 (top panel) and p=0.0007 (bottom panel). In all panels, p-values for comparison between indicated groups were calculated by the Tukey’s multiple comparisons test.
In additional experiments, the ability of MVA-brachyury-TRICOM–infected DCs to stimulate autologous brachyury-specific T cells was also investigated. DCs prepared from PBMCs of several normal donors were infected with MVA-brachyury-TRICOM and used as APCs for in vitro stimulation of autologous PBMCs. At the end of two cycles of stimulation (IVS2), CD4+ T cells were isolated and assayed for proliferation in response to autologous PBMCs pulsed with control HSA protein or purified His-brachyury protein. As shown in Fig. 2B for two representative donors, CD4+ T cells generated in response to MVA-brachyury-TRICOM–infected DCs efficiently proliferated in response to brachyury vs. control HSA protein. Overall, proliferation of autologous CD4+ T cells was observed in 3/5 donors evaluated. Production of IFN-γ by total T cells stimulated for two IVS with autologous MVA-brachyury-TRICOM–infected DCs was also evaluated. As shown in Figure 2C for two donors, autologous T cells stimulated with MVA-brachyury-TRICOM released IFN-γ in response to brachyury but not control HSA (Fig. 2C). Overall, 2/5 normal donors evaluated showed IFN-γ production in response to brachyury protein following stimulation with autologous, MVA-brachyury-TRICOM–infected DCs.
Altogether, these results demonstrated that MVA-brachyury-TRICOM is able to efficiently infect and direct the expression of the encoded transgenes, brachyury, B7.1, ICAM-1 and LFA-3 in human DCs. More importantly, the results demonstrated that the antigen brachyury encoded by the virus is being processed and presented in the surface of infected DCs in the context of MHC-class I and II molecules, leading to the effective activation of brachyury-specific CD8+ and CD4+ T cells, respectively.
Patient demographics
In total, 38 patients (Table 1) were enrolled on the study between July 2014 and March 2015 (DL1, n = 3; DL2, n = 17; DL3; n = 18). On DL2 and DL3, one and two patients, respectively, were not evaluable for safety or immune responses and replaced. Patient baseline characteristics are shown in Table 1. In the expansion cohorts, 13 patients remained on maintenance standard therapy for colorectal cancer, EGFR-mutated lung cancer, and ER+ breast cancer. Median age at enrollment was 60 (range 35–86), 53% (n = 20) female. Excluding the patients on maintenance therapy at enrollment (n = 13 total), 23 of 25 patients had evidence of progressing disease (by imaging, symptoms, or serum markers) within 3 months prior to enrollment on study and had received multiple lines of prior therapy (median 3, range 0–8, Table 1).
Table 1.
Demographic characteristics of enrolled patients
| Dose level | Patient # | Age | Cancer diagnosis | Number of prior treatments |
|---|---|---|---|---|
| 1 | 1 | 60 | Chordoma | 1 |
| 1 | 2 | 58 | Ovarian Ca | 1 |
| 1 | 3 | 65 | Chordoma | 1 |
| 2 | 4 | 65 | Chordoma | 1 |
| 2 | 5 | 58 | Chordoma | 3 |
| 2 | 6 | 84 | Pancreatic Ca | 1 |
| 2 | 7 | 54 | Colon Ca | 4 |
| 2 | 8 | 48 | Chordoma | 0 |
| 2 | 9 | 42 | Chordoma | 2 |
| 2 | 10 | 69 | Chordoma | 1 |
| 2 | 11 | 64 | Lung Ca* | 2 |
| 2 | 15 | 44 | Breast Ca* | 2 |
| 2 | 17 | 64 | Colon Ca* | 5 |
| 2 | 18 | 72 | Breast Ca* | 2 |
| 2 | 20 | 47 | Colon Ca* | 3 |
| 2 | 22 | 75 | Prostate Ca | 1 |
| 2 | 23 | 60 | Colon Ca | 4 |
| 2 | 24 | 60 | Colon Ca* | 3 |
| 2 | 25 | 69 | Breast Ca | 3 |
| 2 | 31 | 53 | Chordoma | 2 |
| 3 | 12 | 65 | Pancreatic Ca | 3 |
| 3 | 13 | 65 | Cholangiocarcinoma | 2 |
| 3 | 14 | 57 | Colon Ca | 2 |
| 3 | 16 | 59 | Chordoma | 2 |
| 3 | 19 | 65 | Prostate Ca | 3 |
| 3 | 21 | 53 | Chordoma | 2 |
| 3 | 26 | 35 | Colon Ca* | 5 |
| 3 | 27 | 51 | Breast Ca* | 2 |
| 3 | 28 | 60 | Lung Ca* | 1 |
| 3 | 29 | 60 | Prostate Ca | 2 |
| 3 | 30 | 60 | Chordoma | 0 |
| 3 | 32 | 51 | Colon Ca* | 5 |
| 3 | 33 | 50 | Colon Ca | 2 |
| 3 | 34 | 86 | Lung Ca* | 1 |
| 3 | 35 | 57 | Chordoma | 3 |
| 3 | 36 | 54 | Lung Ca* | 3 |
| 3 | 37 | 82 | Chordoma | 0 |
| 3 | 38 | 79 | Breast Ca* | 1 |
Denotes patient who was on a maintenance therapy at the time of enrollment. That maintenance therapy is included in the number of prior treatments and was continued on study.
Safety
MVA-brachyury was well tolerated with no dose-limiting toxicities (Table 2). The maximum tolerated dose was not reached. Two deaths occurred on study, both due to complications of rapid disease progression unrelated to vaccine. Two other serious adverse events (AEs) occurred, a hip fracture caused by a fall and a colonic obstruction due to disease progression. No serious AE was related to vaccine. AEs occurring in >2 unique patients included injection-site reaction (78.9%), flu-like symptoms (39.5%), fever (21.1%), and diarrhea (7.9%). One grade 3 AE, diarrhea, was related to vaccine and resolved without intervention after 48 hours. All other AEs related to vaccine were grade 1 or 2 with short duration.
Table 2.
Adverse event profile of MVA-brachyury-TRICOM
| CTC term | Grade 1 | Grade 2 | Grade 3 | All Grades | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||||||
| # distinct patients |
% of all patients (n=38) |
# of Events |
Events as % of doses given (n=107) |
# distinct patients |
% of all patients (n=38) |
# of events |
Events as % of doses given (n=107) |
# distinct patients |
% of all patients (n=38) |
# of events |
Events as % of doses given (n=107) |
# distinct patients |
% of all patients (n=38) |
# of events |
Events as % of doses given (n=107) |
|
| Aspartate aminotransferase increased | 1 | 2.6 | 1 | 0.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2.6 | 1 | 0.9 |
| Diarrhea | 2 | 5.3 | 3 | 2.8 | 0 | 0 | 0 | 0 | 1 | 2.6 | 1 | 0.9 | 3 | 7.9 | 4 | 3.7 |
| Fatigue | 1 | 2.6 | 1 | 0.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2.6 | 1 | 0.9 |
| Fever | 7 | 18.4 | 9 | 8.4 | 1 | 2.6 | 1 | 0.9 | 0 | 0 | 0 | 0 | 8 | 21.1 | 10 | 9.3 |
| Flu like symptoms | 15 | 39.5 | 23 | 21.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 15 | 39.5 | 23 | 21.5 |
| Headache | 1 | 2.6 | 1 | 0.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2.6 | 1 | 0.9 |
| Injection site reaction | 21 | 55.3 | 32 | 29.9 | 19 | 50.0 | 26 | 24.3 | 0 | 0 | 0 | 0 | 30 | 78.9 | 58 | 54.2 |
| Pruritus | 1 | 2.6 | 1 | 0.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2.6 | 1 | 0.9 |
| Rash acneiform | 1 | 2.6 | 1 | 0.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2.6 | 1 | 0.9 |
Clinical activity and outcomes
Most (34/38) patients on study completed all three doses of therapy. Four patients did not complete therapy due to progression (n = 3) or death (n = 1). Two patients died on study. One patient with colon cancer died due to rapid disease progression 36 days after her third dose. The other patient had cholangiocarcinoma and refused treatment when she had sepsis presumably related to common bile duct stent infection, but workup and treatment were stopped based on the patient’s pre-existing wishes. She received two vaccinations and died 5.5 weeks after starting treatment. Twenty-one patients had progressive disease as their best response and 17 had stable disease. As there were only three vaccines offered on study, 13 patients elected to pursue different treatments or to participate in a different clinical trial, and four withdrew due to logistical concerns.
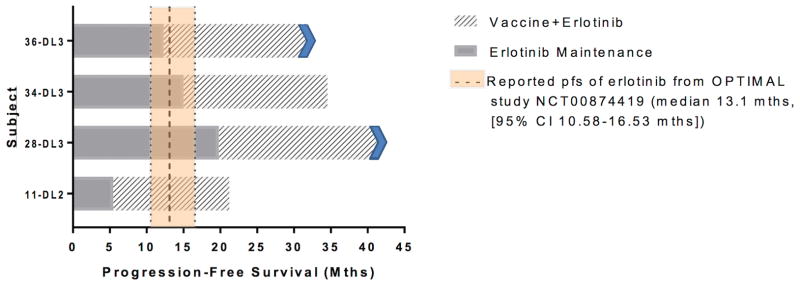
The most notable clinical outcomes were in the four patients with metastatic EGFR-mutated lung cancer in the expansion cohort who enrolled on study while on maintenance erlotinib. Patients 11, 28, 34, and 36 (Table 1) had three, one, two, and four prior therapies, respectively, going on the vaccine study. All had been treated with erlotinib for at least 3 months and had achieved an objective radiographic response or stable disease at the time of vaccine study enrollment. It is interesting to note that all three of the patients treated at DL3 had progression-free survival more than double the median that was seen in prior trials (Figure 3) (46). While it is acknowledged that there is intrinsic ascertainment bias in this very small subset of patients, the safety seen with this combination opens the possibility of a clinical endpoint study of erlotinib with or without vaccine.
Figure 3.
Progression-free survival on combination of vaccine and erlotinib. Patient 11 was treated on vaccine DL2, with time on vaccine of 15.9 months, for a total of 21.5 months on erlotinib. Patients 28, 34, and 36 were treated on vaccine DL3. Patient 28 was on vaccine study for 21.8+ months, and total time on erlotinib was 41.9+ months at the time of data lock for this publication. Patient 34 was on vaccine for 19.8 months, and total time on erlotinib was 35.0 months. Patient 36 was on vaccine study for 20+ months, with a total time on erlotinib of 32+ months at the time of data lock for this publication.
Identification of brachyury-specific T cells
Of the 38 patients enrolled on study, sufficient PBMCs were available from 34 patients to analyze brachyury-specific CD4+ and CD8+ T-cell responses. PBMCs were examined before therapy and after cycle I (day 28, n = 34), cycle II (day 56, n = 34), and cycle III of vaccine (day 84, n = 32), as well as at a later time point (day 168–190, n = 10). The FACS-based assay for T cells expressing the type I cytokines IFN-γ, IL2, TNF-α, and/or the degranulation marker CD107a following stimulation with overlapping peptide pools, is described detail in the Methods section. All assays for a given patient’s samples pre- and post-vaccine were performed in the same controlled experiment.
Including all DLs and all cancer types, 28/34 (82%) patients developed brachyury-specific CD4+ and/or CD8+ T-cell responses after vaccination (Table 3a). Of the 28 patients who developed brachyury-specific responses, 20 (71%) displayed T cells that were positive for the degranulation marker CD107a, which identifies tumor-lytic cells (47). The induction of brachyury-specific T-cell responses was rapid, with responses developing in 17 patients after a single vaccination and in nine patients after two vaccinations. The development of polyfunctional brachyury-specific T cells was seen at all DLs, with 2/3 (66%) patients developing brachyury-specific T cells post-vaccination at DL1, 12/15 (80%) patients at DL2, and 14/16 (88%) patients at DL3. Brachyury-specific T-cell stimulation persisted for at least two consecutive cycles in 0/3 patients at the lowest dose level, 5/15 in the second dose level, and 8/16 patients at the highest dose level. Brachyury-specific T-cell stimulation was observed in >1 timepoint (non-consecutive) in 0/3 patients at the lowest dose level, 8/15 in the second dose level, and 10/16 patients in the highest dose level.
Table 3a.
Development of brachyury-specific T-cell responses by intracellular cytokine staining
| Carcinoma | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical characteristics | Cycle I | Cycle II | Cycle III | Late | Any post | ||||||||||||||||||||||||||||||
| Dose level | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | |||||||||||||||||||||||||||
| Pt | Cancer | CD107a | IFNg | IL2 | TNF | CD107a | IFNg | IL2 | TNF | CD107a | IFNg | IL2 | TNF | CD107a | IFNg | IL2 | TNF | CD107a | IFNg | IL2 | TNF | CD107a | IFNg | IL2 | TNF | CD107a | IFNg | IL2 | TNF | CD107a | IFNg | IL2 | TNF | ||
| 2 | Ovarian | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 17 | 0 | 0/1 (0-%) | ||||||||
| 15 | Breast | 2 | 0 | 0 | 6 | 0 | 0 | 471 | 0 | 894 | 2121 | 0 | 0 | 194 | 0 | 0 | 0 | 458 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7/10 (70%) |
| 18 | Breast | 2 | 0 | 0 | 70 | 0 | 0 | 0 | 79 | 0 | 0 | 0 | 75 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 290 | 0 | 0 | 0 | 32 | 0 | 0 | 0 | 98 | 0 | 0 | 0 | 0 | 0 | |
| 25 | Breast | 2 | 603 | 948 | 0 | 355 | 0 | 395 | 0 | 217 | 1771 | 134 | 0 | 225 | 0 | 268 | 67 | 382 | 0 | 835 | 3480 | 767 | 0 | 0 | 29 | 104 | |||||||||
| 7 | Colon | 2 | 0 | 8 | 0 | 0 | 0 | 0 | 96 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||
| 17 | Colon | 2 | 0 | 0 | 0 | 389 | 0 | 0 | 0 | 446 | 0 | 0 | 0 | 0 | 0 | 191 | 0 | 0 | 87 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 285 | 0 | 44 | 0 | 0 | 135 | 0 | 45 | |
| 23 | Colon | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 56 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||
| 24 | Colon | 2 | 0 | 0 | 0 | 0 | 0 | 366 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||
| 11 | Lung | 2 | 1228 | 4524 | 1120 | 1300 | 71107 | 15652 | 45 | 8323 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 217 | 0 | 46 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 6 | Pancreatic | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 54 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||
| 22 | Prostate | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3400 | 654 | 0 | 2558 | 0 | 0 | 2557 | 0 | 0 | 0 | 0 | 0 | |||||||||
| 27 | Breast | 3 | 0 | 0 | 0 | 136 | 0 | 0 | 0 | 0 | 0 | 256 | 0 | 38 | 0 | 0 | 0 | 0 | 0 | 0 | 121 | 0 | 0 | 0 | 108 | 0 | 11/12 (92%) | ||||||||
| 38 | Breast | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5581 | 0 | 735 | 0 | 8763 | 39149 | 23008 | 5240 | 46867 | 19438 | 2608 | 0 | 10472 | |||||||||
| 14 | Colon | 3 | 19 | 0 | 128 | 0 | 1858 | 335 | 0 | 633 | 0 | 0 | 102 | 0 | 3762 | 391 | 0 | 1286 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 388 | 140 | 0 | 0 | 289 | 0 | 0 | |
| 26 | Colon | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 162 | 0 | 304 | 2150 | 0 | 2500 | 3086 | 96 | 0 | 1259 | 0 | 929 | 0 | 973 | 0 | 223 | 0 | 0 | |||||||||
| 32 | Colon | 3 | 0 | 516 | 0 | 51 | 0 | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 188 | 0 | 0 | 169 | 11 | 0 | 687 | 0 | 0 | 0 | 0 | |||||||||
| 33 | Colon | 3 | 463 | 0 | 146 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2461 | 196 | 0 | 0 | 6971 | 0 | 0 | 0 | |||||||||
| 28 | Lung | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 37 | 0 | 0 | 0 | 122 | 0 | 235 | 857 | 265 | 0 | 0 | 4417 | 507 | 0 | 788 | |||||||||
| 34 | Lung | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||
| 36 | Lung | 3 | 1039 | 250 | 1551 | 1825 | 2894 | 411 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 182 | 0 | 69 | |||||||||
| 12 | Pancreatic | 3 | 404 | 0 | 74 | 0 | 0 | 0 | 0 | 0 | 271 | 348 | 0 | 479 | 1982 | 0 | 10 | 148 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||
| 19 | Prostate | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 394 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1686 | 0 | 0 | 0 | 301 | 0 | |||||||||
| 29 | Prostate | 3 | 0 | 0 | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 128 | 0 | 1116 | 0 | 0 | 0 | 607 | 0 | 58 | 0 | 1276 | 0 | 0 | 0 | |||||||||
| Chordoma | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical characteristics | Cycle I | Cycle II | Cycle III | Late | Any post | ||||||||||||||||||||||||||||||
| Dose level | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | |||||||||||||||||||||||||||
| Pt | Cancer | CD107a | IFNg | IL2 | TNF | CD107a | IFNg | IL2 | TNF | CD107a | IFNg | IL2 | TNF | CD107a | IFNg | IL2 | TNF | CD107a | IFNg | IL2 | TNF | CD107a | IFNg | IL2 | TNF | CD107a | IFNg | IL2 | TNF | CD107a | IFNg | IL2 | TNF | ||
| 1 | Chordoma | 1 | 0 | 0 | 415 | 0 | 2125 | 0 | 0 | 718 | 0 | 0 | 0 | 0 | 0 | 177 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 129 | 2/2 (100%) | ||||||||
| 3 | Chordoma | 1 | 0 | 308 | 0 | 112 | 0 | 0 | 34 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 83 | 0 | 0 | 49 | 0 | 43 | 0 | 0 | 30 | 0 | 0 | 0 | 0 | |
| 4 | Chordoma | 2 | 280 | 0 | 0 | 695 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1592 | 207 | 0 | 804 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 535 | 5/5 (100%) |
| 5 | Chordoma | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 838 | 193 | 38 | 112 | 1148 | 0 | 0 | 442 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 30 | 0 | 11 | 73 | 0 | |
| 8 | Chordoma | 2 | 216 | 0 | 0 | 0 | 0 | 749 | 0 | 1640 | 0 | 0 | 0 | 0 | 0 | 120 | 0 | 120 | 382 | 0 | 0 | 0 | 5278 | 239 | 0 | 302 | |||||||||
| 9 | Chordoma | 2 | 0 | 0 | 0 | 0 | 0 | 253 | 0 | 0 | 0 | 0 | 268 | 0 | 0 | 173 | 0 | 0 | 0 | 0 | 206 | 0 | 0 | 46 | 0 | 0 | |||||||||
| 10 | Chordoma | 2 | 538 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 488 | 0 | 0 | 0 | 3955 | 0 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 16 | Chordoma | 3 | 0 | 0 | 36 | 0 | 1309 | 0 | 0 | 0 | 0 | 248 | 546 | 35 | 6327 | 2079 | 141 | 0 | 1324 | 677 | 0 | 323 | 5761 | 0 | 0 | 0 | 0 | 159 | 0 | 126 | 0 | 0 | 37 | 0 | 3/4 (75%) |
| 21 | Chordoma | 3 | 0 | 0 | 0 | 56 | 0 | 0 | 0 | 0 | 0 | 1640 | 240 | 1566 | 9455 | 57 | 307 | 1554 | |||||||||||||||||
| 30 | Chordoma | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 96 | 0 | 0 | 0 | 0 | |||||||||
| 37 | Chordoma | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1629 | 0 | 0 | 280 | 552 | 0 | 0 | 0 | 3655 | |||||||||
Absolute number of CD4 of CD8 T cells producing cytokine or positive for CD107a per 1×106 cells plated at start of IVS.
Numbers in bold and great are those positive after versus before vaccination.
Gray indicates a patient meeting defined immune response criteria.
Three of the four patients with EGFR-mutated NSCLC, who received maintenance erlotinib while on study, developed brachyury-specific T-cell responses. The responses in these three patients were noted in both the CD4+ and CD8+ T-cell compartments and included the production of multiple cytokines as well as positivity for the degranulation marker CD107a. As noted previously, all values shown in Table 3a are background subtracted for any prior brachyury-specific T cells as well as responses to the HLA control peptides.
Fourteen of 34 patients (41%) had detectable levels of brachyury-specific T-cell responses prior to therapy. These pre-existing brachyury-specific T cells were noted in 8/23 patients (35%) with various types of carcinoma, and 6/11 patients (55%) with chordoma. Of the 14 patients with pre-existing brachyury-specific T-cell responses, only 10 (71%) developed enhanced brachyury-specific T-cell responses after vaccination. Of the 20 patients who had no detectable level of brachyury-specific T cells prior to vaccination, 18 (90%) developed brachyury-specific T cells after vaccination (Table 3b).
Table 3b.
Baseline pre-existing brachyury-specific T-cell responses by intracellular cytokine staining assay
| Carcinoma | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD4 | CD8 | |||||||||
| Pt | Cancer | Dose level | CD107a | IFNγ | IL2 | TNF | CD107a | IFNγ | IL2 | TNF |
| 2 | Ovarian | 1 | PRE | |||||||
| 15 | Breast | 2 | ||||||||
| 18 | Breast | 2 | ||||||||
| 25 | Breast | 2 | ||||||||
| 7 | Colon | 2 | ||||||||
| 17 | Colon | 2 | ||||||||
| 23 | Colon | 2 | PRE | PRE | ||||||
| 24 | Colon | 2 | ||||||||
| 11 | Lung | 2 | ||||||||
| 6 | Pancreatic | 2 | PRE | PRE | ||||||
| 22 | Prostate | 2 | PRE | PRE | PRE | |||||
| 27 | Breast | 3 | ||||||||
| 38 | Breast | 3 | PRE | PRE | PRE | PRE | PRE | |||
| 14 | Colon | 3 | PRE | |||||||
| 26 | Colon | 3 | PRE | PRE | ||||||
| 32 | Colon | 3 | PRE | |||||||
| 33 | Colon | 3 | ||||||||
| 28 | Lung | 3 | ||||||||
| 34 | Lung | 3 | ||||||||
| 36 | Lung | 3 | ||||||||
| 12 | Pancreatic | 3 | ||||||||
| 19 | Prostate | 3 | ||||||||
| 29 | Prostate | 3 | ||||||||
| Baseline response | Developed response | |
|---|---|---|
| Yes | No | |
| YES: 8/23 (35%) | 5/8 (63%) | 3/8 (37%) |
| NO: 15/23 (65%) | 13/15 (87%) | 2/15 (13%) |
| Chordoma | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD4 | CD8 | |||||||||
| Pt | Cancer | Dose level | CD107a | IFNγ | IL2 | TNF | CD107a | IFNγ | IL2 | TNF |
| 1 | Chordoma | 1 | PRE | |||||||
| 3 | Chordoma | 1 | ||||||||
| 4 | Chordoma | 2 | PRE | PRE | ||||||
| 5 | Chordoma | 2 | ||||||||
| 8 | Chordoma | 2 | PRE | PRE | ||||||
| 9 | Chordoma | 2 | ||||||||
| 10 | Chordoma | 2 | PRE | PRE | PRE | |||||
| 16 | Chordoma | 3 | ||||||||
| 21 | Chordoma | 3 | ||||||||
| 30 | Chordoma | 3 | PRE | PRE | ||||||
| 37 | Chordoma | 3 | PRE | |||||||
| Baseline response | Developed response | |
|---|---|---|
| Yes | No | |
| YES: 6/11 (55%) | 5/6 (83%) | 1/6 (17%) |
| NO: 5/11 (45%) | 5/5 (100%) | 0/5 (0%) |
Anti-brachyury antibodies
Three of 34 patients (9%) displayed low levels of antibodies reactive to brachyury at baseline, using the criteria defined in the Methods section. A positive brachyury antibody titer consisted of an absorbance to brachyury protein that was twice that obtained with the negative control protein BSA. One of these patients developed a very slight increase in titer after three cycles of vaccine. In addition, 4/31 patients (13%) who had undetectable levels of antibodies reactive to brachyury at baseline developed barely detectable levels of titer following vaccination.
Discussion
The phenomenon of carcinoma mesenchymalization is now recognized as an important step during the progression of carcinomas towards metastatic disease. Clinical evidence of the association of this phenomenon with tumor progression includes, for example, the observation that mesenchymal circulating tumor cells in breast cancer patients positively associate with disease progression and failure to therapy (48). Distinct from targeting a TAA or a neo-epitope, targeting the process of tumor cells undergoing mesenchymalization represents a unique strategy to minimize tumor dissemination and, more importantly, to prevent the emergence of tumor resistance to therapies. One potential strategy to target the phenomenon of mesenchymalization is immunotherapy. The transcription factor brachyury has been extensively characterized in previous studies (9,14,17,49) in terms of (a) its ability to drive carcinoma cells into a mesenchymal-like invasive phenotype (mesenchymalization); (b) its ability to promote the acquisition of tumor resistance to a range of anticancer therapies; (c) its tumor-restricted pattern of expression with minimal expression in normal adult tissues; and (d) its immunogenicity. Due to the location of transcription factors in the cell nucleus and their lack of a hydrophobic groove, they have been characterized as “non-druggable” or difficult to target by other types of therapeutics. However, prior studies and those reported here demonstrate that the breakdown of brachyury in the cytoplasm can lead to the generation of brachyury peptide MHC complexes in DCs capable of activating brachyury-specific T cells. Prior studies (23) have shown that brachyury-specific T cells can lyse human tumor cells endogenously expressing brachyury. These findings make brachyury a potential vaccine target to eradicate cells undergoing mesenchymalization via an immune-mediated approach. Prior studies (28,50) have also demonstrated that cancer patients receiving either carcinoembryonic antigen (CEA) – or prostate-specific antigen (PSA)–based vaccines develop post-vaccination T-cell responses to brachyury, probably due to cross-presentation of brachyury to APCs as a consequence of some tumor cell destruction. These findings further demonstrated the immunogenicity of brachyury in humans.
Here we demonstrated the ability of MVA-brachyury-TRICOM to successfully infect human DCs in vitro and to direct the expression of brachyury and encoded costimulatory molecules, leading to the activation of both CD8+ and CD4+ T cells specific against brachyury, a self-antigen. The experiment with allogeneic T cells as presented in Figure. 2A was meant only to preliminarily evaluate whether human DCs infected with MVA-brachyury-TRICOM could correctly process and present brachyury peptides in the context of MHC-class I to a previously established brachyury-specific T-cell line. Activation of autologous T cells was conducted with normal donors and the data are presented in Fig. 2B and 2C, where we demonstrate that MVA-brachyury-TRICOM–infected DCs can activate brachyury-specific T cells, as denoted by the proliferation and/or IFN-γ production in response to brachyury protein. These experiments were conducted in a strictly autologous setting. Moreover, the data presented in Table 3 regarding activation of cancer patients’ blood in response to brachyury-specific peptide stimulation in vitro indicate that MVA-brachyury-TRICOM is able to infect APCs in the vaccinated patients, and that the brachyury protein is being expressed, correctly processed and presented by those APCs in an autologous fashion to the patients’ T cells, resulting in expansion of brachyury-specific immune responses, both CD4 and CD8, in 80% of vaccinated patients. These preclinical studies generated the rationale for the phase I trial of MVA-brachyury-TRICOM. MVA has been selected here as a vector due to its infectivity of human DCs and its incapacity to replicate in mammals and its ability to induce similar levels of cellular immune responses to self-antigens and antitumor activity as vaccinia (51). MVA has demonstrated safety in immunosuppressed patients and is being stockpiled as the “safe” anti-smallpox vaccine by the U.S. and other countries (52,53). This clinical trial demonstrates that the MVA-brachyury-TRICOM vaccine can generate both CD8+ and CD4+ brachyury-specific T cells in advanced cancer patients without serious treatment-related AEs.
The FACS-based assay used in the current study to measure the development of antigen- specific T-cell responses employed overlapping peptide pools that span the entire sequence of the TAA, and assessed for the production of cytokines and the degranulation marker CD107a. Due to the low number of T cells specific for brachyury that can be stimulated in vitro in our experimental system with normal donors, unfortunately, we have been unable to perform a lytic assay with brachyury-positive tumor cells or peptide titrations to evaluate functional avidity. However, it is important to point out that the expression of CD107a, a marker of lytic effector function in T cells of patients vaccinated with MVA-brachyury-TRICOM following in vitro restimulation with brachyury-specific peptides, would indicate that the effector T cells have lytic potential and are presumed to be of high avidity. Most patients (82%) were shown to develop brachyury-specific T cells post-vaccination, demonstrating the immunogenicity of this antigen. Additionally, we observed a trend indicating a potential dose response, resulting in the highest dose being selected for use in future phase II trials. One concern is the observation that brachyury-specific immune responses were not maintained for most patients during the trial. Previous studies have shown that the repeated use of a vaccinia or MVA vector for priming and subsequent boosting is not as efficient as a diversified prime and boost approach employing vaccinia or MVA followed by a non-replicative avipox or fowlpox vector to elicit antigen-specific immune responses both in preclinical (51,54,55) and clinical studies (56,57). In this regard, a fowlpox vector encoding for brachyury and TRICOM has now been developed (rF-brachyury-TRICOM) that will be used for boosting of immune responses following MVA-brachyury-TRICOM priming in subsequent studies (Supplementary Fig. S1). This vector was not available at the time of this study. A trial of MVA-brachyury-TRICOM vaccine is planned in which booster doses of rF-brachyury-TRICOM will be employed. Recombinant fowlpox vectors have been evaluated in multiple prior clinical studies with an excellent safety profile. Their inability to replicate in mammalian cells also results in minimal host vector-neutralizing immunity and the ability to administer multiple booster vaccinations.
Because this trial administered only three doses of MVA-brachyury-TRICOM, clinical outcomes were difficult to assess because most patients opted for alternative therapy once vaccination was complete. In the case of EGFR-mutated NSCLC, we were able to enroll four patients who had already responded or had stable disease for at least 3 months on erlotinib. Among those patients, there was preliminary evidence of safety of the combination. Furthermore, while the numbers of patients treated are very small, it is interesting to note that the time on study was 15.9, 19.8, 20.0+, and 21.8+ months with a total time on erlotinib of 21.5, 35.0, 32.0+ and 41.9+ months at the time of data lock for this manuscript, which compares favorably to the 11–12 months progression-free survival typically seen with erlotinib. While there is insufficient numbers of patients to draw any conclusions, the safety of the combination along with preclinical findings (42) demonstrating that human tumor cell lines bearing the EGFR mutation can be rendered more susceptible to brachyury-specific T-cell lysis by treatment with erlotinib provide rationale for a future combination study in NSCLC.
A prior trial of a recombinant yeast-brachyury-targeting vaccine demonstrated immunogenicity, safety, and generation of brachyury-specific T cells, without evidence of autoimmune toxicity (45). Preclinical murine studies (58) have shown that both the recombinant yeast vaccine (yeast-CEA) and the recombinant poxviral vaccine (CEA-TRICOM) platforms generate immune responses directed against different CEA epitopes, most likely due to differences in antigen processing by the two diverse vaccine platforms. Those studies also showed that each vaccine platform generated a unique TCR repertoire and host cytokine profile. The use of both vaccines in combination also resulted in greater antitumor activity than the use of either one alone. One goal of potential future clinical studies would be to employ both MVA and yeast-brachyury vaccine platforms either as combination therapy or in a temporal manner.
The safety and immunogenicity demonstrated with the MVA-brachyury-TRICOM vector described here in advanced and diverse cancer populations in this phase I study provide the evidence for the use of this vaccine in combination immunotherapy studies in more homogeneous and perhaps less advanced cancer settings. One obvious direction is the use of this vaccine in combination with anti-PD-1/PD-L1 checkpoint inhibitor MAbs in patients with so-called “cold tumors.” Other settings may involve the use of this vaccine in the adjuvant setting to attack the metastatic process, in combination with erlotinib in patients with EGFR-mutated NSCLC, or in combination with immune modulators or inhibitors of immunosuppressive entities.
Supplementary Material
Statement of Translational Relevance.
Transcription factors such as brachyury are known to play an important role in the processes of epithelial-to-mesenchymal transition (EMT), stemness, and resistance to therapy of human cancer cells. We describe here the generation of a vector-based vaccine expressing the transgenes for brachyury and three human T-cell costimulatory molecules. The ability of this vaccine to activate CD8+ and CD4+ T cells specific against the self-antigen brachyury, both in vitro and in advanced cancer patients in the absence of a dose-limiting toxicity, provides the rationale to target the EMT process in subsequent phase II studies in combination with checkpoint inhibitor monoclonal antibodies and other immune modulators. Distinct from targeting a tumor-associated antigen or a neo-epitope, targeting the process of tumor cells undergoing mesenchymalization represents a unique strategy to minimize tumor dissemination and, more importantly, to prevent the emergence of tumor resistance to therapies.
Acknowledgments
Funding: Intramural Research Program of the Center for Cancer Research, National Cancer Institute (NCI), NIH, and a Cooperative Research and Development Agreement between the NCI and Bavarian Nordic.
Abbreviations
- AE
adverse event
- APCs
antigen-presenting cells
- CEA
carcinoembryonic antigen
- CRADA
Cooperative Research and Development Agreement
- DCs
dendritic cells
- DLs
dose levels
- EMT
epithelial-to-mesenchymal transition
- GIST
gastrointestinal stromal tumor
- IRB
Institutional Review Board
- IVS
in vitro stimulation MAbs, monoclonal antibodies
- MFI
mean fluorescence intensity
- MOI
multiplicity of infection
- MVA
Modified Vaccinia Ankara
- NCI
National Cancer Institute
- NSCLC
non-small cell lung cancer
- PBMCs
peripheral blood mononuclear cells
- PE
phycoerythrin
- PFU
plaque-forming units
- PMSF
phenylmethanesulfonyl fluoride
- PSA
prostate-specific antigen
- TAA
tumor-associated antigen
- TNBC
triple-negative breast cancer
- WT
wild type
Footnotes
Authors’ Contributions
Conception and design: C.R. Heery, C. Palena, J. Schlom, J.L. Gulley
Development of methodology: C. Palena, R.N. Donahue, U. Dirmeier
Acquisition of data: C.R. Heery, C. Palena, S. McMahon, R.N. Donahue, L.M. Lepone, I. Grenga, U. Dirmeier, L. Cordes, J. Marté2, W. Dahut, H. Singh, R.A. Madan, R.I. Fernando, D.H. Hamilton, J. Schlom, J.L. Gulley
Analysis and interpretation of data: C.R. Heery, C. Palena, R.N. Donahue, Schlom, J.L. Gulley
Writing, review and/or revision of the manuscript: C.R. Heery, C. Palena, R.N. Donahue, Schlom, J.L. Gulley
Study supervision: C.R. Heery, C. Palena, J. Schlom, J.L. Gulley
References
- 1.Herrmann BG, Labeit S, Poustka A, King TR, Lehrach H. Cloning of the T gene required in mesoderm formation in the mouse. Nature. 1990;343:617–22. doi: 10.1038/343617a0. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton DH, Litzinger MT, Fernando RI, Huang B, Palena C. Cancer vaccines targeting the epithelial-mesenchymal transition: tissue distribution of brachyury and other drivers of the mesenchymal-like phenotype of carcinomas. Semin Oncol. 2012;39:358–66. doi: 10.1053/j.seminoncol.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamilton DH, Fernando RI, Schlom J, Palena C. Aberrant expression of the embryonic transcription factor brachyury in human tumors detected with a novel rabbit monoclonal antibody. Oncotarget. 2015;6:4853–62. doi: 10.18632/oncotarget.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roselli M, Fernando RI, Guadagni F, Spila A, Alessandroni J, Palmirotta R, et al. Brachyury, a driver of the epithelial-mesenchymal transition, is overexpressed in human lung tumors: an opportunity for novel interventions against lung cancer. Clin Cancer Res. 2012;18:3868–79. doi: 10.1158/1078-0432.CCR-11-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miettinen M, Wang Z, Lasota J, Heery C, Schlom J, Palena C. Nuclear brachyury expression is consistent in chordoma, common in germ cell tumors and small cell carcinomas, and rare in other carcinomas and sarcomas: an immunohistochemical study of 5229 cases. Am J Surg Pathol. 2015;39:1305–12. doi: 10.1097/PAS.0000000000000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilic N, Feldhaus S, Kilic E, Tennstedt P, Wicklein D, Wasielewski R, et al. Brachyury expression predicts poor prognosis at early stages of colorectal cancer. Eur J Cancer. 2011;47:1080–5. doi: 10.1016/j.ejca.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Du R, Wu S, Lv X, Fang H, Wu S, Kang J. Overexpression of brachyury contributes to tumor metastasis by inducing epithelial-mesenchymal transition in hepatocellular carcinoma. J Exp Clin Cancer Res. 2014;33:105. doi: 10.1186/s13046-014-0105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinto F, Pertega-Gomes N, Pereira MS, Vizcaino JR, Monteiro P, Henrique RM, et al. T-box transcription factor brachyury is associated with prostate cancer progression and aggressiveness. Clin Cancer Res. 2014;20:4949–61. doi: 10.1158/1078-0432.CCR-14-0421. [DOI] [PubMed] [Google Scholar]
- 9.Palena C, Roselli M, Litzinger MT, Ferroni P, Costarelli L, Spila A, et al. Overexpression of the EMT driver brachyury in breast carcinomas: association with poor prognosis. J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamilton DH, Roselli M, Ferroni P, Costarelli L, Cavaliere F, Taffuri M, et al. Brachyury, a vaccine target, is overexpressed in triple-negative breast cancer. Endocr Relat Cancer. 2016;23:783–96. doi: 10.1530/ERC-16-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tirabosco R, Mangham DC, Rosenberg AE, Vujovic S, Bousdras K, Pizzolitto S, et al. Brachyury expression in extra-axial skeletal and soft tissue chordomas: a marker that distinguishes chordoma from mixed tumor/myoepithelioma/parachordoma in soft tissue. Am J Surg Pathol. 2008;32:572–80. doi: 10.1097/PAS.0b013e31815b693a. [DOI] [PubMed] [Google Scholar]
- 12.Vujovic S, Henderson S, Presneau N, Odell E, Jacques TS, Tirabosco R, et al. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol. 2006;209:157–65. doi: 10.1002/path.1969. [DOI] [PubMed] [Google Scholar]
- 13.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 14.Fernando RI, Litzinger M, Trono P, Hamilton DH, Schlom J, Palena C. The T-box transcription factor Brachyury promotes epithelial-mesenchymal transition in human tumor cells. J Clin Invest. 2010;120:533–44. doi: 10.1172/JCI38379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savagner P. Epithelial-mesenchymal transitions: from cell plasticity to concept elasticity. Curr Top Dev Biol. 2015;112:273–300. doi: 10.1016/bs.ctdb.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Nieto MA, Cano A. The epithelial-mesenchymal transition under control: global programs to regulate epithelial plasticity. Semin Cancer Biol. 2012;22:361–8. doi: 10.1016/j.semcancer.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Huang B, Cohen JR, Fernando RI, Hamilton DH, Litzinger MT, Hodge JW, et al. The embryonic transcription factor Brachyury blocks cell cycle progression and mediates tumor resistance to conventional antitumor therapies. Cell Death Dis. 2013;4:e682. doi: 10.1038/cddis.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larocca C, Cohen JR, Fernando RI, Huang B, Hamilton DH, Palena C. An autocrine loop between TGF-beta1 and the transcription factor brachyury controls the transition of human carcinoma cells into a mesenchymal phenotype. Mol Cancer Ther. 2013;12:1805–15. doi: 10.1158/1535-7163.MCT-12-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton DH, Huang B, Fernando RI, Tsang KY, Palena C. WEE1 inhibition alleviates resistance to immune attack of tumor cells undergoing epithelial-mesenchymal transition. Cancer Res. 2014;74:2510–9. doi: 10.1158/0008-5472.CAN-13-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.David JM, Hamilton DH, Palena C. MUC1 upregulation promotes immune resistance in tumor cells undergoing brachyury-mediated epithelial-mesenchymal transition. Oncoimmunology. 2016;5:e1117738. doi: 10.1080/2162402X.2015.1117738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haro A, Yano T, Kohno M, Yoshida T, Koga T, Okamoto T, et al. Expression of Brachyury gene is a significant prognostic factor for primary lung carcinoma. Ann Surg Oncol. 2013;20(Suppl 3):S509–16. doi: 10.1245/s10434-013-2914-9. [DOI] [PubMed] [Google Scholar]
- 22.Pinto F, Campanella NC, Abrahao-Machado LF, Scapulatempo-Neto C, de Oliveira AT, Brito MJ, et al. The embryonic Brachyury transcription factor is a novel biomarker of GIST aggressiveness and poor survival. Gastric Cancer. 2016;19:651–9. doi: 10.1007/s10120-015-0505-0. [DOI] [PubMed] [Google Scholar]
- 23.Palena C, Polev DE, Tsang KY, Fernando RI, Litzinger M, Krukovskaya LL, et al. The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell-mediated cancer immunotherapy. Clin Cancer Res. 2007;13:2471–8. doi: 10.1158/1078-0432.CCR-06-2353. [DOI] [PubMed] [Google Scholar]
- 24.Tucker JA, Jochems C, Boyerinas B, Fallon J, Greiner JW, Palena C, et al. Identification and characterization of a cytotoxic T-lymphocyte agonist epitope of brachyury, a transcription factor involved in epithelial to mesenchymal transition and metastasis. Cancer Immunol Immunother. 2014;63:1307–17. doi: 10.1007/s00262-014-1603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madan RA, Bilusic M, Heery C, Schlom J, Gulley JL. Clinical evaluation of TRICOM vector therapeutic cancer vaccines. Semin Oncol. 2012;39:296–304. doi: 10.1053/j.seminoncol.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gulley JL, Arlen PM, Tsang KY, Yokokawa J, Palena C, Poole DJ, et al. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res. 2008;14:3060–9. doi: 10.1158/1078-0432.CCR-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohebtash M, Tsang KY, Madan RA, Huen NY, Poole DJ, Jochems C, et al. A pilot study of MUC-1/CEA/TRICOM poxviral-based vaccine in patients with metastatic breast and ovarian cancer. Clin Cancer Res. 2011;17:7164–73. doi: 10.1158/1078-0432.CCR-11-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:501–8. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heery CR, Madan RA, Stein MN, Stadler WM, Di Paola RS, Rauckhorst M, et al. Samarium-153-EDTMP (Quadramet(R)) with or without vaccine in metastatic castration-resistant prostate cancer: A randomized Phase 2 trial. Oncotarget. 2016;7:69014–23. doi: 10.18632/oncotarget.10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gulley JL, Arlen PM, Madan RA, Tsang KY, Pazdur MP, Skarupa L, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59:663–74. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arlen PM, Skarupa L, Pazdur M, Seetharam M, Tsang KY, Grosenbach DW, et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol. 2007;178:1515–20. doi: 10.1016/j.juro.2007.05.117. [DOI] [PubMed] [Google Scholar]
- 33.Moorthy VS, McConkey S, Roberts M, Gothard P, Arulanantham N, Degano P, et al. Safety of DNA and modified vaccinia virus Ankara vaccines against liver-stage P. falciparum malaria in non-immune volunteers. Vaccine. 2003;21:1995–2002. doi: 10.1016/s0264-410x(02)00771-5. [DOI] [PubMed] [Google Scholar]
- 34.Amato RJ, Hawkins RE, Kaufman HL, Thompson JA, Tomczak P, Szczylik C, et al. Vaccination of metastatic renal cancer patients with MVA-5T4: a randomized, double-blind, placebo-controlled phase III study. Clin Cancer Res. 2010;16:5539–47. doi: 10.1158/1078-0432.CCR-10-2082. [DOI] [PubMed] [Google Scholar]
- 35.Ramlau R, Quoix E, Rolski J, Pless M, Lena H, Levy E, et al. A phase II study of Tg4010 (Mva-Muc1-Il2) in association with chemotherapy in patients with stage III/IV Non-small cell lung cancer. J Thorac Oncol. 2008;3:735–44. doi: 10.1097/JTO.0b013e31817c6b4f. [DOI] [PubMed] [Google Scholar]
- 36.Scholl SM, Balloul JM, Le Goc G, Bizouarne N, Schatz C, Kieny MP, et al. Recombinant vaccinia virus encoding human MUC1 and IL2 as immunotherapy in patients with breast cancer. J Immunother. 2000;23:570–80. doi: 10.1097/00002371-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 37.von Sonnenburg F, Perona P, Darsow U, Ring J, von Krempelhuber A, Vollmar J, et al. Safety and immunogenicity of modified vaccinia Ankara as a smallpox vaccine in people with atopic dermatitis. Vaccine. 2014;32:5696–702. doi: 10.1016/j.vaccine.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 38.Zitzmann-Roth EM, von Sonnenburg F, de la Motte S, Arndtz-Wiedemann N, von Krempelhuber A, Uebler N, et al. Cardiac safety of Modified Vaccinia Ankara for vaccination against smallpox in a young, healthy study population. PLoS One. 2015;10:e0122653. doi: 10.1371/journal.pone.0122653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwilas AR, Ardiani A, Dirmeier U, Wottawah C, Schlom J, Hodge JW. A poxviral-based cancer vaccine the transcription factor twist inhibits primary tumor growth and metastases in a model of metastatic breast cancer and improves survival in a spontaneous prostate cancer model. Oncotarget. 2015;6:28194–210. doi: 10.18632/oncotarget.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwilas AR, Ardiani A, Gameiro SR, Richards J, Hall AB, Hodge JW. Androgen deprivation therapy sensitizes triple negative breast cancer cells to immune-mediated lysis through androgen receptor independent modulation of osteoprotegerin. Oncotarget. 2016;7:23498–511. doi: 10.18632/oncotarget.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernando RI, Hamilton DH, Dominguez C, David JM, McCampbell KK, Palena C. IL-8 signaling is involved in resistance of lung carcinoma cells to erlotinib. Oncotarget. 2016;7:42031–44. doi: 10.18632/oncotarget.9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dominguez C, Tsang KY, Palena C. Short-term EGFR blockade enhances immune-mediated cytotoxicity of EGFR mutant lung cancer cells: rationale for combination therapies. Cell Death Dis. 2016;7:e2380. doi: 10.1038/cddis.2016.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamilton DH, Griner LM, Keller JM, Hu X, Southall N, Marugan J, et al. Targeting estrogen receptor signaling with fulvestrant enhances immune and chemotherapy-mediated cytotoxicity of human lung cancer. Clin Cancer Res. 2016;22:6204–16. doi: 10.1158/1078-0432.CCR-15-3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hodge JW, Sabzevari H, Yafal AG, Gritz L, Lorenz MG, Schlom J. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59:5800–7. [PubMed] [Google Scholar]
- 45.Heery CR, Singh BH, Rauckhorst M, Marte JL, Donahue RN, Grenga I, et al. Phase I trial of a yeast-based therapeutic cancer vaccine (GI-6301) targeting the transcription factor brachyury. Cancer Immunol Res. 2015;3:1248–56. doi: 10.1158/2326-6066.CIR-15-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 47.Rubio V, Stuge TB, Singh N, Betts MR, Weber JS, Roederer M, et al. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–82. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 48.Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–4. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamilton DH, David JM, Dominguez C, Palena C. Development of cancer vaccines targeting brachyury, a transcription factor associated with tumor epithelial-mesenchymal transition. Cells Tissues Organs. 2017;203:128–38. doi: 10.1159/000446495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bilusic M, Heery CR, Arlen PM, Rauckhorst M, Apelian D, Tsang KY, et al. Phase I trial of a recombinant yeast-CEA vaccine (GI-6207) in adults with metastatic CEA-expressing carcinoma. Cancer Immunol Immunother. 2014;63:225–34. doi: 10.1007/s00262-013-1505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hodge JW, Poole DJ, Aarts WM, Gomez Yafal A, Gritz L, Schlom J. Modified vaccinia virus ankara recombinants are as potent as vaccinia recombinants in diversified prime and boost vaccine regimens to elicit therapeutic antitumor responses. Cancer Res. 2003;63:7942–9. [PubMed] [Google Scholar]
- 52.Earl PL, Americo JL, Wyatt LS, Eller LA, Whitbeck JC, Cohen GH, et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–5. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 53.Meyer H. Summary report on first, second and third generation smallpox vaccines. World Health Organisation; 2013. pp. 1–33. [Google Scholar]
- 54.Hodge JW, McLaughlin JP, Kantor JA, Schlom J. Diversified prime and boost protocols using recombinant vaccinia virus and recombinant non-replicating avian pox virus to enhance T-cell immunity and antitumor responses. Vaccine. 1997;15:759–68. doi: 10.1016/s0264-410x(96)00238-1. [DOI] [PubMed] [Google Scholar]
- 55.Hodge JW, Higgins J, Schlom J. Harnessing the unique local immunostimulatory properties of modified vaccinia Ankara (MVA) virus to generate superior tumor-specific immune responses and antitumor activity in a diversified prime and boost vaccine regimen. Vaccine. 2009;27:4475–82. doi: 10.1016/j.vaccine.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marshall JL, Hoyer RJ, Toomey MA, Faraguna K, Chang P, Richmond E, et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18:3964–73. doi: 10.1200/JCO.2000.18.23.3964. [DOI] [PubMed] [Google Scholar]
- 57.Kaufman HL, Wang W, Manola J, DiPaola RS, Ko YJ, Sweeney C, et al. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:2122–32. doi: 10.1200/JCO.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 58.Boehm AL, Higgins J, Franzusoff A, Schlom J, Hodge JW. Concurrent vaccination with two distinct vaccine platforms targeting the same antigen generates phenotypically and functionally distinct T-cell populations. Cancer Immunol Immunother. 2010;59:397–408. doi: 10.1007/s00262-009-0759-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.