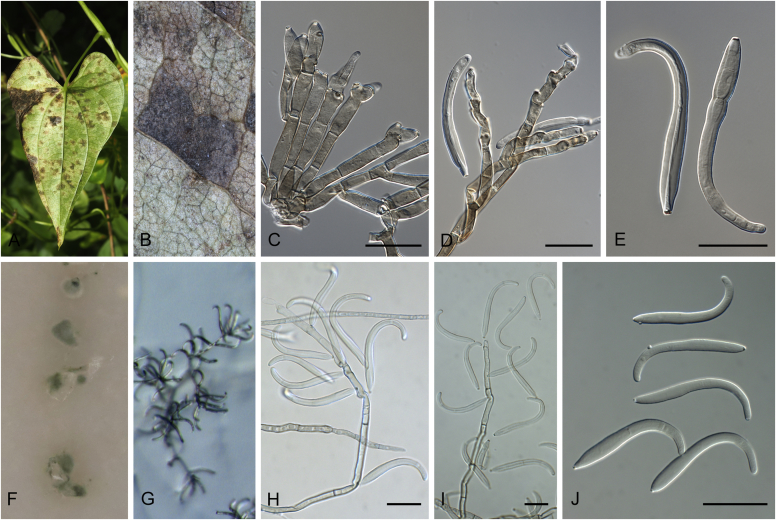
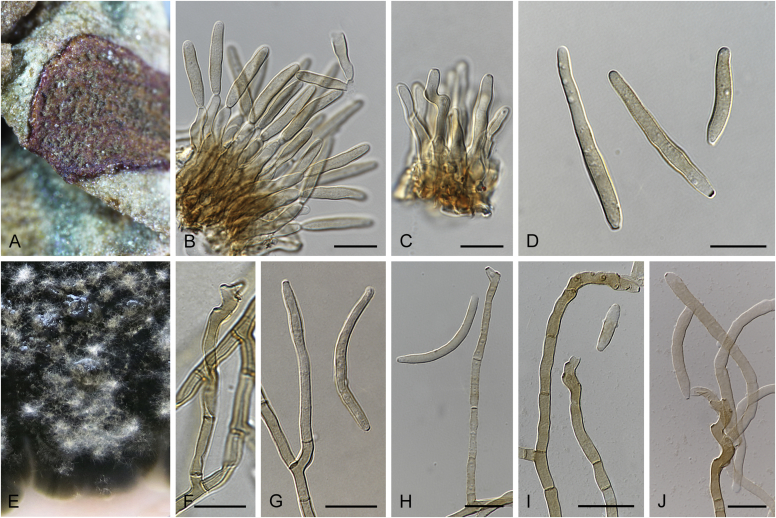
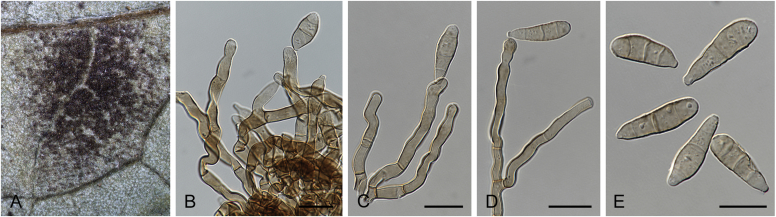
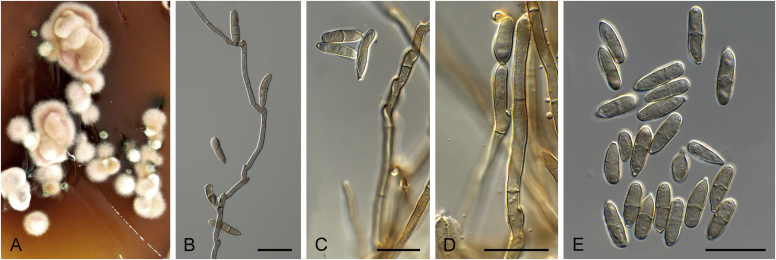
Abstract
The Mycosphaerellaceae represent thousands of fungal species that are associated with diseases on a wide range of plant hosts. Understanding and stabilising the taxonomy of genera and species of Mycosphaerellaceae is therefore of the utmost importance given their impact on agriculture, horticulture and forestry. Based on previous molecular studies, several phylogenetic and morphologically distinct genera within the Mycosphaerellaceae have been delimited. In this study a multigene phylogenetic analysis (LSU, ITS and rpb2) was performed based on 415 isolates representing 297 taxa and incorporating ex-type strains where available. The main aim of this study was to resolve the phylogenetic relationships among the genera currently recognised within the family, and to clarify the position of the cercosporoid fungi among them. Based on these results many well-known genera are shown to be paraphyletic, with several synapomorphic characters that have evolved more than once within the family. As a consequence, several old generic names including Cercosporidium, Fulvia, Mycovellosiella, Phaeoramularia and Raghnildiana are resurrected, and 32 additional genera are described as new. Based on phylogenetic data 120 genera are now accepted within the family, but many currently accepted cercosporoid genera still remain unresolved pending fresh collections and DNA data. The present study provides a phylogenetic framework for future taxonomic work within the Mycosphaerellaceae.
Key words: Multi-gene phylogeny, Mycosphaerella, Plant pathogen, Taxonomy
Taxonomic novelties: New genera: Australosphaerella Videira & Crous; Brunswickiella Videira & Crous; Catenulocercospora C. Nakash., Videira & Crous; Cercoramularia Videira, H.D. Shin, C. Nakash. & Crous; Chuppomyces Videira & Crous; Clarohilum Videira & Crous; Collarispora Videira & Crous; Coremiopassalora U. Braun, C. Nakash., Videira & Crous; Deightonomyces Videira & Crous; Devonomyces Videira & Crous; Distocercosporaster Videira, H.D. Shin, C. Nakash. & Crous; Distomycovellosiella U. Braun, C. Nakash., Videira & Crous; Exopassalora Videira & Crous; Exutisphaerella Videira & Crous; Graminopassalora U. Braun, C. Nakash., Videira & Crous; Hyalocercosporidium Videira & Crous; Hyalozasmidium U. Braun, C. Nakash., Videira & Crous; Madagascaromyces U. Braun, C. Nakash., Videira & Crous; Micronematomyces U. Braun, C. Nakash., Videira & Crous; Neocercosporidium Videira & Crous; Neophloeospora Videira & Crous; Nothopassalora U. Braun, C. Nakash., Videira & Crous; Nothopericoniella Videira & Crous; Nothophaeocryptopus Videira, C. Nakash., U. Braun, Crous; Pachyramichloridium Videira & Crous; Paracercosporidium Videira & Crous; Paramycovellosiella Videira, H.D. Shin & Crous; Parapallidocercospora Videira, Crous, U. Braun, C. Nakash.; Pleopassalora Videira & Crous; Pleuropassalora U. Braun, C. Nakash., Videira & Crous; Pluripassalora Videira & Crous; Pseudopericoniella Videira & Crous; Pseudophaeophleospora U. Braun, C. Nakash., Videira & Crous; Pseudozasmidium Videira & Crous; Rhachisphaerella Videira & Crous; Rosisphaerella Videira & Crous; Sultanimyces Videira & Crous; Virosphaerella Videira & Crous; Xenosonderhenioides Videira & Crous
New species: Cercoramularia koreana Videira, H.D. Shin, C. Nakash. & Crous; Hyalocercosporidium desmodii Videira & Crous; Hyalozasmidium sideroxyli U. Braun, C. Nakash., Videira & Crous; Neoceratosperma legnephoricola U. Braun, C. Nakash., Videira & Crous; Neoceratosperma haldinae U. Braun, C. Nakash., Videira & Crous; Ramulispora sorghiphila U. Braun, C. Nakash., Videira & Crous; Zasmidium elaeocarpi U. Braun, C. Nakash., Videira & Crous; Zasmidium grevilleae U. Braun, C. Nakash., Videira & Crous; Zasmidium hakeae U. Braun, C. Nakash., Videira & Crous; Zasmidium eucalypticola U. Braun, C. Nakash., Videira & Crous; Zasmidium schini U. Braun, C. Nakash., Videira & Crous; Xenosonderhenioides indonesiana C. Nakash., Videira & Crous
New combinations: Amycosphaerella keniensis (Crous & T.A. Cout.) Videira & Crous; Australosphaerella nootherensis (Carnegie) Videira & Crous; Brunswickiella parsonsiae (Crous & Summerell) Videira & Crous; Chuppomyces handelii (Bubák) U. Braun, C. Nakash., Videira & Crous; Catenulocercospora fusimaculans (G.F. Atk.) C. Nakash., Videira & Crous; Cercosporidium californicum (S.T. Koike & Crous) Videira & Crous; Clarohilum henningsii (Allesch.) Videira & Crous; Clypeosphaerella calotropidis (Ellis & Everh.) Videira & Crous; Coremiopassalora eucalypti (Crous & Alfenas) U. Braun, C. Nakash., Videira & Crous; Coremiopassalora leptophlebae (Crous et al.) U. Braun, C. Nakash., Videira & Crous; Collarispora valgourgensis (Crous) Videira & Crous; Deightonomyces daleae (Ellis & Kellerm.) Videira & Crous; Devonomyces endophyticus (Crous & H. Sm. Ter) Videira & Crous; Distocercosporaster dioscoreae (Ellis & G. Martin) Videira, H.D. Shin, C. Nakash. & Crous; Distomycovellosiella brachycarpa (Syd.) U. Braun, C. Nakash., Videira & Crous; Exopassalora zambiae (Crous & T.A. Cout.) Videira & Crous; Exutisphaerella laricina (R. Hartig) Videira & Crous; Fusoidiella anethi (Pers.) Videira & Crous; Graminopassalora graminis (Fuckel) U. Braun, C. Nakash., Videira & Crous; Hyalozasmidium aerohyalinosporum (Crous & Summerell) Videira & Crous; Madagascaromyces intermedius (Crous & M.J. Wingf.) Videira & Crous; Micronematomyces caribensis (Crous & Den Breeÿen) U. Braun, C. Nakash., Videira & Crous; Micronematomyces chromolaenae (Crous & Den Breeÿen) U. Braun, C. Nakash., Videira & Crous; Neocercosporidium smilacis (Thüm.) U. Braun, C. Nakash., Videira & Crous; Neophloeospora maculans (Bérenger) Videira & Crous; Nothopassalora personata (Berk. & M.A. Curtis) U. Braun, C. Nakash., Videira & Crous; Nothopericoniella perseae-macranthae (Hosag. & U. Braun) Videira & Crous; Nothophaeocryptopus gaeumannii (T. Rohde) Videira, C. Nakash., U. Braun, Crous; Pachyramichloridium pini (de Hoog & Rahman) U. Braun, C. Nakash., Videira & Crous; Paracercosporidium microsorum (Sacc.) U. Braun, C. Nakash., Videira & Crous; Paracercosporidium tiliae (Peck) U. Braun, C. Nakash., Videira & Crous; Paramycosphaerella wachendorfiae (Crous) Videira & Crous; Paramycovellosiella passaloroides (G. Winter) Videira, H.D. Shin & Crous; Parapallidocercospora colombiensis (Crous et al.) Videira & Crous; Parapallidocercospora thailandica (Crous et al.) Videira & Crous; Phaeocercospora juniperina (Georgescu & Badea) U. Braun, C. Nakash., Videira & Crous; Pleopassalora perplexa (Beilharz et al.) Videira & Crous; Pleuropassalora armatae (Crous & A.R. Wood) U. Braun, C. Nakash., Videira & Crous; Pluripassalora bougainvilleae (Munt.-Cvetk.) U. Braun, C. Nakash., Videira & Crous; Pseudocercospora convoluta (Crous & Den Breeÿen) U. Braun, C. Nakash., Videira & Crous; Pseudocercospora nodosa (Constant.) U. Braun, C. Nakash., Videira & Crous; Pseudocercospora zambiensis (Deighton) Crous & U. Braun; Pseudopericoniella levispora (Arzanlou, W. Gams & Crous) Videira & Crous; Pseudophaeophleospora atkinsonii (Syd.) U. Braun, C. Nakash., Videira & Crous; Pseudophaeophleospora stonei (Crous) U. Braun, C. Nakash., Videira & Crous; Pseudozasmidium eucalypti (Crous & Summerell) Videira & Crous; Pseudozasmidium nabiacense (Crous & Carnegie) Videira & Crous; Pseudozasmidium parkii (Crous & Alfenas) Videira & Crous; Pseudozasmidium vietnamense (Barber & T.I. Burgess) Videira & Crous; Ragnhildiana ampelopsidis (Peck) U. Braun, C. Nakash., Videira & Crous; Ragnhildiana diffusa (Heald & F.A. Wolf) Videira & Crous; Ragnhildiana ferruginea (Fuckel) U. Braun, C. Nakash., Videira & Crous; Ragnhildiana gnaphaliaceae (Cooke) Videira, H.D. Shin, C. Nakash. & Crous; Ragnhildiana perfoliati (Ellis & Everh.) U. Braun, C. Nakash., Videira & Crous; Ragnhildiana pseudotithoniae (Crous & Cheew.) U. Braun, C. Nakash., Videira & Crous; Rhachisphaerella mozambica (Arzanlou & Crous) Videira & Crous; Rosisphaerella rosicola (Pass.) U. Braun, C. Nakash., Videira & Crous; Sultanimyces vitiphyllus (Speschnew) Videira & Crous; Utrechtiana roumeguerei (Cavara) Videira & Crous; Virosphaerella irregularis (Cheew. et al.) Videira & Crous; Virosphaerella pseudomarksii (Cheew. et al.) Videira & Crous; Zasmidium arcuatum (Arzanlou et al.) Videira & Crous; Zasmidium biverticillatum (Arzanlou & Crous) Videira & Crous; Zasmidium cerophilum (Tubaki) U. Braun, C. Nakash., Videira & Crous; Zasmidium daviesiae (Cooke & Massee) U. Braun, C. Nakash., Videira & Crous; Zasmidium gupoyu (R. Kirschner) U. Braun, C. Nakash., Videira & Crous; Zasmidium iteae (R. Kirschner) U. Braun, C. Nakash., Videira & Crous; Zasmidium proteacearum (D.E. Shaw & Alcorn) U. Braun, C. Nakash. & Crous; Zasmidium pseudotsugae (V.A.M. Mill. & Bonar) Videira & Crous; Zasmidium pseudovespa (Carnegie) U. Braun, C. Nakash., Videira & Crous; Zasmidium strelitziae (Arzanlou et al.) Videira & Crous; Zasmidium tsugae (Dearn.) Videira & Crous; Zasmidium velutinum (G. Winter) Videira & Crous
New names and their replaced synonyms: Exosporium livistonicola U. Braun, Videira & Crous for Distocercospora livistonae U. Braun & C.F. Hill; Pseudocercospora platanigena Videira & Crous for Stigmella platani Fuckel, non Pseudocercospora platani (J.M. Yen) J.M. Yen 1979; Zasmidium musae-banksii Videira & Crous for Ramichloridium australiense Arzanlou & Crous, non Zasmidium australiense (J.L. Mulder) U. Braun & Crous 2013; Zasmidium musigenum Videira & Crous for Veronaea musae Stahel ex M.B. Ellis, non Zasmidium musae (Arzanlou & Crous) Crous & U. Braun 2010
Epitypes: Cercospora brachycarpa Syd., Cercospora smilacis Thüm., Cercospora gomphrenicola Speg., Cercospora microsora Sacc., Cercospora tiliae Peck, Cladosporium bacilligerum Mont. & Fr., Cladosporium chaetomium Cooke, Cladosporium fulvum Cooke, Cladosporium lonicericola Yong H. He & Z.Y. Zhang, Cladosporium personatum Berk. & M.A. Curtis, Clasterosporium degenerans Syd. & P. Syd., Cryptosporium acicola Thüm., Helicoma fasciculatum Berk. & M.A. Curtis., Isariopsis griseola Sacc., Septoria martiniana Sacc
Neotypes: Cercospora cajani Henn., Cercospora mangiferae Koord., Sphaerella laricina R. Hartig
Lectotypes (basionyms): Adelopus gaeumannii T. Rohde, Biharia vangueriae Thirum. & Mishra, Cercospora desmodii Ellis & Kellerm., Cercospora ferruginea Fuckel, Cercospora gnaphaliacea Cooke, Cercospora rosicola Pass., Cercosporidium helleri Earle, Cercospora henningsii Allesch., Cladosporium fulvum Cooke, Cladosporium bacilligerum Mont. & Fr., Cercospora microsora Sacc., Cercospora henningsii Allesch., Coryneum vitiphyllum Speschnew, Cryptosporium acicola Thüm., Isariopsis griseola Sacc., Scolicotrichum roumeguerei Briosi & Cavara, Sphaerella araneosa Rehm, Stictosepta cupularis Petr., Stigmella platani Fuckel, Tapeinosporium viride Bonord
Introduction
Fungi within the Dothideomycetes have a global distribution and occur in diverse habitats, ranging from marine to freshwater or terrestrial. They are mainly characterised by having bitunicate asci, often with fissitunicate dehiscence. The Dothideomycetes currently includes more than 25 orders, 100 families and over 1 500 genera (Schoch et al., 2009, Hyde et al., 2013, Trakunyingcharoen et al., 2014, Crous et al., 2015a, Crous et al., 2015c, van Nieuwenhuijzen et al. 2016, Bezerra et al. 2017). Among them, the order Capnodiales includes nine families, one of which is Mycosphaerellaceae.
Members of Mycosphaerellaceae are able to colonise diverse niches and vary in lifestyle from pathogens to endophytes, saprobes, epiphytes and fungicolous species. Some important plant pathogens in this family include the species associated with Sigatoka disease on banana (Arzanlou et al., 2007, Churchill, 2010, Chang et al., 2016), angular leaf spot of bean (Crous et al. 2006a), tomato leaf mould (de Wit 2016) and Cercospora leaf spot of olive (Ávila et al. 2005).
In addition, several members of Mycosphaerellaceae are quarantine regulated (Quaedvlieg et al. 2012) such as Pseudocercospora angolensis causing fruit and leaf spot disease on citrus (Kirk, 1986, Pretorius et al., 2003), Pseudocercospora pini-densiflorae causing brown needle blight of pine (Deighton, 1987, Crous et al., 1990), Sphaerulina musiva causing canker of poplar (Peace, 1962, Waterman, 1954, Quaedvlieg et al., 2013), Mycosphaerella laricis-leptolepidis causing needle cast of Japanese larch (Peace 1962), Septoria malagutii causing angular leaf spot of potato (Cline & Rossman 2006), Lecanosticta acicula causing brown spot needle blight on Pinus spp. (Quaedvlieg et al. 2012) and Dothistroma spp. causing red band disease of pine (Evans, 1984, Barnes et al., 2004, Barnes et al., 2016). In order to facilitate plant host invasion some species are known to produce fungal toxins such as dothistromin (Bradshaw, 2004, Bradshaw and Zhang, 2006) and cercosporin (Chen et al. 2007) or secrete proteinaceous effectors suppressing host defense responses and facilitating biotrophic growth (de Wit 2016). The potential ability of endophytic species as sources of natural products important in medicine and agriculture is known among taxa of several families (Strobel and Daisy, 2003, Aly et al., 2012, Gond et al., 2014), but is thus far unknown among species within the Mycosphaerellaceae. No species of Mycosphaerellaceae has hitherto been reported as a human pathogen although, in a rare occurrence, a species of Ramularia (R. plurivora) reportedly obtained from bone marrow has shown the ability to grow above 37 °C by changing its filamentous morphology into an arthroconidial yeast (Videira et al. 2015a).
As initially circumscribed Mycosphaerellaceae was polyphyletic (Crous, 2007, Crous et al., 2009a, Crous et al., 2009e) and was later, therefore, split into several families, namely Schizothyriaceae (Batzer et al. 2008), Cladosporiaceae (Schubert et al., 2007, Dugan et al., 2008, Bensch et al., 2012, Bensch et al., 2015), Dissoconiaceae and Teratosphaeriaceae (Crous et al., 2009b, Li et al., 2012, Quaedvlieg et al., 2014). From these results, it became evident that the mycosphaerella-like morphology has evolved multiple times and a new circumscription of Mycosphaerella was urgently required.
Approximately 56 genera have until now been recognised in Mycosphaerellaceae (Wijayawardene et al. 2014), although the mycosphaerella-like sexual morphs are usually morphologically conserved, and hence these genera are chiefly distinguished based on the morphology of their asexual morphs (Crous et al. 2009e). In addition, if one includes all genera that are currently synonymised based on the similarity of morphological characters, a total of 118 generic names can be accounted for in the Mycosphaerellaceae (Braun, 1995, Braun, 1998, Crous and Braun, 2003, Seifert et al., 2011). Mycosphaerella s. str. has Ramularia asexual morphs, which is also the name now applied to members of this genus, while Mycosphaerella s. lat. represents numerous genera distributed over different families. The name Ramularia (1833) is older than Mycosphaerella (1884) and choosing Ramularia over Mycosphaerella required less name changes since most established connections already had species names in Ramularia. Based on the one fungus = one name initiative (Wingfield et al., 2012, Crous et al., 2015b) the name Ramularia was selected over Mycosphaerella and included in a list of protected names (Wijayawardene et al., 2014, Rossman et al., 2015, Videira et al., 2015a, Videira et al., 2015b).
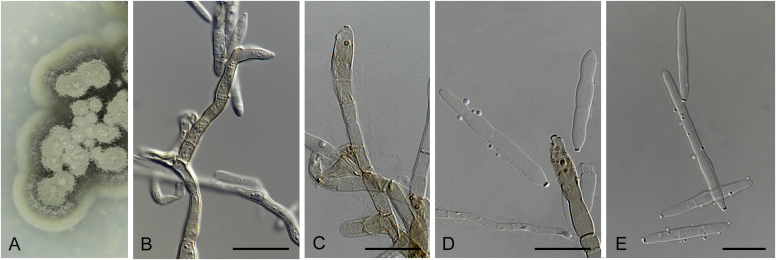
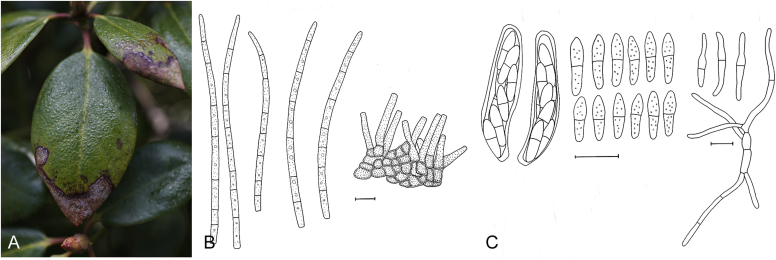
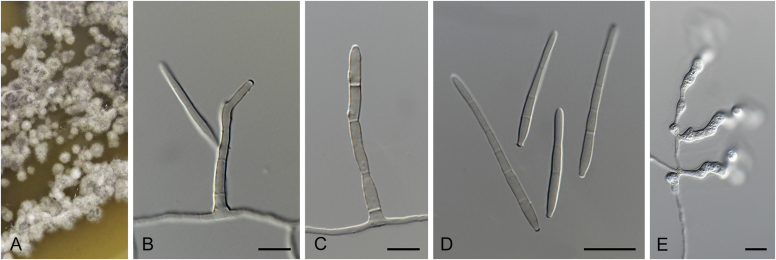
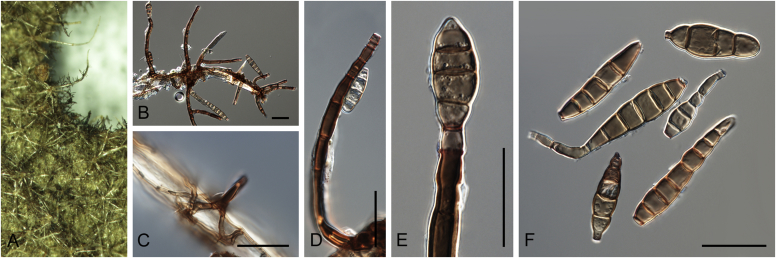
Many asexual morphs linked to mycosphaerella-like sexual morphs are cercosporoid in morphology. Cercosporoid fungi are mostly defined as dematiaceous hyphomycetes with conidiophores formed singly, in groups (fascicles), synnemata or even sporodochia, having integrated, terminal or intercalary conidiogenous cells. Conidiogenesis is holoblastic and generates amerosporous to scolecosporous conidia, which are solitary or in chains (Braun et al. 2013). In a broader sense, it also includes ramularioid fungi that are the hyaline counterparts of cercosporoid fungi, forming conidia singly or in chains. Species in this group are mostly asexual with a relation to mycosphaerella-like sexual morphs, which are characterised by pseudothecial ascomata, with ostiolar periphyses but without interascal tissue, hyaline or slightly pigmented ascospores that are predominantly 1-septate (Barr, 1987, Crous et al., 2009c).
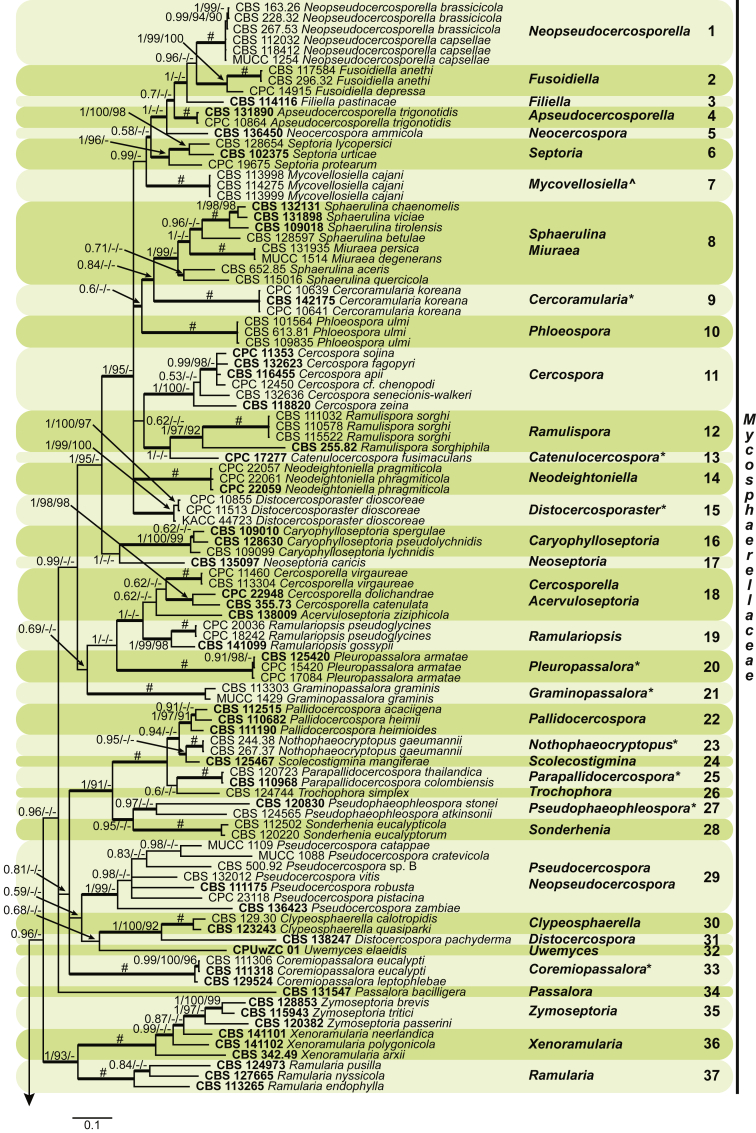
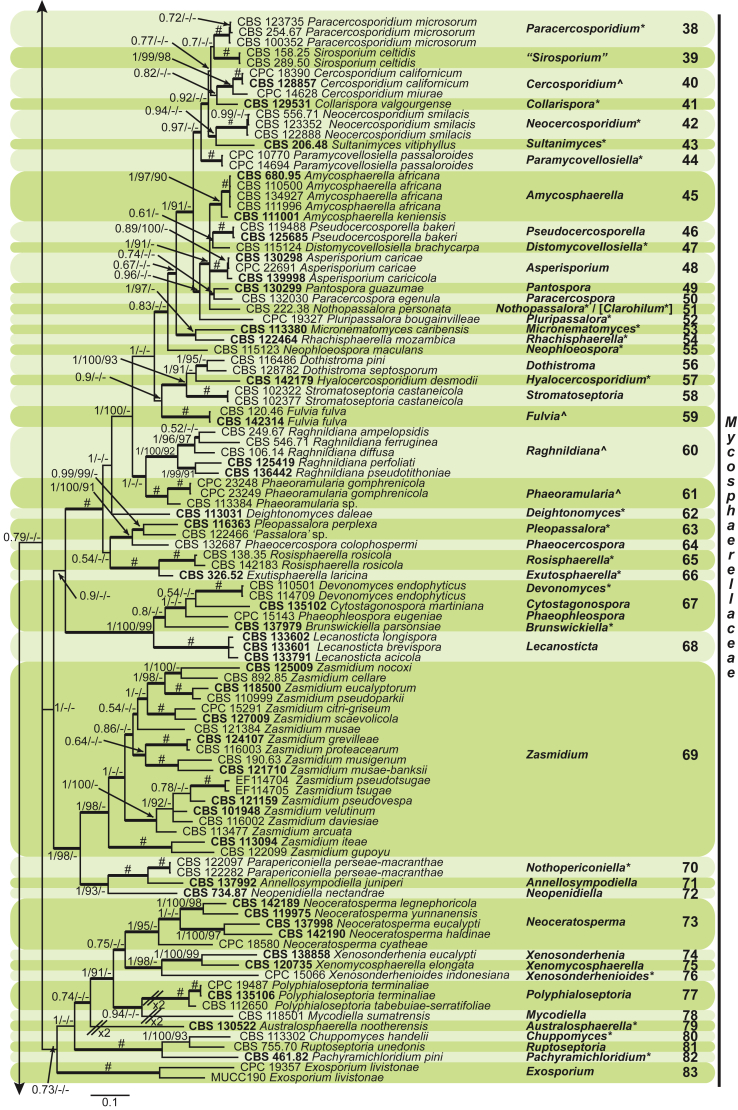
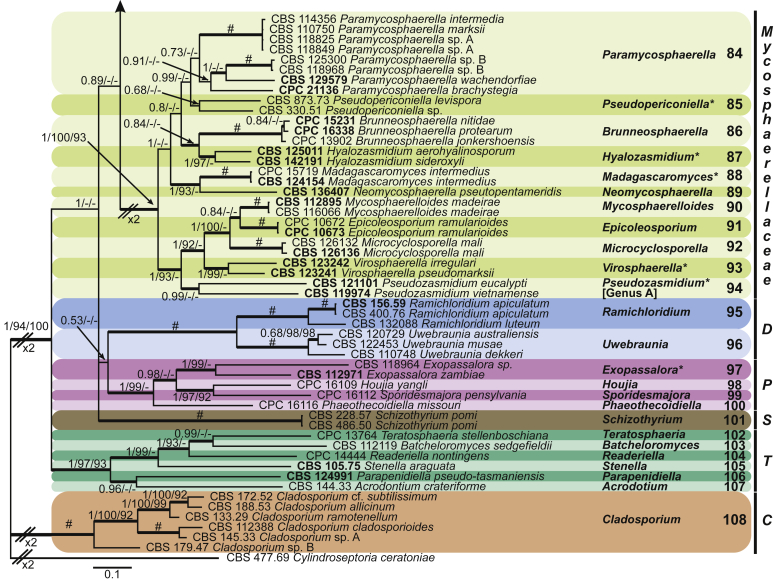
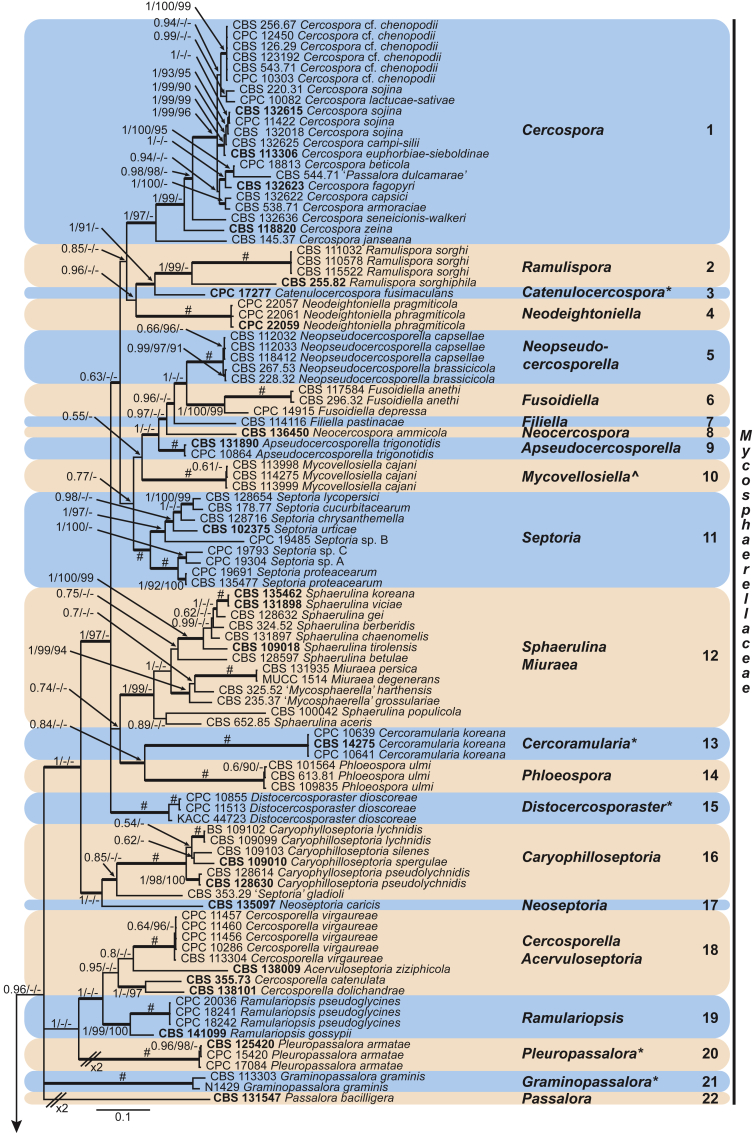
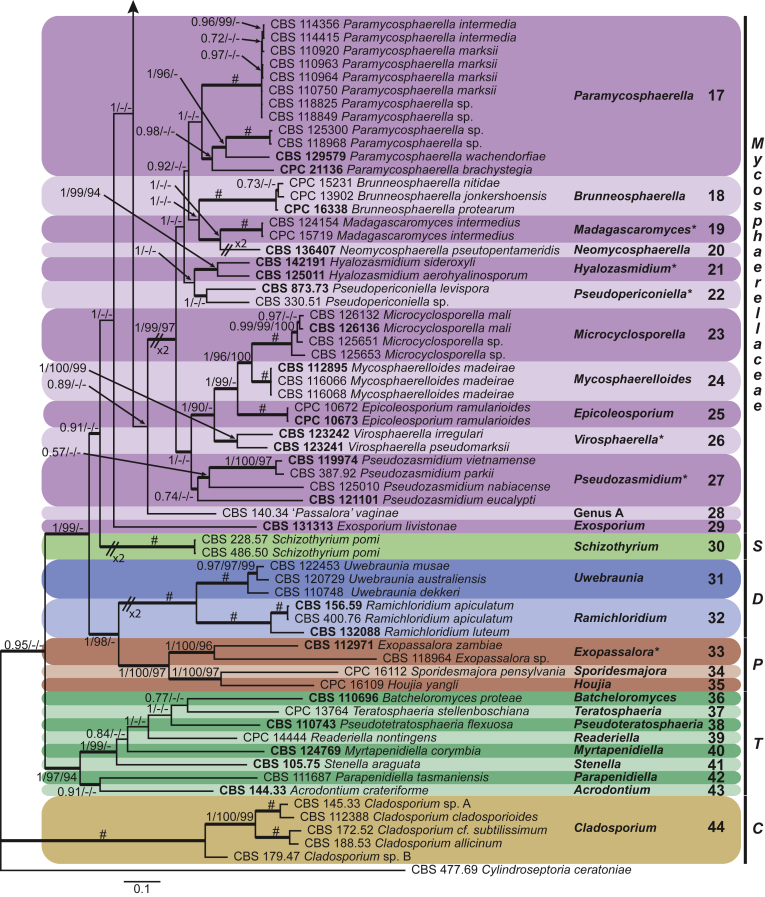
Four genera were initially recognised as true cercosporoid genera, namely Cercospora, Passalora, Pseudocercospora, and Stenella (Crous & Braun 2003). The genus Stenella was allocated to the Teratosphaeriaceae based on the phylogenetic placement of the type species, Stenella araguata, while the stenella-like species remaining in Mycosphaerellaceae were included in the genus Zasmidium (Arzanlou et al., 2007, Braun et al., 2010a, Braun et al., 2013). Currently, the recognised cercosporoid and ramularioid fungi include the latter four and a large assortment of genera that are cercospora-, passalora-, pseudocercospora-, pseudocercosporella-, ramularia- and zasmidium-like in morphology.
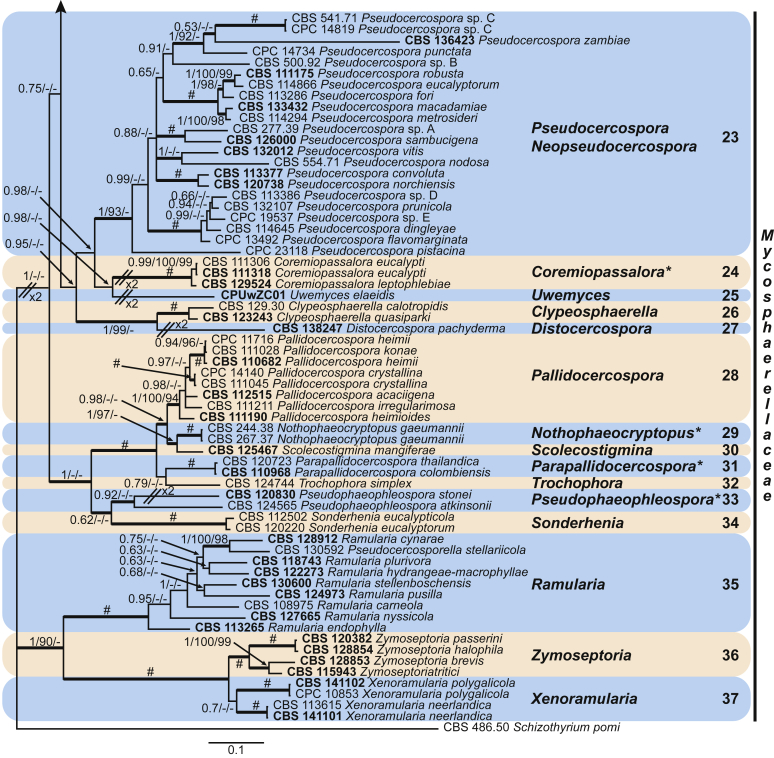
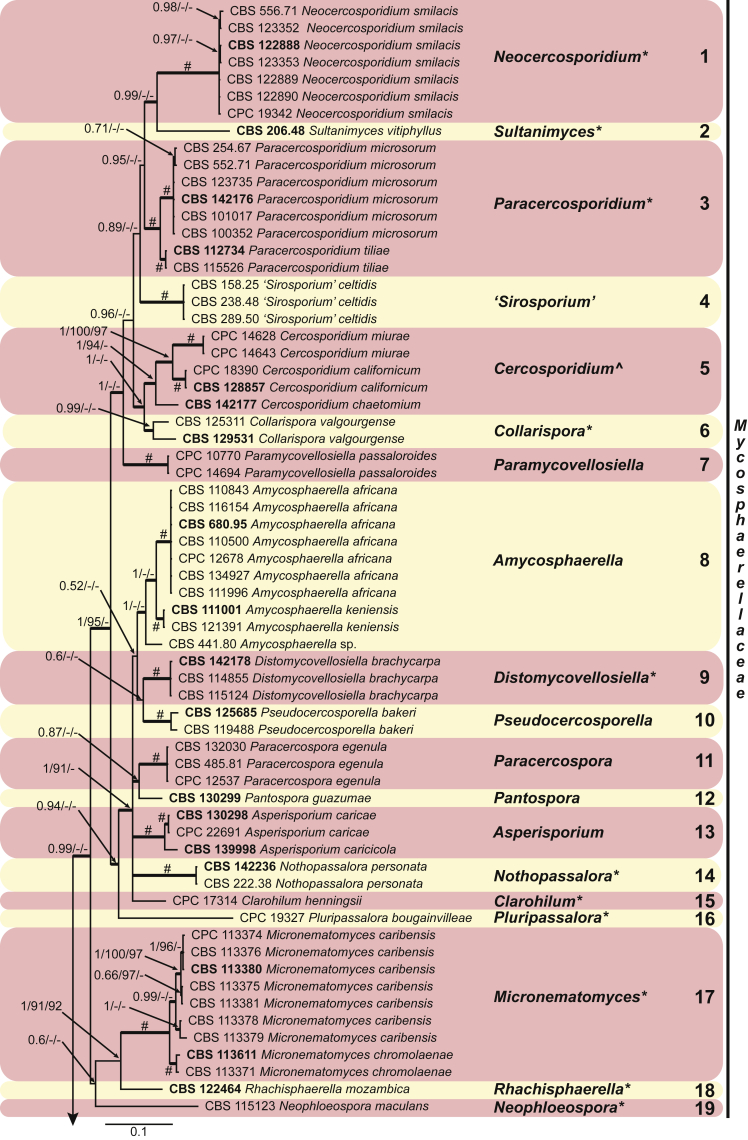
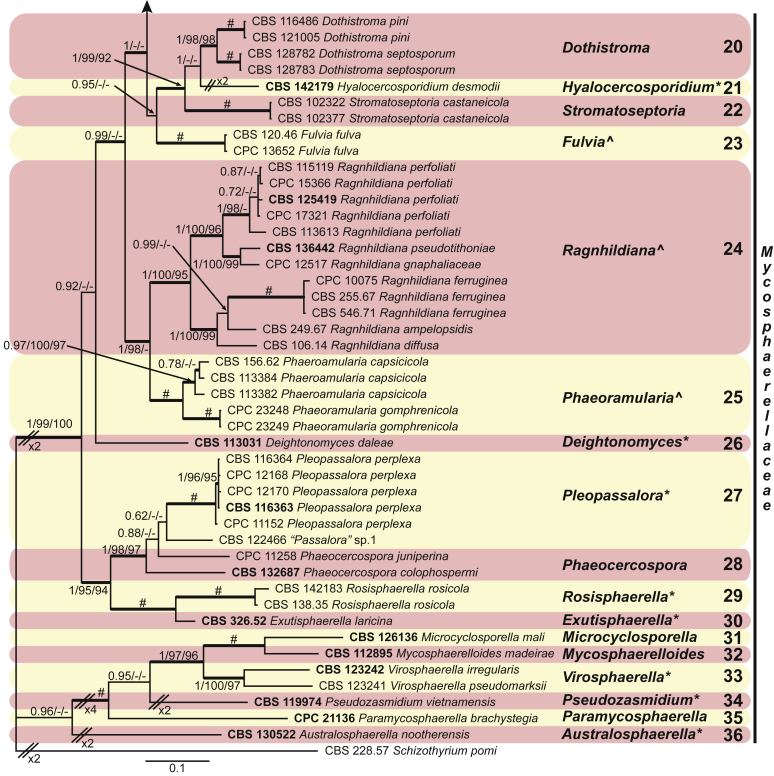
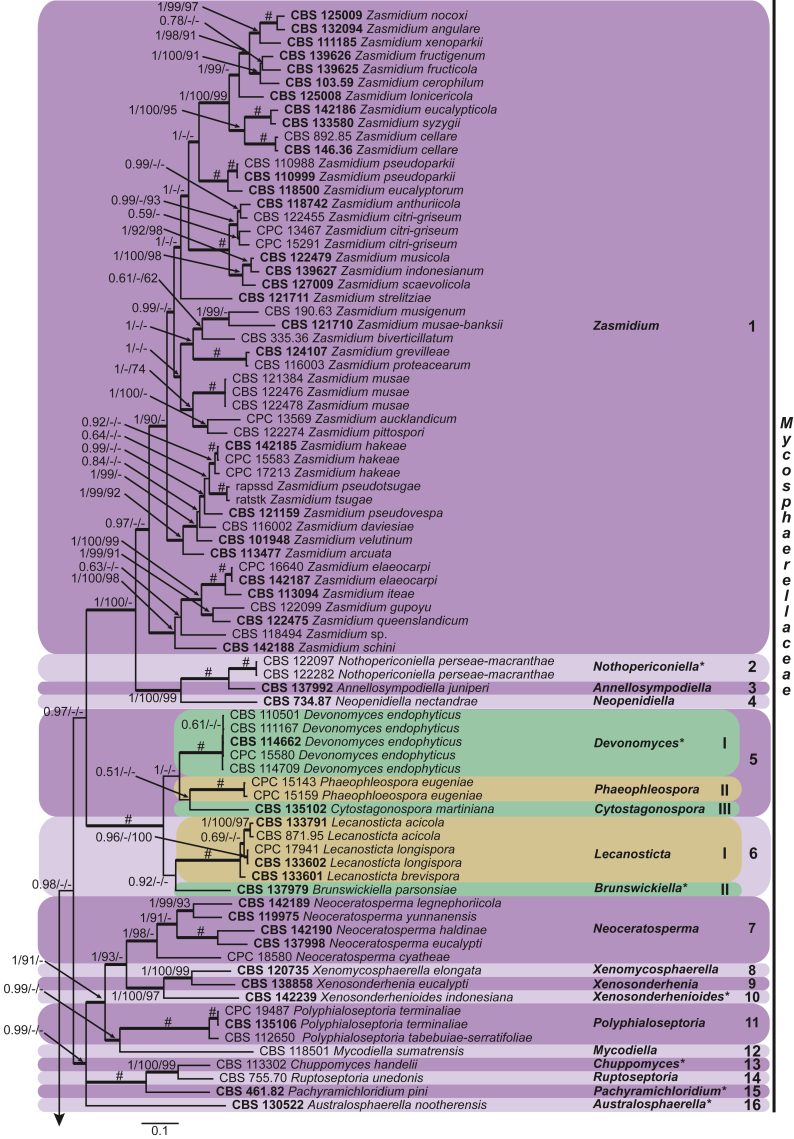
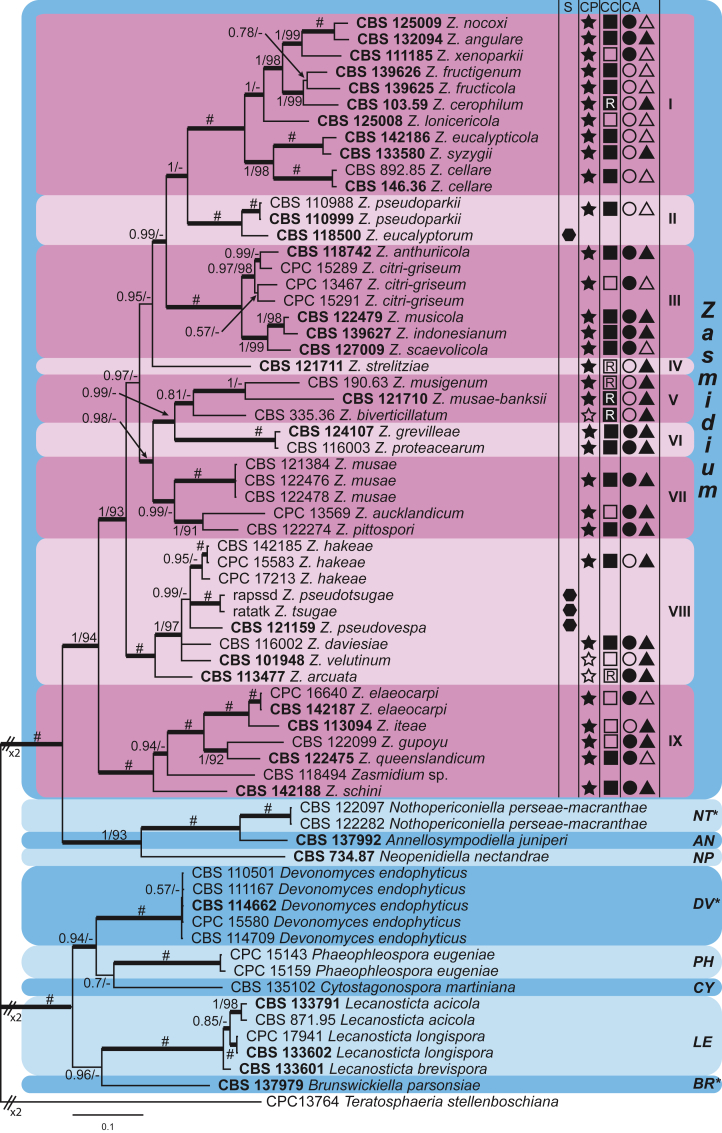
These fungi represent a very large heterogeneous group for which the existing monographs (Chupp, 1954, Braun, 1995, Braun, 1998, Crous and Braun, 2003) are in urgent need of revision (e.g. Braun et al., 2013, Braun et al., 2014, Braun et al., 2015). With the introduction of phylogenetic analyses based on DNA sequences, the Mycosphaerellaceae has been more narrowly defined with names of asexual genera now being used to identify morphologically distinct monophyletic clades, e.g. Cercospora (Groenewald et al. 2013), Pseudocercospora (Crous et al., 2013a, Nakashima et al., 2016), Ramularia (Videira et al. 2016), and Zymoseptoria (Quaedvlieg et al. 2011). However, several genera appear to be paraphyletic, showing that some morphological characters have evolved more than once within the family (e.g. Passalora and Zasmidium). Several accepted cercosporoid genera also have an uncertain status since no suitable type, or ex-type culture, is available (e.g. Distocercospora, Phaeoramularia and Mycovellosiella). Understanding and stabilising the taxonomy of cercosporoid fungi, most of which are plant pathogens, is urgent, given their impact on agriculture, horticulture and forestry. In the present study, we compiled a multigene phylogenetic analysis based on LSU, ITS and rpb2 DNA sequence data, including 415 isolates representing 297 taxa that we have managed to cultivate since this project started in the year 2000. We include ex-type strains when available. Several old generic names are resurrected based on the type species having been recollected, and new genera are described for monophyletic clades where necessary.
Materials and methods
Isolates
The isolates included in this study were obtained from the culture collection of the Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands, which houses the CBS culture collection, and from the working collection of Pedro Crous (CPC), housed at the Westerdijk Institute, or were freshly isolated from a range of different plant hosts (Table 1). Single-conidial and ascospore cultures were obtained using the techniques described for species of Mycosphaerella and associated asexual morphs (Crous et al., 1991, Crous, 1998). Representative cultures of the new species described in this study were deposited in the CBS culture collection.
Table 1.
Collection details and GenBank accession numbers of isolates included in this study. Cultures with a type status are indicated in bold text.
| Family and Current name | Previous name (if different) | Culture accession number(s)1, 2 | Substrate | Country | Collector, Collection date | LSU3 | ITS4 | rpb25 |
|---|---|---|---|---|---|---|---|---|
| Cladosporiaceae | ||||||||
| Cladosporium allicinum | – | CBS 188.53 = IFO 5267 | – | Japan | – | MF951115 | KT600367 | MF951411 |
| C. cf. subtilissimum | Fusicladium effusum | CBS 172.52 = ATCC 11320 | Carya illinoensis | USA | – | EF679390 | EF679390 | MF951412 |
| C. cladosporioides | – | CBS 112388NT | Air | Germany | Ch. Trautmann | KX286982 | HM148003 | KX288432 |
| C. ramotenellum | Fusicladium subsessile | CBS 133.29 = ATCC 36970 | Populus tremuloides | – | MF951116 | MF951281 | MF951413 | |
| Cladosporium sp. A | Fusicladium carpophillum | CBS 145.33 = ATCC 12117 | Prunus persica | USA: Wisconsin | – | MF951117 | MF951282 | MF951414 |
| Cladosporium sp. B | Fusicladium pomi | CBS 179.47 | – | Portugal | – | MF951118 | MF951283 | MF951415 |
| Dissoconiaceae | ||||||||
| Ramichloridium apiculatum | Chloridium apiculatum | CBS 156.59T = ATCC 13211 = IMI 100716 = JCM 6972 = MUCL 15753 = MUCL 7991 = QM 7716 | Forest soil | USA: Georgia | – | EU041848 | EU041791 | MF951416 |
| Rhinocladiella indica | CBS 400.76 = IMI 088021 | Soil | Pakistan | – | EU041851 | EU041794 | KX348077 | |
| R. luteum | – | CBS 132088T = CPC 18961 = ZXR-SD-2 | Malus domestica | China | G.Y. Sun, Oct. 2006 | JQ622099 | EU329730 | MF951417 |
| Uwebraunia australiensis | Dissoconium australiensis | CBS 120729 = CPC 13282 | Eucalyptus platyphylla | Australia: Queensland | P.W. Crous, 26 Aug. 2006 | KF442553 | KF442513 | KX348105 |
| U. dekkeri | Mycosphaerella lateralis | CBS 110748 = CMW 14906 = CPC 825 | Eucalyptus grandis | South Africa: Northern Province | G. Kemp, Oct. 1994 | KF442534 | KF442495 | MF951418 |
| U. musae | Dissoconium musae | CBS 122453 = X1021 | Musa acuminata cv. Nendran (Plantain) AAB | India | I. Buddenhagen, 28 Feb. 2005 | JQ739816 | EU514225 | KX348107 |
| Dothioraceae | ||||||||
| Cylindroseptoria ceratoniae | Septoria ceratoniae | CBS 477.69T = H.A 1731 | Ceratonia siliqua | Spain: Mallorca | H.A. van der Aa, 24 May 1969 | KF251655 | KF251151 | MF951419 |
| Mycosphaerellaceae | ||||||||
| Acervuloseptoria ziziphicola | Acervuloseptoria ziziphicola | CBS 138009T = CPC 23707 | Ziziphus mucronata | South Africa: Northern Cape | J. Roux, Sep. 2013 | KJ869221 | KJ869164 | MF951425 |
| Amycosphaerella africana | Mycosphaerella africana | CBS 680.95T = CPC 796 | Eucalyptus viminalis | South Africa: Western Cape | P.W. Crous, Oct. 1994 | KF902048 | KF901701 | MF951426 |
| Mycosphaerella aurantia | CBS 110500TofMycosphaerella aurantia = CMW 14460 | Eucalyptus globulus | Australia: Western Australia | A. Maxwell, 1 May 2000 | KF901837 | AY725531 | MF951427 | |
| Mycosphaerella ellipsoidea | CBS 110843TofMycosphaerella ellipsoidea = CPC 850 | Eucalyptus cladocalyx | South Africa: Western Cape | P.W. Crous, 7 Nov. 1994 | GQ852602 | AY725545 | MF951431 | |
| Mycosphaerella buckinghamiae | CBS 111996TofMycosphaerella buckinghamiae = CPC 3006 | Buckinghamia sp. | Australia: New South Wales | P.W. Crous & B. Summerell, Aug. 1999 | MF951124 | EU707855 | MF951430 | |
| Mycosphaerella africana | CBS 116154T = CMW 4945 = CPC 794 | Eucalyptus viminalis | South Africa | P.W. Crous, Oct. 1994 | GQ852601 | KF901700 | MF951429 | |
| Mycosphaerella gregaria | CBS 134927TofMycosphaerella gregaria = DAR 72368 | Eucalyptus grandis | Australia: Victoria | A.J. Carnegie, 11 Nov. 1990 | MF951125 | MF951289 | MF951432 | |
| Mycosphaerella aurantia | CPC 12678 | Dracaena draco | New Zealand | M. Braithwaite, 1 Mar. 2004 | MF951123 | MF951288 | MF951428 | |
| A. keniensis | Mycosphaerella keniensis | CBS 111001T = CPC 1084 = CMW 5147 | Eucalyptus grandis litter | Kenya | M.J. Wingfield, May 1995 | GQ852610 | MF951290 | MF951433 |
| Mycosphaerella mozambica | CBS 121391 = UQ 438 = X884 | Musa sp. | Australia | – | MF951126 | EU514258 | MF951434 | |
| Amycosphaerella sp. | Crinipellis perniciosa | CBS 441.80 | Theobroma cacao | Brazil | H.C. Evans | MF951127 | MF951291 | MF951435 |
| Annellosympodiella juniperi | – | CBS 137992T = CPC 23276 | Juniperus procera | Ethiopia | P.W. Crous & A. Assefa, 25 Jun. 2013 | KJ869204 | KJ869204 | MF951436 |
| Apseudocercosporella trigonotidis | Pseudocercosporella sp. | CBS 131890T = CPC 10864 | Trigonotis peduncularis | Republic of Korea | H.-D. Shin, 12 Nov. 2003 | JQ324972 | GU269858 | KX288414 |
| Asperisporium caricae | – | CBS 130298ET | Carica papaya | Brazil | C. Weight, 16 Apr. 2010 | MF951128 | NR_119970 | MF951437 |
| Asperisporium sp. | CPC 22691 | Carica papaya | Brazil | A.C. Alfenas, Mar. 2013 | MF951129 | MF951292 | MF951438 | |
| A. caricicola | – | CBS 139998T = CPC 24348 = TSU:MUMH 11477 | Carica papaya | Republic of Fiji | C. Nakashima, 10 Sep. 2013 | KR611891 | KR611869 | MF951439 |
| Australosphaerella nootherensis | Mycosphaerella nootherensis | CBS 130522T | Corymbia intermedia | Australia: Queensland | A.J. Carnegie, 11 Aug. 2008 | KF901835 | MF951293 | MF951440 |
| Brunneosphaerella jonkershoekensis | – | CPC 13902ET | Protea repens | South Africa: Western Cape | P.W. Crous, Apr. 2007 | JN712503 | JN712439 | MF951441 |
| B. nitidae | – | CBS 130595T = CPC 15231 | Protea nitida leaf litter | South Africa: Western Cape | L. Mostert, 12 Apr. 2008 | GU214396 | GU214625 | MF951442 |
| B. protearum | – | CBS 130597ET = CPC 16338 | Protea sp. | South Africa: Western Cape | P.W. Crous, 13 Jan. 2009 | GU214397 | GU214626 | MF951443 |
| Brunswickiella parsonsiae | – | CBS 137979T = CPC 22537 | Parsonsia straminea | Australia | B.A. Summerell, 9 Mar. 2013 | KJ869188 | KJ869131 | MF951593 |
| Caryophylloseptoria lychnidis | – | CBS 109099 | Silene pratensis | Austria | G. Verkley, 4 Aug. 2000 | KF251791 | KF251287 | MF951444 |
| – | CBS 109102 | Silene pratensis | Austria | G. Verkley, 4 Aug. 2000 | KF251793 | KF251289 | MF951445 | |
| C. pseudolychnidis | – | CBS 128614 = KACC 42904 = SMKC 22691 | Lychnis cognata | Republic of Korea | – | KF251794 | KF251290 | KX348049 |
| – | CBS 128630T = KACC 43866 = SMKC 23519 | Lychnis cognata | Republic of Korea | – | KF251795 | KF251291 | MF951446 | |
| C. silenes | Septoria silenes | CBS 109103 | Silene nutans | Austria | G. Verkley, 3 Aug. 2000 | KF251797 | KF251293 | MF951447 |
| C. spergulae | – | CBS 109010ET | Spergula morisonii | Netherlands | A. Aptroot, 13 Jun. 2000 | KF251798 | KF251294 | MF951448 |
| “Septoria” gladioli | – | CBS 353.29 | – | Netherlands | – | KF251932 | KF251428 | MF951449 |
| Catenulocercospora fusimaculans | Passalora fusimaculans | CPC 17277 | Agrostis sp. | Thailand | P. Pheng, 15 Sep. 2009 | KF251817 | KF251313 | MF951450 |
| Cercoramularia koreana | Phaeoramularia sp. | CBS 142175T = CPC 10709 | Styrax japonicus | Republic of Korea | H.-D. Shin, 17 Sep. 2003 | MF951132 | MF951296 | MF951453 |
| Phaeoramularia sp. | CPC 10639 | Styrax japonicus | Republic of Korea | H.-D. Shin, 2003 | MF951130 | MF951294 | MF951451 | |
| Phaeoramularia sp. | CPC 10641 | Styrax japonicus | Republic of Korea | H.-D. Shin, 2003 | MF951131 | MF951295 | MF951452 | |
| Cercospora apii | – | CBS 116455ET = CPC 11556 | Apium graveolens | Germany | K. Schrameyer, 10 Aug. 2004 | MF951133 | AY840519 | – |
| C. armoraciae | – | CBS 538.71 = IMI 161109 = CPC 5090 | Berteroa incana | Romania | O. Constantinescu, 4 Sep. 1969 | MF951134 | JX143547 | MF951454 |
| C. beticola | – | CPC 18813 | Beta vulgaris | USA: California | S.T. Koike, 1 Nov. 2010 | MF951135 | JX143556 | MF951455 |
| C. campi-silii | – | CBS 132625 = CPC 14585 | Impatiens noli-tangere | Republic of Korea | H.-D. Shin, 29 Sep. 2007 | KX286965 | JX143561 | KX288415 |
| C. capsici | – | CBS 132622 = CPC 14520 | Capsicum annuum | Republic of Korea | H.-D. Shin, 29 Aug. 2005 | MF951136 | JX143568 | MF951456 |
| C. cf. chenopodii | Passalora dubia | CBS 126.29 | – | – | – | MF951139 | MF951299 | MF951459 |
| Passalora dubia | CBS 256.67 | Atriplex hortensis | Romania | – | MF951140 | MF951300 | MF951460 | |
| Passalora dubia | CBS 543.71 = BUCM 2006 | Atriplex oblongifolia | Romania | O. Constantinescu & G. Negrean, 13 Jul. 1970 | MF951141 | MF951301 | MF951461 | |
| Passalora dubia | CBS 123192 = CPC 15387 | Chenopodium album | New Zealand | C.F. Hill, 2 Mar. 2008 | MF951138 | MF951298 | MF951458 | |
| – | CPC 10303 | Chenopodium ficifolium | Republic of Korea | H.-D. Shin, 3 Oct. 2002 | MF951137 | MF951297 | MF951457 | |
| – | CPC 12450 | Chenopodium ficifolium | Republic of Korea | H.-D. Shin, 27 Oct. 2005 | KX286967 | JX143574 | KX288417 | |
| C. euphorbiae-sieboldianae | – | CBS 113306T | Euphorbia sieboldiana | Republic of Korea | H.-D. Shin, 8 May 2003 | MF951142 | JX143593 | MF951462 |
| C. fagopyri | – | CBS 132623NT = CPC 14541 | Fagopyrum esculentum | Republic of Korea | H.-D. Shin | MF951143 | JX143594 | MF951463 |
| C. janseana | Passalora janseana | CBS 145.37 = IMI 303642 | – | USA | – | KF251818 | KF251314 | MF951464 |
| C. lactucae-sativae | – | CPC 10082 | Ixeris chinensis subsp. strigosa (≡ Ixeris strigosa) | Republic of Korea | H.-D. Shin, 11 Oct. 2002 | MF951144 | JX143622 | MF951465 |
| C. senecionis-walkeri | – | CBS 132636 = CPC 19196 | Senecio walkeri | Laos | P. Phengsintham, 20 Feb. 2010 | MF951145 | JX143649 | MF951466 |
| C. sojina | Passalora personata | CBS 220.31 | – | Italy | – | KX286971 | KX287279 | KX288421 |
| – | CBS 132018 = CPC 12322 | Glycine soja | Republic of Korea | H.-D. Shin, 20 Jul. 2004 | GU214655 | GU214655 | MF951467 | |
| – | CBS 132615NT = CPC 11353 | Glycine soja | Republic of Korea | H.-D. Shin, 20 Jul. 2004 | KX286969 | JX143659 | KX288419 | |
| – | CPC 11422 | Glycine soja | Republic of Korea | H.-D. Shin | KX286972 | KX287280 | KX288422 | |
| Cercospora sp. | Passalora dulcamarae | CBS 544.71 = BUCM 2008 | Solanum dulcamara | Romania | O. Constantinescu & G. Negrean, 14 Oct. 1970 | MF951146 | MF951302 | MF951468 |
| C. zeina | – | CBS 118820T = CPC 11995 | Zea mays | South Africa: KwaZulu-Natal | P. Caldwell, 2005 | MF951147 | DQ185081 | MF951469 |
| Cercosporella catenulata | Ramularia deusta var. alba | CBS 355.73T | Phaseolus vulgaris | Rwanda | D. Froment, 10 Jan. 1973 | KX286973 | KX287281 | KX288424 |
| C. dolichandrae | – | CBS 138101T = CPC 22948 | Dolichandra unguiscati | South Africa: KwaZulu-Natal | A. King,15 Nov. 2011 | KJ869197 | KJ869140 | KX288423 |
| C. virgaureae | Cercosporella vergaweae | CBS 113304 | Erigeron annuus | Republic of Korea | H.-D. Shin, 21 May 2003 | KF251805 | GU214658 | KX348051 |
| – | CPC 10286 | Erigeron annuus | Republic of Korea | H.-D. Shin, 9 Oct. 2002 | KX286978 | KX287285 | KX288428 | |
| – | CPC 11456 | Erigeron annuus | Republic of Korea | H.-D. Shin, 1 Jul. 2004 | KX286974 | MF951303 | KX348050 | |
| – | CPC 11457 | Erigeron annuus | Republic of Korea | H.-D. Shin, 1 Jul. 2004 | KX286975 | KX287282 | KX288425 | |
| – | CPC 11460 | Erigeron annuus | Republic of Korea | H.-D. Shin, 1 Jul. 2004 | KX286976 | KX287283 | KX288426 | |
| Cercosporidium californicum | Passalora californica | CBS 128857T = CPC 18389 | Asclepias fascicularis | USA: California | S.T Koike, 19 Jul. 2010 | MF951148 | HQ728115 | MF951470 |
| Passalora californica | CPC 18390 | Asclepias fascicularis | USA: California | S.T Koike, 19 Jul. 2010 | MF951149 | MF951304 | MF951471 | |
| C. chaetomium | Passalora sp. | CBS 142177ET = CPC 18624 | Euphorbia sp. | Canada | P.W. Crous & K. Seifert, 28 Sep. 2010 | MF951151 | MF951306 | MF951474 |
| C. miurae | Passalora miurae | CBS 142235 = CPC 14628 | Metaplexis japonica | Republic of Korea | H.-D. Shin, 1 Oct. 2007 | MF951150 | MF951305 | MF951472 |
| Passalora miurae | CPC 14643 | Metaplexis japonica | Republic of Korea | H.-D. Shin, 22 Sep. 2007 | KJ633268 | KJ633264 | MF951473 | |
| Chuppomyces handelii | Mycosphaerella handelii | CBS 113302 | Rhododendron sp. | Netherlands | P.W. Crous & U. Braun, 2002 | GU214437 | EU167581 | MF951475 |
| Clarohilum henningsii | Passalora henningsii | CPC 17314 | Manihot esculenta | Laos | P. Pheng, 5 May 2006 | MF951152 | MF951307 | MF951476 |
| Clypeosphaerella calotropidis | Passalora calotropidis | CBS 129.30 | Calotropis procera | Egypt | – | MF951153 | MF951308 | MF951477 |
| C. quasiparkii | Mycosphaerella quasiparki | CBS 123243T = CPC 15409 | Eucalyptus sp. | Thailand | P. Suwannawong, Jul. 2007 | KF902128 | KF901771 | MF951478 |
| Collarispora valgourgensis | Passalora sp. | CBS 125311 = CS2 OH3 gH1c | Malus sp. | USA: Ohio | M. Ellis, 29 Sep. 2005 | MF951154 | MF951309 | MF951480 |
| Mycosphaerella valgourgensis | CBS 129531T = CPC 18385 | Yucca sp. | France | P.W. Crous, 15 Jul. 2010 | JF951175 | JF951152 | MF951479 | |
| Coremiopassalora eucalypti | Passalora eucalypti | CBS 111306TofMycovellosiella eucalypti = CPC 1455 = CMW 14907 | Eucalyptus saligna | Brazil | P.W. Crous & A.C. Alfenas, Jun. 1995 | GU253860 | GU269845 | MF951481 |
| Passalora eucalypti | CBS 111318 = CPC 1457 | Eucalyptus saligna | Brazil | P.W. Crous & A.C. Alfenas, Jun. 1995 | GU253860 | GU269845 | MF951482 | |
| C. leptophlebae | Passalora leptophlebiae | CBS 129524T = CPC 18480 | Eucalyptus leptophleba | Brazil | P.W. Crous, A.C. Alfenas, R. Alfenas & O.L. Pereira, 23 Aug. 2010 | KF901939 | MF951310 | MF951483 |
| Cytostagonospora martiniana | Septoria sp. | CBS 135102ET = CPC 17727 | Acacia pycnantha | Australia: Victoria | P.W. Crous, 21 Oct. 2009 | KF251657 | KF251153 | MF951484 |
| Deightonomyces daleae | Passalora daleae | CBS 113031 | Dalea spinosa | Mexico | L.B. Sparrius, Apr. 2003 | MF951155 | EU040236 | MF951485 |
| Devonomyces endophyticus | Phaeophleospora gregaria | CBS 110501 = CMW 14462 | Eucalyptus globulus | Australia: Western Australia | A. Maxwell, 15 Dec. 2000 | EU167580 | EU167580 | MF951589 |
| Phaeophleospora gregaria | CBS 111167 = CPC 1225 | Eucalyptus cladocalyx | South Africa: Western Cape | A.R. Wood, 22 Sep. 1995 | KF902058 | KF901711 | MF951588 | |
| Mycosphaerella endophytica | CBS 114662TofMycosphaerella endophytica = CPC 1193 | Eucalyptus sp. | South Africa: Western Cape | P.W. Crous, Jun. 1995 | KF902060 | KF901713 | MF951590 | |
| Mycosphaerella pseudoellipsoidea | CBS 114709 = CMW 9099 | Eucalyptus nitens | South Africa | – | EU167585 | EU167585 | MF951591 | |
| Stenella sp. | CPC 15580 | Hakea undulata | Australia | A.R. Wood, 2 Aug. 2008 | MF951212 | MF951357 | MF951592 | |
| Distocercospora pachyderma | – | CBS 138247ET = CPC 24144 | Dioscorea sp. | Japan | C. Nakashima & K. Motohashi, 13 Sep. 2010 | MF951156 | MF951311 | MF951486 |
| Distocercosporaster dioscoriae | Passalora dioscoreae | CBS 135460 = CPC 10855 | Dioscorea tokoro | Republic of Korea | H.-D. Shin, 16 Oct. 2003 | GU214665 | GU214665 | MF951488 |
| Passalora dioscoreae | CBS 135463 = CPC 11513 | Dioscorea tenuipes | Republic of Korea | H.-D. Shin, 2003 | KF251815 | KF251311 | MF951489 | |
| Passalora dioscoreae | KACC 44723 | Dioscorea sp. | Republic of Korea | H.-D. Shin | MF951157 | MF951312 | MF951487 | |
| Distomycovellosiella brachycarpa | Passalora brachycarpa | CBS 114855 | – | New Zealand | – | MF951159 | MF951314 | MF951491 |
| Passalora brachycarpa | CBS 115124 | Solanum mauritianum | New Zealand | – | GU214664 | GU214664 | MF951492 | |
| Mycovellosiella brachycarpa | CBS 142178ET = CPC 18381 | Solanum mauritianum | South Africa: KwaZulu-Natal | A.R. Wood, 6 Jul. 2010 | MF951158 | MF951313 | MF951490 | |
| Dothistroma pini | – | CBS 116486 | Pinus nigra | USA: Michigan | G. Adams, 2001 | JX901823 | JX901735 | KX348053 |
| – | CBS 121005 = CMW 24852 | Pinus pallasiana | Russia | T.S. Bulgakov, 8 Oct. 2006 | KF251659 | KF251155 | KX348052 | |
| D. septosporum | – | CBS 128782 = CPC 16798 | Pinus mugo ‘Rostrata’ | Netherlands | W. Quaedvlieg, 1 Jun. 2009 | JX901829 | JX901741 | KX348054 |
| – | CBS 128783 = CPC 16799 | Pinus mugo ‘Rostrata’ | Netherlands | W. Quaedvlieg, 1 Jun. 2009 | JF700938 | JX901742 | MF951493 | |
| Epicoleosporium ramularioides | – | CBS 141103T = CPC 10672 | Coleosporium phellodendri on leaves of Phellodendron amurense | Republic of Korea | H.-D. Shin, 4 Sep. 2003 | GU214688 | GU214688 | KX288433 |
| – | CPC 10673 | Coleosporium phellodendri on leaves of Phellodendron amurense | Republic of Korea | H.-D. Shin, 4 Sep. 2003 | MF951160 | KX287289 | KX288434 | |
| Exosporium livistonae | Passalora sp. | CBS 131313T = CPC 19357 | Livistona benthamii | Australia: Northern Territory | P.W. Crous & B.A. Summerell, 25 Apr. 2011 | JQ044446 | JQ044427 | MF951494 |
| E. livistonicola | – | MUCC 190 | Livistona chinensis | Japan | T. Kobayashi & Y. Ono, 27 Feb. 2003 | MF951161 | MF951315 | MF951495 |
| Exutisphaerella laricina | Mycosphaerella laricina | CBS 326.52NT | Larix decidua | Switzerland | – | GU253693 | GU269643 | MF951496 |
| Filiella pastinacae | Pseudocercosporella pastinacae | CBS 114116 = UPSC 2633 | Laserpitium latifolium | Sweden | K. & L. Holm, 2 Jun. 1988 | KF251832 | KF251328 | KX348056 |
| Fulvia fulva | Passalora fulva | CBS 120.46 = VKM F-3053 | Solanum lycopersicum | Switzerland | – | MF951162 | MF951316 | MF951497 |
| Passalora fulva | CBS 142314ET = CPC 13652 | Solanum lycopersicum | Cuba | B. Summerell, 2006 | MF951163 | MF951317 | MF951498 | |
| Fusoidiella anethi | Passalora puncta | CBS 296.32 | – | Italy | – | MF951164 | MF951318 | MF951499 |
| Passalora puncta | CBS 117584 | Foeniculum vulgare | New Zealand | – | MF951165 | MF951319 | MF951500 | |
| F. depressa | Passalora depressa | CBS 141335 = CPC 14915 | Angelica gigas | Republic of Korea | H.-D. Shin, 18 Oct. 2007 | KF251813 | KF251309 | KX348055 |
| Genus A: “Passalora” vaginae | Passalora vaginae | CBS 140.34 = DSM 1148 = IMI 303641 | Saccharum officinarum | Taiwan | – | MF951166 | MF951320 | – |
| Graminopassalora graminis | Passalora graminis | CBS 113303 | Alopecurus aequalis var. amurensis | Republic of Korea | H.-D. Shin, 24 May 2003 | GU214666 | GU214666 | MF951502 |
| Cercosporidium graminis | MAFF 510604 = MUCC 1429 | Dactylis glomerata | Japan | N. Nishihara, – | MF951167 | MF951321 | MF951501 | |
| Hyalinozasmidium aerohyalinosporum | Zasmidium aerohyalinosporum | CBS 125011TofZasmidium aerohyalinosporum = CPC 14636 | Eucalyptus tectifica | Australia: New South Wales | B.A. Summerell, 23 Sep. 2007 | KF901930 | GQ852839 | MF951504 |
| H. sideroxyli | Zasmidium sp. | CBS 142191T = CPC 23462 | Sideroxylon inerme | South Africa: Eastern Cape | A.R. Wood, 8 May 2013 | MF951169 | MF951323 | MF951505 |
| Hyalocercosporidium desmodii | Passalora sp. | CBS 142179T = CPC 19483 | Desmodium tortuosum | Brazil: Minas Gerais | R.W. Barreto, 2 Aug. 2009 | MF951168 | MF951322 | MF951503 |
| Lecanosticta acicola | – | CBS 871.95 = MPFN 314 | Pinus radiata | France | M. Morelet, Apr. 1995 | GU214663 | GU214663 | MF951506 |
| – | CBS 133791ET = WPF13.12 | Pinus strobus | USA: New Hampshire | B. Ostrofsky, 15 Jun. 2011 | KC013017 | KC012999 | MF951507 | |
| L. brevispora | – | CBS 133601T = CPC 18092 | Pinus sp. | Mexico | M. de Jesús Yáñez-Morales, 24 Oct. 2009 | KF902021 | JX901763 | MF951508 |
| L. longispora | – | CBS 133602ET = CPC 17940 | Pinus sp. | Mexico | M. de Jesús Yáñez-Morales & C. Méndez-Inocencio, 24 Oct. 2009 | JX901858 | JX901766 | MF951510 |
| – | CPC 17941 | Pinus sp. | Mexico | M. de Jesús Yáñez-Morales & C. Méndez-Inocencio, 24 Oct. 2009 | KF902022 | JX901766 | MF951509 | |
| Madagascaromyces intermedius | Passalora intermedia | CBS 124154T = CPC 15745 | Eucalyptus camaldulensis | Madagascar | M.J. Wingfield, Aug. 2007 | FJ790297 | FJ790267 | MF951511 |
| Stenella sp. | CPC 15719 | Eucalyptus camaldulensis | Madagascar | M.J. Wingfield, Oct. 2007 | MF951170 | FJ790251 | MF951512 | |
| Microcyclosporella mali | – | CBS 125651 = RH1 = OH1 34D2a | Malus sp. | USA: Ohio | M. Ellis, 5 Sep. 2005 | FJ031989 | FJ425196 | KX288442 |
| – | CBS 125653 = RH6 = MI3 20F1a | Malus sp. | USA: Michigan | G. Sundin, 1 Sep. 2005 | FJ031994 | FJ425201 | KX288440 | |
| – | CBS 126132 = CPC 16180 | Malus domestica | Slovenia | J. Frank, 17 Oct. 2007 | MF951171 | MF951324 | MF951513 | |
| – | CBS 126136T = CPC 16184 | Malus domestica | Slovenia | J. Frank, 7 Aug. 2007 | GU570547 | GU570535 | KX288436 | |
| Micronematomyces caribensis | Passalora caribensis | CBS 113374 = MJM 1545 = C481 | Chromolaena odorata | Jamaica | M.J. Morris | MF951172 | DQ676512 | MF951514 |
| Passalora caribensis | CBS 113375 = MJM 1543 = C482 | Chromolaena odorata | Jamaica | M.J. Morris | MF951173 | DQ676513 | MF951515 | |
| Passalora caribensis | CBS 113376 = MJM 1539 = C487 | Chromolaena odorata | Cuba | S. Neser, 28 Oct. 1997 | MF951174 | DQ676514 | MF951516 | |
| Passalora perfoliati | CBS 113378 = MJM 1552 = C494 | Chromolaena odorata | Jamaica | M.J. Morris, 1 Nov. 1997 | MF951178 | DQ676520 | MF951520 | |
| Passalora perfoliati | CBS 113379 = MJM 1544 = C495 | Chromolaena odorata | Jamaica | M.J. Morris, 30 Oct. 1997 | MF951177 | DQ676521 | MF951519 | |
| Passalora caribensis | CBS 113380T = MJM 1550 = C498 | Chromolaena odorata | Jamaica | M.J. Morris, 31 Oct. 1997 | MF951175 | DQ676515 | MF951517 | |
| Passalora caribensis | CBS 113381 = MJM 1549 = C500 | Chromolaena odorata | Jamaica | M.J. Morris, 30 Oct. 1997 | MF951176 | DQ676516 | MF951518 | |
| M. chromolaenae | Septoria chromolaenae | CBS 113371 = MJM 1490 = C450 | Chromolaena odorata | Mexico | M.J. Morris, 12 Oct. 1997 | MF951179 | DQ676517 | MF951521 |
| Septoria chromolaenae | CBS 113611T = MJM 1498 = C452 | Chromolaena odorata | Mexico | M.J. Morris, 12 Oct. 1997 | MF951180 | DQ676518 | MF951522 | |
| Miuraea degenerans | Miuraea degenerans | MAFF 239265ET = MUCC 1514 | Prunus mume | Japan | T. Kobayashi, Sep. 2003 | MF951181 | MF951325 | MF951523 |
| M. persica | Miuraea persica | CBS 131935 = CPC 10828 | Prunus armeniaca | Republic of Korea | H.-D. Shin, 7 Oct. 2003 | JQ324939 | GU269844 | MF951524 |
| Mycodiella sumatrensis | Mycosphaerella sumatrensis | CBS 118501 = CPC 11175 | Eucalyptus sp. | Indonesia | M.J. Wingfield, Feb. 2004 | JX901872 | DQ303049 | MF951525 |
| Mycosphaerelloides madeirae | Mycosphaerella madeirae | CBS 112895T = CPC 3745 = CMW 14458 | Eucalyptus globulus | Portugal | S. Denman, Apr. 2000 | KF902017 | AY725553 | KX348057 |
| Mycosphaerella madeirae | CBS 116066 | Quercus robur | Netherlands | – | KX286989 | AY853188 | KX288444 | |
| Mycosphaerella madeirae | CBS 116068 | Quercus robur | Netherlands | – | KX286990 | AY853189 | KX288445 | |
| Mycovellosiella cajani | Passalora sp. | CBS 113998 = CPC 5335 | Cajanus cajan | South Africa: Mpumalanga | L. van Jaarsveld, 17 May 2002 | KF251819 | KF251315 | MF951527 |
| Passalora sp. | CBS 113999 = CPC 5339 | Cajanus cajan | South Africa: Mpumalanga | L. van Jaarsveld, 17 May 2002 | KF251820 | KF251316 | MF951528 | |
| Passalora sp. | CBS 114275 = CPC 5334 | Cajanus cajan | South Africa: Mpumalanga | L. van Jaarsveld, 17 May 2002 | KF251821 | KF251317 | MF951529 | |
| – | CBS 142174NT = CPC 30580 = RWB 2071 | Cajanus cajan | Brazil | R.W. Barreto, 2016 | MF951182 | MF951326 | MF951526 | |
| Neoceratosperma cyatheae | Passalora sp. | CPC 18580 | Cyathea delgadii | Brazil: Rio de Janeiro | R.W. Barreto, 11 Jul. 2009 | KT037580 | KT037539 | MF951530 |
| N. eucalypti | – | CBS 137998T = CPC 23465 | Eucalyptus sp. | Thailand | R. Cheewangkoon, Sep. 2013 | KJ869210 | KJ869153 | MF951531 |
| N. haldinae | Passalora haldinae | CBS 142190T = CPC 19202 | Haldina cordifolia | Laos | P. Pheng | MF951184 | MF951328 | MF951533 |
| N. legnophoricola | Stenella sp. | CBS 142189T = CPC 16411 | Legnephora moorei | Australia: New South Wales | B. Summerell, Mar. 2009 | MF951183 | MF951327 | MF951532 |
| N. yunnanensis | Xenomycosphaerella yunnanensis | CBS 119975T = CMW 23443 = MUCC 410 = PAB 05.05 B2 | Eucalyptus urophylla | China | B. Dell, May 2005 | KF901962 | KF901628 | MF951534 |
| Neocercospora ammicola | – | CBS 136450T = CCTU 1186 | Ammi majus | Iran | M. Arzanlou, Sep. 2012 | KR232405 | KR232407 | KX288446 |
| Neocercosporidium smilacis | Passalora smilacis | CBS 556.71 | Smilax aspera | Italy | W. Gams, 18 May 1971 | KJ633269 | KJ633265 | MF951535 |
| Passalora sp. | CBS 122888ET | Smilax aspera | Portugal | G. Verkley, 23 Jan. 2008 | MF951185 | MF951329 | MF951536 | |
| Passalora sp. | CBS 122889 | Smilax aspera | Portugal | G. Verkley, 23 Jan. 2008 | MF951186 | MF951330 | MF951537 | |
| Passalora sp. | CBS 122890 | Smilax aspera | Portugal | G. Verkley, 23 Jan. 2008 | MF951187 | MF951331 | MF951538 | |
| Passalora sp. | CBS 123352 | Smilax aspera | Portugal | G. Verkley, 23 Jan. 2008 | MF951188 | MF951332 | MF951539 | |
| Passalora sp. | CBS 123353 | Smilax aspera | Portugal | G. Verkley, 23 Jan. 2008 | MF951189 | MF951333 | MF951540 | |
| Passalora sp. | CPC 19342 | Smilax sp. | Italy | W. Gams, 30 Apr. 2011 | MF951190 | MF951334 | MF951541 | |
| Neodeightoniella phragmiticola | – | CBS 136418T = CPC 22059 | Phragmites australis | South Africa: Free State | W.J. Swart, 31 Jan. 2013 | KF777224 | KF777171 | MF951543 |
| – | CPC 22057 | Phragmites australis | South Africa: Free State | W.J. Swart, 31 Jan. 2013 | KF777223 | KF777170 | MF951542 | |
| – | CPC 22061 | Phragmites australis | South Africa: Free State | W.J. Swart, 31 Jan. 2013 | KF777225 | KF777172 | MF951544 | |
| Neomycosphaerella pseudopentameridis | – | CBS 136407T = CPC 21126 | Pseudopentameris macrantha | South Africa: Western Cape | P.W. Crous, 22 Jul. 2012 | KF777226 | KF777173 | MF951545 |
| Neopenidiella nectandrae | Cladosporium ferrugineum | CBS 734.87TofCladosporium ferrugineum = ATCC 200932 = INIFAT 87/45 | Nectandra coriacea | Cuba | R.F. Castañeda & G. Arnold, 24 Jan. 1987 | KF901982 | MF951335 | MF951546 |
| Neophloeospora maculans | Phloeospora maculans | CBS 115123 | Morus alba | New Zealand | – | GU214670 | GU214670 | MF951547 |
| Neopseudocercosporella brassicicola | Mycosphaerella brassicicola | CBS 163.26 | – | – | – | MF951192 | MF951337 | MF951548 |
| Mycosphaerella brassicicola | CBS 228.32 | Brassica oleracea | Denmark | – | KF251808 | KF251304 | KX348058 | |
| Mycosphaerella brassicicola | CBS 267.53 | Brassica oleracea var. acephala subvar. sabelica | Netherlands | – | KF251809 | KF251305 | KX348059 | |
| N. capsellae | Pseudocercosporella capsellae | CBS 112032 = HJS 601 | Brassica sp. | – | – | KF251824 | KF251320 | KX348060 |
| Pseudocercosporella capsellae | CBS 112033 = HJS 600 | Brassica sp. | – | – | KF251810 | KF251306 | KX348061 | |
| Pseudocercosporella capsellae | CBS 118412 | Brassica sp. | New Zealand | – | MF951193 | MF951338 | MF951549 | |
| Pseudocercosporella capsellae | MAFF 237605 = MUCC 1254 | Brassica rapa var. oleifera | Japan | K. Kishi,– | MF951194 | MF951339 | MF951550 | |
| Neoseptoria caricis | – | CBS 135097T = S653 | Carex acutiformis | Netherlands | W. Quaedvlieg, Aug. 2012 | KF251663 | KF251159 | MF951551 |
| Nothopassalora personata | Mycosphaerella berkeleyii | CBS 222.38ITofMycosphaerella berkeleyii | Arachis hypogaea | USA: Georgia | W.A. Jenkins, 23 Jun. 1937 | MF951234 | MF951373 | MF951631 |
| Passalora sp. | CBS 142236ET = CPC 19466 | Arachis hypogaea | Australia: Northern Territory | P.W. Crous, 30 Apr. 2011 | MF951235 | MF951374 | MF951632 | |
| Nothopericoniella persea-macranthae | Periconiella persea-macranthae | CBS 122097 = RoKi 2995 | Machilus zihoensis | Taiwan | R. Kirschner & C.-J. Chen, 18 Mar. 2007 | GU452682 | MF951354 | MF951583 |
| Periconiella persea-macranthae | CBS 122282 = RoKi 3030 | Unidentified Lauraceae | Taiwan | R. Kirschner & C.-J. Chen, 1 Apr. 2007 | GU452681 | MF951355 | MF951584 | |
| Nothophaeocryptopus gaeumannii | Adelopus balsamicola f. douglasii | CBS 244.38 | – | Austria | – | MF951191 | MF951336 | GU357766 |
| Adelopus gaeumannii | CBS 267.37 | Pseudotsuga menziesii | Germany | – | EF114698 | EU700365 | GU357770 | |
| Pachyramichloridium pini | Ramichloridium pini | CBS 461.82T = MUCL 28942 | Pinus contorta | UK: Scotland | – | EU041859 | EU041802 | MF951552 |
| Pallidocercospora acaciigena | Mycosphaerella acaciigena | CBS 112515T = CPC 3837 | Acacia mangium | Venezuela | M.J. Wingfield, May 2000 | KF902166 | KF901805 | KX348062 |
| P. crystallina | – | CBS 111045 = CPC 1179 | Eucalyptus grandis litter | South Africa: KwaZulu-Natal | M.J. Wingfield, 22 Jun. 1995 | KF442659 | KF901704 | KX348063 |
| Passalora sp. | CPC 14140 | Eucalyptus sp. | China | X. Zhao, 1 Mar. 2007 | MF951195 | MF951340 | MF951553 | |
| P. heimii | – | CBS 110682T = CPC 760 | Eucalyptus sp. | Madagascar | P.W. Crous, 16 Apr. 1994 | GQ852604 | KF901671 | MF951554 |
| – | CPC 11716 | – | Brazil | A.C. Alfenas, Jan. 2004 | KF901937 | KF901612 | KX348064 | |
| P. heimioides | Mycosphaerella heimioides | CBS 111190TofMycosphaerella heimioides = CMW 3046 = CPC 1312 | Eucalyptus sp. | Indonesia | M.J. Wingfield, 12 Mar. 1996 | GQ852607 | KF901659 | MF951555 |
| P. irregulariramosa | Mycosphaerella irregulariramosa | CBS 111211T = CPC 1362 | Eucalyptus saligna | South Africa: Northern Province | M.J. Wingfield, Mar. 1996 | KF902053 | KX287297 | KX348065 |
| P. konae | Mycosphaerella konae | CBS 111028T = CPC 2125 | Leucadendron cv. ‘Safari Sunset’ | USA: Hawaii | P.W. Crous & M.E. Palm, 17 Nov. 1998 | KF902158 | KF901798 | KX348066 |
| Pantospora guazumae | – | CBS 130299ET | Guazuma ulmifolia | Mexico | J. Moore, 12 Feb. 2009 | MF951196 | NR_119971 | MF951556 |
| Paracercospora egenula | – | CBS 485.81 | – | India | – | MF951197 | GU269699 | MF951558 |
| – | CBS 132030 = CPC 12537 | Solanum melongena | Republic of Korea | H.-D. Shin, 26 Oct. 2005 | GU253738 | GU269698 | MF951557 | |
| Paracercosporidium microsorum | Mycosphaerella microsora | CBS 254.67 | Tilia tomentosa | Romania | O. Constantinescu, 16 Jun. 1965 | MF951198 | MF951341 | MF951559 |
| Mycosphaerella microsora | CBS 552.71 = BUCM 2014 | Tilia platyphyllos | Romania | O. Constantinescu, 8 Oct. 1969 | MF951199 | MF951342 | MF951560 | |
| Mycosphaerella microsora | CBS 100352 | Tilia cordata | Netherlands | H.A. van der Aa, 19 Oct. 1997 | EU167599 | EU167599 | MF951561 | |
| Mycosphaerella microsora | CBS 101017 | Tilia cordata | Netherlands | H.A. van der Aa, 1 Apr. 1998 | MF951200 | MF951343 | MF951562 | |
| Passalora microsora | CBS 123735 | Tilia sp. | Czech Republic | G. Verkley, 16 Sep. 2008 | KJ633266 | KJ633262 | MF951563 | |
| Passalora microsora | CBS 142176ET = CPC 15550 | Tilia cordata | Ukraine | A. Akulov, 18 Jul. 2008 | MF951201 | MF951344 | MF951564 | |
| P. tiliae | Passalora sp. | CBS 112734ET = CPC 3952 | Tilia americana | Canada | K.A. Seifert | MF951202 | MF951345 | MF951565 |
| Passalora sp. | CBS 115526 = CPC 3953 | Tilia americana | Canada | K.A. Seifert | MF951203 | MF951346 | MF951566 | |
| Paramycosphaerella brachystegiae | – | CBS 136436T = CPC 21136 | Brachystegia sp. | Zimbabwe | J. Roux, 2 Apr. 2012 | KF777230 | KF777178 | MF951567 |
| P. intermedia | – | CBS 114356T = NZFS 301.10 = CMW 7163 = CPC 10902 | Eucalyptus saligna | New Zealand | L. Renney, 30 Jun. 1998 | KF902026 | KF901681 | MF951569 |
| – | CBS 114415 = NZFS 301.13 = CMW 7164 = CPC 10922 | Eucalyptus saligna | New Zealand | K. Dobbie, 12 Aug. 1998 | KF902027 | KF901682 | MF951568 | |
| P. marksii | Pseudocercospora epispermogonia | CBS 110750 = CPC 822 | Eucalyptus grandis | South Africa | G. Kemp, Oct. 1994 | DQ303075 | DQ267596 | MF951573 |
| – | CBS 110963 = CPC 4632 = KS cl 42 | Musa sp. | South Africa: Northern Province | K. Surridge | KF902054 | KF901707 | MF951570 | |
| – | CBS 110964 = CPC 4633 = KS 41 | Musa sp. | South Africa | K. Surridge | KF902055 | KF901708 | MF951571 | |
| Mycosphaerella marksii | CBS 110920 = CPC 935 | Eucalyptus botryoides | Australia: Victoria | A. Carnegie, 14 Oct. 1994 | GU253694 | GU269644 | MF951572 | |
| Paramycosphaerella sp. A | Mycosphaerella colombiensis | CBS 118825 = CMW 10904 | Musa cv. Grand Naine | South Africa | K. Surridge | MF951204 | MF951347 | MF951574 |
| Mycosphaerella colombiensis | CBS 118849 = CMW 10902 | Musa cv. Williams | South Africa | K. Surridge | MF951205 | MF951348 | MF951575 | |
| Paramycosphaerella sp. B | Colletogloeum sp. | CBS 118968 = CUF2d | Malus sp. | USA: Illinois | J. Batzer, Sep. 2000 | MF951206 | MF951349 | MF951576 |
| Colletogloeum sp. | CBS 125300 = NY1 3.2F1c | Malus sp. | USA: New York | D. Rosenberger , 30 Oct. 2005 | MF951207 | MF951350 | MF951577 | |
| P. wachendorfiae | Mycosphaerella wachendorfiae | CBS 129579T = CPC 18338 | Wachendorfia thyrsifolia | South Africa | K.L. Crous & P.W. Crous, 2 May 2010 | JF951163 | JF951143 | MF951578 |
| Paramycovellosiella passaloroides | Mycovellosiella passaloroides | CPC 10770 | Amorpha fruticosa | Republic of Korea | H.-D. Shin, 23 Oct. 2002 | MF951209 | MF951352 | MF951580 |
| Mycovellosiella passaloroides | CPC 14694 | Amorpha fruticosa | Republic of Korea | H.-D. Shin, 30 Oct. 2007 | MF951208 | MF951351 | MF951579 | |
| Parapallidocercospora colombiensis | Mycosphaerella colombiensis | CBS 110968T = CPC 1105 | Eucalyptus urophylla | Colombia | M.J. Wingfield, May 1995 | KF901969 | AY752148 | MF951581 |
| P. thailandica | Pallidocercospora thailandica | CBS 120723 = CPC 13478 | Eucalyptus calmadulensis | Thailand | W. Himaman, Oct. 2006 | KF442667 | MF951353 | MF951582 |
| Passalora bacilligera | – | CBS 131547ET = CPC 19944 | Alnus glutinosa | Poland | D. Karasinski, 20 Sep. 2011 | MF951210 | MF951356 | MF951585 |
| Phaeocercospora colophospermi | – | CBS 132687T = CPC 19812 | Colophospermum mopane | South Africa: Mpumalanga | P.W. Crous, 11 Jul. 2011 | JX069854 | JX069870 | MF951586 |
| P. juniperina | Passalora juniperina | CBS 142238 = CPC 11258 | Juniperus virginiana | USA: North Carolina | C.S. Hodges, 1 Mar. 2004 | MF951211 | GU214667 | MF951587 |
| Phaeophloeospora eugeniae | – | CBS 142184 = CPC 15143 | Eugenia uniflora | Brazil | A.C. Alfenas, 1 Mar. 2008 | FJ493206 | FJ493188 | MF951594 |
| – | CPC 15159 | Eugenia uniflora | Brazil | A.C. Alfenas, 1 Apr. 2008 | FJ493207 | FJ493189 | MF951595 | |
| Phaeoramularia capsicicola | – | CBS 156.62 | Capsicum annuum | Italy | – | KJ633267 | KJ633263 | – |
| Passalora sp. | CBS 113382 = C460 | Chromolaena odorata | USA | M.J. Morris | MF951213 | DQ676522 | MF951596 | |
| Passalora sp. | CBS 113384 = C499 | Chromolaena odorata | Jamaica | M.J. Morris | MF951214 | DQ676524 | MF951597 | |
| P. gomphrenicola | Phaeoramularia gomphrenicola | CBS 142182ET = CPC 23248 = COAD 570 | Pfaffia glomerata | Brazil | R. Barreto, 29 Oct. 2012 | MF951216 | MF951359 | MF951599 |
| Phaeoramularia gomphrenicola | CPC 23249 = COAD571 | Pfaffia glomerata | Brazil | R. Barreto | MF951215 | MF951358 | MF951598 | |
| Phloeospora ulmi | – | CBS 613.81 | Ulmus sp. | Austria | H.A. van der Aa, 21 Sep. 1981 | GU253842 | GU269825 | MF951601 |
| – | CBS 101564 | Ulmus sp. | Netherlands | H.A. van der Aa, 26 Aug. 1998 | KF251703 | KF251200 | MF951602 | |
| – | CBS 109835 | Ulmus sp. | Netherlands | G. Verkley, 27 Aug. 2001 | KF251704 | KF251201 | MF951600 | |
| Pleopassalora perplexa | Passalora perplexa | CBS 116363T = CPC 11147 | Acacia crassicarpa | Indonesia | M.J. Wingfield, Feb. 2004 | MF951220 | AY752162 | MF951606 |
| Passalora perplexa | CBS 116364 = CPC 11150 | Acacia crassicarpa | Indonesia | M.J. Wingfield, Feb. 2004 | GU214459 | AY752163 | MF951607 | |
| Passalora acaciae | CPC 11152 | Acacia crassicarpa | Indonesia | M.J. Wingfield, 1 Mar. 2004 | MF951217 | MF951360 | MF951603 | |
| Passalora perplexa | CPC 12168 | Acacia sp. | Indonesia | M.J. Wingfield, 1 May 2005 | MF951218 | MF951361 | MF951604 | |
| Passalora perplexa | CPC 12170 | Acacia sp. | Indonesia | M.J. Wingfield, 1 May 2005 | MF951219 | MF951362 | MF951605 | |
| “Passalora” sp. 1 | Passalora loranthicola | CBS 122466 = X138 | Citrus sp. | USA: Florida | R. C. Ploetz | MF951221 | EU514280 | MF951608 |
| Pleuropassalora armatae | Passalora armatae | CBS 125420T = CPC 15419 | Dalbergia armata | South Africa: KwaZulu-Natal | A.R Wood, 28 May 2008 | GU214456 | GU214640 | MF951609 |
| Passalora sp. | CPC 15420 | Dalbergia armata | South Africa | A.R Wood, 28 May 2008 | MF951222 | MF951363 | MF951610 | |
| Passalora sp. | CPC 17084 | Dalbergia obovata | South Africa | A.R. Wood, 15 Jun. 2009 | MF951223 | MF951364 | MF951611 | |
| Pluripassalora bougainvilleae | Passalora sp. | CBS 142237 = CPC 19327 | Bougainvillea sp. | Australia: Northern Territory | P.W. Crous, 30 Apr. 2011 | MF951224 | MF951365 | MF951612 |
| Polyphialoseptoria tabebuiae-serratifolia | – | CBS 112650T = CPC 3944 | Tabebuia serratifolia | Brazil | A.C. Alfenas, 1999 | KF251716 | KF251213 | MF951613 |
| P. terminaliae | – | CBS 135106T = CPC 19611 | Terminalia catappa | Brazil | R.W. Barreto, 18 May 2010 | KF251717 | KF251214 | MF951615 |
| – | CBS 135475 = CPC 19487 | Terminalia catappa | Brazil | R.W. Barreto, 18 May 2010 | KF251718 | KF251215 | MF951614 | |
| Pseudocercospora catappae | – | MAFF 238312 = MUCC 1109 | Terminalia catappa | Japan | T. Kobayashi & C. Nakashima, 18 Nov. 1999 | MF951225 | MF951366 | MF951616 |
| P. dingleyae | Pseudocercosporella dingleyae | CBS 114645T | Haloragis erecta | New Zealand | C.F. Hill, 21 Jan. 2001 | KX286997 | KX287299 | KX288454 |
| P. convoluta | Passalora convoluta | CBS 113377T = MJM 1533 = C488 | Chromolaena odorata | Costa Rica | M.J. Morris, 15 Oct. 1997 | MF951226 | DQ676519 | MF951617 |
| P. cratevicola | Prathigada cratevicola | MUCC 1088 | Crataeva falcata | Japan | S. Uematsu & C. Nakashima, – | MF951233 | MF951372 | – |
| P. eucalyptorum | – | CBS 114866T = CPC 11 | Eucalyptus nitens | South Africa: Western Cape | P.W. Crous, Aug. 1988 | JQ739817 | KF901720 | MF951618 |
| P. flavomarginata | – | CBS 124990 = CPC 13492 | Eucalyptus camaldulensis | Thailand | W. Himaman, Oct. 2006 | GU253817 | GU269799 | MF951619 |
| P. fori | – | CBS 113286 = CMW 9096 = BOT 1290 | Eucalyptus sp. | South Africa | J. Roux | KF902068 | KF901721 | KX348072 |
| P. macadamiae | – | CBS 133432ET | Macadamia integrifolia | Australia: Queensland | O.A. Akinsanmi, 12 Nov. 2011 | KX286998 | KX287300 | KX288455 |
| P. metrosideri | Pseudocercosporella metrosideri | CBS 114294 | Metrosideros excelsa | New Zealand | C.F. Hill, 17 Oct. 2003 | KX286999 | KX287301 | KX288456 |
| P. nodosa | Passalora nodosa | CBS 554.71T | Psoralea bituminosa | Romania | O. Constantinescu, 23 Sep. 1966 | MF951227 | MF951367 | MF951620 |
| P. norchiensis | Pseudocercospora schizolobii | CBS 120738T = CPC 13049 | Eucalyptus sp. | Italy | W. Gams, Apr. 2005 | GU253780 | GU269753 | KX348073 |
| P. pistacina | Pseudocercospora pistacina | CPC 23118 | Pistacia vera | Turkey | K. Sarpkaya, 2010 | KF442674 | KF442647 | KX348074 |
| P. prunicola | – | CBS 132107 = CPC 14511 | Prunus yedoensis | Republic of Korea | H.-D. Shin, 2 Oct. 2007 | GU253723 | GU269676 | MF951621 |
| P. punctata | – | CBS 132116 = CPC 14734 | Syzygium sp. | Madagascar | P.W. Crous, 1 Oct. 2007 | GU253791 | GU269765 | MF951622 |
| P. robusta | – | CBS 111175T = CPC 1269 = CMW 5151 | Eucalyptus robusta | Malaysia | M.J. Wingfield, May 1995 | KF442539 | DQ303081 | MF951623 |
| P. sambucigena | – | CBS 126000ET = CPC 14397 | Sambucus nigra | Netherlands | P.W. Crous, 29 Aug. 2007 | GU253823 | GU269805 | MF951624 |
| Pseudocercospora sp. A | Passalora robiniae | CBS 277.39 | Robinia pseudoacacia | USA | – | MF951230 | MF951369 | MF951627 |
| Pseudocercospora sp. B | Tandonella cubensis | CBS 500.92 = INIFAT C92/43-3 | Bauhinia cumanensis | Cuba | R.F. Castañeda | MF951232 | MF951371 | MF951629 |
| Pseudocercospora sp. C | Passalora bolleana | CBS 541.71 = IMI 161111 | Ficus carica | Romania | O. Constantinescu | MF951229 | MF951368 | MF951626 |
| Passalora sp. | CPC 14819 | Ficus carica | Republic of Korea | H.-D. Shin, 14 Nov. 2007 | MF951231 | MF951370 | MF951628 | |
| Pseudocercospora sp. D | – | CBS 113386 = MJM 1511 = C469 | Chromolaena odorata | Guatemala | M.J. Morris | MF951228 | DQ676532 | MF951625 |
| Pseudocercospora sp. E | Cercosporella sp. | CPC 19537 | Eichhornia azurea | Brazil | D.J. Soares, 30 Apr. 2005 | KX287003 | KX287304 | KX288460 |
| P. vitis | – | CBS 132012 = CPC 11595 | Vitis vinifera | Republic of Korea | H.-D. Shin, 30 Sep. 2004 | GU214483 | GU269829 | KX348076 |
| P. zambiae | Neopseudocercospora terminaliae | CBS 136423T = CPC 22686 | Terminalia sp. | Zambia | M. van der Bank, 24 Feb. 2013 | KF777228 | KF777175 | MF951630 |
| Pseudocercosporella bakeri | – | CBS 119488 | Ipomoea indica | New Zealand | C.F. Hill | KX287005 | KX287306 | KX288462 |
| – | CBS 125685ET = CPC 17570 | Ipomoea aquatica | Laos | P. Phengsintham, 8 Sep. 2009 | KX287005 | KX287306 | KX288462 | |
| Pseudopericoniella levispora | Periconiella levispora | CBS 873.73T | Turpinia pomifera | Sri Lanka | W. Gams, Jan. 1973 | EU041837 | EU041780 | MF951633 |
| Pseudopericoniella sp. | Mycosphaerella rosigena | CBS 330.51 | Rosa sp. | Netherlands | – | GU214413 | GU214632 | MF951634 |
| Pseudophaeophleospora atkinsonii | Phaeophleospora atkinsonii | CBS 124565 = ICMP 17860 | Hebe sp. | New Zealand | – | MF951236 | GU214643 | MF951635 |
| P. stonei | Phaeophleospora stonei | CBS 120830T = CPC 13330 | Eucalyptus sp. | Australia: Queensland | P.W. Crous & J. Stone, 19 Aug. 2006 | FJ493210 | EF394856 | MF951636 |
| Pseudozasmidium eucalypti | Zasmidium eucalypti | CBS 121101T = CPC 13302 | Eucalyptus tereticornis | Australia: Queensland | P.W. Crous, 26 Aug. 2006 | KF901931 | KF901606 | MF951637 |
| P. nabiacense | Zasmidium nabiacense | CBS 125010T = CPC 12748 | Eucalyptus sp. | Australia | A.J. Carnegie, 30 Nov. 2005 | KF901933 | GQ852841 | MF951638 |
| P. parkii | Zasmidium parkii | CBS 387.92T = CPC 353 | Eucalyptus grandis | Brazil | M.J. Wingfield, 24 Feb. 1990 | GU214448 | KF901785 | – |
| P. vietnamense | Paramycosphaerella vietnamensis | CBS 119974T = CMW 23441 = MUCC 66 = VTN1 | Eucalyptus grandis | Vietnam | T.I. Burgess, 6 Jul. 2004 | JF700944 | DQ632675 | MF951639 |
| Ragnhildiana ampelopsidis | Passalora ampelopsis | CBS 249.67 = IMI 124968 | Parthenocissus tricuspidata | Romania | – | MF951238 | AY293063 | MF951641 |
| R. diffusa | Sirosporium diffusum | CBS 106.14 | Carya illinoinensis | USA: Georgia | –, 29 Aug. 1911 | MF951239 | MF951375 | MF951642 |
| R. ferruginea | Passalora ferruginea | CBS 255.67 = IMI 124973 | Artemisia vulgaris | Romania | – | MF951241 | MF951377 | MF951644 |
| Passalora ferruginea | CBS 546.71 | Artemisia vulgaris | Romania | – | MF951242 | MF951378 | MF951645 | |
| Mycovellosiella ferruginea | CPC 10075 | Artemisia sylvatica | Republic of Korea | H.-D. Shin, 23 Oct. 2002 | MF951240 | MF951376 | MF951643 | |
| R. gnaphaliaceae | Passalora gnaphaliaceae | CBS 142181 = CPC 12517 | Gnaphalium affine | Republic of Korea | H.-D. Shin, May 2005 | MF951243 | MF951379 | MF951646 |
| R. perfoliati | Passalora sp. | CBS 113613 = MJM 1506 = C486 | Ageratina adenophora | Guatemala | M.J. Morris | MF951246 | DQ676525 | MF951650 |
| Passalora assamensis | CBS 115119 | – | New Zealand | – | MF951244 | MF951380 | MF951648 | |
| Passalora ageratinae | CBS 125419T = CPC 15365 | Ageratina adenophora | South Africa: KwaZulu-Natal | A.R Wood, 28 May 2008 | GU214453 | GU214639 | MF951647 | |
| Passalora perfoliata | CBS 142180 = CPC 17321 | Chromolaena sp. | Laos | P. Pheng, 17 Jun. 2006 | MF951245 | MF951381 | MF951649 | |
| Phaeoramularia sp. | CPC 15366 | Ageratina adenophora | South Africa: KwaZulu-Natal | A.R Wood, 28 May 2008 | MF951247 | MF951382 | MF951651 | |
| R. pseudotithoniae | Passalora pseudotithoniae | CBS 136442T = CPC 21688 | Tithonia diversifolia | Thailand | P.W. Crous, 5 Nov. 2012 | KF777231 | KF777179 | MF951652 |
| Ramularia carneola | – | CBS 108975 | Scrophularia nodosa | Netherlands | G. Verkley, 22 Jun. 2000 | KX287048 | KX287348 | KX288507 |
| R. cynarae | – | CBS 128912T = CPC 18426 | Cynara cardunculus | USA: California | S.T Koike, 10 Aug. 2010 | KX287096 | HQ728117 | KX288554 |
| R. endophylla | Mycosphaerella punctiformis | CBS 113265ET | Quercus robur | Netherlands | G. Verkley, Apr. 2003 | AY490776 | AY490763 | KP894673 |
| R. hydrangeae-macrophyllae | – | CBS 122273T | Hydrangea macrophylla | New Zealand | C.F. Hill, 2 Jul. 2007 | KX287135 | KX287433 | KX288592 |
| R. nyssicola | – | CBS 127665ET = AR 4656 = DM 2 | Nyssa ogeche × sylvatica | USA: Maryland | R. Olsen, 18 Jun. 2009 | KJ504724 | KJ504765 | KJ504636 |
| R. plurivora | – | CBS 118743T = CPC 12207 | Human bone marrow | Netherlands | – | KJ504739 | KJ504780 | KJ504651 |
| R. pusilla | – | CBS 124973ET = RoKi 3143 | Poa annua | Germany | R. Kirschner, 25 Feb. 2008 | KP894141 | KP894248 | KP894687 |
| R. stellariicola | Pseudocercosporella stellariicola | CBS 130592T = CPC 11297 = KACC 42363 | Stellaria aquatica | Republic of Korea | H.-D. Shin & M.J. Park, 3 May 2006 | GU214693 | GU214693 | KX288675 |
| R. stellenboschensis | – | CBS 130600T = CPC 18294 | Protea sp. | South Africa | P.W. Crous, 6 May 2010 | JN712566 | JN712499 | KX288676 |
| Ramulariopsis gossypii | – | CBS 141099ET = CPC 25909 = X30 | Gossypium sp. | Brazil | – | KX287243 | KX287540 | KX288702 |
| R. pseudoglycines | – | CBS 141100T = CPC 18242 | Gossypium sp. | Brazil | –, 2000 | KX287246 | KX287543 | KX288705 |
| – | CPC 18241 | Gossypium sp. | Brazil | – | KX287245 | KX287542 | KX288704 | |
| – | CPC 20036 | Gossypium barbadense | Togo | M. Piatek | KX287244 | KX287541 | KX288703 | |
| Ramulispora sorghi | Cercospora sorghi | CBS 110578 = CPC 905 | Sorghum bicolor | South Africa: KwaZulu-Natal | D. Nowell, Mar. 1995 | GQ852653 | MF951383 | MF951653 |
| Cercospora sorghi | CBS 111032 = CPC 899 = IMI 153076 | Sorghum bicolor | South Africa: KwaZulu-Natal | D. Nowell, Mar. 1995 | MF951248 | MF951384 | MF951654 | |
| Cercospora sorghi | CBS 115522 = CPC 902 | Sorghum bicolor | South Africa: KwaZulu-Natal | D. Nowell, Mar. 1995 | MF951249 | MF951385 | MF951655 | |
| R. sorghiphila | Ramulispora sorghi | CBS 255.82T = IMI 153077 | – | India | –, Oct. 1969 | MF951250 | MF951386 | MF951656 |
| Rhachisphaerella mozambica | Mycosphaerella mozambica | CBS 122464T = X34 | Musa acuminata | Mozambique | A. Viljoen, 2003 | MF951237 | EU514257 | MF951640 |
| Rosisphaerella rosicola | Passalora rosicola | CBS 138.35 = ATCC 52313 | – | USA | – | MF951252 | MF951388 | MF951658 |
| Passalora rosicola | CBS 142183 = CPC 12548 | Rosa hybrid | USA: North Carolina | C.S. Hodges, 2005 | MF951251 | MF951387 | MF951657 | |
| Ruptoseptoria unedonis | Ruptoseptoria unedonis | CBS 755.70 | Arbutus unedo | Croatia | J.A. von Arx, Jul. 1970 | KF251732 | KF251229 | MF951659 |
| Scolecostigmina mangiferae | Scolecostigmina mangiferae | CBS 125467NT = CPC 17351 | Mangifera indica | Australia | P.W. Crous & R.G. Shivas, 10 Aug. 2009 | GU253877 | GU269870 | MF951660 |
| Septoria chrysanthemella | – | CBS 128617 = KACC 43086 = SMKC 22860 | Chrysanthemum morifolium | Republic of Korea | – | KF251882 | KF251378 | MF951661 |
| S. cucurbitacearum | – | CBS 178.77 | Cucurbita maxima | New Zealand | – | KF251903 | KF251399 | MF951662 |
| S. lycopersici | – | CBS 128654 = KACC 42519 = SMKC 22002 | Lycopersicon esculentum | Republic of Korea | – | KF251966 | KF251462 | KX348091 |
| S. protearum | – | CBS 135477 = CPC 19675 | Zantedeschia aethiopica | South Africa: Mpumalanga | P.W. Crous, 15 Jul. 2011 | KF252029 | KF251524 | MF951663 |
| – | CPC 19691 | Zantedeschia aethiopica | South Africa | P.W. Crous, 15 Jul. 2011 | KF252030 | KF251525 | MF951664 | |
| Septoria sp. A | – | CBS 135472 = CPC 19304 | Vigna unguiculata subsp. sesquipedalis | Austria | P.W. Crous, Apr. 2011 | KF252063 | KF251558 | MF951665 |
| Septoria sp. B | – | CBS 135474 = CPC 19485 | Conyza canadensis | Brazil | R.W. Barreto | KF252064 | KF251559 | MF951666 |
| Septoria sp. C | – | CBS 135479 = CPC 19793 | Syzygium cordatum | South Africa | P.W. Crous, 16 Jul. 2011 | KF252066 | KF251561 | MF951667 |
| S. urticae | – | CBS 102375ET | Urtica dioica | Netherlands | H.A. van der Aa & G. Verkley, 14 Oct. 1999 | JN940675 | KF251583 | MF951668 |
| “Sirosporium” celtidis | – | CBS 158.25 | Celtis australis | Algeria | C. Killian, Nov. 1923 | MF951253 | MF951389 | MF951669 |
| – | CBS 238.48 | – | Portugal | – | MF951254 | MF951390 | MF951670 | |
| – | CBS 289.50 | Celtis australis | Italy | V. Mezzetti, Aug. 1949 | MF951255 | MF951391 | MF951671 | |
| Sonderhenia eucalypticola | – | CBS 112502 = CPC 3749 | Eucalyptus sp. | Portugal: Madeira | – | KF902019 | KF901677 | MF951672 |
| S. eucalyptorum | Mycosphaerella swartii | CBS 120220 | Eucalyptus coccifera | Australia: Tasmania | C. Mohammed, Jan. 2006 | DQ923536 | DQ923536 | MF951673 |
| Sphaerulina aceris | Sphaerulina aceris | CBS 652.85 | Acer pseudoplatanus | Netherlands | H.A. van der Aa, 23 Jul. 1985 | MF951258 | MF951394 | MF951676 |
| S. berberidis | Mycosphaerella berberidis | CBS 324.52 | Berberis vulgaris | Switzerland | E. Müller, 2 Jun. 1951 | KF252106 | KF251601 | KX348093 |
| S. betulae | – | CBS 128597 = KACC 43119 = SMKC 23059 | Betula schmidtii | Republic of Korea | – | KF252109 | KF251604 | KX348094 |
| S. chaenomelis | Pseudocercosporella chaenomelis | CBS 131897 = CPC 14795 | Chaenomeles speciosa | Republic of Korea | H.-D. Shin, 14 Nov. 2007 | GU253834 | GU269817 | KX288706 |
| Pseudocercosporella chaenomelis | CBS 132131ETofPseudocercosporella chaenomelis = MUCC 1510 | Chaenomeles sinensis | Japan | C. Nakashima, 29 Oct 2011 | MF951259 | JQ793663 | MF951677 | |
| S. gei | – | CBS 128632 = KACC 44051 = SMKC 23686 | Geum japonicum | Republic of Korea | – | KF252120 | KF251615 | KX348095 |
| S. koreana | Sphaerulina viciae | CBS 131898TofSphaerulina viciae = CPC 11415 | Vicia amurensis | Republic of Korea | H.-D. Shin | KF252144 | KF251639 | KX348096 |
| Pseudocercosporella koreana | CBS 135462TofPseudocercosporella koreana = CPC 11414 | Vicia amurensis | Republic of Korea | H.-D. Shin | GU214683 | GU269852 | KX288707 | |
| S. populicola | – | CBS 100042 | Populus trichocarpa | USA: Washington | – | KF252131 | KF251626 | MF951678 |
| S. quercicola | – | CBS 115016 | Quercus robur | Netherlands | – | KF252133 | KF251628 | MF951679 |
| S. tirolensis | – | CBS 109018T | Rubus idaeus | Austria | G. Verkley | KF252143 | KF251638 | MF951680 |
| “Mycosphaerella” grossulariae | Mycosphaerella grossulariae | CBS 235.37 | Ribes nigrum | Netherlands | M.S.J. Ledeboer | MF951256 | MF951392 | MF951674 |
| “Mycosphaerella” harthensis | Mycosphaerella harthensis | CBS 325.52 | Betula sp. | Switzerland | – | MF951257 | MF951393 | MF951675 |
| Stromatoseptoria castaneicola | – | CBS 102322 | Castanea sativa | Netherlands | G. Verkley, 29 Aug. 1999 | KF251774 | KF251271 | MF951681 |
| – | CBS 102377 | Castanea sativa | Netherlands | G. Verkley, 9 Sep. 1999 | KF251775 | KF251272 | MF951682 | |
| Sultanimyces vitiphyllus | Asperisporium vitiphyllum | CBS 206.48 | Vitis sp. | South Africa | S.J. du Plessis, 1948 | MF951260 | MF951395 | MF951683 |
| Trochophora fasciculata | Trochophora simplex | CBS 124744 = SMKC 21713 | Daphniphyllum macropodum | Republic of Korea | H.-D. Shin, 29 Oct. 2005 | GU253880 | GU269872 | MF951684 |
| Uwemyces elaeidis | – | CPUwZC-01 | Elaeis oleifera | Colombia | G.A. Sarria, May 2013 | KX228356 | KX22829 | KX228371 |
| Virosphaerella irregularis | Mycosphaerella irregulari | CBS 123242T = CPC 15408 | Eucalyptus sp. | Thailand | R. Cheewangkoon, Jul. 2007 | KF901769 | KF902126 | MF951685 |
| V. pseudomarksii | Mycosphaerella pseudomarksii | CBS 123241T = CPC 15410 | Eucalyptus sp. | Thailand | R. Cheewangkoon, Jun. 2007 | KF902127 | KF901770 | MF951686 |
| Xenomycosphaerella elongata | – | CBS 120735T = CPC 13378 | Eucalyptus calmadulensis × urophylla | Venezuela | M.J. Wingfield, Oct. 2006 | JF700942 | EF394833 | MF951687 |
| Xenoramularia arxii | CBS 342.49T | Acorus calamus | Netherlands | J.A. von Arx, 5 Sep. 1949 | KX287258 | KX287552 | KX288720 | |
| X. neerlandica | – | CBS 113615 | Sparganium ramosum | Netherlands | – | KX287259 | KX287553 | KX288721 |
| – | CBS 141101T = CPC 18377 | Iris pseudacorus | Netherlands | P.W. Crous, 26 Jun. 2010 | KX287260 | KX287554 | KX288722 | |
| X. polygonicola | – | CBS 141102T = CPC 10852 | Polygonum sp. | Republic of Korea | H.-D. Shin, 20 Sep. 2003 | GU214695 | GU214695 | KX288723 |
| – | CPC 10853 | Polygonum sp. | Republic of Korea | H.-D. Shin, 20 Sep. 2003 | KX287262 | KX287555 | KX288724 | |
| Xenosonderhenia eucalypti | – | CBS 138858T = CPC 24247 | Eucalyptus urophylla | Mozambique | M.J. Wingfield, 2 Feb. 2014 | KP004485 | KP004457 | MF951688 |
| Xenosonderhenioides indonesiana | Passalora sp. | CBS 142239T = CPC 15066 | Eucalyptus sp. | Indonesia | M.J. Wingfield, 26 Mar. 2008 | MF951261 | MF951396 | MF951689 |
| Zasmidium angulare | – | CBS 132094T = CPC 19042 = GA2 27B1a | Malus domestica | USA: Georgia | M. Wheeler, Aug. 2005 | JQ622096 | JQ622088 | MF951690 |
| Z. anthuriicola | – | CBS 118742T | Anthurium sp. | Thailand | C.F. Hill, 3 Aug. 2005 | FJ839662 | FJ839626 | MF951691 |
| Z. arcuatum | Periconiella arcuata | CBS 113477T | Ischyrolepsis subverticillata | South Africa: Western Cape | S. Lee, 1 May 2001 | EU041836 | EU041779 | MF951692 |
| Z. aucklandicum | Stenella aucklandica | CPC 13569 | Geniostoma rupestre | New Zealand | C.F. Hill, 15 Oct. 2005 | MF951280 | MF951409 | MF951733 |
| Z. biverticillatum | Ramichloridium biverticillatum | CBS 335.36 | Musa sapientum | – | – | EU041853 | EU041796 | – |
| Z. cellare | – | CBS 146.36NT = ATCC 36951 = IFO 4862 = IMI 044943 = LCP 52.402 = LSHB BB274 = MUCL 10089 | Wall in wine cellar | – | – | EU041878 | EU041821 | MF951693 |
| – | CBS 892.85 | Wall in wine cellar | Germany | M. Schlag, Aug. 1985 | MF951262 | MF951397 | KT356875 | |
| Z. cerophillum | Ramichloridium cerophilum | CBS 103.59TofAcrotheca cerophila = MUCL 10034 | Sasa sp. | Japan | –, May 1955 | GU214485 | EU041798 | MF951694 |
| Z. citri-griseum | – | CBS 122455 = CPC 15289 = X126 | Citrus sp. | USA: Florida | R.C. Ploetz, 2003 | KF902151 | KF901792 | MF951695 |
| – | CPC 13467 | Eucalyptus sp. | Thailand | W. Himaman, 2006 | KF251729 | KF251226 | MF951697 | |
| – | CPC 15291 | Citrus sp. | USA: Florida | R.C. Ploetz, 2003 | KF902152 | KF901793 | MF951696 | |
| Z. daviesiae | Verrucisporota daviesiae | CBS 116002 = VPRI 31767 | Daviesia latifolia | Australia: Victoria | V. & R. Beilharz, 30 Dec. 2003 | FJ839669 | FJ839633 | MF951698 |
| Z. elaeocarpi | Stenella sp. | CBS 142187T = CPC 16642 | Elaeocarpus kirtonii | Australia: New South Wales | B. Summerell, 1 Mar. 2009 | MF951263 | MF951398 | MF951699 |
| Stenella sp. | CPC 16640 | Elaeocarpus kirtonii | Australia: New South Wales | B. Summerell, 1 Mar. 2009 | MF951264 | MF951399 | MF951700 | |
| Z. eucalypticola | Stenella sp. | CBS 142186T = CPC 15149 | Eucalyptus sp. | Brazil | A.C. Alfenas, 1 Mar. 2008 | MF951265 | MF951400 | MF951701 |
| Z. eucalyptorum | – | CBS 118500T = CPC 11174 | Eucalyptus urophylla | Indonesia: Sumatra | M.J. Wingfield, Mar. 2004 | MF951266 | KF901652 | MF951702 |
| Z. fructicola | – | CBS 139625T = CPC 24487 = ZJUM 80 | Citrus reticulata | China | X.H. Wang, Jan. 2010 | KP895922 | KP896052 | MF951703 |
| Z. fructigenum | – | CBS 139626T = CPC 24471 = ZJUM 36 | Citrus paradisi × Citrus sp. | China | L. Zhu, Nov. 2009 | KP895926 | KP896056 | MF951704 |
| Z. grevilleae | Verrucisporota grevilleae | CBS 124107T = CPC 14761 | Grevillea decurrens | Australia: Northern Territory | B. Summerell, 22 Sep. 2007 | FJ839670 | FJ839634 | MF951705 |
| Z. gupoyu | Parastenella gupoyu | CBS 122099 = RoKi 3022 | Alocasia odora | Taiwan | R. Kirschner & C.-J. Chen, 31 Mar. 2007 | MF951267 | MF951401 | MF951706 |
| Z. hakeae | Stenella sp. | CBS 142185T = CPC 15577 | Hakea undulata | Australia: Western Australia | A.R. Wood, 2 Aug. 2008 | MF951268 | MF951402 | MF951707 |
| Stenella sp. | CPC 15583 | Hakea undulata | Australia: Western Australia | A.R. Wood, 2 Aug. 2008 | MF951269 | MF951403 | MF951708 | |
| Stenella sp. | CPC 17213 | Leaves in shop (Loma tea) | Australia: Queensland | P.W. Crous, 13 Jul. 2009 | MF951270 | MF951404 | MF951709 | |
| Z. indonesianum | – | CBS 139627T = CPC 15300 | Citrus sp. | Indonesia | M. Arzanlou, 2004 | KF902086 | KF901739 | MF951710 |
| Z. iteae | Stenella iteae | CBS 113094T = RoKi 1279 | Itea parvifolia | Taiwan | R. Kirschner & C.-J. Chen, 2 Jun. 2002 | MF951271 | MF951405 | MF951711 |
| Z. lonicericola | – | CBS 125008ETofCladosporium lonicericola = CPC 11671 | Lonicera japonica | Republic of Korea | H.-D. Shin, 30 Oct. 2004 | KF251787 | KF251283 | MF951712 |
| Z. musae | Stenella musae | CBS 121384 = CIRAD 41 = X877 | Musa sp. | France: Martinique | – | MF951272 | EU514292 | MF951713 |
| Stenella musae | CBS 122476 = X47 | Musa sp. | Netherlands Antilles: Windward Islands | E. Reid, 2003 | MF951273 | EU514288 | MF951714 | |
| Stenella musae | CBS 122478 = X70 | Musa sp. | Netherlands Antilles: Windward Islands | E. Reid, 2003 | MF951274 | EU514290 | MF951715 | |
| Z. musae-banksii | Ramichloridium australiensis | CBS 121710T = X1100 | Musa banksii | Australia: Queensland | P.W. Crous & B. Summerell, Aug. 2006 | EU041852 | EU041795 | MF951716 |
| Z. musicola | Stenella musicola | CBS 122479T = X1019 | Musa cv. Grand Nain | India | I.W. Buddenhagen, 23 Feb. 2005 | MF951275 | EU514294 | MF951717 |
| Z. musigenum | Ramichloridium musae | CBS 190.63 = MUCL 9557 | Musa sapientum | – | – | EU041857 | EU041800 | MF951718 |
| Z. nocoxi | – | CBS 125009T = CPC 14044 | Twig debris of unknown host | USA: Virginia | P.W. Crous, 14 May 2007 | KF251788 | KF251284 | MF951719 |
| Z. pitospori | Stenella pittospori | CBS 122274 = ICMP 17098 | Pittosporum tenuifolium | New Zealand | C.F. Hill, 15 Jul. 2007 | MF951276 | MF951406 | MF951720 |
| Z. proteacearum | Verrucisporota proteacearum | CBS 116003 = VPRI 31812 | Grevillea sp. | Australia: Queensland | J.L. Alcorn, 3 Feb. 2004 | FJ839671 | FJ839635 | MF951721 |
| Z. pseudoparkii | – | CBS 110988 = CPC 1090 | Eucalyptus grandis | Colombia | M.J. Wingfield, May 1995 | KF901975 | DQ303021 | MF951722 |
| – | CBS 110999T = CPC 1087 | Eucalyptus sp. | Colombia | M.J. Wingfield, 1995 | JF700965 | DQ303023 | MF951723 | |
| Z. pseudotsugae | Rasutoria pseudotsugae | rapssd | Pseudotsuga menziesii | USA: Oregon | – | EF114704 | EF114687 | – |
| Z. pseudovespa | Mycosphaerella pseudovespa | CBS 121159T = AC0466 | Eucalyptus biturbinata | Australia: New South Wales | A.J. Carnegie, 14 Apr. 2005 | KF901836 | MF951407 | MF951724 |
| Z. queenslandicum | Stenella queenslandica | CBS 122475T = X1084 | Musa banksii | Australia: Queensland | P.W. Crous, 1 Aug. 2006 | MF951277 | EU514295 | MF951725 |
| Z. scaevolicola | – | CBS 127009T = CPC 17344 | Scaevola taccada | Australia: Queensland | R.G. Shivas & P.W. Crous, 8 Aug. 2009 | KF251789 | KF251285 | MF951726 |
| Z. schini | Stenella sp. | CBS 142188T = CPC 19516 | Schinus terebinthifolius | Brazil | A.B.V. Faria, 1 Sep. 2005 | MF951278 | MF951408 | MF951727 |
| Zasmidium sp. | Mycosphaerella sp. | CBS 118494 = CPC 11004 | Eucalyptus sp. | Colombia | M.J. Wingfield, 2004 | MF951279 | DQ303039 | MF951728 |
| Z. strelitziae | Ramichloridium strelitziae | CBS 121711T = X1029 | Strelitzia sp. | South Africa: KwaZulu-Natal | W. Gams & H. Glen, 5 Feb. 2005 | EU041860 | EU041803 | MF951729 |
| Z. syzygii | – | CBS 133580T = CPC 19792 | Syzigium cordatum | South Africa: Mpumalanga | P.W. Crous, M.K. Crous, M. Crous & K.L. Crous, 16 Jul. 2011 | KC005798 | KC005777 | MF951730 |
| Z. tsugae | Rasutoria tsugae | ratstk | Tsuga heterophylla | USA: Oregon | – | EF114705 | EF114688 | – |
| Z. velutinum | Periconiella velutina | CBS 101948ET = CPC 2262 | Brabejum stellatifolium | South Africa | J.E. Taylor, 21 Jan. 1999 | EU041838 | EU041781 | MF951731 |
| Z. xenoparkii | Stenella xenoparkii | CBS 111185T = CPC 1300 | Eucalyptus grandis | Indonesia | M.J. Wingfield, Mar. 1996 | JF700966 | DQ303028 | MF951732 |
| Zymoseptoria brevis | – | CBS 128853T = CPC 18106 | Phalaris minor | Iran | M. Razavi | JQ739833 | JF700867 | KX348109 |
| Z. halophila | – | CBS 128854T = CPC 18105 = IRAN1483C | Hordeum glaucum | Iran | M. Razavi, 25 Apr. 2007 | KF252150 | KF251645 | KX348110 |
| Z. passerini | – | CBS 120382ET | Hordeum vulgare | USA: North Dakota | S. Goodwin | JQ739843 | JF700877 | KP894763 |
| Z. tritici | – | CBS 115943ET = IPO 323 | Triticum aestivum | Netherlands | R. Daamen, 6 May 1981 | GU214436 | AF181692 | KX348112 |
| Phaeothecoidiellaceae | ||||||||
| Exopassalora sp. | Passalora sp. | CBS 118964 = GTF1a | Malus sp. | USA: Illinois | J. Batzer, Sep. 2000 | MF951119 | MF951284 | MF951420 |
| E. zambiae | Passalora zambiae | CBS 112971T = CMW 14782 = CPC 1227 | Eucalyptus globulus | Zambia | T. Coutinho, 21 Aug. 1995 | EU019273 | AY725523 | MF951421 |
| Houjia pomigena | – | CBS 125224T = CPC 16109 = CMG UIF2b | Malus sp. | USA: Illinois | M. Gleason, Sep. 2000 | MF951120 | MF951285 | MF951422 |
| Phaeothecoidiella missouriensis | – | CBS 125222T = CPC 16116 = CMG AHE7c | Malus sp. | USA: Missouri | M. Gleason, Sep. 2000 | MF951121 | MF951286 | MF951423 |
| Sporidesmajora pennsylvaniensis | – | CBS 125229T = CPC 16112 = CMG PA1-9F1a | Malus sp. | USA: Pennsylvania | J.W. Travis, Sep. 2005 | MF951122 | MF951287 | MF951424 |
| Schizothyriaceae | ||||||||
| Schizothyrium pomi | Schizothyrium pomi | CBS 228.57 | – | Italy | – | EF134947 | EF134947 | MF951734 |
| Schizothyrium pomi | CBS 486.50 | Polygonum sachalinense | Netherlands | – | EF134948 | EF134948 | MF951735 | |
| Teratosphaeriaceae | ||||||||
| Acrodontium crateriforme | Chloridium crateriforme | CBS 144.33T = ATCC 15679 = MUCL 15748 = MUCL 8978 | Associated with Tuberculina maxima | Netherlands | – | KX286952 | MF951410 | KX288399 |
| Batcheloromyces proteae | – | CBS 110696ET = CPC 1518 = CPC 18701 | Protea cynaroides | South Africa: Western Cape | L. Viljoen, 30 Aug. 1996 | EU019247 | JF746163 | MF951736 |
| B. sedgefieldii | – | CBS 112119T = CPC 3026 = JT 851 | Protea repens | South Africa: Western Cape | J.E. Taylor, 10 Aug. 1999 | KF937222 | EU707893 | MF951737 |
| Myrtapenidiella corymbia | Penidiella corymbia | CBS 124769T = CPC 14640 | Corymbia foelscheana | Australia: Northern Territory | B.A. Summerell, 22 Sep. 2007 | KF901838 | GQ303286 | MF951738 |
| Parapenidiella pseudotasmaniensis | – | CBS 124991T = CPC 12400 | Eucalyptus globulus | Australia: Victoria | I.W. Smith, Sep. 2005 | KF901844 | KF901522 | KX348067 |
| P. tasmaniensis | – | CBS 111687T = CMW 14780 = CPC 1555 | Eucalyptus nitens | Australia: Tasmania | M.J. Wingfield, 21 Nov. 1996 | GU214452 | KF901521 | MF951739 |
| Pseudoteratosphaeria flexuosa | Mycosphaerella flexuosa | CBS 110743 = CPC 673 | Eucalyptus globulus | Colombia | M.J. Wingfield, 6 Jul. 1993 | KF902098 | DQ302955 | MF951740 |
| Readeriella nontingens | Readeriella nontingens | CPC 14444 | Eucalyptus oblonga | Australia: New South Wales | B. Summerell, 23 Sep. 2007 | GQ852663 | GQ852786 | MF951741 |
| Stenella araguata | – | CBS 105.75TofCladosporium castellanii | Man, tinea nigra | Venezuela | – | EU019250 | EU019250 | MF951742 |
| Teratosphaeria stellenboschiana | – | CBS 125215 = CPC 13764 | Eucalyptus punctata | South Africa: Gauteng | P.W. Crous, 28 Feb. 2007 | KF937247 | KF901733 | MF951743 |
ATCC: American Type Culture Collection, Virginia, USA; BUCM: Mycological Herbarium of the Institute of Biology, Bucharest, Romania; CBS: Culture collection of the Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; CCTU: Culture Collection of Tabriz University, Tabriz, East Azarbaijan, Iran; CMG: Personal collection of Mark Gleason, Department of Plant Pathology and Microbiology, Iowa State University, Ames, Iowa, USA; CMW: Culture Collection of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa; COAD: Coleção Octávio de Almeida Drumond (COAD), housed at the Universidade Federal de Viçosa, Viçosa, Brazil; CPC: Culture collection of Pedro Crous, housed at the Westerdijk Institute; DAOM: Plant Research Institute, Department of Agriculture (Mycology), Ottawa, Canada; DSM: Deutsche Sammlung von Mikrorrganismen und Zellkulturen GmbH, Braunschweig, Germany; HJS: Personal culture collection of Hans-Josef Schroers, Agricultural institute of Slovenia, Ljubljana, Slovenia; ICMP = PDDCC: International Collection of Micro-organisms from Plants, Landcare Research, Private Bag 92170, Auckland, New Zealand; IFO: Institute for Fermentation, Osaka, Japan; IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, United Kingdom; INIFAT: Alexander Humboldt Institute for Basic Research in Tropical Agriculture, Ciudad de La Habana, Cuba; JCM: Japan Collection of Microorganism, RIKEN BioResource Center, Japan; KACC: Korean Agricultural Culture Collection, National Institute of Agricultural Biotechnology, Rural Development Administration, Suwon, Republic of Korea; LCP: Laboratory of Cryptogamy, National Museum of Natural History, Paris, France; LSHB: London School of Hygiene & Tropical Medicine, London, UK; MAFF: Ministry of Agriculture, Forestry and Fisheries, Tsukuba, Ibaraki, Japan; MPFN: Culture collection at the Laboratoire de Pathologie Forestière, INRA, Centre de Recherches de Nancy, 54280 Champenoux, France; MUCC (in TSU): Culture Collection, Laboratory of Plant Pathology, Mie University, Tsu, Mie Prefecture, Japan; MUCL: Université Catholique de Louvain, Louvain-la-Neuve, Belgium; MUMH: Mycological Herbarium in TSU, Mie University, Tsu, Mie, Japan; QM: Quartermaster Research and Development Center, U.S. Army, Massachusetts, USA; RoKI: Personal culture collection of Roland Kirschner, Department of Life Sciences, National Central University, Taoyuan City, Taiwan; RWB: Personal collection of Robert Barreto, Departmento de Fitopatologia, Universidade Federal de Viçosa, Viçosa, Brazil; SMKC: Culture collection of the Division of Environmental Science and Ecological Engineering, Korea University, Republic of Korea; VKM: All-Russian Collection of Microorganisms, Russian Academy of Sciences, Institute of Biochemistry and Physiology of Microorganisms, 142292 Pushchino, Moscow Region, Russia; X: Personal collection of Mahdi Arzanlou, Tabriz University, Tabriz, East Azarbaijan, Iran; ZJUM: Culture collection at Zhejiang University, China.
Status of the strains: (T) ex-type, (ET) ex-epitype, (NT) ex-neotype, (IT) ex-isotype.
Genbank accession numbers for LSU: large subunit (28S) of the nrRNA gene operon.
Genbank accession numbers for ITS: internal transcribed spacers and intervening 5.8S nrDNA.
Genbank accession numbers for rpb2: partial RNA polymerase II second largest subunit gene; “–” represents a DNA sequence that was not available.
DNA extraction, amplification and sequencing
Fungal mycelium of strains (Table 1) was harvested with a sterile scalpel and the genomic DNA was isolated using the UltraClean Microbial DNA Isolation Kit (MoBio Laboratories, Inc., Solana Beach, CA, USA) following the manufacturers' protocols. Three partial nuclear genes were targeted for PCR amplification and sequencing: 28S nrRNA gene (LSU), internal transcribed spacer regions and intervening 5.8S nrRNA gene (ITS) of the nrDNA operon, RNA polymerase II second largest subunit (rpb2). The primers employed are listed in Table 2, with the respective annealing temperatures used. The PCR amplifications were performed on a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA, USA). The PCR mixtures consisted of 1 μL genomic DNA, 1× NH4 reaction buffer (Bioline, Luckenwalde, Germany), 2 mM MgCl2, 40 μM of each dNTP, 0.2 μM of each primer and 0.5 U Taq DNA polymerase (Bioline) in a total volume of 12.5 μL. The PCR mixture for rpb2 contained 2 μL genomic DNA. The general PCR conditions were: initial denaturation (94 °C, 3 min); 35 cycles amplification [denaturation 94 °C, 30 s; locus-specific annealing temperature (Table 2), 30 s; extension 72 °C, 45 s], and final extension (72 °C, 5 min). To obtain the partial rpb2, a touchdown PCR protocol was used: initial denaturation (94 °C, 3 min), 5 amplification cycles (denaturation 94 °C, 45 s; annealing 60 °C, 45 s; extension 72 °C, 1 min), 5 amplification cycles (denaturation 94 °C, 45 s; annealing 58 °C, 45 s; extension 72 °C, 1 min), 30 amplification cycles (denaturation 94 °C, 45 s; annealing 54 °C, 45 s; extension 72 °C, 1 min) and a final extension (72 °C, 8 min). The resulting fragments were sequenced in both directions using the PCR primers and the BigDye Terminator Cycle Sequencing Kit v. 3.1 (Applied Biosystems Life Technologies, Carlsbad, CA, USA). DNA sequencing amplicons were purified through Sephadex G-50 Superfine columns (Sigma-Aldrich, St. Louis, MO, USA) in MultiScreen HV plates (Millipore, Billerica, MA, USA). Purified sequence reactions were analysed on an Applied Biosystems 3730xl DNA Analyzer (Life Technologies, Carlsbad, CA, USA). The DNA sequences generated were analysed and consensus sequences were computed using the BioNumerics v. 4.61 software package (Applied Maths, St-Martens-Latem, Belgium).
Table 2.
Details of primers used for amplification and sequencing in this study.
| Locus1 | Primer Name | Primer sequence (5′→3′) | Annealing temperature (°C) | Orientation | Reference |
|---|---|---|---|---|---|
| ITS | V9G | TTA CGT CCC TGC CCT TTG TA | 52 | Forward | de Hoog & Gerrits van den Ende (1998) |
| ITS4 | TCC TCC GCT TAT TGA TAT GC | 52 | Reverse | White et al. (1990) | |
| LSU | LSU1Fd | GRA TCA GGT AGG RAT ACC CG | 52 | Forward | Crous et al. (2009c) |
| LR5 | TCC TGA GGG AAA CTT CG | 52 | Reverse | Vilgalys & Hester (1990) | |
| rpb2 | fRPB2-5F | GAY GAY MGW GAT CAY TTY GG | 60→58→54 | Forward | Liu et al. (1999) |
| RPB2-5F2 | GGG GWG AYC AGA AGA AGG C | 60→58→54 | Forward | Sung et al. (2007) | |
| Rpb2-F1 | GGT GTC AGT CAR GTG YTG AA | 60→58→54 | Forward | Videira et al. (2015a) | |
| Rpb2-F4 | GAY YTB GCI GGI CCI YTI ATG GC | 60→58→54 | Forward | Videira et al. (2016) | |
| Rpb2-F5 | GCN ACI GGI AAY TGG GG | 60→58→54 | Forward | This study | |
| Rpb2-F6 | AAR GCI GGT GTI AGY CAR GT | 60→58→54 | Forward | This study | |
| fRPB2-7cR | CCC ATR GCT TGY TTR CCC AT | 60→58→54 | Reverse | Liu et al. (1999) | |
| Rpb2-R1 | TCC TCN GGV GTC ATG ATR ATC AT | 60→58→54 | Reverse | Videira et al. (2015a) | |
| Rpb2-R3 | ATC ATN GMN GGR TGR ATY TC | 60→58→54 | Reverse | This study |
ITS: internal transcribed spacers and intervening 5.8S nrDNA; LSU: large subunit (28S) of the nrRNA gene operon; rpb2 partial RNA polymerase II second largest subunit gene.
Phylogenetic analysis
The generated sequences for each gene were aligned with the online version of MAFFT v. 7 (Katoh & Standley 2013). The alignments were manually checked and improved where necessary using MEGA v. 5 (Tamura et al. 2011) and were concatenated with Mesquite v. 2.75 (Maddison & Maddison 2011). From the strains listed in Table 1, only those with the complete dataset of genes were used in the subsequent phylogenetic analyses, with the exception of Cercospora apii (CBS 116455), “Passalora vaginae” (CBS 140.34), Phaeoramularia capsicicola (CBS 156.62), Prathigada (MUCC 1088), Rasutoria pseudotsugae (rapssd), Rasutoria tsugae (ratstk), Zasmidium biverticillatum (CBS 335.36) and Zasmidium parki (CBS 387.92), which were missing the rpb2 sequence; in those cases, the missing sequences were treated as missing data in the alignments.
The phylogenetic methods used in this study included a Bayesian analysis performed with MrBayes v. 3.2 (Ronquist et al. 2012), a Maximum-Likelihood analysis performed with RAxML v. 7.2.6 (Stamatakis & Alachiotis 2010) and a Parsimony analysis performed with PAUP v. 4.0b10 (Swofford 2003). The phylogenetic analyses were individually applied to four datasets: dataset 1 consisted of a concatenated alignment of LSU and rpb2 sequences from representative strains of most genera currently known to belong in the Mycosphaerellaceae, and from closely related families; datasets 2 to 4 were based on three major clades observed in dataset 1 and consisted of concatenated alignments of LSU, rpb2 and ITS sequences. MrModeltest v. 2.2 (Nylander 2004) was used to determine the best nucleotide substitution model settings for each data partition in order to perform a model-optimised Bayesian phylogenetic reconstruction. The Markov Chain Monte Carlo (MCMC) analysis of four chains started in parallel from a random tree topology, the heat parameter was set at 0.15 and trees were saved every 200 generations until the average standard deviation of split frequencies reached 0.01 (stop value). Burn-in was set to 25 % after which the likelihood values were stationary. The Maximum Likelihood phylogenies performed with RAxML executed 1 000 rapid bootstrap inferences using the GAMMA model and the GTR substitution matrix and produced the best-score maximum-likelihood tree. In the Maximum Parsimony analysis, alignment gaps were treated as fifth character state and all characters were unordered and of unequal weight. A heuristic search option with 100 random taxon additions and tree bisection and reconnection (TBR) as the branch-swapping algorithm was used. Branches of zero length were collapsed and all multiple, equally most parsimonious trees were saved. The robustness of the trees obtained was evaluated by 1 000 bootstrap replications (Hillis & Bull 1993). Other measures calculated included tree length (TL), consistency index (CI), retention index (RI) and rescaled consistency index (RC).
All resulting trees were printed with Geneious v. 7.0.6 (http://www.geneious.com, Kearse et al. 2012). All new sequences generated in this study were deposited in NCBI's GenBank nucleotide database (www.ncbi.nlm.nih.gov) and the accession numbers are listed in Table 1 (GenBank accessions MF951115–MF951743MF951115MF951116MF951117MF951118MF951119MF951120MF951121MF951122MF951123MF951124MF951125MF951126MF951127MF951128MF951129MF951130MF951131MF951132MF951133MF951134MF951135MF951136MF951137MF951138MF951139MF951140MF951141MF951142MF951143MF951144MF951145MF951146MF951147MF951148MF951149MF951150MF951151MF951152MF951153MF951154MF951155MF951156MF951157MF951158MF951159MF951160MF951161MF951162MF951163MF951164MF951165MF951166MF951167MF951168MF951169MF951170MF951171MF951172MF951173MF951174MF951175MF951176MF951177MF951178MF951179MF951180MF951181MF951182MF951183MF951184MF951185MF951186MF951187MF951188MF951189MF951190MF951191MF951192MF951193MF951194MF951195MF951196MF951197MF951198MF951199MF951200MF951201MF951202MF951203MF951204MF951205MF951206MF951207MF951208MF951209MF951210MF951211MF951212MF951213MF951214MF951215MF951216MF951217MF951218MF951219MF951220MF951221MF951222MF951223MF951224MF951225MF951226MF951227MF951228MF951229MF951230MF951231MF951232MF951233MF951234MF951235MF951236MF951237MF951238MF951239MF951240MF951241MF951242MF951243MF951244MF951245MF951246MF951247MF951248MF951249MF951250MF951251MF951252MF951253MF951254MF951255MF951256MF951257MF951258MF951259MF951260MF951261MF951262MF951263MF951264MF951265MF951266MF951267MF951268MF951269MF951270MF951271MF951272MF951273MF951274MF951275MF951276MF951277MF951278MF951279MF951280MF951281MF951282MF951283MF951284MF951285MF951286MF951287MF951288MF951289MF951290MF951291MF951292MF951293MF951294MF951295MF951296MF951297MF951298MF951299MF951300MF951301MF951302MF951303MF951304MF951305MF951306MF951307MF951308MF951309MF951310MF951311MF951312MF951313MF951314MF951315MF951316MF951317MF951318MF951319MF951320MF951321MF951322MF951323MF951324MF951325MF951326MF951327MF951328MF951329MF951330MF951331MF951332MF951333MF951334MF951335MF951336MF951337MF951338MF951339MF951340MF951341MF951342MF951343MF951344MF951345MF951346MF951347MF951348MF951349MF951350MF951351MF951352MF951353MF951354MF951355MF951356MF951357MF951358MF951359MF951360MF951361MF951362MF951363MF951364MF951365MF951366MF951367MF951368MF951369MF951370MF951371MF951372MF951373MF951374MF951375MF951376MF951377MF951378MF951379MF951380MF951381MF951382MF951383MF951384MF951385MF951386MF951387MF951388MF951389MF951390MF951391MF951392MF951393MF951394MF951395MF951396MF951397MF951398MF951399MF951400MF951401MF951402MF951403MF951404MF951405MF951406MF951407MF951408MF951409MF951410MF951411MF951412MF951413MF951414MF951415MF951416MF951417MF951418MF951419MF951420MF951421MF951422MF951423MF951424MF951425MF951426MF951427MF951428MF951429MF951430MF951431MF951432MF951433MF951434MF951435MF951436MF951437MF951438MF951439MF951440MF951441MF951442MF951443MF951444MF951445MF951446MF951447MF951448MF951449MF951450MF951451MF951452MF951453MF951454MF951455MF951456MF951457MF951458MF951459MF951460MF951461MF951462MF951463MF951464MF951465MF951466MF951467MF951468MF951469MF951470MF951471MF951472MF951473MF951474MF951475MF951476MF951477MF951478MF951479MF951480MF951481MF951482MF951483MF951484MF951485MF951486MF951487MF951488MF951489MF951490MF951491MF951492MF951493MF951494MF951495MF951496MF951497MF951498MF951499MF951500MF951501MF951502MF951503MF951504MF951505MF951506MF951507MF951508MF951509MF951510MF951511MF951512MF951513MF951514MF951515MF951516MF951517MF951518MF951519MF951520MF951521MF951522MF951523MF951524MF951525MF951526MF951527MF951528MF951529MF951530MF951531MF951532MF951533MF951534MF951535MF951536MF951537MF951538MF951539MF951540MF951541MF951542MF951543MF951544MF951545MF951546MF951547MF951548MF951549MF951550MF951551MF951552MF951553MF951554MF951555MF951556MF951557MF951558MF951559MF951560MF951561MF951562MF951563MF951564MF951565MF951566MF951567MF951568MF951569MF951570MF951571MF951572MF951573MF951574MF951575MF951576MF951577MF951578MF951579MF951580MF951581MF951582MF951583MF951584MF951585MF951586MF951587MF951588MF951589MF951590MF951591MF951592MF951593MF951594MF951595MF951596MF951597MF951598MF951599MF951600MF951601MF951602MF951603MF951604MF951605MF951606MF951607MF951608MF951609MF951610MF951611MF951612MF951613MF951614MF951615MF951616MF951617MF951618MF951619MF951620MF951621MF951622MF951623MF951624MF951625MF951626MF951627MF951628MF951629MF951630MF951631MF951632MF951633MF951634MF951635MF951636MF951637MF951638MF951639MF951640MF951641MF951642MF951643MF951644MF951645MF951646MF951647MF951648MF951649MF951650MF951651MF951652MF951653MF951654MF951655MF951656MF951657MF951658MF951659MF951660MF951661MF951662MF951663MF951664MF951665MF951666MF951667MF951668MF951669MF951670MF951671MF951672MF951673MF951674MF951675MF951676MF951677MF951678MF951679MF951680MF951681MF951682MF951683MF951684MF951685MF951686MF951687MF951688MF951689MF951690MF951691MF951692MF951693MF951694MF951695MF951696MF951697MF951698MF951699MF951700MF951701MF951702MF951703MF951704MF951705MF951706MF951707MF951708MF951709MF951710MF951711MF951712MF951713MF951714MF951715MF951716MF951717MF951718MF951719MF951720MF951721MF951722MF951723MF951724MF951725MF951726MF951727MF951728MF951729MF951730MF951731MF951732MF951733MF951734MF951735MF951736MF951737MF951738MF951739MF951740MF951741MF951742MF951743). The alignments and respective phylogenetic trees were deposited in TreeBASE, study number 21537.
Taxonomy
Isolates were cultivated for 15–30 d at 21 °C in a 12 h day/night regime. Morphological observations of reproductive structures were determined using a Nikon Eclipse 80i compound microscope with differential interference contrast (DIC) illumination. Slides were prepared using the inclined coverslip method (Kawato & Shinobu 1959, revised in Nugent et al. 2006) and also transparent adhesive tape (Titan Ultra Clear Tape, Conglom Inc., Toronto, Canada) (Bensch et al. 2012). Clear lactic acid was used as mounting medium for microscopic observations of both in vivo and herbarium specimens. The observed isolates were cultivated in synthetic nutrient-poor agar (SNA), V8-juice agar (V8), malt extract agar (MEA) or oatmeal agar (OA) media to produce conidiogenous structures (recipes according to Crous et al. 2009f). The recorded conidial and ascospore measurements represent the minimum and maximum value of 30 individual measurements, for both length and width. For Scanning Electron Microscopy (SEM) observations, dried herbarium specimens were cut into small pieces and mycelial discs were incubated on MEA (Crous et al. 2009f). Both materials were fixed with OsO4 gas at room temperature for 12 h and then coated with gold using an ion-sputter (model E-1010, Hitachi, Tokyo, Japan). Specimens were observed with a SEM (S-4000, Hitachi) at 10–15 kV accelerating voltage. Nomenclatural novelties and descriptions were deposited in MycoBank (Crous et al. 2004a).
Results
DNA amplification
The partial amplification of LSU and ITS was successful for all isolates (Table 1). The partial amplification of rpb2 was difficult with the primer combination fRPB2-f5F and fRPB2-7cR (Liu et al. 1999), but more successful using the forward primer RPB2-5F2 (Sung et al. 2007). Among the used primers, the most successful combination was Rpb2-F4 (Videira et al. 2016) and fRPB2-7cR (Liu et al. 1999). The remaining primers designed in this study were used only in a small number of isolates for which the previously mentioned combinations failed to amplify the gene.
LSU & rpb2 phylogeny: Dataset 1 consisted of a concatenated alignment of two loci (LSU, rpb2) that contained 262 taxa representing several genera known from culture belonging to the Mycosphaerellaceae. A strain of Cylindroseptoria ceratoniae (CBS 477.69; Dothideaceae) was used as outgroup. The final alignment contained a total of 1 471 characters divided in two partitions containing 750 (LSU) and 716 (rpb2) characters respectively, including alignment gaps. From the total alignment five characters that were artificially introduced as spacer between the genes were excluded from the phylogenetic analyses (see alignment in TreeBASE). MrModelTest determined that the Bayesian analysis for both genes (LSU, rpb2) should use dirichlet base frequencies and the GTR+I+G model. The Bayesian analyses of the concatenated two-locus alignment generated 65 562 trees from which 16 390 trees were discarded (25 % burn-in). The posterior probability values (PP) were calculated from the remaining 49 172 trees (Fig. 1; first value: PP ≤1 shown). The alignment contained a total of 811 unique site patterns: 291 (LSU) and 520 (rpb2). The Maximum Likelihood analysis detected 810 distinct patterns and reached a final optimization likelihood of −66911.187183. The bootstrap support values (ML-BS) from the best-scoring tree were mapped on the Bayesian tree as the second value in the tree nodes (Fig. 1; ML-BS ≥ 90 % shown). The Maximum Parsimony (MP) analyses generated the maximum of 1 000 equally most parsimonious trees and the bootstrap support values (MP-BS) were mapped on the Bayesian tree as the third value (Fig. 1; MP-BS ≥ 90 % shown). From the analysed characters, 691 were constant, 100 were variable and parsimony-uninformative and 675 were parsimony-informative. A parsimony consensus tree was calculated from the equally most parsimonious trees and the branches were mapped with a thicker stroke on the Bayesian tree (Fig. 1; Length = 16678, CI = 0.094, RI = 0.647, RC = 0.061, HI = 0.906). The overall parsimony phylogeny supported the same species clades as those presented in the Bayesian phylogeny (Fig. 1). Likewise, the ML analyses of dataset 1 (Fig. 1) separated the strains into the same genus clades as with Bayesian analyses.
Fig. 1.
Phylogenetic tree (50 % majority rule consensus) resulting from a Bayesian analysis of the combined LSU and rpb2 sequence alignment (dataset 1). Bayesian posterior probabilities (PP), maximum likelihood bootstrap support values (≥ 90 %; ML-BS) and maximum parsimony bootstrap support values (≥ 90 %; MP-BS) are indicated at the nodes (PP/ML-BS/MP-BS; a hash (#) symbol denotes fully-supported branches) and the scale bar represents the expected number of changes per site. Branches in a thicker stroke represent the branches present in the strict consensus parsimony tree. Genera clades are delimited in coloured boxes, with the genus name and clade number indicated to the right. All taxa names are written in black, ex-type strains are represented in bold, novel genera denoted with an asterisk (*) and resurrected genera with a circumflex (ˆ). A vertical bar is used to the right of the coloured boxes and encompasses all genera within their respective families. The family name Mycosphaerellaceae is unabbreviated while the rest are abbreviated as follows: D = Dissoconiaceae, P = Phaeothecoidiellaceae, S = Schizothyriaceae, T = Teratosphaeriaceae, C = Cladosporiaceae. The tree was rooted to Cylindroseptoria ceratoniae (CBS 477.69).
Seven families are represented in the tree (Fig. 1): Mycosphaerellaceae (clades 1–94), Dissoconiaceae (clades 95, 96), Phaeothecoidiellaceae (clades 97–100), Schizothyriaceae (clade 101), Teratosphaeriaceae (clades 102–107), Cladosporiaceae (clade 108) and the single strain used as outgroup belonging to the Dothideaceae. The genera included in the Cladosporiaceae (C), Dissoconiaceae (D), Phaeothecoidiellaceae (P) and Teratosphaeriaceae (T) were used to provide an overview of the phylogenetic position of the Mycosphaerellaceae. In addition, some currently include genera that were once considered part of Mycosphaerellaceae, namely Ramichloridium (clade 95), currently in Dissoconiaceae (D), and Stenella (clade 105), presently in Teratosphaeriaceae (T).
LSU, rpb2 and ITS phylogenies (Datasets 2–4): For these analyses, DNA sequence data from LSU, rpb2 and ITS were combined in three datasets (datasets 2–4) corresponding to three large clades from the overview tree with varying outgroup settings. Datasets 2–4 were analysed with the same three phylogenetic methods applied to Dataset 1. The results of the MrModeltest analysis indicated the same priors for the Bayesian analysis for all three partitions (LSU, rpb2 and ITS), as for Dataset 1.
Dataset 2 consisted of clades 1–37 of Fig. 1 with additional taxa, included a total of 166 taxa and used Schizothyrium pomi (CBS 486.50) as outgroup. The final alignment contained a total of 2 113 characters divided in three partitions containing 749 (LSU), 766 (rpb2), 588 (ITS) characters respectively, including alignment gaps. From the total alignment, 10 characters previously introduced as spacers between the genes were excluded from the phylogenetic analysis. The Bayesian analysis generated 33 282 trees from which 8 320 trees were discarded (25 % burnin). The posterior probability values were calculated from the remaining 24 962 trees (Fig. 2; first value: PP ≤1 shown). The alignment contained a total of 988 unique site patterns: 173 (LSU), 496 (rpb2) and 343 (ITS). The Maximum Likelihood analysis detected 984 distinct patterns and reached a final ML optimization likelihood of −43958.897307. The bootstrap support values from the best-scoring tree were mapped on the Bayesian tree as the second value in the tree nodes (Fig. 2; ML-BS ≥ 90 % shown). The Maximum Parsimony analysis generated the maximum of 1 000 equally most parsimonious trees and the bootstrap support values were mapped on the Bayesian tree as the third value (Fig. 2; MP-BS ≥ 90 % shown). From the 2 103 characters, 1 147 were constant, 148 were variable and parsimony-uninformative and 809 were parsimony-informative. A parsimony consensus tree was calculated from the equally most parsimonious trees and the branches were mapped with a thicker stroke on the Bayesian tree (Fig. 2; Length = 10381, CI = 0.174, RI = 0.717, RC = 0.125, HI = 0.826).
Fig. 2.
Phylogenetic tree (50 % majority rule consensus) resulting from a Bayesian analysis of the combined LSU, rpb2 and ITS sequence alignment (dataset 2; representing clades 1–37 of Fig. 1). Bayesian posterior probabilities (PP), maximum likelihood bootstrap support values (≥ 90 %; ML-BS) and maximum parsimony bootstrap support values (≥ 90 %; MP-BS) are indicated at the nodes (PP/ML-BS/MP-BS) and the scale bar represents the expected number of changes per site. Branches in a thicker stroke represent the branches present in the strict consensus parsimony tree. Genera clades are delimited in coloured boxes, with the genus name and clade number are indicated to the right. All taxa names are written in black, ex-type species strains are represented in bold, novel genera with an asterisk (*) and resurrected genera a circumflex (ˆ). A vertical bar is used to the right of the coloured boxes and encompass all genera within their respective family, the Mycosphaerellaceae. The tree was rooted to Schizothyrium pomi (CBS 486.50).
The phylogenetic trees based on dataset 2 (Fig. 2) that were generated with Bayesian and maximum likelihood methods, separated strains into the same generic clades. The phylogenetic trees generated using parsimony differed slightly in the clade order (data not shown, trees deposited on TreeBASE). Twenty-two genera represent stable genera since they maintain their positions in the present analysis as they displayed in previous phylogenetic studies, and most of them are based on their respective ex-type cultures: Cercospora (clade 1), Ramulispora (clade 2), Neodeightoniella (clade 4), Neopseudocercosporella (clade 5), Fusoidiella (clade 6), Filiella (clade 7), Neocercospora (clade 8), Apseudocercosporella (clade 9), Septoria (clade 11), Phloeospora (clade 14), Caryophylloseptoria (clade 16), Neoseptoria (clade 17), Ramulariopsis (clade 19), Uwemyces (clade 25), Clypeosphaerella (clade 26), Pallidocercospora (clade 28), Nothophaeocryptopus (clade 29), Scolecostigmina (clade 30), Trochophora (clade 32), Sonderhenia (clade 34), Ramularia (clade 35), Zymoseptoria (clade 36), and Xenoramularia (clade 37). Three clades have good candidates for epitypification: Mycovellosiella (clade 10), Passalora (clade 22), and Distocercospora (clade 27). Three clades have multiple type species and need to be addressed: Cercosporella and Acervuloseptoria (clade 18), Sphaerulina and Miuraea (clade 12), Pseudocercospora and Neopseudocercospora (clade 23). Seven distinct clades include species that are assigned to new genera: Cercoramularia (clade 13), Distocercosporaster (clade 15), Pleuropassalora (clade 20), Graminopassalora (clade 21), Coremiopassalora (clade 24), Parapallidocercospora (clade 31), Pseudophaeophleospora (clade 33).
Dataset 3 consisted of clades 38–66 of Fig. 1 with additional taxa, included a total of 111 taxa and used Schizothyrium pomi (CBS 486.50) as outgroup. In addition, a total of 7 strains representing 7 taxa from dataset 4 were used for context. The final alignment contained in all 2 067 characters divided in three partitions containing 729 (LSU), 779 (rpb2), 548 (ITS) characters respectively, including alignment gaps. From the complete alignment, 10 characters previously introduced as spacers between the genes were excluded from the phylogenetic analysis. The Bayesian analysis generated 8 242 trees of which 2 060 trees were discarded (25 % burnin). The posterior probability values were calculated from the remaining 6 182 trees (Fig. 3; first value: PP ≤1 % shown). The alignment contained a total of 824 unique site patterns: 125 (LSU), 478 (rpb2) and 233 (ITS). The Maximum-Likelihood analysis detected 821 distinct patterns and reached a final ML optimization likelihood of −23304.617065. The bootstrap support values from the best-scoring tree were mapped on the Bayesian tree as the second value in the tree nodes (Fig. 3; ML-BS ≥ 90 % shown). The Maximum Parsimony analysis generated the maximum 1 000 equally most parsimonious trees and the bootstrap support values were mapped on the Bayesian tree as the third value (Fig. 3, MP-BS ≥ 90 % shown). From the 2 057 characters, 1 227 were constant, 175 were variable and parsimony-uninformative and 655 were parsimony-informative. A parsimony consensus tree was calculated from the equally most parsimonious trees and the branches were mapped with a thicker stroke on the Bayesian tree (Fig. 3; Length = 4806, CI = 0.309, RI = 0.734, RC = 0.227; HI = 0.691).
Fig. 3.
Phylogenetic tree (50 % majority rule consensus) resulting from a Bayesian analysis of the combined LSU, rpb2 and ITS sequence alignment (dataset 3; representing clades 38–66, 79, 84 and 92–94 of Fig. 1). Bayesian posterior probabilities (PP), maximum likelihood bootstrap support values (≥ 90 %; ML-BS) and maximum parsimony bootstrap support values (≥ 90 %; MP-BS) are indicated at the nodes (PP/ML-BS/MP-BS) and the scale bar represents the expected number of changes per site. Branches in a thicker stroke represent the branches present in the strict consensus parsimony tree. Genera clades are delimited in coloured boxes, with the genus name and clade number indicated to the right. All taxa names are written in black, ex-type strains are represented in bold, novel genera with an asterisk (*) and resurrected genera with a circumflex (ˆ). A vertical bar is used to the right of the coloured boxes and encompass all genera within their respective family, the Mycosphaerellaceae. The tree was rooted to Schizothyrium pomi (CBS 228.57).
The phylogenetic trees based on dataset 3 (Fig. 3) that were generated with Bayesian and maximum likelihood methods, separated strains into the same generic clades. The phylogenetic trees generated using parsimony differed slightly in the clade order (data not shown, trees deposited on TreeBASE). Eight genera represent stable genera since they maintain their positions in the present analysis as they displayed in previous phylogenetic studies, and most of them are based on their respective type strain: Amycosphaerella (clade 8), Pseudocercosporella (clade 10), Paracercospora (clade 11), Pantospora (clade 12), Asperisporium (clade 13), Dothistroma (clade 20), Stromatoseptoria (clade 22), and Phaeocercospora (clade 28). Two clades have good candidates for epitypification: Fulvia (clade 23) and Phaeoramularia (clade 25). Eighteen clades have species belonging to Passalora s. lat. that are reassigned into new genera: Neocercosporidium (clade 1), Sultanimyces (clade 2), Paracercosporidium (clade 3), Parasirosporium (clade 4), Cercosporidium (clade 5), Paramycovellosiella (clade 7), Distomycovellosiella (clade 9), Nothopassalora (clade 14), Phanerohilum (clade 15), Pluripassalora (clade 16), Micronematomyces (clade 17), Rhachisphaerella (clade 18), Neophloeospora (clade 19), Hyalocercosporidium (clade 21), Deightonomyces (clade 26), Pleopassalora (clade 27), Rosisphaerella (clade 29), and Exutisphaerella (clade 30). Four clades have species belonging to Passalora s. lat. that are reassigned into resurrected genera: Cercosporidium (clade 5), Fulvia (clade 23), Ragnhildiana (clade 24), and Phaeoramularia (clade 25).
Dataset 4 consisted of clades 66–108 of Fig. 1, which included a total of 147 taxa and used Cylindroseptoria ceratoniae (CBS 477.69) as outgroup. In addition, the final alignment contained a total of 2 121 characters divided in three partitions containing 767 (LSU), 761 (rpb2), 583 (ITS) characters respectively, including alignment gaps. From the total alignment 26 characters were excluded: 10 characters that were previously introduced as spacers between the genes and 16 characters from the ITS that existed only for the outgroup. The Bayesian analysis generated 26 202 trees from which 6 550 trees were discarded (25 % burnin). The posterior probability values were calculated from the remaining 19 652 trees (Fig. 4; first value: PP ≤ 1 shown). The alignment contained altogether 1 209 unique site patterns: 262 (LSU), 557 (rpb2) and 390 (ITS). The Maximum-Likelihood analysis detected 1 187 distinct patterns and reached a final ML optimization likelihood of −57749.224872. The bootstrap support values from the best-scoring tree were mapped on the Bayesian tree as the third value in the tree nodes (Fig. 4; ML-BS ≥ 90 % shown). The Maximum Parsimony analysis generated the maximum of 1 000 equally most parsimonious trees and the bootstrap support values were mapped on the Bayesian tree as the third value (Fig. 4, MP-BS ≥ 90 % shown). From the 2 094 characters, 936 were constant, 127 were variable and parsimony-uninformative and 1 031 were parsimony-informative. A parsimony consensus tree was calculated from the equally most parsimonious trees and the branches were mapped with a thicker stroke on the Bayesian tree (Fig. 4; Length = 14 343, CI = 0.177, RI = 0.666, RC = 0.118, HI = 0.823).
Fig. 4.
Phylogenetic tree (50 % majority rule consensus) resulting from a Bayesian analysis of the combined LSU, rpb2 and ITS sequence alignment (dataset 4; representing clades 67–99 of Fig. 1). Bayesian posterior probabilities (PP), maximum likelihood bootstrap support values (≥ 90 %; ML-BS) and maximum parsimony bootstrap support values (≥ 90 %; MP-BS) are indicated at the nodes (PP/ML-BS/MP-BS) and the scale bar represents the expected number of changes per site. Branches in a thicker stroke represent the branches present in the strict consensus parsimony tree. Genera clades are delimited in coloured boxes, with the genus name and clade number indicated to the right. All taxa names are written in black, ex-type strains are represented in bold, novel genera with an asterisk (*) and resurrected genera with a circumflex (ˆ). A vertical bar is used to the right of the coloured boxes and encompass all genera within their respective families. The family name Mycosphaerellaceae is unnabreviated while the rest are abbreviated as follows: D = Dissoconiaceae, P = Phaeothecoidiellaceae, S = Schizothyriaceae, T = Teratosphaeriaceae, C = Cladosporiaceae. The tree was rooted to Cylindroseptoria ceratoniae (CBS 477.69).
The phylogenetic trees based on dataset 4 (Fig. 4) that were generated with Bayesian and maximum likelihood methods, separated strains into the same generic clades. The phylogenetic trees generated using parsimony differed slightly in the clade order (data not shown, trees deposited on TreeBASE). Seven families are represented in the tree: Mycosphaerellaceae (clades 1–29), Schizothyriaceae (S), Dissoconiaceae (D), Phaeothecoidiellaceae (P), Teratosphaeriaceae (T), Cladosporiaceae (C) and the single strain used as outgroup belonging to the Dothideaceae. Within the Phaeothecoidiellaceae, a new genus is described, namely Exopassalora (clade 31). Within the Mycosphaerellaceae, seventeen genera are stable since they maintain their positions in the present analysis as they displayed in previous phylogenetic studies, and most of them are based on their respective type strain: Annellosympodiella (clade 3), Neopenidiella (clade 4), Phaeophleospora (clade 5), Lecanosticta (clade 6), Neoceratosperma (clade 7), Xenomycosphaerella (clade 8), Xenosonderhenia (clade 9), Polyphialoseptoria (clade 11), Mycodiella (clade 12), Ruptoseptoria (clade 14), Paramycosphaerella (clade 17), Brunneosphaerella (clade 18), Neomycosphaerella (clade 20), Microcyclosporella (clade 23), Mycosphaerelloides (clade 24), Epicoleosporium (clade 25), and Exosporium (clade 29). The genus Zasmidium (clade 1) is redefined as a broader genus and includes species previously belonging to Ramichloridium, Rasutoria, and Periconiella. Eleven clades represent new genera: Nothopericoniella (clade 2), Xenosonderhenioides (clade 10), Chuppomyces (clade 13), Pachyramichloridium (clade 15), Australosphaerella (clade 16), Madagascaromyces (clade 19), Hyalozasmidium (clade 21), Pseudopericoniella (clade 22), Mucosphaerella (clade 26), Pseudozasmidium (clade 27), Saccharosporium (clade 28). The genera Ramichloridium (clade 30) and Stenella (clade 32) cluster outside Mycosphaerellaceae, in the families Dissoconiaceae (D) and Teratosphaeriaceae (T), respectively.
Taxonomy
The taxonomy section is organized in two parts. The first part is organised by clade number based on the tree depicted in Fig. 1 and covers in detail the genera described in the Mycosphaerellaceae for which cultures were available. Untreated taxa whose names are placed under inverted commas are discussed with the genus they were included in, and occur in a coloured box in the trees. The discussed species have a link to the photoplates and trees where they appear. An extra section referring to the genera with uncertain affinity associated with Mycosphaerellaceae can be found at the end of this section. Information about the host and origin of the type specimen is provided along with the most recent reference for a description or illustration in order to motivate the recollection of the species with phylogenetic positions still undetermined. Due to the large number of taxa discussed throughout this manuscript, the taxon names are written in full.
CLADES 1–94: Mycosphaerellaceae
Mycosphaerellaceae Lindau, Nat. Pflanzenfam., Teil I, 1(1): 421. 1897.
Basionym: Sphaerellaceae Nitschke, Verh. Naturhist. Vereins Preuss. Rheinl. 26: 74. 1869, nom. illeg. (Art. 18.3 and 57.1), non Sphaerellaceae (algae).
Synonyms: Ramularieae Sacc., Syll. Fung. 4: 188. 1886.
Septocylindrieae Sacc., Syll. Fung. 4: 188. 1886.
Cercosporaceae Nann., Repert. mic. uomo 4: 507. 1934.
Cercosporellaceae Nann., Repert. mic. uomo 4: 473. 1934.
Ramulariaceae (Sacc.) Nann., Repert. mic. uomo 4: 472. 1934.
Septocylindriaceae (Sacc.) Nann., Repert. mic. uomo 4: 188. 1934.
Septoriaceae W.B. Cooke, Revta Biol. (Lisboa) 12(12): 298. 1983.
Clade 1: Neopseudocercosporella
Neopseudocercosporella Videira & Crous, Stud. Mycol. 83: 80. 2016.
Description (from Videira et al. 2016): Phytopathogenic, causing leaf spots. Mycelium internal, hyaline, septate, branched, stromata almost absent to well-developed. Ascomata pseudothecial, mycosphaerella-like, single to aggregated, black, immersed, becoming erumpent, globose, with apical ostiole; wall of medium brown textura angularis. Asci aparaphysate, fasciculate, bitunicate, subsessile, obovoid to narrowly ellipsoid. Ascospores, straight to fusoid-ellipsoid, hyaline, guttulate, thin-walled, with subobtuse ends, medianly 1-septate. Conidiophores solitary or grouped, erumpent through the cuticle or emerging through stomata, hyaline, sometimes faintly pigmented, smooth, simple, straight, slightly curved or geniculate-sinuous, usually aseptate, i.e. reduced to conidiogenous cells, thin-walled, smooth. Conidiogenous cells hyaline, subcylindrical to geniculate-sinuous, with inconspicuous conidiogenous loci, unthickened, neither darkened nor refractive, mostly truncate. Conidia solitary, hyaline or rarely slightly pigmented, thin-walled, smooth, straight to flexuous, subcylindrical to obclavate, with apex obtuse to subacute and base truncate, sometimes somewhat obconically, one- to multiseptate, hilum not thickened or darkened.
Type species: Neopseudocercosporella capsellae (Ellis & Everh.) Videira & Crous (≡ Cylindrosporium capsellae Ellis & Everh.).
Neopseudocercosporella capsellae (Ellis & Everh.) Videira & Crous, Stud. Mycol. 83: 86. 2016.
Basionym: Cylindrosporium capsellae Ellis & Everh., J. Mycol. 3(11): 130. 1887.
Synonyms: Cercoseptoria capsellae (Ellis & Everh.) H.C. Greene, Trans. Wisconsin Acad. Sci. 47: 127. 1959.
Pseudocercosporella capsellae (Ellis & Everh.) Deighton, Mycol. Pap. 133: 42. 1973.
For additional synonyms see Braun (1995) or MycoBank.
Descriptions and illustrations: Braun, 1995, Videira et al., 2016.
Materials examined: Japan, Miyazaki, on Brassica rapa var. oleifera, unknown date, K. Kishi, culture MAFF 237605 = MUCC 1254. New Zealand, Auckland, Mt. Albert, on Brassica sp., unknown date and collector, isol. C.F. Hill, Jul. 2005, culture CBS 118412. Republic of Korea, Hongcheon, on Capsella bursa-pastoris, 4 Nov. 2005, H.D. Shin, culture CPC 12519; on Draba nemorosa, 30 Oct. 2004, H.D. Shin, culture CBS 135464 = CPC 11677; Namyangju, on Raphanus sativus, 22 Oct. 2007, H.D. Shin, culture CBS 131896 = CPC 14773. Unknown country, on Brassica sp., unknown date and collector, isol. R. Evans, 28 Aug. 2002, cultures CBS 112032 = HJS 601, CBS 112033 = HJS 600. USA, Columbia, Missouri, Boone Co., on Capsella bursa-pastoris, May 1887, Galloway 253 (holotype NY 883641, isotype BPI 399944).
Notes: The genus Neopseudocercosporella was recently established to accommodate two species that were initially placed in Pseudocercosporella, but were not congeneric with the type species Pseudocercosporella bakeri (Videira et al. 2016). Both Neopseudocercosporella capsellae and Neopseudocercosporella brassicae are considered important pathogens of Brassica spp. (e.g. broccoli, cauliflower, Brussels sprout, etc.) and have been reported worldwide. In literature, these pathogens are usually distinguished based on their disease symptoms, morphology of their ascospores, and culture characteristics (Inman et al. 1991). However, based on the DNA similarities of the currently available strains (Fig. 1, clade 1; Fig. 2, clade 5), these species are so similar that more research is required in order to fully understand their identity and biology (Videira et al. 2016).
Clade 2: Fusoidiella
Fusoidiella Videira & Crous, Stud. Mycol. 83: 87. 2016.
Description: Phytopathogenic, causing small yellow to olivaceous green spots on leaves. Mycelium internal. Conidiophores aggregated in dense fascicles, arising through stomata, aseptate, i.e. usually reduced to conidiogenous cells, smooth, brown, subcylindrical to clavate, straight to curved due to thickening of the wall on one side, not geniculate, one to multiple conidiogenous loci located laterally or apically, loci conspicuous, thickened and broad, areolate, darkened and refractive. Conidia solitary, smooth to rough, hyaline to pale brown, thin- to thick-walled, fusiform to obclavate-fusiform, straight to somewhat curved, septate, not constricted at the septa, apex obtuse and base truncate, hilum flattened, thickened, darkened and refractive.
Type species: Fusoidiella depressa (Berk. & Broome) Videira & Crous (≡ Cladosporium depressum Berk. & Broome).
Fusoidiella anethi (Pers.) Videira & Crous, comb. nov. MycoBank MB822818.
Basionym: Sphaeria anethi Pers., Observ. mycol. 1: 67. 1796.
Synonyms: Dothidea anethi (Pers.) Fr., Summa veg. Scand., Sectio Post. 2: 387. 1849.
Azosma punctum Lacroix, Pl. Cryptog. France, Ed. 2, Fasc. XVI, no. 757. 1860.
Mycosphaerella anethi (Pers.) Petr., Ann. Mycol. 25: 229. 1927.
Cercosporidium punctum (Lacroix) Deighton, Mycol. Pap. 112: 48. 1967.
Passalora punctum (Lacroix) Petzoldt (as “puncta”), Nova Hedwigia, Beih. 87: 192. 1987.
For additional synonyms see Deighton (1967) or MycoBank.
Descriptions and illustrations: Deighton (1967) and Crous & Braun (2003).
Materials examined: Italy, unknown host, collector and date, isol. M. Curzi, culture CBS 296.32. New Zealand, Auckland, St. John's, on Foeniculum vulgare, unknown collector and date, isol. C.F. Hill (1099-B), Dec. 2004, culture CBS 117584.
Notes: This species is the pathogenic agent responsible for cercosporoid leaf blight on Foeniculum (fennel), Petroselinum (parsley) and Anethum (dill) (Davis & Raid 2002). The taxonomic history of this species is complex and has been addressed by multiple authors (Deighton 1967, von Arx 1987, Srivastava, 1994, Crous and Braun, 2003, Nakashima et al., 2011). Morphologically the isolates obtained from all three hosts appear to be identical but some varieties may be present. The connection between the sexual morph Mycosphaerella anethi and the asexual morph Passalora punctum has been experimentally proven by Petzoldt, 1989, Petzoldt, 1990. The disease has a worldwide distribution (Africa, Asia, Europe, the Middle East, North America) but this is the first time an isolate was reported from New Zealand. The two strains form a well-supported clade within Fusoidiella represented in both phylogenetic trees (Fig. 1 clade 2; Fig. 2, clade 6).
Fusoidiella depressa (Berk. & Broome) Videira & Crous, Stud. Mycol. 83: 88. 2016.
Basionym: Cladosporium depressum Berk. & Broome, Ann. Mag. Nat. Hist. 7: 99, t. 5: 8. 1851.
Synonyms: Passalora depressa (Berk. & Broome) Sacc., Nuovo Giorn. Bot. Ital. 8(2): 187. 1876.
Cercosporidium depressum (Berk. & Broome) Deighton, Mycol. Pap. 112: 37. 1967.
For additional synonyms see Deighton, 1967, Crous and Braun, 2003 and MycoBank.
Descriptions and illustrations: Deighton, 1967, Crous and Braun, 2003 and Videira et al. (2016).
Material examined: Republic of Korea, Bonghwa, on Angelica gigas, 18 Oct. 2007, H.D. Shin, KUS-F23064 = CBS H-22632, culture CBS 141335 = CPC 14915.
Notes: The genus Fusoidiella was recently established to accommodate Passalora depressa, a species that is not congeneric with Passalora s. str. as defined by the type species Passalora bacilligera. The type species has fusiform conidia that are morphologically very different from the closest phylogenetic species, Neopseudocercosporella capsellae, and fits the description of the authentic specimen (IMI 29181, on Angelica sylvestris, Great Britain; Deighton 1967) (Videira et al. 2016). Based on the phylogenetic analysis the present strains cluster in a well-supported clade by all three phylogenetic methods employed (Fig. 1 clade 2; Fig. 2, clade 6).
Clade 3: Filiella
Filiella Videira & Crous, Stud. Mycol. 83: 88. 2016.
Description (from Videira et al. 2016): Phytopathogenic. Mycelium internal, hyphae hyaline, septate, branched, forming well-developed stromata composed of swollen hyphae. Conidiophores emerging in dense fascicles from stromata, through the cuticle or through stomata, subcylindrical, straight to flexuous, geniculate-sinuous, aseptate, i.e. usually reduced to conidiogenous cells, rarely 1-septate near the base, hyaline to pale yellow at the base, thin-walled, smooth, with inconspicuous conidiogenous loci, unthickened, neither darkened nor refractive. Conidia solitary, acicular, subcylindrical, filiform, narrowly obclavate, hyaline, discretely septate, thin-walled, smooth, apex subacute, base truncate, hila unthickened, not darkened.
Type species: Filiella pastinacae (P. Karst.) Videira & Crous (≡ Cercosporella pastinacae P. Karst.).
Filiella pastinacae (P. Karst.) Videira & Crous, Stud. Mycol. 83: 88. 2016.
Basionym: Cercosporella pastinacae P. Karst., Hedwigia 23: 63. 1884.
Synonyms: Ramularia pastinacae (P. Karst.) Lindr. & Vestergr., Acta Soc. Fauna Fl. Fenn. 22(1): 8. 1902.
Pseudocercosporella pastinacae (P. Karst.) U. Braun, Nova Hedwigia 56(3–4): 444. 1993.
For additional synonyms see Braun (1995) and MycoBank.
Description and illustration: Videira et al. (2016).
Materials examined: Finland, Mustalia, on Pastinaca sativa, 7 Jul. 1867, P. Karsten (holotype H 3921). Germany, Dresden, on Pastinaca sativa, 1866, Rabenh., Fungi Eur. Exs. 1262 (HAL, erroneously designated as “neotype” in Braun 1995). Sweden, Uppland, Uppsala Näs, Vreta, on Laserpitium latifolium, 2 Jun. 1988, K. & L. Holm, culture CBS 114116 = UPSC 2633.
Notes: This monotypic genus was recently established to accommodate Pseudocercosporella pastinacae, since it was not congeneric with Pseudocercosporella s. str. based on Pseudocercosporella bakeri (Videira et al. 2016). This genus is represented by a single-strain lineage in the phylogenetic analyses (Fig. 1, clade 3; Fig. 2, clade 7), and it is closely related to Neopseudocercosporella and Fusoidiella. Morphologically, it can be distinguished by producing acicular-filiform conidia instead of the subcylindrical conidia of Neopseudocercosporella capsellae, or pigmented, fusiform conidia of Fusoidiella depressa. Braun's (1995) designation of a neotype, based on the assumption that the holotype was not preserved, is now obsolete since holotype material of Cercosporella pastinacae has recently been traced at H. The holotype material has been re-examined by U. Braun and found to represent the present species.
Clade 4: Apseudocercosporella
Apseudocercosporella Videira & Crous, Stud. Mycol. 83: 89. 2016.
Description (from Videira et al. 2016): Phytopathogenic. Mycelium composed of hyaline, septate, branched, thin-walled, smooth hyphae. Conidiophores arising from hyphae, simple, and occasionally branched, straight and subcylindrical to flexuous, geniculate-sinuous, septate or aseptate, hyaline, thin-walled, smooth. Conidiogenous cells integrated, terminal or conidiophores often reduced to conidiogenous cells, subcylindrical to geniculate-sinuous conidiogenous loci slightly thickened and darkened. Conidia formed singly, filiform, or subcylindrical, hyaline, thin-walled, smooth, septate or aseptate, base more or less truncate, hilum slightly thickened and darkened.
Type species: Apseudocercosporella trigonotidis Videira et al.
Apseudocercosporella trigonotidis Videira et al., Stud. Mycol. 83: 89. 2016.
Description and illustration: Videira et al. (2016).
Material examined: Republic of Korea, Jeju, on Trigonotis peduncularis, 12 Nov. 2003, H.D. Shin (holotype KUS-F 20054, isotype CBS H-22515, culture ex-isotype CBS 131890 = CPC 10864); idem., culture CPC 10865.
Notes: This monotypic genus was recently established to accommodate a pseudocercosporella-like species that was not congeneric with Pseudocercosporella s. str. based on Pseudocercosporella bakeri. Phylogenetically, this genus is closely related to Filiella and Neopseudocercosporella (Fig. 1, clade 4; Fig. 2, clade 9). Morphologically, it can be distinguished by the conidial hila and conidiogenous loci that are slightly thickened and darkened instead of inconspicuous.
Clade 5: Neocercospora
Neocercospora M. Bakhshi et al., Phytotaxa 213: 28. 2015.
Description (from Bakhshi et al. 2015b): Foliicolous and caulicolous, phytopathogenic. Mycelium internal. Stromata substomatal, weakly to moderately developed, brown. Caespituli amphigenous, punctiform, brown. Conidiophores aggregated in loose to moderately dense fascicles, arising from the upper cells of substomatal to intraepidermal brown stromata; conidiophores aseptate, reduced to conidiogenous cells. Conidiogenous cells unbranched, pale brown to brown, smooth, subcylindrical to cone-shaped, wider at the base, uni- to multilocal, sympodial, subdenticulate; loci conspicuous, thickened, darkened, somewhat refractive, apical or formed on shoulders caused by geniculation. Conidia solitary or catenate, in unbranched chains, hyaline, smooth, guttulate or not, cylindrical, subcylindrical to obclavate-cylindrical, straight to slightly curved, septate; hilum flattened, moderately thickened, darkened and somewhat refractive.
Type species: Neocercospora ammicola M. Bakhshi et al.
Neocercospora ammicola M. Bakhshi et al., Phytotaxa 213: 28. 2015.
Description and illustration: Bakhshi et al. (2015b).
Material examined: Iran, West Azerbeijan, Khoy, Firouragh, on leaves and stems of Ammi majus, Sep. 2012, M. Arzanlou (holotype IRAN 16461 F, culture ex-type CCTU 1186 = CBS 136450).
Notes: The monotypic genus Neocercospora was recently introduced by Bakhshi et al. (2015b) to accommodate a cercospora-like species that is not congeneric with Cercospora s. str. based on Cercospora apii. The most distinctive characteristics are the conidiophores reduced to conidiogenous cells and conidia that can occur in chains. Phylogenetically, this genus forms a single lineage (Fig. 1, clade 5; Fig. 2, clade 8) closely related to Filiella and Neopseudocercosporella.
Clade 6: Septoria
Septoria Sacc., Syll. Fung. 3: 474. 1884.
Description (from Quaedvlieg et al. 2013): Mycelium in vitro slow-growing, pale brown, septate, in vivo immersed. Conidiomata pycnidial, immersed, separate or aggregated (but not confluent), globose, papillate (or not), brown, wall of thin, pale brown textura angularis, inner layer of flattened, hyaline textura angularis, frequently somewhat darker and more thick-walled around the ostiole. Ostiole single, circular, central. Conidiophores reduced to conidiogenous cells. Conidiogenous cells holoblastic, either determinate or indeterminate, proliferating sympodially and/or percurrently, hyaline, smooth, ampulliform, doliiform or lageniform to short cylindrical, without thickened loci. Conidia hyaline, multiseptate, filiform, solitary, smooth, often constricted at septa. Sexual morphs are mycosphaerella-like.
Type species: Septoria cytisi Desm.
Septoria cytisi Desm., Ann. Sci. Nat., Bot., Sér. 3, 8: 24. 1847.
Description and illustration: Quaedvlieg et al. (2013).
Material examined: Slovakia, on leaves of Laburnum anagyroides, 1884, J.A. Baeumler, BPI USO 378994.
Notes: Septoria represents a genus of plant pathogenic fungi with a wide geographic distribution, commonly associated with leaf spots and stem cankers of a broad range of plant hosts. Following a proposal accepted by the International Code of Nomenclature for algae, fungi, and plants (ICN), the generic name Septoria Sacc. was conserved over the older synonym Septaria Fr. (original spelling). Septoria s. str. was circumscribed when Quaedvlieg et al. (2011) managed to obtain sequence data of both ITS (GenBank accession JF700932) and LSU (GenBank accession JF700954) from a Septoria cytisi fungarium specimen (BPI USO 378994). Phylogenetically, Septoria forms a well-supported clade (Fig. 1, clade 6; Fig. 2, clade 11) closely related to Mycovellosiella and Neocercospora.
Clade 7: Mycovellosiella
Mycovellosiella Rangel, Arch. Jard. Bot. Rio de Janeiro 2: 71. 1917.
Synonym: Vellosiella Rangel, Bol. Agric. (São Paulo) 16: 151. 1915, non Baill. 1887.
Description: Phytopathogenic, causing leaf spots. Colonies effuse, greyish olivaceous to olivaceous brown. Stroma absent or poorly developed. Mycelium pale to moderately deep olivaceous, septate, branched, smooth, stromata absent or small; superficial hyphae arising from internal hyphae or stromatic hyphal aggregations, usually emerging through stomata. Conidiophores macronematous, mononematous, solitary, arising from superficial hyphae or in small to medium fascicles, erect, tangled or forming loose ropes resembling synnemata, straight to flexuous, simple or branched, subcylindrical to geniculate-sinuous, thin-walled, continuous to septate, smooth, subhyaline to pigmented. Conidiogenous cells integrated, terminal, intercalary or pleurogenous, straight to geniculate-sinuous, polyblastic, sympodial, with conidiogenous loci thickened, darkened and often protuberant. Conidia solitary to catenate, sometimes in branched chains, ellipsoid-ovoid, subcylindrical-fusiform, obclavate, straight or curved, aseptate or multiseptate (euseptate), subhyaline to pigmented, smooth to slightly verruculose, ends obtuse, rounded, truncate or pointed; hila thickened and darkened; conidial secession schizolytic.
Type species: Mycovellosiella cajani (Henn.) Rangel ex Trotter (≡ Cercospora cajani Henn.).
Mycovellosiella cajani (Henn.) Rangel ex Trotter, Syll. Fung. 25: 942. 1931. Fig. 6.
Fig. 6.
Mycovellosiella cajani (CBS 114275). A–E. Observations in vivo. F–K. Observations in vitro. A, B. Leaf spot symptoms on the host. C, D, G–I. Conidiophores, conidiogenous cells and conidia. E, J–K. Catenate conidia. F. Culture on V8. Scale bars = 10 μm.
Basionym: Cercospora cajani Henn., Hedwigia 41: 309. 1902.
Synonyms: Vellosiella cajani (Henn.) Rangel, Bol. Agric. (São Paulo) 16(2): 145. 1915.
Passalora cajani (Henn.) U. Braun & Crous, in Crous & Braun, CBS Biodiversity Ser. 1: 93. 2003.
Description in vivo and illustrations: Deighton, 1974, Seifert et al., 2011.
Description in vitro (MEA; CBS 114275): Mycelium hyaline to brown, irregular in width, 1.5–3 μm. Conidiophores hyaline to brown, smooth, simple or branched, geniculate-sinuous, irregular in width, 10–50 × 2.5–5(–7.5) μm. Conidiogenous cells integrated, apical, intercalary, pale brown, rarely hyaline, polyblastic, simple or branched, proliferating sympodially, integrated, sometimes reduced to hyphal loci, aseptate, with thickened, darkened and rim-like loci at the apex and shoulders, 1.5–2.5 μm diam. Conidia cylindrical to ellipsoidal, pale brown, solitary to catenate, in single or branched chains, conically truncate at both ends or basal end, rounded at the apex when solitary, 7–25 × 3–7.5 μm, aseptate, with darkened, thickened, and rim-like loci at the both ends or basal end, 1.5–2.5 μm diam.
Materials examined: Brazil, Minas Gerais, Viçosa, on Cajanus cajan, 2016, R.W. Barreto (neotype designated here CBS H-22940, MBT378566, culture ex-neotype CBS 142174 = CPC 30580 = RB 2071A); idem. CPC 31579 = RB 2071B. South Africa, Mpumalanga, Nelspruit, on Cajanus cajan, 17 May 2002, L. van Jaarsveld, cultures CBS 113998 = CPC 5335, CBS 113999 = CPC 5339, CBS 114275 = CPC 5334.
Notes: The type species of Mycovellosiella, Mycovellosiella cajani, was described from leaves of Cajanus indicus (syn. Cajanus cajan), on May 1901 (Puttemans 237) in Brazil (Hennings 1902), but the type material is not preserved in B and could not be located elsewhere. Deighton (1974) examined numerous specimens deposited in IMI and TAI and concluded that two varieties could be distinguished: Mycovellosiella cajani var. cajani (conidia 0–3-septate and 10–35 μm long; South America, West Indies, Mauritius and Africa) and Mycovellosiella cajani var. indica (conidia 0–9-septate 10–129 μm long; India, Pakistan, Bangladesh and Burma). In this study, we obtained a freshly collected sample of Cajanus cajan from Brazil and cultured this fungus. This strain is phylogenetically identical to those from South Africa (Fig. 1, clade 7, Fig. 2, clade 10) and the morphology is identical to the descriptions available in the literature (Deighton, 1974, Braun, 1998), and therefore the specimen is hereby designated as neotype. Mycovellosiella cajani is the causative agent of leaf spot disease of pigeon pea worldwide and, when defoliation occurs before flowering and podding, it causes severe yield losses (up to 85 % in eastern Africa) (Reddy et al. 2012). Mycovellosiella, based on phylogenetic data, is a monotypic genus but with more collections new species may emerge. Previous morphological descriptions of Mycovellosiella s. lat. (Deighton, 1974, Braun, 1998) can no longer be applied to this genus in its current circumscription and the application of this generic name depends on the availability of corresponding phylogenetic data. Mycovellosiella was previously distinguished from Passalora and Phaeoramularia by the formation of superficial mycelium with solitary conidiophores formed in vivo, but these traits are phylogenetically and taxonomically not significant and appear unreliable. Without detailed knowledge of the phylogenetic affinity, species with mycovellosiella-like morphology should tentatively be maintained in or assigned to Passalora s. lat.
Clade 8: Miuraea and Sphaerulina
Miuraea Hara, Byochugai-hoten: 260 & 779. 1948, emend.
Unconfirmed synonyms: Rhopaloconidium Petr. (1952), Hyalodictys Subram. (1962).
Description (adapted from Braun 1995): Leaf spot pathogen of vascular plants. Mycelium hyaline to lightly pigmented, septate, branched, emerging through stomata, thin-walled. Conidiophores little differentiated, semi-macronematous, mononematous, short, sometimes reduced to a conidiogenous cell integrated in the hyphae, with small lateral peg-like protuberances, occasionally subfasciculate and arising from stromatic hyphal aggregations. Conidiogenesis holoblastic, monoblastic, determinate, occasionally polyblastic, proliferation sympodial or percurrent; conidiogenous loci more or less truncate, unthickened or slightly thickened, not darkened. Conidia solitary or catenate, ellipsoid-ovoid, subcylindrical-vermiform, obclavate, subclavate, sometimes somewhat asymmetrical, eu- or distoseptate, pluriseptate, septa transverse, oblique to longitudinal, hyaline to faintly pigmented, thin-walled, old conidia often slightly to moderately thick-walled, hila rounded to truncate, unthickened or slightly thickened, not darkened, conidial secession schizolytic.
Type species: Miuraea degenerans (Syd. & P. Syd.) Hara (≡ Clasterosporium degenerans Syd. & P. Syd.).
Miuraea degenerans (Syd. & P. Syd.) Hara, Byochugai-hoten: 260, 1948. Fig. 7.
Fig. 7.
Miuraea degenerans (MUCC 1514). A–D. Observations in vitro. A. Culture on MEA. B. Olivaceous conidia and short conidiophore. C. Hyaline (left) and pigmented (right) conidia. D. Microcyclic conidia. E–G. Conidiophores and conidia observed using SEM. Scale bars = 10 μm.
Basionym: Clasterosporium degenerans Syd. & P. Syd., Ann. Mycol. 12(2): 164. 1914.
Description in vivo: Braun (1995).
Description in vitro (on MEA; MAFF 239265): Mycelium hyaline, later blackish, aggregated with white-floccose aerial hyphae. Conidiophores short, reduced to conidiogenous cells, hyaline to pale brown, 47–71 × 2–5 μm. Conidiogenous cells determinate, proliferating sympodially and/or percurrently, holoblastic, with slightly thickened loci. Conidia solitary or catenate, oblong to obclavate, hyaline to pale, 10–23 × 6–9 μm, 2–6-eu- or distoseptate, rounded or conically truncate, slightly thickened or unthickened at the base.
Materials examined: Japan, Ibaragi, on Prunus mume, Sep. 2003, T. Kobayashi (epitype designated here TSU MUMH11567, MBT376838, culture ex-type MAFF 239265 = MUCC 1514); Mutsu (= Aomori), on Prunus mume, 1 Nov. 1913, M. Miura (holotype S F41753). Republic of Korea, Chuncheon, on Prunus armeniaca, 7 Oct. 2003, H.D. Shin, CBS H-20840, cultures CBS 131935 = CPC 10828.
Notes: Miuraea degenerans and Miuraea persica are well-known as the causal agents of white mildew or frosty mildew of Prunus spp. in far-east Asian countries. In the present study, the sequences of both Miuraea persicae (sexual morph: Mycosphaerella pruni-persicae) and Miuraea degenerans are quite similar; however, comparison of ITS sequences of several collections identified as either Miuraea degenerans or Miuraea persica show a limited number of nucleotide differences (data not shown), and pending more collections and multigene data we refrain from synonomising these two species. Subramanian's (1962) description of Miuraea degenerans includes Miuraea persicae, but Braun (1995) considers them different species based on the conidial characteristics that are generally longer, with less longitudinal septa and only occasionally constricted at septa in Miuraea persica, while the conidia of Miuraea degenerans are generally broader, with more longitudinal septa and often constricted at septa. Based on the phylogenetic analysis, Miuraea strains cluster among Sphaerulina species and none of the three phylogenetic methods applied provided strong support for their separation (Fig. 1, clade 8; Fig. 2, clade 12). The introduction of more strains of Miuraea species in the future may provide support to the separation of these genera into two independent clades. Morphologically Miuraea is considered intermediate between Pseudocercospora and Pseudocercosporella (Braun 1995), which are hyphomycete genera, while Sphaerulina is a coelomycete genus. Miuraea asiminae, was recently reallocated to Pseudocercospora (Braun & Crous 2008).
Sphaerulina Sacc., Michelia 1(4): 399. 1878.
Unconfirmed synonyms: Ophiocarpella Theiss. & Syd. (1915), Sphaerialea Sousa da Câmara (1926).
Description (adapted from Quaedvlieg et al. 2013): Ascomata pseudothecial, immersed, subepidermal, erumpent at the apex, single to clustered, globose, papillate. Ostiole central, with hyaline periphyses; wall of textura angularis, composed of 2–4 layers of brown cells. Hamathecium dissolving at maturity. Asci bitunicate, fissitunicate, clustered, cylindrical to obclavate, rounded at apex, with or without a shallow apical chamber, short-stipitate or sessile, with 8 bi- to triseriate ascospores. Ascospores subcylindrical to fusiform, rounded at ends, slightly tapered, straight or slightly curved, 1–3-septate, with a primary septum nearly median, hyaline, smooth, without sheath or appendages.
Type species: Sphaerulina myriadea (DC.) Sacc. (≡ Sphaeria myriadea DC.).
Sphaerulina myriadea (DC.) Sacc., Michelia 1(4): 399. 1878.
Basionym: Sphaeria myriadea DC., in de Candolle & Lamarck, Fl. franç., Edn 3 (Paris) 5/6: 145. 1815.
Description and illustration: Crous et al. (2011c).
Materials examined: Germany, Driesen, Lasch, Rabenhorst, Fungi Eur. Exs. no. 149 (L). Japan, Aomori, Tsugaru, Kidukuri, Bense-marsh, on leaves of Quercus dentata, 21 Apr. 2007, K. Tanaka 2243, HHUF 29940, single ascospore culture CBS 124646 = JCM 15565. UK, on leaves of Quercus robur, J.E. Vize, Microfungi Brit. Ex. No. 195, IMI 57186, (= K(M) 167735). USA, California, Sequoia National Park. alt. 2590 m, on leaves of Castanopsis sempervirens, 18 Jun. 1931, H.E. Parks, BPI 623686; Lake Co., Hoberg's Resort, on leaves of Quercus kelloggii, 15 May 1943, V. Miller, BPI 623707; Maryland, Marlboro, on leaves of Quercus alba, 26 Apr. 1929, C.L. Shear, BPI 623705; Texas, Houston, on leaves of Q. alba, 8 Apr. 1869, H.W. Ravenel, BPI 623704.
Notes: The genus Sphaerulina was traditionally separated from Mycosphaerella based on ascospore septation, a trait that was unreliable to infer phylogenetic relatedness (Crous et al., 2003, Crous et al., 2011c). The currently available strains of Sphaerulina myriadea were isolated from several hosts belonging to the Fagaceae originating from various locations. These strains were treated in a previous study where the authors proposed that Sphaerulina myriadea was a species complex and therefore refrained from designating an epitype pending the collection of authentic European material on Quercus from France (Crous et al. 2011c). The genus Sphaerulina was previously found to be phylogenetically close to Septoria (Quaedvlieg et al., 2013, Verkley et al., 2013). In this work, Sphaerulina and Miuraea strains cluster together and none of the three phylogenetic methods applied provided strong support for their separation (Fig. 1, clade 8; Fig. 2, clade 12).
Species clustering in the Sphaerulina clade that need further material to be collected before a formal combination into Sphaerulina can be proposed:
Mycosphaerella grossulariae (Fr.) Lindau, in Engler & Prantl, Nat. Pflanzenfam., Teil I, 1(1): 424. 1897.
Material examined: Netherlands, leaf spot on Ribes nigrum, col. M.S.J. Ledeboer, isol. H.A. Diddens, dep. 1937, culture CBS 235.37.
Notes: The type of Mycosphaerella grossulariae was described from Ribes grossularia collected in Sweden (Aptroot 2006). Tomilin (1979) linked this species to two asexual morphs, Phyllosticta grossulariae and Septoria ribis. According to Eriksson (1992), it is morphologically indistinguishable from Pleospora herbarum (= Stemphylium). The present species is represented by a single strain in the phylogenetic analysis performed (Fig. 2, clade 12). This species needs to be recollected and its phylogenetic position resolved.
Mycosphaerella harthensis (Auersw.) Mig., Krypt.-Fl. Deutschl., Österr. Schweiz, Pilze Vol 10, Theil 3(1): 289. 1912.
Material examined: Switzerland, dead leaves of Betula sp., unknown collector and date, isol. E. Müller, 7 Jun. 1952, culture CBS 325.52.
Notes: The type of Mycosphaerella harthensis was described from Betula collected in Germany and the specimen is probably not preserved (Aptroot 2006). The culture CBS 325.52 is currently sterile. The present species is represented by a single strain in the phylogenetic analysis performed (Fig. 2, clade 12). This species needs to be recollected and neotypified.
Clade 9: Cercoramularia
Cercoramularia Videira, H.D. Shin, C. Nakash. & Crous, gen. nov. MycoBank MB822581.
Etymology: With cercosporidium-like conidiophores and ramularia-like conidia.
Description: Mycelium hyaline to brown. Conidiophores brown to pale brown, emerging from brown hyphae or swollen hyphal cells, smooth, euseptate, straight to geniculate-sinuous, simple or branched, sometimes reduced to conidiogenous cell. Conidiogenous cells integrated, terminal, hyaline to pale brown, monoblastic or proliferating sympodially, with thickened, darkened and refractive conidiogenous loci. Conidia hyaline to pale brown, euseptate, solitary or catenate, holoblastic, fusiform, rounded at the apex when solitary.
Type species: Cercoramularia koreana Videira et al.
Cercoramularia koreana Videira, H.D. Shin, C. Nakash. & Crous, sp. nov. MycoBank MB822710. Fig. 8.
Fig. 8.
Cercoramularia koreana (CPC 10709). A–E. Observations in vivo. A, B. Leaf spot symptoms on the host. C, D. Conidiophores, conidiogenous cells and conidia. E. Catenate conidia. F–L. Observations in vitro. F. Culture on OA. G. Conidiophore and conidiogenous cell. H. Conidiophore, conidiogenous cell and conidium. I, J. Conidiogenous cell and catenate conidia. K, L. Catenate conidia. Scale bars = 10 μm.
Etymology: In honour of the country it was collected from, Republic of Korea.
Description in vivo (CBS H-22941; herb. spec. CPC 10709): Leaf spots small, irregular, 4–10 mm diam, brown to dark brown, distinct. Stromata absent to small, brown, globose. Conidiophores in loose fascicles of 2–12, dark brown, septate, geniculate-sinuous, 23–78 × 2.5–9 μm. Conidiogenous cells integrated, terminal, polyblastic, proliferating sympodially, with thickened, darkened, refractive and rim-like loci at the apex and shoulders, 1.6–2.5 μm diam. Conidia hyaline, solitary or catenate in branched chains, obclavate, cylindrical to filiform, 20–62 × 2.5 μm, 2–5-septate, with thickened and darkened rim-like hila, 1.6–2.5 μm diam, rounded at the apex when solitary.
Description in vitro (SNA; CPC 10639): Mycelium hyaline to brown, 2–2.5 μm diam, with swollen brown cells. Conidiophores pale brown to brown, emerging from brown hyphae or swollen hyphal cells, smooth, straight to geniculous-sinuous, simple or branched, euseptate, 12.5–100 × 2.5–3 μm. Conidiogenous cells integrated, terminal, hyaline to pale brown, monoblastic or polyblastic, proliferating sympodially, with thickened, darkened and refractive loci, 1.8–2.8 μm diam. Conidia hyaline to pale brown, solitary or in chains up to six conidia, fusiform, rounded at the apex when solitary, 1-euseptate, 27–105 × 3–3.7 μm.
Materials examined: Republic of Korea, Seoul, on leaves of Styrax japonica, 17 Sep. 2003, H.D. Shin (holotype CBS H-22941, ex-type culture CBS 142175 = CPC 10709); same location and host, 2003, H.D. Shin, cultures CPC 10639–10641.
Notes: This genus is represented by a single species that is phylogenetically close to Phloeospora and Sphaerulina (Fig. 1, clade 9; Fig. 2, Clade 13). Cercoramularia koreana causes leaf spot symptoms on Styrax japonica, a small tree from the Styracaceae family commonly planted as ornamental.
Clade 10: Phloeospora
Phloeospora Wallr., Fl. Crypt. Germ. 2: 176. 1833.
Synonyms: Septoria Fr., Syst. Orb. Veg. 1: 119. 1825.
Helicobolus Wallr., Fl. Crypt. Germ. 2: 751. 1833.
Phloeochora Höhn., Ber. Deutsch. Bot. Ges. 35: 252. 1917.
Description (from Quaedvlieg et al. 2013): Mycelium immersed, septate, hyaline. Conidiomata acervular, subepidermal, circular, discrete or confluent, composed of hyaline to pale brown, thin-walled textura angularis; dehiscence irregular. Conidiophores reduced to conidiogenous cells or with one or two supporting cells, branched at base or not. Conidiogenous cells holoblastic, annellidic, occasionally also sympodial, discrete, indeterminate hyaline, smooth, cylindrical, with several apical inconspicuous annellations, formed from the upper cells of the acervuli. Conidia solitary, hyaline, septate, smooth, guttulate or not, cylindrical, curved, attenuated towards the apices, apex obtuse to subobtuse, base truncate, with minute marginal frill.
Type species: Phloeospora ulmi (Fr.) Wallr. (≡ Septoria ulmi Fr.).
Phloeospora ulmi (Fr.) Wallr., Fl. Crypt. Germ. 2: 177. 1833.
Basionym: Septoria ulmi Fr. [as ‘Septaria’], Novit. Fl. Svec. 5(cont.): 78. 1819.
Synonyms: Septogloeum ulmi (Fr.) Died., Krypt. Fl. Brandenburg (Leipzig) 9: 836. 1915.
Cylindrosporium ulmi (Fr.) Vassiljevsky, Fungi Imperfecti Parasitici 2: 580. 1950.
Mycosphaerella ulmi Kleb., Z. PflKrankh. 12: 257. 1902.
Sphaerella ulmi (Kleb.) Sacc. & D. Sacc., Syll. Fung. (Abellini) 17: 642. 1905.
Description and illustration: Quaedvlieg et al. (2013).
Materials examined: Austria, Innsbruck, near Hungerburg, on leaves of Ulmus sp., 21 Sep. 1981, H.A. van der Aa, CBS H-14740, CBS H-14861, culture CBS 613.81; Innsbruck, road to Hungerburg, on leaves of Ulmus glabra, 20 Oct. 1996, W. Gams, culture CBS 344.97. Netherlands, Baarn, garden of CBS, Oosterstraat 1, on leaves of Ulmus sp., 26 Aug. 1998, H.A. van der Aa, CBS H-14739, culture CBS 101564; community of Borsele, Schouwersweel near Lisse, on Ulmus sp., 27 Aug. 2001, G. Verkley, culture CBS 109835.
Notes: The generic synonymy has been discussed by Sutton (1977) and the type species described and illustrated by Sutton & Pollack (1974). Phloeospora is based on the type species Phloeospora ulmi, isolated from Ulmus glabra in Europe, but a type specimen could not be located. It can be morphologically distinguished from Septoria by the production of conidia in acervuli, whereas conidiomata in the latter genus are pycnidial. A recent phylogenetic analysis performed to delimit Septoria and allied genera confirmed that Phloeospora (based on Phloeospora ulmi) clusters close to, but separate from Septoria s. str. (Quaedvlieg et al. 2013). This separation is also observed in the phylogenetic analyses performed in this study (Fig. 1, clade 10; Fig. 2, clade 14). The known sexual morphs linked to Phloeospora resemble the concepts of Mycosphaerella, Didymella and Sphaerulina supporting the idea that this genus is heterogenous and in need of revision (Verkley & Priest 2000). In this study, we observed that the strain currently known as Phloeospora maculans is not congeneric with Phloeospora ulmi.
Clade 11: Cercospora
Cercospora Fresen. ex Fuckel, Hedwigia 2(15): 91. 1863 and Fungi Rhen. Exs., Fasc. II: no. 117. 1863, nom. cons. prop.
Unconfirmed synonyms: Virgasporium Cooke (1875), Cercosporina Speg. (1910).
Description (adapted from Braun et al. 2013): Mostly plant pathogenic but also saprobic, usually causing distinct lesions (leaf spots) but sometimes symptomless. Mycelium internal and only rarely external, hyphae usually pigmented but occasionally hyaline, branched, septate, thin-walled, smooth, rarely faintly verruculose. Stromata lacking to well-developed, substomatal, intra-epidermal or immersed, mostly pigmented, composed of textura angulata or globosa. Conidiophores mono- and macronematous, solitary or fasciculate, rarely in sporodochial conidiomata, emerging through stomata or erumpent, erect, continuous to multi-septate, hyaline (subgen. Hyalocercospora) to pigmented, pale olivaceous to dark brown (subgen. Cercospora), wall smooth to slightly rough, thin to moderately thick, sometimes reduced to conidiogenous cells. Conidiogenous cells integrated, terminal or intercalary, usually polyblastic but sometimes monoblastic, proliferation sympodial, rarely percurrent, conidiogenous loci (scars) conspicuous, thickened and darkened-refractive, planate with minute central pore. Conidia solitary, rarely in short chains, mostly scolecosporous, obclavate-cylindrical, acicular, filiform and multi-euseptate, rarely amero- to phragmosporous, broadly ellipsoid-ovoid to broadly obclavate-cylindrical, but always hyaline or subhyaline, thin-walled, smooth or almost so, hila thickened and darkened, conidial secession schizolytic.
Type species: Cercospora apii Fresen. (typ. cons. prop.)
Cercospora apii Fresen., Beitr. Mykol. 3: 91. 1863.
Description: Groenewald et al. (2005).
Materials examined: Austria, Wien, on Beta vulgaris, Jun. 1931, E.W. Schmidt, culture CBS 121.31 = CPC 5073; on Apium sp., 28 Aug. 2003, Institut fur Pflanzengesundheit, culture CBS 114416 = CPC 10925. Germany, Oestrich, garden, on Apium graveolens, Fuckel, Fungi Rhen. Exs. 117 (lectotype selected by Groenewald et al. 2005: HAL); Landwirtschaftsamt, Heilbron, on Apium graveolens, 10 Aug. 2004, K. Schrameyer (epitype designated by Groenewald et al. 2005: preserved as metabolically inactive culture CBS 116455 = CPC 11556); idem. CBS 116504 = CPC 11579, CBS 116507 = CPC 11582. For complete list of existing strains see Groenewald et al. (2013).
Notes: The genus Cercospora contains numerous important plant pathogenic fungi from a diverse range of hosts. The modern taxonomy of this complex began with Chupp (1954) who included all variants in a broadly circumscribed Cercospora. This concept was continuously revised and narrowed by several authors over the years (Deighton, 1976a, Deighton, 1979, von Arx 1983, Braun, 1995, Crous and Braun, 2003). Recent studies based on multi-gene phylogenies have helped to circumscribe Cercospora and to identify new species. No single locus has yet been found as an ideal DNA barcode for the genus, and species identification needs to be based on a combination of gene loci and morphological characters (Groenewald et al., 2013, Bakhshi et al., 2015a, Bakhshi et al., 2015b). The type species of Cercospora, Cercospora depazeoides (= Cercospora penicillata) (see Braun 1995: 41), is a common, widespread cercosporoid fungus on elderberry. Re-examinations of type material and numerous other collections revealed that this species is conspecific with Pseudocercospora sambucigena (Braun et al. 2015), which is a proven species of Pseudocercospora (Crous et al. 2013a). Therefore, Cercospora would formally become the oldest available name for Pseudocercospora, which would be reduced to synonymy with Cercospora. This would be an unpleasant situation with enormous consequences and name changes, which should be avoided. Therefore, a proposal to conserve Cercospora with Cercospora apii as conserved type was recently published (Braun & Crous 2016), which will help to maintain the application of the name Cercospora in the common, generally accepted circumscription.
Cercospora janseana (Racib.) O. Constant., Cryptog. Mycol. 3: 63. 1982.
Basionym: Napicladium janseanum Racib., Parasitische Algen und Pilze Javas 2: 41. 1900.
Synonyms: Passalora janseana (Racib.) U. Braun, Schlechtendalia 5: 39. 2000.
Cercospora oryzae Miyake, Bot Mag. Tokyo 23 (267): 139. 1909.
Sphaerulina oryzina Hara, Diseases of the rice plant (Japan): 144. 1918.
Cercospora oryzae var. rufipogonis R.A. Singh & Pavgi, Sydowia 21: 176. “1967” 1968.
Description and illustration: Chupp, 1954, Braun et al., 2015.
Material examined: USA, unknown collector and date, isol. E.C. Tullis, Aug. 1937, culture CBS 145.37 = IMI 303642.
Notes: The present species is represented by a single strain in the phylogenetic analysis performed (Fig. 2, clade 1). See Braun et al. (2015).
Species clustering in the Cercospora clade that need further material to be collected before its status as species of Cercospora can be confirmed:
Passalora dulcamarae (Peck) U. Braun & Crous, CBS Biodiversity Ser. 1: 167. 2003.
Basionym: Ramularia dulcamarae Peck, Rep. (Annual) New York State Mus. Nat. Hist. 33: 30. 1880.
Synonyms: Cercospora dulcamarae (Peck) Ellis & Everh., J. Mycol. 1(4): 55. 1885.
Mycovellosiella dulcamarae (Peck) U. Braun, Mycotaxon 48: 284. 1993.
Cercospora dulcamaricola Hollós, Ann. Hist. Nat. Mus. Natl. Hung. 4: 370. 1906.
Description and illustration: Chupp (1954).
Materials examined: Romania, Distr. Constanta, Hagieni, on Solanum dulcamara, 14 Oct. 1970, O. Constantinescu & G. Negrean, CBS H-9831, CBS H-9832, culture CBS 544.71 = BUCM 2008.
Notes: Ramularia dulcamarae was described on Solanum dulcamara collected in the USA (New York, Oneida, Verona) and the herbarium specimen is deposited in NYS. The present strain is currently sterile and forms a single strain lineage in the phylogenetic analyses (Fig. 2, clade 1).
Clade 12: Ramulispora
Ramulispora Miura, Bull. S. Manchur. Railway Co. Agr. Exp. Sta. Kunchuling 11: 43. 1920.
Description (adapted from Braun 1995): Graminicolous, causing leaf spots, necrosis, foot-rot, and seedling blight. Mycelium hyaline to faintly pigmented, smooth, septate, branched; stromata absent to well-developed, substomatal to intra-epidermal, hyaline to pigmented. Conidiophores semi-macronematous or macronematous, mononematous, solitary or fasciculate, arising from inner hyphae or stromata, erumpent through the cuticle or emerging through stomata, simple, rarely branched, continuous or sparsely septate, often reduced to conidiogenous cell, straight, subcylindrical to geniculate-sinuous, smooth, hyaline or subhyaline, rarely faintly pigmented. Conidiogenous cells directly arising from hyphae or stromata or integrated, terminal, subcylindrical to geniculate, monoblastic to polyblastic, sympodial, rarely percurrent, with inconspicuous, unthickened, hyaline conidiogenous loci. Conidia solitary, scolecosporous, acicular, subcylindrical, filiform, narrowly obclavate, sometimes with lateral branchlets (microcyclic conidiation), continuous or septate (branchlets mainly produced under humid conditions and in culture when grown on wet, poor media under lights, sometimes developing into secondary conidia which are detached), hyaline, euseptate, multi-septate, smooth, apex blunt to acute, base rounded to truncate, hilum unthickened, hyaline, conidial secession schizolytic.
Type species: Ramulispora sorghi (Ellis & Everh.) L.S. Olive & Lefebvre (≡ Septorella sorghi Ellis & Everh.).
Ramulispora sorghi (Ellis & Everh.) L.S. Olive & Lefebvre, Phytopathology 36: 198. 1946. Fig. 9.
Fig. 9.
Ramulispora sorghi (CBS 110578). A–F. Observations in vitro. A. Culture on OA. B–F. Conidiophore, conidiogenous cell and conidia. Scale bars = 10 μm.
Basionym: Septorella sorghi Ellis & Everh., J. Mycol. 9: 164. 1903.
Synonym: Ramulispora andropogonis Miura, Bull. S. Manchur. Railway Co. Agr. Exp. Sta. Kunchuling: 43. 1920.
Description in vivo and illustrations: Braun (1995).
Description in vitro (SNA, CBS 110578): Mycelium composed of hyaline, smooth, septate, branched hyphae, 1.5 μm wide. Stromata absent to small, pseudoparenchymatous, brown. Conidiophores, conidiogenous cells and conidia hyaline and smooth. Conidiophores solitary or in fascicles, subcylindrical-filiform, sometimes geniculate-sinuous, simple, septate, sometimes reduced to conidiogenous cell, (10–)12–13(–15) × 1.5(–2) μm. Conidiogenous cells terminal, monoblastic or polypblastic, with unthickened and non-refractive loci. Conidia formed singly, filiform, acicular, straight to curved, (11–)39–52(–79.5) × 1.5–2(–3) μm, 4–9-septate, hyaline, smooth, with subacute apex and truncate base, frequently with 1–2 lateral branches.
Materials examined: South Africa, KwaZulu-Natal Province, on Sorghum bicolor, Mar. 1995, coll. D. Nowell, cultures CBS 110578 = CPC 905, CBS 111032 = IMI 153076 = CPC 899, CBS 115522 = CPC 902.
Notes: The genus Ramulispora includes pathogens of gramineous plants (von Arx 1983, Braun 1995) and is typified by Ramulispora sorghi, the causative agent of sorghum sooty stripe disease (Crous et al., 2003, Crous et al., 2009e). It produces numerous microsclerotia on the leaf surface and forms sporodochia with hyaline, transversely euseptate, scolecosporous conidia. A total of 14 species of Ramulispora are known (MycoBank), but without cultures and molecular analyses, their correct phylogenetic position remains unclear. The type species of Ramulispora, Ramulispora sorghi, was described from the host Sorghum halepense, from Tuskeege (Alabama, USA) but a type specimen could not be located. The cultures included in this study were isolated from sorghum from the KwaZulu-Natal Province of South Africa, where the pathogen was associated with a severe outbreak of sooty leaf stripe (Mchau et al. 1996). In a more recent study on the disease, Brady et al. (2011) concluded that differences in disease severity was host genotype-dependent and not due to genetic differences in the local pathogen population. The ITS sequence fragments of Ramulispora sorghi obtained from Kansas (HQ400740–HQ400745HQ400740HQ400741HQ400742HQ400743HQ400744HQ400745) were 100 % identical to those sequences from South Africa (Mchau et al. 1996) which is consistent with the concept that reproduction in Ramulispora sorghi is asexual in the field (Brady et al. 2011). Phylogenetically, Ramulispora forms a well-supported clade (Fig. 1, clade 12; Fig. 2, clade 2), being closely related to Neodeightoniella.
Ramulispora sorghiphila U. Braun, C. Nakash., Videira & Crous, sp. nov. MycoBank MB822717. Fig. 10.
Fig. 10.
Ramulispora sorghiphila (CBS 255.82). A–F. Observations in vitro. A. Culture on V8. B. Stromata. C. Conidiophore and conidia. D–F. Conidia. Scale bars = 10 μm.
Etymology: Composed of the name of the host genus and the Greek adjectival suffix -philum (loving).
Description in vitro (on V8; CBS 255.82): Mycelium composed of hyaline, smooth, septate, branched hyphae, 2–2.5 μm wide. Conidiophores micro- to macronematous, sinuous to geniculous-sinuous, hyaline to pale brown, branched, 30–110 × 2–2.5 μm. Conidiogenous cells integrated, terminal, mono- or polyblastic, proliferating sympodially or percurrently, smooth to verruculose, with unthickened and non-refractive loci. Conidia solitary, rarely catenate, holoblastic, hyaline, filiform, 70–250 × 2–2.5 μm, unthickened and truncate at the base, 2–12 septate.
Materials examined: India, on Sorghum vulgare, Oct. 1969, unknown collector, isol. by G.S. Rawla in 1971, dep. by H.I. Nirenberg in 1982 (holotype IMI 153077, culture ex-type CBS 255.82).
Notes: Differs from Ramulispora sorghi by producing much longer conidiophores and conidia. It is similar to Ramulispora sorghicola by producing very long conidia in culture that are commonly branched but differs by forming sclerotia in culture and not producing conidia in flesh-coloured gelatinous masses. Ramulispora sorghiphila forms a single strain lineage within the Ramulispora genus clade (Fig. 1, clade 12; Fig. 2, clade 2).
Clade 13: Catenulocercospora
Catenulocercospora C. Nakash., Videira & Crous, gen. nov. MycoBank MB822580.
Etymology: Derived from the similarities to the genus Cercospora and the catenulate nature of the conidia.
Description: Phytopathogenic, forming brown rectangular leaf spots. Caespituli amphigenous, mainly hypophyllous, hyaline. Mycelium internal, hyaline. Stromata small to developed, brown, globose. Conidiophores pale brown at the base and turning hyaline towards the apex, septate, straight to geniculate-sinuous. Conidiogenous cells integrated, mono- or polyblastic, with darkened, thickened and refractive rim-like conidiogenous loci. Conidia hyaline, solitary or catenate in branched chains, rounded at the apex when solitary, obclavate or cylindrical to filiform, septate, with rim-like hila that are thickened, darkened and refractive.
Type species: Catenulocercospora fusimaculans (G.F. Atk.) C. Nakash. et al. (≡ Cercospora fusimaculans G.F. Atk.).
Catenulocercospora fusimaculans (G.F. Atk.) C. Nakash., Videira & Crous, comb. nov. MycoBank MB822745. Fig. 11.
Fig. 11.
Catenulocercospora fusimaculans (CPC 17277). A–E. Observations in vivo. A. Leaf spot symptoms on the host. B. Conidia sporulating on the lesion. C, D. Conidiophores, conidiogenous cells and conidia. E. Single and catenate conidia. F–J. Observations in vitro. F. Culture on OA. G–I. Conidiophores, conidiogenous cells and conidia. J. Catenate conidia. Scale bars = 10 μm.
Basionym: Cercospora fusimaculans G.F. Atk., J. Elisha Mitchell Sci. Soc. 8(2): 50. 1892.
Synonyms: Phaeoramularia fusimaculans (G.F. Atk.) X.J. Liu & Y.L. Guo, Acta Phytopathol. Sin. 12 (4): 9. 1982.
Passalora fusimaculans (G.F. Atk.) U. Braun & Crous, in Crous & Braun, Mycosphaerella and Anam.: 192. 2003.
For additional synonyms see Crous and Braun, 2003, Braun et al., 2015 or MycoBank.
Descriptions in vivo and illustrations: Ellis, 1976, Hsieh and Goh, 1990.
Description in vivo (CPC 17277): Leaf spots formed as small streaks, rectangular, 2–6 × 0.5–1 mm, pale brown to dark brown, distinct. Caespituli amphigenous, mainly hypophyllous, white. Mycelium internal, hyphae hyaline, 2.5 μm diam. Stromata small to developed, brown, globose, 27–71 μm diam. Conidiophores in loose fascicles of 2–12, hyaline to pale brown, paler towards the apex, septate, tapered towards the apex, straight to geniculate-sinuous, 23–78 × 2.5–9 μm. Conidiogenous cells integrated, terminal, polyblastic, proliferating sympodially, with conidiogenous loci rim-like, thickened, darkened and refractive and located at the apex and shoulders, 1.6–2.5 μm diam. Conidia hyaline, smooth, solitary or catenate, ocasionaly in branched chains, long-obclavate, cylindrical to filiform, 20–62 × 2.5 μm, 2–5-septate, with thickened and darkened rim-like hila, 1.6–2.5 μm diam.
Description in vitro (on V8; CPC 17277): Mycelium hyaline to pale brown, smooth to rough, delicate, uniform in width, 2.5 μm diam. Conidiophores micronematous, hyaline to pale brown, smooth to verruculose, simple, cylindrical, straight to geniculate-sinuous, 10–100 × 2.5–5 μm. Conidiogenous cells integrated, apical, mono- or polyblastic, proliferating sympodially, with conidiogenous loci thickened, darkened and refractive, 1.5 μm diam. Conidia hyaline, smooth, solitary or catenate, occasionaly in branched chains, long-obclavate, cylindrical to filiform, rounded at the apex when solitary, 17–109 × 2–3.5 μm, 1–7-septate, hila thickened, darkened and refractive, 1.5 μm diam.
Materials examined: Thailand, on Agrostis sp., 15 Sep. 2009, coll. P. Phen, culture CPC 17277. USA, Alabama, Lee County, Auburn, on Panicum dichotomum, 15 Aug. 1891, B.M. Duggar, det. G.F. Atkinson (lectotype designated by Braun et al. 2015: CUP-A-002054#1(AL); isolectotypes CUP-A-2945#2(AL), CUP-A-2945#3(AL)).
Notes: The description of the observed specimen is consistent with the one in literature for the species Cercospora fusimaculans (Ellis 1976). The species Cercospora fusimaculans was recently lectotypified and the species Cercospora agrostidis removed from its synonyms list and tentatively considered a different species. Cercospora fusimaculans, despite the catenate conidia, was tentatively maintained as a Cercospora species (Braun et al. 2015). Phylogenetically, the observed strain forms a single-strain lineage closely related to Ramulispora (Fig. 1, clade 13; Fig. 2, clade 3), but morphologically they are quite distinct from each other (Fig. 11). Therefore, a new genus was introduced to accommodate this species which has a worldwide distribution and affects numerous grass hosts (Poaceae) (Braun et al. 2015). Despite its distribution and host range, it appears to be a mild pathogen susceptible to timely fungicide applications (Smiley 1983).
Clade 14: Neodeightoniella
Neodeightoniella Crous & W.J. Swart, Persoonia 31: 211. 2013.
Description (from Crous et al. 2013b): Foliicolous, plant pathogenic. Conidiophores fasciculate, 3–6, arising from a weakly developed brown stroma composed of a few brown cells, amphigenous. Conidiophores erect, brown, unbranched, finely roughened, straight to slightly flexuous, subcylindrical, septate. Conidiogenous cells terminal and integrated, subcylindrical, brown, finely roughened; conidiogenous loci terminal and lateral on conidiogenous cells, darkened, thickened, protruding, tretic with central pore. Conidia solitary, pale brown, surface finely roughened, fusoid-ellipsoid, straight or gently curved, 1-septate; apical cell globose, with prominent mucoid cap; basal cell funnel-shaped, widest two thirds from basal hilum, tapering prominently to truncate hilum, thickened, darkened, with central pore.
Type species: Neodeightoniella phragmiticola Crous & W.J. Swart.
Neodeightoniella phragmiticola Crous & W.J. Swart, Persoonia 31: 211. 2013.
Description and illustration: Crous et al. (2013b).
Materials examined: South Africa, Free State, Bultfontein, on leaves of Phragmites australis, 31 Jan. 2013, W.J. Swart (holotype CBS H-21427, culture ex-type CBS 136418 = CPC 22059); idem., cultures CPC 22057, CPC 22061.
Notes: Neodeightoniella resembles the genus Deightoniella (based on Deightoniella africana, on Imperata sp., West Africa), in having pale brown, fusoid-ellipsoid, unequally 1-septate conidia arising from brown conidiophores. It is distinct in that conidiophores do not undergo percurrent rejuvenation (seen as nodal swellings in the type of Deightoniella), have prominent apical and lateral conidiogenous loci on the conidiogenous cells, conidia have a prominent mucoid cap, and conidiophores are arranged in fascicles. The genus Deightoniella presently contains a heterogeneous assemblage of taxa, but the type species, Deightoniella africana, probably belongs to the Pyriculariaceae (Klaubauf et al. 2014). Phylogenetically, Neodeightoniella belongs to the Mycosphaerellaceae and is closely related to Ramulispora (Fig. 1, clade 14; Fig. 2, clade 4).
Clade 15: Distocercosporaster
Distocercosporaster Videira, H.D. Shin, C. Nakash. & Crous, gen. nov. MycoBank MB822587.
Etymology: Name composed of the hitherto known genus Distocercospora + -aster (Latin substantival suffix indicating incomplete resemblance).
Description: Foliicolous, plant pathogenic. Mycelium internal, substomatal stromata formed of subhyaline to brown swollen hyphal cells. Conidiophores in small to moderately large fascicles, arising from stromata, through stomata, erect, straight, subcylindrical to geniculate-sinuous, unbranched, pale olivaceous to olivaceous brown, thin-walled, smooth, septate, sometimes reduced to conidiogenous cells. Conidiogenous cells integrated, terminal, with rim-like conidiogenous loci, thickened and darkened. Conidia hyaline to pale olivaceous, thin-walled, smooth to rough, solitary or catenate, in simple or occasionally branched chains, subcylindrical to obclavate-cylindrical, rarely subclavate, apex obtuse, subobtuse to truncate, base short obconically truncate, straight to curved, eu- or distoseptate, hila thickened and darkened.
Type species: Distocercosporaster dioscoreae (Ellis & G. Martin) Videira, H.D. Shin, C. Nakash. & Crous (≡ Cercospora dioscoreae Ellis & G. Martin).
Distocercosporaster dioscoreae (Ellis & G. Martin) Videira, H.D. Shin, C. Nakash. & Crous, comb. nov. MycoBank MB822755. Fig. 12.
Fig. 12.
Distocercosporaster dioscoreae (CPC 11513). A–E. Observations in vivo. A, B. Leaf spot symptoms on the host. C, D. Conidiophores, conidiogenous cells and conidia. E. Catenate conidia. F–L. Observations in vitro. F. Culture on V8. G. Conidiophore and conidiogenous cell. H. Conidiophore, conidiogenous cell, single and catenate conidia. I, J. Catenate conidia. Scale bars = 10 μm.
Basionym: Cercospora dioscoreae Ellis & G. Martin, Amer. Naturalist 16: 1003. 1882.
Synonyms: Phaeoramularia dioscoreae (Ellis & G. Martin) Deighton, More Dematiaceous Hyphomycetes: 319. 1976.
Cercospora nubilosa Ellis & Everh., J. Mycol. 4 (11): 115. 1888.
Cercospora tokoroi Togashi, Bull. Imp. Coll. Agric. (Morioka): 46. 1936.
Passalora dioscoreae (Ellis & G. Martin) U. Braun & Crous, CBS Biodiversity Ser. 1: 162. 2003.
Description in vivo and illustrations: Ellis, 1976, Pons and Sutton, 1988, Guo et al., 2003, Braun et al., 2014.
Description in vitro (on SNA; CPC 11513): Mycelium pale brown to dark brown. Conidiophores micronematous to macronematous, smooth, pale to pale brown, sinuous, irregular in width, 2.5–5(–10) μm, branched. Conidiogenous cells apical, intercalary, polyblastic, proliferating sympodially, often branched, integrated, with thickened and darkened, rim-like conidiogenous loci, 2–2.5 μm diam. Conidia smooth, hyaline to pale brown, single or often catenate, in sigle or branched chains, holoblastic, long-obovoid when single, cylindrical to obclavate when catenate, conical truncate at both ends, straight to strongly sinuous, 12–120 × 3–7.5 μm, 0–5-eu- or distoseptate and occasionally constricted at septa, with hila rim-like, thickened and darkened, 2–2.5 μm diam.
Materials examined: Republic of Korea, on Dioscorea tokoro, 16 Oct. 2003, H.D. Shin, culture CBS 135460 = CPC 10855; on Dioscorea tenuipes, 2003, H.D. Shin, culture CBS 135463 = CPC 11513; on Dioscorea sp., date unknown, H.D. Shin, culture KACC 44723. USA, Pennsylvania, Delaware Co., on Dioscorea villosa, 1 Aug. 1882, W. Trimble (holotype NY 838293, isotype IMI 256891).
Notes: The genus Distocercosporaster is newly introduced to accommodate the species Passalora dioscoreae which is not congeneric with Passalora as defined by the type Passalora bacilligera. The existing strains form a well-supported clade in the phylogenetic analyses (Fig. 1 clade 15; Fig. 2, clade 15). Although the examined strains were collected in the Republic of Korea and the type material is originally from the USA, the observed morphology is consistent with the descriptions found in literature (Braun et al. 2014) and, therefore, these are considered good representatives of this species. Several species of cercosporoid genera have been described from hosts belonging to the plant genus Dioscorea (Crous and Braun, 2003, Braun et al., 2014). The genus Distocercosporaster differs from the genus Distocercospora, in vivo, by forming stromata composed of subhyaline to brown swollen hyphal cells, rather short conidiophores with rim-like and distinctly thickened conidiogenous loci on terminal conidiogenous cells, and frequently catenate conidia.
Clade 16: Caryophylloseptoria
Caryophylloseptoria Verkley et al., Stud. Mycol. 75: 233. 2013.
Description (from Verkley et al. 2013): Conidiomata pycnidial, epiphyllous or predominantly epiphyllous, globose to subglobose, or slightly depressed, with a central ostiolum; wall composed of textura angularis or globulosa-angularis. Conidiogenous cells hyaline, holoblastic, proliferating percurrently one to multiple times with indistinct annellations, or (in addition) proliferating sympodially. Conidia cylindrical, straight, curved or flexuous, multiseptate, not or somewhat constricted around the septa, hyaline, contents with several oil-droplets and granular material in each cell.
Type species: Caryophylloseptoria lychnidis (Desm.) Verkley et al. (≡ Septoria lychnidis Desm.).
Caryophylloseptoria lychnidis (Desm.) Verkley et al., Stud. Mycol. 75: 234. 2013.
Basionym: Septoria lychnidis Desm., Ann. Sci. Nat., Bot., Sér. 3, 11(2): 347. 1849.
For extended synonymy see Shin & Sameva (2004).
Materials examined: Austria, Tirol, Inntal, S of Telfs (W of Innsbruck), along road 171, on living leaves of Silene latifolia subsp. alba (= Melandrium album), 4 Aug. 2000, G. Verkley, CBS H-21161, cultures CBS 109098, CBS 109102; idem., G. Verkley 1048, CBS H-21162, cultures CBS 109099, CBS 109101. Netherlands, Hilversum, on living leaves of Silene dioica (= Melandrium rubrum), 22 Jun. 1985, H.A. van der Aa 9524, CBS H-18112.
Notes: The genus Caryophylloseptoria was recently established to accommodate four septoria-like species infecting hosts belonging to the Caryophyllaceae in Europe and the Republic of Korea (Verkley et al. 2013). The type species, Caryophylloseptoria lychnidis, was originally described from Silene dioica (≡ Lychnis dioica) from France. It has been reported from several species of Silene and the conidial size given by various authors differs considerably (Verkley et al. 2013). In this study, the Caryophylloseptoria strains form a well-supported clade in the phylogeny (Fig. 1, clade 16; Fig. 2 clade 16), closely related to Neoseptoria.
Clade 17: Neoseptoria
Neoseptoria Quaedvlieg et al., Stud. Mycol. 75: 352. 2013.
Description (from Quaedvlieg et al. 2013): Foliicolous. Conidiomata black, immersed, subepidermal, pycnidial, subglobose with central ostiole, exuding creamy conidial mass; wall of 2–3 layers of brown textura angularis. Conidiophores 0–2-septate, subcylindrical, hyaline to pale brown at base, smooth, straight to geniculate-sinuous. Conidiogenous cells phialidic, hyaline, smooth, aggregated, lining the inner cavity, subcylindrical to ampulliform, straight to geniculate-sinuous; proliferating several times percurrently near apex, rarely sympodially. Conidia scolecosporous, hyaline, smooth, flexuous, rarely straight, granular, thin-walled, narrowly obclavate, apex subobtuse, base long obconically truncate, tapering to a truncate hilum, 3- to multi-septate.
Type species: Neoseptoria caricis Quaedvlieg et al.
Neoseptoria caricis Quaedvlieg et al., Stud. Mycol. 75: 352. 2013.
Description and illustration: Quaedvlieg et al. (2013).
Material examined: Netherlands, on leaves of Carex acutiformis, Aug. 2012, W. Quaedvlieg (holotype CBS H-21293, ex-type culture CBS 135097 = S653).
Notes: Neoseptoria is a monotypic genus that is morphologically similar to Septoria but differs in having conidiogenous cells that are mono- to polyphialidic and proliferate percurrently at the apex. In the phylogenetic analyses, it is represented by a single-strain lineage closely related to Caryophylloseptoria (Fig. 1, clade 17; Fig. 2, clade 17).
Clade 18: Acervuloseptoria and Cercosporella
Acervuloseptoria Crous & Jol. Roux, Persoonia 32: 275. 2014.
Description (from Crous et al. 2014a): Plant pathogenic, foliicolous. Conidiomata black, amphigenous, exuding a creamy-white conidial cirrhus, subepidermal, erumpent, multilocular, with upper layer breaking open irregularly and leaving conidioma to have acervular appearance; wall of 3–6 layers of brown textura angularis to textura intricata, basal layers pale brown, roof of conidioma dark brown; in culture conidiomata acervular with elements of conidiomatal roof remaining like brown strands along the sides of conidioma. Conidiophores subcylindrical, straight to once geniculate, pale brown, verruculose, septate, branched or not. Conidiogenous cells terminal and lateral, subcylindrical, pale brown to subhyaline, verruculose to smooth, proliferating sympodially and percurrently. Conidia narrowly obclavate to subcylindrical, flexuous, guttulate, smooth, hyaline, apex subacutely rounded, base obconically truncate, septate.
Type species: Acervuloseptoria ziziphicola Crous & Jol. Roux.
Acervuloseptoria ziziphicola Crous & Jol. Roux, Persoonia 32: 275. 2014.
Description and illustration: Crous et al. (2014a).
Materials examined: South Africa, Northern Cape, Richtersveld National Park, Potjiespram Rest Camp, on leaf spots of Ziziphus mucronata, Sep. 2013, J. Roux (holotype CBS H-21723, culture ex-type CPC 23707 = CBS 138009).
Notes: Acervuloseptoria differs from Septoria and allied genera (Quaedvlieg et al. 2013) in the peculiar conidiomatal morphology, with black, erumpent conidiomata, from which the top layer disintegrates, leaving a conidiomatal body that appears acervular (Crous et al., 2014a, Crous et al., 2015c). The conidiophores are also slightly pigmented and verruculose in their lower part. Phylogenetically, Acervuloseptoria is represented by a single-strain lineage that is closely related to Cercosporella and Ramulariopsis (Fig. 1, clade 18; Fig. 2, clade 18). However, its phylogenetic position is not yet clear since it clustered near Cercosporella in dataset 1 (Fig. 1, clade 18) but clustered among the Cercosporella species when using dataset 2 (Fig. 2, clade 18). In the single-gene Bayesian trees of dataset 2 (data not shown), Acervuloseptoria ziziphicola clusters outside both the Cercosporella and the Ramulariopsis clade with high posterior probability value for LSU (PP = 0.94), with a low support in the case of ITS (PP = 0.54). In the single-gene Bayesian tree of rpb2, Acervuloseptoria ziziphicola sits in a highly supported polytomy (PP = 0.84) including the Cercosporella strains. In both the RAxML and PAUP analyses of dataset 2, Acervuloseptoria ziziphicola appears as a single-strain lineage sister to both Cercosporella and Ramulariopsis. The genus Acervuloseptoria currently includes an additional species, Acervuloseptoria capensis (Crous et al. 2015c). The differences in morphology are significant enough for retaining Acervuloseptoria (a coelomycete) as distinct from Cercosporella (a hyphomycete), pending further collections. The situation of Acervuloseptoria ziziphicola is reminiscent of Pseudocercospora pistacina, which after much debate was placed in the genus Pseudocercospora, although it had pycnidial conidiomata (Crous et al. 2013a).
Cercosporella Sacc., Michelia 2(6): 20. 1880.
Description (from Videira et al. 2016): Phytopathogenic, mostly causing leaf spots. Hyphae restricted to intercellular spaces and forming cup- or bowl-shaped appressoria, 7–17 μm diam that attach to walls of mesophyll cells. Conidiophores emerging through stomata or erumpent through the cuticle, straight, subcylindrical to geniculate-sinuous, hyaline, sometimes lightly pigmented near the base, more or less thin-walled and smooth. Conidiogenous cells integrated, terminal, polyblastic, sympodial, mostly conspicuously geniculate, conidiogenous loci conspicuous, hyaline but refractive, thickened and raised in the shape of a truncated cone (ultrastructure). Conidia formed singly, hyaline, subcylindrical to obclavate, sometimes fusiform, 1- to multi-septate, usually thin-walled and smooth, apex obtuse, base often rounded to truncate or obconically truncate, hilum thickened, not darkened but refractive. Description adapted from Braun (1995) and Kirschner (2009).
Type species: Cercosporella virgaureae (Thüm.) Allesch (≡ Ramularia virgaureae Thüm.).
Cercosporella virgaureae (Thüm.) Allesch., Hedwigia 34: 286. 1895.
Basionym: Ramularia virgaureae Thüm., Fungi Austr. Exs., Cent. 11: no. 1072. 1874.
Synonyms: Cylindrosporium virgaureae (Thüm.) J. Schröt., in Cohn, Krypt.-Fl. Schles. 3: 489. 1897.
Cercosporella cana (Sacc.) Sacc., Michelia 2(6): 20. 1880.
For additional synonymy see Braun (1995) or MycoBank.
Descriptions and illustrations: Braun, 1995, Kirschner, 2009, Videira et al., 2016
Materials examined: Austria, Krems, on Solidago virgaurea, 1871 [Thüm., Fungi Austr. Exs. 1072] (lectotype K, designated by Deighton 1973). Brazil, Guimarania, Minas Gerais, on Conyza canadensis, unknown date, B.S. Vieira, culture CPC 19492. Republic of Korea, Jinju, on Erigeron annuus, 1 Jul. 2004, H.D. Shin, cultures CPC 11456, CPC 11457, CPC 11460, CPC 11461; Namyangju, on Erigeron annuus, 9 Oct. 2002, H.D. Shin, cultures CPC 10286–10288; Chuncheon, on Erigeron annuus, 21 May 2003, H.D. Shin, culture CBS 113304.
Notes: The taxonomic confusion between Cercosporella and Ramularia has been addressed by several authors (Braun, 1995, Braun, 1998, Kirschner, 2009, Videira et al., 2016). Cercosporella and Ramularia are phylogenetically distinct since the LSU sequences of freshly collected isolates of the type species of both genera clustered separately (Kirschner 2009). A later study using both LSU and rpb2 sequences corroborated these results (Videira et al. 2016). Morphologically, Cercosporella can be distinguished from Ramularia by forming an appressorium structure to adhere to the plant cells and by having a distinct ultrastructure of conidiogenous scars that is flat like a truncate cone. The type species, Cercosporella virgaureae, was described from the host Solidago virgaureae collected in Austria. Although the currently available strains of this species are of Brazilean and Korean origin, their morphology is identical to the descriptions available in literature (Braun 1995) and their LSU sequence is 100 % identical to that of a freshly collected isolate of Cercosporella virgaureae from Germany (GenBank EU710894) (Kirschner 2009). Two new species of Cercosporella have recently been introduced, namely Cercosporella dolichandrae (Crous et al. 2014a) and Cercosporella catenulata (Videira et al. 2016), that cluster together with Cercosporella virgaureae (Fig. 1, clade 18; Fig. 2, clade 18).
Clade 19: Ramulariopsis
Ramulariopsis Speg., Anales Mus. Nac. Buenos Aires 20(13): 421 [ser. 3, 13]. 1910.
Description (from Braun 1998): Parasitic on vascular plants, foliicolous, usually forming leaf spots. Mycelium internal, septate, branched, hyaline or almost so, smooth; stromata absent to well-developed, immersed, hyaline to faintly pigmented. Caespituli amphigenous, whitish. Conidiophores macronematous, mononematous, fasciculate, arising from internal hyphae or stromata, through stomata or erumpent, hyaline, septate, smooth, simple or branched. Conidiogenous cells integrated, terminal, intercalary as well as pleurogenous (as short nodulose protuberances or subcylindrical branchlets), polyblastic, sympodial, with cicatrized, thickened and darkened loci. Conidia catenate, in simple as well as branched chains, ellipsoid-ovoid, subcylindrical-fusiform, 0–1- to multi-euseptate, hyaline, with thickened and darkened hila. Conidial secession schizolytic.
Type species: Ramulariopsis cnidoscoli Speg.
Ramulariopsis cnidoscoli Speg., Anales Mus. Nac. Buenos Aires 20: 422. 1911.
Descriptions and illustrations: Braun, 1998, Videira et al., 2016.
Material examined: Argentina, Salta, Orán, on Cnidoscolus vitifolius var. cnicodendron, Apr. 1905, C. Spegazzini (lectotype, designated by Deighton, 1972, LPS 12.851).
Notes: Ramulariopsis was described by Spegazzini (1910) and emended by Deighton (1972). Ramulariopsis differs from Ramularia by producing conidiophores that are frequently branched and conidiogenous cells that are often intercalary or pleurogenous. The type species, Ramulariopsis cnidoscoli, was collected on Cnidoscolus vitifolius in Argentina, and is thus far only known from herbarium material. Five species are currently recognised in this genus (Braun 1998) but only two are known from culture, namely Ramulariopsis gossypii and Ramulariopsis pseudoglycines (Videira et al. 2016). Phylogenetically, these two species cluster in a well-supported clade closely related to Cercosporella (Fig. 1, clade 19; Fig. 2, clade 19). Unfortunatelly, it is still unproven whether Ramulariopsis gossypii is congeneric with Ramulariopsis cnidoscoli. Morphologically, there are slight differences in the structure of the conidiogenous loci between the two species: the loci in Ramulariopsis gossypii are conspicuously thickened and darkened, whereas in Ramulariopsis cnidoscoli these structures are less conspicuous.
Clade 20: Pleuropassalora
Pleuropassalora U. Braun, C. Nakash., Videira & Crous, gen. nov. MycoBank MB822610.
Etymology: Derived from the sporulating arrangement, pleurosporous + its resembling genus, Passalora.
Description: Phytopathogenic. Mycelium internal, smooth, branched, pale brown. Caespituli hypophyllous, fasciculate to synnematous, arising from a pale brown stroma. Conidiophores subcylindrical, unbranched, flexuous, guttulate, pale to medium brown, smooth, septate. Conidiogenous cells terminal, subcylindrical, guttulate, pale to medium brown, finely verruculose, becoming slightly swollen, appearing clavate, with multiple conidiogenous loci, round, darkened, thickened, refractive, prominent, proliferation sympodial. Conidia solitary, pale to medium brown, smooth to finely verruculose, granular to guttulate, thin-walled, ellipsoidal to obovoid, obpyriform, wider basal cell and apical cell elongating into a beak, transversely multiseptate, hilum thickened, darkened and refractive.
Type species: Pleuropassalora armatae (Crous & A.R. Wood) U. Braun et al. (≡ Passalora armatae Crous & A.R. Wood).
Pleuropassalora armatae (Crous & A.R. Wood) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822777.
Basionym: Passalora armatae Crous & A.R. Wood, Stud. Mycol. 64: 35. 2009.
Description in vivo and illustrations: Crous et al. (2009c).
Description in vitro (on SNA; CBS 125420): Mycelium composed of hyaline to pale hyphae, uniform in width, 2–2.5 μm. Conidiophores semimacronematous to macronematous, pale brown to pale olivaceous brown, multiseptate, straight or mildly curved, smooth, 200–740 × 3.8–7.5 μm. Conidiogenous cells integrated, terminal, cylindrical, straight or slightly curved, polyblastic, proliferating sympodially without geniculation, with numerous lateral conidiogenous loci, rim-like, thickened and darkened, 2–2.5 μm. Conidia subhyaline to pale brown, holoblastic, solitary, acropleurogenous, obpyriform with a beak-shape at the apex, obclavate or ellipsoidal, 25–45 × 10–12.5 μm, 1–3-euseptate, not constricted at septa, with distinctly protuberant, thickened, and refractive hilum, 2–2.5 μm diam.
Materials examined: South Africa, KwaZulu-Natal Province, South Coast, Mpenjati Nature Reserve, between Ramsgate and Port Edward, on leaves of Dalbergia armata, 28 May 2008, A.R. Wood (holotype CBS H-20337, culture ex-type CBS 125420 = CPC 15419); idem. cultures CPC 15420, CPC 15421; Kloof Nature Reserve area, on Dalbergia obovata, 15 Jun. 2009, A. Wood, herb. 7/7/2009 (4), culture CPC 17084.
Notes: This genus is proposed in order to accommodate the species Passalora armatae that is not congeneric with Passalora as defined by the type Passalora bacilligera. Pleuropassalora is a monotypic genus that forms a well-supported clade in this study (Fig. 1, clade 20; Fig. 2, clade 20). At the time it was described (Crous et al. 2009c), it was observed that, when in culture, conidia remain attached to conidiogenous cells, giving conidiophores the appearance of small tufts which is very characteristic, but not observed in Passalora s. str.
Clade 21: Graminopassalora
Graminopassalora U. Braun, C. Nakash., Videira & Crous, gen. nov. MycoBank MB822591.
Etymology: Derived from the host family (Poaceae = Gramineae) and similarity to the genus Passalora.
Description: Plant pathogenic, causing leaf spotting symptoms. Mycelium internal, forming stromata of variable shape and size, usually well-developed, substomatal to immersed, brown. Conidiophores in small to very large fascicles, arising from stromata, through stomata or erumpent, erect, subcylindrical, straight to curved, sinuous, slightly geniculate, unbranched, septate, pale to dark brown, thin-walled, smooth to rough-walled, sometimes reduced to conidiogenous cells. Conidiogenous cells integrated, terminal, with a single to several conspicuous conidiogenous loci, circular in outline, thickened and darkened, usually barely protuberant. Conidia formed singly, ellipsoid-ovoid, obovoid, short obclavate, 0–3-septate, occasionally slightly constricted at the septa, subhyaline to pale brownish, thin-walled, smooth to rough-walled, hila rounded, thickened and darkened.
Type species: Graminopassalora graminis (Fuckel) U. Braun, C. Nakash., Videira & Crous (≡ Scolicotrichum graminis Fuckel).
Graminopassalora graminis (Fuckel) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822760. Fig. 13.
Fig. 13.
Graminopassalora graminis (CBS 113303). A–D. Observations in vivo. A, B. Leaf spot symptoms on the host. C. Conidiophores. D. Conidia. Scale bars = 10 μm.
Basionym: Scolicotrichum graminis Fuckel, Hedwigia 2(15): 134. 1863.
Synonym: Passalora graminis (Fuckel) Höhn., Zentrabl. Bakteriol., 2. Abt., 60: 6. 1923.
For additional synonyms see Braun et al. (2015).
Description and illustrations: Braun et al. (2015).
Materials examined: Germany, Rheinland-Pfalz: Mt. Rabenkopf, on grass leaves (exact identity unclear), Fuckel, Fungi Rhen. Exs. 130 (lectotype designated by Braun et al. 2015, HAL; isolectotypes: Fuckel, Fungi Rhen. Exs. 130, e.g. FH, G). Japan, Chiba, on Dactylis glomerata, N. Nishihara, culture MAFF510604 = MUCC 1429. Republic of Korea, Yangyang, on Alopecurus aequalis var. amurensis, 24 May 2003, H.D. Shin, culture CBS 113303.
Notes: The genus Graminopassalora is newly introduced to accommodate Passalora graminis, which is not congeneric with the type of Passalora as defined by the type Passalora bacilligera (Fig. 1, clade 35; Fig. 2, clade 22). The lectotype of Passalora graminis was described from grass leaves of uncertain identity originating from Germany. Passalora graminis is considered a widespread pathogen able to infect a wide range of grass hosts (Poaceae). The existing collections on various hosts are morphologically uniform suggesting this is a single plurivorous species, but more detailed analyses including an ex-type strain are required to ascertain this hypothesis. In this study, two Asian isolates isolated from different hosts were also analysed. Their morphology is identical to the description available in literature (Braun et al. 2015) and they are deemed as good representatives of this species. These isolates formed a well-supported clade by all three phylogenetic methods employed (Fig. 1, clade 21; Fig. 2, clade 21).
Clade 22: Pallidocercospora
Pallidocercospora Crous, Stud. Mycol. 75: 73. 2013.
Description (from Crous et al. 2013a): Foliicolous, phytopathogenic, causing discrete leaf spots. Ascomata single, black, immersed, globose, glabrous; wall of 3–4 layers of medium brown textura angularis. Asci fasciculate, bitunicate, aparaphysate, subsessile, 8-spored, ellipsoid to obclavate or cylindrical, straight or curved, numerous. Ascospores 2- to multi-seriate, oblique, overlapping, straight ellipsoidal to obovoid, hyaline, smooth, 1-septate. Mycelium predominantly immersed, consisting of olivaceous brown hyphae, smooth, branched, septate, 2–4 μm diam. Conidiophores in vivo fasciculate, or occurring singly on superficial mycelium as lateral projections, unbranched or branched, septate, cylindrical, straight to geniculate-sinuous, olivaceous brown. Conidiogenous cells integrated, terminal, cylindrical, straight to geniculate-sinuous, olivaceous brown, proliferating sympodially or percurrently, with unthickened loci, not darker than the surrounding conidiogenous cell. Conidia solitary, straight to irregularly curved, guttulate, pale olivaceous to olivaceous brown, subcylindrical to narrowly obclavate, multi-septate; hila neither thickened nor darkened.
Type species: Pallidocercospora heimii (Crous) Crous (≡ Pseudocercospora heimii Crous).
Pallidocercospora heimii (Crous) Crous, Stud. Mycol. 75: 74. 2013.
Basionym: Pseudocercospora heimii Crous, S. African Forest. J. 172: 4. 1995.
Synonyms: Mycosphaerella heimii Crous, S. African Forest. J. 172: 2. 1995.
Mycosphaerella heimii Bouriquet, Encycl. Mycol. 12: 418. 1946, nom. nud.
Description and illustration: Crous et al. (2013a).
Materials examined: Brazil, Bahia, Teixeira de Freitas, on leaves of Eucalyptus sp., 2004, A.C. Alfenas, culture CPC 11716. Madagascar, Moramanga, on leaves of Eucalyptus sp., 16 Apr. 1994, P.W. Crous (PREM 51749, holotype of sexual morph; PREM 51748, holotype of asexual morph, cultures ex-type CPC 760–761 = CBS 110682).
Notes: The genus Pallidocercospora was established to accommodate the species previously belonging to the Mycosphaerella heimii complex. Pallidocercospora species are morphologically similar to Pseudocercospora s. str. but can be distinguished by the pale olivaceous and smooth conidia and the red crystals they form when cultivated in agar (Crous et al. 2013a). The strains used in this study clustered in a well-supported clade by all three phylogenetic methods employed (Fig. 1, clade 22; Fig. 2, clade 28). At the time this genus was introduced (Crous et al. 2013a), the authors observed two pseudocercospora-like species clustering in the same clade, namely Pseudocercospora thailandica (foliar pathogen of Acacia; Crous et al. 2004c) and Pseudocercospora colombiensis (foliar pathogen of Eucalyptus; Crous 1998), also with mycosphaerella-like sexual morphs. Morphologically, Pseudocercospora thailandica and Pseudocercospora colombiensis were indistinguishable from Pseudocercospora species. In that study (Crous et al. 2013a), the multigene phylogeny strongly supported the clade that included Pallidocercospora, Trochophora, Scolecostigmina and the two mentioned species, but poorly supported their separation, despite their strikingly different morphologies. Based on the morphological differences and poor phylogenetic support, the authors refrained from proposing a formal combination of Pseudocercospora thailandica and Pseudocercospora colombiensis into Pallidocercospora at the time (Crous et al. 2013a). In a recent study, a formal proposal for the combination of these two species into Pallidocercospora was presented on the basis of a multigene phylogeny based on a LSU and ITS alignment (Hyde et al. 2016). In this study, with the introduction of a wider range of species sequences and the rpb2 gene, we find good support for the separation of these two species into their own clade.
Clade 23: Nothophaeocryptopus
Nothophaeocryptopus Videira, C. Nakash. & Crous, gen. nov. MycoBank MB822698.
Etymology: From the greek notho-, meaning false, and the similarity to the genus Phaeocryptopus.
Description: Phytopathogenic. Mycelium internal and superficial, pseudothecia, internal and superficial, emerging through stomata on the lower surface of leaves, black. Ascospores hyaline, ellipsoidal with obtuse ends, 1-septate, slightly constricted at the septa, the basal cell slightly narrower and tapering toward its base. Germinating ascospores develop germ hyphae from polar ends of both cells.
Type species: Nothophaeocryptopus gaeumannii (T. Rohde) Videira et al. (≡ Adelopus gaeumannii T. Rohde).
Nothophaeocryptopus gaeumannii (T. Rohde) Videira, C. Nakash., U. Braun & Crous, comb. nov. MycoBank MB822768.
Basionym: Adelopus gaeumannii T. Rohde, Silva: 51. 1936.
Synonyms: Adelopus balsamicola f. douglasii J. Steiner, Z. Pflanzenkrankh. 47: 184. 1937.
Phaeocryptopus gaeumannii (T. Rohde) Petr., Ann. Mycol. 36(1): 22. 1938.
Description (adapted from Stone et al. 2008): Ascomata pseudothecial, internal, emerging from stomata, on the lower surface of living leaves and dead leaves, less than 0.1 mm diam, black. Superficial, radiating hyphae emerging from developing ascocarps, spreading across the needle surface and re-entering the needle through unoccupied stomata. Ascospores hyaline, ellipsoidal with obtuse ends, 1-septate, slightly constricted at the septa, the basal cell slightly narrower and tapering toward its base, 11–17 × 4–5 μm. Germinating ascospores develop germ hyphae from polar ends of both cells. Germinating hyphae initially hyaline, becoming pale olive brown when up to 20 μm long, then becoming dark brown to black.
Materials examined: Austria, unknown host, date and collector, isol. H. Steiner, dep. in 1938, culture CBS 244.38. Germany, on needles of Pseudotsuga menziesii, unknown date and collector, isol. T. Rhode, deposited in 1937 (lectotype designated here, MBT378568, preserved as metabolically inactive culture CBS 267.37).
Notes: The genus Nothophaeocryptopus is introduced to accommodate the species Phaeocryptopus gaeumannii which is not congeneric with the type of Phaeocryptopus, Phaeocryptopus nudus (Dothideales). The systematic position of Phaeocryptopus gaeumannii was originally determined based on a phylogeny of combined LSU and SSU sequences that placed it within the Mycosphaerellaceae (Capnodiales), followed by a phylogeny of ITS sequences that placed it in the Mycosphaerella heimii complex (Winton et al. 2007). In this study, the phylogenetic results agreed with the previous results of Winton et al. (2007), placing this species in a well-supported clade (Fig. 1, clade 23; Fig. 2, clade 29), closely related with Pallidocercospora. Nothophaeocryptopus gaeumannii is the causal agent of Swiss needle cast disease on Pseudotsuga menziesii (Douglas-fir). The disease symptoms include severe defoliation that leads to reduced height and diameter growth. Publications by Rohde (1937) and Steiner (1937) provided the first insights into the pathogen life-cycle and how it differed from Phaeocryptopus nudus. Although Nothophaeocryptopus gaeumannii grows well on artificial culture media, it behaves as an obligate parasite, reproducing only on living needles of Pseudotsuga menziezii, and no asexual morph has been observed thus far (Stone et al. 2008). Isolates of Nothophaeocryptopus gaeumannii also have been observed to produce diffusing red pigments in culture (Winton et al. 2007), which is a feature also observed in Pallidocercospora.
Clade 24: Scolecostigmina
Scolecostigmina U. Braun, New Zealand J. Bot. 37: 323. 1999.
Description (from Braun et al. 1999): Foliicolous, phytopathogenic, associated with leaf spots. Mycelium immersed, consisting of septate, branched, pigmented hyphae. Sporodochia immersed to erumpent; stromata subglobose to applanate, composed of brown, angular to subglobose cells. Conidiophores numerous, densely aggregated, arising from a stroma, subcylindrical or somewhat tapered towards the apex, occasionally ampulliform, continuous or septate, pigmented, wall somewhat thickened, usually verruculose. Conidiogenous cells integrated, terminal or at times conidiophores reduced to conidiogenous cells, holoblastic, proliferating percurrently via conspicuous annellations. Conidia solitary, scolecosporous, usually subcylindrical-obclavate, transversely pluriseptate, occasionally with few longitudinal or oblique septa, euseptate, rarely with few intermixed distosepta, thick-walled, pigmented, dark, smooth to verrucose, apex obtuse to subacute, base truncate or obconically truncate, hila unthickened, not darkened; secession schizolytic.
Type species: Scolecostigmina mangiferae (Koord.) U. Braun & Mouch. (≡ Cercospora mangiferae Koord.).
Scolecostigmina mangiferae (Koord.) U. Braun & Mouch., New Zealand J. Bot. 37: 323. 1999.
Basionym: Cercospora mangiferae Koord., Verh. Kon. Ned. Akad. Wetensch., Afd. Natuurk., Tweede Sect. 13(4): 236. 1907.
Synonyms: Stigmina mangiferae (Koord.) M.B. Ellis, Mycol. Pap. 72: 49. 1959.
Sciniatosporium mangiferae (Koord.) Morgan-Jones, Canad. J. Bot. 49: 999. 1971.
Descriptions and illustrations: Ellis, 1959, Crous et al., 2013a.
Materials examined: Australia, Queensland, Mareeba, S16°58′75.5″ E145°20′60.8″ on leaves of Mangifera indica, 10 Aug. 2009, P.W. Crous & R.G. Shivas (neotype designated here CBS H-20846, MBT378567, ex-neotype culture CBS 125467 = CPC 17351); idem. CPC 17352. New Caledonia, Port Laguerre (Ec. Agr.), on Mangifera indica, 20 Nov. 1959, Bugnicourt, NC 59.061 a, b (PC).
Notes: The genus Scolecostigmina was introduced by Braun et al. (1999) to accommodate foliicolous stigmina-like hyphomycetes such as the type species Scolecostigmina mangiferae, characterised by producing sporodochial conidiomata with firm stromata, verruculose conidiophores and conidiogenous cells with conspicuous coarse annellations and scolecosporous, pluriseptate, thick-walled conidia. The type material of Cercospora mangiferae could not be traced (Indonesia, Java, on leaves of Mangifera indica, 21 Sep. 1905; Koorders 1907), but various other collections have been examined (Braun et al. 1999). Therefore, we propose the specimen CBS H-20846 as neotype and the strain CBS 125467 = CPC 17351 as ex-neotype culture. In this study, Scolecostigmina is represented by a single-strain lineage in the phylogenetic analysis (Fig. 1, clade 24; Fig. 2, clade 30) and is closely related to Trochophora and Pallidocercospora. Numerous other morphologically similar species assigned to Scolecostigmina are hitherto not known in culture and the affinity of the species concerned to Scolecostigmina mangiferae remains to be proven. Therefore, they are currently only tentatively retained in Scolecostigmina.
Clade 25: Parapallidocercospora
Parapallidocercospora Videira, Crous, U. Braun, C. Nakash., gen. nov. MycoBank MB822604.
Etymology: Similar to the genus Pallidocercospora.
Description: Plant pathogenic. Leaf spots amphigenous, irregular to subcircular. Ascomata pseudothecial, predominantly hypophyllous, black, subglobose to globose, with apical ostiole, walls of 2–3 layers of medium brown textura angularis. Asci fasciculate, bitunicate, subsessile, cylindrical to narrowly ellipsoidal, straight or slightly incurved. Ascospores bi- to multiseriate, overlapping, hyaline, guttulate, thin-walled, straight to slightly curved, fusoid-ellipsoidal, obovoid, medianly 1-septate, not constricted at septum or only slightly constricted, tapering toward both ends but more prominently toward the base. Spermogonia intermixed with the ascomata or with the asexual morph, hyaline and rod-shaped. Mycelium internal and external, hyphae light brown, septate, branched, smooth. Conidiophores arising from superficial mycelium, from the upper cells of a brown stroma; conidiophores light brown, smooth, aseptate or septate, subcylindrical, straight to variously curved, unbranched. Conidiogenous cells terminal, unbranched, light brown, smooth, tapering to flat-tipped apical loci, proliferating sympodially, rarely percurrently near apex. Conidia solitary, light brown, smooth to finely verruculose, septate, guttulate, narrowly obclavate or subcylindrical, tapering towards the base, straight to curved.
Type species: Parapallidocercospora colombiensis (Crous et al.) Videira & Crous (≡ Pseudocercospora colombiensis Crous & M.J. Wingf.).
Parapallidocercospora colombiensis (Crous & M.J. Wingf.) Videira & Crous, comb. nov. MycoBank MB822774.
Basionym: Pseudocercospora colombiensis Crous & M.J. Wingf., Mycol. Mem. 21: 42. 1998.
Synonym: Mycosphaerella colombiensis Crous & M.J. Wingf., Mycol. Mem. 21: 41. 1998.
Description and illustration: Crous (1998).
Materials examined: Colombia, Pinal Farm, on leaves of Eucalyptus urophylla, May 1995, M.J. Wingfield (holotype PREM 54397, ex-type culture CBS 110968 = CPC 1105).
Notes: The genus Parapallidocercospora is hereby introduced in order to accommodate two species, Pseudocercospora colombiensis (foliar pathogen of Eucalyptus; Crous 1998), and Pseudocercospora thailandica (foliar pathogen of Acacia; Crous et al. 2004c). Morphologically, these taxa appear typical members of Pseudocercospora s. str. and are difficult to identify without the use of DNA sequence data. In this study both species clustered in a well-supported clade in the phylogenetic analyses (Fig. 1, clade 25; Fig. 2, clade 31) and are closely related to Pallidocercospora, Scolecostigmina and Trochophora.
Parapallidocercospora thailandica (Crous, Himaman & M.J. Wingfield) Videira & Crous, comb. nov. MycoBank MB822775.
Basionym: Mycosphaerella thailandica Crous et al., Stud. Mycol. 50: 465. 2004.
Synonyms: Pseudocercospora thailandica Crous et al., Stud. Mycol. 50: 465. 2004.
Pallidocercospora thailandica (Crous et al.) Phook. et al., Fungal Diversity 80: 21. 2016.
Descriptions and illustrations: Crous et al., 2004c, Hyde et al., 2016.
Materials examined: Thailand, Chachoengsao Prov., Sanamchaikhet, on leaves of Acacia mangium, 28 May 2003, K. Pongpanich (holotype CBS H-9875, of both M. thailandica and P. thailandica, cultures ex-type CBS 116367 = CPC10547–10549); Thatakiab District, on living leaves of Eucalyptus camaldulensis, Oct. 2006, W. Himaman, culture CBS 120723 = CPC 13478.
Note: See notes on Parapallidocercospora colombiensis and Pallidocercospora.
Clade 26: Trochophora
Trochophora R.T. Moore, Mycologia 47: 90. 1955.
Description (from Crous et al. 2013a): Foliicolous, but pathogenicity unproven. Colonies hypophyllous, medium to dark brown, consisting of fasciculate conidiophores or numerous synnemata. Stroma absent, but with a superficial network of hyphae linking the various synnemata. Conidiophores fasciculate to synnematous, mostly unbranched and straight, or with 1–2 short branches, straight or curved, cylindrical, individual conidiophores tightly aggregated, but separating near the apex, pale to medium brown, smooth. Conidiogenous cells polyblastic, integrated, terminal, determinate to sympodial, with visible unthickened loci, clavate. Conidia solitary, terminal or lateral on conidiogenous cells, prominently curved to helicoid, pale to medium brown, smooth, transversely euseptate with a darkened, thickened band at the septa.
Type species: Trochophora fasciculata (Berk. & M.A. Curtis) Goos (≡ Helicoma fasciculatum Berk. & M.A. Curtis).
Trochophora fasciculata (Berk. & M.A. Curtis) Goos, Mycologia 78: 759. 1986.
Basionym: Helicoma fasciculatum Berk. & M.A. Curtis, Proc. Amer. Acad. Arts Sci. 4: 127. 1858.
Synonyms: Helicosporium fasciculatum (Berk. & M.A. Curtis) Sacc., Syll. Fung. 4: 560. 1886.
Helicomyces fasciculatus (Berk. & M.A. Curtis) Pound & Clem., Minn. Bot. Stud. 9: 658. 1896.
Helicostilbe simplex Petch, Ann. Royal Bot. Gard. Peradeniya 7: 321. 1922.
Trochophora simplex (Petch) R.T. Moore, Mycologia 47: 90. 1955.
Description and illustrations: Ellis, 1971, Zhao et al., 2007, Crous et al., 2013a.
Materials examined: India, Sri Lanka, on Daphniphyllum glaucescens, collector unknown, Apr. 1917 (holotype of Helicostilbe simplex, IMI 87262). Japan, under side of dead leaves, date unknown, C. Wright 142 (holotype of Helicoma fasciculatum, NY 00945981); Shimane, Matsue, on Daphniphyllum macropodum, 26 Apr. 2008, C. Nakashima & I. Araki (epitype designated here TSU MUMH11134, MBT377074, ex-epitype culture MUCC 952). Republic of Korea, Jeju, Halla arboretum, on leaves of Daphniphyllum macropodum, 29 Oct. 2005, H.D. Shin, KACC 42362 = CBS H-20847, culture CBS 124744 = SMKC 21713; Pusan, on leaves of Daphniphyllum macropodum, 13 Nov. 2002, H.D. Shin, KUS-F19414, cultures CPC 10280–10282.
Notes: The genus Trochophora is currently monotypic based on Trochophora fasciculata, a pathogen of Daphniphyllum shrubs and trees in several Asian countries (Zhao et al. 2007). Based on the LSU sequence, the phylogenetic position has been shown to be closely related to Pallidocercospora and Scolecostigmina (Crous et al. 2013a). The phylogenetic results in this study, with the addition of rpb2 and ITS sequences, agreed with the previous observations (Fig. 1, clade 26; Fig. 2, clade 32). Despite the low support, the distinctive morphology observed in Trochophora justifies that it is retained as separate, pending more collections to be added to this clade.
Clade 27: Pseudophaeophleospora
Pseudophaeophleospora C. Nakash., Videira & Crous, gen. nov. MycoBank MB822700.
Etymology: Composed of ‘pseudo’ (resembling but not equalling) + the similar genus, Phaeophleospora.
Description (adapted from Crous et al. 2007c and Wu et al. 1996): Phytopathogenic. Conidiomata amphigenous, globose, wall with up to four layers of dark brown textura angularis, subepidermal, scattered, rarely aggregated, with a central ostiole from where conidia exude in a cirrhus. Conidiophores absent or reduced to only two cells. Conidiogenous cells pale brown, smooth to finely verruculose, ampulliform to doliiform, subcylindrical, proliferating percurrently near apex. Conidia formed singly, pale to dark brown, smooth to slightly verruculose, guttulate, subcylindrical to narrowly obclavate, slightly fusiform, straight, multiseptate, with apical cell tapering into an obtuse apex, widest at basal septum and tapering to a subtruncate base, hilum flattened with minute marginal frill.
Type species: Pseudophaeophleospora stonei (Crous) C. Nakash. et al. (≡ Phaeophleospora stonei Crous).
Pseudophaeophleospora atkinsonii (Syd.) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822781.
Basionym: Scoleciasis atkinsonii Syd., Annls. Mycol. 22(3–6): 312. 1924.
Synonyms: Phaeophleospora atkinsonii (Syd.) Pennycook & McKenzie, Mycotaxon 82: 145. 2002.
Septoria exotica sensu Grove, Brit. Leaf-fung. 1: 415. 1935.
Kirramyces hebes W.P. Wu, B. Sutton & Gange, Mycol. Res. 100: 1208. 1996.
Phaeophleospora hebes (W.P. Wu, B. Sutton & Gange) Crous, F.A. Ferreira & B. Sutton, S. Afr. J. Bot. 63: 113. 1997.
Description and illustration: Wu et al. (1996).
Materials examined: New Zealand, Wellington, York Bay, on Hebe stricta var. atkinsonii, Oct. 1920, E.H. Atkinson (holotype PDD 968); St Johns, Morrin Road, Auckland University Campus, on Hebe sp., unkown date and collector, isol. C.F. Hill, 27 Jan. 2009, PDD 95173, cultures ICMP 17860 = CBS 124565; Grey Lynn, Western Springs Park, Jan. 2007, C.F. Hill, PDD 95176, culture ICMP 17862 = CBS 124566.
Notes: Despite the repeated attempts to induce the available cultures to sporulate on different types of agar medium, no reproductive structures characteristic of this species were formed. This species is transferred to Pseudophaeophleospora based on phylogenetic inference (Fig. 1, clade 27; Fig. 2, clade 33). According to Wu et al. (1996), the conidiophores are reduced to conidiogenous cells that are pale brown, and the conidia are obclavate to cylindrical, which correlate with the type species of Pseudophaeophleospora.
Pseudophaeophleospora stonei (Crous) C. Nakash., Videira & Crous, comb. nov. MycoBank MB822782.
Basionym: Phaeophleospora stonei Crous, Fungal Diversity 26: 169. 2007.
Description and illustration: Crous et al. (2007c).
Description in vitro (on V8; CBS 13330): Mycelium composed of hyaline to pale blackish brown hyphae, uniform in width, 2–2.5 μm diam. Conidiomata absent. Conidiophores micronematous to macronematous, emerging from hyphae, sometimes reduced to conidiogenous cells, pale blackish brown, 10–25 × 2.5–3.8 μm. Conidiogenous cells apical, intercalary, integrated, sometimes reduced to hyphae, proliferating percurrently, with unthickened loci, 2–2.5 μm diam. Conidia solitary, pale blackish brown, smooth, holoblastic, schizolytic, cylindrical to short obclavate, rounded at the apex, 15–32.5 × 3.5–7.5 μm, 1–4-septate, with unthickened and truncate hilum at the base.
Materials examined: Australia, Queensland, Cairns, Kuranda, Karoomba River Walk, S 16° 49′ 08.8″, E 145° 38′ 24.7″, on leaves of Eucalyptus sp., 19 Aug. 2006, P.W. Crous & J. Stone (holotype CBS H-19835, culture ex-type CBS 120830 = CPC 13330); idem. CPC 13331, CPC 13332.
Notes: The genus Phaeophloeospora, based on the ITS sequence of its type species Phaeophloeospora eugeniae, belongs to Mycosphaerellaceae (Crous et al., 2001a, Crous et al., 2001b). Since the ITS sequence of Phaeophloeospora stonei did not cluster with the type of Phaeophleospora, the genus was considered polyphyletic (Crous et al. 2007c). In the present study, the phylogenetic analysis performed based on the sequences of LSU, rpb2 and ITS agrees with the previous work and the strain of Pseudophaeophleospora stonei forms a single strain lineage (Fig. 1, clade 27; Fig. 2, clade 33) that is closely related to Pseudophaeophleospora atkinsonii. The species Phaeophloeospora concentrica (not included in this study), a pathogen of Protea spp., clusters close to Brunneosphaerella (Crous et al. 2009c). Morphologically, Pseudophaeophleospora is very similar to Phaeophleospora, and the two genera can only safely be distinguished by means of DNA data.
Clade 28: Sonderhenia
Sonderhenia H.J. Swart & J. Walker, Trans. Brit. Mycol. Soc. 90: 640. 1988.
Description (from Crous 1998): Foliicolous, phytopathogenic, causing discrete leaf spots. Leaf spots amphigenous, round to confluent and irregular, surrounded by a purple border when young, which becomes dark red to brown and raised with age. Ascomata pseudothecial, amphigenous, on one side of each lesion, often 1–3, intermingled with conidiomata, immersed, black, punctiform, globose to subglobose; apical ostiole substomatal; wall olive brown, of 3–4 layers of textura angularis, subhymenium of 1–2 layers of hyaline cells. Asci fasciculate, bitunicate, subsessile, 8-spored, ovoid to obclavate, straight to incurved. Ascospores 2–3-seriate, hyaline, guttulate, straight or slightly curved, fusiform, 1-septate, widest just above median septum, slightly constricted at septum. Conidiomata pycnidial, amphigenous, subepidermal with central non-projecting ostiole, scattered, black, globose; wall of 2–3 layers of brown cells. Conidiogenous cells minute, olivaceous, proliferating enteroblastically and percurrently, lining the inner pycnidial wall layer. Conidia ellipsoidal to cylindrical or ovoid, straight or bent, brown, 3-distoseptate, not constricted, verruculose, apex obtuse, base truncate with marginal frill.
Type species: Sonderhenia eucalyptorum (Hansf.) H.J. Swart & J. Walker (≡ Hendersonia eucalyptorum Hansf.).
Sonderhenia eucalyptorum (Hansf.) H.J. Swart & J. Walker, Trans. Brit. Mycol. Soc. 90: 640. 1988.
Basionym: Hendersonia eucalyptorum Hansf., Proc. Linn. Soc. N.S.W. 79(3-4): 135. 1954.
Synonym (sexual morph): Mycosphaerella swartii R.F. Park & Keane, Trans. Brit. Mycol. Soc. 83: 99. 1984.
Descriptions and illustrations: Swart and Walker, 1988, Crous, 1998.
Materials examined: Australia, Mt. Gambier, on leaves of Eucalyptus leucoxylon, 9 Dec. 1982, R.F. Park (holotype of Mycosphaerella swartii DAR 45719, isotype IMI 280474, sexual morph); Clare, on leaves of E. leucoxylon, Aug. 1922, T. Osborne (holotype of Hendersonia eucalyptorum, K(M) 137253, WARI 2007, asexual morph); Tasmania, on leaves of Eucalyptus coccifera, Jan. 2006, C. Mohammed, cultures CBS 120220 = CPC 12553, CPC 12554–12555.
Notes: Sonderhenia includes taxa with mycosphaerella-like sexual morphs and pycnidial asexual morphs. The brown conidiogenous cells proliferate percurrently and give rise to brown conidia that are transversely distoseptate. Only two species, Sonderhenia eucalypticola and Sonderhenia eucalyptorum are presently known (Crous et al. 2013a), and they cluster together in a well-supported clade (Fig. 1, clade 28; Fig. 2, clade 34) closely related to Pseudophaeophleospora.
Clade 29: Pseudocercospora, Neopseudocercospora and pseudocercospora-like
Pseudocercospora Speg., Anales Mus. Nac. Hist. Nat. Buenos Aires, Ser. 3, 20: 437. 1910.
Synonym: Neopseudocercospora Crous, Persoonia 31: 219. 2013.
Additional synonyms: See Crous and Braun, 2003, Braun et al., 2013, Crous et al., 2013a.
Description (from Crous et al. 2013a): Foliicolous, chiefly phytopathogenic, but also endophytic; commonly associated with leaf spots, but also occurring on fruits. Mycelium internal and external, consisting of smooth, septate, subhyaline to brown branched hyphae. Stroma absent to well-developed. Conidiophores in vivo arranged in loose to dense fascicles, sometimes forming distinct synnemata or sporodochia, emerging through stomata or erumpent through the cuticle, often arising from substomatal or subcuticular to intraepidermal stromata, or occurring singly on superficial hyphae, short to long, septate or continuous, i.e. conidiophores may be reduced to conidiogenous cells, simple to branched and straight to geniculate-sinuous, subhyaline, pale to dark olivaceous to brown, smooth to finely verruculose. Conidiogenous cells integrated, terminal, occasionally intercalary, polyblastic, sympodial, or monoblastic, proliferating percurrently via inconspicuous or darkened, irregular annellations, subhyaline, olivaceous, pale to dark brown, with inconspicous, or only thickened along the rim, or flat, and unthickened or almost so but refractive or even slightly darkened-refractive loci, but never pronounced. Conidia solitary, rarely in simple chains or disarticulating, subhyaline, olivaceous, pale to dark brown, usually scolecosporous, i.e. obclavate-cylindrical, filiform, acicular, and transversely multi-euseptate, occasionally also with oblique to longitudinal septa, conidia rarely amero- to phragmosporous, short subcylindrical or ellipsoidal-ovoid, aseptate or only with few septa, apex subacute to obtuse, base obconically truncate to truncate, or bluntly rounded, with or without a minute marginal frill, straight to curved, rarely sigmoid, smooth to finely verruculose; hila usually unthickened, not darkened, at most somewhat refractive, occasionally slightly thickened along the rim, or rarely flat, unthickened or almost so, but slightly refractive or even slightly darkened-refractive, but never pronounced.
Type species: Pseudocercospora vitis (Lév.) Speg. (≡ Septonema vitis Lév.).
Pseudocercospora dingleyae U. Braun & C.F. Hill (as ‘dingleyii’), Mycol. Progress 1(1): 23. 2002.
Replaced synonym: Cercospora haloragis Dingley, New Zealand J. Agric. Res. 8(4): 913. 1965, non Pseudocercospora haloragidis (Hansf.) U. Braun 1995.
Materials examined: New Zealand, Auckland, Piha, White's Stream, on Haloragis erecta 31 Jan. 1954, J.M. Dingley (holotype PDD 20086); Auckland, Grey Lynn, Western Springs, on Haloragis erecta, 21 Jan. 2001, C.F. Hill 367, HAL 3239 F, PDD 73036, culture CBS 114645.
Note: The present name was introduced by Braun & Hill (2002) for the species Cercospora haloragidis which had unthickened and undarkened conidiogenous loci and hila, a characteristic of Pseudocercospora.
Pseudocercospora convoluta (Crous & Den Breeÿen) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822778.
Basionym: Passalora convoluta Crous & Den Breeÿen, Fungal Diversity 23: 96. 2006.
Description and illustrations: Den Breeÿen et al. (2006).
Materials examined: Costa Rica, San Isidro between San José and Golfito, on leaves of Chromolaena odorata, 15 Oct. 1997, M.J. Morris (holotype CBS H-19752, ex-type culture CBS 113377 = MJM 1533 = C488).
Notes: The phylogenetic analysis in this study showed that this species clustered within the Pseudocercospora clade (Fig. 1, clade 29; Fig. 2, clade 23). Although in the original description of the species the loci and hila were described as ‘darkened, thickened and refractive’ (Den Breeÿen et al. 2006), observation of the type specimen and culture led to the conclusion that these are within the acceptable range of this genus.
Pseudocercospora metrosideri U. Braun, Fungal Diversity 8: 44. 2001.
Material examined: New Zealand, Auckland, on Metrosideros excelsa, 17 Oct. 2003, C.F. Hill 929, culture CBS 114294.
Note: The present strain was introduced by Braun & Hill (2004) and, although the conidia were shorter and narrower than average, they were still within the range from the original description by Braun (2001).
Pseudocercospora nodosa (Constant.) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822779.
Basionym: Cercospora nodosa Constant., Mycotaxon 3: 122. 1975.
Synonym: Passalora nodosa (Constant.) L.G. Br. & Morgan-Jones, Mycotaxon 4: 303. 1976.
Description and illustration: Brown & Morgan-Jones (1976).
Materials examined: Romania, Bucuresti, on Psoralea bituminosa, 23 Sep. 1966, O. Constantinescu (holotype BUCM 41472, ex-type culture CBS 554.71, wrongly cited as “555.71” in protologue).
Notes: Based on the phylogenetic analyses in this study, this species clustered within Pseudocercospora (Fig. 1, clade 29; Fig. 2, clade 23). Although we did not study the holotype specimen, we examined the ex-type culture. When Constantinescu (1975) proposed this species, his detailed line drawings illustrated “thin”, discrete conidial scars (loci)”. In addition, Brown & Morgan-Jones (1976), who observed the holotype, mentioned that the thin scars, swollen conidiophore apices and basal conidial cells were indicative of its placement in Passalora. However, these characters are also typical characters of Pseudocercospora.
Pseudocercospora vitis (Lév.) Speg., Anales Mus. Nac. Hist. Nat. Buenos Aires 20(13): 438. 1910.
Basionym: Septonema vitis Lév., Ann. Sci. Nat., Bot., Sér. 3, 9: 261. 1848.
For additional synonyms see MycoBank.
Description and illustrations: Deighton (1976a).
Materials examined: Republic of Korea, Namyangju, on Vitis vinifera, 30 Sep. 2004, H.D. Shin, CBS H-20848, CBS 132012 = CPC 11595; on V. vinifera, 1 Oct. 2007, H.D. Shin, cultures CBS 132112 = CPC 14661.
Notes: Type material of Septonema vitis is not preserved, as already noted by Harvey & Wenham (1972), and the designation of a neotype is required, but fresh collections from the type host and location are necessary (France, Bordeaux, on Vitis vinifera). Pseudocercospora is a large cosmopolitan genus of plant pathogenic fungi that is commonly associated with leaf spots and blights on a wide range of plant hosts. Species occur in arid as well as wet environments and in a wide range of climates. The phylogenetic placement of Pseudocercospora has previously been determined and many new species have since been described (Crous et al. 2013a). In this study, the Pseudocercospora clade is well-supported by the phylogenetic analysis (Fig. 1, clade 29; Fig. 2, clade 23) and Pseudocercospora pistacina is basal to the clade. In addition, the type species of Neocercospora, Neocercospora zambiae, is observed to cluster within the Pseudocercospora clade and, therefore, Sutton's reallocation of Sporidesmium zambiense to Pseudocercospora is resurrected as current name.
Pseudocercospora zambiensis (Deighton) B. Sutton, Mycopathologia 125: 61. 1994.
Basionym: Sporidesmium zambiense Deighton, Mycol. Pap. 117: 27. 1969.
Synonyms: Repetophragma zambiense (Deighton) Subram., Proc. Indian Acad. Sci., B, 58: 185. 1992.
Neopseudocercospora terminaliae Crous, Persoonia 31: 219. 2013.
Neopseudocercospora zambiensis (Deighton) Crous & U. Braun, IMA Fungus 5: 204. 2014.
Descriptions and illustrations: Crous et al., 2013a, Braun et al., 2014.
Materials examined: Zambia, on Terminalia sp., 24 Feb. 2013, M. van der Bank (holotype of Neopseudocercospora terminaliae CBS H-21431, culture ex-type CBS 136423 = CPC 22686); idem., culture CPC 22685.
Notes: When Neopseudocercospora was described (Crous et al. 2013a) the phylogenetic analysis performed placed it close to zasmidium-like species based on LSU and ITS sequences. In the present study, when the rpb2 gene is introduced in the phylogenetic analysis, Neopseudocercospora clusters within the genus Pseudocercospora (Fig. 1, clade 29; Fig. 2, clade 23). Conidiogenous cells and conidia of Neopseudocercospora are similar to those of Pseudocercospora in being unthickened and non-pigmented. However, unlike most Pseudocercospora species, it produces solitary conidiophores with conidiogenous cells that proliferate percurrently and conidia with longitudinal septa (sporidesmium-like) (Crous et al., 2013a, Braun et al., 2014).
Species clustering in the Pseudocercospora clade that need further material to be collected before a formal combination into Pseudocercospora can be proposed:
Passalora bolleana (Thüm.) U. Braun, Mycotaxon 55: 228. 1995.
Basionym: Septosporium bolleanum Thüm., Oesterr. Bot. Z. 27 (1): 12.1877.
Synonyms: Cercospora bolleana (Thüm.) Speg., Michelia 1(5): 475. 1879.
Pseudocercospora bolleana (Thüm.) Sivan., The Bitunicate Ascomycetes and their anamorphs: 206. 1984.
For additional synonyms see MycoBank.
Descriptions and illustrations: Ellis, 1976, Sivanesan, 1984.
Materials examined: Romania, on Ficus carica, 21 Oct. 1970, O. Constantinescu, culture CBS 541.71. Republic of Korea, on F. carica, 14 Nov. 2007, H.D. Shin, culture CPC 14819.
Notes: “Passalora bolleana” is widely distributed throughout the world and is known as a typical species of Passalora s. lat. The conidial loci of “Passalora bolleana” are conspicuous, almost unthickened to slightly thickened and somewhat darkened. The present strains, since they cluster in the Pseudocercospora clade, will be treated as Pseudocercospora sp. until more information is available (Table 1).
Passalora robiniae (Shear) S. Hughes, Canad. J. Bot. 31: 572. 1953.
Basionym: Fusicladium robiniae Shear, Bull. Torrey Bot. Club 29: 452. 1902.
Synonyms: Camptomeris robiniae (Shear) Cif., Mycopathol. Mycol. Appl. 6: 25. 1951.
Phaeoisariopsis robiniae (Shear) Deighton, in Ellis, More Dematiaceous Hyphomycetes: 234. 1976.
For additional synonyms see MycoBank.
Description and illustration: Hughes, 1953, Ellis, 1976.
Material examined: USA, on Robinia pseudoacacia, unknown date and collector, isol. and dep. R.W. Davidson, deposited in 1939, culture CBS 277.39.
Notes: The type specimen of Fusicladium robiniae can be found in BPI, together with several isotypes. The specimen from which the culture CBS 277.39 was isolated is likely BPI 424556, based on the specimen metadata agreeing with the culture metadata (USA, Tennessee, Gatlinburg, Great Smoky Mountains National Park, Robinia pseudoacacia, 21 Aug. 1939, R.W. Davidson). Unfortunately, we were unable to study any of the previously mentioned specimens and the examined strain refused to sporulate on various media. Hughes (1953) re-described Passalora robiniae, which typically forms 1(–2)-septate conidia, the lower cell being wider than the upper one. The present strain, since it clusters in the Pseudocercospora clade, will be treated as Pseudocercospora sp. until more information is available (Table 1).
Clade 30: Clypeosphaerella
Clypeosphaerella Guatimosim et al., Persoonia 37: 121. 2016, emend.
Description: Phytopathogenic. Ascomata pseudothecial, epiphyllous, solitary, subcuticular to erumpent, globose, walls of 2–3 layers of brown to dark brown textura angularis, ostiole central. Asci bitunicate, aparaphysate, fasciculate, subsessile, 8-spored, obpyriform to ovoid, hyaline, smooth. Ascospores inordinate, overlapping, fusoid, straight, 1-septate, slightly constricted at the septum, biguttulate, hyaline, thin-walled, smooth; germinating at both ends, remaining hyaline, germ tubes following the main axis of the spore. Conidiophores fasciculate, pale olivaceous, septate, usually curved, rarely branched, geniculate at the apex, conidiogenous cells with conidiogenous loci (scars) thickened and darkened. Conidia solitary, pale brown to olivaceous brown, cylindrical to obclavate, obconic base, bluntly rounded tip, septate, sometimes constricted at the septa, hilum at the base thickened and darkened.
Type species: Clypeosphaerella sticheri Guatimosim et al.
Clypeosphaerella calotropidis (Ellis & Everh.) Videira & Crous, comb. nov. MycoBank MB822749.
Basionym: Cercospora calotropidis Ellis & Everh., Rep. (Annual) Missouri Bot. Gard.: 120. 1898.
Synonyms: Phaeoramularia calotropidis (Ellis & Everh.) Kamal, A.S. Moses & R. Chaudhary, Mycol. Res. 94: 716. 1990.
Pseudocercospora calotropidis (Ellis & Everh.) Haldar & J.B. Ray, J. Mycopathol. Res. 39(1): 43. 2001.
Passalora calotropidis (Ellis & Everh.) U. Braun, Schlechtendalia 5: 60. 2000.
For additional synonyms see Crous & Braun (2003) and MycoBank.
Descriptions and illustrations: Chupp, 1954, Ellis, 1976, Wilkinson et al., 2005.
Material examined: Egypt, on Calotropis procera, unknown date and collector, culture CBS 129.30.
Notes: Braun (2000) transferred Cercospora calotropidis to the genus Passalora based on the observation of numerous specimens, including the type specimen (Bahamas, Fortune Island (Long Cay), on Calotropis procera, Nov. 1890, A.S. Hitchcock, BPI 433953, 433956, NY, IMI 7752, slide). The isolate of Cercospora calotropidis used in our study was sterile and the specimen was unfortunately not preserved. The strain used in the present study has an ITS sequence that is 99 % similar to GenBank AY303969, a Passalora calotropidis strain used by Wilkinson et al. (2005). In Wilkinson et al. (2005), the isolate’s morphology has similar diagnostic characters to those of Passalora calotropidis (Braun 2000) and the phylogenetic analysis based on ITS placed the species in a single-strain lineage closely related to Pseudocercospora. In the present study, based on a multigene analysis, the strain CBS 129.30 clusters in Clypeosphaerella (Fig. 1, clade 30; Fig. 2, clade 26), which is closely related to Pseudocercospora. Based on a BLAST comparison against the alignment, the present species shares 97 % (425/438) similarity based on ITS and 96 % (733/763) similarity based on rpb2 with Clypeosphaerella quasiparkii CBS 123243. Similar values of percentage similarity can be observed, for example, between Zymoseptoria brevis and Zymoseptoria tritici. Therefore, proposing a new genus to include this species would be unreasonable. The main issue is that the previously described species in this genus, Clypeosphaerella sticheri and Clypeosphaerella quasiparki, are only known by their sexual morph and Passalora calotropidis is only known from its asexual morph. Nevertheless, based on the molecular similarities, we propose a tentative combination of the present species in Clypeosphaerella until further morphological studies can be performed.
Clypeosphaerella quasiparkii (Cheew. et al.) Guatimosim et al., Persoonia 37: 121. 2016.
Basionym: Mycosphaerella quasiparkii Cheew. et al., Persoonia 21: 85. 2008.
Description and illustration: Cheew. et al. (2008).
Material examined: Thailand, Burirum, on leaves of Eucalyptus sp., Jul. 2007, P. Suwannawong (holotype CBS H-20132, culture ex-type CBS 123243 = CPC 15409); idem., cultures CPC 15433, CPC 15434.
Note: Clypeosphaerella sticheri is similar to Clypeosphaerella quasiparki but produces smaller ascospores that germinate in a type D pattern (Crous, 1998, Guatimosim et al., 2016).
Clypeosphaerella sticheri Guatimosim et al., Persoonia 37: 121. 2016.
Description and illustration: Guatimosim et al. (2016).
Materials examined: Brazil, Rio de Janeiro, Nova Friburgo, Fazenda Barreto II, Riograndina, ruderal, on fronds of Sticherus bifidus, 11 Feb. 2014, R.W. Barreto (holotype CBS H-22088, isotype VIC 42607, culture ex-type CPC 24705); Minas Gerais, Araponga, Parque Estadual da Serra do Bri- gadeiro, path to Pico do Pato, Atlantic rainforest, on fronds of S. bifidus, 21 Feb. 2014, E. Guatimosim, CBS H-22089, VIC 42516, culture CPC 24733.
Notes: Morphologically, the genus Clypeosphaerella is reminiscent of Mycosphaerella s. lat. (sexual morph) but differs by having the thicker upper wall of the ascomata resembling a pseudoclypeus. The phylogenetic analyses in this study places Clypeosphaerella in a well-supported clade (Fig. 1, clade 30; Fig. 2, clade 26) closely related to Distocercospora.
Clade 31: Distocercospora
Distocercospora N. Pons & B. Sutton, Mycol. Pap. 160: 60. 1988.
Description (from Braun et al. 2013): Foliicolous, plant pathogenic, leaf spotting hyphomycetes (asexual morphs), sexual morphs unknown. Mycelium in vivo internal; hyphae branched, septate, subhyaline to pigmented, thin-walled, smooth. Stromata lacking to well-developed, pigmented, textura angulata to textura globosa. Conidiophores macronematous, mononematous, simple to branched, often strongly branched, septate, pigmented, thin-walled, smooth to rough-walled. Conidiogenous cells integrated, terminal, occasionally intercalary, proliferation sympodial, conidiogenous loci conspicuous, almost unthickened to somewhat thickened and darkened. Conidia formed singly, rarely in short chains, scolecosporous, mostly obclavate to cylindrical, with a single to several transverse distosepta or a mixture of eu- and distosepta, subhyaline to pigmented, wall smooth to rough, hila somewhat thickened and darkened, conidial secession schizolytic.
Type species: Distocercospora pachyderma (Syd. & P. Syd.) N. Pons & B. Sutton (≡ Cercospora pachyderma Syd. & P. Syd.).
Distocercospora pachyderma (Syd. & P. Syd.) N. Pons & B. Sutton, Mycol. Pap. 160: 60. 1988. Fig. 14.
Fig. 14.
Distocercospora pachyderma (CBS 138247). A–E. Observations in vivo. A, B. Leaf spot symptoms on the host. C. Conidiophores and conidiogenous cells. D. Conidiophores, conidiogenous cells and conidia. E. Conidia. F–J. Observations in vitro. F. Culture on OA. G. Conidiophores erect and emerging from hyphae. H–I. Conidiophore, conidiogenous cell and conidia. J. Conidia. Scale bars = 10 μm.
Basionym: Cercospora pachyderma Syd. & P. Syd., Ann. Mycol. 12: 203. 1914.
Synonyms: Cercosporina pachyderma (Syd. & P. Syd.) Sacc., Syll. Fung. 25: 900. 1931.
Cercospora dioscoreae-bulbiferae J.M. Yen & Gilles, Cah. Maboké 9: 105. 1973.
Description and illustration: Braun et al. (2014).
Description in vivo (on V8; CPC 24144): Mycelia composed of hyaline to pale olivaceous, uniform in width, 2.5–3.8 μm, often forming large brown swollen cells, up to 10 μm in size. Conidiophores micro- or macronematous, pale olivaceous, arising from hyphae or swelling cells, smooth, septate, irregular in width, 2.5–7 μm, straight or geniculate, 50–165 × 2.5–7.5 μm. Conidiogenous cells integrated, apical, polyblastic, proliferating percurrently following sympodial sporulation, with darkened, rim-like and thickened loci, 1.25–2.5 μm diam. Conidia solitary, rarely catenate, hyaline to pale olivaceous brown, cylindrical to obclavate, straight, apex rounded and often elongated (beak-like), base long obconically truncate, 28–55 × 2.5–7.5 μm, 2–3-eu- or distoseptate, hila thickened, darkened and refractive.
Materials examined: Japan, Iwate, Morioka, Koma, on Dioscorea sp., 13 Sep. 2010, C. Nakashima & K. Motohashi (epitype designated by Braun et al. 2014, TSU MUMH11476, isotype CBS H-21733, ex-epitype culture CBS 138247 = CPC 24144); Ibaragi, on Dioscorea sp., T. Kobayashi, slide specimen MUCC-PL-185. Fiji, Taveuni, Tabakau, on Dioscorea bulbifera, 22 Dec. 2002, E.H.C. McKenzie, PDD 77375. Philippines, Prov. Laguna, Luzon, Morong Valley, on Dioscorea alata, 9 Nov. 1913, M. B. Raimundo, C.F. Baker 2051 (neotype S F37683); Luzon, Los Baños, on Dioscorea alata, Nov. 1913, C.F. Baker 522 (topotypes: B; BPI 439183, BPI 439184; IMI 256649, S F37682).
Notes: Morphologically, Distocercospora is similar to Passalora with almost unthickened to somewhat thickened, darkened loci and hila and pigmented conidia, but differs in having conidia with a mixture of eu- and distosepta (Fig. 14). The formation of distoseptate conidia occasionally occurs in other genera (e.g. in Pseudocercospora cryptomeriicola) (Nakashima et al. 2007), and may have gone undetected among other cercosporoid fungi due to the difficulty in observing such septa in taxa with thin walls (Braun et al. 2013). The meaning of distoseptation (= pseudoseptation) as character at generic level within the cercosporoid fungi is still unclear (Braun et al. 2015). Morphologically, the genus Distocercospora was evidently characterised by the mode of proliferation of its conidiophores, which are composed of two distinct layers. During proliferation of its conidiogenous cells, first the outer layer of conidiophore is broken by the percurrent proliferation of the inner layer, and secondly, many conidia are formed sympodially. At this point, septa of conidiophores and most of the conidia of Distocercospora pachyderma show the pseudoseptation. The cultures and molecular data based on the type species of Distocercospora (Distocercospora pachyderma) used in this study showed that this species clusters within the Mycosphaerellaceae in a separate clade supported by all the phylogenetic analyses performed (Fig. 1, clade 31; Fig. 2, clade 27). These results support Distocercospora as a separate genus, distinguished from Passalora s. str.
Clade 32: Uwemyces
Uwemyces Hern.-Restr., G.A. Sarria & Crous, Persoonia 36: 455. 2016.
Description (from Crous et al. 2016b): Mycelium immersed and superficial, hyphae branched, septate, hyaline and brown, smooth-walled. Conidiophores fasciculate, simple, dark brown at the base and subhyaline at the apex. Conidiogenous cells cylindrical, sympodial, polytretic, with dark conidiogenous loci, terminal and intercalary, brown. Conidia solitary, straight or curved, cylindrical to obclavate, pale brown to brown, apex subhyaline, verruculose-walled, with a thick, dark brown, truncate scar at the base, septate. Sexual morph unknown.
Type species: Uwemyces elaeidis (Steyaert) M. Hern.-Restr. et al. (≡ Cercospora elaeidis Steyaert).
Uwemyces elaeidis (Steyaert) M. Hern.-Restr. et al. Persoonia 36: 455. 2016.
Basionym: Cercospora elaeidis Steyaert, Bull. Soc. R. Bot. Belg., 80: 35. 1948; as “elaedis”.
Synonym: Pseudospiropes elaeidis (Steyaert) Deighton, Trans. Brit. Mycol. Soc. 85: 739. 1985.
Descriptions and illustrations: Ellis, 1976, Deighton, 1985, Braun et al., 2014, Crous et al., 2016b.
Material examined: Colombia, Barrancabermeja, CENIPALMA, on leaves of Elaeis oleifera, May 2013, coll. G.A. Sarria, culture CPUwZC-01.
Notes: The taxonomic position of Cercospora elaedis was recently discussed by Braun et al. (2014). This species has a wide distribution and seems to be restricted to Elaeis guineensis, (Arecaceae). Phylogenetically, this species is represented by a single-strain lineage closely related to Distocercospora (Fig. 1, clade 31) or to Coremiopassalora (Fig. 2, clade 24). The type material of Cercospora elaeidis (Democratic Republic of the Congo, on Elaeis guineensis) could not be traced and the species needs to be neotypified (Braun et al. 2014). The present strain is unsuitable for neotypification due to its geographical origin (Crous et al. 2016b).
Clade 33: Coremiopassalora
Coremiopassalora U. Braun, C. Nakash., Videira & Crous, gen. nov. MycoBank MB822585.
Etymology: Derived from the arrangement of conidiophores, coremium + resembling the genus Passalora.
Differs from the genus Passalora by synnematous conidiophores and catenate, hyaline to pale olivaceous conidia with distinct, slightly thickened and not darkened loci.
Type species: Coremiopassalora eucalypti (Crous & Alfenas) U. Braun et al. (≡ Mycovellosiella eucalypti Crous & Alfenas).
Coremiopassalora eucalypti (Crous & Alfenas) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822750
Basionym: Mycovellosiella eucalypti Crous & Alfenas, Mycol. Mem. 21: 105. 1998.
Synonym: Passalora eucalypti (Crous & Alfenas) Crous & U. Braun, in Crous & Braun, CBS Biodiversity Ser. 1: 452. 2003.
Description and illustration: Crous (1998).
Description in vitro (on V8; CBS 111318): Mycelium composed of hyaline to pale brown, delicate hyphae, uniform in width, 2.5 μm, often showing a synnematous or cushion-shaped arrangement. Conidiophores straight to sinuous or geniculate, solitary to tightly fasciculate, sometimes appearing as synnemata, simple, 10–33 × 2–2.5 μm. Conidiogenous cells integrated, terminal, polyblastic, proliferating sympodially, conidiogenous loci at the apex and shoulders, protruding and conically truncate, slightly thickened and refractive, 1–2 μm diam. Conidia catenate, occurring in unbranched or branched chains, hyaline, cylindrical, sometimes obclavate, obconically truncate at both ends, 8–40 × 2–2.5 μm, 0–1-septate, sometimes constricted at the centre, hila thickened but not darkened, 1–2 μm diam.
Materials examined: Brazil, São Paulo, on leaves of Eucalyptus saligna, Jun. 1995, P.W. Crous & A.C. Alfenas (holotype PREM 55302, culture ex-type CBS 111306 = CPC 1455); idem., CBS 111318 = CPC 1457; Suzano, on leaves of Eucalyptus saligna, 8 Aug. 1996, P.W. Crous, culture CBS 111306 = CPC 1455.
Notes: The genus Coremiopassalora (Fig. 1 clade 33; Fig. 2, clade 24) includes two species that morphologically can be characterised as Passalora s. lat., but phyllogenetically are not congeneric with the type Passalora bacilligera (Fig. 1 clade 34; Fig. 2, clade 22).
Coremiopassalora leptophlebae (Crous et al.) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822751.
Basionym: Passalora leptophlebae Crous et al. (as “leptophlebiae”), Persoonia 26: 131. 2011.
Description and illustrations: Crous et al. (2011a).
Material examined: Brazil, Minas Gerais, Viçosa, University Forestry Nursery, on leaves of Eucalyptus leptophleba, 23 Aug. 2010, P.W. Crous, A.C. Alfenas, R. Alfenas & O.L. Pereira (holotype CBS H-20585, culture ex-type CBS 129524 = CPC 18480).
Notes: Coremiopassalora leptophlebae is the second species in this genus (Fig. 1, clade 33; Fig. 2, clade 24). The host range and geographic distribution of this taxon are thus far restricted to the type collection.
Clade 34: Passalora
Passalora Fr., Summa Veg. Scand. 2: 500. 1849, emend.
Description: Hyphomycetous, phytopathogenic. Mycelium internal, consisting of hyaline, branched, septate hyphae. Stromata absent or small. Conidiophores emerging through stomata, in fascicles, unbranched or branched, straight to flexuous, at times with a single basal septum, usually up to 3-septate, medium brown, somewhat swollen in the conidiogenous region. Conidiogenous cells integrated, terminal, with flat, somewhat thickened and darkened loci. Conidia solitary, olivaceous to pale brown, thin-walled, smooth, straight or gently curved, mostly didymosporous, constricted at septum, with somewhat thickened, darkened and refractive hila.
Type species: Passalora bacilligera (Mont. & Fr.) Mont. & Fr. (≡ Cladosporium bacilligerum Mont. & Fr.).
Passalora bacilligera (Mont. & Fr.) Mont. & Fr., in Montagne, Sylloge generum specierumque cryptogamarum: 305. 1856. Fig. 15.
Fig. 15.
Passalora bacilligera (CBS 131547). A–G. Observations in vivo. A, B. Leaf spot symptoms on the host. C–E. Conidiophores and conidiogenous cells. F. Conidiogenous cell and conidium. G. Single conidia. Scale bars = 10 μm.
Basionym: Cladosporium bacilligerum Mont. & Fr., in Montagne, Ann. Sci. Nat., Bot., Sér. 2, 6: 31. 1836.
Description in vivo (CBS H-20777): Leaf spots absent or yellowish green, angular, 1–2 mm diam, delimited by leaf veins. Caespituli hypophyllous, olivaceous to pale brown. Mycelium internal, consisting of hyaline, branched, septate, 1–2 μm diam hyphae. Stromata absent or only formed as small aggregations of a few swollen substomatal hyphal cells. Conidiophores medium brown, arising from stomata, in fascicles of up to 12, unbranched or occasionally branched, straight to flexuous, usually up to 3-septate, occasionally with a single basal septum, 40–180 × 3–3.5 μm, geniculate at the apex. Conidiogenous cells integrated, terminal, somewhat swollen, 3–6.5 μm in width, polyblastic, proliferating sympodially, with conidiogenous loci flat, somewhat thickened and darkened, 1–2 μm diam. Conidia solitary, olivaceous to pale brown, thin-walled, smooth, straight or gently curved, basal cell ellipsoid-doliiform and obconical truncate without protruding, apical cell narrowly long-ellipsoid to subcylindrical, 21–68 × 4.5–8.5 μm, (0–)1(–3)-euseptate, constricted at basal septum, with hilum somewhat thickened, darkened and refractive, 1.5–2 μm diam.
Description in vitro (on V8; CBS 131547): Mycelium composed of hyaline to pale olivaceous brown, delicate hyphae, 2–2.5 μm width. Conidiophores macronematous, pale olivaceous brown to brown, simple or branched, straight to sinuous, smooth, paler towards the apex, 25–300 × 2.5–3.3 μm. Conidiogenous cells integrated, terminal, proliferating sympodially, polyblastic, conidiogenous loci located on the shoulders and the apex, slightly thickened and darkened, 2.5 μm diam. Conidia solitary, pale olivaceous brown to brown, cylindrical to obclavate, obconical truncate at the base, rounded or pointed at the apex, 13–37.5 × 2.5–5 μm, (0–)1-euseptate, constricted at the septum, hilum slightly thickened, darkened and refractive, 2.5 μm diam.
Material examined: Poland, Hwozna Protected Area, Bialowieza National Park, on Alnus glutinosa, 20 Sep. 2011, D. Karasinski (epitype designated here CBS H-20777, MBT378570, ex-epitype culture CBS 131547). France, Lyon, on Alnus glutinosa, 1828, Montagne 568 (lectotype designated here, Montagne 568, Ann. Sci. Nat., Bot., Sér. 2, 6: pl. 12, fig. 5. 1836, original illustration, MycoBank, MBT378569).
Notes: Passalora was the first genus introduced for cercosporoid hyphomycetes (Fries 1849) and a review of the taxonomical history of the genus has recently been published by Braun et al. (2013). In one of the most comprehensive examinations on this generic complex, Crous & Braun (2003) concluded that various genera (e.g. Mycovellosiella, Phaeoramularia, Fulvia) should be merged under the oldest name Passalora. After this revision Passalora included cercosporoid species with solitary, fasciculate to synnematous conidiophores and conidia formed singly or in chains, but in all cases with conspicuous (thickened and darkened) conidiogenous loci (scars) and mostly non-scolecosporous, pigmented conidia. This new concept was also supported by first molecular sequence analyses (Crous et al., 2000, Crous et al., 2001b). However, with the addition of more species and more phylogenetic markers, Passalora s. lat. has proven to be para- or polyphyletic (Thomma et al., 2005, Crous et al., 2009b, Crous et al., 2009d, Crous et al., 2013a). In addition, the type species has not been subjected to DNA sequence analyses before, and the passalora-like clades distributed throughout the Mycosphaerellaceae are not clearly connected with morphological groups within Passalora (e.g. mycovellosiella-like). In this study, we propose a good candidate for the epitypification of the type species of Passalora (CBS 131547). Phylogenetically, this strain forms a single species clade in all phylogenetic analyses performed (Fig. 1, clade 34; Fig. 2, clade 22), but without a strong link to other genera. With the additional epitypification of the type species of Fulvia (Fulvia fulva; Fig. 1, clade 59), Mycovellosiella (Mycovellosiella cajani; Fig. 1, clade 7) and Phaeoramularia (Phaeoramularia gomphrenicola; Fig. 1, clade 61), these names are resurrected and applied to different monophyletic clades and are no longer regarded as synonyms of Passalora s. str. The value of features such as mycelium internal and/or external, conidia solitary or in chains, remains doubtful and barely applicable for the discrimination of cercosporoid genera. Morphologically, Passalora s. str. is rather different from common passalora-like species (Fig. 15), in having sparsely septate, flexuous conidiophores, and predominantly smooth, olivaceous, 1–2-septate conidia constricted at the basal septum, with somewhat to distinctly thickened, darkened, and refractive loci. The placement of the hundreds of passalora-like species that are not known from their DNA is not yet possible, and these would for the interim have to be retained in Passalora s. lat. as a wide, morphologically circumscribed genus, pending cultures and results of DNA sequence analyses.
Clade 35: Zymoseptoria
Zymoseptoria Quaedvlieg & Crous, Persoonia 26: 64. 2011.
Description (from Quaedvlieg et al. 2011): Conidiomata pycnidial, semi-immersed to erumpent, dark brown to black, subglobose, with central ostiole; wall of 3–4 layers of brown textura angularis. Conidiophores hyaline, smooth, 1–2-septate, or reduced to conidiogenous cells, lining the inner cavity. Conidiogenous cells tightly aggregated, ampulliform to doliiform or subcylindrical, phialidic with periclinal thickening, or with 2–3 inconspicuous, percurrent proliferations at apex. Type I conidia solitary, hyaline, smooth, guttulate, narrowly cylindrical to subulate, tapering towards acutely rounded apex, with bluntly rounded to truncate base, transversely euseptate, with unthickened and colourless hila. On OA and PDA aerial hyphae disarticulate into phragmospores (Type II conidia), that again give rise to Type I conidia via microcyclic conidiation; yeast-like growth and microcyclic conidiation (Type III conidia) common on agar media.
Type species: Zymoseptoria tritici (Desm.) Quaedvlieg & Crous (≡ Septoria tritici Desm.).
Zymoseptoria tritici (Desm.) Quaedvlieg & Crous, Persoonia 26: 67. 2011.
Basionym: Septoria tritici Desm., Ann. Sci. Nat., Bot., Sér. 2, 17: 107. 1842.
Description and illustration: Quaedvlieg et al. (2011).
Materials examined: France, on Triticum sp. (holotype of Septoria tritici; PC). Germany, Oestrich, on Triticum repens, Fuckel, Fungi Rhen. Exs. no. 1578 (isotype of Mycosphaerella graminicola, L). Netherlands, Brabant West, on Triticum aestivum, coll. R. Daamen, 6 May 1981, isol. as single conidium, W. Veenbaas, 810507/1, 7 May 1981 (epitype designated by Quaedvlieg et al. 2011, CBS H-20545, including sexual morph material on Triticum leaf of heterothallic mating IPO 323 (MAT 1-1) × IPO 94269 (MAT 1-2), culture ex-epitype IPO 323 = CBS 115943).
Notes: Zymoseptoria was introduced to include septoria-like species from graminicolous hosts that did not cluster with the type of Septoria s. str. in the phylogenetic analysis (Quaedvlieg et al. 2011). In addition, Zymoseptoria is morphologically distinct from Septoria by its yeast-like growth in culture, and by producing up to three different conidial types (Type I—pycnidial conidia; Type II—phragmospores on aerial hyphae; Type III—yeast-like growth proliferating via microcyclic conidiation). In the phylogenetic analyses in the present study, Zymoseptoria species cluster within the Mycosphaerellaceae (Fig. 1, clade 35; Fig. 2, clade 36) and close to Ramularia, as observed in previous studies (Quaedvlieg et al., 2013, Stukenbrock et al., 2012, Videira et al., 2016). Zymoseptoria currently comprises seven species including Zymoseptoria tritici, the causal agent of septoria tritici blotch on wheat, and Zymoseptoria passerinii, the causal agent of septoria speckled leaf blotch of barley, which are important crop pathogens responsible for severe yield losses (Stukenbrock et al. 2012).
Clade 36: Xenoramularia
Xenoramularia Videira et al., Stud. Mycol. 83: 96. 2016.
Description (from Videira et al. 2016): Phytopathogenic, causing leaf spots. Mycelium composed of hyaline, septate, branched hyphae. Conidiophores hyaline to pigmented, solitary, simple, straight or slightly curved, often reduced to conidiogenous cells, thin-walled, smooth. Conidiogenous cells hyaline, integrated in the mycelium or terminal in the conidiophores, subcylindrical to geniculate-sinuous, with one or multiple thickened but not darkened conidiogenous loci. Conidia hyaline, thin-walled, smooth, formed singly or catenate, aseptate or 1-septate, subcylindrical, apex obtuse to subacute, base truncate; hila thickened but not darkened.
Type species: Xenoramularia polygonicola Videira et al.
Xenoramularia polygonicola Videira et al., Stud. Mycol. 83: 98. 2016.
Description and illustration: Videira et al. (2016).
Materials examined: Republic of Korea, Pyeongchang, on Polygonum sp., 20 Sep. 2003, H.D. Shin (holotype KUS F19688, isotype CBS H-22541, culture ex-type CBS 141102 = CPC 10852); idem., cultures CPC 10853, CPC 10854.
Notes: The genus Xenoramularia was recently introduced in the Mycosphaerellaceae to accommodate a group of species that was phyllogenetically closely related to Zymoseptoria and Ramularia (Videira et al. 2016) but morphologically distinct. The phylogeny in the present work agrees with the previous results (Fig. 1, clade 36; Fig. 2, clade 37). Xenoramularia can be morphologically distinguished from Ramularia by having conidiogenous loci that are thickened, but not darkened and refractive and differs from Zymoseptoria by not forming acervular conidiomata and producing only one type of conidia.
Clade 37: Ramularia
Ramularia Unger, Exanth. Pflanzen (Wien): 169. 1833. emend. U. Braun (nom. cons.).
Synonyms: Didymaria Corda, Icon. fung. (Prague) 5: 9. 1842.
Phacellium Bonord., in Rabenh., Fungi Eur. Exs., Edn. 2, Ser. 2: no. 288. 1860.
Acrotheca Fuckel, Jahrb. Vereins Naturk. Herzogth. Nassau 15: 43. 1860.
Septocylindrium Bonord. ex Sacc., Michelia 2: 15. 1880.
Ovularia Sacc., Michelia 2: 17. 1880.
Mycosphaerella Johanson, Öfvers. Kongl Vetensk-Akad. Förh,. 41(9): 163. 1884, s. str.
Ophiocladium Cav., Z. Pflanzenkrankh. 3: 26. 1893.
Pseudovularia Speg., Anales Mus. Nac. Buenos Aires, Ser. 3, 20: 418. 1910.
For additional synonyms see Braun (1998) or Videira et al. (2016).
Description (from Videira et al. 2016): Mostly phytopathogenic (leaf spots, chlorosis or necrosis), sometimes saprobic or mycophylic. Conidiophores individual or synnematous, sometimes forming small to sporodochial caespituli, emerging through stomata or through the cuticle, straight, subcylindrical to geniculate-sinuous, continuous or septate, hyaline or in some species with a faintly reddish tinge, occasionally branched, thin-walled, usually smooth but rarely rough. Conidiogenous cells integrated, terminal, polyblastic, sympodially elongating, straight to geniculate-sinuous, conidiogenous loci conspicuously thickened, darkened and refractive, coronate (cladosporoid). Conidia consistently solitary or in simple or branched chains, solitary conidia 0–1-septate, catenate conidia aseptate to multiseptate (mostly 1–4 eusepta), hyaline, in a few species with a faintly reddish tinge, usually ellipsoid-ovoid, cylindrical-fusiform, rarely filiform, occasionally constricted at the septa, thin-walled, smooth to verruculose-echinulate, hila distinct, slightly to conspicuously thickened, darkened, refractive; conidial secession schizolytic.
Type species: Ramularia pusilla Unger.
Ramularia pusilla Unger, Exanth. Pflanzen: 169. 1833.
Synonyms: Caeoma pusilla (Unger) Bonord., Handb. Mykol.: 41. 1851.
Ovularia pusilla (Unger) Sacc., Syll. Fung. 4: 140. 1886.
Ramularia pulchella Ces., Bot. Zeitung (Berlin) 11: 238. 1853.
For additional synonyms see Braun, 1998, Braun et al., 2015 or MycoBank.
Descriptions and illustrations: Braun, 1998, Kirschner, 2009, Braun et al., 2015, Videira et al., 2016.
Materials examined: Austria, on Poa nemoralis, Unger, Exanth. Pfl., Pl. II, fig. 12, (lectotype [iconotype] see Braun 1998). Germany, Frankfurt am Main, Botanical Garden, on leaves of Poa annua, 25 Feb. 2008, R. Kirschner (epitype designated by Videira et al. 2016, CBS H-22527, culture ex-epitype CBS 124973 = RoKi 3143).
Notes: Species of Ramularia are phytopathogenic and mostly cause leaf spots but they can also be endophytic, saprophytic and mycophylic. There are about 325 species accepted in this genus (Braun 1998) of which only six have thus far been experimentally linked to a Mycosphaerella sexual morph (Videira et al. 2015b). Currently Ramularia is accepted as being a host-specific genus of phytopathogenic fungi (Braun 1998), although some exceptions are known (e.g. Ramularia vizellae, Videira et al. 2015b). Ramularia pusilla is the type species of the genus Ramularia and has a broad host range within the family Poaceae and a worldwide distribution (Braun 1998). Phylogenetically, species of Ramularia s. str. cluster in a well-supported clade (Fig. 1, clade 37; Fig. 2, clade 35) as observed in a previous study (Videira et al. 2016).
Clade 38: Paracercosporidium
ParacercosporidiumVideira & Crous, gen. nov. MycoBank MB822601.
Etymology: Morphologically similar to Cercosporidium.
Description: Phytopatogenic. Mycelium internal, hyaline, smooth. Stromata small, composed of few dark brown cells, or medium in size, mainly hypophyllous, substomatal, dark brown. Conidiophores loosely fasciculate, emerging from stromata, pale to dark brown, paler towards the apex, thin- to thick-walled, cylindrical, mildly to strongly geniculate, simple or branched. Conidiogenous cells integrated, terminal or intercalary, polyblastic, proliferating sympodially, with rim-like conidiogenous loci, thickened and darkened, located at the shoulders and apex. Conidia solitary, hyaline to pale olivaceous brown, thick-walled, cylindrical to obclavate, rounded at the apex, usually tapering towards the base, sometimes swollen at the base or truncate, hila rim-like, darkened and refractive.
Type species: Paracercosporidium microsorum (Sacc.) U. Braun et al. (≡ Cercospora microsora Sacc.).
Paracercosporidium microsorum (Sacc.) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822819. Fig. 16.
Fig. 16.
Paracercosporidium microsorum (CPC 15550). A–E. Observations in vivo. A, B. Leaf spot symptoms on the host. C, D. Conidiophores, conidiogenous cells and conidia. E. Conidia. F–I. Observations in vitro. F. Culture on OA. G, H. Conidiophore, conidiogenous cells and conidia. I. Conidiogenous cells and conidia. Scale bars = 10 μm.
Basionym: Cercospora microsora Sacc., Michelia 2(6): 128. 1880.
Synonyms: Passalora microsora (Sacc.) U. Braun, Mycotaxon 55: 233. 1995.
Cercospora microsora var. tiliae-platyphyllae Roum., Rev. Mycol. 16: 109. 1894.
Cercospora exitiosa Syd. & P. Syd., Ann. Mycol. 4(6): 485. 1907.
Cercospora zahariadii Săvul. & Sandu, Hedwigia 75: 226. 1935.
Mycosphaerella microsora Syd. & P. Syd., Ann. Mycol. 38: 465. 1940.
Sphaerella microsora (Syd. & P. Syd.) Sandu, Ciuperci Pyrenomycetes-Sphaeriales din România: 135. 1971.
Description in vivo: Leaf spots scattered, amphigenous, dark brown, later bown with dark brown border, irregular to angular, vein-limited, 1–3 mm in size. Caespituli amphigenous, pale brown, effuse. Mycelium internal, hyphae hyaline, smooth. Stromata small, composed of few dark brown cells, or medium in size and up to 40 μm diam, amphigenous, mainly hypophyllous, substomatal, dark brown. Conidiophores loosely fasciculate, emerging from upper part of stromata, dark brown to pale, paler towards the apex, thick-walled, cylindrical, well-geniculate due to sympodial proliferation, 20–98 × 5–6.5 μm. Conidiogenous cells integrated, terminal or intercalary, polyblastic, proliferating sympodially, with conidiogenous loci rim-like, darkened and thickened, located at the shoulders and apex, 1.5–2.5 μm diam. Conidia solitary, hyaline to pale olivaceous brown, thick-walled, cylindrical to obclavate, obconicaly truncate and thickened at the base, rounded at the apex, 24–66 × 5–7.5 μm, 1–5-septate, hila thickened and darkened, 1.5–2.5 μm diam.
Description in vitro (on SNA; CPC 15550): Mycelium composed of hyaline to pale brown hyphae, uniform in width, smooth, 1.5–2 μm. Conidiophores macronematous, pale to pale brown, smooth, straight to well-geniculate due to sympodial proliferation, simple or branched, 25–90 × 3–5 μm. Conidiogenous cells integrated, terminal or intercalary, proliferating sympodially, mono- or polyblastic, with rim-like conidiogenous loci, thickened, darkened and refractive, located on the shoulders and the apex, 2–2.5 μm diam. Conidia solitary, hyaline to pale brown, cylindrical to obclavate, obconically truncate at the base, rounded at the apex, 1–53 × 3–5 μm, indistinctly 1–6-euseptate, hilum slightly thickened, darkened and refractive, 2–2.5 μm diam.
Materials examined: Czech Republic, Moravia, Veltice, Forest of Rendez Vous, leaf spot on Tilia sp., 16 Sep. 2008, G. Verkley, culture CBS 123735. Netherlands, Z. Flevoland, Zeewolde, Hulkesteynse bos, old leaves of Tilia cordata (after hibernation), 1 Apr. 1998, H.A. van der Aa No. 12451, culture CBS 101017; same location, leaf spot of Tilia cordata, 19 Oct. 1997, H.A. van der Aa No. 12409, culture CBS 100352. Romania, Bucuresti, on Tilia tomentosa, isol. O. Constantinescu, 16 Jun. 1965, culture CBS 254.67; Bucuresti, Mogosoaia, on Tilia platyphyllos, 8 Oct. 1969, O. Constantinescu, CBS H-9853, culture CBS 552.71 = BUCM 2014. Ukraine, Donetsk, Svjatie Gory, vicinities of Svjatogorsk, National Nature Park, flood-plain forest on the left bank of Seversky Donets river, on Tilia cordata, 18 Jul. 2008, A. Akulov (epitype designated here CBS H-22942, MBT378695, ex-epitype culture CBS 142176 = CPC 15550); [lectotype designated here, MycoBank, MBT378694, PAD, Letendre sin. num.; see notes below].
Notes: The genus Paracercosporidium is hereby introduced to accommodate two species from the host Tilia that, due to the obclavate-like morphology of their conidia, were previously placed in Passalora but cluster apart from the type species Passalora bacilligera in a well-supported clade (Fig. 1, clade 38; Fig. 3, clade 3). In literature, only two species of passalora-like fungi have been described from the host Tilia, namely Passalora microsora and Passalora tiliae (Y.L. Guo & X.J. Liu) U. Braun & Crous (≡ Tandonella tiliae Y.L. Guo & X.J. Liu). While the latter is only known from China, the first has a worldwide distribution (Crous & Braun 2003). In the description of Passalora microsora, the size of the conidiophores, 10–40 × 2–3.5(–5) μm, and conidia (20–60 × 2.5–4 μm, rarely 80 × 5 μm, as large as 100 × 6 μm) of observed specimens can vary significantly (Chupp 1954). Based on the phylogenetic analyses, two clades representing two species can be observed, one including strains from Europe and the other with strains from Canada, for which the name Cercospora tiliae, based on type material on Tilia americana collected in Vermont, USA, is available. Morphologically, these two species are quite similar, but differ in vivo as in Paracercosporidium microsorum (Fig. 16) conidiophores are shorter and once abruptly geniculate, while Paracercosporidium tiliae (Fig. 17) has longer conidiophores which are strongly geniculate. The DNA sequences representative of each species clade differ one base pair on LSU, three base pairs on ITS and 21 base pairs on rpb2. According to Klebahn (1918), Passalora microsora is the asexual morph of Mycosphaerella millegrana. However, Sydow (1940) stated that this species is not the asexual morph of Mycosphaerella millegrana and described the true sexual morph as Mycosphaerella microsora (Tomilin 1979). The epitypification requires the citation of the type. However, the typification needs a detailed discussion and clarification. Chupp (1954: 565) mentioned: “No definite type given. Saccardo states it is common on Tilia europaea and Tilia americana in Europe and America.” This is not correct and, although not mentioned by Chupp (l.c.), undoubtedly refers to Saccardo (1886). Saccardo (1880) described this species in a paper dealing with specimens collected by P. Brunaud, Abb. Letendre, A. Malbranche, and J. Therry in Roumeguère's “Mycotheca Gallica” under no. 1041, so that specimens collected in France by the persons concerned represent potential syntypes. However, the number cited in Saccardo (1880) does not refer to “Roum., Fungi Sel. Gall. Exs. 1041” which is a collection of Torula herbarum f. solani-pseudocapsici. A collection of Cercospora microsora was issued as “Roum., Fungi Sel. Gall. Exs. 2062” [France, Parc du Grand-Quévilly (Seine-Inf.), on Tilia × europaea, Automne 1881, Rev. Abb. Letendre (e.g. BPI, FH, PC, PAD)] containing a copy of Saccardo's original description, but this gathering cannot be considered original material since it had been collected in 1881, i.e. one year after Saccardo's original publication. However, there is a sample in Saccardo's herbarium collected by Letendre that can be designated as lectotype.
Fig. 17.
Paracercosporidium tiliae (CBS 112734). A–E. Observations in vivo. A, B. Leaf spot symptoms on the host. C. Conidiophores. D. Conidiophores, conidiogenous cells and conidia. E. Conidia. F–J. Observations in vitro. F. Culture on V8. G, H. Conidiophore, conidiogenous cell and conidia. I. Conidiophore. J. Conidia. Scale bars = 10 μm.
Paracercosporidium tiliae (Peck) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822772. Fig. 17.
Basionym: Cercospora tiliae Peck, Bot. Gaz. 6(10): 277. 1881.
Description in vivo: Leaf spots scattered, amphigenous, dark brown, irregular to angular, vein-limited, 1–3 mm in size. Caespituli amphigenous, pale brown, effuse. Mycelium internal, hyaline, smooth. Stromata composed of a few dark brown cells, up to 30 μm diam, amphigenous, mainly hypophyllous, substomatal. Conidiophores emerging from upper part of stromata in dense fascicles, pale brown, thick-walled, cylindrical, straight or slightly curved, 35–85 × 3–4 μm. Conidiogenous cells integrated, polyblastic, terminal, proliferating sympodially, with rim-like conidiogenous loci, thickened and darkened, located at the shoulders and apex, 1.5–2.6 μm diam. Conidia single, hyaline to pale olivaceous, cylindrical to obclavate, obconicaly truncate at the base, rounded at the apex, thick-walled, 15.5–54 × 2–4 μm, 1–5-septate, 1.5–2 μm diam.
Description in vitro (on SNA; CPC 112734): Mycelium hyaline, hyphae uniform in width, smooth, 1.5–2 μm. Conidiophores macronematous, pale to pale brown, smooth, straight to well-geniculate due to sympodial proliferation, 35–87 × 2.5–3.5 μm. Conidiogenous cells integrated, terminal or intercalary, proliferating sympodially, mono- or polyblastic, with rim-like conidiogenous loci, thickened, darkened and refractive, located on the shoulders and the apex, 2–2.5 μm diam. Conidia solitary, hyaline to pale, cylindrical to obclavate, 15–40 × 2–3 μm, indistinctly 1–6-euseptate, obconically truncate at the base, with slightly thickened and refractive hilum, 1.5–2 μm diam.
Materials examined: Canada, Ottawa, on Tilia americana, 30 Aug. 2000, K.A. Seifert (epitype designated here CBS H-22943, MBT378600, ex-epitype culture CBS 112734 = CPC 3952); idem. culture CBS 115526 = CPC 3953. USA, Vermont, Charlotte, on Tilia americana, June 1881, C.G. Pringle (holotype NYS-F-3187).
Note: See notes under Paracercosporidium microsora.
Clade 39: “Sirosporium”
Sirosporium celtidis (Biv.) M.B. Ellis, Mycol. Pap. 87: 4. 1963.
Basionym: Monilia celtidis Biv., Stirp. Rar. Sicilia 3: 18. 1815.
Synonyms: Gyrocerus celtis (Biv.) Mont. & Ces., Syll. Gen. Sp. Crypt.: 308. 1856.
Helicoceras celtidis (Biv.) Linder, Ann. Missouri Bot. Gard. 18: 3. 1931.
For additional synonyms see MycoBank.
Descriptions and illustrations: Chupp, 1954, Ellis, 1971.
Materials examined: Algeria, on Celtis australis, Nov. 1923, C. Killian, dep. 1925, culture CBS 158.25. Italy, Rome, on C. australis, Aug. 1949, V. Mezzetti, dep. 1950, culture CBS 289.50. Portugal, unknown host, date and collector, dep. Estação Agronómica Nacional (Sacavém), 1948, culture CBS 238.48.
Notes: The species Sirosporium celtidis is based on Monilia celtidis, which was described from the host Celtis australis, probably from Sicily (Italy), although it is not clearly stated in the original publication, and the herbarium specimen could not be located. The species has previously been reported from Algeria, India, Israel, Italy, Japan, Morocco, Portugal, Taiwan and Turkey (Crous & Braun 2003). The cultures observed in this study are presently sterile, but at the time they were collected, the strains CBS 158.25 and CBS 289.50, were subjected to a thorough morphological characterisation (Killian, 1925, Mezzetti, 1950). The morphological description agrees with more recent treatments of the genus Sirosporium (Chupp, 1954, Ellis, 1971). Sirosporium celtidis differs from the type of Sirosporium, Sirosporium antenniforme, by producing conidiophores with thin walls and producing longer and narrower conidia that only rarely show 1–2 longitudinal septa (Ellis 1971). Since there are no cultures available of Sirosporium antenniforme, the precise phylogenetic position of the genus remains unresolved. The present strains cluster in a well supported clade in the pylogenetic analyses (Fig. 1, clade 39; Fig. 3, clade 4).
Clade 40: Cercosporidium
Cercosporidium Earle, Muhlenbergia 1: 16. 1901, emend.
Description: Foliicolous. Mycelium internal, hyaline to pale olivaceous brown, or dark brown. Stromata small to developed, olivaceous brown to brown. Conidiophores solitary or in fascicles, micro- to macronematous, sometimes irregular in width, very pale to olivaceous brown, smooth to rough, simple or branched, straight to geniculate-sinuous, sometimes reduced to conidiogenous cells. Conidiogenous cells terminal, proliferation sympodial or percurrent, mono- or polyblastic, with conidiogenous loci slightly to distinctly thickened and darkened. Conidia solitary in vivo, rarely catenate in vitro, hyaline to pale olivaceous, smooth to verruculose, thick-walled, cylindrical, ovoid, obovoid or obclavate, straight or slightly curved, slightly thickened, truncate or short obconical truncate at the base, broadly rounded or beak-like at the apex, euseptate, hilum thickened, darkened and refractive.
Type species: Scolicotrichum euphorbiae Tracy & Earle (= Cercosporidium chaetomium (Cooke) Deighton; ≡ Cladosporium chaetomium Cooke).
Cercosporidium californicum (S.T. Koike & Crous) Videira & Crous, comb. nov. MycoBank MB822747. Fig. 18.
Fig. 18.
Cercosporidium californicum (CBS 128857). A–D. Observations in vivo. A. Leaf spot symptoms on the host. B. Conidiophores, conidiogenous cells and conidia. C. Conidiophores and conidiogenous cells. D. Conidia. E–J. Observations in vitro. E. Culture on V8. F. Mycelium producing red pigment inside the cells and outside. G, I. Conidiophore, conidiogenous cell and conidia. H, J. Conidia. Scale bars = 10 μm.
Basionym: Passalora californica S.T. Koike & Crous, IMA Fungus 2: 8. 2011.
Description (adapted from Koike et al. 2011): Phytopathogenic, causing black and irregular leaf spots. Stroma amphigenous, globose, brown, 10–30 μm long and 30–100 μm wide. Conidiophores arising from stroma in dense sporodochia, brown, verruculose, subcylindrical, mostly straight, at times geniculate-sinuous, 15–25 × 3–8 μm, occasionally up to 100 μm long and 4–5 μm wide, frequently reduced to conidiogenous cells. Conidiogenous cells terminal, integrated, brown, verruculose, subcylindrical, straight or geniculate-sinuous, usually 10–15 × 4–6 μm, occasionally 15–35 × 4–5 μm, conidiogenous loci apical and lateral, thickened, darkened and refractive, 1–1.5 μm diam. Conidia solitary, brown, verruculose, guttulate, obclavate to subcylindrical, apex obtusely rounded, base obconically truncate, (32–)55–95(–180) × (4–)5–6 μm, (1–)3–5(–9)-septate, hilum darkened, thickened and refractive, 2 μm diam.
Materials examined: USA, California, Santa Clara County, on leaves of Asclepias fascicularis, 19 Jul. 2010, S.T. Koike (holotype CBS H-20512, ex-type cultures CBS 128857 = CPC 18389); idem. cultures CPC 18390, CPC 18391.
Notes: Including Cercosporidium californicum (Fig. 18), several passalora-like species are known from the host genus Asclepias, namely Passalora clavata var. clavata, Passalora clavata var. hansenii, Passalora venturioides and Passalora elaeochroma (Braun and Mel'nik, 1997, Koike et al., 2011). Unfortunately, no cultures of the previous species were available for this study and their phylogenetic position will remain unknown until they are recollected and their DNA analysed. In a phylogenetic analysis based on LSU data, Cercosporidium californicum was described (as Passalora californica) as closely related to Passalora arachidis (as Mycosphaerella arachidis) (Koike et al. 2011). In this study, the phylogenetic analyses place Cercosporidium californicum strains in a well-supported clade closely related to Cercosporidium miurae (Fig. 1, clade 40; Fig. 3, clade 5).
Cercosporidium chaetomium (Cooke) Deighton, Mycol. Pap. 112: 27. 1967. Fig. 19.
Fig. 19.
Cercosporidium chaetomium (CBS 142177). A–D. Observations in vivo. A. Leaf spot symptoms on the host. B. Conidiophores, conidiogenous cells and conidia. C. Conidiophores and conidiogenous cells. D. Single conidia. E–J. Observations in vitro. E. Culture on OA. F. Conidiophore and conidiogenous cell. G–J. Conidiophore, conidiogenous cell and conidia. Scale bars = 10 μm.
Basionym: Cladosporium chaetomium Cooke, Grevillea 17(83): 66. 1889.
Synonyms: Scolicotrichum euphorbiae Tracy & Earle, Bull. Torrey Bot. Club 23(5): 209. 1896.
Pyricularia euphorbiae (Tracy & Earle) G.F. Atk., Bull. Cornell Univ. (Science) 3(1): 40. 1897.
Passalora chaetomium (Cooke) Arx, Proc. K. Ned. Akad. Wet., Ser. C, Biol. Med. Sci. 86(1): 44. 1983.
For additional synonyms see MycoBank.
Description in vivo (CBS H-22944): Phytopathogenic, causing small leaf spots, brown to reddish brown with purplish brown border, circular to subcircular, 2–3 mm diam. Caespituli amphigenous, olivaceous, effuse. Mycelium internal, hyaline, pale to pale olivaceous brown, or dark brown. Stromata amphigenous, substomatal, epidermal, olivaceous brown to brown, small to well developed, 15–90 μm diam. Conidiophores erumpent through the cuticule, or emerging from stomata, solitary or in dense fascicles, smooth, thick-walled, very pale to olivaceous brown, paler towards the apex, simple, cylindrical, straight, sinuous to geniculate, irregular in width, 17–45 × 3.5–8 μm, sometimes reduced to conidiogenous cells. Conidiogenous cells terminal, proliferating sympodially or percurrently, polyblastic, with rim-like conidiogenous loci, slightly thickened and darkened, located on the shoulders and apex, 2–3 μm diam. Conidia solitary, hyaline to pale olivaceous, smooth to verruculose, thick-walled, ovoid, cylindrical, straight or slightly curved, base obconically truncate, apex broadly rounded or beak-shaped, 26–48 × 3.5–5 μm, 0–3-euseptate, hila slightly thickened and darkened, 2–3 μm diam.
Description in vitro (on V8; CPC 18624): Mycelium hyaline to pale olivaceous brown, smooth to rough, uniform to variable in width, 2–3 μm, sometimes constricted at septa. Conidiophores micro- or macronematous, straight or mildly sinuous, simple or branched, pale to pale olivaceous brown, 2.5–250 × 2.5–5 μm. Conidiogenous cells integrated, terminal and intercalary, proliferating sympodially, mono- or polyblastic, with conidiogenous loci slightly thickened and darkened, 2–2.5 μm diam. Conidia solitary or catenate, hyaline to pale olivaceous brown, smooth to rough, cylindrical to obclavate, long-obconically truncate at the base, rounded or beak-like at the apex, 10–75 × 2.5–5 μm, indistinctly 0–5-euseptate, slightly constricted at septa, hila slightly thickened and darkened, 2–2.5 μm diam.
Materials examined: Canada, Ontario, Guelph, on Euphorbia sp., 28 Sep. 2010, P.W. Crous & K.A. Seifert (epitype designated here CBS H-22944, MBT378571, ex-epitype culture CBS 142177 = CPC 18624). USA, New Jersey, Newfield, on leaves of Euphorbia sp., J.B. Ellis No. 2289 (holotype K; isotype IMI 118400).
Notes: Several researchers have discussed the taxonomic position of the genus Cercosporidium to date (Deighton, 1967, Ellis, 1971, von Arx 1983, Braun, 1995, Baker et al., 2000, Crous and Braun, 2003, Braun et al., 2013). The genus was described by Earle (1901: 16) who designated Scoletotrichum euphorbiae as type of the genus [“As the type of this genus I take the species published as Scoletotrichum (?) euphorbiae Tracy & Earle, Bull. Torr. Bot. Club, 23: 209, also as Piricularia euphorbiae (T. & E.) Atkinson, Bull. Cornell univ. 3: 40”]). Deighton (1967) stated that although Earle (1901) designated Scoletotrichum euphorbiae as the type species of Cercosporidium, he did not validly publish the combination in the genus. Deighton (1967) published a revised description of the genus and introduced a combination of the older name Cladosporium chaetomium into Cercosporidium. Subsequent authors followed this treatment (Baker et al. 2000, Crous & Braun 2003). However, Braun et al. (2013) cited Cercosporidium helleri Earle (Fig. 20), described on Sphenoclea zeylanica from Puerto Rico [lectotype (designated here), MBT378572, Puerto Rico, near Añasco, 6 Feb. 1900, A.A. Heller, Plants of Porto Rico 4537 (NY00945749); isolectotypes e.g. in BPI, CHRB, CUP, F, FH, MSC, NEB, NY, UC], as type species of Cercosporidium. Cercosporidium helleri was described as a new species on the same page as the genus was introduced (Earle 1901) and represents the only species in the original publication with description and with a name affiliated with Cercosporidium, which was the source of the error in the type citation in Braun et al. (2013). The status of the genus Cercosporidium was extensively debated over the years with some authors defending Cercosporidium as a synonym of Passalora (von Arx 1983, Castañeda and Braun, 1989, Braun, 1995), while other authors (Pons and Sutton, 1996, Baker et al., 2000) defended Cercosporidium as a recognisably distinct genus (for extensive arguments see Baker et al. 2000). From the results of exhaustive phylogenetic analyses and morphological observations of passalora-like fungi in this study, the genus Cercosporidium is resurrected here (Fig 1, clade 40; Fig. 3, clade 5), typified by Scolicotrichum euphorbiae (= Cercosporidium chaetomium), with a well-developed stroma, and geniculate-sinuous conidiophores with rim-like conidial loci, conidia solitary, subcylindrical to obclavate, pale coloured, relatively thick-walled, smooth to verruculose surface, and darkened hila (Fig. 19).
Fig. 20.
Cercosporidium helleri (NY00945740). A–E. Observations in vivo. A. Leaf spot symptoms on the host. B, D. Conidiophores, conidiogenous cells and conidia. C. Partial conidiophore, conidiogenous cell and conidia. E. Conidia. Scale bars = 10 μm.
Cercosporidium miurae (Syd. & P. Syd.) X.J. Liu & Y.L. Guo, Acta Mycol. Sinica 1(2): 98. 1982. Fig. 21.
Fig. 21.
Cercosporidium miurae (CPC 14628). A–E. Observations in vitro. A. Culture on V8. B–D. Conidiophore, conidiogenous cell and conidia. E. Single conidia. Scale bars = 10 μm.
Basionym: Cercospora miurae Syd. & P. Syd., Ann. Mycol. 11: 117. 1913.
Synonyms: Cercosporiopsis miurae (Syd. & P. Syd.) Miura, Flora of Manchuria and East Mongolia. Part III. Cryptogams, fungi 3: 533. 1928.
Passalora miurae (Syd. & P. Syd.) U. Braun & H.D. Shin, Mycotaxon 49: 354. 1993.
Passalora miurae (Syd. & P. Syd.) Poonam Srivast., J. Living World 1(2): 117. 1994.
Description in vivo: Leaf spots indistinct, yellowish brown, 1–5 mm. Mycelium internal, hyaline to pale brown, smooth. Caespituli hypophyllous, effuse. Stromata lacking or small, composed of few brown cells, stomatal. Conidiophores emerging through the stroma, brown, thick-walled, smooth to rough, often rugged by the forming of numerous loci, straight, flexuous or geniculate, branched, 15–250 × 5 μm. Conidiogenous cells intercalary and terminal, proliferating sympodially, polyblastic, with rim-like conidiogenous loci, thickened and darkened, located on the apex or shoulders, 2–2.5 μm diam. Conidia solitary, pale brown, thick-walled, smooth to rough, obovoid, obclavate, cylindrical, straight to sharply curved, base obconicaly truncate, apex rounded or beak-like, 15–60 × 5–10 μm, 1–3-septate, hilum slightly thickened and darkened, 2–2.5 μm diam.
Description in vivo (on OA; CPC 14628): Mycelium hyaline to brown, uniform in width, 2.5 μm diam. Conidiophores micro- or macronematous, pale brown to pale olivaceous brown, smooth to rough, septate, straight, geniculate-sinuous, 25–180 × 2.5–3.8 μm. Conidiogenous cells integrated, apical and intercalary, mono- or polyblastic, proliferating sympodially, with conidiogenous loci slightly thickened and darkened, 1–2.5 μm diam. Conidia solitary, pale brown to pale olivaceous brown, finely verruculose, ovoid, cylindrical, apex broadly rounded, base obconically truncate, 20–28 × 3.8–10 μm, 1–3-euseptate, occasionally constricted at septa, hilum slightly thickened loci and darkened, 1–2.5 μm diam.
Materials examined: Japan, Hokkaido, Sapporo, Yamahana, on Cynanchum caudatum, 15 Sep. 1907, M. Miura (holotype S F37417; isotype NIAES C-268); Iwate, on Cynanchum caudatum, 25 Sep. 1926, K. Togashi, CNS 426. Republic of Korea, on Metaplexis japonica, 1 Oct. 2007, H.D. Shin, CBS H-22945, culture CBS 142235 = CPC 14628; on Metaplexis japonica, 22 Sep. 2007, H.D. Shin, culture CPC 14643.
Notes: The type species of Cercosporidium miurae was described from Cynanchum caudatum collected in Japan. The morphology of observed specimens and cultures (Fig. 21), originating from Metaplexis japonica, are in agreement with the description available in literature (Chupp 1954), and in line with the Cercosporidium generic description. Both host genera belong to the family Asclepiadaceae. In the phylogenetic analyses, the two available strains cluster in a clade well-supported by all three phylogenetic methods employed (Fig. 1, clade 40; Fig. 3, clade 5).
Clade 41: Collarispora
Collarispora Videira & Crous, gen. nov. MycoBank MB822584.
Etymology: Producing conidia with marginal frill.
Description: Phytopathogenic, causing leaf spots. Ascostromata amphigenous, black, erumpent through epidermis, thick-walled, composed of several layers of textura angularis, ostiole central, periphysate. Asci fasciculate, ellipsoid, straight to incurved, bitunicate, 8-spored, with apical chamber. Ascospores hyaline, smooth, fusoid-ellipsoidal, medianly 1-septate, guttulate, slightly incurved, widest just above septum, tapering towards both acutely rounded ends, thick-walled; ascospores germinate from both ends, germ tubes parallel to the long axis of the spore, lateral branches also developing, becoming constricted at median septum, but remaining hyaline. Mycelium consisting of hyaline, smooth, septate and branched hyphae. Conidiogenous cells terminal on hyphae, hyaline, subcylindrical, smooth, conidiogenous loci not thickened nor darkened. Conidia solitary, subcylindrical to narrowly obclavate, straight to flexuous, apex obtuse, base truncate, multiseptate, hila not thickened nor darkened, with visible marginal frill; with age conidia tend to become pale olivaceous and finely verruculose.
Type species: Collarispora valgourgensis (Crous) Videira & Crous (≡ Mycosphaerella valgourgensis Crous).
Collarispora valgourgensis (Crous) Videira & Crous, comb. nov. MycoBank MB822752.
Basionym: Mycosphaerella valgourgensis Crous, Persoonia 26: 151. 2011.
Description and illustration: Crous et al. (2011a).
Materials examined: France, Ardeche, Valgourge, Domaine Le Fraysse, N44°35.469′ E004°07.710′, on leaves of Yucca sp., 15 Jul. 2010, P.W. Crous (holotype CBS H-20593, culture ex-type CBS 129531 = CPC 18385). USA, Ohio, Columbus, on Malus sp., 29 Sep. 2005, M. Ellis, culture CBS 125311.
Notes: Collarispora valgourgensis was described based on both the sexual morph, which is mycosphaerella-like, and the asexual morph, which is pseudocercospora-like (Crous et al. 2011a, as Mycosphaerella valgourgensis). However, the asexual morph differed from Pseudocercospora by producing subcylindrical to narrowly obclavate conidia that are initially hyaline but later become pale brown and verruculose, with a basal marginal frill. The phylogenetic analyses place this strain in a well-supported clade (Fig. 1, clade 41; Fig. 3, clade 6) that is closely related to Cercosporidium as presently defined. According to Deighton (1967) and the morphological review presented in this study, the conidia in Cercosporidium can be narrowly obclavate and verruculose, but a basal marginal frill has not been observed. In a supplementary phylogenetic analysis performed using a smaller dataset (sequences in dataset 3 corresponding to Fig. 3, clades 1–15), this clade separates from the Cercospordium clade. Based on a BLAST comparison against the alignment, Collarispora valgourgensis CBS 129531 is 100 % (473/473) identical to Amycosphaerella sp. CBS 111001 based on ITS and 92 % (718/780) identical to Cercosporidium chaetomium CPC 18624 based on rpb2. The morphological differences and the instability of the phylogenetic position of these strains indicate that it is rather better to introduce this species into a new genus than combine it into Cercosporidium.
Clade 42: Neocercosporidium
Neocercosporidium Videira & Crous, gen. nov. MycoBank MB822596.
Description: Phytopathogenic. Caespituli amphigenous, punctiform, scattered to dense, dark brown to blackish. Mycelium both internal and external, hyphae branched, septate, subhyaline to medium olivaceous brown, thin-walled, smooth. Stromata well-developed, substomatal to intraepidermal, immersed, brown to dark brown. Conidiophores arising from stromata, occasionally from superficial hyphae, in small to large and loose to dense fascicles, when dense almost coremioid, rarely solitary, smooth, olivaceous to dark olivaceous brown throughout or paler at the tips, thin-walled, erect, straight, subcylindrical to strongly geniculate-sinuous, simple or occasionally branched, sometimes reduced to conidiogenous cells. Conidiogenous cells integrated, terminal, proliferating sympodially, occasionally percurrently, conidiogenous loci minute but slightly thickened, darkened and refractive, front view resembling minute circles. Conidia solitary, subhyaline to pale olivaceous or brownish, smooth, thin-walled, multi-septate, obclavate-cylindrical, apex obtuse to subobtuse, base rounded to short obconically truncate, hila slightly thickened, darkened and refractive.
Type species: Neocercosporidium smilacis (Thüm.) U. Braun et al.
Neocercosporidium smilacis (Thüm.) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822765. Fig. 22.
Fig. 22.
Neocercosporidium smilacis (CPC 19342). A–F. Observations in vitro. A. Culture on V8. B–D. Conidiophore, conidiogenous cell and conidia. E. Conidiogenous cell and conidia. F. Single conidia. Scale bars = 10 μm.
Basionym: Cercospora smilacis Thüm., Contrib. Fl. Mycol. Lusit. 2: 14. 1879.
Synonyms: Passalora smilacis (Thüm.) U. Braun, Arnoldia 14: 30. 1997.
Cercospora smilacina Sacc., Michelia 2(7): 364. 1881.
Cercospora smilacis var. asperae Gonz. Frag., Trab. Mus. Nac. Ci. Nat., Ser. Bot. 9: 66. 1916.
Descriptions and illustrations: Ellis, 1976, Braun et al., 2014.
Description in vitro (on SNA; CPC 19342): Mycelium hyaline to pale brown, 2.5–3 μm diam. Conidiophores emerging from hyphae or bunching large brown cells, micro- or macronematous, pale to pale olivaceous brown, multiseptate, cylindrical, straight to slightly curved, geniculate-sinuous at the apex, often bearing microcyclic conidia, 7.5–125 × 3.5–5 μm. Conidiogenous cells integrated, terminal, intercalary, mono- or polyblastic, proliferating sympodially, apex conically truncate, with conidiogenous loci thickened, darkened and protruding, 1.5–2.5 μm diam. Conidia solitary, subhyaline to pale olivaceous brown, often undergoing microcyclic sporulation, obclavate to long cylindrical, base obconically truncate, apex rounded and long-beak shaped, 32–120 × 3.5–5 μm, 7–10-euseptate, hilum thickened, darkened and refractive, 1.5–2.5 μm diam.
Materials examined: Italy, Sardinia, Monte Ferru, on Smilax aspera, 18 May 1971, W. Gams, CBS H-9864, culture CBS 556.71; Lazio, Viterbo, Selva del Lamone, Il sentiero dei Briganti, on Smilax sp., 30 Apr. 2011, W. Gams, culture CPC 19342. Portugal, Algarve, Carvoeiro, leaf spot on Smilax aspera, 23 Jan. 2008, G. Verkley (epitype designated here MBT378573, CBS 122888, preserved as metabolically inactive); idem. cultures CBS 122889, CBS 122890, CBS 123352, CBS 123353); Coimbra, on Smilax aspera [mauritanica], May 1879, F. Moller, (lectotype designated by Braun et al. 2014, BPI 441368; topotypes [Thüm., Mycoth. Univ. 1670] BPI 441367, 441368, CUP 41239, HAL, LEP).
Notes: Braun et al. (2014) enumerated the cercosporoid species on Smilaceae hosts and provided an identification key for those genera. Cercospora smilacis was allocated to Passalora s. lat. due to its cylindrical-obclavate pigmented conidia and conspicuous conidiogenous loci that look like minute circles in front view (Fig. 22). Passalora s. str. has more prominently obclavate conidia that are single and 1-septate (Fig. 15). The strains used in this study cluster apart from the Passalora type species in a clade well-supported by all the phylogenetic analyses (Fig. 1, clade 42; Fig. 3, clade 1). Based on a BLAST comparison against the alignment, Neocercosporidium smilacis CPC 19342 shares 96 % (455/476) similarity with Paramycovellosiella passaloroides CPC 14694 based on ITS and 91 % (706/780) similarity with Paracercosporidium tiliae CBS 115526 based on rpb2.
Clade 43: Sultanimyces
Sultanimyces Videira & Crous, gen. nov. MycoBank MB822704.
Etymology: Based on “Sultana” (a race of white wine grape) and -myces (fungus).
Description: Phytopathogenic. Caespituli hypophyllous, punctiform, dark brown. Mycelium internal, hyphae almost hyaline. Stroma substomatal, composed of densely packed pale olivaceous hyphae. Conidiophores in fascicles, emerging from stromata, pale to deep olivaceous, straight, smooth. Conidiogenous cells polyblastic, integrated, terminal, more or less clavate, usually continuous above basal septum but sometimes septate and swollen at the base, conidiogenous loci conspicuous and slightly protruding. Conidia solitary, pale to moderate olivaceous, ellipsoid, fusiform, subcylindrical or obclavate, straight, smooth to verruculose, aseptate but usually septate, median septum usually thicker, sometimes slightly constricted at median septum, with conspicuous and slightly protruding hila.
Type species: Sultanimyces vitiphyllus (Speschnew) Videira & Crous (≡ Coryneum vitiphyllum Speschnew).
Sultanimyces vitiphyllus (Speschnew) Videira & Crous, comb. nov. MycoBank MB822802.
Basionym: Coryneum vitiphyllum Speschnew, Trudy Tiflissk. Bot. Sada 5: 177. 1901.
Synonyms: Cercospora roesleri f. vitiphylla (Speschnew) Elenkin, Bolez. Rast.: 68. 1909.
Scolicotrichum vitiphyllum (Speschnew) Karak. & Vassiljevsky, Fungi imperfecti Parasitici. I. Hyphomycetes: 215. 1937.
Cercospora vitiphylla (Speschnew) Barbarin, Ezeg. Sved o Boleznj. Povrezden. Kul't. Dikorast. Polezn. Rast. VII–VIII: 351. 1911–1912.
Asperisporium vitiphyllum (Speschnew) Deighton, Mycol. Pap. 138: 184. 1975.
Exosporium sultanae du Plessis, Ann. Univ. Stellenbosch, Reeks A, 24: 19. 1946.
Stigmina esfandiarii Petr., Sydowia 4(1-6): 35. 1950.
Description in vivo (adapted from Sutton 1975 and Ellis 1976): Caespituli hypophyllous, punctiform, dark brown, spread over light brown lesion. Mycelium internal, hyphae almost hyaline, 2.5–3.5 μm. Stroma substomatal, 40–50 μm high, composed of densely packed, pale olivaceous hyphae about 4 μm wide. Conidiophores in dense fascicles, emerging from stromata, pale to deep olivaceous, straight, smooth, up to 30 × 5–7 μm. Conidiogenous cells polyblastic, integrated, terminal, more or less clavate, usually continuous above basal septum but sometimes 1–3-septate and swollen at the base (up to 9 μm), conidiogenous loci conspicuous and slightly protruding, about 2 μm. Conidia pale to moderate olivaceous, ellipsoid, fusiform, subcylindrical or obclavate, straight, smooth to verruculose, 13–34 × 5.5–8 μm (Sutton 1975) or 15–28 × 7–10 μm (Ellis 1976), mostly 1–3-septate, rarely 5-septate, median septum usually thicker, sometimes slightly constricted at median septum, with hila conspicuous and slightly protruding.
Materials examined: Uzbekistan, Samarkand (Buaki, Fusayne), on living leaves of Vitis vinifera, unknown collector and date (lectotype [iconotype] designated here MBT378577, tab. 2, figs 20–26 in Speschnew 1901). South Africa, Northern Cape Province, Kenhardt district, on Vitis sp. (Sultana vines), 1948, isol. and dep. S.J. du Plessis, culture CBS 206.48.
Notes: Coryneum vitiphyllum was transferred to the genus Asperisporium based on the polyblastic conidiogenous cells with conspicuous scars and euseptate, verrucose conidia with conspicuous hila. A culture of Exosporium sultanae isolated by du Plessis, who described the species (Du Plessis 1946), was analysed and found to be sterile. The strain CBS 206.48 (Fig. 1, clade 43; Fig. 3, clade 2) is not congeneric with the type species of the genus Asperisporium, Asperisporium caricae (Fig. 1, clade 43; Fig. 3, clade 13). Conidiophores of Asperisporium caricae also emerge from dark stromata, are densely arranged and have polyblastic conidiogenous cells, but conidia are shorter and wider (14–22 × 8–13 μm), mostly ellipsoid and typically 1-septate (Minnis et al. 2011). The phylogenetic position of Asperisporium vitiphyllum, among cercosporidium-like species with which it shares few characters (e.g. multiseptate conidia), and apart from Asperisporium caricae, is suggestive that this type of morphology emerged more than once within the Mycosphaerellaceae. Based on a BLAST comparison against the alignment, Sultanimyces vitiphyllus CBS 206.48 shares 95 % (448/474) similarity based on ITS and 91 % (711/780) similarity based on rpb2 with with Paracercosporidium tiliae CBS 115526. Therefore, based on phylogenetic differences and distinctive morphological characters, the new monotypic genus Sultanimyces is hereby introduced to accommodate this species.
Clade 44: Paramycovellosiella
Paramycovellosiella Videira, H.D. Shin & Crous, gen. nov. MycoBank MB822603.
Etymology: Derived from “Para” (similar to) + resembling the genus Mycovellosiella.
Description: Phytopathogenic. Caespituli hypophyllous, occasionally epiphyllous. Mycelium internal and external, olivaceous brown to olivaceous, septate, branched. Stromata lacking or rudimentary, composed only of a few brown swollen hyphal cells. Conidiophores in loose fascicles, emerging through stomata or as lateral branches of external hyphae, pale olivaceous brown throughout or paler at the apex, continuous or septate, straight to geniculate. Conidiogenous cells integrated, terminal or intercalary, pale olivaceous brown, smooth, mono- or polyblastic, determinated or proliferating sympodially, conidiogenous loci small, thickened and darkened, located on apex or shoulders. Conidia solitary or catenate, cylindrical, clavate, obclavate, straight to mildly curved, subhyaline to pale olivaceous brown, aseptate to multiseptate, non-constricted at the septa, hila small, thickened, darkened and slightly protuberant, basal (terminal conidia) or at both ends (intercalary conidia and ramoconidia).
Type species: Paramycovellosiella passaloroides (G. Winter) Videira, H.D. Shin & Crous (≡ Cercospora passaloroides G. Winter).
Paramycovellosiella passaloroides (G. Winter) Videira, H.D. Shin & Crous, comb. nov. MycoBank MB822820. Fig. 23.
Fig. 23.
Paramycovellosiella passaloroides (CPC 14694). A–J. Observations in vitro. A. Culture on SNA. B, G. Conidiophore, conidiogenous cell and conidia. C, D, E. Conidiogenous cell and conidia. F, H. Single and catenate conidia. I. Culture on V8 supplemented with banana leaf promoted the development of black spermogonia and spermatia whitish in mass. J. Hyaline spermatia. Scale bars = 10 μm.
Basionym: Cercospora passaloroides G. Winter, Hedwigia 22: 71. 1883.
Synonyms: Cylindrosporium passaloroides (G. Winter) J.C. Gilman & W.A. Archer, Iowa St. Coll. J. Sci. 3: 334. 1929.
Mycovellosiella passaloroides (G. Winter) J.K. Bai & M.Y. Cheng, Acta Mycol. Sin. 11: 120. 1992.
Passalora passaloroides (G. Winter) U. Braun & Crous, in Crous & Braun, CBS Biodiversity Ser.: 309. 2003.
Descriptions and illustrations: Chupp, 1954, Shin and Kim, 2001.
Description in vitro (on V8; CPC 14694): Mycelium hyaline to pale, hyphae smooth, uniform in width, (1–)2(–3) μm diam. Conidiophores micronematous, erect, simple, straight, pale olivaceous, (30–)79–110(–230) × (2.5–)3–4(–5) μm. Conidiogenous cells integrated, terminal or intercalary, pale olivaceous, smooth, determinate or proliferating sympodially, mono- or polyblastic, with conidiogenous loci small, thickened, darkened and protruding, 1–1.5 μm diam. Conidia catenate (in vivo usually solitary), in single or double chains, pale olivaceous, smooth to verruculose, cylindrical to obclavate, straight to slightly curved, base rounded or obconically truncate, sometimes swollen, apex rounded, sometimes beak-like or swollen, variable in width, (11.5–)20–27(–43.5) × (3.5–)4.5–5(–6) μm, 1–2(–4)-septate, with hila small, thickened, darkened and refractive. Spermogonia formed on the surface of sterilized banana leaf placed on the medium surface, pycnidial, globose, apical ostiole, black. Spermatia hyaline, whitish in mass, smooth-walled, ellipsoid to subcylindrical, with rounded ends, aseptate, 2–4 × 2 μm.
Materials examined: Republic of Korea, Pyeongchang, on Amorpha fructicosa, 29 Sep. 2003, H.D. Shin, culture CPC 10770; on Amorpha fructicosa, 30 Oct. 2007, H.D. Shin, culture CPC 14694.
Notes: The genus Paramycovellosiella is established to accommodate a mycovellosiella-like species that is not congeneric (Fig. 1, clade 44; Fig. 3, clade 7) with the type of Mycovellosiella, Mycovellosiella cajani, as it is circumscribed in this study (Fig. 1, clade 7; Fig. 2, clade 10). Morphologically it is almost indistinguishable from Mycovellosiella. It can be distinguished from the most closely related genera, Cercosporidium, Paracercosporidium and Neocercosporidium, by forming catenate conidia (Fig. 23). The type specimen of Cercospora passaloroides (USA, Illinois, on Amorpha canescens, A.B. Seymour) could not be located and is likely not preserved (Chupp 1954).
Clade 45: Amycosphaerella
Amycosphaerella Quaedvlieg & Crous, Persoonia 33: 22. 2014.
Description (from Quaedvlieg et al. 2014): Foliicolous, plant pathogenic. Ascomata pseudothecial, amphigenous, solitary, black, subepidermal, globose, with central apical ostioles, becoming papillate; walls of 2–3 layers of medium brown textura angularis, subhymenium of 1–2 layers of hyaline cells. Asci obovoid to broadly ellipsoidal, straight or incurved, 8-spored. Ascospores bi- to triseriate, overlapping, hyaline, guttulate, straight, fusoid-ellipsoidal with obtuse ends, widest in middle of apical cells, medianly 1-septate, tapering toward both ends, but more prominently toward base.
Type species: Amycosphaerella africana (Crous & M.J. Wingf.) Quaedvlieg & Crous (≡ Mycosphaerella africana Crous & M.J. Wingf.).
Amycosphaerella africana (Crous & M.J. Wingf.) Quaedvlieg & Crous, Persoonia 33: 23. 2014.
Basionym: Mycosphaerella africana Crous & M.J. Wingf., Mycologia 88: 450. 1996.
Synonyms: Teratosphaeria africana (Crous & M.J. Wingf.) Crous & U. Braun, Stud. Mycol. 58: 8. 2007.
Mycosphaerella aurantia A. Maxwell, Mycol. Res. 107: 353. 2003.
Mycosphaerella ellipsoidea Crous & M.J. Wingf., Mycologia 88: 452. 1996.
Mycosphaerella aggregata Carnegie & Keane, Mycol. Res. 98: 415. 1994, nom. illegit (Art. 53.1). (non Mycosphaerella aggregata (Schwein.) J.A. Stev. 1918).
Mycosphaerella gregaria Carnegie & Keane, Mycol. Res. 101: 843. 1997, nom. inval. (Art. 41.5).
Phaeophleospora gregaria (Carnegie & Keane) Quaedvlieg & Crous, Persoonia 33: 23. 2014, nom. inval. (Art. 41.4).
Mycosphaerella buckinghamiae Crous & Summerell, Australas. Pl. Pathol. 29(4): 272. 2000.
Description and illustration: Crous (1998).
Materials examined: Australia, New South Wales, Mangrove Mountain, on leaves of Buckinghamia sp., Aug. 1999, P.W. Crous & B. Summerell (JT 902, DAR 74865, holotype of Mycosphaerella buckinghamiae, cultures ex-type CBS 111996 = CPC 3006, CPC 3007); Victoria, Nowa Nowa, on leaves of Eucalyptus grandis, 11 Nov. 1990, A.J. Carnegie (holotype of Mycosphaerella gregaria IMI 353729b, isotype VPRI 20739a, cultures ex-type CBS 134927 = DAR 72368); Western Australia, Bunbury, Summerlea plantation of Western Australian Chip and Pulp (WACAP), E115°37′, S33°40′, on Eucalyptus globulus, 1 May 2000, A. Maxwell (holotype of M. aurantia, PERTH 05849543, isotype MURU0001, culture ex-type CBS 110500 = CMW 14460). Colombia, Sinai, on leaves of Eucalyptus grandis, 1995, M.J. Wingfield, PREM 54978. New Zealand, on Dracaena draco, 1 Mar. 2004, M. Braithwaite, culture CPC 12678. Portugal, on leaves of Eucalyptus globulus, Jun. 1995, S. McRae, PREM 54974, cultures CPC 1196–1198. South Africa, Western Cape Province, Stellenbosch, Stellenbosch Mountain, leaves of Eucalyptus viminalis, Oct. 1994, P.W. Crous (holotype of M. africana, PREM 51917, cultures ex-type CPC 794–796 = CBS 116154, 116155, 680.95); on leaves of Eucalyptus deanei, Oct. 1994, P.W. Crous, PREM 51918, culture CPC 816; Rust and Vrede Farm, leaves of Eucalyptus radiata, Nov. 1994, P.W. Crous, cultures CPC 896–898; Darling, Pampoenvlei, leaves of E. globulus, Nov. 1994, P.W. Crous, PREM 51919, cultures CPC 838–840; leaves of E. grandis, Nov. 1994, P.W. Crous, PREM 51920, cultures CPC 833–837; Darling, Pampoenvlei, on leaves of Eucalyptus cladocalyx, 7 Nov. 1994, P.W. Crous (holotype of M. ellipsoidea, PREM 51924, cultures ex-type CPC 849–851, 850 = CBS 110843); Kwazula-Natal Province, Richmond, leaves of Eucalyptus smithii, Nov. 1994, G. Kemp, PREM 51921, cultures CPC 819–821. Zambia, on leaves of E. globulus, Aug. 1995, T. Coutinho, PREM 54973, cultures CPC 1229–1231.
Notes: Only the mycosphaerella-like sexual morph is known for this genus that until now included only one species (Quaedvlieg et al. 2014). Morphologically, Amycosphaerella africana ascospores germinate from both cells and become distorted (though this character was found to vary among different collections, e.g. see Mycosphaerella gregaria). Phylogenetically, Amycosphaerella clusters close to Asperisporium in a clade well-supported by all three phylogenetic analyses (Fig. 1, clade 45; Fig. 3, clade 8). Based on the phylogenetic analyses, the ex-type strain of Mycosphaerella buckinghamiae is identical to Amycosphaerella africana and is therefore considered as a synonym.
Amycosphaerella keniensis (Crous & T.A. Cout.) Videira & Crous, comb. nov. MycoBank MB822738.
Basionym: Mycosphaerella keniensis Crous & T.A. Cout., Mycol. Mem. 21: 74. 1998.
Description and illustration: Crous (1998).
Materials examined: Australia, on Musa sp., unkown collector and date, culture CBS 121391. Kenya, on leaf litter of Eucalyptus grandis, May 1995, M.J. Wingfield (holotype PREM 54402, cultures ex-type CBS 111001 = CPC 1084 = CMW 5147) idem., cultures CPC 1085, CPC 1086.
Notes: The morphological characteristics and phylogenetic position of Mycosphaerella keniensis agree with those of the genus Amycosphaerella, and a new combination is hereby proposed as Amycosphaerella keniensis. Phylogenetically, this species is represented by two strains that cluster in a clade well-supported by all three phylogenetic methods (Fig. 1, clade 45; Fig. 3, clade 8). The strain CBS 121391 was previously classified as Mycosphaerella mozambica based on phylogenetic similarity to the ex-type strain CBS 122464, since it was sterile in culture (Arzanlou et al. 2008). When comparing the type culture of Mycosphaerella mozambica CBS 122464 and strain CBS 121391 using BLAST, they are nearly identical on ITS, but significantly different on actA and his3: on ITS 99 % (496/497) similarity between GenBank EU514257 and GenBank EU514258; on actA 86 % (154/179) similarity and 2 % (5/179) gaps between GenBank EU514318 and GenBank EU514319; on his3 95 % (378/396) similarity and 2 % (8/396) gaps between GenBank EU514371 and GenBank EU514372. The partial rpb2 sequences generated in this study for the same strains showed only 85 % (659/779) similarity. In addition, when comparing the partial rpb2, between the type of Mycosphaerella keniensis CBS 111001 and strain CBS 121391, they are 100 % identical, and the strain is therefore renamed as Amycosphaerella keniensis.
Amycosphaerella sp.
Materials examined: Brazil, Pará, Tomé Acu, on Theobroma cacao, unknown date, H.C. Evans, dep. in 1980, culture CBS 441.80.
Notes: This strain was initially identified as Crinipellis perniciosa, an agaric responsible for the destructive Witches Broom disease on Cocoa (Theobroma cacao). The present strain clusters in the Amycosphaerella clade (Fig. 3, clade 8), therefore it is not a basidiomycete, and is sterile in culture. There is only a single Mycosphaerella species known to infect Theobroma cacao, namely Mycosphaerella theobromae, but it was described from Africa and the whereabouts of the specimen is unkown (Aptroot 2006). Cacao tree pathogens and endophytes have been studied recently (Mejía et al. 2008), but no mycosphaerella-like fungi have been detected so far. Another mycosphaerella-like pathogen known from cacao is Ceratosperma theobromae, but little is known about this pathogen (see section Genera of Mycosphaerellaceae below).
Clade 46: Pseudocercosporella
Pseudocercosporella Deighton, Mycol. Pap. 133: 38. 1973.
Description (from Frank et al. 2010): Colonies in vivo. Mycelium consisting of primary internal and secondary external hyphae, hyaline to pale brown, septate, branched, smooth; stromata lacking or weakly to well-developed, substomatal to intraepidermal. Conidiophores solitary to fasciculate, emerging through stomata or erumpent through the cuticle, arising from inner hyphae or from stromata, sometimes formed as lateral branches of superficial hyphae, or forming crustose to subglobose sporodochia; conidiophores rarely branched, straight and subcylindrical to geniculate-sinuous, hyaline, occasionally faintly pigmented, reduced to conidiogenous cells, or septate. Conidiogenous cells integrated, terminal, mono- to polyblastic, sympodial; conidiogenous loci inconspicuous, unthickened, hyaline. Conidia formed singly, rarely in simple or branched chains, subcylindrical, filiform, somewhat obclavate, euseptate, 1–multi-septate, hyaline, thin-walled, apex obtuse to subacute, subtruncate in catenate conidia, base truncate or subtruncate, hilum unthickened, not darkened, nor refractive.
Type species: Pseudocercosporella bakeri (Syd. & P. Syd.) Deighton (≡ Cylindrosporium bakeri Syd. & P. Syd.).
Pseudocercosporella bakeri (Syd. & P. Syd.) Deighton, Mycol. Pap. 133: 41. 1973.
Basionym: Cylindrosporium bakeri Syd. & P. Syd., Ann. Mycol. 14(5): 372. 1916.
Synonyms: Ramularia ipomoeae F. Stevens, Bull. Bern. Bishop Mus. 19: 150. 1925.
Cercosporella ipomoeae Sawada, Rep. Gov. Agric. Res. Inst. Formosa 86: 161. 1943.
Cercosporella ipomoeicola Sawada, Special Publ. Coll. Agric. Natl. Taiwan Univ. 8: 192. 1959.
Pseudocercosporella ipomoeae Deighton, Mycol. Pap. 133: 38. 1973.
Descriptions and illustrations: Braun, 1995, Frank et al., 2010.
Materials examined: Laos, Vientiane Capital, Xaythany District, Xay Villiage, on Ipomoea sp., 8 Sep. 2009, P. Phengsintham (epitype designated by Frank et al. 2010, CBS H-20409, ex-epitype culture CBS 125685 = CPC 17570). New Zealand, Auckland, St. Johns, Morrin Road, Univ. Campus, on leaf spots on Ipomoea indica, unknown date, C.F. Hill, culture CBS 119488 = Lynfield 1252. Philippines, Los Baños, on Ipomoea sp., Dec. 1915, Baker 4029 (lectotype of Cylindrosporium bakeri, S F40429; isolectotype S F42032, see Braun 1995). Taiwan, Taipei, on Ipomoea indica, 14 Feb. 1913, Y. Fujikuro (isotype of P. ipomoeae TNS-F-220454).
Notes: Based on examination of type materials and additional collections of Pseudocercosporella bakeri and Pseudocercosporella ipomoeae, Braun (1995) concluded that they represented a single taxon. Frank et al. (2010) supported the conclusion of Braun (1995) and designated an epitype for Pseudocercosporella bakeri. Pseudocercosporella, based on Pseudocercosporella bakeri, clusters in a well-supported clade (Fig. 1, clade 46; Fig. 3, clade 10) close to Asperisporium and Amycosphaerella. Based on the single-gene trees of dataset 3 (not shown, in TreeBASE), Pseudocercosporella is reliably distinguished from other genera based on rpb2 sequences while it is less distinct based on LSU and ITS data. Several species that were pseudocercosporella-like in morphology but are phylogenetically not congeneric with the genus type species have been recently assigned to new genera (Videira et al. 2016). As in other cercosporoid genera, morphology alone is insufficient for allocations of new species to the phylogenetically delineated genera.
Clade 47: Distomycovellosiella
Distomycovellosiella U. Braun, C. Nakash., Videira & Crous, gen. nov. MycoBank MB822588.
Etymology: Derived from “Disto-” (referring to distoseptation) + resembling the genus Mycovellosiella.
Description: Phytopathogenic. Caespituli hypophyllous, pale brown or olivaceous, floccose. Mycelium internal composed of hyaline hyphae, external mycelium composed of pale brown to brown hyphae that arise from internal hyphae. Stromata lacking or small, composed of few brown cells. Conidiophores emerging through stomata in loose to dense coremioid fascicles, or arising solitary from external hyphae, brown, straight to geniculate, simple, sometimes branched. Conidiogenous cells integrated, terminal or intercalary, polyblastic, proliferating sympodially, with conidiogenous loci thickened and darkened, flat or protruding. Conidia catenate in unbranched or branched chains, pale brown to pale olivaceous, smooth to verruculose, ovoid, obovoid, obclavate, clavate, cylindrical, fusiform, straight or slightly curved, aseptate, euseptate or distoseptate, hila thickened, darkened and refractive. Differs from the genus Mycovellosiella by forming distoseptate conidia with slightly thickened and refractive loci.
Type species: Distomycovellosiella brachycarpa (Syd.) U. Braun et al. (≡ Cercospora brachycarpa Syd.).
Distomycovellosiella brachycarpa (Syd.) U. Braun, C. Nakash., Videira & Crous, comb. nov. MB822756. Fig. 24.
Fig. 24.
Distomycovellosiella brachycarpa (CPC 18381). A–D. Observations in vivo. A, B. Leaf spot symptoms on the host. C. Catenate and single conidia. D. Conidiophores synnematous-like, conidiogenous cells and conidia. E–G. Observations in vitro. E–G. Conidiophores, conidiogenous cells and conidia. Scale bars = 10 μm.
Basionym: Cercospora brachycarpa Syd., Ann. Mycol. 28: 207. 1930.
Synonyms: Mycovellosiella solanicola (Viégas) Munt.-Cvetk., Lilloa 30: 178. 1960.
Mycovellosiella brachycarpa (Syd.) Deighton, Mycol. Pap. 137: 8. 1974.
Passalora brachycarpa (Syd.) U. Braun & Crous, in Crous & Braun, CBS Biodiversity Ser.: 87. 2003.
For additional synonyms see MycoBank.
Description in vivo (CBS H-22948): Leaf spots circular to subcircular, yellow to pale brown on the upper surface, brown on the lower surface, with indistinct margin, 3–7 mm diam. Caespituli hypophyllous, pale brown or olivaceous, floccose. Mycelium internal composed of hyaline hyphae, external mycelium composed of pale brown to brown hyphae that arise from internal hyphae creeping on the lower leaf surface. Stromata lacking or small, composed of few brown cells. Conidiophores emerging from internal hyphae, bearing through the stomata in loose to dense coremioid fascicles of 4–10 conidiophores, or solitary arising from external hyphae, brown, smooth, branched, straight to geniculate, 11–138 × 2.5–5 μm. Conidiogenous cells integrated, terminal and intercalary, polyblastic, proliferating sympodially, with conidiogenous loci thickened, darkened and somewhat protruding, 1.5–2 μm diam. Conidia catenate in single or branched chains, pale brown to pale olivaceous brown, smooth to verruculose, variable in shape, ovoid, obovoid, obclavate, cylindrical, fusiform, 10–40 × 3–7 μm, 0–3-eu- or distoseptate, hila thickened and darkened, 1.5–2 μm diam.
Description in vitro (on V8; CPC 18381): Mycelium hyaline to pale olivaceous, smooth, uniform in width, 2–2.5 μm. Conidiophores arising from hyphae, macronematous, hyaline to pale brown, smooth, straight, simple or branched, 7.5–215 × 2.5–3 μm. Conidiogenous cells integrated, terminal and intercalary, hyaline to pale brown, smooth, mono- or polyblastic, determinated or proliferating sympodially, conidiogenous loci darkened and thickened, 1–2.5 μm diam. Conidia catenate, in single or branched chains, hyaline to pale olivaceous, smooth to verruculose, guttulate, obovoid, clavate, cylindrical to obclavate, 12.5–55 × 2.5–5 μm, 0–3 indistinctly eu- or distoseptate, hila slightly thickened and darkened.
Materials examined: New Zealand, Coromandel, Thames, on Solanum mauritianum, unknown collector and date, isol. C.F. Hill, Feb. 2004, 1001, MAF, Auckland, culture CBS 115124; same country, unkown host, collector and date, isol. E. McKenzie, 29 Mar. 2003, dep. B.F. Brandwagt, culture CBS 114855. South Africa, KuwaZulu Natal, on Solanum mauritianum, 6 Jul. 2010, A.R. Wood (epitype designated here CBS H-22948, MBT378601, ex-epitype culture CBS 142178 = CPC 18381). Venezuela, D.F., Puerto La Cruz, on Solanum hirtum (= S. obtusifrons), 24 Dec. 1927, H. Sydow 90 (holotype S F23388; isotype IMI 8500).
Notes: Distomycovellosiella is a monotypic genus that is morphologically similar to Mycovellosiella but is not congeneric with its type, Mycovellosiella cajani. Morphologically, Distomycovellosiella differs from Mycovellosiella by forming distoseptate conidia with slightly thickened and refractive loci (Fig. 24). Distomycovellosiella forms a clade well-supported by all three phylogenetic methods (Fig. 1, clade 47; Fig. 3, clade 9) and that is closely related to Pseudocercosporella as defined by its type, Pseudocercosporella bakeri. Based on a BLAST comparison against the alignment, Distomycovellosiella brachycarpa CPC 18381 shares 99 % (470/474) similarity based on ITS with Clarohilum henningsi CPC 17314 and 93 % (723/778) similarity based on rpb2 with Amycosphaerella keniensis CBS 111001. Cultures of collections from South America in general and Venezuela in particular are not yet available, but the collections from New Zealand and South Africa agree with type material of this species and descriptions in literature so that we have decided to fix the application of this species by epitypification.
Clade 48: Asperisporium
Asperisporium Maubl., Bull. Trimestriel Soc. Mycol. France 29: 357. 1913.
Description (from Braun et al. 2014): Usually foliicolous, leaf-spotting hyphomycetes. Mycelium in vivo internal; hyphae branched, septate, hyaline to pigmented, thin-walled, smooth or almost so. Stromata usually well-developed, substomatal to intraepidermal, often somewhat erumpent, pigmented. Conidiophores macronematous, usually densely fasciculate, forming sporodochial conidiomata, continuous to septate, pigmented, wall thin to slightly thickened, smooth or almost so. Conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, usually polyblastic, sympodial, but mostly not strongly geniculate, conidiogenous loci conspicuous, thickened and darkened. Conidia solitary, amero- to phragmosporous (non-scolecosporous), mostly ellipsoid-ovoid, obovoid, fusiform to short cylindrical or obclavate, mostly with 0–3 eusepta, sometimes with a single or several oblique or longitudinal septa, pigmented, distinctly verruculose to coarsely verrucose, basal hilum thickened and darkened, conidial secession schizolytic.
Type species: Asperisporium caricae (Speg.) Maubl (≡ Cercospora caricae Speg.).
Asperisporium caricae (Speg.) Maubl., Bull. Trimestriel Soc. Mycol. France 29: 358. 1913.
Basionym: Cercospora caricae Speg., Anales Soc. Ci. Argent. 22 (4): 215. 1886.
Synonyms: Fusicladium caricae (Speg.) Sacc., Atti Congr. Bot. Palermo: 58. 1902.
Pucciniopsis caricae (Speg.) Höhn., Centralbl. Bakteriol., Abt. II, 60: 5. 1926, nom. illeg (Art. 53.1), non Earle 1902.
Description and illustrations: Minnis et al. (2011).
Materials examined: Brazil, intercepted at USA, Washington, Seattle, entering from Brazil, on fruit of Carica papaya, 16 Apr. 2010, coll. C. Weight, isol. by J.F. Bischoff from BPI 880773 (epitype designated by Minnis et al. 2011, is a dried culture on SDA (BPI 881135), ex-epitype culture CBS 130298); on fruit of Carica papaya, Mar. 2013, A.C. Alfenas, culture CPC 22691. Paraguay, Guarapi, on leaves of Carica papaya, Feb. 1881, B. Balansa, no. 2739 (lectotype designated by Chupp 1954, LPS).
Notes: Morphologically, Asperisporium is passalora-like but with verrucose conidia (Crous & Braun 2003). The phylogenetic position of Asperisporium within the Mycosphaerellaceae has been resolved by Minnis et al. (2011), based on the ITS and LSU of the type species Asperisporium caricae. Asperisporium clusters in a well-supported clade (Fig. 1, clade 48; Fig. 3, clade 13) and is closely related to Amycosphaerella and Paramycovellosiella. Other species assigned to Asperisporum must be individually reassessed.
Asperisporium caricicola Crous & C. Nakash., Sydowia 67: 87. 2015.
Description and illustration: Crous et al. (2015c).
Materials examined: Republic of Fiji, Viti Levu, Navua, on leaves of Carica papaya, 10 Sep. 2013, leg. C. Nakashima (holotype CBS H-22252, isotype TSU: MUMH 11477, cultures ex-holotype CPC 24348 = CBS 139998); idem., culture CPC 24349.
Notes: Asperisporium caricicola is represented by a single strain in the phylogenetic analyses (Fig. 1, clade 48; Fig. 3, clade 13). At the time it was described, Asperisporium caricicola was found to be morphologically very similar to Asperisporium caricae, but phylogenetically distinct based on the partial sequences of LSU and ITS (Crous et al. 2015c). The ITS sequence of Asperisporium caricicola is 97 % (463/477) similar to Asperisporium caricae (GenBank JN190955). The partial sequence of rpb2, however, shares 99 % (778/780) similarity with Asperisporium caricae (GenBank JN190955). More isolates of both species should be analysed in order to determine whether these represent two distinct species or whether they are conspecific with some intraspecific variation.
Clade 49: Pantospora
Pantospora Cif., Ann. Mycol. 36: 242. 1938.
Unconfirmed synonym: Dictyocephala A.G. Medeiros (Medeiros 1962).
Description (from Braun et al. 2013): Foliicolous hyphomycetes, associated with leaf spots. Mycelium internal; hyphae hyaline or almost so. Stromata developed, pigmented. Conidiophores macronematous, in dense coremioid fascicles or synnemata, septate, pigmented, thin-walled, smooth. Conidiogenous cells integrated, terminal, proliferation sympodial and percurrent, with planate to slightly convex, neither thickened nor darkened loci (pseudocercospora-like). Conidia formed singly, shape variable, ellipsoid-ovoid, fusiform, clavate to obclavate, didymo- to scolecosporous, with 1–11 transverse eusepta and often a single or few oblique to longitudinal septa, hila neither thickened nor darkened.
Type species: Pantospora guazumae Cif.
Pantospora guazumae Cif., Ann. Mycol. 36: 242. 1938. Fig. 25.
Fig. 25.
Pantospora guazumae (IMI 59269). A–H. Observations in vivo. A. Leaf spot symptoms on the host. B. Conidiophores and conidia. C–F. Partial conidiophore, conidiogenous cells and conidia. G–H. Conidia. Scale bars = 10 μm.
Synonyms: Cercospora ulmifoliae Obreg.-Bot., Caldasia 1: 51. 1941.
Dictyocephala ulmifoliae (Obreg.-Bot.) A.G. Medeiros, Publ. Inst. Micol. Recife 372: 15. 1962.
Pseudocercospora ulmifoliae (Obreg.-Bot.) U. Braun & Crous, in Crous & Braun, CBS Biodiversity Ser.: 415. 2003.
Description and illustration: Minnis et al. (2011), present study (Fig. 25).
Materials examined: Cuba, Bayamo, on Guazuma ulmifolia (= G. tomentosa), 16 Feb. 1966, R. Urtiaga-Martinez, IMI 117605. Dominican Republic, Valle del Cibao, prov. Santiago, Hato del Yaque, on leaves of Guazuma ulmifolia, 20 Apr. 1930, coll. R. Ciferri & A.M. Borgna Ciferri, Batey no. 1, Mycoflora Domingensis Exsiccata 210 (lectotypus of Pantospora guazumae designated by Deighton 1976a: (IMI 59269). Mexico, intercepted at USA, Arizona, Nogales, entering from Mexico, on leaf of Guazuma ulmifolia, 12 Feb. 2009, coll. J. Moore (epitype designated by Minnis et al. 2011: BPI 880778, culture ex-epitype CBS 130299).
Notes: Pantospora is a monotypic genus with no known sexual morph that is reminiscent of Pseudocercospora but with synnematous conidiomata, percurrent and sympodial conidiogenous cells and frequently dictyosporous conidia (Crous & Braun 2003). Since the formation of dictyosporous conidia also occurred in the type of Pseudocercospora (Pseudocercospora vitis), Crous & Braun (2003) reduced Pantospora to synonymy with Pseudocercospora. The phylogenetic position of Pantospora within the Mycosphaerellaceae has been established by Minnis et al. (2011), based on the ITS and LSU sequences of the epitype culture of the type species Pantospora guazumae. In the present study Pantospora is represented by a single strain lineage (Fig. 1, clade 49; Fig. 3, clade 12) closely related to Paracercospora.
Clade 50: Paracercospora
Paracercospora Deighton, Mycol. Pap. 144: 47. 1979.
Description (from Braun et al. 2013): Mycelium in vivo internal. Conidiophores macronematous, fasciculate, pigmented. Conidiogenous cells integrated, terminal or conidiophores reduced to conidiogenous cells, conidiogenous loci subconspicous by being circular with very slightly thickened and darkened-refractive rim. Conidia solitary, scolecosporous, subhyaline to very pale olivaceous, hila very slightly thickened and darkened-refractive along the rim.
Type species: Paracercospora egenula (Syd.) Deighton (≡ Cercoseptoria egenula Syd.).
Paracercospora egenula (Syd.) Deighton, Mycol. Pap. 144: 48. 1979.
Basionym: Cercoseptoria egenula Syd., Ann. Mycol. 33(3–4): 235. 1935.
Synonyms: Cercospora egenula (Syd.) Chupp & Doidge, Bothalia 4: 885. 1948.
Pseudocercospora egenula (Syd.) U. Braun & Crous, in Crous & Braun, CBS Biodiversity Ser.: 171. 2003.
Cercospora solani-melongenae Chupp, Bothalia 4: 892. 1948.
Descriptions and illustrations: Chupp, 1954, Deighton, 1979, Crous et al., 2013a.
Materials examined: India, on Solanum melongena, N. Ponnapa, No. 109/1981, culture CBS 485.81. Japan, Shimane, on leaves of Solanum melongena, 5 Aug. 1998, T. Mikami, CNS-415, cultures MUCC 883 = MAFF 237766. Republic of Korea, Hongcheon, on leaves of S. melongena, 26 Oct. 2005, H.D. Shin, CBS H-20836, culture CBS 132030 = CPC 12537. South Africa, Gauteng Province, Barberton, on Solanum panduriforme, May 1931, L. Liebenberg No. 25999 (holotype PREM 25999, isotype IMI 89597).
Notes: Paracercospora was introduced by Deighton (1979) in order to accommodate Paracercospora egenula, a pseudocercospora-like species with distinct circular conidiogenous loci (scars) with a slightly thickened dark rim. This type of scar, however, is also present is some species of Pseudocercospora and with further support from earlier phylogenetic works (Stewart et al., 1999, Crous et al., 2000, Crous et al., 2001b), Paracercospora was synonymised with Pseudocercospora (Crous & Braun 2003). When representatives of the type species, Paracercospora egenula, were recollected, their partial LSU DNA sequences placed them apart from Pseudocercospora (Crous et al., 2013a, Vaghefi et al., 2016). Paracercospora is maintained as a separate genus based on the combination of its phylogenetic position (Fig. 1, clade 50, Fig. 3; clade 11), minimal marginal thickening of the conidiogenous loci and subhyaline conidia. Closely related species to Paracercospora egenula include Passalora brachycarpa (pale olivaceous, catenate conidia, prominent, thickened, darkened scars), and Pseudocercospora tibouchinigena (subhyaline conidia, unthickened hila and scars) (Crous et al. 2013a). In the present study, with the addition of the ITS and partial rpb2 sequences to the phylogenetic analysis, the strains of Passalora brachycarpa (now Distomycovellosiella brachycarpa) cluster in a separate clade from Paracercospora egenula. Unfortunately, the phylogenetic position of Pseudocercospora tibouchinigena was not reassessed in this study and it may eventually be shown to represent a distinct genus since phylogenetically, it clusters apart from Pseudocercospora and, morphologically it is neither a species of Pseudocercospora s. str. (subhyaline conidia), nor Paracercospora (lacking any scar thickening). In a recently published paper (Ou et al. 2015), with the description of a new species of Paracercospora, Paracercospora dictamnicola, all three species ‘Pseudocercospora tibouchinigena’, Paracercospora egenula and Paracercospora dictamnicola cluster together in a phylogeny based on LSU and ITS. However, Paracercospora dictamnicola is described as having conidiogenous loci unthickened and not darkened (pseudocercospora-like) and conidia solitary, subhyaline to pale olivaceous (paracercospora-like). Thus, the case of Paracercospora dictamnicola adds to the morphological vs. phylogenetic placement of cercosporoid species dilemma.
Clade 51: Nothopassalora [and Clarohilum]
Nothopassalora U. Braun, C. Nakash., Videira & Crous, gen. nov. MycoBank MB822696.
Etymology: From the greek notho-, meaning false, and resembling the genus Passalora.
Description: Hyphomycetous, phytopathogenic. Mycelium internal, hyaline to pale brown, branched, septate hyphae. Stromata dark, epidermal, substomatal, subglobose. Conidiophores emerging in fascicles from stromata, through stomata, pale to medium brown, smooth to verruculose, simple, straight to flexuous, geniculate-sinuous at the apex, multiseptate, but sometimes with a single basal septum or reduced to conidiogenous cell. Conidiogenous cells integrated, terminal, proliferating sympodially, mono- or polyblastic, conidiogenous loci rim-like, darkened, thickened and refractive. Conidia solitary, pale brown to olivaceous, smooth, thin-walled, cylindrical to long-obclavate, straight or gently curved, apex rounded and sometimes narrowing into a beak, base rounded or obconically truncate, multiseptate, hila thickened, darkened and refractive, sometimes protruding.
Type species: Nothopassalora personata (Berk. & M.A. Curtis) U. Braun et al. (≡ Cladosporium personatum Berk. & M.A. Curtis).
Nothopassalora personata (Berk. & M.A. Curtis) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822766. Fig. 26.
Fig. 26.
Nothopassalora personata (CPC 19466). A–E. Observations in vivo. A, B. Leaf spot symptoms on the host. C, D. Conidiophores and conidiogenous cells. E. Single conidia. F–K. Observations in vitro. F. Culture on SNA. G. Conidiophore and conidiogenous cell. H. Partial conidiophore and conidiogenous cell. I–K. Single conidia. Scale bars = 10 μm.
Basionym: Cladosporium personatum Berk. & M.A. Curtis, Grevillea 3(27): 106. 1875.
Synonyms: Cercospora personata (Berk. & M.A. Curtis) Ellis & Everh., J. Mycol. 1: 63. 1885.
Cercosporiopsis personata (Berk. & M.A. Curtis) Miura, Flora of Manchuria and East Mongolia. Part III. Cryptogams, fungi 3: 529. 1928.
Passalora personata (Berk. & M.A. Curtis) S.A. Khan & M. Kamal, Pakistan J. Sci. Res. 13: 188. 1961.
Cercosporidium personatum (Berk. & M.A. Curtis) Deighton, Mycol. Pap. 112: 71. 1967.
Mycosphaerella berkeleyi Jenkins, J. Agr. Res. 56: 325. 1938.
For additional synonyms see Crous & Braun (2003) or MycoBank.
Description in vivo (CBS H-22946): Leaf spots amphigenous, blackish brown, circular to subcircular, with yellow halo, 5–12 mm diam. Mycelium internal, composed of hyaline to pale brown hyphae, smooth, septate, branching. Stromata amphigenous, mainly hypophyllous, well-developed, 42–165 μm diam, brown to dark brown, epidermal, substomatal, subglobose and composed of textura angularis. Conidiophores emerging from upper part of stromata, densely fasciculate, pale brown to brown olivaceous, smooth to verruculose, erect, simple, straight to sinuous, geniculate-sinuous or conically truncate at the apex, irregular in width, 28–63 × 5–7.3 μm, sometimes only 1-septate. Conidiogenous cells integrated, terminal, mono- or polyblastic, proliferating sympodially, with rim-like conidiogenous loci distinctly thickened, darkened and refractive, located on the shoulders and the apex, 3–4 μm diam. Conidia solitary, pale to pale olivaceous brown, thick-walled, cylindrical to long-obclavate, straight or gently curved, apex rounded and sometimes narrowing into a beak, base rounded or obconicaly truncate, 38–85 × 5–8 μm, 2–7-euseptate, hila thickened, darkened and refractive, 3–4 μm diam.
Description in vitro (on V8; CPC 19466): Mycelium composed of hyaline to olivaceous hyphae, smooth to finely verruculose, septate, branching, uniform in width, 2.5 μm. Conidiophores pale brown to brown, micro- to macronematous, darker at the middle part, and paler towards apex, smooth to rough, cylindrical, geniculate-sinuous at the apex, simple, 50–100 × 3–5 μm. Conidiogenous cells integrated, terminal, mono- or polyblastic, proliferating sympodially, with conidiogenous loci rim-like, thickened, darkened and refractive, located at the shoulders and apex, 2.5 μm diam. Conidia solitary, pale to pale brown, cylindrical to long-obclavate, rounded at the apex and obconicaly truncate at the base, 45–110 × 5–7 μm, 2–10-euseptate, hila thickened, darkened and refractive, 2.5 μm diam.
Materials examined: Australia, Northern Territory, Darwin, on Arachis hypogaea, 30 Apr. 2011, P.W. Crous (epitype designated here, CBS H-22946, MBT378602, ex-epitype culture CBS 142236 = CPC 19466). USA, Georgia, Spalding Co., Georgia Experiment Station, on Arachis hypogaea, 23 Jun. 1937, W.A. Jenkins (CUP-027308, isotype of Mycosphaerella berkeleyii, ex-isotype culture CBS 222.38); South Carolina, Santee River, on Arachis hypogaea, Ravenel 1612 (holotype K, isotype IMI 104552).
Notes: The two major foliar diseases occurring on peanut, Early leaf spot and Late leaf spot, are respectively caused by Cercospora arachidicola (= Mycosphaerella arachidis) and Passalora personata (= Mycosphaerella berkeleyi) (Jenkins, 1938, Kokalis-Burelle et al., 1997). Both diseases occur wherever peanut is grown but is usually manageable with timely fungicide applications (Kokalis-Burelle et al. 1997). The strains used in this study are identical based on their DNA sequences (Fig. 1, clade 51; Fig. 3, clade 14) and closely related with Asperisporium, despite their morphological differences (Fig. 26).
Clarohilum Videira & Crous, gen. nov. MycoBank MB822583.
Etymology: from the greek phaner- that means visible and protruding + hilum.
Description (adapted from Little 1987): Phytopathogenic, causing leaf spots. Ascomata globose, subepidermal, mostly epiphyllous, brown or dark brown, ostiolate, thin wall composed of pseudoparenchymatous cells. Asci cylindrical, tapering towards the base, bitunicate, thick-walled, 8-spored. Ascospores hyaline, tapering at both ends, two-celled with the upper cell slightly broader than the lower. Conidiophores mononematous, pale olivaceous brown, not branched, with slight geniculations, septate. Conidiogenous cells terminal, elongating sympodially, polyblastic, with conidiogenous loci thickened and darkened, located both apical and laterally. Conidia single, pale olivaceous, smooth, obovoid, obclavate, cylindrical to long-obclavate, slightly curved, apex obtuse, base rounded or short obconicaly truncate, septate, hila thickened, darkened and usually protruding.
Type species: Clarohilum henningsii (Allesch.) Videira & Crous Crous (≡ Cercospora henningsii Allesch.).
Clarohilum henningsii (Allesch.) Videira & Crous, comb. nov. MycoBank MB822748. Fig. 27.
Fig. 27.
Clarohilum henningsii (CPC 17314). A–E. Observations in vivo. A, B. Leaf spot symptoms on the host. C, D. Conidiophores and conidiogenous cells. E. Single conidia. F–K. Observations in vitro. F. Culture on OA. G–J. Partial conidiophore, conidiogenous cell and conidia. K. Single conidia. Scale bars = 10 μm.
Basionym: Cercospora henningsii Allesch., Die Pflanzenwelt Ost-Afrikas und der Nachbargebiete. Teil C: 35. 1895.
Synonyms: Cercosporidium henningsii (Allesch.) Deighton, More dematiaceous Hyphomycetes: 295. 1976.
Passalora henningsii (Allesch.) Poonam Srivast., J. Living World 1(2): 116. 1994, nom. inval. (Art. 41.1).
Passalora henningsii (Allesch.) R. F. Castañeda & U. Braun, Cryptog. Bot. 1: 46. 1989.
Cercospora cassavae Ellis & Everh., Bull. Torrey Bot. Club 22: 438. 1895.
Cercospora manihotis Henn., Hedwigia 41: 18. 1902.
Mycosphaerella henningsii Sivan., Trans. Brit. Mycol. Soc. 84: 552. 1985.
For additional synonyms see MycoBank.
Descriptions and illustrations: Ellis, 1976, Little, 1987.
Description in vitro (on V8; CPC 17314): Mycelium hyaline to olivaceous brown, smooth to rough, septate, branching, uniform in width, 2.5–7.5 μm. Conidiophores micro- and macronematous, pale brown to olivaceous brown, paler at the apex, smooth, straight, simple or occasionally branched, cylindrical, geniculate sinuous at the apex, 75–170 × 5–7.5 μm. Conidiogenous cells integrated, terminal, proliferating sympodially, mono- or polyblastic, with conidiogenous loci thickened, darkened and protruding, 2.5 μm diam. Conidia solitary, hyaline to pale brown, smooth, obovoid, cylindrical, obclavate, base rounded or obconically truncate, sometimes sligltly swollen, apex rounded, 30–75 × 5–7 μm, 0–8-euseptate, sometimes slightly constricted at the septa, hila rim-like, thickened, darkened and refractive, sometimes protruding, 2.5 μm diam.
Material examined: Laos, on Manihot esculenta, 5 May 2006, P. Pheng, NOUL 26, culture CPC 17314. Tanzania, Usambara (Amboni), on Manihot esculenta (= M. utilissima), Holst No. 2899 (lectotype of C. henningsii designated here, MBT378578, S F37294; isolectotype S F37295).
Notes: Passalora henningsii is widely distributed in tropical to subtropical regions along with its host plant, Manihot esculenta. Morphologically, the description of the observed strain is similar to that available in literature (Chupp, 1954, Castañeda and Braun, 1989). In this study, caespituli of this species are paler than that of the other species of Passalora s. lat. and has conidia with distinctly protruding hila (Fig. 27). The phylogenetic analyses place this strain in a single-strain lineage (Fig. 3, clade 15) that is closely related to Nothopassalora (Fig. 1, clade 51; Fig. 3, clade 14). Morphologically, a few conidia of Passalora henningsii showed less protruding hila that tapered towards the base like some of the conidia of Nothopassalora personata (Fig. 26). In a supplementary phylogenetic analysis performed using a smaller dataset (sequences in dataset 3 corresponding to Fig. 3, clades 1–16), this single-strain lineage remains apart from the Nothopassalora clade. Based on a BLAST comparison against the alignment, the present strain shares only 95 % (447/473) similarity on ITS and 91 % (686/750) similarity on rpb2 with Nothopassalora personata. The morphological differences and the instability of the phylogenetic position of these strains indicate that it is better to introduce this species into a new genus than combine it into Nothopassalora. Type material of Cercospora manihotis Henn. 1902 (Brazil, Pará, on Manihot esculenta, May 1901, J. Huber 42) is not preserved at B and could not be traced in other herbaria. Syntype material of the illegitimate name Cercospora manihotis Henn., in de Wildemann, Ann. Mus Congo, 5 Sér., Vol. II, Fasc. II: 104. 1907 [non Cercospora manihotis Henn. 1902] is presenved in B and S (Congo, Kwango, Kisantu, May 1906, H. Vanderyst 179). Syntypes of Cercospora cassava are housed in several herbaria, including BPI 434310, 437138, FH 01012118, and S F278433 (USA, Florida, Lake County, Eustis, on Cassava leaves (Manihot sp.), 28 May 1895, Geo. V. Nash).
Clade 52: Pluripassalora
Pluripassalora Videira & Crous, gen. nov. MycoBank MB822611.
Etymology: The name is a combination of pluri- (many) which refers to the multiseptate conidia + passalora, due to the similarity to the Passalora genus.
Description: Phytopathogenic, forming leaf spots. Mycelium internal, septate, smooth, hyaline to pale brown. Stromata amphigenous, mainly hypophyllous, well-developed, epidermal, substomatal, subglobose. Conidiophores emerging from upper part of stromata in dense fascicles, or emerging singly from internal hyphae, pale brown to brown, simple, sinuous, sometimes geniculate, irregular in width, smooth to verruculose, aseptate or septate. Conidiogenous cells integrated, terminal or intercalary, proliferating sympodially, mono- or polyblastic, conidiogenous loci rim-like, thickened, darkened and refractive. Conidia solitary, pale to pale olivaceous brown, paler towards the apex, smooth, thick-walled, mostly obclavate (in host), cylindrical-obclavate (in culture), euseptate, multiseptate, sometimes constricted at the septa (in culture), rounded at the base, beak-like and rounded at the apex, hila thickened, darkened and refractive.
Type species: Pluripassalora bougainvilleae (Munt.-Cvetk.) U. Braun et al. (≡ Cercospora bougainvilleae Munt.-Cvetk.).
Pluripassalora bougainvilleae (Munt.-Cvetk.) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822822. Fig. 28.
Fig. 28.
Pluripassalora bougainvilleae (CPC 19327). A–E. Observations in vivo. A, B. Leaf spot symptoms on the host. C, D. Conidiophores, conidiogenous cells and conidia. E. Single conidia. F–K. Observations in vitro. F. Culture on OA. G–K. Partial conidiophore, conidiogenous cell and conidia. Scale bars = 10 μm.
Basionym: Cercospora bougainvilleae Munt.-Cvetk., Revista Argent. Agron. 24: 84. 1957.
Synonyms: Cercosporidium bougainvilleae (Munt.-Cvetk.) Sobers & C.P. Seym., Proc. Florida State Hort. Soc.: 398. 1969.
Passalora bougainvilleae (Munt.-Cvetk.) R.F. Castañeda & U. Braun, Cryptog. Bot. 2: 291. 1991.
Description and illustration: Ellis (1976), present study (Fig. 28).
Description in vivo (CBS H-22947): Leaf spots amphigenous, brown to whitish brown, circular to subcircular, with a dark brown concentric ring, 2–5 mm diam. Mycelium internal, hyaline to pale brown. Stromata amphigenous, mainly hypophyllous, well-developed, 25–50 μm diam, brown, epidermal, substomatal, subglobose. Conidiophores emerging from upper part of stromata in dense fascicles, or emerging singly from internal hyphae, pale brown to brown, smooth, simple, straight to geniculate-sinuous, irregular in width, 24–62 × 5–6 μm, septate. Conidiogenous cells integrated, terminal and intercalary, mono-or polyblastic, proliferating sympodially, with rim-like conidiogenous loci thickened, darkened and refractive, located on on the shoulders and apex, 2–3 μm diam. Conidia solitary, smooth, pale to pale olivaceous brown, paler towards the apex, thick-walled, mostly obclavate or long-obclavate, base rounded, apex rounded and beak-like, 40–116 × 7–10 μm, 3–10-euseptate, sometimes constricted at the septa, hila distinctly thickened, darkened and refractive, 2–3 μm diam.
Description in vitro (on MEA; CPC 19327): Mycelium composed of hyphae uniform in width, hyaline and 1–2 μm diam when young, pale brown and 3.8–5 μm diam when mature, septate and branching. Conidiophores emerging from large brown aggregated cells 7.5–12.5 μm diam, micro- or macronematous, pale brown, septate, straight to curved in segments, occasionally geniculate-sinuous, uniform in width, 100–150 × 5 μm. Conidiogenous cells integrated, terminal and intercalary, pale brown, straight to mildly geniculate, mono- or polyblastic, conidiogenous loci thickened, darkened and refractive, 2–2.5 μm diam. Conidia solitary, pale brown, smooth, cylindrical-obclavate, long-obclavate, base obconically truncate, apex rounded, beak-like, sometimes swollen, 45–75 × 5–7.5 μm, 3–7-euseptate, slightly to strongly constricted at the septa, hila thickened, darkened and refractive, 2–2.5 μm diam.
Material examined: Australia, Northern Territory, Darwin, on Bougainvillea sp., 30 Apr. 2011, P.W. Crous, CBS H-22947, culture CBS 142237 = CPC 19327.
Notes: The present species was initially described as Cercospora bougainvilleae and was described on the host Bougainvillea stipitata from Argentina (Muntañola-Cvetkovic 1957) but no original material could be traced. The designation of a neotype is necessary, but the present strain is from a different geographical location. In the phylogenetic analyses, this species is represented by a single-strain lineage (Fig. 1, clade 52; Fig. 3, clade 16) closely related to Nothopassalora. Based on a BLAST comparison, Pluripassalora shares 90 % (418/463) similarity on ITS and 87 % (679/780) similarity on rpb2, with Nothopassalora personata CPC 19466. Morphologically, Pluripassalora can be distinguished from Nothopassalora by its obclavate and multiseptate conidia and also differs from Passalora s. str. by its multiseptate conidia.
Clade 53: Micronematomyces
Micronematomyces U. Braun, C. Nakash., Videira & Crous, gen. nov. MycoBank MB822595.
Etymology: Derived from the micronematous conidiophores (micronemato-) and fungus (-myces).
Differs from Passalora in forming short and micronematous to submicronematous conidiophores, and solitary and cylindrical, long-obclavate to filiform conidia.
Type species: Micronematomyces caribensis (Crous & Den Breeÿen) U. Braun, C. Nakash., Videira & Crous (≡ Passalora caribensis Crous & Den Breeÿen).
Micronematomyces caribensis (Crous & Den Breeÿen) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822763.
Basionym: Passalora caribensis Crous & Den Breeÿen, Fungal Diversity 23: 98. 2006.
Description and illustration: Den Breeyën et al. (2006).
Materials examined: Cuba, near Havana, Chromolaena. odorata, 28 Oct. 1997, S. Neser, culture CBS 113376 = MJM 1539 = C487. Jamaica, Central Jamaica, between Guinea corm and John's Hall, on C. odorata, 31 Oct. 1997, M.J. Morris (holotype CBS H-19754, culture ex-type CBS 113380 = MJM 1550 = C498); Kingston, road to Strawberry Hill off Blue Mountain road, on C. odorata, M.J. Morris, 30 Oct. 1997, cultures CBS 113374 = MJM 1545 = C481, CBS 113375 = MJM 1543 = C482; between Maypen and Chapleton, on C. odorata, 30 Oct. 1997, M.J. Morris, culture CBS 113381 = MJM 1549 = C500; on highway to Kingston, between Moneague and Edwarton, on Chromolaena odorata, 1 Nov. 1997, M.J. Morris, culture CBS 113378 = MJM 1552 = C494; Strawberry Hill, on C. odorata, 30 Oct. 1997, M.J. Morris, culture CBS 113379 = MJM 1544 = C495.
Notes: The genus Micronematomyces is phylogenetically and morphologically distinct from Passalora as circumscribed in this study. It encompasses two species, Micronematomyces caribensis and Micronematomyces chromolaenae, that cluster together in a well-supported clade in the phylogenetic analyses performed in this study (Fig. 1, clade 53; Fig. 3, clade 17). Morphologically, species in the genus Micronematomyces differ from Passalora s.str. in forming short conidiophores, and multiseptate conidia that are cylindrical, long-obclavate to filiform. Micronematomyces caribensis can be distinguished from Micronematomyces chromolaenae by its shorter and slightly wider conidia. The strains CBS 113378 and CBS 113379 were identified as Passalora perfoliati based on morphological characters (Den Breeÿen et al. 2006) using the descriptions available in literature (Ellis, 1971, Braun, 1998). Unfortunately, when the cultures were observed in this study they did not sporulate and the fungarium material was depauperate. Based on a BLAST against the entire alignments, these two strains share 99 % (779/780) similarity on rpb2 and 98 % (465/474) similarity on ITS with Micronematomyces caribensis. The type of Passalora perfoliati was isolated from Eupatorium perfoliatum from Wisconsin, USA (Ellis & Everhart 1889; syntypes NY, WIS-F-0003831) while the aforementioned two strains were obtained from a different host (Chromolaena odorata) and from a different location (Jamaica). Therefore, these two strains will henceforth be treated as Micronematomyces caribensis.
Micronematomyces chromolaenae (Crous & Den Breeÿen) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822764.
Basionym: Passalora chromolaenae Crous & Den Breeÿen, Fungal Diversity 23: 98. 2006.
Description and illustration: Den Breeyën et al. (2006).
Materials examined: Mexico, Veracruz Province, Catemaco Lake, on Chromolaena odorata, 12 Oct. 1997, M.J. Morris (holotype CBS H-19753, culture ex-type CBS 113611 = MJM 1498 = C452); Entrada Corretera, on C. odorata, 12 Oct. 1997, M.J. Morris, culture CBS 113371 = MJM 1490 = C450.
Notes: The host Chromolaena odorata (≡ Eupatorium odoratum) is considered to be the one of the most problematic invasive species within protected rainforests in Africa (Struhsaker et al. 2005). Among plant pathogens those considered to be host-specific are considered to be potentially good as biological control agents (Barreto & Evans 1994). Micronematomyces chromolaenae is distinguished from other species occurring on this host by its conidial dimensions (up to 200 μm long and 4 μm wide) and shape that is never curled (Den Breeÿen et al. 2006). The representative strains of this species cluster in a clade well-supported by all three phylogenetic methods employed in this study (Fig. 3, clade 17). They were, unfortunately, sterile in culture at the time this study was performed and the herbarium specimens were depauperate.
Clade 54: Rhachisphaerella
Rhachisphaerella U. Braun, C. Nakash., Videira & Crous, gen. nov. MycoBank MB822702.
Etymology: Derived from the conidiogenous cells forming a rachis and the mycosphaerella-like sexual morph.
Description (adapted from Arzanlou et al. 2008): Phytopathogenic. Ascomata amphigenous, dark brown, subepidermal, becoming erumpent, globose; wall composed of layers of medium brown textura angularis. Asci aparaphysate, fasciculate, bitunicate, subsessile, obovoid to broadly ellipsoid, straight to slightly curved, 8-spored. Ascospores bi- to tri-seriate, overlapping, hyaline, thin-walled, straight to curved, fusoid-ellipsoidal with obtuse ends, widest in middle of apical cell, medianly 1-septate, not to slightly constricted at the septum, tapering towards both ends but more prominently towards the lower end; ascospores becoming distorted upon germination, becoming constricted at the septum, with irregular, wavy germ tubes, growing 90° to the long axis, and not arising from the polar ends of the spore. (In vitro) Mycelium submerged and superficial; submerged hyphae hyaline to subhyaline, thin-walled, smooth or slightly rough; aerial hyphae pale olivaceous, smooth or finely verruculose. Conidiophores arising from hyphae, occasionally reduced to conidiogenous cells, hyaline, subcylindrical. Conidiogenous cells integrated, terminal, proliferating sympodially, polyblastic, conidiogenous loci aggregated, flat, not protuberant (not denticle-like), unthickened, but somewhat darkened. Conidia solitary, hyaline, thin-walled, smooth, obovoid, ellipsoidal, obclavate, aseptate or multiseptate, hilum truncate, flat, broad, unthickened, slightly darkened.
Type species: Rhachisphaerella mozambica (Arzanlou & Crous) Videira & Crous (≡ Mycosphaerella mozambica Arzanlou & Crous).
Rhachisphaerella mozambica (Arzanlou & Crous) Videira & Crous, comb. nov. MycoBank MB822798.
Basionym: Mycosphaerella mozambica Arzanlou & Crous, Persoonia 20: 26. 2008.
Description and illustration: Arzanlou et al. (2008).
Materials examined: Mozambique, Chimoio, Bairro, on leaf of Musa cv., 2003, A. Viljoen (holotype CBS H-20039, culture ex-type CBS 122464); idem. CBS H-20040, CBS H-20041, CBS H-20042.
Notes: Mycosphaerella mozambica is a common pathogen occurring on banana in Mozambique (Arzanlou et al. 2008). The sympodially proliferating conidiogenous cells are reminiscent of Ramichloridium, but the type species of that genus, Ramichloridium apiculatum, has been found to cluster within Dissoconiaceae (Arzanlou et al. 2007). As other ramichloridium-like species within Mycosphaerellaceae, M. mozambica needed to be reassigned into a new genus. Phylogenetically, the representative strain of this species forms a single species lineage closely related to Micronematomyces (Fig. 1, clade 54; Fig. 3, clade 18). Morphologically, Rhachisphaerella mozambica is quite distinct from species of Micronematomyces, since its conidiogenous cells form a rachis with unthickened conidiogenous loci and the conidia are generally obovoid, 0–1-septate with unthickened hila.
Clade 55: Neophloeospora
Neophloeospora U. Braun, C. Nakash., Videira & Crous, gen. nov. MycoBank MB822598.
Etymology: Derived from the similarity to the genus Phloeospora (neo- = new).
Description (adapted from Punithalingam 1990): Phytopathogenic, causing leaf spots. Pseudothecia on overwintered, fallen leaves, initially immersed, later erumpent, epiphyllous, dark brown, spherical, with short necks and circular ostioles, wall composed of several cell layers of textura angularis, the outer cells dark brown, the inner cells hyaline. Asci fasciculate, cylindrical to clavate, hyaline, 8-spored, bitunicate. Ascospores biseriate or irregularly biseriate, ellipsoid, medianly or slightly unequally 1-septate, upper cell slightly wider than the lower cell, guttulate. Conidiomata epiphyllous, acervular, subepidermal, separate or confluent, composed of textura angularis; dehiscence irregular. Conidiogenous cells terminal, hyaline, cylindrical, proliferating percurrently with inconspicuous annellations or sympodially. Conidia hyaline or subhyaline, smooth, cylindrical to obclavate, straight or curved, septate, guttulate, with age becoming darker, constricted at the septa and slightly verruculose.
Type species: Neophloeospora maculans (Bérenger) Videira & Crous (≡ Fusarium maculans Bérenger).
Neophloeospora maculans (Bérenger) Videira & Crous, comb. nov. MycoBank MB822823. Fig. 29.
Fig. 29.
Neophloeospora maculans (CBS 115123). A–E. Observations in vitro. F. Culture on SNA. B, C. Conidiophore and conidia. D, E. Conidia. Scale bars = 10 μm.
Basionym: Fusarium maculans Bérenger, Atti Riunione Sci. Ital. (Milano) 6: 474. 1845 (1844).
Synonyms: Phloeospora maculans (Bérenger) Allesch., in Rabenh., Krypt.-Fl., Edn 2, 1(6): 935. 1900 (1899).
Phloeosporella maculans (Bérenger) Höhn., Mitt. Bot. Lab. T. H. Wien 4(2): 77. 1927.
Cercosporella maculans (Bérenger) F.A. Wolf, J. Elisha Mitchell Sci. Soc. 51: 165.1935.
Septoria mori Lév., Ann. Sci. Nat., Bot., Sér. 3, 5: 279. 1846.
Cheilaria mori (Lév.) Desm., Ann. Sci. Nat., Bot., Sér. 3, 8: 27. 1847.
Phloeospora mori (Lév.) Sacc., Michelia 1(2): 175. 1878.
Septogloeum mori (Lév.) Briosi & Cavara, Fung. Paras. Piante Colt. Util., Fasc. 1: no. 21. 1888.
Cylindrosporium mori (Lév.) Berl., Riv. Patol. Veg., Pavia 5: 205. 1896.
Sphaerella mori Fuckel, Jahrb. Nassauischen Vereins Naturk. 23-24: 106. 1870 (1869–1870).
Mycosphaerella mori (Fuckel) F.A. Wolf, J. Elisha Mitchell Sci. Soc. 51: 165. 1935.
Sphaerella morifolia Pass., Erb. Critt. Ital., Ser. 2, Fasc. 30: no. 1464. 1885.
Mycosphaerella morifolia (Pass.) Cruchet, Bull. Soc. Vaud. Sci. Nat. 55: 43. 1923.
Sphaeria mori Nitschke, Fungi Rhen. Exs.: no. 1784. 1866.
Cercospora pulvinulata f. angulosa Savul. & Sandu, Herb. Mycol. Rom.: no. 188. 1931.
Description and illustration: Punithalingam (1990).
Description in vitro (on OA; CBS 115123): Mycelium hyaline to subhyaline, uniform in width, 2.5–3 μm diam. Conidiophores micronematous, hyaline, smooth, 5–10 × 1–2 μm. Conidiogenous cells terminal, indistinct. Conidia solitary, smooth, hyaline to pale brown, cylindrical to obclavate, subtruncate to truncate at the base, rounded to beak-like at the apex, straight to mildly sinuous, 38–70 × 3–5 μm, 3–10-euseptate, not or only slightly constricted at the septa, with age becoming darker, slightly verruculose, strongly constricted at the septa and wider (5–10 μm).
Material examined: New Zealand, Auckland, Mt. Albert, on Morus alba, isol. CF Hill (996), MAF, Mar. 2004, herbarium material U. Braun, Fungi Sel. Exs. 101, e.g. HAL, PDD 93510, culture CBS 115123.
Notes: This genus is introduced to accommodate the species Phloeospora maculans that is not congeneric with the type of Phloeospora, Phloeospora ulmi, and clusters in a single strain lineage in the phylogenetic analyses performed in this study (Fig. 1, clade 55; Fig. 2, clade 19). Morphologically, Neophloeospora can be distinguished from Phloeospora by the subhyaline to pale brown conidia constricted at the septum (Fig. 29). Neophloeospora maculans is a pathogen causing leaf spot on mulberry (Morus alba), a native tree to China that is commonly used to feed silkworms and is now cultivated worldwide for its berries (Punithalingam, 1990, Hong et al., 2011). The ITS sequence generated here matches those of Hong et al. (2011).
Clade 56: Dothistroma
Dothistroma Hulbary, Bull. Illinois Nat. Hist. Surv. 21: 235. 1941
Description (from Sutton 1980): Mycelium immersed, branched, septate, pale brown to hyaline. Conidiomata sometimes acervular, initially subepidermal later erumpent, composed of pale brown, thin-walled textura angularis, sometimes eustromatic, multilocular and of darker brown, thick-walled tissue. Dehiscence irregular, stromata strongly erumpent and finally pulvinate. Conidiophores absent. Conidiogenous cells holoblastic, discrete, determinate, ampulliform, hyaline, smooth, non-proliferating, formed from the upper cells of stroma or from inner cells of the locular walls. Conidia acrogenous, solitary, hyaline, straight or curved, filiform, 1–5-euseptate, continuous, thin-walled, smooth.
Type species: Dothistroma pini Hulbary.
Dothistroma pini Hulbary, Bull. Illinois Nat. Hist. Surv. 21: 235. 1941.
Descriptions and illustrations: Barnes et al., 2004, Barnes et al., 2016.
Materials examined: Russia, Rostov oblast, Kamensky district, Kamensky timber enterprise, Kamenskoye forestry, 3 km to the east of Staraya Stanitsa village, pine planting, on Pinus pallasiana, 8 Oct. 2006, T.S. Bulgakov, culture CBS 121005 = CMW 24852. USA, Illinois, De Kalb County, on P. nigra subsp. austriaca, 29 Nov. 1938, J. Cedric Carter (holotype ILLS 27093, isotype CBS H-12211); Michigan, Massaukee County, McBain, Riverside Township, on Pinus nigra, Aug. 2001, G. Adams, CBS H-12203, culture CBS 116483 = CMW 14905; Michigan, Montcalm County, Stanton, Evergreen Township, on P. nigra, 2001, G. Adams (epitype designated by Barnes et al. 2016, CBS H-12211, culture ex-epitype CBS 116487 = CMW 10951); idem., culture CBS 116486.
Notes: Dothistroma needle blight is one of the most important diseases of Pinus spp., both in natural forest ecosystems and particularly in plantations of non-native pines. The causal agent of the disease has been narrowed down to two species, Dothistroma septosporum (worldwide) and Dothistroma pini (USA) (Barnes et al., 2004, Groenewald et al., 2007). The type of Dothistroma pini was originaly isolated from Pinus nigra in the USA and an epitype has recently been designated (Barnes et al. 2016). Dothistroma clusters in a clade well-supported by all three phylogenetic methods employed in this study (Fig. 1, clade 56; Fig. 3, clade 20) and is closely related to Stromatoseptoria.
Dothistroma septosporum (Dorog.) M. Morelet, Bull. Soc. Sci. Nat. Archéol. Toulon & Var 177: 9. 1968.
Basionym: Cytosporina septospora Dorog., Bull. Bull. Trimestriel Soc. Mycol. France 27: 106. 1911.
Synonyms: Septoriella septospora (Dorog.) Sacc., Syll. Fung. 25: 480. 1931.
For additional synonyms see MycoBank.
Description and illustrations: Barnes et al., 2004, Barnes et al., 2016.
Materials examined: Brazil, São Paulo, Santo Antonio do Pinhal, on needles of Pinus pinaster, 1974, T. Namekata, culture CBS 543.74. Ecuador, on needles of Pinus radiata, culture CBS 112498 = CPC 3779. France, Meurthe et Moselle, Arboretum d'Amance, on needles of Pinus coulteri, 27 Feb. 1970, culture CBS 383.74. Netherlands, Lunteren, Pinetum Dennenhorst, on needles of Pinus mugo ‘Rostrata’, 1 June 2009, W. Quaedvlieg, cultures CBS 128782 = CPC 16798, CBS 128783 = CPC 16799. Russia, St. Petersburg, Park Sosnovka, from Pinus sylvestris, 14 Nov. 2013, R. Drenkhan & D.L. Musolin (neotype designated by Barnes et al. 2016, CBS H-22299, culture ex-neotype CMW 44656 = CBS 140339 = TAAM 168554A). Poland, Miechów Forest District, Goszcza Forest Unit, on Pinus nigra, Jun. 2003, T. Kowalski, CBS H-12209, cultures CBS 116488 = CMW 13004, CMW 13010. South Africa, Tzaneen, on P. radiata, 2002, M.J. Wingfield, CBS H-12210, culture CBS 116489 = CMW 11372.
Notes: Dothistroma septosporum is one of the causal agents of Dothistroma needle blight (Red band disease of pine) and used to be listed as a species of quarantine importance to Europe (Quaedvlieg et al., 2012, EPPO., 2012). This disease occurs wherever Pinus and Larix species are grown (Groenewald et al. 2007) and can cause varying degrees of damage depending on humidity and temperature (Evans, 1984, Barnes et al., 2004). In the phylogenetic analyses, the strains clustered in a well supported clade (Fig. 1, clade 56; Fig. 3, clade 20). The herbarium material of the holotype was lost and a neotype was recently designated (Barnes et al. 2016).
Clade 57: Hyalocercosporidium
Hyalocercosporidium Videira & Crous, gen. nov. MycoBank MB822592.
Etymology: Similar to Cercosporidium but with hyaline conidia.
Description: Phytopathogenic. Mycelium internal, composed of hyaline to pale brown hyphae. Conidiophores solitary, simple, pale to brown, straight or mildly sinuous, geniculate. Conidiogenous cells terminal and intercalary, geniculate-sinuous, determinate or proliferating sympodially, monoblastic, with conidiogenous loci slightly thickened, darkened and refractive, located on the shoulders and apex. Conidia solitary, hyaline, smooth, obovoid, long-obclavate, straight or slightly curved, base obconical truncate or short obconical truncate, apex rounded, aseptate or euseptate, hila slightly thickened, darkened and refractive.
Type species: Hyalocercosporidium desmodii Videira & Crous.
Hyalocercosporidium desmodii Videira & Crous, sp. nov. MycoBank MB822712. Fig. 30.
Fig. 30.
Hyalocercosporidium desmodii (CPC 19483). A–F. Observations in vitro. A. Culture on OA. B. Mycelium. C–E. Partial conidiophore, conidiogenous cell and conidia. F. Conidia. Scale bars = 10 μm.
Etymology: Named after the genus of the host it was isolated from, Desmodium.
Description in vitro (on MEA; CPC 19483): Mycelium composed of hyaline to pale brown hyphae, smooth to verruculose, septate, branching, 2–3.5 μm diam. Conidiophores pale brown, smooth to lightly verruculose, simple, straight or mildly sinuous, up to 3-geniculate, (52.5–)98–126(–167) × (2.5–)3(–4) μm. Conidiogenous cells integrated, terminal and intercalary, monoblastic, determinate or proliferating sympodially, monoblastic, with conidiogenous loci slightly thickened, darkened and refractive, 1.5 μm diam. Conidia solitary, hyaline, smooth, obovoid, cylindrical to long obclavate, truncate to long-obconically truncate at the base, rounded at the apex, (14.5–)24–30(–40) × (3–)4(–6) μm, aseptate to 3-septate, septa indistinct, hila slightly thickened, darkened and refractive, 1.5 μm diam.
Material examined: Brazil, Minas Gerais, Vale da Lua, Alto Paraiso de Goias, on Desmodium tortuosum, 2 Aug. 2009, R.W. Barreto (holotype CBS H-22949, ex-type culture CBS 142179 = CPC 19483).
Notes: Two Passalora species (s. lat.) are known from Desmodium in literature, namely Passalora desmodii and Passalora atropunctata. From these two species, only the last one has been previously reported from the host Desmodium tortuosum in Brazil (Crous & Braun 2003). Passalora atropunctata produces very pale brown and wider conidia (25–50 × 7–8 μm; Ellis 1976) compared with Hyalocercosporidium desmodii, and Passalora desmodii has multilocal conidiogenous cells with 1–5 minute apical to lateral conidiogenous loci which are unthickened or almost so, only somewhat darkened or refractive and in front view visible as a minute circle [based on comparision with North American material of Passalora desmodii, including Petr., Mycoth. Gen.1220, GZU (lectotype of Cercospora desmodii Ellis & Kellerm., designated here, MBT378579: USA, Kansas, Manhattan, on Desmodium acuminatum, 30 Jul. 1884, W.A. Kellerman 585, BPI 435642; isolectotypes, MU 10493, NY 270695); syntypes: CUP 39659 (only July), NY 838298 (1 July, Kellerman s.n., marked as “type”); topotype collections distributed as Ellis & Everh., N. Amer. Fungi 1501] (Chupp 1954). The original specimen of Hyalocercosporidium desmodii was, unfortunately, not available for morphological examination and a dried culture specimen was prepared. The representative ex-type strain of Hyalocercosporidium desmodii formed a single-strain lineage in the phylogenetic analyses (Fig. 1, clade 57; Fig. 3, clade 21) and is closely related to Dothistroma and Stromatoseptoria. Morphologically, Hyalocercosporidium desmodii cannot be accommodated in Dothistroma or in Stromatoseptoria, since these genera have conidiogenous cells that proliferate percurrently and produce pigmented conidia (Quaedvlieg et al., 2013, Barnes et al., 2004).
Clade 58: Stromatoseptoria
Stromatoseptoria Quaedvlieg et al., Stud. Mycol. 75: 353. 2013.
Description (from Quaedvlieg et al. 2013): Foliicolous, plant pathogenic. Conidiomata pycnidial, hypophyllous, subglobose to lenticular, very pale brown to dark brown, immersed to erumpent, exuding conidia in white cirrhus; ostiolum central, circular, surrounding cells concolorous; conidiomatal wall composed of a homogenous tissue of hyaline to very pale brown, angular to irregular cells. Conidiophores subcylindrical, branched, hyaline, septate. Conidiogenous cells hyaline, discrete or integrated, cylindrical or narrowly ampulliform, holoblastic, often also proliferating percurrently. Conidia solitary, cylindrical, slightly to distinctly curved, broadly rounded apex, attenuated towards a truncate base, transversely euseptate, mostly constricted at septa.
Type species: Stromatoseptoria castaneicola (Desm.) Quaedvlieg et al. (≡ Septoria castaneicola Desm.).
Stromatoseptoria castaneicola (Desm.) Quaedvlieg, Verkley & Crous, Stud. Mycol. 75: 353. 2013.
Basionym: Septoria castaneicola Desm., Ann. Sci. Nat., Bot., Sér. 3, 8: 26. 1847.
Description and illustration: Quaedvlieg et al. (2013).
Material examined: France, on leaves of Castanea sativa, Aug. and Sep. 1843, M. Roberge, ‘Coll. Desmazières 1863, no. 8’ (holotype PC 0084574). Netherlands, Utrecht, Baarn, near Lage Vuursche, on Castanea sativa, 29 Aug. 1999, G. Verkley, CBS H-21200, culture CBS 102322; Mook en Middelaar, St. Jansberg, on on Castanea sativa, 9 Sep. 1999, G. Verkley, No. 932, culture CBS 102377.
Notes: Stromatoseptoria is a monotypic genus that differs from Septoria s. str. by forming a stroma that gives rise to the conidiophores, by producing conidia that are olivaceous in mass and, although hyaline and smooth at first, become olivaceous and verruculose with age (Quaedvlieg et al. 2013). Phylogenetically, Stromatoseptoria clusters within the Mycosphaerellaceae in a clade well-supported by all three phylogenetic methods employed (Fig. 1, clade 58; Fig. 3, clade 22) and is closely related to Dothistroma.
Clade 59: Fulvia
Fulvia Cif., Atti Ist. Bot. Univ. Lab. Crittog. Pavia 10: 246. 1954.
Description (from Ellis 1971): Colonies effuse, velvety, buff to brown or purplish. Stroma present, pale, substomatal. Conidiophores macronematous, mononematous, caespitose, emerging through stomata, unbranched or occasionally branched, straight or flexuous, narrow at the base, thickening towards the apex, with unilateral nodose swellings which may proliferate as short lateral branchlets, very pale to mid pale brown or olivaceous brown, smooth. Conidiogenous cells mono- or polyblastic, integrated, terminal becoming intercalary, sympodial, clavate or cylindrical, cicatrized. Conidia catenate, chains frequently branched, acropleurogenous, simple, cylindrical with rounded ends or ellipsoidal, very pale to mid pale brown or olivaceous brown, smooth, 0–3-septate, hilum sometimes slightly protuberant.
Type species: Fulvia fulva (Cooke) Cif. (≡ Cladosporium fulvum Cooke).
Fulvia fulva (Cooke) Cif., Atti Ist. Bot. Univ. Lab. Crittog. Pavia 10: 245. 1954. Fig. 31.
Fig. 31.
Fulvia fulva (CPC 13652). A–H. Observations in vitro. A. Culture on V8. B, D. Conidiophore reduced to conidiogenous cell and catenate conidia. C. Conidiogenous cell and catenate conidia. E, F. Conidiophore and catenate conidia. G, H. Catenate conidia. Scale bars = 10 μm.
Basionym: Cladosporium fulvum Cooke, Grevillea 12(61): 32. 1883.
Synonyms: Mycovellosiella fulva (Cooke) Arx, Proc. Kon. Ned. Akad. Wetensch., C86(1): 48. 1983.
Passalora fulva (Cooke) U. Braun & Crous, in Crous & Braun, CBS Biodiversity Ser. 1: 453. 2003.
Description and illustrations: Ellis (1971).
Description in vitro (on SNA; CPC 13652): Mycelium composed of hyaline to pale brown hyphae, uniform in width, 2 μm diam. Conidiophores arising from hyphae, pale brown, smooth to rough, micro- or macronematous, multi-septate, simple or short branched, straight or sinuous, often strongly curved at the tip, 20–160 μm × 2.5–10 μm, variable in width, sometimes reduced to conidiogenous cell. Conidiogenous cells integrated, terminal and intercalary, proliferating sympodially, polyblastic, with rim-like conidiogenous loci that are darkened and thickened, 1–2.5 μm. Conidia catenate, often forming branched chains, ovoid, obovoid, ellipsoidal, sphaerical, cylindrical, straight or strongly curved, 10–30 × 5–10 μm, 1–4-septate, hila thickened and darkened, 1–2.5 μm diam.
Materials examined: Cuba, on leaves of Solanum lycopersicum, 2006, B. Summerell (epitype designated here: CBS H-22950, MBT378581, culture ex-epitype CBS 142314 = CPC 13652). Netherlands, unknown host, and collector, 1946, isol. CBS Practicum, culture CBS 119.46. Switzerland, fruit of S. lycopersicum, unknown collector, dep. L. Zobrist, 1946, culture CBS 120.46 = VKM F-3053. USA, South Carolina, Aiken, on S. lycopersicum, H.W. Ravenel, Fungi Amer. Exs. 599 (lectotype, designated here: BPI 426698, MBT378580; isolectotypes, Ravenel, Fungi Amer. Exs. 599, e.g. B, CUP, K, NEB).
Notes: The genus Fulvia is no longer considered a synonym of Passalora as a result of analysis of the type species, Fulvia fulva (≡ Cladosporium fulvum ≡ Passalora fulva), which was recollected and epitypified in this study. Fulvia fulva clusters close to Stromatoseptoria in the phylogenetic analyses (Fig. 1, clade 59; Fig. 3, clade 23). The single-gene trees indicate that both LSU and ITS are able to distinguish this species but rpb2 is more reliable. Fulvia fulva is the causal agent of tomato leaf mould, a disease that affects mostly the leaves of tomato but occasionally also stems, blossoms, petioles and fruit (Butler & Jones 1949, de Wit 1977, 1992, Jones et al. 1997). The interaction between Fulvia fulva and tomato is governed by a gene-for-gene relationship, a characteristic that made this organism an interesting model to study plant-pathogen interactions (de Wit 1981, 1992). The resistance of tomato against Fulvia fulva was genetically determined by the presence of Cf (Cladosporium fulvum) resistance genes of which now five have been cloned. Cf proteins mediate the recognition of effector proteins secreted by Fulvia fulva of which all encoding genes have been cloned (de Wit 2016). Fulvia fulva was once a devastating pathogen of tomato that required treatment with agrochemicals, but since various Cf genes from different wild Solanum species were introduced in commercial tomato cultivars by breeders the pathogen is now under control. Commercially grown tomato cultivars contain up to five different Cf genes (Cf-2, Cf-4, Cf-4E, Cf-5 or Cf-9) (Thomma et al. 2005).
Clade 60: Ragnhildiana
Ragnhildiana Solheim, Mycologia 23: 402. 1931.
Description: Hyphomycetous, phytopathogenic. Mycelium internal and external, composed of hyaline to pigmented hyphae, branched, septate. Stromata lacking or developed, composed of brown pseudoparenchymatal cells. Conidiophores formed in fascicles, sometimes coremioid, emerging through stomata, through the epidermis, or single and arising from external hyphae, olivaceous to brown, septate, simple or branched, straight or geniculate-flexuous, sometimes reduced to conidiogenous cells. Conidiogenous cells integrated, terminal, mono- or polyblastic, with conidiogenous loci somewhat thickened and darkened. Conidia solitary or catenate, chains simple or branched, subhyaline to brown, ellipsoid-ovoid, subcylindrical-fusoid, or obclavate, aseptate to multi-septate, hila somewhat thickened and darkened.
Type species: Ragnhildiana agerati (F. Stevens) F. Stevens & Solheim (≡ Cercospora agerati F. Stevens) = Ragnhildiana perfoliati (Ellis & Everh.) U. Braun, C. Nakash., Videira & Crous
Ragnhildiana ampelopsidis (Peck) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822787. Fig. 32.
Fig. 32.
Ragnhildiana ampelopsidis (CBS 249.67). A–E. Observations in vitro. A. Culture on V8. B. Partial conidiophore, conidiogenous cell and catenate conidia. C. Conidiogenous cell and conidia. D, E. Catenate conidia. Scale bars = 10 μm.
Basionym: Cercospora ampelopsidis Peck, Rep. (Annual) New York State Mus. Nat. Hist. 30: 55. 1877.
Synonyms: Passalora ampelopsidis (Peck) U. Braun, Trudy Bot. Inst. im. V.L. Komarova 20: 38. 1997.
Cercospora pustula Cooke, Grevillea 12: 30. 1883.
Cercospora psedericola Tehon, Mycologia 16: 139. 1924.
Descriptions and illustrations: Chupp, 1954, Braun and Mel'nik, 1997.
Description in vitro (on V8; CBS 249.67): Mycelium composed of hyaline to pale brown hyphae, smooth to verruculose, uniform in width, 1.5–2.5 μm. Conidiophores micro- or macronematous, pale olivaceous brown, smooth to verruculose, simple or branched, strongly geniculated at the apex, 70–160 × 2.5–4 μm. Conidiogenous cells terminal, subhyaline to pale olivaceous brown, smooth, strongly geniculated at the apex, proliferating sympodially, polyblastic, with conidiogenous loci thickened, darkened and protruding, 2 μm diam. Conidia solitary or catenate, in simple or branching chains, subhyaline to pale olivaceous brown, smooth, obovoid, clavate to obclavate, cylindrical, straight or slightly curved, (16–)27–33(–48) × (2.5–)3(–4) μm, 1–4-euseptate, hila thickened, darkened and protruding, 1.5–2 μm diam.
Materials examined: Romania, Simeria, on Parthenocissus tricuspidata, 6 May 1965, unkown collector, isol. O. Constantinescu, culture CBS 249.67 = IMI 124968. USA, New York, Albany, Bethlehem, on Ampelopsis quinquefolia, July, C.H. Peck (holotype NYS-F-000244).
Notes: Braun & Melnik (1997) examined the holotype specimen of Cercospora ampelopsidis, and noted that the conidiophores can occasionally form synnema-like fascicles [20–130 × 3.5–5(–7) μm], and the conidia are formed singly [(20–)30–60(–140) × 4–8 μm]. In culture (CBS 249.67), synnema-like conidiophores were not observed and conidia were catenate and smaller (Fig. 32). Phylogenetically, Ragnhildiana ampelopsidis clusters in the Ragnhildiana clade (Fig. 1, clade 60; Fig. 3, clade 24) as a single-strain lineage.
Ragnhildiana diffusa (Heald & F.A. Wolf) Videira & Crous, comb. nov. MycoBank MB822788.
Basionym: Clasterosporium diffusum Heald & F.A. Wolf, Mycologia 3: 21. 1911.
Synonym: Cercospora fusca F.V. Rand, J. Agric. Res. 1: 318. 1914, nom. nov., non C. diffusa Ellis & Everh., 1888.
Sirosporium diffusum (Heald & F.A. Wolf) Deighton, in Ellis, More Dematiaceous Hyphomycetes: 299. 1976.
Descriptions and illustrations: Chupp, 1954, Ellis, 1976, Poletto et al., 2017.
Material examined: USA, Georgia, Baconton, on Carya illlinoinensis, 29 Aug. 1911, dep. F.V. Rand, culture CBS 106.14; Texas, Gonzales, on Carya illinoinensis, 10 Sep. 1909, F.D. Heald & F.F. Wolf 2695 (holotype of Clasterosporium diffusum [≡ Cercospora fusca], BPI 436535; isotypes CUP 3946, NEB 47510).
Notes: This pathogen is reported to cause reddish brown angular to round spots on leaves of Carya spp. in Cuba, Malawi, Mexico, Mozambique, South Africa, USA, and Venezuela (Ellis, 1976, Crous and Braun, 2003). It has recently been reported from Brazil (Poletto et al. 2017) where it was freshly collected from the same host and examined morphologically and genetically. Both the ITS and tef1-α sequences were identical to the respective sequences of Sirosporium diffusum (CBS 106.14). This culture is an authentic representative of Cercospora fusca, isolated in pure culture by F.V. Rand, on 29 Aug. 1911, from Carya illinoinensis in Baconton, Georgia, USA (Rand 1914). Although this isolate was never observed sporulating in culture, the specimen it was isolated from was compared to the type of Clasterosporium diffusum (basionym to the current name Ragnhildiana diffusa) and considered identical (Rand 1914). Morphologically, the description based on the Brazilian isolate fits well with the published description. Phylogenetically, this strain clusters among Ragnhildiana isolates (Fig. 1, clade 60; Fig. 3, clade 24) that produce catenate conidia, while Sirosporium diffusum produces solitary conidia that are very long and sometimes slightly constricted at the septa. The phylogenetic position of the type species of Sirosporium, Sirosporium antenniforme, is still undetermined (see section Genera of the Mycosphaerellaceae below).
Ragnhildiana ferruginea (Fuckel) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822791. Fig. 33.
Fig. 33.
Ragnhildiana ferruginea (CPC 10075). A–F. Observations in vivo. A, B. Leaf spot symptoms on the host. C. Conidiophores. D, E. Partial conidiophore, conidiogenous cell and conidium. F. Conidia. G–K. Observations in vitro. G. Culture on V8. H, I. Conidiophore, conidiogenous cell and conidia. J. Partial conidiogenous cell with single and catenate conidia. K. Single conidia. Scale bars = 10 μm.
Basionym: Cercospora ferruginea Fuckel, Hedwigia 2(15): 134. 1863 and Fuckel, Fungi Rhen. Exs., Fasc. II: no. 120. 1863.
Synonyms: Mycovellosiella ferruginea (Fuckel) Deighton, Mycol. Pap. 144: 14. 1979.
Passalora ferruginea (Fuckel) U. Braun & Crous, CBS Biodiversity Ser.: 183. 2003.
Cercospora olivacea G.H. Otth, Mitth. Naturf. Ges. Bern 654-683 (1868): 65. 1869.
Helminthosporium absinthii Peck, Rep. (Annual) New York State Mus. Nat. Hist. 30: 54. 1878.
Cercospora absinthii (Peck) Sacc., Syll. Fung. 4: 444. 1886.
Ramularia absinthii Laubert, Centralbl. Bacteriol., 2. Abt., 52: 242. 1920.
Cercosporidium artemisiae Sawada, Rep. Gov. Agric. Res. Inst. Taiwan 86: 164. 1943 (nom. inval.).
Descriptions and illustrations: Deighton, 1979, Shin and Kim, 2001.
Description in vitro (on V8; CBS 546.71): Mycelium composed of hyaline to brown hyphae, smooth to rough, uniform in width, 2–3 μm. Conidiophores micro- or macronematous, pale brown to brown, smooth to faintly verruculose, simple or branched, straight to sinuous, sometimes geniculate-sinuous at the apex, 5–200 × 2.5–5 μm. Conidiogenous cells terminal or intercalary, subhyaline to brown, smooth, geniculate to geniculate-sinuous, proliferating sympodially, polyblastic, with conidiogenous loci thickened, darkened and protruding, 1.5–2 μm diam. Conidia solitary, ocasionally catenate in simple chains, subhyaline to brown, smooth, obovoid, long-obclavate, cylindrical, base long-obconically truncate, apex rounded, straight to mildly curved, 20–75 × 2.5–5 μm, 0–5-euseptate, hila thickened, darkened and protruding, 1.5–2 μm diam.
Materials examined: Germany, Altersand vs. Hostrichiam (Nassau, Oestrich), on Artemisia vulgaris, 1863, Fuckel, Fungi Rhen. Exs. 120 (lectotype, designated here, MBT378582, HAL; isolectotypes, Fuckel, Fungi Rhen. Exs. 120, e.g. BPI 436287, F-C0003573F, FH-01012187, G, S F199142, 267462). Romania, Bucuresti, on Artemisia vulgaris, unknown collector, isol. O. Constantinescu, 6 Apr. 1965, CBS H-9838, culture CBS 255.67 = IMI 124973; unknown host and collector, isol. O. Constantinescu, 20 Jul. 1970, CBS H-9839, culture CBS 546.71. Republic of Korea, Pochon, on Artemisia sylvatica, 23 Oct. 2002, H.D. Shin, cultures CPC 10014, CPC 10075.
Notes: Ragnhildiana ferruginea has a worldwide distribution on hosts from the genera Ambrosia and Artemisia (Asteraceae) (Crous & Braun 2003). It produces mostly single conidia and only rarely catenate conidia in short unbranched chains (Fig. 33) (Shin & Kim 2001). Based on the phylogenetic analyses, Ragnhildiana ferruginea clusters among Ragnhildiana species (Fig. 1, clade 60; Fig. 3, clade 24) in a well-supported clade. Based on a BLAST comparison against the alignment, Ragnhildiana ferruginea CBS 546.71 shared 93 % (441/474) similarity based on ITS and 90 % (674/750) similarity based on rpb2 with Ragnhildiana ampelopsidis CBS 249.67. In addition, it shared only 85 % (664/780) similarity with Ragnhildiana perfoliati CBS 125419 based on rpb2.
Ragnhildiana gnaphaliacea (Cooke) Videira, H.D. Shin, C. Nakash. & Crous, comb. nov. MB822795. Fig. 34.
Fig. 34.
Ragnhildiana gnaphaliacea (CPC 12517). A–E. Observations in vivo. A, B. Leaf spot symptoms on the host. C. Conidiophores. D. Conidiophores, conidiogenous cells and conidia. E. Catenate conidia and single conidia. F–J. Observations in vitro. F. Culture on OA. G–I. Conidiophore, conidiogenous cell and conidia. J. Partial conidiophore, conidiogenous cell and conidia. Scale bars = 10 μm.
Basionym: Cercospora gnaphaliacea Cooke, J. Linn. Soc., Bot. 17: 142. 1880.
Synonyms: Phaeoisariopsis gnaphaliacea (Cooke) Morgan-Jones, Canad. J. Bot. 52: 2635. 1974.
Passalora gnaphaliacea (Cooke) U. Braun & Crous, in Crous & Braun, CBS Biodiversity Ser.: 201. 2003.
Cercospora gnaphalii Harkn., Bull. Calif. Acad. Sci. Bull. 1: 38. 1884.
Description in vivo (CBS H-22952): Leaf spots yellowish to brownish, without definite margin, subcircular to irregular, 3–20 mm. Mycelium internal and external, composed of pale brown to brown hyphae that are septate and smooth to verruculose. Stromata hypophyllous epidermal, submerged, stomatal or erumpent from epidermal cells, small composed of few brown cells to well-developed, pale to dark brown, up to 180 μm diam. Conidiophores solitary to densely fasciculate, emerging from stromata, brown to pale brown, paler towards apex, smooth to verruculose, straight to sinuous-geniculate, simple or branched, 46–75(–240) × 4–6.5 μm. Conidiogenous cells terminal and intercalary, polyblastic, proliferating sympodially, with rim-like conidiogenous loci, thickened and darkened, 2–2.5 μm diam. Conidia solitary or rarely catenate, hyaline to pale olivaceous brown, obovoid, cylindrical, straight or mildly curved, base obconicaly truncate, apex rounded, 18–70 × 6–10 μm, 0–4-euseptate, occasionally constricted at the septa, hila thickened and darkened.
Description in vitro (on SNA; CPC 12517): Mycelium hyaline to olivaceous brown, smooth to verruculose. Conidiophores macronematous, hyaline to pale brown, simple, septate, cylindrical, straight to slightly curved, 30–88 × 2.5–3 μm. Conidiogenous cells integrated, terminal or intercalary, monoblastic, cylindrical, conically truncate at the apex or geniculate-sinuous, determinate or proliferating sympodially, with conidiogenous locus thickened and darkened, located on the shoulder or at the apex, 1.5–2.5 μm diam. Conidia solitary, hyaline to pale brown, smooth to verruculose, long-obovoid, cylindrical to long-obclavate, base obconically truncate, apex rounded, straight to slightly curved, 20–75 × 2.5–5 μm, 0–5-euseptate, sometimes mildly constricted at septa, hila thickened and darkened, 1.5–2.5 μm diam.
Materials examined: Republic of Korea, Jeju, on Gnaphalium affine, May 2005, H.D. Shin, CBS H-22952, culture CBS 142181 = CPC 12517; idem., cultures CPC 10882, CPC 10883. USA, Texas, Houston, Gnaphalium sp., 17 Apr. 1869, H.W. Ravenel 283 (lectotype designated here BPI 436721, MycoBank MBT378599).
Notes: This is the first report of Ragnhildiana gnaphaliacea in Korea (based on Crous and Braun, 2003, Shin and Kim, 2001 and https://nt.ars-grin.gov/fungaldatabases/). Morphologically, the observed isolate description in vivo varies slightly from the one available in literature by producing longer conidiophores (60–90 × 4–5 μm; Morgan-Jones 1974) and shorter conidia [40–65 × 4–5 μm, (2–)3(–5)-septate; Morgan-Jones 1974] (Fig. 34). Phylogenetically, it clusters in the Ragnhildiana clade (Fig. 1, clade 60; Fig. 3, clade 24).
Ragnhildiana perfoliati (Ellis & Everh.) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822824.
Basionym: Cercospora perfoliati Ellis & Everh., J. Mycol. 5: 71. 1889.
Synonyms: Cercospora agerati F. Stevens, Bull. Bern. Bishop Mus. 19: 154. 1925.
Ragnhildiana agerati (F. Stevens) F. Stevens & Solheim, Mycologia 23: 402. 1931.
Cercospora assamensis S. Chowdhury, Lloydia 20(2): 134. 1957.
Passalora perfoliati (Ellis & Everh.) U. Braun & Crous, in Crous & Braun, CBS Biodiversity Ser. 1: 314. 2003.
Passalora assamensis (S. Chowdhury) U. Braun & Crous, in Crous & Braun, CBS Biodiversity Ser. 1: 69. 2003.
Passalora ageratinae Crous & A.R. Wood, Stud. Mycol. 64: 34. 2009.
Description and illustrations: Crous et al. (2009c).
Description in vitro (on V8; CBS 125419): Mycelium hyaline to brown, smooth, uniform in width, 2.5–3 μm diam. Conidiophores micro- to macronematous, cylindrical, subhyaline to brown, smooth, uniform in width, straight to slightly curved, simple, 25–150 × 2.5–5 μm. Conidiogenous cells integrated, apical, mostly monoblastic but sometimes polyblastic, usually determinate but occasionally proliferating sympodially, conically truncate at the apex, with conidiogenous loci slightly but clearly thickened loci at the apex, 2–2.5 μm diam. Conidia catenate, in simple chains, rarely in branched chains, hyaline to brown, smooth to verruculose, variable in shape, long-obovoid, cylindrical to long-obclavate, straight to curved, base and apex short- to medium-obconically truncate in intermediate conidia, apex rounded in terminal conidia, 20–80 × 2.5–5 μm, 0–5-euseptate, ocasionally constricted at septa, hila thickened, darkened and refractive, 2–2.5 μm diam. Strain CPC 15366 on V8 agar produces conidiophores that often form synnematous fascicles and are longer, 10–300 × 2.2–6 μm. Strain CPC 17321 on V8 agar produces conidiophores that are finely verruculose, longer and wider, 20–275 × 2.5–7.5 μm, and wider conidia 26–70 × 3–7.5 μm.
Materials examined: Guatemala, on Ageratina adenophora, unknown date, M.J. Morris, MJM 1506, dep. A. den Breeÿen, culture CBS 113613 = MJM 1506 = C486. Laos, Luang Prabang, on Chromolaena odorata, 17 Jun. 2006, P. Pheng, NOUL P101, culture CBS 142180 = CPC 17321. New Zealand, Cmoromandel, Thames, on Ageratina adenophora, unknown collector and date, isol. CF Hill, MAFF, Auckland, Feb. 2004, culture CBS 115119. South Africa, KwaZulu-Natal Province, Hilton, on leaves of Ageratina adenophora, 28 May 2008, A.R. Wood (holotype of Passalora ageratinae CBS H-20336, ex-type culture CBS 125419 = CPC 15365); idem. CPC 15366, CPC 15367.
Notes: Ragnhildiana was reduced to synonymy with Mycovellosiella by Muntañola (1960), and later both genera were placed in synonymy with Passalora by Crous & Braun (2003). With the recollection of the type species of Passalora, Passalora bacilligera, these three genera were found to be phylogenetically distinct, and hence the name Ragnhildiana is resurrected for this clade of passalora-like fungi. The type of Ragnhildiana, Ragnhildiana agerati was described from Ageratum conyzoides in Hawaii (syntype: ILL00010589, lectotype: ILL00010590). Passalora ageratinae was described from the host Ageratina adenophora from Mexico, and was transported into Hawaii, Australia and South Africa in association with a stem galling fly that was introduced as biocontrol agent for the invasive weed Ageratina adenophora (Dodd, 1961, Morris, 1989, Wang et al., 1997, Zhu et al., 2007, Muniappan et al., 2009). Passalora ageratinae, is similar to “Passalora” assamensis, except for the amphigenous nature of the colonies, the absence of external mycelium and the production of shorter conidiophores. Type material of “Passalora” assamensis was not available for re-examination but other specimens from the same location and host (India, Nepal, Ageratina adenophora) were examined and found to be compatible with the description (Crous & Braun 2003). Based on the phylogenetic analyses, the available strains cluster together in a clade that has a well supported basal branch (Fig. 3, clade 24) and is included in the Ragnhildiana clade (Fig. 1, clade 60). In addition, using a BLAST comparison against the alignment, “Passalora” assamensis CBS 115119 shares 99 % (469/475) similarity on ITS and 99 % (656/657) similarity on rpb2 with “Passalora” ageratinae CBS 125419. The morphological description of “Passalora” perfoliati is also similar to that of “Passalora” ageratinae and, based on a BLAST comparison against the alignment, “Passalora” perfoliati CPC 17321 shares 99 % (468/475) similarity on ITS and 100 % (780/780) similarity on rpb2 with “Passalora” ageratinae CBS 125419. Therefore, we consider them all to be synonyms.
Ragnhildiana pseudotithoniae (Crous & Cheew.) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822797.
Basionym: Passalora pseudotithoniae Crous & Cheew., Persoonia 31: 261. 2013.
Description and illustration: Crous et al. (2013b).
Description (from Crous et al. 2013b): Leaf spots amphigenous, brown, angular, confined by leaf veins, 2–5 mm diam. Conidiophores amphigenous, fasciculate, 40–100 μm tall, 3–4 μm wide, straight to geniculate-sinuous, mostly unbranched, subcylindrical, 1–3-septate, brown, smooth to finely verruculose, arising from a weakly developed brown stroma, up to 50 μm wide and 60 μm tall. Conidiogenous cells integrated, brown, smooth to finely verruculose, terminal, subcylindrical to once geniculate, 15–35 × 3–4.5 μm, with thickened and darkened loci, 2 μm diam, mostly solitary and terminal, but also lateral on conidiogenous cells. Conidia occurring in long branched chains, brown, granular, smooth, subcylindrical to narrowly obclavate, (30–)40–65(–130) × (4–)5(–5.5) μm, 1–6-septate, apex obtuse to truncate, base obconically truncate, thickened and darkened, 2 μm diam.
Materials examined: Thailand, N18°09′24.8″ E98°23′19.6″, Royal Project, on leaves of Tithonia diversifolia (Asteraceae), 5 Nov. 2012, P.W. Crous (holotype CBS H-21453, ex-type culture CBS 136442 = CPC 21688).
Notes: Phylogenetically, Ragnhildiana pseudotithonia clusters in the Ragnhildiana clade (Fig. 1, clade 60; Fig. 3, clade 24) in a single-strain lineage. One other species has been recently described from the same host but originary from Brazil, Passalora stromatica (Fernandes et al. 2013). Based on a BLAST comparison against the alignment, the ITS sequence of Passalora stromatica GenBank KF275128 was closest to Ragnhildiana pseudotithonia CBS 136442, with which it shared 96 % (467/484) similarity, including 2 % (10/484) gaps. Based on the morphological and DNA differences, these are not the same species.
Clade 61: Phaeoramularia
Phaeoramularia Munt.-Cvetk., Lilloa 30: 182. 1960.
Description (from Braun 1998): Phytopathogenic, usually forming leaf spots, occasionally almost symptomless. Mycelium internal, composed of subhyaline to pigmented hyphae, septate, branched, smooth to rough. Stromata almost absent to well-developed, pigmented. Conidiophores macronematous, mononematous, in small to large fascicles, rarely solitary, arising from internal hyphae or stromata, emerging through stomata or erumpent through the cuticle, erect, straight, subcylindrical to flexuous, geniculate-sinuous, simple, rarely branched, continuous to septate, pale yellowish green, olivaceous to brown, smooth to rough, thin-walled. Conidiogenous cells integrated, terminal, occasionally intercalary, sometimes conidiophores reduced to a single conidiogenous cell, polyblastic, proliferation sympodial, rarely percurrent, conidiogenous loci thickened and darkened. Conidia catenate, sometimes in branched chains, ellipsoid-ovoid, subcylindrical, fusiform, continuous to euseptate, subhyaline to pigmented, smooth to rough, ends obtuse, truncate or subacute; hila thickened and darkened; conidial secession schizolytic.
Type species: Phaeoramularia gomphrenicola (Speg.) Munt.-Cvetk. (≡ Cercospora gomphrenicola Speg.).
Phaeoramularia capsicicola (Vassiljevsky) Deighton, More Dematiaceous Hyphomycetes: 323. 1976.
Basionym: Cercospora capsicicola Vassiljevsky, Fungi imperfecti Parasitici. I. Hyphomycetes: 344. 1937.
Synonyms: Cercospora capsici É.J. Marchal & Steyaert, Bull. Soc. Roy. Bot. Belgique 61: 167. 1929.
Cladosporium capsici Kovatsch., Z. Pflanzenkrankh. Pflanzenschutz 48(7): 335. 1938.
Cercospora unamunoi Castell., Rivista Agric. Subtrop. Trop. 42: 20. 1948.
Passalora capsicicola (Vassiljevsky) U. Braun & F.O. Freire, Cryptog. Mycol. 23: 299. 2002.
For additional synonyms see Crous & Braun (2003).
Descriptions and illustrations: Kovachevsky, 1939, Muntañola, 1954, Ellis, 1976, Deighton, 1976b.
Materials examined: Italy, on Capsicum annuum, unknown collector and date, dep. A. Matta, 1962, culture CBS 156.62. Jamaica, on Chromolaena odorata, 2006?, coll. M.J. Morris, dep. A. den Breeÿen, culture CBS 113384 = C499. USA, on C. odorata, 2006?, coll. M.J. Morris, dep. A. den Breeÿen, culture CBS 113382 = C460.
Notes: In August 2011, the occurrence of Passalora capsicicola, the causal agent of a foliar disease on sweet pepper, was reported for the first time in Austria but unfortunately no DNA was extracted (Bedlan et al. 2012). The species Passalora capsicicola is reported to infect hosts of Capsicum sp. (Solanaceae) in tropical and subtropical countries including the USA, Brazil, Romania, Tanzania, China and many others (Crous & Braun 2003). The strains CBS 113384 and CBS 113382 were not described due to the cultures being sterile and the herbarium specimens not being preserved (Den Breeÿen et al. 2006). There is no previous report of Passalora capsicicola being isolated from the host Chromolaena odorata (Asteraceae) (Farr & Rossman, retrieved June 22, 2017, from https://nt.ars-grin.gov/fungaldatabases/). The strain CBS 156.62, identified as Passalora capsicicola, was also sterile in culture and the herbarium specimen could not be traced. Based on the phylogenetic analysis, Passalora capsicicola clusters in the Phaeoramularia clade (Fig. 1, clade 61; Fig. 3, clade 25) in a well supported clade. In addition, based on a BLAST comparison against the alignment, CBS 148.38 shared 99 % (465/472) similarity on ITS and 92 % (587/639) similarity on rpb2 with Phaeoramularia gomphrenicola CPC 23248.
Phaeoramularia gomphrenicola (Speg.) Munt.-Cvetk., Lilloa 30: 209. 1960. Fig. 35.
Fig. 35.
Phaeoramularia gomphrenicola (CPC 23248). A–E. Observations in vivo. A, B. Leaf spot symptoms on the host. C. Conidiophores. D. Partial conidiophore, conidiogenous cells and conidia. E. Catenate conidia. F–J. Observations in vitro. F. Culture on OA. G–I. Partial conidiophore, conidiogenous cell and conidia. J. Single and catenate conidia. Scale bars = 10 μm.
Basionym: Cercospora gomphrenicola Speg., An. Soc. Cient. Argent. 13(1): 29. 1882.
Description in vivo (CBS H-22954): Mycelium internal, composed of hyaline to pale brown hyphae, smooth to finely verruculose. Stromata hypophyllous, epidermal, stomatal, brown to reddish brown, small to well-developed, 20–50 μm diam. Conidiophores emerging from upper part of stromata in dense fascicles, pale brown to brown, smooth to finely verruculose, straight to sinuous, simple or occasionally branched, 25–125(–200) × 5–7.5 μm. Conidiogenous cells integrated, terminal or intercalary, pale brown, smooth to finely verruculose, mono- or polyblastic, proliferating sympodially, with rim-like conidiogenous loci that are thickened and darkened, 2–2.5 μm diam. Conidia catenate, in simple or branched chains, with microcyclic conidiation, pale brown, smooth to finely verruculose, obclavate to cylindrical, base obconically truncate, apex conically truncated in intercalary conidia and rounded in terminal conidia, 20–75 × 5–7.5 μm, (0–)1–3(–4)-septate, hila thickened and darkened, 2–2.5 μm diam.
Description in vitro (on V8; CPC 23248): Mycelium composed of hyaline to olivaceous brown hyphae, smooth to finely verruculose, often constricted at septa, irregular in width, 2.5–7.5 μm. Conidiophores micro- or macronematous, pale brown to pale olivaceous brown, smooth to finely verruculose, constricted at septa, simple or branched, straight or mildly sinuous, 50–250 × 2.5–7.5 μm. Conidiogenous cells integrated, terminal or intercalary, smooth to finely verruculose, mono- or polyblastic, proliferating sympodially, conically truncate at the apex or geniculate-sinuous, with rim-like conidiogenous loci that are slightly thickened and darkened, 2–2.5 μm diam. Conidia catenate in simple chains, rarely in branched chains, pale to pale olivaceous brown, smooth to finely verruculose, obovate, allantoid, cylindrical, base obconically truncate, apex conically truncate in intermediate conidia and rounded in terminal conidia, irregular in width, 18–125 × 3.5–5 μm, 0–4-septate, occasionally constricted at septa, hila slightly thickened and darkened, 2–2.5 μm diam.
Material examined: Argentina, Buenos Aires, Palermo, on Pfaffia glomerata (as Gomphrena glauca), Feb. 1881, C. Spegazzini (holotype LPS 914; isotypes Speg., Hongos Sud-Amer., Dec. Mycol. Argent. 45, e.g. BPI 436740, 722393, FH, PAD, PDD 25866; IMI 7706, slide ex holotype). Brazil, Minas Gerais, Viçosa, on P. glomerata, 29 Oct. 2012, R.W. Barreto (epitype designated here: CBS H-22954, MBT378603, ex-epitype culture CBS 142182 = CPC 23248 = COAD570); idem., culture CPC 23249 = COAD571.
Notes: Phaeoramularia resembles Ramularia by producing catenate conidia but differs by producing pigmented conidiophores and conidia (Braun 1998). In addition, the conidiogenous loci are thickened and rim-like and not coronate. This genus is no longer considered a synonym of Passalora since the type, Phaeoramularia gomphrenicola (Fig. 1, clade 61), clusters apart from the type of Passalora, Passalora bacilligera (Fig. 1, clade 34). Phylogenetically, Phaeoramularia clusters in a clade well-supported by all three phylogenetic methods (Fig. 1, clade 61, Fig. 3, clade 25) and is closely related to Ragnhildiana. Morphologically, it can be distinguished from Ragnhildiana by forming broader conidiophores and conidia, and its conidia can generate new conidia from any segment (Fig. 35). The single-gene trees indicate that both LSU and ITS can distinguish this genus but rpb2 is more reliable. The previously applied phaeoramularioid habit, i.e. internal mycelium in vivo, fasciculate conidiophores and catenate conidia, is not diagnostic any longer since species with this morphology belong to different clades within the Mycosphaerellaceae. Therefore, phylogenetically unproven species should tentatively be maintained in Passalora s. lat.
Clade 62: Deightonomyces
Deightonomyces Videira & Crous, gen. nov. MycoBank MB822586.
Etymology: Name composed of Deighton (F.C. Deighton, British mycologist and pioneer of modern taxonomy of cercosporoid fungi) and -myces (fungus).
Description: Mycelium immersed, hyphae pigmented. Stromata immersed, composed of brown, thick-walled hyphal cells. Conidiophores in dense fascicles, arising from stromata, olivaceous brown, smooth, simple, straight, subcylindrical, slightly geniculate-sinuous. Conidiogenous cells terminal, subhyaline to pale olivaceous, smooth, proliferating sympodially, conidiogenous loci conspicuous, slightly thickened and darkened. Conidia solitary, ellipsoid-ovoid, obclavate-fusiform, subcylindrical, aseptate or septate, subhyaline to pale olivaceous, smooth to verruculose, apex obtuse or subacute, base obconically truncate, hila hardly thickened and somewhat darkened.
Type species: Deightonomyces daleae (Ellis & Kellerm.) Videira & Crous (≡ Cercospora daleae Ellis & Kellerm.).
Deightonomyces daleae (Ellis & Kellerm.) Videira & Crous, comb. nov. MycoBank MB822753.
Basionym: Cercospora daleae Ellis & Kellerm., J. Mycol. 4: 6. 1888.
Synonym: Passalora daleae (Ellis & Kellerm.) U. Braun, Sydowia 48: 208. 1996.
Description and illustration: Braun (1996).
Materials examined: Mexico, Baja California Norte, Catarina, on bark of Dalea spinosa, Apr. 2003, L.B. Sparrius, isol. Aptroot, 2003, culture CBS 113031. USA, Kansas, on stems of Dalea enneandra (= Dalea laxiflora), 10 Dec. 1887, Kellerman 954 (holotype NY00838299).
Notes: The strain of Passalora daleae used in this study forms a single-strain lineage in the phylogenetic analyses (Fig. 1, clade 62; Fig. 4, clade 26). Both morphologically and phylogenetically, this species is not a true Passalora as circumscribed in this study, and therefore a new genus is introduced to accommodate it. When blasted against the individual gene alignments, Deightonomyces daleae CBS 113301 shares 98 % (465/474) similarity with Dothistroma pini CBS 116486 on ITS, 99 % (724/726) similarity with Dothistroma septosporum CBS 128282 on LSU, and only 81 % (633/784) similarity with Phaeoramularia sp. CBS 113382 on rpb2.
Clade 63: Pleopassalora
Pleopassalora Videira & Crous, gen. nov. MycoBank MB822608.
Etymology: Named after its pleomorphic morphology (Greek pleon = more), and its resemblance to Passalora.
Description (adapted from Beilharz et al. 2004): Pleoanamorphic, phytopathogenic, causing leaf spots. Mycelium internal, hyphae smooth, branched, septate, brown. Stromata medium brown, erumpent, protuberant and pulvinate, composed of textura angularis. Conidiomata amphigenous, eustromatic, bearing Type 1 conidiophores and conidia, Type 2 conidiophores and conidia, or both. Type 1 synasexual morph: Conidiophores occasionally solitary, usually in fascicles arising from stromata, pale to medium brown, smooth to rugose, subcylindrical, branched or unbranched, walls slightly thickened, straight to variously curved or geniculate-sinuous, septate. Conidiogenous cells terminal, verruculose or rugose, unbranched, subcylindrical, tapering to rounded apices proliferating sympodially, conidiogenous loci slightly thickened and darkened, refractive, flat or sometimes protuberant. Conidia solitary, pale olivaceous, dry, smooth, rarely finely verruculose, straight or curved, narrowly obclavate to subcylindrical, tapering gradually to an obtuse apex and to a rounded base, often constricted at one or more septa, hila slightly but distinctly thickened, darkened and refractive. Type 2 synasexual morph: Conidiophores reduced, hyaline to sub-hyaline, aseptate or 1-septate, lining a stroma. Conidia hyaline to pale olivaceous, cylindrical, rounded at the apex, truncate at the base, smooth, aseptate to 3-septate, occasionally constricted at septa, hila broad, truncate to slightly convex, not darkened, unthickened, non-refractive. Type 3 synasexual morph: Type 2 conidia develop thick-walled hyphal swellings (reminiscent of chlamydospores), ellipsoid and hyaline, aseptate to 1-septate, that burst free from the cells of the Type 2 conidia, frequently carrying remnants of the conidial wall attached to their hyaline walls.
Type species: Pleopassalora perplexa (Beilharz et al.) Videira & Crous (≡ Passalora perplexa Beilharz et al.).
Pleopassalora perplexa (Beilharz et al.) Videira & Crous, comb. nov. MycoBank MB822776. Fig. 36.
Fig. 36.
Pleopassalora perplexa (CPC 12168). A–H. Observations in vitro. A. Culture on OA. B, C. Conidiophore and conidia type II. D, E. Conidiophore and conidia type I. F. Conidiophore type I. G. Conidia type I, slightly constricted at the septa and swollen cells at the base, and type II, smaller and narrower. H. Conidia type I and type II. Scale bars = 10 μm.
Basionym: Passalora perplexa Beilharz et al., Stud. Mycol. 50: 473. 2004.
Description and illustrations: Beilharz et al. (2004).
Materials examined: Indonesia, South Sumatra, Kerinci, on Acacia crassicarpa, Feb. 2004, M.J. Wingfield (holotype CBS H-9907, culture ex-type CBS 116363 = CPC 11147–11149); idem. CBS H-9908, CBS H-9909, CBS H-9911, cultures derived from CBS H-9911, CBS 116364 = CPC 11150–11151; idem., 1 Mar. 2004, M.J. Wingfield, culture CPC 11152; idem., Acacia sp., 1 May 2005, M.J. Wingfield, cultures CPC 12168, CPC 12170.
Notes: Passalora perplexa is the causal agent of leaf blight in Acacia crassicarpa both in Australia where it is native and also in plantations in Indonesia to where it spread. It is one of few pleoanamorphic cercosporoid fungi described with one morph characterised as a hyphomycete, a second morph described as a coelomycete, and a third morph representing a resting spore form on natural substrates and artificial media (Beilharz et al. 2004). The available strains of Passalora perplexa cluster together in a well-supported clade in the phylogenetic analyses (Fig. 1, clade 63; Fig. 3, clade 27). The phylogenetic analyses supports a clade including strains from Passalora perplexa, Passalora sp. 1, Passalora juniperina and Phaeocercospora colophospermi, but these species vary too significantly in their morphology to be assigned to the same genus.
“Passalora” sp. 1
Description in vitro (V8; CBS 122466): Mycelium composed of hyaline to pale brown hyphae, smooth, uniform in width, 2–2.5 μm. Conidiophores micro- or macronematous, pale to pale brown, simple, rough, straight to mildly sinuous, long to medium conically truncate at the apex, 10–40 × 2.5 μm. Conidiogenous cells integrated, apical, polyblastic, proliferating sympodially, with slightly protruding conidiogenous loci that are somewhat thickened and darkened, 2 μm in diam. Conidia solitary, hyaline to pale brown, finely verruculose, cylindrical to obclavate, base obconically truncate, rounded at the apex, 25–40 × 2–2.5 μm, multi-euseptate, septa indistinct, hila somewhat thickened and darkened, 2 μm diam.
Material examined: USA, Florida, on Citrus sp., unknown date, R.C. Ploetz, culture CBS 122466.
Notes: Phyllogenetically, the present species forms a single strain lineage closely related to Pleopassalora (Fig. 1, clade 63; Fig. 3, clade 27). The present strain was initially identified as Passalora loranthi (Arzanlou et al. 2008) since its DNA was identical to a sequence of Passalora loranthi available on GenBank (GenBank AY348311). Although there is no publication associated with that accession number, many subsequent authors followed this identification (Crous et al., 2004b, Beilharz et al., 2004, Arzanlou et al., 2008, Douanla-Meli et al., 2013, Huang et al., 2015). A description based on the observation of strain CBS 122466 in culture is presented. Unfortunately, the culture became sterile and thus fresh material needs to be collected to fully clarify the taxonomy of this species, which appears to have a wide host range.
Clade 64: Phaeocercospora
Phaeocercospora Crous, Persoonia 28: 171. 2012.
Description (from Crous et al. 2012b): Foliicolous, associated with leaf spots. Caespituli amphigenous, subepidermal, arising from subepidermal, globular fruiting bodies (immature structures with undefined white contents); wall of 2–3 layers of textura angularis, bursting through epidermis, forming grey sporodochia with densely aggregated conidiophores. Conidiophores subcylindrical to ampulliform, brown, finely verruculose, aggregated, 0–2-septate. Conidiogenous cells terminal, brown, finely verruculose, ampulliform, tapering to a truncate apex, proliferating several times percurrently at apex (proliferations irregular, rough), or sympodially. Conidia solitary, brown, finely verruculose, guttulate, subcylindrical to narrowly obclavate, straight to mildly curved, apex subobtuse, base truncate with marginal frill, transversely septate; hila and scars not thickened, nor darkened or refractive.
Type species: Phaeocercospora colophospermi Crous.
Phaeocercospora colophospermi Crous, Persoonia 28: 171. 2012.
Descriptions and illustrations: Crous et al. (2012b).
Material examined: South Africa, Mpumalanga, Kruger Game Reserve, Satara rest camp, on leaves of Colophospermum mopane, 11 Jul. 2011, P.W. Crous & K.L. Crous (holotype CBS H-20966, culture ex-type CBS 132687 = CPC 19812).
Note: Phaeocercospora is a recently introduced genus that was established to accommodate Phaeocercospora colophospermi (Crous et al. 2012b). In the present phylogenetic analyses, Phaeocercospora colophospermi is represented by a single-strain lineage (Fig. 1, clade 64; Fig. 3, clade 28) closely related to Pleopassalora.
Phaeocercospora juniperina (Georgescu & Badea) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822825. Fig. 37.
Fig. 37.
Phaeocercospora juniperina (CPC 11258). A–F. Observations in vivo. A. Leaf spot symptoms on the host. B. Conidiophores and conidia on the lesions. C, E. Conidiogenous cells and conidia. D. Conidiophores and conidia. F. Conidia. G–K. Observations in vitro. G. Culture on SNA. H, I. Conidiophore and conidia. J, K. Conidia. Scale bars = 10 μm.
Basionym: Cercospora juniperina Georgescu & Badea, Analele Inst. Cercet. Exp. Forest. Bucharest I: 37. 1937.
Synonyms: Stigmina juniperina (Georgescu & Badea) M.B. Ellis, Mycol. Pap. 72: 67. 1959.
Sciniatosporium juniperinum (Georgescu & Badea) Morgan-Jones, Canad. J. Bot. 49: 998. 1971.
Asperisporium juniperinum (Georgescu & Badea) B. Sutton & Hodges, Mycologia 82: 317. 1990.
Passalora juniperina (Georgescu & Badea) H. Solheim, Agarica 34: 110. 2014.
Camarosporium juniperinum Georgescu & Badea, Rev. Padurilor, Bucharest: 1. 1935.
Description in vivo (CBS H-22955): Mycelium internal, composed of brown hyphae, septate, branched. Stromata well-developed, brown to dark brown, often with a cavity filled with spermatia, single or aggregate, wall composed of textura angularis, 80–340 μm diam. Conidiophores sporodochial, densely fasciculate, pale brown to brown, smooth, aseptate or septate, cylindrical to geniculate, 16–50 × 4–9 μm, often reduced to conidiogenous cells. Conidiogenous cells integrated, terminal, polyblastic, proliferating percurrently or sympodially, with conidiogenous loci not thickened and not darkened, apical or lateral at the apex, 2–2.5 μm diam. Conidia solitary, pale to pale olivaceous brown, smooth to thinly verruculose, cylindrical to long-obclavate, straight to slightly curved, base obconically truncate, apex rounded, 18–56 × 2.5–3.5 μm, 1–4-septate, hila not thickened and not darkened at the base, 2–2.5 μm diam.
Description in vitro (on SNA; CPC 11258): Mycelium composed of pale brown hyphae, smooth, septate, branched. Stromata absent. Conidiophores emerging from hyphae, pale brown, smooth, erect, cylindrical to geniculate, septate, 11–55 × 3–6 μm. Conidiogenous cells integrated, terminal, polyblastic, proliferating sympodially, conically truncate or geniculate at the apex, with conidiogenous loci not thickened or darkened, apical or lateral at the apex, 2 μm diam. Conidia solitary, pale brown, smooth to thinly verruculose, cylindrical to long-obclavate, straight to slightly curved, base obconically truncate, apex rounded, 25–54 × 2.5–4 μm, 1–4-septate, hila not thickened and not darkened, 2–2.5 μm diam.
Material examined: USA, North Carolina, on Juniperus virginiana, 1 Mar. 2004, C.S. Hodges, CBS H-22955, culture CBS 142238 = CPC 11258.
Notes: Both specimen and culture materials were examined and this fungus has conidiogenous cells proliferating both percurrently with annellations and sympodially with rim-like loci (Fig. 37). Phylogenetically, this strain forms a single-strain lineage closely related to Phaeocercospora colophospermi (Fig. 1, clade 64; Fig. 3, clade 28). Given the phylogenetical proximity and morphological similarities, a combination is proposed in Phaeocercospora until further evidence becomes available.
Clade 65: Rosisphaerella
Rosisphaerella Videira & Crous, gen. nov. MycoBank MB822703.
Etymology: Mycosphaerella-like species from the host genus Rosa.
Description: Phytopathogenic, foliicolous. Mycelium internal, composed of subhyaline to brown hyphae, smooth, septate, branching. Stromata lacking or small, epidermal, substomatal, brown to dark brown. Conidiophores emerging from stromata or few brown cells, solitary to fasciculate, often synnematous, dark olivaceous brown near base and paler toward the tip, smooth, simple, multiseptate, straight to sinuous, usually geniculate-sinuous. Conidiogenous cells integrated, terminal and intercalary, proliferating sympodially, rarely proliferating percurrently, with rim-like conidiogenous loci, somewhat thickened, darkened and protuberant. Conidia solitary, pale to medium olivaceous brown, smooth to finely verruculose, cylindrical to obclavate, straight to mildly curved, septate, obconically truncate at base and rounded at apex, hila somewhat thickened and darkened.
Type species: Rosisphaerella rosicola (Pass.) U. Braun, et al. (≡ Cercospora rosicola Pass.).
Rosisphaerella rosicola (Pass.) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822800. Fig. 38.
Fig. 38.
Rosisphaerella rosicola (CPC 12548). A–E. Observations in vivo. A, B. Leaf spot symptoms on the host. C, D. Conidiophores and conidiogenous cells. E. Single conidia. F–K. Observations in vitro. F. Culture on OA. G–I. Conidiophore, conidiogenous cells and conidia. J. Partial conidiophore, conidiogenous cells and conidia. K. Conidia. Scale bars = 10 μm.
Basionym: Cercospora rosicola Pass., in Thüm., Herb. Mycol. Oecon., Fasc. VII: no. 333. 1875.
Synonyms: Passalora rosicola (Pass.) U. Braun, Mycotaxon 55: 234. 1995.
Cercospora rosicola var. undosa Davis, Trans. Wisconsin Acad. Sci. 20: 405. 1921.
Cercospora rosae J.M. Hook, Proc. Indiana Acad. Sci. 38: 131. 1929.
Cercospora rosae-indianensis J.M. Hook, Proc. Indiana Acad. Sci. 39: 82. 1930.
Mycosphaerella rosicola B.H. Davis, Mycologia 30: 296. 1938.
Description in vivo (CBS H-22956): Leaf spots scattered, circular or irregular when coalescing, singly 1–4 mm diam, uniformly purplish or reddish brown, or greysh white to pale brown at the centre, indistinct on lower leaf surface. Mycelium internal, composed of hyaline and pale brown to brown hyphae, septate, branching, 3–4 μm diam. Stromata lacking or small, epidermal, substomatal, brown to dark brown, 25–52 μm diam. Conidiophores emerging from stromata or agglomerates of a few brown cells, solitary or in fascicles, fascicles loose or dense, often synnematous, dark olivaceous brown near base, paler towards the tip, smooth, simple, multiseptate, straight or sinuous, usually geniculate-sinuous, 20–156 × 3–6 μm. Conidiogenous cells integrated, terminal and intercalary, proliferating sympodially, rarely proliferating percurrently, with rim-like conidiogenous loci, somewhat thickened and darkened, 2–4 μm diam. Conidia solitary, pale to medium olivaceous brown, smooth to finely verruculose, cylindrical to obclavate, straight to mildly curved, base long-obconically truncate, apex rounded, 20–98 × 3–5 μm, 1–6-septate, hila somewhat thickened and darkened, 2–4 μm diam.
Description in vitro (on SNA; CPC 12548): Mycelium composed of pale brown to brown hyphae, uniform in width, 2–3 μm. Conidiophores micro- or macronematous, pale brown to brown, paler at the apex, smooth, erect, simple, septate, geniculate-sinuous, 10–280 × 2.5–5 μm. Conidiogenous cells integrated, terminal and intercalary, pale brown to brown, smooth, mono- or polyblastic, proliferating sympodially, conically truncate at the apex or geniculate-sinuous, with conidiogenous loci thickened and darkened, located protruding at the apex and shoulders, 2–2.5 μm diam. Conidia solitary, subhyaline to pale brown, smooth to finely verruculose, cylindrical to long obclavate, straight or mildly curved, base short obconically truncate, apex rounded, 1–4-euseptate, 20–63 × 2.5–5 μm, hila refractive and slightly thickened and darkened.
Materials examined: Italy, Parma, on Rosa sp. cult., 1874, G. Passerini, Thümen, Herb. Mycol. Oecon. 333 (lectotype designated here, BPI 440506, MBT378584; isolectotypes Thümen, Herb. Mycol. Oecon. 333, e.g. B, K, S). USA, North Carolina, on Rosa sp. hybrid, 2005, C.S. Hodges, CBS H-22956, culture CBS 142183 = CPC 12548; unknown state/city, host, collector and date, dep. LM Massey, 1935, culture CBS 138.35 = ATCC 52313.
Notes: Passalora rosicola is known to cause leaf spot disease on rose worldwide (Davis 1938). Morphologically, the specimens examined (Fig. 38) fit the description available in the literature (Braun 1995). Morphologically, the strain CBS 142183 is a good representative of the species and was isolated from the same host as the type specimen. However, since it was isolated from a different continent, we refrain from proposing an epitype. Phylogenetically, the observed strains cluster in a well-supported clade (Fig. 1, clade 65; Fig. 3 clade 29) closely related to Phaeocercospora and Pleopassalora. When single gene sequences are BLASTed against the alignment, CPC 12548 shares 80 % (623/778) similarity on rpb2 with CPC 11258 Phaeocercospora juniperina, 91 % (438/479) similarity on ITS and 96 % (700/727) similarity on LSU. In Passalora rosicola we did not observe pleomorphic asexual states as in Pleopassalora, nor percurrent conidiation as in Phaeocercospora. Given both the morphological and phylogenetic differences from the closest related genera, we introduce the genus Rosisphaerella to accommodate this species.
Clade 66: Exutisphaerella
Exutisphaerella Videira & Crous, gen. nov. MycoBank MB822590.
Etymology: exutus- meaning “cast off” or “shed” like the disease symptom + sphaerella because of the globose ascomata.
Description: Ascomata pseudothecial, globose to slightly elongated or elliptical, emerging through the epidermis, solitary or gregarious, ostiole apical. Asci club-shaped, stipitate, 8-spored. Ascospores hyaline, oblong, fusiform-elliptical, straight or slightly curved, 1-septate, not constricted at septa, with cells of equal size. Asexual morph acervular-like. Conidiophores ampulliform, in compact bunches. Conidia hyaline, bacillar to allantoid, rounded at the tip, truncate at the base, straight to slightly curved, aseptate to multiseptate. Spermagonia in stromata, barely erumpent to completely exposed, globose to oval or pyriform, apical ostiole. Spermatia bacillar to pyriform.
Type species: Exutisphaerella laricina Videira & Crous (≡ Sphaerella laricina R. Hartig)
Exutisphaerella laricina (R. Hartig) Videira & Crous, comb. nov. MycoBank MB822758.
Basionym: Sphaerella laricina R. Hartig, Forstl.-Naturwiss. Z. 4: 445. 1895.
Synonym: Mycosphaerella laricina (R. Hartig) Mig., Krypt.-Fl. Deutschl. Österr. Schweiz. 3(1): 301. 1912.
Descriptions and illustrations: Hartig (1895), Patton et al. (1983).
Description in vivo (adapted from Hartig 1895 and Patton 1983): Ascomata pseudothecial, globose to slightly elongated or elliptical, emerging through the epidermis, solitary or gregarious, 100–150 μm diam, ostiole apical. Asci club-shaped, stipitate, 40–60 μm long, 8-spored. Ascospores hyaline, oblong, fusiform-elliptical, straight or slightly curved, 1-septate, not constricted at septa, with cells of equal size, 11–17 × 2.5–3 μm. Asexual morph acervulum-like. Conidiophores ampulliform, in compact bunches. Conidia hyaline, bacillar to allantoid, rounded at the tip, truncate at the base, straight to slightly curved, 25–46 × 2–4 μm, (0–)1–4-septate. Spermagonia in stromata, barely erumpent to completely exposed, globose to oval or pyriform, occasionally two spermagonial cavities occur in a single stroma, apical ostiole. Spermatia bacillar to pyriform, 1–3 × 0.5 μm.
Material examined: Switzerland, Kt. Zurich, Horgenberg, on Larix decidua, unknown date and collector, isol. E. Müller, 27 May 1952 (neotype designated here as metabolically inactive culture CBS 326.52, MBT378624).
Notes: Mycosphaerella laricina was first observed infecting the host Larix europaea (Pinaceae) in Germany, and is the causative agent of needle cast disease of European larch wherever it is cultivated. Unfortunately, the type could not be located in any fungaria and a neotype is necessary (Aptroot 2006). The asexual morph is reported as a Cercoseptoria (fide D.F. Farr et al., 1989, Corlett, 1991), currently treated as synonym of Pseudocercospora (needs confirmation based on DNA), or a Leptostroma (fide Tomilin 1979). The asexual morph is characterised by acervular conidiomata, lined with ampulliform conidiophores with truncate apices, producing hyaline and bacillar conidia, 1–4-septate (Patton 1983). The strain used in this study was unfortunately sterile and morphological comparison was impossible. Phylogenetically, this strain forms a single-strain lineage closely related to Rosisphaerella (Fig. 1, clade 66; Fig. 3 clade 30). When the single genes are BLASTed against the alignment, CBS 326.52 shares 90 % (700/774) similarity on rpb2 and 99 % (717/726) similarity on LSU with CPC 12548 Rosiphaerella rosicola, and 98 % (470/480) similarity on ITS with CBS 122466 (Passalora sp.1). Based on the phylogenetic results and the morphological differences in comparison to the closest related species Rosisphaerella rosicola, we introduce this new genus to accommodate the present species.
Clade 67: Brunswickiella, Cytostagonospora, Devonomyces and Phaeophleospora
Brunswickiella Videira & Crous gen. nov. MycoBank MB822694.
Etymology: Named after the nature reserve it was collected from.
Description: Phytopathogenic. Conidiomata pycnidial, epiphyllous, immersed, black and with central ostiole, outer layer with irregular, brown, verruculose hyphae; basal stroma brown, verruculose, giving rise to conidiophores; basal cells brown, verruculose, upper cells hyaline, smooth, septate, subcylindrical, branched below. Conidiogenous cells hyaline, smooth, subcylindrical, terminal and lateral, proliferating percurrently at apex, or with periclinal thickening, intermixed among paraphyses that are branched, similar in length and at times become fertile. Conidia solitary, hyaline, smooth, guttulate, subcylindrical to narrowly fusoid-ellipsoidal, straight to slightly curved, widest in the middle, tapering to subobtuse apex and truncate hilum.
Type species: Brunswickiella parsonsiae (Crous & Summerell) Videira & Crous.
Brunswickiella parsonsiae (Crous & Summerell) Videira & Crous comb. nov. MycoBank MB822740.
Basionym: Phaeophleospora parsonsiae Crous & Summerell (as “parsoniae”), Persoonia 32: 217. 2014.
Description and illustration: Crous et al. (2014a).
Material examined: Australia, New South Wales, Brunswick Heads Nature Reserve, S28°31′90.8″ E153°32′57.0″, on Parsonsia straminea leaves, 9 Mar. 2013, B.A. Summerell (holotype CBS H-21691, culture ex-type CBS 137979 = CPC 22537).
Notes: Brunswickiella parsonsiae forms pycnidial conidiomata with hyaline conidiogenous cells that proliferate percurrently and produce hyaline fusoid-ellipsoid aseptate conidia. At the time it was described, Crous et al. (2014a) assumed it represented a microconidial state of Phaeophleospora. The phylogenetic position of the present strain is outside the Phaeopleospora clade, sitting in a single-strain lineage (Fig. 4, clade 6-II) sister to the clade of Lecanosticta. Based on the morphological differences between this strain and the closest genera and its phylogenetic position we place it in a new genus.
Cytostagonospora Bubák, Ann. Mycol. 14: 150. 1916.
Description (from Sutton 1980): Mycelium immersed, dark brown, branched, septate. Conidiomata pycnidial, amphigenous, separate, globose, dark brown to black, immersed, unilocular, thick-walled, clypeate; walls of dark brown, thick-walled textura angularis to textura globulosa, becoming hyaline towards the conidiogenous region, extending in the upper part to become a circular clypeus of similar thickness to the wall. Ostiole central, circular, papillate to short rostrate, depressed, situated immersed within the clypeus. Conidiophores reduced to conidiogenous cells. Conidiogenous cells holoblastic, determinate, discrete, lageniform, hyaline, smooth, formed from the inner cells of the pycnidial wall. Conidia hyaline, 0–2-euseptate, not constricted at septa, base truncate, apex obtuse, thin-walled, eguttulate, smooth, filiform, often curved.
Type species: Cytostagonospora photiniicola Bubák.
Cytostagonospora martiniana (Sacc.) B. Sutton & H.J. Swart, Trans. Br. mycol. Soc. 87: 99. 1986.
Basionym: Septoria martiniana Sacc., Syll. Fung. (Abellini) 10: 351. 1892.
Synonym: Septoria phyllodiorum Cooke & Massee, Grevillea 19: 47. 1890, non S. phyllodiorum Sacc., Hedwigia 29: 156. 1890.
Description and illlustration: Sutton and Swart, 1986, Quaedvlieg et al., 2013.
Materials examined: Australia, Victoria, Warneet close to Melbourne, S38º13′37.8″ E145º18′25.4″, on leaves of Acacia pycnantha, 21 Oct. 2009, P.W. Crous (epitype designated here CBS H-21297, MBT378691, culture CBS 135102 = CPC 17727); Victoria, on phyllodes of Acacia longifolia, Mrs. Martin 432 (holotype K, slide as IMI 299337).
Notes: According to the phylogenetic analyses in the present study, the strain of Cytostagonospora martiniana forms a single strain lineage (Fig. 1, clade 66; Fig. 4, clade 5-III) closely related to Phaeopleospora species. Cytostagonospora martiniana forms pycnidial to acervular conidiomata, hyaline conidiogenous cells that are polyphialidic with periclinal thickening, proliferate percurrently and produce hyaline, 1–3-septate, scolecosporous conidia (Quaedvlieg et al. 2014). These morphological characters are distinct from the typical generic characters of Phaeophleospora.
Cytostagonospora photiniicola Bubák, Ann. Mycol. 14(3–4): 150. 1916.
Synonym: Cytostaganis photiniicola (Bubák) Clem. & Shear, The genera of Fungi: 367. 1931.
Description and illustration: Quaedvlieg et al. (2013).
Notes: The phylogenetic position of Cytostagonospora (Bubák 1916) is still unclear since material representing the type species, Cytostagonospora photiniicola, has not yet been sequenced. The only Cytostagonospora species of which a strain is available is Cytostagonospora martiniana, which forms a single strain lineage in the phylogenetic analysis (Fig. 4, clade 5-III).
Devonomyces Videira & Crous, gen. nov. MycoBank MB822695.
Etymology: Named after Devon Valley, Stellenbosch, where this taxon was first collected.
Description: Phytopathogenic, foliicolous. Ascomata pseudothecial, amphigenous, subepidermal, becoming erumpent, subglobose to globose, with apical, papillate ostiole; walls of 2–3 layers of medium brown textura angularis, subhymenium of 1–2 layers of hyaline cells. Asci fasciculate, bitunicate, cylindrical to narrowly obovoid, straight or slightly incurved, 8-spored. Ascospores bi- to triseriate, overlapping, hyaline, guttulate, thin-walled, straight, fusoid-ellipsoidal with obtuse ends, medianly 1-septate. Mycelium internal, consisting of septate, branched, hyaline to brown, smooth to verruculose hyphae. Caespituli sporodochial, situated on a brown stroma consisting of verruculose, brown, globose cells and hyphal elements. Conidiophores rarely pigmented and verruculose in lower part, mostly hyaline and smooth throughout, thick-walled, cylindrical, straight to irregularly curved, septate. Conidiogenous cells terminal, hyaline, smooth, unbranched, straight or slightly curved, proliferating sympodially. Conidia solitary, hyaline, smooth, narrowly obclavate, septate, irregularly curved, rarely straight, apex obtuse, base long obconic-truncate, lateral branches common, secondary conidia forming on most mature primary conidia; conidia aggregated in slimy masses.
Type species: Devonomyces endophyticus (Crous & H. Sm. ter) Videira & Crous
Devonomyces endophyticus (Crous & H. Sm. ter) Videira & Crous, comb. nov. MycoBank MB822754.
Basionym: Mycosphaerella endophytica Crous & H. Sm. ter, Mycol. Mem. 21: 54. 1998.
Synonym: Pseudocercosporella endophytica Crous & H. Sm. ter, Mycol. Mem. 21: 55. 1998.
Descriptions and illustrations: Crous (1998).
Materials examined: Australia, Western Australia, Esperance, Chips Plantation (ITC), on Eucalyptus globulus, 15 Dec. 2000, A. Maxwell, MURU0011, culture CBS 110501 = CMW 14462; Pemberton, Steward Road, Banksia woodland, on Hakea undulata, 2 Aug. 2008, A.R. Wood, culture CPC 15580. South Africa, Western Cape Province, De Hoop Nature Reserve, Eucalyptus cladocalyx, 22 Sep. 1995, A.R. Wood, culture CBS 111167 = CPC 1225; Stellenbosch, Devon Valley, on leaves of Eucalyptus sp., Jun. 1995, P.W. Crous (holotype of Mycosphaerella endophytica PREM 54398, culture ex-type CBS 114662 = CPC 1193); Kwazulu-Natal, on Eucalyptus nitens, unknown collector and date, isol. G.C. Hunter, Jun. 2000, culture CBS 114709 = CMW 9099.
Notes: Based on the phylogenetic analyses Devonomyces endophyticus (Fig. 4, clade 5-I) is closely related to Phaeophleospora eugeniae, as observed in a previous study (Quaedvlieg et al. 2014). The pseudocercosporella-like morph of Devonomyces endophyticus is however morphologically distinct from Phaeophleospora (Crous 1998), and thus has to be accommodated in a different genus. The strain CBS 114709 was originally named as Mycosphaerella pseudoellipsoidea but no details of the species description could be found. The strain is currently sterile and is included in Devonomyces endophyticus based on molecular data. The strain CPC 15580 was isolated from the same herbarium material as Periconiella hakeae (CPC 15577), which indicates they may be co-existing in the same lesions.
Phaeophleospora Rangel, Arq. Mus. Nac., Rio de Janeiro 18: 162. 1916.
Description: Foliicolous, plant pathogenic. Conidiomata pycnidial, aggregated or separate, becoming erumpent, lifting the epidermis, pycnidia black, subglobose, unilocular, wall of brown textura epidermoidea in surface view, and of textura angularis to textura intricata in vertical section, base of 2–3 layers, ostiole irregular, central. Conidiophores mostly reduced to conidiogenous cells. Conidiogenous cells terminal, discrete, brown, verruculose, subcylindrical or doliiform, proliferating percurrently, with inconspicuous percurrent proliferations, or at times proliferating sympodially. Conidia solitary, exuded in cirrhus, hyaline to medium brown, smooth to verruculose, granular, thick-walled, subcylindrical to obclavate, straight to irregularly curved, base obconically truncate, apex obtuse, euseptate, hila with a minute marginal frill. Spermatogenous cells developing in conidiomata before the development of conidia, hyaline, ampulliform. Spermatia hyaline, smooth, rod-shaped.
Type species: Phaeophleospora eugeniae Rangel.
Phaeophleospora eugeniae Rangel, Decheniana 18: 162. 1916.
Description and illustration: Crous et al. (1997).
Description in vitro (on V8; CPC 15143): Mycelium hyaline, smooth, uniform in width, 2.5 μm diam. Conidiomata pycnidial, aggregated on mycelial colonies, pale brown, forming 1-layered conidiomatal wall composed of large brown cells (textura intricata), 100–300 μm diam. Conidiogenous cells lining the inner cavity, hyaline to pale brown, ampulliform, monoblastic, determinate or proliferating percurrently, 5–20 × 2.5–3.8 μm, without distinguished loci. Conidia solitary, pale brown to pale olivaceous brown, darker in the center and paler towards both ends, scolecosporous, obclavate, straight or sinuous, base long-obconical, apex pointed, 30–150 × 5–8 μm, 6–25-euseptate, with frill-like hila.
Materials examined: Brazil, Minas Gerais, Viçosa University campus, living leaves of Eugenia uniflora, 8 Jul. 1996, F.A. Ferreira (neotype IMI 372655, designated in Crous et al. 1997; isoneotype PREM 55275, cultures ex-type CPC 1453, CPC 1454); same location and host, 15 Jun. 1990, F.A. Ferreira, PREM 55276; same location and host, 20 Jun. 1989, F.A. Ferreira, PREM 55277; Viçosa, Paraiso, on Eugenia uniflora, 1 Mar. 2008, A.C. Alfenas, CBS H-22957, culture CBS 142184 = CPC 15143; Guaiba, on Eugenia uniflora, 1 Apr. 2008, A.C. Alfenas, culture CPC 15159.
Notes: The genus Phaeophleospora, based on Phaeophleospora eugeniae (on Eugenia uniflora, Brazil), includes species that form pycnidia lined with percurrently proliferating brown conidiogenous cells that give rise to brown, multiseptate, scolecosporous conidia (Crous et al., 1997, Crous et al., 2007a). Based on phylogenetic analyses, Phaeophleospora belongs to the Mycosphaerellaceae (Crous et al., 1997, Crous et al., 2009b; present study Fig. 1, clade 67), and clusters in a well supported clade by the Bayesian analyses (Fig. 4, clade 5-II), being closely related to Lecanosticta. The genus Kirramyces, initially considered a synonym of Phaeophleospora (Crous et al. 1997), is currently considered the asexual morph of Teratosphaeria. The taxa Phaeophleospora scytalidii and Phaeophleospora stramenti were allocated to Phaeophleospora based on phylogenetic inference since only the sexual morph is known (Quaedvlieg et al. 2014). Recently described species in the genus are morphologically variable [e.g. Phaeophleospora pteridivora has a sporodochial hyphomycete asexual morph (Guatimosim et al. 2016); Phaeophleospora hymenocallidis and Phaeophleospora hymenocallidicola produce hyaline conidia (Crous et al. 2015d)], suggesting that this genus needs to be revised.
Clade 68: Lecanosticta
Lecanosticta Syd., Ann. Mycol. 20: 211. 1922.
Description (from Sutton 1980): Mycelium immersed, branched, septate, pale brown. Conidiomata acervular, subepidermal, separate, formed of brown, thin- or thick-walled textura angularis. Dehiscence by pushing back a flap of epidermis that remains attached. Conidiophores hyaline to pale brown, branched, septate, smooth, formed from the upper cells of the pseudoparenchyma. Conidiogenous cells holoblastic, integrated or discrete, indeterminate, cylindrical, hyaline, with 1–2 often widely spaced percurrent proliferations. Conidia acrogenous, straight or curved, fusiform, tapered to the rounded apex and truncate base, l–3-euseptate, continuous, pale brown, verrucose.
Type species: Lecanosticta acicola (Thüm.) Syd. (≡ Cryptosporium acicola Thüm.).
Lecanosticta acicola (Thüm.) Syd., Ann. Mycol. 22: 400. 1924.
Basionym: Cryptosporium acicola Thüm., Flora (Regensburg) 61: 178. 1878.
Synonyms: Septoria acicola (Thüm.) Sacc., Syll. Fung. 3: 507. 1884.
Dothistroma acicola (Thüm.) Schischkina & Tzanava, Novosti Sist. Nizsh. Rast. 1967: 277. 1967.
Lecanosticta pini Syd., Ann. Mycol. 20: 211. 1922.
Oligostroma acicola Dearn., Mycologia 18: 251. 1926.
Scirrhia acicola (Dearn.) Sigg., Phytopathology 29: 1076. 1939.
Systremma acicola (Dearn.) F.A. Wolf & Barbour, Phytopathology 31: 70. 1941.
Mycosphaerella dearnessii M.E. Barr, Contr. Univ. Michigan Herb. 9: 587. 1972.
Description and illustration: Quaedvlieg et al. (2012).
Materials examined: France, Gironde, Le Teich, on needles of Pinus radiata, Apr. 1995, M. Morelet, CBS H-21114, culture CBS 871.95. Lithuania, on needles of Pinus mugo, 2009, S. Markovskaja, A. Kačergius & A. Treigienė, CBS H-21109, cultures LA773A & LA773B = CBS 133790. Mexico, on needles of a Pinus sp., 30 Nov. 2009, M. de Jesús Yáñez-Morales, CBS H-21112, culture CPC 17822 = CBS 133789. USA, South Carolina, Aiken, needles of Pinus caribaea, 1876, H.W. Ravenel (lectotype designated here IMI 91340, MBT378589, isotype of Cryptosporium acicula ex Padova No. 1484); Arkansas, Pike City, alt. 700 ft, needles of Pinus (palustris or taeda), 24 Apr. 1918, coll. J.A. Hughes, det. Sydow (syntypes of Lecanostricta pini, BPI 393329, BPI 393331); Florida, Silver Spring, needles of P. palustris, 27 Feb. 1919, coll. Geo G. Hedgcock, det. J. Dearness (syntype of Oligostroma acicola, BPI 643015); Maine, Bethel, on needles of Pinus strobus, 14 Jun. 2011, coll. B. Ostrofsky, det. K. Broders, WPF4.12; idem., on needles of P. strobus, 15 Jun. 2011, coll. B. Ostrofsky, det. K. Broders, WPF13.12; New Hampshire, Blackwater, on needles of P. strobus, 15 Jun. 2011, coll. B. Ostrofsky, det. K. Broders (epitype of Cryptosporium acicola designated here: CBS H-21113, MBT378591, culture ex-epitype CBS 133791).
Notes: The genus Lecanosticta is closely related to Phaeophleospora based on phylogenetic analyses (Crous et al. 2009c). The phylogenetic analyses in the present study corroborated the previous findings, placing Lecanosticta species in a well-supported clade (Fig. 1, clade 68; Fig. 4, clade 6-I) sister to Phaeophleospora. Species of Lecanosticta have typical phaeophleospora-like conidia, but form acervular conidiomata instead of pycnidial conidiomata. Lecanosticta acicola is the causal agent of brown spot needle blight on Pinus spp. worldwide, a serious disease that leads to defoliation, dieback and finally tree death. For this reason, it is included on the European quarantine list. Lecanosticta acicola was shown to represent a species complex, including Lecanosticta brevispora and Lecanosticta guatemalensis (Quaedvlieg et al. 2012). The epitypification presented by Quaedvlieg et al. was not compliant with the code (Art. 9.8) and a new epitypification is therefore proposed.
Lecanosticta brevispora Quaedvlieg & Crous, Persoonia 29: 109. 2012.
Descriptions and illustrations: Quaedvlieg et al. (2012).
Materials examined: Mexico, on needles of a Pinus sp., 24 Oct. 2009, M. de Jesús Yáñez-Morales (holotype CBS H-21110, cultures ex-type CBS 133601 = CPC 18092).
Notes: Lecanosticta brevispora produces smaller conidia than Lecanosticta acicula (Quaedvlieg et al. 2012). Based on the phylogenetic analyses, Lecanosticta brevispora clusters in the Lecanosticta clade (Fig. 1, clade 68; Fig. 4, clade 6-I) as observed in a previous phylogenetic study (Quaedvlieg et al. 2012).
Lecanosticta longispora Marm., Mycotaxon 76: 395. 2000.
Description and illustration: Quaedvlieg et al. (2012).
Materials examined: Mexico, Nuevo León, Galeana, Cerro del Potosí, on Pinus culminicola, 6 Jun. 1993, J.G. Marmolejo (holotype CFNL); Michoacán State, Zinapecuaro area, on needles of a Pinus sp., 24 Oct. 2009, M. de Jesús Yáñez-Morales & C. Méndez-Inocencio (epitype designated by Quaedvlieg et al. 2012 : CBS H-21111, cultures ex-epitype CBS 133602 = CPC 17940); idem., culture CPC 17941.
Notes: Lecanosticta longispora produces conidia of the same size as Lecanosticta acicola but conidia have only 1–3 septa (Marmolejo 2000). Phylogenetically, Lecanosticta longispora clusters in the Lecanosticta clade (Fig. 1, clade 68; Fig. 4, clade 6-I) as observed in a previous phylogenetic study (Quaedvlieg et al. 2012).
Clade 69: Zasmidium complex (Periconiella, ramichloridium-like, rasutoria-like, stenella-like, Verrucisporota, Zasmidium)
Zasmidium Fr., Summa Veg. Scand. 2: 407. 1849.
Synonyms: Periconiella Sacc., Atti Ist. Veneto Sci. Lett. Arti 3: 727. 1885 (type species: Periconiella velutina (G. Winter) Sacc. 1885).
Biharia Thirum. & Mishra, Sydowia 7: 79. 1953 (type species: Biharia vangueriae Thirum. & Mishra 1953).
Stenellopsis B. Huguenin, Bull. Trimestriel Soc. Mycol. France 81: 695. 1966 (type species: Stenellopsis fagraeae B. Huguenin 1966).
Verrucisporota D.E. Shaw & Alcorn, Austral. Syst. Bot. 6: 273. 1993 (type species: Verrucisporota proteacearum (D.E. Shaw & Alcorn) D.E. Shaw & Alcorn 1993).
Verrucispora D.E. Shaw & Alcorn, Proc. Linn. Soc. New South Wales 92: 171. 1967, nom. illeg. (Art. 53.1).
Description (from Braun et al. 2013): Hyphomycetous (asexual morphs or asexual holomorphs) or Zasmidium with mycosphaerella-like sexual morphs; saprobic or mostly biotrophic, usually foliicolous, symptomless or causing various lesions, ranging from yellowish discolorations to distinct leaf spots. In plant pathogenic species, mycelium mostly immersed as well as superficial, rarely only immersed; hyphae branched, septate, hyaline or almost so to pigmented, pale olivaceous to brown, wall thin to somewhat thickened, immersed hyphae smooth or almost so to faintly rough, external hyphae distinctly verruculose to verrucose (in culture immersed hyphae usually smooth or almost so, aerial hyphae verruculose). Stromata lacking to well-developed, pigmented. Conidiophores solitary, arising from superficial hyphae, lateral, occasionally terminal, in vivo (in plant pathogenic taxa) sometimes also fasciculate, arising from internal hyphae or stromata, semimacronematous to macronematous, in culture occasionally micronematous, cylindrical, filiform, subuliform, straight to strongly geniculate-sinuous, mostly unbranched, aseptate, i.e. reduced to conidiogenous cells, to pluriseptate, subhyaline to pigmented, pale olivaceous to medium dark brown, wall thin to somewhat thickened, smooth to verruculose; conidiogenous cells integrated, terminal, occasionally intercalary, rarely pleurogenous, or conidiophores reduced to conidiogenous cells, mostly polyblastic, sympodial, with conspicuous, somewhat thickened and darkened-refractive, planate loci. Conidia solitary or catenate, in simple or branched acropetal chains, shape and size variable, ranging from amero- to scolecosporous, aseptate to transversely plurieuseptate, subhyaline to pigmented, pale olivaceous to brown, wall thin to somewhat thickened, smooth or almost so to usually distinctly verruculose (in plant pathogenic species without superficial mycelium always verruculose), hila somewhat thickened and darkened-refractive, planate, conidial secession schizolytic.
Type species: Zasmidium cellare (Pers.) Fr. (≡ Racodium cellare Pers.).
Zasmidium angulare Batzer & Crous, Persoonia 28: 123. 2012.
Description and illustration: Li et al. (2012).
Materials examined: USA, Georgia, on fruit surface of Malus domestica, Aug. 2005, M. Wheeler (holotype CBS H-20931, ex-type culture CBS 132094 = CPC 19042 = GA227B1a).
Notes: Zasmidium angulare was the first Zasmidium species described in association with sooty blotch and flyspeck symptoms on apple. Phylogenetically, it is closely related to Zasmidium nocoxi (Fig. 4, clade 1, Fig. 5, clade I) but can morphologically be distinguished in having shorter conidiophores (Li et al. 2012).
Fig. 5.
Phylogenetic tree (50 % majority rule consensus) resulting from a Bayesian analysis of the combined LSU, rpb2 and ITS sequence alignment of the strains in the clades 1–6 from Fig. 4 (clades 67–72 of Fig. 1). Bayesian posterior probabilities (PP) and maximum parsimony bootstrap support values (≥ 90 %; MP-BS) are indicated at the nodes (PP/MP-BS) and the scale bar represents the expected number of changes per site. Branches in a thicker stroke represent the branches present in the strict consensus parsimony tree. Genera clades are delimited in dark and light blue boxes, with the genus name indicated to the right. The genus name Zasmidium is unnabreviated while the rest are abbreviated as follows: NT = Nothopericoniella, AN = Annellosimpodiella, NP = Neopenidiella, DV = Devonomyces, PH = Phaeophleospora, CY = Cytostagonospora, LE = Lecanosticta, BR = Brunswickiella. All taxa names are written in black, ex-type strains are represented in bold and novel genera with an asterisk (*). The dark and light pink coloured boxes, numbered with roman numerals to the right, represent a possible phylogenetic division of the genus Zasmidium based on branch support and/or taxonomic history. Within the pink boxes, the generic name of Zasmidium was abbreviated (Z. = Zasmidium) and a grid representing morphological charaters respective of each taxon is displayed to the right of the taxa and should be interpreted as: S – only sexual morph described (filled hexagon); CP – conidiophores unbranched (filled star), conidiophores branched (empty star); CC – conidiogenous cell terminal (filled square), conidiogenous cell terminal forming rachis (filled square with letter R in white), conidiogenous cell terminal and intercalary (empty square), conidiogenous cell terminal and intercalary forming rachis (empty square with letter R in black); CA – conidia long (>30 μm average; full circle), conidia short (<30 μm average; empty circle), single (full triangle), catenate (empty triangle). The tree was rooted to Teratosphaeria stellenboschiana (CPC 13764).
Zasmidium anthuriicola (U. Braun & C.F. Hill) Crous & U. Braun, Persoonia 23: 104. 2009.
Basionym: Stenella anthuriicola U. Braun & C.F. Hill, Fungal Diversity 22: 33. 2006.
Description and illustration: Braun et al. (2006).
Materials examined: Thailand, (intercepted at Auckland International Airport, New Zealand), on Anthurium sp., 3 Aug. 2005, C.F. Hill 1235 (holotype HAL 1870 F, ex-type culture CBS 118742).
Note: In the present study Zasmidium anthuriicola is phylogenetically close to Zasmidium citri-griseum (Fig. 4, clade 1; Fig. 5, clade III).
Zasmidium arcuatum (Arzanlou et al.) Videira & Crous, comb. nov. MycoBank MB822807.
Basionym: Periconiella arcuata Arzanlou et al., Stud. Mycol. 58: 65. 2007.
Description and illustration: Arzanlou et al. (2007).
Materials examined: South Africa, Western Cape Province, Kogelberg, on dead culms of Ischyrolepis subverticillata, May 2001, S. Lee (holotype CBS H-19927, culture ex-type CBS 113477).
Notes: The present species was previously known as Periconiella arcuata, but the type of Periconiella, Periconiella velutina, is combined into Zasmidium in the present study based on morphology and phylogenetic data (see notes under Zasmidium cellare). Based on the phylogenetic analysis, the present species is represented by a single-strain lineage (Fig. 4, clade 1, Fig. 5, clade VIII). It is unique in producing large obclavate conidia that are pale olive, coarsely verrucose and straight to curved (Arzanlou et al. 2007).
Zasmidium aucklandicum (U. Braun & C.F. Hill) U. Braun, Polish Bot. J. 55: 289. 2010.
Basionym: Stenella aucklandica U. Braun & C.F. Hill, Australas. Pl. Pathol. 32: 96. 2003.
Description and illustration: Braun et al. (2003b).
Materials examined: New Zealand, on Geniostoma rupestre, 15 Oct. 2005, C.F. Hill 6000, culture CPC 13569; Auckland, Grey Lynn, Western Springs Park, on Geniostoma rupestre, 14 Apr. 2001, C.F. Hill 402-A (holotype HAL 1726 F).
Note: Based on the phylogenetic analyses, Zasmidium aucklandicum is closely related to Zasmidium pittospori (Fig. 4, clade 1; Fig. 5, clade VII), which is also found in New Zealand but on a different host (Pittosporum tenuifolium; Pittosporaceae).
Zasmidium biverticillatum (Arzanlou & Crous) Videira & Crous, comb. nov. MycoBank MB822827.
Basionym: Ramichloridium biverticillatum Arzanlou & Crous, Stud. Mycol. 58: 72. 2007.
Synonyms: Ramichloridium musae Stahel, Trop. Agric., Trinidad 14: 43. 1937, nom. inval., Art. 36.
Periconiella musae Stahel ex M.B. Ellis, Mycol. Pap. 111: 5. 1967, non Zasmidium musae (Arzanlou & Crous) Crous & U. Braun, 2010.
Ramichloridium musae (Stahel ex M.B. Ellis) de Hoog, Stud. Mycol. 15: 62. 1977.
Description and illustration: Arzanlou et al. (2007).
Materials examined: Surinam, on Musa sapientum, isol. and dep. G. Stahel, Aug. 1936, culture CBS 335.36.
Notes: The genus Ramichloridium, based on the type Ramichloridium apiculatum, belongs to the Dissoconiaceae (Fig. 1, clade 95; Fig. 4, clade 31). Based on the phylogenetic analyses, the current species belongs to the genus Zasmidium (Fig. 4, clade 1; Fig. 5, clade V). Zasmidium biverticillatum is closely related to Zasmidium musigenum (= Ramichloridium musae), but produces profusely branched conidiophores and smaller conidia (Arzanlou et al. 2007).
Zasmidium cellare (Pers.) Fr., Summa Veg. Scand. 2: 407. 1849.
Basionym: Racodium cellare Pers., Neues Mag. Bot. 1: 123. 1794.
Synonyms: Antennaria cellaris (Pers.) Fr., Syst. Mycol. 3: 229. 1829.
Cladosporium cellare (Pers.) Schanderl, Arch. Hyg. Bakteriol.: 117. 1936.
Rhinocladiella cellaris (Pers.) M.B. Ellis, Dematiaceous Hyphomycetes: 248. 1971.
Rhinocladiella ellisii D. Hawksw., Taxon 26: 208. 1977.
Description and illustration: Arzanlou et al. (2007).
Materials examined: Europe, on wall in wine cellar, unknown collector and date, isol. and dep. H. Schanderl, Jun. 1936 (neotype designated here, preserved as metabolically inactive, CBS 146.36, MBT378698) duplicate cultures are ATCC 36951 = IFO 4862 = IMI 44943 = LCP 52.402 = LSHB BB274 = MUCL 10089, MBT378698; Germany, Lorch am Rhein, on wall in wine cellar, Aug. 1985, M. Schlag, CBS H-3980, culture CBS 892.85.
Notes: Zasmidium was introduced for the stenella-like fungi belonging to the Mycosphaerellaceae, since the type species of Stenella (Stenella araguata) clustered in the Teratosphaeriaceae (Arzanlou et al., 2007, Braun et al., 2010a, Braun et al., 2010b, Kamal, 2010, present study Fig. 1, clade 98; Fig. 4, clade 33). The type specimen of Zasmidium cellare (based on Racodium cellare, from wine cellars in Europe and America) could not be located and the species needed to be neotypified. Morphologically, Stenella and Zasmidium species are very similar and are usually distinguished by the shape of the conidiogenous loci, which is planate in Zasmidium and more pileate in Stenella (Braun et al. 2013).
Based on the phylogenetic analyses of dataset 4, several terminal branches are highly supported but the backbone is usually poorly supported except for a very basal branch that includes various other genera like Verrucisporota, Ramichloridium, Rasutoria, Stenella and Periconiella (Fig. 1, clade 69; Fig. 4, clade 1; Fig. 5, clades I–IX). In order to improve the tree resolution, supplementary phylogenetic analyses were performed including these zasmidium-like and closest related species in dataset 4 (Fig. 4, clades 1–7), using both Bayesian and parsimony methods (PBS), and including three genes (LSU, ITS and rpb2). Based on these analysis, there is strong support from both Bayesian and parsimony methods for keeping these species together (Fig. 5, clades I-IX). Based on the parsimony analysis these clades (Fig. 5, clades I–VIII) cluster in a basal polytomy, with one clade being excluded but closely related (Fig. 5, clade IX). Morphologically, there was also not a clear pattern that could be observed based on the most strongly supported terminal branches that justified the division of this generic complex in multiple genera. Species with a simple conidiophore are more common than with branched conidiophores, a characteristic only found in Fig. 5, clades V (e.g. Zasmidium biverticillatum) and VIII (e.g. Zasmidium velutinum). Conidiogenous cells terminal and forming rachis can be found in Fig. 5, clades I (e.g. Zasmidium cerophilum) and V (e.g. Zasmidium musae-banksii) while conidiogenous cells both terminal and intercalary forming rachis can be found in Fig. 5, clades IV (e.g. Z. strelitziae), V (e.g. Z. musigenum) and VIII (e.g. Zasmidium arcuata). Species with short and catenate conidia are only found on Fig. 5, clades I (e.g. Zasmidium fructicola) and II (e.g. Zasmidium pseudoparkii) while species with shorter but single conidia can be found in Fig. 5, clades I (e.g. Zasmidium syzygii), IV (e.g. Zasmidium strelitziae), V (e.g. Zasmidium musigenum), VIII (e.g. Zasmidium hakeae) and IX (e.g. Zasmidium iteae). Species with single and long-obclavate conidia are less common in Fig. 5, clade I (e.g. Zasmidium angulare) but appear often in Fig. 5, clades III (e.g. Zasmidium citri-griseum), VI (Zasmidium grevilleae), VII (e.g. Zasmidium pittospori), VIII (e.g. Zasmidium daviesiae) and IX (e.g. Zasmidium queenslandicum).
Species of the genus Verrucisporota (Shaw and Alcorn, 1993, Beilharz and Pascoe, 2002) are barely distinguishable from Zasmidium based on morphological traits and phylogenetically cluster with Zasmidium strains (Crous et al. 2009a; present study, Fig. 1, clade 69; Fig. 4, clade 1; Fig. 5, clade VI, clade VIII). Although the exact phylogenetic position of the type species, Verrucisporota proteacearum, is unknown, the fact that a representative strain clustered among Zasmidium species led previous authors to consider the genus Verrucisporota as a synonym of Zasmidium (Braun et al. 2013). Therefore, we propose the combination of these names into Zasmidium.
The genus Ramichloridium was phylogenetically delimited with the sequencing of the type species (Ramichloridium apiculatum) that clustered in Dissoconiaceae (Arzanlou et al. 2007; this study Fig. 1, clade 95; Fig. 4, clade 31). New combinations are proposed for the Ramichloridium species that cluster within the Zasmidium clade, among which are included two species involved in the banana speckle disease, namely Ramichloridium musae and Ramichloridium biverticillatum (Fig. 1, clade 69; Fig. 4, clade 1; Fig. 5, clade V).
The genus Periconiella is based on Periconiella velutina, isolated from Brabejum stellatifolium (South Africa), and is known to be a polyphyletic genus (Arzanlou et al. 2007; present study Fig. 1, clade 69, 70, 85; Fig. 4, clade 1, 2, 21; Fig. 5, clades VIII, X). Morphologically, Periconiella species are zasmidium-like with pigmented conidiophores and conidia, smooth to verruculose, with conidiogenous cells polyblastic and with planate scars and were usually distinguished by producing conidiophores that are prominently branched in the upper part (Arzanlou et al. 2007). Based on the phylogenetic position of the type species Periconiella velutina and the morphological characters of the genus, we propose to reduce Periconiella to synonymy under Zasmidium, which is the older name.
Rasutoria was established by Barr (1987), based on Rasutoria abietis (on Abies amabilis, USA), to accommodate species with hyaline to brown ascospores occurring on Gymnospermae. The genus currently accommodates four species that are only known from their sexual morph. Rasutoria tsugae and Rasutoria pseudotsugae, which are important pathogens of Douglas-fir (Winton et al. 2007), have hyaline ascospores, while Rasutoria abietis and Rasutoria terrieri have pale brown to brown ascospores. Hyaline ascospores is a typical morphological characteristic in the Mycosphaerellaceae (Aptroot 2006). Only Rasutoria pseudotsugae and Rasutoria tsugae have cultures and DNA sequences available that place them among Zasmidium species (Fig. 1, clade 69; Fig. 4, clade 1, Fig. 5, clade VIII), and closely related to Zasmidium pseudovespa (= Mycosphaerella pseudovespa), which also produces hyaline ascospores (Carnegie et al. 2007). Therefore, these two species are placed in the genus Zasmidium, while Rasutoria abietis and Rasutoria terrieri need to be recollected in order to determine their correct phylogenetic position, as well as the position of the genus Rasutoria.
The clade at the bottom of the Zasmidium complex (Fig. 5, clade IX) includes species that are mostly ramichloridium-like, with a straight conidiophore and polyblastic intercalary and terminal conidiogenous cells producing single or short catenate obovoid conidia. However, the species Zasmidium queenslandicum in this clade has a typical Zasmidium morphology, similar to Zasmidium musicola (Fig. 5, clade III) and Zasmidium musae (Fig. 5, clade VIII). Therefore, based on phylogenetic support and morphological similarities these species are considered part of the genus Zasmidium.
Zasmidium cerophilum (Tubaki) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822808.
Basionym: Acrotheca cerophila Tubaki, J. Hattori Bot. Lab. 20: 143. 1958.
Synonyms: Cladosporium cerophilum (Tubaki) Matsush., Icones Microfungorum a Matsushima lectorum: 34. 1975.
Ramichloridium cerophilum (Tubaki) de Hoog, Stud. Mycol. 15: 74. 1977.
Description and illustration: Arzanlou et al. (2007).
Material examined: Japan, on Sasa sp., May 1955, K. Tubaki (holotype preserved in Nagao Institute, culture ex-type of Acrotheca cerophila CBS 103.59 = MUCL 10034).
Notes: The present species is phylogenetically placed within the Zasmidium clade (Fig. 4, clade 1; Fig. 5, clade I) among typical Zasmidium species. Zasmidium cerophilum is closely related to Zasmidium fructigenum, but is more similar morphologically to Zasmidium eucalypticola by producing terminal and short rachis-like conidiogenous cells and secondary conidia (Arzanlou et al. 2007). Zasmidium cerophilum can be morphologically distinguished from Z. musigenum, Zasmidium musae-banksii and Zasmidium biverticillatum by the production of secondary conidia and its distinct conidial hila.
Zasmidium citri-griseum (F.E. Fisher) U. Braun & Crous, IMA Fungus 5: 337. 2014.
Basionym: Cercospora citri-grisea F.E. Fisher, Phytopathology 51: 300. 1961.
Synonyms: Stenella citri-grisea (F.E. Fisher) Sivan., Bitunicate Ascomycetes and their Anamorphs: 226. 1984.
?Mycosphaerella citri Whiteside, Phytopathology 62: 263. 1972.
?Zasmidium citri (Whiteside) Crous, Persoonia 23: 105. 2009.
Descriptions and illustrations: Braun et al., 2014, Huang et al., 2015.
Materials examined: China, Yunnan prov., Mengdian, on leaves with yellow spot of Citrus limon, Jul. 2011, L. Zhu, cultures ZJUM 103 = CPC 24500, ZJUM 104 = CPC 24501; on leaf with yellow spot of Citrus aurantifolia, Jul. 2011, L. Zhu, culture ZJUM 105 = CPC 24502; Zhejiang prov., Cangnan, on leaf with yellow spot of Citrus grandis, Dec. 2009, L. Zhu, culture ZJUM 5 = CPC 24464; Changshan, on leaves of Citrus paradisi × Citrus sp., May 2009, L. Zhu, culture ZJUM 25 = CPC 24468, ZJUM 27 = CPC 24469; Nov. 2011, L. Zhu, culture ZJUM 54 = CPC 24474; Huangyan, on leaf with big round spot of Citrus reticulata, Apr. 2010, L. Zhu, culture ZJUM 81 = CPC 24488; Yuhuan, on leaf with greasy spot of C. grandis, Nov. 2011, L. Zhu, culture ZJUM 97 = CPC 24497; Jiangshan, on leaf with brown small round spot of C. paradisi × Citrus sp., Apr. 2013, F. Huang, culture ZJUM 127 = CPC 24504. Thailand, on living leaves of Eucalyptus sp., 2006, W. Himaman, culture CPC 13467; Chonburi, on living leaves of seedlings of Acacia mangium, 19 Nov. 2002, M.J. Wingfield, culture CPC 10522 = CBS 116366. USA, Florida, Polk County, Babson Park, on C. limon, 15 Jan. 1958, F.E. Fisher (presumably lost); single ascospore isolates, associated with citrus greasy leaf spot disease symptoms, Citrus sp. 2003, R.C. Ploetz, cultures CPC 15289, CPC 15290 = CBS 122455, CPC 15294, CPC 15285, CPC 15291, CPC 15293; on leaves of Musa sp., 2003, J. Cavaletto, culture CBS 116426; Florida, Lake Alfred & Haines City, on Citrus sp., May 1970, F.E. Fisher (neotype designated by Braun et al. 2014: IMI 148810); single ascospore isolates, associated with citrus greasy leaf spot disease symptoms, Citrus sp., 2003, S.N. Mondal (epitype designated by Huang et al. 2015: CBS H-22176, culture ex-epitype CBS 139467 = CPC 15296).
Notes: See Braun et al. (2014) for the detailed description of the neotype and Huang et al. (2015) for the epitype details. Based on the phylogenetic analyses, Zasmidium citri-griseum clusters within the Zasmidium clade (Fig. 1, clade 69; Fig. 4, clade 1; Fig. 5, clade III) and is closely related to Zasmidium anthuricola.
Zasmidium daviesiae (Cooke & Massee) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822828.
Basionym: Cercospora daviesiae Cooke & Massee, Grevillea 18: 7. 1889.
Synonyms: Verrucisporota daviesiae (Cooke & Massee) Beilharz & Pascoe, Mycotaxon 82: 360. 2002.
Mycosphaerella daviesiicola Beilharz & Pascoe, Mycotaxon 82: 364. 2002.
Description and illustration: Chupp, 1954, Beilharz and Pascoe, 2002.
Materials examined: Australia, Victoria, on road from Merimbah to Circuit road, 3.4 km short of Mt. Stirling, on Daviesia mimosoides (= D. cormybosa var. mimosoides), 30 Dec. 2003, V. & R. Beilharz, culture VPRI 31767 = CBS 116002.
Notes: The type of Zasmidium daviesiae, based on Cercospora daviesiae, was isolated from leaves of Daviesia latifolia (Victoria, Australia, K) which is a different host from the examined strain. Phylogenetically, the present specimen clusters among Zasmidium species (Fig. 1, clade 69; Fig. 4, clade 1; Fig. 5, clade VIII), as defined in the present study, and is closely related to Zasmidium velutinum (= Periconiella velutinae), but the latter species produces branched conidiophores with terminal polyblastic conidiogenous cells and shorter conidia (Arzanlou et al. 2007). Morphologically, the examined material is similar to Zasmidium spp. by producing polyblastic, intercalary and terminal conidiogenous cells with conidiogenous loci darkened and planate, which give rise to long-obclavate, multiseptate, verruculose conidia (Beilharz & Pascoe 2002).
Zasmidium elaeocarpi U. Braun, C. Nakash., Videira & Crous, sp. nov. MycoBank MB822718. Fig. 39.
Fig. 39.
Zasmidium elaeocarpi (CPC 16640). A–F. Observations in vitro. A. Culture on OA. B, C. Conidiophore, conidiogenous cells and conidia. D, E. Partial conidiophore, conidiogenous cells and conidia. F. Conidia. Scale bars = 10 μm.
Etymology: Derived from the host genus on which it occurs, Elaeocarpus.
Description in vitro (on SNA): Mycelium composed of hyaline and pale brown to dark blackish brown hyphae, verruculose, septate, branching, uniform in width, 2.5 μm. Conidiophores arising from hyphae, micro- to macronematous, pale olivaceous brown to pale blackish brown, finely verruculose, straight or slightly curved, frequently geniculate, rugged or rugose at the upper part, 25–450 × 3.5–5 μm. Conidiogenous cells integrated, apical or intercalary, polyblastic, proliferating sympodially, with numerous rim-like conidiogenous loci, thickened and darkened, dispersed through the entire cells, forming a single or multicelled rachis (ramichloridium-like), 1–1.5 μm diam. Conidia solitary, occasionally catenate, pale blackish brown to pale olivaceous brown, verruculose, ellipsoidal, cylindrical to obclavate, base obconically truncate and apex rounded, straight or curved, 10–75 × 2.5–4 μm, 0-7-euseptate, sometimes constricted at septa, with hila thickened and darkened, 1–1.5 μm diam.
Materials examined: Australia, New South Wales, north-west of Grafton, North Washpool State Forest, on Elaeocarpus kirtonii, 1 Mar. 2009, B. Summerell (holotype CBS H-22960, ex-type culture CBS 142187 = CPC 16642); idem. culture CPC 16640.
Notes: Zasmidium elaeocarpi is morphologically similar to Zasmidium iteae by producing ramichloridium-like polyblastic conidiogenous cells on a short rachis with thickened and darkened scars, and verruculose conidia that are solitary or catenate. Zasmidium elaeocarpi can be distinguished by producing longer conidiophores and longer and wider conidia than Zasmidium iteae (Kirschner et al. 2004). Based on the phylogenetic analysis, these two species are closely related and cluster in the same clade within the Zasmidium complex (Fig. 4, clade 1; Fig. 5, clade IX).
Zasmidium eucalypticola U. Braun, C. Nakash., Videira & Crous, sp. nov. MycoBank MB822724. Fig. 40.
Fig. 40.
Zasmidium eucalypticola (CPC 15149). A–D. Conidiophores and conidia observed in vivo. Scale bars = 10 μm.
Etymology: Composed of Eucalyptus (host genus) and -cola (dweller).
Description in vitro (on SNA; CPC 15149): Mycelium composed of hyaline, subhyaline, or pale olivaceous brown hyphae, smooth to rough, uniform in width 2–2.5 μm. Conidiophores micro- to macronematous, arising from hyphae, pale olivaceous brown to olivaceous brown, somewhat paler towards the apex, verruculose, simple, septate, straight to slightly curved, uniform in width, rugged or geniculate at the apex, 38–63 × 3–3.5 μm. Conidiogenous cells integrated, apical, polyblastic, proliferating sympodially, with rim-like conidiogenous loci, thickened and darkened, located apically and lateraly as in a short rachis (ramichloridium-like), 1.5–2 μm diam. Conidia solitary, sometimes bearing conidia by microcyclic conidiation, pale olivaceous brown, verruculose, ovoid to cylindrical, base obconically truncate and apex rounded, 7.5–20 × 2.5–4 μm, 0–1-septate, hila thickened and darkened.
Material examined: Brazil, Minas Gerais, Viçosa, Paraíso, on Eucalyptus sp., 1 Mar. 2008, coll. A.C. Alfenas, isol. P.W. Crous (holotype CBS H-22959, ex-type culture CBS 142186 = CPC 15149).
Notes: Phylogenetically, the present species is closely related to Zasmidium syzygii (Fig. 4 clade 1; Fig. 5, clade I) but they are morphologically distinct. Zasmidium eucalypticola produces conidiogenous cells that are rachis-like with broader scars and smaller ovoid conidia. Zasmidium syzygii produces conidiogenous cells with smaller scars and multiseptate conidia that are longer and narrowly obclavate (Crous et al. 2012a). Based on a BLAST comparison against the alignment, Zasmidium eucalypticola shares 99 % (481/486) similarity on ITS and 97 % (714/737) similarity on rpb2 with Zasmidium syzygii.
Zasmidium eucalyptorum (Crous & M.J. Wingf.) Quaedvlieg & Crous, Persoonia 33: 24. 2014.
Basionym: Mycosphaerella eucalyptorum Crous & M.J. Wingf., Stud. Mycol. 55: 112. 2006.
Description and illustration: Crous et al. (2006b).
Material examined: Indonesia, on leaves of Eucalyptus urophylla, Mar. 2004, M.J. Wingfield (holotype CBS H-19689, ex-type culture CBS 118500 = CPC 11174).
Notes: The present species is only known from its sexual morph that is mycosphaerella-like and produces ascospores (12–17 × 3.5–4.5 μm) that germinate in a Type B germination pattern (Crous et al. 2006b). Based on the phylogenetic analyses Zasmidium eucalyptorum is closely related to Zasmidium pseudoparkii (Fig. 1, clade 69; Fig. 4, clade 1; Fig. 5, clade II), which is also a pathogen of Eucalyptus but was originally described from Colombia.
Zasmidium fructicola Crous et al., Mycologia 107: 1165. 2015.
Description and illustration: Huang et al. (2015).
Materials examined: China, Zhejiang Prov., Huangyan, on fruit of Citrus reticulata, Jan. 2010, X.H. Wang, holotype CBS H-22177, culture ex-type ZJUM 80 = CPC 24487 = CBS 139625; Huangyan, on fruit with citrus black spot of Citrus unshiu, Jan. 2010, X.H. Wang, culture ZJUM 84 = CPC 24489; Cangnan, on fruit with greasy spot of Citrus grandis, Oct. 2010, L. Zhu, culture ZJUM 9 = CPC 24465; Changshan, on fruit with yellow spot of Citrus paradisi × Citrus sp., Nov. 2010, L. Zhu, cultures ZJUM 48 = CPC 24472, ZJUM 50 = CPC 24473; on fruit with black dot of C. paradisi × Citrus sp., Dec. 2010, L. Zhu, culture ZJUM 55 = CPC 24475; Linhai, on fruit with black dot of C. sinensis, Nov. 2010, G.Q. Chen, culture ZJUM 89 = CPC 24494); Fujian Prov., on fruit with greasy spot of C. grandis, Nov. 2010, L. Zhu, culture ZJUM 58 = CPC 24477; Nanjing, on fruit with greasy spot of C. grandis, Nov. 2009, L. Zhu, culture ZJUM 90 = CPC 24495; Guangdong Prov., Pingyuan, on fruit with citrus black spot of Citrus sinensis, Nov. 2009, X.H. Wang, culture ZJUM 68 = CPC 24479; Hunan Prov., Jishou, on fruits of C. reticulata, Nov. 2011, X.H. Wang, cultures ZJUM 77 = CPC 24484, ZJUM 78 = CPC 24485, ZJUM 79 = CPC 24486.
Notes: Based on the phylogenetic analyses, Zasmidium fructicola is closely related to Zasmidium fructigenum (Fig. 4, clade 1; Fig. 5, clade I) which agrees with the original assessment by Huang et al. (2015). These two species are morphologically similar, but Zasmidium fructicola produces darker and wider conidia than Zasmidium fructigenum (conidia pale brown, 5–15 × 2 μm; Huang et al. 2015).
Zasmidium fructigenum Crous et al., Mycologia 107: 1165. 2015.
Description and illustration: Huang et al. (2015).
Materials examined: China, Zhejiang Prov., Changshan, on fruit with greasy spot of Citrus paradisi × Citrus sp., Nov. 2009, L. Zhu (holotype CBS H-22178, culture ex-type ZJUM 36 = CPC 24471 = CBS 139626); Yuhuan, on fruits with greasy spot of Citrus grandis, Nov. 2010, L. Zhu, cultures ZJUM 99 = CPC 24498, ZJUM 100 = CPC 24499; Linhai, on fruit with black dot of Citrus reticulata (= Citrus unshiu), Nov. 2010, G.Q. Chen, culture ZJUM 88 = CPC 24493; Jiangxi Prov., on fruit with citrus black spot of Citrus reticulata, Nov. 2010, X.H. Wang, cultures ZJUM 86 = CPC 24491, ZJUM 87 = CPC 24492.
Note: See notes on Zasmidium fructicola.
Zasmidium grevilleae Crous & Summerell, sp. nov. MycoBank MB822721.
Basionym: Verrucisporota grevilleae Crous & Summerell, Persoonia 22: 155. 2009, nom. inval. (Art. 40.6).
Etymology: Derived from the host genus on which it occurs, Grevillea.
Description and illustration: Crous et al. (2009a).
Materials examined: Australia, Northern Territory, Emerald Springs, on leaves of Grevillea decurrens, 22 Sep. 2007, B. Summerell (holotype CBS H-20205, ex-type culture CBS 124107 = CPC 14761); idem. cultures CPC 14762, CPC 14763.
Notes: Crous et al. (2009a) proposed the new species Verrucisporota grevilleae but did not designate the type specimen at the time, making it an invalid name according to Art. 40.6 (Melbourne). Herewith we designate the original specimen as the holotype for Zasmidium grevilleae. Verrucisporota is currently considered a synonym of Zasmidium based on morphological and phylogenetical evidence (Braun et al. 2013). The present species clusters within the genus Zasmidium (Fig. 1, clade 69; Fig. 4, clade 1; Fig. 5, clade VI) as circumscribed in the present study. Zasmidium grevilleae can be distinguished from its closest relative, Verrucisporota proteacearum, by producing shorter conidiophores and narrower and longer conidia (Shaw & Alcorn 1967; Crous et al. 2009a).
Zasmidium gupoyu (R. Kirschner) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822809.
Basionym: Parastenella gupoyu R. Kirschner, Fungal Diversity 40: 42. 2010.
Description and illustration: Kirschner & Chen (2010).
Material examined: Taiwan, Nantou County, Chitou, ca. 1200 m, on senescent lower leaf of Alocasia odora, 19 Mar. 2007, R. Kirschner & S.-H. Wu, 2990-B (holotype TNM, isotypes BPI 878812, FR); Taipei County, Wulai, 300 m, on senescent lower leaf of Alocasia odora, 22 Feb. 2005, R. Kirschner & C.-J. Chen 2279, 3022 (TNM), culture CBS 122099 = RoKi 3022.
Notes: The present species was originally described in the genus Parastenella. However, judging from the SEM photographs (Kirschner & Chen 2010), its loci were distinctly thickened, which is not a character typical of the generic description of Parastenella. Parastenella gupoyu produces erect, unbranched conidiophores and verruculose hyphae and conidia, characters typical of Zasmidium s. lat. The genus Parastenella is based on Parastenella magnolia (on leaves of Magnolia grandiflora, USA) and its current phylogenetic position is unknown because there are no sequence data available. Based on the phylogenetic analyses, the present species clusters in Zasmidium (Fig. 1, clade 63; Fig. 4 clade 1; Fig. 5, clade IX) and is closely related to Zasmidium elaeocarpi. Morphologically, Zasmidium gupoyo can be distinguished from Zasmidium elaeocarpi by producing the conidia in short shoulders mostly in the apical area of the conidiogenous cells and by producing long and single conidia.
Zasmidium hakeae U. Braun, C. Nakash., Videira & Crous, sp. nov. MycoBank MB822723. Fig. 41.
Fig. 41.
Zasmidium hakeae (CPC 15577). A–F. Observations in vivo. A, B. Leaf spot symptoms on the host. C. Partial conidiophore and conidiogenous cells. D–F. Conidia. G–L. Observations in vitro. G. Culture on V8. H–J. Partial conidiophore, conidiogenous cells and conidia. K, L. Conidia. Scale bars = 10 μm.
Etymology: Derived from the host genus on which it occurs, Hakea.
Description in vitro (on SNA; CPC 15577): Mycelium composed of hyaline to subhyaline hyphae, smooth to rough, septate, branching. Conidiophores emerging from hyphae, micro- to macronematous, brown to olivaceous brown, paler towards the apex, verruculose, rugose, straight to slightly curved, simple, strongly geniculate at the apex, 200–250 × 2.5–3.8 μm. Conidiogenous cells integrated, apical, polyblastic, proliferating sympodially, sometimes also percurrently, with rim-like conidiogenous loci, thickened and darkened, located apically and laterally in a short rachis, 1.5–2 μm diam. Conidia solitary, pale to pale olivaceous brown, verruculose, ellipsoid to obclavate, straight to mildly sinuous, obconically truncate at the base, rounded at the apex, 8–32.5 × 2.5–5 μm, 1–9-septate, hila thickened and darkened, 1.5–2 μm diam.
Materials examined: Australia, Western Australia, Pemberton, Steward Road, Banksia woodland, on Hakea undulata, 2 Aug. 2008, A.R. Wood (holotype CBS H-22958, ex-type culture CBS 142185 = CPC 15577); idem., culture CPC 15583; Queensland, Norta Nature Reserve, leaves in shop (Loma tea), 13 Jul. 2009, P.W. Crous, culture CPC 17213.
Notes: Based on the phylogenetic analyses, the present species clusters in Zasmidium (Fig. 4, clade 1; Fig. 5, clade VIII), and is closely related to Zasmidium daviesiae. Morphologically, Zasmidium hakeae produces longer and narrower conidiophores and shorter and narrower conidia with more septa than Zasmidium daviesiae (conidiophores 16–65 × 5–7 μm, conidia 18–56 × 4.5–7 μm, 0–6-septate; Beilharz & Pascoe 2002). There were two different species isolated from the same herbarium specimen in this study, Zasmidium hakeae (CPC 15577) and Devonomyces endophyticus (CPC 15580) which indicates they may be co-existing in the same lesions.
Zasmidium indonesianum Crous et al., Mycologia 107: 1166. 2015.
Description and illustration: Huang et al. (2015).
Materials examined: Indonesia, on leaf spots of Citrus sp., 2004, M. Arzanlou (holotype CBS H-22179, culture ex-type CBS 139627 = CPC 15300); idem., cultures CPC 15301, CPC 15302.
Notes: Based on the phylogenetic analyses, Zasmidium indonesianum clusters in the Zasmidium clade (Fig. 4, clade 1; Fig. 5, clade III), which is in agreement with the original observations by Huang et al. (2015), and is closely related to Zasmidium musicola, a pathogen of Musa sp. Zasmidium indonesianum is a pathogen of Citrus sp. and differs from Zasmidium citri-griseum by producing shorter and narrower conidiophores and conidia (Braun et al., 2014, Huang et al., 2015).
Zasmidium iteae (R. Kirschner) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822810.
Basionym: Stenella iteae R. Kirschner, Fungal Diversity 17: 58. 2004.
Description and illustration: Kirschner et al. (2004).
Materials examined: Taiwan, Pingtung, Nanrenshan, on leaves of Itea parviflora, 2 Jun. 2002, R. Kirschner & C.-J. Chen (holotype TNM, culture ex-type CBS 113094 = RoKi 1279).
Notes: The present species was originally described in the genus Stenella (Kirschner et al. 2004), which is currently accommodated in Teratosphaeriaceae (Fig. 1, clade 98). As a consequence of the circumscription of the genus Stenella based on its type, several stenella-like species in the Mycosphaerellaceae were assigned to the genus Zasmidium (Braun et al. 2010a). Based on the phylogenetic analysis, the present species clusters in Zasmidium (Fig. 1, clade 69; Fig. 4, clade 1; Fig. 5, clade IX), and is closely related to Zasmidium elaeocarpi. Morphologically, Zasmidium iteae can be distinguished from Zasmidium elaeocarpi by producing shorter conidiophores and shorter and narrower conidia (Kirschner et al. 2004).
Zasmidium lonicericola (Y.H. He & Z.Y. Zhang) Crous & U. Braun, Persoonia 23: 140. 2009.
Basionym: Cladosporium lonicericola Yong H. He & Z.Y. Zhang, Mycosystema 20: 469. 2001.
Synonyms: Stenella lonicericola (Yong H. He & Z.Y. Zhang) K. Schub. et al., Fungal Diversity 20: 204. 2005.
Cladosporium lonicerae Sawada, Rep. Gov. Res. Inst. Formosa 86: 163. 1943, nom. inval. (Art. 39.1).
Description and illustrations: See Crous et al. (2009d).
Materials examined: Republic of Korea, Yangpyong, on leaves of Lonicera japonica, 23 Jul. 2004, H.D. Shin, herb. HAL 3240 F; Hongchon, on leaves of Lonicera japonica, 30 Oct. 2004, H.D. Shin [epitype of Cladosporium lonicericola designated here: CBS H-20271, MBT378604, (holotype MHYAU 03533), culture ex-epitype CBS 125008 = CPC 11671]; idem., cultures CPC 11672, CPC11673. Taiwan, Taipei, on leaves of Lonicera japonica var. sempervillosa, 20 Dec. 1914, K. Sawada (authentic material of Cladosporium lonicerae, BPI 427243).
Notes: The taxonomic history of the present species was addressed by several authors (Zhang et al., 2003, Schubert and Braun, 2005, Crous et al., 2009d). The epitypification of Cladosporium lonicericola by Crous et al. (2009d) was not compliant with the code (Art. 9.8) since the holotype was not cited. Based on the phylogenetic analyses this species clusters in Zasmidium (Fig. 4, clade 1; Fig. 5, clade I), and is closely related to Zasmidium cerophilum. The morphological characteristics and scar type (planate instead of pileate), of this species confirms its placement in Zasmidium.
Zasmidium musae (Arzanlou & Crous) Crous & U. Braun, Schlechtendalia 20: 102. 2010.
Basionym: Stenella musae Arzanlou & Crous, Persoonia 20: 31. 2008.
Description and illustration: Arzanlou et al. (2008).
Materials examined: France, Martinique, on Musa sp., unknown collector and date, culture CBS 121384 = CIRAD 41 = X877. Tonga, Aciar Plot, Tongatapu, on Musa cv. TU8 AAAA, Mar. 1990, R.A. Fullerton (holotype of Stenella musae, CBS H-20047, ex-type culture X745 = CBS 122477). Netherlands Antilles, Windward Islands, St Lucia, on Musa cv., 2003, E. Reid, culture X47 = CBS 122476; St. Lucia, on Musa cv., 2003, E. Reid, culture CBS 122478 = X70.
Note: Based on the phylogenetic analyses Zasmidium musae clusters in the Zasmidium clade (Fig. 1, clade 69; Fig. 4, clade 1; Fig. 5, clade VII), and is closely related to Zasmidium aucklandicum, which agrees with previous observations by Arzanlou et al. (2008).
Zasmidium musae-banksii Videira & Crous, nom. nov. MycoBank MB822830.
Replaced synonym: Ramichloridium australiense Arzanlou & Crous, Stud. Mycol. 58: 69. 2007, non Zasmidium australiense (J.L. Mulder) U. Braun & Crous 2010.
Description and illustration: Arzanlou et al. (2007).
Material examined: Australia, Queensland, Mount Lewis, Mount Lewis Road, 16°34′47.2″ S, 145°19′7″ E, 538 m alt., on Musa banksii leaf, Aug. 2006, P.W. Crous & B. Summerell (holotype CBS H-19928, culture ex-type CBS 121710).
Notes: Based on the phylogenetic analyses and morphological characters, the present species belongs to the genus Zasmidium (Fig. 1, clade 69; Fig. 4, clade 1; Fig. 5, clade V). The phylogenetic position of Ramichloridium is defined by the type, Ramichloridium apiculatum, in Dissoconiaceae (Fig. 1, clade 83; Fig. 4, clade 28).
Zasmidium musicola (Arzanlou & Crous) Crous & U. Braun, Schlechtendalia 20: 102. 2010.
Basionym: Stenella musicola Arzanlou & Crous, Persoonia 20: 33. 2008.
Description and illustration: Arzanlou et al. (2008).
Material examined: India, Tamil Nadu, Tiruchirapally, on leaf of Musa cv. Grand Nain AAA (Cav.), 23 Feb. 2005, I. Buddenhagen (holotype CBS H-20046, culture ex-type CBS 122479 = X1019).
Notes: Zasmidium musicola (as Stenella musicola) was described from Musa sp. and found to be both phylogenetically and morphologically close to Zasmidium citri-griseum (Arzanlou et al. 2008). These results are corroborated by the phylogenetic analyses in the present study (Fig. 4, clade 1; Fig. 5, clade III).
Zasmidium musigenum Videira & Crous, nom. nov. MycoBank MB822831.
Replaced synonym: Veronaea musae Stahel ex M.B. Ellis, in Ellis, More Dematiaceous Hyphomycetes: 209. 1976, non Zasmidium musae (Arzanlou & Crous) Crous & U. Braun 2010.
Synonyms: Chloridium musae Stahel, Trop. Agric., Trinidad 14: 43. 1937, nom. inval. (Art. 39.1).
Ramichloridium musae (Stahel ex M.B. Ellis) de Hoog, Stud. Mycol. 15: 62. 1977.
Misapplied name: Chloridium indicum Subram., sensu Batista & Vital, Anais Soc. Biol. Pernambuco 15: 379. 1957.
Description and illustration: Arzanlou et al. (2007).
Materials examined: Cameroon, from Musa sapientum, J.E. Heron, culture CBS 169.61 = ATCC 15681 = IMI 079492 = DAOM 84655 = MUCL 2689. Suriname, Paramaribo, from Musa sapientum leaf, G. Stahel (authentic material of Chloridium musae, CBS H-19933, culture CBS 365.36 = JCM 6973 = MUCL 9556). Unknown, from Musa sapientum, J. Brun, culture CBS 190.63 = MUCL 9557.
Notes: The type specimen of Zasmidium musigenum, based on Veronaea musae, was isolated from Musa sapientum from Jamaica (type IMI 23006), which is a different location from the examined strains. Based on the phylogenetic analysis, Zasmidium musigenum belongs to Zasmidium as circumscribed in the present study (Fig. 1, clade 69; Fig. 4, clade 1; Fig. 5, clade V), and is closely related to Zasmidium musae-banksii. Zasmidium musigenum and Zasmidium musae-banksii are both pathogens of Musa sp. and are morphologically similar, but Zasmidium musigenum produces shorter conidiophores and conidia (Arzanlou et al. 2007).
Zasmidium nocoxi Crous, Persoonia 23: 141. 2009.
Description and illustration: See Crous et al. (2009d).
Material examined: USA, Virginia, Front Royal, on twig debris, 14 May 2007, P.W. Crous (holotype CBS H-20272, cultures ex-type CBS 125009 = CPC 14044).
Notes: Zasmidium nocoxi (Fig. 1, clade 69; Fig. 4, clade 1; Fig. 5, clade I) produces a synasexual morph similar to Hyalozasmidium aerohyalinosporium (Fig. 4, clade 21), revealing this synasexual morph to not be exclusive to the genus Zasmidium.
Zasmidium pittospori (U. Braun) U. Braun, Schlechtendalia 20: 102. 2010.
Basionym: Stenella pittospori U. Braun, Fungal Diversity 26: 68. 2007.
Description and illustration: See Braun & Crous (2007).
Material examined: New Zealand, Auckland, Mt. Albert, on Pittosporum tenuifolium, 15 Jul. 2007, C.F. Hill, culture CBS 122274 = ICMP 17098. China, Sichuan, Dujiangyan, on Pittosporum podocarpum, 20 Sep. 2006, S. Both (holotype HAL 1945 F).
Notes: Based on the phylogenetic analyses Zasmidium pittospori is closely related to Zasmidium aucklandicum and Zasmidium musae (Fig. 4, clade 1; Fig. 5, clade VII). Morphologically, it can be distinguished from Zasmidium musae by producing longer conidiophores, and longer and wider verruculose conidia (Braun & Crous 2007, Arzanlou 2008).
Zasmidium proteacearum (D.E. Shaw & Alcorn) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822812.
Basionym: Verrucispora proteacearum D.E. Shaw & Alcorn, Proc. Linn. Soc. New South Wales. 92: 171. 1967.
Synonym: Verrucisporota proteacearum (D.E. Shaw & Alcorn) D.E. Shaw & Alcorn, Austral. Syst. Bot. 6: 273. 1993.
Description and illustrations: Crous et al. (2009a).
Material examined: Australia, Queensland, Indooroopilly, on Grevillea sp., 3 Feb. 2004, J.L. Alcorn, dep. V. Beilharz, culture CBS 116003 = VPRI 31812.
Notes: The type of Verrucisporota, Verrucisporota proteacearum, was described from the host Finschia chloroxantha from Papua New Guinea (holotype IMI 77905, fide Shaw & Alcorn 1967). The present strain was isolated from a different host and originates from a different country. In addition, it produced wider conidia than those in the original description (Crous et al. 2009a). Therefore, this may be a different species and the precise phylogenetic position of the type of Verrucisporota remains unresolved. Nevertheless, given the morphological similarities with Zasmidium and phylogenetic placement of the existing strains (Fig. 4, clade 1; Fig. 5, clade VI), Verrucisporota was tentatively synonymised with Zasmidium (Braun et al. 2013).
Zasmidium pseudoparkii (Crous & M.J. Wingf.) Crous & U. Braun, Schlechtendalia 20: 102. 2010.
Basionym: Stenella pseudoparkii Crous & M.J. Wingf., Stud. Mycol. 55: 128. 2006.
Description and illustrations: Crous et al. (2006b).
Materials examined: Colombia, Sinai, on leaves of Eucalyptus grandis, May 1995, M.J. Wingfield, culture CBS 110988 = CPC 1090; on leaves of Eucalyptus sp., 1995, M.J. Wingfield (holotype CBS H-19702, culture ex-holotype CBS 110999 = CPC 1087).
Notes: Phylogenetically, Zasmidium pseudoparkii is closely related to Zasmidium eucalyptorum (Fig. 1, clade 69; Fig. 4, clade 1; Fig. 5, clade II). Zasmidium eucalyptorum is only known from its sexual morph and produces ascospores that germinate in a Type C pattern (Crous 1998), while ascospores of Zasmidium pseudoparkii germinate with a Type D pattern (Crous et al. 2006b). The asexual morph of Zasmidium pseudoparkii is morphologically similar to Pseudozasmidium parkii (Fig. 1, clade 94; Fig. 4, clade 27).
Zasmidium pseudotsugae (V.A.M. Mill. & Bonar) Videira & Crous, comb. nov. MycoBank MB822813.
Basionym: Dimeriella pseudotsugae V.A.M. Mill. & Bonar, Univ. Calif. Publ. Bot. 19: 405. 1941.
Synonyms: Epipolaeum pseudotsugae (V.A.M. Mill. & Bonar) Shoemaker, Canad. J. Bot. 43: 637. 1965.
Rasutoria pseudotsugae (V.A.M. Mill. & Bonar) M.E. Barr, Mycotaxon 29: 502. 1987.
Description and illustration: Farr, 1963, Shoemaker, 1965.
Description in vivo (adapted from Shoemaker 1965): Perithecia clustered on hypophyllous superficial mycelium, spherical, 60–80 μm diam, setose; beak rarely perceptible, usually a paler coloured circular area, 10–15 μm diam, composed of 5–8 × 3 μm convergent yellow hyphae; wall 10–15 μm wide, of 2 layers of polygonal cells, 9 × 12 μm. Asci in a basal cluster, bitunicate, saccate to cylindrical, aparaphysate, 30–40 × 6–10 μm, with 8 biseriate ascospores. Ascospores hyaline, smooth, without sheath, 1-septate at middle, wider at upper cell, both cells uninucleate, 9–12(–15) × 2.5–3.5 μm.
Notes: The type specimen of Zasmidium pseudotsugae, based on Dimeriella pseudotsugae, was isolated from Pseudotsuga menziesii from California, USA (holotype UC498795, isotypes in CUP, F, NY, BPI, GAM, ILL, MICH, TENN and WIS). The DNA sequences of Rasutoria pseudotsugae used in this study were available on GenBank (Table 1) (Winton et al. 2007) and no new sequences were generated. See notes on Zasmidium cellare.
Zasmidium pseudovespa (Carnegie) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822814.
Basionym: Mycosphaerella pseudovespa Carnegie, Mycologia 99: 468. 2007.
Description and illustration: Carnegie et al. (2007).
Materials examined: Australia, New South Wales, Urbenville, Reid Plantation, native regeneration within plantation boundary, on living leaves of Eucalyptus biturbinata, 14 Apr. 2005, A.J. Carnegie (holotype DAR 77432, culture ex-type AC0466 = CBS 121159).
Notes: The species Mycosphaerella pseudovespa is commonly associated with wasp galls or leaf spots in Eucalyptus (Carnegie et al. 2007). It was described based solely on the sexual morph which is mycosphaerella-like and produces hyaline ascospores that germinate in a type I pattern (Crous et al. 2008). The phylogenetic analyses showed that it is closely related to Zasmidium velutinum (Fig. 1, clade 69; Fig. 4, clade 1; Fig. 5, clade VIII). See notes on Zasmidium cellare.
Zasmidium queenslandicum (Arzanlou & Crous) Crous & U. Braun, Schlechtendalia 20: 103. 2010.
Basionym: Stenella queenslandica Arzanlou & Crous, Persoonia 20: 34. 2008.
Description and illustration: Arzanlou et al. (2008).
Material examined: Australia, Queensland, Mount Lewis, Mount Lewis Road, 16° 34′ 47.2″ S, 145° 19′ 7″ E, 538 m alt., on leaf of Musa banksii, Aug. 2006, P.W. Crous, W. Gams & B. Summerell (holotype CBS H-20050, culture ex-type CBS 122475 = X1084).
Notes: Based on the phylogenetic analyses, the present species clusters among ramichloridium-like species in the Zasmidium clade (Fig. 4, clade 1; Fig. 5, clade IX) but it is morphologically more similar to Zasmidium musae (Fig. 5, clade VII) and Zasmidium musicola (Fig. 5, clade III). It is characterised by short conidiophores with an apical conidiogenous cell, short geniculate, with darkened and thickened conidiogenous loci, producing single cylindrical-oblong conidia (Arzanlou et al. 2008). Based on a BLAST comparison, Zasmidium queenslandicum shares 97 % (475/491) similarity on ITS with Zasmidium elaeocarpi (CPC 16642) and 89 % (656/735) similarity on rpb2 with Zasmidium gupoyu (CBS 122099).
Zasmidium scaevolicola R.G. Shivas et al., Persoonia 24: 133. 2010.
Description and illustration: Shivas et al. (2010).
Materials examined: Australia, Queensland, Cape Tribulation, 16°04′02″ S 145°27′50.9″ E, on Scaevola taccada, 8 Aug. 2009, R.G. Shivas & P.W. Crous (holotype BRIP 52795, isotype CBS H-20455, culture ex-type CBS 127009 = CPC 17344); Thornton's Beach, 2 Sep. 1977, J.H. Simmonds, BRIP 12368; same loc., 1 Oct. 1979, J.H. Simmonds, BRIP 13098; Cape Tribulation, 30 Sep. 1979, J.H. Simmonds, BRIP 13097; Potters Creek, Wongaling Beach, Sep. 1993, H.Y. Yip, BRIP 21434; same loc., 27 Nov. 1993, H.Y. Yip, BRIP 21479; same loc., 17 Apr. 1994, H.Y. Yip, BRIP 22037; Cape Tribulation, 18 Dec. 2009, R.G. Shivas & A.R. McTaggart, BRIP 50073.
Notes: Zasmidium scaevolicola is morphologically and phylogenetically a Zasmidium species (Fig. 4, clade 1; Fig. 5, clade III) as previously observed by Shivas et al. (2010). In the present phylogenetic analyses, Zasmidium scaevolicola is closely related to Zasmidium indonesianum, a recently described species that infects the host Citrus sp. (Huang et al. 2015). Morphologically, both species produce conidia solitary or catenate, very similar in size and pigmentation, but Zasmidium scaevolicola produces longer conidiophores (Shivas et al., 2010, Huang et al., 2015).
Zasmidium schini U. Braun, C. Nakash., Videira & Crous, sp. nov. MycoBank MB822726. Fig. 42.
Fig. 42.
Zasmidium schini (CPC 19516). A–F. Observations in vitro. A. Culture on V8. B–D. Conidiophore. E, F. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus on which it occurs, Schinus.
Description in vitro (on V8; CPC 19516): Mycelium composed of hyaline, pale olivaceous or pale brown hyphae, rough and uniform in width, 2.5 μm. Conidiophores micro- to macronematous, pale brown to brown, paler towards the apex, rough, straight to mildly sinuous, simple, 45–325 × 2.5–5 μm. Conidiogenous cells integrated, apical, polyblastic, proliferating percurrently and sympodially, with rim-like conidiogenous loci, thickened and somewhat darkened, 2–2.5 μm diam. Conidia solitary, hyaline to pale brown, rough, cylindrical to obclavate, base short-obconically truncate and apex rounded, straight, 17.5–50 × 2.5–4 μm, 0–5-septate, hila darkened and thickened.
Material examined: Brazil, Minas Gerais, Viçosa, Mata da Prefeitura, on Schinus terebinthifolia, 1 Sep. 2005, A.B.V. Faria (holotype CBS H-22961, ex-type culture CBS 142188 = CPC 19516).
Notes: Thus far, only one cercosporoid species was known from this host genus, namely Pseudocercospora schini from Schinus polygama (Argentina) (Braun et al. 2016). The phylogenetic analyses placed the present species in a basal branch to the clade Zasmidium (Fig. 4, clade 1; Fig. 5, clade IX). Based on a BLAST comparison, Zasmidium schini shares 94 % (463/494) similarity, including 2 % (14/494) gaps, on ITS with Zasmidium queenslandicum (CBS 122475) and 84 % (594/708) similarity on rpb2 with Zasmidium iteae (CBS 113094). Morphologically, Zasmidium schini can be distinguished from Zasmidium elaeocarpi, by producing only apical, polyblastic conidiogenous cells and single conidia that are shorter and paler.
Zasmidium sp.
Material examined: Colombia, on Eucalyptus sp., 2004, M.J. Wingfield, culture CBS 118494 = CPC 11004.
Notes: The culture observed was sterile and the fungarium material could not be located. Based on the results of the phylogenetic analyses, it is tentatively assigned to the genus Zasmidium (Fig. 1, clade 63; Fig. 4, clade 2; Fig. 5, clade IX) until it is recollected and morphologically described.
Zasmidium strelitziae (Arzanlou et al.) Videira & Crous, comb. nov. MycoBank MB822815.
Basionym: Ramichloridium strelitziae Arzanlou et al., Stud. Mycol. 58: 74. 2007.
Description and illustrations: Arzanlou et al. (2007).
Materials examined: South Africa, KwaZulu-Natal, Durban, near Réunion, on leaves of Strelitzia nicolai, 5 Feb. 2005, W. Gams & H. Glen (holotype CBS H- 19776, ex-type culture CBS 121711 = X1029).
Notes: Zasmidium strelitziae is the only zasmidium-like species described from the host Strelitzia, an important plant cultivated for its flowers. Phylogenetically, it clusters within the Zasmidium clade (Fig. 4, clade 1; Fig. 5, clade IV) as circumscribed in the present study, and is closely related to Z. musigenum.
Zasmidium syzygii Crous, Persoonia 29: 173. 2012.
Description and illustration: Crous et al. (2012a).
Material examined: South Africa, Mpumalanga, Nelspruit, Lowveld Botanical Garden, on leaves of Syzygium cordatum, 16 Jul. 2011, P.W. Crous, M.K. Crous, M. Crous & K.L. Crous (holotype CBS H-21082, culture ex-type CBS 133580 = CPC 19792).
Notes: Phylogenetically and morphologically the present species belongs to the genus Zasmidium (Fig. 4, clade 1; Fig. 5, clade I). It is closely related to Zasmidium eucalypticola, isolated from the host Eucalyptus sp. (Myrtaceae), but is morphologically distinct (see notes on Zasmidium eucalypticola).
Zasmidium tsugae (Dearn.) Videira & Crous, comb. nov. MycoBank MB822833.
Basionym: Dimerosporium tsugae Dearn., Mycologia 16(4): 153. 1924.
Synonyms: Dimeriella tsugae (Dearn.) Petr., Ber. Schweiz. Bot. Ges. 57: 171. 1947.
Epipolaeum tsugae (Dearn.) Shoemaker, Canad. J. Bot. 43: 635. 1965.
Eudimeriolum tsugae (Dearn.) M.L. Farr, Mycologia 76: 801. 1984.
Rasutoria tsugae (Dearn.) M.E. Barr, Mycotaxon 29: 502. 1987.
Description and illustration: Dearness (1924).
Description in vivo (adapted from Dearness 1924): Mycelium hypophyllous, growing on the surface of the leaf giving it a smoky cast, branched, 3–4 μm thick. Perithecia dark brown, globose, 75–90 μm, gregarious, unappendaged, sometimes with 2 to 3 short rigid mycelioid branches, cells of the wall quadrate, 6–8 μm diam. Asci very variable in shape, clavate to cylindrical, 36–60 × 12–25 μm wide. Ascospores biseriate to conglobate, hyaline, uniseptate, sometimes nucleate in one or both cells, 13–21 × 3.5–5 μm, upper cell usually larger.
Notes: The type specimen of Zasmidium tsugae, based on Dimerosporium tsugae, could not be located (USA, Washington, Pierce Co., on leaves of Tsuga heterophylla, 25 July 1921, J.S. Boyce 832, fide Dearness 1924). The DNA sequences of Rasutoria tsugae used in this study were available on GenBank (Table 1) (Winton et al., 2007, Schoch et al., 2009) and no new sequences were generated. See notes on Zasmidium cellare.
Zasmidium velutinum (G. Winter) Videira & Crous, comb. nov. MycoBank MB822816.
Basionym: Periconia velutina G. Winter, Hedwigia 23: 174. 1884.
Synonym: Periconiella velutina (G. Winter) Sacc., in Saccardo & Berlese, Atti Reale Ist. Veneto Sci. Lett. Arti, Sér. 6, 3: 727. 1885.
Description and illustrations: Arzanlou et al. (2007).
Materials examined: South Africa, Cape Town, on Brabejum stellatifolium (B. stellatum), P. MacOwan, G. Winter herbarium (lectotype selected by Arzanlou et al. 2007: B; isolectotypes PAD, S F42165, S F462166); Stellenbosch, Jonkershoek Nature Reserve, on Brabejum stellatifolium, 21 Jan. 1999, J.E. Taylor (epitype designated by Arzanlou et al. 2007: CBS H-15612, cultures ex-epitype CBS 101948–101950 = CPC 2262–2264).
Note: See notes under Zasmidium cellare.
Zasmidium xenoparkii (Crous & M.J. Wingf.) Crous & U. Braun, Schlechtendalia 20: 103. 2010.
Basionym: Stenella xenoparkii Crous & M.J. Wingf., Stud. Mycol. 55: 129. 2006.
Description and illustration: Crous et al. (2006b).
Materials examined: Indonesia, on leaves of Eucalyptus grandis, Mar. 1996, M.J. Wingfield (holotype PREM 54968, isotype CBS H-19703, culture ex-type CBS 111185 = CPC 1300); idem. Cultures CPC 1299, CPC 1301.
Notes: Zasmidium xenoparkii belongs to the genus Zasmidium both morphologically and phylogenetically (Fig. 4, clade 1; Fig. 5, clade I). In the present phylogenetic analyses, it is closely related to Zasmidium angulare, but morphologically it is more similar to Zasmidium pseudoparkii, which differs by producing longer and wider conidia (Crous et al. 2006b). Zasmidium xenoparkii has a mycosphaerella-like sexual morph and produces hyaline ascospores that germinate in a type D pattern (Crous 1998).
Clade 70: Nothopericoniella
Nothopericoniella Videira & Crous, gen. nov. MycoBank MB822697.
Etymology: From the greek notho-, meaning false, and similarity to the genus Periconiella.
Description: Phytopathogenic. Mycelium mainly superficial, composed of brown and verrucose hyphae, internal mycelium sparsely developed, intracellular, composed of hyaline to brown hyphae, finely verruculose, septate, branched. Conidiophores solitary, arising from superficial hyphae, erect, straight, septate, brown olivaceous, paler at the apex, smooth to verruculose, composed of a main axis with a dichotomously branched apical head, branches terminal, partly lateral, proliferating percurrently and sympodially. Conidiogenous cells integrated, terminal and pleurogenous, polyblastic, sympodial, geniculate or subdenticulate, conidiogenous loci slightly thickened and darkened, truncate, without marginal rim or papillae. Conidia solitary, rarely in short chains, ellipsoid-ovoid, subcylindrical, verruculose, pale olivaceous, apex rounded, base obconically truncate, hila slightly thickened and darkened.
Type species: Nothopericoniella perseae-macranthae (Hosag. & U. Braun) Videira & Crous (≡ Periconiella perseae-macranthae Hosag. & U. Braun).
Nothopericoniella perseae-macranthae (Hosag. & U. Braun) Videira & Crous, comb. nov. MycoBank MB822767.
Basionym: Periconiella perseae-macranthae Hosag. & U. Braun, Indian Phytopathol. 48: 260. 1996 (1995).
Descriptions and illustrations: Hosagoudar and Braun, 1995, Kirschner and Chen, 2010.
Description (adapted from Hosagoudar & Braun 1996 and Kirschner & Chen 2010): Phytopathogenic, producing diffuse leaf spots, colonies hypophyllous, sometimes large and confluent. Mycelium mainly external, composed of superficial brown and verrucose hyphae, internal mycelium sparsely developed, intracellular, hyaline to brown, finely verruculose. Hyphae creeping, septate, branched, occasionally anastomosing, (1–)1.5–2.5(–3.5) μm wide, somewhat darker and wider around the conidiophores. Conidiophores solitary, arising from creeping hyphae, brown olivaceous, paler at the apex, almost smooth to verruculose, septate, erect, straight or slightly curved, 250–800 × 3–5 μm, composed of a very long main axis (about 200–700 μm long) with a 1–3 dichotomously branched apical head, branches terminal, partly lateral, proliferating percurrently and sympodially. Conidiogenous cells integrated, terminal and pleurogenous, often somewhat swollen, polyblastic, sympodial, somewhat geniculate or subdenticulate, conidiogenous loci slightly thickened and darkened, truncate, without marginal rim or papillae. Conidia solitary, rarely in short chains, pale olivaceous to olivaceous brown, verruculose, ellipsoid-ovoid, subcylindrical, base slightly obconically truncate and apex rounded, (8–)10–32 × 3–6 μm, (1–)2–3(–4)-septate, hila slightly thickened and darkened.
Materials examined: India, Tamil Nadu, Coimbatore, Anamalai, Koomati, on leaves of Persea macrantha, 13 Mar. 1994, V.B. Hosagoudar (holotype HAL 1627 F). Taiwan, Taichung County, Dongshi Forest Park, ca. 500 m, on living leaves of Machilus zuihoensis, 18 Mar. 2007, R. Kirschner & C.-J. Chen 2995 (TNM), culture CBS 122097 = RoKi 2995; Taipei County, Wulai, 300 m, 1 Apr. 2007, on living leaves of unidentified Lauraceae, R. Kirschner & C.-J. Chen 3030 (TNM), culture CBS 122282 = RoKi 2995.
Notes: Phylogenetically, Nothopericoniella perseae-macranthae is more closely related to the type of Annellosympodiella (Fig. 1, clade 71; Fig. 4, clade 3) than to the type of Periconiella, Periconiella velutina (Fig. 4, clade 1), the genus in which it was originally described. Morphologically, it is similar to Annellosympodiella by displaying both percurrent and sympodial proliferation, verrucose conidiophores and conidia and conidiogenous scars without marginal rim or papillae but slightly thickened and darkened. Nothopericoniella perseae-macranthae differs from Annellosympodiella nectandrae by forming longer conidiophores (250–800 × 3–5 μm) that rise singly from the external mycelium and are branched at the top instead of forming straight conidiophores (25–50 × 4–7 μm) rising from stromata in densely aggregated bunches. The conidia of Nothopericoniella persea-macranthae are also shorter and narrower (8–32 × 3–6 μm) than those of Annellosympodiella nectandrae (30–70 × 5–7 μm) (Hosagoudar & Braun 1996, Crous et al. 2014a).
Clade 71: Annellosympodiella
Annellosympodiella Crous & Assefa, Persoonia 32: 245. 2014.
Description (from Crous et al. 2014a): Conidiomata sporodochial on leaflets, arising from an erumpent brown stroma, consisting of brown, subcylindrical cells. Conidiophores densely aggregated, subcylindrical, brown, verruculose to warty, rejuvenating percurrently, septate. Conidiogenous cells integrated, terminal, brown, verruculose, proliferating percurrently with irregular annellations, and long, brown, tubular collarettes. Loci formed by sympodial proliferation are also visible on the tubular collarette, circular, thickened, darkened and refractive. Conidia solitary, brown, verruculose to warty, guttulate, subcylindrical to narrowly obclavate, straight to curved, euseptate; hilum truncate, thickened and slightly darkened.
Type species: Annellosympodiella juniperi Crous & Assefa.
Annellosympodiella juniperi Crous & Assefa, Persoonia 32: 245. 2014.
Description and illustration: Crous et al. (2014a).
Materials examined: Ethiopia, Addis Ababa, Mangadishu Forest, on needles of Juniperus procera, 25 Jun. 2013, PW. Crous & A. Assefa (holotype CBS H-21706, ex-type culture CBS 137992 = CPC 23276).
Notes: Annellosympodiella is a monotypic genus similar to Annellophragmia (Ellis 1971) and Annellosympodia (McTaggart et al. 2007) based on their strange mode of percurrent and sympodial proliferation with darkened, thickened scars (Crous et al. 2014a). In the phylogenetic analyses, Annellosympodiella is a single-strain lineage closely related to Neopenidiella and Neopericoniella (Fig. 1, clade 71; Fig. 4, clade 3).
Clade 72: Neopenidiella
Neopenidiella Quaedvlieg & Crous, Persoonia 33: 22. 2014.
Description (from Quaedvlieg et al. 2014): Foliicolous. Conidiophores erect, straight, filiform, pluriseptate throughout, brown, darker below and paler above, thin-walled, smooth, apex penicillate, terminal cell of the conidiophore with short denticle-like loci giving rise to sets of conidiogenous cells or ramoconidia that then form a sequence of new sets of ramoconidia on different levels. Conidiogenous loci terminal or subterminal, usually 1–3(–4), subdenticulate, conical, apically truncate, unthickened or almost so, not to somewhat darkened-refractive. Ramoconidia with truncate base, barely or distinctly attenuated at the truncate base, aseptate, at the apex with 2–3(–4) subdenticulate hila, subcylindrical, very pale olivaceous, olivaceous brown to brown, thin-walled, smooth to faintly verruculose. Conidia in long acropetal chains, narrowly ellipsoid-ovoid, fusiform to cylindrical aseptate, very pale olivaceous, olivaceous brown to brown, thin-walled, smooth to very faintly rough-walled; hila unthickened or almost so, at most slightly darkened-refractive.
Type species: Neopenidiella nectandrae (Crous et al.) Quaedvlieg & Crous (≡ Penidiella nectandrae Crous et al.).
Neopenidiella nectandrae (Crous et al.) Quaedvlieg & Crous, Persoonia 33: 22. 2014.
Basionym: Penidiella nectandrae Crous et al., Stud. Mycol. 58: 20. 2007.
Synonym: Cladosporium ferrugineum R.F. Castañeda, Fungi Cubenses II: 4. 1987, nom. illeg. (Art. 53.1).
Description and illustrations: Crous et al. (2007a).
Material examined: Cuba, Matanzas, San Miguel de los Baños, on living leaves of Nectandrea coriacea, 24 Jan. 1987, R.F. Castañeda & G. Arnold (holotype of Cladosporium ferrugineum INIFAT C87/45, culture ex-type CBS 734.87 = ATCC 200932 = INIFAT 87/45; isotype HAL 2018 F).
Notes: Neopenidiella is currently a monotypic genus that was established to accommodate Neopenidiella nectandrae since it was not congeneric with the type of Penidiella, P. columbiana (Teratosphaeriaceae) (Quaedvlieg et al. 2014). Neopenidiella differs from Penidiella by forming conidiophores that are long and filiform, with a subdenticulate apical cell where long and narrow penicillate ramoconidia are formed. In the phylogenetic analyses performed in this study it forms a single-strain lineage closely related to Annellosympodiella (Fig. 1, clade 72; Fig. 4 clade 4).
Clade 73: Neoceratosperma
Neoceratosperma Crous, Persoonia 32: 257. 2014.
Description (from Crous et al. 2014a): Mycelium consisting of branched, septate, brown, verruculose hyphae turning warty with age. Conidiophores reduced to conidiogenous cells, or septate, erect, brown, verruculose, unbranched, subcylindrical, dark brown and smooth at the base. Conidiogenous cells subcylindrical, brown, verruculose, but conidiogenous apical area smooth, forming a short rachis that proliferates sympodially, with somewhat thickened and darkened loci. Conidia solitary, rarely in unbranched chains, subcylindrical, medium brown, becoming dark brown, verruculose, becoming warty, distoseptate, less obvious when older (dark brown, warty), straight to irregularly curved; apex obtuse, base truncate, but hila somewhat thickened and darkened.
Type species: Neoceratosperma eucalypti Crous & Cheew.
Neoceratosperma cyatheae Guatimosim et al., Persoonia 37: 122. 2016.
Description and illustration: Guatimosim et al. (2016).
Materials examined: Brazil, Rio de Janeiro, Fazenda Barreto II, Rio grandina, on fronds of Cyathea delgadii, 11 Feb. 2014, R.W. Barreto (holotype CBS H-22074; isotype VIC 42605, culture ex-type CPC 24704; Rio de Janeiro, Nova Friburgo, Macaé de Cima, on fronds of C. delgadii, 11 Jul. 2009, R.W. Barreto, CBS H-22078, VIC 42533, cultures CPC 18580 = COAD573.
Notes: Neoceratosperma cyatheae was recently described from a fern host, Cyathea delgadii, originating from Brazil. Only its asexual morph is known, which can easily be distinguished from Neoceratosperma eucalypti by producing smooth conidiophores reduced to conidiogenous cells and solitary conidia (Guatimosim et al. 2016). Based on the phylogenetic analyses it forms a single-strain lineage within the Neoceratosperma clade (Fig. 1, clade 73; Fig. 4, clade 7).
Neoceratosperma eucalypti Crous & Cheew., Persoonia 32: 257. 2014.
Description and illustration: Crous et al. (2014a).
Materials examined: Thailand, Chiang Mai, on living leaves of Eucalyptus sp., Sep. 2013, R. Cheewangkoon (holotype CBS H-21712, culture ex-type CBS 137998 = CPC 23465).
Notes: Neoceratosperma has a zasmidium-like morphology except it produces distoseptate conidia. Neoceratosperma differs from Ceratosperma by forming strongly verruculose conidiophores and conidia, producing conidia in a short sympodial rachis, solitary or in chains and with slightly thickened, darkened hila and scars (Crous et al. 2014a). Phylogenetically, Neoceratospoerma strains cluster in a well-supported clade by both Bayesian and maximum likelihood analyses (Fig. 1, clade 73; Fig. 4, clade 7) and is closely related to Xenomycosphaerella. Neoceratosperma was monotypic, but several species have been recently added by Guatimosim et al. (2016).
Neoceratosperma legnephoricola U. Braun, C. Nakash., Videira & Crous, sp. nov. MycoBank MB822715. Fig. 43.
Fig. 43.
Neoceratosperma legnephoricola (CPC 16411). A–E. Observations in vivo. A. Leaf spot symptoms on the host. B. Partial conidiophore, conidiogenous cells and conidia. C. Conidiophore and conidiogenous cells. D, E. Conidia. F–K. Observations in vitro. F. Culture on V8. G. Conidiophore and conidiogenous cell. H, I. Conidiogenous cell and conidia. J, K. Conidia. Scale bars = 10 μm.
Etymology: Derived from the host genus on which it occurs, Legnephora.
Description in vivo (CBS H-22962): Leaf spots small, brown to dark brown, angular, 2–3 mm diam, later enlarged, circular to subcircular, with 2–3 dark brown concentric ring, 5–10 mm diam. Mycelium internal and external, composed of pale brown to brown hyphae, smooth to verruculose. Caespituli hypophyllous, well-developed, visible in concentric rings, yellowish brown. Stromata hypophyllous, epidermal, substomatal, 20–54 μm diam. Conidiophores often reduced to conidiogenous cells, emerging from stromata and internal/external hyphae, solitary or fasciculate, more than 20, pale brown to brown, verruculose, straight to sinuous, simple, 25–380 × 2–6 μm. Conidiogenous cells terminal or intercalary, pale brown to brown, verruculose, polyblastic, proliferating sympodially, with rim-like conidiogenous loci slightly thickened and darkened, 2–3 μm diam. Conidia solitary, pale brown to brown, verruculose, cylindrical, obclavate to filiform, straight to curved, obconically truncate at the base and apex rounded, 34–260 × 5–11 μm, 0–23-distoseptate, hila slightly thickened and darkened, 2–3 μm diam.
Description in vitro (on SNA; CPC 16411): Mycelium composed of pale brown to olicaveous brown hyphae, smooth to verruculose. Conidiophores single, pale brown to olicaveous brown verruculose, erect, straight, simple, often reduced to conidiogenous cells, 3–120 × 4–5 μm. Conidiogenous cells terminal in conidiophores or integrated in the mycelium, pale brown to olivaceous brown, verruculose, single or polyblastic, proliferating sympodially, with rim-like conidiogenous loci, slightly thickened and darkened. Conidia solitary, occasionally catenate in a single chain, pale brown to olivaceous brown, verruculose, cylindrical, straight or curved, (16–)44–66(–128) × (3.5–)4–5(–5.5) μm, 0–10-distoseptate, obconically truncate at the base and conically truncate at the apex when intercalary, obconically truncate at the base and apex rounded when terminal, hila slightly thickened and darkened, 2–3 μm diam.
Material examined: Australia, New South Wales, North Washpool State Forest, on Legnephora moorei (≡ Cocculus moorei), Mar. 2009, B. Summerell (holotype CBS H-22962, ex-type culture CBS 142189 = CPC 16411).
Notes: This is the first time that a fungus has been described in association with the host Legnephora moorei, an endemic plant of the Australian rainforest. In the phylogenetic analyses it is closely related to Neoceratosperma yunnanensis (Fig. 1, clade 73; Fig. 4, clade 7), but can be morphologically distinguished by producing longer conidiophores and shorter conidia.
Neoceratosperma haldinae U. Braun, C. Nakash., Videira & Crous, sp. nov. MycoBank MB822716. Fig. 44.
Fig. 44.
Neoceratosperma haldinae (CPC 19202). A–F. Observations in vitro. A. Culture on OA. B, C. Conidiophore and conidiogenous cell. D–F. Conidia. Scale bars = 10 μm.
Etymology: Derived from the host genus on which it occurs, Haldina.
Description in vitro (on SNA, CPC 19202): Mycelium composed of pale olivaceous hyphae, verruculose, 2 μm wide. Conidiophores pale olivaceous, finely verruculose, straight, simple, geniculate-sinuous at the apex, (25–)43–53(–76) × 1.5–2 μm, often reduced to conidiogenous cells. Conidiogenous cells pale olivaceous, finely veruculose, proliferating sympodially at the apex, polyblastic, with conidiogenous loci slightly thickened and darkened, 1 μm diam. Conidia solitary, pale olivaceous, finely verruculose, filiform, cylindrical to long obclavate, base short-obconically truncate and apex rounded, (5.5–)17–22.5(–30) × (1.5–)2(–3) μm, 1–5-euseptate, with hila slightly thickened but hardly darkened, 1 μm diam.
Materials examined: Laos, Vientiane, Xanthany, Dong Makkai, on Haldina cordifolia, unknown date, P. Pheng, LC 0408, NUOL P53 (holotype CBS H-22963, culture ex-type CBS 142190 = CPC 19202).
Notes: Neoceratosperma haldinae needs to be compared with Passalora haldinae, which was described from Haldina cordifolia collected in Thailand (Nakashima et al. 2007). The strain CBS 142190 (previously identified as Passalora haldinae), sporulated in culture, and proved to be distinct from Passalora haldinae, which has wider conidiophores (15–63 × 2.8–3.6 μm) that are occasionally branched, and conidia that are smooth, longer and wider (24–80 × 2.7–5 μm, 1–7-septate; Nakashima et al. 2007). Based on the phylogenetic analyses it forms a single-strain lineage within the Neoceratosperma clade (Fig. 1, clade 73; Fig. 4, clade 7).
Neoceratosperma yunnanensis (Barber & T.I. Burgess) Guatimosim et al., Persoonia 37: 123. 2016.
Basionym: Mycosphaerella yunnanensis Barber & T.I. Burgess, Fungal Diversity 24: 150. 2007.
Synonym: Xenomycosphaerella yunnanensis (Barber & T.I. Burgess) Quaedvlieg & Crous, Persoonia 33: 24. 2014.
Description and illustration (sexual morph): Burgess et al. (2007).
Description in vitro (on V8; CBS 119975): Mycelium composed of hyaline to pale olivaceous hyphae, verruculose, 2.5 μm wide. Conidiophores short, reduced to conidiogenous cells, hyaline to pale olivaceous, verruculose, simple, 2.5–5 × 3–4 μm. Conidiogenous cells polyblastic, determinate, rarely proliferating sympodially, with rim-like conidiogenous loci that are slightly thickened and darkened, 1–1.5 μm diam. Conidia solitary, rarely catenate in a single chain, pale to pale olivaceous, verruculose, cylindrical to long obclavate, filiform, base short-obconical truncate and apex rounded, 30–210 × 3–4 μm, 0–6-eu- or distoseptate, hila slightly thickened and darkened, 1–1.5 μm diam.
Materials examined: China, Yunnan, Lancang, on leaves of Eucalyptus urophylla, May 2005, B. Dell (holotype MURU 407, ex-type culture CBS 119975 = CMW 23443 = MUCC 410 = PAB 05.05 B2).
Notes: Until now, Mycosphaerella yunnanensis was only known from its sexual morph, but in this study, we observed the asexual morph in culture using V8 medium with sterilised banana leaves. The morphological features of the asexual morph included short conidiophores reduced to conidiogenous cells and distoseptate scolecospores, which are in agreement with the description of the genus Neoceratosperma. Based on the genes used in this study and the phylogenetic methods employed, Mycosphaerella yunnanensis is included in Neoceratosperma (Fig. 1, clade 73; Fig. 2, clade 7).
Clade 74: Xenosonderhenia
Xenosonderhenia Crous, Persoonia 28: 175. 2012.
Description (from Crous et al. 2012b): Foliicolous, associated with leaf spots. Conidiomata pycnidial, black, globose, substomatal, erumpent, predominantly epiphyllous, with central ostiole, lined with periphyses; wall of 2–3 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells lining the inner cavity, subcylindrical to doliiform; finely verruculose, pale brown, proliferating apically with several percurrent proliferations. Conidia subcylindrical, brown, finely verruculose, apex obtuse, base truncate with visible scar-like hilum, (1–)3-euseptate, but septa with visible central pore. Conidia of synasexual morph intermingled in same conidioma, but conidiogenous cells proliferating percurrently or sympodially; conidia hyaline to subhyaline, narrowly obclavate, apex subobtuse, base truncate, straight to curved, transversely multi-septate. Synasexual morph also hyphomycetous, developing in aerial mycelium; conidiophores subcylindrical, straight to curved, 0–2-septate, hyaline to subhyaline, proliferating sympodially at apex. Conidiophores solitary or fasciculate or forming on a reduced stroma.
Type species: Xenosonderhenia syzygii Crous.
Xenosonderhenia eucalypti Crous & M.J. Wingf., Persoonia 33: 241. 2014.
Description and illustration: Crous et al. (2014b).
Material examined: Mozambique, Forestas de Niassa, leaf spots of Eucalyptus urophylla, 2 Feb. 2014, M.J. Wingfield (holotype CBS H-21991, culture ex-type CPC 24247 = CBS 138858).
Notes: Xenosonderhenia eucalypti was recently described based on the morphological characteristics of the sexual morph. It was placed in Xenosonderhenia due to being phylogenetically closest to Xenosonderhenia syzygii (Crous et al. 2014b). In this study, Xenosonderhenia eucalypti formed a single-strain lineage in the phylogenetic analyses (Fig. 1, clade 74; Fig. 4, clade 9) that is closely related to Xenomycosphaerella elongata with which it shares 98 % (728/740) similarity on LSU, 94 % (449/477) similarity on ITS, and only 84 % (622/737) similarity on rpb2.
Xenosonderhenia syzygii Crous, Persoonia 28: 175. 2012.
Description and illustration: Crous et al. (2012b).
Material examined: South Africa, Mpumalanga, Nelspruit, Lowveld Botanical Garden, on leaves of Syzygium cordatum, 17 Aug. 2011, P.W. Crous, M.K. Crous, M. Crous & K.L. Crous (holotype CBS H-20968, ex-type culture CBS 132688 = CPC 19790).
Notes: Xenosonderhenia currently accommodates two species, Xenosonderhenia syzygii and Xenosonderhenia eucalypti. Xenosonderhenia syzygii is phylogenetically close to Xenomycosphaerella elongata but is unique in being morphologically dimorphic (Crous et al. 2012b). Xenosonderhenia syzygii is easily distinguished from Sonderhenia since species in the latter genus produce distoseptate conidia and form a distinct clade in the Mycosphaerellaceae (Fig. 1, clade 28; Fig. 2, clade 34). It can also be separated from Phaeophleospora since species in the latter genus produce scolecosporous conidia and form a unique clade in the Mycosphaerellaceae (Fig. 1, clade 67; Fig. 4, clade 5). Unfortunately, an rpb2 sequence was not generated for this strain and it was not included in the phylogenetic trees in this study.
Clade 75: Xenomycosphaerella Quaedvlieg & Crous, Persoonia 33: 24. 2014.
Description (from Quaedvlieg et al. 2014): Foliicolous, plant pathogenic. Ascomata pseudothecial, dark brown, subepidermal to erumpent, globose, with an apical ostiole; wall of 2–3 layers of medium brown textura angularis. Asci aparaphysate, fasciculate, bitunicate, subsessile, obovoid to broadly ellipsoidal, straight to slightly curved, 8-spored. Ascospores bi- to multiseriate, overlapping, hyaline, thin- or thick- walled, straight to slightly curved, fusoid-ellipsoidal with obtuse ends, widest in middle of the apical cell, medianly or unequally 1-septate, tapering towards both ends, but more prominently towards the lower end.
Type species: Xenomycosphaerella elongata (Crous & M.J. Wingf.) Quaedvlieg & Crous (≡ Mycosphaerella elongata Crous & M.J. Wingf.).
Xenomycosphaerella elongata (Crous & M.J. Wingf.) Quaedvlieg & Crous, Persoonia 33: 24. 2014.
Basionym: Mycosphaerella elongata Crous & M.J. Wingf., Fungal Diversity 26: 163. 2007.
Description and illustrations: Crous et al. (2007c).
Material examined: Venezuela, El Piñal Lotes farm near Acarigua, on leaves of Eucalyptus calmadulensis × urophylla, Oct. 2006, M.J. Wingfield (holotype CBS H-19824, ex-type culture CBS 120735 = CPC 13378).
Notes: The genus Xenomycosphaerella was introduced to accommodate Mycosphaerella elongata and Mycosphaerella yunnanensis, both species only known from their mycosphaerella-like sexual morph but that were not congeneric with Ramularia (Quaedvlieg et al. 2014). Based on a large phylogenetic analysis based on several genes, Xenomycosphaerella yunnanensis was later combined into Neoceratosperma (Guatimosim et al. 2016). In the present phylogenetic analyses, the genus is represented by its type, Xenomycosphaerella elongata, in a single-strain lineage (Fig. 1, clade 75; Fig. 4, clade 8) that is closely related to Xenosonderhenia. The genera Xenosonderhenia and Xenomycosphaerella are very close phylogenetically, but due to lacking information related to their morphology and the existing differences observed based on the DNA sequences, they should remain separate until more isolates are available for further analysis. The type of Xenosonderhenia, Xenosonderhenia syzygii, is only known by its dimorphic asexual morph while the type of Xenomycosphaerella, Xenomycosphaerella elongata, is only known from its sexual morph. The other known species of Xenosonderhenia, Xenosonderhenia eucalypti, is only known from its sexual morph which can be distinguished from Xenomycosphaerella elongata by forming ascospores not constricted at the septa and widest at one third of the apex of the apical cell (ascospores constricted at the septum and tapering towards both ends but more prominently towards the lower end in Xenomycosphaerella elongata).
Clade 76: Xenosonderhenioides
Xenosonderhenioides Videira & Crous, gen. nov. MycoBank MB822706.
Etymology: Xenos- from the Greek strange + sonderhenioides for the phylogenetic proximity to the genus Sonderhenia.
Description: Mycelium composed of hyaline to pale brown hyphae, smooth, septate, branching. Conidiophores micro- to macronematous, subhyaline to pale brown, smooth to rough, simple, sometimes branched, straight to sinuous. Conidiogenous cells integrated, terminal or intercalary, hyaline to pale brown, proliferating sympodially, polyblastic, with rim-like conidiogenous loci, slightly thickened and darkened. Conidia solitary, rarely catenate in a single chain, hyaline to subhyaline, smooth, oblong, cylindrical to obclavate, straight, base medium-long obconically truncate, apex rounded, aseptate or eu- or distoseptate hila thickened and darkened and protruding at the base or at both ends when catenate.
Type species: Xenosonderhenioides indonesiana C. Nakash., Videira & Crous.
Xenosonderhenioides indonesiana C. Nakash., Videira & Crous, sp. nov. MycoBank MB822728. Fig. 45.
Fig. 45.
Xenosonderhenioides indonesiana (CPC 15066). A–E. Observations in vitro. A. Culture on OA. B, C. Conidiophore and conidiogenous cell. D. Conidiogenous cell and conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Derived from the country where it was collected from, Indonesia.
Description in vitro (on SNA; CPC 15066): Mycelium composed of hyaline to pale brown hyphae, smooth, septate, branched, uniform in width, 2–2.5 μm diam. Conidiophores micro- to macronematous, subhyaline to pale brown, smooth to finely verruculose, simple, straight to sinuous, sometimes geniculate-sinuous at the apex, 20–75 × 2.5–7.5 μm. Conidiogenous cells integrated, terminal and intercalary, hyaline to pale brown, smooth, proliferating sympodially, conical at the apex, mono- or polyblastic, with rim-like conidiogenous loci slightly thickened and darkened, located at the apex or shoulder, sometimes in large number and disperse through the cell, 1.5–2 μm in diam. Conidia solitary, rarely catenate in a single chain, hyaline to subhyaline, smooth, oblong, cylindrical to long-obclavate, base medium-long obconically truncate, apex rounded, 15–50 × 5–6 μm, 0–4-septate, eu- or distosepta, sometimes slightly constricted at the septa, hila slightly thickened and darkened.
Material examined: Indonesia, on Eucalyptus sp., 26 Mar. 2008, M.J. Wingfield (holotype CBS H-19824, ex-type culture CBS 142239 = CPC 15066).
Notes: Phylogenetically, the genus Xenosonderhenioides is represented by a single-strain lineage (Fig. 1, clade 76; Fig. 4, clade 10) that is closely related to Xenosonderhenia. Morphologically, Xenosonderhenioides indonesiana can easily be distinguished from Xenosonderhenia syzygii, which has dimorphic conidia in culture. Due to phylogenetic and morphological differences, we consider that this should represent a unique genus.
Clade 77: Polyphialoseptoria
Polyphialoseptoria Quaedvlieg et al., Stud. Mycol. 75: 355. 2013.
Description (from Quaedvlieg et al. 2013): Foliicolous, plant pathogenic. Conidiomata brown, erumpent, pycnidial (acervular in culture), globose, brown; wall of 3–6 layers of pale brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, smooth, subcylindrical to ampulliform, proliferating sympodially at apex, forming polyphialides with minute periclinal thickening, or as solitary loci on superficial mycelium in culture. Conidia hyaline, smooth, granular to guttulate, scolecosporous, irregularly curved, apex subobtuse, base long obconically truncate, transversely multi- euseptate, in older cultures disarticulating at septa; microcyclic conidiation also common in older cultures.
Type species: Polyphialoseptoria terminaliae Quaedvlieg et al.
Polyphialoseptoria terminaliae Quaedvlieg et al., Stud. Mycol. 75: 356. 2013.
Description and illustration: Quaedvlieg et al. (2013).
Materials examined: Brazil, Minas Gerais, Viçosa, on leaves of Terminalia catappa, 18 May 2010, R.W. Barreto (holotype CBS H-21298, culture ex-type CBS 135106 = CPC 19611); idem. cultures CBS 135475 = CPC 19487.
Notes: Polyphialoseptoria currently includes two species, Polyphialoseptoria terminaliae and Polyphialoseptoria tabebuiae-serratifoliae, both collected from Brazil. It differs from Septoria and Neoseptoria based on the presence of polyphialides. The phylogenetic analyses performed in this study strongly supported the Polyphialoseptoria clade (Fig. 1, clade 77; Fig. 4, clade 11).
Clade 78: Mycodiella
Mycodiella Crous, Persoonia 37: 337. 2016.
Description (from Crous et al. 2016a): Ascomata pseudothecial, brown, erumpent, globose; wall consisting of 2–3 layers of medium brown textura angularis. Asci aparaphysate, fasciculate, bitunicate, subsessile, obovoid, straight to slightly curved, 8-spored. Ascospores multiseriate, overlapping, hyaline, guttulate, thin-walled, straight to slightly curved, fusoid-ellipsoidal with obtuse ends, widest in middle of apical cell, medianly 1-septate.
Type species: Mycodiella eucalypti Crous.
Mycodiella eucalypti Crous, Persoonia 37: 337. 2016.
Description and illustration: Crous et al. (2016a).
Materials examined: Australia, Western Australia, Porongurup, Porongurup National Park, S34°41′18.6″ E117°55′56″, on leaves of Eucalyptus diversicolor, 24 Sep. 2015, P.W. Crous (holotype CBS H-22885, culture ex-type CBS 142097 = CPC 29226); Western Australia, Denmark, Mount Lindesay Walk Trail, Southern Cross, on leaves of Xanthosia rotundifolia, 19 Sep. 2015, P.W. Crous, cultures CBS 142099 = CPC 29525.
Notes: Mycodiella was recently introduced to accommodate Mycodiella eucalypti, a pathogen on Eucalyptus that clustered together with “Mycosphaerella” sumatrensis on Eucalyptus and “Mycosphaerella” laricis-leptolepidis on Larix. All three species are only known from their asexual morph and cluster together in a well-supported clade based on LSU, which supported the combination of all three species into the same genus (Crous et al. 2016a). In this study only a representative of Mycodiella sumatrensis was used and it forms a single-strain lineage closely related to Polyphialoseptoria (Fig. 1, clade 78; Fig. 4, clade 12).
Mycodiella sumatrensis (Crous & M.J. Wingf.) Crous, Persoonia 37: 337. 2016.
Basionym: Mycosphaerella sumatrensis Crous & M.J. Wingf., Stud. Mycol. 55: 124. 2006.
Description and illustration: Crous et al. (2006b).
Material examined: Indonesia, Northern Sumatra, on leaves of Eucalyptus sp., Feb. 2004, M.J. Wingfield (holotype CBS H-19704, cultures ex-type CBS 118499 = CPC 11171); idem. cultures CBS 118501 = CPC 11175, CBS 118502 = CPC 11178.
Note: See Mycodiella eucalypti.
Clade 79: Australosphaerella
Australosphaerella Videira & Crous, gen. nov. MycoBank MB822579.
Etymology: Derived from the country of origin Australia and mycosphaerella-like sexual morph.
Description: Ascomata pseudothecial, black, slightly erumpent, globose. Asci aparaphysate, fasciculate, bitunicate, subsessile, obclavate to ellipsoidal, straight to incurved, 8-spored. Ascospores multiseriate, overlapping, hyaline, straight to rarely curved, fusoid-ellipsoidal with obtuse ends, medianly 1-septate, widest in middle of apical cell, not constricted at septum or only slightly so.
Type species: Australosphaerella nootherensis (Carnegie) Videira & Crous.
Australosphaerella nootherensis (Carnegie) Videira & Crous, comb. nov. MycoBank MB822739.
Basionym: Mycosphaerella nootherensis Carnegie, Austral. Pl. Pathol. 40: 377. 2011.
Description and illustration: Carnegie et al. (2011).
Materials examined: Australia, Queensland, Noosa Heads, on living leaves of Corymbia intermedia, 11 Aug. 2008, A.J. Carnegie (holotype BRIP 52584a, ex-type culture CBS 130522).
Notes: This genus is represented by a single-strain lineage in the phylogenetic analyses (Fig. 1, clade 79; Fig. 4, clade 16), closely related to Mycodiella, but not strongly supported by any of the phylogenetic methods employed, which indicates that it is quite different even from the closest related species. Based on a BLAST search against the alignment, CBS 130522 shares 89 % (428/483) similarity on ITS, including 2 % (10/483) gaps, with Xenosonderhenioides indonesiana CPC 15066 and 76 % (535/704) similarity on rpb2, including 1 % (12/704) gaps, with Polyphialoseptoria terminaliae CBS 135106. Therefore, a new genus is introduced to accommodate this species. Morphologically it is only known from its mycosphaerella-like sexual morph but the ascospores have a distinctive germination pattern with multiple germ tubes growing at various angles from both ends of the ascospore (Carnegie et al. 2011).
Clade 80: Chuppomyces
Chuppomyces Videira & Crous, gen. nov. MycoBank MB822582.
Etymology: In honour of the mycologist Charles Chupp, who produced an extensive work on cercosporoid fungi.
Description: Mycelium composed of hyaline to pale olivaceous brown hyphae, smooth to rough. Conidiophores macronematous, pale olivaceous brown, rough, straight or strongly geniculate, simple. Conidiogenous cells integrated, terminal or intercalary, thickened and darkened, proliferating sympodially, polyblastic, apex short-conically truncate, with rim-like conidiogenous loci, thickened and darkened, located on the apex and shoulders. Conidia solitary, hyaline, smooth, cylindrical to obclavate, septate.
Type species: Chuppomyces handelii (Bubák) U. Braun et al. (≡ Cercospora handelii Bubák).
Chuppomyces handelii (Bubák) U. Braun, C. Nakash., Videira & Crous, comb. nov. MycoBank MB822741. Fig. 46.
Fig. 46.
Chuppomyces handelii (CBS 113302). A. Disease symptoms on the host leaves. B. Drawings of the asexual morph (from Crous & Braun 2003). C. Drawings of the sexual morph (from Crous & Braun 2003).
Basionym: Cercospora handelii Bubák, Ann. Naturhist. Mus. Wien 23: 106. 1909.
Synonyms: Cercoseptoria handelii (Bubák) Deighton, Mycol. Pap. 140: 166. 1976.
Cercospora rhododendri Ferraris, Fl. Ital. Cryptog. I: Fungi, Hyphales: 895. 1910.
Cercospora rhododendri Marchal & Verpl., Bull. Soc. Roy. Bot. Belgique 59: 24. 1927 (1926-1927), nom. illeg. (Art. 53.1).
Pseudocercospora handelii (Bubák) Deighton, Trans. Brit. Mycol. Soc. 88(3): 390. 1987.
Mycosphaerella handelii Crous & U. Braun, CBS Biodiversity Ser. 1: 211. 2003.
Description and illustrations: Chupp, 1954, Ellis, 1976, Deighton, 1976a, Crous and Braun, 2003, present study (Fig. 46).
Description in vitro (on V8; CBS 113302): Mycelium composed of hyaline to pale olivaceous brown hyphae, smooth to rough, uniform in width, 2.5–3 μm. Conidiophores macronematous, pale olivaceous brown, rough, straight or geniculate-sinuous, simple, 30–80 × 3–6 μm. Conidiogenous cells integrated, terminal or intercalary, proliferating sympodially, polyblastic, apex short-conically truncate or geniculate-sinuous, with rim-like conidiogenous loci, thickened and darkened, located on the apex and shoulders, 2–2.5 μm. Conidia solitary, hyaline, smooth, cylindrical to obclavate, base medium-long obconically truncate and apex rounded, straight, 25–125 × 3–5 μm, 1–5-septate, hila thickened and darkened.
Materials examined: Netherlands, Utrecht, Bilthoven, 28 Evert Comelislaan, on Rhododendron sp., 10 Mar. 2003, M. Crous & P.W. Crous (holotype of Mycosphaerella handelii CBS H-6594, culture ex-type CBS 112681); Utrecht, on Rhododendron sp., 2002, P.W. Crous & U. Braun, culture CBS 113302. Turkey, Trabzon District, Fol Koei, on Rhododendron ponticum, 14 Jul. 1907, Handel-Mazzetti (holotype of Cercospora handelii, BPI 437020).
Notes: The culture CBS 113302 was deposited as “Mycosphaerella” handelii (= Pseudocercospora handelii). However, the morphological characters on the V-8 medium are different from that of the genus Pseudocercospora. In the phylogenetic analyses, the present species is closely related to Ruptoseptoria unedonis and Neoamichloridium pini, but morphologically is quite distinct from both (Fig. 1, clade 80; Fig. 4, clade 13). Chuppomyces handelii (Fig. 1, clade 80; Fig. 4, clade 13) forms sympodially proliferating conidiophores and conidia which are hyaline, solitary, cylindrical and multiseptate. Ruptoseptoria unedonis (Fig. 1, clade 81; Fig. 4, clade 14) has convoluted conidiomata that open by irregular rupture and frequently form phialidic conidiogenous cells. Pachyramichloridium pini (Fig. 1, clade 82; Fig. 4, clade 15) has simple conidiophores, plurigenous conidiogenous cells with flat to prominent conidiogenous scars, producing conidia hyaline, obovoid, aseptate with darkened hila. Chuppomyces handelii shares 98 % (729/747) similarity with Ruptoseptoria unedonis and 96 % (712/744) similarity with Pachyramichloridium pini, based on LSU; 95 % (450/475) similarity with Ruptoseptoria unedonis and 91 % (431/476) similarity with Pachyramichloridium pini, based on ITS; 87 % (639/731) similarity with Ruptoseptoria unedonis and 80 % (593/737) similarity with Pachyramichloridium pini, based on rpb2. Despite the strong support on the branch that connects these three strains together based on all three phylogenetic methods employed in this study, the morphological characters are too different to consider joining them in the same genus and, therefore, two new genera are introduced to accommodate them.
Clade 81: Ruptoseptoria
Ruptoseptoria Quaedvlieg et al., Stud. Mycol. 75: 356. 2013.
Description (from Quaedvlieg et al. 2013): Foliicolous, plant pathogenic. Conidiomata black, appressed, elongated, pycnidial, but opening via irregular rupture, convulated; exuding a creamy white conidial mass; outer wall dark brown, crusty, consisting of 6–8 layers of dark brown textura angularis; giving rise to 2–3 inner layers of pale brown to hyaline textura angularis. Conidiophores lining the inner cavity, hyaline, smooth or pale brown, verruculose at base, branched below, septate, subcylindrical. Conidiogenous cells integrated, terminal, subcylindrical, smooth; proliferating sympodially at apex, or apex phialidic with minute periclinal thickening. Conidia solitary, hyaline, smooth, guttulate, subcylindrical to narrowly obclavate, gently to irregularly curved, apex subobtuse, base truncate to narrowly obovoid, transversely septate.
Type species: Ruptoseptoria unedonis (Roberge ex Desm.) Quaedvlieg et al. (≡ Septoria unedonis Roberge ex Desm.).
Ruptoseptoria unedonis (Roberge ex Desm.) Quaedvlieg, Verkley & Crous, Stud. Mycol. 75: 357. 2013.
Basionym: Septoria unedonis Roberge ex Desm., Ann. Sci. Nat., Bot., Sér. 3, 8: 20. 1847.
Synonym: Sphaerella arbuticola Peck, Bull. Torrey Bot. Club 10(7): 75. 1883.
For additional synonyms see MycoBank.
Description and illustration: Quaedvlieg et al. (2013).
Materials examined: Croatia, Rab, city park, leaf spots on Arbutus unedo, Jul. 1970, J.A. von Arx, CBS H-18192, culture CBS 755.70. France, on leaves of Arbutus unedo, Aug. 1986, H.A. van der Aa, CBS H-14645, culture CBS 355.86.
Notes: Morphologically, Ruptoseptoria is very similar to Septoria but differs from the later genus in forming convoluted conidiomata that open by irregular rupture and frequently form phialidic conidiogenous cells. The type species Ruptoseptoria unedonis was described from Arbutus unedo from France, but the type specimen could not be located. The link between the asexual morph Septoria unedonis (CBS 755.70) and the sexual morph Mycosphaerella arbuticola (CBS 355.86) was established based on phylogenetic data (Quaedvlieg et al. 2013). In this study, based on the phylogenetic analyses, Ruptoseptoria forms a single-strain lineage (Fig. 1, clade 81; Fig. 4, clade 14). See also notes on Chuppomyces handelii.
Clade 82: Pachyramichloridium
Pachyramichloridium Videira & Crous, gen. nov. MycoBank MB822600.
Etymology: When noting the differences between Ramichloridium apiculatum and Ramichloridium pini, De Hoog et al. (1983) stated it had: “darker, shorter and stout conidiophores”. The name is formed by the Greek prefix pachy- (stout), and -ramichloridium for its morphological resemblance to the genus.
Description: Mycelium composed by dimorphic hyphae, hyaline to pale olivaceous, or olivaceous to dark brown and thick-walled, verrucose often with irregular clumps of pale olivaceous, capsular material. Conidiophores simple, erect, emerging from hyphae, wall thick and smooth, dark olivaceous brown, aseptate or septate, slightly tapering towards the apex. Conidiogenous cells terminal, subhyaline to brown, with scattered conidiogenous loci, flat or slightly protuberant, slightly darkened. Conidia solitary, pale olivaceous, thin-walled, smooth, obovate to obconical, hila slightly darkened.
Type species: Pachyramichloridium pini (de Hoog & Rahman) C. Nakash., Videira & Crous.
Pachyramichloridium pini (de Hoog & Rahman) C. Nakash., Videira & Crous, comb. nov. MycoBank MB822769.
Basionym: Ramichloridium pini de Hoog & Rahman, Trans. Brit. Mycol. Soc. 81: 485. 1983.
Description in vitro (adapted from De Hoog et al. 1983): Hyphae dimorphic, hyaline to pale olivaceous 0.5–3 μm wide, olivaceous to dark brown, thick-walled, 2–4 μm wide, verrucose, often with irregular clumps of pale olivaceous, capsular material. Conidiophores simple, erect, emerging from hyphae, wall thick and smooth, dark olivaceous brown, 60 × 2–3 μm, slightly tapering towards the rounded apex, aseptate or up to 5-septate. Conidiogenous cells terminal on conidiophore, with scattered conidiogenous loci, flat or slightly protuberant, subhyaline to brown, up to 1 μm wide. Conidia solitary, pale olivaceous, thin wall, mostly smooth, obovate to obconical, 3–8 × 2–3 μm, truncate base, hila slightly darkened.
Material examined: UK, Scotland, Old Aberdeen, branch of Pinus contorta, unknown date and coll., isol. M.A. Rahman, dep. 1982 (holotype CBS 461.82 = MUCL 28942).
Notes: The type species of Ramichloridium, Ramichloridium apiculatum, clusters in a sister clade to Dissoconium (Dissoconiaceae) (Arzanlou et al. 2007; present study Fig. 1, clade 95; Fig.4, clade 31). Other ramichloridium-like species cluster within the Zasmidium complex (Fig. 1, clade 69; Fig. 4, clade 1). The present species forms a single-strain lineage closely related to Ruptoseptoria (Fig. 1, clade 81; Fig. 4, clade 14). Morphological evaluation of the strain CBS 461.82 is impossible since it was sterile (Arzanlou et al. 2007; this study). According to the original description (De Hoog et al. 1983), this species has simple conidiophores, plurigenous conidiogenous cells with flat to prominent conidiogenous scars, producing hyaline obovoid, aseptate conidia. See also notes on Chuppomyces handelii.
Clade 83: Exosporium
Exosporium Link, Mag. Ges. Naturf. Freunde, Berlin 3(1–2): 9. 1809.
Synonyms: Cephaloedium Kunze, Consp. Regni Veget. (Leipzig): 4. 1828.
Cuspidosporium Cif., Sydowia 9: 303. 1955.
Description (from Ellis 1961): Colonies discrete and punctiform or effuse, hairy, brown to black. Mycelium immersed. Stroma usually present, often very well-developed. Setae and hyphopodia absent. Conidiophores macronematous, mononematous, often caespitose, straight or flexuous, unbranched, or very rarely branched, mid to dark brown or olivaceous brown, smooth or verruculose. Conidiogenous cells polytretic, integrated, terminal, becoming intercalary, sympodial, cylindrical, or clavate, cicatrized, conidiogenous loci (scars) often dark and prominent. Conidia usually solitary, short catenate in one species, acropleurogenous, simple, mostly obclavate, pale to dark brown or olivaceous brown, smooth, verrucose or echinulate, distoseptate, generally with a thick, dark hilum at the base.
Type species: Exosporium tiliae Link.
Exosporium livistonae Crous & Summerell, Persoonia 27: 145. 2011.
Description and illustration: Crous et al. (2011a).
Materials examined: Australia, Northern Territory, Litchfield National Park, on leaves of Livistona benthamii, 25 Apr. 2011, P.W. Crous & B.A. Summerell (holotype CBS H-20763, ex-type culture CBS 131313 = CPC 19357).
Note: See notes under Exosporium livistonicola.
Exosporium livistonicola U. Braun, Videira & Crous, nom. nov. MB822834.
Replaced synonym: Distocercospora livistonae U. Braun & C.F. Hill, Fungal Diversity 22: 23. 2006.
Description and illustration: Braun et al. (2006).
Materials examined: Japan, Yonagunijima Is., Livistona chinensis, 27 Feb. 2003, T. Kobayashi & Y. Ono, culture MUCC 190; Hahajima Is., on Livistona chinensis var. boninensis, 17 Mar. 2003, T. Kobayashi & Y. Ono, culture MUCC 194. New Zealand, Auckland, Manurewa, Auckland Regional Botanic Gardens, Hill Road, on Livistona chinensis, 10 Sep. 2005, C.F. Hill 1247 (holotype of Distocercospora livistonae, HAL 1875 F).
Notes: In this study, the type species of the genus Distocercospora, Distocercospora pachyderma, formed an independent clade within Mycosphaerellaceae (Fig. 1, clade 31; Fig. 2, clade 27). Sequences retrieved from cultures of Distocercospora livistonae, isolated from Livistona chinensis and originating from Japan, clustered together with the sequences obtained from the ex-type culture of Exosporium livistonae (Fig. 1, clade 72; Fig. 4, clade 29). The type species of Distocercospora livistonae was described from a different country (New Zealand) but the same host (Livistona chinensis) as the studied material from Japan, which was found to be a good representative of the species. The morphological characters observed for both species, Exosporium livistonae and Distocercospora livistonae, were similar, though the two species differ in conidial width, and this is also to be seen in the phylogeny, where the two taxa are shown to be congeneric, but not conspecific. A further paper on Exosporium species (Nakashima, in prep.) will provide further detail on the genus.
Based on the phylogenetic analyses, the position of Exosporium varies as there is no strong backbone support (Fig. 1, clade 83; Fig. 4, clade 29). Although it sits in the Mycosphaerellaceae in the displayed trees, this position may change when more species are introduced as the current genus occasionally clustered between Schizothyriaceae and Dissononiaceae in different analyses (data not shown). Furthermore, sequences based on the type species of Exosporium, Exosporium tiliae, are not yet available. Therefore, the inclusion of Exosporium livistonae in Exosporium is only tentative until the application of the latter genus based on the phylogeny of its type species will be resolved.
Clade 84: Paramycosphaerella
Paramycosphaerella Crous & Jol. Roux, Persoonia 31: 245. 2013.
Description (from Crous et al. 2013b): Foliicolous, plant pathogenic. Ascomata erumpent, amphigenous, brown, globose, with central ostiole; wall of 2–3 layers of brown textura angularis. Asci fasciculate, bitunicate with apical chamber, 8-spored, subcylindrical to narrowly ellipsoid. Ascospores tri- to multiseriate, thin-walled, guttulate, not to very slightly constricted at septum, obovoid, remaining hyaline.
Type species: Paramycosphaerella brachystegiae Crous & Jol. Roux.
Paramycosphaerella brachystegiae Crous & Jol. Roux (‘brachystegia’), Persoonia 31: 245. 2013.
Description and illustration: Crous et al. (2013b).
Materials examined: Zimbabwe, Mtau forest reserve, near Mvuma, on leaves of Brachystegia sp., 2 Apr. 2012, J. Roux (holotype CBS H-21445, ex-type cultures CBS 136436 = CPC 21136); idem. culture CPC 21137.
Notes: Paramycosphaerella is morphologically mycosphaerella-like, but since Mycosphaerella is restricted to Ramularia asexual morphs, a new genus was established to accommodate the type species Paramycosphaerella brachystegiae (Crous et al. 2013b). Two more species, Paramycosphaerella intermedia (Dick & Dobbie 2001, as Mycosphaerella intermedia) and Paramycosphaerella marksii (Carnegie & Keane 1994, as Mycosphaerella marksii), were later placed in this genus based on phylogenetic inference (Quaedvlieg et al. 2014). In a recent publication (Guatimosim et al. 2016), a large group of species was introduced in this genus, mostly based on phylogenetic inference, including Paramycosphaerella aerohyalinosporum (Crous et al. 2009d, as Zasmidium aerohyalinosporium), Paramycosphaerella blechni (Guatimosim et al. 2016), Paramycosphaerella cyatheae (Guatimosim et al. 2016), Paramycosphaerella dicranopteridis (Kirschner & Liu 2014, as Zasmidium dicranopteridis), Paramycosphaerella dicranopteridis-flexuosae (Guatimosim et al. 2016), Paramycosphaerella gleicheniae (Kirschner & Liu 2014, as Mycosphaerella gleicheniae), Paramycosphaerella irregularis (Cheewangkoon et al. 2008, as Mycosphaerella irregularis), Paramycosphaerella madeirensis (Crous et al. 2004b, as Mycosphaerella madeirae), Paramycosphaerella nabiacense (Crous et al. 2009d, as Zasmidium nabiacense), Paramycosphaerella parkii (Crous et al., 1993, Crous and Alfenas, 1995, as Zasmidium parkii), Paramycosphaerella pseudomarksii (Cheewangkoon et al. 2008, as Mycosphaerella pseudomarksii), Paramycosphaerella sticheri (Guatimosim et al. 2016) and Paramycosphaerella vietnamensis (Burgess et al. 2007, as Mycosphaerella vietnamensis). Morphologically, the majority of these species are only known from their mycosphaerella-like sexual morphs (Mycosphaerella gleicheniae, Mycosphaerella marksii, Mycosphaerella intermedia, Mycosphaerella pseudomarksii, Paramycosphaerella blechni, Paramycosphaerella cyatheae, Paramycosphaerella dicranopteridis-flexuosae, Paramycosphaerella sticheri). Most of the remaining species produce a zasmidium-like asexual morph (Zasmidium aerohyalinosporium, Zasmidium dicranopteridis, Zasmidium nabiacense, Zasmidium parkii). In two cases, both sexual and asexual morphs are known, namely with “Mycosphaerella” madeirensis and “Mycosphaerella” vietnamensis, which have a presumed pseudocercospora-like asexual morph. In a later study (Videira et al. 2016), a new phylogenetic analysis based on LSU and rpb2 placed the strains of Paramycosphaerella madeirensis in a sister clade to Microcyclosporella and, based on their phylogenetic positon and morphological differences, the genus Mycosphaerelloides was erected to accommodate them. In the present study, with the addition of more genera belonging to the Mycosphaerellaceae, we observe the previously defined Paramycosphaerella clade becoming paraphyletic (Fig. 1, clades 84, 87, 93, 94; Fig. 4, clades 17, 21, 22, 26, 27). Consequently, the phylogenetic position of the species Paramycosphaerella blechni, Paramycosphaerella cyatheae and Paramycosphaerella diacranopteridis, that clustered closely related to Mycosphaerelloides (Guatimosim et al. 2016, as Paramycosphaerella madeirensis) need to be re-evaluated based on the rpb2 gene. Based on the phylogenetic analyses, Paramycosphaerella clusters close to Brunneosphaerella in a very heterogeneous clade (Fig. 1, clade 84; Fig. 4, clade 17) suggesting that further analysis is necessary to resolve this group of species.
Paramycosphaerella intermedia (M.A. Dick & K. Dobbie) Quaedvlieg & Crous, Persoonia 33: 23. 2014.
Basionym: Mycosphaerella intermedia M.A. Dick & K. Dobbie, New Zealand J. Bot. 39(2): 272. 2001.
Description and illustration: Dick & Dobbie (2001).
Materials examined: New Zealand, Bay of Plenty, Rotoehu Forest, Kohekohe Road, on living leaves of Eucalyptus saligna, 30 Jun. 1998, L. Renney (holotype NZFRI-M 3831, ex-type cultures NZFS 301.10 = CBS 114356 = CMW 7163 = CPC 10902); Waimana Forest, 12 Aug. 1998, K. Dobbie, culture NZFS 301.13 = CBS 114415 = CMW 7164 = CPC 10922.
Note: See notes on Paramycosphaerella brachystegiae.
Paramycosphaerella marksii (Carnegie & Keane) Quaedvlieg & Crous, Persoonia 33: 23. 2014.
Basionym: Mycosphaerella marksii Carnegie & Keane, Mycol. Res. 98: 414. 1994.
Description and illustration: Carnegie & Keane (1994).
Materials examined: Australia, Victoria, Briagolong, on leaves of Eucalyptus globulus, 14 Oct. 1994, A. Carnegie, culture CBS 110920 = CPC 935. South Africa, Northern Province, Magoebaskloof, Eucalyptus grandis × saligna, Oct. 1994, G. Kemp, cultures CBS 110693 = CPC 823, CBS 110750 = CPC 822 = CMW 14778. Tanzania, Eucalyptus sp., May 1995, M.J. Wingfield, cultures CBS 110981 = CPC 1073.
Notes: The type species of Paramycosphaerella marksii, based on Mycosphaerella marksii, was isolated from Eucalyptus botryoides from Australia (holotype (IMI 353731). See notes on Paramycosphaerella brachystegiae an also Quaedvlieg et al. (2014).
Paramycosphaerella wachendorfiae (Crous) Videira & Crous, comb. nov. MycoBank MB822773.
Basionym: Mycosphaerella wachendorfiae Crous, Persoonia 26: 129. 2011.
Description and illustration: Crous et al. (2011a).
Materials examined: South Africa, Western Cape Province, Hermanus, Fernkloof Nature Reserve, S 34°23′38″ E 19°16′9.7″, on leaves of Wachendorfia thyrsifolia, 2 May 2010, K.L. Crous & P.W. Crous (holotype CBS H-20584, cultures ex-type CBS 129579 = CPC 18338).
Notes: The present strain is phylogenetically closest to the type of Paramycosphaerella, Paramycosphaerella brachystegiae (Fig. 1, clade 84; Fig. 4, clade 17). The morphological characteristics of the sexual morph are compatible with the genus.
Paramycosphaerella sp. A
Materials examined: South Africa, Mpumalanga, on Musa cv. Williams, 27 Jul. 2000, K. Surridge, culture CBS 118825 = CMW 10904; idem. on Musa cv. Grande Naine, 27 Jul. 2000, K. Surridge, culture CBS 118849 = CMW 10902.
Notes: The present strains were originally identified as Mycosphaerella colombiensis based on their ITS sequences originally deposited in GenBank (AY217106 and AY217108, respectively). However, Mycosphaerella colombiensis was described from Eucalyptus in Colombia and is currently a synonym of Parapallidocercospora colombiensis (Fig. 1, clade 25; Fig. 2, clade 31). Phylogenetically, both the present strains cluster in Paramycosphaerella (Fig. 1, clade 84; Fig. 4, clade 17) and were sterile in culture. It is possible that the wrong cultures were deposited in the CBS culture collection. Therefore, they should be treated as Paramycosphaerella sp. until more information becomes available.
Paramycosphaerella sp. B
Materials examined: USA, Illinois, Rockford, apple fruit, Sep. 2000, J. Batzer, culture CBS 118968 = CUF2d; New York, Geneva, on apple fruit, 30 Oct. 2005, D. Rosenberger, culture CBS 125300 = NY1 3.2F1c.
Notes: The present strains were initially identified based on morphological characters as Colletogloeum sp. and, based on an LSU neighbour-joining phylogeny, they clustered closest to Mycosphaerella marksii. They formed a dense, fuliginous mycelial mat with no sclerotium-like bodies, had thick-walled, ovoid to allantoid blastospores that were highly vacuolate, subhyaline, and truncate at the base, measuring 6–19 × 2.5–4.5 μm (strain FG 2.1) or 7–11 × 1–2 μm (strain FG 2.3) on CLA culture media (Batzer et al. 2005). This description is very broad and the present strains are now sterile which makes it impossible to draw further conclusions. The correct phylogenetic placement of the genus Colletogloeum, based on the type Colletogloeum dalbergiae (Pakistan), is unknown, although DNA extracted from a herbarium specimen of Colletogloeum sissoo (IMI 119162) (= Colletogloeum dalbergiae) suggests Colletogloeum to cluster in a sister clade to Pseudocercospora (Crous et al. 2009e). Based on the phylogenetic analysis in the present study, these present strains cluster within the Paramycosphaerella clade (Fig. 1, clade 84; Fig. 4, clade 17), and should be treated as Paramycosphaerella sp. until more information is available.
Clade 85: Pseudopericoniella
Pseudopericoniella Videira & Crous, gen. nov. MycoBank MB822699.
Etymology: From pseudo-, that means resembling but not equalling, and the similarity to the genus Periconiella.
Description: Mycelium composed of submerged hyaline hyphae, smooth and thin-walled, and aerial hyphae subhyaline, later becoming dark brown, smooth and thick-walled. Conidiophores arising from creeping aerial hyphae, erect, dark brown at the base, paler towards the apex, thick-walled, septate, branched in the upper part. Conidiogenous cells integrated, terminal and intercalary, subhyaline, later becoming pale brown, cylindrical, proliferating sympodially, forming a short rachis with conidiogenous loci darkened, slightly thickened and protruding. Conidia solitary, pale olivaceous, smooth, obovoid, ellipsoidal, pyriform to clavate, cylindrical, base long obconicaly truncate and rounded apex, straight to mildly curved, aseptate or septate, sometimes constricted at the septa, with a hilum slightly thickened and darkened.
Type species: Pseudopericoniella levispora (Arzanlou et al.) Videira & Crous (≡ Periconiella levispora Arzanlou et al.).
Pseudopericoniella levispora (Arzanlou et al.) Videira & Crous, comb. nov. MycoBank MB822780.
Basionym: Periconiella levispora Arzanlou et al., Stud. Mycol. 58: 68. 2007.
Description and illustration: Arzanlou et al. (2007).
Materials examined: Sri Lanka, Hakgala Botanic Gardens, on dead leaves of Turpinia pomifera, Jan. 1973, W. Gams (holotype CBS H-15611, culture ex-type CBS 873.73).
Notes: Morphologically, Pseudopericoniella levispora is similar to Periconiella velutina but can be distinguished by producing darker and longer conidia [(7–)8–9(–11) × (2.5–)3(–4) μm, in Periconiella velutina; Arzanlou et al. 2007]. Based on the phylogenetic analyses in the present study, the type of Periconiella, Periconiella velutina, clusters within the Zasmidium complex (Fig. 1, clade 69; Fig. 4, clade 1), while Pseudopericoniella levispora clusters in a unique position (Fig. 1, clade 85; Fig. 4 clade 22) closely related to Hyalozasmidium.
Pseudopericoniella sp.
Material examined: Netherlands, Aalsmeer, leaf spot on Rosa sp., isol. & dep. J.A. von Arx, 1951, culture CBS 330.51.
Notes: The present strain was previously identified as Mycosphaerella rosigena. It is currently sterile and no fungarium material has been preserved. The type specimen of Mycosphaerella rosigena (from Rosa sp., Louisiana, USA, holotype NY) was examined by Aptroot (2006) and combined into Davidiella (currently a synonym of Cladosporium) based on morphological characters. Based on the phylogenetic analysis, the present strain clusters close to Pseudopericoniella levispora (Fig. 1, clade 85; Fig. 4, clade 22), and should be treated as Pseudopericoniella sp. until more information becomes available.
Clade 86: Brunneosphaerella
Brunneosphaerella Crous, Stud. Mycol. 64: 31. 2009.
Description (from Crous et al. 2009c): Ascomata amphigenous, immersed to semi-immersed, black, single, gregarious, substomatal, pyriform or globose with a papillate, periphysate ostiole; peridium consisting of three strata of slightly compressed textura angularis, an outer stratum of dark brown, thick-walled cells, becoming paler in the central stratum, and hyaline, thin-walled in the inner stratum. Asci clavate to cylindro-clavate, often curved, tapering to a pedicel, narrowing slightly to a rounded apex with an indistinct ocular chamber, 8-spored, bitunicate with fissitunicate dehiscence. Pseudoparaphyses absent. Ascospores biseriate, fusiform, broader at the apical end, initially hyaline and 1-septate, becoming yellow-brown and 3-septate at maturity, slightly constricted at median to supra-median septum.
Type species: Brunneosphaerella protearum (Syd. & P. Syd.) Crous (≡ Leptosphaeria protearum Syd. & P. Syd.).
Brunneosphaerella protearum (Syd. & P. Syd.) Crous, Stud. Mycol. 64: 31. 2009.
Basionym: Leptosphaeria protearum Syd. & P. Syd., Ann. Mycol. 10: 441. 1912.
Description and illustration: Crous et al. (2009c).
Material examined: South Africa, Western Cape Province, Wellington, on leaves of Protea lepidocarpodendron (as P. melaleuca), 22 Feb. 1912, E.M. Doidge (holotype PREM 2061); Cape town, Kirstenbosch Botanical Garden, on Protea sp., 13 Jan. 2009, P.W. Crous, (epitype designated by Crous et al. 2011b: CBS H-20335, ex-epitype culture CBS 130597 = CPC 16338); Kirstenbosch Botanical Garden, on leaves of P. coronata, 8 May 2010, P.W. Crous, CBS H-20673, culture CPC 18308 = CBS 130598; Harold Porter Botanical Garden, Betties Bay, on leaves of P. mundii, 4 May 2010, P.W. Crous, CBS H-20683, culture CPC 18328; Bettys' Bay, leaf litter of Protea magnifica, 11 Jul. 2000, S. Marincowitz, PREM 59448; Helderberg Nature Reserve, leaf litter of Protea laurifolia, 14 Aug. 2000, S. Marincowitz, PREM 59482; Helderberg Nature Reserve, leaf litter of Protea obtusifolia, 14 Aug. 2000, S. Marincowitz, PREM 59495; Jonkershoek Nature Reserve, leaf litter of Protea nitida, 6 Jun. 2000, S. Marincowitz, PREM 59442; Jonkershoek Nature Reserve, leaf litter of Protea repens, 6 Jun. 2000, S. Marincowitz, PREM 59450; Jonkershoek Nature Reserve, S33°59′11.2″ E18°57′14.7″ leaves of Protea sp., 1 Apr. 2007, P.W. Crous, CBS H-20330, cultures CPC 13914–13916; Jonkershoek Nature Reserve, S33°59′26.1″ E18°57′59.5″ leaves of P. repens, 1 Apr. 2007, P.W. Crous, CBS H-20331, cultures CPC 13911–13913; Jonkershoek Nature Reserve, leaves of Protea sp., 1 Apr. 2007, P.W. Crous, CBS H-20332, cultures CPC 13908–13910; Jonkershoek Nature Reserve, “Tweede Waterval”, leaves of Protea sp., 1 Apr. 2007, P.W. Crous, CBS H-20333, cultures CPC 13902–13907; Jonkershoek Nature Reserve, leaves of P. nitida, 12 Apr. 2008, L. Mostert, CBS H-20334, cultures CPC 15231–15233; Stellenbosch, J.S. Marais Garden, S33°55′59.3″ E18°52′22.5″, on living leaves of P. magnifica, 1 Apr. 1998, J.E. Taylor, culture CPC 16849.
Notes: The genus Brunneosphaerella was established to accommodate species belonging to the Leptosphaeria protearum complex (Pleosporales) that clustered within the Mycosphaerellaceae (Crous et al., 2009c, Crous et al., 2011b). These species were characterised by having bitunicate asci without pseudoparaphyses, brown, 3-septate ascospores, and a coniothyrium-like asexual morph. Brunneosphaerella protearum is a major leaf spot and blight pathogen of Protea spp. causing severe losses in plantations of South African Protea spp. wherever they are cultivated (Crous et al., 2009c, Crous et al., 2011b). Morphologically Brunneosphaerella is distinct from Leptosphaeria in that its ascospores are always brown at maturity and similar to Phaeophleospora in that conidiogenous cells are brown and proliferate percurrently. The genus Brunneosphaerella currently contains three species that cluster in a well-supported clade (Fig. 1, clade 86; Fig. 4, clade 18) that is closely related to Neomycosphaerella.
Clade 87: Hyalozasmidium
Hyalozasmidium U. Braun, C. Nakash., Videira & Crous, gen. nov. MycoBank MB822593.
Etymology: Derived from the hyaline conidia + resembling the genus Zasmidium.
Description: Mycelium composed of subhyaline to pale brown hyphae, smooth, branched and septate, producing large swollen propagules that occur terminaly or lateraly on hyphal strands. Conidiophores medium to dark brown, unbranched, smooth to verruculose, becoming constricted at septa, eventually disarticulating, with each conidiophore giving rise to a single conidium. Conidiogenous cells apical and intercalary, mono- or polyblastic, straight, proliferating sympodially, with conidiogenous loci unthickened or slightly thickened, located at shoulders and apex. Conidia hyaline, thick-walled, subcylindrical, with multiple transverse septa, developing irregular swellings which can form branches with obtuse ends, body granular, basal cell tapering prominently towards the conidiophore. Differs from the genus Zasmidium, by bearing hyaline conidia.
Type species: Hyalozasmidium aerohyalinosporum (Crous & Summerell) Videira & Crous (≡ Zasmidium aerohyalinosporum Crous & Summerell).
Hyalozasmidium aerohyalinosporum (Crous & Summerell) Videira & Crous, comb. nov. MycoBank MB822761.
Basionym: Zasmidium aerohyalinosporum Crous & Summerell, Persoonia 23: 144. 2009.
Synonym: Paramycosphaerella aerohyalinosporum (Crous & Summerell) Guatimosim et al. Persoonia 37: 124. 2016.
Description and illustration: Crous et al. (2009d).
Materials examined: Australia, New South Wales, Road to Robin Falls, 13°31′01.3″S, 131°16′22.5″E, 126 m, on leaves of Eucalyptus tectifica, 23 Sep. 2007, coll. B.A. Summerell, isol. P.W. Crous (holotype of Zasmidium aerohyalinosporium CBS H-20274, culture ex-type CBS 125011 = CPC 14636); idem., culture CPC 14637.
Notes: In the phylogenetic analyses, the present species is closely related to Neomycosphaerella (Fig. 1, clade 87; Fig. 4, clade 21). See notes in Crous et al. (2009d) and also notes on Paramycosphaerella brachystegiae.
Hyalozasmidium sideroxyli U. Braun, C. Nakash., Videira & Crous, sp. nov. MycoBank MB822713. Fig. 47.
Fig. 47.
Hyalozasmidium sideroxyli (CPC 23462). A– E. Observations in vitro. A. Culture on V8. B, C. Conidiophores and conidiogenous cells. D. Conidia. E. Irregular swollen conidia synanamorph. Scale bars = 10 μm.
Etymology: Named after the host genus on which it occurs, Sideroxylon.
Description in vitro (on SNA; CPC 23462): Mycelium composed of hyaline to subhyaline hyphae, smooth, branched and septate, producing large swollen propagules that occur terminally or laterally on hyphal strands, 1.5–3 μm diam. Conidiophores micro- or macronematous, hyaline to subhyaline, simple or branched, septate, straight to slightly curved, 12.5–60 × 2.5–5 μm. Conidiogenous cells apical and intercalary, mono- or polyblastic, proliferating sympodially, with conidiogenous loci slightly thickened and darkened, located at shoulders and apex, 1.5–2 μm diam. Conidia solitary, sometimes bearing conidia by microcyclic conidiation, hyaline, smooth to rough, cylindrical to obclavate, straight, base obconically truncate and apex rounded, 20–50 × 2–2.5 μm, 0–4-septate, hila slightly thickened and darkened.
Material examined: South Africa, Eastern Cape, Cape St. Francis, on Sideroxylon inerme, 8 May 2013, A.R. Wood (holotype CBS H-22965, ex-type culture CBS 142191 = CPC 23462).
Notes: Based on the phylogenetic analyses, the present strain clusters within the Hyalozasmidium clade (Fig. 1, clade 87; Fig. 4, clade 21). Morphologically, its characteristics are in accordance with the genus description (Fig. 47) and it can be distinguished from Hyalozasmidium aerohyalinosporium by having conidiogenous cells that are polyblastic, and longer, less septate conidia.
Clade 88: Madagascaromyces
Madagascaromyces U. Braun, C. Nakash., Videira & Crous, gen. nov. MycoBank MB822594.
Etymology: Named after the island where the type species was collected, Madagascar.
Description: Mycelium composed of pale to medium brown hyphae, septate, branched, smooth, 2–3 μm. Conidiophores solitary, medium brown, smooth, subcylindrical, simple or branched, straight to variously curved or geniculate-sinuous. Conidiogenous cells terminal and intercalary, proliferating sympodially, with one or multiple conidiogenous loci that are thickened and darkened. Conidia solitary, pale brown, smooth, guttulate, subcylindrical when small, narrowly obclavate when larger, apex subobtuse, base long obconically subtruncate, straight to slightly curved, 1- or multiseptate, with hila thickened and darkened, microcyclic conidiation observed in culture. Spermatogonia forming on OA. Spermatia cylindrical with obtuse ends, smooth, hyaline.
Type species: Madagascaromyces intermedius (Crous & M.J. Wingf.) Videira & Crous (≡ Passalora intermedia Crous & M.J. Wingf.).
Madagascaromyces intermedius (Crous & M.J. Wingf.) Videira & Crous, comb. nov. MycoBank MB822762.
Basionym: Passalora intermedia Crous & M.J. Wingf., Persoonia 22: 88. 2009.
Description and illustrations: Crous et al. (2009g).
Materials examined: Madagascar, Morondavo, on leaf of Eucalyptus calmadulensis, Aug. 2007, M.J. Wingfield (holotype CBS H-20197, ex-type culture CBS 124154 = CPC 15745); on E. calmadulensis, 1 Oct. 2007, M.J. Wingfield, culture CPC 15719.
Notes: The genus Madagascaromyces is monotypic and based on Madagascaromyces intermedius (syn. Passalora intermedia). Morphologically, Madagascaromyces intermedius can be considered intermediate between Pseudocercospora and Passalora, based on the narrowly obclavate conidia with hila that are somewhat thickened and darkened, but not prominently refractive (Crous et al. 2009g). Phylogenetically, strains of the present species cluster in a well-supported clade (Fig. 1, clade 88; Fig. 4, clade 19) that is closely related to Neomycosphaerella. Since the species Madagascaromyces intermedius is only known from its asexual morph, and the species Neomycosphaerella pseudopentameridis is only known by its sexual morph, a direct comparison between both is not possible. Based on a BLAST comparison against the ITS alignment, Madagascaromyces intermedius CPC 15745 shares 92 % (450/489) similarity, including 2 % (13/489) gaps, with Hyalozasmidium sideroxyli CBS 125011 and 90 % (439/488) similarity, including 3 % (16/488) gaps, with Neomycosphaerella pseudopentameridis CBS 136407. Based on a BLAST comparison against the rpb2 alignment, Mad. intermedius CPC 15745 shares 81 % (571/703) similarity with Pseudopericoniella sp. CBS 330.51 using megablast search, and 82 % (589/722) with Neomycosphaerella pseudopentameridis CBS 136407 using a blastn search. Based on the molecular and morphological differences, we decided to keep the present two taxa in single species genera until more information becomes available.
Clade 89: Neomycosphaerella
Neomycosphaerella Crous, Persoonia 31: 195. 2013.
Description (from Crous et al. 2013b): Foliicolous, phytopathogenic. Ascomata immersed, subepidermal, frequently in a brown stroma, unilocular, in rows of 2–4, globose, with central ostiole; wall of 2–4 layers of brown textura angularis. Asci fasciculate, stipitate, 8-spored, with minute ocular chamber, obovoid, straight to slightly curved, hyaline. Ascospores tri- to multiseriate, hyaline, smooth, granular, medianly 1-septate; ascospores becoming brown and verruculose with age.
Type species: Neomycosphaerella pseudopentameridis Crous.
Neomycosphaerella pseudopentameridis Crous, Persoonia 31: 195. 2013.
Description and illustration: Crous et al. (2013b).
Material examined: South Africa, Western Cape Province, Cape Town, Green Point Park, on leaves of Pseudopentameris macrantha, 22 Jul. 2012, P.W. Crous (holotype CBS H-21416, ex-type cultures CBS 136407 = CPC 21126); idem., culture CPC 21127.
Notes: Neomycosphaerella represents a single-strain lineage in the phylogenetic analyses and is closely related to Brunneosphaerella (Fig. 1, clade 89; Fig. 4, clade 20). Morphologically, Neomycosphaerella is only known by its sexual morph, which is mycosphaerella-like. Brunneosphaerella differs from Neomycosphaerella by producing pigmented ascospores, 3-septate, and with mucoid caps (Crous et al. 2013b).
Clade 90: Mycosphaerelloides
Mycosphaerelloides Videira & Crous, Stud. Mycol. 83: 99. 2016.
Description (from Videira et al. 2016): Leaf spots amphigenous, subcircular, 2–15 mm diam, medium brown, surrounded by a slightly raised, red-purple border. Ascomata pseudothecial, predominantly epiphyllous, single, black, immersed, becoming erumpent, globose, up to 120 μm diam; apical ostiole 10–15 μm diam; wall of 2–3 layers of medium brown textura angularis. Asci aparaphysate, fasciculate, bitunicate, subsessile, obovoid to narrowly ellipsoid, straight or slightly incurved, 8-spored, 30–50 × 8–12 μm. Ascospores 3- to multiseriate, overlapping, hyaline, guttulate, thin-walled, straight to slightly curved, fusoid-ellipsoid with subobtuse ends, apex frequently acutely rounded, medianly 1-septate, widest in the middle of the apical cell, not constricted at the septum, tapering towards both ends, but more prominently towards the lower end, (9–)10–13(–15) × 2.5–3(–3.5) μm in vivo. Mycelium internal and external, consisting of smooth, branched, septate, pale to medium brown, 3–6 μm wide hyphae; external mycelium extensive on abaxial leaf surface. Conidiomata fasciculate, hypophyllous, medium brown, up to 90 μm wide and 150 μm high. Conidiophores arising from superficial mycelium, or aggregated in loose fascicles arising from the upper cells of a brown stroma up to 80 μm wide and 90 μm high; conidiophores pale to medium brown, smooth, unbranched or branched, 1–5-septate, subcylindrical, straight to variously curved, 15–45 × 2.5–4 μm; conidiogenous cells terminal or lateral, unbranched, subcylindrical, pale brown, smooth, proliferating sympodially, or 1–4 times percurrently near apex, 7–15 × 2.5–3 μm; conidiogenous loci inconspicuous. Conidia solitary, pale brown, smooth, subcylindrical, but tapering from a subtruncate base towards a subobtuse apex, 3–6- or multiseptate, 35–85 × 2.5–4 μm, hila neither thickened nor darkened-refractive.
Type species: Mycosphaerelloides madeirae (Crous & Denman) Videira & Crous (≡ Mycosphaerella madeirae).
Mycosphaerelloides madeirae (Crous & Denman) Videira & Crous, Stud. Mycol. 83: 100. 2016.
Basionym: Mycosphaerella madeirae Crous & Denman, Stud. Mycol. 50: 204. 2004.
Synonym: Paramycosphaerella madeirae (Crous & Denman) Guatimosim et al., Persoonia 37: 127. 2016, as ‘madeirensis’.
Description and illustrations: Crous et al. (2004b).
Materials examined: Portugal, Madeira, Party Farm, on leaves of Eucalyptus globulus, Apr. 2000, S. Denman (holotype CBS H-9898, culture ex-type CBS 112895 = CPC 3745 = CMW 14458); idem., culture CBS 112301 = CPC 3747. Netherlands, Utrecht, Soest, endophytic on green leaves of Quercus robur, 2002, G. Verkley, cultures CBS 115936, CBS 116068, CBS 116066.
Notes: Mycosphaerelloides is currently a monotypic genus based on Mycosphaerelloides madeirae, which has a mycosphaerella-like sexual morph and a presumed pseudocercospora-like asexual morph (Crous et al., 2004b, Videira et al., 2016). Phylogenetically, the strains of Mycosphaerelloides madeirae cluster in a well-supported clade (Fig. 1, clade 90; Fig. 4, clade 24) that is closely related to Microcyclosporella. Based on a BLAST comparison against the alignment, Mycosphaerelloides madeirae CBS 112895 shares 96 % (467/485) similarity with Microcyclosporella mali CBS 126136 based on ITS and shares 88 % (594/674) similarity with Epicoleosporium ramularioides CPC 10672 based on rpb2.
Clade 91: Epicoleosporium
Epicoleosporium Videira & Crous, Stud. Mycol. 83: 100. 2016.
Description (from Videira et al. 2016): Colonies growing on uredinia of Coleosporium, mycophylic. Mycelium superficial, consisting of hyaline, septate, thin-walled, smooth hyphae. Conidiophores hyaline, loose, straight, subcylindrical, unbranched, septate, thin-walled, smooth. Conidiogenous cells hyaline, terminal in the conidiophore, cylindrical-oblong, proliferation sympodial, with conspicuous conidiogenous loci, thickened, darkened and refractive. Conidia hyaline, smooth, solitary or in short chains, cylindrical-oblong, clavate, obovate, aseptate, thin-walled, smooth, with hila thickened, darkened and refractive.
Type species: Epicoleosporium ramularioides Videira et al.
Epicoleosporium ramularioides Videira et al., Stud. Mycol. 83: 100. 2016.
Description and illustrations: Videira et al. (2016).
Materials examined: Republic of Korea, Pyeongchang, on Coleosporium phellodendri on leaves of Phellodendron amurense, 4 Sep. 2003, H.D. Shin (holotype KUS F19603, isotype CBS H-22542, culture ex-type CBS 141103 = CPC 10672); idem., culture CPC 10673.
Notes: The genus Epicoleosporium is presently monotypic and is based on Epicoleosporium ramularioides, which has a ramularia-like morphology, but is not congeneric with Ramularia as currently circumscribed (Videira et al. 2016). Based on the phylogenetic analyses in the present study, the representative strains cluster in a well-supported clade (Fig. 1, clade 91; Fig. 4, clade 25) and are closely related to the genus Mycosphaerelloides.
Clade 92: Microcyclosporella
Microcyclosporella J. Frank et al., Persoonia 24: 101. 2010.
Description (from Frank et al. 2010): Hyphomycetous. Mycelium consisting of pale brown, smooth to finely verruculose, branched, septate, 2–3.5 μm wide hyphae, at times covered by a mucoid layer, with integrated, lateral, truncate conidiogenous loci. Conidiophores mostly reduced to conidiogenous cells. Conidiogenous cells integrated, intercalary on hyphae, rarely terminal, cylindrical to doliiform, pale brown, but hyaline if occurring in yeast-like sectors of colonies, smooth, mono- or polyblastic, proliferating sympodially, with inconspicuous, truncate, unthickened, not darkened, pale brown to hyaline loci. Conidia solitary, hyaline, smooth, subcylindrical to narrowly obclavate or narrowly fusoid with acutely rounded apex and obconically truncate base, guttulate, 0–6 times transversely septate; microcyclic conidiation common.
Type species: Microcyclosporella mali J. Frank et al.
Microcyclosporella mali J. Frank et al., Persoonia 24: 101. 2010.
Description and illustration: Frank et al. (2010).
Materials examined: Slovenia, Senozeti, Dolsko, on fruit surface Malus domestica, 7 Aug. 2007, J. Frank (holotype CBS H-20413, culture ex-type 300-07 = CBS 126136 = CPC 16184); Mirna, on M. domestica fruit surface, 17 Oct. 2007, J. Frank, culture 174-07 = CPC 16180 = CBS 126132. USA, Michigan, Fennville, on Malus sp., 1 Sep. 2005, G. Sundin, culture CBS 125653 = RH6 = MI3 20F1a; Ohio, Wooster, on Malus sp., 5 Sep. 2005, M. Ellis, culture CBS 125651 = RH1 = OH1 34D2a.
Notes: The genus Microcyclosporella is presently monotypic and is based on Microcyclosporella mali, a species that is associated with sooty blotch and flyspeck (SBFS) lesions on apples. It has a pseudocercosporella-like morphology but is not congeneric with the type of Pseudocercosporella, Pseudocercosporella bakeri (Frank et al., 2010, Videira et al., 2016). Phylogenetically, the present strains clusters in a well-supported clade (Fig. 1, clade 92; Fig. 4, clade 23) that is closely related to Epicoleosporium ramularioides and Mycosphaerella madeirae.
Clade 93: Virosphaerella
Virosphaerella Videira & Crous, gen. nov. MycoBank MB822705.
Etymology: The prefix virus- (= slime) for the germinating ascospores enveloped in a slime sheath + sphaerella (referring to Mycosphaerella).
Description: Phytopathogenic, producing leaf spots or not. Ascomata amphigenous or epiphyllous, black, subepidermal to erumpent, ovoid, globose or subglobose, apical ostiole, wall consisting of 2–3 layers of medium brown textura angularis. Asci aparaphysate, fasciculate, subsessile, subcylindrical to narrowly obovoid, straight to slightly curved, 8-spored. Ascospores bi- to tri-seriate, overlapping, hyaline, guttulate, thin-walled, straight to slightly curved, fusoid, fusoid-ellipsoidal with obtuse ends, medianly 1-septate or slightly longer in the basal cell, slightly constricted at septum, widest just above the septum, or in the middle of the apical cell, tapering toward both ends, but with more prominent taper towards lower end, mucilaginous sheath visible around spore. Ascospore germination from both ends in two patterns (remaining hyaline): Type I (Crous 1998), growing parallel to the long axis of the spore, with lateral branches parallel or perpendicular to the long axis of spore, irregular in width, constricted at the median septum of the spore, slightly distorting; Type B (Crous 1998), germ tube growing parallel to the long axis of the spore, regular in width, not distorting or becoming constricted at septum. Spermatogonia, when present, amphigenous, dark brown, subepidermal to erumpent, globose to subglobose. Spermatia hyaline, smooth, rod-shaped, with obtuse ends.
Type species: Virosphaerella pseudomarksii (Cheewangkoon et al.) Videira & Crous (≡ Mycosphaerella irregularis Cheewangkoon et al.).
Virosphaerella irregularis (Cheewangkoon et al.) Videira & Crous, comb. nov. MycoBank MB822803.
Basionym: Mycosphaerella irregularis Cheewangkoon et al. (as ‘irregulari’), Persoonia 21: 83. 2008.
Synonyms: Paramycosphaerella irregularis (Cheewangkoon et al.) Guatimosim et al., Persoonia 37: 127. 2016.
Description and illustration: Cheewangkoon et al. (2008).
Materials examined: Thailand, Udonthani, on living leaves of Eucalyptus sp., Jul. 2007, R. Cheewangkoon (holotype CBS H-20135, culture ex-type CBS 123242 = CPC 15408); idem., cultures CPC 15431, CPC 15432.
Notes: Ascospores of Virosphaerella irregularis are similar to Amycosphaerella africana, but differ by producing a mucilaginous sheath around the ascospore and by the irregular germ tubes and germination pattern (Cheewangkoon et al. 2008). Phylogenetically, the present species clusters in a well-supported clade with Virosphaerella pseudomarksii (Fig. 1, clade 93; Fig. 4, clade 26), as previously observed by Cheewangkoon et al. (2008) in a phylogeny based only on LSU sequences. Based on a BLAST against the alignment, Virosphaerella irregularis CBS 123242 shares 95 % (472/495) similarity on ITS, including 1 % (7/495) gaps, and 85 % (623/734) similarity on rpb2, with Virosphaerella pseudomarksii CBS 123241.
Virosphaerella pseudomarksii (Cheewangkoon et al.) Videira & Crous, comb. nov. MycoBank MB822806.
Basionym: Mycosphaerella pseudomarksii Cheewangkoon et al., Persoonia 21: 83. 2008.
Synonym: Paramycosphaerella pseudomarksii (Cheewangkoon et al.) Guatimosim et al., Persoonia 37: 127. 2016.
Description and illustration: Cheewangkoon et al. (2008).
Materials examined: Thailand, Chiang Mai, Mae Tang, on living leaves of Eucalyptus sp., Jun. 2007, R. Cheewangkoon (holotype CBS H-20134, ex-type culture CBS 123241 = CPC 15410); idem., cultures CPC 15435, CPC 15436.
Notes: Virosphaerella pseudomarksii ascospore morphology and ascospore germination patterns are similar to Paramycosphaerella marksii (Carnegie & Keane 1994, as Mycosphaerella marksii) but differ by producing a visible mucilaginous sheath around the ascospore (Cheewangkoon et al. 2008). Phylogenetically, the present species clusters in a well-supported clade based on all three phylogenetic methods employed (Fig. 1, clade 93; Fig. 4, clade 26), and is closely related to Virosphaerella irregularis.
Clade 94: Pseudozasmidium [and Genus A]
Pseudozasmidium Videira & Crous, gen. nov. MycoBank MB822701.
Etymology: Derived from pseudo-, that means resembling but not equalling, and the similar genus, Zasmidium.
Description: Phytopathogenic, causing leaf spots. Pseudothecia amphigenous, aggregated, black, immersed and becoming erumpent, wall of 2–3 layers of medium brown textura angularis. Asci aparaphysate, fasciculate, bitunicate, subsessile, narrowly ellipsoid or obclavate to cylindrical, straight or slightly incurved, 8-spored. Ascospores bi-seriate to triseriate, overlapping, hyaline, straight to slightly curved, ellipsoid or fusoid-ellipsoid, with obtuse ends, medianly 1-septate, not constricted to slightly constricted at the septum, symmetrical cells or widest at the middle of the apical cell, tapering towards both ends or more prominently towards lower end. Ascospore germination parallel to perpendicular to the long axis of the spore. Mycelium internal and external, internal hyphae branched, septate, smooth and hyaline, external hyphae verruculose and pale to medium brown, terminal hyphal ends may develop clusters of globose, multi-celled chlamydospore-like structures. Conidiophores pale to medium brown, smooth to verruculose, erect, subcylindrical, straight or curved, branched or unbranched, repeatedly geniculate, septate, sometimes reduced to conidiogenous cells. Conidiogenous cells terminal, smooth to verruculose, pale brown to brown, proliferating sympodially, sometimes repeatedly geniculate, with conidiogenous loci thickened and darkened-refractive. Conidia single, pale brown to olivaceous brown, smooth to verruculose, obclavate, narrowly obclavate to subcylindrical, obtuse apex and obconically truncate base, straight or curved, 1- to multiseptate, hila thickened and darkened-refractive.
Type species: Pseudozasmidium parkii (Crous & Alfenas) Videira & Crous (≡ Stenella parkii Crous & Alfenas).
Pseudozasmidium eucalypti (Crous & Summerell) Videira & Crous comb. nov. MycoBank MB822783.
Basionym: Stenella eucalypti Crous & Summerell, Fungal Diversity 26: 177. 2007.
Synonym: Zasmidium eucalypti (Crous & Summerell) Crous & U. Braun, Schlechtendalia 20: 101. 2010.
Description and illustrations: Crous et al. (2007c).
Description in vitro (on V8; CPC 13302): Mycelium composed of hyaline to subhyaline hyphae, uniform in width, 2–2.5 μm, smooth. Conidiophores macronematous, first cell arising from hypha (foot cell) hyaline, following cells pale brown to dark brown, paler towards the apex, cylindrical, simple, rarely branched, straight to geniculate, 20–80 × 5–7.5 μm. Conidiogenous cells integrated, terminal, polyblastic, proliferating sympodially, occasionally proliferating percurrently, with rim-like conidiogenous loci, somewhat thickened, darkened and protruding, 2–2.5 μm diam. Conidia solitary, hyaline to pale brown, cylindrical to obclavate, obconical truncate at the base and rounded at the apex, 12.5–120 × 3–5 μm, 0–8-septate, hila thickened and darkened, 2–2.5 μm diam.
Materials examined: Australia, Queensland, Cairns, Eureka Creek, 48 km from Mareeba, S17°11′13.2″, E145°02′27.4″, 468 m, on leaves of Eucalyptus tereticornis, 26 Aug. 2006, P.W. Crous (holotype CBS H-19830, ex-type culture CBS 121101 = CPC 13302).
Notes: The present species was initially described in Stenella (Crous et al. 2007c), but was later reallocated to Zasmidium (Braun et al. 2010a). Its asexual morph is zasmidium-like, with brown and verruculose conidiophores and conidia, with hila thickened, darkened and refractive. However, based on the phylogenetic analysis, it is not part of Zasmidium as circumscribed in the present study, but clusters in a poorly resolved clade (Fig. 1, clade 94; Fig. 4, clade 27) that is closely related to Virosphaerella. All three phylogenetic methods support the smaller clade including Pseudozasmidium vietnamense and Pseudozasmidium parkii, but the support for the species Pseudozasmidium eucalypti and Pseudozasmidium nabiacense is very low. Based on the parsimony analysis, Pseudozasmidium eucalypti and Pseudozasmidium nabiacense form a basal polytomy closely related to Pseudozasmidium vietnamense and Pseudozasmidium parkii. Since their morphology is also zasmidium-like, we decided to retain them in the same genus for now. Pseudozasmidium eucalypti is unique among other Pseudozasmidium species in its ability to produce clusters of globose chlamydospore-like structures, frequently surrounded by a mucus sheath, at the terminal ends of hyphae.
Pseudozasmidium nabiacense (Crous & Carnegie) Videira & Crous, comb. nov. MycoBank MB822784.
Basionym: Zasmidium nabiacense Crous & Carnegie, Persoonia 23: 142. 2009.
Synonym: Paramycosphaerella nabiacensis (Crous & Carnegie) Guatimosim et al., Persoonia 37: 127. 2016.
Description and illustrations: Crous et al. (2009d).
Description in vitro (on V8; CPC 12748): Mycelium composed of hyaline to pale olivaceous brown hyphae, verruculose, uniform in width, 2–3 μm. Conidiophores micro- to macronematous, pale olivaceous brown, verruculose, simple, straight to geniculate, 25–58 × 3–4 μm. Conidiogenous cells integrated, terminal, polyblastic, proliferating sympodially, long-conically truncate at the apex and shoulders, with conidiogenous loci somewhat thickened, darkened and protruding, 1.5–2.5 μm diam. Conidia solitary, pale olivaceous brown, verruculose, straight, cylindrical to obclavate, obconically truncate at the base and rounded at the apex, 18–32 × 3–3.5 μm, 0–3-septate, with hila thickened and darkened, 1.5–2.5 μm diam.
Materials examined: Australia, New South Wales, Nabiac, on leaves of Eucalyptus sp. (red gum), 30 Nov. 2005, A.J. Carnegie (holotype CBS H-20273, cultures ex-type CBS 125010 = CPC 12748–CPC 12750).
Notes: Pseudozasmidium nabiacense is only known from its asexual morph, which is zasmidium-like. The phylogenetic analyses in the present study showed Pseudozasmidium nabiacense clustering in a poorly resolved clade (Fig. 1, clade 94; Fig. 4, clade 27) that is closely related to Pseudozasmidium parkii, which agrees with the findings of Crous et al. (2009d). See notes on Pseudozasmidium eucalypti.
Pseudozasmidium parkii (Crous & Alfenas) Videira & Crous, comb. nov. MycoBank MB822785.
Basionym: Stenella parkii Crous & Alfenas, Mycologia 87: 121. 1995.
Synonyms: Zasmidium parkii (Crous & Alfenas) Crous & U. Braun, Schlechtendalia 20: 102. 2010.
Paramycosphaerella parkii (Crous & Alfenas) Guatimosim et al., Persoonia 37: 127. 2016.
Mycosphaerella parkii Crous et al., Mycol. Res. 97: 582. 1993.
Description and illustrations: Crous et al., 1993, Crous and Alfenas, 1995.
Materials examined: Brazil, Aracruz, Florestal nursery, on living leaves of Eucalyptus grandis, 24 Feb. 1990, M.J. Wingfield (holotype of Mycosphaerella parkii, PREM 50668, ex-type culture CBS 387.92); Rio Grande do Sul, on Eucalyptus globulus, 7 Jul. 1993, F.A. Ferreira, PREM 51714, culture CPC 651; São Paulo, on Eucalyptus saligna, Apr. 1993, P.W. Crous (holotype of Stenella parkii, PREM 51713). Indonesia, North of Sumatra, on E. grandis, 22 Nov. 1993, F.A. Alfenas, PREM 51715.
Notes: Pseudozasmidium parkii produces a mycosphaerella-like sexual morph and a zasmidium-like asexual morph, with verruculose hyphae, conidiophores and conidia verruculose and conidiogenous cells with conspicuous, darkened and refractive conidiogenous loci (Crous et al., 1993, Crous and Alfenas, 1995). Based on the phylogenetic analyses, however, Pseudozasmidium clusters apart from the Zasmidium clade, as presently defined by the type species Z. cellare, in a poorly resolved clade including Pseudozasmidium vietnamense, Pseudozasmidium nabiacense and Pseudozasmidium eucalypti (Fig. 1, clade 94; Fig. 4, clade 27). Based on a BLAST search against the alignment, Pseudozasmidium parkii CBS 387.92 shares 99 % (479/485) similarity on ITS with Pseudozasmidium vietnamense CBS 119974. Unfortunately, the rpb2 sequence of Pseudozasmidium parkii failed to amplify and is coded as missing data in the alignments, but the next closest strain on ITS is Virosphaerella irregularis CBS with only 92 % (455/496) similarity and including 3 % (17/496) gaps. See also notes on Pseudozasmidium vietnamense and Paramycosphaerella brachystegiae.
Pseudozasmidium vietnamense (Barber & T.I. Burgess) Videira & Crous, comb. nov. MycoBank MB822786.
Basionym: Mycosphaerella vietnamensis Barber & T. I. Burgess, Fungal Diversity 24: 148. 2007.
Synonym: Paramycosphaerella vietnamensis (Barber & T.I. Burgess) Guatimosim et al., Persoonia 37: 128. 2016.
Description and illustration: Burgess et al. (2007).
Material examined: Vietnam, South East Forestry Institute nursery, on leaves of Eucalyptus grandis hybrid, 6 Jul. 2004, coll. T.I. Burgess, isol. P.A. Barber (holotype MURU 411, ex-type culture CBS 119974 = CMW 23441 = MUCC 66 = VTN1).
Notes: Pseudozasmidium vietnamense was described based on the mycosphaerella-like sexual morph and a presumed pseudocercospora-like asexual morph (Burgess et al. 2007). In previous phylogenetic studies, it always clustered close to Pseudozasmidium parkii (as Mycosphaerella parkii, Burgess et al., 2007, Crous et al., 2009d). The phylogenetic analyses in the present study agrees with the previous works and this species clusters in a poorly resolved clade of zasmidium-like species (Fig. 1, clade 94; Fig. 4, clade 27). Therefore, the presumed pseudocercospora-like asexual morph should not be considered correct. See also notes on Pseudozasmidium parkii and Paramycosphaerella brachystegiae.
Genus A
Passalora vaginae (W. Krüger) U. Braun & Crous, in Crous & Braun, CBS Biodiversity Ser.: 417. 2003.
Basionym: Cercospora vaginae W. Krüger, Ber. Versuchsstat. Zuckerrohr W.-Java, Kagok-Tegal 1: 64. 1890.
Synonyms: Mycovellosiella vaginae (W. Krüger) Deighton, Mycol. Pap. 144: 26. 1979.
Passalora vaginae (W. Krüger) U. Braun & Crous, in Crous & Braun, CBS Biodiversity Ser.: 417. 2003.
Description in vivo (from Braun 2015): Spots mainly on sheaths, sometimes also formed as leaf spots, at first small, subcircular to elliptical, red, margin conspicuous, spots later confluent or increasing to about 15 mm diam, on leaves dark reddish above, indistinct below. Caespituli amphigenous, effuse, dark greyish brown, velvety, mostly in the centre of the lesion. Mycelium internal and external; superficial hyphae sparingly branched, septate, pale, thin-walled, smooth. Stromata sometimes well-developed, substomatal, 10–75 μm diam, dark brown, but without conidiophore fascicles. Conidiophores solitary, arising from superficial hyphae, lateral, at the top of mother cells, occasionally terminal, i.e. at the end of procumbent hyphae, erect to ascending, straight to curved, subcylindrical, conical to geniculate-sinuous, simple or sometimes branched, occasionally entangled, 20–200 × 3–5 μm, 1–5-septate, pale olivaceous brown to darker brown, paler towards the tip, thin-walled, smooth; conidiogenous cells integrated, terminal, with conspicuous conidiogenous loci, about 1–1.5 μm diam. Conidia solitary, cylindrical or obclavate-cylindrical, straight to somewhat curved, 15–55 × 3–6.5 μm, 0–5-septate, occasionally slightly constricted at the septa, hyaline to olivaceous, thin-walled, smooth, apex obtuse, base short obconically truncate, 1–2 μm wide, somewhat thickened and darkened.
Materials examined: Taiwan, on Saccharum officinarum, unknown collector and date, dep. T. Miyake, 1934, culture CBS 140.34 = DSM 1148 = IMI 303641.
Notes: Passalora vaginae causes a foliar disease of sugarcane (Sacharum officinarum) and sorghum (Sorghum vulgare) (Poaceae) and has a worldwide distribution (Crous & Braun 2003). The strain is presently sterile but clusters as a single-strain lineage in the phylogenetic analysis (Fig. 4, clade 28) that represents a potential new genus. The holotype specimen, on Saccharum officinarum, which originates from Java, Indonesia, could not be located, and presently no suitable specimen is available for neotypification (Braun et al. 2015). Therefore, the proposal of a new genus is postponed until suitable material is collected and examined.
CLADES 95–96: Dissoconiaceae
Dissoconiaceae Crous & de Hoog, Stud. Mycol. 64: 36. 2009.
Clade 95: Ramichloridium
Ramichloridium Stahel ex de Hoog, Stud. Mycol.15: 59. 1977.
Note: See Arzanlou et al. (2007).
Clade 96: Uwebraunia
Uwebraunia Crous & M.J. Wingf., Mycologia 88: 446. 1996.
Note: See Crous and Wingfield, 1996, Crous et al., 1999 and Li et al. (2012).
CLADES 97–100: Phaeothecoidiellaceae
Phaeothecoidiellaceae K.D. Hyde & Hongsanan, Mycosphere 8: 140. 2017.
Clade 97: Exopassalora
Exopassalora Videira & Crous, gen. nov. MycoBank MB822589.
Etymology: Exo- meaning outside, as in outside the family Mycosphaerellaceae, where the genus Passalora is included.
Description: Foliicolous, phytopathogenic. Mycelium composed of brown hyphae, smooth to rough, irregularly branched, septate, with dark brown chlamydospore-like hyphal swellings. Conidiophores arising from the mycelium, medium brown, smooth, simple or branched, straight to curved. Conidiogenous cells terminal and intercalary, subcylindrical, pale to medium brown, smooth, proliferating sympodially, conidiogenous loci conspicuous, darkened, refractive. Conidia catenate, in simple or branched chains, medium brown, smooth, narrowly ellipsoidal, tapering to subtruncate, straight or slightly curved, hila slightly thickened and darkened.
Type species: Exopassalora zambiae (Crous & T.A. Cout.) Videira & Crous.
Exopassalora zambiae (Crous & T.A. Cout.) Videira & Crous, comb. nov. MycoBank MB822757.
Basionym: Passalora zambiae Crous & T.A. Cout., Stud. Mycol. 50: 209. 2004.
Description and illustration: Crous et al. (2004b).
Material examined: Zambia, on leaves of Eucalyptus globulus, 21 Aug. 1995, T. Coutinho (holotype CBS H-9895, culture ex-type CBS 112971 = CMW 14782 = CPC 1227); idem., cultures CBS 112970 = CPC 1228).
Notes: This species is phylogenetically distant from other Mycosphaerella spp. known from Eucalyptus (Crous et al. 2004b) and clusters in a well-supported clade (Fig. 1, clade 96; Fig. 4, clade 32) within the recently introduced Phaeothecoidiellaceae family (Hongsanan et al., 2017).
Exopassalora sp.
Material examined: USA, Illinois, Chester, on apple fruits, culture CBS 118964 = GTF1a.
Notes: Based on the phylogenetic analyses this strain is closest to Exopassalora (Fig. 1, clade 96; Fig. 4, clade 32). The present strain shares 97 % (711/736) similarity on LSU, 88 % (288/326) similarity on ITS and 77 % (537/701) similarity on rpb2 with Exopassalora zambiae. Based on the differences observed between the sequences of the partial genes studied, this can be a new genus. Morphological characters from this strain include mycelium on PDA blackish, brown and convoluted, conidia on CLA dark, catenate, with flattened ends (Batzer et al. 2005). Unfortunately, the culture is presently sterile and is tentatively placed in Exopassalora until the morphological characters can be observed and properly described.
Clade 98: Houjia
Houjia G.Y. Sun & Crous, Persoonia 24: 33. 2010.
Note: See Yang et al. (2010).
Clade 99: Sporidesmajora
Sporidesmajora Batzer & Crous, Persoonia 24: 35. 2010.
Note: See Yang et al. (2010).
Clade 100: Phaeothecoidiella
Phaeothecoidiella Batzer & Crous, Persoonia 24: 30. 2010.
Note: See Yang et al. (2010).
CLADE 101: Schizothyriaceae
Schizothyriaceae Höhn. ex Trotter, Sacc., D. Sacc. & Traverso as “Schizothyrieae”, in Saccardo, Syll. fung. (Abellini) 24(2): 1254. 1928.
Synonym: Schizothyrieen Höhn., Ber. Deutsch. Bot. Ges. 35: 417. 1917, nom. inval. (Art. 32.1(b), Art. 18.4).
Clade 101: Schizothyrium
Schizothyrium Desm., Ann. Sci. Nat., Bot., Sér. 3, 11: 360. 1849.
Note: See Batzer et al., 2008, Schoch et al., 2009 and Crous et al. (2009c).
CLADES 102–107: Teratosphaeriaceae
Teratosphaeriaceae Crous & U. Braun, Stud. Mycol. 58: 8. 2007.
Clade 102: Teratosphaeria
Teratosphaeria Syd. & P. Syd., Ann. Mycol. 10: 39. 1912.
Note: See Crous et al. (2009d) and Quaedvlieg et al. (2014).
Clade 103: Batcheloromyces
Batcheloromyces Marasas, P.S. van Wyk & Knox-Dav., S. African J. Bot. 41(1): 41. 1975.
Note: See Crous et al., 2007a, Crous et al., 2008.
Clade 104: Readeriella
Readeriella Syd. & P. Syd., Ann. Mycol. 6: 484. 1908.
Note: See Crous et al. (2009d).
Clade 105: Stenella
Stenella Syd., Ann. Mycol. 28(1–2): 205. 1930.
Note: See Quaedvlieg et al. (2014).
Clade 106: Parapenidiella
Parapenidiella Crous & Summerell, Persoonia 29: 185. 2012.
Note: See Crous et al. (2012a).
Clade 107: Acrodontium
Acrodontium de Hoog, Stud. Mycol. 1: 23. 1972.
Note: See Videira et al. (2016).
CLADE 108: Cladosporiaceae
Cladosporiaceae Castell. & R.G. Archibald, Yearbook of Tropical Medicine and Hygiene: 25. 1915.
Synonyms: Cladosporieae Mathieu, Flore Générale de Belgique: 2. 1854.
Cladosporieae Sacc., Sylloge Fungorum 4: 341. 1886.
Cladosporiaceae Nann., Repertorio sistematico dei miceti dell' uomo e degli animali 4: 404. 1934.
Clade 108: Cladosporium
Cladosporium Link, Mag. Ges. Naturf. Freunde Berlin 7: 37. 1816 [‘1815’].
Note: See Bensch et al. (2015).
Genera of Mycosphaerellaceae
Acervuloseptoria Crous & Jol. Roux, Persoonia 32: 275. 2014.
Note: See treatment in text.
Acrodesmis Syd., Ann. Mycol. 24(5–6): 424. 1926.
Description (adapted from Sydow 1926 and Ellis 1961): Mycelium composed of pale brown to olivaceous brown hyphae, branching and anastomosing, smooth, septate. Stromata composed of dense and irregular dark brown hyphal cells, semiglobose. Conidiophores single or in group, emerging from stromata or from hyphae, erect, straight or flexuous, cylindrical, septate, dark brown, paler towards the tips, densely branched at the apex. Conidiogenous cells terminal, hyaline to pale olivaceous brown, polyblastic, with multiple conidiogenous cells. Conidia single or in short chains, sometimes branched chains, acropleurogenous, pale olivaceous brown, smooth, cylindrical, elliptical or fusiform, aseptate, with minute hila at the base.
Type species: Acrodesmis cestri Syd. [Costa Rica, La Caja, pr. San. Jose, on leaves of Cestrum macrophyllum, 13 Feb. l925, H. Sydow, Fungi Exot. Exs. 650 (syntypes S F12601, S F189761)].
Description and illustration: Ellis, 1967, Ellis, 1971, as Periconiella cestri).
Notes: Unconfirmed synonym of Periconiella. Two species, Acrodesmis cestri and Acrodesmis secunda. No cultures available, and its phylogenetic position remains unresolved.
Acrocladium Petr., Sydowia 3(1–6): 263. 1949.
Description (adapted from Petrak 1949): Mycelium superficial, composed of olivaceous brown hyphae, branched, septate. Conidiophores sparse, brown, long, erect, densely branched at the apex (diverging as in penicillium-like species). Conidia greyish to olivaceous brown, aseptate, acrosporogenous, oblong to ellipsoid.
Type species: Acrocladium andinum Petr.
Description and illustration: Petrak (1949).
Notes: Unconfirmed synonym of Periconiella. Two species, Acrocladium andinum and Acrocladium fragile. No cultures available, and its phylogenetic position remains unresolved.
Achorodothis Syd., Ann. Mycol. 24: 380. 1926.
Description (adapted from Sydow 1926): Stromata mainly intraepidermal, pseudoparenchymatous, dark brown, forming continuous to loose crusts with loculi. Asci sparingly developed, clavate to almost ellipsoid, sessile or with short knob-like stalk, wall firm, apically thickened, 8-spored, immersed in a hyaline, viscous, little differentiated to slightly filamentous, paraphysoid mass. Ascospores 2- to 3-seriate, hyaline, ellipsoid-ovoid, straight or rarely slightly asymmetric, aseptate, slightly attenuated towards the base, both ends rounded, at the base with a colourless bluntly conoid to capped appendage.
Type species: Achorodothis poasensis Syd. [Costa Rica, on Ocotea mollicella (≡ Phoebe mollicella), 15 Jan. 1925 (syntype IMI 18604)].
Description (no illustration): Sydow (1926).
Note: Achorodothis is not known from culture, and its phylogenetic position remains unresolved.
Acrotheca Fuckel, Jahrb. Nassauischen Vereins Naturk.15: 42. 1860.
Type species: Acrotheca gei Fuckel [Austria, Rhenogovia, on Geum urbanum, Fuckel, Fungi Rhen. Exs. 2229, e.g. HAL] = Ramularia gei (A.G. Eliasson) Lindr.
Description and illustration: Hughes, 1951, Braun, 1998, as Ramularia gei).
Note: Acrotheca gei is presently regarded as a species of Ramularia, but this conclusion has not been confirmed based on DNA data.
Allantophomoides S.L. Wei & T.Y. Zhang, Mycosystema 22: 9. 2003.
Description (adapted from Wei & Zhang 2003): Conidiomata pycnidial, immersed, globose to subglobose, unilocular, sometimes slightly papillate, thin-walled, wall composed by 1-3 cells with pale brown to brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells ampulliform to doliiform, hyaline, smooth, covering the entire inside wall, enteroblastic, phialidic with minute collarette. Conidia hyaline, guttulate, allantoid to elongate-ellipsoidal, aseptate or septate.
Type species: Allantophomoides carotae S.L. Wei & T.Y. Zhang [China, Gansu Province, Zhangye, on Daucus carota var. sativa, 10 Oct. 1995 (holotype HSAUP 960001, isotype IMI)].
Description and illustration: Wei & Zhang (2003).
Notes: The most closely related genera are Phoma, Coleophoma and Allantophomopsis. Without molecular data the phylogenetic position of this septoria-like genus remains unresolved.
Amycosphaerella Quaedvlieg & Crous, Persoonia 33: 22. 2014.
Note: See treatment in text.
Anematidium Gronchi, Boll. Ist. Sieroterap. Milan. 10: 242. 1931.
Description (adapted from Gronchi 1931): Mycelium olivaceous, septate, branching. Conidiophores absent. Conidia catenate, in branched chains, integrated in the mycelium, cylindrical. In solid agar media, colonies olivaceous, coalescent, round, convex, with wrinkled and irregular surface, margin dark. Hyphae densely aggregated, olivaceous, branched and septate. In liquid acidic media, hyphae within the liquid media, lax, with very long branches, septate, subhyaline to olivaceous. Conidia catenate, in branched chains, integrated in the mycelium, cylindrical, branching.
Type species: Anematidium oxiphilum Gronchi [Italy, Firenze, growing in a N/10 HCL solution in a laboratory].
Description and illustration: Gronchi (1931).
Notes: This genus is insufficiently known, and its status remains unresolved. The author named the genus Anematidium after the absence of conidiophores and the type species Anematidium oxiphilum after the fungus affinity to the acidic substrate from which it was isolated, a laboratory solution of N/10 HCL (Gronchi 1931).
Anguillosporella U. Braun, A monograph of Cercosporella, Ramularia and allied genera (Phytopathogenic Hyphomycetes) 1: 233. 1995.
Description (adapted from Braun 1995): Mycelium internal, composed of hyaline hyphae, septate and branched. Stromata subcuticular to intraepidermal, often erumpent. Conidiophores hyaline, smooth, arising from stromata, macronematous, single or in fascicles, loose or densely aggregated, simple, continuous or septate, straight, subcylindrical to flexuous. Conidiogenous cells integrated, terminal, monoblastic, determinate, with conidiogenous loci (scars) more or less truncate, unthickened and not darkened, conidial secession schizolytic. Conidia solitary, hyaline, multi-euseptate, scolecosporous, wih apex subacute and base usually with a short appendage.
Type species: Anguillosporella vermiformis (Davis) U. Braun [USA, Wisconsin, on Alnus incana (lectotype BPI 442755, see Braun 1995)].
Descriptions and illustrations: Braun, 1995, Seifert et al., 2011.
Note: The phylogenetic position of Anguillosporella remains unresolved.
Annellophora S. Hughes, Trans. Brit. Mycol. Soc. 34: 544. 1952.
Description (adapted from Ellis 1971): Mycelium superficial or immersed, composed of subhyaline, brown or olivaceous brown hyphae. Conidiophores macronematous, single or in fascicles, brown or dark brown, simple, septate. Conidiogenous cells integrated, terminal, monoblastic, proliferating percurrently. Primary conidia terminal, cylindrical, obclavate or fusiform, subhyaline to brown, smooth, transversely septate or pseudoseptate. Secondary conidia germinating from the apex of primary conidia, one at a time, proliferating percurrently, smaller.
Type species: Annellophora solani (Syd.) S. Hughes (≡ Chaetotrichum solani Syd. 1927).
Description and illustration: Ellis, 1971, Seifert et al., 2011; present study (Fig. 48).
Fig. 48.
Annelophora solani (E00417817). A–F. Observations in vivo. A. Symptoms on host. B, C. Conidiophores erect and developing on the host surface. D. Attachment of the conidiophore to a leaf trichome. E. Conidiogenous cell and conidium. F. Conidia. Scale bars = 10 μm.
Material examined: Costa Rica, Los Angeles de San Ramon, on Solanum erythrotrichum, 30 Jan. 1925 (holotype of Chaetotrichum solani, E 00417817).
Notes: The phylogenetic position of Annellophora is unknown, and its 11 species are only known by their hyphomycetous sporidesmium-like asexual morph (Seifert et al. 2011). Cultures and sequence data are necessary to determine its phylogenetic position.
Annellophragmia Subram., Proc. Indian Acad. Sci., Sect. B, 58: 349. 1963.
Description (adapted from Ellis 1971): Mycelium superficial and immersed. Stroma erumpent, brown and pseudoparenchymatous. Conidiophores macronematous, synnematous, brown, smooth, straight, with each individual stipe unbranched, gathered tightly for most of the length and spreading like a hand fan at the apex. Conidiogenous cells integrated, terminal and intercalary, cylindrical, proliferating sympodially, polyblastic, conidiogenous loci (scars) large, apical and lateral. Conidia solitary, acropleurogenous, pale to dark brown or golden brown, smooth, fusiform to obclavate, truncate at the base, pseudoseptate.
Type species: Annellophragmia coonoorensis (Subram.) Subram. (≡ Arthrobotryum coonoorense Subram.).
Descriptions and illustrations: Ellis, 1971, Seifert et al., 2011; present study (Fig. 49).
Fig. 49.
Annelophragmia coonoorensis (IMI 245197). A–F. Observations in vivo. A. Conidiophores in compact fascicles, erect and emerging from the host. B. Apical area of the conidiophores, with conidiogenous cells and conidia. C, D. Conidiogenous cells and conidia. E, F. Conidiogenous cell and conidium. Scale bars = 10 μm.
Materials examined: India, Madras, Nilgiris, Coonoor, Simm, on leaves of Thysanolaena maxima, 8 Dec. 1953, T.S.S. & C.V. Subramanian (holotype of Arthrobotryum coonoorense (K(M) 180920); Madhya Pradesh, Balaghat, on Thysanolaena maxima, Jan. 1980, S.M. Singh, IMI 245197.
Notes: The phylogenetic position of Annelophragmia is unknown and the genus is only known from its hyphomycetous type species, Annelophragmia coonoorensis (Kirk et al. 2013; genus accepted). Sequence data are necessary to determine its phylogenetic position.
Annellosympodia McTaggart et al., Australas. Pl. Path. 36: 574. 2007.
Description (adapted from McTaggart et al. 2007): Mycelium immersed. Conidiophores reduced to conidiogenous cells. Conidiogenous cells on minute pulvinate sporodochia, macronematous, dark brown, aseptate, verrucose, thick-walled, ampulliform, doliiform or obovoid, mono- or polyblastic, proliferating sympodially (rectilinear), conidiogenous loci ring-like with a central pore, slightly thickened and darkened, apical at first and later displaced laterally. Conidia solitary, brown, coarsely verrucose, cylindrical to ellipsoidal, apex rounded, base truncate with a marginal frill and a dark conspicuous hilum, aseptate or septate, sometimes constricted at the septum; secession rhexolytic.
Type species: Annellosympodia orbiculata McTaggart et al. [Australia, Western Australia, on phyllodes of Acacia sp. (holotype PERTH 03270173)].
Description and illustration: McTaggart et al. (2007).
Note: Annellosympodia is not known from culture, and hence its phylogenetic position remains unresolved.
Annellosympodiella Crous & Assefa
Note: See treatment in text.
Apseudocercosporella Videira & Crous
Note: See treatment in text.
Asperisporium Maubl.
Note: See treatment in text.
Asteromidium Speg., Ann. Soc. Cient. Argent. 26(1): 66. 1888.
Description (from Quaedvlieg et al. 2013, adapted from Sutton 1980): Mycelium immersed, branched, septate, hyaline. Conidiomata acervular, subcuticular, separate or confluent, pulvinate to doliiform, at the base, composed of hyaline to pale brown, thin-walled textura angularis which extends laterally, finally with separate cells dispersed in a mucilaginous matrix to form the overlaying wall; cuticle discoloured and occasionally pseudoparenchymatous, walls adjacent to the upper epidermal wall also discoloured; dehiscence irregular. Conidiogenous cells holoblastic, discrete, indeterminate, ± cylindrical, hyaline, smooth, with 1–2 sympodial proliferations, scars unthickened, flat, formed from the basal and lateral walls. Conidia cylindrical to fusoid, gently tapered at each end, apex obtuse, base truncate, thin-walled, guttulate to granular, hyaline, 3-septate.
Type species: Asteromidium imperspicuum Speg. [Paraguay, on leaves of Sapindaceae, 1883, ex B. Balansa Pl. du Paraguay No. 4085 (syntype K(M) 180228)].
Description and illustration: Quaedvlieg et al. (2013).
Note: See Quaedvlieg et al. (2013).
Berteromyces Cif., Sydowia 8: 267. 1954.
Description (from Ciferri 1954): Biotrophic, external mycelium lacking, internal mycelium with branched hyphae, hyaline, sparingly developed. Conidiophores hyaline or subhyaline, erumpent, with a dense basal stroma, fasciculate, unbranched, erect, distinct. Conidia apical, solitary, hyaline, ovoid, at first continuous, later 1-septate.
Type species: Berteromyces aeneus Cif. [Uganda, Kawanda, on Senna bicapsularis (≡ Cassia bicapsularis), Jul. 1940, Hansford 2751 (neotype designated by Crous & Braun (2003), IMI 8180)] ≡ Passalora aenea (Cif.) U. Braun & Crous.
Description and illustrations: Ciferri, 1954, Deighton, 1967, as Cercosporidium cassiae).
Notes: This genus is seen as part of the Passalora complex, with its type species treated as Passalora aenea (Cif.) U. Braun & Crous. The neotype was selected by Crous & Braun (2003) but as no material is available from which DNA can be extracted, its phylogenetic position remains unresolved.
Australosphaerella Videira & Crous
Note: See treatment in text.
Biharia Thirum. & Mishra, Sydowia 7: 79. 1953.
Description (adapted from Thirumalachar & Mishra 1953): Mycelium yellowish brown, emerging through stoma and developing a stroma, from which conidiophores arise. Conidiophores yellowish brown, smooth, septate, geniculate. Conidiogenous cell terminal, polyblastic, proliferating sympodially. Conidia single, yellowish brown, obclavate or cylindrical, echinulate or rugose, septate, simple or with protrusions at the region of septa.
Type species: Biharia vangueriae Thirum. & Mishra [India, Bihar, on Vangueria spinosa (lectotype designated here IMI 51482, MBT378592)].
Description and illustration: Thirumalachar & Mishra (1953).
Notes: The type species was combined into Stenella by Deighton (1979) and later into Zasmidium by Kamal (2010). It is regarded as part of the Zasmidium complex until sequence data of its type species is available and its phylogenetic position is resolved.
Brunneosphaerella Crous
Note: See treatment in text.
Bryopelta Döbbeler & Poelt, in Döbbeler, Mitt. Bot. Staatssamml. München 14: 126. 1978.
Description (adapted from Döbbeler 1978 and Li et al. 2014): Mycelium composed of hyaline hyphae, septate, branched within the host cells. Ascomata solitary, glabrous, semi-immersed or immersed, globose to subglobose, black, thick-walled, ostiole central, papillate, filled with hyaline to dark brown periphyses. Peridium composed of thick-walled hyaline to dark brown cells of textura angularis to textura porrecta. Hamathecium composed of dense, filamentous, hyaline, septate, unbranched, anastomosing pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindrical to fusiform, obtuse at the tip, slightly widened at base or sometimes with short pedicel, slightly curved. Ascospores multi-seriate, crowded, ellipsoidal, generally 1-septate, asymmetrical, sometimes 1–3-septate, constricted at septa, with a smooth or rough epispore. Mycelium producing black synnemata, with conidiophores directly arising from the basal layers, brown. Conidia hyaline, narrow ellipsoid.
Type species: Bryopelta variabilis Döbbeler & Poelt [Sweden, on Mylia anomala (holotype GZU 000302175)].
Description and illustration: Li et al. (2014).
Notes: The taxonomic history of Bryopelta has been discussed in detail by Li et al. (2014). Bryopelta variabilis is a lichenicolous species with uncertain phylogenetic position due to the lack of sequence data.
Camptomeris Syd., Ann. Mycol. 25: 14. 1927.
Description (adapted from Ellis 1971): Sporodochia mostly hypophyllous, pulvinate, punctiform, dark olivaceous brown to black. Mycelium immersed. Stroma present with one or several swollen cells bearing conidiophores. Conidiophores macronematous, often curved inwards, simple, smooth, pale brown to brown. Conidiogenous cells integrated, terminal, cylindrical, proliferating sympodially, polyblastic, with prominent conidiogenous loci (scars). Conidia solitary, acropleurogenous, pale olivaceous brown or brown, usually verruculose but sometimes smooth, obclavate or oblong, rounded at the ends, aseptate or septate.
Type species: Camptomeris calliandrae Syd. [Costa Rica, on leaves of Calliandra houstoniana var. calothyrsus (= Calliandra similis), 30 Dec. 1924 (slide ex type IMI 7687; fide Hughes 1952)].
Descriptions and illustrations: Ellis, 1971, Seifert et al., 2011; present study (Fig. 50).
Fig. 50.
Camptomeris leucaenae (CBS H-22884). A–F. Observations in vivo. A, B. Leaf spot symptoms on the host. C. Conidiophores emerging from the leaf host with conidiogenous cells. D. Apex of the conidiogenous cells with the conidiogenous scar. E. Conidiogenous cells and conidia. F. Conidia. Scale bars = 10 μm.
Notes: The phylogenetic position of the genus Camptomeris is currently undetermined due to the lack of DNA sequence data from its type species. The cercosporoid nature of the type species suggests an affinity to Mycosphaerellaceae. No species of this genus are presently known from culture.
Camptomeriphila Crous & M.J. Wingf., Persoonia 37: 335. 2016.
Description (from Crous et al. 2016a): Mycelium consisting of branched, septate, smooth, pale brown hyphae, forming thick-walled, brown, verruculose, intercalary chlamydospores. Conidiophores in loose fascicles, erect, branched, flexuous, multiseptate, pale brown, smooth. Conidiogenous cells integrated, terminal and lateral, subcylindrical, pale brown, smooth; scars thickened, darkened, refractive. Conidia solitary, fusoid-ellipsoid, becoming obclavate when mature, subhyaline to pale brown, smooth, apex subobtuse, hilum protruding, truncate, thickened, darkened, refractive.
Type species: Camptomeriphila leucaenae Crous & M.J. Wingf.
Description and illustration: Crous et al. (2016a).
Materials examined: Malaysia, Sabah, growing on Camptomeris leucaenae, on leaves of Leucaena leucocephala, 29 May 2015, M.J. Wingfield (holotype CBS H-22884, culture ex-type CBS 142135 = CPC 27608).
Notes: The present species was observed growing in close association with sporodochia of Camptomeris leucaenae (Fig. 50), which causes a leaf spot disease on Leucaena leucocephala. Morphologically, it is a passalora-like mycophylic fungus, phylogenetically, it is closely related to species of Dothistroma or Pseudophaeophleospora, based on LSU (Crous et al. 2016a). This strain was not included in the present study.
Caryophylloseptoria Verkley, Quaedvlieg & Crous
Note: See treatment in text.
Catenulocercospora C. Nakash., Videira & Crous
Note: See treatment in text.
Ceratosperma Speg., Physis (Buenos Aires) 4(17): 284. 1918.
Description (adapted from Saccardo & Trotter 1913): Ascomata pseudothecial, globose. Asci subglobose, 8-spored, stipitate, aparaphysate. Ascospores oblong, 2–6-septate, constricted at septa, hyaline to olivaceous, smooth.
Type species: Ceratosperma theobromae (Faber) Speg. (≡ Ceratocarpia theobromae Faber) [Cameroon, on Theobroma cacao].
Notes: Very little is known from this genus besides its description. Authentic specimens could not be located and no illustration or recent publication are known. The species needs to be recollected to resolve its phylogenetic position.
Cercocladospora G.P. Agarwal & S.M. Singh, Proc. Natn. Acad. Sci. India, Sect. B, Biol. Sci. 42(4): 439. 1974.
Type species: Cercocladospora adinae G.P. Agarwal & S.M. Singh [India, on leaves of Haldina cordifolia (≡ Adina cordifolia) (IMI 148087), fide Deighton 1976a] = Pseudocercospora adinicola (A.K. Kar & M. Mandal) Deighton.
Note: Both the generic name, Cercocladospora, and the name of the type species, Cercocladospora adinae, were not validly published (Art. 40.1, Art. 40.3, Art. 39.1, Melbourne). Since it was morphologicaly identical to Cercospora adinicola, Deighton (1976a) synonymised both under Pseudocercospora adinicola using the validly published name Cercospora adinicola as the basionym. Although Cercocladospora is treated as a synonym of Pseudocercospora, this conclusion has not been confirmed based on DNA data.
Cercodeuterospora Curzi, Boll. Staz. Patol. Veg. Roma, Ser. 2, 12: 149. 1932.
Type species: Cercodeuterospora trichophila Curzi [Somalia, on Cajanus indicus] = Mycovellosiella cajani (Henn.) Rangel ex Trotter.
Notes: Although no culture is available, Cercodeuterospora is regard as a synonym of Mycovellosiella cajani based on morphology. The latter species also occurs on Cajanus spp. in Africa. Deighton (1974) did not observe the type material when he proposed the combination Mycovellosiella cajani var. trichophila for Cercodeuterospora trichophila, but a specimen from Kenya (IMI 68281) which he deemed very similar to the material illustrated and described by Curzi. We were unable to trace the location of the Curzi specimen.
Cercoramularia Videira, H.D. Shin, C. Nakash. & Crous
Note: See treatment in text.
Cercoseptoria Petr., Ann. Mycol. 23: 69.1925.
Type species: Cercoseptoria chamaesyces (F. Stevens & Dalbey) Petr. (= Septoriopsis chamaesyces F. Stevens & Dalbey) [Puerto Rico, Rio Piedras, on Chamaesyce hypericifolia, F.L. Stevens No. 9445 (holotype ILL00011697)] = Pseudocercospora chamaesyces (F. Stevens & Dalbey) Deighton.
Description and illustration: Stevens & Dalbey (1919, as Septoriopsis chamaesyces); Deighton (1976a, as Cercoseptoria chamaesyces).
Note: Although Cercoseptoria is treated as a synonym of Pseudocercospora, this conclusion has not been confirmed based on DNA data.
Cercosphaerella Kleb., Haupt- und Nebenfruchtformen der Askomyzeten: 132. 1918.
Description (based on Klebahn 1918): “Cercosphaerella. Konidienform Cercospora. Arten: C. millegrana; cerasella” [Cercosphaerella. Conidial form Cercospora. Species: C. millegrana; cerasella].
Type species: Cercosphaerella millegrana (Cooke) Kleb. [Austria, on leaf litter of Carpinus betulus (holotype K(M) 56297)].
Description: Saccardo (1882, as Sphaerella millegrana), Klebahn (1918).
Notes: Klebahn (1918) introduced Cercosphaerella as new genus for Mycosphaerella species with asexual morphs belonging to Cercospora s. lat. and linked Cercospora microsora (≡ Passalora microsora) to Mycosphaerella millegrana, although Sydow (1940) disagreed, and described the sexual morph of Passalora microsora as Mycosphaerella microsora. The name Sphaerella millegrana, based on a Mycosphaerella on leaf litter of Carpinus betulus, was misapplied in Klebahn (1918). Klebahn (1918: 132) placed two species in Cercosphaerella, viz. Cercosphaerella millegrana and Cercosphaerella cerasella (Aderh.) Kleb. (≡ Mycosphaerella cerasella Aderh.). Clements & Shear (1931) cited Cercosphaerella as a subgenus of Mycosphaerella and Mycosphaerella millegrana as lectotype. However, Klebahn (1918: 131) clearly emphasized that Septosphaerella, Ramularisphaerella, and Cercosphaerella were introduced as separate genera. Therefore, it is concluded that the phylogenetic position of Cercosphaerella based on its lectotype species Cercosphaerella millegrana remains unresolved pending the availability of phylogenetic analyses, and an epitypification of the latter species. Cercosphaerella may be available for some unnamed mycosphaerella-like clades.
Cercosperma G. Arnaud ex B. Sutton & Hodges, Nova Hedwigia 35: 798. 1983 [1981].
Description (from Sutton & Hodges 1981): Mycelium mostly superficial, composed of thick-walled, branched, brown, anastomosing, smooth hyphae; hyphopodia and setae absent. Conidiophores micro- to semi-macronematous, mononematous, erect, pale brown, ofen with a single short lateral branch at base. Conidiogenous cells holoblastic, determinate, integrated or discrete, terminal on the main axes or lateral branches, pale brown, smooth, with flattened apex. Conidia solitary, dry, acrogenous, straight to curved, tapered towards apex, truncate at base, distoseptate, alternate septa thickened, lumina reduced, smooth, pale brown.
Type species: Cercosperma arnaudii B. Sutton & Hodges [Brazil, Pará, Monte Dourado, on Eucalyptus leaf litter, 20 Jun. 1974, C.S. Hodges, holotype IMI 186982i].
Note: When Sutton & Hodges (1983) validated Cercosperma, they also pointed out its similarity to Ceratophorum, which is another genus that remains phylogenetically unresolved.
Cercospora Fresen. ex Fuckel.
Note: See treatment in text.
Cercosporella Sacc.
Note: See treatment in text.
Cercosporidium Earle
Note: See treatment in text.
Cercosporina Speg., Anal. Mus. Nac. B. Aires, Ser. 3, 13: 424. 1911.
Type species: Cercosporina asparagicola Speg. [Argentina, La Plata, on Asparagus officinalis, Maj. 1906, holotype LPS 4966; isotype IMI 247001 (slide)] = Cercospora asparagi Sacc.
Description and illustration: Chupp (1954, as Cercospora aparagi).
Note: Cercosporina is currently treated as a synonym of Cercospora.
Cercosporiopsis Miura, Flora of Manchuria and East Mongolia. Part III. Cryptogams, fungi: 527. 1928.
Type species: Cercosporiopsis menispermi (Ellis & Holw.) Miura (≡ Cercospora menispermi Ellis & Holw.) [USA, Iowa, Decorah, on Menispermum canadense, Jun. 1886 (holotype FH-01012294)] = Passalora menispermi (Ellis & Holw.) U. Braun & Crous.
Description and illustration: Chupp (1954, as Cercospora menispermi), Ellis (1976, as Phaeoisariopsis menispermi).
Note: The placement of Cercosporiopsis menispermi in Passalora needs to be confirmed based on DNA data, as this generic name may be available for some of the unnamed passalora-like clades.
Cercostigmina U. Braun, Cryptog. Bot. 4: 107. 1993.
Type species: Cercostigmina concentrica (Cooke & Ellis) U. Braun (≡ Cercospora concentrica Cooke & Ellis) [USA, New Jersey, Gloucester, on Yucca filamentosa, 1 Jun. 1874, W.A. Kellerman, no. 2150 (holotype NY 00838826; isotype: NY 01102862)] = Pseudocercospora concentrica (Cooke & Ellis) U. Braun & Crous.
Description and illustrations: Braun (1993, as Cercostigmina concentrica).
Note: The placement of Cercostigmina concentrica in Pseudocercospora needs to be confirmed based on DNA data.
Chuppomyces Videira & Crous
Note: See treatment in text.
Ciferriella Petr., Ann. Mycol. 28(5–6): 409. 1930.
Type species: Ciferriella domingensis Petr. & Cif. [Dominican Republic, on Vitex umbrosa, 26 May 1929, coll. R. Ciferri, det. F. Petrak (holotype NY 01048475)] = Pseudocercospora domingensis (Petr. & Cif.) Quaedvl., Verkley & Crous.
Description and illustrations: Quaedvlieg et al. (2013).
Note: Ciferriella is currently considered a synonym of Pseudocercospora, see Quaedvlieg et al. (2013).
Cladosporiella Deighton, Mycol. Pap. 101: 34. 1965.
Description (from Braun et al. 2013): Morphologically close to Cladosporium and mycovellosiella-like Passalora species (with superficial hyphae, conidiophores fasciculate or solitary, arising from superficial hyphae, conidiogenous loci conspicuous, thickened and darkened, conidia catenate, pigmented), but the loci and hila are not cladosporium-like (not coronate) and all species assigned to this genus are hyperparasitic.
Type species: Cladosporiella cercosporicola Deighton.
Description and illustrations: Braun et al. (2013); present study (Fig. 51).
Fig. 51.
Cladosporiella cercosporicola (IMI 107538). A–E. Observations in vivo. A. Leaf spot symptoms on the host. B. Conidiophores and conidiogenous cells. C. Conidiophores, conidiogenous cells and conidium. D, E. Catenate and single conidia. Scale bars = 10 μm.
Materials examined: Malaysia, Sabah, Tawau, Quoin Hill, on Passalora koepkei on Saccharum officinarum, 9 May 1964, T.H. Williams (holotype IMI 107538b).
Notes: The hyperparasitic habit is the only character to discriminate this genus from Passalora. However, as the latter is now a generic complex, we tentatively prefer to maintain Cladosporiella as separate genus.
Clarohilum Videira & Crous
Note: See treatment in text.
Clypeispora A.W. Ramaley, Mycotaxon 40: 13. 1991.
Description (from Ramaley 1991): Coelomycetous, phytopathogenic. Mycelium immersed, consisting of branched, septate, hyaline hyphae. Conidiomata pycnidial, immersed, black to subhyaline, substomatal, unilocular, thin-walled, ostiolate, papillate, exuding translucent conidial cirrhus; wall of hyaline to golden brown cells. Conidiophores reduced to conidiogenous cells. Conidiogenous cells holoblastic, discrete, hyaline, golden-brown at base, thin-walled, with thin, thread-like projection giving rise to conidia. Conidia hyaline, allantoid, smooth, aseptate, bluntly rounded at both ends, often with irregular apical, and/or basal appendage.
Type species: Clypeispora angustifoliorum A.W. Ramaley [USA, Colorado, La Plata county, Haflin Creek Trail, on leaves of Populus angustifolia, Sep. 1987, A.W. Ramaley, holotype BPI 1102631)] = Mycosphaerella angustifoliorum A.W. Ramaley [USA, Colorado, La Plata county, Durango, Roosa Avenue, on leaves of Populus angustifolia, Oct. 1988, A.W. Ramaley (holotype BPI 1102629).
Description and illustration: Ramaley (1991).
Note: This species needs to be recollected and its phylogenetic position determined.
Clypeosphaerella Guatimosim, R.W. Barreto & Crous
Note: See treatment in text.
Collarispora Videira & Crous
Note: See treatment in text.
Colletogloeum Petr., Sydowia 7: 368. 1953.
Description (from Sutton 1980): Mycelium immersed, branched, septate, hyaline to pale brown. Conidiomata acervular, epidermal to subepidermal, separate, occasionally confluent, composed of pale brown to hyaline, thin-walled textura angularis. Dehiscence irregular. Conidiophores hyaline or very pale brown, sparsely branched, septate, smooth, cylindrical or slightly irregular, formed from the upper cells of the acervulus. Conidiogenous cells holoblastic, annellidic, integrated or discrete, indeterminate, cylindrical or doliiform, with several percurrent proliferations. Conidia hyaline or pale brown, 0- to multiseptate, straight, curved or irregular, truncate at the base, obtuse at the apex, usually thin-walled, smooth, guttulate or eguttulate.
Type species: Colletogloeum dalbergiae (S. Ahmad) Petr. (≡ Septogloeum dalbergiae S. Ahmad); = Colletogloeum sissoo (Syd.) B. Sutton (= Cercospora sissoo Syd.) [Pakistan, on pods of Dalbergia sissoo (presumed slide ex type collection IMI 8196; authentic for the name C. sissoo IMI 90825, fide Sutton 1964)].
Notes: Colletogloeum was first described by Petrak (1953) based on Septogloeum dalbergiae published earlier in that year. However, Cercospora sissoo Syd. (Sydow & Mitter 1933) provides an earlier epithet for the type and a combination was proposed by Sutton (1964) together with an amendment of the genus description to include only fungi with annellate conidiophores. Colletogloeum differs from Ahmadia only in having epidermal to subepidermal conidiomata as opposed to subcuticular conidiomata. The correct phylogenetic placement of the genus Colletogloeum is unknown, though DNA extracted from a fungarium specimen representative of the type species, C. sissoo (IMI 119162), showed Colletogloeum to be closely related to Pseudocercospora (Crous et al. 2009e), which fits with its morphology.
Coremiopassalora U. Braun, C. Nakash., Videira & Crous
Note: See treatment in text.
Cucurbitariopsis C. Massal., Mém. Accad. Agricolt. Arti Commerc. Verona, Ser. 3, 65: 133. 1889.
Type species: Cucurbitariopsis leptospora C. Massal. [Italy, Veneto, Monte Zevola, Passo Ristele, stem of ‘Clematidis? v. Astragenes?’ (sic. Saccardo, Syll. fung. 10: 396. 1892)] = Rhabdospora leptospora (C. Massal.) Sacc.
Note: Insufficiently known, seen as synonym of Rhabdospora (Saccardo 1892). The type specimen could not be located.
Cyclodothis Syd. & P. Syd., Ann. Mycol. 11: 266. 1913.
Description (adapted from Sydow & Sydow 1913): Stromata erumpent through the epidermis, characteristically annular, with numerous densely arranged small perithecioid loculi, wall distinct, dark brown, composed of small cells, ostiolate. Asci clavate, 8-spored, ascospores 3- to 4-stichous, indistinctly paraphysate. Ascospores oblong cylindrical, colourless, straight, slightly inequilateral, ends obtuse, with a single medial septum.
Type species: Cyclodothis pulchella Syd. & P. Syd. [Philippines, Mindanao, Todaya, Mt. Apo, on leaf spots of Piper celtidiforme, Jul. 1909, A.D.E. Elmer, no. 11163 (syntypes BPI 642231, BPI 642230, S F207022, S F207023)].
Notes: The genus has in recent years been treated as synonym of Mycosphaerella [Mycosphaerella pulchella (Syd. & P. Syd.) Arx]. However, Cyclodothis is insufficiently known, and Aptroot (2006) observed the type specimen to only contain a coelomycete.
Cytostagonospora Bubák, Ann. Mycol. 14: 150. 1916.
Synonym: Cytostaganis Clem. & Shear, Gen. fung., Edn 2 (Minneapolis): 367. 1931.
Description (from Quaedvlieg et al. 2012, adapted from Sutton 1980): Mycelium immersed, dark brown, branched, septate. Conidiomata pycnidial, amphigenous, separate, globose, dark brown to black, immersed, unilocular, thick-walled, clypeate; walls of dark brown, thick-walled textura angularis to textura globulosa, becoming hyaline towards the conidiogenous region, extending in the upper part to become a circular clypeus of similar thickness to the wall. Ostiole central, circular, papillate to short rostrate, depressed, situated immersed within the clypeus. Conidiophores reduced to conidiogenous cells. Conidiogenous cells holoblastic, determinate, discrete, lageniform, hyaline, smooth, formed from the inner cells of the pycnidial wall. Conidia hyaline, 0–2-euseptate, not constricted at septa, base truncate, apex obtuse, thin-walled, eguttulate, smooth, filiform, often curved.
Type species: Cytostagonospora photiniicola Bubák, [Italy, Bozen, Oswald, on Photinia serrulata).
Description and illustration: Quaedvlieg et al. (2013).
Notes: Von Arx (1983) treated Cytostagonospora as a synonym of Septoria, while Sutton (1980) retained it as a separate genus. The genus Cytostaganis Clem. & Shear 1931 is based on the same species as Cytostagonospora, and is thus a homotypic synonym. The type specimen could not be located.
Davisoniella H.J. Swart, Trans. Brit. Mycol. Soc. 90: 289. 1988.
Description (from Swart 1988): Conidiomata in necrotic spots in living leaves, abaxial, single or a few clustered together, stromatic, subepidermal, lifting the epidermis at maturity. Conidiogenous cells holoblastic, percurrent, arising from the inner wall of the locules, flask shaped. Conidia oval, brown, verruculose, apex rounded, base truncate with a marginal frill.
Type species: Davisoniella eucalypti H.J. Swart [Australia, Western Australia, Darling Ranges, Mundlimup Block, on leaves of Eucalyptus marginata, 24 Nov. 1981, F. Tay (holotype DAR 58999)].
Notes: Although Crous et al. (2006b) described a sexual morph on the type material as Mycosphaerella davisoniellae, the link was never confirmed in culture. However, the morphology of both the sexual and asexual morphs suggests that this taxon would be better accommodated in Teratosphaeriaceae (Teratosphaeria) than Mycosphaerellaceae.
Dearnessia Bubák, Hedwigia 58: 25. 1916.
Description (from Quaedvlieg et al. 2013, adapted from Sutton 1980): Mycelium hyaline to brown, branched, septate. Conidiomata pycnidial, amphigenous, separate, globose, immersed, brown; wall of thin-walled textura angularis. Ostiole central, circular, papillate. Setae ostiolar, approximately straight, unbranched, tapered towards apex, dark brown, smooth, thin-walled, septate. Conidiogenous cells holoblastic, determinate, discrete, doliiform to ampulliform, hyaline, smooth and formed from the inner layer of the pycnidial wall. Conidia cylindrical to irregular, hyaline, 1–multi-transversely euseptate, rarely with 1–2 longitudinal eusepta, continuous or constricted, often tapered at the apex, base truncate, thin-walled, smooth, guttulate or not.
Type species: Dearnessia apocyni Bubák [Canada, Ontario, London, on leaves of Apocynum androsaemifolium, 11 Aug. 1910, J. Dearness (holotype F43227)].
Description and illustration: Quaedvlieg et al. (2013).
Notes: The type species needs to be recollected in order to determine the phylogenetic position of this genus. See Quaedvlieg et al. (2013).
Deightoniella S. Hughes, Mycol. Pap. 48: 27. 1952.
Description (adapted from Hughes 1952): Colonies effuse, grey, brown or black. Mycelium immersed, occasionally superficial. Stroma absent. Setae and hyphopodia absent. Conidiophores macronematous, mononematous, torsive or flexuous, unbranched brown, smooth, with characteristic swellings along length of conidiophore, due to percurrent rejuvenation, and elongation of conidiophore, producing conidia at higher levels. Conidiogenous cells monoblastic, integrated, terminal, percurrent, cylindrical. Conidia solitary, acrogenous, obclavate to obpyriform, medium brown, verruculose, transversely 1-septate above the median, with apical cell showing prominent taper towards subobtuse apex; basal scar somewhat darkened and thickened.
Type species: Deightoniella africana S. Hughes.
Description and illustrations: Hughes (1952); present study (Fig. 52).
Fig. 52.
Deightoniella africana (IMI 39675a). A–E. Observations in vivo. A. Conidiophores emerging on the leaf surface. B, C. Conidiophores. D, E. Single conidia. Scale bars = 10 μm.
Materials examined: Ghana, Hohae (Togoland), on leaves of Imperata cylindrica var. africana, 28 May, 1949, S.J. Hughes 913 (holotype IMI 39675a)]; Sierra Leone, Newton (?) colony, on leaves of Imperata cylindrica var. africana,17 Jan. 1950, T.C. Deighton, M3478A, IMI 41188.
Note: See notes under Utrechtiana.
Deightonomyces Videira & Crous
Note: See treatment in text.
Denticularia Deighton, Trans. Brit. Mycol. Soc. 59: 421. 1972.
Description (from Deighton 1972): Parasitic fungi, causing leaf spots. Mycelium immersed. Conidiophores arising from stromata, densely crowded, brown, mostly simple, smooth, thin-walled, continuous or few septate, sympodial, polyblastic, denticulate, not cicatrized, the denticles short and subcylindrical with a truncate unthickened apex. Conidia pale brown, more or less fusiform, catenulate, with the hila and scars unthickened, thin-walled, smooth or very minutely rough-walled, continuous or 1-septate.
Type species: Denticularia modesta (Syd.) Deighton (≡ Cladosporium modestum Syd.). [Sierra Leone, Kenema (Nougowa), on leaves of Anthostema senegalense, 5 Dec. 1938, F.C. Deighton M1681 (holotype IMI 7520)].
Description and illustration: Deighton, 1972, Ellis, 1976; present study (Fig. 53).
Fig. 53.
Denticularia modesta (IMI 62524). A–E. Observations in vivo. A. Leaf spot symptoms on the host. B, C. Conidiophores and conidiogenous cells. D. Conidiophores, conidiogenous cells and conidia. E. Catenate and single conidia. Scale bars = 10 μm.
Material examined: Sierra Leone, Kenema (Nougowa), on Anthostema senegalense, 9 Feb. 1956, C.T. Pyne M6473, IMI 62524.
Notes: Cultures of the type species of this genus and results of molecular analyses are necessary to resolve its phylogenetic position and clarify its relation to Pseudocercospora. It is still unclear and unproven whether this genus belongs in the Mycosphaerellaceae.
Dictyocephala A.G. Medeiros, Publ. Inst. Micol. Recife 372: 13. 1962.
Type species: Dictyocephala ulmifoliae (Obreg.-Bot.) A.G. Medeiros (≡ Cercospora ulmifoliae Obreg.-Bot.) [Colombia, Quipile, on Guazuma ulmifolia, 16 Apr. 1940, R. Obregón-Botero & G.J. Quintana, No. 901] ≡ Pseudocercospora ulmifoliae (Obreg.-Bot.) U. Braun & Crous.
Descriptions and illustrations: Chupp, 1954, Deighton, 1976a.
Note: The synonymy with Pseudocercospora is based on morphology, and needs to be confirmed based on DNA data. The type specimen could not be located.
Dictyodesmium S. Hughes, Mycol. Pap. 36: 29. 1951.
Description (from Ellis 1971): Sporodochia epiphyllous, erumpent, pulvinate, olivaceous brown. Mycelium immersed forming hyphal cushions at the point of origin of the conidiophores but no definite stroma. Setae and hyphopodia absent. Conidiophores mono- and macronematous, caespitose, crowded, straight or flexuous, unbranched, pale brown, smooth. Conidiogenous cells monoblastic, integrated, terminal, determinate, cylindrical. Conidia solitary, acrogenous, simple, fusiform to obclavate, rostrate, truncate at the base, rather pale olivaceous brown, palest at the ends, smooth, with transverse septa throughout and longitudinal and oblique septa in the central 6–9 cells.
Type species: Dictyodesmium ulmicola (Ellis & Kellerm.) S. Hughes (≡ Ceratophorum ulmicola Ellis & Kellerm.).
Descriptions and illustrations: Ellis, 1971, Seifert et al., 2011; present study (Fig. 54).
Fig. 54.
Dictyodesmium ulmicola (NY 00838655). A, C–E. Observations in vivo. A. Leaf spot symptoms on the host. B. Conidiophores and conidia drawing on the specimen envelope. C–E. Conidia. Scale bars = 10 μm.
Materials examined: USA, Kansas, on leaves of Ulmus fulva, Oct. 1987, W.A. Kellerman 1112 (holotype NY 00838655).
Notes: The phylogenetic position of Dictyodesmium is unknown and its four species are only known by their hyphomycetous asexual morph (Seifert et al. 2011). Sequence data are necessary to determine its phylogenetic position.
Didymaria Corda, Icon. fung. 5: 9. 1842.
Type species: Didymaria ungeri Corda [Switzerland, on Ranunculus nemorosus] = Ramularia didyma Unger.
Description and illustration: Braun (1998).
Note: See Braun (1998).
Didymellina Höhn., Ann. Mycol. 16: 66. 1918.
Description: Leaf spots ellipsoid-lenticular, pale brown with dark brown border. Ascomata pseudothecial, black, scattered, subepidermal to erumpent, wall of 2–3 layers of brown textura angularis. Asci aparaphysate, fasciculate, bitunicate, subsessile, obovoid, straight to slightly curved, 8-spored, with visible apical apiculus. Ascospores 3- to multiseriate, hyaline, non-guttulate, thin-walled, straight to slightly curved, fusoid-ellipsoid with obtuse ends, medianly 1-septate, widest in middle of apical cell, not constricted at the septum (but slightly so with age), tapering towards both ends, but slightly more to lower end; ascospores germinating while in ascomata, hyaline, slightly constricted at septum, germinating from both ends with germ tubes parallel to the long axis of the spore, in some cases germinating ascospores becoming 3-septate.
Type species: Didymellina iridis (Desm.) Höhn. (≡ Dothidea iridis Desm.) [France, Trouve a Hermanville, on leaves and capsules of Iris pseudacorus, 1847, M. Roberge (holotype, PC)].
Notes: Didymellina iridis is the type species of the genus Didymellina, which Müller & von Arx (1962) treated as synonym of Mycosphaerella. A short overview of the taxonomic history of this species was presented by (Braun et al. 2003a). Ascospores observed in asci were 1-septate, but 3-septate ascospores were observed at the onset of germination. Based on its morphology, it is considered that this represents a separate genus. However, the taxonomic position of this fungus can only be resolved when fresh material has been obtained.
Didymochora Höhn. Hedwigia 60: 172. 1918.
Description (adapted from von Höhnel 1918): Stromata small, flat, subcuticular, pseudoparenchymatous, carbonaceous, with a vertically arranged successive structure, with a single locus, cover one-layered, irregularly splitting, basal layer pseudoparenchymatous below and palisade-like above. Conidia pigmented, 2-celled, solitary, separated from the tips of the internal palisade-like cell layer by a horizontal septum.
Type species: Didymochora betulina Höhn. (described as asexual morph of Euryachroa betulina (Fr. : Fr.) J. Schröt. ≡ Atopospora betulina (Fr. : Fr.) Petr., without any further details, and placed in the Leptostromaceae.
Notes: The phylogenetic position of Didymochora is unknown and its two species are only known by their stromatic asexual morph. It is currently considered a genus of incertae sedis belonging to the Dothideomycetes (Wijayawardene et al. 2014). Sequence data are necessary to determine its phylogenetic position.
Distocercospora N. Pons & B. Sutton
Note: See treatment in text.
Distocercosporaster Videira, H.D. Shin, C. Nakash. & Crous
Note: See treatment in text.
Distomycovellosiella U. Braun, C. Nakash., Videira & Crous
Note: See treatment in text.
Dothistroma Hulbary
Note: See treatment in text.
Elletevera Deighton, Mycol. Pap. 118: 17. 1969.
Description (from Deighton 1969): Mycelium immersed in the host fungus. Conidiophores dilute brown, smooth, branched, well developed, fasciculate, thin-walled: conidial scars conspicuous, slightly but distinctly thickened, prominent, aggregated towards the apices of the branchlets of the conidiophores. Conidia concolorous with the conidiophores, smooth, thin-walled, mostly cylindric-clavate and 3-septate, sometimes 0–2-septate, the shorter ones very rarely catenulate, sometimes fusoid, rostrate and pluriseptate, with a conspicuous and slightly but distinctly thickened hilum.
Type species: Elletevera parasitica (Ellis & Everh.) Deighton (≡ Pyricularia parasitica Ellis & Everh.).
Description and illustration: Deighton, 1969, Braun et al., 2013; present study (Fig. 55).
Fig. 55.
Elletevera parasitica (IMI 127995). A. Drawing in the specimen envelope. B–E. Observations in vivo. B–D. Conidiophores and conidiogenous cells. E. Conidiogenous cells and conidium. Scale bars = 10 μm.
Material examined: USA, Wisconsin, Kenosha Co., on Phyllachora graminis on Elymus virginicus, 13 Aug. 1893, J.J. Davis 9311, (holotype NY 00928212, isotype BPI 420251, slide ex type collection IMI 129275).
Notes: The present genus was introduced by Deighton (1969) to accomodate hyperparasitic cercosporoid hyphomycetes with distinct conidiogenous loci. Upon re-examination of several specimens, Braun et al. (2013) considered the conidiogenous loci description to be misleading and observed that the denticle-like loci are unthickened and undarkened. Due to the morphological characters this genus may be related to Pseudocercospora but cultures and sequence data are necessary to determine its phylogenetic position.
Epicoleosporium Videira & Crous
Note: See treatment in text.
Eriocercospora Deighton, Mycol. Pap. 118: 5. 1969.
Description (from Deighton 1969): Hyperparasitic hyphomycetes. Mycelium superficial, composed of pale brown, branched, septate, smooth, repent hyphae which bear conidiophores terminally and as lateral branches. Conidiophores pale brown, erect simple or branched, smooth, septate, not geniculate at the old conidial scars. Conidial scars slightly thickened, slightly prominent, the old ones lying more or less flat against the side of the conidiophore. Conidia pale brown, smooth, clavate, fusiform, subcylindric or obclavate, pluriseptate.
Type species: Eriocercospora balladynae (Hansf.) Deighton (≡ Helminthosporium balladynae Hansf.)
Description and illustration: Deighton, 1969, Braun, 1995; present study (Fig. 56).
Fig. 56.
Eriocercospora balladynae (IMI 5293c). A–E. Observations in vivo. A. Leaf spot symptoms on the host. B. Conidiophores, conidiogenous cells and conidia. C. Partial conidiophore, conidiogenous cells and conidium. D, E. Single conidia. Scale bars = 10 μm.
Materials examined: Uganda, Entebbe Road, on Balladynocallia glabra on Grumilea succulenta, Nov. 1943, C.G. Hansford 3264 (holotype of Helminthosporium balladynae, IMI 562a); Entebbe Road (mile 13), on Balladyna sp. on leaves of Pavetta sp., Mar. 1940, C.G. Hansford 2609 (holotype of Cercospora balladynae, IMI 4706c); Entebbe, on Balladynocallia glabra on Pavetta sp., Dec. 1945, C.E. Hansford 3726, (IMI 5293).
Notes: The present genus was introduced by Deighton (1969) who described the conidiogenous loci as mycovellosiella-like. Upon re-examination of several specimens Crous & Braun (2003) considered the conidiogenous loci description to be misleading and observed that the denticle-like loci are neither thickened nor conspicuously darkened. Due to morphological characters this genus may be related to Pseudocercospora but sequence data are necessary to determine its phylogenetic position.
Eriocercosporella Rak. Kumar, A.N. Rai & Kamal ex U. Braun, A monograph of Cercosporella, Ramularia and allied genera (Phytopathogenic Hyphomycetes) 2: 398. 1998.
Description (from Braun et al. 2013): Foliicolous hyphomycetes, associated with leaf spots. Mycelium internal and external, superficial hyphae emerging through stomata, branched, pigmented, septate, thin-walled, smooth. Stromata lacking. Conidiophores macronematous, mononematous, in vivo solitary, arising from superficial hyphae, lateral, simple, occasionally branched, pigmented, septate, thick-walled, smooth; conidiogenous cells integrated, terminal, uni- to multilocal, sympodially or occasionally percurrently proliferating, loci truncate, flat, broad, neither thickened nor darkened, conidiogenesis thalloblastic, i.e. at first blastic, then thallic (base of conidia ± agreeing in width with the diameter of the broad conidiogenous loci). Conidia solitary, cylindrical to subclavate, occasionally disarticulating, plurieuseptate, occasionally with 1–2 additional distosepta, thick-walled, brown, smooth, not attenuated at the base, hila truncate, broad, width ± agreeing with the diameter of the conidiogenous loci, neither thickened nor darkened, conidial secession schizolytic.
Type species: Eriocercosporella indica R. Kumar, A.N. Rai & Kamal ex U. Braun
Description and illustration: Braun, 1998, Braun et al., 2013; present study (Fig. 57).
Fig. 57.
Eriocercosporella indica (IMI 302747). A–I. Observations in vivo. A. Leaf spot symptoms on the host. B, C. Conidiophores. D. Conidiogenous cells proliferating percurrently and conidia. E–H. Conidiophores and conidia. I. Conidia. Scale bars = 10 μm.
Materials examined: India, Uttar Pradesh, Pithoragarh, on Marsdenia roylei, 1985, Kumar (holotype IMI 302747).
Note: Due to its morphological characters this genus may be related to either Pseudocercospora or Sporidesmium, but sequence data are necessary to determine its phylogenetic position.
Euryachora Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 220. 1870 (1869–1870).
Description (from Fuckel 1869): Ascomata pseudothecial. Asci obovoid, sessile, 8-spored. Ascospores obovoid, hyaline, 1-septate.
Type species: Euryachora sedi (Link) Fuckel [as ‘sebi’] (≡ Leptostroma sedi Link) (Austria, on Sedum maximum).
Note: The present genus is based on Euryachora sedi which is only known by the mycosphaerella-like sexual morph. It is currently considered to belong to Mycosphaerellaceae (Lumbsch & Huhndorf 2010) but the type specimen could not be located and no DNA sequence data are available to determine its phylogenetic position.
Exopassalora Videira & Crous
Note: See treatment in text.
Exosporium Link
Note: See treatment in text.
Exutisphaerella Videira & Crous
Note: See treatment in text.
Filiella Videira & Crous
Note: See treatment in text.
Fulvia Cif.
Note: See treatment in text.
Fusicladiella Höhn., Ber. Deutsch. Bot. Ges. 37: 155. 1919.
Description (from Ellis 1971): Colonies suborbicular or angular. Mycelium immersed. Stroma sometimes present in the host cuticle. Setae and hyphopodia absent. Conidiophores macronematous, mononematous, crowded, unbranched, at first erect, straight or slightly curved, cylindrical, almost colourless, later strongly curved, brown or olivaceous brown, pale and thin-walled on one side, dark and thick-walled on the other, the curvature always taking place towards the thin-walled side, smooth or sometimes finely verruculose near the apex. Conidiogenous cells monoblastic, integrated, terminal, determinate, cylindrical, cicatrized, the single apical scar broad and flat. Conidia solitary, dry, aerogenous, straight or slightly curved, often cylindrical, rounded at the apex, truncate with a thin scar at the base, but sometimes clavate, ellipsoidal or obclavate, colourless to pale olive, smooth to finely verruculose, almost always 1-septate, rarely 2-septate.
Type species: Fusicladiella aronici (Sacc.) Höhn. (≡ Fusicladium aronici Sacc.) = Fusicladiella melaena (Fuckel) S. Hughes) [syntypes, Italy, Vette di Feltre, on Doronicum grandiflorum (= Aronicum scorpioides), Aug. 1879, G. Bizzozero (BPI 423776, PAD); Mt. Baldo, Valle delle Pietre, on D. glaciale (= Aronicum doronicum), V. de Cesati, Rabenh., Fungi Eur. 2339 (numerous fungaria including HAL)].
Descriptions and illustrations: Hughes, 1952, Deighton and Pirozynski, 1965, Ellis, 1971, von Arx (1983); present study (Fig. 58).
Fig. 58.
Fusicladiella aronici (IMI 371583). A–H. Observations in vivo. A, B. Leaf spot symptoms on the host. C. Conidiophores. D. Partial conidiophore and conidiogenous cell. E, F. Conidiogenous cells and conidia. G, H. Conidia. Scale bars = 10 μm.
Materials examined: Switzerland, Graubünden, Fimbertal, Silvretta, on leaf of Doronicum grandiflorum, 5 Aug. 1967, J. Poelt & M. Steiner, Reliquiae Petrakianae no. 2565 (IMI 371583). Russia, Moskovsky, St Petersburg, on leaves of Carduus crispus, 2007, V. Melnik. Exsicc. Mycoth. Petropol. 90, UPS:BOT:F-144284.
Notes: Fusicladiella is based on Fusicladiella aronici, which is only known by its hyphomycetous asexual morph. The phylogenetic position of this genus remains obscure due to absence of DNA data.
Fusoidiella Videira & Crous
Note: See treatment in text.
Gillotia Sacc. & Trotter, Syll. Fung. 22: 253. 1913.
Description (Saccardo & Trotter 1913): Ascomata erumpent to superficial, subglobose. Asci saccate, subclavate, aparaphysate, stipitate, 8-spored. Ascospores oblong, 3-septate, straight to slightly curved, hyaline, becoming olivaceous brown.
Type species: Gillotia orbicularis (Syd. & P. Syd.) Sacc. & Trotter (≡ Diplotheca orbicularis Syd. & P. Syd.) [Brazil, São Paulo, Campinas, on Cactus sp., Oct. 1896, F. Noack (holotype S F9063).
Notes: Gillotia is based on Gillotia orbicularis, which is mostly known by its sexual morph. The presence of an asteromella-like asexual morph is indicated by Hyde et al. (2010). The fact that this species produces 3-septate ascospores that become olivaceous brown is suggesting it should not belong in the Mycosphaerellaceae. However, DNA is not available and hence its phylogenetic position remains obscure.
Gloeocercospora D.C. Bain & Edgerton ex Deighton, Trans. Brit. Mycol. Soc. 57: 358. 1971.
Description (from Deighton 1971): Mycelium internal, composed of septate, branched hyphae. Stroma small or absent. Conidiomata sporodochial, suprastomatal, originating from hyphae which emerge through stomata, pulvinate, composed of more or less hyaline, repeatedly branched hyphae with short cells of which the terminal cells act as conidiogenous cells; conidiogenous loci terminal, minute, unthickened. Conidia hyaline, filiform, straight to curved, multi-septate, smooth, in mucoid mass.
Type species: Gloeocercospora sorghi D.C. Bain & Edgerton ex Deighton [USA, Louisiana, on Sorghum vulgaris, Aug. 1943, D.C. Bain (holotype BPI 433333)].
Notes: Gloeocercospora was considered a synonym of Microdochium based on morphological characters (Braun 1995). The ITS sequence of CBS 131812 (unpublished, India, on Sorghum vulgare, Nov. 1971, G.S. Rawla, culture CBS 131812 = IMI 165194) is identical to that of Gloeocercospora sorghi NBRC 7430, currently available in GenBank (ITS and partial LSU: accession LC063852). More data are necessary to resolve the phylogenetic position of this pathogen.
Gomphinaria Preuss, Linnaea 24: 130. 1851.
Description (adapted from Preuss 1851 and Saccardo 1886, as Acrotheca amoena): Caespituli effuse, brown. Conidiophores erect, subulate, simple, below densely septate and brown, above subhyaline, transparent and aseptate. Conidia terminal, subapically formed, oblong, hyaline, aseptate, base acute or subapiculate, hila hyaline.
Type species: Gomphinaria amoena Preuss [Germany, on Alnus glutinosa (holotype in B)].
Description and illustration: Preuss (1851).
Note: Arzanlou et al. (2007) examined the holotype of Gomphinaria amoena Preuss (B), and concluded that without fresh collections, it would not be possible to ascertain the phylogenetic position of this ramichloridium-like hyphomycete.
Graminopassalora U. Braun, C. Nakash., Videira & Crous
Note: See treatment in text.
Haplodothis Höhn., Sitzungsber. Akad. Wiss. Wien, Math.-Naturwiss. Kl., Abt. 1 120: 423 (45 repr.). 1911.
Type species: Haplodothis singularis (Henn.) Höhn. (≡ Lizonia singularis Henn.) [Australia, Western Australia, on Leucopogon hispidus, L. Diels, No. 3055] = Mycosphaerella singularis (Henn.) Arx.
Notes: The genus Haplodothis is based on Haplodothis singularis, which is currently considered a synonym of Mycosphaerella singularis. The type specimen could not be located. Fresh collections and DNA sequence data are necessary to determine if Haplodothis is a real synonym of Mycosphaerella, which is now treated as Ramularia (Videira et al., 2015a, Videira et al., 2015b, Videira et al., 2016).
Haplographium Berk. & Broome, Ann. Mag. Nat. Hist., Ser. 3, 3: 360 (1859).
Type species: Haplographium delicatum Berk. & Broome = Dematioscypha dematiicola (Berk. & Broome) Svrček 1977
Description and illustration: Ellis, 1971, Seifert et al., 2011.
Notes: The genus Haplographium is based on H. delicatum, a hyphomycetous species with a link to the sexual morph Hyaloscypha dematiicola (Berk. & Broome) Nannf. (Ellis 1971), which is a current synonym of Dematioscypha dematiicola (Berk. & Broome) Svrček. The recent work of Han et al. (2014) places a representative strain of Dematioscypha dematiicola (TNS-F17834) in the Leotiomycetes (Helotiales). The work of Crous et al. (2009a) summarizes the taxonomic history of Lauriomyces and Haplographium and shows that the available strains of Haplographium catenulatum (CBS 196.73, CBS 482.67, CBS 739.68) cluster in Hyaloscyphaceae (Leotiomycetes) and apart from the available strains of Lauriomyces bellulus (CBS 517.93) and Lauriomyces heliocephalus (CBS 112054), which are considered to be incertae sedis.
Hawksworthiana U. Braun, Int. J. Mycol. Lichenol. 3: 276. 1988.
Description (from Videira et al. 2016): Lichenicolous, forming gall-like deformations. Mycelium consisting of hyaline, septate, sparsely branched, thin-walled hyphae. Conidiophores reduced to the conidiogenous cells, erumpent, usually ampulliform but sometimes subcylindrical, aseptate, hyaline, thin-walled, mono- or polyblastic, sympodial, conidiogenous loci conspicuous, thickened and darkened. Conidia formed singly, acrogenous, oblong-clavate to subcylindrical, hyaline, thin-walled, smooth, aseptate or 1-septate, hilum conspicuous, thickened and darkened.
Type species: Hawksworthiana peltigericola (D. Hawksw.) U. Braun (≡ Ramularia peltigericola D. Hawskw.) [UK, Scotland, Isle of Mull, Killiemore, on thallus of Peltigera polydactylon, 16 Jun. 1979, Clark (holotype IMI 239715a).
Description and illustration: Braun et al. (1998), Videira et al. (2016).
Notes: Hawksworthiana differs from Ramularia by its lichenicolous habit and some morphological features. Although fresh material has been available, all attempts to grow this fungus in culture have thus far been unsuccessful and no sequence data are available.
Helicomina L.S. Olive, Mycologia 40: 16. 1948.
Type species: Helicomina caperoniae L.S. Olive [USA, Louisiana, Baton Rouge, on Caperonia castaneifolia, 2 Oct. 1946, Q.L. Holdman (holotype BPI 447607)] = Pseudocercospora caperoniae (L.S. Olive) Deighton.
Descriptions and illustrations: Olive, 1948, Ellis, 1971, Deighton, 1976a.
Notes: This genus is currently considered a synonym of Pseudocercospora based on its morphological characters (Crous et al. 2013a). However, the type species needs to be recollected to confirm the generic synonymy based on DNA data.
Hoornsmania Crous, Fungal Planet 11: 1. 2007.
Description (from Crous 2007): Hyphomycetes. Conidiophores solitary, brown, arising from superficial hyphae, septate. Conidiogenous cells brown, smooth to finely verruculose, elongate-ellipsoid to fusoid, with 1–2 truncate loci, somewhat thickened and darkened, but not prominently refractive. Conidia brown, smooth to finely verruculose, broadly ellipsoidal to somewhat fusoidal, occurring in branched, acropetal chains; scars somewhat darkened, thickened, but not refractive; hyperparasitic on Neonectria ditissima.
Type species: Hoornsmania pyrina Crous [Netherlands, Utrecht Prov., Bilthoven, on perithecia of Neonectria ditissima on twigs of Pyrus malus, Jan. 2005, P.W. Crous (holotype CBS H-19769)].
Description and illustration: Crous (2007).
Note: All attempts to cultivate this species, or isolate DNA from freshly collected material, have thus far been unsuccessful.
Hyalodictys Subram., Proc. Indian Acad. Sci., Pl. Sci.: 8. 1962.
Type species: Hyalodictys degenerans (Syd. & P. Syd.) Subram. (≡ Clasterosporium degenerans Syd. & P. Syd.) = Miuraea degenerans (Syd. & P. Syd.) Hara.
Description and illustration: Braun (1995, as Miuraea degenerans).
Notes: The genus Hyalodyctis, based on Hyalodyctis degenerans, is currently considered a synonym of Miuraea based on morphological characters. See treatment of Miuraea in text and Braun (1995).
Hyalocercosporidium Videira & Crous
Note: See treatment in text.
Hyalodothis Pat. & Har., Bull. Soc. Mycol. France 9: 210. 1893.
Description (adapted from Saccardo 1895): Glumicolous. Stromata superficial, encrusting ovaria and fruits, black, effuse-pulvinate, coriaceous-horny or subcarbonaceous, sclerotiform, with numerous immersed little loci. Asci 8-spored. Ascospores oblong, aseptate, hyaline.
Type species: Hyalodothis clavus Pat. & Har. [Democratic Republic of the Congo, on culms of Poaceae].
Descriptions and illustrations: Arnold, 1967, Patouillard and Hariot, 1893.
Notes: Arnold (1967) found that the type specimen contained two distinct species of fungi that were used to generate the description of Hyalodothis, and thus recommended that the genus be considered a nomen confusum, which is currently not part of the ICN. Hence, a lectotypification confining this name to one of the included elements is necessary to clarify the identity of this genus. According to Arnold (1967) the type specimen is part of Patouillard collection (no.597) in FH, but it could not be traced using the online catalog.
Hyalozasmidium U. Braun, C. Nakash., Videira & Crous
Note: See treatment in text.
Isariella Henn., Hedwigia 48: 19. 1908.
Description (adapted from Hennings 1908): “Sporodochia” (fascicles/coremia) parasitic, superficial, fasciculate-fasciate, waxy, composed of hyaline, septate, loosely united, converging “hyphae” (conidiophores). Conidia ellipsoid, aseptate, hyaline.
Type species: Isariella auerswaldiae Henn. [Brazil, São Paulo, Horto Botanico, on stromata of Auerswaldia puttemansia on leaves of Lauraceae, 1902, Puttemans, No. 571 (holotype S F40445)]
Description and illustrations: Hennings, 1908, Seifert et al., 2011.
Notes: The phylogenetic position of Isariella is unknown and its two species are only known by their hyphomycetous asexual morph (Seifert et al. 2011). Sequence data are necessary to determine its phylogenetic position.
Isariopsella Höhn., in Weese, Mitt. Bot. Inst. Tech. Hochsch. Wien 6: 68. 1929.
Type species: Isariopsella vossiana (Thüm.) Höhn. (≡ Ramularia vossiana Thüm.) [Slovenia, Ljubljana (Laibach), on Cirsium oleraceum, Oct. 1879, W. Voss, Thüm., Mycoth. Univ. 1769 (lectotype HAL)] = Phacellium vossianum (Thüm.) U. Braun.
Description and illustration: Braun (1998, as Phacellium vossianum).
Notes: Isariopsella is currently considered a synonym of Phacellium. If Phacellium is synonymous with Ramularia as is expected, the older name Ramularia vossiana will be used for this species. Sequence data are necessary to confirm this hypothesis.
Isariopsis Fresen., Beitr. Mykol. 3: 87. 1863.
Type species: Isariopsis pusilla Fresen. [Germany, on Cerastium holosteoides] = Phacellium alborosellum (Desm.) U. Braun.
Description and illustration: Braun (1998, as Phacellium alborosellum).
Notes: Isariopsis is currently considered a synonym of Phacellium. If Phacellium is synonymous with Ramularia as is expected, the name Ramularia alborosella (Desm.) Gjaerum would be available for the type species of Isariopsis as well as Phacellium (see Braun 1998). Sequence data are necessary to confirm this hypothesis.
Jaczewskiella Murashk., Mater. Mikol. Fitopatol. Rossii 5(2): 5. 1926.
Description (adopted from Shkarupa 1992 and Mel'nik & Popushoj 1992): Saprobic. Conidiomata stromatic, cupulate, with a more or less well developed stalk, sometimes sessile, large, scattered, composed of light brown to brown prismatic or oblong cells, darker and thick-walled towards the periphery. Conidiophores lacking. Conidiogenous cells lining the whole inner surface of the conidiomata, holoblastic, annellidic, indeterminate, discrete, cylindrical, thin-walled, smooth, light brown, with a single percurrent proliferation, margin uneven, fimbriate. Conidia solitary, clavate, obclavate, broad ellipsoid, smooth, with transverse and oblique to vertical septa, constricted at transverse septa, light brown, transparent.
Type species: Jaczewskiella altajensis Murashk. [Russia, Altai, valley of the river Dzhelo, 2200 m alt, on dead branches of Comarum salessowianum, 19 July 1925, S. Antonov (holotype LEP)].
Description and illustration: Shkarupa (1992).
Notes: Jaczewskiella is a coelomycetous genus that was considered a synonym of Stigmina by Sutton (1977). Shkarupa (1992) and Braun & Mel'nik (1996) considered Jaczewskiella to be an independent genus based on cupulate conidiomata and brown phragmo- to dictyoconidia. This genus is insufficiently known, and will have to be recollected and sequenced in order to determine it's true status.
Janetia M.B. Ellis, More Dematiaceous Hyphomycetes (Kew): 33. 1976.
Description (from Ellis 1976): Colonies effuse, thin, dark blackish brown. Mycelium superficial composed of a network of branched and anastomosing septate, olivaceous or dark brown, smooth hyphae. Stroma none; setae and hyphopodia absent. Conidiophores micronematous, mononematous. Conidiogenous cells integrated, mostly intercalary, polyblastic, denticulate; denticles large, flat-topped. Conidia solitary, dry, obclavate, multiseptate, brown, smooth.
Type species: Janetia euphorbiae M.B. Ellis [Tanzania, Ukiriguru Hill, on Euphorbia tirucalli, 13 Nov. 1972, D.L. Ebbels (holotype IMI 163941)].
Description and illustration: Ellis, 1976, Seifert et al., 2011.
Notes: The genus Janetia is characterised by the production of polyblastic, pigmented and denticulate conidiogenous cells that give rise to phragmosporous, disto- or eu-septate conidia. The LSU sequences of two recently described species, Janetia wilsonii and Janetia dimorphandrae-mollis, place the genus in the Mycosphaerellaceae, in close association with species of the Zasmidium complex (Da Silva et al. 2016). However, until sequences from the type species Janetia euphorbia are obtained, the phylogenetic placement of this genus in the Mycosphaerelaceae is only tentative.
Jahniella Petr., Ann Mycol. 18(4/6): 123. 1921. 1920.
Description (from Quaedvlieg et al. 2013, adapted from Sutton 1980): Mycelium branched, immersed, septate, brown. Conidiomata pycnidial, superficial on epidermis, immersed, separate, globose, papillate, dark brown, thick-walled, sclerenchymatic; wall consisting of an outer layer of dark brown, thick-walled textura angularis, a middle layer of 8 cells thick, of hyaline to pale brown, thickwalled cells, and an inner layer of thin-walled, hyaline, irregular cells. Ostiole single, circular, with a distinct channel and hyaline periphysoid cells. Conidiophores reduced to conidiogenous cells. Conidiogenous cells holoblastic, determinate, discrete, hyaline, ampulliform, lining the wall of the pycnidium. Conidia straight or slightly curved, hyaline, thin-walled, smooth, 3–4-euseptate, eguttulate, truncate at the base, slightly tapered to the apex.
Type species: Jahniella bohemica Petr., [Czech Republic, Bohemia, on stems of Scrophularia nodosa, 18 Mar. 1916, J. Jahn (isotype K(M) 180917 (slides) ex BPI).
Description and illustration: Quaedvlieg et al. (2013).
Notes: The type species needs to be recollected in order to determine the phylogenetic position of the genus. See Quaedvlieg et al. (2013).
Laocoön J.C. David, Mycol. Pap. 172: 116. 1997.
Description (from David 1997): Hyphomycetous, phytopathogenic. Mycelium superficial, hyphae creeping, septate, branched, pigmented, smooth. Conidiophores arising from creeping hyphae, macronematous, mononematous, simple, rarely branched, straight to flexuous, septate, densely verruculose, not spirally twisted. Conidiogenous cells integrated, terminal, multilocal, sympodial; conidiogenous loci broad, aggregated, cercospora-like, thickened and darkened, flattened with a rough surface, raised at the edge and with a conspicuous central dome. Conidia solitary, consisting of only one filament, transversely euseptate, pustulate, not proliferating, thin-walled, pigmented; conidial secession schizolytic.
Type species: Laocoön paradoxus (Syd. & P. Syd.) J.C. David (≡ Heterosporium paradoxum Syd. & P. Syd.).
Descriptions and illustrations: David, 1997, Braun, 1998, Seifert et al., 2011; present work (Fig. 59).
Fig. 59.
Laocoön paradoxus (IMI 375866). A–D. Observations in vivo. A. Leaf spot symptoms on the host. B–D. Conidiophores, conidiogenous cells and conidia. Scale bars = 10 μm.
Materials examined: Colombia, Antioquia, Guaca, on Calea glomerata, 12 Sep. 1910, E. Mayor 346 (holotype S F40564, isotype IMI 375866).
Note: Laocoön is a hyphomycetous genus that includes a single species thus far only known from the type locality (Seifert et al. 2011). Sequence data are necessary to determine its phylogenetic position.
Lecanosticta Syd.
Notes: See treatment in text.
Lecanostictopsis B. Sutton & Crous, Mycol. Res. 101: 215. 1997.
Description (from Sutton & Crous 1997): Mycelium immersed, intercellular, branched, septate, dark to reddish brown. Conidiomata epidermal to subepidermal, erumpent, eustromatic, acervular to sporodochial, composed of thick-walled, dark to reddish brown textura angularis. Conidiophores dark to reddish brown, coarsely verrucose, cylindrical, unbranched, septate, formed from the upper cells of the conidiomata. Conidiogenous cells integrated, dark to reddish brown, coarsely verrucose to tuberculate, cylindrical, with several percurrent enteroblastic proliferations. Conidia holoblastic, dark to reddish brown, coarsely verrucose to tuberculate, with 0- to several eusepta, straight to curved, obtuse or acute at apex, truncate at base, cylindrical to fusiform. Conidiogenesis: a succession of conidia is formed by holoblastic conidial ontogeny, delimitation by a transverse septum, schizolytic secession, replacement wall building apex leading to enteroblastic percurrent conidiogenous cell proliferation followed by holoblastic conidial ontogeny, with successive conidia seceding at progressively higher levels.
Type species: Lecanostictopsis kamatii (Ullasa) B. Sutton & Crous (≡ Stigmina kamatii Ullasa) [India, Mysore State, Bettigeri, on leaves of Syzygium aromaticum (type IMI 147817)].
Descriptions and illustrations: Sutton and Crous, 1997, Seifert et al., 2011.
Notes: All attempts to culture species of Lecanostictopsis have thus far proven unsuccessful, even from freshly collected material, therefore its phylogenetic position remains unknown. The taxonomic history of this genus is detailed by Sutton & Crous (1997).
Lembosiopsis Theiss., Ann. Mycol. 15: 422. 1918.
Description (from Hongsanan et al. 2014): Ascomata solitary to clustered, subcuticular, circular, slightly irregular from above, black, shiny, with a central rounded ostiole. Hamathecium lacking pseudoparaphyses. Asci 8-spored, bitunicate, obclavate, tapering towards the apex, apedicellate or with short pedicel, apically rounded with a small ocular chamber. Ascospores 2–3-seriate in the ascus, narrowly ovoid, tapering from the apex to the base, 1-septate slightly above the centre, slightly constricted at the septum, hyaline, surrounded by thin gelatinous sheath, smooth-walled.
Type species: Lembosiopsis andromedae (Tracy & Earle) Theiss. (= Lembosia andromedae Tracy & Earle) [USA, Mississippi, Biloxi, on leaves of Andromeda nitida, 26 May 1895, S.M. Tracy and F.S Earle 4005 (holotype BPI 647155]
Description and illustration: Hongsanan et al. (2014).
Notes: Based on the literature, Lumbsch & Huhndorf (2010) place Lembosiopsis in Asterinaceae, but in a recent review of Asterinales Hongsanan et al. (2014) transferred the genus to the Mycosphaerellaceae based on morphological characters (subcuticular ascomata with a rounded central ostiole, without pseudoparaphyses, and procuding obclavate asci). The phylogenetic placement of this genus is uncertain as DNA sequence data are not available.
Lophiosphaerella Hara, Byogaichu-Hoten (Manual of Pests and Diseases): 778. 1948.
Description (from Li et al. 2014): Parasitic on terrestrial plants, forming conspicuous small, rounded, pale grey leaf spots on both sides of the leaf. Ascomata solitary, scattered, gregarious or confluent, globose or subglobose, semi-immersed or immersed, ostiolate. Ostiole centrally located. Peridium composed of brown to black, thick-walled cells arranged as textura angularis. Pseudoparaphyses absent. Asci 8-spored, bitunicate, fissitunicate, clavate, oblong or elongate, with an ocular chamber. Ascospores multi-seriate or crowded, irregularly arranged in the asci, oblong to fusiform or clavate, 1-septate, slightly constricted at the septum, hyaline, smooth-walled.
Type species: Lophiosphaerella euryae (Syd. & P. Syd.) Hara (≡ Aulographum euryae Syd. & P. Syd.) [Japan, Tokyo, on Eurya chinensis, Jun. 1899, M. Shirai (syntypes S F12246, S F171544)].
Description and illustration: Li et al. (2014).
Notes: Lophiosphaerella was considered incertae sedis by Lumbsch & Huhndorf (2010) but was transferred to Mycosphaerellaceae by Li et al. (2014) based on morphological characters. This genus is insufficiently known, and the type species needs to be recollected and subjected to molecular analysis.
Marcosia Syd. & P. Syd., Ann. Mycol.14: 96. 1916.
Type species: Marcosia ulei Syd. & P. Syd. [Brazil, Brazilia, on leaves of Cynometra bauhiniifolia] ≡ Stigmina ulei (Syd. & P. Syd.) B. Sutton.
Description: Sydow & Sydow (1916).
Notes: Marcosia is based on Marcosia ulei and is considered a synonym of Stigmina ulei. The genus Stigmina is currently considered a synonym of Pseudocercospora (Crous et al. 2013a). However, since no molecular data are available for this species, the current name remains in Stigmina.
Madagascaromyces U. Braun, C. Nakash., Videira & Crous
Note: See treatment in text.
Megaloseptoria Naumov, Bolêz. Rast. 14: 144. 1925.
Description (from Quaedvlieg et al. 2013, adapted from Sutton 1980): Mycelium immersed, branched, septate, brown. Conidiomata pycnidial, separate, globose, slightly papillate, dark brown to black, superficial, sessile, often aggregated in groups, unilocular, thick-walled; wall of several cell layers of brown textura angularis, more darkly pigmented on the outside. Ostiole single, circular. Conidiophores hyaline, branched, septate (mainly at the base), smooth, straight or irregular, formed from the inner cells of the pycnidial wall. Conidiogenous cells enteroblastic, determinate, discrete or integrated, doliiform, ampulliform or irregularly cylindrical, hyaline, smooth, collarette evident, channel wide, periclinal thickening present. Conidia hyaline to pale brown with several transverse eusepta, continuous, tapered near the obtuse apex and truncate base, thin-walled, smooth, cylindrical, straight or slightly curved, often with 2 guttules in each cell.
Type species: Megaloseptoria mirabilis Naumov [Russia, on Picea pungens].
Description and illustration: Quaedvlieg et al. (2013)
Notes: A specimen of Megaloseptoria mirabilis collected by Naomov in Russia was located in BPI (BPI 389179), but was not observed. See also Quaedvlieg et al. (2013).
Melanodothis R.H. Arnold, Canad. J. Bot. 49: 2188. 1972 (1971).
Description (adapted from Arnold 1971): Ascostromata arising from a hypostroma formed within the ovary and perigynum, black, subglobose, multilocular, wall composed of a textura angularis with pseudoparenchymatic cells. Locules in a single layer beneath the surface of the stroma, each with an ostiole. Microconidial locules are formed in the early stages of ascostromata. Microconidia (spermatia) narrowly oblong, hyaline, formed on short projections on the hyaline cells lining the microconidial cavity. Asci oblong to rarely oblong-pyriform, aparaphysate, sessile, 8 spored, arising from a basal cushion of pseudoparenchymatic cells. Ascospores hyaline, one celled, thick-walled, narrowly ellipsoidal, with ends sometimes narrowly and abruptly tapered. Conidiophores indeterminate. Conidiogenous cells holoblastic. Macroconidia ramularia-like, hyaline, smooth, catenulate, branched or unbranched, cylindrical, aseptate or 1 septate, with a disc-like hilum at each end. Blastoconidia formed singly at the apex of hyphae in the periphery of the colony or on the long cylindrical conidia as secondary conidia, one celled, hyaline, smooth, ovoid.
Type species: Melanodothis caricis R.H. Arnold [Canada, on flowers of Carex aquatilis var. dives (= C. sitchensis), (holotype DAOM 116433, ex-type culture CBS 860.72 = ATCC 24309)].
Description and illustration: Arnold (1971).
Notes: The ex-type culture of Melanodothis caricis clusters in Cladosporiaceae, suggesting that Melanodothis is an older name for Davidiella. However, the name presently being used for this genus is that of the asexual morph, Cladosporium (Bensch et al. 2012). Arnold (1971) reported the presence of an ascostroma with pseudothecial locules and this could be consistent with the variation observed in Davidiella (see Schubert et al. 2007). Furthermore, he also reported ramularia-like conidia, which could be Cladosporium, which at times mutates, and produces hyaline conidia with darkened hila only. Furthermore, the relation of this species to the North American Ramularia caricis U. Braun (Braun 1998) has to be proven.
Microcyclosporella Jana Frank, Schroers & Crous
Note: See treatment in text.
Microcyclus Sacc. et al., Ann. Mycol. 2: 165. 1904.
Description (from Monkai et al. 2013): Biotrophic on leaves and stems. Ascostromata pulvinate, irregularly shaped, developing from central basal hypostroma, superficial, multilocular, composed of textura angularis, thick-walled, reddish brown. Ostiole papillate, periphysate. Asci 8-spored, thick-walled, bitunicate, fissitunicate, cylindrical to clavate, with an ocular chamber, with a long pedicel. Ascospores 1–3-seriate, 1-septate, obovoid, upper cell shorter and wider than lower, not or slightly constricted at the septum, smooth wall, granular, hyaline.
Type species: Microcyclus angolensis Sacc. et al. [Angola, on living leaves of Millettia thonningii, Welwitsch (holotype S F8592, isotype S F8593)].
Notes: Monkai et al. (2013) placed the genus in Mycosphaerellaceae, and even though molecular data are lacking, this assumption seems likely. The genus Microcyclus includes an important pathogen on Hevea, Microcyclus ulei, that was recently recollected and transferred to Pseudocercospora based on morphological and molecular data (Da Hora Júnior et al. 2014).
Micronectriella Höhn., Sitzungsber. Kaiserl. Akad. Wiss. Wien, Math.-Naturwiss. Cl., Abt. 1, 115: 1194. 1906.
Type species: Micronectriella pterocarpi (Racib.) Höhn. (≡ Micronectria pterocarpi Racib.) [Indonesia, Java, on leaves of Pterocarpus indicus] = Sphaerulina pterocarpi (Racib.) Arx & E. Müll.
Description and illustration: Von Arx & Müller (1975).
Notes: The genus Sphaerulina was recently shown to be distinct from others in the Mycosphaerellaceae (Quaedvlieg et al. 2013), but fresh collections are required to determine the phylogenetic position of Micronectriella. The type specimen could not be located.
Micronematomyces U. Braun, C. Nakash., Videira & Crous
Note: See treatment in text.
Miuraea Hara
Note: See treatment in text.
Mycodiella Crous
Note: See treatment in text.
Mycoporis Clem., The Genera of Fungi: 50, 173. 1909.
Description (from Thambugala et al. 2014): Parasitic on leaves. Ascomata appearing as black spots on the host surface, gregarious, scattered, superficial, very easily removed from the host surface, globose, uniloculate, ostiolate. Peridium one-layered, composed of dark to brown cells of textura angularis. Haemathecium lacking pseudoparaphyses. Asci eight-spored, bitunicate, broadly cylindrical to fusiform, sessile, with a large ocular chamber. Ascospores overlapping, uniseriate at the apex to tri-seriate near the base, hyaline, 5-septate, strongly constricted at the primary septum, broadly fusiform to cylindrical with broadly rounded ends.
Type species: Mycoporis perexigua (Müll. Arg.) Clem. (≡ Mycoporellum perexiguum Müll. Arg.) [Australia, Queensland, Brisbane, Bailey, on bark (holotype G 00110864)].
Description and illustration: Thambugala et al. (2014).
Notes: Thambugala et al. (2014) allocated the genus to Mycosphaerellaceae based on its ascomatal morphology. Since there are no available DNA sequences the phylogenetic position of Mycoporis remains unresolved.
Mycosphaerelloides Videira & Crous
Note: See treatment in text.
Mycovellosiella Rangel
Note: See treatment in text.
Neoceratosperma Crous & Cheew.
Note: See treatment in text.
Neocercospora M. Bakhshi, Arzanlou, Babai-ahari & Crous
Note: See treatment in text.
Neocercosporidium Videira & Crous
Note: See treatment in text.
Neodeightoniella Crous & W.J. Swart
Note: See treatment in text.
Neomycosphaerella Crous
Note: See treatment in text.
Neoovularia U. Braun, Nova Hedwigia 54: 473. 1992.
Description (from Videira et al. 2016, adapted from Braun 1998): Phytopathogenic, causing leaf spots. Caespituli amphigenous, whitish to pink or ochraceous. Mycelium consisting of hyaline to faintly pigmented, septate, branched, thin-walled hyphae forming well-developed stromata. Conidiophores arising from stromata, emerging through stomata or erumpent through the cuticle, often forming sporodochia, subcylindrical, subclavate, simple, thin-walled, smooth, hyaline or lightly pigmented, continuous or septate. Conidiogenous cells integrated, terminal, straight to moderately geniculate-sinuous, polyblastic and sympodial, conidiogenous loci numerous, conspicuous, bulging, papilla-like, but not thickened and darkened, at most slightly refractive. Conidia formed singly, subglobose, obovoid, ellipsoid, aseptate, hyaline to faintly pigmented, thin-walled, smooth to verruculose, basal hilum not thickened or darkened; conidial secession schizolytic.
Type species: Neoovularia nomuriana (Sacc.) U. Braun (≡ Tuberculina nomuriana Sacc.) [Japan, Kikotaro, on Astragalus sinicus, 1903, Nomura (holotype PAD)].
Descriptions and illustrations: Braun, 1998, Videira et al., 2016.
Notes: The phylogenetic position of Neoovularia remains unresolved since no DNA from the type species is available. See treatment in Braun (1998) and Videira et al. (2016).
Neopenidiella Quaedvlieg & Crous
Note: See treatment in text.
Neophloeospora Videira & Crous
Note: See treatment in text.
Neopseudocercospora Crous
Note: See treatment in text.
Neopseudocercosporella Videira & Crous
Note: See treatment in text and Videira et al. (2016).
Neoramularia U. Braun, Nova Hedwigia 53: 291. 1991.
Description (from Videira et al. 2016, adapted from Braun 1998): Phytopathogenic, causing leaf spots. Mycelium consisting of hyaline or subhyaline, septate, branched, thin-walled hyphae forming stromata or not. Conidiophores macronematous, usually in large fascicles, sometimes forming sporodochial and basistromatic conidiomata, emerging through stomata or erumpent through the cuticle, straight, subcylindrical to geniculate-sinuous, simple, hyaline or faintly pigmented, continuous or septate, thin-walled, smooth or occasionally rough. Conidiogenous cells integrated, terminal, polyblastic, percurrent and sympodial, conidiogenous loci inconspicuous, not thickened or darkened. Conidia solitary or catenate, ellipsoid-ovoid, subcylindrical or fusoid, hyaline or slightly pigmented, aseptate to 3- septate, thin-walled, smooth or almost so, hila unthickened and hyaline, conidial secession schizolytic.
Type species: Neoramularia eurotiae (Gamalizk.) U. Braun (≡ Ramularia eurotiae Gamalizk.) [Kyrgyzstan, Central Tien-Shan, on Krascheninnikovia ceratoides, 5 Jun. 1958, Gamalitzkaya (holotype LE 41968] = Neoramularia kochiae (Woron.) U. Braun (Azerbeijan, on Kochia sp.).
Description and illustration: Braun, 1991, Videira et al., 2016.
Notes: Neoramularia is ramularia-like but differs in having unthickened and not darkened conidiogenous loci and conidial hila, i.e. characteristic Ramularia loci and hila are lacking. Cultures from fresh specimens must be obtained in order to determine the phylogenetic position of this genus based on DNA sequences. See treatment in Videira et al. (2016).
Neoseptoria Quaedvlieg, Verkley & Crous
Note: See treatment in text.
Nothopassalora U. Braun, C. Nakash., Videira & Crous
Note: See treatment in text.
Nothopericoniella Videira & Crous
Note: See treatment in text.
Nothophaeocryptopus Videira, C. Nakash., U. Braun, Crous
Note: See treatment in text.
Oedothea Syd., Ann. Mycol. 28: 202. 1930.
Description (adapted from Sydow 1930): Stromata on leaf veins, forming small gall-like swellings, subepidermal, erumpent through longitudinal fissures, exposed surface dull black-brown, finely pulverulent to floccose by abundant superficial conidia, intramatrical stromata composed of brown hypertrophic cells of the host tissue, interrupted by small to larger cavities, and sparingly developed filamentous, hyaline hyphae. Conidia in small to larger aggregations, broad ovate, ellipsoid to subglobose, with a single median septum, barely constricted, dark brown but transparent.
Type species: Oedothea vismiae Syd.
Description and illustrations: Sydow, 1930, Seifert et al., 2011; present study (Fig. 60).
Fig. 60.
Oedothea vismiae (NY 00945740). A–E. Observations in vivo. A. Leaf spot symptoms on the host. B–D. Conidiophores and conidia. E. Conidia. Scale bars = 10 μm.
Materials examined: Venezuela, Los Naranjos pr. Puerto la Cruz, on leaves of Vismia hamanii, 7 Jan. 1928, H. Sydow 183 (holotype S F42267).
Notes: The phylogenetic position of Oedothea is unknown and only its hyphomycetous asexual morph is known (Seifert et al. 2011). Sequence data are necessary to determine its phylogenetic position.
Ophiocarpella Theiss. & Syd., Ann. Mycol. 13: 644. 1915.
Description (adapted from Theiss & Sydow 1915): Like Montagnella, but paraphyses lacking, ascospores colourless, filiform, septate. Stromata hypophyllous, black, irregular, with dense protuberant loculi, half immersed in the host tissue, connected by vertical hyphal strands, apex free, protuberant through the epidermis. Hyphae greyish brown, with swollen cells, several layers around the loculi forming a kind of wall, dense between loculi, sparingly developed below, loculi globose, non-ostiolate. Asci fasciculate, paraphyses lacking, 8-spored. Ascospores polystichous, colourless, filiform, with a distinct median septum, possibly with several septa when mature.
Type species: Ophiocarpella tarda (Harkn.) Theiss. & Syd. (≡ Ophiodothis tarda Harkn.) (USA, California, San Francisco, on fruit of Rhus diversiloba, H.W. Harkness (holotype BPI 798419)]) ≡ Sphaerulina tarda (Harkn.) M.E. Barr.
Description (no illustration): Theissen & Sydow (1915).
Notes: Based on morphology, Ophiocarpella was considered as a synonym of Sphaerulina. Fresh collections are required to determine the phylogenetic position of Ophiocarpella tarda.
Ophiocladium Cavara, Z. Pflanzenkrankh. 3: 26. 1893.
Type species: Ophiocladium hordei Cavara [Cavara, Z. Pflanzenkrankh. 3: Plate (Tab.) I, Fig. 9, 1893 (lectotype designated by Braun 2017)] [Austria, Reichersberg am Inn, on Hordeum vulgare (epitype designated by Braun 2017, CBS H-22641, culture ex-epitype CBS 101180)] ≡ Ramularia collo-cygni B. Sutton & J.M. Waller.
Description and illustration: See Braun (1998, as Ramularia collo-cygni).
Notes: See treatment in Videira et al. (2016) as Ramularia collo-cygni. The typification of the type species has recently been clarified by Braun (2017).
Oreophylla Cif., Sydowia 8: 253. 1954.
Description (adapted from Ciferi 1954): Biotrophic. Mycelium internal, superficial hyphae lacking. Conidiophores in fascicles, arising from an immersed pseudostromatic base, erect, brown, unbranched, straight to tortuose, septate. Conidia solitary, acrogenous, cylindrical-attenuated, transversely pluriseptate, straight to curved, hyaline.
Type species: Oreophylla angelae-mariae Cif. (as ‘angelaemariae’) [Dominican Republic, Hato del Yaque, on leaves of Gliricidia sepium] = Passalora gliricidiasis (Gonz. Frag. & Cif.) R.F. Castañeda & U. Braun.
Descriptions and illustrations: Ciferri, 1954, Ellis, 1976, as Cercosporidium gliricidiasis).
Notes: Deighton (in Ellis 1976) considered Oreophylla angelae-mariae a synonym of Sirosporium gliricidiae (Syd.) Deighton (≡ Passalora gliricidiae (Syd.) U. Braun & Crous), but Braun et al. (1999) stated that this species has to be reduced to synonymy with Passalora gliricidiasis. Oreophylla was treated as synonym of Passalora s. lat. by Crous & Braun (2003), but as DNA of Passalora gliricidiasis (= Oreophylla angelae-mariae) is not available , the phylogenetic position of Oreophylla remains unresolved.
Ormathodium Syd., Ann. Mycol. 26: 138. 1928.
Description (adapted from Sydow 1928): Leaf spots lacking. Caespituli hypophyllous, regularly spread, loose to dense, mostly on tips of stellate hairs, rarely on the epidermis. Conidiomata superficial, 30–130 μm diam, globose to hemispherical, more rarely irregular, with a basal dense plectenchymatous stroma composed of yellow to olivaceous brown hyphae, equipped with short protuberant free ends [conidiophores] giving rise to simple or dichotomously branched conidial chains. Conidia oblong, often almost cylindrical, more rarely fusiform, olivaceous brown, transversely 1–2-septate, not or only slightly constricted at the septa.
Type species: Ormathodium styracis Syd. [Costa Rica, San José, Rio Torres, on leaves of Styrax argenteus].
Description (no illustration): Sydow (1928).
Notes: The genus Ormathodium was considered a synonym of Mycovellosiella by Muntañola (1960), and subsequently placed in synonymy of Passalora by Crous & Braun (2003). Unfortunatelly, the type material of this genus has not been preserved (Crous & Braun 2003), and this synonymy remains unconfirmed.
Ovosphaerella Laib., Centralbl. Bakteriol., 2. Abth., 55: 293. 1922.
Description (based on Laibach 1922): Introduced for a mycosphaerella-like sexual morph with an Ovularia asexual morph.
Type species: Ovosphaerella lapathi Laib. [Germany, on Rumex sp.) ≡ Mycosphaerella lapathi (Laib.) Petr.
Description: Von Arx (1983, as Mycosphaerella lapathi).
Notes: Laibach (1922) introduced Ovosphaerella as genus for the mycosphaerella-like sexual stage of Ovularia obliqua (current name Ramularia rubella, see Braun 1998). Ramularia obovata, another synonym of Ramularia rubella, was linked to Mycosphaerella lapathi by von Arx (1983). The type material of Ovosphaerella lapathi is probably missing (Aptroot 2006). Fresh collections are required to confirm this relationship, and clarify the phylogenetic position of Ovosphaerella. In case that the connection between these sexual and asexual morphs on Rumex were correct, Ovosphaerella would be a synonym of Ramularia.
Ovularia Sacc., Michelia 2(no. 6): 17. 1880.
Type species: Ovularia obovata (Fuckel) Sacc. (≡ Ramularia obovata Fuckel) [Germany, Erbach, on Rumex crispus, Fuckel, Fungi Rhen. Exs. 1635 (lectotype HAL)] = Ramularia rubella (Bonord.) Nannf.
Description: Braun (1998, as Ramularia rubella).
Note: See Videira et al. (2016) for neotypification details of Ramularia rubella.
Pachyramichloridium Videira & Crous
Note: See treatment in text.
Pallidocercospora Crous
Note: See treatment in text.
Pantospora Cif.
Note: See treatment in text.
Paracercospora Deighton
Note: See treatment in text.
Paracercosporidium Videira & Crous
Note: See treatment in text.
Paramycosphaerella Crous & Jol. Roux
Note: See treatment in text.
Paramycovellosiella Videira, H.D. Shin & Crous
Note: See treatment in text.
Parapallidocercospora Videira, Crous, U. Braun, C. Nakash.
Note: See treatment in text.
Parastenella J.C. David, Mycol. Res. 95: 124. 1991.
Description (from Braun et al. 2013): Dematiaceous hyphomycete genus resembling Zasmidium (in vivo with superficial mycelium, hyphae, conidiophores and solitary conidia pigmented, distinctly verruculose to verrucose), but the conidiogenous cells are terminal and intercalary, denticulate, with lateral short peglike protuberances, conidiogenous loci inconspicuous, neither thickened nor darkened.
Type species: Parastenella magnoliae (Weedon) J.C. David (≡ Heterosporium magnolia Weedon) [USA, Florida, St. Petersburg, on leaves of Magnolia grandiflora, 15 Feb. 1923, A.J. Weedon (holotype ILL 6019, isotypes BPI 443255, 443261, 443270, 443274, K(M), MICH 15715)].
Illustration: Braun et al. (1995).
Notes: The phylogenetic position of this genus is unknown; it should be recollected to resolve this uncertainty. See also notes under Zasmidium gupoyu in text.
Passalora Fr.
Note: See treatment in text.
Periconia Tode, Fung mecklenb. sel. (Lüneburg) 2: 2. 1791.
Description (adapted from Ellis 1971): Colonies effuse, occasionally small and compact, grey, brown, olivaceous brown or black, hairy. Mycelium mostly immersed but sometimes partly superficial. Stroma frequently present, mid to dark brown, pseudoparenchymatous. Setae and hyphopodia absent. Conidiophores micro- and macronematous, mononematous, with a stipe and spherical head, branches present or absent, stipe straight or flexuous, rarely torsive, pale to dark brown or black, smooth or rarely verrucose, apex sometimes sterile and setiform. Conidiogenous cells mono- or polyblastic, discrete on stipe and branches, determinate, ellipsoidal, spherical or subspherical. Conidia catenate, often in branched chains, usually spherical or subspherical, occasionally ellipsoidal, oblong or broadly cylindrical, pale to dark brown, verruculose or echinulate, aseptate.
Type species: Periconia lichenoides Tode.
Descriptions and illustrations: Mason and Ellis, 1953, Ellis, 1971, Seifert et al., 2011.
Notes: Periconia is currently the type genus of the Periconiaceae (Tanaka et al. 2015). The type species is not known from any recent collections and the original material is presumably lost (Ellis, 1971, Tanaka et al., 2015). The type species needs to be recollected to determine the phylogenetic position.
Periconiella Sacc.
Note: See treatment in text.
Phacellium Bonord.
Note: See treatment in text.
Phaeocercospora Crous
Note: See treatment in text.
Phaeoisariopsis Ferraris, Ann. Mycol. 7: 280. 1909.
Type species: Phaeoisariopsis griseola (Sacc.) Ferraris (≡ Isariopsis griseola Sacc.) [Italy, Selva, on Phaseolus vulgaris, Aug. 1877, Saccardo, Mycotheca Veneta 1247 (lectotype designated here, HAL, MBT378593] ≡ Pseudocercospora griseola (Sacc.) Crous & U. Braun [Tanzania, on Phaseolus vulgaris, F.S. Ngulu & C. Mushi (epitype designated here CBS H-19683, MBT378594, culture ex-epitype CBS 119906 = CPC 10468)].
Description and illustration: Crous et al., 2006a, Seifert et al., 2011.
Notes: The present genus has been determined as a synonym of Pseudocercospora by the phylogenetic placement of the type species Phaeoisariopsis griseola (Crous et al. 2006a). The epitype designated by Crous et al. (2006a) did not cite a lectotype, and thus this matter is addressed here.
Phaeophleospora Rangel
Note: See treatment in text.
Phaeophloeosporella Crous & B. Sutton, S. Afr. J. Bot. 63: 281. 1997.
Description (from Crous & Sutton 1997): Associatted with leaf spots. Mycelium immersed, consisting of smooth, hyaline to olivaceous, branched, septate hyphae. Conidiomata amphigenous, separate, palle yellow to light brown, acervular, subepidermal, base consisting of olivaceous cells of textura angularis. Conidiophores pale olivaceous, smooth, simple or branched at the base, septate, cylindrical, erect, formed from the upper cells of the conidioma. Conidiogenous cells integrated, terminal, smooth, pale olivaceous, cylindrical, straight to geniculate-sinuous with a subtruncate apex, proliferating sympodially and holoblastically. Conidia holoblastic, pale olivaceous, smooth, subcylindrical, straight to gently curved, obtuse at apex, and subtruncate at base, guttulate, euseptate, with inconspicuous hila.
Type species: Phaeophloeosporella ekebergiae (Syd. & P. Syd.) Crous & B. Sutton (≡ Cercosporella ekebergiae Syd. & P. Syd.) [South Africa, KwaZulu-Natal, Verulam, on leaves of Ekebergia sp., 1913, J.B. Pole Evans 6799 (holotype S F37999)].
Description and illustration: Crous & Sutton (1997).
Note: This species needs to be recollected to resolve its phylogenetic position.
Phaeoramularia Munt.-Cvetk.
Note: See treatment in text.
Pharcidia Körb., Parerga lichenol. (Breslau) 5: 469. 1865.
Type species: Pharcidia congesta Körb. [Europe, on thallus of Lecanora subfusca, (holotype in L, fide Santesson (1960)] ≡ Stigmidium congestum (Koerb.) Triebel.
Note: See Triebel et al. (1991).
Phloeospora Wallr.
Note: See treatment in text.
Phlyctaeniella Petr., Ann. Mycol. 20(5/6): 323. 1922.
Description (from Quaedvlieg et al. 2013): Mycelium immersed, branched, septate, hyaline. Conidiomata eustromatic, separate, immersed, pale brown, globose, unilocular, scarcely erumpent; side wall and base of several cell layers of hyaline, thin-walled textura angularis, above of larger pale brown tissue. Ostiole indistinct, and dehiscence by rupture of the upper wall. Conidiophores hyaline, smooth, septate, irregularly branched, especially at the base, formed from the inner cells of the stroma wall. Conidiogenous cells phialidic, integrated or discrete, determinate, hyaline, markedly tapered at the apices, smooth, with apical or lateral apertures, collarette minute, with periclinal thickening; only rarely becoming percurrent. Conidia hyaline, smooth, thinwalled, irregularly guttulate, filiform, straight, curved or irregular, multiseptate (Sutton 1980).
Type species: Phlyctaeniella polonica Petr. [Austria, on Aruncus dioicus (= A. silvestris)].
Description and illustration: Quaedvlieg et al. (2013).
Notes: The type specimen of the present species could not be traced. The phylogenetic position of this genus remains unresolved until fresh specimens are collected.
Placocrea Syd., Ann. Mycol. 37: 380. 1939.
Description (from Sydow 1939): Ascomata aggregated in stroma, immersed, globose to ovoid, with papillate ostiole. Asci clavate to cylindrical-clavate, 8-spored. Ascospores biseriate, oblong-clavate to fusoid, medianly 1-septate, constricted at septum, hyaline, pseudoparaphyses present.
Type species: Placocrea pulchella Syd. [Equador, Prov. Pichincha, Mindo, on leaves of Sarcorhachis sydowii, 1937, H. Sydow 252 and 284 (syntypes, NY 01102921, NY 01102922, RMS0017369, S F44505; S F44506; BPI 631051 and Syd., Fungi Exot. Exs. 1200, e.g. S F8589].
Notes: This genus is insufficiently known, and needs to be recollected to resolve its phylogenetic position. Lumbsch & Huhndorf (2010) tentatively place this genus in Mycosphaerellaceae based on its morphological characters.
Pleopassalora Videira & Crous
Note: See treatment in text.
Pleuropassalora U. Braun, C. Nakash., Videira & Crous
Note: See treatment in text.
Pleurovularia R. Kirschner & U. Braun, Mycoscience 43: 16. 2002.
Description (from Kirschner et al. 2002): Phytoparasitic, conidiophores macronematous, mononematous, hyaline, simple or sparsely branched, verruculose at least in the distal part, emerging mainly through the outer wall of epidermal cells of the host, conidiogenous cells intercalary and terminal with slightly thickened, pigmented scars, mono- or polyblastic, producing hyaline conidia with vacuole.
Type species: Pleurovularia polliniae (Henn.) R. Kirschner & U. Braun (≡ Ovularia polliniae Henn.).
Description and illustration: Kirschner et al., 2002, Seifert et al., 2011, present study (Fig. 61).
Fig. 61.
Pleurovularia polliniae (S F43065). A–E. Observations in vivo. A. Leaf spot symptoms on the host. B. Conidiophores, conidiogenous cells and conidium. C, D. Conidiophores and conidiogenous cells. E. Conidia. Scale bars = 10 μm.
Materials examined: Japan, Prov. Tosa, Katakasa-mura, on Pollinia imberbis, Jun. 1901, T. Yoshinaga No. 25 (holotype S F43065).
Notes: The phylogenetic position of Pleurovularia is unknown and only the hyphomycetous asexual morph is known. It is necessary to recollect the type species and obtain cultures to determine the phylogenetic position of Pleurovularia.
Pluripassalora Videira & Crous
Note: See treatment in text.
Polyphialoseptoria Quaedvlieg, R.W. Barreto, Verkley & Crous
Note: See treatment in text.
Polysporella Woron., Izv. Kavkazsk. Muz. 10: 7. 1916.
Description: Ascomata pseudothecial, scattered, immersed, later erumpent through the epidermis, flattened, 120–150 μm diam, 75–90 μm high, parenchyma composed of polygonal cells, 12–15 μm diam. Asci oval, apex thickened, sessile, 60–67 × 30–32 μm, aparaphysate, 24–27(–32?)-spored. Ascospores 1-celled, at first hyaline, late slightly brown, oblong-ovate, aggregated, 20–22 × 7–8 μm.
Type species: Polysporella woronowii Woron. [Turkey (locality historically situated in Russia), eastern Anatolia, Province Kars, Kağızman (‘district Kaghyzman, Novo-Nikolaevka’), on stems of Dianthus crinitus, 7 Jun. 1913, G. Woronow (type TBIP)].
Description and illustration: Woronichin (1916: 7, fig. 3).
Notes: This genus is insufficiently known, and needs to be recollected to resolve its phylogenetic position. The allocation of Polysporella to Mycosphaerelaceae dates back to Lumbsch & Huhndorf (2007: 79, no 4542), with reference to ‘O. Eriksson, in litt.’ However, the position of this genus and its assignment to Mycosphaerelaceae are quite unclear and unproven, which was also confirned by T. Lumbsch and O. Eriksson (pers. comm.). The locality of the holotype was historically located in the Russian Province Kars (Karsskaya Oblast), district Kagizman (Kaghyzman), which nowadays belongs to Turkey (southeast Anatolia, Province Kars, Kağızman). The continued existence and possible current Turkish name of the settlement ‘Novo-Nikolaevka’ could not be clarified.
Polythrincium Kunze, in Kunze & Schmidt, Mykologische Hefte (Leipzig) 1: 13. 1817.
Synonym: Cymadothea F.A. Wolf, Mycologia 27: 71. 1935.
Description (from Ellis 1971): Colonies punctiform or effuse, olivaceous brown. Mycelium immersed. Stroma pseudoparenchymatous, brown to black. Setae and hyphopodia absent. Conidiophores macronematous, mononematous, caespitose, unbranched or with several branches arising at one point, the upper part curved and often thickened on the side away from the curvature, undulate, often torsive, mid pale brown, smooth. Conidiogenous cells polyblastic, integrated, terminal, sympodial, cylindrical, undulate, cicatrized; scars large, flat, unilateral. Conidia solitary, acropleurogenous, simple, cuneiform or pyriform, hyaline to pale brown, smooth or verruculose, 1-septate.
Type species: Polythrincium trifolii Kunze [Germany, on leaves of Trifolium pratense].
Descriptions and illustrations: Sivanesan, 1984, Ellis, 1971, Simon et al., 2009.
Notes: This species is an obligate biotroph, and does not grow in culture. The phylogenetic link between the sexual morph, Cymadothea trifolii, and Polythrincium trifolii was confirmed by Simon et al. (2009). In addition, Simon et al. (2009) determined the phylogenetic position of the genus as belonging in Mycosphaerellaceae by extracting DNA directly from lesion caused by the pathogen on Trifolium repens collected in Germany (CBS H-20110).
Prathigada Subram., J. Madras Univ. 26: 366. 1956.
Type species: Prathigada cratevae (Syd.) Subram. (≡ Napicladium cratevae Syd.) ≡ Pseudocercospora cratevicola C. Nakash. & U. Braun.
Description and illustration: Braun et al. (2013), present study (Fig. 62).
Fig. 62.
Prathigada crataevae (IMI 234117 and IMI 182578). A–G. Observations in vivo. A, B. Leaf spot symptoms on the host. C, D, F. Conidiophores, conidiogenous cells and conidia. E. Conidiogenous cell and conidium. G. Conidia. Scale bars = 10 μm.
Materials examined: India, Madras, Coimbatore, Government Farm, on Crateva religiosa, 5 Feb. 1912, W. McRae 9 (holotype S F42112); Calcuta, on Crateva nurvala, 30 May 1978, J.B. Ray PCC2700 Dep. Botany Presidency College (IMI 234117). Tutkon, on Crateva religiosa, 20 Nov. 1973, Mya Tharng (IMI 182578). Japan, on Crataeva falcata, 18 Sep. 1998, S. Uematsu & C. Nakashima, culture MUCC 1088.
Notes: Braun et al. (2013) examined type material of this species and compared it with conspecific Japanese collections on Crateva formosensis. The morphological characteristics are quite uniform among the observed specimens (Fig. 62). Sequences retrieved from Japanese cultures (MUCC 1088, Table 1, Fig. 1) clusted within the big Pseudocercospora clade close to Pseudocercospora fijiensis. Thus, Prathigada was reduced to synonymy with Pseudocercospora (Braun et al. 2013).
Protostegia Cooke, Grevillea 9(49): 19. 1880.
Description (from Crous et al. 2015a): Conidiomata immersed, becoming somewhat erumpent, solitary, exuding a mucoid conidial cirrhus, pale brown, splitting the leaf surface, with central ostiole; wall of brown textura intricata. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, smooth, lining the inner cavity, lageniform to subcylindrical, proliferating percurrently at apex. Conidia hyaline, smooth, scolecosporous, euseptate.
Type species: Protostegia eucleae Kalchbr. & Cooke [South Africa, on Euclea undulata, (epitype designated by Crous et al. 2015a: PREM 60879, culture ex-epitype CPC 23549 = CBS 137232)].
Description and illustration: Crous et al. (2015a).
Notes: Protostegia is a coelomycetous genus only known from its asexual morph and was recently epitypified (Crous et al. 2015a). Based on the epitype, the phylogenetic position of this genus is close to Cytostagonospora martiniana (Crous et al. 2015a).
Pseudocercospora Speg.
Note: See treatment in text.
Pseudocercosporella Deighton
Note: See treatment in text.
Pseudocercosporidium Deighton
Description (from Braun et al. 2013): Foliicolous, plant pathogenic, leaf spotting hyphomycetes, teleomorph unknown. Mycelium internal. Stromata lacking. Conidiophores in vivo solitary or in small loose fascicles (groups) emerging through stomata, laxly erect, macronematous, frequently branched, septate, pigmented (very pale brown), thin-walled, smooth; conidiogenous cells integrated, terminal, intercalary or pleurogenous (as lateral branchlets), sympodial, polyblastic, conidiogenous loci conspicuous, protruding, convex (papilla-like), but wall of the loci neither thickened nor darkened, only somewhat refractive. Conidia solitary, didymo- to scolecosporous, pigmented (deeper in pigmentation than the conidiophores), thin-walled, smooth or almost so, hila neither thickened nor darkened.
Type species: Pseudocercosporidium venezuelanum (Syd.) Deighton.
Description and illustration: Ellis, 1971, Crous and Braun, 2003, Seifert et al., 2011, Braun et al., 2013, present study (Fig. 63).
Fig. 63.
Pseudocercosporidium venezuelanum (S F38692). A–H. Observations in vivo. A. Leaf spot symptoms on the host. B–D. Conidiophores, conidiogenous cells and conidia. E–G. Conidiogenous cells and conidia. H. Conidia. Scale bars = 10 μm.
Material examined: Venezuela, Aragua, between La Victoria and Guacamaya, on Cordia heterophylla, 3 Jan. 1928, H. Sydow No. 381 (holotype S F38692).
Notes: The phylogenetic position of Pseudocercosporidium venezuelanum is unknown due to the absence of DNA sequence data. Morphologically, Pseudocercosporidium resembles Passalora, but differs in the structure of the conidiogenous loci that are not thickened or darkened (Braun 2013).
Pseudodidymaria U. Braun, Cryptog. Bot. 4: 110. 1993.
Description (from Videira et al. 2016, adapted from Braun 1998): Phytopathogenic, causing leaf spots. Mycelium consisting of hyaline or faintly pigmented, septate, thin-walled and branched hyphae, forming well developed stromata. Conidiomata basistromatic and sporodochial. Conidiophores arranged in palisade-like fascicles, subcylindrical, subclavate, straight to flexuous, sinuous, rarely septate, hyaline to faintly pigmented, thin-walled, smooth, sometimes reduced to conidiogenous cells. Conidiogenous cells integrated, terminal, polyblastic, sympodial, conidiogenous loci bulging, unthickened or with a thickened rim, not darkened but refractive. Conidia formed singly, ellipsoid-obovoid, subclavate, aseptate to 2-septate, base rounded to broadly truncate, hyaline to faintly pigmented, thin-walled, smooth to verruculose, hilum unthickened, not darkened but refractive, conidial secession schizolytic.
Type species: Pseudodidymaria wyethiae (Ellis & Everh.) U. Braun (≡ Marssonina wyethiae Ellis & Everh.) [USA, California, Sonoma, on Wyethia glabra, 25 May 1894, Blasdale (lectotype NY 01087025; isolectotypes Ellis & Everh., Fungi Columb. 589 and Ellis & Everh., N. Amer. Fungi 3184)].
Descriptions and illustrations: Braun, 1998, Videira et al., 2016.
Notes: Pseudodidymaria is tentatively maintained as a separate genus. Molecular data are required to fully resolve its phylogenetic position.
Pseudophaeoramularia U. Braun, Trudy Bot. Inst. im. V.L. Komarova 20: 18. 1997.
Type species: Pseudophaeoramularia geranii (W.B. Cooke & C.G. Shaw) U. Braun (≡ Cercosporella geranii W.B. Cooke & C.G. Shaw) [USA, Washington State, Whiteman Co., on Geranium viscossissimum, 20 Jul. 1948, Shaw & Coheen (holotype WSP 19945)] ≡ Pseudocercospora geranii (W.B. Cooke & C.G. Shaw) U. Braun.
Description and illustration: Braun & Mel'nik (1997).
Notes: Braun & Mel'nik (1997) introduced Pseudophaeoramularia as intermediate between Pseudocercospora and Phaeoramularia. Although the genus has since been treated as synonymous with Pseudocercospora (Crous et al., 2001b, Crous et al., 2013a), phylogenetic proof from the type species is still lacking to confirm this synonymy.
Pseudopericoniella Videira & Crous
Note: See treatment in text.
Pseudophaeophleospora U. Braun, C. Nakash., Videira & Crous
Note: See treatment in text.
Pseudopuccinia Höhn., Mitt. Bot. Inst. Techn. Hochsch. Wien 2: 41. 1925.
Description (adapted from Ellis 1976): Stromata present. Conidiophores with anellations. Conidia pale to brown, verrucose, ellipsoid-obovoid with 1–2 transverse septa and occasionally an oblique septum.
Type species: Pseudopuccinia thermopsidis (Harkn.) Höhn. [as ‘thermopsis’] (≡ Stigmina thermopsidis Harkn.) [USA, California, on Thermopsis californica].
Description and illustration: Ellis (1976, as Stigmina thermopsidis).
Notes: Pseudopuccinia was considered to be a synonym of Stigmina (Seifert et al. 2011). Based on DNA sequence comparisons, the genus Stigmina was treated as synonym of Pseudocercospora (Braun and Crous, 2006, Crous et al., 2013a). However, the phylogenetic position of Pseudopuccinia, a stigmina-like genus, is unknown, pending fresh collections and molecular analyses.
Pseudostigmidium Etayo, Biblioth. Lichenol. 98: 193. 2008.
Description (Etayo & Sancho 2008): Lichenicolous. Ascomata perithecioid, black, subconical or subglobose, immersed to semiimmersed, protruding, periphyses abundant, paraphyses lacking, gelatinuous hymenial mass I+, KI+ red to violaceous. Asci bitunicate, fissitunicate, clavate, broad obovoid to saccate, apically thickened, with an ocular chamber, wall I+, KI+ red to violaceous, 8-spored. Ascospores ellipsoid, ellipsoid-ovoid, fusiform, (0–)1–3-septate, colourless, sometimes becoming somewhat pigmented with age.
Type species: Pseudostigmidium nephromiarium (Linds.) Etayo. (≡ Microthelia nephromiaria Linds.) [Chile, Cape Horn, Hermit Island, on thalus and apothecia of Nephromium cellulosum, Antartic expedition 1839-43, Dr. Hooker.]
Description and illustrations: Etayo & Sancho (2008).
Notes: Pseudostigmidium includes lichenicolous species that are only known by their sexual morph. Hyde et al. (2013) accepted this genus in Mycosphaerellaceae, but it needs to be recollected before its phylogenetic position can be resolved.
Pseudovularia Speg., Anales Mus. Nac. Hist. Nat. Buenos Aires, Ser. 3, 13: 418. 1911.
Type species: Pseudovularia trifolii Speg. [Argentina, Lezama, on Trifolium pratense, 2 Nov. 1904, Spegazzini (holotype LPS 12946)] = Ramularia sphaeroidea Sacc. [Germany, Berlin, Spandau, on Lotus uliginosus, Jul. 1875, Magnus (type PAD)].
Descriptions and illustrations: Spegazzini, 1910, Deighton, 1972.
Notes: Pseudovularia is considered a synonym of Ramularia based on morphological characteristics and Pseudovularia trifolii is currently a synonym of Ramularia sphaeroidea (Braun, 1998, Videira et al., 2016). However, no material originating from the type of Pseudovularia trifolii has thus far been obtained for further DNA studies.
Pseudozasmidium Videira & Crous
Note: See treatment in text.
Quasiphloeospora B. Sutton et al., Mycol. Res. 100: 979. 1996.
Description (from Sutton et al. 1996): Foliicolous, associated with lesions. Mycelium internal, brown, branched, septate. Conidiomata separate, acervular to sporodochial, epidermal to subepidermal, composed of brown textura angularis at the base, and textura prismatica above. Conidiophores brown, verruculose, irregularly branched at the base, septate, cylindrical, formed from the upper cells of the conidiomata. Conidiogenous cells integrated, terminal or lateral, smooth or verruculose, brown, cylindrical, straight, proliferating percurrently and enteroblastically to form annellations or sympodially and holoblastically; conidiogenous loci dark and thickened. Conidia holoblastic, pale brown, smooth, cylindrical, septate, obtuse at the apex and truncate at the base; basal scar dark and thickened.
Type species: Quasiphloeospora saximontanensis (Deighton) B. Sutton et al. (≡ Cercospora saximontanensis Deighton) [USA, Wyoming, Grand Tehon National Park, on leaves of Ribes viscosissimum, 16 Aug. 1937, W.G. & R. Solheim & H.F. House 5369, Solh., Mycofl. Saximon. Exs. 1191 (holotype IMI 98069, isotypes Solh., Mycofl. Saximon. Exs. 1191, e.g. BPI 762561, PUL 25574).
Descriptions and illustrations: Sutton et al., 1996, Seifert et al., 2011.
Notes: Quasiphloeospora is a cercosporoid genus with intricate morphology and complex morphological relations to several other genera, including Cercospora, Passalora and Pseudocercospora (Crous & Braun 2003), but due to very pale, almost hyaline structures, it is also similar to Pseudocercosporella. Sutton et al. (1996) classified the conidiomata as acervuli, although they may better be referred to as sporodochia. The particular characters of Quasiphloeospora saximontanensis, above all the structure of the conidiogenous loci, are intermediate between the three similar genera cited above. A clear affiliation to one of these genera, just based on morphology, is not possible. It is also possible that this species is unrelated to any of the cercosporoid genera. Affinity and position of Quasiphloeospora can only be proven by means of results of molecular sequence analyses, which are, however, not yet available.
Ragnhildiana Solheim
Note: See treatment in text.
Ramularia Unger
Note: See treatment in text.
Ramichloridium Stahel ex de Hoog
Note: See treatment in text under Zasmidium.
Ramulariopsis Speg.
Note: See treatment in text.
Ramularisphaerella Kleb., Haupt- und Nebenfruchtformen der Ascomyzeten (Leipzig) 1: 131. 1918.
Description (from Klebahn 1918): “Ramularisphaerella. Konidienform Ramularia. Arten: R. hieracii, fragariae, punctiformis, maculiformis, tussilaginis” [Ramularisphaerella. Conidial form Ramularia. Species: R. hieracii, fragariae, punctiformis, maculiformis, tussilaginis].
Type species: Ramularisphaerella hieracii (Sacc. & Briard) Kleb. (≡ Sphaerella nebulosa var. hieracii Sacc. & Briard, ≡ Mycosphaerella hieracii (Sacc. & Briard) Jaap) [France, on Hieracium sp.].
Description and illustration: Sivanesan (1984, as Mycosphaerella hieracii and Ramularia hieracii), Braun (1998, as Ramularia hieracii).
Notes: Klebahn (1918) introduced Ramularisphaerella as new genus for a mycosphaerella-like sexual morph on Hieracium that he considered to be linked to Ramularia on hawkweed. The type specimen could not be located (Aptroot 2006), and the status of this genus remains unclear due to the absence of DNA sequence data. Jaap (1908) considered this species to be the sexual morph of Ramularia hieracii (Bäumler) Jaap. Klebahn (1918) has proved the connection between the ascus and conidial state. In case that this connection was correct, Ramularisphaerella would be a synonym of Ramularia.
Ramulispora Miura
Note: See treatment in text.
Rasutoria M.E. Barr, Mycotaxon 29: 501. 1987.
Description (from Barr 1987): Ascomata pseudothecial, globose, superficial, densely clustered on mycelium on the undersides of leaves, dark brown, with numerous hyphal appendages, brown, obtuse, septate ot not. Asci saccate, bitunicate, oblong, paraphysate. Ascospores hyaline to pale brown, obovoid, 1-septate.
Type species: Rasutoria abietis (Dearn.) M.E. Barr (≡ Dimerosporium abietis Dearn.) [USA, Washington, on needles of Abies amabilis (holotype BPI 691065)].
Illustration: Farr (1963).
Notes: A very similar description was also presented by Dearness (1926), as Dimerosporium abietis. See treatment in text under Zasmidium cellare.
Rhabdospora (Durieu & Mont.) Sacc., Syll. fung. (Abellini) 3: 578. 1884.
Description (adapted from Saccardo 1884): Pycnidia (perithecia) subcuticular-erumpent, globose-depressed, papillate, solid, soon subhysterioid, black or brown, usually neither on spots nor on leaves. Spores [conidia] bacilliform or filiform, pluriguttulate or pluriseptate, hyaline. ‘Basidia’ diverse or lacking. Differs from Septoria like Phoma from Phyllosticta.
Type species: Rhabdospora oleandri (Durieu & Mont.) Sacc. (≡ Septoria oleandri Durieu & Mont.) [Algeria, on Nerium oleander].
Description and illustration: Bory de St.-Vincent & Durieu de Maisonneuve (1849).
Notes: Rhabdospora is a poorly known genus from which many species are currently placed in Septoria. The type species needs to be recollected in order to resolve its phyllogenetic position (Quaedvlieg et al. 2013). The type specimen could not be located.
Rhachisphaerella Videira & Crous
Note: See treatment in text.
Rhopaloconidium Petr., Sydowia 6: 300. 1952.
Type species: Rhopaloconidium asiminae (Ellis & Morgan) Petr. (≡ Phloeospora asiminae Ellis & Morgan) [USA, Ohio, Preston, on Asimina triloba, H.P. Morgan 463 (holotype NY 01097272)] ≡ Pseudocercospora asiminae (Ellis & Morgan) U. Braun & Crous.
Description and illustration: Braun (1995, as Miuraea asiminae).
Notes: Braun & Crous (2008) proposed the combination of Phloeospora asiminae into Pseudocercospora. Sequence data authentic for the type species of this genus are necessary to confirm the synonymy of Rhopaloconidium and Pseudocercospora.
Rosisphaerella Videira & Crous
Note: See treatment in text.
Rosenscheldiella Theiss. & Syd., Ann. Mycol. 13: 645. 1915.
Description (adapted from Sultan et al. 2011): Ascomata globose, dark-walled. Pseudothecia develop on stromatic pads of globose cells with thick, dark walls that form amongst thick-walled, multi-lobed hairs on lower surface of leaves. Hamathecium lacking. Asci fissitunicate, cylindrical, 8-spored. Ascospores cylindrical, tapering slightly to rounded ends, 1 median septum, slightly constricted at septum, hyaline.
Type species: Rosenscheldiella styracis (Henn.) Theiss. & Syd. (≡ Naemacyclus styracis Henn.) [Brazil, São Paulo, Morro pelado, on Styrax sp.].
Descriptions and illustrations: Sultan et al. (2011).
Notes: The type species Rosenscheldiella styracis is only known from its sexual morph. The genus Rosenscheldiella is currently accepted in the Mycosphaerellaceae (Wijayawardene et al. 2014) but recollection of the type species is necessary to determine its true phylogenetic position. Two species for which there are cultures available, Rosenscheldiella brachyglottidis and Rosenscheldiella korthalsellae, cluster in the Mycosphaerellaceae and are closely related to Pseudocercospora and Amycosphaerella, respectively (Sultan et al. 2011).
Ruptoseptoria Quaedvlieg, Verkley & Crous
Note: See treatment in text.
Scirrhia Nitschke ex Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 220. 1870 (1869–1870).
Description (from Sivanesan 1984): Stromata subepidermal to erumpent, elongated, depressed globose, rounded, unilocular or with locules in many rows, opening by an apical pore. The stromatic wall is composed of vertically-orientated rows of brown to reddish brown cells of textura globosa or angularis and textura prismatica between locules; the outermost layers composed of black to dark brown cells and the cells of the inner layers brown to hyaline. Asci oblong or clavate, 8-spored, stalked, arising from compressed hyaline tissue at the base of the locule. Ascospores biseriate overlapping in the ascus, hyaline or yellowish, elliptic or obovoid, septate near the middle, not or slightly constricted at the septum, straight or often inequilateral, smooth, sometimes guttulate. Interthecial tissue compressed between asci and intact over the asci.
Type species: Scirrhia rimosa (Alb. & Schwein.) Fuckel (≡ Sphaeria rimosa Alb. & Schwein.) [Germany, Lusatia (Lausitz), on stems of Phragmites australis].
Notes: Scirrhia rimosa is presently not known from available collections. Scirrhia aspidiorum (CBS 204.66) clusters in the Didymellaceae, while Scirrhia brasiliensis (CBS 128762) clusters in Mycosphaerellaceae (Crous et al. 2011d). The status of purported synonyms of Scirrhia, namely Scirrhodothis, Scirrhophragma and Metameris also remains unresolved.
Scolecostigmina U. Braun
Note: See treatment in text.
Semipseudocercospora J.M. Yen, Mycotaxon 17: 361. 1983.
Description (from Braun et al. 2013): Morphologically close to Pseudocercospora (leaf spotting hyphomycetes with unthickened, not darkened conidiogenous loci and hila), but the conidiogenous cells are not geniculate, i.e. not distinctly sympodially proliferating, the conidiogenous loci are distinctly denticle-like, and the solitary conidia are didymo- to phragmosporous, i.e. not scolecosporous.
Type species: Semipseudocercospora peristrophes-acuminatae (J.M. Yen) J.M. Yen (≡ Cercospora peristrophes-acuminatae J.M. Yen).
Description and illustrations: Yen, 1983, Seifert et al., 2011, present study (Fig. 64).
Fig. 64.
Semipseudocercospora peristrophes-acuminatae (IMI 122324). A–E. Observations in vivo. A. Leaf spot symptoms on the host. B–D. Partial conidiophore, conidiogenous cells and conidia. E. Single conidia. Scale bars = 10 μm.
Materials examined: Singapore, Katung, on Peristropha acuminata, 20 Apr. 1964, Sun No. 20 (holotype PC; isotype IMI 122324).
Notes: The phylogenetic position of the type species of this genus and its relation to the Mycosphaerellaceae as well as to the genus Pseudocercospora are still unknown and unproven. Therefore, Semipseudocercospora is tentatively maintained as a separate cercosporoid genus.
Septocylindrium Bonord. ex Sacc., Michelia 2: 15. 1880.
Type species: Septocylindrium bonordenii Sacc., nom. illegit., Art. 52.1 [Italy, Padova, on Galanthus nivalis, Apr. 1876, Sacc., Mycoth. Ven. 1050 (neotype HAL)] = Ramularia septata (Bonord.) Bubák.
Description and illustration: Braun (1998, as Ramularia septata).
Notes: Septocylindrium is currently accepted as a synonym of Ramularia (Braun, 1998, Videira et al., 2016). However, no DNA sequences are available of the type species and that assumption, therefore needs to be re-evaluated.
Septocyta Petr., Ann. Mycol. 25(3/4): 330. 1927.
Description (from Quaedvlieg et al. 2013, adapted from Sutton 1980): Mycelium immersed, branched, septate, hyaline to pale brown. Conidiomata eustromatic, immersed, separate, erumpent, dark brown to black, finally opening widely, unilocular, multilocular or convoluted, thick-walled; wall of pale brown, thin-walled textura angularis except in the dehiscent region which is darker brown and more thick-walled. Ostiole absent, dehiscence by breakdown of the upper wall. Conidiogenous cells are holoblastic, sympodial with 1–3 apical, scarcely protruding, unthickened denticles, indeterminate, discrete, ampulliform to lageniform, hyaline, smooth, formed from the inner cells of the locular walls. Conidia hyaline, 1–3 euseptate, smooth, straight or slightly curved, acicular, apex obtuse, base truncate, often with minute guttules associated with septa.
Type species: Septocyta ramealis (Roberge ex Desm.) Petr. (≡ Septoria ramealis Roberge ex Desm.) [Europe, on stems of Rubus spp.]
Description and illustration: Quaedvlieg et al. (2013).
Notes: The type specimen could not be traced. See Quaedvlieg et al. (2013).
Septopatella Petr., Ann. Mycol. 23(1/2): 128. 1925.
Description (from Quaedvlieg et al. 2013, adapted from Dyko & Sutton 1979 and Sutton 1980): Mycelium immersed, branched, septate, hyaline to subhyaline. Conidiomata superficial, often subtended by a superficial, pale brown, septate, branched mycelium, pulvinate, separate to occasionally aggregated, dark brown to black, finally opening widely, cupulate; basal wall of small-celled, brown, thin-walled textura angularis, becoming textura porrecta as it merges into the periclinal walls; a hypostroma attaches the conidioma to the substrate; Ostiole absent. Conidiophores hyaline, septate, branched at the base, thin-walled, cylindrical, formed from the gelatinized basal wall of the conidioma. Conidiogenous cells holoblastic, sympodial, integrated, indeterminate, cylindrical, hyaline, smooth, produced as 2–3 branches from the apex of the conidiophores. Conidia hyaline, 3–4-euseptate, thin-walled, smooth, minutely guttulate, straight or curved, occasionally irregularly filiform (Dyko and Sutton, 1979, Sutton, 1980).
Type species: Septopatella septata (Jaap) Petr. (≡ Pseudocenangium septatum Jaap) [Austria, Pinus montana, 31 Jul. 1907, O. Jaap (holotype BPI 393484; isotype IMI 225733, slide)].
Description and illustration: Quaedvlieg et al. (2013)
Notes: The present species needs to be recollected and its phylogenetic position determined. The holotype specimen could not be traced. See Quaedvlieg et al. (2013).
Septoria Sacc.
Note: See treatment in text.
Septoriopsis Gonz. Frag. & M.J. Paúl, Bol. Real Soc. Esp. Hist. Nat. 15: 127. 1915.
Description (adapted from Saccardo et al. 1931): Pycnidia on leaf spots, superficial, membraneous-carbonaceous, usually caespitose, globose to conoid. Spores [conidia] bacilliform, hyaline, usually 1-septate, formed at the apex of filiform conidiophores.
Type species: Septoriopsis citri Gonz. Frag. [Spain, Sevilla, Huevar, on Citrus vulgaris, M. de Paul].
Description and illustration: González Fragoso (1915).
Note: Seen as synonym of Septoria, though fresh collections are required to resolve its phylogenetic position.
Septorisphaerella Kleb., Haupt- und Nebenfruchtformen der Ascomyzeten (Leipzig) 1: 131. 1918.
Description (from Klebahn 1918): “Septorisphaerella. Konidienform Septoria oder Phloeospora. Arten: S. hippocastani, populi, ribis, sentina, ulmi, aegopodii, exitialis, jaczewskii, lathyri, nigerristigma” [Ramularisphaerella. Conidial form Septoria or Phloeospora. Species: S. hippocastani, populi, ribis, sentina, ulmi, aegopodii, exitialis, jaczewskii, lathyri, nigerristigma].
Type species: Septorisphaerella hippocastani (Jaap) Kleb. (≡ Sphaerella maculiformis var. hippocastani Jaap), [Germany, Brandenburg, Prignitz, Triglitz, on Aesculus hippocastanum, Mar. 1910, O. Jaap, Fungi Sel. Exs. 423 (syntypes Jaap, Fungi Sel. Exs. 423, e.g. B, HAL, L)] = Mycosphaerella hippocastani Jaap.
Description and illustration: Klebahn (1918).
Notes: Klebahn (1918) introduced Septorisphaerella as genus for sexual mycosphaerella-like morphs associated with septoria-like asexual morphs. Septorisphaerella hippocastani, the type species, was linked to a Septoria on Aesculus which was nomenclaturally discussed in detail, with the conclusion to refer to it as ‘Septoria aesculicola (Fr.) Fuckel’ (including Septorisphaerella hippocastani Berk. & Broome, see Klebahn 1918: 45). Fresh material of the type species needs to be recollected to resolve the phylogenetic position of this genus, above all since Septoria s. lat. has recently been split into several genera (see Verkeley et al. 2013).
Sirosporium Bubák & Serebrian., Hedwigia 52: 273. 1912.
Description (from Braun et al. 2013): Leaf spotting dematiaceous hyphomycetes with internal and external mycelium, superficial hyphae giving rise to solitary conidiophores, lateral and terminal, conidiophores may also be formed in fascicles, conspicuous conidiogenous loci and hila, thickened and darkened, conidia solitary, size, shape and septation variable, but the conidia are relatively thick-walled and at least partly dictyosporous.
Type species: Sirosporium antenniforme (Berk. & M.A. Curtis) Bubák & Serebrian.
Descriptions and illustrations: Ellis, 1971, Seifert et al., 2011; present study (Fig. 65).
Fig. 65.
Sirosporium antenniforme (IMI 1253). A–F. Observations in vivo. A, B. Conidiophores emerging from the host leaf. C–F. Conidia. Scale bars = 10 μm.
Materials examined: USA, Alabama, on leaves of Celtis (microscope slide ex-type of Macrosporium antenniforme, IMI 1253).
Notes: The genus Sirosporium is passalora-like in morphology, but until the type species S. antenniforme has been recollected and its phylogenetic position resolved, its status remains unresolved. Sirosporium has been tentatively treated as a separate genus confined to species with thick-walled dictyosporous conidia (Braun, 1995, Crous and Braun, 2003, Braun et al., 2013). The two Sirosporium species included in this study cluster within the Mycosphaerellaceae but in separate clades, Sirosporium celtidis (Fig. 1, clade 39; Fig. 3, clade 4) and Sirosporium diffusum (Fig. 1; clade 60; Fig. 3, clade 24, as Ragnhildiana diffusa).
Sonderhenia H.J. Swart & J. Walker
Note: See treatment in text.
Sphaerellothecium Zopf, Nova Acta Acad. Caes. Leop.-Carol. German. Nat. Cur. 70: 184. 1897.
Description (adapted from Roux & Triebel 1994 and Knudsen et al. 2009): Lichenicolous, usually distinguished by the formation of a superficial reticulum of dark hyphae occurring on the thallus and apothecia of the host. Ascomata perithecioid, black, immersed to superficial, ostiolate, wall pigmented, hamathecium of unbranched periphyses, but often rudimentary, with colourless interascal filaments (paraphysoids). Asci bitunicate, fissitunicate, 8-spored, clavate, ellipsoid-ovoid, obpyriform, saccate to irregular, with distinct ocular chamber. Ascospores hyaline, sometimes turning brown when mature, 1–3(–5)-septate, smooth-walled.
Type species: Sphaerellothecium araneosum (Rehm) Zopf (≡ Sphaerella araneosa Rehm) [Austria, Tirol, oberhalb der Waldrast (Mattrei), on Ochrolechia tartarea, Aug. 1872, Arnold, ex Herb. Rehm. Ascomyc. nr. 133 (syntype S F45258, designated here as lectotype MBT378595)].
Description: Vouaux (1913, as Discothecium araneosum).
Note: The type species is lichenicholous and, in the absence of DNA, its phylogenetic position remains obscure.
Sphaerulina Sacc.
Note: See treatment in text.
Spilosphaeria Rabenh., Klotzschii Herb. Viv. Mycol., Ed. Nov., Ser. Prima, Cent. 6: no. 559. 1857.
Type species: Not indicated (Rabenhorst assigned eight species to the new genus Spilosphaeria in Cent. 6 of this exsiccatum).
Notes: This genus is insufficiently known, but regarded as synonym of Septoria based on morphology. The status of Spilosphaeria needs to be clarified by lectotypification of the genus and recollection of a lectotype species to determine the phylogenetic position of the genus.
Stenella Syd., Ann. Mycol. 28(1–2): 205. 1930.
Type species: Stenella araguata Syd. [Venezuela, Aragua, La Victoria, on leaves of Pithecellobium lanceolatum, Jan. 1928, H. Sydow (lectotype, designated in Crous et al. 2007b, IMI 15728a; isolectotypes BPI 443420, 443422, S F64888; syntypes Syd., Fungi Exot. Exs. 883, e.g. CUP, MICH 13093, S F64890, Petr., Mycoth. Gen. 1399, e.g. S F64889)].
Description and illustration: Crous et al. (2007b).
Note: Currently assigned to Teratosphaeriaceae (Crous et al., 2007b, Arzanlou et al., 2008, Crous et al., 2009d).
Stenospora Deighton, Mycol. Pap. 118: 22. 1969.
Description (from Deighton 1969): Mycelium hyperparasitic: hyphae colourless, septate. Conidiophores arising as lateral branches of the mycelial hyphae, short, smooth, simple or branched, septate, with conidial scars very slightly but distinctly thickened and refractive and slightly prominent. Conidia colourless, acicular, much resembling those of Cercospora, smooth, pluriseptate, with a very slightly but distinctly thickened and refractive truncate hilum.
Type species: Stenospora uredinicola Deighton [Sierra Leone, Bundulai (Loko Masama), on Puccinia kraussiana on Smilax anceps (= S. kraussiana), 26 Nov. 1951, F.C. Deighton M4515 (holotype IMI 48655b)].
Description and illustration: Deighton (1969).
Note: Stenospora is very similar to Eriocercospora but mucedinaceous (hyaline).
Stenellopsis B. Huguenin, Bull. Trimestriel Soc. Mycol. France 81: 695. 1966.
Description (from Ellis 1971): Colonies effuse, greyish olive, hairy. Mycelium immersed. Stroma rudimentary or prosenchymatous, immersed. Setae and hyphopodia absent. Conidiophores macronematous, mononematous, caespitose, unbranched, usually rather short, straight or flexuous, olivaceous, smooth or verruculose. Conidiogenous cells polyblastic, integrated, terminal, sympodial, cylindrical, cicatrized; scars broad, flat. Conidia solitary, dry, acropleurogenous, simple, cylindrical to obclavate, rounded at the apex, truncate at the base, pale olivaceous brown, verrucose, multiseptate.
Type species: Stenellopsis fagraeae Huguenin.
Descriptions and illustrations: Ellis, 1971, Seifert et al., 2011; present study (Fig. 66).
Fig. 66.
Stenellopsis fragariae (PDD 75945). A–G. Observations in vivo. A. Leaf spot symptoms on the host. B. Conidiophores. C–G. Conidiophores, conidiogenous cells and conidia. Scale bars = 10 μm.
Materials examined: Cook Islands, Rarotonga, Takitumu Conservation Area, on Fagraea berteroana, 14 Jul. 2002, E.H.C. McKenzie EHCM 284 (PDD 75945); Rarotonga, Totokoitu Valley, 19 Oct. 1975, J.M. Dingley (PDD 35381). New Caledonia, Rivière de Thi (St. Louis), on Fagraea berteroana (= F. schlechteri), 24 Nov. 1963, Huguenin, NC 63219 (holotype PC).
Notes: Stenellopsis is morphologically similar to Zasmidium. It has single, conspicuously verrucose conidia with hila that are barely to slightly thickened and somewhat darkened-refractive, but lacks verruculose superficial hyphae (Crous & Braun 2003). The type species needs to be recollected to resolve the phylogenetic position of the genus.
Stictosepta Petr., Sydowia 17: 230. 1964 (1963).
Description (from Quaedvlieg et al. 2013, adapted from Sutton 1980): Mycelium immersed, branched, septate, hyaline. Conidiomata eustromatic, immersed, globose to collabent, papillate, unilocular, often convoluted, hyaline; walls thick, of hyaline, thin-walled textura intricata. Ostiole central and circular, single, furfuraceous. Conidiophores hyaline, septate, branched, anastomosing, formed from the inner cells of the locular wall. Conidiogenous cells sympodial or synchronous, integrated, indeterminate, hyaline, thin-walled, with usually two small, unthickened, apical, slightly protuberant conidiogenous loci. Conidia solitary, hyaline, thin-walled, smooth, multiseptate, slightly constricted at the septa, each cell medianly guttulate, straight or curved, base truncate, apex obtuse.
Type species: Stictosepta cupularis Petr. [Czech Republic, Hranice, Ribar, Fraxinus, 30 Mar. 1927, F. Petrak (syntype BPI 668877, designated here as lectotype MBT378596; syntype IMI 204093 [slide])].
Illustration: Quaedvlieg et al. (2013).
Note: This species needs to be recollected to resolve its phylogenetic position.
Stigmidium Trevis., Conspect. Verruc.: 17. 1860.
Description (adapted from Roux & Triebel 1994): Vegetative hyphae absent. Ascomata perithecioid, black, globose to subglobose, ostiolate, usually half-immersed to sessile. Ascomatal wall dark brownish black in upper part, paler brown in middle or lower part. Periphysoids originating from the upper wall of the ascomatal cavity, hyaline, branched or not. Interascal filaments lacking. Asci originating from the lower wall of the ascomatal cavity, fissitunicate, saccate, 8-spored, with ascospores irregularly arranged. Ascospores 1-septate, hyaline, but occasionally turning brown when overmature.
Type species: Stigmidium schaereri (A. Massal.) Trevis. (≡ Sphaeria schaereri A. Massal.) [Italy, on thalli of Solorina spp.].
Description and illustration: Roux & Triebel (1994).
Notes: The genus Stigmidium is distinguished by ascomata with punctiform ostioles with a hamathecium of periphyses, with periphysoids, and hyaline 1-septate ascospores (rarely turning brown in a few species). The type species is lichenicolous and until DNA data have been generated, the phylogenetic position of the genus remains unresolved.
Stigmina Sacc., Michelia 2: 22. 1880.
Type species: Stigmina platani (Fuckel) Sacc. (≡ Stigmella platani Fuckel) [Greece, Attikis, Kifisia, on Platanus orientalis, 7 Nov. 1869, Th. de Heldreich (syntype BPI 428005, designated here as lectotype, MBT378597] ≡ Pseudocercospora platanigena Videira & Crous, nom. nov. MycoBank MB822835. Replaced synonym: Stigmella platani Fuckel, in Thümen, Bot. Zeitung (Berlin). 29: 27. 1871, non Pseudocercospora platani (J.M. Yen) J.M. Yen, 1979.
Description and illustration: Ellis (1971).
Note: Stigmina is a synonym of Pseudocercospora (Braun and Crous, 2006, Crous et al., 2006a) and a new name is herewith introduced for Stigmina platani.
Stromatoseptoria Quaedvlieg, Verkley & Crous
Note: See treatment in text.
Sultanimyces Videira & Crous
Note: See treatment in text.
Tandonella S.S. Prasad & R.A.B. Verma, Indian Phytopathol. 23: 112. 1970.
Description (from Sutton & Pascoe 1987): Mycelium in vivo immersed and superficial, subhyaline to pigmented, branched, septate, thin-walled. Stromata superficial, small, brown, pseudoparenchymatic. Conidiomata synnematous, synnemata composed of parallel threads, determinate, solitary or grouped, erect, brown, apically lax, splaying out. Individual conidiophores filiform, usualy unbranched, septate, pigmented, smooth to rough-walled; conidiogenous cells integrated, terminal or intercalary (conidiogenous region terminal, rarely lateral or extending down the synnemata), proliferation sympodial, geniculate, cicatrized, conidiogenous loci conspicuous, slightly thickened, darkened-refractive, often protuberant. Conidia holoblastically formed, catenate, in short simple or branched chains, ellipsoid-ovoid, fusiform, cylindrical, aseptate to euseptate, pigmented, rough-walled, hila somewhat thickened and darkened-refractive.
Type species: Tandonella ziziphi S.S. Prasad & R.A.B. Verma [India, Bihar, on leaves of Ziziphus jujuba (holotype IMI 112255c as Cercospora ziziphi)] ≡ Passalora ziziphi (S.S. Prasad & R.A.B. Verma) U. Braun & Crous.
Description and illustration: Sutton & Pascoe (1987).
Notes: Tandonella has currently been treated as a synonym of Passalora (Crous and Braun, 2003, Braun et al., 2013), but the type species Tandonella ziziphi is not known from DNA data. Sutton & Pascoe (1987) added Tandonella oleariae, re-examined and illustrated holotype material of Tandonella ziziphi [IMI 112255c], and published an emended description of the genus Tandonella, which is characterised by a combination of synnematous conidiomata and conspicuous conidiogenous loci (thickened and darkened) giving rise to catenate, pigmented conidia (phaeoramularioid). The species Tandonella cubensis was described by Castañeda & Kendrick (1990) and the holotype was collected from Bauhinia divaricata in Cuba [INIFAT C88/58 (13.IV.1988)]. The strain in this study was collected by the same author from Bauhinia cuyabensis in Cuba and was deposited at the CBS [CBS 500.92, INIFAT C92/43-3 (Nov. 1992), CBS H-18755). The morphology of Tandonella cubensis varied from Tandonella ziziphi mainly in the falcate, lunate or irregular and smooth conidia instead of cylindrical to fusiform and verrucose conidia. Morphologically, Tandonella cubensis (CBS 500.92) differs significantly from Pseudocercospora spp. by the formation of long synnematous fascicles, dark brown at the base and brown above, brown, polyblastic conidiogenous cells and brown, falcate conidia developing in chains. In all phylogenetic analyses performed in this study, this strain clustered within Pseudocercospora.
Tapeinosporium Bonord., Bot. Zeitung (Berlin) 11: 285. 1853.
Description (from Bonorden 1853): Conidial chains multiseptate, arising from aseptate, simple or sometimes branched “stalks” [conidiophores]. Spores [conidia] ovate, 3-septate, greenish, caespitose conidial chains olivaceous or later black.
Type species: Tapeinosporium viride Bonord. [Germany, on Solanum tuberosum (lectotype [iconotype] designated here, MBT378598, Bonorden, Bot. Zeitung (Berlin) 11: Pl. (Tafel) VII, Fig. 6. 1853)] ≡ Septocylindrium tapeinosporum (Bonord.) Sacc.
Description and illustration: Bonorden (1853).
Notes: Saccardo (1886) considered Tapeinosporium a synonym of Septocylindrium, which in turn is considered a synonym of Ramularia (Braun, 1998, Videira et al., 2016), but as emphasized in Braun (1998: 13) Tapeinosporium, described from potato tubers, is a doubtful genus of quite unclear affinity. Type material is not preserved, but Bornorden added an illustration to the original description, which is part of the protologue and has to be used for lectotypification. This illustration does not agree with genuine Ramularia species and could rather pertain to Cladosporium or similar saprobic hyphomycetous genera. New collections from potato tubers are necessary for an epitypification of Tapeinosporium viride and corresponding sequence data for a clarification of its phylogenetic affinity.
Trochophora R.T. Moore
Note: See treatment in text.
Utrechtiana Crous & Quaedvlieg, Persoonia 26: 153. 2011.
Description (from Crous et al. 2011a): Hyphomycetous, associated with leaf spots. Mycelium internal, consisting of septate, smooth, hyaline, branched hyphae. Conidiophores solitary, erect, bursting through epidermis, with circular scar where base of conidiophore is attached to immersed hyphal network; conidiophores dark brown, erect, base subglobose, giving rise to a subcylindrical, brown conidiogenous cell that ends in a clavate, bluntly rounded apex, with truncate, flattened scar; sometimes thickened, not darkened, nor refractive. Conidia solitary, pale brown, ellipsoid, guttulate to granular, smooth to finely verruculose, 1-septate slightly above the conidial median, thin-walled, apex bluntly to acutely rounded, base obtusely rounded with a flattened, darkened and thickened hilum that has a central pore.
Type species: Utrechtiana cibiessia Crous & Quaedvlieg [Netherlands, Utrecht, on leaves of Phragmites australis, 14 Dec. 2010, W. Quaedvlieg (holotype CBS H-20594, cultures ex-type CPC 18917, 18916 = CBS 128780)] = Utrechtiana roumeguerei (Cavara) Videira & Crous [France, Toulouse, on Phragmites australis, undated, coll. C. Roumeguère, Biosi & Cavara, syntypes of Scolicotrichum roumeguerei Briosi & Cavara, Funghi Parass. Piante Colt. Util. Ess. 112 (lectotype in HAL here designated, MBT378701)].
Utrechtiana roumeguerei (Cavara) Videira & Crous, comb. nov. MycoBank MB822836.
Basionym: Scolicotrichum roumeguerei Cavara (as ‘roumegueri’), in Briosi & Cavara, Funghi Parass. Piante Colt. Util. Ess., Fasc. 5: no. 112. 1890.
Synonyms: Deightoniella roumeguerei (Cavara) Constant., Proc. K. Ned. Akad. Wet., Ser. C, Biol. Med. Sci. 86(2): 137. 1983.
Utrechtiana cibiessia Crous & Quaedvl., Persoonia 26: 153. 2011.
Notes: The genus Utrechtiana was regarded as synonymous with Deightoniella by Seifert et al. (2011) based on morphology. The type species, Utrechtiana cibiessia, is synonymous with Deightoniella roumeguerei, which Klaubauf et al. (2014) showed to belong to Pyriculariaceae, a family containing numerous cryptic fungal genera on Poaceae. An examination of the type species of Deightoniella, Deightoniella africana, has shown, however, that Deightoniella is also a generic complex in Pyriculariaceae, meaning that the generic circumscription provided by Ellis (1976) needs to be emended. Deightoniella torulosa, a foliar pathogen of Musa, has been shown to be a species of Corynespora (Crous et al. 2013b). A similar fungus occurring on leaf spots of Phragmites in South Africa, was shown to represent a distinct genus, Neodeightoniella, which lacks conidiophores with percurrent rejuvenation, has well-developed apical and intercalary conidiogenous loci, and conidia with mucoid caps (Crous et al. 2013b). The genus Deightoniella (based on Deightoniella africana), is distinct from Utrechtiana, as the latter lacks torsive to flexuous conidiophores with percurrent rejuvenation and prominent conidiophore swellings. Conidia of Utrechtiana are also very pale brown, smooth to finely roughened, with prominent thickened, darkened scars, while those of Deightoniella are medium brown, verruculose, and obpyriform with prominent apical taper. Fresh material of Deightoniella africana needs to be recollected to facilitate epitypification, and to clarify its phylogenetic relationships.
Uwemyces Hern.-Restr., G.A. Sarria & Crous
Note: See treatment in text.
Verrucisporota D.E. Shaw & Alcorn
Note: See treatment in text under Zasmidium.
Virgasporium Cooke, Grevillea 3(28): 182. 1875.
Type species: Virgasporium maculatum Cooke [Jersey (UK), on leaves of Reseda sp.] = Cercospora resedae Fuckel [Germany, on leaves of Reseda odorata, Fuckel, Fungi Rhen. Exs. nr. 1632 (syntype S F267614)
Description and illustration: Cooke (1875).
Note: Virgasporium is currently considered a synonym of Cercospora based on morphological characteristics (Braun et al. 2013). The type specimen of Virgasporium maculatum could not be traced and the species needs to be recollected in order to confirm its phylogenetic position. A tentative clade of Cercospora cf. resedae is considered in a recent phylogenetic study by Groenewald et al. (2013).
Virosphaerella Videira & Crous
Note: See treatment in text.
Walkeromyces Thaung, Trans. Brit. Mycol. Soc. 66: 213. 1976.
Description (from Thaung 1976): Hyphomycetous, foliicolous, phytopathogenic. Stroma, setae and hyphopodia absent. Mycelium superficial, consisting of brown, branched, septate, creeping hyphae. Conidiophores simple or branched, medium brown, arising from superficial mycelium, straight to flexuous, with intergrated terminal conidiogenous cells. Conidiogenous cells polyblastic, terminal, sympodial, with thickened, darkened scars. Conidia dry, solitary, acropleurogenous, straight or curved, obclavate or fusiform or short navicular, septate, smooth, brown, with thickened, darkened hilum.
Type species: Walkeromyces grewiae Thaung [Myanmar, Maymyo, Kyaukchaw, on Grewia cf. macrophylla, 26 Sep. 1974, M.M. Thaung (holotype IMI 188948)].
Description and illustration: Thaung (1976).
Notes: Walkeromyces is mycovellosiella-like in morphology, and has been treated as synonym of Passalora in the past (Crous & Braun 2003). However, until the type species has been recollected and subjected to molecular comparison, its phylogenetic position remains unknown.
Xenomycosphaerella Quaedvlieg & Crous
Note: See treatment in text.
Xenoramularia Videira, H.D. Shin & Crous
Note: See treatment in text.
Xenosonderhenia Crous
Note: See treatment in text.
Xenosonderhenioides Videira & Crous.
Note: See treatment in text.
Zasmidium Fr.
Note: See treatment in text.
Zymoseptoria Quaedvlieg & Crous
Note: See treatment in text.
Discussion
The Mycosphaerellaceae Lindau (1897), based on Mycosphaerella Johanson (1884) has an intricate taxonomic history spread over many years and numerous publications. From the traditional morphological approaches, to the more recent phylogenetic and genomics studies, species of Mycosphaerellaceae remain as popular among mycologists, due to their morphological diversity, and as infamous among phytopathologists due to the destructive impact some species have on crops that we depend on for food, feed and fuel.
Traditional identification relies on morphological characters in association with the host. The morphology of the sexual morph of Mycosphaerellaceae is extremely uniform and descriptions are mainly based on ascospores size, shape and position of the septa (Aptroot 2006). Believing most species to be host-specific, numerous species were described multiple times under different names only based on the hosts they were isolated from, or their countries of origin. Von Arx (1949) was the first to compare these morphological descriptions and synonymise many species in the genus, a task later continued by Tomilin (1979). Barr (1972) introduced a system of sections to treat the species which was partially followed and improved upon by Aptroot (2006), who provided the most recent revision of Mycosphaerella species based on the study of type material. Many of these specimens contained only immature or over mature material with no ascospores, rendering many species doubtful. As a consequence, only 3000 taxa were estimated to exist in Mycosphaerella out of the total 10000 names (Aptroot 2006), excluding names of thousands of asexual species. The germination pattern of the ascospores was introduced as new character by Park and Keane, 1982a, Park and Keane, 1982b, and was followed by other authors as a diagnostic feature in species recognition (Crous 1998). The morphology of the asexual morphs, on the other hand, is quite distinctive and variable, and many species in the family are also polymorphic.
Two informal asexual taxonomic groups are recognized in Mycosphaerellaceae, namely the hyphomycetes, which produce solitary conidiophores, fascicles or sporodochia, and the coelomycetes, which produce acervuli or pycnidial conidiomata. The coelomycete genera were largely treated by Sutton (1980) and, to a lesser degree, by Nag Raj (1993). The hyphomycetes, however, have been the subject of several monographs. Chupp (1954) and Pollack (1987) took a wide approach and described all cercosporoid fungi in the genus Cercospora. Deighton, 1967, Deighton, 1974, Deighton, 1976a, Deighton, 1979 recognised several genera amid the large Cercospora concept, and was succeeded by Crous & Braun (2003) who narrowed down the true cercosporoid fungi to Cercospora, Pseudocercospora, Stenella and Passalora. The hyaline counterparts of Cercospora, including Ramularia and allied genera, were treated by Braun, 1995, Braun, 1998. The separation of these genera relied on the presence or absence of pigmentation in the conidiogenous structures, arrangement and branching of conidiophores, the conidiogenous cell placement, proliferation and scar type (conidiogenous loci), and conidial formation, shape and septation. Particular emphasis was placed on the nature of the conidiogenous loci and mode of conidiogenesis. However, many difficulties surrounded the definition of these genera based on these characters including intermediate characters, and species that exhibited more than one mode of conidiogenesis (Crous & Braun 2003). Due to the impact of many of these species on agriculture and forestry, many revisions of cercosporoid species have been published based on country or geographical region, e.g. Japan (Katsuki 1965), Taiwan (Hsieh & Goh 1990), China (Guo & Hsieh 1995), South Africa (Crous & Braun, 1996), Russia (Braun & Mel'nik, 1997), Korea (Shin & Kim 2001), India (Kamal 2010), etc. However, the circumscription of genera was not questioned at the time and authors mainly followed the works of Chupp, 1954, Deighton, 1967, Deighton, 1974, Deighton, 1976a, Deighton, 1979, Braun, 1995, Braun, 1998 and Crous & Braun (2003).
Since the first DNA phylogeny paper published on the family (Stewart et al. 1999), the concept of Mycosphaerellaceae and the genera it contains has been significantly revised (Crous et al., 2007a, Crous et al., 2007b, Crous et al., 2009a, Crous et al., 2009c, Crous et al., 2009d, Crous et al., 2009e, Crous et al., 2013a, Quaedvlieg et al., 2011, Quaedvlieg et al., 2013, Quaedvlieg et al., 2014, Verkley et al., 2013, Groenewald et al., 2013, Videira et al., 2015a, Videira et al., 2015b, Videira et al., 2016). The most significant fact was the realisation that Mycosphaerellaceae was poly- and paraphyletic in the Dothideomycetes, and that the same variation also applied to the genera and species. The second milestone was the proof that Mycosphaerella was not the sexual morph of 40 odd genera as formerly believed (Crous 2009), but that these were in fact distinct genera within the Dothideomycetes, for which the names of the asexual genera were available for use.
The widespread use of DNA sequences as an identification tool fuelled an idea that was simmering for a long time among mycologists and plant pathologists alike, namely that dual nomenclature in fungi is superfluous. In its wake came the one fungus = one name initiative, which culminated in the termination of the dual nomenclature system (Hawksworth et al., 2011, Hawksworth, 2012, Wingfield et al., 2012, Crous et al., 2015b). Based on the newly revised International Code of Nomenclature for algae, fungi, and plants (ICN), the asexual morph Ramularia was chosen over that of Mycosphaerella (Wijayawardene et al., 2014, Rossman et al., 2015, Videira et al., 2015b, Videira et al., 2016), and the remaining taxa assigned to existing genera or newly described genera. The ITS was introduced as the official barcode for fungi due to its ease of amplification and its ability to distinguish species across the kingdom (Schoch et al. 2012). Frequently, in Mycosphaerellaceae, the ITS is insufficient to distinguish closely related species and a combination of ITS and a secondary barcode (Stielow et al. 2015) has been proposed for each genus, such as tef1-α or tub2 for Septoria and allied genera (Verkley et al. 2013), rpb2 or actA for Ramularia and allied genera (Videira et al. 2016), cal and his3 for Cercospora (Groenewald et al. 2013), actA and/or tef1-α (Crous et al. 2013a) or rpb2 (Nakashima et al. 2016) for Pseudocercospora, and rpb2 (present study) for many genera in the Mycosphaerellaceae. The rpb2 gene is used to resolve higher levels of classification due to the ease of alignment as the sequence has no introns, and also to discriminate at species level due to the high variability of the sequence data. The main disadvantage of rpb2 is that it is not always easy to amplify. In this regard, the primer RPB2-F4 was revealed to be very effective among numerous genera. Although the coding genes frequently have a higher discriminatory power between species, there are usually less available data in the public databases to compare them to (Quaedvlieg et al. 2013). However, this is slowly being overcome with the increasing amount of newly generated sequence data.
The present study aimed to clarify the phylogenetic position of the genera currently accepted to belong to Mycosphaerellaceae, and thus provides a broad framework and phylogeny for the family, laying a foundation for additional genera and species to be recognised and described. Recent studies have already clearly defined several genera (e.g. Cercospora, Pseudocercospora, Pseudocercosporella, Ramularia, Septoria and Zymoseptoria) but it was clear that genera such as Passalora, Zasmidium, Stenella and Ramichloridium remained para- and polyphyletic (Arzanlou et al., 2007, Arzanlou et al., 2008, Crous et al., 2009c). The sequencing of the type species of Ramichloridium and Stenella revealed them to belong to Teratosphaeriaceae, and the taxa remaining in Mycosphaerellaceae were therefore combined into existing genera (e.g. Zasmidium), or new genera (e.g. Pachyramichloridium).
The genera Phaeoramularia, Fulvia and Mycovellosiella were previously considered synonyms of Passalora (Crous & Braun 2003) since the morphological characters appeared to overlap among them. In the present study, based on the phylogenetic placement of good representative material, these four genera are revived and distinguished from one another. Previous generic definitions can no longer be applied to these genera in their current circumscription, and the description of new species is strongly reliant on the availability of DNA sequence data. Mycovellosiella, based on the present phylogeny is a monotypic genus, but with more collections new species may emerge. Mycovellosiella was previously distinguished from Passalora and Phaeoramularia by the formation of superficial mycelium with solitary conidiophores formed in vivo, but these traits are phylogenetically and taxonomically not significant and deemed unreliable. Other non-type species with mycovellosiella-like morphology cluster in the present trees at quite different positions (e.g. Paramycovellosiella and Distomycovellosiella). The formation of conidia in chains or singly, previously used to differentiate between Passalora (incl. Cercosporidium) and Phaeoramularia, is still somewhat reliable considering the species included in the present phylogeny, since the type of Passalora and species of Cercosporidium produce single conidia, whereas Phaeoramularia and Ragnhildiana produce catenate conidia. With the inclusion of more species this distinguishing character may, however, become less reliable. The genus Passalora is now restricted to species with pale brown conidiophores with apical conidiogenous cell with multiple rim-like conidiogenous loci, thickened and darkened, and single obclavate 1–2-septate conidia with a thickened and darkened hilum. New passalora-like species to be described cannot be assigned without molecular data and, if molecular data are not available, should tentatively be assigned to Passalora s. lat. This interim solution will be necessary considering the large number of species involved globally.
The particularly problematic situation pertaining to the genera Zasmidium, Periconiella, Verrucisporota and Ramichloridium, previously observed by Arzanlou et al. (2008) and Crous et al. (2009a, 2012), was addressed in the present study by taking a broad approach to the generic definition of Zasmidium, due to strong phylogenetic support of the basal branches and morphological similarity of the species involved.
The genus Phaeophleospora appeared to be polyphyletic, as previously observed by Crous et al. (2009b), and the two species that were not congeneric with the type, Phaeophleospora atkinsonii and Phaeophleospora stonei, were reassigned to the new genus Pseudophaeophleospora. The conundrum surrounding Phaeophleospora and Lecanosticta was discussed by Crous et al. (2009c). The genus Lecanosticta produces phaeophleospora-like conidia in acervular conidiomata, in contrast to the pycnidial conidiomata in Phaeophleospora. In the present phylogeny, these two genera cluster in sister clades with a strong basal support to both genera, low support for the Phaeophleospora basal branch, but strong support for the Lecanosticta basal branch. Surprisingly, the species Cytostagonospora martiniana clustered in the Phaeophleospora clade, and Phaeophleospora parsonsiae seemed to have some affinity to Lecanosticta. Until more species and further data are available to clarify this situation, however, we refrain from proposing any new combinations, since our phylogenies will always suffer from undersampling, given the many thousands of taxa included in the family.
One of the major challenges encountered in the present study was that several isolates were sterile (e.g. Sirosporium celtidis and Passalora daleae) irrespective of all the attempts with changing culture media, incubation conditions, and adding plant substrates. This was either due to the age of the isolate, or isolates requiring their respective hosts to sporulate. Fortunately, some isolates could be linked to their original works and respective morphological descriptions (e.g. Asperisporium vitiphyllum). In addition, despite the large number of taxa in the family, many have been described without the deposit of a culture in a public collection, and will therefore need to be recollected in order to resolve their phylogenetic position, which will require an enormous effort. This problem extends in retrospective to many old and obscure genera, and therefore a review of the genera associated with Mycosphaerellaceae has been included in the present study in order to motivate the recollection of these obscure fungi, which will enable us to resolve their phylogenetic relationships.
The present study addresses several problematic taxa in Mycosphaerellaceae in the light of phylogenetic analysis and morphological characterisation. Although the type species of several genera have been reliably identified and typified, many genera remain unresolved or are in need of a more in-depth study (e.g. Paramycosphaerella). The reference cultures used in this paper have, however, been deposited in a public culture collection in order to promote further research on this important family of plant pathogenic fungi. What was known as Mycosphaerella sensu Aptroot (2006), now represents a great number of different genera accommodated in different families within Dothideomycetes. As more cultures become available, new patterns of coevolution with different fungal genera and their associated host families will emerge, which we hope will eventually lead to more clarity.
Acknowledgements
This study was financially supported by the “Fonds Economische Structuurversterking (FES)”, Dutch Ministry of Education, Culture and Science grant BEK/BPR-2009/137964-U, “Making the Tree of Life Work”. We thank the CBS technical staff, Arien van Iperen, Ijda Vlug and Trix Merkx (cultures), Marjan Vermaas (photographic plates), and Mieke Starink-Willemse (partial DNA isolation, amplification and sequencing) for their assistance. We would also like to thank all the mycologists who deposited their specimens in the CBS and CPC collections over the years and made this work possible. We would like to thank the curators and staff of the E, K, M, NY, PDD, S, UC and UPS herbaria for all the specimens sent for study.
Footnotes
Peer review under responsibility of Westerdijk Fungal Biodiversity Institute.
References
- Aly A.H., Debbab A., Proksch P. Fungal endophytes: unique plant inhabitants with great promises. Applied Microbiology Biotechnology. 2012;90:1829–1845. doi: 10.1007/s00253-011-3270-y. [DOI] [PubMed] [Google Scholar]
- Aptroot A. Mycosphaerella and its anamorphs 2. Conspectus of Mycosphaerella. CBS Biodiversity series. 2006;5:1–231. [Google Scholar]
- Arnold R.H. The Nectriella element of “Hyalodothis”. Mycologia. 1967;59:246–254. [Google Scholar]
- Arnold R.H. Melanodothis caricis, n. gen., n. sp. and 'Hyalodothis? caricis'. Canadian Journal of Botany. 1971;49(12):2187–2196. [Google Scholar]
- Arzanlou M., Groenewald J.Z., Fullerton R.A. Multiple gene genealogies and phenotypic characters differentiate several novel species of Mycosphaerella and related anamorphs on banana. Persoonia. 2008;20:19–37. doi: 10.3767/003158508X302212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzanlou M., Groenewald J.Z., Gams W. Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies in Mycology. 2007;58:57–93. doi: 10.3114/sim.2007.58.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ávila A., Groenewald J.Z., Trapero A. Characterisation and epitypification of Pseudocercospora cladosporioides, the causal organism of Cercospora leaf spot of olives. Mycological Research. 2005;109:881–888. doi: 10.1017/s0953756205003503. [DOI] [PubMed] [Google Scholar]
- Baker W.A., Partridge E.C., Morgan-Jones G. Notes on Hyphomycetes. LXXVIII. Asperisporium sequoiae, the causal organism of conifer needle blight, reclassified in Cercosporidium, with comments on the status of the genus. Mycotaxon. 2000;76:247–256. [Google Scholar]
- Bakhshi M., Arzanlou M., Babai-Ahari A. Application of the consolidated species concept to Cercospora spp. from Iran. Persoonia. 2015;34:65–86. doi: 10.3767/003158515X685698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhshi M., Arzanlou M., Babai-Ahari A. Is morphology in Cercospora a reliable reflection of generic affinity? Phytotaxa. 2015;213:22–34. [Google Scholar]
- Barnes I., Crous P.W., Wingfield B.D. Multigene phylogenies reveal that red band needle blight of Pinus is caused by two distinct species of Dothistroma, D. septosporum and D. pini. Studies in Mycology. 2004;50:551–565. [Google Scholar]
- Barnes I., van der Nest A., Mullett M.S. Neotypification of Dothistroma septosporum and epitypification of D. pini, causal agents of Dothistroma needle blight of pine. Forest Pathology. 2016;46:388–407. [Google Scholar]
- Barr M.E. Preliminary studies on the Dothideales in temperate North America. Contributions of the University of Michigan Herbarium. 1972;9:523–638. [Google Scholar]
- Barr M.E. Published by the author; Amherst, Massachusetts: 1987. Prodomus to class Loculoascomycetes. [Google Scholar]
- Barreto R.W., Evans H.C. The mycobiota of the weed Chromolaena odorata in southern Brazil with particular reference to fungal pathogens for biological control. Mycological Research. 1994;98:1107–1116. [Google Scholar]
- Batzer J.C., Arias M.M.D., Harrington T.C. Four species of Zygophiala (Schizothyriaceae, Capnodiales) are associated with the sooty blotch and flyspeck complex on apple. Mycologia. 2008;100:246–258. doi: 10.3852/mycologia.100.2.246. [DOI] [PubMed] [Google Scholar]
- Batzer J.C., Gleason M.L., Harrington T.C. Expansion of the sooty blotch and flyspeck complex on apples based on analysis of ribosomal DNA gene sequences and morphology. Mycologia. 2005;97:1268–1286. doi: 10.3852/mycologia.97.6.1268. [DOI] [PubMed] [Google Scholar]
- Bedlan G., Plenk A., Ambrosch A. Erstnachweis von Passalora capsicicola (Syn. Cladosporium capsici) an Capsicum annuum in Österreich. Journal für Kulturpflanzen. 2012;64(1):29–32. [Google Scholar]
- Beilharz V., Pascoe I.G. Two additional species of Verrucisporota, one with a Mycosphaerella teleomorph, from Australia. Mycotaxon. 2002;82:357–365. [Google Scholar]
- Beilharz V.C., Pascoe I.G., Wingfield M.J. Passalora perplexa, an important pleoanamorphic leaf blight pathogen of Acacia crassicarpa in Australia and Indonesia. Studies in Mycology. 2004;50:471–479. [Google Scholar]
- Bensch K., Braun U., Groenewald J.Z. The genus Cladosporium. Studies in Mycology. 2012;72:1–401. doi: 10.3114/sim0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch K., Groenewald J.Z., Braun U. Common but different: The expanding realm of Cladosporium. Studies in Mycology. 2015;82:23–74. doi: 10.1016/j.simyco.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra J.D.P., Oliveira R.J.V., Paiva L.M. Bezerromycetales and Wiesneriomycetales ord. nov. (class Dothideomycetes), with two novel genera to accommodate endophytic fungi from Brazilian cactus. Mycological Progress. 2017;16(4):297–309. [Google Scholar]
- Bonorden H.F. Beiträge zur Mykologie. Botanische Zeitung. 1853;11:281–296. [Google Scholar]
- Bory de Saint-Vincent Jean-Baptiste-Geneviève-Marcellin Baron de, Durieu de Maisonneuve Michel-Charles (1849). Exploration scientifique de l'Algérie, vol. 1, livraison 14, Imprimerie Impériale, Paris, France
- Bradshaw E.R. Dothistroma (red-band) needle blight of pines and the dothistromin toxin: a review. Forest Pathology. 2004;34:163–185. [Google Scholar]
- Bradshaw E.R., Zhang S. Biosynthesis of dothistromin. Mycopathologia. 2006;162:201–213. doi: 10.1007/s11046-006-0054-5. [DOI] [PubMed] [Google Scholar]
- Brady C.R., Noll L.W., Saleh A.A. Disease severity and microsclerotium properties of the sorghum sooty stripe pathogen, Ramulispora sorghi. Plant Disease. 2011;95:853–859. doi: 10.1094/PDIS-10-10-0742. [DOI] [PubMed] [Google Scholar]
- Braun U. Studies on Ramularia and allied genera (IV) Nova Hedwigia. 1991;53:291–305. [Google Scholar]
- Braun U. New genera of phytopathogenic Deuteromycetes. Cryptogamic Botany. 1993;4:107–114. [Google Scholar]
- Braun U. Vol. 1. IHW-Verlag, Eching; Munich, Germany: 1995. (A monograph of Cercosporella, Ramularia and allied genera (phytopathogenic hyphomycetes)). [Google Scholar]
- Braun U. Taxonomic notes on some species of the Cercospora complex (IV) Sydowia. 1996;48:205–217. [Google Scholar]
- Braun U. Vol. 2. IHW-Verlag, Eching; Munich, Germany: 1998. (A monograph of Cercosporella, Ramularia and allied genera (phytopathogenic hyphomycetes)). [Google Scholar]
- Braun U. Annotated list of Cercospora spp. described by C. Spegazzini. Schlechtendalia. 2000;5:57–79. [Google Scholar]
- Braun U. Taxonomic notes on some species of the Cercospora complex (VII) Fungal Diversity. 2001;8:41–71. [Google Scholar]
- Braun U. Typifications of some species of Ramularia and similar genera (Mycosphaerellaceae) revisited. Schlechtendalia. 2017;32:25–28. [Google Scholar]
- Braun U., Crous P.W. Proposal to conserve the name Pseudocercospora against Stigmina and Phaeoisariopsis (Hyphomycetes) Taxon. 2006;55(3):801–808. [Google Scholar]
- Braun U., Crous P.W. The diversity of cercosporoid hyphomycetes–new species, combinations, names and nomenclatural clarifications. Fungal Diversity. 2007;26:55–72. [Google Scholar]
- Braun U., Crous P.W. Cercosporoid hyphomycetes on hosts of the Annonaceae: Cercospora annonaceae and Isariopsis annonarum revisited. Mycotaxon. 2008;105:207–224. [Google Scholar]
- Braun U., Crous P.W. (2415) Proposal to conserve the name Cercospora (Ascomycota: Mycosphaerellaceae) with a conserved type. Taxon. 2016;65(1):185. [Google Scholar]
- Braun U., Crous P.W., Dugan F. Phylogeny and taxonomy of Cladosporium-like hyphomycetes, including Davidiella gen. nov., the teleomorph of Cladosporium s.str. Mycological Progress. 2003;2(1):3–18. [Google Scholar]
- Braun U., Crous P.W., Nakashima C. Cercosporoid fungi (Mycosphaerellaceae) 2. Species on monocots (Acoraceae to Xyridaceae, excluding Poaceae) IMA Fungus. 2014;5(2):203–390. doi: 10.5598/imafungus.2014.05.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U., Crous P.W., Nakashima C. Cercosporoid fungi (Mycosphaerellaceae) 3. Species on monocots (Poaceae, true grasses) IMA Fungus. 2015;6(1):25–97. doi: 10.5598/imafungus.2015.06.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U., Crous P.W., Nakashima C. Cercosporoid fungi (Mycosphaerellaceae) 5. Species on dicots (Anacardiaceae to Annonaceae) IMA Fungus. 2016;7(1):161–216. doi: 10.5598/imafungus.2016.07.01.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U., Crous P.W., Schubert K. Some reallocations of Stenella species in Zasmidium. Schlechtendalia. 2010;20:99–104. [Google Scholar]
- Braun U., Freire F.O., Urtiaga R. New species and new records of cercosporoid hyphomycetes from Brazil, New Zealand and Venezuela. Polish Botanical Journal. 2010;55(2):281–291. [Google Scholar]
- Braun U., Hill C.F. Some new micromycetes from New Zealand. Mycological Progress. 2002;1(1):19–30. [Google Scholar]
- Braun U., Hill C.F. Some new cercosporoid and related leaf spot diseases from New Zealand and Fiji. Australasian Plant Pathology. 2004;33:485–494. [Google Scholar]
- Braun U., Hill C.F., Dick M. New cercosporoid leaf spot diseases from New Zealand. Australasian Plant Pathology. 2003;32:87–97. [Google Scholar]
- Braun U., Hill C.F., Schubert K. New species and new records of biotrophic micromycetes from Australia, Fiji, New Zealand and Thailand. Fungal Diversity. 2006;22:13–35. [Google Scholar]
- Braun U., Mel'nik V.A. Stigmina caricicola sp. nov., Venturia spiraeicola sp. nov. and notes on Jaczewskiella and Xiphomyces. Mikologiya i Fitopatologiya. 1996;30(3):7–13. [Google Scholar]
- Braun U., Mel'nik V.A. Cercosporoid fungi from Russia and adjacent countries. Trudy Botanicheskogo Instituta Imeni V.L. Komarova. Rossijskaya Akademiya Nauk St. Petersburg. 1997;20:1–130. [Google Scholar]
- Braun U., Mouchacca J., McKenzie E.H.C. Cercosporoid hyphomycetes from New Caledonia and some other South Pacific islands. New Zealand Journal of Botany. 1999;37:297–327. [Google Scholar]
- Braun U., Nakashima C., Crous P.W. Cercosporoid fungi (Mycosphaerellaceae) 1. Species on other fungi, Pteridophyta and Gymnospermae. IMA Fungus. 2013;4(2):265–345. doi: 10.5598/imafungus.2013.04.02.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L.G., Morgan-Jones G. Notes on hyphomycetes XI. Additions to the genera Cercosporidium, Passalora and Phaeoisariopsis. Mycotaxon. 1976;4:299–306. [Google Scholar]
- Bubák F. Achter Beitrag zur Pilzflora von Tirol. Annales Mycologici. 1916;14(3–4):145–158. [Google Scholar]
- Burgess T.I., Barber P.A., Sufaati S. Mycosphaerella spp. on Eucalyptus in Asia; new species, new hosts and new records. Fungal Diversity. 2007;24:135–157. [Google Scholar]
- Butler E.J., Jones S.G. Macmillan; London: 1949. Tomato Leaf Mould, Cladosporium fulvum Cooke. [Google Scholar]
- Carnegie A.J., Burgess T.I., Beilharz V. New species of Mycosphaerella from Myrtaceae in plantations and native forests in eastern Australia. Mycological Research. 2007;99:461–474. doi: 10.3852/mycologia.99.3.461. [DOI] [PubMed] [Google Scholar]
- Carnegie A.J., Keane P.J. Further Mycosphaerella species associated with leaf diseases of Eucalyptus. Mycological Research. 1994;98:413–418. [Google Scholar]
- Carnegie A.J., Pegg G.S., White D. Species within Mycosphaerellaceae and Teratosphaeriaceae from eucalypts in eastern Australia. Australasian Plant Pathology. 2011;40:366–384. [Google Scholar]
- Castañeda R.F., Braun U. Cercospora and allied genera of Cuba (I) Cryptogamic Botany. 1989;1(1):42–55. [Google Scholar]
- Castañeda R.F., Kendrick B. 1990. Conidial fungi from Cuba: I; pp. 1–53. University of Waterloo Biology Series 32. [Google Scholar]
- Chang T.-C., Salvucci A., Crous P.W. Comparative genomics of the Sigatoka disease complex on banana suggests a link between parallel evolutionary changes in Pseudocercospora fijiensis and Pseudocercospora eumusae and increased virulence on the banana host. PLoS Genetics. 2016;12(8):e1005904. doi: 10.1371/journal.pgen.1005904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheewangkoon R., Crous P.W., Hyde K.D. Species of Mycosphaerella and related anamorphs on Eucalyptus leaves from Thailand. Persoonia. 2008;21:77–91. doi: 10.3767/003158508X370857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Lee M.-H., Daub M.E., Chung K.-R. Molecular analysis of the cercosporin biosynthetic gene cluster in Cercospora nicotianae. Molecular Microbiology. 2007;64(3):755–770. doi: 10.1111/j.1365-2958.2007.05689.x. [DOI] [PubMed] [Google Scholar]
- Chupp C. 1954. A monograph of the fungus genus Cercospora. Ithaca, New York. [Google Scholar]
- Churchill A.C.L. Mycosphaerella fijiensis, the black leaf streak pathogen of banana: progress towards understanding pathogen biology and detection, disease development, and the challenges of control. Molecular Plant Pathology. 2010;12:307–328. doi: 10.1111/j.1364-3703.2010.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciferri R. Schedae Mycologicae XII-XXXIV. Sydowia. 1954;8:245–270. [Google Scholar]
- Clements F.E., Shear C.L. H.W. Wilson Co.; New York: 1931. The genera of fungi. [Google Scholar]
- Cline E.T., Rossman A.Y. Septoria malagutii sp. nov., cause of annular leaf spot of potato. Mycotaxon. 2006;98:125–132. [Google Scholar]
- Constantinescu O. Revision of Cercospora species (Hyphomycetes) parasitic on Psoralea. Mycotaxon. 1975;3:119–125. [Google Scholar]
- Cooke M.C. British fungi [cont.] Grevillea. 1875;3(28):177–186. [Google Scholar]
- Corlett M. Vol. 18. J. Cramer Publisher; Berlin, Stuttgart: 1991. (An annotated list of the published names in Mycosphaerella and Sphaerella. Mycologia Memoir). [Google Scholar]
- Crous P.W. Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoir. 1998;21:1–170. [Google Scholar]
- Crous P.W. Westerdijk Fungal Biodiversity Institute; Utrecht, the Netherlands: 2007. Hoornsmania pyrina. Fungal Planet. No. 11. [Google Scholar]
- Crous P.W. Taxonomy and phylogeny of the genus Mycosphaerella and its anamorphs. Fungal Diversity. 2009;38:1–24. [Google Scholar]
- Crous P.W., Alfenas A.C. Mycosphaerella gracilis and other species of Mycosphaerella associated with leaf spots of Eucalyptus in Indonesia. Mycologia. 1995;87:121–126. [Google Scholar]
- Crous P.W., Aptroot A., Kang J.C. The genus Mycosphaerella and its anamorphs. Studies in Mycology. 2000;45:107–121. [Google Scholar]
- Crous P.W., Braun U. Cercosporoid fungi from South Africa. Mycotaxon. 1996;57:233–321. [Google Scholar]
- Crous P.W., Braun U. Westerdijk Fungal Biodiversity Institute; Utrecht, the Netherlands: 2003. Mycosphaerella and its anamorphs: 1. Names published in Cercospora and Passalora. CBS Biodiversity Series No. 1. [Google Scholar]
- Crous P.W., Braun U., Groenewald J.Z. Mycosphaerella is polyphyletic. Studies in Mycology. 2007;58:1–32. doi: 10.3114/sim.2007.58.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Braun U., Hunter G.C. Phylogenetic lineages in Pseudocercospora. Studies in Mycology. 2013;75:37–114. doi: 10.3114/sim0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Braun U., Schubert K., Groenewald J.Z. Delimiting Cladosporium from morphologically similar genera. Studies in Mycology. 2007;58:33–56. doi: 10.3114/sim.2007.58.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Braun U., Wingfield M.J. Phylogeny and taxonomy of obscure genera of microfungi. Persoonia. 2009;22:139–161. doi: 10.3767/003158509X461701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Carris L.M., Giraldo A. The Genera of Fungi – fixing the application of the type species of generic names – G2: Allantophomopsis, Latorua, Macrodiplodiopsis, Macrohilum, Milospium, Protostegia, Pyricularia, Robillarda, Rotula, Septoriella, Torula, and Wojnowicia. IMA Fungus. 2015;6:163–198. doi: 10.5598/imafungus.2015.06.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Ferreira F.A., Sutton B.C. A comparison of the fungal genera Phaeophleospora and Kirramyces (coelomycetes) South African Journal of Botany. 1997;63:111–115. [Google Scholar]
- Crous P.W., Gams W., Stalpers J.A. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology. 2004;50:19–22. [Google Scholar]
- Crous P.W., Groenewald J.Z., Gams W. Eyespot of cereals revisited: ITS phylogeny reveals new species relationships. European Journal of Plant Pathology. 2003;109:841–850. [Google Scholar]
- Crous P.W., Groenewald J.Z., Mansilla J.P. Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. Studies in Mycology. 2004;50(1):195–214. doi: 10.3114/sim.55.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Groenewald J.Z., Pongpanich K. Cryptic speciation and host specificity among Mycosphaerella spp. occurring on Australian Acacia species grown as exotics in the tropics. Studies in Mycology. 2004;50:457–469. [Google Scholar]
- Crous P.W., Groenewald J.Z., Shivas R.G. Fungal Planet Description Sheets: 69–91. Persoonia. 2011;26:108–156. doi: 10.3767/003158511X581723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Groenewald J.Z., Summerell B.A. Co-occurring species of Teratosphaeria on Eucalyptus. Persoonia. 2009;22:38–48. doi: 10.3767/003158509X424333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Hawksworth D.L., Wingfield M.J. Identifying and Naming Plant-Pathogenic Fungi: Past, Present, and Future. Annual Review of Phytopathology. 2015;53:12.1–12.21. doi: 10.1146/annurev-phyto-080614-120245. [DOI] [PubMed] [Google Scholar]
- Crous P.W., Hong L., Wingfield B.D. ITS rDNA phylogeny of selected Mycosphaerella species and their anamorphs occurring on Myrtaceae. Mycological Research. 2001;105:425–431. [Google Scholar]
- Crous P.W., Hong L., Wingfield M.J. Uwebraunia and Dissoconium, two morphologically similar anamorph genera with different teleomorph affinity. Sydowia. 1999;51:155–166. [Google Scholar]
- Crous P.W., Kang J.C., Braun U. A phylogenetic redefinition of anamorph genera in Mycosphaerella based on ITS rDNA sequences and morphology. Mycologia. 2001;93:1081–1101. [Google Scholar]
- Crous P.W., Liebenberg M.M., Braun U. Re-evaluating the taxonomic status of Phaeoisariopsis griseola, the causal agent of angular leaf spot of bean. Studies in Mycology. 2006;55:163–173. doi: 10.3114/sim.55.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Minnis A.M., Pereira O.L. What is Scirrhia? IMA Fungus. 2011;2(2):127–133. doi: 10.5598/imafungus.2011.02.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Schoch C.L., Hyde K.D. Phylogenetic lineages in the Capnodiales. Studies in Mycology. 2009;64:17–47. doi: 10.3114/sim.2009.64.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Schumacher R.K., Wingfield M.J. Fungal Systematics and Evolution: FUSE 1. Sydowia. 2015;67:81–118. [Google Scholar]
- Crous P.W., Shivas R.G., Quaedvlieg W. Fungal Planet description sheets: 214–280. Persoonia. 2014;32:184–306. doi: 10.3767/003158514X682395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.C., Shivas R.G., Wingfield M.J. Fungal Planet description sheets: 128–153. Persoonia. 2012;29:146–201. doi: 10.3767/003158512X661589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Summerell B.A., Carnegie A. Foliicolous Mycosphaerella spp. and their anamorphs on Corymbia and Eucalyptus. Fungal Diversity. 2007;26:143–185. [Google Scholar]
- Crous P.W., Summerell B.A., Carnegie A.J. Novel species of Mycosphaerellaceae and Teratosphaeriaceae. Persoonia. 2009;23:119–146. doi: 10.3767/003158509X479531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Summerell B.A., Carnegie A.J. Unravelling Mycosphaerella: do you believe in genera? Persoonia. 2009;23:99–118. doi: 10.3767/003158509X479487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Summerell B.A., Mostert L. Host specificity and speciation of Mycosphaerella and Teratosphaeria species associated with leaf spots of Proteaceae. Persoonia. 2008;20:59–86. doi: 10.3767/003158508X323949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Summerell B.A., Shivas R.G. Fungal Planet description sheets: 107–127. Persoonia. 2012;28:138–182. doi: 10.3767/003158512X652633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Summerell B.A., Swart L. Fungal pathogens of Proteaceae. Persoonia. 2011;27:20–45. doi: 10.3767/003158511X606239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Sutton B.C. New cercosporoid fungi from southern Africa. South African Journal of Botany. 1997;63(5):280–285. [Google Scholar]
- Crous P.W., Tanaka K., Summerell B.A. Additions to the Mycosphaerella complex. IMA Fungus. 2011;2:49–64. doi: 10.5598/imafungus.2011.02.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Verkley G.J.M., Groenewald J.Z. CBS-KNAW Fungal Biodiversity Centre; Utrecht, The Netherlands: 2009. Fungal Biodiversity. CBS Laboratory Manual Series 1. [Google Scholar]
- Crous P.W., Wingfield M.J. Species of Mycosphaerella and their anamorphs associated with leaf blotch disease of Eucalyptus in South Africa. Mycologia. 1996;88:441–458. [Google Scholar]
- Crous P.W., Wingfield M.J., Burgess T.I. Fungal Planet description sheets: 469–557. Persoonia. 2016;37:218–403. doi: 10.3767/003158516X694499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Ferreira F.A. Mycosphaerella parkii and Phyllosticta eucalyptorum, two new species from Eucalyptus leaves in Brazil. Mycological Research. 1993;97:582–584. [Google Scholar]
- Crous P.W., Wingfield M.J., Groenewald J.Z. Niche sharing reflects a poorly understood biodiversity phenomenon. Persoonia. 2009;22:83–94. doi: 10.3767/003158509X439364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Guarro J. Fungal Planet description sheets: 154–213. Persoonia. 2013;31:188–296. doi: 10.3767/003158513X675925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Guarro J. Fungal Planet description sheets 320–370. Persoonia. 2015;34:167–266. doi: 10.3767/003158515X688433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Mansilla J.P. Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. II. Studies in Mycology. 2006;55:99–131. doi: 10.3114/sim.55.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Park R.F. Mycosphaerella nubilosa, a synonym of M. molleriana. Mycological Research. 1991;95:628–632. [Google Scholar]
- Crous P.W., Wingfield M.J., Richardson D.M. Fungal Planet description sheets: 400–468. Persoonia. 2016;36:316–458. doi: 10.3767/003158516X692185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Schumacher R.K. Fungal Planet description sheets: 281–319. Persoonia. 2014;33:212–289. doi: 10.3767/003158514X685680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Swart W.J. Shoot and needle diseases of pines in South Africa. South African Forestry Journal. 1990;154:60–66. [Google Scholar]
- Da Hora Júnior B.T., de Macedo D.M., Barreto R.W. Erasing the Past: A New Identity for the Damoclean Pathogen Causing South American Leaf Blight of Rubber. PLoS ONE. 2014;9(8):e104750. doi: 10.1371/journal.pone.0104750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva M., Pinho D.B., Pereira O.L. Naming potentially endangered parasites: foliicolous mycobiota of Dimorphandra wilsonii, a highly threatened Brazilian tree species. PLoS ONE. 2016;11(2):e0147895. doi: 10.1371/journal.pone.0147895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J.C. A contribution to the systematics of Cladosporium. Revision of the fungi previously referred to Heterosporium. Mycological Papers. 1997;172:1–157. [Google Scholar]
- Davis B.H. The Cercospora leaf spot of rose caused by Mycosphaerella rosicola. Mycologia. 1938;30(3):282–298. [Google Scholar]
- Davis R.M., Raid R.N. The American Phytopathological Society, APS Press; St. Paul, USA: 2002. Compendium of Umbelliferous Crop Diseases. [Google Scholar]
- De Hoog G.S., Rahman M.A., Boekhout T. Ramichloridium, Veronaea and Stenella: Generic delimitation, new combinations and two new species. Transactions of the British Mycological Society. 1983;81:485–490. [Google Scholar]
- De Wit P.J.G.M. Light and scanning-electron microscopic study of infection of tomato plants by virulent and avirulent races of Cladosporium fulvum. Netherlands Journal Plant Patholology. 1977;83:109–122. [Google Scholar]
- De Wit P.J.G.M. Department, Wageningen University; Netherlands: 1981. Physiological studies on on cultivar-specific resistance of tomato plants to Cladosporium fulvum. PhD dissertation. [Google Scholar]
- De Wit P.J.G.M. Molecular characterization of gene-for-gene systems in plant-fungus interactions and the application of avirulence genes in control of plant pathogens. Annual Review of Phytopathology. 1992;30:391–418. doi: 10.1146/annurev.py.30.090192.002135. [DOI] [PubMed] [Google Scholar]
- De Wit P.J.G.M. Cladosporium fulvum effectors: weapons in the arms race with tomato. Annual Review of Phytopathology. 2016;54:1–23. doi: 10.1146/annurev-phyto-011516-040249. [DOI] [PubMed] [Google Scholar]
- Dearness J. New and Noteworthy Fungi – III. Mycologia. 1924;16:143–176. [Google Scholar]
- Dearness J. New and Noteworthy Fungi – IV. Mycologia. 1926;18:236–255. [Google Scholar]
- Deighton F.C. Studies on Cercospora and allied genera II. Passalora, Cercosporidium, and some species of Fusicladium on Euphorbia. Mycological Papers. 1967;112:1–79. [Google Scholar]
- Deighton F.C. Microfungi. IV. Some hyperparasitic hyphomycetes, and a note on Cercosporella uredinophila Sacc. Mycological Papers. 1969;118:1–41. [Google Scholar]
- Deighton F.C. Validation of the generic name Gloeocercospora and specific names of C. sorghi and C. inconspicua. Transactions of the British Mycological Society. 1971;57(1):358–360. [Google Scholar]
- Deighton F.C. Ramulariopsis Speg., Pseudovularia Speg., Didymariopsis Speg. and Ramularia holci-lanati (Cav.) Lindau. Transactions of the British Mycological Society. 1972;59:185–191. [Google Scholar]
- Deighton F.C. Studies on Cercospora and allied genera IV. Cercosporella Sacc., Pseudocercosporella gen. nov. and Pseudocercosporidium gen. nov. Mycological Papers. 1973;133:1–62. [Google Scholar]
- Deighton F.C. Studies on Cercospora and allied genera V. Mycovellosiella Rangel and a new species of Ramulariopsis. Mycological Papers. 1974;137:1–75. [Google Scholar]
- Deighton F.C. Studies on Cercospora and allied genera VI. Pseudocercospora Speg., Pantospora Cif. And Cercoseptoria Petr. Mycological Papers. 1976;140:1–168. [Google Scholar]
- Deighton F.C. Brown leaf mould of Capsicum caused by Phaeoramularia capsicicola. Transactions of the British Mycological Society. 1976;67(1):140–142. [Google Scholar]
- Deighton F.C. Studies on Cercospora and allied genera VII. New species and redispositions. Mycological Papers. 1979;144:1–56. [Google Scholar]
- Deighton F.C. Three leaf-spotting hyphomycetes on palms. Transactions of the British Mycological Society. 1985;85(4):739–742. [Google Scholar]
- Deighton F.C. New species of Pseudocercospora and Mycovellosiella, and new combinations into Pseudocercospora and Phaeoramularia. Transactions of the British Mycological Society. 1987;88:365–391. [Google Scholar]
- Deighton F.C., Pirozynski K.A. Microfungi. I: African species of Uncinula; some species of Fusicladiella; various hyphomycetes, mainly tropical. Mycological Papers. 1965;101:1–43. [Google Scholar]
- Den Breeÿen A., Groenewald J., G.J.M. V. Morphological and molecular characterisation of Mycosphaerellaceae associated with the invasive weed, Chromolaena odorata. Fungal Diversity. 2006;23:89–110. [Google Scholar]
- Dick M.A., Dobbie K. Mycosphaerella suberosa and M. intermedia sp. nov. on Eucalyptus in New Zealand. New Zealand Journal of Botany. 2001;39(2):269–276. [Google Scholar]
- Döbbeler P. Moosbewohnende Ascomycetes I. Die pyrenocarpen, den Gametophyten besiedelnden Arten in (Germany) Mitteilungen aus der Botanischen Staatssammlung München. 1978;14:1–360. [Google Scholar]
- Dodd A.P. Biological control of Eupatorium adenophorum in Queensland. Australian Journal of Science. 1961;23:356–365. [Google Scholar]
- Douanla-Meli C., Langer E., Mouafo F.T. Fungal endophyte diversity and community patterns in healthy and yellowing leaves of Citrus limon. Fungal Ecology. 2013;6(3):212–222. [Google Scholar]
- Dugan F.M., Braun U., Groenewald J.Z. Morphological plasticity in Cladosporium sphaerospermum. Persoonia. 2008;21:9–16. doi: 10.3767/003158508X334389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Plessis S.J. Die voorkoms en Bestryding van Roetdou op Sultanastokke veroorsaak deur Exosporium sultanae Nov. Spec. Annale van die Universiteit van Stellenbosch. 1946;24(5):1–32. [Google Scholar]
- Dyko B.J., Sutton B.C. Two new and unusual deuteromycetes. Transactions of the British Mycological Society. 1979;72:411–417. [Google Scholar]
- Earle F.S. Some fungi from Porto Rico. Muhlenbergia. 1901;1(2):10–31. [Google Scholar]
- Ellis M.B. Clasterosporium and some allied Dematiaceae - Phragmosporae. II. Mycological Papers. 1959;72:1–72. [Google Scholar]
- Ellis M.B. Dematiaceous Hyphomycetes. III. Mycological Papers. 1961;82:1–55. [Google Scholar]
- Ellis M.B. Dematiaceous Hyphomycetes. VIII. Periconiella, Trichodochium, etc. Mycological Papers. 1967;111:1–46. [Google Scholar]
- Ellis M.B. Commonwealth Mycological Institute; Kew, UK: 1971. Dematiaceous hyphomycetes. Kew, England. [Google Scholar]
- Ellis M.B. Commonwealth Mycological Institute; Kew, UK: 1976. More dematiaceous hyphomycetes. [Google Scholar]
- Ellis J.B., Everhart B.M. New species of hyphomycetous fungi. Journal of Mycology. 1889;5(2):68–72. [Google Scholar]
- EPPO. 2012. EPPO quarantine.http://www.eppo.int [Google Scholar]
- Eriksson O.E. SBT-förlaget; Lund, Sweden: 1992. The non-lichenized pyrenomycetes of Sweden. [Google Scholar]
- Etayo J., Sancho L.G. Hongos liquenícolas del sur de Sudamérica, especialmente de Isla Navarino (Chile) Bibliotheca Lichenologica. 2008;98:1–302. [Google Scholar]
- Evans H.C. The genus Mycosphaerella and its anamorphs Cercoseptoria, Dothistroma and Lecanosticta on pines. Mycological Papers. 1984;153:1–102. [Google Scholar]
- Farr D.F., Bills G.F., Chamuris G.P. APS Press; St. Paul, USA: 1989. Fungi on plants & plant products in the United States. [Google Scholar]
- Farr DF, Rossman AY. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Retrieved June 22, 2017, from https://nt.ars-grin.gov/fungaldatabases/.
- Farr M.L. The systematic position of some Dimeriella species and associated fungi on Pinaceae. Mycologia. 1963;55:226–246. [Google Scholar]
- Fernandes A.F., de Miranda BEC, Duarte L.L. Passalora stromatica sp. nov. associated with leaf spots of Tithonia diversifolia in Brazil. IMA Fungus. 2013;4(2):201–204. doi: 10.5598/imafungus.2013.04.02.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J., Crous P.W., Groenewald J.Z. Microcyclosporella and Microcyclospora: novel genera accommodating epiphytic fungi causing sooty blotch on apple. Persoonia. 2010;24:93–105. doi: 10.3767/003158510X510560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E.M. Typographia Academica; Uppsala: 1849. Summa vegetabilium Scandinaviae. Sectio posterior. [Google Scholar]
- Gond S.K., Kharwar R.N., White J.F., Jr. Will fungi be the new source of the blockbuster drug taxol? Fungal Biology Reviews. 2014;28:77–84. [Google Scholar]
- González Fragoso R. Hongos parásitos de la flórula hispalense, nuevos o poco conocidos. Boletín de la Real Sociedad Española de Historia Natural. 1915;15:120–132. [Google Scholar]
- Groenewald M., Barnes I., Bradshaw R.E. Characterization and distribution of mating type genes in the Dothistroma Needle Blight pathogens. Phytopathology. 2007;97:825–834. doi: 10.1094/PHYTO-97-7-0825. [DOI] [PubMed] [Google Scholar]
- Groenewald M., Groenewald J.Z., Crous P.W. Distinct species exist within the Cercospora apii morphotype. Phytopathology. 2005;95:951–959. doi: 10.1094/PHYTO-95-0951. [DOI] [PubMed] [Google Scholar]
- Groenewald J.Z., Nakashima C., Nishikawa J. Species concepts in Cercospora: spotting the weeds among the roses. Studies in Mycology. 2013;75:115–170. doi: 10.3114/sim0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronchi V. The acidophile property of a fungus isolated from N/10 HC1. Bollettino dell'Istituto Sieroterapico Milanese. 1931;10(5):237–250. [Google Scholar]
- Guatimosim E., Schwartsburd P.B., Barreto R.W. Novel fungi from an ancient niche: Cercosporoid and related sexual morphs on ferns. Persoonia. 2016;37:106–141. doi: 10.3767/003158516X690934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.L., Hsieh W.H. International Academic Publishers; Beijing: 1995. The genus Pseudocercospora in China. [Google Scholar]
- Guo Y.L., Liu X.J., Hsieh W.H. vol. 20. Science Press; Beijing: 2003. Mycovellosiella, Passalora, Phaeoramularia. (Flora Fungorum Sinicorum). [Google Scholar]
- Han J.-G., Hosoya T., Sung G.-H. Phylogenetic reassessment of Hyaloscyphaceae sensu lato (Helotiales, Leotiomycetes) based on multigene analyses. Fungal Biology. 2014;118:150–167. doi: 10.1016/j.funbio.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Hartig R. Der Nadelschüttepilz der Lärche, Sphaerella laricina n. sp. Forstlich-Naturwissenschaftliche Zeitschrift München. 1895;4:445–457. [Google Scholar]
- Harvey I.C., Wenham H.T. A fungal leaf spot disease of grapes caused by Cercospora vitis (Lév.) Sacc. New Zealand Journal of Botany. 1972;10:87–96. [Google Scholar]
- Hawksworth D.L. Managing and coping with names of pleomorphic fungi in a period of transition. Mycosphere. 2012;3(2):143–155. doi: 10.5598/imafungus.2012.03.01.03. IMA Fungus3: 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth D.L., Crous P.W., Redhead S.A. The Amsterdam declaration on fungal nomenclature. IMA Fungus. 2011;2:105–112. doi: 10.5598/imafungus.2011.02.01.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennings P. Fungi S. Paulensis II. a cl. Puttemans collecti. Hedwigia. 1902;41:295–311. [Google Scholar]
- Hennings P. Fungi S. Paulensis IV a cl. Puttemans collecti. Hedwigia. 1908;48:1–20. [Google Scholar]
- Hillis D.M., Bull J.J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology. 1993;42:182–192. [Google Scholar]
- Hong S.K., Kim W.G., Sung G.B. Occurrence of leaf spot on mulberry caused by Phloeospora maculans in Korea. Plant Pathology Journal. 2011;27(2):193. [Google Scholar]
- Hongsanan S., Li Y.-M., Liu J.-K. Revision of genera in Asterinales. Fungal Diversity. 2014;68:1–68. [Google Scholar]
- Hongsanan S., Zhao R.L., Hyde K.D. A new species of Chaetothyrina on branches of mango, and introducing Phaeothecoidiellaceae fam. nov. Mycosphere. 2017;8(1):137–146. [Google Scholar]
- Hosagoudar V.B., Braun U. Two new Indian hyphomycetes. Indian Phytopathology. 1995;48:260–262. [Google Scholar]
- Hsieh W.H., Goh T.K. Maw Chang Book Company; Taipei, Taiwan: 1990. Cercospora and similar fungi from Taiwan. [Google Scholar]
- Huang F., Groenewald J.Z., Zhu L. Cercosporoid diseases of Citrus. Mycologia. 2015;107:1151–1171. doi: 10.3852/15-059. [DOI] [PubMed] [Google Scholar]
- Hughes S.J. Studies on micro-fungi. V. Acrotheca. Mycological Papers. 1951;38:1–8. [Google Scholar]
- Hughes S.J. Studies on Micro-fungi. XIV. Stigmella, Stigmina, Camptomeris, Polythrincium, and Fusicladiella. Mycological Papers. 1952;49:1–25. [Google Scholar]
- Hughes S.J. Some foliicolous hyphomycetes. Canadian Journal of Botany. 1953;31(5):560–576. [Google Scholar]
- Hyde K.D., Gareth E.B.J., Liu J.K. Families of Dothideomycetes. Fungal Diversity. 2013;63:1–313. [Google Scholar]
- Hyde K.D., Hongsanan S., Jeewon R. Fungal diversity notes 367–490: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity. 2016;80:1–270. [Google Scholar]
- Hyde K.D., McKenzie E.H.C., KoKo T.W. Towards incorporating anamorphic fungi in a natural classification – checklist and notes for 2010. Mycosphere. 2010;2:1–88. [Google Scholar]
- Inman A.J., Sivanesan A., Fitt B.D.L. The biology of Mycosphaerella capsellae sp. nov., the teleomorph of Pseudocercosporella capsellae, cause of white leaf spot of oilseed rape. Mycological Research. 1991;95:1334–1342. [Google Scholar]
- Jaap O. Drittes Verzeichnis zu meinesm Exsiccatenwerk “Fungi select exsiccati“, Series IX-XII (Nummern 201-300), nebst Beschreibungen neuer Arten und Bemerkungen. Verhandlungen des Botanischen Vereins der Provinz Brandenburg. 1908;50:29–51. [Google Scholar]
- Jenkins A.E. Two fungi causing leaf spot of peanut. Journal of Agricultural Research. 1938;56:317–332. [Google Scholar]
- Jones J.B., Jones J.P., Stall R.E. APS Press; St. Paul: 1997. Compendium of Tomato Diseases. [Google Scholar]
- Kamal . Bishen Singh Mahendra Pal Singh; Dehra Dun: 2010. Cercosporoid fungi of India. [Google Scholar]
- Katsuki S. Cercosporae of Japan. Transactions of the Mycological Society of Japan. 1965;1(Extra Issue):1–100. [Google Scholar]
- Kawato M., Shinobu R. On Streptomyces herbaricolour, nov. sp., supplement: a simple technique for the microscopic observation. Memoirs of the Osaka University of Liberal Arts and Education B. Natural Sciences. 1959;8:114–119. [Google Scholar]
- Kearse M., Moir R., Wilson A. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian C. Le Gyroceras Celtidis Mont. et Ces. parasite du Celtis australis L. Bulletin de la Société d'histoire naturelle de l'Afrique du nord. 1925;60:274–281. [Google Scholar]
- Kirk P.M. CAB International; Wallingford, UK: 1986. Phaeoramularia angolensis. CMI Descriptions of Pathogenic Fungi and Bacteria No. 843. [Google Scholar]
- Kirk P.M., Stalpers J.A., Braun U. A without-prejudice list of generic names of fungi for protection under the International Code of Nomenclature for algae, fungi, and plants. IMA Fungus. 2013;4(2):381–443. doi: 10.5598/imafungus.2013.04.02.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner R. Cercosporella and Ramularia. Mycologia. 2009;101:110–119. doi: 10.3852/07-038. [DOI] [PubMed] [Google Scholar]
- Kirschner R., Braun U., Chen Z.-C. Pleurovularia, a new genus of hyphomycetes proposed for a parasite on leaves of Microstegium sp. (Poaceae) Mycoscience. 2002;43:15–20. [Google Scholar]
- Kirschner R., Chen C.J. Periconiella species (anamorphic Mycosphaerellaceae) from Taiwan. Fungal Diversity. 2010;44:135–148. [Google Scholar]
- Kirschner R., Liu L.-C. Mycosphaerellaceous fungi and new species of Venustosynnema and Zasmidium on ferns and fern allies in Taiwan. Phytotaxa. 2014;176:309–323. [Google Scholar]
- Kirschner R., Piepenbring M., Chen C.J. Some cercosporoid hyphomycetes from Taiwan, including a new species of Stenella and new reports of Distocercospora pachyderma and Phacellium paspali. Fungal Diversity. 2004;17:57–68. [Google Scholar]
- Klaubauf S., Tharreau D., Fournier E. Resolving the polyphyletic nature of Pyricularia (Pyriculariaceae) Studies in Mycology. 2014;79:85–120. doi: 10.1016/j.simyco.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebahn H. Borntraeger; Leipzig, Germany: 1918. Haupt- und Nebenfruchtformen der Askomyzeten. [Google Scholar]
- Knudsen K., Kocourkova J., Etayo J. A new species of Sphaerellothecium (Mycosphaerellaceae) on Placidium laciniatum. Opuscula Philolichenum. 2009;6:41–44. [Google Scholar]
- Koike S.T., Baameur A., Groenewald J.Z., Crous P.W. Cercosporoid leaf pathogens from whorled milkweed and spineless safflower in California. IMA Fungus. 2011;2:7–12. doi: 10.5598/imafungus.2011.02.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokalis-Burelle N., Porter D.M., Rodríguez-Kábana . The American Phytopathological Society, APS Press; MN, USA: 1997. Compendium of peanut diseases. [Google Scholar]
- Koorders S.H. Botanische Untersuchungen. Verhandelingen Koninklijke Nederlandse Akademie van Wetenschappen Afdeling Natuurkunde. 1907;13(4):1–264. [Google Scholar]
- Kovachevsky I.C. Die Braunfleckenkrankheit der Paprikapflanze in Franz. Marocco. Zeitschrift für Pflanzenkrankheiten, Pflanzenpathologie und Pflanzenschutz. 1939;49:567. [Google Scholar]
- Laibach F. Untersuchungen über einige Ramularia und Ovularia-Arten und ihre Beziehungen zur Ascomyzetengattung Mycosphaerella. II. Centralblatt für Bakteriologie und Parasitenkunde, Zweite Abtheilung. 1922;55:284–293. [Google Scholar]
- Li W.J., Bhat J.D., Hyde K.D. Towards a natural classification of Dothideomycetes 4: The genera Bryopelta, Bryorella, Bryosphaeria, Lophiosphaerella and Maireella (Dothideomycetes incertae sedis) Phytotaxa. 2014;176:28–41. [Google Scholar]
- Li H.Y., Sun G.Y., Zhai X.R. Dissoconiaceae associated with sooty blotch and flyspeck on fruits in China and the United States. Persoonia. 2012;28:113–125. doi: 10.3767/003158512X651157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.J., Whelen S., Hall B.D. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- Little S. CAB International; Wallingford, UK: 1987. Mycosphaerella henningsii. CMI Descriptions of Pathogenic Fungi and Bacteria No. 912. [Google Scholar]
- Lumbsch H.T., Huhndorf S.M. Notes on Ascomycete Systematics. Nos. 4408–4750. Myconet. 2007;13:59–99. [Google Scholar]
- Lumbsch H.T., Huhndorf S.M. Myconet Volume 14. Part One. Outline of Ascomycota – 2009. Part Two. Notes on Ascomycete Systematics. Nos. 4751–5113. Fieldiana Life and Earth Sciences. 2010;1:1–64. [Google Scholar]
- Maddison W.P., Maddison D.R. 2011. Mesquite: a modular system for evolutionary analysis.http://mesquiteproject.org [Google Scholar]
- Marmolejo J.G. The genus Lecanosticta from Nuevo Leon, Mexico. Mycotaxon. 2000;76:393–397. [Google Scholar]
- Mason E.W., Ellis M.B. British species of Periconia. Mycological Papers. 1953;56:1–127. [Google Scholar]
- Mchau G.R.A., Crous P.W., Nowell D.C. Newly recorded foliar diseases on pearl millet (Pennisetum glaucum) and sorghum (Sorghum bicolor) in South Africa. South African Journal of Science. 1996;92(Suppl 1):14. [Google Scholar]
- McTaggart A.R., Shivas R.G., Braun U. Annellosympodia orbiculata gen. et sp. nov. and Scolecostigmina flagellariae sp. nov. from Australia. Australasian Plant Pathology. 2007;36:573–579. [Google Scholar]
- Mejía L.C., Rojas E.I., Maynard Z. Endophytic fungi as biocontrol agents of Theobroma cacao pathogens. Biological Control. 2008;46:4–14. [Google Scholar]
- Mel'nik V.A., Popushoj I.S. 1992. Nesovershennye griby na drevesnykh i kustarnikovykh porodakh. Shtintsa, Kishinev. [Google Scholar]
- Mezzetti A. Observations on the morphology and taxonomy of Gyroceras celtidis (Biv.) Mont. & Ces. Annali della Sperimentazione Agraria. 1950;4:857–867. [Google Scholar]
- Minnis A.M., Kennedy A.H., Grenier D.B. Asperisporium and Pantospora (Mycosphaerellaceae): epitypifications and phylogenetic placement. Persoonia. 2011;27:1–8. doi: 10.3767/003158511X602071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monkai J., Liu J.-K., Boonmee S. Planistromellaceae (Botryosphaeriales) Cryptogamie Mycologie. 2013;34:45–77. [Google Scholar]
- Morgan-Jones G. Notes on Hyphomycetes. VII. An addition to the genus Phaeoisariopsis. Canadian Journal of Botany. 1974;52:2635–2637. [Google Scholar]
- Morris M.J. Host specificity studies of leaf spot fungus, Phaeoramularia sp. for the biological control of crofton weed (Ageratina adenophorum) in South Africa. Phytophylactica. 1989;21:281–283. [Google Scholar]
- Müller E., von Arx J.A. Die Gattungen der didymosporen Pyrenomyceten. Beiträge zur Kryptogamenflora der Schweiz. 1962;11(2):1–922. [Google Scholar]
- Muntañola M. A study of a newly identified pepper disease in the Americas. Phytopathology. 1954;44:233–239. [Google Scholar]
- Muntañola M. Algunos hyphomycetes criticos. Notas y descripciones. Lilloa. 1960;30:183–213. [Google Scholar]
- Muntañola-Cvetkovic Tres especies de “Cercospora” (Deuteromycetae) de Tucúman. Revista Argentina de Agronomia. 1957;24:84. [Google Scholar]
- Muniappan R., Raman A., Reddy G.V.P. Ageratina adenophora (Sprengel) King and Robinson (Asteraceae) In: Muniappan R., Reddy G.V.P., Raman A., editors. Biological control of tropical weeds using arthropods. Cambridge University Press; Cambridge: 2009. pp. 1–16. [Google Scholar]
- Nag Raj T.R. Mycologue publications; Waterloo: 1993. Coelomycetous anamorphs with appendage-bearing conidia. [Google Scholar]
- Nakashima C., Araki I., Kobayashi T. Addition and re-examination of Japanese species belonging to the genus Cercospora and allied genera. X: newly recorded species from Japan (5) Mycoscience. 2011;52:253–259. [Google Scholar]
- Nakashima C., Motohashi K., Chen C.-Y. Species diversity of Pseudocercospora from Far East Asia. Mycological Progress. 2016;15:1093–1117. [Google Scholar]
- Nakashima C., Motohashi K., Meeboon J. Studies on Cercospora and allied genera in northern Thailand. Fungal Diversity. 2007;26:257–270. [Google Scholar]
- Nugent L.K., Sangvichen E., Sihanonth P. A revised method for the observation of conidiogenous structures in fungi. Mycologist. 2006;20:111–114. [Google Scholar]
- Nylander J.A.A. Uppsala University; Uppsala, Sweden: 2004. MrModeltest 2.0. Program distributed by the author. [Google Scholar]
- Olive L.S. Taxonomic notes on Louisiana fungi - I. Mycologia. 1948;40:6–20. [PubMed] [Google Scholar]
- Ou Y.H., Zhou R.J., Fu J.F. Paracercospora dictamnicola sp. nov. from China. Mycological Progress. 2015;14:15. [Google Scholar]
- Park R.F., Keane P.J. Three Mycosphaerella species from leaf diseases of Eucalyptus. Transactions of the British Mycological Society. 1982;79:95–100. [Google Scholar]
- Park R.F., Keane P.J. Leaf diseases of Eucalyptus associated with Mycosphaerella species. Transactions of the British Mycological Society. 1982;79:101–115. [Google Scholar]
- Patouillard N.T., Hariot P. Fungos aliquot novos in regione Congoana collectos. Bulletin de la Société Mycologique de France. 1893;9:206–211. [Google Scholar]
- Patton R.F. Needle cast of European Larch caused by Mycosphaerella laricina in Winsconsin and Iwoa. Plant Disease. 1983;67:1149–1153. [Google Scholar]
- Peace T.R. Clarendon Press; Oxford, UK: 1962. Pathology of trees and shrubs, with special reference to Britain. [Google Scholar]
- Petrak F. Neue Hyphomyzeten-Gattungen aus Ekuador. Sydowia. 1949;3:259–266. [Google Scholar]
- Petrak F. Colletogloeum n. gen., eine neue Melanconieen-Gattung. Sydowia. 1953;7(5–6):367–369. [Google Scholar]
- Petzoldt S. Zur Biologie, Epidemiologie und Schadwirkung des Erregers der Blatt- und Stengelanthraknose (Mycosphaerella anethi Petr.) am Fenchel (Foeniculum vulgare Mill.) - 1. Mitteilung. Drogenreport. 1989;3:49–65. [Google Scholar]
- Petzoldt S. Mycosphaerella anethi – ein Beitrag zur Entwicklungsgeschichte. Boletus. 1990;14(2):49–56. [Google Scholar]
- Poletto T., Muniz M.F.B., Blume E. First report of Sirosporium diffusum causing brown leaf spot on Carya illinoinensis in Brazil. Plant Disease. 2017;101:381. [Google Scholar]
- Pollack F.G. An annotated compilation of Cercospora names. Mycological Memoirs. 1987;12:1–212. [Google Scholar]
- Pons N., Sutton B.C. Cercospora and similar fungi on yams (Dioscorea spp.) Mycological Papers. 1988;160:1–78. [Google Scholar]
- Pons N., Sutton B.C. Cercospora and similar fungi on Heliotropium weeds. Mycological Research. 1996;100:815–820. [Google Scholar]
- Pretorius M.C., Crous P.W., Groenewald J.Z. Phylogeny of some cercosporoid fungi from Citrus. Sydowia. 2003;55:286–305. [Google Scholar]
- Preuss C.G.T. Übersicht untersuchter Pilze, besonders aus der Umgegend von Hoyerswerda. Linnaea. 1851;24:99–153. [Google Scholar]
- Punithalingam E. Mycosphaerella mori. CMI Descriptions of Fungi and Bacteria No. 1014. Mycopathologia. 1990;112:45–47. [Google Scholar]
- Quaedvlieg W., Binder M., Groenewald J.Z. Introducing the consolidated species concept to resolve species in the Teratosphaeriaceae. Persoonia. 2014;33:1–40. doi: 10.3767/003158514X681981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W., Groenewald J.Z., de Jesus Yáñez-Morales M. DNA barcoding of Mycosphaerella species of quarantine importance to Europe. Persoonia. 2012;29:101–115. doi: 10.3767/003158512X661282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W., Kema G.H.J., Groenewald J.Z. Zymoseptoria gen. nov.: a new genus to accommodate Septoria-like species occurring on graminicolous hosts. Persoonia. 2011;26:57–69. doi: 10.3767/003158511X571841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W., Verkley G.J., Shin H.D. Sizing up Septoria. Studies in Mycology. 2013;75:307–390. doi: 10.3114/sim0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaley A.W. Clypeispora and its Mycosphaerella teleomorph. Mycotaxon. 1991;40:13–22. [Google Scholar]
- Reddy M.V., Raju T.N., Sharma S.B. International Crops Research Institute for the Semi-Arid Tropics; India: 2012. Handbook of Pigeonpea Diseases (Revised) Information Bulletin No. 42. Patancheru, AP 502 324. 64 pp. [Google Scholar]
- Rohde T. Über die “Schweizer” Douglasienschütte und ihren vermuteten Erreger Adelopus spec. Mitteilungen aus Forstwirtschaft und Forstwissenschaft. 1937;8:487–514. [Google Scholar]
- Ronquist F., Teslenko M., van der Mark P. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61(3):539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman A.Y., Crous P.W., Hyde K.D. Recommended names for pleomorphic genera in Dothideomycetes. IMA Fungus. 2015;6:507–523. doi: 10.5598/imafungus.2015.06.02.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux C., Triebel D. Révision des espèces de Stigmidium et de Sphaerellothecium (champignons lichénicoles non lichénisés, Ascomycetes) correspondant ou à Pharcidia epicrymatia sensu Keissler ou à Stigmidium schaereri auct. Bulletin de la Société Linéenne de Provence. 1994;45:451–542. [Google Scholar]
- Saccardo P.A. Fungi Gallici lecti a cl. Viris P. Brunaud, Abb. Letendre, A. Malbranche, J. Therry vel editi in Mycotheca Gallica C. Romaguèri. Ser. II. Michelia. 1880;2(6):39–135. [Google Scholar]
- Saccardo P.A. Saccardo; Padua, Italy: 1882. Sylloge Fungorum 1: i–xviii, 1–768. [Google Scholar]
- Saccardo P.A. Saccardo; Padua, Italy: 1884. Sylloge Fungorum 3: i–ii, 1–860. [Google Scholar]
- Saccardo P.A. Saccardo; Padua, Italy: 1886. Sylloge Fungorum 4: i–v, 1–807. [Google Scholar]
- Saccardo P.A. Saccardo; Padua, Italy: 1892. Sylloge Fungorum 10: i–xxx, 1–964. [Google Scholar]
- Saccardo P.A. Saccardo; Padua, Italy: 1895. Sylloge Fungorum 11: i–vii, 1–753. [Google Scholar]
- Saccardo P.A., Trotter A. Saccardo; Patavii, Italy: 1913. Sylloge Fungorum 22: 1–1612. [Google Scholar]
- Saccardo P.A., Saccardo D., Traverso G.B. Saccardo; Abellini, Pergola, Italy: 1931. Sylloge Fungorum 25: i–ii, 1–1093. [Google Scholar]
- Santesson R. Lichenicolous fungi from northern Spain. Svensk Botanisk Tidskrift Utgifven af Svenska Botaniska Foreningen. 1960;54:501–522. [Google Scholar]
- Schoch C.L., Crous P.W., Groenewald J.Z. A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology. 2009;64:1–15. doi: 10.3114/sim.2009.64.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch C.L., Seifert K.A., Huhndorf S. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences (USA) 2012;109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K., Braun U. Taxonomic revision of the genus Cladosporium s.l. 4. Species reallocated to Asperisporium, Dischloridium, Fusicladium, Passalora, Pseudoasperisporium and Stenella. Fungal Diversity. 2005;20:187–208. [Google Scholar]
- Schubert K., Groenewald J.Z., Braun U. Biodiversity in the Cladosporium herbarum complex (Davidiellaceae, Capnodiales), with standardisation of methods for Cladosporium taxonomy and diagnostics. Studies in Mycology. 2007;58:105–156. doi: 10.3114/sim.2007.58.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert K., Morgan-Jones G., Gams W. CBS-KNAW Fungal Biodiversity Centre; Utrecht, the Netherlands: 2011. The genera of Hyphomycetes. CBS Biodiversity Series 9. [Google Scholar]
- Shaw D.E., Alcorn J.L. New name for Verrucispora and its species. Australian Systematic Botany. 1993;6:273–276. [Google Scholar]
- Shin H.D., Kim J.D. Cercospora and allied genera from Korea. Plant Pathogens of Korea. 2001;7:1–303. [Google Scholar]
- Shivas R.G., McTaggart A.R., Young A.J. Zasmidium scaevolicola. Fungal Planet 47. Persoonia. 2010;24:132–133. [Google Scholar]
- Shkarupa A.G. Tselomitsetnyj rod Jaczewskiella Murashk. Mikologiya i Fitopatologiya. 1992;26:30–34. [Google Scholar]
- Shoemaker R.A. Revision of some Dimeriella and Dimerosporium parasites of conifers. Canadian Journal of Botany. 1965;43:631–639. [Google Scholar]
- Simon U.K., Groenewald J.Z., Crous P.W. Cymadothea trifolii, an obligate biotrophic leaf parasite of Trifolium, belongs to Mycosphaerellaceae as shown by nuclear ribosomal DNA analyses. Persoonia. 2009;22:49–55. doi: 10.3767/003158509X425350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivanesan A. Cramer Verlag; Vaduz: 1984. The bitunicate Ascomycetes and their anamorphs. [Google Scholar]
- Shin H.D., Sameva E.F. National Institute of Agricultural Science and Technology; Republic of Korea: 2004. Septoria in Korea (Plant Pathogens of Korea 11) [Google Scholar]
- Smiley R.W. The American Phytopathological Society APS Press; MN, USA: 1983. Compendium of Turfgrass Diseases. [Google Scholar]
- Spegazzini C. Mycetes Argentinenses (Series V) Anales del Museo Nacional de Historia Natural Buenos Aires. 1910;20:329–467. [Google Scholar]
- 303.Speschnew N.N. Fungi transcaspici et turkestanici. Trudy Tiflisskogo Botanicheskogo Sada, Tiflis. 1901;5:177. [Google Scholar]
- Srivastava P. Recombinations in genus Passalora Fries. Journal of Living World. 1994;1:112–119. [Google Scholar]
- Stamatakis A., Alachiotis N. Time and memory efficient likelihood-based tree searches on phylogenomic alignments with missing data. Bioinformatics. 2010;26:132–139. doi: 10.1093/bioinformatics/btq205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H. Adelopus balsamicola (Peck) Theiss. F. Douglasii als Erreger einer Schüttererkrankung der Douglastanne. Zeitschrift für Pflanzenkrankheiten (Pflanzenpathologie) und Pflanzenschutz. 1937;47:164–186. [Google Scholar]
- Stevens F.L., Dalbey N.E. New or noteworthy Porto Rican fungi. Mycologia. 1919;11:4–9. [Google Scholar]
- Stewart E.L., Liu Z., Crous P.W. Phylogenetic relationships among some cercosporoid anamorphs of Mycosphaerella based on rDNA sequence analysis. Mycological Research. 1999;103:1491–1499. [Google Scholar]
- Stielow J.B., Lévesque C.A., Seifert K.A. One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia. 2015;35:242–263. doi: 10.3767/003158515X689135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J.K., Capitano B.R., Kerrigan J.L. The histopathology of Phaeocryptopus gaeumannii on Douglas-fir needles. Mycologia. 2008;100:431–444. doi: 10.3852/07-170r1. [DOI] [PubMed] [Google Scholar]
- Strobel G.A., Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiology and Molecular Biology Reviews. 2003;67:491–502. doi: 10.1128/MMBR.67.4.491-502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhsaker T.T., Struhsaker P.J., Siex K.S. Conserving Africa's rain forests: problems in protected areas and possible solutions. Biological Conservation. 2005;123:45–54. [Google Scholar]
- Stukenbrock E.H., Quaedvlieg W., Javan-Nikhah M. Zymoseptoria ardabiliae and Z. pseudotritici, two progenitor species of the septoria tritici leaf blotch fungus Z. tritici (synonym: Mycosphaerella graminicola) Mycologia. 2012;104:1397–1407. doi: 10.3852/11-374. [DOI] [PubMed] [Google Scholar]
- Subramanian C.V. A classification of the Hyphomycetes. Current Science. 1962;31:409–411. [Google Scholar]
- Sultan A., Johnston P.R., Park D. Two new pathogenic ascomycetes in Guignardia and Rosenscheldiella on New Zealand's pygmy mistletoes (Korthalsella: Viscaceae) Studies in Mycology. 2011;68:237–247. doi: 10.3114/sim.2011.68.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung G.-H., Sung J.-M., Hywel-Jones N.L. A multigene phylogeny of Clavicipitaceae (Ascomycota, Fungi): identification of localized incongruence using a combinational bootstraap approach. Molecular Phylogenetics and Evolution. 2007;44:1204–1223. doi: 10.1016/j.ympev.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Sutton B.C. Coelomycetes. III. Annellolacinia gen. nov., Aristastoma, Phaeocytostroma, Seimatosporium etc. Mycological Papers. 1964;97:1–42. [Google Scholar]
- Sutton B.C. Coelomycetes V. Coryneum. Mycological Papers. 1975;138:1–224. [Google Scholar]
- Sutton B.C. Coelomycetes VI. Nomenclature of generic names proposed for Coelomycetes. Mycological Papers. 1977;141:1–253. [Google Scholar]
- Sutton B.C. Commonwealth Mycological Institute; Kew, UK: 1980. The Coelomycetes. Fungi Imperfecti with Pycnidia Acervuli and Stromata. [Google Scholar]
- Sutton B.C., Crous P.W. Lecanostictopsis gen. nov., and related leaf-spotting fungi on Syzygium species. Mycological Research. 1997;101(2):215–225. [Google Scholar]
- Sutton B.C., Hodges C.S. Eucalyptus microfungi: Cercosperma arnaudii gen. et sp.nov. and Ceratophorum mauiense sp. nov. Nova Hedwigia. 1983;35:793–803. [Google Scholar]
- Sutton B.C., Pascoe I.G. Tandonella oleariae sp. nov. and generic separation of Tandonella and Sclerographiopsis. Australian Journal of Botany. 1987;35:183–191. [Google Scholar]
- Sutton B., Pollack F. Microfungi on Cercocarpus. Mycopathologia. 1974;52:331–351. doi: 10.1007/BF02198763. [DOI] [PubMed] [Google Scholar]
- Sutton B.C., Shamoun S.F., Crous P.W. Two leaf pathogens of Ribes spp. in North America, Quasiphloeospora saximontanensis (Deighton) comb. nov. and Phloeosporella ribis (J.J. Davis) comb. nov. Mycological Research. 1996;100:979–983. [Google Scholar]
- Sutton B.C., Swart H.J. Australian leaf-inhabiting fungi XXIII. Colletogloeum species and similar fungi on Acacia. Transactions of the British Mycological Society. 1986;87:93–102. [Google Scholar]
- Swart H.J. Australian leaf-inhabiting fungi. XXVI. Some noteworthy Coelomycetes on Eucalyptus. Transactions of the British Mycological Society. 1988;90(1–2):279–291. [Google Scholar]
- Swart H.J., Walker J. Australian leaf-inhabiting fungi. XXVIII. Hendersonia on Eucalyptus. Transactions of the British Mycological Society. 1988;90:633–641. [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, Massachusetts: 2003. PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4. [Google Scholar]
- Sydow H. Fungi in intinere costaricensi collecti, pars secunda. Annales Mycologici. 1926;24(5–6):283–426. [Google Scholar]
- Sydow H. Novae fungorum species XIX. Annales Mycologici. 1928;26:132–139. [Google Scholar]
- Sydow H. Fungi venezuelani. Annales Mycologici. 1930;28(1–2):29–224. [Google Scholar]
- Sydow H. Mycotheca germanica. Fasc. LXV-LXVIII (no. 3201-3400) Annales Mycologici. 1940;38:453–473. [Google Scholar]
- Sydow H., Mitter J.H. Fungi indici. I. Annales Mycologici. 1933;31(1–2):84–97. [Google Scholar]
- Sydow H., Sydow P. Novae fungorum species - X. Annales Mycologici. 1913;11:254–271. [Google Scholar]
- Sydow H., Sydow P. Fungi amazonici a cl. E. Ule lecti. Annales Mycologici. 1916;14(1–2):65–97. [Google Scholar]
- Tamura K., Peterson D., Peterson N. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Hirayama K., Yonezawa H. Revision of the Massarineae (Pleosporales, Dothideomycetes) Studies in Mycology. 2015;82:75–136. doi: 10.1016/j.simyco.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thambugala K.M., Ariyawansa H.A., Li Y.-M. Dothideales. Fungal Diversity. 2014;68:105–158. [Google Scholar]
- Thaung M.M. New hyphomycetes from Burma. Transactions of the British Mycological Society. 1976;66(2):211–215. [Google Scholar]
- Theissen F., Sydow H. Die Dothideales. Kritisch-systematische Originaluntersuchungen. Annales Mycologici. 1915;13:147–746. [Google Scholar]
- Thirumalachar M.J., Mishra J.N. Contribution to the study of fungi of Bihar, India - I. Sydowia. 1953;7:29–83. [Google Scholar]
- Thomma B.P., van Esse H.P., Crous P.W. Cladosporium fulvum (syn. Passalora fulva), a highly specialized plant pathogen as a model for functional studies on plant pathogenic Mycosphaerellaceae. Molecular Plant Pathology. 2005;6(4):379–393. doi: 10.1111/j.1364-3703.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- Tomilin B.A. Nauka; Leningrad, Leningradskoe otd-nie: 1979. Opredelitel' gribov roda Mycosphaerella Johans. (in Russian) [Google Scholar]
- Trakunyingcharoen T., Cheewangkoon R., To-anun C. Botryosphaeriaceae associated with diseases of mango (Mangifera indica) Australasian Plant Pathology. 2014;43(4):425–438. [Google Scholar]
- Triebel D., Rambold G., Nash T.H. On lichenicolous fungi from continental North America. Mycotaxon. 1991;42:293–296. [Google Scholar]
- Vaghefi N., Pethybridge S.J., Shivas R.G. Confirmation of Paracercospora egenula causing leaf spot of eggplant in Hawaii. Australasian Plant Disease Notes. 2016;11:35. [Google Scholar]
- Van Nieuwenhuijzen E.J., Miadlikowska J.M., Houbraken J.A.M.P. Wood staining fungi revealed taxonomic novelties in Pezizomycotina: New order Superstratomycetales and new species Cyanodermella oleoligni. Studies in Mycology. 2016;85:107–124. doi: 10.1016/j.simyco.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkley G.J.M., Priest M.J. Septoria and similar coelomycetous anamorphs of Mycosphaerella. Studies in Mycology. 2000;45:123–128. [Google Scholar]
- Verkley G.J.M., Quaedvlieg W., Shin H.D. A new approach to species delimitation in Septoria. Studies in Mycology. 2013;75:213–305. doi: 10.3114/sim0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira S.I.R., Groenewald J.Z., Braun U. All that glitters is not Ramularia. Studies in Mycology. 2016;83:49–163. doi: 10.1016/j.simyco.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira S.I.R., Groenewald J.Z., Kolecka A. Elucidating the Ramularia eucalypti species complex. Persoonia. 2015;34:50–64. doi: 10.3767/003158515X685670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira S.I.R., Groenewald J.Z., Verkley G.J.M. The rise of Ramularia from the Mycosphaerella labyrinth. Fungal Biology. 2015;119:823–843. doi: 10.1016/j.funbio.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Vilgalys R., Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Arx J.A. Mycosphaerella and its anamorphs. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen: Series C: Biological and Medical Sciences. 1983;86(1):15–54. [Google Scholar]
- Von Arx J.A. Plant pathogenic fungi. Beiheft Nova Hedwigia. 1987;87:1–288. [Google Scholar]
- Von Arx J.A., Müller E. A reevaluation of the bitunicate Ascomycetes with keys to families and genera. Studies in Mycology. 1975;9:1–159. [Google Scholar]
- Von Höhnel F. Fungi imperfecti. Beiträge zur Kenntnis derselben. Hedwigia. 1918;60:56–89. [Google Scholar]
- Vouaux L. Synopsis des champignons parasites de lichens. Bulletin de la Société Mycologique de France. 1913;29:33–128. [Google Scholar]
- Wang F., Summerell B.A., Marshall D. Biology and pathology of a species of Phaeoramularia causing a leaf spot of crofton weed. Australasian Plant Pathology. 1997;26:165–172. [Google Scholar]
- Waterman A.M. Department of Agriculture; Washington, USA: 1954. Septoria canker of poplars in the United States. Circular USDA No. 947, U.S. [Google Scholar]
- Wei S.L., Zhang T.Y. Allantophomoides, a new genus in Coelomycetes. Mycosystema. 2003;22:9–11. [Google Scholar]
- White T.J., Bruns T., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylognetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White J.W., editors. A Guide to Molecular Methods and Applications. Academic Press; New York: 1990. pp. 315–322. [Google Scholar]
- Wijayawardene N.N., Crous P.W., Kirk P.M. Naming and outline of Dothideomycetes–2014 including proposals for the protection or suppression of generic names. Fungal Diversity. 2014;69:1–55. doi: 10.1007/s13225-014-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P.M., Thomas-Hall S., Marney T.S. First record of Passalora calotropidis in Australia and its generic position. Australasian Plant Pathology. 2005;34:95–98. [Google Scholar]
- Wingfield M.J., de Beer Z.W., Slippers B. One fungus, one name promotes progressive plant pathology. Molecular Plant Pathology. 2012;13:604–613. doi: 10.1111/j.1364-3703.2011.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winton L.M., Stone J.K., Hansen E.M. The systematic position of Phaeocryptopus gaeumannii. Mycologia. 2007;99:240–252. doi: 10.3852/mycologia.99.2.240. [DOI] [PubMed] [Google Scholar]
- Woronichin N.N. Materialy k mikologicheskoj flore Kavkaza III. Griby iz kollektsii Kavkazskogo Muzeya. Izvestiya Kavkazskogo Muzeya, Tiflis. 1916;10:1–112. [Google Scholar]
- Wu W., Sutton B.C., Gange A.C. Revision of Septoria species on Hebe and Veronica and description of Kirramyces hebes sp. nov. Mycological Research. 1996;100:1207–1217. [Google Scholar]
- Yang H.L., Sun G.Y., Batzer J.C. Novel fungal genera and species associated with the sooty blotch and flyspeck complex on apple in China and the United States. Persoonia. 2010;24:29–37. doi: 10.3767/003158510X492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen J.M. Studies on parasitic fungi from South East Asia, 49. Parasitic fungi from Malaysia, 25: Semipseudocercospora gen. nov. Mycotaxon. 1983;17:361–363. [Google Scholar]
- Zhang Z.Y., Liu Y.L., Zhang T. Cladosporium, Fusicladium, Pyricularia. Flora Fungorum Sinicorum. 2003;14:1–297. [Google Scholar]
- Zhao G., Liu X., Wu W. Helicosporous hyphomycetes from China. Fungal Diversity. 2007;26:313–524. [Google Scholar]
- Zhu L., Sun O.J., Sang W. Predicting the spatial distribution of an invasive plant species (Eupatorium adenophorum) in China. Landscape Ecology. 2007;22:1143–1154. [Google Scholar]