Abstract
Recent evidence places the FRAP/mTOR kinase downstream of the phosphatidyl inositol 3-kinase/Akt-signaling pathway, which is up-regulated in multiple cancers because of loss of the PTEN tumor suppressor gene. We performed biological and biochemical studies to determine whether PTEN-deficient cancer cells are sensitive to pharmacologic inhibition of FRAP/mTOR by using the rapamycin derivative CCI-779. In vitro and in vivo studies of isogenic PTEN+/+ and PTEN−/− mouse cells as well as human cancer cells with defined PTEN status showed that the growth of PTEN null cells was blocked preferentially by pharmacologic FRAP/mTOR inhibition. Enhanced tumor growth caused by constitutive activation of Akt in PTEN+/+ cells also was reversed by CCI-779 treatment, indicating that FRAP/mTOR functions downstream of Akt in tumorigenesis. Loss of PTEN correlated with increased S6 kinase activity and phosphorylation of ribosomal S6 protein, providing evidence for activation of the FRAP/mTOR pathway in these cells. Differential sensitivity to CCI-779 was not explained by differences in biochemical blockade of the FRAP/mTOR pathway, because S6 phosphorylation was inhibited in sensitive and resistant cell lines. These results provide rationale for testing FRAP/mTOR inhibitors in PTEN null human cancers.
Rapamycin is a natural product macrolide that induces G1 growth arrest in yeast, Drosophila, and mammalian cells (1). Genetic and biochemical studies have established that the target of rapamycin is the protein kinase FRAP/mTOR (hereafter called mTOR) (1–5), an evolutionarily conserved member of the phosphoinositide kinase-related kinase family that includes DNA-PK, ATM, and ATR (6). Rapamycin forms a complex with the immunophilin prolyl isomerase FKBP12, which binds specifically to mTOR and inhibits its ability to phosphorylate substrates such as S6 kinase and 4E-BP1. Clinically, rapamycin is an approved immunosuppressive agent, based on its ability to block T cell activation (7, 8). Because rapamycin also can induce growth arrest and apoptosis in certain tumor cells (9), it is under investigation as a potential anticancer drug.
Studies in yeast and mammalian cells suggest that mTOR functions as part of a nutrient-sensing mechanism, regulating the cellular response to starvation conditions such as amino acid deprivation (1, 10). This function is consistent with its biochemical activity in regulating S6 kinase and 4E-BP1, two mTOR targets that play fundamental roles in ribosome biogenesis and cap-dependent translation, respectively (11, 12). S6 kinase and 4E-BP1 are also regulated, in part, through the phosphatidyl inositol 3-kinase (PI3-kinase)/Akt-signaling pathway (13, 14). That mTOR is phosphorylated by Akt (15) raises the possibility of a direct signaling pathway from PI3-kinase/Akt to mTOR. Genetic studies in Drosophila are consistent with this hypothesis, as dTOR is downstream and epistatic to the PI3-kinase/Akt pathway (16, 17). However, dTOR loss gives a more severe phenotype than PI3-kinase, Akt, or S6 kinase loss (18–21). In addition, the Akt phosphorylation site on mTOR is not required for S6 kinase activation (15). Finally, only membrane-targeted alleles of Akt are sufficient to activate S6 kinase, whereas 4E-BP1 phosphorylation appears to be Akt-dependent (22). The collective evidence supports a role for mTOR in PI3-kinase/Akt function, but the relationship is more complex than that of a linear signaling pathway (1).
Genomic amplification of either PI3-kinase or Akt has been reported in cervical, ovarian, and pancreatic cancers (23–25). In addition, mutations in the tumor suppressor phosphatase gene PTEN, which regulates signaling through the PI3-kinase/Akt-signaling pathway, occur commonly in prostate, glioblastoma, and endometrial tumors (26–30). Because mTOR may function in the PI3-kinase/Akt pathway, we examined the potential antitumor properties of mTOR inhibitors in PTEN null tumors. PTEN−/− mouse cells and human tumor lines lacking PTEN were more sensitive than wild-type PTEN cells to the growth-inhibitory effects of CCI-779, an ester of rapamycin. Transformation mediated by expression of activated Akt in cells with an intact PTEN gene also was reversed by CCI-779 treatment. Biochemical analysis of the mTOR target S6 kinase showed CCI-779-dependent constitutive activation in PTEN null cells, indicating up-regulation of the mTOR pathway. These results suggest that drugs that target mTOR may have clinical utility in human cancers lacking PTEN.
Methods
Protein Analysis.
S6 kinase activity was measured by in vitro immune complex assay by using an artificial consensus peptide as substrate as described (31). Phosphorylated (Ser-235/236) and pan-S6 antibodies were provided by Cell Signaling Technology (Beverly, MA). Akt and MAPK activation was measured by immunoblot assay by using phosphospecific antibodies with controls for total Akt and MAPK protein as described (32). Pan-4E-BP1 antibody was provided by N. Sonenberg (McGill, Montreal, Canada). The level of 4E-BP1 bound to eIF4E was measured by precipitation of eIF4E by using 7methyl-GTP Sepharose (Amersham Pharmacia), followed by 4E-BP1 immunoblot as described (14). Cyclin D1 and actin antibodies were obtained from Santa Cruz Biotechnology. eIF4E antibody was obtained from Signal Transduction Laboratories (San Diego).
Tissue Culture Experiments.
PTEN+/+ and PTEN−/− embryonic stem (ES) cells and mouse embryo fibroblasts (MEFs) were derived as described previously (33). 9L rat glioblastoma cells were provided by L. Liau (University of California at Los Angeles). All other cell lines were from American Type Culture Collection. Cell growth was measured by [3H]thymidine uptake and/or cell counts, which were determined by Trypan blue staining. CCI-779 was obtained from Wyeth Ayerst Laboratories (Marietta, PA).
Mouse Experiments.
PTEN+/+ or PTEN−/− ES cells were injected s.c. into nude mice. LAPC-4 and LAPC-9 prostate cancer xenografts were maintained by serial passage in male severe combined immunodeficient (SCID) mice as described and injected as single cell suspensions (34). Tumor growth was measured daily, and mice were randomized to CCI-779 vs. vehicle when tumors reached the size indicated in each experiment. Treatment was given by i.p. injection for 5 consecutive days. Serum prostate-specific antigen levels were measured by ELISA as described (34). Hematoxylin/eosin, Ki-67 antibody, and terminal deoxynucleotidyltransferase-mediated UTP end-labeling staining of tumor tissue was performed as described previously (34). Cell size (in μ2) was measured by using Microcomp scimeasure analytical software, calibrated at ×200 magnification. Statistical analysis was performed by using Student's t test and the ANOVA statistical model (Stata Software, College Station, TX).
Results
Enhanced Sensitivity of PTEN Null Cells to mTOR Inhibition.
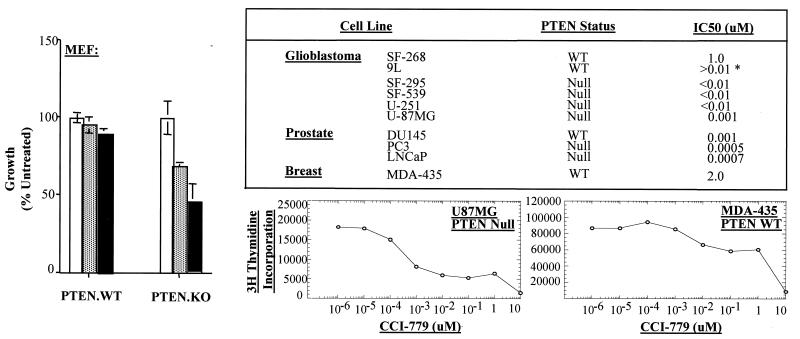
To examine the effect of mTOR pathway inhibition on the growth of PTEN wild-type vs. PTEN-deficient cells, we exposed mouse and human cells with defined PTEN status to varying doses of CCI-779 in vitro. CCI-779 is an ester of rapamycin with equivalent activity and specificity for mTOR.‡‡ First, we examined this question in a genetically defined background by comparing the growth of PTEN+/+ and PTEN−/− MEFs that contain a targeted deletion of the PTEN phosphatase domain (33). The growth of PTEN−/− MEFs was inhibited 40% and 60% by 1.0 nM and 10 nM CCI-779, respectively, whereas PTEN+/+ MEFs were unaffected (Fig. 1 Left). This result cannot be explained by an increased growth rate of PTEN null cells because the doubling times of PTEN null and PTEN wild-type cells were comparable in the absence of CCI-779 (data not shown). We expanded the analysis to a panel of 10 tumor cell lines with defined PTEN status. The concentration of CCI-779 required for 50% growth inhibition (IC50) was less than 10 nM for all of the PTEN null tumor cell lines (Fig. 1; see table and [3H]thymidine uptake curve of U87MG vs. MDA-435). It is important to note that DU145 cells, which have an intact PTEN gene, are relatively sensitive to CCI-779 compared with MDA-435 or SF-268, thereby providing an exception to this correlation. One explanation might be the elevated Akt3 expression and constitutive mTOR phosphorylation reported in these cells (15, 36), suggesting that either PTEN loss or Akt activation may enhance sensitivity to CCI-779. Alternatively, there may be mechanisms independent of the PTEN/Akt pathway that also affect sensitivity to CCI-779.
Figure 1.
PTEN null cells have enhanced sensitivity to mTOR inhibition. Isogenic MEFs derived from PTEN knockout mice were cultured for 72 h in the presence of vehicle alone (open bars), 1.0 nM CCI-779 (hatched bars), or 10 nM CCI-779 (solid bars). Cell growth was measured by cell counts determined by Trypan blue staining. The data are plotted as the number of cells relative to vehicle control for three experiments. The IC50 for growth inhibition of the cell lines in the table was determined by 3H-uptake studies. (*IC50 for 9L was obtained based on lack of growth inhibition by up to 10 nM of CCI-779 as measured by direct cell count.) Examples are shown for U87MG and MDA-435. The PTEN status of these lines has been reported previously (26, 35) with the exception of SF-268, SF-295, and SF-539, which were kindly provided by R. Parsons (personal communication).
Enhanced S6 Kinase Activation in PTEN Null Tumor Lines.
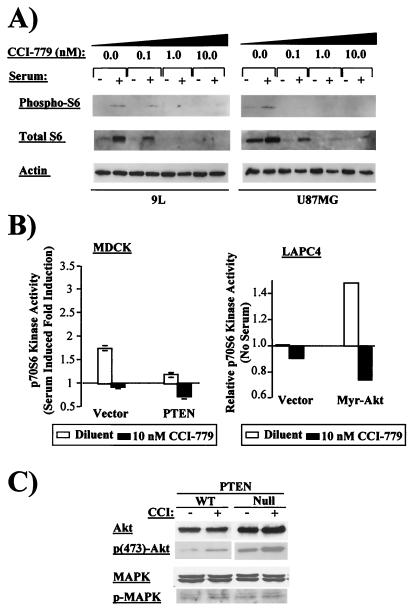
Because PTEN-deficient cells have elevated Akt activation, we asked whether these cells also have enhanced mTOR activity. We explored this question by examining S6 kinase, a downstream target of mTOR. Upon activation by mTOR, S6 kinase phosphorylates the 40S ribosomal protein S6, which functions in translation of mRNAs with a 5′ terminal oligopolypyrimidine (TOP) sequence (37, 38). Because S6 mRNA itself contains a 5′ TOP sequence, both translation and phosphorylation of S6 protein are measures of S6 kinase activity. Under serum starvation conditions, S6 protein level and phosphorylation were increased in PTEN null U87MG cells compared with PTEN wild-type 9L cells. After serum challenge, S6 protein levels and phosphorylation increased in both cell lines and were mTOR-dependent, because treatment with CCI-779 reduced S6 levels and phosphorylation to undetectable levels (Fig. 2A). These data (and data from additional models shown in Figs. 3C and 6) provide evidence that loss of PTEN is accompanied by an increase in mTOR-dependent S6 kinase activity.
Figure 2.
Enhanced S6 kinase activation in PTEN null tumor lines. (A) Phosphorylated (Ser-235/236) and total S6 protein and actin were measured by immunoblot in 9L and U87MG cells treated with vehicle or doses of 0.1, 1.0, and 10 nM CCI-779 for 7 h. Serum challenge was for 15 min. Equal amounts of protein were loaded per lane as determined by Bio-Rad DC protein assay. (B) S6 kinase activity was measured by in vitro kinase assay (31) in MDCK cells stably transfected with vector or wild-type PTEN (Left) and in LAPC-4 prostate cancer cells stably infected with retrovirus expressing myr-Akt or vector control (Right). MDCK cells were serum-starved overnight, pretreated with vehicle (open bars) or 10 nM of CCI-779 (solid bars), and then challenged with serum for 15 min. LAPC-4 cells were treated identically except that no serum challenge was given. Results from two experiments are plotted relative to serum-starved, untreated cells. Expression of PTEN and Akt in the transfectants was confirmed by immunoblot (data not shown). (C) Levels of total and activated Akt and MAPK were measured by immunoblot in lysates of PTEN wild-type (DU145) and PTEN null (PC3) cells by using specified antibodies after 2 h of pretreatment with vehicle or 10 nM CCI-779.
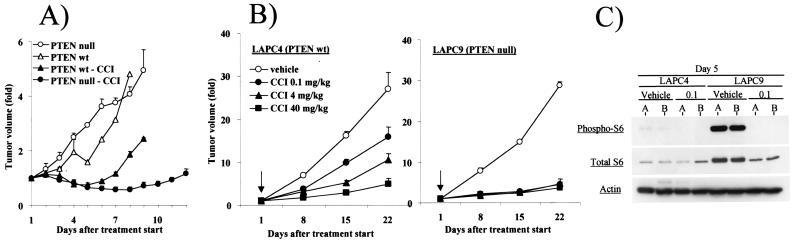
Figure 3.
PTEN null cells have enhanced sensitivity to mTOR inhibition in vivo. (A) PTEN+/+ or PTEN−/− ES cells were injected s.c. into nude mice at a dose of 5 × 105 cells per mouse (n = 20). When tumor volume reached 200 mm3, mice were randomized to treatment with vehicle or 40 mg/kg CCI-779. The fold change in tumor volume in response to treatment was plotted. (B) Single-cell suspensions of LAPC-4 or LAPC-9 prostate cancer xenografts were injected s.c. into male SCID mice (n = 80) at a dose of 106 cells per mouse. When tumors became palpable, mice were randomized (arrow) to treatment with vehicle, 0.1 mg/kg, 4 mg/kg, or 40 mg/kg CCI-779. The fold change in tumor volume from two independent experiments is plotted. (C) Tumors were harvested from mice after 5 days of treatment with vehicle or 0.1 mg/kg CCI-779 and lysed by boiling in 2% SDS buffer. Immunoblots were performed by using antibodies for phosphorylated S6 (Ser-235/236), total S6, and actin.
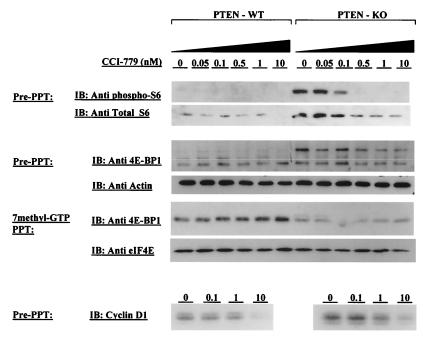
Figure 6.
Analysis of S6, 4E-BP1, and cyclin D1 in PTEN+/+ and PTEN−/− MEFs. PTEN+/+ or PTEN−/− MEFs were treated with the indicated concentrations of CCI-779 for 6 h, lysed by three freeze/thaw cycles in 50 mM Tris, pH 7.5/150 mM KCl mM EDTA/1 mM EGTA/1 mM DTT/50 mM 2-mercaptoethanol, supplemented with protease and phosphatase inhibitors, and probed with antibodies to phosphorylated S6, total S6, total 4E-BP1, or actin. The level of 4E-BP1 bound to eIF4E was measured by precipitation of eIF4E by using 7methyl-GTP Sepharose, followed by 4E-BP1 immunoblot. Comparable precipitation of eIF4E was confirmed by immunoblot.
To directly test the role of the PTEN/Akt pathway in regulating S6 kinase, we engineered PTEN or Akt overexpression in cells with wild-type endogenous PTEN. PTEN overexpression blunted serum-induced S6 kinase activity, as measured by using an immunoprecipitation kinase assay (Fig. 2B Left). Conversely, expression of a constitutively active, membrane-bound allele of Akt (myr-Akt) was sufficient to activate S6 kinase in the absence of serum, and this activation was mTOR-dependent (Fig. 2b Right). The effects of CCI-779 appear to be specific to the mTOR pathway, because no inhibition of Akt or MAPK activation was observed (Fig. 2C). Taken together, these data support the hypothesis that S6 kinase is activated in PTEN null cells.
Enhanced in Vivo Sensitivity of PTEN-Deficient Tumors to CCI-779.
To assess the role of mTOR in tumor growth mediated by PTEN loss, we examined the effect of CCI-779 on the growth of isogenic PTEN+/+ or PTEN−/− tumors produced by s.c. injection of murine ES cells into immunodeficient nude mice. When tumors reached 200 mm3 in size, mice were randomly assigned to treatment with CCI-779 or vehicle. The growth of PTEN+/+ ES tumors was slowed during the 5 days of drug administration but resumed after the drug was stopped (Fig. 3A). In contrast, the growth of PTEN−/− tumors was completely blocked for the duration of the experiment, providing evidence that PTEN-deficient tumors derived from a defined genetic background have enhanced dependence on mTOR for growth.
We explored the potential clinical utility of this observation by using human tumors grown in immunodeficient SCID mice. LAPC-4 (PTEN wild type) and LAPC-9 (PTEN null) (39) are androgen-dependent human prostate cancer xenografts that grow in male SCID mice with defined kinetics and secrete the tumor marker prostate-specific antigen into the serum (34). Because phase I clinical studies of CCI-779 suggest that doses in the 3-mg/kg range are well tolerated in humans and give serum levels well above that required to inhibit mTOR,†† we examined a range of CCI-779 doses. Male mice were injected s.c. with LAPC-4 or LAPC-9 cells and randomly assigned to treatment with three different doses of CCI-779 (0.1 mg/kg, 4.0 mg/kg, or 40 mg/kg) or vehicle when tumors reached ≈200 mm3 in size. CCI-779 impaired the growth of wild-type PTEN LAPC-4 tumors in a dose-dependent fashion, with minimal activity at the 0.1-mg/kg dose, partial activity at 4.0 mg/kg, and nearly complete growth suppression at 40 mg/kg. In contrast, all three doses were equally effective at completely blocking PTEN null LAPC-9 tumor growth in three independent experiments (Fig. 3B). These results from isogenic mouse tumors and human tumor lines establish an increase in sensitivity of PTEN null tumors to mTOR inhibition.
We examined the effect of CCI-779 on S6 kinase inhibition by measuring S6 levels and phosphorylation in tumors harvested on day 5 of treatment with the 0.1-mg/kg dose (Fig. 3C). In vehicle-treated mice, both the level and phosphorylation state of S6 protein were increased in PTEN null LAPC-9 tumors compared with PTEN wild-type LAPC-4 tumors, consistent with the notion that PTEN loss leads to up-regulation of S6 kinase activity. In the CCI-779-treated animals, S6 phosphorylation was almost completely blocked in LAPC-9 tumors and the level of S6 protein was reduced, indicating effective inhibition of mTOR. It is difficult to make similar conclusions for the LAPC-4 tumors because basal S6 phosphorylation is low. The possibility of differential inhibition of mTOR in PTEN wild type vs. PTEN null cells is addressed further in Fig. 6.
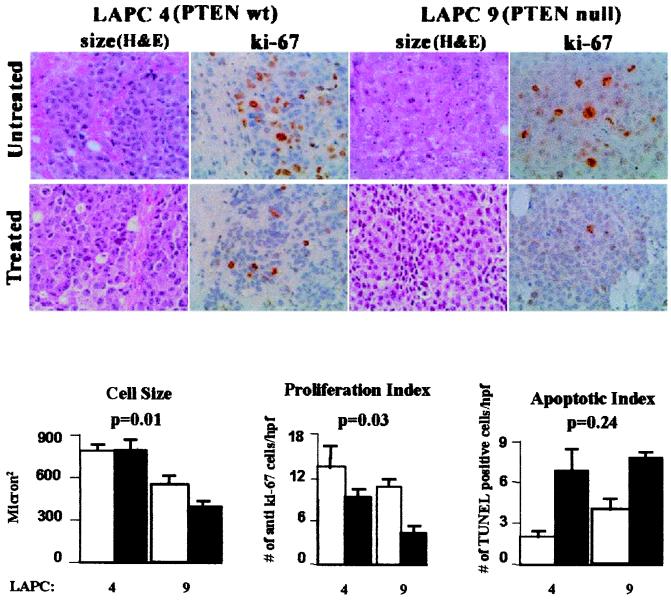
To determine the effect of CCI-779 treatment on growth and apoptosis, we compared proliferation (as determined by anti-Ki-67 staining) and apoptosis (as determined by apoptotic bodies and terminal deoxynucleotidyltransferase-mediated UTP end-labeling staining) in LAPC-4 and LAPC-9 tumors by examining histologic sections obtained after 5 days of treatment with vehicle or 0.1 mg/kg CCI-779. Measurements were based on examination of 10 slides per treatment group and 12–18 high-power fields per slide. The number of Ki-67-positive nuclei fell 1.4-fold in LAPC-4 cells vs. 2.6-fold in LAPC-9 cells, and the number of apoptotic cells increased 3.4-fold in LAPC-4 cells and 1.9-fold in LAPC-9 cells (Fig. 4). Statistical analysis indicated that the decrease in proliferation was significantly greater in the PTEN null LAPC-9 tumor line (P = 0.03), whereas the increase in apoptotic bodies was comparable in both xenografts (P = 0.24).
Figure 4.
Effect of CCI-779 on cell size, proliferation, and apoptosis. Photomicrographs (×200) of hematoxylin/eosin- and Ki-67-stained sections of LAPC-4 and LAPC-9 tumors are shown after 5 days of treatment with vehicle or CCI-779. Cell size was measured by morphometry (1,200 total cells on 20 sections). Proliferation and apoptosis were measured by counting the number of Ki-67-positive nuclei or apoptotic bodies [terminal deoxynucleotidyltransferase-mediated UTP end labeling (TUNEL)-positive] divided by high-power field (hpf) (12–18 hpf/tumor, 5 tumors/group). All values are mean ± SEM. Both cell size and proliferation were reduced significantly in CCI-779-treated LAPC-9 tumors but not LAPC-4 tumors (LAPC-4 size: P = 0.95; LAPC-9 size: P = 0.001; LAPC-4 proliferation: P = 0.212; LAPC-9 proliferation: P = 0.01, Student's t test). Apoptosis was increased significantly in treated LAPC-4 and LAPC-9 tumors (P = 0.01 and P = 0.005, respectively, Student's t test). The relative effect of CCI-779 treatment on size, proliferation, and apoptosis in LAPC-4 vs. LAPC-9 was compared by using an ANOVA model, with P values shown above the bar graphs.
In addition to growth and apoptosis, a third parameter that can affect tumor volume is cell size. Genetic analysis has established that PI3-kinase, dPTEN, dAkt, and S6 kinase regulate cell size in Drosophila (18–21). Similarly, S6 kinase-deficient mice have insulin insufficiency because of small islet cells in the pancreas (40). To determine whether mTOR inhibition affects cell size in tumors, we performed morphometric analysis of 20 tissue sections, in which 1,400 cells were analyzed. CCI-779 treatment led to a 1.4-fold reduction in cell size in PTEN null LAPC-9 tumors but had no effect in PTEN wild-type LAPC-4 tumors (P = 0.01). These findings indicate that CCI-779 can affect at least three parameters that influence tumor volume: growth, apoptosis, and cell size. Although further work is needed to fully account for its antitumor activity, the major difference in the effect of CCI-779 on PTEN null and PTEN wild-type cells appears to be growth suppression and reduction in cell size.
Akt-Mediated Tumor Growth Is mTOR-Dependent.
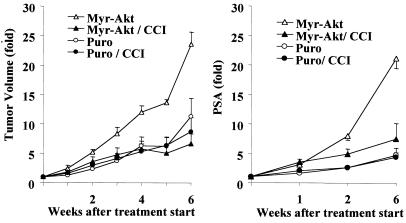
Because Akt is a major effector of transformation caused by PTEN loss, we asked whether introduction of constitutively active Akt into wild-type PTEN cells would affect CCI-779 sensitivity. To test this hypothesis, we introduced a constitutively active allele of Akt (myr-Akt) in wild-type PTEN LAPC-4 cells, which activated S6 kinase in an mTOR-dependent fashion (refer to Fig. 2B). Male SCID mice were injected with LAPC-4/puro or LAPC-4/myr-Akt cells, tumors were allowed to reach 200 mm3 in size, and mice were randomly assigned to treatment with 0.1 mg/kg CCI-779 or vehicle for 5 days. In the absence of treatment, LAPC-4/myr-Akt tumors grew more quickly than LAPC-4/puro tumors, indicating that activation of the Akt pathway enhances tumor growth in this model. The enhanced growth of LAPC-4/myr-Akt tumors was completely inhibited by CCI-779 treatment, whereas this dose had no discernible effect on LAPC-4/puro tumors (Fig. 5 Left). Similar results were obtained by using serum prostate-specific antigen as the treatment endpoint (Fig. 5 Right). These results establish that mTOR is required for the enhanced tumor growth caused by Akt activation.
Figure 5.
Akt-mediated growth in PTEN wild-type tumor cells is mTOR-dependent. LAPC-4/puro or LAPC-4/myr-Akt cells were injected s.c. into male SCID mice (n = 20) at a dose of 106 cells per mouse. When tumor volume reached 50–200 mm3, mice were randomized to treatment with vehicle or 0.1 mg/kg CCI-779, given by i.p. injection for 5 consecutive days in weeks 1 and 4. The fold changes in tumor volume (Left) and in serum prostate-specific antigen (PSA) (Right) are plotted.
Analysis of S6 and 4E-BP1 in PTEN+/+ and PTEN−/− MEFs.
One potential explanation for the differential sensitivity of PTEN wild-type and PTEN null tumor cells to CCI-779 might be a difference in biochemical inhibition of the mTOR pathway. We explored this issue by comparing S6 protein levels and phosphorylation in PTEN+/+ and PTEN−/− MEFs after exposure to concentrations of CCI-779 ranging from 0.05 to 10 nM. As observed previously in tumor cell lines (Fig. 2A) and xenografts (Fig. 3C), the level and phosphorylation state of S6 were markedly elevated in PTEN−/− cells (Fig. 6 Top). S6 phosphorylation and protein level were inhibited in a dose-dependent fashion in PTEN−/− cells. In PTEN+/+ MEFs, S6 protein level was also reduced, but this effect was observed only at the 10-nM dose (S6 phosphorylation was too low in untreated PTEN+/+ MEFs to measure any changes).
We examined this issue further by characterizing the status of 4E-BP1, a protein that blocks translation of 5′ cap mRNAs by binding the initiation factor eIF4E (41). 4E-BP1 phosphorylation, which is modulated by PI3-kinase/Akt and mTOR as well as other pathways, occurs on multiple residues and prevents complex formation with eIF4E (22, 42, 43). The level of hyperphosphorylated 4E-BP1 (detected as a slow-mobility isoform in SDS/PAGE) was elevated in PTEN−/− relative to PTEN+/+ MEFs (Fig. 6 Middle). Conversely, the level of 4E-BP1 (unphosphorylated) bound to eIF4E was increased in PTEN+/+ vs. PTEN−/− cells (Fig. 6 Bottom). Treatment with CCI-779 caused a dose-dependent increase in eIF4E-bound 4E-BP1 in PTEN+/+ cells and a dose-dependent decrease in hyperphosphorylated as well as total 4E-BP1 in PTEN−/− cells, indicating that 4E-BP1 phosphorylation is, in part, mTOR-dependent in both genetic backgrounds. Surprisingly, the decrease in 4E-BP1 phosphorylation in PTEN−/− cells was not accompanied by a detectable increase in eIF4E-bound 4E-BP1. A full explanation of this result and the apparent increase in total levels of 4E-BP1 in PTEN−/− cells require further analysis. We also examined cyclin D1, a potential downstream parameter of 4E-BP1 phosphorylation whose translation is dependent on eIF4E function (and is rapamycin-sensitive) because of complex secondary structure of the 5′ untranslated region of the mRNA (44, 45). Cyclin D1 protein levels were elevated in PTEN−/− compared with PTEN+/+ MEFs and were reduced by CCI-779 treatment in both cell lines (Fig. 6). Because cyclin D1 is also regulated by transcriptional and posttranslational/degradative mechanisms independent of mTOR (46, 47), we cannot make definitive conclusions about how each of these variables influences cyclin D1 levels in PTEN−/− cells. Nevertheless, the collective evidence from analysis of S6, 4E-BP1, and cyclin D1 indicates that doses of CCI-779 (10 nM) that cause selective growth inhibition in PTEN−/− cells block mTOR signaling in PTEN+/+ and PTEN−/− cells.
Discussion
Our studies in human tumor lines and cells from PTEN knockout mice demonstrate that PTEN-deficient cells are sensitive to growth inhibition caused by pharmacologic mTOR blockade. We also show that a membrane-targeted Akt allele, which induces mTOR pathway activation in PTEN wild-type cells, promotes tumor growth in a fashion that is reversed by mTOR inhibition. Although targeted mTOR gene deletion is required to conclusively establish a genetic relationship between PTEN, Akt, and mTOR in tumorigenesis, our data provide pharmacologic evidence that mTOR is an important effector of transformation mediated by perturbation of the PTEN/Akt pathway. This conclusion is consistent with recent work in chicken embryo fibroblasts showing that the mTOR inhibitor rapamycin specifically blocks focus formation induced by oncogenic alleles of PI3-kinase or Akt, but not by Src, Ras, Myc, or other oncogenes (48). Because of evidence from other studies that PI3-kinase/Akt- and mTOR-mediated signals to S6 kinase and 4E-BP1 can be separated (15, 22), it important to stress that the relationship between these signaling pathways is not strictly linear.
Why does loss of PTEN sensitize tumors to mTOR inhibition? Signaling through the PI3-kinase/Akt pathway normally is tightly regulated, such that PIP3 levels are maintained at low concentrations and rise transiently in response to specific growth factor signals. In contrast, PIP3 levels are constitutively elevated in tumors lacking PTEN, raising the possibility that sustained activation of signaling molecules downstream of PIP3 renders cells more dependent on this pathway for growth. If this is the case, cells with PTEN loss could be more sensitive to the biological effects of mTOR inhibition than PTEN wild-type cells, even though biochemical inhibition of the mTOR pathway occurs efficiently in both cell types. Our data showing efficient blockade of S6 phosphorylation in all cell lines tested, regardless of their biological sensitivity to CCI-779, provide support for this hypothesis. A similar conclusion was drawn in the analysis of rapamycin-sensitive and rapamycin-resistant rhabdomyosarcomas (49). In that study, elevated c-myc expression was correlated with rapamycin resistance, raising the possibility of parallel pathways that can rescue cells from mTOR dependence. In preliminary studies, we were unable to demonstrate a similar correlation in PTEN+/+ and PTEN−/− MEFs (M.S.N. and C.L.S, unpublished observations). Identification of these pathways in PTEN+/+ vs. PTEN−/− cells requires further investigation. However, it remains possible that loss of PTEN may predispose cells to a greater degree of biochemical inhibition of the mTOR pathway at very low doses of CCI-779, as suggested by our analysis of S6 protein levels in MEFs. If so, this could have important implications with regard to the degree of mTOR inhibition in clinical situations in which drug levels vary depending on the frequency of dosing.
Aberrant activation of the PI3-kinase/Akt pathway occurs in a large number of human cancers—primarily through PTEN loss, but also through PI3-kinase or Akt gene amplification (23, 50). Phase I clinical trials of mTOR inhibitors in various cancers currently are underway and have shown preliminary evidence of antitumor activity.†† Our findings suggest that PTEN null cancers are one group of human tumors in which mTOR inhibitors may be effective. Because factors other than the PTEN/Akt pathway also can affect mTOR activation, it will be important not to exclude patients with wild-type PTEN tumors from clinical trials.
Acknowledgments
We are grateful to Peter Houghton for sharing data before publication, Nahum Sonenberg for providing 4E-BP1 antibody, Fred Dorey for help with statistics, Ramon Parsons for providing data on PTEN status of cell lines, and Lisa Rose for manuscript preparation. This work was supported by grants from the National Institutes of Health, Department of Defense, and CaP CURE.
Abbreviations
- FRAP/mTOR
mammalian target of rapamycin
- PI3-kinase
phosphatidyl inositol 3-kinase
- MEF
mouse embryo fibroblasts
- ES
embryonic stem
- SCID
severe combined immunodeficient
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 10031.
Gibbons, J. J., Discafani, C., Peterson, R., Hernandez, R., Skotnicki, J. & Fruman, D. (1999) Proc. Am. Assoc. Cancer Res. 40, 301 (abstr.).
Raymond, E., Alexandre, J., Depenbrock, H., Mekhaldi, S., Angevin, E., Hanauske, A., Baudin, E., Escudier, B., Frisch, J., Boni, J., et al. Am. Soc. Clin. Oncol. 36th Annual Meeting, May 19–23, 2000, New Orleans, LA, 728 (abstr.).
References
- 1.Schmelzle T, Hall M N. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- 2.Brown E J, Albers M W, Shin T B, Ichikawa K, Keith C T, Lane W S, Schreiber S L. Nature (London) 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 3.Sabatini D M, Erdjument-Bromage H, Lui M, Tempst P, Snyder S H. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 4.Chiu M I, Katz H, Berlin V. Proc Natl Acad Sci USA. 1994;91:12574–12578. doi: 10.1073/pnas.91.26.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabers C J, Martin M M, Brunn G J, Williams J M, Dumont F J, Wiederrecht G, Abraham R T. J Biol Chem. 1995;270:815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- 6.Keith C T, Schreiber S L. Science. 1995;270:50–51. doi: 10.1126/science.270.5233.50. [DOI] [PubMed] [Google Scholar]
- 7.Schreiber S L. Science. 1991;251:283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- 8.Kuo C J, Chung J, Fiorentino D F, Flanagan W M, Blenis J, Crabtree G R. Nature (London) 1992;358:70–73. doi: 10.1038/358070a0. [DOI] [PubMed] [Google Scholar]
- 9.Dilling M B, Dias P, Shapiro D N, Germain G S, Johnson R K, Houghton P J. Cancer Res. 1994;54:903–907. [PubMed] [Google Scholar]
- 10.Hara K, Yonezawa K, Weng Q-P, Kozlowski M T, Belham C, Avruch J. J Biol Chem. 1998;273:14484–14494. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- 11.Brown E J, Beal P A, Keith C T, Chen J, Shin T B, Schreiber S L. Nature (London) 1995;377:441–446. doi: 10.1038/377441a0. [DOI] [PubMed] [Google Scholar]
- 12.Brown E J, Schreiber S L. Cell. 1996;86:517–520. doi: 10.1016/s0092-8674(00)80125-7. [DOI] [PubMed] [Google Scholar]
- 13.Brunn G J, Williams J, Sabers C, Wiederrecht G, Lawrence J C, Jr, Abraham R T. EMBO J. 1996;15:5256–5267. [PMC free article] [PubMed] [Google Scholar]
- 14.Gingras A C, Kennedy S G, O'Leary M A, Sonenberg N, Hay N. Genes Dev. 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sekulic A, Hudson C C, Homme J L, Yin P, Otterness D M, Karnitz L M, Abraham R T. Cancer Res. 2000;60:3504–3513. [PubMed] [Google Scholar]
- 16.Zhang H, Stallock J P, Ng J C, Reinhard C, Neufeld T P. Genes Dev. 2000;14:2712–2724. doi: 10.1101/gad.835000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oldham S, Montague J, Radimerski T, Thomas G, Hafen E. Genes Dev. 2000;14:2689–2694. doi: 10.1101/gad.845700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goberdhan D C, Paricio N, Goodman E C, Mlodzik M, Wilson C. Genes Dev. 1999;13:3244–3258. doi: 10.1101/gad.13.24.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang H, Potter M, Tao W, Li D M, Brogiolo W, Hafen E, Sun H, Xu T. Development (Cambridge, UK) 1999;126:5365–5372. doi: 10.1242/dev.126.23.5365. [DOI] [PubMed] [Google Scholar]
- 20.Montagne J, Stewart M J, Stocker H, Hafen E, Kozma S C, Thomas G. Science. 1999;285:2126–2129. doi: 10.1126/science.285.5436.2126. [DOI] [PubMed] [Google Scholar]
- 21.Gao X, Neufeld T P, Pan D. Dev Biol. 2000;221:404–418. doi: 10.1006/dbio.2000.9680. [DOI] [PubMed] [Google Scholar]
- 22.Dufner A, Andjelkovic M, Burgering B M, Hemmings B A, Thomas G. Mol Cell Biol. 1999;19:4525–4534. doi: 10.1128/mcb.19.6.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng J Q, Godwin A K, Bellacosa A, Taguchi T, Franke T F, Hamilton T C, Tsichlis P N, Testa J R. Proc Natl Acad Sci USA. 1992;89:9267–9271. doi: 10.1073/pnas.89.19.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng J Q, Ruggeri B, Klein W M, Sonoda G, Altomare D A, Watson D K, Testa J R. Proc Natl Acad Sci USA. 1996;93:3636–3641. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Y Y, Wei S J, Lin Y C, Lung J C, Chang T C, Whang-Peng J, Liu J M, Yang D M, Yang W K, Shen C Y. Oncogene. 2000;19:2739–2744. doi: 10.1038/sj.onc.1203597. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 27.Steck P A, Pershouse M A, Jasser S A, Yung W K, Lin H, Ligon A H, Langford L A, Baumgard M L, Hattier T, Davis T, et al. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 28.Liaw D, Marsh D J, Li J, Dahia P L M, Wang S I, Zheng Z, Bose S, Call K M, Tsou H C, Peacocke M, et al. Nat Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 29.Marsh D J, Dahia P L, Zheng Z, Liaw D, Parsons R, Gorlin R J, Eng C. Nat Genet. 1997;16:333–334. doi: 10.1038/ng0897-333. [DOI] [PubMed] [Google Scholar]
- 30.Cantley L C, Neel B G. Proc Natl Acad Sci USA. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawasome H, Papst P, Webb S, Keller G M, Johnson G L, Gelfand E W, Terada N. Proc Natl Acad Sci USA. 1998;95:5033–5038. doi: 10.1073/pnas.95.9.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu X, Senechal K, Neshat M S, Whang Y E, Sawyers C L. Proc Natl Acad Sci USA. 1998;95:15587–15591. doi: 10.1073/pnas.95.26.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun H, Lesche R, Da-Ming L, Liliental J, Zhang H, Gao J, Gavrilova N, Mueller B, Liu X, Wu H. Proc Natl Acad Sci USA. 1999;96:6199–6204. doi: 10.1073/pnas.96.11.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Craft N, Chhor C, Tran C, Belldegrun A, Dekernion J B, Witte ON, Said J, Reiter R E, Sawyers C L. Cancer Res. 1999;59:5030–5036. [PubMed] [Google Scholar]
- 35.Furnari F B, Lin H, Huang H S, Cavenee W K. Proc Natl Acad Sci USA. 1997;94:12479–12484. doi: 10.1073/pnas.94.23.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakatani K, Thompson D A, Barthel A, Sakaue H, Liu W, Weigel R J, Roth R A. J Biol Chem. 1999;274:21528–21532. doi: 10.1074/jbc.274.31.21528. [DOI] [PubMed] [Google Scholar]
- 37.Terada N, Patel H R, Takase K, Kohno K, Nairn A C, Gelfand E W. Proc Natl Acad Sci USA. 1994;91:11477–11481. doi: 10.1073/pnas.91.24.11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jefferies H B, Reinhard C, Kozma S C, Thomas G. Proc Natl Acad Sci USA. 1994;91:4441–4445. doi: 10.1073/pnas.91.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whang Y E, Wu X, Suzuki H, Reiter R, Tran C, Vessella R L, Said J W, Isaacs W B, Sawyers C L. Proc Natl Acad Sci USA. 1998;95:5246–5250. doi: 10.1073/pnas.95.9.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pende M, Kozma S C, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, Klumperman J, Thorens B, Thomas G. Nature (London) 2000;408:994–997. doi: 10.1038/35050135. [DOI] [PubMed] [Google Scholar]
- 41.Gingras A C, Raught B, Sonenberg N. Genes Dev. 2001;5:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- 42.Beretta L, Gingras A C, Svitkin Y V, Hall M N, Sonenberg N. EMBO J. 1996;15:658–664. [PMC free article] [PubMed] [Google Scholar]
- 43.Gingras A C, Ming X F, Sonenberg N, Thomas G. Proc Natl Acad Sci USA. 1996;93:4076–4080. doi: 10.1073/pnas.93.9.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenwald I B, Kaspar R, Rousseau D, Gehrke L, Leboulch P, Chen J J, Schmidt E V, Sonenberg N, London I M. J Biol Chem. 1995;270:21176–21180. doi: 10.1074/jbc.270.36.21176. [DOI] [PubMed] [Google Scholar]
- 45.Muise-Helmericks R C, Grimes H L, Bellacosa A, Malstrom S E, Tsichlis P N, Rosen N. J Biol Chem. 1998;273:29864–29872. doi: 10.1074/jbc.273.45.29864. [DOI] [PubMed] [Google Scholar]
- 46.Sherr C J. Cancer Res. 2000;60:3689–3695. [PubMed] [Google Scholar]
- 47.Diehl J A, Cheng M, Roussel M F, Sherr C J. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aoki M, Blazek E, Vogt P K. Proc Natl Acad Sci USA. 2001;98:136–141. doi: 10.1073/pnas.011528498. . (First Published December 26, 2000; 10.1073/pnas.011528498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hosoi H, Dilling M B, Liu L N, Danks M K, Shikata T, Sekulic A, Abraham R T, Lawrence JC, Jr, Houghton P J. Mol Pharmacol. 1998;54:815–824. doi: 10.1124/mol.54.5.815. [DOI] [PubMed] [Google Scholar]
- 50.Ali I U, Schriml L M, Dean M. J Natl Cancer Inst. 1999;91:1922–1932. doi: 10.1093/jnci/91.22.1922. [DOI] [PubMed] [Google Scholar]