Summary
Obesity and asthma prevalence has dramatically and concomitantly increased over the last 25 years, and many epidemiological studies have highlighted obesity as an important risk factor for asthma. Although many studies have been performed, the underlying mechanisms remain poorly understood. Innate mechanisms have been involved in both diseases, in particular through the recently described innate lymphoid cells (ILCs). ILCs are subdivided into three groups that are defined by their cytokine production and by their master transcription factor expression, in sharp correlation with their T helper counterparts. However, unlike T helper cells, ILCs do not express antigen‐specific receptors, but respond to damage‐induced signals. ILCs have been found in target tissues of both diseases, and data have implicated these cells in the pathogenesis of both diseases. In particular group 2 ILCs (ILC2) are activated in both the adipose and lung tissues under the effect of interleukin‐33 and interleukin‐25 expression. However, counter‐intuitively to the well‐known association between obesity and asthma, ILC2 are beneficial for obesity but deleterious for asthma. This review will examine the roles of ILCs in each disease and recent data highlighting ILCs as a putative link between obesity and asthma.
Keywords: asthma, innate lymphoid cells, obesity
Abbreviations
- AHR
airway hyper‐responsiveness
- AT
adipose tissue
- CHILP
common helper ILC progenitor
- CLP
common lymphoid progenitor
- DC
dendritic cell
- EILP
early ILC progenitor
- Id2
inhibitor of DNA‐binding protein 2
- IFN
interferon
- ILC
innate lymphoid cell
- ILCP
ILC progenitor
- LTi
lymphoid tissue inducer
- LTip
LTi precursor
- MHC‐II
major histocompatibility complex class II
- NCR
natural cytotoxic receptor
- NK
natural killer
- NKp
natural killer cell precursor
- Th2
T helper type 2
- TSLP
thymic stromal lymphopoietin
Obesity and asthma
Obesity and asthma prevalence has dramatically and concomitantly increased over the last 25 years,1, 2 and a significant proportion of patients with severe or difficult‐to‐control asthma are obese. Although pre‐existing asthma may be complicated by obesity, many epidemiological studies have highlighted obesity as an important risk factor for asthma development3, 4 with a 92% increased risk of asthma when body mass index exceeds 30 kg/m2.5 Moreover, bariatric surgery and dietary restriction improve, respectively, bronchial hyper‐responsiveness,6 and airway inflammation and clinical outcomes in obese patients with asthma,7 pointing towards a functional link between obesity and asthma. Although asthma in adult obese patients is often non‐atopic, associated with corticosteroid‐resistance and with a neutrophilic profile in sputum,8, 9 it has been recently shown that eosinophils are nonetheless predominant in the tissue but not the lumen of obese patients with severe asthma,10 raising the possibility that obese asthmatic patients may be misclassified based on their sputum eosinophils.11 Moreover, increased risks of atopy12, 13, 14 and T helper type 2 (Th2) responses10, 15, 16 are also observed in obese patients. Foremost, obesity contributes to asthma exacerbation in children17 in whom the disease is mostly allergic. However, the underlying mechanisms remain poorly understood.18, 19 Mechanical effects linked to overweight and adipose tissue (AT) accumulation, as well as obesity‐associated inflammation, have been implicated. For example, It has been shown that breathing at low lung volumes20 as well as augmented airway wall thickness,21 increases airway reactivity. On the inflammatory side, increased oxidative stress and modification of nitric oxide metabolism in exhaled breath condensates have been observed in obese patients with asthma.22 Moreover, leptin, a pro‐inflammatory adipokine, increases airway hyper‐responsiveness (AHR) and IgE production in a model of ovalbumin‐sensitized mice,23 whereas administration of the anti‐inflammatory adiponectin almost totally suppresses AHR and Th2 cytokine production in the same model.24 However, reciprocal changes of these adipokines are not always found in obese patients with asthma.25, 26, 27 Some common metabolic pathways may contribute to both diseases such as chitinase 3‐like 1, first described as associated with asthma susceptibility and with allergic airway inflammation,28 and more recently found to be also involved in the development of visceral obesity, through its effect on the Sirtuin1 pathway. This pathway is up‐regulated in both the AT and lung tissue in response to allergen challenge.29 Among other mechanisms, consumption of a high‐fat diet during pregnancy may contribute to the development of asthma in offspring. Indeed, structural changes, higher level of cytokines and increased airway resistance have been observed in rats born from mothers fed a high‐fat diet during gestation.30 Similar structural changes have been observed in lung epithelial cells in adult mice fed with a high‐fat diet.31 Diets low in fibre or high in fat that lead to obesity32 modify the gut microbiota,33, 34 which may be involved in the development of allergic airway disease. Accordingly, increasing fibre consumption in mouse models of asthma modifies the gut microbiota and reduces allergic airway inflammation through mechanisms involving dendritic cells.35 Furthermore, high‐fat diet‐induced dysbiosis results in epigenetic modifications of the Forkhead box P3 (FoxP3) promoter, leading to suppression of regulatory T cells and increased allergic airway inflammation.36
The contribution of adaptive responses triggered by metabolic alterations on the subsequent development of experimental asthma remains controversial. A Th17 response is observed in both diseases,37, 38, 39 whereas the Th2 response is40, 41, 42 or is not43, 44 found in allergen‐induced asthma exacerbated by diet‐induced obesity. Nonetheless, interleukin‐33 (IL‐33) and IL‐25, two pro‐Th2 cytokines produced by both the AT45, 46 and lung epithelial cells47, 48 can activate group 2 innate lymphoid cells (ILC2).49
Innate lymphoid cells
Innate lymphoid cells have been the focus of intense investigation over the last 5 years and have emerged as key players in the immune responses in various tissues, including the visceral AT and the lung.50, 51, 52 ILCs are currently subdivided into three groups that are defined by their cytokine production and by their master transcription factor expression, in sharp correlation with their T helper counterparts49 (Fig. 1). However, unlike T helper cells, ILCs do not express antigen‐specific receptors and lack common cell lineage markers, but respond to damage‐induced signals such as alarmins and cytokines and can shape the adaptive immune response.
Figure 1.
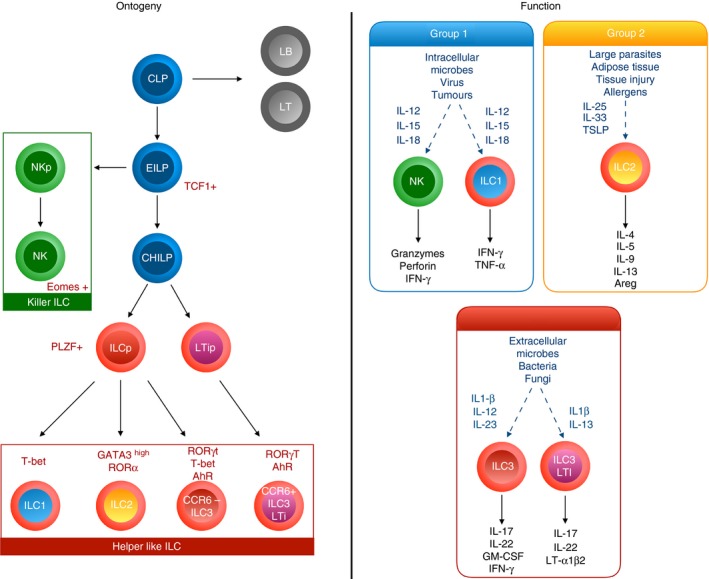
Ontogeny and function of lymphoid lineages. Downstream of the common lymphoid progenitor (CLP), the early innate lymphoid cell (ILC) Progenitor (EILP) initiates TCF1 expression and can differentiate into either natural killer (NK) cells or into an Id2high common helper‐like ILC progenitor (CHILP). According to the expression or not of PLZF, it will give rise to either all helper‐like ILC subsets including CCR6+ ILC3, or to ILC1, ILC2 and CCR6− ILC3. Environmental cytokines as well as ILC differential transcriptional factors programme their cytokine production and function.
Group 1 ILCs (ILC1), include a subgroup of natural killer (NK) cells and of non‐NK ILC1 cells distinguished by a combination of markers including lack of expression of CD127 and expression of eomes for mature NK cells.53 They produce the cytokines interferon‐γ (IFN‐γ) and tumour necrosis factor‐α, express the transcription factor T‐bet and mediate immune responses to pathogens and tumours in response to IL‐12, IL‐15 and IL‐18.54
Group 2 ILCs (ILC2) produce IL‐4, IL‐5, IL‐13, IL‐9 and amphiregulin, express the transcription factors GATA3 and RAR‐related orphan receptor α and mediate responses against parasites and allergens in response to IL‐33, IL‐25 and thymic stromal lymphopoietin (TSLP).52
Group 3 ILCs (ILC3) encompass different subgroups of cells including in mice, the CCR6+ fetal lymphoid tissue inducers (LTi) and adult LTi‐like cells, and the CCR6− natural cytotoxic receptor (NCR)+ or NCR− ILC3. In humans, CCR6+ ILC3 are subdivided according to their expression of NKp44. They produce the cytokines IL‐17 and/or IL‐22, express the transcription factor Retinoid‐related orphan receptor γt and aryl hydrocarbon receptor and mediate mucosal immunity against bacterial and fungal infections, in particular at the intestinal level, in response to IL‐1β and IL‐23.55
All ILC subsets are dependent upon the common γ‐chain receptor signalling and develop from a common lymphoid progenitor found in fetal liver and in bone marrow that will give rise to an early ILC progenitor able to differentiate in either NK cells, or through the up‐regulation of the transcription factor inhibitor of DNA‐binding protein 2 (Id2), into a common helper ILC progenitor (CHILP) (Fig. 1). Downstream commitment to the different ILC lineages is achieved through a wide range of transcription factors including T‐cell factor‐1 (TCF‐1), nuclear factor IL‐3 (NFIL‐3), thymocyte selection associated HMG boxprotein (TOX), GATA‐356, 57, 58, 59, 60 giving rise to either pro‐myelocytic leukaemia zinc finger (PLZF) expressing ILC progenitor (ILCP),61 or to an LTi precursor. ILCP will generate all ILC subgroups except LTi‐like ILC3 (Fig. 1). Interestingly, ILCP able to give rise to all ILC subsets except IL‐17+ ILC3 have recently been described in the peripheral blood in humans, supporting a model of tissue differentiation in response to local environmental cues.62
Recent transcriptomic studies have revealed that ILC subsets exhibit a certain degree of heterogeneity63, 64, 65 and plasticity allowing switching between subsets depending mainly on the local cytokine milieu. For example, ILC1 can evolve towards ILC3 in the presence of IL‐2, IL‐23 and IL‐1β, and CD14+ dendritic cells (DCs) can differentiate ILC3 into ILC1.66 ILC2 have also been shown to rise to ILC1‐like IFN‐γ producer in the presence of IL‐1, IL‐12 and IL‐18,67, 68, 69 and ILC1 can revert to ILC2 in the presence of IL‐4.70 Moreover a potential IL‐25‐induced ILC2 precursor has been reported to give rise to IL‐17‐producing ILC3‐like cells.71
Regulatory pathways also play a role in the control of ILC2 activation. Type I and II IFN, as well as IL‐27, are able to block ILC2 activation in response to IL‐2, IL‐25 and IL‐33 through a signal transducer and activation of transcription 1 (STAT1) ‐dependent pathway,72, 73 opening new avenues for specific targeted therapies.
Lymphoid cells in obesity
Adipose tissue expands with obesity, and initially retains relatively normal metabolic function. In chronic obesity, inflammatory immune cells accumulate in the AT and promote insulin resistance leading to type 2 diabetes. Strikingly, ILC2 have been initially identified in gut mucosa and fat‐associated lymphoid clusters74 and play a key role in metabolic homeostasis of lean healthy AT. Three types of AT are recognized, the most abundant white type is involved in excess energy storage, and the brown and beige types dissipate energy through the production of heat. Type 2 responses promote energy expenditure by inducing beige adipocytes that protect against insulin resistance and type 2 diabetes.75 Interleukin‐5 provided by ILC2 is required for eosinophil activation and their migration to the visceral AT, whereas ILC2‐derived IL‐13 promotes alternately activated macrophages. Altogether, resident visceral AT ILC2 sustain tissue eosinophil and anti‐inflammatory alternately activated macrophage homeostasis as well as beige fat biogenesis,76 all involved in protection against obesity‐induced metabolic dysfunction. In contrast, the absence of ILC2 promotes adiposity and insulin resistance in animals fed a high‐fat diet.46, 77, 78 Residency and activation of ILC2 in AT are promoted by different signals including IL‐33 and IL‐25. Resting AT expresses IL‐33 in particular through endothelial cells, and IL‐33‐deficient mice fed a high‐fat diet exhibit increased whole body adiposity and decreased insulin secretion.79 However, mice lacking IL‐33 signalling still exhibit resident AT ILC2, suggesting that other factors are also involved. Among them, IL‐280 and IL‐2546 may be involved. The importance of IL‐33 and IL‐25 in the activation of ILC2 and visceral AT homeostasis has been confirmed by performing gain and loss of function studies.46, 76, 77, 81 Two mechanisms have been demonstrated. One of them links IL‐5 production by ILC2 to production of IL‐4 by eosinophils and subsequent beiging of adipocytes through their expression of the IL‐4 receptor.81 The second one involves the production of methionine‐enkephalin by ILC2 that up‐regulates the uncoupling protein UCP‐1, inducing the beiging of adipocytes.76 Finally, IL‐4 and IL‐13 production by eosinophils, ILC2 and NKT cells, allows the recruitment of alternately activated macrophages that can regulate energy expenditure by adipocytes.82, 83, 84 It is noteworthy that IL‐33 as well as IL‐2 have been shown to be crucial for T regulatory cell recruitment and expansion within the AT.85, 86, 87 This similar regulation of T regulatory cells and ILC2 suggests that these cell types may cooperate to maintain AT homeostasis. ILC3 have also been observed in visceral AT from lean mice with a slight decrease in diet‐induced obesity,42 but their functionality regarding metabolic homeostasis has still to be established. During chronic obesity, ILC2 within the AT decline. However, little is known about the precise signals allowing ILC2 trafficking to or from the AT. Along the same lines, mechanisms that may restrict AT ILC2 are still unclear. One possible mechanism may involve NK cells, well represented in AT, that have been shown to participate in obesity‐induced adipose tissue inflammation,88 and that highly produce IFN‐γ, a cytokine that can inhibit ILC2 activation and proliferation.89 Moreover, in contrast to adipose‐associated ILC2 that limit inflammation and participate in metabolic homeostasis, it has been recently shown that adipose‐resident ILC1 contribute to disease progression. Experiments in parabiotic mice have shown that both ILC1 and ILC2 maintain long‐term residency. Diet‐induced obesity leads to production of IL‐12 in AT able to drive the local proliferation of ILC1 through the expression of the IL‐12 receptor and of STAT4. Through the production of IFN‐γ, ILC1 induce pro‐inflammatory macrophage polarization and promote obesity‐associated insulin resistance.90 These data may provide mechanistic hypotheses about the diversification of AT ILC infiltration from homeostasis to obesity, such as either an antagonistic balance between ILC1 and ILC2, as IFN‐γ can inhibit ILC2,98 or a potential conversion of ILC2 towards ILC1 during diet‐induced obesity, as such transdifferentiation has been described in response to IL‐12.67 Altogether, AT ILC2, appear to be beneficial in protecting against obesity and insulin resistance, whereas ILC1 appear deleterious (Fig. 2).
Figure 2.
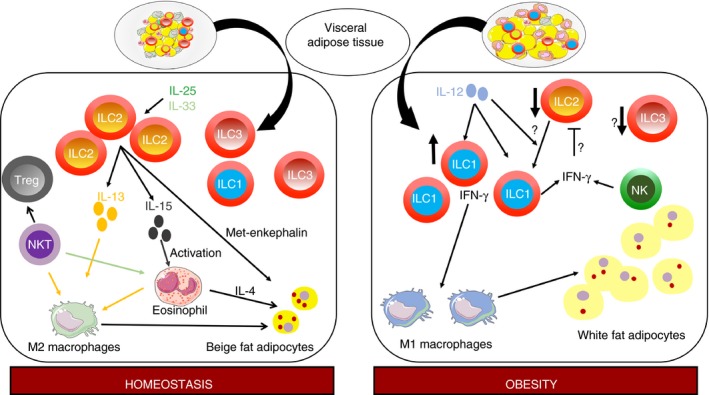
Innate lymphoid cells (ILCs) in the visceral adipose tissue. In homeostasis conditions, the visceral adipose tissue is infiltrated by ILC2 in response to locally produced interleukin‐33 (IL‐33) and IL‐25. Through their production of IL‐5 and IL‐13, they activate on the one hand eosinophils and on the other hand alternately activated M2‐type macrophages, leading to the beiging of adipocytes. Natural killer T cells are also involved in homeostasis through their activation of eosinophils and T regulatory cells. ILC1 and ILC3 are also present although their function at baseline is unclear. In obesity, ILC2 and potentially ILC3 are decreased, whereas ILC1 are increased in response to locally produced IL‐12. ILC1 activate classically activated M1‐type macrophages that promote obesity‐associated insulin resistance. Putatively, decreased ILC2 number may originate from a conversion of ILC2 in ILC1, or from inhibition of ILC2 by ILC1 and natural killer cells through their production of interferon‐γ.
Lymphoid cells in asthma
In agreement with the role of ILC2 in type 2 responses,74, 91, 92 ILC2 contribute to experimental asthma to allergens such as papain, alternaria or house dust mite, by inducing lung eosinophilia, mucus production and AHR, through their rapid production of IL‐13 and IL‐5, upon stimulation by IL‐33, IL‐25 or TSLP mainly derived from epithelial cells.93, 94, 95, 96 In models of persistence of chronic asthma (for more than 6 months), only depletion of ILC2 but not of CD4 T cells abrogates sustained AHR.97 Moreover, in a model of cortico‐resistant airway inflammation, corticosteroids could suppress Th2 cells but not ILC2, the corticoid resistance of ILC2 being induced by TSLP.98
These observations have been confirmed in asthma in humans. ILC2 are increased in peripheral blood from patients with asthma,99, 100, 101, 102 as well as in sputum,102 and bronchoalveolar lavages,97, 103 compared with control subjects. Furthermore, the frequency of ILC2 in blood100 from asthmatic patients was inversely correlated with lung function tests, suggesting a functional link between ILC2 and severity of asthma. ILC2 are also involved in steroid‐resistant asthma. The number of ILC2, and type 2 cytokine‐producing ILC2 is significantly increased in peripheral blood and sputum of systemic steroid‐dependent patients with severe asthma compared with those with mild asthma.102 In contrast, the number of type 2 cytokine‐producing CD4 is similar between the two groups, suggesting that ILC2 rather than CD4 T cells play a significant role in steroid‐resistant asthma.
Remarkably, rag−/− (no T and B cells) but not rag−/− IL2rg−/− (no T, B and ILC) or rag−/− depleted in ILCs, exhibit papain‐induced asthma‐like symptoms,95 suggesting that adaptive immunity is not essential for allergic inflammation development.
It is possible that ILCs act as an early source of cytokines allowing for the development of adaptive immunity as suggested by studies showing decreased sensitization and differentiation of Th2 cells in the absence of ILC2 in models of airway allergic inflammation.104 Major effects of ILC2 depletion might also result from their identified MHC‐II‐restricted antigen presentation properties and to the subsequent IL‐2 production by T cells leading to a mutual increase in type 2 cytokine production.105, 106 In line with this, upon airway allergic inflammation induction, the absence of ILC2 in Rorα sg/sg bone marrow chimeric mice results in a strong decrease in bronchoalveolar lavage eosinophil numbers, IL‐13 lung expression, as well as in IgE levels.107 Adoptive transfer of both ILC2 and CD4+ T cells, but not of each individual population, into Il7ra −/− mice, which lack both T cells and ILC2, results in a robust antigen‐specific Th2 cytokine response and airway inflammation.108 Furthermore, IL‐13 production by ILC2 during a recall response induces interferon‐regulatory‐factor‐4‐expressing DCs in the lung able to drive accumulation of memory Th2 cells.109
The ILC2 can be regulated during lung allergic inflammation in a positive or negative way. For example, basophil‐derived IL‐4 activates lung ILC2,110 whereas IL‐33‐activated mast cells expand T regulatory cells that suppress ILC2 activation.111 In alternaria‐induced lung inflammation, IL‐27 can inhibit tissue‐resident ILC2 but not Th2 cells.73 Finally, lipid mediators such as cysteinyl leukotrienes, prostaglandins and lipoxins, can also, respectively, activate or inhibit ILC2.112, 113, 114
Besides ILC2, a recent work has demonstrated that NCR− ILC3 are also induced in the lungs of mice with house dust mite‐induced airway inflammation.42 In humans, IL‐17‐producing ILC3 have been identified in the bronchoalveolar lavage fluid from patients with asthma.115 For ILC1, no work has yet evaluated their potential role in asthma, except for NK cells, whose main purpose is still unclear.
Altogether, lung ILC2, and putatively ILC3, play a pivotal role in the initiation, exacerbation and chronicity of asthma (Fig. 3), in contrast to obesity where AT ILC2 are beneficial for metabolic homeostasis. However, obesity is associated with increased asthma risk and severity.
Figure 3.
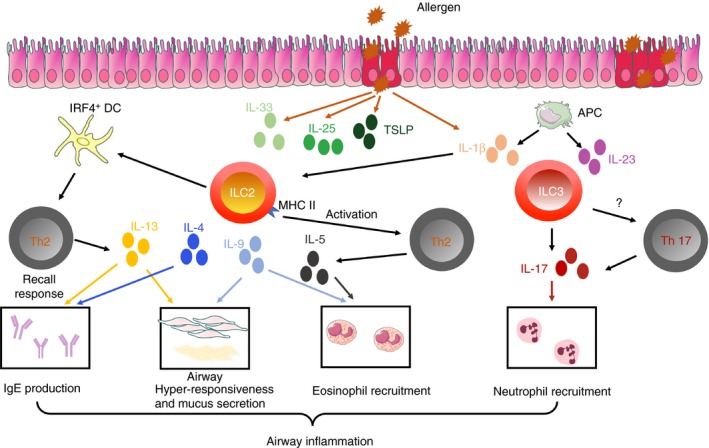
Innate lymphoid cells (ILCs) in allergic airway inflammation. In response to protease‐type allergens, airway epithelial cells release cytokines such as interleukin‐25 (IL‐25), IL33, thymic stromal lymphopoietin (TSLP) and IL‐1β, which all activate ILC2. Activated ILC2 produce type 2 cytokines, such as IL‐4, IL‐5, IL‐9 and IL‐13. Then they activate T helper type 2 (Th2) cells either directly, through MHCII expression, or through dendritic cells. Altogether, the released cytokines promote the different features of asthma, including airway hyper‐responsiveness, eosinophil accumulation and IgE production. IL‐1β produced by epithelial cells or alveolar macrophages can also activate ILC3, leading to the production of IL‐17, promoting the recruitment of neutrophils. ILC3 may also potentially activate Th17 cells.
Lymphoid cell: a link between obesity and asthma?
Some findings suggest that ILCs are also involved in obesity‐associated allergic airway inflammation. Increased numbers of ILC3 have been found in the lungs of obese mice fed a high‐fat diet, in comparison with lean mice.119 These obese mice exhibited AHR in the absence of allergen challenge, which was independent of adaptive immunity, but in relation with ILC3‐derived IL‐17. Indeed, obesity‐induced AHR was decreased in IL‐17−/− mice or rag−/− mice depleted in ILCs, and restored by ILC3 transfer. Interleukin‐17 production by ILC3 was dependent upon the Nlrp3 inflammasome stimulated by macrophage‐derived IL‐1β.115 Ozone exposure has also been shown to result in increased AHR in obese mice compared with lean mice. This effect was induced through increased IL‐33, and induction of IL‐13‐producing ILC2.116
Finally, another paper recently showed in a model of high‐fat diet‐induced obesity followed by house dust mite‐induced airway allergic inflammation that both ILC2 and ILC3 contribute to asthma aggravation by obesity.42 Notably, non‐sensitized obese mice already exhibited increased lung ILC2, ILC3 and tissue (but not airway) eosinophil infiltration compared with lean mice. This contrasts with decreased numbers of ILC2 and eosinophils observed in AT of insulin‐resistant obese animals in previous studies.46, 77, 78 To explain the differential abundance of eosinophils between lung tissue and AT, redistribution of eosinophils from the AT to the lung tissue has been previously suggested.117, 118 This hypothesis may also apply to ILCs, in relation to obesity‐induced systemic inflammation, which might favour ILC migration from the adipose tissue towards the lung, and through their production of Th2 cytokines the recruitment of eosinophils (Fig. 4). Among systemic inflammatory mediators involved in obesity,119 IL‐1β has been involved in the induction of IL‐17 production by ILC3 cells120 and recently in the induction of type 2 cytokines by ILC2.68, 70 Its induction in the lung of non‐sensitized obese mice42, 115 may represent a starting point for the activation of both ILC2 and ILC3 in this context, although this remains to be experimentally evaluated (Fig. 4).
Figure 4.
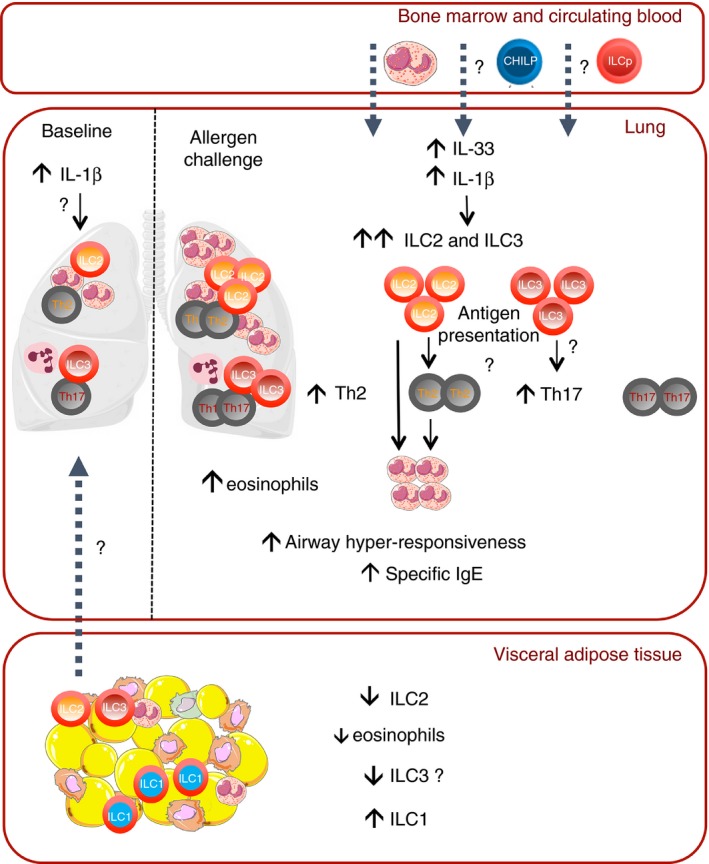
Redistribution of innate lymphoid cells (ILCs) in obesity: a link with asthma? A hypothetic mechanism. At baseline ILC2 and ILC3 are decreased in adipose tissue (AT) but present in lung tissue from obese mice, potentially through redistribution of ILCs from the AT to the lung under the effect of interleukin‐1β (IL‐1β). This leads to a small infiltration of eosinophils in the lung tissue of obese mice. In the context of allergic airway inflammation, there is a further increase in ILC2 and ILC3 at the lung level, that might originate partly from the adipose tissue but also putatively from the bone marrow and from circulating progenitors attracted by the production of IL‐33 and IL‐1β induced by allergen challenge. This increase drives further accumulation of eosinophils, activation of Th2 and Th17 cells potentially through antigen presentation, and aggravation of the features of asthma.
In conditions of allergen challenge, HFD feeding aggravated allergic airway disease features including airway and tissue eosinophilia, AHR, Th2 and Th17 pulmonary profiles, as well as the number of total and cytokine‐expressing lung ILC2 and ILC3 compared to house dust mite‐challenged lean mice.42 These modifications were accompanied by high levels of lung IL‐33 and IL‐1β and decreased ILC markers in visceral AT. Furthermore, depletion of ILCs with an anti‐CD90 antibody, followed by T‐cell reconstitution, led to a profound decrease of allergic airway inflammatory features in obese mice, including Th2 and Th17 infiltration.42 It is of note that ILCs can regulate T cells, and both ILC2 and ILC3 express MHC‐II, and therefore were suggested to directly interact with T cells like antigen‐presenting cells. ILC2 induce expansion of T cells in vitro,106 and are critical for the induction of Th2 responses104, 105, 106, 107 in particular through activation of DCs.109 ILC3 also promote adaptive CD4 responses through MHC‐II expression in the gut,121 and by triggering peripheral IL‐1β production.122 ILC3 are also able to directly induce the death of commensal bacteria‐specific CD4 T cells.123 Lastly, ILC3 can activate DCs through lymphotoxin α 1 β 2, which leads to Th17 cell differentiation.124 Therefore, ILCs may play a predominant role in the activation of Th2 and Th17 cells in obesity associated with asthma.
In other experimental studies of obesity followed by allergen challenge, increased bone marrow eosinophilia, and changes in the trafficking of eosinophils to the airways in high‐fat diet and genetic models of obesity40, 125 have been observed, suggesting that such altered trafficking may participate in asthma development. In conditions of allergen stimulation, which triggers IL‐33 production in the lung, additional sources of ILCs may be recruited to the lung, such as circulating ILC progenitors, recently described in humans,62 or bone marrow progenitors (Fig. 4). Altogether, basal infiltration of ILC2 and ILC3 in the lung of obese mice would provide the framework for the aggravation of asthma under allergen challenge.
Although the migration hypothesis awaits further investigations, it may foster novel therapeutic strategies such as redirecting ILC2 to AT. Indeed, the hallmark of ILC is quick and antigen‐independent activation settling them as putative orchestrators of adaptive responses, and as such are interesting therapeutic targets.
Disclosures
The authors declare having no competing interests.
References
- 1. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C et al Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384:766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK et al Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross‐sectional surveys. Lancet 2006; 368:733–43. [DOI] [PubMed] [Google Scholar]
- 3. Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol 2005; 115:897–909. [DOI] [PubMed] [Google Scholar]
- 4. Sutherland ER. Linking obesity and asthma. Ann N Y Acad Sci 2014; 1311:31–41. [DOI] [PubMed] [Google Scholar]
- 5. Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta‐analysis of prospective epidemiologic studies. Am J Respir Crit Care Med 2007; 175:661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker‐Leclair LA, Griffes LA et al Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol 2011; 128:508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scott HA, Gibson PG, Garg ML, Pretto JJ, Morgan PJ, Callister R et al Dietary restriction and exercise improve airway inflammation and clinical outcomes in overweight and obese asthma: a randomized trial. Clin Exp Allergy 2013; 43:36–49. [DOI] [PubMed] [Google Scholar]
- 8. Scott HA, Gibson PG, Garg ML, Wood LG. Airway inflammation is augmented by obesity and fatty acids in asthma. Eur Respir J 2011; 38:594–602. [DOI] [PubMed] [Google Scholar]
- 9. Telenga ED, Tideman SW, Kerstjens HA, Hacken NH, Timens W, Postma DS et al Obesity in asthma: more neutrophilic inflammation as a possible explanation for a reduced treatment response. Allergy 2012; 67:1060–8. [DOI] [PubMed] [Google Scholar]
- 10. Desai D, Newby C, Symon FA, Haldar P, Shah S, Gupta S et al Elevated sputum interleukin‐5 and submucosal eosinophilia in obese individuals with severe asthma. Am J Respir Crit Care Med 2013; 188:657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peters MC, Fahy JV. Type 2 immune responses in obese individuals with asthma. Am J Respir Crit Care Med 2013; 188:633–4. [DOI] [PubMed] [Google Scholar]
- 12. Forno E, Acosta‐Perez E, Brehm JM, Han YY, Alvarez M, Colon‐Semidey A et al Obesity and adiposity indicators, asthma, and atopy in Puerto Rican children. J Allergy Clin Immunol 2014; 133:1308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hancox RJ, Milne BJ, Poulton R, Taylor DR, Greene JM, McLachlan CR et al Sex differences in the relation between body mass index and asthma and atopy in a birth cohort. Am J Respir Crit Care Med 2005; 171:440–5. [DOI] [PubMed] [Google Scholar]
- 14. Ma J, Xiao L, Knowles SB. Obesity, insulin resistance and the prevalence of atopy and asthma in US adults. Allergy 2010; 65:1455–63. [DOI] [PubMed] [Google Scholar]
- 15. Marijsse GS, Seys SF, Schelpe AS, Dilissen E, Goeminne P, Dupont LJ et al Obese individuals with asthma preferentially have a high IL‐5/IL‐17A/IL‐25 sputum inflammatory pattern. Am J Respir Crit Care Med 2014; 189:1284–5. [DOI] [PubMed] [Google Scholar]
- 16. Sutherland TJ, Cowan JO, Young S, Goulding A, Grant AM, Williamson A et al The association between obesity and asthma: interactions between systemic and airway inflammation. Am J Respir Crit Care Med 2008; 178:469–75. [DOI] [PubMed] [Google Scholar]
- 17. Quinto KB, Zuraw BL, Poon KY, Chen W, Schatz M, Christiansen SC. The association of obesity and asthma severity and control in children. J Allergy Clin Immunol 2011; 128:964–9. [DOI] [PubMed] [Google Scholar]
- 18. Dixon AE, Poynter ME. Mechanisms of asthma in obesity. Pleiotropic aspects of obesity produce distinct asthma phenotypes. Am J Respir Cell Mol Biol 2016; 54:601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rasmussen F, Hancox RJ. Mechanisms of obesity in asthma. Curr Opin Allergy Clin Immunol 2014; 14:35–43. [DOI] [PubMed] [Google Scholar]
- 20. Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest 1995; 96:2393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bates JH, Dixon AE. Potential role of the airway wall in the asthma of obesity. J Appl Physiol 1985; 2015:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Holguin F, Fitzpatrick A. Obesity, asthma, and oxidative stress. J Appl Physiol 1985; 2010:754–9. [DOI] [PubMed] [Google Scholar]
- 23. Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol 2005; 115:103–9. [DOI] [PubMed] [Google Scholar]
- 24. Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen‐induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol 2006; 118:389–95. [DOI] [PubMed] [Google Scholar]
- 25. Jartti T, Saarikoski L, Jartti L, Lisinen I, Jula A, Huupponen R et al Obesity, adipokines and asthma. Allergy 2009; 64:770–7. [DOI] [PubMed] [Google Scholar]
- 26. Sood A. Obesity, adipokines, and lung disease. J Appl Physiol 1985; 2010:744–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sutherland TJ, Sears MR, McLachlan CR, Poulton R, Hancox RJ. Leptin, adiponectin, and asthma: findings from a population‐based cohort study. Ann Allergy Asthma Immunol 2009; 103:101–7. [DOI] [PubMed] [Google Scholar]
- 28. Elias JA, Homer RJ, Hamid Q, Lee CG. Chitinases and chitinase‐like proteins in TH2 inflammation and asthma. J Allergy Clin Immunol 2005; 116:497–500. [DOI] [PubMed] [Google Scholar]
- 29. Ahangari F, Sood A, Ma B, Takyar S, Schuyler M, Qualls C et al Chitinase 3‐like‐1 regulates both visceral fat accumulation and asthma‐like Th2 inflammation. Am J Respir Crit Care Med 2015; 191:746–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Griffiths PS, Walton C, Samsell L, Perez MK, Piedimonte G. Maternal high‐fat hypercaloric diet during pregnancy results in persistent metabolic and respiratory abnormalities in offspring. Pediatr Res 2016; 79:278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leishangthem GD, Mabalirajan U, Singh VP, Agrawal A, Ghosh B, Dinda AK. Ultrastructural changes of airway in murine models of allergy and diet‐induced metabolic syndrome. ISRN Allergy 2013; 2013:261297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wood LG, Gibson PG. Dietary factors lead to innate immune activation in asthma. Pharmacol Ther 2009; 123:37–53. [DOI] [PubMed] [Google Scholar]
- 33. Sonnenburg JL, Backhed F. Diet‐microbiota interactions as moderators of human metabolism. Nature 2016; 535:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity‐associated gut microbiome with increased capacity for energy harvest. Nature 2006; 444:1027–31. [DOI] [PubMed] [Google Scholar]
- 35. Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom‐Bru C et al Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 2014; 20:159–66. [DOI] [PubMed] [Google Scholar]
- 36. Thorburn AN, McKenzie CI, Shen S, Stanley D, Macia L, Mason LJ et al Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun 2015; 6:7320. [DOI] [PubMed] [Google Scholar]
- 37. Mathews JA, Wurmbrand AP, Ribeiro L, Neto FL, Shore SA. Induction of IL‐17A precedes development of airway hyperresponsiveness during diet‐induced obesity and correlates with complement factor D. Front Immunol 2014; 5:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang YH, Wills‐Karp M. The potential role of interleukin‐17 in severe asthma. Curr Allergy Asthma Rep 2011; 11:388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Winer S, Paltser G, Chan Y, Tsui H, Engleman E, Winer D et al Obesity predisposes to Th17 bias. Eur J Immunol 2009; 39:2629–35. [DOI] [PubMed] [Google Scholar]
- 40. Calixto MC, Lintomen L, Schenka A, Saad MJ, Zanesco A, Antunes E. Obesity enhances eosinophilic inflammation in a murine model of allergic asthma. Br J Pharmacol 2010; 159:617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dietze J, Bocking C, Heverhagen JT, Voelker MN, Renz H. Obesity lowers the threshold of allergic sensitization and augments airway eosinophilia in a mouse model of asthma. Allergy 2012; 67:1519–29. [DOI] [PubMed] [Google Scholar]
- 42. Everaere L, Ait‐Yahia S, Molendi‐Coste O, Vorng H, Quemener S, LeVu P et al Innate lymphoid cells contribute to allergic airway disease exacerbation by obesity. J Allergy Clin Immunol 2016; 138:1309–18. [DOI] [PubMed] [Google Scholar]
- 43. de Vries A, Hazlewood L, Fitch PM, Seckl JR, Foster P, Howie SE. High‐fat feeding redirects cytokine responses and decreases allergic airway eosinophilia. Clin Exp Allergy 2009; 39:731–9. [DOI] [PubMed] [Google Scholar]
- 44. Ge XN, Greenberg Y, Hosseinkhani MR, Long EK, Bahaie NS, Rao A et al High‐fat diet promotes lung fibrosis and attenuates airway eosinophilia after exposure to cockroach allergen in mice. Exp Lung Res 2013; 39:365–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wood IS, Wang B, Trayhurn P. IL‐33, a recently identified interleukin‐1 gene family member, is expressed in human adipocytes. Biochem Biophys Res Commun 2009; 384:105–9. [DOI] [PubMed] [Google Scholar]
- 46. Hams E, Locksley RM, McKenzie AN, Fallon PG. Cutting edge: IL‐25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J Immunol 2013; 191:5349–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Prefontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J et al Increased IL‐33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol 2010; 125:752–4. [DOI] [PubMed] [Google Scholar]
- 48. Corrigan CJ, Wang W, Meng Q, Fang C, Eid G, Caballero MR et al Allergen‐induced expression of IL‐25 and IL‐25 receptor in atopic asthmatic airways and late‐phase cutaneous responses. J Allergy Clin Immunol 2011; 128:116–24. [DOI] [PubMed] [Google Scholar]
- 49. Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G et al Innate lymphoid cells – a proposal for uniform nomenclature. Nat Rev Immunol 2013; 13:145–9. [DOI] [PubMed] [Google Scholar]
- 50. Bando JK, Colonna M. Innate lymphoid cell function in the context of adaptive immunity. Nat Immunol 2016; 17:783–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science 2015; 348:aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol 2016; 17:765–74. [DOI] [PubMed] [Google Scholar]
- 53. Spits H, Bernink JH, Lanier L. NK cells and type 1 innate lymphoid cells: partners in host defense. Nat Immunol 2016; 17:758–64. [DOI] [PubMed] [Google Scholar]
- 54. Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD et al Intraepithelial type 1 innate lymphoid cells are a unique subset of IL‐12‐ and IL‐15‐responsive IFN‐γ‐producing cells. Immunity 2013; 38:769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tait Wojno ED, Artis D. Emerging concepts and future challenges in innate lymphoid cell biology. J Exp Med 2016; 213:2229–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Aliahmad P, de la Torre B, Kaye J. Shared dependence on the DNA‐binding factor TOX for the development of lymphoid tissue‐inducer cell and NK cell lineages. Nat Immunol 2010; 11:945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Klose CS, Flach M, Mohle L, Rogell L, Hoyler T, Ebert K et al Differentiation of type 1 ILCs from a common progenitor to all helper‐like innate lymphoid cell lineages. Cell 2014; 157:340–56. [DOI] [PubMed] [Google Scholar]
- 58. Seillet C, Rankin LC, Groom JR, Mielke LA, Tellier J, Chopin M et al Nfil3 is required for the development of all innate lymphoid cell subsets. J Exp Med 2014; 211:1733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Serafini N, Klein Wolterink RG, Satoh‐Takayama N, Xu W, Vosshenrich CA, Hendriks RW et al Gata3 drives development of RORγt+ group 3 innate lymphoid cells. J Exp Med 2014; 211:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang Q, Monticelli LA, Saenz SA, Chi AW, Sonnenberg GF, Tang J et al T cell factor 1 is required for group 2 innate lymphoid cell generation. Immunity 2013; 38:694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature 2014; 508:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lim AI, Li Y, Lopez‐Lastra S, Stadhouders R, Paul F, Casrouge A et al Systemic human ILC precursors provide a substrate for tissue ILC differentiation. Cell 2017; 168:e10. [DOI] [PubMed] [Google Scholar]
- 63. Bjorklund AK, Forkel M, Picelli S, Konya V, Theorell J, Friberg D et al The heterogeneity of human CD127+ innate lymphoid cells revealed by single‐cell RNA sequencing. Nat Immunol 2016; 17:451–60. [DOI] [PubMed] [Google Scholar]
- 64. Koues OI, Collins PL, Cella M, Robinette ML, Porter SI, Pyfrom SC et al Distinct gene regulatory pathways for human innate versus adaptive lymphoid cells. Cell 2016; 165:1134–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Robinette ML, Fuchs A, Cortez VS, Lee JS, Wang Y, Durum SK et al Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat Immunol 2015; 16:306–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bernink JH, Krabbendam L, Germar K, de Jong E, Gronke K, Kofoed‐Nielsen M et al Interleukin‐12 and ‐23 control plasticity of CD127+ group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity 2015; 43:146–60. [DOI] [PubMed] [Google Scholar]
- 67. Lim AI, Menegatti S, Bustamante J, Le Bourhis L, Allez M, Rogge L et al IL‐12 drives functional plasticity of human group 2 innate lymphoid cells. J Exp Med 2016; 213:569–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ohne Y, Silver JS, Thompson‐Snipes L, Collet MA, Blanck JP, Cantarel BL et al IL‐1 is a critical regulator of group 2 innate lymphoid cell function and plasticity. Nat Immunol 2016; 17:646–55. [DOI] [PubMed] [Google Scholar]
- 69. Silver JS, Kearley J, Copenhaver AM, Sanden C, Mori M, Yu L et al Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat Immunol 2016; 17:626–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bal SM, Bernink JH, Nagasawa M, Groot J, Shikhagaie MM, Golebski K et al IL‐1β, IL‐4 and IL‐12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat Immunol 2016; 17:636–45. [DOI] [PubMed] [Google Scholar]
- 71. Huang Y, Guo L, Qiu J, Chen X, Hu‐Li J, Siebenlist U et al IL‐25‐responsive, lineage‐negative KLRG1hi cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat Immunol 2015; 16:161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Duerr CU, McCarthy CD, Mindt BC, Rubio M, Meli AP, Pothlichet J et al Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat Immunol 2016; 17:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Moro K, Kabata H, Tanabe M, Koga S, Takeno N, Mochizuki M et al Interferon and IL‐27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nat Immunol 2016; 17:76–86. [DOI] [PubMed] [Google Scholar]
- 74. Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H et al Innate production of TH2 cytokines by adipose tissue‐associated c‐Kit+ Sca‐1+ lymphoid cells. Nature 2010; 463:540–4. [DOI] [PubMed] [Google Scholar]
- 75. Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH et al Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150:366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF et al Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 2015; 519:242–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A et al Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med 2013; 210:535–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB et al Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 2013; 502:245–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Miller AM, Asquith DL, Hueber AJ, Anderson LA, Holmes WM, McKenzie AN et al Interleukin‐33 induces protective effects in adipose tissue inflammation during obesity in mice. Circ Res 2010; 107:650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Van Gool F, Molofsky AB, Morar MM, Rosenzwajg M, Liang HE, Klatzmann D et al Interleukin‐5‐producing group 2 innate lymphoid cells control eosinophilia induced by interleukin‐2 therapy. Blood 2014; 124:3572–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC et al Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 2015; 160:74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V et al Adipose tissue invariant NKT cells protect against diet‐induced obesity and metabolic disorder through regulatory cytokine production. Immunity 2012; 37:574–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM et al Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014; 157:1292–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wu D, Molofsky AB, Liang HE, Ricardo‐Gonzalez RR, Jouihan HA, Bando JK et al Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 2011; 332:243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hu ZQ, Zhao WH. The IL‐33/ST2 axis is specifically required for development of adipose tissue‐resident regulatory T cells. Cell Mol Immunol 2015; 12:521–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lynch L, Michelet X, Zhang S, Brennan PJ, Moseman A, Lester C et al Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of T(reg) cells and macrophages in adipose tissue. Nat Immunol 2015; 16:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S et al The transcriptional regulators IRF4, BATF and IL‐33 orchestrate development and maintenance of adipose tissue‐resident regulatory T cells. Nat Immunol 2015; 16:276–85. [DOI] [PubMed] [Google Scholar]
- 88. Wensveen FM, Jelencic V, Valentic S, Sestan M, Wensveen TT, Theurich S et al NK cells link obesity‐induced adipose stress to inflammation and insulin resistance. Nat Immunol 2015; 16:376–85. [DOI] [PubMed] [Google Scholar]
- 89. Molofsky AB, Van Gool F, Liang HE, Van Dyken SJ, Nussbaum JC, Lee J et al Interleukin‐33 and interferon‐γ counter‐regulate group 2 innate lymphoid cell activation during immune perturbation. Immunity 2015; 43:161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. O'Sullivan TE, Rapp M, Fan X, Weizman OE, Bhardwaj P, Adams NM et al Adipose‐resident group 1 innate lymphoid cells promote obesity‐associated insulin resistance. Immunity 2016; 45:428–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK et al Nuocytes represent a new innate effector leukocyte that mediates type‐2 immunity. Nature 2010; 464:1367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ et al Systemically dispersed innate IL‐13‐expressing cells in type 2 immunity. Proc Natl Acad Sci USA 2010; 107:11489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP et al Innate IL‐13‐producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol 2012; 129:191–8. [DOI] [PubMed] [Google Scholar]
- 94. Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL‐33‐responsive lineage‐ CD25+ CD44hi lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol 2012; 188:1503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell‐type cytokines in protease allergen‐induced airway inflammation. Immunity 2012; 36:451–63. [DOI] [PubMed] [Google Scholar]
- 96. Klein Wolterink RG, Kleinjan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y et al Pulmonary innate lymphoid cells are major producers of IL‐5 and IL‐13 in murine models of allergic asthma. Eur J Immunol 2012; 42:1106–16. [DOI] [PubMed] [Google Scholar]
- 97. Christianson CA, Goplen NP, Zafar I, Irvin C, Good JT Jr, Rollins DR et al Persistence of asthma requires multiple feedback circuits involving type 2 innate lymphoid cells and IL‐33. J Allergy Clin Immunol 2015; 136:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kabata H, Moro K, Fukunaga K, Suzuki Y, Miyata J, Masaki K et al Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun 2013; 4:2675. [DOI] [PubMed] [Google Scholar]
- 99. Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol 2014; 134:671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Liu T, Wu J, Zhao J, Wang J, Zhang Y, Liu L et al Type 2 innate lymphoid cells: a novel biomarker of eosinophilic airway inflammation in patients with mild to moderate asthma. Respir Med 2015; 109:1391–6. [DOI] [PubMed] [Google Scholar]
- 101. Lombardi V, Beuraud C, Neukirch C, Moussu H, Morizur L, Horiot S et al Circulating innate lymphoid cells are differentially regulated in allergic and nonallergic subjects. J Allergy Clin Immunol 2016; 138:305–8. [DOI] [PubMed] [Google Scholar]
- 102. Smith SG, Chen R, Kjarsgaard M, Huang C, Oliveria JP, O'Byrne PM et al Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol 2016; 137:75–86. [DOI] [PubMed] [Google Scholar]
- 103. Nagakumar P, Denney L, Fleming L, Bush A, Lloyd CM, Saglani S. Type 2 innate lymphoid cells in induced sputum from children with severe asthma. J Allergy Clin Immunol 2016; 137:624–6. [DOI] [PubMed] [Google Scholar]
- 104. Halim TY, Steer CA, Matha L, Gold MJ, Martinez‐Gonzalez I, McNagny KM et al Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell‐mediated allergic lung inflammation. Immunity 2014; 40:425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mirchandani AS, Besnard AG, Yip E, Scott C, Bain CC, Cerovic V et al Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol 2014; 192:2442–8. [DOI] [PubMed] [Google Scholar]
- 106. Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM et al MHCII‐mediated dialog between group 2 innate lymphoid cells and CD4+ T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 2014; 41:283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gold MJ, Antignano F, Halim TY, Hirota JA, Blanchet MR, Zaph C et al Group 2 innate lymphoid cells facilitate sensitization to local, but not systemic, TH2‐inducing allergen exposures. J Allergy Clin Immunol 2014; 133:1142–8. [DOI] [PubMed] [Google Scholar]
- 108. Drake LY, Iijima K, Kita H. Group 2 innate lymphoid cells and CD4+ T cells cooperate to mediate type 2 immune response in mice. Allergy 2014; 69:1300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Halim TY, Hwang YY, Scanlon ST, Zaghouani H, Garbi N, Fallon PG et al Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat Immunol 2016; 17:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Motomura Y, Morita H, Moro K, Nakae S, Artis D, Endo TA et al Basophil‐derived interleukin‐4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity 2014; 40:758–71. [DOI] [PubMed] [Google Scholar]
- 111. Morita H, Arae K, Unno H, Miyauchi K, Toyama S, Nambu A et al An interleukin‐33‐mast cell‐interleukin‐2 axis suppresses papain‐induced allergic inflammation by promoting regulatory T cell numbers. Immunity 2015; 43:175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S et al Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med 2013; 5:174ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol 2013; 132:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Xue L, Salimi M, Panse I, Mjosberg JM, McKenzie AN, Spits H et al Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor‐homologous molecule expressed on TH2 cells. J Allergy Clin Immunol 2014; 133:1184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kim HY, Lee HJ, Chang YJ, Pichavant M, Shore SA, Fitzgerald KA et al Interleukin‐17‐producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity‐associated airway hyperreactivity. Nat Med 2014; 20:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mathews JA, Krishnamoorthy N, Kasahara DI, Cho Y, Wurmbrand AP, Ribeiro L et al IL‐33 drives augmented responses to ozone in obese mice. Environ Health Perspect 2017; 125:246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kim SH, Sutherland ER, Gelfand EW. Is there a link between obesity and asthma? Allergy Asthma Immunol Res 2014; 6:189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lloyd CM, Saglani S. Eosinophils in the spotlight: finding the link between obesity and asthma. Nat Med 2013; 19:976–7. [DOI] [PubMed] [Google Scholar]
- 119. Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity‐related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm 2013; 2013:139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Coccia M, Harrison OJ, Schiering C, Asquith MJ, Becher B, Powrie F et al IL‐1β mediates chronic intestinal inflammation by promoting the accumulation of IL‐17A secreting innate lymphoid cells and CD4+ Th17 cells. J Exp Med 2012; 209:1595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hepworth MR, Monticelli LA, Fung TC, Ziegler CG, Grunberg S, Sinha R et al Innate lymphoid cells regulate CD4+ T‐cell responses to intestinal commensal bacteria. Nature 2013; 498:113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. von Burg N, Chappaz S, Baerenwaldt A, Horvath E, Bose Dasgupta S, Ashok D et al Activated group 3 innate lymphoid cells promote T‐cell‐mediated immune responses. Proc Natl Acad Sci USA 2014; 111:12835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hepworth MR, Fung TC, Masur SH, Kelsen JR, McConnell FM, Dubrot J et al Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria‐specific CD4+ T cells. Science 2015; 348:1031–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tumanov AV, Koroleva EP, Guo X, Wang Y, Kruglov A, Nedospasov S et al Lymphotoxin controls the IL‐22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge. Cell Host Microbe 2011; 10:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lintomen L, Calixto MC, Schenka A, Antunes E. Allergen‐induced bone marrow eosinophilopoiesis and airways eosinophilic inflammation in leptin‐deficient ob/ob mice. Obesity (Silver Spring) 2012; 20:1959–65. [DOI] [PubMed] [Google Scholar]


