Abstract
The endothelium, a monolayer of endothelial cells lining vessel walls, maintains tissue-fluid homeostasis by restricting the passage of the plasma proteins and blood cells into the interstitium. The ion Ca2+, a ubiquitous secondary messenger, initiates signal transduction events in endothelial cells that is critical to control of vascular tone and endothelial permeability. The ion Ca2+ is stored inside the intracellular organelles and released into the cytosol in response to environmental cues. The inositol 1,4,5-trisphosphate (IP3) messenger facilitates Ca2+ release through IP3 receptors which are Ca2+-selective intracellular channels located within the membrane of the endoplasmic reticulum. Binding of IP3 to the IP3Rs initiates assembly of IP3R clusters, a key event responsible for amplification of Ca2+ signals in endothelial cells. This review discusses emerging concepts related to architecture and dynamics of IP3R clusters, and their specific role in propagation of Ca2+ signals in endothelial cells.
Keywords: Microtubule cytoskeleton, End-binding protein 3, Endothelial permeability, Signal transduction, Receptor dynamics
Introduction
In the late 19th century, Wilhelm His Sr. first introduced the term “endothelium” when he described differences between the layers of cells that line the mesoderm. After the inception of the idea of an endothelium as a specific tissue type or organ, the understanding of its exact location spanned many years of debate and technological advancements (reviewed in [1]). Notedly, with the discovery of its form, came the discoveries of its function. In the recent century, we have transitioned from asking, where the endothelium exists to determining the precise function of the endothelium and how this function is regulated in health and disease.
The endothelium is considered as an autocrine, paracrine, and endocrine organ capable of regulating a broad range of vascular functions including tissue-fluid homeostasis, vascular tone, thrombogenesis, inflammation, and vessel growth ([2–4]; reviewed in [5–7]). The large body of data related to the functions of endothelial cells has nowadays centered upon an important feature: vascular permeability. Considering that the inner vascular lining is formed by mature, quiescent endothelial cells, its primary function is not to proliferate or migrate but rather to act as a semi-permeable barrier between the vascular system and surrounding tissues.
Permeability of the endothelial barrier represents one of the hallmark processes for the maintenance of proper tissue-fluid homeostasis [6–8]. Once the endothelial barrier is compromised in response to humoral or mechanical stimuli, the barrier loses its selective control, causing excessive leakage of protein-rich fluids across the barrier. Vascular leakage is not only an integral part of many diseases—including systemic capillary leak syndrome [9], dengue fever [10] and Ebola viruses [11, 12], angioedema [13], anaphylaxis [14], acute lung injury [15, 16], variety of eye [17], and central nervous system [18, 19] disorders—but also the major dose-limiting factor in many immunotherapies [20]. The basis of such an event lies in the disruption of the inter-endothelial junctions, which are finely regulated, in part, by intracellular calcium signaling.
The ion Ca2+ is a secondary messenger paramount to a multitude of physiological and pathological processes in human body—muscle contraction, metabolic regulation, and fertilization to name a few. Within endothelial cells, the ion Ca2+ also plays a fundamental role in the majority of intracellular processes including, but not limited to, cell proliferation, motility, cell–cell adhesion, and cell death. While few recent reviews focus on the role of intracellular calcium in regulation of vascular tone through control of nitric oxide production [21–23], endothelial cell migration and proliferation—a hallmark of angiogenic response of mature endothelium [24, 25] and endothelial progenitor cells [26] to environmental cues, we focus here on the role of IP3R channels, an ion channel whose gating is important to the control of calcium homeostasis. Novel findings in IP3R architecture and dynamics give further insight into regulation of IP3-evoked calcium signaling and, thereby, regulation of endothelial permeability.
The role of intracellular Ca2+ signaling in regulating endothelial barrier function
Endothelial barrier function
Endothelial cells form an inner monolayer, termed the endothelium, lining the vessels of the blood and lymphatic systems, and the endocardium. The endothelium acts as a semi-permeable barrier that regulates passage of fluids, solutes, gases, nutrients, as well as transmigration of blood cells from circulation into surrounding tissues. The passage of macromolecules such as albumin across the endothelial barrier is essential to the maintenance of tissue-fluid homeostasis. Two main pathways, paracellular and transcellular, are involved in this process. The transcellular pathway, also known as transcytosis, uses receptor-mediated vesicular transport to actively distribute macromolecules across the endothelium. In contrast, the paracellular pathway utilizes interendothelial cell–cell junctions that connect adjacent endothelial cells and restrict permeability to macromolecules larger than 3 nm in diameter [27–29]. Hence, albumin, a molecule of 3.8 nm in diameter and 15 nm in length [30, 31], which represents ~60% of all plasma proteins [32, 33], is mainly retained in circulation. This higher concentration of albumin in the circulation compared to the interstitial space creates transendothelial oncotic pressure, a driving force of fluid reabsorption from the interstitium [34, 35]. Loss of interendothelial junctions in disease settings causes proteinaceous tissue edema, a pathological condition associated with leakage of protein-rich fluids in the interstitum [13, 15, 16].
Interendothelial junctions are comprised of adherens junctions (AJs), tight junctions (TJs), and gap junction (GJs) [36–39]. AJs, composed of Vascular Endothelial (VE)–cadherin adhesion complexes, are the primary cell–cell adhesions in the endothelium except for the brain and retinal blood barriers [40–42], also reviewed in [7]. Numerous signaling pathways—including those induced by free cytosolic calcium (Ca2+)—regulate the integrity of AJs [43–47]. The ion Ca2+ is a versatile and ubiquitous secondary messenger implicated in destabilization of endothelial barrier function [48–53]. It participates in signal transduction by binding to intracellular proteins, most of which contain the EF-hand motif [54, 55], and causing conformational changes in their tertiary structure [56, 57]. In most cases, these conformational changes result in activation of protein functions.
The role of calcium-dependent kinases
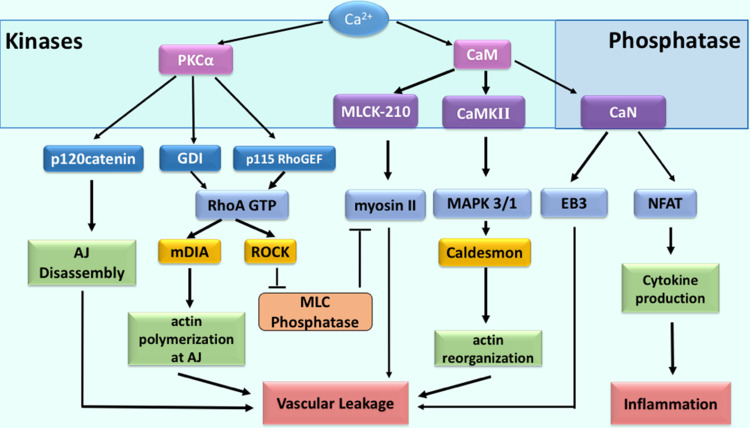
In endothelial cells, the ion Ca2+ orchestrates a set of signal transduction events through activation of calcium-dependent kinases and phosphatases (Fig. 1). These signaling molecules alter endothelial barrier function by multiple convergent mechanisms [6, 7]. The ion Ca2+ activates serine/threonine kinases including protein kinase Cα (PKCα), myosin light chain kinase (MLCK)-210 (endothelial-specific isoform), and Ca2+/calmodulin dependent protein kinase II (CaMKII). In turn, these kinases induce disassembly of AJs, thereby increasing permeability of endothelial barrier to plasma proteins [47, 58–64]. For example, PKCα phosphorylates p120-catenin [47, 65, 66], a protein of the VE–cadherin adhesion complex that binds to the juxtamembrane region of the VE–cadherin [67]. Phosphorylation of p120-catenin at the S879 residue decreases binding affinity of p120-catenin to VE–cadherin, resulting in dissociation of p120-catenin from the VE–cadherin adhesion complex [47]. This event enables binding of an adaptor protein (AP)-2 complex to VE–cadherin [68, 69] and subsequent initiation of internalization [47, 67] and proteolytic processing of VE–cadherin [70, 71].
Fig. 1.
Intracellular Ca2+ signaling in endothelial cells associated with vascular leakage and inflammation. Increase in cytosolic Ca2+ concentration activates specific kinases and phosphatases that, in turn, promote vascular leakage and inflammation. The serine/threonine kinase PKCα induces phosphorylation of p120-catenin and, through this mechanism, contributes to disassembly of AJs. PKCα also promotes reorganization of actin cytoskeleton and acto-myosin contractility by activating RhoA signaling. CaM-dependent activation of both MLCK-210 and CaMKII facilitates reorganization of actin cytoskeleton through the phosphorylation of actin motor myosin-II and caldesmon. In addition, CaM activates the phosphatase CaN, which dephosphorylates the microtubule accessory factor EB3, thereby coordinating reorganization of the actin and microtubule cytoskeleton. CaN also mediates activation of NFAT and promotes cytokine production by endothelium
PKCα is also responsible for the activation of RhoA, a small GTPase that reorganizes the actin cytoskeleton through activation of the downstream effectors, Rho-associated coiled-coil forming protein kinase (ROCK) and Diaphanous-related formin-1 (mDia1) [72–74]. PKCα induces activation of RhoA signaling by phosphorylating both a Guanine Nucleotide Dissociation Inhibitor (GDI) [44, 75] and a Guanine Nucleotide Exchange Factor (GEF) p115RhoGEF [76–78]. Phosphorylation enables p115RhoGEF-mediated GTP exchange by inhibiting GDI binding to RhoA [75, 76]. RhoA activates ROCK, which in turn phosphorylates myosin light chain phosphatase (MLCP) at Thr-695, Ser-894, and Thr-850 [79–81], inhibiting MLCP activity [79]. This process enables phosphorylation of myosin-II, an actin motor that assembles the acto-myosin contractile apparatus, at Ser19/Thr18 by MLCK-210 [82–84]. RhoA also mediates translocation of mDia1 to AJs, allowing polymerization of F-actin filaments [85]. mDia1-dependent assembly of actin filaments is reinforced by CaMKII-dependent activation of Mitogen-activated protein kinase 3/1 (MAPK3/1 or ERK1/2), which phosphorylates actin-binding protein caldesmon at Ser789 to prompt re-organization of the actin cytoskeleton [58, 60, 63].
Another mechanism of endothelial barrier destabilization involves proline-rich tyrosine kinase 2 (Pyk2) that is responsible for phosphorylation of Vascular Endothelial Tyrosine–Protein Phosphatase (VE–PTP), a constituent of AJs [86, 87]. VE–PTP, also known as PTPβ, provides a constitutive mechanism for VE–cadherin dephosphorylation at critical tyrosine residues Y658 and Y685 and, hence, prevents VE–cadherin internalization [88, 89]. Phosphorylation of VE–PTP at Y1981 residue causes a dissociation of VE–PTP from VE–cadherin, thereby increasing endothelial permeability [89]. In addition, Pyk-2 positively regulates Ca2+ entry in endothelial cells by inducing phosphorylation at Y361 of stromal interaction molecule 1 (STIM1), a calcium sensor localized in the endoplasmic reticulum [90–92]. Pyk-2-dependent phosphorylation of STIM1 is required for its interaction with calcium-release-activated calcium channel (Orai1) and Ca2+ entry from extracellular stores [92]. Importantly, phosphorylation of STIM1 at Y361 is required for the development of pulmonary edema in inflammatory lung [92]. Although studies in dermal microvascular endothelial cells isolated from neonatal foreskin challenge the role of Orai1 in agonist-induced barrier disruption [93], further understanding of Orai1 function with respect to intracellular signaling might explain tissue-specific role of Orai1 in microvascular endothelial cells.
The role of calcium-dependent phosphatases
The ion Ca2+ also enables the activation of calcineurin (CaN), a serine/threonine phosphatase [94, 95]. CaN dephosphorylates the microtubule end-binding protein 3 (EB3) at S162 allowing reorganization of the microtubule cytoskeleton [46]. It also dephosphorylates STIM1 and prolongs calcium entry from extracellular stores [96]. In addition, CaN rapidly dephosphorylates the transcription factor nuclear factor of activated T-cells (NFAT) protein to initiate NFAT nuclear import [94, 97, 98]. NFAT signaling in endothelial cells is linked to upregulation of transcripts associated with cytokine–cytokine receptor interaction [C-X-C motif chemokines (CXCL) 10 and 11] and the cell adhesion [vascular cell adhesion protein 1 (VCAM1)] pathways [52]. These factors facilitate entry of neutrophils into pulmonary circulation, augmenting vascular injury. Therefore, calcium signals also contribute to the inflammatory response by inducing expression of specific proteins in activated endothelium (Fig. 1).
Intracellular calcium-release channels in endothelium
Calcium homeostasis is finely regulated in endothelial cells through gating of selective and non-selective cation channels that enable cells to uptake, store, and release Ca2+ ions to manipulate their cytosolic concentration in a spatio-temporal manner [99]. The endoplasmic reticulum (ER) retains about 75% of total stored Ca2+ ions [100, 101], while other stores such as mitochondria, lysosomes, and endosomes hold about 25% [100, 102, 103], recently reviewed in [104]. Movement of Ca2+ ions in and out the cytosol is commonly regulated by channels and pumps [105–107], also reviewed in [108–111]. The channels, when open, enable rapid diffusion of the ion Ca2+ from intracellular or extracellular stores into the cytoplasm downhill concentration gradients. In contrast, the pumps move the ion Ca2+ in an energy-dependent manner against the concentration gradient (reviewed in [104, 112, 113]). The plasma membrane Ca2+ ATPase (PMCA) and the sodium calcium exchanger (NCX) both continuously remove the ion Ca2+ outside of the cells [114–116], whereas the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) moves the ion Ca2+ for storage into ER and the sarcoplasmic reticulum (SR) lumina [117]. These stores release the ion Ca2+ in response to extracellular stimuli to increase the ion Ca2+ concentration and induce calcium signaling in endothelial cells.
There two main types of Ca2+-selective intracellular channels, the inositol 1,4,5-trisphosphate receptors (IP3Rs) and the ryanodine receptors (RyRs). IP3Rs release Ca2+ ions from the ER, whereas RyRs release Ca2+ ions from the SR [118–126], as reviewed in [127, 128]. IP3Rs are ubiquitously expressed IP3-gated channels localized on the membrane of the ER [129]. These channels consist of four ~260 kDa subunits [130–133] that appear to be mostly mobile structures randomly organized into small clusters across the surface of the ER membrane [134–140]. Some evidence from patch clamp experiments using isolated nuclei suggests that IP3R clusters of about four channels are spontaneously induced by IP3 [141, 142]. Although the underlying mechanism of IP3-evoked cluster formation remains unknown, the current view is that binding of IP3 to the IP3Rs may trigger conformational changes within the receptor that favor assembly of the clusters. Interestingly, at basal cytosolic Ca2+ concentrations, clustered IP3Rs have a ~50% reduction in mean open time than lone IP3Rs, suggesting that lone receptors are more active than clustered ones. However, the presence of free Ca2+ ions in cytosol reverses this inhibition [141]. The cytosolic portion of IP3Rs contains a putative Ca2+-sensor region (residues 1933–2271), which may modulate properties of the channels. In the presence of Ca2+ ions, the clustered IP3Rs demonstrate a higher open probability while showing reduced close time as compared to lone receptors; in addition, the receptors within the clusters exhibit simultaneous openings and closing [141], indicative of cooperative behavior. Therefore, the current model proposes that both secondary messengers, IP3 and Ca2+ ion, bind to the IP3Rs and attribute a positive regulation of Ca2+ release. Whereas IP3 rapidly promotes assembly of IP3R clusters, Ca2+ ions allow recruitment of Ca2+ release events through cooperative gating behavior of the receptors within the cluster.
IP3 is generated by a class of membrane-associated enzymes, known as phospholipase C (PLCs), that hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) [143, 144]. These enzymes are classified into six distinct families: PLC-β, γ, δ, ε, ζ, and η [145]. Eight out of thirteen known PLC isoforms have been identified in freshly isolated murine endothelial cells [146] from mesenteric arteries (PLCβ1, β3, and β4; PLCγ1 and γ2; PLCδ1 and δ3; and PLCε), whereas two additional isoforms have been detected in pulmonary (PLCη1 and η2) and middle cerebral arteries (PLCδ4 and PLCη1), indicating tissue-specific diversity of the phosphoinositide signaling pathways across the arterial beds. Whereas a function of PLCδ, PLCε, and PLCη isoforms is mainly unknown in endothelial cells, our previous work indicates that PLCγ1 is a major isoform responsible for maintenance of basal level of IP3 in human lung microvascular endothelial cells [46]. This is consistent with the phenotype of PLCγ1 knock-out mice which die in utero due to loss of both erythroid and endothelial progenitor cells [147, 148]. In contrast, PLCγ2 is responsible for IP3 generation downstream of VE–cadherin adhesion disruption, suggesting a potential role of PLCγ2 in endothelial cell proliferation and migration downstream of inflammatory and pro-angiogenic stimuli [46]. PLCβ isoforms act downstream of G-protein-coupled receptors (GPCRs) coupled to the G-proteins Gq and G11 [149–152] or Vascular Endothelial Growth Factor (VEGF) Receptor 2 signaling [153].
Given the fact that sustained elevation of cytosolic Ca2+ is toxic to cells, several negative feedback mechanisms regulating the channel activity have evolved [154, 155]. As the intracellular ion Ca2+ concentration exceeds a threshold value of 0.92 mM, release of the Ca2+ ions is inhibited due to the Ca2+ binding to the inhibitory sites of IP3Rs [156–159]. To date, seven Ca2+-binding sites have been discovered in the cytosolic portion of IP3R1 [160–163], yet their significance in IP3R gating remains unclear. Mutagenesis analysis of residues overlapping these Ca2+-binding sites has no significant effect on IP3R gating [164]. Another model proposes that luminal concentration of Ca2+ is directly linked to sensitivity of IP3Rs to the Ca2+ ions [165]. As the luminal concentration of Ca2+ falls, IP3R loses its sensitivity to IP3 even in the presence of cytosolic IP3 and Ca2+. This model, however, needs to be supported with further experiments.
It has also been proposed that several proteins including Ca2+-binding proteins containing the tetra EF-hand, calmodulin (CaM), and the calmodulin (CaM)-like neuronal Ca2+-binding proteins (CaBPs), attribute to a negative regulation of the IP3Rs [166–170], recently reviewed in [171]. These proteins bind IP3R1 within the first 128 amino acid sequence [170, 172] and inhibit binding of IP3 to the receptor at 0.15 mM free Ca2+ ions in cytosol leading to blocked Ca2+ release [166, 169]. Hence, cytosolic Ca2+ ions attribute to both positive and negative feedback regulations of Ca2+ release through IP3Rs.
Another type of Ca2+-selective intracellular channel, the ryanodine receptors (RyRs) form Ca2+ channels located on the membrane of the SR. The tetrameric RyR channel consists of subunits ~5000 residues in size [173–176]. There exist three isoforms of RyRs in mammals with RyR1 and RyR2 being preferentially expressed in skeletal muscles and cardiomyocytes [174, 177, 178]. Endothelial cells predominantly express RyR3 isoform [179, 180]. RyRs activity is regulated by ryanodine, a plant alkaloid from Ryania speciosa [181, 182], which induces channel pore opening at nanomolar concentrations and promotes pore closing at micromolar concentrations [154, 183–185]. Furthermore, Ca2+ ions and ryanodine demonstrate cooperative behavior [186, 187]. An increase in free cytosolic Ca2+ facilitates stronger binding of ryanodine to the RyRs, as shown in endothelial cells [188]. In this respect, RyRs and IP3Rs share analogous regulation of Ca2+ release by cytosolic Ca2+. Since our review is mainly focused on the function of IP3Rs in endothelial cells, we refer to recent publications for additional information on structure and function of RyRs [189–191].
Recent studies have also revealed the presence of intracellular two-pore channels (TPCs) in endothelial cells that belong to a family of the voltage-gated ion channels [192–194]. The family is presented by three distantly related proteins (TPC1–3) with TPC2 specifically localized to the lysosomal and TPC1 to the endolysosomal systems [192, 195]. TPC3 is also targeted to acidic organelles as well as the plasma membrane [196, 197], but is lost in human and rodent species [198]. The crystal structure of a two-pore channel from Arabidopsis thaliana suggests that Ca2+ and membrane potential activate two distinct six transmembrane (6-TM) domains [199], indicating potential mechanism for Ca2+ and voltage sensing. It also proposes that luminal Ca2+ might stabilize the second voltage-sensing domain in the resting state and, thereby, shift voltage activation towards more positive potentials.
In endothelial cells, TPCs are mainly involved in regulation of cell proliferation [193, 194]. TPC2, which promotes Ca2+ release from lysosomal stores, potentiates pro-angiogenic effect of VEGF on endothelial cells, suggesting a specific role of TPC2 in VEGFR2-mediated angiogenesis [193]. In contrast, TPC1 is involved in intracellular calcium release from endolysosomal store in response to arachidonic acid and mediates specific calcium signaling associated with proliferation of circulating endothelial colony forming cells [194].
Function of IP3R channels in endothelium
IP3R isoforms
Through cDNA cloning on mammalian cells, three isoforms of IP3Rs (IP3R1, IP3R2, and IP3R3) have been discovered and characterized in various cell culture models [118, 135, 200, 201]. Even though all three isoforms demonstrate about 65–85% sequence similarity, they each produce a different magnitude of Ca2+ release due to their distinct affinities for IP3 [131, 202–205]. The in vitro studies have shown that IP3R2 is the most sensitive to IP3 [135, 206] and produces long-lasting and regular Ca2+ oscillations [203, 207, 208]. IP3R3, the least sensitive for this secondary messenger, instigates monophasic Ca2+ transients [203, 206, 209]. IP3R1 demonstrates intermediate affinity for IP3 and generates Ca2+ oscillations, which appear more irregular when compared with IP3R2-mediated oscillations [206, 207, 209]. These differences in binding affinities for IP3 among receptor types can be further modulated through the interaction of the N-terminus ligand-binding domain (LBD; see “Organization and structure of IP3Rs”) with adenosine triphosphate (ATP), the regulatory proteins—such as CaM, CaMBP, CaN, and STIM 1—and homer protein homolog (HOMER) proteins, as well as through the receptor phosphorylation ([166–170, 209–218], recently reviewed in [128]).
Expression of endothelial IP3R isoforms varies among the different vascular systems [53, 219–221]. Whereas all three isoforms are expressed in the endothelium of the aorta and arteries of systemic circulation in rats and domestic cattle [220, 222–226], IP3R1 is not present in the pulmonary endothelium of either human or mouse [53]. Interestingly, some striking difference in the expression pattern of IP3Rs is also noted among species. This difference might be attributed to specialized function of the receptor isoform in human since in IP3R1 is predominant in endothelial cells of human brain microvasculature and mesenteric artery [219, 220], while IP3R3 and IP3R2 are preferentially expressed in pulmonary microvascular, and aorta endothelial cells [53, 221] as well as in circulating endothelial colony forming cells [227].
The role of IP3R1 in regulating myogenic vascular tone
Interestingly enough, endothelial cell-specific knockout of IP3R1 causes high blood pressure in mice due to disruption of NFAT/endothelial nitric oxide synthase (eNOS) signaling [228]. Consistent with this finding, other studies demonstrate that expression of IP3R1 in aortic endothelial cell is downregulated in spontaneously hypertensive rats [222]. Both IP3R1s and IP3R2s are localized at myo-endothelial projections between endothelial and smooth muscle cells and selectively regulates inter-cellular communications. They are also involved in the control of vascular tone by activating intermediate conductance of calcium-activated potassium channels, thereby hyperpolarizing adjacent smooth muscle cells [224, 229, 230]. It is well known that fluid shear stress induces intracellular Ca2+ oscillations through activation of heterotrimeric Gq/G11 proteins, which is coupled to purinergic receptor [231, 232] or bound to the plasma membrane in endothelial cells [233, 234]. Deficiency in IP3R1 seems to disrupt these adaptive responses to increased blood flow in arteries of systemic circulation and to cause hypertension [228, 235, 236]. Cumulatively, these findings suggest that IP3R1-dependent Ca2+ handling might play a critical role in regulating myogenic vascular tone in arteries. It is plausible that IP3R1 is involved in physiological adaptive responses to hemodynamic changes in endothelium of systemic circulation, whereas it is dispensable in the low-pressure system such as pulmonary circulation.
The role of IP3R2 and IP3R3 in regulating endothelial permeability in lung
The other IP3R isoforms, IP3R2 and IP3R3, expressed in endothelial cells have implications in both increased endothelial permeability and cytokine production during inflammation in the lung [52, 53]. Pro-inflammatory mediators such as serine protease α-thrombin and histamine induce Ca2+ ion release from ER stores. These mediators bind to and activate respective G-protein-coupled receptors (GPCRs), protease-activated receptor 1 (PAR-1) [237, 238], or histamine receptor 1 (H1) [239, 240], at the surface of endothelial cells (Fig. 2).
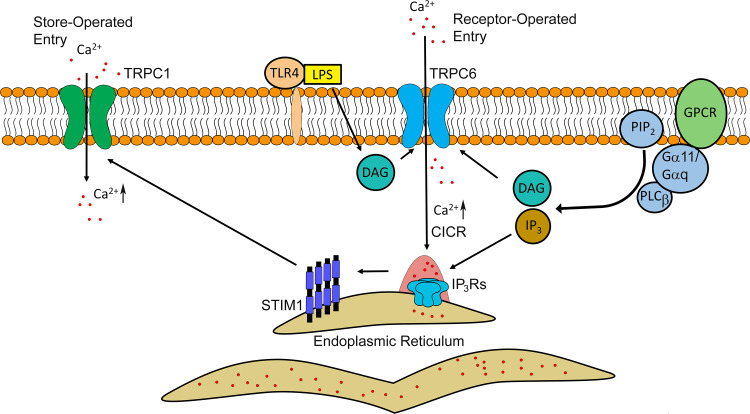
Fig. 2.
Model of receptor-mediated signaling in activating Ca2+ release and entry in endothelial cells during inflammation. The signal transduction begins with the binding of the ligand (hormone, cytokine, etc.) to specific G-protein coupled receptor (GPCR) at the surface of endothelial cells. Initial Gα11/Gαq-coupled activation of phospholipase C (PLC) β directs cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) into the two secondary messengers, diacylglycerol (DAG) and inositol trisphosphate (IP3). IP3 induces clustering and activation of IP3Rs on the ER membrane, whereas DAG instigates Ca2+ entry through transient receptor potential cation channels member 6 (TRPC6) located at the plasma membrane. The latter might contribute to increases in cytosolic Ca2+ concentration igniting global Ca2+ release through IP3Rs. Depletion of the ER Ca2+ stores triggers oligomerization of stromal interaction molecule 1 (STIM1) which is required for activation of TRPC1 channel and Ca2+ entry from extracellular space. Lipopolysaccharide (LPS) also can activate Ca2+ entry through the TRPC6 channel by binding to toll-like receptor 4 (TLR4)
IP3 binds to IP3Rs and initiates Ca2+ release from ER stores [120, 241, 242]. Depletion of intracellular stores, in turn, activates a set of intracellular events that mediate capacitive or store-operated Ca2+ entry (CCE or SOCE) and, thus, replenish Ca2+ in ER (Fig. 2) [243, 244]. The level of Ca2+ ions within ER is sensed by transmembrane proteins localized on the ER membrane, STIM1 [90, 245, 246]. STIM1 binds to the Ca2+ ions through EF-hand motif at the N-terminus, a portion of the protein located in the ER lumen [247, 248]. Depletion of Ca2+ inside the ER leads to oligomerization as well as interaction of STIM1 with both the calcium-release-activated calcium channel proteins (Orai) and transient receptor potential channel 1 (TRPC1) located at the plasma membrane of endothelial cells [249–255].
As mentioned above, distinct stimuli can engage specific IP3R isoforms in activated endothelium. IP3R3 triggers monophasic Ca2+ transients in response to pro-inflammatory mediators such as α-thrombin, and promotes increased permeability of endothelial barrier [53]. IP3R2 induces Ca2+ oscillations in response to endotoxin lipopolysaccharide (LPS), which is found in the outer membrane of Gram-negative bacteria [52]. This isoform is responsible for sustained activation of NFAT-mediated transcripts encoding the proteins associated with inflammation [52, 256]. In addition, DAG promotes Ca2+ influx through receptor-operated Ca2+ channels (ROC), the pathway unique from SOCE (Fig. 2) [257–260]. DAG interacts with TRPC6 at the plasma membrane [259] and permits Ca2+ entry through this non-selective cation channel in endothelial cells [49, 51, 261]. DAG can also contribute to inflammatory responses in endothelial cells through direct activation of protein kinase C (PKC) [44, 47, 59, 262–264] or generation of lipid secondary messengers such as leukotrienes [265–267]. Thus, calcium signaling emanated at the level of ER channels is amplified by Ca2+ flux from extracellular stores through SOCE and ROCE mechanisms.
Organization and structure of IP3Rs
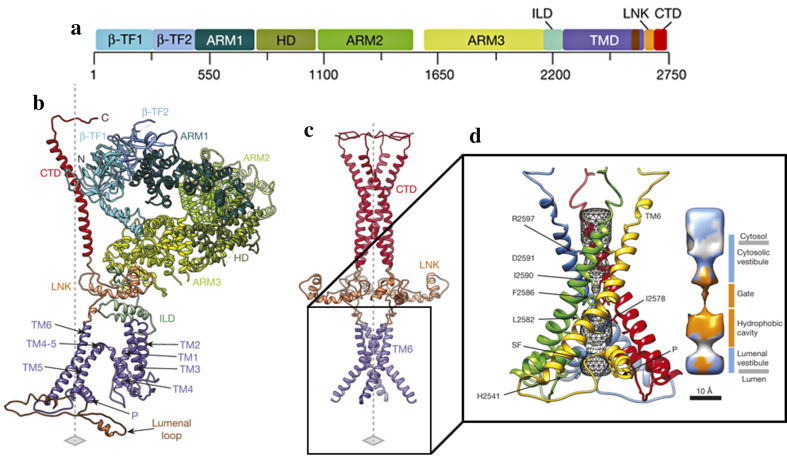
A potential mechanism of channel gating has been suggested based on the recently solved quaternary structure of the IP3R1 channel in non-conducting state [133]. The IP3R1 tetrameric structure consists of four subunits organized around a central axis [133]. Each subunit consists of two β-trefoil domains (β-TF1 and β-TF2), three armadillo solenoid folds (ARM1–ARM3), α-helical domain (HD), intervening lateral domain (ILD), six α-helices (TM1–TM6 or TMD), C-terminal domain (CTD), and a helical linker domain (LNK), which connects the transmembrane and the cytosolic bundles of each subunit (Fig. 3a, b).
Fig. 3.
Domain structure of IP3R1. a Schematic representation of IP3R1 domains with the corresponding amino acids. b Organization of individual IP3R1 molecule within tetrameric channel; different domains are indicated by color as in a. c Structure of the central core of IP3R1 channel (tetramer); axis is indicated by the dashed line. d Bundle of TM6 helices forming the conduction pathway of the IP3R1 channel; four individual TM6 domains are highlighted by color. The P-helices (P) lining the luminal vestibule and selectivity filter (SF) loops are indicated. The pore is formed by chain of hydrophobic residues as indicated by color on the right. β-TF1 and β-TF2 β-trefoil domains, ARM1–ARM3 armadillo solenoid folds, HD α-helical domain, ILD intervening lateral domain, TM1–TM6 or TMD α-helices, CTD C-terminal domain, LNK a helical linker domain.
Modified from Fan et al. [133] with permission of the publisher. Copyright ©2015, Nature Publishing Group
Organization of the permeation pathway
The proposed model suggests that the TMD region is essential for Ca2+ ion conduction [133]. The Ca2+ permeation path is lined by four TM6 α-helices, which are oriented at 37o with respect to the ER membrane (Fig. 3c). A luminal loop between TMD5 and TMD6 α-helices assembles a luminal vestibule. This loop contains both a short P-helix and a selectivity filter comprised of highly conserved residues [133, 268]. An important feature of a luminal vestibule is the presence of a positively charged ring, which is formed by the His2541 residues located within the P-helix of a tetrameric channel (Fig. 3c). This ring is predicted to repel the positively charged Ca2+ ions in the non-conducting channel. In conductive state, the P-helices are anticipated to undergo a structural rearrangement allowing passage of Ca2+ ions beyond a luminal vestibule [133]. The gateway for ion permeation is located at the constriction spot between four of the TM6 helices that each contains several hydrophobic residues oriented towards the permeation pathway (Fig. 3c). In the non-conducting channel, these hydrophobic residues form a pore of 5 Å in diameter that restricts passage of the hydrated Ca2+ ion of 8–10 Å in diameter [133]. In contrast to a luminal vestibule, a cytosolic vestibule formed above the pore is comprised of negatively charged residues, which are anticipated to promote a translocation of the Ca2+ ions into the cytosol [133].
Function of the cytosolic region
The cytosolic part of IP3R1 contains two β-trefoil domains, three ARMs, and an α-helical domain (Fig. 3a, b). The modular organization of armadillo folds predicts their role in assembling different interfaces for binding of IP3, Ca2+ ion, and the regulatory proteins [171]. The β-TF1 domain at the N-terminus (residues 5–225) constitutes a suppressor domain, whereas β-TF2 and two α-helices armadillo repeats of ARM1 assemble the IP3-binding core (IBC) region. These three domains form a triangular structure, also known as ligand-binding domain (LBD) [126, 269, 270], above the CTD bundle. The α-helical, ARM2, and ARM3 domains connect the LBD to the channel-forming region. Even though the exact mechanism underlying IP3-evoked conformational changes within the pore is not fully understood, it is proposed that gating of IP3Rs occurs through a long-range communication between the LBD region and the pore [133].
The current model suggests that the binding of IP3 to the IBC causes a closure of IP3-binding pocket and, sequentially, a twist-like movement of the suppressor domain [133]. The model indicates that the interactions between the suppressor and ARM3 domains carry the signal from the LBD to the pore. Interestingly, the suppressor domain also establishes interaction with β-TF2 and ARM2 domains of adjacent subunit, suggesting that movement of the suppressor domain might transmit the signals to adjacent subunits. Consistent with this model, deletion of the suppressor domain or mutation of the Y167, site involved in the interaction with β-TF2, prevents IP3-evoked gating of the channel [271, 272], indicating an importance of the suppressor domain in the transmission of long-range allosteric changes.
It has also been proposed that CTD domain plays an important role in gating of the IP3R channel. The studies using IP3R mutant lacking the 43 residues from the CTD domain have demonstrated that it is required for channel gating [273]. The CTD domain is comprised of the long helical bundle spanning the cytosolic portion of the receptor [133]. Both biochemical and structural studies indicate that the N-terminal and C-terminal parts of the receptor are closely associated with respect to each other [274]. The quaternary structure of the receptor reveals the presence of the electrostatic interactions between the CTD and β-TF2 domains from the adjacent subunits. The CTD also interacts with LNK, the bridge between the cytosolic and transmembrane parts of the receptor, and possibly transmits the signal to the TMD6 [133].
Therefore, ligand-evoked gating of the channel relies on both extensive inter- and intra-subunit interactions within the receptor comprised of four subunits. The first near-atomic resolution structure of the IP3R1 [133] reveals conservation of the ion-conduction pore among tetrameric channels [275]. The specific features of the IP3R channels are carried through additional domains attached to the pore. A unique architecture in the CTD domain suggests a distinctive mechanism of IP3R gating that requires direct coupling between the CTD and LBD domains of adjacent subunits [133].
IP3-evoked Ca2+ release events
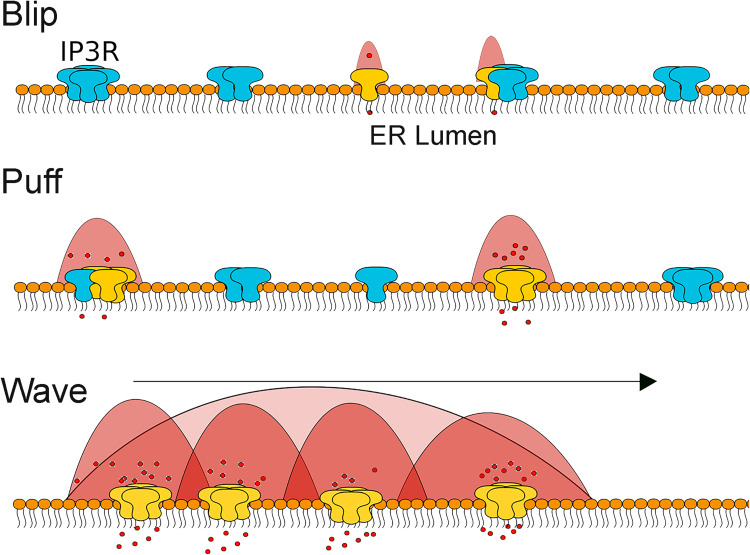
The fundamental question is “How does the ion Ca2+ elicit an array of signaling events alternating from proliferation, survival, and death?” Because diffusion of the ion Ca2+ is altered due to binding to numerous proteins and lipids in the cytosol, in many instances, it is only effective in initiating signal transduction events locally, in the proximity of the ion Ca2+ release or entry. The ion Ca2+ is released from intracellular stores through IP3Rs in three major events called blips, puffs, and waves (Fig. 4). A blip is the elementary release event, a result of a passive diffusion of the Ca2+ ions from the ER through the lone channel (Fig. 4) [276]. This allows movement of the ion Ca2+ along the gradient as the result of a stochastic and autonomous process occurring at intermittent IP3R sites across the ER [276, 277]. Blips typically last for 10 ms and are responsible for basal cytosolic Ca2+ concentration of about 40–80 nM in resting endothelial cells [53].
Fig. 4.
Elementary Ca2+ release events evoked by IP3. Ca2+ release occurs in three major events: blip, puff, and wave. Blips represent release of Ca2+ through a single IP3R channel, which can be lone or part of the cluster. Puffs are characterized by coordinated opening of multiple channels organized into the cluster. Regenerative Ca2+ waves are attributed to activation of IP3R across the entire cell. Both IP3 and Ca2+ ignite Ca2+ release form neighboring IP3R clusters
A greater release of Ca2+ ions leads to puffs, a result of simultaneous openings of 5–6 channels grouped into the clusters lasting for about 100 ms [278–283]. As opposed to blip events, puffs originate within the clusters, suggesting that engagement of lone IP3R channels into multichannel assembly plays a critical role in transition from blip to puff (Fig. 4) [284, 285]. The clusters igniting puffs are ~50 nm wide and distributed at ~3 μm from each other [286]. Multiple studies have experimentally established a direct correlation between frequency of puffs ignition and the number of IP3Rs present within the cluster [276, 285, 287, 288]. This relationship suggests that larger IP3R clusters have a disproportional influence on intracellular Ca2+ signaling and may act as an important ignition switch for regenerative Ca2+ waves (Fig. 4). However, it should be considered that blips and puffs do not solely serve as components of a global Ca2+ release; rather, they are signaling events in themselves.
As IP3 concentration increases, puffs can trigger regenerative Ca2+ waves that spread across cell [286, 289] due to coordinated opening of a numerous channels of IP3Rs within the ER (Fig. 4). It has been proposed that Ca2+ ions trigger higher frequency puffs through activation of neighboring channels. The current model suggests that puff frequency might set up a threshold for regenerative Ca2+ waves, where greater oscillation frequency attributes to higher excitability of the nearest IP3Rs and thus the transition from local (puffs) to global (wave) calcium-release events [290–294]. In this respect, organization and distribution of IP3R clusters become critical features. Below, we discuss the mechanisms regulating the receptor clustering in light of our recent work [53].
Regulation of IP3R clustering in endothelial cells
Dynamics of IP3R clusters in endothelial cells
Regenerative ion Ca2+ release relies on recruitment of neighboring IP3Rs via a process known as Ca2+-induced Ca2+ release (CICR). Therefore, propagation of Ca2+ signals is critically dependent on organization of the IP3Rs as well as optimal distribution of the receptors within the ER membranes. IP3Rs assemble the clusters in unstimulated pulmonary endothelial cells [53] as well as other cell types [134, 135, 139]. IP3R3s form less abundant clusters than IP3R2s in endothelial monolayers, which can be explained by their different binding affinities to IP3. This suggests that local spikes in basal IP3 production that are mediated by PLCγ1 [46, 295] are sufficient to promote clustering of both IP3Rs in unstimulated endothelial cells [53].
Consistent with IP3R distribution in other cell types [134, 135, 138, 139], GFP-IP3R3 form highly mobile structures in resting endothelial monolayers [53]. The IP3R3 clusters appear to be very infrequent structures within the ER membrane that undergo spontaneous assembly and disassembly. Furthermore, consistent with hypothesis of “hot spots” for calcium release [277, 296, 297], some clusters demonstrate sporadic assembly and disassembly at the same location, perhaps in response to changes in local concentrations of IP3. Furthermore, stimulation of the endothelial cells with α-thrombin promptly induces de novo formation of IP3R3 clusters, which demonstrates exponential growth in size [53]. Our recent work identified the microtubule-associated protein EB3 as a key regulator of formation of IP3R3 clusters in endothelial cells [53].
The role of microtubule cytoskeleton in IP3R clustering
The role of the microtubule cytoskeleton in organization of IP3-evoked Ca2+ signals has been established in various cell types [53, 225, 298–303]. IP3Rs are shown to establish the transient associations with microtubules [53, 304], which in turn, actively participate in the redistribution of IP3Rs within the ER membrane [300, 301, 305]. The microtubule cytoskeleton also contributes to initiation and propagation of Ca2+ waves in endothelial cells [225, 299] that may occur through spatial organization and temporal dynamics of IP3R3s within ER membranes [53, 306, 307]. Furthermore, few reports indicate that the microtubule cytoskeleton facilitates delivery of IP3 to IP3Rs [308, 309], possibly contributing to initiation of Ca2+ spikes in secretory cells [310]. More recent studies in endothelial cells indicate that microtubules establish transient interactions with IP3R3 localized at the ER membrane and play a critical role in assembling agonist-evoked IP3R3 clusters [53].
While it is possible that the microtubule cytoskeleton promotes calcium signaling through multiple independent mechanisms, it has become apparent that it contributes to spatio-temporal organization of calcium release from ER stores. Microtubule dynamic is critical to this function because accumulation of specific proteins at the growing microtubule plus ends might provide the mechanism for tethering or stabilizing IP3R clusters at ER membrane.
EB3 is a major regulator of IP3R clusters in stimulated endothelial cells
The EB family consists of three members: EB1, EB2, and EB3 [311]. The EB proteins have demonstrated a high affinity for the outer part of growing microtubules, so-called the ‘+tips’ [312–314]. EBs are involved in establishing a protein network at the ‘+tips’ through recruitment of other proteins. Specifically, EB proteins interact with the CAP-Gly domain proteins (such as cytoplasmic linker and p150-Glued proteins) and proteins containing the short EB-binding motif S/TxIP [315–320].
IP3Rs contain an EB-binding motif within a short unstructured region of the α-helical domain (HD), which connects the LBD to the channel-forming region [133]. The S/TxIP-containing motif conserved among mammalian IP3Rs enables the interaction of IP3Rs with EB3 and, albeit, a much weaker interaction with EB1 [53]. Intriguingly, EB3, but not EB1, is required for α-thrombin-evoked clustering and gating of IP3R3 in endothelial cells. Depletion of EB3 or disruption of the IP3R3 binding interface through a single-point mutation (T804 to A) within EB-binding motif of IP3R3 reduces the number of the IP3R3 (T804A) clusters in response to α-thrombin challenge [53]. The formed clusters are short-lived [53], suggesting that tethering of IP3R3s to microtubule +tips by EB3 might play an essential role in stabilization of IP3R3 intramolecular associations within the cluster. Disruption of this interaction alters organization of IP3R3 clusters and thereby inhibits propagation of calcium signals in endothelial cells.
Our proposed model also suggests that formation of IP3R3 clusters is essential for recruitment of neighboring IP3Rs via Ca2+-induced Ca2+ release since IP3-evoked Ca2+ signals are massively attenuated in EB3-deficient cells. The importance of EB3 in calcium signaling is clear from analyses of isolated lungs, where knockout of EB3 in endothelial cells protects lungs from increased vascular leakage and pulmonary edema [53]. Thus, the microtubule cytoskeleton provides a critical mechanism for assembling stable IP3R clusters and effective Ca2+ signals in endothelium in the context of inflammation. Targeting this interaction with specific inhibitors might provide an attractive strategy for treatment of vascular leakage and pulmonary edema.
Conclusion remarks and future directions
Intracellular Ca2+ signaling is one of the major mechanisms involved in the communication between endothelial cells and the environment. Changes in mechanical (pressure and shear stress) and chemical (growth factors, hormones, and cytokines) stimuli are processed by endothelial cells through receptor-mediated signaling. The ion Ca2+ is a second messenger that is released from ER stores and ignites a myriad of signal transduction pathways upon the activation of endothelial cell surface receptors. The hierarchy of IP3-evoked Ca2+ release events is predicted to differentially regulate the cellular responses. While elementary Ca2+ signals in the form of blips and puffs might be critical to regulation of vascular tone, globalization of Ca2+ signals through regenerative Ca2+ waves might contribute to vascular inflammation and leakage. Understanding a spatial–temporal organization of Ca2+ release in endothelial cells of intact blood vessels remains a major challenge in the field of endothelial biology.
Dynamic reorganization of IP3Rs within ER membrane is crucial in igniting neighboring channels and transitioning from local to global calcium-release events. The microtubule cytoskeleton contributes to assembly of stable IP3R cluster and sets up a positive-feedback loop for the amplification of Ca2+ signals associated with the progression of several pathologies including vascular leakage and inflammation. Increasing our understanding of IP3R regulation at the molecular level will lay a ground work for the design of therapeutic interventions that target specific function of the receptor associated with pathology while leaving intact physiologically important function.
Compliance with ethical standards
Financial support for the author(s)
Supported by NIH Grants R01 HL103922 to Y.A.K; AHA AWARD 13PRE17090090 to M.G.
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Laubichler MD, Aird WC, Maienschein J (2007) The endothelium in history. In: Endothelial biomedicine. Cambridge University Press, Cambridge, pp 3–20
- 2.Busse R, Trogisch G, Bassenge E. The role of endothelium in the control of vascular tone. Basic Res Cardiol. 1985;80:475–490. doi: 10.1007/BF01907912. [DOI] [PubMed] [Google Scholar]
- 3.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 4.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 5.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 6.Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol. 2010;72:463–493. doi: 10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- 7.Komarova YA, Kruse K, Mehta D, Malik AB. Protein interactions at endothelial junctions and signaling mechanisms regulating endothelial permeability. Circ Res. 2017;120:179–206. doi: 10.1161/CIRCRESAHA.116.306534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 9.Xie Z, Ghosh CC, Patel R, Iwaki S, Gaskins D, Nelson C, Jones N, Greipp PR, Parikh SM, Druey KM. Vascular endothelial hyperpermeability induces the clinical symptoms of Clarkson disease (the systemic capillary leak syndrome) Blood. 2012;119:4321–4332. doi: 10.1182/blood-2011-08-375816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avirutnan P, Punyadee N, Noisakran S, Komoltri C, Thiemmeca S, Auethavornanan K, Jairungsri A, Kanlaya R, Tangthawornchaikul N, Puttikhunt C, Pattanakitsakul SN, Yenchitsomanus PT, Mongkolsapaya J, Kasinrerk W, et al. Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J Infect Dis. 2006;193:1078–1088. doi: 10.1086/500949. [DOI] [PubMed] [Google Scholar]
- 11.Wahl-Jensen VM, Afanasieva TA, Seebach J, Stroher U, Feldmann H, Schnittler HJ. Effects of Ebola virus glycoproteins on endothelial cell activation and barrier function. J Virol. 2005;79:10442–10450. doi: 10.1128/JVI.79.16.10442-10450.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang ZY, Duckers HJ, Sullivan NJ, Sanchez A, Nabel EG, Nabel GJ. Identification of the Ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury. Nat Med. 2000;6:886–889. doi: 10.1038/78645. [DOI] [PubMed] [Google Scholar]
- 13.Bouillet L, Mannic T, Arboleas M, Subileau M, Massot C, Drouet C, Huber P, Vilgrain I. Hereditary angioedema: key role for kallikrein and bradykinin in vascular endothelial-cadherin cleavage and edema formation. J Allergy Clin Immunol. 2011;128:232–234. doi: 10.1016/j.jaci.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan AP. Clinical practice. Chronic urticaria and angioedema. N Engl J Med. 2002;346:175–179. doi: 10.1056/NEJMcp011186. [DOI] [PubMed] [Google Scholar]
- 15.Herwig MC, Tsokos M, Hermanns MI, Kirkpatrick CJ, Muller AM. Vascular endothelial cadherin expression in lung specimens of patients with sepsis-induced acute respiratory distress syndrome and endothelial cell cultures. Pathobiology. 2013;80:245–251. doi: 10.1159/000347062. [DOI] [PubMed] [Google Scholar]
- 16.Lee WL, Slutsky AS. Sepsis and endothelial permeability. N Engl J Med. 2010;363:689–691. doi: 10.1056/NEJMcibr1007320. [DOI] [PubMed] [Google Scholar]
- 17.Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood–retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res. 2013;34:19–48. doi: 10.1016/j.preteyeres.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T. Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr Opin Crit Care. 2002;8:101–105. doi: 10.1097/00075198-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Zlokovic BV. Remodeling after stroke. Nat Med. 2006;12:390–391. doi: 10.1038/nm0406-390. [DOI] [PubMed] [Google Scholar]
- 20.Baluna R, Vitetta ES. Vascular leak syndrome: a side effect of immunotherapy. Immunopharmacology. 1997;37:117–132. doi: 10.1016/S0162-3109(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y, Vanhoutte PM, Leung SW. Vascular nitric oxide: beyond eNOS. J Pharmacol Sci. 2015;129:83–94. doi: 10.1016/j.jphs.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Sandoo A, van Zanten JJ, Metsios GS, Carroll D, Kitas GD. The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J. 2010;4:302–312. doi: 10.2174/1874192401004010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tousoulis D, Kampoli AM, Tentolouris C, Papageorgiou N, Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol. 2012;10:4–18. doi: 10.2174/157016112798829760. [DOI] [PubMed] [Google Scholar]
- 24.Munaron L. Intracellular calcium, endothelial cells and angiogenesis. Recent Pat Anticancer Drug Discov. 2006;1:105–119. doi: 10.2174/157489206775246502. [DOI] [PubMed] [Google Scholar]
- 25.Cui C, Merritt R, Fu L, Pan Z. Targeting calcium signaling in cancer therapy. Acta Pharm Sin B. 2017;7:3–17. doi: 10.1016/j.apsb.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovacic JC, Boehm M. Resident vascular progenitor cells: an emerging role for non-terminally differentiated vessel-resident cells in vascular biology. Stem Cell Res. 2009;2:2–15. doi: 10.1016/j.scr.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pappenheimer JR, Renkin EM, Borrero LM. Filtration, diffusion and molecular sieving through peripheral capillary membranes; a contribution to the pore theory of capillary permeability. Am J Physiol. 1951;167:13–46. doi: 10.1152/ajplegacy.1951.167.1.13. [DOI] [PubMed] [Google Scholar]
- 28.Del Vecchio PJ, Siflinger-Birnboim A, Shepard JM, Bizios R, Cooper JA, Malik AB. Endothelial monolayer permeability to macromolecules. Fed Proc. 1987;46:2511–2515. [PubMed] [Google Scholar]
- 29.Siflinger-Birnboim A, Del Vecchio PJ, Cooper JA, Blumenstock FA, Shepard JM, Malik AB. Molecular sieving characteristics of the cultured endothelial monolayer. J Cell Physiol. 1987;132:111–117. doi: 10.1002/jcp.1041320115. [DOI] [PubMed] [Google Scholar]
- 30.Wright AK, Thompson MR. Hydrodynamic structure of bovine serum albumin determined by transient electric birefringence. Biophys J. 1975;15:137–141. doi: 10.1016/S0006-3495(75)85797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters T., Jr Serum albumin. Adv Protein Chem. 1985;37:161–245. doi: 10.1016/S0065-3233(08)60065-0. [DOI] [PubMed] [Google Scholar]
- 32.Huxley VH, Curry FE. Albumin modulation of capillary permeability: test of an adsorption mechanism. Am J Physiol. 1985;248:H264–H273. doi: 10.1152/ajpheart.1985.248.2.H264. [DOI] [PubMed] [Google Scholar]
- 33.Huxley VH, Curry FE. Effect of superfusate albumin on single capillary hydraulic conductivity. Am J Physiol. 1987;252:H395–H401. doi: 10.1152/ajpheart.1987.252.2.H395. [DOI] [PubMed] [Google Scholar]
- 34.Weisberg HF. Osmotic pressure of the serum proteins. Ann Clin Lab Sci. 1978;8:155–164. [PubMed] [Google Scholar]
- 35.Landis EM, Hortenstine JC. Functional significance of venous blood pressure. Physiol Rev. 1950;30:1–32. doi: 10.1152/physrev.1950.30.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Reese TS, Karnovsky MJ. Fine structural localization of a blood–brain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simionescu M, Simionescu N, Palade GE. Segmental differentiations of cell junctions in the vascular endothelium. The microvasculature. J Cell Biol. 1975;67:863–885. doi: 10.1083/jcb.67.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huttner I, Boutet M, More RH. Gap junctions in arterial endothelium. J Cell Biol. 1973;57:247–252. doi: 10.1083/jcb.57.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Firth JA, Bauman KF, Sibley CP. The intercellular junctions of guinea-pig placental capillaries: a possible structural basis for endothelial solute permeability. J Ultrastruct Res. 1983;85:45–57. doi: 10.1016/S0022-5320(83)90115-6. [DOI] [PubMed] [Google Scholar]
- 40.Leach L, Clark P, Lampugnani MG, Arroyo AG, Dejana E, Firth JA. Immunoelectron characterisation of the inter-endothelial junctions of human term placenta. J Cell Sci. 1993;104(Pt 4):1073–1081. doi: 10.1242/jcs.104.4.1073. [DOI] [PubMed] [Google Scholar]
- 41.Tamura K, Shan WS, Hendrickson WA, Colman DR, Shapiro L. Structure–function analysis of cell adhesion by neural (N-) cadherin. Neuron. 1998;20:1153–1163. doi: 10.1016/S0896-6273(00)80496-1. [DOI] [PubMed] [Google Scholar]
- 42.Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E. The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin) J Cell Biol. 1995;129:203–217. doi: 10.1083/jcb.129.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Konstantoulaki M, Kouklis P, Malik AB. Protein kinase C modifications of VE-cadherin, p120, and beta-catenin contribute to endothelial barrier dysregulation induced by thrombin. Am J Physiol Lung Cell Mol Physiol. 2003;285:L434–L442. doi: 10.1152/ajplung.00075.2003. [DOI] [PubMed] [Google Scholar]
- 44.Mehta D, Rahman A, Malik AB. Protein kinase C-alpha signals rho-guanine nucleotide dissociation inhibitor phosphorylation and rho activation and regulates the endothelial cell barrier function. J Biol Chem. 2001;276:22614–22620. doi: 10.1074/jbc.M101927200. [DOI] [PubMed] [Google Scholar]
- 45.Minshall RD, Vandenbroucke EE, Holinstat M, Place AT, Tiruppathi C, Vogel SM, van Nieuw Amerongen GP, Mehta D, Malik AB. Role of protein kinase Czeta in thrombin-induced RhoA activation and inter-endothelial gap formation of human dermal microvessel endothelial cell monolayers. Microvasc Res. 2010;80:240–249. doi: 10.1016/j.mvr.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Komarova YA, Huang F, Geyer M, Daneshjou N, Garcia A, Idalino L, Kreutz B, Mehta D, Malik AB. VE-cadherin signaling induces EB3 phosphorylation to suppress microtubule growth and assemble adherens junctions. Mol Cell. 2012;48:914–925. doi: 10.1016/j.molcel.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vandenbroucke St Amant E, Tauseef M, Vogel SM, Gao XP, Mehta D, Komarova YA, Malik AB. PKCalpha activation of p120-catenin serine 879 phospho-switch disassembles VE-cadherin junctions and disrupts vascular integrity. Circ Res. 2012;111:739–749. doi: 10.1161/CIRCRESAHA.112.269654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen LT, Lum H, Tiruppathi C, Malik AB. Site-specific thrombin receptor antibodies inhibit Ca2+ signaling and increased endothelial permeability. Am J Physiol. 1997;273:C1756–C1763. doi: 10.1152/ajpcell.1997.273.5.C1756. [DOI] [PubMed] [Google Scholar]
- 49.Singh I, Knezevic N, Ahmmed GU, Kini V, Malik AB, Mehta D. Galphaq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin. J Biol Chem. 2007;282:7833–7843. doi: 10.1074/jbc.M608288200. [DOI] [PubMed] [Google Scholar]
- 50.Kini V, Chavez A, Mehta D. A new role for PTEN in regulating transient receptor potential canonical channel 6-mediated Ca2+ entry, endothelial permeability, and angiogenesis. J Biol Chem. 2010;285:33082–33091. doi: 10.1074/jbc.M110.142034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tauseef M, Knezevic N, Chava KR, Smith M, Sukriti S, Gianaris N, Obukhov AG, Vogel SM, Schraufnagel DE, Dietrich A, Birnbaumer L, Malik AB, Mehta D. TLR4 activation of TRPC6-dependent calcium signaling mediates endotoxin-induced lung vascular permeability and inflammation. J Exp Med. 2012;209:1953–1968. doi: 10.1084/jem.20111355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gandhirajan RK, Meng S, Chandramoorthy HC, Mallilankaraman K, Mancarella S, Gao H, Razmpour R, Yang XF, Houser SR, Chen J, Koch WJ, Wang H, Soboloff J, Gill DL, et al. Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J Clin Investig. 2013;123:887–902. doi: 10.1172/JCI65647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geyer M, Huang F, Sun Y, Vogel SM, Malik AB, Taylor CW, Komarova YA. Microtubule-associated protein EB3 regulates IP3 receptor clustering and Ca(2+) signaling in endothelial cells. Cell Rep. 2015;12:79–89. doi: 10.1016/j.celrep.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lewit-Bentley A, Rety S. EF-hand calcium-binding proteins. Curr Opin Struct Biol. 2000;10:637–643. doi: 10.1016/S0959-440X(00)00142-1. [DOI] [PubMed] [Google Scholar]
- 55.Kretsinger RH, Nockolds CE. Carp muscle calcium-binding protein. II. Structure determination and general description. J Biol Chem. 1973;248:3313–3326. [PubMed] [Google Scholar]
- 56.Nelson MR, Chazin WJ. An interaction-based analysis of calcium-induced conformational changes in Ca2+ sensor proteins. Protein Sci. 1998;7:270–282. doi: 10.1002/pro.5560070206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maler L, Blankenship J, Rance M, Chazin WJ. Site-site communication in the EF-hand Ca2+-binding protein calbindin D9k. Nat Struct Biol. 2000;7:245–250. doi: 10.1038/73369. [DOI] [PubMed] [Google Scholar]
- 58.Borbiev T, Verin AD, Shi S, Liu F, Garcia JG. Regulation of endothelial cell barrier function by calcium/calmodulin-dependent protein kinase II. Am J Physiol Lung Cell Mol Physiol. 2001;280:L983–L990. doi: 10.1152/ajplung.2001.280.5.L983. [DOI] [PubMed] [Google Scholar]
- 59.Sandoval R, Malik AB, Minshall RD, Kouklis P, Ellis CA, Tiruppathi C. Ca(2+) signalling and PKCalpha activate increased endothelial permeability by disassembly of VE-cadherin junctions. J Physiol. 2001;533:433–445. doi: 10.1111/j.1469-7793.2001.0433a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borbiev T, Verin AD, Birukova A, Liu F, Crow MT, Garcia JG. Role of CaM kinase II and ERK activation in thrombin-induced endothelial cell barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2003;285:L43–L54. doi: 10.1152/ajplung.00460.2001. [DOI] [PubMed] [Google Scholar]
- 61.Wainwright MS, Rossi J, Schavocky J, Crawford S, Steinhorn D, Velentza AV, Zasadzki M, Shirinsky V, Jia Y, Haiech J, Van Eldik LJ, Watterson DM. Protein kinase involved in lung injury susceptibility: evidence from enzyme isoform genetic knockout and in vivo inhibitor treatment. Proc Natl Acad Sci USA. 2003;100:6233–6238. doi: 10.1073/pnas.1031595100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peng J, He F, Zhang C, Deng X, Yin F. Protein kinase C-alpha signals P115RhoGEF phosphorylation and RhoA activation in TNF-alpha-induced mouse brain microvascular endothelial cell barrier dysfunction. J Neuroinflammation. 2011;8:28. doi: 10.1186/1742-2094-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Z, Ginnan R, Abdullaev IF, Trebak M, Vincent PA, Singer HA. Calcium/calmodulin-dependent protein kinase II delta 6 (CaMKIIdelta6) and RhoA involvement in thrombin-induced endothelial barrier dysfunction. J Biol Chem. 2010;285:21303–21312. doi: 10.1074/jbc.M110.120790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xie L, Chiang ET, Wu X, Kelly GT, Kanteti P, Singleton PA, Camp SM, Zhou T, Dudek SM, Natarajan V, Wang T, Black SM, Garcia JG, Jacobson JR. Regulation of thrombin-induced lung endothelial cell barrier disruption by protein kinase C delta. PLoS One. 2016;11:e0158865. doi: 10.1371/journal.pone.0158865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xia X, Mariner DJ, Reynolds AB. Adhesion-associated and PKC-modulated changes in serine/threonine phosphorylation of p120-catenin. Biochemistry. 2003;42:9195–9204. doi: 10.1021/bi034597h. [DOI] [PubMed] [Google Scholar]
- 66.Brown MV, Burnett PE, Denning MF, Reynolds AB. PDGF receptor activation induces p120-catenin phosphorylation at serine 879 via a PKCalpha-dependent pathway. Exp Cell Res. 2009;315:39–49. doi: 10.1016/j.yexcr.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiao K, Garner J, Buckley KM, Vincent PA, Chiasson CM, Dejana E, Faundez V, Kowalczyk AP. p120-Catenin regulates clathrin-dependent endocytosis of VE-cadherin. Mol Biol Cell. 2005;16:5141–5151. doi: 10.1091/mbc.E05-05-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chiasson CM, Wittich KB, Vincent PA, Faundez V, Kowalczyk AP. p120-catenin inhibits VE-cadherin internalization through a Rho-independent mechanism. Mol Biol Cell. 2009;20:1970–1980. doi: 10.1091/mbc.E08-07-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nanes BA, Chiasson-MacKenzie C, Lowery AM, Ishiyama N, Faundez V, Ikura M, Vincent PA, Kowalczyk AP. p120-catenin binding masks an endocytic signal conserved in classical cadherins. J Cell Biol. 2012;199:365–380. doi: 10.1083/jcb.201205029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nanes BA, Grimsley-Myers CM, Cadwell CM, Robinson BS, Lowery AM, Vincent PA, Mosunjac M, Fruh K, Kowalczyk AP. p120-catenin regulates VE-cadherin endocytosis and degradation induced by the Kaposi sarcoma-associated ubiquitin ligase K5. Mol Biol Cell. 2017;28:30–40. doi: 10.1091/mbc.E16-06-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Su W, Kowalczyk AP. The VE-cadherin cytoplasmic domain undergoes proteolytic processing during endocytosis. Mol Biol Cell. 2017;28:76–84. doi: 10.1091/mbc.E16-09-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakano K, Takaishi K, Kodama A, Mammoto A, Shiozaki H, Monden M, Takai Y. Distinct actions and cooperative roles of ROCK and mDia in Rho small G protein-induced reorganization of the actin cytoskeleton in Madin–Darby canine kidney cells. Mol Biol Cell. 1999;10:2481–2491. doi: 10.1091/mbc.10.8.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geneste O, Copeland JW, Treisman R. LIM kinase and Diaphanous cooperate to regulate serum response factor and actin dynamics. J Cell Biol. 2002;157:831–838. doi: 10.1083/jcb.200203126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsuji T, Ishizaki T, Okamoto M, Higashida C, Kimura K, Furuyashiki T, Arakawa Y, Birge RB, Nakamoto T, Hirai H, Narumiya S. ROCK and mDia1 antagonize in Rho-dependent Rac activation in Swiss 3T3 fibroblasts. J Cell Biol. 2002;157:819–830. doi: 10.1083/jcb.200112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Knezevic N, Roy A, Timblin B, Konstantoulaki M, Sharma T, Malik AB, Mehta D. GDI-1 phosphorylation switch at serine 96 induces RhoA activation and increased endothelial permeability. Mol Cell Biol. 2007;27:6323–6333. doi: 10.1128/MCB.00523-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holinstat M, Mehta D, Kozasa T, Minshall RD, Malik AB. Protein kinase Calpha-induced p115RhoGEF phosphorylation signals endothelial cytoskeletal rearrangement. J Biol Chem. 2003;278:28793–28798. doi: 10.1074/jbc.M303900200. [DOI] [PubMed] [Google Scholar]
- 77.Dubash AD, Wennerberg K, Garcia-Mata R, Menold MM, Arthur WT, Burridge K. A novel role for Lsc/p115 RhoGEF and LARG in regulating RhoA activity downstream of adhesion to fibronectin. J Cell Sci. 2007;120:3989–3998. doi: 10.1242/jcs.003806. [DOI] [PubMed] [Google Scholar]
- 78.Chow CR, Suzuki N, Kawamura T, Hamakubo T, Kozasa T. Modification of p115RhoGEF Ser(330) regulates its RhoGEF activity. Cell Signal. 2013;25:2085–2092. doi: 10.1016/j.cellsig.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, Hartshorne DJ, Nakano T. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem. 1999;274:37385–37390. doi: 10.1074/jbc.274.52.37385. [DOI] [PubMed] [Google Scholar]
- 80.van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res. 2000;87:335–340. doi: 10.1161/01.RES.87.4.335. [DOI] [PubMed] [Google Scholar]
- 81.Fukata M, Kaibuchi K. Rho-family GTPases in cadherin-mediated cell–cell adhesion. Nat Rev Mol Cell Biol. 2001;2:887–897. doi: 10.1038/35103068. [DOI] [PubMed] [Google Scholar]
- 82.Craig R, Smith R, Kendrick-Jones J. Light-chain phosphorylation controls the conformation of vertebrate non-muscle and smooth muscle myosin molecules. Nature. 1983;302:436–439. doi: 10.1038/302436a0. [DOI] [PubMed] [Google Scholar]
- 83.Allen BG, Walsh MP. The biochemical basis of the regulation of smooth-muscle contraction. Trends Biochem Sci. 1994;19:362–368. doi: 10.1016/0968-0004(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 84.Shimokawa H, Seto M, Katsumata N, Amano M, Kozai T, Yamawaki T, Kuwata K, Kandabashi T, Egashira K, Ikegaki I, Asano T, Kaibuchi K, Takeshita A. Rho-kinase-mediated pathway induces enhanced myosin light chain phosphorylations in a swine model of coronary artery spasm. Cardiovasc Res. 1999;43:1029–1039. doi: 10.1016/S0008-6363(99)00144-3. [DOI] [PubMed] [Google Scholar]
- 85.Herzog D, Loetscher P, van Hengel J, Knusel S, Brakebusch C, Taylor V, Suter U, Relvas JB. The small GTPase RhoA is required to maintain spinal cord neuroepithelium organization and the neural stem cell pool. J Neurosci. 2011;31:5120–5130. doi: 10.1523/JNEUROSCI.4807-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, Vestweber D. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J. 2002;21:4885–4895. doi: 10.1093/emboj/cdf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baumer S, Keller L, Holtmann A, Funke R, August B, Gamp A, Wolburg H, Wolburg-Buchholz K, Deutsch U, Vestweber D. Vascular endothelial cell-specific phosphotyrosine phosphatase (VE-PTP) activity is required for blood vessel development. Blood. 2006;107:4754–4762. doi: 10.1182/blood-2006-01-0141. [DOI] [PubMed] [Google Scholar]
- 88.Gong H, Rehman J, Tang H, Wary K, Mittal M, Chaturvedi P, Zhao YY, Komarova YA, Vogel SM, Malik AB. HIF2alpha signaling inhibits adherens junctional disruption in acute lung injury. J Clin Investig. 2015;125:652–664. doi: 10.1172/JCI77701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Soni D, Regmi SC, Wang DM, DebRoy A, Zhao YY, Vogel SM, Malik AB, Tiruppathi C. Pyk2 phosphorylation of VE-PTP downstream of STIM1-induced Ca2+ entry regulates disassembly of adherens junctions. Am J Physiol Lung Cell Mol Physiol. 2017;312:L1003–L1017. doi: 10.1152/ajplung.00008.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yazbeck P, Tauseef M, Kruse K, Amin MR, Sheikh R, Feske S, Komarova Y, Mehta D. STIM1 phosphorylation at Y361 recruits Orai1 to STIM1 puncta and induces Ca2+ entry. Sci Rep. 2017;7:42758. doi: 10.1038/srep42758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stolwijk JA, Zhang X, Gueguinou M, Zhang W, Matrougui K, Renken C, Trebak M. Calcium signaling is dispensable for receptor regulation of endothelial barrier function. J Biol Chem. 2016;291:22894–22912. doi: 10.1074/jbc.M116.756114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 95.Klee CB, Crouch TH, Krinks MH. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci USA. 1979;76:6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vasauskas AA, Chen H, Wu S, Cioffi DL. The serine-threonine phosphatase calcineurin is a regulator of endothelial store-operated calcium entry. Pulm Circ. 2014;4:116–127. doi: 10.1086/675641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jain J, McCaffrey PG, Miner Z, Kerppola TK, Lambert JN, Verdine GL, Curran T, Rao A. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature. 1993;365:352–355. doi: 10.1038/365352a0. [DOI] [PubMed] [Google Scholar]
- 98.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109(Suppl):S67–S79. doi: 10.1016/S0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 99.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 100.Wood PG, Gillespie JI. Evidence for mitochondrial Ca(2+)-induced Ca2+ release in permeabilised endothelial cells. Biochem Biophys Res Commun. 1998;246:543–548. doi: 10.1006/bbrc.1998.8661. [DOI] [PubMed] [Google Scholar]
- 101.Suzuki J, Kanemaru K, Ishii K, Ohkura M, Okubo Y, Iino M. Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat Commun. 2014;5:4153. doi: 10.1038/ncomms5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carafoli E, Crompton M. The regulation of intracellular calcium by mitochondria. Ann N Y Acad Sci. 1978;307:269–284. doi: 10.1111/j.1749-6632.1978.tb41957.x. [DOI] [PubMed] [Google Scholar]
- 103.Katz AM, Repke DI, Fudyma G, Shigekawa M. Control of calcium efflux from sarcoplasmic reticulum vesicles by external calcium. J Biol Chem. 1977;252:4210–4214. [PubMed] [Google Scholar]
- 104.Docampo R, Huang G. Acidocalcisomes of eukaryotes. Curr Opin Cell Biol. 2016;41:66–72. doi: 10.1016/j.ceb.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Domotor E, Abbott NJ, Adam-Vizi V. Na+–Ca2+ exchange and its implications for calcium homeostasis in primary cultured rat brain microvascular endothelial cells. J Physiol. 1999;515(Pt 1):147–155. doi: 10.1111/j.1469-7793.1999.147ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moccia F, Berra-Romani R, Baruffi S, Spaggiari S, Signorelli S, Castelli L, Magistretti J, Taglietti V, Tanzi F. Ca2+ uptake by the endoplasmic reticulum Ca2+-ATPase in rat microvascular endothelial cells. Biochem J. 2002;364:235–244. doi: 10.1042/bj3640235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang X, Reznick S, Li P, Liang W, van Breemen C. Ca(2+) removal mechanisms in freshly isolated rabbit aortic endothelial cells. Cell Calcium. 2002;31:265–277. doi: 10.1016/S0143-4160(02)00075-1. [DOI] [PubMed] [Google Scholar]
- 108.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 109.Patel A, Sharif-Naeini R, Folgering JR, Bichet D, Duprat F, Honore E. Canonical TRP channels and mechanotransduction: from physiology to disease states. Pflugers Arch. 2010;460:571–581. doi: 10.1007/s00424-010-0847-8. [DOI] [PubMed] [Google Scholar]
- 110.Zhu MX, Ma J, Parrington J, Calcraft PJ, Galione A, Evans AM. Calcium signaling via two-pore channels: local or global, that is the question. Am J Physiol Cell Physiol. 2010;298:C430–C441. doi: 10.1152/ajpcell.00475.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ambudkar IS, de Souza LB, Ong HL. TRPC1, Orai1, and STIM1 in SOCE: friends in tight spaces. Cell Calcium. 2016 doi: 10.1016/j.ceca.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Toyoshima C, Cornelius F. New crystal structures of PII-type ATPases: excitement continues. Curr Opin Struct Biol. 2013;23:507–514. doi: 10.1016/j.sbi.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 113.Moller JV, Juul B, le Maire M. Structural organization, ion transport, and energy transduction of P-type ATPases. Biochim Biophys Acta. 1996;1286:1–51. doi: 10.1016/0304-4157(95)00017-8. [DOI] [PubMed] [Google Scholar]
- 114.Hilgemann DW, Yaradanakul A, Wang Y, Fuster D. Molecular control of cardiac sodium homeostasis in health and disease. J Cardiovasc Electrophysiol. 2006;17(Suppl 1):S47–S56. doi: 10.1111/j.1540-8167.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 115.Niggli V, Sigel E, Carafoli E. The purified Ca2+ pump of human erythrocyte membranes catalyzes an electroneutral Ca2+–H+ exchange in reconstituted liposomal systems. J Biol Chem. 1982;257:2350–2356. [PubMed] [Google Scholar]
- 116.Smallwood JI, Waisman DM, Lafreniere D, Rasmussen H. Evidence that the erythrocyte calcium pump catalyzes a Ca2+:nH+ exchange. J Biol Chem. 1983;258:11092–11097. [PubMed] [Google Scholar]
- 117.Sweadner KJ, Donnet C. Structural similarities of Na, K-ATPase and SERCA, the Ca(2+)-ATPase of the sarcoplasmic reticulum. Biochem J. 2001;356:685–704. doi: 10.1042/bj3560685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda N, Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989;342:32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- 119.Mikoshiba K, Changeux JP. Morphological and biochemical studies on isolated molecular and granular layers from bovine cerebellum. Brain Res. 1978;142:487–504. doi: 10.1016/0006-8993(78)90911-3. [DOI] [PubMed] [Google Scholar]
- 120.Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306:67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- 121.Pessah IN, Francini AO, Scales DJ, Waterhouse AL, Casida JE. Calcium-ryanodine receptor complex. Solubilization and partial characterization from skeletal muscle junctional sarcoplasmic reticulum vesicles. J Biol Chem. 1986;261:8643–8648. [PubMed] [Google Scholar]
- 122.Inui M, Saito A, Fleischer S. Isolation of the ryanodine receptor from cardiac sarcoplasmic reticulum and identity with the feet structures. J Biol Chem. 1987;262:15637–15642. [PubMed] [Google Scholar]
- 123.Inui M, Saito A, Fleischer S. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J Biol Chem. 1987;262:1740–1747. [PubMed] [Google Scholar]
- 124.Mauger JP, Claret M, Pietri F, Hilly M. Hormonal regulation of inositol 1,4,5-trisphosphate receptor in rat liver. J Biol Chem. 1989;264:8821–8826. [PubMed] [Google Scholar]
- 125.Chadwick CC, Saito A, Fleischer S. Isolation and characterization of the inositol trisphosphate receptor from smooth muscle. Proc Natl Acad Sci USA. 1990;87:2132–2136. doi: 10.1073/pnas.87.6.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Seo MD, Velamakanni S, Ishiyama N, Stathopulos PB, Rossi AM, Khan SA, Dale P, Li C, Ames JB, Ikura M, Taylor CW. Structural and functional conservation of key domains in InsP3 and ryanodine receptors. Nature. 2012;483:108–112. doi: 10.1038/nature10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moccia F, Berra-Romani R, Tanzi F. Update on vascular endothelial Ca(2+) signalling: a tale of ion channels, pumps and transporters. World J Biol Chem. 2012;3:127–158. doi: 10.4331/wjbc.v3.i7.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Prole DL, Taylor CW. Inositol 1,4,5-trisphosphate receptors and their protein partners as signalling hubs. J Physiol. 2016;594:2849–2866. doi: 10.1113/JP271139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Supattapone S, Worley PF, Baraban JM, Snyder SH. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988;263:1530–1534. [PubMed] [Google Scholar]
- 130.Jiang QX, Thrower EC, Chester DW, Ehrlich BE, Sigworth FJ. Three-dimensional structure of the type 1 inositol 1,4,5-trisphosphate receptor at 24 A resolution. EMBO J. 2002;21:3575–3581. doi: 10.1093/emboj/cdf380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Taylor CW, Genazzani AA, Morris SA. Expression of inositol trisphosphate receptors. Cell Calcium. 1999;26:237–251. doi: 10.1054/ceca.1999.0090. [DOI] [PubMed] [Google Scholar]
- 132.Parys JB, Sernett SW, DeLisle S, Snyder PM, Welsh MJ, Campbell KP. Isolation, characterization, and localization of the inositol 1,4,5-trisphosphate receptor protein in Xenopus laevis oocytes. J Biol Chem. 1992;267:18776–18782. [PubMed] [Google Scholar]
- 133.Fan G, Baker ML, Wang Z, Baker MR, Sinyagovskiy PA, Chiu W, Ludtke SJ, Serysheva II. Gating machinery of InsP3R channels revealed by electron cryomicroscopy. Nature. 2015;527:336–341. doi: 10.1038/nature15249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sheppard CA, Simpson PB, Sharp AH, Nucifora FC, Ross CA, Lange GD, Russell JT. Comparison of type 2 inositol 1,4,5-trisphosphate receptor distribution and subcellular Ca2+ release sites that support Ca2+ waves in cultured astrocytes. J Neurochem. 1997;68:2317–2327. doi: 10.1046/j.1471-4159.1997.68062317.x. [DOI] [PubMed] [Google Scholar]
- 135.Iwai M, Tateishi Y, Hattori M, Mizutani A, Nakamura T, Futatsugi A, Inoue T, Furuichi T, Michikawa T, Mikoshiba K. Molecular cloning of mouse type 2 and type 3 inositol 1,4,5-trisphosphate receptors and identification of a novel type 2 receptor splice variant. J Biol Chem. 2005;280:10305–10317. doi: 10.1074/jbc.M413824200. [DOI] [PubMed] [Google Scholar]
- 136.Mak DO, Foskett JK. Single-channel kinetics, inactivation, and spatial distribution of inositol trisphosphate (IP3) receptors in Xenopus oocyte nucleus. J Gen Physiol. 1997;109:571–587. doi: 10.1085/jgp.109.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cruttwell C, Bernard J, Hilly M, Nicolas V, Tunwell RE, Mauger JP. Dynamics of the Ins(1,4,5)P3 receptor during polarization of MDCK cells. Biol Cell. 2005;97:699–707. doi: 10.1042/BC20040503. [DOI] [PubMed] [Google Scholar]
- 138.Chalmers M, Schell MJ, Thorn P. Agonist-evoked inositol trisphosphate receptor (IP3R) clustering is not dependent on changes in the structure of the endoplasmic reticulum. Biochem J. 2006;394:57–66. doi: 10.1042/BJ20051130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pantazaka E, Taylor CW. Differential distribution, clustering, and lateral diffusion of subtypes of the inositol 1,4,5-trisphosphate receptor. J Biol Chem. 2011;286:23378–23387. doi: 10.1074/jbc.M111.236372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Smith IF, Swaminathan D, Dickinson GD, Parker I. Single-molecule tracking of inositol trisphosphate receptors reveals different motilities and distributions. Biophys J. 2014;107:834–845. doi: 10.1016/j.bpj.2014.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rahman T, Skupin A, Falcke M, Taylor CW. Clustering of InsP3 receptors by InsP3 retunes their regulation by InsP3 and Ca2+ Nature. 2009;458:655–659. doi: 10.1038/nature07763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rahman T. Dynamic clustering of IP3 receptors by IP3. Biochem Soc Trans. 2012;40:325–330. doi: 10.1042/BST20110772. [DOI] [PubMed] [Google Scholar]
- 143.Taylor SJ, Chae HZ, Rhee SG, Exton JH. Activation of the beta 1 isozyme of phospholipase C by alpha subunits of the Gq class of G proteins. Nature. 1991;350:516–518. doi: 10.1038/350516a0. [DOI] [PubMed] [Google Scholar]
- 144.Smrcka AV, Hepler JR, Brown KO, Sternweis PC. Regulation of polyphosphoinositide-specific phospholipase C activity by purified Gq. Science. 1991;251:804–807. doi: 10.1126/science.1846707. [DOI] [PubMed] [Google Scholar]
- 145.Suh PG, Park JI, Manzoli L, Cocco L, Peak JC, Katan M, Fukami K, Kataoka T, Yun S, Ryu SH. Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB Rep. 2008;41:415–434. doi: 10.5483/BMBRep.2008.41.6.415. [DOI] [PubMed] [Google Scholar]
- 146.Beziau DM, Toussaint F, Blanchette A, Dayeh NR, Charbel C, Tardif JC, Dupuis J, Ledoux J. Expression of phosphoinositide-specific phospholipase C isoforms in native endothelial cells. PLoS One. 2015;10:e0123769. doi: 10.1371/journal.pone.0123769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ji QS, Winnier GE, Niswender KD, Horstman D, Wisdom R, Magnuson MA, Carpenter G. Essential role of the tyrosine kinase substrate phospholipase C-gamma1 in mammalian growth and development. Proc Natl Acad Sci USA. 1997;94:2999–3003. doi: 10.1073/pnas.94.7.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liao HJ, Kume T, McKay C, Xu MJ, Ihle JN, Carpenter G. Absence of erythrogenesis and vasculogenesis in Plcg1-deficient mice. J Biol Chem. 2002;277:9335–9341. doi: 10.1074/jbc.M109955200. [DOI] [PubMed] [Google Scholar]
- 149.Muldowney JA, 3rd, Painter CA, Sanders-Bush E, Brown NJ, Vaughan DE. Acute tissue-type plasminogen activator release in human microvascular endothelial cells: the roles of Galphaq, PLC-beta, IP3 and 5,6-epoxyeicosatrienoic acid. Thromb Haemost. 2007;97:263–271. [PubMed] [Google Scholar]
- 150.Seehaus S, Shahzad K, Kashif M, Vinnikov IA, Schiller M, Wang H, Madhusudhan T, Eckstein V, Bierhaus A, Bea F, Blessing E, Weiler H, Frommhold D, Nawroth PP, et al. Hypercoagulability inhibits monocyte transendothelial migration through protease-activated receptor-1-, phospholipase-Cbeta-, phosphoinositide 3-kinase-, and nitric oxide-dependent signaling in monocytes and promotes plaque stability. Circulation. 2009;120:774–784. doi: 10.1161/CIRCULATIONAHA.109.849539. [DOI] [PubMed] [Google Scholar]
- 151.Nussbaum C, Bannenberg S, Keul P, Graler MH, Goncalves-de-Albuquerque CF, Korhonen H, von Wnuck Lipinski K, Heusch G, de Castro Faria Neto HC, Rohwedder I, Gothert JR, Prasad VP, Haufe G, Lange-Sperandio B, et al. Sphingosine-1-phosphate receptor 3 promotes leukocyte rolling by mobilizing endothelial P-selectin. Nat Commun. 2015;6:6416. doi: 10.1038/ncomms7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mikelis CM, Simaan M, Ando K, Fukuhara S, Sakurai A, Amornphimoltham P, Masedunskas A, Weigert R, Chavakis T, Adams RH, Offermanns S, Mochizuki N, Zheng Y, Gutkind JS. RhoA and ROCK mediate histamine-induced vascular leakage and anaphylactic shock. Nat Commun. 2015;6:6725. doi: 10.1038/ncomms7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hoeppner LH, Phoenix KN, Clark KJ, Bhattacharya R, Gong X, Sciuto TE, Vohra P, Suresh S, Bhattacharya S, Dvorak AM, Ekker SC, Dvorak HF, Claffey KP, Mukhopadhyay D. Revealing the role of phospholipase Cbeta3 in the regulation of VEGF-induced vascular permeability. Blood. 2012;120:2167–2173. doi: 10.1182/blood-2012-03-417824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 155.Mak DO, McBride S, Foskett JK. Inositol 1,4,5-trisphosphate [correction of tris-phosphate] activation of inositol trisphosphate [correction of tris-phosphate] receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc Natl Acad Sci USA. 1998;95:15821–15825. doi: 10.1073/pnas.95.26.15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol. 1990;95:1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Finch EA, Turner TJ, Goldin SM. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991;252:443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- 158.Marshall IC, Taylor CW. Biphasic effects of cytosolic Ca2+ on Ins(1,4,5)P3-stimulated Ca2+ mobilization in hepatocytes. J Biol Chem. 1993;268:13214–13220. [PubMed] [Google Scholar]
- 159.Parys JB, Missiaen L, De Smedt H, Casteels R. Loading dependence of inositol 1,4,5-trisphosphate-induced Ca2+ release in the clonal cell line A7r5. Implications for the mechanism of quantal Ca2+ release. J Biol Chem. 1993;268:25206–25212. [PubMed] [Google Scholar]
- 160.Sienaert I, De Smedt H, Parys JB, Missiaen L, Vanlingen S, Sipma H, Casteels R. Characterization of a cytosolic and a luminal Ca2+ binding site in the type I inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1996;271:27005–27012. doi: 10.1074/jbc.271.43.27005. [DOI] [PubMed] [Google Scholar]
- 161.Sienaert I, Missiaen L, De Smedt H, Parys JB, Sipma H, Casteels R. Molecular and functional evidence for multiple Ca2+-binding domains in the type 1 inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1997;272:25899–25906. doi: 10.1074/jbc.272.41.25899. [DOI] [PubMed] [Google Scholar]
- 162.Miyakawa T, Mizushima A, Hirose K, Yamazawa T, Bezprozvanny I, Kurosaki T, Iino M. Ca(2+)-sensor region of IP(3) receptor controls intracellular Ca(2+) signaling. EMBO J. 2001;20:1674–1680. doi: 10.1093/emboj/20.7.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tu H, Nosyreva E, Miyakawa T, Wang Z, Mizushima A, Iino M, Bezprozvanny I. Functional and biochemical analysis of the type 1 inositol (1,4,5)-trisphosphate receptor calcium sensor. Biophys J. 2003;85:290–299. doi: 10.1016/S0006-3495(03)74474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Joseph SK, Brownell S, Khan MT. Calcium regulation of inositol 1,4,5-trisphosphate receptors. Cell Calcium. 2005;38:539–546. doi: 10.1016/j.ceca.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 165.Berridge MJ. Inositol trisphosphate and calcium oscillations. Biochem Soc Symp. 2007;74:1–7. doi: 10.1042/BSS2007c01. [DOI] [PubMed] [Google Scholar]
- 166.Patel S, Morris SA, Adkins CE, O’Beirne G, Taylor CW. Ca2+-independent inhibition of inositol trisphosphate receptors by calmodulin: redistribution of calmodulin as a possible means of regulating Ca2+ mobilization. Proc Natl Acad Sci USA. 1997;94:11627–11632. doi: 10.1073/pnas.94.21.11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Adkins CE, Morris SA, De Smedt H, Sienaert I, Torok K, Taylor CW. Ca2+-calmodulin inhibits Ca2+ release mediated by type-1, -2 and -3 inositol trisphosphate receptors. Biochem J. 2000;345(Pt 2):357–363. doi: 10.1042/bj3450357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hirota J, Michikawa T, Natsume T, Furuichi T, Mikoshiba K. Calmodulin inhibits inositol 1,4,5-trisphosphate-induced calcium release through the purified and reconstituted inositol 1,4,5-trisphosphate receptor type 1. FEBS Lett. 1999;456:322–326. doi: 10.1016/S0014-5793(99)00973-4. [DOI] [PubMed] [Google Scholar]
- 169.Missiaen L, DeSmedt H, Bultynck G, Vanlingen S, Desmet P, Callewaert G, Parys JB. Calmodulin increases the sensitivity of type 3 inositol-1,4, 5-trisphosphate receptors to Ca(2+) inhibition in human bronchial mucosal cells. Mol Pharmacol. 2000;57:564–567. doi: 10.1124/mol.57.3.564. [DOI] [PubMed] [Google Scholar]
- 170.Kasri NN, Holmes AM, Bultynck G, Parys JB, Bootman MD, Rietdorf K, Missiaen L, McDonald F, De Smedt H, Conway SJ, Holmes AB, Berridge MJ, Roderick HL. Regulation of InsP3 receptor activity by neuronal Ca2+-binding proteins. EMBO J. 2004;23:312–321. doi: 10.1038/sj.emboj.7600037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Serysheva II. Toward a high-resolution structure of IP(3)R channel. Cell Calcium. 2014;56:125–132. doi: 10.1016/j.ceca.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sienaert I, Nadif Kasri N, Vanlingen S, Parys JB, Callewaert G, Missiaen L, de Smedt H. Localization and function of a calmodulin–apocalmodulin-binding domain in the N-terminal part of the type 1 inositol 1,4,5-trisphosphate receptor. Biochem J. 2002;365:269–277. doi: 10.1042/bj20020144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- 174.Takeshima H, Nishimura S, Matsumoto T, Ishida H, Kangawa K, Minamino N, Matsuo H, Ueda M, Hanaoka M, Hirose T, et al. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989;339:439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- 175.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 176.des Georges A, Clarke OB, Zalk R, Yuan Q, Condon KJ, Grassucci RA, Hendrickson WA, Marks AR, Frank J. Structural basis for gating and activation of RyR1. Cell. 2016;167:145–157. doi: 10.1016/j.cell.2016.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Marks AR, Tempst P, Hwang KS, Taubman MB, Inui M, Chadwick C, Fleischer S, Nadal-Ginard B. Molecular cloning and characterization of the ryanodine receptor/junctional channel complex cDNA from skeletal muscle sarcoplasmic reticulum. Proc Natl Acad Sci USA. 1989;86:8683–8687. doi: 10.1073/pnas.86.22.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nakai J, Imagawa T, Hakamat Y, Shigekawa M, Takeshima H, Numa S. Primary structure and functional expression from cDNA of the cardiac ryanodine receptor/calcium release channel. FEBS Lett. 1990;271:169–177. doi: 10.1016/0014-5793(90)80399-4. [DOI] [PubMed] [Google Scholar]
- 179.Lesh RE, Marks AR, Somlyo AV, Fleischer S, Somlyo AP. Anti-ryanodine receptor antibody binding sites in vascular and endocardial endothelium. Circ Res. 1993;72:481–488. doi: 10.1161/01.RES.72.2.481. [DOI] [PubMed] [Google Scholar]
- 180.Köhler R, Brakemeier S, Kühn M, Degenhardt C, Buhr H, Pries A, Hoyer J. Expression of ryanodine receptor type 3 and TRP channels in endothelial cells: comparison of in situ and cultured human endothelial cells. Cardiovasc Res. 2001;51:160–168. doi: 10.1016/S0008-6363(01)00281-4. [DOI] [PubMed] [Google Scholar]
- 181.Edwards GA, Weiant EA, Slocombe AG, Roeder KD. The action of ryanodine on the contractile process in striated muscle. Science. 1948;108:330–332. doi: 10.1126/science.108.2804.330. [DOI] [PubMed] [Google Scholar]
- 182.Ciofalo FR. Relationship between 3H-ryanodine uptake and myocardial contractility. Am J Physiol. 1973;225:324–327. doi: 10.1152/ajplegacy.1973.225.2.324. [DOI] [PubMed] [Google Scholar]
- 183.Nagasaki K, Fleischer S. Ryanodine sensitivity of the calcium release channel of sarcoplasmic reticulum. Cell Calcium. 1988;9:1–7. doi: 10.1016/0143-4160(88)90032-2. [DOI] [PubMed] [Google Scholar]
- 184.McGrew SG, Wolleben C, Siegl P, Inui M, Fleischer S. Positive cooperativity of ryanodine binding to the calcium release channel of sarcoplasmic reticulum from heart and skeletal muscle. Biochemistry. 1989;28:1686–1691. doi: 10.1021/bi00430a039. [DOI] [PubMed] [Google Scholar]
- 185.Gyorke S, Fill M. Ryanodine receptor adaptation: control mechanism of Ca(2+)-induced Ca2+ release in heart. Science. 1993;260:807–809. doi: 10.1126/science.8387229. [DOI] [PubMed] [Google Scholar]
- 186.Tripathy A, Xu L, Mann G, Meissner G. Calmodulin activation and inhibition of skeletal muscle Ca2+ release channel (ryanodine receptor) Biophys J. 1995;69:106–119. doi: 10.1016/S0006-3495(95)79880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lai FA, Misra M, Xu L, Smith HA, Meissner G. The ryanodine receptor-Ca2+ release channel complex of skeletal muscle sarcoplasmic reticulum. Evidence for a cooperatively coupled, negatively charged homotetramer. J Biol Chem. 1989;264:16776–16785. [PubMed] [Google Scholar]
- 188.Ziegelstein RC, Spurgeon HA, Pili R, Passaniti A, Cheng L, Corda S, Lakatta EG, Capogrossi MC. A functional ryanodine-sensitive intracellular Ca2+ store is present in vascular endothelial cells. Circ Res. 1994;74:151–156. doi: 10.1161/01.RES.74.1.151. [DOI] [PubMed] [Google Scholar]
- 189.Seo MD, Enomoto M, Ishiyama N, Stathopulos PB, Ikura M. Structural insights into endoplasmic reticulum stored calcium regulation by inositol 1,4,5-trisphosphate and ryanodine receptors. Biochim Biophys Acta. 2015;1853:1980–1991. doi: 10.1016/j.bbamcr.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 190.Van Petegem F. Ryanodine receptors: allosteric ion channel giants. J Mol Biol. 2015;427:31–53. doi: 10.1016/j.jmb.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 191.Clarke OB, Hendrickson WA. Structures of the colossal RyR1 calcium release channel. Curr Opin Struct Biol. 2016;39:144–152. doi: 10.1016/j.sbi.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, Lin P, Xiao R, Wang C, Zhu Y, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Favia A, Desideri M, Gambara G, D’Alessio A, Ruas M, Esposito B, Del Bufalo D, Parrington J, Ziparo E, Palombi F, Galione A, Filippini A. VEGF-induced neoangiogenesis is mediated by NAADP and two-pore channel-2-dependent Ca2+ signaling. Proc Natl Acad Sci USA. 2014;111:E4706–E4715. doi: 10.1073/pnas.1406029111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zuccolo E, Dragoni S, Poletto V, Catarsi P, Guido D, Rappa A, Reforgiato M, Lodola F, Lim D, Rosti V, Guerra G, Moccia F. Arachidonic acid-evoked Ca2+ signals promote nitric oxide release and proliferation in human endothelial colony forming cells. Vascul Pharmacol. 2016;87:159–171. doi: 10.1016/j.vph.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 195.Brailoiu E, Churamani D, Cai X, Schrlau MG, Brailoiu GC, Gao X, Hooper R, Boulware MJ, Dun NJ, Marchant JS, Patel S. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol. 2009;186:201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ruas M, Rietdorf K, Arredouani A, Davis LC, Lloyd-Evans E, Koegel H, Funnell TM, Morgan AJ, Ward JA, Watanabe K, Cheng X, Churchill GC, Zhu MX, Platt FM, et al. Purified TPC isoforms form NAADP receptors with distinct roles for Ca(2+) signaling and endolysosomal trafficking. Curr Biol. 2010;20:703–709. doi: 10.1016/j.cub.2010.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cang C, Aranda K, Ren D. A non-inactivating high-voltage-activated two-pore Na(+) channel that supports ultra-long action potentials and membrane bistability. Nat Commun. 2014;5:5015. doi: 10.1038/ncomms6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Brailoiu E, Hooper R, Cai X, Brailoiu GC, Keebler MV, Dun NJ, Marchant JS, Patel S. An ancestral deuterostome family of two-pore channels mediates nicotinic acid adenine dinucleotide phosphate-dependent calcium release from acidic organelles. J Biol Chem. 2010;285:2897–2901. doi: 10.1074/jbc.C109.081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Guo J, Zeng W, Chen Q, Lee C, Chen L, Yang Y, Cang C, Ren D, Jiang Y. Structure of the voltage-gated two-pore channel TPC1 from Arabidopsis thaliana . Nature. 2016;531:196–201. doi: 10.1038/nature16446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ross CA, Danoff SK, Schell MJ, Snyder SH, Ullrich A. Three additional inositol 1,4,5-trisphosphate receptors: molecular cloning and differential localization in brain and peripheral tissues. Proc Natl Acad Sci USA. 1992;89:4265–4269. doi: 10.1073/pnas.89.10.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mignery GA, Sudhof TC, Takei K, De Camilli P. Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature. 1989;342:192–195. doi: 10.1038/342192a0. [DOI] [PubMed] [Google Scholar]
- 202.Blondel O, Moody MM, Depaoli AM, Sharp AH, Ross CA, Swift H, Bell GI. Localization of inositol trisphosphate receptor subtype 3 to insulin and somatostatin secretory granules and regulation of expression in islets and insulinoma cells. Proc Natl Acad Sci USA. 1994;91:7777–7781. doi: 10.1073/pnas.91.16.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Miyakawa T, Maeda A, Yamazawa T, Hirose K, Kurosaki T, Iino M. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J. 1999;18:1303–1308. doi: 10.1093/emboj/18.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nerou EP, Riley AM, Potter BV, Taylor CW. Selective recognition of inositol phosphates by subtypes of the inositol trisphosphate receptor. Biochem J. 2001;355:59–69. doi: 10.1042/bj3550059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chandrasekhar R, Alzayady KJ, Wagner LE, 2nd, Yule DI. Unique regulatory properties of heterotetrameric inositol 1,4,5-trisphosphate receptors revealed by studying concatenated receptor constructs. J Biol Chem. 2016;291:4846–4860. doi: 10.1074/jbc.M115.705301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Iwai M, Michikawa T, Bosanac I, Ikura M, Mikoshiba K. Molecular basis of the isoform-specific ligand-binding affinity of inositol 1,4,5-trisphosphate receptors. J Biol Chem. 2007;282:12755–12764. doi: 10.1074/jbc.M609833200. [DOI] [PubMed] [Google Scholar]
- 207.Ramos-Franco J, Fill M, Mignery GA. Isoform-specific function of single inositol 1,4,5-trisphosphate receptor channels. Biophys J. 1998;75:834–839. doi: 10.1016/S0006-3495(98)77572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Inoue M, Lin H, Imanaga I, Ogawa K, Warashina A. InsP3 receptor type 2 and oscillatory and monophasic Ca2+ transients in rat adrenal chromaffin cells. Cell Calcium. 2004;35:59–70. doi: 10.1016/S0143-4160(03)00172-6. [DOI] [PubMed] [Google Scholar]
- 209.Tu H, Wang Z, Nosyreva E, De Smedt H, Bezprozvanny I. Functional characterization of mammalian inositol 1,4,5-trisphosphate receptor isoforms. Biophys J. 2005;88:1046–1055. doi: 10.1529/biophysj.104.049593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Cameron AM, Steiner JP, Roskams AJ, Ali SM, Ronnett GV, Snyder SH. Calcineurin associated with the inositol 1,4,5-trisphosphate receptor-FKBP12 complex modulates Ca2+ flux. Cell. 1995;83:463–472. doi: 10.1016/0092-8674(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 211.Smaili SS, Stellato KA, Burnett P, Thomas AP, Gaspers LD. Cyclosporin A inhibits inositol 1,4,5-trisphosphate-dependent Ca2+ signals by enhancing Ca2+ uptake into the endoplasmic reticulum and mitochondria. J Biol Chem. 2001;276:23329–23340. doi: 10.1074/jbc.M100989200. [DOI] [PubMed] [Google Scholar]
- 212.Carmody M, Mackrill JJ, Sorrentino V, O’Neill C. FKBP12 associates tightly with the skeletal muscle type 1 ryanodine receptor, but not with other intracellular calcium release channels. FEBS Lett. 2001;505:97–102. doi: 10.1016/S0014-5793(01)02787-9. [DOI] [PubMed] [Google Scholar]
- 213.Salanova M, Priori G, Barone V, Intravaia E, Flucher B, Ciruela F, McIlhinney RA, Parys JB, Mikoshiba K, Sorrentino V. Homer proteins and InsP(3) receptors co-localise in the longitudinal sarcoplasmic reticulum of skeletal muscle fibres. Cell Calcium. 2002;32:193–200. doi: 10.1016/S0143416002001549. [DOI] [PubMed] [Google Scholar]
- 214.Bruce JI, Shuttleworth TJ, Giovannucci DR, Yule DI. Phosphorylation of inositol 1,4,5-trisphosphate receptors in parotid acinar cells. A mechanism for the synergistic effects of cAMP on Ca2+ signaling. J Biol Chem. 2002;277:1340–1348. doi: 10.1074/jbc.M106609200. [DOI] [PubMed] [Google Scholar]
- 215.Straub SV, Giovannucci DR, Bruce JI, Yule DI. A role for phosphorylation of inositol 1,4,5-trisphosphate receptors in defining calcium signals induced by peptide agonists in pancreatic acinar cells. J Biol Chem. 2002;277:31949–31956. doi: 10.1074/jbc.M204318200. [DOI] [PubMed] [Google Scholar]
- 216.Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley PF. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–789. doi: 10.1016/S0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- 217.Bai GR, Yang LH, Huang XY, Sun FZ. Inositol 1,4,5-trisphosphate receptor type 1 phosphorylation and regulation by extracellular signal-regulated kinase. Biochem Biophys Res Commun. 2006;348:1319–1327. doi: 10.1016/j.bbrc.2006.07.208. [DOI] [PubMed] [Google Scholar]
- 218.Beliveau E, Lessard V, Guillemette G. STIM1 positively regulates the Ca2+ release activity of the inositol 1,4,5-trisphosphate receptor in bovine aortic endothelial cells. PLoS One. 2014;9:e114718. doi: 10.1371/journal.pone.0114718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Haorah J, Knipe B, Gorantla S, Zheng J, Persidsky Y. Alcohol-induced blood–brain barrier dysfunction is mediated via inositol 1,4,5-triphosphate receptor (IP3R)-gated intracellular calcium release. J Neurochem. 2007;100:324–336. doi: 10.1111/j.1471-4159.2006.04245.x. [DOI] [PubMed] [Google Scholar]
- 220.Mountian I, Manolopoulos VG, De Smedt H, Parys JB, Missiaen L, Wuytack F. Expression patterns of sarco/endoplasmic reticulum Ca(2+)-ATPase and inositol 1,4,5-trisphosphate receptor isoforms in vascular endothelial cells. Cell Calcium. 1999;25:371–380. doi: 10.1054/ceca.1999.0034. [DOI] [PubMed] [Google Scholar]
- 221.Sundivakkam PC, Kwiatek AM, Sharma TT, Minshall RD, Malik AB, Tiruppathi C. Caveolin-1 scaffold domain interacts with TRPC1 and IP3R3 to regulate Ca2+ store release-induced Ca2+ entry in endothelial cells. Am J Physiol Cell Physiol. 2009;296:C403–C413. doi: 10.1152/ajpcell.00470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mountian I, Baba-Aïssa F, Jonas JC, Humbert de Smedt, Wuytack F, Parys JB. Expression of Ca(2+) transport genes in platelets and endothelial cells in hypertension. Hypertension. 2001;37:135–141. doi: 10.1161/01.HYP.37.1.135. [DOI] [PubMed] [Google Scholar]
- 223.Grayson TH, Haddock RE, Murray TP, Wojcikiewicz RJ, Hill CE. Inositol 1,4,5-trisphosphate receptor subtypes are differentially distributed between smooth muscle and endothelial layers of rat arteries. Cell Calcium. 2004;36:447–458. doi: 10.1016/j.ceca.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 224.Isakson BE. Localized expression of an Ins(1,4,5)P3 receptor at the myoendothelial junction selectively regulates heterocellular Ca2+ communication. J Cell Sci. 2008;121:3664–3673. doi: 10.1242/jcs.037481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Beliveau E, Guillemette G. Microfilament and microtubule assembly is required for the propagation of inositol trisphosphate receptor-induced Ca2+ waves in bovine aortic endothelial cells. J Cell Biochem. 2009;106:344–352. doi: 10.1002/jcb.22011. [DOI] [PubMed] [Google Scholar]
- 226.Laflamme K, Domingue O, Guillemette BI, Guillemette G. Immunohistochemical localization of type 2 inositol 1,4,5-trisphosphate receptor to the nucleus of different mammalian cells. J Cell Biochem. 2002;85:219–228. doi: 10.1002/jcb.10124. [DOI] [PubMed] [Google Scholar]
- 227.Dragoni S, Laforenza U, Bonetti E, Lodola F, Bottino C, Berra-Romani R, Carlo Bongio G, Cinelli MP, Guerra G, Pedrazzoli P, Rosti V, Tanzi F, Moccia F. Vascular endothelial growth factor stimulates endothelial colony forming cells proliferation and tubulogenesis by inducing oscillations in intracellular Ca2+ concentration. Stem Cells. 2011;29:1898–1907. doi: 10.1002/stem.734. [DOI] [PubMed] [Google Scholar]
- 228.Yuan Q, Yang J, Santulli G, Reiken SR, Wronska A, Kim MM, Osborne BW, Lacampagne A, Yin Y, Marks AR. Maintenance of normal blood pressure is dependent on IP3R1-mediated regulation of eNOS. Proc Natl Acad Sci USA. 2016;113:8532–8537. doi: 10.1073/pnas.1608859113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci USA. 2008;105:9627–9632. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Toussaint F, Charbel C, Blanchette A, Ledoux J. CaMKII regulates intracellular Ca(2)(+) dynamics in native endothelial cells. Cell Calcium. 2015;58:275–285. doi: 10.1016/j.ceca.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 231.Raqeeb A, Sheng J, Ao N, Braun AP. Purinergic P2Y2 receptors mediate rapid Ca(2+) mobilization, membrane hyperpolarization and nitric oxide production in human vascular endothelial cells. Cell Calcium. 2011;49:240–248. doi: 10.1016/j.ceca.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 232.Wang S, Iring A, Strilic B, Albarran Juarez J, Kaur H, Troidl K, Tonack S, Burbiel JC, Muller CE, Fleming I, Lundberg JO, Wettschureck N, Offermanns S. P2Y(2) and Gq/G(1)(1) control blood pressure by mediating endothelial mechanotransduction. J Clin Investig. 2015;125:3077–3086. doi: 10.1172/JCI81067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gudi S, Nolan JP, Frangos JA. Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition. Proc Natl Acad Sci USA. 1998;95:2515–2519. doi: 10.1073/pnas.95.5.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.de la Paz NG, Melchior B, Shayo FY, Frangos JA. Heparan sulfates mediate the interaction between platelet endothelial cell adhesion molecule-1 (PECAM-1) and the Galphaq/11 subunits of heterotrimeric G proteins. J Biol Chem. 2014;289:7413–7424. doi: 10.1074/jbc.M113.542514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Marques FZ, Campain AE, Yang YH, Morris BJ. Meta-analysis of genome-wide gene expression differences in onset and maintenance phases of genetic hypertension. Hypertension. 2010;56:319–324. doi: 10.1161/HYPERTENSIONAHA.110.155366. [DOI] [PubMed] [Google Scholar]
- 236.Edwards JS, Atlas SR, Wilson SM, Cooper CF, Luo L, Stidley CA. Integrated statistical and pathway approach to next-generation sequencing analysis: a family-based study of hypertension. BMC Proc. 2014;8:S104. doi: 10.1186/1753-6561-8-S1-S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Aschner JL, Lennon JM, Fenton JW, 2nd, Aschner M, Malik AB. Enzymatic activity is necessary for thrombin-mediated increase in endothelial permeability. Am J Physiol. 1990;259:L270–L275. doi: 10.1152/ajplung.1990.259.4.L270. [DOI] [PubMed] [Google Scholar]
- 238.Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-V. [DOI] [PubMed] [Google Scholar]
- 239.Ash AS, Schild HO. Receptors mediating some actions of histamine. Br J Pharmacol Chemother. 1966;27:427–439. doi: 10.1111/j.1476-5381.1966.tb01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hill SJ. Histamine receptors branch out. Nature. 1987;327:104–105. doi: 10.1038/327104a0. [DOI] [PubMed] [Google Scholar]
- 241.Burgess GM, Godfrey PP, McKinney JS, Berridge MJ, Irvine RF, Putney JW., Jr The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984;309:63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- 242.Patel S, Joseph SK, Thomas AP. Molecular properties of inositol 1,4,5-trisphosphate receptors. Cell Calcium. 1999;25:247–264. doi: 10.1054/ceca.1999.0021. [DOI] [PubMed] [Google Scholar]
- 243.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 244.Putney JW., Jr TRP, inositol 1,4,5-trisphosphate receptors, and capacitative calcium entry. Proc Natl Acad Sci USA. 1999;96:14669–14671. doi: 10.1073/pnas.96.26.14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Bird GS, Hwang SY, Smyth JT, Fukushima M, Boyles RR, Putney JW., Jr STIM1 is a calcium sensor specialized for digital signaling. Curr Biol. 2009;19:1724–1729. doi: 10.1016/j.cub.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zheng L, Stathopulos PB, Schindl R, Li GY, Romanin C, Ikura M. Auto-inhibitory role of the EF-SAM domain of STIM proteins in store-operated calcium entry. Proc Natl Acad Sci USA. 2011;108:1337–1342. doi: 10.1073/pnas.1015125108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci USA. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Muik M, Frischauf I, Derler I, Fahrner M, Bergsmann J, Eder P, Schindl R, Hesch C, Polzinger B, Fritsch R, Kahr H, Madl J, Gruber H, Groschner K, et al. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem. 2008;283:8014–8022. doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]
- 251.Alicia S, Angélica Z, Carlos S, Alfonso S, Vaca L. STIM1 converts TRPC1 from a receptor-operated to a store-operated channel: moving TRPC1 in and out of lipid rafts. Cell Calcium. 2008;44:479–491. doi: 10.1016/j.ceca.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 252.Cheng KT, Liu X, Ong HL, Ambudkar IS. Functional requirement for Orai1 in store-operated TRPC1-STIM1 channels. J Biol Chem. 2008;283:12935–12940. doi: 10.1074/jbc.C800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kim MS, Zeng W, Yuan JP, Shin DM, Worley PF, Muallem S. Native store-operated Ca2+ influx requires the channel function of Orai1 and TRPC1. J Biol Chem. 2009;284:9733–9741. doi: 10.1074/jbc.M808097200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 256.Savage SR, Bretz CA, Penn JS. RNA-Seq reveals a role for NFAT-signaling in human retinal microvascular endothelial cells treated with TNFalpha. PLoS One. 2015;10:e0116941. doi: 10.1371/journal.pone.0116941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Boulay G, Zhu X, Peyton M, Jiang M, Hurst R, Stefani E, Birnbaumer L. Cloning and expression of a novel mammalian homolog of Drosophila transient receptor potential (Trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. J Biol Chem. 1997;272:29672–29680. doi: 10.1074/jbc.272.47.29672. [DOI] [PubMed] [Google Scholar]
- 258.Zitt C, Obukhov AG, Strubing C, Zobel A, Kalkbrenner F, Luckhoff A, Schultz G. Expression of TRPC3 in Chinese hamster ovary cells results in calcium-activated cation currents not related to store depletion. J Cell Biol. 1997;138:1333–1341. doi: 10.1083/jcb.138.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 260.Okada T, Inoue R, Yamazaki K, Maeda A, Kurosaki T, Yamakuni T, Tanaka I, Shimizu S, Ikenaka K, Imoto K, Mori Y. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca(2+)-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J Biol Chem. 1999;274:27359–27370. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- 261.Weber EW, Han F, Tauseef M, Birnbaumer L, Mehta D, Muller WA. TRPC6 is the endothelial calcium channel that regulates leukocyte transendothelial migration during the inflammatory response. J Exp Med. 2015;212:1883–1899. doi: 10.1084/jem.20150353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ross D, Joyner WL. Resting distribution and stimulated translocation of protein kinase C isoforms alpha, epsilon and zeta in response to bradykinin and TNF in human endothelial cells. Endothelium. 1997;5:321–332. doi: 10.3109/10623329709052596. [DOI] [PubMed] [Google Scholar]
- 263.Hempel A, Maasch C, Heintze U, Lindschau C, Dietz R, Luft FC, Haller H. High glucose concentrations increase endothelial cell permeability via activation of protein kinase C alpha. Circ Res. 1997;81:363–371. doi: 10.1161/01.RES.81.3.363. [DOI] [PubMed] [Google Scholar]
- 264.Ahmmed GU, Mehta D, Vogel S, Holinstat M, Paria BC, Tiruppathi C, Malik AB. Protein kinase Calpha phosphorylates the TRPC1 channel and regulates store-operated Ca2+ entry in endothelial cells. J Biol Chem. 2004;279:20941–20949. doi: 10.1074/jbc.M313975200. [DOI] [PubMed] [Google Scholar]
- 265.Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47:859–866. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- 266.Hui Y, Cheng Y, Smalera I, Jian W, Goldhahn L, Fitzgerald GA, Funk CD. Directed vascular expression of human cysteinyl leukotriene 2 receptor modulates endothelial permeability and systemic blood pressure. Circulation. 2004;110:3360–3366. doi: 10.1161/01.CIR.0000147775.50954.AA. [DOI] [PubMed] [Google Scholar]
- 267.Moos MP, Mewburn JD, Kan FW, Ishii S, Abe M, Sakimura K, Noguchi K, Shimizu T, Funk CD. Cysteinyl leukotriene 2 receptor-mediated vascular permeability via transendothelial vesicle transport. FASEB J. 2008;22:4352–4362. doi: 10.1096/fj.08-113274. [DOI] [PubMed] [Google Scholar]
- 268.Ludtke SJ, Tran TP, Ngo QT, Moiseenkova-Bell VY, Chiu W, Serysheva II. Flexible architecture of IP3R1 by Cryo-EM. Structure. 2011;19:1192–1199. doi: 10.1016/j.str.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Bosanac I, Alattia JR, Mal TK, Chan J, Talarico S, Tong FK, Tong KI, Yoshikawa F, Furuichi T, Iwai M, Michikawa T, Mikoshiba K, Ikura M. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature. 2002;420:696–700. doi: 10.1038/nature01268. [DOI] [PubMed] [Google Scholar]
- 270.Bosanac I, Yamazaki H, Matsu-Ura T, Michikawa T, Mikoshiba K, Ikura M. Crystal structure of the ligand binding suppressor domain of type 1 inositol 1,4,5-trisphosphate receptor. Mol Cell. 2005;17:193–203. doi: 10.1016/j.molcel.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 271.Yamazaki H, Chan J, Ikura M, Michikawa T, Mikoshiba K. Tyr-167/Trp-168 in type 1/3 inositol 1,4,5-trisphosphate receptor mediates functional coupling between ligand binding and channel opening. J Biol Chem. 2010;285:36081–36091. doi: 10.1074/jbc.M110.140129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Uchida K, Miyauchi H, Furuichi T, Michikawa T, Mikoshiba K. Critical regions for activation gating of the inositol 1,4,5-trisphosphate receptor. J Biol Chem. 2003;278:16551–16560. doi: 10.1074/jbc.M300646200. [DOI] [PubMed] [Google Scholar]
- 273.Schug ZT, Joseph SK. The role of the S4–S5 linker and C-terminal tail in inositol 1,4,5-trisphosphate receptor function. J Biol Chem. 2006;281:24431–24440. doi: 10.1074/jbc.M604190200. [DOI] [PubMed] [Google Scholar]
- 274.Boehning D, Joseph SK. Direct association of ligand-binding and pore domains in homo- and heterotetrameric inositol 1,4,5-trisphosphate receptors. EMBO J. 2000;19:5450–5459. doi: 10.1093/emboj/19.20.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 276.Rose HJ, Dargan S, Shuai J, Parker I. ‘Trigger’ events precede calcium puffs in Xenopus oocytes. Biophys J. 2006;91:4024–4032. doi: 10.1529/biophysj.106.088872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Parker I, Choi J, Yao Y. Elementary events of InsP3-induced Ca2+ liberation in Xenopus oocytes: hot spots, puffs and blips. Cell Calcium. 1996;20:105–121. doi: 10.1016/S0143-4160(96)90100-1. [DOI] [PubMed] [Google Scholar]
- 278.Huser J, Blatter LA. Elementary events of agonist-induced Ca2+ release in vascular endothelial cells. Am J Physiol. 1997;273:C1775–C1782. doi: 10.1152/ajpcell.1997.273.5.C1775. [DOI] [PubMed] [Google Scholar]
- 279.Burdyga T, Shmygol A, Eisner DA, Wray S. A new technique for simultaneous and in situ measurements of Ca2+ signals in arteriolar smooth muscle and endothelial cells. Cell Calcium. 2003;34:27–33. doi: 10.1016/S0143-4160(03)00019-8. [DOI] [PubMed] [Google Scholar]
- 280.Duza T, Sarelius IH. Localized transient increases in endothelial cell Ca2+ in arterioles in situ: implications for coordination of vascular function. Am J Physiol Heart Circ Physiol. 2004;286:H2322–H2331. doi: 10.1152/ajpheart.00006.2004. [DOI] [PubMed] [Google Scholar]
- 281.Kansui Y, Garland CJ, Dora KA. Enhanced spontaneous Ca2+ events in endothelial cells reflect signalling through myoendothelial gap junctions in pressurized mesenteric arteries. Cell Calcium. 2008;44:135–146. doi: 10.1016/j.ceca.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Francis M, Waldrup JR, Qian X, Solodushko V, Meriwether J, Taylor MS. Functional tuning of intrinsic endothelial Ca2+ dynamics in swine coronary arteries. Circ Res. 2016;118:1078–1090. doi: 10.1161/CIRCRESAHA.115.308141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Wilson C, Saunter CD, Girkin JM, McCarron JG. Pressure-dependent regulation of Ca2+ signalling in the vascular endothelium. J Physiol. 2015;593:5231–5253. doi: 10.1113/JP271157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Diambra L, Marchant JS. Inositol (1,4,5)-trisphosphate receptor microarchitecture shapes Ca2+ puff kinetics. Biophys J. 2011;100:822–831. doi: 10.1016/j.bpj.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Bootman MD, Berridge MJ, Lipp P. Cooking with calcium: the recipes for composing global signals from elementary events. Cell. 1997;91:367–373. doi: 10.1016/S0092-8674(00)80420-1. [DOI] [PubMed] [Google Scholar]
- 286.Dargan SL, Parker I. Buffer kinetics shape the spatiotemporal patterns of IP3-evoked Ca2+ signals. J Physiol. 2003;553:775–788. doi: 10.1113/jphysiol.2003.054247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Sun XP, Callamaras N, Marchant JS, Parker I. A continuum of InsP3-mediated elementary Ca2+ signalling events in Xenopus oocytes. J Physiol. 1998;509(Pt 1):67–80. doi: 10.1111/j.1469-7793.1998.067bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Smith IF, Parker I. Imaging the quantal substructure of single IP3R channel activity during Ca2+ puffs in intact mammalian cells. Proc Natl Acad Sci USA. 2009;106:6404–6409. doi: 10.1073/pnas.0810799106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Smith IF, Wiltgen SM, Parker I. Localization of puff sites adjacent to the plasma membrane: functional and spatial characterization of Ca2+ signaling in SH-SY5Y cells utilizing membrane-permeant caged IP3. Cell Calcium. 2009;45:65–76. doi: 10.1016/j.ceca.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Marchant J, Callamaras N, Parker I. Initiation of IP(3)-mediated Ca(2+) waves in Xenopus oocytes. EMBO J. 1999;18:5285–5299. doi: 10.1093/emboj/18.19.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Falcke M. On the role of stochastic channel behavior in intracellular Ca2+ dynamics. Biophys J. 2003;84:42–56. doi: 10.1016/S0006-3495(03)74831-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Skupin A, Kettenmann H, Winkler U, Wartenberg M, Sauer H, Tovey SC, Taylor CW, Falcke M. How does intracellular Ca2+ oscillate: by chance or by the clock? Biophys J. 2008;94:2404–2411. doi: 10.1529/biophysj.107.119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Shuai J, Pearson JE, Foskett JK, Mak DO, Parker I. A kinetic model of single and clustered IP3 receptors in the absence of Ca2+ feedback. Biophys J. 2007;93:1151–1162. doi: 10.1529/biophysj.107.108795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ruckl M, Parker I, Marchant JS, Nagaiah C, Johenning FW, Rudiger S. Modulation of elementary calcium release mediates a transition from puffs to waves in an IP3R cluster model. PLoS Comput Biol. 2015;11:e1003965. doi: 10.1371/journal.pcbi.1003965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Gonzales AL, Yang Y, Sullivan MN, Sanders L, Dabertrand F, Hill-Eubanks DC, Nelson MT, Earley S. A PLCgamma1-dependent, force-sensitive signaling network in the myogenic constriction of cerebral arteries. Sci Signal. 2014;7:ra49. doi: 10.1126/scisignal.2004732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Tai YT, Wu CC, Wu GJ, Chang HC, Chen TG, Chen RM, Chen TL. Study of propofol in bovine aortic endothelium: I. Inhibitory effect on bradykinin-induced intracellular calcium immobilization. Acta Anaesthesiol Sin. 2000;38:181–186. [PubMed] [Google Scholar]
- 297.Cseresnyés Z, Schneider MF. Peripheral hot spots for local Ca2+ release after single action potentials in sympathetic ganglion neurons. Biophys J. 2004;86:163–181. doi: 10.1016/S0006-3495(04)74094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Tasaka K, Mio M, Fujisawa K, Aoki I. Role of microtubules on Ca2+ release from the endoplasmic reticulum and associated histamine release from rat peritoneal mast cells. Biochem Pharmacol. 1991;41:1031–1037. doi: 10.1016/0006-2952(91)90211-M. [DOI] [PubMed] [Google Scholar]
- 299.Isshiki M, Ando J, Korenaga R, Kogo H, Fujimoto T, Fujita T, Kamiya A. Endothelial Ca2+ waves preferentially originate at specific loci in caveolin-rich cell edges. Proc Natl Acad Sci USA. 1998;95:5009–5014. doi: 10.1073/pnas.95.9.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Mitsuyama F, Sawai T. The redistribution of Ca2+ stores with inositol 1,4,5-trisphosphate receptor to the cleavage furrow in a microtubule-dependent manner. Int J Dev Biol. 2001;45:861–868. [PubMed] [Google Scholar]
- 301.Vermassen E, Van Acker K, Annaert WG, Himpens B, Callewaert G, Missiaen L, De Smedt H, Parys JB. Microtubule-dependent redistribution of the type-1 inositol 1,4,5-trisphosphate receptor in A7r5 smooth muscle cells. J Cell Sci. 2003;116:1269–1277. doi: 10.1242/jcs.00354. [DOI] [PubMed] [Google Scholar]
- 302.Aguilar-Maldonado B, Gomez-Viquez L, Garcia L, Del Angel RM, Arias-Montano JA, Guerrero-Hernandez A. Histamine potentiates IP(3)-mediated Ca(2+) release via thapsigargin-sensitive Ca(2+) pumps. Cell Signal. 2003;15:689–697. doi: 10.1016/S0898-6568(03)00012-3. [DOI] [PubMed] [Google Scholar]
- 303.Redondo PC, Harper AG, Sage SO, Rosado JA. Dual role of tubulin-cytoskeleton in store-operated calcium entry in human platelets. Cell Signal. 2007;19:2147–2154. doi: 10.1016/j.cellsig.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 304.Takei K, Shin RM, Inoue T, Kato K, Mikoshiba K. Regulation of nerve growth mediated by inositol 1,4,5-trisphosphate receptors in growth cones. Science. 1998;282:1705–1708. doi: 10.1126/science.282.5394.1705. [DOI] [PubMed] [Google Scholar]
- 305.Zhang XF, Forscher P. Rac1 modulates stimulus-evoked Ca(2+) release in neuronal growth cones via parallel effects on microtubule/endoplasmic reticulum dynamics and reactive oxygen species production. Mol Biol Cell. 2009;20:3700–3712. doi: 10.1091/mbc.E08-07-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Aihara Y, Inoue T, Tashiro T, Okamoto K, Komiya Y, Mikoshiba K. Movement of endoplasmic reticulum in the living axon is distinct from other membranous vesicles in its rate, form, and sensitivity to microtubule inhibitors. J Neurosci Res. 2001;65:236–246. doi: 10.1002/jnr.1147. [DOI] [PubMed] [Google Scholar]
- 307.Ferreri-Jacobia M, Mak DO, Foskett JK. Translational mobility of the type 3 inositol 1,4,5-trisphosphate receptor Ca2+ release channel in endoplasmic reticulum membrane. J Biol Chem. 2005;280:3824–3831. doi: 10.1074/jbc.M409462200. [DOI] [PubMed] [Google Scholar]
- 308.Graier WF, Paltauf-Doburzynska J, Hill BJ, Fleischhacker E, Hoebel BG, Kostner GM, Sturek M. Submaximal stimulation of porcine endothelial cells causes focal Ca2+ elevation beneath the cell membrane. J Physiol. 1998;506(Pt 1):109–125. doi: 10.1111/j.1469-7793.1998.109bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Ribeiro CM, Reece J, Putney JW., Jr Role of the cytoskeleton in calcium signaling in NIH 3T3 cells. An intact cytoskeleton is required for agonist-induced [Ca2+]i signaling, but not for capacitative calcium entry. J Biol Chem. 1997;272:26555–26561. doi: 10.1074/jbc.272.42.26555. [DOI] [PubMed] [Google Scholar]
- 310.Fogarty KE, Kidd JF, Turner A, Skepper JN, Carmichael J, Thorn P. Microtubules regulate local Ca2+ spiking in secretory epithelial cells. J Biol Chem. 2000;275:22487–22494. doi: 10.1074/jbc.M909402199. [DOI] [PubMed] [Google Scholar]
- 311.Su LK, Qi Y. Characterization of human MAPRE genes and their proteins. Genomics. 2001;71:142–149. doi: 10.1006/geno.2000.6428. [DOI] [PubMed] [Google Scholar]
- 312.Bieling P, Laan L, Schek H, Munteanu EL, Sandblad L, Dogterom M, Brunner D, Surrey T. Reconstitution of a microtubule plus-end tracking system in vitro. Nature. 2007;450:1100–1105. doi: 10.1038/nature06386. [DOI] [PubMed] [Google Scholar]
- 313.Maurer SP, Bieling P, Cope J, Hoenger A, Surrey T. GTPgammaS microtubules mimic the growing microtubule end structure recognized by end-binding proteins (EBs) Proc Natl Acad Sci USA. 2011;108:3988–3993. doi: 10.1073/pnas.1014758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Duellberg C, Cade NI, Holmes D, Surrey T. The size of the EB cap determines instantaneous microtubule stability. Elife. 2016;5:e13470. doi: 10.7554/eLife.13470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Komarova Y, Lansbergen G, Galjart N, Grosveld F, Borisy GG, Akhmanova A. EB1 and EB3 control CLIP dissociation from the ends of growing microtubules. Mol Biol Cell. 2005;16:5334–5345. doi: 10.1091/mbc.E05-07-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Dixit R, Barnett B, Lazarus JE, Tokito M, Goldman YE, Holzbaur EL. Microtubule plus-end tracking by CLIP-170 requires EB1. Proc Natl Acad Sci USA. 2009;106:492–497. doi: 10.1073/pnas.0807614106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Honnappa S, Gouveia SM, Weisbrich A, Damberger FF, Bhavesh NS, Jawhari H, Grigoriev I, van Rijssel FJ, Buey RM, Lawera A, Jelesarov I, Winkler FK, Wuthrich K, Akhmanova A, et al. An EB1-binding motif acts as a microtubule tip localization signal. Cell. 2009;138:366–376. doi: 10.1016/j.cell.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 318.Slep KC, Rogers SL, Elliott SL, Ohkura H, Kolodziej PA, Vale RD. Structural determinants for EB1-mediated recruitment of APC and spectraplakins to the microtubule plus end. J Cell Biol. 2005;168:587–598. doi: 10.1083/jcb.200410114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Hayashi I, Wilde A, Mal TK, Ikura M. Structural basis for the activation of microtubule assembly by the EB1 and p150Glued complex. Mol Cell. 2005;19:449–460. doi: 10.1016/j.molcel.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 320.Slep KC, Vale RD. Structural basis of microtubule plus end tracking by XMAP215, CLIP-170, and EB1. Mol Cell. 2007;27:976–991. doi: 10.1016/j.molcel.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]