Abstract
Introduction
Loss of renal function is associated with high mortality from cardiovascular disease (CVD). Patients with chronic kidney disease (CKD) have altered circulating adipokine and nonesterified fatty acid concentrations and insulin resistance, which are features of disturbed adipose tissue metabolism. Because dysfunctional adipose tissue contributes to the development of CVD, we hypothesize that adipose tissue dysfunctionality in patients with CKD could explain, at least in part, their high rates of CVD. Therefore we characterized adipose tissue from patients with CKD, in comparison to healthy controls, to search for signs of dysfunctionality.
Methods
Biopsy samples of subcutaneous adipose tissue from 16 CKD patients and 11 healthy controls were analyzed for inflammation, fibrosis, and adipocyte size. Protein composition was assessed using 2-dimensional gel proteomics combined with multivariate analysis.
Results
Adipose tissue of CKD patients contained significantly more CD68-positive cells, but collagen content did not differ. Adipocyte size was significantly smaller in CKD patients. Proteomic analysis of adipose tissue revealed significant differences in the expression of certain proteins between the groups. Proteins whose expression differed the most were α-1-microglobulin/bikunin precursor (AMBP, higher in CKD) and vimentin (lower in CKD). Vimentin is a lipid droplet−associated protein, and changes in its expression may impair fatty acid storage/mobilization in adipose tissue, whereas high levels of AMBP may reflect oxidative stress.
Discussion
These findings demonstrate that adipose tissue of CKD patients shows signs of inflammation and disturbed functionality, thus potentially contributing to the unfavorable metabolic profile and increased risk of CVD in these patients.
Keywords: adipose tissue, alpha-1-microglobulin/bikunin precursor, cardiovascular disease, chronic kidney disease, proteomics, vimentin
Chronic kidney disease (CKD) is a global health problem with increasing prevalence and poor outcome,1 in which cardiovascular disease (CVD) accounts for up to 50% of all-cause mortality.2 The reason for the high CVD risk is unclear, but cannot be explained simply by a decrease in filtering capacity of the kidneys. Indeed patients with a functioning kidney transplant still maintain higher CVD risk than the general population.3 Thus the cause of the metabolic dysregulation observed in CKD is complex, but a role for adipose tissue has been suggested.
Adipose tissue buffers circulating nonesterified fatty acid (NEFA) concentrations, with a failure to do so leading to the development of an atherogenic dyslipidemia4 and lipotoxicity, which results in deterioration of tissue function and impaired lipid and glucose utilization.5, 6 Adipocyte hypertrophy (increased cell size) is often followed by development of tissue fibrosis, hypoxia, and secretion of proinflammatory cytokines (such as tumor necrosis factor [TNF] or interleukin [IL]−6) triggering inflammatory cell recruitment and deterioration of insulin signaling,7 making adipose tissue dysfunctional. Malfunctioning adipose tissue contributes to the development of CVD locally and systemically. Local effects relate mainly to the direct impact on the vessel wall of fatty acids and adipokines released by perivascular adipose tissue, promoting expression of adhesion molecules, inflammatory cytokines, and coagulation factors by endothelial cells,8, 9 and inducing proliferation and migration of vascular smooth muscle cells (VSMC) and macrophage infiltration10, 11 and inflamed visceral fat can directly promote atherosclerotic lesion development in the underlying vessel.12 Systemic effects are attributed to both subcutaneous and visceral depots. Because the subcutaneous abdominal depot is the major determinant of circulating NEFA concentrations,13 its perturbed function promotes development of a proatherogenic lipoprotein profile. Furthermore, a shift toward secretion of more pro-inflammatory adipokines by subcutaneous and/or visceral adipose tissue promotes VSMC migration, proliferation, and osteogenic differentiation; induces a pro-thrombotic phenotype in vascular endothelial cells; and upregulates mediators of vascular inflammation, such as TNF, IL-2, IL-6, and CCL2.14, 15
Malfunctional adipose tissue is believed to promote CVD via the mechanisms described above. Although it is unknown whether these pathways are important contributors to the high rates of CVD in CKD patients, the hypothesis has been proposed.16, 17, 18, 19, 20, 21 The supporting data include the observation that CKD patients have elevated NEFA concentrations, an independent predictor of all-cause and cardiovascular death.21 Furthermore, CKD patients have perturbed circulating adipokine concentrations18, 20 and increased systemic inflammation that correlates with fat mass.16 Indeed, visceral fat mass in peritoneal dialysis patients is an independent predictor of vessel dysfunction and therefore a risk factor for CVD.17 Unfavorable changes in markers reflecting oxidative stress, inflammation, and impaired vessel function have been reported in subcutaneous adipose tissue of patients with kidney failure.22, 23 Furthermore, a uremic milieu increases basal lipolysis rates and decreases expression of the lipid droplet−associated protein perilipin in primary adipocyte cultures,19 which could lead to increased fatty acid release. Finally, adipose lipoprotein lipase activity is decreased in CKD patients, leading to the formation of lipoproteins with a proatherogenic lipid composition.24 Thus a range of studies have suggested that uremic adipose tissue may be dysfunctional, which could position adipose tissue as a contributing factor to the development of CVD in CKD patients. However a beneficial role of adipose tissue in CKD has also been suggested,25 with body mass index (BMI) being associated with better outcome in CKD patients,25, 26 but this was true only when accompanied by normal or high muscle mass, indicating that any beneficial role of obesity is likely attributable to lean mass rather than to adipose tissue.
Many of the studies that indicate a role for adipose tissue in the development of CVD associated with CKD are based on systemic analyses, whereas direct analysis of adipose tissue, especially protein analysis, is largely lacking. Therefore we describe a proteomic approach to characterize the protein composition of subcutaneous adipose tissue in CKD patients, with the aim of better understanding whether adipose tissue is dysfunctional and could contribute to the increased risk of CVD in patients with kidney failure.
Materials and Methods
Subjects
A total of 16 subjects with stage 5 CKD (recipients of kidney transplants) and 11 healthy individuals (kidney donors) were enrolled in the study performed at Karolinska University Hospital (Huddinge). The number of subjects included in the different analyses varied and is indicated accordingly (Table 1). Inclusion criteria were age 18 to 75 years and BMI 18 to 30 kg/m2. Exclusion criteria were hypersensitivity for xylocaine, treated diabetes, and ongoing treatment with heavy immunosuppressives (such as cortisone). Of the 16 CKD patients, prior to kidney transplantation; 7 had received hemodialysis (3.5–4 hours, 3–4 times per week, urea reduction rate >80%); 3 had received peritoneal dialysis, 2 had received both types of dialysis; and 4 had no previous dialysis treatment. The study was approved by the Stockholm Regional Ethics Committee and conducted according to the principles of the Declaration of Helsinki. All patients gave their written consent.
Table 1.
Characteristics of the study subjects
| Parameter | Control | CKD |
|---|---|---|
| Number (M/F) | 11 (3/8) | 16 (9/7) |
| Age (yr) | 45.6 ± 8.6 | 38.8 ± 14.8 |
| BMI (kg/m2) | 26.2 ± 2.9 | 22.7 ± 2.1d |
| GFR (ml/min) | 133 ± 32 | 12 ± 7d |
| CRP (mg/l) | 1.0 ± 0.9 | 7.5 ± 17.9 |
| Glucose (mmol/l)a | 5.2 ± 0.8 | 5.6 ± 1.0 |
| Na (mmol/l) | 141 ± 1 | 139 ± 4 |
| K (mmol/l) | 3.9 ± 0.2 | 4.8 ± 0.7d |
| Creatinine (μmol/l) | 63 ± 21 | 742 ± 284d |
| Urea (mmol/l)b | 4.6 ± 1.2 | 24.4 ± 9.4d |
| Bilirubin (μmol/l)b | 7 ± 2 | 8 ± 2 |
| ASAT (μkat/l) | 0.37 ± 0.07 | 0.33 ± 0.11 |
| Leukocytes (×109/l) | 6.3 ± 1.7 | 7.6 ± 2.4 |
| Hemoglobin (g/l) | 139 ± 10 | 113 ± 20d |
| Triglyceride (mmol/l)c | N/A | 1.7 ± 0.8 |
| Cholesterol (mmol/l)c | N/A | 4.8 ± 1.1 |
| HDL (mmol/l)c | N/A | 1.5 ± 0.6 |
ASAT, aspartate aminotransferase; BMI, body mass index; CKD, chronic kidney disease; CRP, C-reactive protein (measured with a high-sensitivity method); GFR, glomerular filtration rate (for males GFR = (1.23 × [140 − age] × weight)/serum creatinine; for females GFR = (1.04 × [140 − age] × weight)/serum creatinine]; HDL, high-density lipoprotein cholesterol; N/A, data not available.
Values are given as mean ± SD.
Data available from 14 CKD subjects.
Data available from 12 CKD subjects.
Data available from 15 CKD subjects.
P ≤ 0.01 for differences between groups (Student t test).
Subcutaneous adipose tissue biopsy samples from the lower abdominal area were collected during kidney transplantation surgery from both kidney recipients and donors. One part of the tissue was fixed and embedded in paraffin for histological analysis, and one part was placed in AllProtect Reagent (Qiagen, Hilden, Germany) and stored at −20°C for subsequent proteomic analysis. Fasting blood samples were collected 1 day before the operation and analyzed using accredited methods in the clinical chemistry laboratory at Karolinska University Hospital.
A separate group (n = 9, 1 male and 8 females, aged 48.3 ± 7.5 years; 2 patients had diabetes and all had hypertension) of morbidly obese (BMI 41.2 ± 4.4 kg/m2) CKD patients (CKD stage 3–5) admitted to King’s College Hospital, London, UK, for bariatric surgery were enrolled in the study. Subcutaneous adipose tissue biopsies from the abdominal region were collected during surgery and stored in AllProtect Reagent (Qiagen) at −20°C. These biopsy samples were used for proteomic analysis only. The study was approved by the British National Health Service Research Ethics Committee (NHS REC), and the use of biopsy samples in Sweden was approved by the Stockholm Regional Ethics Committee. All patients gave their written consent.
Histological Analyses
Paraffin sections (5 μm) were used for histological analyses. For analysis of inflammation, a mouse CD68 (NCL-CD68-KP1, 1:200, Leica Biosystems Newcastle Ltd., Newcastle Upon Tyne, UK) antibody was used. Sections were counterstained with Harris hematoxylin (Histolab, Gothenburg, Sweden). Numbers of positive cells were counted in 1 entire section of each adipose tissue biopsy sample on 2 occasions by 2 independent observers who were blinded as to the sample status. These numbers were normalized for the total tissue area, measured using the ImageJ application (ImageJ 1.48 [imagej.nih.gov/ij]). For evaluation of fibrosis, adipose tissue sections were stained with saturated picric acid containing 0.1% picrosirius red (Direct Red 80, Sigma-Aldrich, St. Louis, MO), scanned (Hamamatsu NanoZoomer-XR Digital slide scanner C12000), and visualized using Nano Zoomer Digital Pathology viewer software (U12388-01; NDP.view2 Viewing). Representative picrosirius red stainings of a control individual and a CKD patient are shown in Supplementary Figure S1. Collagen content was calculated as the percentage of the total section area. Adipocyte size was measured using the ImageJ application according to the method described previously.27 Representative images of ImageJ-transformed pictures from a control individual and a CKD patient used for size quantification are shown in Supplementary Figure S2.
Proteomics
Protein extraction, 2-dimensional electrophoresis, scanning, analysis, and protein identification with liquid chromatography−tandem mass spectrometry (LC-MS/MS) were performed as described previously.28 Proteins were extracted from approximately 200 mg of adipose tissue. For analytical gels, 5 μg of CyDye-labeled (GE Healthcare, Little Chalfont, UK) total protein was loaded on each gel. The preparative gel was loaded with 1 mg of internal standard (a mixture of adipose tissue protein extracts from all individuals pooled together) and stained with Pierce Silver Stain for Mass Spectrometry (Thermo Fisher Scientific, Waltham, MA). Mass spectrometry analysis was performed by the Clinical Proteomics Mass Spectrometry facility, Karolinska Institutet/Karolinska University Hospital/Science for Life Laboratory.
Western Blot
Protein samples (20 μg) were separated by 10% sodium dodecyl sulfate−polyacrylamide gel electrophoresis under reducing conditions and transferred to a polyvinylidene difluoride membrane (Bio-Rad, Hemel Hempstead, UK). After incubation with 5% nonfat milk in a mixture of Tris-buffered saline and Tween 20, the membrane was incubated with anti-vimentin (SC-66002, 1:500, Santa Cruz Biotechnology, Santa Cruz, CA) or anti−α-1-microglobulin/bikunin precursor (anti-AMBP) (SC-81948, 1:400, Santa Cruz Biotechnology, Santa Cruz, CA) antibody at 4°C for 12 hours. β-Actin was used as a loading control (1:250, Sigma-Aldrich). Membranes were then incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit antibodies (1:50000, Bio-Rad) and developed with ECL Prime (GE Healthcare, Little Chalfont, UK). Detection was performed with the x-ray film Agfa CP-BU new (Agfa Healthcare, Mortsel, Belgium).
Statistical Analysis
Comparisons between CKD patients and healthy controls were performed using a Student t test for continuous variables or Pearson χ2 test with Yates continuity correction for gender distribution. Differences in CD68-positive cell count, collagen content, and adipocyte cell size between groups were compared using a Mann−Whitney U test. Comparison of protein spot volumes between controls, lean CKD patients, and obese CKD patients was performed using analysis of variance and Fisher least significant difference post hoc tests. Analysis of covariance was used to test for differences in adipocyte size between patients and controls with BMI as a covariate. The analysis of the proteomic data (202 distinct protein spots) was performed by multivariate analysis (orthogonal projections to latent structures discriminant analysis [OPLS-DA]) as described previously.28
Results
We obtained subcutaneous abdominal adipose tissue from 16 patients with kidney failure (stage 5 CKD) and 11 healthy individuals (kidney donors). The CKD patients had significantly lower BMI than the control group, and several blood markers reflecting kidney failure and related complications differed significantly between the groups; however, age, gender distribution, and glucose concentrations were not different (Table 1). Concentrations of triglyceride, total cholesterol, and high-density lipoprotein cholesterol in the CKD patients were within normal ranges (Table 1).
Adipose Tissue of CKD Patients Contains More CD68-Positive Cells and Has Smaller Adipocytes Than Healthy Controls
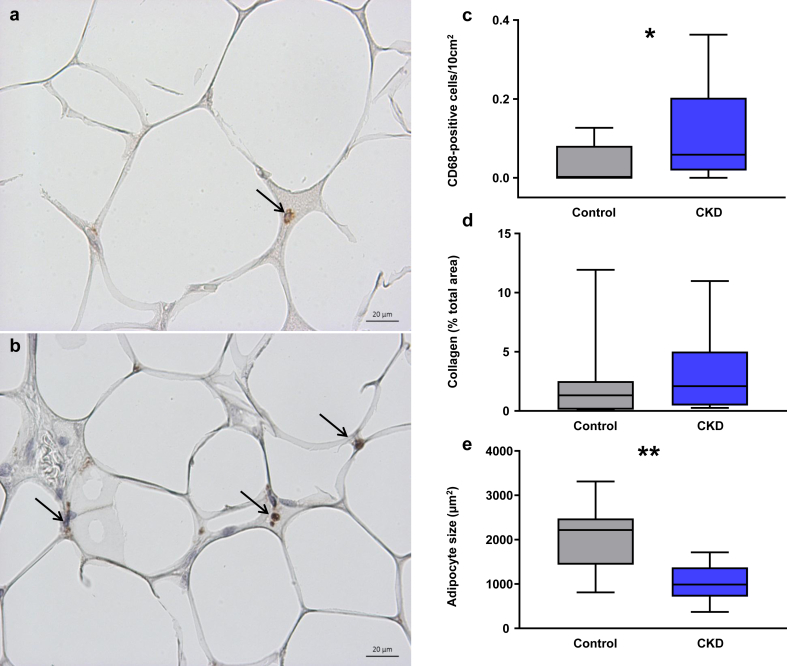
Patients with CKD had significantly more CD68-positive cells in their subcutaneous adipose tissue than controls (Figure 1a−c). To assess whether adipose tissue in patients with CKD was fibrotic, we stained for collagen using picrosirius red (Supplementary Figure S1), but quantification of this staining revealed no significant difference between the groups (Figure 1d). Mean adipocyte size (calculated from tissue sections, Supplementary Figure S2) was significantly smaller in patients with CKD compared to healthy individuals (Figure 1e). This difference in adipocyte size was maintained even after correction for BMI (P = 0.017, data not shown).
Figure 1.
Representative staining for CD68 in subcutaneous adipose tissue sections from (a) a healthy control and (b) a lean chronic kidney disease (CKD) patient. Positive (brown) staining indicated with arrows. Bar = 20 μm. Original magnification ×40. (c) Infiltration of CD68-positive inflammatory cells (number of positive cells per 10-mm2 tissue area) in adipose tissue of control subjects (n = 9, gray fill) and patients with CKD (n = 14, blue fill). *P < 0.05 for the difference between groups. (d) Comparison of collagen content in adipose tissue of controls (n = 10, gray fill) and CKD patients (n = 14, blue fill). (e) Adipocyte size comparison between controls (n = 9, gray fill) and patients with kidney failure (n = 13, blue fill). The average number of cells counted was 71 ± 34 per individual. **P < 0.01 for the difference between groups (Mann−Whitney U test). Box plots represent median (within-box line), minimum, and maximum values (whiskers).
Proteomic Analysis Reveals Differential Protein Composition in Adipose Tissue of Healthy Controls and Patients With Kidney Failure
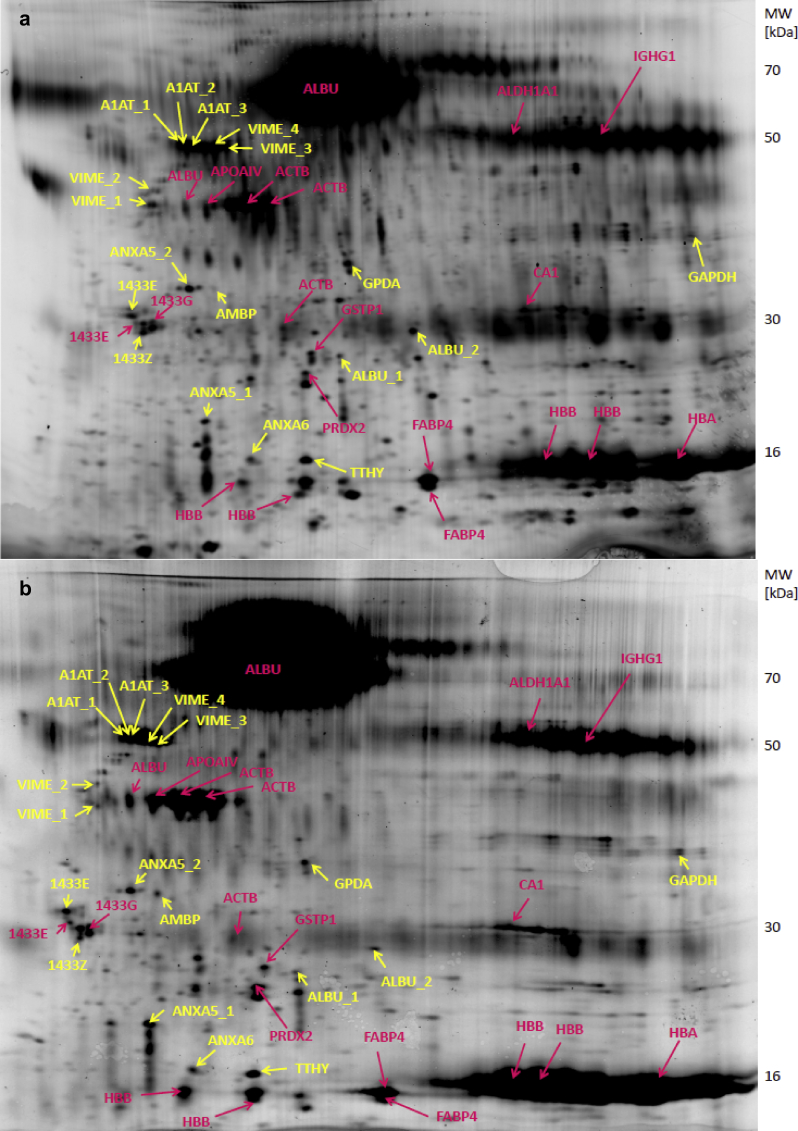
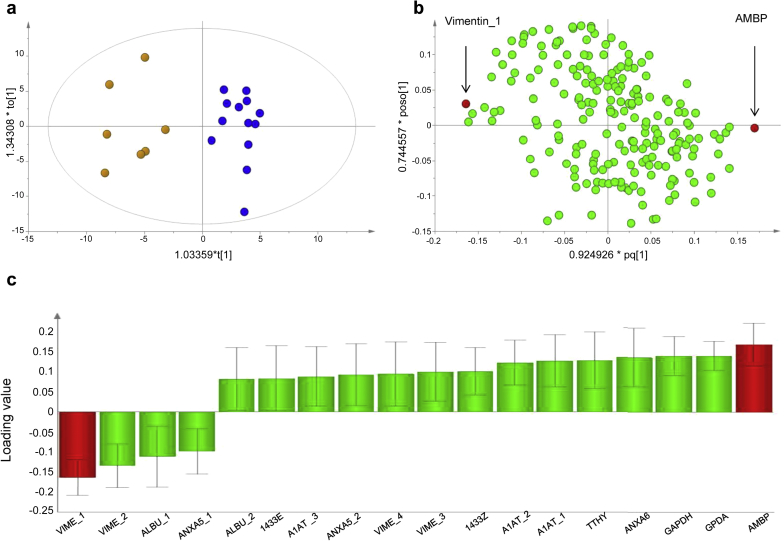
To evaluate whether adipose tissue protein expression patterns differed between individuals with and without CKD, protein extracts from 13 lean CKD patients and 7 control individuals were analyzed using 2-dimensional gel electrophoresis and a total of 202 distinct protein spots were observed (Figure 2). Spot volumes (corresponding to protein expression levels) were measured and used to create a multivariate model (OPLS-DA), which revealed a clear separation of the CKD patients and healthy controls, indicating significant differences in the expression of certain proteins (Figure 3a). A graphical representation of the contribution of the 202 protein spots to the separation between the CKD and the controls is shown in the loading plot (Figure 3b). Quality parameters indicating a robust OPLS-DA model were as follows: number of principal components = 1+1, Rx2 (explained variance) = 0.245, Qy2 (the cumulative fraction of the total variation of Y (presence of CKD) that can be predicted by the model) = 0.556, Ry2 (explained variance for the Y component) = 0.902. Of the 202 spots, 47 contributed significantly to the multivariate model, and 18 of these were sufficiently large to be picked from the preparative gel for subsequent identification (Figure 2 and Figure 3c).
Figure 2.
Representative 2-dimensional gels (Cy5 labeled) of an adipose tissue protein extract from a control individual (a) and a lean patient with chronic kidney disease (CKD) (b). The isoelectric point range is 3 to 10, nonlinear. Spots that were statistically significant in the orthogonal projections to latent structures discriminant analysis (OPLS-DA) model and that were picked for identification are marked in yellow. Spots that were not significant in the model and were identified are marked in pink. 1433E, 14-3-3 protein ε; 1433G, 14-3-3 protein γ; 1433Z, 14-3-3 protein ζ/δ; A1AT, α-1-antitrypsin; ACTB, actin, cytoplasmic 1; ALBU, serum albumin; ALDH1A1, retinal dehydrogenase 1; AMBP, α-1-microglobulin/bikunin precursor; ANXA5, annexin A5; ANXA6, annexin A6; APOAIV, apolipoprotein AIV; CA1, carbonic anhydrase 1; FABP4, fatty acid binding protein 4; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GPDH, glycerol-3-phosphate dehydrogenase; GSTP1, glutathione S-transferase P; HBA1, hemoglobin subunit α; HBB, hemoglobin subunit β; IGHG1, Ig γ-1 chain C; PRDX2, peroxiredoxin 2; TTHY, transthyretin; VIME, vimentin.
Figure 3.
Orthogonal projections to latent structures discriminant analysis (OPLS-DA) analysis of protein spot volumes of adipose tissue from controls and lean patients with chronic kidney disease (CKD). The presence or absence of CKD was used as the Y vector. The analysis was performed on 7 control individuals, 13 CKD patients, and 202 protein spots. The Hotelling T2 (based on 95% confidence level) tolerance ellipse is shown in the score plot (a), which shows all the individuals analyzed (controls yellow, CKD patients blue). Predictive loadings representing the analyzed protein spots are presented as a scatter plot for all protein spots (b) and as a column plot for spots that were identified and were significant in the model (c). In (b) and (c), vimentin_1 and α-1-microglobulin/bikunin precursor (AMBP) are marked in red. For protein name abbreviations, see legend to Figure 2. The significance of the differentially expressed spots was calculated as the difference between the absolute value of the loadings (ABS[loading]) and the absolute value of the corresponding jack-knife confidence interval (ABS[CI]). A positive difference indicates significance.
Vimentin and α-1-Microglobulin/Bikunin Precursor Are the Most Differentially Expressed Proteins Between CKD and Healthy Subjects
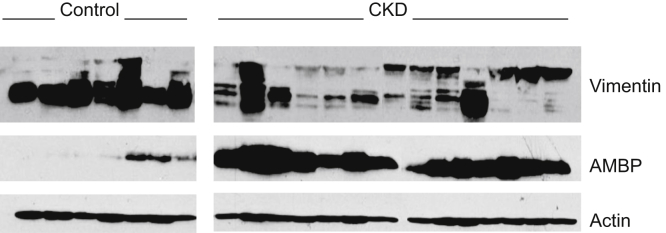
The 18 adipose tissue protein spots that were significant in the OPLS-DA model and that could be picked from the preparative gel were identified by LC-MS/MS (Table 2). Four distinct spots differing slightly in their molecular weight (Figure 2) were identified to be vimentin (annotated vimentin_1–4, with vimentin_1 having the lowest and vimentin_4 the highest molecular weight). Further analysis was focused on the 2 proteins that contributed most strongly to the separation of the control and CKD groups and that were the most significant in the multivariate model, namely vimentin_1 (expression lower in CKD) and α-1-microglobulin/bikunin precursor (AMBP, expression higher in CKD) (Figure 3b, c). Of the other vimentin spots, vimentin_2 was also expressed at lower levels, whereas vimentin_3 and vimentin_4 were expressed at higher levels in CKD compared to controls. However, none of these vimentin spots contributed to the model as strongly as vimentin_1. To confirm differences in expression of vimentin and AMBP, adipose tissue protein extracts from controls and CKD patients were analyzed by Western blot (Figure 4). In agreement with our 2D-gel results and data from others revealing multiple vimentin isoforms in human adipose tissue,29 several vimentin isoforms were also identified by Western blot, as indicated by the multiple bands (Figure 4). In line with the 2D-gel results, the bands on the Western blots corresponding to the lowest-molecular-weight isoforms (similar to vimentin_1 and vimentin_2 on the 2D-gels) had lower expression in the CKD patients than in controls (visual assessment, Figure 4). Inspection of Western blots also clearly confirmed that adipose tissue from CKD patients contained more AMBP than healthy controls (Figure 4).
Table 2.
Adipose tissue protein spots significant in the OPLS-DA analysis and identified by LC-MS/MS.
| Protein name | Protein abbreviation | Normalized spot volume controls | Normalized spot volume CKD | Loading value in OPLS-DA |
|---|---|---|---|---|
| Proteins with lower expression in CKD | ||||
| Vimentin_1 | VIME | 1.58 ± 0.26 | 0.94 ± 0.28a | −0.1675 |
| Vimentin_2 | VIME | 1.29 ± 0.56 | 0.68 ± 0.35a | −0.1372 |
| Serum albumin_1 | ALBU | 2.52 ± 1.05 | 1.31 ± 0.54a | −0.1140 |
| Annexin A5_1 | ANXA5 | 2.15 ± 1.38 | 0.95 ± 0.77b | −0.1003 |
| Proteins with higher expression in CKD | ||||
| α-1-Microglobulin/bikunin precursor | AMBP | 0.53 ± 0.13 | 2.14 ± 0.52a | 0.1734 |
| Glycerol-3-phosphate dehydrogenase [NAD(+)], cytoplasmic | GPDA | 1.02 ± 0.29 | 1.67 ± 0.41a | 0.1438 |
| Glyceraldehyde 3-phosphate dehydrogenase | GAPDH | 1.10 ± 0.42 | 1.88 ± 0.59a | 0.1438 |
| Annexin A6 | ANXA6 | 1.09 ± 0.34 | 2.28 ± 0.70a | 0.1399 |
| Transthyretin | TTHY | 1.17 ± 0.43 | 2.73 ± 0.95a | 0.1323 |
| α-1-Antitrypsin_1 | A1AT | 1.20 ± 0.45 | 1.78 ± 0.51b | 0.1306 |
| α-1-Antitrypsin_2 | A1AT | 1.16 ± 0.44 | 1.66 ± 0.46b | 0.1259 |
| 14-3-3 protein zeta/delta | 1433Z | 1.21 ± 0.32 | 1.74 ± 0.56b | 0.1036 |
| Vimentin_3 | VIME | 1.49 ± 0.72 | 2.08 ± 0.82 | 0.1024 |
| Vimentin_4 | VIME | 1.42 ± 0.62 | 1.91 ± 0.79 | 0.0971 |
| Annexin A5_2 | ANXA5 | 1.11 ± 0.54 | 1.67 ± 0.75 | 0.0947 |
| α-1-Antitrypsin_3 | A1AT | 1.42 ± 0.49 | 1.53 ± 0.50 | 0.0907 |
| 14-3-3 protein epsilon | 1433E | 1.20 ± 0.35 | 1.48 ± 0.42 | 0.0858 |
| Serum albumin_2 | ALBU | 1.13 ± 0.23 | 1.25 ± 0.18 | 0.0839 |
In cases in which 2 or more spots were identified as the same protein, spots are distinguished by successive numbering (e.g., vimentin_1, vimentin_2 etc.).
P ≤ 0.01 for differences between groups (Student t test).
P ≤ 0.05 for differences between groups (Student t test).
Figure 4.
Western blot for vimentin and α-1-microglobulin/bikunin precursor (AMBP) in adipose tissue from controls (n = 7) and lean chronic kidney disease (CKD) patients (n = 13). β-actin was used as a loading control. Half of the CKD samples and all of the control samples were run on 1 gel and the other half of the CKD samples and the same control samples were run on a second gel (space restrictions prevented the loading of all samples on 1 gel). Blots of the control samples are shown only once for ease of understanding. AMBP is consistently higher in the CKD patients, whereas the lower molecular weight forms of vimentin are generally lower in the CKD patients as compared to the controls (visual assessment).
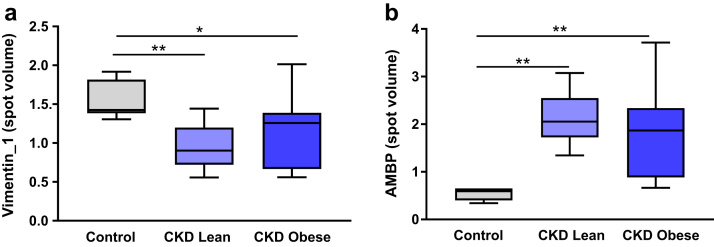
To confirm that the differential expression of vimentin_1 and AMBP proteins was truly related to CKD as opposed to a consequence of the difference in BMI between the groups (lower in CKD), we performed an additional analysis using the same healthy controls and a group of 9 morbidly obese CKD subjects (BMI 41.2 ± 4.4 kg/m2), with greater BMI than the controls and whose adipose tissue had been analyzed by proteomics in an identical fashion. In this new OPLS-DA model (which had model quality parameters reflecting a robust model: number of principal components = 1+1, Rx2 = 0.42, Qy2 = 0.705, Ry2 = 0.907), we found that vimentin_1 expression (spot volume) was significantly lower in obese patients with CKD and AMBP significantly higher in obese patients with CKD compared to healthy controls (Supplementary Figure S3). Direct comparison of the adipose tissue spot volumes of vimentin_1 and AMBP between healthy controls, lean CKD subjects, and obese CKD subjects showed vimentin_1 to be significantly lower (Figure 5a) and AMBP to be significantly higher (Figure 5b) in both CKD groups compared to the controls. These differences persisted even after correction for BMI, age, and gender (control vs. CKD lean: P = 0.002 and P = 0.0006 for vimentin_1 and AMBP respectively; control vs. CKD obese: P = 0.015 and P = 0.004 for vimentin_1 and AMBP respectively; data not shown). Finally, we found no significant correlations between vimentin_1 or AMBP spot volumes and BMI when all 3 groups (lean CKD patients, slightly overweight controls, and morbidly obese CKD patients) were combined (data not shown). These data suggest that the differential expression of vimentin_1 and AMBP proteins is indeed driven by the presence of kidney disease rather than by BMI.
Figure 5.
Comparison of (a) vimentin_1 and (b) α-1-microglobulin/bikunin precursor (AMBP) spot volumes between controls (n = 7, gray fill), lean chronic kidney disease (CKD) patients (n = 13, light blue fill), and obese CKD patients (n = 9, dark blue fill). *P ≤ 0.05, **P ≤ 0.01 for differences between the groups (analysis of variance followed by Fisher least significant difference post hoc tests). Box plots represent median (within-box line), minimum, and maximum values (whiskers).
Discussion
We have characterized subcutaneous abdominal adipose tissue from lean patients with stage 5 CKD in comparison to healthy subjects and have found increased numbers of phagocytic cells (CD68+) and smaller adipocytes in individuals with kidney failure, but no signs of fibrosis. Proteomic analysis revealed different adipose tissue protein profiles between the groups. Our data highlight decreased low-molecular-weight vimentin isoforms and increased AMBP protein expression in adipose tissue as particularly important characteristics of uremic fat. To the best of our knowledge, this is the first study to combine morphological and immunohistochemical characterization with proteomic analysis of adipose tissue from patients with kidney disease.
Systemic inflammation is a feature of CKD, but only recently has adipose tissue inflammation been proposed as a potential contributor to the development of cardiovascular complications in CKD.22, 30 We report here that lean CKD patients have more CD68-positive cells in their adipose tissue than healthy controls, suggesting that adipose inflammation may indeed be a feature of uremic fat. Although adipose tissue fibrosis is a hallmark of perturbed adipose function,31, 32 we found no differences in the degree of adipose tissue fibrosis, assessed by collagen content, between lean CKD patients and controls. This could mean that either adipose tissue fibrosis is not a feature of kidney disease, or that we were not able to detect fibrosis, which might develop in another adipose tissue depot, or that fibrosis is not very apparent in nonobese individuals. Of interest was the significantly smaller adipocyte size in the lean kidney failure patients compared to the controls. Considering the high CVD risk and insulin resistance associated with CKD, one could have expected an increased adipocyte size, which is coupled with blunted insulin signaling in both obese and nonobese individuals.33, 34, 35 On the other hand, the lean CKD subjects had lower BMI than the controls (although the difference in adipocyte size remained significant after correcting for BMI) and individuals with kidney failure experience protein wasting and cachexia,36 but this can be expected to affect primarily lean body mass rather than fat. However, perturbed adipose tissue fatty acid metabolism in CKD is indicated by the increased adipocyte lipolysis and reduced lipogenesis that is induced by a uremic milieu.19, 37 Hence, impaired adipose tissue uptake and storage of fatty acids in CKD, which could be reflected in alterations in adipocyte size, could lead to a proatherogenic blood lipid profile.
The proteomic analysis confirmed our hypothesis that CKD is associated with alterations in the functionality of adipose tissue. The multivariate analysis revealed that the protein composition of subcutaneous adipose tissue could distinguish between subjects with CKD and healthy controls. We identified several proteins whose expression differed significantly between CKD patients and controls. The 2 proteins that contributed most to the multivariate model including healthy controls and lean CKD patients were vimentin and AMBP, which were decreased and increased in CKD, respectively. Although the lean CKD patients had lower BMI than the controls, we attribute alterations in vimentin and AMBP to the presence of CKD, as opposed to differences in obesity, as these alterations were maintained even after correction for BMI. Furthermore, we also quantified these proteins in a group of morbidly obese patients with CKD. The differences in both vimentin and AMBP between healthy controls and CKD patients were the same irrespective of whether the CKD patients were lean or obese, and their expression in adipose tissue did not correlate with BMI, thereby indicating that CKD, rather than obesity, underlies alterations in vimentin and AMBP protein expression in uremic adipose tissue.
Vimentin is a protein present in mesenchymal cells, including adipocytes, with different isoforms that are differentially regulated.38 Correspondingly, our proteomic analysis identified 4 protein spots in adipose tissue as vimentin (vimentin_1-4), with molecular weights in line with those reported previously (46–54 KDa),39 and we confirmed this by Western blot. Of all the protein spots with lower expression in the lean CKD patients, vimentin_1 (the lowest-molecular-weight vimentin spot) contributed most to the separation of the CKD and control subjects in the multivariate model. Western blot analysis confirmed that the lowest-molecular-weight isoforms of vimentin differed the most between controls and lean CKD patients. Vimentin is a lipid-droplet−associated protein40, 41 involved in the regulation of adipocyte lipolysis.39, 42, 43 Primary human adipocytes cultured with uremic serum show increased basal lipolysis and decreased perilipin expression,19 indicating that a uremic environment can alter lipid droplet metabolism, with vimentin having a known role in the latter. Moreover, higher vimentin expression is a hallmark of more insulin-sensitive adipose tissue.29 Thus, vimentin plays an important role in adipose tissue function, and dysregulation of these processes as a result of alterations in vimentin expression is likely to perturb adipose tissue, promoting cardiovascular events such as atherosclerosis4; but it remains to be established whether and how different isoforms of vimentin affect human adipose tissue, and whether they are differentially regulated by the uremic milieu.
Another adipose tissue protein that made a major contribution to the separation between the CKD patients and controls in the multivariate models and whose expression was much higher in the CKD patients was AMBP. Although produced mainly in the liver, AMBP protein is also expressed in adipose tissue (www.genecards.org). AMBP is a precursor protein that is cleaved into 2 functional proteins, namely, α-1-microglobulin (A1M) and bikunin, with all the peptide sequences identified in the present mass spectrometry analysis within A1M. Because A1M binds and degrades free radicals and oxidizing agents, and because oxidative stress in adipose tissue is linked to obesity, inflammation, diabetes, and other diseases,44, 45 the high AMBP protein levels in adipose tissue of CKD patients indicate that the tissue is exposed to the damaging effects of free radicals, which can cause lipid and protein oxidation and cell death, and has a compromised functionality.
We acknowledge some limitations of our study, in particular the small sample size and restriction to subcutaneous adipose tissue. It is possible that alterations in visceral and perivascular adipose tissue depots may have a greater impact on the development of CVD in CKD patients. Finally, the cross-sectional nature of this study restricts our ability to draw causal conclusions. Yet, to the best of our knowledge, this is the first proteomic analysis of adipose tissue from CKD patients; therefore, we believe that it provides relevant information.
In conclusion, we have shown that adipose tissue of patients with CKD has an inflammatory phenotype, has smaller adipocytes, and is exposed to oxidative stress, as manifested by high AMBP levels. Furthermore abnormal vimentin expression may affect lipid droplet metabolism and lipolysis rates. These data provide a novel perspective on how changes in adipose tissue could lead to increased risk of cardiovascular events in CKD.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work received financial support from The Swedish Heart and Lung Foundation, The Swedish Research Council (projects 2015-04622 [RMMB] and 521-2012-1610 [JA]), and Stiftelsen Sigurd och Elsa Goljes Minne. JG was partially supported by faculty funds from the Board of Post-Graduate Education of Karolinska Institutet (KID Award). We would like to thank Professor Peter James and Liselotte Andersson, Department of Immunotechnology, Lund University, for support and technical assistance with the 2D-gel analyses. We also acknowledge Ulrika Jensen, Annika Nilsson, and Ann-Christin Emmoth, Njur-KBC, Karolinska University Hospital Huddinge for their help with collecting biopsy samples.
Footnotes
Figure S1. Representative images of picrosirius red collagen staining of subcutaneous adipose tissue sections from a control individual (a) and a lean chronic kidney disease (CKD) patient (b). Positive staining (red) is visible between adipocytes as reticular fibers as well as clusters of thicker collagen bundles. Bar = 20 μm.
Figure S2. Representative images of transformed ImageJ pictures of subcutaneous adipose tissue sections from a control individual (a) and a lean chronic kidney disease (CKD) patient (b) used for calculation of adipocyte size.
Figure S3. Orthogonal projections to latent structures discriminant analysis (OPLS-DA) analysis of protein spot volumes of adipose tissue from healthy controls and obese patients with chronic kidney disease (CKD). The presence or absence of CKD was used as the Y vector. The analysis was performed on 7 control individuals, 9 obese CKD patients, and 202 protein spots. The Hotelling T2 (based on 95% confidence level) tolerance ellipse is shown in the score plot (a), which shows all the individuals analyzed (controls, yellow; CKD patients, blue). Predictive loadings representing the analyzed protein spots are presented as a scatter plot for all protein spots (b). Vimentin_1 and α-1-microglobulin/bikunin precursor (AMBP) are marked in red.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Representative images of picrosirius red collagen staining of subcutaneous adipose tissue sections from a control individual (a) and a lean chronic kidney disease (CKD) patient (b). Positive staining (red) is visible between adipocytes as reticular fibers as well as clusters of thicker collagen bundles. Bar = 20 μm.
Representative images of transformed ImageJ pictures of subcutaneous adipose tissue sections from a control individual (a) and a lean chronic kidney disease (CKD) patient (b) used for calculation of adipocyte size.
Orthogonal projections to latent structures discriminant analysis (OPLS-DA) analysis of protein spot volumes of adipose tissue from healthy controls and obese patients with chronic kidney disease (CKD). The presence or absence of CKD was used as the Y vector. The analysis was performed on 7 control individuals, 9 obese CKD patients, and 202 protein spots. The Hotelling T2 (based on 95% confidence level) tolerance ellipse is shown in the score plot (a), which shows all the individuals analyzed (controls, yellow; CKD patients, blue). Predictive loadings representing the analyzed protein spots are presented as a scatter plot for all protein spots (b). Vimentin_1 and α-1-microglobulin/bikunin precursor (AMBP) are marked in red.
References
- 1.Jha V., Garcia-Garcia G., Iseki K. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 2.Sarnak M.J. Cardiovascular complications in chronic kidney disease. Am J Kidney Dis. 2003;41:11–17. doi: 10.1016/s0272-6386(03)00372-x. [DOI] [PubMed] [Google Scholar]
- 3.Ojo A.O., Hanson J.A., Wolfe R.A. Long-term survival in renal transplant recipients with graft function. Kidney Int. 2000;57:307–313. doi: 10.1046/j.1523-1755.2000.00816.x. [DOI] [PubMed] [Google Scholar]
- 4.Frayn K., Bernard S., Spalding K. Adipocyte triglyceride turnover is independently associated with atherogenic dyslipidemia. J Am Heart Assoc. 2012;1:e003467. doi: 10.1161/JAHA.112.003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim S., Meigs J.B. Ectopic fat and cardiometabolic and vascular risk. Int J Cardiol. 2013;169:166–176. doi: 10.1016/j.ijcard.2013.08.077. [DOI] [PubMed] [Google Scholar]
- 6.Jocken J.W., Goossens G.H., Boon H. Insulin-mediated suppression of lipolysis in adipose tissue and skeletal muscle of obese type 2 diabetic men and men with normal glucose tolerance. Diabetologia. 2013;56:2255–2265. doi: 10.1007/s00125-013-2995-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mlinar B., Marc J. New insights into adipose tissue dysfunction in insulin resistance. Clin Chem Lab Med. 2011;49:1925–1935. doi: 10.1515/CCLM.2011.697. [DOI] [PubMed] [Google Scholar]
- 8.Hennig B., Meerarani P., Ramadass P. Fatty acid-mediated activation of vascular endothelial cells. Metabolism. 2000;49:1006–1013. doi: 10.1053/meta.2000.7736. [DOI] [PubMed] [Google Scholar]
- 9.Toborek M., Lee Y.W., Garrido R. Unsaturated fatty acids selectively induce an inflammatory environment in human endothelial cells. Am J Clin Nutr. 2002;75:119–125. doi: 10.1093/ajcn/75.1.119. [DOI] [PubMed] [Google Scholar]
- 10.Lamers D., Schlich R., Greulich S. Oleic acid and adipokines synergize in inducing proliferation and inflammatory signalling in human vascular smooth muscle cells. J Cell Mol Med. 2011;15:1177–1188. doi: 10.1111/j.1582-4934.2010.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Police S.B., Thatcher S.E., Charnigo R. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2009;29:1458–1464. doi: 10.1161/ATVBAHA.109.192658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Öhman M.K., Shen Y., Obimba C.I. Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2008;117:798–805. doi: 10.1161/CIRCULATIONAHA.107.717595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen S., Guo Z., Johnson C.M. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y., Tchkonia T., Stout M.B. Inflammation and the depot-specific secretome of human preadipocytes. Obesity (Silver Spring) 2015;23:989–999. doi: 10.1002/oby.21053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maresca F., Di P V., Bevilacqua M. Adipokines, vascular wall, and cardiovascular disease: a focused overview of the role of adipokines in the pathophysiology of cardiovascular disease. Angiology. 2015;66:8–24. doi: 10.1177/0003319713520463. [DOI] [PubMed] [Google Scholar]
- 16.Axelsson J., Møller H.J., Witasp A. Changes in fat mass correlate with changes in soluble sCD163, a marker of mature macrophages, in pateints with CKD. Am J Kidney Dis. 2006;48:916–925. doi: 10.1053/j.ajkd.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 17.Lu Q., Cheng L.T., Wang T. Visceral fat, arterial stiffness, and endothelial function in peritoneal dialysis patients. J Ren Nutr. 2008;18:495–502. doi: 10.1053/j.jrn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Roubicek T., Bartlova M., Krajickova J. Increased production of proinflammatory cytokines in adipose tissue of patients with end-stage renal disease. Nutrition. 2009;25:762–768. doi: 10.1016/j.nut.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Axelsson J., Astrom G., Sjolin E. Uraemic sera stimulate lipolysis in human adipocytes: role of perilipin. Nephrol Dial Transplant. 2011;26:2485–2491. doi: 10.1093/ndt/gfq755. [DOI] [PubMed] [Google Scholar]
- 20.Ambarkar M., Pemmaraju S.V., Gouroju S. Adipokines and their relation to endothelial dysfunction in patients with chronic kidney disease. J Clin Diagn Res. 2016;10:BC04–BC08. doi: 10.7860/JCDR/2016/15867.7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong Z., Xu H., Huang X. Nonesterified fatty acids and cardiovascular mortality in elderly men with CKD. Clin J Am Soc Nephrol. 2015;10:584–591. doi: 10.2215/CJN.08830914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witasp A., Carrero J.J., Heimburger O. Increased expression of pro-inflammatory genes in abdominal subcutaneous fat in advanced chronic kidney disease patients. J Intern Med. 2011;269:410–419. doi: 10.1111/j.1365-2796.2010.02293.x. [DOI] [PubMed] [Google Scholar]
- 23.Witasp A., Ryden M., Carrero J.J. Elevated circulating levels and tissue expression of pentraxin 3 in uremia: a reflection of endothelial dysfunction. PLoS One. 2013;8:e63493. doi: 10.1371/journal.pone.0063493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg A., Sherrard D.J., Brunzell J.D. Adipose tissue lipoprotein lipase in chronic hemodialysis: role in plasma triglyceride metabolism. J Clin Endocrinol Metab. 1978;47:1173–1182. doi: 10.1210/jcem-47-6-1173. [DOI] [PubMed] [Google Scholar]
- 25.Kalantar-Zadeh K., Abbott K.C., Salahudeen A.K. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81:543–554. doi: 10.1093/ajcn/81.3.543. [DOI] [PubMed] [Google Scholar]
- 26.Beddhu S., Pappas L.M., Ramkumar N. Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol. 2003;14:2366–2372. doi: 10.1097/01.asn.0000083905.72794.e6. [DOI] [PubMed] [Google Scholar]
- 27.Cheung L., Gertow J., Werngren O. Human mediastinal adipose tissue displays certain characteristics of brown fat. Nutr Diab. 2013;13:e66. doi: 10.1038/nutd.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kjellqvist S., Maleki S., Olsson T. A combined proteomic and transcriptomic approach shows diverging molecular mechanisms in thoracic aortic aneurysm development in patients with tricuspid- and bicuspid aortic valve. Mol Cell Proteomics. 2013;12:407–425. doi: 10.1074/mcp.M112.021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed M., Neville M.J., Edelmann M.J. Proteomic analysis of human adipose tissue after rosiglitazone treatment shows coordinated changes to promote glucose uptake. Obesity (Silver Spring) 2010;18:27–34. doi: 10.1038/oby.2009.208. [DOI] [PubMed] [Google Scholar]
- 30.Zanetti M., Barazzoni R., Guarnieri G. Inflammation and insulin resistance in uremia. J Ren Nutr. 2008;18:70–75. doi: 10.1053/j.jrn.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Divoux A., Tordjman J., Lacasa D. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59:2817–2825. doi: 10.2337/db10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venteclef N., Guglielmi V., Balse E. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur Heart J. 2015;36:795–805a. doi: 10.1093/eurheartj/eht099. [DOI] [PubMed] [Google Scholar]
- 33.Acosta J.R., Douagi I., Andersson D.P. Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia. 2016;59:560–570. doi: 10.1007/s00125-015-3810-6. [DOI] [PubMed] [Google Scholar]
- 34.Cotillard A., Poitou C., Torcivia A. Adipocyte size threshold matters: link with risk of type 2 diabetes and improved insulin resistance after gastric bypass. J Clin Endocrinol Metab. 2014;99:E1466–E1470. doi: 10.1210/jc.2014-1074. [DOI] [PubMed] [Google Scholar]
- 35.Weyer C., Foley J.E., Bogardus C. Enlarged subcutaneous abdominal adipocyte size, but not obesity iteslf, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43:1498–1506. doi: 10.1007/s001250051560. [DOI] [PubMed] [Google Scholar]
- 36.Obi Y., Qader H., Kovesdy C.P. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr Opin Clin Nutr Metab Care. 2015;18:254–262. doi: 10.1097/MCO.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelletier C.C., Koppe L., Croze M.L. White adipose tissue overproduces the lipid-mobilizing factor zinc alpha2-glycoprotein in chronic kidney disease. Kidney Int. 2013;83:878–886. doi: 10.1038/ki.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buchmaier B.S., Bibi A., Muller G.A. Renal cells express different forms of vimentin: the independent expression alteration of these forms is important in cell resistance to osmotic stress and apoptosis. PLoS One. 2013;8:e68301. doi: 10.1371/journal.pone.0068301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peinado J.R., Quiros P.M., Pulido M.R. Proteomic profiling of adipose tissue from Zmpste24-/- mice, a model of lipodystrophy and premature aging, reveals major changes in mitochondrial function and vimentin processing. Mol Cell Proteomics. 2011;10:M111. doi: 10.1074/mcp.M111.008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heid H., Rickelt S., Zimbelmann R. On the formation of lipid droplets in human adipocytes: the organization of the perilipin-vimentin cortex. PLoS One. 2014;9:e90386. doi: 10.1371/journal.pone.0090386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brasaemle D.L., Dolios G., Shapiro L. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem. 2004;279:46835–46842. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- 42.Shen W.J., Patel S., Eriksson J.E. Vimentin is a functional partner of hormone sensitive lipase and facilitates lipolysis. J Proteome Res. 2010;9:1786–1794. doi: 10.1021/pr900909t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar N., Robidoux J., Daniel K.W. Requirement of vimentin filament assembly for beta3-adrenergic receptor activation of ERK MAP kinase and lipolysis. J Biol Chem. 2007;282:9244–9250. doi: 10.1074/jbc.M605571200. [DOI] [PubMed] [Google Scholar]
- 44.Chattopadhyay M., Khemka V.K., Chatterjee G. Enhanced ROS production and oxidative damage in subcutaneous white adipose tissue mitochondria in obese and type 2 diabetes subjects. Mol Cell Biochem. 2015;399:95–103. doi: 10.1007/s11010-014-2236-7. [DOI] [PubMed] [Google Scholar]
- 45.Marseglia L., Manti S., D'Angelo G. Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci. 2014;16:378–400. doi: 10.3390/ijms16010378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative images of picrosirius red collagen staining of subcutaneous adipose tissue sections from a control individual (a) and a lean chronic kidney disease (CKD) patient (b). Positive staining (red) is visible between adipocytes as reticular fibers as well as clusters of thicker collagen bundles. Bar = 20 μm.
Representative images of transformed ImageJ pictures of subcutaneous adipose tissue sections from a control individual (a) and a lean chronic kidney disease (CKD) patient (b) used for calculation of adipocyte size.
Orthogonal projections to latent structures discriminant analysis (OPLS-DA) analysis of protein spot volumes of adipose tissue from healthy controls and obese patients with chronic kidney disease (CKD). The presence or absence of CKD was used as the Y vector. The analysis was performed on 7 control individuals, 9 obese CKD patients, and 202 protein spots. The Hotelling T2 (based on 95% confidence level) tolerance ellipse is shown in the score plot (a), which shows all the individuals analyzed (controls, yellow; CKD patients, blue). Predictive loadings representing the analyzed protein spots are presented as a scatter plot for all protein spots (b). Vimentin_1 and α-1-microglobulin/bikunin precursor (AMBP) are marked in red.