Abstract
There is limited information regarding the neurobiology underlying non-suicidal self-injury (NSSI) in clinically-referred youth. However, the salience of disturbed interpersonal relationships and disrupted self-processing associated with NSSI suggests the neural basis of social processes as a key area for additional study. Adolescent participants (N=123; M=14.75 years, SD=1.64) were divided into three groups: NSSI plus depression diagnosis (NSSI), depression only (DEP), healthy controls (HC). In the scanner, participants completed an Interpersonal Self-Processing task by taking direct (own) and indirect (mothers’, best friends’, or classmates’) perspectives regarding self-characteristics. Across all perspectives, NSSI showed higher BOLD activation in limbic areas, and anterior and posterior cortical midline structures versus DEP and HC, while HC showed greater activity in rostrolateral, frontal pole and occipital cortex than NSSI and DEP youth. Moreover, NSSI youth showed heightened responses in amygdala, hippocampus, parahippocampus, and fusiform when taking their mothers’ perspective, which were negatively correlated with self-reports of the mother’s support of adolescents’ emotional distress in the NSSI group. NSSI youth also yielded greater precuneus and posterior cingulate cortex activity during indirect self-processing from their classmates’ perspective. Findings suggest a role for disruptions in self- and emotion-processing, and conflicted social relationships in the neurobiology of NSSI among depressed adolescents.
Keywords: neuroimaging, non-suicidal self-injury, self-knowledge, depression, adolescence, limbic and cortical midline structures, emotional invalidation
1. Introduction
Non-suicidal self-injury (NSSI) is the self-inflicted destruction of body tissue without suicidal intent, using socially unsanctioned methods (Nixon and Heath, 2009). This behavior is common among clinically-referred youth, with between 38 and 67% of adolescents with a psychiatric diagnosis reporting NSSI behavior (Brunner et al., 2014; Heath et al., 2009). NSSI is comorbid with both externalizing and internalizing disorders, including borderline personality (BPD), substance use, eating disorders, and depression (Nock et al., 2006). Understanding NSSI within its comorbid disorders is essential, as this behavior portends chronic mental illness (Barrocas et al., 2015), increased suicide risk (Dickstein et al., 2015; Klonsky et al., 2013), and lifetime impairment (Glenn and Klonsky, 2013; In-Albon et al., 2013). Identifying neural signatures distinguishing NSSI from comorbid disorders may elucidate unique neural signatures of higher risk trajectories. For instance, NSSI may facilitate severe negative outcomes such as suicide attempts (Whitlock et al., 2013a), and BPD diagnosis (Groschwitz et al., 2015), which is associated with high societal costs (Brettschneider et al., 2014).
Despite these justifications for examining NSSI behavior’s associated neural signatures there is little neuroimaging research on NSSI in general; moreover, although NSSI’s highest prevalence rates occur in adolescence (Moran et al., 2012) the lack of neuroimaging studies is especially notable within adolescent groups. The few existing imaging studies regarding self-injury show altered emotional processing sub-served by limbic hyperactivity. For instance, Davis and colleagues (2014) found that self-injurers demonstrate more amygdala activation following instructions to regulate emotional responses to negative stimuli compared to healthy controls, even though no such differences were evident in basic emotional reactivity to stimuli. Davis et al. (2014) also reported heightened activity in self-injurers versus depressed non-self-injuring controls in posterior cingulate cortex and prefrontal motor areas (BA8 and BA6) during regulation of negative emotions, perhaps suggestive of greater cognitive effort expended by self-injurers during emotion regulation. In the single neuroimaging study of adolescent-specific NSSI, Plener et al. (2012) reported hyperactivation of the bilateral amygdala, hippocampus, anterior cingulate cortex (ACC), and cerebellum in 9 self-injurers versus 9 healthy controls in response to viewing negative images; however, controlling for depression accounted for these group differences, and this investigation is limited by its small sample size.
While vastly limited in scope, existing neuroimaging research of adult and adolescent NSSI has focused primarily on emotion dysregulation and corresponding limbic hyperactivity during processing of negative stimuli. Nonetheless, the scarcity of existing studies, small sample sizes, and absence of psychiatric, non-NSSI control groups suggest that additional study in this area is urgently needed. Past neuroimaging research has also typically employed methods which are remote from the individual’s own inter- or intra-personal context (i.e., viewing negative images). Thus existing studies have overlooked two relevant and interrelated features of NSSI: interpersonal relationship difficulties and distorted self-processing, both of which are the focus of the present study.
Conflict with parents tends to increase during adolescence (Shanahan et al., 2007), and closeness and warmth within these relationships may decrease during this period (Marceau et al., 2015; Paikoff and Brooks-Gunn, 1991). Although self-injuring youth typically report conflict in both family and peer relationships (Lundh et al., 2009) researchers suggest that a history of conflict with caregivers may play a key role in the emergence of NSSI behavior through the development of poor emotion regulation skills, and that conflict with peers may serve to maintain existing NSSI behavior (Crowell et al., 2009). Specifically, caregiver emotional invalidation, conceptualized as parenting practices which imply the child’s opinions and emotions are invalid, irrational or unimportant (Linehan, 1993), have been linked to emotion dysregulation and NSSI (Adrian et al., 2011; Sturrock and Mellor, 2014; Tan et al., 2014; Yurkowski et al., 2015). Indeed, when accounting for multiple interpersonal characteristics, retrospective self-reports of experiencing maternal antipathy has been shown to be the sole significant predictor of NSSI behavior (Kaess et al., 2013), and observed maternal emotional invalidation and coerciveness have been linked to greater conflict in interactions between adolescent self-injurers and their mothers (Crowell et al., 2013). With respect to peers, conflict in peer relationships is also associated with youth NSSI behavior (e.g., (Hilt et al., 2008), perhaps by placing continued stress on already dysregulated emotional processing systems, or by serving as distressing triggers for NSSI engagement. Consequently, the current study examined how indirect self-processing from both mothers’ and peers’ perspectives engage the neural basis of emotion- and self-processing in self-injurers.
Self-processing is the ability to perceive, evaluate and judge one’s states, traits and abilities. Toward the end of adolescence, self-referential processing results in global cognitive self-representations (self-knowledge) enabled by abstract thinking skills that emerge during this period (Harter, 1999). Researchers have shown that, in addition to dysfunctional interpersonal relationships, negatively biased self-knowledge and unresolved identity formation characterize youth who engage in NSSI. For example among eating disorder (ED) patients, NSSI is significantly and robustly related to greater identity confusion and to less identity coherence (Claes et al., 2015). Similarly, among self-injuring adolescents with ED, poor interoceptive awareness and high interpersonal ineffectiveness are both associated with NSSI (Ross et al., 2009). Lack of self-esteem and low self-efficacy have also each been linked with NSSI in student samples (Tatnell et al., 2014). High self-criticism has been shown to mediate associations between exposure to emotional abuse in childhood and NSSI behavior (Glassman et al., 2007), and is also linked with pain analgesia and higher pain endurance in self-injurers (Glenn et al., 2014; Hooley and St Germain, 2014). Thus, it has been proposed that NSSI may represent a possible manifestation of one’s painful, confused or disrupted self-processing (Claes et al., 2010b; Claes et al., 2015).
The neural substrates of self-processing implicate somewhat distinct systems compared to those involved in emotion processing and regulation (i.e., limbic regions, lateral prefrontal cortex [PFC]). Specifically, processing information regarding the self reliably engages both anterior and posterior cortical midline structures (CMS), including rostral and perigenual anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), precuneus and medial PFC, particularly medial BA10 (Ichikawa et al., 2011; Kircher et al., 2000). Additionally, dorsal ACC recruitment has been noted during social rejection or negative evaluation (Rotge et al., 2015), and when healthy adolescents engage in both direct (self perspective) and indirect (important others’ perspectives) self-referential processing (Jankowski et al., 2014; Pfeifer et al., 2009). Although limited in scope, existing research has shown that self-injuring youth and adults demonstrate hyperactivation of CMS regions (i.e., ACC, PCC) during emotionally distressing tasks (Davis et al., 2014; Plener et al., 2012), perhaps suggesting self-injurers’ atypical processing of self-related information during heightened emotional demand. Hyperactive CMS during direct or indirect self-referential processing is also generally implicated for depressed individuals (Cooney et al., 2010; Ruiz et al., 2013; Zhang et al., 2013), a common comorbid diagnosis of NSSI (Lofthouse et al., 2009). Self-harming adults have also been shown to demonstrate even greater activation of the PCC than depressed (but non-self-harming) controls during emotion processing (Davis et al., 2014). It is also likely there are interactions among the neural systems engaged by self-processing and emotion processing. For example, self-criticism and negative self-concept, which both characterize self-injuring youth (Claes et al., 2010a; Glassman et al., 2007), are also each linked to greater limbic activity during exposure to adjectives of personally-relevant negative content versus exposure to neutral and negative non-self-referential adjectives (Doerig et al., 2014). Overall, the extant literature reviewed here lends support for studying the hypothesis that depressed youth with NSSI may show higher activity in both CMS and limbic areas when engaged in self-processing compared to depressed youth without NSSI and healthy controls.
Given NSSI’s association to both chronic interpersonal difficulties and negatively biased self-processing, the current study sought to compare patterns of neural activation in depressed youth with and without NSSI and psychologically healthy controls during self-processing appraisals both indirectly (from the perspectives of key social others: mother, best friend and classmates), and from their own direct perspective. This research was guided by two overarching hypotheses. First, we hypothesized that NSSI youth would show the greatest hyperactivation of CMS during direct and indirect self-referential processing, regardless of perspective, followed by depressed youth without NSSI, and then psychologically healthy controls. Second, we predicted that self-processing from the mothers’ perspective would elicit greater limbic activity in depressed youth with NSSI compared to both depressed and healthy controls; this hypothesis was based on theory (Linehan, 1993) and research (e.g., Kaess et al., 2013) suggesting the relevance of parental emotional invalidation on dysregulation of emotional processes in self-injuring youth. To further corroborate this putative explanation for variation in neural responses in the NSSI group, we also explored associations between observed neural activation during the mothers’ perspective and mothers’ reports of providing support to adolescents’ experiences of sadness, anger, and fear; we expected that for self-injurers, greater limbic activity would be related to less maternal support of negative emotions.
2. Method
2.1. Participants and general procedure
One-hundred twenty-three adolescents (67 females; Mage=14.75 years, SD=1.64) participated in a larger study concerning the neurobiology of self and social processes in adolescent depression. Participants were recruited from brief crisis inpatient units and from youth assessed for depression at the Universities of Minnesota (40 male, 46 female, Mage=15.18, SD=1.60) and Pittsburgh (16 male, 21 female=21, Mage=14.18, SD=1.58), at local outpatient mental health clinics, and through radio and flyer advertisements. Psychological evaluation and determination of depression diagnosis and NSSI behavior for all participants was completed using the Schedule for Affective Disorders and Schizophrenia for School-Aged Children (K-SADS, Kaufman et al., 1997) and the Child Depression Rating Scale (CDRS, Poznanski et al., 1979). The Pubertal Development Scale (PDS, Petersen et al., 1985) was also completed (M= 3.05, SD = .55), with the majority of participants having mid to late pubertal status. Adolescents also completed measures of anxiety (SCARED, Hale et al., 2005), self-esteem (PCSC, Harter, 1982) and attributional style (CASQ, Conley et al., 2001). Maternal reports of their provision of support to their adolescent child’s emotional experiences of sadness, anger, and fear were obtained via the Emotional Socialization Measure (ESM; Malatesta-Magai, 1991). One to two weeks after psychological evaluation and completion of questionnaires, adolescents completed neuroimaging procedures.
2.2 Participant groups
Participants were divided into three subgroups based on K-SADS depression diagnosis and NSSI behavior. NSSI behavior was determined using the K-SADS interview item regarding self-injury, and was defined as any intentional act of self-injury without suicidal intent that caused tissue damage in the form of bleeding and/or scarring; this included cutting, burning, hitting and scratching. Participants were classified as having engaged in NSSI behavior if they reported at least four instances of self-injury within the last year. Inter-coder agreement was established through double coding of 30 diagnostic interviews (24%), and results showed 98% agreement in primary diagnostic category (depressive disorder), and 100% agreement in presence or absence of NSSI. Fifty adolescents both reported NSSI behavior and met criteria for depression diagnosis (NSSI group), 36 reported no NSSI but met criteria for depression diagnosis (DEP), and 37 had no past or current psychiatric disorder or NSSI behavior (healthy controls, HC). Descriptive statistics and comparative analyses among the three groups for demographic and relevant clinical variables are summarized below and detailed in Table 1.
Table 1.
Demographic and Clinical Differences across Depressed with NSSI, Depressed only, and Healthy Control Groups
| HC n = 37 |
DEP n = 36 |
NSSI n = 50 |
Comparison Statistic | |
|---|---|---|---|---|
| Age | F(2, 120) = 0.83 | |||
| m (sd) | 14.49 (1.53) | 14.77 (1.86) | 14.94 (1.54) | |
| IQ | F (2, 120) = 4.14* | |||
| m (sd) | 117.08 a (12.57) | 109.56 b (20.78) | 107.84 b (12.24) | |
| Sex | χ2 (2) = 3.10 | |||
| Male | 19 (51.35%) | 19 (52.78%) | 18 (36%) | |
| Female | 18 (48.65%) | 17 (47.22%) | 32 (64%) | |
| Ethnicity | χ2 (2) = 3.92c | |||
| White | 28 (75.7%) | 20 (55.6%) | 29 (58.0%) | |
| African American | 1 (2.7%) | 7 (19.4%) | 3 (6.0%) | |
| Hispanic | 1 (2.7%) | 6 (16.7%) | 4 (8.0%) | |
| Indian/Asian | 3 (8.1%) | 0 | 3 (6.0%) | |
| Other | 0 | 1 (2.8%) | 11 (22.0%) | |
| Family Income |
χ2 (4) = 13.77** χ2 (2) =0.26d |
|||
| < $35,000 | 10.8% a | 35.3% a | 32.7% a | |
| $35,000–$75,000 | 21.6% a | 35.3% a | 29.4% a | |
| >$75,000 | 67.6% b | 29.4% a | 34.7% a | |
| Family Structure |
χ2 (2) =7.00* χ2 (1) = 0.25d |
|||
| Cohabiting Parents | 86.5% a | 60.0% b | 65.3% b | |
| Single Parents | 13.5% a | 40.0% b | 34.7% b | |
| Medication | ||||
| Anti-depressants | 0a | 12 (33.3%) a,b | 24 (48%) b | χ2 (2) = 24.07*** |
| Adj. Resid. | −4.7 | 0.6 | 3.8 | χ2 (1) = 1.85d |
| Anti-psychotic | 0a | 0a,b | 6.0b | χ2 (2) = 9.29** |
| Adj. Resid. | 1.6 | 1.6 | 3.0 | χ2 (1) = 2.98d |
| Mood stabilizers | 2.0a | 0a | 1.0a | χ2 (2) = 2.31 |
| Adj. Resid | 1.4 | −1.1 | −.3 | χ2 (1) = 0.73d |
| Stimulant | 0a | 4.0b | 6.0b | χ2 (2) = 4.10 |
| Adj. Resid. | −2.2 | 0.8 | 1.3 | χ2 (1) = 0.02d |
| Anxiolitic | 0a | 1.0a | 5.0a | χ2 (2) = 5.07 |
| Adj. Resid. | −1.6 | −.70 | 2.2 | χ2 (1) = 1.68d |
| Abuse/trauma | 0a | 17 (47.2%)b | 24 (48%)b |
χ2 (2) = 26.47*** χ2 (1) =0.01d |
| Adj. Resid. | −5.1 | 2.1 | 2.9 | |
| Total suicide attempts | 0a | 7.0b | 17.0b | χ2 (2) = 15.62*** |
| Adj. Resid. | −3.6 | 0 | 3.4 | χ2 (1) = 2.20d |
| Current suicide ideation | ||||
| m (se) | 1.16 (.26)a | 3.25 (.26)b | 4.48 (.22)c | F (2, 120) = 46.94*** |
| Depression Severity | F (2, 120) = 154.43*** | |||
| m (se) | 20.65 (2.03)a | 60.53 (2.06)b | 65.12 (1.75)b | |
| Depression Chronicity | F (2, 120) = 29.37*** | |||
| m (se) | 0.43 (.32)a | 2.97 (.33)b | 3.58 (.28)b | |
| Anxiety | ||||
| m (se) | 13.68 (2.49)a | 29.74 (2.56)b | 33.65 (2.17)b | F (2, 118) = 19.51*** |
| Self-esteem | F (2, 120) = 89.91*** | |||
| m (se) | 1.38 (.25)a | 5.22 (.25)b | 5.42 (.21)b | |
| Attributional style | ||||
| m (se) | 6.68 (1.01)a | 1.56 (1.02)b | 0.02 (.87)b | F (2, 120) = 492.34*** |
Note. HC = health control group. DEP = depressed without NSSI group. NSSI_DEP = depressed with NSSI group.
Non-shared subscripts (a, b) indicate significant differences in post-hoc Tukey’s tests comparing the three groups.
Compares groups for Caucasians vs. non-Caucasians due to discrepant cell sizes for additional ethnicity categories.
Statistics represent comparisons between the DEP and NSSI groups only.
p < 0.05.
p < 0.01.
p < 0.001.
Compared to HC, NSSI and DEP groups both yielded lower IQ and family income, and were more likely to be Caucasian. NSSI and DEP groups also reported more abuse/trauma, higher anti-depressant and anti-psychotic medication intake, more frequent suicide attempts, more chronic and severe depression, lower self-esteem and higher anxiety than HC, and more negative attributional styles; however, the NSSI and DEP groups did not differ from one another on any of these variables. The NSSI group reported higher suicidal ideation than both HC and DEP. Finally, scanning site did not differ among NSSI, DEP, and HC groups [χ2 (2)=5.42, p=0.087]. The following variables were thus included as covariates in analyses with extracted brain activity from coordinates that differed significantly among groups using SPSS software: IQ, ethnicity, depression severity and chronicity, medication intake, and suicide ideation.
2.3. Neuroimaging data acquisition
Data were collected using 3.0 Tesla Siemens Trio MRI scanners in both Minneapolis and Pittsburgh. Structural 3D axial MPRAGE images were acquired in the same session (TR/TE=2100/3.31ms; TI: 1050; Flip Angle 8°; Field of View: 256 × 200mm; Slice-Thickness: 1mm; Matrix: 256 × 200; 176 continuous slices). Mean BOLD images were acquired with a gradient echo EPI sequence during 17:02 minutes covering 60 oblique axial slices (2.0mm thick; TR/TE = 3340/30ms; FOV=200×200mm; matrix 80×80; Flip Angle 90°). Temporal signal to noise ratios were calculated using 3dTstats in Afni by dividing mean baseline estimates (signal) by standard deviations of the residual time series (noise); these ratios were then extracted and were statistically similar, t(476)=2.03, p=0.97. The present sample had movement parameters of maximum absolute shifts in xyz axes of < 2.0 mm or absolute rotations < 0.57 radians. Movement parameters did not differ among groups [F (2,117) =0.40, p =0.67] or data collection sites [F(1,117) =1.64, p =0.20], nor were there any significant group by site interaction effects [F(2, 117) =0.26, p=0.77].
2.4. Interpersonal self-processing task
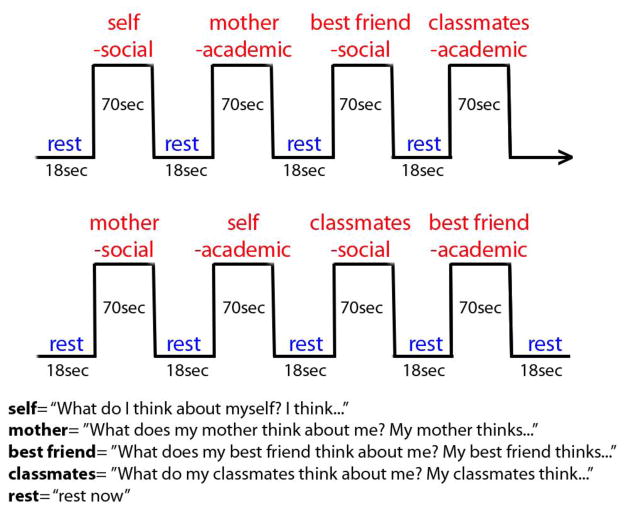
A previously validated Interpersonal Self-Processing task (Pfeifer et al., 2009) assessed both direct and indirect self-processing (see Figure 1). Youth listened to statements about themselves (e.g., “I always have lunch with my friends”, “I am not a good speller”) and responded (via button press) as to whether the statements were self-descriptive, both from their own perspective (direct), and from their mothers’, best friends’, and classmates’ perspectives (indirect). Written reminders of perspective were shown during each condition (e.g., “my mother thinks…” or “I think…”). Further task details are provided in prior studies validating its use to engage the neural basis of self-processing in youth and adults (Pfeifer et al., 2009; Pfeifer and Peake, 2012). Stimulus presentation and recording of responses were obtained using e-prime software version 2.1.
Figure 1.
Direct and reflected/indirect appraisals task: participants endorse or deny self-descriptive phrases from their perspective or the perspective of their mothers, best friend or classmates.
2.5. Analytic plan
Data was previewed before preprocessing with ART, which was also used to correct for unusual scans in the time series (i.e., xyz movement > 2mm), slices and volumes as recommended by Mazaika et al. (2005). Data were preprocessed and analyzed with Statistical Parametric Mapping 8 (SPM8; http://www.fil.ion.ucl.ac.uk/spm). Functional images for each participant were realigned to the first volume in the time series to correct for head motion. Realigned and motion corrected images were co-registered with subjects’ high-resolution anatomical image, segmented, spatially normalized to standard MNI template, re-sliced to voxels of 2mm3 and spatially smoothed with a Gaussian kernel of 7mm full-width at half-maximum (FWHW).
A first-level fixed-effect GLM was constructed for each participant. Hemodynamic response function was applied to model epochs. Scan and predetermined condition effects at each voxel were calculated using a t-statistic, and produced four contrasts of interest for use in second-level models: each perspective (self, mother, best friend, classmates) block minus baseline. A second-level flexible factorial GLM model entered these t-contrast images from the four perspectives (self, mother, best friend, classmates) as a repeated measure within-subjects factor, as recommended to test group by condition effects (Glascher and Gitelman, 2008). This flexible factorial repeated measures ANOVA included two factors (1 between-, 1 within-subjects), Group [3 levels: (NSSI, DEP and HC)] and Perspective [4 levels: self, mother, best friend, and classmate], as well as subject level effects with scanning site as a covariate. See online addendum for reaction time and endorsement of self-descriptors analyses.
To clarify the source of any obtained main effect of group in large cortical midline structure clusters, groups were compared across all self-processing perspectives with contrasts comparing NSSI versus youth without NSSI (i.e., DEP, HC), HC versus depressed youth (i.e., NSSI, DEP), and differences among all three groups. Then, to test our hypothesis regarding perspective effects and, specifically, that NSSI would show higher limbic activity when engaged in self-processing from their mother’s perspective, the flexible factorial repeated measures ANOVA was examined for group differences within each of the four perspectives individually with t-contrasts that were specific to each perspective (e.g., a t-contrast that compared NSSI> DEP > HC for the mother’s perspective only). Labeling of regions was confirmed by the xj-view GUI in SPM8 and Talairach Daemon software.
To correct for multiple comparisons, we calculated whole-brain thresholds via Monte Carlo simulations using the program 3dClustSim in AFNI which yielded a voxel resolution of: 6.86, 6.75, 6.25. Given a voxel-wise threshold of p<.001, a cluster-extent threshold of k=91.5 voxels corresponded with a family-wise-error (FWE) corrected alpha of p < 0.05. This joint magnitude-extent threshold was used in tables and figures. Finally, region of interest (ROI) time series for 7mm spheres centered on coordinates that significantly differed among the groups were extracted using the first eigenvalue function in SPM8. These extractions were used in graphs and additional tests of effects of potential medication, covariates using SPSS software (v. 21). These analyses were Group (3 levels: NSSI, DEP and HC) by Medication (Medication, No Medication) by Brain Activity (ROI) as within participant factors or Group by Brain activity with medication load, number of medications, IQ, suicidal ideation and ethnicity as a covariates. ROI extractions were then also used in correlation analyses with mothers’ reports of support.
3. Results
3.1. Group effects
In the repeated measures ANOVA, a main effect of group was found for five large clusters significant at the whole-brain level. The first spanned occipital and mid-temporal regions including the lingual gyrus, cuneus, and fusiform, [−20 −88 −18], F(2, 476) = 14.49, k=1764; the second was in the precuneus and PCC, [10 −40 48], F (2, 476) = 14.04, k=1037; the third was in the superior frontal gyrus extending across BA8 from lateral to medial dorsal PFC, [32 22 52], F(2, 476)= 4.04, k=445; the fourth encompassed bilateral medial temporal lobe structures and limbic regions including parahippocampus, hippocampus, and amygdala, [30 −38 −22 ], F(2, 476)=13.27, k=602; and the fifth was comprised by the rostral medial prefrontal cortex (rMPFC), including BA10 and subgenual ACC, [−8, 58,14], F(2, 476)=13.17, k=280.
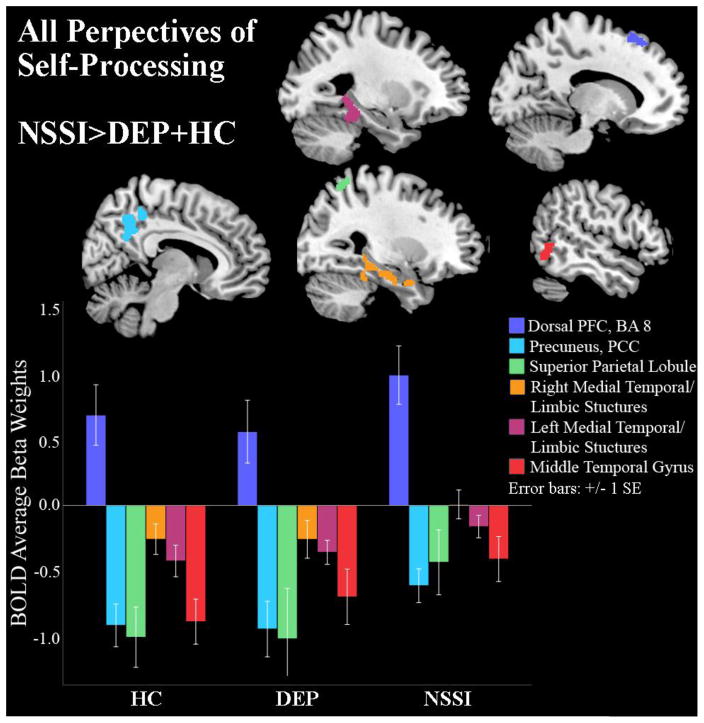
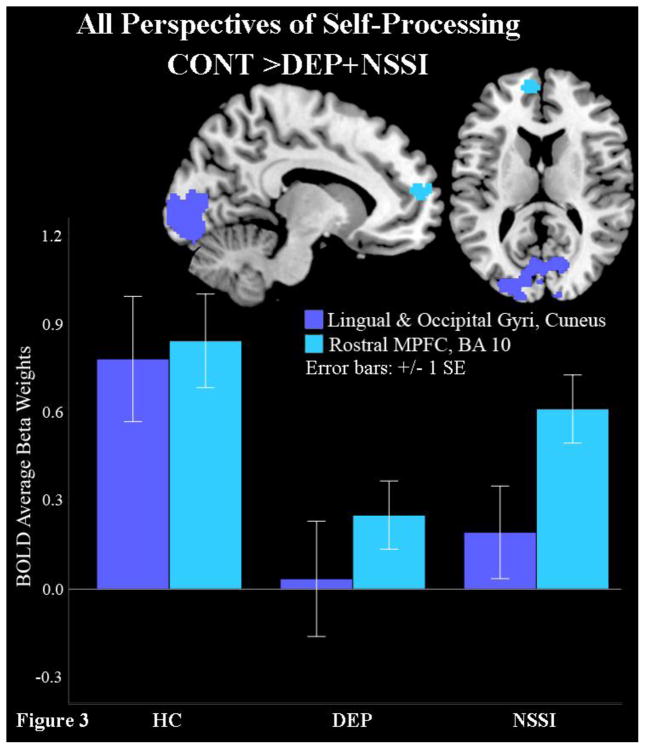
Targeted follow-up contrasts (Table 2) were examined to elucidate the direction of these group effects, collapsed across all four perspectives of self-processing. These contrasts compared (i) NSSI versus both DEP and HC jointly, (ii) HC versus DEP and NSSI jointly and (iii) HC versus NSSI versus DEP separately. First, contrast (i) showed that NSSI had higher BOLD signal than DEP and HC during self-processing in the dorsal prefrontal cortex (BA8), precuneus, PCC, superior parietal lobule, left and right middle temporal limbic structures (amygdala, parahippocampus, and hippocampus), fusiform, and middle temporal gyrus (Figure 2 and Table 2, contrast: NSSI>DEP+HC). Next, contrast (ii) revealed that HC had higher BOLD signal than NSSI and DEP during self-processing in the occipital and rostral medial prefrontal cortex (rMPFC) clusters; however, the ACC activation noted in the omnibus test was absent from the rMPFC cluster (Figure 3, Table 2, contrast: HC>DEP+NSSI). Finally, contrast (iii) showed that, in addition to rMPFC and occipital activation, HC showed less de-activation in the subgenual ACC and caudate compared to NSSI, who in turn had less de-activation in these regions than DEP (see Supplemental Figure). This subgenual ACC cluster fell short of our threshold for significance, but this contrast (iii) was the only test that yielded activity differences previously observed in the omnibus test for the subgenual ACC.
Table 2.
Neural Areas that Distinguish Depressed + NSSI vs. Depressed vs. Healthy Control Adolescents during Self-Referential Processing
| Condition of Self Processing | Contrast | Cluster Size (K) | p(K) | Hemisphere | MNI | t | p | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Across all Points of View: Whole Brain Significant | |||||||||
|
| |||||||||
| Dorsal PFC (BA 8, 6) | NSSI>DEP+HC | 109 | 0.199 | Left | −16 | 32 | 56 | 5.19 | 0.006 |
| Precuneus (BA 7), Mid-Cingulate (BA 31), Posterior Cingulate Cortex (BA 5, 23, 30, 29) | 440 | 0.001 | Right | 12 | −40 | 48 | 5.19 | 0.007 | |
| Superior Parietal Lobule (BA 7, 40) | 111 | 0.191 | Right | 30 | −60 | 60 | 5.06 | 0.012 | |
| Parahippocampus, Fusiform, Hippocampus, Amygdala (BA 20, 37, 36, 38, 28) | 476 | 0.000 | Right | 28 | 2 | −28 | 5.04 | 0.012 | |
| Parahippocampus, Fusiform, Hippocampus (BA 37, 36, 20, 19) | 329 | 0.004 | Left | −30 | −34 | −12 | 5.02 | 0.014 | |
| Middle Temporal Gyrus (BA 37, 19) | 158 | 0.074 | Right | 58 | −60 | 0 | 4.84 | 0.030 | |
|
| |||||||||
| Middle Occipital Gyrus, Lingual Gyrus ( BA 18, 17, 19), Cuneus (BA 23) | HC>DEP+NSSI | 3079 | 0.000 | Left | −20 | −88 | −18 | 5.37 | 0.003 |
| Rostral Medial Prefrontal Cortex (BA 10, 32) | 138 | 0.049 | Left | −8 | 58 | 14 | 4.16 | 0.241 | |
|
| |||||||||
| Mother's Point of View: Whole Brain Significant | |||||||||
|
| |||||||||
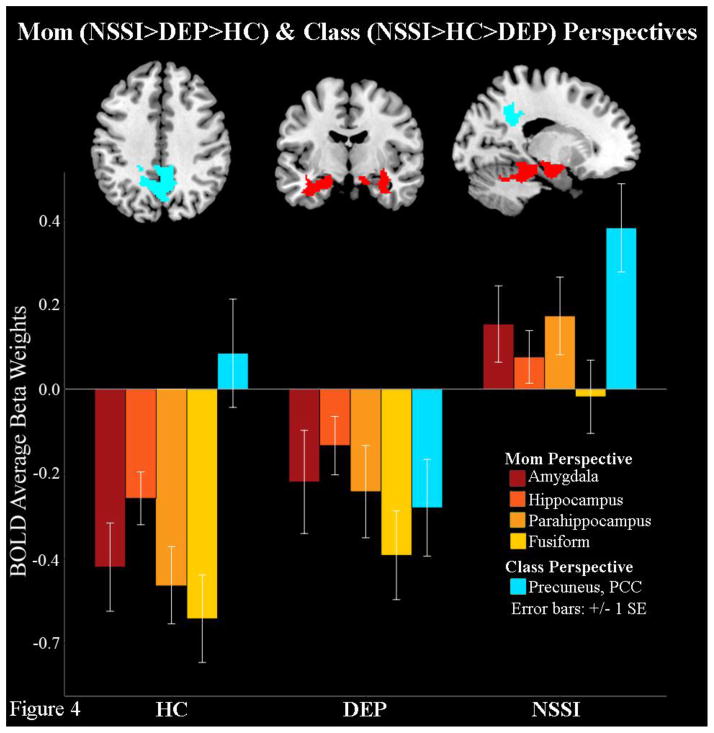
| Parahippocampus, Fusiform, Amygdala, Hippocampus (BA 37, 36, 20, 35, 28, 34, 19) | NSSI>DEP>HC | 1325 | 0.000 | Left and Right | −18 | −32 | −16 | 5.19 | 0.007 |
|
| |||||||||
| Classmates Point of View: Whole Brain Significant | |||||||||
|
| |||||||||
| Precuneus (BA 7, BA 31, BA 40), Posterior Cingulate Cortex (BA 7, 31, 5, 23, 30, 29) | NSSI>HC>DEP | 1493 | 0.024 | Right | 8 | −58 | 30 | 3.93 | 0.026 |
Figure 2.
Depressed youth with NSSI behavior show higher superior frontal gyrus activity than depressed and healthy control youth and less deactivation in the superior parietal lobule, middle temporal gyrus and limbic structures.
Figure 3.
Healthy adolescents show higher frontal pole (BA10), lingual and cuneus activity than both depressed groups.
3.1.1. Medication and covariate effects
For analyses conducted within depressed participants for the extracted significant clusters pertinent to contrast (i) [i.e., superior frontal gyurs (BA8), precuneus, PCC, limbic regions], no significant effects of medication presence, F(1, 116)=0.02, p=0.89, dosage load, F(26, 86)=0.81, p=0.73, or total number of medications, F(3, 113)=0.46, p=0.71, were found. Further, no effects of group interaction with medication presence, F(2, 116)=0.78, p=0.46, dosage load, F(7, 86)=0.50, p=0.83, or total number of medications, F(3, 113)=0.83, p=0.48, were found. Finally, no individual category of medication (antidepressants, anxiolytics, mood stabilizers, stimulants, antipsychotics) significantly interacted with group membership, F(2, 116)=0.001 to 1.90, p=0.45 to 0.97. Similarly, no covariates (IQ, ethnicity, depression severity/chronicity, suicide ideation) were significantly associated with activity from extracted clusters, suggesting that group differences in brain activity were not due to medication effects or to variables that differed among participant groups.
3.2. Group by perspective self-processing effects
To test the second hypothesis, group by perspective interactions were tested with contrasts for each self-processing perspective. Contrast (iv), NSSI>DEP>HC, revealed that when engaged in self-reflection from their mother’s perspective NSSI youth had greater activation in the bilateral amygdala, hippocampus, parahippocampus and fusiform compared to DEP, who in turn activated these areas more than HC (Table 2, Figure 4). Finally, although not hypothesized, group by perspective contrasts also showed that NSSI youth had greater activation in precuneus and PCC compared to HC during the classmates’ perspective, who in turn showed more activity than DEP (Figure 4). No group differences in brain activity were found during self-referential processing from the self or best-friend perspectives.
Figure 4.
Depressed youth with NSSI behaviors show higher bilateral limbic and precuneus activity during self-appraisals from their mothers’ and their classmates’ perspectives respectively, compared to depressed and healthy control youth.
3.2.2. Medication and covariates effects
No significant effects of medication presence, F(1, 117) =0.06, p = .81, dosage load, F(27, 86)=0.83, p = 0.70, or total number of medications, F(3, 114) = 1.00, p=0.40, were found for the extractions of significant neural clusters pertinent to contrast (iv) (i.e., bilateral amygdala, hippocampus, parahippocampus and fusiform). Interactions between group and medication presence, F(2, 117)=0.46, p = 0.63, medication load, F(7, 86)=0.37, p=0.92, and total number of medications, F (3, 114)=0.34, p = 0.80, were also non-significant. Finally, no category of medication (antidepressants, anxiolitic, mood stabilizers, stimulants, antipsychotics) by group interactions were found, F (2, 117)=0.04 to 2.59, p=0.11 to 0.84. None of the included covariates (IQ, ethnicity, depression severity/chronicity, suicide ideation) yielded significant effects, suggesting that group by perspective results were not due to effects of medication or to variables that differed among groups.
3.2.3. Follow-up correlations with maternal support of emotional experience
Additional correlational analyses were conducted to explore our follow-up question regarding associations between BOLD activation in the NSSI group during self-processing from the mothers’ perspective, and mothers’ reports of their provision of support to adolescent’s negative emotions (Table 3). Results showed a consistent pattern of negative correlations between maternal support of adolescent emotions and the NSSI group’s activation in the amygdala and parahippocampus, and marginal negative correlations with fusiform activity. In contrast, maternal support was not associated with BOLD activation in HC and was marginally positively associated with hippocampal and parahippocampal activation in DEP.
Table 3.
Correlations between Mother-reported Supportive Response to Adolescent Emotions a and BOLD Activation in Limbic Regions and Fusiform
| Neural Region of BOLD activity | HC | DEP | NSSI |
|---|---|---|---|
| R Amygdala | 0.01 | 0.19 | −0.29* |
| L Amygdala | 0.07 | 0.15 | −0.20 |
| R Hippocampus | <0.01 | 0.34+ | −0.13 |
| L Hippocampus | −0.01 | 0.25 | −0.08 |
| R Parahippocampus | 0.04 | 0.13 | −0.35** |
| L Parahippocampus | 0.22 | 0.29+ | −0.27* |
| R Fusiform | 0.06 | 0.03 | −0.22 |
| L Fusiform | 0.10 | 0.08 | −0.24+ |
Note. R=right hemisphere. L = left hemisphere. HC = healthy controls. DEP = depressed only. NSSI = depressed + NSSI behavior.
Assessed using the Emotion Socialization Measure, a maternal report of parenting response style to child emotional experiences of sadness, anger, or fear.
p ≤ 0.10.
p < 0.05.
p < 0.01.
4. Discussion
This study aimed to distinguish the neurobiology of self-injuring youth from depressed and psychologically healthy adolescents during direct and indirect self-processing. The majority of hypotheses were supported. First, NSSI youth showed greater limbic and CMS activation during self-referential processing across all perspectives compared to DEP and HC. Second, NSSI was associated with greater limbic activation during self-processing than DEP from the mothers’ perspective, and DEP in turn showed greater limbic activity than HC. Within the NSSI group, greater limbic activation during the mothers’ perspective showed theoretically sound, unique correlations with mother’s lack of support toward adolescents’ negative emotions. Finally, exploratory findings showed higher occipital (lingual and cuneus) and rMPFC activity during self-processing for HC compared to NSSI and DEP, and NSSI yielded higher posterior CMS activity during the classmates’ perspectives versus HC and DEP.
4.1. Group differences in self-processing
Across all four self-processing perspectives, HC showed higher activity than NSSI and DEP in the rMPFC (BA 10) and tended to have more subgenual ACC and caudate activity compared to all depressed youth (supplemental figure). HC also showed higher activity in areas supporting visual, word processing and reading (occipital and lingual gyrus regions) (Bookheimer et al., 1995; Price et al., 1994). Activity in the rMPFC (BA10) is strongly linked to self-processing (Flagan and Beer, 2013). The subgenual ACC is thought to integrate emotional and cognitive signals into decision making processes, integrate conflictive information (Phillips et al., 2003b, c; Phillips et al., 2008), and to support implicit emotion regulation given its interconnections with limbic structures (Bush et al., 2000; Davis et al., 2005). Indeed, rMPFC activation has previously been identified during self-processing in typically-developing adolescents (Dégeilh et al., 2015; Jankowski et al., 2014). Thus, our findings replicate and extend prior research regarding psychologically healthy youth. An identified trend toward less ACC deactivation in HC versus all depressed youth, suggests HC may successfully regulate emotional processes during all conditions of self-processing. However, the marginal significance of these results precludes more precise interpretation.
NSSI youth showed greater dorsal PFC (BA8) activation than both HC and DEP. BA8 is associated with social cognition (Amodio and Frith, 2006; Gusnard et al., 2001) and voluntary emotion regulation (Phillips et al., 2008). As such, these differences suggest that self-injuring, depressed adolescents may exert more conscious regulatory control over their affect (perhaps unsuccessfully, given corresponding limbic hyperactivity) and/or social cognitive effort during self-processing across various social perspectives. However, these findings cannot determine which particular function of the dorsal PFC activity was specifically engaged by NSSI youth, thus this should be further explored in subsequent investigations.
NSSI youth also demonstrated relatively greater precuneus, PCC and superior parietal lobe activity across all perspectives (i.e., less deactivation versus other groups). Relatively higher PCC activation might represent “getting caught up in” one’s experience, given that present-centered awareness has been linked to PCC deactivation (Brewer et al., 2013). Additionally, areas of the superior parietal gyrus are engaged during self-recognition (van Veluw and Chance, 2014), and remembrance of events from the personal past (Spreng and Mar, 2012). Furthermore, both the PCC and precuneus enable episodic memory retrieval (Cavanna and Trimble, 2006) which is also supported by the hippocampus. These findings suggest that, compared to HC and DEP, NSSI youth might be recalling, and becoming relatively more immersed in, past autobiographical memories to process their self-representations from the perspectives of others.
4.2. Group differences in specific indirect self-processing perspectives
As predicted, NSSI youth showed distinct patterns of limbic (i.e., bilateral amygdala, hippocampus, parahippocampus) and fusiform hyperactivation when adopting the perspective of their mothers compared to DEP and HC. Similar limbic hyperactivity has been reported among self-injuring patients during processing of emotional stimuli in prior research (Davis et al., 2014; Osuch et al., 2014; Plener et al., 2012). Prior research suggests amygdala activation is more reliably elicited by negative stimuli (Dickstein and Leibenluft, 2006; Phillips et al., 2003a) or threatening experiences (Ochsner et al., 2012), though this region is active during processing of highly relevant or emotionally salient stimuli, regardless of emotional valence (Cunningham and Brosch, 2012). Amygdala activity is also required for retrieval of fear memories (Erlich et al., 2012) and, with the hippocampus, the amygdala enables retrieval of emotional memories (Phelps, 2004; Richardson et al., 2004). Minimally, the joint hyperactivation of the amygdala, hippocampus, and parahippocampus suggest that NSSI youth may retrieve more emotionally charged and salient memories, regardless of valence, than DEP and HC when taking their mothers’ perspective (Cipolotti and Moscovitch, 2005; Greenberg et al., 2005; Piolino et al., 2004). Finally, the fusiform is part of a network (along with the amygdala and other neocortical areas) believed to be responsible for socioemotional cognition (Adolphs and Spezio, 2006; Allison et al., 2000; Bokde et al., 2006; Fenker et al., 2005). Thus, in combination with high limbic activity, fusiform activation may indicate that self-injuring youths engaged in intense socioemotional processing when taking their mother’s perspective. Future experiments ought to establish whether high limbic and fusiform activity among self-injuring youth during self-appraisals from the mother’s perspective are specifically linked with retrieval of affectively charged memories about salient social relationships (e.g. negatively valenced memories), or with general high emotionality that has been previously noted among patients with NSSI. Additional research should also examine associations between neural activation and informant reports regarding the valence of the emotions and memories recalled during the various conditions of the task.
It is notable that limbic hyperactivity was elicited only by self-processing from the mother’s perspective, and that activation in these regions showed a unique pattern of negative correlations with self-injurers’ mothers’ reports of their low provision of support in response to the child’s negative emotions. Given that prior research in healthy controls has yielded limbic activation in healthy youth while listening to maternal criticism (Lee et al., 2015), the current findings suggest that the mother-child relationship, or at least the youth’s interpretation of how their mother perceives them, may induce strong emotions (as evidenced by high limbic activity) in self-injuring youth (Kaess et al., 2013; Martin et al., 2015; Martin et al., 2016; Nock et al., 2009). Combined with prior research regarding associations between negative parent-child relationships and NSSI (Adrian et al., 2011; Yurkowski et al., 2015), our follow-up analyses demonstrating correlations between low maternal emotional support and high limbic activity suggest that NSSI youths’ limbic hyperactivation during self-referential processing from their mother’s perspective may be linked with maternal emotional invalidation. This notion is bolstered by prior research that has shown hostile mother-child relationships are linked with altered psychophysiology of emotion regulation and with intense anger among self-injurious adolescents (Crowell et al., 2013).
In summary, NSSI youth appear to experience intense emotional activation subserved by high limbic activity during self-processing from the mother’s point of view. That said, additional research specifically targeting adolescents’ reports of their specific emotional experiences while engaging in self-reflection from their mothers’ perspective is required to further inform this interpretation. In turn, high limbic activity was linked to the parent’s report of low support of their child’s experiences of anger, sadness or fear. Together, these results lend credence to the supposition that NSSI is associated with variation in affective processing that can be linked to emotional invalidation by primary caregivers. However, due to the correlational and cross-sectional nature of the current study, this explanation ought to be verified with longitudinal research that links emotion socialization practices to NSSI and brain function over time. Additional investigation should further examine the associations between mother-adolescent relationship challenges and alteration of emotional processing longitudinally, as this area remains vastly understudied.
Finally, depressed youth with NSSI showed greater activation of posterior CMS during self-processing from their classmates’ perspective than DEP, yet resembled HC youth who showed close to zero activity during their classmates’ perspective. Greater similarity between NSSI and HC in these regions while taking classmates’ perspectives, compared to DEP youth, is intriguing. It might be that NSSI attempt (unsuccessfully) to regulate their emotions, whereas DEP youth (who experience less intense affect than NSSI but who also have fewer regulatory skills than HC) engage the posterior CMS to a lesser extent than both NSSI and HC during this perspective. Greater precuneus and PCC activity for NSSI youth resembles findings among self-harming adults during regulation of aversive emotions (Crowell et al., 2013; Davis et al., 2014) and, as scholars have previously suggested, may indicate accessing of autobiographical memories (Lang et al., 1983) or the anticipation of negative stimuli (Scherpiet et al., 2014). Thus, it is possible that NSSI youth hold negative cognitions regarding their peers, or recall negative interactions with peers, when engaging in self-processing from classmates’ perspectives. Alternatively, self-injurers may be isolated from the peer group (Amitai and Apter, 2012; Whitlock et al., 2013b), and greater neural activation may reflect the increased effort required for self-reflection from this particular perspective due to lack of familiarity with peers’ perspectives. Regardless, additional research is needed to corroborate or disconfirm these various possibilities. The lack of limbic activation during self-processing from classmates’ perspectives also bears comment. Specifically, it is possible that peers are more similar to the participants or that reflecting upon the self from peers’ perspectives is less likely to result in strong emotional response (Claes et al., 2010a; Hasking et al., 2013; Prinstein et al., 2010) compared to self-reflection from the mothers’ perspective.
4.3 Limitations
Despite the strengths of this investigation, there are a few limitations to note. First, given the cross-sectional design used, trajectories of depression and self-injury and their corresponding links to neural activity remain unclear. Additional research including such trajectories may reveal processes by which neural disruptions in self-processing develop and progress throughout the duration of depressive illness or engagement in NSSI. Second, as data were collected between two sites, some characteristics unaccounted for could have influenced the results. Nonetheless, key variables did not differ between sites. Third, self-processing from the perspective of other caregivers (e.g., fathers) or measures of family hostility and emotional invalidation may have yielded varying results. Finally, our findings may not be generalizable to all self-injuring youth, particularly those who engage in NSSI and have no psychiatric diagnosis, and the lack of available detail regarding NSSI behavior (i.e., methods, motivations) and other associated symptoms (presence of borderline personality traits) further limit generalizability.
4.4 Conclusions
Our findings suggest that during interpersonal self-processing, particularly from the mother’s perspective, depressed adolescents who engage in NSSI experience greater activation of limbic structures, and may rely more strongly on past autobiographical memories than either depressed youth without NSSI or healthy controls. This study demonstrates the power of fMRI to distinguish depressed adolescents with NSSI from those with depression diagnosis alone using the context of relevant social processes. Future research should continue to study the neurological and physiological underpinnings of this self-damaging behavior using longitudinal methodologies.
Supplementary Material
Supplemental Figure. Healthy adolescents, in addition to higher frontal pole (BA10), lingual and cuneus activity, show less de-activation in the sub-genual ACC than depressed youth with NSSI behaviors who in turn showed less de-activation than depressed youth.
Highlights.
Disrupted emotions, social processes and relationships are salient to NSSI
Identifying neural markers of NSSI can improve assessment and treatment efficiency
NSSI relates to high activity in areas that support socioemotional processing during self-reflection from mother’s perspective
Neural patterns of self-reflection differentiate youth with NSSI are linked to maternal self-report of low emotional support for their teens.
Acknowledgments
This research was supported by a NIMH, K01MH092601 and a NARSAD to the first author and a Social Sciences and Humanities Research Council of Canada Postdoctoral Fellowship to the second author. We are very grateful to Dr. Tom Zeffiro and Dr. Kathleen Thomas, who were key mentors and supporters of the first author’s K award application.
Footnotes
Financial Disclosures
None of the authors report biomedical financial interests or potential conflicts of interest.
Addendum: Behavioral data analyses and results.
Author Contributions
Karina Quevedo: Writing of supporting grant, data collection, primary data analyses, manuscript writing.
Jodi Martin: Assisted in analyses, writing and revision of the manuscript.
Hannah Scott: Data collection, primary data analyses, figures and tables generation.
Garry Smyda: Scripting of fMRI data preprocessing and first level analyses.
Jennifer Pfeifer: Manuscript editing and revision.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R, Spezio M. Role of the amygdala in visual social stimuli Progress in Brain Research. 2006;156:363–378. doi: 10.1016/S0079-6123(06)56020-0. [DOI] [PubMed] [Google Scholar]
- Adrian M, Zeman J, Erdley C, Lisa L, Sim L. Emotional dysregulation and interpersonal difficulties as risk factors for nonsuicidal self-injury in adolescent girls. J Abnorm Child Psychol. 2011;39:389–400. doi: 10.1007/s10802-010-9465-3. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: Role of the STS region. Trends in Cognitive Neurosciences. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Amitai M, Apter A. Social aspects of suicidal behavior and prevention in early life: A review. Internation Journal of Environmental Research and Public Health. 2012;9:985–994. doi: 10.3390/ijerph9030985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Barrocas AL, Giletta M, Hankin BL, Prinstein MJ, Abela JR. Nonsuicidal Self-Injury in Adolescence: Longitudinal Course, Trajectories, and Intrapersonal Predictors. J Abnorm Child Psychol. 2015;43:369–380. doi: 10.1007/s10802-014-9895-4. [DOI] [PubMed] [Google Scholar]
- Bokde AL, Lopez-Bayo P, Meindl T, Pechler S, Born C, Faltraco F, Teipel SJ, Moller HJ, Hampel H. Functional connectivity of the fusiform gyrus during a face-matching task in subjects with mild cognitive impairment. Brain. 2006;129:113–1124. doi: 10.1093/brain/awl051. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Zefro TA, Blaxton T, Gaillard W, Theodore W. Regional cerebral blood flow during object naming and word reading. Human Brain Mapping. 1995;3:93–106. [Google Scholar]
- Brettschneider C, Riedel-Heller S, Konig HH. A systematic review of economic evaluations of treatments for borderline personality disorder. PLoS One. 2014;9:e107748. doi: 10.1371/journal.pone.0107748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Garrison KA, Whitfield-Gabrieli S. What about the “self” is processed in the posterior cingulate cortex? Front Hum Neurosci. 2013;7:647. doi: 10.3389/fnhum.2013.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner R, Kaess M, Parzer P, Fischer G, Carli V, Hoven CW, Wasserman C, Sarchiapone M, Resch F, Apter A, Balazs J, Barzilay S, Bobes J, Corcoran P, Cosmanm D, Haring C, Iosuec M, Kahn JP, Keeley H, Meszaros G, Nemes B, Podlogar T, Postuvan V, Saiz PA, Sisask M, Tubiana A, Varnik A, Wasserman D. Life-time prevalence and psychosocial correlates of adolescent direct self-injurious behavior: a comparative study of findings in 11 European countries. J Child Psychol Psychiatry. 2014;55:337–348. doi: 10.1111/jcpp.12166. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in cognitive sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Moscovitch M. The hippocampus and remote autobiographical memory. Lancet Neurol. 2005;4:792–793. doi: 10.1016/S1474-4422(05)70232-5. [DOI] [PubMed] [Google Scholar]
- Claes L, Houben A, Vandereycken W, Bijttbier P, Muehlenkamp JJ. The association between non-suicidal self-injury, self-concept, and acquaintance with self-injurious peers in a sample of adolescents. Journal of Adolescence. 2010a;33:775–778. doi: 10.1016/j.adolescence.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Claes L, Houben A, Vandereycken W, Bijttebier P, Muehlenkamp J. Brief report: the association between non-suicidal self-injury, self-concept and acquaintance with self-injurious peers in a sample of adolescents. J Adolesc. 2010b;33:775–778. doi: 10.1016/j.adolescence.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Claes L, Luyckx K, Bijttebier P, Turner B, Ghandi A, Smets J, Norre J, Van Assche L, Verheyen E, Goris Y, Hoksbergen I, Schoevaerts K. Non-suicidal self-injury in patients with eating disorder: associations with identity formation above and beyond anxiety and depression. Eur Eat Disord Rev. 2015;23:119–125. doi: 10.1002/erv.2341. [DOI] [PubMed] [Google Scholar]
- Conley CS, Haines BA, Hilt LM, Metalsky GI. The Children’s Attributional Style Interview: Developmental tests of cognitive diathesis-stress theories of depression. Journal of abnormal child psychology. 2001;29:445–463. doi: 10.1023/a:1010451604161. [DOI] [PubMed] [Google Scholar]
- Cooney RE, Joormann J, Eugene F, Dennis EL, Gotlib IH. Neural correlates of rumination in depression. Cognitive, Affective, & Behavioral Neuroscience. 2010;10:470–478. doi: 10.3758/CABN.10.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell SE, Baucom BR, McCauley E, Potapova NV, Fitelson M, Barth H, Smith CJ, Beauchaine TP. Mechanisms of contextual risk for adolescent self-injurers: Invalidation and conflict escalation in mother-child interactions. Journal of Clinical Child & Adolescent Psychology. 2013;42:467–480. doi: 10.1080/15374416.2013.785360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, Linehan MM. biosocial developmental model of borderline personality: Elaborating and extending linehan’s theory. Psychological Bulletin. 2009;135:495–510. doi: 10.1037/a0015616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham WA, Brosch T. Motivational salience amygdala tuning from traits, needs, values, and goals. Current Directions in Psychological Science. 2012;21:54–59. [Google Scholar]
- Davis KD, Taylor KS, Hutchison WD, Dostrovsky JO, McAndrews MP, Richter EO, Lozano AM. Human anterior cingulate cortex neurons encode cognitive and emotional demands. J Neurosci. 2005;25:8402–8406. doi: 10.1523/JNEUROSCI.2315-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TS, Mauss IB, Lumian D, Troy AS, Shallcross AJ, Zarolia P, Ford BQ, McRae K. Emotional reactivity and emotion regulation among adults with a history of self-harm: laboratory self-report and functional MRI evidence. J Abnorm Psychol. 2014;123:499–509. doi: 10.1037/a0036962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dégeilh F, Guillery-Girard B, Dayan J, Gaubert M, Chételat G, Egler PJ, Baleyte JM, Eustache F, Viard A. Neural correlates of self and its interaction with memory in healthy adolescents. Child Development. 2015;86:1966–1983. doi: 10.1111/cdev.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DP, Leibenluft E. Emotion regulation in children and adolescents: Boundaries between normalcy and bipolar disorder. Development & Psychopathology. 2006;18:1105–1131. doi: 10.1017/S0954579406060536. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Puzia ME, Cushman GK, Weissman AB, Wegbreit E, Kim KL, Nock MK, Spirito A. Self-injurious implicit attitudes among adolescent suicide attempters versus those engaged in nonsuicidal self-injury. J Child Psychol Psychiatry. 2015 doi: 10.1111/jcpp.12385. [DOI] [PubMed] [Google Scholar]
- Doerig N, Schlumpf Y, Spinelli S, Spati J, Brakowski J, Quednow BB, Seifritz E, Grosse Holtforth M. Neural representation and clinically relevant moderators of individualised self-criticism in healthy subjects. Soc Cogn Affect Neurosci. 2014;9:1333–1340. doi: 10.1093/scan/nst123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich JC, Bush DE, Ledoux JE. The role of the lateral amygdala in the retrieval and maintenance of fear-memories formed by repeated probabilistic reinforcement. Front Behav Neurosci. 2012;6:16. doi: 10.3389/fnbeh.2012.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenker DB, Schott BH, Richardson-Klavehn A, Heinze HJ, Duzel E. Recapitulating emotional context: activity of amygdala, hippocampus and fusiform cortex during recollection and familiarity. Eur J Neurosci. 2005;21:1993–1999. doi: 10.1111/j.1460-9568.2005.04033.x. [DOI] [PubMed] [Google Scholar]
- Flagan T, Beer JS. Three ways in which midline regions contribute to self-evaluation. Front Hum Neurosci. 2013;7:450. doi: 10.3389/fnhum.2013.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glascher J, Gitelman D. In: Contrast weights in flexible factorial desing with multiple groups of subjects. Sml, editor. SPM@ JISCMAIL; AC. UK: 2008. [Google Scholar]
- Glassman LH, Weierich MR, Hooley JM, Deliberto TL, Nock MK. Child maltreatment, non-suicidal self-injury, and the mediating role of self-criticism. Behav Res Ther. 2007;45:2483–2490. doi: 10.1016/j.brat.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn CR, Klonsky ED. Nonsuicidal Self-Injury Disorder: An Empirical Investigation in Adolescent Psychiatric Patients. J Clin Child Adolesc Psychol. 2013 doi: 10.1080/15374416.2013.794699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn JJ, Michel BD, Franklin JC, Hooley JM, Nock MK. Pain analgesia among adolescent self-injurers. Psychiatry Res. 2014;220:921–926. doi: 10.1016/j.psychres.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Rice HJ, Cooper JJ, Cabeza R, Rubin DC, Labar KS. Co-activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia. 2005;43:659–674. doi: 10.1016/j.neuropsychologia.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Groschwitz RC, Plener PL, Kaess M, Schumacher T, Stoehr R, Boege I. The situation of former adolescent self-injurers as young adults: a follow-up study. BMC Psychiatry. 2015;15:160. doi: 10.1186/s12888-015-0555-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale WW, 3rd, Raaijmakers Q, Muris P, Meeus W. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED) in the general adolescent population. J Am Acad Child Adolesc Psychiatry. 2005;44:283–290. doi: 10.1097/00004583-200503000-00013. [DOI] [PubMed] [Google Scholar]
- Harter S. The Perceived Competence Scale for Children. Child Development. 1982;53:87–97. [PubMed] [Google Scholar]
- Harter S. The construction of the self: A developmental perspective. Guilford Press; New York, NY, US: 1999. [Google Scholar]
- Hasking P, Andrews T, Martin G. The role of exposure to self-injury among peers in predicting later self-injury. Journal of Youth and Adolescence. 2013;42:1543–1556. doi: 10.1007/s10964-013-9931-7. [DOI] [PubMed] [Google Scholar]
- Heath N, Schaub KM, Holly S, Nixon MK. Self-injury today: Review of population and clinical studies in adolescence. In: Nixon MK, Heath N, editors. Self-injury in youth: The essential guide to assessment and intervention. Routledge Press; New York, NY: 2009. pp. 9–27. [Google Scholar]
- Hilt LM, Cha CB, Nolen-Hoeksema S. Nonsuicidal self-injury in young adolescent girls: moderators of the distress-function relationship. J Consult Clin Psychol. 2008;76:63–71. doi: 10.1037/0022-006X.76.1.63. [DOI] [PubMed] [Google Scholar]
- Hooley JM, St Germain SA. Nonsuicidal self-injury, pain, and self-criticism. Clinical Psychological Science. 2014;2:297–305. [Google Scholar]
- Ichikawa N, Siegle GJ, Jones NP, Kamishima K, Thompson WK, Gross JJ, Ohira H. Feeling bad about screwing up: emotion regulation and action monitoring in the anterior cingulate cortex. Cogn Affect Behav Neurosci. 2011;11:354–371. doi: 10.3758/s13415-011-0028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- In-Albon T, Ruf C, Schmid M. Proposed Diagnostic Criteria for the DSM-5 of Nonsuicidal Self-Injury in Female Adolescents: Diagnostic and Clinical Correlates. Psychiatry J. 2013;2013:159208. doi: 10.1155/2013/159208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski KF, Moore WE, Merchant JS, Kahn LE, Pfeifer JH. But do you think I'm cool? Developmental differences in striatal recruitment during direct and reflected social self-evaluations. Developmental cognitive neuroscience. 2014;8:40–54. doi: 10.1016/j.dcn.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaess M, Parzer P, Mattern G, Plener PL, Bifulco A, Resch F, Brunner R. Adverse childhood experiences and their impact on frequency, severity, and the individual function of nonsuicidal self-injury in youth. Psychiatry Research. 2013;206:265–272. doi: 10.1016/j.psychres.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, DW, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Aged Children - Present and Lifetime (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Senior C, Phillips ML, Benson PJ, Bullmore ET, Brammer M, Simmons A, Williams SC, Bartels M, David AS. Towards a functional neuroanatomy of self processing: effects of faces and words. Brain research. 2000;10:133–144. doi: 10.1016/s0926-6410(00)00036-7. [DOI] [PubMed] [Google Scholar]
- Klonsky ED, May AM, Glenn CR. The relationship between nonsuicidal self-injury and attempted suicide: converging evidence from four samples. J Abnorm Psychol. 2013;122:231–237. doi: 10.1037/a0030278. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Levin DN, Miller GA, Kozak MJ. Fear behavior, fear imagery, and the psychophysiology of emotion: The problem of affective response integration. Journal of Abnormal Psychology. 1983;92:276–306. doi: 10.1037//0021-843x.92.3.276. [DOI] [PubMed] [Google Scholar]
- Lee KH, Siegle GJ, Dahl RE, Hooley JM, Silk JS. Neural responses to maternal criticism in healthy youth. Soc Cogn Affect Neurosci. 2015;10:902–912. doi: 10.1093/scan/nsu133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. Guilford Press; New York City, NY: 1993. [Google Scholar]
- Lofthouse N, Muehlenkamp JJ, Adler R. Nonsuicidal self-injury and co-occurrence. In: Nixon MK, Heath N, editors. Self-injury in Youth: The Essential Guide to Assessment and Intervention. Routledge Press; New York, NY: 2009. pp. 59–78. [Google Scholar]
- Lundh LG, Wangby-Lundh M, Ulander J. Emotional tone in young adolescents’ close relationships and its association with deliberate self-harm. Interpersona. 2009;3:111–138. [Google Scholar]
- Malatesta-Magai C. Emotional socialization: Its role in personality and developmental psychopathology. In: Cicchetti D, Toth SL, editors. Rochester Symposium on Developmental Psychopathology: Vol. 3. Internalizing and externalizing expressions of dysfunction. Lawrence Erlbaum Associates, Inc; Hillsdale, NJ: 1991. pp. 203–224. [Google Scholar]
- Marceau K, Ram N, Susman E. Development and Lability in the Parent-Child Relationship During Adolescence: Associations With Pubertal Timing and Tempo. J Res Adolesc. 2015;25:474–489. doi: 10.1111/jora.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Bureau J-F, Lafontaine M-F, Cloutier PF, Hsiao C, Pallanca D, Meinz P. Associations between Non-suicidal Self-injury, Attachment Representations, and Maltreatment. Development & Psychopathology. 2015 doi: 10.1017/S0954579417000050. [DOI] [PubMed] [Google Scholar]
- Martin J, Bureau JF, Yurkowski K, Renaud Fournier T, Lafontaine MF, Cloutier P. Family-based risk factors for non-suicidal self-injury: Influences of maltreatment, adverse family-life experiences, and relational trauma. Journal of Adolescence. 2016 doi: 10.1016/j.adolescence.2016.03.015. [DOI] [PubMed] [Google Scholar]
- Mazaika P, Whitfield S, Cooper J. Detection and Repairof Transient Artifacts in fMRI Data. H BM 2005 [Google Scholar]
- Moran P, Coffey C, Romaniuk H, Olsson C, Borschmann R, Carlin JB, Patton GC. The natural history of self-harm from adolescence to young adulthood: A population-based cohort study. The Lancet. 2012;379:236–243. doi: 10.1016/S0140-6736(11)61141-0. [DOI] [PubMed] [Google Scholar]
- Nixon MK, Heath N. Introduction to non-suicidal self-injury in adolescents. In: Nixon MK, Heath N, editors. Self-injury in youth: The essential guide to assessment and intervention. Routledge Press; New York, NY: 2009. pp. 1–6. [Google Scholar]
- Nock MK, Joiner TE, Jr, Gordon KH, Lloyd-Richardson E, Prinstein MJ. Nonsuicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144:65–72. doi: 10.1016/j.psychres.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Nock MK, Prinstein MJ, Serba SK. Revealing the form and function of self-injurious thoughts and behaviors: A real time ecological momentary assessment study among adolescents and young adults. Journal of Abnormal Psychology. 2009;118:816–827. doi: 10.1037/a0016948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuch E, Ford K, Wrath A, Bartha R, Neufeld R. Functional MRI of pain application in youth who engaged in repetitive non-suicidal self-injury vs. psychiatric controls. Psychiatry Res. 2014;223:104–112. doi: 10.1016/j.pscychresns.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Paikoff RL, Brooks-Gunn J. Do parent-child relationships change during puberty? Psychol Bull. 1991;110:47–66. doi: 10.1037/0033-2909.110.1.47. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Tobin-Richards M, Boxer A. Measuring pubertal status: Reliability and validity of a self-report measure. 1985 doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Borofsky LA, Dapretto M, Fuligni AJ, Lieberman MD. Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Dev. 2009;80:1016–1038. doi: 10.1111/j.1467-8624.2009.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Peake SJ. Self-development: integrating cognitive, socioemotional, and neuroimaging perspectives. Developmental cognitive neuroscience. 2012;2:55–69. doi: 10.1016/j.dcn.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Current opinion in neurobiology. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry. 2003a;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003b;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003c;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:829, 833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piolino P, Giffard-Quillon G, Desgranges B, Chetelat G, Baron JC, Eustache F. Re-experiencing old memories via hippocampus: a PET study of autobiographical memory. Neuroimage. 2004;22:1371–1383. doi: 10.1016/j.neuroimage.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Plener PL, Bubalo N, Fladung AK, Ludolph AG, Lule D. Prone to excitement: adolescent females with Non-suicidal self-injury (NSSI) show altered cortical pattern to emotional and NSSI-related material. Psychiatry Research: Neuroimaging. 2012;203:146–152. doi: 10.1016/j.pscychresns.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Cook SC, Carroll BJ. A depression rating scale for children. Pediatrics. 1979;64:442–450. [PubMed] [Google Scholar]
- Price CJ, Wise R, Watson J, Patterson K, Howard D, Frackowiak RSJ. Brain activity during reading: The effects of task and exposure duration. Brain. 1994;117:1255–1269. doi: 10.1093/brain/117.6.1255. [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, Heilbron N, Guerry JD, Franklin JC, Rancourt D, Simon V, Spirito A. Peer influence and non-suicidal self-injury: Longitudinal results in community and clinically-referred samples. Journal of Abnormal Child Psychology. 2010;38:669–682. doi: 10.1007/s10802-010-9423-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat Neurosci. 2004;7:278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- Ross S, Heath NL, Toste JR. Non-suicidal self-injury and eating pathology in high school students. Am J Orthopsychiatry. 2009;79:83–92. doi: 10.1037/a0014826. [DOI] [PubMed] [Google Scholar]
- Rotge JY, Lemogne C, Hinfray S, Huguet P, Grynszpan O, Tartour E, George N, Fossati P. A meta-analysis of the anterior cingulate contribution to social pain. Soc Cogn Affect Neurosci. 2015;10:19–27. doi: 10.1093/scan/nsu110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz S, Buyukturkoglu K, Rana M, Birbaumer N, Sitaram R. Real-time fMRI brain computer interfaces: Self-regulation of single brain regions to networks. Biol Psychol. 2013 doi: 10.1016/j.biopsycho.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Scherpiet S, Brühle AB, Opialla S, Roth L, Jäncke L, Uwe H. Altered emotion processing circuits during the anticipation of emotional stimuli in women with borderline personality disorder. European Archives of Psychiatry & Clinical Neuroscience. 2014;264:45–60. doi: 10.1007/s00406-013-0444-x. [DOI] [PubMed] [Google Scholar]
- Shanahan L, McHale SM, Osgood DW, Crouter AC. Conflict frequency with mothers and fathers from middle childhood to late adolescence: within- and between-families comparisons. Dev Psychol. 2007;43:539–550. doi: 10.1037/0012-1649.43.3.539. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA. I remember you: a role for memory in social cognition and the functional neuroanatomy of their interaction. Brain Res. 2012;1428:43–50. doi: 10.1016/j.brainres.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturrock B, Mellor D. Perceived emotional invalidation and borderline personality disorder features: a test of theory. Personal Ment Health. 2014;8:128–142. doi: 10.1002/pmh.1249. [DOI] [PubMed] [Google Scholar]
- Tan AC, Rehfuss MC, Suarez EC, Parks-Savage A. Nonsuicidal self-injury in an adolescent population in Singapore. Clin Child Psychol Psychiatry. 2014;19:58–76. doi: 10.1177/1359104512467273. [DOI] [PubMed] [Google Scholar]
- Tatnell R, Kelada L, Hasking P, Martin G. Longitudinal analysis of adolescent NSSI: the role of intrapersonal and interpersonal factors. J Abnorm Child Psychol. 2014;42:885–896. doi: 10.1007/s10802-013-9837-6. [DOI] [PubMed] [Google Scholar]
- van Veluw SJ, Chance SA. Differentiating between self and others: an ALE meta-analysis of fMRI studies of self-recognition and theory of mind. Brain Imaging Behav. 2014;8:24–38. doi: 10.1007/s11682-013-9266-8. [DOI] [PubMed] [Google Scholar]
- Whitlock J, Muehlenkamp J, Eckenrode J, Purington A, Baral Abrams G, Barreira P, Kress V. Nonsuicidal self-injury as a gateway to suicide in young adults. J Adolesc Health. 2013a;52:486–492. doi: 10.1016/j.jadohealth.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Whitlock J, Muehlenkamp JJ, Eckenrode J, Purington A, Baral Abrams G, Barreira P, Kress V. Nonsuicidal self-injury as a gateway to suicide. Journal of Adolescent Health. 2013b;52:486–492. doi: 10.1016/j.jadohealth.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Yates T. Developmental pathways from child maltreatment to non-suicidal self-injury. In: Nock MK, editor. Understanding Non-suicidal Self-injury: Origins, Assessment and Treatment. American Psychological Association; Washington, DC: 2009. pp. 117–138. [Google Scholar]
- Yurkowski K, Martin J, Levesque C, Bureau J-F, Lafontaine M-F, Cloutier PF. Emotion Dysregulation Mediates the Influence of Relationship Difficulties on Nonsuicidal Self-injury Behavior in Young Adults. Psychiatry Research. 2015 doi: 10.1016/j.psychres.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Zhang G, Yao L, Zhang H, Long Z, Zhao X. Improved Working Memory Performance through Self-Regulation of Dorsal Lateral Prefrontal Cortex Activation Using Real-Time fMRI. PLoS One. 2013;8:e73735. doi: 10.1371/journal.pone.0073735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. Healthy adolescents, in addition to higher frontal pole (BA10), lingual and cuneus activity, show less de-activation in the sub-genual ACC than depressed youth with NSSI behaviors who in turn showed less de-activation than depressed youth.