Pediatric OI is common, comprising physiological perturbations as well as diseases. We define, describe, and discuss forms of OI, their treatment, and prognosis.
Abstract
Orthostatic intolerance (OI), having difficulty tolerating an upright posture because of symptoms or signs that abate when returned to supine, is common in pediatrics. For example, ∼40% of people faint during their lives, half of whom faint during adolescence, and the peak age for first faint is 15 years. Because of this, we describe the most common forms of OI in pediatrics and distinguish between chronic and acute OI. These common forms of OI include initial orthostatic hypotension (which is a frequently seen benign condition in youngsters), true orthostatic hypotension (both neurogenic and nonneurogenic), vasovagal syncope, and postural tachycardia syndrome. We also describe the influences of chronic bed rest and rapid weight loss as aggravating factors and causes of OI. Presenting signs and symptoms are discussed as well as patient evaluation and testing modalities. Putative causes of OI, such as gravitational and exercise deconditioning, immune-mediated disease, mast cell activation, and central hypovolemia, are described as well as frequent comorbidities, such as joint hypermobility, anxiety, and gastrointestinal issues. The medical management of OI is considered, which includes both nonpharmacologic and pharmacologic approaches. Finally, we discuss the prognosis and long-term implications of OI and indicate future directions for research and patient management.
Consensus guidelines define orthostatic disorders in adults on the basis of expert opinion and randomized controlled studies.1–3 These conclusions incompletely extend to children, for whom large trials and even small controlled studies are sparse. Pediatric orthostatic intolerance (OI) has drawn increasing attention in recent publications from different groups with different perspectives.4–7 The high and possibly increasing prevalence of orthostatic disorders in teenagers prompted the American Autonomic Society to convene member experts to draft an evidence-based State of the Art document. This document is intended to provide common ground on which to build a better understanding of pathophysiology and treatment.
The Challenge of Orthostasis (Standing Upright)
Humans are bipedal. While upright, our brain is above our heart, whereas 70% of our blood volume is below the heart. If not for autonomic and cardiovascular compensatory mechanisms, hypotension and loss of consciousness would ensue after orthostasis.
Orthostatic Intolerance
OI can be defined as having difficulty tolerating the upright posture because of symptoms that abate when returned to supine. Typical symptoms include a sense of impending loss of consciousness, cognitive deficits (memory loss and decreased reasoning and concentration), visual difficulties, lightheadedness, headache, fatigue, weakness, nausea, abdominal discomfort, tremulousness, exercise intolerance, and reported signs such as pallor, diaphoresis, tachycardia, bradycardia, or hypotension.
This definition encompasses all forms of orthostatic disorders, such as postural faint and orthostatic hypotension (OH), but not others, such as a broken leg or overt muscle disease. The definition is sufficiently broad to encompass postural vertigo, balance issues, and positional headache.
Chronic OI is defined as OI that is present for at least 3 months, although symptoms may wax and wane. An example is postural tachycardia syndrome (POTS).
Acute and subacute OI are defined as OI that has been present for <1 week and <3 months, respectively, or is restricted to recurrent episodes, such as with postural vasovagal syncope (VVS).
Some OI represents an extension of normal physiology, such as transient lightheadedness immediately on standing. Some consider infrequent VVS to be a normal response to central hypovolemia because its lifetime incidence approaches 40%, and it can be universally induced with sufficient orthostatic provocation.2 However, OI that importantly diminishes quality of life deserves treatment.
In what follows, we more specifically define individual entities comprising OI and discuss how best to test for and diagnose OI. We review the epidemiology, predisposing factors, putative causes, and frequent comorbidities of OI and focus on POTS. We discuss management, prognosis, and future directions for research and therapy.
Individual Entities
Initial Orthostatic Hypotension
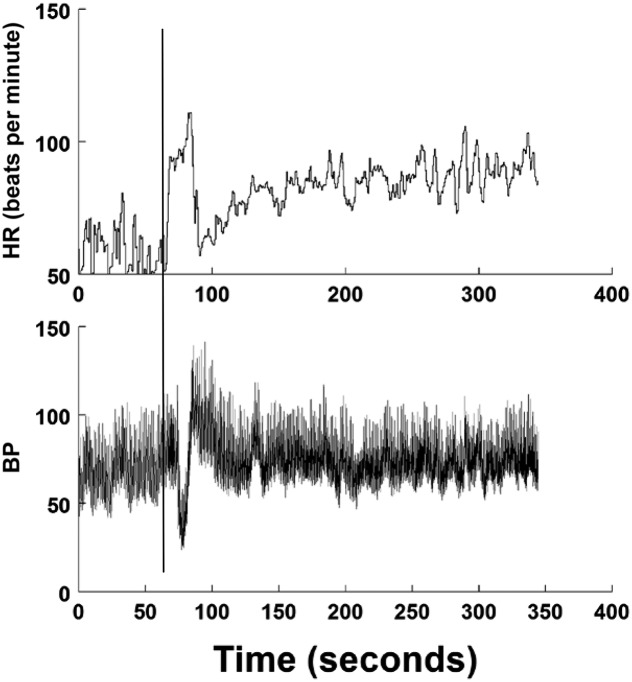
On standing, there is symptomatic hypotension because of the caudal transference of blood because of gravity and a time delay in sympathetic activation.8 Blood pressure (BP) can decrease by >30%, reaching a nadir at 10 to 15 seconds after standing. Lightheadedness and reflex tachycardia occur. BP is restored within 30 seconds, but cerebral blood flow and heart rate (HR) take longer.9–11 Clinically significant initial orthostatic hypotension (IOH) is defined as a decrease in systolic BP of >40 mm Hg or a decrease in diastolic BP of >20 mm Hg.10 The fall in BP is enhanced by the duration of the previous supine position. A history of transient lightheadedness dissipating in <1 minute should be considered as IOH.7,10,12 Syncope is uncommonly reported with IOH. Countermeasures to obviate symptoms include active contraction of lower-body muscles or recumbence.9,10 IOH is the most common form of OI.9,12,13 A representative standing test in which IOH is experienced is shown in Fig 1.
FIGURE 1.
A standing test of IOH is shown. The upper panel shows HR in beats per minute, and the lower panel shows BP in mm Hg. The vertical line indicates standing. BP decreases, and HR increases briefly. Hypotension resolves within 30 seconds. HR stabilizes gradually to its upright, steady-state value.
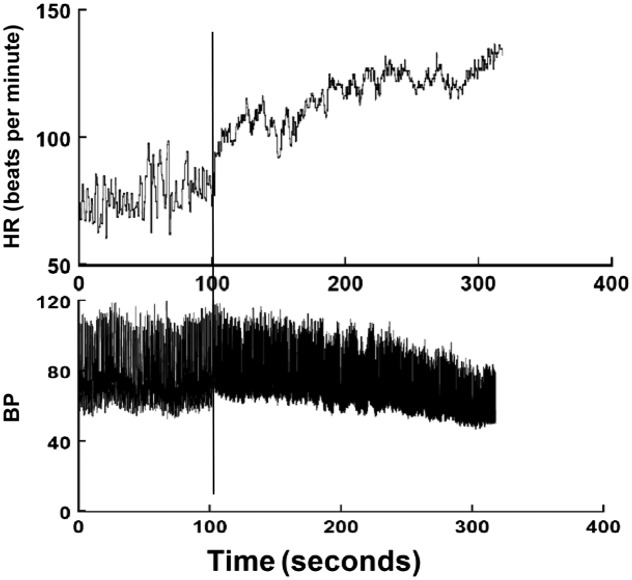
OH is defined by the steady decrease in BP exceeding 20 mm Hg systolic or 10 mm Hg diastolic within 3 minutes of standing from supine.1,7,14 The associated compromise in brain blood flow may cause syncope. OH may be nonneurogenic from severe central hypovolemia or pharmaceutical vasodilation.15 There is supine tachycardia, which increases markedly when upright. OH may be neurogenic (nOH) because of an inadequate release of norepinephrine from sympathetic neurons.1 nOH results from primary autonomic failure or secondary autonomic failure, as in diabetes and amyloidosis.14,16–18 nOH is uncommon in pediatrics but occurs in familial dysautonomia and autoimmune disorders.18,19 There may be little postural HR increase in adults because of frequent, associated cardiac denervation.1,18 In contrast, children typically mount a compensatory tachycardia, suggesting intact cardiac parasympathetic efferents.6 A representative tilt table test in which OH is experienced is shown in Fig 2.
FIGURE 2.
A tilt table test shows true OH. The upper panel shows HR in beats per minute, and the lower panel shows BP in mm Hg. The vertical line indicates tilt. There is a monotonic decrease in BP and a compensatory increase in HR, which can often exceed 40 beats per minute in youngsters.
Postural Vasovagal Syncope
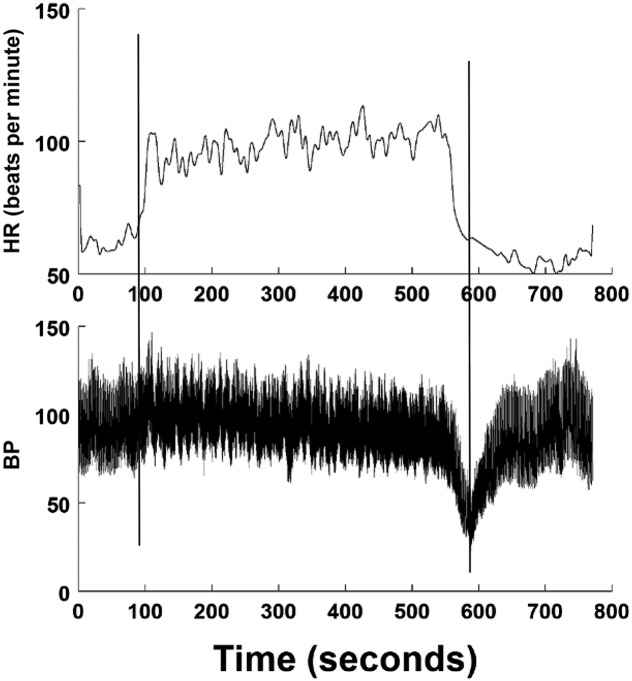
Syncope is defined by a transient loss of consciousness and postural tone because of global cerebral hypoperfusion characterized by rapid onset, short duration (<2 minutes), and spontaneous recovery.20 Postural VVS is syncope occurring after several (>3) minutes upright. Characteristic history features environmental precipitating factors, a prodrome of lightheadedness, diaphoresis, warmth, nausea, hyperventilation, pallor, and a postdrome of fatigue and headache.2,21–23 Cardiac causes for syncope must be ruled out.7,24 Hypotension and bradycardia typically occur together. Postural VVS is aborted by lying down; consciousness returns within seconds. VVS is also provoked by noxious stimuli, such as venipuncture or emotional stress.2,21–23 The most common age of onset is 15 years. VVS occurs twice as often in female adolescents, with sex ratios equalizing later in life.25 Athletic youngsters have an increased prevalence of VVS. Postexercise VVS can occur in patients with postural VVS. Low-serum iron and/or ferritin can contribute to the occurrence of VVS.26,27 VVS can be induced in healthy volunteers in the laboratory but is only clinically important in daily life. Reflex syncope occurs in OH and situational variants, such as deglutition, defecation, micturition, and cough syncope, but rarely from IOH.6,7 Asystolic or convulsive syncope may occur in VVS without prodrome and with tonic posturing after loss of consciousness. Physical injury is common in these patients because of falls and other related trauma. In adults, pacing has been used to prevent these injuries. In patients with presumptive VVS, competing diagnoses include epilepsy and pseudosyncope. A representative tilt table test in which VVS is experienced is shown in Fig 3.
FIGURE 3.
A tilt test shows VVS. The upper panel shows HR in beats per minute, and the lower panel shows BP in mm Hg. Vertical lines indicate the start and end of tilt. On tilt, BP is initially stable then slowly falls because HR rises often by >40 beats per minute. BP then falls rapidly, followed by HR (hypotension bradycardia).
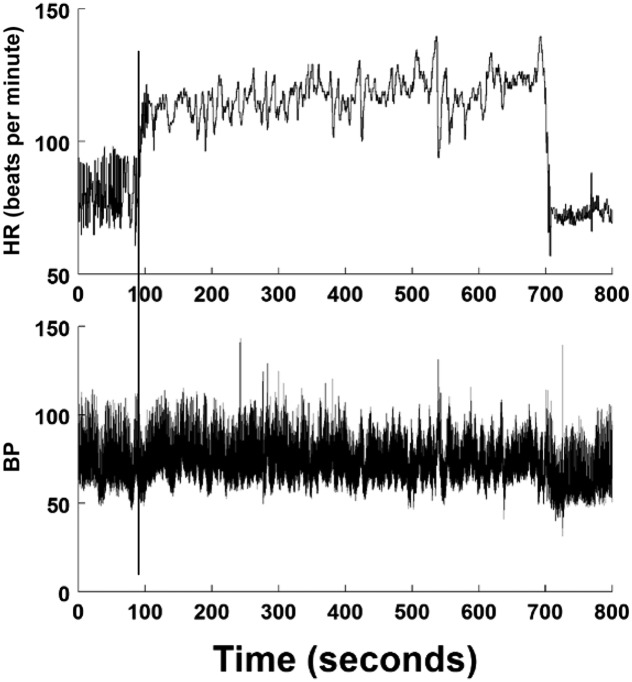
POTS is defined by daily symptoms of chronic OI combined with sustained, excessive upright tachycardia in the absence of postural hypotension. The origins of POTS are heterogeneous. Most cases are in girls (>80%).7,28,29 Symptoms are initiated by an upright posture and abate when supine (ie, they must be postural to be POTS).30 Excessive upright tachycardia is defined in adults as an average sustained increase in HR of >30 beats per minute or to an HR of >120 beats per minute within 10 minutes of standing or upright tilt. Excessive upright tachycardia is defined in adolescents 18 years or younger by an increase in HR of >40 beats per minute.29 By definition, sustained hypotension must be absent. POTS does not cause a transient loss of consciousness, per se, although VVS may also occur in some patients.7 A secondary form of POTS can occur with any central hypovolemic state, such as dehydration or hemorrhage, and can progress to OH.28 Excessive tachycardia also occurs in young VVS patients preceding syncope, but sustained hypotension precludes POTS. POTS can be evoked by excessive upright sympathetic activation or excessive parasympathetic withdrawal. When a cause is identified (eg, Addison disease), some discard the POTS label in favor of the disease name, whereas others prefer to call it secondary POTS. Regardless, POTS is a syndrome, and evaluations for underlying causes, such as dehydration, anemia, hyperthyroidism, and autonomic neuropathies, are necessary in all cases. A representative tilt table test in a patient with POTS is shown in Fig 4.
FIGURE 4.
A tilt test shows POTS. The upper panel shows HR in beats per minute, and the lower panel shows BP in mm Hg. Vertical lines indicate the start and end of tilt. On tilt, BP is initially stable, with a small downward drift during tilt. There is excessive tachycardia but no hypotension.
Prolonged bed rest (>23 hours) induces gravitational deconditioning with concomitant physiologic findings and is distinct from exercise deconditioning; these include a markedly reduced blood volume, blood volume redistribution, trophic cardiac atrophy, baroreflex changes, reduced vasoconstriction to pressor drugs, skeletal muscle atrophy with loss of the skeletal muscle pump, and osteoporosis. The earliest changes are detectable within 24 hours. In a bed-rested patient, OI may take the form of POTS, OH, or VVS, and bed rest worsens these states if they are already present.7,31–35
Hypocaloric Weight Loss
A study of healthy volunteers showed a deterioration of orthostatic tolerance with weight loss of 1% to 4%, which is likely because of a reduced blood volume, the modulation of autonomic function, altered baroreflex function, and altered vascular smooth muscle tone and responsiveness.36 This is potentiated by bed rest.36 OI resembling POTS or OH occurs in anorectic states and during starvation and may precede the well-known terminal bradycardia.37
Testing for OI
Symptoms of OI must be present to merit testing. Autonomic and vasoactive drugs should be stopped for at least 5 half-lives before testing.
Upright tilt table testing is the de facto standard for orthostatic stress tests. The use of the tilt table at angles between 60° and 70° dates to 1930, but its use to diagnose VVS is more recent.38,39 Tilt testing limits the effects of the skeletal muscle pump. Diagnostic criteria for POTS were also developed by using tilt table testing. The standardization of tilt table testing for the diagnosis of VVS has been problematic. The so-called Italian protocol (20 minutes upright followed by sublingual nitroglycerin as pharmacological potentiation) is now commonly used.40,41 Tilt angles of 60° to 70° are advocated because angles of 80° to 90° increase the incidence of false-positive test results.42
Although a 10-minute tilt is standard for POTS, a 5-minute tilt may suffice. There is little consensus on the duration of the supine period preceding the tilt, although it affects results. Extending the tilt past 10 minutes reduces the accuracy of the POTS diagnosis, although it may provide additional information about the nature of OI and the diagnosis of syncope.43 OH can be reliably diagnosed by tilt or standing for 3 minutes.1
Standing is arguably the most physiologic orthostatic test, although it is difficult to standardize. There is little consensus regarding how long a patient should stand, whether movement should be allowed or restricted, and how long a patient should be supine before standing. Validation of a standing test for POTS exists for adults but not yet for children, for whom the tilt table is standard.43 Standing is validated in pediatric patients to diagnose OH and IOH.1,10
Monitoring During Orthostatic Testing
The minimum requirement is an intermittent measurement of HR and BP. Current US Current Procedural Terminology codes require the measurement of beat-to-beat electrocardiogram and BP.9,12,13 Automated autonomic testing is not validated and is not recommended for diagnoses.44 Other monitoring uses nasal capnography, EEG, cerebral blood flow, or cerebral oximetry. Research measurements include regional blood flow and blood volume, pneumotachography, microneurography, and combined modalities.
Prevalence and Phenotype of Pediatric POTS
The prevalence of POTS is not well established.45 Its incidence in pediatrics is not known because screening for OI is not routine. OI affects a broad range of organ systems. Examples of common signs and symptoms are shown in Table 1. Onset may be gradual or acute and often occurs after an infection, immunization, surgery, sepsis, or head trauma.46–49 Infectious diseases exacerbate preexisting OI.50,51
TABLE 1.
Predominant Presenting Symptoms of OI
| Affected System | Symptoms Present While Upright |
|---|---|
| General | Fatigue, heat intolerance, weakness, temperature regulation difficulties |
| Cardiovascular | Lightheadedness, tachycardia, chest pain, palpitations, exercise intolerance, syncope, acrocyanosis, diaphoresis, pallor, flushing |
| Gastrointestinal | Nausea, abdominal pain, dysmotility |
| Respiratory | Dyspnea, hyperpnea |
| Neurologic | Headaches, paresthesias, balance problems |
| Neuropsychological | Memory and attention issues (brain fog) |
Demographic Characteristics
Girls represent >80% of patients.51 BMI is reduced in patients with POTS and hypovolemia.52 Onset is often near puberty, but younger patients have been identified less frequently.48,53 Literature on racial differences is limited: orthostatic tolerance characterizes African Americans, and most reported patients with POTS are white.53–55 Family members of patients with POTS often describe similar symptoms.56 Many young patients with POTS were formerly high achievers.5,53 Yet, with the onset of symptoms, children experienced reduced participation in school, social life, sports, and recreational activities. Psychiatric comorbidities in these patients are also common.
Predisposing and Contributing Factors and Putative Causes of POTS
Up to 50% of case patients have antecedent symptoms that suggest a viral infection with a prolonged course (eg, infectious mononucleosis).57 Others have previous stressors, such as pregnancy, injury, or surgery; the remainder of patients develop symptoms insidiously.58 Inflammatory or autoimmune mechanisms may also be responsible. Patients with prolonged illness may confine themselves to bed, making matters worse.53
Central hypovolemia reduces cardiac venous return, resulting in a compensatory tachycardia. Hypovolemia may be absolute or distributive and accompanies exercise deconditioning and chronic fatigue.59,60 Exercise deconditioning is a result rather than the cause of POTS, and some patients with POTS have normal to supranormal cardiovascular responses to exercise, with a normal stroke volume in 30% of patients.61,62 However, exercise deconditioning can worsen POTS by further reducing blood or plasma volume, heart size, and stroke volume.61
Gravitational deconditioning occurs during microgravity and chronic bed rest and emulates the pathophysiology of POTS.63 Plasma volume decreases by ∼15% within several days, red cell mass declines, and blood flow and blood volume redistribute abnormally from skin and splanchnic reservoirs, reducing cardiac filling, particularly when upright and during exercise.28,51,64–68 Muscle atrophy increases leg venous pooling, contributing to acrocyanosis, impaired vasoconstriction, and loss of the skeletal muscle pump.31,58,68–70 The cardiovascular effects of prolonged bed rest contribute to the pivotal pathophysiology of POTS (central hypovolemia) and potentiate OI and POTS.71 Measures that preserve peak oxygen uptake during exercise do not necessarily reduce OI, supporting the suggestion that POTS and deconditioning each represent separate downstream effects of an inciting cause.63 POTS symptoms are often improved by physical activity, but not all patients with POTS are exercise deconditioned.
Immune Mechanisms
The idea of a limited autonomic neuropathy underlying POTS was suggested in its original description.30 Evidence of partial autonomic denervation, suggestive of a limited autonomic neuropathy in some patients with POTS with a female predominance and preceding viral syndrome with subacute symptom onset, supports an underlying immune system–related cause.56,72,73 This is further supported by the detection of nicotinic ganglionic acetylcholine receptor autoantibodies in a small percentage of patients with OI.47 Recent reports postulate that autoantibodies cross-react with a wide range of cardiac proteins and α- and β-adrenergic receptors in many adult patients with OI, although these findings have thus far not been reproduced.74,75 A study in which evaluate the sera of patients with POTS found organ-specific autoantibodies, particularly thyroid-specific, to be more common in patients with POTS.76 The preliminary nature of these findings prohibits any generalizable recommendations for immunomodulatory or immunosuppressive treatments at this time.
Mast Cell Activation Disorders
POTS was found in patients with mast cell activation disorders (MCADs) 1 decade ago.77 MCADs and POTS are increasingly found together.78 MCADs are symptomatically similar to attenuated mastocytosis, but they are unassociated with increased mast cell numbers. Instead, there is an excessive release of biologically active materials from otherwise normally growing mast cells.79,80 In MCADs, mast cells release histamine, prostaglandins, and leukotrienes, often without a recognizable allergen. Symptoms may be episodic and associated with facial flushing or chronic with fatigue, dizziness, and abdominal discomfort, often posturally induced. Symptomatic flares of MCADs may result in elevated serum tryptase levels. Patients with chronic MCADs may exhibit excessive amounts of urinary N-methylhistamine, leukotriene E4, or 11-β-prostaglandin F2 α.79 Initial treatment targets the chemical being excessively released (aspirin or ibuprofen for 11-β-prostaglandin F2 α, montelukast for leukotriene E4, and antihistamines for histamine).79,80 Many patients require a combination of H1 and H2 antihistamines, antileukotrienes, anti-inflammatory agents, and a mast cell stabilizer (Cromolyn). β-blockers, often used for POTS, may worsen MCAD symptoms.
Comorbidities in Pediatric OI
Several conditions are associated with pediatric OI, including joint hypermobility, functional gastrointestinal disorders (functional abdominal pain, nausea, and cyclic vomiting syndrome), chronic fatigue syndrome, headache, sleep disorders, cognitive dysfunction (brain fog), anxiety, and depression. Comorbidity does not signify direct causation.81 Most researchers evaluate comorbidities in POTS; few address OH and VVS.
Joint hypermobility is associated with POTS, syncope, presyncope, fatigue, heat intolerance, and abnormal autonomic testing scores.82–85 Chronic pain and joint hypermobility are equally present in patients with POTS versus those without.81
Functional gastrointestinal disorders are associated with autonomic dysfunction.86–88 POTS is associated with upper-gastrointestinal symptoms and antroduodenal dysmotility, which exacerbate with standing89 and may improve when OI is treated.90 Both delayed and rapid gastric emptying have been reported.89–92
OI has been identified in both younger and older case patients with cyclic vomiting syndrome,93 with POTS present in 35% of adults.93–95
Symptoms and patterns of orthostatic HR and BP changes occur in adolescents with chronic fatigue syndrome and overlap with those of POTS.96–99
Headache, including migraine, is commonly reported in POTS and VVS.100,101 The association between headaches and OI has been primarily documented in adults. A few studies that include children have shown similar associations.102–104 In a study of adolescent headache patients in a tertiary-care setting, 53% had POTS.105 Although headache can be a symptom of OI, the relationship is unclear. The treatment of POTS has been found to be only partially effective in relieving headache.106
Sleep disorders, such as problematic sleep onset, maintenance, duration, quality, and daytime sleepiness, are consistently reported.5,53,57,107,108 However, sleep studies in adults have shown little differences in sleep characteristics among POTS patients and controls.109–112
Cognitive symptoms or brain fog, which is described as having difficulty with attention, concentration, and memory, are commonly reported in POTS. Triggers include prolonged concentration and sleep disorders.108 Cognitive performance during tilt testing is impaired because of an entrainment of cerebral blood flow by BP that reduces neurovascular coupling, the link between an increase in neural activity in response to a neural activation task, and the resulting increase in cerebral blood flow.113,114 Increased depression and heightened anxiety in patients with POTS negatively impact attention and short-term memory.115
Depression, anxiety, and pain catastrophizing are common in pediatric POTS and VVS, in which they can mirror parental psychiatric symptoms.5,53,116–119 These types of studies are often associative and are complicated by an overlap of psychiatric symptoms with those of OI. Therefore, further research is needed to elucidate the moderating effects of sleep, cognitive, psychiatric, and social-interactional factors on pediatric OI symptoms.
Management
Although pharmacologic and nonpharmacologic interventions have not been compared, it is the opinion of the authors that nonpharmacologic interventions are more important to long-term outcomes, and they are therefore presented first.
Nonpharmacologic Management
Fluids and Exercise
Cell dehydration causes adverse responses, including exaggerated cortisol response to exercise, decreased sympathetic nervous activity, and impaired cognitive and physical performance.120–123 Adequate daily water intake is defined by the National Academy of Medicine (formerly the Institute of Medicine) as 3.7 L for men and 3.0 L for women; however, euhydration is difficult to assess.124
Bed rest emulates POTS with decreased stroke volume and increased HR to orthostasis, which occur in response to central hypovolemia.125 Vigorous salt and water loading eliminates OH after bed rest.126 Symptom severity negatively correlates with urinary sodium excretion.127 Intravenous saline and salt improve symptoms in adolescents.108 Large amounts of dietary salt improve symptoms in adults.128 Some patients with POTS have deficits in plasma and blood volume without increased plasma renin or aldosterone but with increased angiotensin-II.129,130
Target salt and water intakes of 2.5 to 3 L daily in adolescent girls and 3.0 to 3.5 L daily in adolescent boys are recommended, which should be accompanied by >8 g sodium chloride daily. Glucose polymers (eg, maltodextrins) in commercial sports drinks are preferred.131,132 A goal is to achieve urine osmolality of <300 mmol/L or urine sodium >200 mmol per 24 hours. Intravenous hydration may be used acutely (eg, for gastrointestinal so-called influenza) but is strongly discouraged on a chronic basis.
Exercise Training
Four trials in young adults using HR as the target for exercise training showed efficacy in POTS. Exercise can expand blood volume by 20% to 25%.133,134 One approach aims for 60% to 70% of a subject’s HR achieved during maximal exercise testing beginning with semirecumbent exercise; there are 5 minutes of warm-up to achieve target, 15 minutes at target, then 5 minutes of cool down. As exercise tolerance improves, add 5 minutes per session at target until one can complete 30 minutes at the target HR.
Alternative nutritional, psychological, and multidisciplinary therapies have been employed without an evidence base. These have included biofeedback; acupressure and acupuncture; craniosacral therapy; increased fiber intake; gluten avoidance; a diet of avoiding fermentable oligosaccharides, disaccharides, monosaccharides and polyols; and probiotics.
Cognitive behavioral therapy and intensive multidisciplinary rehabilitative programs for individuals with OI, particularly POTS, report improvement in functional outcomes.135 Further research is needed to evaluate the benefits and durability of treatment.
Pharmacologic Management
Studies of pharmacologic interventions in OI are mostly retrospective or single-dose trials. Even documented therapies vary with provider, involve off-label use, and can produce variable clinical responses because of individual differences in drug sensitivity, metabolism, or tolerance to adverse effects.
Key therapies provide circulatory and autonomic support (Table 2), including efforts to increase blood volume. However, we strongly discourage the chronic use of central lines or ports because of the risk of infection, endocarditis, and thrombosis. An enteral intake of fluids and sodium is always preferred.
TABLE 2.
Medications
| Medication | Dose | Side Effects | Comments |
|---|---|---|---|
| Circulatory support | |||
| Fludrocortisone | 0.1–0.2 mg qAM | Peripheral edema, acne, headache, hypokalemia, hypomagnesemia | Monitor basic metabolic panel and magnesium at higher doses90,136 |
| Midodrine | 2.5–10 mg TID q4h | Tingling, goosebumps, headache, hypertension | Check supine BP 30–60 min after a dose137–140 |
| Desmopressin | 0.1–0.4 mg BID | Hyponatremia, headache141 | — |
| Octreotide | 25–100 μg subcutaneously BID | Injection site discomfort, diarrhea, thyroid derangement | Decreased gastrointestinal transit time may be beneficial for some patients138,142,143 |
| Erythropoietin | 10 000–20 000 IU subcutaneously weekly | Hypertension, arthralgias | Ensure hematocrit <50%, ensure adequate iron intake144,145 |
| Acute normal saline infusion | 1–2 L intravenous every 5–7 d | Repeated phlebotomy can lead to scarring of veins | Intermittent rescue use may be beneficial in acute management146 |
| Ivabradine | 2.5–10 mg BID | Bradycardia without hypotension | Inhibits If sinoatrial node, FDA approved for adult CHF. Small trials showed benefit in POTS147,148 |
| Autonomic modulation | |||
| Metoprolol succinate | 12.5–100 mg daily | Lightheadedness, decreased exercise tolerance, fatigue, worsening asthma, depression | Nighttime dosing may decrease lightheadedness139,149 |
| Metoprolol tartrate | 12.5–50 mg BID | ||
| Atenolol | 12.5–50 mg BID | Same as metoprolol succinate | — |
| Nebivolol | 2.5–10 mg daily | Same as metoprolol succinate | Fewer overall side effects because of decreased blood–brain barrier penetration |
| Propranolol | — | Same as metoprolol succinate | — |
| Citalopram | 10–40 mg daily | Nausea, headache, fatigue, increased appetite, suicidal ideation requiring early and frequent monitoring | Causes central sympathetic modulation, reduces abnormal autonomic response150 |
| Escitalopram | 5–20 mg daily | Same as citalopram | — |
| Sertraline | 25–200 mg daily | Same as citalopram | — |
| Clonidine | 0.1–0.3 mg transdermal every 7 d | Contact dermatitis with adhesive, fatigue, dry mouth, headache | Centrally acting α-agonist, may also be used for insomnia151,152 |
| Pyridostigmine | 30–120 mg BID to TID | Abdominal pain, muscle twitch, decreased intestinal transit time | May also be helpful for early satiety and constipation153–155 |
BID, twice daily; CHF, congestive heart failure; FDA, Food and Drug Administration; If, sinus node inward “funny” pacemaker channel; q4h, every 4 hours; qAM, every morning; TID, thrice daily; —, not applicable.
Another common approach targets the treatment of specific symptoms. Often, drugs are employed that successfully improve similar symptoms in other diseases. These can include fatigue, cognitive dysfunction, insomnia, chronic pain, gastrointestinal symptoms, and headaches. Further discussion of specific therapies is beyond the scope of this review.
Prognosis
Patients with VVS improve in their 20s, but symptoms often recur in middle age.25 Recurrent syncope among children and adolescents can negatively affect health-related quality of life.156,157
Little is known about the long-term prognoses for pediatric patients with POTS. Heterogeneous pathophysiology leads to a common phenotype, but prognosis depends on the underlying etiology.30,72,129,158 Comorbidities and treatments can influence short- and long-term outcomes. A survey from a single tertiary center conducted by using a mailed questionnaire suggests a good overall prognosis among adolescent patients with POTS, with 86% reporting symptom improvement or resolution at 5.4 years after diagnosis.159 Another center showed improvements among young adult patients with POTS (aged 20–33 years) over a mean follow-up period of 92 ±41 months.160
Future Directions and Research
Despite an evolving consensus of clinical phenotypes and medical management of OI, underlying mechanisms remain poorly understood. To establish evidence-based diagnostic and treatment strategies, diverse research findings must be integrated to generate hypotheses, develop prognostic and natural history studies, and improve patient care. Uniform, multicenter databases and clinical registries will be important in reaching this goal. This concept has been put into practice in adult patients with POTS.161
Among the challenges in characterizing pediatric patients with OI is defining a consistent method of performing orthostatic stress testing, including a proposed validation of practical standing tests. Also, recent work shows the utility of measuring serum and urinary biomarkers because they relate to different OI phenotypes or because they predict treatment response.161,162
Large prospective randomized controlled studies are needed to determine the impact of treatment strategies ranging from pharmacological regimens, salt and water requirements, and exercise. A better understanding of the effects of treatments on neurocognitive, autonomic, and cardiovascular signs and symptoms underscores the need to incorporate well-trained neuroscientists, psychologists, immunologists, and physiologists in research and therapy. Studies relating diagnostic tools such as functional brain imaging to objectively document neurocognitive assessments may be critical in this regard.
Glossary
- BP
blood pressure
- HR
heart rate
- IOH
initial orthostatic hypotension
- MCAD
mast cell activation disorder
- nOH
neurogenic orthostatic hypotension
- OH
orthostatic hypotension
- OI
orthostatic intolerance
- POTS
postural tachycardia syndrome
- VVS
vasovagal syncope
Footnotes
Dr Stewart introduced the idea of the article, outlined sections, edited drafts and the final manuscript, contributed directly to the first draft of the manuscript, and extensively redacted the draft; Dr T. Chelimsky introduced the idea of the article, outlined sections, edited drafts and the final manuscript, contributed directly to the first draft of the manuscript, and consolidated and secondarily edited the draft; Drs Boris, G. Chelimsky, Fischer, Fortunato, Grubb, Heyer, Jarjour, Numan, Pianosi, Singer, and Tarbell contributed directly to the first draft of the manuscript; Dr Medow contributed directly to the first draft of the manuscript, extensively redacted the draft, and did the bulk of the revision and response to comments; and all authors commented on the draft, approved the final manuscript as submitted, and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Heart, Lung, and Blood Institute (R01HL112736); the National Institute of Neurological Disorders and Stroke (R21NS094644, R01 NS092625, K23 NS075141, and FD004789 [Food and Drug Administration]); the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK083538); Advancing a Healthier Wisconsin (grant 5520298); the Huseby family; the Hohmann Foundation; the US Department of Health and Human Services (HHSA290201500013I); Medtronic; and H. Lundbeck A/S. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Boris has served as a paid expert witness on the subject of postural orthostatic tachycardia syndrome. H. Lundbeck A/S (which underwrote a meeting of the Pediatric Subgroup of the American Autonomic Society) produces Northera (droxidopa) to treat orthostatic intolerance as well as citalopram and escitalopram; the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21(2):69–72 [DOI] [PubMed] [Google Scholar]
- 2.Sheldon RS, Grubb BP II, Olshansky B, et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015;12(6):e41–e63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wieling W, Schatz IJ. The consensus statement on the definition of orthostatic hypotension: a revisit after 13 years. J Hypertens. 2009;27(5):935–938 [DOI] [PubMed] [Google Scholar]
- 4.Jarjour IT. Postural tachycardia syndrome in children and adolescents. Semin Pediatr Neurol. 2013;20(1):18–26 [DOI] [PubMed] [Google Scholar]
- 5.Kizilbash SJ, Ahrens SP, Bruce BK, et al. Adolescent fatigue, POTS, and recovery: a guide for clinicians. Curr Probl Pediatr Adolesc Health Care. 2014;44(5):108–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart JM. Update on the theory and management of orthostatic intolerance and related syndromes in adolescents and children. Expert Rev Cardiovasc Ther. 2012;10(11):1387–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart JM. Common syndromes of orthostatic intolerance. Pediatrics. 2013;131(5):968–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheriff DD, Nådland IH, Toska K. Hemodynamic consequences of rapid changes in posture in humans. J Appl Physiol (1985). 2007;103(2):452–458 [DOI] [PubMed] [Google Scholar]
- 9.Clarke DA, Medow MS, Taneja I, Ocon AJ, Stewart JM. Initial orthostatic hypotension in the young is attenuated by static handgrip. J Pediatr. 2010;156(6):1019.e1–1022.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wieling W, Krediet CT, van Dijk N, Linzer M, Tschakovsky ME. Initial orthostatic hypotension: review of a forgotten condition. Clin Sci (Lond). 2007;112(3):157–165 [DOI] [PubMed] [Google Scholar]
- 11.Carey BJ, Manktelow BN, Panerai RB, Potter JF. Cerebral autoregulatory responses to head-up tilt in normal subjects and patients with recurrent vasovagal syncope. Circulation. 2001;104(8):898–902 [DOI] [PubMed] [Google Scholar]
- 12.Stewart JM, Clarke D. “He’s dizzy when he stands up”: an introduction to initial orthostatic hypotension. J Pediatr. 2011;158(3):499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Wijnen VK, Harms MP, Go-Schön IK, et al. Initial orthostatic hypotension in teenagers and young adults. Clin Auton Res. 2016;26(6):441–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukai S, Lipsitz LA. Orthostatic hypotension. Clin Geriatr Med. 2002;18(2):253–268 [DOI] [PubMed] [Google Scholar]
- 15.Gutkin M, Stewart JM. Orthostatic circulatory disorders: from nosology to nuts and bolts. Am J Hypertens. 2016;29(9):1009–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibao C, Okamoto L, Biaggioni I. Pharmacotherapy of autonomic failure. Pharmacol Ther. 2012;134(3):279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boulton AJ, Vinik AI, Arezzo JC, et al. ; American Diabetes Association . Diabetic neuropathies: a statement by the American diabetes association. Diabetes Care. 2005;28(4):956–962 [DOI] [PubMed] [Google Scholar]
- 18.Mazzeo A, Stancanelli C, Di Leo R, Vita G. Autonomic involvement in subacute and chronic immune-mediated neuropathies. Autoimmune Dis. 2013;2013:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Axelrod FB, Chelimsky GG, Weese-Mayer DE. Pediatric autonomic disorders. Pediatrics. 2006;118(1):309–321 [DOI] [PubMed] [Google Scholar]
- 20.Gowers WR. A lecture on vagal and vasovagal attacks. Lancet. 1907;173:716–724 [Google Scholar]
- 21.Brignole M, Alboni P, Benditt D, et al. ; Task Force on Syncope; European Society of Cardiology . Part 1. The initial evaluation of patients with syncope. Europace. 2001;3(4):253–260 [DOI] [PubMed] [Google Scholar]
- 22.Hainsworth R. Syncope: what is the trigger? Heart. 2003;89(2):123–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medow MS, Stewart JM, Sanyal S, Mumtaz A, Sica D, Frishman WH. Pathophysiology, diagnosis, and treatment of orthostatic hypotension and vasovagal syncope. Cardiol Rev. 2008;16(1):4–20 [DOI] [PubMed] [Google Scholar]
- 24.Rossano J, Bloemers B, Sreeram N, Balaji S, Shah MJ. Efficacy of implantable loop recorders in establishing symptom-rhythm correlation in young patients with syncope and palpitations. Pediatrics. 2003;112(3, pt 1). Available at: www.pediatrics.org/cgi/content/full/112/3/e228 [DOI] [PubMed] [Google Scholar]
- 25.European Society of Cardiology The European Society of Cardiology Guidelines for the diagnosis and management of syncope reviewed by Angel Moya, MD, FESC, Chair of the Guideline Taskforce with J. Taylor, MPhil. Eur Heart J. 2009;30(21):2539–2540 [DOI] [PubMed] [Google Scholar]
- 26.Jarjour IT, Jarjour LK. Low iron storage in children and adolescents with neurally mediated syncope. J Pediatr. 2008;153(1):40–44 [DOI] [PubMed] [Google Scholar]
- 27.Stewart JM. Reduced iron stores and its effect on vasovagal syncope (simple faint). J Pediatr. 2008;153(1):9–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medow MS, Stewart JM. The postural tachycardia syndrome. Cardiol Rev. 2007;15(2):67–75 [DOI] [PubMed] [Google Scholar]
- 29.Singer W, Sletten DM, Opfer-Gehrking TL, Brands CK, Fischer PR, Low PA. Postural tachycardia in children and adolescents: what is abnormal? J Pediatr. 2012;160(2):222–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43(1):132–137 [DOI] [PubMed] [Google Scholar]
- 31.Arbeille P, Kerbeci P, Mattar L, Shoemaker JK, Hughson R. Insufficient flow reduction during LBNP in both splanchnic and lower limb areas is associated with orthostatic intolerance after bedrest. Am J Physiol Heart Circ Physiol. 2008;295(5):H1846–H1854 [DOI] [PubMed] [Google Scholar]
- 32.Blomqvist CG, Buckey JC, Gaffney FA, Lane LD, Levine BD, Watenpaugh DE. Mechanisms of post-flight orthostatic intolerance. J Gravit Physiol. 1994;1(1):122–124 [PubMed] [Google Scholar]
- 33.Convertino VA, Bloomfield SA, Greenleaf JE. An overview of the issues: physiological effects of bed rest and restricted physical activity. Med Sci Sports Exerc. 1997;29(2):187–190 [DOI] [PubMed] [Google Scholar]
- 34.Hurwitz BE, Coryell VT, Parker M, et al. Chronic fatigue syndrome: illness severity, sedentary lifestyle, blood volume and evidence of diminished cardiac function. Clin Sci (Lond). 2009;118(2):125–135 [DOI] [PubMed] [Google Scholar]
- 35.Meck JV, Dreyer SA, Warren LE. Long-duration head-down bed rest: project overview, vital signs, and fluid balance. Aviat Space Environ Med. 2009;80(suppl 5):A1–A8 [DOI] [PubMed] [Google Scholar]
- 36.Florian JP, Baisch FJ, Heer M, Pawelczyk JA. Caloric restriction decreases orthostatic tolerance independently from 6° head-down bedrest. PLoS One. 2015;10(4):e0118812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palla B, Litt IF. Medical complications of eating disorders in adolescents. Pediatrics. 1988;81(5):613–623 [PubMed] [Google Scholar]
- 38.Turner AH, Newton MI, Haynes FW. The circulatory reaction to gravity in healthy young women. Evidence regarding its precision and its instability. Am J Physiol. 1930;94:507–520 [Google Scholar]
- 39.Kenny RA, Ingram A, Bayliss J, Sutton R. Head-up tilt: a useful test for investigating unexplained syncope. Lancet. 1986;1(8494):1352–1355 [DOI] [PubMed] [Google Scholar]
- 40.Raviele A, Menozzi C, Brignole M, et al. Value of head-up tilt testing potentiated with sublingual nitroglycerin to assess the origin of unexplained syncope. Am J Cardiol. 1995;76(4):267–272 [DOI] [PubMed] [Google Scholar]
- 41.Foglia-Manzillo G, Giada F, Fteita N, Nessi I, Santarone M, Raviele A. Tilt testing potentiated with sublingual nitroglycerin in children with unexplained syncope. Eur Heart J. 2007;28(21):2605–2609 [DOI] [PubMed] [Google Scholar]
- 42.Lewis DA, Zlotocha J, Henke L, Dhala A. Specificity of head-up tilt testing in adolescents: effect of various degrees of tilt challenge in normal control subjects. J Am Coll Cardiol. 1997;30(4):1057–1060 [DOI] [PubMed] [Google Scholar]
- 43.Plash WB, Diedrich A, Biaggioni I, et al. Diagnosing postural tachycardia syndrome: comparison of tilt testing compared with standing haemodynamics. Clin Sci (Lond). 2013;124(2):109–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibbons CH, Cheshire WP, Fife TD. Model Coverage Testing Autonomic Testing. Minneapolis, MN: American Academy of Neurology; 2014 [Google Scholar]
- 45.Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci. 1999;317(2):75–77 [DOI] [PubMed] [Google Scholar]
- 46.Kanjwal K, Karabin B, Kanjwal Y, Grubb BP. Postural orthostatic tachycardia syndrome following Lyme disease. Cardiol J. 2011;18(1):63–66 [PubMed] [Google Scholar]
- 47.Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343(12):847–855 [DOI] [PubMed] [Google Scholar]
- 48.Grubb BP, Kanjwal MY, Kosinski DJ. Review: the postural orthostatic tachycardia syndrome: current concepts in pathophysiology diagnosis and management. J Interv Card Electrophysiol. 2001;5(1):9–16 [DOI] [PubMed] [Google Scholar]
- 49.Heyer GL, Fischer A, Wilson J, et al. Orthostatic intolerance and autonomic dysfunction in youth with persistent postconcussion symptoms: a head-upright tilt table study. Clin J Sport Med. 2016;26(1):40–45 [DOI] [PubMed] [Google Scholar]
- 50.Low PA, Schondorf R, Novak V, Sandroni P, Opfer-Gehrking TL, Novak P. Postural tachycardia syndrome. In: Low PA, ed. Clinical Autonomic Disorders, 2nd ed Philadelphia, PA: Lippincott-Raven Publishers; 1997:681–697 [Google Scholar]
- 51.Stewart JM. Chronic orthostatic intolerance and the postural tachycardia syndrome (POTS). J Pediatr. 2004;145(6):725–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stewart JM, Taneja I, Medow MS. Reduced body mass index is associated with increased angiotensin II in young women with postural tachycardia syndrome. Clin Sci (Lond). 2007;113(11):449–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson JN, Mack KJ, Kuntz NL, Brands CK, Porter CJ, Fischer PR. Postural orthostatic tachycardia syndrome: a clinical review. Pediatr Neurol. 2010;42(2):77–85 [DOI] [PubMed] [Google Scholar]
- 54.Goldstein IB, Shapiro D. The cardiovascular response to postural change as a function of race. Biol Psychol. 1995;39(2–3):173–186 [DOI] [PubMed] [Google Scholar]
- 55.Hinds K, Stachenfeld NS. Greater orthostatic tolerance in young black compared with white women. Hypertension. 2010;56(1):75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thieben MJ, Sandroni P, Sletten DM, et al. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc. 2007;82(3):308–313 [DOI] [PubMed] [Google Scholar]
- 57.Benarroch EE. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin Proc. 2012;87(12):1214–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raj SR. Postural tachycardia syndrome (POTS). Circulation. 2013;127(23):2336–2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parsaik AK, Singer W, Allison TG, et al. Orthostatic intolerance without postural tachycardia: how much dysautonomia? Clin Auton Res. 2013;23(4):181–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schondorf R, Freeman R. The importance of orthostatic intolerance in the chronic fatigue syndrome. Am J Med Sci. 1999;317(2):117–123 [DOI] [PubMed] [Google Scholar]
- 61.Joyner MJ, Masuki S. POTS versus deconditioning: the same or different? Clin Auton Res. 2008;18(6):300–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pianosi PT, Goodloe AH, Soma D, Parker KO, Brands CK, Fischer PR. High flow variant postural orthostatic tachycardia syndrome amplifies the cardiac output response to exercise in adolescents. Physiol Rep. 2014;2(8):e12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee SM, Moore AD, Everett ME, Stenger MB, Platts SH. Aerobic exercise deconditioning and countermeasures during bed rest. Aviat Space Environ Med. 2010;81(1):52–63 [DOI] [PubMed] [Google Scholar]
- 64.Greenleaf JE, Kozlowski S. Physiological consequences of reduced physical activity during bed rest. Exerc Sport Sci Rev. 1982;10(1):84–119 [PubMed] [Google Scholar]
- 65.Nixon JV, Murray RG, Bryant C, et al. Early cardiovascular adaptation to simulated zero gravity. J Appl Physiol. 1979;46(3):541–548 [DOI] [PubMed] [Google Scholar]
- 66.Fischer D, Arbeille P, Shoemaker JK, O’Leary DD, Hughson RL. Altered hormonal regulation and blood flow distribution with cardiovascular deconditioning after short-duration head down bed rest. J Appl Physiol (1985). 2007;103(6):2018–2025 [DOI] [PubMed] [Google Scholar]
- 67.Crandall CG, Shibasaki M, Wilson TE, Cui J, Levine BD. Prolonged head-down tilt exposure reduces maximal cutaneous vasodilator and sweating capacity in humans. J Appl Physiol (1985). 2003;94(6):2330–2336 [DOI] [PubMed] [Google Scholar]
- 68.Wilson TE, Shibasaki M, Cui J, Levine BD, Crandall CG. Effects of 14 days of head-down tilt bed rest on cutaneous vasoconstrictor responses in humans. J Appl Physiol (1985). 2003;94(6):2113–2118 [DOI] [PubMed] [Google Scholar]
- 69.Spaak J, Montmerle S, Sundblad P, Linnarsson D. Long-term bed rest-induced reductions in stroke volume during rest and exercise: cardiac dysfunction vs volume depletion. J Appl Physiol (1985). 2005;98(2):648–654 [DOI] [PubMed] [Google Scholar]
- 70.Stewart JM, Medow MS, Montgomery LD, McLeod K. Decreased skeletal muscle pump activity in patients with postural tachycardia syndrome and low peripheral blood flow. Am J Physiol Heart Circ Physiol. 2004;286(3):H1216–H1222 [DOI] [PubMed] [Google Scholar]
- 71.Perhonen MA, Zuckerman JH, Levine BD. Deterioration of left ventricular chamber performance after bed rest: “cardiovascular deconditioning” or hypovolemia? Circulation. 2001;103(14):1851–1857 [DOI] [PubMed] [Google Scholar]
- 72.Jacob G, Costa F, Shannon JR, et al. The neuropathic postural tachycardia syndrome. N Engl J Med. 2000;343(14):1008–1014 [DOI] [PubMed] [Google Scholar]
- 73.Low PA, Opfer-Gehrking TL, Textor SC, et al. Comparison of the postural tachycardia syndrome (POTS) with orthostatic hypotension due to autonomic failure. J Auton Nerv Syst. 1994;50(2):181–188 [DOI] [PubMed] [Google Scholar]
- 74.Li H, Yu X, Liles C, et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc. 2014;3(1):e000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang XL, Chai Q, Charlesworth MC, et al. Autoimmunoreactive IgGs from patients with postural orthostatic tachycardia syndrome. Proteomics Clin Appl. 2012;6(11-12):615–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blitshteyn S. Autoimmune markers and autoimmune disorders in patients with postural tachycardia syndrome (POTS). Lupus. 2015;24(13):1364–1369 [DOI] [PubMed] [Google Scholar]
- 77.Shibao C, Arzubiaga C, Roberts LJ II, et al. Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension. 2005;45(3):385–390 [DOI] [PubMed] [Google Scholar]
- 78.Garland EM, Celedonio JE, Raj SR. Postural tachycardia syndrome: beyond orthostatic intolerance. Curr Neurol Neurosci Rep. 2015;15(9):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Afrin LB, Butterfield JH, Raithel M, Molderings GJ. Often seen, rarely recognized: mast cell activation disease—a guide to diagnosis and therapeutic options. Ann Med. 2016;48(3):190–201 [DOI] [PubMed] [Google Scholar]
- 80.Molderings GJ, Haenisch B, Brettner S, et al. Pharmacological treatment options for mast cell activation disease. Naunyn Schmiedebergs Arch Pharmacol. 2016;389(7):671–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chelimsky G, Kovacic K, Nugent M, Mueller A, Simpson P, Chelimsky TC. Comorbid conditions do not differ in children and young adults with functional disorders with or without postural tachycardia syndrome. J Pediatr. 2015;167(1):120–124 [DOI] [PubMed] [Google Scholar]
- 82.Gazit Y, Nahir AM, Grahame R, Jacob G. Dysautonomia in the joint hypermobility syndrome. Am J Med. 2003;115(1):33–40 [DOI] [PubMed] [Google Scholar]
- 83.Kanjwal K, Saeed B, Karabin B, Kanjwal Y, Grubb BP. Comparative clinical profile of postural orthostatic tachycardia patients with and without joint hypermobility syndrome. Indian Pacing Electrophysiol J. 2010;10(4):173–178 [PMC free article] [PubMed] [Google Scholar]
- 84.Fikree A, Grahame R, Aktar R, et al. A prospective evaluation of undiagnosed joint hypermobility syndrome in patients with gastrointestinal symptoms. Clin Gastroenterol Hepatol. 2014;12(10):1680.e2–1687.e2 [DOI] [PubMed] [Google Scholar]
- 85.Fikree A, Aktar R, Grahame R, et al. Functional gastrointestinal disorders are associated with the joint hypermobility syndrome in secondary care: a case-control study. Neurogastroenterol Motil. 2015;27(4):569–579 [DOI] [PubMed] [Google Scholar]
- 86.Chelimsky G, Hupertz VF, Chelimsky TC. Abdominal pain as the presenting symptom of autonomic dysfunction in a child. Clin Pediatr (Phila). 1999;38(12):725–729 [DOI] [PubMed] [Google Scholar]
- 87.Chelimsky G, Boyle JT, Tusing L, Chelimsky TC. Autonomic abnormalities in children with functional abdominal pain: coincidence or etiology? J Pediatr Gastroenterol Nutr. 2001;33(1):47–53 [DOI] [PubMed] [Google Scholar]
- 88.Safder S, Chelimsky TC, O’Riordan MA, Chelimsky G. Gastric electrical activity becomes abnormal in the upright position in patients with postural tachycardia syndrome. J Pediatr Gastroenterol Nutr. 2010;51(3):314–318 [DOI] [PubMed] [Google Scholar]
- 89.Moak JP, Fabian RR, Clarke LC, Hanumanthaiah S, Desbiens J, Darbari A. Antroduodenal manometry is abnormal in children presenting with orthostatic intolerance and gastrointestinal symptoms. J Pediatr Gastroenterol Nutr. 2016;63(3):329–335 [DOI] [PubMed] [Google Scholar]
- 90.Fortunato JE, Wagoner AL, Harbinson RL, D’Agostino RB Jr, Shaltout HA, Diz DI. Effect of fludrocortisone acetate on chronic unexplained nausea and abdominal pain in children with orthostatic intolerance. J Pediatr Gastroenterol Nutr. 2014;59(1):39–43 [DOI] [PubMed] [Google Scholar]
- 91.Lawal A, Barboi A, Krasnow A, Hellman R, Jaradeh S, Massey BT. Rapid gastric emptying is more common than gastroparesis in patients with autonomic dysfunction. Am J Gastroenterol. 2007;102(3):618–623 [DOI] [PubMed] [Google Scholar]
- 92.Park KJ, Singer W, Sletten DM, Low PA, Bharucha AE. Gastric emptying in postural tachycardia syndrome: a preliminary report. Clin Auton Res. 2013;23(4):163–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chelimsky G, Madan S, Alshekhlee A, Heller E, McNeeley K, Chelimsky T.. A comparison of dysautonomias comorbid with cyclic vomiting syndrome and with migraine. Gastroenterol Res Pract. 2009;2009:701019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chelimsky TC, Chelimsky GG. Autonomic abnormalities in cyclic vomiting syndrome. J Pediatr Gastroenterol Nutr. 2007;44(3):326–330 [DOI] [PubMed] [Google Scholar]
- 95.Tarbell SE, Li BU. Anxiety measures predict health-related quality of life in children and adolescents with cyclic vomiting syndrome. J Pediatr. 2015;167(3):633.e1–638.e1 [DOI] [PubMed] [Google Scholar]
- 96.Stewart JM, Gewitz MH, Weldon A, Arlievsky N, Li K, Munoz J. Orthostatic intolerance in adolescent chronic fatigue syndrome. Pediatrics. 1999;103(1):116–121 [DOI] [PubMed] [Google Scholar]
- 97.Stewart JM, Gewitz MH, Weldon A, Munoz J. Patterns of orthostatic intolerance: the orthostatic tachycardia syndrome and adolescent chronic fatigue. J Pediatr. 1999;135(2, pt 1):218–225 [DOI] [PubMed] [Google Scholar]
- 98.Wyller VB, Saul JP, Walløe L, Thaulow E. Sympathetic cardiovascular control during orthostatic stress and isometric exercise in adolescent chronic fatigue syndrome. Eur J Appl Physiol. 2008;102(6):623–632 [DOI] [PubMed] [Google Scholar]
- 99.Wyller VB, Fagermoen E, Sulheim D, Winger A, Skovlund E, Saul JP. Orthostatic responses in adolescent chronic fatigue syndrome: contributions from expectancies as well as gravity. Biopsychosoc Med. 2014;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khurana RK, Eisenberg L. Orthostatic and nonorthostatic headache in postural tachycardia syndrome. Cephalalgia. 2011;31(4):409–415 [DOI] [PubMed] [Google Scholar]
- 101.Piovesan EJ, Sobreira CF, Scola RH, et al. Episodic migraine associated with postural orthostatic tachycardia syndrome and vasovagal syncope: migraine triggers neuromediated syncope. Arq Neuropsiquiatr. 2008;66(1):77–79 [DOI] [PubMed] [Google Scholar]
- 102.Deb A, Morgenshtern K, Culbertson CJ, Wang LB, Hohler AD. A survey-based analysis of symptoms in patients with postural orthostatic tachycardia syndrome. Proc Bayl Univ Med Cent. 2015;28(2):157–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mack KJ, Johnson JN, Rowe PC. Orthostatic intolerance and the headache patient. Semin Pediatr Neurol. 2010;17(2):109–116 [DOI] [PubMed] [Google Scholar]
- 104.Ojha A, Chelimsky TC, Chelimsky G. Comorbidities in pediatric patients with postural orthostatic tachycardia syndrome. J Pediatr. 2011;158(1):20–23 [DOI] [PubMed] [Google Scholar]
- 105.Heyer GL, Fedak EM, LeGros AL. Symptoms predictive of postural tachycardia syndrome (POTS) in the adolescent headache patient. Headache. 2013;53(6):947–953 [DOI] [PubMed] [Google Scholar]
- 106.Mokri B, Low PA. Orthostatic headaches without CSF leak in postural tachycardia syndrome. Neurology. 2003;61(7):980–982 [DOI] [PubMed] [Google Scholar]
- 107.Lin J, Han Z, Li X, et al. Risk factors for postural tachycardia syndrome in children and adolescents. PLoS One. 2014;9(12):e113625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ross AJ, Medow MS, Rowe PC, Stewart JM. What is brain fog? An evaluation of the symptom in postural tachycardia syndrome. Clin Auton Res. 2013;23(6):305–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bagai K, Wakwe CI, Malow B, et al. Estimation of sleep disturbances using wrist actigraphy in patients with postural tachycardia syndrome. Auton Neurosci. 2013;177(2):260–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bagai K, Peltier AC, Malow BA, et al. Objective sleep assessments in patients with postural tachycardia syndrome using overnight polysomnograms. J Clin Sleep Med. 2016;12(5):727–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Miglis MG, Muppidi S, Feakins C, Fong L, Prieto T, Jaradeh S. Sleep disorders in patients with postural tachycardia syndrome. Clin Auton Res. 2016;26(1):67–73 [DOI] [PubMed] [Google Scholar]
- 112.Pengo MF, Higgins S, Drakatos P, et al. Characterisation of sleep disturbances in postural orthostatic tachycardia syndrome: a polysomnography-based study. Sleep Med. 2015;16(12):1457–1461 [DOI] [PubMed] [Google Scholar]
- 113.Stewart JM, Medow MS, Messer ZR, Baugham IL, Terilli C, Ocon AJ. Postural neurocognitive and neuronal activated cerebral blood flow deficits in young chronic fatigue syndrome patients with postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2011;302(5):H1185–H1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stewart JM, Del Pozzi AT, Pandey A, Messer ZR, Terilli C, Medow MS. Oscillatory cerebral blood flow is associated with impaired neurocognition and functional hyperemia in postural tachycardia syndrome during graded tilt. Hypertension. 2015;65(3):636–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Anderson JW, Lambert EA, Sari CI, et al. Cognitive function, health-related quality of life, and symptoms of depression and anxiety sensitivity are impaired in patients with the postural orthostatic tachycardia syndrome (POTS). Front Physiol. 2014;5:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McTate EA, Weiss KE. Psychosocial dimensions and functioning in youth with postural orthostatic tachycardia syndrome. Clin Pediatr (Phila). 2016;55(10):979–982 [DOI] [PubMed] [Google Scholar]
- 117.Hyphantis TN, Pappas AI, Vlahos AP, Carvalho AF, Levenson JL, Kolettis TM. Depressive symptoms and neurocardiogenic syncope in children: a 2-year prospective study. Pediatrics. 2012;130(5):906–913 [DOI] [PubMed] [Google Scholar]
- 118.Blount RL, Morris JA, Cheng PS, Campbell RM, Brown RT. Parent and child psychological factors in pediatric syncope and other somatic symptoms. J Consult Clin Psychol. 2004;72(4):597–604 [DOI] [PubMed] [Google Scholar]
- 119.Morris JA, Blount RL, Brown RT, Campbell RM. Association of parental psychological and behavioral factors and children’s syncope. J Consult Clin Psychol. 2001;69(5):851–857 [DOI] [PubMed] [Google Scholar]
- 120.Judelson DA, Maresh CM, Yamamoto LM, et al. Effect of hydration state on resistance exercise-induced endocrine markers of anabolism, catabolism, and metabolism. J Appl Physiol (1985). 2008;105(3):816–824 [DOI] [PubMed] [Google Scholar]
- 121.Brown CM, Barberini L, Duplo AG, Montani JP. Cardiovascular responses to water drinking: does osmolality play a role? Am J Physiol Regul Integr Comp Physiol. 2005;289(6):R1687–R1692 [DOI] [PubMed] [Google Scholar]
- 122.Grandjean AC, Grandjean NR. Dehydration and cognitive performance. J Am Coll Nutr. 2007;26(suppl 5):549S–554S [DOI] [PubMed] [Google Scholar]
- 123.Sawka MN. Physiological consequences of hypohydration: exercise performance and thermoregulation. Med Sci Sports Exerc. 1992;24(6):657–670 [PubMed] [Google Scholar]
- 124.Institute of Medicine; Food and Nutrition Board; Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; Panel on Dietary Reference Intakes for Electrolytes and Water . Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, DC: The National Academy Press; 2005 [Google Scholar]
- 125.Levine BD, Zuckerman JH, Pawelczyk JA. Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation. 1997;96(2):517–525 [DOI] [PubMed] [Google Scholar]
- 126.Waters WW, Platts SH, Mitchell BM, Whitson PA, Meck JV. Plasma volume restoration with salt tablets and water after bed rest prevents orthostatic hypotension and changes in supine hemodynamic and endocrine variables. Am J Physiol Heart Circ Physiol. 2005;288(2):H839–H847 [DOI] [PubMed] [Google Scholar]
- 127.Zhang Q, Liao Y, Tang C, Du J, Jin H. Twenty-four–hour urinary sodium excretion and postural orthostatic tachycardia syndrome. J Pediatr. 2012;161(2):281–284 [DOI] [PubMed] [Google Scholar]
- 128.Sandroni P, Opfer-Gehrking TL, McPhee BR, Low PA. Postural tachycardia syndrome: clinical features and follow-up study. Mayo Clin Proc. 1999;74(11):1106–1110 [DOI] [PubMed] [Google Scholar]
- 129.Raj SR, Biaggioni I, Yamhure PC, et al. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111(13):1574–1582 [DOI] [PubMed] [Google Scholar]
- 130.Stewart JM, Glover JL, Medow MS. Increased plasma angiotensin II in postural tachycardia syndrome (POTS) is related to reduced blood flow and blood volume. Clin Sci (Lond). 2006;110(2):255–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Coombes JS, Hamilton KL. The effectiveness of commercially available sports drinks. Sports Med. 2000;29(3):181–209 [DOI] [PubMed] [Google Scholar]
- 132.Coyle EF, Montain SJ. Carbohydrate and fluid ingestion during exercise: are there trade-offs? Med Sci Sports Exerc. 1992;24(6):671–678 [PubMed] [Google Scholar]
- 133.Convertino VA, Brock PJ, Keil LC, Bernauer EM, Greenleaf JE. Exercise training-induced hypervolemia: role of plasma albumin, renin, and vasopressin. J Appl Physiol. 1980;48(4):665–669 [DOI] [PubMed] [Google Scholar]
- 134.Convertino VA. Blood volume response to physical activity and inactivity. Am J Med Sci. 2007;334(1):72–79 [DOI] [PubMed] [Google Scholar]
- 135.Bruce BK, Harrison TE, Bee SM, et al. Improvement in functioning and psychological distress in adolescents with postural orthostatic tachycardia syndrome following interdisciplinary treatment. Clin Pediatr (Phila). 2016;55(14):1300–1304 [DOI] [PubMed] [Google Scholar]
- 136.Freitas J, Santos R, Azevedo E, Costa O, Carvalho M, de Freitas AF. Clinical improvement in patients with orthostatic intolerance after treatment with bisoprolol and fludrocortisone. Clin Auton Res. 2000;10(5):293–299 [DOI] [PubMed] [Google Scholar]
- 137.Chen L, Wang L, Sun J, et al. Midodrine hydrochloride is effective in the treatment of children with postural orthostatic tachycardia syndrome. Circ J. 2011;75(4):927–931 [DOI] [PubMed] [Google Scholar]
- 138.Hoeldtke RD, Horvath GG, Bryner KD, Hobbs GR. Treatment of orthostatic hypotension with midodrine and octreotide. J Clin Endocrinol Metab. 1998;83(2):339–343 [DOI] [PubMed] [Google Scholar]
- 139.Lai CC, Fischer PR, Brands CK, et al. Outcomes in adolescents with postural orthostatic tachycardia syndrome treated with midodrine and beta-blockers. Pacing Clin Electrophysiol. 2009;32(2):234–238 [DOI] [PubMed] [Google Scholar]
- 140.Ross AJ, Ocon AJ, Medow MS, Stewart JM. A double-blind placebo-controlled cross-over study of the vascular effects of midodrine in neuropathic compared with hyperadrenergic postural tachycardia syndrome. Clin Sci (Lond). 2014;126(4):289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Coffin ST, Black BK, Biaggioni I, et al. Desmopressin acutely decreases tachycardia and improves symptoms in the postural tachycardia syndrome. Heart Rhythm. 2012;9(9):1484–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hoeldtke RD, Davis KM, Joseph J, Gonzales R, Panidis IP, Friedman AC. Hemodynamic effects of octreotide in patients with autonomic neuropathy. Circulation. 1991;84(1):168–176 [DOI] [PubMed] [Google Scholar]
- 143.Kanjwal K, Saeed B, Karabin B, Kanjwal Y, Grubb BP. Use of octreotide in the treatment of refractory orthostatic intolerance. Am J Ther. 2012;19(1):7–10 [DOI] [PubMed] [Google Scholar]
- 144.Hoeldtke RD, Streeten DH. Treatment of orthostatic hypotension with erythropoietin. N Engl J Med. 1993;329(9):611–615 [DOI] [PubMed] [Google Scholar]
- 145.Kanjwal K, Saeed B, Karabin B, Kanjwal Y, Sheikh M, Grubb BP. Erythropoietin in the treatment of postural orthostatic tachycardia syndrome. Am J Ther. 2012;19(2):92–95 [DOI] [PubMed] [Google Scholar]
- 146.Moak JP, Leong D, Fabian R, et al. Intravenous hydration for management of medication-resistant orthostatic intolerance in the adolescent and young adult. Pediatr Cardiol. 2016;37(2):278–282 [DOI] [PubMed] [Google Scholar]
- 147.Barzilai M, Jacob G. The effect of ivabradine on the heart rate and sympathovagal balance in postural tachycardia syndrome patients. Rambam Maimonides Med J. 2015;6(3):e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McDonald C, Frith J, Newton JL. Single centre experience of ivabradine in postural orthostatic tachycardia syndrome. Europace. 2011;13(3):427–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wyller VB, Thaulow E, Amlie JP. Treatment of chronic fatigue and orthostatic intolerance with propranolol. J Pediatr. 2007;150(6):654–655 [DOI] [PubMed] [Google Scholar]
- 150.Mar PL, Raj V, Black BK, et al. Acute hemodynamic effects of a selective serotonin reuptake inhibitor in postural tachycardia syndrome: a randomized, crossover trial. J Psychopharmacol. 2014;28(2):155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fagermoen E, Sulheim D, Winger A, et al. Effects of low-dose clonidine on cardiovascular and autonomic variables in adolescents with chronic fatigue: a randomized controlled trial. BMC Pediatr. 2015;15(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gaffney FA, Lane LB, Pettinger W, Blomqvist CG. Effects of long-term clonidine administration on the hemodynamic and neuroendocrine postural responses of patients with dysautonomia. Chest. 1983;83(suppl 2):436–438 [DOI] [PubMed] [Google Scholar]
- 153.Gales BJ, Gales MA. Pyridostigmine in the treatment of orthostatic intolerance. Ann Pharmacother. 2007;41(2):314–318 [DOI] [PubMed] [Google Scholar]
- 154.Kanjwal K, Karabin B, Sheikh M, et al. Pyridostigmine in the treatment of postural orthostatic tachycardia: a single-center experience. Pacing Clin Electrophysiol. 2011;34(6):750–755 [DOI] [PubMed] [Google Scholar]
- 155.Raj SR, Black BK, Biaggioni I, Harris PA, Robertson D. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation. 2005;111(21):2734–2740 [DOI] [PubMed] [Google Scholar]
- 156.Anderson JB, Czosek RJ, Knilans TK, Marino BS. The effect of paediatric syncope on health-related quality of life. Cardiol Young. 2012;22(5):583–588 [DOI] [PubMed] [Google Scholar]
- 157.Rose MS, Koshman ML, Spreng S, Sheldon R. The relationship between health-related quality of life and frequency of spells in patients with syncope. J Clin Epidemiol. 2000;53(12):1209–1216 [DOI] [PubMed] [Google Scholar]
- 158.Stewart JM, Montgomery LD. Regional blood volume and peripheral blood flow in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2004;287(3):H1319–H1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bhatia R, Kizilbash SJ, Ahrens SP, et al. Outcomes of adolescent-onset postural orthostatic tachycardia syndrome. J Pediatr. 2016;173:149–153 [DOI] [PubMed] [Google Scholar]
- 160.Sousa A, Lebreiro A, Freitas J, Maciel MJ. Long-term follow-up of patients with postural tachycardia syndrome. Clin Auton Res. 2012;22(3):151–153 [DOI] [PubMed] [Google Scholar]
- 161.George SA, Bivens TB, Howden EJ, et al. The international POTS registry: evaluating the efficacy of an exercise training intervention in a community setting. Heart Rhythm. 2016;13(4):943–950 [DOI] [PubMed] [Google Scholar]
- 162.Wagoner AL, Shaltout HA, Fortunato JE, Diz DI. Distinct neurohumoral biomarker profiles in children with hemodynamically defined orthostatic intolerance may predict treatment options. Am J Physiol Heart Circ Physiol. 2016;310(3):H416–H425 [DOI] [PMC free article] [PubMed] [Google Scholar]