Abstract
Background:
Coarse particulate matter () is primarily mechanically generated and includes crustal material, brake and tire wear, and biological particles. is associated with pulmonary disease, which can lead to right ventricular (RV) dysfunction. Although RV characteristics have been associated with combustion-related pollutants, relationships with remain unknown.
Objectives:
To quantify cross-sectional associations between RV dysfunction and mass and components among older adults and susceptible populations.
Methods:
We used baseline cardiac magnetic resonance images from 1,490 participants (45–84 y old) from the Multi-Ethnic Study of Atherosclerosis and assigned 5-y residential concentrations of mass, copper, zinc, phosphorus, silicon, and endotoxin, using land-use regression models. We quantified associations with RV mass, end-diastolic volume, and ejection fraction after control for risk factors and copollutants using linear regression. We further examined personal susceptibility.
Results:
We found positive associations of RV mass and, to a lesser extent, end diastolic volume with mass among susceptible populations including smokers and persons with emphysema. After adjustment for copollutants, an interquartile range increase in mass () was associated with (95% CI: 0.0, 1.0), (95% CI: 0.1, 1.7), and (95% CI: 0.4, 2.5) larger RV mass among former smokers, current smokers, and persons with emphysema, respectively. No associations were found with healthy individuals or with ejection fraction.
Conclusions:
Alterations to RV structure may represent a mechanism by which long-term exposure increases risks for adverse respiratory and cardiovascular outcomes, especially among certain susceptible populations. https://doi.org/10.1289/EHP658
Introduction
Air pollution is a well-established risk factor for adverse respiratory outcomes, including chronic lung diseases (Andersen et al. 2011; Karakatsani et al. 2003; Lindgren et al. 2009; Schikowski et al. 2005; Sunyer 2001), hospitalizations (Chen et al. 2005) and death (Dockery et al. 1993; Pope et al. 2002). Most recently, it has been estimated that for 2013 worldwide ambient particulate matter (PM) pollution accounts for nearly 170,000 deaths and nearly 4 million disability-adjusted life years (DALYs) due to chronic respiratory disease (Forouzanfar et al. 2015; IHME 2016).
A common sequela of chronic lung disease is the development of pulmonary hypertension and impairments to the heart, including right ventricular (RV) dysfunction (Freixa et al. 2013). The right ventricle pumps blood through the lungs to allow for its oxygenation. Then the oxygen-rich blood flows to the left ventricle for subsequent distribution to all tissues of the human body. Changes in RV structure and function can therefore result in many similar clinical sequelae of left ventricular (LV) changes, including dyspnea, exercise intolerance, lower-extremity edema, and (at advanced stages) severe heart failure (Voelkel et al. 2006). Although the left ventricle is vulnerable to increased pressures during ejection due to systemic hypertension or valvular disease, reduced blood supply, and hypoxia, the right ventricle may be similarly affected by changes in lung function [e.g., chronic obstructive pulmonary disorder (COPD)], LV function, and hypoxia (e.g., sleep disordered breathing). The RV has been thought to respond to this increased load through structural changes such as hypertrophy (i.e., thickening of the ventricle leading to increased mass), chamber dilation leading to greater end-diastolic volume, and lowered pumping efficiency (i.e., reduced ejection fraction) (Polak et al. 1983; Shah et al. 1986). Although these three manifestations of RV dysfunction are most likely in severe stages of lung disease, the right ventricle can also be affected early in lung disease (Hilde et al. 2013). RV dysfunction has public health importance because it has been linked to poor outcomes among persons with and without preexisting disease, such as COPD and cardiovascular disease (Burgess et al. 2002; France et al. 1988; Kawut et al. 2012).
Long-term exposures to air pollution are believed to affect the same biological mechanisms that lead to COPD and cardiovascular disease. There is evidence that air pollution is associated with greater inflammation (Adar et al. 2015b) and reduced vessel compliance (Brook et al. 2014; Krishnan et al. 2013; Mills et al. 2005); such evidence suggests a plausible link to RV function. In fact, two studies from the Multi-Ethnic Study of Atherosclerosis (MESA) recently linked long-term exposures to two combustion-related air pollutants: nitrogen dioxide () (Leary et al. 2014) and fine PM (aerodynamic diameter , ) (Aaron et al. 2016) to greater RV hypertrophy and lower function. Although PM in the coarse fraction (aerodynamic diameter between 2.5 and , ) has also been associated with adverse respiratory end points (Adar et al. 2014; Brunekreef and Forsberg 2005), no study has investigated associations between and RV characteristics. Understanding the health impacts of independent of other pollutants, including and has importance, given that the U.S. Environmental Protection Agency (EPA) is interested in regulating levels but has struggled with insufficient data in the general population as well as among susceptible individuals (U.S. EPA 2009). Because is generated by very different diverse processes, ranging from crustal material to brake and tire wear, a lack of information on associations between health and indicators of different sources represents another important gap in the literature.
To expand the literature on the health implications of and to better understand environmental risk factors of RV dysfunction, we aimed to quantify cross-sectional associations between and measures of RV function among older adults and susceptible subpopulations. We approached this goal using individual-level long-term estimates of mass and selected source-specific components with multiple measures of RV structure (mass, end-diastolic volume) and function (ejection fraction) in participants of MESA. Some of these results have been previously reported in the form of an abstract (Adar et al. 2015a).
Methods
Study Population
Initiated in 2000, MESA is a multicenter, prospective study examining the progression of subclinical cardiovascular disease among an ethnically diverse population of 6,814 subjects (45–84 y old) who were free of known cardiovascular disease at baseline (Bild et al. 2002). In this analysis, we restricted reporting to participants from Chicago, Illinois, St. Paul, Minnesota, and Winston-Salem, North Carolina, who were part of the MESA Coarse ancillary study (). The MESA Coarse study conducted intensive sampling of concentrations in three of the MESA sites chosen to reflect variability. We further restricted to those who had cardiac magnetic resonance images (MRI) interpreted for RV morphology as part of the MESA RV ancillary study (). After excluding those with missing exposures and covariates, our final sample was 1,490 persons (Figure S1).
All protocols described herein received approval from local and national institutional review boards. Participants also provided informed consent.
Right Ventricle Characteristics
The MESA RV study obtained measures of RV function using cardiac MRIs performed at the baseline exam (Natori et al. 2006). These measures include RV mass at end-diastole, end-diastolic volume, and ejection fraction (Bluemke et al. 2008; Chahal et al. 2010). These measures were estimated by two independent analysts using QMASS software (version 4.2; Medis), is described elsewhere (Chahal et al. 2010). Based on random, blinded rereads from approximately 230 scans, the inter-reader intraclass correlation coefficients were 0.89, 0.96, and 0.80 for RV mass, end-diastolic volume, and ejection fraction, respectively (Kawut et al. 2011).
Exposure Assessment
We used site-specific land-use regression spatial prediction models derived from project-specific measurements and geographic data to predict concentrations of at subjects’ residences. Details of these models have been previously published (Zhang et al. 2014). Briefly, we conducted two spatially intensive 2-wk monitoring campaigns of integrative and samples using paired Harvard Personal Exposure Monitors (HPEMs) in each of three MESA Coarse sites. In each city, approximately 60 locations were targeted to cover the greatest geographic space. Additionally, the locations were selected to capture the variability of hypothesized characteristics associated with mass and components (e.g., vegetation, distance to roads). All samples were weighed in a temperature- and relative humidity-controlled chamber, analyzed for elements by X-ray fluorescence spectroscopy, and total mass and that of chemical components were calculated by difference (U.S. EPA 2009). The specific components of interest were copper, zinc, phosphorus, and silicon as consistent indicators for motor vehicle brake wear, tire wear, fertilized soil/agriculture, and crustal material across all study sites, respectively (Sturtz et al. 2014). We also examined a fifth component of , endotoxin, a major component of the outer membrane of Gram-negative bacteria. Endotoxin was chosen due to its capability to induce inflammation and modulate immune responses (Hadina et al. 2008) and its association with airway disease (Schwartz et al. 1995). We separately derived spatial prediction models for mass and each component using many geographic variables, including land use, population density, vegetation, impervious surface, roadways, railways, and airports, as well as spatial correlation. The cross-validated (CV) for the site-specific models of and chemical species ranged from 0.3 to 0.9. As described elsewhere (Zhang et al 2014), the models performed best for copper (CV , 0.5–0.9) and generally worse for endotoxin (CV ). For our statistical modeling, we selected 5–y average concentrations weighted according to subjects’ residential history preceding subjects’ MRI.
Exposures to and were also estimated for each participant using spatiotemporal models derived from project-specific measurements, land-use characteristics, as well as regulatory monitoring data in the MESA Air study (Gill et al. 2011; Szpiro et al. 2010).
Covariates
All covariates, with the exception of airflow limitation, were assessed at baseline. These included sociodemographic and behavior information obtained via interview, and anthropometric measurements, left ventricle function, and laboratory data from the clinical exam. Comorbidities of hypertension and diabetes were also defined based on blood pressure or glucose measurements, respectively, self-reported medication use, and doctor diagnosis (Genuth et al. 2003; JNC 1997). Through the MESA Lung ancillary study, we had data on percent emphysema from computed tomography (CT) scans (Hoffman et al. 2009) and spirometry (Hankinson et al. 2010). The MESA Neighborhood Study developed a neighborhood socioeconomic scale (NSES) for each participant based on a principal components analysis of 2000 census tract data (U.S. Census Bureau 2002), including median household income, percent of persons in tract with at least a high school degree and median home value (Hajat et al. 2013).
Statistical Analysis
Multivariable linear regression models were used to quantify adjusted cross-sectional associations between and continuous measures of our RV outcomes. All models were adjusted for age, race/ethnicity (White, Chinese, Black, and Hispanic), sex, education (less than high school, high school/some college, college or more), NSES, height, weight, cigarette smoking history (never, former, current), pack-years of smoking (0 pack-y, , , greater than 20), second-hand smoke exposure, hypertension (JNC 1997), diabetes (according to the 2003 American Diabetes Association Fasting Criteria Algorithm: normal, impaired fasting glucose, untreated diabetes, treated diabetes), cholesterol, study site, and an interaction of study site with NSES. Age, height, weight, NSES, and cholesterol were modeled as continuous; all other variables were modeled as categorical. In secondary models, we examined the linearity of these associations using splines and the robustness of our results to adjustment for and in two pollutant models. In secondary models of the chemical species of we also adjusted for total mass as a covariate using a constituent residual model (Mostofsky et al. 2012). We used interaction terms to assess effect modification by age, sex, race/ethnicity, smoking status, emphysema (defined as percent of emphysema-like lung based on CT scans that were greater than the upper limit of normal (Hoffman et al. 2014)), and airflow limitation (). All reported estimates were scaled to the interquartile range (IQR) for each pollutant/species: (), copper (), zinc (), phosphorous (), silicon (), endotoxin (), (7.0 ppb), and ().
In sensitivity analyses, we restricted our analyses to participants who were residentially stable (lived at their current residence for 10 y or longer) and examined additional control for hypertension, diabetes, and cholesterol, as well as measures of LV function and lung disease as potential mediators.
The data analysis for this paper was generated using SAS (version 9.4; SAS Institute Inc.) and R (version 3.3.2; R Development Core Team).
Results
The mean age of the sample at baseline was 61 y; nearly 9% had physician-diagnosed asthma, and 7% had emphysema based on their CT scans (Table 1). Although participants in this sample were more likely to be Chinese, less likely to be black, and more likely to have a graduate degree than the full MESA Coarse cohort, these individuals were otherwise quite similar. Importantly, they did not differ with respect to their air pollution levels for all pollutants except zinc, which was approximately 10% lower in the study sample (Table S1).
Table 1.
Descriptive characteristics of the MESA Coarse participants at the baseline examination (2000–2002), by study site.
| Characteristics | Total | Winston-Salem | St. Paul | Chicago |
|---|---|---|---|---|
| 1490 | 457 | 536 | 497 | |
| Age (y, %) | ||||
| 45–54 | 477 (32%) | 124 (27%) | 196 (37%) | 157 (32%) |
| 55–64 | 437 (29%) | 128 (28%) | 173 (32%) | 136 (27%) |
| 65–74 | 404 (27%) | 150 (33%) | 119 (22%) | 135 (27%) |
| 75–84 | 172 (12%) | 55 (12%) | 48 (9%) | 69 (14%) |
| Female | 795 (53%) | 253 (55%) | 278 (52%) | 264 (53%) |
| Race/ethnicity | ||||
| White | 828 (56%) | 277 (61%) | 327 (61%) | 224 (45%) |
| Chinese | 158 (11%) | 0 (0%) | 0 (0%) | 158 (32%) |
| Black | 293 (20%) | 178 (39%) | 0 (0%) | 115 (23%) |
| Hispanic | 211 (14%) | 2 (0%) | 209 (39%) | 0 (0%) |
| Education | ||||
| 400 (27%) | 128 (28%) | 205 (38%) | 67 (13%) | |
| High school/some college | 440 (30%) | 135 (30%) | 191 (36%) | 114 (23%) |
| 650 (44%) | 194 (42%) | 140 (26%) | 316 (64%) | |
| Smoking status | ||||
| Never | 744 (50%) | 225 (49%) | 255 (48%) | 264 (53%) |
| Former | 556 (37%) | 171 (37%) | 200 (37%) | 185 (37%) |
| Current | 190 (13%) | 61 (13%) | 81 (15%) | 48 (10%) |
| in neighborhood | 1033 (69%) | 281 (61%) | 381 (71%) | 371 (75%) |
| Health | ||||
| BMI () | ||||
| Cholesterol (mg/dl) | ||||
| Hypertension | 584 (39%) | 232 (51%) | 167 (31%) | 185 (37%) |
| Diabetic | 2.3 (1.0) | 1.4 (1.0) | 2.0 (1.0) | 3.8 (1.0) |
| % Emphysema ()a | 81% (0) | 37% (0) | 71% (0) | 134% (0) |
| Airflow limitationb | 220 (22%) | 66 (26%) | 57 (19%) | 97 (22%) |
| Emphysema | 97 (7%) | 15 (3%) | 46 (9%) | 36 (7%) |
| Asthma | 130 (9%) | 36 (8%) | 51 (10%) | 43 (9%) |
| Left Ventricular end-diastolic Mass (g) | ||||
| RV Outcomes | ||||
| RV mass (g) | ||||
| RV ejection fraction (%) | ||||
| RV end-diastolic volume (mL) | ||||
| Pollutants | ||||
| () | ||||
| Copper () | ||||
| Zinc () | ||||
| Silicon () | ||||
| Phosphorous () | ||||
| Endotoxin () | ||||
| () | ||||
| (ppb) |
Note: Values given as (%) or . BMI, body mass index; , nitrogen dioxide; , particulate matter in diameter; , particulate matter between 2.5 and in diameter; RV, right ventricular.
Emphysema is defined as the percent emphysema via computed tomography scan greater than the upper limit of normal.
Airflow limitation is defined as an and was available on only 974 participants.
Average mass concentrations were lowest for Winston-Salem () but similar in St. Paul () and Chicago (). St. Paul had the largest intracity variation (standard deviation: in St. Paul vs. for Chicago and Winston-Salem). With respect to the chemical components, the highest average concentrations of the two traffic-related markers of copper and zinc were in Chicago, whereas Winston-Salem had the highest concentrations of phosphorus. Mean endotoxin levels were generally low () across all locations. In all locations, we observed modest to high correlations (0.46–0.89) between the traffic-related pollutants of copper, zinc, and . In addition, and were also correlated () in all locations. Although the other pollutants did not demonstrate consistent patterns across sites, there were notable () correlations between most pollutants in Chicago (Table S2).
Among all participants, RV mass was positively associated with mass, copper, phosphorus, and silicon in single-pollutant models (Table 2). After controlling for and , however, which were themselves associated with RV mass, the association with copper was eliminated and associations with mass, phosphorus, and silicon were blunted. Apart from copper, adjustment for mass did not strongly affect associations with any chemical components (Figure S2). Results were also robust to more and less control for potential intermediate factors such as hypertension, cholesterol, diabetes, emphysema, airflow limitation, and LV mass and function (Figure S3).
Table 2.
Associations between mass and RV structure and function in single and multipollutant models.
| Model | Mass (g) | Volume (mL) | Ejection Fraction (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Difference | 95% CI | p–Value | Difference | 95% CI | p–Value | Difference | 95% CI | p–Value | |
| Single Pollutant Model | 0.3 | 0.0, 0.5 | 0.06 | 0.4 | , 2.2 | 0.63 | , 0.4 | 0.75 | |
| 0.2 | , 0.5 | 0.14 | 0.3 | , 2.2 | 0.74 | , 0.4 | 0.76 | ||
| 0.2 | , 0.5 | 0.22 | 0.4 | , 2.3 | 0.68 | , 0.4 | 0.72 | ||
| Cu | |||||||||
| Single Pollutant Model | 0.3 | , 0.8 | 0.20 | 0.6 | , 3.6 | 0.71 | 0.1 | , 1.0 | 0.75 |
| 0.0 | , 0.5 | 0.93 | 0.0 | , 3.3 | 0.99 | 0.5 | , 1.4 | 0.32 | |
| , 0.5 | 0.56 | , 4.1 | 0.96 | 0.4 | , 1.6 | 0.56 | |||
| Zn | |||||||||
| Single Pollutant Model | 0.0 | , 0.3 | 0.90 | , 1.3 | 0.51 | , 0.5 | 0.81 | ||
| , 0.1 | 0.16 | , 0.9 | 0.27 | 0.1 | , 0.6 | 0.85 | |||
| , 0.0 | 0.09 | , 0.9 | 0.24 | , 0.6 | 0.81 | ||||
| P | |||||||||
| Single Pollutant Model | 0.5 | 0.0, 1.0 | 0.03 | 0.5 | , 3.6 | 0.75 | , 0.8 | 0.87 | |
| 0.2 | , 0.7 | 0.41 | , 2.9 | 0.83 | 0.0 | , 1.0 | 0.93 | ||
| 0.3 | , 0.8 | 0.25 | 0.0 | , 3.4 | 0.99 | , 0.9 | 0.91 | ||
| Si | |||||||||
| Single Pollutant Model | 0.4 | 0.1, 0.7 | 0.01 | 0.8 | , 2.8 | 0.41 | , 0.4 | 0.54 | |
| 0.2 | , 0.5 | 0.36 | 0.2 | , 2.4 | 0.86 | 0.0 | , 0.6 | 0.95 | |
| 0.3 | , 0.6 | 0.19 | 0.7 | , 3.1 | 0.59 | , 0.4 | 0.49 | ||
| Endotoxin | |||||||||
| Single Pollutant Model | , 0.2 | 0.49 | , 1.9 | 0.82 | , 0.5 | 0.67 | |||
| 0.1 | , 0.4 | 0.64 | 0.1 | , 2.4 | 0.91 | , 0.3 | 0.26 | ||
| 0.0 | , 0.3 | 0.89 | , 2.1 | 0.90 | , 0.5 | 0.59 | |||
| Single Pollutant Model | 0.5 | 0.1, 0.9 | 0.01 | 0.8 | , 3.5 | 0.54 | 0.0 | , 0.7 | 0.93 |
| 0.4 | 0.0, 0.9 | 0.06 | 0.6 | , 3.5 | 0.66 | 0.0 | , 0.8 | 0.96 | |
| 0.2 | , 0.8 | 0.38 | 0.3 | , 3.8 | 0.84 | 0.3 | , 1.3 | 0.53 | |
| Single Pollutant Model | 1.0 | 0.4, 1.6 | 0.001 | 1.8 | , 5.6 | 0.36 | , 0.4 | 0.25 | |
| 0.9 | 0.3, 1.5 | 0.003 | 1.7 | , 5.6 | 0.41 | , 0.5 | 0.28 | ||
| 0.8 | 0.0, 1.5 | 0.043 | 1.5 | , 6.4 | 0.56 | , 0.5 | 0.19 | ||
Note: All models adjusted for age, race, gender, height, weight, neighborhood socioeconomic scale (NSES), NSES, education, smoking status, pack-years, second-hand smoke exposure, hypertension, diabetes, total cholesterol, study site, and site by NSES interaction. Associations scaled to interquartile range (IQR) IQR of pollutant: (), Cu (), Zn (), P (), Si (), endotoxin (), (), (). CI, confidence interval; Cu, copper; , nitrogen dioxide; P, phosphorous; , particulate matter in diameter; , particulate matter between 2.5 and in diameter; Si, silicon; Zn, zinc.
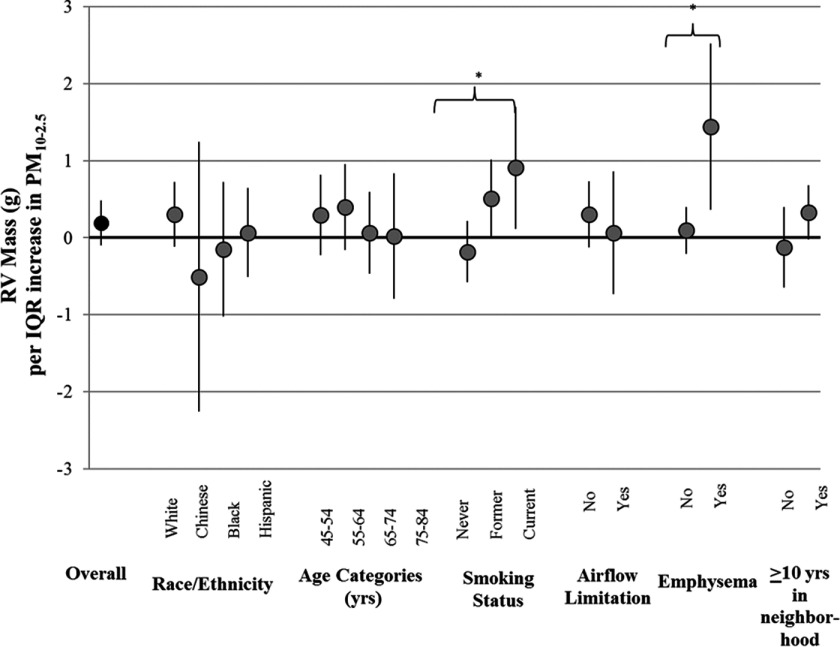
Analysis of effect modification suggested that associations between and RV mass were present in several susceptible populations. These subgroups included: former and current smokers in comparison with nonsmokers (), persons with emphysema in comparison with persons without emphysema (), and residentially stable participants in comparison with participants who had lived at their residences for less than 10 y (). These associations remained even after adjustment for and concentrations (Figure 1) and after adjustment for emphysema (results not shown).
Figure 1.
Effect modification of associations between concentrations and RV end-diastolic mass after control for and [g per interquartile range (IQR) of pollutant, 95% confidence interval (CI)].
*Interaction was statistically significant (). Note: , particulate matter with aerodynamic diameter between 2.5 and ; , nitrogen dioxide.
Although the size and direction of the associations between mass and silicon with RV end-diastolic volume were consistent with RV mass, the confidence intervals were very wide and indistinguishable from no association (Table 2). As with RV mass, associations with RV end-diastolic volume were strongest among current smokers, participants with emphysema, and particpants who were residentially stable, although the precision of these estimates remained large (Figure S4). No associations were observed with ejection fraction in the full cohort or in any subpopulation evaluated.
Discussion
Among a population-based cohort from three U.S. metropolitan areas, we found suggestive evidence of associations between and RV structure after adjustment for confounding by and . Positive associations between total mass concentrations and RV hypertrophy and, to a lesser extent, dilation were driven by relationships among former and current smokers, persons with advanced emphysema, and participants who were residentially stable. Associations were not found among other participants. No associations were found with RV ejection fraction among any group.
This study adds to the literature by expanding our understanding of the health implications of and the environmental risk factors for RV dysfunction. After adjustment for other risk factors such as smoking, height, weight, and co-pollutants previously associated with RV dysfunction, we observed the most robust associations for mass with a (95% CI: 0.4, 2.5) and (95% CI: 0.1, 1.7) larger RV mass among persons with emphysema and current smokers, respectively, per . These associations were on the same order of magnitude as those reported for in the full cohort (Aaron et al. 2016) and in the MESA Coarse cities. These differences are also comparable to differences in RV mass for participants apart in BMI (Chahal et al. 2012) and may be clinically relevant, given that MESA participants with RV hypertrophy have double to triple the risk of heart failure or cardiovascular death (Kawut et al. 2012).
Mechanisms through which exposures might likely affect the right ventricle (Voelkel et al. 2006) include the restructuring of the pulmonary vasculature, increases in RV load (Zangiabadi et al. 2014), hypoxia, inflammation (Chaouat et al. 2009), and autonomic dysfunction (Wensel et al. 2009; Wrobel et al. 2012). Support for these mechanisms comes from a previous study of healthy Mexican children that reported greater pulmonary arterial pressures and serum levels of the vasoconstrictive protein endothelin-1 with larger long-term PM concentrations (Calderón-Garcidueñas et al. 2007). Toxicological research has similarly demonstrated enhanced vasoconstriction and impaired vasodilation of pulmonary arterioles in healthy animals and in animals with chronic bronchitis exposed to PM (Faustini et al. 2012; Peel et al. 2005). Interestingly, the associations with RV mass were robust to control for hypertension, emphysema, airflow limitation, and LV mass and function, suggesting that these factors may not be critical intermediates of our observed associations. However, it is difficult to conclusively assess mediation in this study given our cross-sectional design and the possibility that only advanced cases of emphysema or airflow limitation are critical intermediates, which are limited in number in this population. Our overall null associations with RV ejection fraction were similar to findings in a previous analysis (Kawut et al. 2012) where only RV mass was independently associated with cardiovascular death. These data could suggest that RV hypertrophy is an earlier indicator of increased pressure in the RV than RV ejection fraction, though this has yet to be clearly demonstrated.
Although the observed association between and RV appeared to be independent of -associated lung damage, the interaction with emphysema suggests that individuals with preexisting lung damage may be more susceptible to long-term exposures. This susceptibility is plausible, given that persons with COPD have greater deposition and less mucociliary clearance of particles from their lungs relative to healthy individuals (Bennett et al. 1997; Brown et al. 2002). It is also consistent with epidemiological evidence of enhanced vulnerability of persons with respiratory conditions to short-term air pollution exposures (Faustini et al. 2012; Peel et al. 2005; Sacks et al. 2011), though the findings of the few studies to examine chronic lung disease as an effect modifier of long-term exposures to air pollution have been mixed (Andersen et al. 2012; Jerrett et al. 2009).
We also observed positive associations between RV mass and concentrations among participants who smoke or who have a history of smoking, independent of their emphysema status. One possible explanation may be that individuals who smoke or have smoked are more susceptible to the effects of air pollution because of smoking’s ability to increase inflammation and vasoconstriction (Akishima et al. 2007) and alter immune function, among other effects. However, epidemiologic evidence has also been mixed regarding the interaction between smoking and air pollution (Pope et al. 2011), suggesting that more research is necessary to understand this relationship. In addition, some caution is warranted about the generalizability of these findings as the smokers in MESA are generally healthier than the average smoking population due to our restriction to older adults without cardiovascular disease at baseline.
Our study is not without limitations. First, due to its cross-sectional design, our findings only provide evidence of potential associations that warrant further evaluation. Reverse causality is unlikely, however, and we have adjusted our models for a rich set of personal characteristics to account for between-person differences. Second, despite the highly innovative exposure assessment used, our exposure models are entirely spatial in nature and are assumed to capture the key differences in concentrations across space at different times. Our finding that associations were larger and more precise among persons living at their residences for may, however, suggest that our overall results may be biased towards the null due to inaccuracies in long-term exposures for some participants. On the other hand, compared with individuals who lived in their neighborhood for , residentially stable participants were more likely to be older, have hypertension, and have advanced emphysema, suggesting that these individuals may have been susceptible for other reasons. Another issue is that our models varied in predictive power by pollutant. Thus, differences in the observed strength of association between pollutants may be causal or could simply reflect different measurement errors. For example we found significant associations with and , which, compared with , had substantially better predictive ability due to a greater number of measurements that were collected over a longer period of time. In contrast, no associations were found with endotoxin, which had the lowest in our prediction models. This finding may be the result of smaller errors for and that make them less likely to be biased toward the null in individual pollutant and multipollutant models. Finally, although the exposure estimation methods used in this study allow for individual assessment of outdoor concentrations, we do not have estimates of indoor or personal concentrations.
Despite these limitations, this work has many important strengths. The MESA cohort is an extremely well-characterized population with detailed and standardized measures of outcomes and covariates. The availability of RV measures is unique in such a large sample. Another distinction in this study is our exposure assessment, which improves on existing epidemiology studies of long-term exposures to in the United States. Our model predicts fine-scale spatial variability in exposures using a model derived from intensive air pollution monitoring campaigns in each study community. This methodology is in contrast to previous studies that have relied exclusively on data from relatively sparse national monitoring networks to estimate exposures to (Lipfert et al. 2006; McDonnell et al. 2000; Pope et al. 2002; Puett et al. 2009; Puett et al. 2011). We were also able to control for copollutants ( and ) and demonstrated independent associations with . The availability of chemical component data has particularly important implications for regulatory purposes, given that is generated by both natural and anthropogenic sources. This inclusion has important implications for regulatory purposes, given that is generated by both natural and anthropogenic sources.
Conclusion
This cross-sectional study provided some evidence of a positive association between long-term residential concentrations and RV mass among persons with a history of tobacco-smoke exposures and persons with severe emphysema. If replicated by future work, our findings could suggest a possible mechanism for observed associations between exposures and mortality from respiratory disease.
Supplemental Material
Acknowledgments
This work was supported by supported by grants RD 833741010 and RD 83169701 from the U.S. Environmental Protection Agency (EPA) and the National Institutes of Health (NIH) (R01 HL086719). MESA was further supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-RR-025005 from NCRR. MESA RV was funded by NIH R01-HL086719. MESA Lung was supported by NIH-R01-HL077612 and RC1 HL100543. MESA Neighborhood was supported by 2R01 HL071759. One author (P.S.T.) was supported by NIH P30 ES005605 and another (J.D.K.) by P30 ES07033 and K24 ES013195. Although funded by the U.S. EPA, this publication has not been formally reviewed by the U.S. EPA, and the views expressed in this document are solely the views of the authors. The U.S. EPA also does not endorse any products or commercial services mentioned in this publication.
The authors acknowledge the other investigators, staff, and participants of MESA and MESA Air for their valuable contributions to this work. A full list of MESA investigators and institutions is located at http://www.mesa-nhlbi.org.
References
- Aaron CP, Chervona Y, Kawut SM, Diez Roux AV, Shen M, Bluemke DA, et al. 2016. Particulate matter exposure and cardiopulmonary differences in the multi-ethnic study of atherosclerosis. Environ Health Perspect 124(8):1166–1173, PMID: 26859533, 10.1289/ehp.1409451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adar S, Filigrana P, Clements N, Peel J. 2014. Ambient coarse particulate matter and human health: a systematic review and meta-analysis. Curr Envir Health Rep 1(3):258–274, PMID: 25152864, 10.1007/s40572-014-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adar SD, D'Souza J, Elkayam LR, Sheppard L, Barr RG, Thorne PS, et al. 2015a. Long-term exposures to ambient coarse particulate matter (PM 10-2.5) and the right ventricle [Abstract 3615]. In: Abstracts of the 2015 Conference of the International Society of Environmental Epidemiology, https://ehp.niehs.nih.gov/isee/2015-3615/ [accessed 30 June 2017]. [Google Scholar]
- Adar SD, D’Souza J, Mendelsohn-Victor K, Jacobs DR Jr, Cushman M, Sheppard L, et al. 2015b. Markers of inflammation and coagulation after long-term exposure to coarse particulate matter: a cross-sectional analysis from the Multi-Ethnic Study of Atherosclerosis. Environ Health Perspect, 123:(6):541–548, PMID: 25616153, 10.1289/ehp.1308069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akishima S, Matsushita S, Sato F, Hyodo K, Imazuru T, Enomoto Y, et al. 2007. Cigarette-smoke-induced vasoconstriction of peripheral arteries: evaluation by synchrotron radiation microangiography. Circ J 71(3):418–422, PMID: 17322645, 10.1253/circj.71.418. [DOI] [PubMed] [Google Scholar]
- Andersen ZJ, Hvidberg M, Jensen SS, Ketzel M, Loft S, Sørensen M, et al. 2011. Chronic obstructive pulmonary disease and long-term exposure to traffic-related air pollution a cohort study. Am J Respir Crit Care Med 183(4):455–461, PMID: 20870755, 10.1164/rccm.201006-0937OC. [DOI] [PubMed] [Google Scholar]
- Andersen ZJ, Bonnelykke K, Hvidberg M, Jensen SS, Ketzel M, Loft S, et al. 2012. Long-term exposure to air pollution and asthma hospitalisations in older adults: a cohort study. Thorax 67(1):6–11, PMID: 21890573, 10.1136/thoraxjnl-2011-200711. [DOI] [PubMed] [Google Scholar]
- Bennett WD, Zeman KL, Kim C, Mascarella J. 1997. Enhanced deposition of fine particles in COPD patients spontaneously breathing at rest. Inhal Toxicol 9(1):1–14, 10.1080/089583797198376. [DOI] [Google Scholar]
- Bild DE, Bluemke DA, Burke GL, Detrano R, Roux AVD, Folsom AR, et al. 2002. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 156(9):871–881, 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, et al. 2008. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol 52(25):2148–2155, PMID: 19095132, 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Bard RL, Morishita M, Dvonch JT, Wang L, Yang H, et al. 2014. Hemodynamic, autonomic, and vascular effects of exposure to coarse particulate matter air pollution from a rural location. Environ Health Perspect 122(6):624–630, PMID: 24618231, 10.1289/ehp.1306595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JS, Zeman KL, Bennett WD. 2002. Ultrafine particle deposition and clearance in the healthy and obstructed lung. Am J Respir Crit Care Med 166(9):1240–1247, PMID: 12403694, 10.1164/rccm.200205-399OC. [DOI] [PubMed] [Google Scholar]
- Brunekreef B, Forsberg B. 2005. Epidemiological evidence of effects of coarse airborne particles on health. Eur Respir J 26(2):309–318, PMID: 16055881, 10.1183/09031936.05.00001805. [DOI] [PubMed] [Google Scholar]
- Burgess MI, Mogulkoc N, Bright-Thomas RJ, Bishop P, Egan JJ, Ray SG. 2002. Comparison of echocardiographic markers of right ventricular function in determining prognosis in chronic pulmonary disease. J Am Soc Echocardiogr 15(6):633–639, PMID: 12050605, 10.1067/mje.2002.118526. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Vincent R, Mora-Tiscareño A, Franco-Lira M, Heníquez-Roldán C, Barragán-Mejía G, et al. 2007. Elevated plasma endothelin-1 and pulmonary arterial pressure in children exposed to air pollution. Environ Health Perspect 115(8):1248–1253, PMID: 17687455, 10.1289/ehp.9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal H, Johnson C, Tandri H, Jain A, Hundley WG, Barr RG, et al. 2010. Relation of cardiovascular risk factors to right ventricular structure and function as determined by magnetic resonance imaging (results from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol 106(1):110–116, PMID: 20609657, 10.1016/j.amjcard.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal H, McClelland RL, Tandri H, Jain A, Turkbey EB, Hundley WG, et al. 2012. Obesity and right ventricular structure and function: the MESA-right ventricle study. Chest 141(2):388–395, PMID: 21868467, 10.1378/chest.11-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouat A, Savale L, Chouaid C, Tu L, Sztrymf B, Canuet M, et al. 2009. Role for interleukin-6 in COPD-related pulmonary hypertension. Chest 136(3):678–687, PMID: 19349390, 10.1378/chest.08-2420. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yang Q, Krewski D, Burnett RT, Shi Y, McGrail KM. 2005. The effect of coarse ambient particulate matter on first, second, and overall hospital admissions for respiratory disease among the elderly. Inhal Toxicol 17(12):649–655, PMID: 16087571, 10.1080/08958370500189420. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Pope CA III, Xu X, Spengler JD, Ware JH, Fay ME, et al. 1993. An association between air pollution and mortality in six U.S. cities. N Engl J Med 329(24):1753–1759, PMID: 8179653, 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Faustini A, Stafoggia M, Cappai G, Forastiere F. 2012. Short-term effects of air pollution in a cohort of patients with chronic obstructive pulmonary disease. Epidemiology 23(6):861–879, PMID: 23018970, 10.1097/EDE.0b013e31826767c2. [DOI] [PubMed] [Google Scholar]
- Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, et al. 2015. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386(10010):2287–2323, PMID: 26364544, 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- France AJ, Prescott RJ, Biernacki W, Muir AL, MacNee W. 1988. Does right ventricular function predict survival in patients with chronic obstructive lung disease? Thorax 43(8):621–626, PMID: 3175974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freixa X, Portillo K, Pare C, Garcia-Aymerich J, Gomez FP, Benet M, et al. 2013. Echocardiographic abnormalities in patients with COPD at their first hospital admission. Eur Respir J 41(4):784–791, PMID: 23018914, 10.1183/09031936.00222511. [DOI] [PubMed] [Google Scholar]
- Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. 2003. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 26(11):3160–3167, PMID: 14578255, 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- Gill EA, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. 2011. Air pollution and cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Prog Cardiovasc Dis 53(5):353–360, PMID: 21414470, 10.1016/j.pcad.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadina S, Weiss JP, McCray PB Jr., Kulhankova K, Thorne PS. 2008. MD-2-dependent pulmonary immune responses to inhaled lipooligosaccharides: effect of acylation state. Am J Respir Cell Mol Biol 38(6):647–654, PMID: 18203970, 10.1165/rcmb.2007-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat A, Diez-Roux AV, Adar SD, Auchincloss AH, Auchincloss GS, Lovasi GS, O'Neill MS, et al. 2013. Air pollution and individual and neighborhood socioeconomic status: evidence from the Multi-Ethnic Study of Atherosclerosis (MESA). Environ Health Perspect 121(11–12):1325, PMID: 24076625, 10.1289/ehp.1206337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, Barr RG. 2010. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the Multi-Ethnic Study of Atherosclerosis (MESA) lung study. CHEST 137(1):138–145, PMID: 19741060, 10.1378/chest.09-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilde JM, Skjorten I, Grotta OJ, Hansteen V, Melsom MN, Hisdal J, et al. 2013. Right ventricular dysfunction and remodeling in chronic obstructive pulmonary disease without pulmonary hypertension. J Am Coll Cardiol 62:1103–1111, PMID: 23831444, 10.1016/j.jacc.2013.04.091. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Jiang R, Baumhauer H, Brooks MA, Carr JJ, Detrano R, et al. 2009. Reproducibility and validity of lung density measures from cardiac CT scans–the Multi-Ethnic Study of Atherosclerosis (MESA) lung study. Academic Radiology 16(6):689–699, PMID: 19427979, 10.1016/j.acra.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EA, Ahmed FS, Baumhauer H, Budoff M, Carr JJ, Kronmal R, et al. 2014. Variation in the percent of emphysema-like lung in a healthy, nonsmoking multiethnic sample. The MESA Lung Study. Ann Am Thorac Soc 11(6):898–907, PMID: 24983825, 10.1513/AnnalsATS.201310-364OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IHME (Institute for Health Metrics and Evaluation). 2016. Global Burden of Disease Study 2013 (GBD 2013) results by location, cause, and risk factor, http://ghdx.healthdata.org/global-burden-disease-study-2013-gbd-2013-data-downloads [accessed 1 June 2016].
- Jerrett M, Finkelstein MM, Brook JR, Arain MA, Kanaroglou P, Stieb DM, et al. 2009. A cohort study of traffic-related air pollution and mortality in Toronto, Ontario, Canada. Environ Health Perspect 117(5):772–777, PMID: 19479020, 10.1289/ehp.11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JNC (Joint National Committee). 1997. The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med 157(21):2413–2446, PMID: 9385294, 10.1001/archinte.1997.00440420033005. [DOI] [PubMed] [Google Scholar]
- Karakatsani A, Andreadaki S, Katsouyanni K, Dimitroulis I, Trichopoulos D, Benetou V, et al. 2003. Air pollution in relation to manifestations of chronic pulmonary disease: a nested case-control study in Athens, Greece. Eur J Epidemiol 18(1):45–53, PMID: 12705623, 10.1023/A:1022576028603. [DOI] [PubMed] [Google Scholar]
- Kawut SM, Lima JAC, Barr RG, Chahal H, Jain A, Tandri H, et al. 2011. Sex and race differences in right ventricular structure and function: the Multi-Ethnic Study of Atherosclerosis–right ventricle study. Circulation 123:2542–2551, PMID: 21646505, 10.1161/CIRCULATIONAHA.110.985515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawut SM, Barr RG, Lima JA, Praestgaard A, Johnson WC, Chahal H, et al. 2012. Right ventricular structure is associated with the risk of heart failure and cardiovascular death: the Multi-Ethnic Study of Atherosclerosis (MESA)–right ventricle study. Circulation 126(14):1681–1688, PMID: 22932258, 10.1161/CIRCULATIONAHA.112.095216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan RM, Adar SD, Szpiro AA, Jorgensen NW, Van Hee VC, Barr RG, et al. 2013. Vascular responses to long- and short-term exposure to fine particulate matter: MESA Air (Multi-Ethnic Study of Atherosclerosis and Air Pollution). J Am Coll Cardiol 60(21):2158–2166, PMID: 23103035, 10.1016/j.jacc.2012.08.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary PJ, Kaufman JD, Barr RG, Bluemke DA, Curl CL, Hough CL, et al. 2014. Traffic-related air pollution and the right ventricle. The Multi-Ethnic Study of Atherosclerosis. Am J Respir Crit Care Med 189(9):1093–1100, PMID: 24593877, 10.1164/rccm.201312-2298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren A, Stroh E, Montnemery P, Nihlen U, Jakobsson K, Axmon A. 2009. Traffic-related air pollution associated with prevalence of asthma and COPD/chronic bronchitis. A cross-sectional study in southern Sweden. Int J Health Geogr 8:2, PMID: 19154599, 10.1186/1476-072X-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipfert F, Wyzga R, Baty J, Miller J. 2006. Traffic density as a surrogate measure of environmental exposures in studies of air pollution health effects: long-term mortality in a cohort of U.S. veterans. Atmospheric Environment 40(1):154–169, 10.1016/j.atmosenv.2005.09.027. [DOI] [Google Scholar]
- McDonnell WF, Nishino-Ishikawa N, Petersen FF, Chen LH, Abbey DE. 2000. Relationships of mortality with the fine and coarse fractions of long-term ambient PM10 concentrations in nonsmokers. J Expo Anal Environ Epidemiol 10(5):427–436, PMID: 11051533. [DOI] [PubMed] [Google Scholar]
- Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, et al. 2005. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation 112(25):3930–3936, PMID: 16365212, 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- Mostofsky E, Schwartz J, Coull BA, Koutrakis P, Wellenius GA, Suh HH, et al. 2012. Modeling the association between particle constituents of air pollution and health outcomes. Am J Epidemiol 176(4):317–326, PMID: 22850792, 10.1093/aje/kws018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, et al. 2006. Cardiovascular function in Multi-Ethnic Study of Atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol 186(6 suppl 2):S357–3S365, PMID: 16714609, 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- Peel JL, Tolbert PE, Klein M, Metzger KB, Flanders WD, Todd K, et al. 2005. Ambient air pollution and respiratory emergency department visits. Epidemiology 16(2):164–174, PMID: 15703530. [DOI] [PubMed] [Google Scholar]
- Polak JF, Holman BL, Wynne J, Colucci WS. 1983. Right ventricular ejection fraction: an indicator of increased mortality in patients with congestive heart failure associated with coronary artery disease. J Am Coll Cardiol 2(2):217–224, PMID: 6306086. [DOI] [PubMed] [Google Scholar]
- Pope CA III, Burnett RT, Thun MJ, Calle EE, Krewski D, et al. 2002. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 287(9):1132–1141, PMID: 11879110, 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA III, Burnett RT, Turner MC, Cohen A, Krewski D, Jerrett M, et al. 2011. Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure-response relationships. Environ Health Perspect 119(11):1616–1621, PMID: 21768054, 10.1289/ehp.1103639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puett RC, Hart JE, Yanosky JD, Paciorek C, Schwartz J, Suh H, et al. 2009. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses' Health Study. Environ Health Perspect 117(11):1697–1701, PMID: 20049120, 10.1289/ehp.0900572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puett RC, Hart JE, Suh H, Mittleman M, Laden F. 2011. Particulate matter exposures, mortality, and cardiovascular disease in the health professionals follow-up study. Environ Health Perspect 119(8):1130, PMID: 21454146, 10.1289/ehp.1002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JD, Stanek LW, Luben TJ, Johns DO, Buckley BJ, Brown JS, et al. 2011. Particulate matter-induced health effects: who is susceptible? Environ Health Perspect 119(4):446–454, PMID: 20961824, 10.1289/ehp.1002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikowski T, Sugiri D, Ranft U, Gehring U, Heinrich J, Wichmann HE, et al. 2005. Long-term air pollution exposure and living close to busy roads are associated with COPD in women. Respir Res 6:152, PMID: 16372913, 10.1186/1465-9921-6-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DA, Thorne PS, Yagla SJ, Burmeister LF, Olenchock SA, Watt JL, et al. 1995. The role of endotoxin in grain dust-induced lung disease. Am J Respir Crit Care Med 152(2):603–608, PMID: 7633714, 10.1164/ajrccm.152.2.7633714. [DOI] [PubMed] [Google Scholar]
- Shah PK, Maddahi J, Staniloff HM, Ellrodt AG, Pichler M, Swan HJ, et al. 1986. Variable spectrum and prognostic implications of left and right ventricular ejection fractions in patients with and without clinical heart failure after acute myocardial infarction. Am J Cardiol 58(6):387–393, PMID: 3751905. [DOI] [PubMed] [Google Scholar]
- Sturtz TM, Adar SD, Gould T, Larson TV. 2014. Constrained source apportionment of coarse particulate matter and selected trace elements in three cities from the Multi-Ethnic Study of Atherosclerosis. Atmospheric Environment 84:65–77, PMID: 27468256, 10.1016/j.atmosenv.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunyer J. 2001. Urban air pollution and chronic obstructive pulmonary disease: a review. Eur Respir J 17(5):1024–1033, PMID: 11488305. [DOI] [PubMed] [Google Scholar]
- Szpiro AA, Sampson PD, Sheppard L, Lumley T, Adar SD, Kaufman JD. 2010. Predicting intra-urban variation in air pollution concentrations with complex spatio-temporal dependencies. Environmetrics 21(6):606–631, 10.1002/env.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. 2002. “Census of Population and Housing 2000, Summary File 3.” http://www2.census.gov/census_2000/datasets/Summary_File_3/ [accessed 31 October 2013].
- U.S. EPA (U.S. Environmental Protection Agency). 2009. Integrated science assessment for particulate matter. EPA/600/R-08/139F. Research Triangle Park, NC:NCEA-RTP Office. [PubMed]
- Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, et al. 2006. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 114:1883–1891, 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- Wensel R, Jilek C, Dorr M, Francis DP, Stadler H, Lange T, et al. 2009. Impaired cardiac autonomic control relates to disease severity in pulmonary hypertension. Eur Respir J 34(4):895–901, PMID: 19443531, 10.1183/09031936.00145708. [DOI] [PubMed] [Google Scholar]
- Wrobel JP, Thompson BR, Williams TJ. 2012. Mechanisms of pulmonary hypertension in chronic obstructive pulmonary disease: a pathophysiologic review. J Heart Lung Transplant 31(6):557–564, PMID: 22502811, 10.1016/j.healun.2012.02.029. [DOI] [PubMed] [Google Scholar]
- Zangiabadi A, De Pasquale CG, Sajkov D. 2014. Pulmonary hypertension and right heart dysfunction in chronic lung disease. BioMed Res Int 2014:739674, PMID: 25165714, 10.1155/2014/739674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Larson TV, Gassett A, Szpiro AA, Daviglus M, Burke GL, et al. 2014. Characterizing spatial patterns of airborne coarse particulate () mass and chemical components in three cities: the Multi-Ethnic Study of Atherosclerosis. Environ Health Perspect 122:(8):823–830, PMID: 24642481, 10.1289/ehp.1307287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.