Abstract
Heart disease is the number one cause of human death worldwide. Numerous studies have shown strong connections between obesity and cardiac malfunction in humans, but more tools and research efforts are needed to better elucidate the mechanisms involved. For over a century, the genetically highly tractable model of Drosophila has been instrumental in the discovery of key genes and molecular pathways that proved to be highly conserved across species. Many biological processes and disease mechanisms are functionally conserved in the fly, such as development (e.g., body plan, heart), cancer, and neurodegenerative disease. Recently, the study of obesity and secondary pathologies, such as heart disease in model organisms, has played a highly critical role in the identification of key regulators involved in metabolic syndrome in humans.
Here, we propose to use this model organism as an efficient tool to induce obesity, i.e., excessive fat accumulation, and develop an efficient protocol to monitor fat content in the form of TAGs accumulation. In addition to the highly conserved, but less complex genome, the fly also has a short lifespan for rapid experimentation, combined with cost-effectiveness. This paper provides a detailed protocol for High Fat Diet (HFD) feeding in Drosophila to induce obesity and a high throughput triacylglyceride (TAG) assay for measuring the associated increase in fat content, with the aim to be highly reproducible and efficient for large-scale genetic or chemical screening. These protocols offer new opportunities to efficiently investigate regulatory mechanisms involved in obesity, as well as provide a standardized platform for drug discovery research for rapid testing of the effect of drug candidates on the development or prevention of obesity, diabetes and related metabolic diseases.
Keywords: Genetics, Issue 127, Obesity, metabolic syndrome, screening, genetics, lipids, lipotoxicity
Introduction
We are in a time where obesity, and its associated economic burdens, is a worldwide problem1. Two out of every three Americans are overweight or obese with related heart pathologies, the primary cause of death within the adult population2. New efficient methods are needed to adequately investigate the genetic and molecular components implicated in the regulation of metabolic syndrome using model organisms. For this reason, we choose the fruit fly Drosophila model because it shares the most basic biological processes with mammals, including mice and humans3,4,5,6. Drosophila's genome is highly conserved during evolution but overall much smaller with less gene duplication and metabolic complexity, making it ideal for understanding the fundamental mechanisms implicated in many human diseases4,7,8. Also, characteristic processes carried out by adipose tissue, the gut and pancreas are represented in the fly and mediate regulatory functions in glucose and lipid metabolism, for example, that are similar to humans9,10,11. Moreover, the basic molecular pathways involved in the control of obesity, insulin resistance and diabetes in humans are functionally conserved in Drosophila melanogaster12,13,14,15,16. Like higher organisms, Drosophila has a beating heart that is formed during development by similar processes to that of the mammalian heart3,17. Thus, the development of a reliable HFD feeding protocol and high throughput TAG assay, adapted for efficient screening purposes using the genetic tool box of Drosophila, provide an important means to study and understand the fundamental genetic basis underlying complex metabolic diseases.
The HFD food itself is made from a standard laboratory fly food supplemented with coconut oil, which is composed mostly of saturated fatty acids known to be associated with metabolic syndrome18. While inducing obesity in mammalian models, such as rodents, can take months19,20, our optimized HFD feeding protocol in Drosophila effectively and reproducibly increases organismal fat content in a matter of days12,14. This protocol, in conjunction with a high throughput TAG assay, allows efficient mass screening for the effects of genetic factors, environmental influences and drug candidates to discover new modulators of fat metabolism. In consequence, these protocols are likely relevant to understand and/or combat obesity and obesity-associated human pathologies.
The feeding protocol is versatile and may be applied to study the metabolic and functional effects of single saturated or unsaturated fatty acid. The use of this high throughput TAG assay is not limited to D. melanogaster, but may be adapted to a variety of small model organisms with cuticle or tough extracellular matrices (e.g., other Drosophila species, C. elegans and other emerging invertebrate model organisms) to measure fat content under different environmental, genetic or physiological conditions, at any stage of development, adulthood or phase of metabolic disease. The TAG assay is based on a colorimetric measurement of a series of enzymatic reactions that degrade the TAGs into free fatty acids, glycerol, Glycerol 3-phosphate and finally H2O2 that reacts with 4-aminoantipyrine (4-AAP) and 3,5-dichloro-2-hydroxybenzene sulfonate (3,5 DHBS) to produce a red colored product that is measured using a 96-well spectrophotometer.
Protocol
1. HFD Feeding Protocol
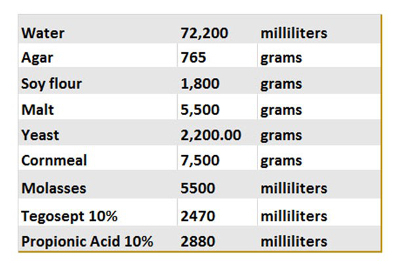 Table 1. Fly food recipe. This table summarizes the different ingredients used to prepare our control food. Once made, 10 mL of the food is poured into vials, cooled and stored at 4 °C for long term storage.
Table 1. Fly food recipe. This table summarizes the different ingredients used to prepare our control food. Once made, 10 mL of the food is poured into vials, cooled and stored at 4 °C for long term storage.
- HFD preparation
- To make 1 kg of high fat food, weigh 700 g of pre-made normal fly food (see Table 1) and 300 g of coconut oil into two separate containers using an analytical balance.
- Heat each container individually in the microwave, until the contents melt completely. Pour the coconut oil into the fly food container. Stir using a whisk until the oil is well mixed into the fly food. NOTE: No chunks of food or oil should be visible. When the fly food is melting in the microwave, stop at intervals of 1 min to mix the food and prevent boiling over.
- Re-weigh the high fat food. If the total weight is less than 1 kg (700 g of food + 300 g of coconut oil), add water (around 10 mL) to compensate for evaporation during heating and to bring back the weight to 1 kg.
- Pour about 10 mL well-mixed high fat food per vial (around 25% of vial volume). Next, cover with cheesecloth to prevent flies' contamination. Cool for 1 h at room temperature then transfer the vials to 4 °C for storage up to 4 weeks. NOTE: The Normal food (NF) is used as a control food. Also pour 10 mL (around 25% of vial volume) of NF per vial. For the HFD food, replace 30% of the NF food with coconut oil. For control and experimental conditions, the flies were given the same amount of NF and HFD (10 mL of food per vial).
Figure 1. HFD feeding in Drosophila. The scheme shows the different feeding steps for flies on a control food (normal diet without addition of coconut oil-NF) or HFD (with the addition of coconut oil). The entire process takes 10 days after the initial collection of adult flies. Please click here to view a larger version of this figure.
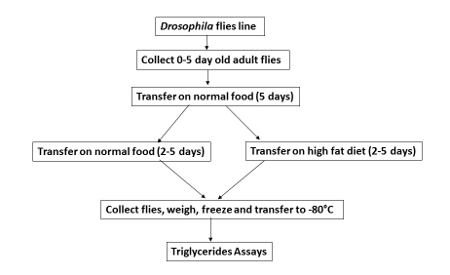
- HFD feeding of D. melanogaster NOTE: This protocol includes control food and HFD feeding procedures that are compatible with any fly line. Control and experimental flies are put in vials containing the same amount food (NF or HFD).
- Use CO2 to anesthetize the flies. NOTE: For neophytes, please read reference21 for an overview on raising and handling D. melanogaster.
- Collect 0-5 day old flies from vials and transfer (by flipping) the flies into new vials containing NF (previously warmed at room temperature). Let the flies age for 5 additional days at 21 °C.
- At the end of the 5 days, take out the vials containing HFD and NF from 4 °C. NOTE: As cold temperatures can stress living flies, let the vials sit for 30 min at room temperature to warm up before use.
- Using wipes, clean and remove excess fluid from the sides of the vials.
- Insert a small piece of filter paper, about 1 cm x 3 cm, into the high fat food to absorb any surplus liquid. NOTE: This step is critical for preventing the flies from sticking to the HFD food.
- Next, transfer the flies onto the high fat food (by flipping). Lay the vials horizontally on their side, (not standing) at 21-22 °C for 5 days. NOTE: Avoid higher temperatures as this can partially melt the high fat food, causing the flies to stick to the food and die.
- After 5 days, transfer (by flipping) the flies from NF and HFD into new vials without food to allow their guts to empty for 30 min. Next, proceed to weighing the flies. NOTE: One of the critical steps in this HFD feeding protocol is to prepare a well-mixed HFD, free of any chunks of fly food or condensed oil. Homogenous mixing and cooling the HFD to 45-30 °C is advisable, before pouring into vials and storing the food at 4 °C. Proper age matching of control and experimental flies is important as well as controlled nutritional and environmental conditions prior to the HFD feeding. During the feeding phase, control and experimental flies must be given the same amount of NF and HFD. Never collect flies from overcrowded vials or bottles (to avoid transgenerational effects). Always put a maximum of 25 flies per vial during NF and HFD feeding to avoid dietary restriction or other crowding effects. The HFD is composed of 30% coconut oil, thus temperatures above 22 °C might partially melt the HFD, causing the flies to stick on the oily food and die. Before putting the flies on the HFD, clean the vials to remove any residual oil and insert a filter paper to absorb any excess fluid in the food. During the incubation of the flies on HFD, the vials are laid on their sides to provide a fat-free horizontal surface for the flies.
- Weighing the flies
- For each genotype and gender to be tested, use an ultra-sensitive scale to record the weights of pre-labeled, empty 1.7 mL tubes.
- Using fly anesthetic or CO2, anesthetize and collect 36 flies of the same genotype, gender, and feeding status with a brush into one pre-weighed 1.7 mL tube.
- Weigh the tube containing flies with the same ultra-sensitive scale.
- Determine the average fly weight following this formula:
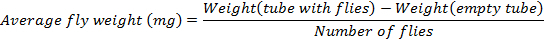 NOTE: The number of flies in this protocol is 36.
NOTE: The number of flies in this protocol is 36. - Flash freeze the 1.7 mL tubes containing flies by plunging into liquid nitrogen for 2 min. Collect and put the tubes into boxes. Next, transfer the boxes to -80 °C (preferable temperature) for storage until use with the TAG assay. CAUTION: Liquid nitrogen is at an extremely low temperature. Please use appropriate personal protective gear.
2. TAG Assay
- Preparation of the TAG standard concentrations
- Prepare 500 mL of PBT (PBS 1X, 0.05% Triton).
- Using 0.5 mL tubes and the TAG solution (Table of Materials), prepare 100 µL of blank (PBT only) and 100 µL of six standard TAG concentrations (2 µg/µL; 1 µg/µL; 0.5 µg/µL; 0.25 µg/µL; 0.125 µg/µL; 0.0625 µg/µL) with the PBT.
- Place the tubes on ice and proceed with grinding the flies.
- Grinding the flies
- Take out the frozen flies from -80 °C (step 1.3). Transfer the tubes containing the flies on ice.
- First, place 2 mm metal grinding balls into a 96-well ball dispenser, use a small paintbrush to remove extra balls.
- Place the 96-well grinding plate upside down on top of the ball dispenser and flip to transfer the metal balls directly into the grinding plate. There should be one ball per well.
- Using a multi-channel pipette, add 600 µL of PBT into each row of the 96-well grinding plate.
- With forceps, add 3 flies per well. Indicate the genotype, gender and food condition of the flies in each row/well of the 96-well grinding plate.
- Tightly place the caps onto the 96-well grinding plate. Tape may be placed on top to ensure that there is no leakage.
- Properly place the 96-well grinding plates into the grinding machine. Set the machine to the highest speed and press "run" to grind the flies for 3 min.
- After 3 min, stop the grinding and proceed with plate centrifugation: centrifuge for 15 min at 4,000 x g, 4 °C. After centrifugation, manage the grinding plate carefully to avoid mixing the supernatant with the pellet.
- Sample loading and TAG content determination
- Take a new 96-well spectrophotometer plate. Using a multi-channel pipette, load 200 µL of the TAG reagent into each row of the plate.
- Load 20 µL of PBT into the first well of the first row, to create a blank. Mix by pipetting up and down. Avoid forming bubbles.
- Load 20 µL of each standard dilution (from step 2.1.2) into the remaining wells of the first row and mix by pipetting up and down. Avoid forming bubbles.
- Using a multi-channel pipette, transfer 20 µL of supernatant from each row of the grinding plate to the corresponding row in the 96-well spectrophotometer plate. Pipette up and down to mix well. Avoid forming bubbles.
- Next, place a gas-permeable foil over the top of the 96-well plate.
- Incubate the plate at 37 °C for 10 min. NOTE: If there are bubbles in the wells, centrifuge the plate for 2 min at 2,000 x g to remove all bubbles. The presence of bubbles will interfere with the absorbance reading.
- Read the absorbance of each well of the plate at 550 nm using a compatible microplate reader. Next, trace the standard curve and determine the concentration [µg/µL] of the unknown samples.
- To determine the amount of TAG content per mean fly weight, use the following formula:
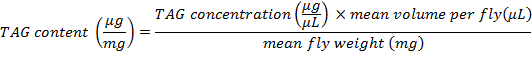 NOTE: In this protocol, 3 flies were minced in 600 µL of PBT; in consequence, the mean volume per fly is 200 µL.
NOTE: In this protocol, 3 flies were minced in 600 µL of PBT; in consequence, the mean volume per fly is 200 µL.
- Bradford assay and normalization of TAG content with protein content
- Prepare seven 100 µL of bovine serum albumin (BSA) standard dilutions (75 µg/mL; 100 µg/mL; 150 µg/mL; 200 µg/mL; 250 µg/mL; 500 µg/mL; 1,000 µg/mL) with the PBT.
- Take a new 96-well plate and load 200 µL of 1X Bradford reagent into each row of the plate.
- In the first well of the first row, add 10 µL of PBT to create a blank. Mix by pipetting up and down. Avoid forming bubbles.
- Add 10 µL of each standard dilution into the remaining wells of the first row and mix by pipetting up and down. Avoid forming bubbles.
- Using a multi-channel pipette, transfer 10 µL of the fly supernatant from each row of the grinding plate into the corresponding row in the 96-well plate. Pipette up and down to mix well. Avoid forming bubbles.
- Place a gas-permeable foil over the top of the 96-well plate.
- Incubate the plate at room temperature for 5 min. If there are bubbles in the wells, centrifuge the plate for 2 min at 2,000 x g to remove them. NOTE: The presence of bubbles will interfere with the absorbance reading.
- Use a spectrophotometer to read the absorbance of the blank, standards and unknown samples at 595 nm. Use the standard curve to determine the concentrations [µg/µL] of the samples in each row.
- Normalize the TAG content with protein level using the following formula:
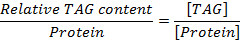
Representative Results
In D. melanogaster, as is the case with other species, there is sexual dimorphism between males and females22. It is well known that females are larger, with more fat in their abdomens, than males22. To test the effectiveness of our protocol, we performed TAG assays to determine the differences in TAG content between males and females of standard laboratory wildtype (w1118) flies. The data show that females have more whole body fat than their male counterparts (Figure 2A, B). The data also showed that the assay is stable, with no variation in TAG quantification over time (up to 50 min after incubation) and no variation depending on biological sample size (3 or 5 flies).
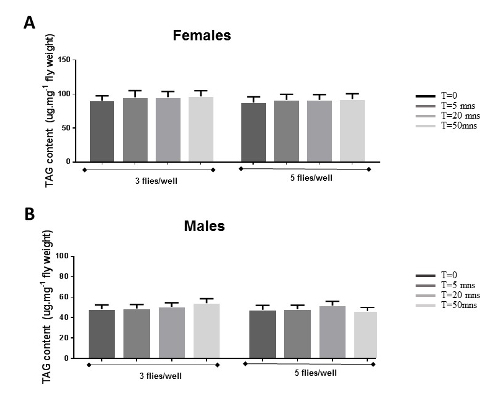
HFD consumption has been shown to cause obesity in human and mouse19,20,23. To test the efficacy of our feeding protocol, we performed TAG assays with our HFD and control fed female flies. We found that consumption of HFD in Drosophila causes increased fat content that progressively accumulates over time (Figure 3A-C). Another important finding is that after only 18 h of HFD feeding (Figure 3C), we were able to induce a significant increase of fat content in these flies. These findings suggest that this genetic model system is an ideal tool for accelerated research into finding novel regulators of HFD-induced obesity.
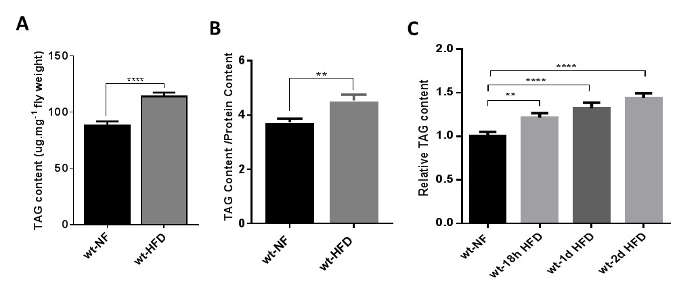
Figure 2. TAG assays in male and female flies. 2-week old flies on a normal diet were collected, grouped by sex (males and females), weighed and ground up (3 or 5 flies per well) for TAG analysis. TAG assays were performed following the procedures described in this paper. The absorbance of each sample at 550 nm was read at different time-points (0 min, 5 min, 20 min and 50 min) to determine eventual fluctuations in TAG quantification over time, fat content variation in different population sizes (3 and 5 flies) of w1118 flies, and differences between male and female TAG contents. The results showed that females accumulate more fat than their male counterparts (A-B), TAG measurements do not fluctuate up to 50 min after the reaction incubation at 37 °C (A-B). Also, the mean TAG levels remain unchanged between TAG assays using 3 or 5 flies (A-B). The data are presented as Mean ± SEM. Statistics: no significant difference. Please click here to view a larger version of this figure.
Figure 3. Effects of HFD on fat accumulation.A-B: 2-week old female flies raised on a normal or HFD for 5 days were collected and the TAG assays were performed to determine levels of fat. TAG content was normalized either with weight (A) or protein levels (B). The data showed that HFD consumption leads to increased fat content with both methods of normalization. C: 2-week old female flies on normal/control food (NF) and 18 h, 1 day or 2 days on HFD are assayed for TAG content. The results show a significant and progressive increase of TAG levels from 18 h to 2 days of exposure to HFD. The data are presented as Mean ± SEM. Statistics: student t-test. *p < 0.05, **p < 0.01, ***p < 0.001. Please click here to view a larger version of this figure.
Discussion
Obesity induction in mice can take months19,20. In flies, this HFD feeding protocol allows for induction of excess fat accumulation in a matter of days or less, causing increases in fat accumulation only after 18 h (see Figure 2). HFD feeding with the described protocol increases glucose content 12 and decreases Bmm lipase and PGC-1 expression24. This is in contrast to fasting of adult Drosophila that causes a fast decrease in both fat and glucose contents25,26 and increased Bmm expression24. Also, elevating Bmm or PGC-1 levels protects against HFD-induced obesity14,24. While 30% HFD has been used in this protocol, feeding flies with 3%, 7%, or 15% fat diet induced obesity in a dose-dependent fashion12, as does increasing the duration of HFD feeding (Figure 3). We also found that feeding single fatty acids of the main components of coconut oil, i.e., 14% lauric acid or 5% myristic acid, causes significantly elevated fat accumulation12.
The accelerated metabolic response with these flies on a HFD regimen is correlated with a reduced lifespan27 as observed in mammals28, but > 80% of the flies survive past 20 days on HFD27. The rapid induction of fat accumulation mediated by conserved cellular and molecular processes controlling lipid and glucose metabolism is advantageous for many obesity-related studies, such as diabetic or lipotoxic cardiomyopathy10,12,13,14,15,16. Key findings in the consequences of metabolic imbalances and related cardiac dysfunctions will likely allow comparative translational studies in humans.
The HFD feeding protocol is compatible for use with other Drosophila species and insects models that share similar diets with D. melanogaster. This HFD feeding protocols could be appropriately adapted for organism like C. elegans or also other insects that require different 'normal' food media. The 30% HFD is not suitable for use in experimentation requiring high temperatures; however, reduced concentrations of coconut oil or single fatty acids could be adequate alternatives12.
This TAG protocol is based on PBS, a non-toxic buffer that allows easy handling and experimentation without a fume hood, in contrast to previously used organic solvents (methanol/chloroform or diethyl ether) in lipid extraction and quantification15,29,30. Another advantage of this buffer is that it can normalize the TAG levels to protein content based on the same initial homogenate, making the fat determination in small tissue samples easily achievable and offering new opportunities to study organ cross-talk in organismal metabolic homeostasis. Also, the same initial fly homogenate obtained after grinding, can be used to perform parallel glucose and glycogen assays, allowing the study of changes in both the lipid and glucose metabolism from the same biological samples. This TAG protocol is less laborious or time-consuming than previous protocols15,29,30, and is rather high throughput, using a 96-well format that allows for rapid testing of close to 100 samples within an hour. This protocol could also be used to quantify fat content under other dietary conditions, including high sugar diet13,31, dietary restriction and starvation, as well as for aging studies to understand age-associated changes in fat metabolism.
Obesity, metabolic syndrome and related pathologies are at an all-time high with disastrous consequences on human health1,2. The combined HFD protocol and the high throughput TAG assay offer a unique platform to use model organisms, such as Drosophila, to rapidly induce obesity and screen for genetic factors, or natural and synthetic chemical compounds, to better understand metabolic imbalance. Ultimately this might greatly contribute to the advancement of scientific discoveries for developing new treatments or cures for metabolic diseases.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We would like to thank Erika Taylor for editing this manuscript. This work was funded by grants from the National Institutes of Health (P01 HL098053, P01 AG033561 and R01 HL054732) to R.B., a post-doctoral research supplement (R01 HL085481) and fellowship (AAUW) to S.B.D., and grants from the American Heart Association to S.B.D. and R.T.B.
References
- Ng M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SL, et al. Mortality in the United States, 2014. NCHS data brief, no 229. 2015. Available from: https://www.cdc.gov/nchs/products/databriefs/db229.htm. [PubMed]
- Bodmer R. Heart development in Drosophila and its relationship to vertebrates. Trends Cardiovasc Med. 1995;5:21–28. doi: 10.1016/1050-1738(94)00032-Q. [DOI] [PubMed] [Google Scholar]
- Brumby AM, Richardson HE. Using Drosophila melanogaster to map human cancer pathways. Nat Rev Cancer. 2005;5:626–639. doi: 10.1038/nrc1671. [DOI] [PubMed] [Google Scholar]
- Chan HY, Bonini NM. Drosophila models of human neurodegenerative disease. Cell Death Differ. 2000;7:1075–1080. doi: 10.1038/sj.cdd.4400757. [DOI] [PubMed] [Google Scholar]
- Levine M, et al. Human DNA sequences homologous to a protein coding region conserved between homeotic genes of Drosophila. Cell. 1984;38:667–673. doi: 10.1016/0092-8674(84)90261-7. [DOI] [PubMed] [Google Scholar]
- Bier E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet. 2005;6:9–23. doi: 10.1038/nrg1503. [DOI] [PubMed] [Google Scholar]
- Bier E, Bodmer R. Drosophila, an emerging model for cardiac disease. Gene. 2004;342:1–11. doi: 10.1016/j.gene.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Noyes BE, et al. Identification and expression of the Drosophila adipokinetic hormone gene. Mol Cell Endocrinol. 1995;109:133–141. doi: 10.1016/0303-7207(95)03492-p. [DOI] [PubMed] [Google Scholar]
- Rajan A, Perrimon N. Of flies and men: insights on organismal metabolism from fruit flies. BMC Biol. 2013;11:38. doi: 10.1186/1741-7007-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson EJ, et al. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 2002;296:1118–1120. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- Birse RT, et al. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metab. 2010;12:533–544. doi: 10.1016/j.cmet.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman LP, et al. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis Models Mech. 2011;4:842–849. doi: 10.1242/dmm.007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diop SB, Bodmer R. Gaining Insights into Diabetic Cardiomyopathy from Drosophila. Trends Endocrinol Metab. 2015;26:618–627. doi: 10.1016/j.tem.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MJ, et al. The Obesity-Linked Gene Nudt3 Drosophila Homolog Aps Is Associated With Insulin Signaling. Mol Endocrinol. 2015;29:1303–1319. doi: 10.1210/ME.2015-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SN, et al. Development of diet-induced insulin resistance in adult Drosophila melanogaster. Biochim Biophys Acta. 2012;1822:1230–1237. doi: 10.1016/j.bbadis.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer R. The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development. 1993;118:719–729. doi: 10.1242/dev.118.3.719. [DOI] [PubMed] [Google Scholar]
- Erkkila A, et al. Dietary fatty acids and cardiovascular disease: an epidemiological approach. Prog Lipid Res. 2008;47:172–187. doi: 10.1016/j.plipres.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Ganz M, et al. High fat diet feeding results in gender specific steatohepatitis and inflammasome activation. World J Gastroenterol. 2014;20:8525–8534. doi: 10.3748/wjg.v20.i26.8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Liao JK. A mouse model of diet-induced obesity and insulin resistance. Methods Mol Biol. 2012;821:421–433. doi: 10.1007/978-1-61779-430-8_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker H, Gallant P. Getting started: an overview on raising and handling Drosophila. Methods Mol Biol. 2008;420:27–44. doi: 10.1007/978-1-59745-583-1_2. [DOI] [PubMed] [Google Scholar]
- Mathews KW, et al. Sexual Dimorphism of Body Size Is Controlled by Dosage of the X-Chromosomal Gene Myc and by the Sex-Determining Gene tra in Drosophila. Genetics. 2017;205:1215–1228. doi: 10.1534/genetics.116.192260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golay A, Bobbioni E. The role of dietary fat in obesity. Int J Obes Relat Metab Disord. 1997;21:2–11. Suppl 3. [PubMed] [Google Scholar]
- Diop SB, et al. PGC-1/Spargel Counteracts High-Fat-Diet-Induced Obesity and Cardiac Lipotoxicity Downstream of TOR and Brummer ATGL Lipase. Cell Rep. 2015;10:1–13. doi: 10.1016/j.celrep.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D, et al. Control of metabolic adaptation to fasting by dILP6-induced insulin signaling in Drosophila oenocytes. Proc Natl Acad Sci U S A. 2014;111:17959–17964. doi: 10.1073/pnas.1409241111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanker L, et al. Drosophila HNF4 regulates lipid mobilization and beta-oxidation. Cell Metab. 2009;9:228–239. doi: 10.1016/j.cmet.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichsen ET, Haddad GG. Role of high-fat diet in stress response of Drosophila. PLoS One. 2012;7:42587. doi: 10.1371/journal.pone.0042587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahara CM, et al. Association between class III obesity (BMI of 40-59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLoS Med. 2014;11:1001673. doi: 10.1371/journal.pmed.1001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis A, et al. A comparison of five lipid extraction solvent systems for lipidomic studies of human LDL. J Lipid Res. 2013;54:1812–1824. doi: 10.1194/jlr.M034330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turne C, et al. Supercritical fluid extraction and chromatography for fat-soluble vitamin analysis. J Chromatogr A. 2001;936:215–237. doi: 10.1016/s0021-9673(01)01082-2. [DOI] [PubMed] [Google Scholar]
- Na J, et al. Drosophila model of high sugar diet-induced cardiomyopathy. PLoS Genet. 2013;9:1003175. doi: 10.1371/journal.pgen.1003175. [DOI] [PMC free article] [PubMed] [Google Scholar]


