Summary
Genetics and neuropathology strongly link α-synuclein aggregation and neurotoxicity to the pathogenesis of Parkinson’s disease and related α-synucleinopathies. Here we describe a new Drosophila model of α-synucleinopathy based on widespread expression of wild type human α-synuclein, which shows robust neurodegeneration, early-onset locomotor deficits, and abundant α-synuclein aggregation. We use results of forward genetic screening and genetic analysis in our new model to demonstrate that α-synuclein expression promotes reorganization of the actin filament network and consequent mitochondrial dysfunction through altered Drp1 localization. Similar changes are present in a mouse α-synucleinopathy model and in postmortem brain tissue from patients with α-synucleinopathy. Importantly, we provide evidence that the interaction of α-synuclein with spectrin initiates pathological alteration of the actin cytoskeleton and downstream neurotoxicity. These findings suggest new therapeutic approaches for α-synuclein induced neurodegeneration.
Keywords: α-synuclein, actin, spectrin, α-synucleinopathy and mitochondria
eTOC Blurb
The synaptic protein α-synuclein has been strongly implicated in neurodegeneration specifically Parkinson’s disease and related disorders. Using a new Drosophila α-synucleinopathy model, Ordonez et al. show that α-synuclein interacts with spectrin to destabilize the actin cytoskeleton and induce mitochondrial dysfunction.
Introduction
Parkinson’s disease, first formally described 200 years ago by James Parkinson (Parkinson, 1817), is the second most common neurodegenerative disorder, after Alzheimer’s disease, and affects 1 in 50 people over the age of 60 (Franco-Iborra et al., 2015). The well-described neuropathological hallmarks of Parkinson’s disease are loss of nigrostriatal dopaminergic neurons and the formation of α-synuclein rich inclusions known as Lewy bodies (Goedert, 2001). We now know that Parkinson’s disease is one of a larger group of neurodegenerative disorders characterized by aggregation of α-synuclein into inclusion bodies in affected brain tissue; these disorders are collectively termed α-synucleinopathies and include dementia with Lewy bodies and multiple system atrophy. α-synucleinopathies are quite common, with dementia with Lewy bodies accounting for up to one-third of all dementia in older individuals (Barker et al., 2002; Zaccai et al., 2005).
Human genetics provides important support for the α-synucleinopathy concept. Most patients with Parkinson’s disease and related α-synucleinopathies have apparently sporadic disease, but rare mutations in the gene encoding α-synuclein can cause penetrant autosomal dominant Parkinson’s disease. Examination of brain tissue from patients with α-synuclein mutations reveals the expected loss of dopaminergic nigral neurons and brainstem Lewy bodies, but also more widespread aggregation of α-synuclein in cortical neurons as characteristically seen in dementia with Lewy bodies, and in some cases even in the glial cytoplasmic inclusions that define multiple system atrophy (Poulopoulos et al., 2012). Importantly, α-synuclein mutations causing familial Parkinson’s disease include duplications and triplications of the SNCA gene (Lin and Farrer, 2014; Hernandez et al., 2016), strongly implicating wild type α-synuclein in neurotoxicity, and supporting the approach taken here and in prior work of modeling Parkinson’s disease and related α-synucleinopathies experimentally by expressing the wild type human protein (Feany and Bender, 2000; Masliah et al., 2000).
In addition to duplications and triplications, SNCA point mutations (A30P, A53T, E46K) also cause dominant, familial Parkinson’s disease (Lin and Farrer, 2014; Hernandez et al., 2016). However, the mechanism(s) by which increasing levels of wild type α-synuclein or expression of mutant forms of α-synuclein promote disease pathogenesis remain incompletely understood despite significant effort (Wong and Krainc, 2017). The impact of familial Parkinson’s disease mutations on the normal function of the protein is also unclear. α-synuclein is a soluble and natively unfolded protein enriched in the presynaptic terminal of neurons. Although a number of studies have suggested that α-synuclein plays a role in regulating synaptic neurotransmitter release (Burre et al., 2010; Bendor et al., 2013), critical interacting partners, molecular conformations and detailed mechanisms remain undefined.
The relationship between α-synuclein and other proteins encoded by genes mutated in familial Parkinson’s disease has also been an important but unresolved issue in Parkinson’s disease research. Recessive mutations in PINK1 and parkin cause early onset forms of familial Parkinson’s disease. From the genetic and cell biological perspectives, the two proteins work in an epistatic pathway to control mitochondrial dynamics, turnover and function (Shulman et al., 2011). While pathological alterations in mitochondrial energetics and morphology have been described in Parkinson’s disease patients (Schapira et al., 1990; Baloyannis et al., 2006), and in experimental models of α-synucleinopathies (Martin et al., 2006; Bose and Beal, 2016), a clear mechanistic connection between α-synuclein and mitochondrial dynamics has not been defined. Here we demonstrate, in an experimentally powerful new widespread Drosophila α-synucleinopathy model, that α-synuclein binds to spectrin, thereby altering F-actin dynamics, promoting mislocalization of the critical mitochondrial fission protein Drp1, and consequently leading to mitochondrial dysfunction and neuronal death.
Results
α-synuclein impairs locomotor ability and causes widespread neurodegeneration
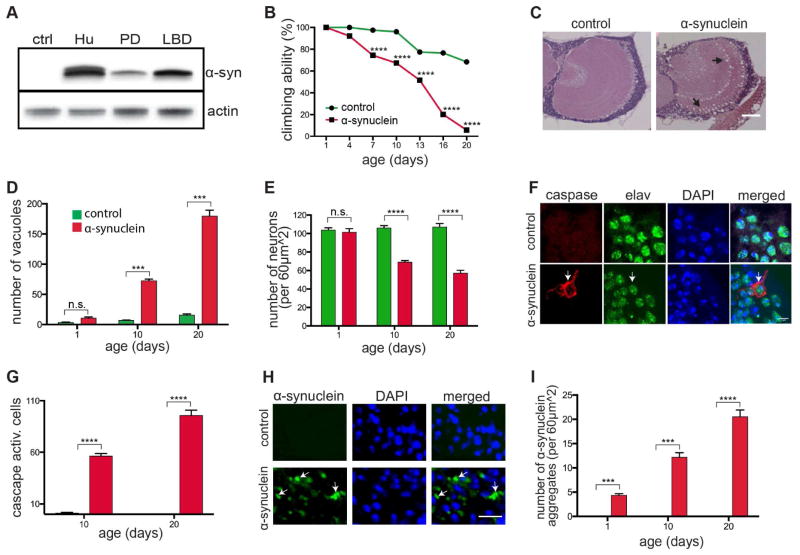
To explore the mechanisms underlying neurotoxicity in the broad group of α-synucleinopathies, we expanded the scope of our previous in vivo Drosophila model of Parkinson’s disease, which showed preferentially degeneration of dopaminergic neurons (Feany and Bender, 2000). We used a recently described binary expression system, the Q system, which relies on the transcriptional activation by the Neurospora protein QF2 to activate transgene expression (Potter et al., 2010). When we expressed wild type human α-synuclein in a panneuronal pattern using the Syb-QF2 driver (Riabinina et al., 2015), we observed higher levels of α-synuclein expression compared with the conventional GAL4/UAS expression system and widespread, robust neurodegeneration (Figure 1). Although expression of α-synuclein was higher than in our previous, more specific, α-synucleinopathy model (compare α-syn PD and α-syn LBD in Figure 1A), levels of α-synuclein expression were still moderate, equivalent to that of human brain homogenate (Figure 1A, Hu). Cortical and neuropil vacuole formation (Figures 1C and 1D, arrows), loss of neurons (Figure 1E) and caspase activation (Figures 1F and 1G, arrows) demonstrated robust neurodegeneration. Correlating with extensive neurodegeneration, we observed loss of locomotor activity (Figures 1B and S1B), as well as reduced lifespan (Figure S1C). To control for nonspecific toxicity of expressing an exogenous protein, we expressed GFP using the same expression system and observed no significant neurotoxicity (Figures S1D and S1E).
Figure 1. Drosophila model of diffuse α-synucleinopathy.
(A) Western blot showing α-synuclein levels in human (Hu) brain homogenate, the previous Parkinson’s disease Drosophila model (PD) and the current α-synucleinopathy Drosophila model (LBD). Flies are 1 day old.
(B) Behavioral analysis of motor deficits using the climbing test indicates loss of locomotor ability with age in α-synuclein transgenic flies. n=60 per genotype.
(C and D) Hematoxylin and eosin staining showing vacuolization in the medulla of 10-day-old α-synuclein transgenic flies (arrows). Scale bar, 50 μm. Quantification (D) reveals an increase in vacuole formation with age in the brains of 1-, 10- and 20-day-old α-synuclein transgenic flies. n=6 per genotype.
(E) Quantification of cortical neurons in the anterior medulla showing decreased neuronal density in 10-day-old α-synuclein transgenic flies. n=6 per genotype.
(F and G) Caspase activation in neurons (marked by elav) in 10-day-old α-synuclein transgenic flies (arrow) and quantification with aging (G). Scale bar, 7 μm. n=6 per genotype. Control is elav-GAL4; UAS-CD8-PARP-Venus/Syb-QF2.
(H and I) α-synuclein aggregates (arrows) in the brain of 10-day-old α-synuclein transgenic flies detected by immunofluorescence. Scale bar, 7 μm. Quantification (I) shows increased numbers of inclusions with age in 1-, 10- and 20-day-old flies. n=6 per genotype.
Asterisks indicate ***p<0.0002 and ****p<0.0001, two-way ANOVA with Student-Newman-Keuls test. Control in (A)–(E), (H), and (I) is Syb-QF2/+. Controls are shown in green and α-synuclein transgenics in red in all bar graphs (D), (E), (G), and (I). Data is represented as mean ± SEM.
See also Figure S1.
α-synuclein aggregates, defining feature of α-synucleinopathies, were abundant in α-synuclein transgenic flies and increased with age (Figures 1H and 1I, arrows). Ubiquitin immunoreactivity is a consistent feature of human Lewy bodies. We therefore determined if α-synuclein aggregates were ubiquitinated in our new model. Double labeling for α-synuclein and ubiquitin showed that the majority of α-synuclein aggregates also contained ubiquitin (Figure S1F, arrows). Dopaminergic neurons are preferentially lost in Parkinson’s disease. We thus examined dopaminergic neurons specifically in our new α-synuclein transgenic model. We stained tissue sections with an antibody against tyrosine hydroxylase and assessed dopaminergic neurons in the medulla, a site of strong expression mediated by Syb-QF2. α-synuclein transgenic flies had significant loss of TH-immunoreactive dopaminergic neurons compared to control flies (Figures S1G and S1H, arrows). We further determined if α-synuclein aggregates are present in dopaminergic neurons. We co-labeled TH-immunoreactive dopaminergic neurons with an α-synuclein antibody. Medullary as well as central brain dopaminergic neurons contained α-synuclein aggregates (Figures S1I and S1J, arrows). Overall, compared to our previous model (Feany and Bender, 2000), widespread neurodegeneration, early-onset of locomotor deficits and the formation of numerous α-synuclein aggregates in our new fly α-synucleinopathy model provide an experimentally facile platform to investigate the mechanisms mediating neurodegeneration induced by α-synuclein.
α-synuclein modulates actin dynamics
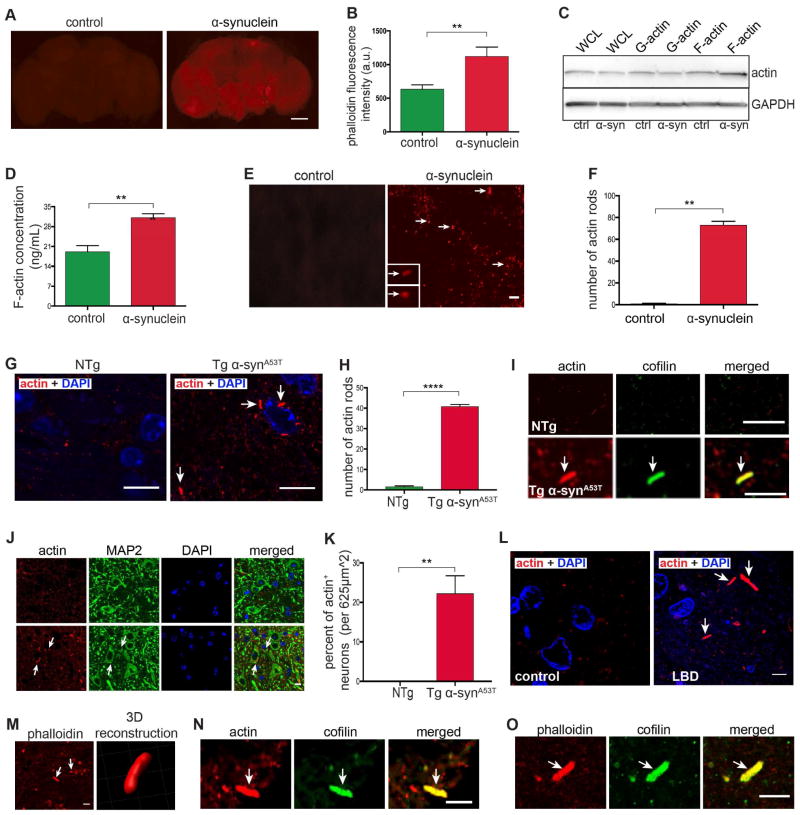
In a forward genetic screen using the retinal degeneration phenotype present in our previously described α-synuclein transgenic model (Feany and Bender, 2000; Chen and Feany, 2005; Chen et al., 2009), we identified a loss of function mutation in Fhos (Formin homology 2 domain containing ortholog), a FHOD class formin, as a suppressor of α-synuclein neurotoxicity (unpublished). Analysis of a well-characterized gene expression reporter (enhancer trap), FhosAA142 (Lammel et al., 2014), supports neuronal expression of Fhos in the adult brain (Figure S3M, arrows). Formins nucleate unbranched actin filaments, and previous work has demonstrated that increased Fhos function promotes actin stress fiber formation (Lammel et al., 2014). We therefore determined if changes in the actin cytoskeleton accompanied neurodegeneration in our α-synuclein transgenic flies. We first stained whole mount brains with fluorescently (Alexa Fluor 532) labeled phalloidin, which specifically stains F-actin, and found a substantial increase in α-synuclein transgenic fly brains compared to controls by 10 days of age (Figures 2A and 2B), although a more modest increase was visible at 1 day of age (Figures S2A and S2B). To monitor F-actin accumulation biochemically, fly head homogenates were prepared in F-actin stabilizing buffer, pelleted by ultracentrifugation, and F-actin levels determined via immunoblotting. Compared to controls, α-synuclein transgenic flies showed increased F-actin levels (Figure 2C). To confirm and quantify our results we used a sensitive F-actin ELISA (Frenzel et al., 2006), which demonstrated that the F-actin concentration in homogenates of α-synuclein transgenic fly heads was significantly higher than in that of control flies (Figure 2D). Finally, we immunostained brain sections with an antibody that recognizes actin and observed frequent rod-shaped actin-rich inclusions, which increased in number from day 1 to day 10 of age (Figures 2E, arrows, 2F, S2C). We have previously demonstrated a correlation between abnormal stabilization of the actin cytoskeleton and actin rod formation (Fulga et al., 2007).
Figure 2. Actin cytoskeletal abnormalities in the brains of α-synuclein transgenic flies, mice, and in α-synucleinopathy patients.
(A and B) F-actin staining of whole mount brains of α-synuclein transgenic flies compared to control flies. Scale bar, 50 μm. Quantification (B) of fluorescence intensity shows an increase in F-actin in α-synuclein transgenic flies. n=6 per genotype.
(C and D) Actin fractionation by high-speed centrifugation and western blotting (C) and ELISA (D) show increased F-actin levels in α-synuclein transgenic flies compared to control flies. n=10 (C) and n=3 (D) per genotype. WCL=whole cell lysate
(E and F) Immunofluorescent staining for actin in tissue sections and quantification (F) reveals numerous actin-rich rods (arrows) in tissue sections from brains of α-synuclein transgenic flies. Scale bar, 20 μm. n=6 per genotype.
(G and H) Immunofluorescent staining of actin-rich rods (arrows) and quantification (H) in brainstem sections of ~11-14-month-old A53T α-synuclein transgenic mice reveals the presence of numerous actin-rich rods. Scale bar, 10 μm. n=4 per genotype.
(I – K) Immunofluorescent staining of actin-rich rods (arrows) in brainstem sections of A53T α-synuclein transgenic mice shows co-labeling with cofilin (I) and localization within MAP2-positive neurons (J and K). Scale bar, 5 μm (I) and 10 μm (J). n=4 per genotype.
(L – O) Immunofluorescent staining of actin-rich rods (arrows) in cingulate cortex of patients with diffuse neocortical Lewy body pathology (arrows) reveals co-labeling with phalloidin (M and O) and cofilin (N and O). Scale bar, 10 μm (L and M, left), 5 μm (N and O).
Asterisks indicate **p<0.001 and ***p<0.0002, unpaired t-test. Data is represented as mean ± SEM. Flies are 10 days old in (A)–(F) and mice are ~11–14 months old (G)–(K). Control in (A)–(F) is Syb-QF2/+ and in (G)–(K) is non-transgenic littermates.
See also Figure S2.
Transgenic mice expressing the Parkinson’s disease-linked A53T mutant form of human α-synuclein under the control of the mouse prion protein promoter develop progressive motor abnormalities and neurodegeneration with accompanying α-synuclein aggregation (Lee et al., 2002). We assessed these α-synuclein transgenic mice for evidence of actin cytoskeletal abnormalities. When we immunostained sagittal mouse brain sections with an antibody to actin we observed multiple rod-shaped actin inclusions in the brainstem, an area of significant pathology in A53T transgenic mice (Figures 2G and 2H, arrows). Colocalization of MAP2 with actin in mutant mice confirmed the presence of actin rods in neurons (Figures 2J and 2K, arrows). We also examined transgenic mice expressing moderate levels of wild type human α-synuclein. These mice do not develop significant neurological or neuropathological abnormalities (Lee et al., 2002), and, accordingly, we did not observe actin-rich rods in these asymptomatic animals.
We next examined sections from the cingulate cortex of patients with diffuse neocortical involvement by Lewy body pathology (McKeith et al., 2005). Similar to results in fly and mouse brains, actin immunostaining revealed rod-shaped, actin-rich inclusions in α-synucleinopathy patients (Figure 2L, arrows), but not in age- and sex-matched controls who had no evidence of α-synucleinopathy clinically or pathologically. We obtained a fresh sample of cingulate cortex at autopsy of a patient with dementia with Lewy bodies and diffuse neocortical Lewy body pathology, fixed the tissue in paraformaldehyde, stained vibratome sections with fluorescent phalloidin and were able to confirm that rods from human patients bound phalloidin (Figure 2M, arrows). 3D reconstruction of phalloidin-stained sections highlighted the rod-shaped morphology of the actin-rich inclusions (Figure 2M, right panel). Mouse (Figure 2I, arrow) and human (Figures 2N and 2O, arrow) actin rods also contained cofilin, as assessed by double label immunofluorescence. All together, we demonstrate parallel actin cytoskeletal abnormalities in brains of α-synuclein transgenic flies, mice and α-synucleinopathy patients.
Genetic manipulation of the actin cytoskeleton rescues α-synuclein induced neurotoxicity
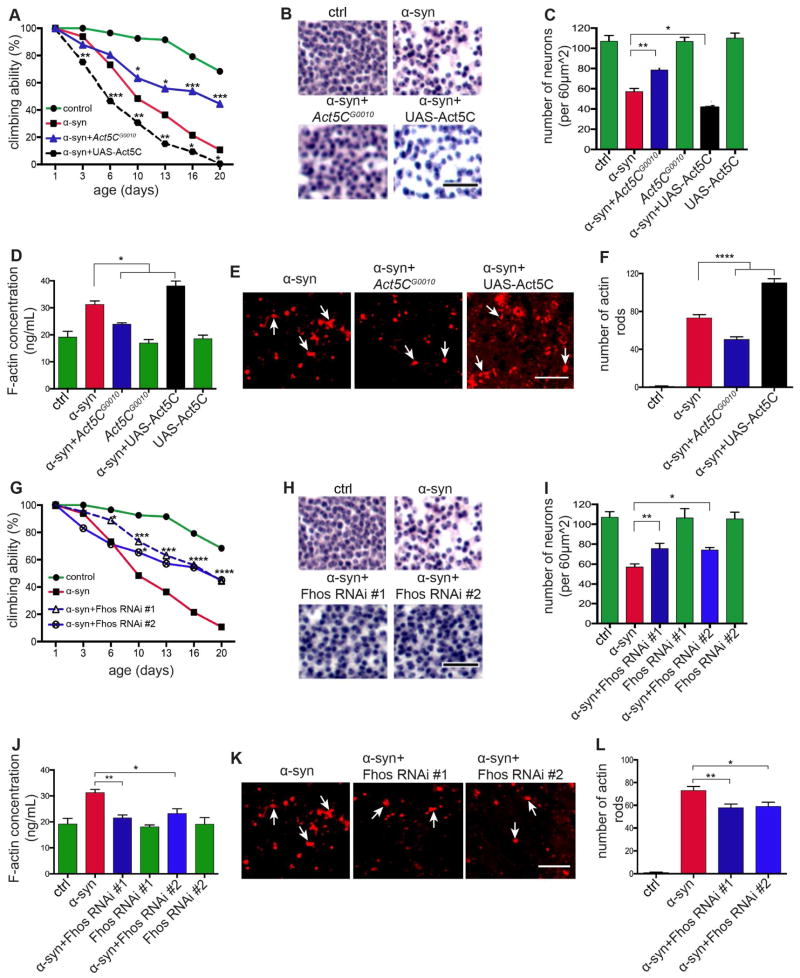
To assess the functional significance of actin cytoskeletal alterations in α-synucleinopathy we performed a series of genetic experiments. We focused on Fhos because we recovered Fhos as a loss of function suppressor in our forward genetic screen, and on actin directly. We have previously demonstrated that manipulating the levels of Actin5C (Act5C), a nervous system enriched cytoplasmic actin isoform (Wagner et al., 2002), using the Act5CG0010 loss of function mutation can rescue neuronal toxicity associated with excess F-actin stabilization mediated by the microtubule associated protein tau (Fulga et al., 2007). When we reduced actin gene dosage using flies heterozygous for Act5CG0010 or expressed either of two non-overlapping transgenic RNAi’s lines directed to Fhos, we observed rescue of the climbing defect (Figures 3A, 3G, S3B and S3G), overall neuronal loss (Figures 3B, 3C, 3H and 3I), dopaminergic neuronal loss (Figure S3N), and vacuole formation (Figures S3C and S3H) in α-synuclein transgenic flies.
Figure 3. Genetic manipulation of the actin cytoskeleton rescues α-synuclein neurotoxicity.
(A – C) Genetic modification of locomotor dysfunction (A) and neuronal degeneration (B and C) in α-synuclein transgenic flies showing rescue by reducing actin in animals heterozygous for Act5CG0010 and enhancement with overexpression of actin (UAS-Act5C). Scale bar, 20 μm. n=60 (A) and n=6 (C) per genotype.
(D) Reduction in F-actin levels in head homogenates of α-synuclein transgenic flies heterozygous for Act5CG0010 and further increase in F-actin levels with overexpression of actin (UAS-Act5C) as determined by F-actin ELISA. n=3 per genotype.
(E and F) Immunostaining of actin-rich rods (E) and quantification (F) showing reduction in actin rods in the brains of α-synuclein transgenic flies heterozygous for Act5CG0010 and further increase in actin rods with overexpression of actin (UAS-Act5C) as determined by immunostaining sections for actin. Scale bar, 5 μm. n=6 per genotype.
(G – I) Genetic rescue of locomotor dysfunction (G) and neuronal degeneration (H and I) in α-synuclein transgenic flies expressing transgenic RNAi directed to Fhos. Scale bar, 20μm. n=60 (G) and n=6 (I) per genotype.
(J) Reduction in F-actin levels in head homogenates of α-synuclein transgenic flies expressing transgenic RNAi directed to Fhos as determined by F-actin ELISA. n=3 per genotype.
(K and L) Immunostaining of actin-rich rods (K) and quantification (L) showing reduction in actin rods in the brains of α-synuclein transgenic flies expressing transgenic RNAi directed to Fhos as determined by immunostaining sections for actin. Scale bar, 5 μm. n=6 per genotype.
Asterisks indicate *p<0.01, **p<0.001, ***p<0.0002 and ****p<0.0001, two-way ANOVA in (A) and (G) and one-way ANOVA in (C), (D), (F), (I), (J), and (L) with Student-Newman-Keuls test. Data is represented as mean ± SEM. Flies are 10 days old in (D), (F), (J) and (L) and 20 days old in (C) and (I). Control (ctrl) is elav-GAL4/+; Syb-QF2/+.
See also Figure S3.
Conversely, increasing levels of actin with a UAS–Act5C–GFP transgene worsened climbing ability (Figures 3A and S3B), neuronal loss (Figures 3B and 3C), and vacuole formation (Figure S3C). Additionally, coexpressing α-synuclein with an EGFP transgene (UAS–EGFP) had no effect on α-synuclein induced neurotoxicity (Figure S3A), showing the specificity of enhancement by overexpression of Act5C. Modulation of Act5C and Fhos did not affect α-synuclein neurotoxicity simply by altering α-synuclein expression (Figures S3D and S3I) or induce toxic effects when expressed in the absence of α-synuclein (Figures 3C, S3E and S3J). Additionally, quantitative real time PCR revealed that actin-related modifiers had reduced expression of Act5C (Figure S3F) and Fhos (Figure S3K).
We next determined the effects of actin-related modifiers on the actin cytoskeleton in α-synuclein transgenic flies. Suppressors, including the Act5C loss of function mutation and RNAi directed to Fhos normalized F-actin concentrations as determined by ELISA (Figures 3D and 3J), while increasing Act5C expression further increased F-actin levels (Figure 3D). Similarly, reducing Act5C levels or Fhos expression decreased the number of actin rods in α-synuclein transgenic flies (Figures 3E, 3F, 3K, 3L and S3L), while a further increase in rods was seen in α-synuclein overexpressing Act5C (Figures 3E, 3F and S3L). These data support a role for abnormal stabilization of the actin cytoskeleton in mediating α-synuclein neurotoxicity, and indicate that the modifiers used exerted their effects by modulating the actin cytoskeleton.
Since genetic modification of the actin cytoskeleton can control both α-synuclein and tau (Fulga et al. 2007) neurotoxicity, we determined if the two neurodegenerative disease-associated proteins interacted in vivo. We began by crossing α-synuclein transgenic flies with flies expressing a mutant human form of tau, tauR406W, found in patients with the familial neurodegenerative disease frontotemporal dementia with parkinsonism linked to chromosome 17. Expression of tauR406W in Drosophila neurons using the panneuronal elav-GAL4 driver creates a level of toxicity amenable to genetic modification and has been widely used as a robust in vivo model of tauopathy (Butzlaff et al., 2015; Duboff et al., 2012; Frost et al., 2014; Fulga et al., 2007; Khurana et al., 2006; Wittmann et al., 2001). Remarkably, expressing α-synuclein and tauR406W together was lethal, indicating significant enhancement of α-synuclein by expression of mutant tau. We then crossed α-synuclein transgenic flies with animals expressing wild type human tau (Wittmann et al., 2001) in neurons. Flies expressing α-synuclein and wild type human tau were recovered, and demonstrated enhanced age-related locomotor decline (Figure S3O) and neurodegeneration (Figure S3P). These findings support a genetic interaction between tau and α-synuclein.
α-synuclein disrupts mitochondrial dynamics via Drp1 mislocalization
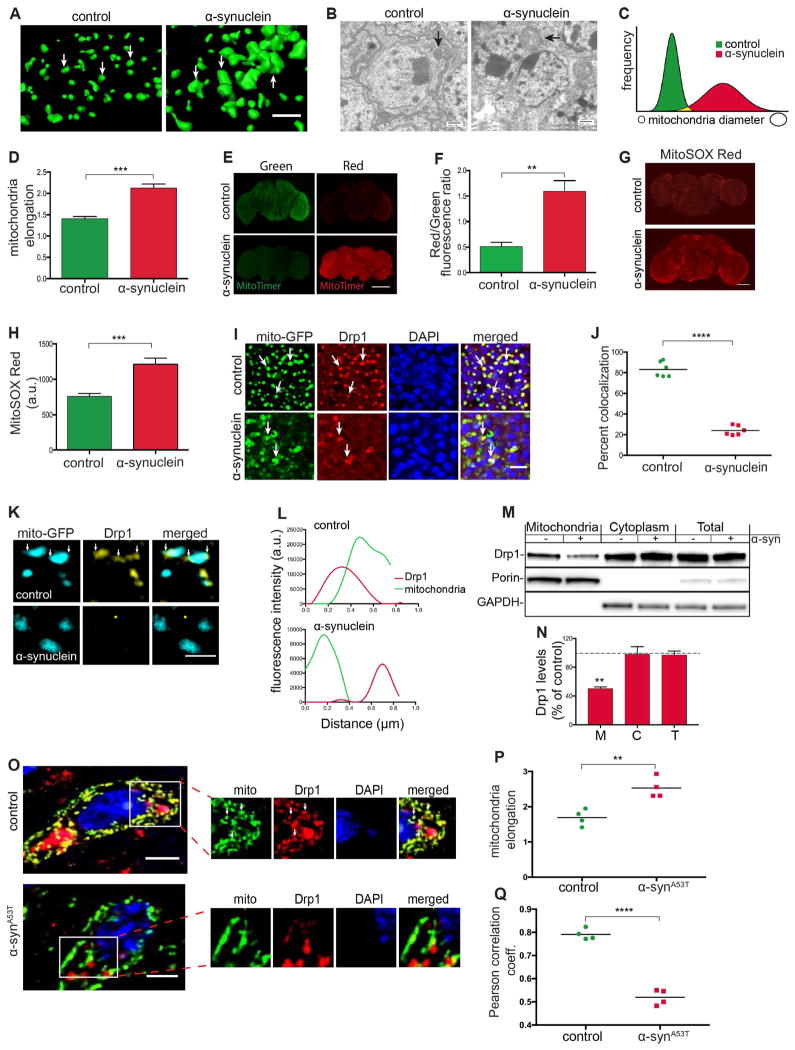
Mitochondrial abnormalities have been implicated in α-synuclein toxicity (Lee et al., 2002; Lin et al., 2012; Martin et al., 2006), and we and others have previously described an important role for the F-actin cytoskeleton in controlling mitochondrial dynamics through the key fission protein Drp1 (Duboff et al., 2012; Korabova et al., 2013; Hatch et al., 2014; Moore et al., 2016). We thus examined mitochondrial morphology in our α-synucleinopathy transgenic flies. Using immunofluorescence 3D reconstruction and electron microscopy we found that mitochondria were significantly enlarged in the brains of α-synuclein transgenic flies (Figures 4A and 4B, arrows). Quantification of mitochondrial diameter (Figure 4C) and elongation (Figures 4D and S4A) confirmed mitochondrial enlargement.
Figure 4. α-synuclein expression induces mitochondrial abnormalities.
(A) 3D reconstruction of immunofluorescence-stained mitochondria showing mitochondrial enlargement in central brain neurons of α-synuclein transgenic flies compared to control flies. Scale bar, 5 μm.
(B) Electron microscopic images of the fly brain cortex showing mitochondrial enlargement in neurons of α-synuclein transgenic flies compared to control flies (arrows). Scale bar, 500 nm.
(C and D) Measurements of mitochondrial diameter and elongation show changes in mitochondrial morphology in α-synuclein transgenic flies. n=6 per genotype.
(E and F) Oxidation of mitochondrial protein is elevated in whole mount brain preparations of α-synuclein transgenic flies compared with controls as monitored by the transition from green to red fluorescence in transgenic MitoTimer protein. Scale bar, 100 μm. n=6 per genotype. Control is elav-GAL4/+; UAS-MitoTimer/Syb-QF2.
(G and H) MitoSOX Red fluorescence (G) and quantification (H) in whole mount brain preparations from α-synuclein transgenic and control flies. Scale bar, 50 μm. n=6 per genotype.
(I and J) Mitochondrial (mito-GFP, green) colocalization with Drp1 (red) is reduced in neurons from α-synuclein transgenic fly brains. Scale bar, 5 μm. n=6 per genotype.
(K and L) Loss of mitochondrial localization of Drp1 as revealed by structured illumination microscopy. Scale bar, 1 μm.
(M and N) Western blot of mitochondria subcellular fractionation probed for Drp1 (M) and quantification (N) showing reduction in Drp1 levels in the mitochondria fraction of α-synuclein flies compared to control. Control is elav-GAL4/+; HA-Drp1/Syb-QF2.
(O – Q) Immunofluorescent staining for mitochondria (ATPVα) and Drp1 (O) shows mitochondrial enlargement (P) and reduced Drp1 colocalization (Q) in ~11-14-month-old A53T human α-synuclein transgenic mice compared to non-transgenic littermates. Scale bar, 5 μm. n=4 per genotype.
Asterisks indicate **p<0.001, ***p<0.0002 and ****p<0.0001. Unpaired t-test. Data is represented as mean ± SEM. Flies are 10 days old in (A)–(L) and 1 day old in (M) and (N). Control in (A)–(D), (G) and (H) is elav-GAL4/+; Syb-QF2. Control in (I)–(L) is elav-GAL4/+; UAS-mito-GFP/+; HA-Drp1/Syb-QF2.
See also Figure S4.
To determine if abnormalities in mitochondrial morphology correlated with indices of mitochondrial dysfunction, we used the reporter gene, MitoTimer (Lacker et al., 2013) to assess mitochondrial health in vivo. MitoTimer encodes a mutant form of DsRed protein that fluoresces green when mitochondria are newly synthesized and shifts irreversibly to red fluorescence following oxidation (Lacker et al., 2013). Compared to controls, brains from 10-day-old α-synuclein transgenic flies had a higher red/green fluorescence ratio, indicating increased mitochondrial oxidation (Figures 4E and 4F), with more modestly increased oxidation present at 1 day of age (Figure S4B). To determine if mitochondrial oxidation correlated with abnormal morphology, we examined red and green fluorescent mitochondria separately for morphological changes and found that enlarged mitochondria are more oxidized (Figure S4D). We next stained freshly dissected whole mount brains with MitoSOX. The MitoSOX dye permeates live cells, where it is targeted to the mitochondria and becomes oxidized by superoxide resulting in strong red fluorescence, which can be detected by microscopy (Robinson et al., 2006). Fluorescence in the brains of α-synuclein transgenic flies was significantly greater than in control flies (Figures 4G and 4H), consistent with elevated levels of mitochondrial superoxide.
The presence of enlarged mitochondria in our α-synuclein transgenic fly and mouse (Martin et al., 2006) α-synucleinopathy models, prompted us to evaluate the subcellular localization of the key mitochondrial fission protein Drp1. Cytoplasmic Drp1 translocates to the mitochondria to drive fission (Li et al., 2014). To visualize Drp1, we used a transgenic line that carries an in-frame rescue construct (FLAG-FIAsH-HA-Drp1) expressed under the control of the endogenous Drp1 promoter (Verstreken et al., 2005). To visualize mitochondria, we used a UAS-Mito-GFP transgene. Drp1 localization to mitochondria was assessed using double label immunofluorescence for HA (Drp1) and GFP (mitochondria). In control flies, Drp1 staining appeared as discrete foci colocalized with mitochondria (Figure 4I, top panels). However, in α-synuclein transgenic flies, the majority of mitochondria did not show Drp1 colocalization (Figure 4I, bottom panels), leading to a significant reduction in percent colocalization by quantitative analysis (Figure 4J). Super-resolution imaging using structured illumination microscopy and mitochondrial subcellular fractionation confirmed Dpr1 mislocalization (Figures 4K and 4M). Note that only a small fraction of total Drp1 localizes to mitochondria (Smirnova et al., 2001), so that the decrease in mitochondrial Drp1 in α-synuclein transgenic flies did not result in a clear increase in cytosolic Drp1 by western blotting of the total cytoplasmic fraction (Figure 4M). Compared to control flies, in α-synuclein transgenic flies the fluorescence spectra of Drp1 measured over a 1 μm distance was dissociated from the mitochondrial spectra (Figure 4L), and Drp1 levels in the mitochondrial fraction were significantly lower (Figure 4N). To ensure that loss of Drp1 localization to mitochondrial protein did not simply reflect reduction in Drp1 protein or changes mitochondrial biogenesis, we performed immunoblotting experiments that demonstrated equivalent levels of Drp1 (Figures 4M and S5G) and mitochondria (Figure S4C) in control and α-synuclein transgenic flies.
To determine if the mitochondrial dynamics machinery was also altered in vertebrates in response to α-synuclein expression we examined A53T mutant α-synuclein transgenic mice. Consistent with our previously published results (Martin et al., 2006), mitochondria had significantly increased size compared to controls (Figures 4O and 4P). In comparison, neurologically and neuropathologically normal transgenic mice expressing wild type human α-synuclein (Lee et al., 2002) did not show changes in mitochondria morphology (Figure S4E).
Drp1 localization to mitochondria in A53T α-synuclein transgenic mice was assessed using double label immunofluorescence for Drp1 and ATPVα (mitochondria). As in flies, human α-synuclein expression in mice resulted in significantly reduced co-immunostaining for Drp1 and mitochondria (Figure 4O, inset). Calculation of the Pearson correlation coefficient confirmed a significant reduction in colocalization of mitochondria and Drp1 in A53T α-synuclein mutant mice compared to control littermates (Figure 4Q). Similar findings in our fly and mouse α-synucleinopathy models suggest that increased levels of α-synuclein promote mitochondrial elongation and dysfunction secondary to lack of Drp1 translocation to the mitochondrial outer membrane in a conserved mechanism of neurotoxicity.
Reversing mitochondrial abnormalities rescues α-synuclein induced neurotoxicity
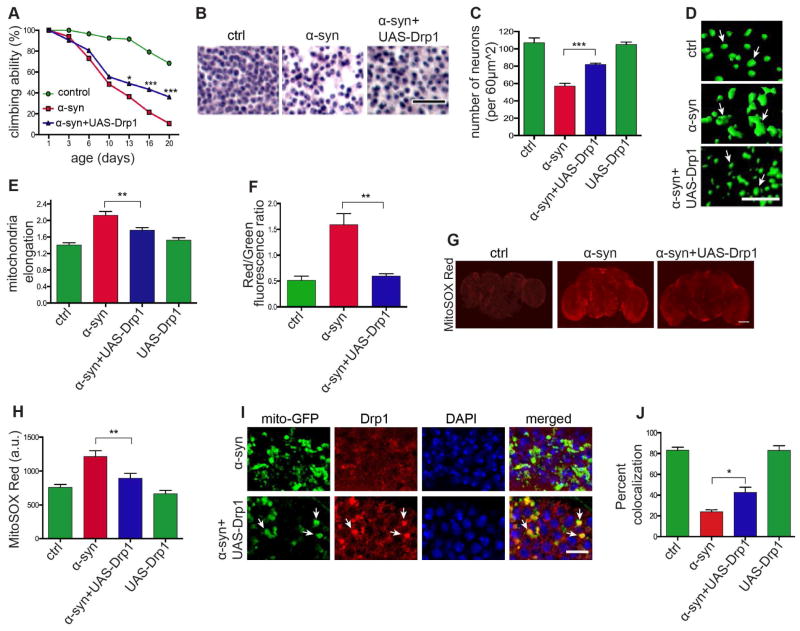
To determine if altered mitochondrial dynamics play a causal role in α-synuclein neurotoxicity, we genetically manipulated mitochondrial dynamics in α-synuclein transgenic flies. We began by assessing the influence of mitochondrial dynamics on α-synuclein toxicity by assaying climbing activity, neuronal loss and vacuole formation. Overexpression of Drp1 in α-synuclein transgenic flies significantly rescued locomotor deficits (Figures 5A and S5A), overall neuronal loss in the medulla (Figures 5B and 5C), dopaminergic neuronal loss (Figure S5H), and vacuole formation (Figure S5B). We then determined if mitochondrial morphological and biochemical abnormalities were also ameliorated in α-synuclein transgenic flies following Drp1 overexpression. We found that increasing expression of Drp1 in α-synuclein transgenic flies normalized mitochondrial size (Figures 5D and 5E, arrows), decreased mitochondrial oxidation (Figure 5F) and reduced mitochondrial superoxide (Figures 5G and 5H). Using double label immunofluorescence, we confirmed that transgenic expression of Drp1 promoted mitochondrial localization of Drp1 in α-synuclein transgenic flies (Figure 5I, arrows). Quantification of the percent localization revealed a significant rescue in colocalization in α-synuclein transgenic flies overexpressing Drp1 (Figure 5J). Overexpression of Drp1 using the moderately expressing transgenic line we have previously characterized and used to normalize mitochondrial dynamics in pathological states (DuBoff et al., 2012) did not alter α-synuclein levels (Figure S5C), locomotor ability (Figure S5D), neuronal density (Figure 5C), mitochondrial elongation (Figure 5E) or Drp1 localization (Figures 5J and S5F, arrows) in the absence of transgenic α-synuclein expression. Increased expression of Drp1 mediated by the UAS-Drp1 transgene was confirmed by quantitative real time PCR (Figure S5E).
Figure 5. Overexpressing Drp1 rescues α-synuclein induced mitochondrial dysfunction and neurodegeneration.
(A – C) Rescue of locomotor deficits (A) and neuronal loss (B and C) by overexpression of Drp1 (UAS-Drp1) in α-synuclein transgenic flies. Scale bar, 20 μm. n=60 (A) and n=6 (C) per genotype. Control (ctrl) is elav-GAL4/+; Syb-QF2/+.
(D and E) Mitochondrial length is normalized by overexpression of Drp1 (UAS-Drp1) in α-synuclein transgenic flies. Scale bar, 10 μm. n=6 per genotype. Control (ctrl) is elav-GAL4/+; UAS-mito-GFP/+; Syb-QF2/+.
(F) Mitochondrial protein oxidation as monitored by the transition from green to red fluorescence in MitoTimer protein is rescued by Drp1 expression (UAS-Drp1) in α-synuclein transgenic flies. n=6 per genotype. Control (ctrl) is elav-GAL4/+; UAS-MitoTimer/Syb-QF2.
(G and H) MitoSOX Red fluorescence is reduced by Drp1 overexpression (UAS-Drp1) in α-synuclein transgenic flies. Scale bar, 50 μm. n=6 per genotype. Control (ctrl) is elav-GAL4/+; Syb-QF2/+.
(I and J) Drp1 localization to the mitochondria, as monitored by HA-tagged Drp1 (HA-Drp1), is restored following Drp1 overexpression (UAS-Drp1) in α-synuclein transgenic flies (arrows). Scale bar, 5 μm. n=6 per genotype. Control is elav-GAL4/+; UAS-Drp1, mito-GFP/+; HA-Drp1/Syb-QF2.
Asterisks indicate *p<0.01, **p<0.001 and ***p<0.002, two-way ANOVA in (A) and one-way ANOVA in (C), (E), (F), (H) and (J) with Student-Newman-Keuls test. Data is represented as mean ± SEM. Flies are 10 days old except for (C) in which flies are 20 days old.
See also Figure S5.
Abnormalities in mitochondria function are downstream of actin stabilization
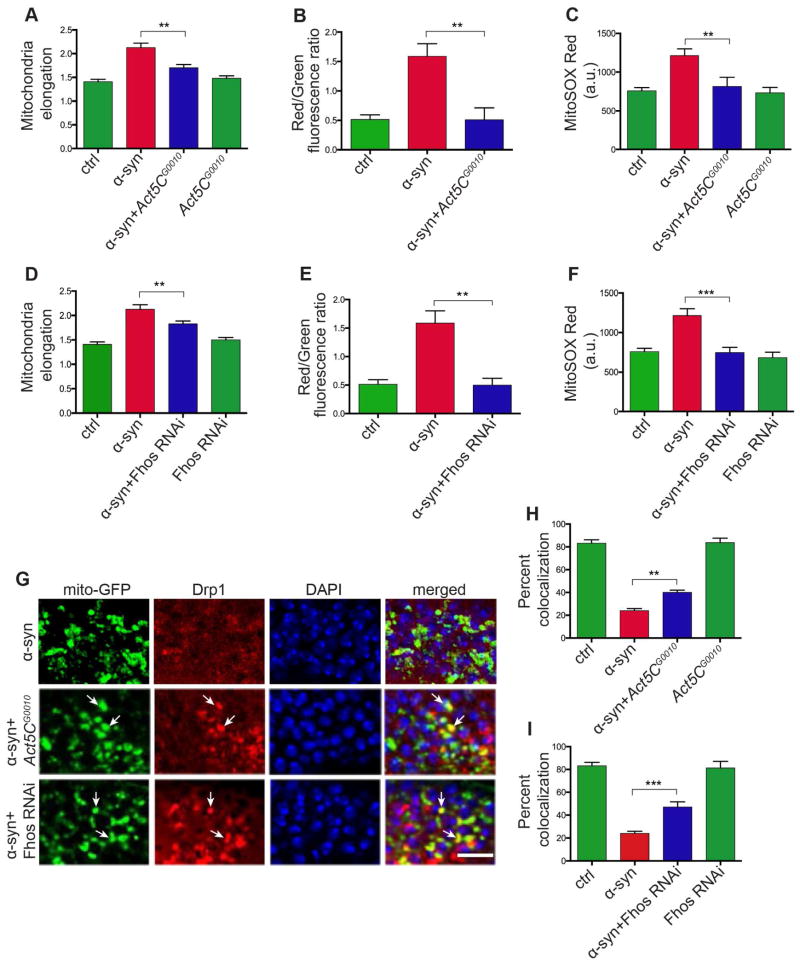
We next took a genetic approach to investigate the relationship between actin cytoskeletal abnormalities and mitochondrial dynamics in α-synuclein transgenic flies. We began by determining if reversing abnormal F-actin stabilization could rescue mitochondrial abnormalities. When we manipulated the F-actin cytoskeleton by removing one copy of Act5C using the Act5CG0010 allele or by expressing transgenic RNAi directed to Fhos, we normalized mitochondria morphology (Figures 6A and 6D). Reducing Act5C and Fhos also rescued mitochondrial biochemical abnormalities in α-synuclein transgenic flies as measured by MitoTimer oxidation (Figures 6B and 6E) and MitoSOX fluorescence (Figures 6C and 6F). We then determined if manipulating actin genetically could influence Drp1 localization. Co-staining for HA-tagged Drp1 and mitochondria (mito-GFP) demonstrated recovery of Drp1 punctate staining (Figure 6G, arrows) and increased mitochondrial colocalization in α-synuclein transgenic flies with actin-related modifiers (Figures 6H, 6I and S6A).
Figure 6. Abnormal F-actin stabilization drives mitochondrial pathology in α-synucleinopathy.
(A – C) Mitochondrial morphology (A), mitochondrial protein oxidation as assessed by fluorescence of the MitoTimer protein (B), and MitoSOX Red fluorescence (C) are all normalized in α-synuclein transgenic flies heterozygous for the Act5CG0010 mutation. n=6 per genotype.
(D – F) Mitochondrial morphology (D), mitochondrial protein oxidation as assessed by fluorescence of the MitoTimer protein (E), and MitoSOX Red fluorescence (F) are all normalized in α-synuclein transgenic flies expressing RNAi directed to Fhos. n=6 per genotype.
(G – I) Drp1 localization to mitochondria is partly restored by heterozygosity for Act5CG0010 (H) or expressing Fhos RNAi (I). Scale bar, 5 μm. n=6 per genotype.
Asterisks indicate **p<0.001 and ***p<0.002, one-way ANOVA with Student-Newman-Keuls test. Data is represented as mean ± SEM. Flies are 10 days old in all panels. Control (ctrl) in (A, D) is elav-GAL4/+; UAS-mito-GFP/+; Syb-QF2/+. Control (ctrl) in (B, E) is elav-GAL4/+; UAS-MitoTimer/Syb-QF2. Control (ctrl) in (C, F) is elav-GAL4/+; Syb-QF2/+. Control in (G–I) is elav-GAL4/+; UAS-mito-GFP/+; HA-Drp1/Syb-QF2.
See also Figure S6.
Additionally, we genetically destabilized actin in α-synuclein transgenic flies and monitored the effects on mitochondrial morphology and Drp1 localization. Overexpression of the actin severing protein Gelsolin (Yin and Stossel, 1979), was used to decrease F-actin levels (Duboff et al., 2010). Increasing levels of Gelsolin (UAS-Gelsolin) reduced mitochondrial elongation (Figure S6B) and rescued Drp1 localization (Figures S6C and S6D, arrows) in α-synuclein transgenic flies.
α-synuclein interacts with spectrin to disrupt the actin cytoskeleton
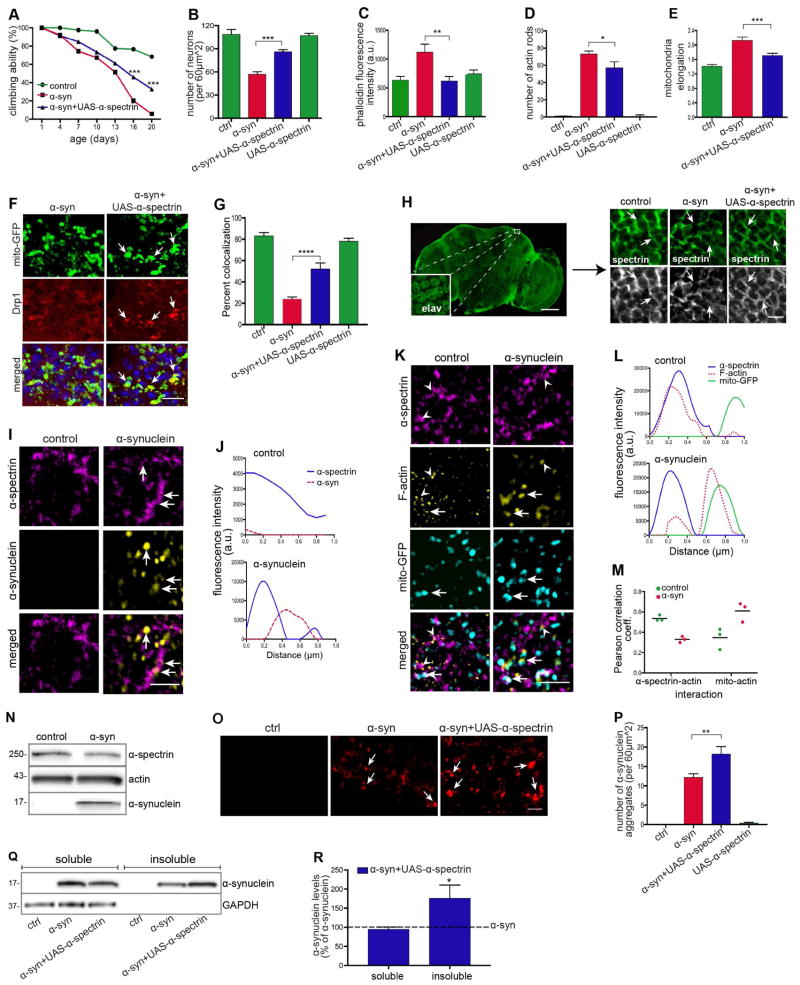
We next addressed the mechanism by which α-synuclein expression altered actin cytoskeletal organization and promoted downstream dysfunction of mitochondria. We focused our attention on spectrins, actin crosslinking proteins that have previously been reported to interact with α-synuclein in vitro (Lee et al., 2012) and in cell culture (Lee et al., 2012; Chung et al., 2017). Spectrins exist primarily as heterotetramers made of α and β subunits. Flies have one α subunit and one conventional β subunit (Dubreuil and Grushko, 1998), facilitating genetic analysis. We first determine if modulating α-spectrin levels could influence α-synuclein neurotoxicity. When we increased expression of α-spectrin, motor deficits (Figures 7A and S7A), overall neuronal loss (Figures 7B and S7B), and dopaminergic neuronal loss (Figure S7I) were rescued in α-synuclein transgenic flies. α-synuclein transgenic flies with α-spectrin overexpression also showed reduced F-actin levels (Figures 7C and S7C) and decreased numbers of actin rods (Figures 7D and S7D, arrows), consistent with an effect of α-spectrin via the actin cytoskeleton. As we would predict given the role of actin in promoting mitochondrial pathology in α-synuclein, we also observed normalization of mitochondrial morphology (Figures 7E and S7E, arrows) and rescue of Drp1 mislocalization (Figures 7F and 7G, arrows) with overexpression of α-spectrin. Importantly, overexpression of α-spectrin did not affect α-synuclein expression levels (Figure S7G) or Drp1 localization to the mitochondria in the absence of transgenic α-synuclein expression (Figure S7F, arrows). We confirmed overexpression of α-spectrin from the UAS-α-spectrin transgene using quantitative real time PCR (Figure S7H).
Figure 7. Spectrin induces actin stabilization and subsequent neurodegeneration.
(A and B) Rescue of locomotor deficits (A) and neuronal loss (B) by overexpression of α-spectrin (UAS-α-spectrin) in α-synuclein transgenic flies. n=60 (A) and n=6 (B) per genotype.
(C) Reduction in F-actin levels in head homogenates of α-synuclein transgenic flies overexpressing α-spectrin as determined by F-actin ELISA. n=3 per genotype.
(D) Reduction in actin rods in the brains of α-synuclein transgenic flies overexpressing α-spectrin as determined by immunostaining sections for actin. n=6 per genotype.
(E) Mitochondrial length is normalized by overexpression of α-spectrin in α-synuclein transgenic flies. n=6 per genotype. Control (ctrl) is elav-GAL4/+; UAS-mito-GFP/+; Syb-QF2/+.
(F and G) Rescue of Drp1 localization to the mitochondria by overexpression of α-spectrin in α-synuclein transgenic flies. Scale bar, 5 μm, n=6 per genotype. Control (ctrl) is elav-GAL4/+; UAS-mito-GFP/+; Syb-QF2/HA-Drp1.
(H) Immunofluorescence staining with an antibody to α-spectrin shows loss of normal subplasmalemmal organization in α-synuclein transgenic flies. Overexpression of α-spectrin restores normal organization. Inset indicates the area of the brain analyzed in (H) and subsequently; elav immunostain identifies neuronal nuclei. Scale bar, 100 μm (inset, 5 μm). Control is elav-GAL4/+; UAS-α-spectrin/+.
(I) Structured illumination microscopy (SIM) immunostaining showing colocalization of α-spectrin with α-synuclein in Kenyon cells of α-synuclein transgenic flies compared to control flies. Scale bar, 1 μm.
(J) Fluorescence spectral z-profile showing association between α-spectrin (blue) and α-synuclein (red) in α-synuclein transgenic flies compared to control flies.
(K) SIM immunostaining showing colocalization of α-spectrin with mitochondria and F-actin in Kenyon cells of α-synuclein transgenic flies compared to control flies. Scale bar, 1 μm.
(L) Fluorescence spectral z-profile shows association between α-spectrin (blue) and F-actin (red) in control flies. In α-synuclein flies, spectrin is disassociated with F-actin (red).
(M) Pearson’s correlation coefficient indicating infrequent association of α-spectrin with actin and increased association with mitochondria in α-synuclein transgenic flies compared to control flies. n=3 per genotype.
(N) F-actin precipitation with biotinylated phalloidin showing biochemical interaction of F-actin with α-spectrin and α-synuclein in α-synuclein transgenic flies compared to control. n=10 per genotype.
(O and P) Immunostaining and quantification (P) of α-synuclein aggregates shows increased numbers of α-synuclein aggregates following α-spectrin overexpression. Scale bar, 3 μm. n=6 per genotype.
(Q and R) Western blot of soluble and insoluble fractions prepared from fly heads (Q) and quantification (R) showing significantly increased α-synuclein levels in insoluble fractions from heads of α-synuclein transgenic flies overexpressing α-spectrin. Both soluble and insoluble fractions were normalized to α-synuclein transgenic flies and compared to α-synuclein transgenic flies overexpressing α-spectrin. Statistical significance was determined based on percent change. n=10 per genotype.
Asterisks indicate *p<0.01, **p<0.001, ***p<0.0002 and ****p<0.0001, two-way ANOVA in (A) and one-way ANOVA in (B–E), (G) and (P) with Student-Newman-Keuls test. Data is represented as mean ± SEM. Flies are 10 days old in all panels. Control (ctrl) in (A–D) and (I–R) is elav-GAL4/+; Syb-QF2/+.
See also Figure S7 and S8.
We also assessed the effects of loss of α-spectrin function on α-synuclein toxicity. Remarkably, expression of human α-synuclein caused lethality in the presence of heterozygosity for each of three loss of function mutations, α-spectrinrg41, α-spectrinlm88 and α-spectrinMI11816. In contrast, each of the α-spectrin alleles were viable and healthy as heterozygotes in otherwise wild type genetic backgrounds, including in the presence of the elav-GAL4 and Syb-QF2 drivers to recapitulate the genetic background of the α-synuclein expression experiment. These findings support a strong genetic interaction between α-synuclein and α-spectrin in vivo.
To investigate the mechanism by which α-spectrin overexpression rescued α-synuclein neurotoxicity in more detail, we immunostained brains from control and α-synuclein transgenic flies with an antibody specific to α-spectrin. Spectrin normally forms a regular subplasmalemmal network that helps maintain the structure and shape of the cell (Machnicka et al., 2012). Accordingly, we observed regular staining for α-spectrin at the neuronal cell periphery in control animals. In contrast, the spectrin network was markedly disrupted in α-synuclein transgenic flies (Figure 7H, arrows). Disruption of the α-spectrin cytoskeleton was not seen in flies expressing serine 129 phosphorylation incompetent (S129A) α-synuclein, but was present with expression of a phosphomimic version (S129D) of α-synuclein (Figure S7L), consistent with prior work showing preferential binding of spectrin to phosphorylated α-synuclein (McFarland et al. 2008). Consistent with rescue of neurotoxicity, normal α-spectrin organization was restored with overexpression of α-spectrin in α-synuclein transgenic flies (Figure 7H, arrows).
We then performed super-resolution imaging with structured illumination microscopy to investigate the organization of α-synuclein and interacting proteins in more detail. Co-labeling for α-synuclein and α-spectrin revealed close association of the two proteins, as shown in the fluorescence spectral z-profile (Figures 7I and 7J, arrows). We next assessed the relationship of mitochondria using mito-GFP, F-actin using fluorescent phalloidin, and α-spectrin using a specific antibody. In control flies, we observed that the majority of F-actin colocalized with subplasmalemmal α-spectrin, as expected. In contrast, in α-synuclein transgenic flies, significant amounts of F-actin were relocalized to mitochondria (Figures 7K and 7L, arrows). The Pearson correlation coefficient demonstrates the relocalization of F-actin to mitochondria in α-synuclein transgenic animals (Figure 7M). We confirmed a close physical interaction between F-actin, α-spectrin, and α-synuclein by precipitating F-actin specifically with biotinylated phalloidin and immunoblotting the resultant precipitate with antibodies against α-spectrin and α-synuclein. We observed co-precipitation of α-spectrin and α-synuclein with F-actin, confirming our imaging data (Figures 7N and S7K).
Since α-spectrin closely associates with α-synuclein (Figures 7I and 7J), we wondered if increasing levels of α-spectrin might alter α-synuclein solubility and thereby modulate neurotoxicity. We thus assessed the number of α-synuclein aggregates using immunofluorescence and the 5G4 α-synuclein monoclonal antibody, which preferentially identifies aggregated forms of α-synuclein (Kovacs et al., 2012). We observed significantly increased numbers of α-synuclein aggregates, and increased size of these aggregates in α-synuclein transgenic flies with α-spectrin overexpression (Figures 7O and 7P, arrows). α-synuclein inclusions contained α-spectrin in α-spectrin overexpressing animals (Figure S7J, arrows). Changes in α-synuclein solubility were also assessed biochemically. We isolated soluble and insoluble fractions from α-synuclein transgenic fly heads and performed immunoblotting with an antibody specific for α-synuclein. We observed significantly higher α-synuclein levels in the insoluble fraction of α-synuclein flies overexpressing α-spectrin compared to α-synuclein flies (Figures 7Q and 7R). These data are consistent with a model in which monomeric or oligomeric α-synuclein binds to and disrupts the spectrin cytoskeleton, disrupting F-actin dynamics and Drp1 localization at mitochondria. Overexpression of α-spectrin promotes aggregation of α-synuclein into larger inclusions that sequester smaller, toxic forms of α-synuclein, restoring F-actin cytoskeletal dynamics and normal mitochondrial function (Figure S8).
Discussion
Despite unequivocal genetic and neuropathological evidence implicating α-synuclein in the pathogenesis of Parkinson’s disease and related α-synucleinopathies, the direct molecular targets of α-synuclein and critical downstream mediators of neurodegeneration have remained elusive. Here we couple a new and powerful experimental genetic model of α-synucleinopathy in Drosophila with analyses in mouse and human tissue to outline a molecular cascade of neurotoxicity beginning with direct binding of α-synuclein to α-spectrin, consequent dysregulation of actin dynamics leading to mitochondrial dysfunction and ultimately to cell death (Figure S8). Our identification of spectrin as a target of α-synuclein in vivo (Figure 7) is supported by prior observations of direct or close proximity spectrin interactions with α-synuclein (Lee et al., 2012; McFarland et al., 2008; Chung et al., 2017), and more generally by previous implication of the actin cytoskeleton in α-synuclein neurotoxicity (Chung et al., 2009; Gillardon et al., 2008; Khurana et at., 2017; Sousa et al., 2009; Xun et al., 2008). α-spectrin has also been described as a component of Lewy bodies (Leverenz et al., 2007), the characteristic protein aggregate of Parkinson’s disease and related α-synucleinopathies. Further, a genome wide association study linked β-spectrin (SPTBN1) to neocortical Lewy body type pathology (Peuralinna et al., 2015), consistent with a causative link between spectrin and the degree of α-synuclein aggregation into inclusion bodies. Our observation that increasing levels of α-spectrin promotes α-synuclein inclusion formation (Figures 7P and 7R) fits well with these human genetic results.
Intriguingly, binding of α-synuclein to spectrin appears to be strongly promoted by phosphorylation of α-synuclein at serine 129 (McFarland et al., 2008), a major disease associated phosphoepitope (Fujiwara et al., 2002). We have previously demonstrated an important role of phosphorylation of serine 129 in promoting neurotoxicity of α-synuclein and regulating aggregation of the protein in vivo (Chen and Feany, 2005). In these studies, we found that formation of large inclusions was correlated with protection from α-synuclein toxicity, consistent with our current observations (Figures 7P and 7R), and the more general implication of smaller, prefibillar toxic species in a wide range of neurodegenerative diseases characterized by abnormal protein aggregation (Karpinar et al., 2009).
Our prior studies on aggregation and neurotoxicity of α-synuclein in Drosophila were conducted in previously described transgenic lines with more modest levels of human α-synuclein expression (Figure 1A) (Feany and Bender, 2000; Chen and Feany, 2005; Chen et al., 2009). These transgenic flies had significant aggregation of α-synuclein, but neurotoxicity was confined to dopaminergic neurons in the brain. In contrast, our current model shows not only widespread aggregation of α-synuclein (Figure 1H), but also marked loss of both dopaminergic and nondopaminergic neurons (Figures 1C–1F and S1G–S1J), and significantly earlier locomotor dysfunction (Figure 1B). Our current model also shows a modest reduction in lifespan (Figure S1C), which was not a consistent feature of our previous α-synucleinopathy model. Thus, we can now expand our previous model to more accurately represent the wider group of neurodegenerative diseases, including dementia with Lewy bodies, associated with α-synuclein aggregation and inclusion formation. Of note, clinical neurologists and neuropathologists have increasingly recognized that even in typical Parkinson’s disease the earliest (Braak et al., 2003) and some of the most devastating clinical manifestations (Shulman et al., 2011) represent the toxicity of α-synuclein to nondopaminergic neuronal populations. The importance of nonmotor symptoms throughout the course of Parkinson’s disease is so significant that these nondopaminergic-derived clinical deficits are now recognized as an important unmet need in Parkinson’s disease (Martinez-Martin et al., 2011). From a practical perspective, the earlier onset and more severe behavioral and neuropathological features of the new model we describe here will enhance the ability to use powerful genetic approaches, and in particularly genome-scale unbiased forward genetics to provide a detailed and comprehensive analysis of the mechanisms controlling α-synuclein neurotoxicity.
Although our prior α-synuclein transgenic model had more modest pathology than the new model we now describe (Figure 1), we were able to a perform forward genetic screen based on the retinal degeneration phenotype (Feany and Bender, 2000), which identified mutations in Fhos as loss of function suppressors of α-synuclein neurotoxicity. These findings prompted us to explore the role of the actin cytoskeleton in mediating α-synuclein toxicity in the current study. Our investigations reveal a robust and previously unexpected dysregulation of the actin cytoskeleton in our Drosophila model, in brain tissue from A53T α-synuclein transgenic mice, and in patients with dementia with Lewy bodies as well (Figure 2). Abnormal stabilization of the actin cytoskeleton in α-synuclein transgenic flies leads to mislocalization of the key mitochondrial fission protein Drp1 and consequent mitochondrial dysfunction in a pathway that we (Duboff et al., 2012) and other investigators (Hatch et al., 2014; Moore et al., 2016) have investigated in detail. In particular, recent studies suggest a critical role for actin dynamics at the mitochondria to target oligomeric Drp1 to fission sites (Ji et al., 2015), consistent with excess stabilization of the actin cytoskeleton in α-synuclein transgenic animals (Figures 2 and 7), including at the mitochondrial membrane (Figures 7K–7M).
Our findings of mitochondria as important downstream targets of α-synuclein neurotoxicity correlate well with observations of abnormal mitochondrial morphology in Parkinson’s disease patients (Baloyannis et al., 2006), longstanding implication of mitochondrial dysfunction in sporadic Parkinson’s disease (Schapira et al., 1990; Bose and Beal, 2016), and prior association of mitochondrial abnormalities in experimental α-synucleinopathies (Choubey et al., 2011; Menges et al., 2017; Nakamura et al., 2011; O’Donnell et al., 2014). In addition, our results suggest intriguing commonalities with autosomal recessive forms of familial Parkinson’s disease caused by mutations in PINK1 and parkin. These two proteins work in a closely connected pathway to promote normal mitochondrial dynamics and mitophagy (Shulman et al., 2011). Perhaps even more intriguingly, our mechanistic (Figures 2 and 4) and neurotoxicity (Figures S3O and S3P) findings suggest a possible mechanistic link to the most common group of neurodegenerative diseases, the tauopathies. Tauopathies, including Alzheimer’s disease, are characterized by the aggregation and deposition of the microtubule binding protein tau in brains from affected patients. We have previously demonstrated that tau binds actin directly (Fulga et al., 2007), stabilizing the actin cytoskeleton and perturbing mitochondrial dynamics (DuBoff et al., 2012). Characterization of a similar pathway that controls α-synuclein neurotoxicity is notable given longstanding recognition of clinical and pathological similarities among α-synucleinopathies and tauopathies. The more recent recovery of the TAU locus in genome wide association studies in Parkinson’s disease (Shulman and Feany, 2011), and observations that a subset of patients with Parkinson’s disease due to mutations in LRRK2 have tauopathy pathology on neuropathological examination (Poulopoulos et al., 2012) further emphasizes the connections among the disorders and supports the possibility of a common mechanism of pathogenesis as outlined here. Our definition of a new pathway mediating neurotoxicity in α-synucleinopathy suggests potential new therapeutic targets for α-synucleinopathies, which may be applicable to a wide range of common human neurodegenerative disorders, including Alzheimer’s disease.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Mel B. Feany (mel_feany@hms.harvard.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Fly stocks and transgenes
Drosophila crosses were performed at 25°C, with the exception of overexpression of actin (UAS-Act5C-GFP), which was performed at 23°C to minimize toxicity. All flies were aged at 25°C for 10 days unless otherwise noted in the figure legend. Male and female flies were used in each experiment. The human wild-type form of α-synuclein was cloned into the pQUAST vector and flies created by embryo injection (BestGene) to generate the QUAS-α-synucleinWT line. The panneuronal drivers elav–GAL4 and Syb-QF2 were used for all experiments. Stocks obtained from the Bloomington Drosophila Stock Center include elav-GAL4, TH-GAL4, Act5CG0010, α-spectrinrg41, α-spectrinlm88, α-spectrinMI11816, QUAS-mCD8-GFP, UAS-mCD8-GFP, UAS-EGFP, UAS-Act5C-GFP, UAS-Fhos RNAi (TRiP.JF01606, TRiP.HMJ21037) and UAS-MitoTimer. The following Drosophila stocks were obtained from the indicated investigators: Syb-QF2, Christopher Potter; UAS-CD8-PARP-Venus, Darren Williams; FLAG-FlAsH-HA-Drp1, Hugo Bellen; UAS-α-Spectrin, Ronald Dubreuil; UAS-Mito-GFP, Thomas Schwarz; Fhos-lacZ, Sven Bogdan. UAS-Drp1, UAS-Gelsolin, UAS-S129D, UAS-S129A and human wild type tau and tauR406W transgenic flies have been described previously (DuBoff et al., 2012; Chen and Feany, 2005; Wittmann et al., 2001) and currently available upon request.
Mouse and human tissue
α-synuclein transgenic mice carrying the familial Parkinson’s disease-associated A53T missense mutation (also called G2-3 line or Hualpha-Syn(A53T) were aged until symptomatic (~11–14 months old). Asymptomatic human wild type α-synuclein transgenic mice were used for all experiments at 12 months of age. Controls were littermates not carrying the human wild type α-synuclein or A53T α-synuclein mutation. Male and female mice were used in all experiments. A total of four animals per genotype were used in each experiment. At the prescribed time point, mouse tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Sections were cut at 7 μm for histological analysis. All experimental protocols involving mice were in strict adherence to the NIH Animal Care and Guidelines and were approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Postmortem human control and α-synucleinopathy brains were collected through the autopsy service at Brigham and Women’s Hospital under the appropriate IRB. Control brains included one female and five males with no significant Lewy pathology, Braak stage I/II, a median age of 68.5 and an age range of 57–84 years. α-synucleinopathy brains were from one female and five males with diffuse neocortical Lewy pathology (McKeith et al., 2005), Braak stage I/II, with a median age of 85 and an age range of 71–94 years. Sections were cut at 6 μm for histological analysis.
METHOD DETAILS
Behavioral and lifespan analysis
For climbing assay, ten flies were placed in individual vials for a total of six vials. Flies were lightly tapped down to the bottom of the vial, and the number of flies climbing above 5 centimeters, within 10 seconds was recorded. A total of 60 flies of each genotype were used for the assay. Each climbing assay was repeated three times and the mean and standard error of the mean calculated. All flies were maintained at 25°C for the duration of the behavioral assay.
For lifespan analysis, 30 flies were aged per vial with a total of 300 flies per genotype. Flies were transferred to fresh food every third day. Aging was performed at 25°C. Mortality was plotted using Kaplan-Meyer anal ysis in GraphPad Prism 7 software.
Immunohistochemistry and histology
Drosophila heads and mouse brains were fixed and embedded in paraffin for immunostaining experiments. The primary antibodies used were anti-actin (1:200, Developmental Studies Hybridoma Bank, JLA20), anti-aggregated-α-synuclein (1:1,000,000, Millipore, 5G4), anti-β-galactosidase (1:100, Promega), anti-cofilin (1:2000, Cytoskeleton), anti-cleaved PARP (1:5000, Abcam, E51), anti-elav (1:5, Developmental Studies Hybridoma Bank), anti-ATPVα (1:200, Novex), anti-GFP (1:2000, Abcam), anti-HA.11 (1:200, BioLegend), anti-tyrosine hydroxylase (1:500, Immunostar), anti-Drp1 (1:50, Santa Cruz), anti-α-spectrin (1:200, Developmental Studies Hybridoma Bank, 3A9), anti-ubiquitin (1:500, DAKO) and anti-MAP2 (1:200, Millipore). Tissue processing was carried out as follows: adult flies were fixed in formalin and embedded in paraffin for sectioning. Serial frontal sections (4 μm) of the entire brain were taken and mounted on slides. Mouse brains were fixed in 4% paraformaldehyde and embedded in paraffin for sectioning. Sagittal sections (7 μm) of paraffin-embedded material were prepared for fluorescent labeling. For antigen retrieval slides were microwaved in sodium citrate buffer for 15 minutes. Immunostaining was performed using secondary antibodies coupled to Alexa Fluor 488 or Alexa Fluor 555. Samples were analyzed on a Zeiss laser-scanning confocal microscope.
For brain morphology, sections were stained using hematoxylin and eosin following a standard protocol. The number of vacuoles in the entire medulla was counted. To assay neuronal density, sections were stained with hematoxylin and the number of neurons in an approximately 60 μm2 area in the anterior medulla was counted. Anterior medulla sections were imaged using the imaging software SPOT and quantitatively analyze using the cell counter plugin in imageJ.
Confocal microscopy
Total F-actin levels were determined in adult fly brains as previously described (Fulga et al., 2007). Briefly, whole brains from adult flies were dissected under a dissection microscope and stained with Alexa Fluor 532 phalloidin (1:1000, Life technologies). Before acquisition, laser parameters were adjusted to obtain nonsaturating conditions. A total of six samples per genotype were processed simultaneously using identical confocal acquisition parameters (laserpower, gain and pinhole settings) on a Zeiss laser-scanning confocal microscope. For quantification of fluorescent staining, average pixel intensity from two-dimensional projections of confocal z-stacks were measured using imageJ.
Following immunofluorescent staining with an actin antibody, the number of actin-rich rods was determined by counting all rod-shaped structures that positively stained for actin in 4 μm frontal brain sections of the entire medulla from flies grown and aged at 25°C. A total of six flies were analyzed per genotype. Actin rod analysis in mice was carried out in the brainstem under the same conditions, in a total of four samples per genotype. For phalloidin staining of human tissue, the cingulate cortex was fixed in 4% paraformaldehyde and sectioned on a vibratome at 30 μm. Sections were then incubated in Alexa Fluor 532 phalloidin for 2 hours and washed with PBS-Triton before mounting. Imaging acquisition was done in a Zeiss laser scanning microscope.
To assess mitochondrial morphology, Kenyon neurons and murine brainstem motor neurons were imaged by confocal microscopy. Mitochondria length and elongation were determined as previously described (Duboff et al., 2012; Dagda et al., 2009). Briefly, in two-dimensional projections of confocal z-stacks, individual mitochondrion diameter was measured by freehand line length in ImageJ. Similarly, mitochondria elongation was determine using the Mito-Morphology micro designed by Ruben K. Dagda at the University of Pittsburg and currently available via ImageJ wiki. A total of 25 mitochondria per sample was used to generate a frequency distribution (diameter) and bar graph (elongation). Mitochondria 3D reconstructions were generated from three-dimensional confocal z-stacks using the Imaris software. Reconstruction parameter were used as provided in the software.
Mitochondria and Drp1 colocalization were analyzed using the coloc2 plugin in ImageJ under the same imaging conditions as described above. To assess mitochondria function, superoxide levels and oxidation were measured in adult fly brains using the MitoSOX Red (Invitrogen) dye and the transgene MitoTimer, respectively. Quantification of MitoSOX and MitoTimer fluorescent was performed by averaging the pixel intensity from two-dimensional projections of confocal z-stacks using ImageJ. A total of six samples per genotype were used in all actin and mitochondrial analysis.
Electron microscopy
For mitochondrial morphology, brains from 20-day-old α-synuclein and control flies were dissected out of the cuticle and fixed in 2% paraformaldehyde and 2.5% glutaraldehyde (Polysciences). Following incubation in 1% osmium tetroxide (Electron Microscopy Sciences) in 1.5% potassium ferrocyanide (MP Biomedicals) for 1 hour, fly brains were incubated in 1% uranyl acetate for 30 minutes, and processed through 70, 90 and 100% ethanol solutions. Brains were then incubated in propylene oxide for 1 hour, embedded in Epon/Araldite mixture, and allowed to polymerize for 2 days at 65°C. Thin sections were cut and examined with a conventional Tecnai G2 Spirit BioTWIN transmission electron microscope.
Structural Illumination Microscopy
To assess mitochondrial, α-synuclein, F-actin, and α-spectrin interactions, 10-day-old fly brains were dissected and fluorescently labeled using standard protocols and mounted using Prolong Diamond antifade mounting medium (Invitrogen). Imaging of Drosophila Kenyon cells was performed using the Zeiss Elyra superresolution microscope. Image deconvolution was carryout using the provided software for the Zeiss Elyra. To assess protein colocalization, we determined the fluorescent spectral of each individual protein across a 1 μm distance in a three-dimensional SIM z-stacks using ImageJ.
F-actin isolation and detection
To determine F-actin concentration in α-synuclein transgenic flies, F-actin isolation was performed using the G-actin/F-actin assay (Cytoskeleton, Inc.). Briefly, ten heads from 10-day-old control and α-synuclein transgenic flies were homogenized in 30 μl of homogenization buffer containing lysis and F-actin stabilization buffer (Cytoskeleton, Inc.), 1 mM ATP and 1X protease inhibitor cocktail (Thermo Scientific). Homogenates were centrifuged (5 minutes at 1000 rpm) to pellet debris followed by ultracentrifugation at 37°C (100,000x g) for 1 hour to pellet F-actin. The pellet was solubilized by incubating in F-actin depolymerizing buffer for 1 hour. Soluble F-actin and G-actin fractions were subsequently immunoblotted. The concentrations of primary and secondary antibody were as described below.
Additionally, we used an F-actin ELISA (MyBiosource) to quantitatively assess F-actin levels. One fly brain was homogenized in 30 μl of homogenizing buffer containing F-actin stabilizing buffer (Cytoskeleton Inc.), 1 mM ATP, 1X protease inhibitor cocktail (Thermo Scientific) and 1X phosphatase inhibitor (Thermo Scientific). The sample was diluted 1:20 in sample diluent and subsequently transferred into a microplate pre-coated with a F-actin specific antibody. The microplate was then incubated with a biotinylated secondary antibody followed by HRP-avidin. F-actin concentrations were determined using the QuantaBlu fluorogenic peroxidase substrate (Thermo Scientific) as described in the manufacturer’s instructions. Each data point is the result of at least three biological replicates each composed of three technical replicates.
Phalloidin Precipitation
To assess α-synuclein, F-actin, and α-spectrin interactions biochemically, F-actin precipitation was performed as previously described in Fulga et al. (2007). Briefly, 10 fly heads were homogenized in F-actin stabilizing buffer (Cytoskeleton Inc.) and centrifuged at 800xg to pellet debris. The supernatant was incubated with 0.3 units biotinylated-phalloidin (Biotin-XX Phalloidin, Life technologies) with rotation for 1 hour at room temperature. Streptavidin-coated magnetic beads (Invitrogen) were blocked for 30 minutes in homogenization buffer, washed and resuspended in homogenization buffer. To isolate biotinylated phalloidin-bound protein complexes, streptavidin-coated beads were added to the extracts and incubated for 1 hour at room temperature with rotation. The precipitated material was then washed five times with homogenization buffer, resuspended in SDS loading buffer and subject to immunoblotting. The concentrations of primary and secondary antibody were as described below.
Mitochondrial fractionation
To determine mitochondrial Drp1 levels we performed mitochondria isolation in adult fly heads as previously described (Duboff et al., 2012). Briefly, 10 fly heads were homogenized in fractionation buffer (250 mM Sucrose, 10 mM Tris-HCl, 1 mM EGTA, pH 7.5) with protease inhibitor cocktail (Thermo Scientific). The homogenate was centrifuged at 800xg to pellet debris, and the supernatant collected and centrifuged at 11,000xg to yield a pellet containing mitochondria and supernatant containing cytoplasmic proteins. Subcellular fractions were subject to immunoblotting for detection of Drp1. The fractionation procedure was carried out three times in 1-day-old α-synuclein and control flies.
Insoluble α-synuclein insolation
Soluble and insoluble α-synuclein isolation was performed as previously described (Lee et al. 2002) with minor changes. Briefly, 10 fly heads were homogenized in TNE buffer (10mM Tris-HCl, pH 7.4, 150mM NaCl and 5mM EDTA) containing protease inhibitor cocktail (Thermo Scientific) and detergent (0.5% Nonidet P-40). The homogenate was centrifuged (5 minutes at 1,000 xg) to pellet debris, followed by ultracentrifugation (100,000 x g) at 4°C for 1 hour. The resulting pellet (P1) and supernatant (S1, soluble) fractions were collected. P1 was washed in TNE buffer containing Triton X-100, and the resulting pellet (P2, nonionic detergent-insoluble) was solubilized in TNE buffer containing 1% SDS. Soluble and insoluble α-synuclein were subsequently immunoblotted. The concentrations of primary and secondary antibody were as described above. α-synuclein fractionation was repeated three times.
Immunoblotting
For western blot analysis, Drosophila heads were homogenized in 15 μl of 2X Laemmli’s buffer (Sigma-Aldrich). Samples were boiled for ten minutes and subjected to SDS-PAGE. Proteins were transferred to nitrocellulose membranes (Bio-Rad), blocked in 2% milk in PBS with 0.05% Tween 20, and immunoblotted following standard protocols. All immunoblots were repeated at least three times with similar results. The following antibodies were used: anti-synuclein (1:100,000, Developmental Studies Hybridoma Bank, H3C), anti-GAPDH (1:100000, Invitrogen), anti-actin (1:10000, Developmental Studies Hybridoma Bank, JLA20), anti-HA.11 (1:1000, BioLegend), anti-porin (1:50000, Abcam) and anti-α-spectrin (1:5000, Developmental Studies Hybridoma Bank, 3A9). The appropriate horseradish peroxidase-conjugated secondary antibody (SouthernBiotech) was applied, and signal was detected by chemiluminescence (Alpha Innotech). Ponceau S staining was used to monitor protein transfer and equivalent protein loading, which was also documented by reprobing with an antibody to GAPDH.
Quantitative real time PCR
For reverse transcription, RNA was extracted from 6 fly heads using QIAZOL (Qiagen). RNA concentrations were measured with a Nanodrop ND-1000 Spectrophotometer and equal amounts of RNA were reverse transcribed using a cDNA Reserve Transcription Kit (Applied Biosystems), followed by qPCR. SYBR Green (Applied Biosystems) based qPCR was performed on an Applied Biosystems StepOnePlus Real-Time PCR System. Primer sequences for Act5C were AGGCCAACCGTGAGAAGATG (forward) and GGGGAAGGGCATAACCCTC (reverse). Primer sequences for Fhos were CCATTCACGCAGTGGATGCT (forward) and ACCCGCCAGAGTTTTGTTAATC (reverse). Primer sequences for Drp1 were ATGGAGGCCCTAATTCCGGT (forward) and GCTCTGACTGCCTAGAACAACA (reverse). Primer sequences for α-spectrin were CGACCGCCCCTATGTAACTA (forward) and CACGCAGTAGTCAGCCATGT (reverse). Each data point is the result of at least three biological replicates each composed of three technical replicates. RPL32 was used as the internal control gene. Primer sequences for RPL32 were GACCATCCGCCCAGCATAC (forward) and CGGCGACGCACTCTGTT (reverse).
QUANTIFICATION AND STATISTICAL ANALYSIS
Non-parametric statistical tests were used for all comparisons. Details regarding each statistical test, biological sample size (n) and p value can be found in the corresponding figure legends. All data is represented as mean ± SEM. SEM represents variance within a group. In all experiments, the control fly genotype can be found in the corresponding legends. Data were collected and processed side by side in randomized order for all experiments, however analysis were not routinely performed blind to the conditions of the experiments. Unpaired, two-tailed t-tests were used for comparison between two groups, with p<0.01 considered significant. For all comparisons involving multiple variables, one-way or two-way ANOVA was performed followed by Student-Newman-Keuls test for multiple comparison using p<0.01 for significance. For all experiments, between 4 and 10 animals per experiment were used, with the number per group stated in each figure legend. No statistical methods were used to predetermine sample sizes but the number of flies used and the number of biological replicates were large enough to obtain a dataset with statistical power and congruent with previously published studies in the field (for example, Fulga et al., 2007; Duboff et al., 2012; Lee et al. 2002). For experiments in mice and human tissue, sample sizes were based on power analysis calculations, which demonstrated that a 30% difference between four (mice) and eight (human) experimental and controls will achieve a power of 80%. In the main figures, locomotor ability was plotted using the mean only, when necessary to show the results clearly. Additional bar graphs have been included in the supplemental figures to show the appropriate statistical information (mean ± SEM). All statistical analyses were preformed using GraphPad Prism.
Supplementary Material
Highlights.
α-synuclein binds to and destabilizes the spectrin cytoskeleton.
Consequent reorganization of actin cytoskeleton impairs actin dynamics.
Actin-mediated Drp1 mislocalization impairs mitochondrial dynamics and function.
Overexpression of spectrin restores normal actin organization and rescues toxicity.
Acknowledgments
Fly stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537), the Vienna Drosophila Resource Center, H. Bellen, S. Bogdan, R. Dubreuil, C. Potter, T. Schwarz, and D. Williams were used in this study. We thank the Transgenic RNAi Project (TRiP) at Harvard Medical School (NIH-NIGMS R01GM084947) for making transgenic RNAi stocks. Monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biology, Iowa City, IA 52242. We thank Ms. Joyce Meints for technical assistance with mouse brain tissue processing. We acknowledge the Harvard Neurodiscovery Center Enhanced Neuroimaging Facility staff for assistance with confocal imaging and 3D reconstruction and the Harvard Center for Biological Imaging staff for insightful comments on super-resolution sample preparation and imaging. The work was supported by grants from the NIH to M.B.F. (R01-NS083391, R01-AG044113) and M.K.L. (R01-NS086074, R01-NS092093) and from the NSF to D.G.O. The authors declare no conflict of interest.
Footnotes
Authors Contribution: Conceptualization, D.G.O. and M.B.F.; Methodology, D.G.O. and M.B.F.; Investigation, D.G.O.; Writing - Original Draft, D.G.O., M.B.F. and M.K.L.; Writing - Review &Editing D.G.O., M.B.F. and M.K.L.; Funding Acquisition, D.G.O., M.K.L. and M.B.F.; Resources, M.B.F. and M.K.L.; Supervision, M.B.F.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baloyannis SJ, Costa V, Baloyannis IS. Morphological alterations of the synapses in the locus coeruleus in Parkinson’s disease. J Neurol Sci. 2006;248(1–2):35–41. doi: 10.1016/j.jns.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, Waters C, Jimison P, Shepherd E, Sevush S, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16(4):203–212. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- Bendor JT, Logan TP, Edwards RH. The function of α-synuclein. Neuron. 2013;79:1044–1066. doi: 10.1016/j.neuron.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose A, Beal MF. Mitochondrial dysfunction in Parkinson’s disease. J Neurochem. 2016;139(Suppl 1):216–231. doi: 10.1111/jnc.13731. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jasen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton M, Sudhof TC. α-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butzlaff M, Hannan SB, Karsten P, Lenz S, Ng J, Vossfeldt H, Prussing K, Pflanz R, Schulz JB, Rasse T, et al. Impaired retrograde transport by the Dynein/Dynactin complex contributes to Tau-induced toxicity. Hum Mol Genet. 2015;24(13):3623–3637. doi: 10.1093/hmg/ddv107. [DOI] [PubMed] [Google Scholar]
- Chen L, Feany MB. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson’s disease. Nat Neurosci. 2005;8(5):657–663. doi: 10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- Chen L, Periquet M, Wang X, Negro A, McLean PJ, Hyman BT, Feany MB. Tyrosine and serine phosphorylation of alpha-synuclein have opposing effects on neurotoxicity and soluble oligomer formation. J Clin Invest. 2009;119(11):3257–3265. doi: 10.1172/JCI39088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choubey V, Safiulina D, Vaarmann A, Cagalinec M, Wareski P, Kuum M, Zharkovsky A, Kaasik A. Mutant A53T alpha-synuclein induces neuronal death by increasing mitochondrial autophagy. J Biol Chem. 2011;286(12):10814–24. doi: 10.1074/jbc.M110.132514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Khurana V, Yi S, Sahni N, Loh KH, Auluck PK, Baru V, Udeshi ND, Freyzon Y, Carr SA, et al. In situ peroxidase labeling and mass-spectrometry connects alpha-Synuclein directly to endocytic trafficking and mRNA metabolism in neurons. Cell Syst. 2017;4:242–250. doi: 10.1016/j.cels.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Koprich JB, Siddiqi H, Isacson O. Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J Neurosci. 2009;29(11):3365–73. doi: 10.1523/JNEUROSCI.5427-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboff B, Gotz J, Feany MB. Tau promotes neurodegeneration via Drp1 mislocalization in vivo. Neuron. 2012;75(4):618–632. doi: 10.1016/j.neuron.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil RR, Grushko T. Genetic studies of spectrin: new life for a ghost protein. Bioessays. 1998;20:1, C4. doi: 10.1002/(SICI)1521-1878(199811)20:11<875::AID-BIES1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Feany MB, Bender W. A Drosophila model of Parkinson’s disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- Franco-Iborra S, Vila M, Perier C. The Parkinson disease mitochondrial hypothesis: Where are we at? The Neuroscientist. 2015 doi: 10.1177/1073858415574600. [DOI] [PubMed] [Google Scholar]
- Frenzel C, Herkel J, Luth S, Galle PR, Schramm C, Lohse AW. Evaluation of F-actin ELISA for the diagnosis of autoimmune hepatitis. Am J Gastroenterology. 2006;101:2731–2736. doi: 10.1111/j.1572-0241.2006.00830.x. [DOI] [PubMed] [Google Scholar]
- Frost B, Hemberg M, Lewis J, Feany MB. Tau promotes neurodegeneration through global chromatin relaxation. Nat Neurosci. 2014;17(3):357–366. doi: 10.1038/nn.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. α-synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- Fulga TA, Elson-Schawb I, Khurana V, Steinhilb ML, Spires TL, Hyman BT, Feany MB. Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nat Cell Biol. 2007;9:139–148. doi: 10.1038/ncb1528. [DOI] [PubMed] [Google Scholar]
- Gillardon F, Mack M, Rist W, Schnack C, Lenter M, Hildebrandt T, Hengerer B. MicroRNA and proteome expression profiling in early-symptomatic α-synuclein(A30P)-transgenic mice. Proteomics Clin Appl. 2008;2(5):697–705. doi: 10.1002/prca.200780025. [DOI] [PubMed] [Google Scholar]
- Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Reviews. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- Hatch AL, Gurel PS, Higgs HN. Novel roles for actin in mitochondrial fission. J Cell Sci. 2014;127(Pt 21):4549–60. doi: 10.1242/jcs.153791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Zhou R, Wu Z, Carrasco MA, Kurshan PT, Farley JE, Simon DJ, Wang G, Han B, Hao J, et al. Prevalent presence of periodic actin–spectrin-based membrane skeleton in a broad range of neuronal cell types and animal species. PNAS. 2010;113:6029–6034. doi: 10.1073/pnas.1605707113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez DG, Reed X, Singleton AB. Genetics in Parkinson’s disease: Mendelian versus non-Medellian inheritance. J Neurochem. 2016;139:59–74. doi: 10.1111/jnc.13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji WK, Hatch AL, Merrill RA, Strack S, Higgs HN. Actin filaments target the oligomeric maturation of the dynamin GTPase Drp1 to mitochondrial fission sites. Elife. 2015;4:e11553. doi: 10.7554/eLife.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinar DP, Balija MBG, Kugler S, Opazo F, Rezaei-Ghaleh N, Wender N, Kim HYY, Taschenberger G, Falkenburger BHH, Heise H. Pre-fibrillar alpha-synuclein variants with impaired beta-structure increase neurotoxicity in Parkinson’s disease models. EMBO J. 2009;28(20):3256–3268. doi: 10.1038/emboj.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korabova F, Ramabhadran V, Higgs HN. An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science. 2013;339:464–467. doi: 10.1126/science.1228360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, Wagner U, Dumont B, Pikkarainen M, Osman AA, Streichenberger N, Leisser I, Verchere J, Baron T, Alafuzoff I, et al. An antibody for high reactivity for disease-assosicated α-synuclein reveals extensive brain pathology. Acta Neuropathol. 2012;124(1):37–50. doi: 10.1007/s00401-012-0964-x. [DOI] [PubMed] [Google Scholar]
- Khurana V, Lu Y, Steinhilb ML, Oldham S, Shulman JM, Feany MB. TOR-mediated cell-cycle activation causes neurodegeneration in a Drosophila tauopathy model. Curr Biol. 2006;16(3):230–241. doi: 10.1016/j.cub.2005.12.042. [DOI] [PubMed] [Google Scholar]
- Khurana V, Peng J, Chung CY, Auluck PK, Fanning S, Tardiff DF, Bartels T, Koeva M, Eichhorn SW, Benyamini H, et al. Genome-scale networks link neurodegenerative disease genes to α-synuclein through specific molecular pathways. Cell Syst. 2017;4(2):157–170. doi: 10.1016/j.cels.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacker AC, Xu P, Ryall KA, Sujkowski A, Kenwood BM, Chain KH, Zhang M, Royal MA, Hoehn KL, Driscoll M, et al. A novel MitoTimer reporter gene for mitochondrial content, structure, stress, and damage in vivo. J Biol Chem. 2013;289(17):12005–12015. doi: 10.1074/jbc.M113.530527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel U, Bechtold M, Risse B, Berh D, Fleige A, Bunse I, Jiang X, Klambt C, Bogdan S. The Drosophila FHOD1-like formin Knittrig acts through Rok to promote stress fiber formation and directed macrophage migration during the cellular immune response. Development. 2014;141(6):1366–1380. doi: 10.1242/dev.101352. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Lee K, Im H. α-synuclein modulates neurite outgrowth by interacting with SPTBN1. Biochem, and Biophis Res Comm. 2012;424:497–502. doi: 10.1016/j.bbrc.2012.06.143. [DOI] [PubMed] [Google Scholar]
- Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG, Jenkins NA, Price DL. Human α-synuclein-harboring familial Parkinson’s disease-linked Ala-53→Thr mutation causes neurodegenerative disease with α-synuclein aggregation in transgenic mice. PNAS. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverenz JB, Umar I, Wang Q, Montine TJ, MacMillan PJ, Tsuang DW, Jin J, Pan C, Shin J, Zhu D, et al. Proteomic identification of novel proteins in cortical lewy bodies. Brain Pathol. 2007;17:139–145. doi: 10.1111/j.1750-3639.2007.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Zhou J, Budhraja A, Hu X, Chen Y, Chen Q, Liu L, Zhou T, Li P, Liu E, et al. Mitochondrial translocation and interaction of cofilin and Drp1 are required for erucin-induced mitochondrial fission and apoptosis. Oncotarget. 2014;6(3):1834–1849. doi: 10.18632/oncotarget.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MK, Farrer MJ. Genetics and genomics of Parkinson’s disease. Genome Med. 2014;6(6):48. doi: 10.1186/gm566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Parisiadou L, Sgobio C, Liu G, Sun L, Shim H, Gu XL, Luo J, Long CX, Ding J, et al. Conditional expression of Parkinson’s disease-related mutant α-synuclein in the midbrain dopaminergic neurons causes progressive neurodegeneration and degradation of transcription factor nuclear receptor related 1. J Neurosci. 2012;32(27):9248–9264. doi: 10.1523/JNEUROSCI.1731-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machnicka B, Czogalla A, Hryniewicz-Jankowska A, Boguslawska DMM, Grochowalska R, Heger E, Sikorski AF. Spectrin-based skeleton as an actor in cell signaling. Cell Mol Life Sci. 2012;69:191–201. doi: 10.1007/s00018-011-0804-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, Price DL, Lee MK. Parkinson’s disease α-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. Neurobiol Dis. 2006;26(1):41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, Chaudhuri KR NMSS Validation Group. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord. 2011;26:399–406. doi: 10.1002/mds.23462. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- McFarland MA, Ellis CE, Markey SP, Nussbaum RL. Proteomics analysis identifies phosphorylation-dependent alpha-synuclein protein interactions. Mol Cell Proteomics. 2008;7:2123–2137. doi: 10.1074/mcp.M800116-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, et al. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- Menges S, Minakaki G, Schaefer PM, Meixner H, Prots I, Schlötzer-Schrehardt U, Friedland K, Winner B, Outeiro TF, Winklhofer KF, et al. Alpha-synuclein prevents the formation of spherical mitochondria and apoptosis under oxidative stress. Sci Rep. 2017;7:42942. doi: 10.1038/srep42942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AS, Wong YC, Simpson CL, Holzbaur EL. Dynamic actin cycling through mitochondrial subpopulations locally regulates the fission-fusion balance within mitochondrial networks. Nat Commun. 2016;7:12886. doi: 10.1038/ncomms12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Nemani VM, Azarbal F, Skibinski G, Levy JM, Egami K, Munishkina L, Zhang J, Gardner B, Wakabayashi J, et al. Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J Biol Chem. 2011;286(23):20710–20726. doi: 10.1074/jbc.M110.213538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell KC, Lulla A, Stahl MC, Wheat ND, Bronstein JM, Sagasti A. Axon degeneration and PGC-1α-mediated protection in a zebrafish model of α-synuclein toxicity. Dis Model Mech. 2014;7(5):571–582. doi: 10.1242/dmm.013185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. An Essay on the Shaking Palsy. Synopsis Nosologiae Methodicae -Tom ii. 1817:195. [Google Scholar]
- Periquet M, Fulga T, Myllykangas L, Schlossmacher MG, Feany MB. Aggregated alpha-synuclein mediates dopaminergic neurotoxicity in vivo. J Neurosci. 2007;27(12):3338–3346. doi: 10.1523/JNEUROSCI.0285-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuralinna T, Myllykangas L, Oinas M, Nalls MA, Keage HA, Isoviita VM, Valori M, Polvikoski T, Paetau A, Sulkava R, et al. Genome-wide association study of neocortical Lewy-related pathology. Ann Clin Transl Neurol. 2015;2(9):920–931. doi: 10.1002/acn3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter CJ, Tasic B, Russler EV, Liang L, Luo L. The Q System: A repressible binary system for transgene expression, lineage tracing and mosaic analysis. Cell. 2010;141(3):536–548. doi: 10.1016/j.cell.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulopoulos M, Levy OA, Alcalay RN. The neuropathology of genetic Parkinson’s disease. Mov Dosord. 2012;27(7):831–842. doi: 10.1002/mds.24962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riabinina O, Luginbuhl D, Marr E, Liu S, Wu MN, Luo L, Potter CJ. Improved and expanded Q-system reagents for genetic manipulations. Nat Methods. 2015;12(3):219–222. doi: 10.1038/nmeth.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, Murphy MP, Beckman JS. Selective fluorescent imaging of superoxide in in vivo using ethidium based probes. PNAS. 2006;103:15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem. 1990;54(3):823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Shulman JM, De Jager PL, Feany MB. Parkinson’s disease: genetics and pathogenesis. Ann Rev Pathol. 2011;6:193–222. doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- Sousa VL, Bellani S, Giannandrea M, Yousuf M, Valtorta F, Meldolesi J, Chieregatti E. α-synuclein and its A30P mutant affect actin cytoskeletal structure and dynamics. Mol Biol Cell. 2009;16:3725–3739. doi: 10.1091/mbc.E08-03-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Wagner CR, Mahowald AP, Miller KG. One of the two cytoplasmic actin isoforms in Drosophila is essential. Proc Natl Acad Sci. 2002;99:8037–8042. doi: 10.1073/pnas.082235499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann CW, Wszolek MF, Shulman JM, Salvaterra PM, Lewis J, Hutton M, Feany MB. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science. 2001;293(5530):711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- Wong YC, Krainc D. α-synuclein toxicity in neurodegeneration: mechanisms and therapeutics strategies. Nat Med. 2017;23:1–13. doi: 10.1038/nm.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xun Z, Sowell RA, Kaufman TC, Clemmer DE. Quantitative proteomics of a presymptomatic A53T alpha-synuclein Drosophila model of Parkinson disease. Mol Cell Proteomics. 2008;7(7):1191–1203. doi: 10.1074/mcp.M700467-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccai J, McCracken C, Brayne C. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age Ageing. 2005;34(6):561–566. doi: 10.1093/ageing/afi190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.