Abstract
Impaired cardiac microvascular function contributes to diabetic cardiovascular complications although effective therapy remains elusive. Empagliflozin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor recently approved for treatment of type 2 diabetes, promotes glycosuria excretion and offers cardioprotective actions beyond its glucose-lowering effects. This study was designed to evaluate the effect of empagliflozin on cardiac microvascular injury in diabetes and the underlying mechanism involved with a focus on mitochondria. Our data revealed that empagliflozin improved diabetic myocardial structure and function, preserved cardiac microvascular barrier function and integrity, sustained eNOS phosphorylation and endothelium-dependent relaxation, as well as improved microvessel density and perfusion. Further study suggested that empagliflozin exerted its effects through inhibition of mitochondrial fission in an adenosine monophosphate (AMP)-activated protein kinase (AMPK)-dependent manner. Empagliflozin restored AMP-to-ATP ratio to trigger AMPK activation, suppressed Drp1S616 phosphorylation, and increased Drp1S637 phosphorylation, ultimately leading to inhibition of mitochondrial fission. The empagliflozin-induced inhibition of mitochondrial fission preserved cardiac microvascular endothelial cell (CMEC) barrier function through suppressed mitochondrial reactive oxygen species (mtROS) production and subsequently oxidative stress to impede CMEC senescence. Empagliflozin-induced fission loss also favored angiogenesis by promoting CMEC migration through amelioration of F-actin depolymerization. Taken together, these results indicated the therapeutic promises of empagliflozin in the treatment of pathological microvascular changes in diabetes.
Keywords: Empagliflozin, Mitochondrial fission, Microvascular, CMECs, AMPK
Highlights
-
•
Empagliflozin alleviates diabetes-induced cardiac microvascular dysfunction.
-
•
Hyperglycemia triggers CMEC dysfunction via mitochondrial fission.
-
•
Empagliflozin inhibits fission and delays CMEC senescence via suppressing mtROS oxidative stress.
-
•
Empagliflozin facilitates CMEC migration and neovascularization by preservation of F-actin homeostasis.
1. Introduction
The cardiac microvasculature, which primarily consists of cardiac microvascular endothelial cell (CMEC) located at the circulatory terminus, governs myocardial perfusion and coronary reserve [1]. Given the direct contact between the microvasculature and blood flow, CMECs are more vulnerable to hyperglycemic damage as opposed to cardiomyocytes. With the onset and development of diabetes mellitus, impaired CMEC viability and cell migration occur and contribute to the compromised endothelial regulation of vascular homeostasis, favoring a pro-inflammatory state that ultimately results in vascular rarefaction and diabetic vasculopathy [2]. In consequence, dampened myocardial perfusion and cardiac ischemia develop due to a shortage of vascular supply to cope with the cardiac demand, predisposing diabetic patients to cardiovascular complications [3]. Therefore, the hunt for means to protect the cardiac microvasculature against hyperglycemic damage is essential to retard or alleviate diabetic macrovascular complications [4].
Empagliflozin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, was recently developed as an anti-diabetic agent to promote urinal glucose excretion or glycosuria in an insulin-independent manner [5], [6]. Empagliflozin may also lower cardiovascular mortality, all-cause mortality and hospitalization for heart failure by 38%, 35% and 32%, respectively, over a median duration of 3.1 years [7]. In addition to the improved glycemic parameters, empagliflozin helps to switch body metabolism towards lipid utilization and reduces systolic blood pressure in the absence of tachycardia [8], thus favoring a cardioprotective property of the SGLT2 inhibitor [9]. Nonetheless, the impact of empagliflozin in diabetic microvascular complications, particularly cardiac microvascular damage, remains largely unknown.
The mitochondrial content is modest in endothelial cells compared to other cell types with a higher energy requirement, indicating a primary role for mitochondria as essential signaling organelles in vascular endothelium [10], [11]. Abnormal mitochondrial dynamics, in particular mitochondrial fission, has been reported to play an important role in the pathogenesis of diabetic nephropathy microvascular complications [12] through mechanism in part associated with transmission of glucose toxic signals [13]. Nonetheless, whether empagliflozin treatment alleviates cardiac microvascular injury in particular by way of mitochondrial fission remains unknown. As a result of excessive mitochondrial fission, cristae disorganization, membrane permeabilization and release of pro-apoptotic proteins [14], [15] develop and contribute to cellular death, en route to the ultimate cardiac-renal injury in hyperglycemia [12]. In addition to cell death, blunted migration and cellular senescence of endothelial cells are also involved in diabetic microvascular damage. However, little is known with regards to the role of mitochondrial fission in CMEC migration and senescence.
Dynamin-related protein 1 (Drp1) serves as a critical effector of mitochondrial fission given the redistribution of GFP-tagged Drp1 from a predominant cytosolic location to predicted sites of division along mitochondrial tubules [16], [17]. Drp1 phosphorylation is a permissive step to facilitate the shift of Drp1 onto mitochondria, a process regulated by post-translational events including phosphorylation, sumoylation, ubiquitination, and nitrosylation [18], [19]. Several upstream signaling molecules were reported to promote Drp1 activation, including Rho-associated coiled-coil containing protein kinase (ROCK1) and AMPK. ROCK triggers fission by phosphorylating Drp1 at the Serine616 (Ser616) residue in kidney podocytes and endothelial cells [12]. In contrast, Drp1 is inactivated via phosphorylation at Ser637 in response to AMPK activation in aortic endothelial cells [20]. These data support a pivotal role for phosphorylation in the regulation of Drp1 and its translocation onto mitochondrial membranes. However, whether empagliflozin affects diabetic microvascular complications and mitochondrial quality through Drp1 modification remains largely unexplored. Here, our data suggested beneficial response of empagliflozin on cardiac microvascular structure and function, revealing an important role for the SGLT2 inhibitor in mitochondrial fission and CMEC viability, migration and proliferation.
2. Methods
2.1. Animal procedure and drug treatment
All experimental procedures described here were in accordance with the National Institutes of Health Guidelines on the Care and Use of Laboratory Animal and were approved by the PLA General Hospital Institutional Animal Care and Use Committee. In brief, 8-week-old C57BL/6J wild-type (WT) mice were intraperitoneally injected with streptozotocin (STZ, 50 mg/kg) for 5 consecutive days. After the first 4 weeks of diabetes (confirmed by blood glucose levels > 16 mmol/L after a six-hour daytime fasting), mice (12-week-old) were treated with empagliflozin (10 mg/kg/d, defined as the empagliflozin group) or the mitochondrial fission inhibitor mdivi-1 (1.2 mg/kg/d, designed as the mdivi1 group) for 20 weeks. At the end of treatment, all mice (32-week-old) were euthanized, and hearts were collected for further experimentations. Empagliflozin was provided by the Boehringer Ingelheim Pharma GmbH & Co. KG, Germany, and was administered via oral gavage (0.5% hydroxyethylcellulose was used as the vehicle).
2.2. Echocardiogram and myocardial contrast echocardiography (MCE)
Echocardiography was performed using echocardiogram (14.0 MHz, Sequoia C512; Acuson, Germany) to monitor changes in cardiac function [14]. Myocardial contrast echocardiography (MCE) was performed using a 14 MHz linear transducer (Acuson Sequoia C512 system) with the constant infusion of microbubbles [10% Perflutren lipid microspheres (Definity, Lantheus Imaging) diluted tenfold in sterile saline] at 20 mL/min as previously described by our group [1].
2.3. Microvascular imaging using gelatin-ink perfusion
Gelatin-ink perfusion was carried out using ink warmed to 37 °C along with 3% gelatin (gelatin-ink staining via jugular vein perfusion). Room temperature was maintained at 25–30 °C. When the limbs turned black, the great vessels of the cardiac base and the superior and inferior vena cava were separated and were ligated. The hearts were subsequently maintained at 4 °C for at least 1 h, prior to heart removal and fixation in 4% paraformaldehyde and processed for cryosectioning [21].
2.4. CMEC culture in vitro
CMECs were isolated from the hearts of normal, diabetic, empagliflozin-treated diabetic mice and diabetic mice treated with Mdivi1 (a mitochondrial fission inhibitor) using the enzyme dissociation method described previously by our group [22]. The purity of cultured cells was assessed by performing CD31 staining and the uptake of acetylated low-density lipoprotein. To induce mitochondrial fission, CMECs obtained from empagliflozin-treated mice were treated with 5 μM FCCP for 2 h.
2.5. CMEC oxidative damage detection
Reactive oxygen species (ROS), an indicator of oxidant status, are involved in the injury of CMECs in the setting of diabetic oxidative stress. Both DCF-DA (10 μM) (Invitrogen, Germany) and MitoSOX™ Red Mitochondrial Superoxide Indicator (Molecular Probes, USA) were used to monitor the change of intracellular ROS and mitochondrial ROS (mtROS), respectively, using a laser confocal microscope (Olympus) [23].
2.6. Flow cytometry
For cell cycle analysis, CMECs were isolated from the heart and were resuspended in phosphate-buffered saline (PBS) followed by fixation with ice-cold 70% ethanol for 24 h. The fixed cells were rinsed and then resuspended in 50 μg/mL propidium iodide for 30 min in the dark [24]. Flow cytometric analysis was performed using a BD FACSCalibur cytometer as described previously [25].
2.7. Migration and wound healing assay
The migration assay of CMECs was evaluated using 24-well transwell chambers (Coring, USA). First, 1 × 105 CMECs were seeded in the upper chamber containing serum-free media. The chemotactic agent SDF-1 (100 ng/mL, Sigma-Aldrich, USA) was added to the lower chamber to induce CMEC migration [26]. After a 12-h incubation at 37 °C, non-migrating cells in the upper chamber were carefully removed using a cotton swab, cells that traversed through the membrane were fixed in methanol, stained with 0.05% crystal violet [27].
As to the wound healing assay, 1 × 106 CMECs were seeded onto 36-mm plates to reach 80–90% confluency. An artificial wound was performed using P200 pipette tip scratching on the confluent cell monolayer [28]. Photomicrograph was taken immediately (time 0 h), then cells were incubated in DMEM containing 1% fetal bovine serum. The mobilization of cells and closing of scratch wound were observed 24 h later.
2.8. Detection of CMEC permeability and transendothelial electrical resistance (TER)
A FITC-dextran clearance assay was performed to monitor changes in CMEC permeability. Cells were incubated with FITC-dextran (final concentration: 1 mg/mL), and were allowed to permeate through the cell monolayer. Two hours later, the FITC content remaining in the plate was measured using a fluorescent plate reader (Bio-Rad, USA) to detect the extent of permeability. TER is a measure of ionic conductance of endothelial cells and is used to assess junctional function. TER decreases when endothelial cells retract or lose adhesion. Using an in vitro Vascular Permeability Assay Kit (ECM640, Millipore, USA), CMECs were seeded onto collagen-coated inserts at a density of 100,000 cells/insert. After reaching confluence, an electrical endothelial resistance system (Millipore, USA) was used to measure TER per our previous description [1].
2.9. Angiogenesis in vitro (capillary tube formation assay)
The in vitro endothelial tube formation assay was performed as previously described [29]. Briefly, 100 μL of Matrigel (BD Bioscience) was added to each well of a 24-well plate and allowed to polymerize at 37 °C for 30 min [30]. CMECs were suspended in FBS-free DMEM medium and seeded in each well at a concentration of 1 × 105 cells/well. Cells were incubated at 37 °C. Each treatment was repeated in triplicates [31]. After 6 h, cells were examined under a light microscope to assess the formation of capillary-like structures. The branch points of the formed tubes, which represent the degree of angiogenesis in vitro, were scanned and quantified in five low-power fields (200×).
2.10. Measurement of endothelial relaxation function in thoracic aorta
Thoracic aorta was immediately dissected and immersed in chilled Krebs–Henseleit (K-H) solution and bubbled with 95% O2 and 5% CO2 (pH 7.4). The descending aortic rings were pre-contracted with U46619 (30 nM). At the plateau of contraction, endothelium-dependent and -independent vasodilation were determined using ACh (10−9–10−5 M) and SNP (10−10–10−6 M), respectively, at the given concentration after 15-min incubation in the bath.
2.11. Histopathological analysis, immunohistochemistry, and immunofluorescence staining
Myocardial tissues were fixed in 4% paraformaldehyde, dehydrated, and embedded in paraffin followed by dehydration in graded ethanol solutions and in toluene. Five-micron-thick sections were stained with hematoxylin and eosin (H.E.) or Masson's stain and examined via light microscopy for histopathological analysis. Microvascular density was observed using a CD31 antibody (1:1500, Abcam), eNOS phosphorylation (Ser1177) (1:1000, Abacm) and endothelial barrier integrity was assessed with VE-cadherin (1:1000, Abcam). For immunofluorescence staining in vitro, cells were fixed with 4% paraformaldehyde, permeabilized with 0.3% Triton X-100, and blocked with 10% goat serum albumin (Invitrogen, USA.). Specimens were subsequently incubated with primary antibodies against Drp1 (1:1000, Abcam) and F-actin (1:1000, Abcam). DAPI staining (Sigma-Aldrich, USA) and mitochondrion-selective MitoFluor™ staining (Molecular Probes, USA) were performed to label nuclei and mitochondria, respectively [32].
2.12. Western blot analysis
Proteins (60–80 µg) were loaded on a 10–15% sodium dodecyl sulfate-polyacrylamide gel. After electrophoresis, proteins were transferred to a PVDF membrane. Membrane were blocked with 5% nonfat dried milk (in TBST) for 2 h at room temperature and then incubated overnight at 4 °C with primary antibodies. The membrane was subsequently washed with TBST (5 min × 3) and incubated with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology) for 1 h at room temperature [33]. After washing with TBST (5 min × 3), bands were detected by enhanced chemiluminescence substrate (Applygen). The antibodies used in the present were as follows: Drp1 (1:1000, Abcam, #ab56788), p-Drp1 (Ser616) (1:1000, Cell Signaling Technology, #3455), p-Drp1 (Ser637) (1:1000, Abcam, #ab193216), Fis1 (1:1000, Abcam, #ab71498), Mfn2 (1:1000, Abcam, #ab56889), Mfn1 (1:1000, Abcam, #ab57602), Mff (1:1000, Cell Signaling Technology, #86668), F-actin (1:1000, Abcam, #ab205), G-actin (1:1000, Abcam, #ab200046), p-eNOS (Ser1117) (1:1000, Abcam, #ab184154), ICAM1 (1:1000, Abcam, #ab119871), VCAM1 (1:1000, Abcam, #ab134047), AMPK (1:1000, Abcam, #ab131512), p-AMPK (1:1000, Abcam, #ab23875) [34].
2.13. Electron microscopy
Tissues were fixed in 2.5% glutaraldehyde at 4 °C. After fixation in 1% osmium tetraoxide, the tissues were dehydrated and then embedded in araldite CY 618. Slides (50 nm) were stained with lead citrate acid and uranium acetate to observe cardiac microvasculature through a Hitachi H600 Electron Microscope (Hitachi, Japan) [24]. Images were at 15,000× magnification.
2.14. Statistical analysis
Data were presented as mean ± standard deviation (SD) from at least three independent experiments and were analyzed using one-way analysis of variance (ANOVA). Statistical significance was set at P < 0.05.
3. Results
3.1. Empagliflozin improved diastolic function and countered myocardial fibrosis in diabetes
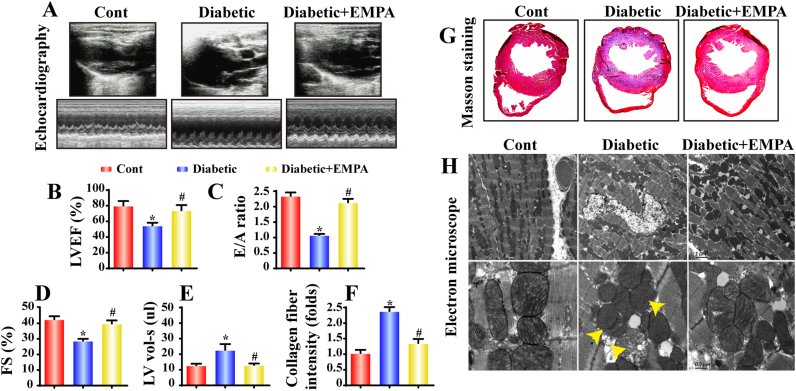
Echocardiography was performed to evaluate changes in cardiac function. Compared with the control group, diabetic mice exhibited overtly dampened diastolic function as manifested by a decline in the E/A ratio and an increased left ventricular volume in systole (LV vol-s), the effect of which was reversed by empagliflozin (Fig. 1A–E). These functional changes were attributable to alterations in cardiac structure. Histological analysis using Masson Trichrome's staining revealed significantly elevated collagen fiber deposition in diabetes, the effect of which was negated by empagliflozin (Fig. 1F–G). In addition, electron microscopy (EM) evaluation revealed overt cardiomyocyte dissolution, muscular fiber twisting and Z line disappearance in diabetes, the effects of which were greatly attenuated by empagliflozin treatment (Fig. 1H). Furthermore, empagliflozin treatment helped to maintain mitochondrial integrity in diabetes as evidenced by the appearance of fragmented mitochondria characterized by crista deformation (yellow arrows presented in Fig. 1H). These data favor a cardioprotective property of empagliflozin on diabetic myocardial structural and functional damages. Furthermore, the effects of empagliflozin on hemodynamics and body weight were evaluated. Diabetic mice exhibited a lower body weight gain and higher levels of systolic blood pressure (SBP) and blood glucose. Not surprisingly, empagliflozin improved body weight gain and decreased the levels of SBP and blood glucose possibly by promoting glycosuria (Table 1).
Fig. 1.
Effect of empagliflozin treatment on cardiac function and morphology in diabetes: Experimental diabetes was induced with STZ and mice were treated with empagliflozin (10 mg/kg/d) for 20 weeks (n = 6/group). A. Representative M-mode echocardiographic images collected using the parasternal long-axis view in each group; B–E. Quantitative analysis of cardiac function performed using echocardiography; F–G. Cardiac fibrosis following empagliflozin treatment assessed using Masson's staining; H. Representative electron microscopy images of cardiomyocytes in each group. Diabetes led to myocyte dissolution, muscular fiber twists and Z line disappearance, the effects of which were reversed by empagliflozin. Diabetes caused mitochondrial swelling and crista fragmentation (yellow arrow), which were in contrast with the clearly visible and regular mitochondria seen in controls. Empagliflozin recovered diabetes-distorted mitochondrial structural integrity. Mean ± SD, *P < 0.05 vs. Control (Cont) group; #P < 0.05 vs. Diabetic group.
Table 1.
Physical and clinical parameters.
| Ctrl | Diabetic | EMPA | |
|---|---|---|---|
| Gain in weight (g) | 5.8 ± 0.6 | 2.4 ± 0.3* | 3.9 ± 0.7** |
| Average blood sugar(mmol/L) | 11.2 ± 0.3 | 21.6 ± 0.4* | 11.8 ± 0.5** |
| Systolic BP (mm Hg) | 118 ± 3 | 122 ± 2 | 119 ± 3 |
| 24 h urine glucose(μmol/day) | 3.6 ± 0.4 | 3125.3 ± 796.2* | 463.2 ± 82.5** |
| Left kidney/body weight ratio (%) | 0.69 ± 0.03 | 0.92 ± 0.04 | 0.83 ± 0.04 |
Data are mean ± standard error of mean. (n = 6/group).
P < 0.05 vs. control group.
P < 0.05 vs. diabetic group.
3.2. Empagliflozin improved myocardial microcirculatory perfusion through increased microvessel density and lessened vascular remodeling
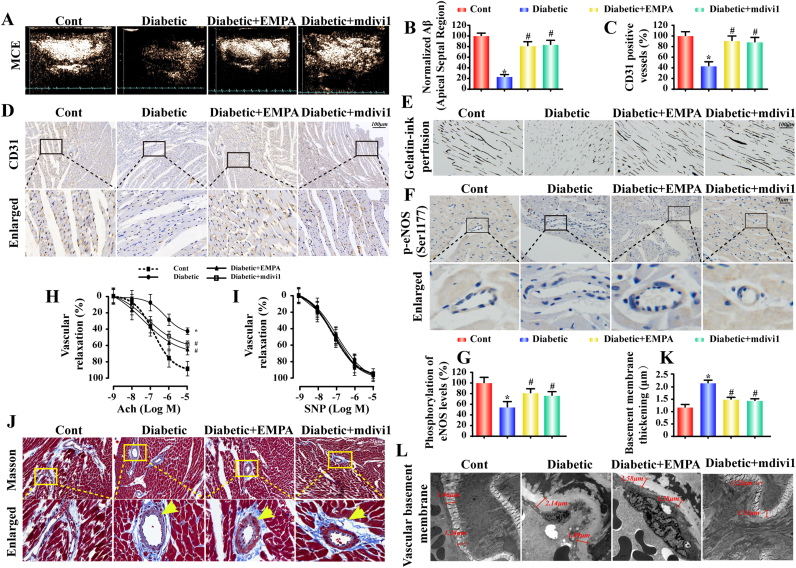
Myocardial function is dependent upon continuous blood flow governed by microcirculation, which is susceptible to hyperglycemic damage. MCE was performed to evaluate myocardial microcirculatory perfusion. Compared to the control group, diabetic mice exhibited a decrease in microvascular perfusion as shown by scattered perfusion defects (Fig. 2A–B). However, mice in empagliflozin-treated diabetic group developed much smaller zones of perfusion defect. Similar results were obtained in mdivi1-treated diabetic mice.
Fig. 2.
Empagliflozin improves myocardial microcirculation perfusion by increasing microvessel density and reducing vascular remodeling. A. Representative MCE images were taken using a constant infusion of microbubbles at 20 mL/min. The signal intensity was determined by capturing a 10-s high-energy sequence at a frame rate of 30 Hz;.B. A quantitative analysis of perfusion was performed using Research-Arena software (Tomtech, Germany). A: plateau intensity, β: flow velocity. Myocardial blood flow (A × β) profiles in the basal septum for different groups (n = 6/group); C–D. Frozen cardiac sections were incubated with CD31 antibody to assess microvascular numbers by performing fluorescence microscopy; E. Microvascular image detection via ink staining; F–G. Immunohistochemistry of p-eNOS (Ser1177) expression; H–I. Endothelial-dependent and endothelial-independent relaxation responses were assessed by applying Ach (10−9–10−5 M) or SNP (10−10–10−6 M). J. Histological analysis of vascular fibrosis with Masson's staining; K–L. TEM was performed to observe changes in the vascular basement membrane. Diabetes induced hyperplasia of the vascular basement, which was reversed by empagliflozin. Mean ± SD, *P < 0.05 vs. Control (Cont) group; #P < 0.05 vs. Diabetic group.
To discern the underlying mechanism, fundamental microcirculatory perfusion factors including microvascular density, vessel stenosis, eNOS phosphorylation (Ser1177) and endothelial relaxation function, were evaluated. As illustrated in Fig. 2C–D, diabetes triggered a drop in the number of CD31+ microvessels, similar to the vascular ink staining depicting the sparse of vascular beds (Fig. 2E), the effects of which were reversed by empagliflozin. Moreover, diabetes-induced decrease in eNOS phosphorylation (Ser1177) was reinstated by empagliflozin (Fig. 2F–G). Changes in eNOS phosphorylation in the microvasculature in response to diabetes and empagliflozin were echoed by the responses in endothelial relaxation in isolated aorta (Fig. 2H–I). In accordance with the functional alterations, diabetes-induced changes in microvascular morphology including fibrosis (Fig. 2J) and basement membrane thickening (Fig. 2K–L) (assessed by Masson's staining and TEM, respectively) were largely ameliorated by empagliflozin, suggesting a role for empagliflozin to retard remodeling in diabetic vasculopathy. Notably, treatment with the mitochondrial fission inhibitor mdivi1 in diabetic mice also increased microvessel density and lessened vascular remodeling.
3.3. Empagliflozin sustained cardiac microvessel integrity and barrier function in diabetes
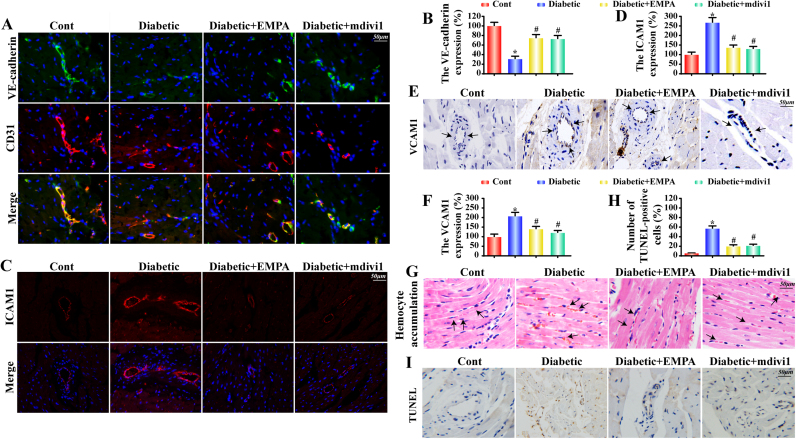
Damage to microvessel integrity and barrier function is considered the initial step in vascular complications in diabetes. VE-cadherins, junctional proteins expressed on endothelial cells, are vital players for endothelial integrity and vascular permeability. Our data revealed that diabetes significantly downregulated VE-cadherin fluorescence in the microvasculature, the effect of which was nullified by empagliflozin (Fig. 3A–B). In addition, diabetes significantly upregulated levels of adhesive proteins including ICAM1 and VCAM1, the effects of which were reversed by empagliflozin (Fig. 3C–F). These diabetes-induced effects should impact the accumulation of erythrocytes in the microvessels due to the stop of turbulent blood flow or the secondary effect owing to the capillary blockage by other cells such as leukocytes. However, empagliflozin considerably retarded such changes (Fig. 3G). These data collectively revealed the beneficial effects of empagliflozin on the microvessel integrity and barrier function in diabetes, favoring a significant decrease for the risk for vascular inflammation and potential microthrombus formation. Similar results were obtained in mdivi1-treated group. Furthermore, the collapse of microvessel integrity and barrier function may be the result of CMEC dysfunction or death given the greater numbers of TUNEL+ cells appeared in diabetic hearts. Interestingly, empagliflozin or mdivi1 prevented diabetes-induced rises in the percentage of TUNEL+ cells (Fig. 3H–I), supporting the pro-survival capacity of empagliflozin on hyperglycemia-mediated CMEC apoptosis.
Fig. 3.
Empagliflozin maintains microvessel integrity and barrier function. A–B. The co-immunofluorescence of VE-cadherin and CD31 indicates the continuity of gap junctional proteins; C–D. Representative fluorescence images for intercellular adhesion molecule (ICAM)-1; E–F. Immunohistochemical staining of vascular cell adhesion molecule (VCAM)-1; G. HE staining to assess red blood cell adhesion to microvessel walls in different groups; H–I. A TUNEL assay was performed to assess CMEC apoptosis. Mean ± SD, *P < 0.05 vs. Control (Cont) group; #P < 0.05 vs. Diabetic group.
3.4. Empagliflozin diminished diabetes-induced mitochondrial fragmentation and regulated the balance of proteins related to mitochondrial fission and fusion
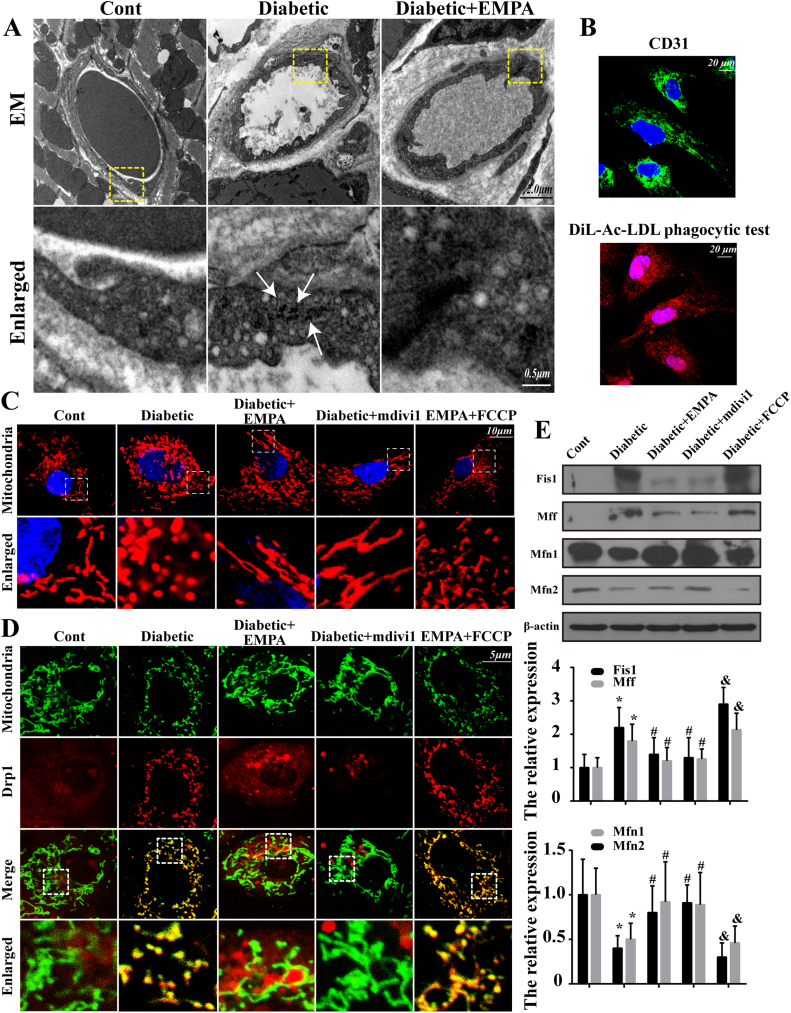
To determine the possible mechanism(s) of action through which empagliflozin protects the microvasculature against diabetic injury, mitochondrial fission was monitored. First, EM was performed to observe mitochondrial fission in the microvasculature in vivo. Our result noted that mitochondria became smaller and punctate with significantly shorter lengths in diabetic microvasculature compared with the control group (white arrows in Fig. 4A). However, the majority of mitochondria in empagliflozin-treated group exhibited a long filamentous morphology, indicating the likelihood ability of empagliflozin to suppress diabetes-induced mitochondrial fission (Fig. 4A). Furthermore, CMECs were isolated from normal, diabetic and empagliflozin-treated diabetic mice to assess mitochondrial fission in vitro. CMECs from diabetic mice treated with Mdivi1 were used as the negative controls. Isolated CMECs were identified by CD31 positivity and by performing a DiL-Ac-LDL phagocytic test (Fig. 4B). As shown in Fig. 4C, the mitochondria in diabetic cells had fewer free ends compared with those in control group, whereas the application of mdivi1 or empagliflozin reduced the numbers of mitochondrial fragments. Numerous bulb-like structures were observed at the base of mitochondrial tubules in empagliflozin-treated cells but not in diabetic cells. However, treatment with the fission activator FCCP (5 μM for 2 h) cancelled off empagliflozin-offered protective effect on fission. Moreover, Drp1 shuttling to the mitochondrial surface is an indispensable process for fission, and the subcellular localization of Drp1 was observed in empagliflozin group. Diabetes increased Drp1 migration onto the fragmented mitochondria carrying greater amounts of free debris (Fig. 4D). However, Drp1 foci on the mitochondria were clearly decreased in CMECs treated with empagliflozin or mdivi1. Notably, FCCP suppressed the protective action of empagliflozin on fission, leading to a greater number of mitochondrial fragments marked with Drp1. These data strongly suggest the ability of empagliflozin to repress excessive mitochondrial fission triggered by long-term diabetes in CMECs. Indeed, mitochondrial fission was manipulated by the balance between fission- and fusion-related proteins. Diabetes significantly diminished the levels of fusion-related proteins, including Mfn1 and Mfn2. However, empagliflozin effectively up-regulated the fusion-related proteins while down-regulating the fission-associated factors such as Fis1 and Mff (Fig. 4E).
Fig. 4.
Empagliflozin diminishes diabetic-induced mitochondrial fragmentation and regulated the balance of proteins responsible for mitochondrial fission and fusion. CMECs were obtained from the control group, diabetic group and empagliflozin group. After isolation, empagliflozin-treated CMECs were treated with FCCP to trigger mitochondrial fission. A. EM was performed to observe mitochondrial fission in vivo. Diabetes induced mitochondrial disintegration into numerous round fragments of varying sizes accompanied by the disappearance of crista (white arrow), unlike the characteristic reticulo-tubular mitochondrial morphology seen in the control group, and these changes were reversed by empagliflozin; B. CD31 immunocytochemistry of CMECs and Dil-acetylated low-density lipoprotein intake assay. (green, CD31; red, Dil-LDL); C. CMECs were isolated and cultured in vitro, and MitoTracker Deep Red was used to label the mitochondria, whose morphology was analyzed using fluorescence microscopy; D. Co-localization of Drp1 and mitochondria. The boxed area under each micrograph represents the amplification of the white square. More Drp1 was located on fragmented mitochondria, while empagliflozin reduced Drp1 migration onto mitochondria; E. Changes in proteins related to mitochondrial fission and fusion. Empagliflozin reduced fission-associated factors and increased fusion-involved protein levels. Mean ± SD, *P < 0.05 vs. Control (Cont) group; #P < 0.05 vs. Diabetic group, &P < 0.05 vs. Diabetic + EMPA group.
3.5. Inhibition of fission protected CMECs against senescence and barrier dysfunction through suppression of mtROS overproduction
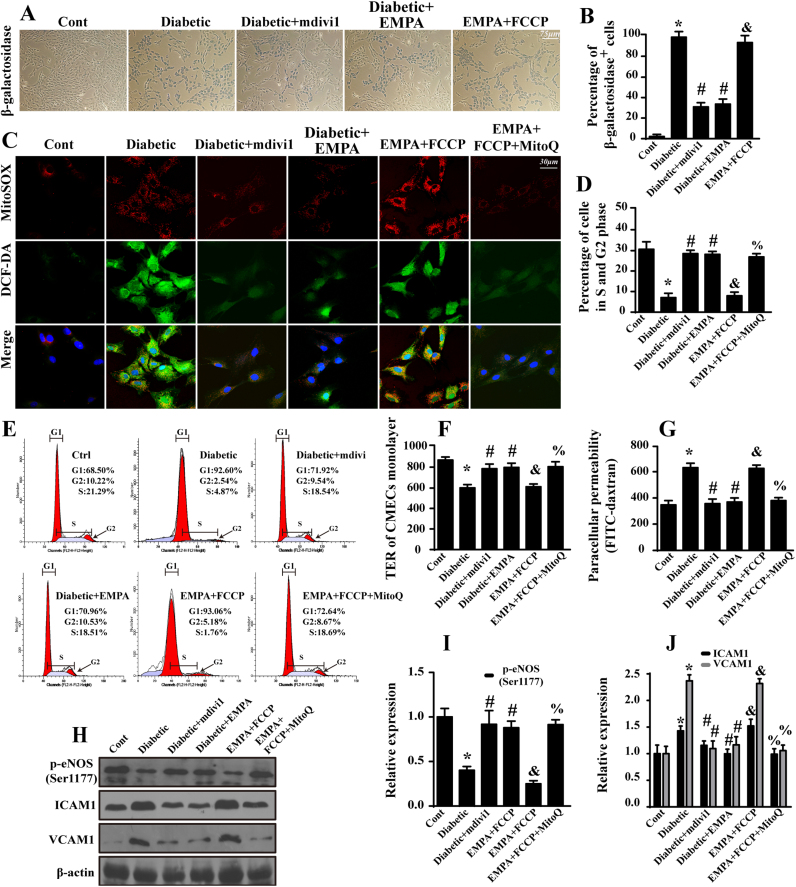
To gain more insight into the role of mitochondrial fission in diabetic microvascular injury, we assessed changes in CMEC senescence by performing β-galactosidase staining. Diabetes evoked a higher percentage of β-gal+ cells compared with that in the control groups (Fig. 5A–B). However, inhibition of fission in diabetic CMECs via the application of mdivi1 reduced the percentage of β-gal+ cells, reminiscent of empagliflozin treatment. In contrast, induction of fission resulted in the rebound of β-gal+ cells in the empagliflozin group, indicating empagliflozin inhibited cellular senescence by suppressing fission (Fig. 5A–B).
Fig. 5.
Empagliflozin protects CMECs against cellular senescence and barrier dysfunction by suppressing fission-mediated mtROS overproduction. A. Representative images of β-galactosidase staining, which was the marker for aged cells. B. Quantification of β-galactosidase-positive endothelial cells. C. The immunofluorescence of intracellular ROS and mtROS as assessed by DCF-DA and MitoSOX™ Red Mitochondrial Superoxide Indicator. Mitoquinone (MitoQ, 2 μM) was applied for 24 h to reduce the cellular oxidative damage. D–E. Cell cycle distribution was detected by performing flow cytometric quantification. Loss of fission due to empagliflozin and mdivi1 contributed to the cell cycle transition from G0/G1 to S phase, suggesting the anti-senescence effects of empagliflozin occur via fission inhibition. F. A TER assay was performed to detect CMEC barrier function during mitochondrial fission. TER increases when endothelial cells adhere and spread out and decreases when endothelial cells retract or lose adhesion, reflecting endothelial barrier integrity. G. FITC-dextran clearance was measured to assess changes in endothelial permeability. FITC-dextran was applied on top of the inserts and allowed to permeate through cell monolayers. The increased endothelial permeability resulted in the retention of more FITC-dextran. Thus, FITC content remaining in the plate indicates the extent of CMEC permeability. H–J. The change of expression of p-eNOS (Ser1177), ICAM1 and VCAM1. Mean ± SD, *P < 0.05 vs. Control (Cont) group; #P < 0.05 vs. Diabetic group, &P < 0.05 vs. Diabetic + EMPA group, %P < 0.05 vs. Diabetic + EMPA + FCCP group.
Oxidative stress serves as the primary cue underlying hyperglycemic cellular senescence, whereas mitochondria function as an important source of ROS. To confirm whether fission aggravated senescence through oxidative stress, an ROS probe was applied. Diabetic CMECs produced higher levels of mtROS and intracellular ROS (Fig. 5C), while mdivi1 and empagliflozin treatment restricted this production. Interestingly, fission activation offset the ROS-clearing effects of empagliflozin, indicating that a permissive role of fission in oxidative stress induction. Excessive ROS hampered the cell cycle transition from G0/G1 to S phase, which was reversed by the application of an mtROS scavenger, empagliflozin and mdivi1 (Fig. 5D–E). Moreover, preventing cellular senescence by neutralizing fission-induced ROS also reduced the residual FITC-dextran content and increased TER values (Fig. 5F–G), illustrating the favorable effects of empagliflozin on CMEC permeability and barrier function via the suppression of mitochondrial fission and subsequent oxidative stress. Moreover, empagliflozin also suppressed oxidative stress-mediated ICAM1 and VCAM1 upregulation while restored the contents of eNOS phosphorylation (Ser1177) (Fig. 5H–J).
3.6. Empagliflozin-induced suppression of fission contributed to CMEC migration and neovascularization by preservation of F-actin homeostasis
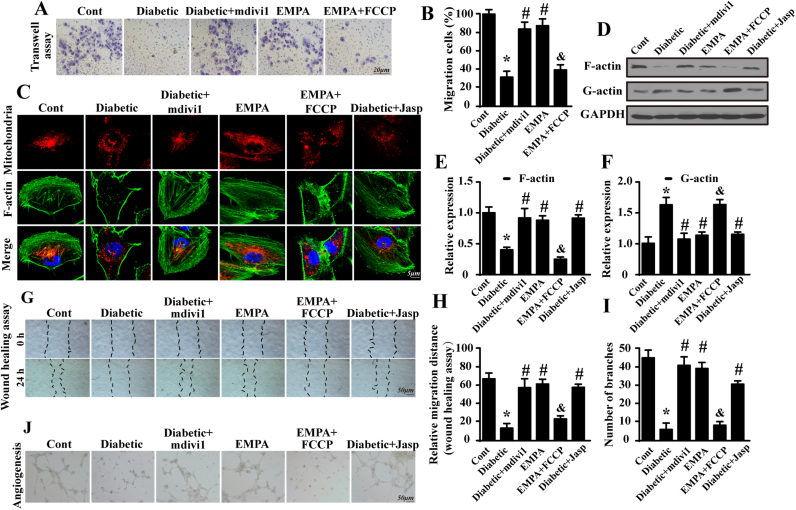
CMEC migration is vital for neovascularization. However, a transwell assay revealed the impaired migration capacity of diabetic CMECs as evidenced by reduced ability of cells to translocate through membrane inserts in response to SDF-1 (Fig. 6A–B). Empagliflozin restored the chemotactic responses of CMECs by limiting fission because fission activation cancelled the positive actions of empagliflozin on CMEC migration. As F-actin is the key stress fiber that directly monitors CMEC mobilization, we speculated that blunted migration arose from fission-involved F-actin dysregulated homeostasis. Presence of fragmented mitochondria concurrently with disappearance of F-actin was supported by the co-immunofluorescence of mitochondria and F-actin (Fig. 6C). Jasplakinolide (Jasp), a F-actin stabilizer was used to stabilize the actin cytoskeleton. The abolition of fission using mdivi1 preserved the filamentary structure of F-actin.
Fig. 6.
Suppression of fission by empagliflozin contributes to CMEC migration and neovascularization by preserving F-actin homeostasis. A–B. The endothelial migration response to SDF-1 was analyzed by performing a transwell assay. C. Co-immunofluorescence of F-actin and mitochondria to establish the role of mitochondrial fission in F-actin homeostasis. Fragmented mitochondria were accompanied by F-actin fiber disorder or dissolution, which was reversed by empagliflozin or mdivi1. D–F. Changes in the expression of F-actin and its metabolite G-actin as assessed by western blotting. G–H. A wound-healing assay was carried out to detect cell motility with F-actin depolymerization. I–J. The influence of mitochondrial fission on the capacity of CMECs to undergo angiogenesis via F-actin was measured by performing a Matrigel assay in vitro. Mean ± SD, *P < 0.05 vs. Control (Cont) group; #P < 0.05 vs. Diabetic group, &P < 0.05 vs. Diabetic + EMPA group.
Furthermore, the decrease in F-actin occurred in parallel with the accumulation of G-actin (Fig. 6D–F), an end-product of F-actin depolymerization, implying fission stimulated the disorder of F-actin synthesis and dissociation. The suppression of mitochondrial fission by empagliflozin reduced F-actin dissolution into G-actin, contributing to the recovery of CMEC migration capacity as evidenced by wound healing assay shown in Fig. 6G–H. Furthermore, these alterations induced by empagliflozin promoted neovascularization in vitro (Fig. 6I–J). If fission activation occurred via FCCP treatment, the constructive effects of empagliflozin on angiogenesis were ablated (Fig. 6I–J). Together, these data confirmed the augmentation of CMEC migration and neovascularization by empagliflozin via interference with fission-associated F-actin collapse.
3.7. Empagliflozin activated AMPK via increased AMP/ATP ratio in contribution to Drp1 inhibition
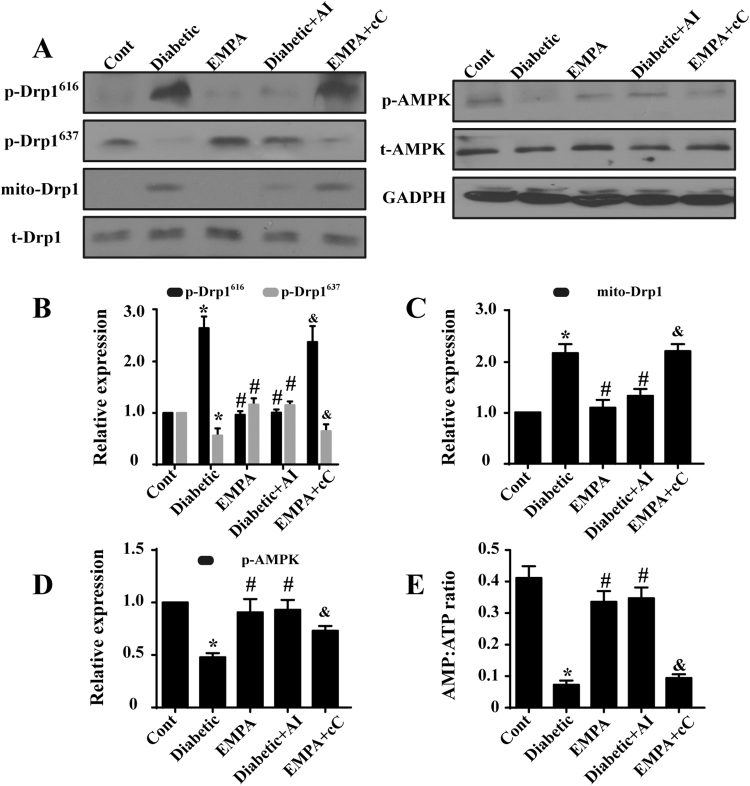
To explore the possible mechanism by which empagliflozin counteracts excessive fission, Drp1 post-transcriptional modification was evaluated. Our results indicated that diabetes promoted Ser616 phosphorylation on Drp1 although it reduced Ser637 phosphorylation (Fig. 7A–B), accompanied with a greater Drp1 accumulation on mitochondria. Empagliflozin promoted Ser637 but attenuated Ser616 phosphorylation of Drp1 in diabetes, leading to a lower mitochondrial Drp1 content (Fig. 7A–C). Next, given the key role of AMPK in the regulation of Drp1 phosphorylation, levels of AMP, ATP and AMPK activation were monitored. First, our data revealed suppressed AMPK phosphorylation in diabetes, the effect of which was reversed by empagliflozin (Fig. 7A–D). Moreover, empagliflozin also elevated the AMP/ATP ratio, favoring activation of AMPK. These data suggested that empagliflozin may promote AMPK activation via regulation of cellular AMP/ATP contents (Fig. 7E). The AMPK pathways activator and inhibitor, AICAR (AI) and compound C (cC), respectively, were used as the positive and negative controls. AICAR promoted AMPK phosphorylation in diabetic group, which was accompanied with increased phosphorylation of Drp1S637 and reduced phosphorylation of Drp1S616 (Fig. 7A–D). In contrast, AMPK inhibition by compound C ameliorated the effects of empagliflozin on Drp1 regulation (Fig. 7A–D). These data suggested that empagliflozin balances Drp1 phosphorylation at both Ser616 and Ser637 residues through AMPK activation, eventually resulting in the failure of Drp1 recruitment to the mitochondria.
Fig. 7.
AMPK signaling is activated by empagliflozin via increases in the AMP/ATP ratio, contributing to Drp1 modifications and subsequently mitochondrial fission. A–D. Empagliflozin increased p-AMPK levels. Activated AMPK was involved in regulating Drp1 phosphorylation. E. Empagliflozin increased the AMP/ATP ratio, which was responsible for AMPK activation. Mean ± SD, *P < 0.05 vs. Control (Cont) group; #P < 0.05 vs. Diabetic group, &P < 0.05 vs. Diabetic+EMPA group. AI: AICAR, cC: compound C.
4. Discussion
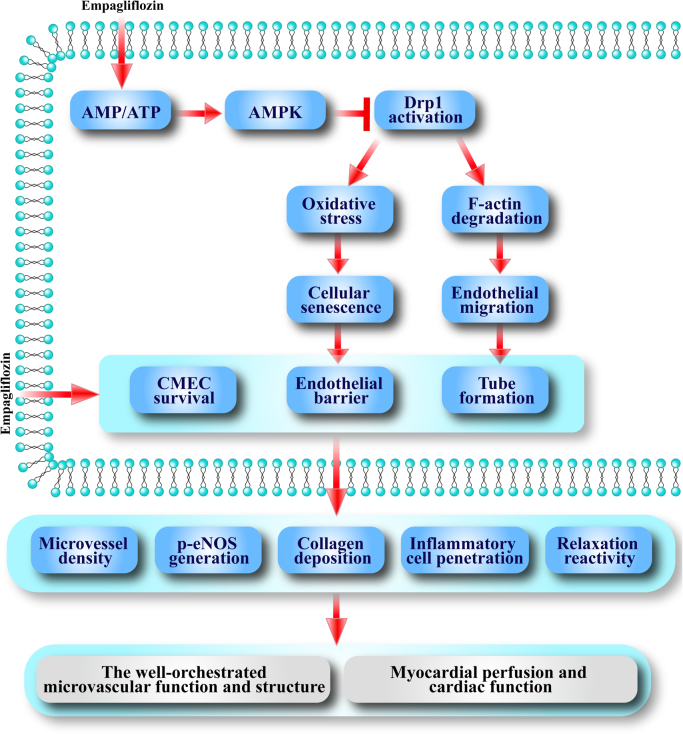
Pharmacological intervention targeting SGLT has recently emerged as a novel therapeutic option for diabetes [7], [35]. Empagliflozin, a specific SGLT2 inhibitor, represents a promising new class of glucose-lowering drugs to speed up glucose excretion in urine independent of insulin. In addition to its well-established glycemic effect, ample evidence has indicated the cardioprotective potential of empagliflozin. The salient findings from our present study revealed that empagliflozin is capable of improving cardiac microvascular perfusion, barrier function, microvessel density, eNOS phosphorylation, endothelial-dependent relaxation and CMEC survival. Our study indicated that empagliflozin may exert its beneficial effects through inhibition of diabetes-induced mitochondrial fission in an AMPK-dependent manner. With AMPK activation, empagliflozin effectively inhibited Drp1 activation and subsequently mitochondrial fission. On one hand, empagliflozin-induced decrease in fission may delay CMEC senescence by suppressing mtROS oxidative stress, leading to improved CMEC viability and barrier function. On the other hand, empagliflozin-induced endothelial migration as a result of F-actin homeostasis may promote angiogenesis (Fig. 8).
Fig. 8.
Scheme depicting proposed mechanisms involved in empagliflozin-offered protection against microvasculature damage in diabetes. Empagliflozin activates AMPK pathways through regulation of the AMP/ATP ratio. Activated AMPK pathways regulates Drp1 posttranscriptional phosphorylation modifications at Ser616 and Ser637, leading to the inability of Drp1 to translocate onto mitochondria and mitochondrial fission impairment. The loss of mitochondrial fission retards cellular senescence and preserves endothelial barrier/permeability by suppressing superfluous ROS. In consequence, endothelial migration and vascularization are improved by balanced F-actin degradation. Moreover, empagliflozin reduces CMEC apoptosis, increases cardiac microvessel density, promotes eNOS phosphorylation and alleviates vascular collagen deposition, leading to improved endothelial function and preserved vascular remodeling, ultimately lower levels of inflammatory cell penetration and better vascular relaxation. Through these aforementioned mechanisms, empagliflozin eventually facilitates diabetic myocardial perfusion and protects the heart against hyperglycemic injury.
During diabetes progression, endothelial damage is detectable early on in the course of disease progression compared with the pathological changes occurred in cardiomyocytes [36], even before overt hyperglycemia ensues. In the present study, we provided evidence for the first time supporting a direct protective role for empagliflozin in diabetic microvascular injury through two distinct mechanisms namely alleviation of endothelial dysfunction and vascular remodeling. First, empagliflozin improved eNOS phosphorylation and barrier function, which alleviated luminal stenosis and inflammatory cell or erythrocyte attachment on microvessels. Second, empagliflozin increased CMEC survival and delayed vascular fibrosis, which greatly alleviated vascular degeneration and enriched microvessel density. Through these mechanisms, empagliflozin increased blood circulation and cardiac perfusion. Unlike great vessels possessing extensive nerve distribution, cardiac microvascular vessels modify their contraction and relaxation by balancing metabolic substances and eNOS phosphorylation [37]. The regulatory role of empagliflozin on eNOS phosphorylation is consolidated by its action on vasomotor tone. Considerable evidence has documented the ability of empagliflozin to reduce SBP through facilitating osmotic diuresis [38]. Our study further bolstered the role of empagliflozin in microvascular diastolic response by promoting eNOS phosphorylation. In addition, beneficial effects of empagliflozin on vascular remodeling was noted, characterized by improved CMEC survival, lower levels of inflammatory related proteins, a thinner basement membrane, and decreased collagen fibrosis. These findings are reminiscent of cardiovascular benefits of empagliflozin against myocardial remodeling and heart failure [39]. Taken together, our data add to previous evidence supporting the defensive and regulatory actions of empagliflozin on cardiac structure and components.
In our study, we confirmed the regulatory role of mitochondrial fission in endothelial cell senescence and migration via mtROS overproduction and F-actin degradation, respectively. This is supported by a number of experimental evidence. First, superoxide overproduction may serve as a unifying mechanism for diabetic microvascular complications [40]. Our previous study identified Nox4 as a source for intracellular ROS overproduction [41]. In the present work, fission was associated with an intracellular ROS outbreak via the induction of mtROS. The mechanism underlying this process involved fission-associated mPTP opening and membrane potential collapse, leading to excessive electron leakage [42]. However, whether enhanced mitochondrial fission triggers other ROS sources, such as Nox1, Nox2, and Nox4, deserves further scrutiny. Second, our findings suggested that fission led to F-actin depolymerization into G-actin, contributing to the inability of CMECs to migrate in response to SDF-1, representing an ultimate obstacle in the formation of new micro-vessels. Indeed, successive mitochondrial fission was dependent on the cascade of event among Drp1, Drp1 receptor and stress fibers [43]. It was reported that transient accumulation of intracellular F-actin on the mitochondrial surface is a prerequisite for mitochondrial division [44]. Under physiological conditions, F-actin is regularly distributed in certain parts of the cytoplasm that control microvascular deformation and vascularization [34], [45]. When mitochondrial fission is initiated, F-actin first decomposes into G-actin that is then reassembled into F-actin at the outer mitochondrial membrane promote the formation of a contractile ring with the help of Drp1 and its receptor [46]. Considering the indispensable nature of F-actin in fission [47], [48], we argue that excessive fission would consume large amounts of cytoplasmic F-actin and also cause the uneven distribution of F-actin [46], ultimately resulting in the dysregulated F-actin homeostasis and impaired migration. More evidence is needed to support this speculation. The suppression of fission by empagliflozin liberates oxidative stress and supports F-actin homeostasis, which hinders cellular age and reverses vascularization potentiality.
Here we provided the first piece of evidence indicating empagliflozin inhibited fission via an AMPK/Drp1-dependent mechanism. Notably, considerable disagreements exist with regards to the effect of AMPK signaling since both inhibitory and stimulatory roles of AMPK have been reported for mitochondrial fission. Previous studies have demonstrated that AMPK, a sensor of cellular energy shortage, promotes mitochondrial fission by activation of Mff, the receptor for Drp1 [49], [50]. Nonetheless, other reports have argued that activation of AMPK represses mitochondrial fission through downregulation of Drp1 [51], [52]. In aortic endothelial cells, Drp1 and mitochondrial fission are inactivated by AMPK-exerted phosphorylation at Ser637 [20]. Moreover, our previous studies reconfirmed that AMPK activation was associated with fission inhibition via suppression of Drp1 [21]. Given the complexity of mitochondrial fission, and existence of several factors in fission regulation apart from Drp1 [53], the precise role of AMPK in the control of fission activation deserves further investigation. Moreover, other signaling pathways may possibly be involved in empagliflozin-related mitochondrial homeostasis. Our previous finding suggested that oxidative stress-induced endothelial mitochondrial damage is resulted from IP3R-triggered cellular overload and subsequently mitochondrial calcium elevation via MCU (mitochondrial calcium uniporter) [54]. Whether IP3R-related calcium homeostasis is regulated by empagliflozin is unknown. Besides, hyperglycemia-triggered mitochondrial fission may be lessened by mitophagy, a repair system for the mitochondrial homeostasis via timely clearance of the mitochondrial debris [15], [53]. Although careful scrutiny has identified mitophagy as an endogenous defender of mitochondrial damage in diabetes [55], [56], whether empagliflozin is capable of using the mitophagy machinery to disrupt the mitochondrial fission under diabetes remains unknown. Further study is warranted to verify such concept.
The clinical implication that can be drawn from our study is multifold. First, our data provided compelling evidence for the clinical application of empagliflozin in diabetic patients with cardiac microvascular dysfunction. Although ample clinical studies have confirmed that empagliflozin can reduce the risk of adverse cardiovascular events in patients with diabetes [57], little information is available with regards to the protective mechanisms involved [58]. In this study, we observed the cardio-protective role of empagliflozin beyond its hypoglycemic effect. Second, empagliflozin regulates the mitochondrial function via an-AMPK-dependent manner based on our experimental findings, offering survival advantage for the cardiac microvascular endothelial cells. Through the improved cardiac blood flow supply, empagliflozin retards the vascular complications and sustains cardiac function in the context of diabetes. To this end, empagliflozin can be considered as a cardiac microvascular-protection drug to maintain cardiac circulatory function and structure upon hyperglycemic insult. In conclusion, empagliflozin alleviated diabetic cardiac microvascular injury by inhibiting mitochondrial fission via the activation of AMPK signaling pathways. Further study is warranted to explore the clinical value of empagliflozin in diabetic vasculopathy.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (Nos. 81030002, 81270186, 81441008, 81102079) and the science technological innovation nursery fund of People Liberation Army General Hospital (No. 16KMZ02). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
Conflict of interest statement
The authors have declared that they have no conflicts of interest.
Contributor Information
Hao Zhou, Email: zhouhao301@outlook.com.
Yundai Chen, Email: yundai@vip.163.com.
Jun Ren, Email: jren@uwyo.edu.
References
- 1.Zhou H., Hu S., Jin Q., Shi C., Zhang Y., Zhu P., Ma Q., Tian F., Chen Y. Mff-dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature ischemia/reperfusion injury via induction of mROS-mediated cardiolipin oxidation and HK2/VDAC1 disassociation-involved mPTP opening. J. Am. Heart Assoc. 2017;6(3) doi: 10.1161/JAHA.116.005328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sawada N., Jiang A., Takizawa F., Safdar A., Manika A., Tesmenitsky Y., Kang K.T., Bischoff J., Kalwa H., Sartoretto J.L., Kamei Y., Benjamin L.E., Watada H., Ogawa Y., Higashikuni Y., Kessinger C.W., Jaffer F.A., Michel T., Sata M., Croce K., Tanaka R., Arany Z. Endothelial PGC-1alpha mediates vascular dysfunction in diabetes. Cell Metab. 2014;19(2):246–258. doi: 10.1016/j.cmet.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamilton S.J., Chew G.T., Watts G.F. Therapeutic regulation of endothelial dysfunction in type 2 diabetes mellitus. Diabetes Vasc. Dis. Res. 2007;4(2):89–102. doi: 10.3132/dvdr.2007.026. [DOI] [PubMed] [Google Scholar]
- 4.Eelen G., De Zeeuw P., Simons M., Carmeliet P. Endothelial cell metabolism in normal and diseased vasculature. Circ. Res. 2015;116(7):1231–1244. doi: 10.1161/CIRCRESAHA.116.302855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steven S., Oelze M., Hanf A., Kroller-Schon S., Kashani F., Roohani S., Welschof P., Kopp M., Godtel-Armbrust U., Xia N., Li H., Schulz E., Lackner K.J., Wojnowski L., Bottari S.P., Wenzel P., Mayoux E., Munzel T., Daiber A. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017;13:370–385. doi: 10.1016/j.redox.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zinman B., Lachin J.M., Inzucchi S.E. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2016;374(11):1094. doi: 10.1056/NEJMc1600827. [DOI] [PubMed] [Google Scholar]
- 7.Zinman B., Wanner C., Lachin J.M., Fitchett D., Bluhmki E., Hantel S., Mattheus M., Devins T., Johansen O.E., Woerle H.J., Broedl U.C., Inzucchi S.E., O E.-R. Investigators, empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 8.Cefalu W.T. Paradoxical insights into whole body metabolic adaptations following SGLT2 inhibition. J. Clin. Invest. 2014;124(2):485–487. doi: 10.1172/JCI74297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixon J.B. Obesity in 2015: advances in managing obesity. Nat. Rev. Endocrinol. 2016;12(2):65–66. doi: 10.1038/nrendo.2015.221. [DOI] [PubMed] [Google Scholar]
- 10.Quintero M., Colombo S.L., Godfrey A., Moncada S. Mitochondria as signaling organelles in the vascular endothelium. Proc. Natl. Acad. Sci. USA. 2006;103(14):5379–5384. doi: 10.1073/pnas.0601026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuhrmann D.C., Brune B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 2017;12:208–215. doi: 10.1016/j.redox.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W., Wang Y., Long J., Wang J., Haudek S.B., Overbeek P., Chang B.H., Schumacker P.T., Danesh F.R. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab. 2012;15(2):186–200. doi: 10.1016/j.cmet.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makino A., Scott B.T., Dillmann W.H. Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia. 2010;53(8):1783–1794. doi: 10.1007/s00125-010-1770-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou H., Zhu P., Guo J., Hu N., Wang S., Li D., Hu S., Ren J., Cao F., Chen Y. Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol. 2017;13:498–507. doi: 10.1016/j.redox.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou H., Du W., Li Y., Shi C., Hu N., Ma S., Wang W., Ren J. Effects of melatonin on fatty liver disease: the role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy. J. Pineal Res. 2018;64(1) doi: 10.1111/jpi.12450. [DOI] [PubMed] [Google Scholar]
- 16.Ding M., Ning J., Feng N., Li Z., Liu Z., Wang Y., Wang Y., Li X., Huo C., Jia X., Xu R., Fu F., Wang X., Pei J. Dynamin-related protein 1-mediated mitochondrial fission contributes to post-traumatic cardiac dysfunction in rats and the protective effect of melatonin. J. Pineal Res. 2018;64(1) doi: 10.1111/jpi.12447. [DOI] [PubMed] [Google Scholar]
- 17.Yang X., Wang H., Ni H.M., Xiong A., Wang Z., Sesaki H., Ding W.X., Yang L. Inhibition of Drp1 protects against senecionine-induced mitochondria-mediated apoptosis in primary hepatocytes and in mice. Redox Biol. 2017;12:264–273. doi: 10.1016/j.redox.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park S.J., Lee H., Jo D.S., Jo Y.K., Shin J.H., Kim H.B., Seo H.M., Rubinsztein D.C., Koh J.Y., Lee E.K., Cho D.H. Heterogeneous nuclear ribonucleoprotein A1 post-transcriptionally regulates Drp1 expression in neuroblastoma cells. Biochim. Biophys. Acta. 2015;1849(12):1423–1431. doi: 10.1016/j.bbagrm.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kashatus D.F. Restraining the divider: a Drp1-phospholipid interaction inhibits Drp1 activity and shifts the balance from mitochondrial fission to fusion. Mol. Cell. 2016;63(6):913–915. doi: 10.1016/j.molcel.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 20.Wang Q., Zhang M., Torres G., Wu S., Ouyang C., Xie Z., Zou M.H. Metformin suppresses diabetes-accelerated atherosclerosis via the inhibition of Drp1-mediated mitochondrial fission. Diabetes. 2017;66(1):193–205. doi: 10.2337/db16-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou H., Zhang Y., Hu S., Shi C., Zhu P., Ma Q., Jin Q., Cao F., Tian F., Chen Y. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J. Pineal Res. 2017;63(1) doi: 10.1111/jpi.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y., Zhou H., Wu W., Shi C., Hu S., Yin T., Ma Q., Han T., Zhang Y., Tian F., Chen Y. Liraglutide protects cardiac microvascular endothelial cells against hypoxia/reoxygenation injury through the suppression of the SR-Ca(2+)-XO-ROS axis via activation of the GLP-1R/PI3K/Akt/survivin pathways. Free Radic. Biol. Med. 2016;95:278–292. doi: 10.1016/j.freeradbiomed.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 23.Lee H.J., Jung Y.H., Choi G.E., Ko S.H., Lee S.J., Lee S.H., Han H.J. BNIP3 induction by hypoxia stimulates FASN-dependent free fatty acid production enhancing therapeutic potential of umbilical cord blood-derived human mesenchymal stem cells. Redox Biol. 2017;13:426–443. doi: 10.1016/j.redox.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das N., Mandala A., Naaz S., Giri S., Jain M., Bandyopadhyay D., Reiter R.J., Roy S.S. Melatonin protects against lipid-induced mitochondrial dysfunction in hepatocytes and inhibits stellate cell activation during hepatic fibrosis in mice. J. Pineal Res. 2017;62(4) doi: 10.1111/jpi.12404. [DOI] [PubMed] [Google Scholar]
- 25.Zhou H., Li D., Shi C., Xin T., Yang J., Zhou Y., Hu S., Tian F., Wang J., Chen Y. Effects of Exendin-4 on bone marrow mesenchymal stem cell proliferation, migration and apoptosis in vitro. Sci. Rep. 2015;5:12898. doi: 10.1038/srep12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou H., Yang J., Xin T., Zhang T., Hu S., Zhou S., Chen G., Chen Y. Exendin-4 enhances the migration of adipose-derived stem cells to neonatal rat ventricular cardiomyocyte-derived conditioned medium via the phosphoinositide 3-kinase/Akt-stromal cell-derived factor-1alpha/CXC chemokine receptor 4 pathway. Mol. Med. Rep. 2015;11(6):4063–4072. doi: 10.3892/mmr.2015.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X., Xu Y., Wang T., Shu D., Guo P., Miskimins K., Qian S.Y. Inhibition of cancer migration and invasion by knocking down delta-5-desaturase in COX-2 overexpressed cancer cells. Redox Biol. 2017;11:653–662. doi: 10.1016/j.redox.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao Y., Xiao X., Zhang C., Yu W., Guo W., Zhang Z., Li Z., Feng X., Hao J., Zhang K., Xiao B., Chen M., Huang W., Xiong S., Wu X., Deng W. Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing PI3K/AKT and NF-kappaB/iNOS signaling pathways. J. Pineal Res. 2017;62(2) doi: 10.1111/jpi.12380. [DOI] [PubMed] [Google Scholar]
- 29.Malinda K.M. In vivo matrigel migration and angiogenesis assay. Methods Mol. Biol. 2009;467:287–294. doi: 10.1007/978-1-59745-241-0_17. [DOI] [PubMed] [Google Scholar]
- 30.Sigala F., Efentakis P., Karageorgiadi D., Filis K., Zampas P., Iliodromitis E.K., Zografos G., Papapetropoulos A., Andreadou I. Reciprocal regulation of eNOS, H2S and CO-synthesizing enzymes in human atheroma: correlation with plaque stability and effects of simvastatin. Redox Biol. 2017;12:70–81. doi: 10.1016/j.redox.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu S., Wang X., Geng P., Tang X., Xiang L., Lu X., Li J., Ruan Z., Chen J., Xie G., Wang Z., Ou J., Peng Y., Luo X., Zhang X., Dong Y., Pang X., Miao H., Chen H., Liang H. Melatonin regulates PARP1 to control the senescence-associated secretory phenotype (SASP) in human fetal lung fibroblast cells. J. Pineal Res. 2017;63(1) doi: 10.1111/jpi.12405. [DOI] [PubMed] [Google Scholar]
- 32.Zhou H., Li D., Zhu P., Hu S., Hu N., Ma S., Zhang Y., Han T., Ren J., Cao F., Chen Y. Melatonin suppresses platelet activation and function against cardiac ischemia/reperfusion injury via PPARgamma/FUNDC1/mitophagy pathways. J. Pineal Res. 2017;63(4) doi: 10.1111/jpi.12438. [DOI] [PubMed] [Google Scholar]
- 33.Jin Q., Li R., Hu N., Xin T., Zhu P., Hu S., Ma S., Zhu H., Ren J., Zhou H. DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol. 2018;14:576–587. doi: 10.1016/j.redox.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi C., Cai Y., Li Y., Li Y., Hu N., Ma S., Hu S., Zhu P., Wang W., Zhou H. Yap promotes hepatocellular carcinoma metastasis and mobilization via governing cofilin/F-actin/lamellipodium axis by regulation of JNK/Bnip3/SERCA/CaMKII pathways. Redox Biol. 2018;14:59–71. doi: 10.1016/j.redox.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaul S. Is the mortality benefit with empagliflozin in type 2 diabetes mellitus too good to be true? Circulation. 2016;134(2):94–96. doi: 10.1161/CIRCULATIONAHA.116.022537. [DOI] [PubMed] [Google Scholar]
- 36.Rosenson R.S., Fioretto P., Dodson P.M. Does microvascular disease predict macrovascular events in type 2 diabetes? Atherosclerosis. 2011;218(1):13–18. doi: 10.1016/j.atherosclerosis.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 37.Katakam P.V., Wappler E.A., Katz P.S., Rutkai I., Institoris A., Domoki F., Gaspar T., Grovenburg S.M., Snipes J.A., Busija D.W. Depolarization of mitochondria in endothelial cells promotes cerebral artery vasodilation by activation of nitric oxide synthase. Arterioscler. Thromb. Vasc. Biol. 2013;33(4):752–759. doi: 10.1161/ATVBAHA.112.300560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chilton R., Tikkanen I., Cannon C.P., Crowe S., Woerle H.J., Broedl U.C., Johansen O.E. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes. Metab. 2015;17(12):1180–1193. doi: 10.1111/dom.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilbert R.E., Krum H. Heart failure in diabetes: effects of anti-hyperglycaemic drug therapy. Lancet. 2015;385(9982):2107–2117. doi: 10.1016/S0140-6736(14)61402-1. [DOI] [PubMed] [Google Scholar]
- 40.Sarwar C.M., Papadimitriou L., Pitt B., Pina I., Zannad F., Anker S.D., Gheorghiade M., Butler J. Hyperkalemia in heart failure. J. Am. Coll. Cardiol. 2016;68(14):1575–1589. doi: 10.1016/j.jacc.2016.06.060. [DOI] [PubMed] [Google Scholar]
- 41.Wang D., Luo P., Wang Y., Li W., Wang C., Sun D., Zhang R., Su T., Ma X., Zeng C., Wang H., Ren J., Cao F. Glucagon-like peptide-1 protects against cardiac microvascular injury in diabetes via a cAMP/PKA/Rho-dependent mechanism. Diabetes. 2013;62(5):1697–1708. doi: 10.2337/db12-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu S., Pi H., Zhang L., Zhang N., Li Y., Zhang H., Tang J., Li H., Feng M., Deng P., Guo P., Tian L., Xie J., He M., Lu Y., Zhong M., Zhang Y., Wang W., Reiter R.J., Yu Z., Zhou Z. Melatonin prevents abnormal mitochondrial dynamics resulting from the neurotoxicity of cadmium by blocking calcium-dependent translocation of Drp1 to the mitochondria. J. Pineal Res. 2016;60(3):291–302. doi: 10.1111/jpi.12310. [DOI] [PubMed] [Google Scholar]
- 43.Bereiter-Hahn J., Voth M., Mai S., Jendrach M. Structural implications of mitochondrial dynamics. Biotechnol. J. 2008;3(6):765–780. doi: 10.1002/biot.200800024. [DOI] [PubMed] [Google Scholar]
- 44.Hatch A.L., Ji W.K., Merrill R.A., Strack S., Higgs H.N. Actin filaments as dynamic reservoirs for Drp1 recruitment. Mol. Biol. Cell. 2016;27(20):3109–3121. doi: 10.1091/mbc.E16-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y., Li H., Bubolz A.H., Zhang D.X., Gutterman D.D. Endothelial cytoskeletal elements are critical for flow-mediated dilation in human coronary arterioles. Med. Biol. Eng. Comput. 2008;46(5):469–478. doi: 10.1007/s11517-008-0331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li S., Xu S., Roelofs B.A., Boyman L., Lederer W.J., Sesaki H., Karbowski M. Transient assembly of F-actin on the outer mitochondrial membrane contributes to mitochondrial fission. J. Cell Biol. 2015;208(1):109–123. doi: 10.1083/jcb.201404050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Preau S., Delguste F., Yu Y., Remy-Jouet I., Richard V., Saulnier F., Boulanger E., Neviere R. Endotoxemia engages the RhoA kinase pathway to impair cardiac function by altering cytoskeleton, mitochondrial fission, and autophagy. Antioxid. Redox Signal. 2016;24(10):529–542. doi: 10.1089/ars.2015.6421. [DOI] [PubMed] [Google Scholar]
- 48.Prudent J., Mcbride H.M. Mitochondrial dynamics: ER actin tightens the Drp1 noose. Curr. Biol. 2016;26(5):R207–R209. doi: 10.1016/j.cub.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Zhang C.S., Lin S.C. AMPK promotes autophagy by facilitating mitochondrial fission. Cell Metab. 2016;23(3):399–401. doi: 10.1016/j.cmet.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 50.Toyama E.Q., Herzig S., Courchet J., Lewis T.L., Jr., Loson O.C., Hellberg K., Young N.P., Chen H., Polleux F., Chan D.C., Shaw R.J. Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science. 2016;351(6270):275–281. doi: 10.1126/science.aab4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang J.W., Hong J.M., Lee S.M. Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride-induced liver fibrosis. J. Pineal Res. 2016;60(4):383–393. doi: 10.1111/jpi.12319. [DOI] [PubMed] [Google Scholar]
- 52.Li J., Wang Y., Wang Y., Wen X., Ma X.N., Chen W., Huang F., Kou J., Qi L.W., Liu B., Liu K. Pharmacological activation of AMPK prevents Drp1-mediated mitochondrial fission and alleviates endoplasmic reticulum stress-associated endothelial dysfunction. J. Mol. Cell. Cardiol. 2015;86:62–74. doi: 10.1016/j.yjmcc.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 53.Rovira-Llopis S., Banuls C., Diaz-Morales N., Hernandez-Mijares A., Rocha M., Victor V.M. Mitochondrial dynamics in type 2 diabetes: pathophysiological implications. Redox Biol. 2017;11:637–645. doi: 10.1016/j.redox.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu H., Jin Q., Li Y., Ma Q., Wang J., Li D., Zhou H., Chen Y. Melatonin protected cardiac microvascular endothelial cells against oxidative stress injury via suppression of IP3R-[Ca(2+)]c/VDAC-[Ca(2+)]m axis by activation of MAPK/ERK signaling pathway. Cell Stress Chaperon. 2017 doi: 10.1007/s12192-017-0827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suarez-Rivero J.M., Villanueva-Paz M., De La Cruz-Ojeda P., De La Mata M., Cotan D., Oropesa-Avila M., De Lavera I., Alvarez-Cordoba M., Luzon-Hidalgo R., Sanchez-Alcazar J.A. Mitochondrial dynamics in mitochondrial diseases. Diseases. 2016;5(1) doi: 10.3390/diseases5010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Onphachanh X., Lee H.J., Lim J.R., Jung Y.H., Kim J.S., Chae C.W., Lee S.J., Gabr A.A., Han H.J. Enhancement of high glucose-induced PINK1 expression by melatonin stimulates neuronal cell survival: involvement of MT2/Akt/NF-kappaB pathway. J. Pineal Res. 2017;63(2) doi: 10.1111/jpi.12427. [DOI] [PubMed] [Google Scholar]
- 57.Bertero E., Prates Roma L., Ameri P., Maack C. Cardiac effects of SGLT2 inhibitors: the sodium hypothesis. Cardiovasc. Res. 2017 doi: 10.1093/cvr/cvx149. [DOI] [PubMed] [Google Scholar]
- 58.Shi X., Verma S., Yun J., Brand-Arzamendi K., Singh K.K., Liu X., Garg A., Quan A., Wen X.Y. Effect of empagliflozin on cardiac biomarkers in a zebrafish model of heart failure: clues to the EMPA-REG OUTCOME trial? Mol. Cell. Biochem. 2017;433(1–2):97–102. doi: 10.1007/s11010-017-3018-9. [DOI] [PubMed] [Google Scholar]