Abstract
The DNA coding sequence of TaqStoffel polymerase was fused with the DNA-binding domain of Pyrococcus furiosus ligase. The resulting novel recombinant gene was cloned and expressed in E. coli. The recombinant enzyme was purified and its enzymatic features were studied. The fusion protein (PfuDBDlig-TaqS) was found to have enhanced processivity as a result of the conversion of the TaqDNA polymerase from a relatively low processive to a highly processive enzyme. The abovementioned processivity enhancement was about threefold as compared to the recombinant TaqStoffel DNA polymerase (TaqS), and the recombinant fusion protein was more thermostable. It had a half-life of 23 min at 99 °C as compared to 10 min for TaqS. The fusion protein also showed a significantly higher resistance to PCR inhibitors such as heparin or lactoferrin and the fusion polymerase-amplified GC-rich templates much more efficiently and was efficient even with 78% GC pairs.
Keywords: PCR, DNA polymerase, Fusion DNA polymerases, Archaeon
Introduction
At the core of PCR-based techniques is the use of a DNA polymerase which plays the major role in molecular biological applications including DNA amplification or sequencing (Terpe 2013). PCR requires a high temperature, and therefore, it is necessary to use a thermostable enzyme for DNA amplification; the most commonly used enzyme is a DNA polymerase derived from the thermophilic Thermus aquaticus. Its native form is produced in Thermus aquaticus and its recombinant form by Escherichia coli. The Taq’s half-life is short compared to that of the other polymerases, e.g., these isolated from Archaea: only 40 min at 95 °C or 9 min at 97.5 °C (Lawyer et al. 1993). Furthermore, the Taq DNA polymerase is not able to synthesize products which are longer than 4 kb (Hamilton et al. 2001). In general, Taq amplification efficiencies for targets shorter than 1 kb, with 45 to 56% CG content, lie at about 80% (Arezi et al. 2003). Usually, product yields decrease when the amplicon size becomes greater than 1 kb because of a relatively low processivity and thermostability of a wild-type enzyme. A novel strategy used to enhance the processivity and performance of the Taq is to fuse it with a thermostable DNA-binding protein such as a Sso7d DNA-binding protein derived from Sulfolobus solfataricus (Wang et al. 2004).
We have tried to improve the properties of the Taq polymerase by creating a fusion protein which contained a highly thermostable DNA-binding domain of the Pyrococcus furiosus ligase, a 6-amino acid linker and a sequence of the TaqStoffel polymerase.
Materials and methods
Construction of recombinant plasmids
The T. aquaticus strain (ATCC25104) was used to isolate a genomic DNA which was used as a template for the amplification of a taqStoffel fragment gene with the use of a standard PCR amplification protocol with the Marathon DNA polymerase (A&A Biotechnology, Gdansk, Poland). A DNA fragment of taqStoffel corresponding to nucleotides 997 to 2626 was obtained in PCR with the use of primers: 5’AATTTTGTTTAACTTTAAGAAGGAGATATACATATGGCCCTGGAGGAGGCCC
(forward) and 5′ GCAAGCTTGTCGACGGAGCTCGAATTCGGATCCTTAatggtggtggtggtggtgCTCCTTGGCGGAGAGCCAG (reverse). The primers contained sequences complementary to the taqStoffel gene (underlined), a sequence complementary to the pET-30 Ek/LIC vector (italics), and a sequence for the oligohistidine tag (lowercase). The stop codon (TTA) was added to the reverse primer immediately after the oligohistidine sequence.
The DNA-binding domain of Pyrococcus furiosus ligase gene was fused with a DNA fragment of taqStoffel (corresponding to nucleotides 997 to 2626 of taq gene) in PCR using primers: 5’TATTGGCTTTCGGAAGCGGAGGGGTCGAC GCCCTGGAGGAGGCCC (forward) and 5′ GCAAGCTTGTCGACGGAGCTCGAATTCGGATCCTTAatggtggtggtggtggtgCTCCTTGGCGGAGAGCCAG (reverse). The primers contained sequences complementary to the taqStoffel gene (underlined), a sequence complementary to the pET-30 Ek/LIC vector (italics), a sequence for a 6-amino acid residue linker (bolded), and a sequence for the oligohistidine tag (lowercase). The stop codon (TTA) was added to the reverse primer immediately after the oligohistidine sequence.
The genomic DNA of Pyrococcus furiosus (DSM 3638) was used as a template for the amplification of the DNA-binding domain of the ligase gene (accession number: NC_003413) using a standard PCR amplification protocol. The forward primer was 5′ ATTTTGTTTAACTTTAAGAAGGAGATATACATATGCCATTGTGGGCTTTATCTTCAACCA (containing a sequence complementary to the DBD fragment of the ligase gene (underlined), and a sequence complementary to the pET-30 Ek/LIC vector (italics) and the reverse primer was 5′.
TCCTCCAGGGCGTCGACCCCTCCGCTTCC GAAAGCCAATAAAGCCAATGCTTGC (containing a sequence complementary to the DBD fragment of the ligase gene (underlined) and a sequence for a 6-amino acid residue linker (bolded). As a result of the PCR amplification, the following two products were obtained: the taqStoffel gene (1703 bp) and the DBD fragment of the ligase gene (685 bp). The PCR products were then mixed together with the DNA of the pET-30 Ek/LIC vector (Novagen, Madison, WI, USA) digested with the use of BamHI and NdeI enzymes (NEB, UK), and the mixture was used for cloning with the use of the OverLap Assembly kit (A&A Biotechnology, Poland). The resulting pET30/PfuDBDlig-TaqS plasmid had a reading frame which contained the DBD sequence of P. furiosus ligase (amino acid residues 218 to 424), a 6-amino acid linker GSGGVD), a sequence of the TaqStoffel polymerase (amino acid residues 317 to 832), and a His tag domain which was necessary for the purification of the recombinant protein by metal affinity chromatography.
The nucleotide sequences of the resulting recombinant plasmids, pET30/TaqS and pET30/PfuDBDlig-TaqS, were confirmed by DNA sequencing (Genomed, Poland).
Expression and purification of TaqS and PfuDBDlig-TaqS polymerases
The pET30/TaqS and pET30/PfuDBDlig-TaqS plasmids were transformed into E. coli BL21 (DE3) RIL (Novagen, USA). The cells which carried the required plasmids were grown at 37 °C in the Luria-Bertani medium, supplemented with 50 μg/ml of kanamycin and 50 μg/ml of chloramphenicol to an OD 600 of 0.4 and were induced by incubation in the presence of IPTG, at a final concentration of 1 mM, for 24 h. The cells were then harvested by centrifugation and the pellets were resuspended in 20 ml of buffer A (50 mM Tris–HCl pH 9, 0.5 M NaCl and 5 mM imidazole). The samples were sonicated three times for 30 s at 4 °C and centrifuged for 15 min at 10000×g. The supernatant was heat-treated for 30 min at 70 °C, and the denatured host proteins were removed by centrifugation. The protein was next purified in a one-step procedure using Ni2+-affinity chromatography. The supernatant containing the protein was loaded directly into a His•Bind Column (Novagen, USA) prepared and equilibrated using the A buffer. The recombinant proteins were washed twice with the washing buffer B (50 mM Tris–HCl pH 9, 0.5 M NaCl and 40 mM imidazole) and then eluted with the elution buffer E (50 mM Tris–HCl pH 9, 0.5 M NaCl and 300 mM imidazole). The eluted fractions were dialyzed three times against the buffer D (100 mM Tris–HCl pH 8, 100 mM KCl, 0.2 mM EDTA). To remove the trace amounts of the genomic bacterial DNA, 25 U of Benzonase® endonuclease (Merck, Darmstadt, Germany) and MgCl2 were added at the final concentration of 5 mM and the preparation was incubated for 1 h at 37 °C. Next, Benzonase was inactivated by incubation for 15 min at 70 °C, and following this, the denatured proteins were removed by centrifugation. Finally, the preparations were dialyzed once against the storage buffer (50 mM Tris–HCl pH 8, 50 mM KCl, 1 mM DTT, 0.1 mM EDTA, 1% Tween 20, 1% Nonidet P-40 and 50% glycerol).
DNA polymerase activity assay
The activity of the DNA polymerase was measured in an isothermal reaction at 72 °C using a real-time PCR apparatus (IT-IS International Ltd., UK) and EvaEZFluorometric Polymerase Activity Assay Kit (Biotium, Hayward, USA) in accordance with the definition of one unit of enzyme activity by Tveit and Kristensen (“One unit of DNA polymerase activity is conventionally defined as the amount of enzyme that will incorporate 10 nmol of nucleotides during a 30-min incubation”). When the DNA polymerase is active, the primer is extended to form a double-stranded product which binds the EvaGreen dye, with the resulting increase in fluorescence. The rate of increase shows a positive correlation with the polymerase activity (Tveit and Kristensen 2001; Driscoll et al. 2014; Ma et al. 2006). The activity was determined using the commercial Taq polymerase (Thermo Scientific, USA) with an activity of 1 U/μl as a reference.
Optimization of PCR amplification
To optimize the amplification process, the polymerase activity was measured using various concentrations of MgCl2, KCl and (NH4)2SO4 in the buffer and various pHs. All reactions were performed using 1 mM of each dNTP, 0.4 mM of each primer and, as a template for PCR, the pET 30 plasmid DNA containing a known target sequence (PCR product of 300 bp). The PCR experiment was performed using 1 U of purified PfuDBDlig-TaqS DNA or TaqS DNA polymerase in 20 μl of the reaction mixture containing 50 ng of the DNA template. The PCR experiment was conducted as follows: 1 min at 94 °C; 25 cycles of 15 s at 94, 55, and 72 °C. To determine the optimum MgCl2 concentration, the PCR was performed at increasing concentrations of MgCl2 (0–9 mM) with the use of the Tris–HCl buffer. Furthermore, the PCR experiment was carried out using various concentrations of KCl (10–90 mM) and (NH4)2SO4 (10–90 mM), at various pHs (7.0–9.0), with the use of the Tris–HCl buffer.
Temperature stability was assayed as described by Dabrowski and Kur (1998). One unit of purified PfuDBDlig-TaqS and TaqS DNA polymerases were heated at 95 and 99 °C for 1, 5, 10, 20, 40, and 60 min. The same amount of enzyme was used to amplify a 300-bp target fragment under the optimal reaction conditions (in the same PCR experiment and under the conditions as determined in the optimization process): 20 mM Tris–HCl (pH 8.0), 4 mM MgCl2, 10 mM (NH4)2SO4, and 10 mM KCl.
Measurements of PCR amplification rates
The PCR amplification rates were measured using the method described by Lee et al. 2010, after some modifications. PfuDBDlig-TaqS and TaqSDNA polymerases were used to amplify the PCR products under the same conditions as those determined in the optimization process and with the use, as a template for PCR, of the pET 30 plasmid DNA containing a known target sequence (PCR product of 300, 500, and 1000 bp). Each PCR cycle started with an initial denaturation at 94 °C for 2 min and included 25 cycles of 15 s at 94 and 55 °C and 25 cycles of 5 to 60 s at 72 °C.
Processivity
The processivity test was carried out as described by Elshawadfy et al. 2014, with some modifications. Eighty-five microliters of reaction buffer (20 mM Tris–HCl pH 8.3, 10 mM KCl, 10 mM (NH4)2SO4, 0.1% Triton X-100), 290 μM of each of the four dNTPs, 40 nM primer template (5′-GGGGATCCTCTAGAGTCGACCTGC and 5′ TATCGGTCCATGAGACAAGCTTGCTTGCCAGCAGGTCGACTCTAGAGGATCCCC), 3 μl of EvaGreen Fluorescent DNA stain (Jena Bioscience, Jena, Germany), and 1 U of tested polymerase were pre-incubated at 50 °C for 5 min. Reactions were initiated by the simultaneous addition of 7.5 μl of 50 mM MgCl2 and 7.5 μl of 0.6 mg/μl of a heparin trap, and the polymerization process was allowed to proceed at 72 °C. Aliquots of 10 μl were withdrawn to cold tubes (4 °C) at 0 (before polymerization process was allowed), 1, 2, 5, and 10 min after polymerization process was allowed and the extension was determined by melting the obtained products with the use of a real-time PCR MyGo instrument (IT-IS International Ltd., GB). The reaction included a pre-melt hold at 95 °C for 10 s (a ramp rate of 5 °C/s), an initial stage at 60 °C for 60 s (a ramp rate of 4 °C/s) and the final stage at 97 °C for 1 s (a ramp rate 0.201 °C/s). The processivity was determined by comparing the melting temperature profiles of the obtained products with the profiles of products with a known length, serving as markers in the reaction. Marker products were obtained in reactions which used the same primer and synthetic templates, which allowed the formation of products which were longer than the primer by 1, 2, 3…22 nt.
Sensitivity
Polymerase sensitivity (affinity for the template) was measured using a PCR in accordance with the protocol described by Halley and Prezioso (2003), after some modifications. The PCR was conducted under conditions optimized for the fusion polymerases PfuDBDlig-TaqS and TaqS. Plasmid DNA pET28a(+) (Novagen, Madison, USA) was used as a template along with the primers GATGCTGCTGGCTACCCTG (forward) and TCAAGAACTCTGTAGCACCGC (reverse), and the product of the reaction was 1264 bp. The reaction was conducted at decreasing template concentrations (serial two-fold dilutions of template) and the reaction included an initial denaturation at 94 °C for 2 min followed by 25 cycles of 15 s at 94 and 55 °C and of 60 s at 72 °C. The amplified products were analyzed in a 1.2 % agarose gel stained with ethidium bromide.
Resistance to inhibitors
The effect of PCR inhibitors such as lactoferrin (Sigma-Aldrich, St. Louis, USA) in a range from 54 to 0.84 μg, heparin (Sigma-Aldrich, St. Louis, USA) in a range from 600 to 4.7 ng, and human blood (from a healthy individual) in a range from 10 to 0.15%, on the catalytic activity of the PfuDBDlig-TaqS and TaqS DNA polymerases, was assessed by a PCR reaction using the genomic DNA of Staphylococcus aureus as a template and primers for the specific nuc gene detection as described by Barski et al. 1996.
Efficiency of a long-range PCR
The efficiency of a long-range PCR was measured as described by Kwona et al. 2016, after some modifications. The PCR experiment was performed with the use of 1 U of purified PfuDBDlig-TaqS DNA polymerase and also with the use of TaqS DNA polymerase which were placed in a 20 μl reaction mixture containing 50 ng of the E. coli genomic DNA as a template. Primers were used to amplify the following four DNA fragments: 5 kbp (5′ GCACCATCAACAATAAAGGCGC and 5′ TTCCGCTAATGCCATGGTGATAG), 8 kbp (5′ GCACCATCAACAATAAAGGCGC and 5′ AACGATGCGATATAGCCGACAC), and 10 kbp (5′ GCACCATCAACAATAAAGGCGC and 5′ AACGATGCGATATAGCCGACAC). The PCR experiment was conducted as follows: 2 min at 94 °C; 30 cycles of 30 s at 94 and 56 °C, and 60 s/kb at 72 °C. The amplified products were analyzed in a 1% agarose gel stained with ethidium bromide.
GC-rich templates
The efficiency of the amplification of the products which are rich in GC pairs was evaluated using a GC-rich template of Mycobacterium tuberculosis. The use of the forward primer CCGCCGTTACCACCCTTACCACCGTT and the reverse primer GCACCGCACCCACCAGCGGC enabled the production of a target with a length of 301 bp and with a GC content of 78% (Kotłowski 2015). The reaction took place under the conditions which were optimized for the fusion polymerase PfuDBDlig-TaqS and reference TaqS at an increasing polymerase activity. It started with 3 min at 95 °C followed by 35 cycles of 15 s at 94, 64 and 72 °C. The amplified products were analyzed in a 2% agarose gel stained with ethidium bromide.
Results
Expression and purification of TaqS and PfuDBDlig-TaqS polymerases
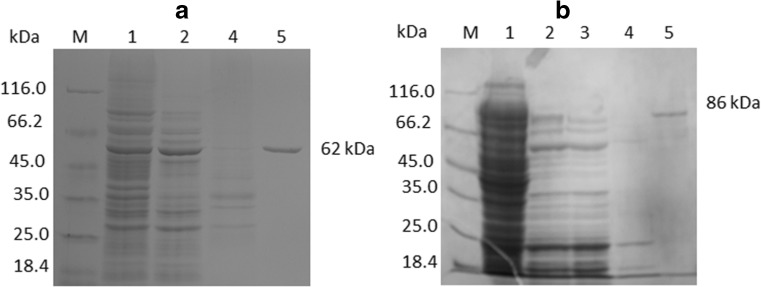
The gene encoding a Stoffel fragment of the Taq polymerase was cloned into a pET-30 Ek/LIC vector to generate a pET30/TaqS plasmid, leading to the expression of the enzyme as a fusion protein with a C-terminal polyhistidine tag. To achieve fusion with the DNA-binding domain of Pyrococcus furiosus ligase gene, two PCR products were mixed together with the DNA of the pET-30 Ek/LIC vector DNA to generate the pET30/PfuDBDlig-TaqS plasmid coding the fusion enzyme with a C-terminal polyhistidine tag. E. coli BL21 (DE3) RIL cultures harboring recombinant plasmids were generated, and cells were harvested and sonicated. The recombinant DNA polymerases were purified by passing the heat-denatured supernatant through a His•BindNi2+ affinity column. The specific activities of the purified TaqS and PfuDBDlig-TaqS polymerases were found to be 1800 and 1600 U/mg, respectively. These results indicated that the PfuDBDlig fusion did not have a negative effect on the catalytic activity of the TaqS DNA polymerase. After each purification step, the purity of the DNA polymerase was monitored by SDS–polyacrylamide gel electrophoresis (Fig. 1) which separated the following major protein bands: 62 or 86 kDa for TaqS and PfuDBDlig-TaqS, respectively; this result was in agreement with the molecular masses of 61.8 or 86.1 kDa which were calculated based on the amino acid sequences. The E. coli overexpression system used in this study enabled the production of 30 mg of TaqS polymerase and 20 mg of PfuDBDlig-TaqS fusion protein per 1 l of induced culture. The production efficiency of the TaqS and PfuDBDlig-TaqS polymerase in this study was quite satisfactory.
Fig. 1.
Expression and purification of the TaqS (a) and the PfuDBDlig-TaqS (b) polymerases. The proteins were analyzed on a 10% polyacrylamide gel (SDS–PAGE). Lane M, unstained protein weight marker 26.610 (14.4–116) (ThermoScientific, USA), with the molecular mass of proteins marked. Lane 1, sonicated extract of induced cells; lane 2, heat treatment; lane 3, fraction of unbound proteins; lane 4, fraction after second washing with buffer B; lane 5, purified protein after elution with buffer E
Characterization of the fusion PfuDBDlig-TaqS DNA polymerase
In our experiments, the activity of 1 U/μl was determined for TaqS and PfuDBDlig-TaqS DNA polymerases and was compared to the commercial Taq polymerase with an activity of 1 U/μl, using the EvaEZFluorometric Polymerase Activity Assay Kit (Biotium, Hayward, USA) in an isothermal reaction at 72 °C on a real-time PCR apparatus (IT-IS International Ltd., UK).
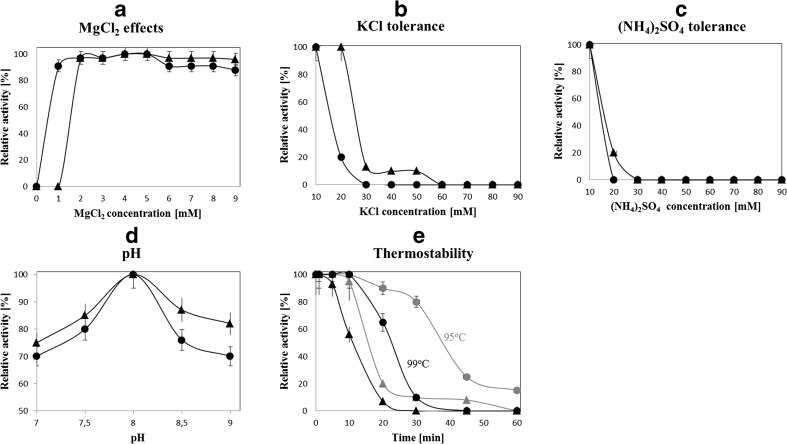
For characterization purposes, the polymerase activity was measured by PCR using various buffer compositions and concentrations of MgCl2, KCl, or (NH4)2SO4 and various pHs (Fig. 2).
Fig. 2.
Characterization of the fusion PfuDBDlig-TaqS DNA polymerase in comparison to the TaqS DNA polymerase. The effect of a MgCl2, b KCl, c (NH4)2SO4, d pH, and e temperature on polymerase activity: gray 95 °C; black 99 °C. Error bars for PfuDBDlig-TaqS DNA polymerase have the end bar, for TaqS DNA polymerase, do not have the end bar
MgCl2 concentration had a strong effect on the activity of TaqS and PfuDBDlig-TaqS, which was optimal for MgCl2 equal 2 to 5 mM and 1 to 5 mM, respectively (Fig. 2a).
The DNA polymerase activity was completely inhibited when KCl concentrations exceeded 60 mM for TaqS and 30 mM for PfuDBDlig-TaqS (Fig. 2b). TaqS and PfuDBDlig-TaqS DNA polymerase activities were also strongly affected by (NH4)2SO4 and were completely inhibited at concentrations of over 30 and 20 mM, respectively (Fig. 2c). The fusion of PfuDBDlig to the TaqS DNA polymerase resulted in a low tolerance to salt inhibition in PCR.
The effect of pH on the activity of TaqS and PfuDBDlig-TaqS DNA polymerases was evaluated using buffers with a various pH ranging from 7 to 9. Both polymerases had the highest enzyme activity at pH 8.0 (Fig. 2d).
Based on the results presented in this study, we found that the optimal PCR buffer for the PfuDBDlig-TaqS DNA polymerase consisted of 20 mM Tris–HCl (pH 8.0), 4 mM MgCl2,10 mM (NH4)2SO4, and 10 mM KCl.
Thermostability of TaqS and PfuDBDlig-TaqS DNA polymerases was tested by measuring the decrease in their activity after preincubation at 95 or 99 °C and was found to be remarkably higher for PfuDBDlig-TaqS. TaqS and PfuDBDlig-TaqS DNA were also found to have a half-life of 10 and 23 min at 99 °C, respectively (Fig. 2e).
PCR amplification rate and processivity
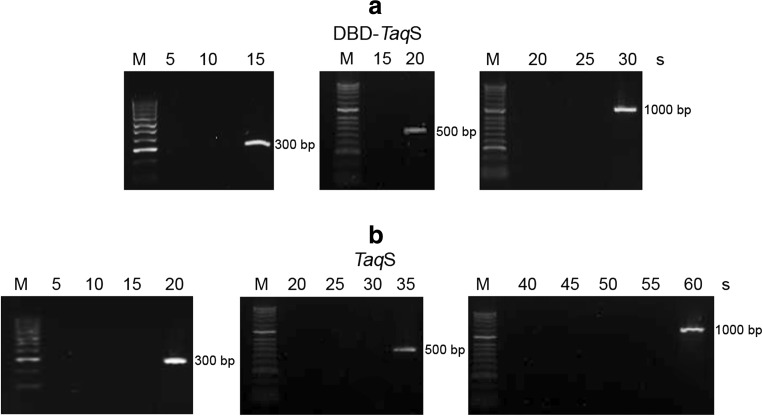
The fusion PfuDBDlig-TaqS DNA polymerase was found to replicate template strands at a faster rate than did the TaqS DNA polymerase (Fig. 3), namely, it replicated a 300-bp template within 15 s, a 500-bp template within 20 s, and a 1000-bp template within 30 s, while the TaqS DNA polymerase required 20 s for a 300-bp template, 35 s for a 500-bp template, and 1 min for a 1000-bp template. This showed that the fusion of the PfuDBDlig protein with the TaqS DNA polymerase was two times more efficient and the amplification of DNA required less time.
Fig. 3.
Comparison of PCR amplification rates of the fusion PfuDBDlig-TaqS DNA polymerase (a) and the TaqS DNA polymerase (b). The elongation times used for PCR amplification are shown at the top. Lane M, DNA molecular size marker (100–1000 bp) (Blirt, Gdańsk, Poland). The amplified products were analyzed in a 1.5% agarose gel stained with ethidium bromide
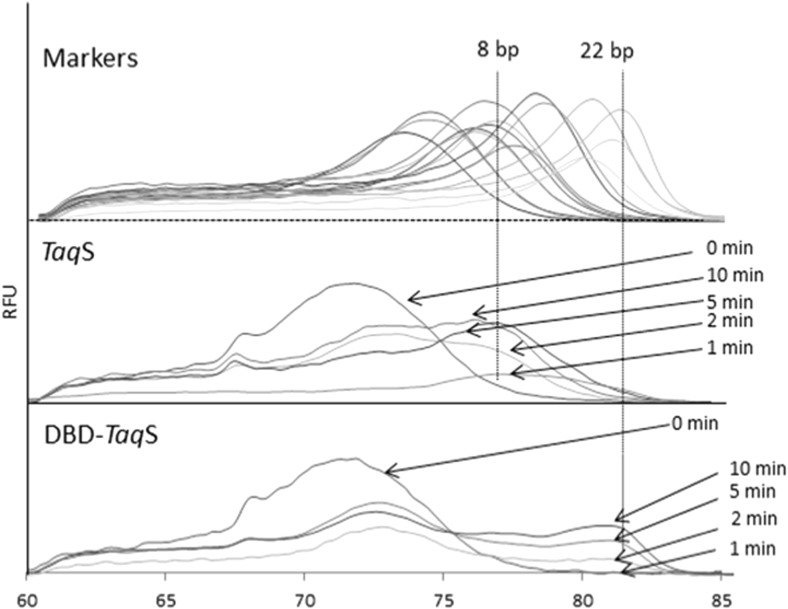
To determine processivity, the primer template and the polymerase were pre-incubated in the absence of Mg2+ and the reaction was initiated by the simultaneous addition of a metal and heparin trap. Next, processivity was determined by comparing the melting temperature profiles of the targets with the profiles of the products of a known length which served as markers in the reaction. In the case of the PfuDBDlig-TaqS DNA polymerase, a prominent melting pick is seen resulting from the incorporation of 22 nucleotides and representing processivity (Fig. 4). The control TaqS DNA polymerase had a prominent melting pick which indicated lower processivity in the order of 8 nucleotides. This shows that a fusion of the TaqS DNA polymerase with the PfuDBDlig strongly improves processivity.
Fig. 4.
Determination of processivity by measuring the melting temperature of DNA products in the presence of a heparin trap
Sensitivity
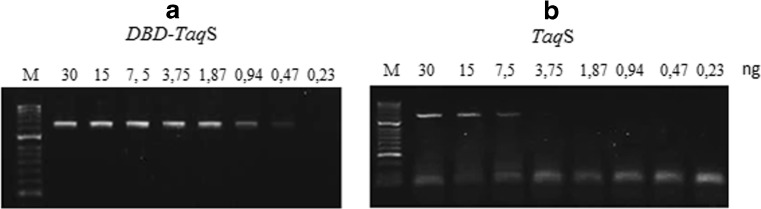
Sensitivity was measured in a PCR with the use of PfuDBDlig-TaqS and TaqS polymerases with two-fold serial dilutions of template, and the size of the PCR product was 1264 bp. The experiment showed that the fusion polymerase was more sensitive than the reference polymerase. In the case of the fusion polymerase, it was sufficient to use 0.47 ng of plasmid DNA, while the TaqS polymerase required at least 7.5 ng of the plasmid. The results are presented in Fig. 5.
Fig. 5.
Electrophoretic separation showing the products of plasmid DNA amplification as a function of a template concentration with the use of the fusion polymerase PfuDBDlig-TaqS polymerase (a) and the TaqS polymerase (b). Lane M, marker HyperLadder II (Bioline, UK). The amplified products were analyzed in a 1.2% agarose gel stained with ethidium bromide
Tolerance of the fusion PfuDBDlig DNA polymerase to PCR inhibitors
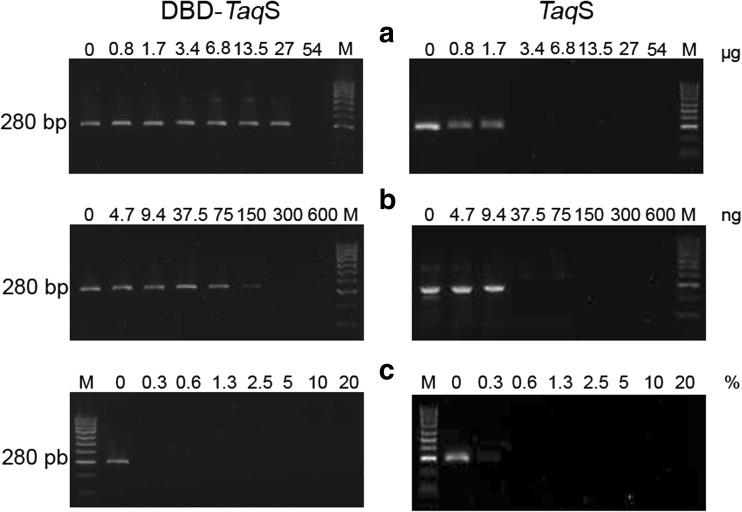
The fusion PfuDBDlig-TaqS enzyme and the TaqS DNA polymerase were PCR-tested for their resistance to serial dilutions of whole human blood, lactoferrin, and heparin, which have been reported to be PCR inhibitors in many publications (Al-Soud and Rådström 1998, 2000, 2001; Yokota et al. 1999; Kermekchiev et al. 2008). It was shown that the fusion PfuDBDlig-TaqSDNA polymerase was significantly more resistant to lactoferrin and heparin than the TaqStoffel enzyme and had no resistance to blood. The PfuDBDlig-TaqS polymerase remained functional in the presence of 0.84–27.0 μg of lactoferrin vs. 1.68 μg for the TaqS enzyme (Fig. 6a) and 4.7–150 ng of heparin vs. 9.4 ng for the TaqS enzyme (Fig. 6b).
Fig. 6.
The effect of lactoferrin (a), heparin (b), and blood (c) inhibitors on DNA amplification using the genomic DNA of S .aureus as a template and primers for S .aureus-specific nuc gene detection. Control reactions were carried out without any inhibitors. Lane M, DNA standards ladder (100–1000 bp) (Blirt, Gdańsk, Poland). The amplified products were analyzed in a 2% agarose gel stained with ethidium bromide
Long-range PCR efficiency
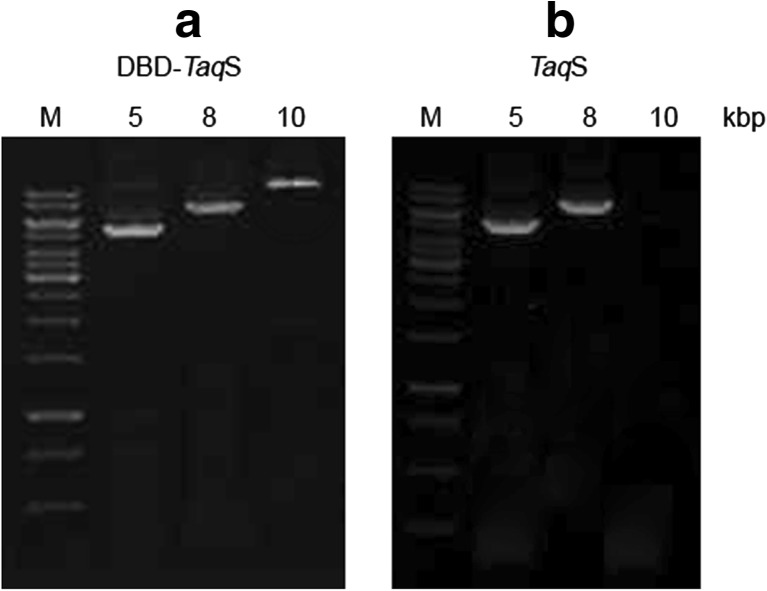
The long-term PCR amplification efficiency of PfuDBDlig-TaqS was tested using DNA fragments of various sizes, including 5-, 8-, and 10-kbp DNA targets, various primer combinations and the abovementioned optimal PCR buffer. The results were compared with what was achieved with the use of the TaqS DNA polymerase. The PCR amplification efficiency of PfuDBDlig-TaqS DNA polymerase using 5 and 8 kbp targets was similar to the efficiency of the TaqS DNA polymerase (Fig. 7). However, the fusion PfuDBDlig-TaqS DNA polymerase amplified longer DNA fragments more efficiently than TaqStoffel: 10 and 8 kbp, respectively.
Fig. 7.
Comparison of the PCR efficiency of the PfuDBDlig-TaqS DNA polymerase (a) and the TaqS DNA polymerase (b). DNA polymerases were used to amplify 5-, 8-, and 10-kbp targets from E. coli genomic DNA with the use of various extension times—72 °C, 1 min/kb. Lane M, GeneRuler DNA ladder SM 1331 (75–20,000 bp) (ThermoScientific, USA) The amplified products were analyzed in a 1% agarose gel stained with ethidium bromide
GC-rich templates
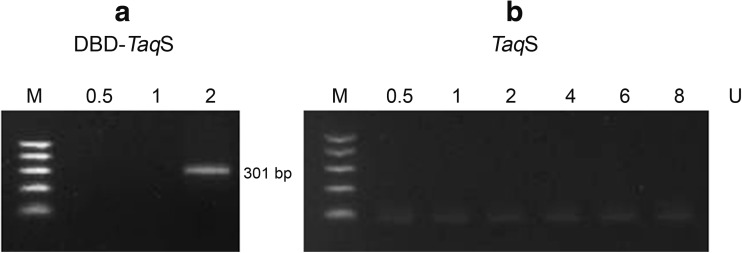
The potential of the fusion polymerase to amplify products with a high GC content was evaluated with the use of PfuDBDlig-TaqS and TaqS under the optimal conditions. A genomic DNA fragment of Mycobacterium tuberculosis with a GC content of 78% was amplified with the use of various polymerase concentrations: 0.5–8 U. Figure 8 presents the results of the experiment. It was shown that the fusion polymerase PfuDBDlig-TaqS had the ability to efficiently amplify a product with a high GC content with the use of only 2 U of polymerase, while the reference TaqS polymerase was shown not to work even where 8 U of polymerase were used.
Fig. 8.
Comparison of PfuDBDlig-TaqS DNA polymerase (a) and the TaqS DNA polymerase (b) PCR efficiency for templates with high content of GC. DNA polymerases were used to amplify 78% GC-rich target from M. tuberculosis genomic DNA for various polymerase concentrations (0.5–8 U). Lane M, DNA ladder (100–500 bp) (Blirt, Gdańsk, Poland). The amplified products were analyzed in a 2% agarose gel stained with ethidium bromide
Discussion
Currently, the PCR method is widely used in diagnostics, molecular biology, and genetic engineering. PCR effectiveness, or in other words amplification yield and accuracy, depends on the type of polymerase and PCR conditions. Many problems are encountered during the PCR amplification of so-called difficult templates. These primarily include large products, products with a high content of high-melting GC pairs or templates of clinical or environmental samples containing numerous DNA polymerase inhibitors. One of the solutions could be the use of a fusion polymerase with an additional DNA-binding domain. Research has indicated (Oscorbin et al. 2015) that the addition of a DNA-binding domain of DNA ligase from Pyrococcus abyssi to a PCR mixture has a positive effect on the activity of the Taq DNA polymerase in the presence of difficult templates. The literature indicates primarily an improvement in the PCR efficiency for long products (4 and 8 kbp) and a considerable reduction in the inhibitory effect of heparin in the reaction mixture.
Our studies have confirmed the results obtained by Oscorbin et al. It was shown that the fusion of a DBD of ligase (from Pyrococcus furiosus) to DNA polymerases improves basic enzyme properties such as thermostability, processivity, or reaction sensitivity. Polymerases containing a DBD of ligase are an ideal solution for PCR amplification of difficult templates.
The half-life of the fusion PfuDBDlig-TaqS polymerase doubled as compared to the reference TaqS, from 10 to 23 min at 99 °C, which predicts that the fusion polymerase will have a greater ability to amplify products with a high GC content. The PfuDBDlig-TaqS polymerase is able to efficiently amplify products with a GC content of up to 78%, while TaqS, which does not contain an additional domain, fails with targets with such a high GC content.
A two-fold increase in the amplification speed (from 16 to 33 nt/s) and an almost three-fold improvement of the processivity (from 8 to 22 nt) were found to increase the fusion polymerase’s ability to amplify large products by 25% (from 8 to 10 kb). Based on the results of the studies, it appears that the TaqStoffel polymerase is able to amplify targets with a size of 8 kb, while the fusion PfuDBDlig-TaqS polymerase, under the same conditions, of up to 10 kb .
Another example of problematic amplification is a PCR carried out in the presence of inhibitors. The fusion polymerase appeared again to have better properties than the reference TaqS. The PfuDBDlig-TaqS polymerase tolerates, in a reaction mixture, an almost 16-fold higher concentration of lactoferrin and an eightfold higher concentration of heparin which are both primary inhibitors found in clinical and environmental samples (Al-Soud and Rådström 1998, 2001; Yokota et al. 1999). Such an improvement may be due to a 16-fold increase in sensitivity of the fusion polymerase which therefore has a higher affinity for the template. It seems that the template is picked up more selectively from the mixture in the presence of a binding domain and the PCR is more efficient even when inhibitors are present.
The fusion PfuDBDlig-TaqS DNA polymerase consisting of a TaqStoffel polymerase and the DNA-binding domain of a ligase obtained from Pyrococcus furiosus exhibits a higher extension rate, higher processivity, thermostability, sensitivity, and a higher tolerance to some PCR inhibitors and is able to amplify templates with a higher GC content and longer PCR products than the TaqStoffel DNA polymerase. For this reason, it may be a better solution to use the PfuDBDlig-TaqS DNA polymerase instead of the Taq DNA polymerase in many PCR applications with difficult targets.
Authors’ contributions
MŚ carried out the cloning, protein expression, and purification experiments, characterized the DBD-TaqS DNA polymerase, and analyzed the data. BK coordinated the study and analyzed the data. BZP took part in the protein expression and purification experiments. MW participated in the preparation of the characterization of polymerases. RP took part in the characterization of polymerases. MO participated in the protein expression and characterization, coordinated the study, drafted the manuscript, and revised the manuscript. All the authors have read and approved the final version of the manuscript.
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Al-Soud WA, Rådström P. Capacity of nine thermostable DNApolymerases to mediate DNA amplification in the presence of PCR inhibiting samples. Appl Environ Microbiol. 1998;64:3748–3753. doi: 10.1128/aem.64.10.3748-3753.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Soud WA, Rådström P. Effect of amplification facilitators on diagnostic PCR in the presence of blood, feces and meat. J Clin Microbiol. 2000;38:4463–4470. doi: 10.1128/jcm.38.12.4463-4470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Soud WA, Rådström P. Purification and characterization of PCR-inhibitory components in blood cells. J Clin Microbiol. 2001;39:485–493. doi: 10.1128/JCM.39.2.485-493.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arezi B, Xing W, Sorge JA, Hogrefe HH. Amplification efficiency of thermostable DNA polymerases. Anal Biochem. 2003;321:226–235. doi: 10.1016/S0003-2697(03)00465-2. [DOI] [PubMed] [Google Scholar]
- Barski P, Piechowicz L, Galiński J, Kur J. Rapid assay for detection of methicillin-resistant Staphylococcus aureus using multiplex PCR. Mol Cell Probes. 1996;10:471–475. doi: 10.1006/mcpr.1996.0066. [DOI] [PubMed] [Google Scholar]
- Dabrowski S, Kur J. Recombinant His-tagged DNA polymerase II. Cloning and purification of Thermus aquaticus recombinant DNA polymerase (Stoffel fragment) Acta Biochim Pol. 1998;45:661–667. [PubMed] [Google Scholar]
- Driscoll MD, Rentergent J, Hay SA. Quantitative fluorescence-based steady-state assay of DNA polymerase. FEBS J. 2014;281(8):2042–2050. doi: 10.1111/febs.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshawadfy AM, Keith BJ, EeOoi H, Kinsman T, Heslop P, Connolly BA. DNA polymerase hybrids derived from the family-B enzymes of Pyrococcus furiosus and Thermococcus kodakarensis: improving performance in the polymerase chain reaction. Front Microbiol. 2014;5:224. doi: 10.3389/fmicb.2014.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halley G, Prezioso V (2003) Eppendorf HotMaster—an innovative hot start/cold stop technology for better PCR* results. Tech Notes (Eppendorff-HotMaster) 76–79
- Hamilton SC, Farchaus JW, Davis MC. DNA polymerases as engines forbiotechnology. BioTechniques. 2001;31:370–383. doi: 10.2144/01312rv01. [DOI] [PubMed] [Google Scholar]
- Kermekchiev MB, Kirilova LI, Vail EE, Barnes WM. Mutants of Taq DNA polymerase resistant to PCR inhibitors allow DNA amplification from whole blood and crude soil samples. Nucleic Acids Res. 2008;37:e40. doi: 10.1093/nar/gkn1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotłowski R. A novel method of Mycobacterium tuberculosis complex strain differentiation using polymorphic GC-rich gene sequences. Acta Biochim Pol. 2015;62:317–322. doi: 10.18388/abp.2015_1037. [DOI] [PubMed] [Google Scholar]
- Kwona KM, Kang SG, Sokolovac TG, Chod SS, Kimb YJ, Kima CH. Characterization of a family B DNA polymerase from Thermococcus barophilus CH5 and its application for long and accurate PCR. Enzyme Microb Technol. 2016;86:117–126. doi: 10.1016/j.enzmictec.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Lawyer FC, Stoffel S, Saiki RK, Chang SY, Landre PA, Abramson RD, Gelfand DH. High-level expression, purification, and enzymatic characterization of full-length Thermus aquaticus DNA polymerase and a truncated form deficient in 5′ to 3′ exonuclease activity. PCR Methods Appl. 1993;2:275–287. doi: 10.1101/gr.2.4.275. [DOI] [PubMed] [Google Scholar]
- Lee J, II, Cho SS, Kil E-J, Kwon S-T. Characterization and PCR application of a thermostable DNA polymerase from Thermococcus pacificus. Enzyme Microb Technol. 2010;47:147–152. doi: 10.1016/j.enzmictec.2010.06.003. [DOI] [Google Scholar]
- Ma C, Tang Z, Wang K, Tan W, Li J, Li W, Li Z, Yang X, Li H, Liu L. Real-time monitoring of DNA polymerase activity using molecular beacon. Anal Biochem. 2006;353(1):141–143. doi: 10.1016/j.ab.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Oscorbin IP, Boyarskikh UA, Zakabunin AI, Khrapov EA, Filipenko ML. DNA-binding domain of DNA ligase from the Thermophilic Archaeon Pyrococcus abyssi: improving long-range PCR and neutralization of Heparin’s inhibitory effect. Appl Biochem Biotechnol. 2015;176(7):1859–1869. doi: 10.1007/s12010-015-1683-2. [DOI] [PubMed] [Google Scholar]
- Terpe K. Overview of thermostable DNA polymerases for classical PCR applications: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol. 2013;97:10243–10254. doi: 10.1007/s00253-013-5290-2. [DOI] [PubMed] [Google Scholar]
- Tveit H, Kristensen T. Fluorescence-based DNA polymerase assay. Anal Biochem. 2001;289(1):96–98. doi: 10.1006/abio.2000.4903. [DOI] [PubMed] [Google Scholar]
- Wang Y, Prosen DE, Mei L, Sullivan JC, Finney M, Vander Horn PB. A novel strategy to engineer DNA polymerases for enhanced processivity and improved performance in vitro. Nucleic Acids Res. 2004;32:1197–1207. doi: 10.1093/nar/gkh271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota M, Tatsumi N, Nathalang O, Yamada T, Tsuda I. Effects of heparin on polymerase chain reaction for blood white cells. J Clin Lab Anal. 1999;13:133–140. doi: 10.1002/(SICI)1098-2825(1999)13:3<133::AID-JCLA8>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]